Abstract
Rho GTPases are highly conserved proteins that play critical roles in many cellular processes including actin dynamics, vesicular trafficking, gene transcription, cell-cycle progression, and cell adhesion. The main mode of regulation of Rho GTPases is through guanine nucleotide binding (cycling between an active GTP-bound form and an inactive GDP-bound form), but transcriptional, post-transcriptional, and post-translational modes of Rho regulation have also been described. In the present review, we summarize recent progress on the mechanisms that control the expression of the three members of the Rho-like subfamily (RhoA, RhoB, and RhoC) at the level of gene transcription as well as their post-transcriptional regulation by microRNAs. We also discuss the progress made in deciphering the mechanisms of cross-talk between Rho proteins and the transforming growth factor β signaling pathway and their implications for the pathogenesis of human diseases such as cancer metastasis and fibrosis.
Keywords: Rho GTPases, RhoA, RhoB, RhoC, Transcriptional regulation, miRNAs, TGFβ, Signaling cross talks
Introduction
Rho proteins are members of the Ras superfamily of small GTPases that play critical roles in many cellular processes including actin dynamics, vesicular trafficking, gene transcription, cell-cycle progression, and cell adhesion [1]. Rho GTPases are highly conserved from lower eukaryotes to plants and mammals. In mammals, the family includes 22 members, divided into eight different subfamilies [2]. The Rho-like subfamily consists of three highly homologous isoforms: RhoA, RhoB, and RhoC. Rho GTPases are molecular switches cycling between an active GTP-bound form and an inactive GDP-bound form. This cycling is regulated by numerous cellular proteins, namely guanosine nucleotide exchange factors (GEFs) that facilitate the exchange of GDP for GTP, GTPase-activating proteins (GAPs) which regulate the GTP hydrolysis of Rho GTPases, and the guanosine nucleotide dissociation inhibitors (GDIs) that bind to the C-terminal prenyl group, preventing the association of Rho to the membrane and sequestering them in the cytoplasm, inhibiting their access to downstream targets. The topic of Rho regulation by effector proteins has been the subject of numerous excellent and very thorough reviews [3–7]. Due to the high amino acid sequence homology among the three Rho-like isoforms especially in the insert region which has been shown to be critical for interaction with GEFs and other effector proteins [7], Rho GEFs and GAPs that show isoform specificity are very few. One example is XPLN, a GEF that belongs to the Dbl-Rho GEF family which shows activity toward RhoA and RhoB but not RhoC due to an amino acid difference at position 43 at the N-terminus of Rho isoforms (Ile vs Val) [8, 9]. Importantly, a RhoC-I43V mutant had increased ovarian cancer cell invasion potential compared to wild type RhoC [9]. Another example of an isoform-specific RhoGEF is SmgGDS which specifically activates RhoA and RhoC but not RhoB or a large panel of other GTPases [10]. Rho GAPs that are able to differentiate among the three Rho-like isoforms have not been identified yet, but specificity of GAPs for Rho versus other members of the Ras family has been reported [11, 12].
In humans, rats and mice Rho-like genes are mapped on different chromosomes and differ in size [13]. The RhoA gene is longer and contains more exons and introns than the other Rho genes, whereas the RhoB gene contains only one exon, possibly derived from reverse transcription [14]. The primary sequences of Rho-like proteins are around 85% identical, with most divergence close to the C-terminus [1].
In addition to their important roles in cell physiology [15], Rho-like proteins also contribute to pathological processes such as cancer cell migration, invasion, metastasis, fibrosis, inflammation, and wound repair [2].
In previous studies, we have revealed the critical role of RhoA and RhoB as well as the downstream effectors (ROCK, LIMK, Cofilin) in TGFβ signaling leading to actin cytoskeleton reorganization [16–21]. We have also shown that RhoB, in contrast to RhoA, is a direct transcriptional target of TGFβ that mediates the long-term effect of TGFβ in actin cytoskeleton [21].
In the present review, we summarize recent progress made on the mechanisms that control the expression of Rho-like proteins (RhoA, RhoB, and RhoC) at the level of gene transcription as well as post-transcriptionally by microRNAs. We also review progress made in deciphering the mechanisms of cross-talk between Rho proteins and TGFβ and their implications in the pathogenesis of human diseases such as cancer and fibrosis.
Transcriptional regulation of the RhoA gene
The role of Rho proteins in cancer and metastasis has been the subject of intense investigation during the past decades [22]. Constitutively active tumorigenic mutations in RhoA are much less common than mutations in the Ras gene, but few of them were identified recently in gastric cancers. In one study, Wang et al. [23] performed whole-genome sequencing and a comprehensive molecular profiling in 100 tumor and non-tumor paired samples from intestinal-type gastric tumors (IGC) and diffuse-type gastric tumors (DGC) followed by resequencing in a larger DGC cohort. RhoA mutations were identified in 14.3% of DGC tumors but not in IGC tumors. Similarly, Kakiuchi et al. [24] performed whole-exome sequencing within 30 DGCs followed by resequencing in another 57 cases and identified RhoA mutations in 25.3% of the cases. These findings were in agreement with the data from The Cancer Genome Atlas (TCGA) project evaluating 295 primary gastric adenocarcinomas [25]. The consortium identified mutations in the RhoA gene in 16 cases which were clustered in two adjacent amino-terminal regions that are predicted to modulate signaling downstream of RhoA [25]. Mutations in RhoA were also identified in a genome-wide association screen of patients with pediatric Burkitt lymphoma [26] and in angioimmunoblastic T-cell lymphoma (AITL), a common type of mature T-cell lymphoma of poor prognosis [27].
Upregulation of RhoA mRNA and protein levels has been well documented in various types of human malignancies [28–30]. Furthermore, the activity of RhoA can be compromised in tumor cells via different mechanisms including its phosphorylation by protein kinase A (PKA) that causes its dissociation from the plasma membrane [31], the proteasomal degradation by E3 ubiquitin ligases [32], inhibition of RhoA GEFs and stimulation of GAPs [33, 34], or the interaction of RhoA with cell-cycle inhibitors that prevent binding of effectors [35].
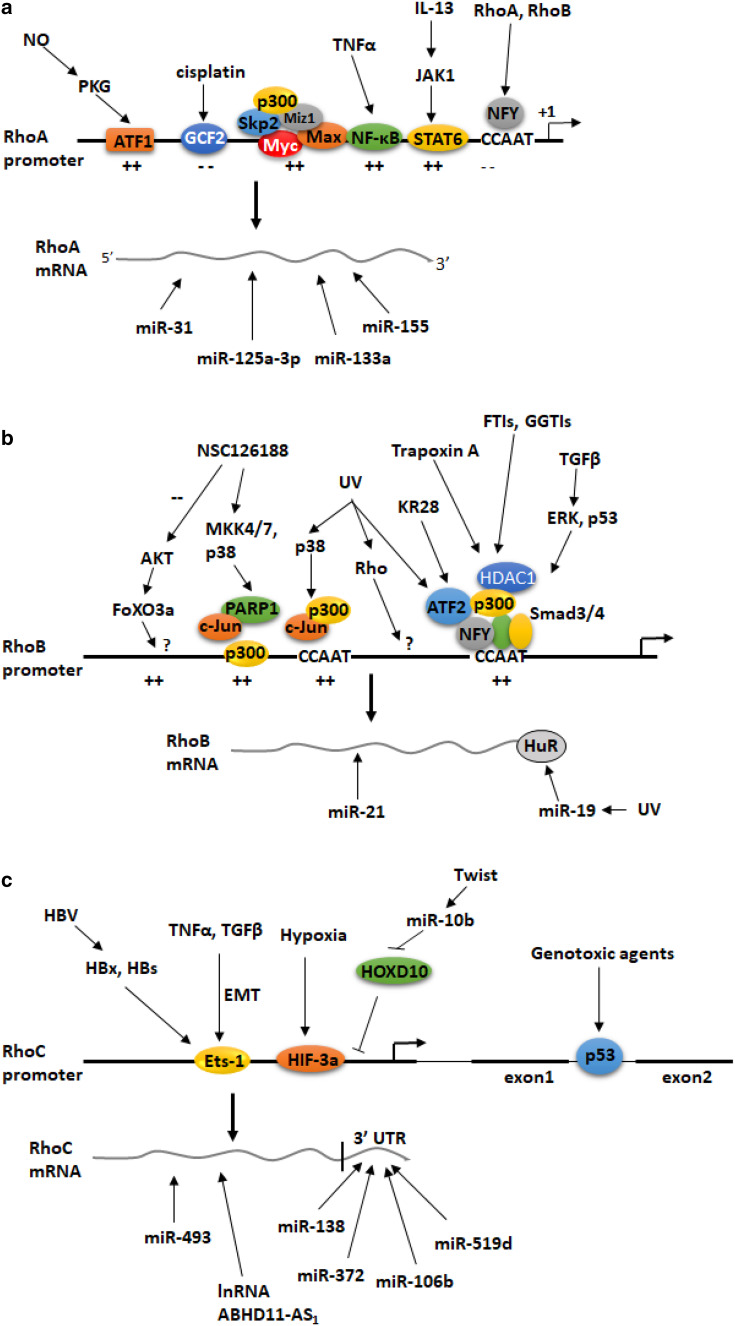
The functions of RhoA in cancer cells can also be modulated at the transcriptional level. The mechanisms that control RhoA gene expression during metastasis have been explored by Chan et al. [36]. They focused on the oncogenic Myc protein, a transcription factor that binds to E-box motives present in target gene promoters and regulates transcription by cooperating with auxiliary factors such as the Myc-interacting zinc finger protein 1 (Miz1) and various coactivator complexes/histone acetyltransferases [37]. The authors showed that Myc cooperates with the E3 ubiquitin ligase S-phase kinase-associated protein 2 (Skp2) to induce RhoA transcription by recruiting Miz1 and p300 to the RhoA promoter independently of Skp1-Cullin-F-box protein containing complex (SCF)–Skp2 E3 ligase activity (Fig. 1a). Myc knockdown decreased RhoA mRNA levels in Rat1 fibroblasts, whereas Myc overexpression induced RhoA expression in MDA-MB-231 breast cancer cells. Importantly, increased levels of the Myc–Skp2–Miz1 complex can be found in metastatic human cancers, whereas deficiency of these proteins not only results in impaired RhoA expression but also inhibits cell migration, invasion, and breast cancer metastasis [36]. In summary, this study revealed, for the first time, the role of the ubiquitin ligase Skp2 in RhoA gene transcription and in cancer metastasis.
