Abstract
Background:
The availability of more accurate point-of-care technology could increase the number of persons aware of their HIV status. The DPP® HIV-1/2 assay is the first dual path platform rapid test (RT) approved in the U.S. that also received the Clinical Laboratory Improvement Amendments (CLIA) waiver for use with oral fluid and fingerstick and venous whole blood.
Objective:
To evaluate the performance of the DPP® HIV-1/2 assay with plasma specimens.
Study design:
Sensitivity and specificity of the assay were calculated from 696 HIV-1 groups M (B and non-B subtypes) and O and HIV-2 (groups A and B) specimens and 505 HIV-negative specimens, respectively. Analysis of the assay performance in HIV-1 early infections was assessed by estimating the relative sensitivity of the RT before the Western blot (WB) becomes positive using a 50% cumulative frequency analysis and by comparing the reactivity with other Food and Drug Administration (FDA)-approved RTs.
Results:
The sensitivity for established infection was 100% for HIV-1 and 100% for HIV-2. The specificity was 100%. The DPP® HIV-1/2 assay performs similarly to most antibody-based RT approved by FDA in early HIV-1 infections.
Conclusions:
The DPP® technology showed no significant improvement for detecting early infections over other lateral-flow RTs used in the U.S. Without more data on the DPP® HIV-1/2 assay, especially from whole blood and oral fluid specimens collected during the early phase of infection, its performance as point-of-care technology remains to be assessed.
Keywords: HIV-1/2, Diagnostics, Rapid test, DPP
1. Background:
HIV rapid tests (RTs) have been widely adopted mainly to increase the number of people who are aware of their HIV status and to provide opportunities for faster linkage to care and treatment for those infected [1–3]. RTs are simple and easy to use, can be performed on different sample types, have fast result turn-around times (usually less than 30 min), and can be used in many settings since they do not require sophisticated equipment.
In the U.S. market, seven Food and Drug Administration (FDA)-approved RTs for detection of HIV antibodies were available prior to the approval of the CHEMBIO DPP® HIV-1/2 assay (DPP® HIV) in December 2012. DPP® HIV is the first RT approved in the U.S. that incorporates the dual path platform (DPP) technology. Although the DPP® HIV received the Clinical Laboratory Improvement Amendments (CLIA) waiver in 2014, this test had been used, mainly with oral fluid (OF), outside of the U.S. since 2009 [4,5]. In addition, in 2013, a RT that detects and distinguishes between HIV antigen and antibody was approved in the US.
The DPP® HIV is a single-use immunochromatographic RT approved for HIV antibody detection with OF, whole blood, serum, or plasma specimens. It employs a combination of antibody binding protein, which is conjugated to colloidal gold dye particles and HIV-1/2 antigens, which are bound to the membrane solid phase. Because of the DPP technology, it contains two wells, one for sample addition and another for the running buffer, which allows the sample and running buffer to flow in two different directions. The sample is added to the SampleTainer™, a bottle containing 1 ml of sample diluent buffer. After mixing, two drops (~65 μl) are dispensed in the first well allowing sample conversation for further testing if needed. After the sample has migrated to the test strip (5 min), the running buffer is added to the second well and allows reagents to flow over the antigen coated strip containing the specimen. In the presence of HIV antibodies, a dye conjugated-immune complex is formed on the HIV antigens immobilized on the membrane. A control line that contains anti-human immunoglobulin G is present to ensure that the sample and reagents have been properly applied. Since the DPP® HIV is approved for multiple specimen types, each kit contains disposable sample loops for whole blood, plasma and serum specimens, OF swabs, and SampleTainerTM and running buffer bottles [6].
Although the DPP® HIV can be used with different specimen types and studies have reported very good sensitivity and specificity for DPP® HIV using OF and whole blood [4,5,7–9], no study has been conducted in early infections and/or using plasma specimens. Results using plasma specimens have been limited to the manufacturer’s clinical trials which reports 99.9% sensitivity for HIV-1 and 99.5% for HIV-1 non-B subtypes, 100% sensitivity for HIV-2, and 99.9% specificity [6].
