Abstract
An emerging concept in intercellular communication in mammals is that communication can be mediated by exchange of genetic material, mainly in the form of RNAs. In this review, we discuss recent studies that describe the trafficking of genetic material with a focus on bone cell communication. Three major carriers are discussed: gap junctions, protein-binding complexes, and genetic material exchange mediated by extracellular vesicles. While protein-level exchange has been well documented, no review has summarized the novel paradigm of cell-to-cell communication by genetic information exchange in bone tissues or its biological relevance in terms of bone homeostasis and bone-related diseases. The purpose of this review is to promote further understanding of this novel discovery regarding bone cell communication and provide references for further investigations.
Keywords: Genetic material, Extracellular vesicles, Gap junction, RNA-binding protein, Bone cells, Cell-to-cell communication
Introduction
Cell-to-cell communication is crucial for cell differentiation, growth, and function as well as morphogenesis. It represents one of the main characters in the multidimensional opera of physiology. Classic cell-to-cell communication is mediated by several methods that fall under either direct or indirect communication; direct communication includes autocrine (self-self) and juxtacrine (between neighboring cells) communication, while indirect communication includes paracrine and synaptic (local and exercised over a short distance) and endocrine (exercised over a longer distance) signaling. Generally, cell-to-cell communication—direct or indirect—involves receptor–ligand interaction, which leads to the activation or suppression of intercellular signaling pathways in target cells [1]. This conventional, largely protein-based (involving growth factors, cytokines, and chemokines, etc.), model has long been considered necessary and sufficient to explain coordinate tissue and organism function.
The exchange of genetic material between eukaryotic cells is a more recently discovered concept [2]. When eukaryotic cells encounter double-stranded RNA (dsRNA), genes carrying a matching sequence are silenced through RNA interference (RNAi). Through experiments involving the injection of dsRNA into C. elegans, researchers observed that dsRNA can be spread throughout the organism, allowing the same gene to be specifically silenced in cells that had not encountered the primary dsRNA [3]. Similarly, in plants, research shows that when a leaf is infected with a plant virus, mobile signals that confer resistance to the virus can be transmitted to other leaves [4]. Furthermore, beyond the virus-induced movement of silencing RNAs (siRNAs), the mobility of endogenous small RNAs (sRNAs) through the plants guiding patterning of leaves and roots has also been demonstrated [5, 6] These findings reveal a new paradigm that cells have a method of communication other than the protein-level information; cells can directly exchange genetic material. Exchange of genetic material can occur through intimate membrane contacts between parental and target cells or, for long-distance communication, through extracellular vesicles (EVs) that contain genetic material and travel via body fluids [7–11]. With growing interest and research in the field, the biological significance of cell-to-cell communication through exchange of genetic material is becoming gradually illuminated.
Bone is a dynamic, homeostatic organ that is constantly remodeled by the balanced and coupled activities of the cells including osteoblasts, osteocytes, and osteoclasts, among others. Closely regulated mechanisms of intercellular communication that permit the cells to sort and migrate, synchronize activity, regulate hormonal responses, and diffuse locally generated signals are crucial [12]. In addition to the classic information exchange methods, both direct and indirect, bone cells can also communicate via an exclusive approach. Research has found that osteoclasts can resorb bone matrix while releasing the growth factors (such as TGF-beta) that are deposited in it, therefore, stimulating osteoblasts and osteoblast precursors [13]. A group of osteoblasts and osteoclasts that cooperate during bone remodeling is referred to as a “basic multicellular unit” (BMU) or “bone remodeling compartment” (BRC). Protein-level information exchange happens mainly within BRCs among cells such as osteoblasts, osteoblast precursors, osteoclasts, osteoclast precursors, osteocytes, microvascular epithelial cells, and neuron sprouts. This information exchange regulates bone resorption, bone formation, and vascular and nerve innervation. Other molecules such as lipids and ions are also transported across cells to participate in the aforementioned processes [14].
After evidence for genetic information exchange was discovered in eukaryotic cells, concepts such as the potential physiological relevance of this type of information exchange and whether bone cells could utilize it became topics of discussion. However, given the complexity of the extracellular environment such as great variance in pH and abundance of enzymes, genetic material such as RNA was ruled out from being able to transfer signals outside of cells because of its high susceptibility to degradation. Of note, the recent discovery that RNAs can be shuttled by extracellular vesicles that protect them from degradation supports the notion that this form of exchange of genetic information may exist in bone tissues [9]. Subsequently, further research found that certain cell-to-cell direct contact structures such as gap junctions, which are common in bone tissue, can also transfer genetic information [15]. These findings convey the intriguing idea that exchange of genetic material may represent a new paradigm in bone cell communications.
Protein-level exchange has been well documented. However, no prior review has summarized the novel paradigm of cell-to-cell communication by genetic information exchange in bone tissues or its biological relevance in terms of bone homeostasis and bone-related diseases. Accordingly, in this review, we discuss recent studies that describe the trafficking of genetic material between bone cells, focusing on EVs and the diverse structures that cells utilize for communication. The purpose of this review is to promote further understanding of this novel discovery regarding bone cell communication and provide references for further investigations.
Genetic transfer in EVs
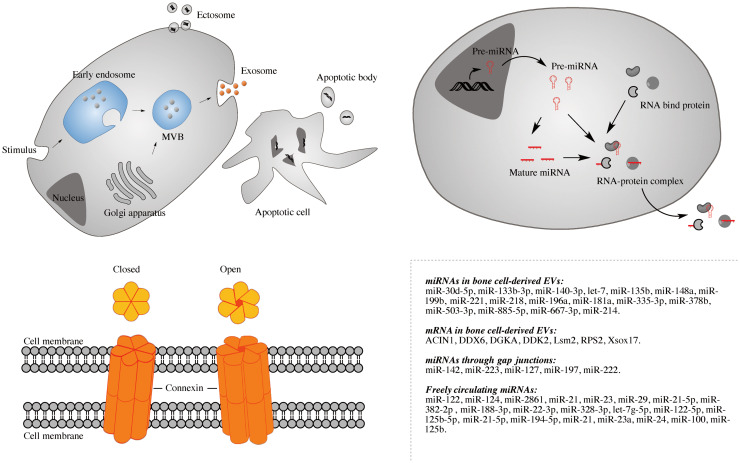
Since the discovery of EVs containing functional mRNA, microRNA (miRNA), and DNA molecules, they have emerged as important vehicles for cell-to-cell communication [16]. EVs also contain other molecules such as lipids, proteins, and ions, but for the purpose of this review, reports on EVs-mediated cell-to-cell transfer of nucleic acids are of particular interest. EVs also contain other molecules such as lipid, proteins, ions, but for the purpose of this review, reports on EVs-mediated cell-to-cell transfer of nucleic acids are of particular interest. Depending on their origin and the size, EVs are categorized as exosomes, shedding vesicles or apoptotic bodies (Fig. 1) [17]. Exosomes (diameter of 30–100 nm) correspond to the intraluminal vesicles (ILVs) that originate from the late endosomal trafficking machinery. Exosomes are gathered into multi-vesicular bodies (MVBs) inside the cell and ultimately released as a result of MVB fusion with the plasma membrane. Ectosomes (also called shedding vesicles), membrane particles, nanoparticles, matrix vesicles, and microvesicles (MVs)—are cell surface-derived EVs that are typically larger than exosomes (with the diameter of 100 nm–1 μm), originating from direct budding from the plasma membrane. Apoptotic bodies are larger than ectosomes or exosomes (> 1 μm in diameter) and are released as blebs of apoptotic cells. They are characterized by phosphatidylserine externalization and contain fragmented DNA.
