Abstract
The 20th Pollutant Responses in Marine Organisms (PRIMO 20) conference provided a forum for scientists from around the world to communicate novel toxicological research findings specifically focused on aquatic organisms, by combining applied and basic research at the intersection of environmental and mechanistic toxicology. The work highlighted in this special issue of Aquatic Toxicology, a special issue of Marine Environmental Research, and presented through posters and presentations, encompass important and emerging topics in freshwater and marine toxicology. This includes multiple types of emerging contaminants including microplastics and UV filtering chemicals. Other studies aimed to further our understanding of the effects of endocrine disrupting chemicals, pharmaceuticals, and personal care products. Further research presented in this virtual issue examined the interactive effects of chemicals and pathogens, while the final set of manuscripts demonstrates continuing efforts to combine traditional biomonitoring, data from -omic technologies, and modeling for use in risk assessment and management. An additional goal of PRIMO meetings is to address the link between environmental and human health. Several articles in this issue of Aquatic Toxicology describe the appropriateness of using aquatic organisms as models for human health, while the keynote speakers, as described in the editorial below, presented research that highlighted bioaccumulation of contaminants such as PFOS and mercury from fish to marine mammals and coastal human populations such as the Gullah/GeeChee near Charleston, South Carolina, USA.
1. History
Pollutant Responses in Marine Organisms (PRIMO) began as an International Symposium in 1981 with a small group of NSF-funded investigators who were addressing questions related to “Chemical Effects and the Health of the Ocean” at a mechanistic level. Key pollutants, global aquatic issues, chemical fate, and most of all mechanisms of action were presented and discussed. Although the word “marine” was used to produce the memorable acronym, the meeting has always included marine and freshwater organisms.
The first PRIMO Symposium was held in Plymouth, UK, in 1981 with the goal of stimulating international scientific interactions and collaborations in aquatic toxicology. The success of the first PRIMO meeting led to a second in 1983 in Woods Hole, USA, and then to biennial meetings held alternately between Europe and the United States. Most meetings have continued to be in Europe or the United States; however there have been meetings in Brazil and Japan (Table 1), and this variety has enhanced scientific quality, diversity of opinion, and greater discussion within this international forum. PRIMO 20, in Charleston, SC USA, was host to scientists from 29 countries. The next two meetings will be in Gothenburg, Sweden in 2021 and Nantes, France in 2023.
Table 1.
Past, present, and future sites of PRIMO meetings.
| Name | Year | Location |
|---|---|---|
| PRIMO 1 | 1981 | Plymouth, UK |
| PRIMO 2 | 1983 | Woods Hole, USA |
| PRIMO 3 | 1985 | Plymouth, UK |
| PRIMO 4 | 1987 | Woods Hole, USA |
| PRIMO 5 | 1989 | Plymouth, UK |
| PRIMO 6 | 1991 | Woods Hole, USA |
| PRIMO 7 | 1993 | Gothenburg, Sweden |
| PRIMO 8 | 1995 | Monterrey, USA |
| PRIMO 9 | 1997 | Bergen, Norway |
| PRIMO 10 | 1999 | Williamsburg, USA |
| PRIMO 11 | 2001 | Plymouth, UK |
| PRIMO 12 | 2003 | Tampa, USA |
| PRIMO 13 | 2005 | Alessandria, Italy |
| PRIMO 14 | 2007 | Florianopolis, Brazel |
| PRIMO 15 | 2009 | Bordeaux, France |
| PRIMO 16 | 2011 | Long Beach, USA |
| PRIMO 17 | 2013 | Algarve, Portugal |
| PRIMO 18 | 2015 | Trondheim, Norway |
| PRIMO 19 | 2017 | Matsuyama, Japan |
| PRIMO 20 | 2019 | Charleston, USA |
| PRIMO 21 | 2021 | Gothenburg, Sweden |
| PRIMO 22 | 2023 | Nantes, France |
The purpose of the meeting is ultimately the dissemination of scientific research progress through presentations and discussions with a strong commitment to participation and recruitment of young researchers through travel grants, presentation opportunities, and networking. Historically, select research was published in a special issue of Marine Environmental Research to facilitate broad dissemation of this important research. More recently, Aquatic Toxicology has been added and primarily publishes molecular and mechanistic studies, while the Marine Environmental Research special issue primarily focuses on more field-based or ecologically relevant studies.
A major thrust historically and currently of PRIMO meetings is the elucidation of underlying molecular mechanisms of toxicity; this extends the relevance of the meeting from impacts in marine/aquatic environments to human health, including the Oceans and Human Health Initiative. At the most recent meeting, PRIMO20, issues such as toxicant absorption, distribution, metabolism and elimination were addressed. In addition, climate change, endocrine disruption, use of -omics, ocean acidification, marine mammals, microplastics, emerging chemicals, genotoxicity, immunotoxicology, mixtures, community and ecosystem level effects, trophic transfer, biomarkers, risk assessment, oil pollution, sustainable development, conservation, genetic diversity, legacy chemicals, pollution in the Arctic, organismal consequences, and more were discussed. This diversity of work fosters comparative and integrative approaches to research and research translation in order to enhance the utility of marine/aquatic organisms as models for environmental health, and human health and disease.
2. Emerging contaminants
Emerging contaminants are aquatic pollutants that are relatively new, recently considered as detrimental to environmental and human health, and typically not regulated by environmental laws. Examples of emerging contaminants include 1,4-dioxane, perfluorinated chemicals (PFCs), polybrominated diphenyl ethers (PBDEs), nanoparticles, pharmaceuticals, personal care products, natural and synthetic hormones with several of these acting as endocrine disrupting chemicals (Salimi et al., 2017). This definition may also consider chemicals that have been on the market but only recently considered contaminants of emerging concern such as microplastics or pharmaceuticals. A large range of emerging chemicals were discussed at PRIMO 20 such as microplastics (Diana et al., 2020; Mancia et al., 2020; Zhu et al., 2020), pharmaceuticals and personal care products (Santonocito et al., 2020), pesticides (De Oliveira et al., 2020; Riegerix et al., 2020), PFCs (Ibor et al., 2020), PBDEs, and endocrine disruptors (Blalock et al., 2020; Ibor et al., 2020) including traditional estrogen-androgen-thyroid (EAT) endocrine disruptors (Blalock et al., 2020; De Oliveira et al., 2020; Ibor et al., 2020). Overall, a significant portion of the meeting discussed emerging contaminants and their adverse effects. Of special note are microplastics (Diana et al., 2020; Mancia et al., 2020; Zhu et al., 2020) and endocrine disruptors (De Oliveira et al., 2020; Ibor et al., 2020), which receive separate sections in this review because of their pervasiveness and adverse effects (LeBlanc and Bain, 1997; LeBlanc et al., 2012; Martyniuk et al., 2020; North and Halden, 2013).
The concept of an emerging contaminant is a moving target as new compounds are produced (Sauvé and Desrosiers, 2014). As compound usage changes, some compounds such as tributyltins become legacy compounds while the arsenical replacements may be chemicals of emerging concern again (Sauvé and Desrosiers, 2014). Location and use patterns can define what chemicals are considered emerging in some areas of the world. This can also lead to prioritization of chemicals of emerging concern for each region. For example, in San Francisco Bay, the chemical classes of primary concern are nonylphenol and nonylphenol ethoxylates, fipronil, perfluorooctane sulfonate (PFOS) and other perfluoroalkyl substances (PFAS). However, the Great Lakes region placed polybrominated diphenyl ethers (PBDEs), PFOS/PFAS, hexabromocyclododecane, and short chain chlorinated paraffins as emerging chemicals of mutual concern for Canada and the United States. Fragrances, pharmaceuticals such as ibuprofen, brominated azo dyes, microplastics, phthaltes, bisphenol A, UV filters, triclosan, pyrethroids etc. have also been placed as emerging chemicals of concern (Hayashi et al., 2008; Lee et al., 2005; Peng et al., 2016; Santonocito et al., 2020; Sutton et al., 2017).
Many pesticides are regarded as legacy compounds such as arsenic, DDT, or organophosphates; however, others such as fipronil, pyrethroids, neonicotinoids, and second generation rodenticides may be considered emerging contaminants (Maruya et al., 2016; Riegerix et al., 2020; Sauvé and Desrosiers, 2014). The toxicity of rodenticides on fish have not been well studied. Furthermore, new generations of rodenticides have recently been produced; however, they are more toxic and more likely to bioaccumulate in the liver of fish. These rodenticides are typically delivered as bait pellets, often air-dropped to remote island areas, with the potential to contaminate coral reefs. Riegerix et al. (Riegerix et al., 2020) describe the acute toxicity of two first generation and one second generation anti-coagulant rodenticides and their effects on clotting times in red-toothed triggerfish (Odonus niger), black triggerfish (Melichthys niger), fathead minnow (Pimephales promelas), and largemouth bass (Micropterus salmoides). The results demonstrated that the saltwater and freshwater fish species studied are far less sensitive to acute toxicity of anti-coagulant rodenticides than are mammals and birds, and while more studies are needed, concerns of acute toxicity of these rodenticides to fish populations and aquatic ecosystems are minimal (Riegerix et al., 2020).
