Abstract
Cigarette smoking is a risk factor for several diseases such as cancer, cardiovascular disease (CVD), and chronic obstructive pulmonary diseases (COPD), however, the underlying mechanisms are not fully understood. Alternative nicotine products with reduced risk potential (RRPs) including tobacco heating products (THPs), and e-cigarettes have recently emerged as viable alternatives to cigarettes that may contribute to the overall strategy of tobacco harm reduction due to the significantly lower levels of toxicants in these products’ emissions as compared to cigarette smoke. Assessing the effects of RRPs on biological responses is important to demonstrate the potential value of RRPs towards tobacco harm reduction. Here, we evaluated the inflammatory and signaling responses of human lung epithelial cells to aqueous aerosol extracts (AqE) generated from the 1R6F reference cigarette, the glo™ THP, and the Vype ePen 3.0 e-cigarette using multiplex analysis of 37 inflammatory and phosphoprotein markers. Cellular exposure to the different RRPs and 1R6F AqEs resulted in distinct response profiles with 1R6F being the most biologically active followed by glo™ and ePen 3.0. 1R6F activated stress-related and pro-survival markers c-JUN, CREB1, p38 MAPK and MEK1 and led to the release of IL-1α. glo™ activated MEK1 and decreased IL-1β levels, whilst ePen 3.0 affected IL-1β levels but had no effect on the signaling activity compared to untreated cells. Our results demonstrated the reduced biological effect of RRPs and suggest that targeted analysis of inflammatory and cell signaling mediators is a valuable tool for the routine assessment of RRPs.
Keywords: In vitro, lung toxicity, aerosol, cigarette, heated tobacco product, electronic cigarette
Graphical Abstract
Highlights
-
•
Cigarette, heated tobacco product (HTP) and electronic cigarette toxicity were assessed.
-
•
Inflammatory and signalling responses in lung cells were evaluated.
-
•
Reference cigarette was significantly more active than the other products tested.
-
•
Approach can be used as part of weight of evidence evaluation of product risk.
1. Introduction
Epidemiological studies demonstrate that cigarette smoking is a cause of several diseases including, but not limited to cancer, cardiovascular diseases (CVD), and chronic obstructive pulmonary disease (COPD) [43], [66]. While smoking cessation provides the best solution for smokers to reduce the health risks related to smoking, many smokers choose not to quit [24], [67]. There has thus been an increasing recognition among public health authorities that encouraging smokers who would not otherwise quit to switch to RRPs [25], [58] can contribute significantly to tobacco harm reduction. RRPs encompass several categories, with snus, electronic cigarettes (e-cigarettes) and tobacco heating products (THPs) among the most widely used and studied. In this context, RRPs should be toxicologically assessed relative to cigarettes [5], [39] with several studies already having provided evidence of reduced toxicity of RRPs compared to cigarettes. By using contemporary and standardized analytical and in vitro toxicological assays, these studies have demonstrated that aerosols from RRPs, whilst effective in delivering nicotine, contain significantly lower levels of harmful chemicals compared to cigarette smoke [20], [71], which may contribute to reduced levels of cellular oxidative stress, DNA damage, mutagenicity, and carcinogenicity [13], [16], [28], [29], [30], [39], [59], [60], [62], [63], [64]. 2D and 3D human in vitro cellular models have also been used to assess smoking-disease related processes such as endothelial migration [10] and integrity and function of airway epithelia, [27] further highlighting reduction in the activity of biological, disease-associated mechanisms in response to RRP aerosols compared to cigarette smoke. While these toxicological responses to RRP emissions are comparably less than those from cigarette smoke, constituents of cigarette smoke such as carbonyl compounds, and nitrosamines can be present in RRPs emissions, albeit at significantly lower concentration levels [50], [34]. Historically, rodent inhalation studies have been used to assess the toxic and pathological effects of cigarette smoke, and more recently, RRPs. However, these models have limitations. For example, it is difficult to induce lung cancer in rodent models using cigarette smoke, despite many years of optimizing exposure protocols and evaluating various rodent strains [15], [55]. Furthermore, as a highly-complex mixture of over 7000 chemicals [54], the effects of all of the individual chemical constituents and the mechanisms by which they induce toxicity and disease are not fully understood.
Recently developed in vitro systems toxicology studies enable the understanding of perturbed cellular processes during exposure to cigarette smoke or PRRP aerosols, and the underlying biological mechanisms involved [22], [23], [27], [28], [29], [30]. Systems approaches generally consider the analysis of gene expression profiles from different cell types exposed to cigarette smoke or RRP aerosols and have demonstrated a reduced effect of RRP on processes commonly affected by cigarette smoke such as oxidative stress, cell proliferation and inflammation. However, these studies are exploratory and are difficult to standardize or increase through-put for routine RRP assessment. At the proteome level, studies are limited to the analysis of inflammatory markers, whilst information at the phosphoprotein level regarding the effect of RRPs on intracellular signaling pathways is lacking. Additionally, the cellular toxicity induced by cigarette smoke may affect protein responses unless experimental conditions are optimized to retain cellular health [13], [63], [64]. Therefore, the development of in vitro assay tools that can provide a simple and robust framework for the toxicological assessment of RRP performance characteristics should be considered.
Here, we propose an experimental platform based on the targeted, multiplex analysis of intracellular signaling markers and secreted inflammatory mediators for the assessment of RRPs (THP and e-cigarette) compared to cigarettes. This is based on the hypothesis that the lower levels of toxicants in aerosols from RRPs relative to cigarette smoke would lead to lower perturbation of cell signaling pathways and less secretion of inflammatory markers. We developed a workflow for exposure of NCI-H292 lung epithelial cells to non-cytotoxic concentrations of aqueous extracts (AqE) generated from cigarette smoke or RRP (THP and e-cigarette) aerosols, followed by multiplex analyses of 37 protein targets. Non-cytotoxic concentrations were selected for primary endpoint analysis so that resulting effects would not be confounded by cytotoxicity. This experimental platform revealed distinct signaling and inflammatory profiles between the different RRP aerosol extracts and cigarette smoke. These data add to the growing weight-of-evidence of the risk continuum for tobacco and nicotine products. Application of this methodology for routine assessment of these and other RRPs, such as tobacco-free nicotine pouches (NPs) can contribute to a better understanding of the complexities involved in the reduced risk assessment of RRPs.
2. Results
2.1. Nicotine analysis of test products
The test products used in this study and the nicotine levels of the respective AqE preparations used for cell exposure are presented in Table 1. As shown in Table 1, the extracts were neither puff-matched nor nicotine-matched. The selection of number of puffs was based on previous data [12] to allow the production of a robust test extract for each product category that would elicit a response in the H292 test system up to the point of cytotoxicity in order to develop the multiplex assays.
Table 1.
Summary of tobacco test products, aerosol generation and AqE nicotine content.
| Test product | Product category | Product Code | Source | Puff Regimen | Puff Number | Puff Volume (ml) | Puff Duration (s) | Repeat Duration (s) | Puff Shape | AqE Nicotine content (μg/ml) |
|---|---|---|---|---|---|---|---|---|---|---|
| Kentucky 1R6F reference cigarette | Cigarette | 1R6F | University of Kentucky | HCI3 | 8 | 55 | 2 | 30 | Bell | 7.07 |
| glo™ with Rich Tobacco Kent Neosticks™1 | Tobacco heating product | THP1.4.HPCTRT | British American Tobacco | HCIm4 | 40 | 55 | 2 | 30 | Bell | 18.03 |
| Vuse ePen 3with Blended Tobacco 18 mg/ml e-liquid2 | Electronic cigarette | EPEN3.0BT18 | British American Tobacco | CRM815 | 200 | 55 | 3 | 30 | Square | 61.31 |
1glo™ g004 is a portable electronic heating device and provides an inhalable aerosol by heating a specific tobacco consumable product to a maximum temperature of 240 °C ± 5 °C.
2a cotton wick system that provides an electrical current to the heating element contained within the disposable cartridge containing e-liquid, producing an inhalable aerosol.
3Health Canada Intense regime
4modified Health Canada Intense regime
5CORESTA CRM81 regime
2.2. Assessment of cell viability in response to cigarette smoke
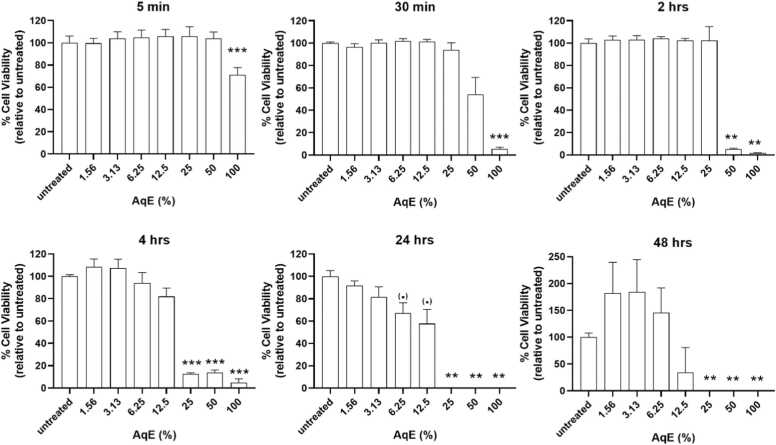
We tested the effect of reference cigarette 1R6F AqE concentration and exposure duration on cell viability with the aim of identifying non-toxic treatment conditions that do not compromise cellular health. We tested 1R6F AqE concentrations of 1.6%-100% v/v for short (5 min up to 4 hrs) and prolonged (24–48 hrs) exposure times. 1R6F AqE at concentrations greater than 25% and exposure times longer than 2 hrs negatively affected cell viability as demonstrated by a decrease in viable cells by >50% (Fig. 1). Prolonged exposure for 24 hrs resulted in statistically significant decrease in cell viability, where >30% cell death at 1R6F AqE concentrations as low as 6.25%. Cell viability also varied after 48 hrs at low concentrations yet decreased significantly at high 1R6F AqE concentrations. Overall, results suggest that 1R6F AqE concentrations no greater than 25%, and exposure times of 2 hrs do not cause a statistically significant decrease in cell viability and are acceptable for assessment of cellular responses without compromising results.
