Abstract
Mammalian arrestins are a family of four highly homologous relatively small ~ 45 kDa proteins with surprisingly diverse functions. The most striking feature is that each of the two non-visual subtypes can bind hundreds of diverse G protein-coupled receptors (GPCRs) and dozens of non-receptor partners. Through these interactions, arrestins regulate the G protein-dependent signaling by the desensitization mechanisms as well as control numerous signaling pathways in the G protein-dependent or independent manner via scaffolding. Some partners prefer receptor-bound arrestins, some bind better to the free arrestins in the cytoplasm, whereas several show no apparent preference for either conformation. Thus, arrestins are a perfect example of a multi-functional signaling regulator. The result of this multi-functionality is that reduction (by knockdown) or elimination (by knockout) of any of these two non-visual arrestins can affect so many pathways that the results are hard to interpret. The other difficulty is that the non-visual subtypes can in many cases compensate for each other, which explains relatively mild phenotypes of single knockouts, whereas double knockout is lethal in vivo, although cultured cells lacking both arrestins are viable. Thus, deciphering the role of arrestins in cell biology requires the identification of specific signaling function(s) of arrestins involved in a particular phenotype. This endeavor should be greatly assisted by identification of structural elements of the arrestin molecule critical for individual functions and by the creation of mutants where only one function is affected. Reintroduction of these biased mutants, or introduction of monofunctional stand-alone arrestin elements, which have been identified in some cases, into double arrestin-2/3 knockout cultured cells, is the most straightforward way to study arrestin functions. This is a laborious and technically challenging task, but the upside is that specific function of arrestins, their timing, subcellular specificity, and relations to one another could be investigated with precision.
Keywords: Arrestin, GPCR, Receptor specificity, Signaling, MAP kinases, Protein engineering
Introduction
Virtually every protein in the cell has more than one function. The proteins’ multi-functionality explains how fairly complex organisms with a multi-stage life cycle can be built with fewer than 30,000 proteins. Even when we call some functions of a particular protein “moonlighting”, this only means that these functions were discovered later then what we call its “main” function. We must keep in mind that in evolution the protein in question always existed as a multi-functional entity: no protein known to man, viral, bacterial, or eukaryotic, is a “one-trick pony”. This appears self-evident and trivial, but in practice we often forget that when targeting an individual protein, we actually affect every one of its functions, those we intend to affect along with many others. Many side effects of drugs are actually “on-target”, direct consequences of affecting the functions of targeted protein that we did not intend to change. Protein knockout, knockdown, and overexpression remain staple methods in the biomedical research. Using these methods, we must keep in mind that changes in protein levels affect every function this protein fulfills, so the results must be interpreted with a proverbial grain of salt. Another common trap is thinking of a particular mutant as “dominant-negative”: this might be true of a particular function, but there are others, where the modified protein is unlikely to be either negative or dominant. Below we discuss arrestin proteins, multi-functionality of which is widely accepted, but we would like to emphasize that arrestins are not unique in this regard, just better studied at the moment.
The discovery of arrestins
Most vertebrates express four arrestin subtypes (only bony fish that underwent an extra whole genome duplication, express more) [33, 44]. Two out of these four are visual: arrestin-1 and arrestin-4 (we use systematic names of arrestin proteins, where the number after the dash indicates the order of cloning: arrestin-1 [historic names S-antigen, 48 kDa protein, visual or rod arrestin], arrestin-2 [β-arrestin or β-arrestin1], arrestin-3 [β-arrestin2 or hTHY-ARRX], and arrestin-4 [cone or X-arrestin]) are primarily expressed in photoreceptors cells, whereas the other two, arrestin-2 and arrestin-3, are expressed ubiquitously. The first arrestin (visual arrestin-1 in photoreceptors) was discovered as an S-antigen causing autoimmune disease uveitis [90], but its actual biological function was later established by Dr. Kuhn’s group: it was shown to preferentially bind light-activated rhodopsin [50]. The binding was enhanced by rhodopsin phosphorylation [51] and was found to suppress rhodopsin coupling to its cognate G protein, transducin, thereby shutting off light-induced signaling [92]. The ability to “arrest” rhodopsin signaling gave the 48 kDa protein described by Dr. Kuhn’s group its modern name, arrestin.
The molecular mechanism of its function turned out to be rather simple: direct competition with the visual G protein, transducin, for light-activated rhodopsin [49, 91]. The first non-visual subtype was purified and cloned as a protein that similarly blocks the signaling of β2-adrenergic receptor (β2AR) [54], another member of a large receptor family now known as G protein-coupled receptors (GPCRs). As it clearly preferred β2AR over rhodopsin, in contrast to the opposite preference of the visual arrestin, it was termed β-arrestin [53, 54]. The second non-visual subtype was cloned three times and given three different names: human protein cloned from thyroid was termed hTHY-ARRX [75], the protein cloned from rat brain was termed β-arrestin2 (with retroactive renaming of β-arrestin into β-arrestin1) [3], and the protein cloned from bovine brain was termed arrestin-3, with proposed introduction of systematic arrestin nomenclature, where the number after the dash indicates the order of cloning [79], by analogy with the systematic nomenclature for G protein-coupled receptor kinases (GRKs), established by that time (reviewed in [23]). The second non-visual arrestin also showed the preference for β2AR over rhodopsin [3]. The last member of the family in mammals, cone-specific arrestin-4, was also cloned twice and termed X-arrestin (because its gene is localized on the X-chromosome) [63] and cone arrestin for obvious reasons [14].
The first function: arresting GPCR signaling via G proteins
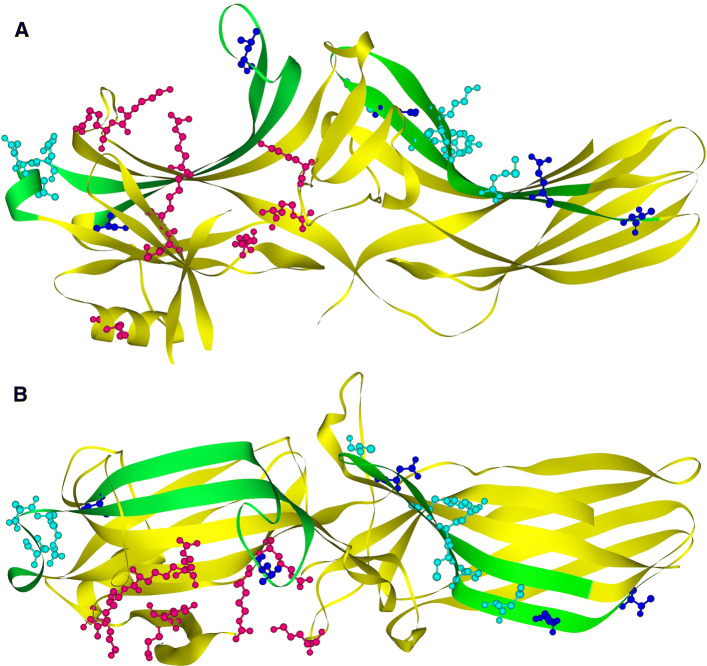
The first arrestin discovered was shown to block G protein-mediated rhodopsin signaling [92]; the second and third blocked the signaling of β2AR [3, 53]. To do that, arrestins must bind GPCRs in a way that precludes G protein interaction. Visual arrestin-1 was found to bind active phosphorylated rhodopsin [51] even before this binding was shown to suppress signal transduction [92]. Structurally, all four vertebrate arrestins are elongated two-domain molecules [20, 34, 42, 61, 80, 96] (Fig. 1). The elements that are engaged by their cognate receptors were extensively studied using a variety of methods in arrestin-1 [24, 25, 27, 37, 39, 45, 66, 68, 71, 81, 83–86, 88, 89, 101, 102], as well as in non-visual arrestin-2 [8, 18, 27, 35, 48, 77, 86] and arrestin-3 [8, 18, 73, 100]. In all cases, receptor-binding elements were localized to the concave side of both domains (Fig. 1). Recent crystal structure of the arrestin-1 complex with rhodopsin confirmed relative orientation of the two molecules [45, 101]. It also supported previous evidence that a single rhodopsin molecule is necessary and sufficient to engage arrestin [4, 38, 82, 87], just like a single molecule of rhodopsin and other GPCRs engages cognate G proteins and specific kinases that phosphorylate active receptors (reviewed in [32]).