This is not the case for UV filtering sunscreens! Because of increasing health concerns regarding epidermal sun damage and skin cancers, UV filtering sunscreen use has increased to about 10,000 tons per year with most sunscreens containing multiple UV filtering chemicals (Danovaro et al., 2008; Gao et al., 2013). The sunscreen, 4-methyl benzylidene camphor (4-MBC), was found in environmental samples on Spanish coasts and Swiss lakes at concentrations from 2 to 125 ng/L (Balmer et al., 2005; Giokas et al., 2007). 4-MBC clearly had an adverse effect on the marine mussel, Ruditapes philippinarum, as it increased sublethal stress responses associated with apoptosis and DNA strand breakages. Furthermore, oxidative stress was increased at concentrations where toxicity was observed with an estimated LC50 of 7.71 μg/L (Santonocito et al., 2020). Typical risk assessments found that 4-MBC is a probable hazard to marine life using a safety factor of 1000 and high risk to marine life using a safety factor of 10,000 (Santonocito et al., 2020). Other studies have come to similar conclusions with the freshwater invertebrate, Daphnia magna, as the test organism (Rodríguez et al., 2015); however, environmental risk was lower in fish (Sang and Leung, 2016). Overall, the environmental risk assessments for sunscreens are disconcerting because other sunscreens such as octocrylene, 3-benzylidene-camphor, and benzophenone-3 (Balmer et al., 2005; Giokas et al., 2007) are also present in the environment and they may act additively on stress responses and lethality (Baldwin and Roling, 2009; Schmidt et al., 2017). In conclusion, sunscreens such as 4-MBC are probable hazards to marine ecosystems and induce stress in marine life (Santonocito et al., 2020).
Solid waste dumpsites and landfills can lead to leachates that enter ground and surface waters with the potential for adverse effects on human health and aquatic life (Arukwe et al., 2012). Simulated leachate from a Nigerian dumpsite increased vitellogenin, Cyp19, and plasma estradiol concentrations in African sharptooth catfish (C. gariepinus), indicating the release of environmental estrogens from the dumpsite. Diethylhexylphthalte and several PFAS chemicals were measured although at lower concentrations that have been measured at other sites such as those found in Norway (Ibor et al., 2020). In summary, dumpsites and landfills are sources of large amounts of emerging contaminants and adverse environmental and human health effects can be measured. Ultimately, new products, changes in use patterns, contaminated sites including Superfund sites, and trophic transfer of common use chemicals are all reasons we will continue to observe novel emerging contaminants.
3. Microplastics
While the problem of plastic pollution in the marine environment has been recognized for almost half a century (Jambeck et al., 2015), the potential effects of microplastics on marine organisms only started to get major attention in the last 10 years (Avio et al., 2017). At the 6th International Conference on Marine Pollution and Ecotoxicology in Hong Kong (May 2010) a number of presentations focused on microplastics in the marine environment, resulting in the first review articles on this topic published in a special issue of Marine Pollution Bulletin (Andrady, 2011; Cole et al., 2011; Zarfl et al., 2011).
Since then, numerous articles have been published on the sources, distribution and effects of microplastics in the marine realm. Sources of microplastics can be plastic microbeads used in a variety of products and applications, or as degradation product of larger plastic objects, including the fragmentation of polymer fibers. The use of microbeads in personal care products is being phased out in a number of countries (Guerranti et al., 2019), but unintended production and distribution of microplastic particles as a result of natural breakdown of larger plastic objects will remain an environmental problem for years to come (Weinstein et al., 2016).
Microplastics can consist of a variety of polymers; well-known examples are polyethylene, polypropylene, polystyrene, polyvinyl chloride, but also abrasion particles from synthetic rubber (tire wear particles) are considered microplastics. While the polymers from which the microplastics are made are generally considered to be relatively non-toxic, additives like plasticizers, stabilizers and fillers are of toxicological concern. Well-studied additives are phthalates and bisphenol A, and polycyclic aromatic hydrocarbons in synthetic rubber (Hermabessiere et al., 2017; LaPlaca and van den Hurk, 2020). Other concerns about microplastics in the aquatic environment are related to the non-polar character of plastics, which enhances the adsorption of poorly water-soluble toxicants. Microplastic particles could therefore function as a vehicle for persistent organic pollutants when the particles are ingested and reside for some time in the intestinal lumen where the toxicants could desorb and be taken up through the intestinal lining (Diana et al., 2020; Koelmans et al., 2016).
At the PRIMO 20 meeting, ten platform presentations and eleven posters addressed various aspects of microplastics (or nanoplastics) in the marine environment. One of the published contributions (Mancia et al., 2020), describe a study in which the ingestion and potential effects of plastics in small-spotted catshark (Scyliorhinus canicula) was investigated. This common shark species lives in shallow coastal habitats and feeds on a wide variety of macrobenthic fauna. Macro- and microplastic particles were found in the GI tract of 80 % of the fish that were collected from 2 locations in the southern part of the central Mediterranean. The presence of plastics, especially macroplastics, in the GI tract was correlated with an increase in the hepatosomatic index and an increased expression of 3 essential immune system genes. It is hypothesized that these effects are induced by additives that are leaching from the ingested plastics.
This potential connection between additives and effects was also suggested by Zhu et al. (Zhu et al., 2020). They describe the effects of chronic dietary exposure to polystyrene microplastics in Japanese medaka (Medaka oryzias). The fish were fed a diet with fluorescent microbeads for 10 weeks during the maturation stage, after which they were analyzed with histological techniques. The results showed mechanical damage to intestinal tissues and gills, combined with changes in gonads, kidney and spleen. Because there was no evidence that the particles passed through the intestinal mucosa, the alterations in internal organs were attributed to leaching of additives from the microplastics.
The third published manuscript on environmental effects of plastics presented at the PRIMO 20 meeting is a study on the responses in sea anemones fed plastic pellets. Individuals of Exaiptasia pallida were presented with polyethylene pellets, which were readily ingested and consequently excreted after about 6 h. When these egested pellets were offered again, the retention time was much shorter, possibly because the taste had changed or because they were covered with an undesired biofilm. While the pellets had low metal concentrations, a significant amount of Pb (10 %) was extracted from the pellets and accumulated into the exposed anemones during residence in the animals (Diana et al., 2020).
In summary, all three studies demonstrated biological effects of ingested macro- or microplastics at very different taxonomic levels of marine organisms. Both effects of mechanical and chemical origin were described, where the chemical effects were attributed to additives that are found in plastics. These results further emphasize that there is an urgent need to reduce the presence of plastics in the marine environment as they are mistaken for food and transfer potentially toxic pollutants to marine and freshwater organisms.
4. Legacy pollutants, biomonitoring and genomics
During the early PRIMO meetings of the 20th century, much of the work focused on PAHs, PCBs, pesticides, and metals - pollutants that are often characterized as legacy pollutants.
During the 21st century, our concerns have in part given way to several emerging pollutants and environmental issues (pharmaceuticals, nanoparticles, plastics, hypoxia, etc.). However, many of the legacy pollutants are still present as part of even more complex mixtures related to anthropogenic impacts (Choo et al., 2020; Hutchinson et al., 2013). Consistent with the strong emphases on sublethal responses, cellular biomarkers were also an important focus that has always made this meeting unique. During the early PRIMOs, important new research on a variety of stress-related proteins (P450s, metallothioneins, heat shock proteins, multi-drug resistant transporters) as well as cellular damage biomarkers (lysosomal destabilization, oxidative damage, DNA damage) was presented (Bello et al., 2001; Moore et al., 2006; Ringwood et al., 1999; Stegeman and Kloepper-Sams, 1987). Then genomic, proteomic, and metabolimic tools presented opportunities for characterizing even broader responses related to individual and multiple pollutants and their resultant effects in aquatic organisms (Campos et al., 2012; Colbourne et al., 2011; Reid et al., 2017; Van Aggelen et al., 2010; Watanabe et al., 2015).
These methods can be used to examine the molecular effects on individuals and populations, to develop biomarkers for risk assessment, assess exposure and recovery to contaminants, and to integrate chemical, nutritional, growth, and biological processes into a single ‘score’ or value. Interest in ecotoxicogenomics began accelerating in the early 2000s with several early studies demonstrating the utility of gene expression profiling in assessing exposures to a single chemical, a class of chemicals, or comparing “chemical signatures” in aquatic organisms collected from impacted versus unimpacted sites (Larkin et al., 2002, 2003; Miracle et al., 2003; Peterson and Bain, 2004; Roling et al., 2007, 2006; Roling and Baldwin, 2006; Snell et al., 2003; Williams et al., 2003).
In recent years, nucleic acid sequencing, mass spectrometry, and NMR technologies have become cheaper and faster, which has allowed investigators to examine changes in transcripts, proteins, and metabolites more rapidly than ever. The use of ‘omic technologies to assess pollutant exposure and effects were discussed at the PRIMO 20 meeting. For example, transcriptomic changes due to PAHs, environmental estrogens, UV filtering chemicals, and pesticides were assessed in model organisms such as the Pacific oyster (Crassostrea gigas), Manilla clam (Ruditapes philippinarum), Japanese medaka, zebrafish (Danio rerio), killifish (Fundulus grandis and Fundulus heteroclitus), sea anemone (Nematostella vectensis), and water flea (Daphnia species). The use of metabolomics in invasive mussels, lipidomics in daphnids, and proteomics in Xenopus was also highlighted at the meeting.
A multifaceted approach with suites of biomarkers (targeted as well as ‘omic responses) will facilitate better tools for characterizing the environmental impacts of pollutant exposures and characterizing the effectivemess of remediation programs. A variety of integrative approaches for developing health index scores continue to be fine-tuned and implemented, and provide valuable translational summaries for government and regulatory agencies. However, the ability to use and apply -‘omic information to risk assessment and risk management has at times been slow to implementation (for recent reviews, see (Van Aggelen et al., 2010), Buesen et al., 2017; Brockmeier et al., 2017; Leung, 2018; Martyniuk et al., 2020). One example of taking this next step is through the DCOD 1.0 program spearheaded by a international group of investigators and PRIMO 20 participants. This program uses a holistic approach to incorporate physiology, toxicology, genomics, and mathematical models to further the use of Atlantic cod (Gadhus morhua) as a model species for environmental monitoring and risk assessment. Currently, DCOD 1.0 is using cod to assess the impacts of industrial discharge, sewage discharge into harbors, endocrine disruption, and oil pollution. During the PRIMO 20 meeting, investigators relied on the Atlantic cod genome (Star et al., 2011; Tørresen et al., 2017) in order to use RNA-Seq to assess the effects of environmental estrogens and PAHs on this species (Yadetie et al., 2018).