Fig. 1.
Three genetic exchange approaches including EVs (exosome, ectosome, and apoptotic body), gap junction, and RNA-binding complex. Genetic material transferred through these approaches associated with bone cells is present. A large amount of miRNAs and mRNAs have been shown to be associated with bone-derived EVs, here only those with reported biological activity are listed. For freely circulating miRNAs listed, prior study showed that these miRNAs are biomarkers for osteoporosis or low bone mass, however direct role in bone cell genetic information exchange has yet to be discovered
EVs as cell-to-cell communication tool
Strictly speaking, to fulfill the definition of cell-to-cell communication, genetic material must be (1) selectively packaged into vectors and (2) retain functionality to exert biological effects in recipient cells. Exosomes can serve as an example of these attributes. First discovered nearly 30 years ago, exosomes were initially considered little more than cellular garbage cans acting to discard unwanted molecular components. In 2007, Jan Lötvall et al. first described the presence of mRNA and miRNA inside the exosomes [18]. Additionally, in a comparison of the mRNAs in exosomes with those in their parental cells, the identified mRNA in exosomes accounted for approximately 8% of the mRNA detected in the parent cells. The gene profile analysis of these mRNAs displayed critical differences in the level of mRNA transcripts in exosomes versus their parental cells. Significantly, the most abundant transcripts in the exosomes were generally different from the most abundant transcripts in the parental cells. A subset of some specific targeting mRNA sequences was controllably enriched in the released vesicles. Such observations rule out the now-outdated idea that the presence of mRNA in exosomes results from random contaminations.
miRNAs are endogenous ~ 22-nucleotide noncoding small RNAs that cause mRNA cleavage or suppression of protein synthesis by binding to the 3′-untranslated region (3′-UTR) of the target mRNAs [19]. Current evidence also suggests that the export profile of miRNAs in exosomes is not representative of the parent cell and is distinct in terms of both abundance and content [20–22]. Additionally, the exosome repertoires of miRNA patterns are associated with cellular processes, reflecting the nature and even the state of the parental cells [23–25]. Given these findings, a sorting mechanism must exist that provides exosomes with a unique subset of RNA. Some developments have been made in elucidating these mechanisms: certain pathways such as sumoylated heterogeneous ribonucleoprotein A2B1 (hnRNPA2B1), annexin-2 have been reported to control RNA sorting [26–28]. The endosomal sorting complex required for transport (ESCRT) has been shown to play a role in sorting of proteins into exosomes, but the idea that the ESCRT has a similar function in the sorting of RNA is speculation at this point, if an attractive possibility [29].
Further research demonstrates that mRNAs present in EVs could be translated into functional proteins in target cells [30]. Other studies have found that DNA can be transferred from tumor cells to healthy ones through EVs, spreading oncogenes such as c-myc [31]. These findings experimentally confirmed that EV transportation is a method of genetic material exchange across cells.
Regarding exosome-mediated miRNA-based intercellular communication, the observations from Tewari et al. raise questions regarding whether there are enough microRNAs present in EVs to account for biological activity. In their study, less than one molecule of a given miRNA, even for the most abundant miRNAs (mean ± SD across six exosome sources: 0.00825 ± 0.02 miRNA molecules/exosome), was found per exosome, regardless of the source [32]. As commented by Théry et al., this finding suggested that either very few miRNA molecules are present within each small EVs or, more likely, that only a restricted subtype of EVs contain significant amounts of miRNA molecules and thus are capable of transferring miRNA-based information [10]. To delineate these mechanisms, the necessary technical advances and subsequent understanding of the various and fundamental roles of each type of EV is urgently required. Research by Alexander et al. also confirmed the low content of miRNA copy per exosome. In their study, approximately one copy of miR-146a per exosome was observed. However, it has also been witnessed that one bone marrow-derived dendritic cell (BMDC) produces ~ 500 exosomes after 24 h of culture, indicating that each cell is able to release at least hundreds of copies of miR-146a in exosomes to be delivered to recipient BMDCs, and is enough to exert physiological effects in recipient cells [33]. Thus, it seems that the large numbers of exosomes produced per cell allows for the loading of low miRNA numbers per exosome to achieve functional relevance.
Characterization of bone cell-derived EVs
Most studies reporting transportation of EVs in bone focus on osteoblasts or osteoclasts [34]. This is due to the unique role of these cells in bone homeostasis as well as their frequent and dynamic interactions with each other. Jess Morhayim et al. applied a proteomics approach to characterize EVs secreted by both mineralizing and non-mineralizing osteoblasts [35]. This study used a simian virus 40-immortalized human osteoblast cell line (SV-HFO cells) established from normal human fetal calvarias. This study found classic vesicle proteins such as annexins (ANXA2, ANXA6), tetraspanins (CD9, CD81), and metabolic proteins (GAPDH, LDHA) as well as four uniquely detected proteins in EVs derived from non-mineralizing osteoblasts and 26 uniquely detected proteins in EVs derived from mineralizing osteoblasts. Interestingly, the enzyme alkaline phosphatase, tissue-nonspecific isozyme (ALPL), was found to be continuously enriched in EVs in increasing abundance along with mineralization extension [35].
Another group of researchers analyzed the exosomes from a mouse osteoblast cell line MC3T3, but this study did not provide unique protein markers for exosomes released from osteoblasts [36]. Using the stromal/osteoblastic cell line UAMS-32P, Deng et al. demonstrated that microvesicles shed from osteoblasts contain receptor activator of nuclear factor κ-B ligand (RANKL) and could transfer it to osteoclast precursors through receptor–ligand (RANKL–RANK), leading to the stimulation of RANKL–RANK signaling to facilitate osteoclast formation [37]. This observation was further confirmed by Cappariello et al., who found that osteoblast-derived EVs were enriched in RANKL protein and that RANKL levels increased further after treatment with parathyroid hormone (PTH) through use of primary osteoblasts [38]. RANKL is primarily a transmembrane protein expressed by osteoblasts and osteoclasts, and binds its receptor, RANK, on the surface of osteoclasts and monocytes. The RANKL/RANK axis is a pivotal mediator of osteoclastogenesis, osteoclast function and osteoclast survival [38]. For osteoclast-derived EVs, Huynh et al. demonstrated that the exosome-associated markers CD63 and epithelial cell adhesion molecule (EpCAM) were enriched in the EVs while markers of Golgi and endoplasmic reticulum were not detected. Additionally, RANK was found to be enriched in osteoclast-derived EVs [39]. Similarly, study by Nikolett et al. identified two tetraspanins (CD9, CD63) and two cytosolic (syntenin-1, TSG-101) in EVs isolated from human CD14+ monocytes (recognized as osteoclast precursors) while the negative EXO marker, endoplasmic reticulum-derived calnexin, was absent [40]. Still another group of researchers, Sun et al., demonstrated that osteoclast-derived exosomes specifically recognized osteoblasts through interaction between ephrinA2 and EphA2, a ligand/receptor pair originally found on the surface of osteoblasts [41, 42]. However, EphA2 is widely expressed on EVs derived from various tumor cells, so it would be inaccurate to refer to EphA2 as a unique marker for bone cell-derived EVs [43].
