Abstract
Mesoderm posterior 1 is one of earliest markers of the nascent mesoderm. Its best-known function is driving the onset of the cardiovascular system. In the past decade, new evidence supports that Mesp1 acts earlier with greater breadth in cell fate decisions, and through cell-autonomous and cell non-autonomous mechanisms. This review summarizes these new aspects, with an emphasis on the upstream and downstream regulation around Mesp1 and how they may guide cell fate reprogramming.
Keywords: Stem cell, Cell fate, Mesoderm, Reprogramming, Cardiomyocyte
Introduction
Mesoderm posterior 1 (Mesp1) was first cloned from the base of the allantois of 7.5 dpc mouse embryos two decades ago [1]. It contains a basic helix–loop–helix domain, hence designated bHLHc5, with close cousins being Myod1 (bHLHc1), Myf5 (bHLHc2), Myog (bHLHc3), Myf6 (bHLHc4), Mesp2 (bHLHc6) and FIGLA (bHLHc8) [2]. Mouse genetic models and ectopic expression studies in embryonic stem cells (ESCs) established Mesp1 as a high hierarchical regulator of the cardiovascular system [1, 3–8]. Mesp1+ cells derive major cardiovascular cell types including cardiomyocytes (CMs), vascular smooth muscle cells (SMCs) and endothelial cells (ECs). They contribute to the myocardium, endocardium as well as epicardium. Mesp1 is used in some reprogramming cocktails to convert human fibroblasts into CM-like cells, in experimental attempts to repair injured hearts [9–11].
There has been substantial advancement in our understanding of this early transcription factor (TF). Its cell-fate-specifying role is tracked to as early as the epiblast stage. It directly induces or indirectly “poises” a range of important cell types beyond the cardiovascular system. The new knowledge is valuable for our understanding of embryonic development and human congenital diseases, and may be employed to help regenerating critical human tissues. Here, I summarize these new findings, and refer the readers to two excellent reviews for broader coverage of earlier literatures [4, 12].
Mesp1 as the master regulator of cardiovascular development
Mesp1 in mouse cardiovascular development
Mesp1 first appears at the onset of gastrulation, in the nascent mesoderm ingressed at the end of the primitive streak [1]. Mesp1+ cells move laterally toward the anterior region, but the expression of Mesp1 quickly disappears. With a Cre recombinase knocked into the Mesp1 locus, the lineage of Mesp1-expressing cells was tracked to the mesoderm component of the amnion and the heart tube. Homologous disruption of the Mesp1 gene results in cardia bifida and embryonic lethality at E9.5, due to delayed migration of Mesp1-lineage cells from the primitive streak to the heart field [4, 5]. The expression of Mesp1 in the developing embryo is highly transitory, starting at the onset of gastrulation (E6.25) and almost completely disappearing before the appearance of cardiac crescent (E7.5). Therefore, Mesp1 is not required for later stages of cardiac development.
Mouse Mesp1 gene is located on chromosome 7, back-to-back to the gene of its closest cousin, Mesp2. Both Mesp1 and Mesp2 are expressed during gastrulation and together play essential roles in somitogenesis [13–16]. Mesp2 compensates for the function of Mesp1 when Mesp1 is knocked out [3, 5]. Ablation of both Mesp1 and Mesp2 leads to the absence of most posterior structures (heart, somites and gut) and lethality at E9.5 [3]. In chimeric embryos, Mesp1/Mesp2 double-mutant cells rarely contribute to the anterior-cephalic and heart mesoderm, but they participate in the formation of the somites and gut. Thus, their function in cranial-cardiac mesoderm is intrinsic and cell-autonomous, while their roles in other posterior structures are secondary and non-autonomous. Mesp1 and Mesp2 proteins share almost identical bHLH motifs, and their functions may be indistinguishable, as knocking one gene into the other’s locus rescues the otherwise knockout phenotypes [17].
