Abstract
Axonal outgrowth and guidance require numerous extracellular cues and intracellular mediators that transduce signals in the growth cone to regulate cytoskeletal dynamics. However, the way in which cytoskeletal effectors respond to these signals remains elusive. Here, we demonstrate that Porf-2, a neuron-expressed RhoGTPase-activating protein, plays an essential role in the inhibition of initial axon growth by restricting the expansion of the growth cone in a cell-autonomous manner. Furthermore, the EphB1 receptor is identified as an upstream controller that binds and regulates Porf-2 specifically upon extracellular ephrin-B stimulation. The activated EphB forward signal deactivates Rac1 through the GAP domain of Porf-2, which inhibits growth cone formation and brakes axon growth. Our results therefore provide a novel GAP that regulates axon growth and braking sequentially through Eph receptor-independent and Eph receptor-dependent pathways.
Electronic supplementary material
The online version of this article (10.1007/s00018-018-2858-0) contains supplementary material, which is available to authorized users.
Keywords: Axon growth, Porf-2, EphB, GAP, Vilse
Introduction
The formation of precise neuronal connectivity during the development of the central nervous system depends on axon growth and targeting, which are regulated by diverse families of cytoskeletal-related proteins [1–3]. Determining how these signaling pathways function to regulate axon growth and branching will enhance our understanding of wiring specificity in the nervous system and various neural developmental disorders. Although important progress has been made in identifying proteins involved in such processes [1, 4], little is known regarding the molecular mechanisms that link extracellular signals to the dynamic reorganization of actin-based structures upon axon–cell contact.
As a result of their ability to modulate the dynamic changes and rearrangement of the cytoskeleton, the Rho GTPases (among which RhoA, Cdc42 and Rac1 are the best characterized) have been highlighted as significant contributors to the orchestration of neuronal development [5]. In response to upstream signaling during different developmental stages, the Rho GTPases are precisely and coordinately regulated by corresponding regulatory proteins. Rho guanine nucleotide exchange factors (RhoGEFs) promote the release of bound GDP and facilitate the binding of GTP to RhoGTPases [6]. Rho GTPase-activating proteins (RhoGAPs) stimulate the intrinsic GTP hydrolysis activity of Rho GTPases, resulting in the inactive GDP-bound state [7, 8]. To date, approximately 70 RhoGAPs have been identified, but only a small fraction have been thoroughly investigated [8]. Preoptic regulatory factor-2 (Porf-2), also known as CrGAP/Vilse/ARHGAP39, is a multi-domain protein containing WW domain, MyTH4 (myosin tail homolog 4) domain, and RhoGAP domain [8]. Previous study showed that Porf-2 is involved in the Roundabout (Robo)-mediated midline crossing of axons in Drosophila [9, 10]. Furthermore, Porf-2 is involved in dendritic spine formation by linking CNK2 (a scaffold protein) via its WW domain to downstream cytoskeletal mediators [11]. Recent studies demonstrate that Porf-2 is essential for synaptic plasticity and spatial memory [12]. However, the function and molecular mechanisms of Porf-2 with multi-domains underlying axonal development in mammals remain unclear.
In this study, we demonstrate that Porf-2 plays an essential role in the inhibition of axon growth during postnatal development. We further reveal that Porf-2 is capable of binding to the EphB1 receptor, causing the outgrowth of axons to stop and retract upon EphB forward signal transduction within the axon growth cone. Porf-2 serves as a mediator of EphB1–Rac1 signaling by regulating growth cone dynamics through its GAP domain. Taken together, our results indicate that Porf-2 serves as a novel GAP that restricts axon extension in a cue-independent manner and brakes axon growth and branching through EphB1 signaling.
Materials and methods
Animals
All animal experiments conducted in this study were approved and monitored by the Animal Care Committee of the Shanghai Jiao Tong University School of Medicine. The EphB1−/− mice were kindly provided by Professor Mark Henkemeyer Lab at UT Southwestern Medical Center. The mutant and WT mouse line with the same background was used in our previous studies [13, 14] and also in the present study.
Plasmids
The Porf-2 DNA constructs were generated as previously described [15]. Briefly, the Porf-2 gene was amplified from hippocampal cDNA via PCR. Next, the Porf-2 gene was digested with the Xbal1/Not1 enzymes and inserted into the pLVX-IRES-ZsGREEN1 vector. All mutant forms of Porf-2 (ΔWW-Porf-2 (amino acids 65–99 deleted), ΔMyth4-Porf-2 (amino acids 717–902 deleted), and ΔGAP-Porf-2 (amino acids 914–1099 deleted)) were generated from Porf-2 via PCR and ligated into the pLVX-IRES-ZsGreen1 vector.
All EphA1, EphA2, EphA3, EphA4, EphB1, EphB2, and EphB3 plasmids were kindly provided by Nan-Jie Xu. The EphB1-ΔC mutant was generated from WT EphB1 via PCR and ligated into pCDNA3.1. The Porf-2 shRNA sequences were as follows: shRNA1: CCCTTGATTCCTCATGAAT; shRNA2: CTGCGAGATCTTCAAGCTA; shRNA3: CAAAGTGACACAGCACATA; and Control shRNA (shCtrl): TTCTCCGAACGTGTCACGT. All shRNAs were constructed in the PLKO.1 vector.
Cell culture and plasmids transfections
Primary hippocampus neuron cultures were prepared from mice as previously described [13]. Briefly, hippocampal neurons were isolated from E18 mice. Dissociated neurons were seeded onto poly-d-lysine (PDL) and cultured in neurobasal medium containing a B27 supplement and GlutaMAX. After 48 h of culture, the neurons were transfected with the indicated plasmids using a Ca2+ transfection system. NG108 cells were cultured in DMEM containing 10% FBS, 1% HT supplement, and 1% l-glutamine. For axon growth experiments, NG108 cells were transfected using a Ca2+ transfection system (Invitrogen) in 24-well dishes and were then induced to extend neurites by the addition of 1 mM dibutyryl cAMP as previously described [13].
Immunofluorescence staining
Both primary mouse hippocampal neurons and NG108 cells were fixed in PBS containing 4% PFA and 4% sucrose for 30 min at 4 °C. For brain slice immunofluorescence assay, after perfusion of mice with 4% PFA and fixation with 4% PFA at 4 °C overnight, 30 µm brain slices were generated. After washing in PBS, both the cells and brain slices were blocked and permeabilized in 1× PBS plus 0.3% Triton X-100 and 10% normal donkey serum (Jackson ImmunoResearch, 122346) for 60 min at room temperature, followed by incubation with the primary antibodies (diluted in blocking buffer) at 4 °C overnight. After washing three times (10 min for each wash) in 1× PBS plus 0.1% Triton X-100, the brain slices or cells were incubated in fluorescence-conjugated secondary antibodies (1:500 dilution in 1× PBS) plus DAPI for 1 h at RT. After washing three times, the chamber slides were then mounted with mounting medium and imaged. Images were captured using a Zeiss LSM 710.
As primary antibodies, we used mouse antibodies against β-tubulin III (Tuj1) (1:1000, Beyotime, AT809), GFAP (1:3000, Millipore, AB5806), and tau (1:3000, Sigma, T9450) and rabbit anti-Porf-2 (1:1000, Abcam). Cell nuclei were stained with DAPI.
For F-actin staining, NG108 cells or hippocampal neurons were cultured and fixed as described above and then stained with phalloidin (1:400, Thermo Fisher Scientific, R415). Images of both GFP-positive and GFP-negative cells in each group were obtained from at least four independent preparations.
