Abstract
The comprehensive systems-biology database (CSB.DB) was used to reveal brassinosteroid (BR)-related genes from expression profiles based on co-response analyses. Genes exhibiting simultaneous changes in transcript levels are candidates of common transcriptional regulation. Combining numerous different experiments in data matrices allows ruling out outliers and conditional changes of transcript levels. CSB.DB was queried for transcriptional co-responses with the BR-signalling components BRI1 and BAK1: 301 out of 9694 genes represented in the nasc0271 database showed co-responses with both genes. As expected, these genes comprised pathway-involved genes (e.g. 72 BR-induced genes), because the BRI1 and BAK1 proteins are required for BR-responses. But transcript co-response takes the analysis a step further compared with direct approaches because BR-related non BR-responsive genes were identified. Insights into networks and the functional context of genes are provided, because factors determining expression patterns are reflected in correlations. Our findings demonstrate that transcript co-response analysis presents a valuable resource to uncover common regulatory patterns of genes. Different data matrices in CSB.DB allow examination of specific biological questions. All matrices are publicly available through CSB.DB. This work presents one possible roadmap to use the CSB.DB resources.
INTRODUCTION
Brassinosteroids (BRs) are highly potent growth-promoting sterol derivatives. BR-deficient and BR-insensitive mutants in Arabidopsis, pea, tomato, barley and rice show dwarfism (1). The growth-promoting effect of BR was assigned to changes in transcript levels of genes involved in cell wall modifications such as xyloglucan endotransglucosylase/hydrolases and expansins. Other BR-regulated genes point to further mechanisms contributing to growth. BR apparently coordinates diverse processes, partly through interactions with other phytohormones. Enhanced resistance of BR-treated plants to temperature, salt, water, phytopathogens and other environmental stresses has been reported (2–4). However, underlying molecular mechanisms are unknown. The growth effects of exogenous BR are light-dependent. Arabidopsis mutants such as det2, cpd and bri1 display short hypocotyls, opened cotyledons, and emergence of primary leaves in darkness. These findings suggest a cross-talk between photomorphogenesis and steroid signal transduction (5).
In several studies, BR-responsive gene expression in Arabidopsis was analysed (6–10). Comparisons of expression profiling experiments revealed that the majority of identified genes do not show consistent BR-dependent expression in different genotypes, environmental conditions, developmental stages and tissues, and upon BR-treatment (11,12). Thus, gene expression patterns are conditional, and the identified genes probably present only a subset of genes involved in BR-responses. Another reason for the incomplete discovery of genomic effects is the hitherto limited number of experiments, as gene expression varies even under highly controlled conditions. Many genes fail to meet the stringent selection criteria routinely applied in expression profiling experiments.
Cross-experiment co-response analysis provides an alternative approach which is based on the assumption that common transcriptional control of genes should be reflected in synchronous changes in transcript levels. Co-response analysis describes common changes of transcript levels among gene pairs. Publicly available expression profiles represent a rich resource for cross-experiment investigations. CSB.DB [http://csbdb.mpimp-golm.mpg.de/ (13)] provides access to co-response analysis based on numerous independent expression profiling experiments.
Here, we describe a strategy to identify BR-associated genes using the BRI1 and BAK1 genes. In the following, these anchors for subsequent co-response analyses will be termed ‘guide genes’ and correlated genes will be termed ‘associated genes’. We demonstrate means of in silico cross-checking and confirmation using the publicly available Affymetrix expression profiles provided by the AtGenExpress consortium. In addition, 44 cell wall and growth-related genes were selected for wet-lab experimental validation and subsequent real-time RT–PCR.
MATERIALS AND METHODS
Co-response analyses
Transcript co-responses were retrieved from CSB.DB [a comprehensive systems-biology database; http://csbdb.mpimp-golm.mpg.de (13)] for the data matrices nasc0271, nasc0272 and nasc0273. A total of 123 expression profiles from 22 experiments were obtained from NASCarrays [http://affymetrix.arabidopsis.info/ (14)] and used for generation of the data matrices [supplement.XLS sheet 11 (nasc profiles)]. All profiles were based on 22k Affymetrix ATH1 GeneChips (Affymetrix, La Jolla, CA). The number of Present and Marginal calls (according to the MAS 5.0 Detection algorithm) was calculated for each profile. In most cases, 2 or 3 profiles per experiment with the highest numbers of Present and Marginal calls were selected for nasc0271. Nasc0271 covers the expression of 9694 genes that were well measured in at least 85% of the underlying expression profiles. The nasc0272 and nasc0273 matrices were generated from profiles which ranked 2nd and 3rd according to the numbers of Present and Marginal calls. The nasc0272 matrix was based on 51 profiles and represents 8927 genes. The nasc0273 matrix was based on 49 experiments and represents 8691 genes. Correlations were based on non-parametric Spearman's rank-order correlation (rs). Positive transcriptional co-responses with an uncorrected probability for multiple comparisons of <0.01 (99%) and a power of test of >0.7 (70%) were retrieved. From the initial result with the nasc0271 matrix (which covers 301 associated genes), 135 genes were selected and used for statistical in-depth analyses. To test for influence of individual or small subsets of underlying expression profiles, transcriptional co-responses were confirmed by bootstrap analyses. Bootstrap spearman correlation, probability, confidence interval and power of test were re-computed using the statistical software environment R (http://www.r-project.org) by non-parametric bootstrap analyses with 2000 numbers of bootstrap samples (15) on the log base 2 and range normalized signal intensities.
Distribution of functional categories
Assignments of genes into bins were taken from the MapMan software (16). Obtained assignments were slightly modified for bins that cover few genes. Merging of bins is illustrated in Table SI (Supplementary information). Genes without assignment or with unclear classification were termed ‘unclassified’. The relative impact (ri) of a gene with multiple assignments (nassign) onto each category was defined as ri = 1/nassign. The distribution of retrieved genes into functional categories was computed by adding-up the relative assignment coefficient for each gene per category.
Plant material and growth conditions for real-time RT–PCR
Two growth conditions were applied. Arabidopsis thaliana cv. C24 (wild-type), dwf1–6 (cbb1), cbb2 and cbb3 mutants were grown in half-concentrated MS medium supplemented with 1% sucrose and solidified with 0.7% agar under a 16 h day (140 μE, 22°C)/8 h (22°C) night regime. Plants were harvested 14 or 19 days after sowing. Roots were discarded. Alternatively, Arabidopsis thaliana cv. C24, dwf1–6 and CPD-antisense plants were grown in soil under long day conditions (16 h fluorescent light, 180 μE, 20°C, 70% relative humidity/8 h dark, 16°C and 75% relative humidity). Aerial organs were harvested 28 days after sowing.
Real-time RT–PCR analysis
Total RNA was isolated using the Invisorb Spin Plant RNA kit (Invitek, Berlin, Germany). One microgram of total RNA was reverse-transcribed with Superscript II reverse transcriptase (Invitrogen) in a reaction volume of 28.5 μl to generate first-strand cDNA. Real-time RT–PCR was performed with 1 μl of the 1:3.5 diluted first-strand cDNA reaction and the SYBR Green reagent (Applied Biosystems, Foster City, CA) in a 25 μl volume. Primer sequences are given in Table 1. Data were normalized to the eIF1a gene (At5g60390) and then compared according to the formula (considering as example the KCS1 gene):
Amplification efficiency (E) was checked for all primer pairs. Briefly, E-values were derived from the log slope of fluorescence versus cycle number curve for a particular primer pair, using the Equation (1 + E) = 10slope. E-values for all primer pairs are summarized in Table 1 and used to calculate normalized fold change values, using the Equation (1 + E)ΔCT. Control experiments showed that use of different control genes [either eIF1α or eIF4A1 (At3g13920, primers: ACAATGTGGTTGTCGAAGAGCTG and GCAGAGCAAACACAGCAACAGAA)] did not bias results.
Table 1.
