Abstract
The purpose of this review is to explore immune-mediated mechanisms of stress surveillance in cancer, with particular emphasis on the idea that all cancers have classical hallmarks (Hanahan and Weinberg in Cell 100:57–70, 67; Cell 144:646–674, 68) that could be interrelated. We postulate that hallmarks of cancer associated with cellular stress pathways (Luo et al. in Cell 136:823–837, 101) including oxidative stress, proteotoxic stress, mitotic stress, DNA damage, and metabolic stress could define and modulate the inflammatory component of cancer. As such, the overarching goal of this review is to define the types of cellular stress that cancer cells undergo, and then to explore mechanisms by which immune cells recognize, respond to, and are affected by each stress response.
Keywords: Cancer immune surveillance, Cancer immunity, Cancer-associated stress, Cancer inflammation, Tumor microenvironment, ER stress, Unfolded protein response, Immunogenic cell death, Danger-associated molecular patterns (DAMPs), Chromosome instability (CIN), Hyperploidy, Senescence-associated secretory phenotype (SASP), DNA damage response (DDR), Oncometabolites, Mitochondrial stress
Introduction
Almost all tumors have an inflammatory component that contributes a significant portion of the cellularity to the growing mass. This inflammatory component consists of resident and/or infiltrating immune cells and has been categorized as a bonafide “hallmark” of cancer [68]. The activity of these immune cells can either promote cancer growth, e.g., “cancer related inflammation” or inhibit cancer progression, e.g., “cancer immune surveillance”. Given these disparate activities of immunity on cancer progression, it is important to understand the characteristics of cancer cells that regulate the inflammatory constituents that inhabit almost all cancers. Indeed, the field of cancer immune therapy, heralded as a “Breakthrough of the Year” [33], critically relies on mobilizing anti-tumor immune effectors while diminishing the activity of pro-tumor inflammation. In this review, we provide a summary of how intrinsic cellular stress modulates immune cell infiltration or activity. We focus on oxidative stress, proteotoxic stress, mitotic stress, DNA damage and metabolic stress as key cancer intrinsic hallmarks [101] that can impact on cancer’s extrinsic hallmark of immunity and inflammation. Figure 1 shows that the stresses that cancer cells experience and sense can result in both pro- or anti-tumor outcomes. All of these stress pathways are closely interrelated and can directly cause or be caused by one another (Fig. 2). This review focuses largely on the downstream effects of these five stressors, and we acknowledge that the transformed state, chemotherapies, and radiotherapies all can induce some form of cellular stress.
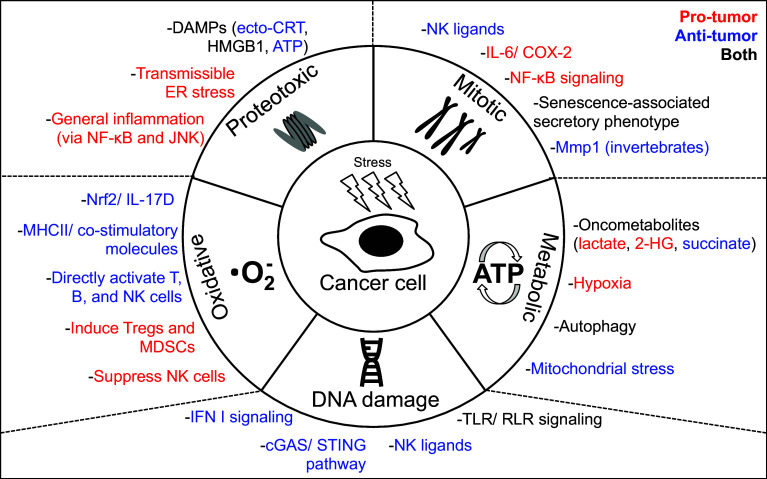
Fig. 1.
Regulation of immune surveillance by intrinsic stress pathways in cancer. Shown are the five unique stress pathways that occur in cancer cells (inner circle) and the effects of each stress on immunity (outer circle). These effects are broad reaching and can promote (red font) and/or inhibit (blue font) anti-tumor immunity, or both (black font).
Adapted from Luo et al. [101]
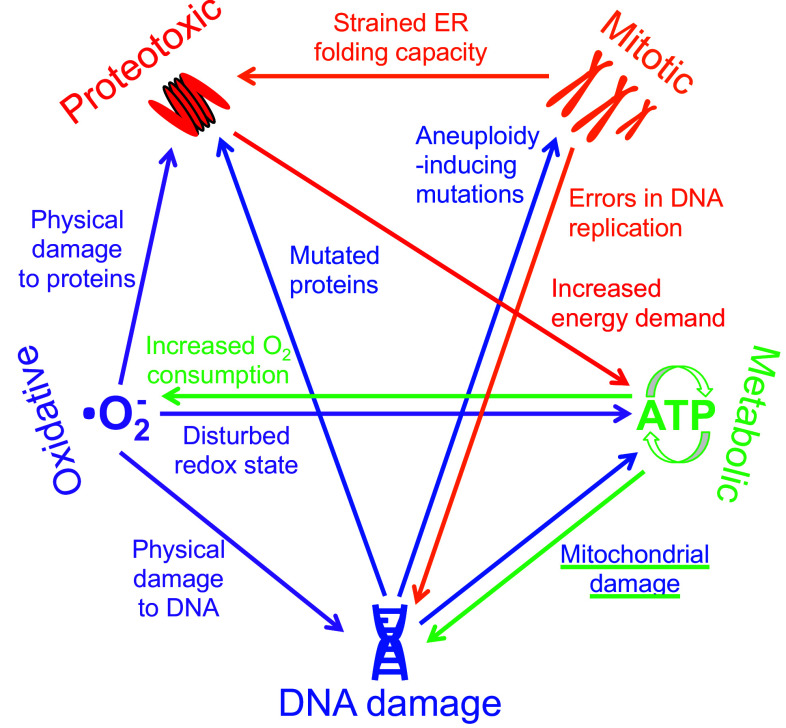
Fig. 2.
Interrelation of stress pathways. The five stress pathways that occur in cancer cells are highly interrelated and can each directly cause at least one or more other stress response. The arrows demonstrate direct (rather than indirect) interconnections between each of the five stress pathways, and the text next to each arrow describes how each direct effect is mediated
Oxidative stress
Oxidative stress occurs in cancer when the balance between reactive oxygen species (ROS) and the ability of the cell and its microenvironment to detoxify them is disturbed, resulting in the accumulation of free oxygen radicals such as hydrogen peroxide (H2O2), the superoxide radical (O2 −) or the hydroxyl radical (OH). Due to the toxic effects of ROS on different components of a cell, oxidative stress is closely related to each of the other four forms of stress discussed in this review (DNA damage, proteotoxic, mitotic, and metabolic). For example, it can directly cause DNA and protein damage or cause and be caused by metabolic stress (Fig. 2). On the other hand, ROS are important second messengers during cell signaling and homeostasis, and a tightly regulated balance of their production and their scavenging is needed for transformation-free cell survival.
Because of ROS’ cytotoxic and DNA-damaging effects, they are closely associated with tumorigenesis, which has been reviewed elsewhere [129, 148, 149]. However, the overall impact of ROS on cancer progression is difficult to predict, as ROS can exert both pro- and anti-tumor effects. Moreover, ROS also impact positively and negatively on immune function, and thus can indirectly affect cancer progression via their control of cancer immune surveillance. In this section, we focus on the impact of ROS on immune cells and will interpret these effects in the context of cancer progression.
Although ROS are abundant in the tumor microenvironment, their origin has not been completely dissected. Phagocytes such as neutrophils and macrophages are a major source of ROS, but excessive ROS production by tumor cells has also been widely accepted and may even be regarded as a hallmark of cancer [129]. ROS from tumor cells can influence the immune microenvironment and the ROS status of surrounding immune cells because increased intracellular ROS in the T cells from tumor-bearing hosts has been described [14]. Cytokines that are abundant in the tumor microenvironment such as tumor necrosis factor (TNF), interleukin (IL)-1β, interferon (IFN)-γ, transforming growth factor (TGF)-β and IL-6 have been shown to increase both intracellular and extracellular ROS production from epithelial, smooth muscle and pancreatic cells [38, 165, 194]. Thus, strategies to treat cancer via oxidative or antioxidative drugs should take into account the wide panoply of both positive and negative effects of ROS on immunity and cancer progression.
Multiple studies have found a significant role for ROS to control leukocyte recruitment by serving as direct chemoattractants. ROS can directly recruit immune cells in inflamed zebrafish tissue [122] or induce proteins such as thioredoxin that are chemotactic for monocytes, neutrophils and T cells [13]. However, neither of these works used cancer immune surveillance models. Our group has recently linked oxidative stress pathways directly to leukocyte recruitment in cancer [141]. In a study using transplantable melanoma and sarcoma mouse models, we found that activating the antioxidant transcription factor nuclear factor (erythroid-derived 2)-like 2 (Nrf2) induced the cytokine IL-17D, which mediated the recruitment of cancer-eliminating natural killer (NK) cells. Indeed, treatment of cancer cells with Nrf2 agonists led to secretion of IL-17D, NK cell recruitment, and cancer elimination. To our knowledge, this is the first study showing that intrinsic oxidative stress of the tumor cell itself activates mechanisms that lead to immune cell recruitment and immune cell-mediated cancer elimination [141]. Thus, it is possible that cancer cells initiate mechanisms (such as transcriptional activation of antioxidant genes) to overcome oxidative stress, but that the same mechanisms lead eventually to their elimination by the immune system. It is interesting to speculate that the Nrf2/IL-17D pathway may have evolved as the immune system’s refutation to the cancer’s increased defense mechanisms against oxidative stress. Moreover, ROS might not only influence immune cell infiltration, but also retention and survival at the site of inflammation or cancer, since ROS derived from myeloperoxidase activity can promote paracrine neutrophil survival [86, 156].
ROS can also directly limit tumor progression by augmenting the function of phagocytes and antigen-presenting cells (APCs), often leading to increased anti-tumor T-cell activation. The killing capacity of activated macrophages towards tumor cells, for example, largely depends on ROS production from macrophages [116, 119], that could be further induced if tumor cells were coated with eosinophil peroxidase generating H2O2 [118]. H2O2 can increase major histocompatibility complex (MHC) class II and co-stimulatory molecules on human dendritic cells (DCs), thereby enhancing T-cell proliferation and activation [140]. Moreover, DCs generate ROS during antigen presentation to T cells [106], and antigen presentation is also influenced by ROS, thus indirectly affecting T-cell stimulation [110]. More evidence that APC-mediated T-cell activation is influenced by ROS activity came from studies showing that macrophages can modulate their secretion of the antioxidant glutathione, which facilitates T-cell activation [5, 55, 120, 154]. On the other hand, the production of glutathione by tumor cells has been suggested as a cancer defense mechanism against macrophage-mediated killing [117].
ROS can also directly activate lymphocytes, including T, B, and NK cells. Oxidation of human ovarian epithelial cancer cells, for example, was shown to enhance T-cell activation from patients in an MHC class I- and II-restricted manner [26]. In line with that, antioxidants directly inhibit T-cell activation, proliferation and IL-2 receptor expression [24, 25, 123]. Moreover, the oxidative status of antigens can modify T-cell receptor (TCR) binding to the antigenic peptide [182], and TCR ligation induces ROS production from T cells [42]. Oxidative stress has also been suggested to promote T-cell polarization into a Th2 phenotype [85]. In B cells, ROS are important for B cell receptor (BCR) signaling [158].
In other instances, ROS can be immune suppressive by their ability to influence or be released from immune suppressive regulatory T cells (TReg) and myeloid-derived suppressor cells (MDSCs). TRegs can be induced by ROS [90] and are more resistant to ROS than effector T cells [112], which might be a mechanism of cancer immune evasion by favoring an immune suppressive tumor microenvironment. Although most studies found ROS to induce TRegs, one showed that ROS from MDSCs inhibited the maturation of TRegs using murine breast and lung carcinoma cancer models [20]. Some of TReg cell suppressive functions towards other T cells are mediated by their secretion of ROS [51] or indirectly by their ability to suppress glutathione release from DCs [193]. H2O2 has been found to directly inhibit nuclear factor κB (NF-κB)-induced cytokine expression from activated T cells [95, 104]. ROS can also regulate T-cell apoptosis and thereby contribute to T-cell balance under homeostatic and disease conditions [172].
MDSCs are another major suppressive cell type producing and reacting to ROS in the tumor microenvironment. Several tumor-derived cytokines trigger ROS production from MDSCs, which might account for some of MDSC’s immunosuppressive functions [59] and maintain them in an undifferentiated state [94]. In a mouse lymphoma model, MDSCs were able to suppress T-cell proliferation and IFNγ production by disrupting the TCR/CD8 complex, which was mediated by overproduction of ROS [113]. MDSCs also suppress T cells by depletion of cysteine and arginine (which are crucial for T-cell activation and proliferation), production of peroxynitrite (which are cytotoxic to T cells), and upregulation of the ROS-generating enzyme cyclooxygenase (COX)-2 in T cells [17, 59, 84, 164]. Additionally, in advanced cancer patients, H2O2 derived from granulocytes is suggested to suppress cytokine release from T cells [144].
Early studies suggest that ROS production from NK cells is a necessary event for NK cell cytotoxicity against cancer [49, 50, 136, 167]. On the other hand, monocyte-derived ROS downregulate the expression of activating receptors NKG2D and NKp46 on a certain subtype of NK cells with high cytotoxic ability [138]. Moreover, H2O2 in the cancer microenvironment decreases the recruitment of this NK cell subtype [80], suggesting that ROS production is a mechanism of cancer cells to evade NK cell-mediated immunity. Monocyte-derived ROS can also inhibit activation, proliferation and IFNγ secretion as well as induce apoptosis of NK cells [69, 72, 150]. ROS might also play a role in the dysfunction and depletion of NK cells in myelogenous leukemia patients [108]. H2O2 from macrophages isolated from melanoma-bearing patients was shown to downregulate CD3 zeta on T cells and NK cells as well as their cytotoxic activity [88]. The documented inhibitory activity of ROS on NK cells implies that the role of Nrf2, a known master regulator of antioxidant responses and an inducer of IL-17D and NK cell recruitment [141], could be to remove ROS in order to promote anti-tumor activities of NK cells.
