Abstract
Methods for isolating mitochondria from different rodent tissues have been established for decades. Although the general principles for crude mitochondrial preparations are largely shared across tissues – tissue disruption followed by differential centrifugation – critical differences exist for isolation from different tissues to optimize mitochondrial yield and function. This protocol offers a unified resource for preparations of isolated mitochondria from mouse liver, kidney, heart, brain, skeletal muscle, and brown and white adipose tissue suitable for functional analysis.
Keywords: Mitochondria, liver, kidney, brain, heart, skeletal muscle, brown adipose tissue, white adipose tissue, oxidative phosphorylation, bioenergetics
1. INTRODUCTION
Isolation of mitochondria from rodent tissues has been conducted for many decades and is a foundational experimental technique in metabolism research [1, 2]. Although most isolation protocols were historically optimized for rat tissue given the greater tissue mass and mitochondrial yield per animal, mitochondrial isolation from tissue of a single mouse is frequently required during studies of genetically modified animals. Fortunately, microplate-based methods have been established for essential functional analyses in isolated mitochondria (e.g. measurements of oxygen consumption [3, 4], membrane potential [5], and superoxide production [6, 7]) and enable meaningful results to be obtained from small mitochondrial populations.
In this chapter, we describe protocols to isolate mitochondria from seven mouse tissues: liver, kidney, heart, brain, skeletal muscle, brown adipose tissue (BAT), and white adipose tissue (WAT). Importantly, mitochondrial isolation protocols are not uniform across tissues. Although each preparation relies on principles of differential centrifugation (Fig. 1), most tissues critically differ in the composition of isolation buffers used, method of tissue disruption and speeds of centrifugation steps. Other specific steps, such as the use of a protease to isolate skeletal muscle mitochondria or the use of digitonin to permeabilize synaptosomal membranes while isolating brain mitochondria, are sometimes required to optimize quality and yield. The protocols provided yield crude preparations ideal for functional analysis, as opposed to more stringent protocols using gradient purification which often optimize purity at the expense of function and yield. We would like to acknowledge our colleagues and mentors Martin Brand, José Antonio Enríquez, Marc Liesa, Giovanni Manfredi, and Anne Murphy for their guidance over the years in helping us refine these protocols.
FIGURE 1.
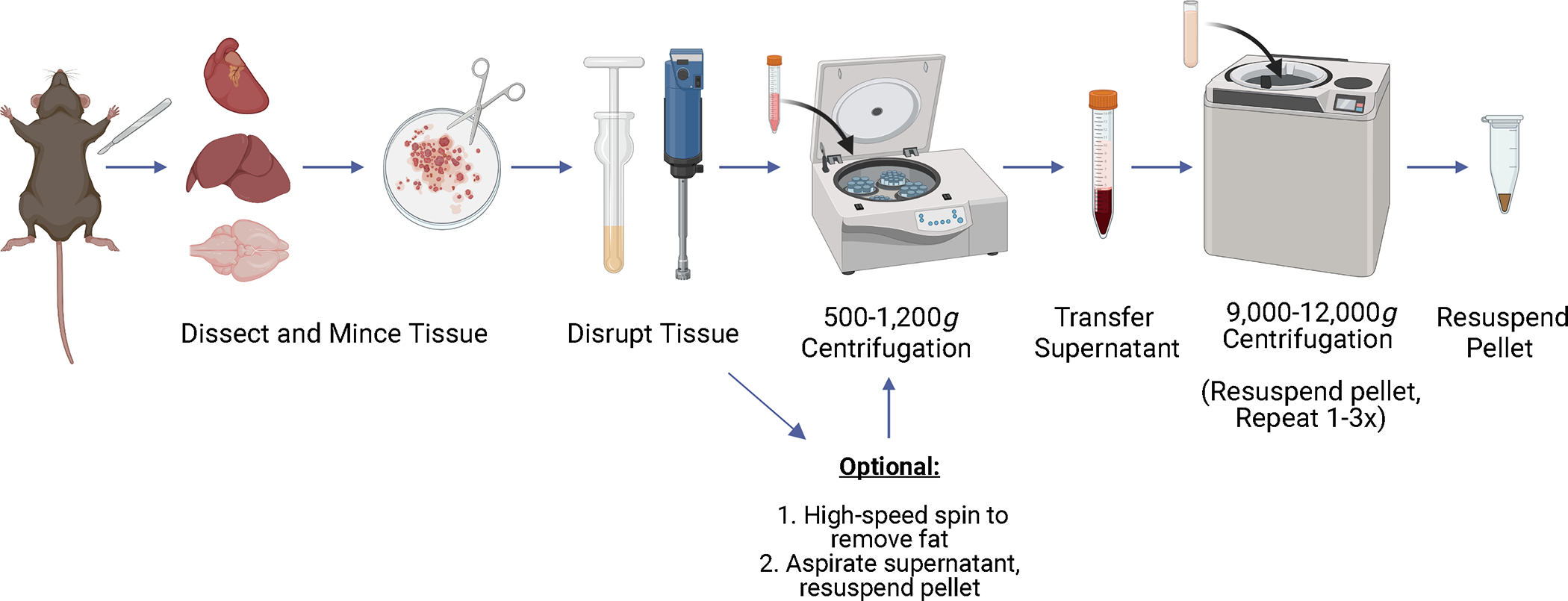
Schematic representation of the protocol steps to isolate mitochondria from different mouse tissues. The mitochondrial isolation protocols provided here all rely on differential centrifugation. However, isolation procedures for different tissues often vary critically in the composition of isolation buffer, method of tissue disruption, and centrifugation speeds.
2. MATERIALS
Unless stated otherwise, all materials may be purchased from Sigma-Aldrich
2.1 –. MATERIALS REQUIRED FOR MITOCHONDRIAL ISOLATIONS FOR ALL MOUSE TISSUES
Animals – C57BL/6 mice aged 20 – 28 weeks. (see Notes 1 and 2)
Dissection tools – Sharp-point operating scissors; straight and curved forceps; razor blades.
Solutions and reagents – Buffer recipes are listed below. All buffers should be made with tissue culture-grade water.
Common equipment – Spray-bottle of 70% (w/v) ethanol; absorbent underpads; two layers of cheesecloth or muslin pre-cut into 4 in. x 4 in. squares; plastic (or glass) beakers of various sizes for mincing tissue and decanting medium; pH meter; ice buckets.