Primers used for real-time RT–PCR analysis
| Gene | Sense primer | Antisense primer | E ± SD |
|---|---|---|---|
| At1g01120 | ACCGAAGCTAAGGGTCGGGTTA | GTAACTCTTTCCAAACCGCACT | 0.838 ± 0.007 |
| At1g01620 | CGGAACCTTCGTCCTTGTCT | ATTGGGAGTGGTGCCAAAAT | 0.936 ± 0.007 |
| At1g03870 | GGTGAGATGTTCGGAGAGCA | TGACTTATCCGACGGTGACG | 0.862 ± 0.003 |
| At1g27600 | CGACGTCCCTTTTCACATCC | TCCATCTCGCTTTCATCAGC | 0.906 ± 0.003 |
| At1g31310 | TGTGGAGGAGGTTGGACGTA | CTCTCCCTAATCGCGTTTGC | 0.857 ± 0.005 |
| At1g55330 | CAGCTCCAAGCCCAACTTCT | TGAAACCAGATGCCAAAGCA | 0.896 ± 0.049 |
| At1g70210 | ACGAGAGCCCTGAGACTTGG | GGCCATCGCTTTCATCAGTC | 0.861 ± 0.012 |
| At2g06850 | CTGAACAATGGCGTCGTCTC | CTGGCATAACCGGGAACCTA | 0.894 ± 0.016 |
| At2g14890 | TTGGATCTGTTCTCGTCTGG | TCCCAAGCAATACAGTGGAA | 0.936 ± 0.002 |
| At2g16850 | AGAAGTGCCCGTGACTCTCA | CCAAAGCTTCTGGCTGGATT | 0.868 ± 0001 |
| At2g22840 | GCGATGCGTGGATCAGATAA | GAAGCCCCTCGAGAATTTTG | 0.757 ± 0.011 |
| At2g36400 | TTTTGGTGGTGGTGGTGGTA | TCTTGCTTCATCTCCGAACA | 0.833 ± 0.021 |
| At2g36830 | TCGCTTGCCTCATCCTTAAA | TCGAAAACGAAAGCGTTCAA | 0.981 ± 0000 |
| At2g45960 | CCAAGAGAAACGCTCGTGAC | CCAGTAATGGGGATGGTTGC | 0.898 ± 0.036 |
| At3g05910 | CGGTGGCAATAGCTGTAGGA | CACCAAGTTGTGGCAGCTCT | 0.874 ± 0.011 |
| At3g16240 | CACAGTCATCACCGGAGTTT | GATCCTAGTCCAGCCGCAAC | 0.934 ± 0.012 |
| At3g24480 | ACCGCCAGTCCATCACAGTT | GTGGTGGGGGAGATGCATAG | 0.892 ± 0.018 |
| At3g26520 | GTTTCTGGCCGTTGGATCAT | TTGCACAAAAGCCTTCCAGA | 0.991 ± 0.000 |
| At3g28180 | GCAACAACATCGTTGGCATT | CGCCTTCTTCCAAACCGATA | 0.907 ± 0.005 |
| At3g53420 | CCACCAATTCGTTCTGAGAG | GTGTTTAGACGTTGGCAGCA | 0.887 ± 0.014 |
| At3g57790 | TTACAGTGCAGGGCTTGTGG | AACCCGTTCACATGCTCCAT | 0.917 ± 0.014 |
| At3g61430 | GACCATTCCTGGGATGACCA | TGGCTCTGATGACAACCACA | 0.953 ± 0.026 |
| At4g02500 | CGGTGTTCGAAGCTGATGAT | GAATCCCCCAGTAACCGTGA | 0.952 ± 0.006 |
| At4g12420 | TCGGACACCCTGACAATGTT | CGTGAATCCAATGCTCTTCG | 0.854 ± 0.003 |
| At4g12730 | CGTGGCATTTACCTCGCTTT | TTCCAAATCTCCACACCAAG | 0.899 ± 0.000 |
| At4g13340 | AGGACCACTACCACCGGTCA | CGATCTGTTCGTGTCACGTC | 0.791 ± 0.004 |
| At4g14130 | CTGGAGACCCCAACACATCA | TGGGTTGACTCTTTGGGAAA | 0.842 ± 0.013 |
| At4g18670 | GCCGTCGTCACCTAGTCCTC | GGTGGGGATGGAGGAGAGTA | 0.724 ± 0.001 |
| At4g19410 | CTTGGTCCGGTGACAAAGGT | ATGCACTCCGGCCATAAAAC | 0.704 ± 0.000 |
| At4g23400 | ATTAACCCGGCCAGGAGTCT | GATGGTACAGAGCAGCAAGC | 0.917 ± 0.001 |
| At4g25260 | TTGAGCAATTCGCTGAAGGA | GCGGTCTCATCTGTCAGTGC | 0.897 ± 0.000 |
| At4g25620 | ACGGGTCGTCAAGGCATAAT | AACCGGAACGGTTTAGCTTG | 0.833 ± 0.001 |
| At4g31590 | CGGGACCTATGCAGCTTTTC | TCAGATTCGCCTTCTTCCAT | 0.890 ± 0.001 |
| At5g01210 | GGGGAAAACCGTTAGCTGTG | ACTTCCAGATCCACGCTTCC | 0.832 ± 0.008 |
| At5g05170 | ATTGCCAGCCGTTTGTCTCT | TATACCCGTGGCGAAAATGG | 0.949 ± 0.007 |
| At5g07830 | CAGCTACGGGTTTACGCACA | CCACGTTTATGCCATTGCTG | 0.870 ± 0.022 |
| At5g12250 | GGACAATGAAGCCCTTTACG | CCGGGAACCTAAGACAGCAT | 0.773 ± 0.042 |
| At5g19770 | TCTCCGTCCGTCGAAGAAGT | GACCTGGATCCCAGCTTGTC | 0.884 ± 0.001 |
| At5g20250 | AACTCGCGATTGTTTGTTCG | CACGCCAAGAACTCCAGTGT | 0.892 ± 0.016 |
| At5g26670 | GGAGCTGTCACAATCTGGTC | ACGAATGCAGCAAAATGTCA | 0.838 ± 0.007 |
| At5g43760 | AATCTTGGTGGAATGGGATG | GCGTATGAGTTTGGTTGCAC | 0.872 ± 0.027 |
| At5g55730 | TAATGTCGGCTCATGGATGC | CATTGCATCATCTCCTGGAC | 0.986 ± 0.002 |
| At5g60390 | TTGACAGGCGTTCTGGTAAGG | CAGCGTCACCATTCTTCAAAAA | Not determined |
| At5g64740 | CCCTGCCATCTGTCTTCTCA | AGAAGAGCGCCATGAAGAGG | 0.884 ± 0.006 |
| At5g67260 | CCGTATGTTTCTCGATGTGC | ATTGTAGCCGATGGCCGATA | 0.822 ± 0.009 |
The amplification efficiency (E) was determined for each primer pair [see Materials and Methods and (34)].
Analysis of Affymetrix expression profiles
Normalization and expression analysis were performed with the MAS 5.0 and GCOS software (Affymetrix). Output of all experiments was multiplied by a scaling factor to adjust its average intensity to a target intensity of 100. Results of Absolute and Comparison expression analysis were imported into MS Access2003 and screened for significant changes. The Detection algorithm calculates detection p-values and assigns Present, Marginal or Absent calls. Standard parameters were applied to remove genes with Absent and Marginal calls. Induced genes were expected to be Present in experiments representing higher phytohormone levels (‘experimental’ profiles). Repressed genes were expected to be Present in experiments representing lower phytohormone levels (‘baseline’ profiles). Simultaneously, Affymetrix Change and Signal Log Ratio algorithms were used in order to identify changes with high reliability. The Change algorithm is based on Wilcoxon's signed rank test and produces a final change p-value. This p-value ranges from 0.0 to 1.0. The signal log ratio estimates the magnitude and direction of change of a transcript. Significant increases of transcript levels in comparisons of two profiles are indicated by change p-values of <0.01 and signal log ratios of ≥0.8. Significant decreases are indicated by change p-values of >0.99 and signal log ratios of ≤−0.8. Interchanging ‘experimental’ and ‘baseline’ profiles mirrors results. Change p-values of <0.01 and signal log ratios of ≥0.8 become >0.99 and ≤−0.8, respectively (for details see www.affymetrix.com).
Table 2 specifies AtGenExpress CEL files used for this study. Plant material was grown in liquid MS medium for 7 days at 23°C prior to the BL-, CS-, BRZ-, GA- and PAC-treatments. Two additional profiles were used [7 h EBL and control treatment of wild-type (Col-0) plants grown in half-concentrated MS medium supplemented with 1% sucrose and solidified with 0.7% agar] (17).
Table 2.
CEL files from AtGenExpress used in this study
| Experiment | Genotype | Experiment | Baseline |
|---|---|---|---|
| 10 nM BL | Wild-type (Col-0) | ||
| 30 min | BL30-2 and BL30-3 | mock 30-2 and mock 30-3 | |
| 1 h | BL1-2 and BL1-3 | mock 1-2 and mock 1-3 | |
| 3 h | BL3-2 and BL3-3 | mock 3-2 and mock 3-3 | |
| 10 nM BL | det2-1 | ||
| 30 min | det2BL30-1 and det2BL30-2 | det2m30-1 and det2m30-2 | |
| 1 h | det2BL1-1 and det2BL1-2 | det2m1-1 and det2m1-2 | |
| 3 h | det2BL3-1 and det2BL3-2 | det2m3-1 and det2m3-2 | |
| 1 μM GA3 | Wild-type (Col-0) | ||
| 30 min | GA3 30-2 and GA3 30-3 | mock 30-2 and mock 30-3 | |
| 1 h | GA3 1-2 and GA3 1-3 | mock 1-2 and mock 1-3 | |
| 3 h | GA3 3-2 and GA3 3-3 | mock 3-2 and mock 3-3 | |
| 1 μM GA3 | ga1-5 | ||
| 30 min | GA1-5G30-1 and GA1-5G30-2 | GA1-5m30-1 and GA1-5m30-2 | |
| 1 h | GA1-5G1-1 and GA1-5G1-2 | GA1-5m1-1 and GA1-5m1-2 | |
| 3 h | GA1-5G3-1 and GA1-5G3-2 | GA1-5m3-1 and GA1-5m3-2 | |
| 100 nM CS | det2-1 | CS3h-1 and CS3h-2 | m3h-1 and m3h-2 |
| 10 μM BRZ220 | Wild-type (Col-0) | ||
| 3 h | mock 3h-1 and mock 3h-2 | 2203h-1 and 2203h | |
| 12 h | mock12h-1 and mock 12h-2 | 220 12h-1 and 220 12h-2 | |
| 10 μM BRZ91 | Wild-type (Col-0) | ||
| 3 h | mock 3h-1 and mock 3h-2 | 91 3h-1 and 91 3h-2 | |
| 12 h | mock12h-1 and mock 12h-2 | 91 12h-1 91 12h-2 | |
| 10 μM PAC | Wild-type (Col-0) | ||
| 3 h | mock 3h-1 and mock 3h-2 | pac 3h-1 and pac 3h-2 | |
| 12 h | mock12h-1 and mock 12h-2 | pac 12h-1 and pac 12h-2 |
RESULTS
Transcript co-response analysis: a novel approach to identify BR-related genes
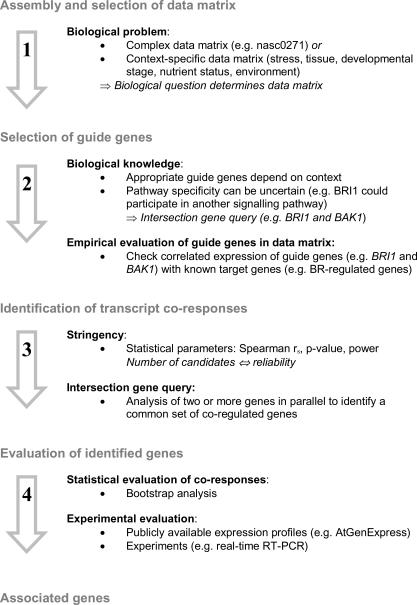
Central part of CSB.DB is a set of co-response databases (CoR.DBs) which are based on publicly available transcript profiles. The basic assumption is that common transcriptional control of genes is accompanied by synchronous changes of transcript levels. Scanning for best co-responses among changing transcript levels allows the deduction of hypotheses about common regulation of genes (18). We propose a general strategy to exploit CSB.DB. The strategy is illustrated in Figure 1. The biological question determines the choice of the type of data matrix. Complex data matrices identify genes which are ‘constitutively’ associated with the guide genes. Specific matrices (e.g. representing certain tissues or stress conditions) identify genes within a specific context. After the selection of a suitable data matrix, useful guide genes have to be identified. The expression pattern and function determine the suitability of guide genes. Therefore, biological knowledge is required. The guide genes are used to screen for genes which show similar expression patterns in the profiles underlying a data matrix. Statistical methods and experiments allow testing of the identified genes. In the following section, the strategy is demonstrated using BR-related genes.