F-actin/G-actin assay
The F-actin-to-G-actin ratio was determined by western blotting. Briefly, the two forms of actin differ in that F-actin is insoluble, whereas G-actin is soluble. NG108 cells transfected with indicated plasmids were homogenized in cold lysis buffer (10 mM K2HPO4, 100 mM NaF, 50 mM KCl, 2 mM MgCl2, 1 mM EGTA, 0.2 mM DTT, 0.5% Triton X-100, and 1 mM sucrose, pH 7.0), followed by centrifugation at 15,000×g for 30 min. Soluble actin (G-actin) was measured in the supernatant. The insoluble F-actin in the pellet was re-suspended in lysis buffer plus an equal volume of buffer 2 (1.5 mM guanidine hydrochloride, 1 mM sodium acetate, 1 mM CaCl2, 1 mM ATP, and 20 mM Tris–HCl, pH 7.5), followed by incubation on ice for 1 h to convert F-actin into soluble G-actin, with gentle mixing every 15 min. The samples were then centrifuged at 15,000×g for 30 min, and F-actin was measured in this supernatant. Samples from the supernatant (G-actin) and pellet (F-actin) fractions were proportionally loaded and analyzed via western blotting using a specific anti-β-actin antibody (1:5000, Thermo Fisher Scientific, MA5-15739).
Western blotting, Co-IP, pull-down, and affinity purification proteomics
For western blotting and co-immunoprecipitation, the procedures were performed as previously described [13]. Briefly, cells were lysed in 1% NP-40 buffer (20 mM Tris pH 8.0, 137 mM NaCl, 10% glycerol, 1% NP-40 alternative, 0.15 U/mL aprotinin, 1 mM PMSF, and 0.5 mM sodium vanadate), and the lysates were clarified via centrifugation. Equivalent amounts of protein lysate were incubated with the appropriate antibody and Protein G Sepharose beads for 3 h at 4 °C. Complexes were washed extensively with 1% NP-40 buffer and examined through immunoblotting analysis.
For pull-down assay and affinity purification proteomics, NG108 cells were transfected with Flag-Porf-2 or vector, and proteins were extracted from the cells. After co-incubation of the NG108 protein lysates with protein extracts from the mouse hippocampus for 4 h, equivalent amounts of the protein lysates were incubated with the Flag antibody and Protein G Sepharose beads for 3 h at 4 °C. The obtained complexes were washed extensively with 1% NP-40 buffer and examined through immunoblotting analysis. The proteins pulled down were stained with silver for visualization, and the differential bands were excised and subjected to mass spectrometry to identify the proteins. The mass spectrometry was conducted by Applied Protein Technology Co. Ltd. The proteins pulled down were also detected by immunoblotting with indicated antibodies.
As primary antibodies, we used mouse anti-GAPDH (1:5000, Sigma-Aldrich, G8795), mouse anti-β-actin (1:5000, Thermo Fisher Scientific, MA5-15739), mouse anti-HA (1:1000, Cell Signaling Technology, 2367s), goat anti-EphB2 (1:500, R&D Systems, P54763), mouse anti-Flag (1:1000, Sigma, A3682), and rabbit anti-Porf-2 (1:1000, Abcam, 93780).
Cell stimulation
NG108 cells were transfected with the corresponding plasmids. After subjecting NG108 cells to serum starvation for 6 h, they were stimulated with 5 µg/mL pre-clustered ephrin-B2-Fc (R&D Systems) or unconjugated Fc as a control for 30 min and then lysed with ice-cold lysis buffer for Co-IP or fixed for immunofluorescence staining.
Rac1/Cdc42 activation assay
Rac1/Cdc42 activity was assessed using the Rac1/Cdc42 Activation Assay Kit (Millipore, 17-283) according to the manufacturer’s instructions. The GST fusion protein corresponding to the p21-binding domain (PBD, residues 67–150) of human PAK-1 was expressed in Escherichia coli. This protein can specifically bind to and precipitate Rac-GTP and Cdc42-GTP from cell lysates. Briefly, cell lysates were clarified via centrifugation at 14,000×g at 4 °C for 10 min. Equal volumes of the lysates were then incubated with the Rac1/Cdc42 Assay Reagent (PAK-1 p21-binding domain PBD, agarose beads) to pull down activated Rac1 and Cdc42 proteins. After incubation at 4 °C for 1 h, the beads were washed three times with cold MLB buffer. After elution with sample buffer, the precipitated fraction, together with the total lysate, was subsequently subjected to sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) followed by western blotting analysis with either anti-Rac1 or anti-Cdc42 antibody, respectively (Millipore).
Axon and growth cone morphology assay and growth cone collapse assay
For measurements of axon length and number, images were obtained from at least four independent preparations of NG108 cells and cultured neurons. GFP-positive cells were assessed, and the longest neurite from each neuron or NG108 cells was measured from the initiation to the terminus of the axons using ImageJ software. For axon branching analysis, GFP-positive neurons were assessed regarding the number of branches projecting from the soma of the cells. For growth cone morphology, the F-actin staining area and number of protrusions were quantified using ImageJ in the growth cones of both GFP and non-GFP cells. The F-actin staining area was normalized to the control group. All conditions were examined in at least four independent experiments.
For growth cone collapse assay, the quantification was performed as described previously [16, 17]. A growth cone is considered to be in a collapsed status when forming a varicosity devoid of lamellipodia and exhibiting no more than two filopodia.
Stripe assay
The stripe assay was performed according to the method reported by Zhang et al. [18]. PDMS model substrates were patterned in 60 µm-wide parallel stripes separated by 90 µm gaps. A PDMS mold was reversibly sealed on PDL-coated glass bottom dishes (35 mm dish with a 20 mm bottom well), and micro-channels were formed between the PDMS mold and the dish well. A mixture of FITC-BSA and ephrin-B2-Fc or Con-Fc was added to one end of the micro-channels, and a vacuum was applied to the other end of the micro-channels to ensure that FITC-BSA/ephrin-B2-Fc or FITC-BSA/Con-Fc filled all micro-channels. The PDMS molds were removed after drying overnight. The stripe-coated dishes were then plated with dissociated primary cultured neurons to study axonal repulsion. Two days later, the neurons were infected with the indicated lentivirus (shCtrl, shPorf-2, ΔGAP-Porf-2). After 48 h, the cells were fixed on cover glasses and stained with an anti-tau antibody. Randomly selected images were obtained with a Zeiss 710 confocal microscope. All conditions were measured in at least four independent experiments. The number of axons that did not cross the adjacent FITC stripe and the total number of axons (including those crossing and not crossing the stripe) were quantified using ImageJ. The axonal repulsion index was calculated as follows: Repulsion index (%) = number of non-crossed axons/total axon number × 100.
Statistical analysis
All experiments were performed at least three times in triplicate. The results are presented as the mean ± SEM. Significant differences were determined with Student’s t test for two-group comparisons or ANOVA followed by Tukey’s test for multiple comparisons among more than two groups.
Results
Porf-2 restricts normal axonal growth
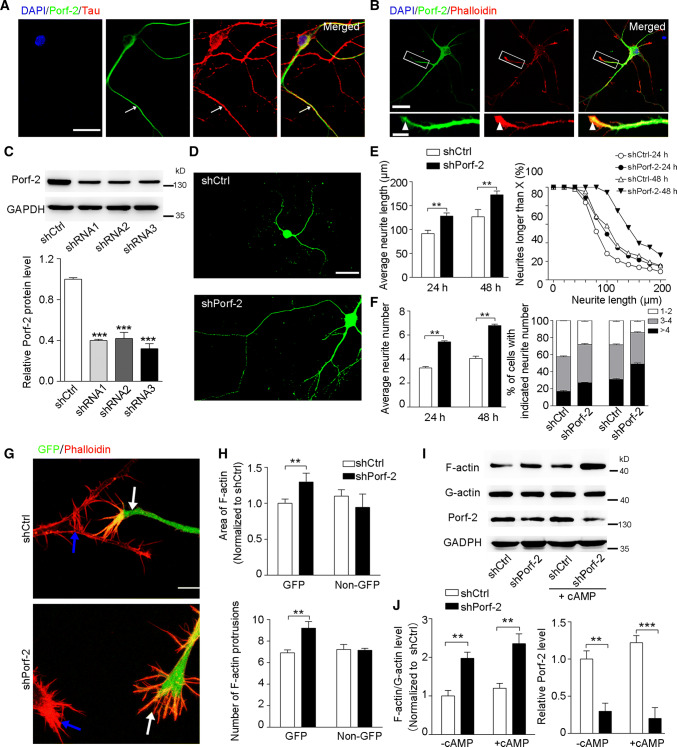
As Porf-2 is highly expressed in brain [12, 19], we further explored the expression pattern of Porf-2 in neural cells. We found that Porf-2 co-localized with tau (Fig. 1a) and phalloidin (Fig. 1b), which are markers of axon and growth cone F-actin, respectively. These indicate that Porf-2 is expressed in axon terminals.