Fig. 1.
Summary of the mechanisms that control the expression of the small GTPases RhoA (a), RhoB (b) and RhoC (c) genes at the transcriptional and the post-transcriptional level. Symbols + and − indicate positive and negative effects on gene expression, respectively
RhoA has been implicated in the pathogenesis of allergic bronchial asthma because its upregulation causes Ca2+ sensitization of bronchial smooth muscles (BSMs). However, the mechanisms and the transcription factors that mediate this RhoA upregulation were poorly understood. Goto et al. showed that RhoA gene expression and the activity of the RhoA promoter were induced by treatment of BSMs with the pro-inflammatory cytokines Tumor necrosis factor α (TNFα) and interleukin 13 (IL-13) [38]. A promoter deletion analysis showed that the proximal RhoA promoter between nucleotide position -112 and the transcription start site contains binding sites for the transcription factors signal transducer and activator of transcription 6 (STAT6) at position − 78/− 70 bp and nuclear factor kappa B (NF-κB) at position − 84/− 74 bp. Mutations in these sites abolished the upregulation of the RhoA promoter by the two cytokines suggesting a critical role of the two pro-inflammatory transcription factors in RhoA gene regulation during asthma. Similar results were found by Chiba et al. who showed that the rat RhoA gene promoter contains an STAT6-binding region and that RhoA expression may be regulated by the IL-13–JAK1–STAT6 signaling pathway in BSMs of asthmatic rats (Fig. 1a) [39].
It was reported previously that cells that were resistant to the chemotherapeutic drug cisplatin had decreased levels of RhoA, disorganized cytoskeleton, and as a consequence, displayed with reduced uptake of drugs from cell-surface transporters [40]. However, the mechanism that could account for RhoA gene downregulation in cisplatin-resistant cells remained unknown. In a follow-up study, the same group showed that the transcription factor GCF2 (GC-binding factor 2) is a transcriptional repressor of the RhoA gene [41]. Specifically, they showed that overexpression of GCF2 abolished RhoA expression and disrupted the actin–filamin network; and as a result, membrane transporter MRP1 was translocated from the cell membrane to the cytoplasm rendering cells resistant to cisplatin. In contrast, siRNA-mediated silencing of GCF2 restored RhoA expression and actin microfilament organization [41].
Nitric oxide (NO) plays an important role in vessel wall homeostasis via many functions including the transcriptional activation of target gene in endothelial or smooth muscle cells [42]. Sauzeau et al. [43] showed that in arterial smooth muscle cells, RhoA mRNA and protein levels were increased by treatment with agents that serve as precursors for NO (sodium nitroprusside) or raise the intracellular cGMP [8-(2-chlorophenylthio)-cGMP] and this upregulation was inhibited by the cGMP-dependent protein kinase (PKG) inhibitor (Rp)-8-bromo-phenyl-1,N2-ethenoguanosine 3:5-phosphorothioate. Promoter analysis revealed that the NO/PKG pathway triggered the phosphorylation of activating transcription factor 1 (ATF-1) and its binding to a cAMP-response element present on the RhoA promoter (Fig. 1a). In agreement with these findings, they showed that chronic inhibition of NO synthesis decreased RhoA mRNA and protein expression in the aorta and the pulmonary artery of rats and this was associated with inhibition of RhoA-mediated Ca2+ sensitization. These data suggested a critical role of a NO/PKG/ATF-1/RhoA signaling pathway in vascular smooth muscle cells [43].
Transcriptional regulation of the RhoB gene
RhoA and RhoC proteins are often upregulated in human tumors and their expression correlates with tumor aggressiveness whereas the levels and functions of RhoB in human cancers are context-dependent. Although expression of RhoB inversely correlates with disease progression in several epithelial cancers, recent data suggest that RhoB may support malignant phenotypes in certain cancer types including T-acute lymphoblastic leukemia [44], lung adenocarcinoma [45], and glioblastoma [46, 47].
It has been proposed that RhoB can work as a tumor suppressor as it is activated in response to several stress stimuli including DNA damage or hypoxia; and it has been reported to inhibit tumor growth, cell migration, and invasion, and have proapoptotic functions in cells [48, 49]. In addition, we have shown previously that RhoB, similar to RhoA, is a major membrane androgen receptor effector regulating actin cytoskeleton and apoptosis in various tumor cells [50–53].
RhoB expression is further regulated by extracellular stimuli such as UV irradiation [54–56], growth factors (epidermal growth factor and platelet-derived growth factor in Rat-2 fibroblasts) [57], cytokines, and oncogenes [54–58].
The two CCAAT boxes (proximal and distal) that are present in the RhoB promoter mediate RhoB gene regulation by genotoxic stress (Fig. 1b). It was shown that NFY that binds to the proximal CCAAT box of the RhoB promoter mediates RhoB gene induction by the binding of activating transcription factor 2 (ATF-2) and inhibition of histone deacetylase 1 (HDAC1) [49, 56]. More recently, it was shown that the p38 mitogen-activated protein kinase (MAPK) regulates the recruitment of the proto-oncogene c-Jun and the histone acetyltransferase p300 to the more distal CCAAT box of the RhoB promoter leading to upregulation of RhoB gene expression and induction of apoptosis of UV-irradiated human T lymphocyte (Jurkat) cells [59].
The p38 MAPK/ATF-2 complex is also responsible for the upregulation of the RhoB gene by the piperazine alkyl anticancer compound KR28 in human prostate carcinoma PC-3 cells and this upregulation was shown to require p300 and the CCAAT box of the RhoB promoter (Fig. 1b) [60]. HDAC1 is also involved in the upregulation of the RhoB gene by farnesyltransferase inhibitors (FTIs) and geranylgeranyltransferase I inhibitors (GGTIs). Specifically, treatment of cancer cells with FTIs and GGTIs resulted in HDAC1 dissociation, HAT association, and histone acetylation of the RhoB promoter, which led to increased RhoB expression [61].
Several studies focused on the mechanism by which the piperazine alkyl derivative NSC126188 induces apoptosis in several cancer cell types (Fig. 1b). In one study, it was shown that in stomach carcinoma NUGC-3 cells, NSC126188 induced the activity of the RhoB promoter by increasing the expression of p300 and c-Jun in a poly(ADP-ribose) polymerase-1 (PARP-1)-dependent manner [62]. The signaling pathway that was activated by this drug involved the MAP kinases MKK4/7 and p38 and the transcription factor c-Jun [63]. In another study, it was found that NSC126188 induced apoptosis in prostate cancer PC-3 cells by interfering with membrane recruitment of AKT, resulting in dephosphorylation of AKT and of the forkhead box transcription factor FoxO3a, which increased the transcription of the RhoB gene [64]. NSC126188 also induced apoptosis of the cervical carcinoma HeLa cells by inducing the transcriptional activation of RhoB [65].
Pharmaceutical agents including the alkylating agent N-methyl-N-nitrosourea (MNU), the cytostatic drug cisplatin, hydroxyurea, and dexamethasone elicited RhoB gene induction in NIH3T3 cells [55]. Gallic acid was shown to inhibit gastric cancer cell metastasis and invasive growth via increased expression of RhoB through suppressing the PI3K/AKT pathway [66]. Aloe emodin (AE), a natural anthraquinone compound, was reported to have antiproliferative activity in various cancer cell lines. AE suppressed the phorbol-12-myristyl-13-acetate (PMA)-induced migration and invasion of tumor cells and downregulated RhoB gene expression indicating the involvement of RhoB in the antimigratory property of AE in colon cancer cells [67].
RhoB transcription is also negatively regulated by oncogenes including H-Ras N-Ras, K-Ras, EGFR, and ErbB2 in NIH 3T3 cells and human cancer cell lines derived from lung, pancreatic, and cervical tumors [58, 68].
The transcription of the RhoB gene is regulated in a tissue- and age-specific manner. Yoon et al. [69] measured the RhoB mRNA levels in 4-week-old mice and observed high transcriptional levels in liver, skeletal muscle, kidney, and lung with the highest levels of expression in the brain. Furthermore, the RhoB mRNA levels gradually decreased with age in lung and skeletal muscles but not in the other tissues. These changes in RhoB expression were not due to alterations in DNA methylation but rather due to the differential binding of HDAC1 to the CCAAT boxes of RhoB promoter. Specifically, histone H3 and histone H4 acetylation levels of the RhoB CCAAT boxes decreased; histone H3 lysine 9 trimethylation levels and recruitment of HP1b (heterochromatin protein 1b) increased, whereas histone H3 lysine 4 demethylation levels gradually decreased with aging [69]. It was concluded that the chromatin structure of the RhoB promoter gradually changes during aging in a tissue-specific manner [69].
Transcriptional regulation of the RhoC gene
Similar to RhoA, RhoC was found to be upregulated in many types of tumors and it was proposed that inhibition of RhoC could be a promising antitumor strategy [70–72]. RhoC is an essential factor for invasion and metastasis [73]. Overexpression of RhoC in breast cancer cells indicates poor prognosis. In a very recent study Xu et al. investigated the possible anticancer potential of small-interfering RNA (siRNA) targeting RhoC in breast cancer cells [74]. The authors showed that the RhoC-specific siRNA inhibited cancer cell proliferation and invasion, increased cell apoptosis, and induced cell-cycle arrest. Furthermore, intra-tumoral injection of the RhoC siRNA inhibited tumor growth and increased survival rate in BALB/c-nu mice [74].