EVs in bone cells communication
Morhayim et al. carried out next generation sequencing to characterize human osteoblast (SV-HFO cells)-derived EV mRNAs under non-mineralizing status [44]. Results showed that the majority of mRNAs were shared between parent osteoblasts and EVs, but a small yet significant fraction of mRNAs is exclusive to either the osteoblasts (5.9%, 693 mRNAs) or the EVs (5.2%, 608 mRNAs). To determine whether or not mRNAs are selectively packaged into EVs, distribution of mRNA abundance and biological significance of the most abundant mRNAs were compared between parental cells and EVs. This study found that the top 100 highly expressed genes account for 58.25% of all mapped EV mRNA read counts, but only account for 34.04% of mapped mRNAs in the parental cells. These results combined with functional analysis indicated that even though most EV mRNA contents mirrors the transcriptome of its donor cells, EVs exhibit a unique set of mRNAs that may support intercellular communication. These findings clearly indicate that osteoblasts do not incorporate mRNAs into EVs randomly during vesicular trafficking and extracellular release but selectively sort mRNAs into EVs. Moreover, this study also found that osteoblast-derived EVs contain 49 unique mRNAs that have not been previously detected in the EVs secreted by other cells or present in body fluids.
Study on miRNA-containing osteoblast-derived EVs found that five miRNAs (miR-199b, miR-218, miR-148a, miR-135b, and miR-221) were differentially expressed depending on the stage of osteogenic differentiation. These differentially expressed miRNAs are involved in RNA degradation, mRNA surveillance pathway, Wnt signaling pathway, and RNA transport pathway, all of which play important roles in osteoblast differentiation [45]. This information not only implies that miRNAs are selectively sorted into EVs by their parental cells, but that EV-mediated genetic information transfer may be a form of autocrine signaling. Unfortunately, no previous studies have reported mRNA or miRNA characterization for bone cells such as osteoclast- and osteocyte-derived EVs.
It has been shown that mRNA that remains in the exosomes and can be translated into proteins in target cells in many tissues. However, most studies on the effects of RNA from EVs on recipient cells have focused on miRNA function. A landmark event in the field came in 2016 when two research groups, Sun et al. and Li et al., both reported that osteoclast-derived miR-214 was transferred to osteoblasts through exosomes and inhibit osteoblast activity [41, 46]. These studies found that miR-214 can negatively impact osteoblastic bone formation by directly targeting the 3′-UTR in the mRNA of an important osteogenic transcription factor called cyclic AMP-dependent transcription factor (ATF4). This cell-to-cell communication was accomplished through exosomes recognizing recipient osteoblasts via the aforementioned ephrinA2/EphA2 axis. Notably, miR-214 has previously been reported to use autocrine signaling to affect osteoclast differentiation. It also plays a crucial role in osteoclastogenesis of bone marrow-derived macrophage (BMM) cells through the PI3K/Akt pathway [47]. However, it is not known whether or not this autocrine effect also involves EVs.
Davis et al. performed a mouse study on EVs in bone cell-to-cell communication and found that even though the size and concentration of bone cell-derived EVs was comparable between young and aged mice, the miRNA contents differed between the two age groups. Specifically, nine miRNAs (miR-183-5p, miR-96-5p, miR-182-5p, miR-335-5p, miR467e-5p, miR-291a-3p, miR-10b-5p, miR-196a-5p, and miR-155-5p) are found to be consistently upregulated in EVs of aged mice [48]. Further investigation demonstrated that miR-183-5p suppresses bone marrow stromal (stem) cell proliferation and induces stem cell senescence. Given that hydrogen peroxide (H2O2) treatment of bone marrow-derived stem cells (BMSCs) increases levels of miR-183-5p in BMSC-derived EVs, this particular form of EV-mediated communication may also have an autocrine effect. Further research is required to determine whether or not this is true. Noticeably, a large number of miRNAs are found to exist in bone cells such as osteoblasts and osteoclasts, and these miRNAs coordinate these two types of cells to maintain bone homeostasis under different conditions [49, 50]. Many of these miRNAs, including miR-503-3p, for example—have recently been found in bone cell-derived EVs [51]. It would follow that at least some of these miRNAs may also be involved in EV-mediated cell-to-cell genetic material exchange and thereby play a role in coordinating bone cell communication.
Genetic material transfer through direct cell-to-cell contact
Gap junctions
Gap junctions are formed by hexameric connexin oligomers that directly connect adjacent cells, forming a direct cell-to-cell communication channel that allows transfer of small molecules such as ions, second messengers, and other small metabolites. Gap junctions are regulated gates that rest in either an open or closed state. Their status is regulated by post-translational modifications of connexins (such as redox-mediated regulation or phosphorylation) or variations in transmembrane physical–chemical conditions (such as transmembrane voltage, pH, and extracellular cation concentration). As mentioned previously, the proteins that form gap junctions are part of a family called connexins (or Cxs). So far, twenty-one members of the Cx family have been cloned and sequenced from mammalian tissues. Cxs can be classified based on their predicted protein molecular weights from corresponding cDNA sequences. For instance, a gap junction protein of 46 kDa was named “connexin46” or “Cx46” [11, 15].
Although gap junction-mediated signal transfer and the crucial role of Cx family in this process have long been known, gap junction-mediated transfer of genetic material—mainly through the action on miRNAs—is an emerging field. Research by Valimas et al. tested whether or not a specific siRNA for DNA polymerase β (pol β) can move from one cell to another via gap junctions and inhibit gene expression in the recipient cell [52]. By introducing fluorescently labeled oligonucleotide probes into one cell of a pair using a patch pipette, they found that these probes can move from one cell to another through gap junctions which consist of connexin 43 (Cx43). They also discovered that the rate of transfer declined as the length of the oligonucleotide probe increased. Further quantitative analysis of the co-culture of pol β knockdown cells with wild-type cells showed that gap junction-transferred siRNAs can function in recipient cells, causing a significant reduction in pol β levels in WT cells [52].