2. Objective
To evaluate the performance of the DPP® HIV using subsets of plasma specimens characterized in four different studies performed at CDC from individuals with HIV-1 and HIV-2 established infections, uninfected individuals, and HIV-1 seroconverters [10–13].
3. Study design
3.1. HIV assay
The DPP® HIV-1/2 assay (CHEMBIO Diagnostic Systems, Inc., Medford, NY) was performed as indicated in the package insert for plasma samples [6]. All specimens were tested at ambient temperature in singlet and repeated only if invalid results were obtained. Briefly, 10 μl of plasma specimens were added into the SampleTainer™, mixed, and 2 drops (~65 μl) were used to run the assay. Results were read between 10 and 25 min after the addition of the running buffer.
3.2. Sample sets
Subsets of previously characterized plasma specimens were used in this study to assess sensitivity and specificity of DPP® HIV. For sensitivity, a total of 696 plasma specimens from individuals with HIV-established infections (HIV Western blot (WB)-positive) were tested with DPP® HIV. The HIV-1 subsets included 487 of 2202 characterized in the Wesolowski [9] study from the U.S. and 127 of 621 from the Owen [11] study from the U.S. and Cameroon. Five-hundred specimens from the U.S. were presumably infected with HIV-1 subtype B virus [10,11], but 114 specimens from Cameroon were sequenced in the p17 and gp41 regions, 111 were HIV-1 group M subtypes A, G, F1, F2, D, CRF 01, CRF 02, CRF 11, CRF 09, and CRF 13 and 3 were HIV-1 group O [12]. The HIV-2 subset included 82 of 86 HIV-2 WB-positive plasma specimens from Ivory Coast (Boca Biolistics, Inc., Coconut Creek, FL) [12]. HIV-2 specimens were tested by Multispot HIV-1/HIV-2 rapid test (Bio-Rad Laboratories, Redmond, WA) in the field and further characterized at CDC with HIV-2 WB (MP Diagnostics). Sequence analysis in the integrase region was conducted on 30 of the 82 HIV-2 specimens: 7 were group A and 23 were group B [12]. For specificity, a total of 505 HIV-uninfected plasma specimens were also tested. The subsets included 480 of 1517 that were identified as negative during antibody and nucleic acid testing in a blood donation center and further characterized in Wesolowski study [10], 20 of 513 from Owen [11] study, and a false-positive (Multispot) antibody panel (n = 5; BBI-SeraCare Diagnostics, West Bridgewater, MA).
In addition, to assess performance for detecting early infection, well-characterized HIV-1 seroconversion panels from the US (presumably subtype B) were obtained from Zeptometrix, Inc. (Buffalo, NY) and BBI-SeraCare Diagnostics [12–14]. As previously reported, each panel had at least one specimen that was WB indeterminate (9 seroconverters with 64 total specimens) or WB positive (17 seroconverters with 166 total specimens) and all panels had at least one specimen that was HIV-1 NAT-only positive. Serial plasma specimens from 17 seroconverters (n = 166) that met the criteria of positive WB were used to estimate the relative sensitivity of the RT. The test reactivity was compared with data previously obtained on other tests with this same panels in the 50% cumulative frequency analysis by calculating the days at which 50% of each test became positive relative to when the first positive WB [11–13].
A pair comparison statistical test (McNemar’s test with one degree of freedom and continuity correction; 95% confidence interval) was used to analyze the differences in reactivity during early infections among 26 seroconverters (n = 230 specimens) between DPP® HIV and other FDA-approved tests.
Because all specimens used in this study were unlinked from personal identifiers, this study was determined by the CDC to be research not involving human subjects.
4. Results
4.1. Sensitivity and specificity of the DPP® HIV-1/2 assay
The sensitivity of the DPP® HIV for all 614 established HIV-1 infections (WB-positive) was 100% (95% CI: 99.4–100%), while the sensitivity for 82 HIV-2 infections was 100% (95% CI: 95.5–100%). The reactivity of the DPP® HIV in different sample sets is shown in Table 1. The assay detected all infections with HIV-1 groups M (subtypes B and non-B) and O, and HIV-2 groups A and B.
