Abstract
Studies over the past decades have elucidated the critical role of autophagy in human health and diseases. Although the processes of autophagy in the cytoplasm have been well studied, the posttranscriptional and epigenetic regulation mechanisms of autophagy are still poorly understood. Protein methylation, including histone methylation and non-histone protein methylation, is the most important type of posttranscriptional and epigenetic modification. Recent studies have shown that protein methylation is associated with effects on autophagosome formation, autophagy-related protein expression, and signaling pathway activation, but the details are still unclear. Thus, it is important to summarize the current status and discuss the future directions of research on protein methylation in the context of autophagy.
Keywords: Autophagy, Protein methylation, Non-histone protein methylation, Histone methylation, Methyltransferase, Demethylase, Posttranslational modification, Autophagy-related proteins
Introduction
Ashford and Porter first discovered a “self-digestion” process in human hepatic cells associated with lysosomes as early as 1962, and this phenomenon was first termed “autophagy” by de Duve [1]. Autophagy is a catabolic homeostasis process, wherein cytoplasmic contents, including damaged organelles, long-lived or aggregated proteins, and pathogens, are surrounded by multilayered autophagosomes, which fuse with lysosomes to enable degradation of the sequestered contents [2–4]. Autophagy is also an adaptive cellular process that occurs in response to different forms of stress to eliminate harmful cytosolic components to maintain cellular homeostasis [5]. Due to the fundamental importance of autophagy, the dysregulation of autophagy has been implicated in various human diseases, including infection [6], immune disease [7], pulmonary disease [8], kidney disease [9], metabolic disease [10], neurodegeneration [11], cancer [12], and cardiovascular diseases [13]. However, advances in autophagy research have led to the current conventional wisdom that autophagy is a double-edged sword. Thus, further clarification of the regulatory mechanisms of autophagy is important to enable better understanding and control of autophagy.
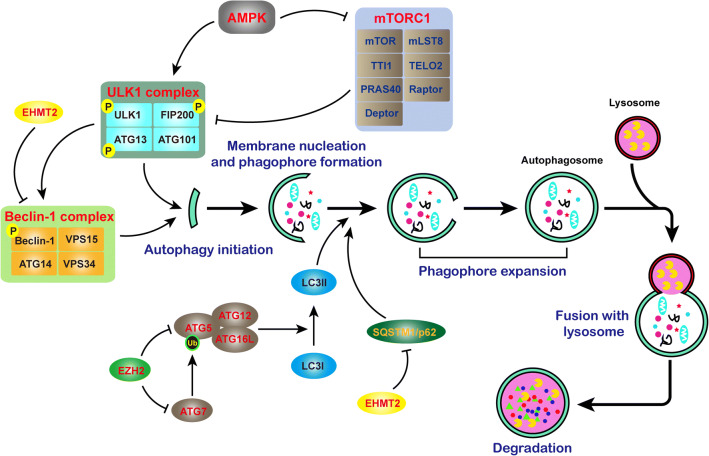
To date, at least three types of autophagy have been recognized: microautophagy, chaperone-mediated autophagy, and macroautophagy (hereafter referred to as autophagy). Autophagy is a finely regulated and highly conserved biological process that includes five sequential steps: autophagy initiation, membrane nucleation and phagophore formation, phagophore expansion, lysosomal fusion, and degradation (Fig. 1) [14, 15]. To date, no less than 38 autophagy-related (ATG) genes have been identified in yeast and higher eukaryotes [16]. These ATGs usually interact with other proteins to form complexes and jointly regulate the autophagy process. For example, Unc-51-like autophagy-activating kinase 1 (ULK1) complex in mammals, commonly comprising ULK1–RB1CC1/FIP200–ATG13–ATG101, is indispensable for autophagy induction [5]. The activity of the ULK1 complex is regulated by the mammalian target of rapamycin (mTOR) complex and AMP-activated protein kinase (AMPK). mTORC1 is a complex that can sense nutritional status and control protein synthesis [17]. Under normal conditions, mTORC1 is phosphorylated and active and promotes ULK1 and ATG13 phosphorylation to suppress autophagy. While under starvation or other specific cellular stress, mTORC1 is inactivated, which results in rapid dephosphorylation of ATG13 and ULK1 to induce autophagy [5, 16].
Fig. 1.
Specific process of autophagy and the genes involved in it. Autophagy is a multi-stage biological process, including initiation, elongation, closure, maturation, and degradation. The ATG family is the most investigated genes that involved in autophagy
From the example of the ULK1 complex, it is not difficult to discern that protein posttranslational modification (PTM) is a critical way to regulate autophagy. Aside from phosphorylation, O-linked glycosylation, ubiquitination, and acetylation are important for autophagy regulation, and recently published reviews have given very detailed descriptions of these processes [16, 17]. In addition, phosphatidylethanolamine (PE)-modified Atg8/LC3 (Atg8-PE/LC3-II) is necessary for phagophore elongation. The ATG12–ATG5–ATG16L1 conjugation system, containing the core proteins ATG5, ATG12, ATG7, ATG10, and ATG16L1, promotes LC3 conversion to the PE-conjugated LC3-II form in an E3-like manner [18]. CSNK2 phosphorylates ATG16L1 on Ser139 to positively regulate autophagy [19]. Substrates to be degraded by autophagy require selective receptors (e.g., SQSTM1/p62, BNIP3L, NBR1, and OPTN) and scaffold proteins, which usually bind LC3-II and bring SQSTM1-containing protein aggregates to the autophagosome for degradation [20].
Over the past several decades, although it has been reported that signaling pathway-associated autophagy regulation is complicated, the identity of the overarching “gatekeeper” of autophagy has remained elusive. It has been documented that posttranscriptional and epigenetic regulation are critical for autophagy processes [21]. In addition to the abovementioned protein modifications, protein methylation is a burgeoning research topic. Protein methylation, including histone methylation and non-histone protein methylation, is one of the most important form of posttranscriptional and epigenetic modifications associated with effects on autophagosome formation, autophagy-related protein expression, and signaling pathway activation and potentially functions as the PTM switch to regulate autophagy [21]. Similar to phosphorylation, methylation is also a reversible biological process, and it mediated by methyltransferases and demethylases [22]. However, methylation can be found not only at different amino-acid sites, but also in different forms, such as mono-, di-, or trimethylation, which indicates that methylation is flexible and diverse [22]. Methylation has been found to affect protein activity, protein–protein interactions, and interplay with other PTMs [22]. Although the regulation of autophagy by protein methylation is far from clear, existing studies have shown that both histone methylation and non-histone methylation have important regulatory effects on autophagy. Therefore, it is urgent to summarize related studies to help researchers discover new scientific problems and research directions. Thus, this review focuses on protein methylation in the context of autophagy along with the responsible enzymes and their effects on various biological processes (Table 1).
Table 1.
