Abstract
Objective
The modeled CA-125 ELIMination rate constant K (KELIM) has been validated as a marker of response to chemotherapy in >12,000 patients with advanced epithelial ovarian carcinoma (EOC) treated in first-line setting enrolled in >12 clinical trials. Patient KELIM is calculable online https://www.biomarker-kinetics.org/presentation. The objective was to investigate the prognostic value of KELIM in a large real-life national cancer registry with non-selected patients.
Methods
We investigated 4,025 EOC patients from the Netherlands Cancer Registry treated with neoadjuvant chemotherapy (NACT) ± followed by interval debulking surgery (IDS). Patient KELIM values were calculated in patients with ≥ 3 CA-125 measurements during NACT. KELIM was standardized with a pre-specified cut-off and scored as unfavorable/favorable (<1.0/≥1.0). KELIM’s prognostic value regarding radiological response, completeness of IDS, progression-free survival (PFS), and overall survival (OS) was assessed using univariate/multivariate analyses.
Results
The data from 1,582 patients treated with heterogeneous chemotherapy regimens and sequences were assessable. KELIM was prognostic for radiological response and the likelihood of complete IDS after NACT (odds ratio=2.59; 95% confidence interval [CI]=2.04–3.29). Moreover, KELIM was independently associated with PFS (hazard ratio [HR]=0.76; 95% CI=0.66–0.87), and OS (HR=0.79; 95% CI=0.69–0.91). Combining KELIM with the completeness of the IDS resulted in 3 prognostic groups (satisfactory, intermediate, and poor) with significant OS differences, namely a good, intermediate, and poor survival respectively.
Conclusion
The value of KELIM, as a pragmatic indicator of response to chemotherapy, was maintained in a large real-life population-based cohort, highlighting its applicability in routine conditions.
Keywords: Antigen, CA-125; Kinetics; Ovarian Neoplasms; Models, Theoretical; Survival; Prognosis
Synopsis
CA-125 ELIMination rate constant K (KELIM) depicts the longitudinal CA-125 kinetics. KELIM was validated as a chemosensitivity marker in advanced ovarian cancer in a nationwide cohort. KELIM is prognostic for survival and complete debulking in advanced ovarian cancer after neoadjuvant chemotherapy.
INTRODUCTION
Patients with advanced-stage (International Federation of Obstetrics and Gynecologists [FIGO] stage IIB-IV) epithelial ovarian cancer (EOC) not amenable to primary debulking surgery (PDS) are typically treated with platinum-based neoadjuvant chemotherapy (NACT) with the objective of a complete interval debulking surgery (IDS) [1]. This strategy is implemented for the majority of patients with advanced ovarian carcinoma [2]. NACT is meant to induce regression of tumor load to increase the odds of a complete IDS without postoperative residual lesions, which has been recognized as a major prognostic factor for survival [3]. As a consequence, the medical-and-surgical treatment success is strongly dependent on the tumor’s sensitivity to chemotherapy, which is therefore of high interest for disease management. As acknowledged by the European Society for Medical Oncology and the European Society of Gynaecological Oncology, reproducible indicators of chemosensitivity are needed [3].
Many indicators of treatment efficacy based on the CA-125 kinetics were reported in the literature, with inconsistent outcomes [4,5,6,7]. The Gynecologic Cancer Intergroup (GCIG) defined the CA-125 response as a 50% reduction in CA-125 maintained for at least 28 days in patients with recurrent disease only [8]. However, several studies have depicted the limited prognostic and predictive value of this parameter [9,10,11]. Two timepoint-based kinetic strategies are limited by high inter-and intra-individual variability of assays, and therefore might not apply to other settings than those initially assessed in [12]. To overcome these limitations, the modeled CA-125 ELIMination rate constant K (KELIM) was developed to assess the mathematical equations of the longitudinal CA-125 kinetics during treatment. KELIM is calculated with ≥3 CA-125 measurements during the first 100 days of chemotherapy. A higher value should be understood as a faster CA-125 elimination rate and therefore higher chemosensitivity. The prognostic value of KELIM regarding progression-free survival (PFS) and overall survival (OS) was found to be reproducible on the data from more than 12,000 patients in 12 randomized trials treated with adjuvant or neoadjuvant chemotherapy [7,11,13,14,15,16]. These results consistently show that KELIM is an indicator of the tumor’s intrinsic chemosensitivity, with a strong and independent prognostic value regarding the likelihood of successful medical-and-surgical treatment.
To ensure the accessibility to the KELIM score calculation in routine practice for clinicians, an online calculator was created. Patient KELIM score can easily be calculated with a minimum of 3 CA-125 measurements during the first 3 cycles of chemotherapy using the online calculator (on https://www.biomarker-kinetics.org/CA-125-neo for patients treated with NACT, and on https://www.biomarker-kinetics.org/CA-125 for patients treated with adjuvant chemotherapy). However, the model was built based on results obtained from clinical trials encompassing highly selected patients. Therefore, assessment of the utility of KELIM in real-life non-selected patients is needed to ensure its possible extrapolation to the clinical setting.
The aim of this study, performed on the Netherlands Cancer Registry (NCR), was to confirm the independent prognostic value of KELIM regarding tumor response after NACT, the likelihood of complete IDS, PFS, and OS, in a nationwide cohort of patients with advanced-stage EOC treated with NACT. Furthermore, the present study aimed to explore the prognostic value of KELIM concerning BReast CAncer gene (BRCA) mutational status.
MATERIALS AND METHODS
Patient data were extracted from the NCR; a population-based registry comprising all newly diagnosed malignancies in the Netherlands. Patients diagnosed with advanced-stage EOC between January 1, 2008, and December 31, 2011, treated with NACT (every 3 weeks or weekly regimen), and with at least 3 serum CA-125 measurements during the first 100 days of chemotherapy, were selected. Patients with a CA-125 that remained <35 IU/L were excluded. Patients could have potentially been treated with subsequent IDS after NACT (varying from 3 up to 11 chemotherapy cycles) or received systemic treatments only (varying from 2 up to 20 cycles, in case of weekly regimens). CA-125 concentrations were determined by local laboratories.