Fig. 1.
Porf-2 regulates axon growth and growth cone dynamics. a Representative images show the co-localization of Porf-2 with axonal marker tau in primary cultured neurons. The arrows indicate Porf-2 and tau co-localization. Scale bar: 20 μm. b Cultured neurons were stained with antibodies against Porf-2 and phalloidin to visualize F-actin in the growth cone. The arrowheads show the co-localization of Porf-2 with F-actin. Scale bar: 20 μm in the upper panel and 5 μm in the lower panel. c Immunoblots show Porf-2 knockdown with shRNAs. Quantification of the blotting data in shRNA knockdown assay. ***P < 0.001 compared to shCtrl. d Representative images of cultured neurons after shCtrl or shPorf-2 transfection. Scale bar: 25 μm. e–f Quantification of the neurite length and number in the shCtrl and shPorf-2 groups after 24 and 48 h transfection. g Representative images of phalloidin staining in the shCtrl and shPorf-2 groups of NG108 cells. The white and blue arrows indicate GFP-positive and -negative (non-GFP) cells, respectively. Scale bar: 10 μm. h Quantification of the growth cone F-actin staining areas and number of protrusions. i The F-actin level was measured via immunoblotting in both cAMP-treated and -untreated conditions. j Quantification of the band intensity in i. The data were normalized to the shCtrl (-cAMP) group. All experiments were performed ≥ 3 times independently. Error bars represent ± SEM; **P < 0.01. ***P < 0.01
The expression of Porf-2 in axons suggested a possible function in modulating axonal development. We next used shRNAs that can knock down Porf-2 expression in cultured neurons to determine if Porf-2 plays an essential role in axon growth and branching (Fig. 1c). As shown in Fig. 1c, the shRNA3 has the highest knockdown efficiency, which was used in the following study. The axonal morphology was detected after shRNA transfection (Fig. 1d). Compared with those shCtrl neurons, we found that Porf-2 knockdown resulted in an increased axon length after 24 and 48 h transfection (Fig. 1e). Similarly, a greater number of neurites were observed after Porf-2 knockdown (Fig. 1f). Considering the role and expression pattern of Porf-2 at axon terminals, we hypothesized that Porf-2 mediates the process of growth cone dynamics. To verify the hypothesis, the growth cone morphology was detected and revealed by phalloidin staining in shCtrl- and shPorf-2-expressing cells (Fig. 1g). The data showed that both the F-actin staining area and protrusion number were increased in shPorf-2-expressing cells compared with those of shCtrl cells (Fig. 1h). By measuring the level of F-actin/G-actin, we confirmed that the F-actin/G-action ratio was significantly upregulated upon Porf-2 knockdown (Fig. 1i, j),
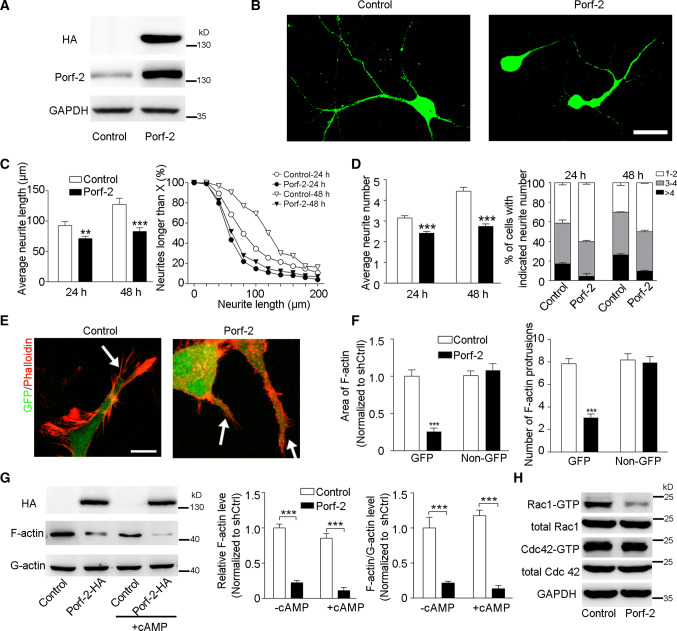
Conversely, the role of Porf-2 overexpression in axon growth was also assessed. First, immunoblot was done to verify the overexpression in neurons (Fig. 2a). The axonal morphology was detected after Porf-2 overexpression (Fig. 2b). We found that overexpression of Porf-2 in neurons led to a decreased neurite length (Fig. 2c) and reduced neurite number (Fig. 2d) compared with those control neurons. Similar data were also obtained in differentiated NG108 cells (data not shown). Furthermore, overexpression of Porf-2 was sufficient to disturb the growth cone structure (Fig. 2e). The quantitative data demonstrated that Porf-2 overexpression resulted in fewer areas of F-actin staining and less protrusion number in NG108 cells (Fig. 2f). Consistently, the growth cone collapse rate was significantly increased after Porf-2 overexpression (Fig. S1). Immunoblot data also showed that Porf-2 overexpression reduced total F-actin level (Fig. 2g) and decreased F-actin/G-actin ratio (Fig. 2g).
Fig. 2.
Porf-2 overexpression inhibits axon outgrowth and growth cone expansion. a Immunoblotting with Porf-2 and HA antibodies to confirm the overexpression of Porf-2. b Porf-2-overexpressed neurons showed decreased neurite length and number compared with control neurons. Scale bar: 25 μm. c, d Quantification of the neurite length and number in the control and Porf-2 groups at 24 and 48 h. e Representative image of F-actin staining in the control and Porf-2 groups of NG108 cells. The arrows indicate the growth cone in GFP-positive cells. Scale bar: 10 μm. f Quantification of the F-actin staining areas and number of protrusions in growth cone of both GFP and non-GFP cells in each group. The areas were normalized to the control group. g The F-actin level was measured via immunoblotting in both cAMP-treated and untreated conditions. The band intensity was quantified in each group. h GST pull-down to detect the Rho GTPase activity upon Porf-2 overexpression. All experiments were performed ≥ 3 times independently. Error bars represent ± SEM; **P < 0.01, ***P < 0.001
Considering the potential RhoGAP function of Porf-2 on Rho activity which is important in mediating axonal morphogenesis and actin dynamics, we evaluated the effect of Porf-2 on RhoGTPase activity (Fig. 2h). We found that Porf-2 overexpression decreased the Rac1-GTP level without affecting Cdc42-GTP (Fig. 2h), indicating that Porf-2 can inactivate Rac1 but exert no effect on Cdc42.
These results indicate that Porf-2 inhibits normal axon growth/branching and growth cone expansion during neuronal development through the inactivation of Rac1/F-actin signaling.