Ultimately, understanding fundamental cellular biomarker responses is essential for establishing robust biomonitoring programs to characterize pollutant effects. Many of these issues were addressed in the paper by Blalock et al., “Assessing legacy and endocrine disrupting pollutants in Boston Harbor with transcriptomic biomarkers.” This work, presented at the PRIMO 20 meeting (Blalock et al., 2020), involved an important partnership between UMass-Boston and Massachusetts Water Resource Authority aimed at characterizing impacts of improvements in water treatment facilities using a high density microarray to characterize differential gene expression as well as tissue contaminants from caged mussels (Blalock et al., 2018, 2020). An integrated C-BED biomarker (Coastal Biosensor for Endocrine Disruption assay) based on a suite of genes related to the toxicological modes of action of endocrine disrupting chemicals was used for the field studies and also in laboratory-exposure studies with 17α-ethinylestradiol (Blalock et al., 2018). Significant site-specific effects related to sewage and EDCs were identified as well as correlations with tissue PAHs and PCBs, indicating the continued impacts of persistent pollutants (Blalock et al., 2020). Their results indicate that the expression several genes involved in signal transduction, oxidation/reduction, and cilium assembly correlate with levels of PAHs and PCBs at the sites. The group incorporated their findings into an Integrated Biomarker Response (IBR) star plot value, thus allowing for easier interpretation of multiple transcript responses due to contaminant exposure (Blalock et al., 2020). These kinds of integrated field and laboratory exposures are essential for validating and promoting transcriptomic biomarkers for biomonitoring programs (Akbarzadeh et al., 2018; Antczak et al., 2013; Roling et al., 2004; Van Aggelen et al., 2010).
Biomarker and biomonitoring studies with wide-spread bioindicator species and model laboratory species such as mussels, oysters, cod, zebrafish, medaka, killifish, trout, flatfish, etc. presented at PRIMO meetings have facilitated important insights regarding species-specific differences and the development of tools that can be broadly used (Burnett et al., 2007; Canesi et al., 2012; Dondero et al., 2006; Reid et al., 2017; Tørresen et al., 2016). With this strong background, it is now recognized that many of these tools can be transferred to other species, especially for local species or in habitats where the more traditional bioindicators are not present, such as tropical habitats. This important topic is addressed in the paper by van den Hurk et al., “Lionfish (Pterois volitans) as biomonitoring species for oil pollution effects in coral reef ecosystems.” A suite of well-established biomarkers of PAH exposure (bile fluorescence, EROD for cytochrome P450—1A induction, glutathione S-transferase activity) was used in laboratory studies with lionfish exposed to Louisiana Sweet Crude oil (van den Hurk et al., 2020). The results indicated sensitivity to low levels of PAHs, comparable to that observed in other fish species, verifying the potential value of lionfish as a bioindicator species. Field studies were conducted with lionfish that were collected from the Florida Keys over multiple seasons did not indicate significant oil pollution effects, and generally the biomarker responses were similar across all seasons, providing important baseline response data. These kinds of studies are essential for establishing the relative sensitivity and defining expected unperturbed responses of a potential bioindicator species for localized biomonitoring programs with resident species.
One outcome from genomic technologies is the development of biomarkers that can be used to study the effects of toxicants on organisms in a more direct way. In this issue, the article by Santonocito and colleagues (Santonocito et al., 2020) illustrates the use of gene expression biomarkers to aid in risk assessment. The group exposed Manila clams (Ruditapes philippinarum) to 4-methylbenzylidenecamphor (4-MBC), which is used as a UV filter in sunscreen, and examined the expression levels of fourteen different genes involved in detoxification and apoptosis. After a seven-day exposure, there were increases in glutathione S-transferase (GST) transcript expression, along with increases in the apoptosis genes Bcl2 and GADD45. Even with the changes in gene expression, the clams were not able to protect themselves against the deleterious effects of 4-MBC. Using the data to perform an environmental risk assessment, the authors conclude that levels of 4-MBC found in Cadiz Bay, in the southern part of Spain, are sufficiently high to warrant concern (Santonocito et al., 2020).
While both these studies, along with others presented at the PRIMO 20 meeting, illustrate the benefit of using genomic technologies in ecotoxicology, there are number of challenges with their use. In the ecosystem, fluctuating environmental factors (temperature, salinity, pH, etc), deciding which transcriptomic changes are relevant to the organism and toxicant being observed, and how to appropriately incorporate these changes into a risk assessment (Bahamonde et al., 2016; Brockmeier et al., 2017; Buesen et al., 2017; Martyniuk, 2018) are all challenges that need to be addressed to fully apply the power of these technologies
5. Immunotoxicology
Chemically-induced perturbations in the immune system can increase inflammation, inflammatory associated disease, and increase the likelihood of infectious disease in humans (Cao et al., 2016; Corsini et al., 2011; Furman et al., 2019) and marine life (Bossart et al., 2017; Segner et al., 2012). The effects of contaminants or environmental stressors on immune-related responses were addressed in multiple abstracts and three published manuscripts in the PRIMO20 virtual special issues (Mancia et al., 2020; Mello et al., 2020; Riegerix et al., 2020). Some of these studies concern emerging contaminants including microplastics and therefore are discussed in more detail above.
Mello et al. (Mello et al., 2020) describes the direct effects of glutathione depletion in oysters (Crassostrea gigas) as they relate to hemocyte numbers and function, as well as susceptibility to several Vibrio species. The manuscript provides a substantial historical background on the importance of bivalves as sentinel species for evaluating the environmental health of local environments across the globe. As pointed out, several contaminants, and especially metals, are known to deplete glutathione reserves in bivalves leading to susceptibility to oxidative stress, reduced metabolism of electrophilic compounds, and impaired DNA and protein synthesis. The importance of glutathione in pathogen resistance is clearly documented, which set the stage for experimentally depleting glutathione in oysters using injections of the glutathione synthesis inhibitor buthionine sulfoximine (BSO). Total glutathione was measured in gills, digestive gland, and hemocytes over a six-day period, and was demonstrated to be reduced in all three tissues. Moreover, BSO pre-treatment increased the mortality of oysters exposed to cumene hydrogen peroxide (CHP) and hydrogen peroxide (H2O2). Hemocytes from BSO-treated oysters were examined using several functional assays, yet no differences were noted in cell-type distribution, viability, phagocytosis of latex beads, and percentage of adherent cells. Ultimately, the effects of glutathione depletion by BSO treatments were examined in pathogen challenge trials against several species of Vibrio sp. Under the conditions of these studies BSO-treatments reduced survivability after challenge with V. anguillarum, V. alginolyticus, and V. harveyi. This study indicates that disturbances in natural habitats or in industrialized aquaculture environments may lead to impaired glutathione metabolism with resulting susceptibility to disease outbreaks (Mello et al., 2020).
Mancia et al. (Mancia et al., 2020) describe a large study aiming to characterize the environmental distribution of plastics, by type and size (micro- vs macro), in populations of small-spotted catshark (Scyliorhinus canicular), a very abundant elasmobranch and key predator of the Mediterranean, south of Mazara del Vallo and south of Lampedusa, Sicily, Italy. Data from this study reveal a surprising amount of microplastics (1 μm to < 1 mm) in all samples, and macro-plastics (> 1 cm) in approximately 18 % of samples. This study correlated microplastic load and type with the expression of genes TCR-β, TCR-δ, and IgM heavy chain from splenic tissues. The data suggest that microplastics may not lead to the activation of these immune response genes, but macro-plastic burdens do correlate with increased expression of these three genes (Mancia et al., 2020). This descriptive study sets the stage for further studies examining functional parameters of immune responses, and especially from the various lymphoid organs of elasmobranchs. Thymic expression of the two TCR genes would be most informative, as well as circulating IgM levels. Ultimately, we need a much better understanding of the effects of toxicants on immune functions and disease susceptiblity in fish, including elasmobranchs.
6. Endocrine disruption
Increasing incidents of disorders such as obesity, diabetes, metabolic syndrome, reproductive dysfunction, and neuro-developmental abnormalities in some human populations have raised concern that disruption of key endocrine-signaling pathways by exposure to environmental chemicals may be involved (Heindel and Blumberg, 2019; LeBlanc et al., 2012; Lupu et al., 2020; Mimoto et al., 2017; Papalou et al., 2019; Repouskou et al., 2020). Similarly, many endocrine active chemicals have been identified in surface waters globally which has raised significant concern over the past few decades regarding their impacts on wildlife populations (Alvarez et al., 2009; Cotter et al., 2015; Jeffries et al., 2010; Kolpin et al., 2002). Aquatic environments contaminated with endocrine disrupting chemicals (EDC), particularly estrogenic EDC’s, have been linked to a number of adverse effects in wildlife, including the aberrant expression of vitellogenin (VTG), a female specific egg yolk protein, in male fish (Cotter et al., 2015; Hutchinson et al., 2013; White et al., 1994), and feminization and/or demasculinization within oviparous populations, which may be linked to decreased reproductive output, compromised immunity, altered sex ratios, and ultimately population collapse (Kidd et al., 2007; Nash et al., 2004; Woodling et al., 2006). Accordingly, the World Health Organization (WHO) has defined an endocrine disruptor as an exogenous substance or mixture that alters functions(s) of the endocrine system and, consequently, causes adverse health effects in an intact organism, or its progeny, or (sub)populations.
