Abstract
Aims:
The management of cranial chordomas is controversial. We provide a comprehensive review of the evolving patterns of care of cranial chordomas in the USA.
Materials and methods:
We analysed the National Cancer Database (NCDB) and the Surveillance, Epidemiology, and End Results (SEER) database between 2004 and 2014 for clinical characteristics and long-term survival, and the National Surgical Quality Improvement Program (NSQIP) dataset between 2005 and 2016 for perioperative characteristics and surgical morbidity.
Results:
In total, 936 patients were identified from the NCDB, 405 patients from SEER and 64 patients from the NSQIP. Most patients were men (56.2, 54.8 and 57.8% in NCDB, SEER and NSQIP, respectively) and White (80.9 and 83.2% in NCDB and SEER, respectively). Surgery was the preferred treatment modality (87.3% in NCDB and 86.2% in SEER). Surgery was carried out alone (41.8% in NCDB and 40.7% in SEER) or in combination with radiation (42.1% in NCDB and 45.4% in SEER). Proton therapy was the most common type of radiation (32.2% in NCDB), particularly after 2011. The median operative time, median hospital length and postoperative morbidity were significantly higher in chordoma patients compared with patients who underwent other skull-base procedures. The 5-year survival rate was 79.8% in NCDB and 76.9% in SEER. There was a trend towards longer survival in patients receiving surgery and radiation, which has been increasingly used since 2004. Patients younger than 60 years had a decreased risk of mortality.
Conclusions:
Our analysis reflects patterns of care in the USA. The use of surgery and radiation is increasing, with a trend towards longer survival. Surgery is complicated with long operative time, hospital stay and a higher rate of complications.
Keywords: Cranial chordoma, morbidity, outcomes, patterns of care
Introduction
Chordomas are slow-growing and locally destructive neoplasms that originate from the remnants of the notochord along the neural axis [1–3]. These tumours represent less than 4% of all bone malignancies [4] and have an incidence rate of 0.08 per 100 000 population [5]. Around 30–40% of chordomas are located in the cranium and are generally associated with a poor prognosis [1,6]. Chordomas are typically divided into three variants based on histological features: classical, chondroid or dedifferentiated chordomas. The diagnosis of chordoma is confirmed with immunohistochemical studies showing its epithelial origin (cytokeratin, epithelial membrane antigen and brachyury) [7,8].
Large tumour extension and proximity to eloquent brain structures frequently limit gross total resection [9]. High-dose radiation therapy after surgical resection has shown a significant survival advantage [9–11]. However, it is limited due to the high doses needed and the risk for neurotoxicity [2]. Chemotherapy has not shown effectiveness as a first course of treatment, but there are some investigations for targeted therapies at recurrence [12–15].
Descriptions of perioperative morbidity for chordoma surgery are limited to single-centre experiences. Similarly, previous studies describing outcomes and patterns of care either combined chordomas and chondrosarcomas together or were conducted using patient data recorded over a decade ago [5,6,11,16,17]. An updated description is warranted, as radiation and surgical techniques have improved significantly allowing for improved local control and survival [18,19]. This study provides a comprehensive description of patterns of care, operative characteristics and survival using the National Cancer Database (NCDB), the Surveillance, Epidemiology, and End Results (SEER) database and the National Surgical Quality Improvement Program (NSQIP) dataset.
Materials and Methods
We carried out an analysis of the NCDB, NSQIP and SEER to evaluate and compare the demographics, clinical characteristics and survival of patients with a diagnosis of chordoma. The NSQIP and the NCDB are hospital-based databases maintained by the American College of Surgeons as quality improvement projects. The NCDB collects cancer-based information from 1500 hospitals in the USA, whereas the NSQIP collects surgical data from 688 hospitals. SEER is a population-based cancer registry. These databases have been described previously and have been used extensively in brain tumour research [20–26]. Informed consent was waived as data are de-identified per NCDB, SEER and NSQIP before data analysis.
We analysed the 2015 NCDB, which includes patients from 2004 to 2014 and collects over 70% of all newly diagnosed cancer patients in the USA. Chordoma patients were found in the brain/central nervous system and bone participant user files. In SEER, 18 registries were queried from 2004 to 2014 that cover around 28% of the US population. Due to the similarities in data collection of both databases, some cases are expected to be captured by both datasets. The degree of case overlapping is unknown due to data de-identification. Cranial chordomas were identified using the International Classification of Disease for Oncology third edition (ICD-O-3) codes 9370, 9371 and 9372 in the NCDB. Cases located within the cranium were selected. Spinal cases were excluded (C70.1, C72.0, C72.1). The type of radiation was derived from the Regional Treatment Modality variable in the NCDB and was divided into four groups: intensity-modulated radiation (IMRT), stereotactic radiosurgery (SRS), other photon and proton therapy. The median income (census median income quartiles 2007–2012), education status and residency area were derived from the NCDB based on the patient’s residential zip code at the time of diagnosis. Education was assessed by the percentage of non-high school graduates in the patient’s residential zip code (≤7%, 7–12.9%, 13–20.9%, ≥21%) [27]. Income and education from SEER are defined using the American Community Survey 2007–2011. Living regions in SEER are defined using the Rural Urban Continuum Codes.
The NSQIP was queried from 2005 to 2016 for all skull-base procedures using the CPT codes: 61580–61598. Chordomas were identified using the following postoperative diagnosis codes: an ICD-9 code of 170.0 or an ICD-10 code of 41.0. Patients with a diagnosis of chordoma were compared with patients who underwent skull-base surgery for other pathologies. The NSQIP and the hospitals participating are the source of the data used herein; they have not verified and are not responsible for the statistical validity of the data analysis or the conclusions derived by this study. In the NSQIP, morbidity was defined as one of the following 30-day outcomes: any surgical site infection (incisional, deep or organ/space), wound dehiscence, pneumonia, blood transfusion, urinary tract infection, unplanned intubation, mechanical ventilation for greater than 48 h, sepsis/septic shock, renal failure or insufficiency, pulmonary embolism, deep venous thrombosis, cardiac arrest, myocardial infarction or stroke, as previously described by other groups. Critical care complications were defined as one of the following 30-day outcomes: unplanned intubation, mechanical ventilation for greater than 48 h, sepsis/septic shock, renal failure or insufficiency, cardiac arrest, myocardial infarction, stroke, pulmonary embolism, deep venous thrombosis or organ/space surgical site infection (SSI).
Categorical variables were reported using counts and percentages and compared using chi-squared and Fisher’s exact tests. Medians and interquartile ranges are presented for continuous variables from the NSQIP data. Survival estimates were assessed using data from the NCDB by Kaplan–Meier and Cox proportional hazards models. Analyses from the NSQIP were carried out in R 3.5.0 (R Core Team; Vienna, Austria), whereas analyses from the NCDB and SEER were carried out using SAS software version 9.4 (SAS Statistical Institute, Cary, NC, USA). Statistical significance was set at P < 0.05.
Results
Demographics and Characteristics of Cranial Chordomas in the USA
Queries identified 936 patients from the NCDB and 405 patients from SEER. The median age at diagnosis was 47 years in the NCDB and 50 years in SEER. Paediatric patients (0e20 years) accounted for 10.3% of patients in the NCDB and for 8.4% of patients in SEER (Table 1). Patients older than 60 years represented 26.8% of the patients in the NCDB and 28.4% in SEER. Most patients were men (56.2% in NCDB and 54.8% in SEER), White (80.9% in NCDB and 83.2% in SEER) and non-Hispanic (78.6% in NCDB and 77.8% in SEER).