Protein methylation and their effects on autophagy
| HMTs | Targets | Effectors molecules | Impact to autophagy | Tissue/cell | Consequence | References |
|---|---|---|---|---|---|---|
| EZH2 | H3K27me3 | TSC2, RHOA, DEPTOR, FKBP11, RGS16 and GPI | Inhibition | HeLa, MCF-7, HCT116, LOVO, U2OS, PC3, H719, HT29, and SW620 cell lines | Cell growth retardation | [30] |
| LC3B, ATG7 | Inhibition | HCT116, LOVO, HCT-15, and DLD-1 cell lines | Cell survival | [33] | ||
| H3K27me3 | p14ARF, p16INK4a, p53, pRb, and p21, CDK1, CDK2 | Activation | SKOV3/DDP cell line | Cell death | [34] | |
| H3K27me2/3 | ATG5, ATG7 | Inhibition | Aortic VSMCs | Cell growth | [36] | |
| EHMT2 | H3K9me2 | LC3B, WIPI1 and TP53INP2/DOR | Inhibition | Naïve T cell activation | Naïve T cell activation stimulation | [40, 41] |
| H3K9me2 | Beclin-1 | Inhibition | MCF-7, and HS578T cell lines | Poor prognosis | [46] | |
| SMYD3 | H3K4me2/me3 | BCLAF1 | Activation | Bladder cancer | Poor prognosis | [50] |
| LSD1 | H3K4me2 | LC3II, p62 | Inhibition | HEK293, MEF, HCT116, and U2OS cell lines | Cell proliferation | [52] |
| CARM1 | H3R17me2 | TFEB | Activation | MEFs, HeLa, HepG2 | Cell survival | [54] |
| DOT1L | H3K79me2 | CD9, MMP9 | Inhibition | RAW264.7 | Osteoclast dysregulation | [57] |
| SETD7 | ATG16L1 (K151me1) | Inhibition | Cardiomyocytes | Cell death/apoptosis | [63] |
Histone methylation and methyltransferases linked to the regulation of autophagy
Histone methylation mainly occurs on lysine [23] and arginine [24] and is carried out by protein lysine methyltransferases (PKMTs or PLMTs) and protein arginine methyltransferases (PRMTs), including members of the PRMT family, SET gene family, and non-SET gene family [25–27]. Lysine can be monomethylated (me1), dimethylated (me2), or trimethylated (me3), while arginine can be monomethylated (me1), symmetrically dimethylated (me2s), or asymmetrically dimethylated (me2a) on its guanidine group [28]. It has been identified that a plethora of methyltransferases and demethylases mediate the addition or removal of methyl groups from different residues on histones [29]. These histone markers potentially recruit nuclear factors that either activate or repress transcription directly or by altering chromatin states [28]. Histone methylation can serve as specific binding sites for transcription factors and coregulators [21]. Thus, histone methylation sites form hubs on chromatin that can accommodate specific transcription factors or cofactors linking upstream signals to downstream gene expression. In view of the critical role that histone methylation plays during cellular events, it is important to investigate the role of histone methylation during autophagy and the potential mechanism. Accumulating evidence indicates that histone methylation contributes to the control of cell fate and to the maintenance or suppression of autophagy, which is a new area of study. Here, we summarize various histone methylation markers that directly or indirectly control autophagy (Fig. 2).
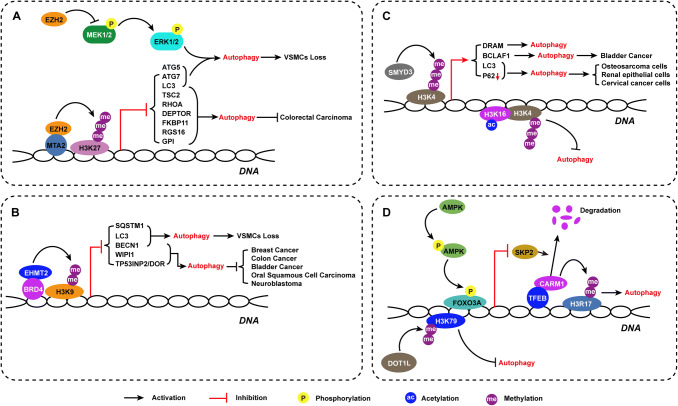
Fig. 2.
Histone methylation enzymes control over autophagy regulation. Multiple histone methylation markers, along with its responsible enzymes, regulate autophagy activation in various cells lines and contribute to determine that the cell fate is presented
H3K27 trimethylation by EZH2
Although autophagy is universally recognized as cytoprotective, dysregulation of autophagy can also result in cell death. Thus, understanding how autophagy holds the switch of cell survival and death is quite important. Emerging evidence has shown that EZH2, which catalyzes H3K27me2/3 and induces transcriptional repression, plays a determinant role in autophagy regulation. Wei et al. demonstrated that EZH2 inhibited autophagy by epigenetically repressing several negative regulators of the mTOR pathway, including TSC2, RHOA, DEPTOR, FKBP11, RGS16, and GPI, in colorectal carcinoma cell lines under serum starvation conditions and contributed to slowing of tumor growth [30]. EZH2 was recruited to the gene promoter via metastasis-associated 1 family, member 2 (MTA2) to silence target genes, such as TSC2 [30]. EZH2 associates with specific target gene promoters and catalyzes H3K27me3 by docking histones through MTA2 recruitment, revealing a transcriptional repressive model mediated by multiple types of histone modifications [30]. Over the past few years, several potent inhibitors of EZH2 have been discovered [31]. Liu et al. showed that GSK343, an S-adenosyl-l-methionine (SAM)-competitive inhibitor of EZH2, induced autophagy and enhanced drug sensitivity in cancer cells, including hepatocellular carcinoma cells, in in silico and in vitro experimental analyses [32]. It is reported that UNC1999, a selective inhibitor of EZH2, functions as an autophagy inducer by upregulating LC3B gene expression in a partially ATG7-dependent manner and causes cell death [33]. Although EZH2 knockdown and EZH2 inhibition with selective inhibitors induce autophagy in different cancer cell lines, knockdown of EZH2 results in slowing of cell growth, while UNC1999 promotes cell survival [30, 33]. These results indicate that UNC1999 may have side effects independent of its effects on EZH2. However, Sun et al. reported a completely opposite finding after transfecting an EZH2 RNA interference plasmid into the human ovarian cancer cell line SKOV3/DDP [34]. They found that EZH2 inhibition significantly inhibited autophagy and increased the expression of the cellular senescence-signaling proteins p14, p16, p53, and pRb, which was followed by cell death [34]. As cellular senescence is tightly related to ovarian cancer cell sensitivity to cisplatin [35], these results suggest that knockdown of EZH2 can inhibit ovarian cancer cell autophagy and reverse resistance to chemotherapy. Furthermore, EZH2 inhibitors inhibit autophagy with an efficiency similar to that of EZH2 knockdown in ovarian cancer cells [34]. Studies on the various roles of EZH2 have predominantly focused on tumors; however, EZH2-induced autophagy is also a key factor in cardiovascular disease. Our recent research demonstrated that EZH2 manages vascular smooth muscle cell (VSMC) survival by suppressing autophagic cell death associated with aortic dissection by catalyzing H3K27me2/3 and inhibiting the MEK–ERK1/2-signaling pathway [36]. Remarkably, our results showed that the inhibition of EZH2 activity by UNC1999 or knockdown of EZH2 resulted in VSMC loss accompanied by increased expression of LC3B, ATG5, and ATG7 and formation of autophagosomes; in contrast, ATG5 or ATG7 knockdown resulted in autophagy inhibition that virtually eliminated the VSMC loss induced by EZH2 inhibition or knockdown. According to the molecular definitions of cell death processes, uncontrolled autophagy activation leads to cell death, which is defined as “autophagic cell death (ACD)”, and is also known as “type II programmed cell death”; notably, we were the first to discover that EZH2 inhibition induces autophagy-related VSMC loss [37]. Our results elucidated the role of EZH2 inhibition-induced autophagy during cell death. Although further studies are needed, treatments targeting to EZH2 or selective inhibitors of EZH2 will not only prevent silencing of tumor suppressor genes, but also activate autophagy, which will be an interesting area of research for autophagy-related disease treatment. Notably, when using EZH2 inhibitors in the treatment of tumors, one should consider their side effects on the cardiovascular system, especially the aorta.
H3K9 dimethylation by EHMT2
H3K9me2 is the most studied histone modification related to autophagy regulation. H3K9 methylation levels are managed by histone methyltransferases (HMTs) such as SUV39H1 (suppressor of variegation 3–9 homolog 1) and G9a/EHMT2, and by histone lysine demethylases (KDMs), such as KDM4/JMJD2 (Jumonji domain-containing 2) [38, 39]. Recent studies have described that EHMT2 is enriched on the promoters of key genes during autophagy, including LC3B, WIPI1, and TP53INP2/DOR, as a transcriptional repressor [40, 41]. Nutrient deprivation leads to dissociation of EHMT2 from the promoter and enables dimethylation as well as acetylation of H3K9, resulting in transcriptional activation [40]. EHMT2 also acts as a co-factor of BRD4 (bromodomain-containing 4) and performs transcriptional repression, regulating autophagy and lysosome-related gene expression [40]. Selective inhibitors of H3K9 methylation, such as BRD4770 and BIX01294, can effectively enhance autophagy in various tumors, such as breast cancer [42], colon cancer [42], bladder cancer [43], oral squamous cell carcinoma [44], and neuroblastoma tumors [45]. Park et al. demonstrated that EHMT2 inhibition by BIX01294 reduces H3K9me2 and induces the dissociation of EHMT2 and H3K9me2 from the promoter of Beclin-1; RNA polymerase II and NF-κB are then recruited to the promoter, resulting in transcriptional activation that accelerates autophagy [46]. Our unpublished data also confirm that the inhibition of EHMT2 by gene knockdown or BIX01294 treatment is capable of activating autophagy via regulation of SQSTM1/p62 and Beclin-1 expression to cause VSMC loss. However, BRD4770, reported to be an inhibitor of EHMT2, suppresses VSMC proliferation by regulating G2/M phase arrest and decreases the expression levels of major regulators of cell-cycle G2/M checkpoints in an autophagy- and EHMT2-independent manner. Taken together, the findings suggest that EHMT2 likely acts as an epigenetic repressor of autophagy and that the expression level or function of EHMT2 acts as the switch for autophagy.
