Abstract
Objective:
DR/ER-MPH (formerly HLD200) is an evening-dosed delayed-release and extended-release methylphenidate approved for the treatment of ADHD in patients ≥6 years. Post hoc analyses of two pivotal Phase 3 trials: HLD200-107 (NCT02493777) and HLD200-108 (NCT02520388) evaluated emotional lability (EL) with DR/ER-MPH treatment.
Methods:
Differences in Conners Global Index—Parent (CGI-P) EL subscale scores and age- and gender-adjusted T-scores over an open-label titration phase (HLD200-107) and between treatment and placebo groups at endpoint (HLD200-108) were evaluated.
Results:
In HLD200-107 (N = 117) mean CGI-P EL subscale scores improved from 5.3 to 1.3 (p < .0001) after 6 weeks; in HLD200-108 significant improvements were observed in the treatment group (n = 81) versus placebo (n = 80; 3.11 vs. 4.08; p = .0053). T-scores showed an improvement with DR/ER-MPH treatment in both trials. Few emotional adverse events (AEs) were reported.
Conclusion:
DR/ER-MPH treatment resulted in statistically significant improvements in EL to the level of non-ADHD peers as contextualized by T-scores.
Keywords: ADHD, methylphenidate, emotional lability
Introduction
ADHD, characterized by symptoms of inattention, hyperactivity, and impulsivity across multiple settings resulting in functional impairment (American Psychiatric Association, 2022), is a neurodevelopmental disorder estimated to affect 11% of children and 4.4% of adults in the United States; furthermore, approximately 90% of those diagnosed with ADHD in childhood go on to experience symptoms at some point during adulthood (Kessler et al., 2006; Sibley et al., 2022; Visser et al., 2014).
Emotional lability (EL) is defined as sudden changes in emotions and behaviors of inappropriately high intensity, and is associated with distress, impaired functioning, and lowered quality of life (A. C. Childress & Sallee, 2015). EL is common in children, youth, and adults with ADHD, with parent-reported prevalence rates ranging from 11% to 47% (Anastopoulos et al., 2011; Skirrow & Asherson, 2013; Stringaris & Goodman, 2009); despite the frequency of EL in individuals with ADHD, EL is not included in the DSM-5 diagnostic criteria used to diagnose ADHD (American Psychiatric Association, 2022). EL may also occur in individuals with other conditions such as oppositional defiant disorder (ODD) and autism spectrum disorder (ASD; Bennett et al., 2017 A. C. Childress & Sallee, 2015; A. C. Childress & Sallee, 2015).
Emotional terms including dysregulation and lability are often used interchangeably in different sources, with the majority of these terms sharing reference to excessive positive and negative emotional and behavioral responses (Carlson & Kelly, 1998; Faraone et al., 2019; Maedgen & Carlson, 2000). However, other sources are more careful to outline EL as a distinct type of emotional dysregulation characterized by rapid changes in emotional states (A. C. Childress & Sallee, 2015). Regardless, the severity of these emotional and behavioral changes has been observed to positively correlate with ADHD symptom severity, particularly in individuals who exhibit the hyperactive-impulsive sub-type of ADHD, impacting social, daily living, and adaptive skills (Anastopoulos et al., 2011; Sobanski et al., 2010).
Children with ADHD and emotional dysregulation are more likely to see their ADHD symptoms persist into adulthood and are overall less likely to experience symptom remission, even when they are treated (Biederman et al., 2012). This may translate into an overall lower quality of life with 44.7% of 585 surveyed individuals reporting that their symptoms had a negative impact on their overall wellbeing (Schein et al., 2023); individuals also have more difficulty with social adjustment and compromised marital status compared to those who have ADHD without emotional dysregulation (C. B. Surman et al., 2013). Additionally, ADHD combined with severe EL increases the likelihood of certain comorbidities, such as oppositional defiant, affective, and substance use disorders, which make identifying an appropriate treatment regime more challenging due to varying symptom profiles and complex medication regimens (Jensen et al., 2001; Sobanski et al., 2010). Beyond difficulties identifying appropriate treatment, detecting EL itself poses a unique challenge, with EL often being confused for stimulant treatment-related “rebound” which refers to the worsening of symptoms, including emotional dysregulation, beyond a baseline state because of medication wearing off (Barkley et al., 1990).
It has been proposed that the assessment and treatment of EL is essential for successful treatment outcomes in ADHD (A. C. Childress & Sallee, 2015), however the relationship between stimulant treatment and EL in individuals with ADHD remains unclear (Posner et al., 2014). Stimulant medications such as methylphenidate (MPH) are known to be effective treatments for ADHD (Biederman et al., 2003) and some studies have reported improvements in EL with stimulant treatment, including MPH (Baweja et al., 2021; A. C. Childress et al., 2014; López et al., 2017). These reports have been further demonstrated by several meta-analyses and reviews of clinical trials for stimulant and non-stimulant treatments (Lenzi et al., 2017; Moukhtarian et al., 2017; C. B. H. Surman & Walsh, 2022). However, other studies indicate that medications may blunt emotions and make individuals more irritable, with irritability contributing to the onset and worsening of EL; this can however be altered by changing a patient’s medication formulation (Ahmann et al., 1993; Gillberg et al., 1997; Kratochvil et al., 2007; Perwien et al., 2008; Posner et al., 2014). In order to ascertain the impact of treatment on EL, tools are required to aid in the measurement of EL severity at baseline and throughout the course of treatment. Rating scales such as the three-item Emotional Lability subscale of the Conners Global Index—Parent (CGI-P EL), a validated measure of emotional lability, have been developed to assess EL in individuals with ADHD, however despite their clinical utility, these scales are few and none have been adopted as a standard clinical practice (Conners, 1997; Faraone et al., 2019).
DR/ER-MPH (formerly HLD200; trade name: JORNAY PM® [Ironshore, Grand Cayman, Cayman Islands]) is an evening-dosed delayed-release and extended-release MPH indicated for the treatment of ADHD in individuals aged ≥6 years. EL was measured in two phase 3 clinical trials of DR/ER MPH using the CGI-P EL subscale. Herein, we describe the findings of these two randomized, double-blind, phase 3 trials of DR/ER-MPH treatment in children with ADHD (HLD200-107 [NCT02493777] and HLD200-108 [NCT02520388]) to evaluate the effect of treatment on EL.