The endocrine system is comprised of multiple signaling pathways that function through an intricate network of feedforward and feedback pathways and through multiple organ systems that comprise specific endocrine “axes”. These pathways are intricately involved in either organizational events during fetal development or later in life during activational maintenance of homeostasis in the adult organism (Guillette et al., 1995; LeBlanc et al., 1997). Exposures during early windows of development appear to impact organ system development and effects can be permanent. In contrast, exposures within the activational phase may be mitigated after termination of exposure and are typically associated with a biological compensatory response that facilitates a reduction in exposure or effect. Mounting evidence has demonstrated that the endocrine system is a highly susceptible and sensitive target of exogenous chemicals and this was a topic of significance within two focused sessions with presentations and posters covering EDC species sensitivities, mechanisms of action and epigenetic/transgenerational effects at PRIMO 20. The utility of aquatic models to examine the impacts of EDCs were presented over the course of these sessions, with a strong focus on teleosts, and marine invertebrates.
Several presentations addressed the ability of early life exposure to EDCs to illicit multigenerational and transgenerational effects. One study demonstrated that select pesticide exposure impacted sex ratio, phenotypic outcomes, and DNA methylation across multiple generations. Integral projection modeling of these outcomes illustrated the ability to predict longer-term effects on population variability that could be utilized for risk assessments. Another study in Japanese medaka assessed DNA methylation dynamics during BPA-induced transgenerational inheritance of reproductive and developmental phenotypes, including epigenetic reprogramming of medaka embryos and their primordial germ cells (PGCs) and surveyed BPA-induced epimutations in PGCs and sperm across multiple generations. illustrate the utility of aquatic models to assess early life stage EDC exposures and the ability to initiate functionally relevant epigenetic changes and link these events to apical outcomes that may have long lasting impacts on unexposed and exposed future populations.
Several studies addressed the role of hormone nuclear receptors (NRs) as major targets of endocrine disrupting chemicals (EDCs) and the ability of EDCs to function as receptor ligands. Studies demonstrate that EDCs disrupt NR function through their ability to mimic natural hormones as receptor agonists (activation) or block activity as antagonists (inhibition). NRs from multiple fresh water and marine species including retinoid X receptor (RXR), vitamin D receptor (VDR), peroxisome proliferator activated receptors (PPAR) and estrogen receptor (ER) were discussed and demonstrated to serve as molecular initiating events (MIE) leading to adverse effects following EDC exposures.
Estrogenic EDCs represent a subclass of endocrine active compounds that comprise a multitude of chemical classes including: natural estrogens (e.g., 17β-estradiol), synthetic estrogens (17α-ethynylestradiol and diethylstilbestrol) and estrogen mimics (e.g., nonylphenol and bisphenol A). Several studies exploited the linkage between ER activation and expression of vitellogenin (VTG); a female specific egg yolk protein, that is expressed in male fish following ER activation. Using both Gal4-based luciferase systems and precision-cut liver slices from Atlantic Cod (G. morhua) as part of DCOD 1.0, investigators were able to assess endocrine disrupting activities of bisphenol analogs; some worse than bisphenol A. Another study investigated the effects of EDCs that target glucocorticoid receptor (GR) signaling in zebrafish embryos. This model proved to be highly responsive to singular GR agonists and laboratory and environmental mixtures of GR acting compounds. Results suggest the ability of glucocorticoids to activate transcriptional networks indicative of GR signaling, and that mixtures of compounds target GR function in an additive nature in this model. Results of this study suggest that GR signaling may be a sensitive target in aquatic environments. Lastly, one study examined the evolutionary dynamics of NR sensitivities to EDC ligands across metazoans. Investigators indicate the importance of genome duplication events, gene loss and ligand co-evolution as critical components establishing the molecular landscape of NR mediated transcription and functional physiological outcomes.
Presentations on the utility of invertebrate models to assess EDC activities and mechanisms were also presented. For example, researchers utilized cherry shrimp (Neocaridina davidi) to establish mechanisms of juvenile hormone analogue (JHA) insecticides effects on crustacean development and reproduction through disruption of JHA and ecdysone receptor (EcR) signaling, molting, and disruption of biosynthetic and degradative pathways associated with juvenile hormone and EcR signaling. Another focused on development of Adverse Outcome Pathways (AOPs) for EDCs in aquatic arthropods and the application of this approach to risk assessment through EcR signaling and molting. Computational and experimental evidence was compiled providing linkages between disruption in EcR signaling as molecular initiating event and subsequent apical adverse outcomes that could be exploited for screening/prioritization and risk assessment determinations.
Three EDC studies presented at PRIMO20 focused on biomonitoring were recently published in the Aquatic Toxicicology special issue (Blalock et al., 2020; De Oliveira et al., 2020; Ibor et al., 2020). Blalock et al., 2020 (Blalock et al., 2020) preseted on the transcriptomic-based Coastal Biosensor of Endocrine Disruption (C-BED) assay using (Mytilus edulis). This assay incorporates a species specific microarray approach to demonstrate that transcriptomic biomarkers can be used to recognize organismal responses from EDCs and legacy contaminants found in Boston Harbor (Blalock et al., 2020). Similarly, Ibor et al., 2020 (Ibor et al., 2020) utilized a biomarker approach to assess emerging contaminants emanating from a from solid waste dumpsite. Using juvenile African sharptooth catfish (C. gariepinus) as a sentinel, researchers investigated if a simulated leachate would induce measurable biomarkers markers of organismal stress and toxicity. The study demonstrated a concentration-dependent increases in mRNA transcripts for estrogenic markers, their corresponding functional protein products, and changes in plasma hormone levels. Changes in gene/protein expression were attributed to the presence select PFAS’s and DEHP in the leachate. Finally, De Oliveira et al., 2020 (De Oliveira et al., 2020) reported on the utility of a transgenic zebrafish line for the detection of endocrine disrupting contaminants. The assay incorporated a transgenic zebrafish line expressing eGFP under the control of the cyp19a1a promoter in conjunction with the OECD Fish Short Term Reproduction Assay. Fish were exposed to select reference substances and gonadal aromatase flurescence assessed in relation to reproductive output and vitellogenin expression. Results from the study suggest that assessment of gonadal aromatase via fluorsence of a transgenic reporter is an excellent compliment to OECD Fish Short Term Reproduction Assay endpoints. The study illustrates the feasibility of this approach and provides further complimentary mechanistic information that can be useful for enhancing assessment of toxicity and putative risk.
7. Conclusion
There are multiple issues that adversely affect our surface waters, freshwater and marine. Key issues covered at PRIMO20 include new technologies and biomarkers of exposure and effect, emerging contaminants, endocrine disruption, legacy pollutants, microplastics, climate change, interaction of chemical exposures with climate change or disease state, cause of disease in marine mammals, bioaccumulation and bioconcentration, and trophic transfer. Ultimately, bioconcentration and bioaccumulation of pollutants that lead to trophic transfer continues to be a major problem for legacy pollutants, endocrine disruptors, emerging contamiants, and others. This issue does not just affect the fish and invertebrates in our surface waters, but ultimately disrupts the health of higher organisms and humans, including indigenous populations (Fig. 1). This was highlighted in plenary talks by both Dr. Patricia Fair (Medical University of South Carolina) and Marquetta L. Goodwine (Queen Quet of the Gullah/Geechee Nation).
Fig. 1. Crucial issues to marine/aquatic toxicology and ecosystem health and their potential for trophic transfer and perturbation of human health.
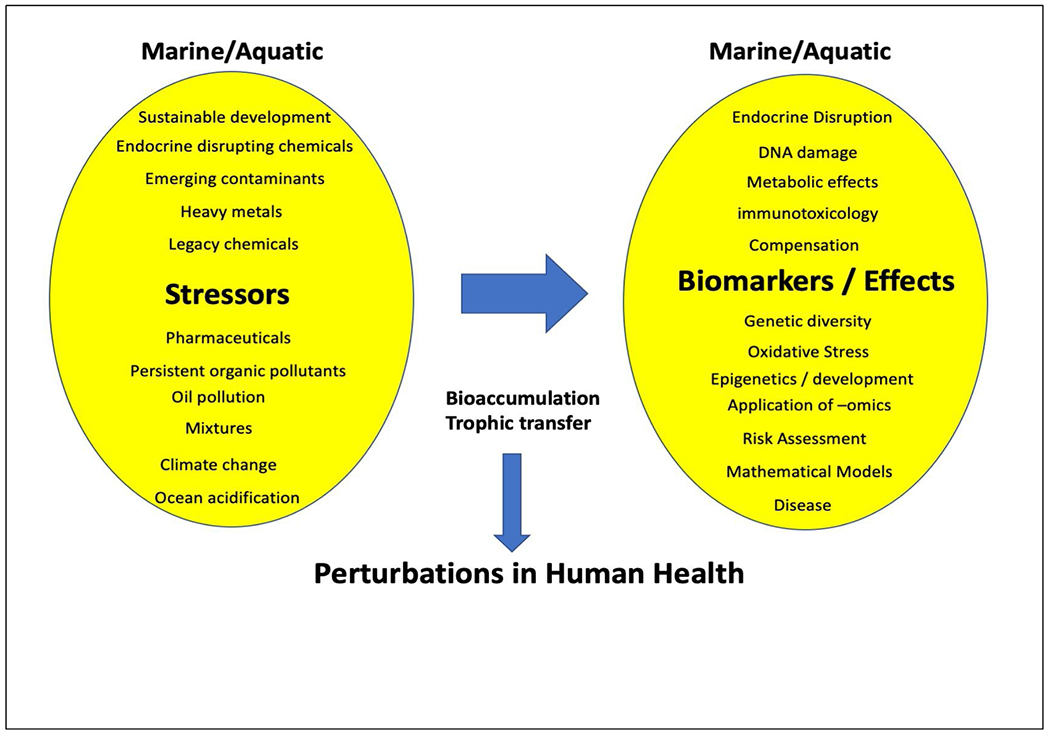
Several pollutants and other stressors were discussed that have significant effects on marine /aquatic organisms. These pollutants also bioaccumulate into higher trophic levels including coastal human populations with the potential for adverse effects.
