Abstract
Exosomes represent an important group of extracellular vesicles with a defined size between 40 and 150 nm and cup-shaped construction which have a pivotal role in elimination of intracellular debris and intercellular signaling networks. A line of evidence revealed the impact of different types of exosomes in initiation, progression, and metastasis of gastric cancer (GC). These bioactive vesicles mediate tumor and stromal communication network through modulation of cell signaling for carcinogenesis and pre-metastatic niche formation in distant organs. Exosomes contain various cargos including DNAs (mitochondrial and genomic), proteins, transposable elements, and RNAs (coding and noncoding) with different compositions related to functional status of origin cells. In this review, we summarize the main roles of key exosomal cargos in induction of exosome-mediated signaling in cancer cells. Body fluids are employed frequently as the source of exosomes released by tumor cells with a potential role in early diagnosis of GC and chemoresistance. These vesicles as non-toxic and non-immunogenic carriers are also found to be applied for novel drug delivery systems.
Keywords: MiRNA, Long non-coding RNA, Signaling pathways, Biomarker, Chemoresistance, Exosome-dependent drug delivery
Introduction
Gastric cancer (GC) is the fourth prevalent malignancy and the second prominent cause of cancer-related death worldwide which is recognized as a multifactorial disease with a heterogeneous nature. Five-year survival rate of GC patients is reported 10–30% due to late diagnosis with deprive curative resections [1, 2]. Surgery removal of the tumors beside pre- or post-operative chemo-, chemo radio- and adjuvant therapies are the main strategies to boost survival rate [3, 4]. Metastasis as the main cause of cancer death is orchestrated by multistep intrinsic and extrinsic molecular cascades in tumoral and stromal cells. GC dissemination targets both local tissues via direct invasion and distant organs by seeding for pre-metastatic niches through secreted elements [5, 6]. Advances in high-resolution imaging introduced a novel class of extracellular vesicles in the cell secretome recognized as exosomes with data trafficking and cell reprogramming capability to contribute in signal transduction of pathophysiological conditions [7, 8]. This study is an overview of the recent knowledge on the general structure of exosomes and their role in initiation and progression of malignancies, particularly in GC. Since established characteristics of exosomes strongly depend upon the cells of the origin and status of development, key modulating molecules, related signaling pathways and clinical relevance to GC will be reviewed. Furthermore, exosome contents, as potential diagnostic biomarkers with possible role in induction of chemoresistance followed by their application in exosomes-based strategies to overcome this resistance will be discussed.
Structure and biological functions of exosomes
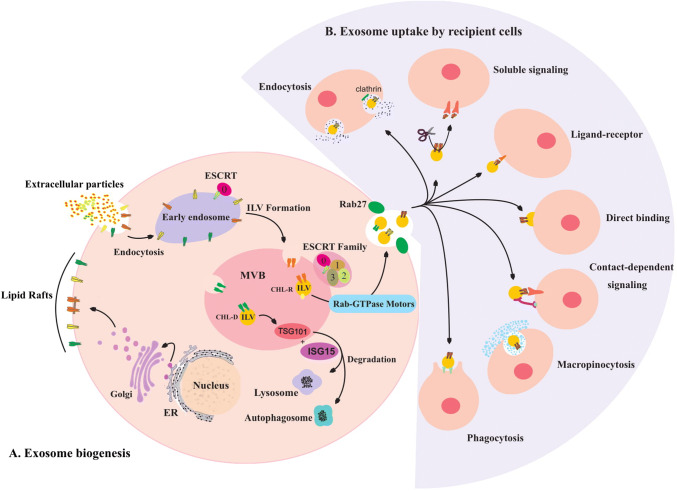
Exosomes are nanometer-sized bioactive extracellular vesicles, span 40–150 nm in diameter and encompass cytoplasm fractions without any organelles by a lipid bilayer membrane with similar orientation of origin cell. These vesicles have spherical or cup-shaped construction which are released to the extracellular space by multitude of cells into the extracellular space with a central role in initiation and progression of intercellular signaling networks [9–12]. The main endosomal proteins of the vesicles which are known as exosome surface markers include CD9, CD81, and CD82. Exosome biogenesis is initiated by membrane invagination of early endosomes to form intracellular multivesicular bodies (MVBs) which organize intraluminal vesicles under control of the endosomal sorting complex that is required for the transport (ESCRT) signaling. Cholesterol-rich MVBs are conveyed into the cellular membrane under traction of Rab GTPases 27A/B molecular motors to dock, fuse and exocytose exosomes into the extracellular space, while cholesterol-deficient MVBs are recycled by lysosome and/or autophagosome complexes under regulation of ISG15-ISGylation on tumor susceptibility gene 101 protein (TSG101). Exosomes contain various cargos including DNAs (mitochondrial and genomic), proteins, transposable elements, and RNAs (coding and noncoding) with different compositions originated from functional status of source cells [13–16]. Exosome organotrophy is planned by patterns of transmembrane integrins, whereas preferential uptake is mediated by macropinocytosis, clathrin-dependent and -independent endocytosis, phagocytosis, ligand-receptor interaction and direct binding. These vesicles can also induce various signaling pathways without cellular internalization through soluble and contact-dependent (juxtacrine) signal transduction [14, 17] (Fig. 1).
Fig. 1.
a Lipid rafts are produced by Golgi apparatus and facilitate endocytosis which provides extrinsic exosome cargos. Endosomal and cytosolic contents are encompassed in exosomes during invagination of intraluminal vesicles (ILVs) inside the multivesicular bodies (MVBs) under control of endosomal sorting complex required for the transport (ESCRT) family signals. Cholesterol-rich (CHL-R) pre-exosomes are convened in the cellular membrane by Rab-GTPase motors to be released in cooperation with Rab-27, but cholesterol deficient (CHL-D) pre-exosomes are degraded under control of ISG15-ISGylation on TSG101 through lysosome or autophagosome complexes. b Exosome uptake is mediated by several mechanisms including: macropinocytosis, clathrin-dependent and -independent endocytosis, phagocytosis, ligand-receptor interaction and direct binding through soluble and contact-dependent signaling
Generally, cells remove hazardous and redundant intracellular constituents including different drugs through exosomes in conserved condition with minimum susceptibility of degradation to avoid local contamination [18]. Exact physiologic features of exosomes are largely unexplored except in a few insights on their role in modulation of immune response by antigen presenting capability, synaptic physiology, and cellular hemostasis.
In the period of cellular stress, elevated exosome secretion subscribes to maintain cellular hemostasis by preventing cytosolic nuclear DNA accumulation to restrain innate immune response for cell cycle arrest or apoptosis in normal cells [19]. Constant presence of exosomes in body fluids (saliva, breast milk, plasma, bronchial lavage, amniotic fluid, abdominal cavity effusion, urine, and cerebrospinal fluid), extracellular spaces, and different tissues postulate their role as the important modulators in signal transduction pathways accompanied with cell–cell contact and secretome signaling [20]. The role of these vesicles in initiation and progression of inflammation, autoimmune and neurological diseases, infectious disease, and cancers are well known. Small non-coding RNAs (MiRNAs) are known as the most abundant and crucial biomolecules derived from exosomes which play a pivotal role in tumor regulation [21–23]. Excessive amount of circulating exosomes along with exosomal cargo in cancer, suggest possible contribution of these subcellular secretory nanoparticles to establish complex cross-talk networks in dependent signaling pathways for initiation, progression and dissemination of tumors.