At the transcriptional level, there are very few studies addressing the mechanisms that control the regulation of the expression of the RhoC gene. In one study, it was shown that the RhoC gene is induced by various genotoxic agents in cancer cell lines and that activated p53 binds to a consensus p53-binding element present in the second intron of the RhoC gene (Fig. 1c) [75]. Thus, RhoC is a direct p53 target gene that is induced during genotoxic stress to mediate the pro-survival functions of p53.
RhoC is also involved in epithelial-to-mesenchymal transition (EMT). It was shown that the levels of RhoC expression and activity are induced during EMT in colon cancer cells and that its expression can be regulated by the Ets-1 transcription factor which binds to multiple sites on the RhoC promoter (Fig. 1c) [76].
The expression of RhoC is significantly increased in hepatocellular carcinoma (HCC). Hepatitis B virus (HBV) was shown to upregulate RhoC expression through enhancing the activity of its promoter [77]. It was subsequently shown that the HBV proteins, HBx and HBs, induced the expression levels of Ets-1 which activated the RhoC promoter (Fig. 1c) [78]. These findings provide a novel insight into HBV-induced HCC metastasis.
In a very recent study, Luo et al. [79] found that the mRNA levels of RhoC are increased in Jurkat acute lymphoblastic leukemia cells by stromal cell-derived factor-1 (SDF-1) signaling which is important for the maintenance and progression of T-cell acute lymphoblastic leukemia. In addition to inducing RhoC expression, SDF-1 activated RhoC signaling towards reactive oxygen species (ROS) production and the subsequent cytoskeleton redistribution and assembly which are important for cell migration [79].
Hypoxia and hypoxia-inducible factors (HIF) play critical roles in pancreatic cancer metastasis. In a recent study, Zhou et al. [80] investigated the mechanism by which HIF-3a controls invasion and metastasis in pancreatic cells. They found that HIF-3a overexpression increased RhoC mRNA levels and promoted tumor cell invasion in transwell and wound healing assays which was compromised by siRNAs targeting RhoC. Using chromatin immunoprecipitation and luciferase reporter assays, they found that endogenous HIF-3a binds to various regions on the promoter of the RhoC gene under hypoxic but not under normoxic conditions (Fig. 1c). It was proposed that targeting the HIF-3a/RhoC signaling pathway may be a novel therapeutic approach for the treatment of pancreatic cancer invasion and metastasis [80].
Phagocytosis via phagosome formation of macrophages plays an essential role in the host defense mechanism and in tissue remodeling. Very recently, using live-cell imaging combined with RNAi-based knockdown and CRISPR/Cas-mediated knockout (KO), Egami et al. [81] showed that RhoC is implicated in the regulation of phagosome formation in macrophages by modifying actin cytoskeletal remodeling via mDia1.
Regulation of Rho genes by the transforming growth factor β (TGFβ) and TGFβ/Rho signaling cross talks
In a previous study, we showed that the transforming growth factor β (TGFβ) upregulates RhoB transcription via activation of cytoplasmic Smad3/4 and MEK/ERK pathways in human HaCaT keratinocytes [21]. Activation of the MEK/ERK pathway by TGFβ as well as functional p53 was required for the binding of Smad3 to a non-classical binding site in the proximal RhoB promoter which overlaps a CCAAT box that constitutively binds nuclear factor Y (Fig. 1b) [21]. Importantly, we showed that TGFβ/Smad signaling had no effect on the RhoA gene despite the fact that both RhoA and RhoB are critical for the rapid non-genomic cell responses to TGFβ toward actin cytoskeleton reorganization [21].
In a follow-up study, we showed that short-term TGFβ treatment of HaCaT keratinocytes induced the RhoA-specific guanine nucleotide exchange factor Net1 isoform2 (Net1A) [18] and this induction was essential for the activation of RhoA GTPase activity by TGFβ. The signaling pathways that were found to facilitate Net1A upregulation by TGFβ were the Smads and the MAPK/ERK kinase (MEK)/extracellular signal-regulated kinase (ERK) pathway. Furthermore, we showed that miR-24 was a post-transcriptional regulator of Net1A expression. miR-24 was found to be implicated in the regulation of the EMT program in response to TGFβ and was shown to be directly involved in the TGFβ-induced breast cancer cell invasiveness through Net1A regulation. It was concluded that Net1 isoform 2 plays a critical role in the short- and long-term TGFβ-mediated regulation of EMT [18].
Recent work has extended our understanding of how TGFβ/Rho signaling operates in multiple cell models. In one study, the role of Rho/Rock in TGFβ1-induced lung fibroblasts differentiation was examined [82]. They showed that Rho/Rock and TGFβ/Smad inhibitors suppressed TGFβ1- induced lung fibroblast differentiation. RhoA, RhoC, ROCK1, Smad2, and tissue inhibitor of metalloproteinase-1 were upregulated by TGFβ1 stimulation. The Rho/Rock inhibitor downregulated Smad2 expression and the TGFβ/Smad inhibitor downregulated RhoA, RhoC, and ROCK1 expression. Therefore, the Rho/Rock pathway and Smad signaling were involved in the process of lung fibroblasts transformation, induced by TGFβ1 to myofibroblasts [82].
In another study, the role of small GTPases Rac1 and RhoA in the NADPH oxidase 4 (Nox4)-dependent generation of ROS during TGFβ1-induced kidney myofibroblast activation was investigated [83]. It was shown that TGFβ induced the expression and the activity of Nox4 as well as the expression of α-smooth muscle actin (SMA) and the fibronectin variant Fn-EIIIA in kidney myofibroblasts in an RhoA-dependent manner. Downregulation of RhoA using siRNAs or inhibition of ROCK compromised the effect of TGFβ1 on the expression of the above genes, whereas inhibition of Nox4 inhibited TGFβ1-induced α-SMA and Fn-EIIIA expression, indicating that RhoA is upstream of ROS generation. RhoA/ROCK also regulated polymerase (DNA-directed) δ-interacting protein 2 (Poldip2), a newly discovered Nox4 enhancer protein, suggesting that induction of redox signaling in kidney myofibroblast activation is mediated by RhoA/ROCK upstream of Poldip2-dependent Nox4 oxidase. The findings may have broad implications in the pathophysiology of renal fibrosis [83].
Very recently, the involvement of Nox4 in epithelial-to-amoeboid transition in the highly metastatic hepatocellular carcinoma was investigated [84]. Crosas-Molist et al. showed that Nox4 gene deletions are frequently found in HCC patients correlating with higher tumor grade. They found an inverse association between Nox4 expression and the levels of RhoC and Cdc42 in HCC, and the low Nox4/high RhoC phenotype was associated with worse cancer prognosis. Importantly, overexpression of Nox4 caused a downregulation in RhoC and Cdc42, it maintained parenchymal structures, increased cell–substrate adhesion and suppressed actomyosin contractility. [84].
Bravo-Nuevo et al. [85] examined the potential involvement of RhoB in thymocyte development using RhoB-deficient mice. The showed that mice lacking RhoB were characterized by thymic atrophy, i.e. small thymus weight and cellularity, beginning as early as 5 weeks of age. These mice also had enhanced expression of TGFβ receptor type II (TGFβRII) in thymic medullary epithelium as well as enhanced fibronectin. These data support a role of RhoB in the regulation of thymus development through inhibition of TGFβ signaling in thymic medullary epithelium [85].
Epithelial–mesenchymal transition (EMT) is one of the critical steps in cancer metastasis and is regulated by multiple factors including TGFβ and the small Rho GTPases [16, 86]. The molecular mechanisms that control this cellular transformation are not yet clear. Toward this goal, Menezes et al. [87] showed that silencing of MDA-9/syntenin (SDCBP) in mesenchymal metastatic breast cancer cells triggered a transition to a more epithelial-like morphology which was confirmed by changes in EMT markers. In contrast, overexpression of MDA-9 in epithelial cells induced EMT, cytoskeleton reorganization and invasion. Importantly, they found that MDA-9 upregulated both RhoA and Cdc42 via TGFβ1, and they showed that MDA-9 binds to TGFβ1 via its PDZ1 domain. Finally, they showed that silencing the expression of MDA-9 resulted in decreased lung metastasis. These findings support the importance of MDA-9 in EMT in breast cancer and suggest that MDA-9 could be explored as a potential therapeutic target against metastatic diseases [87].
Mechanisms of autoregulation of RhoA and RhoB genes
In a recent study, we showed that the activity of the RhoA and RhoB promoters is subject to autoregulation [88]. Specifically, we showed that overexpression of RhoA inhibits the activity of the RhoB and RhoA promoters and that the CCAAT box of the RhoB promoter is essential for this autoregulation (Fig. 1a). We also showed that the pan-Rho inhibitor C3 increased the mRNA levels of the RhoB gene in a time-dependent manner but not of the RhoA gene. These data could also explain, at least in part, the low levels of expression of RhoB gene in cells that express high levels of RhoA such as the stem cell lines CGR8 and P-19 [88]. Similar type of Rho autoregulation has been observed in mouse fibroblasts [54], in adenocarcinoma cells [89] as well as in PC3 prostate cancer cells and MDA-MB-231 breast cancer cells [90].
Post-transcriptional regulation of RhoA, RhoB, and RhoC genes by miRNAs
MicroRNAs (miRNAs) function as modulators of gene expression at the post-transcriptional level and play a wide range of physiological and pathological roles [91, 92]. They are small non-coding RNA molecules that bind to complementary mRNAs expressed from target genes and inhibit their translation by the ribosomes or enhance their degradation. Several microRNAs were shown to regulate the expression of small Rho GTPases including RhoA, RhoB, and RhoC, and to affect the functions of different cell types. These miRNAs are discussed below.
