Abstract
The Hippo tumor suppressor pathway, which is well conserved from Drosophila to humans, has emerged as the master regulator of organ size, as well as major cellular properties, such as cell proliferation, survival, stemness, and tissue homeostasis. The biological significance and deregulation of the Hippo pathway in tumorigenesis have received a surge of interest in the past decade. In the current review, we present the major discoveries that made substantial contributions to our understanding of the Hippo pathway and discuss how Hippo pathway components contribute to cellular signaling, physiology, and their potential implications in anticancer therapeutics.
Keywords: Hippo pathway, YAP/TAZ, TEAD, Cancer, Therapeutic target
Overview of the Hippo pathway
The core Hippo components in Drosophila
Since it was first established as a genetic model by Thomas Hunt Morgan, Drosophila melanogaster has made a large contribution to our understanding of metazoan development [1]. The genetic mosaic screening of Drosophila using tissue-specific loss-of-function assays by the flippase/FRT system is a powerful tool to investigate genetic pathways [2]. Surprisingly, this led to the discovery of almost all components of the Hippo tumor suppressor pathway in experiments designed to identify growth suppressors. Gene inactivation in this pathway resulted in massive tissue overgrowth in the developing eyes or wings. In 1995, two studies discovered that homozygous loss of Warts (Wts), which encodes a nuclear Dbf-2-related (NDR) family Ser/Thr kinase, caused large outgrowth of multiple tissues in Drosophila [3, 4]. These initial findings indicated that Wts regulates tissue growth and functions as a tumor suppressor gene. Following the discovery of Warts, Hariharan, Halder, and collaborators reported that Salvador (Sav; also known as Shar-Pei), a WW domain-containing protein, genetically and physically interacts with Wts and that a Sav mutant clone showed excess interommatidial cells in Drosophila eye development via elevated cyclin E-induced cell cycle progression [5, 6]. Moreover, Sav-deficient cells induced DIAP1 (Drosophila inhibitor of apoptosis protein 1), which attenuates apoptosis by inhibiting caspase, resulting in cell proliferation and organ growth. These results suggested that Wts–Sav restrain massive cell growth during development and may have critical roles in tumor suppression.
In 2003, five independent groups identified Hippo (Hpo) from genetic mosaic screens for mutant clones that show excess overgrowth phenotypes in Drosophila [7–11]. Interestingly, all five reports noticed that the phenotypic characteristics of Hpo mutants were identical to those of Wts or Sav loss-of-function mutants. Hpo deletion increased the number of interommatidial cells in Drosophila eye disc via robust cyclin E [12–14] and DIAP [15] expression. In contrast, overexpression of Hpo showed a loss of eye, head, and wing tissue phenotype during imaginal disc development through cell cycle arrest and apoptosis. Collectively, these findings indicated that Hpo, Sav, and Wts control cell cycle exit and apoptosis in Drosophila, and thus function as negative growth regulators, identifying Salvador/Warts/Hippo (SWH) signaling as a tumor suppressor pathway [16]. Another core component, Mats (Mob as tumor suppressor), was later identified as a Wts-interacting partner that enhances Wts kinase activity [17]. Loss of Mats also results in an excessive overgrowth phenotype similar to Hpo or Wts mutation in Drosophila.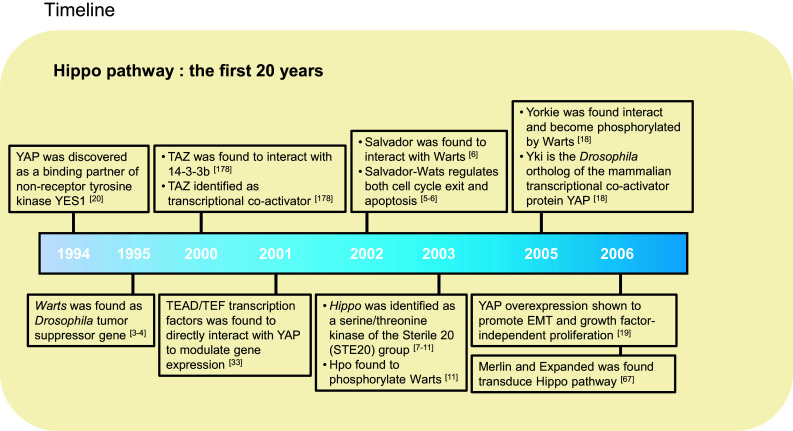
Notably, in 2005, Pan’s group discovered Yorkie (Yki), a transcriptional co-activator that acts as a Wts-binding partner [18]. Overexpression of Yki recapitulates the tissue overgrowth phenotype similar to Sav, Wts, or Hpo mutants, while Yki mutants suppress tissue proliferation. Moreover, Yki mutants combined with Hpo, Sav, or Wts mutants did not show any phenotypic difference when compared with the Yki mutation alone. These results suggested that Yki is genetically epistatic to Hpo, Wts, and Sav and that the Sav/Wts/Hpo complex possibly acts upstream of Yki. Two years later, Pan and colleagues reported that Wts phosphorylates Yki at serine 168 residue, which induces cytoplasmic retention of Yki via interaction with the 14-3-3 protein, thus providing the first mechanism of Yki regulation by Wts [19] (Fig. 1).
Fig. 1.
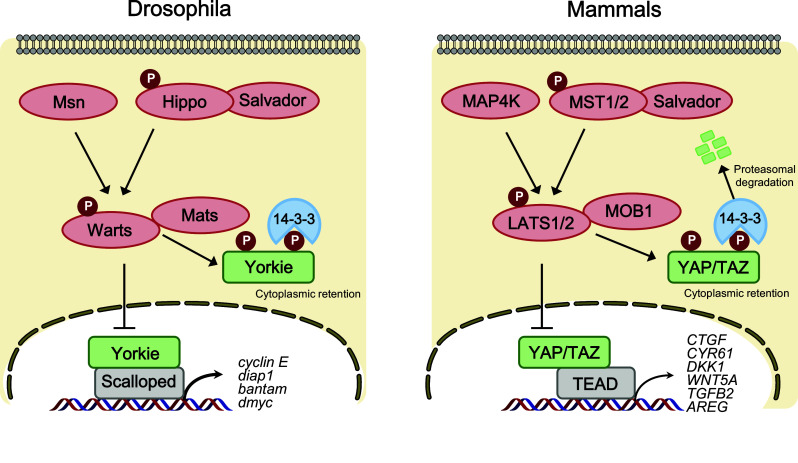
Core Hippo pathway components in Drosophila and mammals. In Drosophila (left), Hippo (Hpo) and Misshapen (Msn) kinase phosphorylate and activate Warts (Wts) kinase, which in turn inactivate the transcriptional co-activator Yorkie (Yki). Phosphorylation of Yki leads to cytoplasmic retention via a 14-3-3 interaction. Upon Hippo pathway inactivation, dephosphorylated Yki translocates to the nucleus and binds the transcription factor Scalloped (Sd) to induce gene expression involved in cell proliferation and anti-apoptosis. In mammals (right), the core Hippo pathway components are evolutionarily conserved. MST1/2 kinase phosphorylates LATS1/2, which in turn phosphorylates and inhibits YAP/TAZ. Phosphorylation of YAP/TAZ leads to cytoplasmic sequestration and proteasomal degradation
The Yki transcription co-activator lacks a DNA-binding domain, which prompted research to identify the transcription factors that mediate Yki target gene expression [18, 20]. In 2008, independent groups observed that non-phosphorylated Yki localizes in the nucleus and interacts with the Scalloped (Sd) transcription factor [21–24], which in turn induces Sd target genes. Yki–Sd-induced Bantam microRNA expression, which promotes the overgrowth phenotype, and overexpression of bantam partially rescued the growth defect phenotype, caused by the Yki loss-of-function mutation [25, 26]. Bantam also links the epidermal growth factor receptor (EGFR) and Hippo pathway-mediated tissue growth during development [27]. Yki also induces gene expression of the proto-oncoprotein and transcription factor dMyc [28, 29], as well as cell cycle regulator E2F [24]. In addition to Sd, Homothorax (Hth), and Teashirt (Tsh) are reported to interact with Yki, and mediate the transcriptional output of Hippo growth-regulatory pathway [30].
Collectively, this kinase cascade has been grouped into a new signal transduction called the “Hippo pathway”, which was named after the Hpo mutant Drosophila overgrowth head phenotype that resembles the folded skin around the neck of a hippopotamus.
The Hippo signaling pathway in mammals
The Hippo signaling pathway is evolutionarily well conserved from flies to mammals. Hence, most of the core components of the Drosophila Hippo pathway have mammalian homologs. These include mammalian STE20-like protein kinase 1/2 (MST1/2) (Hippo homolog, also known as STK4 and STK3), Salvador family WW domain-containing protein 1 (SAV1) (Salvador homolog), large tumor suppressor 1/2 (LATS1/2) (Warts homolog), MOB kinase activator 1A/B (MOB1A/B) (Mats homolog), YAP [20] and TAZ (Yorkie homologs), and TEA domain family members 1–4 (TEAD1–4) (Scalloped homolog). Similar to the Drosophila Hippo pathway, the mammalian Hippo pathway is also considered a tumor suppressor pathway that is mainly regulated by a phosphorylation-dependent protein kinase cascade. For example, upon receiving various upstream signals, activated MST1/2 phosphorylates and activates the downstream kinases LATS1/2, MOB1, and SAV1. MST1/2 interacts with SAV1, which enhances MST1/2 kinase activity to interact and phosphorylate LATS1/2. Activation of LATS1/2 subsequently phosphorylates and inhibits YAP, and its paralog TAZ. Therefore, the final physiological output of the Hippo kinase cascade is to restrict the transcriptional activity of YAP/TAZ and TEAD (Fig. 1).
Regulation of the Hippo pathway
YAP/TAZ regulation by the Hippo pathway
The core Hippo pathway consists of the cytoplasmic kinase modules MST1/2 and LATS1/2, which are serine–threonine kinases with tumor suppressive functions, and the nuclear transcriptional modules YAP/TAZ and TEAD, which harbor oncogenic functions. Upon Hippo pathway inactivation, YAP/TAZ translocate into the nucleus, which leads to enhanced gene transcription via interactions with TEAD1–4 transcription factors [21, 31–33]. In contrast, when YAP/TAZ is sequestered in the cytoplasm by LATS1/2-mediated phosphorylation, TEAD activity is suppressed by Vestigial-like 4 (VGLL4), which acts as a tumor suppressor, and the TEAD–VGLL4 interaction is dissociated by YAP/TAZ–TEAD activation [34–37]. In addition to TEAD, YAP/TAZ can interact with diverse transcription factors via their WW domain–PPXY motif, including FoxO1, ErbB4, TBX5, Pax, Smads, p73, and RUNX1/2 to regulate multiple aspects of tissue growth and cell differentiation [38]. Through interacting with TEAD and other transcription factors, YAP/TAZ promote the expression of target genes including CTGF, CYR61, WNT5A, TGFB2, NOTCH2, IL6, and AREG, which are involved in cell proliferation, anti-apoptosis, and tumorigenesis [21, 39–44].
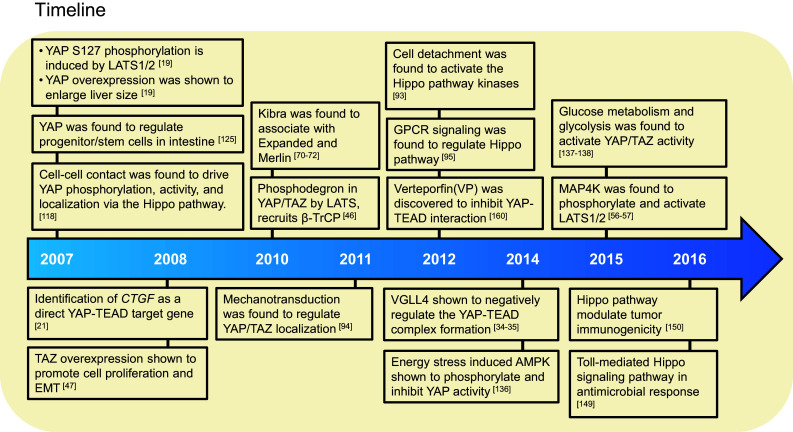
Regulation of protein stability by post-translational modifications is a common mechanism to alter transcriptional activity. In Drosophila, Leash E3 ubiquitin ligase induces Yki degradation via Hippo protein–protein interaction network [45]. In addition, Zhao and colleagues showed that Hippo signaling promotes YAP/TAZ cytoplasmic retention via YAP/TAZ phosphorylation and 14-3-3 interaction, followed by β-TrCP E3 ubiquitin ligase-mediated ubiquitination and proteasomal degradation [46–49]. YAP/TAZ degradation depends on LATS1/2-mediated direct phosphorylation of YAP/TAZ at multiple sites. YAP S127 phosphorylation by LATS1/2 is essential for YAP to associate with 14-3-3, while phosphorylation on YAP S381 triggers subsequent phosphorylation by Casein kinase 1δ/ε, thus creating a phospho-degron motif for β-TrCP, which leads to YAP/TAZ ubiquitination and degradation. Conversely, upon Hippo pathway inactivation, dephosphorylated YAP/TAZ translocates into the nucleus and interacts with the TEAD family transcription factors, to activate the transcription of genes encoding proteins involved in cell proliferation and tissue growth [46]. Consistently, mouse models with deletion of upstream Hippo components, MST1/2, NF2 (Merlin homolog), SAV1, MOB1A/B, or LATS1/2, all exhibit hyperactivated YAP-induced progenitor cell expansion and tumorigenesis [50]. Therefore, YAP/TAZ phosphorylation by the Hippo pathway is a critical process that restricts their transcriptional activity. In addition to phosphorylation and ubiquitination, multiple post-translational modifications such as acetylation, methylation, sumoylation, and glycosylation have been recently identified to regulate the Hippo pathway (for a review, see [51]).