Fig. 1.
Cell viability following exposure to 1R6F AqE. Each graph corresponds to a different exposure time. Data from the CellTiter-Glo assay were first transformed into % viable cells by comparing the data from each 1R6F AqE concentration (% v/v from stock solution) to the untreated cells. Untreated cells were considered as 100% viable. Mean ± standard deviation from three independent replicates is presented. (.) p = 0.05, *p<0.05, **p<0.01, ***p<0.001.
2.3. Comparative assessment of RRPs’ biological effects
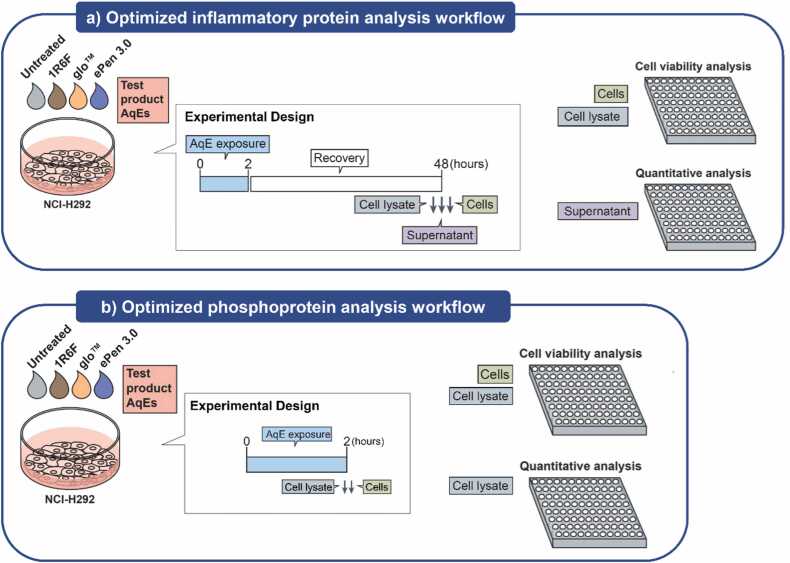
The experimental design used for assessment of biological responses of test products is schematically presented in Fig. 2. Selection of AqE exposure conditions was based on optimization experiments using 1R6F AqE and are collectively presented in Supplementary Material. Briefly, the conclusion from the optimization experiments was that 25% 1R6F applied for 2 hrs with a 48 hrs recovery phase was the optimum treatment condition for protein analysis (phosphoprotein analysis performed on the cells immediately following the treatment, and cytokine analysis performed after the recovery period) and was carried forward for the THP and e-cigarette test articles.
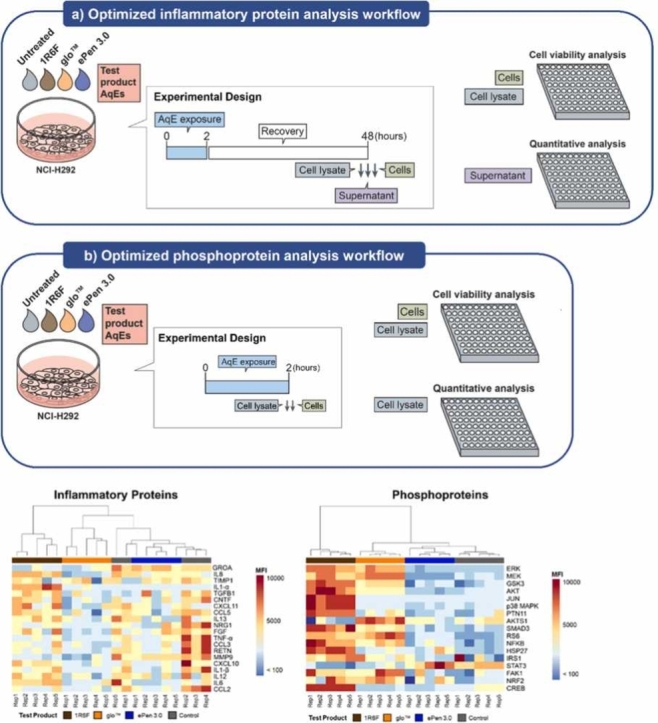
Fig. 2.
Optimized workflows used to assess inflammatory and cell signaling proteins in H292 cells exposed to AqEs from different tobacco product categories. A) Cells aimed for inflammatory protein analysis were exposed for 2 hrs to AqE from the test products, followed by recovery in complete culture medium for 48 hrs. At the end of the recovery period, the culture supernatant was kept for multiplex quantitative analysis. B) Cells aimed for phosphoprotein analysis were exposed for 2 hrs to AqE from the test products and the cell lysates were immediately harvested for multiplex semi-quantitative analysis. In both cases, cells were assessed for viability by CellTiter-Glo.
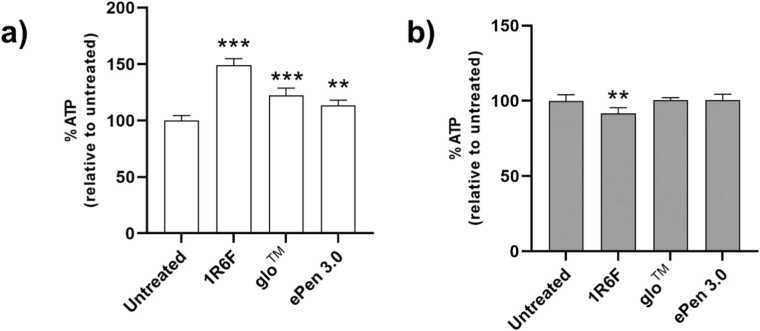
In the cell viability assay, a 2 hrs exposure to 1R6F, glo™ and ePen3 AqE resulted in a significant increase in ATP levels by 49% (p<0.001), 22% (p<0.001), and 13% (p<0.01), respectively, compared to the untreated control (Fig. 3a). After 48 hrs of cellular recovery (the time point of collection of cell supernatants used for inflammatory protein analysis), cells across all treatments had similar ATP levels compared to the untreated control, with 1R6F AqE exposure showing only a marginal decrease by 8% (p <0.01) (Fig. 3b). Overall, the data confirmed the conditions and concentrations used for protein analysis were non-cytotoxic, which ensured that cytokine secretion and cell signaling proteins could be assessed without the confounding factor of cytotoxicity.
Fig. 3.
Cell viability following exposure to 1R6F and RRP AqEs. Intracellular ATP levels measured by CellTiter-Glo immediately after 2 hrs exposure to 25% AqE of each test product (a) or after 2 hrs exposure and 48 hrs recovery (b) are presented as % relative to untreated cells. Relative ATP levels for each product AqEs are mean ± standard deviation from 3 independent replicates. **p<0.01, ***p<0.001.
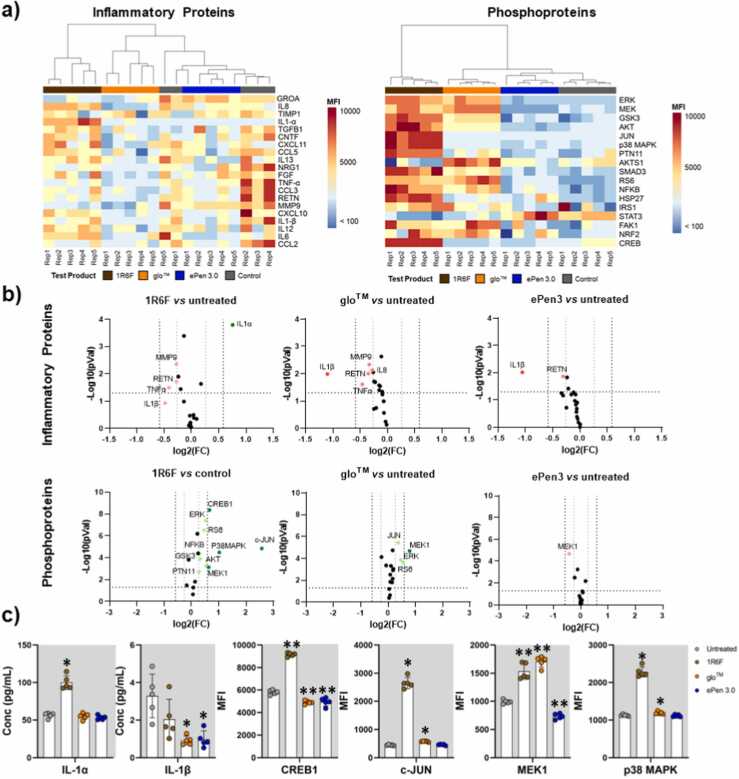
Responses from 17 signaling (Table 2) and 20 inflammatory markers (Table 3) were analyzed in total. Hierarchical clustering of relative protein levels revealed distinct patterns across all test products (Fig. 4a). Specifically, relative phosphoprotein levels showed a distinct activatory cell signaling profile following 1R6F AqE exposure, compared to RRPs and the untreated control. Samples from 1R6F AqE-exposed H292 cells clustered furthest from the untreated controls, followed by glo™ and ePen 3.0. We also observed a similar pattern of clustering in the case of secreted inflammatory molecules, with ePen 3.0 and untreated samples forming one uniform cluster, while glo™ and 1R6F samples forming distinct groups.