Figure 1.
Strategy of transcript co-response analyses.
Assembly and selection of data matrices
Complex data matrices can be assembled by selecting transcript profiles representing many different experimental conditions. Complex data matrices include different genotypes, environmental conditions and developmental stages. To identify BR-related genes, the complex nasc0271 database was used. The matrix comprised 51 expression profiles [http://csbdb.mpimp-golm.mpg.de/csbdb/home/matrices/ath_nasc0271.html; supplement.XLS sheet 11 (nasc profiles)] representing a wide range of experimental conditions with a minimal overlap of identical experiments. All expression profiles were normalized using the MAS 5.0 software. Transcript measurements were required to have high quality, i.e. a detection call of Marginal or Present (according to standard parameters of the MAS 5.0 Detection algorithm) in at least 85% of the experiments. Thus, the nasc0271 matrix contained only 9694 accessible genes. Two other complex matrices (nasc0272 and nasc0273) were established using additional expression profiles [supplement.XLS sheet 11 (nasc profiles)]. These matrices were used to test and confirm the results obtained with the nasc0271 matrix. The nasc0272 and nasc0273 matrices consisted of 51 and 49 expression profiles and represented 8927 and 8691 genes, respectively.
Selection of guide genes and identification of transcript co-responses
Biological knowledge is required for the selection of useful guide genes. The screen for BR-related genes shows that different options emerge. Three different classes of genes could be used: either known BR-responsive genes or BR-biosynthesis genes, or genes involved in BR-signalling.
Use of known BR-responsive genes for co-response analyses reveals further BR-responsive genes (Table 3; statistical parameters below). However, BR-responsive genes are also likely to be regulated by other factors, and the functional context is unclear. For example, several BR-regulated such as BEE1, DRT100, IAA3, MSS3, SAUR-AC1, SAUR16 and TCH4 are auxin-regulated (9,19), and the use of these genes will result in the identification of further auxin-related genes.
The use of BR-biosynthesis genes, such as CPD, DWF4 and CYP85A1, should result in higher specificity. Transcript co-responses with the CPD gene recovered several known BR-regulated genes (Table 3). However, transcripts of other BR-biosynthesis genes were excluded from the data matrices because of quality concerns and thus could not be tested.
Use of BR-signalling components presents a third alternative. BR responses depend on signalling components such as BRI1, BAK1, BIN2, BZR1 and BES1. BRI1 is an essential receptor component for BR-responses. BR-insensitivity of bri1 mutants (20–22) indicates that major BR-responses depend on BRI1. BAK1 is a receptor-like kinase which forms a heterodimer with BRI1 (23,24). BAK1 was identified independently by a yeast two-hybrid screen for BRI1-interacting proteins and as suppressor of a weak bri1 allele. A null allele of BAK1 results in reduced (but not abolished) sensitivity to BR (23). In the presence of BR, the BRI1 and BAK1 proteins initiate a phosphorylation cascade which regulates BR-responsive genes. Downstream components such as BZR1 and BES1 regulate subsets of BR-responsive genes (10). However, BZR1 and BES1 could mediate responses to other stimuli as well, because the complex phosphorylation cascade between the BRI1/BAK1 and BZR/BES1 proteins is likely to receive additional input signals and thereby modulate BR-responses. In fact, BRI1 and BAK1 identified more known BR-regulated genes in the matrices than other signalling components (Table 3).
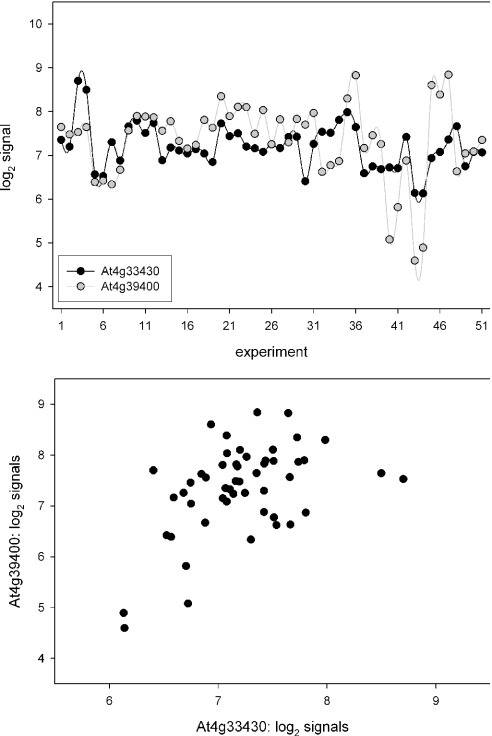
The BRI1 protein is the major BR-receptor component in Arabidopsis. However, Arabidopsis BRI1 may bind other ligands. For example, tomato BRI1 perceives both BR and the peptide hormone systemin (25,26). Therefore, BAK1 was included as a second guide gene. BRI1 and BAK1 showed variable and correlated transcript levels throughout the set of expression profiles (Figure 2). Thus, the requirements for subsequent intersection analysis were met. Transcript co-response calculations are based on changes in mRNA levels within the underlying expression profiles. Spearman's non-parametric rank correlation coefficient (rs), p-value and power were used to retrieve transcript co-responses. Exclusion criteria for the statistical parameters were rs > 0.35, p < 0.01 and power >0.7, respectively.
Table 3.
Recovery of known BR-regulated genes by transcript co-response analyses
| Gene | Function | References | nasc0271 | nasc0272 | nasc0273 | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Present | %rec | %spec | Present | %rec | %spec | Present | %rec | %spec | ||||
| BR-signalling | ||||||||||||
| At1g19350 | BES1 | BR-signalling positive regulator | (10) | n.a. | n.a. | n.a. | ||||||
| At1g55610 | BRL1 | Protein kinase | (35) | n.a. | n.a. | n.a. | ||||||
| At1g75080 | BZR1 | BR-signalling positive regulator | (36) | x | 0.0 | 0.0 | x | 4.5 | 0.1 | x | 9.1 | 0.2 |
| At3g13380 | BRL3 | Protein kinase | (35) | n.a. | n.a. | n.a. | ||||||
| At4g18710 | BIN2 | Shaggy-like kinase | (37) | x | 30.4 | 0.6 | x | 22.7 | 0.8 | x | 18.2 | 0.4 |
| At4g33430 | BAK1 | Receptor kinase | (23,24) | x | 47.8 | 1.5 | x | 31.8 | 1.8 | x | 36.4 | 1.7 |
| At4g39400 | BRI1 | Receptor kinase | (20) | x | 47.8 | 0.9 | x | 45.5 | 1.7 | x | 45.5 | 1.0 |
| BR-biosynthesis | ||||||||||||
| At3g50660 | DWF4 | Cytochrome P450 90B1 | (38) | n.a. | n.a. | n.a. | ||||||
| At4g36380 | ROT3 | Cytochrome P450 90C1 | (39) | n.a. | n.a. | n.a. | ||||||
| At5g05690 | CPD | Cytochrome P450 90A1 | (40) | x | 43.5 | 0.7 | x | 40.9 | 0.8 | x | 45.5 | 0.8 |
| At5g38970 | CYP85A1 | Cytochrome P450 85A1 | (41) | n.a. | n.a. | n.a. | ||||||
| BR-upregulated | ||||||||||||
| At1g01120 | KCS1 | Fatty acid elongase | (7,17) | x | 45.5 | 1.0 | x | 42.9 | 1.0 | x | 42.9 | 1.0 |
| At1g04240 | IAA3/SHY2 | Auxin-responsive protein | (6,7) | x | 36.4 | 0.8 | x | 33.3 | 0.6 | x | 33.3 | 0.7 |
| At1g04610 | YUCCA 3 | Flavin-containing monooxygenase | (10) | n.a. | n.a. | n.a. | ||||||
| At1g15580 | IAA5 | Auxin-responsive protein | (7,9) | n.a. | n.a. | n.a. | ||||||
| At1g18400 | BEE1 | Basic helix–loop–helix family protein | (19,29) | x | 40.9 | 0.8 | x | 38.1 | 0.7 | x | 42.9 | 0.9 |
| At1g20440 | COR47 | Dehydrin | (6) | x | 4.5 | 0.3 | x | 28.6 | 1.3 | x | 33.3 | 1.9 |
| At1g21820 | Unknown protein | (10,19) | x | 54.5 | 1.0 | x | 42.9 | 0.9 | x | 28.6 | 0.7 | |
| At1g33240 | GTL1 | Plant transcriptional activator | (6,19) | x | 54.5 | 1.2 | x | 38.1 | 0.8 | x | 33.3 | 0.9 |
| At1g65310 | Xyloglucan endotransglycosylase | (7,19) | n.a. | n.a. | n.a. | |||||||
| At1g67730 | GLOSSY8 | Putative β-keto acyl reductase | (6) | x | 40.9 | 0.8 | x | 23.8 | 0.6 | x | 23.8 | 0.6 |
| At1g73830 | BEE3 | Basic helix–loop–helix family protein | (19,29) | n.a. | n.a. | n.a. | ||||||
| At1g76680 | OPR1 | 12-Oxophytodienoate reductase | (7,9) | n.a. | n.a. | n.a. | ||||||
| At2g14900 | Unknown protein | (10,19) | n.a. | n.a. | n.a. | |||||||
| At2g26710 | BAS1/CYP72B1 | Cytochrome P450 | (7,19) | n.a. | n.a. | n.a. | ||||||
| At2g27690 | CYP94C1 | Cytochrome P450 | (7,19) | n.a. | n.a. | n.a. | ||||||
| At2g31730 | Unknown protein | (7,19) | n.a. | n.a. | n.a. | |||||||
