Abstract
The metabolic syndrome (MetS) includes a group of medical conditions such as insulin resistance (IR), dyslipidemia and hypertension, all associated with an increased risk for cardiovascular disease. Increased visceral and ectopic fat deposition are also key features in the development of IR and MetS, with pathophysiological sequels on adipose tissue, liver and muscle. The recent recognition of aquaporins (AQPs) involvement in adipose tissue homeostasis has opened new perspectives for research in this field. The members of the aquaglyceroporin subfamily are specific glycerol channels implicated in energy metabolism by facilitating glycerol outflow from adipose tissue and its systemic distribution and uptake by liver and muscle, unveiling these membrane channels as key players in lipid balance and energy homeostasis. Being involved in a variety of pathophysiological mechanisms including IR and obesity, AQPs are considered promising drug targets that may prompt novel therapeutic approaches for metabolic disorders such as MetS. This review addresses the interplay between adipose tissue, liver and muscle, which is the basis of the metabolic syndrome, and highlights the involvement of aquaglyceroporins in obesity and related pathologies and how their regulation in different organs contributes to the features of the metabolic syndrome.
Keywords: Aquaporins, Aquaglyceroporins, Glycerol, Metabolic syndrome, Obesity
Introduction to the metabolic syndrome
The International Diabetes Federation estimates that a quarter of the adults worldwide suffer from metabolic syndrome (MetS), a cluster of atherosclerotic cardiovascular disease risk factors including visceral adiposity, insulin resistance (IR) and dyslipidemia [1, 2]. The main pathophysiological mechanism involved is IR (MetS is also known as IR syndrome) where liver, skeletal muscle and adipose tissue cells become progressively less sensitive to insulin, and consequently, pancreatic beta cells must secrete more insulin to maintain normoglycemia, which ultimately leads to their progressive dysfunction and eventual loss of function [3, 4].
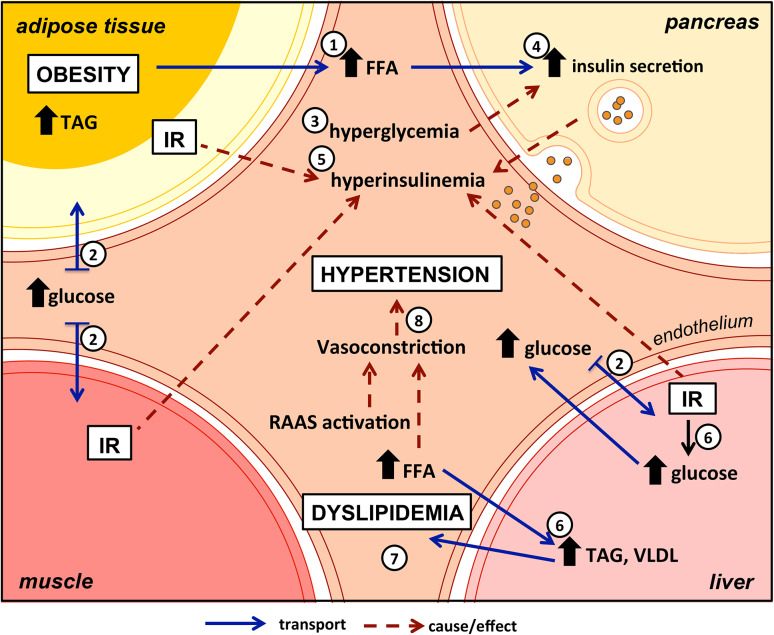
In normal physiological conditions, glucose homeostasis is tightly controlled and the fasting plasma glucose concentration is maintained within a very narrow range. In the postabsorptive state, the increase in plasma glucose stimulates insulin secretion by pancreatic beta cells. Hyperglycemic challenges are tightly regulated by a normal amount of secreted insulin where hyperglycemia plus hyperinsulinemia combine to suppress hepatic glucose production and stimulate glucose uptake by splanchnic and peripheral (primarily muscle) tissues to restore normoglycemia [5]. However, under some pathological conditions glucose homeostasis may be disturbed. IR, implying depressed cellular sensitivity to insulin, is a presumed state of insufficient insulin production and insulin secretory dysfunction despite high metabolic demand. A central feature of IR is the inability to suppress free fatty acids (FFA) production and release from adipocytes by the normal levels of insulin. Indeed, adipocyte resistance to the antilipolytic effect of insulin and the consequent elevated plasma free fatty acid levels (Fig. 1, (1)) may play an important role in the development of IR in muscle and other target tissues. Consequently, the ability of insulin to augment glucose uptake in adipose tissue, liver and muscle (Fig. 1, (2)) is impaired. The resultant hyperglycemia (Fig. 1, (3)) presents a stimulus to pancreatic beta cells (Fig. 1, (4)), which being demanded to secrete large amounts of insulin as a compensatory mechanism eventually become exhausted, resulting in deregulation in the pathways of insulin action and hyperinsulinemia (Fig. 1, (5)). In the liver, glucose production and lipid storage in the form of triacylglycerol (TAG) are increased (Fig. 1, (6)). The enlarged TAG accumulation results in increased secretion of very-low-density lipoproteins (VLDLs) into the systemic circulation and consequent dyslipidemia (Fig. 1, (7)), an important criterion for the diagnosis of the MetS [2, 6, 7].
Fig. 1.
MetS results from the interplay between adipose tissue, skeletal muscle, liver and pancreas. Obesity development and adipose tissue dysfunction lead to high levels of circulating FFA (1). Decreased insulin sensitivity in liver and muscle results in impaired glucose uptake (2) and hyperglycemia (3). Over-stimulation of insulin release by endocrine pancreas (4) ends in hyperinsulinemia (5) and consequently IR. In the liver, glucose production, lipid storage in the form of triacylglycerol and VLDL secretion are increased (6) contributing to dyslipidemia (7). Lack of insulin action combined with high levels of FFA activates the RAAS, leading to vasoconstriction and causing hypertension (8). FFA free fatty acids, IR insulin resistance, RAAS renin angiotensin aldosterone system, TAG triacylglycerols, VLDL very-low-density lipoproteins
Hypertension is another symptom in MetS but its relation to the syndrome is complex, although it is almost consensual that increased blood pressure is one of the conditions in diagnostic criteria [8]. The kidney plays a crucial role in controlling blood pressure, particularly through the regulation of sodium excretion [9] and through various vasoactive systems such as the renin–angiotensin–aldosterone system (RAAS). Adipocytes also produce angiotensin-converting enzyme and cathepsins, affecting angiotensin conversion and catabolism, respectively. By increasing angiotensinogen production in adipose tissue, an enlarged fat mass may contribute to an increase in blood pressure [10]. In addition, insulin is a strong enhancer of sodium reabsorption in the kidney, and simultaneously, causes a release of nitric oxide and subsequent vasodilation. Instead, IR and concomitant hyperinsulinemia activate the RAAS and reduce nitric oxide-mediated vasodilation resulting in vasoconstriction and hypertension [11, 12] (Fig. 1, (8)). Moreover, the increase of FFA in circulation can induce angiotensinogen production and may promote oxidative stress in endothelial cells, contributing to the pathogenesis of hypertension [10].
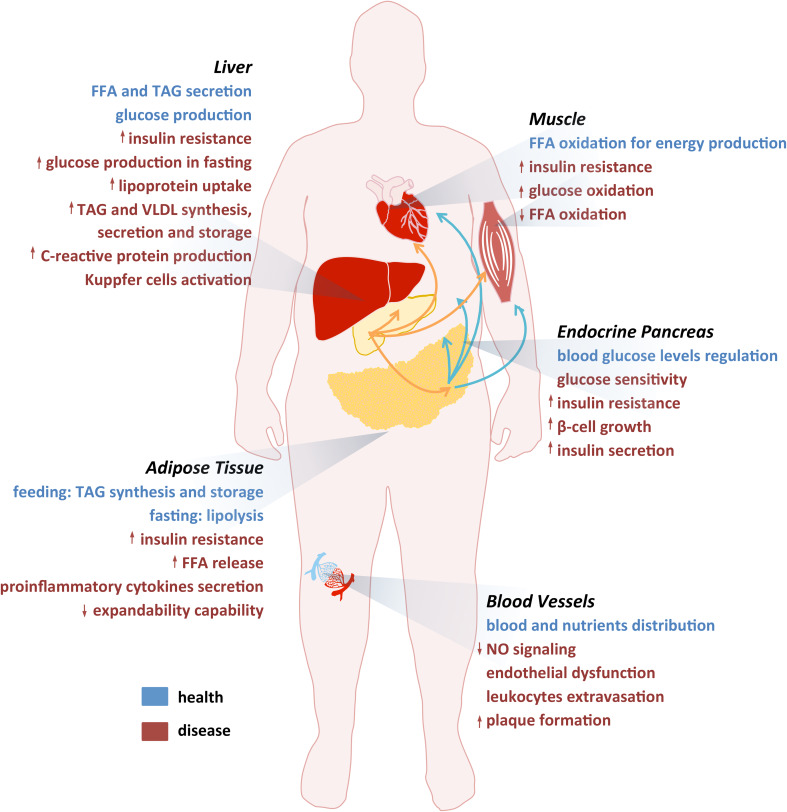
In sum, the MetS results from interplay between insulin-targeted tissues such as adipose tissue, liver and skeletal muscle (Fig. 1). The involvement of these tissues in energy metabolism and metabolic dysfunction is illustrated in Fig. 2 and will be individually addressed in the next sections.
Fig. 2.
The most significant events associated with MetS. Summary of the most significant events in healthy (blue) and in disease (red) conditions affecting the main tissues implicated in MetS (adipose tissue, liver, muscle, endocrine pancreas and vessels)
Adipose tissue stores energy and regulates metabolism through the release of adipokines
Adipose tissue is a particularly flexible tissue capable of reduction, expansion or alteration under appropriate stimulations. According to its cellular and endocrine functions, the adipose tissue is divided into two major types, the white adipose tissue (WAT) and the brown adipose tissue (BAT). While white fat stores energy, brown fat expends it [13]. The current classification of adipose tissue includes a third category, the beige adipose tissue. It shows thermogenic properties similar to BAT and has been detected in humans when stimulated by cold stress or β3-adrenoceptor agonists that mimic cold stress [14]. The three types of adipose tissues have different morphology, distribution, gene expression, and metabolic functions [15]. Being the main function of WAT the storage of energy in the form of TAG, its dysfunction is deeply implicated in obesity and related metabolic disorders.
WAT is composed by adipocytes, vascular tissue and immune cells, all enclosed in an extracellular matrix formed by proteins such as collagen. Fully matured adipocytes develop from preadipocytes after undergoing differentiation [16]. In situations of reduced energy expenditure or increased food intake, which are characterized as positive energy balance, mature adipocytes experience hyperplasia (increase in number) and hypertrophy (increase in size) to store excess lipid in the form of TAG with consequent change in their morphology [17–19]. Adipocyte enlargement occurs in parallel with extracellular matrix adjustment, by the action of proteases that hydrolyze collagen to allow cell hypertrophy, and with the formation of new vessels (angiogenesis) [20]. Adipocytes have origin in adipose stem cells located in the vicinity of the adipose microvasculature [21] that early in postnatal life or even prenatally, differentiate into preadipocytes and adipocytes by adipose vasculature stimuli [21]. Adipose tissue is an important endocrine and paracrine organ that communicates with many other organs in the body by secreting signaling proteins collectively known as adipokines [22]. In addition, adipose tissue constitutes an important source of circulating exosomal micro RNAs (miRNAs), which can regulate gene expression in distant tissues, and thereby serve as a form of adipokine [23]. Overall, adipose tissue contributes to the maintenance of energy, lipid and glucose homeostasis, mediating multiple biological processes such as inflammation, immunity and metabolism [24–26].
