Abstract
Objective
Early iatrogenic menopause in gynecological cancer survivors and BRCA mutation (BRCAm) carriers undergoing risk-reducing salpingo-oophorectomy (RRSO) is a major health concern. Hormone replacement therapy (HRT) is the most effective remedy, but remains underused in clinical practice. The Multicenter Italian Trials in Ovarian cancer and gynecologic malignancies (MITO) group promoted a national survey to investigate the knowledge and attitudes of healthcare professionals regarding the prescription of HRT.
Methods
The survey consisted of a self-administered, multiple-choice 45-item questionnaire, available online to all MITO members for 2 months starting from January 2022.
Results
A total of 61 participants completed the questionnaire (47 out of 180 MITO centers; compliance: 26.1%). Most respondents were female (73.8%), younger than 50 years (65.6%), and gynecologic oncologists (55.7%), working in public general hospitals (49.2%). An 84.4% of specialists actively discuss HRT with patients and 51.0% of patients ask the specialist for an opinion on HRT. The rate of specialists globally in favor of prescribing HRT was 22.9% for ovarian cancer, 49.1% for cervical cancer, and 8.2% for endometrial cancer patients. Most respondents (70.5%) believe HRT is safe for BRCA-mutated patients after RRSO. Nearly 70% of physicians prescribe systemic HRT, while 23.8% prefer local HRT. Most specialists recommend HRT for as long as there is a benefit and generally for up to 5 years.
Conclusion
Real-world data suggest that many healthcare professionals still do not easily prescribe HRT for gynecological cancer survivors and BRCA mutation carriers after RRSO. Further efforts are required to implement the use of HRT in clinical practice and to support both clinicians in recommending HRT and patients in accepting it.
Keywords: Ovarian Neoplasms, Uterine Neoplasms, Hormone Replacement Therapy, Menopause, Surgery, Survivors
Synopsis
Iatrogenic menopause-related morbidity and mortality are major health issues, although often underestimated. Hormone replacement therapy is effective but remains underused in gynecological cancer and BRCA-mutated patients. Further efforts are required to fully implement the use of hormone replacement therapy in clinical practice for selected patients.
INTRODUCTION
With the growing population aging, the number of menopausal women is constantly increasing to the point that women now spend more than one-third of their life in the postmenopausal phase [1]. The menopause-related morbidity and mortality are major health issues, although often underestimated [2]. These affect not only the general population experiencing natural menopause but even more women undergoing early (before age 45) iatrogenic menopause, such as patients who have had or are at risk of developing gynecological cancer [3].
Gynecological cancers affect a growing number of women worldwide. Significant improvements in diagnostics and treatment opportunities have led to a significant increase in survival rates, raising even more attention on the menopausal issues resulting from oncological treatments, such as surgery, chemoradiotherapy-induced ovarian failure, and the anti-estrogenic effects of endocrine therapy [4]. In addition, given the spread of testing for pathogenic gene variants, such as BRCA mutations (BRCAm), more women are aware of their higher lifetime cancer risk and undergo risk-reducing salpingo-oophorectomy (RRSO) at a premenopausal age [5].
Dealing with iatrogenic menopause in gynecological cancer survivors and BRCAm carriers is particularly challenging as the well-recognized benefits of hormone replacement therapy (HRT) must be balanced with oncological safety [6,7]. Iatrogenic menopause may result not only in the abrupt onset of early symptoms, but also in increased long-term morbidity (e.g., osteoporosis, cardiovascular disease, dementia) and all-cause mortality compared with physiologic menopause [8,9]. Notably, BRCAm carriers undergoing RRSO are healthy women, and preserving their quality of life is crucial [10]. Not all menopausal symptoms require treatment, but when they do, HRT is the most effective. However, the well-recognized role of hormone receptors in many gynecological cancers poses a huge challenge to the management of menopause. Despite reassuring data and comprehensive international guidelines on oncological safety in various settings, HRT remains inexplicably underused in daily practice [2,3,4,5,6,11,12].
The Multicenter Italian Trials in Ovarian cancer and gynecologic malignancies (MITO) group promoted a national survey to deepen the knowledge and attitudes of different healthcare professionals regarding the prescription of HRT to gynecological cancer survivors and BRCAm carriers after RRSO.
MATERIALS AND METHODS
This nationwide survey consisted of a self-administered, multiple-choice questionnaire. A structured list of questions was designed by I.P. and V.D.D. and independently tested for readability and clarity by a group of physicians not directly involved in the survey design. The questionnaire was then reviewed by the MITO scientific committee and approved by the MITO Internal Review Board. An Internet program and hosting site (www.surveymonkey.com) was used to develop an online survey whose link was emailed to all MITO members and remained available from January 3 to March 1, 2022. Each member was assigned a unique survey link, which allowed for the survey to be completed only once. The target population included physicians working in the field of gynecologic oncology who were members of the MITO group at the time of the survey.