Shuttling of miRNA through gap junctions has further been reported to occur between various cells, including cardiac cells [53, 54], bone marrow stromal and tumor cells [55], and glioma cells [56, 57] and that the delivery of miRNA from a donor cell to the recipient cell, through Cx43 gap junctions can affect the function of a target gene. For instance, a study by Lim et al. demonstrated that transfer of appropriate miRNAs targeting stromal cell-derived factor 1 (CXCL12) from BMSC to breast cancer cells resulted in an inhibition of cell division in the breast cancer cells [55]. Additionally, research by Aucher et al. showed that human macrophages can transfer miRNAs to hepatocarcinoma cells (HCCs) through gap junctions [58]. Two miRNAs—miR-142 and miR-223—that are both endogenously expressed in macrophages but not in HCCs were transferred efficiently between these cells. These miRNAs influenced the post-transcriptional regulation of proteins in HCCs and successfully inhibited proliferation of these cancerous cells. Furthermore, in light of the fact that the resultant gap junctions exhibit specific charge and size permeability as a result of the composition of the Cx proteins, Zong et al. performed a study in which they compared differences in Cx proteins in terms of permeability to miRNAs. In the tested connexin family members, the permeability to miRNAs was found to be as follows: Cx43 > Cx26/30 > Cx26 > Cx31 > Cx30 = Cx-null with Cx43 having highest permeability and Cx30 with lowest permeability to microRNAs [59].
All of these observations suggest a novel function of gap junctions in mediating direct cell-to-cell communication. This function is the direct transfer of genetic information in the form of miRNAs and other nucleic acids at a level sufficient to elicit a biological response in recipient cells. This effect can be aborted through action of dominant negative mutant forms of Cx43 that prevent transfer of the delivery molecule or an application of a gap junction channel uncoupler such as carbenoxolone or octanol.
Gap junction plays a critical role in the coordinated function and activity of nearly all skeletal cells. Its function in bone physiology has been widely discussed and summarized elsewhere [60, 61]. To summarize, gap junctions play a significant role in signal transduction that affects bone modeling and remodeling processes. These signal transduction pathways are ordinarily stimulated by mechanical stresses, but can also occur in response to changes in parathyroid hormone (PTH) levels. The bone modeling and remodeling processes are crucial to bone development, maintenance of bone homeostasis, fracture healing, and other physiological and pathological processes.
Expression of three connexins has been observed in bone and bone cells, with Cx43 being the most prominent in both expression levels and functionality. The other two, Cx45 and Cx26, are also expressed in bone, but their functions have not been elucidated. As mentioned above, Cx43 has the highest permeability to miRNAs, and as such, existing studies in gap junction-mediated miRNA transfer report on this isoform more than other members of the Cx family. It has been reported that BMSCs, the osteoblast progenitors, are able to target CXCL12 expression in target cells through transfer of miRNAs such as miR-127, -197, -222, and -223 via gap junctions [55]. CXC chemokine ligand 12 (CXCL12) has broad effects on cell proliferation and differentiation, and also acts as a chemotactic agent to direct cell migration [62]. Research by Mohammad Shahnazari et al. showed the chemoattractant effect of CXCL12 on osteoblast migration as well as on osteoclast precursor populations, influencing osteoblast/osteoclast recruitment and translocation to the functional site of bone surface [63]. These observations may imply that gap junction-mediated miRNA transfer may represent a means of cell-to-cell communication between BMSCs on an autocrine level and between BMSCs and OB/OC on a paracrine level.
Moreover, as mentioned earlier, Aucher et al. used peripheral blood monocytes (PBMCs) to demonstrate that miR-142 and miR-223 can be transferred via gap junctions, resulting in inhibition of HCC proliferation [58]. PBMCs are a known progenitor of osteoclasts. Addition of sRANKL (30 ng/mL) and M-CSF (25 ng/mL) to PBMC culture medium has stimulated osteoclast differentiation [64]. Noticeably, miR-142 was reported to promote osteoblast differentiation by modulating Wnt signaling and overexpression of miR-223 in osteoclast promotes osteoclastogenesis [65, 66]. Therefore, genetic material may also be transferred through gap junctions among osteoclasts and between osteoclasts and osteoblasts. As of now, direct evidence supporting cell-to-cell movement of siRNA or miRNA through gap junctions between bone cells—especially during certain physiological or pathological processes such as bone remodeling—is scarce. Fortunately, evidence from other tissue cells lays groundwork for the basic theory and methodology that can be applied to bone cells; for instance, use of dual whole patch clamps and fluorescent or radiolabeled probes to investigate gap junction-mediated genetic material transfer may be beneficial to further study of bone cell communication.
Genetic material transfer through RNA-binding proteins
In addition to EVs and gap junctions transfer, some proteins such as Argonaute-2 (Ago2), nucleophosmin 1 (NPM1), and high-density lipoprotein (HDL) are also evidenced to carry genetic material—mostly RNAs—between cells. These proteins form complexes with RNAs and protect them from degradation (Fig. 1). NPM1 was considered the first protein responsible for miRNA transport, having been implicated in this role by the research of Wang et al., who incubated miR-122 with NPM1; this resulted in miR-122 being protected from RNase A [67]. Subsequently, HDL was also found to participate in cell-to-cell communication involving the transport and transfer of miRNAs [68]. The knowledge of when and where the HDL–miRNA complex is formed in vivo has not yet been elucidated.
The protein argonaute2 (Ago2) can also assist in the transport of RNAs between cells. Ago2, a key effector component of miRNA-mediated silencing complex that mediates mRNA repression, forms circulating ribonucleoprotein complexes with miRNAs. The miRNA–Ago2 complex is the main component of RNA-induced silencing complex (RISC) [69]. Since the discovery of RISC in blood plasma by Arroyo et al. attention has been increasingly drawn to this putative method of gene transfer as it is possible that cells release a functional miRNA-induced silencing complex [70]. These RNA–protein complexes have been found to circulate in the body fluids. While the physiological significance of these complexes is still being investigated, they may represent potential biomarkers for certain diseases [69]. While a number of miRNA–protein complexes—particularly the miRNA–Ago2 complex—have been reported to play an important role in intracellular bone cell communication, it is not yet known whether or not these complexes are involved in intercellular bone cell communication. In addition, investigation in blood (both serum and plasma) from patients with osteoporosis or low bone mass showed that a variety of freely circulating miRNAs are associated with bone homeostasis [71–76].
Conclusion/perspective
Evidence is mounting that genetic material, primarily in the form of regulatory RNAs, can be exchanged between cells as a form of information transfer. Extracellular vesicles, gap junctions, and RNA-binding proteins have been named as three major players in this form of cell-to-cell communication. While direct exchange of genetic information is an exciting new paradigm in the field of intercellular communication between bone cells, major questions still remain regarding its biological significance, exact mechanism, and bone tissue-specific characteristics.
In vitro experiments involving overexpression of mRNA and miRNA have shown that both these forms of RNA are functional in recipient cells. The exact role and significance of this form of communication remain unclear in many physiological and pathological processes. As such, the outstanding challenges of elucidating the role of genetic material transfer are to determine (1) whether or not it occurs at endogenous levels in vivo and (2) its physiological importance. These challenges are particularly pressing in light of the fact that protein-mediated intercellular information transfer is much better understood. For example, it is well known that EVs contain not only genetic information but also proteins and lipids. A large number of studies have demonstrated that these EV-derived molecules affect biological responses. As such, a direct demonstration that functional EV-mediated RNA transfer—not EV-mediated protein/lipid transfer—is the exclusive relevant mechanism in certain biological processes is necessary and requires further research. Additionally, as mentioned above, question on whether there are enough microRNAs present in EVs to account for biological activity requires the necessary technical advances in EV isolation and detection, and subsequent understanding of the various and fundamental roles of each type of EV.
