Abstract
Graphene oxide nanomaterials are being developed for wide-ranging applications but are associated with potential safety concerns for human health. We conducted a double-blind randomized controlled study to determine how the inhalation of graphene oxide nanosheets affects acute pulmonary and cardiovascular function. Small and ultrasmall graphene oxide nanosheets at a concentration of 200 μg m−3 or filtered air were inhaled for 2 h by 14 young healthy volunteers in repeated visits. Overall, graphene oxide nanosheet exposure was well tolerated with no adverse effects. Heart rate, blood pressure, lung function and inflammatory markers were unaffected irrespective of graphene oxide particle size. Highly enriched blood proteomics analysis revealed very few differential plasma proteins and thrombus formation was mildly increased in an ex vivo model of arterial injury. Overall, acute inhalation of highly purified and thin nanometre-sized graphene oxide nanosheets was not associated with overt detrimental effects in healthy humans. These findings demonstrate the feasibility of carefully controlled human exposures at a clinical setting for risk assessment of graphene oxide, and lay the foundations for investigating the effects of other two-dimensional nanomaterials in humans. Clinicaltrials.gov ref: NCT03659864.
Subject terms: Nanoparticles, Nanostructures
Assessment of the health risks of exposure to anthropogenic nanomaterials is crucial to maximize their potential applications. This double-blind, randomized controlled study in healthy humans evaluates the impact of inhalation of graphene oxide nanosheets on acute pulmonary and cardiovascular functions.
Main
Two-dimensional (2D) nanomaterials are defined as flat, non-spherically shaped substances with a least one dimension <100 nm, and have generated worldwide interest for a variety of potential applications including building materials, car tyres, inks, food preservatives, sun-screens and anti-corrosion and lubricating products. Graphene is an archetypal 2D nanomaterial of a single layer or few layers of carbon lattice. Its unique structure, strength, flexibility, transparency and electrical conductance properties make it attractive for a wide range of applications1. There is also intense interest in further developing such materials for biomedical applications, including diagnostic and drug-delivery agents2,3. The oxidized form of graphene, graphene oxide (GO), has shown promise in the biomedical setting due to its hydrophilicity, high surface area for chemical functionalization, reasonable colloidal stability in biologically relevant solutions and compatibility with blood cells4. However, like other manufactured nanomaterials, the safety profile and limitations of GO on human exposure need to be determined before widespread use. There are limited and inconsistent toxicological data available for GO, often arising from differences among the many different sources of the material and their notable variability in dimensions and chemical properties that do not allow confident conclusions to be reached regarding its safety5. We have systematically synthesized GO nanosheets with high control and homogeneity of size and minimal trace metal and no endotoxin contaminations6 that do not exert the overt toxicity reported for many commercial sources of other graphene material types (such as graphene nanoplatelets)7–9. These highly purified GO materials have been thoroughly investigated using in vitro and in vivo models by different laboratories during the past decade10,11. A recent repeated and long-term pulmonary exposure study of these GO nanosheets in mice found that large (micrometre range) lateral size and high doses induced pulmonary inflammation (although substantially less than long, rigid carbon nanotubes) and more persistent granulomas12. Smaller (nanometre range) GO nanosheet exposures have demonstrated a transient inflammatory response that resolved rapidly post-exposure12–14.
Parallels can be drawn between manufactured nanomaterials and the small particulate matter in air pollution15. Particulate matter exposure has been linked to adverse health effects in almost every organ of the body16, although the respiratory and cardiovascular effects drive the substantial morbidity and mortality associated with particulate matter. Ultrafine (nano-sized) particulate matter is likely to contribute to these effects, given the high deposition in the alveoli of the lungs, the high reactive surface area for a given mass and the penetration to systemic organs16. While different classes of manufactured nanomaterial have distinct properties, there are commonalities with ultrafine particulate matter with respect to some physiochemical features and the pathways by which they induce toxicological effects, such as inflammation and oxidative stress15. Although there is an expanse of large-cohort epidemiological data linking particulate matter in air pollution with adverse effects, human data on the biological actions of manufactured nanomaterials are confined to cultures of human cell lines and biomonitoring in occupational settings or isolated accidental exposures in small groups of individuals17. Inhalation is the primary route of unintended pulmonary exposure to manufactured nanomaterials, but it also represents a promising route of administration for nanomedicines used for diagnosis and drug delivery for respiratory conditions. Therefore, human data are urgently needed to assess risk assessment and realize the true potential of these materials.
Controlled human exposure studies offer several advantages for assessing the acute biological effects of xenobiotics17. Unlike epidemiological studies, substances can be tested in isolation at defined doses. Real-world confounders, such as other environmental stressors (for example noise, stress, heat, exercise or medication) can be minimized or standardized across participants and study visits. The design can be tailored to include relevant control exposures, with repeated measure designs allowing each participant to be their own control. The use of a controlled exposure environment at clinics or laboratory facilities can broaden the range of endpoints to include a greater range of subclinical and mechanistic endpoints and has been used successfully to determine the respiratory and cardiovascular effects of combustion-derived nanoparticles such as those in diesel exhaust emissions16,18,19. However, only a handful of studies have tested the actions of manufactured nanomaterials, and no studies have been performed with non-spherical materials such as graphene17.
In this study we aimed to understand the potential for GO to have detrimental health effects, principally from the viewpoint of unintended exposure (for example occupationally or from public exposure with increasing use of nanomaterials in real-world applications) but also from the perspective of the development of safe forms of GO for intended human exposure by inhalation (for example for diagnostic imaging of the lung or drug delivery to or via the lung). Using a randomized controlled double-blind crossover design, we investigated the cardiorespiratory effects of acute inhalation of GO nanosheets in human volunteers. We hypothesized that inhalation of our high-purity, thin GO would have only modest effects on cardiorespiratory function and blood markers of inflammation and coagulability, the magnitude of which would be lower the smaller the lateral dimensions of the nanosheets. The findings of this controlled human inhalation exposure to GO in human volunteers lay the foundations for future investigations to establish which properties of graphene materials determine their biological actions to provide a formal risk assessment and allow safe-and-sustainable-by-design development for various applications.
Synthesis and characterization of GO nanosheets
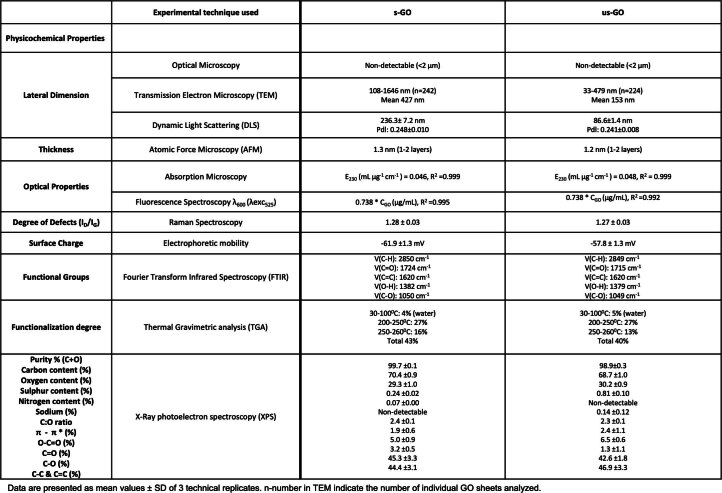
Thin, highly purified metal-free and endotoxin-free GO materials were synthesized using a modified Hummers’ method and comprehensively characterized (Fig. 1, Extended Data Table 1 and Extended Data Fig. 1)6. GO nanosheets were of tightly defined size, with no metallic or other elemental contamination, residues or indicators of bacterial contamination10. Two lateral dimensions (maintaining all other physicochemical characteristics almost identical) were selected for the study: small GO (s-GO) and ultrasmall GO (us-GO). Both types of nanosheet have demonstrated no acute or longitudinal adverse effects in our previous pre-clinical (rodent) studies6, contrary to ‘large’ GO sheets that were thus excluded from this work as a safety precaution.
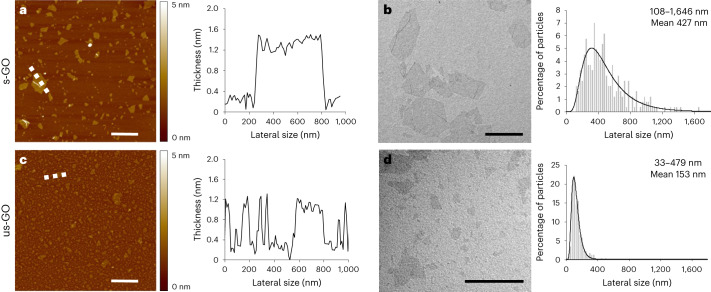
Fig. 1. GO nanosheet size distributions.
a–d, Morphological characterizations of s-GO (a,b) and us-GO (c,d). The images (left) and spectra (right) are representative of the technical replicates performed. Atomic force microscopy height images (left; a and c) with the corresponding cross-section analysis along the indicated dashed white lines (right; a and c). The colour bars indicate the height intensity range from 0 to 5 nm. Transmission electron microscopy micrographs (left; b and d) with the size distribution analyses using a Gaussian single peak fitting depicted with solid lines (right; b and d) are shown. Scale bars are 1 µm. The lateral size range and means given in b and d are an average of 242 and 224 individual GO sheets, respectively (see a summary table in Extended Data Table 1).
Extended Data Table 1.
