Abstract
The heart is regarded as an endocrine organ as well as a pump for circulation, since atrial natriuretic peptide (ANP) and brain natriuretic peptide (BNP) were discovered in cardiomyocytes to be secreted as hormones. Both ANP and BNP bind to their receptors expressed on remote organs, such as kidneys and blood vessels; therefore, the heart controls the circulation by pumping blood and by secreting endocrine peptides. Cardiomyocytes secrete other peptides besides natriuretic peptides. Although most of such cardiomyocyte-derived peptides act on the heart in autocrine/paracrine fashions, several peptides target remote organs. In this review, to overview current knowledge of endocrine properties of the heart, we focus on cardiomyocyte-derived peptides (cardiomyokines) that act on the remote organs as well as the heart. Cardiomyokines act on remote organs to regulate cardiovascular homeostasis, systemic metabolism, and inflammation. Therefore, through its endocrine function, the heart can maintain physiological conditions and prevent organ damage under pathological conditions.
Keywords: sPLA2, FSTL1, ET1, CHGA, FGF21
Introduction
The heart functions not only as an essential pump but also as an endocrine organ to maintain homeostasis of the circulatory system [1]. The discovery of atrial natriuretic peptide (ANP) indicates the heart as an endocrine organ. In the middle of 20th century, researchers who used electron microscopes observed granules in atrial cardiomyocytes that resembled those found in endocrine glands. These observations let them consider the possibility that atrial cardiomyocytes might function as hormone-secreting cells [2]. In 1981, de Bold et al. demonstrated endocrine properties of the heart [3]. The extract of rat atrial cardiomyocytes contained peptides that exerted potent natriuretic and diuretic effects when it was injected in rats. The peptide was identified by several groups and named ANP or atrial natriuretic factor (ANF) [4–7]. The identification of ANP acting on kidneys revealed that the heart functioned as an endocrine organ. After the discovery of ANP, structurally and functionally related peptides including brain natriuretic peptide (BNP) and C-type natriuretic peptide (CNP) were identified [8, 9]. These peptides are referred to as the natriuretic peptide (NP) family.
Cardiomyocytes also secrete other peptide hormones besides natriuretic peptides (NPs) through secretory granules [10]. In addition to peptides, lipids and genetic materials including mRNAs, DNAs, and non-coding RNAs are secreted from cardiomyocytes through extracellular vesicles [11]. The word ‘cardiokine’ is used to describe proteins secreted from cardiomyocytes, cardiac fibroblasts, endothelial cells, and smooth muscle cells in response to changes in the cardiac environment [10, 12]. Particularly, proteins secreted from cardiomyocytes are referred to as ‘cardiomyokines’ [13]. Therefore, ANP and BNP are considered to belong to cardiomyokines. Cardiomyokines are predicted to play physiological and pathological roles in the heart and remote organs. Although most of cardiomyokines act on the heart in autocrine or paracrine fashions, some of them exert endocrine actions (Fig. 1). In this review, by focusing on the regulation and function of cardiomyokines, we overview the current knowledge of the heart as an endocrine organ.
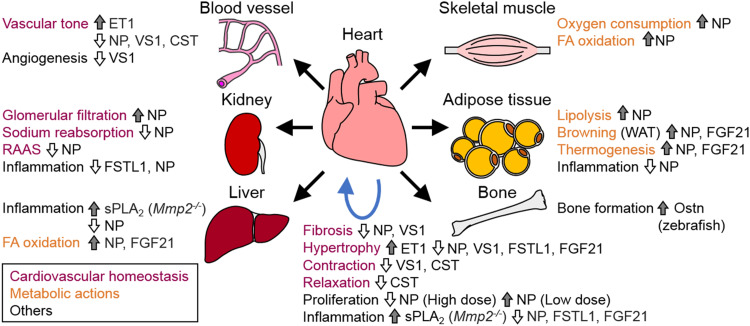
Fig. 1.
Target organs of cardiomyokines. Cardiomyokines act on blood vessels, kidney, liver, skeletal muscle, and adipose tissue. Cardiomyocyte-derived osteocrin (Ostn) might act on bone in zebrafish. Cardiokines exert biological functions (cardiovascular homeostasis and metabolic actions) in an endocrine manner (black arrows) and in an autocrine/paracrine manner (blue arrow). CST catestatin, ET1 endothelin 1, FA fatty acid, FGF21 fibroblast growth factor 21, FSTL1 follistatin-like 1, Mmp2 matrix metalloproteinase 2, NP natriuretic peptide, sPLA 2 secreted phospholipase A2, RAAS renin angiotensin aldosterone system, VS1 vasostatin 1, WAT white adipose tissue
Natriuretic peptides
Natriuretic peptides are mainly secreted from the cardiovascular system. Although they are primarily known as natriuretic, diuretic, and vasodilator factors, NPs also possess metabolic activities [14]. ANP is the first cardiac endocrine factor identified in rat hearts [4, 5] and human atrial tissues [6]. Following the discovery of ANP, BNP was identified in the brain [8]. Although isolated from the porcine brain, BNP is mainly produced in the heart [15]. Because ANP and BNP are expressed predominantly in the heart, they are called cardiac NPs [1]. CNP was identified as the third NP from the porcine brain [9]. CNP is mainly expressed in vascular endothelial cells [16] and the central nervous system [17, 18]. CNP acts locally as an autocrine/paracrine factor [19].
NP receptors and signal transduction
NP receptor family consists of NP receptor 1 (NPR1), NPR2, and NPR3 [20–23]. Because NPR1 and NPR2 have guanylate cyclase (GC) domain in their cytoplasmic domain, upon binding to those receptors, NPs increase intracellular cyclic guanosine monophosphate (cGMP) and subsequently activate cGMP-dependent protein kinase (PKG). On the other hand, NPR3 does not induce intracellular cGMP accumulation, because it lacks the GC domain and instead has a short 37-amino acid cytoplasmic domain. NPR3 acts as a clearance receptor for NPs by binding to all NPs. It is still controversial whether NPR3 triggers the intracellular signaling via Gi/o proteins [24]. NPs have 17-amino acid ring structure formed by a disulfide cysteine bridge between two cysteine residues that is necessary for binding to NPR1 and NPR2 [1]. Both ANP and BNP preferentially bind to NPR1, while CNP binds to NPR2 [25]. All the NPs bind to NPR3. Binding affinities are ranked as follows; NPR1 = ANP > BNP ≫ CNP; NPR2 = CNP ≫ ANP > BNP; NPR3 = ANP > CNP > BNP [26].
Secretion of ANP
Cardiomyocytes store ANP in secretory granules. ANP is secreted through the classical secretory pathway, a secretory pathway dependent on the endoplasmic reticulum (ER), coat protein complex II-coated vesicles, and the Golgi apparatus [10, 27]. ANP is secreted in a basal manner as well as inductive manners, such as agonist-stimulated and stretch-stimulated manners [1]. ANP secretion is induced by the stimulation of agonists including endothelin1 (ET1) and alpha adrenergic agents [28, 29]. Agonist-stimulated ANP secretion is mediated through the activation of Gq proteins [30]. Mechanical stretch also promotes the release of ANP from cardiomyocytes [31]. Although the mechanisms how stretch stimulation exerts secretion of cardiac granules have not been fully clarified yet, the secretion is mediated through pertussis toxin-sensitive Gi/o proteins [30]. Stretch-sensitive ion channels function as mechanosensors in cardiomyocytes [32, 33]. Stretch-activated non-selective cation channels and swelling-activated Cl− channels contribute to stretch-activated ANP secretion [32, 34].
ANP secretion from cardiomyocytes is increased under pathological conditions, such as ventricular hypertrophy, hypertension, heart failure, and myocardial infarction (MI) [35–38]. In these conditions, myocardial stretch and hypoxia induce ANP secretion. Hypoxic conditions stabilize hypoxia inducible factor 1 alpha (HIF1A). HIF1A promotes ANP transcription in neonatal rat cardiomyocytes [39]. In addition, cardiomyocytes in hypoxic conditions develop intracellular acidosis. The intracellular H+ increment increases intracellular Na+ and Ca2+ via H+–Na+ and Na+–Ca2+ exchange [40], which is also observed in hyperosmolar conditions. The resulting ion imbalance results in an increase of ANP secretion [41, 42].
Secretion of BNP
ANP and BNP are co-stored in the same secretory granules in cardiomyocytes [43]. They are co-released from cardiomyocytes, suggesting common mechanisms for the secretion of ANP and BNP [44]. On the other hand, in physiological conditions, plasma BNP is much lower than plasma ANP. Thus, separate NP-specific mechanisms of secretion and synthesis must be present. Plasma BNP reflects the severity of the pathological conditions [45]. Specific cytokines that are increased in pathological conditions including tumor necrosis factor alpha (TNFα) and interleukin 1 beta (IL1β) selectively promote BNP expression through a p38 mitogen-activated protein kinase-dependent (p38MAPK-dependent) pathway [46]. Moreover, BNP synthesis and secretion might be regulated by ER stress. ER stress under pathological conditions induces the secretion of various cardiomyokines from cardiomyocytes. ER stress promotes the expression of transcription factors, such as activating transcription factor 4 (ATF4), ATF6, and X-box binding protein 1 (XBP1), thereby inducing the expression of the genes involved in protein folding and secretion as well as the genes encoding cardiomyokines [10, 47–49]. In failing human hearts, the expression of active XBP1 and BNP is increased. In cultured neonatal rat cardiomyocytes, pharmacological ER stress induces BNP expression through an XBP1-dependent pathway [50].
NPs inhibit cardiac hypertrophy in autocrine/paracrine fashions
ANP inhibits hypertrophy in cultured neonatal rat cardiomyocytes [51]. NPR1-deficient (Npr1 −/−) mice show cardiac hypertrophy as well as systemic hypertension [52]. Moreover, although cardiac-specific Npr1 −/− mice show cardiac hypertrophy, the blood pressure of these Npr1 −/− mice is slightly below wild type. These data suggest that NPs directly inhibit cardiac hypertrophy [53].
NPs also inhibit cardiac inflammation. Pro-inflammatory cytokines are associated with the development of cardiac hypertrophy. Expression of pro-inflammatory cytokines is increased in the Npr1 −/− mouse hearts, while it is decreased in the NPR1 gene-duplicated mouse hearts [54]. The NPR1-dependent signaling pathway suppresses the expression of pro-inflammatory cytokines through the inhibition of the transcription mediated by nuclear factor kappa B (NFκB) and activator protein 1 [54].
NPs regulate cardiomyocyte proliferation in autocrine/paracrine fashions
Natriuretic peptides show an anti-proliferative effect on cardiomyocytes. ANP suppresses angiotensin II-stimulated (ANGII-stimulated) proliferation of cultured fetal sheep cardiomyocytes through a cGMP-dependent pathway [55]. On the other hand, there are controversial reports demonstrating that NPs promote cardiomyocyte proliferation probably through an NPR3/Gi/o protein-mediated signaling pathway. In developing zebrafish, cardiomyocyte proliferation is increased by simultaneous knockdown of npr1 and npr2, whereas it is decreased by knockdown of npr3. In cultured neonatal rat cardiomyocytes, a low concentration (10 nM) of ANP triggers an NPR3-dependent pathway to promote cardiomyocyte proliferation, while a high concentration (10 μM) of ANP induces NPR1- and NPR2-dependent pathways to inhibit cardiomyocyte proliferation [56].
NPs inhibit cardiac fibrosis in paracrine fashions
Natriuretic peptides inhibit proliferation of cardiac fibroblasts and their collagen syntheses in a cGMP-dependent manner [57, 58]. The endogenous ANP released from cultured cardiomyocytes inhibits collagen synthesis in cardiac fibroblasts [59]. Consistently, both Npr1 −/− mice and BNP-deficient (Nppb −/−) mice show more cardiac fibrosis than the control [52, 60]. Cardiac fibrosis is also regulated by the expression of matrix metalloproteinases (MMPs). The expression of MMP2 and MMP9 is increased in the Npr1 −/− mouse hearts. The treatment of a MMP inhibitor reduces cardiac fibrosis in Npr1 −/− mice [61]. These data suggest that ANP suppresses cardiac fibrosis by the inhibition of MMP expression.