The final survey contained 45 multiple-choice questions (Table 1) divided into 3 sections. The first section (4 questions) assessed the personal characteristics of the respondents (sex, age, specialty, work center). The second section (22 questions) assessed physician knowledge and confidence in prescribing HRT to gynecological cancer survivors, while the third section (19 questions) specifically addressed the subset of BRCAm carriers (without prior history of breast cancer or other BRCA-related cancers) undergoing RRSO.
Table 1. Survey questions.
| Section | Questions |
|---|---|
| Section 1: Personal characteristics of respondents | 1. Sex |
| 2. Age | |
| 3. Institutional affiliation | |
| 4. Medical speciality | |
| Section 2: HRT in gynecological cancer survivors | 5. How many post-menopausal gynecological cancer patients refer to your center every year? |
| 6. Of these patients, how many of them are aged <40, 40–44, 45–49, and 50–55? | |
| 7. In your experience, how often gynecological cancer patients experience menopausal symptoms? | |
| 8. How many patients ask you for a consult about HRT? | |
| 9. Do you consider safe using HRT in patients with ovarian cancer? Cervical cancer? Endometrial cancer? | |
| 10. How often do you spend time to discuss about iatrogenic menopause? | |
| 11. What do patients most often complain about? | |
| 12. How often do you offer HRT to menopausal patients with previous ovarian cancer? | |
| 13. How often do you offer HRT to menopausal patients with previous cervical cancer? | |
| 14. How often do you offer HRT to menopausal patients with previous endometrial cancer? | |
| 15. In case of previous answer “always/often”, why? | |
| 16. In case of previous answer “sometimes/never”, why? | |
| 17. In case of previous answer “it depends”, which factors lead your decision? | |
| 18. What type of HRT (systemic and/or local) do you prescribe? | |
| 19. How often so you ask for an expert opinion to prescribe HRT? | |
| 20. How many patients accept HRT when you offer it? | |
| 21. How long should patients receive HRT for? | |
| 22. Why do patients refuse HRT? | |
| 23. How long do patients use HRT for? | |
| 24. How often do patients ask for a change of HRT? | |
| 25. Are patients under HRT globally satisfied? | |
| 26. Why patients interrupt HRT? | |
| Section 3: HRT in BRCAm patients (without prior breast cancer) | 27. How many BRCAm patients undergoing RRSO refer to your center every year? |
| 28. Do you consider safe using HRT in BRCAm patients after RRSO without hysterectomy? | |
| 29. Do you consider safe using HRT in BRCAm patients after RRSO plus hysterectomy? | |
| 30. How often do you spend time to discuss about iatrogenic menopause after RRSO? | |
| 31. How many BRCAm patients ask you for an opinion about HRT after RRSO? | |
| 32. What do patients most often complain about? | |
| 33. How often do you offer HRT to BRCAm patients after RRSO? | |
| 34. In case of previous answer “always/often”, why? | |
| 35. In case of previous answer “sometimes/never”, why? | |
| 36. In case of previous answer “it depends”, which factors lead your decision? | |
| 37. What type of HRT (systemic and/or local) do you prescribe? | |
| 38. How often do you ask for an expert opinion to prescribe HRT for BRCAm patients? | |
| 39. How many BRCAm patients accept HRT when you offer it? | |
| 40. How long should BRCAm patients receive HRT for? | |
| 41. Why do BRCAm patients refuse HRT? | |
| 42. How long should BRCAm patients use HRT? | |
| 43. How often do BRCAm patients ask for a change of HRT? | |
| 44. Are BRCAm patients under HRT globally satisfied? | |
| 45. Why BRCAm patients interrupt HRT? |
BRCAm, BRCA mutation; HRT, hormone replacement therapy; RRSO, risk-reducing salpingo-oophorectomy.
The authors and respondents declared no conflicts of interest and no financial support. Ethical approval was not required, and all responses were anonymized. The online format allowed for automatic data import into an Excel database ready for statistical analysis. Data processing was performed at the Department of Maternal and Child Health and Urological Sciences at the Sapienza University of Rome. Survey data are presented using descriptive statistics. Categorical and continuous variables are reported as frequencies and percentages. Response percentages refer to the number of respondents to each question. All analyses were performed with SPSS statistical software, version 20.0 (SPSS Statistics; IBM Corp., Armonk, NY, USA) for Mac.
RESULTS
A total of 61 participants completed the online survey. Detailed answers to each question are summarized in Tables S1, S2, S3. Briefly, questionnaires were compiled in 47 of 180 MITO centers (compliance: 26.2%). Most respondents were female (73.8%), less than 50 years old (65.6%), gynecologic oncologists (55.8%) and working at public general hospitals (49.2%).
