Abstract
Nonalcoholic fatty liver disease (NAFLD) is a main hepatic manifestation of metabolic syndrome. It represents a wide spectrum of histopathological abnormalities ranging from simple steatosis to nonalcoholic steatohepatitis (NASH) with or without fibrosis and, eventually, cirrhosis and hepatocellular carcinoma. While hepatic simple steatosis seems to be a rather benign manifestation of hepatic triglyceride accumulation, the buildup of highly toxic free fatty acids associated with insulin resistance-induced massive free fatty acid mobilization from adipose tissue and the increased de novo hepatic fatty acid synthesis from glucose acts as the “first hit” for NAFLD development. NAFLD progression seems to involve the occurrence of “parallel, multiple-hit” injuries, such as oxidative stress-induced mitochondrial dysfunction, endoplasmic reticulum stress, endotoxin-induced, TLR4-dependent release of inflammatory cytokines, and iron overload, among many others. These deleterious factors are responsible for the triggering of a number of signaling cascades leading to inflammation, cell death, and fibrosis, the hallmarks of NASH. This review is aimed at integrating the overwhelming progress made in the characterization of the physiopathological mechanisms of NAFLD at a molecular level, to better understand the factor influencing the initiation and progression of the disease.
Keywords: Nonalcoholic fatty liver disease, Nonalcoholic steatohepatitis, Lipotoxicity, Oxidative stress, Liver fibrosis, Hepatocellular death
Introduction
Nonalcoholic fatty liver disease (NAFLD) is defined as a clinico-pathological syndrome, with the main feature being fat accumulation in hepatic tissue greater than 5% of liver weight, which cannot be explained by excessive alcohol consumption (i.e., > 30 g per day) [1]. This entity remained unidentified until the 1980s, but it is recognized nowadays as the most common chronic liver disease in most parts of the world, and the first one in Western countries [2]. Its prevalence rates ranges from 6 to 35% in the general population from different countries, with a median of 20% [3]; this high variability is attributed to racial and ethnical factors, as well as to differences in patient’s selection, diagnostic methods, and dietary/lifestyle customs. The disease has dramatically increased its prevalence worldwide; the percentage of NAFLD among chronic liver diseases increased from 47 to 75% in the last 20 years [4]. As a consequence of this high prevalence, NAFLD is expected to become the leading cause of end-stage liver disease, liver transplantation, and HCC over the next decade [5].
A reason for the high prevalence of NAFLD is that it is strongly linked to obesity, a global epidemic mainly associated with hypercaloric diet and low physical activity, as well as with their direct consequences, insulin resistance (IR) and dyslipidemia; actually, among obese individuals, prevalence ranges from 57 to 98% [3]. NAFLD patients usually have an unhealthy dietary pattern characterized by high consumption of fat esterified with saturated free fatty acids (FFAs), cholesterol, and fructose and, on the other hand, low intake of antioxidant vitamins and fat esterified with polyunsaturated FFAs [6]. However, fatty liver can also be found in lean patients with IR and atherogenic dyslipidemia, referred to as ‘metabolically obese normal weight patients’, as well as in patients with genetic liver metabolic disorders leading to macro- or microvesicular steatosis (e.g., lipodystrophy, hypobetalipoproteinemia, Weber-Christian, and glycogen storage diseases), nutritional alterations (e.g., severe weight loss, total parenteral nutrition, and starvation), or as a secondary consequence of drug-induced fatty liver (e.g., amiodarone, tamoxifen, corticosteroids, tamoxifen, and antiretroviral therapy), among other additional factors [7–9]; the prevalence of non-obese fatty liver ranges from 4.2 to 27.5%, with the higher values found in the Asian population [7].
Epidemiological, familial, and twin studies have provided conclusive evidence for an elevated component of hereditability of liver fat content and NAFLD progression to nonalcoholic steatohepatitis (NASH) [8, 9]; actually, common genetic variants associated with the pathogenesis of NAFLD are currently deemed the major contributors of the disease risk [10].
NAFLD is more prevalent in people of Hispanic origin than in Europeans, and less common in African-Americans, a difference that is not accounted for by obesity and diabetes [9, 11], but by a genetic susceptibility to this condition (33% vs. 14%) [12]. In addition, family studies demonstrated a clear heritability of NASH. Finally, high heritability of abnormalities in liver function tests reflecting NAFLD has been demonstrated in twins. Overall, there is compelling evidence that about half of steatosis variability, determined either by biochemical indices or noninvasive assessment, is inherited [13], with a range from 20 to 70%, depending on the ethnicity, study design, and diagnosis methodology [14]. Furthermore, studies in twins demonstrated that liver fatty content and transaminase serum levels are strongly heritable characters, with genetic factors accounting for up to 60% of this variability [15]. Therefore, genetic susceptibility may be a crucial factor explaining why only a limited number of subjects with NAFLD ever progress beyond steatosis to significant steatohepatitis, fibrosis, and hepatocarninogenesis [13, 16, 17].
Physiopathological mechanisms of NAFLD
NAFLD has a wide histological spectrum, from pure triglyceride (TG) accumulation in droplets within hepatocytes associated with negligible, if any, inflammation (simple, macrovesicular steatosis) to overt inflammation (with or without fibrosis), a disorder referred to as NASH; this latter condition is present in 3–15% of the general population [18]. NASH may eventually evolve to cirrhosis and hepatocellular carcinoma (HCC); 9–20% of patients with early stage NASH progress to cirrhosis over a period of 5–10 years [1], and some of them develop HCC (5-year cumulative incidence of 20% in patients with F3/F4-staged liver fibrosis) [3, 19]; HCC can also arise without cirrhosis [20].
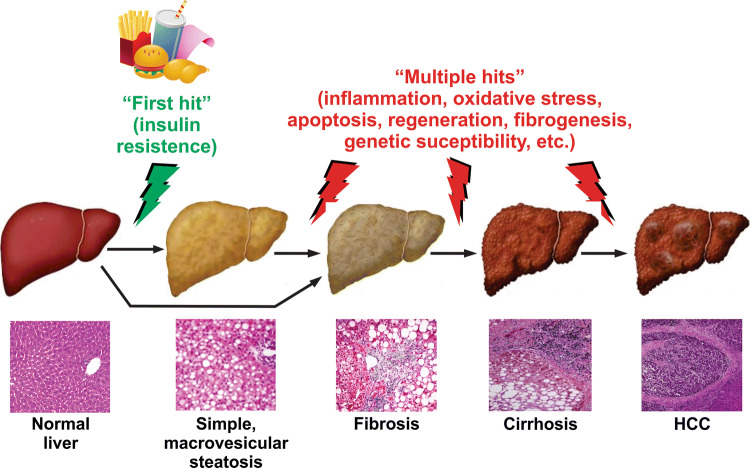
This progression was formerly explained by Day et al. almost two decade ago by the “two-hit hypothesis” [21]. According to this theory, the ‘first hit’ is triggered by the lipid accumulation in hepatocytes (hepatic macrovesicular steatosis), a trait in which exacerbated fat intake and IR would play a key role. This condition would enhance the vulnerability of the liver to other damaging factors, collectively referred to as ‘second hit’, such as oxidative stress (OS), genetic polymorphisms that increase susceptibility to the disease, activation of inflammatory pathways by the release of pro-inflammatory cytokines by Kupffer cells or adipokines from adipocytes, dysregulated hepatocyte apoptosis, and hepatic stellate cells (HSC) activation, among others. They constitute the ‘second hit’ which, when acting separately, would drive the progress from macrovesicular steatosis to NASH. This progression includes gradual liver injury associated with hepatocyte apoptosis, liver inflammation, and fibrogenesis; fibrosis progresses from periportal to bridging fibrosis, and eventually, cirrhotic remodeling with liver failure and, finally, hepatocarcinogenesis [22] (Fig. 1).
Fig. 1.
‘Multiple-hit’ theory of clinical progression of NAFLD. NAFLD comprises a wide spectrum of liver damage, ranging from simple macrovesicular steatosis to steatohepatitis, with combined inflammation, fibrosis and liver injury (steatohepatitis), progressing to cirrhosis and eventually to hepatocellular carcinoma (HCC). The initial hit is insulin resistance, leading to an increased uptake and synthesis of free fatty acids (FFAs) stored as triglycerides, resulting in simple steatosis. Further multiple hits acting as superimposed insults may be involved in the progression to more advanced stages of the disease, including oxidative stress, inflammatory mediators from hepatic origin (e.g., Kupffer cell-derived inflammatory cytokines) and from extrahepatic sources (e.g., adipokines and lipopolysaccharides of gut microbiota), apoptotic insults and the resulting compensatory regeneration, acquisition of a pro-fibrotic phenotype, and prosteatotic genetic factors, among others. NASH may occur even without steatosis, thus supporting the view that steatosis is not a causal factor in NASH development and progression
Over time, this concept has evolved, and this progression has been more recently explained by a ‘parallel multiple-hit theory’ [23]. According to this view, IR, which is an independent risk factor for NAFLD severity [24], would be the ‘first hit’ that triggers the disease, leading to hepatocellular elevations of FFAs; these molecules would be the main pathogenic factors that make the liver extremely vulnerable to the further hits stated above. This model conceptualizes NASH as a condition which is preceded by IR (from a pathophysiological point of view) and by simple, macrovesicular steatosis (from a histological point of view), with progression from pure steatosis to NASH being driven by a plethora of parallel hepatic injuries gradually accumulating over time [23]. However, a more recent ‘distinct-hit’ theory has emerged, which proposes that NASH and pure fatty liver are two independent twin conditions caused by IR, with steatosis in NASH being a benign epiphenomenon rather than a causal factor of inflammation and fibrosis, the two main hallmarks of NASH [25]. According to this new hypothesis, the differential timing and the combinations of pathogenic molecular events rather than the plain accumulation of hepatotoxic insults would result in the differential activation of these distinct pathways leading to simple steatosis and/or NASH, regarded as two separate pathological entities [25]. In line with this view, NASH patients may present without any or much steatosis [23, 26]. Furthermore, hepatocyte stress due to inflammation in NASH may cause lipid accumulation, so that, in certain cases, inflammation even precedes steatosis rather than been a consequence of it [25].
This paradigm change agrees well with the currently well-documented evidence that FFAs rather than TGs accumulated in lipid droplets are the main culprit factors for the inflammatory liver damage in NASH. Hepatic metabolism of FFAs leads to the formation of toxic metabolites (e.g., diacylglycerol and ceramides), and they are mainly responsible for OS generation, inflammation, and liver parenchyma injury [27–31]. On the other hand, hepatic TG accumulation in the liver is nowadays considered a nontoxic, safer form of storage for lipids in the liver, and an epiphenomenon reflecting changes in hepatocyte FFA flux balance and cellular stress [32]; thus, steatosis can be considered an early adaptive response to hepatocyte stress due to increased caloric consumption, through which potentially lipotoxic FFAs are partitioned into relatively inert intracellular TG molecules [23]. In line with this, inhibition of TG synthesis counteracted hepatic steatosis, but exacerbated liver damage and fibrosis in an obese mouse model of NASH [33].
Metabolic basis of NAFLD pathogenesis
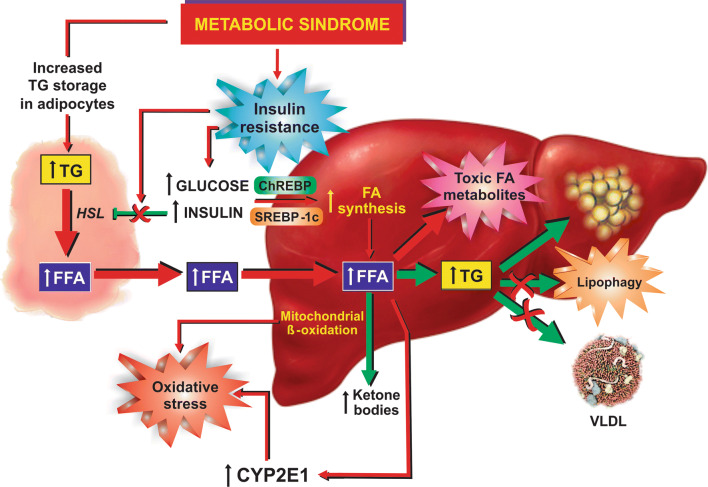
Excessive hepatic FFA accumulation in NAFLD results from an imbalance between increased FFA availability due to hepatic FFA synthesis and uptake and, on the other hand, reduced FFA removal via mitochondrial β-oxidation, very low density lipoprotein (VLDL) exportation, and lipophagy [34, 35] (Fig. 2).
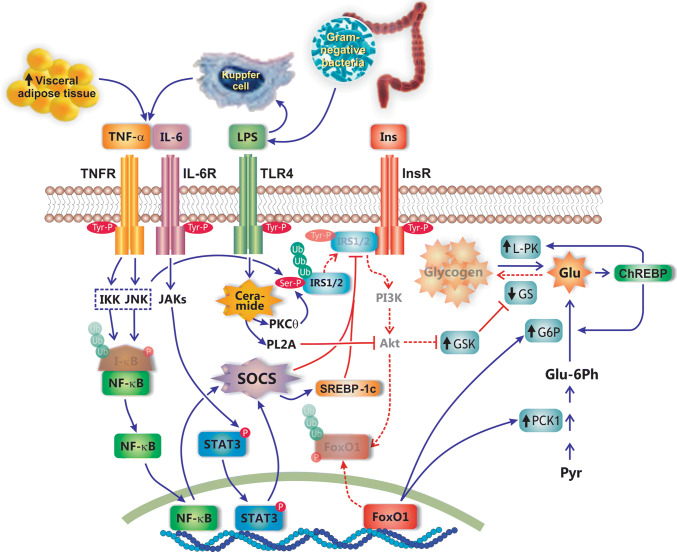
Fig. 2.
Metabolic basis of NAFLD pathogenesis. Insulin resistance associated with metabolic syndrome leads to unbalance between gain (red arrows) and loss (green arrows) of fat in liver. Insulin resistance in adipocyte tissue leads to massive mobilization of fatty acids (FFAs) from fat-overloaded adipocytes into liver, due to the suppression of the inhibitory effect of insulin on hormone-sensitive lipase (HLS), the rate-limiting enzyme catalyzing adipocyte TG lipolysis. Peripheral insulin resistance also leads to an overload of glucose (not internalized by peripheral tissues) and insulin (due to compensatory hyperinsulinaemia). Delayed insulin resistance in liver as compared with other tissues allows for the exacerbated insulin- and glucose-induced hepatic effects, with overproduction of hepatic FFAs via activation of the transcription factors sterol regulatory binding protein-1c (SREBP-1c) and carbohydrate response element binding protein (ChREBP), respectively. FFAs are stored as TGs in lipid droplets, exported as very low density lipoprotein (VLDL), and oxidized by mitochondrial β-oxidation, as compensatory mechanisms to the increased FFA uptake and synthesis. Lipophagy helps to degrade TGs from lipid droplets and to deliver FFAs for these exportation routes. However, VLDL production and lipophagy are impaired in NAFLD, and enhanced mitochondrial FFA β-oxidation leads to oxidative stress due to exacerbated leakage of electrons from the electron transport chain. Oxidative stress is aggravated by the concomitant FFA-mediated induction of CYP2E1, a cytochrome that performs futile cycles with release of electrons into the cytosol
Dietary fat represents the primary supply of adipocyte FFAs, which are further released and re-esterified in the liver [36]. IR, predisposing to both lipolysis of peripheral fat with mobilization of FFAs to the liver and exacerbated hepatic lipogenesis, is the most important and frequent underlying factor involved in hepatic FFA accumulation [36, 37]. Actually, circulating FFAs derived from dysregulated lipolysis in adipose tissue contributes to more than 80% of the total circulating FFA pool in NAFLD patients; most of the hepatic fat comes from this FFA pool (59%), whereas a lower fraction comes from de novo lipogenesis (26%) and from diet (15%) [34].
Both type-2 diabetes and obesity are strongly associated with IR, and interlinked with each other: anyone who is obese has IR to some extent [38], and central nervous IR induces obesity [39]. Peripheral and hepatic IR leads to a compensatory increase in pancreatic β-cell insulin secretion, which eventually induces β-cell secretory failure and, subsequently, overt type-2 diabetes [40]. Thus, this disease develops in those obese individuals with insufficient secretion of insulin to overcome their IR degree [38].
Obesity leading to IR and the subsequent chronically elevated FFAs in serum can disturb different metabolic pathways and induce IR in many tissues, including the adipose one. Adipose tissue is the unique physiological lipid depot and the repository of the excess energy derived from food intake. However, the massive accumulation of fat in adipose tissue associated with obesity leads to dysfunctional adipocytes, with abnormal release of adipokines [41], including inflammatory cytokines such as tumor necrosis factor-α (TNF-α), interleukin (IL)-6, plasminogen activator inhibitor 1, resistin, and monocyte chemoattractant protein 1 (MCP-1), which impair adipocyte responsiveness to insulin [18, 42]. Since insulin inhibits hormone-sensitive lipase, the rate-limiting enzyme catalyzing TG lipolysis in adipocyte tissue, IR in this tissue leads to failure in insulin-mediated inhibition of adipocyte lipolysis [43]. This, in turn, leads to massive mobilization of FFAs from adipose tissue to other non-adipose tissues, thus causing ectopic intracellular accumulation of FFAs. This fact, together with the long-distance action of fat-derived cytokines, leads to generalized IR [44].
Ectopic FFA accumulation in skeletal muscle has been regarded as an early and major source of IR in NAFLD [45], whereas hepatic IR may be a further event [46]. When the IR-induced impairment of insulin uptake predominates in extrahepatic tissues over that in liver, the latter organ receives an overload of glucose (not internalized by peripheral tissues) and insulin (due to compensatory hyperinsulinaemia) [47]. Under such a condition, both insulin (through activation of the transcription factor sterol regulatory binding protein-1c, SREBP-1c) and glucose (through activation of the transcription factor carbohydrate response element binding protein, ChREBP) induce liver synthesis of FFAs [48]. SREBP-1c activates the transcription of genes codifying a plethora of enzymes/transporters involved in FFA metabolism, including those required for FFA uptake (e.g., CD36), FFA synthesis (e.g., acetyl-CoA carboxylase and FFA synthase), and TG synthesis (e.g., glycerol-3-phosphate acyltransferase) [48, 49]. ChREBP also stimulates transcription of many lipogenic genes and induces the expression of both liver-type pyruvate kinase (L-PK), a key regulator of glycolysis, and glucose-6 phosphatase (G6P), a critical enzyme involved in gluconeogenesis [48, 50–52]. Dietary factors may aggravate this situation further. SREBP-1c is induced by dietary cholesterol, via activation of the ligand-activated nuclear receptor liver X receptor (LXR); this transcription factor binds to oxysterols, which are intermediate metabolites in the bile acid biosynthetic pathways from cholesterol [53]. The pro-stetotic proteins ChREBP, stearoyl-CoA desaturase (rate-limiting step of monounsaturated FFAs), and FFA synthase are also activated by LXR [54]. In addition, the kind of fatty acids contained in the diet may also impact on SREBP-1c expression, with saturated ones upregulating and polyunsaturated ones down-regulating this transcription factor [55]. Finally, chronic fructose intake activates both SREBP-1c and ChREBP [56, 57]. Prolonged fructose intake leads to sustained SREBP1c activation by promoting liver buildup of advanced glycation end products that activate SREBP-cleavage activating protein (SCAP) [58], whereas it can also activate ChREBP both directly through fructose-derived intermediate products and, indirectly, via activation of glycogen kinase (GK) [59].
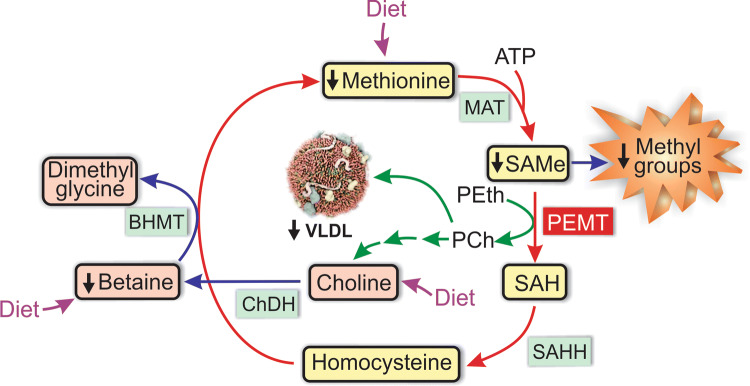
Exacerbated liver insulin signaling also impairs the hepatic metabolic utilization of FFAs. The synthesis of apolipoprotein (apo) B, the main protein cofactor of VLDL, is repressed by insulin, and this impairs exportation of hepatic lipids via this lipoprotein [60]. Another pathomechanism that affects VLDL exportation is the impairment in the methyl group metabolism associated with the shortage of the main human methyl donor, S-adenosylmethionine (SAMe) [61, 62]. As shown in Fig. 3, SAMe is the methyl donor for phosphatidylcholine synthesis from phosphatidylethanolamine catalyzed by the endoplasmic reticulum (ER) enzyme phosphatidylethanolamine N-methyltransferase (PEMT). The de novo synthesis of phosphatidylcholine by this enzyme is a critical prerequisite for VLDL formation and stability; newly synthesized phosphatidylcholine associates with apo B in early stages of hepatic VLDL assembly in the ER lumen, and lack of phosphatidylcholine affects this assembly, leading to increased degradation of nascent VLDLs [63]. In addition, a normal PEMT activity seems to be required for secretion of apo B-containing VLDLs [64]. Betaine, an oxidized metabolite of choline, indirectly influences PEMT-mediated phosphatidylcholine synthesis by being involved in the synthesis of SAMe (Fig. 3) [65]. Betaine levels are decreased in NASH patients as compared with both controls and individuals with pure steatosis, and this may play a role in the progression of the disease by impairing TG export via VLDL secretion [61].
Fig. 3.
