Abstract
Alcoholic liver disease (ALD) remains to be a worldwide health problem. It is generally accepted that oxidative stress plays critical roles in the pathogenesis of ALD, and antioxidant therapy represents a logical strategy for the prevention and treatment of ALD. Nuclear factor erythroid-derived 2-like 2 (NFE2L2 or Nrf-2) is essential for the antioxidant responsive element (ARE)-mediated induction of endogenous antioxidant enzymes such as heme oxygenase 1 (HO-1) and glutamate–cysteine ligase [GCL, the rate-limiting enzyme in the synthesis of glutathione (GSH)]. Activation of Nrf-2 pathway by genetic manipulation or pharmacological agents has been demonstrated to provide protection against ALD, which suggests that targeting Nrf-2 may be a promising approach for the prevention and treatment of ALD. Herein, we review the relevant literature about the potential hepatoprotective roles of Nrf-2 activation against ALD.
Keywords: Alcoholic liver disease, Nuclear factor erythroid-derived 2-like 2 (Nrf-2), Oxidative stress, p62, Autophagy
Introduction
Excessive ethanol consumption can cause a progressively aggravated liver disease, namely alcoholic liver disease (ALD). ALD presents as a broad spectrum of disorders, ranging from simple fatty liver (steatosis) to alcoholic hepatitis (AH), alcoholic fibrosis (AF), alcoholic cirrhosis (AC), and the superimposed hepatocellular carcinoma (HCC) [1]. ALD is highly prevalent and is listed among the top 20 causes of death worldwide [2]. In Europe, it is estimated that more than 2,370,000 years of life are lost from liver diseases before the age of 50, and about 60–80% of these deaths are ethanol related [3]. In the USA, ethanol abuse is also the leading cause of death from liver diseases [4]. Although the number of newly hepatitis B virus (HBV)-infected patients in China is significantly declined due to the establishment of the expanded program on immunization in 1992, the number of ALD patients is rising at an alarming rate with the prevalence of ALD ranging from 2.3 to 6.1% in different local areas [5]. Thus, ALD has been becoming a worldwide health problem. Unfortunately, in contrast to the steady progress in the clarification of the pathogenic mechanisms of ALD, no significant advance has been made in the management of ALD, especially in the field of long-term treatments [6]. Currently, abstinence remains to be the cornerstone of ALD treatment which is largely dependent on patient’s willingness and compliance [7]. Due to the increasing prevalence of ALD and the lack of effective therapeutic agents, there is an urgent need to develop effective and safe pharmacological interventions for patients with ALD [8].
Nuclear factor erythroid-derived 2-like 2 (NFE2L2 or Nrf-2) is a transcription factor that plays a key role in the activation of cellular antioxidant enzymes in response to oxidative stress. Nrf-2 belongs to the Cap“n”Collar (CNC)-bZip (basic leucine zipper) family, which includes Nrf-1, Nrf-2, Nrf-3, and p45 NFE2 [9, 10]. After heterodimerization with one of three small Maf proteins, the indispensable partners of CNC-bZip transcription factors, Nrf-2 binds to the Maf recognition element-related sequence, namely antioxidant or electrophile response element (ARE/EpRE) [11, 12]. ARE has been identified in the transcriptional regulatory regions of antioxidant and xenobiotic-metabolizing enzyme genes including glutamate–cysteine ligase (GCL), the rate-limiting enzyme in the synthesis of glutathione (GSH), heme oxygenase-1 (HO-1), glutathione S-transferase (GST) family, and NAD(P)H quinone oxidoreductase 1 (NQO-1). Nrf-2 has been suggested to be a potential target for new therapeutics in various liver diseases [13, 14]. Activation of Nrf-2 signaling by pharmacological agents is considered as a promising strategy for protecting against liver injury induced by various chemicals including ethanol [15].
Critical roles of oxidative stress in the pathogenesis of ALD
ALD is a multifactorial disease involving complicated mechanisms, among which oxidative stress has been demonstrated to play critical roles [16]. As early as 1964, the pioneer study by Diluzio demonstrated that simultaneous supplementation of antioxidants could prevent acute ethanol-induced fatty liver, proposing the possible relationship between ethanol-induced steatosis and oxidative stress [17]. Using electron spin resonance (ESR) spectroscopy technique, increasing production of several reactive oxygen species (ROS) including nitroxyl radical, hydroxyl radicals, and hydroxyethyl free radicals has been detected [18, 19]. In addition, a variety of studies have provided evidence that both chronic and binge drinking could result in the enhancement of lipid peroxidation (shown as the elevation of malondialdehyde (MDA) and 4-hydroxynonenal (4-HNE) levels) and protein carbonyl formation, and the impairment of the hepatic antioxidant defense system including reduced GSH level and superoxide dismutase (SOD) activity [16, 20]. Interestingly, ethanol-induced liver injury could be significantly attenuated by various kinds of antioxidants including N-acetyl cysteine (NAC), resveratrol, silymarin, quercetin, and organosulfur compounds derived from garlic [21–26]. Furthermore, overexpression of the Cu/Zn-SOD or Mn-SOD gene with adenovirus suppressed alcohol-induced early liver injury in rats [27, 28], while ethanol-induced liver damage was aggravated in Cu/Zn-SOD-deficient mice and glutathione peroxidase (GPX)/catalase double-knockout mice [29–31]. Lastly, cytochrome P4502E1 (CYP2E1) and NADPH oxidase (NOX) have been demonstrated to be the two major sources of ethanol-generated ROS in ALD models [24, 32, 33]. Collectively, these results provide solid evidence for the causal roles of oxidative stress in the pathogenesis of ALD.
The canonical and non-canonical activation of Nrf-2/Keap-1 pathway
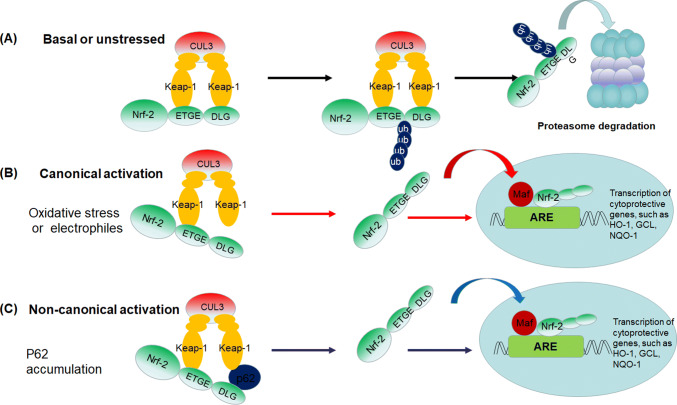
Under basal or unstressed conditions, Nrf-2 is kept in the cytoplasm by a cluster of proteins including Kelch like-ECH-associated protein 1 (Keap-1) and Cullin 3 (Cul3). Keap-1 contains three major domains: a N-terminal BTB (broad complex, tram track, and bric-a-brac) domain, a linker region, and a C-terminal Kelch domain. The Kelch domain contains six conserved Kelch repeat sequences and binds to the Neh2 domain of Nrf-2, while the BTB domain is responsible for the homodimerization of the Keap-1 protein. The linker region is a cysteine-rich domain which is demonstrated to be indispensable for the activity of Keap-1 [10, 34, 35]. Nrf-2 has six Neh (Nrf-2-ECH homology) domains (Neh1–Neh6). The Neh2 domain has two different motifs (the ETGE and the DLG motifs) which can bind Keap-1, resulting in an Nrf-2–Keap-1 complex of 1:2 stoichiometry with two binding sites [36, 37]. Cul3 ubiquitinates Nrf-2, while Keap-1 is a substrate adaptor protein for the Cul3 E3 ubiquitin (Ub) ligase complex that facilitates the reaction. The ubiquitinated Nrf-2 then transports to the proteasome for degradation, ensuring the low basal levels of Nrf-2 [38].
Two activation models of Nrf-2, the canonical activation and non-canonical activation, have been proposed (Fig. 1). Upon attack by ROS or electrophiles, the sensory cysteines of Keap-1 can be modified, resulting in a conformational change which could prevent Nrf-2 from ubiquitination. The newly synthesized Nrf-2 escapes from Keap-1-mediated repression and translocates to the nucleus, and then binds to small Maf protein and turns on the transcription of ARE-controlled genes to maintain cellular redox homeostasis. This mechanism of Keap-1 cysteine-dependent Nrf-2 activation is termed canonical activation [10, 38–40]. The non-canonical activation of Nrf-2 is mediated by the interactions between Keap-1 and p62 (also known as sequestosome-1, SQSTM1), which localizes to sites of autophagosome formation and acts as a receptor for ubiquitinated proteins and organelles in autophagy [41]. p62 is considered to be a predictor of autophagy flux, as the protein level of p62 is usually inversely correlated with autophagy activity [42]. p62 is a multifunctional protein, which contains more than ten domains and putative binding sites including a Keap-1-interacting region in the C terminus [41]. Keap-1 can interact with p62 via the STGE motif, leading to the stabilization, nucleus translocation and activation of Nrf-2, namely the non-canonical activation of Nrf-2 [43, 44]. Although the STGE motif in the Keap-1-interacting region of p62 has lower affinity for Keap-1 compared with the Nrf-2 ETGE motif; however, the affinity could markedly increase by some post-translational modification of p62 [45, 46]. For example, it has been demonstrated that the phosphorylation of serine 349 in the STGE motif of p62 by mammalian target of rapamycin (mTOR) and phosphorylation of serine 24 in the PB1 domain of p62 could increase its affinity to Keap-1 [47, 48]. Interestingly, the expression of p62 is regulated in an Nrf-2-dependent manner during oxidative stress, thus forming a positive feedback loop [46]. However, it should be noted that the activation of Nrf-2 by p62-mediated Keap-1 dissociation may be associated with some negative effects such as the tumorigenesis and the resistance to chemotherapy [49, 50]. More recently, Hu and colleagues demonstrated that inhibitor of apoptosis stimulating protein of p53 (iASPP) could compete with Keap-1 for Nrf-2 binding, leading to decreased Nrf-2 ubiquitination and increased Nrf-2 accumulation and antioxidative transactivation [51]. Results of this study expand our understanding of the antioxidant Nrf-2/Keap-1 pathway, which is needed to be further studied.
Fig. 1.
The canonical and non-canonical models for the activation of Nrf-2/Keap-1 system. a Under basal or unstressed conditions, Keap-1 dimer binds Nrf-2 via the DLG and ETGE motifs of Nedh2 domain, leading to the ubiquitination of the lysine residues located between the ETGE and DLG motifs and subsequent degradation of Nrf-2 by proteasome. b The canonical activation of Nrf-2. Oxidants or electrophiles can modify cysteine residues of Keap-1, resulting in a conformational change in Keap-1 leading to detachment of the weaker binding DLG motif and the termination of Nrf-2 ubiquitination. Nrf-2 then translocates to nucleus and activates the transcription of a battery of antioxidant enzymes and phase II detoxifying enzymes. c The non-canonical activation of Nrf-2. p62 can compete with Nrf-2 to bind Keap-1, resulting in the liberation of Nrf-2 from Keap-1-dependent ubiquitination and degradation in the cytoplasm
In addition to the above two activation models, a number of studies have provided evidence that Nrf-2 activity could be modulated by several putative kinases including protein kinase C (PKC), mitogen-activated protein kinase (MAPK), and Fyn kinase [52]. It has been demonstrated that PKC could phosphorylate Nrf-2 at serine 40 which is a critical signaling event leading to ARE-mediated cellular antioxidant response [53, 54]. p38MAPK could directly phosphorylate the recombinant GST-tagged Nrf-2 protein, promoting the interaction between recombinant protein and endogenous Keap-1 in vitro [55], whereas results of another study suggested that Nrf-2 phosphorylation by MAPKs might have minimal effects on Nrf-2 stability or its subcellular localization [56]. In addition, Jain and colleagues demonstrated that Fyn kinase could phosphorylate Nrf-2 protein at tyrosine 568 by glycogen synthase kinase-3β (GSK-3β) and promote its nuclear export and degradation, thereby contributing to the suppression of ARE-mediated gene expression [57, 58].