Fig. 1.
Arrestin structure and receptor-binding elements. a Arrestin view from the side. b The view of the receptor-binding surface (down the cavities of both domains). In both images, the elements responsible for receptor preference (30) are shown in green. Individual residues with known functions in receptor binding are shown as scaled ball-and-stick models, colored, as follows: residues engaging receptor-attached phosphates [(23,32,43,100) (Lys10, Lys11, Arg25, Lys107, Lys160, Lys161, Arg165, Arg169, Lys294)] are shown in red, residues that play a role in receptor preference (29,40,41,80,108) are shown in blue, critical ones (Asp68, Ser86, Asp240, Asp259, Thr261) in dark blue, supporting ones (Leu48, Glu50, Arg51, Tyr238, Cys242, Lys250, Cys251, Pro252, Met255) in light blue. Images are based on 1G4M structure of arrestin-2 [monomer A in (18)] and generated by DS ViewerPro 6.0
The first non-receptor partners: trafficking proteins
The role of receptor phosphorylation and non-visual arrestins in GPCR endocytosis was discovered in mid-1990s [15, 16]. The molecular basis of this role was described soon thereafter: it was shown that receptor-associated non-visual arrestins directly bind clathrin [19] and clathrin adaptor AP2 [52], the key components of the internalization machinery of the coated pit. Arrestin elements that engage these partners were then identified [46]. Both were localized in the arrestin C-terminus, which was shown by a variety of methods to be released upon receptor binding [37, 69, 84, 103]. The fact that in free arrestins the C-terminus is tucked into the cavity of the N-domain and not particularly accessible makes perfect sense, as it prevents the competition of free arrestins with the arrestin-receptor complexes for the internalization machinery (reviewed in [28]). Arrestins bind yet another trafficking protein, N-ethylmaleimide sensitive fusion protein (NSF) [59] and, in case of visual arrestin-1, the interaction with NSF was found to regulate synaptic vesicle trafficking [43]. The discovery of these interactions added several more arrestin functions to the previously known. Thus, since mid-1990s arrestins have been regarded as multi-functional proteins.
Other arrestin-binding partners: their name is Legion
The discovery in 1999 that non-visual arrestins bind c-Src and facilitate its activation [55] was the first instance when arrestins were shown to interact with non-GPCR signaling proteins. The others came in rapid succession: ASK1 and JNK3 in 2000 [58], c-Raf1 and ERK1/2 in 2001 [56], and so on. A comprehensive proteomics study in 2007 identified more than a hundred binding partners of arrestin-2 and arrestin-3 [94], many of which interacted with both non-visual subtypes, but some specifically bound only one of the two. Even after that seemingly comprehensive list was published, additional arrestin-binding partners were discovered, including ubiquitin ligases AIP4 [5] and parkin [1], etc.
Importantly, in many cases functional significance of these interactions has been established. Non-visual arrestins were shown to facilitate the activation of several protein kinases that play a key role in the regulation of cell proliferation and death, such as c-Src [55], JNK3 [6, 58, 62, 76, 78], ubiquitous JNK1 and JNK2 isoforms [47], p38 [7], as well as ERK1/2 [6, 13, 56]. Even though ERK1/2 activation upon GPCR stimulation appears to require G protein signaling and can proceed without arrestins [2, 21, 65], arrestins facilitate it [21, 57]. The mechanism of c-Src activation by arrestins was recently studied by biophysical methods [95]. The activation appears to be direct: arrestin-2 “unwinds” the auto-inhibitory conformation of c-Src. Even though direct binding of arrestin-2, presumably in its basal conformation, enhanced c-Src activity, the effect was much stronger when arrestin-2 bound phosphorylated receptor peptide or phosphorylated active GPCR [95]. In case of MAP kinases, it appears pretty well established that arrestins bind all members of these three-tiered kinase cascades [78], thereby facilitating the signaling by bringing them in close proximity to each other, as originally proposed [56, 58]. Interestingly, while the arrestin-assisted activation of ERK1/2 requires arrestin binding to GPCRs, as originally proposed [56], arrestin-dependent activation of JNKs does not: it can be observed with purified proteins in the absence of any GPCRs [47, 97]. Moreover, arrestin-3 mutant defective in GPCR binding activates JNK3 as efficiently as WT arrestin-3 [6, 78], whereas the mutant with enhanced GPCR binding does not [6]. Most importantly, a short 25-residue N-terminal arrestin-3 peptide lacking most GPCR-binding elements promotes the activation of JNK3 in vitro and in cells [99]. Interestingly, this peptide is one of the three JNK3-binding arrestin-3 elements, which were all identified in the absence of GPCRs [98].
Identification of partner’s binding sites
Thus, arrestins bind many different partners, including GPCRs and other signaling proteins. Biologically, it is important to know what fraction of arrestin in the cell at any given moment interacts with each partner. It is equally important to establish which interactions are mutually exclusive, i.e., involve the same or overlapping sites. It is also important to know which interactions promote the binding of other partners and how this is achieved. For example, arrestin binding to a GPCR enhances arrestin interaction with ERK1/2 [12], as well as with clathrin and AP2 [46]. In contrast, specific arrestin interactions could be independent of one another (e.g., GPCR and JNK3 binding do not appear to affect each other [98]), and the reason for this independence needs to be established. This information is necessary to reveal what arrestins do in the cell and how their functions are regulated. From therapeutic perspective, it is clear that manipulating the expression of multi-functional WT arrestins would affect too many signaling pathways to be usable. Thus, targeted intervention requires either arrestin mutants where only one of a few select functions is disabled or the construction of arrestin-derived monofunctional elements that would not interfere with the other “jobs” endogenous arrestins perform. Precise identification of the binding sites of individual partners and conformational requirements of every interaction is necessary in both cases.