Summary of the physicochemical properties of s-GO and us-GO
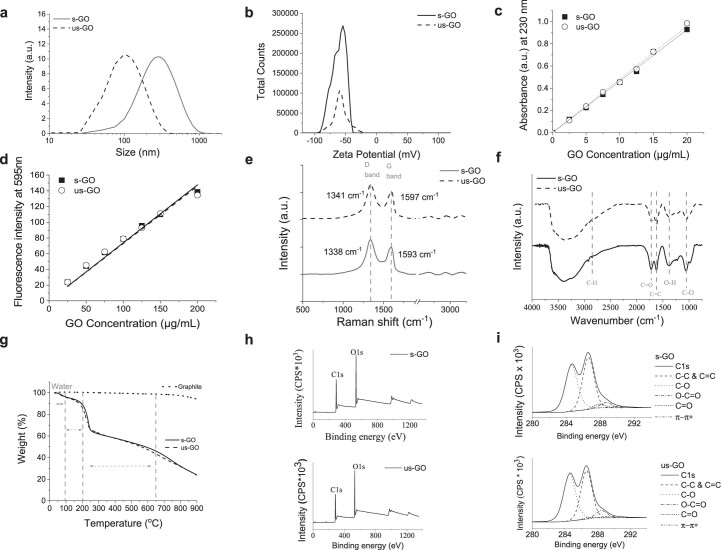
Extended Data Fig. 1. Physicochemical properties of s-GO and us-GO.
a. Dynamic light scattering (DLS) confirms the size reduction from s-GO to us-GO; b. Electrophoretic light scattering (ELS) shows highly negatively charged particles, suggesting a high colloidal stability; c. Absorption spectroscopy confirms the C=C grapheneous backbone signature through its absorbance linearity at 230 nm; d. Upon excitation with λexc = 525 nm (fluorescence spectroscopy), a maximum band at λem ~ 595 nm is generated by electrons transitioning between the oxidized and non-oxidized carbon atoms of the GO sheets; e. Raman spectroscopy highlights a GO-specific spectral signature through D and G bands, an indicator of functional groups (defects) presence onto the grapheneous backbone; f. Fourier-transform infrared spectroscopy (FT-IR) confirms the presence of functional groups such as OH, CH, C=O; C=C and C-O; g. Thermal gravimetric analysis (TGA) also confirms the oxygenated nature of s-GO and us-GO, which show particular degradation steps that are associated with the disruption of COOH, C=O, OH, C-O-C, as opposed to graphite which is stable at temperatures below 600 °C; h. X-Ray photoelectron spectroscopy (XPS) for the survey spectra indicate highly-pure materials of rich oxygen content. i. X-Ray photoelectron spectroscopy (XPS) for the high-resolution C1s spectra mainly involved in C-O, C=O and O-C-O covalent bonds. a.u. = arbitrary units; CPS = counts per second; eV = electronvolt. Data are presented as mean values ± SEM. n-numbers. Representative data of the technical replicates performed are shown.
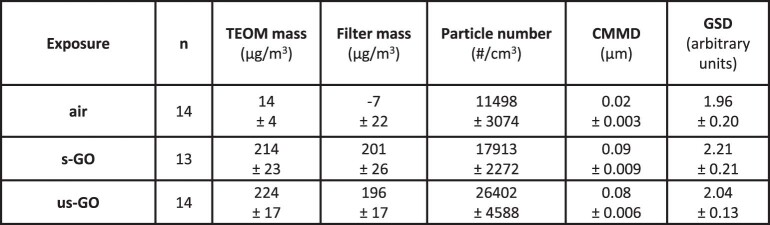
GO nanosheets were aerosolized for exposure of volunteers through inhalation via a face mask with a target mass concentration of 200 μg m−3. The actual concentrations of s-GO and us-GO were 214 ± 23 μg m−3 and 224 ± 17 μg m−3, respectively (Extended Data Table 2) and were maintained at a constant level throughout the 2 h exposure (Supplementary Fig. 1a). As anticipated, the us-GO had a greater particle number than the s-GO for the same mass. The GO agglomerated to airborne sizes with a median value of 80–90 nm and a high size distribution homogeneity (Supplementary Fig. 1b). The levels of GO used here (200 μg m−3) were substantially higher than concentrations of graphene materials found in many workplaces that handle/process these materials (0.4–50 μg m−3)20–22 and are relevant to proposed occupational guidance for graphene nanoplatelets (212 μg m−3)23.
Extended Data Table 2.
Particle characteristics in the exposure aerosol
Clinical effects of inhaled GO
This study was performed in accordance with the Declaration of Helsinki and rigorous ethical review at the University of Edinburgh and relevant UK National Health Service research and development office. Volunteers inhaled either GO or filtered air for 2 h under carefully controlled conditions while intermittently cycling (used to standardize respiratory rates between individuals based on exercise workload determined at the screening visit) (Fig. 2 and Supplementary Fig. 2). Of the 14 participants, 13 completed all 3 visits; one participant was unable to attend their third visit within the time frame of the study. All exposures were well tolerated, with participants reporting no symptoms related to exposure and only mild transient fatigue related to the exercise. No symptoms were reported throughout each study day, and there were no clinical adverse events of any description throughout the study. The lack of overt effects of acute inhalation of high-purity GO highlights the feasibility of controlled exposures of graphene-based nanomaterials for risk assessment in humans.
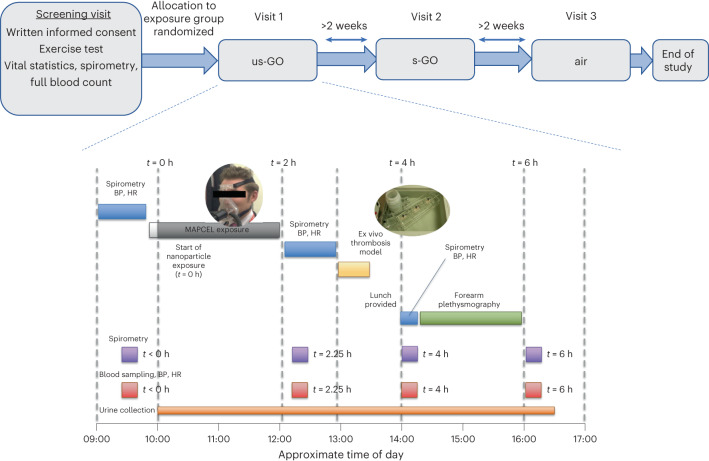
Fig. 2. Study design and study day schedule.
Top: timeline of randomized study visits. Bottom: study day schedule. Inset images show a volunteer inhaling exposures through a tubed face mask (left) and the ex vivo thrombosis chamber (right; see Fig. 4c). BP, blood pressure; HR, heart rate; MAPCEL, mobile ambient particle concentrator exposure laboratory.
Lung function
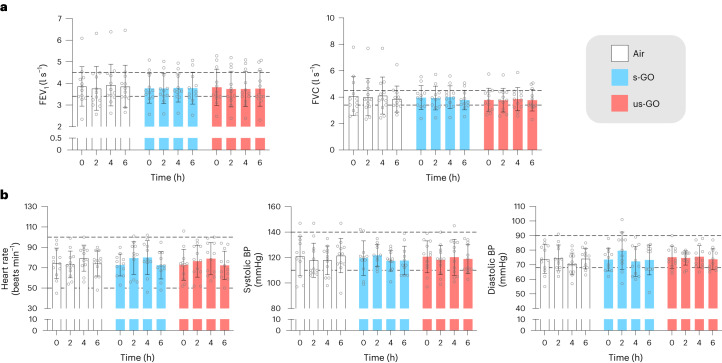
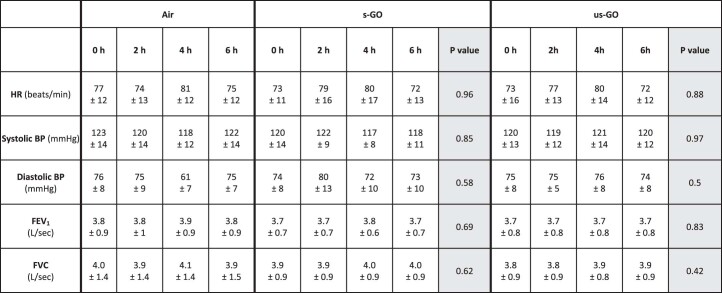
Spirometry is widely used to monitor changes in, or sensitization to, airway reactivity to assess the risk and progression of respiratory conditions such as asthma and chronic obstructive pulmonary disease. The forced expiratory volume in 1 s and forced vital capacity varied little across the time points within each exposure, and there were no differences in either of these parameters between different exposures (P > 0.42 for all comparisons; Fig. 3 and Extended Data Table 3). The findings demonstrate that the GO nanosheet exposures did not directly alter lung function after acute exposure. While studies in animal models have not found effects of GO on airway reactivity, GO has been found to induce pulmonary inflammation (see the Supplementary Information for a discussion). Biomarkers of pulmonary inflammation would be a valuable addition to future human-controlled nanomaterial exposures. Toxicological studies in mice with the same GO materials as the current study found that pulmonary exposure induced a mild and transient pulmonary inflammation12–14. It is possible that GO with greater lateral dimensions could have induced greater inflammation and changes to lung function, or that effects would have been observed in susceptible individuals, such as those with asthma.
Fig. 3. Lung function and cardiovascular vital signs for exposure to air, s-GO and us-GO.
a, Lung function as determined by the forced expiratory volume in 1 s (FEV1, left) and forced vital capacity (FVC, right). b, Cardiovascular vital signs: heart rate (left), systolic BP (middle) and diastolic BP (right). The dotted lines represent the upper and lower limits of expected normal values and the coloured bars and error bars show the mean ± s.d. (n = 12 for air and us-GO groups, n = 11 for s-GO groups; biologically independent measurements). No significant differences were found between treatments (two-way analysis of variance followed by Tukey’s post hoc test).
Extended Data Table 3.
Cardiovascular vital indicators and lung function
Systemic haemodynamic effects
Heart rate and blood pressure were measured at rest at the same time points as lung function. There were no differences in heart rate (P > 0.88) and systolic (P > 0.85) or diastolic (P > 0.5) blood pressure (Fig. 3 and Extended Data Table 3). The lack of effect was anticipated, as our previous volunteer studies did not find consistent increases in heart rate or blood pressure after acute exposure to diesel exhaust nanoparticles19,24,25. Only one study has investigated the effects of graphene material on blood pressure26, finding that dextran-coated graphene nanoplatelets had no effect on blood pressure in mice after high-dose (up to 250 mg kg−1) intravenous exposure. Many manufactured nanomaterials have oxidative properties that stimulate cellular and tissue oxidative stress. In small arterioles, oxidative stress would be expected to increase blood pressure via constriction of blood vessels and impairment of vasodilatory capacity. The lack of effect of GO on blood pressure and the absence of substantive changes in plasma isoprostanes following GO exposure in preliminary lipidomic analyses (see below) suggest that there was no induction of systemic oxidative stress in this study. This is supported by studies using cultured vascular endothelial cells that have demonstrated that while GO is internalized by the cells, it did not generate intracellular superoxide27. Nonetheless, further exploration of these mechanisms is warranted in future human studies, especially for the assessment of the risk of emerging graphene materials with different redox properties.