H3K4 di- and trimethylation
H3K4 methylation, a common euchromatin marker for active transcription, is mediated by methyltransferases, such as SMYD3, and demethylases, such as KDM1A/LSD1 [47]. Ni et al. observed that H3K4me3 was enriched at the core promoter region of the damage-regulated autophagy modulator (DRAM) gene in a time-dependent manner upon serum deprivation [48]. DRAM is a lysosomal membrane protein that is associated with p53-mediated autophagy [49]. Increased H3K4me3-induced DRAM expression is required for serum deprivation-induced autophagy activation [48]. In addition, Shen et al. discovered that SMYD3 overexpression in bladder cancer cells promoted autophagy activation which was associated with bladder cancer progression and poor prognosis [50]. Mechanistically, SMYD3 upregulated Bcl2-associated transcription factor 1 (BCLAF1) expression by enriching di- and trimethylation of H3K4 at the BCLAF1 locus [50]. Interestingly, Füllgrabe et al. achieved the opposite result. They observed that H3K4me3 reductions accompanied H4K16 deacetylation in both mammalian and yeast cells during autophagy [51]. Wang et al. found that H3K4 demethylase inhibitor treatment induced autophagy in multiple mammalian cell lines [52]. They treated osteosarcoma cells, human renal epithelial cells and cervical cancer cells with 2-PCPA and GSK–LSDS1, two inhibitors of the H3K4 demethylase KDM1A/LSD1, and autophagy activation was observed in all the cell lines [52]. The two inhibitors induced LC3II accumulation, autophagosome and autolysosome formation, and p62 degradation [52]. Moreover, they found four new inhibitors, including UNC1215 for the histone methylation reader L3MBTL3, ML324 for the H3K9 and H3K36 demethylase JMJD2, GSK-J4 for the H3K27 demethylases UTX and JMJD3, and PBIT for the H3K4 demethylase KDM5B that were able to induce accumulation of LC3II in cells [52]. Consistently, it is reported that the inhibition of H3K4 methylation in hepatic stellate cells occurs in the Hif-1 transcriptional complex and suppresses Hif-1 nuclear transport and autophagosome formation [53]. Although the specific mechanism of the paradoxical role of H3K4me2/3 in autophagy is still unknown, there is no doubt that H3K4me2/3 levels are closely associated with the autophagy process.
H3R17 dimethylation
H3R17 dimethylation (H3R17me2), which is associated with transcriptional activation, is mediated by coactivator-associated arginine methyltransferase 1 (CARM1, also called PRMT4). A recent study identified CARM1-mediated H3R17me2 as a crucial component of autophagy in mammals [54]. Autophagy activation induced by glucose starvation increased H3R17me2 levels resulting from CARM1 induction in the nucleus, and CARM1 exerted transcriptional coactivator effects on autophagy-related and lysosomal genes through transcription factor EB (TFEB) to regulate autophagy [54]. Notably, CARM1 is degraded by the SKP2-containing SCF (SKP1-cullin1-F-box protein) E3 ubiquitin ligase in the nucleus under glucose rich conditions [54]. When glucose starvation persists, AMPKα2 and phosphorylated AMPK, the activated form of AMPK, accumulate in the nucleus to phosphorylate FOXO3a, which acts as a transcriptional repressor of SKP2 [54]. Nutrient starvation-induced activation of AMPK downregulates SKP2 expression in the nucleus and enables CARM1 to escape degradation by SCF E3 ubiquitin ligase and stabilization [54]. Given that ellagic acid is an effective H3R17me2 blocker [55], application of ellagic acid is able to almost completely block autophagy activation both in vitro and in vivo [54]. These findings identify CARM1-mediated histone arginine methylation as a critical nuclear event in the regulation of autophagy and elucidate the potential therapeutic value of targeting the recently identified AMPK–SKP2–CARM1 signaling axis in autophagy-related diseases.
H3K79 dimethylation
H3K79me2 is strongly correlated with active gene transcription. DOT1/DOT1L is an evolutionarily conserved histone methyltransferase that mediates H3K79me2/3 [56]. Methylation of H3K79 is involved in the regulation of telomeric silencing, cellular development, cell-cycle checkpoints, DNA repair, and transcription [56]. It has been reported that DOT1L-mediated H3K79me2 is also involved in autophagy. Gao et al. discovered that DOT1L and H3K79me2 expression increased during osteoclast differentiation [57]. DOT1L inhibition increased autophagic activity, which was associated with osteoclast differentiation and resorption capability [57]. Their work indicates that autophagy is potentially a target of DOT1L, although further study is needed to reveal the specific mechanisms of DOT1L in the autophagy process.
Non-histone protein methylation associated with autophagy regulation
Methylation of lysine and arginine residues on non-histone proteins has emerged as a prevalent PTM and as an important regulator of cellular signal transduction mediated by multiple signaling pathways [58, 59]. Crosstalk between methylation and other types of PTMs, and between histone and non-histone protein methylation frequently occurs and affects cellular functions such as chromatin remodeling, gene transcription, protein synthesis, signal transduction, and DNA repair [60]. It is now firmly established that non-histone protein methylation is an integral part of cellular biology and an important regulator of the physiological or pathological state of a cell [61] (Fig. 3). Emerging studies have illustrated that protein methylation acts as a “gatekeeper” in autophagy regulation.
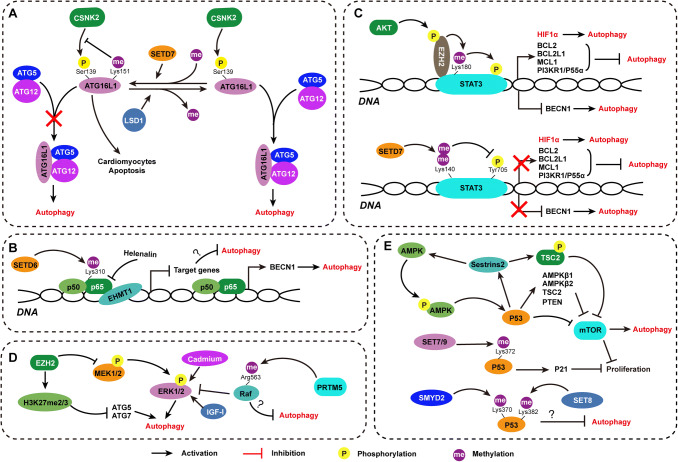
Fig. 3.
Non-histone protein methylation acted as “gatekeeper” in autophagy regulation. Known non-histone protein methylation associated with autophagy regulation by manipulating ATG gene expression and autophagy-related signaling pathway activation is depicted
Posttranslational modifications of autophagy-related (ATG) genes
ATG proteins are required for the management of the formation of autophagosomes [62]. These ATG proteins are recruited and constitute a cluster of functional protein complexes that are activated sequentially. The ULK1 complex is responsible for autophagy induction, and the class III phosphatidylinositol (PtdIns) 13-kinase–Beclin-1 complex controls autophagosome nucleation. Finally, the ATG12–ATG5–ATG16L1 complexes recruit and activate the E2-like protein ATG3, thereby promoting conjugation of LC3 to phosphatidylethanolamine (PE) and subsequently converting LC3B-I to LC3B-II to induce extension and closure of autophagosome membranes [5]. Song et al. presented evidence that ATG16L1 methylation at lysine 151 induced by SETD7, an SET domain-containing lysine methyltransferase, impaired the binding of ATG16L1 to the ATG12–ATG5 conjugate, leading to autophagy inhibition and exacerbating hypoxia/reoxygenation (H/R)-treated cardiomyocyte apoptosis, while lysine demethylase 1A (LSD1/KDM1A) removed the methyl mark from ATG16L1 [63]. Under H/R or conditions of enhanced autophagy activation, SETD7 expression was significantly suppressed, while KDM1A expression was enhanced [63]. In addition, methylation at lysine 151 inhibits phosphorylation of ATG16L1 at S139 by casein kinase 2 (CSNK2), which was previously shown to be critical for autophagy maintenance [19]. These findings reveal the correlation between ATG16L1 methylation and autophagy initiation and suggest a working model of “two-way” crosstalk in which methylation and phosphorylation at different residues of ATG16L1 influence each other.