Population studies have shown an association between abdominal obesity and IR. However, the specific molecular mechanisms that lead obesity to related metabolic pathologies remain unclear. Several hypotheses have been proposed to explain the development of adipose tissue dysfunction and obesity. The adipose tissue expandability hypothesis has been corroborated by both clinical and experimental data and is based on the limitation of the adipose tissue to expand above a given threshold for a specific individual [27]. When an individual increases in fat mass, the adipose tissue expands until a certain point where it reaches its limit of storage and is no longer able to accumulate more fat. At this point, ectopic accumulation of bloodstream lipids in non-adipose tissues, such as liver, skeletal muscle and pancreas occurs. Contrary to adipocytes, these tissues are poorly adjustable to store lipids, triggering a lipid-induced toxicity that culminates in failure of cellular function and results in inflammation and IR [28, 29]. The maximal capacity of the adipose tissue is dependent on the type of lipid depots [25], subcutaneous or visceral, the first being more adipogenic and with greater expansion capacity (hyperplasia) and the latter metabolically more active, expanding mainly by hypertrophy with huge infiltration of macrophages and regulated by a large number of glucocorticoid receptors and a lower number of insulin receptors. It is well accepted that in humans, peripheral subcutaneous adiposity is not harmful and may even be protective, acting as lipid-buffering tissue that helps in the maintenance of the daily lipid fluxes homeostasis, whereas increased visceral fat is associated with metabolic complications due to its proximity to the liver through the portal vein together with its diminished expansion capability [24, 27]. Therefore, the risk of MetS progression is closely associated with visceral obesity [30]. Moreover, the evidence that the individual adipose expandability threshold is determined by genetic and environmental factors, may explain why both apparently lean and obese people are prone to develop IR [31].
Another recognized mechanism linking obesity to IR is the adipose tissue inflammation mediated by overproduction of proinflammatory and anti-adipogenic cytokines [32]. Hypertrophic adipocytes secrete high levels of proinflammatory adipokines, such as leptin, and FFA that induce macrophage infiltration into the adipose tissue and their activation. In turn, activated macrophages secrete anti-adipogenic cytokines [tumor necrosis factor-α (TNF-α), interleukin-6 (IL-6) and interleukin-1β (IL-1β)] that inhibit insulin action [33]. Insulin-resistant adipocytes continue releasing FFA, activating macrophages to destroy compromised adipocytes that in turn secrete even more anti-adipogenic cytokines, consequently increasing IR in mature adipocytes and blocking preadipocytes maturation [27].
Liver regulates systemic energy homeostasis
The liver plays a central and critical biochemical role in metabolism. In addition to the main function of glucose uptake, production and storage as glycogen, liver is also involved in processes such as biotransformation and excretion, through bile secretion, granting a protective role from exogenous and potentially toxic substances [34]. Overall, liver acts as a buffer, regulating blood glucose levels to provide this substrate to other tissues such as brain and skeletal muscle.
In response to feeding, nutrients absorbed into the bloodstream reach the liver through the portal vein. Glucose is then converted into pyruvate, through glycolysis, in the cytoplasm of hepatocytes, being oxidized through the tricarboxylic acid cycle (TCA) and oxidative phosphorylation in the mitochondria to produce ATP. If energy is not needed, glucose is stored as glycogen, through glycogenesis, or converted into FFA and amino acids. By esterification of FFA with glycerol, TAG are generated and stored in lipid droplets or secreted into circulation as VLDL, while amino acids are used to synthesize proteins, glucose or other biomolecules [35]. These pathways are controlled by insulin, which stimulates the uptake of glucose by peripheral tissues causing a rapid removal of glucose from the blood. Insulin induces energy storage and anabolic reactions, such as fatty acid synthesis in liver and adipose tissue [36]. Moreover, insulin stimulates glycolysis, and in the liver, it blocks glycogenolysis and gluconeogenesis and stimulates lipogenesis [35].
During fasting, fuel substrates are released from the liver into the circulation to be metabolized in peripheral tissues. Glucagon, produced by alpha cells of the pancreas, induces an increase in hepatic intracellular cyclic adenosine monophosphate (cAMP) that promotes glycogenolysis and gluconeogenesis [37]. The net outcome of these pathways leads to an increase in hepatic glucose production.
The state of hepatic IR refers to impaired insulin suppression of gluconeogenesis. In fact, type 2 diabetics are unable to decrease glucagon appropriately upon increase in plasmatic glucose concentration [38]. Moreover, there are reports that T2D is associated with defects in insulin signaling pathway. Liver-specific insulin receptor knockout mice exhibit dramatic insulin resistance and severe glucose intolerance [39] while muscle-specific insulin receptor knockout mice display elevated fat mass, serum TAG and FFA, but blood glucose, serum insulin, and glucose tolerance are normal [40], suggesting a major role of liver in the development of hyperglycemia, T2D and MetS.
Lipid metabolism in the liver is also affected in the setting of IR. In IR condition, lipolysis in adipocytes is enhanced and an excess of FFA released from adipose tissue is delivered to the liver and muscle. In the liver, FFA are primarily used to form TAG that may accumulate causing steatosis, also known as fatty liver [8]. Due to excess FFA in the blood or inefficient FFA oxidation, TAG can also accumulate in the muscle [8]. In addition, Insulin also stimulates lipogenesis in the liver directly. There are two contrasting theories that explain the role of IR in increasing VLDL production. It has been suggested that VLDL overproduction is a response to hyperinsulinaemia, inducing de novo hepatic synthesis of fatty acids. In fact, it was shown in obese mice that chronic hyperinsulinaemia downregulates insulin receptor substrate-2 (IRS-2), a crucial receptor in insulin signaling pathway. However, insulin continues to induce sterol regulatory element-binding protein 1c (SREBP-1c), activating fatty acid synthesis [41]. In this case, there is a combination of IR, due to increased gluconeogenesis, and insulin sensitivity, represented by elevated lipogenesis, the natural insulin response. Thus, an aggravated state of hyperinsulinaemia and consequent IR are observed. On the other hand, other study suggested that the major contributor is the increased supply of FFA to the liver, released from adipose tissue, correlating the augment in plasma FFA with the enhanced production of VLDL [42].
Skeletal muscle consumes most of the systemic glucose in an insulin-dependent manner
Skeletal muscle is a key insulin-targeted tissue, constituting up to 50% of total body mass. In resting conditions, it requires small amount of glucose, while 80% of blood glucose is metabolized by brain, gut and red blood cells in an insulin-independent manner [43]. However, after insulin stimulation, skeletal muscle accounts for 85% of glucose utilization [44–46].
Energy for muscle contraction in the form of ATP is mostly used by myosin heads and ion pumps, especially Ca2+ pumps in the sarcoplasmic reticulum [47]. Insulin induces the uptake of glucose and has an antilipolytic function. In the feeding state, insulin suppresses lipolysis in the adipose tissue, declining the concentration of FFA in plasma. Under such circumstances, muscle uses mostly glucose, which uptake is stimulated, entering the cell through the glucose transporter GLUT4, whose translocation from intracellular vesicles to the plasma membrane is induced by insulin [48].
On the other hand, under fasting conditions, muscle uses mostly FFA for energy production, as these are in high concentrations in plasma due to adipose tissue lipolysis, increasing muscle fatty acid oxidation [49]. High-fat diets play a role in the development of IR in muscle. In fact, a study performed in rats showed that an increased plasma concentration of fatty acids leads to an increased intracellular concentration of fatty acyl-coA which in turn blocks insulin activation of insulin receptor substrate-1 (IRS-1) [50]. As a final result, this will cause a decrease in insulin sensitivity. IR is characterized by a decreased but also delayed muscle glucose uptake [51]. Moreover, it was reported that in IR states such as obesity, the rate of FFA oxidation is reduced during fasting despite similar plasmatic concentrations [52]. Impaired fat oxidation can be associated to mitochondrial defects since it occurs in this organelle. In fact, patients with controlled T2D show reduced muscular ATP synthesis [53] and decreased activity of several mitochondrial enzymes [54], reflecting problems in oxidative phosphorylation. The increased FFA influx and decreased mitochondrial fat oxidation occurring in IR alters the dynamic equilibrium between fatty acid intake and oxidation and may be reflected in elevated muscular lipid content.
Aquaporins: new players in the development of MetS
Aquaporins (AQPs) are a family of membrane protein channels, present in all kinds of organisms [55] that facilitate the transport of water and small molecules such as glycerol and urea through the plasma membrane driven by osmotic or solute gradients [56, 57]. The members of the aquaporin family present high homology in protein sequence [58] and so far, 13 isoforms (AQP0-12) were described in mammals with tissue- and subcellular-specific localization [59] suggesting a link between site of expression and function [60]. According to their primary sequence and channel selectivity, AQPs are divided into three subfamilies: orthodox AQPs (AQP0, 1, 2, 4, 5, 6, 8) are mainly selective for water, aquaglyceroporins (AQP3, 7, 9, 10) facilitate the permeation of other small-uncharged solutes such as glycerol in addition to water, and S-aquaporins (AQP11, 12) found mostly intracellularly, with lower sequence homology and permeability still unclear [61].
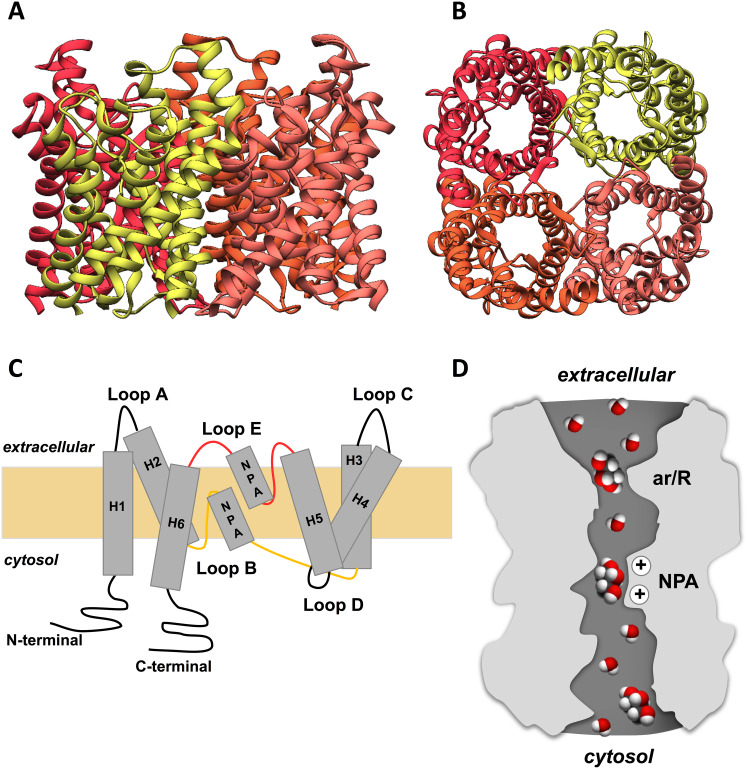
AQPs are architected in membranes as tetramers formed by four identical monomers (Fig. 3a, b), each behaving as a channel and sharing a conserved typical hourglass fold (Fig. 3d). Each monomer is composed by around 320 amino acid residues with approximately 28 kDa, characterized by a topology of six highly hydrophobic transmembrane domains (1–6) connected by five loops (A–E) with the N- and C-terminal sequences facing the cytosol, and two half-helixes containing highly conserved asparagine–proline–alanine (NPA) motifs that are part of the protein family signature [55] (Fig. 3c). To achieve the channel selectivity, the pore region contains two selective filters with size constrictions and charge characteristics that enable water or glycerol to permeate, while preventing the passage of charged molecules such as protons or any solute above the pore size [62]. Starting from the periplasmic side of the channel, the first selectivity filter is a constriction site formed by aromatic/arginine residues (ar/R) near the extracellular entrance [63] that determines the size of molecules allowed to pass through (pore size of ~ 2.8 Å in orthodox aquaporins and ~ 3.4 Å, in aquaglyceroporins). The second constriction site is formed by the two highly preserved NPA motifs that behave as dipoles, preventing ions to permeate the channel [64, 65] (Fig. 3d).
