Abstract
Although we witnessed considerable progress in the prevention and treatment of cancer during the past few decades, a number of cancers remain difficult to treat. The main reasons for this are a lack of effective biomarkers necessary for an early detection and inefficient treatments for cancer that are diagnosed at late stages of the disease. Because of their alarmin-like properties and their protumorigenic role during cancer progression, members of the galectin family are uniquely positioned to provide information that could be used for the exploration of possible avenues for the treatment of high fatality cancer (HFC). A rapid overview of studies that examined the expressions and functions of galectins in cancer cells reveals that they play a central role in at least three major features that characterize HFCs: (1) induction of systemic and local immunosuppression, (2) chemoresistance of cancer cells, and (3) increased invasive behavior. Defining the galectinome in HFCs will also lead to a better understanding of tumor heterogeneity while providing critical information that could improve the accuracy of biomarker panels for a more personalized treatment of HFCs. In this review, we discuss the relevance of the galectinome in HFC and its possible contribution to providing potential solutions.
Keywords: Galectins, High fatality cancer, Biomarker, Galectinome
Introduction on high fatality cancer
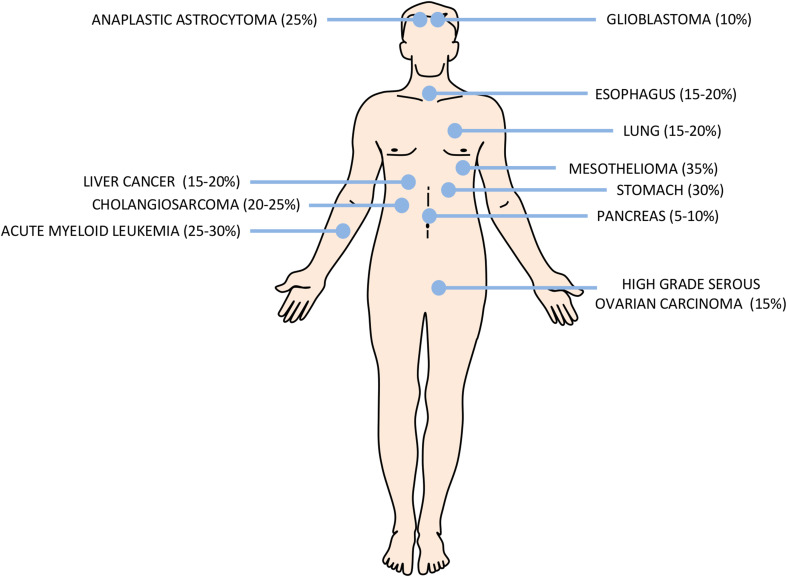
The definition of high fatality cancer (HFC) remains unclear and is often debated. For many, it is related to cancers for which a diagnosis can be a death sentence. In practice, and for the purpose of this review, HFCs are defined as cancers for which the five-year survival rate after diagnosis is below 25–30% (Fig. 1). They compose most of what is sometimes called “high mortality cancers”, which include cancers with survival rates of less than 50%, as defined by the National Cancer Institute in President Obama’s High Mortality Cancer Bill of 2013. Of course, these rates will vary according to both intrinsic (e.g., genetic) and extrinsic factors (e.g., lifestyle, environmental factors). Yet, HFCs can be arbitrarily classified into two broad categories: those in which little progress has been made (i.e., pancreatic, liver, non-small cell lung cancer, mesothelioma, gastric and glioblastoma), and high fatality subtypes (“bad” cancers among the “good”, i.e., triple negative breast cancer (TNBC), high grade serous ovarian cancer (HGSC), acute myeloid leukemia (AML), or castrate resistant prostate cancer). The former category often refers to cancers that remain relatively rare. For example, there are approximately less than 2–5 gallbladder cancer cases per 100,000 persons, making it the 20th most frequent cancer in the world (http://www.wcrf.org). Unfortunately, others, such as lung cancer (30–50 cases per 100,000 persons) and breast cancer (more than 100 cases per 100,000 persons in more developed countries), are more frequent and largely responsible for high mortality rates. Clearly, there is an urgent need to develop new biomarkers that can be used to identify people at risk and to develop treatment with greater efficacy.
Fig. 1. High fatality cancer (HFCs) according to body location.
Illustration of the major HFCs and the percentages indicate the approximative 5-year survival rate according to the 2017 annual report of the American Cancer Society (http://www.cancer.org)
Galectins
Galectins are multifunctional proteins that belong to the animal lectin family. All galectins share similar binding affinities to β-galactosides and display high amino acid sequence homology among their carbohydrate-recognition domains (CRDs) [1]. In mammals, 19 different members of this family have been identified, with 13 of them being expressed in humans (galectin-5, -6, -11, -15, -16, -19, and -20 are not found in humans). Galectins are divided into three sub-groups according to their structure: prototypic galectins containing one CRD (Gal-1, -2, -5, -7, -10, -11, -13, -14, -15, -16, -17, -19, and -20), tandem-repeat galectins containing two covalently linked CRDs (Gal-4, -6, -8, -9 and -12) and chimera-type galectins containing multiple CRDs linked by their amino-terminal domain (Gal-3). While these proteins perform homeostatic functions inside normal cells, under pathological or stress conditions, galectins are released either passively from dead cells or actively via non-classical secretion pathways. Once released into the extracellular milieu, they bind to repeating units of high density N- and O-glycans on the peptide backbone of membrane receptors via their CRD. This ability of galectins to promote the packing of glycosylated receptors into an ordered cross-linked lattice at the cell surface is facilitated by their inherent multivalency. Such cross-linking of glycosylated receptors triggers signals that are critical for the regulation of cell fate.
Galectins as potential therapeutic targets in HFC?
While the main reasons for the high mortality rates for HFCs are linked to a late diagnosis, other factors that contribute to their aggressiveness include an elevated growth rate, sequestration from the immune system, lack of effective treatments, therapeutic resistance, metastasis, tumor microenvironment, dormancy and heterogeneity of the tumor. Because galectins have been shown to modulate most if not all these processes and can thus play a crucial role at different stages of cancer progression, their potential as therapeutic targets in HFC is high. The revived interest in designing new and effective immunotherapies for cancer treatment has further placed the galectin under the projector. This is largely due to recent studies showing that almost all secreted galectins share the ability to build an immunosuppressive microenvironment that helps tumor cells escape cancer-killing immune cells [2]. Such immunosuppressive activity represents a major obstacle to cancer treatment and slows down the pace of progress in cancer immunotherapy, a promising avenue for the treatment of aggressive cancers for which there are limited treatment options. This immunosuppressive function of galectins has been well-described during pregnancy, in which placental galectins have been shown to be essential for establishing immune tolerance that protects the fetus from an aggressive maternal allogeneic response [3, 4]. Such new paradigm attracts the interest of many researchers involved in the development of novel immunotherapies that target immune checkpoints, a valuable strategy for the treatment of HFC, most notably for those harboring an immune phenotype. The role of galectins in controlling immunological homeostasis explains that they are often considered alarmin-like proteins, a family of structurally unrelated proteins that are released from intracellular compartments in the milieu in response to stress signals or cell damage [5, 6]. However, the role of galectins in cancer is by far not limited to their immunomodulatory role. There is a large amount of literature establishing galectins as a group of proteins that induce resistance to drug-induced cell death or promote metastasis by facilitating cell-to-cell and cell-to-matrix adhesion. In the section below, we briefly review some of the key findings and recent advances illustrating the emergence of galectins as potential therapeutic targets in HFC.
Pancreatic cancer
Pancreatic ductal adenocarcinoma is the preeminent subtype of pancreatic cancer. The 5-year survival is less than 10% in the USA and the overall survival after being diagnosed varies between 3 and 6 months if no treatment is given (http://www.cancer.org). The main reason for pancreatic cancer having a poor prognosis is the late stage at which the disease is discovered. It is an asymptomatic disease that comes with an early metastasis and recurrence risk, as well as chemoresistance and radioresistance problems. At present, most of the studies on the role of galectins in pancreatic cancer have focused on gal-1 and gal-3. The role of gal-1 in pancreatic cancer has been mostly linked to its immunomodulatory properties and the potential for targeting extracellular gal-1 to restore the immunological barrier to cancer has been relatively well-documented, making gal-1 a strong candidate for pancreatic cancer therapy [7]. Gal-3, like gal-1, is also expressed at abnormally high levels in human pancreatic tumor tissue [8, 9]. The role of gal-3 in pancreatic cancer, however, is not linked to its extracellular form but to its ability to modulate intracellular signaling events by increasing Ras activity, thereby stimulating growth and invasive behavior of pancreatic cancer cells [10]. Such a role of gal-3 in pancreatic cell migration and invasion has also been reported by Kobayashi et al. [11]. The ability of gal-3 to modulate key signaling pathways, including the Akt pathway, also increases the resistance of pancreatic cancer cells to chemotherapy-induced apoptosis [12]. Two recent studies have shown, however, that galectins may not always be protumorigenic in pancreatic cancer. Van Die and colleagues recently reported that gal-4, which is absent in healthy pancreatic tissue but expressed at high levels in pancreatic cancer cells, has tumor suppressive functions [13, 14]. The authors showed that de novo expression of gal-4 interferes with the Wnt/β-catenin pathway and inhibits the invasive behavior of human pancreatic cancer cell lines and primary pancreatic ductal adenocarcinoma cells. However, although there are indications suggesting that galectins are important for pancreatic cancer progression, clearly, additional knowledge of their expressions and functions in pancreatic cancer cells is deeply needed, especially for the less well-known galectins.
Lung cancer
Because lung cancer is the deadliest form of cancer in terms of the number of victims worldwide [15–17], there has been considerable research on the role of galectins in lung cancer. There are two main subtypes of lung cancer; 85% are non-small cell lung cancer (NSCLC) and 15% are small cell lung cancer (SCLC), and they are associated with a 5-year overall survival rate of approximately 15 and 7%, respectively [15, 18]. The SCLC form is the more aggressive subtype, with an estimated life expectancy of 7 months if no treatment is given [18, 19]. The advanced stage at which it is diagnosed, the lack of an efficient early diagnosis technique, the rapid metastasis formation and the molecular complexity of this disease are the major factors that make SCLC an HFC [18]. These factors are also an obstacle to the survival of NSCLC patients, for whom the median survival is between 8 and 10 months [20–22]. Similarly to pancreatic cancer and other cancers, most of the research on galectins in lung cancer has focused on gal-1 and gal-3. Again, the role of gal-1 in modulating the antitumoral response and suppressing cancer-killing immune cells is well-documented [23–25]. This is not, however, the only role of gal-1 in lung cancer, as a number of studies have shown that it also promotes cancer progression by increasing the invasive behavior of lung cancer cells (Table 1). A protumorigenic role of gal-3 in lung cancer has also been attributed to intracellular gal-3 and its ability to modulate key signaling pathways, such as the β-catenin pathway [26]. However, while the roles of gal-1 and gal-3 in lung cancer generally mirror their roles in pancreatic cancer, this is certainly not the case for gal-4. Using biopsies collected from a cohort of more than 700 patients with lung adenocarcinoma, Hayashi and colleagues [27] showed that expression of intracellular gal-4 is inversely associated with clinicopathologic variables of disease progression. Such an anti-tumorigenic role of galectins in lung cancer is not unique to gal-4 as it has also been shown for gal-9 [28, 29], illustrating, again, their well-documented double-edged swords for their function [3]. Such opposing roles for galectins in HFC are also observed in stomach cancer. Again, while gal-1 is generally associated with tumor progression of stomach cancer, gal-7 and gal-9 express anti-tumorigenic properties [23, 25].