The alteration of Nrf-2 activity in ALD models
Nrf-2 is theoretically to be activated after ethanol exposure via the canonical activation mechanism, as ethanol could induce ROS production. Indeed, a couple of studies have suggested that ethanol exposure led to the activation of Nrf-2 pathway, which might act as a compensatory or adaptive mechanism to suppress ethanol-induced oxidative injury [20]. For example, Gong et al. found that the mRNA and protein levels of Nrf-2 significantly increased in the liver of liquid diet (ethanol accounting for 35% of the total calories)-fed male C57BL/6 mice and in the isolated hepatocytes of ethanol-containing liquid diet-fed rats, which was thought to be mediated by CYP2E1-generated ROS [59]. The study by Bardag-Gorce et al. also reported that Nrf-2 mRNA level in ethanol-fed rats was significantly increased compared with that of dextrose-fed animals. However, the authors found that proteasome inhibitor (PS-341) could protect against ethanol-induced liver injury in rats via regulating the ARE by activating transcription factor 4 (ATF-4), but not Nrf-2, as the combination of proteasome inhibitor and ethanol led to a significant decrease of Nrf-2 expression [60]. Yeligar et al. demonstrated that ethanol exposure by intragastric infusion (9–16 g/kg body weight/day) augmented Nrf-2-mediated transcription of HO-1 in rat Kupffer cells (KCs) [61]. Similarly, short-term ethanol treatment resulted in the induction of HO-1 and NQO-1 in liver tissues of mice [62]. Results of these studies are consistent with the canonical activation theory: ethanol metabolism-associated ROS may lead to the modulation of cysteine residues of Keap-1, resulting in the nucleus translocation and activation of Nrf-2; the activated Nrf-2 then upregulates important antioxidant genes and detoxification enzymes, helping to maintain cellular protective pathways [59].
In contrast to the results of above studies, some studies reported that Nrf-2 expression was not altered [63, 64] or even decreased in the livers of ethanol-exposed animals/hepatocytes [65–67]. At present, it remains unclear why Nrf-2 responded differently in different studies. Anyway, the difference in the experimental animals (such as the species, ages, and sexes), mode of ethanol delivery, and diet composition may be responsible for these contradictory results. For example, the liquid diet-induced chronic mice model was used in the study by Gong et al. [59], while binge drinking-induced acute ALD mice models were used in the study by Choi et al. and the study by Zhou et al. [63, 65]. Thus, it may be speculated that Nrf-2 activation in ethanol-treated mice is time dependent. Although the same strains of rats (Sprague–Dawley rats) were used in the studies by Lu et al. and in the study by Gong et al.; however, the former study used gavage model (56%, v/v, 10 ml/kg body weight, once daily) for 9 weeks [66–68], while the study by Gong et al. used a liquid diet feeding model for 2 months [59]. Therefore, the discrepancy in ethanol delivery may also account for these contradictory reports.
Nrf-2 deficiency aggravates ethanol-induced liver damage
Lamle et al. found that Nrf-2−/− mice displayed dramatically increased mortality, significantly reduced ability in detoxifying acetaldehyde, marked steatosis, upregulation of sterol regulatory element binding protein 1c (SREBP-1c), depletion of total and mitochondrial GSH, and aggravated inflammatory response, when exposed to ethanol at a dose which was tolerated by wild-type mice [62]. Wu et al. compared acute ethanol-induced liver toxicity in Nrf-2 null mice, wild-type mice, Keap-1-knockdown (Keap-1 KD) mice, and Keap-1-hepatocyte knockout (Keap-1-H-KO) mice [69]. They found that acute ethanol-induced increase of serum alanine transaminase (ALT) and lactate dehydrogenase (LDH) activities, triglyceride (TG) and thiobarbituric acid-reactive substances (TBARS) contents in Nrf-2-null and wild-type mice, but not in Nrf-2-enhanced mice (Keap-1-KD and Keap-1-H-KO mice). Besides, acute ethanol-induced decrease of mitochondrial GSH level and increase of ROS in hepatocytes disappeared in Nrf-2-enhanced mice. Furthermore, the basal mRNA and protein levels of SREBP-1c, the major nuclear transcription factor regulating the transcription of a battery of genes involved in fatty acid synthesis, were decreased with graded Nrf-2 activation. These results suggest that Nrf-2 activation can prevent acute ethanol-induced oxidative stress and accumulation of free fatty acids in liver by increasing genes involved in antioxidant defense and decreasing genes involved in lipogenesis [69].
Nrf-2 activators significantly attenuate ethanol-induced liver injury
Over the past few years, many bioactive natural compounds including sulforaphane, quercetin, curcumin, and diallyl disulfide have been shown to exhibit hepatoprotective effects against ALD, which may be related with the induction of Nrf-2 (Table 1).
Table 1.
Effects of various phytochemical compounds/extracts on ethanol-induced liver injury via regulating Nrf-2 signaling pathways
| Compounds | ALD models | Improved parameters | Evidences of Nrf-2 in the protection against ALD | References |
|---|---|---|---|---|
| Sulforaphane | Sprague–Dawley rats exposed to ethanol (5 g/kg bw) twice daily for 16 days | Serum ALT, AST, LDH; histological changes | Increased Nrf-2 nucleus translocation; increased mRNA and protein levels of HO-2, NQO-1, and GST-P | [70] |
| Sulforaphane | CYP2E1-expressing HepG2; Male SV129 humanized CYP2E1 knockin mice exposed to 3 g/kg bw ethanol twice daily for 5 days | Liver TC and TG levels; liver steatosis | Increased protein levels of Nrf-2 and HO-1; increased Nrf-2 activity | [64] |
| Curcumin | Sprague–Dawley rats exposed to ethanol (56%, 10 ml/kg bw) twice daily for 4 weeks; LO2 cells exposed to 100 mM ethanol | Necroptosis; serum ALT | Nrf-2 knockdown by shRNA lentivirus abrogated the hepatoprotective effect of curcumin | [66] |
| Curcumin | Sprague–Dawley rats exposed to ethanol (56%, 10 ml/kg bw) once daily for 9 weeks; LO2 cells exposed to 100 mM ethanol | Serum ALT, AST, ALP, LDH, TG, TC, LDL-C, HDL-C; Liver TG, TC; ALT, AST, and LDH in culture medium; Cellular lipid level; Steatosis | Increased protein levels of Nrf-2; Nrf-2 siRNA attenuated the protective effects, while Nrf-2 overexpression enhanced the protection. | [67] |
| Curcumin | Male Balb/C mice exposed to ethanol (56%, 10 ml/kg bw) orally for 6 weeks | Serum ALT, AST; Liver TG, TC, LDL-C, HDL-C; Steatosis | Increased Nrf-2 nucleus translocation; increased mRNA levels of NQO1, HO-1, and GCLc | [80] |
| Quercetin | LO2 cells exposed to ethanol | Cell viability | Induced nucleus translocation of Nrf-2 and p62; increased expression of GCL and HO-1; p62 siRNA blocked the protective effects | [76] |
| Quercetin | Human hepatocytes exposed to ethanol | GSH and MDA, LDH and AST activity in culture medium, and EC50 of ethanol | Nrf-2/HO-1 inhibitor abrogated the protective effects of quercetin | [75] |
| Diallyl disulfide | Male Kunming mice exposed to 3 doses of ethanol (5 g/kg bw); LO2 cells exposed to 25–200 μM ethanol | LDH, AST in culture medium; apoptosis | Increased the Nrf-2 nucleus translocation; increased protein and mRNA levels of HO-1; HO-1 inhibitor abrogated the protective effects of DADS | [25] |
| Dihydromyricetin | C57BL/6 mice fed with Lieber–DeCarli liquid diet for 6 weeks | Hepatic enzyme release, lipid peroxidation; TG deposition; inflammatory cytokines; hepatic pathological changes | Increased the protein levels of Nrf-2 and p62 | [97] |
| Wuzhi tablet | Male C57BL/6 mice exposed to chronic-plus-binge model; or exposed to 6 g/kg bw ethanol for 3 times | Serum ALT and AST; Liver steatosis | Increase the protein levels of Nrf-2, GCLc, GCLm and HO-1 | [99] |
| Triticum aestivum sprout-derived polysaccharide | Male C57BL/6 mice were exposed to ethanol for 10 days | Serum ALT and AST; Liver TG and TG; steatosis; apoptosis | Upregulated the expression of Nrf-2 and HO-1 | [96] |
| Ligustrazine | Male ICR mice exposed to ethanol (56%, v/v, 10 ml/kg bw) once daily for 4 weeks; LO2 cell exposed to ethanol (100 mM) | Serum ALT, AST, ALP, LDH; liver inflammation and steatosis | Nrf-2 knockdown abrogated the hepatoprotective effects | [91] |
| Polydatin | Male Wistar rats exposed to ethanol (7 ml/kg bw) orally every 12 h at 5 different time points | Serum ALT, AST, ALP, LDH; liver inflammation, steatosis, necrosis, apoptosis | Increased protein levels of Nrf-2 and Nrf-2-targeted HO-1 | [65] |
| Baicalin | Chronic-plus-binge model (Gao-Bin model) | Serum ALT and AST; liver TG; liver steatosis, inflammation, apoptosis, necrosis | Enhanced nuclear translocation of Nrf-2 and increased mRNA levels of Nrf-2 target genes including HO-1 and NQO-1 | [92] |
| Hoveniae semen cum fructus extracts | Male C57BL/6 mice exposed to ethanol (5 g/kg bw) for 14 days | Serum ALT, AST, albumin, ALP, TG, γ-GT (γ-glutamyl transferase); liver TG, TNF-α; liver steatosis | Suppressed ethanol-induced decline of Nrf2 mRNA level and the decrease of hepatic GSH level and SOD and CAT activity | [93] |
| Glycycoumarin | Chronic plus binge drinking-induced chronic ALD and acute ALD model in C57BL/6 mice | Serum ALT and AST; liver TG level | Increased the protein levels of Nrf-2, HO-1, and GCLc; Nrf-2 activation lead to upregulation of p62. | [102] |
| Ethanolic extract of Sida cordifolia | Male Sprague–Dawley rats were gavaged with ethanol (4 g/kg bw) for 90 days | Serum ALT, AST, GGT; liver ALT, AST | Increased the nucleus translocation of Nrf-2 and the mRNA level of γ-GCS. | [94] |
| Tetramethylpyrazine | Ethanol-exposed LO2 cells | Cell viability, ALT and AST in culture medium; cellular TG, TC; apoptosis, | Increased Nrf-2 expression and nucleus translocation; overexpression of Nrf-2 enhanced the protective effects, while Nrf-2 siRNA eliminated the protective effects. | [68] |
| Citrus aurantium extract | Male C57BL/6 mice exposed to 5 g/kg bw ethanol for 3 doses | Serum ALT, AST, TG; liver steatosis, necrosis, apoptosis | Increased the protein levels of Nrf-2, NQO-1 and γ-GCSc | [63] |
| Chlorella ethanol extract | Sprague–Dawley rats were gavaged with ethanol 5 g/kg bw twice daily for 16 days | Serum ALT, AST, γ-GT, LDH | Increased Nrf-2 nucleus translocation and increased mRNA and protein levels of HO-2, NQO-1, and GST-P. | [70] |
| Oleanolic acid | Sprague–Dawley rats were gavaged with 4 g/kg bw ethanol for 30 days | Serum ALT, AST; liver ATP, TG, MDA | Increased the nucleus translocation of Nrf-2, and the protein expression of HO-1, SOD, and GR | [70] |
| Antroquinonol | Ethanol-exposed HepG2 cells | ALT, AST, ROS, MDA, NO | Increased the mRNA and protein levels of HO-1, Nrf-2 nucleus translocation, and ARE binding activity | [100] |
| Lucidone | Ethanol-exposed HepG2 cells | ALT, AST, NO, TNF-α, MDA, ROS | Increased the mRNA and protein levels of HO-1, Nrf-2 nucleus translocation and ARE binding activity | [101] |
ALD alcoholic liver disease; ALP alkaline phosphatase; ALT alanine aminotransferase; ARE antioxidant response element; AST aspartate aminotransferase; CAT catalase; GCL glutamate–cysteine ligase; GR glutathione reductase; GSH glutathione; GST glutathione S-transferase; γ-GT γ-glutamyl transferase; HDL-C high-density lipoprotein cholesterol; HO-1 heme oxygenase 1; LDH lactic dehydrogenase; LDL-C low-density lipoprotein cholesterol; MDA malondialdehyde; NO nitrogen oxide; NQO-1 NAD(P)H quinone oxidoreductase 1; Nrf-2 nuclear factor erythroid-derived 2-like 2; ROS reactive oxygen species; SOD superoxide dismutase; TG triglyceride; TC total cholesterol; TNF-α tumor necrosis factor α
Sulforaphane Sulforaphane is a well-known Nrf-2 activator affecting the cysteine residues in Keap-1 and affecting the phosphorylation of Nrf-2 [52]. The study by Zhou et al. demonstrated that sulforaphane could prevent binge drinking (3 g/kg body weight, twice daily, for 5 days)-induced liver steatosis by upregulating Nrf-2-mediated antioxidant defense and increasing autophagy activity in mice [64]. Another study showed that sulforaphane increased the Nrf-2 nucleus translocation, the protein and mRNA levels of HO-1, NQO-1, and GST-P in Hepa1c1c7 cells, and attenuated chronic ethanol (5 g/kg boy weight, twice daily, for 27 days)-induced increase of serum ALT and aspartate transaminase (AST) activities and improved the hepatic pathological changes (steatosis, necrosis, lymphocyte infiltration, loss of cellular boundaries) in male Sprague–Dawley rats [70].