Table 1.
Comparison of the demographics of patients with a diagnosis of chordomas in the National Cancer Database (NCDB) and Surveillance, Epidemiology, and End Results (SEER) 2004–2014
| Variables | NCDB n = 936 |
SEER n = 405 |
|
|---|---|---|---|
| n (%) | n (%) | ||
|
| |||
| Age groups | 0–20 years | 96(10.3%) | 34 (8.4%) |
| 21–59 years | 589 (62.9%) | 256 (63.2%) | |
| 60 years | 251 (26.8%) | 115 (28.4%) | |
| Gender | Male | 526 (56.2%) | 222 (54.8%) |
| Female | 410 (43.8%) | 183 (45.2%) | |
| Race | White | 757 (80.9%) | 337 (83.2%) |
| Black | 83 (8.9%) | 23 (5.7%) | |
| Other | 79 (8.4%) | 40 (9.9%) | |
| Unknown | 17 (1.8%) | 5 (1.2%) | |
| Spanish Hispanic origin | Unknown | 47 (5.0%) | 0 |
| No | 736 (78.6%) | 315 (77.8%) | |
| Yes | 153 (16.3%) | 90 (22.2%) | |
| Histology | Chordoma, NOS | 831 (88.8%) | 361 (89.1%) |
| Chondroid chordoma | 96(10.3%) | 42 (10.4%) | |
| Dedifferentiated chordoma | 9 (1.0%) | 2 (0.5%) | |
| Size of tumour | Unknown | 257 (27.5%) | 102 (25.2%) |
| 0–2 cm | 664 (70.9%) | 299 (73.8%) | |
| 2–5 cm | 13 (1.4%) | 3 (0.7%) | |
| 5 + cm | 1 (0.1%) | 1 (0.3%) | |
| Census median income quartiles 2007–2012 | Unknown | 15 (1.6%) | 0 |
| <$38 000 | 158 (16.9%) | 11 (2.7%) | |
| $38 000–47 999 | 207 (22.1%) | 52 (12.8%) | |
| $48 000–62 999 | 233 (24.9%) | 234 (57.8%) | |
| ≥$68 000 | 323 (34.5%) | 105 (26.7%) | |
| Percentage with no high school degree 2007–2012 | Unknown | 15 (1.6%) | 0 |
| ≥21% | 181 (19.3%) | 101 (24.9%) | |
| 13.0–20.9% | 252 (26.9%) | 154 (38.0%) | |
| 7.0–12.9% | 268 (28.6%) | 137 (33.8%) | |
| <7.0% | 220 (23.5%) | 13 (3.2%) | |
| Urban/rural 2013 | Unknown | 35 (3.7%) | 0 |
| Metropolitan | 785 (83.9%) | 380 (93.8%) | |
| Urban | 78 (8.3%) | 24 (5.9%) | |
| Rural | 38 (4.1%) | 1 (0.3%) | |
| Surgery | Unknown | 12 (1.3%) | 1 (0.2%) |
| None | 107 (11.4%) | 55 (13.6%) | |
| Yes | 817 (83.4%) | 349 (87.2%) | |
| Radiation | Unknown | 17 (1.8%) | 0 |
| No | 481 (51.4%) | 221 (54.6%) | |
| Yes | 438 (46.8%) | 184 (45.4%) | |
| Type of radiation | IMRT | 99 (10.6%) | - |
| Other photon therapy | 104(11.1%) | - | |
| Protons | 141 (15.1%) | - | |
| SRS | 87 (9.3%) | - | |
| Unknown radiotherapy/unknown type | 25 (2.67%) | - | |
| Chemotherapy | Unknown | 42 (4.5%) | - |
| No | 879 (93.9%) | 395 (97.53%)* | |
| Yes | 15 (1.6%) | 10(2.5%) | |
| Treatment combination | Unknown | 54 (5.8%) | 1 (0.2%) |
| None | 66(7.1%) | 54(13.3%) | |
| Radiotherapy + chemotherapy + surgery | 3 (0.3%) | 7 (1.7%) | |
| Radiotherapy + chemotherapy | 2 (0.2%) | 0 (0.0%) | |
| Radiotherapy + surgery | 391 (41.8%) | 177 (43.7%) | |
| Chemotherapy + surgery | 6 (0.6%) | 2 (0.5%) | |
| Surgery only | 385 (41.1%) | 163 (40.2%) | |
| Radiotherapy only | 28 (3.0%) | 0 (0.0%) | |
| Chemotherapy only | 1 (0.1%) | 1 (0.2%) | |
IMRT, intensity-modulated radiotherapy; NOS, not otherwise specified; SRS, stereotactic radiosurgery.
The patients categorized as “unknown to have received chemotherapy”, and “no chemotherapy received” were combined in SEER.
Conventional chordomas (chordomas, not otherwise specified) accounted for 88.8% of the patients in the NCDB and 89.1% in SEER, chondroid chordomas for 10.3% in the NCDB and 10.4% in SEER and dedifferentiated chordomas for 1.0% in the NCDB and 0.5% in SEER. We found no significant difference in demographics between the three histological groups. Tumour size was available for 72.5% of the cases in the NCDB and for 74.8% in SEER. Tumour size was between 0 and 2 cm in 70.9% of patients in the NCDB and 73.8% in SEER. Only 14 (1.5%) patients in the NCDB and four (1.0%) patients in SEER had reported measurements over 2 cm.
About 60% of patients in the NCDB and over 84% of patients in SEER lived in areas with a median annual income of over $48 000. Patients with an annual income under $38 000 accounted for 16.9% in the NCDB and 2.7% in SEER. Over 19% of patients in the NCDB and around 25% of patients in SEER lived in areas with over 21% of non-high school graduates (Table 1).
Patterns of Care
Surgery was the preferred treatment modality, with 87.2% of the patients in the NCDB and 86.2% of the patients in SEER undergoing any type of surgical procedure (Table 1). Surgery was carried out alone (41.1% in NCDB and 40.2% in SEER) or in combination with radiation (41.8% in NCDB and 43.7% in SEER) (Figure 1). Twenty-eight (3.0%) were treated with radiation only in the NCDB compared with none receiving radiation only in SEER. Watchful waiting was the first course of treatment in 7.1% of patients in the NCDB and 13.3% of patients in SEER. Only 15 (1.6%) patients in the NCDB and 10 (2.5%) in SEER received chemotherapy as the first course of treatment. There was no significant difference in terms of treatment between the three histology subgroups in both databases.
Fig 1.