Enzymatic pathways of the methyl group metabolism involved in VLDL formation. The primary methyl donor S-adenosylmethionine (SAMe) provides methyl groups for numerous methyltransferase reactions, and is regenerated by a number of interrelated metabolic pathways. Following SAMe-dependent transmethylations, SAMe is converted into S-adenosylhomocysteine (SAH) by phosphatidylethanolamine N-methyltransferase (PEMT), and SAH is further converted into homocysteine by SAH hydrolase (SAHH). Homocysteine can be remethylated back to methionine using betaine as a methyl donor, with the additional formation of dimethylglycine, in a reaction catalyzed by betaine-homocysteine S-methyltransferase (BHMT). The methionine thus formed, together with that provided by the diet, regenerates SAMe after receiving an adenosyl group from ATP, in a reaction catalyzed by methionine adenosyltransferase (MAT). Betaine is provided by the diet, and it is also produced from diet-derived choline by the enzyme choline dehydrogenase (ChDH), which represents the final step in a catabolic pathway initiated by the formation of phosphatidylcholine (PCh) from phosphatidylethanolamine (PEth), catalyzed by PEMT. Alternatively, PCh can be incorporated with other lipids and apolipoprotein (apo) B into nascent VLDL in the ER, where it plays a critical role in VLDL assembly and secretion. In NAFLD, there is a shortage of SAMe due to the fact that betaine levels are decreased in NASH patients, thus affecting VLDL exportation
Another source of hepatic lipids is that provided by LDL endocytosis. Catabolism of LDL-derived cholesterol esters and TGs involves LDL trafficking to lysosomes and further lysosomal hydrolysis into free cholesterol and FFAs by lysosomal acid lipase (lipophagy) [66]; interestingly, NAFLD patients have lower activity of lysosomal acid lipase [67]. Therefore, the inhibition of this process induces intralysosomal lipid accumulation, and the consequent reduction of FFAs and free cholesterol in cytosol, which can promote (1) increase of the activity of SREBPs, leading to increased lipogenesis and cholesterol biosynthesis, and (2) reduction in the LXR expression, which leads to reduced efflux of cholesterol and HDL production [68]. Interestingly, insulin is known to inhibit autophagy in general (and lipophagy in particular) by activating mTOR complex 1 (mTORC1), which inhibits autophagosome assembly by inactivating via phosphorylation a multiprotein complex composed of Unc-51 like autophagy activating kinase 1/2 (ULK1/2), autophagy-related protein 13 (ATG13), and FAK family interacting protein of 200 kDa (FIP200); this prevents this complex from activating beclin 1 and its further interactions with phosphatidylinositol 3-kinase catalytic subunit type 3 (PIK3C3), which nucleates the autophagosome membrane formation by generating PI-3,4,5-phosphate [69].
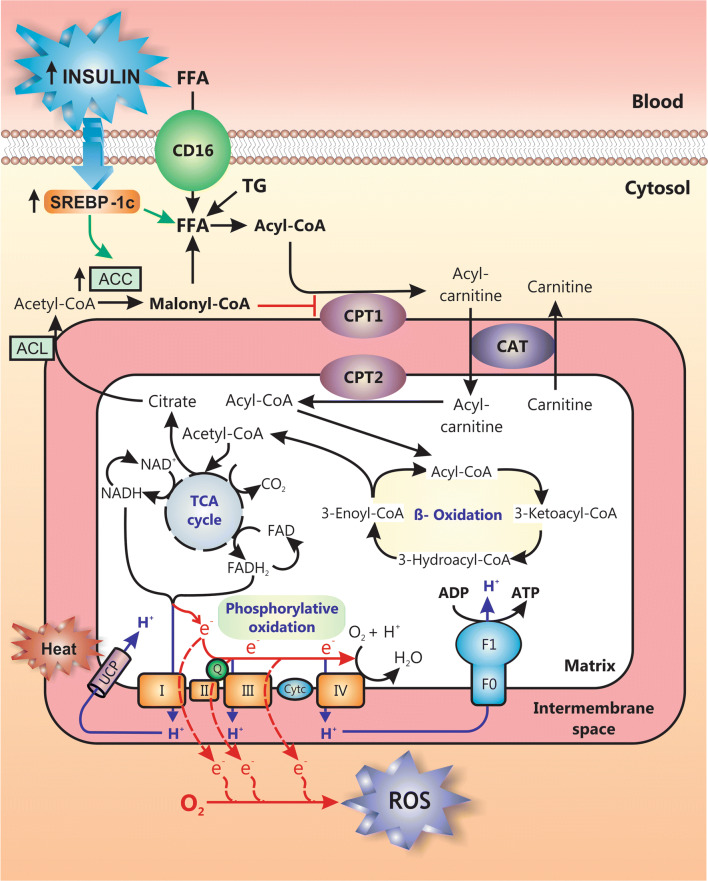
Aggravating the hepatic accumulation of lipid products further, in advanced NAFLD there is an impairment of mitochondrial β-oxidation, a metabolic process that converts FFAs into ketone bodies, which are both excreted into urine and exhaled (Fig. 4). This impairment is due to (1) SREBP-1c-mediated induction of acetyl-CoA carboxylase 2 (ACC2) with further overproduction of malonyl-CoA, a metabolite that inhibits the β-oxidation regulatory enzyme carnitine palmitoyltransferase 1 (CPT1) [70]; (2) hepatic accumulation of atypical toxic lipids that inhibit mitochondrial β-oxidation, such as oxidized cardiolipin [71, 72] and ceramides [30, 73]; (3) the OS that develops within mitochondria due to this metabolic process, which can damage complexes of the mitochondrial respiratory chain or mitochondrial DNA encoding some of these components; this latter event eventually impairs β-oxidation, since it hampers the re-oxidation of NADH and FADH2 into NAD+ and FAD, thus leading to the inhibition of mitochondrial β-oxidation and the tricarboxylic acid cycle, which require NAD+ and FAD to occurs [74].
Fig. 4.
Mitochondrial dysfunction in NAFLD. Long-chain free fatty acids (FFAs) from different sources, including uptake of FFAs mobilized from adipocyte tissue via CD16, de novo lipogenesis stimulated by insulin via sterol regulatory binding protein-1c (SREBP-1c) activation and triglyceride (TG) hydrolysis, are metabolized by mitochondria to supply energy. Acyl-CoA resulting from FFA activation penetrates the mitochondrial directly (for short and medium chain acyl-CoA) or via the carnitine shuttle (for long chain acyl-CoA), a process consisting of its conversion into carnitine-CoA catalyzed by the mitochondrial outer membrane enzyme carnitine palmitoyltransferase 1 (CPT1) and its further reconversion to acyl-CoA by the inner membrane enzyme CPT2; this makes acyl-CoA available for β-oxidation in the matrix. Acetyl-CoA resulting from β-oxidation enters the tricarboxylic acid (TCA) cycle, which renders NADH and FADH2 molecules. These reduced mediators carry their electrons to the respiratory electron carriers (complexes I-IV) in the mitochondrial inner membrane, which pumps protons (H+) through this membrane to generate the electrochemical H+ gradient that drives mitochondrial ATP production by the ATP synthase (F1F0 complex), a process known as ‘oxidative phosphorylation’. Alternatively, the electrochemical H+ gradient can be dissipated as heat via the uncoupling protein (UCP). Leakage of electrons at complexes I-III results in the formation of reactive oxygen species (ROS), such as superoxide (O2−) or H2O2; this may damage the organelle, leading to impaired mitochondrial function and to a decrease in FFA metabolization. Failure in FFA β-oxidation is aggravated further by the inhibition of CPT1 by malonyl-CoA, which is produced from TCA cycle-derived citrate, via conversion to acetyl-CoA by the ATP citrate lyase (ACL) and further carboxylation by the acetyl coenzyme A carboxylase (ACC); the latter enzyme is induced by SREBP-1c
Hepatic FFA β-oxidation, however, can be augmented in later stages of NASH, as a compensatory mechanism to the increased uptake and synthesis of FFAs [75]; this involves both activation of peroxisome proliferator-activated receptor-α (PPAR-α) and the consequent enhancement of CPT1 activity, as well as loss of affinity of CPT1 for its inhibitor, malonyl-CoA [76, 77]. Other alternative cellular FFA oxidation mechanisms, including microsomal (ω-oxidation) and peroxisomal (β-oxidation), may be also upregulated to decrease FFA accumulation, with the latter increasing hydrogen peroxide production in peroxisomes [78]. However, these mechanisms might not overcome the increased rates of liver FFA synthesis.
In addition to extrahepatic IR, it is now well recognized that IR at the hepatic level also plays a major role in NAFLD/NASH [46]; furthermore, NAFLD itself can promote hyperinsulinemia because of impaired insulin degradation [79]. Hepatic IR occurs as a consequence of multiple mechanisms, mainly as a secondary consequence of the inflammatory condition (Fig. 5). Hepatic FFA accumulation, with the consequent production of FFA toxic metabolites (e.g., ceramides), interferes with the activation of insulin receptor substrates 1 and 2 (IRS1/2) by protein kinase C-θ (PKCθ), which phosphorylates IRS1/2 at a serine residue, thus leading to its ubiquitination and further proteosomal degradation [80, 81]. This involves activation by lipopolysaccharide (LPS) from intestinal origin of the toll-like receptor 4 (TLR4)/myeloid differentiation primary response 88 (MyD88) pathway, the TLR4-mediated enhancement of de novo ceramide synthesis, and the further ceramide-induced activation of protein phosphatase 2A (PP2A), which inhibits insulin signaling by counteracting Akt phosphorylation [82, 83]. Cytokines, particularly TNF-α and IL-6, which are produced both by hypertrophic visceral adipose tissue and Kupffer cells activated by LPS, also contribute to hepatic IR induction. After interacting with its membrane receptor, IL-6 activates Janus-activated kinases (JAKs), which in turn activates by phosphorylation the signal transducer and activator of transcription 3 (STAT3), an inducer of the negative regulators of cytokine action suppressor of cytokine signaling proteins (SOCS); similarly, TNF-α activates SOCS via nuclear factor-κB (NF-κB)-dependent mechanisms involving c-Jun N-terminal kinase (JNK)- and IκB kinase (IKK)-dependent mechanisms. SOCS induce IR via inhibition of insulin receptor-mediated IRS1/2 activation by different mechanisms: (1) by inducing SREBP-1c, which suppresses IRS1/2 synthesis, (2) by inhibiting the tyrosine kinase activity of the insulin receptor, and (3) by competing with IRS1/2 for the insulin receptor binding sites required for IRS1/2 activation via tyrosine phosphorylation. Lack of IRS1/2 activation impedes IRS1/2-mediated activation of the phosphoinositide 3-kinase (PI3K)/Akt signaling pathway, and targets IRS1/2 to degradation [80, 81, 84–86].
Fig. 5.
Mechanisms of hepatic insulin resistance in NAFLD. This pathology occurs in a context of sub-acute inflammation (e.g., obesity, type 2 mellitus diabetes), which promotes insulin resistance via the influx of pro-inflammatory cytokines, such as TNF-α and IL-6, from both the adipose tissue and Kupffer cells. After interaction with their respective membrane receptors, TNFR and IL-6R, these cytokines activate different signaling pathways that result in ubiquitin-mediated proteosomal degradation of the insulin receptor substrates 1 and 2 (IRS1/2), two signaling molecules required for insulin transduction via the phosphoinositide 3-kinase (PI3K)/Akt pathway. TNF-α and IL-6 promote IRS1/2 degradation by inducing synthesis of suppressor of cytokine signaling (SOCS), via activation of the transcription factors NF-κB and the signal transducer and activator of transcription 3 (STAT3), respectively. NF-κB is activated by TNF-α via inhibitor of κB (I-κB) kinase (IKK)- and c-Jun N-terminal kinase (JNK)-mediated phosphorylation, and further proteosomal degradation of the NF-κB inhibitor I-κB. IL-6 activates STAT3 via phosphorylation of this transcription factor by Janus activated kinase (JAK). Alternatively, proteosomal degradation of IRS1/2 is promoted by lipopolysaccharide (LPS) from intestinal microbiota. LPS interacts with the toll-like receptor (TLR4) to activate the ceramide/PKCθ signaling pathway, which induces, in turn, serine phosphorylation of IRS1/2 and further proteosomal degradation; ceramide also activates phospholipase-A2 (PLA2), which inhibits Akt activation. Lack of Akt signaling impairs Akt-mediated phosphorylation and further ubiquitin-mediated proteosomal degradation of forkhead box protein O1 (FoxO1), a transcription factor whose activation induces upregulation of gluconeogenic enzymes, such as phosphoenolpyruvate carboxykinase (PCK1) and glucokinase-6 phosphatase (G6P). The resulting increase in hepatocellular glucose (Glu) levels stimulates the transcription factor carbohydrate response element-binding protein (ChREBP), which in turn reinforces G6P activation and induces upregulation of enzymes involved in glycogenolysis, such as liver pyruvate kinase (L-PK), thus resulting in a higher hepatocellular glucose increase. Glycogenolysis is exacerbated by lack of Akt-mediated glycogen synthase kinase (GSK), an enzyme that inhibits glycogen synthase (GS). Blue, continuous lines: activation pathways. Red, continuous lines: inhibitory pathways. Red, dashed lines: inactivated pathways
The addition of IR at the hepatic level does not modify the unbalance between gain and loss of fat in liver. This is due, in part, to the increase in glucose bioavailability for lipogenesis. Indeed, apart from the sustained uptake of glucose, which is insulin independent in liver, under hepatic IR conditions there is an exacerbated hepatic glucose synthesis (gluconeogenesis), an inhibited glucose recruitment via glycogen synthesis (glycogenogenesis), and a stimulated glycogen degradation (glycogenolysis) [87] (Fig. 5). Induction of gluconeogenesis involves upregulation of genes encoding gluconeogenic enzymes, otherwise downregulated by insulin, as for example phosphoenolpyruvate carboxykinase (PCK1) and G6P; insulin-mediated upregulation of these enzymes involves, in turn, lack of insulin receptor-mediated activation of the phosphoinositide 3-kinase (PI3K)/Akt signaling pathway, and the subsequent lack of Akt-mediated phosphorylation and further ubiquitin-mediated degradation of transcription factors involved in gluconeogenesis enzyme synthesis, such as forkhead box protein-O1 (Fox-O1) [88, 89]. Inhibition of gluconeogenesis occurs via inactivation of glycogen synthase (GS) by glycogen synthase kinase (GSK), which is in turn activated by the lack of repression induced by the insulin-mediated PI3K/Akt signaling pathway [88]. Finally, glucose also activates the transcription factor ChREBP, which induces genes involved in both gluconeogenesis (G6P) and glycogenolysis (L-PK) [48, 50–52].
As for lipogenesis, the glucose molecules from hepatic origin, in concert with the extrahepatic ones which are augmented due to peripheral IR, stimulate de novo FFA synthesis via ChREBP [90]. Likewise, the lipogenic transcription factor SREBP-1c can also be activated in an insulin-independent manner by SOCS, which are induced by pro-inflammatory cytokines, as described above.
Excessive FFA accumulation in liver can trigger pro-oxidizing, pro-inflammatory, pro-fibrotic, and pro-apoptotic signal pathways that account for the characteristic features of NASH, i.e., OS, inflammation, fibrosis, and apoptosis. FFA accumulation may induce: (1) OS, due mainly to early mitochondrial overfunction [91, 92] and further mitochondrial dysfunction [76], as well as induction of cytochrome P450 family 2 subfamily E member 1 (CYP2E1) [93, 94]; (2) systemic and hepatic inflammation, with upregulation of pro-inflammatory cytokines, such as TNF-α and different interleukins, which are pro-steatotic, pro-inflammatory, pro-fibrotic and pro-apoptotic in nature [95]; (3) production of transforming growth factor β (TGF-β) by Kupffer cells, which activates HSCs to produce collagen, leading to fibrosis [96]; and (4) upregulation of hepatocyte apoptosis via OS- and cytokine-mediated mechanisms [97]. All these events are intertwined, and vicious cycles can arise between them.
OS in NASH
OS is a critical for NAFLD pathogenesis [98]. Elevated OS levels are well documented in NAFLD patients using different markers of oxidized cellular components, including proteins (e.g., 3-nitrotyrosine) [75], lipids (e.g., 4-hydroxy-2-noneal) [99], and DNA (e.g., 8-hydroxydeoxyguanosine) [99]. Interestingly, NASH patients have higher OS levels than those with pure steatosis [100]. Since oxidative damage to lipid, protein, and DNA molecules can trigger inflammatory and fibrogenic signaling pathways, OS can play a crucial role in NASH development and progression [101].
Although primarily aimed to counteract the excessive FFA liver content [91, 92], enhanced FFA metabolism via mitochondrial β-oxidation is a critical pro-oxidizing factor in NASH [76, 102]. Long-chain FFAs (14–24 carbons) are converted into long-chain fatty acyl-coenzyme A (CoA) and further transported inside the mitochondrial matrix as acyl-carnitine by the CPT1, via the carnitine shuttle carnitine acetyl transferase (CAT), the rate-limiting step for mitochondrial FFA β-oxidation. Unlike long-chain FFAs, short/medium chain FFAs diffuse passively, whereas very long-chain FFAs (> 24 carbons) are oxidized in peroxisomes until they attain a 24-carbon chain length for mitochondrial oxidation. Once inside the mitochondria, they undergo β-oxidation, which yields one molecule of acetyl-CoA per oxidation cycle (Fig. 4). The further metabolism of acetyl-CoA by the tricarboxylic acid cycle produces NADH and FADH2, and these molecules are, in turn, oxidized by transferring their electrons to the mitochondrial respiratory chain to ultimately render ATP. However, at I and III complexes, electrons can prematurely escape and interact with oxygen molecules, thus resulting in radical oxygen species (ROS) production [103]; 2% of the oxygen consumed by hepatocytes is transformed into ROS by mitochondria under basal conditions [104]. Exacerbated OS in the mitochondria environment by excess FFAs as substrates may further damage the organelle function; this establishes a vicious circle, since ROS generation by the damaged respiratory chain is augmented, which further damages the integrity of the respiratory chain [74, 103]. Lower mtDNA levels have also been reported in NASH patients [105], but not in simple steatosis [106]. In addition, different genetic mtDNA alterations such as deletions, point mutations, and increased 8-hydroxydeoxyguanosine levels (a marker of mitochondrial oxidized DNA) have been reported in the livers of NASH patients [107, 108], which appear to account for the alterations in expression/function of mitochondrially encoded subunit I and subunit II of complex I and complex IV, respectively [109]. The complex I function also depends on the integrity of cardiolipin, a normal phospholipid component of the inner mitochondrial membrane that it is excessively oxidized in NASH, thus contributing to mitochondrial dysfunction [71]. Impaired mitochondrial function is also associated with decreased production of the beneficial adipocytokine adiponectin by the hypertrophic adipocyte [110, 111]. Adiponectin stimulates hepatic β-oxidation of FFAs by activating PPAR-α in hepatocytes [112], and PPAR-α, in turn, inhibits LXR, a transcription factor that stimulates FFA synthesis via SREBP-1c. Therefore, lack of this adiponectin-activated signaling leads to increased levels of FFAs, thus aggravating liver damage [113]. Taken together, these findings support the concept that functionally and structurally altered mitochondria are a main source of OS in NASH, irrespective of whether FFA β-oxidation is increased or decreased [74].
Another main source of ROS in NASH is CYP2E1, a main enzyme of the microsomal oxidizing system that metabolizes, and is induced, by many endo- and xenobiotics, including ethanol and FFAs [94, 114–116]. Furthermore, the excess ketone bodies produced by the liver in NAFLD may not be totally utilized or cleared by extrahepatic tissues, and, consequently, they increase in liver, which also enhances CYP2E1 activation [116]. This cytochrome performs futile cycles, with release of electrons to the cytosol and the consequent ROS formation; thus, during CYP2E1-induced FFA oxidation, high-reactive carbonyl free radicals are produced [94, 114, 115]. Insulin has an inhibitory effect on CYP2E1 expression and, therefore, IR may play a role in CYP2E1 induction [117, 118]. Furthermore, CYP2E1 activity positively correlates with the steatosis degree [119], and hepatic CYP2E1 protein expression is higher in patients with steatohepatitis compared to both normal individuals and patients with simple steatosis [93, 94]; this correlates with liver injury severity [120–122], thus suggesting that CYP2E1 induction contributes to NASH progression.
Recently, NADPH oxidase 4 (NOX4), a membrane-integrated enzyme that generates superoxide anion (O2−) and hydrogen peroxide (H2O2) from molecular oxygen using NADPH as an electron donor, has been identified as another producer of ROS in NAFLD [123]. NOX4 is mainly expressed in liver both in hepatocytes [124] and in HSCs [125]. Whereas NOX4 activation in HSCs has pro-fibrogenic effects (see Section "HSC activation and fibrogenesis in NASH"), in hepatocytes, it prolongs OS-mediated ER stress, a main mechanism of hepatocellular apoptosis in NASH (see Section “Hepatocellular death in NASH”) [123]. The mechanism of NOX4 activation in NAFLD is unclear, but direct induction by saturated FFAs has been reported in mouse hepatocytes [123]. In addition, fructose intake was found to induce mitochondrial OS via uric acid-induced mitochondria translocation and activation of NADPH oxidase [126]; fructose is metabolized in the liver by fructokinase C, which results in the breakdown of adenosine monophosphate to inosine monophosphate, and further uric acid generation [127].
Iron is a well-known pro-oxidant metal and there is an association between NAFLD and body iron overload [128]; this impacts particularly on the liver since its is the major iron-storage organ, thus resulting in mild iron accumulation in both Kupffer cells and hepatocytes [129]. Excessive iron accumulation in NAFLD has been associated with downregulations of ferroportin-1 (FP-1) and hemojuvelin (HJV), an iron-export transporter and an iron-sensing protein, respectively [130].
Hepatic iron accumulation may contribute to the initial development of macrovesicular steatosis and to the progression to NASH [131]. A large study in NAFLD patients showed that hepatocellular iron was associated with increased likelihood of higher fibrosis stages [118]. In part, this effect has been attributed to the iron pro-oxidizing damage related to its role as a catalyst of ROS production through the Fenton reaction [131]. However, exacerbation of IR through alteration of insulin signaling in both adipocytes [132] and hepatocytes [133], as well as a ferritin-dependent impairment of hepatocyte lipid exportation via VLDL [134], can also be contributing factors.
Antioxidant enzymes, such as superoxide dismutase and catalase, are overexpressed in NAFLD, indicating that antioxidant pathways are activated in an attempt to neutralize ROS [78]. However, a progressive diminution of the antioxidant capacity occurs in NASH patients by both overconsume-induced depletion of antioxidant molecules (e.g., glutathione and coenzyme Q10) and oxidative inhibition of the activity of certain antioxidant enzymes (e.g., superoxide dismutase, catalase, and glutathione S-transferase), a deficit that correlates with the severity of the disease [101, 120]. Finally, not only activity but also the expression of these antioxidant enzymes is decreased in patients with cirrhotic-stage NASH [135].
