Abstract
Parkinson’s disease (PD) is a progressive neurodegenerative disorder implicitly marked by the substantia nigra dopaminergic neuron degeneration and explicitly characterized by the motor and non-motor symptom complexes. Apart from the nigrostriatal dopamine depletion, the immune and endocrine study findings are also frequently reported, which, in fact, have helped to broaden the symptom spectrum and better explain the pathogenesis and progression of PD. Nevertheless, based on the neural, immune, and endocrine findings presented above, it is still difficult to fully recapitulate the pathophysiologic process of PD. Therefore, here, in this review, we have proposed the neuroimmunoendocrine (NIE) modulatory network in PD, aiming to achieve a more comprehensive interpretation of the pathogenesis and progression of this disease. As a matter of fact, in addition to the classical motor symptoms, NIE modulatory network can also underlie the non-motor symptoms such as gastrointestinal, neuropsychiatric, circadian rhythm, and sleep disorders in PD. Moreover, the dopamine (DA)–melatonin imbalance in the retino-diencephalic/mesencephalic-pineal axis also provides an alternative explanation for the motor complications in the process of DA replacement therapy. In conclusion, the NIE network can be expected to deepen our understanding and facilitate the multi-dimensional management and therapy of PD in future clinical practice.
Keywords: Neuroinflammation, Neuroendocrine, α-Synuclein (SNCA), Dopaminergic receptor (DR), Hypothalamus, Pineal gland
Introduction
Parkinson’s disease (PD), the main entity of Parkinsonism, is symptomatically characterized by bradykinesia, muscular rigidity, static tremor, and postural disturbance, with the progressive degeneration of nigrostriatal dopaminergic (NSD) system and presence of Lewy bodies (LBs) in remnant neurons as the typical pathological hallmarks [1]. As a result of the confirmative dopamine (DA) depletion and remarkable symptom relief upon DA replacement therapy, PD has long been conceptualized as a neurodegenerative disease resulting from the NSD system degeneration. Nevertheless, in spite of the current diagnostic and therapeutic emphases largely on the motor symptomatology, the gradual understanding of non-motor symptom complexes [2–9] has increasingly interpreted PD as a multisystem involved syndrome [7, 10], more than a simple nigrostriatal DA-deficiency disease. To be specific, the non-motor symptoms suffered by PD patients mainly include neuropsychiatric symptoms (depression, anhedonia, hallucination, delusions, and dementia), autonomic symptoms (orthostatic hypotension, bladder disturbance, and sexual dysfunction), gastrointestinal (GI) symptoms (constipation, ptyalism, and dysphagia), sensory symptoms (paraesthesia and hyposmia), sleep disorders (restless leg and periodic limb movements, rapid eye movement sleep behavior disorder (RBD), excessive daytime somnolence, and insomnia), etc. [3].
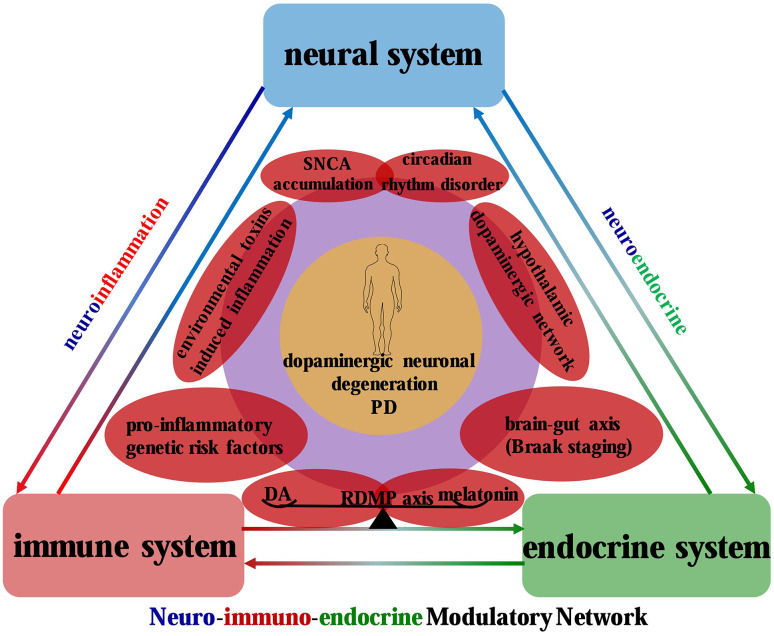
Given this broad motor and non-motor symptom spectrums, we may then wonder what indeed results in these multisystemic symptoms in PD. Up to now, multitudinous cellular and molecular mechanisms have been confirmed to be involved in the neurodegenerative disorder, including mitochondrial dysfunction, oxidative stress, neuroinflammation, neuroendocrine dysregulation, circadian rhythm dysfunction, endoplasmic reticulum stress, protein aggregates toxicity, protein degradation impairment, etc. [11–18]. However, the pathogenic mechanisms presented above still fail to sufficiently explain the complex motor and non-motor symptom matrix. To this end, the neuronimmunoendocrine (NIE) modulatory network has been proposed in this review to provide a comprehensive interpretation (Fig. 1).
Fig. 1.
NIE modulatory network in PD: a confluence of neuroinflammation and neuroendocrine systems. The neuroinflammation and neuroendocrine systems recapitulate the pathophysiologic processes of PD. Specifically, the former is a convergent point for pro-inflammatory genetic factors, environmental toxin-induced inflammation, DA depletion, and SNCA accumulation, which collectively contribute to the neuroinflammatory events in the pathogenesis of PD. In contrast, the hypothalamic dopaminergic network dysfunction, circadian rhythm disorder, and brain–gut axis-mediated pathological dissemination execute as the neuroendocrine nodes to incorporate the neural and endocrine systems into a symptomatic entity in PD, with DA and melatonin as the connectors of the NIE modulatory network
As mentioned above, multiple systems have been confirmed to be involved in the symptom spectrum of PD, which indeed can be further divided into neural, immune, and endocrine subcategories. Since the up-regulation of major histocompatibility complex (MHC) was confirmed in PD patients by Whitton [19], neuroinflammation has been increasingly recognized to be capable of compromising NSD neuron survival and hastening disease progression in PD patients and models [20–24]. Besides, the sustained inflammatory responses, massive lymphocyte T infiltration, and remarkable glial cell activation collectively indicate that the PD is a neuroinflammation-involved non-dopaminergic neuron autonomous process [25, 26]. Moreover, the fact that parkinsonian symptoms can be relieved or even reversed by interventional remedies targeting neuroinflammation also corroborates the supposition [18]. Apart from that, several neuroendocrine dysregulation events, such as circadian rhythm disorder [27], hypothalamic–pituitary–adrenal (HPA) axis dyshomeostasis [28], and retino-diencephalic/mesencephalic-pineal (RDMP) axis disequilibrium [12], have also been proven to contribute to the motor fluctuation and numerous non-motor symptoms of PD. Therefore, based on this, it can be speculated that the neural, endocrine, and immune system can act in concert to form an integrated circuit by virtue of shared signaling molecules and receptors [28, 29], thus precipitating and propelling the pathogenesis and progression of PD.
Here, in this review, we overview the implication of neuroendocrine and neuroinflammatory dysregulation events in detail in the context of PD. Moreover, from a perspective of NIE modulatory network, the synergistic effects of neuroendocrine and neuroinflammation implied in the pathophysiologic process of PD are illustrated as well (Figs. 1, 5). We expect that the NIE network proposed in this review can be used to deepen our understanding of PD and further facilitate the multi-dimensional management and therapy in future clinical practice.
Fig. 5.
Implication of NIE modulatory network in the motor and non-motor symptom complexes of PD. Parkinsonian symptom complex encompasses a broad array of motor and non-motor symptoms, with neuroimmune and neuroendocrine networks converging to form an NIE modulatory network. In particular, the neuroinflammation begins at the peripheral olfactory bulb and enteric plexus, and proceeding into the CNS in a Braak disseminating style. While in the RDMP axis, the dysfunctional SCN, hypothalamus, pineal gland, and resultant DA–melatonin imbalance help to interpret the Parkinsonian neuroendocrine abnormities. Based on the neuroendocrine and neuroimmune networks, the four dopaminergic projection pathways (nigrostriatal, mesolimbic, mesocortical, and TIDA pathways) underlie the motor and non-motor symptom complexes in PD
Neuroinflammation: a convergent loop implied in the pathogenesis of PD
Neuroinflammation, a closely regulated host-defense mechanism, is mainly committed to remove noxious agents and neutralize exogenous insults. However, researchers have been once trapped in a dilemma when interpreting the role of inflammatory responses in neuronal degeneration, since many of the responses can either promote or inhibit the neurodegenerative process [30]. Besides, the PD-related iconic molecules, α-synuclein (SNCA) and DA, have been proposed to be able to exert neuromodulatory effects [31–35] depending on a specific condition. Moreover, neuroinflammation has also been supposed to be a connector in the complex interaction between gene and environment, predisposing susceptible people to the development of PD [36]. As a matter of fact, closely modulated neuroinflammation can neutralize pathogenic triggers and deter neurodegenerative process, while maladjusted or persistent inflammation can otherwise contribute to a cascade of events contributing to neuronal degeneration including PD. Therefore, maladjusted neuroinflammation can be proposed as a convergent loop implied in the complex of genetic risk factors, environment, DA, and SNCA, executing as a backstage perpetrator to propel the pathogenesis and progression of PD (Fig. 2).
Fig. 2.
Microglial activation and dopaminergic neuron death induced by LPS and MPTP: different pathways and common outcomes. Microglial activation can be categorized into two types: (1) direct microglial phenotypic and functional activation by LPS and (2) delayed reactive microgliosis secondary to MPTP-induced dopaminergic neuron death. LPS can be specifically recognized by microglial TLR4 and then proceeds to activate downstream pro-inflammatory pathways, resulting in neuroinflammation-induced dopaminergic neuron death and amplified microglial activation. In comparison, MPTP is primitively metabolized into MPP+ by astrocytes and then taken up into dopaminergic neurons via DAT, ultimately leading to mitochondrial damage, reactive microgliosis, and neuronal death
Genetic risk factors and neuroinflammation
Specific gene mutations, revealed by several genetic studies, have been confirmed to be correlated with the pathogenesis of PD partly via neuroinflammation modulation, which includes mutations in SNCA/PARK1, Parkin/PARK2, PINK-1/PARK6, DJ-1/PARK7, LRRK2/PARK8, and so on [16, 37–39]. In fact, among the numerous PD-related genes, at least half of which have been identified to be associated with neuroimmune responses [39, 40]. Besides, several nuclear receptors also emerge as neuroinflammatory regulators, contributing to the dopaminergic neuronal degeneration [41]. Therefore, it can be speculated that neuroinflammation can execute as a convergent downstream pathway for the multifarious genetic risk factor-mediated onset and progression of PD.
Genes and neuroinflammation
The SNCA gene, either copy multiplication or missense point mutation, has been proven to be involved in the familial and sporadic form of PD [42–49]. In the context of immune system, SNCA acts as a danger-associated molecular pattern (DAMP) and is capable of stimulating Toll-like receptors (TLRs) [40]. In particular, misfolded and fibrillar forms of SNCA are confirmed to activate microglia via TLR2 and TLR4 [50, 51]. Moreover, it should be noted that the SNCA gene is also expressed in a variety of immune cells such as microglia, lymphocyte, and NK cell [33–35]. In addition, microglia from SNCA null mice display a more activated phenotype in terms of morphology and cytokines secretion other than decreased phagocytic ability [52]. Collectively, these studies demonstrate that SNCA gene can instigate and foster a pro-inflammatory milieu, thereby contributing to the neuronal loss as corroborated in PD (Table 1).
Table 1.
Neuroinflammation relevant genes in PD
| Gene | Locus | Location | Inheritance | Phenotype | Gene function | Neuropathology | Reference |
|---|---|---|---|---|---|---|---|
| SNCA | PARK1/4 | 4q22.1 |
AD Sporadic |
Early-onset PD |
1. Presynaptic vesicular neurotransmission 2. Neuroinflammation modulation |
1. Facilitating pro-inflammatory milieu formation 2. Neurodegeneration in SN 3. Widespread LBs formation |
[33–35, 40, 50–52] |
| LRRK2 | PARK8 | 12q12 |
AD Sporadic |
Classical PD |
1. Neuroinflammation modulation 2. Dynamic cytoskeletal regulation 3. Autophagy accommodation |
1. Microglial phenotype switch (M2–M1) 2. Inflammation exacerbation 3. LBs formation and nigral neurons loss |
[54–57] |
| Parkin | PARK2 | 6q25.2-7 |
AR Sporadic |
Juvenile and early onset PD |
1. Encoding protein containing E3 ligase activity 2. Involved in UPS 3. Preventing protein aggregation and promoting mitophagy 4. Adaptive immunity regulation |
1. Increase susceptibility to inflammation-induced neurodegeneration 2. Absence of LBs 3. Dopaminergic neuronal loss in SN |
[58–61] |
| PINK1 | PARK6 | 1p36.12 | AR | Early-onset PD |
1. Encoding PTEN-induced putative kinase 1 (mitochondrial kinase) 2. Stabilize mitochondrial function during episodes of cellular stress 3. Adaptive immunity regulation |
1. Increase vulnerability to neuroinflammation 2. Dopaminergic neuron loss in SN 3. Far-ranging LBs formation |
[61, 62] |
| DJ-1 | PARK7 | 1p36.23 | AR | Early-onset PD |
1. Encoding the redox sensor DJ-1 2. Protecting cell from oxidative stress response 3. Associate with HSP70 to mediate PTM or repair misfolded protein 4. Involved in PD-related cellular pathway as a RNA binding protein |
1. Increase susceptibility to PD-related environmental toxins 2. Enable dopaminergic neurons sensitive to oxidative stress and proteasomal inhibition |
[65–72] |
| ATP13A2 | PARK9 | 1p36 | AR | Early-onset levodopa responsive parkinsonism (KRS) |
1. Encoding transmembrane lysosomal P5 type ATPase 2. Involved in ALP 3. Facilitating lysosome function and preventing protein aggregation 4. Mediating neuroinflammation via NLRP3 inflammasome |
1. Dopaminergic neurons loss in SN 2. Enable lysosome dysfunction and protein aggregates accumulation 3. Inducing NLRP3 inflammasome-mediated dopaminergic neurodegeneration |
[73, 74, 78] |
AD autosomal dominant, AR autosomal recessive, PTM post-transcriptional modification, UPS ubiquitin–proteasome system, ALP autophagy lysosome pathway, KRS Kulfor–Rakeb syndrome
Leucine-rich repeat kinase 2 (LRRK2), a kinase identified in both autosomal-dominantly inherited and sporadic PD cases, has been demonstrated to possess remarkable capability to modulate inflammation in response to different pathological stimuli. Moreover, LRRK2 has sizable homology to the receptor-interacting protein kinases, a kinase family with confirmed roles in immunity [53]. In the context of PD, abnormal LRRK2 activities or mutations can induce microglial cells to transform into a pro-inflammatory phenotype following the pathways as follows: (1) modulating microglia cell activation or phagocytosis via hyperphosphorylation and hyperpolymerization of cytoskeleton components such as actin and β-tublin and (2) accommodating membrane receptors (CD11b and MHC-II) delivery and inflammatory cytokines expression through the regulation of transcription factors such as nuclear factor-kappa B (NF-κB) and interaction and phosphorylation of vesicle-associated proteins [54]. Hence, LRRK2 has been perceived as a main perpetrator to sensitize microglias into a pro-inflammatory state, thereby resulting in exacerbated inflammation and even consequent neurodegeneration (Table 1). Apart from that, mutation variants at the LRRK2 locus have been demonstrated to be capable of conferring increased risk to inflammatory bowel disease (Crohn’s Disease and Ulcerative Colitis) and leprosy [55–57], two category of disease with remarkable inflammatory reaction, which further strengthens the pro-inflammatory features of LRRK2.
The Parkin gene, encoding a multi-domain protein that contains E3 ligase activity, is mainly involved in a role of preventing protein aggregation and promoting mitophagy. In addition, the function loss of Parkin gene causes autosomal recessive form of juvenile PD [58]. Parkin knockout mice have been revealed to demonstrate increased vulnerability to inflammation-related degeneration. Moreover, persistent peripheral intraperitoneal injection of low dose of lipopolysaccharide (LPS) in Parkin knockout mice induces fine motor deficits and dopaminergic neuron loss in SN [59, 60]. Furthermore, Parkin and PINK1 (PTEN-induced putative kinase 1), two PD-associated mitochondrial protein, can regulate adaptive immunity via active inhibition of mitochondrial-derived vesicle (MDV) formation and mitochondrial antigen presentation (MitAP) [61], thus suppressing the immune response pathway-induced inflammation. In fact, just as dopaminergic neurons become “visible” to the immune system when expressing MHC class I molecules on their surface in the presence of pro-inflammatory stimuli [62], the MitAP activation in Parkin (−/−) dopaminergic neurons would engage recognition by established mitochondrial antigen specific T cell so as to trigger a cytotoxic response and lead to neuronal cell death ultimately. In addition, Parkin has been postulated to function as a transcription factor that regulates p53 expression [63], while specifically modified p53 can execute as a novel regulator of Parkin-mediated neuronal cell death in sporadic PD [64]. Therefore, the evidences displayed above indicate an involvement of Parkin/PINK1 in inflammation-induced neurodegeneration (Table 1).
The DJ-1 (PARK7) gene, encoding a putative redox sensor protein that associates with chaperone HSP70 [65] and D2 receptor [66], is proposed to function as a survival factor and anti-oxidant protein [67], as well as a RNA binding protein involved in multiple PD-related cellular pathways [68]. DJ-1(−/−) mice have been reported to be hypersensitive to 1-methyl-4-phenyl-1,2,3,6-tetrahydropyrindine (MPTP) [69] and to display dopaminergic neuronal deficits when exposed to environmental toxins [70]. Besides, DJ-1(−/−) or siRNA-mediated knockdown of DJ-1 mRNA in primary embryonic midbrain dopaminergic neurons also demonstrates increased sensitivity to oxidative stress and proteasomal inhibition [71]. Moreover, LPS exposure can cause astrocytes derived from DJ-1(−/−) mice to generate ten times more nitric oxide than that derived from wild-type mice [72]. These studies suggest that loss-of-function mutations in DJ-1 can affect both neuronal and non-neuronal cells and result in enhanced microglial activation upon neuroinflammatory insults (Table 1).
The transmembrane lysosomal P5-type ATPase (ATP13A2), encoded by Atp13a2 gene, is highly expressed in SN, a region that displays progressive dopaminergic neuronal degeneration in PD [73, 74]. In addition, the missense and truncation mutations in Atp13a2 gene is associated with lysosomal dysfunction and aggregates accumulation as well [73], resulting in an autosomal recessive levodopa responsive early-onset Parkinsonism, also known as Kulfor–Rakeb syndrome (KRS). As is known to all, neuroinflammation is involved in the pathogenesis of PD [11, 21, 75], while the lysosomal dysfunction and consequent protein aggregate accumulation further aggravates the already terrible status. In addition, the NLRP3 (nucleotide binding oligomerization domain, leucine-rich repeat, and pyrin domain containing protein 3) inflammasome-mediated neuroinflammation is proven to participate in PD as well [31, 76, 77]. Most recently, a novel role of ATP13A2 has been revealed to modulate astrocyte-mediated neuroinflammation via NLRP3 inflammasome activation following MPP+ treatment, thus indicating Atp13a2 gene as a potential mediator in neuroinflammation-induced dopaminergic neuron degeneration in PD (Table 1) [78].
Nuclear receptors and neuroinflammation
Apart from the pro-inflammatory PD-related genes, nuclear receptor (NR) superfamily also provokes a broad interest in a range of inflammation-associated neurodegenerative disorders. NRs are ligand-activated transcription factors that regulate genes, involved in physiological, metabolic, and developmental process, via their association with sequence specific elements within the promoter region of target genes [41, 79]. At present, certain NRs such as nuclear receptor-related receptor 1 (Nurr1), peroxisome proliferator-activated receptors (PPARs), glucocorticoid receptor (GR), and retinoic acid receptors (RARs) have been confirmed to function in several modulatory aspects of neurodegeneration, including the dopaminergic neuronal degeneration in PD (Table 2) [41].
Table 2.
Neuroinflammation-related nuclear receptors in PD
| Nuclear receptor | Superfamily | Ligand | Anti-inflammatory effects | References |
|---|---|---|---|---|
| Nurr1 | NR4A | N/A |
Inhibiting pro-inflammatory factors expression Alleviating neuroinflammation-mediated dopaminergic neuron death |
[80, 81] |
| PPARs | NR1C |
Pioglitazone Rosiglitazone |
Modulating Pro-inflammatory pathway (e.g., NF-κB pathway) Repressing excessive oxidative stress response Prevent dopaminergic neuron loss and DA depletion in SN |
[84–93] |
| GR | NR3C | Dexamethasone |
Repressing systemic inflammation upon toxin insult Preventing toxin-induced neurotoxicity and microgliosis |
[96–100] |
| RA/RAR | NR1B | N/A |
Promoting axon outgrowth, nerve regeneration and neural patterning Suppressing neuroinflammation via inhibition of NF-κB nuclear translocation Prevent dopaminergic neuron death and DA depletion in SN |
[102–104] |
N/A not available
Nurr1, a member of the nuclear receptor family of intracellular transcription factors, has been confirmed to express in microglia [80, 81] and astrocytes [81], and it indicates that Nurr1 can inhibit the pro-inflammatory mediator expression, thus potentially protecting from inflammation-mediated dopaminergic neuronal death. Similarly, nerve growth factor IB (Nur77) has been shown to compromise dopaminergic neuron survival via mitochondrial impairment and potential neuroinflammation [82, 83].