Quercetin Quercetin is one of the most abundant dietary flavonoids, which has been demonstrated to protect against ethanol-induced oxidative damage in a variety of studies [21, 71–76]. Mechanism studies revealed that quercetin increased nucleus translocation of Nrf-2 and the activation of HO-1, which could be blocked by SB203580 (p38MAPK inhibitor) and PD98059 (ERK inhibitor), suggesting that p38MAPK and ERK-mediated Nrf-2/HO-1 activation might account for the protective effects of quercetin against ALD [75]. A recent study revealed that quercetin could prevent ethanol-induced hepatotoxicity by inducing p62-mediated non-canonical activation of Nrf-2 pathway, as p62 siRNA abrogated quercetin-associated hepatoprotection against ALD [76].
Curcumin Curcumin, extracted from dry rhizome of Curcuma longa, attenuated chronic ethanol-induced liver injury by attenuating oxidative stress and suppressing the expression of nuclear factor kappa-light-chain enhancer of activated B cells (NF-κB) [77–79]. A series of studies have been conducted to investigate the roles of Nrf-2 activation and the hepatoprotective effects of curcumin against ALD [66, 67, 80]. Lu et al. found that curcumin could suppress ethanol-induced disturbance of SREBP-1c and peroxisome proliferator-activated receptor α (PPAR-α), and simultaneously induce the expression of Nrf-2 and farnesoid X receptor (FXR) in liver; the gain- and loss-of-function analyses in LO2 hepatocytes revealed Nrf-2 and FXR mediated the effect of curcumin on cellular lipid deposition, and curcumin modulated the expression of FXR by Nrf-2 [67]. Their following study showed that curcumin dose dependently ameliorated ethanol-caused hepatocyte necroptosis, which was blocked by Nrf-2 knockdown using shRNA lentivirus [66].
Organosulfur compounds from garlic Garlic is one of the most widely used herbal medicines in the world and is honored as “nature’s protection against physiological threats” [81, 82]. Many organosulfur compounds in garlic including diallyl sulfide, dially disulfide and diallyl trisulfide have all been demonstrated to induce Nrf-2 activation and could protect against ALD [24, 83–90]. In one of our studies, we found that diallyl disulfide could suppress ethanol-induced elevation of LDH and AST activities, decrease of GSH level, and increase of MDA level, and apoptosis in LO2 cell, which could be blocked by Nrf-2/HO-1 inhibitor, ZnPPIX. The in vivo study showed that diallyl disulfide dose dependently increased the protein levels of HO-1 in mice liver [25].
Other phytochemical compounds/extracts A large number of other phytochemical compounds/extracts including oleanolic acid, polymethoxy flavonoid-containing citrus aurantium extract (CAE), tetramethylpyrazine (TMP), ethanolic extract of sida cordifolia, hoveniae semen cum fructus extract, baicalin, polydatin, ligustrazine, triticum aestivum sprout-derived polysaccharide (TASP), dihydromyricetin, baccharis trimera, and schisandra sphenanthera extract have been demonstrated to attenuate binge or chronic ethanol-induced liver/hepatocytes injury in various ALD models, which might be associated with the activation of Nrf-2 antioxidant system [63, 65, 68, 70, 91–102]. However, it should be noted that whether the hepatoprotective effects of these compounds/extracts are mainly attributed to Nrf-2 activation remains to be elucidated. As the authors only detected the activation of Nrf-2 antioxidant system (such as the increased nucleus translocation of Nrf-2 and the increased mRNA and protein levels of Nrf-2 targeted genes including HO-1, GCL, NQO-1) in many studies, the involvement of other mechanisms cannot be completely excluded.
Nrf-2 activation on the gut–liver axis and the adipose–liver axis in ALD
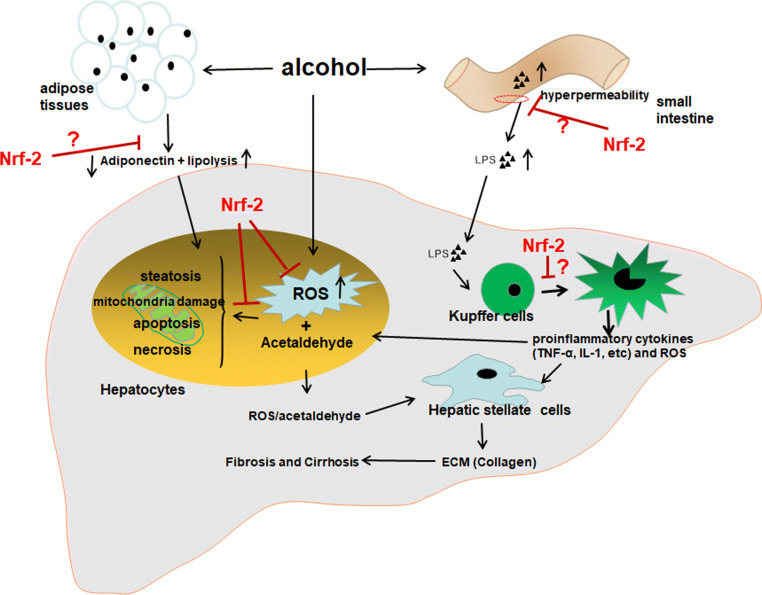
In addition to the direct impairment on hepatocytes, the deleterious effects of ethanol on adipose tissues and the hepatic resident macrophages (the Kupffer cells, KCs) have also been demonstrated to play crucial roles in ethanol-induced liver injury. Ethanol could stimulate lipolysis in adipose tissues, and the adipose TG then transports and deposits in liver forming steatosis [103, 104]. Besides, ethanol could impair the secretion of adiponectin, a 30-kD protein hormone, which has been demonstrated to provide protection against ALD via adiponectin–sirtuin-1(SIRT1)–AMP-activated kinase (AMPK) pathway [105, 106]. In addition, ethanol exposure could lead to intestinal hyperpermeability of intestinal mucosa and alter the gut microbiota favoring the production of pro-inflammatory endotoxin/lipopolysaccharide (LPS) [107, 108]. LPS translocates to liver and activates the toll-like receptor 4 (TLR-4) signaling pathway in KCs. The M1-type-polarized KCs can produce a large amount of ROS and pro-inflammatory cytokines including tumor necrosis factor α (TNF-α) and interleukin 1β (IL-1β) [109, 110]. Animal studies showed that suppressing LPS-producing bacteria by probiotics, intestinal sterilization by antibiotics, and knockout of LPS receptor could suppress ethanol-induced liver injury, which supports that the gut–liver axis plays critical roles in the pathogenesis of ALD [111–114], and thus, pharmacological intervention targeting M2 type polarization of KCs has been considered as an attractive strategy for the limitation of ethanol-induced inflammation and hepatocyte injury [115, 116]. Previous studies suggest that oxidative stress could impair adiponectin secretion and promote lipolysis in adipose tissues, and is crucial for LPS-mediated KCs M1 type polarization [117–120]. Therefore, it appears plausible that the activation of Nrf-2 in adipose tissues may be beneficial for ALD protection by rescuing the adiponectin secretion and blocking lipolysis, while the Nrf-2 activation in KCs may suppress the activation of KCs and the production of pro-inflammatory cytokines. The effects of Nrf-2 on the deleterious effects of ethanol on adipose tissues and hepatic KCs need to be further investigated in future studies (Fig. 2).
Fig. 2.
Potential molecular targets for the hepatoprotection of Nrf-2 activators against alcoholic liver disease (ALD). (1) Nrf-2 activation in hepatocytes could eliminate ethanol-induced reactive oxygen species (ROS) and mitigate the subsequent deleterious effects of ROS in hepatocytes; (2) Nrf-2 activation in adipose tissues may block ethanol-induced lipolysis and the decreased secretion of adiponectin; (3) Nrf-2 activation in intestine may suppress ethanol-induced intestinal damage, and thus reduce the translocation of gut-sourced lipopolysaccharide (LPS) to liver; (4) garlic may suppress LPS-induced Kupffer cells (KCs) activation and reduce the production of pro-inflammatory cytokines such as tumor necrosis factor α (TNF-α)
The crosstalk between Nrf-2/Keap-1 pathway and autophagy in ALD
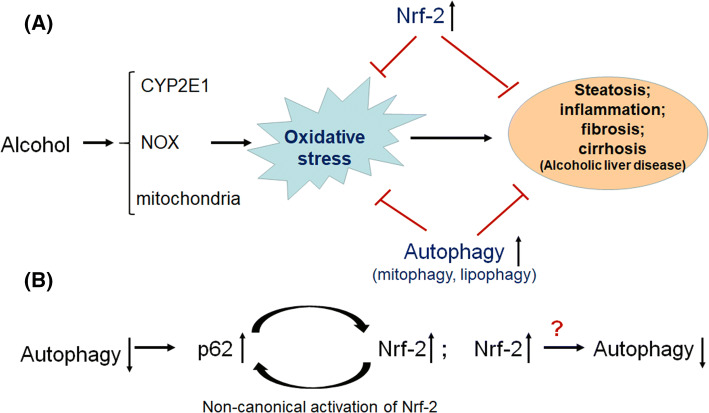
Complementing the Nrf-2/Keap-1 pathway, autophagy (macrophage) is another important defense mechanism against cellular oxidative stress [121]. Autophagy is a highly conserved intracellular catabolic pathway which is responsible for the degradation of oxidatively modified proteins, accumulated lipids and damaged organelles [122, 123]. Interestingly, accumulating evidence indicate a crosstalk between autophagy and Nrf-2/Keap-1 pathway. As described before, p62 could competitively bind with Keap-1, leading to the non-canonical activation of Nrf-2 [43, 44]. Therefore, autophagy blockage, either via genetic ablation of key autophagy initiation proteins (Beclin-1, ATG5, or ATG7) or exposure to some environmental toxicants such as arsenate, results in the activation of Nrf-2 [47]. On the other hand, Nrf-2/Keap-1 pathway may also regulate the activity of autophagy [124]. p62 and nuclear dot protein 2 (NDP52) have been demonstrated to be targets of Nrf-2 [125, 126]. Furthermore, Keap-1 binding to p62 may be involved in p62-mediated autophagy of unbiquitinated proteins, as genetic ablation of Keap-1 led to accumulation of ubiquitin aggregates and defective activation of autophagy [127]. However, Nrf-2 activity seems to negatively regulate the autophagy activity, although some opposite results also exist [124, 128–130]. The negative regulation of Nrf-2 on autophagy activity may be not unexpected, as both Nrf-2 and autophagy play similar roles in mitigating oxidative stress. If the cellular antioxidant system is at a higher level due to the activation of Nrf-2, then it would be reasonable that autophagy, another antioxidant pathway, may maintain at a relatively lower level (Fig. 3).
Fig. 3.
The crosstalk between Nrf-2/Keap-1 pathway and autophagy in ALD. (1) Accumulating evidence demonstrates that both Nrf-2 activation and autophagy activation can significantly alleviate ethanol-induced oxidative stress and the subsequent liver damage; (2) autophagy suppression resulted in the non-canonical activation of Nrf-2 via accumulated p62, while Nrf-2 may negatively regulate the autophagy activity
Similar to the alteration of Nrf-2 activity in ALD, the activities of autophagy in ALD models remain inconsistent [131]. However, pharmacological activation of autophagy could attenuate ethanol-induced liver injury, suggesting that autophagy plays protective roles against ALD [131, 132]. Specially, PTEN-induced putative kinase 1 (PINK-1) and Parkin-associated mitophagy (responsible for degradation of damaged mitochondria) have been demonstrated to play critical roles in the protection against ALD by removing damaged mitochondria, maintaining a healthy mitochondria population for the efficient β-oxidation in the hepatocytes [133–136]. Much more works are needed to clarify the interactions between Nrf-2/Keap-1 pathway and PINK–Parkin-induced mitophagy pathway.