Proteotoxic stress
Proteotoxic stress [also referred to as “endoplasmic reticulum (ER) stress”] is characterized by the accumulation of misfolded and/or unfolded proteins within the ER of a cell [179]. ER stress occurs when the amount of proteins entering the ER (input) exceeds the ER’s processing capacity (output), leading to dysregulation of post-translational modifications that occur within the ER [16]. Because post-translational modifications help proteins form tertiary and quaternary structures, the lack of appropriate modification results in misfolded proteins that accumulate within the secretory pathway, leading to proteotoxic stress [147].
As it relates to cancer, proteotoxic stress can arise from a number of different sources. In fact, many of the “hallmarks of cancer” directly induce proteotoxic stress. For example, aneuploidy induced by mitotic stress can directly contribute to proteotoxic stress through the presence of excess copies of wild type (WT) proteins, which accumulate within the ER and disrupt the organelle’s folding capacity [48]. Furthermore, the presence of abnormal proteins that do not fold properly or cannot be modified can also cause proteins to build up within the ER and trigger proteotoxic stress. Abnormal proteins can arise either directly from genetic mutations or indirectly from ROS (oxidative stress) that damage WT proteins (Fig. 2). Clearly there are many different stressors present in cancer cells that have the capability to over-burden the secretory pathway and directly lead to proteotoxic stress.
The accumulation of misfolded proteins and proteotoxic stress leads to the activation of the unfolded protein response (UPR). Although the UPR signaling network does not directly activate immune responses, when the UPR fails to restore ER homeostasis, a form of apoptotic cell death occurs that is characterized by the release of immune-stimulating molecules [176]. This phenomenon is referred to as immunogenic cell death (ICD), distinct from classical apoptosis, which is considered tolerogenic. ICD, on the other hand, is a powerful stimulator of immune cells by causing the release of danger-associated molecular patterns (DAMPs) into the extracellular environment. Examples of DAMPs include proteins such as calreticulin (CRT) and high-mobility group box 1 (HMGB1), and small molecules, like ATP [175]. DAMPs can directly or indirectly stimulate many different types of immune cells, including macrophages, DCs, and T cells [176]. The specific mechanisms by which each immune cell subset is affected by and responds to DAMPs will be discussed throughout this section. ICD is induced when the UPR fails to restore ER homeostasis, often due to the use of chemotherapeutics, such as anthracyclines, oxaliplatin, bortezomib, radiotherapy, and photodynamic therapy [92]. Thus, at the “basal” state, cancer cells can exhibit proteotoxic stress, but when cancer cells are exposed to a higher level of proteotoxic stress, they can undergo cell death and potently activate the immune system through release of DAMPs.
Perhaps the best-explored DAMP is CRT, a normally intracellular transmembrane protein that is translocated to the cell surface (ecto-CRT) during proteotoxic stress. For example, when anthracycline treatment is used to model proteotoxic stress, ecto-CRT is exposed at the cell surface and leads to phagocytosis of the dying cells by immune cells, leading to their activation [124]. In this model system ecto-CRT is both sufficient and necessary to promote immune surveillance of anthracycline-induced proteotoxic stress in colon cancer. These results were corroborated and shown to require the UPR system [128]. Ecto-CRT signals through low density lipoprotein receptor-related protein 1 (LRP1, also known as CD91), a pattern recognition receptor (PRR) that is shared with heat shock proteins. CD91 is expressed on immune phagocytes, and CRT-CD91 signaling promotes phagocytosis and induces the secretion of pro-inflammatory cytokines that ultimately aid in the presentation of tumor antigen [11]. Therefore, this signaling pathway is important for eliciting robust T-cell responses that are critical for tumor elimination.
HMGB1, a nuclear protein involved in chromatin organization, is a DAMP that is released passively into the extracellular environment during ICD. HMGB1 can signal through toll-like receptor 4 (TLR4) expressed on DCs, resulting in MyD88-mediated pro-inflammatory cytokine production [6]. In addition, the receptor for advanced glycation end-products (RAGE), expressed by macrophages, can also recognize HMGB1 and induce the production and secretion of pro-inflammatory cytokines [87]. Each of these signaling events is important for eliciting anti-tumor immune responses and therefore plays important roles in the immune surveillance of cancer cells in a manner dependent on antigen-presenting cells. On the other hand, HMGB1-RAGE signaling can also induce inflammatory responses that promote tumor progression and are associated with worse prognosis in certain patients [121, 157]. Because it is released by dying cells rather than induced by the UPR, HMGB1 presumably could activate the immune system when released by stressors other than proteotoxic stress.
ATP is another DAMP that is released into the extracellular environment in the event of ICD. During proteotoxic stress, ATP is actively secreted from cells, where it can either induce local inflammation or act as an “eat me” signal. Secreted ATP can be recognized by the purinergic receptor P2X7 that is expressed on DCs. This signal induces inflammasome activation and drives the secretion of IL-1β, a highly pro-inflammatory cytokine [64]. Furthermore, secreted ATP can also act as a “find me” signal by recruiting phagocytic cells [53]. In either case, it is clear that secreted ATP acts to promote immune recognition and clearance of cells undergoing proteotoxic stress.
In addition to DAMPs, proteotoxic stress can stimulate general inflammation through the activation of NF-κB and Jun N-terminal kinase (JNK). NF-kB is a master regulator of inflammation, and upon activation, induces the production of a number of pro-inflammatory genes. Proteotoxic stress has been shown to activate NF-kB in several model systems. In HeLa cells, 2-deoxyglucose can induce ROS and misfolded proteins, leading to proteotoxic stress and NF-κB activation [126]. In primary fibroblasts, thapsigargin-induced ER stress can promote NF-κB activation by attenuating translation. This increases the ratio of NF-κB to IκB (owing to the short half-life of IκB), thereby freeing NF-κB to translocate into the nucleus when the UPR is activated [40]. Proteotoxic stress also activates JNK directly via interaction between JNK and components of the UPR [173].
Although the action of tumor-derived proteotoxic stress on immune cells is generally considered anti-tumorigenic, in certain contexts it has also been shown to promote tumor growth. For example, activation of NF-κB is well known to promote cancer progression [82] and in some circumstances, HMGB1-RAGE signaling can provide “wound healing” signals to also facilitate cancer growth. In addition, several groups have demonstrated a pro-tumorigenic role for proteotoxic stress by showing that it acts in a cell extrinsic manner on myeloid cells to facilitate tumor growth [35, 198]. Evidence for this phenomenon is that macrophages cultured in the conditioned medium of ER-stressed breast, lung, or melanoma cancer cells become activated and begin secreting pro-inflammatory/tumorigenic cytokines and enzymes that suppress T-cell tumoricidal activity [103]. Furthermore, DCs cultured in the presence of conditioned media of ER-stressed cancer cells downregulate cross-presentation of high-affinity antigens and fail to effectively cross-prime CD8+ T cells, leading to diminished CD8+ T-cell infiltration in vivo [102]. Finally, it has also been demonstrated that transmissible ER stress is pro-angiogenic, as macrophages cultured in the presence of conditioned media of ER-stressed breast cancer cells express the angiogenic factor vascular endothelial growth factor (VEGF) in vitro [36]. Together, these results show that ER stress within the tumor microenvironment has the capacity to reshape myeloid cells to promote tumor growth.
Mitotic stress
Mitotic stress is characterized by the duplication or deletion of whole or partial chromosomes from the genome of a cell, which can occur through a variety of means. Most commonly, mitotic stress is mediated via chromosome instability (CIN). CIN refers to the ability of cells to rapidly lose or gain chromosomes during cell division [89]. This occurs due to mis-segregation of individual chromosomes during cellular replication, wherein DNA is distributed unequally to daughter cells [48]. As a result, cells are generated that possess an abnormal, or “non-diploid” number of chromosomes, a state referred to as “aneuploidy”. Other mechanisms that induce aneuploidy include failure in cytokinesis (endoreplication), where DNA is duplicated but the cell does not undergo cytokinesis, and cell–cell fusion, where two cells physically connect their plasma membranes and cytosol and combine DNA. Both of these mechanisms give rise to daughter cells that contain twice the amount of DNA than parental cells and feature mitotic stress [89].
Aneuploidy and CIN are closely related, each being an extremely common feature in cancer. It has been estimated that greater than 70% of all cancers display aneuploidy [111], although whether this is a cause or consequence of the disease is highly debated. CIN can occur through three major mechanisms: mitotic checkpoint defects (resulting in premature chromosome segregation), centrosome overduplication (resulting in improper attachments between microtubules and the kinetochore and frequent chromosome mis-segregation), and faulty sister chromatid cohesion (causing premature separation of sister chromatids) [48]. Many genes involved in these processes are direct transcriptional targets of E2F and/or p53 [89]. Aberrations in these pathways caused by either mutations or overexpression/gene duplication have the potential to induce mitotic stress and CIN that culminates in aneuploidy, which can be sensed by immune cells in a variety of different ways.
A direct consequence of mitotic stress induced by CIN and aneuploidy is that it causes imbalances in the composition of cellular proteins, which affects the folding capacity of the ER and leads to proteotoxic stress (Fig. 2). In this sense, all of the surveillance mechanisms relevant in proteotoxic stress also occur during mitotic stress (see “Proteotoxic stress”). Specifically, hyperploidy is known to be immunogenic by inducing surface expression of the DAMP calreticulin (ecto-CRT) [22]. Tetraploid colon, lung, and fibrosarcoma cancer cells readily proliferate and maintain their increased DNA content and ecto-CRT expression in immunodeficient, but not in immunocompetent, mice. In immune competent mice, growth of tetraploid cancer cells is delayed, and tumors that do grow exhibit reduced DNA content and ecto-CRT exposure relative to tumors grown from immune-deficient mice [151]. These results suggest an active immunoediting mechanism operates against mitotically stressed cells. Furthermore, colon cancer cells are susceptible to drug-induced tetraploidization only in the absence of the tumor suppressor Tp53, and tetraploid Tp53 −/− colon cancer cells are only able to form tumors in immune deficient, but not immune competent, mice [15]. This result suggests that the mitotic stress induced by tetraploidy is particularly oncogenic in the context of deficient immune surveillance, and that tetraploidy (and associated mitotic stress) is immunogenic. Finally, it has also been shown that hyperploid cancer cells stimulate NK cell-mediated anti-cancer immunity. Specifically, hyperploid human erythroleukemic, colon and liver cancer cells can activate the cytotoxic activity of NK cells via the expression of ligands for NK activating receptors such as NKG2D and DNAX accessory molecule (DNAM-1) [1]. Together, these findings strongly suggest that mitotic stress associated with hyperploidy (specifically tetraploidization) is immunogenic and induces anti-tumor immune surveillance by NK cells, and that this phenomenon is broadly conserved across multiple cancer types.
Mitotic stress can also promote cancer progression through the induction of inflammatory cytokines that support tumor growth [135]. In a mouse model of colon tumorigenesis whereby mice are haplosufficient for Shugoshin-1 (Sgo1 +/−), a gene involved in the maintenance of chromosome cohesion during cellular replication, CIN and DNA damage results, leading to the secretion of pro-inflammatory cytokines that promote tumorigenesis. Specifically, Sgo1 +/− mice display increased expression of the pro-inflammatory factors COX-2 and IL-6, each of which has been shown to have a role in promoting colon cancer formation. This cytokine response is dependent on DNA damage response proteins, supporting the role of mitotic stress in mediating this response [191]. Indeed, even in human cancers, a similar finding has been reported, whereby CIN-induced DNA damage signaling leads to the secretion of pro-tumor inflammatory cytokines [137]. In a slightly different mouse model of CIN-induced tumorigenesis whereby the gene flap endonuclease 1 (Fen1) is mutated to induce genomic instability, CIN is associated with tumor progression through predisposition to chronic inflammation mediated by NF-kB. Specifically, mice harboring the mutant Fen1 showed significantly higher levels of inflammatory NF-kB target genes compared to WT mice [201].
Mitotic stress can also trigger immune surveillance through the induction of cellular senescence, which has been shown to be immunogenic in certain circumstances. Senescence is a permanent state of cellular growth arrest that can be triggered by various stressors. In cancer, senescence acts as a tumor suppressive mechanism by inhibiting cellular proliferation and tumor growth, but can also favor tumor growth in certain instances by promoting inflammation [32]. Mitotic stress occurring in cancer cells can induce senescence by activating pathways that promote both cell cycle arrest and survival [174]. Introducing an oncogenic activating H-Ras mutation (H-RasV12) into human fibroblasts resulted in enhanced survival of cells with mitotic spindle and chromatin defects. These cells also featured induction of the key senescence effectors p21 and p16, further supporting that mitotic disruption and enhanced survival are linked during senescence [46]. As it relates to immune surveillance, senescence is immunogenic by causing the secretion of inflammatory cytokines, which can both promote and inhibit tumor progression. This is referred to as the senescence-associated secretory phenotype (SASP) [29]. The SASP is mediated primarily by the transcription factors NF-kB and CCAAT/enhancer binding protein beta (C/EBPβ) and consists of a broad range of secreted factors, including chemokines/cytokines, growth factors, and matrix-remodeling enzymes, among many others (reviewed in [96]). Together, these secreted factors produce a rich pro-inflammatory microenvironment that recruits immune cells that can either promote or inhibit tumor growth, depending on the circumstance. For example, in an oncogene-induced model of senescence in murine hepatocytes, senescent cells are subject to CD4+ T-cell-mediated immune clearance that is also dependent on monocytes/macrophages, and in the absence of immune surveillance, pre-malignant hepatocytes develop into hepatocellular carcinomas [81]. NK cells also mediate immune surveillance of senescent cells. NKG2D-dependent elimination of hepatocellular carcinomas can be mediated by p53-dependent chemokine production by senescent tumor cells [76]. Alternatively, the SASP has also been demonstrated to promote tumor progression. Using a model of DNA damage-induced senescence on pre-malignant epithelial cells, the SASP induced epithelial-to-mesenchymal transition and invasiveness, two hallmarks of malignancy. These phenotypic changes were dependent on the inflammatory cytokines IL-6 and IL-8 [30]. These examples clearly demonstrate how senescence induces immune surveillance that ultimately can either promote or inhibit tumor progression, depending on the context.