Table 1.
Sensitivity of DPP® HIV-1/2 rapid test.
| DPP® HIV assay |
|||||
|---|---|---|---|---|---|
| Total | Reactive | Non-reactive | Sensitivity (%) | 95% Confidence intervals | |
|
HIV-1 Group M HIV-1 B subtype |
500 | 500 | 0 | 100 | 99.24–100 |
| HIV-1 non-B subtype | 111 | 111 | 0 | 100 | 96.65–100 |
| HIV-1 Group O | 3 | 3 | 0 | 100 | 43.85–100 |
| HIV-2 | 82 | 82 | 0 | 100 | 95.52–100 |
Specimens from all 500 HIV-uninfected individuals and all members of the false-positive panel were non-reactive with the DPP® HIV. The specificity of the DPP® HIV was 100% (95% CI: 99.3–100%).
4.2. DPP® HIV-1/2 assay performance in early HIV-1 infections
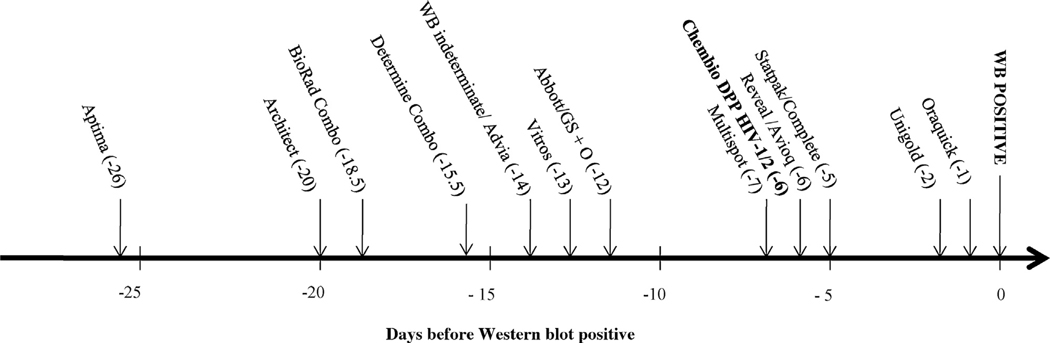
The relative sensitivity of the DPP® HIV in 166 plasma specimens was estimated by calculating the 50% cumulative frequency as described previously [11–13]. The sequence of test reactivity expressed as the number of days before the first positive WB is shown in Fig. 1. The DPP® HIV was estimated to be positive 6 days before the WB becomes positive. The analysis, using the historically obtained data with the same specimen set placed the DPP® HIV with Reveal (flow through test), between Multispot (flow through test), Statpak and Complete (lateral flow tests) and 4 and 5 days before Unigold and Oraquick, respectively, two lateral flow RTs.
Fig. 1.