| At2g32560 | F-box family protein | (10,19) | x | 4.5 | 0.1 | x | 0 | 0 | x | 4.8 | 0.1 | |
| At2g36220 | Unknown protein | (9,19) | n.a. | n.a. | n.a. | |||||||
| At2g40610 | EXP8 | Expansin | (7,10) | n.a. | n.a. | n.a. | ||||||
| At2g41940 | ZFP8 | Zinc finger protein | (6,19) | n.a. | n.a. | n.a. | ||||||
| At2g43290 | MSS3 | Calmodulin-like protein | (7,19) | x | 22.7 | 0.4 | x | 14.3 | 0.3 | x | 14.3 | 0.3 |
| At3g12610 | DRT100 | DNA-damage-repair/toleration protein | (19) | x | 59.1 | 1.2 | x | 42.9 | 0.9 | x | 42.9 | 1.1 |
| At3g15540 | IAA19 | Auxin-responsive protein | (6,7) | n.a. | n.a. | n.a. | ||||||
| At3g16240 | TIP2.1/d-TIP | Aquaporin | (6,17) | x | 63.6 | 1.5 | x | 52.4 | 1.3 | x | 52.4 | 1.6 |
| At3g29030 | EXP5 | Expansin | (6,19) | n.a. | n.a. | n.a. | ||||||
| At3g30775 | ERD5/PRO1 | Proline oxidase | (6,19) | x | 50.0 | 1.1 | x | 42.9 | 1.3 | x | 33.3 | 0.8 |
| At3g46090 | ZAT7 | Zinc finger protein | (7,9) | n.a. | n.a. | n.a. | ||||||
| At3g47340 | ASN1 | Asparagine synthetase | (6) | x | 40.9 | 1.0 | x | 28.6 | 0.7 | x | 33.3 | 1.0 |
| At4g00360 | CYP86A2 | Cytochrome P450 | (7,19) | n.a. | n.a. | n.a. | ||||||
| At4g01950 | Phospholipid/glycerol acyltransferase | (10,19) | n.a. | n.a. | n.a. | |||||||
| At4g08950 | EXO | Transcriptional regulator | (7,17) | x | 40.9 | 0.8 | n.a. | n.a. | ||||
| At4g09460 | MYB6 | myb Family transcription factor | (6,19) | x | 54.5 | 0.9 | x | 52.4 | 1.3 | x | 52.4 | 1.3 |
| At4g09890 | Unknown protein | (9,19) | x | 18.2 | 0.7 | x | 4.8 | 0.1 | x | 0.0 | 0.0 | |
| At4g20780 | Calcium-binding protein | (10,19) | x | 4.5 | 0.3 | x | 4.8 | 0.1 | x | 0.0 | 0.0 | |
| At4g25810 | XTR6 | Xyloglucan endotransglycosylase | (7,19) | n.a. | n.a. | n.a. | ||||||
| At4g34160 | CYCD3 | Cyclin | (42) | x | 22.7 | 0.5 | x | 9.5 | 0.4 | x | 0.0 | 0.0 |
| At4g36540 | BEE2 | Basic helix–loop–helix family protein | (29) | n.a. | n.a. | n.a. | ||||||
| At4g37390 | GH3-2/YDK1 | Auxin-responsive protein | (7,9) | n.a. | n.a. | n.a. | ||||||
| At4g38850 | SAUR-AC1 | Auxin-responsive protein | (7,19) | n.a. | n.a. | n.a. | ||||||
| At4g38860 | SAUR16 | Auxin-responsive protein | (7,19) | n.a. | n.a. | n.a. | ||||||
| At5g04190 | Phytochrome kinase substrate-related | (19) | n.a. | n.a. | n.a. | |||||||
| At5g06720 | ATPA2 | Peroxidase | (7,19) | x | 9.1 | 0.3 | x | 4.8 | 0.1 | x | 0.0 | 0.0 |
| At5g10430 | AGP4 | Arabinogalactan-protein | (6,7) | x | 45.5 | 1.0 | x | 47.6 | 1.0 | x | 38.1 | 1.0 |
| At5g13870 | EXGT-A4 | Xyloglucan endotransglycosylase | (10) | n.a. | n.a. | n.a. | ||||||
| At5g37770 | TCH2 | Calmodulin-related protein | (7,19) | x | 18.2 | 0.4 | x | 14.3 | 0.4 | x | 9.5 | 0.2 |
| At5g47370 | HAT2 | Homeobox-leucine zipper protein | (6) | x | 36.4 | 0.9 | x | 23.8 | 0.6 | x | 9.5 | 0.2 |
| At5g51460 | TPPA | Trehalose-6-phosphate phosphatase | (6) | n.a. | n.a. | n.a. | ||||||
| At5g57560 | TCH4 | Xyloglucan endotransglycosylase | (6,43) | x | 40.9 | 0.8 | x | 28.6 | 0.6 | x | 23.8 | 0.6 |
| At5g64000 | SAL2 | 3′(2′),5′-Bisphosphate nucleotidase | (6) | n.a. | n.a. | n.a. | ||||||
| At5g66400 | RAB18 | Dehydrin | (6) | n.a. | n.a. | n.a. | ||||||
In the nasc0271, nasc0272 and nasc0273 matrices, 23, 22 and 22 published BR-regulated genes were present, respectively. BR-signalling genes, BR-biosynthesis genes and BR-regulated genes were used for co-response analyses. BR-regulated genes were termed ‘recovered’ in case significant correlations that were calculated (criteria as mentioned in the text). ‘%rec’ gives the percentage of the known BR-regulated genes (present in the respective matrix) identified by transcript co-response analysis; ‘%spec’ gives the percentage of identified known BR-regulated genes relating to the total number of genes showing significant co-responses. Underlying data are provided as Supplementary Material [supplement.XLS sheet 8 (nasc0271)], sheet 9 (nasc0272) and sheet 10 (nasc0273)]. ‘n.a.’: Gene not analysable. ‘x’: Gene present in data matrix.
Figure 2.
BRI1 (At4g39400) and BAK1 (At4g33430) transcript levels denoted as log2 signals in 51 expression profiles underlying the nasc0271 data matrix (upper panel). Lines are drawn to aid interpretation. Scatter plot of log2 signals (lower panel).
The BRI1 gene was associated with 1179 genes in the nasc0271 matrix (see below). These transcript co-responses identified 11 of 23 known BR-induced genes present in the nasc0271 matrix [Table 3 and supplement.XLS sheet 8 (nasc0271)]. We define this observation as a co-response recovery of 47.8% and a specificity of 0.9% (for definitions and calculations, see Table 3). A total of 720 genes showed co-responses with the BAK1 gene. The test for co-responses with known BR-induced genes resulted in 47.8% recovery and 1.5% specificity [Table 3 and supplement.XLS sheet 8 (nasc0271)].
The intersection gene query with BRI1 and BAK1 resulted in the identification of 301 co-responding genes. Public databases contained information that allowed functional categorization of >50% of these genes [supplement.XLS sheet 1 (301 genes); supplementary data (Figure S1 and Table SI)]. In agreement with high correlation of BRI1 and BAK1 expression, 8 of 23 known BR-regulated genes were still present after intersection analysis in the nasc0271 matrix resulting in 34.8% recovery and 2.7% specificity (Table 4).
Table 4.
Intersection gene queries with BR-signalling components
| Gene combination | Recovery | Maximum | %recovery | Intersection | %specificity | |
|---|---|---|---|---|---|---|
| nasc271 | ||||||
| At4g39400 + At4g33430 | BRI1 + BAK1 | 8 | 23 | 34.8 | 301 | 2.7 |
| At4g39400 + At4g18710 | BRI1 + BIN2 | 3 | 23 | 13.0 | 227 | 1.3 |
| At4g39400 + At1g75080 | BRI1 + BZR1 | 0 | 23 | 0.0 | 109 | 0.0 |
| At4g33430 + At4g18710 | BAK1 + BIN2 | 3 | 23 | 13.0 | 234 | 1.3 |
| At4g33430 + At1g75080 | BAK1 + BZR1 | 0 | 23 | 0.0 | 1 | 0.0 |
| At4g18710 + At1g75080 | BIN2 + BZR1 | 0 | 23 | 0.0 | 18 | 0.0 |
| nasc0272 | ||||||
| At4g39400 + At4g33430 | BRI1 + BAK1 | 4 | 22 | 18.2 | 144 | 2.8 |
| At4g39400 + At4g18710 | BRI1 + BIN2 | 1 | 22 | 4.5 | 184 | 0.5 |
| At4g39400 + At1g75080 | BRI1 + BZR1 | 0 | 22 | 0.0 | 373 | 0.0 |
| At4g33430 + At4g18710 | BAK1 + BIN2 | 3 | 22 | 13.6 | 301 | 1.0 |
| At4g33430 + At1g75080 | BAK1 + BZR1 | 0 | 22 | 0.0 | 6 | 0.0 |
| At4g18710 + At1g75080 | BIN2 + BZR1 | 0 | 22 | 0.0 | 4 | 0.0 |
| nasc0273 | ||||||
| At4g39400 + At4g33430 | BRI1 + BAK1 | 5 | 22 | 22.7 | 161 | 3.1 |
| At4g39400 + At4g18710 | BRI1 + BIN2 | 1 | 22 | 4.5 | 194 | 0.5 |
| At4g39400 + At1g75080 | BRI1 + BZR1 | 0 | 22 | 0.0 | 229 | 0.0 |
| At4g33430 + At4g18710 | BAK1 + BIN2 | 3 | 22 | 13.6 | 231 | 1.3 |
| At4g33430 + At1g75080 | BAK1 + BZR1 | 0 | 22 | 0.0 | 3 | 0.0 |
| At4g18710 + At1g75080 | BIN2 + BZR1 | 0 | 22 | 0.0 | 3 | 0.0 |
Recovery of known BR-regulated genes (Table 3) was tested in intersection gene queries of 2 signalling components. Intersections of BR-biosynthesis genes could not be tested because only the CPD gene was present in the matrices (Table 3). ‘recovery’ gives the number of BR-regulated genes identified by means of the respective intersection gene query; ‘maximum’ gives the number of BR-regulated genes present in the matrix (see Table 3); ‘%recovery’ gives the percentage of BR-regulated genes identified in the query; ‘intersection’ gives the number of genes showing significant co-responses with both genes; ‘%specificity’ gives the percentage of BR-regulated genes in the intersection.