Mitotic stress and CIN are also known to induce immune surveillance in invertebrates [152]. Indeed, inducing CIN in proliferating Drosophila larval tissue resulted in the activation of innate cellular signaling in cells with CIN. Included in this innate signaling was the activation of matrix metalloproteinase 1, which is responsible for recruiting hemocytes (innate insect immune cells) to the site of CIN and providing the necessary signals for effective elimination of CIN cells [99]. This pathway appears to be mediated by JNK, as knockdown of JNK signaling resulted in death of CIN cells [187]. These studies demonstrate a cell-intrinsic role for mitotic stress and CIN in inducing innate immunity in insects, suggesting a conserved mechanism for eukaryotic organisms for responding to mitotically stressed cells.
DNA damage
DNA damage is a change in DNA structure that can occur in cancer cells intrinsically during the “stress” of extensive replication or as a direct result of mitotic and/or oxidative stress (Fig. 2). In addition, it can be caused by extrinsic stresses such as viral infection, radiation, UV light or chemotherapy [98]. Apart from causing mutations that can lead to the formation of neoantigens activating the immune system, DNA damage can also result in the accumulation of ectopic DNA particles that can function as DAMPs [83] as well as in the upregulation of stress ligands activating immune receptors [62]. This section will focus on the DNA damage-induced expression of stress ligands as well as on immune surveillance activities induced by DNA damage-associated DAMPs rather than the well-described formation of neoantigens that has been reviewed elsewhere [98].
DNA damage can directly alert the immune system by inducing MHC class I-like ligands of activating receptors present on immune cells [21]. NK cells, γδ T cells, αβ CD8+ T cells and NKT cells express the receptor NKG2D that can bind to stress ligands [100], which become upregulated after stress signals, especially in cancer cells [161]. DNA-damaging conditions such as ionizing radiation (IR), damaging agents or synthesis inhibitors can induce the expression of several of these ligands, and this depends on the DNA damage response (DDR) machinery [62, 63]. Similarly, ligands of the activating receptor DNAM-1 expressed by NK and T cells were found to be upregulated by chemotherapeutic treatment of multiple myeloma cells, and this was counteracted by inhibiting members of the DDR machinery [162, 163]. Recently, it has been suggested that fibroblasts can acquire APC-like functions by their ability to activate naïve CD8+ T cells in response to DNA damage. Treatment of fibroblasts with a DNA-damaging agent induced their expression of MHC class I molecules as well as multiple NKG2D and DNAM-1 ligands [169]. In addition to DNA damage alerting the immune system via stress ligands, the damaged DNA itself can be sensed by the DDR, leading to a senescent state in which inflammatory cytokines are secreted [137]. Indeed, agents that promote double stranded (ds) DNA breaks have been shown to induce inflammatory genes [18]. Moreover, inhibiting the DDR machinery impairs cytokine induction [127] and NK and T-cell dependent tumor regression [168].
If enough damage occurs, DNA can undergo fragmentation and leak into the cytosol or extracellular milieu. In this scenario, DNA itself is a DAMP that is sensed by PRRs resulting in the downstream production of cytokines such as type I IFNs that normally act to initiate anti-viral responses [77]. In typical anti-viral immune responses, PRRs recognize viral nucleic acids, leading to the production of type I IFNs and activation of T-cell responses. It is now believed that the same responses can also be initiated from sensing of endogenous DNA particles that are found in ectopic locations (extranuclear or extracellular), which can occur during cancer as a result of DNA damage. Cytosolic DNA resulting from extensive replication or defects in the DDR in the cancer cell itself can bind to cancer cell-expressed receptors that activate immune surveillance pathways. Extracellular DNA resulting from DNA damage-induced apoptosis, necrosis or leakage can be sensed by receptors on immune cells or non-immune cells in the tumor microenvironment, inducing innate and subsequent adaptive immunity. Type I IFNs produced by either malignant cells or DCs in the tumor microenvironment are therefore mediators of the pathways underlying cancer immune surveillance in response to DNA damage, underlined by their requirement for an optimal anti-cancer response after radiation or chemotherapy [160]. In contrast, induction of type I IFNs by DNA damage might also favor tumor growth because of IFN’s known ability to upregulate tumor programmed cell death ligand 1 (PD-L1), an immune suppressive molecule [12, 196].
Since DNA is sensed by PRRs, the role of DNA damage in controlling immune responses in cancer has been studied by examining the role of specific PRRs or their signaling pathways in cancer progression. We review below the following PRRs/signaling pathways: endosomal receptors—TLR3, TLR7, TLR8, and TLR9; cytosolic receptors—cyclic GMP–AMP synthase (cGAS)/stimulator of IFN genes (STING), absent in melanoma (AIM) 2, and retinoic acid-inducible gene (RIG) I-like receptors (RLRs).
TLR3 binds to endosomal dsRNA and serves to alert the immune system to viral infection, but can also promote cancer clearance. For example, signaling through TLR3 in DCs and other APCs can activate anti-tumor NK cells, presumably due to dsRNA released by cancer cells [3, 107]. Tlr3 −/− mice featured an increased tumor burden in a mouse model of prostate cancer, which could be counteracted by administration of the TLR3 ligand polyinosinic-polycytidylic acid [poly(I:C)], leading to immune surveillance by T and NK cells [27]. Poly(I:C) administration has also been shown to reduce lung cancer growth, mediated by Th1 and Th17 immunity [57] and is currently investigated in clinical trials as a cancer vaccine adjuvant [114]. Chemotherapeutic agents can induce the production of type I IFNs in response to TLR3 signaling, resulting in chemokine release [160]. Moreover, treatment of prostate cancer cell lines in vitro with TLR3 agonists induces inflammatory molecules that had the potential to recruit immune cells, suggesting that a TLR3-mediated anti-cancer immune response could be directly initiated by signaling inside the cancer cell itself [60].
The closely related TLRs 7 and 8, which recognize single-stranded (ss) RNA, are popular targets for cancer immune therapy. Their agonists induce cytokine and chemokine secretion, macrophage activation and cellular immunity through pathways downstream of the transcription factor NF-κB [146]. Although exogenously used in cancer immune therapy, TLR7/8-mediated immune surveillance has not been documented. However, TLR7 has been suggested to promote chemoresistance when expressed by cancer cells instead of antigen-presenting cells, even in Tlr7 −/− mice [23].
TLR9 binds to endosomal CpG DNA or oligodeoxynucleotides and its expression has been detected in a number of cancer cell lines and human cancer biopsies. High expression of TLR9 in cancer has been associated with both poor (glioma, prostate cancer, esophageal adenocarcinoma) and good (triple-negative breast cancer, renal cell carcinoma) prognosis [142]. The mechanisms of TLR9’s opposing roles in cancer cells have not been fully elucidated, and it is unclear if they are completely immune-related. However, TLR9 agonists are currently investigated for use in cancer immune therapy because of their ability to directly induce activation and maturation of plasmacytoid (p)DCs and subsequent downstream adaptive immunity, and to enhance differentiation of B cells into plasma cells [91]. On the other hand, one recent study found that TLR9 signaling in tumor-infiltrating myeloid cells promoted tumor re-growth after radiation by inducing tumor-promoting inflammation and re-vascularization [61].
The cGAS/STING pathway detects cytosolic DNA accumulated in response to DNA damage [78, 79]. One hypothesis for STING-mediated anti-cancer immunity is that DNA from necrotic tumor cells is engulfed by DCs and triggers STING signaling inside the DCs. Accordingly, STING-deficient mice feature defective T-cell responses in melanoma [188] and glioma [125], and STING is required for type I IFN-mediated anti-tumor effects after radiation [41]. Additionally, loss of STING has been suggested as an escape mechanism of damaged or pre-malignant cells to evade immune surveillance [2, 190, 202]. Moreover, STING agonists are proposed to have potential as a cancer immune therapy agent through their activation of DCs and production of IFNs [97].
Absent in melanoma (AIM) 2 is an intracellular dsDNA sensor that is part of a unique multiprotein complex called the inflammasome, which mediates the secretion of IL-1β and IL-18. Since these two cytokines strongly promote inflammatory responses, AIM2 has been studied in the context of inflammatory cancers, especially those in the gut, where it was shown that Aim2 −/− mice develop more colitis-associated cancer [105, 186]. As its name implies, AIM2 is downregulated in a variety of cancers and cancer cell lines [43, 45, 132], presumably because it prevents cancer progression, and cancer cells must lose expression of AIM2 to survive anti-tumor responses. Indeed, a recent study proposes that its expression renders mice less resistant to DNA ds-breaks caused by IR and chemotherapeutic agents, which could point towards an inflammasome-mediated role in cancer cell susceptibility to IR and chemotherapy [73].
RLRs recognize cytoplasmic RNA, increasingly present after IR. They include RIG-I, melanoma differentiation-associated protein (MDA)-5 and laboratory of genetics and physiology 2 (LGP2), which is a negative regulator of the former two [185]. Their signaling pathways converge on the recruitment of NF-κB and IRF3, subsequently activating type I IFN production. It has been demonstrated that RIG-I became activated by binding to tumor-endogenous RNA translocating to the cytoplasm after IR and chemotherapy, which resulted in IFN production that was blocked by LGP2 [134, 185]. Ectopic expression of MDA-5 in prostate cancer cells led to eradication of established tumors by activating innate and adaptive immunity via IFN [197]. These studies support the use of RLR agonists for cancer immune therapy [52, 107, 130, 145] although some of the effects are attributed to indirect immune activation by cancer cell apoptosis rather than direct activation of the immune system [47, 93].
Metabolic stress
Cancer cells feature a number of alterations in their metabolism and on the other hand can also influence the metabolic status of their environment. Due to their rapid and extensive replication, cancer cells are in high need for metabolic nutrients and oxygen, creating an altered microenvironment of hypoxia, low pH and/or nutrient deprivation. Metabolic stress can be defined as any sort of cellular stress caused by increased need for ATP, elevated biosynthesis of macromolecules or altered redox balance [19]. Thus, it is closely correlated with the before-mentioned forms of stress such as oxidative, proteotoxic, mitotic or DNA damage stress (Fig. 2). One well-characterized metabolic effect occurring in cancer is the Warburg effect, which refers to a shift from oxidative phosphorylation to oxygen-independent glycolysis, a faster but less efficient way to generate ATP under hypoxic conditions [180]. As a result, a tumor cell is in abnormally high demand for glucose uptake from the surrounding tissue. This section will focus on the control of immunity by several key processes resulting from metabolic stress, including hypoxic pathways, autophagy, mitochondrial stress, and oncometabolites.
As mentioned above, changes in metabolism can cause and be caused by hypoxia in the tumor microenvironment of solid cancers, which can influence infiltrated immune cells in different, mainly suppressive, ways. Hypoxia can directly inhibit the cytotoxic activity of NK cells [143], or lead to downregulation of stress ligands on the tumor cell surface [155]. It also decreases T-cell survival [166], IL-2 secretion [203] and increases the expression of the inhibitory ligand PD-L1 on tumor cells [10]. Under hypoxic conditions, tumor cells release a number of immune suppressive cytokines such as TGF-β, which inhibits T-cell proliferation and activation, promotes suppressive TReg development, inhibits antigen presentation by DCs and decreases the expression of activating NK cell receptors [189]. Together with IL-10 also released in response to hypoxia, TGF-β induces the differentiation of macrophages into a tumor-promoting M2 phenotype [70]. VEGF induced by hypoxia suppresses DC maturation and antigen presentation [58], increases their expression of PD-L1 [37] and promotes the accumulation of MDSCs in tumor tissue [58]. Moreover, it was shown in an ovarian cancer model that tumor cells can secrete the TReg-recruiting chemokine CCL28 under hypoxic conditions [54]. COX-2 expression is upregulated in cancer cells in response to hypoxia [66], resulting in effector T cell and DC suppression as well as in TReg and MDSC activation [159, 184, 195]. Hypoxia might not only be immune suppressive. Hypoxia-experiencing tumor cells release higher amounts of ATP, which can serve as a DAMP for inflammasome-induced immune responses [70], as described in the section about “DNA damage”.
Another direct result of metabolic stress is autophagy, the process in which a cell degrades, reassembles and recycles its components to survive under nutrient and energy starvation conditions [7]. Because autophagy modulates the cancer cell secretome and surface proteome, it can result in the release of immune-activating DAMPs such as ATP, ecto-CRT, or HMGB1 [177], and other secreted proteins such as cytokines [177] (see “DNA damage” and “Proteotoxic stress”). Autophagy can also promote DC and T-cell recruitment into the tumor bed and initiate immune responses [109]. In immune cells, autophagy can promote proliferation, antigen presentation, cell activation and cytokine secretion [74, 133, 177]. Cancers can also use hypoxia-induced intrinsic autophagy as an immune evasion mechanism because increased autophagy suppresses anti-tumor immune responses [4]. Additionally, it has recently been shown that cancer cells use autophagy-mediated degradation of granzyme B secreted from NK cells to evade lysis [9].