Exosome and gastric cancer
Tumor development requires continuous carcinogenic reprogramming to establish malignant characteristics in the cells. Exosomes are potential communicative vectors to share oncosignals with dual role in tumorigenesis due to cell of the origin. Normal cell exosomes block the corresponding cancer signaling pathways by tumor-suppressive profile, however, oncogenic contents of tumor exosomes (TEXs) provoke recipient cells to acquire malignant characteristics [24, 25]. For instance, normal cells secrete exosomes containing miR-101, as an anti-tumor molecule, into the systemic circulation to suppress GC tumor cells. During early stages of the tumorigenesis, downregulation of anti-tumoral miRNAs in cancer cells and insufficient exosomal supplement of miR-101 by microenvironment of residential cells stimulate GC development [26]. Tumor suppressor genes are generally targeted by Onco-exomiRs in recipient cells, several exomiRs are known to trigger carcinogenesis in GC, including exomiR-Let7 of AZ-P7a cells that inhibit expression of common tumor suppressors and high mobility group at-hook-2 to establish tumors. Moreover, mesenchymal stem cells (MSCs)-derived exosomes from gastric tumor biopsies contain exomiR-221, which stimulates HGC-27 cells proliferation. ExomiR-217 is able to target and downregulate cadherin-1, a tumor suppressor gene, which is responsible for maintaining cell–cell adhesion, this downregulation causes tumor proliferation. Downregulation of cadherin-1 predispose cells for tumor initiation, meanwhile declined load of cadherin-1 in host cell derived exosomes facilitate establishment of malignancy in the cells within cross-talk networks [27–29]. Exosomes of primary tumors adjacent to gastric cells also provide cancer initiation. Three-dimensional culture system of stem cells revealed that esophageal cancer-derived exo-miR-25 and -210 effectively reprogram gastric organoids that provides cancer initiation by inhibition of phosphatase and tensin homolog (PTEN) and apoptosis inducing factor mitochondria associated-3 (AIFM3) genes expression. PTEN and AIFM3 are apoptotic genes which their down-expression roll out apoptosis for uncontrolled cell proliferation [30]. Mitogen-activated protein kinase/extracellular-signal-regulated kinase (MAPK/ERK) signaling pathway is mostly involved in tumor initiation and progression which are regulated by exosomes. Tumor cell-derived exosomes partially activate MAPK/ERK and PI3K/Akt pathways in SGC7901 and BGC823 GC cells without any alteration in extracellular signal-regulated kinases-1/2 and Akt cytoplasmic level, suggesting epigenetic reprogramming through induction of phosphorylation in Akt and ERK1/2 to promote cancer. Exosomes which contain CD97 including human bone marrow MSC exosomes activate MAPK and hedgehog signaling pathways in SGC-7901 GC cells that elevate cellular proliferation to initiate cancerous features [31–33]. These vesicles also transform recipient cells to expand malignant characteristics, orchestrate cell transcriptome and proteome landscapes to co-opt bi-directionally with the surrounding cell derived exosomes in the tumor microenvironment to stimulate cancer invasion and progression into the distant organs.
Exosomes/ExomiRs and GC invasion and metastasis
During multiple steps of metastasis procedure, cells loose adhesion into the stroma and migrate into the bloodstream to reach pre-metastatic niches followed by establishment of secondary tumors. Notably, only 0.01% of circulating tumor cells successfully develop distant organ metastasis [34]. GC microenvironment includes various types of stromal cells corresponding for tumor metabolism. The multidirectional communication network of cancer and stromal cells based on secreted compounds comprising exosomes, participate in cancer metastasis [35]. For example, extracted exosomes of KatoIII and MKN45 GC cell lines elevate MKN45 cell invasion and migration through upregulation of fibronectin-1 (FN1) and laminin Subunit Gamma-1 (LAMC1). Similarly, in vivo studies revealed enhanced adhesion of mesothelial cells into the GC cells during peritoneal metastasis with similar pattern of elevated FN1 and LAMC1. Moreover, in vitro treatment of mesothelial cells by malignant pleural effusion derived exosomes shows similar raise in expression pattern of FN1 and LAMC1 in MKN45 cells, but the effect is not reciprocal [36, 37]. Overexpression of oncogenic exomiR-21, -320c, -1225-5p, -1202, -4270, -1207 in the peritoneal lavage fluid revealed possible molecular reprogramming mechanism for the cancer spread into the mesothelial cells [38]. Also, exosomal delivery of hypoxia-inducible factor-1α (HIF-1α) into the GC cells educates them to adapt hypoxic microenvironment of the abdominal cavity for feasing peritoneal dissemination [37]. GC cell-derived exosomes induce pro-tumor phenotype in neutrophils through exosomal induction of NF-κB pathway by interaction of high mobility group box-1 and Toll-like receptor 4 molecules to provoke GC cell migration [39]. Angiogenesis is an essential part of metastasis which is mediated by exosomes, GC exomiR-130a induce angiogenesis in vascular–endothelial cells by modification of c-myb proto-oncogene expression [40, 41].
Epithelial–mesenchymal transition (EMT) conducts tumor escape from the primary sites of tumors in cooperation with reversible mesenchymal–epithelial transition (MET) in the distant metastatic niches to embed and establish secondary tumors [42]. EMT stimulates tumor cells to loose apical–basal polarity, cell–cell junction and render mesenchymal features with low rate of proliferation beside high motility, invasiveness, and survival rate. Loss of epithelial E-cadherin and acquirement of mesenchymal vimentin, N-cadherin, and spindle-like cellular shape are the main hallmarks of EMT [43]. ExomiR-423-5p induces EMT in GC cell lines by downregulation of suppressor of fused protein gene (SUFU), a tumor suppressor gene, to promote tumor migration and proliferation. ExomiR-191 and let7a are also abundant cargos in GC exosomes which modulate EMT to support cancer promotion [44, 45]. MSC exosomes increase the expression of mesenchymal markers and decrease the expression of epithelial markers in GC cells which result in acceleration of EMT to elevate migration and invasion of HGC-27 cells by predominant activation of protein kinase B signaling pathway through octamer-binding transcription factor 4, sex determining region Y-box 2 and Lin28B [46].
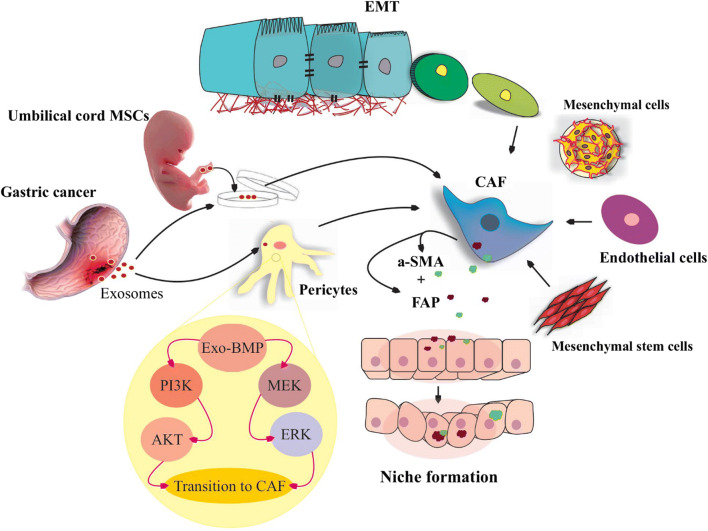
Cancer-associated fibroblasts (CAFs) are abundant and heterogeneous spindle-shaped group of stromal cells mainly originate local fibroblasts and several other cells (mesenchymal stem cells, epithelial, endothelial, adipocytes and pericytes cells) under effect of exosomes to modulate tumor growth, angiogenesis, metastasis, and chemoresistance [47]. GC exosomes contain exomiR-27a which remarkably reprograms residential fibroblasts to transform into CAFs. Furthermore, GC-derived exosomes transform umbilical cord MSCs into the CAFs through direct delivery of exosomal transforming growth factor-beta (TGF-β) and initiation of TGF-ß/Smad cascades, for cancer promotion [48, 49]. Residential MSCs of the GC tumor secret exosomes that transfer exomiR-221 and -222 into the gastric tumor cells to support migration and metastasis of the cells [24]. Also, exosomal bone morphogenetic protein (BMP) induces transition of pericytes into CAFs through PI3K/AKT and MEK/ERK signaling pathways. CAFs create niches in remote organs by secretion of α-smooth muscle actin and fibroblast-activating proteins to support metastasis [50] (Fig. 2). Bone marrow-derived MSC secret exosomes that regulate tumor proliferation and progression of GC through activation of Hedgehog signaling pathway by exomiR-221 [33, 51]. Also, these vesicles promote angiogenesis via activation of ERK1/2 and MAPK pathways for tumor growth in vivo [52].