OS in NAFLD can be often aggravated by drug-induced liver injury, since polypharmacy is very frequent in NAFLD obese patients to treat their comorbidities [136]. Drugs may exacerbate ROS production in NAFLD by further impairing mitochondrial function [102, 136]. This may occur by inhibition of enzymes of the respiratory chain, by uncoupling of oxidative phosphorylation, or by exacerbating glutathione depletion due to glutathione consume after drug conjugation with the peptide; this is critical for mitochondrial integrity, since mitochondria lack catalase, and glutathione is their only resource to scavenge H2O2 [137]. In addition, drugs may aggravate the impairment of FFA ß-oxidation occurring in NASH via inhibition of key mitochondrial enzymes involved in this process [138]. Finally, FFA-induced CYP2E1 overexpression may enhance CYP2E1-mediated biotransformation of drugs into highly toxic metabolites [139].
Liver inflammation in NASH
NAFLD is a pro-inflammatory condition, and inflammation is critical for NASH development and progression. Actually, whereas “pure” steatosis does not adversely affect NAFLD outcome, inflammation and its main consequence, fibrosis, is a key determinant of the long-term prognosis of the disease [140].
Inflammation in NASH is associated with the exacerbated production of inflammatory factors originated from both extrahepatic sites (e.g., adipose tissue, gut) or, within the liver, with the activation of Kupffer cells and by hepatocytes themselves, as a secondary consequence of the hepatic lipotoxic attack [23, 141] (Fig. 6).
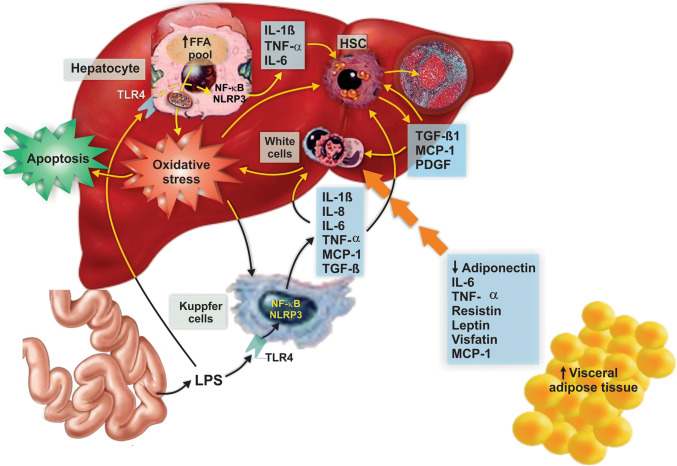
Fig. 6.
Main pro-inflammatory mechanisms involved in NASH pathogenesis. NAFLD is associated with activation of a plethora of inflammatory pathways, with mediators of this process arising from multiple origins. Hypertrophic, dysregulated adipocytes release an increased amount of several pro-inflammatory chemokines and cytokines, and a reduced amount of the insulin-sensitizing and anti-inflammatory adipocytokine, adiponectin. Intestine contributes to the inflammatory process by releasing bacterial products such as lipopolysaccharide (LPS), which targets and activates the TLR4 receptor in Kupffer cells and hepatocytes, with the consequent production of cytokines and chemokines via activation of both the transcription factor nuclear factor-κB (NF-κB) and the nucleotide oligomerization domain (NOD)-like receptor protein 3 (NLRP3) inflammasome. These cyto/chemokines promote inflammation and hepatocellular apoptosis via specific receptors in hepatocytes and immunocompetent cells, thus accelerating NAFLD progression to NASH. In addition, the increase in free fatty acids (FFAs) in hepatocytes produces sustained oxidative damage that also induces apoptosis via redox-sensitive pro-apoptotic pathways. These cyto/chemokines, particularly transforming growth factor β (TGF-β), also promote fibrogenesis by activating hepatic stellate cells (HSCs). Activated HSCs produce collagen, which is deposited in the space of Disse, and release fibrogenic cytokines with autocrine and paracrine effects, including transforming growth factor β (TGF-β), which perpetuate the fibrotic injury
Adipose tissue is an endocrine organ, and produces and releases a number of hormones (e.g., leptin, resistin, and visfatin), proinflammatory cytokines (e.g., TNF-α, IL-1 β, IL-6, and IL-8), and chemokines (e.g., MCP-1), collectively known as adipokines [142]. Enlarged adipose tissue in obese patients suffers dysregulation of adipokine production [143], with enhanced production of inflammatory chemokines and cytokines and decreased production of beneficial ones, thus contributing to peripheral and hepatic IR [144] (see Section “Metabolic basis of NAFLD pathogenesis”). Macrophages, whose amount in adipocyte tissue is positively correlated with both body mass and adipocyte size, also contribute to inflammatory cytokine secretion [145]; obesity induces a switch in macrophages from an anti-inflammatory, M2 phenotype to a pro-inflammatory, M1 one [146]. On the other hand, both serum [110, 147] and hepatic [148] levels of the beneficial adipokine adiponectin are decreased in NASH patients; adiponectin regulates FFA β-oxidation, reduces lipid accumulation in both adipose and hepatic tissues, and regulates glucose homeostasis and liver insulin sensitivity [149].
Pro-inflammatory adipokines have receptors in liver parenchyma, which traduce signaling cascades involved in IR (through suppressors of cytokine signaling [SOCS] activation), proinflammatory cytokine production (through nuclear factor-κB [NF-κB] activation), and HCC development (through JAK/STAT signaling) [23]. For example, leptin, an archetypical adipokine that suppresses food intake and enhances energy expenditure, activates macrophages, presumably through JAK/STAT signaling [150]. Fibrogenic and inflammatory effects of leptin have also been observed in HSCs [151] and Kupffer cells [152], respectively.
Certain dietary and microbiota factors that reach the liver after intestinal absorption have also a pro-inflammatory role in NASH [153]. Diets enriched in fat and fructose alter intestinal motility; this induces dysbiosis, including bacterial overgrowth, and disrupts intestinal barrier by increasing intestinal permeability to gut-derived products [154]. This alteration promotes the release of pro-inflammatory dietary components into blood, such as trans-FFAs, fructose, and aryl hydrocarbon receptor (AhR) ligands (e.g., indolo-(3,2-b)-carbazole and 3,3′-diindolylmethane) [23]. The wall of Gram-negative bacteria present in the microbiota (i.e., Bacteroidetes, Cyanobacteria, and Verrucomicrobia) has the potent pro-inflammatory agent LPS (endotoxin), and high-fat or high-carbohydrate diet affects bacterial endotoxin metabolism, which results in enhanced LPS serum plasma levels [155]. Studies in rodents demonstrated that the subgroup of animals that failed to display hyperglycemia and systemic inflammation when on a high-fat diet has a distinct gut microbiota at different taxonomic ranks, such as phylum, genus, and species [156]. Increased intake of certain nutrients, such as fructose, enhances the profusion of some otherwise minor Gram-negative bacteria, such as Enterobacteriaceae and Desulfovibrionaceae [157], thus promoting bacterial overgrowth and, hence, LPS release [158]. In addition, and beyond the effect of a specific nutrient, patients with NAFLD have increased intestinal permeability by disruption of tight junctions, which further helps to increase endotoxin intestinal leakage, and even bacterial translocation [154, 159, 160].
Endotoxin from the gut is sensed in liver by TLR4 from Kupffer cells, hepatocytes, and HSCs [161], whose expression is enhanced in NAFLD [162]. In all these cells, but particularly in Kupffer cells which have the highest TLR4 levels [163], LPS activates different signaling cascades, leading to pro-inflammatory cytokine synthesis by activation of MAPKs, and the further activation of pro-inflammatory transcription factors, such as NF-κB and adaptor protein-1 (AP-1), through a signal initiated in its interacting adaptor molecule MyD88 [141, 161, 164]. The resulting release of cytokines, particularly TGF-β and IL-8, has chemoattractant effects, and promotes the recruitment of immune effector cells such as neutrophils, with subsequent hepatocyte injury via OS-mediated mechanisms [161, 163, 165].
Although hepatocytes express TLR4 and are responsive to LPS, the response is weak as compared with the other hepatic cell types [166]. In hepatocytes, accumulated FFAs rather than LPS are the main culprit mediators of the inflammatory response. FFAs act as danger-associated molecular patterns (DAMPs) and activate the NACHT, LRR, and PYD domains-containing protein 3 (NALP3) inflammasome. NALP3 is a large caspase 1-activating multiprotein complex that senses FFAs through intracellular NOD-like receptors (NLRs) and that activates pro-caspase 1 through its inflammasome adaptor molecule apoptotic speck-like protein containing caspase-1 activation and recruitment domain (ASC); caspase-1 induces the cleavage and, therefore, maturation of pro-inflammatory cytokines, mainly IL-1β, to promote and sustain inflammation [167]. In addition, increased FFAs in hepatocytes induces lysosomal translocation of Bax, a pore-forming protein that triggers lysosomal destabilization and release of the lysosomal cysteine protease cathepsin B into cytosol, an event that enhances TNF-α expression via NF-κB activation [168]. Finally, increased ROS activate NF-κB by directly interacting with the small G-protein p21ras, and further activation of a signaling pathway involving sequentially TGF-β activated kinase-1 (TAK1), PI3K, and mitogen activated protein kinase 1 (MEK1) [169]. NF-κB upregulates the expression of several pleiotropic cytokines, including TGF-β, IL-6, IL-8, TNF-α, and Fas ligand (FasL), which are considered the primary mediators of the inflammatory, apoptotic, and fibrogenic responses that drive the appearance and progression of NASH [110]. It is noteworthy that circulating saturated FFAs activate or amplify signaling through TLR4 in a synergic manner with LPS [170], and that this effect occurs in many pro-inflammatory cells involved in NAFLD pathogenesis (e.g., adipocytes and macrophages) [171]; this points saturated FFAs as pleiotropic inflammatory agents that play a key role in NASH development and progression.
Hepatocellular death in NASH
Apoptosis is a key morphologic and pathogenic feature in patients with NASH [97, 172–174] and a biological event that unequivocally distinguishes NASH from simple fatty liver [175]. Actually, the extent of hepatocyte apoptosis well correlates with the liver injury degree in this disease [97]. Furthermore, the gradual hepatocyte death and altered replication of mature hepatocytes in NASH have been proposed to trigger a compensatory progenitor cell expansion, thus promoting cirrhosis and predisposing to HCC [176, 177].
The strong association between hepatocellular apoptosis and excess lipid accumulation makes this phenomenon a form of “lipoapoptosis”, with FFAs being its main triggering factors [28, 31]. The pathogenic mechanisms accounting for the increased hepatocyte apoptosis in NASH are multiple, and virtually involve all known pathways leading to this kind of cell death, namely the intrinsic (mitochondrial) pathway, the extrinsic pathways, the ER pathway, and the lysosomal pathway [28, 31] (Fig. 7).
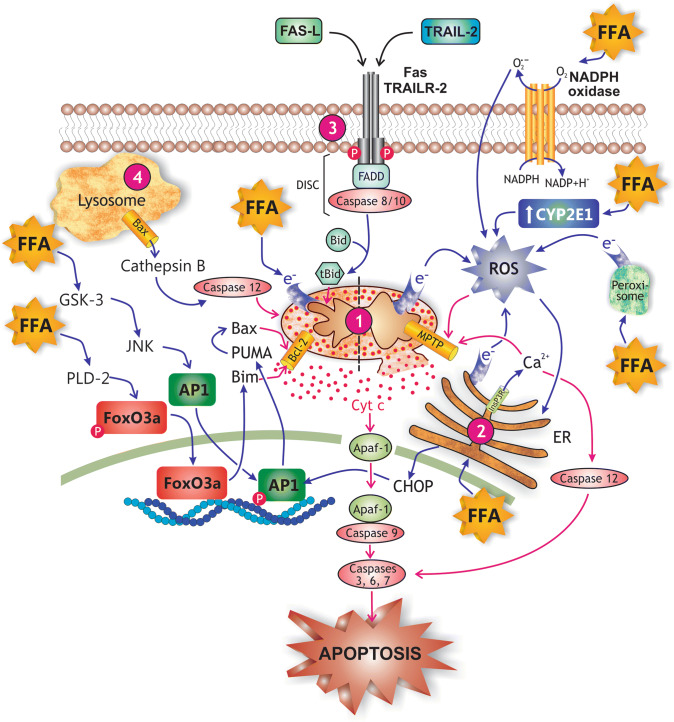
Fig. 7.
Main mechanisms of free fatty acid (FFA)-induced hepatocellular apoptosis in NASH. FFAs promote apoptosis by virtually all known apoptotic pathways. (1) The intrinsic or mitochondrial pathway of apoptosis, which is activated by FFA-induced cellular stress, and mediated by the release of cytochrome c (Cyt c) from the mitochondrial intermembraneous space to cytosol; Cyt c promotes binding of pro-caspase 9 to apoptosis protease-activating factor-1 (APAF-1), pro-caspase 9 activation by autocatalysis, and finally, recruitment and activation by caspase 9 of the executioner caspases 3, 6, and 7. FFAs activate this pro-apoptotic pathway by a activating the glycogen synthase kinase-3 (GSK-3)/JNK and the protein phosphatase 2A (PP2A) pathways, which causes the transcription factors FoxOa3 and AP-1 to translocate to the nucleus and to induce transcription of genes encoding pro-apoptotic proteins of the Bcl-2 family (PUMA, Bim); Bim is a pore-forming protein whereas PUMA activates the pore-forming protein Bax by binding inhibitory members of the Bcl-2 family, which release Bax from this inhibitory constraint (see mitochondrion, left side), and b by promoting the formation of mitochondrial permeability transition pores (MPTP), a process facilitated by reactive oxygen species (ROS) generated from leakage of electrons from the respiratory chain in mitochondria, from oxidases in peroxisomes and endoplasmic reticulum (ER) involved in FFA β- and ω-oxidations, and from induction of pro-oxidizing enzymes, such as NADPH oxidase 4 (NOX4) and CYP2E1 (see mitochondrion, right side); mitochondria permeabilization also exacerbates the leakage of electrons from the mitochondrial electron transport chain and further cytosolic ROS generation. (2) The endoplasmic reticulum (ER) stress pathway of apoptosis, which is induced by both FFAs and ROS, and mediated by a inositol 1,4,5-triphosphate receptor (Ins3PR)-induced Ca2+ release to cytosol and further Ca2+-dependent activation of the executor caspase 12, b Ca2+-dependent MPTP formation in mitochondria, and c ER stress-induced CCAAT-enhancer-binding protein homologous protein (CHOP) expression, which enhances AP-1 transcriptional activation. (3) The extrinsic pathway of apoptosis, which is induced sequentially by a binding of pro-inflammatory cytokines (e.g., Fas-L and TRAIL) to their respective plasma membrane receptors (Fas and TRAILR), b autophosphorylation of these receptors, c association of the activated receptors with FADD and with pro-caspases 8 and 10 to form the death complex DISC, d proteolytic activation of these pro-caspases, and e caspase-dependent cleavage of Bid to truncated Bid (tBid), which promotes Bcl-2-dependent mitochondrial pore formation. (4) The lysosomal pathway of apoptosis, which involves FFA-induced Bax translocation to lysosomes and further cytosolic release of cathepsin B; this protease activates caspases 12, which would interact directly with mitochondria to induce membrane permeabilization
The intrinsic mitochondrial pathway is originated by intracellular stimuli associated with cellular stress, resulting in mitochondrial outer membrane permeabilization and release to cytosol of pro-apoptotic proteins, including cytochrome c, second mitochondria-derived activator of caspases (SMAC)/direct inhibitor of apoptosis protein binding protein with low pI (DIABLO), and apoptosis-inducing factor (AIF). Cytochrome c is associated with apoptotic protease-activating factor 1 (Apaf-1) in cytosol to form the apoptosome; this complex recruits pro-caspase 9 to facilitate its autoactivation, and the further activation of the executioner caspases 3 and 7 by caspase 9-mediated cleavage of their pro-caspase precursors [178]. Smac/DIABLO activates these caspases by antagonizing inhibitors of apoptosis proteins (IAPs) [179], whereas AIF traffics to the nucleus, where it prompts, in a caspase-independent manner, DNA breaks and chromatin condensation, two main features of apoptosis [180]. This mitochondrial apoptotic cascade is initiated by the activation of pro-apoptotic members of the B-cell lymphoma-2 (Bcl-2) protein family, such as the pore-inducing Bcl-2 homology-3 (BH3)-only Bcl-2 proteins (e.g., BH3 interacting-domain death agonist [Bid], BCL-2-interacting mediator of cell death [Bim], Bcl-2 antagonist of cell death [Bad], p53 upregulated modulator of apoptosis [PUMA], and Noxa [for “noxious”]) and multi-domain Bcl-2 proteins (e.g., Bcl-2-associated X protein [Bax] and Bcl-2-associated X killer [Bak]). On the other hand, this apoptotic mechanism is antagonized by the anti-apoptotic members of the same family (e.g., Bcl-2, B-cell lymphoma-extra large [Bcl-xL], and myeloid cell leukemia sequence-1 [Mcl-1]) [181]. Certain long-chain saturated FFAs (e.g., palmitic and stearic acids) enhance the expression of Bim by dephosphorylating and further activating the transcription factor Fox-O3a in a PP2A-dependent manner [182]. These saturated FFAs also activate the pro-apoptotic proteins Bax [183] and PUMA [184], via a cascade of events involving JNK activation, namely: (1) FFA-derived lysophosphatidyl choline activates GSK-3; (2) GSK-3 activates JNK [185]; (3) JNK phosphorylates c-Jun, which is dimerized either with itself or with other proteins (e.g., c-Fos, ATF1, and CREB) to form the AP-1 complex; (4) c-Jun phosphorylation enhances DNA binding and transactivation of AP-1; (5) AP-1 induces PUMA synthesis [186]; (6) PUMA activates Bax by binding inhibitory members of the Bcl-2 family, which releases Bax from this inhibitory constraint [187].
In NASH, there is an exacerbated demand of protein synthesis to cope with the regeneration process secondary to liver injury, and this leads to disruption of ER homeostasis and ER stress, another major mechanism of apoptosis [188, 189]. In addition, saturated FFAs induce directly ER stress [190]. This ER-mediated pro-apoptotic pathway involves sequentially: (1) disruption of ER Ca2+ homeostasis, (2) ER Ca2+ release, (3) Ca2+-dependent activation of calpains, (4) calpain-dependent pro-caspase 12 activation, and (5) caspase 12-induced activation of executioner caspases 3, 6, and 7 [191, 192]. Ca2+ release from the ER is facilitated by Bax and Bak, which can localize also at the ER membrane under ER stress conditions, where they regulate the Ca2+ channel inositol 1,4,5-triphosphate receptor 1 (InsP3R1) [193]. Finally, stressed ER can activate the JNK-mediated signaling pathway, which in turn enhances the expression of the pro-apoptotic transcription factor CCAAT/enhancer-binding protein homologous protein (CHOP) and the dimerization with phosphorylated-c-Jun to render the AP-1 complex; AP-1 enhances PUMA expression, with subsequent Bax activation, mitochondrial membrane permeabilization, caspase activation, and cell death [186].
NASH is an overt pro-inflammatory condition, and the extrinsic apoptotic pathway is activated by pro-inflammatory cytokines released during the inflammatory process (See Section “Liver inflammation in NASH”). This desth pathway is classically triggered by the external activation of plasma membrane cytokine receptors to their cytokine ligands, e.g., TNFR (by TNF-α), Fas (by FasL), and TRAILR (by TRAIL). Activation of these receptors leads to apoptosis through the following sequential events: (1) binding-dependent homo-oligomerization of the receptor, (2) formation of the death-inducing signaling complex (DISC), (3) DISC-induced activation of pro-caspases 8 and 10, and (4) caspase 8- and 10-induced activation of the executioner caspases 3, 6, and 7 [194]; caspases 8 and 10 also cleave Bid, and truncated Bid recruits Bax and Bak to the mitochondria, with the consequent activation of the intrinsic apoptosis pathway [195, 196].
Fas/FasL and TRAILR/TRAIL systems have been both implicated in the pathogenesis of NASH. On the other hand, TNFR/TNF-α system seems not to be critically involved, since its pro-apoptotic role is counterbalanced by its simultaneous capability to activate NF-κB expression, a transcription factor that induces genes involved in survival pathways in the NAFLD context [31]. Fas [97] and TRAILR [197, 198] expressions are increased in livers of patients with NASH via direct FFA-mediated mechanisms; actually, FFA-treated cell lines show an increased sensibility towards FasL- [199] and TRAIL-induced apoptosis [197], in part by induction of the expression of their respective receptors, Fas and TRAILR. This indicates that FFAs are pro-apoptotic per se via the extrinsic apoptotic pathway, irrespective of the additional role for inflammation in this process.
As for the lysosomal pathway of apoptosis, it has recently been suggested that long-chain-saturated FFAs induce lysosomal destabilization and cytosolic release of cathepsin B, a lysosomal cysteine protease [168, 200], via translocation of Bax to lysosomes [201]. Cytosolic release of cathepsin B leads to apoptosis through the intrinsic, mitochondrial pathway [200], presumably by proteolytic activation of pro-caspase 2, whose active form would interact directly with mitochondria to induce membrane permeabilization [202].
OS is a key event in the establishment and progress of NASH (see Section “OS in NASH”) and presumably a major triggering factor of apoptosis under this condition (Fig. 7). Increased mitochondrial permeabilization by the formation of mitochondrial permeability transition pores (MPTPs) is an important mechanism of apoptosis by OS in NAFLD [72]. MPTPs increase the mitochondrial inner membrane permeability to low molecular weight solutes, which induces swelling and further rupture of the outer mitochondrial membrane, with cytosolic release of mitochondrial pro-apoptotic factors, including cytochrome c [203]. MPTP formation may also lead to lytic necrosis, depending on the number of mitochondria affected. Since apoptosis is an energy-demanding process, it occurs when the number of mitochondria affected is low so that ATP levels are preserved [204]. Contrarily, necrosis is a passive process triggered by massive mitochondrial affectation, and leads to cytolysis by impairment of plasma membrane integrity [204].
OS-induced MPTP formation is critically dependent on Ca2+, through a Ca2+/calmodulin/calcium- and calmodulin-dependent protein kinase II (CaMKII)-dependent mechanism [205], which involves the concerted downstream activation of MAPKs of the Erk1/2, p38MAPK, and JNK1/2 types [206]. Dysfunctional mitochondria are, in turn, a source of ROS. This establishes a vicious circle by which ROS induce elevations in mitochondrial Ca2+, which in turn promotes mitochondrial ROS generation. Ca2+ induces ROS in mitochondria by stimulating the delivery of electrons to simultaneously blocked electron transport complexes, which dramatically increase the leakage of electrons from the electron transport chain. Indeed, Ca2+ stimulates the Krebs cycle, the principal source of electrons for the respiratory chain, but simultaneously blocks complex III (via MPTP-mediated release of cytochrome c), and complex IV (by the inhibitory effect of NO, whose levels are increased by the Ca2+-dependent stimulation of NO synthase) [207]. An important source of Ca2+ from outside the mitochondria is the ER, a neighbor organelle that releases Ca2+ under stress conditions [189, 207, 208]. Promotion of mitochondrial dysfunction by ER Ca2+ release has been associated with direct lipotoxicity, as has been shown in palmitic acid-overloaded hepatocytes [209].