Methods
Participants
Details for both study HLD200-107 and HLD200-108 have been previously described in detail (A. C. Childress et al., 2020; Pliszka et al., 2017). Briefly, children (aged 6–12 years) were enrolled in both trials if they met the defined study inclusion and exclusion criteria. Key inclusion criteria included: (i) diagnosis of ADHD based on the Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition; (ii) baseline ADHD Rating Scale-IV (ADHD-RS-IV) total score of ≥26 at baseline, and baseline ADHD-RS-IV score ≥90th percentile normalized for sex and age in total score for HLD200-108 and in at least one of the following categories: inattentive, hyperactive-impulsive, or total score for HLD200-107; (iii) Clinical Global Impression of Severity (CGI-S) score ≥4 and Conners’ Global Index—Parent (CGI-P) score >10 at baseline; (iv) at least partial clinical response to MPH by investigator judgment (HLD200-108 and HLD200-107) or treatment with the same dose of MPH and clinical response with acceptable tolerability to MPH for ≥2 weeks prior to screening (HLD200-107); and (v) parent/guardian confirmation of before-school functional impairment and difficulties performing morning routine (with regular weekday morning routine of ≥30 minutes). Key exclusion criteria included: (i) history of current medical condition or laboratory result that could interfere with study participation, participant safety, or satisfactory completion of the study; (ii) any cardiac problems that may place the participant at increased vulnerability to the sympathomimetic effects of a stimulant drug; (iii) history of psychosis, bipolar disorder, anorexia nervosa, bulimia, or suicide attempt; and (iv) current depression, anxiety, conduct disorder, substance use disorder, or other psychiatric condition. ODD was not an exclusion criterion in either study.
Clinical Trial Study Designs
HLD200-107 was a parallel-group, laboratory classroom study of DR/ER-MPH in children with ADHD which was conducted in three distinct phases: A screening/washout phase lasting up to 4 weeks, an open-label, treatment-optimization phase lasting 6 weeks and a 1 week double-blind, randomized, placebo-controlled, parallel-group phase ending with a laboratory classroom test day. During the 6-week treatment-optimization phase, participants were initiated on either 20 or 40 mg at the discretion of the investigator, dependent on treatment history. Dose titrations were permitted in 20- or 40-mg increments or decrements until an optimal daily dose was achieved or a maximum daily dose of 100 or 3.7 mg/kg was reached (A. C. Childress et al., 2020). HLD200-108 was a parallel-group, forced-dose titration trial of DR/ER-MPH in children with ADHD conducted in two phases: a screening/washout phase lasting up to 2 weeks followed by a 3 weeks, randomized, placebo-controlled test phase. Dosing was initiated at 40 mg/day for 1 week, with scheduled titration, as tolerated, over the subsequent 2 weeks to 60 mg/day (Week 2) and 80 mg/day (Week 3). The maximum allowable dose was 3.7 mg/kg and one down-titration step (ie, 20-mg decrement) was permitted for safety or tolerability reasons (Pliszka et al., 2017). In both studies, evening-dosed DR/ER-MPH was initiated at 8:00 PM ±30 minutes, with weekly adjustments between 6:30 and 9:30 PM permitted in 30- or 60-minute increments in order to optimize treatment (A. C. Childress et al., 2020; Pliszka et al., 2017).
EL Assessments
The CGI-P is a parent/caregiver-completed assessment of ADHD symptom control with two subscales: the Restless-Impulsive subscale (seven items) and the EL subscale (three items; Conners, 1997). The three items which comprise the EL subscale are: Temper outbursts, Cries often and easily, and Mood changes quickly and drastically, which correspond to the definition of EL as a distinct type of emotional dysregulation characterized by rapid changes in emotional states (A. C. Childress & Sallee, 2015). Each CGI-P item is rated from 0 (never/seldom) to 3 (very frequently). Possible CGI-P EL subscale scores range from 0 (least severe) to 9 (most severe). CGI-P scores were assessed at the following time points: baseline and at each weekly visit during the open-label, treatment-optimization phase (Weeks 1–6) in HLD200-107 (CGI-P was used as a criterion to optimize dosage during this phase); screening, baseline, and endpoint (Week 3) in HLD200-108 (CGI-P at Week 3 was pre-specified as an exploratory endpoint). CGI-P was not rated over any specific timeframe in either study; however, it would be expected that the scale likely reflects a home setting during the early mornings and evenings, as those are the times when parents/caregivers are most likely to observe their children.
Safety
Safety and tolerability have previously been reported in detail (A. C. Childress et al., 2020; Pliszka et al., 2017). The frequency of emotion-related treatment-emergent adverse events (TEAEs) as reported in response to a general query was assessed; these included affect lability, aggression, agitation, apathy, dysphoria, emotional poverty, and irritability.
Analyses
In HLD200-107, mean scores at baseline and at each Week in the 6-week, open-label, treatment-optimization phase were calculated for the CGI-P EL subscale. A paired t-test was used to determine significance of the difference between baseline and visit scores. In HLD200-108, least squares (LS) mean scores at baseline and at the study endpoint (Week 3) were calculated in the DR/ER-MPH and placebo groups for the CGI-P EL subscale. An ANCOVA model was used to determine significance of the difference between the DR/ER-MPH and placebo group scores, with treatment as the main effect and study center and baseline score as covariates. Change score distributions were also determined in both studies.
Raw CGI-P EL subscale scores for each participant were also converted to age- and gender-adjusted T-scores to assess severity compared to a representative US sample for that age and gender (Conners, 1997). All T-scores have a mean of 50 (typical for age and gender) and a standard deviation of 10. T-scores falling below 60 are considered Average, the lowest severity achievable per the Conners’ manual, and generally suggest typical or absent concerns for a child’s age and gender. T-scores between 60 and 64 are considered High Average, indicating slightly more concerns than typical. T-scores between 65 and 69 are considered Elevated scores (more concerns than typically reported). T-scores of ≥70 are considered Very Elevated and denote many more concerns than typically reported.
Pairwise changes from baseline to Week 3 in HLD200-108 and from baseline to Week 6 of the open-label phase of HLD200-107 were plotted for each participant that had completed assessments at both timepoints. Of participants who reported T-scores ≥60 at baseline (indicating greater than Average levels of concern), the proportion achieving T-scores <60 (indicating Average levels of concern) was calculated. The proportion of participants with EL concerns at baseline (ie, ≥High Average) who achieved Average scores at endpoint were determined. Treatment group differences were determined using the Fisher Exact Test. No imputation for missing values was performed.
Results
Participant Disposition and Characteristics
A total of 125 children were enrolled (enrolled safety population) in HLD200-107, 119 were randomized to either DR/ER-MPH or placebo (randomized safety population), and 117 were included in the efficacy analysis (intent-to-treat [ITT] population; DR/ER-MPH, n = 64; placebo, n = 53). The enrolled safety population was 68% male with a mean age of 9.4 years. At baseline 42% of participants had a CGI-S score of 5 (markedly ill). At the end of the 6-week, open-label, treatment-optimization phase, the mean optimized dose of DR/ER-MPH was 66.2 mg and the most common optimized administration time (64.1%) was 8:00 PM (A. C. Childress et al., 2020). A psychiatric history of ODD was reported in 15% of the population.
In HLD200-108, 163 children were enrolled and 161 were included in the safety and ITT populations (DR/ER-MPH, n = 81; placebo, n = 80). The safety/ITT population was 70% male, with a mean age of 9.3 years, and a baseline CGI-S score of 5 (markedly ill) in 66% of participants. After 3 weeks of treatment, mean DR/ER-MPH dose was 68.1 mg and the most common administration time (84%) was 8:00 PM (Pliszka et al., 2017). A psychiatric history of ODD was reported in 13% of the population.