Centrifuges – Refrigerated centrifuges capable of operating between 500g and 12,000g and centrifuge tubes compatible with the appropriate rotor.
Protein assay – Commercial protein assay kit such as the bicinchoninic acid (BCA) protein assay (see Note 3); Multi-well plate reader or spectrophotometer for absorbance readings.
2.2 –. MATERIALS REQUIRED FOR ISOLATION OF MITOCHONDRIA FROM LIVER AND KIDNEY
Specialized equipment – Drill-driven Teflon-on-glass homogenizer (see Note 4)
Solutions and reagents – MSHE with 0.2% BSA: 210 mM mannitol, 70 mM sucrose, 5 mM HEPES, 1 mM EGTA, 0.2% (w/v) fatty acid-free (Fraction V) BSA, pH 7.4 to 4°C with KOH (see Notes 5–7); MSHE without added BSA is required for the final spin and resuspension of the final pellet, so set aside 20–30 mLs of medium prior to adding BSA on the day of the assay.
2.3 –. MATERIALS REQUIRED FOR ISOLATION OF MITOCHONDRIA FROM HEART
Specialized equipment – 7 mL glass-on-glass Dounce homogenizer with loose- and tight-fitting pestles; Six-well plate such as those used for cell culture; (Optional) Hand-held mechanical homogenizer or tissue disruptor (e.g. from Kinematica™ or IKA™)
Solutions and reagents – Relaxation Buffer: 100 mM KCl, 5 mM sodium pyrophosphate, 5 mM HEPES, 5 mM EGTA, pH 7.4 to 4°C with KOH; SHE: 250 mM sucrose, 5 mM HEPES, 1 mM EGTA, pH 7.4 to 4°C with KOH (see Notes 5,7)
2.4 –. MATERIALS REQUIRED FOR ISOLATION OF MITOCHONDRIA FROM BRAIN
Specialized equipment – 7 mL glass-on-glass Dounce homogenizer with loose- and tight-fitting pestles; petri dish or polypropylene cutting board; (Optional) guillotine; rongeurs or surgical pliers.
Solutions and reagents – MSHE with and without 0.2% BSA (see 2.2.2); 10% (w/v) digitonin solution (see Note 8)
2.5 –. MATERIALS REQUIRED FOR ISOLATION OF MITOCHONDRIA FROM SKELETAL MUSCLE
Specialized equipment – 15 mL glass-on-glass Dounce homogenizer with loose- and tight-fitting pestles; petri dish or polypropylene cutting board; (Optional) Hand-held mechanical homogenizer or tissue disruptor (e.g. from Kinematica™ or IKA™)
Solutions and reagents – Chappell-Perry buffer (CP1): 100 mM KCl, 50 mM Tris-HCl, 5 mM MgCl2, 2 mM EGTA, 1 mM ATP, pH 7.4 to 4°C with KOH; CP2: CP1 supplemented with 0.5% (w/v) fatty acid-free (Fraction V) BSA (see Notes 5–7); Type VIII Protease (see Note 9). SHE: 250 mM sucrose, 5 mM HEPES, 1 mM EGTA, pH 7.4 to 4°C with KOH
2.6 –. MATERIALS REQUIRED FOR ISOLATION OF MITOCHONDRIA FROM BROWN AND WHITE ADIPOSE TISSUE
Specialized equipment – 7 mL glass-on-glass Dounce with loose- and tight-fitting pestles; petri dish or polypropylene cutting board.
Solutions – SHE with 1% BSA: 250 mM sucrose, 5 mM HEPES, 1 mM EGTA, 1% (w/v) fatty acid-free (Fraction V) BSA, pH 7.4 to 4°C with KOH (see Notes 5–7); SHE without added BSA is required for the final spin and resuspension of the final pellet, so set aside 20–30 mLs of medium prior to adding BSA on the day of the assay.
3. METHODS
The isolation protocols here are written for those with access to a refrigerated floor-model centrifuge and rotor(s) capable of spinning 15–18 mL centrifuge tubes up to 12,000g. However, it is possible to adapt the protocol to use a refrigerated benchtop microcentrifuge with multiple microcentrifuge tubes for the fast-speed spins between 10,000g and 12,000g. Indications of where to combine pellets from multiple microfuge tubes into a single tube are given in the protocol.
Additionally, the protocols provided are intended to be broadly accessible and therefore rely on relatively inexpensive Dounce or Potter-Elvehjem tissue homogenizers. Where appropriate, references are provided for those who have access to specialized equipment such as a hand-held mechanical tissue disruptor such as a Kinematica POLYTRON® or IKA ULTRA-TURRAX®. The yield and quality of the mitochondria will vary considerably based on the degree of homogenization: over-disruption of the tissue results in preparations with poor function and low respiratory control, and incomplete tissue disruption results in a suboptimal yield. Everything must be kept ice-cold throughout the isolation procedure, and the steps from animal sacrifice to the initial high-speed spin to pellet the mitochondria should be conducted as quickly as possible to preserve optimal mitochondrial function.
3.1 –. ISOLATION OF MITOCHONDRIA FROM MOUSE LIVER
This protocol is adapted from [3] and images of the preparation and representative oxygen consumption data from mouse liver mitochondria isolated with this method are available in [8].
Pre-chill centrifuges to 4°C and place homogenizers, glassware and plasticware, isolation buffers, and all tubes on ice. Ensure everything remains as cold as possible throughout the isolation procedure. Add BSA to the MSHE medium freshly on the day of isolation, reserving 30–50 mL of MSHE without BSA for the final spin step and resuspension of the mitochondrial pellet.
Euthanize the animal in accordance with institutional IACUC guidelines by anesthetization with isoflurane followed by cervical dislocation. Spray the carcass with ethanol to mat the fur. Using scissors, open the peritoneal wall by making a U-shaped incision in the lower abdomen, being careful not to pierce any of the organs. Remove the liver with forceps and scissors as quickly as possible, placing the tissue in a 50 mL beaker with enough MSHE (with BSA) to cover the tissue.
Mince the tissue with scissors, keeping the beaker submerged in ice the entire time. At frequent intervals, let the tissue quickly settle and decant the medium containing blood, fat, and any connective tissue. Replace with fresh MSHE (with BSA) and repeat the process of mincing and draining the tissue until the tissue is finely minced and the medium is clear.