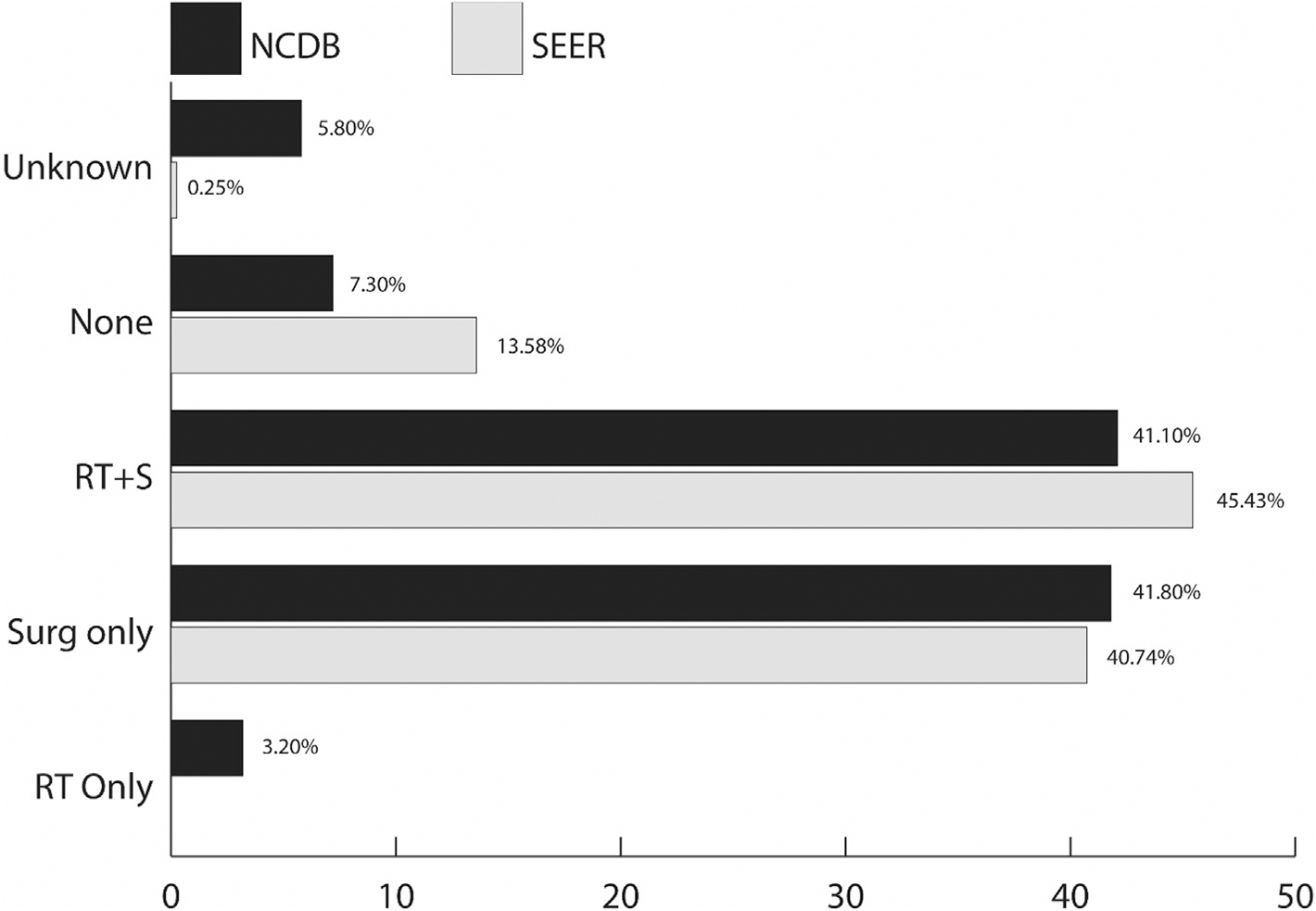
Most common treatment combination in patients with a diagnosis of chordomas, National Cancer Database (NCDB) and Surveillance, Epidemiology, and End Results (SEER) 2004–2014. RT, radiotherapy; S, surgery.
The type of radiation was reported for 431 of the 438 patients who received radiation in the NCDB. Proton therapy was the most common, accounting for 32.2% of patients, followed by other photon therapy (23.7%), IMRT (22.6%) and SRS (19.9%) (Table 1). There was an increasing trend for the use of proton and other photon therapy, with a decreasing use of SRS (Supplementary Figure S1).
We analysed patterns of care throughout the years of diagnosis (Figure 2). We found an increasing trend towards using surgery and radiation, and a decreasing use of surgery alone in both the NCDB and SEER. The trend was marked after 2010 and was especially noticeable in the NCDB (Figure 2A). The use of watchful waiting has been stable throughout the years, with a slight decreasing trend (Figure 2).
Fig 2.
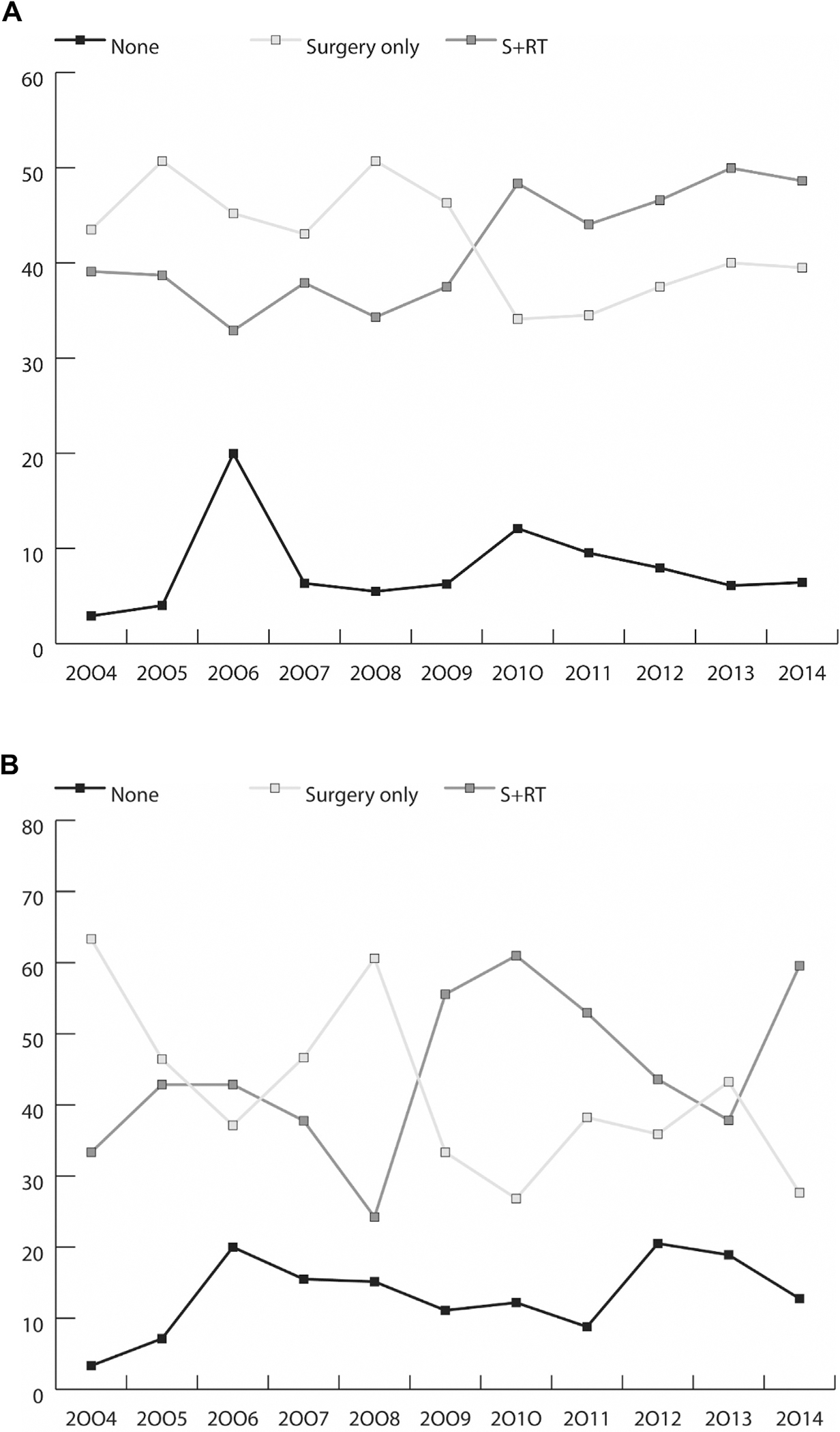
(A) Percentage of treatment received (none, surgery alone or radiation and surgery) by year of diagnosis, National Cancer Database (NCDB) 2004–2014. (B) Percentage of treatment received (none, surgery alone or radiation and surgery) by year of diagnosis, Surveillance, Epidemiology, and End Results (SEER) 2004–2014.