CGI-P EL Subscale Scores
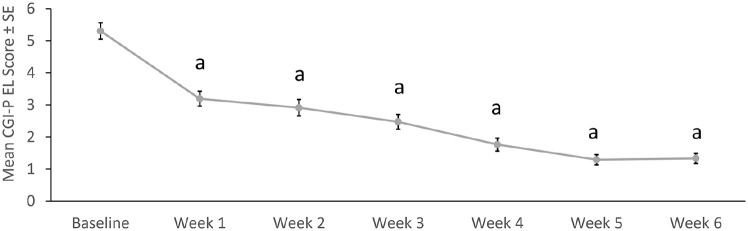
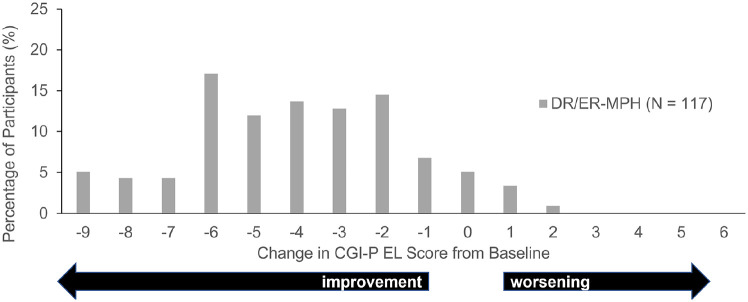
Mean CGI-P EL subscale scores during HLD200-107 improved over the open-label, treatment-optimization phase, from 5.3 at baseline to 1.3 at Week 6 (p < .0001 for each Week vs. baseline; Figure 1). A mean score of 1.3 indicates that only one item on the CGI-P EL would be rated as 1 = happened occasionally. The distribution of change scores demonstrated that almost all participants showed an improvement from baseline to Week 6 (Figure 2).
Figure 1.
Mean CGI-P EL scores in the open-label, treatment-optimization phase of HLD200-107.
Note. CGI-P = Conners’ Global Index—Parent; EL = emotional lability; SE = standard error.
ap < .0001 versus baseline.
Figure 2.
CGI-P EL score change from baseline to week 6 in HLD200-107.
Note. CGI-P = Conners’ Global Index—Parent; DR/ER-MPH = delayed-release and extended-release methylphenidate; EL = emotional lability.
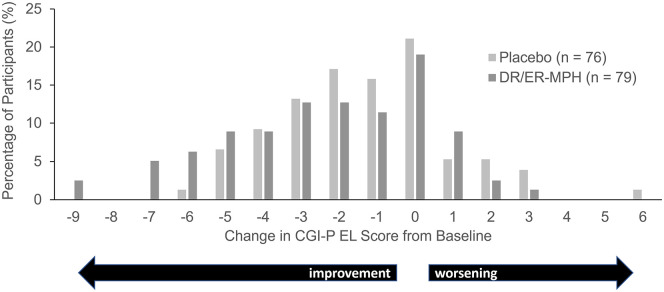
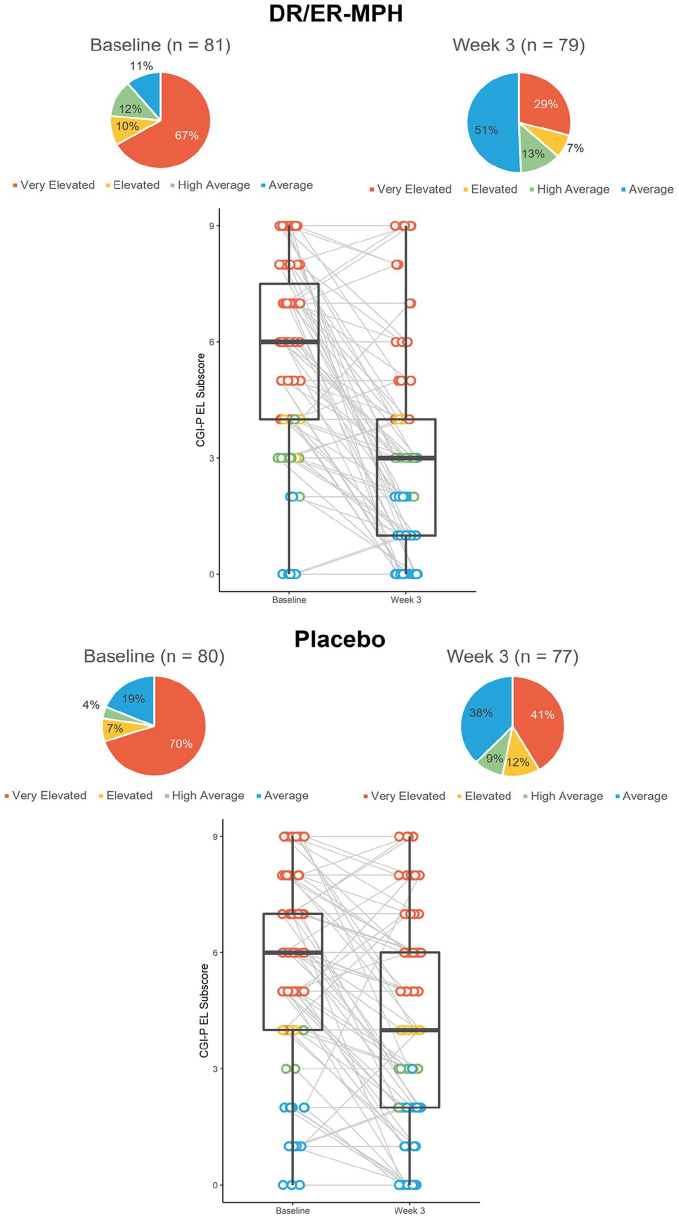
Baseline CGI-P EL subscale scores for HLD200-108 were similar between the placebo and DR/ER-MPH groups (5.3 and 5.5, respectively; Figure 3). LS mean CGI-P EL subscale scores were significantly improved in the DR/ER-MPH group versus placebo after 3 weeks of treatment (p = .0053; Figure 3). Figure 4 demonstrates a shift in the distribution of change scores, such that more participants in the DR/ER-MPH group achieved larger improvements in CGI-P EL scores compared with those on placebo.
Figure 3.
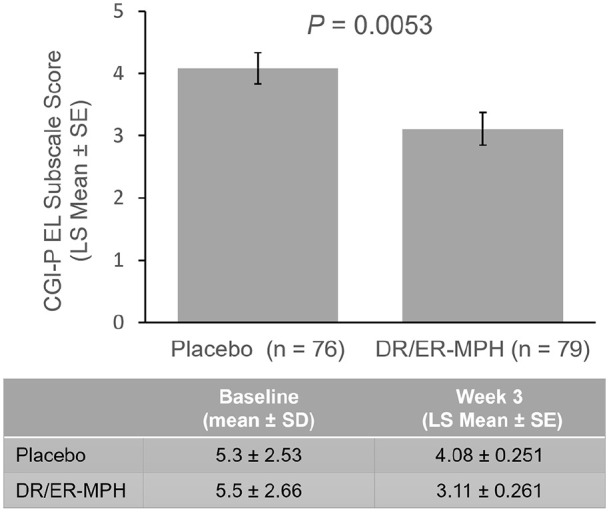
Mean CGI-P EL scores in HLD200-108 at endpoint (week 3).