Sensitivity of assay reactivity during early HIV-1 infections as number of days before first positive WB when 50% of specimens tested with each test became positive. The names, abbreviations, and sources, of the HIV assays previously evaluated 11,12 are as follows: APTIMA HIV-1 Quantitative assay (Aptima, Gen-Probe, Inc., San Diego, CA); ARCHITECT® HIV Ag/Ab Combo assay (Architect; Abbott Diagnostics, Wiesbaden Germany; CE marked version was used as the US version was not available when testing was conducted); GS HIV Combo Ag/Ab (BioRad Combo; Bio-Rad Laboratories, Redmond, WA); DetermineTM HIV-1/2 Ag/Ab Combo (Determine Combo (rapid test); Alere Medical Co., Ltd. Scarborough, ME); GS HIV-1/HIV-2 PLUS O EIA (GS + O; Bio-Rad Laboratories, Redmond, WA); VITROS anti-HIV 1 + 2 assay (Vitros; Ortho-Clinical Diagnostics, Buckinghamshire, UK); ADVIA Centaur HIV 1/O/2 enhanced assay (Advia; Bayer, Tarrytown, NY); Abbott HIVAB HIV-1/2 (rDNA) EIA (Abbott; Abbott Laboratories, Abbott Park, IL); Avioq HIV-1 Microelisa system (Avioq; Avioq, Inc, Rockville, MD); Multispot HIV-1/HIV-2 rapid test (Multispot (rapid test); Bio-Rad Laboratories); Clearview HIV-1/2 STAT-PAK (Statpak (rapid test); Inverness Medical, Princeton, NJ); Clearview Complete HIV-1/2 (Complete (rapid test); Inverness Medical); Reveal G2 and G3 Rapid HIV-1 antibody tests (Reveal G2 or G3 (rapid test); MedMira Laboratories, Inc.; Halifax, Nova Scotia, Canada); OraQuick ADVANCE Rapid HIV-1/2 antibody test (Oraquick (rapid test); OraSure Technologies, Inc.; Bethlehem, PA); Uni-Gold Recombigen HIV (Unigold (rapid test); Trinity Biotech USA, St. Louis, MO). These assays have manufacturer reported point estimates for sensitivity ranging from 99.60% to 100.00% and point estimates for specificity ranging from 98.60% to 99.90%. The Genetic Systems HIV-1 Western blot (WB; Bio-Rad Laboratories) and Cambridge Biotech HIV-1 Western blot (WB; Maxim Biomedical Inc., Rockville, MD) have been shown to give concordant interpretations in studies conducted to qualify use in our clinical laboratory and were used interchangeably.
The paired comparison analysis of antibody reactivity using 230 plasma specimens from 26 individuals in early stages of HIV-1 infections showed that DPP® HIV performs similarly to Reveal, Unigold and Statpak (Table 2). In contrast, DPP® HIV performed significantly better than Complete and Oraquick, and significantly less well than Multispot.
Table 2.
Comparison of reactivity of DPP® HIV and other antibody-based rapid tests during early stages of HIV-1 infection.
| DPP® HIV assay |
|||
|---|---|---|---|
| Reactive(n) | Non-Reactive(n) | p value | |
| Oraquick-R | 62 | 1 | 0.0001* |
| Oraquick-NR | 19 | 148 | |
| Complete-R | 72 | 1 | 0.0269* |
| Complete-NR | 9 | 148 | |
| Unigold-R | 62 | 10 | 0.1374 |
| Unigold-NR | 19 | 139 | |
| Statpak-R | 74 | 1 | 0.0771 |
| Statpak-NR | 7 | 148 | |
| Reveal-R | 75 | 5 | 1 |
| Reveal-NR | 6 | 144 | |
| Multispot-R | 78 | 12 | 0.039* |
| Multispot-NR | 3 | 137 | |
The numbers (n) reflect the paired comparison analysis of 230 plasma specimens from 26 seroconverters. The reactivity of DPP® HIV was compared to historical data generated with each of the following FDA-approved rapid tests: OraQuick ADVANCE (Oraquick), Uni-Gold Recombigen HIV (Unigold), Reveal G2 and G3 Rapid HIV-1 antibody test (Reveal), Multispot HIV-1/HIV-2 rapid test (Multispot), Clearview HIV-1/2 STAT-PAK (Statpak), and Clearview HIV-12/ Complete (Complete). The p values were obtained from the McNemar’s statistical analysis. The values with an asterisk indicate a statistically significant difference in reactivity between tests during early stages of HIV-1 infection. R: reactive; NR: non-reactive.
5. Discussion
After approval by FDA, this is the first performance evaluation of the CHEMBIO DPP® HIV-1/2 assay among well-characterized plasma specimens showing that overall HIV-1 and HIV-2 sensitivities and specificity were within the reported values in the package insert [6]. Results from field evaluations in Nigeria and Mozambique indicate that the assay performs well in OF and whole blood [4,8] specimens from these countries which predominantly have subtypes A, C, CRF-02 AG, and G. Cameroon specimens of these subtypes were also detected using plasma in our study. However, a recent study showed a lower sensitivity in OF among specimens with low viral load and/or high CD4 counts [5]. DPP® HIV detected all HIV-2 specimens (group A and B) from Ivory Coast, so despite the fact that the assay does not distinguish between HIV-1 and HIV-2, detection of HIV-2 is not a problem.