MiR-31 was identified as one of the highly upregulated miRNAs during osteoclast development and controls cytoskeleton organization in osteoclasts for optimal bone resorption activity by targeting RhoA [93]. miR-125a-3p functions as a tumor suppressor that inhibits the migration and invasion of lung cancer cells [94]. miR-125a-3p decreased the RhoA protein levels, while the levels of RhoA mRNA remain unchanged. Negative regulation of RhoA protein expression has also been demonstrated by miR-133a in cardiomyocytes [95] and human bronchial smooth muscle cells [96]. The microRNA miR-151 suppresses expression of RhoGDI A, resulting in increased basal activation of RhoA, Rac1, and Cdc42 [97] in hepatocellular carcinoma, while RhoA protein expression is negatively regulated by miR-155 in breast cancer cells [98] (Fig. 1a).
Sabatel et al. [99] showed that miR-21 acts as a negative modulator of angiogenesis. miR-21 overexpression reduced endothelial cell proliferation, migration, and the organization of actin into stress fibers. They showed that RhoB gene expression and activity is decreased in miR-21 overexpressing cells and that RhoB silencing impaired endothelial cell migration and tubulogenesis, thus providing a possible mechanism for miR-21-mediated inhibition of angiogenesis [99]. In another study, Connolly et al. [100] showed miR-21 targets the 3′ untranslated region of the RhoB gene and that loss of miR-21 is associated with an elevation of RhoB in hepatocellular carcinoma cell lines Huh-7 and HepG2 and in the metastatic breast cancer cell line MDA-MB-231. Using in vitro models of distinct stages of metastasis, they showed that loss of miR-21 also causes a reduction in migration, invasion, and cell elongation which could be mimicked by overexpression of RhoB [100]. Finally, Liu et al. [101] showed that the expression of miR-21 in HEK293 and several colorectal cancer cells was inversely correlated with the levels of RhoB expression. miR-21 overexpression mimicked the effect of RhoB knockdown in promoting proliferation and invasion and inhibiting apoptosis (Fig. 1b).
Glorian et al. [102] showed that miR-19 regulates the expression of RhoB in keratinocytes upon exposure to UV radiation. miR-19-mediated regulation requires the binding of human antigen R (HuR), an AU-rich element binding protein, to the 3-untranslated region of the RhoB mRNA. It was suggested that downregulation of RhoB by miR-19 potentiates UV-induced apoptosis (Fig. 1b) [102].
Two papers demonstrated the role of miR-138 in cancer migration and metastasis via RhoC downregulation. In one paper, Jiang et al. [103] showed that miR-138 suppressed migration and invasion of tongue squamous cell carcinoma (TSCC) cells by directly targeting the 3′ untranslated region of RhoC mRNA. Reduced expression of RhoC was associated with morphological changes including reorganization of the stress fibers to a round bleb-like shape as well as the suppression of cell migration and invasion whereas knockdown of miR-138 in TSCC cells enhanced the expression of RhoC and accelerated cell migration and invasion. In the second study, Islam et al. [104] showed similar roles of miR-138 and RhoC in head and neck squamous cell carcinoma (HNCCC) cells. They also showed that inhibition of RhoC by miR-138 caused the downregulation of FAK, Src and Erk1/2 signaling molecules (Fig. 1c).
Liu et al. [105] examined the role of miR-372 in the pathogenesis of endometrial adenocarcinoma (EC). They showed that miR-372 levels are lower in EC than normal endometrial specimens and that miR-372 overexpression reduced the expression of RhoC via its 3′ untranslated region and suppressed cell proliferation, migration, and invasion suggesting that miR-372 could be a novel therapeutic target in EC (Fig. 1c).
Another miR that regulates RhoC gene in various cancer cells is miR-10b. Recent studies showed that miR-10b was highly expressed in metastatic breast cancer cells and enhanced cell migration and invasion via a mechanism that involves activation of miR-10b expression by the transcription factor Twist and the subsequent repression of the translation of the messenger RNA encoding homeobox D10 (HOXD10) which is an inhibitor of RhoC [106] resulting in increased expression of RhoC [107, 108]. RhoC regulation by miR-10b via HOXD10 was reported in the case of colorectal cancer cells [109] and in malignant glioma cells [110].
The role of RhoC in the pathogenesis of ovarian cancer was studied by Wu et al. [111]. They found that the levels of RhoC in ovarian cancer cells were increased by the overexpression of the long-coding RNA ABHD11-AS1 and the two molecules were co-immunoprecipitated suggesting a direct interaction. They also showed that silencing of RhoC compromises the cancer-promoting effects of ABHD11-AS1. The findings suggested a potential role of this long-coding RNA in anticancer therapies. In the same cancer type (ovarian cancer) Sang et al. [112] showed that miR-519d binds directly to the 3′ UTR of the RhoC mRNA and inhibits its expression suggesting that the RhoC mRNA is a direct target of miR-519d. A negative correlation between the levels of miR519d and RhoC was also found in vivo using the nude mouse xenograft model [112] (Fig. 1c).
Zhou et al. [113] showed that levels of miR-493 were strongly downregulated in gastric cancer and were associated with clinical stage and the presence of lymph node metastases. They found that upregulation of miR-493 inhibited the proliferation and metastasis of gastric cancer cells, in vitro and in vivo and that miR-493 directly targeted RhoC, which resulted in a marked reduction of the expression of mRNA and protein. This effect, in turn, led to a decreased ability of growth, invasion and metastasis in gastric cancer cells (Fig. 1c).
Yau et al. [114] established an orthotopic hepatocellular carcinoma (HCC) metastasis animal model to identify miRNAs that could associated with the development of metastasis in vivo. They found 15 miRNAs, including miR-106b, which were differentially expressed in 2 metastatic cell lines compared with the primary tumor cell lines. They showed that miR-106b enhanced cell migration and stress fiber formation by inducing the expression of the small GTPases RhoA and RhoC and these effects were associated with activation of epithelial–mesenchymal transition (EMT). A role of miR-106b in RhoC regulation was also found in epithelial ovarian cancer (Fig. 1c) [115]. Table 1 summarizes the transcriptional and post-transcriptional Rho modulators the mechanisms involved, the associated diseases and the relevant cell types in which these mechanisms were studied.
Table 1.
Modulators and mechanisms of transcriptional and post-transcriptional regulation of the RhoA, RhoB and RhoC genes
| Gene | Modulator | Mechanism | Associated disease | Cell type | Refs. |
|---|---|---|---|---|---|
| RhoA | Skp2 | Activation of RhoA gene expression by the Myc–Skp2–Miz1 complex in metastatic human cancers is correlated with RhoA expression | Cancer metastasis | 293T, Rat1, MDA-MB-231 | [36] |
| RhoA | TNFα, IL-13 | Binding of STAT6 and NF-κB to the proximal RhoA promoter | Bronchial asthma | Bronchial smooth muscle cells | [38, 39] |
| RhoA | Cisplatin | Inhibition of RhoA gene transcription by the repressor GCF2 | Resistance to anticancer drugs | Human cisplatin-resistant cancer cell lines | [41] |
| RhoA | NO | Phosphorylation of ATF-1 by PKG and ATF-1 binding to the RhoA promoter | Cardiovascular diseases | Arterial smooth muscle cells, Swiss 3T3 fibroblasts | [43] |
| RhoA | miR-31 | Decreases RhoA | Cytoskeleton organization | Osteoclasts | [93] |
| RhoA | miR-125a-3p | Decreases RhoA | Cancer | Lung cancer cells | [94] |
| RhoA | miR-133a | Decreases RhoA | Cardiovascular disease | Cardiomyocytes, hBSM cells | [95] |
| RhoA | miR-151 | Suppresses Rho GDI, increases RhoA, Rac, cdc42 | Cancer | Hepatocellular carcinoma | [97] |
| RhoA | miR-155 | Decreases RhoA | Cancer | Breast cancer cells | [98] |
| RhoB | Histone deacetylase inhibitors | Repression of HDAC1 activity via a CCAAT box element | Cancer | Human nonsmall lung carcinoma cell line | [49] |
| RhoB | UV | Formation of NFY/ATF-2 complexes on the CCAAT box of the RhoB promoter | Genotoxic stress | NIH3T3 cells, Jurkat cells | [56] |
| RhoB | FTIs, GGTIs | HDAC1 dissociation, HAT association and histone acetylation of the RhoB promoter lead to RhoB upregulation | Cancer | Various human cancer cell lines | [61] |
| RhoB | UV | p38 MAPK upregulation of RhoB (c-Jun and p300 recruitment) | Cancer | Jurkat cells/prostate cancer PC-3 cells | [59] |
| RhoB | KR28 | p38/ATF2/p300 complex formation on the CCAAT box | Cancer | Human prostate cancer PC-3 cells | [60] |