Two other data matrices were used for an intersection gene query with the BRI1 and BAK1 genes. Intersection gene queries with the nasc0272 and nasc0273 matrices resulted in the identification of 144 and 161 genes, respectively: 69% and 71% of these genes were also present in the BRI1/BAK1 intersection of the nasc0271 matrix [including several known BR-regulated genes; Table 3 and supplement.XLS sheet 9 (nasc0272) and sheet 10 (nasc0273)]. Therefore, the nasc0271, nasc0272 and nasc0273 data matrices produced similar results.
EVALUATION OF IDENTIFIED GENES
Statistical evaluation of co-responses
Non-parametric bootstrap analysis with 2000 bootstrap samples was exemplarily performed with 135 associated genes, which could be functionally classified (including BRI1 and BAK1). The resulting Spearman correlation coefficients (rs), p-values and power values confirmed correlated behaviour of both the BAK1 gene and the BRI1 gene with the respective other 134 genes. A complete matrix of all gene correlations is provided [supplement.XLS sheet 2 (Spearman rs), sheet 3 (p-value) and sheet 4 (power)].
Affymetrix expression profiling experiments
The BRI1/BAK1 intersection gene query was expected to identify associated genes which we call ‘BR-related’ genes. The BR-related genes comprise different classes of genes, namely BR-responsive genes and genes which mirror the functional context of BRI1 and BAK1.
Altered transcript levels in plants with altered BR-levels or altered BR-sensitivity confirm the BR-responsiveness of genes. Publicly available expression profiles were used for in silico cross-checking of the 301 associated genes identified with the nasc0271 matrix. AtGenExpress is a multinational coordinated effort to uncover the transcriptome of Arabidopsis (http://www.uni-frankfurt.de/fb15/botanik/mcb/AFGN/atgenex.htm). For this study, expression profiles established by Hideki Goda (Plant Science Center and Plant Functions Laboratory, RIKEN, Japan) and two own profiles (17) were used. Expression profiles were analyzed using the stringent settings of the statistical algorithms of the MAS5.0 and GCOS software (see Materials and Methods).
Thirty expression profiles of brassinolide (BL)-, 24-epibrassinolide (EBL)-, castasterone (CS)- or control-treated wild-type and BR-deficient det2 plants were used for 15 comparisons. Results were screened for the overlap with the 301 associated genes: 55 genes showed stronger expression upon BR-treatment in at least two independent situations [supplement.XLS sheet 6 (Affx results BRs, BRZ)]; 14 genes including BRI1 and a BRI1 homolog (At1g72180) revealed weaker expression in at least two independent experiments. These up- or down-regulated genes did not show conflicting expression patterns (i.e. were not inversely regulated in other situations).
Exogenously applied BR strongly promotes growth. The observed transcript co-responses could be related to growth rather than to specific BR action. In this case, expression may be induced by other growth-promoting compounds such as GA. Therefore, 24 expression profiles of GA3- or control-treated wild-type and ga1–5 plants were used for 12 comparisons. Genes with significantly altered transcript levels were compared with the 301 candidate genes. Only one gene showed stronger expression upon GA-treatment [supplement.XLS sheet 7 (Affx results GA3, PAC)].
Reduced BR-levels should result in weaker expression of BR-induced genes. Brassinazole (BRZ) is a specific BR biosynthesis inhibitor: 12 expression profiles of BRZ- or control-treated wild-type plants were used for 8 comparisons; 17 (of 301) genes showed reduced expression in the presence of BRZ in at least two independent situations [supplement.XLS sheet 6 (Affx results BRs, BRZ)]. In order to test the effects of reduced GA-levels, eight expression profiles of paclobutrazol (PAC)- or control-treated wild-type plants were used for four comparisons. Only two genes (of 301) showed weaker expression in presence of PAC [supplement.XLS sheet 7 (Affx results GA3, PAC)]. Thus, transcript levels of the identified genes were marginally affected by GA. This implies that the genes are not secondarily regulated by growth, and dissection of the genomic basis of BR-promoted growth is feasible.
Real-time RT–PCR analysis of growth-related genes
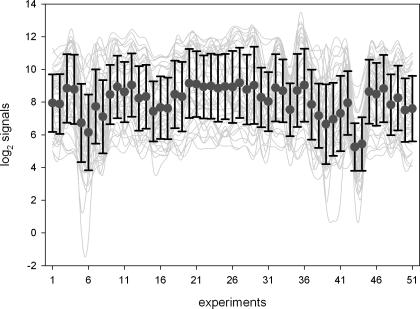
Transcript co-responses reveal the functional context of guide genes. The most obvious BR-effect is promotion of growth, and the identified associated genes should mirror this BR-function. The associated genes were manually screened and 44 genes with predicted or known functions in growth processes were identified and selected for real-time RT–PCR analysis. The correlated behaviour of these genes in the nasc0271 data matrix is illustrated in Figure 3.
Figure 3.
Transcript levels of 44 cell wall and growth-related genes denoted as log2 signals in 51 expression profiles underlying the nasc0271 data matrix. Dots and bars give average log2 signals of all genes and SD values, respectively. Lines are drawn to aid interpretation.
BR-induced genes were expected to have reduced transcript levels in BR-mutants and increased transcript levels in BR-treated plants. Three sets of experiments were performed: (i) Wild-type, dwf1–6, cbb3 and cbb2 plants (all C24 background, mutants allelic to the dim, cpd and bri1 mutants) (21) were grown in half-concentrated Murashige and Skoog medium. Plant material was harvested 14 or 19 days after sowing. (ii) Wild-type, dwf1–6 and CPD-antisense plants (27) were grown in soil. Plant material was harvested 28 days after sowing. (iii) Wild-type and dwf1–6 plants were grown in half-concentrated Murashige and Skoog medium and either treated with a control solution or 300 nM EBL.
Transcript levels were determined using real-time RT–PCR. Eight-fold change values were established (Tables 5 and 6). Fold change values >1.0 indicate a positive BR-effect on transcript levels. CT values are provided in Table SII (Supplementary Material).
Table 5.
Real-time RT–PCR analysis of cell wall and growth-related genes
| Gene | Function | Sterile culture | Sterile culture | Soil | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| WT vs dwf1-6 (14 days) | WT vs cbb2 (19 days) | WT vs cbb3 (19 days) | WT vs dwf1-6: controls (19 days) | WT: BR vs control (19 days) | dwf1-6: BR vs control (19 days) | WT vs dwf1-6 (28 days) | WT vs αCPD (28 days) | |||
| Weaker expression in at least two mutants, stronger expression upon BR-application | ||||||||||
| KCS1 | At1g01120 | Fatty acid elongase | 1.7 | 1.5 | 2.2 | 1.1 | 1.6 | 1.4 | 3.1 | 1.6 |
| At1g27600 | Glycosyltransferase | 2.0 | 0.9 | 1.6 | 1.9 | 2.3 | 1.4 | 1.4 | 2.0 | |
| At3g24480 | Leucine-rich repeat extensin | 1.4 | 1.6 | 1.7 | 1.4 | 2.5 | 1.8 | 1.6 | 1.3 | |
| CSLC4 | At3g28180 | Cellulose synthase | 1.4 | 0.9 | 1.9 | 2.5 | 1.5 | 3.0 | 0.8 | 1.1 |
| At3g57790 | Polygalacturonase/pectinase | 1.3 | 1.5 | 1.5 | 7.3 | 2.1 | 3.9 | 2.6 | 1.8 | |
| At4g13340 | Leucine-rich repeat extensin | 1.6 | 1.1 | 2.9 | 1.2 | 1.7 | 1.9 | 0.8 | 1.7 | |
| Weaker expression in at least two mutants | ||||||||||
| PIP1.3 | At1g01620 | Aquaporin | 3.7 | 1.4 | 1.8 | 2.5 | 0.9 | 1.6 | 0.9 | 0.8 |
| AGP21 | At1g55330 | Arabinogalactan-protein | 2.1 | 3.7 | 4.1 | 1.9 | 1.0 | 1.7 | 1.2 | 2.3 |
| EXT | At2g06850 | Xyloglucan endotransglycosylase | 2.5 | 1.3 | 2.0 | 1.6 | 1.2 | 1.2 | 1.0 | 0.8 |
| AGP9 | At2g14890 | Arabinogalactan-protein | 1.8 | 1.9 | 1.7 | 1.6 | 0.7 | 1.0 | 1.2 | 2.0 |
| PIP2.8 | At2g16850 | Aquaporin | 3.6 | 2.3 | 3.7 | 2.5 | 0.8 | 1.5 | 0.8 | 1.2 |
| TIP1.1 | At2g36830 | Aquaporin | 1.4 | 1.6 | 1.2 | 1.7 | 0.7 | 1.2 | 0.7 | 1.2 |
| PIP1.2 | At2g45960 | Aquaporin | 1.9 | 1.8 | 1.8 | 1.8 | 0.7 | 1.0 | 1.5 | 1.0 |
| At3g05910 | Pectinacetylesterase | 1.2 | 1.2 | 1.5 | 1.6 | 1.1 | 1.6 | 1.7 | 1.4 | |
| TIP2.1 | At3g16240 | Aquaporin | 2.8 | 1.5 | 2.3 | 1.9 | 0.9 | 1.3 | 0.8 | 1.0 |
| TIP1.2 | At3g26520 | Aquaporin | 1.6 | 2.2 | 1.7 | 1.8 | 1.5 | 1.2 | 1.4 | 1.8 |
| PIP2.1 | At3g53420 | Aquaporin | 2.6 | 1.4 | 1.9 | 1.5 | 0.7 | 0.6 | 1.7 | 1.0 |
| PIP1.1 | At3g61430 | Aquaporin | 2.7 | 1.5 | 1.7 | 1.9 | 0.9 | 1.0 | 1.6 | 1.1 |
| SKU5 | At4g12420 | Pectinesterase | 1.4 | 1.3 | 2.0 | 1.0 | 1.1 | 1.0 | 0.8 | 0.9 |
| FLA2 | At4g12730 | Fasciclin-like arabinogalactan-protein | 1.5 | 2.5 | 2.7 | 0.8 | 1.2 | 1.0 | 1.3 | 1.7 |
| At4g18670 | Leucine-rich repeat extensin | 1.1 | 1.5 | 2.1 | 1.4 | 0.8 | 1.0 | 1.0 | 1.2 | |
| At4g25260 | Invertase/pectin methylesterase inhibitor | 1.3 | 1.9 | 1.1 | 1.3 | 0.7 | 0.9 | 1.0 | 1.9 | |
| At5g43760 | β-Ketoacyl-CoA synthase | 1.8 | 1.6 | 1.7 | 1.3 | 0.9 | 1.0 | 0.8 | 0.7 | |
Wild-type plants and different BR mutants were grown under aseptic conditions (columns 1–6) or in soil (columns 7 and 8) and harvested after 14 days (column 1), 19 days (columns 2–6) and 28 days (columns 7 and 8), respectively. Plant material was treated with a control solution or 300 nM 24-epibrassinolide and harvested 5 h after treatment (columns 5 and 6). Control treatments were also used to compare basal transcript levels in wild-type and dwf1-6 plants (column 4). Fold change ratios were calculated taking into account the amplification efficiencies of primers (Table 1). RT–PCR data (i.e. CT values) are given in Table SII (Supplementary Material).