Blood coagulability
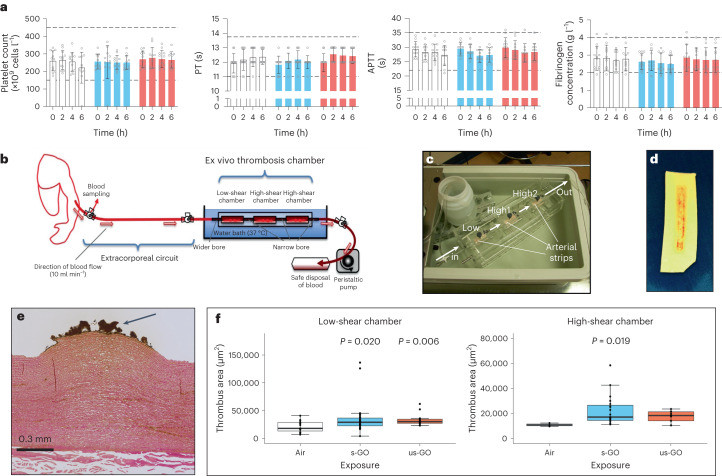
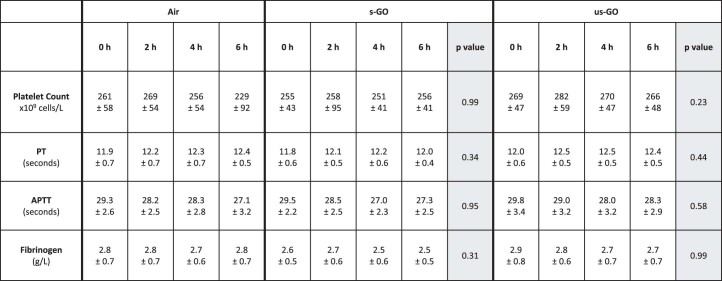
An increased propensity of the blood to clot increases the risk of thrombosis within a blood vessel and concomitant cardiovascular events, such as a heart attack or ischaemic stroke. Human exposure to particulate matter in ambient air pollution is associated with raised markers of blood coagulation, such as fibrinogen, platelet activity and in vitro coagulation assays, although the data are inconsistent28. In this study, blood platelet counts and activated partial thromboplastin times were similar within each exposure (Fig. 4a and Extended Data Table 4). Although a marginal increase was observed in the prothrombin time after exposure (t = 2 h) in the us-GO group (P = 0.02), this increase was transient and remained within normal limits. There were no differences between exposures for any of these parameters. Blood fibrinogen, an essential glycoprotein of the coagulation cascade that is also an acute-phase response factor, was unchanged between exposures. See the Supplementary Information for further discussion.
Fig. 4. Blood coagulability parameters.
a, Platelet counts and coagulability markers for exposure to air (white); s-GO (blue); or us-GO (red). The dotted lines represent the expected upper and lower limits of normal values. The colour bars and error bars show the mean ± s.d. (n = 12 for air and us-GO groups, n = 11 for s-GO groups; biologically independent measurements). No significant differences were found between treatments (two-way analysis of variance followed by Tukey’s post hoc test). b, Schematic of the ex vivo thrombosis chamber set-up for measuring blood thrombogenicity. c, Photograph of the chamber containing the three strips of porcine artery (pink) with the direction of blood flow across the chamber marked. Low, low-shear chamber; High1, first high-shear chamber; High2, second high-shear chamber. d, Porcine strip (white) taken from the chamber with adhering blood (vertical red line) seen running down the centre of the strip. A corner of the strip is removed to identify the direction of blood flow. e, A representative histological section of the strip (pink) with blood (brown, arrow) adhering to the intimal surface. f, Inhalation of either s-GO (P = 0.02) or us-GO (P = 0.006) led to a greater blood thrombogenicity compared with air exposure in the low-shear chamber. A similar pattern was observed in the high-shear chamber, although the effect was only demonstrable for s-GO (P = 0.019; for us-GO, P = 0.07). There was no statistically significant difference in thrombogenicity between s-GO and us-GO. The central lines of the box plots show the median, box ranges show the 25–75th percentiles and whiskers show the minima (25th percentile − 1.5 × interquartile range) and maxima (75th percentile + 1.5 × interquartile range); each point represents a single section from the arterial strip. Biologically independent measurements are from 13 volunteers (low-shear chamber) and 8 volunteers (high-shear chamber). Comparisons with air were made by one-tailed Kruskal–Wallis tests. APTT, activated partial thromboplastin time; PT, prothrombin time.
Extended Data Table 4.
Platelet counts and coagulation markers
One limitation of individual biomarkers and in vitro coagulation assays is that they overlook the interaction of blood with the vessel wall, an essential aspect of thrombosis. For this, we employed an ex vivo model of arterial thrombosis incorporating denuded porcine arterial strips, with different chambers that have flow conditions representing patent (low-shear chamber) and stenosed (high-shear chamber) human coronary arteries. Our previous studies in healthy volunteers demonstrated that acute exposure to diesel exhaust nanoparticles promoted the thrombogenicity of the blood in the absence of changes in individual biomarkers of coagulation29,30. In this study, inhalation of either s-GO (P = 0.02) or us-GO (P = 0.006) led to a greater blood thrombogenicity compared with air exposure in the low-shear chamber (Fig. 4b–f). A similar pattern was observed in the high-shear chamber, although the effect was only demonstrable for s-GO (P = 0.019; for us-GO, P = 0.07). No differences were observed when directly comparing s-GO with us-GO. The findings highlight that traditional coagulation markers are crude indicators of thrombogenicity, and that the inclusion of physiological conditions, such as the injured vessel wall, should be used to address the potential for thrombosis in vivo. The magnitude of the greater thrombogenicity after GO exposure was relatively mild and would be potentially of limited consequence, even in patients with pre-existing heart disease. Nevertheless, we recommend that characterization of the thrombogenic potential is included in experimental models used to develop risk assessments for graphene materials that may be of higher risk, or to consider higher exposure scenarios.
Systemic inflammation
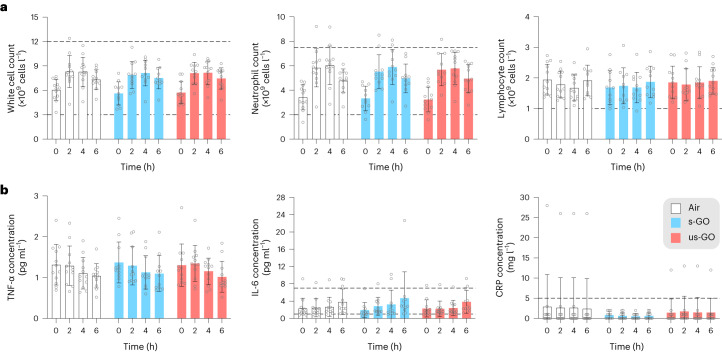
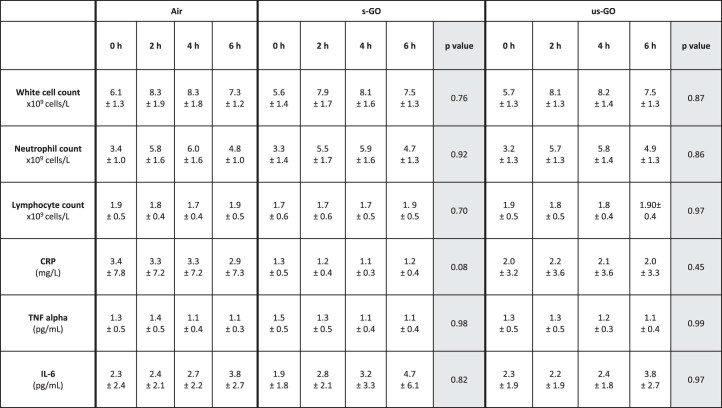
Inflammation is a key mechanism underlying the cardiovascular effects of particles in air pollution in humans31 and various carbon-manufactured nanomaterials in animal models32. While white blood cell and neutrophil counts increased over the exposure period (P < 0.001 for all groups), the effect was identical in all the exposure groups, including filtered air (Fig. 5a and Extended Data Table 5). The lack of impact of GO in white blood cell counts aligns with those of a murine study of graphene nanoplatelet exposure26. There were no changes in the concentrations of the inflammatory cytokines: tumour necrosis factor alpha (TNF-α) or interleukin-6 (IL-6) over time or between different exposures (Fig. 5b and Extended Data Table 5). Minor differences in the baseline serum concentrations of the acute response protein C-reactive protein (CRP) were observed between exposure groups, but there were no changes between exposures when expressed as a percentage change from the baseline (P = 0.08 for s-GO versus air and P = 0.45 for us-GO versus air). The results suggest that physical exercise during the exposure increased the mobilization of white blood cells in the systemic circulation33. The exposure itself did not influence inflammatory cell number or activation.
Fig. 5. White blood cell counts and markers of inflammation.
a,b, Inflammatory cell counts (a) and inflammatory cytokine concentrations (b) for exposure to air, s-GO and us-GO. The dotted lines represent the expected upper and lower limits of normal values. The coloured bars and error bars show mean ± s.d. (n = 12 for air and us-GO groups, n = 11 for s-GO groups; biologically independent measurements). No significant differences were found between treatments (two-way analysis of variance followed by Tukey’s post hoc test).
Extended Data Table 5.
Blood inflammatory cell counts and biomarkers
Controlled exposure to diesel exhaust emissions can be associated with increased in markers of systemic inflammation in humans18,25,34, whereas exposure to spark-generated carbon black particles did not induce a systemic inflammatory response in healthy volunteers29. This is also in keeping with the available pre-clinical evidence: inhalation of GO in mice induced only mild increases in circulatory inflammatory cells or cytokines9,35 at high doses (>3 mg m−3) that are likely to be associated with lung overload and do not extrapolate to anticipated real-life exposure scenarios in humans20,36. The lack of effect of GO on markers of inflammation and the acute-phase response may reflect the purity of the GO, although the profile of the response at later time points remains to be confirmed (see the Supplementary Information for further discussion). Studies that make a direct comparison of our high-purity materials and commercial sources of GO that are typically less pure and more heterogenous in their size distribution would be valuable.
Blood proteomic and lipidomic profiles
High-fidelity blood proteomics was used to probe for unanticipated acute systemic effects and further explore underlying pathways. A previously developed plasma enrichment approach (NanoOmics pipeline37) was employed before liquid chromotography tandem mass spectrometry analysis to minimize the masking effects from highly abundant plasma proteins (such as albumin and immunoglobulins) and to increase the dynamic range of the plasma proteomics data detected38. Lipid-based nanoparticles were used to adsorb proteins from the extracted plasma sample, forming a protein corona that can be recovered and purified from unbound proteins. Plasma from the baseline (t = 0 h) and end of the protocol (t = 6 h) were chosen for analysis, as the later time point was deemed the most likely to coincide with any inflammatory response to the GO exposure. A total number of 692 proteins were identified in the plasma of s-GO-exposed volunteers, of which only 3 were found to be differentially abundant (1 downregulated, 2 upregulated; Fig. 6) compared with the baseline (t = 0 h).