Pour the tissue into the pre-chilled Teflon-on-glass homogenizer receptacle, washing out any remaining pieces with MSHE. For a single mouse liver, use roughly 10–12 mLs of isolation buffer in a 15 mL (or 40 mL) homogenizer, taking care not to overfill the homogenizer past the narrow part of the receptacle (see Note 10).
Disrupt the tissue with a drill-driven Teflon pestle in 2–3 strokes, ensuring the mixture is homogenous and free of large debris. Transfer this mixture into pre-chilled centrifuge tubes, filling the tubes to approximately 80% of the volume with MSHE (with BSA). Depending on the size of the centrifuge and rotors available, this will usually either be one large (40–50 mL) tube or two smaller (15–18 mL) tubes.
To remove any remaining fat, spin at 12,000g for 10 min at 4°C. After centrifugation, the fat will float to the top of the tube or stick to the sides and can be aspirated/cleaned. The pellet can then be resuspended with MSHE (with BSA) into a homogenous solution and the isolation procedure can continue (see Note 11).
Spin the resuspended homogenate in a fresh tube for 800g for 5 min at 4°C.
Decant the supernatant through two layers of cheesecloth pre-wet with MSHE and into a prechilled 50 mL conical tube or large centrifuge tube.
Centrifuge the supernatant at 12,000g for 10 min at 4°C.
Aspirate the supernatant and resuspend the pellet(s) in a minimal amount (<1mL) of MSHE (with BSA). Gently mix and pipet along the side of the centrifuge tube while keeping the tube on ice as much as possible (see Note 12). Try to exclude any unbroken, contaminating red blood cells that may be visible in the pellet. If multiple microfuge tubes were used for Step 8, combine the pellets into a single tube. Fill the tube to approximately 80% of volume with MSHE (with BSA), gently mix so the solution is homogeneous, and centrifuge with a balance tube at 12,000g for 10 min at 4°C.
Aspirate the supernatant as well as any ‘fluffy,’ light layer sitting atop the dark brown pellet as much as possible (See Note 13). Resuspend the pellet in a minimal amount (<1mL) of MSHE without BSA. Gently mix and pipet along the side of the centrifuge tube while keeping the tube on ice as much as possible. Fill the tube to approximately 80% of volume with MSHE without BSA, gently mix so the solution is homogeneous, and again centrifuge with a balance tube at 12,000g for 10 min at 4°C.
Resuspend the final pellet in a very small volume of MSHE without BSA (generally 50μL-100μL based on the size of the pellet) so as to keep the mitochondrial suspension as concentrated as possible (See Notes 14–15).
Measure the mitochondrial protein concentration using a commercial protein assay such as the BCA assay. From a single mouse liver, an expected yield from this preparation is 15–30mg mitochondrial protein and is dependent on several factors such as the initial amount of tissue, thoroughness of homogenization, degree of light, ‘fluffy’ layer removed, etc.
3.2 –. ISOLATION OF MITOCHONDRIA FROM MOUSE KIDNEY
The mitochondrial isolation presented for mouse kidney presented here is based on that for liver presented in 3.1, and follows historical precedent for using similar isolation protocols for these tissues [9]. Far less tissue is obtained, so the protocol requires a smaller volume of isolation medium and fewer passes with the homogenizer and fast-speed centrifugation steps.
Pre-chill centrifuges to 4°C and place homogenizers, glassware and plasticware, isolation buffers, and all tubes on ice. Ensure everything remains as cold as possible throughout the isolation procedure. Add BSA to the MSHE medium freshly on the day of isolation, reserving 20–30 mL of MSHE without BSA for the final spin step and resuspension of the mitochondrial pellet.
Euthanize the animal in accordance with institutional IACUC guidelines by anesthetization with isoflurane followed by cervical dislocation. Spray the carcass with ethanol to mat the fur. Using scissors, open the peritoneal wall by making a U-shaped incision in the lower abdomen, being careful not to pierce any of the organs.
Remove both kidneys and place in a petri dish kept on ice. Remove the kidney capsule by gently squeezing the organ with the thumb and forefinger. Place both kidneys (cortex and medulla) in a small beaker (usually between 10 and 50 mL) with enough MSHE (with BSA) to cover the tissue.
Mince the tissue with scissors, keeping the beaker submerged in ice the entire time. At frequent intervals, let the tissue quickly settle and decant the medium to remove any residual blood, fat, or connective tissue. Replace with fresh MSHE (with BSA) and repeat the process of mincing and draining the tissue until the tissue is finely minced and the medium is clear.
Pour the tissue into the pre-chilled Teflon-on-glass homogenizer receptacle, washing out any remaining pieces with MSHE (with BSA). For two mouse kidneys, use roughly 6–8 mLs of isolation buffer in a 15 mL homogenizer, taking care not to overfill the homogenizer past the narrow part of the receptacle (see Note 10).
Disrupt the tissue with a drill-driven Teflon pestle in 1–2 strokes, ensuring the mixture is homogenous and free of large debris. Transfer this mixture into a pre-chilled centrifuge tube, filling the tube to approximately 80% of the volume with MSHE (with BSA).
Centrifuge for 800g for 5 min at 4°C (see Note 11).
Decant the supernatant through two layers of cheesecloth pre-wet with MSHE and into a prechilled 50 mL conical tube or centrifuge tube.
Centrifuge the supernatant at 11,600g for 10 min at 4°C.
Aspirate the supernatant and resuspend the pellet in a minimal amount (<1mL) of MSHE without BSA. Gently mix and pipet along the side of the centrifuge tube while keeping the tube on ice as much as possible (see Note 12). Try to exclude any unbroken, contaminating red blood cells that may be visible in the pellet. If multiple microfuge tubes were used for Step 9, combine the pellets into a single tube. Fill the tube to approximately 80% of volume with MSHE without BSA, gently mix so the solution is homogeneous, and centrifuge with a balance tube at 11,600g for 10 min at 4°C.
Aspirate the supernatant as well as any apparent ‘fluffy,’ light layer of the dark brown pellet as much as possible. (see Note 13).
Resuspend the final pellet in a very small volume of MSHE without BSA (generally 20μL-40μL based on the size of the pellet) so as to keep the mitochondrial suspension as concentrated as possible (see Notes 14–15).