Table 1.
Functional effects of galectins in HFCs
| Cancer type | Subtypes | Galectins | Effect | References |
|---|---|---|---|---|
| Liver | Hepatocellular carcinoma | Gal-1 | ↑ Tumor progression | [47–54] |
| Contribute to resistance to antibody-mediated killing of cancer cells | [55] | |||
| Contribute to Sorafenib and Cisplatin chemoresistance | [49, 56] | |||
| Gal-3 | ↑ Tumor progression | [41–43, 57–62] | ||
| Gal-4 | ↑ Tumor progression | [63] | ||
| Gal-9 | Interact with Tim-3 to promote degeneration of T cells | [64] | ||
| ↓ Tumor progression | [44, 65] | |||
| Gal-9 expression correlates with recurrence of cancer | [66] | |||
| Cholangiocarcinoma | Gal-1 | ↑ Tumor progression | [67] | |
| Gal-3 | ↑ Anti-apoptosis capacities of cancer cells | [68] | ||
| Contribute to the chemoresistance | ||||
| Involved in the preneoplasic and neoplasic transformation | [67] | |||
| Gal-9 | ↓ Tumor progression | [45] | ||
| General | Gal-9 | Gal-9 induced lymphocyte apoptosis and tumor cell immune escape | [46] | |
| Brain | Glioblastomas | Gal-1 | ↑ Tumor progression | [28, 34, 69–72] |
| Contribute to the chemoresistance | [29] | |||
| Reduces motility | [73] | |||
| Gal-3 | ↑ Tumor progression | [71] | ||
| Enhances the adhesion of homotypic tumor cell | [74] | |||
| Gal-8 | ↑ Tumor progression | [71] | ||
| Astrocytomas | Gal-1 | ↑ Tumor progression | [71, 72] | |
| Gal-3 | ↑ Tumor progression | [71, 75] | ||
| Gal-8 | ↑ Tumor progression | [71] | ||
| General | Gal-1 | ↑ tumor progression | [76, 77] | |
| Silencing of tumor-derived gal-1 increased survival | [34] | |||
| Gal-3 | ↑ Tumor progression | [78, 79] | ||
| Ovaries | Serous high grade | Gal-3 | ↓ Cellular proliferation of Clear Carcinoma cancer cells | [80] |
| Contribute to the chemoresistance to CDDP | ||||
| Gal-7 | ↑ Tumor progression | [81, 82] | ||
| Breast | Triple negative | Gal-3 | ↑ Tumor progression | [83] |
| Gal-7 | ↑ Tumor progression | [158] | ||
| Pancreatic | Undefined | Gal-1 | ↑ Tumor progression and tumor evasion | [7, 84–91] |
| Gal-3 | ↑ Tumor progression and chemoresistance | [10, 12, 89, 92, 93] | ||
| Gal-4 | ↓ The tumors progression | [13, 14] | ||
| Lung | Undefined | Gal-1 | ↑ Tumor progression, chemoresistance and tumor evasion | [23, 24, 94–98] |
| Gal-3 | ↑ Tumor progression and chemoresistance | [26, 99–106] | ||
| Gal-4 | ↑ Tumor progression | [27] | ||
| Gal-9 | ↓ Tumor progression | [107, 108] | ||
| Oesophagus | Undefined | Gal-3 | Nuclear gal-3 inversely correlates with vascular invasion | [109] |
| ↑ Tumor progression | ||||
| ↑ Chemoresistance to Gefitinib treatment | [110] | |||
| Gal-7 | ↑ Tumor progression | [111] | ||
| Stomach | Undefined | Gal-1 | ↑ Tumor progression | [112, 113] |
| Gal-3 | ↑ Tumor progression | [114–119] | ||
| ↓ Metastasis formation | [120] | |||
| Contribute to the chemotherapy resistance of cancer cells | [119] | |||
| Gal-7 | ↓ Tumor progression | [121] | ||
| Gal-9 | ↓ Tumor progression | [122–124] |
Brain cancer
The most common type of brain cancer diagnosed, glioblastoma multiforme, is also the most aggressive [30, 31]. The incidence of this grade IV astrocytoma is approximately 3 per 100,000 persons. Glioblastoma multiforme displays a median survival of 14.6 months [31, 32]. Another type of brain tumor with a low survival rate is anaplastic astrocytoma, a grade III astrocytoma with a 5-year survival rate of approximately 10% for people older than 55-years of age. An important restriction for the treatment of brain cancers is the blood–brain barrier, which restricts the passage of drugs. The heterogeneity of the disease and the higher proportion of recurrence or therapy resistance are among the reasons leading to this unfavorable prognosis [30, 33]. In this case, the role of galectins is unequivocal since most, if not all, studies on gal-1, -3, and -8 have reported these galectins as having a protumorigenic role (Table 1). The role of gal-1 secreted by glioma cancer cells in inhibiting infiltration of myeloid-derived suppressor cells confirms the importance of this galectin in controlling the anti-cancer immune response [34]. The ability of gal-1 and gal-3 to promote the migration and invasion of cancer cells is also a central theme in studies aimed at defining the role of galectins in brain cancer. There is, however, much to be learned about the role of galectins in brain cancer since all but one study have focused almost exclusively on gal-1 and gal-3.
Liver cancer
Liver cancer has the second highest mortality rate worldwide. Hepatocellular carcinoma (HCC) is the most common malignant hepatic disease [35]. The 5-year overall survival is approximately 10–20% but increases to 70% after a surgery [36–39]. Liver transplantation and other resection surgeries are effective procedures for this disease, but the late stage at which it is diagnosed, limited availability of organ donation, problem of recurrence and other associated liver dysfunctions are important obstacles to the successful therapy of HCC [36, 40]. What is unique to liver cancer is the dominant roles of gal-1 and gal-3 in conferring resistance of cancer cells to cell death induced by either antibodies or chemotherapeutic drugs (Table 1). A fair amount of studies on galectins in liver cancer have focused on gal-3 and its role in migration and invasion [41–43]. A number of studies have also focused on gal-9, which seems to have an anti-proliferative effect on hepatocarcinoma and cholangiocarcinoma cells [44–46].
Other HFCs
Very few studies have examined the role of galectins in other HFCs, including gallbladder cancer, a relatively rare (a global incidence of 2.2 per 100,000) [125, 126] but deadly disease. The 5-year overall survival rate of gallbladder cancer does not exceed 5% and it is associated with an average survival of 6 months [127]. Surprisingly, to our knowledge, there is only one study published on the roles of gal-1 and gal-3, the most commonly studied galectins in cancer. Yang et al. [128] have shown that an increased expression of gal-3 was associated with a decreased overall survival of patients with gallbladder adenocarcinoma. However, a recent study by a group in Japan has shown that gal-9 suppresses the growth of cancer cells and their resistance to apoptosis [129]. Such an antitumoral role of gal-9 has also been found in gastric cancer [130], the fourth most commonly diagnosed cancer and the second leading cause of cancer-related deaths worldwide, with an incidence rate that varies greatly (from 2 to 3 in Egypt, and up to 65.9 in men and 25.9 in women in Korea) [131, 132]. On average, people diagnosed with stomach cancer have a 25% chance of living at least 5 years after their diagnosis. In contrast, the protumorigenic role of gal-1 and gal-3 in promoting invasive behavior and a resistance to drug-induced apoptosis in gastric cancer is relatively well-documented (Table 1). Preliminary reports have been published on the expressions of gal-4, gal-7 and gal-8 in gastric cancer [121, 133, 134]. However, functional data are lacking with regards to their role in tumor progression. On the other hand, a number of studies are now focusing on high mortality (“bad”) subtypes of cancer that have a relatively high 5-year survival rate. This is the case, for example, for high grade serous ovarian carcinoma (HGSC), which has a 5-year survival rate of less than 15% while the overall survival rate of ovarian cancer is approximately 46%, in the US (http://www.cancer.org). Recent studies have shown that gal-3 and gal-7 may confer epithelial ovarian cancer cells with resistance to drug-induced apoptosis [80–82]. This field of research is likely to rapidly progress as we are now to able to refine the classification of cancer subtypes and identify novel aggressive molecular subtypes with the help of comparative transcriptome analysis of large cohort of patients.