Fig. 6. Blood proteome comparison from plasma samples using the NanoOmics enrichment pipeline.
a, Heat map of normalized abundance values of all corona proteins identified by LC-MS/MS in samples collected before and after exposure to s-GO (left) and us-GO (right). A total of 692 (772) corona proteins were identified in s-GO (us-GO) exposure samples. Protein columns are sorted according to the abundance values (from highest to lowest) of the first sample. b, Volcano plots displaying the differential abundance of significantly different proteins before and after exposure to s-GO and us-GO (top) and between GO exposure groups and air at 6 h (bottom). Blue data points represent downregulated proteins and red data point upregulated proteins. The n values shown are from biologically independent samples. Filled dots represent the n = 1 common differentially abundant protein (SRI) between the two time points. The peptide intensities were compared between groups by one-way analysis of variance (ANOVA). Only proteins with P < 0.05 are shown.
Similarly, out of 772 proteins identified in the plasma of us-GO-exposed volunteers, the abundance of only 4 proteins was shown to be affected (all upregulated) versus the baseline (t = 0 h). The proteomic analysis (Table 1) revealed that sorcin protein was upregulated after exposure to both s-GO and us-GO. Although previous studies have not associated sorcin protein with nanoparticle exposure, inflammation or toxicity, other studies have implicated sorcin in neurodegenerative diseases39 and calcium signalling in heart tissues40. Comparing between exposure groups, a total of 10 and 7 differentially abundant proteins were identified between s-GO and air exposures and between us-GO and air exposures, respectively. Of all the differentially abundant proteins, factor XII (air versus us-GO exposure groups) was the only protein identified that had immediate relevance to cardiorespiratory parameters of particulates, being implicated in both the complement and coagulation pathways41,42. In general, unlike previous diesel exhaust particulate exposure studies that have shown distinct plasma protein changes43, our analysis revealed only subtle changes in a very small number of plasma proteins.
Table 1.
Identification of differentially abundant proteins from nano-enriched proteomic analysis of plasma samples
| Description | Gene name | P value | Maximum fold change |
|---|---|---|---|
| s-GO: 0 h versus 6 h (n = 3) | |||
| Downregulated | |||
| cDNA FLJ26936 fis, clone RCT06808 | 0.019 | 5.52 | |
| Upregulated | |||
| Platelet endothelial cell adhesion molecule | PECAM1 | 0.016 | 2.05 |
| Sorcin | SRI | 0.043 | 15.07 |
| us-GO: 0 h versus 6 h (n = 3) | |||
| Upregulated | |||
| Fibroblast growth factor-binding protein 2 | FGFBP2 | 0.024 | 1.91 |
| Annexin | 0.027 | 2.55 | |
| Proteoglycan 1, secretory granule, isoform CRA_a | PRG1 | 0.041 | 4.14 |
| Sorcin | SRI | 0.046 | 21.16 |
| Air versus s-GO (6 h) (n = 10) | |||
| Downregulated | |||
| Tubulin alpha-4A chain | TUBA4A | 0.007 | 3.99 |
| Immunoglobulin G heavy chain | 0.015 | 20.71 | |
| Prolactin-inducible protein | PIP | 0.017 | 2.22 |
| Alpha-1-antichymotrypsin (fragment) | SERPINA3 | 0.036 | 2.76 |
| HRV Fab 026-VL (fragment) | 0.042 | 1.53 | |
| 60S ribosomal protein L18 (fragment) | RPL18 | 0.045 | 515.74 |
| Upregulated | |||
| Uncharacterized protein DKFZp686N02209 | DKFZp686N02209 | 0.031 | 1.36 |
| Phosphatidylinositol 3,4,5-trisphosphate 5-phosphatase 1 | INPP5D | 0.031 | 1.83 |
| Collagen alpha-1(IV) chain | COL4A1 | 0.036 | 1.74 |
| Putative ciliary rootlet coiled-coil protein 2 | CROCC2 | 0.040 | 1.48 |
| Air versus us-GO (6 h) (n = 7) | |||
| Downregulated | |||
| Coagulation factor XII | F12 | 0.006 | 5.27 |
| Apolipoprotein B protein (fragment) | APOB | 0.006 | 3.87 |
| Plasma protease C1 inhibitor | SERPING1 | 0.02 | 1.46 |
| Alpha-2-HS-glycoprotein | AHSG | 0.02 | 1.66 |
| cDNA FLJ41054 fis, clone STOMA1000189 | 0.03 | 9.00 | |
| cDNA FLJ90052 fis, clone HEMBA1002767, highly similar to Beta-1,4-galactosyltransferase 2 (EC 2.4.1.-) | 0.049 | 1.70 | |
| Immunoglobulin heavy chain variable region (fragment) | 0.049 | 18.05 | |
The listed values are from independent biological samples for each group (from top to bottom table: n = 3, n = 4, n = 10, n = 7). Data were filtered in Pyrogenesis LC-MC to present a 1% false discovery rate. Comparisons between groups conducted using one-way analysis of variance, with only proteins demonstrating P < 0.05 shown. cDNA, copyDNA.
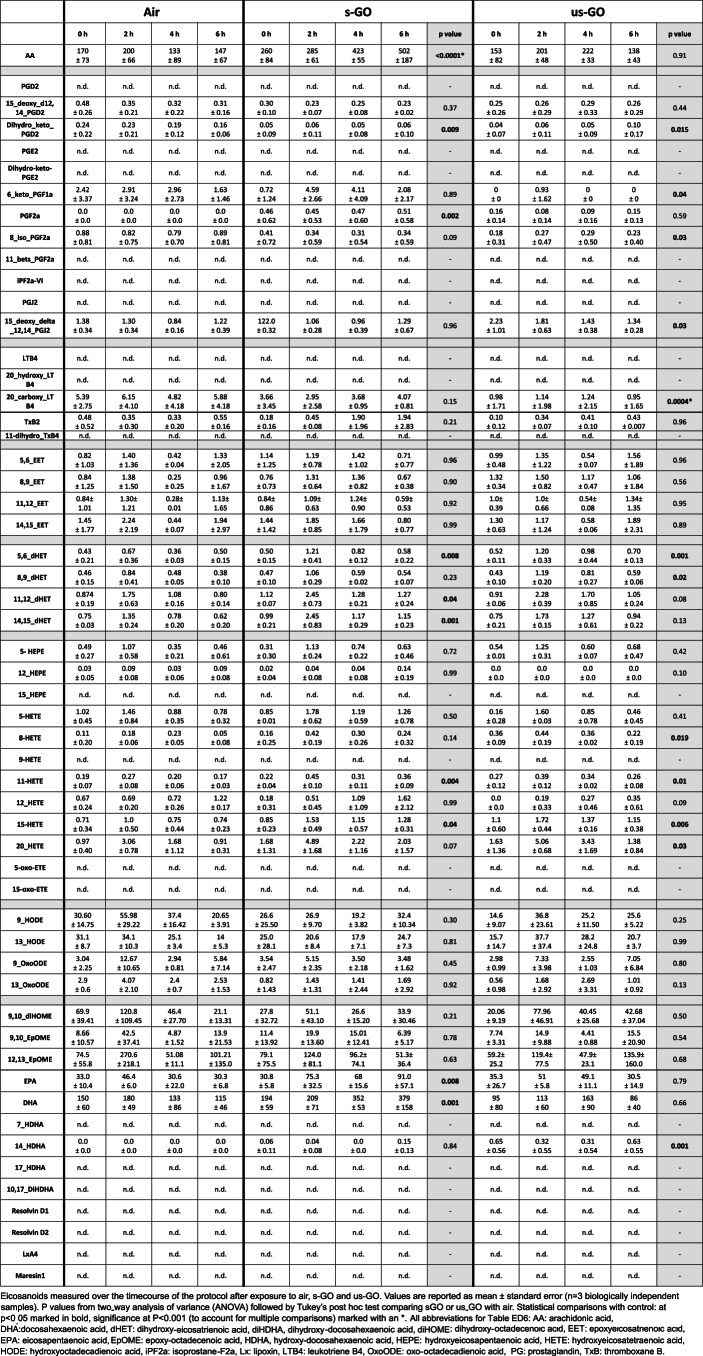
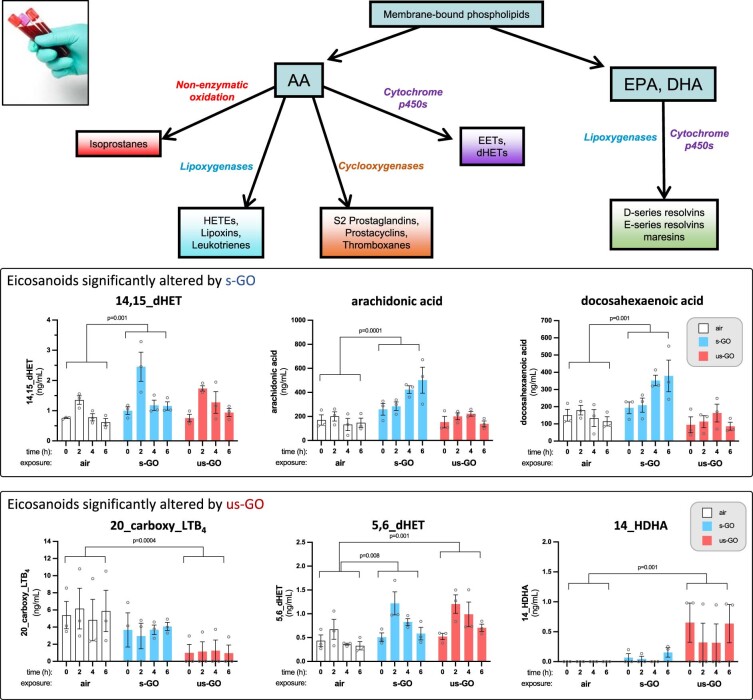
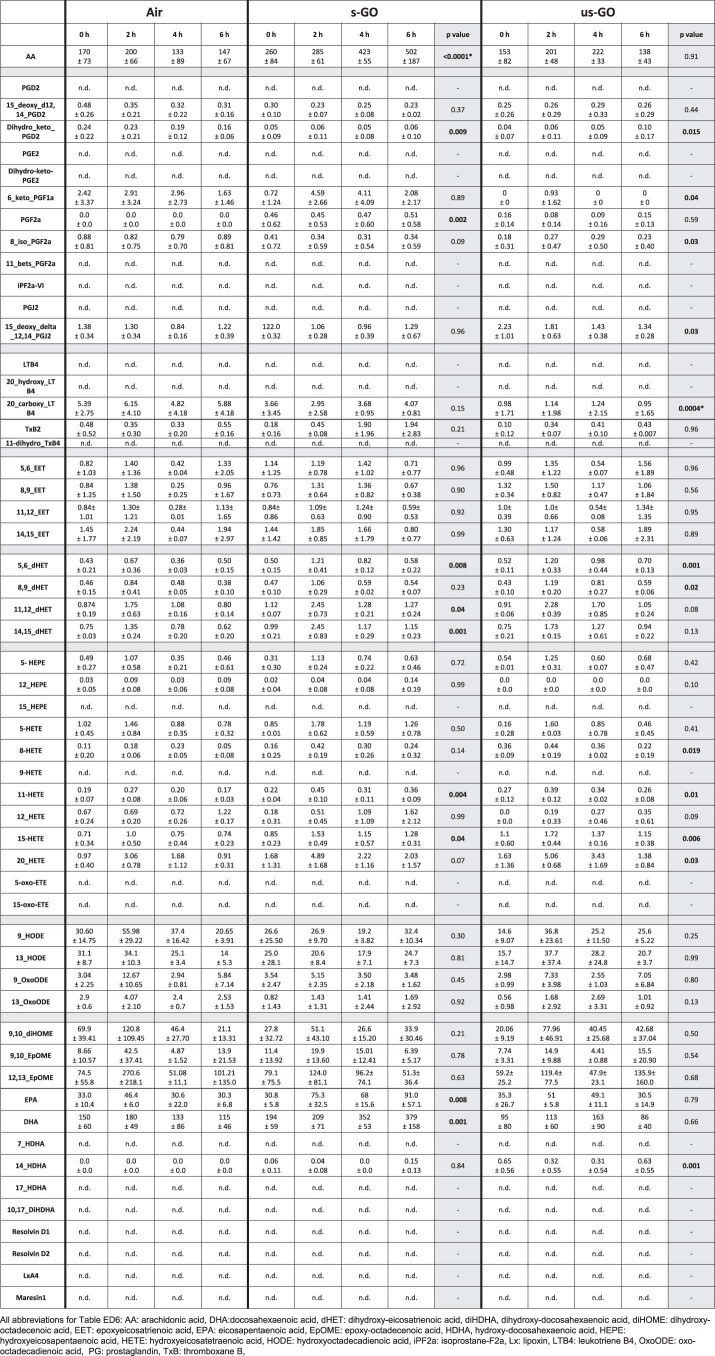
Lastly, using lipidomic analysis of the extracted plasma samples we profiled eicosanoid species that may represent a means to detect more subtle changes in biological function that precede inflammatory biomarkers or functional changes in organ systems44. A preliminary exploration of plasma eicosanoids using targeted lipidomics found limited differences between some factors across the exposure groups (Extended Data Fig. 2 and Extended Data Table 6). Overall, the proteomic and lipidomic analyses corroborated the limited and mild effects of GO exposure on cardiorespiratory parameters and inflammatory mediators.