Measure the mitochondrial protein concentration using a commercial protein assay such as the BCA assay. From two mouse kidney cortices, an expected yield from this preparation is 4–8 mg mitochondrial protein and is dependent on several factors such as the initial amount of tissue, thoroughness of homogenization, degree of light, ‘fluffy’ layer removed, etc.
3.3 –. ISOLATION OF MITOCHONDRIA FROM MOUSE HEART
This isolation procedure for mitochondria from mouse heart presented here uses a relaxation buffer and hand-driven glass-on-glass homogenizer and does not require specialized equipment [10, 11]. Other isolation protocols use hand-held mechanical tissue disruptors that may be cost-prohibitive for some laboratories [12].
Pre-chill centrifuges to 4°C and place homogenizers, glassware and plasticware, isolation buffers, and all tubes on ice. Ensure everything remains as cold as possible throughout the isolation Add 3 mL of freshly prepared Relaxation Buffer to three wells of a six-well plate and keep on ice.
Euthanize the animal in accordance with institutional IACUC guidelines by anesthetization with isoflurane followed by cervical dislocation. Spray the carcass with ethanol to mat the fur. Using scissors, make a small incision in the lower abdomen and cut the skin upwards along the midline of the chest to expose the ribcage. Remove the heart as quickly as possible, as best results are obtained when the heart is still beating upon removal.
Immediately immerse the still-beating heart in 3 mL of Relaxation Buffer in one well of the six-well dish on ice. Squeeze the heart with tweezers to remove blood from the interior of the organ.
Move the heart to a new well with fresh Relaxation Buffer and incubate for 3–5 minutes on ice. Make initial cuts to the tissue to remove more blood.
Move the cut pieces of heart into a new well containing 3 mL of Relaxation Buffer, leaving behind the blood, mince the tissue into roughly 0.1 cm pieces.
Collect the minced tissue with curved tweezers (or a 1 mL pipette with the end of the tip cut off to increase the aperture) and transfer to the barrel of an ice-cold 7 mL glass-on-glass Dounce homogenizer containing 2–3 mLs SHE (see Note 10 & Note 16).
Homogenize the tissue with 20 strokes of a loose pestle while the homogenizer is on ice. If a vacuum is formed during homogenization and/or it is exceedingly difficult to homogenize, this indicates the tissue pieces are too large.
Perform an additional 20 strokes with a tight pestle. The resulting solution should be a pink-colored homogenate with no large chunks of tissue.
Centrifuge the homogenate 900g for 10 minutes at 4°C to pellet cellular debris, nuclei, and unhomogenized tissue (see Note 11). Fill the centrifuge tube up to about 80% of the total volume with SHE.
Pipette the supernatant to a clean centrifuge tube (or tubes if using microfuge) and discard the pellet. If any of the pellet is mistakenly included during the pipetting, Step 9 can be repeated.
Centrifuge the supernatant at 9000g for 10 minutes at 4°C. A brown mitochondrial pellet should be clearly visible after centrifugation (see Notes 12–13).
Resuspend the pellet in a small amount of SHE buffer (<1 mL) until homogenous with no clumps, then fill the centrifuge tube to about 80% of the total volume with SHE and centrifuge at 9000g for 10 minutes at 4°C. If using microfuge tubes, resuspend the pellets in a small volume (50 – 100 μL) and combine the resuspended pellets into a single tube prior to centrifugation.
Resuspend the pellet in a very small volume of SHE (generally 20μL-40μL based on the size of the pellet) so as to keep the mitochondrial suspension as concentrated as possible (see Note 14). Transfer the mitochondria to a small glass vial or 500μL microfuge tube and keep on ice.
Measure the mitochondrial protein concentration using a commercial protein assay such as the BCA assay. From a single mouse heart, an expected yield from this preparation is roughly 0.6–1.0 mg mitochondrial protein, but is dependent on several factors such as the initial amount of tissue, degree of homogenization, etc.
3.4 –. ISOLATION OF MITOCHONDRIA FROM MOUSE BRAIN
This protocol is based on that published previously [13]. It follows similar differential centrifugation protocols to liver and kidney with a notable exception of a spin step with isolation buffer containing a small amount of the plant sterol glycoside digitonin. The use of a very small [0.02% (w/v)] amount of detergent will permeabilize unbroken synaptosomes – re-sealed synaptic terminals – and yield a preparation containing both synaptosomal and non-synaptosomal mitochondria.
Pre-chill centrifuges to 4°C and place homogenizers, glassware and plasticware, isolation buffers, and all tubes on ice. Ensure everything remains as cold as possible throughout the isolation procedure. Add BSA to the MSHE medium freshly on the day of isolation, reserving 20–30 mL of MSHE without BSA for the final spin step and resuspension of the mitochondrial pellet.
Euthanize the animal in accordance with institutional IACUC guidelines. For brain mitochondria, ideally anesthetize the animal with isoflurane and, once the blinking reflex is lost and the animal is refractory to toe pinch, quickly remove from the induction chamber and place the head (right behind the ears) into the guillotine. Decapitate in one swift motion. If a guillotine is unavailable, cervical dislocation can be used.
Harvest the brain from the skull by first spraying the head with 70% EtOH to mat the fur followed by cutting the skin from the top of the head down the midline from posterior to anterior (to nose). Use rongeurs or curved scissors and forceps to carefully break off pieces of the skull, making sure not to dig out chunks of tissue. When the whole brain is exposed, use a spatula to scoop the brain out from anterior to posterior, cutting the optic nerves to remove the brain. Place brain on an inverted petri dish kept on ice.
Working quickly, remove the brain stem and any easily accessible meninges or connective tissue.
Place the tissue in a small beaker (25–50 mL) with a small volume of MSHE (with BSA) just covering the tissue. Quickly chop the brain into small pieces in the MSHE. occasionally decanting and replacing the medium to remove any connective tissue or fat. Unlike with other tissues, there should be minimal contamination from blood.
Transfer the tissue to a 7 mL glass-on-glass Dounce homogenizer, filling the barrel with 4–5 mL of MSHE (with BSA) (see Note 10).
Homogenize with 6 strokes of the loose pestle and six strokes of the tight pestle.