Recent studies discovered that Tao Kinases 1–3 (TAOK1/2/3) and Misshapen (Msn) function similar to Hpo kinase and are responsible for Wts activation. The sterile 20 family kinase, TAO1/3, directly phosphorylates MST1/2 and LATS1/2 via NF2 and RHOA upstream signaling [52–54]. Msn is conserved in mammals as MAP4K4, and its activation induces phosphorylation of LATS1/2 [55], which in turn inhibits YAP/TAZ activity [56]. In addition, two recent studies revealed that multiple MAP4K family kinases, including MAP4K1/2/3/5 (Happyhour in Drosophila) and MAP4K4/6/7 (Msn in Drosophila), could directly phosphorylate and activate LATS1/2 [57, 58]. Thus, MST1/2 and MAP4Ks act in parallel to phosphorylate and activate LATS1/2, and loss of both MST1/2 and MAP4Ks can completely inhibit the Hippo pathway by turning off LATS1/2 hydrophobic motif phosphorylation and activation [55, 57, 58].
Regulation of the Hippo pathway by cell polarity and cell adhesion
Apical–basal polarity separates two complementary membrane domains, the adherens junctions, and tight junctions (adherens and septate junctions in Drosophila). Cell polarity is connected to diverse signaling pathways, and controls development and tissue growth [59]. Genetic studies showed that two protocadherins Fat (Ft) and Dachsous (Ds), both of which are highly involved in cell polarity and planar-cell polarity (PCP), regulate the Hippo–Yki pathway during Drosophila wing growth [60, 61]. The Drosophila Zyxin family gene, Zyx102 (Zyx), a downstream target of Ds, interacts with Wts and inhibits its kinase activity, linking Fat signaling to the Hippo pathway [62, 63]. Moreover, the Drosophila Crumbs (Crb) protein has been identified as a cell surface Hippo signaling regulator [64–67]. Crb interacts with FERM (4.1, Ezrin, Radxin, and Moesin) domain proteins, Merlin (Mer) and Expanded (Ex), which are homologs of NF2 and Willin/FRMD6 in mammals, respectively, and function as tumor suppressors by interacting with Tao-1 through Schip1, which activates the Hippo pathway [68, 69]. Crb also inhibits Yki by regulating the Ex level through ubiquitin-mediated degradation [70]. Kibra (WW and C2 domain-containing protein 1) interacts with Mer and Ex, which in turn activate Wts to suppress Yki activity [71–73]. Inactivation of Kibra by forming a complex with Par3/aPKC promotes cancer metastasis via Hippo inactivation [74]. Thus, the Mer/Ex/Kibra complex recruits the Hippo pathway kinases to the apical plasma membrane for activation.
In mammals, tight junction (TJ)-associated scaffold protein angiomotin (Amot) has emerged as a critical regulator of the Hippo pathway. Amot has both PY- and PDZ-binding motifs, which interact with diverse proteins involved in cell polarity and junction formation [75–77]. Zhao et al. reported that Amot family proteins directly interact with YAP/TAZ through PPXY motif and WW domain interactions [78]. Amot inhibits YAP/TAZ activity by promoting their cytoplasmic translocation [78–80]. In addition, Amot can bind both LATS1/2 and MST1/2 to promote LATS kinase activity [81], which in turn suppresses YAP/TAZ activity. Scribble, a polarity protein localized to the epithelial junctions, has been shown to physically interact with and inhibit TAZ. Upon EMT epithelial-to-mesenchymal transition (EMT), Scribble delocalization activates TAZ, which induces cancer stem cell traits [82]. Another tight junction cytoplasmic scaffolding protein, Zonula occludens-2 (ZO-2) interacts with the C-terminal PDZ domain of YAP/TAZ, and promotes YAP/TAZ nuclear translocation [83, 84] (Fig. 2).
Fig. 2.
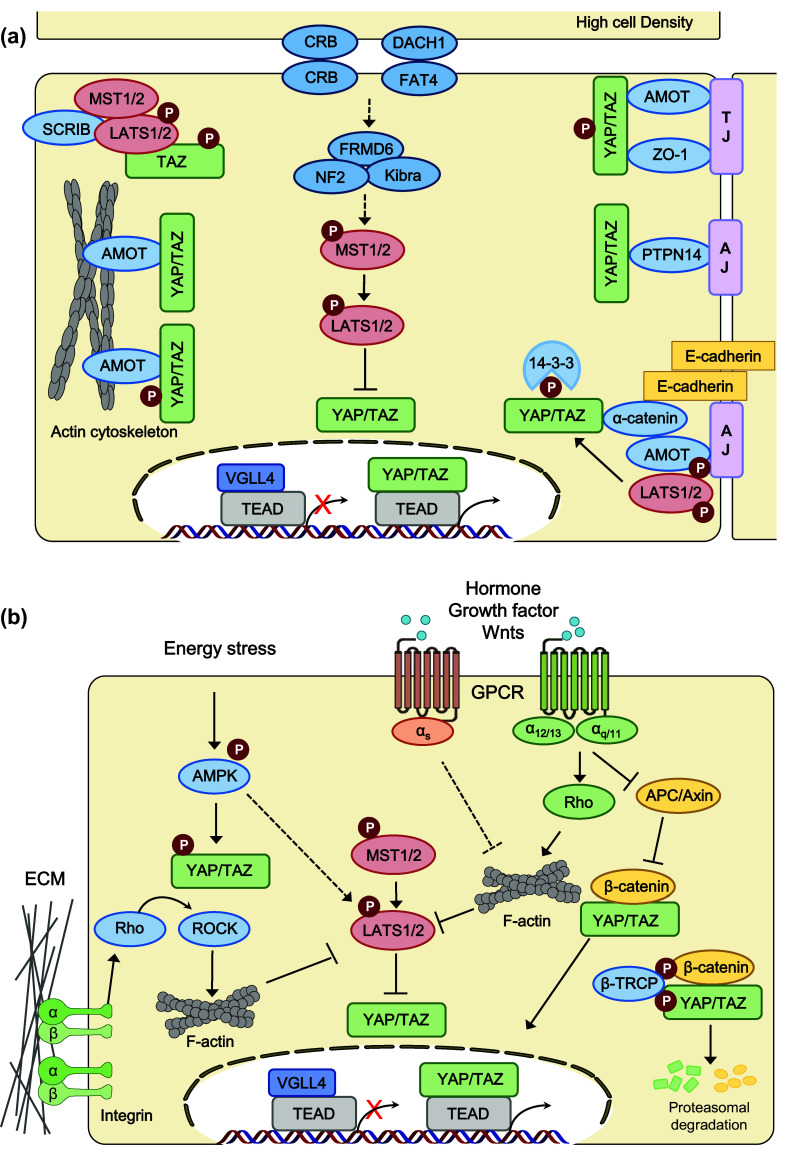
Regulators and regulations of the Hippo pathway. Hippo pathway components in mammals are shown in various colors. Pointed arrows indicate activation, and blunt-ended lines indicate inhibition. Hippo cascade kinases are shown in red and inhibitory regulators of YAP/TAZ activity are shown in blue. a Hippo pathway is regulated by cell polarity (Crumbs, DACH1–FAT4) and cell–cell junctions (adherens junction, tight junction). AMOT angiomotin, AJ adherens junction, CRB Crumbs homolog, DACH1 Dachous-1, FRMD6 FERM domain-containing protein 6, LATS large tumor suppressor homolog, MST mammalian STE20-like protein kinase, NF2 neurofibromin 2 (also known as Merlin), PTPN14 protein tyrosine phosphatase, non-receptor type 14, SCRIB scribbled planar-cell polarity protein, TAZ transcriptional co-activator with PDZ-binding motif, TJ tight junction, VGLL4 vestigial-like protein 4, YAP Yes-associated protein, ZO zona occludens protein. b Hippo pathway is regulated by extracellular ligands, stress responses, and mechanotransduction. AMPK 5′ AMP-activated protein kinase, APC adenomatous polyposis coli, β-TRCP β-transducin repeat-containing E3 ubiquitin protein ligase, ECM extracellular matrix, GPCR G protein-coupled receptor, Rho Ras homolog gene family, ROCK Rho-associated protein kinase
α-Catenin is a major component of adherens junctions (AJs), and acts as a linker for the cadherin complex and actin cytoskeleton [85]. α-Catenin functions as a tumor suppressor by negatively regulating YAP activity during epidermal stem cell proliferation and tissue expansion [86]. Consistently, Silvis et al. reported that deletion of α-catenin in the hair follicle stem cells led to skin cancer because of the constitutive nuclear localization of YAP [87]. In addition, the Hippo pathway is required for E-cadherin/catenin-dependent contact inhibition of proliferation via YAP inhibition [88]. Another AJ component, protein tyrosine phosphatase 14 (PTPN14), also directly interacts with YAP and contributes to YAP cytoplasmic retention [89–92] (Fig. 2). Therefore, the apical cell polarity complex, cadherin/catenin, and adherens junction proteins are important regulators of Hippo signaling to suppress cell proliferation and tumorigenesis.
Regulation of the Hippo pathway by extracellular ligands and cellular stress
The Rho family of GTPase are molecular switches that play an essential role in converting external cues into signal transduction pathways that regulate actin cytoskeleton polymerization, membrane transport, and cell migration [93]. Importantly, Rho GTPases, including RhoA, Rac1, and Cdc42, play pivotal roles in Hippo pathway regulation linking extracellular stimuli, such as membrane receptor signaling and mechanotransduction, to YAP/TAZ activity [94–96]. First, various G protein-coupled receptors (GPCRs) and their associated heterotrimeric G protein signaling regulate Hippo pathway activity via Rho GTPases. Serum-borne lysophosphatidic acid (LPA) and sphingosine 1-phosphate (S1P) act as ligands for the Gα12/13–coupled receptor, and its downstream RhoA signaling to inhibit LATS1/2 [96]. Thrombin, thromboxane A2, and Kaposi sarcoma-associated herpesvirus also activate YAP via Rho GTPases. Similarly, Gαq/11-coupled GPCR ligands such as endothelin-1 and estrogen can activate YAP/TAZ via Rho GTPases [97, 98]. Moreover, constitutively active YAP/TAZ resulting from Gαq/11 mutations is oncogenic drivers in patients with uveal melanoma [99, 100]. In contrast, upon stimulation of the Gαs-coupled receptor, activation of PKA by cAMP inhibits Rho GTPases, which in turn activates LATS kinase activity [99, 101, 102]. Rho GTPases link ligand stimuli to the Hippo pathway through F-actin formation. Both in Drosophila and mammalian cells, modulating actin polymerization by Rho GTPases and actin-capping proteins affect YAP/TAZ and Yorkie activities [103, 104]. However, how the actin cytoskeleton controls the core Hippo kinase activity requires further investigation.
Wnt signaling has been intensively studied as a YAP/TAZ upstream regulator. YAP/TAZ interacts with β-catenin, which is a key transcriptional co-activator in canonical Wnt signaling. Imajo et al. found that YAP inhibits Wnt signaling by sequestering β-catenin in the cytoplasm [105]. Piccolo and colleagues identified that YAP and TAZ are essential components of the β-catenin destruction complex [106, 107]. In addition, alternative Wnt signaling by Wnt ligands, including Wnt3 and Wnt5a/b, activates YAP/TAZ through the Gα12/13–Rho–LATS signaling pathway by interacting with the Frizzled/ROR receptors [40]. Amino acids regulate YAP/TAZ activity through the TSC–mTORC1 pathway. TSC1/2 deficiency potentiates YAP through mTORC1-mediated inhibition of autophagosome degradation [108]. YAP in turn activates mTORC1. YAP-induced miR-29 downregulates PTEN, which in turn activates the PI3K–mTOR pathway [109]. YAP/TAZ also promotes mTORC1 activation via a TEAD-induced LAT1 amino acid transporter under low nutrient conditions [110]. In Drosophila, positive crosstalk between Yki and insulin/Tor pathway also suggested coordinated regulation of these two oncogenic pathways in development and cancer [111].
MAPK, including JNK and p38, link stress responses to the Hippo pathway. During would healing and tumor growth, JNK increases Yki and YAP activity by promoting the binding of Ajuba family proteins to Warts and LATS [112]. When activated by UV irradiation, JNK and p38 MAPK phosphorylate and potentiate YAP [113, 114]. Moreover, p38 promotes TEAD cytoplasmic translocation and suppresses YAP-driven cancer cell growth upon various cellular stress signals [115]. NLK also phosphorylates YAP on Ser128 during osmotic stress and disrupts 14-3-3 interactions, thereby promoting nuclear accumulation of YAP [116, 117]. Stress caused by cytokinesis failure has been shown to activate LATS2, which in turn inactivates YAP/TAZ and stabilizes p53 [118]. Hippo pathway activation by cellular stresses is considered an important tumor suppression mechanism.