The PPAR (also NR1C) subfamily compromises three isoforms, PPARα (NR1C1), PPARβ/δ (NR1C2), and PPARγ (NR1C3), all of which are shown to exert anti-inflammatory effects by trans-repressing NF-κB or by modulating the oxidative stress pathway [84–86]. PPARγ is the most widely studied isoform, and the effects of PPARγ agonists have been assessed in PD animal models. Pioglitazone and rosiglitazone, two common synthetic PPARγ agonists administrated for type 2 diabetes, have also been revealed to possess neuroprotective effects on dopaminergic neurons by preventing inflammation, oxidative damage, and apoptosis [86–89]. In the context of PD, PPARα/β/δ agonist have been described to beneficially modulate neuroinflammation as well [90–93], thus protecting against dopaminergic neuronal loss and microglial activation upon environmental toxin insult.
GR, glucocorticoid receptor (also called NR3C1), belongs to the steroid hormone receptor family and is activated by a class of steroid hormones called glucocorticoid. Apart from ubiquitous expression in the periphery tissues, GR is also widely expressed in several brain regions including SN [94]. In the context of PD patients, the expression of GR has been reported to be decreased in post-mortem SN compared with healthy controls, but with significantly higher level of cortisol [95]. In fact, when exposed to inflammatory reaction or to stress, the HPA axis is stimulated to increase the systemic level of glucocorticoids, resulting in a consequent repression of inflammation involving NF-κB [96, 97]. Besides, in LPS- and MPTP-induced rodent models, the administration of dexamethasone can prevent environmental toxin-induced neurotoxicity and microglial activation [98, 99]. Moreover, pharmacological antagonism of GR has been proven to aggravate LPS-induced inflammatory reaction and exacerbate consequent neurodegeneration as well, thus suggesting that glucocorticoids exert a neuroprotective effect during inflammatory responses [100]. Collectively, it can be concluded that the glucocorticoid/GR pathway dysregulation could be responsible for the sustained inflammatory processes-mediated dopaminergic neuronal death in PD [96, 101].
RA, a derivative of vitamin A (retinol), is involved in regulating numerous physiological functions such as vision, vertebrate development, cell proliferation, nutrient metabolism, and immunity [102]. RA has been demonstrated to be involved in the development and maintenance of nigrostriatal pathway [103], primarily by promoting axon outgrowth, nerve regeneration, and neural patterning. In addition, the anti-inflammatory effects of RA are correlated with the enhanced expression of retinoic acid receptor (RAR) and transforming growth factor beta 1 (TGFβ-1) as well as the inhibition of NF-κB nuclear translocation [104]. Therefore, these data described above suggest that the RA/RAR pathway can be developed as an alternative therapeutic target in PD.
Environmental toxins and neuroinflammation
Apart from genetic risk factors, environmental toxins are also closely implicated in the process of PD onset and progression, to a great extent initiated and exacerbated by neuroinflammation. Microglia, the resident innate immune cells in central nervous system, has been perceived as the main executor of neuroinflammation, providing the first defense line whenever injury or disease occurs [105]. In physiological state, microglia density varies between different brain regions, with relatively higher concentration being confined to SN, hippocampus, basal ganglion, and olfactory telencephalon [106, 107]. Thus, in the context of PD, dopaminergic neurons in the SN may be particularly vulnerable to inflammatory insults compared to other brain tissue as a result of regionally concentrated microglia. Upon acute or persistent stress insult, neuroinflammation can be initiated or even extended when reaching specific threshold, demonstrated by the microglial phenotype switch and synthesis of a range of pro-inflammatory cytokines and mediators. More specifically, a wide range of stimuli including endogenous proteins (SNCA, damaged cell debris, cytosolic components, etc.) and a variety of exogenous environmental toxins (LPS, MPTP, paraquat, rotenone, etc.) have been proven to amplify ongoing microglial activation and even induce neuronal death. In particular, as for environmental toxin-induced neuroinflammation, microglial activation can be classified into two categories depending on the toxin types: (1) direct microglial phenotypic and functional activation upon toxin insult and (2) delayed reactive microgliosis secondary to neuronal injury or death, both of which eventually converges to induce common deleterious downstream effectors, including NADPH oxidase, ROS, superoxide, and multiple pro-inflammatory cytokines (Fig. 2) [11, 108–110].
LPS: direct stimulant of microglia
Microglia has evolved to express multiple membrane receptors, also termed pattern recognition receptors (PRRs), which are generally constitutively expressed to identify and bind pathogen-associated molecular patterns (PAMPs) in relation to microbial pathogens, as well as damage-associated molecular patterns (DAMPs) correlated with cellular components released from damaged neurons [111]. Contrary to the DAMPs, PAMPs are usually small molecular motifs existed within a class of microbes that mainly activate innate immune system and protect host from external infection. Bacterial LPS, the polysaccharide component derived from Gram-negative bacterial wall, are considered to be the prototypical class of PAMPs. In the context of PD, several LPS [112–116] as well as paraquat [117–119] regimens have been implemented to model inflammatory signaling and microglia-induced dopaminergic neuron loss in rodents. Microglial activation and neuroinflammation have been proven to be involved in the pathology of PD as well. In particular, several studies have demonstrated that LPS is neurotoxic to neurons only in the presence of microglia and microglia-induced neuronal cytotoxicity is only feasible via a proximity-dependent mechanism [120, 121]. Moreover, the inhibition of microglial activation is shown to alleviate microglia-mediated dopaminergic neuron injury [122–125]. In fact, LPS can be specifically recognized by TLR4 expressed on microglia and then proceeds to activate the downstream NF-κB signaling pathway, thus fostering a pro-inflammatory milieu to induce neuronal death and amplify ongoing microglial activation (Fig. 2).
MPTP and rotenone: the reactive microgliosis secondary to neuronal lesions
The microglial response secondary to direct neuronal lesions, also termed reactive microgliosis, is a process involving increased proliferation, recruitment, and activation of microglia [126, 127]. In physiological condition, moderately activated microglia exert neuroprotective influence in the CNS by phagocytizing excess neurotoxins, scavenging dying cells and cellular debris [128], and promoting the post-traumatic repairment process [129]. Hyperactivated microglia, however, exert cytotoxic effects which, in turn, facilitate neuronal death by synthesizing and releasing a plethora of neurotoxic mediators that include reactive oxygen species (ROS), free radicals, and pro-inflammatory cytokines [130, 131]. Indeed, reactive microgliosis has been demonstrated to be involved in a variety of neurodegenerative diseases including PD [131–134]. The MPTP-induced PD model, in particular, best characterizes the involvement of reactive microgliosis in the onset and progression of neurodegenerative processes. In fact, unlike the direct microglial stimulation by LPS, MPTP is primitively metabolized to 1-methyl-4-phenylpyridinium (MPP+) by glial cells once exposed to CNS, which is then taken up by dopaminergic neurons via the dopamine transporter, ultimately leading to mitochondrial damage, neuronal death, and reactive microgliosis (Fig. 2) [135, 136]. The activation of microglia has been observed to facilitate dopaminergic neuronal degeneration in the SNpc [137], suggesting that reactive microgliosis may contribute to the dopaminergic neuronal loss [131]. In addition, persistent microglial activation can be detected in the SNpc of human [138] and nonhuman primate [139] even years after the initial MPTP exposure. Moreover, blockade of microglial activation with minocycline prevents nigrostriatal dopaminergic neurodegeneration induced by MPTP in PD mouse model [140–142]. Apart from MPTP, rotenone [143–145] is also implicated in the same scenario. Hence, a vicious cycle may develop among environmental toxins exposure, reactive microgliosis, and neuronal degeneration, thus resulting in the progressive dopaminergic neurodegeneration in PD (Fig. 2).
DA and neuroinflammation: a cross talk between neural and immune system
Except for the conventional roles of neurotransmitters in neural communication, multifarious evidence indicates that neurotransmitters can also mediate cross talk between the neural and immune system [146]. Among neurotransmitters of this kind, DA is a typical representative. Apart from regulating behavior, movement, endocrine, cardiovascular, renal, and gastrointestinal functions [147, 148], DA can also function as an important molecule bridging the neural and immune system (Fig. 3) [32, 148]. The DA receptors, further classified into D1 and D2 classes, are present in almost all immune cell subpopulations [148], including microglia, lymphocytes, dendritic cells, and so on [149–151]. By acting on the corresponding receptors, DA and the dopaminergic agonists are proven to modulate the activation, proliferation, and cytokines secretion in immune cells [148, 152]. Moreover, dopaminergic innervation of lymphoid tissue through sympathetic nerve also suggests the immunomodulatory role of DA [153]. Hence, DA can execute as an intermediary between neural and immune systems, implying the cross talk between neural and immune systems in PD.
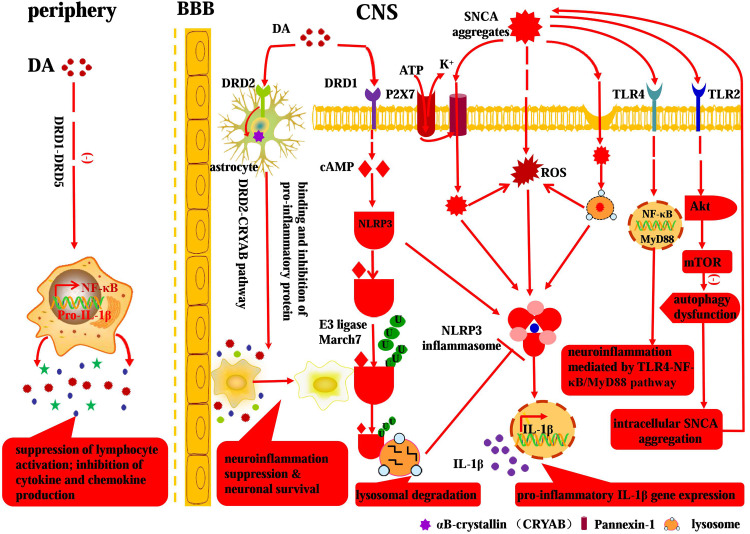
Fig. 3.
Involvement of DA and SNCA in neuroinflammation: a crosstalk between neural and immune system. Peripheral DA can modulate the immune network via DR (DRD1–DRD5), leading to the inhibition of cytokine and chemokine production, interruption of lymphocyte activation, and attenuation of inflammation intensity. While the anti-inflammatory effect of DA in CNS is mediated by the DRD2–CRYAB pathway and DRD1–cAMP–NLRP3/autophagy–lysosome pathway. Extracellular SNCA aggregates can be endocytosed or internalized via receptor independent and receptor-mediated patterns, respectively. In particular, the endocytosed SNCA aggregates can directly and indirectly induce NLRP3 inflammasome activation and subsequent pro-inflammatory IL-1β gene expression. Moreover, the SNCA aggregates can act on specific TLR-mediated pro-inflammatory signaling pathways (e.g., TLR2–Akt–mTOR&TLR4–NF-κB), thus resulting in pervasive SNCA accumulation, persistent neuroinflammation, and ultimate dopaminergic neuron death in PD
DA–dopaminergic receptor (DR) signaling complex: a neuroinflammation modulatory pathway
DA exerts its effects by binding to the activating receptors located on the cell surface. The D1 and D2 classes of DRs can be further categorized into five subtypes: DRD1–DRD5. The D1 class includes the DRD1 and DRD5 subtypes, which on activation mediates the downstream cyclic adenosine monophosphate (cAMP) increase [148, 150]. In contrast, the D2 class encompasses DRD2–DRD4 subtypes, which inhibits intracellular cAMP on stimulation [148, 150]. Inflammasomes are involved in diverse inflammatory diseases such as type 2 diabetes, atherosclerosis, gout, and PD, so the activation of inflammasome needs to be tightly controlled to prevent excessive inflammation [77, 154]. It has been demonstrated that DA inhibits NLRP3 inflammasome activation via DRD1, while DRD1 signaling negatively regulates NLRP3 inflammasome via a second messenger cAMP which binds to NLRP3 and promotes its ubiquitination and degradation [31]. Moreover, this study demonstrates DA and DRD1 signaling prevents NLRP3 inflammasome-dependent inflammation such as neurotoxin-induced neuroinflammation, LPS-induced systemic inflammation, and monosodium urate crystal-induced peritoneal inflammation as well [31]. In addition, DRD2 knockout mice display a remarkable neuroinflammatory response in multiple CNS regions and increase the vulnerability of nigral dopaminergic neurons to MPTP-induced neurotoxicity, suggesting that DA–DRD2 signaling possesses an anti-inflammatory function partly via αB-crystallin [155]. Moreover, deficient DRD2 receptor function increases renal expression of pro-inflammatory and profibrotic factors [156, 157], which can be ameliorated by retrograde renal infusion of adeno-associated virus (AAV) vector with DRD2 [156]. In contrast, DRD3 expressed on CD4+ T cells is crucial for the dopaminergic neurons destruction in SN and DRD3-deficient mice are proven to be protective against dopaminergic neuron loss and microglial activation upon MPTP insult [158]. Furthermore, the DRD3 expression alteration is demonstrated to be correlated with disease severity in PD patients [158]. Therefore, these study findings above indicate that DA and DA receptor signaling complex may represent a neuroinflammation modulatory pathway, while the disruption of this pathway can contribute to the pathogenesis of PD (Fig. 3).
Peripherally retained DA: a neuroinflammation moderator
Large quantities of inflammatory mediators are released upon endotoxin insult, which can in turn stimulate the sympathetic nervous system to synthesize and release catecholamines, ultimately modulating inflammation-induced impairment. Among the released catecholamine, DA receives much more attention. Apart from the common hemodynamic effects, DA itself can modulate the neuroinflammatory network and thereby regulate both suppressive and stimulatory effects on immune responses [32, 159], which leads to the inhibition of cytokine and chemokine production, interruption of lymphocyte activation, and attenuation of inflammation intensity (Fig. 3). Furthermore, sciatic nerve activation via electroacupuncture has been reported to control systemic inflammation and rescue mice from polymicrobial peritonitis [152]. In fact, the electroacupuncture can induce the activation of DOPA decarboxylase to produce more DA in the adrenal medulla, thus leading to the confinement of inflammation scope [152]. Similarly, a recent study has proven that acupuncture stimulation can transmit signals into the vagus nerve and mediate anti-inflammatory effects in internal organs as well [160]. Neuroinflammation has been proven to be involved in the pathogenesis of PD, and the implication of peripheral inflammation in PD gradually comes into researchers’ sight. Epidemiological studies have shown that incidence of idiopathic PD is about 50% lower in regular users of non-steroid anti-inflammatory drugs (NSAIDs) or cyclooxygenase (COX) inhibitors than in age-matched nonusers [161–163]. Moreover, peripheral inflammation has also been demonstrated to enhance the degeneration of nigrostriatal dopaminergic system, possibly by the recruitment of peripheral monocytes into the CNS via defective blood brain barrier (BBB), thus synergizing with other insults to exacerbate the progression of PD [164–166]. Therefore, given the immunomodulatory effect of DA, it can be proposed that the therapeutic effect of DA replacement therapy in PD may be partially attributed to the modulation of peripheral inflammation (Fig. 3), more than just replenishing the nigrostriatal DA depletion.
SNCA: neuroinflammatory initiator implied in PD
PD has been proven to be a chronic inflammatory disease, characterized by widespread inflammation and extensive microgliosis which contributes to the nigral DA depletion and consequent dopaminergic neuron loss. SNCA, a presynaptic protein with a propensity to aggregate into oligomers of multifarious morphology, is central to the pathogenesis and progression of PD. The aggregation form of SNCA—LB or Lewy neurite (LN)—is a neuropathological feature that defines a spectrum of disorders collectively termed synucleinopathies, among which PD is undoubtedly the best characterized. Moreover, LBs and LNs are the major component implied in the microglial activation, thus bestowing neuroinflammatory capabilities on the SNCA aggregates to initiate and promote the pathogenesis of PD. During this process, microglia is generally regarded as the executor and its activation largely contributes to the inflammation-induced neurodegeneration. In this section, the interaction between SNCA aggregates and microglia and downstream inflammatory cascades will be illustrated.
Internalization patterns between SNCA aggregates and microglia
SNCA is indeed an intraneuronal protein, while the SNCA aggregates—LBs or LNs—can be released into the extraneuronal milieu upon dopaminergic neuron death. Then, the released SNCA can be internalized by microglia to initiate the neuroinflammatory process [167–171]. Among which the internalization of SNCA aggregates by microglia is a premise for microglial activation and subsequent neuroinflammation process. As for the internalization pattern between SNCA aggregates and microglia, several modes have been proposed which can be further divided into receptor independent (exosome transmission, passive transmembrane diffusion, classical endocytosis, etc.) and receptor-mediated styles (Fig. 3).
Receptor independent internalization pattern In general, the receptor independent internalization pattern between SNCA aggregates and microglia includes exosome transmission, passive transmembrane diffusion, direct transmembrane pore formation, and classical endocytosis (Fig. 3). Several studies have reported that SNCA aggregates can be released by exosome in a calcium-dependent manner [172–174], which is further exacerbated by lysosomal dysfunction [175]. Conceivably, the SNCA aggregate-loaded exosome can be internalized by the microglia, triggering microglial activation, and downstream pro-inflammatory reaction. SNCA has been proven to associate with membranous compartments in vivo and in vitro as well [176]. The transmembrane permeability of SNCA aggregates is partly dependent on the specific assembly state of this protein [177, 178], thus suggesting a conformation-dependent internalization style. In contrast, monomeric SNCA can passively diffuse across the plasma membrane. In addition, SNCA is proven to interact with lipid layers via conformational inversion to alpha helical structure [179], which, to some extent, mediates the internalization between SNCA aggregates and microglia as well. Moreover, it has been shown that A53T mutation-induced SNCA aggregates can mediate faster internalization process than the wild-type counterparts with direct transmembrane pore formation [180]. Apart from that, endocytosis, a classical protein transferring method between cells, is involved in the receptor independent internalization process as well [171, 178, 181, 182].
Receptor-mediated internalization pattern Apart from the receptor independent endocytosis, it has been proven that aggregated forms of SNCA, both fibrils and oligomers, can penetrate into microglia via specific receptor-mediated internalization patterns. Moreover, the fact that the conserved SNCA N-terminal sequence can regulate membrane translocation efficiency, but not significantly affected by endocytosis inhibitors and indirectly corroborates that SNCA aggregates can be internalized by specific receptors [183] (Fig. 3). Here, we present evidence that TLRs (e.g., TLR2 and TLR4) and NLRP3 inflammasome are involved in the internalization process.
TLRs belong to the family of pattern recognition receptors (PRR) and are crucial players in the innate immune response. TLRs are expressed on innate immune system cells, including microglial and astroglial cells, which can recognize PAMP and endogenous molecules such as misfolded proteins [51, 184, 185]. As for the receptor-mediated SNCA internalization and downstream neuroinflammation, there exist conflicting results in the context of TLRs. Neuron-released extracellular SNCA oligomers are shown to be able to execute as endogenous agonist for microglial TLR2, triggering downstream inflammatory response in microglia [50]. However, only specific-type SNCA oligomers are involved in this paracrine interaction with microglial TLR2 [50]. In comparison, extracellular SNCA can induce neuroinflammation via pro-inflammatory TLR4 pathway as well, whereas the SNCA internalization is independent of TLR4 [186–188]. In spite of the controversies above, TLRs are still typical mediator for SNCA internalization and subsequent neuroinflammation.
The NLRP3 inflammasome, a fully characterized inflammasome consisting of NLRP3 scaffold, the ASC (PYCARD) adaptor, and caspase-1, can be activated upon exposure to whole pathogens, as well as a number of structurally diverse PAMPs, DAMPs, and environmental irritants [189]. As for the NLRP3 inflammasome activation pattern, there have been proposed three models to illustrate it [189]: (1) extracellular ATP stimulates the purogenic P2X7 ATP-gated ion channel [190], triggering K+ efflux and inducing the pannexin-1-mediated transmembrane pore formation which then allows extracellular agnoists such as DAMPs and PAMPs to enter the cytosol and engage NLRP3 (Fig. 3) [191]. (2) Particular NLRP3 agnoists (e.g., amyloid-β and silica) are engulfed, wherein the engulfment results in rupture and release of lysosomal contents that are somehow sensed by the NLRP3 inflammasome [192, 193]. (3) Specific types of PAMPs and DAMPs induce the generation of reactive oxygen species (ROS), and then, an ROS-dependent common pathway triggers the NLRP3 inflammasome activation [194, 195]. In the context of PD, SNCA aggregates are a typical represent of DAMPs which hold great potential to be internalized and induce NLRP3 inflammasome activation via the proposed pathways above. Moreover, it has been demonstrated that insoluble SNCA fibrils can induce monocytes to release IL-1β following the NLRP3 inflammasome activation, which is a strong and convincing evidence for the involvement of NLRP3 inflammasome in prion-associated inflammation [196] (Fig. 3).
SNCA-mediated downstream pro-inflammatory pathways in microglia
Apart from the morphological changes and cell surface expression alterations, microglial activation induced by SNCA aggregates is likely to evoke multiple pro-inflammatory events, ranging from the nuclear translocation of inflammation regulating elements, up-regulation of pro-inflammatory genes expression, and release of inflammatory cytokines. Indeed, all the cellular pro-inflammatory events above corroborate the activation of multiple pro-inflammatory pathways as well.
NF-κB transcription factor acts as a pleiotropic regulator of target genes in the CNS controlling physiological function [197] as well as pathological processes associated with neurodegeneration [198, 199]. NF-κB signaling cascade has been proven by several studies to be involved in monomeric, oligomeric, aggregated, and nitrated SNCA-induced microglial activation, highlighting its role in the regulation of pro-inflammatory mediators [34]. It has recently been proven that TLR4 plays a modulatory role on glial pro-inflammatory responses and ROS production induced by SNCA [51], thus demonstrating a pro-inflammatory SNCA–TLR4–NF-κB pathway (Fig. 3). In addition, SNCA is demonstrated to induce the expression of matrix metalloproteinases (MMPs), while the latter can lead to the activation of protease-activated receptor-1, MAPK, and NF-κB which mediate the downstream inflammation [200]. Beyond the signaling pathways above, the p38–ERK1/2/MAPK–NF-κB pathway [201], TLR4–MyD88 pathway [51], and TLR2–Akt–mTOR pathway [202] are also associated with SNCA-induced neuroinflammation (Fig. 3). In fact, studies on the multiple pro-inflammatory pathways not only improve our understanding of pathogenesis of PD, but also provide candidates for therapeutic intervention in patients with PD [203].