Alteration of signaling pathway activation induced by non-histone protein methylation
Various signaling pathways have been reported to be enabled by protein methylation, such as the NF-κB [64], STAT3, MAPK [65], Akt [66], p53 [67], and Wnt [68] -signaling pathways. It is noteworthy that multiple signaling pathways involved in autophagy regulation could be governed by protein methylation. Hence, we here summarize the protein methylation-induced signaling pathway alterations related to autophagy (Fig. 3).
NF-κB-signaling pathway
Transcriptional regulation of autophagy, such as that occurring through the NF-κB-signaling pathway, has been directly implicated in death and survival signaling. The NF-κB family is an important transcription factor family consisting of Rel, p65/RelA, RelB, p105/p50, and p100/p52 that target autophagy-related genes [69]. NF-κB plays a protective role in many pathological processes by enhancing autophagy. Kretowski et al. found that NF-κB was activated by the anti-apoptotic chaperone ORP150, which increased autophagic mechanisms to protect MCF-7 cells against apoptosis [70]. Copetti et al. revealed that the NF-κB family member p65/RelA increased Beclin-1 expression levels in different cellular systems and enhanced autophagy by binding to the Beclin-1 promoter [71]. Lim’s results showed that the repression of NF-κB expression by helenalin contributed to autophagic cell death [72]. On the other hand, there is also evidence that NF-κB suppresses autophagy via activation of mTOR. It is reported that NF-κB-targeted cell signaling is involved in advanced glycation end product-mediated autophagy impairment in p53-negative/null cells [73]. The activity of NF-κB is under the control of posttranslational modifications of signaling components [74], and phosphorylation is the most common modification. Furthermore, NF-κB can be methylated reversibly on lysine or arginine residues by histone lysine and arginine methyltransferases and demethylases [75]. NF-κB methylation status can overwhelmingly affect the functions of NF-κB by altering its stability, transactivation ability, and affinity for DNA [76]. For example, methylation of p65/RelA by SETD6 induces recruitment of the histone methyltransferase GLP (also called EHMT1) to chromatin, resulting in chromatin condensation and repression of NF-κB target gene expression [77]. Lu et al. demonstrated that differential methylation of NF-κB on K37 and K218/221 is able to guide differential activation of NF-κB, causing it to bind to specific promoters. Their work may explain the conflicting effects of NF-κB in autophagy regulation.
STAT3-signaling pathway
Signal transducer and activator of transcription 3 (STAT3) is a transcription factor that mediates the expression of a variety of genes in response to cell stimuli and thus plays a key role in many cellular processes, such as apoptosis, cell growth, and autophagy [78]. STAT3 is predominantly activated by phosphorylation of a tyrosine residue [79]. STAT3 carries out antiautophagic functions by promoting the expression of negative regulators of autophagy, such as BCL2, BCL2L1, MCL1, and PI3KR1/P55α, or by inhibiting the expression of autophagy-related genes, such as Beclin-1 [80]. The anti-apoptotic BCL2 family members, including BCL2, BCL2L1, and MCL1, are important negative regulators of Beclin-1. After activation, STAT3 upregulates BCL2 expression and leads to inhibition of autophagy [81]. On the other hand, STAT3 has been reported to transcriptionally upregulate HIF1a [82]. HIF1a is stabilized and expressed under hypoxic conditions and induces autophagy [82]. Thus, the switch-governing STAT3 activation is quite important for autophagy regulation. Kim et al. found that the activation of STAT3 at the tyrosine site is positively regulated by STAT3 methylation on Lys180, which is mediated by EZH2 [83]. A conflicting report by Kong et al. showed that STAT3 dimethylation at Lys140 by SETD7 decreased STAT3 phosphorylation at Tyr705, thus negatively regulating STAT3 activity [84]. Overall, the activation of STAT3 is regulated by multiple interactions between methylation and phosphorylation modifications, although further study is needed to figure out the determinant factor that maintains the switch of STAT3 activation by posttranslational modifications.
MAPK-signaling pathway
The enzymes of the mitogen-activated protein kinases (MAPK) family are activated through a sequential phosphorylation cascade to amplify and transduce signals from the membrane to the nucleus [85]. Extracellular-regulated protein kinase 1/2 (ERK1/2, also known as p42/p44MAPK) is the key member of the MAPK family and communicate extracellular signals to affect nuclear event. Emerging studies have revealed that the MAPK/ERK-signaling pathway contributes to autophagic cell death [86]. We found that the inhibition of the MEK–ERK1/2 pathway mediated by EZH2-attenuated aortic VSMC autophagic cell death [36]. Treatment with a selective MEK inhibitor mostly abolished EZH2 inhibition caused VSMC loss [36]. Our discovery is supported by several other studies. It has been reported that the activation of ERK by active Raf, cadmium, and the IGF-I receptor induces excessive formation of autophagosomes, leading to cell death [87]. A flurry of studies has demonstrated that the methylation status of MAPK plays a vital role in signaling pathway activation by either reinforcing or antagonizing phosphorylation effects. For example, PRMT5 overexpression leads to methylation of RAF, an MAPK kinase kinase, at Arg563 along with inhibition of ERK1/2 phosphorylation, which not only affects the duration of ERK signaling, but also alters P12 cell behavior from proliferation to differentiation [88]. Is it possible that modifications in the MAPK-signaling pathway, such as methylation, limit autophagy to keep it from changing from a prosurvival mechanism to a cell death mechanism?
p53-signaling pathway
p53, as tumor suppressor, is a crucial regulator of apoptosis, cell-cycle arrest, senescence, DNA repair, and cell metabolism that exert its effects via DNA binding or protein interactions [89]. Accumulating evidence has demonstrated that p53 is a critical component of the autophagy regulation machinery [90]. Both nutrient deprivation and genotoxic stress induce p53-dependent autophagy [91]. Feng et al. showed that DNA-damaging agents induced p53 expression in mouse embryo fibroblasts and enhanced autophagy, probably because genotoxic stress induced the activation of AMPK and subsequently promoted p53-dependent inhibition of mTOR [91]. In addition, AMPKβ1, AMPKβ2, TSC2, and PTEN, which are negative regulators of mTOR, can be transactivated by p53 [92]. Genotoxic stress-induced p53 dependent upregulation of sestrins 1 and 2, which function as AMPK activators, results in inhibition of mTOR [93]. Nutrient starvation significantly increases the expression of Sestrin2, and loss of Sestrin2 markedly reduces p53-mediated autophagy [93]. p53 is a transcription factor that both activates and represses a plethora of target genes with an exquisitely complicated network, and PTMs regulate its activation [89, 94]. In addition to the traditional view that acetylation and ubiquitination compete for p53’s C-terminal lysines, methylation at specific sites also contributes to p53 promoter specificity, thereby affecting the ability of p53 to bind DNA [89]. p53 monomethylation by SET7/9 at lysine372 (K372) promotes transactivation of p21 [95]. p53 monomethylation at K370 and K382 by SMYD2 and SET8, respectively, can repress p53 activity [96]. Research from Fan et al. demonstrated that SMYD2 transcriptionally inhibits p53 target gene expression and prohibits BIX01294-induced autophagy-related cell death [97]. Although the details of the roles of p53 methylation in autophagy are still ambiguous, the previous studies have offered convincing evidence that particular p53 methylation statuses are capable of regulating autophagy by stimulating autophagy-related genes and signaling pathways.
p53 also mediates transcription-independent extranuclear functions. Tasdemir et al. proved that the inhibition of p53-induced autophagy was detrimental to cell survival in enucleated cells under multiple experimental conditions [98]. Various autophagy inducers, such as starvation and mTOR inhibition, contributed to proteasome-mediated degradation of p53, while inhibition of p53 degradation prevented the activation of autophagy in several cell lines. Accordingly, human p53−/− colon cancer cells are characterized by excessive autophagy activation, which can be decreased by reintroduction of wild-type p53 [99]. Moreover, the suppression of autophagy by p53 correlates with its nuclear-to-cytosolic redistribution. It is reported that sumoylation promotes the localization of p53 to the cytoplasm [100]. Given the crosstalk between modifications [101, 102], it is reasonable to assume that p53 methylation is involved in autophagy regulation and that this involvement is associated with the effects of sumoylation on p53 translocation.