For example, Dr. Fair presented research indicating that dolphin health in southeastern U.S. populations is deteriorating because of a combination of disease and pollutants. (Bossart et al., 2017; Reif et al., 2017). Dr. Fair and collaborators have found that PFAS concentrations in Charleston Harbor dolphins, near the site of the PRIMO 20 meeting, are on the same order of magnitude as occupationally exposed humans (Fair et al., 2012, 2013), making them some of the highest concentrations ever measured in marine mammals.. Charleston Harbor dolphins also had extremely high levels of DDT, PCBs, triclosan, and antibiotic resistance was common in the dolphins, which is most likely due to high use of human and veterinary antibiotics that enter through sewage systems.
In contrast, dolphins from Indian River Lagoon in Florida, USA had high levels of mercury, most likely due to the consumption of fish and shellfish in the area. Not surprisingly, coastal human population in this region also have high levels of mercury. Mercury is a neurotoxin that causes cognitive and developmental impairments in developing fetuses (Chang et al., 2008; Morris et al., 2018). Both of these dolphin populations are being stressed by antropogenic chemical release and there are consequences (Reif et al., 2017), including increased incidence of diseases such as cetacean morbillivirus, West Nile virus, papilloma virus with possible cancer formation, fungal infections, and lobomycosis. Some of these diseases are specific to cetaceans while others effect both humans and dolphins. About 33–50% of the dolphins in Charleston Harbor or Indian River are considered healthy. Clearly, humans are having an adverse effect on dolphins due to coastal pollution, possible immunosuppression, and introduction of disease (Bossart et al., 2017). These impacts are likely also effecting other coastal inhabitants such as humans.
As Queen Quet presented, the Gullah/Geechee are coastal inhabitants that within rural populations use the ocean for substinence and in turn are affected by the oceans, good and bad (Ellis et al., 2014; Goodwine and Quet, 2015). PFOS and other PFAS are emerging, persistent, endocrine disruptors with immunotoxic effects that can trophically transfer, bioaccumulate and affect multiple levels of organization as they rise through the food chain. The Gullah/Geechee population has significant exposure to PFOS and other PFAS via bioaccumulation through seafood from the Charleston Harbor area with serum PFOS concentrations averaging 53.3 ng/g (Gribble et al., 2015). Overall, seafood consumption was associated with serum PFAS concentrations. Further, serum PFOS concentrations were 50 % higher in individuals with autoimmune disease (75.1 ng/mL) than those without disease (48.2) as were PFNA (perfluorononanoic acid) (3.2 ng/mL vs. 2.1) concentrations (Kamen et al., 2012). The good news is that PFAS concentrations are dropping in the Gullah population (Gribble et al., 2015). Other coastal indigenous populations also show high levels of PFAS including Greenlandic inuits (Lindh et al., 2012) with effects on X: Y chromosome ratios (Kvist et al., 2012) and potential effects on time to pregnancy (Jørgensen et al., 2014).
The primary goals of PRIMO 20 were: (1) to bring together cutting-edge scientists and their students from around the world to present and discuss their latest findings concerning exposures to chemical contaminants and others stressors, their mechanisms of toxicity, and their potential consequences in marine and other aquatic organisms; (2) to enhance interdisciplinary research, international collaborations, and graduate education among cell and molecular biologists, environmental scientists, and toxicologists; and (3) to foster comparative and integrative biology approaches to research and research translation in order to enhance the utility of marine/aquatic organisms as models for human health and disease. PRIMO 20 accomplished each of these major goals.
Acknowledgements
PRIMO20 was funded in part by grants or donations from the University of South Carolina Arnold School of Public Health, National Institutes of Health R13 ES030621, Guy Harvey Ocean Foundation, Clemson University College of Science, Clemson University Department of Biological Sciences, Society of Toxicology, Duke University Superfund Research Center, South Carolina SeaGrant Consortium, National Oceanic and Atmospheric Administration, NIEHS Centers for Oceans and Human Health and Climate Change Interactions at the University of South Carolina, and Rachel Carson Center for Environment and Society. We would like to thank presenters, participants, and the scientific and local organizing committees for an excellent PRIMO 20 meeting in Charleston, SC.
Contributor Information
William S. Baldwin, Biological Sciences, Clemson University, Clemson, SC 29631, United States.
Lisa J. Bain, Biological Sciences, Clemson University, Clemson, SC 29631, United States
Richard Di Giulio, Nicholas School of the Environment, Duke University, Durham, NC 27708, United States.
Seth Kullman, Biological Sciences, North Carolina State University, Raleigh, NC 27695, United States.
Charles D. Rice, Biological Sciences, Clemson University, Clemson, SC 29631, United States
Amy H. Ringwood, Biological Sciences, University of North Carolina-Charlotte, Charlotte, NC 28223, United States
Peter van den Hurk, Biological Sciences, Clemson University, Clemson, SC 29631, United States.
References
- Akbarzadeh A, Günther OP, Houde AL, Li S, Ming TJ, Jeffries KM, Hinch SG, Miller KM, 2018. Developing specific molecular biomarkers for thermal stress in salmonids. BMC Genomics 19, 749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alvarez DA, Cranor WL, Perkins SD, Schroeder VL, Iwanowicz LR, Clark RC, Guy CP, Pinkney AE, Blazer VS, Mullican JE, 2009. Reproductive health of bass in the Potomac, U.S.A., drainage: part 2. Seasonal occurrence of persistent and emerging organic contaminants. Environ. Toxicol. Chem 28, 1084–1095. [DOI] [PubMed] [Google Scholar]
- Andrady AL, 2011. Microplastics in the marine environment. Mar. Pollut. Bull 62, 1596–1605. [DOI] [PubMed] [Google Scholar]
- Antczak P, Jo HJ, Seonock W, Scanlan L, Poynton H, Loguinov A, Chan S, Falciani F, Vulpe C, 2013. Molecular toxicity identification evaluation (mTIE) approach predicts chemical exposure in Daphnia magna. Environ. Sci. Technol 47, 11747–11756. [DOI] [PubMed] [Google Scholar]
- Arukwe A, Eggen T, Möder M, 2012. Solid waste deposits as a significant source of contaminants of emerging concern to the aquatic and terrestrial environments—a developing country case study from Owerri. Nigeria.. Sci Total Environ 438, 94–102. [DOI] [PubMed] [Google Scholar]
- Avio CG, Gorbi S, Regoli F, 2017. Plastics and microplastics in the oceans: from emerging pollutants to emerged threat. Mar. Environ. Res 128, 2–11. [DOI] [PubMed] [Google Scholar]
- Bahamonde PA, Feswick A, Isaacs MA, Munkittrick KR, Martyniuk CJ, 2016. Defining the role of omics in assessing ecosystem health: perspectives from the Canadian environmental monitoring program. Environ. Toxicol. Chem 35, 20–35. [DOI] [PubMed] [Google Scholar]
- Baldwin WS, Roling JA, 2009. A concentration addition model for the activation of the constitutive androstane receptor by xenobiotic mixtures. Toxicol. Sci 107, 93–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balmer ME, Buser HR, Muller MD, Poiger T, 2005. Occurrence of some organic UV filters in wastewater, in surface waters, and in fish from swiss lakes. Environ. Sci. Technol 39, 953–962. [DOI] [PubMed] [Google Scholar]
- Bello SM, Franks DG, Stegeman JJ, Hahn ME, 2001. Acquired resistance to Ah receptor agonists in a population of atlantic killifish (Fundulus heteroclitus) inhabting a marine superfund site: in vivo and in vitro studies on the inducibility of xenobiotic metabolizing enzymes. Toxicol. Sci 60, 77–91. [DOI] [PubMed] [Google Scholar]
- Blalock BJ, Robinson WE, Loguinov A, Vulpe CD, Krick KS, Poynton HC, 2018. Transcriptomic and network analyses reveal mechanistic-based biomarkers of endocrine disruption in the marine mussel, Mytilus edulis. Environ. Sci. Technol 52, 9419–9430. [DOI] [PubMed] [Google Scholar]
- Blalock BJ, Robinson WE, Poynton HC, 2020. Assessing legacy and endocrine disrupting pollutants in Boston Harbor with transcriptomic biomarkers. Aquat. Toxicol 220, 105397. [DOI] [PubMed] [Google Scholar]