Table 2.
Phosphoproteins included in multiplex assay panel A (phospho-plex).
| Protein Name | Uniprot Entry | Phospho Residue | Description | Function |
|---|---|---|---|---|
| AKT (pan) |
P31749 P31751 Q9Y243 |
S473 | Rac-alpha/beta/gamma serine-threonine protein kinase | Metabolism, proliferation, cell survival, cell growth, angiogenesis |
| GSK-3α/β |
P49840 P49841 |
S21/9 | Glygogen synthase kinase alpha & beta | Glugose homeostasis, Wnt signalling, regulation of transcription factors, apoptosis |
| MEK1 | Q02750 | S218/222 | Dual specificity mitogen-activated protein kinase kinase 1 | Cell growth, survival, differentiation |
| ERK1/2 |
P28482 P27361 |
T202/Y204 | Mitogen-activated protein kinase-1 & 3 | Cell growth, adhesion, survival, differentiation |
| AKT1S1 (PRAS40) | Q96B36 | T246 | Proline-rich AKT1 substrate 1 | Cell growth, survival |
| c-JUN | P05412 | S63 | Transcription factor AP-1 | Cell cycle progression, proliferation, angiogenesis, apoptosis, response to stress |
| SMAD3 | P84022 | S423/425 | Mothers against decapentaplegic homolog 3 | Wound healing, cell differentiation, apoptosis, inflammation |
| NFkB | P19838 | S536 | Nuclear factor NF-kappa-B p105 subunit | inflammation, immunity, differentiation, cell growth, tumorigenesis and apoptosis |
| p38 MAPK | P53778 | T180/Y182 | Mitogen-activated protein kinase 12 | DNA-damage response, response to stress, cell cycle arrest |
| RS6 | P62753 | S235/236 | 40 S ribosomal protein S6 | Cell growth, proliferation, apoptosis |
| STAT3 | P40763 | Y705 | Signal transducer and activator of transcription 3 | Cell growth, proliferation, migration, differentiation, apoptosis, inflammation, defense response |
| IRS1 | P35568 | S636/639 | Insulin receptor substrate 1 | Glucose homeostasis, PI3K signaling |
| FAK1 | Q05397 | Y397 | Focal adhesion kinase-1 | Cell migration, adhesion, cell cycle, progression, proliferation, apoptosis |
| CREB1 | P16220 | S133 | Cyclic AMP-responsive element-binding protein 1 | Proliferation, migration, tumour suppression, response to nicotine |
| HSP27 | P04792 | S78/82 | Heat shock protein beta-1 | Oxidative stress response, apoptosis, angiogenesis, chemotaxis |
| NRF2 | Q60795 | S40 | Nuclear factor erythroid 2-related factor 2 | Oxidative stress response, angiogenesis |
| PTN11 | Q06124 | Y542 | Tyrosine-protein phosphatase non-receptor type 11 | MAPK signaling, cell adhesion, DNA damage response |
Table 3.
Inflammatory proteins included in multiplex panel B (cyto-plex).
| Gene Name / Protein Name | Uniprot Entry | Protein Names | Function | Reference |
|---|---|---|---|---|
| CCL2/MCP1 | P13500 | C-C motif chemokine 2, Monocyte chemotactic protein 1 | Angiogenesis, chemotaxis | [14], [37] |
| CCL3/MIP-1α | P10147 | C-C motif chemokine 3, Macrophage inflammatory protein 1-alpha | Monokine with inflammatory and chemokinetic properties | [73] |
| CCL5/RANTES | P13501 | C-C motif chemokine 5 | Chemoattractant | [44] |
| CNTF | P26441 | Ciliary neurotrophic factor | Axon regeneration | NA |
| CXCL10/IP-10 | P02778 | C-X-C motif chemokine 10, 10 kDa interferon gamma-induced protein | chemotaxis, differentiation, activation of peripheral immune cells, cell growth, apoptosis, modulation of angiostatic effects | [30] |
| CXCL11/I-TAC | O14625 | C-X-C motif chemokine 11, Interferon-inducible T-cell alpha chemoattractant | Chemotaxis, skin immune responses | NA |
| FGF-1 | P05230 | Fibroblast growth factor 1 | cell survival, cell division, angiogenesis, cell differentiation, cell migration | NA |
| CXCL1/GRO-α | P09341 | Growth-regulated alpha protein | Chemotaxis, inflammatory response | [26] |
| IL-12A | P29459 | Interleukin-12 subunit alpha | Cytokine, growth factor | [7] |
| IL-13 | P35225 | Interleukin-13 | Cytokine, inflammatory response | NA |
| IL-1α | P01583 | Interleukin-1 alpha | Inflammatory response, angiogenesis, cell division | [30] |
| IL-1β | P01584 | Interleukin-1 beta | Inflammatory response, angiogenesis, cell division, cell migration | [26], [30], [35] |
| IL-6 | P05231 | Interleukin-6 | Acute-phase response, immunity, tissue regeneration, metabolism | [7], [37] |
| CXCL8/IL-8 | P10145 | Interleukin-8 | Chemotaxis, angiogenesis, inflammatory response | [7], [26], [41], [44]] |
| MMP9 | P14780 | Matrix metalloproteinase-9 | Extracellular matrix organization, apoptosis, response to stress | [7] |
| NRG1 | Q02297 | Pro-neuregulin-1, membrane-bound isoform, heregulin | Cell growth, differentiation, wound healing | NA |
| RETN | Q9HD89 | Resistin | Hormone, fat cell differentiation | NA |
| TGF-β1 | P01137 | Transforming growth factor beta-1 proprotein | Cell growth, migration, differentiation, chemotaxis, inflammatory response, vasculogenesis | [33] |
| TIMP1 | P01033 | Metalloproteinase inhibitor 1, tissue inhibitor of metalloproteinases 1 | Extracellular matrix disassembly, cell differentiation, migration, cell death | [70] |
| TNF-α | P01375 | Tumor necrosis factor | Cell death, cell proliferation, angiogenesis, inflammatory response | [73] |
Fig. 4.
Inflammatory protein and cell signaling profiles of H292 cells exposed to 25% AqEs from 1R6F and RRP aerosols. (a) Heat maps showing MFI values of 20 inflammatory markers (left) and 17 phosphoproteins (right) normalized by subtracting background noise data from analysis of RPMI media or lysis buffer alone, respectively). (b) Volcano plots comparing MFI values between AqE-exposed and untreated cells for each test product category. Proteins showing significant fold-changes (higher than 1.5 or lower than −1.5) compared to the untreated control occur outside a pair of vertical black dotted lines and are highlighted in green and red to indicate increase and decrease, respectively. Proteins showing a tendency in fold change difference compared to the untreated control (higher than 1.2 or lower than −1.2) occur outside a pair of grey vertical dotted threshold lines and are highlighted in light green or light red respectively. Proteins with statistically significant changes compared to untreated control (p < 0.05, n=5, Mann-Whitney test) occur above the horizontal dotted black lines. Black dots correspond to proteins with statistically non-significant changes or with changes < 1.5 relative to untreated controls. (c) Concentration (pg/ml) of individual cytokines and MFI values of cell signaling phosphoproteins with > 1.5-fold change and p <0.05 from (b). Mean ± standard deviation from 5 independent replicates is presented. FC: fold change, *p<0.05, **p<0.01.
We further assessed the statistical significance and extent of the changes from exposure to the three different test products compared to untreated control (Fig. 4b-c). Results from the three contrasts (summarized in volcano plots, Fig. 4b) showed that exposure to 1R6F AqE had the strongest effect on the levels of the protein markers tested followed by glo™ and ePen 3.0 (p-value < 0.05). Six proteins exhibited fold changes > 1.5 following exposure to one or more test products (Fig. 4c). Specifically, 1R6F resulted in the significant activation of signaling markers MEK1, CREB1, cJUN and p38 MAPK. glo™ had markedly reduced effect on these markers compared to 1R6F, except for MEK1 which was activated at levels similar to 1R6F. ePen 3.0 exposure did not activate any of the signaling markers tested and resulted in a minor decrease on MEK1 phosphorylation. In the case of inflammatory markers, 1R6F resulted in the significant release of IL-1α compared to the other product categories and the untreated control (1R6F = 99.94 pg/ml, glo™ =55.00 pg/ml, ePen 3.0 =53.26 pg/ml, and untreated =56.45 pg/ml). glo™ and ePen 3.0 exposure led to the significant decrease in IL-1β levels compared to untreated cells (1R6F = 2.04 pg/ml, THP = 0.91 pg/ml, EC = 0.98 pg/ml, and untreated = 3.29 pg/ml) (Fig. 4c).