Conclusion and perspectives
Methylation is an important protein posttranslational modification that not only regulates target gene transcription and expression, but also controls the activity of various signaling pathways. Protein methylation alone or in concert with other modifications is capable of altering gene expression or activity, which possibly underlies the switch of autophagy from a prosurvival to antisurvival mechanism. Thus far, our understanding of the patterns and mechanisms of protein modification crosstalk has remained largely limited. However, it is indisputable that comprehensive and meticulous investigation of the effects of protein methylation modifications and specific inhibitors may provide new insights and understanding aiding the exploration of new therapeutic targets or treatments for autophagy-related diseases.
Acknowledgements
This work was supported by Grants from the National Natural Science Foundation of China (nos. 81600188, 81670050), Tongji Hospital Fund for Distinguished Young Scholars (no. 2016YQ02), and Integrated Innovative Team for Major Human Diseases Program of Tongji Medical College, HUST.
Compliance with ethical standards
Conflict of interest
The authors declare that they have no competing interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Ashford TP, Porter KR. Cytoplasmic components in hepatic cell lysosomes. J Cell Biol. 1962;12:198–202. doi: 10.1083/jcb.12.1.198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mei Y, Glover K, Su M, Sinha SC. Conformational flexibility of BECN1: essential to its key role in autophagy and beyond. Protein Sci. 2016;25(10):1767–1785. doi: 10.1002/pro.2984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Xie Z, Klionsky DJ. Autophagosome formation: core machinery and adaptations. Nat Cell Biol. 2007;9(10):1102–1109. doi: 10.1038/ncb1007-1102. [DOI] [PubMed] [Google Scholar]
- 4.Mizushima N, Levine B, Cuervo AM, Klionsky DJ. Autophagy fights disease through cellular self-digestion. Nature. 2008;451(7182):1069–1075. doi: 10.1038/nature06639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Klionsky DJ, Abdelmohsen K, Abe A, Abedin MJ, Abeliovich H, Acevedo Arozena A, et al. Guidelines for the use and interpretation of assays for monitoring autophagy (3rd edition) Autophagy. 2016;12(1):1–222. doi: 10.1080/15548627.2015.1100356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ahmad L, Mashbat B, Leung C, Brookes C, Hamad S, Krokowski S, Shenoy AR, Lorenzo L, Levin M, O’Hare P, Zhang SY, Casanova JL, Mostowy S, Sancho-Shimizu V. Human TANK-binding kinase 1 is required for early autophagy induction upon herpes simplex virus 1 infection. J Allergy Clin Immunol. 2018 doi: 10.1016/j.jaci.2018.09.013. [DOI] [PubMed] [Google Scholar]
- 7.Kato H, Perl A. Blockade of Treg cell differentiation and function by the interleukin-21-mechanistic target of rapamycin axis via suppression of autophagy in patients with systemic lupus erythematosus. Arthritis Rheumatol. 2018;70(3):427–438. doi: 10.1002/art.40380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Liu H, Fang S, Wang W, Cheng Y, Zhang Y, Liao H, Yao H, Chao J. Macrophage-derived MCPIP1 mediates silica-induced pulmonary fibrosis via autophagy. Part Fibre Toxicol. 2016;13(1):55. doi: 10.1186/s12989-016-0167-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Duan X, Kong Z, Mai X, Lan Y, Liu Y, Yang Z, Zhao Z, Deng T, Zeng T, Cai C, Li S, Zhong W, Wu W, Zeng G. Autophagy inhibition attenuates hyperoxaluria-induced renal tubular oxidative injury and calcium oxalate crystal depositions in the rat kidney. Redox Biol. 2018;16:414–425. doi: 10.1016/j.redox.2018.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhang Y, Whaley-Connell AT, Sowers JR, Ren J. Autophagy as an emerging target in cardiorenal metabolic disease: from pathophysiology to management. Pharmacol Ther. 2018;191:1–22. doi: 10.1016/j.pharmthera.2018.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Menzies FM, Fleming A, Caricasole A, Bento CF, Andrews SP, Ashkenazi A, Fullgrabe J, Jackson A, Jimenez Sanchez M, Karabiyik C, Licitra F, Lopez Ramirez A, Pavel M, Puri C, Renna M, Ricketts T, Schlotawa L, Vicinanza M, Won H, Zhu Y, Skidmore J, Rubinsztein DC. Autophagy and neurodegeneration: pathogenic mechanisms and therapeutic opportunities. Neuron. 2017;93(5):1015–1034. doi: 10.1016/j.neuron.2017.01.022. [DOI] [PubMed] [Google Scholar]
- 12.Sun CY, Zhang QY, Zheng GJ, Feng B. Autophagy and its potent modulators from phytochemicals in cancer treatment. Cancer Chemother Pharmacol. 2018 doi: 10.1007/s00280-018-3707-4. [DOI] [PubMed] [Google Scholar]
- 13.Tai S, Hu XQ, Peng DQ, Zhou SH, Zheng XL. The roles of autophagy in vascular smooth muscle cells. Int J Cardiol. 2016;211:1–6. doi: 10.1016/j.ijcard.2016.02.128. [DOI] [PubMed] [Google Scholar]
- 14.Mizushima N. Autophagy: process and function. Genes Dev. 2007;21(22):2861–2873. doi: 10.1101/gad.1599207. [DOI] [PubMed] [Google Scholar]
- 15.Hansen M, Rubinsztein DC, Walker DW. Autophagy as a promoter of longevity: insights from model organisms. Nat Rev Mol Cell Biol. 2018;19(9):579–593. doi: 10.1038/s41580-018-0033-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Xie Y, Kang R, Sun X, Zhong M, Huang J, Klionsky DJ, Tang D. Posttranslational modification of autophagy-related proteins in macroautophagy. Autophagy. 2015;11(1):28–45. doi: 10.4161/15548627.2014.984267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wani WY, Boyer-Guittaut M, Dodson M, Chatham J, Darley-Usmar V, Zhang J. Regulation of autophagy by protein post-translational modification. Lab Invest. 2015;95(1):14–25. doi: 10.1038/labinvest.2014.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kuma A, Mizushima N, Ishihara N, Ohsumi Y. Formation of the approximately 350-kDa Apg12–Apg5.Apg16 multimeric complex, mediated by Apg16 oligomerization, is essential for autophagy in yeast. J Biol Chem. 2002;277(21):18619–18625. doi: 10.1074/jbc.m111889200. [DOI] [PubMed] [Google Scholar]
- 19.Song H, Pu J, Wang L, Wu L, Xiao J, Liu Q, Chen J, Zhang M, Liu Y, Ni M, Mo J, Zheng Y, Wan D, Cai X, Cao Y, Xiao W, Ye L, Tu E, Lin Z, Wen J, Lu X, He J, Peng Y, Su J, Zhang H, Zhao Y, Lin M, Zhang Z. ATG16L1 phosphorylation is oppositely regulated by CSNK2/casein kinase 2 and PPP1/protein phosphatase 1 which determines the fate of cardiomyocytes during hypoxia/reoxygenation. Autophagy. 2015;11(8):1308–1325. doi: 10.1080/15548627.2015.1060386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pankiv S, Clausen TH, Lamark T, Brech A, Bruun JA, Outzen H, Overvatn A, Bjorkoy G, Johansen T. p62/SQSTM1 binds directly to Atg8/LC3 to facilitate degradation of ubiquitinated protein aggregates by autophagy. J Biol Chem. 2007;282(33):24131–24145. doi: 10.1074/jbc.M702824200. [DOI] [PubMed] [Google Scholar]
- 21.Baek SH, Kim KI. Epigenetic control of autophagy: nuclear events gain more attention. Mol Cell. 2017;65(5):781–785. doi: 10.1016/j.molcel.2016.12.027. [DOI] [PubMed] [Google Scholar]
