Abstract
The chemical variability of the intestinal lumen requires the presence of molecular receptors detecting the various substances naturally occurring in the diet and as a result of the activity of the microbiota. Despite their early discovery, intestinal bitter taste receptors (Tas2r) have not yet been assigned an unambiguous physiological function. Recently, using a CRE-recombinant approach we showed that the Tas2r131 gene is expressed in a subset of mucin-producing goblet cells in the colon of mice. Moreover, we also demonstrated that the expression of the Tas2r131 locus is not restricted to this region. In the present study we aimed at characterizing the presence of positive cells also in other gastrointestinal regions. Our results show that Tas2r131+ cells appear in the jejunum and the ileum, and are absent from the stomach and the duodenum. We identified the positive cells as a subpopulation of deep-crypt Paneth cells in the ileum, strengthening the notion of a defensive role for Tas2rs in the gut. To get a broader perspective on the expression of bitter taste receptors in the alimentary canal, we quantified the expression of all 35 Tas2r genes along the gastrointestinal tract by qRT-PCR. We discovered that the number and expression level of Tas2r genes profoundly vary along the alimentary canal, with the stomach and the colon expressing the largest subsets.
Electronic supplementary material
The online version of this article (doi:10.1007/s00018-017-2621-y) contains supplementary material, which is available to authorized users.
Keywords: Tas2r, Paneth cell, Small intestine, Bitter taste receptor, Taste
Introduction
Taste receptors of the Tas1r and Tas2r GPCR families are regarded as the molecular sensors evaluating the composition of food in the oral cavity before it enters the gastrointestinal (GI) tract [1]. They provide information on the presence of energy-rich substrates such as mono- and disaccharides, l-amino acids, and the occurrence of potentially toxic bitter compounds [2–4]. Mono- and disaccharides as well as l-amino acids are recognized by the receptor heteromers Tas1r2-Tas1r3 and Tas1r1-Tas1r3 [3, 5, 6] underlying the taste qualities of sweet and umami, respectively. Bitter compounds are recognized by the Tas2r receptors [7–12], of which humans possess 25 and rodents 35 [13–15]. In addition to these three GPCR-dependent taste qualities, salty and sour recognition is mediated by ion channels, however, the molecular identity of the corresponding receptors are less well understood [16].
In recent years it has become clear that the role of taste receptors is not restricted to the oral cavity. An ever-growing number of reports provided evidence for the presence of Tas1r and Tas2r receptors and taste signalling components in a variety of extragustatory tissues (for a recent review see [17]), hinting that they might serve diverse physiological functions. The first reports on the occurrence of taste GPCRs in gastrointestinal tissues were devoted to the expression of Tas1rs and Tas2rs in gastric and small-intestinal mucosa [18, 19]. Meanwhile it was demonstrated that Tas1r1, Tas1r2 and Tas1r3 are expressed in enteroendocrine K and L cells in the small intestine [20–23], and successively also in enterochromaffin cells in the colon [24]. In these tissues Tas1r receptors control the secretion of gastrointestinal hormones such as cholecystokinin (CCK) [21], glucagon-like peptide 1 and 2 (GLP-1, GLP-2) [22, 23, 25] and serotonin (5-HT) [24] through which they modulate plasma insulin level, glucose absorption and colonic peristaltic movements [21–24, 26, 27].
On the contrary, the role of Tas2rs is less clear. Initial studies reported the expression of subsets of Tas2rs at mRNA level in all regions of the gastrointestinal tract from the stomach to the large intestine [19, 28–31], hinting at a role in the physiology of these tissues. It was indeed observed that bitter compounds influence different gastrointestinal parameters. A delay in gastric emptying [32] and a modulation of food intake partially mediated by α-gustducin-dependent release of ghrelin from enteroendocrine ghrelin-producing cells was observed after gavage of bitter compounds in rodents [33]. In both the small and the large intestine, the bitter agonists quinine and 6-PTU (6-n-propyl-2-thiouracil) elicit anion and fluid secretion from the gastrointestinal mucosa [29, 34]. In line with the putative protective role of Tas2r receptors in the oral cavity, these mechanisms are thought to limit the absorption of ingested noxious bitter compounds by the GI mucosa either by slowing down their entry in the intestine from the stomach or by flushing them off the intestinal mucosa consistent with the proposed chemofensor function for Tas2rs [35].
Unlike these findings, others reported that, similarly to Tas1r receptors, also gastrointestinal Tas2rs might control glucose metabolism. This is mostly supported by the fact that enteroendocrine cell lines of gastrointestinal origin express bitter taste receptor mRNA and that their stimulation with bitter compounds elicits elevation of intracellular calcium and release of gastrointestinal hormones such as CCK and GLP-1 [19, 30, 31, 36–40]. These observations are in line with a human in vivo study in which a non-functional variant of the bitter taste receptor TAS2R9 is associated to parameters linked to glucose deregulation such as high glucose and insulin plasma levels after glucose oral tolerance test and type II diabetes incidence [28]. A functional explanation was provided by demonstrating that the same receptor haplotype completely blunts the elevation of intracellular calcium in a recombinant HEK cell assay after receptor stimulation with its cognate agonist [28]. This indicates that the carriers of this haplotype might have an impaired capacity to release GLP-1 and therefore to properly regulate their glucose metabolism.
However, it has been difficult to associate one of the observed functions to a specific intestinal cell type because of the lack of reliable antisera or mouse models to demonstrate the in situ expression of Tas2r genes. Only recently, few reports demonstrated GI cell types expressing Tas2rs. However, the use of an antiserum directed against the mouse bitter taste receptor Tas2r138 resulted in contrasting evidence on the expression in enteroendocrine cells [38, 41, 42].
Using a knock-in mouse model conferring high-sensitive detection of Tas2r131 expression in GI tissues we previously identified a small subpopulation of colonic mucus-producing goblet cells in which the Tas2r131 gene was expressed [41]. In this study Tas2r131-expressing cells were also found in jejunum and in ileum, but not further investigated.
In this report we examined cell types expressing Tas2r131-driven fluorescent marker proteins in more proximal small intestinal tissues using the same mouse model. In order to investigate the overall bitter sensing strategy in gastrointestinal tissues we performed an expression profiling of all 35 mouse genes in GI tract tissues by qRT-PCR.
Materials and methods
Animals
All mice used in this study were kept and treated in accordance with the guidelines of and approved by the animal welfare committee of the Ministry of Environment, Health and Consumer Protection of the State of Brandenburg (permit number: 23-2347-A-1-1-2010, application number: 32-2347/5 + 1#59904/2007). Mice were kept in polycarbonate cages on regular light/dark cycle conditions of 12 h of light starting at 6 am every day. The temperature and humidity of the rooms were kept constant at 20 °C and 55 ± 5%, respectively. During their stay in the animal facility, mice were fed standard chow ad libitum and had free access to water. For experiments both genetically modified and wild type C57BL/6 J mice were used. A description of the generation of the genetically modified mice was already provided [41, 43–45]. Briefly, a construct including homologous 5′ and 3′ flanking regions of the Tas2r131 gene and containing a modified version of barley lectin, an internal ribosome entry site, the coding sequence of the Cre recombinase and a neomycin resistance cassette flanked by FRT sites 3′ of the Cre recombinase sequence was introduced into embryonic stem cells of a 129/Sv mouse strain. Neomycin-resistant clones were selected and injected in blastocysts of C57BL/6 J mice, which were in turn implanted in the uteri of (C57BL/6 × DBA) F1 foster mothers to generate Tas2r131F/MBLF/M−IRES−Cre neo+ (short: Tas2r131BLiC neo+) chimeric mice. Males of this progeny were then backcrossed to females with C57Bl/6 wild-type background. Subsequent breeding with FLP recombinase deleter mouse strain [46] eliminated the neomycin resistance cassette and resulted in mice with Tas2r131BliC background. These animals were crossed with Rosa26:stoppfloxedtdRFP [47] mice. The resulting breeds present heterozygous Tas2r131+/BLiC/Rosa26+/tdRFP (short: Tas2r131BLiC/ROSA26-tdRFP) background and express the tandem dimer Red Fluorescent Protein (tdRFP) [48]. For some histological experiments, a related line of transgenic mice expressing tauGFP instead of tdRFP under the control of the Tas2r131 gene promoter was used [43].
Tissue preparation
To prepare tissues for histology, mice underwent transcardial perfusion as described elsewhere [41]. Tongue, stomach, duodenum (0–3 cm post pylorus), proximal jejunum (5–10 cm post pylorus), middle jejunum (12–16 cm post pylorus), distal jejunum (23–27 cm post pylorus), ileum (3 cm from caecum), caecum and colon (distal 4 cm-piece from rectum) were excised, rolled, fixated, post-fixated, cryoprotected and sectioned as previously described [41]. To prepare tissues for molecular biology, mice were anesthetized and sacrificed as previously described [41]. Pieces of rear tongue containing vallate papilla (VP), stomach (entire organ), duodenum (0–3 cm post pylorus), jejunum (8–10 cm post pylorus), ileum (0–3 cm from caecum), caecum (entire length), colon (0–2.5 cm post caecum), liver (one-third of the organ) were extracted and immediately frozen in liquid nitrogen.
Histological staining procedures
For nuclear staining of tissue cryostat sections alimentary canal segments were fixated in 4% w/v PFA in PBS for 5 min, then washed 5 min in PBS. 4′,6-diamidino-2-phenylindole (DAPI) staining was performed by incubating tissue sections in DAPI 1:1000 in PBS for 10 min, followed by 2× rinsing steps in PBS of 5 min each. Slides were mounted with Dako Fluorescent Mounting Medium (Dako, Hamburg, Germany) and glass coverslips.
For detecting chromogranin A, villin, mucin-2, lysozyme, and PLCβ2 in ileum, the primary and secondary antibodies, blocking peptides and the experimental protocols are identical to the ones already described [41]. For detecting lysozyme we used a primary goat polyclonal antibody (1:1000, Santa Cruz, ref. number sc-27958) and a mouse anti-goat secondary antibody AlexaFluor488-conjugated (1:2000, Molecular Probes, ref. number A-10680). Specificity of the anti-lysozyme antibody reaction was controlled by preincubation with its immunogenic peptide (Santa Cruz, ref. number sc-27958 P). Briefly, cryostat sections of Tas2r131BLiC/ROSA26-tdRFP ileum were fixated in 4% paraformaldehyde in PBS, and then rinsed 3 times in PBS. An antigen retrieval step (10 min, 80 °C, 10 mM sodium citrate, pH 6) was performed for chromogranin A, villin, mucin-2 and lysozyme staining procedures. After cooling, sections were washed 3 times in PBS, and incubated with blocking solution (5% normal horse serum, 0.5% Triton X-100 in PBS), followed by overnight incubation at 4 °C with primary antibodies diluted in PBS containing 5% normal horse serum and 0.2% Triton X-100. Next, sections were washed 3 times in PBS and secondary antibodies were applied for 1 h, followed by 3 washes in PBS. Sections were counter-stained with DAPI, washed twice in PBS and mounted as above.
Sections were analysed using a MIRAX MIDI system with filter sets for FITC, Cy3 and DAPI (Zeiss). Digital images were first analysed using Mirax Viewer (Zeiss), and then modified for brightness and contrast, and finally assembled using CorelDraw Graphic Suite.