Could Nrf-2 activators be used for the prevention and therapeutic treatment of human ALD?
The intricacy of the human anatomy, along with the existence of many other variables in association with alcohol abuse in humans, makes it extremely difficult to replicate all facets of human drinking in laboratory models [137]. It is well known that the rodents (rats and mice) are more resistant to the effects of alcohol as compared with humans [138]. The currently available ALD animals models using ethanol-containing liquid diet or by intragastric feeding ethanol could only induce the early stages of ALD (e.g., steatosis, steatohepatitis, mild fibrosis), while the late stage of ALD (e.g., severe fibrosis, cirrhosis and hepatic carcinoma) could not be induced without the addition of secondary or multiple insults [137, 139–142]. Therefore, although a significant number of studies have illustrated the protective role of Nrf-2 against ALD in vitro and in animal models, whether these experimental data can be directly translated to human beings should be questioned due to the lack of clinical trials.
However, there has been accumulating evidence indicating the possible causative involvement of oxidative stress in the pathophysiology of human ALD [20]. For example, ethanol consumption increased the oxidative stress biomarkers including F2-isoprostanes and 4-HNE in the serum and urine of ALD patients [143, 144]. Besides, alkylation of proteins by hydroxyethyl radicals was detected in patients with AC [145]. Unexpectedly, a randomized, double-blind, placebo-controlled clinical trial found that S-adenosylmethionine (SAM), a well-characterized antioxidant, was not effective than placebo in the treatment of ALD [146], although an earlier study showed that SAM could improve survival or delay liver transplantation in patients with AC, especially in those with less advanced liver disease [147]. Other antioxidants including vitamin E and NAC also failed to show their efficacy in improving alcoholic hepatitis [148, 149]. The poor response of these conventional antioxidants in human ALD may be related to the reduced efficiency in enhancing the antioxidant activity in ALD patients. For example, there has been evidence for the less efficiency of reasonable levels of supplementation with vitamin E in enhancing the antioxidative status of healthy persons [150, 151]. In addition, clinical trials usually enrolled ALD patient with severe hepatitis, fibrosis, and/or cirrhosis. The severely impaired hepatocytes in patients with advanced ALD may not make full use of these exogenous antioxidants and thus poorly respond to the supplementation of antioxidants [146].
Interestingly, several studies have suggested that Nrf-2 activators could induce antioxidant enzymes in humans and ameliorate several chronic diseases which were associated with oxidative stress and inflammation. For example, protandim, a composition consisting of extracts of five widely studied medicinal plants, has been demonstrated to induce endogenous antioxidant enzymes (including SOD and catalase) and lowered oxidative blood markers in runners [152, 153]. Bardoxolone methyl, a novel synthetic Nrf2 activator, has been demonstrated to improve the kidney function in patients with advanced chronic kidney disease and type 2 diabetes in a phase II double-blind, randomized, placebo-controlled clinical trial, although a follow-up phase III trial was terminated due to undisclosed safety concerns [154, 155]. Another Nrf-2 activator, compound BG-12 (dimethyl fumarate), reduced brain magnetic resonance imaging activity and lesions associated with multiple sclerosis as compared with patients who received placebo in a phase IIb clinical trial [156].
Collectively, induction of endogenous Nrf-2-regulated antioxidant system may represent a promising approach for the prevention and treatment of human ALD. Considering the less efficiency of conventional antioxidants such as vitamin E and other potential therapeutic drugs such as TNF-α inhibitors (e.g., pentoxifylline, infliximab, and etanercept), well-designed clinical trials are warranted to investigate the roles of Nrf-2 activators in ALD patients [109, 153, 157–159].
Nrf-2 and other liver diseases
Non-alcoholic fatty liver disease (NAFLD) shares similar mechanisms with ALD, and the general consensus is that the gut microbiota, oxidative stress and mitochondrial damage may play key roles in the pathogenesis of both ALD and NAFLD [160, 161]. Knockout of Nrf-2 in mice profoundly exacerbated NAFLD, while activation of Nrf-2 by knockdown of Keap-1 or by pharmacological agents protected NAFLD [162–168]. However, there are some studies providing conflicting results. For example, genetic activation of Nrf-2 in mice by knockdown of Keap-1 aggravated NAFLD induced by long-term high-fat diet feeding [169]. A more recent study showed that oxidative stress-induced Nrf-2 might be responsible for the upregulation of hepatic very low density lipoprotein (VLDL), which plays an important role in the development of hepatic steatosis [170]. Why Nrf-2 plays different roles in different NAFLD models remains to be elucidated.
The roles of Nrf-2 in the protection of chemical-induced liver injury have also been proposed, as oxidative stress serves as a common and important mechanism for the liver injury induced by various chemicals. It has been demonstrated that Nrf-2 activation offered significant protection against the liver injury caused by carbon tetrachloride (CCl4), acetaminophen, microcystin, cadmium, and diquat [171–174]. Furthermore, some recent studies have provided clues that Nrf-2 may be also involved in the deleterious effects of HBV and hepatitis C virus (HCV) on liver. In hepatocytes, HBV could stimulate the expression of glucose-6-phosphate dehydrogenase (G6PD) by promoting HBV x protein (HBx) expression in an Nrf-2-dependent manner, which might play important roles in the development of HBV-associated hepatocarcinoma [175]. HCV could interfere with the crosstalk between Nrf-2/Keap-1 pathway, elevated ROS levels and autophagy, which was required for the release of infectious viral particles [176].
Conclusions and future research perspectives
Nrf-2-regulated antioxidant system has been demonstrated to play core roles in mitigating ethanol-induced oxidative stress. Nrf-2 activation by genetic manipulation or pharmacological compounds could effectively attenuate both binge and chronic ethanol-induced hepatocytes/liver damage in vitro and in animal studies, which suggest that Nrf-2 is a promising targeting molecule for the prevention and treatment of human ALD. However, there are some issues which should be addressed in future studies.
First, it should be noted that the currently available data are obtained from animal studies of ethanol-induced early stage of ALD or from in vitro studies. These results may be interpreted as Nrf-2 activator could prevent ethanol-induced early liver disease. Therefore, it remains to study whether Nrf-2 activator can improve advanced stage of ALD such as the fibrosis and cirrhosis. It may be necessary to use primate models to investigate the roles of the Nrf-2 activator in the pathogenesis of alcoholic fibrosis and cirrhosis, as excessive ethanol consumption alone could result in liver fibrosis and cirrhosis in baboon [137, 141]. Additionally, hybrid rats/mice models of fibrosis/cirrhosis induced by ethanol and other hits such as high-fat diet or hepatotoxicants (e.g., CCl4) can be also considered, as both CCl4 and ethanol may induce hepatocyte damage through some common mechanisms [142, 177–179].
Second, the roles of Nrf-2 in the hepatoprotection of many phytochemical compounds/extracts against ALD need to be further confirmed. Many studies only reported the increase of Nrf-2 nucleus translocation and increased expression of Nrf-2 target antioxidant genes. Whether Nrf-2 activation played the major roles for the protection of these compounds/extract against ALD remained to be clarified, as other mechanisms such as reducing ROS production, maintaining the intestinal barrier integrity, and improving the adipose–liver axis may be also involved. For example, DADS has been suggested to suppress CYP2E1 activity in human hepatocytes and also induce the activation of Nrf-2 [84, 180]. Besides, the isolation and preparation of these hepatoprotective compounds/extracts should be standardized and the bioavailability of these compounds/extracts must be evaluated, as uncharacterized crude extracts may lead to the difficulty in reproducing the results [181]. This is particularly important as there is an increasing public and scientific interest in this natural-derived substance [182]. In addition, it will be interesting to investigate the roles of combination of these Nrf-2 activators and KCs polarizing modulators in ALD models, as M1-type-polarized KC induced by gut-sourced LPS has been demonstrated to be another key contributor to ALD. Furthermore, well-designed clinical trials are urgently needed to evaluate the efficiency of these Nrf-2 activators on ALD patients.
Third, it has been demonstrated that the non-canonical activation of Nrf-2 may be associated with some negative outcomes such as tumor progression and chemotherapy resistance, namely the “dark side” of Nrf-2 [49, 183, 184]. For example, autophagy deficiency led to the formation of protein aggregates, liver fibrosis, inflammation and tumorigenesis, which could be blocked by knockout of p62 or Nrf-2 [49, 185]. As such, long-term use of these Nrf-2 non-canonical activators should be carefully considered.
Acknowledgements
This work was supported by the National Natural Science Foundation of China (Grant No. 81473004 and 81102153), the key Research and Develop Project of Shandong Province (Grant No. 2017GSF18122), the Young Scholars Program of Shandong University (Grant No. 2015WLJH52), and the Project for the Traditional Chinese Medicine of Shandong Province (Grant No. 2013-167).