Despite the profusion of known arrestin-binding proteins, the elements of arrestins engaged by most of them remain unknown. So far, we can be fairly sure where receptors bind: a large area on the concave sides of both arrestin domains (Fig. 1) is the GPCR footprint. This has been shown by a variety of methods: peptide competition [74], spin label immobilization in arrestin-1 [37, 84] and arrestin-2 [35], chimera construction [27, 86], site-directed mutagenesis [17, 18, 24–26, 39, 85, 89], and NMR [102]. However, even this identification is not free from controversy. In the structure of the arrestin-1 complex with rhodopsin [45, 101], only a part of the presumed receptor-binding surface is seen engaged by the receptor, whereas much of the C-domain does not contact it. Yet several residues in the region that do not directly contact rhodopsin in the structure were immobilized upon receptor binding in both arrestin-1 [37] and arrestin-2 [35], which suggest that they do participate in the receptor biding. Moreover, mutations in these parts of the C-domain dramatically change receptor preference of arrestin-3 [17, 18], suggesting that they do contact GPCRs. Mutations of arrestin-3 residues homologous to those in arrestin-1 that are in direct contact with rhodopsin also change receptor binding in the GPCR subtype-specific manner [73, 100]. Thus, the most likely explanation is that crystal structure captured only one “flavor” of the receptor-arrestin complex out of several existing in equilibrium. Pulse EPR technique double electron–electron resonance (DEER) detects multiple distances between selected positions in arrestin-1 and rhodopsin in the complex, only one of which matches the crystal structure [45, 101], which supports this idea of the existence of more than one conformation of the complex.
Only two non-receptor partners were found to engage the parts overlapping with GPCR footprint: microtubules [36] and calmodulin [93], whereas others appear to interact with the non-receptor-binding side of the molecule. It stands to reason that any partner binding the arrestin-receptor complex must interact with arrestin elements that are not engaged by the receptor (reviewed in [28]).
Signaling-biased arrestins and arrestin-derived monofunctional tools
Considering how many signaling effects non-visual arrestins have in cells (reviewed in [22, 29, 72]), it is obvious that overexpression, knockdown, or knockout of either subtype changes the signaling in too many pathways to yield interpretable data [31]. Thus, to elucidate the role of arrestins in the cell, two types of molecular tools are necessary: arrestin mutants where a few and preferably only one of many functions is changed and monofunctional structural elements distilled from these multi-functional proteins. The identification of the binding sites for particular partners is necessary to make both types of these molecular tools.
The first arrestin mutants of that category were the mutants that do not bind clathrin and/or clathrin adaptor AP2 constructed following the identification of the clathrin and AP2-binding sites and then shown to suppress arrestin-mediated GPCR internalization via coated pits [67]. Arrestin mutants that appear to be normal in other regards, where the interaction with individual kinases in the cRaf-MEK1-ERK1/2 cascade, c-Raf [13] and MEK1 [60] is reduced, have been created. These mutations identified at least critical elements of respective binding sites. As could be expected, in both cases these mutations impeded arrestin-dependent facilitation of the activation of the downstream kinases in this cascade, ERK1/2 [13, 60]. One of the non-visual subtypes, arrestin-3, also scaffolds ASK1-MKK4/7-JNK3 cascade, thereby facilitating JNK3 activation in cells [58, 70]. Several mutants where this function was compromised were described, the most potent of which was a point mutant V343T [76]. Interestingly, this mutant appears to bind all kinases of the cascade similar to WT arrestin-3 but, apparently, holds them in the way that is not conducive to the JNK3 activation [76]. It is important to note that all these mutants were tested for a few (out of many) arrestin functions, so that we cannot be sure whether the mutations inadvertently changed other functions that simply have not been tested. There is an example of these inadvertent changes in arrestins. The substitution of 12 key residues involved in GPCR binding (KNC mutants) yields arrestin species that do not bind receptors [17, 85]. Venus-tagged arrestin-3-KNC is routinely used as a control in cell-based BRET assays of Venus-tagged arrestin interaction with Luc-tagged GPCRs, as it only yields non-specific “bystander” BRET [17, 73, 100]. Unexpectedly, it turned out that arrestin-3-KNC is also defective in JNK3 activation, even though it appears to bind all kinases in the pathway, some even better than WT arrestin-3 [6]. In fact, in cells arrestin-3-KNC acted as a dominant-negative silent scaffold, suppressing JNK3 activation facilitated by WT arrestin-3 [6]. While it is clear that there are allosteric interactions between the receptor-binding side of arrestins and their opposite side engaging most non-receptor partners [9], we still cannot predict the consequences of manipulation of one side on the functional capabilities of the other. Thus, we cannot be sure that only one function was affected in the mutants described above.
An alternative approach to the creation of signaling-biased arrestins is the construction of arrestin-derived peptides able to support only one select arrestin function. Such monofunctional peptide elements of arrestins have an advantage: in these cases, we can be fairly sure that the other functions are lacking. However, the construction of the molecular tools of this type requires precise identification of arrestin elements involved in particular interactions, which so far has been achieved for very few partners (reviewed in [30]). However, two monofunctional arrestin-derived peptides have been constructed and tested. One is the separated arrestin-2 C-terminus carrying clathrin and AP2-binding sites [67]. In cells, this fragment apparently binds clathrin and AP2 outcompeting the GPCR complexes with endogenous arrestins and, thereby, suppressing arrestin-mediated receptor internalization [67]. Another is a short 25-resiude peptide T1A representing the N-terminus of arrestin-3 [99]. It binds the kinases of the ASK1-MKK4/7-JNK3 cascade [70, 99] and productively scaffolds this pathway, facilitating JNK3 activation in vitro and in cells [99]. Importantly, these 25 residues do not contain other arrestin-3 elements with known functions, so that it likely retained only one—scaffolding the ASK1-MKK4/7-JNK3 cascade. Although we cannot be 100% sure, these small peptide tools likely have fewer surprises than full-length proteins with mutations.
GPCR-independent functions of arrestins
For most of their lifetime, arrestins exist as free molecules in the cytoplasm, not in complex with GPCRs. Like all proteins, arrestins are flexible in any state. This is clearly indicated by the widths of distance distributions between any two points in both free or receptor-bound arrestins measured by DEER (24,54,92) [reviewed in (93)]. Still, by way of oversimplification, we can assume that arrestins exist in at least three groups of conformations: free (often called basal), receptor-bound (usually considered active), and microtubule-bound (83,94). It is a common misconception that arrestins only signal when they are “activated” by GPCRs. It is true that most arrestin partners bind to the elements that are not shielded by bound receptor [reviewed in (83)]. However, there are exceptions even to this rule: both microtubules (37) and Ca-liganded calmodulin (82) bind to the same side as GPCRs. Many arrestin-binding partners have preference for the GPCR-bound (presumably “active”) arrestin conformation, the most striking example being ERK1/2. ERK1/2 has such a low affinity for the free arrestin in the cytoplasm that its interaction with arrestin can only be captured by co-immunoprecipitation without cross-linking upon GPCR activation (59,63,95). However, there are partners that show preference for the basal arrestin conformation over receptor-bound, such as E3 ubiquitin ligases Mdm2 (96,97) and parkin (62). Finally, some partners, such as JNK3, appear to bind equally well to both states of arrestin-3 (96,97).
There are also non-GPCR molecules inducing conformational changes similar to those induced by the receptor. For example, the release of the arrestin C-terminus, that is induced by its binding to GPCRs (42,43), is also facilitated by poly-anions, such as heparin and inositol-hexaphosphate (IP6) (53,81,98,99). Structural data show that the phosphates in IP6 bind positive charges in arrestin-3 (81) homologous to those engaged by the receptor-attached phosphates in the arrestin-1 complex with rhodopsin (43), as well as the phosphates on the multi-phosphorylated angiotensin receptor C-terminal peptide in its complex with the arrestin-2 (100). Moreover, IP6 binding induces many of the same conformational rearrangements in arrestin-3, including domain rotation, as receptor binding (81).