Quantitative real time PCR (qRT-PCR)
Total RNA was extracted using TRIzol reagent (Invitrogen). DNase I digestion was performed prior to reverse-transcription using Superscript II and random primers (Invitrogen) as described before [41]. To control for the presence of contaminating genomic DNA, reverse transcriptase was omitted in -RT samples. Amplification of the target sequences of each Tas2r gene was achieved by using specific primer pairs and TaqMan fluorescent probes (Online Resource 1) designed with Primer Express 3.0 software (Applied Biosystems) and synthesized by Eurofins MWG Operon as already described [41] or purchased as ready-to-use assays (Applied Biosystems). Housekeeping gene expression (β-actin-sequence, Online Resource 1) served as internal control. Real-time PCR was performed using 7500 Fast Real-Time PCR System (Applied Biosystems). The reaction was performed in a total volume of 10 µl. The reaction mixture was constituted by 5 µl of cDNA corresponding to 25 ng of initial total RNA and 5 µl of assay mixture consisting of 1 X TaqMan Gene Expression Master Mix (Applied Biosystems), 0.5 µM TaqMan fluorescent probe and 1.25 µM of forward and reverse primer. Cycling parameters of the Real-time quantitative PCR QuantStudio 12 K Flex software (Applied Biosystems) were set as follows: 50 °C for 2 min for preincubation, and 95 °C for 10 min for polymerase activation and initial denaturation; followed by 40 cycles of 15 s at 95 °C for denaturation, and 1 min at 60 °C for annealing and elongation. Each +RT sample was run in triplicate. −RT and water controls were run in parallel for each reaction. The QuantStudioTM 12 K Flex Software (Applied Biosystems) was set to use the experimental property “Quantitation-Comparative CT (∆∆CT)” to give threshold cycle (C T) values. Raw data were further analysed using Microsoft Excel. Mean values of CT triplets were obtained when the standard deviation (SD) was <1, otherwise the deviating well was excluded. 2−∆CT values were calculated using mean C T of β-actin as a reference (∆C T = C T Tas2r − C T β-actin). Data were plotted as mean 2−∆CT ± SD of three biological replicates by using GraphPad Prism (GraphPad Software).
For the comparisons of tongue vs. GI tract cDNAs, samples analyzed on separate plates were used. An average of the 2-deltaCT of the VP samples from the 3 animals was calculated and used to normalize the individual 2-deltaCT values obtained for the GI tissue samples of each animal before averaging.
Prediction of transcription factor binding sites
Analysis of the putative promoter regions of GI expressed mouse Tas2r genes was carried out to identify putative cis-regulatory elements (cis-REs) bound by known transcription factors. For this purpose, a 1 kbp sequence in the putative proximal promoter region upstream of the ATG start codon of each gene was retrieved from the UCSC Genome Browser (https://genome-euro.ucsc.edu) and entered in the online tool ALGGEN PROMO (http://alggen.lsi.upc.es/cgi-bin/promo_v3/promo/promoinit.cgi?dirDB=TF_8.3) [49, 50] based on the TRANSFAC database [51]. 5% was chosen as dissimilarity to the consensus sequence, and mouse motifs and factors were selected.
Results
The GI tract of mice harbours Tas2r131+ cells
To visualize the presence of Tas2r131 expressing cells in the GI tract of mice as assessed by tdRFP expression, cryostat sections of the VP (vallate papilla as a positive control), stomach, duodenum, jejunum, ileum and colon of genetically modified Tas2r131BLiC/ROSA26-tdRFP animals were DAPI-stained and analysed under the fluorescent microscope. In parallel, cryostat sections of Tas2r131wt/ROSA26-tdRFP control animals were used as a control for the absence of CRE-independent expression of the fluorescent marker tdRFP. The results show that, as expected, the tdRFP marks several taste bud cells in the VP (cf. [45]) (Fig. 1). On the contrary, VP taste buds of Tas2r131wt/ROSA26-tdRFP animals were devoid of detectable signals (Fig. 1), thus indicating that the tdRFP is produced in a Cre-dependent fashion and truly marks cells in which the Tas2r131 locus has been activated [41]. In the GI tissues, individual Tas2r131+ cells are scattered in the mucosa of the jejunum, ileum and colon (Fig. 1). In the small intestine these cells are always found at the crypt’s base and present a triangular bulky shape (Fig. 1, jejunum and ileum inserts). The corpus of the stomach (including the limiting ridge), the duodenum, and the caecum do not show tdRFP signals (not shown).
Fig. 1.
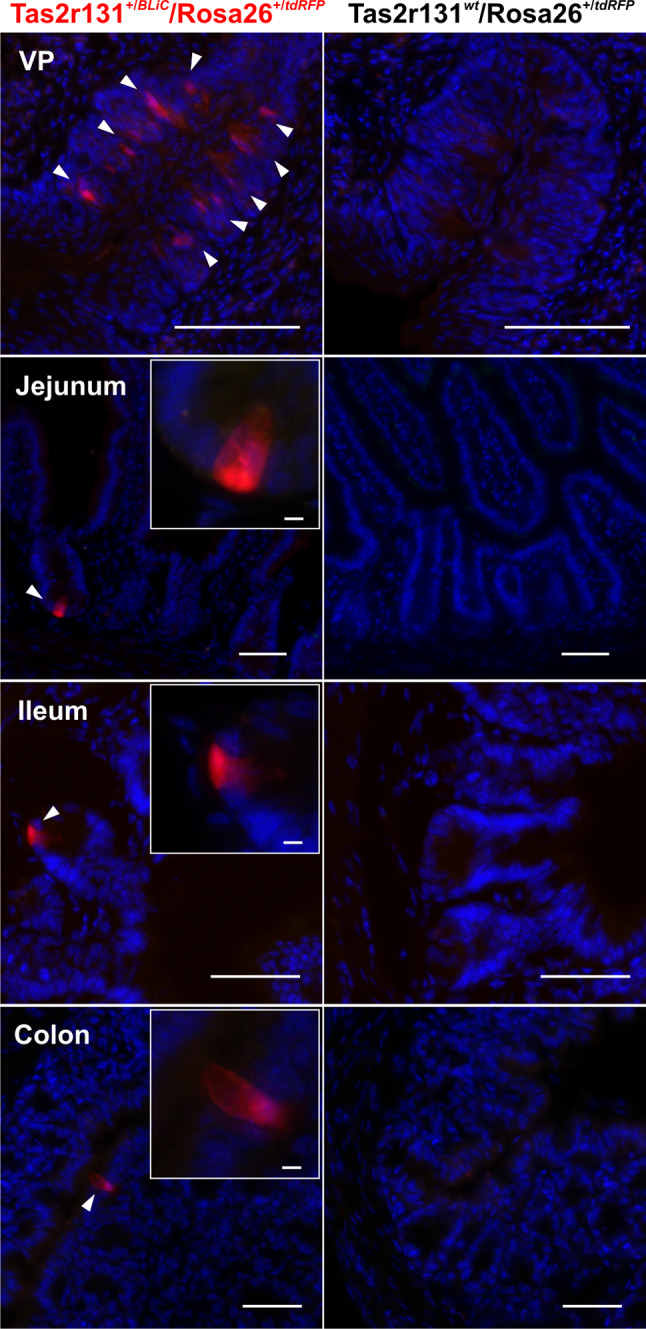
Histochemical characterization of GI tissues from Tas2r131+/BLiC/Rosa26+/tdRFP and Tas2r131wt/Rosa26+/tdRFP control animals. Cryostat sections of 14 μm thickness were stained with DAPI and subjected to fluorescent scans with a MIRAX Midi system. Arrowheads point to Tas2r131+ cells in red (Cy3 filter), DAPI-stained nuclei are depicted in blue (DAPI filter). Green (FITC filter) was used as background. The vallate papillae (VP) served as a positive control for the expression of the Tas2r131 gene in taste cells. Tas2r131+ cells are found in the jejunum, ileum, and colon as already reported (Prandi et al., 2013). Scale bars 50 µm. Inserts, scale bar 5 µm, show a magnification of the Tas2r131+ cells
The Tas2r131+ cells in ileum are Paneth cells
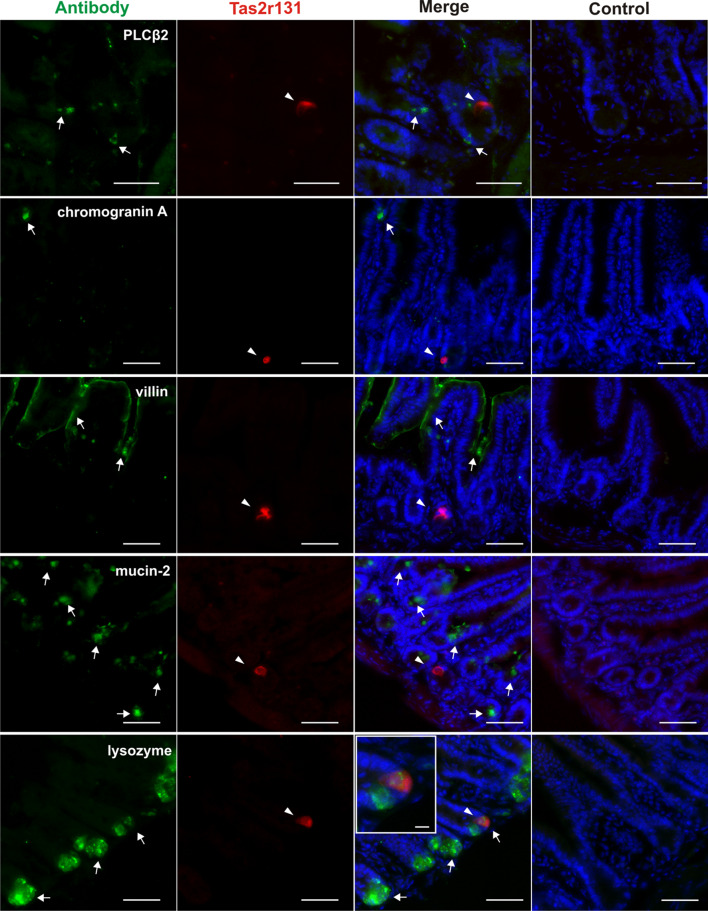
To identify the type of Tas2r131+ cells in the small intestine, we employed an immunohistochemical approach [41] using antisera against specific molecular markers of the main small intestinal cell types. Therefore, we performed immunohistochemical experiments with an anti-PLCβ2 antibody, as this known taste cell marker [52] is also expressed in intestinal mucosal enteroendocrine L and tuft cells [22, 53, 54]. However, the results in Fig. 2 show that the crypt Tas2r131+ cells do not co-express PLCβ2, a situation similar to the Tas2r131+ colonic goblet cells, which do not express taste markers [41]. As a general marker for enteroendocrine cells, we chose chromogranin A [42, 55]. Enteroendocrine cells are known to express taste-related proteins including Tas1r and Tas2r as well as components of the canonical taste transduction cascade [21–23, 42, 56]. Therefore, they are candidate cells for Tas2r131 gene expression. However, we observed no overlap between the signal obtained with the antibody and that of tdRFP (Fig. 2). Hence, enteroendocrine cells do not express Tas2r131.
Fig. 2.
Immunohistochemical identification of the Tas2r131+ cells with intestinal-specific cell and taste markers in the ileum of Tas2r131+/BliC/Rosa26+/tdRFP animals. Representative microphotographs showing ileal Tas2r131+ cells appearing in the mucosal layer of the intestinal wall, and the main mucosal cell types as identified by specific antibodies. Cryostat sections of 14 μm thickness were stained with the corresponding antibodies (and corresponding controls) for known markers and then subjected to fluorescent scan with a MIRAX Midi system. Arrowheads point to Tas2r131+ cells in red (Cy3 filter), arrows to antibody-labelled cells in green (FITC filter), DAPI-stained nuclei are depicted in blue (DAPI filter). The insert shows a magnification of a Tas2r131+ cell positive for lysozyme labelling. Control reactions for each antibody (blocking peptide for villin and lysozyme, minus antibody control for chromogranin A, mucin-2, and PLCβ2) are shown on the right column. Scale bars 50 µm, 10 µm (inserts)
Enterocytes are the most represented cell type in the small intestinal mucosa (~80%) [57]. Villin is a marker protein residing in the enterocytes’ apical brush-border where the microvilli are located [58]. Additionally, it is present in the tuft of apical microvilli of intestinal tuft cells [59]. Our experiments document that villin does not co-stain tdRFP+ cells (Fig. 2), therefore enterocytes and tuft cells do not express the Tas2r131 gene.