References
- 1.Gao B, Bataller R (2011) Alcoholic liver disease: pathogenesis and new therapeutic targets. Gastroenterology 141:1572–1585 [DOI] [PMC free article] [PubMed]
- 2.Livero F, Acco A. Molecular basis of alcoholic fatty liver disease: from incidence to treatment. Hepatol Res. 2016;46:111–123. doi: 10.1111/hepr.12594. [DOI] [PubMed] [Google Scholar]
- 3.Sheron N. Alcohol and liver disease in Europe-Simple measures have the potential to prevent tens of thousands of premature deaths. J Hepatol. 2016;64:957–967. doi: 10.1016/j.jhep.2015.11.006. [DOI] [PubMed] [Google Scholar]
- 4.Beier JI, Arteel GE, McClain CJ. Advances in alcoholic liver disease. Curr Gastroenterol Rep. 2011;13:56–64. doi: 10.1007/s11894-010-0157-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wang FS, Fan JG, Zhang Z, et al. The global burden of liver disease: the major impact of China. Hepatology. 2014;60:2099–2108. doi: 10.1002/hep.27406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rosato V, Abenavoli L, Federico A, et al. Pharmacotherapy of alcoholic liver disease in clinical practice. Int J Clin Pract. 2016;70:119–131. doi: 10.1111/ijcp.12764. [DOI] [PubMed] [Google Scholar]
- 7.Kim MS, Ong M, Qu X. Optimal management for alcoholic liver disease: conventional medications, natural therapy or combination? World J Gastroenterol. 2016;22:8–23. doi: 10.3748/wjg.v22.i1.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Singal AK, Anand BS (2013) Recent trends in the epidemiology of alcoholic liver disease. Clin Liver Dis 2:53–56 [DOI] [PMC free article] [PubMed]
- 9.Loboda A, Damulewicz M, Pyza E, et al. Role of Nrf2/HO-1 system in development, oxidative stress response and diseases: an evolutionarily conserved mechanism. Cell Mol Life Sci. 2016;73:3221–3247. doi: 10.1007/s00018-016-2223-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhang DD. Mechanistic studies of the Nrf2-Keap1 signaling pathway. Drug Metab Rev. 2006;38:769–789. doi: 10.1080/03602530600971974. [DOI] [PubMed] [Google Scholar]
- 11.Blank V. Small Maf proteins in mammalian gene control: mere dimerization partners or dynamic transcriptional regulators? J Mol Biol. 2008;376:913–925. doi: 10.1016/j.jmb.2007.11.074. [DOI] [PubMed] [Google Scholar]
- 12.Ohtsuji M, Katsuoka F, Kobayashi A, et al. Nrf1 and Nrf2 play distinct roles in activation of antioxidant response element-dependent genes. J Biol Chem. 2008;283:33554–33562. doi: 10.1074/jbc.M804597200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bataille AM, Manautou JE (2012) Nrf2: a potential target for new therapeutics in liver disease. Clin Pharmacol Ther 92:340–348 [DOI] [PMC free article] [PubMed]
- 14.Tang W, Jiang YF, Ponnusamy M, et al. Role of Nrf2 in chronic liver disease. World J Gastroenterol. 2014;20:13079–13087. doi: 10.3748/wjg.v20.i36.13079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Klaassen CD, Reisman SA. Nrf2 the rescue: effects of the antioxidative/electrophilic response on the liver. Toxicol Appl Pharmacol. 2010;244:57–65. doi: 10.1016/j.taap.2010.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cederbaum AI, Lu YWuD. Role of oxidative stress in alcohol-induced liver injury. Arch Toxicol. 2009;83:519–548. doi: 10.1007/s00204-009-0432-0. [DOI] [PubMed] [Google Scholar]
- 17.Diluzio NR. Prevention of the acute ethanol-induced fatty liver by the simultaneous administration of antioxidants. Life Sci. 1964;3:113–118. doi: 10.1016/0024-3205(64)90189-4. [DOI] [PubMed] [Google Scholar]
- 18.Albano E, French SW, Ingelman-Sundberg M (1999) Hydroxyethyl radicals in ethanol hepatotoxicity. Front Biosci 4:D533–D540 [DOI] [PubMed]
- 19.Rashba-Step J, Turro NJ, Cederbaum AI (1993) Increased NADPH- and NADH-dependent production of superoxide and hydroxyl radical by microsomes after chronic ethanol treatment. Arch Biochem Biophys 300:401–408 [DOI] [PubMed]
- 20.Zhu H, Jia Z, Misra H, et al. Oxidative stress and redox signaling mechanisms of alcoholic liver disease: updated experimental and clinical evidence. J Dig Dis. 2012;13:133–142. doi: 10.1111/j.1751-2980.2011.00569.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tang Y, Gao C, Xing M, et al. Quercetin prevents ethanol-induced dyslipidemia and mitochondrial oxidative damage. Food Chem Toxicol. 2012;50:1194–1200. doi: 10.1016/j.fct.2012.02.008. [DOI] [PubMed] [Google Scholar]
- 22.Song Z, Deaciuc I, Song M, et al. Silymarin protects against acute ethanol-induced hepatotoxicity in mice. Alcohol Clin Exp Res. 2006;30:407–413. doi: 10.1111/j.1530-0277.2006.00063.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dou X, Li S, Wang Z, et al. Inhibition of NF-kappaB activation by 4-hydroxynonenal contributes to liver injury in a mouse model of alcoholic liver disease. Am J Pathol. 2012;181:1702–1710. doi: 10.1016/j.ajpath.2012.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Guan MJ, Zhao N, Xie KQ, et al. Hepatoprotective effects of garlic against ethanol-induced liver injury: a mini-review. Food Chem Toxicol. 2018;111:467–473. doi: 10.1016/j.fct.2017.11.059. [DOI] [PubMed] [Google Scholar]
- 25.Zeng T, Zhang CL, Song FY, et al. The activation of HO-1/Nrf-2 contributes to the protective effects of diallyl disulfide (DADS) against ethanol-induced oxidative stress. Biochim Biophys Acta. 2013;1830:4848–4859. doi: 10.1016/j.bbagen.2013.06.028. [DOI] [PubMed] [Google Scholar]
- 26.Ajmo JM, Liang X, Rogers CQ, et al. Resveratrol alleviates alcoholic fatty liver in mice. Am J Physiol Gastrointest Liver Physiol. 2008;295:G833–G842. doi: 10.1152/ajpgi.90358.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wheeler MD, Kono H, Yin M, et al. Delivery of the Cu/Zn-superoxide dismutase gene with adenovirus reduces early alcohol-induced liver injury in rats. Gastroenterology. 2001;120:1241–1250. doi: 10.1053/gast.2001.23253. [DOI] [PubMed] [Google Scholar]
- 28.Wheeler MD, Nakagami M, Bradford BU, et al. Overexpression of manganese superoxide dismutase prevents alcohol-induced liver injury in the rat. J Biol Chem. 2001;276:36664–36672. doi: 10.1074/jbc.M105352200. [DOI] [PubMed] [Google Scholar]
- 29.Kessova IG, Ho YS, Thung S, et al. Alcohol-induced liver injury in mice lacking Cu, Zn-superoxide dismutase. Hepatology. 2003;38:1136–1145. doi: 10.1053/jhep.2003.50450. [DOI] [PubMed] [Google Scholar]
- 30.Curry-McCoy TV, Osna NA, Nanji AA, et al. Chronic ethanol consumption results in atypical liver injury in copper/zinc superoxide dismutase deficient mice. Alcohol Clin Exp Res. 2010;34:251–261. doi: 10.1111/j.1530-0277.2009.01088.x. [DOI] [PubMed] [Google Scholar]
- 31.Kim SJ, Lee JW, Jung YS, et al. Ethanol-induced liver injury and changes in sulfur amino acid metabolomics in glutathione peroxidase and catalase double knockout mice. J Hepatol. 2009;50:1184–1191. doi: 10.1016/j.jhep.2009.01.030. [DOI] [PubMed] [Google Scholar]
- 32.Lu Y, Zhuge J, Wang X, et al. Cytochrome P450 2E1 contributes to ethanol-induced fatty liver in mice. Hepatology. 2008;47:1483–1494. doi: 10.1002/hep.22222. [DOI] [PubMed] [Google Scholar]
- 33.Kono H, Rusyn I, Uesugi T, et al. Diphenyleneiodonium sulfate, an NADPH oxidase inhibitor, prevents early alcohol-induced liver injury in the rat. Am J Physiol Gastrointest Liver Physiol. 2001;280:G1005–G1012. doi: 10.1152/ajpgi.2001.280.5.G1005. [DOI] [PubMed] [Google Scholar]
- 34.Ogura T, Tong KI, Mio K, et al. Keap1 is a forked-stem dimer structure with two large spheres enclosing the intervening, double glycine repeat, and C-terminal domains. Proc Natl Acad Sci USA. 2010;107:2842–2847. doi: 10.1073/pnas.0914036107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhang DD, Hannink M (2003) Distinct cysteine residues in Keap1 are required for Keap1-dependent ubiquitination of Nrf2 and for stabilization of Nrf2 by chemopreventive agents and oxidative stress. Mol Cell Biol 23:8137–8151 [DOI] [PMC free article] [PubMed]
- 36.Kansanen E, Jyrkkanen HK, Levonen AL (2012) Activation of stress signaling pathways by electrophilic oxidized and nitrated lipids. Free Radic Biol Med 52:973–982 [DOI] [PubMed]
- 37.Tong KI, Katoh Y, Kusunoki H, et al. Keap1 recruits Neh2 through binding to ETGE and DLG motifs: characterization of the two-site molecular recognition model. Mol Cell Biol. 2006;26:2887–2900. doi: 10.1128/MCB.26.8.2887-2900.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tao S, Liu P, Luo G, et al. p97 negatively regulates NRF2 by extracting ubiquitylated NRF2 from the KEAP1-CUL3 E3 complex. Mol Cell Biol. 2017;37:e00660-16. doi: 10.1128/MCB.00660-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Itoh K, Chiba T, Takahashi S, et al. An Nrf2/small Maf heterodimer mediates the induction of phase II detoxifying enzyme genes through antioxidant response elements. Biochem Biophys Res Commun. 1997;236:313–322. doi: 10.1006/bbrc.1997.6943. [DOI] [PubMed] [Google Scholar]
- 40.Katsuoka F, Yamamoto M (2016) Small Maf proteins (MafF, MafG, MafK): History, structure and function. Gene 586:197–205 [DOI] [PMC free article] [PubMed]
- 41.Long M, Li X, Li L, et al. Multifunctional p62 effects underlie diverse metabolic diseases. Trends Endocrinol Metab. 2017;28:818–830. doi: 10.1016/j.tem.2017.09.001. [DOI] [PubMed] [Google Scholar]
- 42.Bjorkoy G, Lamark T, Pankiv S, et al. Monitoring autophagic degradation of p62/SQSTM1. Methods Enzymol. 2009;452:181–197. doi: 10.1016/S0076-6879(08)03612-4. [DOI] [PubMed] [Google Scholar]
- 43.Lau A, Wang XJ, Zhao F, et al. A noncanonical mechanism of Nrf2 activation by autophagy deficiency: direct interaction between Keap1 and p62. Mol Cell Biol. 2010;30:3275–3285. doi: 10.1128/MCB.00248-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Komatsu M, Kurokawa H, Waguri S, et al. The selective autophagy substrate p62 activates the stress responsive transcription factor Nrf2 through inactivation of Keap1. Nat Cell Biol. 2010;12:213–223. doi: 10.1038/ncb2021. [DOI] [PubMed] [Google Scholar]
- 45.de la Vega MR, Dodson M, Chapman E, et al. NRF2-targeted therapeutics: new targets and modes of NRF2 regulation. Curr Opin Toxicol. 2016;1:62–70. doi: 10.1016/j.cotox.2016.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Itoh K, Ye P, Matsumiya T, et al. Emerging functional cross-talk between the Keap1-Nrf2 system and mitochondria. J Clin Biochem Nutr. 2015;56:91–97. doi: 10.3164/jcbn.14-134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ichimura Y, Waguri S, Sou YS, et al. Phosphorylation of p62 activates the Keap1-Nrf2 pathway during selective autophagy. Mol Cell. 2013;51:618–631. doi: 10.1016/j.molcel.2013.08.003. [DOI] [PubMed] [Google Scholar]
- 48.Christian F, Krause E, Houslay MD, et al. PKA phosphorylation of p62/SQSTM1 regulates PB1 domain interaction partner binding. Biochim Biophys Acta. 2014;1843:2765–2774. doi: 10.1016/j.bbamcr.2014.07.021. [DOI] [PubMed] [Google Scholar]
- 49.Ni HM, Woolbright BL, Williams J, et al. Nrf2 promotes the development of fibrosis and tumorigenesis in mice with defective hepatic autophagy. J Hepatol. 2014;61:617–625. doi: 10.1016/j.jhep.2014.04.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lau A, Whitman SA, Jaramillo MC, et al. Arsenic-mediated activation of the Nrf2-Keap1 antioxidant pathway. J Biochem Mol Toxicol. 2013;27:99–105. doi: 10.1002/jbt.21463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ge W, Zhao K, Wang X, et al. iASPP Is an antioxidative factor and drives cancer growth and drug resistance by competing with Nrf2 for Keap1 binding. Cancer Cell. 2017;32(561–573):e6. doi: 10.1016/j.ccell.2017.09.008. [DOI] [PubMed] [Google Scholar]