Metabolic stress can also result in damage of mitochondrial (mt) DNA due to hyperactive mitochondria in response to increased energetic requirements of cancer cells. Compared to nuclear DNA, mtDNA is more susceptible to damage because it is not associated with histones and constantly exposed to high ROS levels [178]. Therefore, immune responses similar to the ones activated in response to nuclear DNA damage (see “DNA damage”) can also be initiated by mtDNA damage, including activation of TLR9 [181, 199], the NLRP3 inflammasome [115, 153], and cGAS/STING [183].
Another result of the unique metabolism observed in cancer cells is the production of metabolic byproducts that are different from those of normal cells. These “oncometabolites” include lactate (or lactic acid), succinate, and 2-hydroxyglutarate (2-HG), among others [31]. Oncometabolites accumulate within the tumor microenvironment and have the capability to affect healthy host cells residing there. As described below, oncometabolites have been shown to regulate immune-mediated surveillance of cancer cells by directly affecting a broad range of immune cells, including macrophages, monocytes, NK cells, MDSCs, CD8+ T cells, and DCs.
One prominent effect of the altered metabolic state of cancer cells is the production and accumulation of lactate. This depends on lactate dehydrogenase (LDH), the enzyme responsible for catalyzing the formation of lactate from pyruvate in the final step of the glycolytic pathway [8]. LDH is frequently upregulated in various cancers and acts as an important control point for metabolic regulation in cancer cells. Lactate has been shown to promote tumor growth by negatively affecting immune surveillance in a number of different contexts. For example, tumor-associated macrophages can be functionally polarized toward a pro-tumor M2 phenotype by lactate derived from murine lung cancer cells [28]. This phenotypic change is mediated through HIF1α and appears to be critical for tumor growth, as lung tumors grown in mice lacking pro-tumor M2 macrophages grew significantly slower than tumors in WT mice [28]. This is in line with similar reports showing that during wound healing, extracellular lactate stimulates the production of the immunosuppressive M2 factors VEGF and TGF-β from macrophages [171]. Lactate also suppresses production of the pro-inflammatory cytokine TNF. Co-culturing monocytes with melanoma cells reduced the ability of monocytes to produce TNF, but this effect was not observed if the melanoma cells were pre-treated with oxamic acid, an LDH inhibitor that prevents the production of lactate [44].
NK cells are also negatively affected by tumor-derived lactate, both directly and indirectly via MDSCs [75]. In murine pancreatic cancer cells, knockdown of LdhA (and subsequent lack of lactate production) delays tumor growth relative to WT tumors, and NK cells from knockdown tumors have improved cytolytic function compared to NK cells from WT tumors. Matching this result, in vitro treatment of NK cells with lactate inhibits cytolytic function and decreases expression of cytolytic granules and the activating receptor NKp46. Furthermore, lactate appears to stimulate the development of MDSCs, a cell type capable of directly inhibiting NK cytotoxicity. MDSCs are present in higher abundance in mice bearing WT pancreatic tumors compared to mice bearing tumors that cannot produce lactate, and in vitro lactate treatment increases the generation of MDSCs [75]. These data show how tumor growth can be promoted by lactate through the functional impairment of NK cells and induction of MDSCs.
Tumor-derived lactate can also inhibit the function of CD8+ T cells. Co-culturing human CD8+ T cells with lactate-producing melanoma cells reduces T-cell proliferation and production of pro-inflammatory cytokines, but this effect is not observed using melanoma cells that have been pre-treated with oxamic acid and cannot produce lactate. Similarly, in human cancer patients, serum lactate levels and tumor burden are positively correlated, suggesting that lactate provides a positive signal for tumor growth [56]. Together, these results support that tumor-derived lactate induces inhibitory effects on adaptive immune cells and acts to suppress immune surveillance and promote tumor growth.
LDH, the enzyme responsible for producing lactate in tumor cells, has also been implicated in regulating immune–cancer interactions [8]. Indeed, glioma-derived LDH isoform 5 induces the expression of NKG2D ligands on myeloid cells, which subsequently decreases the expression of NKG2D on NK cells themselves. As a result of these interactions, NK cell-mediated killing of glioma cells is decreased [34]. Clearly tumor-derived lactate can have broad-reaching effects on many different types of immune cells and generally acts to inhibit immune-mediated cancer surveillance.
The oncometabolite 2-HG can promote tumor growth by acting cell-intrinsically to induce epigenetic reprogramming in cancer cells that can affect their ability to be recognized by immune cells. In gliomas, these epigenetic changes influence genes that regulate immune surveillance. The majority (~80%) of gliomas feature gain-of-function mutations in the enzyme IDH1 or 2, which causes the enzymes to produce the oncometabolite 2-HG instead of NADPH [192]. In gliomas harboring this activating mutation, NKG2D ligands are down regulated via epigenetic silencing, and these cancers thereby acquire resistance to NK cells in a manner dependent on 2-HG [200].
Not all oncometabolites are immune suppressive like lactate and 2-HG. Succinate, an intermediate in the citric acid cycle, has been shown to stimulate immune cells and could potentially induce immune surveillance. The accumulation of succinate occurs in rare cancers with mutations in genes coding for succinate dehydrogenase, such as paragangliomas and pheochromocytomas [39, 65]. In these cancers, levels of succinate are elevated, and so are HIF-1α and HIF-1α-related genes, suggesting that hypoxic pathways are also activated in these cancers [131]. Succinate is a known inflammatory signal that induces IL-1β secretion from macrophages in the context of LPS-induced activation [170]. Succinate can also act cell-extrinsically by signaling through its receptor GPR91 [71], leading to production of pro-inflammatory cytokines in DCs [139]. While it has not yet been empirically tested if these effects promote or inhibit cancer progression, one could speculate that the immune stimulatory effects of succinate would act to enhance immune surveillance and inhibit tumor growth. In this sense, succinate appears to oppose the immune inhibitory, tumor-promoting effects of lactate and 2-HG.
Concluding remarks
Immune cells make up a surprisingly large component of a tumor mass, and the activity of these cells plays an important role in dictating the outcome of cancer (i.e., rejection, equilibrium, progressive growth, metastasis, etc.). Here, we have focused on how the activity of immune cells is regulated by intrinsic stress occurring in cancer cells, and how this regulation subsequently affects tumor progression. We have defined five unique stress pathways that occur in cancer and described how each pathway affects immune surveillance of cancer. These stress pathways are highly interrelated, have broad-reaching effects on many different immune cells, and can both promote and inhibit anti-tumor immunity, depending on the context (Figs. 1, 2). Clearly, the regulation of immune cells by tumor cells is complex and can ultimately either inhibit or support tumor progression. In this sense, it becomes increasingly important to fully understand the intricate and sometimes paradoxical relationship between cancer and immune cells, since these interactions could become the basis for future cancer therapies.
Footnotes
Ruth Seelige and Stephen Searles contributed equally.
References
- 1.Acebes-Huerta A, Lorenzo-Herrero S, Folgueras AR, Huergo-Zapico L, Lopez-Larrea C, Lopez-Soto A, Gonzalez S. Drug-induced hyperploidy stimulates an antitumor NK cell response mediated by NKG2D and DNAM-1 receptors. Oncoimmunology. 2016;5:e1074378. doi: 10.1080/2162402X.2015.1074378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ahn J, Konno H, Barber GN. Diverse roles of STING-dependent signaling on the development of cancer. Oncogene. 2015;34:5302–5308. doi: 10.1038/onc.2014.457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Akazawa T, Ebihara T, Okuno M, Okuda Y, Shingai M, Tsujimura K, Takahashi T, Ikawa M, Okabe M, Inoue N, et al. Antitumor NK activation induced by the Toll-like receptor 3-TICAM-1 (TRIF) pathway in myeloid dendritic cells. Proc Natl Acad Sci USA. 2007;104:252–257. doi: 10.1073/pnas.0605978104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Amaravadi RK, Lippincott-Schwartz J, Yin XM, Weiss WA, Takebe N, Timmer W, DiPaola RS, Lotze MT, White E. Principles and current strategies for targeting autophagy for cancer treatment. Clin Cancer Res. 2011;17:654–666. doi: 10.1158/1078-0432.CCR-10-2634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Angelini G, Gardella S, Ardy M, Ciriolo MR, Filomeni G, Di Trapani G, Clarke F, Sitia R, Rubartelli A. Antigen-presenting dendritic cells provide the reducing extracellular microenvironment required for T lymphocyte activation. Proc Natl Acad Sci USA. 2002;99:1491–1496. doi: 10.1073/pnas.022630299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Apetoh L, Ghiringhelli F, Tesniere A, Obeid M, Ortiz C, Criollo A, Mignot G, Maiuri MC, Ullrich E, Saulnier P, et al. Toll-like receptor 4-dependent contribution of the immune system to anticancer chemotherapy and radiotherapy. Nat Med. 2007;13:1050–1059. doi: 10.1038/nm1622. [DOI] [PubMed] [Google Scholar]
- 7.Ashford TP, Porter KR. Cytoplasmic components in hepatic cell lysosomes. J Cell Biol. 1962;12:198–202. doi: 10.1083/jcb.12.1.198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Augoff K, Hryniewicz-Jankowska A, Tabola R. Lactate dehydrogenase 5: an old friend and a new hope in the war on cancer. Cancer Lett. 2015;358:1–7. doi: 10.1016/j.canlet.2014.12.035. [DOI] [PubMed] [Google Scholar]
- 9.Baginska J, Viry E, Berchem G, Poli A, Noman MZ, van Moer K, Medves S, Zimmer J, Oudin A, Niclou SP, et al. Granzyme B degradation by autophagy decreases tumor cell susceptibility to natural killer-mediated lysis under hypoxia. Proc Natl Acad Sci USA. 2013;110:17450–17455. doi: 10.1073/pnas.1304790110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Barsoum IB, Smallwood CA, Siemens DR, Graham CH. A mechanism of hypoxia-mediated escape from adaptive immunity in cancer cells. Cancer Res. 2014;74:665–674. doi: 10.1158/0008-5472.CAN-13-0992. [DOI] [PubMed] [Google Scholar]
- 11.Basu S, Binder RJ, Ramalingam T, Srivastava PK. CD91 is a common receptor for heat shock proteins gp96, hsp90, hsp70, and calreticulin. Immunity. 2001;14:303–313. doi: 10.1016/S1074-7613(01)00111-X. [DOI] [PubMed] [Google Scholar]
- 12.Benci JL, Xu B, Qiu Y, Wu TJ, Dada H, Twyman-Saint Victor C, Cucolo L, Lee DS, Pauken KE, Huang AC, et al. Tumor interferon signaling regulates a multigenic resistance program to immune checkpoint blockade. Cell. 2016;167(1540–1554):e1512. doi: 10.1016/j.cell.2016.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bertini R, Howard OM, Dong HF, Oppenheim JJ, Bizzarri C, Sergi R, Caselli G, Pagliei S, Romines B, Wilshire JA, et al. Thioredoxin, a redox enzyme released in infection and inflammation, is a unique chemoattractant for neutrophils, monocytes, and T cells. J Exp Med. 1999;189:1783–1789. doi: 10.1084/jem.189.11.1783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bhattacharyya S, Mandal D, Sen GS, Pal S, Banerjee S, Lahiry L, Finke JH, Tannenbaum CS, Das T, Sa G. Tumor-induced oxidative stress perturbs nuclear factor-kappaB activity-augmenting tumor necrosis factor-alpha-mediated T-cell death: protection by curcumin. Cancer Res. 2007;67:362–370. doi: 10.1158/0008-5472.CAN-06-2583. [DOI] [PubMed] [Google Scholar]
- 15.Boileve A, Senovilla L, Vitale I, Lissa D, Martins I, Metivier D, van den Brink S, Clevers H, Galluzzi L, Castedo M, Kroemer G. Immunosurveillance against tetraploidization-induced colon tumorigenesis. Cell Cycle. 2013;12:473–479. doi: 10.4161/cc.23369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Braakman I, Bulleid NJ. Protein folding and modification in the mammalian endoplasmic reticulum. Annu Rev Biochem. 2011;80:71–99. doi: 10.1146/annurev-biochem-062209-093836. [DOI] [PubMed] [Google Scholar]