| RhoB | TGFβ | TGFβ1 upregulates RhoB transcription via Smad3/4 (p53-dependent) and MEK/ERK | Cancer | HaCaT | [21] |
| RhoB | Rho GTPases and effectors | Induction/inhibition of RhoB promoter activity, autoregulation | Genotoxic stress | Fibroblasts | [54] |
| RhoB | Ras, EGFR, ErbB2 | Suppression of RhoB | Cancer | NIH 3T3 cells/lung, pancreatic and cervical cancer cell lines | [58, 68] |
| RhoB | NSC126188 | RhoB induction via p300, c-Jun, JNK, MKK4/7, AKT dephosphorylation and FoxO3a activation | Cancer | Stomach carcinoma NUGC-3 cells/prostate cancer PC-3 cells/HeLa | [62–65] |
| RhoB | TSA | RhoB gene induction | Cancer | Ovarian cancer | [116] |
| RhoB | MNU, cisplatin, hydroxyurea, dexamethasone | RhoB gene induction | Cancer | NIH3T3 cells | [55] |
| RhoB | Gallic acid | RhoB gene induction through PI3 K/AKT suppression | Metastasis | Gastric cancer cells | [66] |
| RhoB | Aloe emodin | Downregulation of RhoB, suppression of PMA | Cancer | Cancer cell lines | [67] |
| RhoB | miR-21 | RhoB suppression | Angiogenesis, cancer | Endothelial cells, hepatic, breast, colorectal cancer cells | [99–101] |
| RhoB | miR-19 | RhoB suppression | Genotoxic stress | Keratinocytes | [102] |
| RhoC | Genotoxic stress | Binding of p53 to the 2nd intron of the RhoC gene | Cancer | Cancer cell lines | [75] |
| RhoC | EMT induced by TGFβ and TNFα | Binding of Ets-1 to various sites on the RhoC promoter | Cancer | LIM 1863 colon cancer cell line | [76] |
| RhoC | Stromal cell-derived factor-1 (SDF-1) | Induction of RhoC | T-cell acute lymphoblastic leukemia | Jurkat cells | [79] |
| RhoC | Hypoxia | Binding of HIF-3a to the RhoC promoter | Pancreatic cancer | Pancreatic cancer cell lines | [80] |
| RhoC | Hepatitis B virus | Binding of Ets-1 to the RhoC promoter | Hepatocellular carcinoma | HepG2 cells | [77, 78] |
| RhoC | miR-138 | RhoC suppression |
Tongue squamous cell carcinoma (TSCC) and head and neck squamous Cell carcinoma (HNSCC) |
TSCC and HNSCC cell lines | [103, 104] |
| RhoC | miR-372 | RhoC suppression | Endometrial adenocarcinoma (EC) | EC cells | [105] |
| RhoC | miR-10b | RhoC induction via suppression of homeobox D10 (HOXD10) | Breast cancer, colorectal cancer, malignant glioma | Breast cancer cell lines, colorectal cancer cell lines, glioblastoma cell lines | [107–110] |
| RhoC | lncRNA ABHD11-AS1 | RhoC upregulation | Ovarian cancer | Ovarian carcinoma cell lines | [111] |
| RhoC | miR-519d | RhoC suppression | Ovarian cancer | Ovarian carcinoma cell lines | [112] |
| RhoC | miR-493 | RhoC suppression | Gastric cancer | Gastric cancer cell lines | [113] |
| RhoC | miR-106b | RhoC upregulation | Hepatocellular carcinoma, ovarian cancer | HCC cell lines, ovarian cancer cell lines | [114, 115] |
Conclusions
In conclusion, investigation of the mechanisms that control the expression of RhoA and RhoB small GTPases in various cell types and conditions during the past decade revealed the existence of a core group of transcription factors and coactivators that are involved in different cellular responses such as genotoxic stress, cytokines, and drugs. These factors are the nuclear factor Y, the leucine bZip proteins c-Jun and ATF2, and the coactivators p300 and HDACs. Formation of protein complexes on specific regulatory elements of the RhoA and RhoB promoters, including the CCAAT boxes, appears to coordinate the regulation of their activity and to increase (in the majority of the cases) the mRNA levels of the two genes. Dysregulation of RhoA, RhoB and RhoC gene expression or inhibition of their expression by a variety of microRNAs can be causal to pathological conditions such as cancer and asthma. Positive or negative cross talks between Rho GTPases and TGFβ have been shown to regulate cell responses to TGFβ such as EMT, migration, myofibroblast activation and thymus development. Understanding in depth the mechanisms that fine tune the expression of Rho GTPases and their cross talks with other signaling pathways combined with the critical roles that these proteins play in disease pathogenesis will lead to the development of novel, optimized therapies for the treatment of devastating disease such as cancer, asthma or fibrosis.
Acknowledgements
This work was supported by a Grant from the Hellenic Ministry for Education, Research and Religious Affairs (THALIS MIS 380334) to DK and CS. EN and ML were supported by a doctoral fellowship from IKY-Siemens Research Grants.
Abbreviations
- ATF-1
Activating transcription factor 1
- BSMs
Bronchial smooth muscles
- PKG
cGMP-dependent protein kinase
- EC
Endometrial carcinoma
- EMT
Epithelial-to-mesenchymal transition
- ERK
Extracellular signal-regulated kinase
- FTIs
Farnesyltransferase inhibitors
- GGTIs
Geranylgeranyltransferase I inhibitors
- GAPs
GTPase-activating proteins
- GDIs
Guanosine nucleotide dissociation inhibitors
- GEFs
Guanosine nucleotide exchange factors
- HCC
Hepatocellular carcinoma
- HDAC1
Histone deacetylase 1
- HOXD10
Homeobox D10
- HIF
Hypoxia inducible factor
- IL-13
Interleukin 13
- miRNAs
MicroRNAs
- MAPK
Mitogen-activated protein kinase
- Miz1
Myc-interacting zinc finger protein 1
- NO
Nitric oxide
- NF-κB
Nuclear factor kappa B
- NF-Y
Nuclear factor Y
- PARP-1
Poly(ADP-ribose) polymerase-1
- PMA
Phorbol-12-myristyl-13-acetate
- ROS
Reactive oxygen species
- STAT6
Signal transducer and activator of transcription 6
- siRNAs
Small interfering RNAs
- Skp2
S-phase kinase-associated protein 2
- SDF-1
Stromal cell-derived factor-1
- TGFβ
Transforming growth factor β
- TNFα
Tumor necrosis factor α
References
- 1.Bishop AL, Hall A. Rho GTPases and their effector proteins. Biochem J. 2000;348(Pt 2):241–255. doi: 10.1042/bj3480241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Vega FM, Ridley AJ. Rho GTPases in cancer cell biology. FEBS Lett. 2008;582:2093–2101. doi: 10.1016/j.febslet.2008.04.039. [DOI] [PubMed] [Google Scholar]
- 3.Hall A, Nobes CD. Rho GTPases: molecular switches that control the organization and dynamics of the actin cytoskeleton. Philos Trans R Soc Lond B Biol Sci. 2000;355:965–970. doi: 10.1098/rstb.2000.0632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Haga RB, Ridley AJ. Rho GTPases: regulation and roles in cancer cell biology. Small GTPases. 2016;7:207–221. doi: 10.1080/21541248.2016.1232583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hodge RG, Ridley AJ. Regulating Rho GTPases and their regulators. Nat Rev Mol Cell Biol. 2016;17:496–510. doi: 10.1038/nrm.2016.67. [DOI] [PubMed] [Google Scholar]
- 6.Lawson CD, Ridley AJ. Rho GTPase signaling complexes in cell migration and invasion. J Cell Biol. 2018;217:447–457. doi: 10.1083/jcb.201612069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schaefer A, Reinhard NR, Hordijk PL. Toward understanding RhoGTPase specificity: structure, function and local activation. Small GTPases. 2014;5:6. doi: 10.4161/21541248.2014.968004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Arthur WT, Ellerbroek SM, Der CJ, Burridge K, Wennerberg K. XPLN, a guanine nucleotide exchange factor for RhoA and RhoB, but not RhoC. J Biol Chem. 2002;277:42964–42972. doi: 10.1074/jbc.M207401200. [DOI] [PubMed] [Google Scholar]
- 9.Sloan CM, Quinn CV, Peters JP, Farley J, Goetzinger C, Wernli M, DeMali KA, Ellerbroek SM. Divergence of Rho residue 43 impacts GEF activity. Small GTPases. 2012;3:15–22. doi: 10.4161/sgtp.19557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hamel B, Monaghan-Benson E, Rojas RJ, Temple BR, Marston DJ, Burridge K, Sondek J. SmgGDS is a guanine nucleotide exchange factor that specifically activates RhoA and RhoC. J Biol Chem. 2011;286:12141–12148. doi: 10.1074/jbc.M110.191122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ridley AJ, Self AJ, Kasmi F, Paterson HF, Hall A, Marshall CJ, Ellis C. rho family GTPase activating proteins p190, bcr and rhoGAP show distinct specificities in vitro and in vivo. EMBO J. 1993;12:5151–5160. doi: 10.1002/j.1460-2075.1993.tb06210.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lazarini M, et al. ARHGAP21 is a RhoGAP for RhoA and RhoC with a role in proliferation and migration of prostate adenocarcinoma cells. Biochim Biophys Acta. 2013;1832:365–374. doi: 10.1016/j.bbadis.2012.11.010. [DOI] [PubMed] [Google Scholar]
- 13.Cannizzaro LA, Madaule P, Hecht F, Axel R, Croce CM, Huebner K. Chromosome localization of human ARH genes, a ras-related gene family. Genomics. 1990;6:197–203. doi: 10.1016/0888-7543(90)90557-B. [DOI] [PubMed] [Google Scholar]
- 14.Karnoub AE, Symons M, Campbell SL, Der CJ. Molecular basis for Rho GTPase signaling specificity. Breast Cancer Res Treat. 2004;84:61–71. doi: 10.1023/B:BREA.0000018427.84929.5c. [DOI] [PubMed] [Google Scholar]