Galectins as biomarkers in HFCs
Currently, considerable efforts are being dedicated to the development of predictive biomarkers for the early detection of HFC and for the initiation of treatment in the early stages of progression before metastasis. The benefit of early detection using imaging procedures, routine clinical exams, cytology screening, or blood tests has been well-established for several cancers, including breast, colon, prostate and cervical cancers. Biomarkers are also used to assess disease susceptibility and risk, grading severity of the disease, the determination of an optimal treatment or predicting outcomes to a specific treatment. They are at the center of precision medicine, which requires better stratification of patients. Ideally, they are used as companion tests in harmony with a given therapeutic drug. A case in point is testing for HER-2 expression, which classifies patients with breast cancer into two categories: responders and non-responders to targeted therapy with Trastuzumab (Herceptin). This immunohistochemistry test detects the expression of HER-2 membrane proteins at the surface of epithelial breast cancer cells. Although the “Her-2 test”, which was approved in 1998, has survived numerous obstacles linked to reproducibility and quantification, the general view is that measurements of plasma biomarkers by ELISA testing are better suited not only because of the ease and its relatively non-invasive nature but also because this assay is more quantifiable and reproducible. Not surprisingly, given the soluble nature of galectins and their release outside of cells, plasma levels of galectins are now commonly used as a predictive biomarker in many diseases. The best-known galectin plasma biomarker is probably gal-13, also known as PP-13, which is specifically expressed in placental tissues where it plays a central role in maternal–fetal immune tolerance [135]. Its potential as a biomarker alone or in combination with other biomarkers for detecting pre-eclampsia in the first trimester has been well-documented and tested clinically [136–138]. Another good example is plasmatic gal-3, which is commonly used as a biomarker in patients with various vascular diseases, thereby helping in the prognosis of these patients [139]. Measurements of plasma galectin-3 were approved by the US Food and Drug Administration in 2011 as helping in the prognosis of patients with heart failure. Abnormally high levels of galectins have also been reported in many other diseases, including colorectal cancer [140], acute intracerebral hemorrhage [141], pulmonary arterial hypertension [142], prediabetes and diabetes [143, 144], systemic lupus erythematosus [145] and viral infections [146]. Galectin levels have also been shown to be elevated in patients with HFCs such as pancreatic cancer [147]. Clearly, levels of galectins in plasma samples and other liquid biopsies can be used for prognostic purposes. However, given their dual role in cancer, it is logical to believe that plasma levels of a given galectin may not necessarily correlate with the cancer’s aggressiveness. This is well-illustrated in malignant pleural mesothelioma (MPM), the most frequent type of mesothelioma, which has a median survival time of approximately 12 months after diagnosis [148]. Gal-3 concentrations in pleural fluids are significantly lower in MPM than in lung adenocarcinoma. Gal-3 can thus be used to differentiate MPM from lung adenocarcinoma and as a negative marker to exclude a diagnosis of MPM [149, 150]. This is in contrast to gal-1. In this case, high concentrations of gal-1 in pleural fluids correlates with a lower overall survival [151], which is consistent with its well-documented role in creating an immunosuppressive tumor microenvironment [152]. Whether other galectins are present in pleural effusion or other liquid biopsies (including cerebrospinal fluids, which contains several biomarkers for different forms of brain cancer) remains unknown. The potential of using galectins in liquid biopsies as a predictive tool in cancer patients, however, is a rapidly evolving field of research investigation, and there are reasons to believe that they may be useful, alone or in combination, for prognostic purposes or for predicting responses to treatment. A good example is the combination of galectins and MUC-1 as potential biomarkers for metastatic breast cancer [153], reinforcing the association between galectins and MUC-1, a highly glycosylated cell surface receptor expressed on the surface of cancer cells [154, 155].
Predictive value of intracellular galectins
Although most of the attention on galectins has historically focused on their extracellular functions, their intracellular patterns of expression are often significantly altered in cancers compared to normal cells. In cancer cells, they can be found almost anywhere, including in cytosolic, nuclear, and mitochondrial compartments, where they accomplish distinct and often contradictory functions [156]. Such distinct patterns of expression can be exploited for the development of biomarkers for risk prediction in cancer patients. A good example is galectin-8, a galectin known to shuttle between the nucleus and the cytosol in cancer cells [157]. Our recent studies showed that nuclear, but not cytosolic, gal-8 is associated with a good prognosis in patients diagnosed with TNBC, one of the most aggressive subtypes of breast cancer. TNBC patients with nuclear gal-8 have a significantly better disease-free survival, metastasis, and overall survival [158]. This is in contrast to nuclear gal-1, which is rather associated with a poor prognosis. Interestingly, in TNBC patients who expressed both nuclear gal-1 and gal-8, the phenotype of nuclear gal-8 is clearly dominant. Indeed, despite having nuclear gal-1, the 5-year survival rate of TNBC patients expressing nuclear both gal-1 and gal-8 is 100% [158]. Such findings illustrate the importance of looking at the overall galectinome when examining the predictive values of galectins in patients with cancer. Such shuttling between intracellular compartments in cancer cells has also been well-documented for gal-3, which translocates to the nucleus to modulate β-catenin regulated transcriptional activity [159]. Although there are indications that nuclear gal-3 is expressed in pancreatic and gallbladder cancer cells, its potential as a predictive factor for these HFCs remains unknown [128, 160]. The potential of nuclear gal-3 as a predictive biomarker in HFC has, however, been reported for at least two other HFCs. These include lung and esophageal carcinomas [109, 161, 162].
Galectin inhibitors for the treatment of HFCs?
Because of their critical role in cancer, considerable efforts have been directed towards the development of carbohydrate-based inhibitors that would limit the binding of galectins to glycosylated residues on cell surface receptors. Up to now, however, most of these efforts have focused on targeting the glycan binding site of extracellular gal-1 and gal-3 using either modified mono- and disaccharides, synthetic glycodendrimers and modified complex glycans, and peptide inhibitors, such as Anginex [163–166]. Such inhibitors have shown great potential against HFCs. For example, a polysaccharide purified from the flower of Panax notoginseng has shown strong antiproliferative activity against pancreatic cancer cells in vitro by disrupting the interaction between Gal-3 and EGFR [167]. Despite decades of research, however, the progression in this field has been relatively slow. Achieving specificity and high affinity for these compounds, most notably considering our limited knowledge on less well-characterized galectins, is a true challenge. Consequently, the benefits of using these inhibitors for the treatment of cancer are currently shadowed by their putative off-target effects. More specific inhibitors are alternative strategies are thus urgently needed. The use of antisense- and short hairpin RNA-based strategies is one possibility. We and others have shown that such approach is an interesting option to inhibit the pro-tumoral activity of galectins, most notably in glioblastoma, pancreatic cancer and highly aggressive forms of lymphoid malignancies [12, 28, 93, 168–170]. Yet, another consideration to take into account in the design of inhibitors is the need to target intracellular galectins. This is not trivial as we found that accumulation of galectins occurs in several intracellular compartments and that their pro-tumoral role with depend on their intracellular localization [156]. A better understanding of the roles of intracellular galectins is thus needed to determine how galectins collaboratively modulate cancer progression from within the cells. This is especially important as it is increasingly clear that is the cellular context, as defined by the balance of intracellular and extracellular signaling events, that dictates whether galectins will spare the cancer cell or lead to its apoptotic demise.
Conclusions