It is also worth reiterating that the exact mechanism for RNA transfer remains unknown. As more attention has been paid to the field of EV-mediated intercellular communication, the mechanism has gradually been illuminated. The understanding of RNA transfer at gap junctions is still in its infancy. Further studies are needed to determine the specifics of the molecular pathways regulating the movement of RNA through gap junctions. For circulating RNA–protein complexes, the major question lies in understanding how and why these proteins interact more frequently with certain miRNAs than others. This should lead to better understanding of how RNA–protein complexes cause specific biological effects in recipient cells.
Additionally, even though involvement of the described miRNA is generally well-supported, direct demonstration that functional EV-mediated miRNA transfer is the relevant mechanism is still difficult to achieve. One major concern is that all EVs isolation techniques potentially co-isolate other RNA-binding structures, such as aforementioned miRNA–Ago2 complex [77]. Thus, there is a discussion on whether miRNAs that are actually carried inside the vesicles or those co-isolated with the exosomes on RNA-binding proteins account for the biological activity. Facing such concern, in 2013, the International Society for Extracellular Vesicles (ISEV) recommended in the position paper that treatment of RNA nucleases (RNase) may somehow help discriminate EV-RNA species from extracellular RNA not associated with EVs [78]. Since EV-RNA should be protected from nucleases activity in the environment by the EV lipid bilayer, RNase treatment has, therefore, been used to degrade RNAs co-isolated with the EVs. However, as mentioned in the position paper, it should be noted that some RNA-binding complexes is not RNase-sensitive (such as aforementioned NPM1 protecting miR-122 from RNase A). Additionally, proteins on the surface of EVs may bind and protect RNA from degradation. Thus RNase treatment may be effective in removing contaminating RNA molecules that are passively released by dead cells and which may non-specifically stick to EVs in environments that are low in nuclease content. Subsequently, in 2014, the other position paper by ISEV highlighted that additional steps of separation of EVs from other structures, e.g., by floatation into density gradients or by immuno-isolation via specific antibodies, are necessary before claiming specific EV-mediated miRNA transfer [79] In addition, knockdown or inhibition of sphingomyelinases (SMases) by small-molecule inhibitors, which results in impaired ceramide formation, has often been used to inhibit exosome/EV secretion. Therefore, making use of such systematic negative controls to provide insights into the “background” extracellular miRNAs’ functional activity or signal may possibly help distinguish the proportion of functional “activity” present in the soluble versus that present inside of EVs [80]. However, the specificity of this effect for EV secretion remains unclear, as opposed to other secretions including RNA-binding complex.
Finally, in terms of bone and bone cells, some of the means of genetic information exchange—for instance, RNA–protein complexes—still require further verification. Furthermore, it remains unknown whether or not there are novel forms of genetic information transfer that are exclusive to bone tissue and cells. For instance, previous studies have demonstrated that bone cells can communicate by releasing molecules that are deposited in the bone matrix, and the possibility that genetic material can be transferred through such a mode of communication merits further investigation. In conclusion, more effort is still required to solve the specific puzzle of this new paradigm in bone cell communication.
Potentially clinical significance
As a result of advances in next generation sequencing technology, diseases such as cancer, Parkinson’s, rheumatoid arthritis, and Alzheimer’s etc. have all had many of their genetic components revealed, bringing us closer than ever to ‘personalized medicine’. Thus far, investigation in capturing the genetic messages shared by cells has been adapted for diagnostic use. For instance, the so-called “liquid biopsy” into circulating RNA has been adapted into a useful diagnostic tool in tumors. Kits or microfluidic chips designed for diagnosing certain disease have been merchandized and benefit both doctors and patients in point-of-care situation. The knowledge in this area also has shown the potential in therapeutic application. Nonetheless, it still has a long way to go before fully being translated to pharmaceutical interventions addressing certain diseases. Specifically, as for bone diseases, the topic is still new and progresses are making in assembling the puzzle of genetic information communications across the bone cells.
Acknowledgements
The article was funded by National Natural Science Foundation of China (81702176).
Compliance with ethical standards
Conflict of interest
Pengbin Yin, Houchen Lv, Yi Li, Deng Yuan, Licheng Zhang, Peifu Tang declare that they have no conflict of interest.
Footnotes
Pengbin Yin and Yi Li contributed equally to this paper.
Contributor Information
Licheng Zhang, Phone: 86-10-66938201, Email: zhanglcheng218@126.com.
Peifu Tang, Phone: 86-10-66938201, Email: pftang301@126.com.
References
- 1.Alberts B, Johnson A, Lewis J, Raff M, Roberts K, Walter P (2017) General principles of cell communication. https://www.ncbi.nlm.nih.gov/books/NBK26813/. Cited 10 Nov 2017
- 2.Mittelbrunn M, Sánchez-Madrid F. Intercellular communication: diverse structures for exchange of genetic information. Nat Rev Mol Cell Biol. 2012;13(5):328–335. doi: 10.1038/nrm3335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fire A, Mello CC, Montgomery MK, Driver SE, Xu S, Kostas SA. Potent and specific genetic interference by double-stranded RNA in Caenorhabditis elegans. Nature. 1998;391(6669):806. doi: 10.1038/35888. [DOI] [PubMed] [Google Scholar]
- 4.Brosnan CA, Voinnet O. Cell-to-cell and long-distance siRNA movement in plants: mechanisms and biological implications. Curr Opin Plant Biol. 2011;14(5):580–587. doi: 10.1016/j.pbi.2011.07.011. [DOI] [PubMed] [Google Scholar]