- 52.Keum YS. Regulation of the Keap1/Nrf2 system by chemopreventive sulforaphane: implications of posttranslational modifications. Ann N Y Acad Sci. 2011;1229:184–189. doi: 10.1111/j.1749-6632.2011.06092.x. [DOI] [PubMed] [Google Scholar]
- 53.Huang HC, Nguyen T, Pickett CB (2002) Phosphorylation of Nrf2 at Ser-40 by protein kinase C regulates antioxidant response element-mediated transcription. J Biol Chem 277:42769–42774 [DOI] [PubMed]
- 54.Numazawa S, Ishikawa M, Yoshida A, et al. Atypical protein kinase C mediates activation of NF-E2-related factor 2 in response to oxidative stress. Am J Physiol Cell Physiol. 2003;285:C334–C342. doi: 10.1152/ajpcell.00043.2003. [DOI] [PubMed] [Google Scholar]
- 55.Keum YS, Yu S, Chang PP, et al. Mechanism of action of sulforaphane: inhibition of p38 mitogen-activated protein kinase isoforms contributing to the induction of antioxidant response element-mediated heme oxygenase-1 in human hepatoma HepG2 cells. Cancer Res. 2006;66:8804–8813. doi: 10.1158/0008-5472.CAN-05-3513. [DOI] [PubMed] [Google Scholar]
- 56.Sun Z, Huang Z, Zhang DD (2009) Phosphorylation of Nrf2 at multiple sites by MAP kinases has a limited contribution in modulating the Nrf2-dependent antioxidant response. PLoS One 4:e6588 [DOI] [PMC free article] [PubMed]
- 57.Jain AK, Jaiswal AK (2006) Phosphorylation of tyrosine 568 controls nuclear export of Nrf2. J Biol Chem 281:12132–12142 [DOI] [PubMed]
- 58.Jain AK, Jaiswal AK (2007) GSK-3beta acts upstream of Fyn kinase in regulation of nuclear export and degradation of NF-E2 related factor 2. J Biol Chem 282:16502–16510 [DOI] [PubMed]
- 59.Gong P, Cederbaum AI (2006) Nrf2 is increased by CYP2E1 in rodent liver and HepG2 cells and protects against oxidative stress caused by CYP2E1. Hepatology 43:144–153 [DOI] [PubMed]
- 60.Bardag-Gorce F, Oliva J, Lin A, et al. Proteasome inhibitor up regulates liver antioxidative enzymes in rat model of alcoholic liver disease. Exp Mol Pathol. 2011;90:123–130. doi: 10.1016/j.yexmp.2010.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Yeligar SM, Machida K, Kalra VK. Ethanol-induced HO-1 and NQO1 are differentially regulated by HIF-1alpha and Nrf2 to attenuate inflammatory cytokine expression. J Biol Chem. 2010;285:35359–35373. doi: 10.1074/jbc.M110.138636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Lamle J, Marhenke S, Borlak J, et al. Nuclear factor-eythroid 2-related factor 2 prevents alcohol-induced fulminant liver injury. Gastroenterology. 2008;134:1159–1168. doi: 10.1053/j.gastro.2008.01.011. [DOI] [PubMed] [Google Scholar]
- 63.Choi BK, Kim TW, Lee DR, et al. A polymethoxy flavonoids-rich Citrus aurantium extract ameliorates ethanol-induced liver injury through modulation of AMPK and Nrf2-related signals in a binge drinking mouse model. Phytother Res. 2015;29:1577–1584. doi: 10.1002/ptr.5415. [DOI] [PubMed] [Google Scholar]
- 64.Zhou R, Lin J, Wu D. Sulforaphane induces Nrf2 and protects against CYP2E1-dependent binge alcohol-induced liver steatosis. Biochim Biophys Acta. 2014;1840:209–218. doi: 10.1016/j.bbagen.2013.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Huang QH, Xu LQ, Liu YH, et al. Polydatin Protects Rat Liver against Ethanol-Induced Injury: involvement of CYP2E1/ROS/Nrf2 and TLR4/NF-kappaB p65 Pathway. Evid Based Complement Alternat Med. 2017;2017:7953850. doi: 10.1155/2017/7953850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Lu C, Xu W, Zhang F, et al. Nrf2 knockdown disrupts the protective effect of curcumin on alcohol-induced hepatocyte necroptosis. Mol Pharm. 2016;13:4043–4053. doi: 10.1021/acs.molpharmaceut.6b00562. [DOI] [PubMed] [Google Scholar]
- 67.Lu C, Zhang F, Xu W, et al. Curcumin attenuates ethanol-induced hepatic steatosis through modulating Nrf2/FXR signaling in hepatocytes. IUBMB Life. 2015;67:645–658. doi: 10.1002/iub.1409. [DOI] [PubMed] [Google Scholar]
- 68.Lu C, Jiang Y, Zhang F, et al. Tetramethylpyrazine prevents ethanol-induced hepatocyte injury via activation of nuclear factor erythroid 2-related factor 2. Life Sci. 2015;141:119–127. doi: 10.1016/j.lfs.2015.08.018. [DOI] [PubMed] [Google Scholar]
- 69.Wu KC, Liu J, Klaassen CD. Role of Nrf2 in preventing ethanol-induced oxidative stress and lipid accumulation. Toxicol Appl Pharmacol. 2012;262:321–329. doi: 10.1016/j.taap.2012.05.010. [DOI] [PubMed] [Google Scholar]
- 70.Byun HG, Lee JK. Chlorella ethanol extract induced phase II enzyme through NFE2L2 (nuclear factor [erythroid-derived] 2-like 2, NRF2) activation and protected ethanol-induced hepatoxicity. J Med Food. 2015;18:182–189. doi: 10.1089/jmf.2014.3159. [DOI] [PubMed] [Google Scholar]
- 71.Liu S, Hou W, Yao P, et al. Quercetin protects against ethanol-induced oxidative damage in rat primary hepatocytes. Toxicol In Vitro. 2010;24:516–522. doi: 10.1016/j.tiv.2009.03.006. [DOI] [PubMed] [Google Scholar]
- 72.Liu CM, Zheng YL, Lu J, et al. Quercetin protects rat liver against lead-induced oxidative stress and apoptosis. Environ Toxicol Pharmacol. 2010;29:158–166. doi: 10.1016/j.etap.2009.12.006. [DOI] [PubMed] [Google Scholar]
- 73.Kahraman A, Çakar H, Köken T. The protective effect of quercetin on long-term alcohol consumption-induced oxidative stress. Mol Biol Rep. 2012;39:2789–2794. doi: 10.1007/s11033-011-1037-2. [DOI] [PubMed] [Google Scholar]
- 74.Liu S, Hou W, Yao P, et al. Quercetin protects against ethanol-induced oxidative damage in rat primary hepatocytes. Toxicol In Vitro. 2010;24:516–522. doi: 10.1016/j.tiv.2009.03.006. [DOI] [PubMed] [Google Scholar]
- 75.Yao P, Nussler A, Liu L, et al. Quercetin protects human hepatocytes from ethanol-derived oxidative stress by inducing heme oxygenase-1 via the MAPK/Nrf2 pathways. J Hepatol. 2007;47:253–261. doi: 10.1016/j.jhep.2007.02.008. [DOI] [PubMed] [Google Scholar]
- 76.Ji LL, Sheng YC, Zheng ZY, et al. The involvement of p62-Keap1-Nrf2 antioxidative signaling pathway and JNK in the protection of natural flavonoid quercetin against hepatotoxicity. Free Radic Biol Med. 2015;85:12–23. doi: 10.1016/j.freeradbiomed.2015.03.035. [DOI] [PubMed] [Google Scholar]
- 77.Bao W, Li K, Rong S, et al. Curcumin alleviates ethanol-induced hepatocytes oxidative damage involving heme oxygenase-1 induction. J Ethnopharmacol. 2010;128:549–553. doi: 10.1016/j.jep.2010.01.029. [DOI] [PubMed] [Google Scholar]
- 78.Samuhasaneeto S, Thong-Ngam D, Kulaputana O, et al. Curcumin decreased oxidative stress, inhibited NF-kappaB activation, and improved liver pathology in ethanol-induced liver injury in rats. J Biomed Biotechnol. 2009;2009:981963. doi: 10.1155/2009/981963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Nanji AA, Jokelainen K, Tipoe GL, et al. Curcumin prevents alcohol-induced liver disease in rats by inhibiting the expression of NF-kappa B-dependent genes. Am J Physiol Gastrointest Liver Physiol. 2003;284:G321–G327. doi: 10.1152/ajpgi.00230.2002. [DOI] [PubMed] [Google Scholar]
- 80.Xiong ZE, Dong WG, Wang BY, et al. Curcumin attenuates chronic ethanol-induced liver injury by inhibition of oxidative stress via mitogen-activated protein kinase/nuclear factor E2-related factor 2 pathway in mice. Pharmacogn Mag. 2015;11:707–715. doi: 10.4103/0973-1296.165556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Butt MS, Sultan MT, Iqbal J. Garlic: nature’s protection against physiological threats. Crit Rev Food Sci Nutr. 2009;49:538–551. doi: 10.1080/10408390802145344. [DOI] [PubMed] [Google Scholar]
- 82.Zeng T, Zhang CL, Zhao XL, et al. The roles of garlic on the lipid parameters: a systematic review of the literature. Crit Rev Food Sci Nutr. 2013;53:215–230. doi: 10.1080/10408398.2010.523148. [DOI] [PubMed] [Google Scholar]
- 83.Gong P, Hu B, Cederbaum AI. Diallyl sulfide induces heme oxygenase-1 through MAPK pathway. Arch Biochem Biophys. 2004;432:252–260. doi: 10.1016/j.abb.2004.09.024. [DOI] [PubMed] [Google Scholar]
- 84.Chen C, Pung D, Leong V, et al. Induction of detoxifying enzymes by garlic organosulfur compounds through transcription factor Nrf2: effect of chemical structure and stress signals. Free Radical Biol Med. 2004;37:1578–1590. doi: 10.1016/j.freeradbiomed.2004.07.021. [DOI] [PubMed] [Google Scholar]
- 85.Zeng T, Zhang CL, Song FY, et al. Garlic oil alleviated ethanol-induced fat accumulation via modulation of SREBP-1, PPAR-alpha, and CYP2E1. Food Chem Toxicol. 2012;50:485–491. doi: 10.1016/j.fct.2011.11.030. [DOI] [PubMed] [Google Scholar]
- 86.Zeng T, Zhang CL, Zhu ZP, et al. Diallyl trisulfide (DATS) effectively attenuated oxidative stress-mediated liver injury and hepatic mitochondrial dysfunction in acute ethanol-exposed mice. Toxicology. 2008;252:86–91. doi: 10.1016/j.tox.2008.07.062. [DOI] [PubMed] [Google Scholar]
- 87.Zeng T, Zhang CL, Pan GB, et al. The protective effects of garlic oil on acute ethanol-induced oxidative stress in the liver of mice. J Sci Food Agric. 2008;88:2238–2243. doi: 10.1002/jsfa.3336. [DOI] [Google Scholar]
- 88.Zeng T, Guo FF, Zhang CL, et al. The anti-fatty liver effects of garlic oil on acute ethanol-exposed mice. Chem Biol Interact. 2008;176:234–242. doi: 10.1016/j.cbi.2008.07.004. [DOI] [PubMed] [Google Scholar]
- 89.Raghu R, Liu CT, Tsai MH, et al. Transcriptome analysis of garlic-induced hepatoprotection against alcoholic fatty liver. J Agric Food Chem. 2012;60:11104–11119. doi: 10.1021/jf303800p. [DOI] [PubMed] [Google Scholar]
- 90.Kim MH, Kim MJ, Lee JH, et al. Hepatoprotective effect of aged black garlic on chronic alcohol-induced liver injury in rats. J Med Food. 2011;14:732–738. doi: 10.1089/jmf.2010.1454. [DOI] [PubMed] [Google Scholar]
- 91.Lu C, Xu W, Shao J, et al. Nrf2 activation is required for ligustrazine to inhibit hepatic steatosis in alcohol-preferring mice and hepatocytes. Toxicol Sci. 2017;155:432–443. doi: 10.1093/toxsci/kfw228. [DOI] [PubMed] [Google Scholar]
- 92.He P, Wu Y, Shun J, et al. Baicalin ameliorates liver injury induced by chronic plus binge ethanol feeding by modulating oxidative stress and inflammation via CYP2E1 and NRF2 in mice. Oxid Med Cell Longev. 2017;2017:4820414. doi: 10.1155/2017/4820414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Cho I, Kim J, Jung J, et al. Hepatoprotective effects of hoveniae semen cum fructus extracts in ethanol intoxicated mice. J Exerc Nutr Biochem. 2016;20:49–64. doi: 10.20463/jenb.2016.03.20.1.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Rejitha S, Prathibha P, Indira M. Nrf2-mediated antioxidant response by ethanolic extract of Sida cordifolia provides protection against alcohol-induced oxidative stress in liver by upregulation of glutathione metabolism. Redox Rep. 2015;20:75–80. doi: 10.1179/1351000214Y.0000000108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Liu J, Wang X, Liu R, et al. Oleanolic acid co-administration alleviates ethanol-induced hepatic injury via Nrf-2 and ethanol-metabolizing modulating in rats. Chem Biol Interact. 2014;221:88–98. doi: 10.1016/j.cbi.2014.07.017. [DOI] [PubMed] [Google Scholar]