Table 6.
Real-time RT–PCR analysis of cell wall- and growth-related genes
| Gene | Function | Sterile culture | Sterile culture | Soil | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| WT vs dwf1-6 (14 days) | WT vs cbb2 (19 days) | WT vs cbb3 (19 days) | WT vs dwf1-6: controls (19 days) | WT: BR vs control (19 days) | dwf1-6: BR vs control (19 days) | WT vs dwf1-6 (28 days) | WT vs αCPD (28 days) | |||
| No consistent BR-dependent or variable expression | ||||||||||
| FLA9 | At1g03870 | Fasciclin-like arabinogalactan-protein | 1.6 | 1.1 | 0.8 | 1.0 | 1.5 | 1.5 | 1.1 | 1.0 |
| At1g31310 | Hydroxyproline-rich glycoprotein | 0.9 | 0.5 | 0.6 | 1.1 | 1.8 | 1.1 | 0.6 | 1.0 | |
| CYCD1.1 | At1g70210 | Cyclin | 2.3 | 0.8 | 1.2 | 2.8 | 0.4 | 0.8 | 0.9 | 1.0 |
| At4g02500 | Galactosyl transferase | 1.1 | 1.0 | 1.1 | 0.8 | 0.8 | 1.1 | 1.2 | 0.8 | |
| At4g19410 | Pectinacetylesterase | 1.1 | 0.9 | 1.1 | 1.1 | 2.3 | 1.1 | 1.0 | 1.5 | |
| PIP1.5 | At4g23400 | aquaporin | 2.0 | 1.7 | 1.4 | 1.3 | 0.7 | 0.5 | 0.7 | 0.7 |
| At4g25620 | Hydroxyproline-rich glycoprotein | 1.0 | 0.7 | 1.1 | 1.0 | 1.1 | 0.8 | 0.6 | 0.6 | |
| CSLC5 | At4g31590 | Cellulose synthase | 1.2 | 1.0 | 1.5 | 1.8 | 0.5 | 0.7 | 1.5 | 1.0 |
| At5g01210 | Transferase | 1.0 | 1.1 | 1.0 | 0.5 | 1.1 | 0.7 | 1.1 | 0.9 | |
| CESA3 | At5g05170 | Cellulose synthase | 1.4 | 1.0 | 1.3 | 1.1 | 1.0 | 1.0 | 1.3 | 1.2 |
| At5g07830 | Glycosyl hydrolase | 0.8 | 1.0 | 1.2 | 1.0 | 1.0 | 1.4 | 1.8 | 1.4 | |
| TUB6 | At5g12250 | Tubulin | 1.2 | 1.2 | 1.4 | 1.3 | 0.9 | 1.0 | 1.5 | 1.7 |
| TUA3 | At5g19770 | Tubulin | 1.2 | 1.3 | 1.1 | 1.3 | 0.6 | 1.1 | 1.5 | 1.8 |
| DIN10 | At5g20250 | Glycosyl hydrolase | 1.7 | 1.4 | 2.2 | 0.5 | 2.3 | 1.3 | 0.4 | 1.4 |
| At5g26670 | Pectinacetylesterase | 1.0 | 0.8 | 1.3 | 1.5 | 0.8 | 0.8 | 1.2 | 1.2 | |
| FLA1 | At5g55730 | Fasciclin-like arabinogalactan-protein | 1.0 | 1.2 | 1.0 | 0.8 | 1.1 | 0.6 | 1.3 | 1.8 |
| CESA6 | At5g64740 | Cellulose synthase | 1.2 | 1.1 | 1.8 | 1.4 | 1.0 | 1.2 | 1.2 | 0.7 |
| CYCD3.2 | At5g67260 | Cyclin | 1.5 | 1.0 | 1.1 | 1.7 | 0.5 | 0.7 | 0.7 | 1.0 |
| Stronger expression in mutants or weaker expression upon BR-application | ||||||||||
| GRL1 | At2g22840 | Transcription activator | 1.2 | 1.1 | 1.6 | 1.4 | 0.5 | 0.7 | 0.6 | 0.6 |
| GRL3 | At2g36400 | Transcription activator | 1.2 | 1.0 | 1.3 | 1.2 | 0.6 | 0.6 | 0.4 | 0.5 |
| XTR7 | At4g14130 | Xyloglucan endotransglycosylase | 0.4 | 0.4 | 1.1 | 0.2 | 3.8 | 0.3 | 0.1 | 0.6 |
Details given in legend of Table 5.
Twenty-three genes showed reduced transcript levels in at least two BR-mutants (Table 5). These genes include known BR-responsive genes such as KCS1 and TIP2.1 (δ-TIP) (17), four further aquaporins [TIP1.1 (At2g36830), PIP1.2 (At2g45960), TIP2.1 (At3g16240) and TIP1.2 (At3g26520)], a KCS1 homologue (At5g43760), and genes presumably involved in cell wall modifications [At1g27600, AGP21 (At1g55330), At2g06850, AGP9 (At2g14890), At3g05910, At3g24480, At3g28180, At3g57790, FLA2 (At4g12730), At4g13340 and At4g18670]. Fold changes of growth-related genes were more pronounced in 14- and 19-day-old plants in comparison to 28-day-old plants, probably due to reduced growth rates in older plants (Table 5). Six (of 23) genes also displayed higher mRNA levels in BR-treated plants (Table 5). Three genes displayed stronger expression in BR-mutants or weaker expression upon BR-treatment (Table 6). Eighteen genes did not exhibit BR-responsive expression or showed variable transcript levels (Table 6).
Transcript co-responses point to additional BR functions
Positive effects of BR on plant growth upon cold stress and salt stress have been reported (28). The ERD4 (EARLY RESPONSIVE TO DEHYDRATION 4), ERD6, ERD15 and At1g29470 (similar to ERD3) genes showed co-responses with BRI1 and BAK1 [Supplementary EXCEL file sheet 1 (301 genes)]. Genes involved in auxin responses [IAA7, IAA14, IAA16, TIR1 homologs (At3g26810 and At4g03190) and GRH1 (At4g03190)], auxin transport (PIN3 and AUX1) and ethylene responses (EIL1, At3g11930, ERF7 and EIN2) were identified, thus providing further evidence for phytohormone interactions. Expression of the IAA14 and At3g11930 genes was reported to be weaker in roots of the dwf1–6 mutant in comparison to roots of wild-type plants (8). Transcription factors such as BEE1, GTL1 and MYC1 showed co-responses. These genes are induced upon BR-treatment (6,29). Co-responses of genes encoding ubiquitin-conjugating enzymes (At1g63800 and At1g64230), the ubiquitin-ligase RMA1 and F-box proteins (At1g30200, At1g67480, At2g18280, At3g06380, At3g61060, At5g27920 and At5g60570) may indicate that BR promotes specific protein degradation and thus modifies signalling pathways.
Interestingly, several genes showing high sequence similarity to BR-signalling components also showed co-responses [genes similar to BRI1: At1g28440, At1g72180, and At2g01950 (BRL2, 35); BAK1: At1g07650 and At3g14840; BRZ1/BES1: At1g78700). These genes could be involved in the mediation of BR-responses.
DISCUSSION
Transcript co-response analyses: a novel approach
Transcript co-response analysis differs from direct approaches in several ways.
Transcript co-response analysis allows ruling out outliers and conditional changes of transcript levels. The genotype, developmental stage, environmental conditions and tissue determine specific expression patterns. Consequently, BR-induced or BR-repressed genes depend on the experimental conditions (6–12). The data matrices of CSB.DB combine numerous experiments. Thus, statistically significant transcript co-responses mark robust expression patterns.
Transcript co-response analysis describes the functional context of genes and provides insights into networks. The statistical approach allows the identification of pathway-involved genes, but also highlights the functional context of genes.
CSB.DB is an open access resource. The proposed strategy can easily be adapted to other biological problems.
To demonstrate the value of this approach, we established and used the nasc0271, nasc0272 and nasc0273 data matrices for the identification of BR-related genes.