Fig. 2.
Niche formation. Different cells contribute to transit into cancer-associated-fibroblasts (CAFs) in tumor microenvironment. Several cells are transformed into the CAFs including mesenchymal stem cells (MSCa), mesenchymal cells, endothelial cells, and also epithelial cells which are under epithelial mesenchymal transition (EMT). Gastric cancer (GC)-derived exosomes are able to promote pericytes and umbilical cord MSCs transition into the CAFs. GC exosomes delivering bone-morphogenetic-proteins (BMP) into the pericytes and BMP activates PI3K/AKT and MEK/ERK signaling pathways. Subsequent CAFs secret a-smooth muscle actin (a-SMA) and fibroblast-activating proteins (FAP) in target cells environment and provoke niche formation for GC cell metastasis
Macrophages are the most abundant fraction of stromal leukocytes with a dual activity, M1cells act as pro-inflammatory with tumoricidal activity and M2 cells function as anti-inflammatory cells which are linked to cancer progression and metastasis [53]. GC exosomes promote inflammation by induction of M2 subtype of macrophages through NF-κB pathway that causes tumor proliferation, migration and invasion. NF-κB pathway is epigenetically activated by exosomes, and none of the engaged molecules are delivered directly by exosomes [54]. Exosomes derived from SGC7901 and BGC823 gastric cancer cell lines are able to educate monocytes to transit into the programmed cell death-1 (PD-1) positive tumor-associated macrophages with M2 features for upregulation of interleukin-10 secretion that interferes with CD8+ T cell function which leads to GC progression [55]. Mass spectrometry revealed that M2 macrophages release exosomes containing apolipoprotein E that activates PI3K-Akt signaling pathway in recipient GC cells which results in cytoskeleton remodeling to establish EMT and promotion of metastasis [56].
Exosomal DNAs and gastric cancer
Exosomal DNA (exoDNA) generally appears as double strand fragments that may extend more than 10,000 base pair in length. Tumor-methylated DNA comprises great portion of TEXs DNA. In vitro analysis of exoDNA fragments for long interspersed nuclear elements-1 (LINE-1) and SOX-17 indicates same methylation pattern in nuclear and exoDNA. Furthermore, in vivo analysis of LINE-1 shows slightly lower rate of methylation in exoDNA in contrast to nuclear DNA which may arise non-cancerous circulating exosomes. Exosomal SOX-17 methylation pattern in exosomal and nuclear DNA is concordant with in vitro investigations [57]. BarH-like 2 homeobox protein (BARHL2) expression level in lavage is significantly shifted at the early stage of GC with independent pattern of Helicobacter pylori (H. pylori) infection. The exosomal-DNA derived from the gastric juice of patients is used for evaluation of BARHL2 methylation pattern demonstrates similar pattern of biopsy samples, free DNA in gastric juice and GC cell lines. BARHL2 methylation is gradually increases by development of GC suggesting exosomal-BARHL2 methylation pattern as a promising diagnostic and prognostic biomarker in GC [58].
Exosomal proteins and gastric cancer
Exosomes contain a large number of proteins as internal and surface molecules which probably suggest an important role for these proteins in exosome navigation and function. GC TEX-proteins are mainly participated in promotion of the cancer, for instance, exosomes of gastrointestinal stromal tumors contain tyrosine kinase proteins which transform progenitor cell-derived smooth muscle cells into the pre-metastatic cells. Exosomal CD97 induces proliferation of GC cells by activation of MAPK signaling pathways in combination with miRNAs. Exosomal CD9 of CAFS also stimulate metastatic migration in scirrhous-type GC cells [32, 59, 60]. On the other hand, lack of certain exosomal molecules may promote cell viability and proliferation. The absence of gastrokine-1, as a tumor suppressor protein, in exosomes provides GC cell lines proliferation. Low concentration of Tripartite Motif Containing 3 (TRIM3) in serum exosomes of GC patients promote growth and progression of GC as a key regulator of stem cell factors and EMT [61, 62]. CAFs are able to control EMT by secretory TGF-1 through activation of TGF-1-SMAD signaling pathway. These molecules also provoke EMT by exosomal delivery of TGF-1. Gastroepiploic vein of GC patients contain circulating exosomes which are rich in TGF-β1 that significantly modulate host immune-surveillance by transforming of naive T cells into FOXP3+ T regulatory cells and prepare tumor escape to promote lymphatic metastasis [63]. Exosomal-tetraspanin 8 is another molecule that increases the invasion rate in recipient GC cells by activation of ERK/MAPK pathway that promotes angiogenesis and metastasis [64]. Exosomes are also able to conduct targeted metastasis for different organs. Exosomal epidermal growth factor receptors in GC cells mediate organotropism metastasis into the liver through activation of hepatocyte growth factor (HGF) signaling pathway by suppressing of miR-26a/b in GC [65]. HIF-1α expression is directly associated with GC peritoneal dissemination; it is an adaptation key in the hypoxic microenvironment of the abdominal cavity for GC cells which exert its effect by β1 integrin as mediator of HIF-1α to increase cell adhesion. Exosomal delivery of HIF-1α in hypoxia condition into the GC cells promote the cancer dissemination by upregulation of EMT-associated transcription factors [66].
Exosomal long non-coding RNAs in gastric cancer
Non-coding genes comprise approximately 98% of the human genome which are involved in regulation of various cellular processes. Long non-coding RNAs (LncRNAs) are defined as longer RNAs than 200 bp without protein coding capability. LncRNAs target preferential transcriptomes directly by base–base complementation or regulate up- or down-stream of gene transcription cascades [67, 68]. Exosomal transportation of these molecules in GC is repeatedly reported. Overexpression of serum-exosomal hoxa transcript at the distal tip (HOTTIP) is indirectly correlated with greater tumor-size, advanced pathological stage and extensive metastasis beside shorter overall survival rate of GC patients. HOTTIP targets miR-331-3p as competitive endogenous RNA to reduce expression of HER2 gene which results in gastric tumor proliferation and invasion [69, 70]. Upregulation of circulating-exosomal long intergenic non-protein-coding RNA 152 (LINC00152) and zinc finger antisense1 (ZFAS1) serve as prognostic biomarkers, they are capable to mediate clinical status of patients by promoting lymphatic metastasis [71, 72]. LINC00152 downregulates miR-193a-3p to elevate expression of MCL1 gene and also targets PI3K/Akt signaling pathway to induce GC proliferation and invasion. MCL1 expression is a trigger factor for EMT to induce metastasis [73, 74]. Also, ZFAS1 is a modulatory factor in notch signaling pathway that affects EMT which results in GC dissemination. Competitive sponging of miR-150, miR-200b or miR-200c by ZFAS1 leads to overexpression of ZEB1/2, MMP-14/16, BMI1, and Sp1 gene profile that stimulates proliferation and invasion in GC cells [75, 76]. UFC1 promotes GC by sponging of miR-498 that represses expression of Lin28b as tumor suppressor [77].