- 15.Wennerberg K, Rossman KL, Der CJ. The Ras superfamily at a glance. J Cell Sci. 2005;118:843–846. doi: 10.1242/jcs.01660. [DOI] [PubMed] [Google Scholar]
- 16.Kardassis D, Murphy C, Fotsis T, Moustakas A, Stournaras C. Control of transforming growth factor beta signal transduction by small GTPases. FEBS J. 2009;276:2947–2965. doi: 10.1111/j.1742-4658.2009.07031.x. [DOI] [PubMed] [Google Scholar]
- 17.Papadimitriou E, Kardassis D, Moustakas A, Stournaras C. TGFbeta-induced early activation of the small GTPase RhoA is Smad2/3-independent and involves Src and the guanine nucleotide exchange factor Vav2. Cell Physiol Biochem. 2011;28:229–238. doi: 10.1159/000331734. [DOI] [PubMed] [Google Scholar]
- 18.Papadimitriou E, Vasilaki E, Vorvis C, Iliopoulos D, Moustakas A, Kardassis D, Stournaras C. Differential regulation of the two RhoA-specific GEF isoforms Net1/Net1A by TGF-beta and miR-24: role in epithelial-to-mesenchymal transition. Oncogene. 2012;31:2862–2875. doi: 10.1038/onc.2011.457. [DOI] [PubMed] [Google Scholar]
- 19.Vardouli L, Moustakas A, Stournaras C. LIM-kinase 2 and cofilin phosphorylation mediate actin cytoskeleton reorganization induced by transforming growth factor-beta. J Biol Chem. 2005;280:11448–11457. doi: 10.1074/jbc.M402651200. [DOI] [PubMed] [Google Scholar]
- 20.Vardouli L, Vasilaki E, Papadimitriou E, Kardassis D, Stournaras C. A novel mechanism of TGFbeta-induced actin reorganization mediated by Smad proteins and Rho GTPases. FEBS J. 2008;275:4074–4087. doi: 10.1111/j.1742-4658.2008.06549.x. [DOI] [PubMed] [Google Scholar]
- 21.Vasilaki E, Papadimitriou E, Tajadura V, Ridley AJ, Stournaras C, Kardassis D. Transcriptional regulation of the small GTPase RhoB gene by TGFβ-induced signaling pathways. FASEB J. 2010;24:891–905. doi: 10.1096/fj.09-134742. [DOI] [PubMed] [Google Scholar]
- 22.Jansen S, Gosens R, Wieland T, Schmidt M. Paving the Rho in cancer metastasis: Rho GTPases and beyond. Pharmacol Ther. 2018;183:1–21. doi: 10.1016/j.pharmthera.2017.09.002. [DOI] [PubMed] [Google Scholar]
- 23.Wang K, et al. Whole-genome sequencing and comprehensive molecular profiling identify new driver mutations in gastric cancer. Nat Genet. 2014;46:573–582. doi: 10.1038/ng.2983. [DOI] [PubMed] [Google Scholar]
- 24.Kakiuchi M, et al. Recurrent gain-of-function mutations of RHOA in diffuse-type gastric carcinoma. Nat Genet. 2014;46:583–587. doi: 10.1038/ng.2984. [DOI] [PubMed] [Google Scholar]
- 25.Cancer Genome Atlas Research Network Comprehensive molecular characterization of gastric adenocarcinoma. Nature. 2014;513:202–209. doi: 10.1038/nature13480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Richter J, et al. Recurrent mutation of the ID3 gene in Burkitt lymphoma identified by integrated genome, exome and transcriptome sequencing. Nat Genet. 2012;44:1316–1320. doi: 10.1038/ng.2469. [DOI] [PubMed] [Google Scholar]
- 27.Yoo HY, et al. A recurrent inactivating mutation in RHOA GTPase in angioimmunoblastic T cell lymphoma. Nat Genet. 2014;46:371–375. doi: 10.1038/ng.2916. [DOI] [PubMed] [Google Scholar]
- 28.Bellizzi A, et al. RhoA protein expression in primary breast cancers and matched lymphocytes is associated with progression of the disease. Int J Mol Med. 2008;22:25–31. [PubMed] [Google Scholar]
- 29.Faried A, Faried LS, Usman N, Kato H, Kuwano H. Clinical and prognostic significance of RhoA and RhoC gene expression in esophageal squamous cell carcinoma. Ann Surg Oncol. 2007;14:3593–3601. doi: 10.1245/s10434-007-9562-x. [DOI] [PubMed] [Google Scholar]
- 30.Horiuchi A, Imai T, Wang C, Ohira S, Feng Y, Nikaido T, Konishi I. Up-regulation of small GTPases, RhoA and RhoC, is associated with tumor progression in ovarian carcinoma. Lab Invest. 2003;83:861–870. doi: 10.1097/01.LAB.0000073128.16098.31. [DOI] [PubMed] [Google Scholar]
- 31.Lang P, Gesbert F, Delespine-Carmagnat M, Stancou R, Pouchelet M, Bertoglio J. Protein kinase A phosphorylation of RhoA mediates the morphological and functional effects of cyclic AMP in cytotoxic lymphocytes. EMBO J. 1996;15:510–519. doi: 10.1002/j.1460-2075.1996.tb00383.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chen Y, et al. Cullin mediates degradation of RhoA through evolutionarily conserved BTB adaptors to control actin cytoskeleton structure and cell movement. Mol Cell. 2009;35:841–855. doi: 10.1016/j.molcel.2009.09.004. [DOI] [PubMed] [Google Scholar]
- 33.Alberts AS, Qin H, Carr HS, Frost JA. PAK1 negatively regulates the activity of the Rho exchange factor NET1. J Biol Chem. 2005;280:12152–12161. doi: 10.1074/jbc.M405073200. [DOI] [PubMed] [Google Scholar]
- 34.Callow MG, Zozulya S, Gishizky ML, Jallal B, Smeal T. PAK4 mediates morphological changes through the regulation of GEF-H1. J Cell Sci. 2005;118:1861–1872. doi: 10.1242/jcs.02313. [DOI] [PubMed] [Google Scholar]
- 35.Besson A, Gurian-West M, Schmidt A, Hall A, Roberts JM. p27Kip1 modulates cell migration through the regulation of RhoA activation. Genes Dev. 2004;18:862–876. doi: 10.1101/gad.1185504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chan CH, et al. Deciphering the transcriptional complex critical for RhoA gene expression and cancer metastasis. Nat Cell Biol. 2010;12:457–467. doi: 10.1038/ncb2047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Adhikary S, Eilers M. Transcriptional regulation and transformation by Myc proteins. Nat Rev Mol Cell Biol. 2005;6:635–645. doi: 10.1038/nrm1703. [DOI] [PubMed] [Google Scholar]
- 38.Goto K, Chiba Y, Matsusue K, Hattori Y, Maitani Y, Sakai H, Kimura S, Misawa M. The proximal STAT6 and NF-kappaB sites are responsible for IL-13- and TNF-alpha-induced RhoA transcriptions in human bronchial smooth muscle cells. Pharmacol Res. 2010;61:466–472. doi: 10.1016/j.phrs.2009.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chiba Y, Goto K, Matsusue K, Kimura S, Misawa M. Identification and characterization of rat RhoA gene promoter. J Pharmacol Sci. 2010;112:467–472. doi: 10.1254/jphs.09346SC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Shen DW, Su A, Liang XJ, Pai-Panandiker A, Gottesman MM. Reduced expression of small GTPases and hypermethylation of the folate binding protein gene in cisplatin-resistant cells. Br J Cancer. 2004;91:270–276. doi: 10.1038/sj.bjc.6601956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Shen DW, Pouliot LM, Gillet JP, Ma W, Johnson AC, Hall MD, Gottesman MM. The transcription factor GCF2 is an upstream repressor of the small GTPAse RhoA, regulating membrane protein trafficking, sensitivity to doxorubicin, and resistance to cisplatin. Mol Pharm. 2012;9:1822–1833. doi: 10.1021/mp300153z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Somlyo AP, Somlyo AV. Signal transduction by G-proteins, rho-kinase and protein phosphatase to smooth muscle and non-muscle myosin II. J Physiol. 2000;522(Pt 2):177–185. doi: 10.1111/j.1469-7793.2000.t01-2-00177.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sauzeau V, Rolli-Derkinderen M, Marionneau C, Loirand G, Pacaud P. RhoA expression is controlled by nitric oxide through cGMP-dependent protein kinase activation. J Biol Chem. 2003;278:9472–9480. doi: 10.1074/jbc.M212776200. [DOI] [PubMed] [Google Scholar]
- 44.Bhavsar PJ, Infante E, Khwaja A, Ridley AJ. Analysis of Rho GTPase expression in T-ALL identifies RhoU as a target for Notch involved in T-ALL cell migration. Oncogene. 2013;32:198–208. doi: 10.1038/onc.2012.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Luis-Ravelo D, et al. RHOB influences lung adenocarcinoma metastasis and resistance in a host-sensitive manner. Mol Oncol. 2014;8:196–206. doi: 10.1016/j.molonc.2013.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Forget MA, Desrosiers RR, Del M, Moumdjian R, Shedid D, Berthelet F, Beliveau R. The expression of rho proteins decreases with human brain tumor progression: potential tumor markers. Clin Exp Metastasis. 2002;19:9–15. doi: 10.1023/A:1013884426692. [DOI] [PubMed] [Google Scholar]
- 47.Ma Y, Gong Y, Cheng Z, Loganathan S, Kao C, Sarkaria JN, Abel TW, Wang J. Critical functions of RhoB in support of glioblastoma tumorigenesis. Neuro Oncol. 2015;17:516–525. doi: 10.1093/neuonc/nou228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Huang M, Prendergast GC. RhoB in cancer suppression. Histol Histopathol. 2006;21:213–218. doi: 10.14670/HH-21.213. [DOI] [PubMed] [Google Scholar]
- 49.Wang S, et al. Histone deacetylase 1 represses the small GTPase RhoB expression in human nonsmall lung carcinoma cell line. Oncogene. 2003;22:6204–6213. doi: 10.1038/sj.onc.1206653. [DOI] [PubMed] [Google Scholar]