Mucin-2 is a marker for goblet cells [60, 61]. These are responsible for the production of the mucus layer covering and protecting the intestinal mucosa [62, 63]. They also sample luminal antigens in order to develop a tolerogenic response to them [64]. A small subpopulation of these cells was recently shown to express the Tas2r131 gene in the colon [41]. Therefore, we stained ileal sections with a mucin-2 antibody. However, in the small intestine no mucin-2-positive cell expresses the tdRFP (Fig. 2).
Paneth cells represent a cell type specific to the small intestine [65]. They are interdigitated among the stem cells at the crypt’s bottom where they control their growth and differentiation into all intestinal cell types [66]. An established marker for Paneth cells is lysozyme, an antibacterial molecule contained in the large granules of their cell body [67]. Our experiments with an anti-lysozyme antibody finally resulted in the staining of the Tas2r131+ cell at the bottom of the crypts. The insert in Fig. 2 shows that the staining is granule-like and only involves the area of the cell body above the nucleus. All controls resulted in negative staining confirming the specificity of the antisera used in the study (Fig. 2).
In summary, we have not observed any PLCβ2, mucin-2, chromogranin A or villin-positive cell co-expressing tdRFP, while 8 of 9 Tas2r131+ cells found in the ileum were positive for the Paneth cell marker lysozyme.
The Tas2r genes are differentially expressed in GI tract tissues
The results presented so far confirmed our previous findings [41] that the bitter taste receptor gene Tas2r131 exhibits a highly restricted expression pattern in GI tissues. In order to test if this applies to all Tas2r genes, we investigated the distribution of the entire Tas2r gene repertoire in mouse GI tissues by means of qRT-PCR. As expected, the VP of wild type mice used as a positive control expresses all 35 Tas2r genes (Fig. 3) confirming our previous observations [10]. However, from a quantitative perspective they are expressed at different levels: Tas2r108, Tas2r126, Tas2r135, Tas2r137 and Tas2r143 are most abundant, whereas Tas2r103, Tas2r107, Tas2r109, Tas2r114, Tas2r122, Tas2r140 and Tas2r144 are least abundant. In between these two groups lie the remaining genes, although they still retain a high variability in their expression levels.
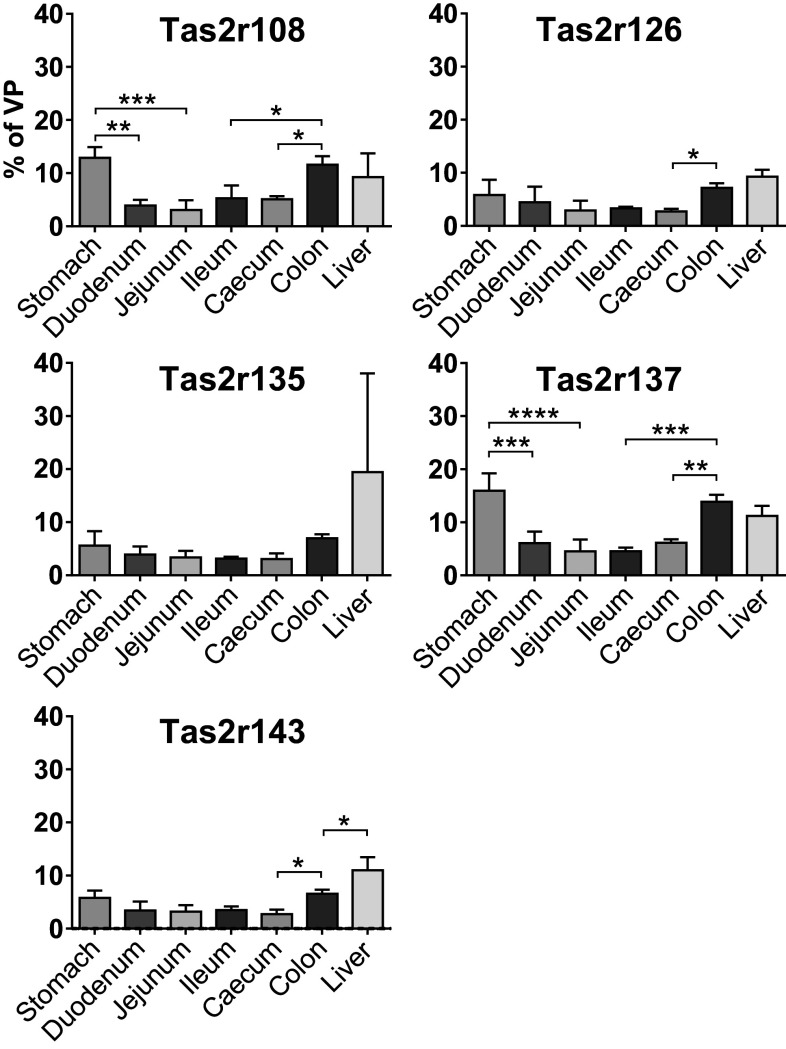
Fig. 3.
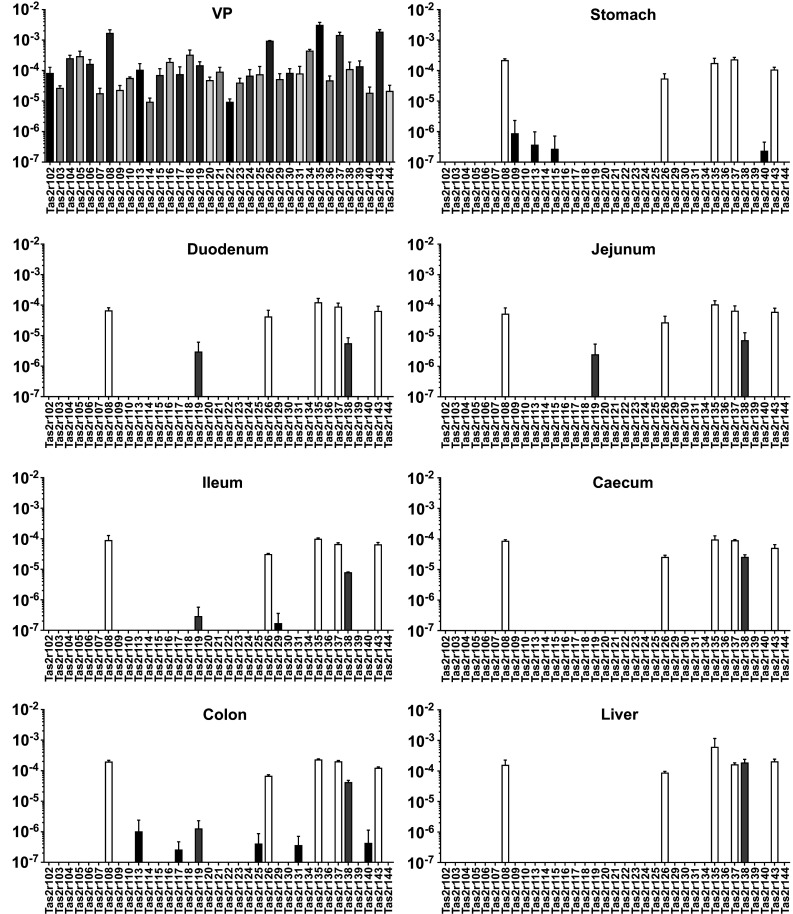
Expression levels of Tas2r genes in mouse GI tissues of wild type mice. cDNA corresponding to 25 ng RNA was subjected to amplification of gene-specific sequences by means of qRT-PCR in the presence of fluorescently labelled TaqMan probes. Detection and computation of the fluorescent signals generated after the depolymerisation of the TaqMan probe by the polymerase contained in the reaction mixture were used to calculate the expression level for each gene. The broadly expressed genes are depicted in white, the intestine-specific genes in gray and the GI region-specific genes in black. Values (y axis) are expressed in logarithmic scale as mean 2−∆CT ± SD relative to β-actin from three biological replicates (n = 3)
Within the detection limits of our study, the GI tissues, unlike the VP, exhibit a much more restricted Tas2r expression that varies both in the number of expressed genes and their expression levels. The colon with 12 Tas2r genes is the GI region with the highest number of detected Tas2rs, whereas the caecum expresses only 6 genes. We detected 9 Tas2r mRNAs in samples from stomach tissue, 7 Tas2rs were detected in the duodenum and the jejunum. Ileal tissue allowed the identification of 8 Tas2rs. Despite of the detected gene number, all the tissues harbour 5 commonly expressed transcripts (Tas2r108, Tas2r126, Tas2r135, Tas2r137 and Tas2r143) (white bars in Fig. 3), which also present the highest expression levels (~1000-fold of the other genes).
The colon and the stomach are the GI regions where most restrictedly expressed genes (Fig. 3, black bars) are found. These are the stomach-specific Tas2r109 and Tas2r115, and the colon-specific Tas2r117, Tas2r125 and Tas2r131. In addition, colon and stomach share the expression of Tas2r113 and Tas2r140. These transcripts are expressed at a very low level, presumably at the detection limit, since we could detect them in only one or two animals (biological replicates). The fact that these receptors are concentrated at the proximal and distal ends of the investigated alimentary canal regions strengthens a putatively important role of bitter taste receptors in these two regions as already proposed by us and others [29, 30, 33, 41, 68, 69].
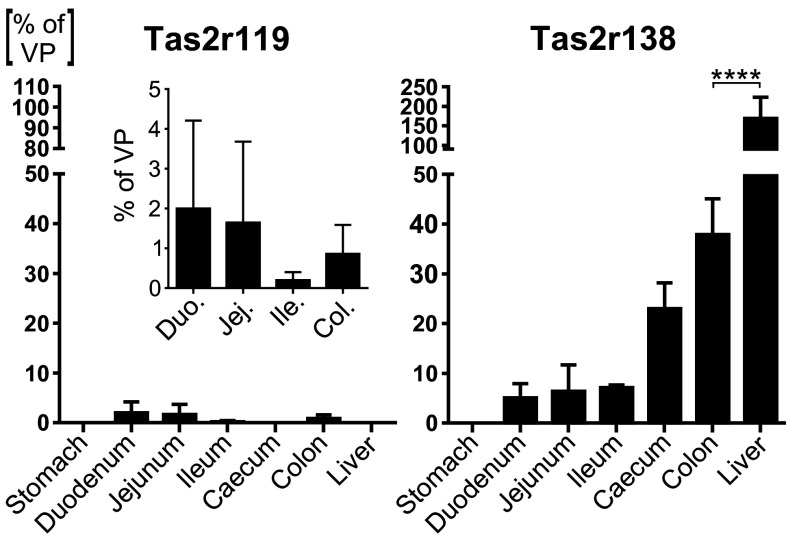
The small intestine and the colon also harbour transcripts of the Tas2r119 and Tas2r138 genes (Fig. 3, gray bars). These genes exhibit intermediate expression levels in between the broadly and specifically expressed ones, and an intermediate spatial distribution. In fact, the expression of the Tas2r138 occurs only in the small and large intestine, and not in the stomach. The Tas2r138 is also expressed in the liver. Different is the case for Tas2r119, the expression of which is restricted to the small intestine and the colon. It does not appear in the stomach and the caecum.
At last, it is interesting to point out that the ileum is the only portion of the small intestine where Tas2r129 is found. This observation may point to a specialized function for this receptor in this region.
Thus, RNA-expression data shows that the Tas2r gene expression profile differs from tissue to tissue in the digestive system. Interestingly, the expressed Tas2r genes can be grouped according to their expression levels and distributions.
The Tas2r genes have precise expression patterns along the GI tract
After showing that GI tissues express subsets of Tas2r genes, we analysed the quantitative relationships among the different genes in different GI regions. Therefore, we expressed the 2−∆CT values as % of VP in order to directly compare the gene expression with the VP chosen as a reference tissue.