Extended Data Fig. 2. Eicosanoid measurements in blood.
Eicosanoids measured over the time course of the protocol after exposure to air (white), small graphene oxide (s-GO, blue) and ultra-small graphene oxide (us-GO, red). Values are reported as mean ± standard error (n=3 biologically independent samples). dHET: dihydroxy-eicosatrienoic acid, EET: epoxyeicosatrienoic acid, EPA: eicosapentaenoic acid, HDHA: hydroxy-docosahexaenoic acid, HETE: hydroxyeicosatetraenoic acid, LTB4: leukotriene B4, PG: prostaglandin. Significance compared to air control, *P<0.05, **P<0.01, ***P<0.001. To account for multiple testing, P≤0.001 taken as statistically significant.
Extended Data Table 6.
Eicosanoid measurements in blood
Study limitations
While the use of controlled exposures in human participants has many advantages17, we acknowledge that our study has several limitations. First, the number of participants for this foundational study was only powered to detect changes in biological parameters based on our previous work with diesel exhaust nanoparticles. However, participant numbers may be insufficient to detect more subtle effects of GO inhalation. Second, we were only able to test a single dose of GO. For safety reasons, the dose was carefully chosen to avoid overt physiological effects, and it is possible that higher concentrations or longer durations of GO exposure could have actions that were not apparent in the current study. However, we note that our previous exposures to diesel exhaust used similar concentrations (100–300 μg m−3) of particulates and were accompanied by cardiovascular dysfunction measured using similar parameters19,25,29. Lastly, human-controlled exposure studies are limited to the exploration of acute exposure and restricted measurement periods. The study protocol could not be extended to more than 6 h after the start of the exposure and, therefore, slow-onset responses, such as some inflammatory pathways, would not have been captured.
Conclusions
We found that the two GO sheets (s-GO and us-GO) with nanometre-sized lateral dimensions, minute thickness (1–2 nm) and high purity (in terms of both metallic and endotoxin contaminants) were largely innocuous in healthy volunteers at the dose and duration tested. This GO material was neither associated with acute changes in respiratory or cardiovascular function, nor systemic inflammation. While there was no major effect on blood coagulability, there was a mild increase in thrombogenicity in an ex vivo model of vascular injury, highlighting the need for comprehensive and subtle profiling of cardiovascular parameters to assess the actions of inhaled manufactured nanomaterials fully. This study lays the foundation for subsequent human studies investigating GO in larger numbers of individuals that could include differences in the degree of oxidation of GO (and surface oxygen content), purity and doses, as well as additional health parameters, time points and exploration of potential susceptible groups such as individuals with asthma and those with a greater risk of blood clotting. Great care should be taken to avoid generalizations from these results as the structural and surface characteristics of the GO materials and their purity, along with extensive pre-clinical investigations and knowledge, allowed the safe and ethical translation into the human exposures undertaken here. These studies could constitute a major advancement towards a comprehensive risk assessment of graphene and 2D nanomaterials to adopt a safe-by-design approach to harness the true potential of this unique material.
Methods
See the Supplementary Information for full details.
GO synthesis
Aqueous dispersions of s-GO and us-GO were prepared as described in our previous studies6,45 by a modified Hummers’ method coupled with sonication. We used depyrogenized glassware, handled under endotoxin-free conditions. Graphite powder was mixed with sodium nitrate and sulfuric acid by rigorous stirring at low temperature, followed by potassium permanganate and dropwise addition of water for injections. The mixture was stirred for 30 min at 98 °C before the reaction was stopped with hydrogen peroxide. Water for injections was used to neutralize the pH, remove impurities and separate the GO from the graphitic residues. GO was exfoliated by vortexing, and solubilized with warm water for injections from the orange gel layer. Any graphitic residues still present in the dispersion were removed by an additional centrifugation step 24 h post-reaction. Size reduction to small and ultrasmall flakes was carried out by sonication for 5 min and 4 h, respectively.
Characterization of GO nanosheets
GO was comprehensively characterized by atomic force microscopy, transmission electron microscopy, hydrodynamic diameter and surface charge (zeta potential) measurements, UV–visible spectroscopy, fluorescence spectroscopy, Raman spectroscopy, Fourier transform infrared spectroscopy, thermogravimetric analysis and X-ray photoelectron spectroscopy (see the Supplementary Information for full details).
Nanoparticle exposure and characterization
Nanoparticles exposures were performed in a mobile exposure laboratory positioned outside the Royal Infirmary of Edinburgh (Supplementary Fig. 2), under the supervision of an experienced exposure technician. Stock suspensions of GO (2 mg ml−1 for s-GO; 1.3 mg ml−1 for us-GO) were made in sterile distilled water that was free of any bacterial contamination, confirmed using a previously published method10. s-GO was diluted to 1.3 mg/ ml−1 in sterile saline in aseptic conditions, aliquoted and stored at 4 °C until use.
GO was transferred to a 5 ml syringe, placed on the syringe pump and aerosolized using a Schlick compressed air nebulizer (model 970/S Untersiemau, Dusen-Schlick), with in-line diluted with HPLC-grade water. The compressed pre-heated (60 °C) airflow of the Schlick nebulizer was 12 l m−1. The aerosol was dried in a heated mixing glass tube, then diluted with HEPA-filtered room air to the desired concentration and humidified to 50–60% relative humidity using an ultrasonic nebulizer (Omron Ultrasonic Nebulizer NE-U12). The aerosol was fed into a 200 l mixing chamber and delivered to the volunteer by an exposure mask placed over the mouth and nose, under a constant temperature and relative humidity (50%). GO was delivered at exposure concentrations between 100 and 300 μg m−3, with a target average concentration of 200 μg m−3. This dose range was chosen based on our previous controlled exposure studies with dilute diesel exhaust, which were associated with impairment of a range of cardiovascular parameters without adverse effects19,30 and with carbon and gold nanoparticles that did not alter cardiovascular parameters29,46. The concentration could be adjusted by altering the speed of the syringe pump delivering the suspension based on tapered element oscillating microbalance (model 1400 A, Thermo Scientific) readings, monitored and maintained by the exposure technicians.
The particle concentration in the aerosol was measured in the middle of the 200 l mixing chamber by an stainless steel tube. The particle characteristics measured were: particle mass (tapered element oscillating microbalance, as well as by gravimetric filter based analyses), particle number (condensation particle counter, model 3022 A CPC, TSI Inc.) and particle size distribution (PALAS differential electrical mobility classifier (U-DEMC model 2200) and an optical particle sizer (model 3330,TSI Inc.)). The particle mass was also determined post-exposure by calculating the accumulated mass on pre-weighed telfon filters taken from the metal tubing close to the volunteer exposure mask.
Ethics statement
This study was designed with rigorous ethical review, with procedures being run by experienced clinicians and nursing support, and performed at a major hospital with the necessary emergency facilities should an adverse event have occurred. The study was performed in accordance with the Declaration of Helsinki, favourable ethical opinion of the University of Edinburgh, NHS Academic and Clinical Central Office for Research and Development (ACCORD), Research Ethics Committee (18-HV-084) and with written informed consent from all participants. The study has been registered on Clinicaltrials.gov under reference number NCT03659864.
Participants and eligibility criteria
Fifteen healthy volunteers were recruited by advertising the study by posters and e-mails in the hospital and university campus, as approved by local ethical review. The data from 14 participants were included as one participant was unable to complete the exposure visits in the time frame of the study. The target of 15 individuals was chosen according to our previous controlled exposure studies with air pollutants based on changes to vascular reactivity and inflammatory cytokines in the blood from diesel exhaust exposure due to a lack of other controlled exposure studies of a 2D material for comparisons. A 1 h exposure to diluted diesel exhaust produced an ~32% reduction in forearm blood flow to 1 nmol min−1 bradykinin (~16 ± 2 versus ~19 ± 2.5 ml per 100 ml tissue per min (±s.d.) for diesel exhaust versus filtered air control, respectively19. A 2 h exposure to diluted diesel exhaust produced a 12.5% increase in plasma TNF-α (0.99 ±;0.07 versus 0.88 ± 0.007 pg ml−1 (±s.d.) for diesel exhaust versus filtered air, respectively25. On the basis of these figures, 12 and 10 volunteers, respectively, would be needed to detect these changes with significance of P < 0.05 with an 80% power. As other studies have not tested the effects of an inhaled 2D material, as an additional precautionary step, the decision was taken to not increase group sizes beyond 15 for this initial study.