- 96.Nepali S, Ki HH, Lee JH, et al. Triticum aestivum sprout-derived polysaccharide exerts hepatoprotective effects against ethanol-induced liver damage by enhancing the antioxidant system in mice. Int J Mol Med. 2017;40:1243–1252. doi: 10.3892/ijmm.2017.3095. [DOI] [PubMed] [Google Scholar]
- 97.Qiu P, Dong Y, Li B, et al. Dihydromyricetin modulates p62 and autophagy crosstalk with the Keap-1/Nrf2 pathway to alleviate ethanol-induced hepatic injury. Toxicol Lett. 2017;274:31–41. doi: 10.1016/j.toxlet.2017.04.009. [DOI] [PubMed] [Google Scholar]
- 98.Rabelo ACS, de Padua Lucio K, Araujo CM, et al. Baccharis trimera protects against ethanol induced hepatotoxicity in vitro and in vivo. J Ethnopharmacol. 2017;215:1–13. doi: 10.1016/j.jep.2017.12.043. [DOI] [PubMed] [Google Scholar]
- 99.Zeng X, Li X, Xu C, et al. Schisandra sphenanthera extract (Wuzhi Tablet) protects against chronic-binge and acute alcohol-induced liver injury by regulating the NRF2-ARE pathway in mice. Acta Pharm Sin B. 2017;7:583–592. doi: 10.1016/j.apsb.2017.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Kumar KJ, Chu FH, Hsieh HW, et al. Antroquinonol from ethanolic extract of mycelium of Antrodia cinnamomea protects hepatic cells from ethanol-induced oxidative stress through Nrf-2 activation. J Ethnopharmacol. 2011;136:168–177. doi: 10.1016/j.jep.2011.04.030. [DOI] [PubMed] [Google Scholar]
- 101.Senthil Kumar KJ, Liao JW, Xiao JH, et al. Hepatoprotective effect of lucidone against alcohol-induced oxidative stress in human hepatic HepG2 cells through the up-regulation of HO-1/Nrf-2 antioxidant genes. Toxicol In Vitro. 2012;26:700–708. doi: 10.1016/j.tiv.2012.03.012. [DOI] [PubMed] [Google Scholar]
- 102.Song X, Yin S, Huo Y, et al. Glycycoumarin ameliorates alcohol-induced hepatotoxicity via activation of Nrf2 and autophagy. Free Radic Biol Med. 2015;89:135–146. doi: 10.1016/j.freeradbiomed.2015.07.006. [DOI] [PubMed] [Google Scholar]
- 103.Zhong W, Zhao Y, Tang Y, et al. Chronic alcohol exposure stimulates adipose tissue lipolysis in mice: role of reverse triglyceride transport in the pathogenesis of alcoholic steatosis. Am J Pathol. 2012;180:998–1007. doi: 10.1016/j.ajpath.2011.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Zhao YY, Yang R, Xiao M, et al. Kupffer cells activation promoted binge drinking-induced fatty liver by activating lipolysis in white adipose tissues. Toxicology. 2017;390:53–60. doi: 10.1016/j.tox.2017.09.001. [DOI] [PubMed] [Google Scholar]
- 105.Shen Z, Liang X, Rogers CQ, et al. Involvement of adiponectin-SIRT1-AMPK signaling in the protective action of rosiglitazone against alcoholic fatty liver in mice. Am J Physiol Gastrointest Liver Physiol. 2010;298:G364–374. doi: 10.1152/ajpgi.00456.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.You M, Liang X, Ajmo JM, et al. Involvement of mammalian sirtuin 1 in the action of ethanol in the liver. Am J Physiol Gastrointest Liver Physiol. 2008;294:G892–G898. doi: 10.1152/ajpgi.00575.2007. [DOI] [PubMed] [Google Scholar]
- 107.Keshavarzian A, Farhadi A, Forsyth CB, et al. Evidence that chronic alcohol exposure promotes intestinal oxidative stress, intestinal hyperpermeability and endotoxemia prior to development of alcoholic steatohepatitis in rats. J Hepatol. 2009;50:538–547. doi: 10.1016/j.jhep.2008.10.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Mutlu E, Keshavarzian A, Engen P, et al. Intestinal dysbiosis: a possible mechanism of alcohol-induced endotoxemia and alcoholic steatohepatitis in rats. Alcohol Clin Exp Res. 2009;33:1836–1846. doi: 10.1111/j.1530-0277.2009.01022.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Zeng T, Zhang C-L, Xiao M, et al. Critical roles of Kupffer cells in the pathogenesis of alcoholic liver disease: from basic science to clinical trials. Front Immunol. 2016;7:538. doi: 10.3389/fimmu.2016.00538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Zeng T, Zhao YY, Xie KQ. Does intestinal microbiota protect mice against acute/binge drinking-induced liver injury? Alcohol Clin Exp Res. 2016;40:1788–1790. doi: 10.1111/acer.13130. [DOI] [PubMed] [Google Scholar]
- 111.Adachi Y, Moore LE, Bradford BU, et al. Antibiotics prevent liver injury in rats following long-term exposure to ethanol. Gastroenterology. 1995;108:218–224. doi: 10.1016/0016-5085(95)90027-6. [DOI] [PubMed] [Google Scholar]
- 112.Yin M, Bradford BU, Wheeler MD, et al. Reduced early alcohol-induced liver injury in CD14-deficient mice. J Immunol. 2001;166:4737–4742. doi: 10.4049/jimmunol.166.7.4737. [DOI] [PubMed] [Google Scholar]
- 113.Uesugi T, Froh M, Arteel GE, et al. Toll-like receptor 4 is involved in the mechanism of early alcohol-induced liver injury in mice. Hepatology. 2001;34:101–108. doi: 10.1053/jhep.2001.25350. [DOI] [PubMed] [Google Scholar]
- 114.Han SH, Suk KT, Kim DJ, et al. Effects of probiotics (cultured Lactobacillus subtilis/Streptococcus faecium) in the treatment of alcoholic hepatitis: randomized-controlled multicenter study. Eur J Gastroenterol Hepatol. 2015;27:1300–1306. doi: 10.1097/MEG.0000000000000458. [DOI] [PubMed] [Google Scholar]
- 115.Wan J, Benkdane M, Teixeira-Clerc F, et al. M2 Kupffer cells promote M1 Kupffer cell apoptosis: a protective mechanism against alcoholic and nonalcoholic fatty liver disease. Hepatology. 2014;59:130–142. doi: 10.1002/hep.26607. [DOI] [PubMed] [Google Scholar]
- 116.Smith K. Liver disease: kupffer cells regulate the progression of ALD and NAFLD. Nat Rev Gastroenterol Hepatol. 2013;10:503. doi: 10.1038/nrgastro.2013.140. [DOI] [PubMed] [Google Scholar]
- 117.Tang H, Sebastian BM, Axhemi A, et al. Ethanol-induced oxidative stress via the CYP2E1 pathway disrupts adiponectin secretion from adipocytes. Alcohol Clin Exp Res. 2012;36:214–222. doi: 10.1111/j.1530-0277.2011.01607.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Zhang X, Wang Z, Li J, et al. Increased 4-hydroxynonenal formation contributes to obesity-related lipolytic activation in adipocytes. PLoS ONE. 2013;8:e70663. doi: 10.1371/journal.pone.0070663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Matsuzawa A, Saegusa K, Noguchi T, et al. ROS-dependent activation of the TRAF6-ASK1-p38 pathway is selectively required for TLR4-mediated innate immunity. Nat Immunol. 2005;6:587–592. doi: 10.1038/ni1200. [DOI] [PubMed] [Google Scholar]
- 120.Lu Y, Wah LM. Oxidative stress augments the production of matrix metalloproteinase-1, cyclooxygenase-2, and prostaglandin E2 through enhancement of NF-kappa B activity in lipopolysaccharide-activated human primary monocytes. J Immunol. 2005;175:5423–5429. doi: 10.4049/jimmunol.175.8.5423. [DOI] [PubMed] [Google Scholar]
- 121.Dodson M, Redmann M, Rajasekaran NS, et al. KEAP1-NRF2 signalling and autophagy in protection against oxidative and reductive proteotoxicity. Biochem J. 2015;469:347–355. doi: 10.1042/BJ20150568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Singh R, Kaushik S, Wang Y, et al. Autophagy regulates lipid metabolism. Nature. 2009;458:1131–1135. doi: 10.1038/nature07976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Lemasters JJ. Variants of mitochondrial autophagy: types 1 and 2 mitophagy and micromitophagy (Type 3) Redox Biol. 2014;2:749–754. doi: 10.1016/j.redox.2014.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Digaleh H, Kiaei M, Khodagholi F. Nrf2 and Nrf1 signaling and ER stress crosstalk: implication for proteasomal degradation and autophagy. Cell Mol Life Sci. 2013;70:4681–4694. doi: 10.1007/s00018-013-1409-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Jain A, Lamark T, Sjottem E, et al. p62/SQSTM1 is a target gene for transcription factor NRF2 and creates a positive feedback loop by inducing antioxidant response element-driven gene transcription. J Biol Chem. 2010;285:22576–22591. doi: 10.1074/jbc.M110.118976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Jo C, Gundemir S, Pritchard S, et al. Nrf2 reduces levels of phosphorylated tau protein by inducing autophagy adaptor protein NDP52. Nat Commun. 2014;5:3496. doi: 10.1038/ncomms4496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Fan W, Tang Z, Chen D, et al. Keap1 facilitates p62-mediated ubiquitin aggregate clearance via autophagy. Autophagy. 2010;6:614–621. doi: 10.4161/auto.6.5.12189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Chung SD, Lai TY, Chien CT, et al. Activating Nrf-2 signaling depresses unilateral ureteral obstruction-evoked mitochondrial stress-related autophagy, apoptosis and pyroptosis in kidney. PLoS One. 2012;7:e47299. doi: 10.1371/journal.pone.0047299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Rao VA, Klein SR, Bonar SJ, et al. The antioxidant transcription factor Nrf2 negatively regulates autophagy and growth arrest induced by the anticancer redox agent mitoquinone. J Biol Chem. 2010;285:34447–34459. doi: 10.1074/jbc.M110.133579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Zhou Y, Wang HD, Zhu L, et al. Knockdown of Nrf2 enhances autophagy induced by temozolomide in U251 human glioma cell line. Oncol Rep. 2013;29:394–400. doi: 10.3892/or.2012.2115. [DOI] [PubMed] [Google Scholar]
- 131.Wang L, Khambu B, Zhang H, et al. Autophagy in alcoholic liver disease, self-eating triggered by drinking. Clin Res Hepatol Gastroenterol. 2015;39(Suppl 1):S2–S6. doi: 10.1016/j.clinre.2015.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Lin CW, Zhang H, Li M, et al. Pharmacological promotion of autophagy alleviates steatosis and injury in alcoholic and non-alcoholic fatty liver conditions in mice. J Hepatol. 2013;58:993–999. doi: 10.1016/j.jhep.2013.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Williams JA, Ding WX. A mechanistic review of mitophagy and its role in protection against alcoholic liver disease. Biomolecules. 2015;5:2619–2642. doi: 10.3390/biom5042619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Ding WX, Li M, Yin XM. Selective taste of ethanol-induced autophagy for mitochondria and lipid droplets. Autophagy. 2011;7:248–249. doi: 10.4161/auto.7.2.14347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Williams JA, Ni HM, Ding Y, et al. Parkin regulates mitophagy and mitochondrial function to protect against alcohol-induced liver injury and steatosis in mice. Am J Physiol Gastrointest Liver Physiol. 2015;309:G324–G340. doi: 10.1152/ajpgi.00108.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Ding WX, Li M, Chen X, et al. Autophagy reduces acute ethanol-induced hepatotoxicity and steatosis in mice. Gastroenterology. 2010;139:1740–1752. doi: 10.1053/j.gastro.2010.07.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.D’Souza El-Guindy NB, Kovacs EJ, De Witte P, et al. Laboratory models available to study alcohol-induced organ damage and immune variations: choosing the appropriate model. Alcohol Clin Exp Res. 2010;34:1489–1511. doi: 10.1111/j.1530-0277.2010.01234.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Li HH, Tyburski JB, Wang YW, et al. Modulation of fatty acid and bile acid metabolism by peroxisome proliferator-activated receptor alpha protects against alcoholic liver disease. Alcohol Clin Exp Res. 2014;38:1520–1531. doi: 10.1111/acer.12424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Bertola A, Mathews S, Ki SH, et al. Mouse model of chronic and binge ethanol feeding (the NIAAA model) Nat Protoc. 2013;8:627–637. doi: 10.1038/nprot.2013.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Tsukamoto H, Mkrtchyan H, Dynnyk A. Intragastric ethanol infusion model in rodents. Methods Mol Biol. 2008;447:33–48. doi: 10.1007/978-1-59745-242-7_3. [DOI] [PubMed] [Google Scholar]