Mechanotransduction and the Hippo pathway
Organ and cell growth are constantly subjected to mechanical stresses, extra cellular matrix (ECM) stiffness, cell adhesion, and cell–matrix interaction. For instance, cell–cell contact at high density results in a growth inhibitory signal, which is an important cue for Hippo pathway activation [31, 32, 119]. Recently, YAP/TAZ has emerged as critical sensors of mechanotransduction, demonstrating that ECM stiffness, cell shape, or tension of cells controls YAP/TAZ activity and localization in the context of stem cell differentiation and tumorigenesis [95, 120, 121] (Fig. 2). Small adhesive areas or soft ECM conditions retain YAP/TAZ in the cytoplasm. Conversely, large adhesive areas or a stiffer ECM induces nuclear localization of YAP/TAZ and enhances TEAD transcriptional activity [95]. Wada et al. showed that stress fiber-dependent F-actin signaling suppresses YAP phosphorylation and induces YAP nuclear translocation [121]. Consistent findings showed that F-actin formation induced a strong overgrowth phenotype in Drosophila imaginal discs by inhibiting the Hippo pathway [104]. Aragona et al. showed that F-actin-capping/severing proteins Cofilin, CapZ, and Gelsolin inhibit YAP/TAZ transcriptional activity during cell contact inhibition by sensing mechanical forces [122]. Mechanical regulation promotes LIMD1–LATS1 binding through activation of c-Jun N-terminal kinase (JNK), which in turn suppresses LATS activity, leading to YAP transcriptional activity [123].
The Hippo pathway in cancer biology
The dramatic overgrowth observed upon Hippo pathway dysregulation has led to the investigation of its role in cancer development. Numerous reports indicate that hyperactivation of YAP/TAZ or TEAD confers proliferative advantage, promotes cell invasion and migration, enhances cancer stem cell traits, and elicits metastasis and drug resistance [124, 125]. One of the early studies was performed in mouse liver. Liver-specific induction of YAP, or depletion of upstream Hippo components, caused aberrant tissue expansion and a dramatic increase in liver size, which subsequently developed into liver tumors [19, 126]. Deletion of Mst1/2 or SAV1 in the mouse intestinal epithelium also resulted in crypt hyperplasia and tumorigenesis through hyperactivation of YAP [127–129]. Moreover, loss of the APC tumor suppressor in colon cancer led to YAP/TAZ activation [106, 130]. YAP also promotes resistance to anticancer drugs targeting RAF and MEK in tumor cells harboring BRAF, KRAS, and NRAS mutations, which is a major clinical challenge [131]. Indeed, the protein levels and nuclear localization of YAP/TAZ and TEAD are elevated in many human cancers, such as lung carcinoma, thyroid, ovarian, colorectal, prostate, pancreatic, esophageal, liver, and breast cancer [125, 132]. Higher YAP/TAZ protein levels positively correlate with poorly differentiated tumors and are associated with shorter patient overall survival [125]. In addition to mammals, the Hippo pathway in Drosophila also plays an important role in tumorigenesis. Nutritional cues activate salt-inducible kinase (SIK), which inhibits the Hippo pathway and activates Yorkie in Ras/Src-induced tumorigenesis [133]. In addition, Hippo pathway inhibition by mitochondrial dysfunction has been shown to drive non-autonomous tumor progression via JNK/Ras signaling [134]. Therefore, the conserved components and tumorigenic phenotypes in various genetic models, as well as in humans, underscore the role of the Hippo pathway in cancer biology.
The Hippo pathway in cancer metabolism
Cancer cells utilize different metabolism from that of normal tissues, because they require ample amount of cellular building blocks, such as glucose, amino acids, and fatty acids for their rapid and unrestricted proliferation. Importantly, Warburg showed that under aerobic conditions, cancer cells metabolize a significantly higher amount of glucose into lactate than normal tissues do a phenomenon called the Warburg effect [135]. In addition to altered glucose metabolism or glycolysis, cancer-associated metabolic changes in amino acid uptake, increased demand for nitrogen via glutamine, and the use of intermediate metabolites for biosynthesis are reported [136]. Recent studies highlighted the role of the Hippo pathway in several aspects of these altered metabolisms in cancer cells.
Glucose metabolism and the cellular energy level have emerged as critical upstream regulators of the Hippo pathway. The AMP-activated protein kinase (AMPK), a major energy sensor, directly phosphorylates angiomotin-like protein 1(AMOTL1) at Ser 793, which in turn stabilizes AMOTL1 to inhibit YAP activity via the Hippo pathway [137]. Two independent groups explored whether AMPK directly phosphorylates YAP on multiple residues, including S94, and should that these additional phosphorylations diminished the YAP–TEAD interaction and YAP-driven cancer cell growth [138, 139]. Moreover, YAP increases glucose uptake in cancer cells by increasing the transcription of mRNA encoding the GLUT3 transporter [139]. Phosphofructokinase (PFK1), which catalyzes the first rate-limiting step of glycolysis, interacts with TEAD in the nucleus to promote the YAP–TEAD interaction and transcription activity [140]. In Drosophila, liver kinase B1 (LKB1) directly activates AMPK, which further attenuates Yki activity [141]. In addition, SIKs sense nutrients such as glucose to activate Yki during tumorigenesis [133, 142]. Two studies also indicate that glucose stimulates O-GlcNAcylation of YAP via O-GlcNAC transferase (OGT) at residues S109 and T241, which is critical for high glucose-induced tumorigenesis [143, 144]. Collectively, these studies suggest that glucose metabolism is a critical regulator of YAP transcriptional activity in cancer cells.
The mevalonate pathway or the cholesterol synthesis pathway activates YAP/TAZ via Rho GTPases. Inhibition of this pathway by statins and bisphosphonates suppresses YAP nuclear localization and YAP-driven tumor growth [145, 146]. Reliance on Glutamine is considered as another hallmark of cancer cell metabolism. YAP can directly enhance glutamine synthase (GLUL) expression to elevate intracellular glutamine levels and stimulate nucleotide biosynthesis, which is required for liver growth and tumorigenesis [147]. Thus, further understanding of the role of the Hippo pathway in both normal and cancer cell metabolism will lead to novel therapeutic strategies to treat cancer.
The Hippo pathway in cancer immunity
Immunotherapy provides novel therapeutic approaches for clinical oncology, and recently, several studies have demonstrated the importance of Hippo signaling in host immune responses and the tumor microenvironment. YAP activation in tumor-initiating cells (TICs) recruits M2 macrophages via TEAD-dependent Ccl2 and Csf1 expression, which protects the TIC from immune clearance, and thus enhances tumor growth [148]. By contrast, YAP activation in cancer cells promotes myeloid-derived suppressor cell (MDSC) recruitment by inducing TEAD-dependent CXCL5 expression, thus suppressing tumor progression [149]. Further investigation may reveal how YAP-induced secreted ligands trigger context-dependent effects in the tumor microenvironment and immune cell infiltration. Similarly, YAP activation by LATS1/2 deletion in cancer cells triggers an anti-tumor immune response via activation of the TLR–MYD88/TRIF pathway by secreting nucleic-acid-rich extracellular vesicles (EVs) [150, 151]. Recent studies indicate that these nucleic-acid-rich EVs might also trigger an antiviral immune response in neighboring cells via the Hippo pathway. Independent studies demonstrate that MST1 and YAP/TAZ are critical regulators of the cytosolic RNA/DNA, sensing immune response by inhibiting TBK1 activity [152, 153]. Notably, YAP/TAZ has recently been shown to directly regulate PD-L1 expression in lung cancer cells. In EGFR–TKI-resistant lung cancer cells, YAP induces the transcription of PD-L1 [154]. In addition, TAZ activation is responsible for lactate-rich tumor microenvironment-induced PD-L1 expression in lung cancer cells, which led to apoptosis of T cells in vitro [155]. Therefore, the Hippo pathway may provide new avenues for the development of immunotherapy targeting the PD1/PD-L1 pathway.
Next, in immune cells, the Hippo pathway plays a role in tumor immunity. Liu et al. reported that Gram-positive bacteria activate Hippo–Yki signaling through a Toll–Myd88–Pelle/Cha cascade in the Drosophila immune organ [150]. Several reports demonstrate that the Hippo pathway plays pivotal roles in T-cell differentiation and inflammation. Activation of CD8+ T cells induces the expression of core Hippo pathway components, including YAP and TEAD, through CTLA-4, which regulates T-cell terminal differentiation [156]. The Hippo pathway is also involved in TH17 cell differentiation, which plays an important role in cancer immunity. Geng et al. showed that the TAZ/RORγt transcription complex is required for TH17 differentiation and TH17 cell-mediated inflammatory diseases, whereas the TAZ/TEAD complex promotes Treg-cell differentiation [157]. The core Hippo kinase Mst1-null mice exhibit a low number of mature T cells by inhibiting naïve T-cell proliferation [158]. MST1 in dendritic cells inhibits TH17-cell differentiation and regulates autoimmune diseases via p38 MAPK signaling [159]. Therefore, the status of the Hippo pathway may be a critical factor in both innate and adaptive immune responses in the tumor microenvironment. Interestingly, unique to the hematopoietic system, YAP/TAZ expression is restricted to the hematopoietic stem cell (HSC) fraction, whereas upstream Hippo components are expressed at high levels across all fractions, including mature and lineage positive cells [160]. Further investigation may delineate the critical functions of YAP/TAZ and the Hippo pathway in immune cells and their role in cancer immunity.
Therapeutics targeting the Hippo pathway
The core Hippo pathway functions as a tumor suppressor pathway by inhibiting YAP/TAZ-dependent tumor growth. Therefore, current therapeutic approaches are mostly focused on inactivating YAP/TAZ oncogenic activity, but also negative upstream regulators of the pathway. Here, we focus on small molecules that are designed to target Hippo pathway components themselves, as well as various upstream regulators.
Targeting protein–protein interactions (PPIs)
Recent studies demonstrated that disrupting the formation of the YAP/TAZ–TEAD complex might be a potential strategy to develop anticancer therapeutics. In 2002, a screening of FDA-approved drugs by Pan and colleagues led to the identification of verteporfin (VP) and related porphyrin compounds as inhibitors of YAP–TEAD interactions [161] (Fig. 3). Verteporfin is a small molecule that inhibits YAP-induced tumor progression and suppresses liver overgrowth in mice, thereby demonstrating the therapeutic significance of interrupting YAP–TEAD interactions in cancer cells [99, 100, 161]. However, verteporfin has been reported to be toxic in non-malignant cells and has poor solubility, which may limit its specificity and bioavailability for cancer patients [162].
Fig. 3.
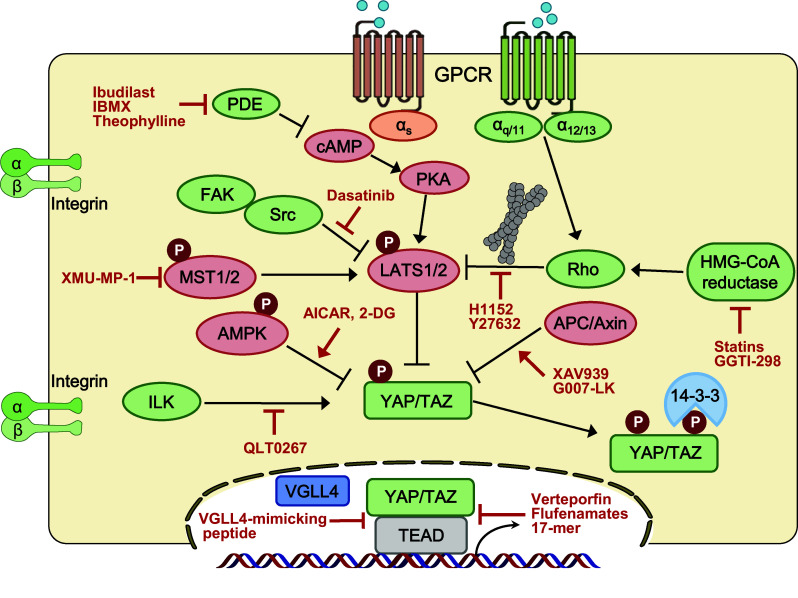
Putative targets for therapeutic intervention in the Hippo pathway. Summary of the positive and negative regulators of Hippo kinases and YAP/TAZ, respectively. Activating components’ upstream of YAP/TAZ is in green, and inhibitory components are in red
Vestigial-like family member 4 (VGLL4) is a transcriptional regulator that has two Tondu (TDU) domains. VGLL4 was initially reported to have interacted with TEAD1 and suppresses TEAD1-dependent α1 adrenergic activation in cardiac myocytes [163]. Recent reports identified VGLL4 as an antagonist of YAP/TAZ–TEAD transcriptional activity by directly interacting with TEADs via its TDU domains. VGLL4 is downregulated in gastric, lung, and colorectal cancer, and is considered as a potential tumor suppressor and prognostic marker [34, 35, 164]. In addition, based on the structure of the VGLL4–TEAD interacting domain, Jiao and colleagues developed a new therapeutic strategy against YAP-driven human cancers: a VGLL4-mimicking inhibitor peptide termed “Super-TDU,” which disrupts the YAP–TEAD interaction [35]. In addition, Jiao et al. reported that VGLL4 targets the TEAD4–TCF4 complex to interfere with the functional interplay between TEAD4 and TCF4 [155]. Similarly, cyclic YAP-like peptide (17mer) was designed to disrupt the YAP–TEAD interaction [165]. Flufenamic acid, a non-steroidal anti-inflammatory drug (NSAID), binds to the YAP-binding domain of TEADs and inhibits YAP–TEAD-dependent transcription and cell proliferation [166]. Interestingly, the structure of the TEAD–flufenamic complex showed that the flufenamic acid-contacting residue (C380 of TEAD2) is palmitoylated. Two studies indicated that palmitoylation of TEAD at evolutionarily conserved cysteine residues is required for TEAD stability and the YAP/TAZ interaction [167, 168]. Whether flufenamic acid affects TEAD palmitoylation requires further investigation. Mo and colleagues reported that AMP-activated protein kinase (AMPK) directly phosphorylates YAP at residue S94 and disrupts the YAP–TEAD interaction. 5-Aminoimidazole-4-carboxamide ribonucleotide (AICAR) is a cell-permeable activator of AMPK that inhibits YAP activity [138]. Src family kinase inhibitor, Dasatinib, inhibits the kinase activity of YES, which disrupts the YAP–β-catenin–TBX5 complex, and thus attenuates cancer progression in both cell lines and animal models [169].