Neuroendocrine: a missing link bridging the systemic gap and expanding the symptomatic spectrum in PD
PD, as is known to all, is pathologically marked by DA deficiency in the nigrostriatal system and, therefore, symptomatically characterized by bradykinesia, rigidity, static tremor, and postural disturbance. Apart from the classical role in motor coordination, in fact, DA is also a neurotransmitter distributed in hypothalamus and other tissues, peripheral and CNS, participating in the modulation of neuropsychiatric activity, reward, cognition, sleep, and so on [3, 204, 205]. In particular, the hypothalamic–hypophysial network, a pivotal constituent of classical endocrine system, has been proven to be also implicated in the pathophysiology of several neurodegenerative diseases [206–209]. Moreover, DA can exert auto-receptor regulatory effect on the hypothalamic dopaminergic network [204]. In addition, circadian rhythm desynchronization is also involved in sleep disorders of PD, including excessive daytime sleep and insomnia, which has been recognized as a non-negligible part of the symptom complex of PD [12]. Therefore, the neuroendocrine dysregulation events, in the context of PD, potentiate to bridge the systemic gap and expand the symptomatic spectrum as well (Fig. 4).
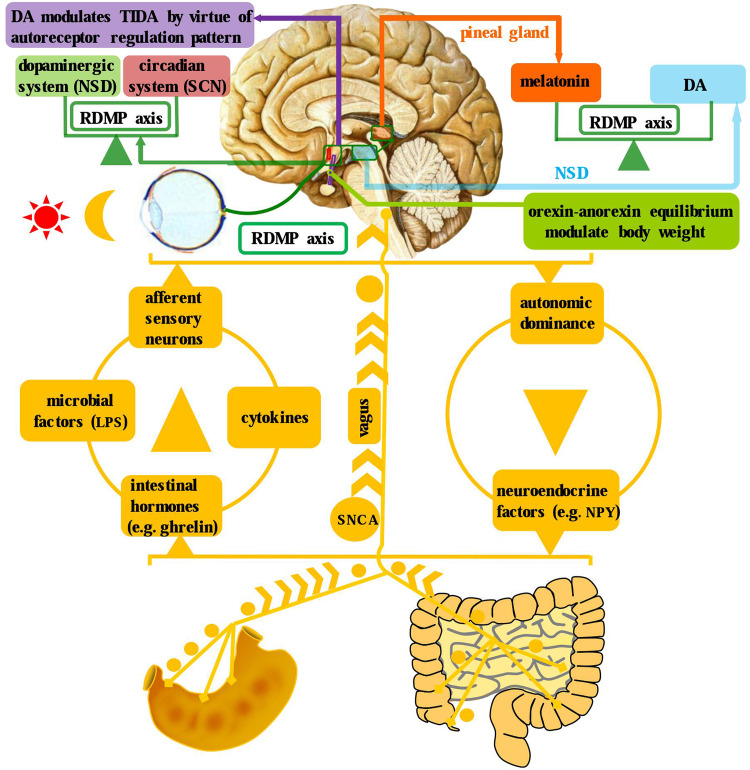
Fig. 4.
Brain–gut axis, dopaminergic system, and circadian system in the neuroendocrine context of PD. There exist bidirectional communications between brain and gut, which enable these two entities to be an integrated complex. In particular, there are four ascending pathways from the gut to brain: afferent sensory neurons, microbial factors, intestinal hormones, and cytokines. While there are two avenues from the brain to gut: autonomic dominance and neuroendocrine factors. Besides, a cross talk is identified between the circadian (SCN) and dopaminergic (NSD) systems, specifically manifesting as the DA and melatonin yoking in the RDMP axis. Moreover, the infundibular orexin–anorexin equilibrium and dopaminergic auto-receptor regulation of TIDA is illustrated as well
Neuroendocrine dysregulation of hypothalamic dopaminergic network
Hypothalamus, a confluent pivot linking the nervous and endocrine system by virtue of hypophysis, is an advanced nervous center modulating body temperature, feeding, mood, sleep, isohydria, circadian rhythm, and so on. What is interesting is that several experimental and post-mortem studies have demonstrated the involvement of hypothalamus in PD. Initially, Javoy-Agid et al. [210] reported that hypothalamic DA concentrations were reduced in PD, indicating that the deficient hypothalamic DA transmission may partly contribute to the autonomic and endocrine abnormalities of this disorder. Then, Shannak et al. [211] revealed that a mild-to-moderate reduction of DA levels is identified in the hypothalamus of idiopathic PD patients. Later, an 18F-dopa positron emission tomography (PET) study has shown a significant presynaptic DA storage reduction and postsynaptic dopaminergic dysfunction [212] in the hypothalamus. In addition, a most recent study reveals that DA modulates the tubero-infundibular dopaminergic axis (TIDA) via an auto-receptor regulation pattern [204]. In addition, the TIDA impairment has been found to be precisely correlated with the progression of motor dysfunction in PD [213]. Moreover, the main pathological hallmark of PD, LB formation, is also found in the hypothalamic nuclei along with SN and other brainstem nuclei as well [214]. Therefore, it can be concluded that the primary DA deficiency and LB, in the context of PD, can result in hypothalamic dopaminergic network dysregulation via an auto-receptor modulatory pattern (Fig. 4). Given the widespread hypothalamic neuroendocrine connection, it is, therefore, reasonable to propose that neuroendocrine network bridges the systematic gap and expands the symptomatic spectrum in PD.
Neuroendocrine pathways implied in brain–gut axis
The term “brain–gut axis” refers to the bidirectional communication between the brain and gut. In addition, the gut microbiota can communicate with the brain via neuroimmune or neuroendocrine pathways, which comprise the brain–gut axis. While in the context of PD, the brain–gut axis dysregulation may be associated with GI manifestations frequently preceding motor symptoms, as well as with the pathogenesis of PD itself [215], supporting the Braak staging hypothesis that the pathological process is spread from the gut to the brain along this axis. In general, there exist four communication pathways—afferent sensory neurons, gut hormones, immune mediators, and microbial signaling molecules—from the gut to the brain, where they can modify cerebral function and behavior (Fig. 4). Similarly, there are two pathways—autonomic and neuroendocrine output signals—from the brain to the gut [216]. In fact, among the microbial, immune, endocrine, and neural signaling pathways implied in brain–gut axis, the neuroendocrine pathway, denoted by numerous biologically active peptides, may occupy the pivotal status [216].
Bioactive peptides, particularly neuropeptides, play crucial roles in the bidirectional communication between the gut and the brain. Neuropeptides comprise a class of evolutionarily well-conserved molecules that, by definition, operate as transmitters in the enteric, peripheral, and central nervous systems and share transduction mechanisms with other bioactive peptides such as gut hormones [216–221]. As a result of the simultaneous existence and effect in brain and gut, the neuropeptides are also called brain–gut peptide and is frequently difficult to distinguish between their function as neuropeptides or gut hormones. In fact, neurons as well as endocrine, immune, interstitial, muscle, epithelial, and microbial cells can respond to these signaling molecules by expressing specific peptide receptors. Ghrelin, a gut hormone synthesized in the stomach to modulate appetite and energy balance, has been demonstrated to mediate a neuroprotective effect upon MPTP insult in PD mouse models through downstream AMPK signaling pathway [217] (Fig. 4). In addition, it has been proven that acylated, but not des-acyl ghrelin, is neuroprotective in MPTP mouse models by attenuating dopaminergic neuron loss and glial activation [218]. Besides, neuropeptide Y (NPY), a neurotransmitter in CNS (especially hypothalamus) and a gut hormone in autonomic nervous system, has been confirmed as a neuroprotective agent, as a neural stem cell proliferative agent, as an agent that increases trophic support, as a stimulator of autophagy, and as an inhibitor of excitotoxicity and neuroinflammation, thus enabling it as a therapeutic target in neurodegenerative disease [219, 220]. Moreover, NPY can mediate the stimulatory effect of ghrelin to form a synergistic effect on autophagy activation [221]. In addition, LPS and other bacterial factors can promote the generation and release of pro-inflammatory cytokines by stimulating TLR4 which is distributed in several levels of brain–gut axis, thus to some extent advancing the progression of neurodegeneration [222]. In conclusion, the studies above show that the brain–gut peptides can modulate the brain and gut activity simultaneously by virtue of neuroendocrine pathways, implying its involvement in brain–gut axis modulation in PD.
Neuroendocrine concept of weight loss in PD
Several studies have conformably demonstrated that PD patients have lower body weights when compared with age-matched control groups [223–225]. For example, Chen et al. have proven a remarkable weight loss until shortly before the diagnosis and then continue to decline following that in 468 PD patients [224]. Besides, a meta-analysis including 871 patients revealed an overall weight reduction of 1.73 kg/m2 in PD patients, with a positive association with disease severity but not disease duration [225]. Up to now, a variety of factors are proven to contribute to the weight loss phenomenon in PD, involving chewing difficulty, dysphagia, intestinal hypomotility, decreased rewarding in dopaminergic mesolimbic regions, etc. However, while viewing from a neuroendocrine perspective, hypothalamus indeed regulates the homeostasis between orexia and anorexia (Fig. 4). Specifically, the infundibular neurons produce two different kinds of hormones to, respectively, modulate the orexigenic and anorexigenic activities [226]. Nevertheless, it had been reported that there existed hypocretin (orexin) cell loss in PD [227]; moreover, decreased orexin level in cerebrospinal fluid was revealed in advanced PD as well [228]. Therefore, based on the evidences above, it can be speculated that neuroendocrine factors are implicated in the weight loss context of PD (Fig. 4).
Neuroendocrine disorder implicit in circadian system
Circadian rhythms are physiological and behavioral cycles generated by an endogenous biological clock, the suprachiasmatic nucleus (SCN). The circadian system exerts an effect on majority physiological processes, including sleep–wake homeostasis, cognitive performance, motor control, mental health, and metabolism [229]. A growing body of evidences has demonstrated that circadian dysfunction is a common symptom of neurodegenerative disease such as PD, which might in turn exacerbate the disease process as well [12, 230, 231]. Moreover, it has been proven that DA is a neurotransmitter of great importance at several levels of the circadian system, and its metabolism and signaling activity are also strongly influenced by the circadian rhythm [232] (Fig. 4). In fact, the functional effectuation of circadian system is largely implemented by neuroendocrine pathway. As a result of the pervasiveness of neuroendocrine modulation, the disruption of the circadian system is expected to have extensive effects throughout the body and may in turn to exacerbate the deteriorative situation. In the context of PD, circadian dysfunction is implicated not only in the dysregulation of sleep–wake cycle, but also in cognitive, neuropsychiatric, motor, and non-motor manifestations of this disease via an involvement of neuroendocrine pathway.
Involvement of DA in the circadian system
Dopaminergic neurotransmission has been implied at several levels of the circadian system, stemming from retina to the circadian center. As a matter of fact, there exists a crosstalk between the circadian clock components and dopaminergic signaling pathway at several aspects (Fig. 4). Apart from the light adaption role in the retina [233], DA also participates in the rhythmic expression of melanopsin, a photopigment expressed intrinsically at retinal ganglion cells, which is implied in circadian entrainment [234]. Moreover, the retinal dopaminergic cells can express circadian clock genes and core components of the molecular clock such as Per, Cry, Clock, and Bmall, which might help to sense the environmental illumination changes and accommodate to achieve optimal photic response [235]. In contrast, circadian clock genes can modulate the biosynthesis, transmission, and turnover of DA [236–239]. Moreover, the circadian nuclear receptor REV–ERBα has been proven to repress tyrosine hydroxylase (TH) gene transcription via competition with Nurr1, another nuclear receptor crucial for dopaminergic neuronal function, thus driving subsequent circadian TH expression through a target-dependent antagonistic mechanism [239]. In addition, DA and dopaminergic drugs, prescribed to alleviate the motor and non-motor symptoms of PD, have been demonstrated to exert circadian modulatory effects as well [240–243], more than simply replacing deficient DA. Therefore, this raise the presumption that circadian dysfunction suffered by PD patients may not merely be a subsidiary of the motor symptom, but an integral part of the disease which can further hasten the pathological process implied in PD.
Participation of melatonin in the circadian system
Melatonin, an organic substance with indole structure (N-acetyl-5-methoxytryptamine) synthesized predominantly in the pineal gland, has been proven to possess circadian, hypnotic, free-radical scavenging, SNCA aggregation inhibitory, and anti-inflammatory properties [244–247]. Melatonin secretion is closely modulated by the retino-diencephalic/mesencephalic-pineal (RDMP) axis integrating the NSD system and retinal hypothalamic tract (RHT), whereby photic information conducted by the RHT to the SCN and then from there to the pineal gland and other brain regions [12, 244]. As a widespread endogenous synchronous hormone, melatonin can stabilize and coordinate secondary internal circadian rhythms and hence modulate many biological processes, including sleep–wake cycle, hormone secretion, core body temperature, cognitive performance, and mood as well [244, 248]. In addition, exogenous melatonin has circadian resetting and restoration effects, thus enabling it applicable to various biorhythm disorders implicit in neurodegenerative disorders. Moreover, the distribution of melatonin ranges along the RDMP axis from the retina to the pineal gland, bridging the NSD system and RHT to form a neuroendocrine network [12] (Fig. 4). In the context of PD, melatonin has been demonstrated to execute as a neuroprotective agent to attenuate MPTP-induced neurotoxicity via preventing CDK5-mediated autophagy and SNCA aggregation [249, 250]. Besides, chronic low-dose melatonin treatment can also alleviate the dopaminergic neurons loss and improve the sleep disorders, especially combined with DA replacement, in PD models [251–253]. Furthermore, the melatonin administration in PD patients is proven to help to improve motor and non-motor manifestation, cognitive impairment, and mood disorder as well [247, 254, 255]. The extensive effects of melatonin on PD symptom matrix, in fact, can be largely attributed to the RDMP axis, which links the neural and endocrine system (Fig. 4). In conclusion, it can be speculated that melatonin holds great promise to be included in the anti-parkinsonian regimens, in particular, combined with levodopa preparations, more than just a circadian resetting agent.
DA–melatonin imbalance implied in the circadian disorders: viewing from a neuroendocrine perspective
Apart from the classical role of DA and melatonin in neural, endocrine, and immune systems described above, the relationship between DA and melatonin implicit in the NSD system also needs to be further explored. Generally speaking, DA and melatonin sit in functional opposition to each other in the RDMP axis, executing respective neuroendocrine roles simultaneously [12] (Fig. 4). To be specific, melatonin performs a contra-regulatory role with DA amid the day–night cycle. During the day, melatonin is reduced and DA is up; conversely, during the night, melatonin is increased, while DA is reduced [12]. Besides, circadian-related heteromerization of adrenergic and dopamine D4 receptors has been proven to modulate the synthesis and release of melatonin in pineal gland [256]. Based on this, it can be concluded that these two systems appear to be functionally yoked in healthy condition (Fig. 4), while the imbalance, more than DA deficiency, can send the system into disarray. Currently, DA replacement still occupies the first-class therapeutic regimen, but the wearing-off phenomenon and levodopa-induced dyskinesia have driven it into a dilemma. The attempt to replace deficient DA, in fact, is often a matter of hit or miss, because the DA–melatonin imbalance is implicit in a dynamic RDMP axis which is not easily rectified by simply replenishing deficient DA. Moreover, this has also been confirmed by the phenomenon that the patient inevitably experiences wearing-off phenomenon with long-term DA replacement therapy. Coincidental with this, the patients with wearing-off phenomenon also present elevated plasma melatonin [257]. Equally interesting is the occurrence of dyskinesia with prolonged exposure to high-dose DA and the ratio of DA to melatonin is severely imbalanced as well. By contrast, melatonin administration has been found to reduce dyskinesia in experimental PD models [258], which further highlight the importance of obtaining DA–melatonin balance rather than merely replacing deficient DA. Therefore, it can be concluded that the DA–melatonin imbalance is distributed concomitantly across the RDMP axis in PD (Fig. 4), which precisely interprets the extensive symptom spectrum stretching across the neuroendocrine system.
NIE modulatory network: confluence of neuroinflammation and neuroendocrine in motor and non-motor symptom complexes of PD
As demonstrated above, neuroinflammatory and neuroendocrine dysregulations, to a large extent, interpret the pathophysiologic processes of PD. To be specific, neuroinflammation can be regarded as a convergent point for genetic factors, environmental toxins, DA, and SNCA, while hypothalamic dopaminergic network, brain–gut axis, and circadian rhythm regulatory system can execute as neuroendocrine nodes to link the neural and endocrine systems and expand the PD symptomatic spectrum as well (Figs. 1, 5). Moreover, DA and melatonin can serve as the connector to incorporate the neuroinflammatory and neuroendocrine processes in PD. Therefore, NIE modulatory network, deriving from the confluence of neural, immune, and endocrine systems, sufficiently recapitulates the motor and non-motor symptom complexes of PD (Fig. 5).
DA and melatonin: coupler of the neuroinflammatory and neuroendocrine systems
Corresponding to the multisystemic symptom spectrum of PD, DA functions as an iconic molecule stretching across neural, immune, and endocrine systems. Apart from the conventional motor control, rewarding, and vasoactive effects, DA has also been proven to be involved in the processes of neuroinflammation and neuroendocrine modulation [32, 148, 204, 259].
DA and DRs are expressed in numerous immune cells such as T cells, B cells, neutrophils, eosinophils, and monocytes [260]. The proliferation, differentiation, and function of immune cells can be regulated by DA in an autocrine or paracrine pattern [148, 152, 261]. The DA–DR signaling complex has also been shown to modulate neuroinflammation via DA–DRD1, DA–DRD2, and DA–DRD3 pathways in PD [31, 155–158]. In particular, the pro-inflammatory environment enables active immune cells to adhere to blood vessel and infiltrate into brain, which can subsequently induce neuroinflammation and finally contribute to the neurodegenerative processes [108, 262]. Besides, peripheral inflammation has also been proven to aggravate the NSD degeneration, which may result from the recruitment of activated immune cells into CNS [164–166]. Moreover, epidemiological studies have shown that the incidence of idiopathic PD is about 50% lower in populations with regular use of NSAIDs or COX inhibitors than in age-matched nonusers [161–163]. In light of the neuroinflammation evidence in PD and the immunomodulatory properties of DA, it can be, therefore, speculated that the therapeutic effect of DA may partly result from neuroinflammation modulation other than replenishing the deficient DA in NSD system.
The neuroendocrine modulation of DA mainly embodies at the hypothalamic dopaminergic network and circadian system. Previously, several studies have demonstrated a mild-to-moderate DA reduction in hypothalamus [210–212]. Given the pivotal role of hypothalamus in endocrine system, it can be presumed that the hypothalamic DA deficiency may partly contribute to the autonomic and endocrine abnormalities in PD. Beyond that, though, DA is also implied at several levels of the circadian system: from retina to the pineal gland [235]. For example, the retinal dopaminergic cells can express circadian clock genes to help precisely sense the illumination changes and thus better adjust to photic stimulation [235]. Besides, DA and the dopaminergic agents are also proven to exert circadian modulatory effects as well [240, 243]. Moreover, DA and iron metabolism can underlie the symptomatic circadian fluctuation of restless leg syndrome, a non-negligible non-motor complication of PD [263].
Melatonin (N-acetyl-5-methoxytryptamine) and its metabolites can easily permeate blood brain barrier, which have been previously reported to possess anti-inflammation, anti-oxidant, anti-SNCA aggregation, anti-apoptotic, free-radical scavenging, and sleep-adjusting properties [244–247]. Under normal circumstances, melatonin has no pineal storage and is readily released into blood, so the circulating melatonin concentrations can indirectly mirror the integral functional status of circadian system [264]. The secretion pattern of melatonin in the previous studies also reflected the disease stage of PD and therapeutic response to DA [257, 265]. Besides, the diminished amplitude of serum melatonin secretion revealed in PD patients receiving dopaminergic therapies has also been demonstrated to be correlated with excessive daytime sleeping [248] and sleep structure disorders [266]. Nevertheless, exogenous melatonin administration was also shown to improve sleep disorders [267] and ameliorate motor symptoms [258, 268] in PD patients and models. In particular, a cross-over trial indicated that melatonin (50 mg/d) could significantly improve subjective sleep disturbance, sleep quantity, and daytime sleepiness when compared with daily 5 mg or placebo regimen [269]. Moreover, systemic administrated melatonin could remarkably improve catecholaminergic neurotoxin 6-OHDA-induced hemi-parkinsonian symptoms via a restoration of the mitochondrial complex I dysfunction upon 6-OHDA insult [268].
Apart from the modification of circadian system and Parkinsonian symptoms, melatonin has also been shown to modulate neuroinflammation and immune response [270]. The immunomodulatory effect of melatonin varies at different inflammatory stages: a pro-inflammatory role at early phase and an antagonist role at later phase [271]. At early phase of inflammation, melatonin can facilitate the immune response via activation of pro-inflammatory mediators such as phospholipase A2 (PLA2), 5-lipoxygenase (LOX), IL-1, and TNF-α. In contrast, when it comes to the chronic and later phase, melatonin can suppress the inflammation by virtue of down-regulating the pro-inflammatory mediators, inhibiting ROS production, scavenging free radicals, and inducing pro-survival signaling pathways [271, 272]. In addition, melatonin can prevent the nuclear translocation of NF-κB and its subsequent binding to DNA, thus reducing the production of pro-inflammatory cytokines [273, 274]. Moreover, melatonin can also inhibit the expression of adhesion molecules, reduce the leukocyte–endothelial interaction, and thereby attenuate the transendothelial cell migration and secondary inflammatory responses [275].
Therefore, based on the study findings above, it can be concluded that DA and melatonin can integrate the neuroinflammatory and neuroendocrine systems to participate in the NIE modulatory network, which fully recapitulates the neural, immune, and circadian symptoms of PD (Figs. 1, 5).