Porf-2 modulates axon morphogenesis via its GAP domain
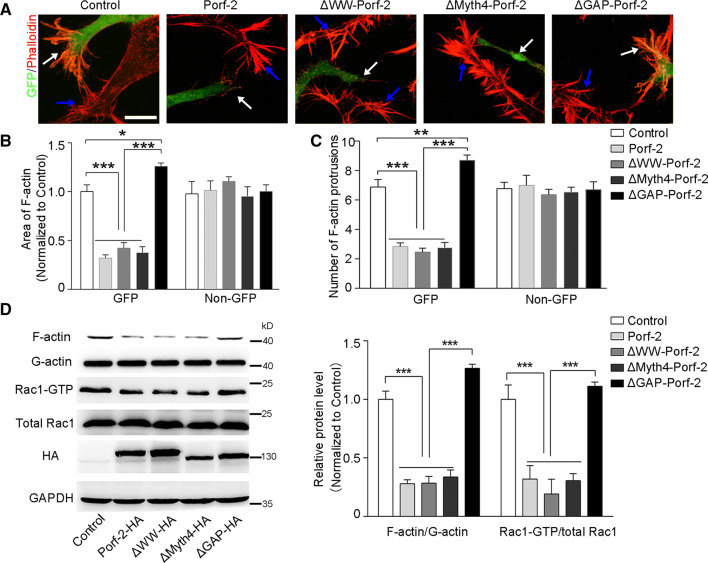
We next asked how Porf-2 carries out its inhibitory role during neuronal development. Since Porf-2 exhibits three conserved domains (WW, Myth4 (myosin tail homology) and GAP domains), we constructed three domain-deleted mutants of Porf-2 to determine the essential domain(s) (Fig. 3a). Immunoblot with anti-HA antibody was used to confirm the expression of the mutants (Fig. 3b). Similarly, the morphological features were detected after the expression of the mutants in neurons (Fig. 3c). We observed that ΔGAP-Porf-2-expressing neurons showed a significantly longer axon length than the Porf-2 group or control group, whereas ΔWW-Porf-2 or ΔMyth4-Porf-2 mutant-transfected neurons showed a short neurite length, similar to Porf-2-transfected neurons (Fig. 3d), suggesting that the GAP domain is the functional domain for axon growth inhibition (Fig. 3d). The average neurite number was also quantified in each group (Fig. 3e). The data showed that ΔGAP-Porf-2-expressing neurons showed more neurites than the Porf-2 group or control group, whereas ΔWW-Porf-2 or ΔMyth4-Porf-2 mutant-transfected neurons showed less neurite number, similar to Porf-2-transfected neurons (Fig. 3e). All the data demonstrated that the GAP domain is the key functional domain for axon growth inhibition (Fig. 3d, e). Similar results were observed in differentiated NG108 cells, which further validated our findings (data not shown).
Fig. 3.
Porf-2 inhibits axon outgrowth via its GAP domain. a The construct scheme of the domain-deleted plasmids of Porf-2. b Verification of domain-deleted plasmids via immunoblotting with the HA antibody. The same blot was probed with β-actin antibody as a loading control. c Representative images of neurons in different groups. Scale bar: 25 μm. d, e Quantification of the neurite length and number in each group. All experiments were performed ≥ 3 times independently. Error bars represent ± SEM; **P < 0.01, ***P < 0.001
Regarding growth cone morphology, the Porf-2 mutants, Porf-2 and control were expressed into the differentiated NG108 cells, respectively (Fig. 4a). We found that ΔGAP-Porf-2 showed an increased F-actin staining area (Fig. 4b) and number of protrusions (Fig. 4c), whereas both ΔWW-Porf-2 and ΔMyth4-Porf-2 cells exhibited a decreased F-actin staining area (Fig. 4b) and number of protrusions (Fig. 4c) in the growth cone, similar to Porf-2-expressing cells. The Rac1 activity and F-actin/G-actin were also evaluated in each group (Fig. 4d). We found that ΔGAP-Porf-2 expression significantly increased the Rac1-GTP/total Rac1 and F-actin/G-actin level, while ΔWW-Porf-2 and ΔMyth4-Porf-2 did not (Fig. 4d). Therefore, mutation of GAP domain lost its inhibitory effect on Rac1 activity, F-actin expression (Fig. 4d), as well as growth cone expansion (Fig. 4b, c). These data indicate that the GAP domain is required for Porf-2 activity in the growth cone for axon morphogenesis.
Fig. 4.
Porf-2 inhibits growth cone expansion through GAP domain-mediated inactivation Rac1/F-actin signaling. a Representative images of phalloidin staining in the growth cone of NG108 cells after transfected with mutant Porf-2. The white arrows indicate the GFP cells in each group and the blue arrows indicate the non-GFP cells. Scale bar: 10 μm. b, c Quantification of the F-actin staining area and number of protrusions in the growth cone of different groups. d Rac1 activity was measured via GST pull-down assay in each group. F-actin level was also measured via immunoblotting in each group. The band intensity of immunoblotting was quantified and normalized to the control group. All experiments were performed ≥ 3 times independently. Error bars represent ± SEM; *P < 0.05, **P < 0.01, ***P < 0.001
Porf-2 is highly affiliated with EphB1
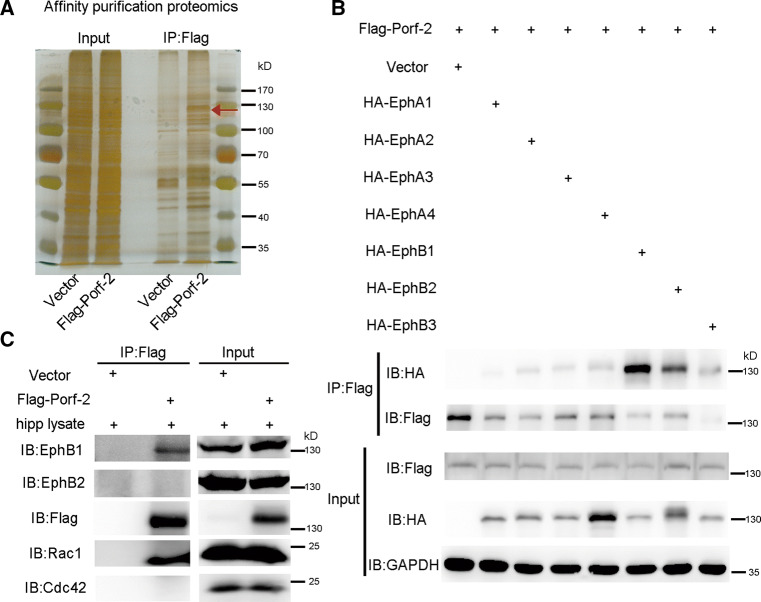
Given that axonal guidance is initiated by extracellular cues to stimulate receptor signaling and regulate the actin cytoskeleton in growth cones [20, 21], we next asked whether the Porf-2/Rac1 pathway serves as a downstream effector of this receptor signaling. We performed affinity purification proteomics to explore the potential interacting proteins of Porf-2 (Fig. 5a). The proteins of NG108 cells in which Flag-Porf-2 was expressed were immobilized on beads via Flag antibody to pull down the hippocampal proteins interacting with Porf-2 (Fig. 5a). The proteins pull-down by Flag-Porf-2 were subject to mass spectrometry analysis (Fig. 5a). EphB1 receptor, a key member of the EphB tyrosine receptor kinase family involved in axonal development [22, 23], was identified as a Porf-2 binding protein (Fig. S2), which differs from the findings of previous work [9, 10], and suggests a common signaling pathway shared by both the slit/Robo and ephrin/Eph families.
Fig. 5.
EphB1 is identified as a Porf-2 binding protein. a The proteins of NG108 cells transfected with Flag-Porf-2 or vector were immobilized on beads via Flag antibody. The immobilized NG108 proteins were used to pull down the proteins from the hippocampal lysate. The proteins pull-down were subject to sliver staining and further identified by mass spectrometry analysis. The red arrow indicates the band of EphB1 protein which was pulled down by anti-Flag antibody. b Screening the interaction of Porf-2 with EphA and EphB family members in NG108 cells. c Pull-down assay to verify the interaction of Porf-2 with endogenous EphB1 in the brain. The proteins of NG108 cells transfected with Flag-Porf-2 or vector were used to pull down the hippocampal proteins, which were further detected by indicated antibodies
To further elucidate the binding specificity of the possible Eph receptor members, we performed in vitro IP analysis of Porf-2 with all EphA and EphB members that are highly expressed in the brain (Fig. 5b). We found that EphB1 was the member with the highest binding affinity in vitro (Fig. 5b; Fig. S3). Furthermore, we detect the binding property of Porf-2 with endogenous EphB members EphB1 and EphB2, and found that Porf-2 predominantly binds to EphB1 (Fig. 5c). The results indicate EphB1 may serve as an upstream receptor protein that regulates Porf-2 in the brain.