- 50.Papadopoulou N, Charalampopoulos I, Alevizopoulos K, Gravanis A, Stournaras C. Rho/ROCK/actin signaling regulates membrane androgen receptor induced apoptosis in prostate cancer cells. Exp Cell Res. 2008;314:3162–3174. doi: 10.1016/j.yexcr.2008.07.012. [DOI] [PubMed] [Google Scholar]
- 51.Papadopoulou N, Papakonstanti EA, Kallergi G, Alevizopoulos K, Stournaras C. Membrane androgen receptor activation in prostate and breast tumor cells: molecular signaling and clinical impact. IUBMB Life. 2009;61:56–61. doi: 10.1002/iub.150. [DOI] [PubMed] [Google Scholar]
- 52.Lang F, Alevizopoulos K, Stournaras C. Targeting membrane androgen receptors in tumors. Expert Opin Ther Targets. 2013;17:951–963. doi: 10.1517/14728222.2013.806491. [DOI] [PubMed] [Google Scholar]
- 53.Stournaras C, Gravanis A, Margioris AN, Lang F. The actin cytoskeleton in rapid steroid hormone actions. Cytoskeleton (Hoboken) 2014;71:285–293. doi: 10.1002/cm.21172. [DOI] [PubMed] [Google Scholar]
- 54.Fritz G, Kaina B. rhoB encoding a UV-inducible Ras-related small GTP-binding protein is regulated by GTPases of the Rho family and independent of JNK, ERK, and p38 MAP kinase. J Biol Chem. 1997;272:30637–30644. doi: 10.1074/jbc.272.49.30637. [DOI] [PubMed] [Google Scholar]
- 55.Fritz G, Kaina B, Aktories K. The ras-related small GTP-binding protein RhoB is immediate-early inducible by DNA damaging treatments. J Biol Chem. 1995;270:25172–25177. doi: 10.1074/jbc.270.42.25172. [DOI] [PubMed] [Google Scholar]
- 56.Fritz G, Kaina B. Transcriptional activation of the small GTPase gene rhoB by genotoxic stress is regulated via a CCAAT element. Nucleic Acids Res. 2001;29:792–798. doi: 10.1093/nar/29.3.792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Jahner D, Hunter T. The ras-related gene rhoB is an immediate-early gene inducible by v-Fps, epidermal growth factor, and platelet-derived growth factor in rat fibroblasts. Mol Cell Biol. 1991;11:3682–3690. doi: 10.1128/MCB.11.7.3682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Jiang K, Sun J, Cheng J, Djeu JY, Wei S, Sebti S. Akt mediates Ras downregulation of RhoB, a suppressor of transformation, invasion, and metastasis. Mol Cell Biol. 2004;24:5565–5576. doi: 10.1128/MCB.24.12.5565-5576.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ahn J, Choi JH, Won M, Kang CM, Gyun MR, Park HM, Kim CH, Chung KS. The activation of p38 MAPK primarily contributes to UV-induced RhoB expression by recruiting the c-Jun and p300 to the distal CCAAT box of the RhoB promoter. Biochem Biophys Res Commun. 2011;409:211–216. doi: 10.1016/j.bbrc.2011.04.121. [DOI] [PubMed] [Google Scholar]
- 60.Chung KS, et al. A novel antitumor piperazine alkyl compound causes apoptosis by inducing RhoB expression via ROSmediated cAbl/p38 MAPK signaling. Cancer Chemother Pharmacol. 2013;72:1315–1324. doi: 10.1007/s00280-013-2310-y. [DOI] [PubMed] [Google Scholar]
- 61.Delarue FL, et al. Farnesyltransferase and geranylgeranyltransferase I inhibitors upregulate RhoB expression by HDAC1 dissociation, HAT association and histone acetylation of the RhoB promoter. Oncogene. 2007;26:633–640. doi: 10.1038/sj.onc.1209819. [DOI] [PubMed] [Google Scholar]
- 62.Kim BK, et al. p300 cooperates with c-Jun and PARP-1 at the p300 binding site to activate RhoB transcription in NSC126188-mediated apoptosis. Biochim Biophys Acta. 2014;1839:364–373. doi: 10.1016/j.bbagrm.2014.03.004. [DOI] [PubMed] [Google Scholar]
- 63.Kim BK, et al. Upregulation of RhoB via c-Jun N-terminal kinase signaling induces apoptosis of the human gastric carcinoma NUGC-3 cells treated with NSC12618. Carcinogenesis. 2011;32:254–261. doi: 10.1093/carcin/bgq244. [DOI] [PubMed] [Google Scholar]
- 64.Won KJ, et al. NSC126188 induces apoptosis of prostate cancer PC-3 cells through inhibition of Akt membrane translocation, FoxO3a activation, and RhoB transcription. Apoptosis. 2014;19:179–190. doi: 10.1007/s10495-013-0905-8. [DOI] [PubMed] [Google Scholar]
- 65.Kim BK, et al. NSC126188, a piperazine alkyl derivative, induces apoptosis via upregulation of RhoB in HeLa cells. Invest New Drugs. 2011;29:853–860. doi: 10.1007/s10637-010-9433-3. [DOI] [PubMed] [Google Scholar]
- 66.Ho HH, Chang CS, Ho WC, Liao SY, Lin WL, Wang CJ. Gallic acid inhibits gastric cancer cells metastasis and invasive growth via increased expression of RhoB, downregulation of AKT/small GTPase signals and inhibition of NF-kappaB activity. Toxicol Appl Pharmacol. 2013;266:76–85. doi: 10.1016/j.taap.2012.10.019. [DOI] [PubMed] [Google Scholar]
- 67.Suboj P, Babykutty S, Gopi DRV, Nair RS, Srinivas P, Gopala S. Aloe emodin inhibits colon cancer cell migration/angiogenesis by downregulating MMP-2/9, RhoB and VEGF via reduced DNA binding activity of NF-kappaB. Eur J Pharm Sci. 2012;45:581–591. doi: 10.1016/j.ejps.2011.12.012. [DOI] [PubMed] [Google Scholar]
- 68.Jiang K, Delarue FL, Sebti SM. EGFR, ErbB2 and Ras but not Src suppress RhoB expression while ectopic expression of RhoB antagonizes oncogene-mediated transformation. Oncogene. 2004;23:1136–1145. doi: 10.1038/sj.onc.1207236. [DOI] [PubMed] [Google Scholar]
- 69.Yoon YS, Choo JH, Yoo T, Kang K, Chung JH. RhoB is epigenetically regulated in an age-and tissue-specific manner. Biochem Biophys Res Commun. 2007;362:164–169. doi: 10.1016/j.bbrc.2007.08.002. [DOI] [PubMed] [Google Scholar]
- 70.Mokady D, Meiri D. RhoGTPases–a novel link between cytoskeleton organization and cisplatin resistance. Drug Resist Updat. 2015;19:22–32. doi: 10.1016/j.drup.2015.01.001. [DOI] [PubMed] [Google Scholar]
- 71.Guan X, Chen S, Zhao Y. The role of RhoC in malignant tumor invasion, metastasis and targeted therapy. Histol Histopathol. 2018;33(3):255–260. doi: 10.14670/HH-11-915. [DOI] [PubMed] [Google Scholar]
- 72.Ridley AJ. RhoA, RhoB and RhoC have different roles in cancer cell migration. J Microsc. 2013;251:242–249. doi: 10.1111/jmi.12025. [DOI] [PubMed] [Google Scholar]
- 73.Lang S, Busch H, Boerries M, Brummer T, Timme S, Lassmann S, Aktories K, Schmidt G. Specific role of RhoC in tumor invasion and metastasis. Oncotarget. 2017;8:87364–87378. doi: 10.18632/oncotarget.20957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Xu XD, Shen HB, Zhu L, Lu JQ, Zhang L, Luo ZY, Wu YQ. Anti-RhoC siRNAs inhibit the proliferation and invasiveness of breast cancer cells via modulating the KAI1, MMP9, and CXCR4 expression. Onco Targets Ther. 2017;10:1827–1834. doi: 10.2147/OTT.S93164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Croft DR, et al. p53-mediated transcriptional regulation and activation of the actin cytoskeleton regulatory RhoC to LIMK2 signaling pathway promotes cell survival. Cell Res. 2011;21:666–682. doi: 10.1038/cr.2010.154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Bellovin DI, Simpson KJ, Danilov T, Maynard E, Rimm DL, Oettgen P, Mercurio AM. Reciprocal regulation of RhoA and RhoC characterizes the EMT and identifies RhoC as a prognostic marker of colon carcinoma. Oncogene. 2006;25:6959–6967. doi: 10.1038/sj.onc.1209682. [DOI] [PubMed] [Google Scholar]
- 77.Tian Y, Liu Y, Qu J, Li K, Qin D, Huang A, Tang H. HBV regulated RhoC expression in HepG2.2.15 cells by enhancing its promoter activity. J Basic Microbiol. 2013;53:461–468. doi: 10.1002/jobm.201200063. [DOI] [PubMed] [Google Scholar]
- 78.Qin D, Li K, Qu J, Wang S, Zou C, Sheng Y, Huang A, Tang H. HBx and HBs regulate RhoC expression by upregulating transcription factor Ets-1. Arch Virol. 2013;158:1773–1781. doi: 10.1007/s00705-013-1655-1. [DOI] [PubMed] [Google Scholar]
- 79.Luo J, Li D, Wei D, Wang X, Wang L, Zeng X. RhoA and RhoC are involved in stromal cell-derived factor-1-induced cell migration by regulating F-actin redistribution and assembly. Mol Cell Biochem. 2017;436:13–21. doi: 10.1007/s11010-017-3072-3. [DOI] [PubMed] [Google Scholar]
- 80.Zhou X, Guo X, Chen M, Xie C, Jiang J. HIF-3alpha promotes metastatic phenotypes in pancreatic cancer by transcriptional regulation of the RhoC-ROCK1 signaling pathway. Mol Cancer Res. 2018;16:124–134. doi: 10.1158/1541-7786.MCR-17-0256. [DOI] [PubMed] [Google Scholar]
- 81.Egami Y, Kawai K, Araki N. RhoC regulates the actin remodeling required for phagosome formation during FcgammaR-mediated phagocytosis. J Cell Sci. 2017;130:4168–4179. doi: 10.1242/jcs.202739. [DOI] [PubMed] [Google Scholar]