The search for finding new and effective biomarkers and treatment options that would offer hope for patients with HFCs needs to be conducted using new approaches, given the distinct characteristics of HFCs. A rapid overview of studies that examined the expressions and functions of galectins in cancer cells reveals that they play a central role in at least three major features that characterize HFCs: (1) induction of systemic and local immunosuppression, (2) chemoresistance of cancer cells, and (3) increased invasive behavior. It is important to note, however, that galectins are not exclusively associated with increased aggressiveness. Many of them do have anti-tumorigenic functions and do inhibit cancer progression. Because we do find a relatively large and heterogeneous galectinome in cancer tissues, the use of galectins as therapeutic targets will need to be well-thought and ideally involved companion diagnostic testings. This is, especially true as most of the studies have focused on a limited number of galectins and we still know very little information about the less well-known galectins. Exploiting our knowledge on galectins to fight HFCs will thus require both the identification of the specific galectinome expressed in cancer tissues and the development of highly specific galectin inhibitors. Defining the galectinome in HFCs will also lead to a better understanding of tumor heterogeneity while providing critical information that could improve the accuracy of biomarker panels for a more personalized treatment of HFCs.
Abbreviations
- HFC
High fatality cancer
- TNBC
Triple-negative breast cancer
- HGSC
High grade serous ovarian carcinoma
- CRD
Carbohydrate-recognition domains
- NSCLC
Non-small cell lung cancer
- SCLC
Small cell lung cancer
- HCC
Hepatocellular carcinoma
- MPM
Malignant pleural mesothelioma
- AML
Acute myeloid leukemia
References
- 1.Barondes SH, Castronovo V, Cooper DN, Cummings RD, Drickamer K, Feizi T, Gitt MA, Hirabayashi J, Hughes C, Kasai K, et al. Galectins: a family of animal beta-galactoside-binding lectins. Cell. 1994;76:597–598. doi: 10.1016/0092-8674(94)90498-7. [DOI] [PubMed] [Google Scholar]
- 2.Liu FT, Rabinovich GA. Galectins as modulators of tumour progression. Nat Rev Cancer. 2005;5:29–41. doi: 10.1038/nrc1527. [DOI] [PubMed] [Google Scholar]
- 3.Than NG, Romero R, Balogh A, Karpati E, Mastrolia SA, Staretz-Chacham O, Hahn S, Erez O, Papp Z, Kim CJ. Galectins: double-edged swords in the cross-roads of pregnancy complications and female reproductive tract inflammation and neoplasia. J Pathol Transl Med. 2015;49:181–208. doi: 10.4132/jptm.2015.02.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bildner AG, Rabinovich GA. ‘Sweetening’ pregnancy: galectins at the fetomaternal interface. Am J Reprod Immunol. 2013;69:369–382. doi: 10.1111/aji.12090. [DOI] [PubMed] [Google Scholar]
- 5.Mishra BB, Li Q, Steichen AL, Binstock BJ, Metzger DW, et al. Galectin-3 functions as an alarmin: pathogenic role for sepsis development in murine respiratory tularemia. PLoS One. 2013;8:e59616. doi: 10.1371/journal.pone.0059616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Steichen AL, Simonson TJ, Salmon SL, Metzger DW, Mishra BB, et al. Alarmin function of galectin-9 in murine respiratory tularemia. PLoS One. 2015;10:e0123573. doi: 10.1371/journal.pone.0123573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Martinez-Bosch N, Navarro P. Targeting galectin-1 in pancreatic cancer: immune surveillance on guard. Oncoimmunology. 2014;3:e952201. doi: 10.4161/21624011.2014.952201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Terris B, Blaveri E, Crnogorac-Jurcevic T, Jones M, Missiaglia E, Ruszniewski P, Sauvanet A, Lemoine NR. Characterization of gene expression profiles in intraductal papillary-mucinous tumors of the pancreas. Am J Pathol. 2002;160:1745–1754. doi: 10.1016/S0002-9440(10)61121-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Grutzmann R, Pilarsky C, Ammerpohl O, Luttges J, Bohme A, Sipos B, Foerder M, Alldinger I, Jahnke B, Schackert HK, et al. Gene expression profiling of microdissected pancreatic ductal carcinomas using high-density DNA microarrays. Neoplasia. 2004;6:611–622. doi: 10.1593/neo.04295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Song S, Ji B, Ramachandran V, Wang H, Hafley M, Logsdon C, Bresalier RS. Overexpressed galectin-3 in pancreatic cancer induces cell proliferation and invasion by binding Ras and activating Ras signaling. PLoS One. 2012;7:e42699. doi: 10.1371/journal.pone.0042699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kobayashi T, Ishida J, Musashi M, Ota S, Yoshida T, Shimizu Y, Chuma M, Kawakami H, Asaka M, Tanaka J, et al. p53 transactivation is involved in the antiproliferative activity of the putative tumor suppressor RBM5. Int J Cancer. 2011;128:304–318. doi: 10.1002/ijc.25345. [DOI] [PubMed] [Google Scholar]
- 12.Kobayashi T, Shimura T, Yajima T, Kubo N, Araki K, Wada W, Tsutsumi S, Suzuki H, Kuwano H, Raz A. Transient silencing of galectin-3 expression promotes both in vitro and in vivo drug-induced apoptosis of human pancreatic carcinoma cells. Clin Exp Metastasis. 2011;28:367–376. doi: 10.1007/s10585-011-9376-x. [DOI] [PubMed] [Google Scholar]
- 13.Belo AI, van der Sar AM, Tefsen B, van Die I. Galectin-4 reduces migration and metastasis formation of pancreatic cancer cells. PLoS One. 2013;8:e65957. doi: 10.1371/journal.pone.0065957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Maftouh M, Belo AI, Avan A, Funel N, Peters GJ, Giovannetti E, Van Die I. Galectin-4 expression is associated with reduced lymph node metastasis and modulation of Wnt/beta-catenin signalling in pancreatic adenocarcinoma. Oncotarget. 2014;5:5335–5349. doi: 10.18632/oncotarget.2104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chen Z, Fillmore CM, Hammerman PS, Kim CF, Wong KK. Non-small-cell lung cancers: a heterogeneous set of diseases. Nat Rev Cancer. 2014;14:535–546. doi: 10.1038/nrc3775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Islami F, Torre LA, Jemal A. Global trends of lung cancer mortality and smoking prevalence. Transl Lung Cancer Res. 2015;4:327–338. doi: 10.3978/j.issn.2218-6751.2015.08.04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Czyzykowski R, Polowinczak-Przybylek J, Potemski P. Nicotine-induced resistance of non-small cell lung cancer to treatment—possible mechanisms. Postepy Hig Med Dosw (Online) 2016;70:186–193. doi: 10.5604/17322693.1196391. [DOI] [PubMed] [Google Scholar]
- 18.Byers LA, Rudin CM. Small cell lung cancer: where do we go from here? Cancer. 2015;121:664–672. doi: 10.1002/cncr.29098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wao H, Mhaskar R, Kumar A, Miladinovic B, Djulbegovic B. Survival of patients with non-small cell lung cancer without treatment: a systematic review and meta-analysis. Syst Rev. 2010;2:10. doi: 10.1186/2046-4053-2-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Barr Kumarakulasinghe N, Nv Zanwijk, Soo RA. Molecular targeted therapy in the treatment of advanced stage non-small cell lung cancer (NSCLC) Respirology. 2015;20:370–378. doi: 10.1111/resp.12490. [DOI] [PubMed] [Google Scholar]
- 21.Chan BA, Hughes BG. Targeted therapy for non-small cell lung cancer: current standards and the promise of the future. Transl Lung Cancer Res. 2015;4:36–54. doi: 10.3978/j.issn.2218-6751.2014.05.01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Reck M, Heigener DF, Mok T, Soria JC, Rabe KF. Management of non-small-cell lung cancer: recent developments. Lancet. 2013;382:709–719. doi: 10.1016/S0140-6736(13)61502-0. [DOI] [PubMed] [Google Scholar]
- 23.Kuo PL, Hung JY, Huang SK, Chou SH, Cheng DE, Jong YJ, Hung CH, Yang CJ, Tsai YM, Hsu YL, et al. Lung cancer-derived galectin-1 mediates dendritic cell anergy through inhibitor of DNA binding 3/IL-10 signaling pathway. J Immunol. 2011;186:1521–1530. doi: 10.4049/jimmunol.1002940. [DOI] [PubMed] [Google Scholar]
- 24.Kuo PL, Huang MS, Cheng DE, Hung JY, Yang CJ, Chou SH. Lung cancer-derived galectin-1 enhances tumorigenic potentiation of tumor-associated dendritic cells by expressing heparin-binding EGF-like growth factor. J Biol Chem. 2012;287:9753–9764. doi: 10.1074/jbc.M111.321190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hsu YL, Hung JY, Chiang SY, Jian SF, Wu CY, Lin YS, Tsai YM, Chou SH, Tsai MJ, Kuo PL. Lung cancer-derived galectin-1 contributes to cancer associated fibroblast-mediated cancer progression and immune suppression through TDO2/kynurenine axis. Oncotarget. 2016;7:27584–27598. doi: 10.18632/oncotarget.8488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chung LY, Tang SJ, Wu YC, Sun GH, Liu HY, Sun KH. Galectin-3 augments tumor initiating property and tumorigenicity of lung cancer through interaction with beta-catenin. Oncotarget. 2015;6:4936–4952. doi: 10.18632/oncotarget.3210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hayashi T, Saito T, Fujimura T, Hara K, Takamochi K, Mitani K, Mineki R, Kazuno S, Oh S, Ueno T, et al. Galectin-4, a novel predictor for lymph node metastasis in lung adenocarcinoma. PLoS One. 2013;8:e81883. doi: 10.1371/journal.pone.0081883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Camby I, Decaestecker C, Lefranc F, Kaltner H, Gabius HJ, Kiss R. Galectin-1 knocking down in human U87 glioblastoma cells alters their gene expression pattern. Biochem Biophys Res Commun. 2005;335:27–35. doi: 10.1016/j.bbrc.2005.07.037. [DOI] [PubMed] [Google Scholar]
- 29.Le Mercier M, Mathieu V, Haibe-Kains B, Bontempi G, Mijatovic T, Decaestecker C, Kiss R, Lefranc F. Knocking down galectin 1 in human hs683 glioblastoma cells impairs both angiogenesis and endoplasmic reticulum stress responses. J Neuropathol Exp Neurol. 2008;67:456–469. doi: 10.1097/NEN.0b013e318170f892. [DOI] [PubMed] [Google Scholar]
- 30.Pointer KB, Clark PA, Zorniak M, Alrfaei BM, Kuo JS. Glioblastoma cancer stem cells: biomarker and therapeutic advances. Neurochem Int. 2014;71:1–7. doi: 10.1016/j.neuint.2014.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jackson CM, Lim M, Drake CG. Immunotherapy for brain cancer: recent progress and future promise. Clin Cancer Res. 2014;20:3651–3659. doi: 10.1158/1078-0432.CCR-13-2057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Thakkar JP, Dolecek TA, Horbinski C, Ostrom QT, Lightner DD, Barnholtz-Sloan JS, Villano JL. Epidemiologic and molecular prognostic review of glioblastoma. Cancer Epidemiol Biomark Prev. 2014;23:1985–1996. doi: 10.1158/1055-9965.EPI-14-0275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.DeAngelis LM. Anaplastic glioma: how to prognosticate outcome and choose a treatment strategy. [corrected] J Clin Oncol. 2009;27:5861–5862. doi: 10.1200/JCO.2009.24.5985. [DOI] [PubMed] [Google Scholar]