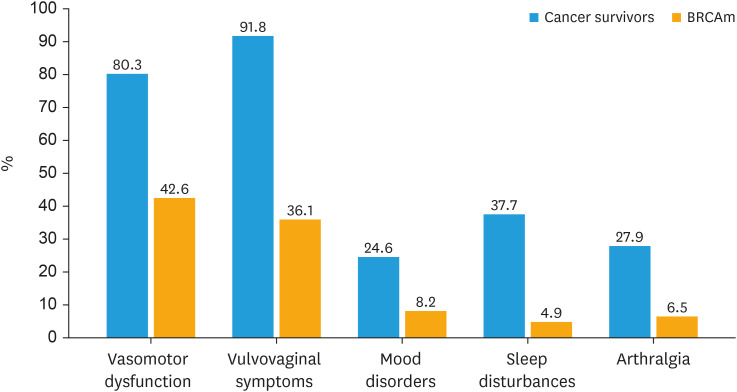
Overall, 84.4% of specialists (64.8% gynecologic oncologists and 35.2% medical oncologists) reported actively discussing HRT with patients (including both cancer survivors and BRCAm), 7.4% at the patient’s request, while 6.6% of them rarely find the time to discuss HRT. On average 51.0% of patients ask the specialist for an opinion on HRT. The most commonly reported menopausal symptoms are vulvovaginal disorders (91.8%) in gynecological cancer survivors and vasomotor symptoms (42.6%) in BRCA-mutated patients (Fig. 1).
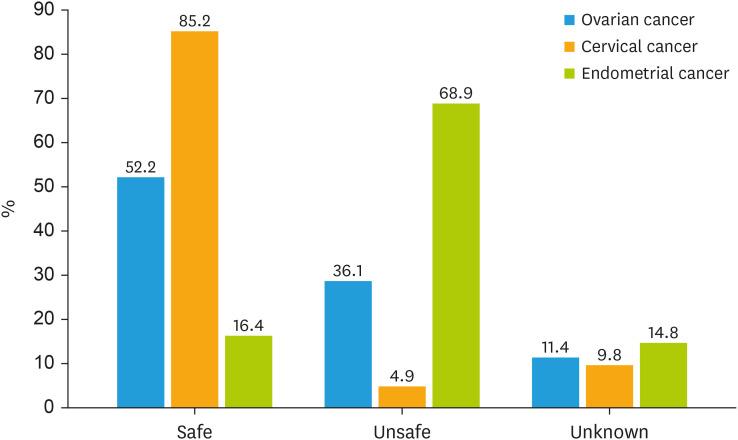
Fig. 1. Safety of HRT after gynecological cancers.
HRT, hormone replacement therapy.
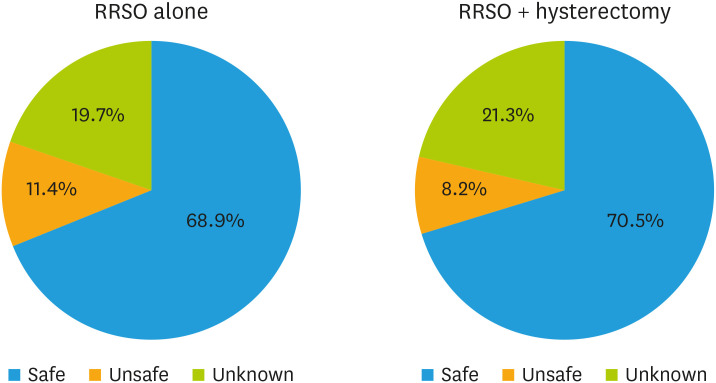
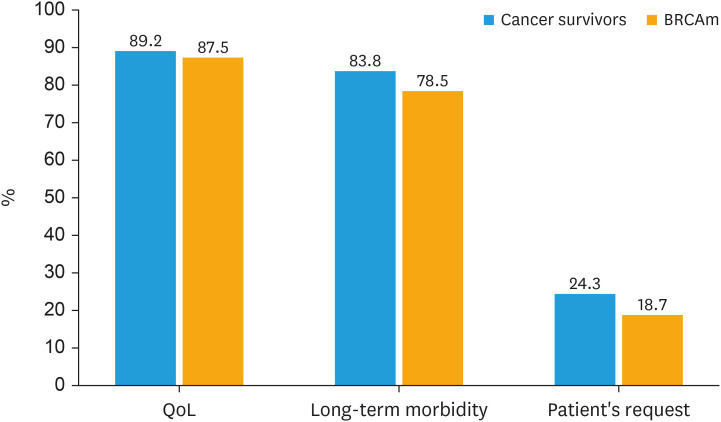
Depending on the cancer histotype, the rate of respondents globally in favor of prescribing HRT was 22.9% for ovarian cancer, 49.1% for cervical cancer, and 8.2% for endometrial cancer patients (Fig. 2). Most respondents considered HRT to be safe for BRCA-mutated patients after prophylactic surgery (68.9% for RRSO alone and 70.5% for RRSO plus hysterectomy), while a lower percentage considered it unsafe (11.4% and 8.2%, respectively) or did not know the answer (19.7% and 21.3%, respectively) (Fig. 3). The main reasons for prescribing HRT include improving in quality of life (89.2% for cancer survivors and 87.5% for BRCA-mutated patients) and reducing long-term morbidity associated with menopause (83.8% for cancer survivors and 78.5% for BRCA-mutated patients) (Fig. 4). The main reasons for not prescribing HRT include concerns about the oncological safety (57.4% for cancer survivors and 50.0% for BRCA-mutated patients) and lack of explicit patient request (39.1% for cancer survivors and 50.0% for BRCA-mutated patients) (Fig. S1).