Fig. 3.
General structure of aquaporins. a Side and b intracellular views of the homotetrameric representation of the glycerol channel GlpF based on its X-ray structure (PDB code: 1FX8). Figures were generated with UCSF Chimera software. c Representation of AQP membrane topography, showing the monomer comprising six membrane-spanning α-helices (H1–6) connected by five loops (A–E), the conserved asparagine–proline–alanine (NPA) motifs embed in the membrane. In the functional monomer, the hydrophilic loops B and E are bent back into the cavity formed by the helices. The two loops meet in the middle to form the water-selective gate that contains two consensus NPA motifs (Asn–Pro–Ala). The hydrogen bonding properties of the polar side groups of the two Asn residues are thought to constitute the permeation barrier. d Illustration of water and glycerol molecules permeating the aquaporin pore, with the two selectivity filters ar/R and NPA represented
AQPs have been associated with a variety of important physiological roles including transepithelial fluid transport, brain water homeostasis, osmoregulation, cell migration and proliferation [57, 66] and have been suggested as potential targets for drug development [67–70]. In particular, due to their ability to transport glycerol through the plasma membrane regulating cellular glycerol content, aquaglyceroporins are involved in skin hydration [71] and fat metabolism [72], and are emerging as important players in adipose tissue homeostasis and insulin response with possible implications in metabolic disorders such as obesity and MetS [69, 70].
Aquaglyceroporins in glycerol metabolism and energy homeostasis
Glycerol is the carbon backbone for the de novo synthesis of TAG and phospholipids and it is also an important intermediate in both carbohydrate and lipid metabolism. Intracellular glycerol accumulation activates glycerol kinase (GK) promoting the synthesis of glycerol-3-phosphate (G3P), a by-product from glycolysis and a key molecule in the regeneration of NAD+ from NADH [73, 74]. In the liver, G3P is used in glycolysis and gluconeogenesis, and in muscle, it is an energy substrate via the G3P shuttle, which has a key role in oxidizing glucose rapidly and generating adenosine triphosphate (ATP) in the mitochondria through the oxidation of G3P [75]. Under negative energy balance conditions (fasting or exercise), TAG stored in WAT are hydrolyzed releasing FFA and glycerol into the bloodstream, that can be used by other tissues as an energy source [76, 77]. Being a highly hydrophilic molecule, glycerol diffusion across tissues’ membranes is facilitated through protein channels that allow its rapid transfer and promote its availability for intracellular metabolism. In this regard, the members of the aquaglyceroporin subfamily have been recognized as the main membrane channels involved in glycerol permeation. Regulation of glycerol fluxes via aquaglyceroporins plays a key role in the control of metabolic processes and energy homeostasis [74, 78, 79]. A list of the aquaglyceroporins expressed in organs and tissues involved in energy homeostasis and their implication in glycerol fluxes is presented in Table 1.
Table 1.
Expression of aquaglyceroporins involved in energy homeostasis by organ/tissue
| Organ/tissue | Aquaglyceroporin | Role | References |
|---|---|---|---|
| Adipose tissue | AQP3 | Glycerol metabolism | [74] |
| AQP7 | Main glycerol transporter; control glycerol uptake and release | [72, 80–83] | |
| AQP9 | Glycerol influx | [74] | |
| AQP10 | Maintain normal glycerol levels in blood | [82] | |
| AQP11 | Mediate intracellular glycerol movements | [84] | |
| Muscle | AQP3 | Glycerol transport for energy production in skeletal muscle | [75, 85, 86] |
| AQP7 | Glycerol transport for energy production mainly in cardiac muscle | [75, 85–87] | |
| Liver | AQP9 | Glycerol uptake for glucose production | [88] |
| Endocrine pancreas | AQP7 | Involvement in insulin exocytosis | [89, 90] |
AQP7 is the main glycerol gateway in adipocytes
Adipose tissue constitutes the most important source of glycerol in the body. AQP7 is the most representative glycerol channel and the first identified in human and rodents adipose tissue [91–93], adipocytes [80, 81] and adipose endothelial cells [82, 94–96]. Nevertheless, alternative glycerol pathways have also been detected in human adipocytes, such as AQP3 [74, 81, 97], AQP9 [98], AQP10 [82] and AQP11 [84].
The fundamental role of AQP7 in facilitating glycerol efflux from the adipocyte into the bloodstream was achieved after its characterization as a glycerol channel [80]. Obese insulin-resistant db+/db+ mice showed higher AQP7 expression [80], and similarly, an increase in AQP7 mRNA was also observed in adipose tissue of a rodent model of T2D with obesity [99]. This suggests that AQP7 dysregulation may lead to an augmented input of glycerol for hepatic gluconeogenesis, and consequently, to increased glucose in the bloodstream, in T2D [91].
Furthermore, AQP7 knockout mice showed adipocyte hypertrophy and early obesity onset due to the accumulation of glycerol, which stimulates GK, leading to TAG accumulation [72, 83]. Controversially, susceptibility for obesity was not observed in other AQP7 knockout mice [96, 100]. Despite the different phenotypes described in distinct AQP7 knockout mice, all confirmed the involvement of AQP7 in glycerol metabolism. Moreover, although no obvious correlation was found between adipose AQP7 expression/glycerol metabolism and related metabolic complications in humans [101], genome-wide analysis found AQP7 gene linked to T2D [102], MetS [103] and obesity, the latter just for female participants [104]. Gender differences concerning the role of AQP7 in adipose tissue metabolism have also been reported, where higher fasting circulating levels of glycerol have been found in women comparing to men. Obese women showed higher AQP7 expression than men in both subcutaneous and visceral adipose tissue, suggesting an association between impaired AQP7 expression and obesity, at least in women [95, 105].
AQP7 gene expression is upregulated in mice and human in the fasting state or during exercise, whereas during the feeding state it is downregulated and its abundance is inversely related with plasma insulin levels [80]. Transcription of AQP7 gene is inhibited by the increase of plasma insulin levels through a negative insulin response element (IRE) located in the promoter region of AQP7 gene [106, 107] and by blockage of the phosphatidylinositol-3 kinase (PI3K) pathway [98, 107]. On the other hand, peroxisome proliferator-activated receptor gamma (PPARɣ) was demonstrated to upregulate AQP7 [79, 101, 107]. PPARɣ is also essential for adipocyte differentiation by regulating the transcription of several adipose genes, including AQP7, and, in differentiating adipocytes, an increase in AQP7 expression along with adipocyte differentiation was observed, suggesting a cell differentiation-dependent regulation [80, 108]. Indeed, thiazolidinediones (synthetic PPARɣ) and insulin sensitizers were reported to upregulate AQP7 [99, 106, 107], whereas insulin resistance inducers such as leptin [98, 105], TNF-α, adrenergic agonists and steroids, downregulate AQP7 expression [109]. Lipogenic hormones, such as ghrelin, also control AQP7 regulation, decreasing its expression while promoting TAG accumulation [110].
During fasting, low plasma insulin levels and catecholamine induce AQP7 gene transcription and AQP7 translocation to the plasma membrane, promoting glycerol release from adipocytes [80]. A recent study in human primary adipocytes proposes a molecular mechanism explaining how glycerol release is controlled in adipocytes by interaction of AQP7 with perilipin 1 (PLIN1) and protein kinase A (PKA). In lipogenic conditions, AQP7 and PLIN1 physically interact in the adipocyte, whereas in the lipolytic state, catecholamine-activated PKA phosphorylation of the N terminus of AQP7 reduces the complexation favoring AQP7 translocation to the plasma membrane [111].
Studies with obese individuals showed a differential regulation of AQP7 depending on the type of adipose depot, subcutaneous or visceral; low AQP7 expression was detected in subcutaneous fat leading to fat accumulation and adipocyte hypertrophy. In contrast, increased AQP7 levels were observed in visceral fat, which can be correlated with increased lipolysis [74, 79]. Knowing that subcutaneous adipose tissue is more insulin sensitive than visceral adipose tissue, AQP7 downregulation might represent a mechanism of protection attempting to prevent lipid depletion and consequent lipotoxicity to peripheral organs [79].
AQP9 facilitates glycerol uptake in the liver for gluconeogenesis and TAG synthesis
Glycerol has a central role in the equilibrium of hepatic gluconeogenesis and lipid accumulation in adipose tissue. In the liver, AQP9 is the channel responsible for glycerol influx both in the fed or fasting states [74, 88, 112] where it is converted to G3P used for gluconeogenesis and synthesis of TAG [98].
In addition to AQP9 expressed in the basolateral sinusoidal membrane and AQP11 in the endoplasmic reticulum, aquaglyceroporins AQP3 and AQP7 were also found, but their exact location and physiological roles are still unclear [74, 98, 113, 114].
The role of AQP9 as the major glycerol channel in the liver and its implication in glycerol metabolism was elucidated through experiments with AQP9-knockout mice [88] that showed increased plasma glycerol and TAG due to impaired glycerol metabolism. In healthy conditions, during the feeding state, insulin leads to a reduction in lipolysis and AQP7 downregulation that results in impaired glycerol release from adipose tissue, and parallel liver AQP9 downregulation and impaired glycerol uptake, a coordinated regulation mechanism able to prevent liver gluconeogenesis [115, 116]. Liver AQP9 appears to be downregulated only in male rodents, whereas in female, downregulation is prevented by estrogen [117]. Gender-specific differences in glycerol metabolism were also reported in healthy humans probably due to hormonal regulation of adipose and liver aquaglyceroporins, which may impact on the relative risk of metabolic diseases in men and women [105].
The role of AQP9 in fat accumulation liver disorders was investigated [116, 118–120]. Using a non-alcoholic fatty liver disease (NAFLD) cell model, expression of AQP9 led to steatosis due to increase of intracellular TAG, FFA and glycerol contents and importantly, steatosis was reversed by AQP9 suppression [120]. Although correlation between hepatic AQP9 expression and steatosis in obese patients could not be found [121], downregulation of hepatic AQP9 along with NAFLD or non-alcoholic steatohepatitis was reported in obese patients with IR or diabetes, suggesting a compensatory mechanism to avoid TAG, FFAs and glycerol accumulation and gluconeogenesis [119]. Further investigation is needed to clarify the role of AQP9 in liver metabolic homeostasis and fat liver disease.
AQP3 and AQP7 transport glycerol for energy production in muscle
In mammalian cardiac and skeletal muscle, aquaglyceroporins facilitate glycerol uptake for energy production [75, 87]. As an energetic substrate, glycerol is dispensable under physiological conditions but becomes important in the stressed heart [75]. Expression of AQP1, 3, 4 and 7 was found in cardiac muscle [75, 85, 86], where AQP1 and 4 are involved in water permeation and AQP3 and 7 have a role in glycerol transport particularly for energy production [85]. While AQP3 mRNA was identified in human and rat heart tissues, AQP3 protein was weakly expressed or absent in human cardiomyocytes [75, 86, 122].
So far, AQP7 is the only aquaglyceroporin known to be involved in glycerol permeation in mammalian heart muscle, confined to myocytes and fibroblasts [75, 86, 96, 123]. AQP7-knockout mice showed lower glycerol and ATP contents in cardiomyocytes and cardiac muscle along with lower glycerol uptake, and in pressure overload condition, developed excessive cardiac hypertrophy with higher mortality [75]. These results supported AQP7 involvement in cardiac muscle glycerol uptake as well as glycerol relevance as a source for cardiac energy [75, 87].
The preferred metabolic substrate for heart muscle energy in diabetes mellitus is glucose instead of FFA and glycerol, due to activation of the genes responsible for glucose oxidation and downregulation of genes related with FFA oxidation [124, 125]. The increased levels of blood glycerol and its continuous removal, mainly via AQP7, as well as the fact that it is not being used for oxidative phosphorylation, may explain the high TAG accumulation and storage inside myocytes.