Interested participants were provided with a participant information sheet that they were asked to read and consider for at least 24 h before agreeing to be involved in the study. For study visits, participants abstained from alcohol for 24 h and from food and caffeine containing beverages for at least 12 h before the study visit. Participants were invited for an initial screening visit to ensure that they met the inclusion criteria (Supplementary Table 2). The participants were asked about their occupation on their screening visit to rule out obvious exposures to particulates. We did not ask participants to wear a face masks outside of the study visits, as low compliance would have added an additional source of variability between participants (the study was run before the coronavirus pandemic, before mask wearing became common in the United Kingdom), and even occupational face masks have been shown to vary greatly in their removal of inhaled particles during different modes of activity47. Importantly, each volunteer acted as their own control and received each exposure in a random order, minimizing variation from both intrinsic biology and lifestyle factors.
Study design
See Fig. 2. A screening visit was used to confirm eligibility criteria with the participant, followed by taking written consent and assignment of a participant code. Height, weight, heart rate, blood pressure and lung function were measured, and a 3 ml blood sample was taken for a full blood cell count. If parameters were within the normal range for young healthy individuals, participants were taken forwards to full study days. Participants also took a graded cardiorespiratory exercise stress test on a bicycle ergonometer to determine the workload required to generate a ventilation rate of 25 l min−1 m−2.
Two lateral dimensions of GO (maintaining all other physicochemical characteristics almost identical) were selected for the study: s-GO and us-GO. Both types of nanosheet have demonstrated no acute or longitudinal adverse effects in our previous pre-clinical (rodent) studies6, contrary to ‘large’ GO sheets that were thus excluded from this work as a safety precaution. A double-blind randomized crossover study design was used for the study visits, whereby the order of exposures (filtered air, s-GO, us-GO) were randomized. All study visits were organized at least 2 weeks apart to allow a wash-out period between different exposures. The volunteer and clinician performing the study were blinded to the identity of the exposure group. All researchers involved with collating and analysing the raw data were blinded to the exposure group, with unblinding occurring only when ready for grouping by exposure.
Before exposures (time t = 0 h), heart rate, blood pressure and lung function were measured, and blood taken. Participants were asked to empty their bladders and then given a urine container to collect any urine over the course of the study visit. Participants were then taken to the exposure laboratory based at the Royal Infirmary of Edinburgh site for the duration of the study. An experienced research clinician and exposure technician were present throughout the exposure, with the same researcher and nursing support present during the rest of the protocol.
In the exposure laboratory, participants wore a face mask through which nanoparticles could be delivered by inhalation. Volunteers were asked to cycle at the workload required to increase respiratory rate to 25 l min−1 m−2 (pre-determined by exercise testing at the screening visit) and rest alternately for 15 min periods across the 2 h exposure. After exposure, the subject returned to the Clinical Research Facility for assessment of biological parameters.
Vital signs, lung function and blood collected pre-exposure (t = 0 h), were repeated at t = 2.25, 4 and 6 h (that is, 15 min, 2 h and 4 h after exposure). For ease of reading, the 2.25 h time point is referred to as t = 2 h throughout). The ex vivo model of deep arterial injury was performed at 1–1.5 h post-exposure, and forearm plethsymography performed at 2–4 h post-exposure (see below). A light lunch was provided that was identical for all volunteers and all study visits. As an additional safety measure, a shortened protocol (without the ex vivo thrombosis assay, forearm plethysmography or 4 h measurements) was performed for the first exposure of each group. The study visits for the subsequent volunteers with the full protocol were scheduled only after it was confirmed that there were no adverse events and no marked changes in blood biomarkers. Volunteers were compensated for their time and travel expenses, which was approved by the ethics committee.
Lung function and vital signs
The participants were asked to rest in a sitting position for 15 min before measurement of vital signs and lung function. Lung function was measured by spirometry (Vitalograph Alpha III), with the optimal breathing techniques demonstrated at the screening visit. FEV1 and FVC were then measured, and a mean of two closely concurring consecutive runs were used. The participants were allowed to rest for a further 5 min before measurement of blood pressure and heart rate by sphygmomanometry.
Vascular function
The clinical protocol was designed to include measurement of vascular function in response to vasodilator drugs by venous occlusion plethysmography19 between t = 4 h and t = 6 h. However, due to technical and staffing difficulties we were unable to obtain reliable data from sufficient volunteers to make meaningful conclusions, thus the data were omitted. Further details of the technique can be found in the Supplementary Information.
Blood biomarkers
Blood was sampled before nanoparticle exposure (t = 0 h) and at 2, 4 and 6 h. A 17-gauge cannula was inserted into a large antecubital vein of both arms, and flushed with sterile saline. First, 1 ml of blood was discarded and approximately 27 ml was then collected for analysis. EDTA-treated blood was used for measurements of blood cell differentials, citrate-treated blood was used for coagulation markers (activated partial thromboplastin time, prothrombin time, fibrinogen) and clotted blood was used to collect serum for C-reactive protein (CRP) and cytokines (IL-6, TNF-α). Blood measurements were performed by the Clinical Biochemistry Unit at the NHS Royal Infirmary of Edinburgh by the standard methodology. Cytokines were measured using enzyme-linked immunosorbent assay (ELISA) (R&D Systems), with limits of detection of 0.022 pg ml−1 for TNF-α and 0.031 pg ml−1 for IL-6. Subsamples of blood and urine were frozen at −80 °C for biobanking.
Ex vivo thrombosis
The coagulability of blood was measured ex vivo using a model of thrombosis on deep arterial injury (Fig. 6). We have used this technique extensively in our clinical studies following exposure of volunteers to diesel exhaust30,48 and testing of antithrombotic medication49,50. Blood was withdrawn from an antecubital vein via a pump set at a flow rate of 10 ml min−1. The first 5 ml of blood was discarded before the cannula was connected using non-coagulation tubing (Masterflex Tygon, Cole Parmer) to three sequential cylindrical perfusion chambers maintained at 37 °C in a water bath. Strips of porcine aorta (Pel-freez) were prepared by carefully removing the intima and a thin layer of media to act as a thrombogenic substrate, and mounted in the chamber according to the physiological direction of blood flow. The rheological conditions in the first chamber simulated those of patent coronary arteries (low-shear rate, ~212 s−1), whereas those in the second and third chambers simulate those of mildly stenosed coronary arteries (high-shear rate, ~1,690 s−1). The model thus acts as one of deep coronary arterial injury. Each chamber run lasted for 5 min after which saline was perfused over the strip to remove non-adherent blood. The porcine strips with thrombus attached were removed and fixed in 4% paraformaldehyde. Strips were cut into eight cross-sections, wax-embedded, histologically sectioned and endogenous hydrogen peroxide activity blocked with 3% hydrogen peroxide solution. Sections were then incubated at room temperature for 1 h with polyclonal rabbit anti-human fibrin(ogen) antibody (1.2 μg ml−1; Cat. No. A0080, Dako) and monoclonal mouse anti-human CD61 antibody (1.28 μg ml−1; Cat. No. M0753, Dako). Antigen visualization was performed using a Bond Polymer refine detection kit (Leica Microsystems GmbH) and treatment with 3,3′-diaminobenzidine substrate chromogen (66 mM, Dako). Finally, sections were counterstained with haematoxylin followed by direct red 80 (0.1% sirius red). A semi-automated slide scanner (Axioscan Z1, Zeiss) and image analysis software (QuPath 0.2.3)51 were used by a blinded researcher to quantify thrombus area. High-resolution classifiers based on colour were established to detect total thrombus area.
High-fidelity nanoproteomics analysis of plasma samples
Preparation of liposomal nanoparticles and enrichment of plasma proteins
Hyrogenated soybean phosphatidylcholine (HSPC): Cholesterol (Chol):- 1,2-distearoyl-sn-glycero-3-phosphoethanolamine-polyethylene glycol2000 (DSPE-PEG2000) were prepared by thin lipid film hydration followed by extrusion, as previously described52. Lipids were dissolved in a 1:1 volume ratio of chloroform:methanol and evaporated under a vacuum. The lipid films were hydrated with ammonium sulfate to produce large multilammer liposomes. Small unilamellar liposomes were produced by extrusion through polycarbonate and extrusion filters (Whatman) using a mini-Extruder (Avanti Polar Lipids).
Liposomes and human plasma were incubated in an orbital shaker, and protein-coated liposomes were separated from excess plasma proteins following a previously described method53 using a two-step purification protocol that included size exclusion chromatography and membrane ultrafiltration. Bound proteins were mixed with S-trap lysis buffer containing 5% SDS triethylammonium bicarbonate to allow protein solubilization. Samples were reduced with dithiothreitol, and alkylated with iodoacetamide and dithiothreitol. Protein lysates were mixed with phosphoric acid and S-trap binding buffer to trap proteins in the columns, then digested with trypsin. Peptide samples were extracted and then desalted using oligo R3 beads. Samples were analysed by liquid chromatography mass spectrometry/mass spectrometry using an UltiMate 3000 Rapid Separation lipid chromatography platform (Dionex Corporation) coupled to a Q Exactive Hybrid Quadrupole-Orbitrap mass spectrometer (Thermo Fisher Scientific). The data analysis of the peptide samples is outlined in the Supplementary Information.
Eicosanoids and related bioactive lipid mediators
Targeted lipidomic analysis was undertaken using a panel of >50 eicosanoids that included prostaglandins (PGD2, PGE2, PGF2α, 13,14-dihydro-15-keto-PGD2, 13,14-dihydro-15-keto-PGE2, 11-beta-PGF2α, 6-keto-PGF1α, 15-deoxy-∆12,14-PGD2, 15-deoxy-∆12,14-PGJ2); thromboxanes (TxB2, 11-dehydro-TxB2); hydroxy-eicosatetraenoic acids (5-HETE, 8-HETE, 9-HETE, 11-HETE, 12-HETE, 15-HETE, 20-HETE); leukotrienes (LTB4, 20-carboxy-LTB4); epoxy-eicosatrienoic acids (5,6-EET, 8,9-EET, 11,12-EET, 14,15-EET; 5-OxoETE, 15-OxoETE); dihydroxy-eicosatrienoic acids (5,6-DHET, 8,9-DHET, 11,12-DHET, 14,15-DHET), hydroxy-eicosapentaenoics acids (5-HEPE, 15-HEPE), octadecadienoic acids (9-HODE; 13-HODE; 9-Oxo-ODE, 13-Oxo-ODE), epoxyoctadecamonoenoic acids (9,10-EpOME, 12,13-EpOME), pro-resolving mediators (lipoxin A4 - LXA4 and resolvins, RvD1, RvD2); isoprostanes (8-iso-PGF2α) and fatty acids (arachidonic acid, eicosapentaenoic acid (EPA), docosahexaenoic acid (DHA) and its metabolites, 7-HDHA; 14-HDHA; 17-HDHA; 10,17-DiHDHA).