Fig. 2. Safety of HRT after RRSO ± hysterectomy in BRCAm patients.
BRCAm, BRCA mutation; HRT, hormone replacement therapy; RRSO, risk-reducing salpingo-oophorectomy.
Fig. 3. Menopausal symptoms in gynecological cancer survivors and BRCAm patients.
BRCAm, BRCA mutation.
Fig. 4. Main reasons for prescribing HRT.
BRCAm, BRCA mutation; HRT, hormone replacement therapy.
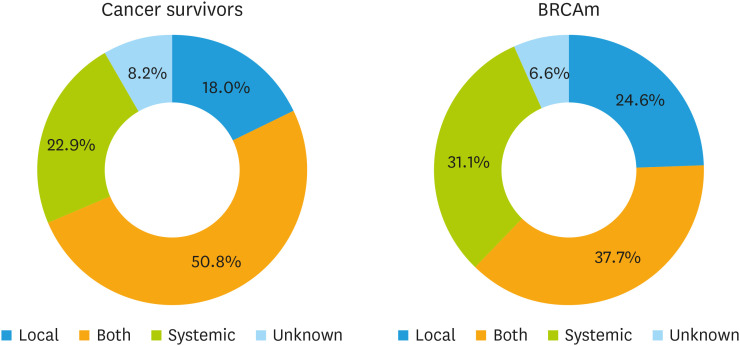
Nearly 70% of physicians prescribe systemic HRT (44.3% systemic plus local and 24.6% systemic only), while 23.8% prefer local HRT alone. Physicians prescribing systemic HRT (either alone or with local HRT) were gynecologic oncologists in 63.9% of cases and medical oncologists in 30.9%, while those preferring local HRT alone were 37.9% and 58.6%, respectively. The rates of systemic vs local HRT stratified for cancer survivors and BRCAm patients are shown in Fig. 5.
Fig. 5. Type of HRT prescribed.
BRCAm, BRCA mutation; HRT, hormone replacement therapy.
Overall, 65.5% of physicians reported that HRT, when offered, was accepted by more than 50% of patients (including both cancer survivors and BRCAm). The main reason for refusing HRT is concern about the oncological risk (67.2% for cancer survivors and 31.1% for BRCA-mutated patients) (Fig. S2). The vast majority of respondents (85.2%) reported that patients were generally satisfied with the use of HRT. Regarding the duration of HRT, 14.5% recommend it for less than 2 years, 24.1% for up to 5 years, and 27.1% for as long as it provides benefit, while the decision is shared with the patient in 23.8% of cases.
DISCUSSION
The idea to conduct a survey on HRT for gynecological cancer survivors and BRCAm carriers after RRSO arose from outpatient follow-up experience with postmenopausal women experiencing distressing symptoms that severely impact their quality of life and couple relationships. Too often, patients do not spontaneously report their menopausal symptoms unless specifically asked by the specialist [13].
The role of HRT in the general female population is still largely debated, and it becomes even more challenging when referring to patients who have had, or are at risk of developing, gynecological cancer. In addition, younger patients undergoing iatrogenic menopause experience more severe symptoms than women undergoing natural menopause [8,9]. The clinical benefits of HRT extend beyond the management of early symptoms and include long-term complications (e.g., osteoporosis, cardiovascular disease, dementia) and all-cause mortality, especially in the case of premature menopause [14,15]. Although international menopause societies recommend HRT for young symptomatic patients, there seems to be a large gap between the encouraging research data and its actual use in real-life settings. Therefore, the present nationwide survey aimed to better understand the underlying possible reasons and to draw attention to a somewhat uncomfortable taboo topic by directly asking experts in the field.
The majority of respondents were young gynecologic oncologists as they are likely to be more comfortable prescribing HRT. Indeed, the older MITO members certainly remember the damaging stigma surrounding HRT after the publication and press release of the Women’s Health Initiative (WHI) results in 2002 [16], which has been overcome with great effort in recent years. The initial misinterpretation of the WHI findings, combined with media attention and miscommunication led to fear and confusion about the use of HRT, resulting in a dramatic reduction in HRT prescriptions for symptomatic postmenopausal women worldwide. The WHI trial was limited by low adherence, inadequate power to detect risks for some outcomes, and evaluation of old formulations and few regimens. Moreover, the WHI studies were weighted toward women who were 60 or older, so the results may not be applicable to younger women who tend to be healthier and have more menopausal symptoms. Subsequent studies by the WIH and others have clearly shown that younger women and those nearing menopause have a favorable benefit-risk ratio, with a protective effect on coronary heart disease and no significantly increased risk of venous thrombosis, ischemic stroke, and breast cancer [17].