Targeting upstream components of YAP/TAZ
The Hippo pathway receives a wide range of upstream signals that lead to the regulation of YAP/TAZ. Thus, the primary targets for small-molecule therapeutics are modulators of YAP/TAZ cellular localization and activity.
Recent reports underscore the metabolic regulation of YAP/TAZ activity by the mevalonate pathway and energy stress. Inhibitors of the mevalonate pathway, such as statins, HMG–CoA reductase inhibitors, as well as the geranylgeranyl transferase inhibitor, GGTI-298, suppress YAP/TAZ activity via Rho GTPase inhibition [145, 146, 170] (Fig. 3). In addition, energy stress conditions inhibit YAP activity through AMPK and LATS kinase activity. AMPK directly suppresses the YAP–TEAD interaction, as mentioned above; however, it also promotes YAP cytoplasmic retention by activating Hippo kinase LATS1/2 [138, 139]. In addition, AMPK phosphorylates AMOTL1, which then facilitates YAP phosphorylation by LATS [137]. Thus, targeting the components of metabolic signaling might efficiently regulate YAP/TAZ activity.
Next, targeting GPCRs and their downstream effectors are promising approaches to modulate YAP/TAZ activity. Recent studies indicated that YAP/TAZ is activated through Gα12/13 or Gαq/11-coupled GPCR ligands by Rho GTPases [40, 96, 171]. Hippo pathway activity is strongly affected by actin dynamics through Rho GTPases and their downstream effector ROCK. Thus, ROCK inhibitors, such as fasudil and Y27632, may be potent inhibitors of YAP/TAZ. In contrast, activation of Gαs-coupled GPCR signaling by glucagon and epinephrine inhibits YAP/TAZ activity [96, 101, 102]. Similarly, phosphodiesterase (PDE) inhibitors elevate the cAMP level, which lead to LATS1/2 activation and suppression of YAP/TAZ [101]. Membrane proximal components are also targets for therapeutic approaches. Dasatinib, a Src family inhibitor, inhibits focal adhesion kinase (FAK)–Src–PI3K signaling, which in turn suppress YAP by promoting cytoplasmic retention [172].
Tankyrase inhibitors target YAP/TAZ by Hippo-independent mechanisms. XAV939, which is a small-molecule inhibitor of Wnt/β-catenin signaling that acts through axin stabilization [173], not only decreases β-catenin stability, but also the TAZ protein level via a destruction complex [107]. In addition, a tankyrase inhibitor attenuated TEAD transcriptional activity by stabilizing angiomotin and sequestering YAP in the cytoplasm [174, 175]. QLT0267, a small-molecule inhibitor targeting integrin-linked kinase (ILK), reduces tumor growth by inhibiting YAP/TAZ [176]. In addition, dobutamine, a sympathomimetic drug that antagonizes the β-adrenergic receptor, prevents YAP nuclear accumulation and YAP-dependent gene transcription [177] (Fig. 3). The core Hippo kinases are poorly druggable anticancer targets, because very few small-molecule kinase activators are available. By contrast, inhibition of the Hippo pathway may be valuable for wound healing and tissue regeneration. XMU-MP-1 has been identified as an MST1/2 inhibitor, which encourages multiple tissue repair and regeneration through YAP activation [178].
Conclusion/perspective
Hippo signaling has emerged as a pivotal regulatory pathway in mammalian development and tissue growth. Recent advances in research on the deregulation of Hippo pathway have attracted significant attention from researchers interested in all the stages of tumorigenesis from EMT, metastasis, and drug resistance to those involved in cancer metabolism and immunity. Accumulating evidence suggests that perturbation of YAP/TAZ activity has profound effects on various cancer types. Notably, since driver mutations of the core components of the Hippo pathway are relatively rare, and development of small-molecule activators of the Hippo kinases is difficult, it is important to identify a wide range of upstream regulators, as well as downstream targets, of Hippo–YAP signaling. In addition, identifying crosstalk with other oncogenic signal transduction pathways might provide valuable targets to modulate the Hippo pathway activity. Therefore, understanding the cellular determinants of the Hippo pathway and its role in tumorigenesis and regeneration will be critical to classify cancers susceptible to YAP/TAZ modulation and to develop anticancer therapeutics.
Acknowledgements
We apologize to those colleagues, whose work has not been cited because of space limitations. This work was supported by a grant from the Korea Health Technology R&D Project through the Korea Health Industry Development Institute (KHIDI), funded by the Ministry of Health and Welfare, Republic of Korea (Grant Number: HI17C1560), and the National Research Foundation of Korea (NRF) Grant funded by the Korea government (2017R1A4A1015328 and 2018R1C1B6004301), and funded by the Yonsei University Future-leading Research Initiative of 2017 (2017-22-0071) to H.W.P. In addition, S.H.M and S.Y.P were supported by the Brain Korea (BK21) PLUS Program.
References
- 1.Gonzalez C. Drosophila melanogaster: a model and a tool to investigate malignancy and identify new therapeutics. Nat Rev Cancer. 2013;13(3):172–183. doi: 10.1038/nrc3461. [DOI] [PubMed] [Google Scholar]
- 2.St Johnston D. The art and design of genetic screens: drosophila melanogaster. Nat Rev Genet. 2002;3(3):176–188. doi: 10.1038/nrg751. [DOI] [PubMed] [Google Scholar]
- 3.Justice RW, Zilian O, Woods DF, Noll M, Bryant PJ. The Drosophila tumor suppressor gene warts encodes a homolog of human myotonic dystrophy kinase and is required for the control of cell shape and proliferation. Genes Dev. 1995;9(5):534–546. doi: 10.1101/gad.9.5.534. [DOI] [PubMed] [Google Scholar]
- 4.Xu T, Wang W, Zhang S, Stewart RA, Yu W. Identifying tumor suppressors in genetic mosaics: the Drosophila lats gene encodes a putative protein kinase. Development. 1995;121(4):1053–1063. doi: 10.1242/dev.121.4.1053. [DOI] [PubMed] [Google Scholar]
- 5.Tapon N, Harvey KF, Bell DW, Wahrer DC, Schiripo TA, Haber D, Hariharan IK. Salvador promotes both cell cycle exit and apoptosis in Drosophila and is mutated in human cancer cell lines. Cell. 2002;110(4):467–478. doi: 10.1016/S0092-8674(02)00824-3. [DOI] [PubMed] [Google Scholar]
- 6.Kango-Singh M, Nolo R, Tao C, Verstreken P, Hiesinger PR, Bellen HJ, Halder G. Shar-pei mediates cell proliferation arrest during imaginal disc growth in Drosophila. Development. 2002;129(24):5719–5730. doi: 10.1242/dev.00168. [DOI] [PubMed] [Google Scholar]
- 7.Harvey KF, Pfleger CM, Hariharan IK. The Drosophila Mst ortholog, hippo, restricts growth and cell proliferation and promotes apoptosis. Cell. 2003;114(4):457–467. doi: 10.1016/S0092-8674(03)00557-9. [DOI] [PubMed] [Google Scholar]
- 8.Jia J, Zhang W, Wang B, Trinko R, Jiang J. The Drosophila Ste20 family kinase dMST functions as a tumor suppressor by restricting cell proliferation and promoting apoptosis. Genes Dev. 2003;17(20):2514–2519. doi: 10.1101/gad.1134003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pantalacci S, Tapon N, Leopold P. The Salvador partner Hippo promotes apoptosis and cell-cycle exit in Drosophila. Nat Cell Biol. 2003;5(10):921–927. doi: 10.1038/ncb1051. [DOI] [PubMed] [Google Scholar]
- 10.Udan RS, Kango-Singh M, Nolo R, Tao C, Halder G. Hippo promotes proliferation arrest and apoptosis in the Salvador/Warts pathway. Nat Cell Biol. 2003;5(10):914–920. doi: 10.1038/ncb1050. [DOI] [PubMed] [Google Scholar]
- 11.Wu S, Huang J, Dong J, Pan D. Hippo encodes a Ste-20 family protein kinase that restricts cell proliferation and promotes apoptosis in conjunction with salvador and warts. Cell. 2003;114(4):445–456. doi: 10.1016/S0092-8674(03)00549-X. [DOI] [PubMed] [Google Scholar]
- 12.Richardson HE, O’Keefe LV, Reed SI, Saint R. A Drosophila G1-specific cyclin E homolog exhibits different modes of expression during embryogenesis. Development. 1993;119(3):673–690. doi: 10.1242/dev.119.3.673. [DOI] [PubMed] [Google Scholar]
- 13.Richardson H, O’Keefe LV, Marty T, Saint R. Ectopic cyclin E expression induces premature entry into S phase and disrupts pattern formation in the Drosophila eye imaginal disc. Development. 1995;121(10):3371–3379. doi: 10.1242/dev.121.10.3371. [DOI] [PubMed] [Google Scholar]
- 14.Knoblich JA, Sauer K, Jones L, Richardson H, Saint R, Lehner CF. Cyclin E controls S phase progression and its down-regulation during Drosophila embryogenesis is required for the arrest of cell proliferation. Cell. 1994;77(1):107–120. doi: 10.1016/0092-8674(94)90239-9. [DOI] [PubMed] [Google Scholar]
- 15.Wang SL, Hawkins CJ, Yoo SJ, Muller HA, Hay BA. The Drosophila caspase inhibitor DIAP1 is essential for cell survival and is negatively regulated by HID. Cell. 1999;98(4):453–463. doi: 10.1016/S0092-8674(00)81974-1. [DOI] [PubMed] [Google Scholar]
- 16.Ryoo HD, Steller H. Hippo and its mission for growth control. Nat Cell Biol. 2003;5(10):853–855. doi: 10.1038/ncb1003-853. [DOI] [PubMed] [Google Scholar]
- 17.Lai ZC, Wei X, Shimizu T, Ramos E, Rohrbaugh M, Nikolaidis N, Ho LL, Li Y. Control of cell proliferation and apoptosis by mob as tumor suppressor, mats. Cell. 2005;120(5):675–685. doi: 10.1016/j.cell.2004.12.036. [DOI] [PubMed] [Google Scholar]
- 18.Huang J, Wu S, Barrera J, Matthews K, Pan D. The Hippo signaling pathway coordinately regulates cell proliferation and apoptosis by inactivating Yorkie, the Drosophila Homolog of YAP. Cell. 2005;122(3):421–434. doi: 10.1016/j.cell.2005.06.007. [DOI] [PubMed] [Google Scholar]
- 19.Dong J, Feldmann G, Huang J, Wu S, Zhang N, Comerford SA, Gayyed MF, Anders RA, Maitra A, Pan D. Elucidation of a universal size-control mechanism in Drosophila and mammals. Cell. 2007;130(6):1120–1133. doi: 10.1016/j.cell.2007.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sudol M. Yes-associated protein (YAP65) is a proline-rich phosphoprotein that binds to the SH3 domain of the Yes proto-oncogene product. Oncogene. 1994;9(8):2145–2152. [PubMed] [Google Scholar]
- 21.Zhao B, Ye X, Yu J, Li L, Li W, Li S, Yu J, Lin JD, Wang CY, Chinnaiyan AM, Lai ZC, Guan KL. TEAD mediates YAP-dependent gene induction and growth control. Genes Dev. 2008;22(14):1962–1971. doi: 10.1101/gad.1664408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhang L, Ren F, Zhang Q, Chen Y, Wang B, Jiang J. The TEAD/TEF family of transcription factor Scalloped mediates Hippo signaling in organ size control. Dev Cell. 2008;14(3):377–387. doi: 10.1016/j.devcel.2008.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wu S, Liu Y, Zheng Y, Dong J, Pan D. The TEAD/TEF family protein Scalloped mediates transcriptional output of the Hippo growth-regulatory pathway. Dev Cell. 2008;14(3):388–398. doi: 10.1016/j.devcel.2008.01.007. [DOI] [PubMed] [Google Scholar]
- 24.Goulev Y, Fauny JD, Gonzalez-Marti B, Flagiello D, Silber J, Zider A. SCALLOPED interacts with YORKIE, the nuclear effector of the hippo tumor-suppressor pathway in Drosophila. Curr Biol. 2008;18(6):435–441. doi: 10.1016/j.cub.2008.02.034. [DOI] [PubMed] [Google Scholar]
- 25.Thompson BJ, Cohen SM. The Hippo pathway regulates the bantam microRNA to control cell proliferation and apoptosis in Drosophila. Cell. 2006;126(4):767–774. doi: 10.1016/j.cell.2006.07.013. [DOI] [PubMed] [Google Scholar]