Perioperative Characteristics and Surgical Morbidity
We identified 2362 patients undergoing skull-base procedures, of which 64 were defined as chordomas. We compared patients who underwent skull-base surgery for chordomas with patients who had skull-base surgery for any other pathology (Supplementary Tables S1 and S2). We found no significant difference in age, gender, comorbidities or preoperative laboratory values. No patient who underwent skull-base procedures in the NSQIP database presented with ascites or severe preoperative renal failure. No patient was functionally dependent, ventilator dependent, had a history of chronic heart failure, bleeding disorder, preoperative sepsis or received dialysis at the time of surgery in the chordoma cohort (Supplementary Table S1).
The median operative time was significantly higher in patients with chordomas (497 min compared with 320 min in patients with any other indications, P < 0.0001). The American Society of Anesthesiologists’ (ASA) classification did not differ between the two groups. In patients with chordomas, the most common ASA class was ASA III (60.9%), followed by ASA I–II (28.1%). Over 10% of patients had an ASA of IV–V. The median hospital length of stay was also significantly longer in patients with chordomas (7 days versus 4 days, P = 0.0001). Most patients were discharged home (82.9%). One patient died within 30 days of surgery.
Overall postoperative morbidity was higher in the chordoma group (43.8% versus 22.5%, P = 0.0001). It was statistically significant for superficial and deep surgical site infection, wound disruption and blood transfusion. Critical care complications did not vary significantly between groups (Supplementary Table S2).
Survival
The median survival for cranial chordomas could not be assessed in both the NCDB and SEER as the survival rates were higher than 50% at the end of the follow-up period (Figure 3A,B). The 5-year survival rate was 79.8% in the NCDB and 76.9% in SEER (Table 2). We found no statistically significant difference in overall survival between histology groups in both the NCDB and SEER. The 5-year survival rates for conventional chordoma, chondroid chordoma and dedifferentiated chordoma were 79.7, 82.7 and 64.8%, respectively, in the NCDB, with 75.3, 82.5 and 50.0%, respectively, in SEER.
Fig 3.
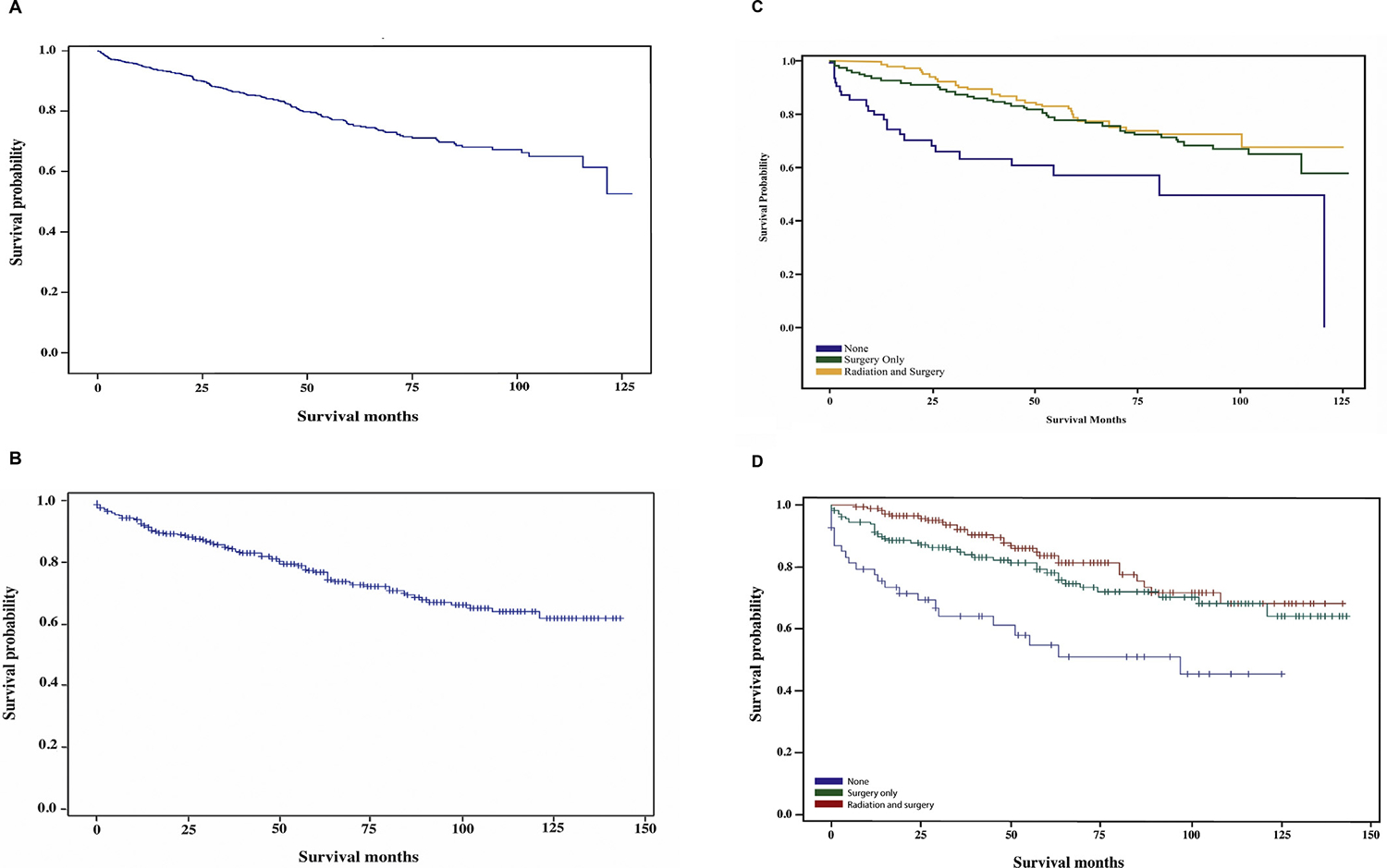
(A) Overall survival, National Cancer Database (NCDB) 2004–2014. (B) Overall survival, Surveillance, Epidemiology, and End Results (SEER) 2004–2014. (C) Survival by treatment modality comparing no treatment, surgery only and surgery plus radiation, NCDB 2004–2014. (D) Survival by treatment modality comparing no treatment, surgery only and surgery plus radiation, SEER 2004–2014.
Table 2.