- 34.Verschuere T, Toelen J, Maes W, Poirier F, Boon L, Tousseyn T, Mathivet T, Gerhardt H, Mathieu V, Kiss R, et al. Glioma-derived galectin-1 regulates innate and adaptive antitumor immunity. Int J Cancer. 2014;134:873–884. doi: 10.1002/ijc.28426. [DOI] [PubMed] [Google Scholar]
- 35.Li S, Yang F, Ren X. Immunotherapy for hepatocellular carcinoma. Drug Discov Ther. 2015;9:363–371. doi: 10.5582/ddt.2015.01054. [DOI] [PubMed] [Google Scholar]
- 36.Jiang W, Li J, Guo Q, Sun J, Chen C, Shen Z. Liver transplantation for hepatocellular carcinoma. Drug Discov Ther. 2015;9:331–334. doi: 10.5582/ddt.2015.01048. [DOI] [PubMed] [Google Scholar]
- 37.Canadian Cancer Society's Advisory Committee on Cancer Statistics (2016) Canadian Cancer Statistics 2016. Canadian Cancer Society, Toronto, ON. https://www.cancer.ca/en/cancer-information/cancer-type/liver/prognosis-and-survival/survival-statistics/?region=on#ixzz4xg2Gp06L. Accessed 16 Oct 2017
- 38.American Cancer Society (2016) Cancer Facts & Figures 2016. American Cancer Society, Atlanta, Ga
- 39.Cancer Research UK (2016) Liver cancer survival statistics, Cancer Research UK, London, UK
- 40.Lin S, Hoffmann K, Schemmer P. Treatment of hepatocellular carcinoma: a systematic review. Liver Cancer. 2012;1:144–158. doi: 10.1159/000343828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zheng D, Hu Z, He F, Gao C, Xu L, Zou H, Wu Z, Jiang X, Wang J. Downregulation of galectin-3 causes a decrease in uPAR levels and inhibits the proliferation, migration and invasion of hepatocellular carcinoma cells. Oncol Rep. 2014;32:411–418. doi: 10.3892/or.2014.3170. [DOI] [PubMed] [Google Scholar]
- 42.Eisa NH, Ebrahim MA, Ragab M, Eissa LA, El-Gayar AM. Galectin-3 and matrix metalloproteinase-9: perspective in management of hepatocellular carcinoma. J Oncol Pharm Pract. 2015;21:323–330. doi: 10.1177/1078155214532698. [DOI] [PubMed] [Google Scholar]
- 43.Serizawa N, Tian J, Fukada H, Baghy K, Scott F, Chen X, Kiss Z, Olson K, Hsu D, Liu F-T, et al. Galectin 3 regulates HCC cell invasion by RhoA and MLCK activation. Lab Invest. 2015;95:1145–1156. doi: 10.1038/labinvest.2015.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Fujita K, Iwama H, Sakamoto T, Okura R, Kobayashi K, Takano J, Katsura A, Tatsuta M, Maeda E, Mimura S, et al. Galectin-9 suppresses the growth of hepatocellular carcinoma via apoptosis in vitro and in vivo. Int J Oncol. 2015;46:2419–2430. doi: 10.3892/ijo.2015.2941. [DOI] [PubMed] [Google Scholar]
- 45.Kobayashi K, Morishita A, Iwama H, Fujita K, Okura R, Fujihara S, Yamashita T, Fujimori T, Kato K, Kamada H, et al. Galectin-9 suppresses cholangiocarcinoma cell proliferation by inducing apoptosis but not cell cycle arrest. Oncol Rep. 2015;34:1761–1770. doi: 10.3892/or.2015.4197. [DOI] [PubMed] [Google Scholar]
- 46.Yang Q, Jiang W, Zhuang C, Geng Z, Hou C, Huang D, Hu L, Wang X. microRNA-22 downregulation of galectin-9 influences lymphocyte apoptosis and tumor cell proliferation in liver cancer. Oncol Rep. 2015;34:1771–1778. doi: 10.3892/or.2015.4167. [DOI] [PubMed] [Google Scholar]
- 47.Spano D, Russo R, Di Maso V, Rosso N, Terracciano LM, Roncalli M, Tornillo L, Capasso M, Tiribelli C, Iolascon A. Galectin-1 and its involvement in hepatocellular carcinoma aggressiveness. Mol Med. 2010;16:102–115. doi: 10.2119/molmed.2009.00119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yeh CC, Hsu CH, Shao YY, Ho WC, Tsai MH, Feng WC, Chow LP. Integrated Stable Isotope Labeling by Amino Acids in Cell Culture (SILAC) and Isobaric Tags for Relative and Absolute Quantitation (iTRAQ) Quantitative Proteomic Analysis Identifies Galectin-1 as a Potential Biomarker for Predicting Sorafenib Resistance in Liver Cancer. Mol Cell Proteom. 2015;14:1527–1545. doi: 10.1074/mcp.M114.046417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zhang PF, Li KS, Shen YH, Gao PT, Dong ZR, Cai JB, Zhang C, Huang XY, Tian MX, Hu ZQ, et al. Galectin-1 induces hepatocellular carcinoma EMT and sorafenib resistance by activating FAK/PI3 K/AKT signaling. Cell Death Dis. 2016;7:e2201. doi: 10.1038/cddis.2015.324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Espelt MV, Croci DO, Bacigalupo ML, Carabias P, Manzi M, Elola MT, Munoz MC, Dominici FP, Wolfenstein-Todel C, Rabinovich GA, et al. Novel roles of galectin-1 in hepatocellular carcinoma cell adhesion, polarization, and in vivo tumor growth. Hepatology. 2011;53:2097–2106. doi: 10.1002/hep.24294. [DOI] [PubMed] [Google Scholar]
- 51.Bacigalupo ML, Manzi M, Espelt MV, Gentilini LD, Compagno D, Laderach DJ, Wolfenstein-Todel C, Rabinovich GA, Troncoso MF. Galectin-1 triggers epithelial-mesenchymal transition in human hepatocellular carcinoma cells. J Cell Physiol. 2015;230:1298–1309. doi: 10.1002/jcp.24865. [DOI] [PubMed] [Google Scholar]
- 52.Manzi M, Bacigalupo ML, Carabias P, Elola MT, Wolfenstein-Todel C, Rabinovich GA, Espelt MV, Troncoso MF. Galectin-1 controls the proliferation and migration of liver sinusoidal endothelial cells and their interaction with hepatocarcinoma cells. J Cell Physiol. 2016;231:1522–1533. doi: 10.1002/jcp.25244. [DOI] [PubMed] [Google Scholar]
- 53.Inufusa H, Nakamura M, Adachi T, Aga M, Kurimoto M, Nakatani Y, Wakano T, Miyake M, Okuno K, Shiozaki H, et al. Role of galectin-3 in adenocarcinoma liver metastasis. Int J Oncol. 2001;19:913–919. doi: 10.3892/ijo.19.5.913. [DOI] [PubMed] [Google Scholar]
- 54.Wu H, Chen P, Liao R, Li YW, Yi Y, Wang JX, Sun TW, Zhou J, Shi YH, Yang XR, et al. Overexpression of galectin-1 is associated with poor prognosis in human hepatocellular carcinoma following resection. J Gastroenterol Hepatol. 2012;27:1312–1319. doi: 10.1111/j.1440-1746.2012.07130.x. [DOI] [PubMed] [Google Scholar]
- 55.Zhang P, Shi B, Gao H, Jiang H, Kong J, Yan J, Pan X, Li K, Zhang P, Yao M, et al. An EpCAM/CD3 bispecific antibody efficiently eliminates hepatocellular carcinoma cells with limited galectin-1 expression. Cancer Immunol Immunother. 2014;63:121–132. doi: 10.1007/s00262-013-1497-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Su YC, Davuluri GV, Chen CH, Shiau DC, Chen CC, Chen CL, Lin YS, Chang CP. Galectin-1-induced autophagy facilitates cisplatin resistance of hepatocellular carcinoma. PLoS One. 2016;11:e0148408. doi: 10.1371/journal.pone.0148408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Fang QQ, Ni RZ, Xiao MB, Jiang F, Lu CH. Serum and tissue expressions of galectin-3 in hepatocellular carcinoma and the clinical significances. Zhonghua Gan Zang Bing Za Zhi. 2011;19:527–531. doi: 10.3760/cma.j.issn.1007-3418.2011.07.014. [DOI] [PubMed] [Google Scholar]
- 58.Jiang SS, Weng DS, Wang QJ, Pan K, Zhang YJ, Li YQ, Li JJ, Zhao JJ, He J, Lv L, et al. Galectin-3 is associated with a poor prognosis in primary hepatocellular carcinoma. J Transl Med. 2014;12:273. doi: 10.1186/s12967-014-0273-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Matsuda Y, Yamagiwa Y, Fukushima K, Ueno Y, Shimosegawa T. Expression of galectin-3 involved in prognosis of patients with hepatocellular carcinoma. Hepatol Res. 2008;38:1098–1111. doi: 10.1111/j.1872-034X.2008.00387.x. [DOI] [PubMed] [Google Scholar]
- 60.Lin L, Han MM, Wang F, Xu LL, Yu HX, Yang PY. CXCR7 stimulates MAPK signaling to regulate hepatocellular carcinoma progression. Cell Death Dis. 2014;5:e1488. doi: 10.1038/cddis.2014.392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Yu Q, Shen W, Zhou H, Dong W, Gao D. Knockdown of LI-cadherin alters expression of matrix metalloproteinase-2 and -9 and galectin-3. Mol Med Rep. 2016;13:4469–4474. doi: 10.3892/mmr.2016.5069. [DOI] [PubMed] [Google Scholar]
- 62.Hsu DK, Dowling CA, Jeng KC, Chen JT, Yang RY, Liu FT. Galectin-3 expression is induced in cirrhotic liver and hepatocellular carcinoma. Int J Cancer. 1999;81:519–526. doi: 10.1002/(SICI)1097-0215(19990517)81:4<519::AID-IJC3>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- 63.Cai Z, Zeng Y, Xu B, Gao Y, Wang S, Zeng J, Chen L, Huang A, Liu X, Liu J. Galectin-4 serves as a prognostic biomarker for the early recurrence/metastasis of hepatocellular carcinoma. Cancer Sci. 2014;105:1510–1517. doi: 10.1111/cas.12536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Li H, Wu K, Tao K, Chen L, Zheng Q, Lu X, Liu J, Shi L, Liu C, Wang G, et al. Tim-3/galectin-9 signaling pathway mediates T-cell dysfunction and predicts poor prognosis in patients with hepatitis B virus-associated hepatocellular carcinoma. Hepatology. 2012;56:1342–1351. doi: 10.1002/hep.25777. [DOI] [PubMed] [Google Scholar]
- 65.Zhang ZY, Dong JH, Chen YW, Wang XQ, Li CH, Wang J, Wang GQ, Li HL, Wang XD. Galectin-9 acts as a prognostic factor with antimetastatic potential in hepatocellular carcinoma. Asian Pac J Cancer Prev. 2012;13:2503–2509. doi: 10.7314/APJCP.2012.13.6.2503. [DOI] [PubMed] [Google Scholar]
- 66.Liang Y, Chen J, Zhang Y, Zhang W. Expression of galectin-9 mRNA in hepatocellular carcinoma. Int J Clin Exp Pathol. 2015;8:15100–15105. [PMC free article] [PubMed] [Google Scholar]
- 67.Shimonishi T, Miyazaki K, Kono N, Sabit H, Tuneyama K, Harada K, Hirabayashi J, Kasai K, Nakanuma Y. Expression of endogenous galectin-1 and galectin-3 in intrahepatic cholangiocarcinoma. Hum Pathol. 2001;32:302–310. doi: 10.1053/hupa.2001.22767. [DOI] [PubMed] [Google Scholar]