- 22.Wu Z, Connolly J, Biggar KK. Beyond histones—the expanding roles of protein lysine methylation. FEBS J. 2017;284(17):2732–2744. doi: 10.1111/febs.14056. [DOI] [PubMed] [Google Scholar]
- 23.Murray K. The occurrence of epsilon-N-methyl lysine in histones. Biochemistry. 1964;3:10–15. doi: 10.1021/bi00889a003. [DOI] [PubMed] [Google Scholar]
- 24.Byvoet P, Shepherd GR, Hardin JM, Noland BJ. The distribution and turnover of labeled methyl groups in histone fractions of cultured mammalian cells. Arch Biochem Biophys. 1972;148(2):558–567. doi: 10.1016/0003-9861(72)90174-9. [DOI] [PubMed] [Google Scholar]
- 25.Trievel RC, Beach BM, Dirk LM, Houtz RL, Hurley JH. Structure and catalytic mechanism of a SET domain protein methyltransferase. Cell. 2002;111(1):91–103. doi: 10.1016/S0092-8674(02)01000-0. [DOI] [PubMed] [Google Scholar]
- 26.Lee S, Oh S, Jeong K, Jo H, Choi Y, Seo HD, Kim M, Choe J, Kwon CS, Lee D. Dot1 regulates nucleosome dynamics by its inherent histone chaperone activity in yeast. Nat Commun. 2018;9(1):240. doi: 10.1038/s41467-017-02759-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Blanc RS, Richard S. Arginine methylation: the coming of age. Mol Cell. 2017;65(1):8–24. doi: 10.1016/j.molcel.2016.11.003. [DOI] [PubMed] [Google Scholar]
- 28.Greer EL, Shi Y. Histone methylation: a dynamic mark in health, disease and inheritance. Nat Rev Genet. 2012;13(5):343–357. doi: 10.1038/nrg3173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mosammaparast N, Shi Y. Reversal of histone methylation: biochemical and molecular mechanisms of histone demethylases. Annu Rev Biochem. 2010;79:155–179. doi: 10.1146/annurev.biochem.78.070907.103946. [DOI] [PubMed] [Google Scholar]
- 30.Wei FZ, Cao Z, Wang X, Wang H, Cai MY, Li T, Hattori N, Wang D, Du Y, Song B, Cao LL, Shen C, Wang L, Wang H, Yang Y, Xie D, Wang F, Ushijima T, Zhao Y, Zhu WG. Epigenetic regulation of autophagy by the methyltransferase EZH2 through an MTOR-dependent pathway. Autophagy. 2015;11(12):2309–2322. doi: 10.1080/15548627.2015.1117734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tan JZ, Yan Y, Wang XX, Jiang Y, Xu HE. EZH2: biology, disease, and structure-based drug discovery. Acta Pharmacol Sin. 2014;35(2):161–174. doi: 10.1038/aps.2013.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Liu TP, Hong YH, Tung KY, Yang PM. In silico and experimental analyses predict the therapeutic value of an EZH2 inhibitor GSK343 against hepatocellular carcinoma through the induction of metallothionein genes. Oncoscience. 2016;3(1):9–20. doi: 10.18632/oncoscience.285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hsieh YY, Lo HL, Yang PM. EZH2 inhibitors transcriptionally upregulate cytotoxic autophagy and cytoprotective unfolded protein response in human colorectal cancer cells. Am J Cancer Res. 2016;6(8):1661–1680. [PMC free article] [PubMed] [Google Scholar]
- 34.Sun Y, Jin L, Liu JH, Sui YX, Han LL, Shen XL. Interfering EZH2 expression reverses the cisplatin resistance in human ovarian cancer by inhibiting autophagy. Cancer Biother Radiopharm. 2016;31(7):246–252. doi: 10.1089/cbr.2016.2034. [DOI] [PubMed] [Google Scholar]
- 35.Wang Z, Liu H, Xu C. Cellular senescence in the treatment of ovarian cancer. Int J Gynecol Cancer. 2018 doi: 10.1097/igc.0000000000001257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Li R, Yi X, Wei X, Huo B, Guo X, Cheng C, Fang ZM, Wang J, Feng X, Zheng P, Su YS, Masau JF, Zhu XH, Jiang DS. EZH2 inhibits autophagic cell death of aortic vascular smooth muscle cells to affect aortic dissection. Cell Death Dis. 2018;9(2):180. doi: 10.1038/s41419-017-0213-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Galluzzi L, Vitale I, Abrams JM, Alnemri ES, Baehrecke EH, Blagosklonny MV, Dawson TM, Dawson VL, El-Deiry WS, Fulda S, Gottlieb E, Green DR, Hengartner MO, Kepp O, Knight RA, Kumar S, Lipton SA, Lu X, Madeo F, Malorni W, Mehlen P, Nunez G, Peter ME, Piacentini M, Rubinsztein DC, Shi Y, Simon HU, Vandenabeele P, White E, Yuan J, Zhivotovsky B, Melino G, Kroemer G. Molecular definitions of cell death subroutines: recommendations of the Nomenclature Committee on Cell Death 2012. Cell Death Differ. 2012;19(1):107–120. doi: 10.1038/cdd.2011.96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Shinkai Y. Regulation and function of H3K9 methylation. Subcell Biochem. 2007;41:337–350. [PubMed] [Google Scholar]
- 39.Jin Q, Yu LR, Wang L, Zhang Z, Kasper LH, Lee JE, Wang C, Brindle PK, Dent SY, Ge K. Distinct roles of GCN5/PCAF-mediated H3K9ac and CBP/p300-mediated H3K18/27ac in nuclear receptor transactivation. EMBO J. 2011;30(2):249–262. doi: 10.1038/emboj.2010.318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Artal-Martinez de Narvajas A, Gomez TS, Zhang JS, Mann AO, Taoda Y, Gorman JA, Herreros-Villanueva M, Gress TM, Ellenrieder V, Bujanda L, Kim DH, Kozikowski AP, Koenig A, Billadeau DD. Epigenetic regulation of autophagy by the methyltransferase G9a. Mol Cell Biol. 2013;33(20):3983–3993. doi: 10.1128/MCB.00813-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Collins PL, Oltz EM. Histone methylation keeps the brakes on autophagy. Mol Cell Biol. 2013;33(20):3974–3975. doi: 10.1128/MCB.01033-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Morgan C, Hibben M, Esan O, John S, Patel V, Weiss HA, Murray RM, Hutchinson G, Gureje O, Thara R, Cohen A. Searching for psychosis: INTREPID (1): systems for detecting untreated and first-episode cases of psychosis in diverse settings. Soc Psychiatry Psychiatr Epidemiol. 2015;50(6):879–893. doi: 10.1007/s00127-015-1013-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Li F, Zeng J, Gao Y, Guan Z, Ma Z, Shi Q, Du C, Jia J, Xu S, Wang X, Chang L, He D, Guo P. G9a inhibition induces autophagic cell death via AMPK/mTOR pathway in bladder transitional cell carcinoma. PLoS One. 2015;10(9):e0138390. doi: 10.1371/journal.pone.0138390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ren A, Qiu Y, Cui H, Fu G. Inhibition of H3K9 methyltransferase G9a induces autophagy and apoptosis in oral squamous cell carcinoma. Biochem Biophys Res Commun. 2015;459(1):10–17. doi: 10.1016/j.bbrc.2015.01.068. [DOI] [PubMed] [Google Scholar]
- 45.Ke XX, Zhang D, Zhu S, Xia Q, Xiang Z, Cui H. Inhibition of H3K9 methyltransferase G9a repressed cell proliferation and induced autophagy in neuroblastoma cells. PLoS One. 2014;9(9):e106962. doi: 10.1371/journal.pone.0106962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Park SE, Yi HJ, Suh N, Park YY, Koh JY, Jeong SY, Cho DH, Kim CS, Hwang JJ. Inhibition of EHMT2/G9a epigenetically increases the transcription of Beclin-1 via an increase in ROS and activation of NF-kappaB. Oncotarget. 2016;7(26):39796–39808. doi: 10.18632/oncotarget.9290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Shilatifard A. Molecular implementation and physiological roles for histone H3 lysine 4 (H3K4) methylation. Curr Opin Cell Biol. 2008;20(3):341–348. doi: 10.1016/j.ceb.2008.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ni P, Xu H, Chen C, Wang J, Liu X, Hu Y, Fan Q, Hou Z, Lu Y. Serum starvation induces DRAM expression in liver cancer cells via histone modifications within its promoter locus. PLoS One. 2012;7(12):e50502. doi: 10.1371/journal.pone.0050502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rosenstein DI, Chiodo GT, Bartley MH. Treating recurrent aphthous ulcers in patients with AIDS. J Am Dent Assoc. 1991;122(10):64, 67–68. doi: 10.14219/jada.archive.1991.0299. [DOI] [PubMed] [Google Scholar]