Transfer the homogenate to a centrifuge tube kept on ice. Use a small amount of MSHE (with BSA) to rinse the homogenizer and maximize the yield.
Centrifuge at 1,200g for 5 min at 4° (see Note 11).
Place two layers of cheesecloth pre-wet with cold MSHE over a centrifuge tube and pass the supernatant through the cheesecloth with a serological pipet or careful pouring. This step can also be completed by using a 50 mL conical tube as a receptacle followed by transferring the homogenate to the appropriate centrifuge tube(s).
Centrifuge at 12,000g for 10 min at 4°C.
While waiting for the spin to complete, heat the pre-made 10% (w/v) digitonin stock on a heat block to re-dissolve after storage, and place at room temperature once back in solution (see Note 17). Add 40 μL of 10% digitonin into 20 mL of MSHE for a final concentration of 0.02% (w/v) for each mouse brain.
Aspirate the supernatant (leaving the white layer) and resuspend the pellet in a small amount of MSHE with 0.02% digitonin until homogenous (<1mL) and then fill the centrifuge tube with the MSHE with 0.02% digitonin (See Note 12).
Centrifuge at 12,000g for 10 min at 4°C.
Aspirate the supernatant along with most of the white, fluffy layer sitting atop the darker brown mitochondrial pellet. Resuspend the mitochondrial pellet in 100 μL of MSHE without BSA. If using multiple microfuge tubes, combine all the pellets into a single tube at this point.
Fill the centrifuge tube to roughly four-fifths of the height of the centrifuge tube with MSHE without BSA, making sure the mixture is homogenous.
Centrifuge at 12,000g for 10 min at 4°C.
Aspirate the supernatant and any remaining white layer of the pellet that can be removed without disturbing the mitochondrial pellet.
Resuspend brown pellet in 20–40 μL of MSHE medium without BSA so as to keep the mitochondrial suspension as concentrated as possible (See Notes 14–15, 18).
Measure the mitochondrial protein concentration using a commercial protein assay such as the BCA assay. From a single mouse brain, an expected yield from this preparation is 1–1.5 mg mitochondrial protein but is dependent on several factors such as the initial amount of tissue, degree of homogenization, amount of lighter layer removed, etc.
3.5 –. ISOLATION OF MITOCHONDRIA FROM MOUSE SKELETAL MUSCLE
Of the tissues presented in this chapter, isolation of mitochondria from skeletal muscle is the procedure requiring the most upfront optimization due to the use of protease to digest fibrous tissue. As indicated in Note 9, several methods are available, some of which have been optimized to enrich for mitochondrial subpopulations (e.g. subsarcolemmal vs. interfibrillar) [14, 15]. The amount of protease and duration of exposure should be optimized in each laboratory to maximize yield while minimizing organelle damage. Overexposure to protease is evident by a gluey, gelatinous tissue suspension (indicating destruction of the nuclear envelope), a ‘fluffy,’ light mitochondrial pellet, and poor respiratory control in oxygen consumption experiments.
Pre-chill centrifuges to 4°C and place homogenizers, glassware and plasticware, isolation buffers, and all tubes on ice. Ensure everything remains as cold as possible throughout the isolation procedure. Add MgCl2 and ATP to the CP1 medium, and BSA to the CP2 medium freshly on the day of isolation. SHE without BSA (30–50 mL) is required for the final spin step and resuspension of the mitochondrial pellet.
Euthanize the animal in accordance with institutional IACUC guidelines by anesthetization with isoflurane followed by cervical dislocation. Spray the carcass with ethanol to mat the fur. Use dissection tools to skin the hindlimbs and excise the gastrocnemius and muscles from the quadriceps. Place the pieces in CP1 buffer kept in a beaker on ice.
On a chilled petri dish or polypropylene cutting board, trim the muscle of connective tissue and fat, at least as much as possible while preserving the muscle yield. Mince the cleaned muscle using scissors in a beaker with CP1 or, alternatively, using a razor blade or a hand-held rolling slicer on an ice-cold polypropylene cutting board.
Transfer minced tissue to CP1 with protease (0.3 mg protease/g tissue) and incubate for 5 min. This incubation occurs off ice at room temperature but beginning with ice-cold medium, but each laboratory should optimize the amount of protease relative to tissue as well as the duration of exposure that yields optimal results (see Notes 9,19).
Homogenize with a glass-on-glass Dounce homogenizer, with the first 10 strokes using the loose pestle and 10–20 strokes with a tight pestle (see Notes 10, 20).
Dilute the homogenized solution 1:1 with CP2 buffer and centrifuge at 600g for 10 min at 4°C to separate any unbroken tissue or debris (see Note 11).
Decant the supernatant through two layers of cheesecloth pre-wet with CP1 and into a prechilled 50 mL conical tube or centrifuge tube.
Centrifuge at 10,000g for 10 min at 4°C to pellet the mitochondria.
Resuspend the pellet in a minimal amount of CP2 buffer (<1mL) and ensure the suspension is homogenous. Fill the centrifuge tube to 80% of the volume with CP2 (see Note 12).
Repeat Step 9, centrifuging at 10,000g for 10 min at 4°C.
Resuspend the pellet in a minimal amount of SHE buffer (<1mL) and ensure the suspension is homogenous. If using multiple microfuge tubes, resuspend the individual pellets in a smaller volume (50–100 μL) and consolidate the resuspensions into a single tube for the final spin. Fill the tube to 80% of the total volume in SHE buffer and centrifuge at 10,000g for 10 min at 4°C.
Resuspend dark brown pellet (see Note 13) in 20–100 μL of SHE medium without BSA so as to keep the mitochondrial suspension as concentrated as possible (see Notes 14–15).
Measure the mitochondrial protein concentration using a commercial protein assay such as the BCA assay. From a single mouse, an expected yield from this preparation is 1–2 mg per g of initial tissue, but is dependent on several factors, notably the degree of proteolysis and homogenization.
3.6 –. ISOLATION OF MITOCHONDRIA FROM MOUSE BROWN ADIPOSE TISSUE
Isolation of mitochondria from brown and white adipose tissue requires an initial high-speed spin step to remove fat from the mitochondrial suspension, and a high concentration of BSA to sequester free fatty acids. Recently, intracellular mitochondrial heterogeneity has been described within the brown adipocyte, and these different mitochondrial populations can be enriched [16].