- 26.Nolo R, Morrison CM, Tao C, Zhang X, Halder G. The bantam microRNA is a target of the hippo tumor-suppressor pathway. Curr Biol. 2006;16(19):1895–1904. doi: 10.1016/j.cub.2006.08.057. [DOI] [PubMed] [Google Scholar]
- 27.Herranz H, Hong X, Cohen SM. Mutual repression by bantam miRNA and Capicua links the EGFR/MAPK and Hippo pathways in growth control. Curr Biol. 2012;22(8):651–657. doi: 10.1016/j.cub.2012.02.050. [DOI] [PubMed] [Google Scholar]
- 28.Neto-Silva RM, de Beco S, Johnston LA. Evidence for a growth-stabilizing regulatory feedback mechanism between Myc and Yorkie, the Drosophila homolog of Yap. Dev Cell. 2010;19(4):507–520. doi: 10.1016/j.devcel.2010.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ziosi M, Baena-Lopez LA, Grifoni D, Froldi F, Pession A, Garoia F, Trotta V, Bellosta P, Cavicchi S, Pession A. dMyc functions downstream of Yorkie to promote the supercompetitive behavior of hippo pathway mutant cells. PLoS Genet. 2010;6(9):e1001140. doi: 10.1371/journal.pgen.1001140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Peng HW, Slattery M, Mann RS. Transcription factor choice in the Hippo signaling pathway: homothorax and yorkie regulation of the microRNA bantam in the progenitor domain of the Drosophila eye imaginal disc. Genes Dev. 2009;23(19):2307–2319. doi: 10.1101/gad.1820009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ota M, Sasaki H. Mammalian Tead proteins regulate cell proliferation and contact inhibition as transcriptional mediators of Hippo signaling. Development. 2008;135(24):4059–4069. doi: 10.1242/dev.027151. [DOI] [PubMed] [Google Scholar]
- 32.Nishioka N, Inoue K, Adachi K, Kiyonari H, Ota M, Ralston A, Yabuta N, Hirahara S, Stephenson RO, Ogonuki N, Makita R, Kurihara H, Morin-Kensicki EM, Nojima H, Rossant J, Nakao K, Niwa H, Sasaki H. The Hippo signaling pathway components Lats and Yap pattern Tead4 activity to distinguish mouse trophectoderm from inner cell mass. Dev Cell. 2009;16(3):398–410. doi: 10.1016/j.devcel.2009.02.003. [DOI] [PubMed] [Google Scholar]
- 33.Vassilev A, Kaneko KJ, Shu H, Zhao Y, DePamphilis ML. TEAD/TEF transcription factors utilize the activation domain of YAP65, a Src/Yes-associated protein localized in the cytoplasm. Genes Dev. 2001;15(10):1229–1241. doi: 10.1101/gad.888601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhang W, Gao Y, Li P, Shi Z, Guo T, Li F, Han X, Feng Y, Zheng C, Wang Z, Li F, Chen H, Zhou Z, Zhang L, Ji H. VGLL4 functions as a new tumor suppressor in lung cancer by negatively regulating the YAP–TEAD transcriptional complex. Cell Res. 2014;24(3):331–343. doi: 10.1038/cr.2014.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jiao S, Wang H, Shi Z, Dong A, Zhang W, Song X, He F, Wang Y, Zhang Z, Wang W, Wang X, Guo T, Li P, Zhao Y, Ji H, Zhang L, Zhou Z. A peptide mimicking VGLL4 function acts as a YAP antagonist therapy against gastric cancer. Cancer Cell. 2014;25(2):166–180. doi: 10.1016/j.ccr.2014.01.010. [DOI] [PubMed] [Google Scholar]
- 36.Koontz LM, Liu-Chittenden Y, Yin F, Zheng Y, Yu J, Huang B, Chen Q, Wu S, Pan D. The Hippo effector Yorkie controls normal tissue growth by antagonizing scalloped-mediated default repression. Dev Cell. 2013;25(4):388–401. doi: 10.1016/j.devcel.2013.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Guo T, Lu Y, Li P, Yin MX, Lv D, Zhang W, Wang H, Zhou Z, Ji H, Zhao Y, Zhang L. A novel partner of Scalloped regulates Hippo signaling via antagonizing Scalloped-Yorkie activity. Cell Res. 2013;23(10):1201–1214. doi: 10.1038/cr.2013.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Moroishi T, Hansen CG, Guan KL. The emerging roles of YAP and TAZ in cancer. Nat Rev Cancer. 2015;15(2):73–79. doi: 10.1038/nrc3876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lai D, Ho KC, Hao Y, Yang X. Taxol resistance in breast cancer cells is mediated by the hippo pathway component TAZ and its downstream transcriptional targets Cyr61 and CTGF. Cancer Res. 2011;71(7):2728–2738. doi: 10.1158/0008-5472.CAN-10-2711. [DOI] [PubMed] [Google Scholar]
- 40.Park HW, Kim YC, Yu B, Moroishi T, Mo JS, Plouffe SW, Meng Z, Lin KC, Yu FX, Alexander CM, Wang CY, Guan KL. Alternative Wnt Signaling Activates YAP/TAZ. Cell. 2015;162(4):780–794. doi: 10.1016/j.cell.2015.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Seo E, Kim WY, Hur J, Kim H, Nam SA, Choi A, Kim YM, Park SH, Chung C, Kim J, Min S, Myung SJ, Lim DS, Kim YK. The Hippo-Salvador signaling pathway regulates renal tubulointerstitial fibrosis. Sci Rep. 2016;6:31931. doi: 10.1038/srep31931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tschaharganeh DF, Chen X, Latzko P, Malz M, Gaida MM, Felix K, Ladu S, Singer S, Pinna F, Gretz N, Sticht C, Tomasi ML, Delogu S, Evert M, Fan B, Ribback S, Jiang LJ, Brozzetti S, Bergmann F, Dombrowski F, Schirmacher P, Calvisi DF, Breuhahn K. Yes-associated protein up-regulates jagged-1 and activates the NOTCH pathway in human hepatocellular carcinoma. Gastroenterology. 2013;144(7):1530-U1368. doi: 10.1053/j.gastro.2013.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kim T, Yang SJ, Hwang D, Song J, Kim M, Kyum Kim S, Kang K, Ahn J, Lee D, Kim MY, Kim S, Seung Koo J, Seok Koh S, Kim SY, Lim DS. A basal-like breast cancer-specific role for SRF-IL6 in YAP-induced cancer stemness. Nat Commun. 2015;6:10186. doi: 10.1038/ncomms10186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zhang J, Ji JY, Yu M, Overholtzer M, Smolen GA, Wang R, Brugge JS, Dyson NJ, Haber DA. YAP-dependent induction of amphiregulin identifies a non-cell-autonomous component of the Hippo pathway. Nat Cell Biol. 2009;11(12):1444–1450. doi: 10.1038/ncb1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kwon Y, Vinayagam A, Sun X, Dephoure N, Gygi SP, Hong P, Perrimon N. The Hippo signaling pathway interactome. Science. 2013;342(6159):737–740. doi: 10.1126/science.1243971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zhao B, Li L, Tumaneng K, Wang CY, Guan KL. A coordinated phosphorylation by Lats and CK1 regulates YAP stability through SCF(beta-TRCP) Genes Dev. 2010;24(1):72–85. doi: 10.1101/gad.1843810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lei QY, Zhang H, Zhao B, Zha ZY, Bai F, Pei XH, Zhao S, Xiong Y, Guan KL. TAZ promotes cell proliferation and epithelial-mesenchymal transition and is inhibited by the hippo pathway. Mol Cell Biol. 2008;28(7):2426–2436. doi: 10.1128/MCB.01874-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Liu CY, Zha ZY, Zhou X, Zhang H, Huang W, Zhao D, Li T, Chan SW, Lim CJ, Hong W, Zhao S, Xiong Y, Lei QY, Guan KL. The hippo tumor pathway promotes TAZ degradation by phosphorylating a phosphodegron and recruiting the SCF{beta}-TrCP E3 ligase. J Biol Chem. 2010;285(48):37159–37169. doi: 10.1074/jbc.M110.152942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kanai F, Marignani PA, Sarbassova D, Yagi R, Hall RA, Donowitz M, Hisaminato A, Fujiwara T, Ito Y, Cantley LC, Yaffe MB. TAZ: a novel transcriptional co-activator regulated by interactions with 14-3-3 and PDZ domain proteins. EMBO J. 2000;19(24):6778–6791. doi: 10.1093/emboj/19.24.6778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Meng Z, Moroishi T, Guan KL. Mechanisms of Hippo pathway regulation. Genes Dev. 2016;30(1):1–17. doi: 10.1101/gad.274027.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.He M, Zhou Z, Shah AA, Hong Y, Chen Q, Wan Y. New insights into posttranslational modifications of Hippo pathway in carcinogenesis and therapeutics. Cell Div. 2016;11:4. doi: 10.1186/s13008-016-0013-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Boggiano JC, Vanderzalm PJ, Fehon RG. Tao-1 phosphorylates Hippo/MST kinases to regulate the Hippo-Salvador-Warts tumor suppressor pathway. Dev Cell. 2011;21(5):888–895. doi: 10.1016/j.devcel.2011.08.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Poon CL, Lin JI, Zhang X, Harvey KF. The sterile 20-like kinase Tao-1 controls tissue growth by regulating the Salvador–Warts–Hippo pathway. Dev Cell. 2011;21(5):896–906. doi: 10.1016/j.devcel.2011.09.012. [DOI] [PubMed] [Google Scholar]
- 54.Plouffe SW, Meng Z, Lin KC, Lin B, Hong AW, Chun JV, Guan KL. Characterization of Hippo pathway components by gene inactivation. Mol Cell. 2016;64(5):993–1008. doi: 10.1016/j.molcel.2016.10.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Li Q, Li S, Mana-Capelli S, Roth Flach RJ, Danai LV, Amcheslavsky A, Nie Y, Kaneko S, Yao X, Chen X, Cotton JL, Mao J, McCollum D, Jiang J, Czech MP, Xu L, Ip YT. The conserved misshapen-warts-Yorkie pathway acts in enteroblasts to regulate intestinal stem cells in Drosophila. Dev Cell. 2014;31(3):291–304. doi: 10.1016/j.devcel.2014.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Mohseni M, Sun J, Lau A, Curtis S, Goldsmith J, Fox VL, Wei C, Frazier M, Samson O, Wong KK, Kim C, Camargo FD. A genetic screen identifies an LKB1-MARK signalling axis controlling the Hippo-YAP pathway. Nat Cell Biol. 2014;16(1):108–117. doi: 10.1038/ncb2884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zheng Y, Wang W, Liu B, Deng H, Uster E, Pan D. Identification of Happyhour/MAP4K as alternative Hpo/Mst-like kinases in the Hippo kinase cascade. Dev Cell. 2015;34(6):642–655. doi: 10.1016/j.devcel.2015.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Meng Z, Moroishi T, Mottier-Pavie V, Plouffe SW, Hansen CG, Hong AW, Park HW, Mo JS, Lu W, Lu S, Flores F, Yu FX, Halder G, Guan KL. MAP4K family kinases act in parallel to MST1/2 to activate LATS1/2 in the Hippo pathway. Nat Commun. 2015;6:8357. doi: 10.1038/ncomms9357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Rodriguez-Boulan E, Macara IG. Organization and execution of the epithelial polarity programme. Nat Rev Mol Cell Biol. 2014;15(4):225–242. doi: 10.1038/nrm3775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Zecca M, Struhl G. A feed-forward circuit linking wingless, fat-dachsous signaling, and the warts-hippo pathway to Drosophila wing growth. PLoS Biol. 2010;8(6):e1000386. doi: 10.1371/journal.pbio.1000386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Vrabioiu AM, Struhl G. Fat/Dachsous signaling promotes drosophila wing growth by regulating the conformational state of the NDR kinase warts. Dev Cell. 2015;35(6):737–749. doi: 10.1016/j.devcel.2015.11.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Renfranz PJ, Siegrist SE, Stronach BE, Macalma T, Beckerle MC. Molecular and phylogenetic characterization of Zyx102, a Drosophila orthologue of the zyxin family that interacts with Drosophila Enabled. Gene. 2003;305(1):13–26. doi: 10.1016/S0378-1119(02)01173-3. [DOI] [PubMed] [Google Scholar]
- 63.Rauskolb C, Pan G, Reddy BV, Oh H, Irvine KD. Zyxin links fat signaling to the hippo pathway. PLoS Biol. 2011;9(6):e1000624. doi: 10.1371/journal.pbio.1000624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Chen CL, Gajewski KM, Hamaratoglu F, Bossuyt W, Sansores-Garcia L, Tao C, Halder G. The apical-basal cell polarity determinant Crumbs regulates Hippo signaling in Drosophila. Proc Natl Acad Sci USA. 2010;107(36):15810–15815. doi: 10.1073/pnas.1004060107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Grzeschik NA, Parsons LM, Allott ML, Harvey KF, Richardson HE. Lgl, aPKC, and Crumbs regulate the Salvador/Warts/Hippo pathway through two distinct mechanisms. Curr Biol. 2010;20(7):573–581. doi: 10.1016/j.cub.2010.01.055. [DOI] [PubMed] [Google Scholar]