- 82.Ji H, Tang H, Lin H, Mao J, Gao L, Liu J, Wu T. Rho/Rock cross-talks with transforming growth factor-beta/Smad pathway participates in lung fibroblast-myofibroblast differentiation. Biomed Rep. 2014;2:787–792. doi: 10.3892/br.2014.323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Manickam N, Patel M, Griendling KK, Gorin Y, Barnes JL. RhoA/Rho kinase mediates TGF-beta1-induced kidney myofibroblast activation through Poldip2/Nox4-derived reactive oxygen species. Am J Physiol Renal Physiol. 2014;307:F159–F171. doi: 10.1152/ajprenal.00546.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Crosas-Molist E, Bertran E, Rodriguez-Hernandez I, Herraiz C, Cantelli G, Fabra A, Sanz-Moreno V, Fabregat I. The NADPH oxidase NOX4 represses epithelial to amoeboid transition and efficient tumour dissemination. Oncogene. 2017;36:3002–3014. doi: 10.1038/onc.2016.454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Bravo-Nuevo A, O’Donnell R, Rosendahl A, Chung JH, Benjamin LE, Odaka C. RhoB deficiency in thymic medullary epithelium leads to early thymic atrophy. Int Immunol. 2011;23:593–600. doi: 10.1093/intimm/dxr064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Ungefroren H, Witte D, Lehnert H. The role of small GTPases of the Rho/Rac family in TGF-beta-induced EMT and cell motility in cancer. Dev Dyn. 2018;247:451–461. doi: 10.1002/dvdy.24505. [DOI] [PubMed] [Google Scholar]
- 87.Menezes ME, Shen XN, Das SK, Emdad L, Sarkar D, Fisher PB. MDA-9/Syntenin (SDCBP) modulates small GTPases RhoA and Cdc42 via transforming growth factor beta1 to enhance epithelial–mesenchymal transition in breast cancer. Oncotarget. 2016;7:80175–80189. doi: 10.18632/oncotarget.13373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Nomikou E, Stournaras C, Kardassis D. Functional analysis of the promoters of the small GTPases RhoA and RhoB in embryonic stem cells. Biochem Biophys Res Commun. 2017;491:754–759. doi: 10.1016/j.bbrc.2017.07.114. [DOI] [PubMed] [Google Scholar]
- 89.Ho TT, Merajver SD, Lapiere CM, Nusgens BV, Deroanne CF. RhoA-GDP regulates RhoB protein stability potential involvement of RhoGDIalpha. J Biol Chem. 2008;283:21588–21598. doi: 10.1074/jbc.M710033200. [DOI] [PubMed] [Google Scholar]
- 90.Vega FM, Fruhwirth G, Ng T, Ridley AJ. RhoA and RhoC have distinct roles in migration and invasion by acting through different targets. J Cell Biol. 2011;193:655–665. doi: 10.1083/jcb.201011038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Bartel DP. MicroRNAs: target recognition and regulatory functions. Cell. 2009;136:215–233. doi: 10.1016/j.cell.2009.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Bartel DP. MicroRNAs: genomics, biogenesis, mechanism, and function. Cell. 2004;116:281–297. doi: 10.1016/S0092-8674(04)00045-5. [DOI] [PubMed] [Google Scholar]
- 93.Mizoguchi F, Murakami Y, Saito T, Miyasaka N, Kohsaka H. miR-31 controls osteoclast formation and bone resorption by targeting RhoA. Arthritis Res Ther. 2013;15:R102. doi: 10.1186/ar4282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Huang B, Luo W, Sun L, Zhang Q, Jiang L, Chang J, Qiu X, Wang E. MiRNA-125a-3p is a negative regulator of the RhoA-actomyosin pathway in A549 cells. Int J Oncol. 2013;42:1734–1742. doi: 10.3892/ijo.2013.1861. [DOI] [PubMed] [Google Scholar]
- 95.Care A, et al. MicroRNA-133 controls cardiac hypertrophy. Nat Med. 2007;13:613–618. doi: 10.1038/nm1582. [DOI] [PubMed] [Google Scholar]
- 96.Chiba Y, Tanabe M, Goto K, Sakai H, Misawa M. Down-regulation of miR-133a contributes to up-regulation of Rhoa in bronchial smooth muscle cells. Am J Respir Crit Care Med. 2009;180:713–719. doi: 10.1164/rccm.200903-0325OC. [DOI] [PubMed] [Google Scholar]
- 97.Ding J, et al. Gain of miR-151 on chromosome 8q24.3 facilitates tumour cell migration and spreading through downregulating RhoGDIA. Nat Cell Biol. 2010;12:390–399. doi: 10.1038/ncb2039. [DOI] [PubMed] [Google Scholar]
- 98.Kong W, Yang H, He L, Zhao JJ, Coppola D, Dalton WS, Cheng JQ. MicroRNA-155 is regulated by the transforming growth factor beta/Smad pathway and contributes to epithelial cell plasticity by targeting RhoA. Mol Cell Biol. 2008;28:6773–6784. doi: 10.1128/MCB.00941-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Sabatel C, et al. MicroRNA-21 exhibits antiangiogenic function by targeting RhoB expression in endothelial cells. PLoS One. 2011;6:e16979. doi: 10.1371/journal.pone.0016979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Connolly EC, Van Doorslaer K, Rogler LE, Rogler CE. Overexpression of miR-21 promotes an in vitro metastatic phenotype by targeting the tumor suppressor RHOB. Mol Cancer Res. 2010;8:691–700. doi: 10.1158/1541-7786.MCR-09-0465. [DOI] [PubMed] [Google Scholar]
- 101.Liu M, Tang Q, Qiu M, Lang N, Li M, Zheng Y, Bi F. miR-21 targets the tumor suppressor RhoB and regulates proliferation, invasion and apoptosis in colorectal cancer cells. FEBS Lett. 2011;585:2998–3005. doi: 10.1016/j.febslet.2011.08.014. [DOI] [PubMed] [Google Scholar]
- 102.Glorian V, Maillot G, Poles S, Iacovoni JS, Favre G, Vagner S. HuR-dependent loading of miRNA RISC to the mRNA encoding the Ras-related small GTPase RhoB controls its translation during UV-induced apoptosis. Cell Death Differ. 2011;18:1692–1701. doi: 10.1038/cdd.2011.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Jiang L, Liu X, Kolokythas A, Yu J, Wang A, Heidbreder CE, Shi F, Zhou X. Downregulation of the Rho GTPase signaling pathway is involved in the microRNA-138-mediated inhibition of cell migration and invasion in tongue squamous cell carcinoma. Int J Cancer. 2010;127:505–512. doi: 10.1002/ijc.25320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Islam M, Datta J, Lang JC, Teknos TN. Down regulation of RhoC by microRNA-138 results in de-activation of FAK, Src and Erk1/2 signaling pathway in head and neck squamous cell carcinoma. Oral Oncol. 2014;50:448–456. doi: 10.1016/j.oraloncology.2014.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Liu BL, Sun KX, Zong ZH, Chen S, Zhao Y. MicroRNA-372 inhibits endometrial carcinoma development by targeting the expression of the Ras homolog gene family member C (RhoC) Oncotarget. 2016;7:6649–6664. doi: 10.18632/oncotarget.6544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Myers C, Charboneau A, Cheung I, Hanks D, Boudreau N. Sustained expression of homeobox D10 inhibits angiogenesis. Am J Pathol. 2002;161:2099–2109. doi: 10.1016/S0002-9440(10)64488-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Ma L, Teruya-Feldstein J, Weinberg RA. Tumour invasion and metastasis initiated by microRNA-10b in breast cancer. Nature. 2007;449:682–688. doi: 10.1038/nature06174. [DOI] [PubMed] [Google Scholar]
- 108.Knirsh R, Ben-Dror I, Modai S, Shomron N, Vardimon L. MicroRNA 10b promotes abnormal expression of the proto-oncogene c-Jun in metastatic breast cancer cells. Oncotarget. 2016;7:59932–59944. doi: 10.18632/oncotarget.11000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Wang YF, Li Z, Zhao XH, Zuo XM, Zhang Y, Xiao YH, Li J, Peng ZH. MicroRNA-10b is upregulated and has an invasive role in colorectal cancer through enhanced Rhoc expression. Oncol Rep. 2015;33:1275–1283. doi: 10.3892/or.2015.3737. [DOI] [PubMed] [Google Scholar]
- 110.Sasayama T, Nishihara M, Kondoh T, Hosoda K, Kohmura E. MicroRNA-10b is overexpressed in malignant glioma and associated with tumor invasive factors, uPAR and RhoC. Int J Cancer. 2009;125:1407–1413. doi: 10.1002/ijc.24522. [DOI] [PubMed] [Google Scholar]
- 111.Wu DD, Chen X, Sun KX, Wang LL, Chen S, Zhao Y. Role of the lncRNA ABHD11-AS1 in the tumorigenesis and progression of epithelial ovarian cancer through targeted regulation of RhoC. Mol Cancer. 2017;16:138. doi: 10.1186/s12943-017-0709-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Sang XB, Zong ZH, Wang LL, Wu DD, Chen S, Liu BL, Zhao Y. E2F-1 targets miR-519d to regulate the expression of the ras homolog gene family member C. Oncotarget. 2017;8:14777–14793. doi: 10.18632/oncotarget.14833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Zhou W, Zhang C, Jiang H, Zhang Z, Xie L, He X. MiR-493 suppresses the proliferation and invasion of gastric cancer cells by targeting RhoC. Iran J Basic Med Sci. 2015;18:1027–1033. [PMC free article] [PubMed] [Google Scholar]
- 114.Yau WL, et al. Over-expression of miR-106b promotes cell migration and metastasis in hepatocellular carcinoma by activating epithelial–mesenchymal transition process. PLoS One. 2013;8:e57882. doi: 10.1371/journal.pone.0057882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Chen S, Chen X, Xiu YL, Sun KX, Zhao Y. Inhibition of ovarian epithelial carcinoma tumorigenesis and progression by microRNA 106b mediated through the RhoC pathway. PLoS One. 2015;10:e0125714. doi: 10.1371/journal.pone.0125714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Liu Y, Song N, Ren K, Meng S, Xie Y, Long Q, Chen X, Zhao X. Expression loss and revivification of RhoB gene in ovary carcinoma carcinogenesis and development. PLoS One. 2013;8:e78417. doi: 10.1371/journal.pone.0078417. [DOI] [PMC free article] [PubMed] [Google Scholar]