A few AQPs were identified in mammalian skeletal muscle. AQP3 was first described in rat skeletal muscle [126] and its expression was confirmed in human [122, 127]. Along with AQP4, AQP3 was suggested to participate in the maintenance of water homeostasis in myofibers of skeletal tissue [128]. AQP9 was found weakly expressed in mice skeletal muscle but its role has not been defined [129]. AQP7 was found expressed in human and mice skeletal muscle [127, 130]; however, a later study described its location in capillaries instead of in sarcolemma of skeletal muscle fibers [96]. Although the functional role of AQP7 in human skeletal muscle is still not clear, the fact that insulin decreases its expression in mice adipose tissue in parallel with TAG content in muscle [131] supports AQP7 glycerol channeling as a mechanism involved in skeletal muscle metabolic processes.
AQP7 is implicated in the signaling cascade for insulin exocytosis
Pancreatic β-cells are responsible for synthesizing, storing and secreting insulin in response to an increase in blood nutrients such as glucose, amino acids and FFAs. It was demonstrated that a raise in blood glucose induces pancreatic β-cell swelling, activates volume-regulated anion channels (VRAC) [132]. The subsequent chloride efflux and cell membrane depolarization opens voltage-sensitive calcium channels allowing calcium influx and triggering a signalization cascade that results in insulin secretion [89, 90, 133, 134].
Due to the basal low intracellular GK concentration, glycerol is not likely to directly induce insulin secretion. However, similar to the effect of glucose, pancreatic islets confronted to an isosmotic or hyperosmotic solution of glycerol undergo cell swelling, activation of VRAC and depolarization with subsequent insulin release [89].
The putative role of glycerol and aquaporin expression in regulation of insulin secretion was intensively studied. AQP7 is the main glycerol channel in the endocrine pancreas and mediates the rapid entry of extracellular glycerol into β-cells [100, 135]. Insulin secretion was impaired in pancreatic islets from AQP7 knockout mice by the addition of glycerol or increasing d-glucose concentrations. Exposure of β-cells to hypotonic or hypertonic medium caused a similar degree of swelling and comparable pattern of electrical activity in cells from wild type and AQP7 knockout mice. However, when glycerol was added to the extracellular medium these events were only reduced in AQP7 knockout cells [90]. On the other hand, AQP7 knockout mice exhibit increased intra-islet glycerol and TG content with a concomitant increase of GK activity, increased pancreatic insulin mRNA levels and hyperinsulinemia [100], suggesting that AQP7 reduction favors intracellular glycerol accumulation for TAG biosynthesis, insulin biosynthesis and secretion and may play an important role in the exocytosis pathway of insulin [134].
Recently, AQP12 was also found expressed in β-cells of the Langerhans islets, both in samples of rat pancreas and in RIN-m5F β-cells, a widely used cell line based on its high insulin secretion rate [136]. However, AQP12 expression was not correlated with TAG accumulation or with insulin release [136].
Endothelial aquaporins: the gateway between bloodstream and adjacent tissues
The endothelium, a confluent layer of endothelial cells, is the biological gateway between the blood and organs, and is the source of many factors that are critical to health and disease. In particular, glycerol obtained from dietary fat or from adipocyte TAG hydrolysis must cross endothelial membranes to reach the tissues where it will be processed. It has been proposed that aquaglyceroporins expressed in endothelia might be involved in glycerol transmembrane flow. AQP7 was found in mice capillary endothelia of adipose tissue (and cardiac and striated muscle) and was upregulated in streptozotocin-induced diabetes mellitus [96], suggesting that insulin effect on adipose tissue also involves the control of glycerol efflux out of endothelial microvessels. Accordingly, human AQP7 was found localized at the plasma membrane of capillary endothelial cells and adjacent adipocytes [97], and may represent the main route for endothelial transmembrane glycerol fluxes. However, our recent study in human endothelial primary cells (HUVEC) revealed AQP3 as the predominant aquaglyceroporin accounting for glycerol fluxes in these cells, suggesting that AQP3 is also an important glycerol gateway between bloodstream and adjacent tissues [137].
Impaired glycerol fluxes in the onset of MetS
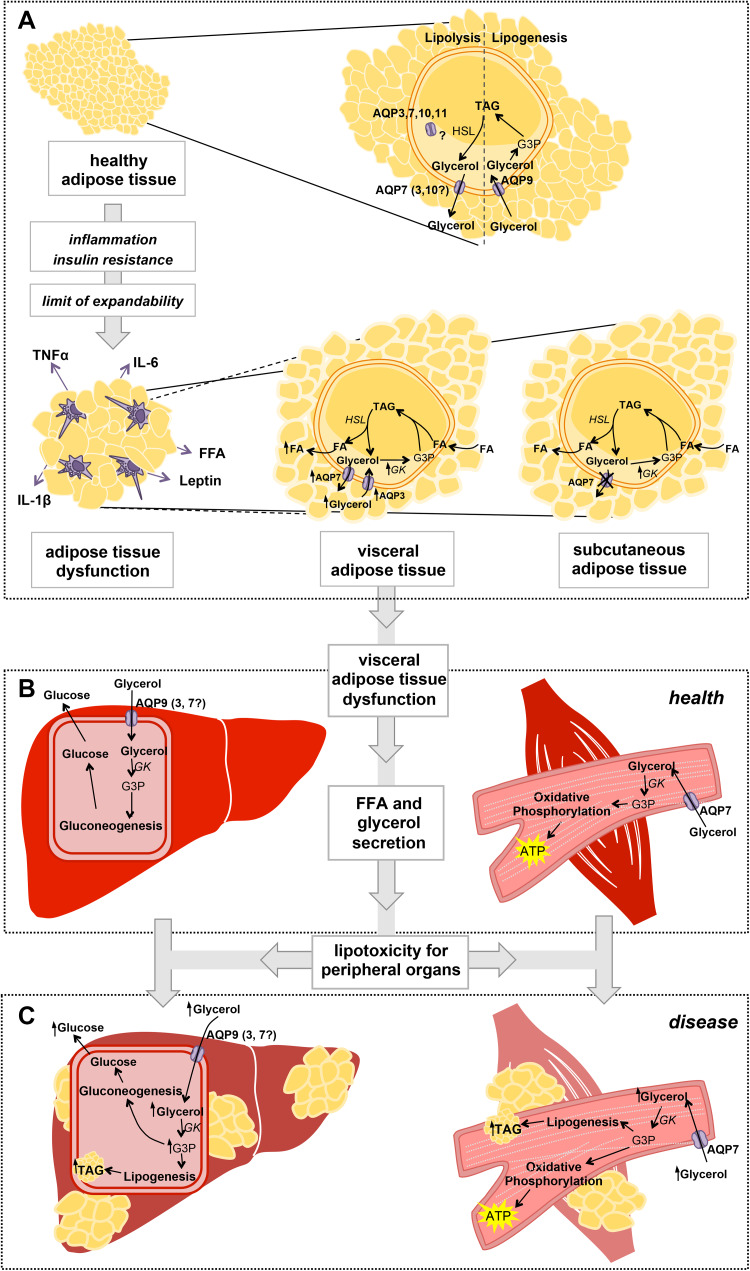
The involvement of aquaglyceroporins in adipose tissue dysfunction and consequent lipotoxicity-induced complications has been in debate in the last years. Putative mechanisms for the role of aquaglyceroporins in the onset of the metabolic syndrome include their altered expression and coordinated regulation in the tissues involved, such as adipose tissue, liver and muscle. A possible mechanism in which impaired glycerol fluxes and metabolism are key factors to the development of MetS is illustrated in Fig. 4. In the adipose tissue, during fasting, glucagon promotes lipolysis with glycerol release into the bloodstream via AQP7, 3 and 10, and in response to feeding insulin promotes lipogenesis with storage of glycerol as TAG. The balance of lipolysis and lipogenesis is crucial to maintain a healthy adipose reservoir. However, in case of high food intake and sedentary lifestyle when body energetic demands are largely overcome, adipose tissue TAG storage reaches its limit of expandability and lipotoxicity occurs. Overproduction of proinflammatory adipokines such as TNFα, IL-6, IL-1β and leptin, and reduced production of anti-inflammatory and insulin sensitizing adipokines lead to adipose tissue dysfunction [26, 138], affecting aquaglyceroporin regulation differently in subcutaneous and visceral fat. In subcutaneous adipose tissue, AQP7 downregulation and impairment of glycerol release leads to fat accumulation. In visceral adipose tissue, AQP7 and AQP3 are upregulated allowing increased effluxes of glycerol and FFA, hormones and proinflammatory cytokines to the portal vein to be delivered to the liver, where they interact with hepatocytes and various immune cells such as Kupffer cells [139]. In the liver, excessive glycerol levels imported via AQP9 will be used for de novo lipogenesis in addition to gluconeogenesis. High glycerol in the blood has also a lipotoxic effect in muscle. In the myocyte, glycerol imported via AQP7 is an energy source through oxidative phosphorylation but in case glycerol is increased in the blood, it is also used for TAG synthesis. Accumulation of TAG in liver and muscle, which are not appropriate fat storage depots, leads to cell dysfunction and failure of their tissue-specific functions.
Fig. 4.
Involvement of aquaglyceroporins in adipose tissue dysfunction and lipotoxicity in liver and muscle. a In healthy conditions, during fasting when blood nutrient levels are low, TAG hydrolysis in the adipocytes yields FFA and glycerol that is released to the blood stream mainly via AQP7. In the feeding state, when plasma glycerol reaches high concentrations, glycerol is taken up by adipocytes possibly via AQP9, and is converted to TAG that is stored in lipid droplets. When adipose tissue expandability reaches its limits, macrophage infiltration and activation by secretion of proinflammatory molecules (TNFα, IL-1β, IL-6, FFA, leptin) prompt inflammation, loss of insulin sensitivity and adipose tissue dysfunction. In disease, AQP7 downregulation in the subcutaneous adipose tissue results in increase in intracellular glycerol supply for TAG synthesis, which accumulates in lipid droplets with consequent adipocyte hypertrophy. When adipocytes reach their limit of fat accumulation, upregulation of AQP3 and AQP7 in the visceral adipose tissue facilitates the efflux of glycerol into the bloodstream that together with high levels of FFA trigger lipotoxicity in peripheral organs. b In healthy conditions, blood glycerol is taken up by AQP9 in the liver (and possibly by AQP3 and 7) expressed in the basolateral sinusoidal membrane of hepatocytes, and is converted in G3P to be used in gluconeogenesis. In cardiac and skeletal muscle, glycerol is taken up by AQP7 and it is used to generate reductive powder in oxidative phosphorylation for ATP production. c In disease, higher blood glycerol levels stimulate a rise in intracellular G3P in the liver and muscle that can be used for de novo lipogenesis with resultant intracellular TAG accumulation and lipotoxicity. FFA free fatty acids, G3P glycerol-3-phosphate, GK glycerol kinase, TAG triacylglycerols
Final remarks
MetS is a complex disorder associated with obesity, IR and hypertension with strong risk factor for cardiovascular disease. The pathophysiological mechanisms of the disease involve glucose and lipid metabolism, insulin action, and several proinflammatory and anti-adipogenic cytokines. Although highly prevalent, MetS remains under-diagnosed and undertreated. Besides dramatic changes in lifestyle and eating habits, therapeutic approaches to MetS treatment are targeted to individual components of the syndrome rather than to the complex disease pathogenesis [140]. New advanced therapeutic strategies and drug targets should be paramount.