As evident from the results shown in Fig. 4, the expression level of the broadly expressed genes is much lower than in the VP. On average, the expression levels of these Tas2r genes is below 10% of the levels observed in the VP, although with some exceptions (Tas2r108: 12.88% in the stomach, 11.57% in the colon; Tas2r135: 19.94% in the liver, but with a large SD; Tas2r137: 15.94% in the stomach, 13.85% in the colon, 11.21% in the liver). Moreover, it is evident that the relative expression levels of these genes follow a similar pattern along the antero-posterior axis of the GI tract. The two most anterior and posterior regions of the investigated alimentary canal tissues, namely the stomach and the colon, are the two regions in which the expression reaches the highest level for every gene. We already showed that these are the two tissues with the highest variety of detected Tas2r genes. Therefore, this finding suggests that bitter taste receptors play a more prominent role in these tissues. After the stomach, the relative expression level is lower in the duodenum and further decreases in the jejunum and the ileum. In the caecum, the amount of transcript increases again in some cases (Tas2r108, Tas2r135, Tas2r137). Regarding the liver, the amounts of Tas2r transcripts are comparable to the stomach and the colon.
Fig. 4.
Expression of the five broadly expressed genes along the GI tract and the liver. cDNA corresponding to 25 ng RNA was subjected to amplification of gene-specific sequences by means of qRT-PCR in the presence of fluorescently labelled TaqMan probe. Detection and computation of the fluorescent signals generated after the depolymerisation of the TaqMan probe by the polymerase contained in the reaction mixture were used to calculate the expression level for each gene. Values are expressed in linear scale as mean % 2−∆CT of VP ± SD from three biological replicates (n = 3)
Regarding the intermediately expressed genes, they present a pattern different from the broadly expressed ones. The Tas2r119 follows a distal-to-proximal expression gradient along the GI tract and it is not found in the stomach, caecum and liver (Fig. 5, left panel). An opposite trend is followed by the Tas2r138, which is expressed at intermediate level in the intestinal tissues, whereas we detected no expression in the stomach (Fig. 5, right panel). In the small intestine, it slightly shows an increase along the antero-posterior axis, whereas in the colon its level abruptly increases by up to ~ 8 times, hinting at a more important role in this GI segment. The liver confirms to be a site of high expression and, probably, functional relevance for Tas2rs.
Fig. 5.
Expression of the two intermediately expressed genes along the GI tract and the liver. The cDNA corresponding to 25 ng RNA was subjected to amplification of gene-specific sequences by means of qRT-PCR in the presence of fluorescently labelled TaqMan probe. Detection and computation of the fluorescent signals generated after the depolymerisation of the TaqMan probe by the polymerase contained in the reaction mixture were used to calculate the expression level for each gene. Values are expressed in linear scale as mean % 2−∆CT of VP ± SD from three biological replicates (n = 3)
Taken together, this analysis of RNA-expression data demonstrates that the expression levels of Tas2r genes in GI tissues is much lower than in the VP. It also shows that, within each expression group, specific patterns of detectable expression can be recognized. This indicates that common determinants might shape the expression of the Tas2r genes in each expression group.
The expression pattern of Tas2r genes in the GI tract reflects their genomic localization
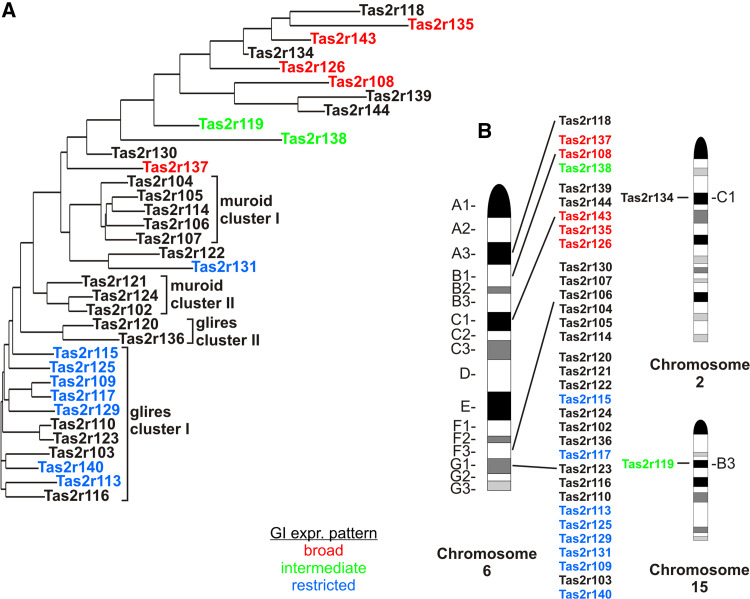
After showing the differential expression of Tas2r genes in GI tissues, we wondered whether there are common determinants shaping the expression profile in each group as observed in the RNA-expression study. It was previously suggested that the expression of Tas2r genes is coordinated in different tissues, including the heart, according to which gene cluster they belong to [70]. To check whether this notion might also apply to the GI tissues, we investigated, on the one hand the phylogenetic relationship among the mouse Tas2r genes, which may point to similarities extending into their promoter regions, as well as, on the other hand, the positions of the expressed Tas2r genes on the mouse chromosomes (Fig. 6). The comparison of the sequence relationships among the coding sequences of the 35 mouse Tas2r genes revealed that all of the broadly expressed Tas2rs (Tas2r108, Tas2r126, Tas2r135, Tas2r137, Tas2r143) as well as the two intermediately expressed Tas2rs, Tas2r119 and Tas2r138, represent one-to-one orthologs among the Euarchontoglires clade [10, 13] (Fig. 6a, red). On the contrary, all restrictedly expressed Tas2rs (Tas2r109, Tas2r113, Tas2r115, Tas2r117, Tas2r125, Tas2r131, Tas2r140), except for Tas2r131, belong to a cluster of genes (glires cluster I) expanded in the glires clade including lagomorphs and rodents, whereas primate species did not show expansion of the corresponding Tas2r genes [13]. This may indicate that the restricted expression of homologous glires cluster I genes in GI tissues is due to sequence similarities extending into their promoter regions, whereas the broad and intermediate expression pattern of the less homologous one-to-one orthologous genes could be influenced by their chromosomal positions. Indeed, all broadly expressed genes are situated in the B1 and C1 bands of the chromosome 6, whereas all the restrictedly expressed ones map on the G1 band (Fig. 6b). Therefore, it appears likely that the different expression patterns result from the presence of different regulatory sequences that control the expression of these two spatially segregated gene groups. Interestingly, the intermediately expressed Tas2r138 is situated in the B1 band together with broadly expressed genes. Therefore, it might be that its expression is governed by the same sequences controlling the expression of the broadly expressed ones. Interestingly, we did not detect any of the genes belonging to the clusters in the A3 (Tas2r118) and F3 (Tas2r104, Tas2r105, Tas2r106, Tas2r107, Tas2r114, Tas2r130) bands on the chromosome 6, and the C1 (Tas2r134) band on the chromosome 2, respectively. This further hints that the gene transcription in GI tissues involves the common regulation of gene clusters confirming the notion that Tas2r gene expression is coordinately regulated [70].
Fig. 6.
Phylogenetic relationships and chromosomal clustering of mouse Tas2r genes. Gene symbols are colored in red, green or blue to indicate whether their expression pattern is broad, intermediate or restricted, respectively, as assessed in the qRT-PCR profiling. Unlabelled gene symbols indicate that no expression was detected for these genes. The phylogenetic tree (a) was generated from the coding sequences of the 35 mouse Tas2r genes with the program AlignX of the software package Vector NTI using the Kimura correction option. The chromosomal position (b) of each gene in the corresponding band was assessed with www.ncbi.nlm.nih.gov/gene. Pictures of mouse chromosomes were taken from www.pathology.washington.edu
In order to assess whether this holds true also for gastrointestinal tissues, we ran an analysis of the putative proximal promoter regions of the Tas2r genes detected in our qRT-PCR screening (Fig. 3). By means of the online tool ALGGEN PROMO we searched for common cis-regulatory motifs likely to bind known transcription factors collected in the TRANSFAC database. We found that a number of putative cis-regulatory elements putatively binding ubiquitous transcription factors such as YY1, C/EBPα, C/EBPβ, c-Fos, c-Jun, JunD, NF-kβ [71–76] are shared among the different expression groups, and probably represent the determinants of their basal expression (not shown). In addition to these, putative binding sites for transcription factors belonging to the POU family [77] are massively situated in the proximal promoters of the broadly expressed genes and Tas2r138, the latter distributed all along the intestine (Fig. 4). On the contrary, the restrictedly expressed gene promoters seem to harbour different binding motifs for factors that might be even more specific. Among these, HNF-3 and -6 (hepatocyte nuclear factor) transcription factors have been shown to regulate expression of genes in intestine and digestive organs [78] and their putative binding motifs have been found in the promoter of Tas2r109 (not shown). Pax5 (paired box protein 5) binding motifs and AhR (aryl hydrocarbon receptor) are situated in the promoter regions of Tas2r125 and Tas2r131, respectively. Both transcription factors have been shown to regulate gene expression in gastrointestinal tissue [79, 80]. These predictions are particularly interesting because these genes were detected only in selected GI regions (Fig. 3) and therefore the specific binding motifs might be the determinants of their expression in these selected tissues.
Discussion
In this study we showed that bitter taste receptor genes are differentially expressed in the murine GI tract. Using a genetically modified mouse model, we observed fluorescent cells expressing the Tas2r131 receptor scattered in the mucosa of the jejunum, ileum and, as previously shown [41], in the colon. On the contrary, the stomach and the duodenum are devoid of detectable fluorescent cells. Subsequently, we identified by immunohistochemical experiments the ileal Tas2r131+ cells as deep crypt Paneth cells. The qRT-PCR profiling of all 35 mouse Tas2rs revealed that specific subsets of the genes are expressed in GI tract tissues and in the liver. These can be grouped in broadly, intermediately and specifically expressed genes according to their distribution and expression level.
The Tas2r131 is expressed in individual cells in the mucosa of the distal GI tract
Our results show that isolated scattered cells express the Tas2r131 gene in the mucosa of the jejunum, ileum and colon (Fig. 1). This agrees with previous observations demonstrating that cells expressing bitter taste receptor genes are situated in the mucosal layer of intestinal tissues [41, 42, 56], where they might sense the content of the intestinal lumen [81]. On the contrary, no cells were found in the mucosa of the stomach and duodenum, although Tas2r-expressing cells were reported in these tissues [19, 31, 38, 42, 68, 82]. The qRT-PCR profiling presented in this study (Fig. 3) confirm our previous observations showing that the frequency of the Tas2r131+ cells increases in the distal regions of the GI tract and peaks in the colon, where the Tas2r131+ cells have been identified as mucus-producing goblet cells [41].
In this report, we provided evidence of positive cells also in the jejunum and the ileum (Fig. 1). The apparent discrepancy between the occurrence of fluorescently labelled Tas2r131+ cells in jejunum and ileum with our qRT-PCR results obtained from these tissues (Fig. 3) likely reflects the decreased expression frequency from colon over ileum to jejunum. A salient feature of the Tas2r131+ cells is that they are all located at the crypt base, where the columnar intestinal stem cells reside and give birth to all differentiated mucosal cell types [83–85]. Here, the stem cells are in close contact with Paneth cells, which are found only in this crypt compartment supporting the stem cells in their growth and differentiation [83–85]. By means of an anti-lysozyme antiserum, we demonstrated that the deep crypt Tas2r131 + cells are Paneth cells (Fig. 2, for additional examples see online resource 2). However, it is evident that they represent a small fraction of the total Paneth cell number, as most lysozyme+ cells are Tas2r131−. This parallels our previous observation that the number of Tas2r131+ goblet cells is small in comparison with the total number of mucin 2+ cells in the colon of mouse [41].