- 5.Carlsbecker A, Lee J-Y, Roberts CJ, Dettmer J, Lehesranta S, Zhou J, et al. Cell signalling by microRNA165/6 directs gene dose-dependent root cell fate. Nature. 2010;465(7296):316–321. doi: 10.1038/nature08977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Melnyk CW, Molnar A, Baulcombe DC. Intercellular and systemic movement of RNA silencing signals. EMBO J. 2011;30(17):3553–3563. doi: 10.1038/emboj.2011.274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Roberts CT, Kurre P. Vesicle trafficking and RNA transfer add complexity and connectivity to cell–cell communication. Cancer Res. 2013;73(11):3200–3205. doi: 10.1158/0008-5472.CAN-13-0265. [DOI] [PubMed] [Google Scholar]
- 8.Chen X, Liang H, Zhang J, Zen K, Zhang C-Y. Secreted microRNAs: a new form of intercellular communication. Trends Cell Biol. 2012;22(3):125–132. doi: 10.1016/j.tcb.2011.12.001. [DOI] [PubMed] [Google Scholar]
- 9.Théry C (2011) Exosomes: secreted vesicles and intercellular communications. F1000 Biol Rep [Internet]. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3155154/. Cited 7 Nov 2017 [DOI] [PMC free article] [PubMed]
- 10.Tkach M, Théry C. Communication by extracellular vesicles: where we are and where we need to go. Cell. 2016;164(6):1226–1232. doi: 10.1016/j.cell.2016.01.043. [DOI] [PubMed] [Google Scholar]
- 11.Brink PR, Valiunas V, Gordon C, Rosen MR, Cohen IS. Can gap junctions deliver? Biochim Biophys Acta BBA Biomembr. 2012;1818(8):2076–2081. doi: 10.1016/j.bbamem.2011.09.025. [DOI] [PubMed] [Google Scholar]
- 12.Park Y-E, Musson DS, Naot D, Cornish J. Cell–cell communication in bone development and whole-body homeostasis and pharmacological avenues for bone disorders. Curr Opin Pharmacol. 2017;34(Suppl C):21–35. doi: 10.1016/j.coph.2017.04.001. [DOI] [PubMed] [Google Scholar]
- 13.Pfeilschifter J, Mundy GR. Modulation of type beta transforming growth factor activity in bone cultures by osteotropic hormones. Proc Natl Acad Sci USA. 1987;84(7):2024–2028. doi: 10.1073/pnas.84.7.2024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Matsuo K, Irie N. Osteoclast–osteoblast communication. Arch Biochem Biophys. 2008;473(2):201–209. doi: 10.1016/j.abb.2008.03.027. [DOI] [PubMed] [Google Scholar]
- 15.Aasen T, Mesnil M, Naus CC, Lampe PD, Laird DW. Gap junctions and cancer: communicating for 50 years. Nat Rev Cancer. 2016;16(12):nrc.2016.105. doi: 10.1038/nrc.2016.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Colombo M, Raposo G, Théry C. Biogenesis, secretion, and intercellular interactions of exosomes and other extracellular vesicles. Annu Rev Cell Dev Biol. 2014;30(1):255–289. doi: 10.1146/annurev-cellbio-101512-122326. [DOI] [PubMed] [Google Scholar]
- 17.Ciardiello C, Cavallini L, Spinelli C, Yang J, Reis-Sobreiro M, de Candia P, et al. Focus on extracellular vesicles: new frontiers of cell-to-cell communication in cancer. Int J Mol Sci. 2016;17(2):175. doi: 10.3390/ijms17020175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bossios A, Valadi H, Lee JJ, Lötvall JO, Ekström K, Sjöstrand M. Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nat Cell Biol. 2007;9(6):654. doi: 10.1038/ncb1596. [DOI] [PubMed] [Google Scholar]
- 19.Bartel DP. MicroRNAs: genomics, biogenesis, mechanism, and function. Cell. 2004;116(2):281–297. doi: 10.1016/S0092-8674(04)00045-5. [DOI] [PubMed] [Google Scholar]
- 20.Ciaudo C, Gibbings DJ, Erhardt M, Voinnet O. Multivesicular bodies associate with components of miRNA effector complexes and modulate miRNA activity. Nat Cell Biol. 2009;11(9):1143. doi: 10.1038/ncb1929. [DOI] [PubMed] [Google Scholar]
- 21.Nolte-’t Hoen ENM, Buermans HPJ, Waasdorp M, Stoorvogel W, Wauben MHM, ’t Hoen PAC. Deep sequencing of RNA from immune cell-derived vesicles uncovers the selective incorporation of small non-coding RNA biotypes with potential regulatory functions. Nucleic Acids Res. 2012;40(18):9272–9285. doi: 10.1093/nar/gks658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kharaziha P, Ceder S, Li Q, Panaretakis T. Tumor cell-derived exosomes: a message in a bottle. Biochim Biophys Acta. 2012;1826(1):103–111. doi: 10.1016/j.bbcan.2012.03.006. [DOI] [PubMed] [Google Scholar]
- 23.van Balkom BWM, de Jong OG, Smits M, Brummelman J, den Ouden K, de Bree PM, et al. Endothelial cells require miR-214 to secrete exosomes that suppress senescence and induce angiogenesis in human and mouse endothelial cells. Blood. 2013;121(19):3997–4006. doi: 10.1182/blood-2013-02-478925. [DOI] [PubMed] [Google Scholar]
- 24.Bernad A, Villarroya-Beltri C, Gutiérrez-Vázquez C, Sánchez-Cabo F, Sánchez-Madrid F, González MÁ, et al. Unidirectional transfer of microRNA-loaded exosomes from T cells to antigen-presenting cells. Nat Commun. 2011;19(2):282. doi: 10.1038/ncomms1285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Montecalvo A, Larregina AT, Shufesky WJ, Stolz DB, Sullivan MLG, Karlsson JM, et al. Mechanism of transfer of functional microRNAs between mouse dendritic cells via exosomes. Blood. 2012;119(3):756–766. doi: 10.1182/blood-2011-02-338004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pascual-Montano A, Villarroya-Beltri C, Gutiérrez-Vázquez C, Pérez-Hernández D, Martinez-Herrera DJ, Sánchez-Cabo F, et al. Sumoylated hnRNPA2B1 controls the sorting of miRNAs into exosomes through binding to specific motifs. Nat Commun. 2013;20(4):2980. doi: 10.1038/ncomms3980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Filipenko NR, MacLeod TJ, Yoon C-S, Waisman DM. Annexin A2 is a novel RNA-binding protein. J Biol Chem. 2004;279(10):8723–8731. doi: 10.1074/jbc.M311951200. [DOI] [PubMed] [Google Scholar]
- 28.Villarroya-Beltri C, Baixauli F, Gutiérrez-Vázquez C, Sánchez-Madrid F, Mittelbrunn M. Sorting it out: regulation of exosome loading. Semin Cancer Biol. 2014;28(Suppl C):3–13. doi: 10.1016/j.semcancer.2014.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Babst M. MVB vesicle formation: ESCRT-dependent, ESCRT-independent and everything in between. Curr Opin Cell Biol. 2011;23(4):452–457. doi: 10.1016/j.ceb.2011.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Skog J, Wurdinger T, van Rijn S, Meijer D, Gainche L, Sena-Esteves M, et al. Glioblastoma microvesicles transport RNA and protein that promote tumor growth and provide diagnostic biomarkers. Nat Cell Biol. 2008;10(12):1470–1476. doi: 10.1038/ncb1800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bergsmedh A, Castro J, Ehnfors J, Holmgren L, Yang L, Kost-Alimova M, et al. Horizontal transfer of tumor DNA to endothelial cells in vivo. Cell Death Differ. 2009;16(5):749. doi: 10.1038/cdd.2009.7. [DOI] [PubMed] [Google Scholar]