Direct approaches versus transcript co-response analyses
Direct approaches analysed plants with altered BR-levels or impaired BR-sensitivity (6–12). However, altered transcript levels in these plants do not necessarily reflect normal physiological events, because physiological networks are disrupted. Expression profiling experiments of BR-mutants might be compromised by secondary effects that arise due to the long-term BR deficiency, resulting in physiological aberrations that in turn cause changes in expression patterns rather unrelated to the primary action of BR. For example, BR-mutants show altered light and stress responses, delayed development, impaired photosynthesis, reduced root systems and reduced fertility.
BR treatment of plants (the status of which is determined by the genotype, developmental stage and environment) results in an artificial status in which established networks are overlaid. The rate of uptake and the degree of distribution of the exogenously applied BR are unknown and thus is the actual dose of BR and the tissues reached. Long-term application of higher concentrations of active BRs results in severe developmental alterations and can be lethal. Consequently, BR-treatments trigger antagonistic events, which are switched off in the presence of physiological BR-levels (i.e. in the experiments used for the nasc0271 matrix). Antagonistic events probably serve to limit responses and counter toxic BR-effects. BR-treatments repress BR-biosynthetic genes such as CPD and DWF4 (30,31) and BR-signalling genes such as BRI1, and induce genes involved in the inactivation of BR such as BAS1 (7,32). Thus, BR-repressed genes partly represent components required for BR-action.
Due to conditional disturbances of networks, previous expression profiling approaches identified widely different sets of BR-regulated genes (11,12). Transcript co-response analysis allows to analyse BR-related genes in plants with intact BR-biosynthesis and BR-signalling pathways. In fact, the analysis of BR-related genes is barely possible in a data matrix based on expression profiles of BR-mutants: BR-mutants do not show BR-dependent expression patterns, correlations of BR-related genes are disrupted. Similarly, correlations of BR-related genes are at least partly disrupted in BR-treated plants because of antagonistic events.
The combination of many different experiments accounts for the fact that only 23 or 22 genes were present in the complex matrices used in this study. It also explains that the BRI1 and BAK1 guide genes identified only a subset of these genes. Different context-specific data matrices probably would raise the recovery.
Identification of BR-related genes: BR-regulated genes and the functional context
Our statistical approach was based on a wide range of experiments. A total of 51 expression profiles were included in the nasc0271 matrix. The BRI1 and BAK1 genes were selected to screen for BR-related genes. One group of genes was identified which showed positively correlated expression in the nasc0271 matrix [Supplementary EXCEL file sheet 1 (301 genes)]. Experimental evaluation using BR-mutants, BR- and BRZ-treated plants divided this group into three subgroups: BR-induced genes [in total 72 genes (24%)], BR-repressed genes [in total 16 genes (5%)] and genes which were not affected by altered BR-levels or showed variable expression [213 genes (71%)]. The identification of BR-repressed genes and the large number of genes which were not affected by BR-treatments appear to contradict BR-induction inferred from positive correlations with BRI1 and BAK1. However, as mentioned above the identification of common expression patterns in correlation matrices constitutes a context analysis.
On one hand, transcript co-response analysis identifies pathway-involved genes, because BRI1 and BAK1 are required for BR-responses. The specificity of the guide genes was demonstrated by the finding that the identified genes were virtually unaffected by GA. On the other hand, transcript co-responses provide insights into networks. Gene expression patterns are determined by various factors, and these factors are mirrored in correlations. In theory, the observed transcript co-responses could (also) represent co-responses with such unknown factors. Co-response analyses in different matrices could hold the potential to identify upstream factors which determine BRI1 and BAK1 expression.
The functional context comprises physiological pathways, which are (also) regulated by other signalling pathways. Growth-related genes exemplify one aspect of the functional context of the BRI1 and BAK1 genes. BR promotes growth in all plant organs at early developmental stages. There is increasing evidence for involvement of several molecular mechanisms such as cell wall loosening, acidification of wall space, carbohydrate allocation, carbon assimilation and control of aquaporin activity (33). BR apparently coordinates and integrates diverse processes required for growth. Transcript levels of growth-related genes were analysed in BR-mutants and BR- or BRZ-treated plants. A subset of these genes could not be identified as BR-responsive despite the fact that a large set of experiments was performed.
Another example is the identification of auxin-signalling components (e.g. IAA genes), which are not BR-responsive. The BR–auxin interaction is an important aspect in growth processes (19,33). Thus, the transcript co-responses provided additional information about the growth processes in which BR is involved that could not be derived from direct approaches.
Potentials and limitations of transcript co-response analyses
Transcript co-responses can help to learn more about physiological pathways. The results critically depend on the guide genes and the data matrix. Biological knowledge is required for selection of suitable guide genes, and co-responses for a given gene cannot be expected in all data matrices. In this study, the BR-signalling components BRI1 and BAK1 were used as guide genes in combination with the complex nasc0271 matrix. However, BRI1 and BAK1 may not represent suitable guide genes in other data matrices. For example, BRI1 or BAK1 homologs could perform better in tissue or stress-specific matrices.
This implies that specific data matrices are necessary to identify co-responses for genes with context-specific expression. For example, numerous stress-related genes are only expressed under stress. Therefore, co-response analysis with stress-signalling components results in incomplete coverage of target genes in a complex matrix. Tissue-specific matrices hold the potential to analyse expression patterns which are hidden in whole plant profiles.
Statistical approaches have limitations. Correlations do not allow discrimination between primary and secondary events and are not equal to causal relationships. Thus, direct approaches are still necessary for the functional characterization of genes. Other limitations are shared with direct approaches. Reliable measurements of all transcripts are not feasible, and there is an ongoing debate about optimal normalization procedures. In this study, the Affymetrix MAS5.0 and GCOS software were used to normalize the expression profiles. For specific data matrices, alternative normalization procedures will be implemented (e.g. RMA for tissue-specific matrices).
CSB.DB: a valuable public resource to uncover genomic effects
In this study, we focused on genes showing positive transcript co-responses. CSB.DB also allows screening for genes which display negative correlation coefficients. Following the presented approach, 404 genes would have had negative transcript co-responses with both BRI1 and BAK1 [rs < (−0.35), p-value < 0.01 and power > 0.7]. Future work shall address the analysis and experimental verification of negative co-responses. We currently construct subsets of expression experiments which will allow screening for co-responses under specific experimental conditions. All data matrices will be publicly available.
SUPPLEMENTARY MATERIAL
Supplementary Material is available at NAR Online.
Supplementary Material
Acknowledgments
We acknowledge the AtGenExpress consortium (Lutz Nover, Thomas Altmann and Detlef Weigel) for supply of expression profiles. We thank Björn Usadel for assisting us in statistical analyses. We acknowledge NASCarrays and all scientists who submitted transcript profiles. We thank Dirk Büssis for critical reading of the manuscript. We are grateful to the anonymous reviewers for helpful comments. This work was supported by a grant from the Deutsche Forschungsgemeinschaft (DFG), MU 1738/2-3. Funding to pay the Open Access publication charges for this article was provided by the Max-Planck-Institute of Molecular Plant Physiology.
Conflict of interest statement. None declared.
REFERENCES
- 1.Bishop G.J. Brassinosteroid mutants of crops. J. Plant Growth Regul. 2003;22:325–335. doi: 10.1007/s00344-003-0064-1. [DOI] [PubMed] [Google Scholar]
- 2.Sasse J. Physiological actions of brassinosteroids. In: Sakurai A., Yokota T., Clouse S.D., editors. Brassinosteroids: Steroidal Plant Hormones. Tokyo: Springer Verlag; 1999. pp. 137–161. [Google Scholar]
- 3.Khripach V., Zhabinskii V., De Groot A. Twenty years of brassinosteroids: steroidal plant hormones warrant better crops for the XXI century. Ann. Bot. 2000;86:441–447. [Google Scholar]
- 4.Nakashita H., Yasuda M., Nitta T., Asami T., Fujioka S., Arai Y., Sekimata K., Takatsuto S., Yamaguchi I., Yoshida S. Brassinosteroid functions in a broad range of disease resistance in tobacco and rice. Plant J. 2003;33:887–898. doi: 10.1046/j.1365-313x.2003.01675.x. [DOI] [PubMed] [Google Scholar]