Cardiolipin oxidation is another contributing factor of mitochondrial-associated apoptosis in NASH [71, 72]. Oxidized cardiolipin makes mitochondria more vulnerable to Ca2+-induced MPTP generation by interacting with MPTP components, as has been shown in rats with steatohepatitis [210].
Other pro-apoptotic lipids that are overproduced in NAFLD/NASH are ceramides [30]. These compounds are precursors of sphingolipids, and FFAs fuel sphingolipid de novo biosynthesis [30]. Hepatocellular ceramide levels increment in different animal models of diet-induced steatohepatitis, and they both impare mitochondrial FFA β-oxidation by antagonizing electron transport chain complex function [73] and trigger apoptosis via the ER–mitochondria axis [211].
Hepatocellular autophagy in NASH
Autophagy is a lysosomal, self-degradative cell death mechanism that promotes cell survival of the remaining cells by supplying them energy under conditions of cellular stress, and also an adaptive mechanism to cellular damage, by allowing for the engulfment and further removal of damaged organelles and misfolded/aggregate-prone proteins [212]; thus, under autophagy-deficient conditions, cells would more easily succumb to death when challenged with a death stimulus [213]. Autophagy also regulates the breakdown of lipids contained in hepatocellular droplets and, hence, its impairment may result in hepatic steatosis [214, 215]. In addition, autophagy would restrain tumor initiation by blocking cellular injury or by promoting the removal of tumorigenic initiated cells and, hence, its impairment may be a causal factor of HCC development in advanced NASH.
The main two kinds of autophagy whose impairment has been implicated in NAFLD pathogenesis are macroautophagy and chaperone-mediated autophagy. In macroautophagy, cellular organelles, such as mitochondria (‘mitoautophagy’) or lipid droplets (‘lipophagy’), are sequestered in double-membrane structures referred to as ‘autophagosomes’, which fuse to lysosomes resulting in lysosomal degradation [212, 214]. In chaperone-mediated autophagy, soluble proteins containing a specific pentapeptide moiety bind to a chaperone protein for lysosomal translocation, with the catabolic products being released into the cytosol for either reuse or energy supply [216].
In response to fasting, the number of autophagosomes containing lipid cargo increases, thus supporting the role for macroautophagy in the disposal of lipids [217]; the FFAs thus released fuel mitochondrial β-oxidation for energy supply [215]. On the contrary, inhibition of hepatocellular macroautophagy results in reduced lipolytic breakdown and excessive TG accumulation [215, 218]. Although there are currently no reliable methods to ascertain macroautophagy activity in human liver samples, reports exist on decreased macroautophagy in human NAFLD based upon surrogated parameters [219–221]. Experimental evidences also exist that genetically and nutritionally generated obesity impairs autophagy [215, 222, 223]. The inhibition of autophagy in NAFLD seems to be multifactorial, and has been attributed to the downregulation of autophagy genes and decreased levels of lysosomal enzymes [214, 224], as well as altered fusion of autophagic vacuoles with lysosomes [225, 226]. The hyperinsulinemia occurring in the NAFLD context seems to be a contributive causal factor [223]. Finally, the impairment of the signaling pathway initiated by the protein tyrosine phosphatase receptor type O (PTPRO), a regulatory protein that is much decreased in human steatohepatitis [227], has been recently suggested to be at least in part responsible for the impairment of autophagy [227]. This impairment involves (1) downregulation of the protein tyrosine phosphatase receptor type O (PTPRO), a negative regulator of the PI3K signaling, (2) activation of the PI3K/Akt signaling pathway, (3) Akt-dependent stabilization of the MDM4/MDM2 complex, a regulator of the p53 cytoplasmic levels via p53 poly-ubiquitination, and (4) cytoplasmic accumulation of p53, a well-recognized inhibitor of autophagy function [227] (Fig. 8). Another possible target of the PTPRO/PI3K/Akt signaling pathway relevant to autophagy is the mammalian target of rapamycin (mTOR), which inhibits autophagy by blocking the activation of Ulk1 by AMP-activated protein kinase (AMPK) [228]; this latter kinase is an autophagy positive regulator, by acting as a key cell energy sensor of ATP depletion or glucose starvation [229], and its hepatic levels are decreased in rodent fatty liver models [230, 231] (Fig. 8).
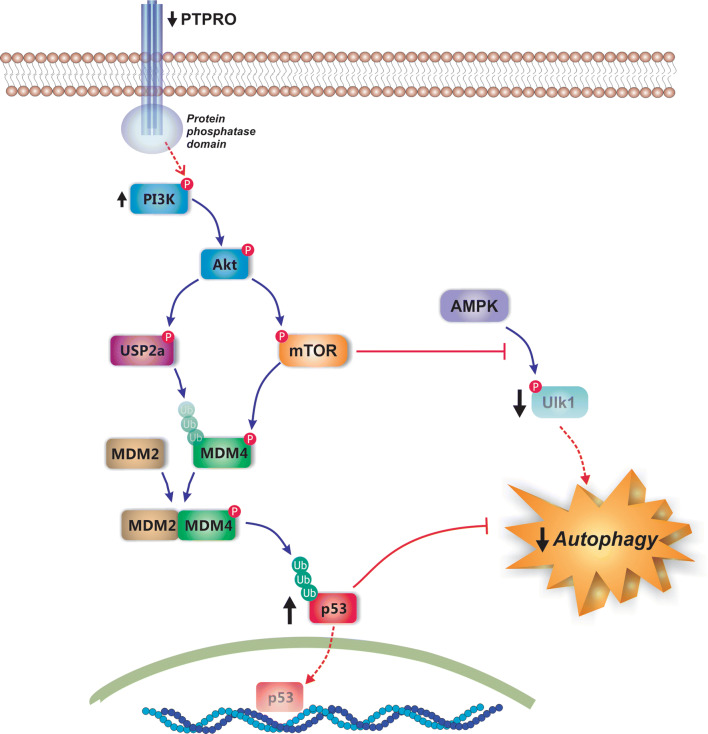
Fig. 8.
Molecular mechanisms of autophagy inhibition in NAFLD. Downregulation of the protein tyrosine phosphatase receptor type O (PTPRO) in NAFLD leads to impairment of signaling pathways involved in autophagy activation. Since PTPRO is involved in inactivation of the phosphoinositide 3-kinase (PI3K)/Akt signaling pathway, its downregulation leads to activation of this pathway and the further Akt-dependent stabilization of MDM4 via both mammalian target of rapamycin (mTOR)-mediated phosphorylation and ubiquitin-specific cysteine protease 2a (USP2a)-mediated deubiquitination. After stabilization, MDM4 forms the MDM4/MDM2 complex, a regulator of the p53 cytoplasmic levels via p53 poly-ubiquitination; this leads to cytoplasmic accumulation of p53, a well-recognized inhibitor of autophagy function. Akt-mediated mTOR activation also inhibits autophagy by blocking the activation of the pro-autophagic kinase unc-51 like autophagy activating kinase 1 (Ulk1) by AMP-activated protein kinase (AMPK)
Impaired TG catabolism and its accumulation in lipid droplets due to impaired macroautophagy can be regarded as a beneficial rather than a detrimental factor, since it prevents cytosolic release of toxic FFAs. However, reduced macroautophagy may contribute to other features of NAFLD of true pathogenic importance, such as IR and ER stress, the latter due to a reduced ability to remove damaged proteins; these two factors are causally intertwined, since the ER stress aggravated by macroautophagy failure promotes IR, and IR further aggravates ER stress [222, 232, 233]. In addition, macroautophagy impairment may aggravate NASH injury by lack of clearance of damaged organelles or proteins that contribute to cellular dysfunction [214]; interestingly, hepatocytes with inhibited macroautophagy are sensitized to OS-induced apoptosis [234], a phenomenon likely due to the failure to remove dysfunctional mitochondria, which are more prone to trigger apoptosis after a pro-oxidant insult [235]. Furthermore, macroautophagy controls activation of immunocompetent cells and release of inflammatory cytokines, so that a failure in this process may contribute to the inflammation underlying NAFLD pathogenesis [236]. Finally, macroautophagy dysfunction may be a causal factor in the progression to HCC due to hepatocellular accumulation of the oncogenic protein insulin-like growth factor 2 (IGF2) mRNA-binding protein-2 (IGF2BP2-2, aka p62), a selective substrate for autophagy; this progression would involve (1) shift of macrophage polarization to the M1, pro-inflammatory phenotype associated with autophagy inhibition as NAFLD severity increases, (2) aggregation of M1-polarized macrophages around hepatocytes containing large lipid droplets, (3) formation of Mallory-Denk bodies containing p62, and (4) p62-mediated survival of stressed HCC-initiating cells [237].
Impairment of chaperone-mediated autophagy may also play a role in NAFLD pathogenesis. Mice with knockout of a gene involved in this autophagic mechanism develop hepatic steatosis together with abnormalities in carbohydrate/lipid metabolism, due to the failure to degrade certain metabolic enzymes [238]. In addition, impairment of chaperone-mediated autophagy may affect the catabolism of perilipin 2 and perilipin 3, two lipid droplet proteins whose degradation is required for lipolysis [239]. Of interest, these two proteins are upregulated in human fatty livers [240], and their experimental genetic ablation in mice alleviates steatosis [241–243], in part by counteracting SREBP activation [243].
HSC activation and fibrogenesis in NASH
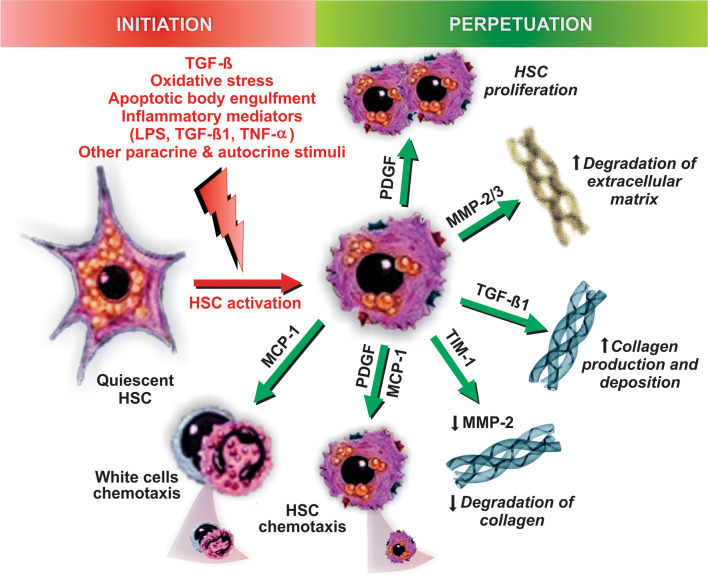
Liver inflammation is strongly associated with chronic hepatic injury and with the resulting hepatic fibrosis, a reversible wound healing process consisting of the rapid synthesis and deposition of extracellular matrix components, mainly collagen fibers, to maintain tissue integrity during repair [174]. Fibrosis has been observed in 37–84% of NASH patients [244, 245], and its is associated with worse prognosis of liver failure [246].
Fibrogenesis requires the concerted action of many different cell types, with HSCs being the main collagen-producing effector cells, and Kupffer cells and hepatocytes being the main regulatory actors. However, paracrine regulation is also provided by other neighboring cell types, including platelets and sinusoidal endothelial cells [247, 248]. Once activated, HSCs themselves are also critical actors in the perpetuation of the pro-fibrotic phenotype, via autocrine mechanisms [248] (Fig. 9).
Fig. 9.
Pathways of initiation and perpetuation of fibrosis mediated by hepatic stellate cell (HSC) activation. Following liver injury, a number of hormonal, paracrine, and autocrine stimuli induce HSC activation, a process initiated by the transition from quiescent, vitamin A-rich HSCs into fibrogenic, proliferative ones; TGF-β produced by Kupffer cells is the main stimulus that drives this phenotypic change. This ‘initiation phase’ is followed by the ‘perpetuation phase’, in which this phenotype is preserved and potentiated by additional stimuli that promote HSC proliferation (via the platelet-derived growth factor [PDGF]), sustained collagen production and deposition (via TGF-β), matrix degradation (via matrix metalloproteinases 1 and 3 [MMP-1/3]), inhibition of collagen degradation (via the MMP-2 inhibitor, tissue inhibitor of metalloproteinase-1 [TIM-1]), HSC chemotaxis (via PDGF and monocyte chemoattractant protein-1 [MCP-1]), and white cell chemoattraction (via MCP-1)
In the normal liver, hepatic HSCs are quiescent, but in response to liver injury, they activate to become the major extracellular matrix-producing cell type [249]. Kupffer cells initiate fibrogenesis by secreting TGF-β. This cytokine promotes both HSC proliferation and maintenance of the myofibroblastic phenotype, as well as collagen synthesis via the TGF-β/SMAD3-signaling pathway [250]. There are a number of pro-fibrotic mediators downstream of TGF-β/SMAD3, most importantly NOX4 [251] and connective tissue growth factor (CTGF) [252]; the latter can be also directly activated by glucose and insulin [253]. Migration and proliferation of activated HSCs, other two essential features required for fibrosis, are also TGF-β-dependent, and stimulated by guanosine triphosphate (Rho GTPase) signaling [254] and platelet-derived growth factor (PDGF) receptor-β expression [255], respectively, among many others autocrine and paracrine stimuli. In addition, ROS derived from both outside and inside the HSCs, the latter secondary to TGF-β-dependent NOX4 activation, can directly activate HSCs. This involves the redox-sensitive Ras/extracellular signal-regulated kinase (Erk) pathway, which stimulates HSC proliferation, migration, and procollagen type I expression [247, 256–259].
Phagocytosis of apoptotic bodies has been shown to have a role in HSC activation and fibrogenesis [260], and in the activation of Kupffer cells as well [261]. This provides a link between apoptosis-associated liver injury and fibrogenesis; actually, apoptosis alone has been considered sufficient to elicit a pro-fibrogenic response in the liver [262]. Similarly to the direct activation of HSCs by ROS, HSC activation by apoptotic body engulfment depends on the Erk-mediated signaling pathway [260]. This is somewhat expected, since HSC apoptotic body phagocytosis induces NOX4, which is known to activate the Erk-mediated signaling pathway to promote the pro-fibrogenic response [263].
Similarly to what happens with the inflammatory response, TLR4-mediated signaling induced by LPS is an important regulator of fibrosis. TLR4 is expressed in HSCs [161], where it triggers an inflammatory-like response, with NF-κB activation and further NF-κB-dependent gene expression [264]. Although this inflammatory signal does not directly promote activation of HSCs, it enhances the pro-fibrogenic response of HSCs to TGF-β [161].
Perpetuation of the fibrosis process involves a number of phenotypic changes in HSCs mediated by the effect of cytokines and chemokines, which favor the remodeling of the extracellular matrix [265, 266] (Fig. 9). This involves: (1) HSC proliferation in response to growth factors, such as PDGF, which transduces mitogenic signals via Ras/Erk and Ras/PI3K signaling pathways [267, 268]; (2) HSC chemotaxis, with PDGF and MCP-1 released by both Kupffer cells and hypertrophic adipocytes being the most important chemoattractants of activated HSCs [268, 269]; (3) extracellular matrix degradation, as part of the initial remodeling where the subendothelial extracellular matrix is degraded by the Zn2+-dependent matrix metalloproteinase (MMP)-2 and -3 released early by activated HSCs, and replaced by collagen fibers [270]; (4) collagen fiber preservation, via upregulation of tissue inhibitor of metalloproteinase-1 (TIMP-1), an MMP inhibitor released later by activated HSCs to stabilize the fibrotic tissue [270]; (5) leukocyte chemoattraction, via the release of neutrophil and monocyte chemoattractants, mainly MCP-1 [248]; (6) release of cytokines (e.g., TGF-β1, PDGF, hepatocyte growth factor, fibroblast growth factor, and endothelin-1), which amplify inflammation, thus perpetuating HSC activation via an autocrine mechanism [248].
There is compelling evidence for a major role for a dysregulation of the hedgehog (Hh) signaling pathway in the NAFLD progression to NASH, fibrosis, cirrhosis, and hepatocarcinogenesis [271, 272]. Actually, the activity of the Hh pathway correlates with portal inflammation and severity of fibrosis in NAFLD [273], and Hh biomarkers are expressed in HCC tumors [274, 275]. Normally, hepatocytes do not produce Hh ligands and, hence, this signaling pathway is virtually inactive in normal liver, and even in the ‘pure’ steatotic one [276]. On the other hand, this signaling pathway is activated in NASH-related liver injury, and is a key orchestrator of the wound healing response resulting from hepatocyte lost [271, 272]. After the lipotoxic insult, hepatocytes upregulate Hh ligand expression and are a main source of these ligands when they die [277]. This prompts accumulation of myofibroblasts, immune cells, activated sinusoidal endothelial cells, and resident liver progenitor/stem cells, which are necessary for optimal liver regeneration and are also a source of Hh ligands [271, 272]. Although this process is aimed to repair the damaged liver tissue, its overstimulation in NASH halts the process at the initial, fibrogenic stage, thus causing progressive fibrogenesis without hepatocyte replenishment. This failure involves both lipotoxicity-induced shift from proliferation to Fas-dependent apoptosis of mature hepatocytes [278] and increased proliferation but impaired hepatocytic differentiation of liver progenitor cells [276, 279, 280]. This represents actually a pro-neoplastic condition, which is reinforced by Hh-signaling-induced triggering of precursor cancerous cell proliferation through induction of pro-mitotic proteins [281], inhibition of apoptosis [282], and promotion of epithelial to mesenchymal transition in HCC cells, thus favoring migration and invasiveness [283].
Conclusions and perspectives
Given the robust association of NAFLD with metabolic syndrome and the worldwide epidemic of obesity, the prevalence of NAFLD is increasing at an alarming rate, making this hepatopathy the most frequent liver disease nowadays.
NAFLD comprises a heterogeneous group of hepatic disorders that share common features, including hepatic fat infiltration (from a histological point of view) and IR (from a pathogenic point of view). NAFLD pathogenesis is particularly complex, since it involves the interplay between genetics and environmental factors, many of them having been elucidated recently. There is general agreement that NAFLD progression occurs when adaptive mechanisms aimed to counteract hepatic FFA-induced lipotoxicity are overwhelmed, leading to OS, ER stress, mitochondrial damage, and hepatocellular dysfunction. Eventually, the resulting cellular injury triggers immune-mediated hepatocellular damage and apoptotic/necrotic cell death mechanisms, leading to HSC activation and fibrogenesis, further development of cirrhosis and, eventually, HCC.
The extensive information that has accumulated in the past few years on the molecular mechanisms underlying NAFLD pathogenesis has led to the identification of a number of promising targets for therapeutics. Consequently, this has been accompanied by several clinical trials exploring novel therapeutic alternatives, mostly derived from preclinical studies.
Despite these clear advances, there are nowadays no licensed therapies for this highly prevalent condition. Robust biomarkers to predict evolution or to select patients for treatment and to monitor therapeutic response are also lacking; their development and validation are eagerly awaited, since liver biopsy is generally not recommended. Therefore, further studies are clearly needed to identify patients at higher risk of progression by genetic/epigenetic typification together with omics technology, thus allowing a stratified approach for management of NAFLD patients, and for identification of those who would benefit most from current and future therapies. We hope this review helps to evoke pivotal questions about NAFLD pathogenesis to be explored in cellular, animal, and human models of this liver disease, so that to eventually use this new knowledge to envisage new therapeutic targets, as well as to establish new diagnostic and predictive indicators of this worldwide epidemic disease.
Abbreviations
- ACC2
Acetyl-CoA carboxylase 2
- AhR
Aryl hydrocarbon receptor
- AIF
Apoptosis-inducing factor
- AMPK
AMP-activated protein kinase
- AP-1
Adaptor protein-1
- Apaf-1
Apoptotic protease-activating factor 1
- Apo
Apolipoprotein
- ASC
Apoptotic speck-like protein containing caspase-1 activation and recruitment domain
- ATG13
Autophagy-related protein 13
- Bad
Bcl-2 antagonist of cell death
- Bak
Bcl-2-associated X killer
- Bax
Bcl-2-associated X protein
- Bcl-2
B cell lymphoma-2
- Bcl-xL
B-cell lymphoma-extra large
- BH3
Bcl-2 homology-3
- Bim
BCL-2-interacting mediator of cell death
- CaMKII
Calcium- and calmodulin-dependent protein kinase II
- CAT
Carnitine acetyl transferase
- CHOP
CCAAT/enhancer-binding protein homologous protein
- ChREBP
Carbohydrate response element binding protein
- CoA
Coenzyme A
- CPT1
Carnitine palmitoyltransferase 1
- CTGF
Connective tissue growth factor
- CYP2E1
Cytochrome P450 family 2 subfamily E member 1
- DAMP
Danger-associated molecular pattern
- DIABLO
Direct inhibitor of apoptosis protein binding protein with low pi
- DISC
Death-inducing signaling complex
- ER
Endoplasmic reticulum
- Erk
Ras/extracellular signal-regulated kinase
- FasL
Fas ligand
- FIP200
FAK family-interacting protein of 200 kDa
- FoxO1
Forkhead box protein O1
- FP-1
Ferroportin-1
- FFA
Free fatty acid
- G6P
Glucose-6 phosphatase
- GK
Glycogen kinase
- GSK
Glycogen synthase kinase
- HCC
Hepatocellular carcinoma
- Hh
Hedgehog
- HJV
Hemojuvelin
- HSC
Hepatic stellate cell
- IAP
Inhibitors of apoptosis protein
- IGF2BP2-2
Insulin-like growth factor 2 (IGF2) mRNA-binding protein-2
- I-κB
Inhibitor of κB
- IKK
IκB kinase
- IL
Interleukin
- InsP3R1
Inositol 1,4,5-triphosphate receptor 1
- IR
Insulin resistance
- IRS1/2
Insulin receptor substrates 1/2
- JAK
Janus activated kinase
- JNK
c-Jun N-terminal kinase
- LPS
Lipopolysaccharide
- LXR
Liver X receptor
- L-PK
Liver-type pyruvate kinase
- Mcl-1
Myeloid cell leukemia sequence-1
- MCP-1
Monocyte chemoattractant protein 1
- MEK1
Mitogen activated protein kinase 1
- MMP
Matrix metalloproteinase
- MPTP
Mitochondrial permeability transition pore
- mTOR
Mammalian target of rapamycin
- MyD88
Myeloid differentiation primary response 88
- NAFLD
Nonalcoholic fatty liver disease
- NALP3
NACHT, LRR and PYD domains-containing protein 3
- NASH
Nonalcoholic steatohepatitis
- NLR
NOD-like receptor
- NOX4
NADPH oxidase 4
- NF-κB
Nuclear factor-κB
- PCK1
Phosphoenolpyruvate carboxykinase
- OS
Oxidative stress
- PUMA
p53 upregulated modulator of apoptosis
- PDGF
Platelet-derived growth factor
- PEMT
Phosphatidylethanolamine N-methyltransferase
- PI3K
Phosphoinositide 3-kinase
- PKCθ
Protein kinase C-θ
- PP2A
Protein phosphatase 2A
- PPAR-α
Peroxisome proliferator-activated receptor-α
- PTPRO
Protein tyrosine phosphatase receptor type O
- ROS
Radical oxygen species
- SAMe
S-adenosylmethionine
- SCAP
SREBP-cleavage activating protein
- SMAC
Second mitochondria-derived activator of caspases
- SOCS
Suppressor of cytokine signaling
- SREBP-1c
Sterol regulatory binding protein-1c
- STAT3
Signal transducer and activator of transcription 3
- TAK1
TGF-β activated kinase-1
- TG
Triglyceride
- TGF-β
Transforming growth factor-β
- TIMP-1
Tissue inhibitor of metalloproteinase-1
- TLR4
Toll-like receptor 4
- TNF-α
Tumor necrosis factor-α
- Ulk1/2
Unc-51 like autophagy activating kinase 1/2
- VLDL
Very low density lipoprotein
Footnotes
Fernando Bessone and María Valeria Razori have contributed equally to this work.