Five-year survival rate comparison by demographics and treatment receipt, National Cancer Database (NCDB) and Surveillance, Epidemiology, and End Results (SEER) 2004–2014
| NCDB |
SEER |
||
|---|---|---|---|
| 5-year survival (95% CI) | 5-year survival (95% CI) | ||
|
| |||
| Overall | 79.8% (76.8–82.8%) | 76.9% (72.3–81.6%) | |
| Age groups | 0–20 years | 87.4% (80.0–94.8%) | 74.6% (59.2–90.0%) |
| 21–59 years | 84.5% (81.1–88.0%) | 85.0% (79.8–90.2%) | |
| 60 + years | 65.8% (58.9–72.7%) | 61.5% (51.7–71.3%) | |
| Gender | Female | 81.3% (76.9–85.7%) | 78.9% (72.1–85.7%) |
| Male | 78.6% (74.5–82.7%) | 75.4% (68.9–81.3%) | |
| Race | Black | 81.5% (72.2–90.8%) | 95.0% (85.5–104.5%) |
| Other | 85.1% (75.1–95.2%) | 78.1% (63.3–92.85) | |
| Unknown | 86.9% (69.8–100%) | 100.0% | |
| White | 79.0% (75.6–82.3%) | 75.6% (70.3–80.8%) | |
| Hispanic | No | 79.6% (76.2–83.0%) | 75.2% (69.8–80.5%) |
| Unknown | 78.3% (65.6–91.0%) | - | |
| Yes | 81.0% (73.5–88.4%) | 84.6% (75.3–93.8%) | |
| Chemotherapy | No | 80.0% (77.0–83.1%) | 77.8% (73.1–82.5%) |
| Yes | 83.1% (68.9–97.3%) | 48.0% (15.9–80.1%)* | |
| Unknown | 56.2% (26.0–86.4%) | - | |
| Treatment combination | None | 61.1% (47.5–74.6%) | 54.5% (54.4–54.7%) |
| Radiation only | 48.9% (29.7–68.2%) | - | |
| Surgery only | 80.2% (75.7–84.8%) | 78.9% (78.9–79.0%) | |
| Surgery + radiation | 84.8% (80.5–89.0%) | 83.4% (83.4–83.5%) | |
| Unknown | 81.8% (69.0–94.5%) | 100% (100–100%)† | |
CI, confidence interval.
Unknown to have received chemotherapy, and no chemotherapy received were combined in SEER.
Only one case was reported as unknown.
Patients older than 60 years of age had shorter 5-year survival estimates compared with any other age group (Table 2). Patients who received chemotherapy in the NCDB had a 5-year survival rate of 56.2% compared with 48% in SEER. The 5-year survival rates for surgery and radiation combination treatment were comparable between both databases. Patients treated with radiation only had a 5-year survival rate of 48.9% in the NCDB compared with 61.1% in patients who did not receive any form of initial treatment. The 1- and 5-year survival rates were highest with proton therapy, followed closely by IMRT and other photon therapy (Supplementary Table S3).
Kaplan–Meier survival analyses showed a lower median survival in patients who received radiation only (4 months in the NCDB) (Figure 3C,D). There was a trend towards longer survival in patients who received surgery and radiation in both the NCDB and SEER (Figure 3C,D). Median survival did not differ by type of radiation modality used (Supplementary Figure S2). We carried out a multivariate analysis for independent risk factors for mortality (Table 3). Patients younger than 60 years had a decreased risk of mortality. Lower socioeconomic status and a higher number of comorbidities showed a trend towards a higher risk of mortality but it was not statistically significant.
Table 3.
Multivariate analysis for risk factors for mortality, National Cancer Database (NCDB) 2004–2014 and Surveillance, Epidemiology, and End Results (SEER) 2004–2014
| NCDB |
SEER |
||||
|---|---|---|---|---|---|
| Hazard ratio | 95% confidence interval | Hazard ratio | 95% confidence interval | ||
|
| |||||
| Age groups | 0–20 years | 0.36 | 0.18–0.72 | 0.473 | 0.22–1.00 |
| 21–59 years | 0.47 | 0.32–0.71 | 0.332 | 0.22–0.50 | |
| >60 years | Reference | - | Reference | - | |
| Gender | Female | 0.89 | 0.65–1.21 | 0.832 | 0.55–1.25 |
| Male | Reference | - | Reference | - | |
| Race | Black | 0.65 | 0.38–1.12 | 0.159 | 0.02–1.14 |
| Other | 0.79 | 0.41–1.52 | 0.738 | 0.35–1.52 | |
| White | Reference | - | Reference | - | |
| Hispanic | No | 1.13 | 0.70–1.81 | 1.52 | 0.85–2.73 |
| Yes | Reference | - | Reference | - | |
| Median income | <$38 000 | 1.69 | 0.95–3.00 | 2.178 | 0.75–6.33 |
| $38 000–47 999 | 1.30 | 0.83–2.05 | 0.859 | 0.39–1.87 | |
| $48 000–62 999 | 1.31 | 0.83–2.05 | 1.397 | 0.86–2.27 | |
| ≥$68 000 | Reference | - | Reference | - | |
| Percentage no high school | 13.0–20.9% | 1.03 | 0.66–1.60 | 0.893 | 0.53–1.50 |
| 7.0–12.9% | 1.08 | 0.64–1.82 | 0.960 | 0.57–1.62 | |
| <7.0% | 0.74 | 0.38–1.41 | 1.665 | 0.63–4.37 | |
| ≥21% | Reference | - | Reference | - | |
Discussion
Cranial chordomas were most common in White people with median age of 47–56 years, which matches previous demographic descriptions [6,28]. There was a male gender majority in both the NCDB and SEER, which is different from a previous analysis of SEER (1975–2004), which reported a female predominance [6]. There was a higher percentage of females and Hispanic patients in SEER. Conventional chordomas was the most common histology group (88.8% in NCDB and 89.1% in SEER), followed by chondroid chordomas (10.3% in NCDB and 10.4% in SEER) and dedifferentiated chordomas (1.0% in NCDB and 0.5% in SEER), which is similar to previous epidemiological descriptions [28]. Most published studies have reported that most patients had a tumour size between 2 and 6 cm. However, in our analysis of both the NCDB and SEER, tumours <2 cm represented 59.5% and 73.8% of chordomas [28]. This may be due to reporting bias, as the vast majority of previous studies were single-centre retrospective studies. Of note, tumour size was missing in 27.5% of NCDB patients and in 25.2% of SEER patients. Smaller tumours may allow for increasing rates of surgery, more extensive resections and fewer complications.
Treatment protocols for cranial chordomas are controversial due to the relative radio-resistance of chordomas [29] and the limitations of surgical resection. Most studies agree on the paramount importance of radical surgical resection as initial therapy [22,30], with the extent of the resection being the most important survival predictor [16,31,32]. However, due to the involvement of main neurovascular structures at the skull base, resection is often limited [9]. Strategies to achieve local control often include adjuvant high-dose focused radiation (proton beam, carbon ion or SRS).
The use of combinatorial treatment of surgery and radiation has shown survival benefits over surgery alone in single-centre experiences [19,33]. Previous analyses of the SEER database did not show higher survival in patients who received surgery and radiation over patients who underwent surgery only [6]. Our results show a trend towards longer overall survival in patients receiving surgery and radiation. It is of note that comparing our results with SEER descriptions from 1975–2004 [6], the survival of patients receiving surgery and radiation has increased significantly. This may reflect a higher use of radiation for patients who were not offered the option in previous years, improved imaging techniques and the advent of modern radiation techniques, such as IMRT, SRS and proton therapy, which enhance delivery of tumoricidal doses of radiation.
Radiation for chordomas requires very high doses to achieve effectiveness [34]. In patients with clival chordomas receiving photon beam radiation with doses around 60 Gy, 5-year local control has been reported to be between 28 and 39% [35–37], whereas at a median dose of 66.6 Gy, 5-year control rates are reported to be 50% [38]. The doses of radiation are individualised based on tumour location and the extent of the resection; recommended doses range from 70 to 78 Gy [39]. An investigation using the NCDB showed that doses higher than 70 Gy were associated with survival in both chordomas and chondrosarcomas [40].