Note. CGI-P = Conners’ Global Index—Parent; DR/ER-MPH = delayed-release and extended-release methylphenidate; EL = emotional lability; LS = least squares; SD = standard deviation; SE = standard error.
Figure 4.
CGI-P EL score change from baseline to week 3 in HLD200-108.
Note. CGI-P = Conners’ Global Index—Parent; DR/ER-MPH = delayed-release and extended-release methylphenidate; EL = emotional lability.
CGI-P EL T-Scores
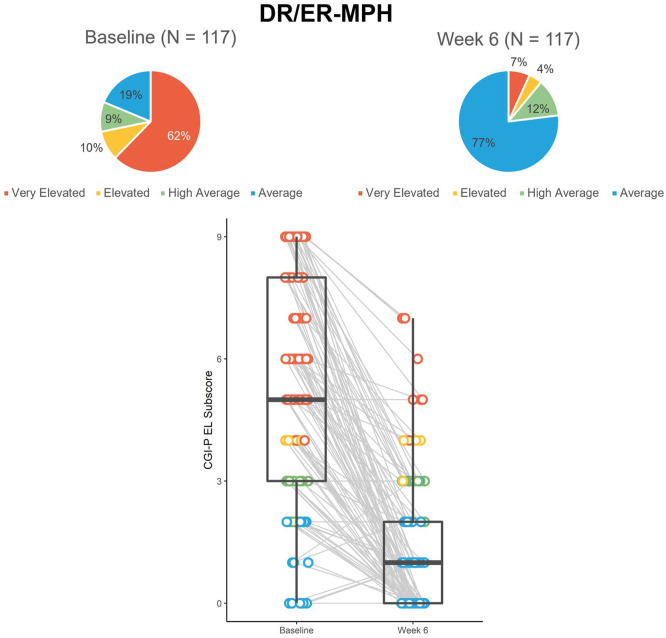
At baseline in HLD200-107, the majority of participants (62%) had Very Elevated EL scores with only 19% of participants considered to have Average (typical levels of concern) EL scores (Figure 5). Of the 95 participants who had EL scores of concern (≥High Average) at baseline, 73% achieved Average scores by the end of the 6-week, open-label, treatment-optimization phase.
Figure 5.
Proportion and distribution of CGI-P EL scores color-coded by severity from the open-label phase of study HLD200-107.a
Note. CGI-P = Conners’ Global Index—Parent; DR/ER-MPH = delayedrelease and extended-release methylphenidate; EL = emotional lability.
aBox-and-whisker plots show the distribution of the dataset. The box shows the median (middle of the data points), upper quartile (25% of data points are greater than this value), and lower quartile (25% of data points are lower than this value). The tails show the minimum and maximum values.
In HLD200-108, 67% and 70% reported Very Elevated EL scores in the DR/ER-MPH and placebo groups, respectively at baseline; only 11% and 19% of DR/ER-MPH and placebo groups had Average EL scores (Figure 6). Among the 70 DR/ER-MPH and 63 placebo participants with EL concerns at baseline (ie, ≥High Average), a significantly greater proportion had achieved Average levels of concern after 3 weeks of treatment with DR/ER-MPH versus placebo, respectively (44% vs. 27%, p = .04).
Figure 6.
Proportion and distribution of CGI-P EL scores color-coded by severity from study HLD200-108.a
Note. CGI-P = Conners’ Global Index—Parent; DR/ER-MPH = delayed-release and extended-release methylphenidate; EL = emotional lability.
aBox-and-whisker plots show the distribution of the dataset. The box shows the median (middle of the data points), upper quartile (25% of data points are greater than this value), and lower quartile (25% of data points are lower than this value). The tails show the minimum and maximum values.
Safety
Of participants reporting TEAEs with DR/ER-MPH, most had described their maximum severity as mild or moderate (95% in HLD200-108 and 94% and 100% in HLD200-107 open-label and double-blind phases, respectively) and the types of TEAEs reported were similar to those seen with other methylphenidates (A. C. Childress et al., 2020; Pliszka et al., 2017).
During the open-label phase of HLD200-107, 37 participants reported 41 emotion-related TEAEs (9 of whom had an emotion-related condition in their medical history), with 24 instances being mild and 17 moderate; 1 participant randomized to the placebo group reported 1 moderate emotion-related TEAE during the double-blind phase and no participants randomized to DR/ER-MPH reported emotion-related TEAEs during the double-blind phase. Two participants discontinued due to EL-related TEAEs during the open-label phase; one as a result of affect lability and one as a result of agitation/aggression.
In HLD200-108, eight participants reported ten emotion-related TEAEs in the DR/ER-MPH group compared to five participants reporting six emotion-related TEAEs in the placebo group (three in each group had an EL-related condition in their medical history). The range of severities of emotion-related TEAEs were similar between the DR/ER-MPH (three mild, five moderate, and two severe) and placebo (five moderate and one severe) groups, and the number of discontinuations due to EL-associated TEAEs were also similar (two in the placebo group as a result of irritability and one in the DR/ER-MPH group as a result of mood swings).
Discussion
The relationship between stimulant treatment and EL is unclear because of significant inter-patient variability in EL in response to stimulant treatment, which makes clinical decision making difficult when EL is present; many studies imply a beneficial effect of stimulant treatment on EL while some patients experience increased irritability as a result of treatment which may induce or worsen EL (Posner et al., 2014). Negative emotion-related side effects of stimulant medications such as worsened EL has been cited in several studies as the main cause of treatment cessation (Brinkman et al., 2012; Findling et al., 2011; Meaux et al., 2006), therefore understanding the impact of a medication on EL prior to starting a new regimen is important. Despite literature describing the negative impact of stimulants on emotional symptoms, several meta-analyses and reviews assessing the results of double-blind randomized control trials describe positive emotional outcomes for treatments such as atomoxetine, lisdexamfetamine, methylphenidate transdermal system, and OROS-MPH versus placebo in adults with ADHD (Lenzi et al., 2017; Moukhtarian et al., 2017; C. B. H. Surman & Walsh, 2022); while the majority of these studies target adult ADHD populations, several studies also indicate a positive impact of stimulant and non-stimulant treatment on emotional symptoms in children and adolescents (Kutlu et al., 2017; López et al., 2017; Shih et al., 2019). Consistent with these findings, this post hoc analysis investigating two randomized, double-blind, phase 3 trials of DR/ER-MPH treatment, demonstrated a statistically significant improvement in EL versus placebo in children with ADHD, alongside a relatively low incidence of emotion-related TEAEs reported and few discontinuations. This shows a consistent effect of DR/ER-MPH despite different study designs and small differences in patient characteristics.