In addition, we also screened the potential binding site of Porf-2 with EphB1. We found that domain mutant Porf-2, including ΔWW-Porf-2, ΔMyth4-Porf-2 and ΔGAP-Porf-2, can still bind well with EphB1 (Fig. S4a, b). This suggests that the three conventional domains of Porf-2 are not the binding part with EphB1. We further studied two mutants (Fig. S4c). We found that 1-333Porf-2 lost its high binding ability with EphB1, while 1-667Porf-2 and full-length Porf-2 did not (Fig. S4d). The data indicate that the binding site lies in the 333–667 amino acid of Porf-2 protein (Fig. S4d).
Porf-2 responds to and transduces EphB forward signaling
As EphB receptor signaling is stimulated by ephrin-B ligands upon cell–cell contact, we next asked whether Porf-2 functions in response to extracellular stimulation. To mimic ephrin-B–EphB-mediated cell–cell cross talk, NG108 cells co-transfected with EphB1-HA and Flag-Porf-2 were exposed to pre-clustered ephrin-B2-Fc, and the protein–protein interactions between EphB1 and Porf-2 were analyzed. Our results showed that the binding between Porf-2 and EphB1 was increased in response to external ephrin-B2-Fc stimulation (Fig. 6a). We further detected endogenous EphB1 and Porf-2 expressions in primary cultured neurons in response to external ephrin-B2-Fc stimulation using anti-EphB1 and anti-Porf-2 antibodies (Fig. 6b). Both EphB1 and Porf-2 were scattered in Con-Fc treated neurons (Fig. 6b). After ephrin-B2-Fc treatment, both EphB1 and Porf-2 can form into clusters (Fig. 6b). The clustered EphB1 puncta also co-localized with clustered Porf-2 (Fig. 6b). The quantification data showed that co-localized cluster puncta was significantly increased after ephrin-B2-Fc treatment in comparison with Con-Fc treatment (Fig. 6b). The data indicate that activated EphB1 can recruit and form into complex with Porf-2.
Fig. 6.
Porf-2 responds to ephrin-B-EphB signal transduction. a CO-IP assay to explore the binding ability of Porf-2 with EphB1 in response to ephrin-B2-Fc treatment in NG108 cells transfected with Flag-Porf-2 and EphB1-HA. b Endogenous Porf-2 formed clusters (arrowheads) and co-localized with EphB1 clusters (arrowheads) after ephrin-B2-Fc stimulation in primary cultured neuron. The white arrowheads indicate the clustered Porf-2 and EphB1. Scale bar: 5 μm. The co-localized cluster puncta was quantified in each group. c Co-IP assay shows the binding of Porf-2 with EphB1 but not EphB1-ΔC in NG108 cells. d Transfected Porf-2 formed clusters and co-localized with EphB1 clusters but not EphB1-ΔC clusters after ephrin-B2-Fc stimulation in NG108 cells. Scale bar: 10 μm. The red arrowhead in the upper panel indicates no Porf-2 clusters and EphB1 clusters after Con-Fc treatment. The whiter arrows in the middle panel indicate the co-localization of Porf-2 clusters and EphB1 clusters after ephrin-B2-Fc treatment. The white arrowheads in the lower panel indicate the EphB1-ΔC clusters, but no Porf-2 clusters after ephrin-B2-Fc treatment
To examine whether the intracellular segment of EphB1 is required for EphB1/Porf-2 interactions, we constructed an intracellular domain mutant of EphB1 (EphB1-ΔC) and found that Porf-2 failed to bind to EphB1-ΔC (Fig. 6c). Furthermore, Porf-2 failed to form clusters with EphB1-ΔC in response to ephrin-B2-Fc treatment (Fig. 6d). These data indicate that EphB1 is capable of recruiting Porf-2 through its intracellular segment and forms a complex with Porf-2 to transduce forward signaling upon ephrin-B2-Fc stimulation.
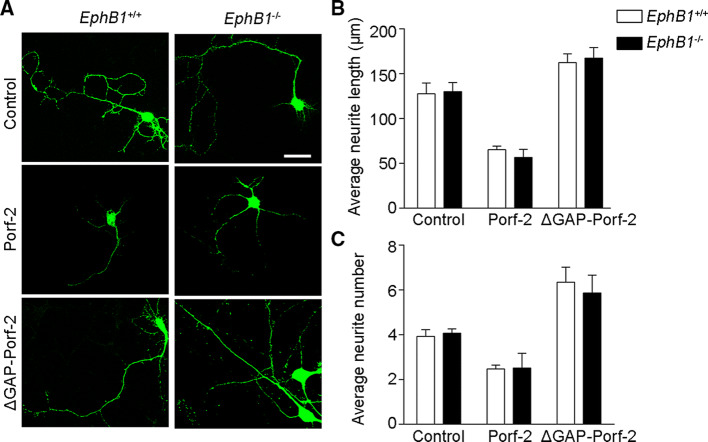
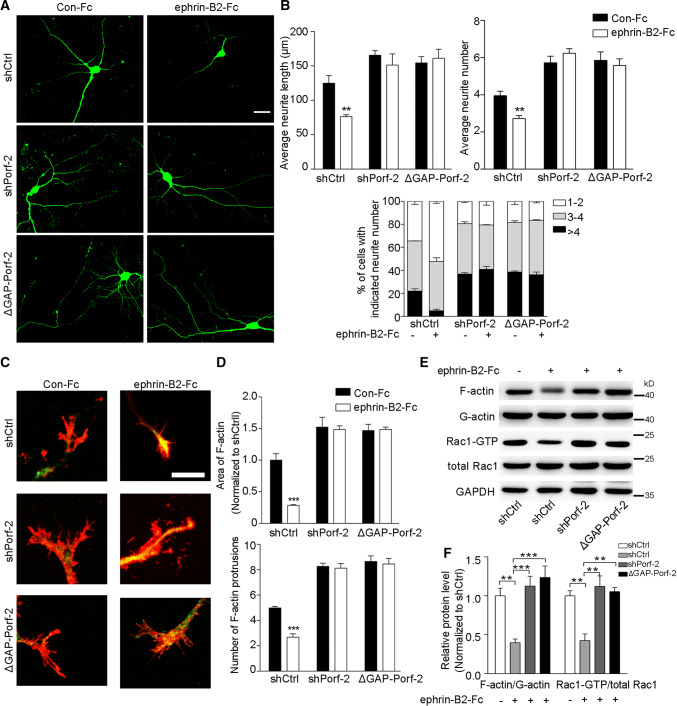
We next examined the requirement of EphB1 signaling for Porf-2 mediation of cytoskeleton regulatory pathways during different conditions of axonal growth. We compared axon morphogenesis in EphB1−/− and EphB1+/+ (wild-type) neurons (Fig. 7a). When the axons initially grew and extended in the dish, we found no differences in neurite length or number between EphB1−/− and EphB1+/+ neurons (Fig. 7b, c), suggesting that EphB1 per se has no effect on initial axon growth. Overexpression of Porf-2, but not ΔGAP-Porf-2, decreased neurite length and number in both EphB1−/− and EphB1+/+ neurons (Fig. 7b, c). Upon ephrin-B2-Fc treatment to mimic cell–cell contact, the axon morphology was detected (Fig. 8a). We found that upon ephrin-B2-Fc treatment, both the axon length and number were significantly reduced in shCtrl neurons (Fig. 8b). However, neurons transfected with shPorf-2 or ΔGAP-Porf-2 failed to respond to ephrin-B2-Fc treatment and exhibted a greater neurite length and neurite number (Fig. 8b). Similarly, the growth cone morphology was detected upon ephrin-B2-Fc treatment (Fig. 8c). The F-actin staining area and protrusion number were reduced upon ephrin-B2-Fc treatment in shCtrl neurons, but not in shPorf-2- or ΔGAP-Porf-2-transfected neurons (Fig. 8d). The growth cone collapse rate was significantly increased upon ephrin-B2-Fc treatment in shCtrl neurons (Fig. S5). However, knockdown or mutation of Porf-2 reversed or blocked the ephrin-B2-Fc induced growth cone collapse, exhibiting a lower collapse rate (Fig. S5). Consistently, the F-actin/G-actin level and Rac1 activity were also decreased upon ephrin-B2-Fc treatment in shCtrl neurons, but not in shPorf-2- or ΔGAP-Porf-2-transfected neurons (Fig. 8e, f). These data indicate the requirement of Porf-2 in both the control of initial intrinsic axon growth and subsequent EphB1 signal-dependent axon repulsion.