- 68.Wongkham S, Junking M, Wongkham C, Sripa B, Chur-In S, Araki N. Suppression of galectin-3 expression enhances apoptosis and chemosensitivity in liver fluke-associated cholangiocarcinoma. Cancer Sci. 2009;100:2077–2084. doi: 10.1111/j.1349-7006.2009.01304.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Fortin S, Le Mercier M, Camby I, Spiegl-Kreinecker S, Berger W, Lefranc F, Kiss R. Galectin-1 is implicated in the protein kinase C epsilon/vimentin-controlled trafficking of integrin-beta1 in glioblastoma cells. Brain Pathol. 2010;20:39–49. doi: 10.1111/j.1750-3639.2008.00227.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Le Mercier M, Lefranc F, Mijatovic T, Debeir O, Haibe-Kains B, Bontempi G, Decaestecker C, Kiss R, Mathieu V. Evidence of galectin-1 involvement in glioma chemoresistance. Toxicol Appl Pharmacol. 2008;229:172–183. doi: 10.1016/j.taap.2008.01.009. [DOI] [PubMed] [Google Scholar]
- 71.Camby I, Belot N, Rorive S, Lefranc F, Maurage CA, Lahm H, Kaltner H, Hadari Y, Ruchoux MM, Brotchi J, et al. Galectins are differentially expressed in supratentorial pilocytic astrocytomas, astrocytomas, anaplastic astrocytomas and glioblastomas, and significantly modulate tumor astrocyte migration. Brain Pathol. 2001;11:12–26. doi: 10.1111/j.1750-3639.2001.tb00377.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Toussaint LG, Nilson AE, Goble JM, Ballman KV, James CD, Lefranc F, Kiss R, Uhm JH. Galectin-1, a gene preferentially expressed at the tumor margin, promotes glioblastoma cell invasion. Mol Cancer. 2012;11:32. doi: 10.1186/1476-4598-11-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Debray C, Vereecken P, Belot N, Teillard P, Brion JP, Pandolfo M, Pochet R. Multifaceted role of galectin-3 on human glioblastoma cell motility. Biochem Biophys Res Commun. 2004;325:1393–1398. doi: 10.1016/j.bbrc.2004.10.181. [DOI] [PubMed] [Google Scholar]
- 74.Tews DS, Nissen A. Expression of adhesion factors and degrading proteins in primary and secondary glioblastomas and their precursor tumors. Invasion Metastasis. 1998;18:271–284. doi: 10.1159/000024520. [DOI] [PubMed] [Google Scholar]
- 75.Rorive S, Belot N, Decaestecker C, Lefranc F, Gordower L, Micik S, Maurage CA, Kaltner H, Ruchoux MM, Danguy A, et al. Galectin-1 is highly expressed in human gliomas with relevance for modulation of invasion of tumor astrocytes into the brain parenchyma. Glia. 2001;33:241–255. doi: 10.1002/1098-1136(200103)33:3<241::AID-GLIA1023>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- 76.Jung TY, Jung S, Ryu HH, Jeong YI, Jin YH, Jin SG, Kim IY, Kang SS, Kim HS. Role of galectin-1 in migration and invasion of human glioblastoma multiforme cell lines. J Neurosurg. 2008;109:273–284. doi: 10.3171/JNS/2008/109/8/0273. [DOI] [PubMed] [Google Scholar]
- 77.Yamaoka K, Mishima K, Nagashima Y, Asai A, Sanai Y, Kirino T. Expression of galectin-1 mRNA correlates with the malignant potential of human gliomas and expression of antisense galectin-1 inhibits the growth of 9 glioma cells. J Neurosci Res. 2000;59:722–730. doi: 10.1002/(SICI)1097-4547(20000315)59:6<722::AID-JNR4>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- 78.Ikemori RY, Machado CM, Furuzawa KM, Nonogaki S, Osinaga E, Umezawa K, de Carvalho MA, Verinaud L, Chammas R. Galectin-3 up-regulation in hypoxic and nutrient deprived microenvironments promotes cell survival. PLoS One. 2014;9:e111592. doi: 10.1371/journal.pone.0111592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Bresalier RS, Yan PS, Byrd JC, Lotan R, Raz A. Expression of the endogenous galactose-binding protein galectin-3 correlates with the malignant potential of tumors in the central nervous system. Cancer. 1997;80:776–787. doi: 10.1002/(SICI)1097-0142(19970815)80:4<776::AID-CNCR17>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- 80.Oishi T, Itamochi H, Kigawa J, Kanamori Y, Shimada M, Takahashi M, Shimogai R, Kawaguchi W, Sato S, Terakawa N. Galectin-3 may contribute to Cisplatin resistance in clear cell carcinoma of the ovary. Int J Gynecol Cancer. 2007;17:1040–1046. doi: 10.1111/j.1525-1438.2007.00916.x. [DOI] [PubMed] [Google Scholar]
- 81.Kim HJ, Jeon HK, Lee JK, Sung CO, Do IG, Choi CH, Kim TJ, Kim BG, Bae DS, Lee JW. Clinical significance of galectin-7 in epithelial ovarian cancer. Anticancer Res. 2013;33:1555–1561. [PubMed] [Google Scholar]
- 82.Labrie M, Vladoiu MC, Grosset AA, Gaboury L, St-Pierre Y. Expression and functions of galectin-7 in ovarian cancer. Oncotarget. 2014;5:7705–7721. doi: 10.18632/oncotarget.2299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Zhang H, Luo M, Liang X, Wang D, Gu X, Duan C, Gu H, Chen G, Zhao X, Zhao Z, et al. Galectin-3 as a marker and potential therapeutic target in breast cancer. PLoS One. 2014;9:e103482. doi: 10.1371/journal.pone.0103482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Tang D, Zhang J, Yuan Z, Gao J, Wang S, Ye N, Li P, Gao S, Miao Y, Wang D, et al. Pancreatic satellite cells derived galectin-1 increase the progression and less survival of pancreatic ductal adenocarcinoma. PLoS One. 2014;9:e90476. doi: 10.1371/journal.pone.0090476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Ansari D, Aronsson L, Sasor A, Welinder C, Rezeli M, Marko-Varga G, Andersson R. The role of quantitative mass spectrometry in the discovery of pancreatic cancer biomarkers for translational science. J Transl Med. 2014;12:87. doi: 10.1186/1479-5876-12-87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Chen R, Dawson DW, Pan S, Ottenhof NA, de Wilde RF, Wolfgang CL, May DH, Crispin DA, Lai LA, Lay AR, et al. Proteins associated with pancreatic cancer survival in patients with resectable pancreatic ductal adenocarcinoma. Lab Invest. 2015;95:43–55. doi: 10.1038/labinvest.2014.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Masamune A, Satoh M, Hirabayashi J, Kasai K, Satoh K, Shimosegawa T. Galectin-1 induces chemokine production and proliferation in pancreatic stellate cells. Am J Physiol Gastrointest Liver Physiol. 2006;290:G729–G736. doi: 10.1152/ajpgi.00511.2005. [DOI] [PubMed] [Google Scholar]
- 88.Xue X, Lu Z, Tang D, Yao J, An Y, Wu J, Li Q, Gao W, Xu Z, Qian Z, et al. Galectin-1 secreted by activated stellate cells in pancreatic ductal adenocarcinoma stroma promotes proliferation and invasion of pancreatic cancer cells: an in vitro study on the microenvironment of pancreatic ductal adenocarcinoma. Pancreas. 2011;40:832–839. doi: 10.1097/MPA.0b013e318217945e. [DOI] [PubMed] [Google Scholar]
- 89.Berberat PO, Friess H, Wang L, Zhu Z, Bley T, Frigeri L, Zimmermann A, Buchler MW. Comparative analysis of galectins in primary tumors and tumor metastasis in human pancreatic cancer. J Histochem Cytochem. 2001;49:539–549. doi: 10.1177/002215540104900414. [DOI] [PubMed] [Google Scholar]
- 90.Tang D, Gao J, Wang S, Yuan Z, Ye N, Chong Y, Xu C, Jiang X, Li B, Yin W, et al. Apoptosis and anergy of T cell induced by pancreatic stellate cells-derived galectin-1 in pancreatic cancer. Tumour Biol. 2015;36:5617–5626. doi: 10.1007/s13277-015-3233-5. [DOI] [PubMed] [Google Scholar]
- 91.Tang D, Yuan Z, Xue X, Lu Z, Zhang Y, Wang H, Chen M, An Y, Wei J, Zhu Y, et al. High expression of Galectin-1 in pancreatic stellate cells plays a role in the development and maintenance of an immunosuppressive microenvironment in pancreatic cancer. Int J Cancer. 2012;130:2337–2348. doi: 10.1002/ijc.26290. [DOI] [PubMed] [Google Scholar]
- 92.Jiang HB, Xu M, Wang XP. Pancreatic stellate cells promote proliferation and invasiveness of human pancreatic cancer cells via galectin-3. World J Gastroenterol. 2008;14:2023–2028. doi: 10.3748/wjg.14.2023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Kobayashi T, Shimura T, Yajima T, Kubo N, Araki K, Tsutsumi S, Suzuki H, Kuwano H, Raz A. Transient gene silencing of galectin-3 suppresses pancreatic cancer cell migration and invasion through degradation of beta-catenin. Int J Cancer. 2011;129:2775–2786. doi: 10.1002/ijc.25946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Hsu YL, Wu CY, Hung JY, Lin YS, Huang MS, Kuo PL. Galectin-1 promotes lung cancer tumor metastasis by potentiating integrin alpha6beta4 and Notch1/Jagged2 signaling pathway. Carcinogenesis. 2013;34:1370–1378. doi: 10.1093/carcin/bgt040. [DOI] [PubMed] [Google Scholar]
- 95.Wu MH, Hong TM, Cheng HW, Pan SH, Liang YR, Hong HC, Chiang WF, Wong TY, Shieh DB, Shiau AL, et al. Galectin-1-mediated tumor invasion and metastasis, up-regulated matrix metalloproteinase expression, and reorganized actin cytoskeletons. Mol Cancer Res. 2009;7:311–318. doi: 10.1158/1541-7786.MCR-08-0297. [DOI] [PubMed] [Google Scholar]
- 96.Chung LY, Tang SJ, Sun GH, Chou TY, Yeh TS, Yu SL, Sun KH. Galectin-1 promotes lung cancer progression and chemoresistance by upregulating p38 MAPK, ERK, and cyclooxygenase-2. Clin Cancer Res. 2012;18:4037–4047. doi: 10.1158/1078-0432.CCR-11-3348. [DOI] [PubMed] [Google Scholar]
- 97.Zhou X, Li D, Wang X, Zhang B, Zhu H, Zhao J. Galectin-1 is overexpressed in CD133+ human lung adenocarcinoma cells and promotes their growth and invasiveness. Oncotarget. 2015;6:3111–3122. doi: 10.18632/oncotarget.3076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Hsu YL, Hung JY, Chiang SY, Jian SF, Wu CY, Lin YS, Tsai YM, Chou SH, Tsai MJ, Kuo PL. Lung cancer-derived galectin-1 contributes to cancer associated fibroblast-mediated cancer progression and immune suppression through TDO2/kynurenine axis. Oncotarget. 2016;7:25784–27798. doi: 10.18632/oncotarget.8488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Raz A, Zhu DG, Hogan V, Shah N, Raz T, Karkash R, Pazerini G, Carmi P. Evidence for the role of 34-kDa galactoside-binding lectin in transformation and metastasis. Int J Cancer. 1990;46:871–877. doi: 10.1002/ijc.2910460520. [DOI] [PubMed] [Google Scholar]