The early mouse genetic analyses of Mesp1 were performed by Saga et al. Their work laid a solid foundation for our current knowledge of Mesp1, and their Mesp1Cre (Cre recombinase knocked into the Mesp1 genomic locus) mouse strain has been widely used in subsequent investigations to track the Mesp1 lineage.
Mesp1 orthologs
Since Yumiko Saga’s pioneering work in mouse, orthologs of Mesp1 have been uncovered in several other species. Like the Mesp pair in mouse, two types of Mesp often co-exist: Meso-1 and Meso-2 in chicken [18, 19]; mespa and mespb in xenopus laevis [20, 21]; and mespaa/ab (mesp-a) and mespba/mespbb (mesp-b) in zebrafish [22–24]. There is only one Mesp in chordate ascidians (for instance, Cs-Mesp in Ciona savignyi) [25]. Chicken Meso-1 and Meso-2 have comparable expression patterns to their mouse counterparts, respectively, but both Meso-1 and Meso-2 are mainly involved in somitogenesis [18, 19]. In zebrafish, overexpression of mespaa promotes cardiac fates in the animal cap, a region that is normally non-cardiogenic. Unlike murine Mesp1, mespaa is dispensable for cardiac development [22]. In xenopus, mespa induces the animal cap to express both cardiac transcription factors and structural genes. Loss of mespa results in impaired cardiac gene expression. However, mespa showed a punctate expression pattern, while potential downstream targets, isl1 and nkx2-5, appeared more condensed. This suggests that either mespa does not regulate these TFs directly, or through cell non-autonomous mechanisms [20].
The invertebrate chordate, Ciona intestinalis, represents an ideal model for studying early cardiogenesis. Early embryos of Ciona intestinalis are extremely simple. Gastrulation starts at the 110 cell stage with two blastomeres (B7.5) expressing Ci-Mesp [26, 27]. Mesp drives expression of Ets1/2 in all descendants of the B7.5 blastomeres. FGF signaling activates Ets1/2 in the smaller rostral daughters, leading to the expression of FoxF and the specification of cardiac fate, while the larger caudal daughters form the anterior tail muscles [28, 29]. Collectively, mesp orthologs in lower species conserve the task distribution between the two mesps: one for cardiac development and the other for somitogenesis. Mesp1 orthologs act early, before or at the beginning of gastrulation, regulating prerequisite steps of cardiac specification.
Mesp1 in cardiomyocyte reprogramming
Because human myocardium does not possess sufficient regenerating power to repair damages, generating cardiomyocytes from other cell types poses a promising strategy in cardiovascular medicine. Several transcription factors (Gata4, Mef2c, Tbx5, or GMT plus Hand2) were shown to convert rodent fibroblast into cardiomyocyte in culture dishes [30], and most strikingly, convert cardiac fibroblast into cardiomyocyte in vivo after ischemic injury [31, 32]. Other reprogramming cocktails have since been developed, including a recent 9-small molecule formula [33]. Given the importance of Mesp1 in cardiac development, it is almost always included in reprogramming screenings. In fact, Mesp1 is a popular component of current human cardiomyocyte reprogramming cocktails: Islas et al. used Mesp1 and Ets2 [9], Wada et al. used GMT plus Mesp1 and Myocd [10], Fu et al. used GMT plus Mesp1 and Esrrg [11]. Only one formula (Gata4, Hand2, Tbx5, Myocd, miR-1 and miR-133) does not include Mesp1 [34]. Comparing to the mouse counterpart, the process of human cardiomyocyte reprogramming is longer and the resulting cardiomyocytes are only partially reprogrammed: the best evidence is often the broad transcriptome shift toward cardiomyocyte, but contractile events are rare or absent. The benefits of Mesp1 are not fully understood. In one of the studies, endogenous Mesp1 was activated at a low level in most of the converted cells [11]. However, cardiomyocytes do not express Mesp1. Mesp1 strongly induced ryanodine receptor 2 (Ryr2) expression and spontaneous Ca2+ oscillations [10], but the mechanistic basis has not been established in normal embryonic development. The recent bodies of knowledge about Mesp1, to be discussed in the following sections, may shed light on using this factor in human cardiomyocyte reprogramming and improving efficiency and fidelity.