References
- 1.Angulo P. Nonalcoholic fatty liver disease. N Engl J Med. 2002;346:1221–1231. doi: 10.1056/NEJMra011775. [DOI] [PubMed] [Google Scholar]
- 2.Tolman KG, Dalpiaz AS. Treatment of non-alcoholic fatty liver disease. Ther Clin Risk Manag. 2007;3:1153–1163. doi: 10.1136/pgmj.2005.042200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Vernon G, Baranova A, Younossi ZM. Systematic review: the epidemiology and natural history of non-alcoholic fatty liver disease and non-alcoholic steatohepatitis in adults. Aliment Pharmacol Ther. 2011;34:274–285. doi: 10.1111/j.1365-2036.2011.04724.x. [DOI] [PubMed] [Google Scholar]
- 4.Younossi ZM, Stepanova M, Afendy M, Fang Y, Younossi Y, Mir H, Srishord M. Changes in the prevalence of the most common causes of chronic liver diseases in the United States from 1988 to 2008. Clin Gastroenterol Hepatol. 2011;9:524–530. doi: 10.1016/j.cgh.2011.03.020. [DOI] [PubMed] [Google Scholar]
- 5.Calzadilla Berlot L, Adams LA. The natural course of non-alcoholic fatty liver disease. Int J Mol Sci. 2016;17:E774. doi: 10.3390/ijms17050774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Musso G, Gambino R, De MF, Cassader M, Rizzetto M, Durazzo M, Faga E, Silli B, Pagano G. Dietary habits and their relations to insulin resistance and postprandial lipemia in nonalcoholic steatohepatitis. Hepatology. 2003;37:909–916. doi: 10.1053/jhep.2003.50132. [DOI] [PubMed] [Google Scholar]
- 7.Kim D, Kim WR. Nonobese fatty liver disease. Clin Gastroenterol Hepatol. 2017;15:474–485. doi: 10.1016/j.cgh.2016.08.028. [DOI] [PubMed] [Google Scholar]
- 8.Schwimmer JB, Celedon MA, Lavine JE, Salem R, Campbell N, Schork NJ, Shiehmorteza M, Yokoo T, Chavez A, Middleton MS, Sirlin CB. Heritability of nonalcoholic fatty liver disease. Gastroenterology. 2009;136:1585–1592. doi: 10.1053/j.gastro.2009.01.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Guerrero R, Vega GL, Grundy SM, Browning JD. Ethnic differences in hepatic steatosis: an insulin resistance paradox? Hepatology. 2009;49:791–801. doi: 10.1002/hep.22726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Manolio TA, Collins FS, Cox NJ, Goldstein DB, Hindorff LA, Hunter DJ, McCarthy MI, Ramos EM, Cardon LR, Chakravarti A, Cho JH, Guttmacher AE, Kong A, Kruglyak L, Mardis E, Rotimi CN, Slatkin M, Valle D, Whittemore AS, Boehnke M, Clark AG, Eichler EE, Gibson G, Haines JL, Mackay TF, McCarroll SA, Visscher PM. Finding the missing heritability of complex diseases. Nature. 2009;461:747–753. doi: 10.1038/nature08494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Williams CD, Stengel J, Asike MI, Torres DM, Shaw J, Contreras M, Landt CL, Harrison SA. Prevalence of nonalcoholic fatty liver disease and nonalcoholic steatohepatitis among a largely middle-aged population utilizing ultrasound and liver biopsy: a prospective study. Gastroenterology. 2011;140:124–131. doi: 10.1053/j.gastro.2010.09.038. [DOI] [PubMed] [Google Scholar]
- 12.Wagenknecht LE, Scherzinger AL, Stamm ER, Hanley AJ, Norris JM, Chen YD, Bryer-Ash M, Haffner SM, Rotter JI. Correlates and heritability of nonalcoholic fatty liver disease in a minority cohort. Obesity (Silver Spring) 2009;17:1240–1246. doi: 10.1038/oby.2009.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dongiovanni P, Anstee QM, Valenti L. Genetic predisposition in NAFLD and NASH: impact on severity of liver disease and response to treatment. Curr Pharm Des. 2013;19:5219–5238. doi: 10.2174/13816128113199990381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sookoian S, Pirola CJ. Genetic predisposition in nonalcoholic fatty liver disease. Clin Mol Hepatol. 2017;23:1–12. doi: 10.3350/cmh.2016.0109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Makkonen J, Pietilainen KH, Rissanen A, Kaprio J, Yki-Jarvinen H. Genetic factors contribute to variation in serum alanine aminotransferase activity independent of obesity and alcohol: a study in monozygotic and dizygotic twins. J Hepatol. 2009;50:1035–1042. doi: 10.1016/j.jhep.2008.12.025. [DOI] [PubMed] [Google Scholar]
- 16.Severson TJ, Besur S, Bonkovsky HL. Genetic factors that affect nonalcoholic fatty liver disease: a systematic clinical review. World J Gastroenterol. 2016;22:6742–6756. doi: 10.3748/wjg.v22.i29.6742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Macaluso FS, Maida M, Petta S. Genetic background in nonalcoholic fatty liver disease: a comprehensive review. World J Gastroenterol. 2015;21:11088–11111. doi: 10.3748/wjg.v21.i39.11088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hardy T, Oakley F, Anstee QM, Day CP. Nonalcoholic fatty liver disease: pathogenesis and disease spectrum. Annu Rev Pathol. 2016;11:451–496. doi: 10.1146/annurev-pathol-012615-044224. [DOI] [PubMed] [Google Scholar]
- 19.Levene AP, Goldin RD. The epidemiology, pathogenesis and histopathology of fatty liver disease. Histopathology. 2012;61:141–152. doi: 10.1111/j.1365-2559.2011.04145.x. [DOI] [PubMed] [Google Scholar]
- 20.Piscaglia F, Svegliati-Baroni G, Barchetti A, Pecorelli A, Marinelli S, Tiribelli C, Bellentani S. Clinical patterns of hepatocellular carcinoma in nonalcoholic fatty liver disease: a multicenter prospective study. Hepatology. 2016;63:827–838. doi: 10.1002/hep.28368. [DOI] [PubMed] [Google Scholar]
- 21.Day CP, James OF. Steatohepatitis: a tale of two “hits”? Gastroenterology. 1998;114:842–845. doi: 10.1016/S0016-5085(98)70599-2. [DOI] [PubMed] [Google Scholar]
- 22.Brunt EM. Grading and staging the histopathological lesions of chronic hepatitis: the Knodell histology activity index and beyond. Hepatology. 2000;31:241–246. doi: 10.1002/hep.510310136. [DOI] [PubMed] [Google Scholar]
- 23.Tilg H, Moschen AR. Evolution of inflammation in nonalcoholic fatty liver disease: the multiple parallel hits hypothesis. Hepatology. 2010;52:1836–1846. doi: 10.1002/hep.24001. [DOI] [PubMed] [Google Scholar]
- 24.Eguchi Y, Eguchi T, Mizuta T, Ide Y, Yasutake T, Iwakiri R, Hisatomi A, Ozaki I, Yamamoto K, Kitajima Y, Kawaguchi Y, Kuroki S, Ono N. Visceral fat accumulation and insulin resistance are important factors in nonalcoholic fatty liver disease. J Gastroenterol. 2006;41:462–469. doi: 10.1007/s00535-006-1790-5. [DOI] [PubMed] [Google Scholar]
- 25.Yilmaz Y. Review article: is non-alcoholic fatty liver disease a spectrum, or are steatosis and non-alcoholic steatohepatitis distinct conditions? Aliment Pharmacol Ther. 2012;36:815–823. doi: 10.1111/apt.12046. [DOI] [PubMed] [Google Scholar]
- 26.Tiniakos DG, Vos MB, Brunt EM. Nonalcoholic fatty liver disease: pathology and pathogenesis. Annu Rev Pathol. 2010;5:145–171. doi: 10.1146/annurev-pathol-121808-102132. [DOI] [PubMed] [Google Scholar]
- 27.Kasumov T, Li L, Li M, Gulshan K, Kirwan JP, Liu X, Previs S, Willard B, Smith JD, McCullough A. Ceramide as a mediator of non-alcoholic Fatty liver disease and associated atherosclerosis. PLoS One. 2015;10:e0126910. doi: 10.1371/journal.pone.0126910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mota M, Banini BA, Cazanave SC, Sanyal AJ. Molecular mechanisms of lipotoxicity and glucotoxicity in nonalcoholic fatty liver disease. Metabolism. 2016;65:1049–1061. doi: 10.1016/j.metabol.2016.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hirsova P, Ibrahim SH, Gores GJ, Malhi H. Lipotoxic lethal and sublethal stress signaling in hepatocytes: relevance to NASH pathogenesis. J Lipid Res. 2016;57:1758–1770. doi: 10.1194/jlr.R066357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chaurasia B, Summers SA. Ceramides—lipotoxic inducers of metabolic disorders. Trends Endocrinol Metab. 2015;26:538–550. doi: 10.1016/j.tem.2015.07.006. [DOI] [PubMed] [Google Scholar]
- 31.Malhi H, Gores GJ. Molecular mechanisms of lipotoxicity in nonalcoholic fatty liver disease. Semin Liver Dis. 2008;28:360–369. doi: 10.1055/s-0028-1091980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cortez-Pinto H, de Moura MC, Day CP. Non-alcoholic steatohepatitis: from cell biology to clinical practice. J Hepatol. 2006;44:197–208. doi: 10.1016/j.jhep.2005.09.002. [DOI] [PubMed] [Google Scholar]
- 33.Yamaguchi K, Yang L, McCall S, Huang J, Yu XX, Pandey SK, Bhanot S, Monia BP, Li YX, Diehl AM. Inhibiting triglyceride synthesis improves hepatic steatosis but exacerbates liver damage and fibrosis in obese mice with nonalcoholic steatohepatitis. Hepatology. 2007;45:1366–1374. doi: 10.1002/hep.21655. [DOI] [PubMed] [Google Scholar]
- 34.Donnelly KL, Smith CI, Schwarzenberg SJ, Jessurun J, Boldt MD, Parks EJ. Sources of fatty acids stored in liver and secreted via lipoproteins in patients with nonalcoholic fatty liver disease. J Clin Invest. 2005;115:1343–1351. doi: 10.1172/JCI23621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kwanten WJ, Martinet W, Michielsen PP, Francque SM. Role of autophagy in the pathophysiology of nonalcoholic fatty liver disease: a controversial issue. World J Gastroenterol. 2014;20:7325–7338. doi: 10.3748/wjg.v20.i23.7325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tamura S, Shimomura I. Contribution of adipose tissue and de novo lipogenesis to nonalcoholic fatty liver disease. J Clin Invest. 2005;115:1139–1142. doi: 10.1172/JCI24930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Marchesini G, Brizi M, Morselli-Labate AM, Bianchi G, Bugianesi E, McCullough AJ, Forlani G, Melchionda N. Association of nonalcoholic fatty liver disease with insulin resistance. Am J Med. 1999;107:450–455. doi: 10.1016/S0002-9343(99)00271-5. [DOI] [PubMed] [Google Scholar]
- 38.Al-Goblan AS, Al-Alfi MA, Khan MZ. Mechanism linking diabetes mellitus and obesity. Diabetes Metab Syndr Obes. 2014;7:587–591. doi: 10.2147/DMSO.S67400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pliquett RU, Fuhrer D, Falk S, Zysset S, von Cramon DY, Stumvoll M. The effects of insulin on the central nervous system–focus on appetite regulation. Horm Metab Res. 2006;38:442–446. doi: 10.1055/s-2006-947840. [DOI] [PubMed] [Google Scholar]
- 40.Ye J. Mechanisms of insulin resistance in obesity. Front Med. 2013;7:14–24. doi: 10.1007/s11684-013-0262-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Baranova A, Gowder SJ, Schlauch K, Elariny H, Collantes R, Afendy A, Ong JP, Goodman Z, Chandhoke V, Younossi ZM. Gene expression of leptin, resistin, and adiponectin in the white adipose tissue of obese patients with non-alcoholic fatty liver disease and insulin resistance. Obes Surg. 2006;16:1118–1125. doi: 10.1381/096089206778392149. [DOI] [PubMed] [Google Scholar]
- 42.Hotamisligil GS, Arner P, Caro JF, Atkinson RL, Spiegelman BM. Increased adipose tissue expression of tumor necrosis factor-alpha in human obesity and insulin resistance. J Clin Invest. 1995;95:2409–2415. doi: 10.1172/JCI117936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Siddle K, Hales CN. Hormonal control of adipose tissue lipolysis. Proc Nutr Soc. 1975;34:233–239. doi: 10.1079/PNS19750044. [DOI] [PubMed] [Google Scholar]
- 44.Shulman GI. Cellular mechanisms of insulin resistance. J Clin Invest. 2000;106:171–176. doi: 10.1172/JCI10583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Guilherme A, Virbasius JV, Puri V, Czech MP. Adipocyte dysfunctions linking obesity to insulin resistance and type 2 diabetes. Nat Rev Mol Cell Biol. 2008;9:367–377. doi: 10.1038/nrm2391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yki-Jarvinen H. Fat in the liver and insulin resistance. Ann Med. 2005;37:347–356. doi: 10.1080/07853890510037383. [DOI] [PubMed] [Google Scholar]
- 47.Lonardo A, Lombardini S, Ricchi M, Scaglioni F, Loria P. Review article: hepatic steatosis and insulin resistance. Aliment Pharmacol Ther. 2005;22:64–70. doi: 10.1111/j.1365-2036.2005.02600.x. [DOI] [PubMed] [Google Scholar]
- 48.Xu X, So JS, Park JG, Lee AH. Transcriptional control of hepatic lipid metabolism by SREBP and ChREBP. Semin Liver Dis. 2013;33:301–311. doi: 10.1055/s-0033-1358523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Horton JD, Goldstein JL, Brown MS. SREBPs: activators of the complete program of cholesterol and fatty acid synthesis in the liver. J Clin Invest. 2002;109:1125–1131. doi: 10.1172/JCI0215593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Uyeda K, Yamashita H, Kawaguchi T. Carbohydrate responsive element-binding protein (ChREBP): a key regulator of glucose metabolism and fat storage. Biochem Pharmacol. 2002;63:2075–2080. doi: 10.1016/S0006-2952(02)01012-2. [DOI] [PubMed] [Google Scholar]
- 51.Yamashita H, Takenoshita M, Sakurai M, Bruick RK, Henzel WJ, Shillinglaw W, Arnot D, Uyeda K. A glucose-responsive transcription factor that regulates carbohydrate metabolism in the liver. Proc Natl Acad Sci USA. 2001;98:9116–9121. doi: 10.1073/pnas.161284298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ishii S, Iizuka K, Miller BC, Uyeda K. Carbohydrate response element binding protein directly promotes lipogenic enzyme gene transcription. Proc Natl Acad Sci USA. 2004;101:15597–15602. doi: 10.1073/pnas.0405238101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Enjoji M, Yasutake K, Kohjima M, Nakamuta M. Nutrition and nonalcoholic fatty liver disease: the significance of cholesterol. Int J Hepatol. 2012;2012:925807. doi: 10.1155/2012/925807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zhang X, Han J, Man K, Li X, Du J, Chu ES, Go MY, Sung JJ, Yu J. CXC chemokine receptor 3 promotes steatohepatitis in mice through mediating inflammatory cytokines, macrophages and autophagy. J Hepatol. 2016;64:160–170. doi: 10.1016/j.jhep.2015.09.005. [DOI] [PubMed] [Google Scholar]
- 55.Jump DB. Dietary polyunsaturated fatty acids and regulation of gene transcription. Curr Opin Lipidol. 2002;13:155–164. doi: 10.1097/00041433-200204000-00007. [DOI] [PubMed] [Google Scholar]
- 56.Fisher FM, Kim M, Doridot L, Cunniff JC, Parker TS, Levine DM, Hellerstein MK, Hudgins LC, Maratos-Flier E, Herman MA. A critical role for ChREBP-mediated FGF21 secretion in hepatic fructose metabolism. Mol Metab. 2017;6:14–21. doi: 10.1016/j.molmet.2016.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Jegatheesan P, De Bandt JP. Fructose and NAFLD: the multifaceted aspects of fructose metabolism. Nutrients. 2017;9:E230. doi: 10.3390/nu9030230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Mastrocola R, Collino M, Rogazzo M, Medana C, Nigro D, Boccuzzi G, Aragno M. Advanced glycation end products promote hepatosteatosis by interfering with SCAP-SREBP pathway in fructose-drinking mice. Am J Physiol Gastrointest Liver Physiol. 2013;305:G398–G407. doi: 10.1152/ajpgi.00450.2012. [DOI] [PubMed] [Google Scholar]
- 59.Geidl-Flueck B, Gerber PA. Insights into the hexose liver metabolism-glucose versus fructose. Nutrients. 2017;9:E1026. doi: 10.3390/nu9091026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Charlton M, Sreekumar R, Rasmussen D, Lindor K, Nair KS. Apolipoprotein synthesis in nonalcoholic steatohepatitis. Hepatology. 2002;35:898–904. doi: 10.1053/jhep.2002.32527. [DOI] [PubMed] [Google Scholar]
- 61.Sookoian S, Puri P, Castano GO, Scian R, Mirshahi F, Sanyal AJ, Pirola CJ. Nonalcoholic steatohepatitis is associated with a state of betaine-insufficiency. Liver Int. 2017;37:611–619. doi: 10.1111/liv.13249. [DOI] [PubMed] [Google Scholar]
- 62.Noureddin M, Mato JM, Lu SC. Nonalcoholic fatty liver disease: update on pathogenesis, diagnosis, treatment and the role of S-adenosylmethionine. Exp Biol Med (Maywood) 2015;240:809–820. doi: 10.1177/1535370215579161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Fast DG, Vance DE. Nascent VLDL phospholipid composition is altered when phosphatidylcholine biosynthesis is inhibited: evidence for a novel mechanism that regulates VLDL secretion. Biochim Biophys Acta. 1995;1258:159–168. doi: 10.1016/0005-2760(95)00116-T. [DOI] [PubMed] [Google Scholar]
- 64.Noga AA, Zhao Y, Vance DE. An unexpected requirement for phosphatidylethanolamine N-methyltransferase in the secretion of very low density lipoproteins. J Biol Chem. 2002;277:42358–42365. doi: 10.1074/jbc.M204542200. [DOI] [PubMed] [Google Scholar]
- 65.Obeid R. The metabolic burden of methyl donor deficiency with focus on the betaine homocysteine methyltransferase pathway. Nutrients. 2013;5:3481–3495. doi: 10.3390/nu5093481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Fasano T, Pisciotta L, Bocchi L, Guardamagna O, Assandro P, Rabacchi C, Zanoni P, Filocamo M, Bertolini S, Calandra S. Lysosomal lipase deficiency: molecular characterization of eleven patients with Wolman or cholesteryl ester storage disease. Mol Genet Metab. 2012;105:450–456. doi: 10.1016/j.ymgme.2011.12.008. [DOI] [PubMed] [Google Scholar]
- 67.Baratta F, Pastori D, Del BM, Polimeni L, Labbadia G, Di SS, Piemonte F, Tozzi G, Violi F, Angelico F. Reduced Lysosomal acid lipase activity in adult patients with non-alcoholic fatty liver disease. EBioMedicine. 2015;2:750–754. doi: 10.1016/j.ebiom.2015.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Dubland JA, Francis GA. Lysosomal acid lipase: at the crossroads of normal and atherogenic cholesterol metabolism. Front Cell Dev Biol. 2015;3:3. doi: 10.3389/fcell.2015.00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Zechner R, Madeo F, Kratky D. Cytosolic lipolysis and lipophagy: two sides of the same coin. Nat Rev Mol Cell Biol. 2017;18:671–684. doi: 10.1038/nrm.2017.76. [DOI] [PubMed] [Google Scholar]
- 70.Fon Tacer K, Rozman D. Nonalcoholic Fatty liver disease: focus on lipoprotein and lipid deregulation. J Lipids. 2011;2011:783976. doi: 10.1155/2011/783976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Petrosillo G, Portincasa P, Grattagliano I, Casanova G, Matera M, Ruggiero FM, Ferri D, Paradies G. Mitochondrial dysfunction in rat with nonalcoholic fatty liver Involvement of complex I, reactive oxygen species and cardiolipin. Biochim Biophys Acta. 2007;1767:1260–1267. doi: 10.1016/j.bbabio.2007.07.011. [DOI] [PubMed] [Google Scholar]
- 72.Paradies G, Paradies V, Ruggiero FM, Petrosillo G. Oxidative stress, cardiolipin and mitochondrial dysfunction in nonalcoholic fatty liver disease. World J Gastroenterol. 2014;20:14205–14218. doi: 10.3748/wjg.v20.i39.14205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Raichur S, Wang ST, Chan PW, Li Y, Ching J, Chaurasia B, Dogra S, Ohman MK, Takeda K, Sugii S, Pewzner-Jung Y, Futerman AH, Summers SA. CerS2 haploinsufficiency inhibits beta-oxidation and confers susceptibility to diet-induced steatohepatitis and insulin resistance. Cell Metab. 2014;20:687–695. doi: 10.1016/j.cmet.2014.10.007. [DOI] [PubMed] [Google Scholar]
- 74.Fromenty B, Robin MA, Igoudjil A, Mansouri A, Pessayre D. The ins and outs of mitochondrial dysfunction in NASH. Diabetes Metab. 2004;30:121–138. doi: 10.1016/S1262-3636(07)70098-8. [DOI] [PubMed] [Google Scholar]