Involvement of NIE modulatory network in motor symptom complex of PD
Motor symptoms, externally marked by bradykinesia, muscle rigidity, and static tremor and internally characterized by nigrostriatal dopaminergic degeneration, occupy prominent and salient status in the symptom complex of PD. Accumulating evidences suggest that dysregulated NIE modulatory network may contribute to the compromise of the nigrostriatal dopaminergic system, resulting in typical Parkinsonian motor symptoms. As previously discussed, circadian disruption, in large part mediated by melatonin secretion rhythm alteration, is a common feature in PD, implicating not only in sleep disorders but also in cognitive, neuropsychiatric, and other non-motor symptoms of this disease. In fact, circadian disruption can also contribute to motor deficits and motor skills learning inability by virtue of triggering robust neuroinflammatory reactions [17]. For example, circadian rhythm disruption (20/4 h light and dark cycles for 60 days) can produce more severe neurotoxic effects in MPTP exposed mice models, which finally translates into motor deficit exacerbation, severe dopaminergic cell loss, and intense astrocytes activation [17]. Moreover, the neuroinflammatory reactions revealed in neurodegenerative disease models have been proven to be attenuated by melatonin receptor [276, 277] or specific RAGE/NF-κB/JNK signaling pathway [278]. In addition, given the pivotal role of hypothalamus in neuroendocrine system [279], the hypothalamic volume loss and reduced melatonin output revealed in PD [280] also indicate the implication of neuroendocrine dysfunction in PD.
As is known to all, DA replacement therapy has been the first-class therapeutic alternative for PD by virtue of direct replenishment of nigrostriatal DA deficiency and considerable alleviation of Parkinsonian motor symptoms. However, beyond that, DA receptors and dopaminergic innervation are identified in lymphocytes and lymphoid tissues [148, 152, 153], and several DA–DRs (e.g., DRD1 and DRD2) signaling pathways have also been proven to alleviate neuroinflammation-induced neurotoxicity, dopaminergic neuron loss, and motor deficits upon MPTP insult [31, 155], which collectively suggest the immunomodulatory role of DA. Besides, a meta-analysis research has found that several peripheral inflammatory cytokines are significantly higher in PD patients than that of healthy controls [281], which further strengthens the hypothesis that PD is an inflammatory response-related neurodegenerative disease. Moreover, the incidence of PD is lower in populations regularly taking NSAIDs or COX inhibitors [161–163] also corroborate the hypothesis. In addition, as we all know, hypothalamus occupies the pivotal position of neuroendocrine system, but researches have demonstrated that the overall DA level, presynaptic DA storage, and postsynaptic transmission present considerable reduction in the hypothalamus of PD patients [211, 212]. In addition, numerous previous studies have indicated that neuroinflammatory reaction and neuroendocrine disorders can directly and indirectly aggravate the symptoms of PD, including motor deficits [17, 282, 283]. Therefore, the DA replacement therapy may implicate the neuroinflammatory and neuroendocrine modulatory network in the process of motor symptom alleviation, more than solely replenishing deficient DA.
In conclusion, based on the study finding centering on DA, melatonin, circadian rhythm disruption, and Parkinsonian motor deficits, it can be concluded that neuroinflammatory and neuroendocrine dysregulation, i.e., NIE modulatory network, are involved in the pathophysiological processes of PD motor phenotypes (Fig. 5).
Implication of NIE modulatory network in non-motor symptom complex of PD
Non-motor symptoms of PD, presenting across all the stages of this disease are non-negligible part of the symptom complex and are key determinants of patients’ life quality. As a matter of fact, the occurrence and progression of non-motor symptoms conform to the Braak staging strategy and correlate closely with LB pathology in PD [170]. For example, it has been postulated that the Parkinsonian pathology begins at periphery such as enteric plexus, olfactory bulb, and vermiform appendix [284, 285], then proceeds upward into the midbrain nigrostriatal system, and finally diffusely encroaches the functional cortexes [7, 170]. Correspondingly, rapid eye movement (REM) sleep behavior disorder (RBD), constipation, and hyposmia can precede the onset of motor symptoms with several years, while apathy, psychotic symptoms, and dementia can emerge sequentially with the progression of PD from early to late stages [286]. Here, in this part, we will discuss the implication of neural, immune, and endocrine networks in the non-motor symptom complex of PD.
Dopaminergic neurons loss and dopaminergic pathway dysfunction are typical pathological features identified in PD, and, in turn, DA replacement therapy has been the first-class treatment approach. On the whole, the key dopaminergic area is mainly located at SNpc, ventral tegmental area, and hypothalamus, from which the output fibers project extensively to form four main pathways: nigrostriatal, mesolimbic, mesocortical, and TIDA pathways [5]. In the context of NIE modulatory network, these four pathways serve to mediate the symptom complex of PD, especially non-motor symptoms such as gastrointestinal dysfunction (constipation), sleep disorders, neuropsychosis, etc. (Fig. 5).
Here, we take depression of PD, for example. Depression in PD is a neuropsychosis characterized by specific core symptoms such as pessimism, interest absence, and anhedonia. Currently, it has been proven that the serotoninergic, norepinephrinergic, and dopaminergic pathway dysfunctions in SN, locus coeruleus, and limbic system are implicated [287]. Based on this, the selective serotonin re-uptake inhibitors (SSRIs) are routinely introduced to treat depression in PD. Nevertheless, pramipexole and ropinirole, two dopaminergic receptor agonists administrated to alleviate motor deficits, have also been demonstrated to possess anti-depressant effect in several clinical studies [288–290], and the possible therapeutic effect is attributed to mesolimbic DRD3 agonism [288]. As previously discussed, DA and dopaminergic receptor complex can mediate neuroinflammation modulatory pathway, and disruption of this pathway can partly contribute to the pathogenesis of PD. In addition, several evidences suggest that inflammatory–immune responses are closely correlated with non-motor symptoms of PD such as depression [291, 292]. In addition, SSRIs are also proven to prevent dopaminergic neuron loss via inhibition of neuroinflammation in PD models [293]. Moreover, as the pivot of neuroendocrine and circadian regulatory systems in the RDMP axis, hypothalamus-pituitary–target gland axis and melatonin dysfunctions sufficiently underlie the Parkinsonian non-motor symptoms including depression from a neuroendocrine perspective [226, 294]. Therefore, on the basis of the evidences above, it can be speculated that NIE modulatory network is involved in the pathogenesis of depression of PD (Fig. 5).
Similarly, dopaminergic and neuroimmune dysfunction are also relevant to PD-related sleep disorders such as RBD. Studies in human have revealed that the degeneration of the direct and indirect sublaterodorsal projections to the spinal interneurons has been associated with the pathophysiologic processes of RBD [295]. SN is also involved in the REM and non-REM sleep circuits [5], and pramipexole is proven to decrease the muscle atonia time of RBD [296]. Besides, abnormal iron metabolic and neuroinflammatory biomarkers identified in cerebrospinal fluid and serum can predict the occurrence of RBD as well [297]. Moreover, hypothalamus is closely correlated with hypocretin effect in the RDMP axis, while the latter modulates RBD via activation of locus coeruleus neurons [298]. Therefore, as one previous study proposed, dysfunctional neural, immune, and endocrine network can execute as precipitating factor to induce the occurrence of non-motor symptoms in PD [7]. In fact, apart from depression and RBD, GI dysfunction, cognitive impairments, and other non-motor symptoms also implicate NIE modulatory network in its pathophysiologic processes, respectively [7, 226].
Based on the evidences listed above, it can be concluded that the non-motor symptoms, spanning from prodromal to late-stage PD, are implicated in neural, immune, and endocrine (NIE) modulatory networks (Fig. 5), sharing specific common pathophysiologic features. Hence, in the near future, the treatment of non-motor symptoms of PD should convert to multi-dimensional strategy, more than solely anti-parkinsonism or symptomatic therapy.
Conclusions
Parkinson’s disease (PD) has long been described as a clinical syndrome with a broad array of motor and non-motor symptom spectrum, implicitly marked by progressive substantia nigra dopaminergic neuron degeneration and explicitly characterized by bradykinesia, static tremor, muscle rigidity, hyposmia, constipation, neuropsychosis, sleep disorders, etc. Apart from the nigrostriatal dopamine depletion, neuroimmune and neuroendocrine dysfunctions are also frequently reported, which have helped to broaden the symptom spectrum. Therefore, here, in this review, we have briefly overviewed the neuroendocrine and neuroinflammatory study findings in relation to PD, thereby proposing that NIE network is involved in the pathogenesis and progression of PD.
As a matter of fact, the proposed NIE network has transcended conventional view of nigrostriatal DA deficiency and further incorporated the neuroendocrine and neuroinflammation perspectives into the pathophysiological process of PD (Figs. 1, 5). Besides, DA and melatonin are presumed to execute as connectors of the NIE modulatory network, and the DA–melatonin imbalance theory in the RDMP axis also provides an alternative explanation for the wearing-off phenomenon in the process of DA replacement therapy. Moreover, the preliminary researches targeting neuroinflammation and neuroendocrine dysfunction lead to considerable therapeutic effects, though further studies in humans are mandatory. Therefore, the future anti-Parkinsonism therapy should transform from simple DA replacement, dopaminergic activation, and symptomatic therapy to multi-dimensional therapeutic strategies, allowing for neural, immune, and endocrine participation in PD. Given the evidences presented above, it can be predicted that NIE network holds great promise to deepen our understanding of PD and further facilitate the multi-dimensional management and therapy of PD in future clinical practice.
Acknowledgements
This work was supported by Grants 31171211, 81471305, and 81671260 from the National Natural Science Foundation of China (to TW), Grant 81200983 from the National Natural Science Foundation of China (to NX), Grant 81301082 from the National Natural Science Foundation of China (to JSH), Grant 2012B09 from China Medical Foundation (to NX), and Grant 0203201343 from Hubei Molecular Imaging Key Laboratory (to NX). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Abbreviations
- CNS
Central nervous system
- DA
Dopamine
- DAT
Dopamine transporter
- DAMP
Danger-associated molecular pattern
- DR
Dopaminergic receptor
- GI
Gastrointestinal
- GR
Glucocorticoid receptor
- HPA
Hypothalamic–pituitary–adrenal
- LB
Lewy body
- LN
Lewy neurite
- LPS
Lipopolysaccharide
- LRRK2
Leucine-rich repeat kinase 2
- MHC
Major histocompatibility complex
- MPTP
1-Methyl-4-phenyl-1,2,3,6-tetrahydropyrindine
- NIE
Neuroimmunoendocrine
- NR
Nuclear receptor
- NLRP3
Nucleotide binding oligomerization domain, leucine-rich repeat, and pyrin domain containing protein 3
- NF-κB
Nuclear factor-kappa B
- NSD
Nigrostriatal dopaminergic
- PD
Parkinson’s disease
- PRR
Pattern recognition receptor
- PAMP
Pathogen-associated molecular pattern
- RBD
Rapid eye movement sleep behavior disorder
- RDMP
Retino-diencephalic/mesencephalic pineal
- REM
Rapid eye movement sleep
- ROS
Reactive oxygen species
- SCN
Suprachiasmatic nucleus
- SNCA
α-Synuclein
- SN
Substantia nigra
- SNpc
Substantia nigra pars compacta
- SSRIs
Selective serotonin re-uptake inhibitors
- TLR
Toll-like receptor
- TIDA
Tubero-infundibular dopaminergic axis
Compliance with ethical standards
Conflict of interest
The authors declare that there is no conflict of commercial or financial interests regarding the publication of this paper. Our research team has never received any payment or services from a third party for any aspects of the submitted work. We collectively declare that we do not have any financial relationship with any entities that can potentially influence the conception, creation, and submission of the manuscript. We confirm that there are not any patents and copyrights disputes that are relevant to this manuscript.
References
- 1.Samii A, Nutt JG, Ransom BR. Parkinson’s disease. Lancet. 2004;363(9423):1783–1793. doi: 10.1016/S0140-6736(04)16305-8. [DOI] [PubMed] [Google Scholar]
- 2.Witjas T, Kaphan E, Azulay JP, Blin O, Ceccaldi M, Pouget J, Poncet M, Cherif AA. Nonmotor fluctuations in Parkinson’s disease: frequent and disabling. Neurology. 2002;59(3):408–413. doi: 10.1212/WNL.59.3.408. [DOI] [PubMed] [Google Scholar]
- 3.Chaudhuri KR, Healy DG, Schapira AH, National Institute for Clinical E Non-motor symptoms of Parkinson’s disease: diagnosis and management. Lancet Neurol. 2006;5(3):235–245. doi: 10.1016/S1474-4422(06)70373-8. [DOI] [PubMed] [Google Scholar]
- 4.Poewe W. Non-motor symptoms in Parkinson’s disease. Eur J Neurol. 2008;15(Suppl 1):14–20. doi: 10.1111/j.1468-1331.2008.02056.x. [DOI] [PubMed] [Google Scholar]
- 5.Chaudhuri KR, Schapira AH. Non-motor symptoms of Parkinson’s disease: dopaminergic pathophysiology and treatment. Lancet Neurol. 2009;8(5):464–474. doi: 10.1016/S1474-4422(09)70068-7. [DOI] [PubMed] [Google Scholar]
- 6.Chen W, Xu ZM, Wang G, Chen SD. Non-motor symptoms of Parkinson’s disease in China: a review of the literature. Parkinsonism Relat Disord. 2012;18(5):446–452. doi: 10.1016/j.parkreldis.2012.02.002. [DOI] [PubMed] [Google Scholar]
- 7.Barnum CJ, Tansey MG. Neuroinflammation and non-motor symptoms: the dark passenger of Parkinson’s disease? Curr Neurol Neurosci Rep. 2012;12(4):350–358. doi: 10.1007/s11910-012-0283-6. [DOI] [PubMed] [Google Scholar]
- 8.Rana AQ, Ahmed US, Chaudry ZM, Vasan S. Parkinson’s disease: a review of non-motor symptoms. Expert Rev Neurother. 2015;15(5):549–562. doi: 10.1586/14737175.2015.1038244. [DOI] [PubMed] [Google Scholar]
- 9.Ou R, Yang J, Cao B, Wei Q, Chen K, Chen X, Zhao B, Wu Y, Song W, Shang H. Progression of non-motor symptoms in Parkinson’s disease among different age populations: a two-year follow-up study. J Neurol Sci. 2016;360:72–77. doi: 10.1016/j.jns.2015.11.047. [DOI] [PubMed] [Google Scholar]
- 10.Fahn S. Description of Parkinson’s disease as a clinical syndrome. Ann N Y Acad Sci. 2003;991:1–14. doi: 10.1111/j.1749-6632.2003.tb07458.x. [DOI] [PubMed] [Google Scholar]
- 11.Hirsch EC, Hunot S. Neuroinflammation in Parkinson’s disease: a target for neuroprotection? Lancet Neurol. 2009;8(4):382–397. doi: 10.1016/S1474-4422(09)70062-6. [DOI] [PubMed] [Google Scholar]
- 12.Willis GL. Parkinson’s disease as a neuroendocrine disorder of circadian function: dopamine-melatonin imbalance and the visual system in the genesis and progression of the degenerative process. Rev Neurosci. 2008;19(4–5):245–316. doi: 10.1515/revneuro.2008.19.4-5.245. [DOI] [PubMed] [Google Scholar]
- 13.Cali T, Ottolini D, Brini M. Mitochondria, calcium, and endoplasmic reticulum stress in Parkinson’s disease. BioFactors. 2011;37(3):228–240. doi: 10.1002/biof.159. [DOI] [PubMed] [Google Scholar]
- 14.Haddad D, Nakamura K. Understanding the susceptibility of dopamine neurons to mitochondrial stressors in Parkinson’s disease. FEBS letters. 2015;589(24 Pt A):3702–3713. doi: 10.1016/j.febslet.2015.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cook C, Stetler C, Petrucelli L. Disruption of protein quality control in Parkinson’s disease. Cold Spring Harbor Perspect Med. 2012;2(5):a009423. doi: 10.1101/cshperspect.a009423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lindholm D, Makela J, Di Liberto V, Mudo G, Belluardo N, Eriksson O, Saarma M. Current disease modifying approaches to treat Parkinson’s disease. Cell Mol Life Sci CMLS. 2016;73(7):1365–1379. doi: 10.1007/s00018-015-2101-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lauretti E, Di Meco A, Merali S, Pratico D. Circadian rhythm dysfunction: a novel environmental risk factor for Parkinson’s disease. Mol Psychiatry. 2016 doi: 10.1038/mp.2016.47. [DOI] [PubMed] [Google Scholar]
- 18.Pinto M, Nissanka N, Peralta S, Brambilla R, Diaz F, Moraes CT. Pioglitazone ameliorates the phenotype of a novel Parkinson’s disease mouse model by reducing neuroinflammation. Mol Neurodegener. 2016;11:25. doi: 10.1186/s13024-016-0090-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Whitton PS. Inflammation as a causative factor in the aetiology of Parkinson’s disease. Br J Pharmacol. 2007;150(8):963–976. doi: 10.1038/sj.bjp.0707167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Block ML, Hong JS. Microglia and inflammation-mediated neurodegeneration: multiple triggers with a common mechanism. Prog Neurobiol. 2005;76(2):77–98. doi: 10.1016/j.pneurobio.2005.06.004. [DOI] [PubMed] [Google Scholar]
- 21.Pfeiffer RF. Neuroinflammation and Parkinson disease: the silent battleground. Neurology. 2009;73(18):1434–1435. doi: 10.1212/WNL.0b013e3181c2f07d. [DOI] [PubMed] [Google Scholar]
- 22.Ouchi Y, Yagi S, Yokokura M, Sakamoto M. Neuroinflammation in the living brain of Parkinson’s disease. Parkinsonism Relat Disord. 2009;15(Suppl 3):S200–S204. doi: 10.1016/S1353-8020(09)70814-4. [DOI] [PubMed] [Google Scholar]
- 23.Tansey MG, Goldberg MS. Neuroinflammation in Parkinson’s disease: its role in neuronal death and implications for therapeutic intervention. Neurobiol Dis. 2010;37(3):510–518. doi: 10.1016/j.nbd.2009.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Joers V, Tansey MG, Mulas G, Carta AR. Microglial phenotypes in Parkinson’s disease and animal models of the disease. Prog Neurobiol. 2016 doi: 10.1016/j.pneurobio.2016.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rama Rao KV, Kielian T. Neuron-astrocyte interactions in neurodegenerative diseases: role of neuroinflammation. Clin Exp Neuroimmunol. 2015;6(3):245–263. doi: 10.1111/cen3.12237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hirsch EC, Jenner P, Przedborski S. Pathogenesis of Parkinson’s disease. Mov Disord. 2013;28(1):24–30. doi: 10.1002/mds.25032. [DOI] [PubMed] [Google Scholar]