- 17.Bronte V, Serafini P, Mazzoni A, Segal DM, Zanovello P. l-Arginine metabolism in myeloid cells controls T-lymphocyte functions. Trends Immunol. 2003;24:302–306. doi: 10.1016/S1471-4906(03)00132-7. [DOI] [PubMed] [Google Scholar]
- 18.Brzostek-Racine S, Gordon C, Van Scoy S, Reich NC. The DNA damage response induces IFN. J Immunol. 2011;187:5336–5345. doi: 10.4049/jimmunol.1100040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cairns RA, Harris IS, Mak TW. Regulation of cancer cell metabolism. Nat Rev Cancer. 2011;11:85–95. doi: 10.1038/nrc2981. [DOI] [PubMed] [Google Scholar]
- 20.Centuori SM, Trad M, LaCasse CJ, Alizadeh D, Larmonier CB, Hanke NT, Kartchner J, Janikashvili N, Bonnotte B, Larmonier N, Katsanis E. Myeloid-derived suppressor cells from tumor-bearing mice impair TGF-beta-induced differentiation of CD4 + CD25 + FoxP3 + Tregs from CD4 + CD25 − FoxP3 − T cells. J Leukoc Biol. 2012;92:987–997. doi: 10.1189/jlb.0911465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cerboni C, Fionda C, Soriani A, Zingoni A, Doria M, Cippitelli M, Santoni A. The DNA damage response: a common pathway in the regulation of NKG2D and DNAM-1 ligand expression in normal, infected, and cancer cells. Front Immunol. 2014;4:508. doi: 10.3389/fimmu.2013.00508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chan CJ, Smyth MJ, Martinet L. Molecular mechanisms of natural killer cell activation in response to cellular stress. Cell Death Differ. 2014;21:5–14. doi: 10.1038/cdd.2013.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chatterjee S, Crozet L, Damotte D, Iribarren K, Schramm C, Alifano M, Lupo A, Cherfils-Vicini J, Goc J, Katsahian S, et al. TLR7 promotes tumor progression, chemotherapy resistance, and poor clinical outcomes in non-small cell lung cancer. Cancer Res. 2014;74:5008–5018. doi: 10.1158/0008-5472.CAN-13-2698. [DOI] [PubMed] [Google Scholar]
- 24.Chaudhri G, Clark IA, Hunt NH, Cowden WB, Ceredig R. Effect of antioxidants on primary alloantigen-induced T cell activation and proliferation. J Immunol. 1986;137:2646–2652. [PubMed] [Google Scholar]
- 25.Chaudhri G, Hunt NH, Clark IA, Ceredig R. Antioxidants inhibit proliferation and cell surface expression of receptors for interleukin-2 and transferrin in T lymphocytes stimulated with phorbol myristate acetate and ionomycin. Cell Immunol. 1988;115:204–213. doi: 10.1016/0008-8749(88)90174-8. [DOI] [PubMed] [Google Scholar]
- 26.Chiang CL, Ledermann JA, Aitkens E, Benjamin E, Katz DR, Chain BM. Oxidation of ovarian epithelial cancer cells by hypochlorous acid enhances immunogenicity and stimulates T cells that recognize autologous primary tumor. Clin Cancer Res. 2008;14:4898–4907. doi: 10.1158/1078-0432.CCR-07-4899. [DOI] [PubMed] [Google Scholar]
- 27.Chin AI, Miyahira AK, Covarrubias A, Teague J, Guo B, Dempsey PW, Cheng G. Toll-like receptor 3-mediated suppression of TRAMP prostate cancer shows the critical role of type I interferons in tumor immune surveillance. Cancer Res. 2010;70:2595–2603. doi: 10.1158/0008-5472.CAN-09-1162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Colegio OR, Chu NQ, Szabo AL, Chu T, Rhebergen AM, Jairam V, Cyrus N, Brokowski CE, Eisenbarth SC, Phillips GM, et al. Functional polarization of tumour-associated macrophages by tumour-derived lactic acid. Nature. 2014;513:559–563. doi: 10.1038/nature13490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Coppe JP, Desprez PY, Krtolica A, Campisi J. The senescence-associated secretory phenotype: the dark side of tumor suppression. Annu Rev Pathol. 2010;5:99–118. doi: 10.1146/annurev-pathol-121808-102144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Coppe JP, Patil CK, Rodier F, Sun Y, Munoz DP, Goldstein J, Nelson PS, Desprez PY, Campisi J. Senescence-associated secretory phenotypes reveal cell-nonautonomous functions of oncogenic RAS and the p53 tumor suppressor. PLoS Biol. 2008;6:2853–2868. doi: 10.1371/journal.pbio.0060301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Corrado M, Scorrano L, Campello S. Changing perspective on oncometabolites: from metabolic signature of cancer to tumorigenic and immunosuppressive agents. Oncotarget. 2016;7:46692–46706. doi: 10.18632/oncotarget.8727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Courtois-Cox S, Jones SL, Cichowski K. Many roads lead to oncogene-induced senescence. Oncogene. 2008;27:2801–2809. doi: 10.1038/sj.onc.1210950. [DOI] [PubMed] [Google Scholar]
- 33.Couzin-Frankel J. Breakthrough of the year 2013. Cancer immunotherapy. Science. 2013;342:1432–1433. doi: 10.1126/science.342.6165.1432. [DOI] [PubMed] [Google Scholar]
- 34.Crane CA, Austgen K, Haberthur K, Hofmann C, Moyes KW, Avanesyan L, Fong L, Campbell MJ, Cooper S, Oakes SA, et al. Immune evasion mediated by tumor-derived lactate dehydrogenase induction of NKG2D ligands on myeloid cells in glioblastoma patients. Proc Natl Acad Sci USA. 2014;111:12823–12828. doi: 10.1073/pnas.1413933111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cubillos-Ruiz JR, Bettigole SE, Glimcher LH. Tumorigenic and immunosuppressive effects of endoplasmic reticulum stress in cancer. Cell. 2017;168:692–706. doi: 10.1016/j.cell.2016.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cullen SJ, Fatemie S, Ladiges W. Breast tumor cells primed by endoplasmic reticulum stress remodel macrophage phenotype. Am J Cancer Res. 2013;3:196–210. [PMC free article] [PubMed] [Google Scholar]
- 37.Curiel TJ, Wei S, Dong H, Alvarez X, Cheng P, Mottram P, Krzysiek R, Knutson KL, Daniel B, Zimmermann MC, et al. Blockade of B7-H1 improves myeloid dendritic cell-mediated antitumor immunity. Nat Med. 2003;9:562–567. doi: 10.1038/nm863. [DOI] [PubMed] [Google Scholar]
- 38.da Silva Krause M, Bittencourt A, de Bittencourt PIH, Jr McClenaghan NH, Flatt PR, Murphy C, Newsholme P. Physiological concentrations of interleukin-6 directly promote insulin secretion, signal transduction, nitric oxide release, and redox status in a clonal pancreatic beta-cell line and mouse islets. J Endocrinol. 2012;214:301–311. doi: 10.1530/JOE-12-0223. [DOI] [PubMed] [Google Scholar]
- 39.Dahia PL, Ross KN, Wright ME, Hayashida CY, Santagata S, Barontini M, Kung AL, Sanso G, Powers JF, Tischler AS, et al. A HIF1alpha regulatory loop links hypoxia and mitochondrial signals in pheochromocytomas. PLoS Genet. 2005;1:72–80. doi: 10.1371/journal.pgen.0010008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Deng J, Lu PD, Zhang Y, Scheuner D, Kaufman RJ, Sonenberg N, Harding HP, Ron D. Translational repression mediates activation of nuclear factor kappa B by phosphorylated translation initiation factor 2. Mol Cell Biol. 2004;24:10161–10168. doi: 10.1128/MCB.24.23.10161-10168.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Deng L, Liang H, Xu M, Yang X, Burnette B, Arina A, Li XD, Mauceri H, Beckett M, Darga T, et al. STING-dependent cytosolic DNA sensing promotes radiation-induced type I interferon-dependent antitumor immunity in immunogenic tumors. Immunity. 2014;41:843–852. doi: 10.1016/j.immuni.2014.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Devadas S, Zaritskaya L, Rhee SG, Oberley L, Williams MS. Discrete generation of superoxide and hydrogen peroxide by T cell receptor stimulation: selective regulation of mitogen-activated protein kinase activation and fas ligand expression. J Exp Med. 2002;195:59–70. doi: 10.1084/jem.20010659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.DeYoung KL, Ray ME, Su YA, Anzick SL, Johnstone RW, Trapani JA, Meltzer PS, Trent JM. Cloning a novel member of the human interferon-inducible gene family associated with control of tumorigenicity in a model of human melanoma. Oncogene. 1997;15:453–457. doi: 10.1038/sj.onc.1201206. [DOI] [PubMed] [Google Scholar]
- 44.Dietl K, Renner K, Dettmer K, Timischl B, Eberhart K, Dorn C, Hellerbrand C, Kastenberger M, Kunz-Schughart LA, Oefner PJ, et al. Lactic acid and acidification inhibit TNF secretion and glycolysis of human monocytes. J Immunol. 2010;184:1200–1209. doi: 10.4049/jimmunol.0902584. [DOI] [PubMed] [Google Scholar]
- 45.Dihlmann S, Tao S, Echterdiek F, Herpel E, Jansen L, Chang-Claude J, Brenner H, Hoffmeister M, Kloor M. Lack of Absent in Melanoma 2 (AIM2) expression in tumor cells is closely associated with poor survival in colorectal cancer patients. Int J Cancer. 2014;135:2387–2396. doi: 10.1002/ijc.28891. [DOI] [PubMed] [Google Scholar]
- 46.Dikovskaya D, Cole JJ, Mason SM, Nixon C, Karim SA, McGarry L, Clark W, Hewitt RN, Sammons MA, Zhu J, et al. Mitotic stress is an integral part of the oncogene-induced senescence program that promotes multinucleation and cell cycle arrest. Cell Rep. 2015;12:1483–1496. doi: 10.1016/j.celrep.2015.07.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Duewell P, Steger A, Lohr H, Bourhis H, Hoelz H, Kirchleitner SV, Stieg MR, Grassmann S, Kobold S, Siveke JT, et al. RIG-I-like helicases induce immunogenic cell death of pancreatic cancer cells and sensitize tumors toward killing by CD8(+) T cells. Cell Death Differ. 2014;21:1825–1837. doi: 10.1038/cdd.2014.96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Duijf PH, Benezra R. The cancer biology of whole-chromosome instability. Oncogene. 2013;32:4727–4736. doi: 10.1038/onc.2012.616. [DOI] [PubMed] [Google Scholar]
- 49.Duwe AK, Roder JC. Involvement of hydroxyl free radical, but not superoxide, in the cytolytic pathway of natural killer cells. Revision of an earlier hypothesis. Med Biol. 1984;62:95–100. [PubMed] [Google Scholar]
- 50.Duwe AK, Werkmeister J, Roder JC, Lauzon R, Payne U. Natural killer cell-mediated lysis involves an hydroxyl radical-dependent step. J Immunol. 1985;134:2637–2644. [PubMed] [Google Scholar]
- 51.Efimova O, Szankasi P, Kelley TW. Ncf1 (p47phox) is essential for direct regulatory T cell mediated suppression of CD4+ effector T cells. PLoS One. 2011;6:e16013. doi: 10.1371/journal.pone.0016013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ellermeier J, Wei J, Duewell P, Hoves S, Stieg MR, Adunka T, Noerenberg D, Anders HJ, Mayr D, Poeck H, et al. Therapeutic efficacy of bifunctional siRNA combining TGF-beta1 silencing with RIG-I activation in pancreatic cancer. Cancer Res. 2013;73:1709–1720. doi: 10.1158/0008-5472.CAN-11-3850. [DOI] [PubMed] [Google Scholar]
- 53.Elliott MR, Chekeni FB, Trampont PC, Lazarowski ER, Kadl A, Walk SF, Park D, Woodson RI, Ostankovich M, Sharma P, et al. Nucleotides released by apoptotic cells act as a find-me signal to promote phagocytic clearance. Nature. 2009;461:282–286. doi: 10.1038/nature08296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Facciabene A, Peng X, Hagemann IS, Balint K, Barchetti A, Wang LP, Gimotty PA, Gilks CB, Lal P, Zhang L, Coukos G. Tumour hypoxia promotes tolerance and angiogenesis via CCL28 and T(reg) cells. Nature. 2011;475:226–230. doi: 10.1038/nature10169. [DOI] [PubMed] [Google Scholar]
- 55.Fidelus RK, Ginouves P, Lawrence D, Tsan MF. Modulation of intracellular glutathione concentrations alters lymphocyte activation and proliferation. Exp Cell Res. 1987;170:269–275. doi: 10.1016/0014-4827(87)90305-3. [DOI] [PubMed] [Google Scholar]
- 56.Fischer K, Hoffmann P, Voelkl S, Meidenbauer N, Ammer J, Edinger M, Gottfried E, Schwarz S, Rothe G, Hoves S, et al. Inhibitory effect of tumor cell-derived lactic acid on human T cells. Blood. 2007;109:3812–3819. doi: 10.1182/blood-2006-07-035972. [DOI] [PubMed] [Google Scholar]
- 57.Forte G, Rega A, Morello S, Luciano A, Arra C, Pinto A, Sorrentino R. Polyinosinic-polycytidylic acid limits tumor outgrowth in a mouse model of metastatic lung cancer. J Immunol. 2012;188:5357–5364. doi: 10.4049/jimmunol.1103811. [DOI] [PubMed] [Google Scholar]
- 58.Gabrilovich D. Mechanisms and functional significance of tumour-induced dendritic-cell defects. Nat Rev Immunol. 2004;4:941–952. doi: 10.1038/nri1498. [DOI] [PubMed] [Google Scholar]
- 59.Gabrilovich DI, Nagaraj S. Myeloid-derived suppressor cells as regulators of the immune system. Nat Rev Immunol. 2009;9:162–174. doi: 10.1038/nri2506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Galli R, Starace D, Busa R, Angelini DF, Paone A, De Cesaris P, Filippini A, Sette C, Battistini L, Ziparo E, Riccioli A. TLR stimulation of prostate tumor cells induces chemokine-mediated recruitment of specific immune cell types. J Immunol. 2010;184:6658–6669. doi: 10.4049/jimmunol.0902401. [DOI] [PubMed] [Google Scholar]