The number of patients requesting HRT is still too low, highlighting the need to further raise public awareness and unhinge old, unfounded prejudices on this topic. Although up to 90% of postmenopausal women report symptoms, only about one in two patients typically ask their doctor about the HRT. Even when the HRT is considered safe and actively offered by specialists, one in two patients refuses to start it, mainly because of the oncological risk. However, it is the professionals who need to change their attitudes on this crucial issue before the patients. In up to one-third of the cases in this survey, HRT counseling was omitted because it was not explicitly requested by the patient. Our survey perfectly outlines the gap between theory and practice, as the number of physicians who prescribe HRT in daily practice is lower than the number of physicians who declare HRT is safe. In line with recent recommendations on the safety of HRT based on gynecological cancer histotype, our respondents consider HRT to be generally safe in cervical cancer patients and BRCA-mutated women, unsafe in endometrial cancer, while HRT in ovarian cancer patients is discussed on a case-by-case basis. In fact, HRT is contraindicated in the following cases: 1) breast cancer (except for the triple-negative histotype) [18,19,20]; 2) uterine cancer (except for the serous, clear cell, and undifferentiated histotypes) [21,22]; 3) low-grade serous and endometrioid ovarian cancer and sex cord stromal ovarian tumors [23,24,25]. Surprisingly, although the survey was addressed to experts in the field of gynecologic oncology, a relevant percentage of respondents did not know whether HRT is safe in gynecological cancer survivors (11.4% in ovarian cancer, 9.8% in cervical cancer, and 14.9% in endometrial cancer) and in BRCA-mutated patients after RRSO (20% of respondents). Notably, the lower the activity/patient volume, the lower the HRT prescription, suggesting the importance of referring these patients to centers with expertise in this area.
The role of HRT is crucial in the healthy BRCAm carriers undergoing RRSO, as they need to preserve the survival benefit and their quality of life [26,27,28,29]. Indeed, it has been demonstrated that healthy women without BRCAm who underwent bilateral salpingo-oophorectomy before the age of 45 years and did not use HRT had an increased risk of death compared with matched controls (hazard ratio=1.67; 95% confidence interval=1.16–2.40) [30]. The main concern is the potential increase in the already elevated risk for breast cancer. However, international guidelines are quite reassuring and recommend short-term (<5 years) HRT in all BRCA-mutated women without a personal history of breast cancer [31]. If the uterus has been removed, estrogens alone should be preferred to the estro-progestin combination to further reduce the risk of breast cancer [32]. Micronized progesterone has been shown to be safer when progestin use is necessary [33]. The role of hysterectomy in BRCA-mutated patients is still largely controversial. Apart from reducing the risk of serous endometrial cancer (especially in the case of BRCA1 mutation) [34,35], the main advantage is the possibility to use estrogen-only HRT [32,36]. However, this must be balanced against the increased surgical complications and costs [37]. Pending further evidence, hysterectomy with RRSO should be considered in the setting of concomitant benign uterine disease, Lynch syndrome, and in breast cancer patients on tamoxifen maintenance.
Regarding the type of HRT, gynecologic oncologists generally prescribe systemic HRT, either alone or combined with local HRT, while medical oncologists prefer to offer vaginal HRT alone, as they are likely to be more concerned with oncologic safety. Most specialists agree that HRT should be used as long as there is a benefit, preferably for a maximum of 5 years, depending on the overall risk-benefit ratio.
Limitations of this survey include the low response rate, as only around one-quarter of all invited MITO centers participated, leaving room for speculation about the actual attention given to this issue in clinical practice. Moreover, the respondents are members of the MITO group and mostly experts in the field of gynecologic oncology; therefore, the results of this survey may not accurately reflect the general awareness and attitudes of physicians.
Much of the evidence on the risks and benefits of HRT comes from studies of older women undergoing natural menopause, and these data are not necessarily relevant to the question of HRT after surgical menopause. The current evidence should be strengthened because it includes few prospective studies with small sample sizes, short follow-up, heterogeneity between studies (e.g., hormone regimens), and often analysis of old formulations (more than 15 years old) that used higher doses of estrogen. Further research on the use of HRT in higher-risk subpopulations, including BRCAm carriers, is warranted. Several ongoing clinical trials are currently investigating the role of different therapeutic approaches for the treatment of menopausal symptoms, such as micronized progesterone with estrogen (NCT05586724), bazedoxifene plus conjugated estrogens (NCT04821141), transdermal estradiol (NCT03556800), local silicone gel (VITAL-E study; NCT05672901), local dehydroepiandrosterone (DHEA) (NCT05586711), resveratrol (NCT05410093), vaginal hyaluronic acid treatment and autologous platelet rich plasma treatment (NCT05571527), acupuncture (Acu-HOTFLASH study; NCT05760222), and EMBr Wave® technology (NCT05086705).