NPs inhibit hypertension in endocrine fashions
When NPs are given to animals, NPs exert natriuretic and vasodilator effects, thereby decreasing intravascular volume and blood pressure. NPs reduce vascular tone through a relaxant effect on vascular smooth muscle cells [62]. NPs also reduce intravascular volume through a direct effect on endothelial permeability [63]. Furthermore, NPs regulate blood pressure by counteracting the renin–angiotensin–aldosterone system, by reducing sympathetic tone, and by suppressing the secretion of ET1, a potent vasoconstrictor [35]. In the kidney, NPs regulate electrolyte and fluid balance by increasing glomerular filtration rate [64, 65] and by decreasing sodium reabsorption in the proximal tubes and the collecting duct [66, 67]. NPs reduce renin release, peritubular ANGII, and ANGII-dependent reuptake of sodium [64]. These mechanisms lead to natriuresis that was the origin used for the naming of these peptides.
NPs regulate metabolism in endocrine fashions
Natriuretic peptides directly act on adipose tissues, resulting in the inhibition of the proliferation of human primary adipocytes [68]. NPs induce the synthesis of free fatty acid (FFA) through promoting lipolysis in adipose tissues by NPR1/cGMP/PKG-mediated activation of hormone-sensitive lipase [69, 70]. Indeed, ANP infusion increases plasma FFA and glycerol in young men. These increments are independent of the activation of the sympathetic nervous system [71]. Moreover, exercise training improves ANP-induced lipolysis in human adipose tissues [72, 73].
NPs modulate thermogenesis. Adipose tissues consist of white adipose tissue (WAT) and brown adipose tissue (BAT). The former is the main fat reservoir, while the latter is another fat reservoir and is able to generate heat through mitochondria-uncoupled respiration. NPs induce a transition from WAT to BAT-like tissue. In both BAT and WAT, NPs increase the expression of thermogenic genes, such as peroxisome proliferator activated receptor gamma coactivator 1 alpha (PGC1α) and uncoupling protein 1 (UCP1). The expression of those genes by NPs in the adipocytes is mediated by an NPR1/cGMP/PKG/p38 MAPK and subsequent ATF2-dependent pathway [74]. These data suggest that NPs might increase energy expenditure through the regulation of lipolysis and thermogenesis. The heart needs a huge energy supply to maintain continuous beating. To this end, the adult heart mainly uses either FFA or glucose as its energy source to generate ATP. Under normal conditions, most of ATP is generated from mitochondrial oxidation of FFA [75]. NPs increase FFA availability and mitochondrial biogenesis. These effects might contribute to more efficient FFA oxidation in the heart [76].
NPs also regulate skeletal muscle oxidative capacity. Skeletal muscle in which either BNP or PKG is overexpressed in mice shows higher oxygen consumption, greater FFA oxidation, and higher expression of mitochondrial oxidative genes [77]. Interestingly, exercise training up-regulates NPR1 transcripts in human skeletal muscles [78]. Therefore, NP signaling may also contribute to exercise training-induced fat oxidation in skeletal muscle.
NPs control satiety
Natriuretic peptides might regulate the gastro-intestinal system through modulating satiety hormone levels. Ghrelin, a gut-derived hormone, increases appetite and energy balance [79–81]. Plasma ghrelin is decreased after an administration of somatostatin, a peptide produced in the gastric oxyntic mucosa [82]. ANP stimulates somatostatin secretion via NPR1 activation [83]. Considering these data, NPs might indirectly inhibit ghrelin secretion through somatostatin secretion. Indeed, intravenous BNP administration inhibits the fasting-induced increment of plasma ghrelin in healthy volunteers [82]. In addition, BNP decreases the subjective rating of hunger and increases the feeling of satiety [84]. These results support the existence of a mutual regulation mediated by peptide hormones between the heart and gut.
NPs suppress inflammation in an endocrine fashion
Natriuretic peptides exhibit anti-inflammatory actions. As nitric oxide (NO) produced in the activated macrophages is toxic for microorganisms [85], an excess of NO production can cause damage in neighboring tissues. ANP inhibits the lipopolysaccharide-induced expression of inducible nitric oxide synthase (iNOS) that is required for NO production in macrophages [86]. In addition, ANP stimulation inhibits the secretion of pro-inflammatory cytokines, chemokines, and adipokines from cultured human adipose tissues [87]. Similarly, BNP has protective effects on acute lung, kidney, and intestinal tissue injury by down-regulating the expression of pro-inflammatory cytokines, such as TNFα and IL6. These anti-inflammatory effects of NPs are mediated through the suppression of NFκB inhibitor (IκB) phosphorylation and NFκB expression [88–91]. Because the chronic low-grade inflammation state is a risk factor for cardiovascular and metabolic disease [87], those anti-inflammatory effects of NPs seem to be beneficial to suppress the onset of those diseases.
Secreted phospholipase A2
Secreted phospholipase A2 (sPLA2) is a class of enzyme that catalyze sn-2 ester of glycerophospholipids to release FFAs and lysophospholipids. PLA2 family consists of intracellular PLA2 and sPLA2 [92]. In mammals, there are 11 sPLA2s (groups IB, IIA, IIC, IID, IIE, IIF, III, V, X, XIIA, and XIIB) [93].
Secretion of sPLA2s
sPLA2s are stored in secretory granules. sPLA2s have distinct tissue distributions. Group V sPLA2 (PLA2G5) is expressed mainly in the heart [94]. Cardiomyocytes secrete sPLA2s through the classical secretory pathway. The release of sPLA2 from hearts is enhanced by the ex vivo incubation with pro-inflammatory C-C motif chemokine ligand 7 (CCL7). CCL7 binds to and is inactivated by MMP2. In MMP2-deficient (Mmp2 −/−) mice, the plasma sPLA2 that is presumably released from cardiomyocytes is elevated [95, 96].
sPLA2s induce inflammation in autocrine/paracrine fashions
Cardiomyocyte-derived sPLA2s function as autocrine/paracrine factors. The elevation of cardiomyocyte-derived sPLA2s in the Mmp2 −/− mice induces cardiac inflammation. The expression of pro-inflammatory cytokines is increased in the Mmp2 −/− mouse hearts. The pro-inflammatory cytokine expression induced in the Mmp2 −/− mice is down-regulated by the knockdown of Pla2g5 gene and the treatment of a pan-sPLA2 inhibitor, respectively [95]. Therefore, cardiomyocyte-derived sPLA2s induce cardiac inflammation.
sPLA2s induce inflammation and metabolic dysregulation in endocrine fashions
In addition, cardiomyocyte-derived sPLA2s work as endocrine factors. sPLA2s derived from the Mmp2 −/− mouse hearts induce hepatic inflammation and metabolic dysregulation. A pan-sPLA2 inhibitor normalizes the expression of lipid metabolic genes and pro-inflammatory cytokines in the liver of Mmp2 −/− mice [96]. Therefore, liver functions are regulated by cardiac sPLA2s in Mmp2 −/− mice.
Follistatin-like 1
Follistatin-like 1 (FSTL1), also referred to transforming growth factor beta-stimulated (TGFβ-stimulated) clone-36 (TSC-36), is a secreted glycoprotein identified originally as a molecule induced by TGFβ stimulation [97]. FSTL1 belongs to follistatin family, because it shares a domain structure, which is called the FS domain [98]. Although other members of follistatin family proteins antagonize the binding of TGFβ superfamily proteins to their receptors, FSTL1 does not act on cells through its ability to inhibit the actions of TGFβ superfamily proteins [99].
FSTL1 receptor and signal transduction
Despite of inhibiting TGFβ superfamily proteins, FSTL1 activates Disco-interacting protein 2 homolog A, a cell surface receptor, and induces AKT serine/threonine kinase (AKT) phosphorylation [99]. In neonatal rat cardiomyocytes, FSTL1 induces extracellular signal-regulated kinase1/2 (ERK1/2) phosphorylation and AMP-activated protein kinase (AMPK) activation [100, 101].
Secretion of FSTL1
FSTL1 is secreted from cardiomyocytes through the classical secretory pathways [10]. The expression of Fstl1 is ubiquitous in early mouse embryos, whereas it becomes restricted and is mostly found in the mesenchymal tissue later during development [102]. Although FSTL1 is expressed in epicardial cells of the normal adult mouse heart, FSTL1 is expressed in cardiomyocytes and disappears from epicardial cells in the mouse heart with MI [103]. Serum FSTL1 is increased in mice with MI [101]. FSTL1 protein in the heart and plasma is increased in the mice with transverse aortic constriction (TAC) and ischemia/reperfusion (I/R) injury [100, 104]. Furthermore, FSTL1 expression is increased in patients with heart failure [105].
FSTL1 improves cardiomyocyte survival and inhibits cardiac hypertrophy in autocrine/paracrine fashions
FSTL1 secreted from the cardiomyocytes inhibits myocardial apoptosis and hypertrophy. FSTL1 knockdown exacerbates hypoxia/reoxygenation-induced apoptosis in neonatal rat cardiomyocytes through an AKT-dependent mechanism [101]. Cardiac-specific FSTL1-deficiency exacerbates cardiac hypertrophy following TAC. Cardiac FSTL1 inhibits TAC-induced cardiac hypertrophy through an AMPK-dependent mechanism [104]. On the other hand, epicardial cell- but not cardiomyocyte-derived FSTL1 improves cardiac functions after MI [103]. Although the role of cardiomyocyte-derived FSTL1 remains to be resolved, these data indicate the cardio-protective role for FSTL1.
FSTL1 inhibits systemic inflammation in endocrine fashions
FSTL1 works as an endocrine factor. In mice with kidney injury, plasma FSTL1 is increased. An increase of FSTL1 seems to be dependent on its secretion from cardiomyocytes. Given that cardiac-specific FSTL1-deficient mice show the exacerbation of renal injury after nephrectomy and that cardiomyocyte-derived FSTL1 exerts anti-inflammatory effects in kidneys via an AMPK-dependent mechanism, FSTL1 might function as a mediator involved in inter-organ communication between the heart and kidney [106].
Endothelin 1
ET1 is the most potent and long acting vasoconstrictor [107]. In 1988, ET1 was identified as an endothelium-derived blood vessel-constricting factor using the supernatant of cultured porcine aortic endothelial cells [108]. The ET peptide family consists of three structurally similar 21-amino acid peptides, ET1, ET2, and ET3 [109].
ET1 receptors and signal transduction
In 1990, two ET receptors, endothelin receptor type A (ENDRA) and type B (ENDRB), were identified [110, 111]. ENDRA has higher affinity to ET1 and ET2 than ET3. ENDRB has equal affinity to all ETs. Both ET receptors belong to the G-protein-coupled receptor family. ET receptors activate phospholipase C, inositol triphosphate, diacylglycerol, and intracellular calcium-dependent pathways [112].
Secretion of ET1
Cardiovascular systems predominantly express ET1 [113]. In coronary endothelial cells, ET1 is stored in secretory granules. ET1 secretion follows the classical secretory pathway [114]. Although the primary source of ET1 is vascular endothelial cells [115], ET1 is also expressed in cardiomyocytes [116]. Mechanical stretch and low oxygen conditions lead to ET1 secretion in cultured neonatal rat cardiomyocytes [117, 118]. Plasma ET1 concentration and cardiac ET1 synthesis are increased in patients with ischemic cardiomyopathy [119].
ET1 promotes cardiac hypertrophy and cell survival in autocrine/paracrine fashions
Autocrine/paracrine effects of cardiomyocyte-derived ET1 have been demonstrated by cardiac-specific ET1-deficient mice. ET1 exacerbates tri-iodothyronine-induced cardiac hypertrophy [120]. On the contrary, ET1 is essential for aged mice to survive and to maintain cardiac functions [121].
ET1 controls blood pressure
ENDRA is expressed on vascular smooth muscle cells and cardiomyocytes. The activation of ENDRA results in vasoconstriction and vascular smooth muscle cell proliferation through a phospholipase C-dependent pathway. On the other hand, ENDRB is expressed on endothelial cells and is involved in the clearance of ETs. ENDRB also shows a vasodilator effect through the release of NO and prostacyclin from endothelial cells [112, 122]. Although the role of cardiomyocyte-derived ET1 for the systemic regulation remains to be resolved, it appears to work as an autocrine or a paracrine factor in the organs where ET1 is produced.
Chromogranin A
Chromogranin A (CHGA) is an ubiquitously expressed acidic secretory protein, which belongs to the granin family. Because CHGA has several cleavage sites, the proteolytic cleavage of CHGA results in several biologically active CHGA-derived peptides, such as vasostatin1 (VS1), pancreastin, and catestatin (CST) [123].