The following parameters were collected in the NCR: pathological subtype; tumor grade; FIGO stage [17]; germline or somatic BRCA mutational status if available (no mutation, BRCA1 mutation, or BRCA2 mutation); treatment regimen (carboplatin-paclitaxel doublet, carboplatin as a single agent, carboplatin-paclitaxel combined with another drug, or other chemotherapy regimens); completeness of IDS based on post-operative residual lesions as judged by the surgeon (complete with no visible disease [CC0 score], optimal with residues less than 1 cm maximum diameter, or sub-optimal with residues of 1 cm or more [CC1-CC2]); radiological tumor response to NACT (satisfactory response with complete/partial response or unsatisfactory response with stable/progressive diseases); disease-risk group (high-risk in the case of stage IV, or incompletely resected stage III disease; and low-risk in the other cases); PFS and OS. The study design, data extraction, and protocols were approved by the NCR review board (reference number; K19.365). The requested dataset was considered anonymous, and the use is therefore exempt from ethics review board approval according to Dutch legislation.
1. Mathematical modeling of longitudinal CA-125 kinetics, estimation of patient KELIM, and standardization
At least 3 CA-125 values during the first 100 days of NACT were required to ensure an accurate assessment of KELIM by the model. To normalize the distribution and eliminate right-skewness, CA-125 levels were log-transformed. The mathematical modeling of early CA-125 kinetics with a non-linear mixed-effect model and details about the semi-mechanistic kinetic-pharmacodynamic (K-PD) model adjustment and qualification were previously described [7,11,15,18].
Individual KELIM values were estimated with the same model implemented in the online calculator. Individual KELIM values were computed using Empirical Bayes Estimates (EBE). As assessed in previous studies [15,19], KELIM was standardized by the optimized cut-off in the neoadjuvant setting (pre-specified optimized cut-off, 0.05/days) to provide easy reading of patient KELIM outcome. The standardization was calculated by: Standardized (std) KELIM = KELIM Estimated by the Model/Cut-Off. As a consequence, std KELIM was a continuous covariate centered on 1.0. To help the interpretation of KELIM for prognostic analyses, KELIM was dichotomized into a KELIM score: std KELIM <1.0 was considered unfavorable, whilst std KELIM ≥1.0 was considered favorable.
2. Associations between KELIM, complete IDS, and radiological response
The distributions of std KELIM among patients with or without complete IDS were assessed using box plots. The predictive value of std KELIM regarding the likelihood of complete IDS (no, suboptimal and optimal debulking; vs. yes, complete debulking) was assessed using multivariate logistic regression models, integrating other known prognostic factors, including disease stage (FIGO stage II, III, or IV); tumor histology based on the International Classification of Diseases for Oncology 3rd edition (ICD-O-3) (clear cell and mucinous vs other histotypes including endometrioid and serous); tumor grade (I, II, or III); and radiological tumor response (complete/partial response, or stable/progressive disease). Analyses of the deviance, along with C-index analyses, were used to assess the improvement related to the incorporation of KELIM and the other covariates in the logistic regression models predicting the likelihood of complete surgery. Repeated 10-fold cross-validation was used to assess the final model accuracy. The statistical association between std KELIM and the radiological tumor response was assessed using box plots.
3. Association between KELIM, OS, and PFS
The prognostic value of std KELIM score regarding PFS and OS were assessed with univariate Kaplan-Meier analysis, Log-rank tests, and multivariate Cox models. The other prognostic factors implemented in the univariate analyses were the same as described above, along with the completeness of IDS (complete vs. incomplete); and disease-risk group (low-risk vs. high-risk disease). Those found significant in univariate analyses with a p<0.1, were included in the multivariate Cox model and assessed using backward selections. As previously done on the GCIG meta-analysis dataset and ICON-8 trial, both the completeness of debulking surgery and KELIM score were combined into prognostic groups regarding PFS and OS [13,20].
Survival was calculated as the interval between the date of diagnosis and death. If the patients were alive, the date of the last check of the municipal population register (February 1, 2022) was used, and patients were right censored thereafter. All survival analyses were implemented with a landmark time point set at 100 days after the start of NACT or at the surgery date, whichever occurred first. CA-125 was modeled from day 0 to 100, and exclusion of the early progressions observed during the first 100 days avoided the biases related to the links between early progressions and CA-125 kinetics, or radiological tumor response [21].
4. Associations between KELIM and BRCA mutational status
The distributions of std KELIM among patients with known somatic or germline BRCA1 mutation, BRCA2 mutation, or wild-type status were assessed using box plots. The predictive value of std KELIM regarding the likelihood of BRCA1 or BRCA2 mutations was assessed using univariate and multivariate logistic regression models, together with the following covariates: disease histology (clear cell and mucinous vs other), tumor grade (I, II, or III), and disease stage (stage II and III vs IV).
5. Statistics and computing process
All tests were implemented using a two-sided 0.05 alpha risk. NONMEM 7.4 (ICON Development Solutions, Ellicott City, MD, USA) software was used to fit the semi-mechanistic model to CA-125 kinetic data [22]. The XPOSE4 program was used for the graphical evaluation of model fits [23]. Logistic analyses, cross-validation, survival analyses, and concordance probability (C-index) were obtained in R software version 3.6.2 (R Foundation for Statistical Computing, Vienna, Austria).
RESULTS
1. Assessable patients
Out of 4,025 patients who received NACT between 2008 and 2015 in the Netherlands, 2,369 were excluded due to insufficient numbers of CA-125 values and 74 due to baseline CA-125 values <35, resulting in 1,582 assessable patients (Fig. S1). Patient characteristics are presented in Table 1. The median number of CA-125 values per patient was 3 measurements (interquartile range [IQR], 1), with a median of 21 days between measurements (IQR, 17–21). Whilst most patients were treated with the standard regimen, as shown in Table 1, there was heterogeneity in the neo-adjuvant chemotherapy regimens administered to patients (carboplatin-paclitaxel regimen, 88%; carboplatin as a single agent, 6%; and triplet combination regimen, 4%). This was also the case for medical-surgical treatment sequences (NACT followed by IDS, 71%; incomplete PDS followed by chemotherapy before another attempt of IDS, 5%; no surgery, 24%).