In conclusion, this survey provided interesting real-life insights into the inadequate approach to HRT in patients with, or at risk of, gynecological cancer. There is still a large gap between theory and routine clinical practice. International consensus guidelines should be implemented to further stress the efficacy and safety of HRT and support experts in recommending it. More efforts are required to further raise public awareness and properly educate people about the real risks and benefits of HRT. Multidisciplinarity and a solid collaboration between gynecologic oncologists and medical oncologists are crucial to tailor the treatment strategy and improve patient care.
ACKNOWLEDGEMENTS
The authors thank all the members of Multicenter Italian Trials in Ovarian cancer and gynecologic malignancies (MITO) group for the support.
Footnotes
Conflict of Interest: No potential conflict of interest relevant to this article was reported.
- Conceptualization: P.I.
- Data curation: P.I., C.G., D.V., T.C., S.A., P.G., G.R., G.A., S.V., B.G., T.F., G.D., G.G., M.A., M.L., P.S.
- Formal analysis: P.I., C.G., D.V., T.C.
- Investigation: P.I., C.G., D.V., T.C.
- Methodology: P.I., C.G., D.V., T.C., S.A., P.G., G.R., G.A., S.V., B.G., T.F., G.D., G.G., M.A., M.L., P.S.
- Project administration: P.I.
- Resources: P.I.
- Software: P.I., C.G., D.V., T.C.
- Supervision: P.I., T.C.
- Validation: P.I., C.G., D.V., T.C.
- Visualization: P.I., C.G., D.V., T.C., S.A., G.R., G.A., S.V., B.G., T.F., G.D., G.G., M.A., M.L., P.S.
- Writing - original draft: P.I., D.V., T.C., P.G., C.G.
- Writing - review & editing: P.I., C.G., D.V., T.C., S.A., P.G., G.R., G.A., S.V., B.G., T.F., G.D., G.G., M.A., M.L., P.S.
SUPPLEMENTARY MATERIALS
Section 1: Personal characteristics of respondents (n=61)
Section 2: HRT in gynecological cancer survivors (n=61)
Section 3: HRT in BRCAm patients (n=61)
Main reasons for not prescribing HRT.
Main reasons why patients refuse HRT.
References
- 1.Auro K, Joensuu A, Fischer K, Kettunen J, Salo P, Mattsson H, et al. A metabolic view on menopause and ageing. Nat Commun. 2014;5:4708. doi: 10.1038/ncomms5708. [DOI] [PubMed] [Google Scholar]
- 2.de Villiers TJ, Hall JE, Pinkerton JV, Pérez SC, Rees M, Yang C, et al. Revised global consensus statement on menopausal hormone therapy. Maturitas. 2016;91:153–155. doi: 10.1016/j.maturitas.2016.06.001. [DOI] [PubMed] [Google Scholar]
- 3.Rees M, Angioli R, Coleman RL, Glasspool R, Plotti F, Simoncini T, et al. European Menopause and Andropause Society (EMAS) and International Gynecologic Cancer Society (IGCS) position statement on managing the menopause after gynecological cancer: focus on menopausal symptoms and osteoporosis. Maturitas. 2020;134:56–61. doi: 10.1016/j.maturitas.2020.01.005. [DOI] [PubMed] [Google Scholar]
- 4.Sinno AK, Pinkerton J, Febbraro T, Jones N, Khanna N, Temkin S, et al. Hormone therapy (HT) in women with gynecologic cancers and in women at high risk for developing a gynecologic cancer: a Society of Gynecologic Oncology (SGO) clinical practice statement: this practice statement has been endorsed by The North American Menopause Society. Gynecol Oncol. 2020;157:303–306. doi: 10.1016/j.ygyno.2020.01.035. [DOI] [PubMed] [Google Scholar]
- 5.Brennan A, Brennan D, Rees M, Hickey M. Management of menopausal symptoms and ovarian function preservation in women with gynecological cancer. Int J Gynecol Cancer. 2021;31:352–359. doi: 10.1136/ijgc-2020-002032. [DOI] [PubMed] [Google Scholar]
- 6.Brennan A, Hickey M. The use of menopausal hormone therapy after cancer. Best Pract Res Clin Obstet Gynaecol. 2022;81:22–30. doi: 10.1016/j.bpobgyn.2021.11.005. [DOI] [PubMed] [Google Scholar]
- 7.Szabo RA, Marino JL, Hickey M. Managing menopausal symptoms after cancer. Climacteric. 2019;22:572–578. doi: 10.1080/13697137.2019.1646718. [DOI] [PubMed] [Google Scholar]
- 8.Rodriguez M, Shoupe D. Surgical Menopause. Endocrinol Metab Clin North Am. 2015;44:531–542. doi: 10.1016/j.ecl.2015.05.003. [DOI] [PubMed] [Google Scholar]