An obvious question is why only a few Paneth cells express the Tas2r131 gene. A possible answer could be that only these cells express transcriptional regulators that make them competent for Tas2r131 expression. Unfortunately, the identity of Tas2r gene regulators is largely unknown. Only one report showed that the sterol-responsive-element-binding-protein 2 (SREBP-2) binds a specific sequence upstream of the transcription initiation site regulating the transcription of many Tas2r genes in mice under a sterol-depleted diet [38]. However, the Tas2r131 gene does not undergo a transcriptional regulation under these conditions in both the small and the large intestine [38]. Furthermore, our bioinformatic prediction of cis-regulatory elements did not report any putative SREBP-2 binding sites.
In conclusion, the identity of the factors regulating the transcription of the Tas2r131 and other bitter taste receptor genes is unknown and hence, an involvement of gene regulatory elements and factors in the mosaic expression pattern of Tas2rs in cells of the GI mucosa remains to be determined.
Nevertheless, the observed expression pattern could indicate the existence of subpopulations of Tas2r-expressing Paneth cells with different taste-related chemoresponsive abilities related to the expression of the other Tas2r genes detected in the GI tract (Fig. 3). In fact, a previous study pointed to the existence of subpopulations of Paneth cells expressing Tas1r1 and Tas1r3, as well as α-gustducin and α-transducin, in the murine small intestine, suggesting the possibility that Paneth cells with different chemosensory properties might exist [86]. Recently, Gu et al. [82] reported the expression of Tas2r105 in cells at the base of the small intestinal crypts in a region seemingly overlapping with the Paneth cell zone. However, the cell type(s) expressing Tas2r105 was not identified. Since we have only analysed one of the 35 putatively functional mouse Tas2r genes represented by our genetically modified mouse model and it is known that individual bitter taste receptor cells express only subsets of the entire bitter taste receptor gene repertoire [87], it could be speculated that a much larger, rather heterogeneous Tas2r expressing Paneth cell population exists in mice.
The Tas2r genes are differentially expressed in GI tissues
In a second set of experiments analysing the Tas2r gene expression in GI tissues, a qRT-PCR screening of all murine Tas2r genes was performed to assess the putative bitter sensing capacity in GI tissues.
The results show that, within the detection limits of our study, GI tissues express only subsets of Tas2r genes. This differs from the VP, where transcripts of all genes were detected (Fig. 3). Furthermore, the expression levels within the VP presented in this study are somewhat lower than those presented in a recent report [10]. The most likely reason is that two different VP preparations were used: taste epithelium [10] and the entire taste papilla (this study), which differ in the proportion of non-taste tissue used for RNA extraction.
The proximal and the distal GI tract are the most prominent sites of Tas2r gene expression, as indicated by the number of detected genes (9 in the stomach and 12 in the colon) and by their expression levels (Tas2r108, Tas2r126, Tas2r135, Tas2r137, Tas2r143 and Tas2r138 have the highest expression), hinting at important physiological functions in these GI regions. Indeed, it was already reported that the rodent stomach expresses several bitter taste receptor genes [19, 31] and functional experiments showed that gavage of bitter agonists affects stomach emptying and food intake [32, 33]. The Tas2r109 and Tas2r115 are expressed stomach-specifically (Fig. 3) and were previously not described in this organ. On the contrary, Tas2r108 and Tas2r119 (Fig. 3) were already reported [19, 31], which is in good agreement with our study. As a further confirmation, a recent report [41] demonstrated that the Tas2r108, Tas2r119 and Tas2r138 have distributions and expression levels similar to those presented in this work, not only in the small but also in the large intestine. Furthermore, another recent study [42] demonstrated via qRT-PCR that the Tas2r108 and the Tas2r138 exhibit a distribution and relative expression levels along the entire GI tract in line with our results. However, they and Wu and colleagues [31] could detect a weak expression of the Tas2r138 in the stomach, which is in contrast with our data (Fig. 5). This can be due to technical reasons: those authors sub-divided the stomach in fundus and corpus before RNA extraction, whereas we used whole stomach tissue for the analyses probably diluting rare mRNA species.
The colon is the tissue with the highest number of detected genes (Fig. 3). Among these, we could confirm the presence of the Tas2r131, already shown by qRT-PCR and by the presence of Tas2r131+ cells ([41]; Fig. 1). In addition, the Tas2r117 and the Tas2r125 are also specifically detected in this tissue (Fig. 3). However, we were not able to confirm the presence of the Tas2r118 [41]. A possible reason is that the expression of this gene was extremely low and it was not possible to detect it in every animal analysed [41]. The Tas2r138 presents a clear proximal-to-distal gradient of expression with a peak of expression in the colon reaching about 40% of VP expression level (Fig. 5). The importance of bitter taste receptors in colonic physiology was already suggested by Rozengurt et al. [30] who found the majority of TAS2R gene transcripts in biopsies of the human large intestine. Indeed, electrophysiological recordings suggested that bitter compounds evoke ion and fluid secretion in the large intestine mucosa of human and rodents [29]. Although it remains to be formally proven that these effects are mediated by bitter taste receptors, these results indicate that sensing mechanisms for bitter compounds are prominently present in the large intestine.
Unlike the above-mentioned studies, where only subsets of Tas2r genes were analysed, Jeon et al. [38] and Gu et al. [82] examined the expression of all mouse Tas2r genes by qRT-PCR. They reported that the proximal small intestine expresses 27 [38] and 34 [82] Tas2r genes, respectively, while the distal part expresses only six [38]. Moreover, the expression of the different genes compared to each other and with our study are not in agreement. As we can exclude strain- and sex-related effects because in all studies male wild type C57BL/6 mice were used, different RNA extraction and qRT-PCR protocols might account for such differences. Since it has been demonstrated that blood cells express detectable levels of Tas2r gene transcripts [41, 88], it might be that the protocols used in our studies led to a different extraction of RNA from blood cells present in intestinal tissues.
Among other possible confounders, the diet might play an important role. Indeed, it was shown that the diet specifically regulates the expression of bitter taste receptor genes in GI tissues [38]. Furthermore, it was also shown that different diets (cholesterol-deprived and high fat) differentially modulate the expression of Tas2r108 and Tas2r138 in different parts of the gut [42]. Therefore, it is possible that differences in the diet given to the animals account to some extent for the discrepancies among these studies [38, 82] and ours.
Interestingly, we could identify genes with broad, intermediate and restricted expression patterns (Figs. 3, 4, 5) and noticed that they exhibit discrete phylogenetic relationships and match different gene clusters on the chromosomes (Fig. 6). Intriguingly, we noticed that most Tas2r genes of glires cluster I which represents genes that have been expanded specifically in the glires clade whereas, e.g. primates do not show multiplication of the corresponding Tas2rs, show a restricted expression pattern in GI tissues. As it is believed that such species-specific Tas2r gene expansions allow adaptations to specific nutritional needs and/or habitats, while one-to-one orthologous genes show functional conservation for the recognition of important bitter compounds shared by more diverse species [15], GI tissue may, analogous to the gustatory system, dynamically adapt to different dietary habits.
Also Foster and colleagues observed a gene cluster-dependent expression for Tas2r genes in heart tissue [43]. More recently, another report showed that bitter taste receptor genes belonging to the same gene cluster share a similar expression pattern in multiple tissues [70]. Areas with putatively high transcriptional activity, as identified by histone and DNase I marks, were found in genome regions in the vicinity of the Tas2r143, Tas2r135 and Tas2r126 genes in many different tissues [70] including the heart and the intestine. Also in the VP partially similar mechanisms of gene transcription as in the GI tissues might take place because the broadly expressed genes, as defined in GI tissues, are also prominently expressed in the VP (Fig. 3). However, the fact that all of the genes are expressed and present at different expression levels indicates that additional mechanisms govern the transcription of bitter taste receptor genes in the VP, as proposed by Lossow and colleagues [10].
Possible functional significance of Tas2r gene expression in GI tissues
A crucial point regards the possible functional impact of the differential expression of the Tas2r genes as assessed by our qRT-PCR profiling. The expression of the entire Tas2r repertoire in taste tissue, which is located at the entrance of the GI tract, is necessary to detect the multitude of bitter compounds present in the diet of omnivore animals. Since strong bitter taste is believed to prevent ingestion of potentially noxious compounds, it is conceivable that fewer and/or less concentrated bitter substances normally reach the GI tract and therefore not all Tas2rs need to be highly expressed in all regions of the alimentary canal beyond the oral cavity checkpoint. In line with this hypothesis is the finding that the 5 broadly expressed receptors from genetic loci in the B1 and C1 bands on chromosome 6 respond in vitro to 30 compounds in comparison to the 24 recognized by the 8 restrictedly expressed receptors sitting in the gene cluster in the G1 band on chromosome 6 [10]. Indeed, among the widely expressed receptors, Tas2r135 is endowed with broad tuning properties, while Tas2r108, Tas2r126 and Tas2r137 share intermediate tuning properties. None of them is a “specialist”. On the contrary, among the restrictedly expressed ones there are three “specialist” receptors (Tas2r113, Tas2r115, Tas2r125), three receptors with intermediate tuning properties (Tas2r109, Tas2r117, Tas2r140) and two orphans (Tas2r129, Tas2r131) [10]. Thus, it seems that within the GI tract more broadly tuned receptors exhibit a less restricted spatial expression pattern compared to the more narrowly tuned receptors. Moreover, the higher abundance of Tas2r transcripts in the stomach and, in particular, in the colon suggests an elevated need for bitter compound recognition in these regions of the GI tract, perhaps due to chemical and enzymatic (stomach) as well as microbial (colon) modifications of compounds. Outside the alimentary canal, the liver has the most restricted variety of expressed Tas2rs and at the same time the highest expression levels (Fig. 3). This might indicate that hepatocytes and cholangiocytes need to detect a narrow array of bitter substances, whose detection might have a significant role in liver physiology.
The knowledge of the Tas2r gene family expression pattern alone is however not enough to understand its potential physiological consequences in GI tissues. It is also of fundamental importance to know what compounds bitter taste receptors actually detect. The recent deorphanisation of most mouse Tas2rs [10] was achieved by using an in vitro recombinant system [10]. Some of these compounds exerted measurable effects in physiological experiments and are ligands of Tas2rs expressed in GI tissues (Online Resource 3, Online Resource 4), indicating that their observed physiological effects are mediated by bitter taste receptors. In order to understand the putative physiological role of bitter taste receptors in the GI tract it is likewise important to have a closer look on those receptors that are not expressed in parts or the entire alimentary canal. Recently, we reported that among the mouse Tas2rs 3 receptors respond to the bile acid taurocholic acid [10]. The most sensitive of these receptors was the Tas2r117, which exhibited a tenfold lower threshold concentration than the other two receptors Tas2r123 and Tas2r144. Bile acids are secreted from the gall bladder through the common bile duct into the duodenal lumen. High concentrations of bile acids are maintained in the gut lumen until active reabsorption of ~95% in the terminal ileum reduces their concentration. Only 3–5% of bile acids reach the colon (for a review see [89]). Hence, any bile acid-sensitive Tas2r located at the luminal side of the intestinal mucosa between the duodenum and the terminal ileum would likely be useless or even detrimental due to chronic activation. Consequently, our data reporting the absence of the 3 bile acid-sensitive receptors from duodenum, jejunum, ileum and caecum (Fig. 3) is in good agreement with the occurrence of high luminal bile acid concentrations. Intriguingly, we detected the most sensitive taurocholic acid receptor, Tas2r117, in colon tissue, where only residual bile acids are present. This does not necessarily imply that Tas2r117 in the colon acts as a bile acid sensing receptor as another GPCR, the TGR5 (also known as GPBAR1) [90], is devoted to this role, however, Tas2r117 could be available to sense one of its other agonists in the absence of overwhelming bile acid concentrations. Of course, a complementary role of Tas2r117 for bile acid sensing could be envisaged as well.