In early HIV-1 infections, the analysis of the sensitivity relative to other RTs using the same sample set was limited to RTs that detect HIV antibody only that were available from previous evaluations. No comparison was done with the Determine™ HIV-1/2 Ag/Ab Combo RT which detects and distinguishes HIV antigen and antibody and has been shown to detect more early infections than any other RTs in the same specimen set [12]. DPP® HIV performs similarly to other antibody RTs that are flow through (Reveal) or lateral flow (Stat-Pak and Unigold). However, the analysis in longitudinal samples from seroconverters showed that DPP® HIV detected antibodies against HIV-1 significantly earlier in the infections than Oraquick, which is less sensitive than other RTs in the cumulative frequency analysis. One of the limitations of the analysis that may have contributed to the differences observed in test reactivity during early infection is that in some cases not every RT result remained reactive after the first reactive result. In addition, testing in early stages of infection challenges the analytical sensitivity of any assay. In this case, negative results from singlet testing (which represents clinical sensitivity) in the seroconversion panels may reflect that the sample contains levels of antibodies that are close to the threshold of the assay’s analytical sensitivity thus missed by the assay and may be detected if repeated. However, the performance of the DPP® HIV, an IgG-only RT that requires 10 μl of serum or plasma, was not substantially different compared to other RTs that either require approximately 2.5 times more sample volume (Multispot) or detect IgM and IgG (Unigold). Another limitation of the study is that no data in the same sample set were available for comparison with INSTI™ HIV-1 Antibody Test (bioLytical, Richmond, BC, Canada), another flow through FDA-approved RT.
It is desired for new HIV assays to improve detection of early infections so that infected persons can be linked to care and treatment. According to the manufacturer, the DPP format is designed to improve sensitivity by separating sample and reagent flow. Previous reports have shown that the assay performs well in high- and low-risk populations from Nigeria, Mozambique and the U.S. using OF or whole blood [4,7–9]. However, published data also suggest that the performance of the DPP® HIV with OF from individuals in early stages of infection or on antiretroviral therapy may not be as sensitive [5]. In our study with plasma specimen, despite the earlier detection of HIV-1 antibodies by Multispot, the FDA-approved DPP® HIV RT has a sensitivity similar to most FDA-approved antibody-based RTs in early and established HIV infections when used with serum and plasma specimens. Furthermore, our data show similar performance characteristics for plasma samples (subtype-B, non-subtype B and seroconversion panels) to that reported in the current DPP® HIV package insert. Additional studies to evaluate its performance at point-of-care, especially with whole blood and OF from individuals in early stages of infection or on antiretroviral therapy, are needed to further characterize this assay.
Funding
Centers for Disease Control intramural funding.
Footnotes
Competing interest
No financial disclosures were reported by the authors of this paper.
Ethical approval
No ethical approval was sought because all specimens used in this study were unlinked from personal identifiers, so this study was determined by the CDC to be research not involving human subjects.
Disclaimer
The findings and conclusions in this report are ours and do not necessarily represent the views of the Centers for Disease Control and Prevention. Use of brand names is for identification purposes and does not imply endorsements by the US Department of Health and Human.