3. Discussion
The growing interest to further tobacco harm reduction by encouraging smokers who would not otherwise quit to switch to RRPs has contributed to rapid growth in THPs and e-cigarettes [43]. Given the diversity of RRPs, in vitro assessment of their biological effects should be part of their routine toxicological evaluation [43], [58], [29]. The focus of this study was to compare the inflammatory responses and cell signaling effects between a combustible reference cigarette (1R6F) and two RRPs (glo™ and ePen3), at non-cytotoxic conditions, using an experimental multiplex proteomics platform.
Experiment conditions such as exposure time, cell recovery time, and AqE concentration were optimized to ensure that biologically relevant responses were not obscured by cell death due to test product toxicity and that the most potent effects were captured. Our approach enabled the discrimination between the different RRP categories based on differential inflammatory and cell signaling profiles. Hierarchical clustering analysis demonstrated the heterogeneous effect of AqE exposure on cytokine and phosphoprotein signatures in H292 cells, as evident from the different clustering patterns observed. H292 cells exposed to AqE from RRPs displayed significant reductions in both inflammatory and intracellular signaling activity compared to 1R6F AqE exposure. This adds to the growing body of evidence that RRP aerosol exposure may result in reduced biological outcomes in vitro when compared to reference cigarette smoke.
Cytokines commonly serve as biomarkers for inflammation and the subsequent oxidative stress. In this study we devised a panel of cytokines, focusing on markers that are shown in the literature to be linked to specific pathophysiology related to cigarette smoke.
For example, MCP1 (Monocyte chemoattractant protein-1), also known as Chemokine (CC-motif) ligand 2 (CCL2) is a proinflammatory cytokine, known to enhance the migration of inflammatory cells to the inflammatory site. It is involved in the pathogenesis of several diseases, such as cancers, CVD, and idiopathic pulmonary fibrosis (IPF). This is particularly relevant as IPF is shown to be substantially increased in smokers [56], [6]. Similarly, MIP-1α is involved in inflammatory cell migration and the pathology of several inflammatory diseases, specifically, MIP-1α levels are increased in several inflammatory lung diseases [9]. Other cytokines in our inflammatory panel (Table 3) included MMP9 and TIMP1 which are implicated in the regulation of extracellular matrix degradation in pulmonary fibrosis [11], [70]. A group of interleukins which have been shown to be impacted by cigarette smoke but not by other RRPs in in vitro studies (IL-1α, IL-1β, IL-6, IL-8) [7], [30] were also included in our panel aiming to reproduce common patterns of inflammatory responses that can differentiate between combustible cigarette and RRPs. Interestingly, in a recent study by Desai et al. [17] considerable increases in the levels of some of the abovementioned markers (MCP1, MIP-1α, MMP9, IL-1β) were found in the bronchoalveolar lavage of rats exposed to cigarette smoke in comparison to rats exposed to filtered air or e-cigarette vape which were associated with pulmonary inflammation in rats. These findings further showcase the relevance of studying these markers in the context of establishing the risk continuum of RRPs.
Differences in inflammatory responses between 1R6F and RRP AqEs were mostly limited to differential levels of IL-1α and IL-1β markers found in the culture supernatants of H292 cells. IL-1 has emerged as a key component in immune defense against inhaled noxious agents, including cigarette smoke, [38] and cancer-related inflammation through its role in activating cytokines and chemokines involved in pro-tumoral microenvironments [3], [68]. For instance, highly metastatic human lung cancer cell lines are known to express higher levels of IL-1α compared to low-metastatic cell lines [69]. IL-1 s also have demonstrated their roles in the different phenotypes found in COPD [45], [46]. The levels of IL-1 s may also aid in differentiating COPD phenotypes [74]. Our findings show that cells exposed to 1R6F AqEs release more IL-1α and IL-1β than those exposed to the RRP AqEs, supporting the evidence that RRP may reduce the risk of developing abovementioned inflammatory diseases via a decrease in pro-inflammatory molecule activation when compared to conventional cigarette smoking. However, the caveats of utilizing cell lines for such experiments must also be considered, as other studies using in vitro cytokine profiling have shown that exposing human monocytic (THP.1) cells to potential toxins can cause cytotoxic events despite an absence of pro-inflammatory markers [8]. Based on this, and our assay’s primary focus on the listed 20 inflammatory cytokines, it is difficult to conclude whether the relative alterations in cytokine levels are an accurate reflection of the comprehensive toxicity-related inflammatory events in H292s during exposure to RRP AqEs. Moreover, it should be noted that, while studies have shown that IL-1β accumulation has pro-tumorigenic and pro-inflammatory roles in most cells, IL-1β has also shown an antagonistic effect on tumor growth and has shown tumor inhibiting effects [21], [2]. Finally, the absence of an effect on the other markers with previously proven dependency to cigarette smoke may be a limitation of the in vitro cellular model used. Whilst some markers may be secreted by the studied cells, others may only be found to be secreted under certain conditions in vivo in the bronchoalveolar lavage.
Phosphorylation of intracellular proteins is critical for cell signal transduction, and perturbations in signaling pathways could result in an increase in disease phenotypes [4]. Therefore, the relative differences in the phosphorylated forms of intracellular phosphoproteins upon exposure to AqEs from the reference cigarette and the RRPs enable better understanding of the relationships between signal transduction patterns and tobacco and nicotine product usage. The increase (≥1.5-fold) in phosphorylation of several signaling molecules upon 1R6F AqE exposure indicated widespread signaling activity caused by cigarette smoke as compared to RRP aerosol extracts. Some of the hyperphosphorylated markers identified in this study have been previously linked to smoking-related diseases. The proto-oncogene c-JUN is a known mediator of growth factor signals in non-small cell lung cancer. Recently, the role of c-JUN in relation to cigarette smoke extracts (CSEs) and development of COPD was described. Mitani et al. [42] provided evidence of the role of c-JUN in COPD corticosteroid insensitivity by inhibition of its activator, mTOR, using rapamycin. Additionally, their results showed that an increase in immature dendritic cells found in COPD patients may correlate with CSE induced DNMT3a regulation via the c-JUN pathway. Studies have also pointed out the contribution of the MEK/Erk1/2 pathway on lung fluid homeostasis through the cystic fibrosis transmembrane conductance regulator (CFTR) [72]. Cigarette smoke negatively regulates CFTR expression through the activation of the MEK pathway, thereby resulting in a cigarette-smoke induced decrease of airway surface liquid in the lungs, which is typically seen in regular smokers with COPD [72]. Here, MEK1 was found to be increased in cells exposed to reference cigarette (1R6F) and THP (glo™) AqEs, but not in cells exposed to e-cigarette (ePen3) AqE. This illustrates that the relative changes identified in cell signaling levels in toxicological studies of tobacco and nicotine product categories occur in a risk continuum. Finally, the ERK/p38 MAPK-CREB1 signaling axis may implicate intracellular mechanisms that mediate mucin hypersecretion in cells of the human airway epithelia due to inflammatory activity (IL-1β) in the respiratory tract [57]. These signaling and cytokine markers were affected in concerted patterns in cells exposed to 1R6F AqEs, but not in those exposed to RRP AqEs.
In conclusion, the results presented here are consistent with historical data and previous studies wherein combustible cigarettes are positioned at one end of the risk continuum with the highest level of associated risk, followed by THPs, and e-cigarettes, in order of their potential risk reduction profiles [43], [12], [40]. The simultaneous detection of inflammatory and intracellular cell signaling markers could help to provide comparative risk assessments of RRPs by highlighting the changes to markers associated with smoking-related disease mechanisms and functional networks. Multiplex proteomics can generate rapid, quantifiable, and high-throughput analysis of in vitro toxicological profiles that is compatible with other datasets, i.e., chemical, in vivo, clinical, and population studies. We propose this proteomic multiplex approach as a robust and reliable tool for screening RRPs such as THPs and e-cigarettes for comparative biological effects. The identified inflammatory and phosphoproteomic responses in H292 cells enable the ‘benchmarking’ of a known/expected response to be generated when contrasting across RRPs and may possibly be replicated across different cell types.
4. Materials and methods
4.1. Test products
The tobacco and nicotine products used are summarized in Table 1.
4.2. Reagents
Cell culture reagents were purchased from Fisher Scientific. MagPlex® magnetic microspheres (beads) were from Luminex Corp. Recombinant standard proteins were purchased from Peprotech and Bio-techne. Buffers for cell lysis and multiplex assays were prepared in-house using reagents from Sigma-Aldrich and Santa Cruz Biotechnology.
4.3. AqE preparation
AqE generation was performed as previously described [12]. Prior to use 1R6F cigarettes and THP consumables were conditioned for at least 48 hrs at 22 ± 1 °C and 60% ± 3% relative humidity in accordance with ISO 3402:1999 ISO [31] and generation conducted under ISO laboratory conditions. 1R6F cigarette AqE extracts were produced using the Health Canada Intense (HCI) machine puffing regime (55 ml puff taken over 2 s in 30 s intervals, bell-shaped puffing profile) on a Borgwaldt-KC RM20H rotary 20-port smoking machine (Borgwaldt GmBH, Hamburg, Germany) with cigarettes vent blocked. glo™ AqE extracts were also produced on a Borgwaldt-KC RM20H rotary 20-port smoking machine with no vent blocking using the modified Health Canada Intense (HCIm) machine puffing regime (55 ml puff taken over 2 s in 30 s intervals, bell-shaped puffing profile). ePen3 e-cigarette aerosol extracts were machine-puffed using the CORESTA Recommended Method No. 81 machine puffing regime (Borgwaldt, Richmond, VA, USA) (55 ml puff taken over 3 s in 30 s intervals, square wave puffing profile). AqE samples were produced by bubbling 8 puffs from a single 1R6F cigarette, 40 puffs for the THP variants, and 200×55 for the e-cigarette through 20 ml supplemented serum-free RPMI 1640 media The generated 100% AqE stock solution was used to prepare test concentrations for cell exposure. AqE stock solutions were stored at −80 °C immediately and used within one month. To prevent “dry-wicking” conditions in e-cigarette samples, devices were puffed at 45⁰C and cartomisers changed every 100 puffs [19].