Table 1. Characteristics of patients.
| Covariates | Global population (n=4,025) | Included patients (n=1,582) | ||
|---|---|---|---|---|
| Age (yr) | 67 (15) | 67 (14) | ||
| Tumor histology | ||||
| Clear cell and mucinous | 125 (3.10) | 50 (3.20) | ||
| Others | 3,900 (96.9) | 1,532 (96.8) | ||
| Tumor grade | ||||
| Grade I | 138 (3.4) | 52 (3.3) | ||
| Grade II | 303 (7.5) | 134 (8.5) | ||
| Grade III | 2,091 (52.0) | 826 (52.2) | ||
| Missing | 1,493 (37.1) | 570 (36.0) | ||
| FIGO tumor stage | ||||
| IIB | 29 (0.7) | 4 (0.3) | ||
| IIC | 16 (0.4) | 5 (0.3) | ||
| IIIA | 19 (0.5) | 3 (0.2) | ||
| IIIB | 100 (2.5) | 26 (1.6) | ||
| IIIC | 2,356 (58.5) | 891 (56.3) | ||
| IV | 1,505 (37.4) | 653 (41.3) | ||
| Cytoreductive surgery performed | ||||
| No | 955 (23.7) | 327 (21) | ||
| Yes | 3,070 (76.3) | 1,255 (79) | ||
| Post-operative lesions after IDS* | ||||
| Suboptimal (>1 cm residual) | 354 (11.5) | 157 (12.5) | ||
| Optimal (<1 cm residual) | 1,097 (35.7) | 475 (37.8) | ||
| Complete | 1,553 (50.6) | 598 (47.6) | ||
| Missing | 66 (2.1) | 25 (2.0) | ||
| Completeness of IDS† | ||||
| Incomplete IDS | 1,451 (47.3) | 632 (50.4) | ||
| Complete IDS | 1,553 (50.6) | 598 (47.6) | ||
| Missing | 66 (2.1) | 25 (2.0) | ||
| Tumor radiological response | ||||
| Complete or Partial response | 2,650 (65.8) | 1,141 (72.1) | ||
| Stable or progressive disease | 565 (14.0) | 235 (14.9) | ||
| Missing | 810 (20.1) | 206 (13.0) | ||
| Treatment type | ||||
| NACT followed by IDS | 2,864 (71.2) | 1,186 (75.0) | ||
| PDS followed by chemotherapy and second cytoreductive surgery | 207 (5.1) | 69 (4.4) | ||
| Chemotherapy only, and no surgery | 954 (23.7) | 327 (20.7) | ||
| Chemotherapy regimens | ||||
| Carboplatin + paclitaxel | 3,532 (87.8) | 1,381 (87.3) | ||
| Carboplatin monotherapy | 233 (5.8) | 95 (6.0) | ||
| Carboplatin + paclitaxel + other chemotherapy | 160 (4.0) | 86 (5.4) | ||
| Other regimens | 100 (2.5) | 20 (1.3) | ||
| Risk-disease groups† | ||||
| Low-risk disease | 1,094 (27.2) | 384 (24.3) | ||
| High-risk disease | 1,929 (47.9) | 852 (53.9) | ||
| Missing | 1,002 (24.9) | 346 (21.9) | ||
| BRCA1 mutation | ||||
| No | 1,078 (26.8) | 439 (27.7) | ||
| Yes | 191 (4.7) | 70 (4.4) | ||
| Missing/not tested | 2,756 (68.5) | 1,073 (67.8) | ||
| BRCA2 mutation | ||||
| No | 1,172 (29.1) | 472 (29.8) | ||
| Yes | 97 (2.4) | 37 (2.3) | ||
| Missing/not tested | 2,756 (68.5) | 1,073 (67.8) | ||
| BRCA1-2 mutation | ||||
| No | 981 (24.4) | 402 (25.4) | ||
| Yes | 288 (7.2) | 107 (6.8) | ||
| Missing/not tested | 2,756 (68.5) | 1,073 (67.8) | ||
Values are presented as median (interquartile range) or number of patients (%).
BRCA, BReast CAncer gene; FIGO, International Federation of Obstetrics and Gynecologists; IDS, interval debulking surgery; NACT, neoadjuvant chemotherapy; PDS, primary debulking surgery.
*From patients who received debulking surgery. †High-risk group: Stage IV and incompletely resected stage III diseases; BRCA1-2 mutation = at least one BRCA1 or BRCA2 mutation.
2. Model qualification
Typical parameter estimates, along with the qualification analyses from the final semi-mechanistic model, are presented in the supplementary materials (Data S1, Table S1, Fig. S2).
3. Standardization of KELIM
A complete IDS was obtained in 48% of patients receiving IDS. The discriminative ability of KELIM regarding the likelihood of complete IDS estimated with the receiver operating characteristic curve (ROC) curve analysis was: area under the ROC curve=0·66; 95% confidence interval (CI)= 0.63–0.69; sensitivity=74%; specificity=48% (Fig. S3). In all further analyses, std KELIM was calculated as patient KELIM/0.05.
4. Confirmation of the value of KELIM as a marker of response to chemotherapy
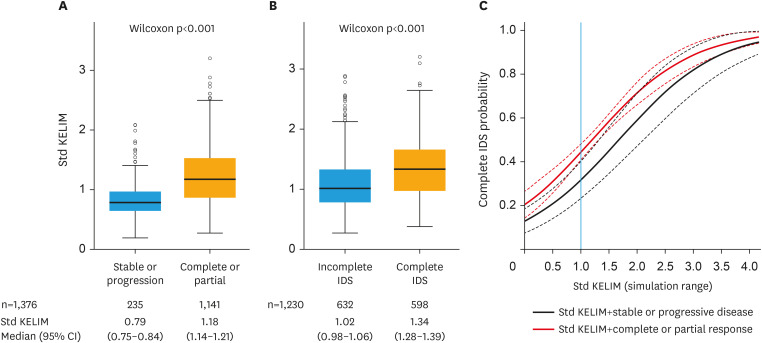
An association was found between higher std KELIM values and favorable radiological tumor response after neoadjuvant chemotherapy (Fig. 1A). Moreover, the median std KELIM was significantly higher in patients with a complete IDS compared to an incomplete IDS (1.34 vs. 1.02; p<0.001, respectively) (Fig. 1B). Three parameters were significantly associated with the likelihood of a complete IDS in univariate logistic regression: std KELIM as a continuous covariate (odds ratio [OR]=3.27; 95% CI=2.53–4.24; C-Index=0.66; 95% CI=0.63–0.69), KELIM score (favorable vs. unfavorable, OR=2.59; 95% CI=2.04–3.29; C-Index=0.61; 95% CI=0.58–0.63), and radiological response (complete/partial response vs stable/progressive disease, OR=2.50; 95% CI=1.67–3.75; C-Index=0.54; 95% CI=0.52–0.56) (Table S2). Std KELIM (continuous or dichotomized) was associated with the highest C-Index improvement, suggesting that this covariate was the most predictive marker among those tested (Table S2). In the final multivariate logistic regression model, both std KELIM and radiological tumor response remained significant (Table 2, Fig. 1C). The analysis of deviance confirmed the improvement in the prediction of IDS completeness related to the integration of KELIM (Table S3).