- 27.Videnovic A, Lazar AS, Barker RA, Overeem S. ‘The clocks that time us’—circadian rhythms in neurodegenerative disorders. Nat Rev Neurol. 2014;10(12):683–693. doi: 10.1038/nrneurol.2014.206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Herrero MT, Estrada C, Maatouk L, Vyas S. Inflammation in Parkinson’s disease: role of glucocorticoids. Front Neuroanat. 2015;9:32. doi: 10.3389/fnana.2015.00032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fragala MS, Kraemer WJ, Denegar CR, Maresh CM, Mastro AM, Volek JS. Neuroendocrine-immune interactions and responses to exercise. Sports Med. 2011;41(8):621–639. doi: 10.2165/11590430-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 30.Wyss-Coray T, Mucke L. Inflammation in neurodegenerative disease—a double-edged sword. Neuron. 2002;35(3):419–432. doi: 10.1016/S0896-6273(02)00794-8. [DOI] [PubMed] [Google Scholar]
- 31.Yan Y, Jiang W, Liu L, Wang X, Ding C, Tian Z, Zhou R. Dopamine controls systemic inflammation through inhibition of NLRP3 inflammasome. Cell. 2015;160(1–2):62–73. doi: 10.1016/j.cell.2014.11.047. [DOI] [PubMed] [Google Scholar]
- 32.Beck G, Brinkkoetter P, Hanusch C, Schulte J, van Ackern K, van der Woude FJ, Yard BA. Clinical review: immunomodulatory effects of dopamine in general inflammation. Crit Care. 2004;8(6):485–491. doi: 10.1186/cc2879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Su AI, Wiltshire T, Batalov S, Lapp H, Ching KA, Block D, Zhang J, Soden R, Hayakawa M, Kreiman G, Cooke MP, Walker JR, Hogenesch JB. A gene atlas of the mouse and human protein-encoding transcriptomes. Proc Natl Acad Sci USA. 2004;101(16):6062–6067. doi: 10.1073/pnas.0400782101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Austin SA, Floden AM, Murphy EJ, Combs CK. Alpha-synuclein expression modulates microglial activation phenotype. J Neurosci. 2006;26(41):10558–10563. doi: 10.1523/JNEUROSCI.1799-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wu C, Orozco C, Boyer J, Leglise M, Goodale J, Batalov S, Hodge CL, Haase J, Janes J, Huss JW, 3rd, Su AI. BioGPS: an extensible and customizable portal for querying and organizing gene annotation resources. Genome Biol. 2009;10(11):R130. doi: 10.1186/gb-2009-10-11-r130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Appel SH. Inflammation in Parkinson’s disease: cause or consequence? Mov Disord. 2012;27(9):1075–1077. doi: 10.1002/mds.25111. [DOI] [PubMed] [Google Scholar]
- 37.Klein C, Westenberger A. Genetics of Parkinson’s disease. Cold Spring Harbor Perspect Med. 2012;2(1):a008888. doi: 10.1101/cshperspect.a008888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mullin S, Schapira A. The genetics of Parkinson’s disease. Br Med Bull. 2015;114(1):39–52. doi: 10.1093/bmb/ldv022. [DOI] [PubMed] [Google Scholar]
- 39.Kumaran R, Cookson MR. Pathways to Parkinsonism Redux: convergent pathobiological mechanisms in genetics of Parkinson’s disease. Hum Mol Genet. 2015;24(R1):R32–R44. doi: 10.1093/hmg/ddv236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dzamko N, Geczy CL, Halliday GM. Inflammation is genetically implicated in Parkinson’s disease. Neuroscience. 2015;302:89–102. doi: 10.1016/j.neuroscience.2014.10.028. [DOI] [PubMed] [Google Scholar]
- 41.Nolan YM, Sullivan AM, Toulouse A. Parkinson’s disease in the nuclear age of neuroinflammation. Trends Mol Med. 2013;19(3):187–196. doi: 10.1016/j.molmed.2012.12.003. [DOI] [PubMed] [Google Scholar]
- 42.Polymeropoulos MH, Lavedan C, Leroy E, Ide SE, Dehejia A, Dutra A, Pike B, Root H, Rubenstein J, Boyer R, Stenroos ES, Chandrasekharappa S, Athanassiadou A, Papapetropoulos T, Johnson WG, Lazzarini AM, Duvoisin RC, Di Iorio G, Golbe LI, Nussbaum RL. Mutation in the alpha-synuclein gene identified in families with Parkinson’s disease. Science. 1997;276(5321):2045–2047. doi: 10.1126/science.276.5321.2045. [DOI] [PubMed] [Google Scholar]
- 43.Chartier-Harlin MC, Kachergus J, Roumier C, Mouroux V, Douay X, Lincoln S, Levecque C, Larvor L, Andrieux J, Hulihan M, Waucquier N, Defebvre L, Amouyel P, Farrer M, Destee A. Alpha-synuclein locus duplication as a cause of familial Parkinson’s disease. Lancet. 2004;364(9440):1167–1169. doi: 10.1016/S0140-6736(04)17103-1. [DOI] [PubMed] [Google Scholar]
- 44.Singleton AB, Farrer M, Johnson J, Singleton A, Hague S, Kachergus J, Hulihan M, Peuralinna T, Dutra A, Nussbaum R, Lincoln S, Crawley A, Hanson M, Maraganore D, Adler C, Cookson MR, Muenter M, Baptista M, Miller D, Blancato J, Hardy J, Gwinn-Hardy K. alpha-Synuclein locus triplication causes Parkinson’s disease. Science. 2003;302(5646):841. doi: 10.1126/science.1090278. [DOI] [PubMed] [Google Scholar]
- 45.Kruger R, Kuhn W, Muller T, Woitalla D, Graeber M, Kosel S, Przuntek H, Epplen JT, Schols L, Riess O. Ala30Pro mutation in the gene encoding alpha-synuclein in Parkinson’s disease. Nat Genet. 1998;18(2):106–108. doi: 10.1038/ng0298-106. [DOI] [PubMed] [Google Scholar]
- 46.Zarranz JJ, Alegre J, Gomez-Esteban JC, Lezcano E, Ros R, Ampuero I, Vidal L, Hoenicka J, Rodriguez O, Atares B, Llorens V, Gomez Tortosa E, del Ser T, Munoz DG, de Yebenes JG. The new mutation, E46K, of alpha-synuclein causes Parkinson and Lewy body dementia. Ann Neurol. 2004;55(2):164–173. doi: 10.1002/ana.10795. [DOI] [PubMed] [Google Scholar]
- 47.Lesage S, Anheim M, Letournel F, Bousset L, Honore A, Rozas N, Pieri L, Madiona K, Durr A, Melki R, Verny C, Brice A, French Parkinson’s Disease Genetics Study G G51D alpha-synuclein mutation causes a novel parkinsonian-pyramidal syndrome. Ann Neurol. 2013;73(4):459–471. doi: 10.1002/ana.23894. [DOI] [PubMed] [Google Scholar]
- 48.Proukakis C, Dudzik CG, Brier T, MacKay DS, Cooper JM, Millhauser GL, Houlden H, Schapira AH. A novel alpha-synuclein missense mutation in Parkinson disease. Neurology. 2013;80(11):1062–1064. doi: 10.1212/WNL.0b013e31828727ba. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ross OA, Braithwaite AT, Skipper LM, Kachergus J, Hulihan MM, Middleton FA, Nishioka K, Fuchs J, Gasser T, Maraganore DM, Adler CH, Larvor L, Chartier-Harlin MC, Nilsson C, Langston JW, Gwinn K, Hattori N, Farrer MJ. Genomic investigation of alpha-synuclein multiplication and parkinsonism. Ann Neurol. 2008;63(6):743–750. doi: 10.1002/ana.21380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kim C, Ho DH, Suk JE, You S, Michael S, Kang J, Joong Lee S, Masliah E, Hwang D, Lee HJ, Lee SJ. Neuron-released oligomeric alpha-synuclein is an endogenous agonist of TLR2 for paracrine activation of microglia. Nat Commun. 2013;4:1562. doi: 10.1038/ncomms2534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Fellner L, Irschick R, Schanda K, Reindl M, Klimaschewski L, Poewe W, Wenning GK, Stefanova N. Toll-like receptor 4 is required for alpha-synuclein dependent activation of microglia and astroglia. Glia. 2013;61(3):349–360. doi: 10.1002/glia.22437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Austin SA, Rojanathammanee L, Golovko MY, Murphy EJ, Combs CK. Lack of alpha-synuclein modulates microglial phenotype in vitro. Neurochem Res. 2011;36(6):994–1004. doi: 10.1007/s11064-011-0439-9. [DOI] [PubMed] [Google Scholar]
- 53.Zhang D, Lin J, Han J. Receptor-interacting protein (RIP) kinase family. Cell Mol Immunol. 2010;7(4):243–249. doi: 10.1038/cmi.2010.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Russo I, Bubacco L, Greggio E. LRRK2 and neuroinflammation: partners in crime in Parkinson’s disease? J Neuroinflamm. 2014;11:52. doi: 10.1186/1742-2094-11-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zhang FR, Huang W, Chen SM, Sun LD, Liu H, Li Y, Cui Y, Yan XX, Yang HT, Yang RD, Chu TS, Zhang C, Zhang L, Han JW, Yu GQ, Quan C, Yu YX, Zhang Z, Shi BQ, Zhang LH, Cheng H, Wang CY, Lin Y, Zheng HF, Fu XA, Zuo XB, Wang Q, Long H, Sun YP, Cheng YL, Tian HQ, Zhou FS, Liu HX, Lu WS, He SM, Du WL, Shen M, Jin QY, Wang Y, Low HQ, Erwin T, Yang NH, Li JY, Zhao X, Jiao YL, Mao LG, Yin G, Jiang ZX, Wang XD, Yu JP, Hu ZH, Gong CH, Liu YQ, Liu RY, Wang DM, Wei D, Liu JX, Cao WK, Cao HZ, Li YP, Yan WG, Wei SY, Wang KJ, Hibberd ML, Yang S, Zhang XJ, Liu JJ. Genomewide association study of leprosy. N Engl J Med. 2009;361(27):2609–2618. doi: 10.1056/NEJMoa0903753. [DOI] [PubMed] [Google Scholar]
- 56.Barrett JC, Hansoul S, Nicolae DL, Cho JH, Duerr RH, Rioux JD, Brant SR, Silverberg MS, Taylor KD, Barmada MM, Bitton A, Dassopoulos T, Datta LW, Green T, Griffiths AM, Kistner EO, Murtha MT, Regueiro MD, Rotter JI, Schumm LP, Steinhart AH, Targan SR, Xavier RJ, Consortium NIG. Libioulle C, Sandor C, Lathrop M, Belaiche J, Dewit O, Gut I, Heath S, Laukens D, Mni M, Rutgeerts P, Van Gossum A, Zelenika D, Franchimont D, Hugot JP, de Vos M, Vermeire S, Louis E, Belgian-French IBDC. Wellcome Trust Case Control C. Cardon LR, Anderson CA, Drummond H, Nimmo E, Ahmad T, Prescott NJ, Onnie CM, Fisher SA, Marchini J, Ghori J, Bumpstead S, Gwilliam R, Tremelling M, Deloukas P, Mansfield J, Jewell D, Satsangi J, Mathew CG, Parkes M, Georges M, Daly MJ. Genome-wide association defines more than 30 distinct susceptibility loci for Crohn’s disease. Nat Genet. 2008;40(8):955–962. doi: 10.1038/ng.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Umeno J, Asano K, Matsushita T, Matsumoto T, Kiyohara Y, Iida M, Nakamura Y, Kamatani N, Kubo M. Meta-analysis of published studies identified eight additional common susceptibility loci for Crohn’s disease and ulcerative colitis. Inflamm Bowel Dis. 2011;17(12):2407–2415. doi: 10.1002/ibd.21651. [DOI] [PubMed] [Google Scholar]
- 58.Bonifati V. Autosomal recessive parkinsonism. Parkinsonism Relat Disord. 2012;18(Suppl 1):S4–S6. doi: 10.1016/S1353-8020(11)70004-9. [DOI] [PubMed] [Google Scholar]
- 59.Goldberg MS, Fleming SM, Palacino JJ, Cepeda C, Lam HA, Bhatnagar A, Meloni EG, Wu N, Ackerson LC, Klapstein GJ, Gajendiran M, Roth BL, Chesselet MF, Maidment NT, Levine MS, Shen J. Parkin-deficient mice exhibit nigrostriatal deficits but not loss of dopaminergic neurons. J Biol Chem. 2003;278(44):43628–43635. doi: 10.1074/jbc.M308947200. [DOI] [PubMed] [Google Scholar]
- 60.Frank-Cannon TC, Tran T, Ruhn KA, Martinez TN, Hong J, Marvin M, Hartley M, Trevino I, O’Brien DE, Casey B, Goldberg MS, Tansey MG. Parkin deficiency increases vulnerability to inflammation-related nigral degeneration. J Neurosci. 2008;28(43):10825–10834. doi: 10.1523/JNEUROSCI.3001-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Matheoud D, Sugiura A, Bellemare-Pelletier A, Laplante A, Rondeau C, Chemali M, Fazel A, Bergeron JJ, Trudeau LE, Burelle Y, Gagnon E, McBride HM, Desjardins M. Parkinson’s disease-related proteins PINK1 and Parkin repress mitochondrial antigen presentation. Cell. 2016;166(2):314–327. doi: 10.1016/j.cell.2016.05.039. [DOI] [PubMed] [Google Scholar]
- 62.Cebrian C, Zucca FA, Mauri P, Steinbeck JA, Studer L, Scherzer CR, Kanter E, Budhu S, Mandelbaum J, Vonsattel JP, Zecca L, Loike JD, Sulzer D. MHC-I expression renders catecholaminergic neurons susceptible to T-cell-mediated degeneration. Nat Commun. 2014;5:3633. doi: 10.1038/ncomms4633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Alves da Costa C, Checler F. Parkin: much more than a simple ubiquitin ligase. Neuro-degener Dis. 2012;10(1–4):49–51. doi: 10.1159/000332803. [DOI] [PubMed] [Google Scholar]
- 64.Sunico CR, Nakamura T, Rockenstein E, Mante M, Adame A, Chan SF, Newmeyer TF, Masliah E, Nakanishi N, Lipton SA. S-Nitrosylation of parkin as a novel regulator of p53-mediated neuronal cell death in sporadic Parkinson’s disease. Mol Neurodegener. 2013;8:29. doi: 10.1186/1750-1326-8-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Li HM, Niki T, Taira T, Iguchi-Ariga SM, Ariga H. Association of DJ-1 with chaperones and enhanced association and colocalization with mitochondrial Hsp70 by oxidative stress. Free Radic Res. 2005;39(10):1091–1099. doi: 10.1080/10715760500260348. [DOI] [PubMed] [Google Scholar]
- 66.Goldberg MS, Pisani A, Haburcak M, Vortherms TA, Kitada T, Costa C, Tong Y, Martella G, Tscherter A, Martins A, Bernardi G, Roth BL, Pothos EN, Calabresi P, Shen J. Nigrostriatal dopaminergic deficits and hypokinesia caused by inactivation of the familial Parkinsonism-linked gene DJ-1. Neuron. 2005;45(4):489–496. doi: 10.1016/j.neuron.2005.01.041. [DOI] [PubMed] [Google Scholar]
- 67.Canet-Aviles RM, Wilson MA, Miller DW, Ahmad R, McLendon C, Bandyopadhyay S, Baptista MJ, Ringe D, Petsko GA, Cookson MR. The Parkinson’s disease protein DJ-1 is neuroprotective due to cysteine-sulfinic acid-driven mitochondrial localization. Proc Natl Acad Sci USA. 2004;101(24):9103–9108. doi: 10.1073/pnas.0402959101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.van der Brug MP, Blackinton J, Chandran J, Hao LY, Lal A, Mazan-Mamczarz K, Martindale J, Xie C, Ahmad R, Thomas KJ, Beilina A, Gibbs JR, Ding J, Myers AJ, Zhan M, Cai H, Bonini NM, Gorospe M, Cookson MR. RNA binding activity of the recessive parkinsonism protein DJ-1 supports involvement in multiple cellular pathways. Proc Natl Acad Sci USA. 2008;105(29):10244–10249. doi: 10.1073/pnas.0708518105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kim RH, Smith PD, Aleyasin H, Hayley S, Mount MP, Pownall S, Wakeham A, You-Ten AJ, Kalia SK, Horne P, Westaway D, Lozano AM, Anisman H, Park DS, Mak TW. Hypersensitivity of DJ-1-deficient mice to 1-methyl-4-phenyl-1,2,3,6-tetrahydropyrindine (MPTP) and oxidative stress. Proc Natl Acad Sci USA. 2005;102(14):5215–5220. doi: 10.1073/pnas.0501282102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Yang W, Chen L, Ding Y, Zhuang X, Kang UJ. Paraquat induces dopaminergic dysfunction and proteasome impairment in DJ-1-deficient mice. Hum Mol Genet. 2007;16(23):2900–2910. doi: 10.1093/hmg/ddm249. [DOI] [PubMed] [Google Scholar]
- 71.Martinat C, Shendelman S, Jonason A, Leete T, Beal MF, Yang L, Floss T, Abeliovich A. Sensitivity to oxidative stress in DJ-1-deficient dopamine neurons: an ES- derived cell model of primary Parkinsonism. PLoS Biol. 2004;2(11):e327. doi: 10.1371/journal.pbio.0020327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Waak J, Weber SS, Waldenmaier A, Gorner K, Alunni-Fabbroni M, Schell H, Vogt-Weisenhorn D, Pham TT, Reumers V, Baekelandt V, Wurst W, Kahle PJ. Regulation of astrocyte inflammatory responses by the Parkinson’s disease-associated gene DJ-1. FASEB J. 2009;23(8):2478–2489. doi: 10.1096/fj.08-125153. [DOI] [PubMed] [Google Scholar]
- 73.Ramirez A, Heimbach A, Grundemann J, Stiller B, Hampshire D, Cid LP, Goebel I, Mubaidin AF, Wriekat AL, Roeper J, Al-Din A, Hillmer AM, Karsak M, Liss B, Woods CG, Behrens MI, Kubisch C. Hereditary parkinsonism with dementia is caused by mutations in ATP13A2, encoding a lysosomal type 5 P-type ATPase. Nat Genet. 2006;38(10):1184–1191. doi: 10.1038/ng1884. [DOI] [PubMed] [Google Scholar]
- 74.Usenovic M, Tresse E, Mazzulli JR, Taylor JP, Krainc D. Deficiency of ATP13A2 leads to lysosomal dysfunction, alpha-synuclein accumulation, and neurotoxicity. J Neurosci. 2012;32(12):4240–4246. doi: 10.1523/JNEUROSCI.5575-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Deleidi M, Gasser T. The role of inflammation in sporadic and familial Parkinson’s disease. Cell Mol Life Sci CMLS. 2013;70(22):4259–4273. doi: 10.1007/s00018-013-1352-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Wang Q, Liu Y, Zhou J. Neuroinflammation in Parkinson’s disease and its potential as therapeutic target. Transl Neurodegener. 2015;4:19. doi: 10.1186/s40035-015-0042-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Lamkanfi M, Dixit VM. Inflammasomes and their roles in health and disease. Annu Rev Cell Dev Biol. 2012;28:137–161. doi: 10.1146/annurev-cellbio-101011-155745. [DOI] [PubMed] [Google Scholar]
- 78.Qiao C, Yin N, Gu HY, Zhu JL, Ding JH, Lu M, Hu G. Atp13a2 Deficiency Aggravates Astrocyte-Mediated Neuroinflammation via NLRP3 Inflammasome Activation. CNS Neurosci Ther. 2016;22(6):451–460. doi: 10.1111/cns.12514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Dong J, Li S, Mo JL, Cai HB, Le WD. Nurr1-based therapies for Parkinson’s Disease. CNS Neurosci Ther. 2016;22(5):351–359. doi: 10.1111/cns.12536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Fan X, Luo G, Ming M, Pu P, Li L, Yang D, Le W. Nurr1 expression and its modulation in microglia. NeuroImmunoModulation. 2009;16(3):162–170. doi: 10.1159/000204229. [DOI] [PubMed] [Google Scholar]
- 81.Saijo K, Winner B, Carson CT, Collier JG, Boyer L, Rosenfeld MG, Gage FH, Glass CK. A Nurr1/CoREST pathway in microglia and astrocytes protects dopaminergic neurons from inflammation-induced death. Cell. 2009;137(1):47–59. doi: 10.1016/j.cell.2009.01.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.No H, Bang Y, Lim J, Kim SS, Choi HS, Choi HJ. Involvement of induction and mitochondrial targeting of orphan nuclear receptor Nur77 in 6-OHDA-induced SH-SY5Y cell death. Neurochem Int. 2010;56(4):620–626. doi: 10.1016/j.neuint.2010.01.005. [DOI] [PubMed] [Google Scholar]
- 83.Wei X, Gao H, Zou J, Liu X, Chen D, Liao J, Xu Y, Ma L, Tang B, Zhang Z, Cai X, Jin K, Xia Y, Wang Q. Contra-directional coupling of Nur77 and Nurr1 in neurodegeneration: a novel mechanism for memantine-induced anti-inflammation and anti-mitochondrial impairment. Mol Neurobiol. 2016;53(9):5876–5892. doi: 10.1007/s12035-015-9477-7. [DOI] [PubMed] [Google Scholar]
- 84.Blanquart C, Barbier O, Fruchart JC, Staels B, Glineur C. Peroxisome proliferator-activated receptors: regulation of transcriptional activities and roles in inflammation. J Steroid Biochem Mol Biol. 2003;85(2–5):267–273. doi: 10.1016/S0960-0760(03)00214-0. [DOI] [PubMed] [Google Scholar]
- 85.Park MH, Park JY, Lee HJ, Kim DH, Chung KW, Park D, Jeong HO, Kim HR, Park CH, Kim SR, Chun P, Byun Y, Moon HR, Chung HY. The novel PPAR alpha/gamma dual agonist MHY 966 modulates UVB-induced skin inflammation by inhibiting NF-kappaB activity. PLoS One. 2013;8(10):e76820. doi: 10.1371/journal.pone.0076820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Chen YC, Wu JS, Tsai HD, Huang CY, Chen JJ, Sun GY, Lin TN. Peroxisome proliferator-activated receptor gamma (PPAR-gamma) and neurodegenerative disorders. Mol Neurobiol. 2012;46(1):114–124. doi: 10.1007/s12035-012-8259-8. [DOI] [PubMed] [Google Scholar]
- 87.Swanson CR, Joers V, Bondarenko V, Brunner K, Simmons HA, Ziegler TE, Kemnitz JW, Johnson JA, Emborg ME. The PPAR-gamma agonist pioglitazone modulates inflammation and induces neuroprotection in parkinsonian monkeys. J Neuroinflamm. 2011;8:91. doi: 10.1186/1742-2094-8-91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Carta AR. PPAR-gamma: therapeutic prospects in Parkinson’s disease. Curr Drug Targets. 2013;14(7):743–751. doi: 10.2174/1389450111314070004. [DOI] [PubMed] [Google Scholar]
- 89.Pisanu A, Lecca D, Mulas G, Wardas J, Simbula G, Spiga S, Carta AR. Dynamic changes in pro- and anti-inflammatory cytokines in microglia after PPAR-gamma agonist neuroprotective treatment in the MPTPp mouse model of progressive Parkinson’s disease. Neurobiol Dis. 2014;71:280–291. doi: 10.1016/j.nbd.2014.08.011. [DOI] [PubMed] [Google Scholar]