4.4. Nicotine measurement and analysis
Nicotine concentration was measured for each cigarette or RRP AqE, as previously described, as a QC measure to ensure reproducible extract generation [12]. Briefly, samples were spiked with d4-nicotine (Sigma Aldrich, St Louis, USA) to a final concentration of 10 ng/ml, evaporated under a vacuum, and dissolved in a 5% acetonitrile:water solution (volume:volume). Nicotine quantification was carried out using a Waters Acquity UPLC (Waters, Milford, USA) linked to an Applied Biosciences 4000QTrap mass spectrometer (Applied Biosystems, Foster City, USA); the UPLC method was adapted from Adamson et al. [1]
4.5. Candidate biomarkers selection and multiplex assay development
Two multiplex assay panels were prepared for this study. Panel A (phospho-plex, Table 2) was used for semi-quantitative analysis of the phosphorylation status of 17 phosphoproteins and panel B (cyto-plex, Table 3) was used for quantitative analysis of 20 extracellular inflammatory proteins (cytokines, chemokines, and growth factors). Phosphoproteins were selected based on their involvement on key signaling pathways driving cellular processes known to be affected by cigarette smoke. Inflammatory proteins were selected based on previous studies [26], [32], [35], [37], [41], [44], [47], [65] and included inflammatory mediators and markers of extracellular matrix organization found to be perturbed in response to cigarette smoke and/or smoking-related pathological conditions such as COPD and idiopathic pulmonary fibrosis [32], [35], [47].
Assays were developed by Protavio as previously described [18]. For preparation of multiplex assay reagents, each bead ID was coupled to a different capture antibody at a ratio of 30 μg antibody per 6.25 million beads and bead IDs were mixed to a final concentration of 50 beads/μl per bead ID (bead mix). Detection antibodies for each assay were biotinylated and mixed according to internally pre-determined concentrations to generate the detection antibody mix. For the cyto-plex panel, recombinant standard proteins were mixed at a final concentration of 40 ng/ml and the protein mix was used for generation of the assay calibration curves. Assay performance characteristics are presented in Supplementary Material.
4.6. Cell culture and AqE treatment
NCI-H292 human lung carcinoma cells (#CRL-1848, ATCC) were cultured in RPMI-1640 medium supplemented with 10% fetal bovine serum (FBS), 1% penicillin/streptomycin and 1% L-glutamine (complete medium) for two weeks, followed by seeding into 96-well plates at a density of 25,000 cells/well. Treatment with AqE was optimized for AqE concentration, time of exposure and time of recovery (for analysis of inflammatory markers). Optimization experiments are presented in detail in Supplementary Material. The final optimized workflow is presented in Fig. 2. Cells were treated for 2hrs with 100 μl/well of 25% AqE (expressed as % v/v of the stock solution) prepared in RPMI-1640 medium supplemented with penicillin/streptomycin and L-glutamine only (dilution medium). Dilution medium was used as mock treatment (untreated control). For phosphoprotein analysis, cellular protein lysates were collected immediately after exposure. For inflammatory protein analysis, AqEs were removed, and cells were washed once in dilution medium and left to recover in complete medium for 48 hours. The culture medium supernatant was collected and stored at −80°C until the day of analysis.
4.7. Protein lysate preparation and multiplex protein analysis
Cellular lysates were generated by washing cells in ice-cold PBS followed by lysis in 60 μl/well of lysis buffer containing protease and phosphatase inhibitors (Protavio) using a freeze-thaw cycle. Proteins were extracted by centrifugation at 4000 x g for 10 min and stored at −80°C until the day of analysis. Total protein content was measured using the Pierce BCA Protein Assay (Thermo Fisher Scientific) following the manufacturer’s instructions.
Multiplex assay measurements were performed in 96-well plates as described previously [49]. Briefly, 35 μl of each undiluted sample, control lysate (panel A) or standard (panel B) was incubated with 50 μl of the bead mix (2500 beads per bead ID) for 90 min at room temperature with shaking. Beads were washed twice with assay buffer to remove unbound material and further incubated with 20 μl of the detection antibody mix for 60 min at room temperature with shaking. Beads were washed again with assay buffer to remove unbound antibodies and incubated with 35 μl of streptavidin-phycoerythrin at a 1:200 dilution for 15 min at room temperature with shaking. Beads were washed once, resuspended in 130 μl assay buffer and measured in a FlexMAP 3D instrument (Luminex Corp, Austin, Texas, USA) using a minimum of 50 counts, high PMT setting and DD gating of 3000–20,000. Median Fluorescence Intensity (MFI)values were used as input data for subsequent statistical analysis. For absolute quantification of cytokine levels, net MFI values (blank-subtracted) were used and a 5PL logistic regression was applied for generation of calibration curves.
4.8. Cell viability assay
The CellTiter-Glo® Luminescent assay (Promega) was used to determine the number of viable cells upon treatment with the aqueous extracts [[51], [52], [53]]. Cells were washed once with PBS to remove AqE extracts and incubated for 10 min with 100 μl/well of the assay reagent diluted 1:2 with RPMI-1640 medium supplemented with penicillin/streptomycin and L-glutamine. Luminescence was measured on a Varioskan™ LUX multimode microplate reader (Thermo Scientific) using 100 msec acquisition time.
4.9. Statistical analysis
Statistical analysis for Fig. 1, Fig. 3 was performed in GraphPad Prism (v. 9.0.0). For each dataset, homoscedasticity was tested using Bartlett’s and Brown-Forsythe tests and the residuals distribution was visually inspected through Quantile-Quantile plot, residual histogram and residual vs. fitted values plot. If the assumptions of normality and homoscedasticity were met, data were analysed using ordinary one-way ANOVA followed by Dunnett’s post hoc tests to compare each treatment versus the ‘untreated’ control. If the assumptions were not met data were analysed using Welch’s ANOVAs followed by Dunnett’s T3 multiple comparison tests (treatments vs. ‘untreated’ control).
Statistical analysis for Fig. 4 was performed in R using the package “PMCMRplus”[48]. Test products were compared against the untreated control using nonparametric two-tailed Mann–Whitney tests followed by a multiple test correction by means of Benjamini-Hochberg false discovery rate. P-values < 0.05 were considered statistically significant.
The effect of AqE exposure on phosphoprotein and inflammatory protein levels changes was plotted using a volcano plot with the negative log10 transformed p values on the y-axis against the log2 fold change between the two conditions on the x-axis. Proteins were considered as showing differential expression if they showed statistically significant differential levels (Mann-Whitney test, see above) and with fold changes in expression levels higher than 1.5 or lower than −1.5 compared to the untreated control. MFI values in response to the different test products were compiled into a heatmap with unsupervised visual hierarchical clustering using Pearson correlation and average linkage with scaled protein values using R package “Pheatmap”[36] in R v. 4.1.1[61] and RStudio IDE (v. 1.4).
CRediT authorship contribution statement
Vaia Pliaka: Data curation, Formal analysis, Investigation. Asier Antoranz: Data curation, Formal analysis, Investigation, Methodology. Emma Bishop: Writing – original draft, Writing – review & editing. Fabio Miazzi: Visualization, Writing – original draft, Writing – review & editing. Linsey E Haswell: Conceptualization, Investigation, Methodology, Project administration, Writing – original draft, Writing – review & editing. Nikos Tsolakos: Conceptualization, Investigation, Methodology, Project administration, Writing – original draft, Writing – review & editing. Damien Breheny: Conceptualization, Supervision, Writing – original draft, Writing – review & editing. Marianna Gaça: Methodology, Resources, Writing – original draft, Writing – review & editing. Leonidas G Alexopoulos: Conceptualization, Funding acquisition, Project administration, Supervision, Writing – original draft, Writing – review & editing. Angeliki Minia: Investigation, Supervision, Data curation, Formal analysis.
Code Availability
The R code and the datasets generated and analyzed from this study are available from the corresponding authors upon reasonable request.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
We thank Tahseen Jilani for input on the statistical analysis, Fan Yu and Rhian Evans for their critical comments and suggestions.
Handling editor: Lawrence Lash
Footnotes
Supplementary data associated with this article can be found in the online version at doi:10.1016/j.toxrep.2024.04.006.
Appendix A. Supplementary material
Supplementary material
Data availability
Data will be made available on request.