Infection-induced exosomes in gastric cancer
Pathogenic organisms generally utilize endocytic-pathways of host cells to alter cellular activity and also hijack exosome content and membrane structure. Infected cell-derived exosomes are potential transmitters of pathogen substances to spread the infection. H. pylori and Epstein–Barr virus (EBV) are the main infectious agents in gastric epithelium which are associated with GC initiation. CagA is the main virulence factor of H. pylori that promotes GC establishment. Exosomal-CagA delivery supports GC metastasis by modulation of Src family kinases in recipient cells through activation of NF-κB pathway and induction of MET proto-oncogene sorting over generated exosomes to educate residential macrophages for tumor progression through secretion of pro-inflammatory cytokine like IL-1β [78–80]. EBV infection, as a latent virus, is detectable in about 90% of population worldwide. Exosomal latent membrane protein-1 (LMP1) of EBV stimulates tumor-related signaling pathways including EGFR/PI3 K and AKT/ERK beside upregulation of ICAM-1 or CD54 which cause promotion of GC by suppressing of T cell proliferation and natural killer cell cytotoxicity. Also, miR-BART15-3p is transferred by exosomes in EBV-infected GC cells, supporting inhibition of apoptosis with overexpression of BRUCE in neighbor immune cells to provide GC progression. miR-BART6-3p is EBV-specific molecule that directly suppresses host miRNA biogenesis by targeting of host Dicer which is transposable through exosome secretion [81].
Exosomes as potential diagnostic and prognostic biomarkers in gastric cancer
Enigmatic origin of free circulating non-coding RNAs and their poor association with tumoral expression profile beside expression impressibility in blood stream by hypoxia, diet, exercise, circulating blood cells secretome, and infections remarkably candidate them as debatable biomarkers [82, 83]. Cytosolic content of exosomes properly resembles metabolic status of the origin cell. Abundance of exosomes in body fluids, selective cargo sorting, accessibility, circulation stability and reproducibility in different stages of the disease, suggesting these vesicles as emerging non- or semi-invasive diagnostic and prognostic targets in cancer biomarker investigation. ExomiRs expression pattern (not exosomal mRNA) supports potential signature for discrimination of origin and subtypes of various cancers including GC [84, 85]. Combined quadri-plasma derived exomiRs (miR10b-5p, miR195-5p, miR20a-3p, and miR296-5p) pattern is suggested as a diagnostic biomarker for GC which indicates similar correlation with gastric tumors. Upregulation of miR10b-5p or miR296-5p afterward adjuvant chemotherapy tends to reduce overall survival rate of the patients [86]. Elevated peritoneal lavage fluid derived exomiR-21, -320c and -1225-5p beside plasma exomiR-23b are proposed as prognostic biomarkers in evaluation of peritoneal GC recurrence [87, 88]. Different exosomal cargos including DNAs, proteins, miRNAs, and LncRNAs are previously described as potential GC diagnostic and prognostic biomarkers which are summarized in Table 1.
Table 1.
Exosomal biomarkers in gastric cancer
| Biomarker type | Molecules (signatures) | Exosome origin | References |
|---|---|---|---|
| miRNA | miR10b-5p, miR195-5p, miR20a-3p, and miR296-5p | Plasma | [86] |
| miR-21, miR-320c, miR1225-5p, miR-1202, miR-4270, miR-1207-5p | Peritoneum lavage fluid | [34] | |
| (miR-23b, mir-210, mir-25, miR-92b) | Plasma | [85] | |
| miR-19b-3p, miR-17-5p, miR-30a-5p, and miR-106a-5p | Serum | [86] | |
| (miR-92b, miR-25, miR-20a, miR-185, miR-210) | Plasma | [87] | |
| Let-7 | Cell line | [29] | |
| miR-101 | Serum | [26] | |
| miR-217 | Plasma | [28] | |
| miR-423-5p | Serum | [44] | |
| miR‑23b | Plasma | [88] | |
| miR-101 | Plasma | [26] | |
| miR-221 | Peripheral blood | [51] | |
| Long None coding RNA | HOTTIP | Plasma and serum | [69] |
| LINC00152 | Plasma and serum | [73] | |
| ZFAS1 | Plasma and serum | [75] | |
| lncUEGC1 | Serum | [89] | |
| UFC1 | Serum | [77] | |
| lncRNA HOTAIR | Serum | [90] | |
| DNA | BARHL2 | Gastric juice | [54] |
| LINE-1 and SOX-17 | In vivo and in vitro | [53] | |
| Protein | Tetraspanin 8 | Plasma | [61] |
| CD97 | Serum | [90] | |
| Glypican-3 | Serum | [91] | |
| GRN | Cell line | [60] | |
| HER-2/neu and CCR6 | Blood exosome surface | [92] | |
| TRIM3 | Serum | [91] | |
| Gastrokine 1 | HFE-145 cell line | [61] | |
| CD9 | In vitro | [60] |
Exosomes and gastric cancer therapy and chemoresistance
Modest achievements of chemotherapy in GC treatment are highly under influence of the late diagnosis. Cisplatin as the main chemotherapy treatment is common approach in solid tumors including GC which triggers DNA damage in rapidly proliferating cells that causes massive cell death by inducing apoptosis. Cisplatin resistance in tumor cells is established by several mechanisms: decreased drug-uptake, raised activity of efflux pumps, reduction of active drug agents in cancer cells, subsequent modifications of molecular targets of drugs, improved DNA damage repair, and reduced pro-apoptotic factors or upregulated anti-apoptotic genes. Exosomes are also novel players that interfering drug metabolism by exporting drugs or sharing anti-apoptotic agents in cancer cells to promote chemoresistance [92–95]. MSC exosomes modulate drug efflux and drug delivery into the recipient cells by transferring of certain proteins (MRP2, ATP7A, and ATP7B) and miRNAs (miR-100, m-222, miR-30a, miR-17) to activate apoptosis escaping pathways beside CaM-Ks/Raf/MEK/ERK signaling pathways that provoke 5-fluorouracil chemoresistance in GC cells [96]. Dysregulated miR-101 expression in gastric tumors promotes the malignancy, and therefore, exosomal delivery of miR-101 by blood circulation promotes elimination of established tumors [26]. Cancer stem cells (CSCs) take role both in cancer seeding and induction of chemo/radio therapy resistance, hence targeted delivery of the therapeutic agents into the CSCs is beneficial for cancer restriction. Line of evidence confirms that transformation of non-CSCs into CSCs is mediated by sharing stemness and EMT-related factors through exosomes, Snail is an EMT promoting factor which is shared by GC exosomes to establish gastric carcinoma cells with CSC characteristics [97, 98]. M2 macrophages derived from peripheral blood mononuclear cells produce exosomes that containing mir-21 which triggers downregulation of PTEN, and activation of PI3K/AKT pathway in GC cells and establish cisplatin resistance [99]. MiRNAs efficiently interrupt cellular functions by dysregulation of expression networks in the cells, precisely in cancers. Directed exosomal delivery of miRNAs, to modify recipient cell metabolism is a novel approach which is growing fast. Mir-214 overexpression in GC cells is associated with anti-apoptotic behavior of cells leading to invasion and metastasis with poor prognosis. Also, it is shown that mir-214 interrupt PTEN function in cancer cells and tumor microenvironment to induce cisplatin resistance. Exosomal-anti-mir-214 delivery to GC tumor cells recovers cisplatin activity in cisplatin refractory GC patients [100]. Delivery of tumor-suppressive miRNAs to chemo-resistant cancer cells is another approach to retrieve drug effectiveness in chemoresistance cells. Mir-122 concentration increases gradually by cancer cell proliferation and causes GC cell resistance to cisplatin. It has been suggested that exosomal delivery of anti-miR-122 is a valuable approach to overcome this chemoresistance in vitro and in vivo [100]. Exosomal miR-21, a tumor promoter, downregulates PDCD4 gene expression to bypasses apoptosis in benefit of tumor progression, exosomal antibody delivery against miR-21 effectively stimulates cell apoptosis [101]. Exosomal delivery of overexpressed TRIM3 can suppress GC growth and metastasis by arresting cell cycle at G0/G1 checkpoints in vitro and in vivo [62]. Trastuzumab emtansine (T-DM1) is a conjugated antibody with drug complex which transfers cytotoxic part (DM1) to HER2+ tumor cells through targeted activity of antibody. Strong attachment of T-DM1 to exosomes with origin of HER2+ cells induces out spreading of T-DM1 through exosomes by circulation followed by decrease in viability of target cells [102]. Angiogenesis as crucial part of metastasis is mediated by overexpression of HGF/c-Met signaling pathway in GC that upregulates VEGF expression through activation of MAPK, PIK3 and Stat3 which leads to tumor spread. Exosomal HGF siRNA delivery efficiently suppresses cell proliferation through blocking of vasculation [103]. In a recent report, milk exosomes were introduced as resistant carriers to human digestive system which enabled exosome uptake in gastrointestinal (GI) tract cells which is suggesting exosomes as promising oral delivery systems for wide range of therapeutics which are encapsulated in these vesicles without any modification or degradation to target GI cells [104]. These findings support promising role of exosomes in induction of chemoresistance to transfer and deliver anti-resistant factors to various cells.