The fact that many bitter compounds do exert physiological effects also in absence of their cognate bitter taste receptor confirms that bitter tasting molecules have a broad pharmacological activity that involve different targets other than Tas2r receptors (Online Resource 4).
Functional significance of Tas2r gene expression in Paneth cells
Bitter taste receptors have been associated with different functions in the GI tract ranging from food intake regulation [33, 68], metabolic control [28] to defensive roles [29, 37, 41]. In this study, we demonstrated that the Tas2r131 gene is expressed in a subpopulation of Paneth cells in the ileum (Fig. 2). On the one hand, this confirms our previous findings that the Tas2r131 gene is expressed in isolated cells in the gastrointestinal mucosa [41] (Figs. 1, 3) and might therefore serve the detection of substances in the intestinal lumen. On the other hand, it points to a defensive function for Tas2r131 in the small intestine, as it was already proposed in general [35] and for the colon specifically [41].
Paneth cells are situated in a critical position because the adjacent Lgr5+ stem cells need a stable environment to ensure the proper fuelling to the intestinal epithelium in terms of new epithelial cells [91]. This environment must be protected against perturbing agents such as toxicants or microorganisms. In the small intestine, Paneth cells produce antimicrobial molecules such as defensins and lysozyme [92] and are therefore regarded as the guardians of crypt sterility [93]. It seems that in vivo, Paneth cells are able to directly sense bacteria through MyD88-dependent TLR signalling and then start an antimicrobial transcriptional program that limits the diffusion of commensals and pathogens through the mucus barrier [94]. The ligands of TLR receptors are the so-called pathogen-associated molecular patterns (PAMPs) found in microbial macromolecules and structures such as nucleic acids, lipids, proteins and cell wall [95]. Therefore, bitter taste receptors are likely not involved in the response to such stimuli, as they recognize low molecular weight ligands [2, 96].
However, bacteria also produce and secrete low molecular weight molecules, such as the quorum-sensing (QS) molecules, to monitor cell population density and coordinate gene expression accordingly [97]. Bacterial pathogens use QS systems to coordinate their activity during infections [98–100]. On the contrary, commensal bacteria of the intestine do not seem to produce any known QS molecules, as they have not been detected by chemical methods in the intestine of several mammals [101]. It was shown by ex vivo and in vitro experiments that cells expressing Tas2rs respond to QS molecules. In mouse, nasal SCCs expressing bitter taste signalling components trigger mucosal inflammation when exposed to acyl-homoserine-lactones [102], a well-known class of QS molecules [97]. A recent study confirmed these findings by overexpressing mouse Tas2rs in a HEK cell recombinant system, showing that the generalist Tas2r105 is the only receptor responding to the QS homoserine lactones used [10]. Unfortunately, the Tas2r131 remained orphan in this study [10], and we could not detect the Tas2r105 in our intestinal RNA samples (Fig. 3). This, however, does not exclude that the Tas2r131, as well as other receptors such as the ileum-specific Tas2r129, may be involved in the detection of QS molecules. Paneth cells indeed respond to pathogenic bacteria by releasing defensins to prevent them to spread [94, 103, 104]. Interestingly, bitter taste receptors in the airway mucosa were proposed to act as detectors of QS molecules from growing pathogens before these reach population densities that allow them to create a dangerous biofilm [102]. It might well be that also intestinal Tas2r fulfil a similar function.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
We thank Dr. H. J. Fehling for providing the ROSA26tdRFP mouse strain, S. Demgensky for the perfusion of mouse tissues and the genotyping of the mice, Dr. S. Huebner and J. Wuerfel for establishing the qRT-PCR. This work was supported by the German Ministry of Education and Research (BMBF, #0315669, to WM and MB).
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
References
- 1.Lindemann B. Taste reception. Physiol Rev. 1996;76:718–766. doi: 10.1152/physrev.1996.76.3.719. [DOI] [PubMed] [Google Scholar]
- 2.Meyerhof W. Elucidation of mammalian bitter taste. Rev Physiol Biochem Pharmacol. 2005;154:37–72. doi: 10.1007/s10254-005-0041-0. [DOI] [PubMed] [Google Scholar]
- 3.Nelson G, Chandrashekar J, Hoon MA, Feng L, Zhao G, Ryba NJ, Zuker CS. An amino-acid taste receptor. Nature. 2002;416:199–202. doi: 10.1038/nature726. [DOI] [PubMed] [Google Scholar]
- 4.Vigues S, Dotson CD, Munger SD. The receptor basis of sweet taste in mammals. Results Probl Cell Differ. 2009;47:187–202. doi: 10.1007/400_2008_9. [DOI] [PubMed] [Google Scholar]
- 5.Behrens M, Meyerhof W, Hellfritsch C, Hofmann T. Sweet and umami taste: natural products, their chemosensory targets, and beyond. Angew Chem Int Ed Engl. 2011;50:2220–2242. doi: 10.1002/anie.201002094. [DOI] [PubMed] [Google Scholar]
- 6.Nelson G, Hoon MA, Chandrashekar J, Zhang Y, Ryba NJ, Zuker CS. Mammalian sweet taste receptors. Cell. 2001;106:381–390. doi: 10.1016/S0092-8674(01)00451-2. [DOI] [PubMed] [Google Scholar]
- 7.Adler E, Hoon MA, Mueller KL, Chandrashekar J, Ryba NJ, Zuker CS. A novel family of mammalian taste receptors. Cell. 2000;100:693–702. doi: 10.1016/S0092-8674(00)80705-9. [DOI] [PubMed] [Google Scholar]
- 8.Behrens M, Korsching SI, Meyerhof W. Tuning properties of avian and frog bitter taste receptors dynamically fit gene repertoire sizes. Mol Biol Evol. 2014;31:3216–3227. doi: 10.1093/molbev/msu254. [DOI] [PubMed] [Google Scholar]
- 9.Chandrashekar J, Mueller KL, Hoon MA, Adler E, Feng L, Guo W, Zuker CS, Ryba NJ. T2Rs function as bitter taste receptors. Cell. 2000;100:703–711. doi: 10.1016/S0092-8674(00)80706-0. [DOI] [PubMed] [Google Scholar]
- 10.Lossow K, Hubner S, Roudnitzky N, Slack JP, Pollastro F, Behrens M, Meyerhof W. Comprehensive analysis of mouse bitter taste receptors reveals different molecular receptive ranges for orthologous receptors in mice and humans. J Biol Chem. 2016;291:15358–15377. doi: 10.1074/jbc.M116.718544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Matsunami H, Montmayeur JP, Buck LB. A family of candidate taste receptors in human and mouse. Nature. 2000;404:601–604. doi: 10.1038/35007072. [DOI] [PubMed] [Google Scholar]
- 12.Meyerhof W, Batram C, Kuhn C, Brockhoff A, Chudoba E, Bufe B, Appendino G, Behrens M. The molecular receptive ranges of human TAS2R bitter taste receptors. Chem Senses. 2010;35:157–170. doi: 10.1093/chemse/bjp092. [DOI] [PubMed] [Google Scholar]
- 13.Hayakawa T, Suzuki-Hashido N, Matsui A, Go Y. Frequent expansions of the bitter taste receptor gene repertoire during evolution of mammals in the Euarchontoglires clade. Mol Biol Evol. 2014;31:2018–2031. doi: 10.1093/molbev/msu144. [DOI] [PubMed] [Google Scholar]
- 14.Li D, Zhang J. Diet shapes the evolution of the vertebrate bitter taste receptor gene repertoire. Mol Biol Evol. 2014;31:303–309. doi: 10.1093/molbev/mst219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shi P, Zhang J, Yang H, Zhang YP. Adaptive diversification of bitter taste receptor genes in Mammalian evolution. Mol Biol Evol. 2003;20:805–814. doi: 10.1093/molbev/msg083. [DOI] [PubMed] [Google Scholar]
- 16.Chaudhari N, Roper SD. The cell biology of taste. J Cell Biol. 2010;190:285–296. doi: 10.1083/jcb.201003144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Behrens M, Prandi S, Meyerhof W (2017) Taste receptor gene expression outside the gustatory system. In: Krautwurst D (ed) Topics in medicinal chemistry 23: Taste and smell. Springer International Publishing, pp 1–34
- 18.Dyer J, Salmon KS, Zibrik L, Shirazi-Beechey SP. Expression of sweet taste receptors of the T1R family in the intestinal tract and enteroendocrine cells. Biochem Soc Trans. 2005;33:302–305. doi: 10.1042/BST0330302. [DOI] [PubMed] [Google Scholar]
- 19.Wu SV, Rozengurt N, Yang M, Young SH, Sinnett-Smith J, Rozengurt E. Expression of bitter taste receptors of the T2R family in the gastrointestinal tract and enteroendocrine STC-1 cells. Proc Natl Acad Sci USA. 2002;99:2392–2397. doi: 10.1073/pnas.042617699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Daly K, Al-Rammahi M, Arora DK, Moran AW, Proudman CJ, Ninomiya Y, Shirazi-Beechey SP. Expression of sweet receptor components in equine small intestine: relevance to intestinal glucose transport. Am J Physiol Regul Integr Comp Physiol. 2012;303:R199–R208. doi: 10.1152/ajpregu.00031.2012. [DOI] [PubMed] [Google Scholar]
- 21.Daly K, Al-Rammahi M, Moran A, Marcello M, Ninomiya Y, Shirazi-Beechey SP. Sensing of amino acids by the gut-expressed taste receptor T1R1–T1R3 stimulates CCK secretion. Am J Physiol Gastrointest Liver Physiol. 2013;304:G271–G282. doi: 10.1152/ajpgi.00074.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jang HJ, Kokrashvili Z, Theodorakis MJ, Carlson OD, Kim BJ, Zhou J, Kim HH, Xu X, Chan SL, Juhaszova M, Bernier M, Mosinger B, Margolskee RF, Egan JM. Gut-expressed gustducin and taste receptors regulate secretion of glucagon-like peptide-1. Proc Natl Acad Sci USA. 2007;104:15069–15074. doi: 10.1073/pnas.0706890104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Margolskee RF, Dyer J, Kokrashvili Z, Salmon KS, Ilegems E, Daly K, Maillet EL, Ninomiya Y, Mosinger B, Shirazi-Beechey SP. T1R3 and gustducin in gut sense sugars to regulate expression of Na+ -glucose cotransporter 1. Proc Natl Acad Sci USA. 2007;104:15075–15080. doi: 10.1073/pnas.0706678104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kendig DM, Hurst NR, Bradley ZL, Mahavadi S, Kuemmerle JF, Lyall V, DeSimone J, Murthy KS, Grider JR. Activation of the umami taste receptor (T1R1/T1R3) initiates the peristaltic reflex and pellet propulsion in the distal colon. Am J Physiol Gastrointest Liver Physiol. 2014;307:G1100–G1107. doi: 10.1152/ajpgi.00251.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shirazi-Beechey SP, Moran AW, Batchelor DJ, Daly K, Al-Rammahi M. Glucose sensing and signalling; regulation of intestinal glucose transport. Proc Nutr Soc. 2011;70:185–193. doi: 10.1017/S0029665111000103. [DOI] [PubMed] [Google Scholar]
- 26.Gerspach AC, Steinert RE, Schonenberger L, Graber-Maier A, Beglinger C. The role of the gut sweet taste receptor in regulating GLP-1, PYY, and CCK release in humans. Am J Physiol Endocrinol Metab. 2011;301:E317–E325. doi: 10.1152/ajpendo.00077.2011. [DOI] [PubMed] [Google Scholar]