Fig. 1. Association between KELIM and neoadjuvant chemotherapy efficacy.
(A) Radiological tumor response according to std KELIM (KELIM/0.05). (B) Completeness of IDS according to std KELIM. (C) Likelihood of complete surgery according to radiological response and std KELIM values. Dashed line 95% CI; Black curve: logistic probability over std KELIM values for stable or progression radiological response; Red curve: logistic probability over std KELIM values for a complete or partial radiological response. Blue line: Std KELIM threshold at 1.
CI, confidence interval; IDS, interval debulking surgery; KELIM, CA-125 ELIMination rate constant K; std, standardized.
Table 2. Final logistic regression analyses regarding the likelihood of complete interval debulking surgery (n=1,107).
| Variables | Estimate | OR (95% CI) | p | C-Index (95% CI) | |
|---|---|---|---|---|---|
| Intercept | −1.89 | 0.15 (0.09–0.24) | <0.001 | 0.67 (0.63–0.70) | |
| Std KELIM* | 1.13 | 3.10 (2.33–4.12) | <0.001 | ||
| Radiological tumor response | 0.012 | ||||
| Stable or progression | REF | REF | |||
| Complete or partial | 0.54 | 1.71 (1.12–2.61) | |||
CI, confidence interval; KELIM, CA-125 ELIMination rate constant K; OR, odds-ratio; std, standardized.
*Std KELIM = KELIM/0.05.
5. KELIM score and completeness of IDS to identify 3 prognostic groups regarding PFS and OS
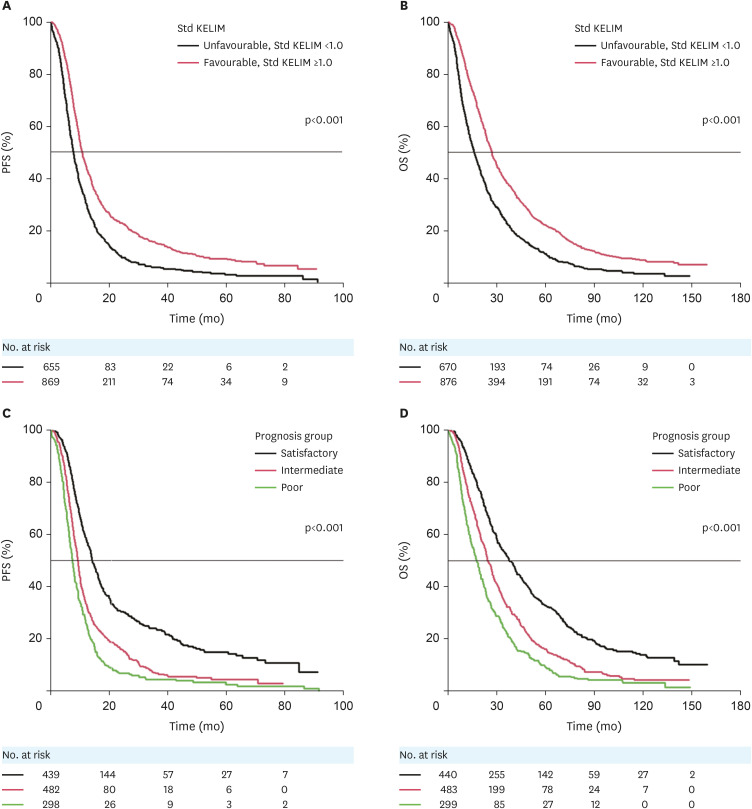
The median follow-up for OS was 25.2 months (95% CI=23.6–26.5). A total of 1,524 and 1,546 patients were assessable for PFS and OS analyses, respectively (Fig. S1). The results of the univariate and multivariate survival analyses for PFS and OS are presented in Table 3. Multivariable analysis showed that disease stage, completeness of IDS, disease-risk groups, and KELIM were significantly, and independently, associated with PFS, whilst completeness of IDS, disease-risk groups, and KELIM were significantly, and independently, associated with overall survival. Analyses of the deviance confirmed the strong and independent prognostic value of KELIM compared to the other covariates. The Kaplan-Meier curves of PFS and OS according to KELIM are presented in Fig. 2A and B.
Table 3. Univariate Log-rank tests and final multivariate Cox model for PFS and OS.