- 5.Turk E.M., Fujioka S., Seto H., Shimada Y., Takatsuto S., Yoshida S., Denzel M.A., Torres Q.I., Neff M.M. CYP72B1 inactivates brassinosteroid hormones: an intersection between photomorphogenesis and plant steroid signal transduction. Plant Physiol. 2003;133:1643–1653. doi: 10.1104/pp.103.030882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Müssig C., Fischer S., Altmann T. Brassinosteroid-regulated gene expression. Plant Physiol. 2002;129:1241–1251. doi: 10.1104/pp.011003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Goda H., Shimada Y., Asami T., Fujioka S., Yoshida S. Microarray analysis of brassinosteroid-regulated genes in Arabidopsis. Plant Physiol. 2002;130:1319–1334. doi: 10.1104/pp.011254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Müssig C., Shin G.-H., Altmann T. Brassinosteroids promote root growth in Arabidopsis. Plant Physiol. 2003;133:1261–1271. doi: 10.1104/pp.103.028662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Goda H., Shinichiro S., Asami T., Fujioka S., Shimada Y., Yoshida S. Comprehensive comparison of auxin-regulated and brassinosteroid-regulated genes in Arabidopsis. Plant Physiol. 2004;134:1555–1573. doi: 10.1104/pp.103.034736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yin Y.H., Wang Z.Y., Mora-Garcia S., Li J.M., Yoshida S., Asami T., Chory J. BES1 accumulates in the nucleus in response to brassinosteroids to regulate gene expression and promote stem elongation. Cell. 2002;109:181–191. doi: 10.1016/s0092-8674(02)00721-3. [DOI] [PubMed] [Google Scholar]
- 11.Nemhauser J.L., Chory J. BRing it on: new insights into the mechanism of brassinosteroid action. J. Exp. Bot. 2004;55:265–270. doi: 10.1093/jxb/erh024. [DOI] [PubMed] [Google Scholar]
- 12.Müssig C., Altmann T. Genomic brassinosteroid effects. J. Plant Growth Regul. 2003;22:313–324. doi: 10.1007/s00344-003-0061-4. [DOI] [PubMed] [Google Scholar]
- 13.Steinhauser D., Usadel B., Lüdemann A., Thimm O., Kopka J. CSB.DB: a Comprehensive Systems-Biology Database. Bioinformatics. 2004;20:3647–3651. doi: 10.1093/bioinformatics/bth398. [DOI] [PubMed] [Google Scholar]
- 14.Craigon D.J., James N., Okyere J., Higgins J., Jotham J., May S. NASCArrays: a repository for microarray data generated by NASC's transcriptomics service. Nucleic Acids Res. 2004;32:D575–D577. doi: 10.1093/nar/gkh133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Efron B., Tibshirani R. An introduction to the Bootstrap. New York, London: Chapman and Hall; 1993. [Google Scholar]
- 16.Thimm O., Bläsing O., Gibon Y., Nagel A., Meyer S., Krüger P., Selbig J., Müller L.A., Seung Y.R., Stitt M. MAPMAN: a user-driven tool to display genomics data sets onto diagrams of metabolic pathways and other biological processes. Plant J. 2004;37:914–939. doi: 10.1111/j.1365-313x.2004.02016.x. [DOI] [PubMed] [Google Scholar]
- 17.Coll-Garcia D., Mazuch J., Altmann T., Müssig C. EXORDIUM regulates brassinosteroid-responsive genes. FEBS Lett. 2004;563:82–86. doi: 10.1016/S0014-5793(04)00255-8. [DOI] [PubMed] [Google Scholar]
- 18.Steinhauser D., Junker B.H., Luedemann A., Selbig J., Kopka J. Hypothesis-driven approach to predict transcriptional units from gene expression data. Bioinformatics. 2004;20:1928–1939. doi: 10.1093/bioinformatics/bth182. [DOI] [PubMed] [Google Scholar]
- 19.Nemhauser J.L., Mockler T.C., Chory J. Interdependency of brassinosteroid and auxin signaling in Arabidopsis. PLoS Biol. 2004;2:1460–1471. doi: 10.1371/journal.pbio.0020258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Clouse S.D., Langford M., McMorris T.C. A brassinosteroid-insensitive mutant in Arabidopsis thaliana exhibits multiple defects in growth and development. Plant Physiol. 1996;111:671–678. doi: 10.1104/pp.111.3.671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kauschmann A., Jessop A., Koncz C., Szekeres M., Willmitzer L., Altmann T. Genetic evidence for an essential role of brassinosteroids in plant development. Plant J. 1996;9:701–713. [Google Scholar]
- 22.Li J., Chory J. A putative leucine-rich repeat receptor kinase involved in brassinosteroid signal transduction. Cell. 1997;90:929–938. doi: 10.1016/s0092-8674(00)80357-8. [DOI] [PubMed] [Google Scholar]
- 23.Li J., Wen J.Q., Lease K.A., Doke J.T., Tax F.E., Walker J.C. BAK1, an Arabidopsis LRR receptor-like protein kinase, interacts with BRI1 and modulates brassinosteroid signaling. Cell. 2002;110:213–222. doi: 10.1016/s0092-8674(02)00812-7. [DOI] [PubMed] [Google Scholar]
- 24.Nam K.H., Li J.M. BRI1/BAK1, a receptor kinase pair mediating brassinosteroid signaling. Cell. 2002;110:203–212. doi: 10.1016/s0092-8674(02)00814-0. [DOI] [PubMed] [Google Scholar]
- 25.Montoya T., Nomura T., Farrar K., Kaneta T., Yokota T., Bishop G.J. Cloning the tomato Curl3 gene highlights the putative dual role of the leucine-rich repeat receptor kinase tBRI1/SR160 in plant steroid hormone and peptide hormone signaling. Plant Cell. 2002;14:3163–3176. doi: 10.1105/tpc.006379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Scheer J.M., Ryan C.A. The systemin receptor SR160 from Lycopersicon peruvianum is a member of the LRR receptor kinase family. Proc. Natl Acad. Sci. USA. 2002;99:9585–9590. doi: 10.1073/pnas.132266499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schlüter U., Köpke D., Altmann T., Müssig C. Analysis of carbohydrate metabolism of CPD antisense plants and the brassinosteroid-deficient cbb1 mutant. Plant Cell Environ. 2002;25:783–791. [Google Scholar]
- 28.Kamuro Y., Takatsuto S. Practical application of brassinosteroids in agricultural fields. In: Sakurai A., Yokota T., Clouse S.D., editors. Brassinosteroids: Steroidal Plant Hormones. Tokyo: Springer Verlag; 1999. pp. 223–241. [Google Scholar]
- 29.Friedrichsen D.M., Nemhauser J., Muramitsu T., Maloof J.N., Alonso J., Ecker J.R., Furuya M., Chory J. Three redundant brassinosteroid early response genes encode putative bHLH transcription factors required for normal growth. Genetics. 2002;162:1445–1456. doi: 10.1093/genetics/162.3.1445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mathur J., Molnár G., Fujioka S., Takatsuto S., Sakurai A., Yokota T., Adam G., Voigt B., Nagy F., Maas C., et al. Transcription of the Arabidiopsis CPD gene, encoding a steroidogenic cytochrome P450, is negatively controlled by brassinosteroids. Plant J. 1998;14:593–602. doi: 10.1046/j.1365-313x.1998.00158.x. [DOI] [PubMed] [Google Scholar]
- 31.Bancos S., Nomura T., Sato T., Molnár G., Bishop G.J., Koncz C., Yokota T., Nagy F., Szekeres M. Regulation of transcript levels of the Arabidopsis cytochrome P450 genes involved in brassinosteroid biosynthesis. Plant Physiol. 2002;130:504–513. doi: 10.1104/pp.005439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Neff M.M., Nguyen S.M., Malancharuvil E.J., Fujioka S., Noguchi T., Seto H., Tsubuki M., Honda T., Takatsuto S., Yoshida S., et al. BAS1: a gene regulating brassinosteroid levels and light responsiveness in Arabidopsis. Proc. Natl Acad. Sci. USA. 1999;96:15316–15323. doi: 10.1073/pnas.96.26.15316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Müssig C. Brassinosteroid-promoted growth. Plant Biol. 2005;7:110–117. doi: 10.1055/s-2005-837493. [DOI] [PubMed] [Google Scholar]
- 34.Czechowski T., Bari R.P., Stitt M., Scheible W.-R., Udvardi M.K. Real-time RT-PCR profiling of over 1400 Arabidopsis transcription factors: unprecedented sensitivity reveals novel root- and shoot-specific genes. Plant J. 2004;38:366–379. doi: 10.1111/j.1365-313X.2004.02051.x. [DOI] [PubMed] [Google Scholar]
- 35.Cano-Delgado A., Yin Y., Yu C., Vafeados D., Mora-Garcia S., Cheng J.-C., Nam K.H., Li J., Chory J. BRL1 and BRL3 are novel brassinosteroid receptors that function in vascular differentiation in Arabidopsis. Development. 2004;131:5341–5351. doi: 10.1242/dev.01403. [DOI] [PubMed] [Google Scholar]
- 36.Wang Z.-Y., Nakano T., Gendron J., He J., Chen M., Vafeados D., Yang Y., Fujioka S., Yoshida S., Asami T., et al. Nuclear-localized BZR1 mediates brassinosteroid-induced growth and feedback suppression of brassinosteroid biosynthesis. Dev. Cell. 2002;2:505–513. doi: 10.1016/s1534-5807(02)00153-3. [DOI] [PubMed] [Google Scholar]
- 37.He J.-X., Gendron J.M., Yang Y., Li J., Wang Z.-Y. The GSK3-like kinase BIN2 phosphorylates and destabilizes BZR1, a positive regulator of the brassinosteroid signaling pathway in Arabidopsis. Proc. Natl Acad. Sci. USA. 2002;99:10185–10190. doi: 10.1073/pnas.152342599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Choe S., Dilkes B.P., Fujioka S., Takatsuto S., Sakurai A., Feldmann K.A. The DWF4 gene of Arabidopsis encodes a cytochrome P450 that mediates multiple 22α-hydroxylation steps in brassinosteroid biosynthesis. Plant Cell. 1998;10:231–243. doi: 10.1105/tpc.10.2.231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kim G.-T., Fujioka S., Kozuka T., Tax F.E., Takatsuto S., Yoshida S., Tsukaya H. CYP90C1 and CYP90D1 are involved in different steps in the brassinosteroid biosynthesis pathway in Arabidopsis thaliana. Plant J. 2005;41:710–721. doi: 10.1111/j.1365-313X.2004.02330.x. [DOI] [PubMed] [Google Scholar]
- 40.Szekeres M., Nemeth K., Koncz-Kalman Z., Mathur J., Kauschmann A., Altmann T., Redei G.P., Nagy F., Schell J., Koncz C. Brassinosteroids rescue the deficiency of CYP90, a cytochrome P450, controlling cell elongation and de-etiolation in Arabidopsis. Cell. 1996;85:171–182. doi: 10.1016/s0092-8674(00)81094-6. [DOI] [PubMed] [Google Scholar]
- 41.Shimada Y., Fujioka S., Miyauchi N., Kushiro M., Takatsuto S., Nomura T., Yokota T., Kamiya Y., Bishop G.J., Yoshida S. Brassinosteroid-6-oxidases from Arabidopsis and tomato catalyze multiple C-6 oxidations in brassinosteroid biosynthesis. Plant Physiol. 2001;126:770–779. doi: 10.1104/pp.126.2.770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hu Y., Bao F., Li J. Promotive effect of brassinosteroids on cell division involves a distinct CycD3-induction pathway in Arabidopsis. Plant J. 2000;24:693–710. doi: 10.1046/j.1365-313x.2000.00915.x. [DOI] [PubMed] [Google Scholar]
- 43.Xu W., Purugganan M.M., Polisensky D.H., Antosiewicz D.W., Fry S.C., Braam J. Arabidopsis TCH4, regulated by hormones and the environment, encodes a xyloglucan endotransglycosylase. Plant Cell. 1995;7:1555–1567. doi: 10.1105/tpc.7.10.1555. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.