- 9.Marino JL, Saunders CM, Emery LI, Green H, Doherty DA, Hickey M. Nature and severity of menopausal symptoms and their impact on quality of life and sexual function in cancer survivors compared with women without a cancer history. Menopause. 2014;21:267–274. doi: 10.1097/GME.0b013e3182976f46. [DOI] [PubMed] [Google Scholar]
- 10.Stuursma A, van Driel CM, Wessels NJ, de Bock GH, Mourits MJ. Severity and duration of menopausal symptoms after risk-reducing salpingo-oophorectomy. Maturitas. 2018;111:69–76. doi: 10.1016/j.maturitas.2018.01.012. [DOI] [PubMed] [Google Scholar]
- 11.Vermeulen RF, Korse CM, Kenter GG, Brood-van Zanten MM, Beurden MV. Safety of hormone replacement therapy following risk-reducing salpingo-oophorectomy: systematic review of literature and guidelines. Climacteric. 2019;22:352–360. doi: 10.1080/13697137.2019.1582622. [DOI] [PubMed] [Google Scholar]
- 12.Gordhandas S, Norquist BM, Pennington KP, Yung RL, Laya MB, Swisher EM. Hormone replacement therapy after risk reducing salpingo-oophorectomy in patients with BRCA1 or BRCA2 mutations; a systematic review of risks and benefits. Gynecol Oncol. 2019;153:192–200. doi: 10.1016/j.ygyno.2018.12.014. [DOI] [PubMed] [Google Scholar]
- 13.Campfield Bonadies D, Moyer A, Matloff ET. What I wish I’d known before surgery: BRCA carriers’ perspectives after bilateral salipingo-oophorectomy. Fam Cancer. 2011;10:79–85. doi: 10.1007/s10689-010-9384-z. [DOI] [PubMed] [Google Scholar]
- 14.Faubion SS, Kuhle CL, Shuster LT, Rocca WA. Long-term health consequences of premature or early menopause and considerations for management. Climacteric. 2015;18:483–491. doi: 10.3109/13697137.2015.1020484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shuster LT, Rhodes DJ, Gostout BS, Grossardt BR, Rocca WA. Premature menopause or early menopause: long-term health consequences. Maturitas. 2010;65:161–166. doi: 10.1016/j.maturitas.2009.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rossouw JE, Anderson GL, Prentice RL, LaCroix AZ, Kooperberg C, Stefanick ML, et al. Risks and benefits of estrogen plus progestin in healthy postmenopausal women: principal results From the Women’s Health Initiative randomized controlled trial. JAMA. 2002;288:321–333. doi: 10.1001/jama.288.3.321. [DOI] [PubMed] [Google Scholar]
- 17.Lobo RA. Where are we 10 years after the Women’s Health Initiative? J Clin Endocrinol Metab. 2013;98:1771–1780. doi: 10.1210/jc.2012-4070. [DOI] [PubMed] [Google Scholar]
- 18.Holmberg L, Iversen OE, Rudenstam CM, Hammar M, Kumpulainen E, Jaskiewicz J, et al. Increased risk of recurrence after hormone replacement therapy in breast cancer survivors. J Natl Cancer Inst. 2008;100:475–482. doi: 10.1093/jnci/djn058. [DOI] [PubMed] [Google Scholar]
- 19.Faubion SS, Larkin LC, Stuenkel CA, Bachmann GA, Chism LA, Kagan R, et al. Management of genitourinary syndrome of menopause in women with or at high risk for breast cancer: consensus recommendations from the North American Menopause Society and the International Society for the Study of Women’s Sexual Health. Menopause. 2018;25:596–608. doi: 10.1097/GME.0000000000001121. [DOI] [PubMed] [Google Scholar]
- 20.SOGC. Use of hormonal replacement therapy after treatment of breast cancer. Number 142, May 2004. Int J Gynaecol Obstet. 2005;88:216–221. doi: 10.1016/j.ijgo.2004.11.002. [DOI] [PubMed] [Google Scholar]
- 21.Londero AP, Parisi N, Tassi A, Bertozzi S, Cagnacci A. Hormone replacement therapy in endometrial cancer survivors: a meta-analysis. J Clin Med. 2021;10:3165. doi: 10.3390/jcm10143165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Edey KA, Rundle S, Hickey M. Hormone replacement therapy for women previously treated for endometrial cancer. Cochrane Database Syst Rev. 2018;5:CD008830. doi: 10.1002/14651858.CD008830.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pergialiotis V, Pitsouni E, Prodromidou A, Frountzas M, Perrea DN, Vlachos GD. Hormone therapy for ovarian cancer survivors: systematic review and meta-analysis. Menopause. 2016;23:335–342. doi: 10.1097/GME.0000000000000508. [DOI] [PubMed] [Google Scholar]
- 24.Power L, Lefas G, Lambert P, Kim D, Evaniuk D, Lotocki R, et al. Hormone use after nonserous epithelial ovarian cancer: overall and disease-free survival. Obstet Gynecol. 2016;127:837–847. doi: 10.1097/AOG.0000000000001396. [DOI] [PubMed] [Google Scholar]