| Variables | PFS (n=1,524; events=1,358; median [95% CI]=9.6 [9.2–9.9]) | OS (n=1,546; events=1,416; median [95% CI]=22.6 [21.2–23.7]) | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Univariate log-rank | Final multivariate Cox model | Univariate log-rank | Final multivariate Cox model | ||||||||||||
| Subject | Survival (mo) | p | HR (95% CI) | p | Analysis of deviance p (>|χ|)‡ | C-Index (95% CI) | Subject | Survival (mo) | p | HR (95% CI) | p | Analysis of deviance p (>|χ|)‡ | C-Index (95% CI) | ||
| n | Medians (95% CI) | n | Medians (95% CI) | ||||||||||||
| Std KELIM* | <0.001 | <0.001 | <0.001 | 0.61 (0.60–0.63) | <0.001 | 0.002 | 0.003 | 0.60 (0.59–0.62) | |||||||
| Unfavorable score, std KELIM <1.0 | 655 | 7.8 (7.3–8.3) | REF | 670 | 16.2 (14.6–18.4) | REF | |||||||||
| Favorable score, std KELIM ≥1.0 | 869 | 10.9 (10.2–11.6) | 0.79 (0.69–0.90) | 876 | 27.1 (25.3–29.4) | 0.83 (0.73–0.93) | |||||||||
| Tumor histology | 0.183 | NS | 0.173 | NS | |||||||||||
| Clear cell &Mucinous | 34 | 7.9 (5.0–12.3) | 35 | 18.1 (11.4–25.4) | |||||||||||
| Others | 1,490 | 9.6 (9.2–10.0) | 1,511 | 22.8 (21.4–23.8) | |||||||||||
| Tumor grade | 0.233 | NS | 0.490 | NS | |||||||||||
| Grade I | 50 | 9.5 (7.8–14.6) | 51 | 31.9 (21.8–50.2) | |||||||||||
| Grade II | 132 | 11.0 (9.5–12.8) | 132 | 25.9 (21.9–30.4) | |||||||||||
| Grade III | 810 | 9.8 (9.3–10.6) | 817 | 24.5 (22.9–26.6) | |||||||||||
| FIGO Tumor Stage | <0.001 | 0.016 | 0.016 | 0.001 | NS | ||||||||||
| II + III | 904 | 10.1 (9.6–10.8) | REF | 912 | 24.4 (23.2–26.5) | ||||||||||
| IV | 620 | 8.7 (8.1–9.4) | 1.16 (1.03–1.32) | 634 | 19.6 (17.9–21.0) | ||||||||||
| Completeness of IDS | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | |||||||||
| Incomplete IDS | 623 | 8.2 (7.7–8.9) | REF | 625 | 20.1 (18.7–22.4) | REF | |||||||||
| Complete IDS | 596 | 13.7 (12.3–14.6) | 0.53 (0.47–0.60) | 597 | 36.8 (32.6–41.0) | 0.53 (0.47–0.61) | |||||||||
| Radiological tumor response | <0.001 | NS | <0.001 | NS | |||||||||||
| Stable or Progression | 213 | 7.3 (6.4–8.0) | 223 | 11.9 (9.9–13.7) | |||||||||||
| Complete or Partial | 1,121 | 10.0 (9.5–10.6) | 1,128 | 24.6 (23.2–26.7) | |||||||||||
| Disease risk group† | <0.001 | <0.001 | |||||||||||||
| Low-risk | 383 | 14.3 (13.5–16.3) | 384 | 39.2 (34.5–45.3) | |||||||||||
| High-risk | 842 | 9.0 (8.4–9.4) | 844 | 22.4 (20.6–23.7) | |||||||||||
CI, confidence interval; HR, hazard ratio; KELIM, CA-125 ELIMination rate constant K; IDS, interval debulking surgery; NS, not significant; OS, overall survival; PFS, progression free survival; std, standardized.
*Std KELIM = KELIM/0.05. †High-risk group: Stage IV and incompletely resected stage III diseases. Not assessed in multivariate analyses. ‡Analysis of deviance table, likelihood-ratio χ2 test: comparison of the log-likelihood between the full final model and the model without the interest covariate.
Fig. 2. Prognostic value of KELIM score without/with the completeness of IDS regarding PFS and OS. (A) Kaplan-Meier curve of PFS according to KELIM score (unfavorable <1.0, or favorable ≥1.0). (B) Kaplan-Meier curve of OS according to KELIM score (unfavorable <1.0, or favorable ≥1.0). (C) Kaplan-Meier curve of PFS according to the 3 prognostic groups based on KELIM score (unfavorable <1.0, or favorable ≥1.0) and completeness of IDS (complete vs. incomplete). (D) Kaplan-Meier curve of OS according to the three prognostic groups based on KELIM score (unfavorable <1.0, or favorable ≥1.0) and completeness of IDS (complete, vs incomplete).
IDS, interval debulking surgery; KELIM, CA-125 ELIMination rate constant K; OS, overall survival; PFS, progression-free survival; std, standardized.
KELIM score was combined with the completeness of IDS to define 3 prognostic groups (Fig. 2C and D). These groups showed statistically significant survival differences, in agreement with previous results [13,20], and were defined as followed: 1) A group with a good prognosis, with a favorable KELIM score and complete IDS (median PFS, 14.3 months; 95% CI=13.4–16.0 and median OS, 37.6 months; 95% CI=32.8–42.2); 2) A group with an intermediate prognosis, with either a favorable KELIM score and incomplete IDS, or an unfavorable KELIM score and complete IDS (median PFS, 9.3 months; 95% CI=8.8–9.8; hazard ratio [HR]=1.74; 95% CI=1.51–2.01, p<0.001 and median OS, 24.5 months; 95% CI=22.8–27.3; HR=1.61; 95% CI=1.40–1.84; p<0.001); and 3) A group with a poor prognosis, with an unfavorable KELIM score and incomplete IDS (median PFS, 7.4 months; 95% CI=6.8–8.1; HR=2.38; 95% CI=2.03–2.79; p<0.001 and median OS, 17.6 months; 95% CI=15.0–20.1; HR=2.24; 95% CI=1.92–2.62; p<0.001).
6. Exploratory analysis on associations between KELIM and BRCA mutational status
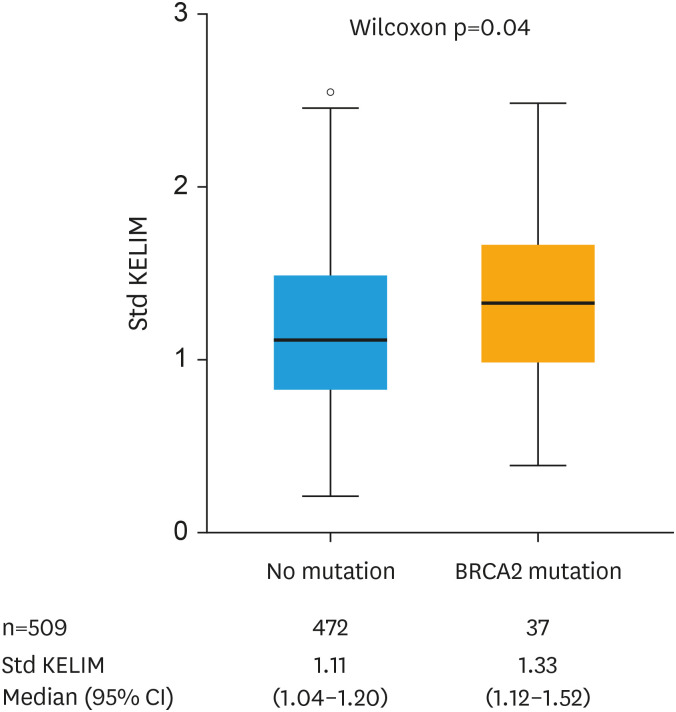
The somatic or germline BRCA mutational status was available in 509 patients (32.2%) with the following features: at least one BRCA mutation in 107 patients (21.0%); BRCA1 mutation in 70 patients (13.8%); and BRCA2 mutation in 37 patients (7.3%) (Table 1). Using logistic regression models, std KELIM was significantly associated with the probability of carrying a BRCA mutation (OR=1.61; 95% CI=1.05–2.47) (Table S4). This association was mainly driven by BRCA2 mutation. Std KELIM was higher in patients with BRCA1 mutation compared to those with wild-type status, but not statistically significant (1.20 vs. 1.13, p=0.383), whilst it was significant in those with BRCA2 mutation compared to patients with wild-type status (1.33 vs. 1.11, p=0.039) (Fig. 3).