- 61.Gao C, Kozlowska A, Nechaev S, Li H, Zhang Q, Hossain DM, Kowolik CM, Chu P, Swiderski P, Diamond DJ, et al. TLR9 signaling in the tumor microenvironment initiates cancer recurrence after radiotherapy. Cancer Res. 2013;73:7211–7221. doi: 10.1158/0008-5472.CAN-13-1314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Gasser S, Orsulic S, Brown EJ, Raulet DH. The DNA damage pathway regulates innate immune system ligands of the NKG2D receptor. Nature. 2005;436:1186–1190. doi: 10.1038/nature03884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Gasser S, Raulet DH. The DNA damage response arouses the immune system. Can Res. 2006;66:3959–3962. doi: 10.1158/0008-5472.CAN-05-4603. [DOI] [PubMed] [Google Scholar]
- 64.Ghiringhelli F, Apetoh L, Tesniere A, Aymeric L, Ma Y, Ortiz C, Vermaelen K, Panaretakis T, Mignot G, Ullrich E, et al. Activation of the NLRP3 inflammasome in dendritic cells induces IL-1beta-dependent adaptive immunity against tumors. Nat Med. 2009;15:1170–1178. doi: 10.1038/nm.2028. [DOI] [PubMed] [Google Scholar]
- 65.Gimenez-Roqueplo AP, Favier J, Rustin P, Mourad JJ, Plouin PF, Corvol P, Rotig A, Jeunemaitre X. The R22X mutation of the SDHD gene in hereditary paraganglioma abolishes the enzymatic activity of complex II in the mitochondrial respiratory chain and activates the hypoxia pathway. Am J Hum Genet. 2001;69:1186–1197. doi: 10.1086/324413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Greenhough A, Smartt HJ, Moore AE, Roberts HR, Williams AC, Paraskeva C, Kaidi A. The COX-2/PGE2 pathway: key roles in the hallmarks of cancer and adaptation to the tumour microenvironment. Carcinogenesis. 2009;30:377–386. doi: 10.1093/carcin/bgp014. [DOI] [PubMed] [Google Scholar]
- 67.Hanahan D, Weinberg RA. The hallmarks of cancer. Cell. 2000;100:57–70. doi: 10.1016/S0092-8674(00)81683-9. [DOI] [PubMed] [Google Scholar]
- 68.Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 69.Hansson M, Asea A, Ersson U, Hermodsson S, Hellstrand K. Induction of apoptosis in NK cells by monocyte-derived reactive oxygen metabolites. J Immunol. 1996;156:42–47. [PubMed] [Google Scholar]
- 70.Hao NB, Lu MH, Fan YH, Cao YL, Zhang ZR, Yang SM. Macrophages in tumor microenvironments and the progression of tumors. Clin Dev Immunol. 2012;2012:948098. doi: 10.1155/2012/948098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.He W, Miao FJ, Lin DC, Schwandner RT, Wang Z, Gao J, Chen JL, Tian H, Ling L. Citric acid cycle intermediates as ligands for orphan G-protein-coupled receptors. Nature. 2004;429:188–193. doi: 10.1038/nature02488. [DOI] [PubMed] [Google Scholar]
- 72.Hellstrand K, Asea A, Dahlgren C, Hermodsson S. Histaminergic regulation of NK cells. Role of monocyte-derived reactive oxygen metabolites. J Immunol. 1994;153:4940–4947. [PubMed] [Google Scholar]
- 73.Hu B, Jin C, Li HB, Tong J, Ouyang X, Cetinbas NM, Zhu S, Strowig T, Lam FC, Zhao C, et al. The DNA-sensing AIM2 inflammasome controls radiation-induced cell death and tissue injury. Science. 2016;354:765–768. doi: 10.1126/science.aaf7532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Hubbard VM, Valdor R, Patel B, Singh R, Cuervo AM, Macian F. Macroautophagy regulates energy metabolism during effector T cell activation. J Immunol. 2010;185:7349–7357. doi: 10.4049/jimmunol.1000576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Husain Z, Huang Y, Seth P, Sukhatme VP. Tumor-derived lactate modifies antitumor immune response: effect on myeloid-derived suppressor cells and NK cells. J Immunol. 2013;191:1486–1495. doi: 10.4049/jimmunol.1202702. [DOI] [PubMed] [Google Scholar]
- 76.Iannello A, Thompson TW, Ardolino M, Lowe SW, Raulet DH. p53-dependent chemokine production by senescent tumor cells supports NKG2D-dependent tumor elimination by natural killer cells. J Exp Med. 2013;210:2057–2069. doi: 10.1084/jem.20130783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Isaacs A, Lindenmann J. Virus interference. I. The interferon. Proc R Soc Lond B Biol Sci. 1957;147:258–267. doi: 10.1098/rspb.1957.0048. [DOI] [PubMed] [Google Scholar]
- 78.Ishikawa H, Barber GN. STING is an endoplasmic reticulum adaptor that facilitates innate immune signalling. Nature. 2008;455:674–678. doi: 10.1038/nature07317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Ishikawa H, Ma Z, Barber GN. STING regulates intracellular DNA-mediated, type I interferon-dependent innate immunity. Nature. 2009;461:788–792. doi: 10.1038/nature08476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Izawa S, Kono K, Mimura K, Kawaguchi Y, Watanabe M, Maruyama T, Fujii H. H(2)O(2) production within tumor microenvironment inversely correlated with infiltration of CD56(dim) NK cells in gastric and esophageal cancer: possible mechanisms of NK cell dysfunction. Cancer Immunol Immunother. 2011;60:1801–1810. doi: 10.1007/s00262-011-1082-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Kang TW, Yevsa T, Woller N, Hoenicke L, Wuestefeld T, Dauch D, Hohmeyer A, Gereke M, Rudalska R, Potapova A, et al. Senescence surveillance of pre-malignant hepatocytes limits liver cancer development. Nature. 2011;479:547–551. doi: 10.1038/nature10599. [DOI] [PubMed] [Google Scholar]
- 82.Karin M. NF-kappaB as a critical link between inflammation and cancer. Cold Spring Harb Perspect Biol. 2009;1:a000141. doi: 10.1101/cshperspect.a000141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Kawai T, Akira S. The role of pattern-recognition receptors in innate immunity: update on Toll-like receptors. Nat Immunol. 2010;11:373–384. doi: 10.1038/ni.1863. [DOI] [PubMed] [Google Scholar]
- 84.Khaled YS, Ammori BJ, Elkord E. Myeloid-derived suppressor cells in cancer: recent progress and prospects. Immunol Cell Biol. 2013;91:493–502. doi: 10.1038/icb.2013.29. [DOI] [PubMed] [Google Scholar]
- 85.King MR, Ismail AS, Davis LS, Karp DR. Oxidative stress promotes polarization of human T cell differentiation toward a T helper 2 phenotype. J Immunol. 2006;176:2765–2772. doi: 10.4049/jimmunol.176.5.2765. [DOI] [PubMed] [Google Scholar]
- 86.Klebanoff SJ. Myeloperoxidase: friend and foe. J Leukoc Biol. 2005;77:598–625. doi: 10.1189/jlb.1204697. [DOI] [PubMed] [Google Scholar]
- 87.Kokkola R, Andersson A, Mullins G, Ostberg T, Treutiger CJ, Arnold B, Nawroth P, Andersson U, Harris RA, Harris HE. RAGE is the major receptor for the proinflammatory activity of HMGB1 in rodent macrophages. Scand J Immunol. 2005;61:1–9. doi: 10.1111/j.0300-9475.2005.01534.x. [DOI] [PubMed] [Google Scholar]
- 88.Kono K, Salazar-Onfray F, Petersson M, Hansson J, Masucci G, Wasserman K, Nakazawa T, Anderson P, Kiessling R. Hydrogen peroxide secreted by tumor-derived macrophages down-modulates signal-transducing zeta molecules and inhibits tumor-specific T cell-and natural killer cell-mediated cytotoxicity. Eur J Immunol. 1996;26:1308–1313. doi: 10.1002/eji.1830260620. [DOI] [PubMed] [Google Scholar]
- 89.Kops GJ, Weaver BA, Cleveland DW. On the road to cancer: aneuploidy and the mitotic checkpoint. Nat Rev Cancer. 2005;5:773–785. doi: 10.1038/nrc1714. [DOI] [PubMed] [Google Scholar]
- 90.Kraaij MD, Savage ND, van der Kooij SW, Koekkoek K, Wang J, van den Berg JM, Ottenhoff TH, Kuijpers TW, Holmdahl R, van Kooten C, Gelderman KA. Induction of regulatory T cells by macrophages is dependent on production of reactive oxygen species. Proc Natl Acad Sci USA. 2010;107:17686–17691. doi: 10.1073/pnas.1012016107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Krieg AM. Toll-like receptor 9 (TLR9) agonists in the treatment of cancer. Oncogene. 2008;27:161–167. doi: 10.1038/sj.onc.1210911. [DOI] [PubMed] [Google Scholar]
- 92.Kroemer G, Galluzzi L, Kepp O, Zitvogel L. Immunogenic cell death in cancer therapy. Annu Rev Immunol. 2013;31:51–72. doi: 10.1146/annurev-immunol-032712-100008. [DOI] [PubMed] [Google Scholar]
- 93.Kubler K, tho Pesch C, Gehrke N, Riemann S, Dassler J, Coch C, Landsberg J, Wimmenauer V, Polcher M, Rudlowski C, et al. Immunogenic cell death of human ovarian cancer cells induced by cytosolic poly(I:C) leads to myeloid cell maturation and activates NK cells. Eur J Immunol. 2011;41:3028–3039. doi: 10.1002/eji.201141555. [DOI] [PubMed] [Google Scholar]
- 94.Kusmartsev S, Gabrilovich DI. Inhibition of myeloid cell differentiation in cancer: the role of reactive oxygen species. J Leukoc Biol. 2003;74:186–196. doi: 10.1189/jlb.0103010. [DOI] [PubMed] [Google Scholar]
- 95.Lahdenpohja N, Savinainen K, Hurme M. Pre-exposure to oxidative stress decreases the nuclear factor-kappa B-dependent transcription in T lymphocytes. J Immunol. 1998;160:1354–1358. [PubMed] [Google Scholar]
- 96.Lasry A, Ben-Neriah Y. Senescence-associated inflammatory responses: aging and cancer perspectives. Trends Immunol. 2015;36:217–228. doi: 10.1016/j.it.2015.02.009. [DOI] [PubMed] [Google Scholar]
- 97.Li T, Cheng H, Yuan H, Xu Q, Shu C, Zhang Y, Xu P, Tan J, Rui Y, Li P, Tan X. Antitumor activity of cGAMP via stimulation of cGAS-cGAMP-STING-IRF3 mediated innate immune response. Sci Rep. 2016;6:19049. doi: 10.1038/srep19049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Liontos M, Anastasiou I, Bamias A, Dimopoulos MA. DNA damage, tumor mutational load and their impact on immune responses against cancer. Ann Transl Med. 2016;4:264. doi: 10.21037/atm.2016.07.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Liu D, Shaukat Z, Saint RB, Gregory SL. Chromosomal instability triggers cell death via local signalling through the innate immune receptor Toll. Oncotarget. 2015;6:38552–38565. doi: 10.18632/oncotarget.6035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Lopez-Larrea C, Suarez-Alvarez B, Lopez-Soto A, Lopez-Vazquez A, Gonzalez S. The NKG2D receptor: sensing stressed cells. Trends Mol Med. 2008;14:179–189. doi: 10.1016/j.molmed.2008.02.004. [DOI] [PubMed] [Google Scholar]
- 101.Luo J, Solimini NL, Elledge SJ. Principles of cancer therapy: oncogene and non-oncogene addiction. Cell. 2009;136:823–837. doi: 10.1016/j.cell.2009.02.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Mahadevan NR, Anufreichik V, Rodvold JJ, Chiu KT, Sepulveda H, Zanetti M. Cell-extrinsic effects of tumor ER stress imprint myeloid dendritic cells and impair CD8(+) T cell priming. PLoS One. 2012;7:e51845. doi: 10.1371/journal.pone.0051845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Mahadevan NR, Rodvold J, Sepulveda H, Rossi S, Drew AF, Zanetti M. Transmission of endoplasmic reticulum stress and pro-inflammation from tumor cells to myeloid cells. Proc Natl Acad Sci USA. 2011;108:6561–6566. doi: 10.1073/pnas.1008942108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Malmberg KJ, Arulampalam V, Ichihara F, Petersson M, Seki K, Andersson T, Lenkei R, Masucci G, Pettersson S, Kiessling R. Inhibition of activated/memory (CD45RO(+)) T cells by oxidative stress associated with block of NF-kappaB activation. J Immunol. 2001;167:2595–2601. doi: 10.4049/jimmunol.167.5.2595. [DOI] [PubMed] [Google Scholar]
- 105.Man SM, Zhu Q, Zhu L, Liu Z, Karki R, Malik A, Sharma D, Li L, Malireddi RK, Gurung P, et al. Critical role for the DNA sensor AIM2 in stem cell proliferation and cancer. Cell. 2015;162:45–58. doi: 10.1016/j.cell.2015.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Matsue H, Edelbaum D, Shalhevet D, Mizumoto N, Yang C, Mummert ME, Oeda J, Masayasu H, Takashima A. Generation and function of reactive oxygen species in dendritic cells during antigen presentation. J Immunol. 2003;171:3010–3018. doi: 10.4049/jimmunol.171.6.3010. [DOI] [PubMed] [Google Scholar]
- 107.McCartney S, Vermi W, Gilfillan S, Cella M, Murphy TL, Schreiber RD, Murphy KM, Colonna M. Distinct and complementary functions of MDA5 and TLR3 in poly(I:C)-mediated activation of mouse NK cells. J Exp Med. 2009;206:2967–2976. doi: 10.1084/jem.20091181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Mellqvist UH, Hansson M, Brune M, Dahlgren C, Hermodsson S, Hellstrand K. Natural killer cell dysfunction and apoptosis induced by chronic myelogenous leukemia cells: role of reactive oxygen species and regulation by histamine. Blood. 2000;96:1961–1968. [PubMed] [Google Scholar]