Using IMRT and SRS, 5-year control rates are between 62 and 93% [41–46]. Particle therapy, such as proton and carbon ion therapy, allow for controlled dose distribution and are a promising option in the treatment of cranial chordomas. The use of carbon ion radiotherapy for skull-based chordomas have reported 5-year local control rates over 70% in single-centre studies [47–50] and was found to be superior to gamma knife radiosurgery in a meta-analysis [16]. However, a recent meta-analysis did not show significant differences between SRS, proton therapy and carbon ion therapy at 3- and 5-year survival [51]. The 10-year survival data showed a benefit of proton therapy [51]. Carbon ions have a higher biological effectiveness and deliver larger mean energy per unit length compared with photons and protons, but it is a technique that is less widely available.
Particle therapy is considered to be the standard of care, due to its ability to provide higher doses associated with the Bragg peak; allowing dose deposit within the targeted volume with limited or no radiation to distal tissues and a safer profile compared with SRS [39]. Proton beam radiation series have described a 5-year survival rate of over 60–80% [10,34,40,52–56]. Due to the high costs associated with carbon ion and proton radiation therapy, facilities are not widely available, and no published clinical trial has compared SRS with proton therapy or proton therapy with carbon ions [57,58]. Our 5-year survival rates with radiation plus surgery are similar to those described in recent adjuvant radiation series and our results did not suggest a benefit of one radiation modality over the other. Radiation techniques can also combine photon and proton therapy, and this may not be assessed from the data available in the NCDB. The use of photon beam radiation after proton beam therapy for skull-base chordomas remains under investigation, but may be halted due to the high cost of proton beam radiation and its limited availability.
Our results showed a higher use of radiation in patients reported by SEER compared with the NCDB. This may be associated with socioeconomic differences, as there was a significantly lower percentage of patients in SEER earning less than $38 000 a year (2.7% in SEER compared with 16.9% in NCDB) and a lower percentage of patients living in rural areas (0.3% in SEER compared with 4.1% in NCDB).
There is no established role for chemotherapy in the initial management of chordomas. Epidermal growth factor receptor expression has been confirmed in a series of 12 chordoma patients [59] and whole-transcriptome analyses have identified T (brachyury), LMX1A, Z1C4, LHX4 and HOXA1 to be potential biomarkers for chordomas [60]. At the time of progression, evidence suggests some degree of activity using targeted therapies, specifically lapatinib, cetuximab, gefinitib, imitinib and sunitinib [15,61,62]. Finding targetable markers for chordomas is an area of active investigation.
Several approaches have been described in the literature to improve surgical time and patient outcomes, including endoscopic approaches [63–66]. Our results suggested that chordoma surgery was significantly more morbid compared with any other skull-base procedure, with relatively high superficial and deep surgical site infections, as well as haemorrhage requiring blood transfusions. Cerebrospinal fluid leak after extensive skull-base surgery is known to be associated with surgical site infection and meningitis [67]. Bleeding has also been reported to be high in skull-base tumours displacing or encasing the internal carotid artery or its branches. Cerebrospinal fluid leak is commonly described in the chordoma literature, in up to 26% of patients, followed by 12% intraoperative vessel injury and 12% perioperative death [17,66].
The median survival in both the NCDB and SEER 2004–2014 was indeterminate, but is expected to be over 12 years, which is significantly longer than the 6–9 years previously described using SEER [5,6]. The median survival for patients who did not receive initial treatment was 8 years, but the median survival could not be assessed for patients who had surgery and radiation or surgery only. We believe that this is in part due to improved surgical and radiation techniques and the wide availability of radiation [34,52]. Previous single-centre studies and SEER analyses have suggested longer survival in younger patients without statistical significance [6,37]. Our analysis showed that younger patients had a decreased risk of mortality compared with patients older than 60 years. Age was the only significant risk factor identified.
Some of the limitations of our analysis included the lack of availability of details about clinical presentation, surgical approach, type of radiation, recurrence and complications management. We have provided a comprehensive review on the management of a rare tumour using the available data for the first line of treatment. Patients reported in SEER were more likely to receive radiation and more likely to be followed expectantly, suggesting differences that may be bound to facility location and socioeconomic status. Patterns of care are changing, with a trend towards longer survival and relatively acceptable post-treatment complications with surgery and radiation. Surgery is complicated with a long operative time, hospital stay and a higher rate of complications.
Supplementary Material
Acknowledgement
This study was supported by the Biostatistics and Bioinformatics Shared Resource of the University of Kentucky Markey Cancer Center (P30CA177558).
Footnotes
Conflict of Interest
The authors declare no conflicts of interest.
Appendix A. Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.clon.2019.06.004.
References
- [1].Heffelfinger MJ, Dahlin DC, MacCarty CS, Beabout JW. Chordomas and cartilaginous tumors at the skull base. Cancer 1973;32:410–420. [DOI] [PubMed] [Google Scholar]
- [2].Walcott BP, Nahed BV, Mohyeldin A, Coumans JV, Kahle KT, Ferreira MJ. Chordoma: current concepts, management, and future directions. Lancet Oncol 2012;13:e69–e76. [DOI] [PubMed] [Google Scholar]
- [3].Horten BC, Montague SR. In vitro characteristics of a sacrococcygeal chordoma maintained in tissue and organ culture systems. Acta Neuropathol 1976;35:13–25. [DOI] [PubMed] [Google Scholar]
- [4].Healey JH, Lane JM. Chordoma: a critical review of diagnosis and treatment. Orthop Clin North Am 1989;20:417–426. [PubMed] [Google Scholar]
- [5].McMaster ML, Goldstein AM, Bromley CM, Ishibe N, Parry DM. Chordoma: incidence and survival patterns in the United States, 1973e1995. Cancer Causes Control 2001;12:1–11. [DOI] [PubMed] [Google Scholar]
- [6].Chambers KJ, Lin DT, Meier J, Remenschneider A, Herr M, Gray ST. Incidence and survival patterns of cranial chordoma in the United States. Laryngoscope 2014;124:1097–1102. [DOI] [PubMed] [Google Scholar]