Conclusion
Exosomes as natural nanocarriers continuously departure from various cell types to preserve cellular hemostasis and intracellular signaling networks. In contrast to other family of extracellular vesicles, exosomes biogenesis is result of multivesicular bodies’ fusion with plasma cell membrane instead of direct shedding under control of strict loading mechanisms. Certain biological roles are introduced for exosome in normal condition, genetic material exchange and excretion of redundant waste substances in conserved manner are main roles in various investigations. Distribution of different biological molecules through exosomes during pathological conditions including cancers, suggesting a considerable role for modulation of cancer initiation, progression and metastasis. These vesicles can easily present in different body fluids due to their nanoscale, their ability for modifications of recipient cells, and detectability of exosomes in the majority of body organs which candidate them as potential targets for non-invasive biomarker studies. Exosomes are persistent part of tumor microenvironment with origin of tumoral and normal cells. Various tumor cells tend to release set of exosomes with oncogenic activity to promote the cancer in the battle of normal exosomes. Exosomes provide distant metastasis by modulating of target organs which suggesting possible monitoring of metastasis procedure. Exosomes also modify metastatic cell adaption into the target tissue microenvironment through reprogramming of cells’ domestic metabolism. Exosomal HIF was reported to modify GC abdominal metastasis by mediating hypoxia adaption, targeting of exosomal HIF delivery in GC may serve as potential approach to limit GC invasion into adjacent tissues. Infectious agents hijack exosome loading machinery in host cells a beneficial system to spread the pathogenic organism in the host cells. They also deliver genotoxic and carcinogenic elements into the recipient cells which may cause cancer initiation, as the mechanism of CagA in H. pylori. These findings suggest that exosome biogenesis and loading can be controlled by extrinsic modifiers such as infectious agents. Also, exosome content screening may be a considerable approach to detect carcinogenic molecules during infections. The role of exosomes in chemoresistance is emerging finding, they exert chemotherapy compounds outward the cells and also share key molecules for regulation of target cells in benefit of chemoresistance by epigenetic reprogramming and direct delivery of functional molecules. The natural structure of these vesicles makes them candidate as non-immunogenic vectors to deliver chemotherapy compounds and recover therapeutic activity. Overall, exosomes are multipotent carriers to participate in tumor initiation, progression and chemoresistance. These vesicles can also be applied early detection of tumor initiation and targeted therapy.
Acknowledgements
This work was supported by Tabriz university of medical sciences under Grant no. IR.TBZMED.REC.1396.910.
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Sitarz R, Skierucha M, Mielko J, et al. Gastric cancer: epidemiology, prevention, classification, and treatment. Cancer Manag Res. 2018;10:239–248. doi: 10.2147/CMAR.S149619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jeddi F, Soozangar N, Sadeghi MR, et al. Nrf2 overexpression is associated with P-glycoprotein upregulation in gastric cancer. Biomed Pharmacother. 2018;97:286–292. doi: 10.1016/j.biopha.2017.10.129. [DOI] [PubMed] [Google Scholar]
- 3.Smyth EC, Verheij M, Allum W, et al. Gastric cancer: ESMO clinical practice guidelines for diagnosis, treatment and follow-up†. Ann Oncol. 2016;27:v38–v49. doi: 10.1093/annonc/mdw350. [DOI] [PubMed] [Google Scholar]
- 4.Yan Y, Fu G, Ye Y, Ming L. Exosomes participate in the carcinogenesis and the malignant behavior of gastric cancer. Scand J Gastroenterol. 2017;52:499–504. doi: 10.1080/00365521.2016.1278458. [DOI] [PubMed] [Google Scholar]
- 5.Orditura M, Galizia G, Sforza V, et al. Treatment of gastric cancer. World J Gastroenterol. 2014;20:1635–1649. doi: 10.3748/wjg.v20.i7.1635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shimizu D, Kanda M, Kodera Y. Emerging evidence of the molecular landscape specific for hematogenous metastasis from gastric cancer. World J Gastrointest Oncol. 2018;10:124–136. doi: 10.4251/wjgo.v10.i6.124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Maia J, Caja S, Strano Moraes MC, et al. Exosome-based cell-cell communication in the tumor microenvironment. Front cell Dev Biol. 2018;6:18. doi: 10.3389/fcell.2018.00018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Harding CV, Heuser JE, Stahl PD. Exosomes: looking back three decades and into the future. J Cell Biol. 2013;200:367–371. doi: 10.1083/jcb.201212113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vlassov AV, Magdaleno S, Setterquist R, Conrad R. Exosomes: current knowledge of their composition, biological functions, and diagnostic and therapeutic potentials. Biochim Biophys Acta. 2012;1820:940–948. doi: 10.1016/j.bbagen.2012.03.017. [DOI] [PubMed] [Google Scholar]
- 10.Rajagopal C, Harikumar KB. The origin and functions of exosomes in cancer. Front Oncol. 2018;8:66. doi: 10.3389/fonc.2018.00066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Skotland T, Sandvig K, Llorente A. Lipids in exosomes: current knowledge and the way forward. Prog Lipid Res. 2017;66:30–41. doi: 10.1016/j.plipres.2017.03.001. [DOI] [PubMed] [Google Scholar]
- 12.Kowal J, Arras G, Colombo M, et al. Proteomic comparison defines novel markers to characterize heterogeneous populations of extracellular vesicle subtypes. Proc Natl Acad Sci USA. 2016;113:E968–E977. doi: 10.1073/pnas.1521230113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ruivo CF, Adem B, Silva M, Melo SA. The biology of cancer exosomes: insights and new perspectives. Cancer Res. 2017;77:6480–6488. doi: 10.1158/0008-5472.CAN-17-0994. [DOI] [PubMed] [Google Scholar]
- 14.Hessvik NP, Llorente A. Current knowledge on exosome biogenesis and release. Cell Mol Life Sci. 2017 doi: 10.1007/s00018-017-2595-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yuyama K, Sun H, Mitsutake S, Igarashi Y. Sphingolipid-modulated Exosome secretion promotes clearance of amyloid-β by microglia. J Biol Chem. 2012;287:10977–10989. doi: 10.1074/jbc.M111.324616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Villarroya-Beltri C, Baixauli F, Mittelbrunn M, et al. ISGylation controls exosome secretion by promoting lysosomal degradation of MVB proteins. Nat Commun. 2016;7:13588. doi: 10.1038/ncomms13588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.McKelvey KJ, Powell KL, Ashton AW, et al. Exosomes: mechanisms of uptake. J Circ biomarkers. 2015;4:7. doi: 10.5772/61186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhang H-G, Grizzle WE. Exosomes: a novel pathway of local and distant intercellular communication that facilitates the growth and metastasis of neoplastic lesions. Am J Pathol. 2014;184:28–41. doi: 10.1016/j.ajpath.2013.09.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li X, Wang Y, Wang Q, et al. Exosomes in cancer: small transporters with big functions. Cancer Lett. 2018;435:55–65. doi: 10.1016/j.canlet.2018.07.037. [DOI] [PubMed] [Google Scholar]