- 109.Michaud M, Martins I, Sukkurwala AQ, Adjemian S, Ma Y, Pellegatti P, Shen S, Kepp O, Scoazec M, Mignot G, et al. Autophagy-dependent anticancer immune responses induced by chemotherapeutic agents in mice. Science. 2011;334:1573–1577. doi: 10.1126/science.1208347. [DOI] [PubMed] [Google Scholar]
- 110.Mishell RI, Dutton RW. Immunization of normal mouse spleen cell suspensions in vitro. Science. 1966;153:1004–1006. doi: 10.1126/science.153.3739.1004. [DOI] [PubMed] [Google Scholar]
- 111.Mitelman F, Johansson B, Mertens F (eds) (2017) Mitelman database of chromosome aberrations and gene fusions in cancer. http://cgap.nci.nih.gov/Chromosomes/Mitelman
- 112.Mougiakakos D, Johansson CC, Jitschin R, Bottcher M, Kiessling R. Increased thioredoxin-1 production in human naturally occurring regulatory T cells confers enhanced tolerance to oxidative stress. Blood. 2011;117:857–861. doi: 10.1182/blood-2010-09-307041. [DOI] [PubMed] [Google Scholar]
- 113.Nagaraj S, Gupta K, Pisarev V, Kinarsky L, Sherman S, Kang L, Herber DL, Schneck J, Gabrilovich DI. Altered recognition of antigen is a mechanism of CD8+ T cell tolerance in cancer. Nat Med. 2007;13:828–835. doi: 10.1038/nm1609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Nagato T, Lee YR, Harabuchi Y, Celis E. Combinatorial immunotherapy of polyinosinic-polycytidylic acid and blockade of programmed death-ligand 1 induce effective CD8 T-cell responses against established tumors. Clin Cancer Res. 2014;20:1223–1234. doi: 10.1158/1078-0432.CCR-13-2781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Nakahira K, Haspel JA, Rathinam VA, Lee SJ, Dolinay T, Lam HC, Englert JA, Rabinovitch M, Cernadas M, Kim HP, et al. Autophagy proteins regulate innate immune responses by inhibiting the release of mitochondrial DNA mediated by the NALP3 inflammasome. Nat Immunol. 2011;12:222–230. doi: 10.1038/ni.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Nathan C, Cohn Z. Role of oxygen-dependent mechanisms in antibody-induced lysis of tumor cells by activated macrophages. J Exp Med. 1980;152:198–208. doi: 10.1084/jem.152.1.198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Nathan CF, Arrick BA, Murray HW, DeSantis NM, Cohn ZA. Tumor cell anti-oxidant defenses. Inhibition of the glutathione redox cycle enhances macrophage-mediated cytolysis. J Exp Med. 1981;153:766–782. doi: 10.1084/jem.153.4.766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Nathan CF, Klebanoff SJ. Augmentation of spontaneous macrophage-mediated cytolysis by eosinophil peroxidase. J Exp Med. 1982;155:1291–1308. doi: 10.1084/jem.155.5.1291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Nathan CF, Silverstein SC, Brukner LH, Cohn ZA. Extracellular cytolysis by activated macrophages and granulocytes. II. Hydrogen peroxide as a mediator of cytotoxicity. J Exp Med. 1979;149:100–113. doi: 10.1084/jem.149.1.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Nathan CF, Terry WD. Differential stimulation of murine lymphoma growth in vitro by normal and BCG-activated macrophages. J Exp Med. 1975;142:887–902. doi: 10.1084/jem.142.4.887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Nguyen AH, Detty SQ, Agrawal DK. Clinical Implications of high-mobility group box-1 (HMGB1) and the receptor for advanced glycation end-products (RAGE) in cutaneous malignancy: a systematic review. Anticancer Res. 2017;37:1–7. doi: 10.21873/anticanres.11282. [DOI] [PubMed] [Google Scholar]
- 122.Niethammer P, Grabher C, Look AT, Mitchison TJ. A tissue-scale gradient of hydrogen peroxide mediates rapid wound detection in zebrafish. Nature. 2009;459:996–999. doi: 10.1038/nature08119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Novogrodsky A, Ravid A, Rubin AL, Stenzel KH. Hydroxyl radical scavengers inhibit lymphocyte mitogenesis. Proc Natl Acad Sci USA. 1982;79:1171–1174. doi: 10.1073/pnas.79.4.1171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Obeid M, Tesniere A, Ghiringhelli F, Fimia GM, Apetoh L, Perfettini JL, Castedo M, Mignot G, Panaretakis T, Casares N, et al. Calreticulin exposure dictates the immunogenicity of cancer cell death. Nat Med. 2007;13:54–61. doi: 10.1038/nm1523. [DOI] [PubMed] [Google Scholar]
- 125.Ohkuri T, Ghosh A, Kosaka A, Zhu J, Ikeura M, David M, Watkins SC, Sarkar SN, Okada H. STING contributes to antiglioma immunity via triggering type I IFN signals in the tumor microenvironment. Cancer Immunol Res. 2014;2:1199–1208. doi: 10.1158/2326-6066.CIR-14-0099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Pahl HL, Baeuerle PA. Activation of NF-kappa B by ER stress requires both Ca2+ and reactive oxygen intermediates as messengers. FEBS Lett. 1996;392:129–136. doi: 10.1016/0014-5793(96)00800-9. [DOI] [PubMed] [Google Scholar]
- 127.Pamment J, Ramsay E, Kelleher M, Dornan D, Ball KL. Regulation of the IRF-1 tumour modifier during the response to genotoxic stress involves an ATM-dependent signalling pathway. Oncogene. 2002;21:7776–7785. doi: 10.1038/sj.onc.1205981. [DOI] [PubMed] [Google Scholar]
- 128.Panaretakis T, Kepp O, Brockmeier U, Tesniere A, Bjorklund AC, Chapman DC, Durchschlag M, Joza N, Pierron G, van Endert P, et al. Mechanisms of pre-apoptotic calreticulin exposure in immunogenic cell death. EMBO J. 2009;28:578–590. doi: 10.1038/emboj.2009.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Panieri E, Santoro MM. ROS homeostasis and metabolism: a dangerous liason in cancer cells. Cell Death Dis. 2016;7:e2253. doi: 10.1038/cddis.2016.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Poeck H, Besch R, Maihoefer C, Renn M, Tormo D, Morskaya SS, Kirschnek S, Gaffal E, Landsberg J, Hellmuth J, et al. 5′-Triphosphate-siRNA: turning gene silencing and Rig-I activation against melanoma. Nat Med. 2008;14:1256–1263. doi: 10.1038/nm.1887. [DOI] [PubMed] [Google Scholar]
- 131.Pollard PJ, Briere JJ, Alam NA, Barwell J, Barclay E, Wortham NC, Hunt T, Mitchell M, Olpin S, Moat SJ, et al. Accumulation of Krebs cycle intermediates and over-expression of HIF1alpha in tumours which result from germline FH and SDH mutations. Hum Mol Genet. 2005;14:2231–2239. doi: 10.1093/hmg/ddi227. [DOI] [PubMed] [Google Scholar]
- 132.Ponomareva L, Liu H, Duan X, Dickerson E, Shen H, Panchanathan R, Choubey D. AIM2, an IFN-inducible cytosolic DNA sensor, in the development of benign prostate hyperplasia and prostate cancer. Mol Cancer Res. 2013;11:1193–1202. doi: 10.1158/1541-7786.MCR-13-0145. [DOI] [PubMed] [Google Scholar]
- 133.Pua HH, Dzhagalov I, Chuck M, Mizushima N, He YW. A critical role for the autophagy gene Atg5 in T cell survival and proliferation. J Exp Med. 2007;204:25–31. doi: 10.1084/jem.20061303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Ranoa DR, Parekh AD, Pitroda SP, Huang X, Darga T, Wong AC, Huang L, Andrade J, Staley JP, Satoh T, et al. Cancer therapies activate RIG-I-like receptor pathway through endogenous non-coding RNAs. Oncotarget. 2016;7:26496–26515. doi: 10.18632/oncotarget.8420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Rao CV, Asch AS, Yamada HY. Emerging links among chromosome instability (CIN), cancer, and aging. Mol Carcinog. 2017;56:791–803. doi: 10.1002/mc.22539. [DOI] [PubMed] [Google Scholar]
- 136.Roder JC, Helfand SL, Werkmeister J, McGarry R, Beaumont TJ, Duwe A. Oxygen intermediates are triggered early in the cytolytic pathway of human NK cells. Nature. 1982;298:569–572. doi: 10.1038/298569a0. [DOI] [PubMed] [Google Scholar]
- 137.Rodier F, Coppe JP, Patil CK, Hoeijmakers WA, Munoz DP, Raza SR, Freund A, Campeau E, Davalos AR, Campisi J. Persistent DNA damage signalling triggers senescence-associated inflammatory cytokine secretion. Nat Cell Biol. 2009;11:973–979. doi: 10.1038/ncb1909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Romero AI, Thoren FB, Brune M, Hellstrand K. NKp46 and NKG2D receptor expression in NK cells with CD56dim and CD56bright phenotype: regulation by histamine and reactive oxygen species. Br J Haematol. 2006;132:91–98. doi: 10.1111/j.1365-2141.2005.05842.x. [DOI] [PubMed] [Google Scholar]
- 139.Rubic T, Lametschwandtner G, Jost S, Hinteregger S, Kund J, Carballido-Perrig N, Schwarzler C, Junt T, Voshol H, Meingassner JG, et al. Triggering the succinate receptor GPR91 on dendritic cells enhances immunity. Nat Immunol. 2008;9:1261–1269. doi: 10.1038/ni.1657. [DOI] [PubMed] [Google Scholar]
- 140.Rutault K, Alderman C, Chain BM, Katz DR. Reactive oxygen species activate human peripheral blood dendritic cells. Free Radic Biol Med. 1999;26:232–238. doi: 10.1016/S0891-5849(98)00194-4. [DOI] [PubMed] [Google Scholar]
- 141.Saddawi-Konefka R, Seelige R, Gross ET, Levy E, Searles SC, Washington A, Jr, Santosa EK, Liu B, O’Sullivan TE, Harismendy O, Bui JD. Nrf2 induces IL-17D to mediate tumor and virus surveillance. Cell Rep. 2016;16:2348–2358. doi: 10.1016/j.celrep.2016.07.075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Sandholm J, Selander KS. Toll-like receptor 9 in breast cancer. Front Immunol. 2014;5:330. doi: 10.3389/fimmu.2014.00330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Sarkar S, Germeraad WT, Rouschop KM, Steeghs EM, van Gelder M, Bos GM, Wieten L. Hypoxia induced impairment of NK cell cytotoxicity against multiple myeloma can be overcome by IL-2 activation of the NK cells. PLoS One. 2013;8:e64835. doi: 10.1371/journal.pone.0064835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Schmielau J, Finn OJ. Activated granulocytes and granulocyte-derived hydrogen peroxide are the underlying mechanism of suppression of t-cell function in advanced cancer patients. Cancer Res. 2001;61:4756–4760. [PubMed] [Google Scholar]
- 145.Schnurr M, Duewell P. Breaking tumor-induced immunosuppression with 5′-triphosphate siRNA silencing TGFbeta and activating RIG-I. Oncoimmunology. 2013;2:e24170. doi: 10.4161/onci.24170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Schon MP, Schon M. TLR7 and TLR8 as targets in cancer therapy. Oncogene. 2008;27:190–199. doi: 10.1038/sj.onc.1210913. [DOI] [PubMed] [Google Scholar]
- 147.Schroder M, Kaufman RJ. ER stress and the unfolded protein response. Mutat Res. 2005;569:29–63. doi: 10.1016/j.mrfmmm.2004.06.056. [DOI] [PubMed] [Google Scholar]
- 148.Schumacker PT. Reactive oxygen species in cancer cells: live by the sword, die by the sword. Cancer Cell. 2006;10:175–176. doi: 10.1016/j.ccr.2006.08.015. [DOI] [PubMed] [Google Scholar]
- 149.Schumacker PT. Reactive oxygen species in cancer: a dance with the devil. Cancer Cell. 2015;27:156–157. doi: 10.1016/j.ccell.2015.01.007. [DOI] [PubMed] [Google Scholar]
- 150.Seaman WE, Gindhart TD, Blackman MA, Dalal B, Talal N, Werb Z. Suppression of natural killing in vitro by monocytes and polymorphonuclear leukocytes: requirement for reactive metabolites of oxygen. J Clin Invest. 1982;69:876–888. doi: 10.1172/JCI110527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Senovilla L, Vitale I, Martins I, Tailler M, Pailleret C, Michaud M, Galluzzi L, Adjemian S, Kepp O, Niso-Santano M, et al. An immunosurveillance mechanism controls cancer cell ploidy. Science. 2012;337:1678–1684. doi: 10.1126/science.1224922. [DOI] [PubMed] [Google Scholar]
- 152.Shaukat Z, Liu D, Gregory S. Sterile inflammation in Drosophila . Mediat Inflamm. 2015;2015:369286. doi: 10.1155/2015/369286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Shimada K, Crother TR, Karlin J, Dagvadorj J, Chiba N, Chen S, Ramanujan VK, Wolf AJ, Vergnes L, Ojcius DM, et al. Oxidized mitochondrial DNA activates the NLRP3 inflammasome during apoptosis. Immunity. 2012;36:401–414. doi: 10.1016/j.immuni.2012.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Sido B, Lasitschka F, Giese T, Gassler N, Funke B, Schroder-Braunstein J, Brunnemer U, Meuer SC, Autschbach F. A prominent role for mucosal cystine/cysteine metabolism in intestinal immunoregulation. Gastroenterology. 2008;134:179–191. doi: 10.1053/j.gastro.2007.11.001. [DOI] [PubMed] [Google Scholar]
- 155.Siemens DR, Hu N, Sheikhi AK, Chung E, Frederiksen LJ, Pross H, Graham CH. Hypoxia increases tumor cell shedding of MHC class I chain-related molecule: role of nitric oxide. Cancer Res. 2008;68:4746–4753. doi: 10.1158/0008-5472.CAN-08-0054. [DOI] [PubMed] [Google Scholar]
- 156.Simon HU. Neutrophil apoptosis pathways and their modifications in inflammation. Immunol Rev. 2003;193:101–110. doi: 10.1034/j.1600-065X.2003.00038.x. [DOI] [PubMed] [Google Scholar]