Pre-chill centrifuges to 4°C and place homogenizers, glassware and plasticware, isolation buffers, and all tubes on ice. Ensure everything remains as cold as possible throughout the isolation procedure. Add BSA to the SHE medium on the day of isolation, reserving 20–30 mL without BSA for the final spin step and resuspension of the mitochondrial pellet.
Euthanize the animal in accordance with institutional IACUC guidelines by anesthetization with isoflurane followed by cervical dislocation. Place the animal face-down and spray the carcass with 70% (w/v) ethanol to mat the fur. Make an incision in the middle of the back and make a sagittal cut up the spine exposing the scapula. Dissect interscapular brown adipose tissue (BAT) and immediately place in ice-cold SHE buffer (with BSA).
Place the tissue on an ice-cold petri dish wet with SHE (with BSA) and remove any skeletal muscle, white adipose tissue, and connective tissue from the BAT.
Weigh the pooled BAT and mince into ~0.2 cm pieces with scissors or a razor blade. Resuspend the pieces in SHE (with BSA) and place in a 7 or 15 mL glass-on-glass Dounce homogenizer (see Note 10). Disrupt the tissue with 9–10 strokes using the tight pestle until the liquid appears homogenous and the Dounce head moves smoothly through the tube.
Centrifuge the tube at 12,000g for 10 min at 4°C. Discard the supernatant and fat layer and resuspend the mitochondrial pellet in SHE (with BSA). Transfer to a new centrifuge tube taking care to avoid any contamination from the fat layer (see Note 12).
Fill the new centrifuge tube containing the pellet to ~80% of the volume, mixing the homogenate with SHE (with BSA), and centrifuge at 900g for 10 min at 4°C.
Carefully collect the supernatant, leaving behind the pellet containing unbroken cells and other debris.
Centrifuge the supernatant and fat at 10,000g for 10 min at 4°C.
Remove the supernatant and any residual fat, although none should be present at this step. Resuspend the pellet in a minimal amount (<1mL) of SHE (with BSA) by gently mixing and pipetting along the side of the centrifuge tube while keeping the tube on ice as much as possible (see Note 12). If any undesired contaminants are present in the tube (e.g. residual fat or red blood cells to be kept separate from the pellet), transfer the resuspended pellet to a new centrifuge tube.
Fill the tube to approximately 80% of volume with SHE (with BSA), gently mix so the solution is homogeneous, and again centrifuge at 10,000g for 10 min at 4°C.
Repeat Steps 9–10 (resuspending the pellet and centrifuging at 10,000g) but use SHE without BSA.
Measure the mitochondrial protein concentration using a commercial protein assay such as the BCA assay. Resuspend the brown pellet in 50–100 μL of SHE medium without BSA to keep the mitochondrial suspension as concentrated as possible (see Notes 14–15). With BAT from a single mouse, an expected yield from this preparation is 0.25–0.40 mg mitochondrial protein but is dependent on several factors such as the age of the animal, initial amount of tissue, degree of homogenization, etc.
3.7 –. ISOLATION OF MITOCHONDRIA FROM MOUSE WHITE ADIPOSE TISSUE
The mitochondrial yield from WAT is much lower than from other tissues, and it is often advised to pool WAT depots from more than one animal for single preparation.
Pre-chill centrifuges to 4°C and place homogenizers, glassware and plasticware, isolation buffers, and all tubes on ice. Ensure everything remains as cold as possible throughout the isolation procedure. Add BSA to the SHE medium on the day of isolation, reserving 20–30 mL of SHE without BSA for the final spin step and resuspension of the mitochondrial pellet.
Euthanize the animal in accordance with institutional IACUC guidelines by anesthetization with isoflurane followed by cervical dislocation. Spray the carcass with 70% (w/v) ethanol to mat the fur. Dissect out the epididymal fat pads and (if desired) subcutaneous white adipose tissue and place immediately into a Petri dish kept on ice and wet with SHE (with BSA).
Place the tissue on an ice-cold petri dish wet with SHE (with BSA) and remove any debris, connective tissue, or organs (e.g. testes or lymph nodes in proximity to perigonadal fat).
Weigh the pooled WAT and mince into ~0.2 cm pieces with scissors or a razor blade. Resuspend the pieces in SHE (with BSA) and place in a 7 or 15 mL glass-on-glass Dounce homogenizer (see Note 10). Disrupt the tissue with 5–7 strokes using the loose pestle followed by 5–7 strokes with the tight pestle until the liquid appears homogenous and the Dounce head moves smoothly through the tube.
Pass the homogenate through a 630 micron nylon mesh to remove any undigested connective tissue and collect in an ice-cold centrifuge or conical tube.
(Steps 5–11 from the BAT mitochondria prep are shared and reproduced here as steps 6–12) Centrifuge the tube at 12,000g for 10 min at 4°C. Discard the supernatant and fat layer and resuspend the mitochondrial pellet in SHE (with BSA). Transfer to a new centrifuge tube taking care to avoid any contamination from the fat layer (see Note 12).
Fill the new centrifuge tube containing the pellet to ~80% of the volume, mixing the homogenate with SHE (with BSA), and centrifuge at 900g for 10 min at 4°C.
Carefully collect the supernatant, leaving behind the pellet containing unbroken cells and other debris.
Centrifuge the supernatant and fat at 10,000g for 10 min at 4°C.
Remove the supernatant and any residual fat, although none should be present at this step. Resuspend the pellet in a minimal amount (<1mL) of SHE (with BSA) by gently mixing and pipetting along the side of the centrifuge tube while keeping the tube on ice as much as possible (see Note 12). If any undesired contaminants are present in the tube (e.g. residual fat or red blood cells to be kept separate from the pellet), transfer the resuspended pellet to a new centrifuge tube.
Fill the tube to approximately 80% of volume with SHE (with BSA), gently mix so the solution is homogeneous, and again centrifuge at 10,000g for 10 min at 4°C.
Repeat Steps 9–10 (resuspending the pellet and centrifuging at 10,000g) but use SHE without BSA.
Measure the mitochondrial protein concentration using a commercial protein assay such as the BCA assay. Resuspend the pellet in 20–40 μL of SHE medium without BSA to keep the mitochondrial suspension as concentrated as possible (see Notes 14–15). With WAT from a single mouse, an expected yield from this preparation is 0.05 – 0.10 mg mitochondrial protein but is dependent on several factors such as the initial amount of tissue, degree of homogenization, etc.