Fig. 3. BRCA2 mutation according to standardized KELIM. Std KELIM = KELIM/0.05.
BRCA, BReast CAncer gene; CI, confidence interval; KELIM, CA-125 ELIMination rate constant K; std, standardized.
DISCUSSION
The prognostic and predictive marker KELIM has been developed and validated on the data of more than 12,000 patients who were highly selected for randomized clinical trials [14,24]. Whilst online KELIM calculation has been made possible, the prognostic value of KELIM regarding response to chemotherapy in non-selected patients in a real-life setting still needed to be assessed.
In the present study, the prognostic value of KELIM regarding the response to NACT was confirmed in real-life patients registered in the NCR, as was its prognostic value regarding PFS and OS. These outcomes were observed despite the heterogeneity of the used CA-125 assays, the chemotherapy regimens, the number of cycles, and the medical-and-surgical treatment sequences, inherent to routine management.
Consistent with the literature, the rate of complete IDS in this study was close to 50% of all debulked patients [10,15]. The probability of obtaining a complete IDS in patients with a favorable KELIM was doubled compared to patients with an unfavorable KELIM, as seen in CHIVA and ICON-8 trials [15]. KELIM score may therefore be of interest for decision making regarding IDS attempt when the feasibility of complete IDS is uncertain, or the risk of post-operative morbidity is high. Moreover, whilst the prognostic value of KELIM was confirmed regarding PFS and OS, the lower hazard ratio observed in the present study (up to 0.8) compared to those reported in previous studies (up to 0.5) may be related to the lower accuracy in KELIM estimation, due to the lack of available data necessary for precise KELIM estimation. For example, the exact chemotherapy administration dates were not available in the database, although this data is integrated into the mathematical formula. Despite its lower accuracy, the prognostic value of KELIM was statistically and clinically significant, with large overall survival differences. As already done in ICON-8 and GCIG trial meta-analysis datasets, KELIM score and completeness of debulking surgery could be successfully combined into 3 prognostic groups [13,20]: 1) A good prognostic group, including patients with favorable KELIM score operated with complete IDS (median OS, 37 months); 2) An intermediate prognostic group, comprising patients with either favorable KELIM score, or complete IDS (median OS, 24 months); and 3) A poor prognostic group, including patients with unfavorable KELIM score operated with incomplete IDS (median OS, 18 months). The development of innovative approaches meant to increase chemosensitivity is warranted for the patients belonging to the poor prognostic group, as a way of improving their prognosis. Different strategies of chemosensitization could be considered, such as chemotherapy dosage adjustment with fractionated dose-dense chemotherapy as shown in ICON-8 trial [20], the addition of bevacizumab as demonstrated in ICON-7 trial, and validated in GOG-0218 trial [25], or addition of cell cycle checkpoint inhibitors [26].
BRCA status was available in 32% of patients. KELIM was significantly associated with the probability of a BRCA mutation, which was mainly driven by the association between KELIM and BRCA2. A higher KELIM value in BRCA mutated patients is probably a reflection of the higher chemosensitivity in such patients [27,28]. Interestingly, a strong association between veliparib-related PFS benefit and KELIM has been found in a post-hoc analysis of the VELIA phase III trial, suggesting that KELIM might be complementary to homologous recombination status for selecting which patients benefit from poly (ADP-ribose) polymerase (PARP) inhibitors [16]. Of note, the present study was done with the data from patients treated before the broad use of these drugs, therefore the association between PARP inhibitors and KELIM could not be assessed.
This study contains some limitations. In addition to the heterogeneity of patient characteristics, biology assays, and treatment, inherent to the real-life feature of the dataset, the radiological tumor response rate was not assessed using the RECIST 1.1 criteria but based on the treating physician’s observation with the use of radiology reports, thereby limiting its comparison with clinical trials [29]. Also, BRCA mutation status was only available in 32% of patients, as the present study included patients treated before BRCA mutation status was more routinely tested, preventing the integration of BRCA in multivariable analyses.
Despite these limitations, the results from this large nationwide dataset confirm that KELIM, calculated in non-selected patients treated with NACT followed by IDS, exhibits an independent prognostic value regarding chemotherapy efficacy, along with an independent prognostic value regarding PFS and OS, complementary to the prognostic value of surgery completeness. We believe that the need for a predictor of tumor chemosensitivity acknowledged by the European consensus conferences is addressed by this pragmatic kinetic parameter.3 KELIM, which is easily calculable online (https://www.biomarker-kinetics.org/CA-125-neo), could be integrated into the disease management algorithm as a complementary tool for predicting the likelihood of complete IDS after NACT when needed, and for identifying patients who warrant innovative treatment to improve their poor prognosis. In total, the heterogeneity of this cohort does not only offer limitations but also confirms the robustness and applicability of KELIM in a real-life setting.
ACKNOWLEDGEMENTS
The authors thank the Netherlands Comprehensive Cancer Organization for the collection of data for the Netherlands Cancer Registry.
Footnotes
Funding: This work was supported by the Dutch Cancer Society (IKNL2014-6838). The funder of the study had a role in the data collection.
Presentation: The outcomes of this study were presented at the 2020 European Society for Medical Oncology Virtual Meeting, abstract #4562.
Conflict of Interest: Y.B. received personal fees for advisory board participation from AZ, GSK, NOVARTIS, BAYER, ROCHE, CLOVIS, AMGEN, MSD, ECS Progastrin. Furthermore, Y.B. received personal fees from Lyon University Hospital and Lyon University. S.G.S. reports institutional research support from AstraZeneca, Merck, Novartis, and Roche. All other authors declare no competing interests.
Data Sharing Statement: The datasets analyzed during the current study are not publicly available due to patient anonymity. Data sharing of anonymous data from the Netherlands Cancer Registry (NCR) will be considered for non-commercial, research, or statistical-based use on a case-by-case basis (to be requested and approved by the NCR; gegevensaanvraag@iknl.nl).
- Conceptualization: W.L., Y.B.
- Formal analysis: W.L., C.O., Y.B.