- 20.Ludwig A-K, Giebel B. Exosomes: small vesicles participating in intercellular communication. Int J Biochem Cell Biol. 2012;44:11–15. doi: 10.1016/j.biocel.2011.10.005. [DOI] [PubMed] [Google Scholar]
- 21.De Toro J, Herschlik L, Waldner C, Mongini C. Emerging roles of exosomes in normal and pathological conditions: new insights for diagnosis and therapeutic applications. Front Immunol. 2015;6:203. doi: 10.3389/fimmu.2015.00389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rana S, Malinowska K, Zöller M. Exosomal tumor microRNA modulates premetastatic organ cells. Neoplasia. 2013;15:281–295. doi: 10.1593/neo.122010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Eyvazi S, Hejazi MS, Kahroba H, et al. Cdk9 as an appealing target for therapeutic interventions. Curr Drug Targets. 2018 doi: 10.2174/1389450119666181026152221. [DOI] [PubMed] [Google Scholar]
- 24.Seo N, Shirakura Y, Tahara Y, et al. Activated CD8 + T cell extracellular vesicles prevent tumour progression by targeting of lesional mesenchymal cells. Nat Commun. 2018;9:435. doi: 10.1038/s41467-018-02865-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Brinton L, Sloane H, Kester M, Kelly K. Formation and role of exosomes in cancer. Cell Mol Life. 2015;72:659–671. doi: 10.1007/s00018-014-1764-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Imamura T, Komatsu S, Ichikawa D, et al. Low plasma levels of miR-101 are associated with tumor progression in gastric cancer. Oncotarget. 2017;8:106538–106550. doi: 10.18632/oncotarget.20860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wang M, Zhao C, Shi H, et al. Deregulated microRNAs in gastric cancer tissue-derived mesenchymal stem cells: novel biomarkers and a mechanism for gastric cancer. Br J Cancer. 2014;110:1199. doi: 10.1038/bjc.2014.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Li W, Gao Y-Q. MiR-217 is involved in the carcinogenesis of gastric cancer by down-regulating CDH1 expression. Kaohsiung J Med Sci. 2018;34:377–384. doi: 10.1016/j.kjms.2018.02.003. [DOI] [PubMed] [Google Scholar]
- 29.Ohshima K, Inoue K, Fujiwara A, et al. Let-7 MicroRNA family is selectively secreted into the extracellular environment via exosomes in a metastatic gastric cancer cell line. PLoS One. 2010;5:e13247. doi: 10.1371/journal.pone.0013247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ke X, Yan R, Sun Z, et al. Esophageal adenocarcinoma-derived extracellular vesicle microRNAs induce a neoplastic phenotype in gastric organoids. Neoplasia. 2017;19:941–949. doi: 10.1016/j.neo.2017.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Qu J-L, Qu X-J, Zhao M-F, et al. Gastric cancer exosomes promote tumour cell proliferation through PI3K/Akt and MAPK/ERK activation. Dig Liver Dis. 2009;41:875–880. doi: 10.1016/j.dld.2009.04.006. [DOI] [PubMed] [Google Scholar]
- 32.Li C, Liu D, Li G, et al. CD97 promotes gastric cancer cell proliferation and invasion through exosome-mediated MAPK signaling pathway. World J Gastroentrol. 2015;21:6215. doi: 10.3748/wjg.v21.i20.6215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Qi J, Zhou Y, Jiao Z, et al. Exosomes derived from human bone marrow mesenchymal stem cells promote tumor growth through hedgehog signaling pathway. Cell Physiol Biochem. 2017;42:2242–2254. doi: 10.1159/000479998. [DOI] [PubMed] [Google Scholar]
- 34.Valastyan S, Weinberg RA. Tumor metastasis: molecular insights and evolving paradigms. Cell. 2011;147:275–292. doi: 10.1016/j.cell.2011.09.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sawayama H, Ishimoto T, Baba H. Microenvironment in the pathogenesis of gastric cancer metastasis. J Cancer Metastasis Treat. 2018;4:10. doi: 10.20517/2394-4722.2017.79. [DOI] [Google Scholar]
- 36.Arita T, Ichikawa D, Konishi H, et al. Tumor exosome-mediated promotion of adhesion to mesothelial cells in gastric cancer cells. Oncotarget. 2016;7:56855–56863. doi: 10.18632/oncotarget.10869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chen K-B, Chen J, Jin X-L, et al. Exosome-mediated peritoneal dissemination in gastric cancer and its clinical applications. Biomed Rep. 2018;8:503–509. doi: 10.3892/br.2018.1088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tokuhisa M, Ichikawa Y, Kosaka N, et al. Exosomal miRNAs from peritoneum lavage fluid as potential prognostic biomarkers of peritoneal metastasis in gastric cancer. PLoS One. 2015;10:e0130472. doi: 10.1371/journal.pone.0130472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhang X, Shi H, Yuan X, et al. Tumor-derived exosomes induce N2 polarization of neutrophils to promote gastric cancer cell migration. Mol Cancer. 2018;17:146. doi: 10.1186/s12943-018-0898-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yang H, Zhang H, Ge S, et al. Exosome-derived miR-130a activates angiogenesis in gastric cancer by targeting C-MYB in vascular endothelial cells. Mol Ther. 2018;26:2466–2475. doi: 10.1016/j.ymthe.2018.07.023. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 41.Fallah A, Sadeghinia A, Kahroba H, et al. Therapeutic targeting of angiogenesis molecular pathways in angiogenesis-dependent diseases. Biomed Pharmacother. 2019;110:775–785. doi: 10.1016/j.biopha.2018.12.022. [DOI] [PubMed] [Google Scholar]
- 42.Tsai JH, Yang J. Epithelial-mesenchymal plasticity in carcinoma metastasis. Genes Dev. 2013;27:2192–2206. doi: 10.1101/gad.225334.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Diepenbruck M, Christofori G. Epithelial–mesenchymal transition (EMT) and metastasis: yes, no, maybe? Curr Opin Cell Biol. 2016;43:7–13. doi: 10.1016/j.ceb.2016.06.002. [DOI] [PubMed] [Google Scholar]
- 44.Yang H, Fu H, Wang B, et al. Exosomal miR-423-5p targets SUFU to promote cancer growth and metastasis and serves as a novel marker for gastric cancer. Mol Carcinog. 2018 doi: 10.1002/mc.22838. [DOI] [PubMed] [Google Scholar]
- 45.Steinbichler TB, Dudás J, Riechelmann H, Skvortsova I-I. The role of exosomes in cancer metastasis. Semin Cancer Biol. 2017;44:170–181. doi: 10.1016/j.semcancer.2017.02.006. [DOI] [PubMed] [Google Scholar]
- 46.Gu H, Ji R, Zhang X, Wang M. Exosomes derived from human mesenchymal stem cells promote gastric cancer cell growth and migration via the activation of the Akt pathway. Mol Med Rep. 2016;14:3452–3458. doi: 10.3892/mmr.2016.5625. [DOI] [PubMed] [Google Scholar]
- 47.Tao L, Huang G, Song H, et al. Cancer associated fibroblasts: an essential role in the tumor microenvironment. Oncol Lett. 2017;14:2611–2620. doi: 10.3892/ol.2017.6497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wang J, Guan X, Zhang Y, et al. Exosomal miR-27a derived from gastric cancer cells regulates the transformation of fibroblasts into cancer-associated fibroblasts. Cell Physiol Biochem. 2018;49:I. doi: 10.1159/000493218. [DOI] [PubMed] [Google Scholar]