- 27.Steinert RE, Gerspach AC, Gutmann H, Asarian L, Drewe J, Beglinger C. The functional involvement of gut-expressed sweet taste receptors in glucose-stimulated secretion of glucagon-like peptide-1 (GLP-1) and peptide YY (PYY) Clin Nutr. 2011;30:524–532. doi: 10.1016/j.clnu.2011.01.007. [DOI] [PubMed] [Google Scholar]
- 28.Dotson CD, Zhang L, Xu H, Shin YK, Vigues S, Ott SH, Elson AE, Choi HJ, Shaw H, Egan JM, Mitchell BD, Li X, Steinle NI, Munger SD. Bitter taste receptors influence glucose homeostasis. PLoS One. 2008;3:e3974. doi: 10.1371/journal.pone.0003974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kaji I, Karaki S, Fukami Y, Terasaki M, Kuwahara A. Secretory effects of a luminal bitter tastant and expressions of bitter taste receptors, T2Rs, in the human and rat large intestine. Am J Physiol Gastrointest Liver Physiol. 2009;296:G971–G981. doi: 10.1152/ajpgi.90514.2008. [DOI] [PubMed] [Google Scholar]
- 30.Rozengurt N, Wu SV, Chen MC, Huang C, Sternini C, Rozengurt E. Colocalization of the alpha-subunit of gustducin with PYY and GLP-1 in L cells of human colon. Am J Physiol Gastrointest Liver Physiol. 2006;291:G792–G802. doi: 10.1152/ajpgi.00074.2006. [DOI] [PubMed] [Google Scholar]
- 31.Wu SV, Chen MC, Rozengurt E. Genomic organization, expression, and function of bitter taste receptors (T2R) in mouse and rat. Physiol Genom. 2005;22:139–149. doi: 10.1152/physiolgenomics.00030.2005. [DOI] [PubMed] [Google Scholar]
- 32.Glendinning JI, Yiin YM, Ackroff K, Sclafani A. Intragastric infusion of denatonium conditions flavor aversions and delays gastric emptying in rodents. Physiol Behav. 2008;93:757–765. doi: 10.1016/j.physbeh.2007.11.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Janssen S, Laermans J, Verhulst PJ, Thijs T, Tack J, Depoortere I. Bitter taste receptors and alpha-gustducin regulate the secretion of ghrelin with functional effects on food intake and gastric emptying. Proc Natl Acad Sci USA. 2011;108:2094–2099. doi: 10.1073/pnas.1011508108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yang N, Lei Z, Li X, Zhao J, Liu T, Ning N, Xiao A, Xu L, Li J. Chloroquine stimulates Cl– secretion by Ca2+ activated Cl– channels in rat ileum. PLoS One. 2014;9:e87627. doi: 10.1371/journal.pone.0087627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Green BG. Chemesthesis and the chemical senses as components of a “chemofensor complex”. Chem Senses. 2012;37:201–206. doi: 10.1093/chemse/bjr119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chen MC, Wu SV, Reeve JR, Jr, Rozengurt E. Bitter stimuli induce Ca2+ signaling and CCK release in enteroendocrine STC-1 cells: role of L-type voltage-sensitive Ca2+ channels. Am J Physiol Cell Physiol. 2006;291:C726–C739. doi: 10.1152/ajpcell.00003.2006. [DOI] [PubMed] [Google Scholar]
- 37.Jeon TI, Seo YK, Osborne TF. Gut bitter taste receptor signalling induces ABCB1 through a mechanism involving CCK. Biochem J. 2011;438:33–37. doi: 10.1042/BJ20110009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jeon TI, Zhu B, Larson JL, Osborne TF. SREBP-2 regulates gut peptide secretion through intestinal bitter taste receptor signaling in mice. J Clin Invest. 2008;118:3693–3700. doi: 10.1172/JCI36461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kim KS, Egan JM, Jang HJ. Denatonium induces secretion of glucagon-like peptide-1 through activation of bitter taste receptor pathways. Diabetologia. 2014;57:2117–2125. doi: 10.1007/s00125-014-3326-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Masuho I, Tateyama M, Saitoh O. Characterization of bitter taste responses of intestinal STC-1 cells. Chem Senses. 2005;30:281–290. doi: 10.1093/chemse/bji022. [DOI] [PubMed] [Google Scholar]
- 41.Prandi S, Bromke M, Hubner S, Voigt A, Boehm U, Meyerhof W, Behrens M. A subset of mouse colonic goblet cells expresses the bitter taste receptor Tas2r131. PLoS One. 2013;8:e82820. doi: 10.1371/journal.pone.0082820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Vegezzi G, Anselmi L, Huynh J, Barocelli E, Rozengurt E, Raybould H, Sternini C. Diet-induced regulation of bitter taste receptor subtypes in the mouse gastrointestinal tract. PLoS One. 2014;9:e107732. doi: 10.1371/journal.pone.0107732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Foster SR, Porrello ER, Purdue B, Chan HW, Voigt A, Frenzel S, Hannan RD, Moritz KM, Simmons DG, Molenaar P, Roura E, Boehm U, Meyerhof W, Thomas WG. Expression, regulation and putative nutrient-sensing function of taste GPCRs in the heart. PLoS One. 2013;8:e64579. doi: 10.1371/journal.pone.0064579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Voigt A, Hubner S, Doring L, Perlach N, Hermans-Borgmeyer I, Boehm U, Meyerhof W. Cre-mediated recombination in Tas2r131 cells-a unique way to explore bitter taste receptor function inside and outside of the taste system. Chem Senses. 2015;40:627–639. doi: 10.1093/chemse/bjv049. [DOI] [PubMed] [Google Scholar]
- 45.Voigt A, Hubner S, Lossow K, Hermans-Borgmeyer I, Boehm U, Meyerhof W. Genetic labeling of Tas1r1 and Tas2r131 taste receptor cells in mice. Chem Senses. 2012;37:897–911. doi: 10.1093/chemse/bjs082. [DOI] [PubMed] [Google Scholar]
- 46.Rodriguez CI, Buchholz F, Galloway J, Sequerra R, Kasper J, Ayala R, Stewart AF, Dymecki SM. High-efficiency deleter mice show that FLPe is an alternative to Cre-loxP. Nat Genet. 2000;25:139–140. doi: 10.1038/75973. [DOI] [PubMed] [Google Scholar]
- 47.Luche H, Weber O, Nageswara Rao T, Blum C, Fehling HJ. Faithful activation of an extra-bright red fluorescent protein in “knock-in” Cre-reporter mice ideally suited for lineage tracing studies. Eur J Immunol. 2007;37:43–53. doi: 10.1002/eji.200636745. [DOI] [PubMed] [Google Scholar]
- 48.Campbell RE, Tour O, Palmer AE, Steinbach PA, Baird GS, Zacharias DA, Tsien RY. A monomeric red fluorescent protein. Proc Natl Acad Sci USA. 2002;99:7877–7882. doi: 10.1073/pnas.082243699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Farre D, Roset R, Huerta M, Adsuara JE, Rosello L, Alba MM, Messeguer X. Identification of patterns in biological sequences at the ALGGEN server: PROMO and MALGEN. Nucleic Acids Res. 2003;31:3651–3653. doi: 10.1093/nar/gkg605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Messeguer X, Escudero R, Farre D, Nunez O, Martinez J, Alba MM. PROMO: detection of known transcription regulatory elements using species-tailored searches. Bioinformatics. 2002;18:333–334. doi: 10.1093/bioinformatics/18.2.333. [DOI] [PubMed] [Google Scholar]
- 51.Wingender E, Chen X, Hehl R, Karas H, Liebich I, Matys V, Meinhardt T, Pruss M, Reuter I, Schacherer F. TRANSFAC: an integrated system for gene expression regulation. Nucleic Acids Res. 2000;28:316–319. doi: 10.1093/nar/28.1.316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Rossler P, Kroner C, Freitag J, Noe J, Breer H. Identification of a phospholipase C beta subtype in rat taste cells. Eur J Cell Biol. 1998;77:253–261. doi: 10.1016/S0171-9335(98)80114-3. [DOI] [PubMed] [Google Scholar]
- 53.Bezencon C, Furholz A, Raymond F, Mansourian R, Metairon S, Le Coutre J, Damak S. Murine intestinal cells expressing Trpm5 are mostly brush cells and express markers of neuronal and inflammatory cells. J Comp Neurol. 2008;509:514–525. doi: 10.1002/cne.21768. [DOI] [PubMed] [Google Scholar]
- 54.Bezencon C, le Coutre J, Damak S. Taste-signaling proteins are coexpressed in solitary intestinal epithelial cells. Chem Senses. 2007;32:41–49. doi: 10.1093/chemse/bjl034. [DOI] [PubMed] [Google Scholar]
- 55.Facer P, Bishop AE, Lloyd RV, Wilson BS, Hennessy RJ, Polak JM. Chromogranin: a newly recognized marker for endocrine cells of the human gastrointestinal tract. Gastroenterology. 1985;89:1366–1373. doi: 10.1016/0016-5085(85)90657-2. [DOI] [PubMed] [Google Scholar]
- 56.Park J, Kim KS, Kim KH, Lee IS, Jeong HS, Kim Y, Jang HJ. GLP-1 secretion is stimulated by 1,10-phenanthroline via colocalized T2R5 signal transduction in human enteroendocrine L cell. Biochem Biophys Res Commun. 2015;468:306–311. doi: 10.1016/j.bbrc.2015.10.107. [DOI] [PubMed] [Google Scholar]
- 57.van der Flier LG, Clevers H. Stem cells, self-renewal, and differentiation in the intestinal epithelium. Annu Rev Physiol. 2009;71:241–260. doi: 10.1146/annurev.physiol.010908.163145. [DOI] [PubMed] [Google Scholar]
- 58.Robine S, Huet C, Moll R, Sahuquillo-Merino C, Coudrier E, Zweibaum A, Louvard D. Can villin be used to identify malignant and undifferentiated normal digestive epithelial cells? Proc Natl Acad Sci USA. 1985;82:8488–8492. doi: 10.1073/pnas.82.24.8488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Gerbe F, Legraverend C, Jay P. The intestinal epithelium tuft cells: specification and function. Cell Mol Life Sci. 2012;69:2907–2917. doi: 10.1007/s00018-012-0984-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kim YS, Ho SB. Intestinal goblet cells and mucins in health and disease: recent insights and progress. Curr Gastroenterol Rep. 2010;12:319–330. doi: 10.1007/s11894-010-0131-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Van der Sluis M, De Koning BA, De Bruijn AC, Velcich A, Meijerink JP, Van Goudoever JB, Buller HA, Dekker J, Van Seuningen I, Renes IB, Einerhand AW. Muc2-deficient mice spontaneously develop colitis, indicating that MUC2 is critical for colonic protection. Gastroenterology. 2006;131:117–129. doi: 10.1053/j.gastro.2006.04.020. [DOI] [PubMed] [Google Scholar]
- 62.Johansson ME, Phillipson M, Petersson J, Velcich A, Holm L, Hansson GC. The inner of the two Muc2 mucin-dependent mucus layers in colon is devoid of bacteria. Proc Natl Acad Sci USA. 2008;105:15064–15069. doi: 10.1073/pnas.0803124105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.McGuckin MA, Linden SK, Sutton P, Florin TH. Mucin dynamics and enteric pathogens. Nat Rev Microbiol. 2011;9:265–278. doi: 10.1038/nrmicro2538. [DOI] [PubMed] [Google Scholar]
- 64.McDole JR, Wheeler LW, McDonald KG, Wang B, Konjufca V, Knoop KA, Newberry RD, Miller MJ. Goblet cells deliver luminal antigen to CD103+ dendritic cells in the small intestine. Nature. 2012;483:345–349. doi: 10.1038/nature10863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Oullette AJ (2012) Paneth cells. In: Ghishan FK, Kaunitz JD, Merchant JL, Said HM, Wood JD (eds) Physiology of the gastrointestinal tract, 5th edn. Academic Press, Boston, pp 1211–1228