Secretion of CHGA
Chromogranin A is found as a chromaffin granule protein secreted from the catecholamine- stimulated adrenal medulla [124, 125]. CHGA is co-stored and co-secreted with other secretory factors, such as catecholamines and NPs. Immunohistochemical analyses show that CHGA co-exists with ANP and BNP in cardiac secretory granules [126, 127]. Circulating CHGA levels are increased in patients with heart failure and essential hypertension [128, 129].
Vasostatin1
VS1 is an N-terminal fragment of CHGA. CHGA fragments containing VS1 are detected in rat heart extracts [130]. VS1 binds to heparin sulfate proteoglycans and activates phosphoinositide 3-kinase (PI3K) through a caveolae endocytosis-dependent mechanism [131].
VS1 inhibits cardiomyocyte hypertrophy in autocrine/paracrine fashions
VS1 treatment shows anti-adrenergic effects in cardiomyocytes. VS1 abolishes the isoproterenol-induced (ISO-induced) positive inotropism in the perfused rat heart [132]. Consistent with this action, chronic VS1 treatment inhibits ISO-induced cardiomyocyte hypertrophy in rats [133]. The anti-adrenergic effect of VS1 might depend on the activation of a PI3K/endothelial NOS (eNOS)/NO-dependent signaling pathway in endothelial cells rather than in cardiomyocytes [134]. Cardiomyocyte-derived VS1 might work as a paracrine factor and inhibits cardiomyocyte hypertrophy by targeting endothelial cells.
VS1 inhibits hypertension and angiogenesis
Although VS1 treatment suppresses vascular tension and inhibits angiogenesis [135, 136], it is unclear whether cardiomyocyte-derived VS1 acts as an endocrine factor.
Catestatin
CST is a C-terminal fragment of CHGA. CHGA fragments containing CST are detected in the mouse heart [137]. CST acts as a nicotinic cholinergic antagonist to inhibit catecholamine release [138]. While plasma CHGA is increased, plasma CST is decreased in patients with cardiovascular disease, such as hypertension [139] and heart failure [140]. Therefore, low plasma CST might increase the risk for cardiovascular disease.
CST controls heart function
In the Langendorff-perfused rat heart, CST stimulation counteracts ISO-induced positive inotropism and lusitropism through a Gi/o protein and eNOS-dependent mechanism [141].
CST inhibits hypertension
CST Catestatin prevents blood pressure elevation. Intravenous administration of CST results in a decrease of blood pressure in rats through an increment of plasma histamine, a potent vasodilator [142]. CHGA-deficient mice show high blood pressure, increased heart rate, and high plasma catecholamines. This phenotype is rescued by exogenous injection of CST [143, 144]. Although these data suggest that CST is involved in the cardiovascular maintenance, it is unclear whether cardiomyocyte-derived CHGA acts as an endocrine factor.
Fibroblast growth factors
Fibroblast growth factor (FGF) family consists of 22 FGFs, FGF1–FGF23 (FGF15 and FGF19 are orthologous peptides). FGFs can be classified as paracrine, endocrine, and intracrine FGFs [145]. Seven major FGF receptors (FGFRs) are translated from 4 FGFR genes, FGFR1-FGFR4 [146]. Paracrine FGFs including FGF1-FGF10, FGF16-FGF18, FGF20, and FGF22 bind to FGFRs with heparin/heparin sulfate as a cofactor. Endocrine FGFs comprising FGF15/19, FGF21, and FGF23 require either αKlotho or βKlotho as a cofactor to bind to FGFRs. Because of their low heparin-binding affinity, endocrine FGFs are capable of targeting remote organs through the blood stream [147]. Intracrine FGFs, FGF11–FGF14, are intracellular proteins that regulate voltage gated sodium channels through intracrine fashions. Among FGFs, FGF3, FGF8, FGF9, FGF10, FGF15/19, and FGF16 work as paracrine factors during heart development. On the other hand, FGF2, FGF9, FGF10, FGF16, and FGF21 act on the heart in pathological conditions as paracrine factors [148].
FGF21 receptors and signal transduction
FGF21, an endocrine FGF, activates FGFR1c, FGFR2c, and FGFR3c with βKlotho [145]. Cardiomyocytes express both FGFR1 and βKlotho [148]. FGF21 activates ERK1/2, p38MAPK, AMPK, and PI3K/AKT pathways in mouse hearts and in rat cardiomyocytes [149, 150].
Secretion of FGF21
FGF21 is mainly expressed in the liver and acts as a metabolic regulator [151, 152]. FGF21 increases glucose uptake [153], regulates lipid metabolism [154, 155], and improves insulin sensitivity in the liver and adipose tissues [156]. Because FGF21 is also produced in cardiomyocytes, it is regarded as a cardiomyokine [157]. Its expression is up-regulated in H9C2 cardiomyotubes by ER stress and in mouse hearts by mitochondrial dysfunction, respectively [158, 159]. FGF21 mRNAs in mouse hearts are also up-regulated after ISO-induced cardiac hypertrophy, TAC, and MI [160].
FGF21 inhibits cardiac hypertrophy in an autocrine/paracrine fashion
Cardiomyocyte-derived FGF21 works as an autocrine/paracrine factor. It prevents cardiac hypertrophy through the inhibition of metabolic dysregulation and pro-inflammatory signaling in cardiomyocytes. FGF21 activates FFA oxidation through an ERK1/2-, cAMP-responsive element binding protein-, and PGC1α-dependent pathway. FGF21 suppresses pro-inflammatory gene expression through the inhibition of NFκB activity [160].
FGF21 protects cardiomyocytes in autocrine/paracrine fashions
FGF21 protects the heart from oxidative stress through up-regulating antioxidant factors, including UCP2, UCP3, and superoxide dismutase 2 [161]. In addition, FGF21 inhibits cardiac apoptosis in an ERK1/2-, p38 MAPK-, and AMPK-dependent manner [150].
FGF21 controls systemic metabolism in endocrine fashions
Although the endocrine function of cardiomyocyte-derived FGF21 remains to be elucidated, mice overexpressing FGF21 in the heart show a decrease in body weight [158]. Moreover, mitochondrial dysfunction-induced FGF21 up-regulation in the mouse heart seems to be responsible for systemic changes in metabolism [159]. These data suggest that cardiomyocyte-derived FGF21 has a potential to work as an endocrine factor.
Osteocrin
Osteocrin (OSTN) was originally identified in muscles and bones by signal-sequence trap methods [162, 163]. OSTN is proposed to belong to the NP family, because it has two NP-like motifs. OSTN binds to NPR3, but not NPR1 and NPR2, because NP-like motifs of OSTN lack disulfide cysteine bridges that are essential for the circle structure of NPs [164, 165].
Cardiomyocyte-derived Ostn might promote bone formation in zebrafish
Recently, we reported that Ostn is expressed in zebrafish cardiomyocytes and that Ostn-deficient fish show the shortening of membranous bone and cartilage lengths. This impairment of bone growth was rescued by the myocardium-specific overexpression of Ostn. Although it is unclear whether the amount of endogenous cardiomyocyte-derived Ostn is enough to regulate bone formation, cardiomyocyte-derived Ostn might contribute to bone formation [166]. These data suggest that the cardiomyocyte-derived peptide has a potential to regulate bone growth at least in zebrafish.
Cardiomyokines have a potential to regulate bone formation
In mammals, OSTN is mainly expressed in bones and skeletal muscles, whereas subtle expression is detected in cardiomyocytes. Besides Ostn, several peptides known to regulate bone formation have been reported to be secreted from cardiomyocytes, although they are thought to work as autocrine factors. TGFβ superfamily peptides, such as activin A, bone morphogenetic protein 2 (BMP2), growth differentiation factor 15 (GDF15), and myostatin (MSTN) are secreted from cardiomyocytes [167–170]. Cardiomyocyte-derived TGFβ superfamily peptides might target bones, because some of them contribute to systemic increment of these peptide levels in pathological conditions. Cardiomyocyte-derived MSTN contributes to skeletal muscle atrophy in heart failure [171]. An increase of GDF15 in the blood that is presumably derived from cardiomyocytes acts on the liver to inhibit body growth [172]. In addition, FGFs have a potential to regulate bone formation. Cardiomyocyte-derived FGF21 might affect bone formation, because it has been reported to enhance the osteogenic activity of BMP2 [173]. Moreover, parathyroid hormone like hormone, a peptide known to regulate bone formation, has been reported to be secreted from cardiomyocytes [174]. Therefore, bone formation might also be regulated by cardiomyokines in mammals.
Secretion through cardiac extracellular vesicles
Besides the secretory pathway released from secretory granules, cardiomyocytes secrete proteins using extracellular vesicles, such as exosomes and micro-vesicles [11].
Cardiomyocyte exosome-derived proteins work as paracrine factors
Under hypoxic conditions, cultured adult rat cardiomyocytes secrete HSP20 via exosomes. Serum HSP20 in mice are increased after myocardial I/R. Cardiomyocyte-specific HSP20-overexpressing mice show increased circulating HSP20 and enhanced capillary density in hearts. Cardiomyocyte-derived HSP20 might induce angiogenesis through its paracrine effects [175].
Cardiomyocyte exosome-derived microRNAs work as paracrine factors
Extracellular vesicles carry not only proteins but also other bioactive mediators, such as lipids, DNAs, mRNAs, and non-coding RNAs including microRNAs (miRNAs) [11]. Exosomal miRNAs work as paracrine factors. In adult Goto-Kakizaki rats, an animal model of type 2 diabetes, cardiomyocytes release exosomes containing miR-320. Cardiomyocyte exosomal miR-320 regulates its target genes and inhibits proliferation, migration, and tube formation of cultured cardiac endothelial cells [176].
Cardiomyocyte exosome-derived microRNAs are detected in systemic blood
miRNAs are reported to play roles in progression of cardiovascular diseases and are recognized as potential biomarkers and novel drug targets [177]. miR-1, miR-133a/b, miR-208a, and miR-499 are highly expressed in cardiomyocytes. Plasma levels of these miRNAs are increased after acute MI [178]. These miRNAs regulate cardiac function. For example, miR-1 and 133 inhibit cardiac hypertrophy in mice [179]. On the other hand, the inhibition of miR-208a improves cardiac function and survival during hypertension-induced heart failure [180]. Although the function of circulating miRNA remains to be resolved, the systemic elevation of cardiomyocyte-derived miRNAs has the potential to target remote organs.
Conclusion
The fact that the heart is an endocrine organ was demonstrated by the discovery of ANP. Besides ANP, several peptides have been reported to be secreted from cardiomyocytes. Although most of such cardiomyokines act as autocrine or paracrine factors, several cardiomyokines, including those introduced in this review, target remote organs as endocrine factors. These peptides act on not only blood vessels and kidneys, but also liver, skeletal muscles, and adipose tissues. In addition, bone formation might also be regulated through cardiomyokines. Therefore, these peptides regulate various biological functions, such as cardiovascular homeostasis and metabolism. Cardiomyokines are synthesized in stress environments, such as pressure overload and ischemia through hypoxia- and ER stress-induced up-regulation of transcripts and are subsequently released in stretch- and agonist-induced manners. The heart contributes to general homeostasis of the body by regulating circulation and by secreting peptides. Although there are many cardiomyokines that have potentials to work as endocrine factors, their endocrine function is not fully demonstrated because of the lack of cardiac-specific knockout data. Further investigation will lead to a better understanding of the heart as an endocrine organ.
Acknowledgements
This work was partly supported by the Japan Society for the Promotion of Science KAKENHI Grants (22122003 and 16H02618), Japan Agency for Medical Research and Development AMED-CREST Grant (13414779 to N.M.), by Health and Labor Sciences.