- 100.Iurisci I, Tinari N, Natoli C, Angelucci D, Cianchetti E, Iacobelli S. Concentrations of galectin-3 in the sera of normal controls and cancer patients. Clin Cancer Res. 2000;6:1389–1393. [PubMed] [Google Scholar]
- 101.O’Driscoll L, Linehan R, Liang YH, Joyce H, Oglesby I, Clynes M. Galectin-3 expression alters adhesion, motility and invasion in a lung cell line (DLKP), in vitro. Anticancer Res. 2002;22:3117–3125. [PubMed] [Google Scholar]
- 102.Abdel-Aziz HO, Murai Y, Takasaki I, Tabuchi Y, Zheng HC, Nomoto K, Takahashi H, Tsuneyama K, Kato I, Hsu DK, et al. Targeted disruption of the galectin-3 gene results in decreased susceptibility to NNK-induced lung tumorigenesis: an oligonucleotide microarray study. J Cancer Res Clin Oncol. 2008;134:777–788. doi: 10.1007/s00432-007-0345-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Dange MC, Srinivasan N, More SK, Bane SM, Upadhya A, Ingle AD, Gude RP, Mukhopadhyaya R, Kalraiya RD. Galectin-3 expressed on different lung compartments promotes organ specific metastasis by facilitating arrest, extravasation and organ colonization via high affinity ligands on melanoma cells. Clin Exp Metastasis. 2014;31:661–673. doi: 10.1007/s10585-014-9657-2. [DOI] [PubMed] [Google Scholar]
- 104.Wu SW, Yu L, Zhou L, Cheng ZN, Tao YS. Expression of Gal-3 and CD82/KAI1 proteins in non-small cell lung cancer and their clinical significance. Zhonghua Zhong Liu Za Zhi. 2013;35:124–128. doi: 10.3760/cma.j.issn.0253-3766.2013.02.010. [DOI] [PubMed] [Google Scholar]
- 105.Kuo HY, Hsu HT, Chen YC, Chang YW, Liu FT, Wu CW. Galectin-3 modulates the EGFR signalling-mediated regulation of Sox2 expression via c-Myc in lung cancer. Glycobiology. 2016;26:155–165. doi: 10.1093/glycob/cwv088. [DOI] [PubMed] [Google Scholar]
- 106.Buttery R, Monaghan H, Salter DM, Sethi T. Galectin-3: differential expression between small-cell and non-small-cell lung cancer. Histopathology. 2004;44:339–344. doi: 10.1111/j.1365-2559.2004.01815.x. [DOI] [PubMed] [Google Scholar]
- 107.Nobumoto A, Nagahara K, Oomizu S, Katoh S, Nishi N, Takeshita K, Niki T, Tominaga A, Yamauchi A, Hirashima M. Galectin-9 suppresses tumor metastasis by blocking adhesion to endothelium and extracellular matrices. Glycobiology. 2008;18:735–744. doi: 10.1093/glycob/cwn062. [DOI] [PubMed] [Google Scholar]
- 108.Kadowaki T, Arikawa T, Shinonaga R, Oomizu S, Inagawa H, Soma G, Niki T, Hirashima M. Galectin-9 signaling prolongs survival in murine lung-cancer by inducing macrophages to differentiate into plasmacytoid dendritic cell-like macrophages. Clin Immunol. 2012;142:296–307. doi: 10.1016/j.clim.2011.11.006. [DOI] [PubMed] [Google Scholar]
- 109.Shibata T, Noguchi T, Takeno S, Takahashi Y, Fumoto S, Kawahara K. Impact of nuclear galectin-3 expression on histological differentiation and vascular invasion in patients with esophageal squamous cell carcinoma. Oncol Rep. 2005;13:235–239. [PubMed] [Google Scholar]
- 110.Cui G, Cui M, Li Y, Liang Y, Li W, Guo H, Zhao S. Galectin-3 knockdown increases gefitinib sensitivity to the inhibition of EGFR endocytosis in gefitinib-insensitive esophageal squamous cancer cells. Med Oncol. 2015;32:124. doi: 10.1007/s12032-015-0570-6. [DOI] [PubMed] [Google Scholar]
- 111.Zhu X, Ding M, Yu ML, Feng MX, Tan LJ, Zhao FK. Identification of galectin-7 as a potential biomarker for esophageal squamous cell carcinoma by proteomic analysis. BMC Cancer. 2010;10:290. doi: 10.1186/1471-2407-10-290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Chen J, Zhou SJ, Zhang Y, Zhang GQ, Zha TZ, Feng YZ, Zhang K. Clinicopathological and prognostic significance of galectin-1 and vascular endothelial growth factor expression in gastric cancer. World J Gastroenterol. 2013;19:2073–2079. doi: 10.3748/wjg.v19.i13.2073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.He XJ, Tao HQ, Hu ZM, Ma YY, Xu J, Wang HJ, Xia YJ, Li L, Fei BY, Li YQ, et al. Expression of galectin-1 in carcinoma-associated fibroblasts promotes gastric cancer cell invasion through upregulation of integrin beta1. Cancer Sci. 2014;105:1402–1410. doi: 10.1111/cas.12539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Yang ZM, Wu XT, He T, Da MX, Luo T, Qian K. Expression of galectin-3 mRNA in gastric cancer with peritoneal metastasis. Sichuan Da Xue Xue Bao Yi Xue Ban. 2006;37:105–108. [PubMed] [Google Scholar]
- 115.Dong WG, Yu QF, Xu Y, Fan LF. Li-cadherin is inversely correlated with galectin-3 expression in gastric cancer. Dig Dis Sci. 2008;53:1811–1817. doi: 10.1007/s10620-007-0080-2. [DOI] [PubMed] [Google Scholar]
- 116.Kim SJ, Shin JY, Cheong TC, Choi IJ, Lee YS, Park SH, Chun KH. Galectin-3 germline variant at position 191 enhances nuclear accumulation and activation of beta-catenin in gastric cancer. Clin Exp Metastasis. 2011;28:743–750. doi: 10.1007/s10585-011-9406-8. [DOI] [PubMed] [Google Scholar]
- 117.Kim SJ, Choi IJ, Cheong TC, Lee SJ, Lotan R, Park SH, Chun KH. Galectin-3 increases gastric cancer cell motility by up-regulating fascin-1 expression. Gastroenterology. 2010;138(1035–1045):e1031–e1032. doi: 10.1053/j.gastro.2009.09.061. [DOI] [PubMed] [Google Scholar]
- 118.Kim SJ, Shin JY, Lee KD, Bae YK, Choi IJ, Park SH, Chun KH. Galectin-3 facilitates cell motility in gastric cancer by up-regulating protease-activated receptor-1 (PAR-1) and matrix metalloproteinase-1 (MMP-1) PLoS One. 2011;6:e25103. doi: 10.1371/journal.pone.0025103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Cheong TC, Shin JY, Chun KH. Silencing of galectin-3 changes the gene expression and augments the sensitivity of gastric cancer cells to chemotherapeutic agents. Cancer Sci. 2010;101:94–102. doi: 10.1111/j.1349-7006.2009.01364.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Leal MF, Calcagno DQ, Chung J, de Freitas VM, Demachki S, Assumpcao PP, Chammas R, Burbano RR, Smith MC. Deregulated expression of annexin-A2 and galectin-3 is associated with metastasis in gastric cancer patients. Clin Exp Med. 2015;15:415–420. doi: 10.1007/s10238-014-0299-0. [DOI] [PubMed] [Google Scholar]
- 121.Kim SJ, Hwang JA, Ro JY, Lee YS, Chun KH. Galectin-7 is epigenetically-regulated tumor suppressor in gastric cancer. Oncotarget. 2013;4:1461–1471. doi: 10.18632/oncotarget.1219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Jiang J, Jin MS, Kong F, Cao D, Ma HX, Jia Z, Wang YP, Suo J, Cao X. Decreased galectin-9 and increased Tim-3 expression are related to poor prognosis in gastric cancer. PLoS One. 2013;8:e81799. doi: 10.1371/journal.pone.0081799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Yang J, Zhu L, Cai Y, Suo J, Jin J. Role of downregulation of galectin-9 in the tumorigenesis of gastric cancer. Int J Oncol. 2014;45:1313–1320. doi: 10.3892/ijo.2014.2494. [DOI] [PubMed] [Google Scholar]
- 124.Cho SJ, Kook MC, Lee JH, Shin JY, Park J, Bae YK, Choi IJ, Ryu KW, Kim YW. Peroxisome proliferator-activated receptor gamma upregulates galectin-9 and predicts prognosis in intestinal-type gastric cancer. Int J Cancer. 2015;136:810–820. doi: 10.1002/ijc.29056. [DOI] [PubMed] [Google Scholar]
- 125.Rakic M, Patrlj L, Kopljar M, Klicek R, Kolovrat M, Loncar B, Busic Z. Gallbladder cancer. Hepatobiliary Surg Nutr. 2014;3:221–226. doi: 10.3978/j.issn.2304-3881.2014.09.03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Bizama C, García P, Espinoza JA, Weber H, Leal P, Nervi B, Roa JC. Targeting specific molecular pathways holds promise for advanced gallbladder cancer therapy. Cancer Treat Rev. 2015;41:222–234. doi: 10.1016/j.ctrv.2015.01.003. [DOI] [PubMed] [Google Scholar]
- 127.Hundal R, Shaffer EA. Gallbladder cancer: epidemiology and outcome. Clin Epidemiol. 2014;6:99–109. doi: 10.2147/CLEP.S37357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Yang LP, Jiang S, Liu JQ, Miao XY, Yang ZL. Up-regulation of galectin-3 and Sambucus nigra agglutinin binding site is associated with invasion, metastasis and poor-progression of the gallbladder adenocarcinoma. Hepatogastroenterology. 2012;59:2089–2094. doi: 10.5754/hge12129. [DOI] [PubMed] [Google Scholar]
- 129.Tadokoro T, Morishita A, Fujihara S, Iwama H, Niki T, Fujita K, Akashi E, Mimura S, Oura K, Sakamoto T, et al. Galectin-9: an anticancer molecule for gallbladder carcinoma. Int J Oncol. 2016;48:1165–1174. doi: 10.3892/ijo.2016.3347. [DOI] [PubMed] [Google Scholar]
- 130.Takano J, Morishita A, Fujihara S, Iwama H, Kokado F, Fujikawa K, Fujita K, Chiyo T, Tadokoro T, Sakamoto T, et al. Galectin-9 suppresses the proliferation of gastric cancer cells in vitro. Oncol Rep. 2016;35:851–860. doi: 10.3892/or.2015.4452. [DOI] [PubMed] [Google Scholar]
- 131.Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global Cancer Statistics, 2012. CA Cancer J Clin. 2015;65:87–108. doi: 10.3322/caac.21262. [DOI] [PubMed] [Google Scholar]
- 132.Jemal A, Center MM, DeSantis C, Ward EM. Global patterns of cancer incidence and mortality rates and trends. Cancer Epidemiol Biomark Prev. 2010;19:1893–1907. doi: 10.1158/1055-9965.EPI-10-0437. [DOI] [PubMed] [Google Scholar]
- 133.Kocevar N, Hudler P, Komel R. The progress of proteomic approaches in searching for cancer biomarkers. N Biotechnol. 2013;30:319–326. doi: 10.1016/j.nbt.2012.11.011. [DOI] [PubMed] [Google Scholar]
- 134.Danguy A, Rorive S, Decaestecker C, Bronckart Y, Kaltner H, Hadari YR, Goren R, Zich Y, Petein M, Salmon I, et al. Immunohistochemical profile of galectin-8 expression in benign and malignant tumors of epithelial, mesenchymatous and adipous origins, and of the nervous system. Histol Histopathol. 2001;16:861–868. doi: 10.14670/HH-16.861. [DOI] [PubMed] [Google Scholar]
- 135.Baumann MU, Bersinger NA, Surbek DV. Serum markers for predicting pre-eclampsia. Mol Aspects Med. 2007;28:227–244. doi: 10.1016/j.mam.2007.04.002. [DOI] [PubMed] [Google Scholar]