- 90.Iwashita A, Muramatsu Y, Yamazaki T, Muramoto M, Kita Y, Yamazaki S, Mihara K, Moriguchi A, Matsuoka N. Neuroprotective efficacy of the peroxisome proliferator-activated receptor delta-selective agonists in vitro and in vivo. J Pharmacol Exp Ther. 2007;320(3):1087–1096. doi: 10.1124/jpet.106.115758. [DOI] [PubMed] [Google Scholar]
- 91.Kreisler A, Gele P, Wiart JF, Lhermitte M, Destee A, Bordet R. Lipid-lowering drugs in the MPTP mouse model of Parkinson’s disease: fenofibrate has a neuroprotective effect, whereas bezafibrate and HMG-CoA reductase inhibitors do not. Brain Res. 2007;1135(1):77–84. doi: 10.1016/j.brainres.2006.12.011. [DOI] [PubMed] [Google Scholar]
- 92.Barbiero JK, Santiago R, Tonin FS, Boschen S, da Silva LM, Werner MF, da Cunha C, Lima MM, Vital MA. PPAR-alpha agonist fenofibrate protects against the damaging effects of MPTP in a rat model of Parkinson’s disease. Prog Neuropsychopharmacol Biol Psychiatry. 2014;53:35–44. doi: 10.1016/j.pnpbp.2014.02.009. [DOI] [PubMed] [Google Scholar]
- 93.Das NR, Gangwal RP, Damre MV, Sangamwar AT, Sharma SS. A PPAR-beta/delta agonist is neuroprotective and decreases cognitive impairment in a rodent model of Parkinson’s disease. Curr Neurovasc Res. 2014;11(2):114–124. doi: 10.2174/1567202611666140318114037. [DOI] [PubMed] [Google Scholar]
- 94.Morimoto M, Morita N, Ozawa H, Yokoyama K, Kawata M. Distribution of glucocorticoid receptor immunoreactivity and mRNA in the rat brain: an immunohistochemical and in situ hybridization study. Neurosci Res. 1996;26(3):235–269. doi: 10.1016/S0168-0102(96)01105-4. [DOI] [PubMed] [Google Scholar]
- 95.Ros-Bernal F, Hunot S, Herrero MT, Parnadeau S, Corvol JC, Lu L, Alvarez-Fischer D, Carrillo-de Sauvage MA, Saurini F, Coussieu C, Kinugawa K, Prigent A, Hoglinger G, Hamon M, Tronche F, Hirsch EC, Vyas S. Microglial glucocorticoid receptors play a pivotal role in regulating dopaminergic neurodegeneration in parkinsonism. Proc Natl Acad Sci USA. 2011;108(16):6632–6637. doi: 10.1073/pnas.1017820108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Silverman MN, Sternberg EM. Glucocorticoid regulation of inflammation and its functional correlates: from HPA axis to glucocorticoid receptor dysfunction. Ann N Y Acad Sci. 2012;1261:55–63. doi: 10.1111/j.1749-6632.2012.06633.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Lamers F, Vogelzangs N, Merikangas KR, de Jonge P, Beekman AT, Penninx BW. Evidence for a differential role of HPA-axis function, inflammation and metabolic syndrome in melancholic versus atypical depression. Mol Psychiatry. 2013;18(6):692–699. doi: 10.1038/mp.2012.144. [DOI] [PubMed] [Google Scholar]
- 98.Castano A, Herrera AJ, Cano J, Machado A. The degenerative effect of a single intranigral injection of LPS on the dopaminergic system is prevented by dexamethasone, and not mimicked by rh-TNF-alpha, IL-1beta and IFN-gamma. J Neurochem. 2002;81(1):150–157. doi: 10.1046/j.1471-4159.2002.00799.x. [DOI] [PubMed] [Google Scholar]
- 99.Kurkowska-Jastrzebska I, Litwin T, Joniec I, Ciesielska A, Przybylkowski A, Czlonkowski A, Czlonkowska A. Dexamethasone protects against dopaminergic neurons damage in a mouse model of Parkinson’s disease. Int Immunopharmacol. 2004;4(10–11):1307–1318. doi: 10.1016/j.intimp.2004.05.006. [DOI] [PubMed] [Google Scholar]
- 100.Nadeau S, Rivest S. Glucocorticoids play a fundamental role in protecting the brain during innate immune response. J Neurosci. 2003;23(13):5536–5544. doi: 10.1523/JNEUROSCI.23-13-05536.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Sun XC, Ren XF, Chen L, Gao XQ, Xie JX, Chen WF. Glucocorticoid receptor is involved in the neuroprotective effect of ginsenoside Rg1 against inflammation-induced dopaminergic neuronal degeneration in substantia nigra. J Steroid Biochem Mol Biol. 2016;155(Pt A):94–103. doi: 10.1016/j.jsbmb.2015.09.040. [DOI] [PubMed] [Google Scholar]
- 102.Duong V, Rochette-Egly C. The molecular physiology of nuclear retinoic acid receptors. From health to disease. Biochem Biophys Acta. 2011;1812(8):1023–1031. doi: 10.1016/j.bbadis.2010.10.007. [DOI] [PubMed] [Google Scholar]
- 103.Maden M. Retinoic acid in the development, regeneration and maintenance of the nervous system. Nat Rev Neurosci. 2007;8(10):755–765. doi: 10.1038/nrn2212. [DOI] [PubMed] [Google Scholar]
- 104.Dheen ST, Jun Y, Yan Z, Tay SS, Ling EA. Retinoic acid inhibits expression of TNF-alpha and iNOS in activated rat microglia. Glia. 2005;50(1):21–31. doi: 10.1002/glia.20153. [DOI] [PubMed] [Google Scholar]
- 105.Glass CK, Saijo K, Winner B, Marchetto MC, Gage FH. Mechanisms underlying inflammation in neurodegeneration. Cell. 2010;140(6):918–934. doi: 10.1016/j.cell.2010.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Mittelbronn M, Dietz K, Schluesener HJ, Meyermann R. Local distribution of microglia in the normal adult human central nervous system differs by up to one order of magnitude. Acta Neuropathol. 2001;101(3):249–255. doi: 10.1007/s004010000284. [DOI] [PubMed] [Google Scholar]
- 107.Kim WG, Mohney RP, Wilson B, Jeohn GH, Liu B, Hong JS. Regional difference in susceptibility to lipopolysaccharide-induced neurotoxicity in the rat brain: role of microglia. J Neurosci. 2000;20(16):6309–6316. doi: 10.1523/JNEUROSCI.20-16-06309.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Block ML, Zecca L, Hong JS. Microglia-mediated neurotoxicity: uncovering the molecular mechanisms. Nat Rev Neurosci. 2007;8(1):57–69. doi: 10.1038/nrn2038. [DOI] [PubMed] [Google Scholar]
- 109.Zhang W, Wang T, Pei Z, Miller DS, Wu X, Block ML, Wilson B, Zhang W, Zhou Y, Hong JS, Zhang J. Aggregated alpha-synuclein activates microglia: a process leading to disease progression in Parkinson’s disease. FASEB J. 2005;19(6):533–542. doi: 10.1096/fj.04-2751com. [DOI] [PubMed] [Google Scholar]
- 110.Xu L, He D, Bai Y. Microglia-mediated inflammation and neurodegenerative disease. Mol Neurobiol. 2016;53(10):6709–6715. doi: 10.1007/s12035-015-9593-4. [DOI] [PubMed] [Google Scholar]
- 111.Akira S, Uematsu S, Takeuchi O. Pathogen recognition and innate immunity. Cell. 2006;124(4):783–801. doi: 10.1016/j.cell.2006.02.015. [DOI] [PubMed] [Google Scholar]
- 112.Qin L, Wu X, Block ML, Liu Y, Breese GR, Hong JS, Knapp DJ, Crews FT. Systemic LPS causes chronic neuroinflammation and progressive neurodegeneration. Glia. 2007;55(5):453–462. doi: 10.1002/glia.20467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Burguillos MA, Hajji N, Englund E, Persson A, Cenci AM, Machado A, Cano J, Joseph B, Venero JL. Apoptosis-inducing factor mediates dopaminergic cell death in response to LPS-induced inflammatory stimulus: evidence in Parkinson’s disease patients. Neurobiol Dis. 2011;41(1):177–188. doi: 10.1016/j.nbd.2010.09.005. [DOI] [PubMed] [Google Scholar]
- 114.Hoban DB, Connaughton E, Connaughton C, Hogan G, Thornton C, Mulcahy P, Moloney TC, Dowd E. Further characterisation of the LPS model of Parkinson’s disease: a comparison of intra-nigral and intra-striatal lipopolysaccharide administration on motor function, microgliosis and nigrostriatal neurodegeneration in the rat. Brain Behav Immun. 2013;27(1):91–100. doi: 10.1016/j.bbi.2012.10.001. [DOI] [PubMed] [Google Scholar]
- 115.Qin L, Liu Y, Hong JS, Crews FT. NADPH oxidase and aging drive microglial activation, oxidative stress, and dopaminergic neurodegeneration following systemic LPS administration. Glia. 2013;61(6):855–868. doi: 10.1002/glia.22479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Chien CH, Lee MJ, Liou HC, Liou HH, Fu WM. Microglia-derived Cytokines/Chemokines are involved in the enhancement of LPS-induced loss of nigrostriatal dopaminergic neurons in DJ-1 knockout mice. PLoS One. 2016;11(3):e0151569. doi: 10.1371/journal.pone.0151569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Wu XF, Block ML, Zhang W, Qin L, Wilson B, Zhang WQ, Veronesi B, Hong JS. The role of microglia in paraquat-induced dopaminergic neurotoxicity. Antioxid Redox Signal. 2005;7(5–6):654–661. doi: 10.1089/ars.2005.7.654. [DOI] [PubMed] [Google Scholar]
- 118.Mangano EN, Litteljohn D, So R, Nelson E, Peters S, Bethune C, Bobyn J, Hayley S. Interferon-gamma plays a role in paraquat-induced neurodegeneration involving oxidative and proinflammatory pathways. Neurobiol Aging. 2012;33(7):1411–1426. doi: 10.1016/j.neurobiolaging.2011.02.016. [DOI] [PubMed] [Google Scholar]
- 119.Purisai MG, McCormack AL, Cumine S, Li J, Isla MZ, Di Monte DA. Microglial activation as a priming event leading to paraquat-induced dopaminergic cell degeneration. Neurobiol Dis. 2007;25(2):392–400. doi: 10.1016/j.nbd.2006.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Gibbons HM, Dragunow M. Microglia induce neural cell death via a proximity-dependent mechanism involving nitric oxide. Brain Res. 2006;1084(1):1–15. doi: 10.1016/j.brainres.2006.02.032. [DOI] [PubMed] [Google Scholar]
- 121.Le W, Rowe D, Xie W, Ortiz I, He Y, Appel SH. Microglial activation and dopaminergic cell injury: an in vitro model relevant to Parkinson’s disease. J Neurosci. 2001;21(21):8447–8455. doi: 10.1523/JNEUROSCI.21-21-08447.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Li R, Huang YG, Fang D, Le WD. (-)-Epigallocatechin gallate inhibits lipopolysaccharide-induced microglial activation and protects against inflammation-mediated dopaminergic neuronal injury. J Neurosci Res. 2004;78(5):723–731. doi: 10.1002/jnr.20315. [DOI] [PubMed] [Google Scholar]
- 123.Sharma N, Nehru B. Apocyanin, a microglial NADPH oxidase inhibitor prevents dopaminergic neuronal degeneration in lipopolysaccharide-induced Parkinson’s disease model. Mol Neurobiol. 2016;53(5):3326–3337. doi: 10.1007/s12035-015-9267-2. [DOI] [PubMed] [Google Scholar]
- 124.Liu RP, Zou M, Wang JY, Zhu JJ, Lai JM, Zhou LL, Chen SF, Zhang X, Zhu JH. Paroxetine ameliorates lipopolysaccharide-induced microglia activation via differential regulation of MAPK signaling. J Neuroinflamm. 2014;11:47. doi: 10.1186/1742-2094-11-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Radler ME, Hale MW, Kent S. Calorie restriction attenuates lipopolysaccharide (LPS)-induced microglial activation in discrete regions of the hypothalamus and the subfornical organ. Brain Behav Immun. 2014;38:13–24. doi: 10.1016/j.bbi.2013.11.014. [DOI] [PubMed] [Google Scholar]
- 126.Streit WJ, Walter SA, Pennell NA. Reactive microgliosis. Prog Neurobiol. 1999;57(6):563–581. doi: 10.1016/S0301-0082(98)00069-0. [DOI] [PubMed] [Google Scholar]
- 127.Gao HM, Hong JS. Why neurodegenerative diseases are progressive: uncontrolled inflammation drives disease progression. Trends Immunol. 2008;29(8):357–365. doi: 10.1016/j.it.2008.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Nakamura Y. Regulating factors for microglial activation. Biol Pharm Bull. 2002;25(8):945–953. doi: 10.1248/bpb.25.945. [DOI] [PubMed] [Google Scholar]
- 129.Batchelor PE, Liberatore GT, Wong JY, Porritt MJ, Frerichs F, Donnan GA, Howells DW. Activated macrophages and microglia induce dopaminergic sprouting in the injured striatum and express brain-derived neurotrophic factor and glial cell line-derived neurotrophic factor. J Neurosci. 1999;19(5):1708–1716. doi: 10.1523/JNEUROSCI.19-05-01708.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Liu B, Hong JS. Role of microglia in inflammation-mediated neurodegenerative diseases: mechanisms and strategies for therapeutic intervention. J Pharmacol Exp Ther. 2003;304(1):1–7. doi: 10.1124/jpet.102.035048. [DOI] [PubMed] [Google Scholar]
- 131.Levesque S, Wilson B, Gregoria V, Thorpe LB, Dallas S, Polikov VS, Hong JS, Block ML. Reactive microgliosis: extracellular micro-calpain and microglia-mediated dopaminergic neurotoxicity. Brain J Neurol. 2010;133(Pt 3):808–821. doi: 10.1093/brain/awp333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Wang T, Zhang W, Pei Z, Block M, Wilson B, Reece JM, Miller DS, Hong JS. Reactive microgliosis participates in MPP+-induced dopaminergic neurodegeneration: role of 67 kDa laminin receptor. FASEB J. 2006;20(7):906–915. doi: 10.1096/fj.05-5053com. [DOI] [PubMed] [Google Scholar]
- 133.Hu X, Zhang D, Pang H, Caudle WM, Li Y, Gao H, Liu Y, Qian L, Wilson B, Di Monte DA, Ali SF, Zhang J, Block ML, Hong JS. Macrophage antigen complex-1 mediates reactive microgliosis and progressive dopaminergic neurodegeneration in the MPTP model of Parkinson’s disease. J Immunol. 2008;181(10):7194–7204. doi: 10.4049/jimmunol.181.10.7194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Chen SH, Oyarzabal EA, Hong JS. Critical role of the Mac1/NOX2 pathway in mediating reactive microgliosis-generated chronic neuroinflammation and progressive neurodegeneration. Curr Opin Pharmacol. 2016;26:54–60. doi: 10.1016/j.coph.2015.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Dauer W, Przedborski S. Parkinson’s disease: mechanisms and models. Neuron. 2003;39(6):889–909. doi: 10.1016/S0896-6273(03)00568-3. [DOI] [PubMed] [Google Scholar]
- 136.Langston JW, Ballard P, Tetrud JW, Irwin I. Chronic Parkinsonism in humans due to a product of meperidine-analog synthesis. Science. 1983;219(4587):979–980. doi: 10.1126/science.6823561. [DOI] [PubMed] [Google Scholar]
- 137.Jackson-Lewis V, Jakowec M, Burke RE, Przedborski S. Time course and morphology of dopaminergic neuronal death caused by the neurotoxin 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine. Neurodegener J Neurodegener Disord Neuroprot Neuroregener. 1995;4(3):257–269. doi: 10.1016/1055-8330(95)90015-2. [DOI] [PubMed] [Google Scholar]
- 138.Langston JW, Forno LS, Tetrud J, Reeves AG, Kaplan JA, Karluk D. Evidence of active nerve cell degeneration in the substantia nigra of humans years after 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine exposure. Ann Neurol. 1999;46(4):598–605. doi: 10.1002/1531-8249(199910)46:4<598::AID-ANA7>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- 139.McGeer PL, Schwab C, Parent A, Doudet D. Presence of reactive microglia in monkey substantia nigra years after 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine administration. Ann Neurol. 2003;54(5):599–604. doi: 10.1002/ana.10728. [DOI] [PubMed] [Google Scholar]
- 140.Du Y, Ma Z, Lin S, Dodel RC, Gao F, Bales KR, Triarhou LC, Chernet E, Perry KW, Nelson DL, Luecke S, Phebus LA, Bymaster FP, Paul SM. Minocycline prevents nigrostriatal dopaminergic neurodegeneration in the MPTP model of Parkinson’s disease. Proc Natl Acad Sci USA. 2001;98(25):14669–14674. doi: 10.1073/pnas.251341998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Wu DC, Jackson-Lewis V, Vila M, Tieu K, Teismann P, Vadseth C, Choi DK, Ischiropoulos H, Przedborski S. Blockade of microglial activation is neuroprotective in the 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine mouse model of Parkinson disease. J Neurosci. 2002;22(5):1763–1771. doi: 10.1523/JNEUROSCI.22-05-01763.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Peng J, Xie L, Stevenson FF, Melov S, Di Monte DA, Andersen JK. Nigrostriatal dopaminergic neurodegeneration in the weaver mouse is mediated via neuroinflammation and alleviated by minocycline administration. J Neurosci. 2006;26(45):11644–11651. doi: 10.1523/JNEUROSCI.3447-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Gao HM, Liu B, Hong JS. Critical role for microglial NADPH oxidase in rotenone-induced degeneration of dopaminergic neurons. J Neurosci. 2003;23(15):6181–6187. doi: 10.1523/JNEUROSCI.23-15-06181.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Emmrich JV, Hornik TC, Neher JJ, Brown GC. Rotenone induces neuronal death by microglial phagocytosis of neurons. FEBS J. 2013;280(20):5030–5038. doi: 10.1111/febs.12401. [DOI] [PubMed] [Google Scholar]
- 145.Klintworth H, Garden G, Xia Z. Rotenone and paraquat do not directly activate microglia or induce inflammatory cytokine release. Neurosci Lett. 2009;462(1):1–5. doi: 10.1016/j.neulet.2009.06.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Sternberg EM. Neural-immune interactions in health and disease. J Clin Investig. 1997;100(11):2641–2647. doi: 10.1172/JCI119807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Chakroborty D, Chowdhury UR, Sarkar C, Baral R, Dasgupta PS, Basu S. Dopamine regulates endothelial progenitor cell mobilization from mouse bone marrow in tumor vascularization. J Clin Investig. 2008;118(4):1380–1389. doi: 10.1172/JCI33125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Sarkar C, Basu B, Chakroborty D, Dasgupta PS, Basu S. The immunoregulatory role of dopamine: an update. Brain Behav Immun. 2010;24(4):525–528. doi: 10.1016/j.bbi.2009.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.McKenna F, McLaughlin PJ, Lewis BJ, Sibbring GC, Cummerson JA, Bowen-Jones D, Moots RJ. Dopamine receptor expression on human T- and B-lymphocytes, monocytes, neutrophils, eosinophils and NK cells: a flow cytometric study. J Neuroimmunol. 2002;132(1–2):34–40. doi: 10.1016/S0165-5728(02)00280-1. [DOI] [PubMed] [Google Scholar]
- 150.Missale C, Nash SR, Robinson SW, Jaber M, Caron MG. Dopamine receptors: from structure to function. Physiol Rev. 1998;78(1):189–225. doi: 10.1152/physrev.1998.78.1.189. [DOI] [PubMed] [Google Scholar]
- 151.Meredith EJ, Chamba A, Holder MJ, Barnes NM, Gordon J. Close encounters of the monoamine kind: immune cells betray their nervous disposition. Immunology. 2005;115(3):289–295. doi: 10.1111/j.1365-2567.2005.02166.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Torres-Rosas R, Yehia G, Pena G, Mishra P, del Rocio Thompson-Bonilla M, Moreno-Eutimio MA, Arriaga-Pizano LA, Isibasi A, Ulloa L. Dopamine mediates vagal modulation of the immune system by electroacupuncture. Nat Med. 2014;20(3):291–295. doi: 10.1038/nm.3479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Mignini F, Streccioni V, Amenta F. Autonomic innervation of immune organs and neuroimmune modulation. Auton Autacoid Pharmacol. 2003;23(1):1–25. doi: 10.1046/j.1474-8673.2003.00280.x. [DOI] [PubMed] [Google Scholar]
- 154.Davis BK, Wen H, Ting JP. The inflammasome NLRs in immunity, inflammation, and associated diseases. Annu Rev Immunol. 2011;29:707–735. doi: 10.1146/annurev-immunol-031210-101405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Shao W, Zhang SZ, Tang M, Zhang XH, Zhou Z, Yin YQ, Zhou QB, Huang YY, Liu YJ, Wawrousek E, Chen T, Li SB, Xu M, Zhou JN, Hu G, Zhou JW. Suppression of neuroinflammation by astrocytic dopamine D2 receptors via alphaB-crystallin. Nature. 2013;494(7435):90–94. doi: 10.1038/nature11748. [DOI] [PubMed] [Google Scholar]
- 156.Konkalmatt PR, Asico LD, Zhang Y, Yang Y, Drachenberg C, Zheng X, Han F, Jose PA, Armando I. Renal rescue of dopamine D2 receptor function reverses renal injury and high blood pressure. JCI Insight. 2016;1(8):e85888. doi: 10.1172/jci.insight.85888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Zhang Y, Cuevas S, Asico LD, Escano C, Yang Y, Pascua AM, Wang X, Jones JE, Grandy D, Eisner G, Jose PA, Armando I. Deficient dopamine D2 receptor function causes renal inflammation independently of high blood pressure. PLoS One. 2012;7(6):e38745. doi: 10.1371/journal.pone.0038745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Gonzalez H, Contreras F, Prado C, Elgueta D, Franz D, Bernales S, Pacheco R. Dopamine receptor D3 expressed on CD4+ T cells favors neurodegeneration of dopaminergic neurons during Parkinson’s disease. J Immunol. 2013;190(10):5048–5056. doi: 10.4049/jimmunol.1203121. [DOI] [PubMed] [Google Scholar]