- 66.Ling C, Zheng Y, Yin F, Yu J, Huang J, Hong Y, Wu S, Pan D. The apical transmembrane protein Crumbs functions as a tumor suppressor that regulates Hippo signaling by binding to Expanded. Proc Natl Acad Sci USA. 2010;107(23):10532–10537. doi: 10.1073/pnas.1004279107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Robinson BS, Huang J, Hong Y, Moberg KH. Crumbs regulates Salvador/Warts/Hippo signaling in Drosophila via the FERM-domain protein Expanded. Curr Biol. 2010;20(7):582–590. doi: 10.1016/j.cub.2010.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.McCartney BM, Kulikauskas RM, LaJeunesse DR, Fehon RG. The neurofibromatosis-2 homologue, Merlin, and the tumor suppressor expanded function together in Drosophila to regulate cell proliferation and differentiation. Development. 2000;127(6):1315–1324. doi: 10.1242/dev.127.6.1315. [DOI] [PubMed] [Google Scholar]
- 69.Badouel C, Gardano L, Amin N, Garg A, Rosenfeld R, Le Bihan T, McNeill H. The FERM-domain protein Expanded regulates Hippo pathway activity via direct interactions with the transcriptional activator Yorkie. Dev Cell. 2009;16(3):411–420. doi: 10.1016/j.devcel.2009.01.010. [DOI] [PubMed] [Google Scholar]
- 70.Ribeiro P, Holder M, Frith D, Snijders AP, Tapon N. Crumbs promotes expanded recognition and degradation by the SCF(Slimb/beta-TrCP) ubiquitin ligase. Proc Natl Acad Sci USA. 2014;111(19):E1980–1989. doi: 10.1073/pnas.1315508111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Baumgartner R, Poernbacher I, Buser N, Hafen E, Stocker H. The WW domain protein Kibra acts upstream of Hippo in Drosophila. Dev Cell. 2010;18(2):309–316. doi: 10.1016/j.devcel.2009.12.013. [DOI] [PubMed] [Google Scholar]
- 72.Genevet A, Wehr MC, Brain R, Thompson BJ, Tapon N. Kibra is a regulator of the Salvador/Warts/Hippo signaling network. Dev Cell. 2010;18(2):300–308. doi: 10.1016/j.devcel.2009.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Yu J, Zheng Y, Dong J, Klusza S, Deng WM, Pan D. Kibra functions as a tumor suppressor protein that regulates Hippo signaling in conjunction with Merlin and Expanded. Dev Cell. 2010;18(2):288–299. doi: 10.1016/j.devcel.2009.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Zhou PJ, Xue W, Peng J, Wang Y, Wei L, Yang Z, Zhu HH, Fang YX, Gao WQ. Elevated expression of Par3 promotes prostate cancer metastasis by forming a Par3/aPKC/KIBRA complex and inactivating the hippo pathway. J Exp Clin Cancer Res. 2017;36(1):139. doi: 10.1186/s13046-017-0609-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Bratt A, Wilson WJ, Troyanovsky B, Aase K, Kessler R, Van Meir EG, Holmgren L. Angiomotin belongs to a novel protein family with conserved coiled-coil and PDZ binding domains. Gene. 2002;298(1):69–77. doi: 10.1016/S0378-1119(02)00928-9. [DOI] [PubMed] [Google Scholar]
- 76.Sugihara-Mizuno Y, Adachi M, Kobayashi Y, Hamazaki Y, Nishimura M, Imai T, Furuse M, Tsukita S. Molecular characterization of angiomotin/JEAP family proteins: interaction with MUPP1/Patj and their endogenous properties. Genes Cells. 2007;12(4):473–486. doi: 10.1111/j.1365-2443.2007.01066.x. [DOI] [PubMed] [Google Scholar]
- 77.Wells CD, Fawcett JP, Traweger A, Yamanaka Y, Goudreault M, Elder K, Kulkarni S, Gish G, Virag C, Lim C, Colwill K, Starostine A, Metalnikov P, Pawson T. A Rich1/Amot complex regulates the Cdc42 GTPase and apical-polarity proteins in epithelial cells. Cell. 2006;125(3):535–548. doi: 10.1016/j.cell.2006.02.045. [DOI] [PubMed] [Google Scholar]
- 78.Zhao B, Li L, Lu Q, Wang LH, Liu CY, Lei Q, Guan KL. Angiomotin is a novel Hippo pathway component that inhibits YAP oncoprotein. Genes Dev. 2011;25(1):51–63. doi: 10.1101/gad.2000111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Chan SW, Lim CJ, Chong YF, Pobbati AV, Huang C, Hong W. Hippo pathway-independent restriction of TAZ and YAP by angiomotin. J Biol Chem. 2011;286(9):7018–7026. doi: 10.1074/jbc.C110.212621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Wang W, Huang J, Chen J. Angiomotin-like proteins associate with and negatively regulate YAP1. J Biol Chem. 2011;286(6):4364–4370. doi: 10.1074/jbc.C110.205401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Paramasivam M, Sarkeshik A, Yates JR, 3rd, Fernandes MJ, McCollum D. Angiomotin family proteins are novel activators of the LATS2 kinase tumor suppressor. Mol Biol Cell. 2011;22(19):3725–3733. doi: 10.1091/mbc.e11-04-0300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Cordenonsi M, Zanconato F, Azzolin L, Forcato M, Rosato A, Frasson C, Inui M, Montagner M, Parenti AR, Poletti A, Daidone MG, Dupont S, Basso G, Bicciato S, Piccolo S. The Hippo transducer TAZ confers cancer stem cell-related traits on breast cancer cells. Cell. 2011;147(4):759–772. doi: 10.1016/j.cell.2011.09.048. [DOI] [PubMed] [Google Scholar]
- 83.Oka T, Remue E, Meerschaert K, Vanloo B, Boucherie C, Gfeller D, Bader GD, Sidhu SS, Vandekerckhove J, Gettemans J, Sudol M. Functional complexes between YAP2 and ZO-2 are PDZ domain-dependent, and regulate YAP2 nuclear localization and signalling. Biochem J. 2010;432(3):461–472. doi: 10.1042/BJ20100870. [DOI] [PubMed] [Google Scholar]
- 84.Remue E, Meerschaert K, Oka T, Boucherie C, Vandekerckhove J, Sudol M, Gettemans J. TAZ interacts with zonula occludens-1 and -2 proteins in a PDZ-1 dependent manner. FEBS Lett. 2010;584(19):4175–4180. doi: 10.1016/j.febslet.2010.09.020. [DOI] [PubMed] [Google Scholar]
- 85.Kobielak A, Fuchs E. Alpha-catenin: at the junction of intercellular adhesion and actin dynamics. Nat Rev Mol Cell Biol. 2004;5(8):614–625. doi: 10.1038/nrm1433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Schlegelmilch K, Mohseni M, Kirak O, Pruszak J, Rodriguez JR, Zhou D, Kreger BT, Vasioukhin V, Avruch J, Brummelkamp TR, Camargo FD. Yap1 acts downstream of alpha-catenin to control epidermal proliferation. Cell. 2011;144(5):782–795. doi: 10.1016/j.cell.2011.02.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Silvis MR, Kreger BT, Lien WH, Klezovitch O, Rudakova GM, Camargo FD, Lantz DM, Seykora JT, Vasioukhin V. Alpha-catenin is a tumor suppressor that controls cell accumulation by regulating the localization and activity of the transcriptional coactivator Yap1. Sci Signal. 2011;4(174):ra33. doi: 10.1126/scisignal.2001823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Kim NG, Koh E, Chen X, Gumbiner BM. E-cadherin mediates contact inhibition of proliferation through Hippo signaling-pathway components. Proc Natl Acad Sci USA. 2011;108(29):11930–11935. doi: 10.1073/pnas.1103345108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Huang JM, Nagatomo I, Suzuki E, Mizuno T, Kumagai T, Berezov A, Zhang H, Karlan B, Greene MI, Wang Q. YAP modifies cancer cell sensitivity to EGFR and survivin inhibitors and is negatively regulated by the non-receptor type protein tyrosine phosphatase 14. Oncogene. 2013;32(17):2220–2229. doi: 10.1038/onc.2012.231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Liu X, Yang N, Figel SA, Wilson KE, Morrison CD, Gelman IH, Zhang J. PTPN14 interacts with and negatively regulates the oncogenic function of YAP. Oncogene. 2013;32(10):1266–1273. doi: 10.1038/onc.2012.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Michaloglou C, Lehmann W, Martin T, Delaunay C, Hueber A, Barys L, Niu H, Billy E, Wartmann M, Ito M, Wilson CJ, Digan ME, Bauer A, Voshol H, Christofori G, Sellers WR, Hofmann F, Schmelzle T. The tyrosine phosphatase PTPN14 is a negative regulator of YAP activity. PLoS One. 2013;8(4):e61916. doi: 10.1371/journal.pone.0061916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Wang W, Huang J, Wang X, Yuan J, Li X, Feng L, Park JI, Chen J. PTPN14 is required for the density-dependent control of YAP1. Genes Dev. 2012;26(17):1959–1971. doi: 10.1101/gad.192955.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Hodge RG, Ridley AJ. Regulating Rho GTPases and their regulators. Nat Rev Mol Cell Biol. 2016;17(8):496–510. doi: 10.1038/nrm.2016.67. [DOI] [PubMed] [Google Scholar]
- 94.Zhao B, Li L, Wang L, Wang CY, Yu J, Guan KL. Cell detachment activates the Hippo pathway via cytoskeleton reorganization to induce anoikis. Genes Dev. 2012;26(1):54–68. doi: 10.1101/gad.173435.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Dupont S, Morsut L, Aragona M, Enzo E, Giulitti S, Cordenonsi M, Zanconato F, Le Digabel J, Forcato M, Bicciato S, Elvassore N, Piccolo S. Role of YAP/TAZ in mechanotransduction. Nature. 2011;474(7350):179–183. doi: 10.1038/nature10137. [DOI] [PubMed] [Google Scholar]
- 96.Yu FX, Zhao B, Panupinthu N, Jewell JL, Lian I, Wang LH, Zhao J, Yuan H, Tumaneng K, Li H, Fu XD, Mills GB, Guan KL. Regulation of the Hippo-YAP pathway by G-protein-coupled receptor signaling. Cell. 2012;150(4):780–791. doi: 10.1016/j.cell.2012.06.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Wang Z, Liu P, Zhou X, Wang TX, Feng X, Sun YP, Xiong Y, Yuan HX, Guan KL. Endothelin promotes colorectal tumorigenesis by activating YAP/TAZ. Cancer Res. 2017;77(9):2413–2423. doi: 10.1158/0008-5472.CAN-16-3229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Zhou X, Wang SY, Wang Z, Feng X, Liu P, Lv XB, Li FL, Yu FX, Sun YP, Yuan HX, Zhu HG, Xiong Y, Lei QY, Guan KL. Estrogen regulates Hippo signaling via GPER in breast cancer. J Clin Investig. 2015;125(5):2123–2135. doi: 10.1172/JCI79573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Yu FX, Luo J, Mo JS, Liu GB, Kim YC, Meng ZP, Zhao L, Peyman G, Ouyang H, Jiang W, Zhao JG, Chen X, Zhang LF, Wang CY, Bastian BC, Zhang K, Guan KL. Mutant Gq/11 promote uveal melanoma tumorigenesis by activating YAP. Cancer Cell. 2014;25(6):822–830. doi: 10.1016/j.ccr.2014.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Feng XD, Degese MS, Iglesias-Bartolome R, Vaque JP, Molinolo AA, Rodrigues M, Zaidi MR, Ksander BR, Merlino G, Sodhi A, Chen QM, Gutkind JS. Hippo-independent activation of YAP by the GNAQ uveal melanoma oncogene through a trio-regulated Rho GTPase signaling circuitry. Cancer Cell. 2014;25(6):831–845. doi: 10.1016/j.ccr.2014.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Yu FX, Zhang Y, Park HW, Jewell JL, Chen Q, Deng Y, Pan D, Taylor SS, Lai ZC, Guan KL. Protein kinase A activates the Hippo pathway to modulate cell proliferation and differentiation. Genes Dev. 2013;27(11):1223–1232. doi: 10.1101/gad.219402.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Kim M, Kim M, Lee S, Kuninaka S, Saya H, Lee H, Lee S, Lim DS. cAMP/PKA signalling reinforces the LATS-YAP pathway to fully suppress YAP in response to actin cytoskeletal changes. EMBO J. 2013;32(11):1543–1555. doi: 10.1038/emboj.2013.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Fernandez BG, Gaspar P, Bras-Pereira C, Jezowska B, Rebelo SR, Janody F. Actin-capping protein and the Hippo pathway regulate F-actin and tissue growth in Drosophila. Development. 2011;138(11):2337–2346. doi: 10.1242/dev.063545. [DOI] [PubMed] [Google Scholar]