3.8 –. MITOCHONDRIAL FUNCTIONAL ASSAYS
A suite of functional assays can be conducted with mitochondria once isolated. Of course, the breadth of mitochondrial functions in cellular biology (energy transduction, biosynthesis, calcium homeostasis, metabolite generation for cell signaling, etc.) means that there is no one-size-fits-all functional test [17]. Well established protocols for isolated mitochondria are available to measure respiration [3, 8], mitochondrial membrane potential [18], superoxide production [18, 19], mitochondrial swelling and calcium retention capacity [20], and patch-clamp electrophysiology across the inner membrane [21]. Additionally, frozen mitochondrial samples can be used for studies of individual respiratory chain enzymology [22, 23] and supercomplex formation and function [24].
4. NOTES
The age of the animal should be thoughtfully considered prior to beginning the experiment. For example, it is often advised to use adult animals when isolating mitochondria from most tissues as the tissue mass and subsequent mitochondrial yield will be larger. However, brown adipose tissue depots will decrease with age, so younger animals may be advised for these studies.
C57BL/6J mice supplied by the Jackson Laboratory carry a deletion in the gene encoding the mitochondrial nicotinamide nucleotide transhydrogenase (NNT) and the supercomplex assembly factor SCAFI [25].
The protein concentration can depend on the particular detection method used (e.g. biuret, Bradford, Lowry, BCA, etc.). The use of detergents or other contaminants may interfere in sample preparation, and the buffer in which samples are resuspended should be considered when controlling for signal background.
Every effort should be made to establish a setup with a drill-driven Teflon- or PFTE-on-glass homogenizer. Isolating liver mitochondria with a hand-driven homogenizer is possible but results in lower yield and poorer quality.
Isolation buffers such as MSHE and SHE without BSA can be filter-sterilized and stored at 4°C for weeks on end. The pH should be checked prior to use, and BSA must be added freshly on the day of use. A base medium for CP1 consisting of KCl, Tris, and EGTA can also be made in advance, filter-sterilized, and stored at 4°C, with the other additives added on the day of the assay.
A solution of fatty acid-free (Fraction V) BSA can be made in advance with tissue culture-grade water at 10% (w/v), filter-sterilized, and stored at 4°C. BSA should be added freshly on the day of the assay to the isolation medium.
When required, all mitochondria isolation medium should be adjusted with KOH and not NaOH.
A 10% (w/v) digitonin solution in tissue culture-grade water can be made in advance and stored at 4°C until needed on the day of the assay. Preparation of the solution should account for the fact that many commercial preparations are impure, and adjust for this when making the solution [i.e. 1 mL of a 10% (w/v) digitonin solution requires 200 mg of digitonin powder with 50% purity]. When redissolving from cold storage, the digitonin solution can be warmed using a modular heating block until the solution is transparent with no insoluble detergent.
Several different types of proteases have been used for the isolation of skeletal muscle mitochondria including nagarse (Type XXVII protease; [26]), subtilisin A (Type VIII protease; [18, 27], trypsin [28], and dispase [15]. The amount of protease and duration of treatment should be optimized prior to conducting experiments.
As a general rule for mitochondrial isolations from all tissues, use 1 mL of isolation buffer for every 100 mg of tissue to be disrupted.
The isolation protocol for liver mitochondria includes an initial high-speed spin similar to mitochondrial isolation from BAT and WAT to remove any contaminating fat. Minimizing contamination from free fatty acids is particularly important for functional measurements to minimize free fatty acid-induced uncoupling and maximize respiratory control. This initial high-speed spin step can be used for mitochondrial isolations from any tissue.
Avoid resuspending the mitochondrial pellet in too large of an initial volume, as this can lead to a heterogenous, ‘broken’ suspension with the pellet not fully resuspended. Resuspend in a small volume initially for a homogenous mix and sequentially dilute this mixture.
The mitochondrial pellet is brown “café au lait” color. For liver mitochondrial preparations, it will often sit below a looser, tan “light” layer that should be aspirated [3, 29]. In preparations from skeletal muscle, the mitochondrial pellet may sit below a gluey, gelatinous layer indicative of excessive homogenization or proteolysis. This layer should be removed and can be avoided with careful optimization of the protocol and additional spins [15].
The final mitochondrial resuspension should be kept highly concentrated to maximize the duration in which functional studies can be conducted. Mitochondrial suspensions should be no lower than 20 mg/mL. Most suspensions should be kept between 20–50 mg/mL, though for larger yields such as those from mouse liver, concentrations of ~100 mg/mL are preferred. Try to avoid bubbles as much as possible during the resuspension.
BSA must be excluded from the final resuspension to avoid any contribution to the protein determination. BSA is excluded from the final spin step in many protocols for this reason.
Although this protocol does not use BSA in the isolation medium, others do [12] and this should be considered if there is reason to think fat tissue is contaminating the mitochondrial isolation or functional measurements suggest a high degree of mitochondrial uncoupling.
Digitonin should be added freshly to the MSHE medium immediately before use.
The mitochondrial pellet from brain mitochondria is often lighter in color than those obtained from other tissues.
Although it may seem best to standardize the amount of protease to units of activity rather than mass of protease, in our experience we have found this can be unreliable. We have found success with a value of 0.3 mg protease/g tissue in line with literature consensus.
The number of strokes should be optimized to fully homogenize the tissue without damaging the mitochondria or nuclear envelope. A hand-held mechanical tissue disruptor can also be used here to help break apart fibrous muscle tissue.