To make matters even more complicated, arrestins-1, -2, and -3 form oligomers, each in its own way [10, 11]. Oligomerization of these subtypes is differentially affected by an abundant cytoplasmic molecule IP6 [41]. The only common feature of all arrestin oligomers is that receptor-binding surface in them is invariably shielded by sister protomers, so that only monomeric arrestins can bind GPCRs [40]. Monomeric and oligomeric arrestins likely exist in the cell in a complex conformational equilibrium, which is affected by their binding partners, including GPCRs, microtubules, IP6, and other molecules. While different conformations most likely have distinct functional capabilities, the “activity” of arrestins in a particular pathway does not necessarily depend on their binding to GPCRs.
Several functions of non-visual arrestins were shown beyond reasonable doubt not to depend on GPCRs. The most studied is the scaffolding of the ASK1-MKK4/7-JNK1/2/3 signaling cascades. While the first study suggested that arrestin-3 performs this function in response to receptor activation, soon thereafter it was discovered that it can facilitate JNK3 activation independently of receptors (101). Arrestin-3 mutant that does not bind GPCRs because it lacks most of the inter-domain hinge was found to facilitate JNK3 activation as efficiently as WT arrestin-3 (63), whereas “pre-activated” mutant that was expected to exist in a more receptor bound-like conformation did not (65). The ability of arrestin-3 to facilitate signaling in the MKK4-JNK3 (75), MKK7-JNK (76), as well as in the MKK4/7-JNK1/2 modules (67) of these cascades in the system reconstituted from purified proteins in vitro in the absence of any GPCRs further supported this idea. Finally, effective scaffolding of this cascade with the resulting activation of JNK3 by a short 25-residue arrestin-3-derived peptide lacking most of the receptor-binding elements of arrestin-3 in vitro and in cells (66) proved receptor independence of this arrestin-3 function.
Another receptor-independent function was described for arrestin-2. The product of its cleavage by caspases, arrestin-2-(1–380), was shown to translocate to mitochondria (where there are no GPCRs) in several cell types and facilitate cytochrome c release induced by caspase-cleaved tBid, thereby promoting apoptotic cell death (102). Interestingly, caspase-generated arrestin-3-(1–366) appears to have an opposite function, enhancing cell survival, likely due to its inability to activate JNK family kinases (103). Both non-visual subtypes play a role in focal adhesion disassembly (104) and regulation of small G proteins involved in cell spreading and motility (105). Receptor binding-deficient mutants of non-visual arrestins perform these functions just as effectively as WT proteins (104,105). Arrestins recruit Mdm2 and ERK1/2 to microtubules, thereby directing Mdm2 activity to microtubule-associated substrates and suppressing ERK1/2 activation in cells, likely because arrestins do not recruit its upstream activator cRaf to that the compartment (37). The latter example of arrestin-dependent redistribution of signaling molecules between subcellular compartments illustrates how the signaling functions of arrestins could be regulated by the interplay of their biding to receptors or non-receptor partners leading to re-localization of arrestin and, consequently, of arrestin-dependent signaling within the cells. Another example of such redistribution of arrestin driven by alternative binding to microtubules and active GPCR is the light-dependent translocation of arrestin-1 in rod photoreceptors [64].
Conclusions (for now—the science does not end here)
The two ubiquitously expressed non-visual arrestins have numerous biological functions. Some of these require arrestin binding to an active GPCR phosphorylated by GRKs, whereas others do not. Elucidation of arrestin functions requires a variety of molecular tools. These tools include arrestin-2/3 mutants where only one function is disabled by the mutation(s). The downside of this approach is that it is hardly feasible to test constructed mutants for 100 + functional interactions the parental arrestins have, so we can never be sure that only the targeted function was affected. The other type of arrestin-based molecular tools is more promising: short peptides that retain only one (possibly a few) functions and lack all other capabilities of parental proteins mediated by the other parts of arrestins. These tools have the potential to channel cell signaling in desired directions, doing so in the language the cell cannot ignore, which makes them suitable for research and therapeutic purposes. The construction of these tools requires precise identification of arrestin elements responsible for each functional interaction, which is an enormous task. Where such identification has been achieved, it resulted in the design of powerful tools: peptides suppressing GPCR internalization or facilitating JNK signaling. These results are encouraging, but we are making only the first steps on a long road of identification of elements critical for numerous functions of arrestins and exploiting this knowledge by creating molecular tools to regulate cellular signaling.
Acknowledgements
This study was supported in part by NIH Grants RO1 EY011500, R35 GM122491, and Cornelius Vanderbilt Endowed Chair (VVG), and RO1s NS065868 and DA030103 (EVG).
Abbreviations
- GPCR
G protein-coupled receptor
- NSF
N-ethylmaleimide sensitive fusion protein
- EPR
Electron paramagnetic resonance
- DEER
Double electron–electron resonance
- NMR
Nuclear magnetic resonance
- ERK
Extracellular signal-regulated kinase
- JNK
c-Jun N-terminal kinase
- ASK
Apoptosis signal-regulating kinase
- MAPK
Mitogen-activated protein kinase
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Ahmed MR, Zhan X, Song X, Kook S, Gurevich VV, Gurevich EV. Ubiquitin ligase parkin promotes Mdm2-arrestin interaction but inhibits arrestin ubiquitination. Biochemistry. 2011;50:3749–3763. doi: 10.1021/bi200175q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alvarez-Curto E, Inoue A, Jenkins L, Raihan SZ, Prihandoko R, Tobin AB, Milligan G. Targeted elimination of G proteins and arrestins defines their specific contributions to both intensity and duration of G protein-coupled receptor signaling. J Biol Chem. 2016;291:27147–27159. doi: 10.1074/jbc.M116.754887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Attramadal H, Arriza JL, Aoki C, Dawson TM, Codina J, Kwatra MM, Snyder SH, Caron MG, Lefkowitz RJ. Beta-arrestin2, a novel member of the arrestin/beta-arrestin gene family. J Biol Chem. 1992;267:17882–17890. [PubMed] [Google Scholar]