Interestingly, glycerol is a key molecule for metabolic reactions in cells that are involved in energy balance and fat metabolism. By facilitating glycerol permeation through cell membranes and establishing a glycerol network between different tissues and organs, aquaglyceroporins are emerging as key players in lipid balance and energy homeostasis. In fact, these membrane channels represent routes for glycerol absorption and reabsorption in the intestine and kidney, for glycerol efflux in adipose tissue during fasting, and for glycerol uptake in the liver for gluconeogenesis. Circulating plasma glycerol is also channeled by aquaglyceroporins to be used as fuel by the heart and skeletal muscle. Aquaglyceroporins in the capillaries may function as channels modulating glycerol delivery to the whole organism.
Broad range of evidence indicates that AQPs can be important therapeutic targets and that their modulation can be used for treatment of several pathologies [69, 70]. Due to their crucial role in maintaining the control of fat accumulation in adipose tissue and liver, as well as whole-body glucose homeostasis, aquaglyceroporins may indeed represent potential therapeutic targets in the management of obesity, T2D and associated metabolic disorders such as MetS.
Acknowledgements
Financial support from Fundação para a Ciência e a Tecnologia (FCT-MEC, Portugal) through PhD fellowship to I. V. S. (PD/BD/113634/2015) and iMed.ULisboa (UID/DTP/04138/2013).
Abbreviations
- AQPs
Aquaporins
- ATP/ADP
Adenosine triphosphate/adenosine diphosphate
- BAT
Brown adipose tissue
- cAMP
Cyclic adenosine monophosphate
- FFA
Free fatty acids
- GK
Glycerol kinase
- IR
Insulin resistance
- MetS
Metabolic syndrome
- PLIN1
Perilipin 1
- PKA
Protein kinase A
- RAAS
Renin–angiotensin–aldosterone system
- TAG
Triacylglycerols
- T2D
Type 2 diabetes
- VLDL
Very-low-density lipoproteins
- VRAC
Volume-regulated anion channels
- WAT
White adipose tissue
Footnotes
Joana P. G. Miranda and Graça Soveral supervised this work.
References
- 1.Ford ES, Giles WH, Dietz WH. Prevalence of the metabolic syndrome among US adults: findings from the third National Health and Nutrition Examination Survey. JAMA. 2002;287(3):356–359. doi: 10.1001/jama.287.3.356. [DOI] [PubMed] [Google Scholar]
- 2.Eckel RH, Grundy SM, Zimmet PZ. The metabolic syndrome. Lancet. 2005;365(9468):1415–1428. doi: 10.1016/S0140-6736(05)66378-7. [DOI] [PubMed] [Google Scholar]
- 3.Reaven GM. Banting lecture 1988. Role of insulin resistance in human disease. Diabetes. 1988;37(12):1595–1607. doi: 10.2337/diab.37.12.1595. [DOI] [PubMed] [Google Scholar]
- 4.Reaven G. The metabolic syndrome or the insulin resistance syndrome? Different names, different concepts, and different goals. Endocrinol Metab Clin N Am. 2004;33(2):283–303. doi: 10.1016/j.ecl.2004.03.002. [DOI] [PubMed] [Google Scholar]
- 5.DeFronzo RA, Ferrannini E. Regulation of hepatic glucose metabolism in humans. Diabetes Metab Rev. 1987;3(2):415–459. doi: 10.1002/dmr.5610030204. [DOI] [PubMed] [Google Scholar]
- 6.Sparks JD, Sparks CE, Adeli K. Selective hepatic insulin resistance, VLDL overproduction, and hypertriglyceridemia. Arterioscler Thromb Vasc Biol. 2012;32(9):2104–2112. doi: 10.1161/ATVBAHA.111.241463. [DOI] [PubMed] [Google Scholar]
- 7.Armani A, Berry A, Cirulli F, Caprio M. Molecular mechanisms underlying metabolic syndrome: the expanding role of the adipocyte. FASEB J. 2017;31(10):4240–4255. doi: 10.1096/fj.201601125RRR. [DOI] [PubMed] [Google Scholar]
- 8.Miranda PJ, DeFronzo RA, Califf RM, Guyton JR. Metabolic syndrome: definition, pathophysiology, and mechanisms. Am Heart J. 2005;149(1):33–45. doi: 10.1016/j.ahj.2004.07.013. [DOI] [PubMed] [Google Scholar]
- 9.Hall JE, Guyton AC, Smith MJ, Jr, Coleman TG. Blood pressure and renal function during chronic changes in sodium intake: role of angiotensin. Am J Physiol. 1980;239(3):F271–F280. doi: 10.1152/ajprenal.1980.239.3.F271. [DOI] [PubMed] [Google Scholar]
- 10.Egan BM, Greene EL, Goodfriend TL. Insulin resistance and cardiovascular disease. Am J Hypertens. 2001;14(6 Pt 2):116S–125S. doi: 10.1016/S0895-7061(01)02078-7. [DOI] [PubMed] [Google Scholar]
- 11.Sowers JR. Insulin resistance and hypertension. Am J Physiol Heart Circ Physiol. 2004;286(5):H1597–H1602. doi: 10.1152/ajpheart.00026.2004. [DOI] [PubMed] [Google Scholar]
- 12.Horita S, Seki G, Yamada H, Suzuki M, Koike K, Fujita T. Insulin resistance, obesity, hypertension, and renal sodium transport. Int J Hypertens. 2011;2011:391762. doi: 10.4061/2011/391762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lowell BB, Flier JS. Brown adipose tissue, beta 3-adrenergic receptors, and obesity. Annu Rev Med. 1997;48:307–316. doi: 10.1146/annurev.med.48.1.307. [DOI] [PubMed] [Google Scholar]
- 14.Wu J, Bostrom P, Sparks LM, Ye L, Choi JH, Giang AH, Khandekar M, Virtanen KA, Nuutila P, Schaart G, Huang K, Tu H, van Marken Lichtenbelt WD, Hoeks J, Enerback S, Schrauwen P, Spiegelman BM. Beige adipocytes are a distinct type of thermogenic fat cell in mouse and human. Cell. 2012;150(2):366–376. doi: 10.1016/j.cell.2012.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Saely CH, Geiger K, Drexel H. Brown versus white adipose tissue: a mini-review. Gerontology. 2012;58(1):15–23. doi: 10.1159/000321319. [DOI] [PubMed] [Google Scholar]
- 16.Lefterova MI, Lazar MA. New developments in adipogenesis. Trends Endocrinol Metab TEM. 2009;20(3):107–114. doi: 10.1016/j.tem.2008.11.005. [DOI] [PubMed] [Google Scholar]
- 17.Hirsch J, Batchelor B. Adipose tissue cellularity in human obesity. Clin Endocrinol Metab. 1976;5(2):299–311. doi: 10.1016/S0300-595X(76)80023-0. [DOI] [PubMed] [Google Scholar]
- 18.Ryden M, Andersson DP, Bergstrom IB, Arner P. Adipose tissue and metabolic alterations: regional differences in fat cell size and number matter, but differently: a cross-sectional study. J Clin Endocrinol Metab. 2014;99(10):E1870–E1876. doi: 10.1210/jc.2014-1526. [DOI] [PubMed] [Google Scholar]
- 19.Muir LA, Neeley CK, Meyer KA, Baker NA, Brosius AM, Washabaugh AR, Varban OA, Finks JF, Zamarron BF, Flesher CG, Chang JS, DelProposto JB, Geletka L, Martinez-Santibanez G, Kaciroti N, Lumeng CN, O’Rourke RW. Adipose tissue fibrosis, hypertrophy, and hyperplasia: correlations with diabetes in human obesity. Obesity (Silver Spring) 2016;24(3):597–605. doi: 10.1002/oby.21377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Corvera S, Gealekman O. Adipose tissue angiogenesis: impact on obesity and type-2 diabetes. Biochem Biophys Acta. 2014;1842(3):463–472. doi: 10.1016/j.bbadis.2013.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tang W, Zeve D, Suh JM, Bosnakovski D, Kyba M, Hammer RE, Tallquist MD, Graff JM. White fat progenitor cells reside in the adipose vasculature. Science. 2008;322(5901):583–586. doi: 10.1126/science.1156232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rodriguez A, Ezquerro S, Mendez-Gimenez L, Becerril S, Fruhbeck G. Revisiting the adipocyte: a model for integration of cytokine signaling in the regulation of energy metabolism. Am J Physiol Endocrinol Metab. 2015;309(8):E691–E714. doi: 10.1152/ajpendo.00297.2015. [DOI] [PubMed] [Google Scholar]
- 23.Thomou T, Mori MA, Dreyfuss JM, Konishi M, Sakaguchi M, Wolfrum C, Rao TN, Winnay JN, Garcia-Martin R, Grinspoon SK, Gorden P, Kahn CR. Adipose-derived circulating miRNAs regulate gene expression in other tissues. Nature. 2017;542(7642):450–455. doi: 10.1038/nature21365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rodriguez A, Catalan V, Gomez-Ambrosi J, Fruhbeck G. Visceral and subcutaneous adiposity: are both potential therapeutic targets for tackling the metabolic syndrome? Curr Pharm Des. 2007;13(21):2169–2175. doi: 10.2174/138161207781039599. [DOI] [PubMed] [Google Scholar]
- 25.Ebbert JO, Jensen MD. Fat depots, free fatty acids, and dyslipidemia. Nutrients. 2013;5(2):498–508. doi: 10.3390/nu5020498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.da Silva IV, Soveral G. Aquaporins in obesity. Adv Exp Med Biol. 2017;969:227–238. doi: 10.1007/978-94-024-1057-0_15. [DOI] [PubMed] [Google Scholar]
- 27.Virtue S, Vidal-Puig A. Adipose tissue expandability, lipotoxicity and the metabolic syndrome—an allostatic perspective. Biochem Biophys Acta. 2010;1801(3):338–349. doi: 10.1016/j.bbalip.2009.12.006. [DOI] [PubMed] [Google Scholar]
- 28.Lara-Castro C, Garvey WT. Intracellular lipid accumulation in liver and muscle and the insulin resistance syndrome. Endocrinol Metab Clin N Am. 2008;37(4):841–856. doi: 10.1016/j.ecl.2008.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Krssak M, Roden M. The role of lipid accumulation in liver and muscle for insulin resistance and type 2 diabetes mellitus in humans. Rev Endocr Metab Disord. 2004;5(2):127–134. doi: 10.1023/B:REMD.0000021434.98627.dc. [DOI] [PubMed] [Google Scholar]
- 30.Grundy SM. Adipose tissue and metabolic syndrome: too much, too little or neither. Eur J Clin Investig. 2015;45(11):1209–1217. doi: 10.1111/eci.12519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Virtue S, Vidal-Puig A. It’s not how fat you are, it’s what you do with it that counts. PLoS Biol. 2008;6(9):e237. doi: 10.1371/journal.pbio.0060237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Romacho T, Elsen M, Rohrborn D, Eckel J. Adipose tissue and its role in organ crosstalk. Acta Physiol (Oxf) 2014;210(4):733–753. doi: 10.1111/apha.12246. [DOI] [PubMed] [Google Scholar]
- 33.Trayhurn P, Wood IS. Signalling role of adipose tissue: adipokines and inflammation in obesity. Biochem Soc Trans. 2005;33(Pt 5):1078–1081. doi: 10.1042/BST20051078. [DOI] [PubMed] [Google Scholar]