- 49.Gu J, Qian H, Shen L, et al. Gastric cancer exosomes trigger differentiation of umbilical cord derived mesenchymal stem cells to carcinoma-associated fibroblasts through TGF-β/Smad pathway. PLoS One. 2012;7:e52465. doi: 10.1371/journal.pone.0052465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ning X, Zhang H, Wang C, Song X. Exosomes released by gastric cancer cells induce transition of pericytes into cancer-associated fibroblasts. Med Sci Monit. 2018;24:2350–2359. doi: 10.12659/MSM.906641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ma M, Chen S, Liu Z, et al. miRNA-221 of exosomes originating from bone marrow mesenchymal stem cells promotes oncogenic activity in gastric cancer. Onco Targets Ther. 2017;10:4161–4171. doi: 10.2147/OTT.S143315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zhu W, Huang L, Li Y, et al. Exosomes derived from human bone marrow mesenchymal stem cells promote tumor growth in vivo. Cancer Lett. 2012;315:28–37. doi: 10.1016/j.canlet.2011.10.002. [DOI] [PubMed] [Google Scholar]
- 53.Zheng X, Turkowski K, Mora J, et al. Redirecting tumor-associated macrophages to become tumoricidal effectors as a novel strategy for cancer therapy. Oncotarget. 2017;8:4843–48452. doi: 10.18632/oncotarget.17061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wu L, Zhang X, Zhang B, et al. Exosomes derived from gastric cancer cells activate NF-κB pathway in macrophages to promote cancer progression. Tumor Biol. 2016;37:12169–12180. doi: 10.1007/s13277-016-5071-5. [DOI] [PubMed] [Google Scholar]
- 55.Wang F, Li B, Wei Y, et al. Tumor-derived exosomes induce PD1 + macrophage population in human gastric cancer that promotes disease progression. Oncogenesis. 2018;7:41. doi: 10.1038/s41389-018-0049-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zheng P, Luo Q, Wang W, et al. Tumor-associated macrophages-derived exosomes promote the migration of gastric cancer cells by transfer of functional Apolipoprotein E. Cell Death Dis. 2018;94(9):434. doi: 10.1038/s41419-018-0465-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Yamamoto H. Detection of DNA methylation of gastric juice-derived exosomes in gastric cancer. Integr Mol Med. 2014;1:17–21. doi: 10.15761/IMM.1000105. [DOI] [Google Scholar]
- 58.Yamamoto H, Watanabe Y, Oikawa R, et al. BARHL2 methylation using gastric wash DNA or gastric juice exosomal DNA is a useful marker for early detection of gastric cancer in an H. pylori-independent manner. Clin Transl Gastroenterol. 2016;7:e184. doi: 10.1038/ctg.2016.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Chen Y, Xie Y, Xu L, et al. Protein content and functional characteristics of serum-purified exosomes from patients with colorectal cancer revealed by quantitative proteomics. Int J Cancer. 2017;140:900–913. doi: 10.1002/ijc.30496. [DOI] [PubMed] [Google Scholar]
- 60.Miki Y, Yashiro M, Okuno T, et al. CD9-positive exosomes from cancer-associated fibroblasts stimulate the migration ability of scirrhous-type gastric cancer cells. Br J Cancer. 2018;118:867–877. doi: 10.1038/bjc.2017.487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Yoon JH, Ham I-H, Kim O, et al. Gastrokine 1 protein is a potential theragnostic target for gastric cancer. Gastric Cancer. 2018 doi: 10.1007/s10120-018-0828-8. [DOI] [PubMed] [Google Scholar]
- 62.Fu H, Yang H, Zhang X, et al. Exosomal TRIM3 is a novel marker and therapy target for gastric cancer. J Exp Clin Cancer Res. 2018;37:162. doi: 10.1186/s13046-018-0825-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Yen E-Y, Miaw S-C, Yu J-S, Lai I-R. Exosomal TGF-β1 is correlated with lymphatic metastasis of gastric cancers. Am J Cancer Res. 2017;7:2199–2208. [PMC free article] [PubMed] [Google Scholar]
- 64.Anami K, Oue N, Noguchi T, et al. TSPAN8, identified by Escherichia coli ampicillin secretion trap, is associated with cell growth and invasion in gastric cancer. Gastric Cancer. 2016;19:370–380. doi: 10.1007/s10120-015-0478-z. [DOI] [PubMed] [Google Scholar]
- 65.Zhang H, Deng T, Liu R, et al. Exosome-delivered EGFR regulates liver microenvironment to promote gastric cancer liver metastasis. Nat Commun. 2017;8:15016. doi: 10.1038/ncomms15016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Chen K-B, Chen J, Jin X-L, et al. Exosome-mediated peritoneal dissemination in gastric cancer and its clinical applications. Biomed reports. 2018;8:503–509. doi: 10.3892/br.2018.1088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Wang J-P, Tang Y-Y, Fan C-M, et al. The role of exosomal non-coding RNAs in cancer metastasis. Oncotarget. 2018;9:12487–12502. doi: 10.18632/oncotarget.23552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Majidinia M, Yousefi B. Long non-coding RNAs in cancer drug resistance development. DNA Repair (Amst) 2016;45:25–33. doi: 10.1016/j.dnarep.2016.06.003. [DOI] [PubMed] [Google Scholar]
- 69.Zhao R, Zhang Y, Zhang X, et al. Exosomal long noncoding RNA HOTTIP as potential novel diagnostic and prognostic biomarker test for gastric cancer. Mol Cancer. 2018;17:68. doi: 10.1186/s12943-018-0817-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Liu X, Sun M, Nie F, et al. Lnc RNA HOTAIR functions as a competing endogenous RNA to regulate HER2 expression by sponging miR-331-3p in gastric cancer. Mol Cancer. 2014;13:92. doi: 10.1186/1476-4598-13-92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Li Q, Shao Y, Zhang X, et al. Plasma long noncoding RNA protected by exosomes as a potential stable biomarker for gastric cancer. Tumor Biol. 2015;36:2007–2012. doi: 10.1007/s13277-014-2807-y. [DOI] [PubMed] [Google Scholar]
- 72.Pan L, Liang W, Fu M, et al. Exosomes-mediated transfer of long noncoding RNA ZFAS1 promotes gastric cancer progression. J Cancer Res Clin Oncol. 2017;143:991–1004. doi: 10.1007/s00432-017-2361-2. [DOI] [PubMed] [Google Scholar]
- 73.Huang Y, Luo H, Li F et al (2018) LINC00152 down-regulated miR-193a-3p to enhance MCL1 expression and promote gastric cancer cells proliferation. Biosci Rep 38:BSR20171607. 10.1042/BSR20171607 [DOI] [PMC free article] [PubMed] [Retracted]
- 74.Huang Y, Zhang J, Hou L, et al. LncRNA AK023391 promotes tumorigenesis and invasion of gastric cancer through activation of the PI3K/Akt signaling pathway. J Exp Clin Cancer Res. 2017;36:194. doi: 10.1186/s13046-017-0666-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Dong D, Mu Z, Zhao C, Sun M. ZFAS1: a novel tumor-related long non-coding RNA. Cancer Cell Int. 2018;18:125. doi: 10.1186/s12935-018-0623-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Majidinia M, Darband SG, Kaviani M, et al. Cross-regulation between Notch signaling pathway and miRNA machinery in cancer. DNA Repair (Amst) 2018;66–67:30–41. doi: 10.1016/j.dnarep.2018.04.002. [DOI] [PubMed] [Google Scholar]