References
- 1.Ogawa T, de Bold AJ. The heart as an endocrine organ. Endocr Connect. 2014;3:R31–R44. doi: 10.1530/EC-14-0012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.de Bold AJ. Atrial natriuretic factor: a hormone produced by the heart. Science. 1985;230:767–770. doi: 10.1126/science.2932797. [DOI] [PubMed] [Google Scholar]
- 3.de Bold AJ, Borenstein HB, Veress AT, Sonnenberg H. A rapid and potent natriuretic response to intravenous injection of atrial myocardial extract in rats. Life Sci. 1981;28:89–94. doi: 10.1016/0024-3205(81)90370-2. [DOI] [PubMed] [Google Scholar]
- 4.de Bold AJ, Flynn TG. Cardionatrin I—a novel heart peptide with potent diuretic and natriuretic properties. Life Sci. 1983;33:297–302. doi: 10.1016/0024-3205(83)90390-9. [DOI] [PubMed] [Google Scholar]
- 5.Flynn TG, de Bold ML, de Bold AJ. The amino acid sequence of an atrial peptide with potent diuretic and natriuretic properties. Biochem Biophys Res Commun. 1983;117:859–865. doi: 10.1016/0006-291X(83)91675-3. [DOI] [PubMed] [Google Scholar]
- 6.Kangawa K, Matsuo H. Purification and complete amino acid sequence of alpha-human atrial natriuretic polypeptide (alpha-hANP) Biochem Biophys Res Commun. 1984;118:131–139. doi: 10.1016/0006-291X(84)91077-5. [DOI] [PubMed] [Google Scholar]
- 7.Kennedy BP, Marsden JJ, Flynn TG, de Bold AJ, Davies PL. Isolation and nucleotide sequence of a cloned cardionatrin cDNA. Biochem Biophys Res Commun. 1984;122:1076–1082. doi: 10.1016/0006-291X(84)91201-4. [DOI] [PubMed] [Google Scholar]
- 8.Sudoh T, Kangawa K, Minamino N, Matsuo H. A new natriuretic peptide in porcine brain. Nature. 1988;332:78–81. doi: 10.1038/332078a0. [DOI] [PubMed] [Google Scholar]
- 9.Sudoh T, Minamino N, Kangawa K, Matsuo H. C-type natriuretic peptide (CNP): a new member of natriuretic peptide family identified in porcine brain. Biochem Biophys Res Commun. 1990;168:863–870. doi: 10.1016/0006-291X(90)92401-K. [DOI] [PubMed] [Google Scholar]
- 10.Doroudgar S, Glembotski CC. The cardiokine story unfolds: ischemic stress-induced protein secretion in the heart. Trends Mol Med. 2011;17:207–214. doi: 10.1016/j.molmed.2010.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chistiakov DA, Orekhov AN, Bobryshev YV. Cardiac extracellular vesicles in normal and infarcted heart. Int J Mol Sci. 2016;17:E63. doi: 10.3390/ijms17010063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shimano M, Ouchi N, Walsh K. Cardiokines: recent progress in elucidating the cardiac secretome. Circulation. 2012;126:e327–e332. doi: 10.1161/CIRCULATIONAHA.112.150656. [DOI] [PubMed] [Google Scholar]
- 13.Glembotski CC. Functions for the cardiomyokine, MANF, in cardioprotection, hypertrophy and heart failure. J Mol Cell Cardiol. 2011;51:512–517. doi: 10.1016/j.yjmcc.2010.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schlueter N, de Sterke A, Willmes DM, Spranger J, Jordan J, Birkenfeld AL. Metabolic actions of natriuretic peptides and therapeutic potential in the metabolic syndrome. Pharmacol Ther. 2014;144:12–27. doi: 10.1016/j.pharmthera.2014.04.007. [DOI] [PubMed] [Google Scholar]
- 15.Mukoyama M, Nakao K, Hosoda K, Suga S, Saito Y, Ogawa Y, Shirakami G, Jougasaki M, Obata K, Yasue H. Brain natriuretic peptide as a novel cardiac hormone in humans. Evidence for an exquisite dual natriuretic peptide system, atrial natriuretic peptide and brain natriuretic peptide. J Clin Investig. 1991;87:1402–1412. doi: 10.1172/JCI115146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Suga S, Nakao K, Itoh H, Komatsu Y, Ogawa Y, Hama N, Imura H. Endothelial production of C-type natriuretic peptide and its marked augmentation by transforming growth factor-beta. Possible existence of “vascular natriuretic peptide system”. J Clin Investig. 1992;90:1145–1149. doi: 10.1172/JCI115933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Komatsu Y, Nakao K, Suga S, Ogawa Y, Mukoyama M, Arai H, Shirakami G, Hosoda K, Nakagawa O, Hama N. C-type natriuretic peptide (CNP) in rats and humans. Endocrinology. 1991;129:1104–1106. doi: 10.1210/endo-129-2-1104. [DOI] [PubMed] [Google Scholar]
- 18.Stepan H, Leitner E, Bader M, Walther T. Organ-specific mRNA distribution of C-type natriuretic peptide in neonatal and adult mice. Regul Pept. 2000;95:81–85. doi: 10.1016/S0167-0115(00)00141-5. [DOI] [PubMed] [Google Scholar]
- 19.Kishimoto I, Tokudome T, Nakao K, Kangawa K. Natriuretic peptide system: an overview of studies using genetically engineered animal models. FEBS J. 2011;278:1830–1841. doi: 10.1111/j.1742-4658.2011.08116.x. [DOI] [PubMed] [Google Scholar]
- 20.Chang MS, Lowe DG, Lewis M, Hellmiss R, Chen E, Goeddel DV. Differential activation by atrial and brain natriuretic peptides of two different receptor guanylate cyclases. Nature. 1989;341:68–72. doi: 10.1038/341068a0. [DOI] [PubMed] [Google Scholar]
- 21.Chinkers M, Garbers DL, Chang MS, Lowe DG, Chin HM, Goeddel DV, Schulz S. A membrane form of guanylate cyclase is an atrial natriuretic peptide receptor. Nature. 1989;338:78–83. doi: 10.1038/338078a0. [DOI] [PubMed] [Google Scholar]
- 22.Fuller F, Porter JG, Arfsten AE, Miller J, Schilling JW, Scarborough RM, Lewicki JA, Schenk DB. Atrial natriuretic peptide clearance receptor. Complete sequence and functional expression of cDNA clones. J Biol Chem. 1988;263:9395–9401. [PubMed] [Google Scholar]
- 23.Schulz S, Singh S, Bellet RA, Singh G, Tubb DJ, Chin H, Garbers DL. The primary structure of a plasma membrane guanylate cyclase demonstrates diversity within this new receptor family. Cell. 1989;58:1155–1162. doi: 10.1016/0092-8674(89)90513-8. [DOI] [PubMed] [Google Scholar]
- 24.Anand-Srivastava MB, Sehl PD, Lowe DG. Cytoplasmic domain of natriuretic peptide receptor-C inhibits adenylyl cyclase. Involvement of a pertussis toxin-sensitive G protein. J Biol Chem. 1996;271:19324–19329. doi: 10.1074/jbc.271.32.19324. [DOI] [PubMed] [Google Scholar]
- 25.Koller KJ, Lowe DG, Bennett GL, Minamino N, Kangawa K, Matsuo H, Goeddel DV. Selective activation of the B natriuretic peptide receptor by C-type natriuretic peptide (CNP) Science. 1991;252:120–123. doi: 10.1126/science.1672777. [DOI] [PubMed] [Google Scholar]
- 26.Suga S, Nakao K, Hosoda K, Mukoyama M, Ogawa Y, Shirakami G, Arai H, Saito Y, Kambayashi Y, Inouye K. Receptor selectivity of natriuretic peptide family, atrial natriuretic peptide, brain natriuretic peptide, and C-type natriuretic peptide. Endocrinology. 1992;130:229–239. doi: 10.1210/endo.130.1.1309330. [DOI] [PubMed] [Google Scholar]
- 27.Nickel W, Rabouille C. Mechanisms of regulated unconventional protein secretion. Nat Rev Mol Cell Biol. 2009;10:148–155. doi: 10.1038/nrm2617. [DOI] [PubMed] [Google Scholar]
- 28.Bruneau BG, Piazza LA, de Bold AJ. Alpha 1-adrenergic stimulation of isolated rat atria results in discoordinate increases in natriuretic peptide secretion and gene expression and enhances Egr-1 and c-Myc expression. Endocrinology. 1996;137:137–143. doi: 10.1210/endo.137.1.8536605. [DOI] [PubMed] [Google Scholar]
- 29.Fukuda Y, Hirata Y, Yoshimi H, Kojima T, Kobayashi Y, Yanagisawa M, Masaki T. Endothelin is a potent secretagogue for atrial natriuretic peptide in cultured rat atrial myocytes. Biochem Biophys Res Commun. 1988;155:167–172. doi: 10.1016/S0006-291X(88)81064-7. [DOI] [PubMed] [Google Scholar]
- 30.Bensimon M, Chang AI, de Bold ML, Ponce A, Carreras D, De Bold AJ. Participation of G proteins in natriuretic peptide hormone secretion from heart atria. Endocrinology. 2004;145:5313–5321. doi: 10.1210/en.2004-0698. [DOI] [PubMed] [Google Scholar]
- 31.Bruneau BG, Piazza LA, de Bold AJ. BNP gene expression is specifically modulated by stretch and ET-1 in a new model of isolated rat atria. Am J Physiol. 1997;273:H2678–H2686. doi: 10.1152/ajpheart.1997.273.6.H2678. [DOI] [PubMed] [Google Scholar]
- 32.Han JH, Bai GY, Park JH, Yuan K, Park WH, Kim SZ, Kim SH. Regulation of stretch-activated ANP secretion by chloride channels. Peptides. 2008;29:613–621. doi: 10.1016/j.peptides.2007.12.003. [DOI] [PubMed] [Google Scholar]
- 33.Laine M, Arjamaa O, Vuolteenaho O, Ruskoaho H, Weckstrom M. Block of stretch-activated atrial natriuretic peptide secretion by gadolinium in isolated rat atrium. J Physiol. 1994;480(Pt 3):553–561. doi: 10.1113/jphysiol.1994.sp020383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhang YH, Youm JB, Earm YE. Stretch-activated non-selective cation channel: a causal link between mechanical stretch and atrial natriuretic peptide secretion. Prog Biophys Mol Biol. 2008;98:1–9. doi: 10.1016/j.pbiomolbio.2008.05.005. [DOI] [PubMed] [Google Scholar]
- 35.Clerico A, Giannoni A, Vittorini S, Passino C. Thirty years of the heart as an endocrine organ: physiological role and clinical utility of cardiac natriuretic hormones. Am J Physiol Heart Circ Physiol. 2011;301:H12–H20. doi: 10.1152/ajpheart.00226.2011. [DOI] [PubMed] [Google Scholar]
- 36.Burnett JC, Jr, Kao PC, Hu DC, Heser DW, Heublein D, Granger JP, Opgenorth TJ, Reeder GS. Atrial natriuretic peptide elevation in congestive heart failure in the human. Science. 1986;231:1145–1147. doi: 10.1126/science.2935937. [DOI] [PubMed] [Google Scholar]
- 37.Nagaya N, Nishikimi T, Goto Y, Miyao Y, Kobayashi Y, Morii I, Daikoku S, Matsumoto T, Miyazaki S, Matsuoka H, Takishita S, Kangawa K, Matsuo H, Nonogi H. Plasma brain natriuretic peptide is a biochemical marker for the prediction of progressive ventricular remodeling after acute myocardial infarction. Am Heart J. 1998;135:21–28. doi: 10.1016/S0002-8703(98)70338-2. [DOI] [PubMed] [Google Scholar]
- 38.Nishikimi T, Yoshihara F, Morimoto A, Ishikawa K, Ishimitsu T, Saito Y, Kangawa K, Matsuo H, Omae T, Matsuoka H. Relationship between left ventricular geometry and natriuretic peptide levels in essential hypertension. Hypertension. 1996;28:22–30. doi: 10.1161/01.HYP.28.1.22. [DOI] [PubMed] [Google Scholar]
- 39.Chun YS, Hyun JY, Kwak YG, Kim IS, Kim CH, Choi E, Kim MS, Park JW. Hypoxic activation of the atrial natriuretic peptide gene promoter through direct and indirect actions of hypoxia-inducible factor-1. Biochem J. 2003;370:149–157. doi: 10.1042/bj20021087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Teshima Y, Akao M, Jones SP, Marban E. Cariporide (HOE642), a selective Na+–H+ exchange inhibitor, inhibits the mitochondrial death pathway. Circulation. 2003;108:2275–2281. doi: 10.1161/01.CIR.0000093277.20968.C7. [DOI] [PubMed] [Google Scholar]