- 34.Mazzoleni G, Steimberg N. New models for the in vitro study of liver toxicity: 3D culture systems and the role of bioreactors. In: Tyshenko MG, editor. The Continuum of Health Risk Assessement. Croatia: InTechOpen; 2012. [Google Scholar]
- 35.Rui L. Energy metabolism in the liver. Compr Physiol. 2014;4(1):177–197. doi: 10.1002/cphy.c130024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Desvergne B, Michalik L, Wahli W. Transcriptional regulation of metabolism. Physiol Rev. 2006;86(2):465–514. doi: 10.1152/physrev.00025.2005. [DOI] [PubMed] [Google Scholar]
- 37.Jiang G, Zhang BB. Glucagon and regulation of glucose metabolism. Am J Physiol Endocrinol Metab. 2003;284(4):E671–E678. doi: 10.1152/ajpendo.00492.2002. [DOI] [PubMed] [Google Scholar]
- 38.Meshkani R, Adeli K. Hepatic insulin resistance, metabolic syndrome and cardiovascular disease. Clin Biochem. 2009;42(13–14):1331–1346. doi: 10.1016/j.clinbiochem.2009.05.018. [DOI] [PubMed] [Google Scholar]
- 39.Michael MD, Kulkarni RN, Postic C, Previs SF, Shulman GI, Magnuson MA, Kahn CR. Loss of insulin signaling in hepatocytes leads to severe insulin resistance and progressive hepatic dysfunction. Mol Cell. 2000;6(1):87–97. doi: 10.1016/S1097-2765(05)00015-8. [DOI] [PubMed] [Google Scholar]
- 40.Bruning JC, Michael MD, Winnay JN, Hayashi T, Horsch D, Accili D, Goodyear LJ, Kahn CR. A muscle-specific insulin receptor knockout exhibits features of the metabolic syndrome of NIDDM without altering glucose tolerance. Mol Cell. 1998;2(5):559–569. doi: 10.1016/S1097-2765(00)80155-0. [DOI] [PubMed] [Google Scholar]
- 41.Shimomura I, Matsuda M, Hammer RE, Bashmakov Y, Brown MS, Goldstein JL. Decreased IRS-2 and increased SREBP-1c lead to mixed insulin resistance and sensitivity in livers of lipodystrophic and ob/ob mice. Mol Cell. 2000;6(1):77–86. doi: 10.1016/S1097-2765(05)00010-9. [DOI] [PubMed] [Google Scholar]
- 42.Kissebah AH, Alfarsi S, Adams PW, Wynn V. Role of insulin resistance in adipose tissue and liver in the pathogenesis of endogenous hypertriglyceridaemia in man. Diabetologia. 1976;12(6):563–571. doi: 10.1007/BF01220632. [DOI] [PubMed] [Google Scholar]
- 43.Ehrenborg E, Krook A. Regulation of skeletal muscle physiology and metabolism by peroxisome proliferator-activated receptor delta. Pharmacol Rev. 2009;61(3):373–393. doi: 10.1124/pr.109.001560. [DOI] [PubMed] [Google Scholar]
- 44.DeFronzo RA, Ferrannini E, Sato Y, Felig P, Wahren J. Synergistic interaction between exercise and insulin on peripheral glucose uptake. J Clin Investig. 1981;68(6):1468–1474. doi: 10.1172/JCI110399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Shulman GI, Rothman DL, Jue T, Stein P, DeFronzo RA, Shulman RG. Quantitation of muscle glycogen synthesis in normal subjects and subjects with non-insulin-dependent diabetes by 13C nuclear magnetic resonance spectroscopy. N Engl J Med. 1990;322(4):223–228. doi: 10.1056/NEJM199001253220403. [DOI] [PubMed] [Google Scholar]
- 46.Jensen J, Rustad PI, Kolnes AJ, Lai YC. The role of skeletal muscle glycogen breakdown for regulation of insulin sensitivity by exercise. Front Physiol. 2011;2:112. doi: 10.3389/fphys.2011.00112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Homsher E. Muscle enthalpy production and its relationship to actomyosin ATPase. Annu Rev Physiol. 1987;49:673–690. doi: 10.1146/annurev.ph.49.030187.003325. [DOI] [PubMed] [Google Scholar]
- 48.de Luca C, Olefsky JM. Inflammation and insulin resistance. FEBS Lett. 2008;582(1):97–105. doi: 10.1016/j.febslet.2007.11.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Abdul-Ghani MA, DeFronzo RA. Pathogenesis of insulin resistance in skeletal muscle. J Biomed Biotechnol. 2010;2010:476279. doi: 10.1155/2010/476279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yu C, Chen Y, Cline GW, Zhang D, Zong H, Wang Y, Bergeron R, Kim JK, Cushman SW, Cooney GJ, Atcheson B, White MF, Kraegen EW, Shulman GI. Mechanism by which fatty acids inhibit insulin activation of insulin receptor substrate-1 (IRS-1)-associated phosphatidylinositol 3-kinase activity in muscle. J Biol Chem. 2002;277(52):50230–50236. doi: 10.1074/jbc.M200958200. [DOI] [PubMed] [Google Scholar]
- 51.DeFronzo RA, Gunnarsson R, Bjorkman O, Olsson M, Wahren J. Effects of insulin on peripheral and splanchnic glucose metabolism in noninsulin-dependent (type II) diabetes mellitus. J Clin Investig. 1985;76(1):149–155. doi: 10.1172/JCI111938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kelley DE, Goodpaster B, Wing RR, Simoneau JA. Skeletal muscle fatty acid metabolism in association with insulin resistance, obesity, and weight loss. Am J Physiol. 1999;277(6 Pt 1):E1130–E1141. doi: 10.1152/ajpendo.1999.277.6.E1130. [DOI] [PubMed] [Google Scholar]
- 53.Szendroedi J, Schmid AI, Chmelik M, Toth C, Brehm A, Krssak M, Nowotny P, Wolzt M, Waldhausl W, Roden M. Muscle mitochondrial ATP synthesis and glucose transport/phosphorylation in type 2 diabetes. PLoS Med. 2007;4(5):e154. doi: 10.1371/journal.pmed.0040154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Mogensen M, Sahlin K, Fernstrom M, Glintborg D, Vind BF, Beck-Nielsen H, Hojlund K. Mitochondrial respiration is decreased in skeletal muscle of patients with type 2 diabetes. Diabetes. 2007;56(6):1592–1599. doi: 10.2337/db06-0981. [DOI] [PubMed] [Google Scholar]
- 55.King LS, Kozono D, Agre P. From structure to disease: the evolving tale of aquaporin biology. Nat Rev Mol Cell Biol. 2004;5(9):687–698. doi: 10.1038/nrm1469. [DOI] [PubMed] [Google Scholar]
- 56.Agre P. Aquaporin water channels (Nobel Lecture) Angew Chem Int Ed Engl. 2004;43(33):4278–4290. doi: 10.1002/anie.200460804. [DOI] [PubMed] [Google Scholar]
- 57.Carbrey JM, Agre P. Discovery of the aquaporins and development of the field. Handb Exp Pharmacol. 2009;190:3–28. doi: 10.1007/978-3-540-79885-9_1. [DOI] [PubMed] [Google Scholar]
- 58.Wang F, Feng XC, Li YM, Yang H, Ma TH. Aquaporins as potential drug targets. Acta Pharmacol Sin. 2006;27(4):395–401. doi: 10.1111/j.1745-7254.2006.00318.x. [DOI] [PubMed] [Google Scholar]
- 59.Magni F, Sarto C, Ticozzi D, Soldi M, Bosso N, Mocarelli P, Kienle MG. Proteomic knowledge of human aquaporins. Proteomics. 2006;6(20):5637–5649. doi: 10.1002/pmic.200600212. [DOI] [PubMed] [Google Scholar]
- 60.Takata K, Matsuzaki T, Tajika Y. Aquaporins: water channel proteins of the cell membrane. Prog Histochem Cytochem. 2004;39(1):1–83. doi: 10.1016/j.proghi.2004.03.001. [DOI] [PubMed] [Google Scholar]
- 61.Ishibashi K, Tanaka Y, Morishita Y. The role of mammalian superaquaporins inside the cell. Biochem Biophys Acta. 2014;1840(5):1507–1512. doi: 10.1016/j.bbagen.2013.10.039. [DOI] [PubMed] [Google Scholar]
- 62.Eriksson UK, Fischer G, Friemann R, Enkavi G, Tajkhorshid E, Neutze R. Subangstrom resolution X-ray structure details aquaporin–water interactions. Science. 2013;340(6138):1346–1349. doi: 10.1126/science.1234306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Sui H, Han BG, Lee JK, Walian P, Jap BK. Structural basis of water-specific transport through the AQP1 water channel. Nature. 2001;414(6866):872–878. doi: 10.1038/414872a. [DOI] [PubMed] [Google Scholar]
- 64.Wu B, Steinbronn C, Alsterfjord M, Zeuthen T, Beitz E. Concerted action of two cation filters in the aquaporin water channel. EMBO J. 2009;28(15):2188–2194. doi: 10.1038/emboj.2009.182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.de Groot BL, Frigato T, Helms V, Grubmuller H. The mechanism of proton exclusion in the aquaporin-1 water channel. J Mol Biol. 2003;333(2):279–293. doi: 10.1016/j.jmb.2003.08.003. [DOI] [PubMed] [Google Scholar]
- 66.Verkman AS. Aquaporins at a glance. J Cell Sci. 2011;124(Pt 13):2107–2112. doi: 10.1242/jcs.079467. [DOI] [PubMed] [Google Scholar]
- 67.Verkman AS, Anderson MO, Papadopoulos MC. Aquaporins: important but elusive drug targets. Nat Rev Drug Discov. 2014;13(4):259–277. doi: 10.1038/nrd4226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Beitz E, Golldack A, Rothert M, von Bulow J. Challenges and achievements in the therapeutic modulation of aquaporin functionality. Pharmacol Ther. 2015;155:22–35. doi: 10.1016/j.pharmthera.2015.08.002. [DOI] [PubMed] [Google Scholar]
- 69.Soveral G, Nielsen S, Casini A. Aquaporins in health and disease: new molecular targets for drug discovery. Boca Raton: CRC Press, Taylor & Francis Group; 2016. [Google Scholar]
- 70.Soveral G, Casini A. Aquaporin modulators: a patent review (2010–2015) Expert Opin Ther Pat. 2017;27(1):49–62. doi: 10.1080/13543776.2017.1236085. [DOI] [PubMed] [Google Scholar]
- 71.Ma T, Hara M, Sougrat R, Verbavatz JM, Verkman AS. Impaired stratum corneum hydration in mice lacking epidermal water channel aquaporin-3. J Biol Chem. 2002;277(19):17147–17153. doi: 10.1074/jbc.M200925200. [DOI] [PubMed] [Google Scholar]
- 72.Hibuse T, Maeda N, Funahashi T, Yamamoto K, Nagasawa A, Mizunoya W, Kishida K, Inoue K, Kuriyama H, Nakamura T, Fushiki T, Kihara S, Shimomura I. Aquaporin 7 deficiency is associated with development of obesity through activation of adipose glycerol kinase. Proc Natl Acad Sci USA. 2005;102(31):10993–10998. doi: 10.1073/pnas.0503291102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Brisson D, Vohl MC, St-Pierre J, Hudson TJ, Gaudet D. Glycerol: a neglected variable in metabolic processes? BioEssays News Rev Mol Cell Dev Biol. 2001;23(6):534–542. doi: 10.1002/bies.1073. [DOI] [PubMed] [Google Scholar]