References
- 1.Adamson J., Li X., Cui H., Thorne D., Xie F., Gaca M.D. Nicotine quantification in vitro: a consistent dosimetry marker for e-cigarette aerosol and cigarette smoke generation. Appl. Vitr. Toxicol. 2017;3:14–27. [Google Scholar]
- 2.Allen I.C., Tekippe E.M., Woodford R.M., Uronis J.M., Holl E.K., Rogers A.B., Herfarth H.H., Jobin C., Ting J.P. The NLRP3 inflammasome functions as a negative regulator of tumorigenesis during colitis-associated cancer. J. Exp. Med. 2010;207:1045–1056. doi: 10.1084/jem.20100050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Apte R.N., Dotan S., Elkabets M., White M.R., Reich E., Carmi Y., Song X., Dvozkin T., Krelin Y., Voronov E. The involvement of IL-1 in tumorigenesis, tumor invasiveness, metastasis and tumor-host interactions. Cancer Metastas-.-. Rev. 2006;25:387–408. doi: 10.1007/s10555-006-9004-4. [DOI] [PubMed] [Google Scholar]
- 4.Ardito F., Giuliani M., Perrone D., Troiano G., Lo Muzio L. The crucial role of protein phosphorylation in cell signaling and its use as targeted therapy (Review) Int J. Mol. Med. 2017;40:271–280. doi: 10.3892/ijmm.2017.3036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Azzopardi D., Patel K., Jaunky T., Santopietro S., Camacho O.M., Mcaughey J., Gaca M. Electronic cigarette aerosol induces significantly less cytotoxicity than tobacco smoke. Toxicol. Mech. Methods. 2016;26:477–491. doi: 10.1080/15376516.2016.1217112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bae W., Lee C.H., Lee J., Kim Y.W., Han K., Choi S.M. Impact of smoking on the development of idiopathic pulmonary fibrosis: results from a nationwide population-based cohort study. Thorax. 2022;77:470–476. doi: 10.1136/thoraxjnl-2020-215386. [DOI] [PubMed] [Google Scholar]
- 7.Banerjee A., Haswell L.E., Baxter A., Parmar A., Azzopardi D., Corke S., Thorne D., Adamson J., Mushonganono J., Gaca M.D., Minet E. Differential gene expression using RNA sequencing profiling in a reconstituted airway epithelium exposed to conventional cigarette smoke or electronic cigarette aerosols. Appl. Vitr. Toxicol. 2017;3:84–98. [Google Scholar]
- 8.Bhattacharya K., Kilic G., Costa P.M., Fadeel B. Cytotoxicity screening and cytokine profiling of nineteen nanomaterials enables hazard ranking and grouping based on inflammogenic potential. Nanotoxicology. 2017;11:809–826. doi: 10.1080/17435390.2017.1363309. [DOI] [PubMed] [Google Scholar]
- 9.Bhavsar I., Miller C.S., Al-Sabbagh M. Macrophage inflammatory protein-1 alpha (MIP-1 alpha)/CCL3: as a biomarker. Gen. Methods Biomark. Res. their Appl. 2015:223–249. doi: 10.1007/978-94-007-7696-8_27. 2015 Jun 1. [DOI] [Google Scholar]
- 10.Bishop E., Breheny D., Hewitt K., Taylor M., Jaunky T., Camacho O.M., Thorne D., Gaca M. Evaluation of a high-throughput in vitro endothelial cell migration assay for the assessment of nicotine and tobacco delivery products. Toxicol. Lett. 2020;334:110–116. doi: 10.1016/j.toxlet.2020.07.011. [DOI] [PubMed] [Google Scholar]
- 11.Bormann T., Maus R., Stolper J., Tort Tarres M., Brandenberger C., Wedekind D., Jonigk D., Welte T., Gauldie J., Kolb M., Maus U.A. Role of matrix metalloprotease-2 and MMP-9 in experimental lung fibrosis in mice. Respir. Res. 2022;23:180. doi: 10.1186/s12931-022-02105-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bozhilova S., Baxter A., Bishop E., Breheny D., Thorne D., Hodges P., Gaca M. Optimization of aqueous aerosol extract (AqE) generation from e-cigarettes and tobacco heating products for in vitro cytotoxicity testing. Toxicol. Lett. 2020;335:51–63. doi: 10.1016/j.toxlet.2020.10.005. [DOI] [PubMed] [Google Scholar]
- 13.Breheny D., Oke O., Pant K., Gaca M. Comparative tumor promotion assessment of e-cigarette and cigarettes using the in vitro Bhas 42 cell transformation assay. Environ. Mol. Mutagen. 2017;58:190–198. doi: 10.1002/em.22091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Brunssen C., Giebe S., Hofmann A., Brux M., Morawietz H. Evalulation of the cytotoxic, oxidative and pro-inflammatory effects of aqueous cigarette extract on human monocytes: a potential model system for assessment of next-generation tobacco and nicotine products. Appl. Vitr. Toxicol. 2017;3:121–130. [Google Scholar]
- 15.Coggins C.R. A further review of inhalation studies with cigarette smoke and lung cancer in experimental animals, including transgenic mice. Inhal. Toxicol. 2010;22:974–983. doi: 10.3109/08958378.2010.501831. [DOI] [PubMed] [Google Scholar]
- 16.Czekala L., Simms L., Stevenson M., Tschierske N., Maione A.G., Walele T. Toxicological comparison of cigarette smoke and e-cigarette aerosol using a 3D in vitro human respiratory model. Regul. Toxicol. Pharm. 2019;103:314–324. doi: 10.1016/j.yrtph.2019.01.036. [DOI] [PubMed] [Google Scholar]
- 17.Desai R.W., Demir K., Tsolakos N., Moir-Savitz T.R., Gaworski C.L., Weil R., Oldham M.J., Lalonde G. Comparison of the toxicological potential of two JUUL ENDS products to reference cigarette 3R4F and filtered air in a 90-day nose-only inhalation toxicity study. Food Chem. Toxicol. 2023;179 doi: 10.1016/j.fct.2023.113917. [DOI] [PubMed] [Google Scholar]
- 18.Doulamis I.P., Konstantopoulos P., Tzani A., Antoranz A., Minia A., Daskalopoulou A., Charalampopoulos A., Alexopoulos L., Perrea D.N., Menenakos E. Visceral white adipose tissue and serum proteomic alternations in metabolically healthy obese patients undergoing bariatric surgery. Cytokine. 2019;115:76–83. doi: 10.1016/j.cyto.2018.11.017. [DOI] [PubMed] [Google Scholar]
- 19.Farsalinos K., Voudris V., Poulas K. Response to Shihadeh et al. (2015): e-cigarettes generate high levels of aldehydes only in 'dry puff' conditions. Addiction. 2015;110:1862–1864. doi: 10.1111/add.13078. [DOI] [PubMed] [Google Scholar]
- 20.Forster M., Fiebelkorn S., Yurteri C., Mariner D., Liu C., Wright C., Mcadam K., Murphy J., Proctor C. Assessment of novel tobacco heating product THP1.0. Part 3: comprehensive chemical characterisation of harmful and potentially harmful aerosol emissions. Regul. Toxicol. Pharm. 2018;93:14–33. doi: 10.1016/j.yrtph.2017.10.006. [DOI] [PubMed] [Google Scholar]
- 21.Haabeth O.A., Lorvik K.B., Yagita H., Bogen B., Corthay A. Interleukin-1 is required for cancer eradication mediated by tumor-specific Th1 cells. Oncoimmunology. 2016;5 doi: 10.1080/2162402X.2015.1039763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Haswell L.E., Baxter A., Banerjee A., Verrastro I., Mushonganono J., Adamson J., Thorne D., Gaca M., Minet E. Reduced biological effect of e-cigarette aerosol compared to cigarette smoke evaluated in vitro using normalized nicotine dose and RNA-seq-based toxicogenomics. Sci. Rep. 2017;7:888. doi: 10.1038/s41598-017-00852-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Haswell L.E., Corke S., Verrastro I., Baxter A., Banerjee A., Adamson J., Jaunky T., Proctor C., Gaca M., Minet E. In vitro RNA-seq-based toxicogenomics assessment shows reduced biological effect of tobacco heating products when compared to cigarette smoke. Sci. Rep. 2018;8:1145. doi: 10.1038/s41598-018-19627-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hughes J.R., Keely J., Naud S. Shape of the relapse curve and long-term abstinence among untreated smokers. Addiction. 2004;99:29–38. doi: 10.1111/j.1360-0443.2004.00540.x. [DOI] [PubMed] [Google Scholar]
- 25.Institute Of Medicine . The National Academies Press; Washington, DC: 2012. Scientific Standards for Studies on Modified Risk Tobacco Products. [Google Scholar]
- 26.Ishikawa S., Ito S. Repeated whole cigarette smoke exposure alters cell differentiation and augments secretion of inflammatory mediators in air-liquid interface three-dimensional co-culture model of human bronchial tissue. Toxicol. Vitr. 2017;38:170–178. doi: 10.1016/j.tiv.2016.09.004. [DOI] [PubMed] [Google Scholar]