To better contextualize these findings, raw CGI-P EL scores were converted to age- and gender-adjusted T-scores, which place individual impairment ratings within groups from Average up to Very Elevated scores. In both studies, at baseline the majority of participants had Very Elevated T-scores (the most severe rating), emphasizing the frequency and severity of EL in untreated individuals with ADHD in these studies; the high incidence of severe EL observed in the untreated study population here may be linked to the inclusion requirements including parental/caregiver confirmation of their child’s early morning impairment and an ADHD-RS-IV severity ≥90th percentile at baseline for age and sex.
Of HLD200-107 participants who reported T-scores of concern (≥High Average) at baseline, the majority achieved Average scores by the end of the 6-week open-label treatment optimization phase; while in HLD200-108 significantly more participants in the treatment group had achieved Average levels of EL than placebo by Week 3. With EL known to aggravate ADHD and impair various aspects of life quality, these studies show that DR/ER-MPH treatment can alleviate these daily challenges by restoring EL to the level of non-ADHD peers (Anastopoulos et al., 2011; A. C. Childress & Sallee, 2015; Jensen et al., 2001). Furthermore, alongside the improvements in EL with DR/ER-MPH treatment, few participants also experienced emotion-related TEAEs in both studies, indicating that DR/ER-MPH treatment does not exacerbate EL symptoms in this population. While the majority of participants experienced improved EL in response to DR/ER-MPH, a small proportion did not, highlighting the clinical utility of using multiple therapeutic approaches, with combination therapies such as concurrent psychotherapy and MPH treatment also shown to improve EL (Faraone et al., 2019).
The importance of targeting EL when treating ADHD is clear. In order to do so, it has been recommended that healthcare practitioners (HCPs) utilize an appropriate scale to detect EL (A. C. Childress & Sallee, 2015). However, definitive methodologies for assessing EL changes in practice and clinical trials are as yet unestablished (Manos et al., 2011); indeed, while routine scales used in pediatric practice to assess ADHD may include some degree of emotional assessment, they lack validated subscales to determine EL. The CGI-P EL subscale is one of few validated scales developed to assess EL (Faraone et al., 2019) and offers a simple yet comprehensive three-item measurement of EL severity. In the present study, improvements in CGI-P EL scores increased with treatment optimization over time, with greater improvements seen in the 6-week open-label phase of HLD200-107 compared to the 3-week forced-titration design of HLD200-108. This finding suggests that the CGI-P EL subscale may be an effective tool for HCPs to monitor changes in EL while titrating their patients to an optimal dose. Further to this, the application of T-scores to raw CGI-P EL data can also provide useful clinical insight as to the level of EL relative to non-ADHD peers, improving the scale’s clinical utility. This insight can be particularly useful when presented as individual datapoints, emphasizing here that the overall effect of DR/ER-MPH treatment on mean EL improvements was not driven by a small subset of participants in either study as shown in Figures 5 and 6.
Those with ADHD have symptoms upon awakening and throughout the entire day into the evening (Harpin, 2005). These symptoms vary from individual to individual, but may include EL (A. C. Childress & Sallee, 2015). DR/ER-MPH has a smooth pharmacokinetic profile whereby the active ingredient is biologically available upon awakening and throughout the entire day, with a prolonged elimination phase such that >50% of MPH absorption occurs after peak plasma concentrations are reached (A. Childress et al., 2018). Studies with DR/ER-MPH have shown significant improvements in ADHD functional impairment in both the morning and evening using validated rating scales such as the Parent Rating of Evening and Morning Behavior Scale—Revised (A. C. Childress et al., 2020; Pliszka et al., 2017). Mornings provide a particularly hectic challenge for those with ADHD where many tasks must be completed in a timely manner during a typically untreated portion of the day. Most morning-dosed stimulant medications having a lag time between administration and effect, which results in mornings being more difficult for the individual with ADHD but also for their caregivers/families (A. C. Childress et al., 2023). Individuals treated with morning-dosed stimulants report EL symptoms in the mornings, including frequent temper tantrums and sudden changes to their mood and feelings (Sallee, 2015). Exacerbation of morning symptoms has been observed in classroom studies of morning-administered stimulants, where treated children have worse behavior than placebo-treated children in the early morning before their medication is administered (Swanson et al., 2004). As DR/ER-MPH is evening-dosed, there is no lag time between awakening and onset of effect, which reduces overall ADHD functional impairment in the early morning (A. C. Childress et al., 2020; Pliszka et al., 2017). There were no time-specific measurements of EL in the present study, however earlier studies indicate morning to evening improvements in overall ADHD symptoms and functional impairment with DR/ER-MPH treatment. This may imply that the extended exposure window of DR/ER-MPH due to colonic absorption alongside availability upon awakening could provide relief of EL symptoms in the morning, throughout the day, and evening.
Limitations
While the benefit of DR/ER-MPH treatment on EL was clearly outlined in this analysis, the beneficial effect compared with other stimulants remains to be tested; furthermore investigating the impact of DR/ER-MPH treatment on other population groups including adults, those without prior MPH experience, and those with comorbid conditions such as ASD would prove beneficial. Additionally, while the CGI-P EL subscale provides an effective measure of EL, not all HCPs are able to access copyrighted material in practice; in such instances, alternative non-proprietary scales may be utilized to assess EL. When treating adults, the Wender-Reimherr Adult Attention Deficit Disorder Scale (Marchant et al., 2013) may be appropriate; for children and adolescents, while not validated in an ADHD population, the Emotional Dysregulation Inventory (Mazefsky et al., 2018) provides a useful measure of EL, underpinned by population norms (Mazefsky et al., 2021). Understanding EL improvements throughout the course of the day at different timepoints could also provide further insight into the therapeutic benefits of treatment and risks for exacerbation of EL. Further work should apply measurements in the mornings, afternoon, and evenings as an all-day therapeutic effect should be the goal.
Conclusions
In a post hoc analysis, DR/ER-MPH treatment improved mean parent-rated CGI-P EL subscale scores in two phase 3 trials of children (6–12 years) with ADHD. As the scale is parent-rated, EL is being measured at times of day when parents are interacting with their children; thus, the observed improvements in EL could also lead to an improved home environment (eg, improved parent-child and/or family relationships). These scores were contextualized by applying T-score severity thresholds. The proportion of participants who achieved T-scores indicating Average EL severity, the lowest achievable threshold, increased with longer duration of treatment; this suggests that persevering with treatment soon after prescribing in the face of lower efficacy or even tolerable TEAEs may be an effective course of action for the longer-term treatment of EL. It has been proposed that the assessment and treatment of EL is essential for successful treatment outcomes in ADHD; this post hoc analysis demonstrated that reduction of EL to the level of non-ADHD peers is an achievable target when individuals with ADHD are appropriately dosed with DR/ER-MPH.