- 74.Rodriguez A, Catalan V, Gomez-Ambrosi J, Fruhbeck G. Aquaglyceroporins serve as metabolic gateways in adiposity and insulin resistance control. Cell Cycle. 2011;10(10):1548–1556. doi: 10.4161/cc.10.10.15672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Hibuse T, Maeda N, Nakatsuji H, Tochino Y, Fujita K, Kihara S, Funahashi T, Shimomura I. The heart requires glycerol as an energy substrate through aquaporin 7, a glycerol facilitator. Cardiovasc Res. 2009;83(1):34–41. doi: 10.1093/cvr/cvp095. [DOI] [PubMed] [Google Scholar]
- 76.Duncan RE, Ahmadian M, Jaworski K, Sarkadi-Nagy E, Sul HS. Regulation of lipolysis in adipocytes. Annu Rev Nutr. 2007;27:79–101. doi: 10.1146/annurev.nutr.27.061406.093734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Fruhbeck G, Mendez-Gimenez L, Fernandez-Formoso JA, Fernandez S, Rodriguez A. Regulation of adipocyte lipolysis. Nutr Res Rev. 2014;27(1):63–93. doi: 10.1017/S095442241400002X. [DOI] [PubMed] [Google Scholar]
- 78.Hibuse T, Maeda N, Nagasawa A, Funahashi T. Aquaporins and glycerol metabolism. Biochem Biophys Acta. 2006;1758(8):1004–1011. doi: 10.1016/j.bbamem.2006.01.008. [DOI] [PubMed] [Google Scholar]
- 79.Madeira A, Moura TF, Soveral G. Aquaglyceroporins: implications in adipose biology and obesity. Cell Mol Life Sci. 2015;72(4):759–771. doi: 10.1007/s00018-014-1773-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Kishida K, Kuriyama H, Funahashi T, Shimomura I, Kihara S, Ouchi N, Nishida M, Nishizawa H, Matsuda M, Takahashi M, Hotta K, Nakamura T, Yamashita S, Tochino Y, Matsuzawa Y. Aquaporin adipose, a putative glycerol channel in adipocytes. J Biol Chem. 2000;275(27):20896–20902. doi: 10.1074/jbc.M001119200. [DOI] [PubMed] [Google Scholar]
- 81.Miranda M, Escote X, Ceperuelo-Mallafre V, Alcaide MJ, Simon I, Vilarrasa N, Wabitsch M, Vendrell J. Paired subcutaneous and visceral adipose tissue aquaporin-7 expression in human obesity and type 2 diabetes: differences and similarities between depots. J Clin Endocrinol Metab. 2010;95(7):3470–3479. doi: 10.1210/jc.2009-2655. [DOI] [PubMed] [Google Scholar]
- 82.Laforenza U, Scaffino MF, Gastaldi G. Aquaporin-10 represents an alternative pathway for glycerol efflux from human adipocytes. PLoS One. 2013;8(1):e54474. doi: 10.1371/journal.pone.0054474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Hara-Chikuma M, Sohara E, Rai T, Ikawa M, Okabe M, Sasaki S, Uchida S, Verkman AS. Progressive adipocyte hypertrophy in aquaporin-7-deficient mice: adipocyte glycerol permeability as a novel regulator of fat accumulation. J Biol Chem. 2005;280(16):15493–15496. doi: 10.1074/jbc.C500028200. [DOI] [PubMed] [Google Scholar]
- 84.Madeira A, Fernandez-Veledo S, Camps M, Zorzano A, Moura TF, Ceperuelo-Mallafre V, Vendrell J, Soveral G. Human aquaporin-11 is a water and glycerol channel and localizes in the vicinity of lipid droplets in human adipocytes. Obesity (Silver Spring) 2014;22(9):2010–2017. doi: 10.1002/oby.20792. [DOI] [PubMed] [Google Scholar]
- 85.Rutkovskiy A, Valen G, Vaage J. Cardiac aquaporins. Basic Res Cardiol. 2013;108(6):393. doi: 10.1007/s00395-013-0393-6. [DOI] [PubMed] [Google Scholar]
- 86.Butler TL, Au CG, Yang B, Egan JR, Tan YM, Hardeman EC, North KN, Verkman AS, Winlaw DS. Cardiac aquaporin expression in humans, rats, and mice. Am J Physiol Heart Circ Physiol. 2006;291(2):H705–H713. doi: 10.1152/ajpheart.00090.2006. [DOI] [PubMed] [Google Scholar]
- 87.Gladka M, El Azzouzi H, De Windt LJ, da Costa Martins PA. Aquaporin 7: the glycerol aquaeductus in the heart. Cardiovasc Res. 2009;83(1):3–4. doi: 10.1093/cvr/cvp147. [DOI] [PubMed] [Google Scholar]
- 88.Rojek AM, Skowronski MT, Fuchtbauer EM, Fuchtbauer AC, Fenton RA, Agre P, Frokiaer J, Nielsen S. Defective glycerol metabolism in aquaporin 9 (AQP9) knockout mice. Proc Natl Acad Sci USA. 2007;104(9):3609–3614. doi: 10.1073/pnas.0610894104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Best L, Brown PD, Yates AP, Perret J, Virreira M, Beauwens R, Malaisse WJ, Sener A, Delporte C. Contrasting effects of glycerol and urea transport on rat pancreatic beta-cell function. Cell Physiol Biochem. 2009;23(4–6):255–264. doi: 10.1159/000218172. [DOI] [PubMed] [Google Scholar]
- 90.Louchami K, Best L, Brown P, Virreira M, Hupkens E, Perret J, Devuyst O, Uchida S, Delporte C, Malaisse WJ, Beauwens R, Sener A. A new role for aquaporin 7 in insulin secretion. Cell Physiol Biochem. 2012;29(1–2):65–74. doi: 10.1159/000337588. [DOI] [PubMed] [Google Scholar]
- 91.Rojek A, Praetorius J, Frokiaer J, Nielsen S, Fenton RA. A current view of the mammalian aquaglyceroporins. Annu Rev Physiol. 2008;70:301–327. doi: 10.1146/annurev.physiol.70.113006.100452. [DOI] [PubMed] [Google Scholar]
- 92.Kuriyama H, Kawamoto S, Ishida N, Ohno I, Mita S, Matsuzawa Y, Matsubara K, Okubo K. Molecular cloning and expression of a novel human aquaporin from adipose tissue with glycerol permeability. Biochem Biophys Res Commun. 1997;241(1):53–58. doi: 10.1006/bbrc.1997.7769. [DOI] [PubMed] [Google Scholar]
- 93.Ishibashi K, Yamauchi K, Kageyama Y, Saito-Ohara F, Ikeuchi T, Marumo F, Sasaki S. Molecular characterization of human aquaporin-7 gene and its chromosomal mapping. Biochem Biophys Acta. 1998;1399(1):62–66. doi: 10.1016/s0167-4781(98)00094-3. [DOI] [PubMed] [Google Scholar]
- 94.Miyauchi T, Yamamoto H, Abe Y, Yoshida GJ, Rojek A, Sohara E, Uchida S, Nielsen S, Yasui M. Dynamic subcellular localization of aquaporin-7 in white adipocytes. FEBS Lett. 2015;589(5):608–614. doi: 10.1016/j.febslet.2015.01.025. [DOI] [PubMed] [Google Scholar]
- 95.Lebeck J, Ostergard T, Rojek A, Fuchtbauer EM, Lund S, Nielsen S, Praetorius J. Gender-specific effect of physical training on AQP7 protein expression in human adipose tissue. Acta Diabetol. 2012;49(Suppl 1):S215–S226. doi: 10.1007/s00592-012-0430-1. [DOI] [PubMed] [Google Scholar]
- 96.Skowronski MT, Lebeck J, Rojek A, Praetorius J, Fuchtbauer EM, Frokiaer J, Nielsen S. AQP7 is localized in capillaries of adipose tissue, cardiac and striated muscle: implications in glycerol metabolism. Am J Physiol Renal Physiol. 2007;292(3):F956–F965. doi: 10.1152/ajprenal.00314.2006. [DOI] [PubMed] [Google Scholar]
- 97.Madeira A, Camps M, Zorzano A, Moura TF, Soveral G. Biophysical assessment of human aquaporin-7 as a water and glycerol channel in 3T3-L1 adipocytes. PLoS One. 2013;8(12):e83442. doi: 10.1371/journal.pone.0083442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Rodriguez A, Catalan V, Gomez-Ambrosi J, Garcia-Navarro S, Rotellar F, Valenti V, Silva C, Gil MJ, Salvador J, Burrell MA, Calamita G, Malagon MM, Fruhbeck G. Insulin- and leptin-mediated control of aquaglyceroporins in human adipocytes and hepatocytes is mediated via the PI3K/Akt/mTOR signaling cascade. J Clin Endocrinol Metab. 2011;96(4):E586–E597. doi: 10.1210/jc.2010-1408. [DOI] [PubMed] [Google Scholar]
- 99.Lee DH, Park DB, Lee YK, An CS, Oh YS, Kang JS, Kang SH, Chung MY. The effects of thiazolidinedione treatment on the regulations of aquaglyceroporins and glycerol kinase in OLETF rats. Metabolism. 2005;54(10):1282–1289. doi: 10.1016/j.metabol.2005.04.015. [DOI] [PubMed] [Google Scholar]
- 100.Matsumura K, Chang BH, Fujimiya M, Chen W, Kulkarni RN, Eguchi Y, Kimura H, Kojima H, Chan L. Aquaporin 7 is a beta-cell protein and regulator of intraislet glycerol content and glycerol kinase activity, beta-cell mass, and insulin production and secretion. Mol Cell Biol. 2007;27(17):6026–6037. doi: 10.1128/MCB.00384-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Ceperuelo-Mallafre V, Miranda M, Chacon MR, Vilarrasa N, Megia A, Gutierrez C, Fernandez-Real JM, Gomez JM, Caubet E, Fruhbeck G, Vendrell J. Adipose tissue expression of the glycerol channel aquaporin-7 gene is altered in severe obesity but not in type 2 diabetes. J Clin Endocrinol Metab. 2007;92(9):3640–3645. doi: 10.1210/jc.2007-0531. [DOI] [PubMed] [Google Scholar]
- 102.Lindgren CM, Mahtani MM, Widen E, McCarthy MI, Daly MJ, Kirby A, Reeve MP, Kruglyak L, Parker A, Meyer J, Almgren P, Lehto M, Kanninen T, Tuomi T, Groop LC, Lander ES. Genomewide search for type 2 diabetes mellitus susceptibility loci in Finnish families: the Botnia study. Am J Hum Genet. 2002;70(2):509–516. doi: 10.1086/338629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Loos RJ, Katzmarzyk PT, Rao DC, Rice T, Leon AS, Skinner JS, Wilmore JH, Rankinen T, Bouchard C, Study HF Genome-wide linkage scan for the metabolic syndrome in the HERITAGE Family Study. J Clin Endocrinol Metab. 2003;88(12):5935–5943. doi: 10.1210/jc.2003-030553. [DOI] [PubMed] [Google Scholar]
- 104.Prudente S, Flex E, Morini E, Turchi F, Capponi D, De Cosmo S, Tassi V, Guida V, Avogaro A, Folli F, Maiani F, Frittitta L, Dallapiccola B, Trischitta V. A functional variant of the adipocyte glycerol channel aquaporin 7 gene is associated with obesity and related metabolic abnormalities. Diabetes. 2007;56(5):1468–1474. doi: 10.2337/db06-1389. [DOI] [PubMed] [Google Scholar]
- 105.Rodriguez A, Marinelli RA, Tesse A, Fruhbeck G, Calamita G. Sexual dimorphism of adipose and hepatic aquaglyceroporins in health and metabolic disorders. Front Endocrinol. 2015;6:171. doi: 10.3389/fendo.2015.00171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Kondo H, Shimomura I, Kishida K, Kuriyama H, Makino Y, Nishizawa H, Matsuda M, Maeda N, Nagaretani H, Kihara S, Kurachi Y, Nakamura T, Funahashi T, Matsuzawa Y. Human aquaporin adipose (AQPap) gene. Genomic structure, promoter analysis and functional mutation. Eur J Biochem. 2002;269(7):1814–1826. doi: 10.1046/j.1432-1033.2002.02821.x. [DOI] [PubMed] [Google Scholar]