- Bossart GD, Fair P, Schaefer AM, Reif JS, 2017. Health and environmental risk assessment project for bottlenose dolphins Tursiops truncatus from the southeastern USA. I. Infectious diseases. Dis Aquat Org 125, 141. [DOI] [PubMed] [Google Scholar]
- Brockmeier EK, Hodges G, Hutchinson TH, Butler E, Hecker M, Tollefsen KE, Garcia-Reyero N, Kille P, Becker D, Chipman K, Colbourne J, Collette TW, Cossins A, Cronin M, Graystock P, Gutsell S, Knapen D, Katsiadaki I, Lange A, Marshall S, Owen SF, Perkins EJ, Plaistow S, Schroeder A, Taylor D, Viant M, Ankley G, Falciani F, 2017. The role of omics in the application of adverse outcome pathways for chemical risk assessment. Toxicol. Sci 158, 252–262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buesen R, Chorley BN, da Silva Lima B, Daston G, Deferme L, Ebbels T, Gant TW, Goetz A, Greally J, Gribaldo L, Hackermüller J, Hubesch B, Jennen D, Johnson K, Kanno J, Kauffmann HM, Laffont M, McMullen P, Meehan R, Pemberton M, Perdichizzi S, Piersma AH, Sauer UG, Schmidt K, Seitz H, Sumida K, Tollefsen KE, Tong W, Tralau T, van Ravenzwaay B, Weber RJM, Worth A, Yauk C, Poole A, 2017. Applying’ omics technologies in chemicals risk assessment: report of an ECETOC workshop. Regul. Toxicol. Pharmacol 91, S3–S13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burnett KG, Bain LJ, Baldwin WS, Callard GV, Cohen S, Di Giulio RT, Evans DH, Gomez-Chiarri M, Hahn ME, Hoover CA, Karchner SI, Katoh F, MacLatchy DL, Marshall WS, Meyer JN, Nacci DE, Oleksiak MF, Rees BB, Singer TD, Stegeman JJ, Towle DW, Van Veld PA, Vogelbein WK, Whitehead A, Winn RN, Crawford DL, 2007. Fundulus as the premier teleost model in environmental biology: opportunities for new insights using genomics. Comp. Biochem. Physiol. Part D Genomics Proteomics 2, 257–286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campos A, Tedesco S, Vasconcelos V, Cristobal S, 2012. Proteomic research in bivalves. Towards the identification of molecular markers of aquatic pollution. J. Proteomics 75, 4346–4359. [DOI] [PubMed] [Google Scholar]
- Canesi L, Ciacci C, Fabbri R, Marcomini A, Pojana G, Gallo G, 2012. Bivalve molluscs as a unique target group for nanoparticle toxicity. Mar. Environ. Res 76, 16–21. [DOI] [PubMed] [Google Scholar]
- Cao J, Xu X, Hylkema MN, Zeng EY, Sly PD, Suk WA, Bergman A, Huo X, 2016. Early-life exposure to widespread environmental toxicants and health risk: a focus on the immune and respiratory systems. Ann. Glob. Health 82, 119–131. [DOI] [PubMed] [Google Scholar]
- Chang J-W, Pai M-C, Chen H-L, Guo H-R, Su H-J, Lee C-C, 2008. Cognitive function and blood methylmercury in adults living near a deserted chloralkali factory. Environ. Res 108, 334–339. [DOI] [PubMed] [Google Scholar]
- Choo G, Wang W, Cho H-S, Kim KB, Park K, Oh J-E, 2020. Legacy and emerging persistent organic pollutants in the freshwater system: relative distribution, contamination trends, and bioaccumulation. Environ. Int 135, 105377. [DOI] [PubMed] [Google Scholar]
- Colbourne JK, Pfrender ME, Gilbert D, Thomas WK, Tucker A, Oakley TH, Tokishita S, Aerts A, Arnold GJ, Basu MK, Bauer DJ, Caceres CE, Carmel L, Casola C, Choi J-H, Detter JC, Dong Q, Dusheyko S, Eads BD, Frohlich T, Geiler-Samerotte KA, Gerlach D, Hatcher P, Jogdeo S, Krijgsveld J, Kriventseva EV, Kultz D, Laforsch C, Lindquist E, Lopez J, Manak R, Muller J, Pangilinan J, Patwardhan RP, Pitluck S, Pritham EJ, Rechtsteiner A, Rho M, Rogozin IB, Sakarya O, Salamov A, Schaack S, Shapiro H, Shiga Y, Skalitzky C, Smith Z, Souvorov A, Sung W, Tang Z, Tsuchiya D, Tu H, Vos H, Wang M, Wolf YI, Yamagata H, Yamada T, Ye Y, Shaw JR, Andrews J, Crease TJ, Tang H, Lucas SM, Robertson HM, Bork P, Zdobnov EM, Grigoriev IV, Lynch M, Boore JL, 2011. The ecoresponsive genome of Daphnia pulex. Science 331, 555–561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole M, Lindeque P, Halsband C, Galloway TS, 2011. Microplastics as contaminants in the marine environment: a review. Mar. Pollut. Bull 62, 2588–2597. [DOI] [PubMed] [Google Scholar]
- Corsini E, Avogadro A, Galbiati V, dell’Agli M, Marinovich M, Galli CL, Germolec DR, 2011. In vitro evaluation of the immunotoxic potential of perfluorinated compounds (PFCs). Toxicol. Appl. Pharmacol 250, 108–116. [DOI] [PubMed] [Google Scholar]
- Cotter KA, Nacci D, Champlin D, Chuprin J, Callard GV, 2015. Cloning of multiple ERα mRNA variants in killifish (Fundulus heteroclitus), and differential expression by tissue type, stage of reproduction, and estrogen exposure in fish from polluted and unpolluted environments. Aquat. Toxicol 159, 184–197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danovaro R, Bongiorni L, Corinaldesi C, Giovannelli D, Damiani E, Astolfi P, Greci L, Puscddu A, 2008. Sunscreens cause coral bleaching by promoting viral infections. Environ. Health Perspect 118, 441–447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Oliveira J, Chadili E, Piccini B, Turiesa C, Maillot-Maréchal E, Palluel O, Pardon P, Budzinski H, Cousin X, Brion F, Hinfray N, 2020. Refinement of an OECD test guideline for evaluating the effects of endocrine disrupting chemicals on aromatase gene expression and reproduction using novel transgenic cyp19a1a-eGFP zebrafish. Aquat. Toxicol 220, 105403. [DOI] [PubMed] [Google Scholar]
- Diana Z, Sawickij N, Rivera NAJ, Hsu-Kim H, Rittschof D, 2020. Plastic pellets trigger feeding responses in Sea Anemones. Aquat. Toxicol 222, 105447. [DOI] [PubMed] [Google Scholar]
- Dondero F, Piacentini L, Marsano F, Rebelo M, Vergani L, Venier P, Viarengo A, 2006. Gene transcription profiling in pollutant exposed mussels (Mytilusspp.)Using a new low-density oligonucleotide microarray. Gene 376, 24–36. [DOI] [PubMed] [Google Scholar]
- Ellis JH, Friedman DB, Puett R, Scott GI, Porter DE, 2014. A qualitative exploration of fishing and fish consumption in the Gullah/Geechee culture. J. Community Health 39, 1161–1170. [DOI] [PubMed] [Google Scholar]
- Fair PA, House M, Hulsey TC, Bossart GD, Adams J, Balthis L, Muir DCG, 2012. Assessment of perfluorinated compounds (PFCs) in plasma of bottlenose dolphins from two southeast US estuarine areas: relationship with age, sex and geographic locations. Mar. Pollut. Bull 64, 66–74. [DOI] [PubMed] [Google Scholar]
- Fair PA, Romano T, Schaefer AM, Reif JS, Bossart GD, Houde M, Muir D, Adams J, Rice C, Hulsey TC, Peden-Adams M, 2013. Associations between perfluoroalkyl compounds and immune and clinical chemistry parameters in highly exposed bottlenose dolphins (Tursiops Truncatus). Environ. Toxicol. Chem 32, 736–746. [DOI] [PubMed] [Google Scholar]
- Furman D, Campisi J, Verdin E, Carrera-Bastos P, Targ S, Franceschi C, Ferrucci L, Gilroy DW, Fasano A, Miller GW, Miller AH, Mantovani A, Weyand CM, Barzilai N, Goronzy JJ, Rando TA, Effros RB, Lucia A, Kleinstreuer N, Slavich GM, 2019. Chronic inflammation in the etiology of disease across the life span. Nat. Med 25, 1822–1832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao L, Yuan T, Zhou C, Cheng P, Bai Q, Ao J, Wang W, Zhang H, 2013. Effects of four commonly used UV filters on the growth, cell viability and oxidative stress responses of the Tetrahymena thermophila. Chemosphere 93, 2507–2513. [DOI] [PubMed] [Google Scholar]
- Giokas DL, Salvador A, Chisvert A, 2007. UV filters : from sunscreens to human body and the environment. Trac Trends Anal Chem 26, 360–374. [Google Scholar]
- Goodwine ML, Quet Q, 2015. WEBE Gullah/Geechee: Cultural Capital and Collaboration Anthology. Kinship Publicarions, Brooklyn, NY. [Google Scholar]
- Gribble MO, Bartell SM, Kannan K, Wu Q, Fair PA, Kamen DL, 2015. Longitudinal measures of perfluoroalkyl substances (PFAS) in serum of Gullah African Americans in South Carolina: 2003–2013. Environ. Res 143, 82–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guerranti C, Martellini T, Perra G, Scopetani C, Cincinelli A, 2019. Microplastics in cosmetics: environmental issues and needs for global bans. Environ. Toxicol. Pharmacol 68, 75–79. [DOI] [PubMed] [Google Scholar]
- Guillette LJJ, Crain DA, Rooney AA, Pickford DB, 1995. Organization versus activation: the role of endocrine-disrupting contaminants (EDCs) during embryonic development in wildlife. Environ. Health Perspect 103, 157–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi Y, Heekmann L-H, Callaghan A, Sibly RM, 2008. Reproduction recovery of the crustacean Daphnia magna after chronic exposure to ibuprofen. Ecotoxicology 17, 246–251. [DOI] [PubMed] [Google Scholar]