- 136.Nicolaides KH, Bindra R, Turan OM, Chefetz I, Sammar M, Meiri H, Tal J, Cuckle HS. A novel approach to first-trimester screening for early pre-eclampsia combining serum PP-13 and Doppler ultrasound. Ultrasound Obstet Gynecol. 2006;27:13–17. doi: 10.1002/uog.2686. [DOI] [PubMed] [Google Scholar]
- 137.Romero R, Kusanovic JP, Than NG, Erez O, Gotsch F, Espinoza J, Edwin S, Chefetz I, Gomez R, Nien JK, et al. First-trimester maternal serum PP13 in the risk assessment for preeclampsia. Am J Obstet Gynecol. 2008;199:122 e111–122 e121. doi: 10.1016/j.ajog.2008.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Grill S, Rusterholz C, Zanetti-Dallenbach R, Tercanli S, Holzgreve W, Hahn S, Lapaire O. Potential markers of preeclampsia—a review. Reprod Biol Endocrinol. 2009;7:70. doi: 10.1186/1477-7827-7-70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Bosnjak I, Selthofer-Relatic K, Vcev A. Prognostic value of galectin-3 in patients with heart failure. Dis Mark. 2005;2015:690205. doi: 10.1155/2015/690205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Watanabe M, Takemasa I, Kaneko N, Yokoyama Y, Matsuo E, Iwasa S, Mori M, Matsuura N, Monden M, Nishimura O. Clinical significance of circulating galectins as colorectal cancer markers. Oncol Rep. 2011;25:1217–1226. doi: 10.3892/or.2011.1198. [DOI] [PubMed] [Google Scholar]
- 141.Yan X-J, Yu G-F, Jie Y-Q, Fan X-F, Huang Q, Dai W-M. Role of galectin-3 in plasma as a predictive biomarker of outcome after acute intracerebral hemorrhage. J Neurol Sci. 2016;368:121–127. doi: 10.1016/j.jns.2016.06.071. [DOI] [PubMed] [Google Scholar]
- 142.Calvier L, Legchenko E, Grimm L, Sallmon H, Hatch A, Plouffe BD, Schroeder C, Bauersachs J, Murthy SK, Hansmann G. Galectin-3 and aldosterone as potential tandem biomarkers in pulmonary arterial hypertension. Heart. 2016;102:390–396. doi: 10.1136/heartjnl-2015-308365. [DOI] [PubMed] [Google Scholar]
- 143.Yilmaz H, Cakmak M, Inan O, Darcin T, Akcay A. Increased levels of galectin-3 were associated with prediabetes and diabetes: new risk factor? J Endocrinol Invest. 2015;38:527–533. doi: 10.1007/s40618-014-0222-2. [DOI] [PubMed] [Google Scholar]
- 144.Pugliese G, Iacobini C, Ricci C, Blasetti Fantauzzi C, Menini S. Galectin-3 in diabetic patients. Clin Chem Lab Med. 2014;52:1413–1423. doi: 10.1515/cclm-2014-0187. [DOI] [PubMed] [Google Scholar]
- 145.Nielsen CT, Lood C, Ostergaard O, Iversen LV, Voss A, Bengtsson A, Jacobsen S, Heegaard NH. Plasma levels of galectin-3-binding protein reflect type I interferon activity and are increased in patients with systemic lupus erythematosus. Lupus Sci Med. 2004;1:e000026. doi: 10.1136/lupus-2014-000026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Chagan-Yasutan H, Ndhlovu LC, Lacuesta TL, Kubo T, Leano PS, Niki T, Oguma S, Morita K, Chew GM, Barbour JD, et al. Galectin-9 plasma levels reflect adverse hematological and immunological features in acute dengue virus infection. J Clin Virol. 2013;58:635–640. doi: 10.1016/j.jcv.2013.10.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Fredriksson S, Horecka J, Brustugun OT, Schlingemann J, Koong AC, Tibshirani R, Davis RW. Multiplexed proximity ligation assays to profile putative plasma biomarkers relevant to pancreatic and ovarian cancer. Clin Chem. 2008;54:582–589. doi: 10.1373/clinchem.2007.093195. [DOI] [PubMed] [Google Scholar]
- 148.Robinson BW, Musk AW, Lake RA. Malignant mesothelioma. Lancet. 2005;366:397–408. doi: 10.1016/S0140-6736(05)67025-0. [DOI] [PubMed] [Google Scholar]
- 149.Gueugnon F, Leclercq S, Blanquart C, Sagan C, Cellerin L, Padieu M, Perigaud C, Scherpereel A, Gregoire M. Identification of novel markers for the diagnosis of malignant pleural mesothelioma. Am J Pathol. 2011;178:1033–1042. doi: 10.1016/j.ajpath.2010.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Blanquart C, Gueugnon F, Nguyen JM, Roulois D, Cellerin L, Sagan C, Perigaud C, Scherpereel A, Gregoire M. CCL2, galectin-3, and SMRP combination improves the diagnosis of mesothelioma in pleural effusions. J Thorac Oncol. 2012;7:883–889. doi: 10.1097/JTO.0b013e31824c9272. [DOI] [PubMed] [Google Scholar]
- 151.Mundt F, Johansson HJ, Forshed J, Arslan S, Metintas M, Dobra K, Lehtio J, Hjerpe A. Proteome screening of pleural effusions identifies galectin 1 as a diagnostic biomarker and highlights several prognostic biomarkers for malignant mesothelioma. Mol Cell Proteom. 2014;13:701–715. doi: 10.1074/mcp.M113.030775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Rabinovich GA. Galectin-1 as a potential cancer target. Br J Cancer. 2005;92:1188–1192. doi: 10.1038/sj.bjc.6602493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Ideo H, Hinoda Y, Sakai K, Hoshi I, Yamamoto S, Oka M, Maeda K, Maeda N, Hazama S, Amano J, et al. Expression of mucin 1 possessing a 3′-sulfated core1 in recurrent and metastatic breast cancer. Intl J Cancer. 2015;137:1652–1660. doi: 10.1002/ijc.29520. [DOI] [PubMed] [Google Scholar]
- 154.Tanida S, Mori Y, Ishida A, Akita K, Nakada H. Galectin-3 binds to MUC1-N-terminal domain and triggers recruitment of beta-catenin in MUC1-expressing mouse 3T3 cells. Biochim Biophys Acta. 2014;1840:1790–1797. doi: 10.1016/j.bbagen.2014.02.008. [DOI] [PubMed] [Google Scholar]
- 155.Kolbl AC, Andergassen U, Jeschke U. The role of glycosylation in breast cancer metastasis and cancer control. Front Oncol. 2015;5:219. doi: 10.3389/fonc.2015.00219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Vladoiu MC, Labrie M, St-Pierre Y. Intracellular galectins in cancer cells: potential new targets for therapy (Review) Int J Oncol. 2014;44:1001–1014. doi: 10.3892/ijo.2014.2267. [DOI] [PubMed] [Google Scholar]
- 157.Elola MT, Ferragut F, Cardenas Delgado VM, Nugnes LG, Gentilini L, Laderach D, Troncoso MF, Compagno D, Wolfenstein-Todel C, Rabinovich GA. Expression, localization and function of galectin-8, a tandem-repeat lectin, in human tumors. Histol Histopathol. 2014;29:1093–1105. doi: 10.14670/HH-29.1093. [DOI] [PubMed] [Google Scholar]
- 158.Grosset AA, Labrie M, Vladoiu MC, Yousef EM, Gaboury L, St-Pierre Y. Galectin signatures contribute to the heterogeneity of breast cancer and provide new prognostic information and therapeutic targets. Oncotarget. 2016;7:18183–18203. doi: 10.18632/oncotarget.7784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Funasaka T, Raz A, Nangia-Makker P. Nuclear transport of galectin-3 and its therapeutic implications. Semin Cancer Biol. 2014;27:30–38. doi: 10.1016/j.semcancer.2014.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Schaffert C, Pour PM, Chaney WG. Localization of galectin-3 in normal and diseased pancreatic tissue. Int J Pancreatol. 1998;23:1–9. doi: 10.1007/BF02787497. [DOI] [PubMed] [Google Scholar]
- 161.Puglisi F, Minisini AM, Barbone F, Intersimone D, Aprile G, Puppin C, Damante G, Paron I, Tell G, Piga A, et al. Galectin-3 expression in non-small cell lung carcinoma. Cancer Lett. 2004;212:233–239. doi: 10.1016/j.canlet.2004.03.006. [DOI] [PubMed] [Google Scholar]
- 162.Mathieu A, Saal I, Vuckovic A, Ransy V, Vereerstraten P, Kaltner H, Gabius HJ, Kiss R, Decaestecker C, Salmon I, et al. Nuclear galectin-3 expression is an independent predictive factor of recurrence for adenocarcinoma and squamous cell carcinoma of the lung. Mod Pathol. 2005;18:1264–1271. doi: 10.1038/modpathol.3800416. [DOI] [PubMed] [Google Scholar]
- 163.Griffioen AW, Van Der Schaft DW, Barendsz-Janson AF, Andrew COX, Boudier HA, Hillen HF. Anginex, a designed peptide that inhibits angiogenesis. Biochem J. 2001;354:233–242. doi: 10.1042/bj3540233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Leffler H, Nilsson UJ (2012) Low-molecular weight inhibitors of galectins. In: Galectins and disease implications for targeted therapeutics, vol 1115. American Chemical Society, Washington, DC, pp 47–59
- 165.Blanchard H, Yu X, Collins PM, Bum-Erdene K. Galectin-3 inhibitors: a patent review (2008–present) Expert Opin Ther Pat. 2014;24:1053–1065. doi: 10.1517/13543776.2014.947961. [DOI] [PubMed] [Google Scholar]
- 166.Blanchard H, Bum-Erdene K, Bohari MH, Yu X. Galectin-1 inhibitors and their potential therapeutic applications: a patent review. Expert Opin Ther Pat. 2016;26(5):537–554. doi: 10.1517/13543776.2016.1163338. [DOI] [PubMed] [Google Scholar]
- 167.Zhang L, Wang P, Qin Y, Cong Q, Shao C, Du Z, Ni X, Li P, Ding K. RN1, a novel galectin-3 inhibitor, inhibits pancreatic cancer cell growth in vitro and in vivo via blocking galectin-3 associated signaling pathways. Oncogene. 2016;36:1297–1308. doi: 10.1038/onc.2016.306. [DOI] [PubMed] [Google Scholar]
- 168.Camby I, Belot N, Lefranc F, Sadeghi N, De Launoit Y, Kaltner H, Musette S, Darro F, Danguy A, Salmon I, Gabius HJ. Galectin-1 modulates human glioblastoma cell migration into the brain through modifications to the actin cytoskeleton and levels of expression of small GTPases. J Neuropathol Exp Neurol. 2002;61:585–596. doi: 10.1093/jnen/61.7.585. [DOI] [PubMed] [Google Scholar]
- 169.Demers M, Biron-Pain K, Hébert J, Lamarre A, Magnaldo T, St-Pierre Y. Galectin-7 in lymphoma: elevated expression in human lymphoid malignancies and decreased lymphoma dissemination by antisense strategies in experimental model. Cancer Res. 2007;67:2824–2829. doi: 10.1158/0008-5472.CAN-06-3891. [DOI] [PubMed] [Google Scholar]
- 170.Song S, Mazurek N, Liu C, Sun Y, Ding QQ, Liu K, Hung MC, Bresalier RS. Galectin-3 mediates nuclear β-catenin accumulation and Wnt signaling in human colon cancer cells by regulation of glycogen synthase kinase-3β activity. Cancer Res. 2009;69:1343–1349. doi: 10.1158/0008-5472.CAN-08-4153. [DOI] [PMC free article] [PubMed] [Google Scholar]