- 32.Chevillet JR, Kang Q, Ruf IK, Briggs HA, Vojtech LN, Hughes SM, et al. Quantitative and stoichiometric analysis of the microRNA content of exosomes. Proc Natl Acad Sci. 2014;111(41):14888–14893. doi: 10.1073/pnas.1408301111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Alexander M, Hu R, Runtsch MC, Kagele DA, Mosbruger TL, Tolmachova T, et al. Exosome-delivered microRNAs modulate the inflammatory response to endotoxin. Nat Commun. 2015;18(6):7321. doi: 10.1038/ncomms8321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Liu J, Li D, Wu X, Dang L, Lu A, Zhang G. Bone-derived exosomes. Curr Opin Pharmacol. 2017;1(34):64–69. doi: 10.1016/j.coph.2017.08.008. [DOI] [PubMed] [Google Scholar]
- 35.Morhayim J, van de Peppel J, Demmers JAA, Kocer G, Nigg AL, van Driel M, et al. Proteomic signatures of extracellular vesicles secreted by nonmineralizing and mineralizing human osteoblasts and stimulation of tumor cell growth. FASEB J. 2015;29(1):274–285. doi: 10.1096/fj.14-261404. [DOI] [PubMed] [Google Scholar]
- 36.Ge M, Ke R, Cai T, Yang J, Mu X. Identification and proteomic analysis of osteoblast-derived exosomes. Biochem Biophys Res Commun. 2015;467(1):27–32. doi: 10.1016/j.bbrc.2015.09.135. [DOI] [PubMed] [Google Scholar]
- 37.Deng L, Wang Y, Peng Y, Wu Y, Ding Y, Jiang Y, et al. Osteoblast-derived microvesicles: a novel mechanism for communication between osteoblasts and osteoclasts. Bone. 2015;1(79):37–42. doi: 10.1016/j.bone.2015.05.022. [DOI] [PubMed] [Google Scholar]
- 38.Cappariello A, Loftus A, Muraca M, Maurizi A, Rucci N, Teti A. Osteoblast-derived extracellular vesicles are biological tools for the delivery of active molecules to bone. J Bone Miner Res. 2017 doi: 10.1002/jbmr.3332. [DOI] [PubMed] [Google Scholar]
- 39.Huynh N, VonMoss L, Smith D, Rahman I, Felemban MF, Zuo J, et al. Characterization of regulatory extracellular vesicles from osteoclasts. J Dent Res. 2016;95(6):673–679. doi: 10.1177/0022034516633189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Marton N, Kovács OT, Baricza E, Kittel Á, Győri D, Mócsai A, et al. Extracellular vesicles regulate the human osteoclastogenesis: divergent roles in discrete inflammatory arthropathies. Cell Mol Life Sci. 2017;74(19):3599–3611. doi: 10.1007/s00018-017-2535-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sun W, Zhao C, Li Y, Wang L, Nie G, Peng J, et al. Osteoclast-derived microRNA-containing exosomes selectively inhibit osteoblast activity. Cell Discov. 2016;2:celldisc201615. doi: 10.1038/celldisc.2016.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Matsuo K, Otaki N. Bone cell interactions through Eph/ephrin. Cell Adhes Migr. 2012;6(2):148–156. doi: 10.4161/cam.20888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pasquale EB. Exosomes expand the sphere of influence of Eph receptors and ephrins. J Cell Biol. 2016;214:5–7. doi: 10.1083/jcb.201606074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Morhayim J, van de Peppel J, Dudakovic A, Chiba H, van Wijnen AJ, van Leeuwen JP. Molecular characterization of human osteoblast-derived extracellular vesicle mRNA using next-generation sequencing. Biochim Biophys Acta BBA Mol Cell Res. 2017;1864(7):1133–1141. doi: 10.1016/j.bbamcr.2017.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Xu J-F, Yang G, Pan X-H, Zhang S-J, Zhao C, Qiu B-S, et al. Altered MicroRNA expression profile in exosomes during osteogenic differentiation of human bone marrow-derived mesenchymal stem cells. PLoS One. 2014;9(12):e114627. doi: 10.1371/journal.pone.0114627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lu A, Qian A, Shaikh AB, Zhang B-T, Jiang B, Guo B, et al. Osteoclast-derived exosomal miR-214-3p inhibits osteoblastic bone formation. Nat Commun. 2016;7(7):10872. doi: 10.1038/ncomms10872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhao C, Sun W, Zhang P, Ling S, Li Y, Zhao D, et al. miR-214 promotes osteoclastogenesis by targeting Pten/PI3K/Akt pathway. RNA Biol. 2015;12(3):343–353. doi: 10.1080/15476286.2015.1017205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Davis C, Dukes A, Drewry M, Helwa I, Johnson MH, Isales CM, et al. MicroRNA-183-5p increases with age in bone-derived extracellular vesicles, suppresses bone marrow stromal (stem) cell proliferation, and induces stem cell senescence. Tissue Eng Part A. 2017;23(21–22):1231–1240. doi: 10.1089/ten.tea.2016.0525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.van Wijnen AJ, Stein GS, Lian JB, Stein JL, Hassan MQ, Gaur T, et al. MicroRNA control of bone formation and homeostasis. Nat Rev Endocrinol. 2012;8(4):212. doi: 10.1038/nrendo.2011.234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tang P, Xiong Q, Ge W, Zhang L. The role of microRNAs in osteoclasts and osteoporosis. RNA Biol. 2014;11(11):1355–1363. doi: 10.1080/15476286.2014.996462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Xie Y, Chen Y, Zhang L, Ge W, Tang P. The roles of bone-derived exosomes and exosomal microRNAs in regulating bone remodelling. J Cell Mol Med. 2017;21(5):1033–1041. doi: 10.1111/jcmm.13039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Valiunas V, Polosina YY, Miller H, Potapova IA, Valiuniene L, Doronin S, et al. Connexin-specific cell-to-cell transfer of short interfering RNA by gap junctions. J Physiol. 2005;568(2):459–468. doi: 10.1113/jphysiol.2005.090985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hosoda T, Zheng H, Cabral-da-Silva M, Sanada F, Ide-Iwata N, Ogórek B, et al. Human cardiac stem cell differentiation is regulated by a mircrine mechanism. Circulation. 2011;123(12):1287–1296. doi: 10.1161/CIRCULATIONAHA.110.982918. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 54.Cingolani E, Kizana E, Marbán E. Non-cell-autonomous effects of vector-expressed regulatory RNAs in mammalian heart cells. Gene Ther. 2009;16(9):1163. doi: 10.1038/gt.2009.64. [DOI] [PubMed] [Google Scholar]
- 55.Lim PK, Bliss SA, Patel SA, Taborga M, Dave MA, Gregory LA, et al. Gap junction-mediated import of microRNA from bone marrow stromal cells can elicit cell cycle quiescence in breast cancer cells. Cancer Res. 2011;71(5):1550–1560. doi: 10.1158/0008-5472.CAN-10-2372. [DOI] [PubMed] [Google Scholar]
- 56.Suzhi Z, Liang T, Yuexia P, Lucy L, Xiaoting H, Yuan Z, et al. Gap junctions enhance the antiproliferative effect of microRNA-124-3p in glioblastoma cells. J Cell Physiol. 2015;230(10):2476–2488. doi: 10.1002/jcp.24982. [DOI] [PubMed] [Google Scholar]