- 27.Iskandar A.R., Gonzalez-Suarez I., Majeed S., Marescotti D., Sewer A., Xiang Y., Leroy P., Guedj E., Mathis C., Schaller J.P., Vanscheeuwijck P., Frentzel S., Martin F., Ivanov N.V., Peitsch M.C., Hoeng J. A framework for in vitro systems toxicology assessment of e-liquids. Toxicol. Mech. Methods. 2016;26:389–413. doi: 10.3109/15376516.2016.1170251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Iskandar A.R., Mathis C., Schlage W.K., Frentzel S., Leroy P., Xiang Y., Sewer A., Majeed S., Ortega-Torres L., Johne S., Guedj E., Trivedi K., Kratzer G., Merg C., Elamin A., Martin F., Ivanov N.V., Peitsch M.C., Hoeng J. A systems toxicology approach for comparative assessment: biological impact of an aerosol from a candidate modified-risk tobacco product and cigarette smoke on human organotypic bronchial epithelial cultures. Toxicol. Vitr. 2017;39:29–51. doi: 10.1016/j.tiv.2016.11.009. [DOI] [PubMed] [Google Scholar]
- 29.Iskandar A.R., Titz B., Sewer A., Leroy P., Schneider T., Zanetti F., Mathis C., Elamin A., Frentzel S., Schlage W.K., Martin F., Ivanov N.V., Peitsch M.C., Hoeng J. Systems toxicology meta-analysis of in vitro assessment studies: biological impact of a candidate modified-risk tobacco product aerosol compared with cigarette smoke on human organotypic cultures of the aerodigestive tract. Toxicol. Res (Camb. ) 2017;6:631–653. doi: 10.1039/c7tx00047b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Iskandar A.R., Zanetti F., Marescotti D., Titz B., Sewer A., Kondylis A., Leroy P., Belcastro V., Torres L.O., Acali S., Majeed S., Steiner S., Trivedi K., Guedj E., Merg C., Schneider T., Frentzel S., Martin F., Ivanov N.V., Peitsch M.C., Hoeng J. Application of a multi-layer systems toxicology framework for in vitro assessment of the biological effects of Classic Tobacco e-liquid and its corresponding aerosol using an e-cigarette device with MESH technology. Arch. Toxicol. 2019;93:3229–3247. doi: 10.1007/s00204-019-02565-9. [DOI] [PubMed] [Google Scholar]
- 31.Iso . International Organization for Standardization; Geneva, Switzerland: 1999. Tobacco and Tobacco Products—Atmosphere for Conditioning and Testing. [Google Scholar]
- 32.Khalil N., O'connor R.N., Unruh H.W., Warren P.W., Flanders K.C., Kemp A., Bereznay O.H., Greenberg A.H. Increased production and immunohistochemical localization of transforming growth factor-beta in idiopathic pulmonary fibrosis. Am. J. Respir. Cell Mol. Biol. 1991;5:155–162. doi: 10.1165/ajrcmb/5.2.155. [DOI] [PubMed] [Google Scholar]
- 33.Khalil N., O'connor R.N., Unruh H.W., Warren P.W., Flanders K.C., Kemp A., Bereznay O.H., Greenberg A.H. Increased production and immunohistochemical localization of transforming growth factor-α in idiopathic pulmonary fibrosis. Am. J. Respir. Cell Mol. Biol. 1991;5:155–162. doi: 10.1165/ajrcmb/5.2.155. [DOI] [PubMed] [Google Scholar]
- 34.Kogel U., Titz B., Schlage W.K., Nury C., Martin F., Oviedo A., Lebrun S., Elamin A., Guedj E., Trivedi K., Ivanov N.V., Vanscheeuwijck P., Peitsch M.C., Hoeng J. Evaluation of the Tobacco Heating System 2.2. Part 7: systems toxicological assessment of a mentholated version revealed reduced cellular and molecular exposure effects compared with mentholated and non-mentholated cigarette smoke. Regul. Toxicol. Pharm. 2016;81(Suppl 2):S123–S138. doi: 10.1016/j.yrtph.2016.11.001. [DOI] [PubMed] [Google Scholar]
- 35.Kolb M., Margetts P.J., Anthony D.C., Pitossi F., Gauldie J. Transient expression of IL-1beta induces acute lung injury and chronic repair leading to pulmonary fibrosis. J. Clin. Invest. 2001;107:1529–1536. doi: 10.1172/JCI12568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kolde, R. 2019. pheatmap: Pretty Heatmaps. R package version 1.0.12 ed.
- 37.Lagente V., Planquois J.M., Leclerc O., Schmidlin F., Bertrand C.P. Oxidative stress is an important component of airway inflammation in mice exposed to cigarette smoke or lipopolysaccharide. Clin. Exp. Pharm. Physiol. 2008;35:601–605. doi: 10.1111/j.1440-1681.2007.04848.x. [DOI] [PubMed] [Google Scholar]
- 38.Laniado-Laborin R. Smoking and chronic obstructive pulmonary disease (COPD). Parallel epidemics of the 21 century. Int J. Environ. Res Public Health. 2009;6:209–224. doi: 10.3390/ijerph6010209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Margham J., Mcadam K., Forster M., Liu C., Wright C., Mariner D., Proctor C. Chemical composition of aerosol from an e-cigarette: a quantitative comparison with cigarette smoke. Chem. Res Toxicol. 2016;29:1662–1678. doi: 10.1021/acs.chemrestox.6b00188. [DOI] [PubMed] [Google Scholar]
- 40.Mcewan M., Gale N., Ebajemito J.K., Camacho O.M., Hardie G., Proctor C.J., Murphy J. A randomized controlled study in healthy participants to explore the exposure continuum when smokers switch to a tobacco heating product or an E-cigarette relative to cessation. Toxicol. Rep. 2021;8:994–1001. doi: 10.1016/j.toxrep.2021.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mio T., Romberger D.J., Thompson A.B., Robbins R.A., Heires A., Rennard S.I. Cigarette smoke induces interleukin-8 release from human bronchial epithelial cells. Am. J. Respir. Crit. Care Med. 1997;155:1770–1776. doi: 10.1164/ajrccm.155.5.9154890. [DOI] [PubMed] [Google Scholar]
- 42.Mitani A., Ito K., Vuppusetty C., Barnes P.J., Mercado N. Restoration of corticosteroid sensitivity in chronic obstructive pulmonary disease by inhibition of mammalian target of rapamycin. Am. J. Respir. Crit. Care Med. 2016;193:143–153. doi: 10.1164/rccm.201503-0593OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Murphy J., Gaca M., Lowe F., Minet E., Breheny D., Prasad K., Camacho O., Fearon I.M., Liu C., Wright C., Mcadam K., Proctor C. Assessing modified risk tobacco and nicotine products: description of the scientific framework and assessment of a closed modular electronic cigarette. Regul. Toxicol. Pharm. 2017;90:342–357. doi: 10.1016/j.yrtph.2017.09.008. [DOI] [PubMed] [Google Scholar]
- 44.Oltmanns U., Chung K.F., Walters M., John M., Mitchell J.A. Cigarette smoke induces IL-8, but inhibits eotaxin and RANTES release from airway smooth muscle. Respir. Res. 2005;6:74. doi: 10.1186/1465-9921-6-74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Osei E.T., Noordhoek J.A., Hackett T.L., Spanjer A.I., Postma D.S., Timens W., Brandsma C.A., Heijink I.H. Interleukin-1alpha drives the dysfunctional cross-talk of the airway epithelium and lung fibroblasts in COPD. Eur. Respir. J. 2016;48:359–369. doi: 10.1183/13993003.01911-2015. [DOI] [PubMed] [Google Scholar]
- 46.Pauwels N.S., Bracke K.R., Dupont L.L., Van Pottelberge G.R., Provoost S., Vanden Berghe T., Vandenabeele P., Lambrecht B.N., Joos G.F., Brusselle G.G. Role of IL-1alpha and the Nlrp3/caspase-1/IL-1beta axis in cigarette smoke-induced pulmonary inflammation and COPD. Eur. Respir. J. 2011;38:1019–1028. doi: 10.1183/09031936.00158110. [DOI] [PubMed] [Google Scholar]
- 47.Piguet P.F., Ribaux C., Karpuz V., Grau G.E., Kapanci Y. Expression and localization of tumor necrosis factor-alpha and its mRNA in idiopathic pulmonary fibrosis. Am. J. Pathol. 1993;143:651–655. [PMC free article] [PubMed] [Google Scholar]
- 48.Pohlert, T. 2022. PMCMRplus: Calculate Pairwise Multiple Comparisons of Mean Rank Sums Extended. R package version 1.9.4 ed.
- 49.Poussin C., Mathis C., Alexopoulos L.G., Messinis D.E., Dulize R.H., Belcastro V., Melas I.N., Sakellaropoulos T., Rhrissorrakrai K., Bilal E., Meyer P., Talikka M., Boue S., Norel R., Rice J.J., Stolovitzky G., Ivanov N.V., Peitsch M.C., Hoeng J. The species translation challenge-a systems biology perspective on human and rat bronchial epithelial cells. Sci. Data. 2014;1 doi: 10.1038/sdata.2014.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Reinikovaite V., Rodriguez I.E., Karoor V., Rau A., Trinh B.B., Deleyiannis F.W.-B., Taraseviciene-Stewart L. The effects of electronic cigarette vapour on the lung: direct comparison to tobacco smoke. Eur. Respir. J. 2018;51 doi: 10.1183/13993003.01661-2017. [DOI] [PubMed] [Google Scholar]
- 51.Riss, T., Niles, A., Moravec, R., Karassina, N. & Vidugiriene, J. 2004a. Cytotoxicity Assays: In Vitro Methods to Measure Dead Cells. In: MARKOSSIAN, S., SITTAMPALAM, G.S., GROSSMAN, A., BRIMACOMBE, K., ARKIN, M., AULD, D., AUSTIN, C.P., BAELL, J., CAAVEIRO, J.M.M., CHUNG, T.D.Y., COUSSENS, N.P., DAHLIN, J.L., DEVANARYAN, V., FOLEY, T.L., GLICKSMAN, M., HALL, M.D., HAAS, J.V., HOARE, S.R.J., INGLESE, J., IVERSEN, P.W., KAHL, S.D., KALES, S.C., KIRSHNER, S., LAL-NAG, M., LI, Z., MCGEE, J., MCMANUS, O., RISS, T., SARADJIAN, P., TRASK, O.J., J.R., WEIDNER, J.R., WILDEY, M.J., XIA, M. & XU, X. (eds.) Assay Guidance Manual. Bethesda (MD).