- 41.Chen YF. Atrial natriuretic peptide in hypoxia. Peptides. 2005;26:1068–1077. doi: 10.1016/j.peptides.2004.08.030. [DOI] [PubMed] [Google Scholar]
- 42.Schiebinger RJ, Joseph CM, Li Y, Cragoe EJ., Jr Mechanism of hyperosmolality stimulation of ANP secretion: its dependency on calcium and sodium. Am J Physiol. 1995;268:E476–E483. doi: 10.1152/ajpendo.1995.268.3.E476. [DOI] [PubMed] [Google Scholar]
- 43.Nakamura S, Naruse M, Naruse K, Kawana M, Nishikawa T, Hosoda S, Tanaka I, Yoshimi T, Yoshihara I, Inagami T. Atrial natriuretic peptide and brain natriuretic peptide coexist in the secretory granules of human cardiac myocytes. Am J Hypertens. 1991;4:909–912. doi: 10.1093/ajh/4.11.909. [DOI] [PubMed] [Google Scholar]
- 44.Bialik GM, Abassi ZA, Hammel I, Winaver J, Lewinson D. Evaluation of atrial natriuretic peptide and brain natriuretic peptide in atrial granules of rats with experimental congestive heart failure. J Histochem Cytochem. 2001;49:1293–1300. doi: 10.1177/002215540104901012. [DOI] [PubMed] [Google Scholar]
- 45.Mukoyama M, Nakao K, Saito Y, Ogawa Y, Hosoda K, Suga S, Shirakami G, Jougasaki M, Imura H. Increased human brain natriuretic peptide in congestive heart failure. N Engl J Med. 1990;323:757–758. doi: 10.1056/NEJM199009133231114. [DOI] [PubMed] [Google Scholar]
- 46.Ma KK, Ogawa T, de Bold AJ. Selective upregulation of cardiac brain natriuretic peptide at the transcriptional and translational levels by pro-inflammatory cytokines and by conditioned medium derived from mixed lymphocyte reactions via p38 MAP kinase. J Mol Cell Cardiol. 2004;36:505–513. doi: 10.1016/j.yjmcc.2004.01.001. [DOI] [PubMed] [Google Scholar]
- 47.Belmont PJ, Tadimalla A, Chen WJ, Martindale JJ, Thuerauf DJ, Marcinko M, Gude N, Sussman MA, Glembotski CC. Coordination of growth and endoplasmic reticulum stress signaling by regulator of calcineurin 1 (RCAN1), a novel ATF6-inducible gene. J Biol Chem. 2008;283:14012–14021. doi: 10.1074/jbc.M709776200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ron D, Walter P. Signal integration in the endoplasmic reticulum unfolded protein response. Nat Rev Mol Cell Biol. 2007;8:519–529. doi: 10.1038/nrm2199. [DOI] [PubMed] [Google Scholar]
- 49.Thuerauf DJ, Marcinko M, Gude N, Rubio M, Sussman MA, Glembotski CC. Activation of the unfolded protein response in infarcted mouse heart and hypoxic cultured cardiac myocytes. Circ Res. 2006;99:275–282. doi: 10.1161/01.RES.0000233317.70421.03. [DOI] [PubMed] [Google Scholar]
- 50.Sawada T, Minamino T, Fu HY, Asai M, Okuda K, Isomura T, Yamazaki S, Asano Y, Okada K, Tsukamoto O, Sanada S, Asanuma H, Asakura M, Takashima S, Kitakaze M, Komuro I. X-box binding protein 1 regulates brain natriuretic peptide through a novel AP1/CRE-like element in cardiomyocytes. J Mol Cell Cardiol. 2010;48:1280–1289. doi: 10.1016/j.yjmcc.2010.02.004. [DOI] [PubMed] [Google Scholar]
- 51.Horio T, Nishikimi T, Yoshihara F, Matsuo H, Takishita S, Kangawa K. Inhibitory regulation of hypertrophy by endogenous atrial natriuretic peptide in cultured cardiac myocytes. Hypertension. 2000;35:19–24. doi: 10.1161/01.HYP.35.1.19. [DOI] [PubMed] [Google Scholar]
- 52.Oliver PM, Fox JE, Kim R, Rockman HA, Kim HS, Reddick RL, Pandey KN, Milgram SL, Smithies O, Maeda N. Hypertension, cardiac hypertrophy, and sudden death in mice lacking natriuretic peptide receptor A. Proc Natl Acad Sci USA. 1997;94:14730–14735. doi: 10.1073/pnas.94.26.14730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Holtwick R, van Eickels M, Skryabin BV, Baba HA, Bubikat A, Begrow F, Schneider MD, Garbers DL, Kuhn M. Pressure-independent cardiac hypertrophy in mice with cardiomyocyte-restricted inactivation of the atrial natriuretic peptide receptor guanylyl cyclase-A. J Clin Investig. 2003;111:1399–1407. doi: 10.1172/JCI17061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Vellaichamy E, Das S, Subramanian U, Maeda N, Pandey KN. Genetically altered mutant mouse models of guanylyl cyclase/natriuretic peptide receptor-A exhibit the cardiac expression of proinflammatory mediators in a gene-dose-dependent manner. Endocrinology. 2014;155:1045–1056. doi: 10.1210/en.2013-1416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.O’Tierney PF, Chattergoon NN, Louey S, Giraud GD, Thornburg KL. Atrial natriuretic peptide inhibits angiotensin II-stimulated proliferation in fetal cardiomyocytes. J Physiol. 2010;588:2879–2889. doi: 10.1113/jphysiol.2010.191098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Becker JR, Chatterjee S, Robinson TY, Bennett JS, Panakova D, Galindo CL, Zhong L, Shin JT, Coy SM, Kelly AE, Roden DM, Lim CC, MacRae CA. Differential activation of natriuretic peptide receptors modulates cardiomyocyte proliferation during development. Development. 2014;141:335–345. doi: 10.1242/dev.100370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kapoun AM, Liang F, O’Young G, Damm DL, Quon D, White RT, Munson K, Lam A, Schreiner GF, Protter AA. B-type natriuretic peptide exerts broad functional opposition to transforming growth factor-beta in primary human cardiac fibroblasts: fibrosis, myofibroblast conversion, proliferation, and inflammation. Circ Res. 2004;94:453–461. doi: 10.1161/01.RES.0000117070.86556.9F. [DOI] [PubMed] [Google Scholar]
- 58.Redondo J, Bishop JE, Wilkins MR. Effect of atrial natriuretic peptide and cyclic GMP phosphodiesterase inhibition on collagen synthesis by adult cardiac fibroblasts. Br J Pharmacol. 1998;124:1455–1462. doi: 10.1038/sj.bjp.0701994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Maki T, Horio T, Yoshihara F, Suga S, Takeo S, Matsuo H, Kangawa K. Effect of neutral endopeptidase inhibitor on endogenous atrial natriuretic peptide as a paracrine factor in cultured cardiac fibroblasts. Br J Pharmacol. 2000;131:1204–1210. doi: 10.1038/sj.bjp.0703679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Tamura N, Ogawa Y, Chusho H, Nakamura K, Nakao K, Suda M, Kasahara M, Hashimoto R, Katsuura G, Mukoyama M, Itoh H, Saito Y, Tanaka I, Otani H, Katsuki M. Cardiac fibrosis in mice lacking brain natriuretic peptide. Proc Natl Acad Sci USA. 2000;97:4239–4244. doi: 10.1073/pnas.070371497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Vellaichamy E, Khurana ML, Fink J, Pandey KN. Involvement of the NF-kappa B/matrix metalloproteinase pathway in cardiac fibrosis of mice lacking guanylyl cyclase/natriuretic peptide receptor A. J Biol Chem. 2005;280:19230–19242. doi: 10.1074/jbc.M411373200. [DOI] [PubMed] [Google Scholar]
- 62.Holtwick R, Gotthardt M, Skryabin B, Steinmetz M, Potthast R, Zetsche B, Hammer RE, Herz J, Kuhn M. Smooth muscle-selective deletion of guanylyl cyclase-A prevents the acute but not chronic effects of ANP on blood pressure. Proc Natl Acad Sci USA. 2002;99:7142–7147. doi: 10.1073/pnas.102650499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Sabrane K, Kruse MN, Fabritz L, Zetsche B, Mitko D, Skryabin BV, Zwiener M, Baba HA, Yanagisawa M, Kuhn M. Vascular endothelium is critically involved in the hypotensive and hypovolemic actions of atrial natriuretic peptide. J Clin Investig. 2005;115:1666–1674. doi: 10.1172/JCI23360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Burnett JC, Jr, Granger JP, Opgenorth TJ. Effects of synthetic atrial natriuretic factor on renal function and renin release. Am J Physiol. 1984;247:F863–F866. doi: 10.1152/ajprenal.1984.247.5.F863. [DOI] [PubMed] [Google Scholar]
- 65.Marin-Grez M, Fleming JT, Steinhausen M. Atrial natriuretic peptide causes pre-glomerular vasodilatation and post-glomerular vasoconstriction in rat kidney. Nature. 1986;324:473–476. doi: 10.1038/324473a0. [DOI] [PubMed] [Google Scholar]
- 66.Sonnenberg H, Honrath U, Chong CK, Wilson DR. Atrial natriuretic factor inhibits sodium transport in medullary collecting duct. Am J Physiol. 1986;250:F963–F966. doi: 10.1152/ajprenal.1986.250.6.F963. [DOI] [PubMed] [Google Scholar]
- 67.Zeidel ML, Kikeri D, Silva P, Burrowes M, Brenner BM. Atrial natriuretic peptides inhibit conductive sodium uptake by rabbit inner medullary collecting duct cells. J Clin Investig. 1988;82:1067–1074. doi: 10.1172/JCI113663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Sarzani R, Marcucci P, Salvi F, Bordicchia M, Espinosa E, Mucci L, Lorenzetti B, Minardi D, Muzzonigro G, Dessi-Fulgheri P, Rappelli A. Angiotensin II stimulates and atrial natriuretic peptide inhibits human visceral adipocyte growth. Int J Obes (Lond) 2008;32:259–267. doi: 10.1038/sj.ijo.0803724. [DOI] [PubMed] [Google Scholar]
- 69.Sengenes C, Berlan M, De Glisezinski I, Lafontan M, Galitzky J. Natriuretic peptides: a new lipolytic pathway in human adipocytes. FASEB J. 2000;14:1345–1351. doi: 10.1096/fasebj.14.10.1345. [DOI] [PubMed] [Google Scholar]
- 70.Sengenes C, Bouloumie A, Hauner H, Berlan M, Busse R, Lafontan M, Galitzky J. Involvement of a cGMP-dependent pathway in the natriuretic peptide-mediated hormone-sensitive lipase phosphorylation in human adipocytes. J Biol Chem. 2003;278:48617–48626. doi: 10.1074/jbc.M303713200. [DOI] [PubMed] [Google Scholar]
- 71.Birkenfeld AL, Boschmann M, Moro C, Adams F, Heusser K, Tank J, Diedrich A, Schroeder C, Franke G, Berlan M, Luft FC, Lafontan M, Jordan J. Beta-adrenergic and atrial natriuretic peptide interactions on human cardiovascular and metabolic regulation. J Clin Endocrinol Metab. 2006;91:5069–5075. doi: 10.1210/jc.2006-1084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Moro C, Pasarica M, Elkind-Hirsch K, Redman LM. Aerobic exercise training improves atrial natriuretic peptide and catecholamine-mediated lipolysis in obese women with polycystic ovary syndrome. J Clin Endocrinol Metab. 2009;94:2579–2586. doi: 10.1210/jc.2009-0051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Moro C, Pillard F, De Glisezinski I, Harant I, Riviere D, Stich V, Lafontan M, Crampes F, Berlan M. Training enhances ANP lipid-mobilizing action in adipose tissue of overweight men. Med Sci Sports Exerc. 2005;37:1126–1132. doi: 10.1249/01.mss.0000170124.51659.52. [DOI] [PubMed] [Google Scholar]