- 75.Sanyal AJ, Campbell-Sargent C, Mirshahi F, Rizzo WB, Contos MJ, Sterling RK, Luketic VA, Shiffman ML, Clore JN. Nonalcoholic steatohepatitis: association of insulin resistance and mitochondrial abnormalities. Gastroenterology. 2001;120:1183–1192. doi: 10.1053/gast.2001.23256. [DOI] [PubMed] [Google Scholar]
- 76.Begriche K, Igoudjil A, Pessayre D, Fromenty B. Mitochondrial dysfunction in NASH: causes, consequences and possible means to prevent it. Mitochondrion. 2006;6:1–28. doi: 10.1016/j.mito.2005.10.004. [DOI] [PubMed] [Google Scholar]
- 77.Kerner J, Hoppel C. Fatty acid import into mitochondria. Biochim Biophys Acta. 2000;1486:1–17. doi: 10.1016/S1388-1981(00)00044-5. [DOI] [PubMed] [Google Scholar]
- 78.Kohjima M, Enjoji M, Higuchi N, Kato M, Kotoh K, Yoshimoto T, Fujino T, Yada M, Yada R, Harada N, Takayanagi R, Nakamuta M. Re-evaluation of fatty acid metabolism-related gene expression in nonalcoholic fatty liver disease. Int J Mol Med. 2007;20:351–358. doi: 10.3892/ijmm.20.3.351. [DOI] [PubMed] [Google Scholar]
- 79.Angelico F, Del BM, Conti R, Francioso S, Feole K, Fiorello S, Cavallo MG, Zalunardo B, Lirussi F, Alessandri C, Violi F. Insulin resistance, the metabolic syndrome, and nonalcoholic fatty liver disease. J Clin Endocrinol Metab. 2005;90:1578–1582. doi: 10.1210/jc.2004-1024. [DOI] [PubMed] [Google Scholar]
- 80.Larter CZ, Farrell GC. Insulin resistance, adiponectin, cytokines in NASH: which is the best target to treat? J Hepatol. 2006;44:253–261. doi: 10.1016/j.jhep.2005.11.030. [DOI] [PubMed] [Google Scholar]
- 81.Johnston AM, Pirola L, Van OE. Molecular mechanisms of insulin receptor substrate protein-mediated modulation of insulin signalling. FEBS Lett. 2003;546:32–36. doi: 10.1016/S0014-5793(03)00438-1. [DOI] [PubMed] [Google Scholar]
- 82.Galbo T, Perry RJ, Jurczak MJ, Camporez JP, Alves TC, Kahn M, Guigni BA, Serr J, Zhang D, Bhanot S, Samuel VT, Shulman GI. Saturated and unsaturated fat induce hepatic insulin resistance independently of TLR-4 signaling and ceramide synthesis in vivo. Proc Natl Acad Sci USA. 2013;110:12780–12785. doi: 10.1073/pnas.1311176110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Holland WL, Bikman BT, Wang LP, Yuguang G, Sargent KM, Bulchand S, Knotts TA, Shui G, Clegg DJ, Wenk MR, Pagliassotti MJ, Scherer PE, Summers SA. Lipid-induced insulin resistance mediated by the proinflammatory receptor TLR4 requires saturated fatty acid-induced ceramide biosynthesis in mice. J Clin Invest. 2011;121:1858–1870. doi: 10.1172/JCI43378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Ueki K, Kadowaki T, Kahn CR. Role of suppressors of cytokine signaling SOCS-1 and SOCS-3 in hepatic steatosis and the metabolic syndrome. Hepatol Res. 2005;33:185–192. doi: 10.1016/j.hepres.2005.09.032. [DOI] [PubMed] [Google Scholar]
- 85.Farrell GC. Signalling links in the liver: knitting SOCS with fat and inflammation. J Hepatol. 2005;43:193–196. doi: 10.1016/j.jhep.2005.04.004. [DOI] [PubMed] [Google Scholar]
- 86.Emanuelli B, Peraldi P, Filloux C, Sawka-Verhelle D, Hilton D, Van OE. SOCS-3 is an insulin-induced negative regulator of insulin signaling. J Biol Chem. 2000;275:15985–15991. doi: 10.1074/jbc.275.21.15985. [DOI] [PubMed] [Google Scholar]
- 87.Michael MD, Kulkarni RN, Postic C, Previs SF, Shulman GI, Magnuson MA, Kahn CR. Loss of insulin signaling in hepatocytes leads to severe insulin resistance and progressive hepatic dysfunction. Mol Cell. 2000;6:87–97. doi: 10.1016/S1097-2765(05)00015-8. [DOI] [PubMed] [Google Scholar]
- 88.Radziuk J, Pye S. Hepatic glucose uptake, gluconeogenesis and the regulation of glycogen synthesis. Diabetes Metab Res Rev. 2001;17:250–272. doi: 10.1002/dmrr.217. [DOI] [PubMed] [Google Scholar]
- 89.Oh KJ, Han HS, Kim MJ, Koo SH. CREB and FoxO1: two transcription factors for the regulation of hepatic gluconeogenesis. BMB Rep. 2013;46:567–574. doi: 10.5483/BMBRep. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Davies MN, O’Callaghan BL, Towle HC. Glucose activates ChREBP by increasing its rate of nuclear entry and relieving repression of its transcriptional activity. J Biol Chem. 2008;283:24029–24038. doi: 10.1074/jbc.M801539200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Iozzo P, Bucci M, Roivainen A, Nagren K, Jarvisalo MJ, Kiss J, Guiducci L, Fielding B, Naum AG, Borra R, Virtanen K, Savunen T, Salvadori PA, Ferrannini E, Knuuti J, Nuutila P. Fatty acid metabolism in the liver, measured by positron emission tomography, is increased in obese individuals. Gastroenterology. 2010;139:846–856. doi: 10.1053/j.gastro.2010.05.039. [DOI] [PubMed] [Google Scholar]
- 92.Sunny NE, Parks EJ, Browning JD, Burgess SC. Excessive hepatic mitochondrial TCA cycle and gluconeogenesis in humans with nonalcoholic fatty liver disease. Cell Metab. 2011;14:804–810. doi: 10.1016/j.cmet.2011.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Weltman MD, Farrell GC, Hall P, Ingelman-Sundberg M, Liddle C. Hepatic cytochrome P450 2E1 is increased in patients with nonalcoholic steatohepatitis. Hepatology. 1998;27:128–133. doi: 10.1002/hep.510270121. [DOI] [PubMed] [Google Scholar]
- 94.Chalasani N, Gorski JC, Asghar MS, Asghar A, Foresman B, Hall SD, Crabb DW. Hepatic cytochrome P450 2E1 activity in nondiabetic patients with nonalcoholic steatohepatitis. Hepatology. 2003;37:544–550. doi: 10.1053/jhep.2003.50095. [DOI] [PubMed] [Google Scholar]
- 95.Diehl AM. Tumor necrosis factor and its potential role in insulin resistance and nonalcoholic fatty liver disease. Clin Liver Dis. 2004;8:619–638. doi: 10.1016/j.cld.2004.04.012. [DOI] [PubMed] [Google Scholar]
- 96.Koek GH, Liedorp PR, Bast A. The role of oxidative stress in non-alcoholic steatohepatitis. Clin Chim Acta. 2011;412:1297–1305. doi: 10.1016/j.cca.2011.04.013. [DOI] [PubMed] [Google Scholar]
- 97.Feldstein AE, Canbay A, Angulo P, Taniai M, Burgart LJ, Lindor KD, Gores GJ. Hepatocyte apoptosis and fas expression are prominent features of human nonalcoholic steatohepatitis. Gastroenterology. 2003;125:437–443. doi: 10.1016/S0016-5085(03)00907-7. [DOI] [PubMed] [Google Scholar]
- 98.Spahis S, Delvin E, Borys JM, Levy E. Oxidative stress as a critical factor in nonalcoholic fatty liver disease pathogenesis. Antioxid Redox Signal. 2017;26:519–541. doi: 10.1089/ars.2016.6776. [DOI] [PubMed] [Google Scholar]
- 99.Seki S, Kitada T, Sakaguchi H. Clinicopathological significance of oxidative cellular damage in non-alcoholic fatty liver diseases. Hepatol Res. 2005;33:132–134. doi: 10.1016/j.hepres.2005.09.020. [DOI] [PubMed] [Google Scholar]
- 100.Feldstein AE, Lopez R, Tamimi TA, Yerian L, Chung YM, Berk M, Zhang R, McIntyre TM, Hazen SL. Mass spectrometric profiling of oxidized lipid products in human nonalcoholic fatty liver disease and nonalcoholic steatohepatitis. J Lipid Res. 2010;51:3046–3054. doi: 10.1194/jlr.M007096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Liu W, Baker SS, Baker RD, Zhu L. Antioxidant mechanisms in nonalcoholic fatty liver disease. Curr Drug Targets. 2015;16:1301–1314. doi: 10.2174/1389450116666150427155342. [DOI] [PubMed] [Google Scholar]
- 102.Begriche K, Massart J, Robin MA, Borgne-Sanchez A, Fromenty B. Drug-induced toxicity on mitochondria and lipid metabolism: mechanistic diversity and deleterious consequences for the liver. J Hepatol. 2011;54:773–794. doi: 10.1016/j.jhep.2010.11.006. [DOI] [PubMed] [Google Scholar]
- 103.Pessayre D, Fromenty B, Berson A, Robin MA, Letteron P, Moreau R, Mansouri A. Central role of mitochondria in drug-induced liver injury. Drug Metab Rev. 2012;44:34–87. doi: 10.3109/03602532.2011.604086. [DOI] [PubMed] [Google Scholar]
- 104.Boveris A, Chance B. The mitochondrial generation of hydrogen peroxide. General properties and effect of hyperbaric oxygen. Biochem J. 1973;134:707–716. doi: 10.1042/bj1340707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Sookoian S, Rosselli MS, Gemma C, Burgueno AL, Fernandez GT, Castano GO, Pirola CJ. Epigenetic regulation of insulin resistance in nonalcoholic fatty liver disease: impact of liver methylation of the peroxisome proliferator-activated receptor gamma coactivator 1α promoter. Hepatology. 2010;52:1992–2000. doi: 10.1002/hep.23927. [DOI] [PubMed] [Google Scholar]
- 106.Chiappini F, Barrier A, Saffroy R, Domart MC, Dagues N, Azoulay D, Sebagh M, Franc B, Chevalier S, Debuire B, Dudoit S, Lemoine A. Exploration of global gene expression in human liver steatosis by high-density oligonucleotide microarray. Lab Invest. 2006;86:154–165. doi: 10.1038/labinvest.3700374. [DOI] [PubMed] [Google Scholar]
- 107.Caldwell SH, Swerdlow RH, Khan EM, Iezzoni JC, Hespenheide EE, Parks JK, Parker WD., Jr Mitochondrial abnormalities in non-alcoholic steatohepatitis. J Hepatol. 1999;31:430–434. doi: 10.1016/S0168-8278(99)80033-6. [DOI] [PubMed] [Google Scholar]
- 108.Fujita N, Miyachi H, Tanaka H, Takeo M, Nakagawa N, Kobayashi Y, Iwasa M, Watanabe S, Takei Y. Iron overload is associated with hepatic oxidative damage to DNA in nonalcoholic steatohepatitis. Cancer Epidemiol Biomarkers Prev. 2009;18:424–432. doi: 10.1158/1055-9965.EPI-08-0725. [DOI] [PubMed] [Google Scholar]
- 109.Kawahara H, Fukura M, Tsuchishima M, Takase S. Mutation of mitochondrial DNA in livers from patients with alcoholic hepatitis and nonalcoholic steatohepatitis. Alcohol Clin Exp Res. 2007;31:S54–S60. doi: 10.1111/j.1530-0277.2006.00287.x. [DOI] [PubMed] [Google Scholar]
- 110.Hui JM, Hodge A, Farrell GC, Kench JG, Kriketos A, George J. Beyond insulin resistance in NASH: tNF-alpha or adiponectin? Hepatology. 2004;40:46–54. doi: 10.1002/hep.20280. [DOI] [PubMed] [Google Scholar]
- 111.Bugianesi E, Pagotto U, Manini R, Vanni E, Gastaldelli A, Gentilcore E, Natale S, Cassader M, Rizzetto M, Pasquali R, Marchesini G. Plasma adiponectin in nonalcoholic fatty liver is related to hepatic insulin resistance and hepatic fat content, not to liver disease severity. J Clin Endocrinol Metab. 2005;90:3498–3504. doi: 10.1210/jc.2004-2240. [DOI] [PubMed] [Google Scholar]
- 112.Kaser S, Moschen A, Cayon A, Kaser A, Crespo J, Pons-Romero F, Ebenbichler CF, Patsch JR, Tilg H. Adiponectin and its receptors in non-alcoholic steatohepatitis. Gut. 2005;54:117–121. doi: 10.1136/gut.2003.037010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Yoshikawa T, Ide T, Shimano H, Yahagi N, Amemiya-Kudo M, Matsuzaka T, Yatoh S, Kitamine T, Okazaki H, Tamura Y, Sekiya M, Takahashi A, Hasty AH, Sato R, Sone H, Osuga J, Ishibashi S, Yamada N. Cross-talk between peroxisome proliferator-activated receptor (PPAR) alpha and liver X receptor (LXR) in nutritional regulation of fatty acid metabolism. I. PPARs suppress sterol regulatory element binding protein-1c promoter through inhibition of LXR signaling. Mol Endocrinol. 2003;17:1240–1254. doi: 10.1210/me.2002-0190. [DOI] [PubMed] [Google Scholar]
- 114.Osmundsen H, Bremer J, Pedersen JI. Metabolic aspects of peroxisomal beta-oxidation. Biochim Biophys Acta. 1991;1085:141–158. doi: 10.1016/0005-2760(91)90089-Z. [DOI] [PubMed] [Google Scholar]
- 115.Robertson G, Leclercq I, Farrell GC. Nonalcoholic steatosis and steatohepatitis. II. Cytochrome P-450 enzymes and oxidative stress. Am J Physiol Gastrointest Liver Physiol. 2001;281:G1135–G1139. doi: 10.1152/ajpgi.2001.281.5.G1135. [DOI] [PubMed] [Google Scholar]
- 116.Zangar RC, Novak RF. Effects of fatty acids and ketone bodies on cytochromes P450 2B, 4A, and 2E1 expression in primary cultured rat hepatocytes. Arch Biochem Biophys. 1997;337:217–224. doi: 10.1006/abbi.1996.9785. [DOI] [PubMed] [Google Scholar]
- 117.Woodcroft KJ, Novak RF. Insulin effects on CYP2E1, 2B, 3A, and 4A expression in primary cultured rat hepatocytes. Chem Biol Interact. 1997;107:75–91. doi: 10.1016/S0009-2797(97)00075-6. [DOI] [PubMed] [Google Scholar]
- 118.Valenti L, Fracanzani AL, Bugianesi E, Dongiovanni P, Galmozzi E, Vanni E, Canavesi E, Lattuada E, Roviaro G, Marchesini G, Fargion S. HFE genotype, parenchymal iron accumulation, and liver fibrosis in patients with nonalcoholic fatty liver disease. Gastroenterology. 2010;138:905–912. doi: 10.1053/j.gastro.2009.11.013. [DOI] [PubMed] [Google Scholar]
- 119.Chtioui H, Semela D, Ledermann M, Zimmermann A, Dufour JF. Expression and activity of the cytochrome P450 2E1 in patients with nonalcoholic steatosis and steatohepatitis. Liver Int. 2007;27:764–771. doi: 10.1111/j.1478-3231.2007.01524.x. [DOI] [PubMed] [Google Scholar]
- 120.Videla LA, Rodrigo R, Orellana M, Fernandez V, Tapia G, Quinones L, Varela N, Contreras J, Lazarte R, Csendes A, Rojas J, Maluenda F, Burdiles P, Diaz JC, Smok G, Thielemann L, Poniachik J. Oxidative stress-related parameters in the liver of non-alcoholic fatty liver disease patients. Clin Sci (Lond) 2004;106:261–268. doi: 10.1042/CS20030285. [DOI] [PubMed] [Google Scholar]
- 121.Leclercq IA. Antioxidant defence mechanisms: new players in the pathogenesis of non-alcoholic steatohepatitis? Clin Sci (Lond) 2004;106:235–237. doi: 10.1042/CS20030368. [DOI] [PubMed] [Google Scholar]
- 122.Orellana M, Rodrigo R, Varela N, Araya J, Poniachik J, Csendes A, Smok G, Videla LA. Relationship between in vivo chlorzoxazone hydroxylation, hepatic cytochrome P450 2E1 content and liver injury in obese non-alcoholic fatty liver disease patients. Hepatol Res. 2006;34:57–63. doi: 10.1016/j.hepres.2005.10.001. [DOI] [PubMed] [Google Scholar]
- 123.Bettaieb A, Jiang JX, Sasaki Y, Chao TI, Kiss Z, Chen X, Tian J, Katsuyama M, Yabe-Nishimura C, Xi Y, Szyndralewiez C, Schroder K, Shah A, Brandes RP, Haj FG, Torok NJ. Hepatocyte nicotinamide adenine dinucleotide phosphate reduced oxidase 4 regulates stress signaling, fibrosis, and insulin sensitivity during development of steatohepatitis in mice. Gastroenterology. 2015;149:468–480. doi: 10.1053/j.gastro.2015.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.de Mochel NS, Seronello S, Wang SH, Ito C, Zheng JX, Liang TJ, Lambeth JD, Choi J. Hepatocyte NAD(P)H oxidases as an endogenous source of reactive oxygen species during hepatitis C virus infection. Hepatology. 2010;52:47–59. doi: 10.1002/hep.23671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Jiang JX, Chen X, Serizawa N, Szyndralewiez C, Page P, Schroder K, Brandes RP, Devaraj S, Torok NJ. Liver fibrosis and hepatocyte apoptosis are attenuated by GKT137831, a novel NOX4/NOX1 inhibitor in vivo. Free Radic Biol Med. 2012;53:289–296. doi: 10.1016/j.freeradbiomed.2012.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Lanaspa MA, Sanchez-Lozada LG, Choi YJ, Cicerchi C, Kanbay M, Roncal-Jimenez CA, Ishimoto T, Li N, Marek G, Duranay M, Schreiner G, Rodriguez-Iturbe B, Nakagawa T, Kang DH, Sautin YY, Johnson RJ. Uric acid induces hepatic steatosis by generation of mitochondrial oxidative stress: potential role in fructose-dependent and -independent fatty liver. J Biol Chem. 2012;287:40732–40744. doi: 10.1074/jbc.M112.399899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Smith CM, Rovamo LM, Raivio KO. Fructose-induced adenine nucleotide catabolism in isolated rat hepatocytes. Can J Biochem. 1977;55:1237–1240. doi: 10.1139/o77-185. [DOI] [PubMed] [Google Scholar]
- 128.Zelber-Sagi S, Nitzan-Kaluski D, Halpern Z, Oren R. NAFLD and hyperinsulinemia are major determinants of serum ferritin levels. J Hepatol. 2007;46:700–707. doi: 10.1016/j.jhep.2006.09.018. [DOI] [PubMed] [Google Scholar]
- 129.Turlin B, Mendler MH, Moirand R, Guyader D, Guillygomarc’h A, Deugnier Y. Histologic features of the liver in insulin resistance-associated iron overload. A study of 139 patients. Am J Clin Pathol. 2001;116:263–270. doi: 10.1309/WWNE-KW2C-4KTW-PTJ5. [DOI] [PubMed] [Google Scholar]
- 130.Aigner E, Theurl I, Theurl M, Lederer D, Haufe H, Dietze O, Strasser M, Datz C, Weiss G. Pathways underlying iron accumulation in human nonalcoholic fatty liver disease. Am J Clin Nutr. 2008;87:1374–1383. doi: 10.1093/ajcn/87.5.1374. [DOI] [PubMed] [Google Scholar]
- 131.Nelson JE, Klintworth H, Kowdley KV. Iron metabolism in nonalcoholic fatty liver disease. Curr Gastroenterol Rep. 2012;14:8–16. doi: 10.1007/s11894-011-0234-4. [DOI] [PubMed] [Google Scholar]
- 132.Green A, Basile R, Rumberger JM. Transferrin and iron induce insulin resistance of glucose transport in adipocytes. Metabolism. 2006;55:1042–1045. doi: 10.1016/j.metabol.2006.03.015. [DOI] [PubMed] [Google Scholar]
- 133.Niederau C, Berger M, Stremmel W, Starke A, Strohmeyer G, Ebert R, Siegel E, Creutzfeldt W. Hyperinsulinaemia in non-cirrhotic haemochromatosis: impaired hepatic insulin degradation? Diabetologia. 1984;26:441–444. doi: 10.1007/BF00262217. [DOI] [PubMed] [Google Scholar]
- 134.Hevi S, Chuck SL. Ferritins can regulate the secretion of apolipoprotein B. J Biol Chem. 2003;278:31924–31929. doi: 10.1074/jbc.M303081200. [DOI] [PubMed] [Google Scholar]
- 135.Sreekumar R, Rosado B, Rasmussen D, Charlton M. Hepatic gene expression in histologically progressive nonalcoholic steatohepatitis. Hepatology. 2003;38:244–251. doi: 10.1053/jhep.2003.50290. [DOI] [PubMed] [Google Scholar]
- 136.Bessone F, Dirchwolf M, Rodil MA, Razori MV, Roma MG. Review article drug-induced liver injury in the context of nonalcoholic fatty liver disease—a physiopathological and clinical integrated view. Aliment Pharmacol Ther. 2018 doi: 10.1111/apt.14952. [DOI] [PubMed] [Google Scholar]
- 137.Mari M, Morales A, Colell A, Garcia-Ruiz C, Fernandez-Checa JC. Mitochondrial glutathione, a key survival antioxidant. Antioxid Redox Signal. 2009;11:2685–2700. doi: 10.1089/ars.2009.2695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Fromenty B, Pessayre D. Inhibition of mitochondrial beta-oxidation as a mechanism of hepatotoxicity. Pharmacol Ther. 1995;67:101–154. doi: 10.1016/0163-7258(95)00012-6. [DOI] [PubMed] [Google Scholar]
- 139.Abdelmegeed MA, Ha SK, Choi Y, Akbar M, Song BJ. Role of CYP2E1 in mitochondrial dysfunction and hepatic injury by alcohol and non-alcoholic substances. Curr Mol Pharmacol. 2017;10:207–225. doi: 10.2174/1874467208666150817111114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Dam-Larsen S, Franzmann M, Andersen IB, Christoffersen P, Jensen LB, Sorensen TI, Becker U, Bendtsen F. Long term prognosis of fatty liver: risk of chronic liver disease and death. Gut. 2004;53:750–755. doi: 10.1136/gut.2003.019984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Tilg H, Diehl AM. Cytokines in alcoholic and nonalcoholic steatohepatitis. N Engl J Med. 2000;343:1467–1476. doi: 10.1056/NEJM200011163432007. [DOI] [PubMed] [Google Scholar]