Fig. 7.
EphB1 per se has no effect on normal axon growth. a Representative images of neuronal morphology in WT (EphB1+/+) and EphB1−/− neurons after Porf-2 or mutant Porf-2 overexpression. Scale bar: 25 μm. b, c Neurite length and number were quantified in each group. The data are expressed as the mean ± SEM. The experiments were performed in triplicate independently
Fig. 8.
Porf-2 is required for EphB1/Rac1 signal transduction upon ephrin-B2 treatment. a Representative images of neuronal morphology in the shCtrl, shPorf-2 and ΔGAP-Porf-2 groups upon ephrin-B2-Fc treatment. Scale bar: 25 μm. b Quantification of the neurite length and number in each group. c Representative images of phalloidin staining in growth cone of shCtrl-, shPorf-2- and ΔGAP-Porf-2-transfected neurons upon ephrin-B2-Fc treatment. Scale bar: 7.5 μm. d Quantification of the F-actin staining area and number of protrusions in different groups. e The levels of F-actin and Rac1-GTP were detected via immunoblotting in shCtrl, shPorf-2 and ΔGAP-Porf-2 groups upon ephrin-B2-Fc treatment. f The band intensity of immunoblotting in e was quantified and normalized to the shCtrl group. All experiments were performed ≥ 3 times independently. Error bars represent ± SEM; **P < 0.01, ***P < 0.001
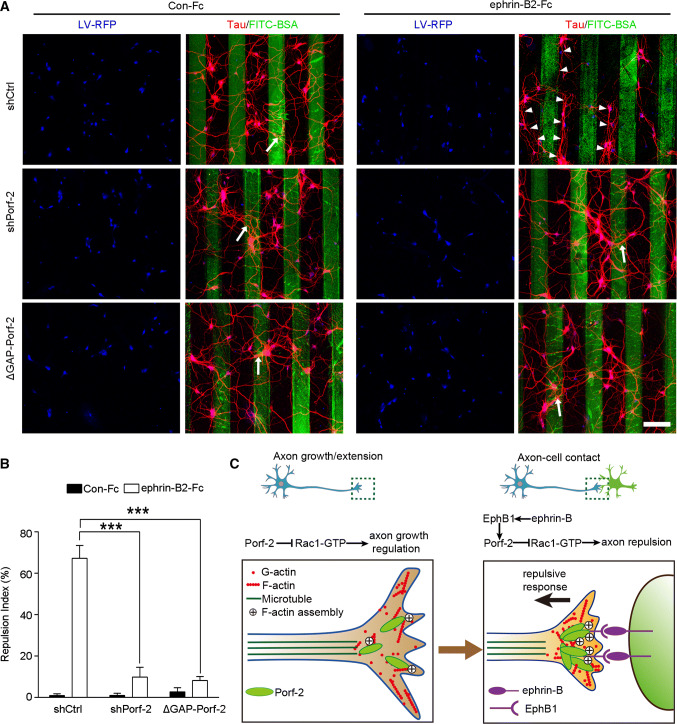
To further explore the precise role of Porf-2 in EphB1-mediated axon repulsion, we performed a stripe assay in which ephrin-B2-Fc repulsive borders were created. We first optimized the concentration of ephrin-B2-Fc in the stripe and found that 50 µg/mL of ephrin-B2 significantly repelled axons (data not shown). As shown in Fig. 9a, the shCtrl-transfected neurons initiated spontaneous outgrowth of axons on Con-Fc stripes, but preferred to grow shrinking away from ephrin-B2-Fc stripes. However, in the shPorf-2 and ΔGAP-Porf-2 groups, the axons grew crossing the ephrin-B2-Fc stripes frequently, suggesting that knockdown of Porf-2 abolished the repulsive effects of ephrin-B2 borders (Fig. 9a). The high repulsion index of ephrin-B2-Fc-containing stripes was decreased significantly in the shPorf-2 and ΔGAP-Porf-2 groups (Fig. 9b). These data support our conclusion that Porf-2 is required for EphB1 signal-mediated axon repulsion.
Fig. 9.
Porf-2 is required for EphB1 mediated axon repulsion. a Representative images of axon morphology in shCtrl-, shPorf-2- or ΔGAP-Porf-2-transfected neurons on ephrin-B2-Fc- or Con-Fc-containing stripes. Scale bar: 100 μm. The cells were transfected with indicated virus (blue) and stained with tau (red). Arrows indicate axons crossing the stripe and arrowheads indicate axon bundles that grow in the space between the stripes. b The quantification of the axonal repulsion index. Repulsion index (%) = non-crossed axon number/total axon number × 100. All experiments were performed ≥ 3 times independently. Error bars represent ± SEM; ***P < 0.001. c Schematic drawing demonstrating a previously uncharacterized mechanism for Porf-2 through inactivation of Rac1/F-actin via its GAP domain either in inhibiting filopodia formation and neurite branching during initial axon growth/extension (left panel), or in transduing EphB-mediated forward signaling upon axon–cell contact, which brakes axon growth and leads to growth cone repuslion (right panel)
Taken together, our findings indicate that Porf-2 serves not only as an intrinsic controller of initial axon growth inhibition in a cell-autonomous manner but also as a brake by inducing growth termination and retraction via transducing ephrin-B–EphB forward signaling upon cell–cell contact (Fig. 9c).
Discussion
In the current study, we showed that Porf-2, a neuron-specific RhoGAP, inhibited axon growth and branching during neuron development. The function of Porf-2 is exerted through Rac1/F-actin cytoskeletal signals in growth cones, either in a cell-autonomous manner or via ephrin-B-EphB signaling-mediated transcellular regulation (Fig. 9c). To the best of our knowledge, this is the first study to elucidate the role of Porf-2 in axon growth and EphB receptor-mediated axon guidance.
Porf-2 has been reported to be highly expressed in the brain [12, 19, 24]. The function of Porf-2 in brain development has remained largely unknown. Porf-2 has been reported to inhibit NSC proliferation by enhancing p21 protein levels followed by G1 phase arrest, and to play a pro-apoptotic role involved in an increase of p53 and Bax [25]. We have previously demonstrated that Porf-2 can regulate the Wnt/β-catenin pathway to mediate NSC proliferation and targeting Porf-2 may be a possible avenue for mediating NSC-based transplantation in optic nerve injury [15, 26]. In addition to its role in NSCs, Porf-2 has been reported to regulate the midline crossing of axonal and tracheal branches through a direct interaction with Robo in Drosophila [9, 10]. Our present work demonstrates that Porf-2 plays an inhibitory role in axon growth and branching (Figs. 1e, f, 2c, d). These studies indicate that Porf-2 possesses diverse function in the brain, showing distinct roles in different neural cell types. In a recent published literature, Porf-2 knockdown mice exhibited abnormal synaptic transmission and plasticity as well as impaired spatial memory [12], suggesting its essential role for brain development. Considering the previous report and the important role of Porf-2 in axon development and growth cone dynamics revealed in our study, Porf-2 servers as a pivotal protein in mediating neuronal connectivity and plasticity during CNS development.