- 74.Bordicchia M, Liu D, Amri EZ, Ailhaud G, Dessi-Fulgheri P, Zhang C, Takahashi N, Sarzani R, Collins S. Cardiac natriuretic peptides act via p38 MAPK to induce the brown fat thermogenic program in mouse and human adipocytes. J Clin Investig. 2012;122:1022–1036. doi: 10.1172/JCI59701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Lionetti V, Stanley WC, Recchia FA. Modulating fatty acid oxidation in heart failure. Cardiovasc Res. 2011;90:202–209. doi: 10.1093/cvr/cvr038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Sarzani R, Salvi F, Dessi-Fulgheri P, Rappelli A. Renin–angiotensin system, natriuretic peptides, obesity, metabolic syndrome, and hypertension: an integrated view in humans. J Hypertens. 2008;26:831–843. doi: 10.1097/HJH.0b013e3282f624a0. [DOI] [PubMed] [Google Scholar]
- 77.Miyashita K, Itoh H, Tsujimoto H, Tamura N, Fukunaga Y, Sone M, Yamahara K, Taura D, Inuzuka M, Sonoyama T, Nakao K. Natriuretic peptides/cGMP/cGMP-dependent protein kinase cascades promote muscle mitochondrial biogenesis and prevent obesity. Diabetes. 2009;58:2880–2892. doi: 10.2337/db09-0393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Engeli S, Birkenfeld AL, Badin PM, Bourlier V, Louche K, Viguerie N, Thalamas C, Montastier E, Larrouy D, Harant I, de Glisezinski I, Lieske S, Reinke J, Beckmann B, Langin D, Jordan J, Moro C. Natriuretic peptides enhance the oxidative capacity of human skeletal muscle. J Clin Investig. 2012;122:4675–4679. doi: 10.1172/JCI64526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Date Y, Kojima M, Hosoda H, Sawaguchi A, Mondal MS, Suganuma T, Matsukura S, Kangawa K, Nakazato M. Ghrelin, a novel growth hormone-releasing acylated peptide, is synthesized in a distinct endocrine cell type in the gastrointestinal tracts of rats and humans. Endocrinology. 2000;141:4255–4261. doi: 10.1210/endo.141.11.7757. [DOI] [PubMed] [Google Scholar]
- 80.Kojima M, Hosoda H, Date Y, Nakazato M, Matsuo H, Kangawa K. Ghrelin is a growth-hormone-releasing acylated peptide from stomach. Nature. 1999;402:656–660. doi: 10.1038/45230. [DOI] [PubMed] [Google Scholar]
- 81.Tschop M, Smiley DL, Heiman ML. Ghrelin induces adiposity in rodents. Nature. 2000;407:908–913. doi: 10.1038/35038090. [DOI] [PubMed] [Google Scholar]
- 82.Shimada M, Date Y, Mondal MS, Toshinai K, Shimbara T, Fukunaga K, Murakami N, Miyazato M, Kangawa K, Yoshimatsu H, Matsuo H, Nakazato M. Somatostatin suppresses ghrelin secretion from the rat stomach. Biochem Biophys Res Commun. 2003;302:520–525. doi: 10.1016/S0006-291X(03)00178-5. [DOI] [PubMed] [Google Scholar]
- 83.Gower WR, Jr, McCuen RW, Arimura A, Coy DA, Dietz JR, Landon CS, Schubert ML. Reciprocal paracrine pathways link atrial natriuretic peptide and somatostatin secretion in the antrum of the stomach. Regul Pept. 2003;110:101–106. doi: 10.1016/S0167-0115(02)00206-9. [DOI] [PubMed] [Google Scholar]
- 84.Vila G, Grimm G, Resl M, Heinisch B, Einwallner E, Esterbauer H, Dieplinger B, Mueller T, Luger A, Clodi M. B-type natriuretic peptide modulates ghrelin, hunger, and satiety in healthy men. Diabetes. 2012;61:2592–2596. doi: 10.2337/db11-1466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Murray PJ, Wynn TA. Protective and pathogenic functions of macrophage subsets. Nat Rev Immunol. 2011;11:723–737. doi: 10.1038/nri3073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Kiemer AK, Vollmar AM. Autocrine regulation of inducible nitric-oxide synthase in macrophages by atrial natriuretic peptide. J Biol Chem. 1998;273:13444–13451. doi: 10.1074/jbc.273.22.13444. [DOI] [PubMed] [Google Scholar]
- 87.Moro C, Klimcakova E, Lolmede K, Berlan M, Lafontan M, Stich V, Bouloumie A, Galitzky J, Arner P, Langin D. Atrial natriuretic peptide inhibits the production of adipokines and cytokines linked to inflammation and insulin resistance in human subcutaneous adipose tissue. Diabetologia. 2007;50:1038–1047. doi: 10.1007/s00125-007-0614-3. [DOI] [PubMed] [Google Scholar]
- 88.Li N, Jin HX, Song Z, Bai CZ, Cui Y, Gao Y. Protective effect of recombinant human brain natriuretic peptide on acute renal injury induced by endotoxin in canines. Cell Biochem Biophys. 2014;70:1317–1324. doi: 10.1007/s12013-014-0057-7. [DOI] [PubMed] [Google Scholar]
- 89.Song Z, Cui Y, Ding MZ, Jin HX, Gao Y. Protective effects of recombinant human brain natriuretic peptide against LPS-induced acute lung injury in dogs. Int Immunopharmacol. 2013;17:508–512. doi: 10.1016/j.intimp.2013.05.028. [DOI] [PubMed] [Google Scholar]
- 90.Song Z, Zhao X, Liu M, Jin H, Wang L, Hou M, Gao Y. Recombinant human brain natriuretic peptide attenuates trauma-/haemorrhagic shock-induced acute lung injury through inhibiting oxidative stress and the NF-kappaB-dependent inflammatory/MMP-9 pathway. Int J Exp Pathol. 2015;96:406–413. doi: 10.1111/iep.12160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Yang H, Song Z, Jin H, Cui Y, Hou M, Gao Y. Protective effect of rhBNP on intestinal injury in the canine models of sepsis. Int Immunopharmacol. 2014;19:262–266. doi: 10.1016/j.intimp.2014.01.023. [DOI] [PubMed] [Google Scholar]
- 92.Lambeau G, Gelb MH. Biochemistry and physiology of mammalian secreted phospholipases A2. Annu Rev Biochem. 2008;77:495–520. doi: 10.1146/annurev.biochem.76.062405.154007. [DOI] [PubMed] [Google Scholar]
- 93.Murakami M, Taketomi Y. Secreted phospholipase A2 and mast cells. Allergol Int. 2015;64:4–10. doi: 10.1016/j.alit.2014.07.005. [DOI] [PubMed] [Google Scholar]
- 94.Murakami M, Koduri RS, Enomoto A, Shimbara S, Seki M, Yoshihara K, Singer A, Valentin E, Ghomashchi F, Lambeau G, Gelb MH, Kudo I. Distinct arachidonate-releasing functions of mammalian secreted phospholipase A2 s in human embryonic kidney 293 and rat mastocytoma RBL-2H3 cells through heparan sulfate shuttling and external plasma membrane mechanisms. J Biol Chem. 2001;276:10083–10096. doi: 10.1074/jbc.M007877200. [DOI] [PubMed] [Google Scholar]
- 95.Berry E, Hernandez-Anzaldo S, Ghomashchi F, Lehner R, Murakami M, Gelb MH, Kassiri Z, Wang X, Fernandez-Patron C. Matrix metalloproteinase-2 negatively regulates cardiac secreted phospholipase A2 to modulate inflammation and fever. J Am Heart Assoc. 2015;4:e001868. doi: 10.1161/JAHA.115.001868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Hernandez-Anzaldo S, Berry E, Brglez V, Leung D, Yun TJ, Lee JS, Filep JG, Kassiri Z, Cheong C, Lambeau G, Lehner R, Fernandez-Patron C. Identification of a novel heart-liver axis: matrix metalloproteinase-2 negatively regulates cardiac secreted phospholipase A2 to modulate lipid metabolism and inflammation in the liver. J Am Heart Assoc. 2015;4:e002553. doi: 10.1161/JAHA.115.002553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Shibanuma M, Mashimo J, Mita A, Kuroki T, Nose K. Cloning from a mouse osteoblastic cell line of a set of transforming-growth-factor-beta 1-regulated genes, one of which seems to encode a follistatin-related polypeptide. Eur J Biochem. 1993;217:13–19. doi: 10.1111/j.1432-1033.1993.tb18212.x. [DOI] [PubMed] [Google Scholar]
- 98.Tanaka M, Murakami K, Ozaki S, Imura Y, Tong XP, Watanabe T, Sawaki T, Kawanami T, Kawabata D, Fujii T, Usui T, Masaki Y, Fukushima T, Jin ZX, Umehara H, Mimori T. DIP2 disco-interacting protein 2 homolog A (Drosophila) is a candidate receptor for follistatin-related protein/follistatin-like 1—analysis of their binding with TGF-beta superfamily proteins. FEBS J. 2010;277:4278–4289. doi: 10.1111/j.1742-4658.2010.07816.x. [DOI] [PubMed] [Google Scholar]
- 99.Ouchi N, Asaumi Y, Ohashi K, Higuchi A, Sono-Romanelli S, Oshima Y, Walsh K. DIP2A functions as a FSTL1 receptor. J Biol Chem. 2010;285:7127–7134. doi: 10.1074/jbc.M109.069468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Ogura Y, Ouchi N, Ohashi K, Shibata R, Kataoka Y, Kambara T, Kito T, Maruyama S, Yuasa D, Matsuo K, Enomoto T, Uemura Y, Miyabe M, Ishii M, Yamamoto T, Shimizu Y, Walsh K, Murohara T. Therapeutic impact of follistatin-like 1 on myocardial ischemic injury in preclinical models. Circulation. 2012;126:1728–1738. doi: 10.1161/CIRCULATIONAHA.112.115089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Oshima Y, Ouchi N, Sato K, Izumiya Y, Pimentel DR, Walsh K. Follistatin-like 1 is an Akt-regulated cardioprotective factor that is secreted by the heart. Circulation. 2008;117:3099–3108. doi: 10.1161/CIRCULATIONAHA.108.767673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Adams D, Larman B, Oxburgh L. Developmental expression of mouse Follistatin-like 1 (Fstl1): dynamic regulation during organogenesis of the kidney and lung. Gene Expr Patterns. 2007;7:491–500. doi: 10.1016/j.modgep.2006.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Wei K, Serpooshan V, Hurtado C, Diez-Cunado M, Zhao M, Maruyama S, Zhu W, Fajardo G, Noseda M, Nakamura K, Tian X, Liu Q, Wang A, Matsuura Y, Bushway P, Cai W, Savchenko A, Mahmoudi M, Schneider MD, van den Hoff MJ, Butte MJ, Yang PC, Walsh K, Zhou B, Bernstein D, Mercola M, Ruiz-Lozano P. Epicardial FSTL1 reconstitution regenerates the adult mammalian heart. Nature. 2015;525:479–485. doi: 10.1038/nature15372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Shimano M, Ouchi N, Nakamura K, van Wijk B, Ohashi K, Asaumi Y, Higuchi A, Pimentel DR, Sam F, Murohara T, van den Hoff MJ, Walsh K. Cardiac myocyte follistatin-like 1 functions to attenuate hypertrophy following pressure overload. Proc Natl Acad Sci USA. 2011;108:E899–E906. doi: 10.1073/pnas.1108559108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.El-Armouche A, Ouchi N, Tanaka K, Doros G, Wittkopper K, Schulze T, Eschenhagen T, Walsh K, Sam F. Follistatin-like 1 in chronic systolic heart failure: a marker of left ventricular remodeling. Circ Heart Fail. 2011;4:621–627. doi: 10.1161/CIRCHEARTFAILURE.110.960625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Hayakawa S, Ohashi K, Shibata R, Kataoka Y, Miyabe M, Enomoto T, Joki Y, Shimizu Y, Kambara T, Uemura Y, Yuasa D, Ogawa H, Matsuo K, Hiramatsu-Ito M, van den Hoff MJ, Walsh K, Murohara T, Ouchi N. Cardiac myocyte-derived follistatin-like 1 prevents renal injury in a subtotal nephrectomy model. J Am Soc Nephrol. 2015;26:636–646. doi: 10.1681/ASN.2014020210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Barton M, Yanagisawa M. Endothelin: 20 years from discovery to therapy. Can J Physiol Pharmacol. 2008;86:485–498. doi: 10.1139/Y08-059. [DOI] [PubMed] [Google Scholar]
- 108.Yanagisawa M, Kurihara H, Kimura S, Goto K, Masaki T. A novel peptide vasoconstrictor, endothelin, is produced by vascular endothelium and modulates smooth muscle Ca2+ channels. J Hypertens Suppl. 1988;6:S188–S191. doi: 10.1097/00004872-198812040-00056. [DOI] [PubMed] [Google Scholar]