Plasma was prepared from EDTA-treated blood. The following internal standards were used: PGE2-d4, 15-HETE-d8, LTB4-d4, 14,15- EET-d11, 14,15-dHET-d11, 9,10-EpOME-d4, 9,10-DiHOME-d4, RvD2-d5, EPA-d5 and 8-iso-PGF2α-d4 (Cayman Chemical). See the Supplementary Information for details of the processing of the samples. Eicosanoids were separated on a Hypersil GOLD C18 column (Thermo) using a Shimadzu Nexera-X2 ultra high performance liquid chromatography system. The effluent was directed into an IonTurbo source of a Sciex QTRAP 6500 mass spectrometer operated in negative-ion mode using multiple reaction monitoring. Eicosanoids were identified on the basis of their characteristic precursor/product ion pair transitions and matching retention time with authentic standards. Data were acquired and analysed using Sciex Analyst54 software v1.6. Concentrations of eicosanoids were determined by comparison to a calibration curve run in parallel for each compound and adjusted for recovery by reference to amounts of the appropriate internal standards.
General data and statistical analysis
Data were analysed using Excel 201055, R 3.2.2 (ref. 56) and Prism 9.3 (ref. 57). Data in Table 1 and Extended Data Tables 1–6 are presented as mean ± s.d., unless otherwise indicated. Continuous data are presented as means and s.d. Statistical significance within groups and between groups was tested using two-way analysis of variance with Tukey’s honest significant difference post hoc test. Parametric assumptions (normal distribution and equal variances) were confirmed using the statistical packages above; where data were not normally distributed a non-parametric alternative (for example, the Kruskal–Wallis test) was used.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Online content
Any methods, additional references, Nature Portfolio reporting summaries, source data, extended data, supplementary information, acknowledgements, peer review information; details of author contributions and competing interests; and statements of data and code availability are available at 10.1038/s41565-023-01572-3.
Supplementary information
Supplementary Methods; Results and Discussion; References; Table 1 and Figs. 1 and 2.
Source data
Statistical source data.
Statistical source data.
Statistical source data.
Statistical source data.
Statistical source data.
Statistical source data.
Acknowledgements
We thank all the staff at the Edinburgh Wellcome Trust Clinical Research Facility and M. Doris for their assistance with the participant study days. We also thank J. MacLeod for performing the screening visit exercise tests and the NHS Royal Infirmary of Edinburgh staff for the clinical biochemistry measurements. We also thank F. Howie and K. Wilson for their assistance in measuring TNF-α and IL-6, and the members of the University of Edinburgh SuRF histology laboratories for the processing and imaging of Badimon strips. We gratefully acknowledge the assistance of the SSC2b medical students (J. Garcia Fernandez, L. Buijs, S. Henry and N. Cameron) who participated in the initial data processing. We thank the staff at the Manchester Mass Spectrometry Facility, FTIR Facility, EM Facility and Bio-AFM Facility for assistance and advice regarding instrumentation. The University of Manchester Bioimaging Facility microscopes (EM, AFM) used in the present study were purchased with grants from the UK Research and Innovation (UKRI) Biotechnology and Biological Sciences Research Council, the Wellcome Trust and the University of Manchester Strategic Fund. We also acknowledge the UK Research and Innovation (UKRI) Engineering and Physical Sciences Research Council (EPSRC) Harwell XPS facility based at Cardiff University for the provision of X-ray photoelectron spectrometric data. Finally, we thank all the volunteers taking part in the study, which would not have been possible without them. For the purpose of open access, the author has applied a Creative Commons Attribution (CC BY) licence to any Author Accepted Manuscript version arising from this submission. This work was funded by the British Heart Foundation (grant numberSP/15/8/31575, M.R.M.), a UKRI and Engineering and Physical Sciences Research Council EPSRC 2D-Health Programme Grant (UKRI-EPSRC EP/P00119X/1) and the University of Manchester Strategic Fund studentship (both K.K.). The ICN2 is funded by the CERCA programme/Generalitat de Catalunya and has been supported by the Severo Ochoa Centres of Excellence programme (grant number SEV-2017-0706) and is currently supported by the Severo Ochoa Centres of Excellence programme, grant number CEX2021-001214-S, both funded by MCIN/AEI/10.13039.501100011033. S.S.J. and D.E.N. are supported by the British Heart Foundation (grant numbers FS/CRTF/20/24087, CH/09/002, RG/05/003, RG/10/9/28286, PG/03/017/15071, RG/16/10/32375 and RE/18/5/34216, all D.E.N.). D.E.N. is the recipient of a Wellcome Trust Senior Investigator Award (WT103782AIA).
Extended data
Author contributions
J.P.M.A., E.T. and M.B.S. recruited participants, monitored their welfare and performed the clinical measurements during the study visits. H.C. was the principal clinical research facility nursing support. S.S.J. performed the data analysis and image analysis and drafted the manuscript. L.E.C. and N.L. synthesized and characterized the graphene oxide nanosheets. P.H.B.F., A.J.F.B. and D.L.A.C.L. performed the exposures and the exposure characterization. L.B. and R.D. were involved in the pre-clinical work used in the human ethics application. C.A.P. performed the human dose extrapolations from pre-clinical data. J.B.R. assisted with the preparation of the Badimon experiments. E.O. and M.H. designed and performed the blood proteomics analysis of the plasma samples. I.L.M., P.D.W., K.Z. and S.T. designed and performed the eicosanoid analysis. C.B. characterized the GO material and performed pre-clinical studies with the material. F.R.C. contributed to the design of the study, oversaw the exposure work and provided critical analysis. D.E.N. contributed to the design of the study and provided critical analysis of the clinical measurements. K.K. contributed to the conception and design of the study, oversaw the graphene synthesis and characterization, pre-clinical studies and proteomics analyses and provided critical analysis of the findings. M.R.M. conceived and designed the study, was the Chief Investigator for the clinical study, contributed to the analysis and assisted with the drafting of the manuscript. All authors read and provided input on the writing of the manuscript.
Peer review
Peer review information
Nature Nanotechnology thanks Jörg Radnik, Stefania Sabella, Ulla Vogel and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Data availability
Data are available through the University of Edinburgh online data repository at 10.7488/ds/7545. Source data are provided with this paper. Correspondence and requests for materials should be addressed to the cooresponding authors M.R.M. or K.K.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Jack P. M. Andrews, Shruti S. Joshi.
These authors jointly supervised this work: Kostas Kostarelos, Mark R. Miller.
Contributor Information
Kostas Kostarelos, Email: kostas.kostarelos@manchester.ac.uk.
Mark R. Miller, Email: mark.miller@ed.ac.uk
Extended data
is available for this paper at 10.1038/s41565-023-01572-3.
Supplementary information
The online version contains supplementary material available at 10.1038/s41565-023-01572-3.
References
- 1.Ferrari AC, et al. Science and technology roadmap for graphene, related two-dimensional crystals, and hybrid systems. Nanoscale. 2015;7:4598–4810. doi: 10.1039/C4NR01600A. [DOI] [PubMed] [Google Scholar]
- 2.Bitounis D, Ali-Boucetta H, Hong BH, Min D-H, Kostarelos K. Prospects and challenges of graphene in biomedical applications. Adv. Mater. 2013;25:2258–2268. doi: 10.1002/adma.201203700. [DOI] [PubMed] [Google Scholar]
- 3.Kostarelos K, Novoselov KS. Exploring the interface of graphene and biology. Science. 2014;344:261–263. doi: 10.1126/science.1246736. [DOI] [PubMed] [Google Scholar]
- 4.Sasidharan A, et al. Hemocompatibility and macrophage response of pristine and functionalized graphene. Small. 2012;8:1251–1263. doi: 10.1002/smll.201102393. [DOI] [PubMed] [Google Scholar]
- 5.Fadeel B, et al. Safety assessment of graphene-based materials: focus on human health and the environment. ACS Nano. 2018;12:10582–10620. doi: 10.1021/acsnano.8b04758. [DOI] [PubMed] [Google Scholar]
- 6.Rodrigues AF, et al. A blueprint for the synthesis and characterisation of thin graphene oxide with controlled lateral dimensions for biomedicine. 2D Mater. 2018;5:035020. doi: 10.1088/2053-1583/aac05c. [DOI] [Google Scholar]
- 7.Ma-Hock L, et al. Comparative inhalation toxicity of multi-wall carbon nanotubes, graphene, graphite nanoplatelets and low surface carbon black. Part. Fibre Toxicol. 2013;10:23. doi: 10.1186/1743-8977-10-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schinwald A, Murphy FA, Jones A, MacNee W, Donaldson K. Graphene-based nanoplatelets: a new risk to the respiratory system as a consequence of their unusual aerodynamic properties. ACS Nano. 2012;6:736–746. doi: 10.1021/nn204229f. [DOI] [PubMed] [Google Scholar]