- [7].Vujovic S, Henderson S, Presneau N, Odell E, Jacques TS, Tirabosco R, et al. Brachyury, a crucial regulator of notochordal development, is a novel biomarker for chordomas. J Pathol 2006;209:157–165. [DOI] [PubMed] [Google Scholar]
- [8].Salisbury JR, Isaacson PG. Demonstration of cytokeratins and an epithelial membrane antigen in chordomas and human fetal notochord. Am J Surg Pathol 1985;9:791–797. [DOI] [PubMed] [Google Scholar]
- [9].Tzortzidis F, Elahi F, Wright D, Natarajan SK, Sekhar LN. Patient outcome at long-term follow-up after aggressive microsurgical resection of cranial base chordomas. Neurosurgery 2006;59:230–237. [DOI] [PubMed] [Google Scholar]
- [10].Hug EB, Loredo LN, Slater JD, DeVries A, Grove RI, Schaefer RA, et al. Proton radiation therapy for chordomas and chondrosarcomas of the skull base. J Neurosurg 1999;91:432–439. [DOI] [PubMed] [Google Scholar]
- [11].Crockard HA, Steel T, Plowman N, Singh A, Crossman J, Revesz T, et al. A multidisciplinary team approach to skull base chordomas. J Neurosurg 2001;95:175–183. [DOI] [PubMed] [Google Scholar]
- [12].George S, Merriam P, Maki RG, Van den Abbeele AD, Yap JT, Akhurst T, et al. Multicenter phase II trial of sunitinib in the treatment of nongastrointestinal stromal tumor sarcomas. J Clin Oncol 2009;27:3154–3160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Stacchiotti S, Marrari A, Tamborini E, Palassini E, Virdis E, Messina A, et al. Response to imatinib plus sirolimus in advanced chordoma. Ann Oncol 2009;20:1886–1894. [DOI] [PubMed] [Google Scholar]
- [14].Casali PG, Messina A, Stacchiotti S, Tamborini E, Crippa F, Gronchi A, et al. Imatinib mesylate in chordoma. Cancer 2004; 101:2086–2097. [DOI] [PubMed] [Google Scholar]
- [15].Stacchiotti S, Tamborini E, Lo Vullo S, Bozzi F, Messina A, Morosi C, et al. Phase II study on lapatinib in advanced EGFR-positive chordoma. Ann Oncol 2013;24:1931–1936. [DOI] [PubMed] [Google Scholar]
- [16].Di Maio S, Temkin N, Ramanathan D, Sekhar LN. Current comprehensive management of cranial base chordomas: 10-year meta-analysis of observational studies. J Neurosurg 2011;115:1094–1105. [DOI] [PubMed] [Google Scholar]
- [17].Sekhar LN, Pranatartiharan R, Chanda A, Wright DC. Chordomas and chondrosarcomas of the skull base: results and complications of surgical management. Neurosurg Focus 2001;10:E2. [DOI] [PubMed] [Google Scholar]
- [18].Rassi MS, Hulou MM, Almefty K, Bi WL, Pravdenkova S, Dunn IF, et al. Pediatric clival chordoma: a curable disease that conforms to Collins’ Law. Neurosurgery 2018;82:652–660. [DOI] [PubMed] [Google Scholar]
- [19].Borba LA, Al-Mefty O, Mrak RE, Suen J. Cranial chordomas in children and adolescents. J Neurosurg 1996;84:584–591. [DOI] [PubMed] [Google Scholar]
- [20].Surawicz TS, Davis F, Freels S, Laws ER Jr, Menck HR. Brain tumor survival: results from the National Cancer Data Base. J Neuro-oncol 1998;40:151–160. [DOI] [PubMed] [Google Scholar]
- [21].Boffa DJ, Rosen JE, Mallin K, Loomis A, Gay G, Palis B, et al. Using the National Cancer Database for outcomes research: a review. JAMA Oncol 2017;3:1722–1728. [DOI] [PubMed] [Google Scholar]
- [22].Sen C, Triana AI, Berglind N, Godbold J, Shrivastava RK. Clival chordomas: clinical management, results, and complications in 71 patients. J Neurosurg 2010;113:1059–1071. [DOI] [PubMed] [Google Scholar]
- [23].Raval MV, Bilimoria KY, Stewart AK, Bentrem DJ, Ko CY. Using the NCDB for cancer care improvement: an introduction to available quality assessment tools. J Surg Oncol 2009;99:488–490. [DOI] [PubMed] [Google Scholar]
- [24].Steinberg SM, Popa MR, Michalek JA, Bethel MJ, Ellison EC. Comparison of risk adjustment methodologies in surgical quality improvement. Surgery 2008;144:662–667. [DOI] [PubMed] [Google Scholar]
- [25].McCarthy BJ, Shibui S, Kayama T, Miyaoka E, Narita Y, Murakami M, et al. Primary CNS germ cell tumors in Japan and the United States: an analysis of 4 tumor registries. Neuro-oncology 2012;14:1194–1200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Garcia CR, Slone SA, Pittman T, St Clair WH, Lightner DD, Villano JL. Comprehensive evaluation of treatment and outcomes of low-grade diffuse gliomas. PLoS One 2018;13:e0203639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Chiu CH, Tsai CM, Chen YM, Chiang SC, Liou JL, Perng RP. Gefitinib is active in patients with brain metastases from non-small cell lung cancer and response is related to skin toxicity. Lung Cancer 2005;47:129–138. [DOI] [PubMed] [Google Scholar]
- [28].Bohman LE, Koch M, Bailey RL, Alonso-Basanta M, Lee JY. Skull base chordoma and chondrosarcoma: influence of clinical and demographic factors on prognosis: a SEER analysis. World Neurosurg 2014;82:806–814. [DOI] [PubMed] [Google Scholar]
- [29].Henderson FC, McCool K, Seigle J, Jean W, Harter W, Gagnon GJ. Treatment of chordomas with CyberKnife: Georgetown University experience and treatment recommendations. Neurosurgery 2009;64:A44–A53. [DOI] [PubMed] [Google Scholar]
- [30].Al-mefty O, Almefty R. Chordomas: a personal perspective. New York: Thieme; 2017. [Google Scholar]
- [31].Maira G, Pallini R, Anile C, Fernandez E, Salvinelli F, La Rocca LM, et al. Surgical treatment of clival chordomas: the transsphenoidal approach revisited. J Neurosurg 1996;85:784–792. [DOI] [PubMed] [Google Scholar]
- [32].Sen C, Triana A. Cranial chordomas: results of radical excision. Neurosurg Focus 2001;10:E3. [DOI] [PubMed] [Google Scholar]
- [33].Potluri S, Jefferies SJ, Jena R, Harris F, Burton KE, Prevost AT, et al. Residual postoperative tumour volume predicts outcome after high-dose radiotherapy for chordoma and chondrosarcoma of the skull base and spine. Clin Oncol 2011;23:199–208. [DOI] [PubMed] [Google Scholar]
- [34].Nguyen QN, Chang EL. Emerging role of proton beam radiation therapy for chordoma and chondrosarcoma of the skull base. Curr Oncol Rep 2008;10:338–343. [DOI] [PubMed] [Google Scholar]
- [35].Rich TA, Schiller A, Suit HD, Mankin HJ. Clinical and pathologic review of 48 cases of chordoma. Cancer 1985;56:182–187. [DOI] [PubMed] [Google Scholar]
- [36].Zorlu F, Gurkaynak M, Yildiz F, Oge K, Atahan IL. Conventional external radiotherapy in the management of clivus chordomas with overt residual disease. Neurol Sci 2000;21:203–207. [DOI] [PubMed] [Google Scholar]
- [37].Forsyth PA, Cascino TL, Shaw EG, Scheithauer BW, O’Fallon JR, Dozier JC, et al. Intracranial chordomas: a clinicopathological and prognostic study of 51 cases. J Neurosurg 1993;78:741–747. [DOI] [PubMed] [Google Scholar]
- [38].Debus J, Schulz-Ertner D, Schad L, Essig M, Rhein B, Thillmann CO, et al. Stereotactic fractionated radiotherapy for chordomas and chondrosarcomas of the skull base. Int J Radiat Oncol Biol Phys 2000;47:591–596. [DOI] [PubMed] [Google Scholar]