- 109.Inoue A, Yanagisawa M, Kimura S, Kasuya Y, Miyauchi T, Goto K, Masaki T. The human endothelin family: three structurally and pharmacologically distinct isopeptides predicted by three separate genes. Proc Natl Acad Sci USA. 1989;86:2863–2867. doi: 10.1073/pnas.86.8.2863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Arai H, Hori S, Aramori I, Ohkubo H, Nakanishi S. Cloning and expression of a cDNA encoding an endothelin receptor. Nature. 1990;348:730–732. doi: 10.1038/348730a0. [DOI] [PubMed] [Google Scholar]
- 111.Sakurai T, Yanagisawa M, Takuwa Y, Miyazaki H, Kimura S, Goto K, Masaki T. Cloning of a cDNA encoding a non-isopeptide-selective subtype of the endothelin receptor. Nature. 1990;348:732–735. doi: 10.1038/348732a0. [DOI] [PubMed] [Google Scholar]
- 112.Barst RJ. A review of pulmonary arterial hypertension: role of ambrisentan. Vasc Health Risk Manag. 2007;3:11–22. [PMC free article] [PubMed] [Google Scholar]
- 113.Masaki T. The discovery of endothelins. Cardiovasc Res. 1998;39:530–533. doi: 10.1016/S0008-6363(98)00153-9. [DOI] [PubMed] [Google Scholar]
- 114.Russell FD, Skepper JN, Davenport AP. Evidence using immunoelectron microscopy for regulated and constitutive pathways in the transport and release of endothelin. J Cardiovasc Pharmacol. 1998;31:424–430. doi: 10.1097/00005344-199803000-00014. [DOI] [PubMed] [Google Scholar]
- 115.Davenport AP, Hyndman KA, Dhaun N, Southan C, Kohan DE, Pollock JS, Pollock DM, Webb DJ, Maguire JJ. Endothelin. Pharmacol Rev. 2016;68:357–418. doi: 10.1124/pr.115.011833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Suzuki T, Kumazaki T, Mitsui Y. Endothelin-1 is produced and secreted by neonatal rat cardiac myocytes in vitro. Biochem Biophys Res Commun. 1993;191:823–830. doi: 10.1006/bbrc.1993.1291. [DOI] [PubMed] [Google Scholar]
- 117.Kagamu H, Suzuki T, Arakawa M, Mitsui Y. Low oxygen enhances endothelin-1 (ET-1) production and responsiveness to ET-1 in cultured cardiac myocytes. Biochem Biophys Res Commun. 1994;202:1612–1618. doi: 10.1006/bbrc.1994.2117. [DOI] [PubMed] [Google Scholar]
- 118.Yamazaki T, Komuro I, Kudoh S, Zou Y, Shiojima I, Hiroi Y, Mizuno T, Maemura K, Kurihara H, Aikawa R, Takano H, Yazaki Y. Endothelin-1 is involved in mechanical stress-induced cardiomyocyte hypertrophy. J Biol Chem. 1996;271:3221–3228. doi: 10.1074/jbc.271.6.3221. [DOI] [PubMed] [Google Scholar]
- 119.Serneri GG, Cecioni I, Vanni S, Paniccia R, Bandinelli B, Vetere A, Janming X, Bertolozzi I, Boddi M, Lisi GF, Sani G, Modesti PA. Selective upregulation of cardiac endothelin system in patients with ischemic but not idiopathic dilated cardiomyopathy: endothelin-1 system in the human failing heart. Circ Res. 2000;86:377–385. doi: 10.1161/01.RES.86.4.377. [DOI] [PubMed] [Google Scholar]
- 120.Shohet RV, Kisanuki YY, Zhao XS, Siddiquee Z, Franco F, Yanagisawa M. Mice with cardiomyocyte-specific disruption of the endothelin-1 gene are resistant to hyperthyroid cardiac hypertrophy. Proc Natl Acad Sci USA. 2004;101:2088–2093. doi: 10.1073/pnas.0307159101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Zhao XS, Pan W, Bekeredjian R, Shohet RV. Endogenous endothelin-1 is required for cardiomyocyte survival in vivo. Circulation. 2006;114:830–837. doi: 10.1161/CIRCULATIONAHA.105.577288. [DOI] [PubMed] [Google Scholar]
- 122.Paradis A, Zhang L. Role of endothelin in uteroplacental circulation and fetal vascular function. Curr Vasc Pharmacol. 2013;11:594–605. doi: 10.2174/1570161111311050004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Helle KB, Corti A, Metz-Boutigue MH, Tota B. The endocrine role for chromogranin A: a prohormone for peptides with regulatory properties. Cell Mol Life Sci. 2007;64:2863–2886. doi: 10.1007/s00018-007-7254-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Banks P, Helle K. The release of protein from the stimulated adrenal medulla. Biochem J. 1965;97:40C–41C. doi: 10.1042/bj0970040C. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Blaschko H, Comline RS, Schneider FH, Silver M, Smith AD. Secretion of a chromaffin granule protein, chromogranin, from the adrenal gland after splanchnic stimulation. Nature. 1967;215:58–59. doi: 10.1038/215058a0. [DOI] [PubMed] [Google Scholar]
- 126.Pieroni M, Corti A, Tota B, Curnis F, Angelone T, Colombo B, Cerra MC, Bellocci F, Crea F, Maseri A. Myocardial production of chromogranin A in human heart: a new regulatory peptide of cardiac function. Eur Heart J. 2007;28:1117–1127. doi: 10.1093/eurheartj/ehm022. [DOI] [PubMed] [Google Scholar]
- 127.Tota B, Cerra MC, Gattuso A. Catecholamines, cardiac natriuretic peptides and chromogranin A: evolution and physiopathology of a ‘whip-brake’ system of the endocrine heart. J Exp Biol. 2010;213:3081–3103. doi: 10.1242/jeb.027391. [DOI] [PubMed] [Google Scholar]
- 128.Ceconi C, Ferrari R, Bachetti T, Opasich C, Volterrani M, Colombo B, Parrinello G, Corti A. Chromogranin A in heart failure; a novel neurohumoral factor and a predictor for mortality. Eur Heart J. 2002;23:967–974. doi: 10.1053/euhj.2001.2977. [DOI] [PubMed] [Google Scholar]
- 129.Takiyyuddin MA, Cervenka JH, Hsiao RJ, Barbosa JA, Parmer RJ, O’Connor DT. Chromogranin A. Storage and release in hypertension. Hypertension. 1990;15:237–246. doi: 10.1161/01.HYP.15.3.237. [DOI] [PubMed] [Google Scholar]
- 130.Glattard E, Angelone T, Strub JM, Corti A, Aunis D, Tota B, Metz-Boutigue MH, Goumon Y. Characterization of natural vasostatin-containing peptides in rat heart. FEBS J. 2006;273:3311–3321. doi: 10.1111/j.1742-4658.2006.05334.x. [DOI] [PubMed] [Google Scholar]
- 131.Ramella R, Boero O, Alloatti G, Angelone T, Levi R, Gallo MP. Vasostatin 1 activates eNOS in endothelial cells through a proteoglycan-dependent mechanism. J Cell Biochem. 2010;110:70–79. doi: 10.1002/jcb.22510. [DOI] [PubMed] [Google Scholar]
- 132.Cerra MC, De IL, Angelone T, Corti A, Tota B. Recombinant N-terminal fragments of chromogranin-A modulate cardiac function of the Langendorff-perfused rat heart. Basic Res Cardiol. 2006;101:43–52. doi: 10.1007/s00395-005-0547-2. [DOI] [PubMed] [Google Scholar]
- 133.Wang D, Shan Y, Huang Y, Tang Y, Chen Y, Li R, Yang J, Huang C. Vasostatin-1 stops structural remodeling and improves calcium handling via the eNOS-NO-PKG pathway in rat hearts subjected to chronic beta-adrenergic receptor activation. Cardiovasc Drugs Ther. 2016;30:455–464. doi: 10.1007/s10557-016-6687-9. [DOI] [PubMed] [Google Scholar]
- 134.Gallo MP, Levi R, Ramella R, Brero A, Boero O, Tota B, Alloatti G. Endothelium-derived nitric oxide mediates the antiadrenergic effect of human vasostatin-1 in rat ventricular myocardium. Am J Physiol Heart Circ Physiol. 2007;292:H2906–H2912. doi: 10.1152/ajpheart.01253.2006. [DOI] [PubMed] [Google Scholar]
- 135.Aardal S, Helle KB. The vasoinhibitory activity of bovine chromogranin A fragment (vasostatin) and its independence of extracellular calcium in isolated segments of human blood vessels. Regul Pept. 1992;41:9–18. doi: 10.1016/0167-0115(92)90509-S. [DOI] [PubMed] [Google Scholar]
- 136.Pike SE, Yao L, Jones KD, Cherney B, Appella E, Sakaguchi K, Nakhasi H, Teruya-Feldstein J, Wirth P, Gupta G, Tosato G. Vasostatin, a calreticulin fragment, inhibits angiogenesis and suppresses tumor growth. J Exp Med. 1998;188:2349–2356. doi: 10.1084/jem.188.12.2349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Biswas N, Curello E, O’Connor DT, Mahata SK. Chromogranin/secretogranin proteins in murine heart: myocardial production of chromogranin A fragment catestatin (Chga(364–384)) Cell Tissue Res. 2010;342:353–361. doi: 10.1007/s00441-010-1059-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Mahata SK, O’Connor DT, Mahata M, Yoo SH, Taupenot L, Wu H, Gill BM, Parmer RJ. Novel autocrine feedback control of catecholamine release. A discrete chromogranin a fragment is a noncompetitive nicotinic cholinergic antagonist. J Clin Investig. 1997;100:1623–1633. doi: 10.1172/JCI119686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.O’Connor DT, Kailasam MT, Kennedy BP, Ziegler MG, Yanaihara N, Parmer RJ. Early decline in the catecholamine release-inhibitory peptide catestatin in humans at genetic risk of hypertension. J Hypertens. 2002;20:1335–1345. doi: 10.1097/00004872-200207000-00020. [DOI] [PubMed] [Google Scholar]
- 140.Ottesen AH, Carlson CR, Louch WE, Dahl MB, Sandbu RA, Johansen RF, Jarstadmarken H, Bjoras M, Hoiseth AD, Brynildsen J, Sjaastad I, Stridsberg M, Omland T, Christensen G, Rosjo H. Glycosylated chromogranin A in heart failure: implications for processing and cardiomyocyte calcium homeostasis. Circ Heart Fail. 2017;10:e003675. doi: 10.1161/CIRCHEARTFAILURE.116.003675. [DOI] [PubMed] [Google Scholar]
- 141.Angelone T, Quintieri AM, Brar BK, Limchaiyawat PT, Tota B, Mahata SK, Cerra MC. The antihypertensive chromogranin a peptide catestatin acts as a novel endocrine/paracrine modulator of cardiac inotropism and lusitropism. Endocrinology. 2008;149:4780–4793. doi: 10.1210/en.2008-0318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Kennedy BP, Mahata SK, O’Connor DT, Ziegler MG. Mechanism of cardiovascular actions of the chromogranin A fragment catestatin in vivo. Peptides. 1998;19:1241–1248. doi: 10.1016/S0196-9781(98)00086-2. [DOI] [PubMed] [Google Scholar]