- 9.Kim YH, et al. Short-term inhalation study of graphene oxide nanoplates. Nanotoxicology. 2018;12:224–238. doi: 10.1080/17435390.2018.1431318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mukherjee SP, et al. Detection of endotoxin contamination of graphene based materials using the TNF-α expression test and guidelines for endotoxin-free graphene oxide production. PLoS ONE. 2016;11:e0166816. doi: 10.1371/journal.pone.0166816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Peng G, et al. Graphene oxide elicits microbiome-dependent type 2 immune responses via the aryl hydrocarbon receptor. Nat. Nanotechnol. 2023;18:42–48. doi: 10.1038/s41565-022-01260-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Loret T, et al. Innate but not adaptive immunity regulates lung recovery from chronic exposure to graphene oxide nanosheets. Adv. Sci. 2022;9:2104559. doi: 10.1002/advs.202104559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rodrigues AF, et al. Size‐dependent pulmonary impact of thin graphene oxide sheets in mice: toward safe‐by‐design. Adv. Sci. 2020;7:1903200. doi: 10.1002/advs.201903200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.de Luna LAV, et al. Lung recovery from DNA damage induced by graphene oxide is dependent on size, dose and inflammation profile. Part. Fibre Toxicol. 2022;19:62. doi: 10.1186/s12989-022-00502-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Stone V, et al. Nanomaterials versus ambient ultrafine particles: an opportunity to exchange toxicology knowledge. Environ. Health Perspect. 2017;125:106002. doi: 10.1289/EHP424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Miller MR, Newby DE. Air pollution and cardiovascular disease: car sick. Cardiovasc. Res. 2019;116:279–294. doi: 10.1093/cvr/cvz228. [DOI] [PubMed] [Google Scholar]
- 17.Miller MR, Poland CA. Nanotoxicology: the need for a human touch? Small. 2020;16:2001516. doi: 10.1002/smll.202001516. [DOI] [PubMed] [Google Scholar]
- 18.Holgate ST, et al. Health effects of acute exposure to air pollution. Part I: healthy and asthmatic subjects exposed to diesel exhaust. Res. Rep. Health Eff. Inst. 2003;1–30:51–67. [PubMed] [Google Scholar]
- 19.Mills NL, et al. Diesel exhaust inhalation causes vascular dysfunction and impaired endogenous fibrinolysis. Circulation. 2005;112:3930–3936. doi: 10.1161/CIRCULATIONAHA.105.588962. [DOI] [PubMed] [Google Scholar]
- 20.Lee JH, et al. Exposure monitoring of graphene nanoplatelets manufacturing workplaces. Inhal. Toxicol. 2016;28:281–291. doi: 10.3109/08958378.2016.1163442. [DOI] [PubMed] [Google Scholar]
- 21.Pelin M, Sosa S, Prato M, Tubaro A. Occupational exposure to graphene based nanomaterials: risk assessment. Nanoscale. 2018;10:15894–15903. doi: 10.1039/C8NR04950E. [DOI] [PubMed] [Google Scholar]
- 22.Lovén K, et al. Emissions and exposures of graphene nanomaterials, titanium dioxide nanofibers, and nanoparticles during down-stream industrial handling. J. Expo. Sci. Environ. Epidemiol. 2021;31:736–752. doi: 10.1038/s41370-020-0241-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Spinazzè A, et al. Probabilistic approach for the risk assessment of nanomaterials: a case study for graphene nanoplatelets. Int. J. Hyg. Environ. Health. 2019;222:76–83. doi: 10.1016/j.ijheh.2018.08.011. [DOI] [PubMed] [Google Scholar]
- 24.Mills NL, et al. Ischemic and thrombotic effects of dilute diesel-exhaust inhalation in men with coronary heart disease. N. Engl. J. Med. 2007;357:1075–1082. doi: 10.1056/NEJMoa066314. [DOI] [PubMed] [Google Scholar]
- 25.Törnqvist H, et al. Persistent endothelial dysfunction in humans after diesel exhaust inhalation. Am. J. Respir. Crit. Care Med. 2007;176:395–400. doi: 10.1164/rccm.200606-872OC. [DOI] [PubMed] [Google Scholar]
- 26.Kanakia S, et al. Dose ranging, expanded acute toxicity and safety pharmacology studies for intravenously administered functionalized graphene nanoparticle formulations. Biomaterials. 2014;35:7022–7031. doi: 10.1016/j.biomaterials.2014.04.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cao Y, Xiao W, Li S, Qiu D. A comparative study of toxicity of graphdiyne and graphene oxide to human umbilical vein endothelial cells. J. Appl. Toxicol. 2021;41:2021–2030. doi: 10.1002/jat.4182. [DOI] [PubMed] [Google Scholar]
- 28.Robertson S, Miller MR. Ambient air pollution and thrombosis. Part. Fibre Toxicol. 2018;15:1. doi: 10.1186/s12989-017-0237-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mills NL, et al. Combustion-derived nanoparticulate induces the adverse vascular effects of diesel exhaust inhalation. Eur. Heart J. 2011;32:2660–2671. doi: 10.1093/eurheartj/ehr195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lucking AJ, et al. Diesel exhaust inhalation increases thrombus formation in man. Eur. Heart J. 2008;29:3043–3051. doi: 10.1093/eurheartj/ehn464. [DOI] [PubMed] [Google Scholar]
- 31.Arias-Pérez RD, et al. Inflammatory effects of particulate matter air pollution. Environ. Sci. Pollut. Res. 2020;27:42390–42404. doi: 10.1007/s11356-020-10574-w. [DOI] [PubMed] [Google Scholar]
- 32.Rezaee M, Behnam B, Banach M, Sahebkar A. The Yin and Yang of carbon nanomaterials in atherosclerosis. Biotechnol. Adv. 2018;36:2232–2247. doi: 10.1016/j.biotechadv.2018.10.010. [DOI] [PubMed] [Google Scholar]
- 33.Neves PRDS, et al. Acute effects of high- and low-intensity exercise bouts on leukocyte counts. J. Exerc. Sci. Fit. 2015;13:24–28. doi: 10.1016/j.jesf.2014.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Salvi S, et al. Acute inflammatory responses in the airways and peripheral blood after short-term exposure to diesel exhaust in healthy human volunteers. Am. J. Respir. Crit. Care Med. 1999;159:702–709. doi: 10.1164/ajrccm.159.3.9709083. [DOI] [PubMed] [Google Scholar]
- 35.Han SG, et al. Pulmonary responses of Sprague-Dawley rats in single inhalation exposure to graphene oxide nanomaterials. BioMed. Res. Int. 2015;2015:376756. doi: 10.1155/2015/376756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Vaquero C, Wendelbo R, Egizabal A, Gutierrez-Cañas C, López de Ipiña J. Exposure to graphene in a pilot production plant. J. Phys. Conf. Ser. 2019;1323:012005. doi: 10.1088/1742-6596/1323/1/012005. [DOI] [Google Scholar]
- 37.Gardner L, Kostarelos K, Mallick P, Dive C, Hadjidemetriou M. Nano-omics: nanotechnology-based multidimensional harvesting of the blood-circulating cancerome. Nat. Rev. Clin. Oncol. 2022;19:551–561. doi: 10.1038/s41571-022-00645-x. [DOI] [PubMed] [Google Scholar]
- 38.Hadjidemetriou M, et al. The human in vivo biomolecule corona onto PEGylated liposomes: a proof-of-concept clinical study. Adv. Mater. 2019;31:1803335. doi: 10.1002/adma.201803335. [DOI] [PubMed] [Google Scholar]
- 39.Genovese I, et al. Sorcin is an early marker of neurodegeneration, Ca2+ dysregulation and endoplasmic reticulum stress associated to neurodegenerative diseases. Cell Death Dis. 2020;11:861. doi: 10.1038/s41419-020-03063-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zamparelli C, et al. Activation of the cardiac Na+–Ca2+ exchanger by sorcin via the interaction of the respective Ca2+-binding domains. J. Mol. Cell. Cardiol. 2010;49:132–141. doi: 10.1016/j.yjmcc.2010.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jiang L, et al. Silica nanoparticles induced the pre-thrombotic state in rats via activation of coagulation factor XII and the JNK-NF-κB/AP-1 pathway. Toxicol. Res. 2015;4:1453–1464. doi: 10.1039/C5TX00118H. [DOI] [Google Scholar]
- 42.E K, et al. Factor XII activation is essential to sustain the procoagulant effects of particulate matter. J. Thromb. Haemost. 2011;9:1359–1367. doi: 10.1111/j.1538-7836.2011.04280.x. [DOI] [PubMed] [Google Scholar]
- 43.Mehus AA, et al. Comparison of acute health effects from exposures to diesel and biodiesel fuel emissions. J. Occup. Environ. Med. 2015;57:705. doi: 10.1097/JOM.0000000000000473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Dennis EA, Norris PC. Eicosanoid storm in infection and inflammation. Nat. Rev. Immunol. 2015;15:511–523. doi: 10.1038/nri3859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Jasim DA, Lozano N, Kostarelos K. Synthesis of few-layered, high-purity graphene oxide sheets from different graphite sources for biology. 2D Mater. 2016;3:014006. doi: 10.1088/2053-1583/3/1/014006. [DOI] [Google Scholar]
- 46.Miller MR, et al. Inhaled nanoparticles accumulate at sites of vascular disease. ACS Nano. 2017;11:4542–4552. doi: 10.1021/acsnano.6b08551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Cherrie JW, et al. Effectiveness of face masks used to protect Beijing residents against particulate air pollution. Occup. Environ. Med. 2018;75:446–452. doi: 10.1136/oemed-2017-104765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lucking AJ, et al. Particle traps prevent adverse vascular and prothrombotic effects of diesel engine exhaust inhalation in men. Circulation. 2011;123:1721–1728. doi: 10.1161/CIRCULATIONAHA.110.987263. [DOI] [PubMed] [Google Scholar]
- 49.Wilson SJ, et al. Exosite 1 thrombin inhibition with JNJ-64179375 inhibits thrombus formation in a human translational model of thrombosis. Cardiovasc. Res. 2019;115:669–677. doi: 10.1093/cvr/cvy227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Meah MN, et al. Antithrombotic effects of combined PAR (Protease-Activated Receptor)-4 antagonism and factor Xa inhibition. Arterioscler. Thromb. Vasc. Biol. 2020;40:2678–2685. doi: 10.1161/ATVBAHA.120.314960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bankhead, P. et al. QuPath: open source software for digital pathology image analysis. Sci. Rep. 10.1038/s41598-017-17204-5 (2017). [DOI] [PMC free article] [PubMed]
- 52.Hadjidemetriou M, et al. In vivo biomolecule corona around blood-circulating, clinically used and antibody-targeted lipid bilayer nanoscale vesicles. ACS Nano. 2015;9:8142–8156. doi: 10.1021/acsnano.5b03300. [DOI] [PubMed] [Google Scholar]
- 53.Hadjidemetriou M, et al. Nano-scavengers for blood biomarker discovery in ovarian carcinoma. Nano Today. 2020;34:100901. doi: 10.1016/j.nantod.2020.100901. [DOI] [Google Scholar]
- 54.Sciex Analyst v1.6 (Sciex, 2019).
- 55.Microsoft Excel 10 (Microsoft, 2010).
- 56.R Core Team R: A Language and Environment for Statistical Computing (R Foundation for Statistical Computing, 2021).
- 57.Prism 9.3 (GraphPad, 2021).
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Methods; Results and Discussion; References; Table 1 and Figs. 1 and 2.
Statistical source data.
Statistical source data.
Statistical source data.
Statistical source data.
Statistical source data.
Statistical source data.
Data Availability Statement
Data are available through the University of Edinburgh online data repository at 10.7488/ds/7545. Source data are provided with this paper. Correspondence and requests for materials should be addressed to the cooresponding authors M.R.M. or K.K.