- [39].Mercado CE, Holtzman AL, Rotondo R, Rutenberg MS, Mendenhall WM. Proton therapy for skull base tumors: a review of clinical outcomes for chordomas and chondrosarcomas. Head Neck 2019;41:536–541. [DOI] [PubMed] [Google Scholar]
- [40].Palm RF, Oliver DE, Yang GQ, Abuodeh Y, Naghavi AO, Johnstone PAS. The role of dose escalation and proton therapy in perioperative or definitive treatment of chondrosarcoma and chordoma: an analysis of the National Cancer Data Base. Cancer 2019;125:642–651. [DOI] [PubMed] [Google Scholar]
- [41].Sahgal A, Chan MW, Atenafu EG, Masson-Cote L, Bahl G, Yu E, et al. Image-guided, intensity-modulated radiation therapy (IG-IMRT) for skull base chordoma and chondrosarcoma: preliminary outcomes. Neuro-oncology 2015;17:889–894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Hasegawa T, Ishii D, Kida Y, Yoshimoto M, Koike J, Iizuka H. Gamma Knife surgery for skull base chordomas and chondrosarcomas. J Neurosurg 2007;107:752–757. [DOI] [PubMed] [Google Scholar]
- [43].Yoneoka Y, Tsumanuma I, Fukuda M, Tamura T, Morii K, Tanaka R, et al. Cranial base chordoma – long term outcome and review of the literature. Acta Neurochir 2008;150:773–778. [DOI] [PubMed] [Google Scholar]
- [44].Choy W, Terterov S, Ung N, Kaprealian T, Trang A, DeSalles A, et al. Adjuvant stereotactic radiosurgery and radiation therapy for the treatment of intracranial chordomas. J Neurol Surg B 2016;77:38–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].White EC, Ajlan AC, Kumar KA, Gibbs IC, Chang SD, Harsh GR, et al. Stereotactic radiosurgery for newly diagnosed and recurrent chordomas. Int J Radiat Oncol Biol Phys 2016;96:E89. [Google Scholar]
- [46].Jung EW, Jung DL, Balagamwala EH, Angelov L, Suh JH, Djemil T, et al. Single-fraction spine stereotactic body radiation therapy for the treatment of chordoma. Technol Cancer Res Treat 2017;16:302–309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Mizoe JE, Hasegawa A, Takagi R, Bessho H, Onda T, Tsujii H. Carbon ion radiotherapy for skull base chordoma. Skull Base 2009;19:219–224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Uhl M, Mattke M, Welzel T, Roeder F, Oelmann J, Habl G, et al. Highly effective treatment of skull base chordoma with carbon ion irradiation using a raster scan technique in 155 patients: first long-term results. Cancer 2014;120:3410–3417. [DOI] [PubMed] [Google Scholar]
- [49].Schulz-Ertner D, Karger CP, Feuerhake A, Nikoghosyan A, Combs SE, Jakel O, et al. Effectiveness of carbon ion radiotherapy in the treatment of skull-base chordomas. Int J Radiat Oncol Biol Phys 2007;68:449–457. [DOI] [PubMed] [Google Scholar]
- [50].Takahashi S, Kawase T, Yoshida K, Hasegawa A, Mizoe JE. Skull base chordomas: efficacy of surgery followed by carbon ion radiotherapy. Acta Neurochir 2009;151:759–769. [DOI] [PubMed] [Google Scholar]
- [51].Zhou J, Yang B, Wang X, Jing Z. Comparison of the effectiveness of radiotherapy with photons and particles for chordoma after surgery: a meta-analysis. World Neurosurg 2018;117:46–53. [DOI] [PubMed] [Google Scholar]
- [52].Hug EB. Review of skull base chordomas: prognostic factors and long-term results of proton-beam radiotherapy. Neurosurg Focus 2001;10:E11. [DOI] [PubMed] [Google Scholar]
- [53].Munzenrider JE, Liebsch NJ. Proton therapy for tumors of the skull base. Strahlenther Onkol 1999;175(Suppl. 2):57–63. [DOI] [PubMed] [Google Scholar]
- [54].Deraniyagala RL, Yeung D, Mendenhall WM, Li Z, Morris CG, Mendenhall NP, et al. Proton therapy for skull base chordomas: an outcome study from the University of Florida proton therapy institute. J Neurol Surg B 2014;75:53–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Grosshans DR, Zhu XR, Melancon A, Allen PK, Poenisch F, Palmer M, et al. Spot scanning proton therapy for malignancies of the base of skull: treatment planning, acute toxicities, and preliminary clinical outcomes. Int J Radiat Oncol Biol Phys 2014;90:540–546. [DOI] [PubMed] [Google Scholar]
- [56].Weber DC, Malyapa R, Albertini F, Bolsi A, Kliebsch U, Walser M, et al. Long term outcomes of patients with skull-base low-grade chondrosarcoma and chordoma patients treated with pencil beam scanning proton therapy. Radiother Oncol 2016;120:169–174. [DOI] [PubMed] [Google Scholar]
- [57].Uhl M, Edler L, Jensen AD, Habl G, Oelmann J, Roder F, et al. Randomized phase II trial of hypofractionated proton versus carbon ion radiation therapy in patients with sacrococcygeal chordoma – the ISAC trial protocol. Radiat Oncol 2014;9:100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Nikoghosyan AV, Karapanagiotou-Schenkel I, Munter MW, Jensen AD, Combs SE, Debus J. Randomised trial of proton vs. carbon ion radiation therapy in patients with chordoma of the skull base, clinical phase III study HIT-1-Study. BMC Cancer 2010;10:607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Weinberger PM, Yu Z, Kowalski D, Joe J, Manger P, Psyrri A, et al. Differential expression of epidermal growth factor receptor, c-Met, and HER2/neu in chordoma compared with 17 other malignancies. Arch Otolaryngol Head Neck Surg 2005;131:707–711. [DOI] [PubMed] [Google Scholar]
- [60].Bell D, Raza SM, Bell AH, Fuller GN, DeMonte F. Whole-transcriptome analysis of chordoma of the skull base. Virchows Archiv 2016;469:439–449. [DOI] [PubMed] [Google Scholar]
- [61].Hof H, Welzel T, Debus J. Effectiveness of cetuximab/gefitinib in the therapy of a sacral chordoma. Onkologie 2006;29:572–574. [DOI] [PubMed] [Google Scholar]
- [62].Alan O, Akin Telli T, Ercelep O, Tanrikulu Simsek E, Basoglu Tuylu T, Mutis A, et al. Chordoma: a case series and review of the literature. J Med Case Rep 2018;12:239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Chibbaro S, Cornelius JF, Froelich S, Tigan L, Kehrli P, Debry C, et al. Endoscopic endonasal approach in the management of skull base chordomas – clinical experience on a large series, technique, outcome, and pitfalls. Neurosurg Rev 2014;37:217–224. discussion 24–5. [DOI] [PubMed] [Google Scholar]
- [64].Holzmann D, Reisch R, Krayenbuhl N, Hug E, Bernays RL. The transnasal transclival approach for clivus chordoma. Min Invasive Neurosurg 2010;53:211–217. [DOI] [PubMed] [Google Scholar]
- [65].Singh H, Harrop J, Schiffmacher P, Rosen M, Evans J. Ventral surgical approaches to craniovertebral junction chordomas. Neurosurgery 2010;66:96–103. [DOI] [PubMed] [Google Scholar]
- [66].Al-Mefty O, Kadri PA, Hasan DM, Isolan GR, Pravdenkova S. Anterior clivectomy: surgical technique and clinical applications. J Neurosurg 2008;109:783–793. [DOI] [PubMed] [Google Scholar]
- [67].Fang C, Zhu T, Zhang P, Xia L, Sun C. Risk factors of neurosurgical site infection after craniotomy: a systematic review and meta-analysis. Am J Infect Control 2017;45. e123–e34. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