Author Biographies
Valerie K. Arnold, MD, DFAPA, DFAACAP, ACPsych, is the Chief of Child and Adolescent Psychiatry at the University of Tennessee Health Science Center.
Frank A. López, MD, is a Neurodevelopmental Pediatrician currently practicing at the Pediatrix Neurology and Epilepsy Research Center in Winter Park, FL.
Ann C. Childress, MD, is a Psychiatrist and President at the Center for Psychiatry and Behavioral Medicine. She also has adjunct faculty appointments at the University of Nevada Las Vegas School of Medicine and Touro University of Nevada College of Medicine and is a past president of the American Professional Society for ADHD and Related Disorders.
Michelle D. Po, PhD, is the AVP of Global Medical Communications at Ironshore.
Cassandra L. Uchida, PhD, is a Senior Medical Writer at Ironshore.
Lewis Cuthbertson, PhD, is a Medical Writer at Ironshore.
Floyd R. Sallee, MD, PhD, is the Vice President of Medical Affairs at Ironshore.
Bev Incledon, PhD, is the Chief Scientific Officer at Ironshore.
Footnotes
The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: VKA: Advisory Board/Consultant—Ironshore, Neos, Rho, Shire; Speakers Bureau—Ironshore Pharmaceuticals Inc., Lundbeck/Takeda. FAL: Consultant, Speaker, and/or Research Support—Cingulate, Corium, Eli Lilly, GSK, Ironshore, Neos, Novartis, Noven, Pfizer, Rhodes, Shionogi, Shire, Sunovion, Supernus, Tris. ACC: Research Support—Acadia, Akili Interactive Labs, Allergan, Arbor Pharmaceuticals, LLC, Axial, Cingulate, Corium, Emalex, Forest Laboratories, Ironshore, KemPharm, Inc., Lumos, Neos Therapeutics, Neurocentria, Otsuka America Pharmaceutical, Inc., Purdue Pharma, Rhodes Pharmaceuticals, Servier, Shire, Sunovion Pharmaceuticals, Inc., Supernus Pharmaceuticals, Inc., Takeda Pharmaceutical Company Ltd., Tris Pharma, Advisory Board—Adlon, Akili Interactive Labs, Arbor Pharmaceuticals, LLC, Cingulate Therapeutics, Otsuka America Pharmaceutical, Inc., Pfizer, Purdue Pharma, Shire, Sunovion Pharmaceuticals, Inc., Supernus Pharmaceuticals, Inc., Takeda Pharmaceutical Company Ltd., Tris Pharma; Consultant—Arbor Pharmaceuticals, LLC, Aytu, Attentive Therapeutics, Corium, Ironshore, KemPharm, Inc., Lumos, Neos Therapeutics, Neurocentria, ., Purdue Pharma, Sunovion Pharmaceuticals, Inc., Supernus Pharmaceuticals, Inc., Tris Pharma; Speakers Bureau—Ironshore, Takeda Pharmaceutical Company Ltd., Tris Pharma; Writing Support—Arbor Pharmaceuticals, LLC, Ironshore, Neos Therapeutics, Pfizer, Purdue Pharma, Rhodes Pharmaceuticals, Shire, Sunovion Pharmaceuticals, Inc., Takeda Pharmaceutical Company Ltd., Tris Pharma. MDP, CLU, LC, and BI: Employees—Ironshore. FRS: Employee—Ironshore; Advisory Board/Board of Directors—P2D Bioscience.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This research was funded by Ironshore.
ORCID iDs: Michelle D. Po  https://orcid.org/0000-0002-6808-6416
https://orcid.org/0000-0002-6808-6416
Cassandra L. Uchida  https://orcid.org/0000-0002-6537-4092
https://orcid.org/0000-0002-6537-4092
Lewis Cuthbertson  https://orcid.org/0000-0001-6077-5615
https://orcid.org/0000-0001-6077-5615
References
- Ahmann P. A., Waltonen S. J., Olson K. A., Theye F. W., Van Erem A. J., LaPlant R. J. (1993). Placebo-controlled evaluation of Ritalin side effects. Pediatrics, 91(6), 1101–1106. 10.1542/peds.91.6.1101 [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association. (2022). Diagnostic and statistical manual of mental disorders (5th ed., text rev.). 10.1176/appi.books.9780890425787 [DOI]
- Anastopoulos A. D., Smith T. F., Garrett M. E., Morrissey-Kane E., Schatz N. K., Sommer J. L., Kollins S. H., Ashley-Koch A. (2011). Self-regulation of emotion, functional impairment, and comorbidity among children with AD/HD. Journal of Attention Disorders, 15(7), 583–592. 10.1177/1087054710370567 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barkley R. A., McMurray M. B., Edelbrock C. S., Robbins K. (1990). Side effects of methylphenidate in children with attention deficit hyperactivity disorder: A systemic, placebo-controlled evaluation. Pediatrics, 86(2), 184–192. 10.1542/peds.86.2.184 [DOI] [PubMed] [Google Scholar]
- Baweja R., Waschbusch D. A., Pelham W. E., 3rd, Pelham W. E., Jr, Waxmonsky J. G. (2021). The impact of persistent irritability on the medication treatment of paediatric attention deficit hyperactivity disorder. Frontiers in Psychiatry, 12, 699687. 10.3389/fpsyt.2021.699687 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett R. H., Somandepalli K., Roy A. K., Di Martino A. (2017). The neural correlates of emotional lability in children with autism spectrum disorder. Brain Connectivity, 7(5), 281–288. 10.1089/brain.2016.0472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biederman J., Quinn D., Weiss M., Markabi S., Weidenman M., Edson K., Karlsson G., Pohlmann H., Wigal S. (2003). Efficacy and safety of Ritalin LA, a new, once daily, extended-release dosage form of methylphenidate, in children with attention deficit hyperactivity disorder. Paediatric Drugs, 5(12), 833–841. 10.2165/00148581-200305120-00006 [DOI] [PubMed] [Google Scholar]
- Biederman J., Spencer T. J., Petty C., Hyder L. L., O’Connor K. B., Surman C. B., Faraone S. V. (2012). Longitudinal course of deficient emotional self-regulation CBCL profile in youth with ADHD: Prospective controlled study. Neuropsychiatry Disease and Treatment, 8, 267–276. 10.2147/NDT.S29670 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brinkman W. B., Sherman S. N., Zmitrovich A. R., Visscher M. O., Crosby L. E., Phelan K. J., Donovan E. F. (2012). In their own words: Adolescent views on ADHD and their evolving role managing medication. Academic Pediatrics, 12(1), 53–61. 10.1016/j.acap.2011.10.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlson G. A., Kelly K. L. (1998). Manic symptoms in psychiatrically hospitalized children–what do they mean? Journal of Affective Disorders, 51(2), 123–135. 10.1016/s0165-0327(98)00211-0 [DOI] [PubMed] [Google Scholar]
- Childress A. C., Arnold V., Adeyi B., Dirks B., Babcock T., Scheckner B., Lasser R., Lopez F. A. (2014). The effects of lisdexamfetamine dimesylate on emotional lability in children 6 to 12 years of age with ADHD in a double-blind placebo-controlled trial. Journal of Attention Disorders, 18(2), 123–132. 10.1177/1087054712448252 [DOI] [PubMed] [Google Scholar]