- 143.Mahapatra NR, O’Connor DT, Vaingankar SM, Hikim AP, Mahata M, Ray S, Staite E, Wu H, Gu Y, Dalton N, Kennedy BP, Ziegler MG, Ross J, Mahata SK. Hypertension from targeted ablation of chromogranin A can be rescued by the human ortholog. J Clin Investig. 2005;115:1942–1952. doi: 10.1172/JCI24354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Gayen JR, Gu Y, O’Connor DT, Mahata SK. Global disturbances in autonomic function yield cardiovascular instability and hypertension in the chromogranin a null mouse. Endocrinology. 2009;150:5027–5035. doi: 10.1210/en.2009-0429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Itoh N, Ohta H. Pathophysiological roles of FGF signaling in the heart. Front Physiol. 2013;4:247. doi: 10.3389/fphys.2013.00247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Zhang X, Ibrahimi OA, Olsen SK, Umemori H, Mohammadi M, Ornitz DM. Receptor specificity of the fibroblast growth factor family. The complete mammalian FGF family. J Biol Chem. 2006;281:15694–15700. doi: 10.1074/jbc.M601252200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Goetz R, Beenken A, Ibrahimi OA, Kalinina J, Olsen SK, Eliseenkova AV, Xu C, Neubert TA, Zhang F, Linhardt RJ, Yu X, White KE, Inagaki T, Kliewer SA, Yamamoto M, Kurosu H, Ogawa Y, Kuro-o M, Lanske B, Razzaque MS, Mohammadi M. Molecular insights into the klotho-dependent, endocrine mode of action of fibroblast growth factor 19 subfamily members. Mol Cell Biol. 2007;27:3417–3428. doi: 10.1128/MCB.02249-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Itoh N, Ohta H, Nakayama Y, Konishi M. Roles of FGF signals in heart development, health, and disease. Front Cell Dev Biol. 2016;4:110. doi: 10.3389/fcell.2016.00110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Patel V, Adya R, Chen J, Ramanjaneya M, Bari MF, Bhudia SK, Hillhouse EW, Tan BK, Randeva HS. Novel insights into the cardio-protective effects of FGF21 in lean and obese rat hearts. PLoS One. 2014;9:e87102. doi: 10.1371/journal.pone.0087102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Zhang C, Huang Z, Gu J, Yan X, Lu X, Zhou S, Wang S, Shao M, Zhang F, Cheng P, Feng W, Tan Y, Li X. Fibroblast growth factor 21 protects the heart from apoptosis in a diabetic mouse model via extracellular signal-regulated kinase 1/2-dependent signalling pathway. Diabetologia. 2015;58:1937–1948. doi: 10.1007/s00125-015-3630-8. [DOI] [PubMed] [Google Scholar]
- 151.Badman MK, Pissios P, Kennedy AR, Koukos G, Flier JS, Maratos-Flier E. Hepatic fibroblast growth factor 21 is regulated by PPARalpha and is a key mediator of hepatic lipid metabolism in ketotic states. Cell Metab. 2007;5:426–437. doi: 10.1016/j.cmet.2007.05.002. [DOI] [PubMed] [Google Scholar]
- 152.Inagaki T, Dutchak P, Zhao G, Ding X, Gautron L, Parameswara V, Li Y, Goetz R, Mohammadi M, Esser V, Elmquist JK, Gerard RD, Burgess SC, Hammer RE, Mangelsdorf DJ, Kliewer SA. Endocrine regulation of the fasting response by PPARalpha-mediated induction of fibroblast growth factor 21. Cell Metab. 2007;5:415–425. doi: 10.1016/j.cmet.2007.05.003. [DOI] [PubMed] [Google Scholar]
- 153.Ge X, Chen C, Hui X, Wang Y, Lam KS, Xu A. Fibroblast growth factor 21 induces glucose transporter-1 expression through activation of the serum response factor/Ets-like protein-1 in adipocytes. J Biol Chem. 2011;286:34533–34541. doi: 10.1074/jbc.M111.248591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Fisher FM, Kleiner S, Douris N, Fox EC, Mepani RJ, Verdeguer F, Wu J, Kharitonenkov A, Flier JS, Maratos-Flier E, Spiegelman BM. FGF21 regulates PGC-1alpha and browning of white adipose tissues in adaptive thermogenesis. Genes Dev. 2012;26:271–281. doi: 10.1101/gad.177857.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Potthoff MJ, Inagaki T, Satapati S, Ding X, He T, Goetz R, Mohammadi M, Finck BN, Mangelsdorf DJ, Kliewer SA, Burgess SC. FGF21 induces PGC-1alpha and regulates carbohydrate and fatty acid metabolism during the adaptive starvation response. Proc Natl Acad Sci USA. 2009;106:10853–10858. doi: 10.1073/pnas.0904187106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Coskun T, Bina HA, Schneider MA, Dunbar JD, Hu CC, Chen Y, Moller DE, Kharitonenkov A. Fibroblast growth factor 21 corrects obesity in mice. Endocrinology. 2008;149:6018–6027. doi: 10.1210/en.2008-0816. [DOI] [PubMed] [Google Scholar]
- 157.Planavila A, Redondo-Angulo I, Villarroya F. FGF21 and cardiac physiopathology. Front Endocrinol (Lausanne) 2015;6:133. doi: 10.3389/fendo.2015.00133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Brahma MK, Adam RC, Pollak NM, Jaeger D, Zierler KA, Pocher N, Schreiber R, Romauch M, Moustafa T, Eder S, Ruelicke T, Preiss-Landl K, Lass A, Zechner R, Haemmerle G. Fibroblast growth factor 21 is induced upon cardiac stress and alters cardiac lipid homeostasis. J Lipid Res. 2014;55:2229–2241. doi: 10.1194/jlr.M044784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Dogan SA, Pujol C, Maiti P, Kukat A, Wang S, Hermans S, Senft K, Wibom R, Rugarli EI, Trifunovic A. Tissue-specific loss of DARS2 activates stress responses independently of respiratory chain deficiency in the heart. Cell Metab. 2014;19:458–469. doi: 10.1016/j.cmet.2014.02.004. [DOI] [PubMed] [Google Scholar]
- 160.Planavila A, Redondo I, Hondares E, Vinciguerra M, Munts C, Iglesias R, Gabrielli LA, Sitges M, Giralt M, van Bilsen M, Villarroya F. Fibroblast growth factor 21 protects against cardiac hypertrophy in mice. Nat Commun. 2013;4:2019. doi: 10.1038/ncomms3019. [DOI] [PubMed] [Google Scholar]
- 161.Planavila A, Redondo-Angulo I, Ribas F, Garrabou G, Casademont J, Giralt M, Villarroya F. Fibroblast growth factor 21 protects the heart from oxidative stress. Cardiovasc Res. 2015;106:19–31. doi: 10.1093/cvr/cvu263. [DOI] [PubMed] [Google Scholar]
- 162.Nishizawa H, Matsuda M, Yamada Y, Kawai K, Suzuki E, Makishima M, Kitamura T, Shimomura I. Musclin, a novel skeletal muscle-derived secretory factor. J Biol Chem. 2004;279:19391–19395. doi: 10.1074/jbc.C400066200. [DOI] [PubMed] [Google Scholar]
- 163.Thomas G, Moffatt P, Salois P, Gaumond MH, Gingras R, Godin E, Miao D, Goltzman D, Lanctot C. Osteocrin, a novel bone-specific secreted protein that modulates the osteoblast phenotype. J Biol Chem. 2003;278:50563–50571. doi: 10.1074/jbc.M307310200. [DOI] [PubMed] [Google Scholar]
- 164.Moffatt P, Thomas G, Sellin K, Bessette MC, Lafreniere F, Akhouayri O, St-Arnaud R, Lanctot C. Osteocrin is a specific ligand of the natriuretic peptide clearance receptor that modulates bone growth. J Biol Chem. 2007;282:36454–36462. doi: 10.1074/jbc.M708596200. [DOI] [PubMed] [Google Scholar]
- 165.Potter LR, Abbey-Hosch S, Dickey DM. Natriuretic peptides, their receptors, and cyclic guanosine monophosphate-dependent signaling functions. Endocr Rev. 2006;27:47–72. doi: 10.1210/er.2005-0014. [DOI] [PubMed] [Google Scholar]
- 166.Chiba A, Watanabe-Takano H, Terai K, Fukui H, Miyazaki T, Uemura M, Hashimoto H, Hibi M, Fukuhara S, Mochizuki N. Osteocrin, a peptide secreted from the heart and other tissues, contributes to cranial osteogenesis and chondrogenesis in zebrafish. Development. 2017;144:334–344. doi: 10.1242/dev.143354. [DOI] [PubMed] [Google Scholar]
- 167.Kempf T, Eden M, Strelau J, Naguib M, Willenbockel C, Tongers J, Heineke J, Kotlarz D, Xu J, Molkentin JD, Niessen HW, Drexler H, Wollert KC. The transforming growth factor-beta superfamily member growth-differentiation factor-15 protects the heart from ischemia/reperfusion injury. Circ Res. 2006;98:351–360. doi: 10.1161/01.RES.0000202805.73038.48. [DOI] [PubMed] [Google Scholar]
- 168.Oshima Y, Ouchi N, Shimano M, Pimentel DR, Papanicolaou KN, Panse KD, Tsuchida K, Lara-Pezzi E, Lee SJ, Walsh K. Activin A and follistatin-like 3 determine the susceptibility of heart to ischemic injury. Circulation. 2009;120:1606–1615. doi: 10.1161/CIRCULATIONAHA.109.872200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Tokola H, Rysa J, Pikkarainen S, Hautala N, Leskinen H, Kerkela R, Ilves M, Aro J, Vuolteenaho O, Ritvos O, Ruskoaho H. Bone morphogenetic protein-2—a potential autocrine/paracrine factor in mediating the stretch activated B-type and atrial natriuretic peptide expression in cardiac myocytes. Mol Cell Endocrinol. 2015;399:9–21. doi: 10.1016/j.mce.2014.09.003. [DOI] [PubMed] [Google Scholar]
- 170.Zimmers TA, Davies MV, Koniaris LG, Haynes P, Esquela AF, Tomkinson KN, McPherron AC, Wolfman NM, Lee SJ. Induction of cachexia in mice by systemically administered myostatin. Science. 2002;296:1486–1488. doi: 10.1126/science.1069525. [DOI] [PubMed] [Google Scholar]
- 171.Heineke J, Auger-Messier M, Xu J, Sargent M, York A, Welle S, Molkentin JD. Genetic deletion of myostatin from the heart prevents skeletal muscle atrophy in heart failure. Circulation. 2010;121:419–425. doi: 10.1161/CIRCULATIONAHA.109.882068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Wang T, Liu J, McDonald C, Lupino K, Zhai X, Wilkins BJ, Hakonarson H, Pei L. GDF15 is a heart-derived hormone that regulates body growth. EMBO Mol Med. 2017;9:1150–1164. doi: 10.15252/emmm.201707604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Ishida K, Haudenschild DR. Interactions between FGF21 and BMP-2 in osteogenesis. Biochem Biophys Res Commun. 2013;432:677–682. doi: 10.1016/j.bbrc.2013.02.019. [DOI] [PubMed] [Google Scholar]
- 174.Deftos LJ, Burton DW, Brandt DW. Parathyroid hormone-like protein is a secretory product of atrial myocytes. J Clin Investig. 1993;92:727–735. doi: 10.1172/JCI116643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Zhang X, Wang X, Zhu H, Kranias EG, Tang Y, Peng T, Chang J, Fan GC. Hsp20 functions as a novel cardiokine in promoting angiogenesis via activation of VEGFR2. PLoS One. 2012;7:e32765. doi: 10.1371/journal.pone.0032765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Wang X, Huang W, Liu G, Cai W, Millard RW, Wang Y, Chang J, Peng T, Fan GC. Cardiomyocytes mediate anti-angiogenesis in type 2 diabetic rats through the exosomal transfer of miR-320 into endothelial cells. J Mol Cell Cardiol. 2014;74:139–150. doi: 10.1016/j.yjmcc.2014.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Vegter EL, van der Meer P, de Windt LJ, Pinto YM, Voors AA. MicroRNAs in heart failure: from biomarker to target for therapy. Eur J Heart Fail. 2016;18:457–468. doi: 10.1002/ejhf.495. [DOI] [PubMed] [Google Scholar]
- 178.Oliveira-Carvalho V, da Silva MM, Guimaraes GV, Bacal F, Bocchi EA. MicroRNAs: new players in heart failure. Mol Biol Rep. 2013;40:2663–2670. doi: 10.1007/s11033-012-2352-y. [DOI] [PubMed] [Google Scholar]
- 179.Care A, Catalucci D, Felicetti F, Bonci D, Addario A, Gallo P, Bang ML, Segnalini P, Gu Y, Dalton ND, Elia L, Latronico MV, Hoydal M, Autore C, Russo MA, Dorn GW, Ellingsen O, Ruiz-Lozano P, Peterson KL, Croce CM, Peschle C, Condorelli G. MicroRNA-133 controls cardiac hypertrophy. Nat Med. 2007;13:613–618. doi: 10.1038/nm1582. [DOI] [PubMed] [Google Scholar]
- 180.Montgomery RL, Hullinger TG, Semus HM, Dickinson BA, Seto AG, Lynch JM, Stack C, Latimer PA, Olson EN, van Rooij E. Therapeutic inhibition of miR-208a improves cardiac function and survival during heart failure. Circulation. 2011;124:1537–1547. doi: 10.1161/CIRCULATIONAHA.111.030932. [DOI] [PMC free article] [PubMed] [Google Scholar]