- 25.Saeaib N, Peeyananjarassri K, Liabsuetrakul T, Buhachat R, Myriokefalitaki E. Hormone replacement therapy after surgery for epithelial ovarian cancer. Cochrane Database Syst Rev. 2020;1:CD012559. doi: 10.1002/14651858.CD012559.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chan JL, Senapati S, Johnson LN, DiGiovanni L, Voong C, Butts SF, et al. Risk factors for sexual dysfunction in BRCA mutation carriers after risk-reducing salpingo-oophorectomy. Menopause. 2019;26:132–139. doi: 10.1097/GME.0000000000001176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Finch AP, Lubinski J, Møller P, Singer CF, Karlan B, Senter L, et al. Impact of oophorectomy on cancer incidence and mortality in women with a BRCA1 or BRCA2 mutation. J Clin Oncol. 2014;32:1547–1553. doi: 10.1200/JCO.2013.53.2820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Marchetti C, De Felice F, Palaia I, Perniola G, Musella A, Musio D, et al. Risk-reducing salpingo-oophorectomy: a meta-analysis on impact on ovarian cancer risk and all cause mortality in BRCA 1 and BRCA 2 mutation carriers. BMC Womens Health. 2014;14:150. doi: 10.1186/s12905-014-0150-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Madalinska JB, van Beurden M, Bleiker EM, Valdimarsdottir HB, Hollenstein J, Massuger LF, et al. The impact of hormone replacement therapy on menopausal symptoms in younger high-risk women after prophylactic salpingo-oophorectomy. J Clin Oncol. 2006;24:3576–3582. doi: 10.1200/JCO.2005.05.1896. [DOI] [PubMed] [Google Scholar]
- 30.Rocca WA, Grossardt BR, de Andrade M, Malkasian GD, Melton LJ., 3rd Survival patterns after oophorectomy in premenopausal women: a population-based cohort study. Lancet Oncol. 2006;7:821–828. doi: 10.1016/S1470-2045(06)70869-5. [DOI] [PubMed] [Google Scholar]
- 31.Sessa C, Balmaña J, Bober SL, Cardoso MJ, Colombo N, Curigliano G, et al. Risk reduction and screening of cancer in hereditary breast-ovarian cancer syndromes: ESMO Clinical Practice Guideline. Ann Oncol. 2023;34:33–47. doi: 10.1016/j.annonc.2022.10.004. [DOI] [PubMed] [Google Scholar]
- 32.Chlebowski RT, Rohan TE, Manson JE, Aragaki AK, Kaunitz A, Stefanick ML, et al. Breast cancer after use of estrogen plus progestin and estrogen alone: analyses of data from 2 Women’s Health Initiative randomized clinical trials. JAMA Oncol. 2015;1:296–305. doi: 10.1001/jamaoncol.2015.0494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Stute P, Wildt L, Neulen J. The impact of micronized progesterone on breast cancer risk: a systematic review. Climacteric. 2018;21:111–122. doi: 10.1080/13697137.2017.1421925. [DOI] [PubMed] [Google Scholar]
- 34.Matanes E, Volodarsky-Perel A, Eisenberg N, Rottenstreich M, Yasmeen A, Mitric C, et al. Endometrial cancer in germline BRCA mutation carriers: a systematic review and meta-analysis. J Minim Invasive Gynecol. 2021;28:947–956. doi: 10.1016/j.jmig.2020.11.023. [DOI] [PubMed] [Google Scholar]
- 35.de Jonge MM, Mooyaart AL, Vreeswijk MP, de Kroon CD, van Wezel T, van Asperen CJ, et al. Linking uterine serous carcinoma to BRCA1/2-associated cancer syndrome: A meta-analysis and case report. Eur J Cancer. 2017;72:215–225. doi: 10.1016/j.ejca.2016.11.028. [DOI] [PubMed] [Google Scholar]
- 36.Anderson GL, Chlebowski RT, Aragaki AK, Kuller LH, Manson JE, Gass M, et al. Conjugated equine oestrogen and breast cancer incidence and mortality in postmenopausal women with hysterectomy: extended follow-up of the Women’s Health Initiative randomised placebo-controlled trial. Lancet Oncol. 2012;13:476–486. doi: 10.1016/S1470-2045(12)70075-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Aarts JW, Nieboer TE, Johnson N, Tavender E, Garry R, Mol BW, et al. Surgical approach to hysterectomy for benign gynaecological disease. Cochrane Database Syst Rev. 2015;2015:CD003677. doi: 10.1002/14651858.CD003677.pub5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Section 1: Personal characteristics of respondents (n=61)
Section 2: HRT in gynecological cancer survivors (n=61)
Section 3: HRT in BRCAm patients (n=61)
Main reasons for not prescribing HRT.
Main reasons why patients refuse HRT.