- 52.Riss T.L., Moravec R.A. Use of multiple assay endpoints to investigate the effects of incubation time, dose of toxin, and plating density in cell-based cytotoxicity assays. Assay. Drug Dev. Technol. 2004;2:51–62. doi: 10.1089/154065804322966315. [DOI] [PubMed] [Google Scholar]
- 53.Riss, T.L., Moravec, R.A., Niles, A.L., Duellman, S., Benink, H.A., Worzella, T.J. & Minor, L. 2004b. Cell Viability Assays. In: MARKOSSIAN, S., SITTAMPALAM, G.S., GROSSMAN, A., BRIMACOMBE, K., ARKIN, M., AULD, D., AUSTIN, C.P., BAELL, J., CAAVEIRO, J.M.M., CHUNG, T.D.Y., COUSSENS, N.P., DAHLIN, J.L., DEVANARYAN, V., FOLEY, T.L., GLICKSMAN, M., HALL, M.D., HAAS, J.V., HOARE, S.R.J., INGLESE, J., IVERSEN, P.W., KAHL, S.D., KALES, S.C., KIRSHNER, S., LAL-NAG, M., LI, Z., MCGEE, J., MCMANUS, O., RISS, T., SARADJIAN, P., TRASK, O.J., J.R., WEIDNER, J.R., WILDEY, M.J., XIA, M. & XU, X. (eds.) Assay Guidance Manual. Bethesda (MD).
- 54.Rodgman A., Perfetti T.A. CRC Press; 2016. The Chemical Components of Tobacco and Tobacco Smoke. [Google Scholar]
- 55.Seiler C.L., Song J.U.M., Kotandeniya D., Chen J., Kono T.J.Y., Han Q., Colwell M., Auch B., Sarver A.L., Upadhyaya P., Ren Y., Faulk C., De Flora S., La Maestra S., Chen Y., Kassie F., Tretyakova N.Y. Inhalation exposure to cigarette smoke and inflammatory agents induces epigenetic changes in the lung. Sci. Rep. 2020;10:11290. doi: 10.1038/s41598-020-67502-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Singh S., Anshita D., Ravichandiran V. MCP-1: Function, regulation, and involvement in disease. Int Immunopharmacol. 2021;101 doi: 10.1016/j.intimp.2021.107598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Song K.S., Lee W.J., Chung K.C., Koo J.S., Yang E.J., Choi J.Y., Yoon J.H. Interleukin-1 beta and tumor necrosis factor-alpha induce MUC5AC overexpression through a mechanism involving ERK/p38 mitogen-activated protein kinases-MSK1-CREB activation in human airway epithelial cells. J. Biol. Chem. 2003;278:23243–23250. doi: 10.1074/jbc.M300096200. [DOI] [PubMed] [Google Scholar]
- 58.Stratton K., Shetty P., Wallace R., Bondurant S. National Academies Press (US); Washington (DC): 2001. Institute of Medicine Committee to Assess the Science Base for Tobacco Harm, Reduction. Clearing the Smoke: Assessing the Science Base for Tobacco Harm Reduction. Copyright 2001 by the National Academy of Sciences. All rights reserved. [PubMed] [Google Scholar]
- 59.Taylor M., Carr T., Oke O., Jaunky T., Breheny D., Lowe F., Gaca M. E-cigarette aerosols induce lower oxidative stress in vitro when compared to tobacco smoke. Toxicol. Mech. Methods. 2016;26:465–476. doi: 10.1080/15376516.2016.1222473. [DOI] [PubMed] [Google Scholar]
- 60.Taylor M., Thorne D., Carr T., Breheny D., Walker P., Proctor C., Gaca M. Assessment of novel tobacco heating product THP1.0. Part 6: a comparative in vitro study using contemporary screening approaches. Regul. Toxicol. Pharm. 2018;93:62–70. doi: 10.1016/j.yrtph.2017.08.016. [DOI] [PubMed] [Google Scholar]
- 61.Team R.C. R Foundation for Statistical Computing; Vienna, Austria: 2014. R: A Language and Environment for Statistical Computing. [Google Scholar]
- 62.Thorne D., Breheny D., Proctor C., Gaca M. Assessment of novel tobacco heating product THP1.0. Part 7: comparative in vitro toxicological evaluation. Regul. Toxicol. Pharm. 2018;93:71–83. doi: 10.1016/j.yrtph.2017.08.017. [DOI] [PubMed] [Google Scholar]
- 63.Thorne D., Crooks I., Hollings M., Seymour A., Meredith C., Gaca M. The mutagenic assessment of an electronic-cigarette and reference cigarette smoke using the Ames assay in strains TA98 and TA100. Mutat. Res. 2016;812:29–38. doi: 10.1016/j.mrgentox.2016.10.005. [DOI] [PubMed] [Google Scholar]
- 64.Thorne D., Larard S., Baxter A., Meredith C., Gaa M. The comparative in vitro assessment of e-cigarette and cigarette smoke aerosols using the gammaH2AX assay and applied dose measurements. Toxicol. Lett. 2017;265:170–178. doi: 10.1016/j.toxlet.2016.12.006. [DOI] [PubMed] [Google Scholar]
- 65.Tsai J.S., Guo F.R., Chen S.C., Lue B.H., Lee L.T., Huang K.C., Chen C.Y., Hung S.H., Chuang L.M., Chen C.Y. Changes of serum adiponectin and soluble intercellular adhesion molecule-1 concentrations after smoking cessation. Clin. Chem. Lab Med. 2012;50:1063–1069. doi: 10.1515/cclm-2011-0852. [DOI] [PubMed] [Google Scholar]
- 66.Us Department Of Health And Human Services 2010. How Tobacco Smoke Causes Disease: The Biology and Behavioral Basis for Smoking-Attributable Disease. In: PREVENTION, C. F. D. C. A. (ed.). Atlanta, GA. [PubMed]
- 67.Us Department Of Health And Human Services . Centers for Disease Control and Prevention; Atlanta, GA: 2014. The Health Consequences of Smoking—50 Years of Progress: A Report of the Surgeon General. [PubMed] [Google Scholar]
- 68.Voronov E., Shouval D.S., Krelin Y., Cagnano E., Benharroch D., Iwakura Y., Dinarello C.A., Apte R.N. IL-1 is required for tumor invasiveness and angiogenesis. Proc. Natl. Acad. Sci. USA. 2003;100:2645–2650. doi: 10.1073/pnas.0437939100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Watari K., Shibata T., Kawahara A., Sata K., Nabeshima H., Shinoda A., Abe H., Azuma K., Murakami Y., Izumi H., Takahashi T., Kage M., Kuwano M., Ono M. Tumor-derived interleukin-1 promotes lymphangiogenesis and lymph node metastasis through M2-type macrophages. PLoS One. 2014;9 doi: 10.1371/journal.pone.0099568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Watson A.M., Benton A.S., Rose M.C., Freishtat R.J. Cigarette smoke alters tissue inhibitor of metalloproteinase 1 and matrix metalloproteinase 9 levels in the basolateral secretions of human asthmatic bronchial epithelium in vitro. J. Invest. Med. 2010;58:725–729. doi: 10.231/JIM.0b013e3181db874e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Wieczorek R., Phillips G., Czekala L., Trelles Sticken E., O'connell G., Simms L., Rudd K., Stevenson M., Walele T. A comparative in vitro toxicity assessment of electronic vaping product e-liquids and aerosols with tobacco cigarette smoke. Toxicol. Vitr. 2020;66 doi: 10.1016/j.tiv.2020.104866. [DOI] [PubMed] [Google Scholar]
- 72.Xu X., Balsiger R., Tyrrell J., Boyaka P.N., Tarran R., Cormet-Boyaka E. Cigarette smoke exposure reveals a novel role for the MEK/ERK1/2 MAPK pathway in regulation of CFTR. Biochim Biophys. Acta. 2015;1850:1224–1232. doi: 10.1016/j.bbagen.2015.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Zhao J., Li X., Xie F., Yang Z., Pan X., Zhu M., Shang P., Nie C., Liu H., Xie J. Immunomodulatory effects of cigarette smoke condensate in mouse macrophage cell line. Int J. Immunopathol. Pharm. 2017;30:315–321. doi: 10.1177/0394632017716370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Zou Y., Chen X., Liu J., Zhou D.B., Kuang X., Xiao J., Yu Q., Lu X., Li W., Xie B., Chen Q. Serum IL-1beta and IL-17 levels in patients with COPD: associations with clinical parameters. Int J. Chron. Obstruct Pulmon Dis. 2017;12:1247–1254. doi: 10.2147/COPD.S131877. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material
Data Availability Statement
Data will be made available on request.