Porf-2 has been predicted to have three conserved domains according to its protein sequence. The WW domain of Porf-2 is reported to be a binding domain that can bind with the membrane receptor Robo and the scaffold protein CNK2, to regulate axon guidance and spine development, respectively [9, 11]. The Myth4 domain has been identified as a conserved domain in the tails of several different unconventional myosins involved in intracellular trafficking, cell division, and muscle contraction [27–29]. The GAP domain mediates intrinsic GTPase activity, leading to the inactive state of the GTPase, and is involved in multiple cellular processes via actin cytoskeleton rearrangement, including axon guidance, growth, and branching [30]. In the present study, we determined that the GAP domain is required for the regulation of axonal development (Fig. 3c, d), resulting in restricted growth cone expansion (Fig. 4b, c). The role of Porf-2 as a GAP is to inactivate Rac1 (Fig. 4d), although both Rac1 and Cdc42 are reported to modulate axon growth through actin dynamics. Our findings, therefore, demonstrate that Porf-2 acts as a neuronal Rac1-specific GAP during axonal development, which is consistent with previous reports [9, 10].
Notably, we found that EphB receptors, comprising the largest family of receptor tyrosine kinases [31], are able to interact with Porf-2 in neurons (Fig. 5b; Fig. S2), and this interaction is further enhanced upon ephrin-B stimulation (Fig. 6a). EphB signaling plays key roles in axon bundling, growth, guidance, and targeting via cell–cell contacts during brain development [22, 23, 32–34]. Furthermore, the EphB-dependent recruitment of both GEFs and GAPs acts in concert to regulate Rho GTPase signaling precisely for spinogenesis [35, 36]. In the current study, we reveal that upon ephrin-B stimulation, Porf-2 becomes an effector of the EphB1 receptor and is recruited and bound to EphB1 (Fig. 6a, b). Porf-2–EphB1 binding results in EphB forward signal transduction to the downstream effector Rac1 in growth cone dynamics (Fig. 8c–f; Fig. S5). These data suggest a new EphB1 downstream cytoskeletal regulator that is predominant in contacting growth cones to regulate Rac1/F-actin and cytoskeletal rearrangement to brake axon growth and branching (Fig. 9c).
Our current study showing that Porf-2 participates in EphB signaling to inhibit axon growth and branching is distinct from previous studies in the following ways. First, our work is focused on the developmental mechanism of axon guidance in mammalian neurons, which differs from that in Drosophila neurons [9] and tracheal cells [10], or dendritic spine morphogenesis [11]. Second, we reveal a dual function of Porf-2 in axon growth that is exerted sequentially via guidance cue-independent and EphB receptor-dependent pathways (Figs. 7, 8). During initial axonal growth, Porf-2 plays a restrictive role to prevent potential excessive growth in an EphB1-independent manner (Fig. 7a, b). After axons grow to some extent, they can sense outside stimulation by interacting with other axons or cells, which then guide these axons via processes of adhesion/attraction or retraction/repulsion. During these processes (cell–cell contact), we found that Porf-2 serves as a downstream effector of EphB1 (Fig. 6) and transduces EphB1-dependent cytoskeletal dynamics in axon terminals to mediate axon growth inhibition (Fig. 8a–d) and repulsion (Fig. 9a). This dual role of Porf-2 has not been revealed in the previous studies. Third, Porf-2-mediated EphB1 signaling occurs upon cell–cell contact, and its regulation is restricted in a short-distance manner, in contrast to Porf-2-mediated slit-Robo signals, which rely on the formation of ligand gradients for long-distance regulation [9, 10]. Last, the mechanism of protein–protein interaction or signaling transduction is different from previous reports. The conventional WW domain was identified to be the binding domain with Robo to transduce its upstream signaling [9, 10]. In the EphB1-Porf-2 signaling transduction, we found that the binding site is not WW domain (Fig. S4a, b). The different binding pattern implies the different function of Porf-2 in transducing upstream signaling in different conditions. Therefore, our data from the present study provide evidence of the integration of intracellular signaling and extracellular conditions during initial axon growth and guidance, suggesting temporally and spatially specific modulation in the developing brain (Fig. 9c).
In summary, these findings define a molecular mechanism underlying the function of Porf-2 in axonal development and reveal a new downstream effector of EphB1 forward signaling through the Rac1/F-actin pathway. Our analysis may lead to a greater understanding of the molecular mechanisms by which the assembly of functional axonal structures is regulated in the developing brain.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
We thank Prof. Jialin C. Zheng for providing the PDMS stamp model used in the stripe assay.
Abbreviations
- GAPs
GTPase-activating proteins
- GEFs
Guanine nucleotide exchange factors
- Porf-2
Preoptic regulatory factor-2
- MS
Mass spectrometry
- WT
Wild type
- Robo
Roundabout
- Co-IP
Co-immunoprecipitation
- shRNA
Short hairpin RNA
- cAMP
Cyclic adenosine monophosphate
- PDL
Poly-d-lysine
- NSC
Neural stem cell
- PFA
Paraformaldehyde
- CNK2
Connector enhancer of KSR-2
- GFP
Green fluorescent protein
- FITC
Fluorescein isothiocyanate
- BSA
Bovine serum albumin
- GST
Glutathione S-transferase
- PBD
p21-binding domain
Author contributions
G-HH and LG designed and performed the experiments and wrote the manuscript. LZ, X-DL, Z-LS and H-JL performed the data and statistical analysis. N-JX and D-FF designed the experiments and revised the manuscript critically. All authors agree that all the questions related to the accuracy or integrity of the paper have been appropriately investigated and resolved and have given the final approval of the version to be published.
Funding
This study was supported by the National Natural Science Foundation of China (81772059 to D.-F.F, 31671062 and 31371097 to N.-J.X.), National Basic Research Program of China (Program 973 Grant 2014CB965002), 1000-Talents Program, Grants of Shanghai Brain-Intelligence Project from STCSM (16JC1420501), and the Program for Professor of Special Appointment (Eastern Scholar) at Shanghai Institutions of Higher Learning (No. 2013-25) to N.-J.X.
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
Footnotes
Guo-Hui Huang and Lin Guo contributed equally to this work.
Contributor Information
Nan-Jie Xu, Phone: +86 021-34696293, Email: xunanjie@sjtu.edu.cn.
Dong-Fu Feng, Email: drneuro@163.com.