- 50.Shen B, Tan M, Mu X, Qin Y, Zhang F, Liu Y, Fan Y. Upregulated SMYD3 promotes bladder cancer progression by targeting BCLAF1 and activating autophagy. Tumour Biol. 2016;37(6):7371–7381. doi: 10.1007/s13277-015-4410-2. [DOI] [PubMed] [Google Scholar]
- 51.Fullgrabe J, Lynch-Day MA, Heldring N, Li W, Struijk RB, Ma Q, Hermanson O, Rosenfeld MG, Klionsky DJ, Joseph B. The histone H4 lysine 16 acetyltransferase hMOF regulates the outcome of autophagy. Nature. 2013;500(7463):468–471. doi: 10.1038/nature12313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wang Z, Long QY, Chen L, Fan JD, Wang ZN, Li LY, Wu M, Chen X. Inhibition of H3K4 demethylation induces autophagy in cancer cell lines. Biochim Biophys Acta 1864. 2017;12:2428–2437. doi: 10.1016/j.bbamcr.2017.08.005. [DOI] [PubMed] [Google Scholar]
- 53.Hong F, Wan L, Liu J, Huang K, Xiao Z, Zhang Y, Shi C. Histone methylation regulates Hif-1 signaling cascade in activation of hepatic stellate cells. FEBS Open Bio. 2018;8(3):406–415. doi: 10.1002/2211-5463.12379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Shin HJ, Kim H, Oh S, Lee JG, Kee M, Ko HJ, Kweon MN, Won KJ, Baek SH. AMPK–SKP2–CARM1 signalling cascade in transcriptional regulation of autophagy. Nature. 2016;534(7608):553–557. doi: 10.1038/nature18014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Selvi BR, Batta K, Kishore AH, Mantelingu K, Varier RA, Balasubramanyam K, Pradhan SK, Dasgupta D, Sriram S, Agrawal S, Kundu TK. Identification of a novel inhibitor of coactivator-associated arginine methyltransferase 1 (CARM1)-mediated methylation of histone H3 Arg-17. J Biol Chem. 2010;285(10):7143–7152. doi: 10.1074/jbc.M109.063933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Farooq Z, Banday S, Pandita TK, Altaf M. The many faces of histone H3K79 methylation. Mutat Res Rev Mutat Res. 2016;768:46–52. doi: 10.1016/j.mrrev.2016.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Gao Y, Ge W. The histone methyltransferase DOT1L inhibits osteoclastogenesis and protects against osteoporosis. Cell Death Dis. 2018;9(2):33. doi: 10.1038/s41419-017-0040-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hoeflich KP, Gray DC, Eby MT, Tien JY, Wong L, Bower J, Gogineni A, Zha J, Cole MJ, Stern HM, Murray LJ, Davis DP, Seshagiri S. Oncogenic BRAF is required for tumor growth and maintenance in melanoma models. Cancer Res. 2006;66(2):999–1006. doi: 10.1158/0008-5472.CAN-05-2720. [DOI] [PubMed] [Google Scholar]
- 59.Xu J, Wang AH, Oses-Prieto J, Makhijani K, Katsuno Y, Pei M, Yan L, Zheng YG, Burlingame A, Bruckner K, Derynck R. Arginine methylation initiates BMP-induced smad signaling. Mol Cell. 2013;51(1):5–19. doi: 10.1016/j.molcel.2013.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Biggar KK, Li SS. Non-histone protein methylation as a regulator of cellular signalling and function. Nat Rev Mol Cell Biol. 2015;16(1):5–17. doi: 10.1038/nrm3915. [DOI] [PubMed] [Google Scholar]
- 61.Wu D, Pan W. GSK3: a multifaceted kinase in Wnt signaling. Trends Biochem Sci. 2010;35(3):161–168. doi: 10.1016/j.tibs.2009.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Mizushima N, Yoshimori T, Ohsumi Y. The role of Atg proteins in autophagosome formation. Annu Rev Cell Dev Biol. 2011;27:107–132. doi: 10.1146/annurev-cellbio-092910-154005. [DOI] [PubMed] [Google Scholar]
- 63.Song H, Feng X, Zhang M, Jin X, Xu X, Wang L, Ding X, Luo Y, Lin F, Wu Q, Liang G, Yu T, Liu Q, Zhang Z. Crosstalk between lysine methylation and phosphorylation of ATG16L1 dictates the apoptosis of hypoxia/reoxygenation-induced cardiomyocytes. Autophagy. 2018;14(5):825–844. doi: 10.1080/15548627.2017.1389357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wen C, Xu M, Mo C, Cheng Z, Guo Q, Zhu X. JMJD6 exerts function in neuropathic pain by regulating NFkappaB following peripheral nerve injury in rats. Int J Mol Med. 2018 doi: 10.3892/ijmm.2018.3613. [DOI] [PubMed] [Google Scholar]
- 65.Hsu JM, Chen CT, Chou CK, Kuo HP, Li LY, Lin CY, Lee HJ, Wang YN, Liu M, Liao HW, Shi B, Lai CC, Bedford MT, Tsai CH, Hung MC. Crosstalk between Arg 1175 methylation and Tyr 1173 phosphorylation negatively modulates EGFR-mediated ERK activation. Nat Cell Biol. 2011;13(2):174–181. doi: 10.1038/ncb2158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Jiang H, Zhou Z, Jin S, Xu K, Zhang H, Xu J, Sun Q, Wang J, Xu J. PRMT9 promotes hepatocellular carcinoma invasion and metastasis via activating PI3K/Akt/GSK-3beta/Snail signalling. Cancer Sci. 2018 doi: 10.1111/cas.13598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Raposo AE, Piller SC. Protein arginine methylation: an emerging regulator of the cell cycle. Cell Div. 2018;13:3. doi: 10.1186/s13008-018-0036-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Wils LJ, Bijlsma MF. Epigenetic regulation of the Hedgehog and Wnt pathways in cancer. Crit Rev Oncol Hematol. 2018;121:23–44. doi: 10.1016/j.critrevonc.2017.11.013. [DOI] [PubMed] [Google Scholar]
- 69.Nandy A, Lin L, Velentzas PD, Wu LP, Baehrecke EH, Silverman N. The NF-kappaB factor relish regulates Atg1 expression and controls autophagy. Cell Rep. 2018;25(8):2110–2120 e2113. doi: 10.1016/j.celrep.2018.10.076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kretowski R, Borzym-Kluczyk M, Stypulkowska A, Branska-Januszewska J, Ostrowska H, Cechowska-Pasko M. Low glucose dependent decrease of apoptosis and induction of autophagy in breast cancer MCF-7 cells. Mol Cell Biochem. 2016;417(1–2):35–47. doi: 10.1007/s11010-016-2711-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Copetti T, Bertoli C, Dalla E, Demarchi F, Schneider C. p65/RelA modulates BECN1 transcription and autophagy. Mol Cell Biol. 2009;29(10):2594–2608. doi: 10.1128/MCB.01396-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Lim CB, Fu PY, Ky N, Zhu HS, Feng X, Li J, Srinivasan KG, Hamza MS, Zhao Y. NF-kappaB p65 repression by the sesquiterpene lactone, Helenalin, contributes to the induction of autophagy cell death. BMC Complement Altern Med. 2012;12:93. doi: 10.1186/1472-6882-12-93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Verma N, Manna SK. Advanced glycation end products (AGE) potentiates cell death in p53 negative cells via upregulaion of NF-kappa B and impairment of autophagy. J Cell Physiol. 2017;232(12):3598–3610. doi: 10.1002/jcp.25828. [DOI] [PubMed] [Google Scholar]
- 74.Gilmore TD. Introduction to NF-kappaB: players, pathways, perspectives. Oncogene. 2006;25(51):6680–6684. doi: 10.1038/sj.onc.1209954. [DOI] [PubMed] [Google Scholar]