- 104.Sansores-Garcia L, Bossuyt W, Wada K, Yonemura S, Tao C, Sasaki H, Halder G. Modulating F-actin organization induces organ growth by affecting the Hippo pathway. EMBO J. 2011;30(12):2325–2335. doi: 10.1038/emboj.2011.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Imajo M, Miyatake K, Iimura A, Miyamoto A, Nishida E. A molecular mechanism that links Hippo signalling to the inhibition of Wnt/beta-catenin signalling. EMBO J. 2012;31(5):1109–1122. doi: 10.1038/emboj.2011.487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Azzolin L, Panciera T, Soligo S, Enzo E, Bicciato S, Dupont S, Bresolin S, Frasson C, Basso G, Guzzardo V, Fassina A, Cordenonsi M, Piccolo S. YAP/TAZ incorporation in the beta-catenin destruction complex orchestrates the Wnt response. Cell. 2014;158(1):157–170. doi: 10.1016/j.cell.2014.06.013. [DOI] [PubMed] [Google Scholar]
- 107.Azzolin L, Zanconato F, Bresolin S, Forcato M, Basso G, Bicciato S, Cordenonsi M, Piccolo S. Role of TAZ as mediator of Wnt signaling. Cell. 2012;151(7):1443–1456. doi: 10.1016/j.cell.2012.11.027. [DOI] [PubMed] [Google Scholar]
- 108.Liang N, Zhang C, Dill P, Panasyuk G, Pion D, Koka V, Gallazzini M, Olson EN, Lam H, Henske EP, Dong Z, Apte U, Pallet N, Johnson RL, Terzi F, Kwiatkowski DJ, Scoazec JY, Martignoni G, Pende M. Regulation of YAP by mTOR and autophagy reveals a therapeutic target of tuberous sclerosis complex. J Exp Med. 2014;211(11):2249–2263. doi: 10.1084/jem.20140341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Tumaneng K, Schlegelmilch K, Russell RC, Yimlamai D, Basnet H, Mahadevan N, Fitamant J, Bardeesy N, Camargo FD, Guan KL. YAP mediates crosstalk between the Hippo and PI(3)K-TOR pathways by suppressing PTEN via miR-29. Nat Cell Biol. 2012;14(12):1322–1329. doi: 10.1038/ncb2615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Hansen CG, Ng YL, Lam WL, Plouffe SW, Guan KL. The Hippo pathway effectors YAP and TAZ promote cell growth by modulating amino acid signaling to mTORC1. Cell Res. 2015;25(12):1299–1313. doi: 10.1038/cr.2015.140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Strassburger K, Tiebe M, Pinna F, Breuhahn K, Teleman AA. Insulin/IGF signaling drives cell proliferation in part via Yorkie/YAP. Dev Biol. 2012;367(2):187–196. doi: 10.1016/j.ydbio.2012.05.008. [DOI] [PubMed] [Google Scholar]
- 112.Sun G, Irvine KD. Ajuba family proteins link JNK to Hippo signaling. Sci Signal. 2013;6(292):ra81. doi: 10.1126/scisignal.2004324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Tomlinson V, Gudmundsdottir K, Luong P, Leung KY, Knebel A, Basu S. JNK phosphorylates Yes-associated protein (YAP) to regulate apoptosis. Cell Death Dis. 2010;1:e29. doi: 10.1038/cddis.2010.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Lee KK, Yonehara S. Identification of mechanism that couples multisite phosphorylation of Yes-associated protein (YAP) with transcriptional coactivation and regulation of apoptosis. J Biol Chem. 2012;287(12):9568–9578. doi: 10.1074/jbc.M111.296954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Lin KC, Moroishi T, Meng Z, Jeong HS, Plouffe SW, Sekido Y, Han J, Park HW, Guan KL. Regulation of Hippo pathway transcription factor TEAD by p38 MAPK-induced cytoplasmic translocation. Nat Cell Biol. 2017;19(8):996–1002. doi: 10.1038/ncb3581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Hong AW, Meng Z, Yuan HX, Plouffe SW, Moon S, Kim W, Jho EH, Guan KL. Osmotic stress-induced phosphorylation by NLK at Ser128 activates YAP. EMBO Rep. 2017;18(1):72–86. doi: 10.15252/embr.201642681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Moon S, Kim W, Kim S, Kim Y, Song Y, Bilousov O, Kim J, Lee T, Cha B, Kim M, Kim H, Katanaev VL, Jho EH. Phosphorylation by NLK inhibits YAP-14-3-3-interactions and induces its nuclear localization. EMBO Rep. 2017;18(1):61–71. doi: 10.15252/embr.201642683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Ganem NJ, Cornils H, Chiu SY, O’Rourke KP, Arnaud J, Yimlamai D, Thery M, Camargo FD, Pellman D. Cytokinesis failure triggers hippo tumor suppressor pathway activation. Cell. 2014;158(4):833–848. doi: 10.1016/j.cell.2014.06.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Zhao B, Wei X, Li W, Udan RS, Yang Q, Kim J, Xie J, Ikenoue T, Yu J, Li L, Zheng P, Ye K, Chinnaiyan A, Halder G, Lai ZC, Guan KL. Inactivation of YAP oncoprotein by the Hippo pathway is involved in cell contact inhibition and tissue growth control. Genes Dev. 2007;21(21):2747–2761. doi: 10.1101/gad.1602907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Driscoll TP, Cosgrove BD, Heo SJ, Shurden ZE, Mauck RL. Cytoskeletal to nuclear strain transfer regulates YAP signaling in mesenchymal stem cells. Biophys J. 2015;108(12):2783–2793. doi: 10.1016/j.bpj.2015.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Wada K, Itoga K, Okano T, Yonemura S, Sasaki H. Hippo pathway regulation by cell morphology and stress fibers. Development. 2011;138(18):3907–3914. doi: 10.1242/dev.070987. [DOI] [PubMed] [Google Scholar]
- 122.Aragona M, Panciera T, Manfrin A, Giulitti S, Michielin F, Elvassore N, Dupont S, Piccolo S. A mechanical checkpoint controls multicellular growth through YAP/TAZ regulation by actin-processing factors. Cell. 2013;154(5):1047–1059. doi: 10.1016/j.cell.2013.07.042. [DOI] [PubMed] [Google Scholar]
- 123.Codelia VA, Sun G, Irvine KD. Regulation of YAP by mechanical strain through Jnk and Hippo signaling. Curr Biol. 2014;24(17):2012–2017. doi: 10.1016/j.cub.2014.07.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Kim MH, Kim J. Role of YAP/TAZ transcriptional regulators in resistance to anti-cancer therapies. Cell Mol Life Sci. 2017;74(8):1457–1474. doi: 10.1007/s00018-016-2412-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Zanconato F, Cordenonsi M, Piccolo S. YAP/TAZ at the roots of cancer. Cancer Cell. 2016;29(6):783–803. doi: 10.1016/j.ccell.2016.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Camargo FD, Gokhale S, Johnnidis JB, Fu D, Bell GW, Jaenisch R, Brummelkamp TR. YAP1 increases organ size and expands undifferentiated progenitor cells. Curr Biol. 2007;17(23):2054–2060. doi: 10.1016/j.cub.2007.10.039. [DOI] [PubMed] [Google Scholar]
- 127.Lee JH, Kim TS, Yang TH, Koo BK, Oh SP, Lee KP, Oh HJ, Lee SH, Kong YY, Kim JM, Lim DS. A crucial role of WW45 in developing epithelial tissues in the mouse. EMBO J. 2008;27(8):1231–1242. doi: 10.1038/emboj.2008.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Zhou D, Zhang Y, Wu H, Barry E, Yin Y, Lawrence E, Dawson D, Willis JE, Markowitz SD, Camargo FD, Avruch J. Mst1 and Mst2 protein kinases restrain intestinal stem cell proliferation and colonic tumorigenesis by inhibition of Yes-associated protein (Yap) overabundance. Proc Natl Acad Sci USA. 2011;108(49):E1312–1320. doi: 10.1073/pnas.1110428108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Lee KP, Lee JH, Kim TS, Kim TH, Park HD, Byun JS, Kim MC, Jeong WI, Calvisi DF, Kim JM, Lim DS. The Hippo-Salvador pathway restrains hepatic oval cell proliferation, liver size, and liver tumorigenesis. Proc Natl Acad Sci USA. 2010;107(18):8248–8253. doi: 10.1073/pnas.0912203107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Cai J, Maitra A, Anders RA, Taketo MM, Pan D. beta-Catenin destruction complex-independent regulation of Hippo-YAP signaling by APC in intestinal tumorigenesis. Genes Dev. 2015;29(14):1493–1506. doi: 10.1101/gad.264515.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Lin L, Sabnis AJ, Chan E, Olivas V, Cade L, Pazarentzos E, Asthana S, Neel D, Yan JJ, Lu X, Pham L, Wang MM, Karachaliou N, Cao MG, Manzano JL, Ramirez JL, Torres JM, Buttitta F, Rudin CM, Collisson EA, Algazi A, Robinson E, Osman I, Munoz-Couselo E, Cortes J, Frederick DT, Cooper ZA, McMahon M, Marchetti A, Rosell R, Flaherty KT, Wargo JA, Bivona TG. The Hippo effector YAP promotes resistance to RAF- and MEK-targeted cancer therapies. Nat Genet. 2015;47(3):250–256. doi: 10.1038/ng.3218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Lin KC, Park HW, Guan KL. Regulation of the Hippo pathway transcription factor TEAD. Trends Biochem Sci. 2017;42(11):862–872. doi: 10.1016/j.tibs.2017.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Hirabayashi S, Cagan RL. Salt-inducible kinases mediate nutrient-sensing to link dietary sugar and tumorigenesis in Drosophila. Elife. 2015;4:e08501. doi: 10.7554/eLife.08501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Ohsawa S, Sato Y, Enomoto M, Nakamura M, Betsumiya A, Igaki T. Mitochondrial defect drives non-autonomous tumour progression through Hippo signalling in Drosophila. Nature. 2012;490(7421):547–551. doi: 10.1038/nature11452. [DOI] [PubMed] [Google Scholar]
- 135.Vander Heiden MG, Cantley LC, Thompson CB. Understanding the Warburg effect: the metabolic requirements of cell proliferation. Science. 2009;324(5930):1029–1033. doi: 10.1126/science.1160809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Pavlova NN, Thompson CB. The emerging hallmarks of cancer metabolism. Cell Metab. 2016;23(1):27–47. doi: 10.1016/j.cmet.2015.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.DeRan M, Yang J, Shen CH, Peters EC, Fitamant J, Chan P, Hsieh M, Zhu S, Asara JM, Zheng B, Bardeesy N, Liu J, Wu X. Energy stress regulates hippo-YAP signaling involving AMPK-mediated regulation of angiomotin-like 1 protein. Cell Rep. 2014;9(2):495–503. doi: 10.1016/j.celrep.2014.09.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Mo JS, Meng Z, Kim YC, Park HW, Hansen CG, Kim S, Lim DS, Guan KL. Cellular energy stress induces AMPK-mediated regulation of YAP and the Hippo pathway. Nat Cell Biol. 2015;17(4):500–510. doi: 10.1038/ncb3111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Wang W, Xiao ZD, Li X, Aziz KE, Gan B, Johnson RL, Chen J. AMPK modulates Hippo pathway activity to regulate energy homeostasis. Nat Cell Biol. 2015;17(4):490–499. doi: 10.1038/ncb3113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Enzo E, Santinon G, Pocaterra A, Aragona M, Bresolin S, Forcato M, Grifoni D, Pession A, Zanconato F, Guzzo G, Bicciato S, Dupont S. Aerobic glycolysis tunes YAP/TAZ transcriptional activity. EMBO J. 2015;34(10):1349–1370. doi: 10.15252/embj.201490379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Gailite I, Aerne BL, Tapon N. Differential control of Yorkie activity by LKB1/AMPK and the Hippo/Warts cascade in the central nervous system. Proc Natl Acad Sci USA. 2015;112(37):E5169–5178. doi: 10.1073/pnas.1505512112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Wehr MC, Holder MV, Gailite I, Saunders RE, Maile TM, Ciirdaeva E, Instrell R, Jiang M, Howell M, Rossner MJ, Tapon N. Salt-inducible kinases regulate growth through the Hippo signalling pathway in Drosophila. Nat Cell Biol. 2013;15(1):61–71. doi: 10.1038/ncb2658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Peng C, Zhu Y, Zhang W, Liao Q, Chen Y, Zhao X, Guo Q, Shen P, Zhen B, Qian X, Yang D, Zhang JS, Xiao D, Qin W, Pei H. Regulation of the Hippo-YAP pathway by glucose sensor O-GlcNAcylation. Mol Cell. 2017;68(3):591–604 e595. doi: 10.1016/j.molcel.2017.10.010. [DOI] [PubMed] [Google Scholar]