- 141.Lieber CS, DeCarli LM, Sorrell MF. Experimental methods of ethanol administration. Hepatology. 1989;10:501–510. doi: 10.1002/hep.1840100417. [DOI] [PubMed] [Google Scholar]
- 142.Bataller R, Gao B. Liver fibrosis in alcoholic liver disease. Semin Liver Dis. 2015;35:146–156. doi: 10.1055/s-0035-1550054. [DOI] [PubMed] [Google Scholar]
- 143.Aleynik SI, Leo MA, Aleynik MK, et al. Increased circulating products of lipid peroxidation in patients with alcoholic liver disease. Alcohol Clin Exp Res. 1998;22:192–196. doi: 10.1111/j.1530-0277.1998.tb03637.x. [DOI] [PubMed] [Google Scholar]
- 144.Meagher EA, Barry OP, Burke A, et al. Alcohol-induced generation of lipid peroxidation products in humans. J Clin Invest. 1999;104:805–813. doi: 10.1172/JCI5584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Clot P, Bellomo G, Tabone M, et al. Detection of antibodies against proteins modified by hydroxyethyl free radicals in patients with alcoholic cirrhosis. Gastroenterology. 1995;108:201–207. doi: 10.1016/0016-5085(95)90025-X. [DOI] [PubMed] [Google Scholar]
- 146.Medici V, Virata MC, Peerson JM, et al. S-adenosyl-l-methionine treatment for alcoholic liver disease: a double-blinded, randomized, placebo-controlled trial. Alcohol Clin Exp Res. 2011;35:1960–1965. doi: 10.1111/j.1530-0277.2011.01547.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Mato JM, Camara J, Fernandez de Paz J, et al. S-adenosylmethionine in alcoholic liver cirrhosis: a randomized, placebo-controlled, double-blind, multicenter clinical trial. J Hepatol. 1999;30:1081–1089. doi: 10.1016/S0168-8278(99)80263-3. [DOI] [PubMed] [Google Scholar]
- 148.Moreno C, Langlet P, Hittelet A, et al. Enteral nutrition with or without N-acetylcysteine in the treatment of severe acute alcoholic hepatitis: a randomized multicenter controlled trial. J Hepatol. 2010;53:1117–1122. doi: 10.1016/j.jhep.2010.05.030. [DOI] [PubMed] [Google Scholar]
- 149.Mezey E, Potter JJ, Rennie-Tankersley L, et al. A randomized placebo controlled trial of vitamin E for alcoholic hepatitis. J Hepatol. 2004;40:40–46. doi: 10.1016/S0168-8278(03)00476-8. [DOI] [PubMed] [Google Scholar]
- 150.Meagher EA, Barry OP, Lawson JA, et al. Effects of vitamin E on lipid peroxidation in healthy persons. JAMA. 2001;285:1178–1182. doi: 10.1001/jama.285.9.1178. [DOI] [PubMed] [Google Scholar]
- 151.Jacob RA, Aiello GM, Stephensen CB, et al. Moderate antioxidant supplementation has no effect on biomarkers of oxidant damage in healthy men with low fruit and vegetable intakes. J Nutr. 2003;133:740–743. doi: 10.1093/jn/133.3.740. [DOI] [PubMed] [Google Scholar]
- 152.Ueberschlag SL, Seay JR, Roberts AH, et al. The effect of protandim(R) supplementation on athletic performance and oxidative blood markers in runners. PLoS One. 2016;11:e0160559. doi: 10.1371/journal.pone.0160559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Nelson SK, Bose SK, Grunwald GK, et al. The induction of human superoxide dismutase and catalase in vivo: a fundamentally new approach to antioxidant therapy. Free Radic Biol Med. 2006;40:341–347. doi: 10.1016/j.freeradbiomed.2005.08.043. [DOI] [PubMed] [Google Scholar]
- 154.Pergola PE, Raskin P, Toto RD, et al. Bardoxolone methyl and kidney function in CKD with type 2 diabetes. N Engl J Med. 2011;365:327–336. doi: 10.1056/NEJMoa1105351. [DOI] [PubMed] [Google Scholar]
- 155.Chin MP, Wrolstad D, Bakris GL, et al. Risk factors for heart failure in patients with type 2 diabetes mellitus and stage 4 chronic kidney disease treated with bardoxolone methyl. J Cardiac Fail. 2014;20:953–958. doi: 10.1016/j.cardfail.2014.10.001. [DOI] [PubMed] [Google Scholar]
- 156.Kappos L, Gold R, Miller DH, et al. Efficacy and safety of oral fumarate in patients with relapsing-remitting multiple sclerosis: a multicentre, randomised, double-blind, placebo-controlled phase IIb study. (vol 372, pg 1463, 2008) Lancet. 2009;373:1340. doi: 10.1016/S0140-6736(09)60776-5. [DOI] [PubMed] [Google Scholar]
- 157.Singh S, Murad MH, Chandar AK, et al. Comparative effectiveness of pharmacological Interventions for severe alcoholic hepatitis: a systematic review and network meta-analysis. Gastroenterology. 2015;149(958–70):e12. doi: 10.1053/j.gastro.2015.06.006. [DOI] [PubMed] [Google Scholar]
- 158.Naveau S, Chollet-Martin S, Dharancy S, et al. A double-blind randomized controlled trial of infliximab associated with prednisolone in acute alcoholic hepatitis. Hepatology. 2004;39:1390–1397. doi: 10.1002/hep.20206. [DOI] [PubMed] [Google Scholar]
- 159.Boetticher NC, Peine CJ, Kwo P, et al. A randomized, double-blinded, placebo-controlled multicenter trial of etanercept in the treatment of alcoholic hepatitis. Gastroenterology. 2008;135:1953–1960. doi: 10.1053/j.gastro.2008.08.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Borrelli A, Bonelli P, Tuccillo FM, et al. Role of gut microbiota and oxidative stress in the progression of non-alcoholic fatty liver disease to hepatocarcinoma: current and innovative therapeutic approaches. Redox Biol. 2018;15:467–479. doi: 10.1016/j.redox.2018.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Sumida Y, Yoneda M (2018) Current and future pharmacological therapies for NAFLD/NASH. J Gastroenterol 53:362–376 [DOI] [PMC free article] [PubMed]
- 162.Chowdhry S, Nazmy MH, Meakin PJ, et al. Loss of Nrf2 markedly exacerbates nonalcoholic steatohepatitis. Free Radic Biol Med. 2010;48:357–371. doi: 10.1016/j.freeradbiomed.2009.11.007. [DOI] [PubMed] [Google Scholar]
- 163.Sugimoto H, Okada K, Shoda J, et al. Deletion of nuclear factor-E2-related factor-2 leads to rapid onset and progression of nutritional steatohepatitis in mice. Am J Physiol Gastrointest Liver Physiol. 2010;298:G283–G294. doi: 10.1152/ajpgi.00296.2009. [DOI] [PubMed] [Google Scholar]
- 164.Okada K, Warabi E, Sugimoto H, et al. Deletion of Nrf2 leads to rapid progression of steatohepatitis in mice fed atherogenic plus high-fat diet. J Gastroenterol. 2013;48:620–632. doi: 10.1007/s00535-012-0659-z. [DOI] [PubMed] [Google Scholar]
- 165.Zhang YK, Yeager RL, Tanaka Y, et al. Enhanced expression of Nrf2 in mice attenuates the fatty liver produced by a methionine- and choline-deficient diet. Toxicol Appl Pharmacol. 2010;245:326–334. doi: 10.1016/j.taap.2010.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Okada K, Warabi E, Sugimoto H, et al. Nrf2 inhibits hepatic iron accumulation and counteracts oxidative stress-induced liver injury in nutritional steatohepatitis. J Gastroenterol. 2012;47:924–935. doi: 10.1007/s00535-012-0552-9. [DOI] [PubMed] [Google Scholar]
- 167.Sharma RS, Harrison DJ, Kisielewski D, et al. Experimental nonalcoholic steatohepatitis and liver fibrosis are ameliorated by pharmacologic activation of Nrf2 (NF-E2 p45-related factor 2) Cell Mol Gastroenterol Hepatol. 2018;5:367–398. doi: 10.1016/j.jcmgh.2017.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Feng X, Yu W, Li X, et al. Apigenin, a modulator of PPARgamma, attenuates HFD-induced NAFLD by regulating hepatocyte lipid metabolism and oxidative stress via Nrf2 activation. Biochem Pharmacol. 2017;136:136–149. doi: 10.1016/j.bcp.2017.04.014. [DOI] [PubMed] [Google Scholar]
- 169.More VR, Xu J, Shimpi PC, et al. Keap1 knockdown increases markers of metabolic syndrome after long-term high fat diet feeding. Free Radic Biol Med. 2013;61:85–94. doi: 10.1016/j.freeradbiomed.2013.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Zarei M, Barroso E, Palomer X, et al. Hepatic regulation of VLDL receptor by PPARbeta/delta and FGF21 modulates non-alcoholic fatty liver disease. Mol Metab. 2018;8:117–131. doi: 10.1016/j.molmet.2017.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Liu J, Wu KC, Lu YF, et al. Nrf2 protection against liver injury produced by various hepatotoxicants. Oxid Med Cell Longev. 2013;2013:305861. doi: 10.1155/2013/305861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Wu KC, Zhang Y, Klaassen CD (2012) Nrf2 protects against diquat-induced liver and lung injury. Free Radic Res 46:1220–1229 [DOI] [PubMed]
- 173.Wu KC, Liu JJ, Klaassen CD (2012) Nrf2 activation prevents cadmium-induced acute liver injury. Toxicol Appl Pharmacol 263:14–20 [DOI] [PubMed]
- 174.Reisman SA, Buckley DB, Tanaka Y, et al. CDDO-Im protects from acetaminophen hepatotoxicity through induction of Nrf2-dependent genes. Toxicol Appl Pharmacol. 2009;236:109–114. doi: 10.1016/j.taap.2008.12.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Liu B, Fang M, He Z, et al. Hepatitis B virus stimulates G6PD expression through HBx-mediated Nrf2 activation. Cell Death Dis. 2015;6:e1980. doi: 10.1038/cddis.2015.322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Medvedev R, Ploen D, Spengler C, et al. HCV-induced oxidative stress by inhibition of Nrf2 triggers autophagy and favors release of viral particles. Free Radic Biol Med. 2017;110:300–315. doi: 10.1016/j.freeradbiomed.2017.06.021. [DOI] [PubMed] [Google Scholar]
- 177.Chiang DJ, Roychowdhury S, Bush K, et al. Adenosine 2A receptor antagonist prevented and reversed liver fibrosis in a mouse model of ethanol-exacerbated liver fibrosis. PLoS One. 2013;8:e69114. doi: 10.1371/journal.pone.0069114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Jeong WI, Park O, Gao B. Abrogation of the antifibrotic effects of natural killer cells/interferon-gamma contributes to alcohol acceleration of liver fibrosis. Gastroenterology. 2008;134:248–258. doi: 10.1053/j.gastro.2007.09.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Lazaro R, Wu R, Lee S, et al. Osteopontin deficiency does not prevent but promotes alcoholic neutrophilic hepatitis in mice. Hepatology. 2015;61:129–140. doi: 10.1002/hep.27383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Shimada M, Liu L, Nussler N, et al. Human hepatocytes are protected from ethanol-induced cytotoxicity by DADS via CYP2E1 inhibition. Toxicol Lett. 2006;163:242–249. doi: 10.1016/j.toxlet.2005.11.003. [DOI] [PubMed] [Google Scholar]
- 181.Aqil F, Munagala R, Jeyabalan J, et al. Bioavailability of phytochemicals and its enhancement by drug delivery systems. Cancer Lett. 2013;334:133–141. doi: 10.1016/j.canlet.2013.02.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Kumar V. Potential medicinal plants for CNS disorders: an overview. Phytother Res. 2006;20:1023–1035. doi: 10.1002/ptr.1970. [DOI] [PubMed] [Google Scholar]
- 183.Dodson M, Zhang DD. Non-canonical activation of NRF2: New insights and its relevance to disease. Curr Pathobiol Rep. 2017;5:171–176. doi: 10.1007/s40139-017-0131-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Lau A, Zheng Y, Tao S, et al. Arsenic inhibits autophagic flux, activating the Nrf2-Keap1 pathway in a p62-dependent manner. Mol Cell Biol. 2013;33:2436–2446. doi: 10.1128/MCB.01748-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Komatsu M, Waguri S, Koike M, et al. Homeostatic levels of p62 control cytoplasmic inclusion body formation in autophagy-deficient mice. Cell. 2007;131:1149–1163. doi: 10.1016/j.cell.2007.10.035. [DOI] [PubMed] [Google Scholar]