- Heindel JJ, Blumberg B, 2019. Environmental obesogens: mechanisms and controversies. Annu. Rev. Pharmacol. Toxicol 59, 89–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hermabessiere L, Dehaut A, Paul-Pont I, Lacroix C, Jezequel R, Soudant P, Duflos G, 2017. Occurrence and effects of plastic additives on marine environments and organisms: a review. Chemosphere 182, 781–793. [DOI] [PubMed] [Google Scholar]
- Hutchinson TH, Lyons BP, Thain JE, Law RJ, 2013. Evaluating legacy contaminants and emerging chemicals in marine environments using adverse outcome pathways and biological effects-directed analysis. Mar. Pollut. Bull 74, 517–525. [DOI] [PubMed] [Google Scholar]
- Ibor OR, Andem AB, Enia G, Arong GA, Adeougn AO, Arukwe A, 2020. Contaminant levels and endocrine disruptive effects in Clarias gariepinus exposed to simulated leachate from a solid waste dumpsite in Calabar. Nigeria. Aquat Toxicol 219, 105375. [DOI] [PubMed] [Google Scholar]
- Jeffries S, Adams SM, Greeley MSJ, Ryon MG, 2010. Evaluating effects of contaminants on fish health at multiple levels of biological organization: extrapolating from lower to higher levels. Hum. Ecol. Risk Assess: Int. J 6, 15–27. [Google Scholar]
- Jørgensen KT, Specht IO, Lenters V, Bach CC, Rylander L, Jönsson BAG, Lindh CH, Giwercman A, Heederik D, Toft G, Bonde JP, 2014. Perfluoroalkyl substances and time to pregnancy in couples from Greenland, Poland and Ukraine. Environ. Health 13, 116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamen DL, Peden-Adams MM, Vena JE, Gilkeson GS, Hulsey TC, Moultrie L, Stevens BE, 2012. Seafood consumption and persistent organic pollutants as triggers of autoimmunity among Gullah African Americans. Arthritis Res. Ther 14, A19. [Google Scholar]
- Kidd KA, Blanchfield PJ, Mills KH, Palace VP, Evans RE, Lazorchak JM, Flick RW, 2007. Collapse of a fish population after exposure to a synthetic estrogen. Proc. Natl. Acad. Sci. U.S.A 104, 8897–8901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koelmans AA, Bakir A, Burton GA, Janssen CR, 2016. Microplastic as a vector for chemicals in the aquatic environment: critical review and model-supported reinterpretation of empirical studies. Environ. Sci. Technol 50, 3315–3326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolpin DW, Furlong ET, Meyer MT, Thurman EM, Zaugg SD, Barber LB, Buxton HT, 2002. Pharmaceuticals, hormones, and other organic wastewater contaminants in U.S. streams, 1999-2000: a national reconnaissance. Environ. Sci. Technol 36 (1202), 1211. [DOI] [PubMed] [Google Scholar]
- Kvist L, Giwercman YL, Jönsson BAG, Lindh CH, Bonde J-P, Toft G, Strucinski P, Pedersen HS, Zvyezday V, Giwercman A, 2012. Serum levels of perfluorinated compounds and sperm Y:x chromosome ratio in two European populations and in Inuit from Greenland. Reprod. Toxicol 34, 644–650. [DOI] [PubMed] [Google Scholar]
- LaPlaca SB, van den Hurk P, 2020. Toxicological effects of micronized tire crumb rubber on mummichog (Fundulus heteroclitus) and fathead minnow (Pimephales promelas). Ecotoxicology 29, 524–534. [DOI] [PubMed] [Google Scholar]
- Larkin P, Folmar LC, Hemmer MJ, Poston AJ, Lee HS, Denslow ND, 2002. Array technology as a tool to monitor exposure of fish to xenoestrogens. Mar. Environ. Res 54, 395–399. [DOI] [PubMed] [Google Scholar]
- Larkin P, Knoebl I, Denslow ND, 2003. Differential gene expression analysis in fish exposed to endocrine disrupting compounds. Comp. Biochem. Physiol. B, Biochem. Mol. Biol 136, 149–161. [DOI] [PubMed] [Google Scholar]
- LeBlanc GA, Bain LJ, 1997. Chronic toxicity of environmental contaminants: sentinels and biomarkers. Environ. Health Perspect 105 (Suppl 1), 65–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeBlanc GA, Bain LJ, Wilson VS, 1997. Pesticides: multiple mechanisms of demasculinization. Mol. Cell. Endocrinol 126, 1–5. [DOI] [PubMed] [Google Scholar]
- LeBlanc GA, Norris DO, Kloas W, Kullman SW, Baldwin WS, Greally JM, 2012. Detailed Review Paper on the State of the Science on Novel In Vitro and In Vivo Screening and Testing Methods and Endpoints for Evaluating Endocdrine Disrupters Series on Testing & Assessment: No. 178. Organisation for Economic Co-Operation and Development, Paris, p. 213. [Google Scholar]
- Lee HB, Peart TE, Svoboda ML, 2005. Determination of endocrine-disrupting phenols, acidic pharmaceuticals, and personal-care products in sewage by solid-phase extraction and gas chromatography-mass spectrometry. J. Chromatogr. A 1094, 122–129. [DOI] [PubMed] [Google Scholar]
- Lindh CH, Rylander L, Toft G, Axmon A, Rignell-Hydbom A, Giwercman A, Pedersen HS, Góalczyk K, Ludwicki JK, Zvyezday V, Vermeulen R, Lenters V, Heederik D, Bonde JP, Jönsson BAG, 2012. Blood serum concentrations of perfluorinated compounds in men from Greenlandic Inuit and European populations. Chemosphere 88, 1269–1275. [DOI] [PubMed] [Google Scholar]
- Lupu D, Andersson P, Bornehag C-G, Demeneix B, Fritsche E, Gennings C, Lictensteiger W, Leist M, Leonards PEG, Ponsonby A-L, Scholze M, Testa G, Tresguerres JAF, Westerink RHS, Zalc B, Ruegg J, 2020. The ENDpoiNTs project: novel testing strategies for endocrine disruptors linked to developmental neurotoxicity. Int. J. Mol. Sci 21, 3978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mancia A, Chenet T, Bono G, Geraci ML, Vaccaro C, Munari C, Mistri M, Cavazzini A, Pasti L, 2020. Adverse effects of plastic ingestion on the mediterranean small-spotted catshark (Scyliorhinus canicula). Mar. Environ. Res 155, 104876. [DOI] [PubMed] [Google Scholar]
- Martyniuk CJ, 2018. Are we closer to the vision? A proposed framework for incorporating omics into environmental assessments. Environ. Toxicol. Pharmacol 59, 87–93. [DOI] [PubMed] [Google Scholar]
- Martyniuk CJ, Mehinto AC, Denslow ND, 2020. Organochlorine pesticides: agrochemicals with potent endocrine-disrupting properties in fish. Mol. Cell. Endocrinol 507, 110764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maruya KA, Dodder NG, Sengupta A, Smith DJ, Lyons JM, Heil AT, Drewes JE, 2016. Multimedia screening of contaminants of emerging concern (CECS) in Coastal Urban Watersheds in Southern California (USA). Environ. Toxicol. Chem 35, 1986–1994. [DOI] [PubMed] [Google Scholar]
- Mello DF, Trevisan R, Danielli NM, Dafre AL, 2020. Vulnerability of glutathione-depleted Crassostrea gigas oysters to Vibrio species. Mar. Environ. Res 154, 104870. [DOI] [PubMed] [Google Scholar]
- Mimoto MS, Nadal A, Sargis RM, 2017. Polluted pathways: mechanisms of metabolic disruption by endocrine disrupting chemicals. Curr. Environ. Health Rep 4, 208–222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miracle AL, Toth GP, Lattier DL, 2003. The path from molecular indicators of exposure to describing dynamic biological systems in an aquatic organism: microarrays and the fathead minnow. Ecotoxicology 12, 457–462. [DOI] [PubMed] [Google Scholar]
- Moore MN, Allen JI, McVeigh A, 2006. Environmental prognostics: an integrated model supporting lysosomal stress responses as predictive biomarkers of animal health status. Mar. Environ. Res 61, 278–304. [DOI] [PubMed] [Google Scholar]
- Morris G, Puri BK, Frye RE, Maes M, 2018. The putative role of environmental mercury in the pathogenesis and pathophysiology of autism spectrum disorders and subtypes. Mol. Neurobiol 55, 4834–4856. [DOI] [PubMed] [Google Scholar]
- Nash JP, Kime DE, Van der Ven LTM, Wester PW, Brion F, Maack G, Stahlschmidt-Allner P, Tyler CR, 2004. Long-term exposure to environmental concentrations of the pharmaceutical ethynylestradiol causes reproductive failure in fish. Environ. Health Perspect 112, 1725–1733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- North EJ, Halden RU, 2013. Plastics and environmental health: the road ahead. Rev. Environ. Health 28, 108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papalou O, Kandaraki EA, Papadakis G, Diamanti-Kandarakis E, 2019. Endocrine disrupting chemicals: an occult mediator of metabolic disease. Front Endocrinol 10, 112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng H, Saunders DMV, Sun J, Jones PD, Wong CKC, Liu H, Giesy JP, 2016. Mutagenic azo dyes, rather than flame retardants, are the predominant brominated compounds in house dust. Environ. Sci. Technol 50, 12669–12677. [DOI] [PubMed] [Google Scholar]
- Peterson JS, Bain LJ, 2004. Differential gene expression in anthracene-exposed mummichogs (Fundulus heteroclitus). Aquat. Toxicol 66, 345–355. [DOI] [PubMed] [Google Scholar]