- 66.Sato T, van Es JH, Snippert HJ, Stange DE, Vries RG, van den Born M, Barker N, Shroyer NF, van de Wetering M, Clevers H. Paneth cells constitute the niche for Lgr5 stem cells in intestinal crypts. Nature. 2011;469:415–418. doi: 10.1038/nature09637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Cadwell K, Liu JY, Brown SL, Miyoshi H, Loh J, Lennerz JK, Kishi C, Kc W, Carrero JA, Hunt S, Stone CD, Brunt EM, Xavier RJ, Sleckman BP, Li E, Mizushima N, Stappenbeck TS, Virgin HWt. A key role for autophagy and the autophagy gene Atg16l1 in mouse and human intestinal Paneth cells. Nature. 2008;456:259–263. doi: 10.1038/nature07416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Avau B, Bauters D, Steensels S, Vancleef L, Laermans J, Lesuisse J, Buyse J, Lijnen HR, Tack J, Depoortere I. The gustatory signaling pathway and bitter taste receptors affect the development of obesity and adipocyte metabolism in mice. PLoS One. 2015;10:e0145538. doi: 10.1371/journal.pone.0145538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Avau B, Rotondo A, Thijs T, Andrews CN, Janssen P, Tack J, Depoortere I. Targeting extra-oral bitter taste receptors modulates gastrointestinal motility with effects on satiation. Sci Rep. 2015;5:15985. doi: 10.1038/srep15985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Foster SR, Porrello ER, Stefani M, Smith NJ, Molenaar P, dos Remedios CG, Thomas WG, Ramialison M. Cardiac gene expression data and in silico analysis provide novel insights into human and mouse taste receptor gene regulation. Naunyn Schmiedebergs Arch Pharmacol. 2015;388:1009–1027. doi: 10.1007/s00210-015-1118-1. [DOI] [PubMed] [Google Scholar]
- 71.Becker KG, Swergold GD, Ozato K, Thayer RE. Binding of the ubiquitous nuclear transcription factor YY1 to a cis regulatory sequence in the human LINE-1 transposable element. Hum Mol Genet. 1993;2:1697–1702. doi: 10.1093/hmg/2.10.1697. [DOI] [PubMed] [Google Scholar]
- 72.Chen F, Castranova V, Shi X, Demers LM. New insights into the role of nuclear factor-kappaB, a ubiquitous transcription factor in the initiation of diseases. Clin Chem. 1999;45:7–17. [PubMed] [Google Scholar]
- 73.Hatada EN, Chen-Kiang S, Scheidereit C. Interaction and functional interference of C/EBPbeta with octamer factors in immunoglobulin gene transcription. Eur J Immunol. 2000;30:174–184. doi: 10.1002/1521-4141(200001)30:1<174::AID-IMMU174>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- 74.Jain J, Nalefski EA, McCaffrey PG, Johnson RS, Spiegelman BM, Papaioannou V, Rao A. Normal peripheral T-cell function in c-Fos-deficient mice. Mol Cell Biol. 1994;14:1566–1574. doi: 10.1128/MCB.14.3.1566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Legraverend C, Antonson P, Flodby P, Xanthopoulos KG. High level activity of the mouse CCAAT/enhancer binding protein (C/EBP alpha) gene promoter involves autoregulation and several ubiquitous transcription factors. Nucleic Acids Res. 1993;21:1735–1742. doi: 10.1093/nar/21.8.1735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Mensah-Osman EJ, Veniaminova NA, Merchant JL. Menin and JunD regulate gastrin gene expression through proximal DNA elements. Am J Physiol Gastrointest Liver Physiol. 2011;301:G783–G790. doi: 10.1152/ajpgi.00160.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Zhao FQ. Octamer-binding transcription factors: genomics and functions. Front Biosci (Landmark Ed) 2013;18:1051–1071. doi: 10.2741/4162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Shaw-Smith CJ, Walters JR. Regional expression of intestinal genes for nutrient absorption. Gut. 1997;40:5–8. doi: 10.1136/gut.40.1.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Kawajiri K, Kobayashi Y, Ohtake F, Ikuta T, Matsushima Y, Mimura J, Pettersson S, Pollenz RS, Sakaki T, Hirokawa T, Akiyama T, Kurosumi M, Poellinger L, Kato S, Fujii-Kuriyama Y. Aryl hydrocarbon receptor suppresses intestinal carcinogenesis in ApcMin/+mice with natural ligands. Proc Natl Acad Sci USA. 2009;106:13481–13486. doi: 10.1073/pnas.0902132106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Xu H, Zhang B, Li J, Chen H, Wang C, Ghishan FK. Transcriptional inhibition of intestinal NHE8 expression by glucocorticoids involves Pax5. Am J Physiol Gastrointest Liver Physiol. 2010;299:G921–G927. doi: 10.1152/ajpgi.00227.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Rozengurt E. Taste receptors in the gastrointestinal tract. I. Bitter taste receptors and alpha-gustducin in the mammalian gut. Am J Physiol Gastrointest Liver Physiol. 2006;291:G171–G177. doi: 10.1152/ajpgi.00073.2006. [DOI] [PubMed] [Google Scholar]
- 82.Gu F, Liu X, Liang J, Chen J, Chen F, Li F. Bitter taste receptor mTas2r105 is expressed in small intestinal villus and crypts. Biochem Biophys Res Commun. 2015;463:934–941. doi: 10.1016/j.bbrc.2015.06.038. [DOI] [PubMed] [Google Scholar]
- 83.Barker N, van Es JH, Kuipers J, Kujala P, van den Born M, Cozijnsen M, Haegebarth A, Korving J, Begthel H, Peters PJ, Clevers H. Identification of stem cells in small intestine and colon by marker gene Lgr5. Nature. 2007;449:1003–1007. doi: 10.1038/nature06196. [DOI] [PubMed] [Google Scholar]
- 84.Bjerknes M, Cheng H. The stem-cell zone of the small intestinal epithelium. V. Evidence for controls over orientation of boundaries between the stem-cell zone, proliferative zone, and the maturation zone. Am J Anat. 1981;160:105–112. doi: 10.1002/aja.1001600109. [DOI] [PubMed] [Google Scholar]
- 85.Clevers H. The intestinal crypt, a prototype stem cell compartment. Cell. 2013;154:274–284. doi: 10.1016/j.cell.2013.07.004. [DOI] [PubMed] [Google Scholar]
- 86.Mace OJ, Affleck J, Patel N, Kellett GL. Sweet taste receptors in rat small intestine stimulate glucose absorption through apical GLUT2. J Physiol. 2007;582:379–392. doi: 10.1113/jphysiol.2007.130906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Behrens M, Foerster S, Staehler F, Raguse JD, Meyerhof W. Gustatory expression pattern of the human TAS2R bitter receptor gene family reveals a heterogenous population of bitter responsive taste receptor cells. J Neurosci. 2007;27:12630–12640. doi: 10.1523/JNEUROSCI.1168-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Malki A, Fiedler J, Fricke K, Ballweg I, Pfaffl MW, Krautwurst D. Class I odorant receptors, TAS1R and TAS2R taste receptors, are markers for subpopulations of circulating leukocytes. J Leukoc Biol. 2015;97:533–545. doi: 10.1189/jlb.2A0714-331RR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Roberts MS, Magnusson BM, Burczynski FJ, Weiss M. Enterohepatic circulation: physiological, pharmacokinetic and clinical implications. Clin Pharmacokinet. 2002;41:751–790. doi: 10.2165/00003088-200241100-00005. [DOI] [PubMed] [Google Scholar]
- 90.Kawamata Y, Fujii R, Hosoya M, Harada M, Yoshida H, Miwa M, Fukusumi S, Habata Y, Itoh T, Shintani Y, Hinuma S, Fujisawa Y, Fujino M. A G protein-coupled receptor responsive to bile acids. J Biol Chem. 2003;278:9435–9440. doi: 10.1074/jbc.M209706200. [DOI] [PubMed] [Google Scholar]
- 91.Barker N. Adult intestinal stem cells: critical drivers of epithelial homeostasis and regeneration. Nat Rev Mol Cell Biol. 2014;15:19–33. doi: 10.1038/nrm3721. [DOI] [PubMed] [Google Scholar]
- 92.Bevins CL, Salzman NH. Paneth cells, antimicrobial peptides and maintenance of intestinal homeostasis. Nat Rev Microbiol. 2011;9:356–368. doi: 10.1038/nrmicro2546. [DOI] [PubMed] [Google Scholar]
- 93.Clevers HC, Bevins CL. Paneth cells: maestros of the small intestinal crypts. Annu Rev Physiol. 2013;75:289–311. doi: 10.1146/annurev-physiol-030212-183744. [DOI] [PubMed] [Google Scholar]
- 94.Vaishnava S, Behrendt CL, Ismail AS, Eckmann L, Hooper LV. Paneth cells directly sense gut commensals and maintain homeostasis at the intestinal host-microbial interface. Proc Natl Acad Sci USA. 2008;105:20858–20863. doi: 10.1073/pnas.0808723105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Wells JM, Rossi O, Meijerink M, van Baarlen P. Epithelial crosstalk at the microbiota-mucosal interface. Proc Natl Acad Sci USA. 2011;108(Suppl 1):4607–4614. doi: 10.1073/pnas.1000092107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Belitz HD, Wieser H. Bitter compounds: occurrence and structure-activity relationships. Food Rev Int. 1985;1:271–354. doi: 10.1080/87559128509540773. [DOI] [Google Scholar]
- 97.Ng WL, Bassler BL. Bacterial quorum-sensing network architectures. Annu Rev Genet. 2009;43:197–222. doi: 10.1146/annurev-genet-102108-134304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Christensen LD, Moser C, Jensen PO, Rasmussen TB, Christophersen L, Kjelleberg S, Kumar N, Hoiby N, Givskov M, Bjarnsholt T. Impact of Pseudomonas aeruginosa quorum sensing on biofilm persistence in an in vivo intraperitoneal foreign-body infection model. Microbiology. 2007;153:2312–2320. doi: 10.1099/mic.0.2007/006122-0. [DOI] [PubMed] [Google Scholar]
- 99.Nadell CD, Xavier JB, Levin SA, Foster KR. The evolution of quorum sensing in bacterial biofilms. PLoS Biol. 2008;6:e14. doi: 10.1371/journal.pbio.0060014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Winson MK, Camara M, Latifi A, Foglino M, Chhabra SR, Daykin M, Bally M, Chapon V, Salmond GP, Bycroft BW, et al. Multiple N-acyl-l-homoserine lactone signal molecules regulate production of virulence determinants and secondary metabolites in Pseudomonas aeruginosa. Proc Natl Acad Sci USA. 1995;92:9427–9431. doi: 10.1073/pnas.92.20.9427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Swearingen MC, Sabag-Daigle A, Ahmer BM. Are there acyl-homoserine lactones within mammalian intestines? J Bacteriol. 2013;195:173–179. doi: 10.1128/JB.01341-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Tizzano M, Gulbransen BD, Vandenbeuch A, Clapp TR, Herman JP, Sibhatu HM, Churchill ME, Silver WL, Kinnamon SC, Finger TE. Nasal chemosensory cells use bitter taste signaling to detect irritants and bacterial signals. Proc Natl Acad Sci USA. 2010;107:3210–3215. doi: 10.1073/pnas.0911934107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Rumio C, Besusso D, Palazzo M, Selleri S, Sfondrini L, Dubini F, Menard S, Balsari A. Degranulation of Paneth cells via toll-like receptor 9. Am J Pathol. 2004;165:373–381. doi: 10.1016/S0002-9440(10)63304-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Tanabe H, Ayabe T, Bainbridge B, Guina T, Ernst RK, Darveau RP, Miller SI, Ouellette AJ. Mouse Paneth cell secretory responses to cell surface glycolipids of virulent and attenuated pathogenic bacteria. Infect Immun. 2005;73:2312–2320. doi: 10.1128/IAI.73.4.2312-2320.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.