- 157.Sims GP, Rowe DC, Rietdijk ST, Herbst R, Coyle AJ. HMGB1 and RAGE in inflammation and cancer. Annu Rev Immunol. 2010;28:367–388. doi: 10.1146/annurev.immunol.021908.132603. [DOI] [PubMed] [Google Scholar]
- 158.Singh DK, Kumar D, Siddiqui Z, Basu SK, Kumar V, Rao KV. The strength of receptor signaling is centrally controlled through a cooperative loop between Ca2+ and an oxidant signal. Cell. 2005;121:281–293. doi: 10.1016/j.cell.2005.02.036. [DOI] [PubMed] [Google Scholar]
- 159.Sinha P, Clements VK, Fulton AM, Ostrand-Rosenberg S. Prostaglandin E2 promotes tumor progression by inducing myeloid-derived suppressor cells. Cancer Res. 2007;67:4507–4513. doi: 10.1158/0008-5472.CAN-06-4174. [DOI] [PubMed] [Google Scholar]
- 160.Sistigu A, Yamazaki T, Vacchelli E, Chaba K, Enot DP, Adam J, Vitale I, Goubar A, Baracco EE, Remedios C, et al. Cancer cell-autonomous contribution of type I interferon signaling to the efficacy of chemotherapy. Nat Med. 2014;20:1301–1309. doi: 10.1038/nm.3708. [DOI] [PubMed] [Google Scholar]
- 161.Smyth MJ, Swann J, Cretney E, Zerafa N, Yokoyama WM, Hayakawa Y. NKG2D function protects the host from tumor initiation. J Exp Med. 2005;202:583–588. doi: 10.1084/jem.20050994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Soriani A, Fionda C, Ricci B, Iannitto ML, Cippitelli M, Santoni A. Chemotherapy-elicited upregulation of NKG2D and DNAM-1 ligands as a therapeutic target in multiple myeloma. Oncoimmunology. 2013;2:e26663. doi: 10.4161/onci.26663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Soriani A, Zingoni A, Cerboni C, Iannitto ML, Ricciardi MR, Di Gialleonardo V, Cippitelli M, Fionda C, Petrucci MT, Guarini A, et al. ATM-ATR-dependent up-regulation of DNAM-1 and NKG2D ligands on multiple myeloma cells by therapeutic agents results in enhanced NK-cell susceptibility and is associated with a senescent phenotype. Blood. 2009;113:3503–3511. doi: 10.1182/blood-2008-08-173914. [DOI] [PubMed] [Google Scholar]
- 164.Srivastava MK, Sinha P, Clements VK, Rodriguez P, Ostrand-Rosenberg S. Myeloid-derived suppressor cells inhibit T-cell activation by depleting cystine and cysteine. Cancer Res. 2010;70:68–77. doi: 10.1158/0008-5472.CAN-09-2587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Sturrock A, Cahill B, Norman K, Huecksteadt TP, Hill K, Sanders K, Karwande SV, Stringham JC, Bull DA, Gleich M, et al. Transforming growth factor-beta1 induces Nox4 NAD(P)H oxidase and reactive oxygen species-dependent proliferation in human pulmonary artery smooth muscle cells. Am J Physiol Lung Cell Mol Physiol. 2006;290:L661–L673. doi: 10.1152/ajplung.00269.2005. [DOI] [PubMed] [Google Scholar]
- 166.Sun J, Zhang Y, Yang M, Zhang Y, Xie Q, Li Z, Dong Z, Yang Y, Deng B, Feng A, et al. Hypoxia induces T-cell apoptosis by inhibiting chemokine C receptor 7 expression: the role of adenosine receptor A(2) Cell Mol Immunol. 2010;7:77–82. doi: 10.1038/cmi.2009.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Suthanthiran M, Solomon SD, Williams PS, Rubin AL, Novogrodsky A, Stenzel KH. Hydroxyl radical scavengers inhibit human natural killer cell activity. Nature. 1984;307:276–278. doi: 10.1038/307276a0. [DOI] [PubMed] [Google Scholar]
- 168.Tang ML, Gasser S. ATM activation mediates anticancer immunosurveillance by natural killer and T cells. Oncoimmunology. 2013;2:e24438. doi: 10.4161/onci.24438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Tang ML, Khan MK, Croxford JL, Tan KW, Angeli V, Gasser S. The DNA damage response induces antigen presenting cell-like functions in fibroblasts. Eur J Immunol. 2014;44:1108–1118. doi: 10.1002/eji.201343781. [DOI] [PubMed] [Google Scholar]
- 170.Tannahill GM, Curtis AM, Adamik J, Palsson-McDermott EM, McGettrick AF, Goel G, Frezza C, Bernard NJ, Kelly B, Foley NH, et al. Succinate is an inflammatory signal that induces IL-1beta through HIF-1alpha. Nature. 2013;496:238–242. doi: 10.1038/nature11986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Trabold O, Wagner S, Wicke C, Scheuenstuhl H, Hussain MZ, Rosen N, Seremetiev A, Becker HD, Hunt TK. Lactate and oxygen constitute a fundamental regulatory mechanism in wound healing. Wound Repair Regen. 2003;11:504–509. doi: 10.1046/j.1524-475X.2003.11621.x. [DOI] [PubMed] [Google Scholar]
- 172.Tripathi P, Hildeman D. Sensitization of T cells to apoptosis—a role for ROS? Apoptosis. 2004;9:515–523. doi: 10.1023/B:APPT.0000038033.14925.02. [DOI] [PubMed] [Google Scholar]
- 173.Urano F, Wang X, Bertolotti A, Zhang Y, Chung P, Harding HP, Ron D. Coupling of stress in the ER to activation of JNK protein kinases by transmembrane protein kinase IRE1. Science. 2000;287:664–666. doi: 10.1126/science.287.5453.664. [DOI] [PubMed] [Google Scholar]
- 174.van Deursen JM. The role of senescent cells in ageing. Nature. 2014;509:439–446. doi: 10.1038/nature13193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.van Vliet AR, Martin S, Garg AD, Agostinis P. The PERKs of damage-associated molecular patterns mediating cancer immunogenicity: from sensor to the plasma membrane and beyond. Semin Cancer Biol. 2015;33:74–85. doi: 10.1016/j.semcancer.2015.03.010. [DOI] [PubMed] [Google Scholar]
- 176.Verfaillie T, Garg AD, Agostinis P. Targeting ER stress induced apoptosis and inflammation in cancer. Cancer Lett. 2013;332:249–264. doi: 10.1016/j.canlet.2010.07.016. [DOI] [PubMed] [Google Scholar]
- 177.Viry E, Paggetti J, Baginska J, Mgrditchian T, Berchem G, Moussay E, Janji B. Autophagy: an adaptive metabolic response to stress shaping the antitumor immunity. Biochem Pharmacol. 2014;92:31–42. doi: 10.1016/j.bcp.2014.07.006. [DOI] [PubMed] [Google Scholar]
- 178.Wallace DC. Diseases of the mitochondrial DNA. Annu Rev Biochem. 1992;61:1175–1212. doi: 10.1146/annurev.bi.61.070192.005523. [DOI] [PubMed] [Google Scholar]
- 179.Walter P, Ron D. The unfolded protein response: from stress pathway to homeostatic regulation. Science. 2011;334:1081–1086. doi: 10.1126/science.1209038. [DOI] [PubMed] [Google Scholar]
- 180.Warburg O. On the origin of cancer cells. Science. 1956;123:309–314. doi: 10.1126/science.123.3191.309. [DOI] [PubMed] [Google Scholar]
- 181.Wei X, Shao B, He Z, Ye T, Luo M, Sang Y, Liang X, Wang W, Luo S, Yang S, et al. Cationic nanocarriers induce cell necrosis through impairment of Na(+)/K(+)-ATPase and cause subsequent inflammatory response. Cell Res. 2015;25:237–253. doi: 10.1038/cr.2015.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Weiskopf D, Schwanninger A, Weinberger B, Almanzar G, Parson W, Buus S, Lindner H, Grubeck-Loebenstein B. Oxidative stress can alter the antigenicity of immunodominant peptides. J Leukoc Biol. 2010;87:165–172. doi: 10.1189/jlb.0209065. [DOI] [PubMed] [Google Scholar]
- 183.West AP, Khoury-Hanold W, Staron M, Tal MC, Pineda CM, Lang SM, Bestwick M, Duguay BA, Raimundo N, MacDuff DA, et al. Mitochondrial DNA stress primes the antiviral innate immune response. Nature. 2015;520:553–557. doi: 10.1038/nature14156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Whiteside TL, Mandapathil M, Schuler P. The role of the adenosinergic pathway in immunosuppression mediated by human regulatory T cells (Treg) Curr Med Chem. 2011;18:5217–5223. doi: 10.2174/092986711798184334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Widau RC, Parekh AD, Ranck MC, Golden DW, Kumar KA, Sood RF, Pitroda SP, Liao Z, Huang X, Darga TE, et al. RIG-I-like receptor LGP2 protects tumor cells from ionizing radiation. Proc Natl Acad Sci USA. 2014;111:E484–E491. doi: 10.1073/pnas.1323253111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Wilson JE, Petrucelli AS, Chen L, Koblansky AA, Truax AD, Oyama Y, Rogers AB, Brickey WJ, Wang Y, Schneider M, et al. Inflammasome-independent role of AIM2 in suppressing colon tumorigenesis via DNA-PK and Akt. Nat Med. 2015;21:906–913. doi: 10.1038/nm.3908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Wong HW, Shaukat Z, Wang J, Saint R, Gregory SL. JNK signaling is needed to tolerate chromosomal instability. Cell Cycle. 2014;13:622–631. doi: 10.4161/cc.27484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Woo SR, Fuertes MB, Corrales L, Spranger S, Furdyna MJ, Leung MY, Duggan R, Wang Y, Barber GN, Fitzgerald KA, et al. STING-dependent cytosolic DNA sensing mediates innate immune recognition of immunogenic tumors. Immunity. 2014;41:830–842. doi: 10.1016/j.immuni.2014.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Wrzesinski SH, Wan YY, Flavell RA. Transforming growth factor-beta and the immune response: implications for anticancer therapy. Clin Cancer Res. 2007;13:5262–5270. doi: 10.1158/1078-0432.CCR-07-1157. [DOI] [PubMed] [Google Scholar]
- 190.Xia T, Konno H, Ahn J, Barber GN. Deregulation of STING signaling in colorectal carcinoma constrains DNA damage responses and correlates with tumorigenesis. Cell Rep. 2016;14:282–297. doi: 10.1016/j.celrep.2015.12.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Yamada HY, Yao Y, Wang X, Zhang Y, Huang Y, Dai W, Rao CV. Haploinsufficiency of SGO1 results in deregulated centrosome dynamics, enhanced chromosomal instability and colon tumorigenesis. Cell Cycle. 2012;11:479–488. doi: 10.4161/cc.11.3.18994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Yan H, Parsons DW, Jin G, McLendon R, Rasheed BA, Yuan W, Kos I, Batinic-Haberle I, Jones S, Riggins GJ, et al. IDH1 and IDH2 mutations in gliomas. N Engl J Med. 2009;360:765–773. doi: 10.1056/NEJMoa0808710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Yan Z, Garg SK, Kipnis J, Banerjee R. Extracellular redox modulation by regulatory T cells. Nat Chem Biol. 2009;5:721–723. doi: 10.1038/nchembio.212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Yang D, Elner SG, Bian ZM, Till GO, Petty HR, Elner VM. Pro-inflammatory cytokines increase reactive oxygen species through mitochondria and NADPH oxidase in cultured RPE cells. Exp Eye Res. 2007;85:462–472. doi: 10.1016/j.exer.2007.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Yang L, Yamagata N, Yadav R, Brandon S, Courtney RL, Morrow JD, Shyr Y, Boothby M, Joyce S, Carbone DP, Breyer RM. Cancer-associated immunodeficiency and dendritic cell abnormalities mediated by the prostaglandin EP2 receptor. J Clin Invest. 2003;111:727–735. doi: 10.1172/JCI16492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Yang X, Zhang X, Fu ML, Weichselbaum RR, Gajewski TF, Guo Y, Fu YX. Targeting the tumor microenvironment with interferon-beta bridges innate and adaptive immune responses. Cancer Cell. 2014;25:37–48. doi: 10.1016/j.ccr.2013.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Yu X, Wang H, Li X, Guo C, Yuan F, Fisher PB, Wang XY. Activation of the MDA-5-IPS-1 viral sensing pathway induces cancer cell death and type I IFN-dependent antitumor immunity. Cancer Res. 2016;76:2166–2176. doi: 10.1158/0008-5472.CAN-15-2142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Zanetti M, Rodvold JJ, Mahadevan NR. The evolving paradigm of cell-nonautonomous UPR-based regulation of immunity by cancer cells. Oncogene. 2016;35:269–278. doi: 10.1038/onc.2015.108. [DOI] [PubMed] [Google Scholar]
- 199.Zhang Q, Raoof M, Chen Y, Sumi Y, Sursal T, Junger W, Brohi K, Itagaki K, Hauser CJ. Circulating mitochondrial DAMPs cause inflammatory responses to injury. Nature. 2010;464:104–107. doi: 10.1038/nature08780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Zhang X, Rao A, Sette P, Deibert C, Pomerantz A, Kim WJ, Kohanbash G, Chang Y, Park Y, Engh J, et al. IDH mutant gliomas escape natural killer cell immune surveillance by downregulation of NKG2D ligand expression. Neuro Oncol. 2016;18:1402–1412. doi: 10.1093/neuonc/now061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Zheng L, Dai H, Zhou M, Li M, Singh P, Qiu J, Tsark W, Huang Q, Kernstine K, Zhang X, et al. Fen1 mutations result in autoimmunity, chronic inflammation and cancers. Nat Med. 2007;13:812–819. doi: 10.1038/nm1599. [DOI] [PubMed] [Google Scholar]
- 202.Zhu Q, Man SM, Gurung P, Liu Z, Vogel P, Lamkanfi M, Kanneganti TD. Cutting edge: STING mediates protection against colorectal tumorigenesis by governing the magnitude of intestinal inflammation. J Immunol. 2014;193:4779–4782. doi: 10.4049/jimmunol.1402051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Zuckerberg AL, Goldberg LI, Lederman HM. Effects of hypoxia on interleukin-2 mRNA expression by T lymphocytes. Crit Care Med. 1994;22:197–203. doi: 10.1097/00003246-199402000-00008. [DOI] [PubMed] [Google Scholar]