References
- 1.O’Donnell M, Chance RK, Bashaw GJ. Axon growth and guidance: receptor regulation and signal transduction. Annu Rev Neurosci. 2009;32:383–412. doi: 10.1146/annurev.neuro.051508.135614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cheng PL, Poo MM. Early events in axon/dendrite polarization. Annu Rev Neurosci. 2012;35:181–201. doi: 10.1146/annurev-neuro-061010-113618. [DOI] [PubMed] [Google Scholar]
- 3.Heng JI, Chariot A, Nguyen L. Molecular layers underlying cytoskeletal remodelling during cortical development. Trends Neurosci. 2010;33:38–47. doi: 10.1016/j.tins.2009.09.003. [DOI] [PubMed] [Google Scholar]
- 4.Bilimoria PM, Bonni A. Molecular control of axon branching. Neuroscientist. 2013;19:16–24. doi: 10.1177/1073858411426201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Heasman SJ, Ridley AJ. Mammalian Rho GTPases: new insights into their functions from in vivo studies. Nat Rev Mol Cell Biol. 2008;9:690–701. doi: 10.1038/nrm2476. [DOI] [PubMed] [Google Scholar]
- 6.Miller MB, Yan Y, Eipper BA, Mains RE. Neuronal Rho GEFs in synaptic physiology and behavior. Neuroscientist. 2013;19:255–273. doi: 10.1177/1073858413475486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Moon SY, Zheng Y. Rho GTPase-activating proteins in cell regulation. Trends Cell Biol. 2003;13:13–22. doi: 10.1016/S0962-8924(02)00004-1. [DOI] [PubMed] [Google Scholar]
- 8.Huang GH, Sun ZL, Li HJ, Feng DF. Rho GTPase-activating proteins: regulators of Rho GTPase activity in neuronal development and CNS diseases. Mol Cell Neurosci. 2017;80:18–31. doi: 10.1016/j.mcn.2017.01.007. [DOI] [PubMed] [Google Scholar]
- 9.Hu H, Li M, Labrador JP, McEwen J, Lai EC, Goodman CS, Bashaw GJ. Cross GTPase-activating protein (CrossGAP)/Vilse links the Roundabout receptor to Rac to regulate midline repulsion. Proc Natl Acad Sci USA. 2005;102:4613–4618. doi: 10.1073/pnas.0409325102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lundstrom A, Gallio M, Englund C, Steneberg P, Hemphala J, Aspenstrom P, Keleman K, Falileeva L, Dickson BJ, Samakovlis C. Vilse, a conserved Rac/Cdc42 GAP mediating Robo repulsion in tracheal cells and axons. Genes Dev. 2004;18:2161–2171. doi: 10.1101/gad.310204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lim J, Ritt DA, Zhou M, Morrison DK. The CNK2 scaffold interacts with vilse and modulates Rac cycling during spine morphogenesis in hippocampal neurons. Curr Biol. 2014;24:786–792. doi: 10.1016/j.cub.2014.02.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lee JY, Lee LJ, Fan CC, Chang HC, Shih HA, Min MY, Chang MS. Important roles of Vilse in dendritic architecture and synaptic plasticity. Sci Rep. 2017;7:45646. doi: 10.1038/srep45646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Xu NJ, Henkemeyer M. Ephrin-B3 reverse signaling through Grb4 and cytoskeletal regulators mediates axon pruning. Nat Neurosci. 2009;12:268–276. doi: 10.1038/nn.2254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhu XN, Liu XD, Zhuang H, Henkemeyer M, Yang JY, Xu NJ. Amygdala EphB2 signaling regulates glutamatergic neuron maturation and innate fear. J Neurosci. 2016;36:10151–10162. doi: 10.1523/JNEUROSCI.0845-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Huang GH, Yang XT, Chen K, Xing J, Guo L, Zhu L, Li HJ, Li XC, Zhang SY, Feng DF. Porf-2 inhibits neural stem cell proliferation through Wnt/beta-catenin pathway by its GAP domain. Front Cell Neurosci. 2016;10:85. doi: 10.3389/fncel.2016.00085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yue X, Son AI, Zhou R. Growth cone collapse assay. Methods Mol Biol. 2013;1018:221–227. doi: 10.1007/978-1-62703-444-9_21. [DOI] [PubMed] [Google Scholar]
- 17.Meyer LA, Kaselis A, Satkauskas S, Bagnard D. Analysis of semaphorin-induced growth cone collapse and axon growth inhibition. Methods Mol Biol. 2017;1493:171–183. doi: 10.1007/978-1-4939-6448-2_12. [DOI] [PubMed] [Google Scholar]
- 18.Zhang M, Song A, Lai S, Qiu L, Huang Y, Chen Q, Zhu B, Xu D, Zheng JC. Applications of stripe assay in the study of CXCL12-mediated neural progenitor cell migration and polarization. Biomaterials. 2015;72:163–171. doi: 10.1016/j.biomaterials.2015.08.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Diez-Roux G, Banfi S, Sultan M, Geffers L, Anand S, Rozado D, Magen A, Canidio E, Pagani M, Peluso I, et al. A high-resolution anatomical atlas of the transcriptome in the mouse embryo. PLoS Biol. 2011;9:e1000582. doi: 10.1371/journal.pbio.1000582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Huber AB, Kolodkin AL, Ginty DD, Cloutier JF. Signaling at the growth cone: ligand–receptor complexes and the control of axon growth and guidance. Annu Rev Neurosci. 2003;26:509–563. doi: 10.1146/annurev.neuro.26.010302.081139. [DOI] [PubMed] [Google Scholar]
- 21.Dent EW, Gupton SL, Gertler FB. The growth cone cytoskeleton in axon outgrowth and guidance. Cold Spring Harb Perspect Biol. 2011;3:a001727. doi: 10.1101/cshperspect.a001800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Robichaux MA, Chenaux G, Ho HY, Soskis MJ, Dravis C, Kwan KY, Sestan N, Greenberg ME, Henkemeyer M, Cowan CW. EphB receptor forward signaling regulates area-specific reciprocal thalamic and cortical axon pathfinding. Proc Natl Acad Sci USA. 2014;111:2188–2193. doi: 10.1073/pnas.1324215111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Egea J, Klein R. Bidirectional Eph-ephrin signaling during axon guidance. Trends Cell Biol. 2007;17:230–238. doi: 10.1016/j.tcb.2007.03.004. [DOI] [PubMed] [Google Scholar]
- 24.Nowak FV. Preoptic regulatory factor-2, a Rhogap domain protein that modifies cell cycle progression and apoptosis in the CNS. In: Hayat MA, editor. Stem cells and cancer stem cells, vol 12: Therapeutic applications in disease and injury. Dordrecht: Springer; 2014. pp. 219–230. [Google Scholar]
- 25.Ma S, Nowak FV. The RhoGAP domain-containing protein, Porf-2, inhibits proliferation and enhances apoptosis in neural stem cells. Mol Cell Neurosci. 2011;46:573–582. doi: 10.1016/j.mcn.2010.12.008. [DOI] [PubMed] [Google Scholar]
- 26.Yang XT, Huang GH, Li HJ, Sun ZL, Xu NJ, Feng DF. Rac1 guides Porf-2 to Wnt pathway to mediate neural stem cell proliferation. Front Mol Neurosci. 2017;10:172. doi: 10.3389/fnmol.2017.00172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kerber ML, Cheney RE. Myosin-X: a MyTH-FERM myosin at the tips of filopodia. J Cell Sci. 2011;124:3733–3741. doi: 10.1242/jcs.023549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lu Q, Li J, Zhang M. Cargo recognition and cargo-mediated regulation of unconventional myosins. Acc Chem Res. 2014;47:3061–3070. doi: 10.1021/ar500216z. [DOI] [PubMed] [Google Scholar]
- 29.Weck ML, Grega-Larson NE, Tyska MJ. MyTH4-FERM myosins in the assembly and maintenance of actin-based protrusions. Curr Opin Cell Biol. 2017;44:68–78. doi: 10.1016/j.ceb.2016.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Koh CG. Rho GTPases and their regulators in neuronal functions and development. Neurosignals. 2006;15:228–237. doi: 10.1159/000101527. [DOI] [PubMed] [Google Scholar]
- 31.Flanagan JG, Vanderhaeghen P. The ephrins and Eph receptors in neural development. Annu Rev Neurosci. 1998;21:309–345. doi: 10.1146/annurev.neuro.21.1.309. [DOI] [PubMed] [Google Scholar]
- 32.Chen ZY, Sun C, Reuhl K, Bergemann A, Henkemeyer M, Zhou R. Abnormal hippocampal axon bundling in EphB receptor mutant mice. J Neurosci. 2004;24:2366–2374. doi: 10.1523/JNEUROSCI.4711-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Marler KJ, Becker-Barroso E, Martinez A, Llovera M, Wentzel C, Poopalasundaram S, Hindges R, Soriano E, Comella J, Drescher U. A TrkB/EphrinA interaction controls retinal axon branching and synaptogenesis. J Neurosci. 2008;28:12700–12712. doi: 10.1523/JNEUROSCI.1915-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Xu NJ, Sun S, Gibson JR, Henkemeyer M. A dual shaping mechanism for postsynaptic ephrin-B3 as a receptor that sculpts dendrites and synapses. Nat Neurosci. 2011;14:1421–1429. doi: 10.1038/nn.2931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Penzes P, Beeser A, Chernoff J, Schiller MR, Eipper BA, Mains RE, Huganir RL. Rapid induction of dendritic spine morphogenesis by trans-synaptic ephrinB–EphB receptor activation of the Rho-GEF kalirin. Neuron. 2003;37:263–274. doi: 10.1016/S0896-6273(02)01168-6. [DOI] [PubMed] [Google Scholar]
- 36.Um K, Niu S, Duman JG, Cheng JX, Tu YK, Schwechter B, Liu F, Hiles L, Narayanan AS, Ash RT, et al. Dynamic control of excitatory synapse development by a Rac1 GEF/GAP regulatory complex. Dev Cell. 2014;29:701–715. doi: 10.1016/j.devcel.2014.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.