- 159.Oberbeck R, Schmitz D, Wilsenack K, Schuler M, Husain B, Schedlowski M, Exton MS. Dopamine affects cellular immune functions during polymicrobial sepsis. Intensive Care Med. 2006;32(5):731–739. doi: 10.1007/s00134-006-0084-y. [DOI] [PubMed] [Google Scholar]
- 160.Lim HD, Kim MH, Lee CY, Namgung U. Anti-Inflammatory effects of acupuncture stimulation via the vagus nerve. PLoS One. 2016;11(3):e0151882. doi: 10.1371/journal.pone.0151882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Chen H, Zhang SM, Hernan MA, Schwarzschild MA, Willett WC, Colditz GA, Speizer FE, Ascherio A. Nonsteroidal anti-inflammatory drugs and the risk of Parkinson disease. Arch Neurol. 2003;60(8):1059–1064. doi: 10.1001/archneur.60.8.1059. [DOI] [PubMed] [Google Scholar]
- 162.Chen H, Jacobs E, Schwarzschild MA, McCullough ML, Calle EE, Thun MJ, Ascherio A. Nonsteroidal antiinflammatory drug use and the risk for Parkinson’s disease. Ann Neurol. 2005;58(6):963–967. doi: 10.1002/ana.20682. [DOI] [PubMed] [Google Scholar]
- 163.Esposito E, Di Matteo V, Benigno A, Pierucci M, Crescimanno G, Di Giovanni G. Non-steroidal anti-inflammatory drugs in Parkinson’s disease. Exp Neurol. 2007;205(2):295–312. doi: 10.1016/j.expneurol.2007.02.008. [DOI] [PubMed] [Google Scholar]
- 164.Armentero MT, Levandis G, Nappi G, Bazzini E, Blandini F. Peripheral inflammation and neuroprotection: systemic pretreatment with complete Freund’s adjuvant reduces 6-hydroxydopamine toxicity in a rodent model of Parkinson’s disease. Neurobiol Dis. 2006;24(3):492–505. doi: 10.1016/j.nbd.2006.08.016. [DOI] [PubMed] [Google Scholar]
- 165.Machado A, Herrera AJ, Venero JL, Santiago M, De Pablos RM, Villaran RF, Espinosa-Oliva AM, Arguelles S, Sarmiento M, Delgado-Cortes MJ, Maurino R, Cano J. Peripheral inflammation increases the damage in animal models of nigrostriatal dopaminergic neurodegeneration: possible implication in Parkinson’s disease incidence. Parkinson’s Dis. 2011;2011:393769. doi: 10.4061/2011/393769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Grozdanov V, Bliederhaeuser C, Ruf WP, Roth V, Fundel-Clemens K, Zondler L, Brenner D, Martin-Villalba A, Hengerer B, Kassubek J, Ludolph AC, Weishaupt JH, Danzer KM. Inflammatory dysregulation of blood monocytes in Parkinson’s disease patients. Acta Neuropathol. 2014;128(5):651–663. doi: 10.1007/s00401-014-1345-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Fung VS, Kordower JH. Parkinson’s disease and prion disease: straining the comparison. Mov Disord. 2015;30(13):1727. doi: 10.1002/mds.26437. [DOI] [PubMed] [Google Scholar]
- 168.Goedert M. NEURODEGENERATION. Alzheimer’s and Parkinson’s diseases: the prion concept in relation to assembled Abeta, tau, and alpha-synuclein. Science. 2015;349(6248):1255555. doi: 10.1126/science.1255555. [DOI] [PubMed] [Google Scholar]
- 169.Olanow CW, Brundin P. Parkinson’s disease and alpha synuclein: is Parkinson’s disease a prion-like disorder? Mov Disord. 2013;28(1):31–40. doi: 10.1002/mds.25373. [DOI] [PubMed] [Google Scholar]
- 170.Braak H, Del Tredici K, Rub U, de Vos RA, Jansen Steur EN, Braak E. Staging of brain pathology related to sporadic Parkinson’s disease. Neurobiol Aging. 2003;24(2):197–211. doi: 10.1016/S0197-4580(02)00065-9. [DOI] [PubMed] [Google Scholar]
- 171.Desplats P, Lee HJ, Bae EJ, Patrick C, Rockenstein E, Crews L, Spencer B, Masliah E, Lee SJ. Inclusion formation and neuronal cell death through neuron-to-neuron transmission of alpha-synuclein. Proc Natl Acad Sci USA. 2009;106(31):13010–13015. doi: 10.1073/pnas.0903691106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Emmanouilidou E, Melachroinou K, Roumeliotis T, Garbis SD, Ntzouni M, Margaritis LH, Stefanis L, Vekrellis K. Cell-produced alpha-synuclein is secreted in a calcium-dependent manner by exosomes and impacts neuronal survival. J Neurosci. 2010;30(20):6838–6851. doi: 10.1523/JNEUROSCI.5699-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Lee HJ, Patel S, Lee SJ. Intravesicular localization and exocytosis of alpha-synuclein and its aggregates. J Neurosci. 2005;25(25):6016–6024. doi: 10.1523/JNEUROSCI.0692-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Danzer KM, Kranich LR, Ruf WP, Cagsal-Getkin O, Winslow AR, Zhu L, Vanderburg CR, McLean PJ. Exosomal cell-to-cell transmission of alpha synuclein oligomers. Mol Neurodegener. 2012;7:42. doi: 10.1186/1750-1326-7-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Alvarez-Erviti L, Seow Y, Schapira AH, Gardiner C, Sargent IL, Wood MJ, Cooper JM. Lysosomal dysfunction increases exosome-mediated alpha-synuclein release and transmission. Neurobiol Dis. 2011;42(3):360–367. doi: 10.1016/j.nbd.2011.01.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.McLean PJ, Kawamata H, Ribich S, Hyman BT. Membrane association and protein conformation of alpha-synuclein in intact neurons. Effect of Parkinson’s disease-linked mutations. J Biol Chem. 2000;275(12):8812–8816. doi: 10.1074/jbc.275.12.8812. [DOI] [PubMed] [Google Scholar]
- 177.Kayed R, Sokolov Y, Edmonds B, McIntire TM, Milton SC, Hall JE, Glabe CG. Permeabilization of lipid bilayers is a common conformation-dependent activity of soluble amyloid oligomers in protein misfolding diseases. J Biol Chem. 2004;279(45):46363–46366. doi: 10.1074/jbc.C400260200. [DOI] [PubMed] [Google Scholar]
- 178.Lee HJ, Suk JE, Bae EJ, Lee JH, Paik SR, Lee SJ. Assembly-dependent endocytosis and clearance of extracellular alpha-synuclein. Int J Biochem Cell Bio. 2008;40(9):1835–1849. doi: 10.1016/j.biocel.2008.01.017. [DOI] [PubMed] [Google Scholar]
- 179.Recasens A, Dehay B. Alpha-synuclein spreading in Parkinson’s disease. Front Neuroanat. 2014;8:159. doi: 10.3389/fnana.2014.00159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Tsigelny IF, Sharikov Y, Wrasidlo W, Gonzalez T, Desplats PA, Crews L, Spencer B, Masliah E. Role of alpha-synuclein penetration into the membrane in the mechanisms of oligomer pore formation. FEBS J. 2012;279(6):1000–1013. doi: 10.1111/j.1742-4658.2012.08489.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Hansen C, Angot E, Bergstrom AL, Steiner JA, Pieri L, Paul G, Outeiro TF, Melki R, Kallunki P, Fog K, Li JY, Brundin P. alpha-Synuclein propagates from mouse brain to grafted dopaminergic neurons and seeds aggregation in cultured human cells. J Clin Investig. 2011;121(2):715–725. doi: 10.1172/JCI43366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Volpicelli-Daley LA, Luk KC, Patel TP, Tanik SA, Riddle DM, Stieber A, Meaney DF, Trojanowski JQ, Lee VM. Exogenous alpha-synuclein fibrils induce Lewy body pathology leading to synaptic dysfunction and neuron death. Neuron. 2011;72(1):57–71. doi: 10.1016/j.neuron.2011.08.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Ahn KJ, Paik SR, Chung KC, Kim J. Amino acid sequence motifs and mechanistic features of the membrane translocation of alpha-synuclein. J Neurochem. 2006;97(1):265–279. doi: 10.1111/j.1471-4159.2006.03731.x. [DOI] [PubMed] [Google Scholar]
- 184.Akira S. Toll-like receptors and innate immunity. Adv Immunol. 2001;78:1–56. doi: 10.1016/S0065-2776(01)78001-7. [DOI] [PubMed] [Google Scholar]
- 185.Lehnardt S. Innate immunity and neuroinflammation in the CNS: the role of microglia in Toll-like receptor-mediated neuronal injury. Glia. 2010;58(3):253–263. doi: 10.1002/glia.20928. [DOI] [PubMed] [Google Scholar]
- 186.Rannikko EH, Weber SS, Kahle PJ. Exogenous alpha-synuclein induces toll-like receptor 4 dependent inflammatory responses in astrocytes. BMC Neurosci. 2015;16:57. doi: 10.1186/s12868-015-0192-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Kim C, Lee HJ, Masliah E, Lee SJ. Non-cell-autonomous Neurotoxicity of alpha-synuclein Through Microglial Toll-like Receptor 2. Exp Neurobiol. 2016;25(3):113–119. doi: 10.5607/en.2016.25.3.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Stefanova N, Fellner L, Reindl M, Masliah E, Poewe W, Wenning GK. Toll-like receptor 4 promotes alpha-synuclein clearance and survival of nigral dopaminergic neurons. Am J Pathol. 2011;179(2):954–963. doi: 10.1016/j.ajpath.2011.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Schroder K, Tschopp J. The inflammasomes. Cell. 2010;140(6):821–832. doi: 10.1016/j.cell.2010.01.040. [DOI] [PubMed] [Google Scholar]
- 190.Kahlenberg JM, Dubyak GR. Mechanisms of caspase-1 activation by P2X7 receptor-mediated K+ release. Am J Physiol Cell Physiol. 2004;286(5):C1100–C1108. doi: 10.1152/ajpcell.00494.2003. [DOI] [PubMed] [Google Scholar]
- 191.Kanneganti TD, Lamkanfi M, Kim YG, Chen G, Park JH, Franchi L, Vandenabeele P, Nunez G. Pannexin-1-mediated recognition of bacterial molecules activates the cryopyrin inflammasome independent of Toll-like receptor signaling. Immunity. 2007;26(4):433–443. doi: 10.1016/j.immuni.2007.03.008. [DOI] [PubMed] [Google Scholar]
- 192.Halle A, Hornung V, Petzold GC, Stewart CR, Monks BG, Reinheckel T, Fitzgerald KA, Latz E, Moore KJ, Golenbock DT. The NALP3 inflammasome is involved in the innate immune response to amyloid-beta. Nat Immunol. 2008;9(8):857–865. doi: 10.1038/ni.1636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Hornung V, Bauernfeind F, Halle A, Samstad EO, Kono H, Rock KL, Fitzgerald KA, Latz E. Silica crystals and aluminum salts activate the NALP3 inflammasome through phagosomal destabilization. Nat Immunol. 2008;9(8):847–856. doi: 10.1038/ni.1631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Cassel SL, Eisenbarth SC, Iyer SS, Sadler JJ, Colegio OR, Tephly LA, Carter AB, Rothman PB, Flavell RA, Sutterwala FS. The Nalp3 inflammasome is essential for the development of silicosis. Proc Natl Acad Sci USA. 2008;105(26):9035–9040. doi: 10.1073/pnas.0803933105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Dostert C, Petrilli V, Van Bruggen R, Steele C, Mossman BT, Tschopp J. Innate immune activation through Nalp3 inflammasome sensing of asbestos and silica. Science. 2008;320(5876):674–677. doi: 10.1126/science.1156995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Codolo G, Plotegher N, Pozzobon T, Brucale M, Tessari I, Bubacco L, de Bernard M. Triggering of inflammasome by aggregated alpha-synuclein, an inflammatory response in synucleinopathies. PLoS One. 2013;8(1):e55375. doi: 10.1371/journal.pone.0055375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Crampton SJ, O’Keeffe GW. NF-kappaB: emerging roles in hippocampal development and function. Int J Biochem Cell Biol. 2013;45(8):1821–1824. doi: 10.1016/j.biocel.2013.05.037. [DOI] [PubMed] [Google Scholar]
- 198.Baiguera C, Alghisi M, Pinna A, Bellucci A, De Luca MA, Frau L, Morelli M, Ingrassia R, Benarese M, Porrini V, Pellitteri M, Bertini G, Fabene PF, Sigala S, Spillantini MG, Liou HC, Spano PF, Pizzi M. Late-onset Parkinsonism in NFkappaB/c-Rel-deficient mice. Braina J Neurol. 2012;135(Pt 9):2750–2765. doi: 10.1093/brain/aws193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Shih RH, Wang CY, Yang CM. NF-kappaB signaling pathways in neurological inflammation: a mini review. Front Mol Neurosci. 2015;8:77. doi: 10.3389/fnmol.2015.00077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Lee EJ, Woo MS, Moon PG, Baek MC, Choi IY, Kim WK, Junn E, Kim HS. Alpha-synuclein activates microglia by inducing the expressions of matrix metalloproteinases and the subsequent activation of protease-activated receptor-1. J Immunol. 2010;185(1):615–623. doi: 10.4049/jimmunol.0903480. [DOI] [PubMed] [Google Scholar]
- 201.Wilms H, Rosenstiel P, Romero-Ramos M, Arlt A, Schafer H, Seegert D, Kahle PJ, Odoy S, Claasen JH, Holzknecht C, Brandenburg LO, Deuschl G, Schreiber S, Kirik D, Lucius R. Suppression of MAP kinases inhibits microglial activation and attenuates neuronal cell death induced by alpha-synuclein protofibrils. Int J Immunopathol Pharmacol. 2009;22(4):897–909. doi: 10.1177/039463200902200405. [DOI] [PubMed] [Google Scholar]
- 202.Kim C, Rockenstein E, Spencer B, Kim HK, Adame A, Trejo M, Stafa K, Lee HJ, Lee SJ, Masliah E. Antagonizing neuronal toll-like receptor 2 prevents synucleinopathy by activating autophagy. Cell Rep. 2015;13(4):771–782. doi: 10.1016/j.celrep.2015.09.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Wang G, Pan J, Chen SD. Kinases and kinase signaling pathways: potential therapeutic targets in Parkinson’s disease. Prog Neurobiol. 2012;98(2):207–221. doi: 10.1016/j.pneurobio.2012.06.003. [DOI] [PubMed] [Google Scholar]
- 204.Stagkourakis S, Kim H, Lyons DJ, Broberger C. Dopamine autoreceptor regulation of a hypothalamic dopaminergic network. Cell Rep. 2016 doi: 10.1016/j.celrep.2016.03.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Politis M, Piccini P, Pavese N, Koh SB, Brooks DJ. Evidence of dopamine dysfunction in the hypothalamus of patients with Parkinson’s disease: an in vivo 11C-raclopride PET study. Exp Neurol. 2008;214(1):112–116. doi: 10.1016/j.expneurol.2008.07.021. [DOI] [PubMed] [Google Scholar]
- 206.Du X, Pang TY. Is dysregulation of the HPA-Axis a core pathophysiology mediating co-morbid depression in neurodegenerative diseases? Front Psychiatry. 2015;6:32. doi: 10.3389/fpsyt.2015.00032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Seifried C, Boehncke S, Heinzmann J, Baudrexel S, Weise L, Gasser T, Eggert K, Fogel W, Baas H, Badenhoop K, Steinmetz H, Hilker R. Diurnal variation of hypothalamic function and chronic subthalamic nucleus stimulation in Parkinson’s disease. Neuroendocrinology. 2013;97(3):283–290. doi: 10.1159/000343808. [DOI] [PubMed] [Google Scholar]
- 208.Aziz NA, Pijl H, Frolich M, van der Graaf AW, Roelfsema F, Roos RA. Increased hypothalamic-pituitary-adrenal axis activity in Huntington’s disease. J Clin Endocrinol Metab. 2009;94(4):1223–1228. doi: 10.1210/jc.2008-2543. [DOI] [PubMed] [Google Scholar]
- 209.Batalha VL, Ferreira DG, Coelho JE, Valadas JS, Gomes R, Temido-Ferreira M, Shmidt T, Baqi Y, Buee L, Muller CE, Hamdane M, Outeiro TF, Bader M, Meijsing SH, Sadri-Vakili G, Blum D, Lopes LV. The caffeine-binding adenosine A2A receptor induces age-like HPA-axis dysfunction by targeting glucocorticoid receptor function. Sci Rep. 2016;6:31493. doi: 10.1038/srep31493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Javoy-Agid F, Ruberg M, Pique L, Bertagna X, Taquet H, Studler JM, Cesselin F, Epelbaum J, Agid Y. Biochemistry of the hypothalamus in Parkinson’s disease. Neurology. 1984;34(5):672–675. doi: 10.1212/WNL.34.5.672. [DOI] [PubMed] [Google Scholar]
- 211.Shannak K, Rajput A, Rozdilsky B, Kish S, Gilbert J, Hornykiewicz O. Noradrenaline, dopamine and serotonin levels and metabolism in the human hypothalamus: observations in Parkinson’s disease and normal subjects. Brain Res. 1994;639(1):33–41. doi: 10.1016/0006-8993(94)91761-2. [DOI] [PubMed] [Google Scholar]
- 212.Moore RY, Whone AL, Brooks DJ. Extrastriatal monoamine neuron function in Parkinson’s disease: an 18F-dopa PET study. Neurobiol Dis. 2008;29(3):381–390. doi: 10.1016/j.nbd.2007.09.004. [DOI] [PubMed] [Google Scholar]
- 213.Cusimano G, Capriani C, Bonifati V, Meco G. Hypothalamo-pituitary function and dopamine dependence in untreated parkinsonian patients. Acta Neurol Scand. 1991;83(3):145–150. doi: 10.1111/j.1600-0404.1991.tb04666.x. [DOI] [PubMed] [Google Scholar]
- 214.Langston JW, Forno LS. The hypothalamus in Parkinson disease. Ann Neurol. 1978;3(2):129–133. doi: 10.1002/ana.410030207. [DOI] [PubMed] [Google Scholar]
- 215.Felice VD, Quigley EM, Sullivan AM, O’Keeffe GW, O’Mahony SM. Microbiota-gut-brain signalling in Parkinson’s disease: implications for non-motor symptoms. Parkinsonism Relat Disord. 2016;27:1–8. doi: 10.1016/j.parkreldis.2016.03.012. [DOI] [PubMed] [Google Scholar]
- 216.Holzer P, Farzi A. Neuropeptides and the microbiota-gut-brain axis. Adv Exp Med Biol. 2014;817:195–219. doi: 10.1007/978-1-4939-0897-4_9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Bayliss JA, Lemus MB, Stark R, Santos VV, Thompson A, Rees DJ, Galic S, Elsworth JD, Kemp BE, Davies JS, Andrews ZB. Ghrelin-AMPK signaling mediates the neuroprotective effects of calorie restriction in Parkinson’s disease. J Neurosci. 2016;36(10):3049–3063. doi: 10.1523/JNEUROSCI.4373-15.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Bayliss JA, Lemus M, Santos VV, Deo M, Elsworth JD, Andrews ZB. Acylated but not des-acyl ghrelin is neuroprotective in an MPTP mouse model of Parkinson’s disease. J Neurochem. 2016;137(3):460–471. doi: 10.1111/jnc.13576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Duarte-Neves J, Pereira de Almeida L, Cavadas C. Neuropeptide Y (NPY) as a therapeutic target for neurodegenerative diseases. Neurobiol Dis. 2016;95:210–224. doi: 10.1016/j.nbd.2016.07.022. [DOI] [PubMed] [Google Scholar]
- 220.Zhang L, Nguyen AD, Lee IC, Yulyaningsih E, Riepler SJ, Stehrer B, Enriquez RF, Lin S, Shi YC, Baldock PA, Sainsbury A, Herzog H. NPY modulates PYY function in the regulation of energy balance and glucose homeostasis. Diabetes Obes Metab. 2012;14(8):727–736. doi: 10.1111/j.1463-1326.2012.01592.x. [DOI] [PubMed] [Google Scholar]
- 221.Ferreira-Marques M, Aveleira CA, Carmo-Silva S, Botelho M, Pereira de Almeida L, Cavadas C. Caloric restriction stimulates autophagy in rat cortical neurons through neuropeptide Y and ghrelin receptors activation. Aging. 2016;8(7):1470–1484. doi: 10.18632/aging.100996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Arroyo DS, Soria JA, Gaviglio EA, Rodriguez-Galan MC, Iribarren P. Toll-like receptors are key players in neurodegeneration. Int Immunopharmacol. 2011;11(10):1415–1421. doi: 10.1016/j.intimp.2011.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Beyer PL, Palarino MY, Michalek D, Busenbark K, Koller WC. Weight change and body composition in patients with Parkinson’s disease. J Am Diet Assoc. 1995;95(9):979–983. doi: 10.1016/S0002-8223(95)00269-3. [DOI] [PubMed] [Google Scholar]
- 224.van der Marck MA, Dicke HC, Uc EY, Kentin ZH, Borm GF, Bloem BR, Overeem S, Munneke M. Body mass index in Parkinson’s disease: a meta-analysis. Parkinsonism Relat Disord. 2012;18(3):263–267. doi: 10.1016/j.parkreldis.2011.10.016. [DOI] [PubMed] [Google Scholar]
- 225.Chen H, Zhang SM, Hernan MA, Willett WC, Ascherio A. Weight loss in Parkinson’s disease. Ann Neurol. 2003;53(5):676–679. doi: 10.1002/ana.10577. [DOI] [PubMed] [Google Scholar]
- 226.De Pablo-Fernandez E, Breen DP, Bouloux PM, Barker RA, Foltynie T, Warner TT. Neuroendocrine abnormalities in Parkinson’s disease. J Neurol Neurosurg Psychiatry. 2017;88(2):176–185. doi: 10.1136/jnnp-2016-314601. [DOI] [PubMed] [Google Scholar]