References
- [1].Cherutich P, Bunnell R, Mermin J, HIV testing: current practice and future directions, Curr. HIV/AIDS Rep. 10 (2013) 134–141. [DOI] [PubMed] [Google Scholar]
- [2].Martin EG, Salaru G, Paul SM, Cadoff EM, Use of a rapid testing algorithm to improve linkage to care, J. Clin. Virol. 52S (2011) S11–S15. [DOI] [PubMed] [Google Scholar]
- [3].Patel P, Bennet B, Sullivan T, Parker MM, Heffelfinger JD, Sullivan PS, CDC AHI Study Group. Rapid HIV. Screening: missed opportunities for HIV diagnosis and prevention, J. Clin. Virol. 54 (2012) 42–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Semá Baltazar C, Raposso C, Correia D, Jani I, Shodell D, Nelson L, et al. , Evaluation of performance and acceptability of two rapid oral fluid tests for HIV detection in Mozambique, J. Clin. Microbiol. 52 (2014) 3544–3548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Jaspar M, Le Moal G, Saberan-Roncato M, Plainchamp D, Langlois A, Camps P, et al. , Finger-stick whole blood HIV-1/2 home-use tests are more sensitive than oral fluid-based in-home HIV tests, PLoS One 9 (2014) e101148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Chembio Diagnostic Systems, INC. DPP® HIV1/2 Assay package insert; 2012. [Google Scholar]
- [7].Cappello JM, Gunasekera A, Gunasekera D, Esfandiari J, Ippolito T, A multicenter performance evaluation of the DPP® HIV-1/2 assay for the detection of HIV antibodies in various HIV testing algorithms, J. Clin. Virol. 58S (2013) e59–e64. [DOI] [PubMed] [Google Scholar]
- [8].Iregbu KC, Esfandiari J, Nnorom J, Sonibare SA, Uwaezuoke SN, Eze SO, et al. , Dual path platform HIV ½ assay: evaluation of a novel rapid test using oral fluids for HIV screening at the National Hospital in Abuja, Nigeria, J. Diag. Microbio. 69 (2011) 405–409. [DOI] [PubMed] [Google Scholar]
- [9].Sigismondi L, Ippolito T, Millbrandt J, Gunasekera D, Esfandiari J. Performance evaluation of a novel HIV-1/2 rapid test for the detection of HIV-1/2 antibodies in oral fluid and whole blood. In: HIV Diagnostics conference. 2012. Available: https://custom.cvent.com/ADE0EB81B3184D618E2FB8340F1EC28E/files/29f3717707a44f91859f65feb4cefec6.pdf. [Google Scholar]
- [10].Wesolowski LG, Delaney KP, Hart C, Dawson C, Owen SM, Candal D, et al. , Performance of an alternative laboratory-based algorithm for diagnosis of HIV infection utilizing a third generation immunoassay, a rapid HIV-1/HIV-2 differentiation test and a DNA or RNA-based nucleic acid amplification test in persons with established HIV-1 infection and blood donors, J. Clin. Virol. 52S (2011) S45–S49. [DOI] [PubMed] [Google Scholar]
- [11].Owen SM, Yang C, Spira T, Ou CY, Pau CP, Parekh BS, Candal D, Kuehl D, Kennedy MS, Rudolph D, Luo W, Delatorre N, Masciotra S, Kalish ML, Cowart F, Barnett T, Lal RB, McDougal JS, Alternative algorithms for human immunodeficiency virus infection diagnosis using tests that are licensed in the United States, J. Clin. Microbiol. 46 (2008) 1588–1595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Masciotra S, Luo W, Youngpairoj AS, Kennedy MS, Wells S, Ambrose K, et al. , Performance of the Alere DetermineTM HIV-1/2 Ag/Ab Combo rapid test with specimens from HIV-1 seroconverters from the US and HIV-2 infected individuals from Ivory Coast, J. Clin. Virol. 58S (2013) S54–S58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Masciotra S, McDougal JS, Feldman J, Sprinkle P, Wesolowski L, Owen SM, Evaluation of an alternative HIV diagnostic algorithm using specimens from seroconversion panels and persons with established HIV infections, J. Clin. Virol. 52S (2011) S17–S22. [DOI] [PubMed] [Google Scholar]
- [14].Nasrullah M, Wesolowski LG, Meyer III WA, Owen M, Masciotra S, Vorwald C, et al. , Performance of a fourth-generation HIV screening assay and an alternative HIV diagnostic testing algorithm, AIDS 27 (2012) 731–737. [DOI] [PMC free article] [PubMed] [Google Scholar]