- 4.Bayburt TH, Vishnivetskiy SA, McLean M, Morizumi T, Huang C-C, Tesmer JJ, Ernst OP, Sligar SG, Gurevich VV. Rhodopsin monomer is sufficient for normal rhodopsin kinase (GRK1) phosphorylation and arrestin-1 binding. J Biol Chem. 2011;286:1420–1428. doi: 10.1074/jbc.M110.151043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bhandari D, Trejo J, Benovic JL, Marchese A. Arrestin-2 interacts with the ubiquitin-protein isopeptide ligase atrophin-interacting protein 4 and mediates endosomal sorting of the chemokine receptor CXCR5. J Biol Chem. 2007;282:36971–36979. doi: 10.1074/jbc.M705085200. [DOI] [PubMed] [Google Scholar]
- 6.Breitman M, Kook S, Gimenez LE, Lizama BN, Palazzo MC, Gurevich EV, Gurevich VV. Silent scaffolds: inhibition of c-Jun N-terminal kinase 3 activity in the cell by a dominant-negative arrestin-3 mutant. J Biol Chem. 2012;287:19653–19664. doi: 10.1074/jbc.M112.358192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bruchas MR, Macey TA, Lowe JD, Chavkin C. Kappa opioid receptor activation of p38 MAPK is GRK3- and arrestin-dependent in neurons and astrocytes. J Biol Chem. 2006;281:18081–18089. doi: 10.1074/jbc.M513640200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Celver J, Vishnivetskiy SA, Chavkin C, Gurevich VV. Conservation of the phosphate-sensitive elements in the arrestin family of proteins. J Biol Chem. 2002;277:9043–9048. doi: 10.1074/jbc.M107400200. [DOI] [PubMed] [Google Scholar]
- 9.Chen Q, Iverson TM, Gurevich VV. Structural basis of arrestin-dependent signal transduction. Trends Biochem Sci. 2018;43:412–423. doi: 10.1016/j.tibs.2018.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen Q, Perry NA, Vishnivetskiy SA, Berndt S, Gilbert NC, Zhuo Y, Singh PK, Tholen J, Ohi MD, Gurevich EV, et al. Structural basis of arrestin-3 activation and signaling. Nat Commun. 2017;8:1427. doi: 10.1038/s41467-017-01218-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chen Q, Zhuo Y, Kim M, Hanson SM, Vishnivetskiy SA, Altenbach C, Klug CS, Hubbell WL, Gurevich VV. Self-association of arrestin family members. Handb Exp Pharmacol. 2014;219:205–223. doi: 10.1007/978-3-642-41199-1_11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Coffa S, Breitman M, Hanson SM, Callaway K, Kook S, Dalby KN, Gurevich VV. The effect of arrestin conformation on the recruitment of c-Raf1, MEK1, and ERK1/2 activation. PLoS One. 2011;6:e28723. doi: 10.1371/journal.pone.0028723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Coffa S, Breitman M, Spiller BW, Gurevich VV. A single mutation in arrestin-2 prevents ERK1/2 activation by reducing c-Raf1 binding. Biochemistry. 2011;50:6951–6958. doi: 10.1021/bi200745k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Craft CM, Whitmore DH, Wiechmann AF. Cone arrestin identified by targeting expression of a functional family. J Biol Chem. 1994;269:4613–4619. [PubMed] [Google Scholar]
- 15.Ferguson SS, Downey WE, 3rd, Colapietro AM, Barak LS, Ménard L, Caron MG. Role of beta-arrestin in mediating agonist-promoted G protein-coupled receptor internalization. Science. 1996;271:363–366. doi: 10.1126/science.271.5247.363. [DOI] [PubMed] [Google Scholar]
- 16.Ferguson SS, Ménard L, Barak LS, Koch WJ, Colapietro AM, Caron MG. Role of phosphorylation in agonist-promoted beta 2-adrenergic receptor sequestration. Rescue of a sequestration-defective mutant receptor by beta ARK1. J Biol Chem. 1995;270:24782–24789. doi: 10.1074/jbc.270.42.24782. [DOI] [PubMed] [Google Scholar]
- 17.Gimenez LE, Babilon S, Wanka L, Beck-Sickinger AG, Gurevich VV. Mutations in arrestin-3 differentially affect binding to neuropeptide Y receptor subtypes. Cell Signal. 2014;26:1523–1531. doi: 10.1016/j.cellsig.2014.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gimenez LE, Vishnivetskiy SA, Baameur F, Gurevich VV. Manipulation of very few receptor discriminator residues greatly enhances receptor specificity of non-visual arrestins. J Biol Chem. 2012;287:29495–29505. doi: 10.1074/jbc.M112.366674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Goodman OB, Jr, Krupnick JG, Santini F, Gurevich VV, Penn RB, Gagnon AW, Keen JH, Benovic JL. Beta-arrestin acts as a clathrin adaptor in endocytosis of the beta2-adrenergic receptor. Nature. 1996;383:447–450. doi: 10.1038/383447a0. [DOI] [PubMed] [Google Scholar]
- 20.Granzin J, Wilden U, Choe HW, Labahn J, Krafft B, Buldt G. X-ray crystal structure of arrestin from bovine rod outer segments. Nature. 1998;391:918–921. doi: 10.1038/36147. [DOI] [PubMed] [Google Scholar]
- 21.Grundmann M, Merten N, Malfacini D, Inoue A, Preis P, Simon K, Rüttiger N, Ziegler N, Benkel T, Schmitt NK, et al. Lack of beta-arrestin signaling in the absence of active G proteins. Nat Commun. 2018;9:341. doi: 10.1038/s41467-017-02661-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gurevich EV, Gurevich VV. Arrestins are ubiquitous regulators of cellular signaling pathways. Genome Biol. 2006;7:236. doi: 10.1186/gb-2006-7-9-236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gurevich EV, Tesmer JJ, Mushegian A, Gurevich VV. G protein-coupled receptor kinases: more than just kinases and not only for GPCRs. Pharmacol Ther. 2012;133:40–46. doi: 10.1016/j.pharmthera.2011.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gurevich VV, Benovic JL. Visual arrestin interaction with rhodopsin: sequential multisite binding ensures strict selectivity towards light-activated phosphorylated rhodopsin. J Biol Chem. 1993;268:11628–11638. [PubMed] [Google Scholar]
- 25.Gurevich VV, Benovic JL. Visual arrestin binding to rhodopsin: diverse functional roles of positively charged residues within the phosphorylation-recignition region of arrestin. J Biol Chem. 1995;270:6010–6016. doi: 10.1074/jbc.270.11.6010. [DOI] [PubMed] [Google Scholar]
- 26.Gurevich VV, Benovic JL. Mechanism of phosphorylation-recognition by visual arrestin and the transition of arrestin into a high affinity binding state. Mol Pharmacol. 1997;51:161–169. doi: 10.1124/mol.51.1.161. [DOI] [PubMed] [Google Scholar]
- 27.Gurevich VV, Dion SB, Onorato JJ, Ptasienski J, Kim CM, Sterne-Marr R, Hosey MM, Benovic JL. Arrestin interaction with G protein-coupled receptors. Direct binding studies of wild type and mutant arrestins with rhodopsin, b2-adrenergic, and m2 muscarinic cholinergic receptors. J Biol Chem. 1995;270:720–731. doi: 10.1074/jbc.270.2.720. [DOI] [PubMed] [Google Scholar]
- 28.Gurevich VV, Gurevich EV. The new face of active receptor bound arrestin attracts new partners. Structure. 2003;11:1037–1042. doi: 10.1016/S0969-2126(03)00184-9. [DOI] [PubMed] [Google Scholar]