- Investigation: W.L., C.O., A.M.A., Y.B.
- Methodology: W.L., C.O.
- Resources: W.L.
- Supervision: Y.B.
- Visualization: C.O.
- Writing - original draft: V.L., C.O., Y.B.
- Writing - review & editing: C.O., A.M.A., F.G., S.G.S., K.R.F.P.M.
SUPPLEMENTARY MATERIALS
Typical parameter estimates, along with the qualification analyses from the final semi-mechanistic model
Typical parameter estimates from the final semi-mechanistic model (n=1,582)
Outcomes of the univariate logistic analyses regarding the likelihood of complete interval debulking surgery (n=1,230)
Analysis of deviance table for the final logistic regression analyses regarding the likelihood of complete interval debulking surgery (n=1,107)
Univariate and multivariate logistic analyses regarding the probability of at least one BRCA1 or BRCA2 mutation (BRCA1/2 mutation)
Flowchart of the study.
Goodness-of-fit plots. (A) Individual predictions versus observed CA-125 concentrations; Black line: identity line. (B) NPDE; Red line: theorical density, Blue line: NPDE density. (C) Visual predictive checks. Purple areas represent the 95% confidence intervals of the 10th, 50th, and 90th percentiles of simulated data. Red lines represent the median (solid line), and the 10th and 90th percentiles (dashed lines) of the observations.
Receiver operating characteristic curve for the IDS endpoint.
References
- 1.Wright AA, Bohlke K, Armstrong DK, Bookman MA, Cliby WA, Coleman RL, et al. Neoadjuvant chemotherapy for newly diagnosed, advanced ovarian cancer: Society of Gynecologic Oncology and American Society of Clinical Oncology Clinical Practice Guideline. J Clin Oncol. 2016;34:3460–3473. doi: 10.1200/JCO.2016.68.6907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Melamed A, Rauh-Hain JA, Knisely AT, St. Clair CM, Tergas AI, Khoury Collado F, et al. The effect of liberal versus restrictive use of neoadjuvant chemotherapy (NACT) for ovarian cancer on postoperative mortality and long-term survival: a quasi-experimental study. Gynecol Oncol. 2020;159:81. [Google Scholar]
- 3.Colombo N, Sessa C, du Bois A, Ledermann J, McCluggage WG, McNeish I, et al. ESMO-ESGO consensus conference recommendations on ovarian cancer: pathology and molecular biology, early and advanced stages, borderline tumours and recurrent disease. Ann Oncol. 2019;30:672–705. doi: 10.1093/annonc/mdz062. [DOI] [PubMed] [Google Scholar]
- 4.Rutten MJ, van de Vrie R, Bruining A, Spijkerboer AM, Mol BW, Kenter GG, et al. Predicting surgical outcome in patients with International Federation of Gynecology and Obstetrics stage III or IV ovarian cancer using computed tomography: a systematic review of prediction models. Int J Gynecol Cancer. 2015;25:407–415. doi: 10.1097/IGC.0000000000000368. [DOI] [PubMed] [Google Scholar]
- 5.Rodriguez N, Rauh-Hain JA, Shoni M, Berkowitz RS, Muto MG, Feltmate C, et al. Changes in serum CA-125 can predict optimal cytoreduction to no gross residual disease in patients with advanced stage ovarian cancer treated with neoadjuvant chemotherapy. Gynecol Oncol. 2012;125:362–366. doi: 10.1016/j.ygyno.2012.02.006. [DOI] [PubMed] [Google Scholar]
- 6.Lee YJ, Lee IH, Kim YJ, Chung YS, Lee JY, Nam EJ, et al. Evaluation of various kinetic parameters of CA-125 in patients with advanced-stage ovarian cancer undergoing neoadjuvant chemotherapy. PLoS One. 2018;13:e0203366. doi: 10.1371/journal.pone.0203366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.You B, Colomban O, Heywood M, Lee C, Davy M, Reed N, et al. The strong prognostic value of KELIM, a model-based parameter from CA 125 kinetics in ovarian cancer: data from CALYPSO trial (a GINECO-GCIG study) Gynecol Oncol. 2013;130:289–294. doi: 10.1016/j.ygyno.2013.05.013. [DOI] [PubMed] [Google Scholar]
- 8.Rustin GJ, Vergote I, Eisenhauer E, Pujade-Lauraine E, Quinn M, Thigpen T, et al. Definitions for response and progression in ovarian cancer clinical trials incorporating RECIST 1.1 and CA 125 agreed by the Gynecological Cancer Intergroup (GCIG) Int J Gynecol Cancer. 2011;21:419–423. doi: 10.1097/IGC.0b013e3182070f17. [DOI] [PubMed] [Google Scholar]
- 9.Lee CK, Friedlander M, Brown C, Gebski VJ, Georgoulopoulos A, Vergote I, et al. Early decline in cancer antigen 125 as a surrogate for progression-free survival in recurrent ovarian cancer. J Natl Cancer Inst. 2011;103:1338–1342. doi: 10.1093/jnci/djr282. [DOI] [PubMed] [Google Scholar]
- 10.Morgan RD, McNeish IA, Cook AD, James EC, Lord R, Dark G, et al. Objective responses to first-line neoadjuvant carboplatin-paclitaxel regimens for ovarian, fallopian tube, or primary peritoneal carcinoma (ICON8): post-hoc exploratory analysis of a randomised, phase 3 trial. Lancet Oncol. 2021;22:277–288. doi: 10.1016/S1470-2045(20)30591-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Colomban O, Tod M, Leary A, Ray-Coquard I, Lortholary A, Hardy-Bessard AC, et al. Early modeled longitudinal CA-125 kinetics and survival of ovarian cancer patients: a GINECO AGO MRC CTU study. Clin Cancer Res. 2019;25:5342–5350. doi: 10.1158/1078-0432.CCR-18-3335. [DOI] [PubMed] [Google Scholar]
- 12.Almufti R, Wilbaux M, Oza A, Henin E, Freyer G, Tod M, et al. A critical review of the analytical approaches for circulating tumor biomarker kinetics during treatment. Ann Oncol. 2014;25:41–56. doi: 10.1093/annonc/mdt382. [DOI] [PubMed] [Google Scholar]
- 13.Corbaux P, You B, Glasspool RM, Yanaihara N, Tinker AV, Lindemann K, et al. Survival prognostic and surrogate values of the early modeled CA-125 KELIM in newly diagnosed advanced ovarian cancer: data from the GCIG meta-analysis group. Ann Oncol. 2021;32:S744. [Google Scholar]