- 77.Zhang X, Liang W, Liu J, et al. Long non-coding RNA UFC1 promotes gastric cancer progression by regulating miR-498/Lin28b. J Exp Clin Cancer Res. 2018;37:134. doi: 10.1186/s13046-018-0803-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Shimoda A, Ueda K, Nishiumi S. Exosomes as nanocarriers for systemic delivery of the Helicobacter pylori virulence factor CagA. Sci Rep. 2016;6:18346. doi: 10.1038/srep18346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Zhang W, Jiang X, Bao J, et al. Exosomes in pathogen infections: a bridge to deliver molecules and link functions. Front Immunol. 2018;9:90. doi: 10.3389/fimmu.2018.00090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Che Y, Geng B, Xu Y, et al. Helicobacter pylori-induced exosomal MET educates tumour-associated macrophages to promote gastric cancer progression. J Cell Mol Med. 2018;22:5708–5719. doi: 10.1111/jcmm.13847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Polakovicova I, Jerez S, Wichmann IA, et al. Role of microRNAs and exosomes in Helicobacter pylori and Epstein-barr virus associated gastric cancers. Front Microbiol. 2018;9:636. doi: 10.3389/fmicb.2018.00636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Wang J, Wang Q, Liu H, et al. MicroRNA expression and its implication for the diagnosis and therapeutic strategies of gastric cancer. Cancer Lett. 2010;29:7137–7143. doi: 10.1016/j.canlet.2010.07.018. [DOI] [PubMed] [Google Scholar]
- 83.Jarry J, Schadendorf D, Greenwood C, et al. The validity of circulating microRNAs in oncology: five years of challenges and contradictions. Mol Oncol. 2014;8:819–829. doi: 10.1016/j.molonc.2014.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Ueda T, Volinia S, Okumura H, et al. Relation between microRNA expression and progression and prognosis of gastric cancer: a microRNA expression analysis. Lancet Oncol. 2010;11:136–146. doi: 10.1016/S1470-2045(09)70343-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Rosenfeld N, Aharonov R, Meiri E, et al. MicroRNAs accurately identify cancer tissue origin. Nat Biotechnol. 2008;26:462–469. doi: 10.1038/nbt1392. [DOI] [PubMed] [Google Scholar]
- 86.Huang Z, Zhu D, Wu L, et al. Six serum-based miRNAs as potential diagnostic biomarkers for gastric cancer. Cancer Epidemiol Biomarkers Prev. 2017;26:188–196. doi: 10.1158/1055-9965.EPI-16-0607. [DOI] [PubMed] [Google Scholar]
- 87.Tokuhisa M, Ichikawa Y, Kosaka N, Ochiya T. Exosomal miRNAs from peritoneum lavage fluid as potential prognostic biomarkers of peritoneal metastasis in gastric cancer. PLoS One. 2015;10:e130472. doi: 10.1371/journal.pone.0130472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Kumata Y, Iinuma H, Suzuki Y, et al. Exosome-encapsulated microRNA-23b as a minimally invasive liquid biomarker for the prediction of recurrence and prognosis of gastric cancer patients in each tumor stage. Oncol Rep. 2018;40:319–330. doi: 10.3892/or.2018.6418. [DOI] [PubMed] [Google Scholar]
- 89.Lin L-Y, Yang L, Zeng Q, et al. Tumor-originated exosomal lncUEGC1 as a circulating biomarker for early-stage gastric cancer. Mol Cancer. 2018;17:84. doi: 10.1186/s12943-018-0834-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Berrondo C, Flax J, Kucherov V, et al. Expression of the long non-coding RNA HOTAIR correlates with disease progression in bladder cancer and is contained in bladder cancer patient urinary exosomes. PLoS One. 2016;11:e0147236. doi: 10.1371/journal.pone.0147236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Li W, Li C, Zhou T, et al. Role of exosomal proteins in cancer diagnosis. Mol Cancer. 2017;16:145. doi: 10.1186/s12943-017-0706-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Abak A, Abhari A, Rahimzadeh S. Exosomes in cancer: small vesicular transporters for cancer progression and metastasis, biomarkers in cancer therapeutics. Peer J. 2018;6:e4763. doi: 10.7717/peerj.4763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Rocha CRR, Silva MM, Quinet A, et al. DNA repair pathways and cisplatin resistance: an intimate relationship. Clinics (Sao Paulo) 2018;73:e478s. doi: 10.6061/clinics/2018/e478s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Jeddi F, Soozangar N, Sadeghi MR, et al. Contradictory roles of Nrf2/Keap1 signaling pathway in cancer prevention/promotion and chemoresistance. DNA Repair (Amst) 2017;54:13–21. doi: 10.1016/j.dnarep.2017.03.008. [DOI] [PubMed] [Google Scholar]
- 95.Ratti M, Lampis A, Hahne JC, et al. Microsatellite instability in gastric cancer: molecular bases, clinical perspectives, and new treatment approaches. Cell Mol Life Sci. 2018;75:4151–4162. doi: 10.1007/s00018-018-2906-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Zhou J, Tan X, Tan Y, et al. Mesenchymal stem cell derived exosomes in cancer progression, metastasis and drug delivery: a comprehensive review. J Cancer. 2018;9:3129–3137. doi: 10.7150/jca.25376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Hu Y, Yan C, Mu L, et al. Fibroblast-derived exosomes contribute to chemoresistance through priming cancer stem cells in colorectal cancer. PLoS One. 2015;10:e0125625. doi: 10.1371/journal.pone.0125625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Dianat-Moghadam H, Heydarifard M, Jahanban-Esfahlan R, et al. Cancer stem cells-emanated therapy resistance: implications for liposomal drug delivery systems. J Control Release. 2018;288:62–83. doi: 10.1016/j.jconrel.2018.08.043. [DOI] [PubMed] [Google Scholar]
- 99.Zheng P, Chen L, Yuan X, et al. Exosomal transfer of tumor-associated macrophage-derived miR-21 confers cisplatin resistance in gastric cancer cells. J Exp Clin Cancer Res. 2017;36:53. doi: 10.1186/s13046-017-0528-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Wang X, Zhang H, Bai M, et al. Exosomes serve as nanoparticles to deliver anti-miR-214 to reverse chemoresistance to cisplatin in gastric cancer. Mol Ther. 2018;26:774–783. doi: 10.1016/j.ymthe.2018.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Wang J-J, Wang Z-Y, Chen R, et al. Macrophage-secreted Exosomes delivering miRNA-21 inhibitor can regulate BGC-823 cell proliferation. Asian Pac J Cancer Prev. 2015;16:4203–4209. doi: 10.7314/APJCP.2015.16.10.4203. [DOI] [PubMed] [Google Scholar]
- 102.Barok M, Puhka M, Vereb G, et al. Cancer-derived exosomes from HER2-positive cancer cells carry trastuzumab-emtansine into cancer cells leading to growth inhibition and caspase activation. BMC Cancer. 2018;18:504. doi: 10.1186/s12885-018-4418-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Zhang H, Wang Y, Bai M, et al. Exosomes serve as nanoparticles to suppress tumor growth and angiogenesis in gastric cancer by delivering hepatocyte growth factor siRNA. Cancer Sci. 2018;109:629–641. doi: 10.1111/cas.13488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Kahn S, Liao Y, Du X, et al. Exosomal MicroRNAs in milk from mothers delivering preterm infants survive in vitro digestion and are taken up by human intestinal cells. Mol Nutr Food Res. 2018;62:1701050. doi: 10.1002/mnfr.201701050. [DOI] [PubMed] [Google Scholar]