- 29.Gurevich VV, Gurevich EV. The structural basis of arrestin-mediated regulation of G protein-coupled receptors. Pharm Ther. 2006;110:465–502. doi: 10.1016/j.pharmthera.2005.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gurevich VV, Gurevich EV. Synthetic biology with surgical precision: targeted reengineering of signaling proteins. Cell Signal. 2012;24:1899–1908. doi: 10.1016/j.cellsig.2012.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gurevich VV, Gurevich EV. Analyzing the roles of multi-functional proteins in cells: the case of arrestins and GRKs. Crit Rev Biochem Mol Biol. 2015;50:440–452. doi: 10.3109/10409238.2015.1067185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gurevich VV, Gurevich EV. GPCRs and signal transducers: interaction stoichiometry. Trends Pharmacol Sci. 2018;39:672–684. doi: 10.1016/j.tips.2018.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gurevich VV, Gurevich EV, Cleghorn WM. Arrestins as multi-functional signaling adaptors. Handb Exp Pharmacol. 2008;186:15–37. doi: 10.1007/978-3-540-72843-6_2. [DOI] [PubMed] [Google Scholar]
- 34.Han M, Gurevich VV, Vishnivetskiy SA, Sigler PB, Schubert C. Crystal structure of beta-arrestin at 1.9 A: possible mechanism of receptor binding and membrane translocation. Structure. 2001;9:869–880. doi: 10.1016/S0969-2126(01)00644-X. [DOI] [PubMed] [Google Scholar]
- 35.Hanson SM, Cleghorn WM, Francis DJ, Vishnivetskiy SA, Raman D, Song X, Nair KS, Slepak VZ, Klug CS, Gurevich VV. Arrestin mobilizes signaling proteins to the cytoskeleton and redirects their activity. J Mol Biol. 2007;368(2):375–387. doi: 10.1016/j.jmb.2007.02.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hanson SM, Francis DJ, Vishnivetskiy SA, Klug CS, Gurevich VV. Visual arrestin binding to microtubules involves a distinct conformational change. J Biol Chem. 2006;281:9765–9772. doi: 10.1074/jbc.M510738200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hanson SM, Francis DJ, Vishnivetskiy SA, Kolobova EA, Hubbell WL, Klug CS, Gurevich VV. Differential interaction of spin-labeled arrestin with inactive and active phosphorhodopsin. Proc Natl Acad Sci USA. 2006;103:4900–4905. doi: 10.1073/pnas.0600733103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hanson SM, Gurevich EV, Vishnivetskiy SA, Ahmed MR, Song X, Gurevich VV. Each rhodopsin molecule binds its own arrestin. Proc Nat Acad Sci USA. 2007;104:3125–3128. doi: 10.1073/pnas.0610886104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hanson SM, Gurevich VV. The differential engagement of arrestin surface charges by the various functional forms of the receptor. J Biol Chem. 2006;281:3458–3462. doi: 10.1074/jbc.M512148200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hanson SM, Van Eps N, Francis DJ, Altenbach C, Vishnivetskiy SA, Arshavsky VY, Klug CS, Hubbell WL, Gurevich VV. Structure and function of the visual arrestin oligomer. EMBO J. 2007;26:1726–1736. doi: 10.1038/sj.emboj.7601614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hanson SM, Vishnivetskiy SA, Hubbell WL, Gurevich VV. Opposing effects of inositol hexakisphosphate on rod arrestin and arrestin2 self-association. Biochemistry. 2008;47:1070–1075. doi: 10.1021/bi7021359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hirsch JA, Schubert C, Gurevich VV, Sigler PB. The 2.8 A crystal structure of visual arrestin: a model for arrestin’s regulation. Cell. 1999;97:257–269. doi: 10.1016/S0092-8674(00)80735-7. [DOI] [PubMed] [Google Scholar]
- 43.Huang SP, Brown BM, Craft CM. Visual Arrestin 1 acts as a modulator for N-ethylmaleimide-sensitive factor in the photoreceptor synapse. J Neurosci. 2010;30:9381–9391. doi: 10.1523/JNEUROSCI.1207-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Indrischek H, Prohaska SJ, Gurevich VV, Gurevich EV, Stadler PF. Uncovering missing pieces: duplication and deletion history of arrestins in deuterostomes. BMC Evol Biol. 2017;17:163. doi: 10.1186/s12862-017-1001-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kang Y, Zhou XE, Gao X, He Y, Liu W, Ishchenko A, Barty A, White TA, Yefanov O, Han GW, et al. Crystal structure of rhodopsin bound to arrestin determined by femtosecond X-ray laser. Nature. 2015;523:561–567. doi: 10.1038/nature14656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kim YM, Benovic JL. Differential roles of arrestin-2 interaction with clathrin and adaptor protein 2 in G protein-coupled receptor trafficking. J Biol Chem. 2002;277:30760–30768. doi: 10.1074/jbc.M204528200. [DOI] [PubMed] [Google Scholar]
- 47.Kook S, Zhan X, Kaoud TS, Dalby KN, Gurevich VV, Gurevich EV. Arrestin-3 binds JNK1α1 and JNK2α2 and facilitates the activation of these ubiquitous JNK isoforms in cells via scaffolding. J Biol Chem. 2013;288:37332–37342. doi: 10.1074/jbc.M113.510412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kovoor A, Celver J, Abdryashitov RI, Chavkin C, Gurevich VV. Targeted construction of phosphorylation-independent b-arrestin mutants with constitutive activity in cells. J Biol Chem. 1999;274:6831–6834. doi: 10.1074/jbc.274.11.6831. [DOI] [PubMed] [Google Scholar]
- 49.Krupnick JG, Gurevich VV, Benovic JL. Mechanism of quenching of phototransduction. Binding competition between arrestin and transducin for phosphorhodopsin. J Biol Chem. 1997;272:18125–18131. doi: 10.1074/jbc.272.29.18125. [DOI] [PubMed] [Google Scholar]
- 50.Kuhn H. Light-regulated binding of rhodopsin kinase and other proteins to cattle photoreceptor membranes. Biochemistry. 1978;17:4389–4395. doi: 10.1021/bi00614a006. [DOI] [PubMed] [Google Scholar]
- 51.Kuhn H, Hall SW, Wilden U. Light-induced binding of 48-kDa protein to photoreceptor membranes is highly enhanced by phosphorylation of rhodopsin. FEBS Lett. 1984;176:473–478. doi: 10.1016/0014-5793(84)81221-1. [DOI] [PubMed] [Google Scholar]
- 52.Laporte SA, Oakley RH, Zhang J, Holt JA, Ferguson SSG, Caron MG, Barak LS. The 2-adrenergic receptor/arrestin complex recruits the clathrin adaptor AP-2 during endocytosis. Proc Nat Acad Sci USA. 1999;96:3712–3717. doi: 10.1073/pnas.96.7.3712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lohse MJ, Andexinger S, Pitcher J, Trukawinski S, Codina J, Faure JP, Caron MG, Lefkowitz RJ. Receptor-specific desensitization with purified proteins. Kinase dependence and receptor specificity of beta-arrestin and arrestin in the beta 2-adrenergic receptor and rhodopsin systems. J Biol Chem. 1992;267:8558–8564. [PubMed] [Google Scholar]
- 54.Lohse MJ, Benovic JL, Codina J, Caron MG, Lefkowitz RJ. beta-Arrestin: a protein that regulates beta-adrenergic receptor function. Science. 1990;248:1547–1550. doi: 10.1126/science.2163110. [DOI] [PubMed] [Google Scholar]