- 144.Zhang X, Qiao Y, Wu Q, Chen Y, Zou S, Liu X, Zhu G, Zhao Y, Chen Y, Yu Y, Pan Q, Wang J, Sun F. The essential role of YAP O-GlcNAcylation in high-glucose-stimulated liver tumorigenesis. Nat Commun. 2017;8:15280. doi: 10.1038/ncomms15280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Sorrentino G, Ruggeri N, Specchia V, Cordenonsi M, Mano M, Dupont S, Manfrin A, Ingallina E, Sommaggio R, Piazza S, Rosato A, Piccolo S, Del Sal G. Metabolic control of YAP and TAZ by the mevalonate pathway. Nat Cell Biol. 2014;16(4):357–366. doi: 10.1038/ncb2936. [DOI] [PubMed] [Google Scholar]
- 146.Wang Z, Wu Y, Wang H, Zhang Y, Mei L, Fang X, Zhang X, Zhang F, Chen H, Liu Y, Jiang Y, Sun S, Zheng Y, Li N, Huang L. Interplay of mevalonate and Hippo pathways regulates RHAMM transcription via YAP to modulate breast cancer cell motility. Proc Natl Acad Sci USA. 2014;111(1):E89–98. doi: 10.1073/pnas.1319190110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Cox AG, Hwang KL, Brown KK, Evason K, Beltz S, Tsomides A, O’Connor K, Galli GG, Yimlamai D, Chhangawala S, Yuan M, Lien EC, Wucherpfennig J, Nissim S, Minami A, Cohen DE, Camargo FD, Asara JM, Houvras Y, Stainier DYR, Goessling W. Yap reprograms glutamine metabolism to increase nucleotide biosynthesis and enable liver growth. Nat Cell Biol. 2016;18(8):886–896. doi: 10.1038/ncb3389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Guo X, Zhao Y, Yan H, Yang Y, Shen S, Dai X, Ji X, Ji F, Gong XG, Li L, Bai X, Feng XH, Liang T, Ji J, Chen L, Wang H, Zhao B. Single tumor-initiating cells evade immune clearance by recruiting type II macrophages. Genes Dev. 2017;31(3):247–259. doi: 10.1101/gad.294348.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Wang G, Lu X, Dey P, Deng P, Wu CC, Jiang S, Fang Z, Zhao K, Konaparthi R, Hua S, Zhang J, Li-Ning-Tapia EM, Kapoor A, Wu CJ, Patel NB, Guo Z, Ramamoorthy V, Tieu TN, Heffernan T, Zhao D, Shang X, Khadka S, Hou P, Hu B, Jin EJ, Yao W, Pan X, Ding Z, Shi Y, Li L, Chang Q, Troncoso P, Logothetis CJ, McArthur MJ, Chin L, Wang YA, DePinho RA. Targeting YAP-dependent MDSC infiltration impairs tumor progression. Cancer Discov. 2016;6(1):80–95. doi: 10.1158/2159-8290.CD-15-0224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Liu B, Zheng Y, Yin F, Yu J, Silverman N, Pan D. Toll receptor-mediated Hippo signaling controls innate immunity in Drosophila. Cell. 2016;164(3):406–419. doi: 10.1016/j.cell.2015.12.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Moroishi T, Hayashi T, Pan WW, Fujita Y, Holt MV, Qin J, Carson DA, Guan KL. The Hippo pathway kinases LATS1/2 suppress cancer immunity. Cell. 2016;167(6):1525–1539 e1517. doi: 10.1016/j.cell.2016.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Meng F, Zhou R, Wu S, Zhang Q, Jin Q, Zhou Y, Plouffe SW, Liu S, Song H, Xia Z, Zhao B, Ye S, Feng XH, Guan KL, Zou J, Xu P. Mst1 shuts off cytosolic antiviral defense through IRF3 phosphorylation. Genes Dev. 2016;30(9):1086–1100. doi: 10.1101/gad.277533.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Zhang Q, Meng F, Chen S, Plouffe SW, Wu S, Liu S, Li X, Zhou R, Wang J, Zhao B, Liu J, Qin J, Zou J, Feng XH, Guan KL, Xu P. Hippo signalling governs cytosolic nucleic acid sensing through YAP/TAZ-mediated TBK1 blockade. Nat Cell Biol. 2017;19(4):362–374. doi: 10.1038/ncb3496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Lee BS, Park DI, Lee DH, Lee JE, Yeo MK, Park YH, Lim DS, Choi W, Lee DH, Yoo G, Kim HB, Kang D, Moon JY, Jung SS, Kim JO, Cho SY, Park HS, Chung C. Hippo effector YAP directly regulates the expression of PD-L1 transcripts in EGFR-TKI-resistant lung adenocarcinoma. Biochem Biophys Res Commun. 2017;491(2):493–499. doi: 10.1016/j.bbrc.2017.07.007. [DOI] [PubMed] [Google Scholar]
- 155.Feng J, Yang H, Zhang Y, Wei H, Zhu Z, Zhu B, Yang M, Cao W, Wang L, Wu Z. Tumor cell-derived lactate induces TAZ-dependent upregulation of PD-L1 through GPR81 in human lung cancer cells. Oncogene. 2017;36(42):5829–5839. doi: 10.1038/onc.2017.188. [DOI] [PubMed] [Google Scholar]
- 156.Thaventhiran JE, Hoffmann A, Magiera L, de la Roche M, Lingel H, Brunner-Weinzierl M, Fearon DT. Activation of the Hippo pathway by CTLA-4 regulates the expression of Blimp-1 in the CD8+ T cell. Proc Natl Acad Sci USA. 2012;109(33):E2223–2229. doi: 10.1073/pnas.1209115109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Geng J, Yu S, Zhao H, Sun X, Li X, Wang P, Xiong X, Hong L, Xie C, Gao J, Shi Y, Peng J, Johnson RL, Xiao N, Lu L, Han J, Zhou D, Chen L. The transcriptional coactivator TAZ regulates reciprocal differentiation of TH17 cells and Treg cells. Nat Immunol. 2017;18(7):800–812. doi: 10.1038/ni.3748. [DOI] [PubMed] [Google Scholar]
- 158.Zhou D, Medoff BD, Chen L, Li L, Zhang XF, Praskova M, Liu M, Landry A, Blumberg RS, Boussiotis VA, Xavier R, Avruch J. The Nore1B/Mst1 complex restrains antigen receptor-induced proliferation of naive T cells. Proc Natl Acad Sci USA. 2008;105(51):20321–20326. doi: 10.1073/pnas.0810773105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Li C, Bi Y, Li Y, Yang H, Yu Q, Wang J, Wang Y, Su H, Jia A, Hu Y, Han L, Zhang J, Li S, Tao W, Liu G. Dendritic cell MST1 inhibits Th17 differentiation. Nat Commun. 2017;8:14275. doi: 10.1038/ncomms14275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Jansson L, Larsson J. Normal hematopoietic stem cell function in mice with enforced expression of the Hippo signaling effector YAP1. PLoS One. 2012;7(2):e32013. doi: 10.1371/journal.pone.0032013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Liu-Chittenden Y, Huang B, Shim JS, Chen Q, Lee SJ, Anders RA, Liu JO, Pan D. Genetic and pharmacological disruption of the TEAD-YAP complex suppresses the oncogenic activity of YAP. Genes Dev. 2012;26(12):1300–1305. doi: 10.1101/gad.192856.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Dasari VR, Mazack V, Feng W, Nash J, Carey DJ, Gogoi R. Verteporfin exhibits YAP-independent anti-proliferative and cytotoxic effects in endometrial cancer cells. Oncotarget. 2017;8(17):28628–28640. doi: 10.18632/oncotarget.15614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Chen HH, Mullett SJ, Stewart AF. Vgl-4, a novel member of the vestigial-like family of transcription cofactors, regulates alpha1-adrenergic activation of gene expression in cardiac myocytes. J Biol Chem. 2004;279(29):30800–30806. doi: 10.1074/jbc.M400154200. [DOI] [PubMed] [Google Scholar]
- 164.Jiao S, Li C, Hao Q, Miao H, Zhang L, Li L, Zhou Z. VGLL4 targets a TCF4–TEAD4 complex to coregulate Wnt and Hippo signalling in colorectal cancer. Nat Commun. 2017;8:14058. doi: 10.1038/ncomms14058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Zhou Z, Hu T, Xu Z, Lin Z, Zhang Z, Feng T, Zhu L, Rong Y, Shen H, Luk JM, Zhang X, Qin N. Targeting Hippo pathway by specific interruption of YAP–TEAD interaction using cyclic YAP-like peptides. FASEB J. 2015;29(2):724–732. doi: 10.1096/fj.14-262980. [DOI] [PubMed] [Google Scholar]
- 166.Pobbati AV, Han X, Hung AW, Weiguang S, Huda N, Chen GY, Kang C, Chia CS, Luo X, Hong W, Poulsen A. Targeting the central pocket in human transcription factor TEAD as a potential cancer therapeutic strategy. Structure. 2015;23(11):2076–2086. doi: 10.1016/j.str.2015.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Chan P, Han X, Zheng B, DeRan M, Yu J, Jarugumilli GK, Deng H, Pan D, Luo X, Wu X. Autopalmitoylation of TEAD proteins regulates transcriptional output of the Hippo pathway. Nat Chem Biol. 2016;12(4):282–289. doi: 10.1038/nchembio.2036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Noland CL, Gierke S, Schnier PD, Murray J, Sandoval WN, Sagolla M, Dey A, Hannoush RN, Fairbrother WJ, Cunningham CN. Palmitoylation of TEAD transcription factors is required for their stability and function in Hippo pathway signaling. Structure. 2016;24(1):179–186. doi: 10.1016/j.str.2015.11.005. [DOI] [PubMed] [Google Scholar]
- 169.Rosenbluh J, Nijhawan D, Cox AG, Li X, Neal JT, Schafer EJ, Zack TI, Wang X, Tsherniak A, Schinzel AC, Shao DD, Schumacher SE, Weir BA, Vazquez F, Cowley GS, Root DE, Mesirov JP, Beroukhim R, Kuo CJ, Goessling W, Hahn WC. beta-Catenin-driven cancers require a YAP1 transcriptional complex for survival and tumorigenesis. Cell. 2012;151(7):1457–1473. doi: 10.1016/j.cell.2012.11.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Mi W, Lin Q, Childress C, Sudol M, Robishaw J, Berlot CH, Shabahang M, Yang W. Geranylgeranylation signals to the Hippo pathway for breast cancer cell proliferation and migration. Oncogene. 2015;34(24):3095–3106. doi: 10.1038/onc.2014.251. [DOI] [PubMed] [Google Scholar]
- 171.Mo JS, Yu FX, Gong R, Brown JH, Guan KL. Regulation of the Hippo-YAP pathway by protease-activated receptors (PARs) Genes Dev. 2012;26(19):2138–2143. doi: 10.1101/gad.197582.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Kim NG, Gumbiner BM. Adhesion to fibronectin regulates Hippo signaling via the FAK-Src-PI3K pathway. J Cell Biol. 2015;210(3):503–515. doi: 10.1083/jcb.201501025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Huang SM, Mishina YM, Liu S, Cheung A, Stegmeier F, Michaud GA, Charlat O, Wiellette E, Zhang Y, Wiessner S, Hild M, Shi X, Wilson CJ, Mickanin C, Myer V, Fazal A, Tomlinson R, Serluca F, Shao W, Cheng H, Shultz M, Rau C, Schirle M, Schlegl J, Ghidelli S, Fawell S, Lu C, Curtis D, Kirschner MW, Lengauer C, Finan PM, Tallarico JA, Bouwmeester T, Porter JA, Bauer A, Cong F. Tankyrase inhibition stabilizes axin and antagonizes Wnt signalling. Nature. 2009;461(7264):614–620. doi: 10.1038/nature08356. [DOI] [PubMed] [Google Scholar]
- 174.Troilo A, Benson EK, Esposito D, Garibsingh RA, Reddy EP, Mungamuri SK, Aaronson SA. Angiomotin stabilization by tankyrase inhibitors antagonizes constitutive TEAD-dependent transcription and proliferation of human tumor cells with Hippo pathway core component mutations. Oncotarget. 2016;7(20):28765–28782. doi: 10.18632/oncotarget.9117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Wang W, Li N, Li X, Tran MK, Han X, Chen J. Tankyrase inhibitors target YAP by stabilizing angiomotin family proteins. Cell Rep. 2015;13(3):524–532. doi: 10.1016/j.celrep.2015.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Serrano I, McDonald PC, Lock F, Muller WJ, Dedhar S. Inactivation of the Hippo tumour suppressor pathway by integrin-linked kinase. Nat Commun. 2013;4:2976. doi: 10.1038/ncomms3976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Bao Y, Nakagawa K, Yang Z, Ikeda M, Withanage K, Ishigami-Yuasa M, Okuno Y, Hata S, Nishina H, Hata Y. A cell-based assay to screen stimulators of the Hippo pathway reveals the inhibitory effect of dobutamine on the YAP-dependent gene transcription. J Biochem. 2011;150(2):199–208. doi: 10.1093/jb/mvr063. [DOI] [PubMed] [Google Scholar]
- 178.Fan F, He Z, Kong LL, Chen Q, Yuan Q, Zhang S, Ye J, Liu H, Sun X, Geng J, Yuan L, Hong L, Xiao C, Zhang W, Sun X, Li Y, Wang P, Huang L, Wu X, Ji Z, Wu Q, Xia NS, Gray NS, Chen L, Yun CH, Deng X, Zhou D. Pharmacological targeting of kinases MST1 and MST2 augments tissue repair and regeneration. Sci Transl Med. 2016;8(352):352ra108. doi: 10.1126/scitranslmed.aaf2304. [DOI] [PubMed] [Google Scholar]