5. REFERENCES
- 1.Chappell JB, Hansford RG (1972) PREPARATION OF MITOCHONDRIA FROM ANIMAL TISSUES AND YEASTS. Subcellular Components 77–91. 10.1016/B978-0-408-70360-4.50009-2 [DOI] [Google Scholar]
- 2.Brand MD, Nicholls DG (2011) Assessing mitochondrial dysfunction in cells. Biochemical Journal [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rogers GW, Brand MD, Petrosyan S, et al. (2011) High throughput microplate respiratory measurements using minimal quantities of isolated mitochondria. PLoS One. 10.1371/journal.pone.0021746 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Divakaruni AS, Jastroch M (2022) A practical guide for the analysis, standardization and interpretation of oxygen consumption measurements. Nat Metab 4:. 10.1038/S42255-022-00619-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Toime LJ, Brand MD (2010) Uncoupling protein-3 lowers reactive oxygen species production in isolated mitochondria. Free Radic Biol Med 49:606–611. 10.1016/J.FREERADBIOMED.2010.05.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wong HS, Monternier PA, Orr AL, Brand MD (2018) Plate-Based Measurement of Superoxide and Hydrogen Peroxide Production by Isolated Mitochondria. Methods Mol Biol 1782:287–299. 10.1007/978-1-4939-7831-1_16 [DOI] [PubMed] [Google Scholar]
- 7.Dröse S, Brandt U (2008) The mechanism of mitochondrial superoxide production by the cytochrome bc1 complex. J Biol Chem 283:21649–21654. 10.1074/JBC.M803236200 [DOI] [PubMed] [Google Scholar]
- 8.Yang K, Doan MT, Stiles L, Divakaruni AS (2021) Measuring CPT-1-mediated respiration in permeabilized cells and isolated mitochondria. STAR Protoc 2:. 10.1016/j.xpro.2021.100687 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Johnson D, Lardy H (1967) [15] Isolation of liver or kidney mitochondria. Methods Enzymol 10:94–96. 10.1016/0076-6879(67)10018-9 [DOI] [Google Scholar]
- 10.Liesa M, Luptak I, Qin F, et al. (2011) The mitochondrial transporter ABC-me (ABCB10) is a novel gene required for cardiac recovery after ischemia-reperfusion. Circulation 124:806. 10.1161/CIRCULATIONAHA.110.003418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sahin E, Colla S, Liesa M, et al. (2011) Telomere dysfunction induces metabolic and mitochondrial compromise. Nature 470:359. 10.1038/NATURE09787 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Woodall BP, Orogo AM, Najor RH, et al. (2019) Parkin does not prevent accelerated cardiac aging in mitochondrial DNA mutator mice. JCI Insight 5:. 10.1172/JCI.INSIGHT.127713 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kushnareva Y, Murphy AN, Andreyev A (2002) Complex I-mediated reactive oxygen species generation: modulation by cytochrome c and NAD(P)+ oxidation–reduction state. Biochemical Journal 368:545–553. 10.1042/BJ20021121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cogswell AM, Stevens RJ, Hood DA (1993) Properties of skeletal muscle mitochondria isolated from subsarcolemmal and intermyofibrillar regions. 10.1152/ajpcell19932642C383264:. [DOI] [PubMed]
- 15.Lai N, M. Kummitha C, Rosca MG, et al. (2019) Isolation of mitochondrial subpopulations from skeletal muscle: Optimizing recovery and preserving integrity. Acta Physiol (Oxf) 225:. 10.1111/APHA.13182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Benador IY, Veliova M, Mahdaviani K, et al. (2018) Mitochondria Bound to Lipid Droplets Have Unique Bioenergetics, Composition, and Dynamics that Support Lipid Droplet Expansion. Cell Metab 27:869–885.e6. 10.1016/j.cmet.2018.03.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jones AE, Sheng L, Acevedo A, et al. (2021) Forces, fluxes, and fuels: Tracking mitochondrial metabolism by integrating measurements of membrane potential, respiration, and metabolites. Am J Physiol Cell Physiol 320:C80–C91. 10.1152/ajpcell.00235.2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Affourtit C, Quinlan CL, Brand MD (2012) Measurement of proton leak and electron leak in isolated mitochondria. Methods in Molecular Biology. 10.1007/978-1-61779-382-0_11 [DOI] [PubMed] [Google Scholar]
- 19.Starkov AA (2010) Measurement of mitochondrial ROS production. Methods Mol Biol 648:245–255. 10.1007/978-1-60761-756-3_16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bhosale G, Duchen MR (2019) Investigating the Mitochondrial Permeability Transition Pore in Disease Phenotypes and Drug Screening. Curr Protoc Pharmacol 85:. 10.1002/CPPH.59 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bertholet AM (2021) The Use of the Patch-Clamp Technique to Study the Thermogenic Capacity of Mitochondria. J Vis Exp 2021:. 10.3791/62618 [DOI] [PubMed] [Google Scholar]
- 22.Acin-Perez R, Benador IY, Petcherski A, et al. (2020) A novel approach to measure mitochondrial respiration in frozen biological samples. EMBO J 39:. 10.15252/EMBJ.2019104073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Spinazzi M, Casarin A, Pertegato V, et al. (2012) Assessment of mitochondrial respiratory chain enzymatic activities on tissues and cultured cells. Nat Protoc 7:1235–1246. 10.1038/NPROT.2012.058 [DOI] [PubMed] [Google Scholar]
- 24.Acín-Pérez R, Hernansanz-Agustín P, Enríquez JA (2020) Analyzing electron transport chain supercomplexes. Methods Cell Biol 155:181–197. 10.1016/BS.MCB.2019.12.002 [DOI] [PubMed] [Google Scholar]
- 25.Enríquez JA (2019) Mind your mouse strain. Nat Metab 1:5–7. 10.1038/S42255-018-0018-3 [DOI] [PubMed] [Google Scholar]
- 26.Nedergaard J, Cannon B (1979) Overview--preparation and properties of mitochondria from different sources. Methods Enzymol 55:3–28. 10.1016/0076-6879(79)55003-4 [DOI] [PubMed] [Google Scholar]
- 27.White AT, Philp A, Fridolfsson HN, et al. (2014) High-fat diet-induced impairment of skeletal muscle insulin sensitivity is not prevented by SIRT1 overexpression. Am J Physiol Endocrinol Metab 307:E764–E772. 10.1152/AJPENDO.00001.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Frezza C, Cipolat S, Scorrano L (2007) Organelle isolation: functional mitochondria from mouse liver, muscle and cultured filroblasts. Nature Protocols 2007 2:2 2:287–295. 10.1038/nprot.2006.478 [DOI] [PubMed] [Google Scholar]
- 29.Heisler CR (1991) Mitochondria from rat liver: Method for rapid preparation and study. Biochem Educ 19:35–38. 10.1016/0307-4412(91)90145-X [DOI] [Google Scholar]