- 55.Luttrell LM, Ferguson SS, Daaka Y, Miller WE, Maudsley S, Della Rocca GJ, Lin F, Kawakatsu H, Owada K, Luttrell DK, et al. Beta-arrestin-dependent formation of beta2 adrenergic receptor-Src protein kinase complexes. Science. 1999;283:655–661. doi: 10.1126/science.283.5402.655. [DOI] [PubMed] [Google Scholar]
- 56.Luttrell LM, Roudabush FL, Choy EW, Miller WE, Field ME, Pierce KL, Lefkowitz RJ. Activation and targeting of extracellular signal-regulated kinases by beta-arrestin scaffolds. Proc Natl Acad Sci USA. 2001;98:2449–2454. doi: 10.1073/pnas.041604898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Luttrell LM, Wang J, Plouffe B, Smith JS, Yamani L, Kaur S, Jean-Charles P-Y, Gauthier C, Lee M-H, Pani B, et al. Manifold roles of beta-arrestins in GPCR signaling elucidated with siRNA and CRISPR/Cas9. Sci Signal. 2018;11:eaat7650. doi: 10.1126/scisignal.aat7650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.McDonald PH, Chow CW, Miller WE, Laporte SA, Field ME, Lin FT, Davis RJ, Lefkowitz RJ. Beta-arrestin 2: a receptor-regulated MAPK scaffold for the activation of JNK3. Science. 2000;290:1574–1577. doi: 10.1126/science.290.5496.1574. [DOI] [PubMed] [Google Scholar]
- 59.McDonald PH, Cote NL, Lin FT, Premont RT, Pitcher JA, Lefkowitz RJ. Identification of NSF as a beta-arrestin1-binding protein. Implications for beta2-adrenergic receptor regulation. J Biol Chem. 1999;274:10677–10680. doi: 10.1074/jbc.274.16.10677. [DOI] [PubMed] [Google Scholar]
- 60.Meng D, Lynch MJ, Huston E, Beyermann M, Eichhorst J, Adams DR, Klusmann E, Houslay MD, Baillie GS. MEK1 binds directly to betaarrestin1, influencing both its phosphorylation by ERK and the timing of its isoprenaline-stimulated internalization. J Biol Chem. 2009;284:11425–11435. doi: 10.1074/jbc.M806395200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Milano SK, Pace HC, Kim YM, Brenner C, Benovic JL. Scaffolding functions of arrestin-2 revealed by crystal structure and mutagenesis. Biochemistry. 2002;41:3321–3328. doi: 10.1021/bi015905j. [DOI] [PubMed] [Google Scholar]
- 62.Miller WE, McDonald PH, Cai SF, Field ME, Davis RJ, Lefkowitz RJ. Identification of a motif in the carboxyl terminus of beta -arrestin2 responsible for activation of JNK3. J Biol Chem. 2001;276:27770–27777. doi: 10.1074/jbc.M102264200. [DOI] [PubMed] [Google Scholar]
- 63.Murakami A, Yajima T, Sakuma H, McLaren MJ, Inana G. X-arrestin: a new retinal arrestin mapping to the X chromosome. FEBS Lett. 1993;334:203–209. doi: 10.1016/0014-5793(93)81712-9. [DOI] [PubMed] [Google Scholar]
- 64.Nair KS, Hanson SM, Mendez A, Gurevich EV, Kennedy MJ, Shestopalov VI, Vishnivetskiy SA, Chen J, Hurley JB, Gurevich VV, et al. Light-dependent redistribution of arrestin in vertebrate rods is an energy-independent process governed by protein-protein interactions. Neuron. 2005;46:555–567. doi: 10.1016/j.neuron.2005.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.O’Hayre M, Eichel K, Avino S, Zhao X, Steffen DJ, Feng X, Kawakami K, Aoki J, Messer K, Sunahara R, et al. Genetic evidence that β-arrestins are dispensable for the initiation of β2-adrenergic receptor signaling to ERK. Sci Signal. 2017;10:484. doi: 10.1126/scisignal.aal3395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ohguro H, Palczewski K, Walsh KA, Johnson RS. Topographic study of arrestin using differential chemical modifications and hydrogen/deuterium exchange. Protein Sci. 1994;3:2428–2434. doi: 10.1002/pro.5560031226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Orsini MJ, Benovic JL. Characterization of dominant negative arrestins that inhibit beta2-adrenergic receptor internalization by distinct mechanisms. J Biol Chem. 1998;273:34616–34622. doi: 10.1074/jbc.273.51.34616. [DOI] [PubMed] [Google Scholar]
- 68.Ostermaier MK, Peterhans C, Jaussi R, Deupi X, Standfuss J. Functional map of arrestin-1 at single amino acid resolution. Proc Natl Acad Sci USA. 2014;111:1825–1830. doi: 10.1073/pnas.1319402111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Palczewski K, Pulvermuller A, Buczylko J, Hofmann KP. Phosphorylated rhodopsin and heparin induce similar conformational changes in arrestin. J Biol Chem. 1991;266:18649–18654. [PubMed] [Google Scholar]
- 70.Perry NA, Kaoud TS, Ortega OO, Kaya AI, Marcus DJ, Pleinis JM, Berndt S, Chen Q, Zhan X, Dalby KN, et al. Arrestin-3 scaffolding of the JNK3 cascade suggests a mechanism for signal amplification. Proc Natl Acad Sci USA. 2019;116:810–815. doi: 10.1073/pnas.1819230116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Peterhans C, Lally CC, Ostermaier MK, Sommer ME, Standfuss J. Functional map of arrestin binding to phosphorylated opsin, with and without agonist. Sci Rep. 2016;6:28686. doi: 10.1038/srep28686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Peterson YK, Luttrell LM. The diverse roles of arrestin scaffolds in G protein-coupled receptor signaling. Pharmacol Rev. 2017;69:256–297. doi: 10.1124/pr.116.013367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Prokop S, Perry NA, Vishnivetskiy SA, Toth AD, Inoue A, Milligan G, Iverson TM, Hunyady L, Gurevich VV. Differential manipulation of arrestin-3 binding to basal and agonist-activated G protein-coupled receptors. Cell Signal. 2017;36:98–107. doi: 10.1016/j.cellsig.2017.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Pulvermuller A, Schroder K, Fischer T, Hofmann KP. Interactions of metarhodopsin II. Arrestin peptides compete with arrestin and transducin. J Biol Chem. 2000;275:37679–37685. doi: 10.1074/jbc.M006776200. [DOI] [PubMed] [Google Scholar]
- 75.Rapoport B, Kaufman KD, Chazenbalk GD. Cloning of a member of the arrestin family from a human thyroid cDNA library. Mol Cell Endocrinol. 1992;84:R39–R43. doi: 10.1016/0303-7207(92)90038-8. [DOI] [PubMed] [Google Scholar]
- 76.Seo J, Tsakem EL, Breitman M, Gurevich VV. Identification of arrestin-3-specific residues necessary for JNK3 activation. J Biol Chem. 2011;286:27894–27901. doi: 10.1074/jbc.M111.260448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Shukla AK, Manglik A, Kruse AC, Xiao K, Reis RI, Tseng WC, Staus DP, Hilger D, Uysal S, Huang LY, et al. Structure of active beta-arrestin-1 bound to a G-protein-coupled receptor phosphopeptide. Nature. 2013;497:137–141. doi: 10.1038/nature12120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Song X, Coffa S, Fu H, Gurevich VV. How does arrestin assemble MAPKs into a signaling complex? J Biol Chem. 2009;284:685–695. doi: 10.1074/jbc.M806124200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Sterne-Marr R, Gurevich VV, Goldsmith P, Bodine RC, Sanders C, Donoso LA, Benovic JL. Polypeptide variants of beta-arrestin and arrestin3. J Biol Chem. 1993;268:15640–15648. [PubMed] [Google Scholar]