- 14.You B, Freyer G, Gonzalez-Martin A, Lheureux S, McNeish I, Penson RT, et al. The role of the tumor primary chemosensitivity relative to the success of the medical-surgical management in patients with advanced ovarian carcinomas. Cancer Treat Rev. 2021;100:102294. doi: 10.1016/j.ctrv.2021.102294. [DOI] [PubMed] [Google Scholar]
- 15.You B, Robelin P, Tod M, Louvet C, Lotz JP, Abadie-Lacourtoisie S, et al. CA-125 ELIMination rate constant K (KELIM) is a marker of chemosensitivity in patients with ovarian cancer: results from the phase II CHIVA trial. Clin Cancer Res. 2020;26:4625–4632. doi: 10.1158/1078-0432.CCR-20-0054. [DOI] [PubMed] [Google Scholar]
- 16.You B, Fleming G, Bookman M, Moore KN, Steffensen KD, Coleman RL. Prognostic value and association with veliparib benefit of modeled CA-125 elimination kinetics (KELIM) in patients with newly diagnosed ovarian cancer: analysis from the VELIA/GOG-3005 study. Int J Gynecol Cancer. 2020;30:A24–A25. [Google Scholar]
- 17.Prat J FIGO Committee on Gynecologic Oncology. Staging classification for cancer of the ovary, fallopian tube, and peritoneum. Int J Gynaecol Obstet. 2014;124:1–5. doi: 10.1016/j.ijgo.2013.10.001. [DOI] [PubMed] [Google Scholar]
- 18.Dartois C, Brendel K, Comets E, Laffont CM, Laveille C, Tranchand B, et al. Overview of model-building strategies in population PK/PD analyses: 2002-2004 literature survey. Br J Clin Pharmacol. 2007;64:603–612. doi: 10.1111/j.1365-2125.2007.02975.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Van Wagensveld L, Colomban O, Van Der AA M, Tod M, Sonke GS, Kruitwagen R, et al. 847P The prognostic value of chemosensitivity, estimated by the modeled CA-125 KELIM, in ovarian cancer patients treated with neo-adjuvant chemotherapy in the Netherlands. Ann Oncol. 2020;31:S633. [Google Scholar]
- 20.You B, Clamp A, Cook A, McNeish IA, Colomban O. Differential benefit from fractionated dose-dense first-line chemotherapy for epithelial ovarian cancer (EOC) according to KELIM-evaluated tumor primary chemosensitivity: exploratory analyses of ICON-8 trial. J Clin Oncol. 2021;39:5530. [Google Scholar]
- 21.Heller G, McCormack R, Kheoh T, Molina A, Smith MR, Dreicer R, et al. Circulating tumor cell number as a response measure of prolonged survival for metastatic castration-resistant prostate cancer: a comparison with prostate-specific antigen across five randomized phase III clinical trials. J Clin Oncol. 2018;36:572–580. doi: 10.1200/JCO.2017.75.2998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Beal S, Sheiner LB, Boeckmann R, Bauer RJ. NONMEM User’s Guides (1989–2009) Ellicott City, MD: Icon; 2009. [Google Scholar]
- 23.Jonsson EN, Karlsson MO. Xpose--an S-PLUS based population pharmacokinetic/pharmacodynamic model building aid for NONMEM. Comput Methods Programs Biomed. 1999;58:51–64. doi: 10.1016/s0169-2607(98)00067-4. [DOI] [PubMed] [Google Scholar]
- 24.Lauby A, Colomban O, Corbaux P, Peron J, Van Wagensveld L, Gertych W, et al. The increasing prognostic and predictive roles of the tumor primary chemosensitivity assessed by CA-125 ELIMination rate constant K (KELIM) in ovarian cancer: a narrative review. Cancers (Basel) 2021;14:98. doi: 10.3390/cancers14010098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.You B, Purdy C, Swisher EM, Bookman MA, Fleming GF, Coleman RL, et al. Identification of the ovarian cancer patients experiencing the highest benefit from bevacizumab in first-line setting based on their tumor intrinsic chemosensitivity (KELIM): GOG-0218 validation study. J Clin Oncol. 2022;40:5553. doi: 10.1200/JCO.22.01207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mirza MR, Coleman RL, González-Martín A, Moore KN, Colombo N, Ray-Coquard I, et al. The forefront of ovarian cancer therapy: update on PARP inhibitors. Ann Oncol. 2020;31:1148–1159. doi: 10.1016/j.annonc.2020.06.004. [DOI] [PubMed] [Google Scholar]
- 27.Hollis RL, Churchman M, Gourley C. Distinct implications of different BRCA mutations: efficacy of cytotoxic chemotherapy, PARP inhibition and clinical outcome in ovarian cancer. Onco Targets Ther. 2017;10:2539–2551. doi: 10.2147/OTT.S102569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gorodnova TV, Sokolenko AP, Ivantsov AO, Iyevleva AG, Suspitsin EN, Aleksakhina SN, et al. High response rates to neoadjuvant platinum-based therapy in ovarian cancer patients carrying germ-line BRCA mutation. Cancer Lett. 2015;369:363–367. doi: 10.1016/j.canlet.2015.08.028. [DOI] [PubMed] [Google Scholar]
- 29.Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1) Eur J Cancer. 2009;45:228–247. doi: 10.1016/j.ejca.2008.10.026. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Typical parameter estimates, along with the qualification analyses from the final semi-mechanistic model
Typical parameter estimates from the final semi-mechanistic model (n=1,582)
Outcomes of the univariate logistic analyses regarding the likelihood of complete interval debulking surgery (n=1,230)
Analysis of deviance table for the final logistic regression analyses regarding the likelihood of complete interval debulking surgery (n=1,107)
Univariate and multivariate logistic analyses regarding the probability of at least one BRCA1 or BRCA2 mutation (BRCA1/2 mutation)
Flowchart of the study.
Goodness-of-fit plots. (A) Individual predictions versus observed CA-125 concentrations; Black line: identity line. (B) NPDE; Red line: theorical density, Blue line: NPDE density. (C) Visual predictive checks. Purple areas represent the 95% confidence intervals of the 10th, 50th, and 90th percentiles of simulated data. Red lines represent the median (solid line), and the 10th and 90th percentiles (dashed lines) of the observations.
Receiver operating characteristic curve for the IDS endpoint.