- 227.Thannickal TC, Lai YY, Siegel JM. Hypocretin (orexin) cell loss in Parkinson’s disease. Brain J Neurol. 2007;130(Pt 6):1586–1595. doi: 10.1093/brain/awm097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Drouot X, Moutereau S, Nguyen JP, Lefaucheur JP, Creange A, Remy P, Goldenberg F, d’Ortho MP. Low levels of ventricular CSF orexin/hypocretin in advanced PD. Neurology. 2003;61(4):540–543. doi: 10.1212/01.WNL.0000078194.53210.48. [DOI] [PubMed] [Google Scholar]
- 229.Mohawk JA, Green CB, Takahashi JS. Central and peripheral circadian clocks in mammals. Annu Rev Neurosci. 2012;35:445–462. doi: 10.1146/annurev-neuro-060909-153128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Bruguerolle B, Simon N. Biologic rhythms and Parkinson’s disease: a chronopharmacologic approach to considering fluctuations in function. Clin Neuropharmacol. 2002;25(4):194–201. doi: 10.1097/00002826-200207000-00002. [DOI] [PubMed] [Google Scholar]
- 231.Videnovic A, Willis GL. Circadian system—a novel diagnostic and therapeutic target in Parkinson’s disease? Mov Disord. 2016;31(3):260–269. doi: 10.1002/mds.26509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Videnovic A, Golombek D. Circadian and sleep disorders in Parkinson’s disease. Exp Neurol. 2013;243:45–56. doi: 10.1016/j.expneurol.2012.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Witkovsky P. Dopamine and retinal function. Doc Ophthalmol Adv Ophthalmol. 2004;108(1):17–40. doi: 10.1023/B:DOOP.0000019487.88486.0a. [DOI] [PubMed] [Google Scholar]
- 234.Sakamoto K, Liu C, Kasamatsu M, Pozdeyev NV, Iuvone PM, Tosini G. Dopamine regulates melanopsin mRNA expression in intrinsically photosensitive retinal ganglion cells. Eur J Neurosci. 2005;22(12):3129–3136. doi: 10.1111/j.1460-9568.2005.04512.x. [DOI] [PubMed] [Google Scholar]
- 235.Dorenbos R, Contini M, Hirasawa H, Gustincich S, Raviola E. Expression of circadian clock genes in retinal dopaminergic cells. Vis Neurosci. 2007;24(4):573–580. doi: 10.1017/S0952523807070538. [DOI] [PubMed] [Google Scholar]
- 236.McClung CA, Sidiropoulou K, Vitaterna M, Takahashi JS, White FJ, Cooper DC, Nestler EJ. Regulation of dopaminergic transmission and cocaine reward by the Clock gene. Proc Natl Acad Sci USA. 2005;102(26):9377–9381. doi: 10.1073/pnas.0503584102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Hampp G, Ripperger JA, Houben T, Schmutz I, Blex C, Perreau-Lenz S, Brunk I, Spanagel R, Ahnert-Hilger G, Meijer JH, Albrecht U. Regulation of monoamine oxidase A by circadian-clock components implies clock influence on mood. Curr Biol CB. 2008;18(9):678–683. doi: 10.1016/j.cub.2008.04.012. [DOI] [PubMed] [Google Scholar]
- 238.Spencer S, Torres-Altoro MI, Falcon E, Arey R, Marvin M, Goldberg M, Bibb JA, McClung CA. A mutation in CLOCK leads to altered dopamine receptor function. J Neurochem. 2012;123(1):124–134. doi: 10.1111/j.1471-4159.2012.07857.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Chung S, Lee EJ, Yun S, Choe HK, Park SB, Son HJ, Kim KS, Dluzen DE, Lee I, Hwang O, Son GH, Kim K. Impact of circadian nuclear receptor REV-ERBalpha on midbrain dopamine production and mood regulation. Cell. 2014;157(4):858–868. doi: 10.1016/j.cell.2014.03.039. [DOI] [PubMed] [Google Scholar]
- 240.Hood S, Cassidy P, Cossette MP, Weigl Y, Verwey M, Robinson B, Stewart J, Amir S. Endogenous dopamine regulates the rhythm of expression of the clock protein PER2 in the rat dorsal striatum via daily activation of D2 dopamine receptors. J Neurosci. 2010;30(42):14046–14058. doi: 10.1523/JNEUROSCI.2128-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Glass JD, Brager AJ, Stowie AC, Prosser RA. Cocaine modulates pathways for photic and nonphotic entrainment of the mammalian SCN circadian clock. Am J Physiol Regul Integr Comp Physiol. 2012;302(6):R740–R750. doi: 10.1152/ajpregu.00602.2011. [DOI] [PubMed] [Google Scholar]
- 242.Honma K, Honma S. The SCN-independent clocks, methamphetamine and food restriction. Eur J Neurosci. 2009;30(9):1707–1717. doi: 10.1111/j.1460-9568.2009.06976.x. [DOI] [PubMed] [Google Scholar]
- 243.Rye DB. The two faces of Eve: dopamine’s modulation of wakefulness and sleep. Neurology. 2004;63(8 Suppl 3):S2–S7. doi: 10.1212/WNL.63.8_suppl_3.S2. [DOI] [PubMed] [Google Scholar]
- 244.Trotti LM, Karroum EG. Melatonin for sleep disorders in patients with neurodegenerative diseases. Curr Neurol Neurosci Rep. 2016;16(7):63. doi: 10.1007/s11910-016-0664-3. [DOI] [PubMed] [Google Scholar]
- 245.Ono K, Mochizuki H, Ikeda T, Nihira T, Takasaki J, Teplow DB, Yamada M. Effect of melatonin on alpha-synuclein self-assembly and cytotoxicity. Neurobiol Aging. 2012;33(9):2172–2185. doi: 10.1016/j.neurobiolaging.2011.10.015. [DOI] [PubMed] [Google Scholar]
- 246.Pandi-Perumal SR, BaHammam AS, Brown GM, Spence DW, Bharti VK, Kaur C, Hardeland R, Cardinali DP. Melatonin antioxidative defense: therapeutical implications for aging and neurodegenerative processes. Neurotox Res. 2013;23(3):267–300. doi: 10.1007/s12640-012-9337-4. [DOI] [PubMed] [Google Scholar]
- 247.Permpoonputtana K, Govitrapong P. The anti-inflammatory effect of melatonin on methamphetamine-induced proinflammatory mediators in human neuroblastoma dopamine SH-SY5Y cell lines. Neurotox Res. 2013;23(2):189–199. doi: 10.1007/s12640-012-9350-7. [DOI] [PubMed] [Google Scholar]
- 248.Videnovic A, Noble C, Reid KJ, Peng J, Turek FW, Marconi A, Rademaker AW, Simuni T, Zadikoff C, Zee PC. Circadian melatonin rhythm and excessive daytime sleepiness in Parkinson disease. JAMA neurology. 2014;71(4):463–469. doi: 10.1001/jamaneurol.2013.6239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Singhal NK, Srivastava G, Agrawal S, Jain SK, Singh MP. Melatonin as a neuroprotective agent in the rodent models of Parkinson’s disease: is it all set to irrefutable clinical translation? Mol Neurobiol. 2012;45(1):186–199. doi: 10.1007/s12035-011-8225-x. [DOI] [PubMed] [Google Scholar]
- 250.Jeong JK, Moon MH, Lee YJ, Seol JW, Park SY. Melatonin-induced autophagy protects against human prion protein-mediated neurotoxicity. J Pineal Res. 2012;53(2):138–146. doi: 10.1111/j.1600-079X.2012.00980.x. [DOI] [PubMed] [Google Scholar]
- 251.Belaid H, Adrien J, Karachi C, Hirsch EC, Francois C. Effect of melatonin on sleep disorders in a monkey model of Parkinson’s disease. Sleep Med. 2015;16(10):1245–1251. doi: 10.1016/j.sleep.2015.06.018. [DOI] [PubMed] [Google Scholar]
- 252.Carriere CH, Kang NH, Niles LP. Chronic low-dose melatonin treatment maintains nigrostriatal integrity in an intrastriatal rotenone model of Parkinson’s disease. Brain Res. 2016;1633:115–125. doi: 10.1016/j.brainres.2015.12.036. [DOI] [PubMed] [Google Scholar]
- 253.Naskar A, Prabhakar V, Singh R, Dutta D, Mohanakumar KP. Melatonin enhances L-DOPA therapeutic effects, helps to reduce its dose, and protects dopaminergic neurons in 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-induced Parkinsonism in mice. J Pineal Res. 2015;58(3):262–274. doi: 10.1111/jpi.12212. [DOI] [PubMed] [Google Scholar]
- 254.Bassani TB, Gradowski RW, Zaminelli T, Barbiero JK, Santiago RM, Boschen SL, da Cunha C, Lima MM, Andreatini R, Vital MA. Neuroprotective and antidepressant-like effects of melatonin in a rotenone-induced Parkinson’s disease model in rats. Brain Res. 2014;1593:95–105. doi: 10.1016/j.brainres.2014.09.068. [DOI] [PubMed] [Google Scholar]
- 255.Emre M, Ford PJ, Bilgic B, Uc EY. Cognitive impairment and dementia in Parkinson’s disease: practical issues and management. Mov Disord. 2014;29(5):663–672. doi: 10.1002/mds.25870. [DOI] [PubMed] [Google Scholar]
- 256.Gonzalez S, Moreno-Delgado D, Moreno E, Perez-Capote K, Franco R, Mallol J, Cortes A, Casado V, Lluis C, Ortiz J, Ferre S, Canela E, McCormick PJ. Circadian-related heteromerization of adrenergic and dopamine D(4) receptors modulates melatonin synthesis and release in the pineal gland. PLoS Biol. 2012;10(6):e1001347. doi: 10.1371/journal.pbio.1001347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Bordet R, Devos D, Brique S, Touitou Y, Guieu JD, Libersa C, Destee A. Study of circadian melatonin secretion pattern at different stages of Parkinson’s disease. Clin Neuropharmacol. 2003;26(2):65–72. doi: 10.1097/00002826-200303000-00005. [DOI] [PubMed] [Google Scholar]
- 258.Cotzias GC, Tang LC, Miller ST, Ginos JZ. Melatonin and abnormal movements induced by l-dopa in mice. Science. 1971;173(3995):450–452. doi: 10.1126/science.173.3995.450. [DOI] [PubMed] [Google Scholar]
- 259.Yujnovsky I, Hirayama J, Doi M, Borrelli E, Sassone-Corsi P. Signaling mediated by the dopamine D2 receptor potentiates circadian regulation by CLOCK:BMAL1. Proc Natl Acad Sci USA. 2006;103(16):6386–6391. doi: 10.1073/pnas.0510691103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.Pacheco R, Prado CE, Barrientos MJ, Bernales S. Role of dopamine in the physiology of T-cells and dendritic cells. J Neuroimmunol. 2009;216(1–2):8–19. doi: 10.1016/j.jneuroim.2009.07.018. [DOI] [PubMed] [Google Scholar]
- 261.Levite M. Dopamine and T cells: dopamine receptors and potent effects on T cells, dopamine production in T cells, and abnormalities in the dopaminergic system in T cells in autoimmune, neurological and psychiatric diseases. Acta Physiol. 2016;216(1):42–89. doi: 10.1111/apha.12476. [DOI] [PubMed] [Google Scholar]
- 262.Rangel-Barajas C, Coronel I, Floran B. Dopamine receptors and neurodegeneration. Aging Dis. 2015;6(5):349–368. doi: 10.14336/AD.2015.0330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263.Baier PC, Trenkwalder C. Circadian variation in restless legs syndrome. Sleep Med. 2007;8(6):645–650. doi: 10.1016/j.sleep.2006.10.011. [DOI] [PubMed] [Google Scholar]
- 264.Claustrat B, Brun J, Chazot G. The basic physiology and pathophysiology of melatonin. Sleep Med Rev. 2005;9(1):11–24. doi: 10.1016/j.smrv.2004.08.001. [DOI] [PubMed] [Google Scholar]
- 265.Fertl E, Auff E, Doppelbauer A, Waldhauser F. Circadian secretion pattern of melatonin in Parkinson’s disease. J Neural Transm Park Dis Dement Sect. 1991;3(1):41–47. doi: 10.1007/BF02251135. [DOI] [PubMed] [Google Scholar]
- 266.Breen DP, Vuono R, Nawarathna U, Fisher K, Shneerson JM, Reddy AB, Barker RA. Sleep and circadian rhythm regulation in early Parkinson disease. JAMA Neurol. 2014;71(5):589–595. doi: 10.1001/jamaneurol.2014.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267.Medeiros CA, Carvalhedo de Bruin PF, Lopes LA, Magalhaes MC, de Lourdes Seabra M, de Bruin VM. Effect of exogenous melatonin on sleep and motor dysfunction in Parkinson’s disease. A randomized, double blind, placebo-controlled study. J Neurol. 2007;254(4):459–464. doi: 10.1007/s00415-006-0390-x. [DOI] [PubMed] [Google Scholar]
- 268.Dabbeni-Sala F, Di Santo S, Franceschini D, Skaper SD, Giusti P. Melatonin protects against 6-OHDA-induced neurotoxicity in rats: a role for mitochondrial complex I activity. FASEB J. 2001;15(1):164–170. doi: 10.1096/fj.00-0129com. [DOI] [PubMed] [Google Scholar]
- 269.Dowling GA, Mastick J, Colling E, Carter JH, Singer CM, Aminoff MJ. Melatonin for sleep disturbances in Parkinson’s disease. Sleep Med. 2005;6(5):459–466. doi: 10.1016/j.sleep.2005.04.004. [DOI] [PubMed] [Google Scholar]
- 270.Reiter RJ, Calvo JR, Karbownik M, Qi W, Tan DX. Melatonin and its relation to the immune system and inflammation. Ann N Y Acad Sci. 2000;917:376–386. doi: 10.1111/j.1749-6632.2000.tb05402.x. [DOI] [PubMed] [Google Scholar]
- 271.Radogna F, Diederich M, Ghibelli L. Melatonin: a pleiotropic molecule regulating inflammation. Biochem Pharmacol. 2010;80(12):1844–1852. doi: 10.1016/j.bcp.2010.07.041. [DOI] [PubMed] [Google Scholar]
- 272.Mayo JC, Sainz RM, Tan DX, Antolin I, Rodriguez C, Reiter RJ. Melatonin and Parkinson’s disease. Endocrine. 2005;27(2):169–178. doi: 10.1385/ENDO:27:2:169. [DOI] [PubMed] [Google Scholar]
- 273.Mohan N, Sadeghi K, Reiter RJ, Meltz ML. The neurohormone melatonin inhibits cytokine, mitogen and ionizing radiation induced NF-kappa B. Biochem Mol Biol Int. 1995;37(6):1063–1070. [PubMed] [Google Scholar]
- 274.Chuang JI, Mohan N, Meltz ML, Reiter RJ. Effect of melatonin on NF-kappa-B DNA-binding activity in the rat spleen. Cell Biol Int. 1996;20(10):687–692. doi: 10.1006/cbir.1996.0091. [DOI] [PubMed] [Google Scholar]
- 275.Bertuglia S, Marchiafava PL, Colantuoni A. Melatonin prevents ischemia reperfusion injury in hamster cheek pouch microcirculation. Cardiovasc Res. 1996;31(6):947–952. doi: 10.1016/S0008-6363(96)00030-2. [DOI] [PubMed] [Google Scholar]
- 276.Wongprayoon P, Govitrapong P. Melatonin attenuates methamphetamine-induced neuroinflammation through the melatonin receptor in the SH-SY5Y cell line. Neurotoxicology. 2015;50:122–130. doi: 10.1016/j.neuro.2015.08.008. [DOI] [PubMed] [Google Scholar]
- 277.Hardeland R, Cardinali DP, Brown GM, Pandi-Perumal SR. Melatonin and brain inflammaging. Prog Neurobiol. 2015;127–128:46–63. doi: 10.1016/j.pneurobio.2015.02.001. [DOI] [PubMed] [Google Scholar]
- 278.Ali T, Badshah H, Kim TH, Kim MO. Melatonin attenuates D-galactose-induced memory impairment, neuroinflammation and neurodegeneration via RAGE/NF-K B/JNK signaling pathway in aging mouse model. J Pineal Res. 2015;58(1):71–85. doi: 10.1111/jpi.12194. [DOI] [PubMed] [Google Scholar]
- 279.Hagenauer MH, Lee TM. The neuroendocrine control of the circadian system: adolescent chronotype. Front Neuroendocrinol. 2012;33(3):211–229. doi: 10.1016/j.yfrne.2012.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 280.Breen DP, Nombela C, Vuono R, Jones PS, Fisher K, Burn DJ, Brooks DJ, Reddy AB, Rowe JB, Barker RA. Hypothalamic volume loss is associated with reduced melatonin output in Parkinson’s disease. Mov Disord. 2016;31(7):1062–1066. doi: 10.1002/mds.26592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 281.Qin XY, Zhang SP, Cao C, Loh YP, Cheng Y. Aberrations in Peripheral inflammatory cytokine levels in Parkinson disease: a systematic review and meta-analysis. JAMA Neurol. 2016;73(11):1316–1324. doi: 10.1001/jamaneurol.2016.2742. [DOI] [PubMed] [Google Scholar]
- 282.Manocha GD, Floden AM, Puig KL, Nagamoto-Combs K, Scherzer CR, Combs CK. Defining the contribution of neuroinflammation to Parkinson’s disease in humanized immune system mice. Mol Neurodegener. 2017;12(1):17. doi: 10.1186/s13024-017-0158-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 283.Liu M, Bachstetter AD, Cass WA, Lifshitz J, Bing G. Pioglitazone attenuates neuroinflammation and promotes dopaminergic neuronal survival in the nigrostriatal system of rats after diffuse brain injury. J Neurotrauma. 2017;34(2):414–422. doi: 10.1089/neu.2015.4361. [DOI] [PubMed] [Google Scholar]
- 284.Mendes A, Goncalves A, Vila-Cha N, Moreira I, Fernandes J, Damasio J, Teixeira-Pinto A, Taipa R, Lima AB, Cavaco S. Appendectomy may delay Parkinson’s disease Onset. Mov Disord. 2015;30(10):1404–1407. doi: 10.1002/mds.26311. [DOI] [PubMed] [Google Scholar]
- 285.Svensson E, Horvath-Puho E, Stokholm MG, Sorensen HT, Henderson VW, Borghammer P. Appendectomy and risk of Parkinson’s disease: a nationwide cohort study with more than 10 years of follow-up. Mov Disord. 2016;31(12):1918–1922. doi: 10.1002/mds.26761. [DOI] [PubMed] [Google Scholar]
- 286.Poewe W, Seppi K, Tanner CM, Halliday GM, Brundin P, Volkmann J, Schrag AE, Lang AE. Parkinson disease. Nat Rev Dis Primers. 2017;3:17013. doi: 10.1038/nrdp.2017.13. [DOI] [PubMed] [Google Scholar]
- 287.Remy P, Doder M, Lees A, Turjanski N, Brooks D. Depression in Parkinson’s disease: loss of dopamine and noradrenaline innervation in the limbic system. Brain J Neurol. 2005;128(Pt 6):1314–1322. doi: 10.1093/brain/awh445. [DOI] [PubMed] [Google Scholar]
- 288.Rektorova I, Rektor I, Bares M, Dostal V, Ehler E, Fanfrdlova Z, Fiedler J, Klajblova H, Kulist’ak P, Ressner P, Svatova J, Urbanek K, Veliskova J. Pramipexole and pergolide in the treatment of depression in Parkinson’s disease: a national multicentre prospective randomized study. Eur J Neurol. 2003;10(4):399–406. doi: 10.1046/j.1468-1331.2003.00612.x. [DOI] [PubMed] [Google Scholar]
- 289.Barone P, Scarzella L, Marconi R, Antonini A, Morgante L, Bracco F, Zappia M, Musch B, Depression/Parkinson Italian Study G Pramipexole versus sertraline in the treatment of depression in Parkinson’s disease: a national multicenter parallel-group randomized study. J Neurol. 2006;253(5):601–607. doi: 10.1007/s00415-006-0067-5. [DOI] [PubMed] [Google Scholar]
- 290.Pahwa R, Stacy MA, Factor SA, Lyons KE, Stocchi F, Hersh BP, Elmer LW, Truong DD, Earl NL, Investigators E-PAS. Ropinirole 24-hour prolonged release: randomized, controlled study in advanced Parkinson disease. Neurology. 2007;68(14):1108–1115. doi: 10.1212/01.wnl.0000258660.74391.c1. [DOI] [PubMed] [Google Scholar]
- 291.Pessoa Rocha N, Reis HJ, Vanden Berghe P, Cirillo C. Depression and cognitive impairment in Parkinson’s disease: a role for inflammation and immunomodulation? NeuroImmunoModulation. 2014;21(2–3):88–94. doi: 10.1159/000356531. [DOI] [PubMed] [Google Scholar]
- 292.Lindqvist D, Hall S, Surova Y, Nielsen HM, Janelidze S, Brundin L, Hansson O. Cerebrospinal fluid inflammatory markers in Parkinson’s disease–associations with depression, fatigue, and cognitive impairment. Brain Behav Immun. 2013;33:183–189. doi: 10.1016/j.bbi.2013.07.007. [DOI] [PubMed] [Google Scholar]
- 293.Chung YC, Kim SR, Jin BK. Paroxetine prevents loss of nigrostriatal dopaminergic neurons by inhibiting brain inflammation and oxidative stress in an experimental model of Parkinson’s disease. J Immunol. 2010;185(2):1230–1237. doi: 10.4049/jimmunol.1000208. [DOI] [PubMed] [Google Scholar]
- 294.Sandyk R. Pineal melatonin functions and the depression of Parkinson’s disease: a hypothesis. Int J Neurosci. 1990;51(1–2):73–77. doi: 10.3109/00207459009000510. [DOI] [PubMed] [Google Scholar]
- 295.Boeve BF, Silber MH, Saper CB, Ferman TJ, Dickson DW, Parisi JE, Benarroch EE, Ahlskog JE, Smith GE, Caselli RC, Tippman-Peikert M, Olson EJ, Lin SC, Young T, Wszolek Z, Schenck CH, Mahowald MW, Castillo PR, Del Tredici K, Braak H. Pathophysiology of REM sleep behaviour disorder and relevance to neurodegenerative disease. Brain J Neurol. 2007;130(Pt 11):2770–2788. doi: 10.1093/brain/awm056. [DOI] [PubMed] [Google Scholar]
- 296.Fantini ML, Gagnon JF, Filipini D, Montplaisir J. The effects of pramipexole in REM sleep behavior disorder. Neurology. 2003;61(10):1418–1420. doi: 10.1212/WNL.61.10.1418. [DOI] [PubMed] [Google Scholar]
- 297.Hu Y, Yu SY, Zuo LJ, Piao YS, Cao CJ, Wang F, Chen ZJ, Du Y, Lian TH, Liu GF, Wang YJ, Chan P, Chen SD, Wang XM, Zhang W. Investigation on abnormal iron metabolism and related inflammation in Parkinson disease patients with probable RBD. PLoS One. 2015;10(10):e0138997. doi: 10.1371/journal.pone.0138997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 298.Bourgin P, Huitron-Resendiz S, Spier AD, Fabre V, Morte B, Criado JR, Sutcliffe JG, Henriksen SJ, de Lecea L. Hypocretin-1 modulates rapid eye movement sleep through activation of locus coeruleus neurons. J Neurosci. 2000;20(20):7760–7765. doi: 10.1523/JNEUROSCI.20-20-07760.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]