- 107.Kishida K, Shimomura I, Nishizawa H, Maeda N, Kuriyama H, Kondo H, Matsuda M, Nagaretani H, Ouchi N, Hotta K, Kihara S, Kadowaki T, Funahashi T, Matsuzawa Y. Enhancement of the aquaporin adipose gene expression by a peroxisome proliferator-activated receptor gamma. J Biol Chem. 2001;276(51):48572–48579. doi: 10.1074/jbc.M108213200. [DOI] [PubMed] [Google Scholar]
- 108.Maeda N. Implications of aquaglyceroporins 7 and 9 in glycerol metabolism and metabolic syndrome. Mol Asp Med. 2012;33(5–6):665–675. doi: 10.1016/j.mam.2012.02.004. [DOI] [PubMed] [Google Scholar]
- 109.Fasshauer M, Klein J, Lossner U, Klier M, Kralisch S, Paschke R. Suppression of aquaporin adipose gene expression by isoproterenol, TNFalpha, and dexamethasone. Horm Metab Res. 2003;35(4):222–227. doi: 10.1055/s-2003-39478. [DOI] [PubMed] [Google Scholar]
- 110.Rodriguez A, Gomez-Ambrosi J, Catalan V, Gil MJ, Becerril S, Sainz N, Silva C, Salvador J, Colina I, Fruhbeck G. Acylated and desacyl ghrelin stimulate lipid accumulation in human visceral adipocytes. Int J Obes (Lond) 2009;33(5):541–552. doi: 10.1038/ijo.2009.40. [DOI] [PubMed] [Google Scholar]
- 111.Hansen JS, Krintel C, Hernebring M, Haataja TJ, de Mare S, Wasserstrom S, Kosinska-Eriksson U, Palmgren M, Holm C, Stenkula KG, Jones HA, Lindkvist-Petersson K. Perilipin 1 binds to aquaporin 7 in human adipocytes and controls its mobility via protein kinase A mediated phosphorylation. Metabolism. 2016;65(12):1731–1742. doi: 10.1016/j.metabol.2016.09.004. [DOI] [PubMed] [Google Scholar]
- 112.Calamita G, Gena P, Ferri D, Rosito A, Rojek A, Nielsen S, Marinelli RA, Fruhbeck G, Svelto M. Biophysical assessment of aquaporin-9 as principal facilitative pathway in mouse liver import of glucogenetic glycerol. Biol Cell. 2012;104(6):342–351. doi: 10.1111/boc.201100061. [DOI] [PubMed] [Google Scholar]
- 113.Portincasa P, Calamita G. Water channel proteins in bile formation and flow in health and disease: when immiscible becomes miscible. Mol Asp Med. 2012;33(5–6):651–664. doi: 10.1016/j.mam.2012.03.010. [DOI] [PubMed] [Google Scholar]
- 114.Gregoire F, Lucidi V, Zerrad-Saadi A, Virreira M, Bolaky N, Delforge V, Lemmers A, Donckier V, Deviere J, Demetter P, Perret J, Delporte C. Analysis of aquaporin expression in liver with a focus on hepatocytes. Histochem Cell Biol. 2015;144(4):347–363. doi: 10.1007/s00418-015-1341-3. [DOI] [PubMed] [Google Scholar]
- 115.Kuriyama H, Shimomura I, Kishida K, Kondo H, Furuyama N, Nishizawa H, Maeda N, Matsuda M, Nagaretani H, Kihara S, Nakamura T, Tochino Y, Funahashi T, Matsuzawa Y. Coordinated regulation of fat-specific and liver-specific glycerol channels, aquaporin adipose and aquaporin 9. Diabetes. 2002;51(10):2915–2921. doi: 10.2337/diabetes.51.10.2915. [DOI] [PubMed] [Google Scholar]
- 116.Rodriguez A, Moreno NR, Balaguer I, Mendez-Gimenez L, Becerril S, Catalan V, Gomez-Ambrosi J, Portincasa P, Calamita G, Soveral G, Malagon MM, Fruhbeck G. Leptin administration restores the altered adipose and hepatic expression of aquaglyceroporins improving the non-alcoholic fatty liver of ob/ob mice. Sci Rep. 2015;5:12067. doi: 10.1038/srep12067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Lebeck J, Gena P, O’Neill H, Skowronski MT, Lund S, Calamita G, Praetorius J. Estrogen prevents increased hepatic aquaporin-9 expression and glycerol uptake during starvation. Am J Physiol Gastrointest Liver Physiol. 2012;302(3):G365–G374. doi: 10.1152/ajpgi.00437.2011. [DOI] [PubMed] [Google Scholar]
- 118.Gu LY, Qiu LW, Chen XF, Lv L, Mei ZC. Expression of aquaporin 3 and aquaporin 9 is regulated by oleic acid through the PI3K/Akt and p38 MAPK signaling pathways. Zhonghua Gan Zang Bing Za Zhi. 2013;21(10):753–758. doi: 10.3760/cma.j.issn.1007-3418.2013.10.008. [DOI] [PubMed] [Google Scholar]
- 119.Rodriguez A, Gena P, Mendez-Gimenez L, Rosito A, Valenti V, Rotellar F, Sola I, Moncada R, Silva C, Svelto M, Salvador J, Calamita G, Fruhbeck G. Reduced hepatic aquaporin-9 and glycerol permeability are related to insulin resistance in non-alcoholic fatty liver disease. Int J Obes (Lond) 2014;38(9):1213–1220. doi: 10.1038/ijo.2013.234. [DOI] [PubMed] [Google Scholar]
- 120.Wang C, Lv ZL, Kang YJ, Xiang TX, Wang PL, Jiang Z. Aquaporin-9 downregulation prevents steatosis in oleic acid-induced non-alcoholic fatty liver disease cell models. Int J Mol Med. 2013;32(5):1159–1165. doi: 10.3892/ijmm.2013.1502. [DOI] [PubMed] [Google Scholar]
- 121.Miranda M, Ceperuelo-Mallafre V, Lecube A, Hernandez C, Chacon MR, Fort JM, Gallart L, Baena-Fustegueras JA, Simo R, Vendrell J. Gene expression of paired abdominal adipose AQP7 and liver AQP9 in patients with morbid obesity: relationship with glucose abnormalities. Metabolism. 2009;58(12):1762–1768. doi: 10.1016/j.metabol.2009.06.004. [DOI] [PubMed] [Google Scholar]
- 122.Mobasheri A, Wray S, Marples D. Distribution of AQP2 and AQP3 water channels in human tissue microarrays. J Mol Histol. 2005;36(1–2):1–14. doi: 10.1007/s10735-004-2633-4. [DOI] [PubMed] [Google Scholar]
- 123.Sjoholm K, Palming J, Olofsson LE, Gummesson A, Svensson PA, Lystig TC, Jennische E, Brandberg J, Torgerson JS, Carlsson B, Carlsson LM. A microarray search for genes predominantly expressed in human omental adipocytes: adipose tissue as a major production site of serum amyloid A. J Clin Endocrinol Metab. 2005;90(4):2233–2239. doi: 10.1210/jc.2004-1830. [DOI] [PubMed] [Google Scholar]
- 124.Taegtmeyer H, McNulty P, Young ME. Adaptation and maladaptation of the heart in diabetes: part I: general concepts. Circulation. 2002;105(14):1727–1733. doi: 10.1161/01.CIR.0000012466.50373.E8. [DOI] [PubMed] [Google Scholar]
- 125.Stanley WC, Lopaschuk GD, Hall JL, McCormack JG. Regulation of myocardial carbohydrate metabolism under normal and ischaemic conditions. Potential for pharmacological interventions. Cardiovasc Res. 1997;33(2):243–257. doi: 10.1016/S0008-6363(96)00245-3. [DOI] [PubMed] [Google Scholar]
- 126.Umenishi F, Verkman AS, Gropper MA. Quantitative analysis of aquaporin mRNA expression in rat tissues by RNase protection assay. DNA Cell Biol. 1996;15(6):475–480. doi: 10.1089/dna.1996.15.475. [DOI] [PubMed] [Google Scholar]
- 127.Wakayama Y, Jimi T, Inoue M, Kojima H, Shibuya S, Murahashi M, Hara H, Oniki H. Expression of aquaporin 3 and its localization in normal skeletal myofibres. Histochem J. 2002;34(6–7):331–337. doi: 10.1023/A:1023382609541. [DOI] [PubMed] [Google Scholar]
- 128.Shibuya S, Wakayama Y, Inoue M, Kojima H, Oniki H. The relationship between intramembranous particles and aquaporin molecules in the plasma membranes of normal rat skeletal muscles: a fracture-label study. J Electron Microsc (Tokyo) 2006;55(2):63–68. doi: 10.1093/jmicro/dfl017. [DOI] [PubMed] [Google Scholar]
- 129.Yang B, Verbavatz JM, Song Y, Vetrivel L, Manley G, Kao WM, Ma T, Verkman AS. Skeletal muscle function and water permeability in aquaporin-4 deficient mice. Am J Physiol Cell Physiol. 2000;278(6):C1108–C1115. doi: 10.1152/ajpcell.2000.278.6.C1108. [DOI] [PubMed] [Google Scholar]
- 130.Wakayama Y, Inoue M, Kojima H, Jimi T, Shibuya S, Hara H, Oniki H. Expression and localization of aquaporin 7 in normal skeletal myofiber. Cell Tissue Res. 2004;316(1):123–129. doi: 10.1007/s00441-004-0857-y. [DOI] [PubMed] [Google Scholar]
- 131.Chen X, Yu QQ, Zhu YH, Bi Y, Sun WP, Liang H, Cai MY, He XY, Weng JP. Insulin therapy stimulates lipid synthesis and improves endocrine functions of adipocytes in dietary obese C57BL/6 mice. Acta Pharmacol Sin. 2010;31(3):341–346. doi: 10.1038/aps.2010.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Miley HE, Sheader EA, Brown PD, Best L. Glucose-induced swelling in rat pancreatic beta-cells. J Physiol. 1997;504(Pt 1):191–198. doi: 10.1111/j.1469-7793.1997.00191.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Drews G, Krippeit-Drews P, Dufer M. Electrophysiology of islet cells. Adv Exp Med Biol. 2010;654:115–163. doi: 10.1007/978-90-481-3271-3_7. [DOI] [PubMed] [Google Scholar]
- 134.Delporte C. Aquaporins in salivary glands and pancreas. Biochem Biophys Acta. 2014;1840(5):1524–1532. doi: 10.1016/j.bbagen.2013.08.007. [DOI] [PubMed] [Google Scholar]
- 135.Delporte C, Virreira M, Crutzen R, Louchami K, Sener A, Malaisse WJ, Beauwens R. Functional role of aquaglyceroporin 7 expression in the pancreatic beta-cell line BRIN-BD11. J Cell Physiol. 2009;221(2):424–429. doi: 10.1002/jcp.21872. [DOI] [PubMed] [Google Scholar]
- 136.Mendez-Gimenez L, Becerril S, Camoes SP, da Silva IV, Rodrigues C, Moncada R, Valenti V, Catalan V, Gomez-Ambrosi J, Miranda JP, Soveral G, Fruhbeck G, Rodriguez A. Role of aquaporin-7 in ghrelin- and GLP-1-induced improvement of pancreatic beta-cell function after sleeve gastrectomy in obese rats. Int J Obes (Lond) 2017;41(9):1394–1402. doi: 10.1038/ijo.2017.135. [DOI] [PubMed] [Google Scholar]
- 137.da Silva IV, Barroso M, Moura T, Castro R, Soveral G. Endothelial aquaporins and hypomethylation: potential implications for atherosclerosis and cardiovascular disease. Int J Mol Sci. 2018 doi: 10.3390/ijms19010130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Gregor MF, Hotamisligil GS. Inflammatory mechanisms in obesity. Annu Rev Immunol. 2011;29:415–445. doi: 10.1146/annurev-immunol-031210-101322. [DOI] [PubMed] [Google Scholar]
- 139.Lafontan M, Girard J. Impact of visceral adipose tissue on liver metabolism. Part I: heterogeneity of adipose tissue and functional properties of visceral adipose tissue. Diabetes Metab. 2008;34(4 Pt 1):317–327. doi: 10.1016/j.diabet.2008.04.001. [DOI] [PubMed] [Google Scholar]
- 140.Mehta NN, Reilly MP. Mechanisms of the metabolic syndrome. Drug Discov Today Dis Mech. 2004;1(2):187–194. doi: 10.1016/j.ddmec.2004.09.010. [DOI] [Google Scholar]