- 142.El Husseny MW, Mamdouh M, Shaban S, Ibrahim AA, Zaki MM, Ahmed OM, Abdel-Daim MM. Adipokines: potential therapeutic targets for vascular dysfunction in type II diabetes mellitus and obesity. J Diabetes Res. 2017;2017:8095926. doi: 10.1155/2017/8095926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Skurk T, Alberti-Huber C, Herder C, Hauner H. Relationship between adipocyte size and adipokine expression and secretion. J Clin Endocrinol Metab. 2007;92:1023–1033. doi: 10.1210/jc.2006-1055. [DOI] [PubMed] [Google Scholar]
- 144.Rotter V, Nagaev I, Smith U. Interleukin-6 (IL-6) induces insulin resistance in 3T3-L1 adipocytes and is, like IL-8 and tumor necrosis factor-α, overexpressed in human fat cells from insulin-resistant subjects. J Biol Chem. 2003;278:45777–45784. doi: 10.1074/jbc.M301977200. [DOI] [PubMed] [Google Scholar]
- 145.Weisberg SP, McCann D, Desai M, Rosenbaum M, Leibel RL, Ferrante AW., Jr Obesity is associated with macrophage accumulation in adipose tissue. J Clin Invest. 2003;112:1796–1808. doi: 10.1172/JCI200319246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Lumeng CN, Bodzin JL, Saltiel AR. Obesity induces a phenotypic switch in adipose tissue macrophage polarization. J Clin Invest. 2007;117:175–184. doi: 10.1172/JCI29881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Polyzos SA, Toulis KA, Goulis DG, Zavos C, Kountouras J. Serum total adiponectin in nonalcoholic fatty liver disease: a systematic review and meta-analysis. Metabolism. 2011;60:313–326. doi: 10.1016/j.metabol.2010.09.003. [DOI] [PubMed] [Google Scholar]
- 148.Moschen AR, Molnar C, Wolf AM, Weiss H, Graziadei I, Kaser S, Ebenbichler CF, Stadlmann S, Moser PL, Tilg H. Effects of weight loss induced by bariatric surgery on hepatic adipocytokine expression. J Hepatol. 2009;51:765–777. doi: 10.1016/j.jhep.2009.06.016. [DOI] [PubMed] [Google Scholar]
- 149.Ghadge AA, Khaire AA, Kuvalekar AA. Adiponectin: a potential therapeutic target for metabolic syndrome. Cytokine Growth Factor Rev. 2018;39:151–158. doi: 10.1016/j.cytogfr.2018.01.004. [DOI] [PubMed] [Google Scholar]
- 150.La Cava A, Matarese G. The weight of leptin in immunity. Nat Rev Immunol. 2004;4:371–379. doi: 10.1038/nri1350. [DOI] [PubMed] [Google Scholar]
- 151.Ikejima K, Okumura K, Kon K, Takei Y, Sato N. Role of adipocytokines in hepatic fibrogenesis. J Gastroenterol Hepatol. 2007;22:S87–S92. doi: 10.1111/j.1440-1746.2007.04961.x. [DOI] [PubMed] [Google Scholar]
- 152.Shen J, Sakaida I, Uchida K, Terai S, Okita K. Leptin enhances TNF-alpha production via p38 and JNK MAPK in LPS-stimulated Kupffer cells. Life Sci. 2005;77:1502–1515. doi: 10.1016/j.lfs.2005.04.004. [DOI] [PubMed] [Google Scholar]
- 153.Alisi A, Ceccarelli S, Panera N, Nobili V. Causative role of gut microbiota in non-alcoholic fatty liver disease pathogenesis. Front Cell Infect Microbiol. 2012;2:132. doi: 10.3389/fcimb.2012.00132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Spruss A, Bergheim I. Dietary fructose and intestinal barrier: potential risk factor in the pathogenesis of nonalcoholic fatty liver disease. J Nutr Biochem. 2009;20:657–662. doi: 10.1016/j.jnutbio.2009.05.006. [DOI] [PubMed] [Google Scholar]
- 155.Amar J, Burcelin R, Ruidavets JB, Cani PD, Fauvel J, Alessi MC, Chamontin B, Ferrieres J. Energy intake is associated with endotoxemia in apparently healthy men. Am J Clin Nutr. 2008;87:1219–1223. doi: 10.1093/ajcn/87.5.1219. [DOI] [PubMed] [Google Scholar]
- 156.Le Roy T, Llopis M, Lepage P, Bruneau A, Rabot S, Bevilacqua C, Martin P, Philippe C, Walker F, Bado A, Perlemuter G, Cassard-Doulcier AM, Gerard P. Intestinal microbiota determines development of non-alcoholic fatty liver disease in mice. Gut. 2013;62:1787–1794. doi: 10.1136/gutjnl-2012-303816. [DOI] [PubMed] [Google Scholar]
- 157.Miura K, Ohnishi H. Role of gut microbiota and Toll-like receptors in nonalcoholic fatty liver disease. World J Gastroenterol. 2014;20:7381–7391. doi: 10.3748/wjg.v20.i23.7381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Thuy S, Ladurner R, Volynets V, Wagner S, Strahl S, Konigsrainer A, Maier KP, Bischoff SC, Bergheim I. Nonalcoholic fatty liver disease in humans is associated with increased plasma endotoxin and plasminogen activator inhibitor 1 concentrations and with fructose intake. J Nutr. 2008;138:1452–1455. doi: 10.1093/jn/138.8.1452. [DOI] [PubMed] [Google Scholar]
- 159.Miele L, Valenza V, La TG, Montalto M, Cammarota G, Ricci R, Masciana R, Forgione A, Gabrieli ML, Perotti G, Vecchio FM, Rapaccini G, Gasbarrini G, Day CP, Grieco A. Increased intestinal permeability and tight junction alterations in nonalcoholic fatty liver disease. Hepatology. 2009;49:1877–1887. doi: 10.1002/hep.22848. [DOI] [PubMed] [Google Scholar]
- 160.Bergheim I, Weber S, Vos M, Kramer S, Volynets V, Kaserouni S, McClain CJ, Bischoff SC. Antibiotics protect against fructose-induced hepatic lipid accumulation in mice: role of endotoxin. J Hepatol. 2008;48:983–992. doi: 10.1016/j.jhep.2008.01.035. [DOI] [PubMed] [Google Scholar]
- 161.Pradere JP, Troeger JS, Dapito DH, Mencin AA, Schwabe RF. Toll-like receptor 4 and hepatic fibrogenesis. Semin Liver Dis. 2010;30:232–244. doi: 10.1055/s-0030-1255353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Cani PD, Amar J, Iglesias MA, Poggi M, Knauf C, Bastelica D, Neyrinck AM, Fava F, Tuohy KM, Chabo C, Waget A, Delmee E, Cousin B, Sulpice T, Chamontin B, Ferrieres J, Tanti JF, Gibson GR, Casteilla L, Delzenne NM, Alessi MC, Burcelin R. Metabolic endotoxemia initiates obesity and insulin resistance. Diabetes. 2007;56:1761–1772. doi: 10.2337/db06-1491. [DOI] [PubMed] [Google Scholar]
- 163.Baffy G. Kupffer cells in non-alcoholic fatty liver disease: the emerging view. J Hepatol. 2009;51:212–223. doi: 10.1016/j.jhep.2009.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Seki E, Brenner DA. Toll-like receptors and adaptor molecules in liver disease: update. Hepatology. 2008;48:322–335. doi: 10.1002/hep.22306. [DOI] [PubMed] [Google Scholar]
- 165.Carter-Kent C, Zein NN, Feldstein AE. Cytokines in the pathogenesis of fatty liver and disease progression to steatohepatitis: implications for treatment. Am J Gastroenterol. 2008;103:1036–1042. doi: 10.1111/j.1572-0241.2007.01709.x. [DOI] [PubMed] [Google Scholar]
- 166.Matsumura T, Degawa T, Takii T, Hayashi H, Okamoto T, Inoue J, Onozaki K. TRAF6-NF-κB pathway is essential for interleukin-1-induced TLR2 expression and its functional response to TLR2 ligand in murine hepatocytes. Immunology. 2003;109:127–136. doi: 10.1046/j.1365-2567.2003.01627.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Csak T, Ganz M, Pespisa J, Kodys K, Dolganiuc A, Szabo G. Fatty acid and endotoxin activate inflammasomes in mouse hepatocytes that release danger signals to stimulate immune cells. Hepatology. 2011;54:133–144. doi: 10.1002/hep.24341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Feldstein AE, Werneburg NW, Canbay A, Guicciardi ME, Bronk SF, Rydzewski R, Burgart LJ, Gores GJ. Free fatty acids promote hepatic lipotoxicity by stimulating TNF-alpha expression via a lysosomal pathway. Hepatology. 2004;40:185–194. doi: 10.1097/00005176-200406001-00042. [DOI] [PubMed] [Google Scholar]
- 169.Chen L, Xiong S, She H, Lin SW, Wang J, Tsukamoto H. Iron causes interactions of TAK1, p21ras, and phosphatidylinositol 3-kinase in caveolae to activate IκB kinase in hepatic macrophages. J Biol Chem. 2007;282:5582–5588. doi: 10.1074/jbc.M609273200. [DOI] [PubMed] [Google Scholar]
- 170.Schilling JD, Machkovech HM, He L, Sidhu R, Fujiwara H, Weber K, Ory DS, Schaffer JE. Palmitate and lipopolysaccharide trigger synergistic ceramide production in primary macrophages. J Biol Chem. 2013;288:2923–2932. doi: 10.1074/jbc.M112.419978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Shi H, Kokoeva MV, Inouye K, Tzameli I, Yin H, Flier JS. TLR4 links innate immunity and fatty acid-induced insulin resistance. J Clin Invest. 2006;116:3015–3025. doi: 10.1172/JCI28898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Ribeiro PS, Cortez-Pinto H, Sola S, Castro RE, Ramalho RM, Baptista A, Moura MC, Camilo ME, Rodrigues CM. Hepatocyte apoptosis, expression of death receptors, and activation of NF-κB in the liver of nonalcoholic and alcoholic steatohepatitis patients. Am J Gastroenterol. 2004;99:1708–1717. doi: 10.1111/j.1572-0241.2004.40009.x. [DOI] [PubMed] [Google Scholar]
- 173.Alkhouri N, Carter-Kent C, Feldstein AE. Apoptosis in nonalcoholic fatty liver disease: diagnostic and therapeutic implications. Expert Rev Gastroenterol Hepatol. 2011;5:201–212. doi: 10.1586/egh.11.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Susca M, Grassi A, Zauli D, Volta U, Lenzi M, Marchesini G, Bianchi FB, Ballardini G. Liver inflammatory cells, apoptosis, regeneration and stellate cell activation in non-alcoholic steatohepatitis. Dig Liver Dis. 2001;33:768–777. doi: 10.1016/S1590-8658(01)80694-0. [DOI] [PubMed] [Google Scholar]
- 175.Ulukaya E, Acilan C, Yilmaz Y. Apoptosis: why and how does it occur in biology? Cell Biochem Funct. 2011;29:468–480. doi: 10.1002/cbf.1774. [DOI] [PubMed] [Google Scholar]
- 176.Diehl AM. Lessons from animal models of NASH. Hepatol Res. 2005;33:138–144. doi: 10.1016/j.hepres.2005.09.022. [DOI] [PubMed] [Google Scholar]
- 177.Jou J, Choi SS, Diehl AM. Mechanisms of disease progression in nonalcoholic fatty liver disease. Semin Liver Dis. 2008;28:370–379. doi: 10.1055/s-0028-1091981. [DOI] [PubMed] [Google Scholar]
- 178.Pop C, Salvesen GS. Human caspases: activation, specificity, and regulation. J Biol Chem. 2009;284:21777–21781. doi: 10.1074/jbc.R800084200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Adrain C, Creagh EM, Martin SJ. Apoptosis-associated release of Smac/DIABLO from mitochondria requires active caspases and is blocked by Bcl-2. EMBO J. 2001;20:6627–6636. doi: 10.1093/emboj/20.23.6627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Cande C, Cecconi F, Dessen P, Kroemer G. Apoptosis-inducing factor (AIF): key to the conserved caspase-independent pathways of cell death? J Cell Sci. 2002;115:4727–4734. doi: 10.1242/jcs.00210. [DOI] [PubMed] [Google Scholar]
- 181.Siddiqui WA, Ahad A, Ahsan H. The mystery of BCL2 family: Bcl-2 proteins and apoptosis: an update. Arch Toxicol. 2015;89:289–317. doi: 10.1007/s00204-014-1448-7. [DOI] [PubMed] [Google Scholar]
- 182.Barreyro FJ, Kobayashi S, Bronk SF, Werneburg NW, Malhi H, Gores GJ. Transcriptional regulation of Bim by FoxO3A mediates hepatocyte lipoapoptosis. J Biol Chem. 2007;282:27141–27154. doi: 10.1074/jbc.M704391200. [DOI] [PubMed] [Google Scholar]
- 183.Malhi H, Bronk SF, Werneburg NW, Gores GJ. Free fatty acids induce JNK-dependent hepatocyte lipoapoptosis. J Biol Chem. 2006;281:12093–12101. doi: 10.1074/jbc.M510660200. [DOI] [PubMed] [Google Scholar]
- 184.Cazanave SC, Mott JL, Elmi NA, Bronk SF, Werneburg NW, Akazawa Y, Kahraman A, Garrison SP, Zambetti GP, Charlton MR, Gores GJ. JNK1-dependent PUMA expression contributes to hepatocyte lipoapoptosis. J Biol Chem. 2009;284:26591–26602. doi: 10.1074/jbc.M109.022491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Kakisaka K, Cazanave SC, Fingas CD, Guicciardi ME, Bronk SF, Werneburg NW, Mott JL, Gores GJ. Mechanisms of lysophosphatidylcholine-induced hepatocyte lipoapoptosis. Am J Physiol Gastrointest Liver Physiol. 2012;302:G77–G84. doi: 10.1152/ajpgi.00301.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Cazanave SC, Elmi NA, Akazawa Y, Bronk SF, Mott JL, Gores GJ. CHOP and AP-1 cooperatively mediate PUMA expression during lipoapoptosis. Am J Physiol Gastrointest Liver Physiol. 2010;299:G236–G243. doi: 10.1152/ajpgi.00091.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Hikisz P, Kilianska ZM. PUMA, a critical mediator of cell death–one decade on from its discovery. Cell Mol Biol Lett. 2012;17:646–669. doi: 10.2478/s11658-012-0032-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Lee J, Ozcan U. Unfolded protein response signaling and metabolic diseases. J Biol Chem. 2014;289:1203–1211. doi: 10.1074/jbc.R113.534743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Bozaykut P, Sahin A, Karademir B, Ozer NK. Endoplasmic reticulum stress related molecular mechanisms in nonalcoholic steatohepatitis. Mech Ageing Dev. 2016;157:17–29. doi: 10.1016/j.mad.2016.07.001. [DOI] [PubMed] [Google Scholar]
- 190.Wei Y, Wang D, Topczewski F, Pagliassotti MJ. Saturated fatty acids induce endoplasmic reticulum stress and apoptosis independently of ceramide in liver cells. Am J Physiol Endocrinol Metab. 2006;291:E275–E281. doi: 10.1152/ajpendo.00644.2005. [DOI] [PubMed] [Google Scholar]
- 191.Faitova J, Krekac D, Hrstka R, Vojtesek B. Endoplasmic reticulum stress and apoptosis. Cell Mol Biol Lett. 2006;11:488–505. doi: 10.2478/s11658-006-0040-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Lamkanfi M, Kalai M, Vandenabeele P. Caspase-12: an overview. Cell Death Differ. 2004;11:365–368. doi: 10.1038/sj.cdd.4401364. [DOI] [PubMed] [Google Scholar]
- 193.Scorrano L, Oakes SA, Opferman JT, Cheng EH, Sorcinelli MD, Pozzan T, Korsmeyer SJ. BAX and BAK regulation of endoplasmic reticulum Ca2+: a control point for apoptosis. Science. 2003;300:135–139. doi: 10.1126/science.1081208. [DOI] [PubMed] [Google Scholar]
- 194.Waring P, Mullbacher A. Cell death induced by the Fas/Fas ligand pathway and its role in pathology. Immunol Cell Biol. 1999;77:312–317. doi: 10.1046/j.1440-1711.1999.00837.x. [DOI] [PubMed] [Google Scholar]
- 195.Li H, Zhu H, Xu CJ, Yuan J. Cleavage of BID by caspase 8 mediates the mitochondrial damage in the Fas pathway of apoptosis. Cell. 1998;94:491–501. doi: 10.1016/S0092-8674(00)81590-1. [DOI] [PubMed] [Google Scholar]
- 196.Milhas D, Cuvillier O, Therville N, Clave P, Thomsen M, Levade T, Benoist H, Segui B. Caspase-10 triggers Bid cleavage and caspase cascade activation in FasL-induced apoptosis. J Biol Chem. 2005;280:19836–19842. doi: 10.1074/jbc.M414358200. [DOI] [PubMed] [Google Scholar]
- 197.Malhi H, Barreyro FJ, Isomoto H, Bronk SF, Gores GJ. Free fatty acids sensitise hepatocytes to TRAIL mediated cytotoxicity. Gut. 2007;56:1124–1131. doi: 10.1136/gut.2006.118059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Volkmann X, Fischer U, Bahr MJ, Ott M, Lehner F, Macfarlane M, Cohen GM, Manns MP, Schulze-Osthoff K, Bantel H. Increased hepatotoxicity of tumor necrosis factor-related apoptosis-inducing ligand in diseased human liver. Hepatology. 2007;46:1498–1508. doi: 10.1002/hep.21846. [DOI] [PubMed] [Google Scholar]
- 199.Feldstein AE, Canbay A, Guicciardi ME, Higuchi H, Bronk SF, Gores GJ. Diet associated hepatic steatosis sensitizes to Fas mediated liver injury in mice. J Hepatol. 2003;39:978–983. doi: 10.1016/S0168-8278(03)00460-4. [DOI] [PubMed] [Google Scholar]
- 200.Li Z, Berk M, McIntyre TM, Gores GJ, Feldstein AE. The lysosomal-mitochondrial axis in free fatty acid-induced hepatic lipotoxicity. Hepatology. 2008;47:1495–1503. doi: 10.1002/hep.22183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Feldstein AE, Werneburg NW, Li Z, Bronk SF, Gores GJ. Bax inhibition protects against free fatty acid-induced lysosomal permeabilization. Am J Physiol Gastrointest Liver Physiol. 2006;290:G1339–G1346. doi: 10.1152/ajpgi.00509.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Guicciardi ME, Bronk SF, Werneburg NW, Yin XM, Gores GJ. Bid is upstream of lysosome-mediated caspase 2 activation in tumor necrosis factor alpha-induced hepatocyte apoptosis. Gastroenterology. 2005;129:269–284. doi: 10.1053/j.gastro.2005.05.022. [DOI] [PubMed] [Google Scholar]
- 203.Kinnally KW, Peixoto PM, Ryu SY, Dejean LM. Is mPTP the gatekeeper for necrosis, apoptosis, or both? Biochim Biophys Acta. 2011;1813:616–622. doi: 10.1016/j.bbamcr.2010.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Lemasters JJ, Nieminen AL, Qian T, Trost LC, Elmore SP, Nishimura Y, Crowe RA, Cascio WE, Bradham CA, Brenner DA, Herman B. The mitochondrial permeability transition in cell death: a common mechanism in necrosis, apoptosis and autophagy. Biochim Biophys Acta. 1998;1366:177–196. doi: 10.1016/S0005-2728(98)00112-1. [DOI] [PubMed] [Google Scholar]
- 205.Toledo FD, Perez LM, Basiglio CL, Ochoa JE, Sanchez Pozzi EJ, Roma MG. The Ca2+-calmodulin-Ca2+/calmodulin-dependent protein kinase II signaling pathway is involved in oxidative stress-induced mitochondrial permeability transition and apoptosis in isolated rat hepatocytes. Arch Toxicol. 2014;88:1695–1709. doi: 10.1007/s00204-014-1219-5. [DOI] [PubMed] [Google Scholar]
- 206.Toledo FD, Basiglio CL, Barosso IR, Boaglio AC, Zucchetti AE, Sanchez Pozzi EJ, Roma MG. Mitogen-activated protein kinases are involved in hepatocanalicular dysfunction and cholestasis induced by oxidative stress. Arch Toxicol. 2017;91:2391–2403. doi: 10.1007/s00204-016-1898-1. [DOI] [PubMed] [Google Scholar]
- 207.Camello-Almaraz C, Gomez-Pinilla PJ, Pozo MJ, Camello PJ. Mitochondrial reactive oxygen species and Ca2+ signaling. Am J Physiol Cell Physiol. 2006;291:C1082–C1088. doi: 10.1152/ajpcell.00217.2006. [DOI] [PubMed] [Google Scholar]
- 208.Malhotra JD, Kaufman RJ. Endoplasmic reticulum stress and oxidative stress: a vicious cycle or a double-edged sword? Antioxid Redox Signal. 2007;9:2277–2293. doi: 10.1089/ars.2007.1782. [DOI] [PubMed] [Google Scholar]
- 209.Egnatchik RA, Leamy AK, Jacobson DA, Shiota M, Young JD. ER calcium release promotes mitochondrial dysfunction and hepatic cell lipotoxicity in response to palmitate overload. Mol Metab. 2014;3:544–553. doi: 10.1016/j.molmet.2014.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Teodoro JS, Rolo AP, Duarte FV, Simoes AM, Palmeira CM. Differential alterations in mitochondrial function induced by a choline-deficient diet: understanding fatty liver disease progression. Mitochondrion. 2008;8:367–376. doi: 10.1016/j.mito.2008.07.008. [DOI] [PubMed] [Google Scholar]
- 211.Martinez L, Torres S, Baulies A, Alarcon-Vila C, Elena M, Fabrias G, Casas J, Caballeria J, Fernandez-Checa JC, Garcia-Ruiz C. Myristic acid potentiates palmitic acid-induced lipotoxicity and steatohepatitis associated with lipodystrophy by sustaning de novo ceramide synthesis. Oncotarget. 2015;6:41479–41496. doi: 10.18632/oncotarget.6286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Mehrpour M, Esclatine A, Beau I, Codogno P. Autophagy in health and disease. 1. Regulation and significance of autophagy: an overview. Am J Physiol Cell Physiol. 2010;298:C776–C785. doi: 10.1152/ajpcell.00507.2009. [DOI] [PubMed] [Google Scholar]