- Childress A. C., Sallee F. R. (2015). Emotional lability in patients with attention-deficit/hyperactivity disorder: Impact of pharmacotherapy. CNS Drugs, 29(8), 683–693. 10.1007/s40263-015-0264-9 [DOI] [PubMed] [Google Scholar]
- Childress A. C., Cutler A. J., Marraffino A., McDonnell M. A., Turnbow J. M., Brams M., DeSousa N. J., Incledon B., Sallee F. R., Wigal S. B. (2020). A randomized, double-blind, placebo-controlled study of HLD200, a delayed-release and extended-release methylphenidate, in children with attention-deficit/hyperactivity disorder: An evaluation of safety and efficacy throughout the day and across settings. Journal of Child and Adolescent Psychopharmacology, 30(1), 2–14. 10.1089/cap.2019.0070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Childress A. C., Yu K. R., Cuthbertson L. (2023). Early morning ADHD symptoms and functional impairment: Impact on patients and caregivers, and pharmacological approaches to management. CNS Drugs, 37(1), 31–44. 10.1007/s40263-022-00978-2 [DOI] [PubMed] [Google Scholar]
- Childress A., Mehrotra S., Gobburu J., McLean A., DeSousa N. J., Incledon B. (2018). Single-dose pharmacokinetics of HLD200, a delayed-release and extended-release methylphenidate formulation, in healthy adults and in adolescents and children with attention-deficit/hyperactivity disorder. Journal of Child and Adolescent Psychopharmacology, 28(1), 10–18. 10.1089/cap.2017.0044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conners C. K. (1997). Conners’ rating scales-revised technical manual. Multi-Health Systems. [Google Scholar]
- Faraone S. V., Rostain A. L., Blader J., Busch B., Childress A. C., Connor D. F., Newcorn J. H. (2019). Practitioner review: Emotional dysregulation in attention-deficit/hyperactivity disorder - implications for clinical recognition and intervention. The Journal of Child Psychology and Psychiatry, 60(2), 133–150. 10.1111/jcpp.12899 [DOI] [PubMed] [Google Scholar]
- Findling R. L., Brams M., Childress A. C., López F. A., Manos M. J., Jensen P. S. (2011). Changes in emotions related to medication used to treat ADHD. Part II: Clinical approaches. Journal of Attention Disorders, 15(2), 113–121. 10.1177/1087054710381232 [DOI] [PubMed] [Google Scholar]
- Gillberg C., Melander H., von Knorring A. L., Janols L. O., Thernlund G., Hägglöf B., Eidevall-Wallin L., Gustafsson P., Kopp S. (1997). Long-term stimulant treatment of children with attention-deficit hyperactivity disorder symptoms. A randomized, double-blind, placebo-controlled trial. Archives of General Psychiatry, 54(9), 857–864. 10.1001/archpsyc.1997.01830210105014 [DOI] [PubMed] [Google Scholar]
- Harpin V. A. (2005). The effect of ADHD on the life of an individual, their family, and community from preschool to adult life. Archives of Disease in Childhood, 90 Suppl 1(1), i2–i7. 10.1136/adc.2004.059006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen P. S., Hinshaw S. P., Kraemer H. C., Lenora N., Newcorn J. H., Abikoff H. B., March J. S., Arnold L. E., Cantwell D. P., Conners C. K., Elliott G. R., Greenhill L. L., Hechtman L., Hoza B., Pelham W. E., Severe J. B., Swanson J. M., Wells K. C., Wigal T., Vitiello B. (2001). ADHD comorbidity findings from the MTA study: Comparing comorbid subgroups. Journal of the American Academy of Child and Adolescent Psychiatry, 40(2), 147–158. 10.1097/00004583-200102000-00009 [DOI] [PubMed] [Google Scholar]
- Kessler R. C., Adler L., Barkley R., Biederman J., Conners C. K., Demler O., Faraone S. V., Greenhill L. L., Howes M. J., Secnik K., Spencer T., Ustun T. B., Walters E. E., Zaslavsky A. M. (2006). The prevalence and correlates of adult ADHD in the United States: Results from the National Comorbidity Survey Replication. The American Journal of Psychiatry, 163(4), 716–723. 10.1176/ajp.2006.163.4.716 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kratochvil C. J., Faries D., Vaughan B., Perwien A., Busner J., Saylor K., Kaplan S., Buermeyer C., Swindle R. (2007). Emotional expression during attention-deficit/hyperactivity disorders treatment: Initial assessment of treatment effects. Journal of Child and Adolescent Psychopharmacology, 17(1), 51–62. 10.1089/cap.2006.0018 [DOI] [PubMed] [Google Scholar]
- Kutlu A., Akyol Ardic U., Ercan E. S. (2017). Effect of Methylphenidate on Emotional Dysregulation in Children With Attention-Deficit/Hyperactivity Disorder + Oppositional Defiant Disorder/Conduct Disorder. Journal of clinical psychopharmacology, 37(2), 220–225. 10.1097/JCP.0000000000000668 [DOI] [PubMed] [Google Scholar]
- Lenzi F., Cortese S., Harris J., Masi G. (2018). Pharmacotherapy of emotional dysregulation in adults with ADHD: A systematic review and meta-analysis. Neuroscience and Biobehavioral Reviews, 84, 359–367. 10.1016/j.neubiorev.2017.08.010 [DOI] [PubMed] [Google Scholar]
- López F. A., Childress A., Adeyi B., Dirks B., Babcock T., Scheckner B., Lasser R. A., Shepski J., Arnold V. (2017). ADHD symptom rebound and emotional lability with lisdexamfetamine dimesylate in children aged 6 to 12 years. Journal of Attention Disorders, 21(1), 52–61. 10.1177/1087054712474685 [DOI] [PubMed] [Google Scholar]
- Maedgen J. W., Carlson C. L. (2000). Social functioning and emotional regulation in the attention deficit hyperactivity disorder subtypes. Journal of Clinical Child Psychology, 29(1), 30–42. 10.1207/S15374424jccp2901_4 [DOI] [PubMed] [Google Scholar]
- Manos M. J., Brams M., Childress A. C., Findling R. L., López F. A., Jensen P. S. (2011). Changes in emotions related to medication used to treat ADHD. Part I: Literature review. Journal of Attention Disorders, 15(2), 101–112. 10.1177/1087054710381230 [DOI] [PubMed] [Google Scholar]
- Marchant B. K., Reimherr F. W., Robison D., Robison R. J., Wender P. H. (2013). Psychometric properties of the Wender-Reimherr Adult Attention Deficit Disorder Scale. Psychological assessment, 25(3), 942–950. 10.1037/a0032797 [DOI] [PubMed] [Google Scholar]