- 75.Xia L, Tan S, Zhou Y, Lin J, Wang H, Oyang L, Tian Y, Liu L, Su M, Wang H, Cao D, Liao Q. Role of the NFkappaB-signaling pathway in cancer. Onco Targets Ther. 2018;11:2063–2073. doi: 10.2147/OTT.S161109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Ea CK, Baltimore D. Regulation of NF-kappaB activity through lysine monomethylation of p65. Proc Natl Acad Sci USA. 2009;106(45):18972–18977. doi: 10.1073/pnas.0910439106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Mukherjee N, Cardenas E, Bedolla R, Ghosh R. SETD6 regulates NF-kappaB signaling in urothelial cell survival: implications for bladder cancer. Oncotarget. 2017;8(9):15114–15125. doi: 10.18632/oncotarget.14750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Yuan ZL, Guan YJ, Wang L, Wei W, Kane AB, Chin YE. Central role of the threonine residue within the p + 1 loop of receptor tyrosine kinase in STAT3 constitutive phosphorylation in metastatic cancer cells. Mol Cell Biol. 2004;24(21):9390–9400. doi: 10.1128/MCB.24.21.9390-9400.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Hu W, Lv J, Han M, Yang Z, Li T, Jiang S, Yang Y. STAT3: the art of multi-tasking of metabolic and immune functions in obesity. Prog Lipid Res. 2018;70:17–28. doi: 10.1016/j.plipres.2018.04.002. [DOI] [PubMed] [Google Scholar]
- 80.You L, Wang Z, Li H, Shou J, Jing Z, Xie J, Sui X, Pan H, Han W. The role of STAT3 in autophagy. Autophagy. 2015;11(5):729–739. doi: 10.1080/15548627.2015.1017192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Feng Y, Ke C, Tang Q, Dong H, Zheng X, Lin W, Ke J, Huang J, Yeung SC, Zhang H. Metformin promotes autophagy and apoptosis in esophageal squamous cell carcinoma by downregulating Stat3 signaling. Cell Death Dis. 2014;5:e1088. doi: 10.1038/cddis.2014.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Mazure NM, Pouyssegur J. Hypoxia-induced autophagy: cell death or cell survival? Curr Opin Cell Biol. 2010;22(2):177–180. doi: 10.1016/j.ceb.2009.11.015. [DOI] [PubMed] [Google Scholar]
- 83.Kim E, Kim M, Woo DH, Shin Y, Shin J, Chang N, Oh YT, Kim H, Rheey J, Nakano I, Lee C, Joo KM, Rich JN, Nam DH, Lee J. Phosphorylation of EZH2 activates STAT3 signaling via STAT3 methylation and promotes tumorigenicity of glioblastoma stem-like cells. Cancer Cell. 2013;23(6):839–852. doi: 10.1016/j.ccr.2013.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Kong J, Kong F, Gao J, Zhang Q, Dong S, Gu F, Ke S, Pan B, Shen Q, Sun H, Zheng L, Sun W. YC-1 enhances the anti-tumor activity of sorafenib through inhibition of signal transducer and activator of transcription 3 (STAT3) in hepatocellular carcinoma. Mol Cancer. 2014;13:7. doi: 10.1186/1476-4598-13-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Cargnello M, Roux PP. Activation and function of the MAPKs and their substrates, the MAPK-activated protein kinases. Microbiol Mol Biol Rev. 2011;75(1):50–83. doi: 10.1128/MMBR.00031-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Martinez-Lopez N, Singh R. ATGs: scaffolds for MAPK/ERK signaling. Autophagy. 2014;10(3):535–537. doi: 10.4161/auto.27642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Cagnol S, Chambard JC. ERK and cell death: mechanisms of ERK-induced cell death—apoptosis, autophagy and senescence. FEBS J. 2010;277(1):2–21. doi: 10.1111/j.1742-4658.2009.07366.x. [DOI] [PubMed] [Google Scholar]
- 88.Andreu-Perez P, Esteve-Puig R, de Torre-Minguela C, Lopez-Fauqued M, Bech-Serra JJ, Tenbaum S, Garcia-Trevijano ER, Canals F, Merlino G, Avila MA, Recio JA. Protein arginine methyltransferase 5 regulates ERK1/2 signal transduction amplitude and cell fate through CRAF. Sci Signal. 2011;4(190):ra58. doi: 10.1126/scisignal.2001936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Kruse JP, Gu W. Modes of p53 regulation. Cell. 2009;137(4):609–622. doi: 10.1016/j.cell.2009.04.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Maiuri MC, Galluzzi L, Morselli E, Kepp O, Malik SA, Kroemer G. Autophagy regulation by p53. Curr Opin Cell Biol. 2010;22(2):181–185. doi: 10.1016/j.ceb.2009.12.001. [DOI] [PubMed] [Google Scholar]
- 91.Feng Z, Zhang H, Levine AJ, Jin S. The coordinate regulation of the p53 and mTOR pathways in cells. Proc Natl Acad Sci USA. 2005;102(23):8204–8209. doi: 10.1073/pnas.0502857102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Feng Z, Hu W, de Stanchina E, Teresky AK, Jin S, Lowe S, Levine AJ. The regulation of AMPK beta1, TSC2, and PTEN expression by p53: stress, cell and tissue specificity, and the role of these gene products in modulating the IGF-1-AKT-mTOR pathways. Cancer Res. 2007;67(7):3043–3053. doi: 10.1158/0008-5472.CAN-06-4149. [DOI] [PubMed] [Google Scholar]
- 93.Budanov AV, Karin M. p53 target genes sestrin1 and sestrin2 connect genotoxic stress and mTOR signaling. Cell. 2008;134(3):451–460. doi: 10.1016/j.cell.2008.06.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Appella E, Anderson CW. Post-translational modifications and activation of p53 by genotoxic stresses. Eur J Biochem. 2001;268(10):2764–2772. doi: 10.1046/j.1432-1327.2001.02225.x. [DOI] [PubMed] [Google Scholar]
- 95.Chuikov S, Kurash JK, Wilson JR, Xiao B, Justin N, Ivanov GS, McKinney K, Tempst P, Prives C, Gamblin SJ, Barlev NA, Reinberg D. Regulation of p53 activity through lysine methylation. Nature. 2004;432(7015):353–360. doi: 10.1038/nature03117. [DOI] [PubMed] [Google Scholar]
- 96.Huang J, Perez-Burgos L, Placek BJ, Sengupta R, Richter M, Dorsey JA, Kubicek S, Opravil S, Jenuwein T, Berger SL. Repression of p53 activity by Smyd2-mediated methylation. Nature. 2006;444(7119):629–632. doi: 10.1038/nature05287. [DOI] [PubMed] [Google Scholar]
- 97.Fan JD, Lei PJ, Zheng JY, Wang X, Li S, Liu H, He YL, Wang ZN, Wei G, Zhang X, Li LY, Wu M. The selective activation of p53 target genes regulated by SMYD2 in BIX-01294 induced autophagy-related cell death. PLoS One. 2015;10(1):e0116782. doi: 10.1371/journal.pone.0116782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Tasdemir E, Maiuri MC, Galluzzi L, Vitale I, Djavaheri-Mergny M, D’Amelio M, Criollo A, Morselli E, Zhu C, Harper F, Nannmark U, Samara C, Pinton P, Vicencio JM, Carnuccio R, Moll UM, Madeo F, Paterlini-Brechot P, Rizzuto R, Szabadkai G, Pierron G, Blomgren K, Tavernarakis N, Codogno P, Cecconi F, Kroemer G. Regulation of autophagy by cytoplasmic p53. Nat Cell Biol. 2008;10(6):676–687. doi: 10.1038/ncb1730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Morselli E, Tasdemir E, Maiuri MC, Galluzzi L, Kepp O, Criollo A, Vicencio JM, Soussi T, Kroemer G. Mutant p53 protein localized in the cytoplasm inhibits autophagy. Cell Cycle. 2008;7(19):3056–3061. doi: 10.4161/cc.7.19.6751. [DOI] [PubMed] [Google Scholar]
- 100.Carter S, Bischof O, Dejean A, Vousden KH. C-terminal modifications regulate MDM2 dissociation and nuclear export of p53. Nat Cell Biol. 2007;9(4):428–435. doi: 10.1038/ncb1562. [DOI] [PubMed] [Google Scholar]
- 101.Venne AS, Kollipara L, Zahedi RP. The next level of complexity: crosstalk of posttranslational modifications. Proteomics. 2014;14(4–5):513–524. doi: 10.1002/pmic.201300344. [DOI] [PubMed] [Google Scholar]
- 102.Gu B, Zhu WG. Surf the post-translational modification network of p53 regulation. Int J Biol Sci. 2012;8(5):672–684. doi: 10.7150/ijbs.4283. [DOI] [PMC free article] [PubMed] [Google Scholar]