- 213.Wang Z, Han W, Sui X, Fang Y, Pan H. Autophagy: a novel therapeutic target for hepatocarcinoma (Review) Oncol Lett. 2014;7:1345–1351. doi: 10.3892/ol.2014.1916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Czaja MJ. Function of autophagy in nonalcoholic fatty liver disease. Dig Dis Sci. 2016;61:1304–1313. doi: 10.1007/s10620-015-4025-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Singh R, Kaushik S, Wang Y, Xiang Y, Novak I, Komatsu M, Tanaka K, Cuervo AM, Czaja MJ. Autophagy regulates lipid metabolism. Nature. 2009;458:1131–1135. doi: 10.1038/nature07976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Orenstein SJ, Cuervo AM. Chaperone-mediated autophagy: molecular mechanisms and physiological relevance. Semin Cell Dev Biol. 2010;21:719–726. doi: 10.1016/j.semcdb.2010.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Kwanten WJ, Vandewynckel YP, Martinet W, De Winter BY, Michielsen PP, Van Hoof VO, Driessen A, Timmermans JP, Bedossa P, Van VH, Francque SM. Hepatocellular autophagy modulates the unfolded protein response and fasting-induced steatosis in mice. Am J Physiol Gastrointest Liver Physiol. 2016;311:G599–G609. doi: 10.1152/ajpgi.00418.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Xiong X, Tao R, DePinho RA, Dong XC. The autophagy-related gene 14 (Atg14) is regulated by forkhead box O transcription factors and circadian rhythms and plays a critical role in hepatic autophagy and lipid metabolism. J Biol Chem. 2012;287:39107–39114. doi: 10.1074/jbc.M112.412569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Fukuo Y, Yamashina S, Sonoue H, Arakawa A, Nakadera E, Aoyama T, Uchiyama A, Kon K, Ikejima K, Watanabe S. Abnormality of autophagic function and cathepsin expression in the liver from patients with non-alcoholic fatty liver disease. Hepatol Res. 2014;44:1026–1036. doi: 10.1111/hepr.12282. [DOI] [PubMed] [Google Scholar]
- 220.Kashima J, Shintani-Ishida K, Nakajima M, Maeda H, Unuma K, Uchiyama Y, Yoshida K. Immunohistochemical study of the autophagy marker microtubule-associated protein 1 light chain 3 in normal and steatotic human livers. Hepatol Res. 2014;44:779–787. doi: 10.1111/hepr.12183. [DOI] [PubMed] [Google Scholar]
- 221.Gonzalez-Rodriguez A, Mayoral R, Agra N, Valdecantos MP, Pardo V, Miquilena-Colina ME, Vargas-Castrillon J, Lo IO, Corazzari M, Fimia GM, Piacentini M, Muntane J, Bosca L, Garcia-Monzon C, Martin-Sanz P, Valverde AM. Impaired autophagic flux is associated with increased endoplasmic reticulum stress during the development of NAFLD. Cell Death Dis. 2014;5:e1179. doi: 10.1038/cddis.2014.162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Yang L, Li P, Fu S, Calay ES, Hotamisligil GS. Defective hepatic autophagy in obesity promotes ER stress and causes insulin resistance. Cell Metab. 2010;11:467–478. doi: 10.1016/j.cmet.2010.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Liu HY, Han J, Cao SY, Hong T, Zhuo D, Shi J, Liu Z, Cao W. Hepatic autophagy is suppressed in the presence of insulin resistance and hyperinsulinemia: inhibition of FoxO1-dependent expression of key autophagy genes by insulin. J Biol Chem. 2009;284:31484–31492. doi: 10.1074/jbc.M109.033936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Czaja MJ. Functions of autophagy in hepatic and pancreatic physiology and disease. Gastroenterology. 2011;140:1895–1908. doi: 10.1053/j.gastro.2011.04.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Koga H, Kaushik S, Cuervo AM. Altered lipid content inhibits autophagic vesicular fusion. FASEB J. 2010;24:3052–3065. doi: 10.1096/fj.09-144519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Park HW, Park H, Semple IA, Jang I, Ro SH, Kim M, Cazares VA, Stuenkel EL, Kim JJ, Kim JS, Lee JH. Pharmacological correction of obesity-induced autophagy arrest using calcium channel blockers. Nat Commun. 2014;5:4834. doi: 10.1038/ncomms5834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Zhang W, Hou J, Wang X, Jiang R, Yin Y, Ji J, Deng L, Huang X, Wang K, Sun B. PTPRO-mediated autophagy prevents hepatosteatosis and tumorigenesis. Oncotarget. 2015;6:9420–9433. doi: 10.18632/oncotarget.3353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Kim J, Kundu M, Viollet B, Guan KL. AMPK and mTOR regulate autophagy through direct phosphorylation of Ulk1. Nat Cell Biol. 2011;13:132–141. doi: 10.1038/ncb2152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Hardie DG. AMPK and autophagy get connected. EMBO J. 2011;30:634–635. doi: 10.1038/emboj.2011.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Muse ED, Obici S, Bhanot S, Monia BP, McKay RA, Rajala MW, Scherer PE, Rossetti L. Role of resistin in diet-induced hepatic insulin resistance. J Clin Invest. 2004;114:232–239. doi: 10.1172/JCI200421270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Yu X, McCorkle S, Wang M, Lee Y, Li J, Saha AK, Unger RH, Ruderman NB. Leptinomimetic effects of the AMP kinase activator AICAR in leptin-resistant rats: prevention of diabetes and ectopic lipid deposition. Diabetologia. 2004;47:2012–2021. doi: 10.1007/s00125-004-1570-9. [DOI] [PubMed] [Google Scholar]
- 232.Singh R. Autophagy and regulation of lipid metabolism. Results Probl Cell Differ. 2010;52:35–46. doi: 10.1007/978-3-642-14426-4_4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Kim KH, Jeong YT, Oh H, Kim SH, Cho JM, Kim YN, Kim SS, Kim DH, Hur KY, Kim HK, Ko T, Han J, Kim HL, Kim J, Back SH, Komatsu M, Chen H, Chan DC, Konishi M, Itoh N, Choi CS, Lee MS. Autophagy deficiency leads to protection from obesity and insulin resistance by inducing Fgf21 as a mitokine. Nat Med. 2013;19:83–92. doi: 10.1038/nm.3014. [DOI] [PubMed] [Google Scholar]
- 234.Wang Y, Singh R, Xiang Y, Czaja MJ. Macroautophagy and chaperone-mediated autophagy are required for hepatocyte resistance to oxidant stress. Hepatology. 2010;52:266–277. doi: 10.1002/hep.23645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Lemasters JJ. Selective mitochondrial autophagy, or mitophagy, as a targeted defense against oxidative stress, mitochondrial dysfunction, and aging. Rejuvenation Res. 2005;8:3–5. doi: 10.1089/rej.2005.8.3. [DOI] [PubMed] [Google Scholar]
- 236.Levine B, Mizushima N, Virgin HW. Autophagy in immunity and inflammation. Nature. 2011;469:323–335. doi: 10.1038/nature09782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Fukushima H, Yamashina S, Arakawa A, Taniguchi G, Aoyama T, Uchiyama A, Kon K, Ikejima K, Watanabe S. Formation of p62-positive inclusion body is associated with macrophage polarization in non-alcoholic fatty liver disease. Hepatol Res. 2018;48:757–767. doi: 10.1111/hepr.13071. [DOI] [PubMed] [Google Scholar]
- 238.Schneider JL, Suh Y, Cuervo AM. Deficient chaperone-mediated autophagy in liver leads to metabolic dysregulation. Cell Metab. 2014;20:417–432. doi: 10.1016/j.cmet.2014.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Kaushik S, Cuervo AM. Degradation of lipid droplet-associated proteins by chaperone-mediated autophagy facilitates lipolysis. Nat Cell Biol. 2015;17:759–770. doi: 10.1038/ncb3166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Carr RM, Dhir R, Mahadev K, Comerford M, Chalasani NP, Ahima RS. Perilipin staining distinguishes between steatosis and nonalcoholic steatohepatitis in adults and children. Clin Gastroenterol Hepatol. 2017;15:145–147. doi: 10.1016/j.cgh.2016.08.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Imai Y, Varela GM, Jackson MB, Graham MJ, Crooke RM, Ahima RS. Reduction of hepatosteatosis and lipid levels by an adipose differentiation-related protein antisense oligonucleotide. Gastroenterology. 2007;132:1947–1954. doi: 10.1053/j.gastro.2007.02.046. [DOI] [PubMed] [Google Scholar]
- 242.Carr RM, Patel RT, Rao V, Dhir R, Graham MJ, Crooke RM, Ahima RS. Reduction of TIP47 improves hepatic steatosis and glucose homeostasis in mice. Am J Physiol Regul Integr Comp Physiol. 2012;302:R996–R1003. doi: 10.1152/ajpregu.00177.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Libby AE, Bales E, Orlicky DJ, McManaman JL. Perilipin-2 deletion impairs hepatic lipid accumulation by interfering with sterol regulatory element-binding protein (SREBP) activation and altering the hepatic lipidome. J Biol Chem. 2016;291:24231–24246. doi: 10.1074/jbc.M116.759795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Angulo P, Keach JC, Batts KP, Lindor KD. Independent predictors of liver fibrosis in patients with nonalcoholic steatohepatitis. Hepatology. 1999;30:1356–1362. doi: 10.1002/hep.510300604. [DOI] [PubMed] [Google Scholar]
- 245.Wong VW, Wong GL, Choi PC, Chan AW, Li MK, Chan HY, Chim AM, Yu J, Sung JJ, Chan HL. Disease progression of non-alcoholic fatty liver disease: a prospective study with paired liver biopsies at 3 years. Gut. 2010;59:969–974. doi: 10.1136/gut.2009.205088. [DOI] [PubMed] [Google Scholar]
- 246.Angulo P, Kleiner DE, Dam-Larsen S, Adams LA, Bjornsson ES, Charatcharoenwitthaya P, Mills PR, Keach JC, Lafferty HD, Stahler A, Haflidadottir S, Bendtsen F. Liver fibrosis, but no other histologic features, is associated with long-term outcomes of patients with nonalcoholic fatty liver disease. Gastroenterology. 2015;149:389–397. doi: 10.1053/j.gastro.2015.04.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Sanchez-Valle V, Chavez-Tapia NC, Uribe M, Mendez-Sanchez N. Role of oxidative stress and molecular changes in liver fibrosis: a review. Curr Med Chem. 2012;19:4850–4860. doi: 10.2174/092986712803341520. [DOI] [PubMed] [Google Scholar]
- 248.Friedman SL. Cytokines and fibrogenesis. Semin Liver Dis. 1999;19:129–140. doi: 10.1055/s-2007-1007105. [DOI] [PubMed] [Google Scholar]
- 249.Friedman SL. Hepatic stellate cells: protean, multifunctional, and enigmatic cells of the liver. Physiol Rev. 2008;88:125–172. doi: 10.1152/physrev.00013.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Fabregat I, Moreno-Caceres J, Sanchez A, Dooley S, Dewidar B, Giannelli G, Ten DP. TGF-beta signalling and liver disease. FEBS J. 2016;283:2219–2232. doi: 10.1111/febs.13665. [DOI] [PubMed] [Google Scholar]
- 251.Sancho P, Mainez J, Crosas-Molist E, Roncero C, Fernandez-Rodriguez CM, Pinedo F, Huber H, Eferl R, Mikulits W, Fabregat I. NADPH oxidase NOX4 mediates stellate cell activation and hepatocyte cell death during liver fibrosis development. PLoS One. 2012;7:e45285. doi: 10.1371/journal.pone.0045285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Liu Y, Liu H, Meyer C, Li J, Nadalin S, Konigsrainer A, Weng H, Dooley S, Ten DP. Transforming growth factor-β (TGF-β)-mediated connective tissue growth factor (CTGF) expression in hepatic stellate cells requires Stat3 signaling activation. J Biol Chem. 2013;288:30708–30719. doi: 10.1074/jbc.M113.478685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Paradis V, Perlemuter G, Bonvoust F, Dargere D, Parfait B, Vidaud M, Conti M, Huet S, Ba N, Buffet C, Bedossa P. High glucose and hyperinsulinemia stimulate connective tissue growth factor expression: a potential mechanism involved in progression to fibrosis in nonalcoholic steatohepatitis. Hepatology. 2001;34:738–744. doi: 10.1053/jhep.2001.28055. [DOI] [PubMed] [Google Scholar]
- 254.Li L, Wang JY, Yang CQ, Jiang W. Effect of RhoA on transforming growth factor β1-induced rat hepatic stellate cell migration. Liver Int. 2012;32:1093–1102. doi: 10.1111/j.1478-3231.2012.02809.x. [DOI] [PubMed] [Google Scholar]
- 255.Shah R, Reyes-Gordillo K, Arellanes-Robledo J, Lechuga CG, Hernandez-Nazara Z, Cotty A, Rojkind M, Lakshman MR. TGF-β1 up-regulates the expression of PDGF-beta receptor mRNA and induces a delayed PI3K-, AKT-, and p70(S6K) -dependent proliferative response in activated hepatic stellate cells. Alcohol Clin Exp Res. 2013;37:1838–1848. doi: 10.1111/acer.12167. [DOI] [PubMed] [Google Scholar]
- 256.Galli A, Svegliati-Baroni G, Ceni E, Milani S, Ridolfi F, Salzano R, Tarocchi M, Grappone C, Pellegrini G, Benedetti A, Surrenti C, Casini A. Oxidative stress stimulates proliferation and invasiveness of hepatic stellate cells via a MMP2-mediated mechanism. Hepatology. 2005;41:1074–1084. doi: 10.1002/hep.20683. [DOI] [PubMed] [Google Scholar]
- 257.Svegliati Baroni G, D’Ambrosio L, Ferretti G, Casini A, Di SA, Salzano R, Ridolfi F, Saccomanno S, Jezequel AM, Benedetti A. Fibrogenic effect of oxidative stress on rat hepatic stellate cells. Hepatology. 1998;27:720–726. doi: 10.1002/hep.510270313. [DOI] [PubMed] [Google Scholar]
- 258.Casini A, Ceni E, Salzano R, Biondi P, Parola M, Galli A, Foschi M, Caligiuri A, Pinzani M, Surrenti C. Neutrophil-derived superoxide anion induces lipid peroxidation and stimulates collagen synthesis in human hepatic stellate cells: role of nitric oxide. Hepatology. 1997;25:361–367. doi: 10.1002/hep.510250218. [DOI] [PubMed] [Google Scholar]
- 259.Nieto N, Friedman SL, Cederbaum AI. Cytochrome P450 2E1-derived reactive oxygen species mediate paracrine stimulation of collagen I protein synthesis by hepatic stellate cells. J Biol Chem. 2002;277:9853–9864. doi: 10.1074/jbc.M110506200. [DOI] [PubMed] [Google Scholar]
- 260.Lee TF, Lin YL, Huang YT. Kaerophyllin inhibits hepatic stellate cell activation by apoptotic bodies from hepatocytes. Liver Int. 2011;31:618–629. doi: 10.1111/j.1478-3231.2011.02485.x. [DOI] [PubMed] [Google Scholar]
- 261.Canbay A, Feldstein AE, Higuchi H, Werneburg N, Grambihler A, Bronk SF, Gores GJ. Kupffer cell engulfment of apoptotic bodies stimulates death ligand and cytokine expression. Hepatology. 2003;38:1188–1198. doi: 10.1053/jhep.2003.50472. [DOI] [PubMed] [Google Scholar]
- 262.Guicciardi ME, Gores GJ. Apoptosis as a mechanism for liver disease progression. Semin Liver Dis. 2010;30:402–410. doi: 10.1055/s-0030-1267540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263.Zhan SS, Jiang JX, Wu J, Halsted C, Friedman SL, Zern MA, Torok NJ. Phagocytosis of apoptotic bodies by hepatic stellate cells induces NADPH oxidase and is associated with liver fibrosis in vivo. Hepatology. 2006;43:435–443. doi: 10.1002/hep.21093. [DOI] [PubMed] [Google Scholar]
- 264.Paik YH, Schwabe RF, Bataller R, Russo MP, Jobin C, Brenner DA. Toll-like receptor 4 mediates inflammatory signaling by bacterial lipopolysaccharide in human hepatic stellate cells. Hepatology. 2003;37:1043–1055. doi: 10.1053/jhep.2003.50182. [DOI] [PubMed] [Google Scholar]
- 265.Henderson NC, Iredale JP. Liver fibrosis: cellular mechanisms of progression and resolution. Clin Sci (Lond) 2007;112:265–280. doi: 10.1042/CS20060242. [DOI] [PubMed] [Google Scholar]
- 266.Friedman SL. Molecular regulation of hepatic fibrosis, an integrated cellular response to tissue injury. J Biol Chem. 2000;275:2247–2250. doi: 10.1074/jbc.275.4.2247. [DOI] [PubMed] [Google Scholar]
- 267.Pinzani M, Marra F, Carloni V. Signal transduction in hepatic stellate cells. Liver. 1998;18:2–13. doi: 10.1111/j.1600-0676.1998.tb00120.x. [DOI] [PubMed] [Google Scholar]
- 268.Marra F, Gentilini A, Pinzani M, Choudhury GG, Parola M, Herbst H, Dianzani MU, Laffi G, Abboud HE, Gentilini P. Phosphatidylinositol 3-kinase is required for platelet-derived growth factor’s actions on hepatic stellate cells. Gastroenterology. 1997;112:1297–1306. doi: 10.1016/S0016-5085(97)70144-6. [DOI] [PubMed] [Google Scholar]
- 269.Marra F, Romanelli RG, Giannini C, Failli P, Pastacaldi S, Arrighi MC, Pinzani M, Laffi G, Montalto P, Gentilini P. Monocyte chemotactic protein-1 as a chemoattractant for human hepatic stellate cells. Hepatology. 1999;29:140–148. doi: 10.1002/hep.510290107. [DOI] [PubMed] [Google Scholar]
- 270.Roeb E. Matrix metalloproteinases and liver fibrosis (translational aspects) Matrix Biol. 2018;68–69:463–473. doi: 10.1016/j.matbio.2017.12.012. [DOI] [PubMed] [Google Scholar]
- 271.Verdelho MM, Diehl AM. The hedgehog pathway in nonalcoholic fatty liver disease. Crit Rev Biochem Mol Biol. 2018;53:264–278. doi: 10.1080/10409238.2018.1448752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 272.Verdelho MM, Diehl AM. Role of hedgehog signaling pathway in NASH. Int J Mol Sci. 2016;17:857. doi: 10.3390/ijms17060857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 273.Guy CD, Suzuki A, Zdanowicz M, et al. Hedgehog pathway activation parallels histologic severity of injury and fibrosis in human nonalcoholic fatty liver disease. Hepatology. 2012;55:1711–1721. doi: 10.1002/hep.25559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 274.Dugum M, Hanouneh I, McIntyre T, et al. Sonic hedgehog signaling in hepatocellular carcinoma: a pilot study. Mol Clin Oncol. 2016;4:369–374. doi: 10.3892/mco.2016.728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 275.Al-Bahrani R, Nagamori S, Leng R, Petryk A, Sergi C. Differential expression of sonic hedgehog protein in human hepatocellular carcinoma and intrahepatic cholangiocarcinoma. Pathol Oncol Res. 2015;21:901–908. doi: 10.1007/s12253-015-9918-7. [DOI] [PubMed] [Google Scholar]
- 276.Sicklick JK, Li YX, Melhem A, et al. Hedgehog signaling maintains resident hepatic progenitors throughout life. Am J Physiol Gastrointest Liver Physiol. 2006;290:G859–G870. doi: 10.1152/ajpgi.00456.2005. [DOI] [PubMed] [Google Scholar]
- 277.Kakisaka K, Cazanave SC, Werneburg NW, et al. A hedgehog survival pathway in ‘undead’ lipotoxic hepatocytes. J Hepatol. 2012;57:844–851. doi: 10.1152/ajpgi.00456.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 278.Sommerfeld A, Reinehr R, Haussinger D. Free fatty acids shift insulin-induced hepatocyte proliferation towards CD95-dependent apoptosis. J Biol Chem. 2015;290:4398–4409. doi: 10.1074/jbc.M114.617035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 279.Syn WK, Jung Y, Omenetti A, et al. Hedgehog-mediated epithelial-to-mesenchymal transition and fibrogenic repair in nonalcoholic fatty liver disease. Gastroenterology. 2009;137:1478–1488. doi: 10.1053/j.gastro.2009.06.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 280.Jung Y, Witek RP, Syn WK, et al. Signals from dying hepatocytes trigger growth of liver progenitors. Gut. 2010;59:655–665. doi: 10.1136/gut.2009.204354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 281.Cai H, Li H, Li J, et al. Sonic hedgehog signaling pathway mediates development of hepatocellular carcinoma. Tumour Biol. 2016;37:16199–16205. doi: 10.1007/s13277-016-5463-6. [DOI] [PubMed] [Google Scholar]
- 282.Zhang DW, Li HY, Lau WY, et al. Gli2 silencing enhances TRAIL-induced apoptosis and reduces tumor growth in human hepatoma cells in vivo. Cancer Biol Ther. 2014;15:1667–1676. doi: 10.4161/15384047.2014.972286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 283.Chen JS, Li HS, Huang JQ, et al. Down-regulation of Gli-1 inhibits hepatocellular carcinoma cell migration and invasion. Mol Cell Biochem. 2014;393:283–291. doi: 10.1007/s11010-014-2071-x. [DOI] [PubMed] [Google Scholar]