- Mazefsky C. A., Day T. N., Siegel M., White S. W., Yu L., Pilkonis P. A., & Autism and Developmental Disabilities Inpatient Research Collaborative. (2018). Development of the emotion dysregulation inventory: A PROMIS®ing method for creating sensitive and unbiased questionnaires for autism spectrum disorder. Journal of Autism and Developmental Disorders, 48(11), 3736–3746. 10.1007/s10803-016-2907-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazefsky C. A., Yu L., Pilkonis P. A. (2021). Psychometric properties of the emotion dysregulation inventory in a nationally representative sample of youth. Journal of Clinical Child and Adolescent Psychology, 50(5), 596–608. 10.1080/15374416.2019.1703710 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meaux J. B., Hester C., Smith B., Shoptaw A. (2006). Stimulant medications: A trade-off? The lived experience of adolescents with ADHD. Journal for Specialists in Pediatric Nursing: JSPN, 11(4), 214–226. 10.1111/j.1744-6155.2006.00063.x [DOI] [PubMed] [Google Scholar]
- Moukhtarian T. R., Cooper R. E., Vassos E., Moran P., Asherson P. (2017). Effects of stimulants and atomoxetine on emotional lability in adults: A systematic review and meta-analysis. European Psychiatry: The Journal of the Association of European Psychiatrists, 44, 198–207. 10.1016/j.eurpsy.2017.05.021 [DOI] [PubMed] [Google Scholar]
- Perwien A. R., Kratochvil C. J., Faries D., Vaughan B., Busner J., Saylor K. E., Buermeyer C. M., Kaplan S., Swindle R. (2008). Emotional expression in children treated with ADHD medication: Development of a new measure. Journal of Attention Disorders, 11(5), 568–579. 10.1177/1087054707306117 [DOI] [PubMed] [Google Scholar]
- Pliszka S. R., Wilens T. E., Bostrom S., Arnold V. K., Marraffino A., Cutler A. J., López F. A., DeSousa N. J., Sallee F. R., Incledon B., Newcorn J. H. (2017). Efficacy and safety of HLD200, delayed-release and extended-release methylphenidate, in children with attention-deficit/hyperactivity disorder. Journal of Child and Adolescent Psychopharmacology, 27(6), 474–482. 10.1089/cap.2017.0084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Posner J., Kass E., Hulvershorn L. (2014). Using stimulants to treat ADHD-related emotional lability. Current Psychiatry Reports, 16(10), 478. 10.1007/s11920-014-0478-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sallee F. R. (2015). Early morning functioning in stimulant-treated children and adolescents with attention-deficit/hyperactivity disorder, and its impact on caregivers. Journal of Child and Adolescent Psychopharmacology, 25(7), 558–565. 10.1089/cap.2014.0160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schein J., Cloutier M., Gauthier-Loiselle M., Bungay R., Guerin A., Childress A. (2023). Symptoms associated with ADHD/treatment-related adverse side effects and their impact on quality of life and work productivity in adults with ADHD. Current Medical Research and Opinion, 39(1), 149–159. 10.1080/03007995.2022.2122228 [DOI] [PubMed] [Google Scholar]
- Shih H. H., Shang C. Y., Gau S. S. (2019). Comparative efficacy of methylphenidate and atomoxetine on emotional and behavioral problems in Youths with Attention-Deficit/Hyperactivity Disorder. Journal of Child and Adolescent Psychopharmacology, 29(1), 9–19. 10.1089/cap.2018.0076 [DOI] [PubMed] [Google Scholar]
- Sibley M. H., Arnold L. E., Swanson J. M., Hechtman L. T., Kennedy T. M., Owens E., Molina B. S. G., Jensen P. S., Hinshaw S. P., Roy A., Chronis-Tuscano A., Newcorn J. H., Rohde L. A., & MTA Cooperative Group. (2022). Variable patterns of remission from ADHD in the Multimodal Treatment Study of ADHD. The American Journal of Psychiatry, 179(2), 142–151. 10.1176/appi.ajp.2021.21010032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skirrow C., Asherson P. (2013). Emotional lability, comorbidity and impairment in adults with attention-deficit hyperactivity disorder. Journal of Affective Disorders, 147(1–3), 80–86. 10.1016/j.jad.2012.10.011 [DOI] [PubMed] [Google Scholar]
- Sobanski E., Banaschewski T., Asherson P., Buitelaar J., Chen W., Franke B., Holtmann M., Krumm B., Sergeant J., Sonuga-Barke E., Stringaris A., Taylor E., Anney R., Ebstein R. P., Gill M., Miranda A., Mulas F., Oades R. D., Roeyers H., Rothenberger A., Steinhausen H-C., Faraone S. V. (2010). Emotional lability in children and adolescents with attention deficit/hyperactivity disorder (ADHD): Clinical correlates and familial prevalence. The Journal of Child Psychology and Psychiatry, 51(8), 915–923. 10.1111/j.1469-7610.2010.02217.x [DOI] [PubMed] [Google Scholar]
- Stringaris A., Goodman R. (2009). Mood lability and psychopathology in youth. Psychological Medicine, 39(8), 1237–1245. 10.1017/S0033291708004662 [DOI] [PubMed] [Google Scholar]
- Surman C. B., Biederman J., Spencer T., Miller C. A., McDermott K. M., Faraone S. V. (2013). Understanding deficient emotional self-regulation in adults with attention deficit hyperactivity disorder: A controlled study. ADHD Attention Deficit Hyperactivity Disorders, 5(3), 273–281. 10.1007/s12402-012-0100-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Surman C. B. H., Walsh D. M. (2022). Do treatments for adult ADHD improve emotional behavior? A systematic review and analysis. Journal of Attention Disorders, 26(14), 1822–1832. 10.1177/10870547221110926 [DOI] [PubMed] [Google Scholar]
- Swanson J. M., Wigal S. B., Wigal T., Sonuga-Barke E., Greenhill L. L., Biederman J., Kollins S., Nguyen A. S., DeCory H. H., Hirshe Dirksen S. J., Hatch S. J., & COMACS Study Group. (2004). A comparison of once-daily extended-release methylphenidate formulations in children with attention-deficit/hyperactivity disorder in the laboratory school (the Comacs Study). Pediatrics, 113(3 Pt 1), e206–e216. 10.1542/peds.113.3.e206 [DOI] [PubMed] [Google Scholar]
- Visser S. N., Danielson M. L., Bitsko R. H., Holbrook J. R., Kogan M. D., Ghandour R. M., Perou R., Blumberg S. J. (2014). Trends in the parent-report of health care provider-diagnosed and medicated attention-deficit/hyperactivity disorder: United States, 2003-2011. Journal of the American Academy of Child and Adolescent Psychiatry, 53(1), 34–46.e2. 10.1016/j.jaac.2013.09.001 [DOI] [PMC free article] [PubMed] [Google Scholar]