- 80.Sutton RB, Vishnivetskiy SA, Robert J, Hanson SM, Raman D, Knox BE, Kono M, Navarro J, Gurevich VV. Crystal structure of cone arrestin at 2.3A: evolution of receptor specificity. J Mol Biol. 2005;354:1069–1080. doi: 10.1016/j.jmb.2005.10.023. [DOI] [PubMed] [Google Scholar]
- 81.Szczepek M, Beyriere F, Hofmann KP, Elgeti M, Kazmin R, Rose A, Bartl FJ, von Stetten D, Heck M, Sommer ME, et al. Crystal structure of a common GPCR-binding interface for G protein and arrestin. Nat Commun. 2014;5:4801. doi: 10.1038/ncomms5801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Tsukamoto H, Sinha A, Dewitt M, Farrens DL. Monomeric rhodopsin is the minimal functional unit required for arrestin binding. J Mol Biol. 2010;399:501–511. doi: 10.1016/j.jmb.2010.04.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Vishnivetskiy SA, Baameur F, Findley KR, Gurevich VV. Critical role of the central 139-loop in stability and binding selectivity of arrestin-1. J Biol Chem. 2013;288:11741–11750. doi: 10.1074/jbc.M113.450031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Vishnivetskiy SA, Francis DJ, Van Eps N, Kim M, Hanson SM, Klug CS, Hubbell WL, Gurevich VV. The role of arrestin alpha-helix I in receptor binding. J Mol Biol. 2010;395:42–54. doi: 10.1016/j.jmb.2009.10.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Vishnivetskiy SA, Gimenez LE, Francis DJ, Hanson SM, Hubbell WL, Klug CS, Gurevich VV. Few residues within an extensive binding interface drive receptor interaction and determine the specificity of arrestin proteins. J Biol Chem. 2011;286:24288–24299. doi: 10.1074/jbc.M110.213835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Vishnivetskiy SA, Hosey MM, Benovic JL, Gurevich VV. Mapping the arrestin-receptor interface: structural elements responsible for receptor specificity of arrestin proteins. J Biol Chem. 2004;279:1262–1268. doi: 10.1074/jbc.M308834200. [DOI] [PubMed] [Google Scholar]
- 87.Vishnivetskiy SA, Ostermaier MK, Singhal A, Panneels V, Homan KT, Glukhova A, Sligar SG, Tesmer JJ, Schertler GF, Standfuss J, et al. Constitutively active rhodopsin mutants causing night blindness are effectively phosphorylated by GRKs but differ in arrestin-1 binding. Cell Signal. 2013;25:2155–2162. doi: 10.1016/j.cellsig.2013.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Vishnivetskiy SA, Paz CL, Schubert C, Hirsch JA, Sigler PB, Gurevich VV. How does arrestin respond to the phosphorylated state of rhodopsin? J Biol Chem. 1999;274:11451–11454. doi: 10.1074/jbc.274.17.11451. [DOI] [PubMed] [Google Scholar]
- 89.Vishnivetskiy SA, Schubert C, Climaco GC, Gurevich YV, Velez M-G, Gurevich VV. An additional phosphate-binding element in arrestin molecule: implications for the mechanism of arrestin activation. J Biol Chem. 2000;275:41049–41057. doi: 10.1074/jbc.M007159200. [DOI] [PubMed] [Google Scholar]
- 90.Wacker WB, Donoso LA, Kalsow CM, Yankeelov JA, Jr, Organisciak DT. Experimental allergic uveitis. Isolation, characterization, and localization of a soluble uveitopathogenic antigen from bovine retina. J Immunol. 1977;119:1949–1958. [PubMed] [Google Scholar]
- 91.Wilden U. Duration and amplitude of the light-induced cGMP hydrolysis in vertebrate photoreceptors are regulated by multiple phosphorylation of rhodopsin and by arrestin binding. Biochemistry. 1995;34:1446–1454. doi: 10.1021/bi00004a040. [DOI] [PubMed] [Google Scholar]
- 92.Wilden U, Hall SW, Kühn H. Phosphodiesterase activation by photoexcited rhodopsin is quenched when rhodopsin is phosphorylated and binds the intrinsic 48-kDa protein of rod outer segments. Proc Natl Acad Sci. 1986;83:1174–1178. doi: 10.1073/pnas.83.5.1174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Wu N, Hanson SM, Francis DJ, Vishnivetskiy SA, Thibonnier M, Klug CS, Shoham M, Gurevich VV. Arrestin binding to calmodulin: a direct interaction between two ubiquitous signaling proteins. J Mol Biol. 2006;364:955–963. doi: 10.1016/j.jmb.2006.09.075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Xiao K, McClatchy DB, Shukla AK, Zhao Y, Chen M, Shenoy SK, Yates JR, Lefkowitz RJ. Functional specialization of beta-arrestin interactions revealed by proteomic analysis. Proc Natl Acad Sci USA. 2007;104:12011–12016. doi: 10.1073/pnas.0704849104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Yang F, Xiao P, Qu C-X, Liu Q, Wang L-Y, Liu Z-X, He Q-T, Liu C, Xu J-Y, Li R-R, et al. Allosteric mechanisms underlie GPCR signaling to SH3-domain proteins through arrestin. Nat Chem Biol. 2018;14:876–886. doi: 10.1038/s41589-018-0115-3. [DOI] [PubMed] [Google Scholar]
- 96.Zhan X, Gimenez LE, Gurevich VV, Spiller BW. Crystal structure of arrestin-3 reveals the basis of the difference in receptor binding between two non-visual arrestins. J Mol Biol. 2011;406:467–478. doi: 10.1016/j.jmb.2010.12.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Zhan X, Kaoud TS, Dalby KN, Gurevich VV. Non-visual arrestins function as simple scaffolds assembling MKK4- JNK3α2 signaling complex. Biochemistry. 2011;50:10520–10529. doi: 10.1021/bi201506g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Zhan X, Perez A, Gimenez LE, Vishnivetskiy SA, Gurevich VV. Arrestin-3 binds the MAP kinase JNK3α2 via multiple sites on both domains. Cell Signal. 2014;26:766–776. doi: 10.1016/j.cellsig.2014.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Zhan X, Stoy H, Kaoud TS, Perry NA, Chen Q, Vucak G, Perez A, Els-Heindl S, Slagis JV, Iverson TM, et al. Peptide mini-scaffold facilitates JNK3 activation in cells. Sci Rep. 2016;6:21025. doi: 10.1038/srep21025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Zheng C, Tholen J, Gurevich VV. Critical role of the finger loop in arrestin binding to the receptors. PLoS ONE. 2019;14:e0213792. doi: 10.1371/journal.pone.0213792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Zhou XE, He Y, de Waal PW, Gao X, Kang Y, Van Eps N, Yin Y, Pal K, Goswami D, White TA, et al. Structural identification of phosphorylation codes for arrestin recruitment by G protein-coupled receptors. Cell. 2017;170:457–469. doi: 10.1016/j.cell.2017.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Zhuang T, Chen Q, Cho M-K, Vishnivetskiy SA, Iverson TM, Gurevich VV, Sanders CR. Involvement of distinct arrestin-1 elements in binding to different functional forms of rhodopsin. Proc Nat Acad Sci USA. 2013;110:942–947. doi: 10.1073/pnas.1215176110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Zhuo Y, Vishnivetskiy SA, Zhan X, Gurevich VV, Klug CS. Identification of receptor binding-induced conformational changes in non-visual arrestins. J Biol Chem. 2014;289:20991–21002. doi: 10.1074/jbc.M114.560680. [DOI] [PMC free article] [PubMed] [Google Scholar]