- 57.Katakowski M, Buller B, Wang X, Rogers T, Chopp M. Functional microRNA is transferred between glioma cells. Cancer Res. 2010;70(21):8259–8263. doi: 10.1158/0008-5472.CAN-10-0604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Aucher A, Rudnicka D, Davis DM. MicroRNAs transfer from human macrophages to hepato-carcinoma cells and inhibit proliferation. J Immunol. 2013;191(12):6250–6260. doi: 10.4049/jimmunol.1301728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zong L, Zhu Y, Liang R, Zhao H-B (2016) Gap junction mediated miRNA intercellular transfer and gene regulation: a novel mechanism for intercellular genetic communication. Sci Rep [Internet]. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4728487/. Cited 20 Nov 2017 [DOI] [PMC free article] [PubMed]
- 60.Stains JP, Civitelli R. Gap junctions in skeletal development and function. Biochim Biophys Acta BBA Biomembr. 2005;1719(1–2):69–81. doi: 10.1016/j.bbamem.2005.10.012. [DOI] [PubMed] [Google Scholar]
- 61.Loiselle AE, Jiang JX, Donahue HJ. Gap junction and hemichannel functions in osteocytes. Bone. 2013;54(2):205–212. doi: 10.1016/j.bone.2012.08.132. [DOI] [PubMed] [Google Scholar]
- 62.Sun X, Cheng G, Hao M, Zheng J, Zhou X, Zhang J, et al. CXCL12/CXCR4/CXCR7 chemokine axis and cancer progression. Cancer Metastasis Rev. 2010;29(4):709–722. doi: 10.1007/s10555-010-9256-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Shahnazari M, Chu V, Wronski TJ, Nissenson RA, Halloran BP. CXCL12/CXCR4 signaling in the osteoblast regulates the mesenchymal stem cell and osteoclast lineage populations. FASEB J. 2013;27(9):3505–3513. doi: 10.1096/fj.12-225763. [DOI] [PubMed] [Google Scholar]
- 64.Li C, Zheng C, Li D, Ning G, Qu G, Lin H, et al. LGR4 is a receptor for RANKL and negatively regulates osteoclast differentiation and bone resorption. Nat Med. 2016;22(5):539. doi: 10.1038/nm.4076. [DOI] [PubMed] [Google Scholar]
- 65.Hu W, Ye Y, Zhang W, Wang J, Chen A, Guo F. miR-142-3p promotes osteoblast differentiation by modulating Wnt signaling. Mol Med Rep. 2013;7(2):689–693. doi: 10.3892/mmr.2012.1207. [DOI] [PubMed] [Google Scholar]
- 66.M’Baya-Moutoula E, Louvet L, Metzinger-Le Meuth V, Massy ZA, Metzinger L. High inorganic phosphate concentration inhibits osteoclastogenesis by modulating miR-223. Biochim Biophys Acta BBA Mol Basis Dis. 2015;1852(10, part A):2202–2212. doi: 10.1016/j.bbadis.2015.08.003. [DOI] [PubMed] [Google Scholar]
- 67.Wang K, Zhang S, Weber J, Baxter D, Galas DJ. Export of microRNAs and microRNA-protective protein by mammalian cells. Nucleic Acids Res. 2010;38(20):7248–7259. doi: 10.1093/nar/gkq601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Vickers KC, Palmisano BT, Shoucri BM, Shamburek RD, Remaley AT. MicroRNAs are transported in plasma and delivered to recipient cells by high-density lipoproteins. Nat Cell Biol. 2011;13(4):423–433. doi: 10.1038/ncb2210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Creemers EE, Tijsen AJ, Pinto YM. Circulating microRNAs: novel biomarkers and extracellular communicators in cardiovascular disease? Circ Res. 2012;110(3):483–495. doi: 10.1161/CIRCRESAHA.111.247452. [DOI] [PubMed] [Google Scholar]
- 70.Arroyo JD, Chevillet JR, Kroh EM, Ruf IK, Pritchard CC, Gibson DF, et al. Argonaute2 complexes carry a population of circulating microRNAs independent of vesicles in human plasma. Proc Natl Acad Sci. 2011;108(12):5003–5008. doi: 10.1073/pnas.1019055108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Seeliger C, Karpinski K, Haug AT, Vester H, Schmitt A, Bauer JS, et al. Five freely circulating miRNAs and bone tissue miRNAs are associated with osteoporotic fractures. J Bone Miner Res. 2014;29(8):1718–1728. doi: 10.1002/jbmr.2175. [DOI] [PubMed] [Google Scholar]
- 72.Meng J, Zhang D, Pan N, Sun N, Wang Q, Fan J, et al. Identification of miR-194-5p as a potential biomarker for postmenopausal osteoporosis. PeerJ. 2015;21(3):e971. doi: 10.7717/peerj.971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Panach L, Mifsut D, Tarín JJ, Cano A, García-Pérez MÁ. Serum circulating microRNAs as biomarkers of osteoporotic fracture. Calcif Tissue Int. 2015;97(5):495–505. doi: 10.1007/s00223-015-0036-z. [DOI] [PubMed] [Google Scholar]
- 74.Weilner S, Skalicky S, Salzer B, Keider V, Wagner M, Hildner F, et al. Differentially circulating miRNAs after recent osteoporotic fractures can influence osteogenic differentiation. Bone. 2015;1(79):43–51. doi: 10.1016/j.bone.2015.05.027. [DOI] [PubMed] [Google Scholar]
- 75.Heilmeier U, Hackl M, Skalicky S, Weilner S, Schroeder F, Vierlinger K, et al. Serum miRNA signatures are indicative of skeletal fractures in postmenopausal women with and without type 2 diabetes and influence osteogenic and adipogenic differentiation of adipose tissue-derived mesenchymal stem cells in vitro. J Bone Miner Res. 2016;31(12):2173–2192. doi: 10.1002/jbmr.2897. [DOI] [PubMed] [Google Scholar]
- 76.Yavropoulou MP, Anastasilakis AD, Makras P, Tsalikakis DG, Grammatiki M, Yovos JG. Expression of microRNAs that regulate bone turnover in the serum of postmenopausal women with low bone mass and vertebral fractures. Eur J Endocrinol. 2017;176(2):169–176. doi: 10.1530/EJE-16-0583. [DOI] [PubMed] [Google Scholar]
- 77.Palma J, Yaddanapudi SC, Pigati L, Havens MA, Jeong S, Weiner GA, et al. MicroRNAs are exported from malignant cells in customized particles. Nucleic Acids Res. 2012;40(18):9125–9138. doi: 10.1093/nar/gks656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Hill AF, Pegtel DM, Lambertz U, Leonardi T, O’Driscoll L, Pluchino S, et al. ISEV position paper: extracellular vesicle RNA analysis and bioinformatics. J Extracell Vesicles. 2013;2(1):22859. doi: 10.3402/jev.v2i0.22859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Lötvall J, Hill AF, Hochberg F, Buzás EI, Vizio DD, Gardiner C, et al. Minimal experimental requirements for definition of extracellular vesicles and their functions: a position statement from the International Society for Extracellular Vesicles. J Extracell Vesicles. 2014;3(1):26913. doi: 10.3402/jev.v3.26913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Trajkovic K, Hsu C, Chiantia S, Rajendran L, Wenzel D, Wieland F, et al. Ceramide triggers budding of exosome vesicles into multivesicular endosomes. Science. 2008;319(5867):1244–1247. doi: 10.1126/science.1153124. [DOI] [PubMed] [Google Scholar]