- Reid NM, Jackson CE, Gilbert D, Minx P, Montague MJ, Hampton TH, Helfrich LW, King BL, Nacci DE, Aluru N, Karchner SI, Colbourne JK, Hahn ME, Shaw JR, Oleksiak MF, Crawford DL, Warren WC, Whitehead A, 2017. The landscape of extreme genomic variation in the highly adaptable atlantic killifish. Genome Biol. Evol 9, 659–676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reif JS, Schaefer AM, Bossart GD, Fair PA, 2017. Health and Environmental Risk Assessment Project for bottlenose dolphins Tursiops truncatus from the southeastern USA. II. Environmental aspects. Dis. Aquat. Org 125, 155. [DOI] [PubMed] [Google Scholar]
- Repouskou A, Papadopoulou A-K, Panagiotidou E, Trichas P, Lindh C, Bergman A, Gennings C, Bornehag C-G, Riiegg J, Kitraki E, Stamatakis A, 2020. Long term transcriptional and behavioral effects in mice developmentally exposed to a mixture of endocrine disruptors associated with delayed human neurodevelopment. Sci. Rep 10, 9367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riegerix RC, Tanner M, Gale R, Tillett DE, 2020. Acute toxicity and clotting times of anticoagulant rodenticides to red-toothed (Odonus niger) and black (Melichthys niger) triggerfish, fathead minnow (Pimephales promelas), and largemouth bass (Micropterus salmoides). Aquat. Toxicol 221, 105429. [DOI] [PubMed] [Google Scholar]
- Ringwood AH, Conners DE, Keppler CJ, DiNovo AA, 1999. Biomarker studies with juvenile oysters (Crassostrea virginica) deployed in situ. Biomarkers 4, 400–415. [DOI] [PubMed] [Google Scholar]
- Rodríguez AS, Sanz MR, Rodriguez JRB, 2015. Occurrence of eight UV filters in beaches of Gran Canaria (Canary Islands). An approach to environmental risk assessment. Chemosphere 131, 85–90. [DOI] [PubMed] [Google Scholar]
- Roling JA, Baldwin WS, 2006. Alterations in hepatic gene expression by trivalent chromium in Fundulus heteroclitus. Mar. Environ. Res 62, S122–S127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roling JA, Bain LJ, Baldwin WS, 2004. Differential gene expression in mummichogs (Fundulus heteroclitus) following treatment with pyrene: comparison to a creosote contaminated site. Mar. Environ. Res 57, 377–395. [DOI] [PubMed] [Google Scholar]
- Roling JA, Bain LJ, Gardea-Torresdey J, Bader J, Baldwin WS, 2006. Hexavalent chromium reduces larval growth and alters gene expression in mummichog (Fundulus heteroclitus). Environ. Toxicol. Chem 25, 2725–2733. [DOI] [PubMed] [Google Scholar]
- Roling JA, Bain LJ, Gardea-Torresdey J, Key PB, Baldwin WS, 2007. Using mummichog (Fundulus heteroclitus) arrays to monitor the effectiveness of remediation at a superfund site in Charleston, South Carolina, USA. Environ. Toxicol. Chem 26, 1205–1213. [DOI] [PubMed] [Google Scholar]
- Salimi M, Esrafili A, Gholami M, Jafari AJ, Kalantary RR, Farzadkia M, Kermani M, Sobhi HR, 2017. Contaminants of emerging concern: a review of new approach in AOP technologies. Environ. Monit. Assess 189, 414. [DOI] [PubMed] [Google Scholar]
- Sang Z, Leung KS, 2016. Environmental occurrence and ecological risk assessment of organic UV fi lters in marine organisms from Hong Kong coastal waters. Sci Tot Environ 566-567, 489–498. [DOI] [PubMed] [Google Scholar]
- Santonocito M, Salerno B, Trombini C, Tonini F, Pintado-Herrera MG, Martínez-Rodríguez G, Blasco J, Lara-Martín PA, Hampel M, 2020. Stress under the sun: effects of exposure to low concentrations of UV-filter 4-methylbenzylidene camphor (4-MBC) in a marine bivalve filter feeder, the Manila clam Ruditapes philippinarum. Aquat. Toxicol 221, 105418. [DOI] [PubMed] [Google Scholar]
- Sauvé S, Desrosiers M, 2014. A review of what is an emerging contaminant. Chem. Cent. J 8, 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt AM, Sengupta N, Noorai RE, Saski CA, Baldwin WS, 2017. RNA sequencing indicates that atrazine induces multiple detoxification genes in Daphnia magna and this is a potential sources of its mixtures interactions with other chemicals. Chemosphere 189, 699–708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segner H, Wenger M, Moller AM, Kollner B, Casanova-Nakayama A, 2012. Immunotoxic effects of environmental toxicants in fish — how to assess them? Environ Sci and Pollut Res 19, 2465–2476. [DOI] [PubMed] [Google Scholar]
- Snell TW, Brogdon SE, Morgan MB, 2003. Gene expression profiling in ecotoxicology. Ecotoxicology 12, 475–483. [DOI] [PubMed] [Google Scholar]
- Star B, Nederbragt AJ, Jentoft S, Grimholt U, Malmstrøm M, Gregers TF, Rounge TB, Paulsen J, Solbakken MH, Sharma A, Wetten OF, Lanzén A, Winer R, Knight J, Vogel JH, Aken B, Andersen O, Lagesen K, Tooming-Klunderud A, Edvardsen RB, Tina KG, Espelund M, Nepal C, Previti C, Karlsen BO, Mourn T, Skage M, Berg PR, Gjøen T, Kuhl H, Thorsen J, Malde K, Reinhardt R, Du L, Johansen SD, Searle S, Lien S, Nilsen F, Jonassen I, Omholt SW, Stenseth NC, Jakobsen KS, 2011. The genome sequence of Atlantic cod reveals a unique immune system. Nature 477, 207–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stegeman JJ, Kloepper-Sams PJ, 1987. Cytochrome P-450 isozymes and monooxygenase activity in aquatic animals. Environ. Health Perspect 71, 87–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutton R, Sedlak M, Sun J, Lin D, 2017. Contaminants of emerging concern in San Francisco Bay: a strategy for future investigations. San Francisco Esturate Institute, p. 76. [Google Scholar]
- Tørresen OK, Rise ML, Jin X, Star B, MacKenzie S, Jakobsen KS, Nederbragt AJ, Jentoft S, 2016. An improved version of the Atlantic cod genome and advancements in functional genomics: implications for the future of cod farming. Genomics in Aquaculture 45–72. [Google Scholar]
- Tørresen OK, Star B, Jentoft S, Reinar WB, Grove H, Miller JR, Walenz BP, Knight J, Ekholm JM, Peluso P, Edvardsen RB, Tooming-Klunderud A, Skage M, Lien S, Jakobsen KS, Nederbragt AJ, 2017. An improved genome assembly uncovers prolific tandem repeats in Atlantic Cod. BMC Genomics 18, 95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Aggelen G, Ankley GT, Baldwin WS, Bearden DW, Benson WH, Chipman JK, Collette TW, Craft JA, Denslow ND, Embry MR, Falciani F, George SG, Helbing CC, Hoekstra PF, Iguchi T, Kagami Y, Katsiadaki I, Kille P, Liu L, Lord PG, McIntyre T, O’Neill A, Osachoff H, Perkins EJ, Santos EM, Skirrow RC, Snape JR, Tyler CR, Versteeg D, Viant MR, Volz DC, Williams TD, Yu L, 2010. Integrating omic technologies into aquatic ecological risk assessment and environmental monitoring: hurdles, achievements, and future outlook. Environ. Health Perspect 118, 1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Hurk P, Edhlund I, Davis R, Hahn JJ, McComb MJ, Rogers EL, Pisarski E, Chung K, DeLorenzo M, 2020. Lionfish (Pterois Volitans) as biomonitoring species for oil pollution effects in coral reef ecosystems. Mar. Environ. Res 156, 104915. [DOI] [PubMed] [Google Scholar]
- Watanabe M, Meyer KA, Jackson TM, Schock TB, Johnson WE, Bearden DW, 2015. Application of NMR-based metabolomics for environmental assessment in the Great Lakes using zebra mussel (Dreissena polymorpha). Metabolomics 11, 1302–1315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinstein JE, Crocker BK, Gray AD, 2016. From macroplastic to microplastic: degradation of high-density polyethylene, polypropylene, and polystyrene in a salt marsh habitat. Environ. Toxicol. Chem 35, 1632–1640. [DOI] [PubMed] [Google Scholar]
- White R, Jobling S, Hoare SA, Sumpter JP, Parker MG, 1994. Environmentally persistent alkylphenolic compounds are estrogenic. Endocrinology 135, 175–182. [DOI] [PubMed] [Google Scholar]
- Williams TD, Gensberg K, Minchin SD, Chipman JK, 2003. A DNA expression array to detect toxic stress response in European flounder (Plactichthys flesus). Aquat. Toxicol 65, 141–157. [DOI] [PubMed] [Google Scholar]
- Woodling JD, Lopez EM, Maldonado TA, Norris DO, Vajda AM, 2006. Intersex and other reproductive disruption of fish in wastewater effluent dominated Colorado streams. Comp. Biochem. Physiol. C Toxicol. Pharmacol 144, 10–15. [DOI] [PubMed] [Google Scholar]
- Yadetie F, Zhang X, Hanna EM, Aranguren-Abadía L, Eide M, Blaser N, Brun M, Jonassen I, Goksøyr A, Karlsen OA, 2018. RNA-seq analysis of transcriptome responses in Atlantic Cod (Gadus Morhua) precision-cut liver slices exposed to benzo [a]pyrene and 17α-ethynylestradiol. Aquat. Toxicol 201, 174–186. [DOI] [PubMed] [Google Scholar]
- Zarfl C, Fleet D, Fries E, Galgani F, Gerdts G, Hanke G, Matthies M, 2011. Microplastics in oceans. Mar. Pollut. Bull 62, 1589–1591. [DOI] [PubMed] [Google Scholar]
- Zhu M, Chernick M, Rittschof D, Hinton DE, 2020. Chronic dietary exposure to polystyrene microplastics in maturing Japanese medaka (Oryzias latipes). Aquat. Toxicol 220, 105396. [DOI] [PubMed] [Google Scholar]