Tracking the earliest Mesp1-expressing cells at clonal level
Mesp1 expression has been considered the first event of cardiovascular differentiation. Tracking the earliest Mesp1-expressing cells would allow delineation of the temporal order and special organization of cardiac progenitor allocation [35, 36]. Earlier works predict the existence of an early and specific multipotent progenitor for all anatomic and cellular components of the heart [37–40]. Retrospective lineage analysis supports that segments of the heart arise from distinctive precursor pools [39, 41, 42]. Aiming to determine when the early mesodermal cells are specified to the cardiac fate, and when they become destined to an anatomical location, several groups have tracked the Mesp1-lineage at clonal level.
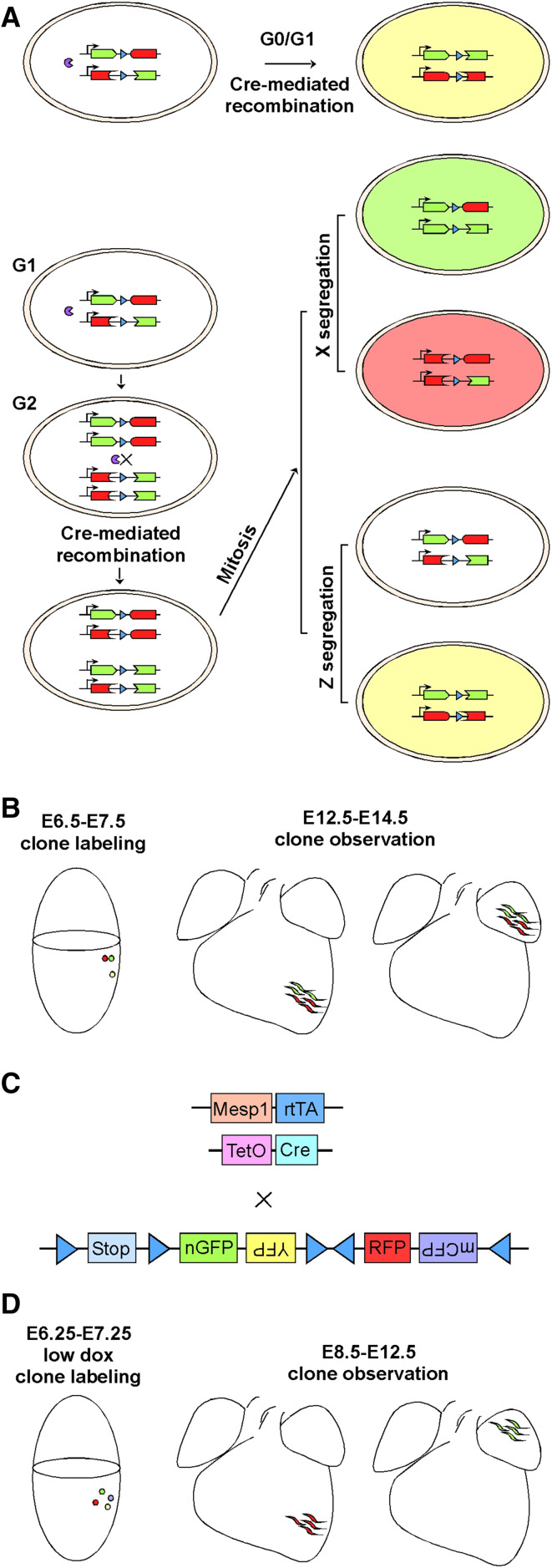
Bruneau’s group performed clonal analyses using the mosaic analysis with double markers (MADM) system, which relies on Cre-mediated recombination to restore fluorescent proteins and a mitotic event to generate “twin” red and green daughter cells (Fig. 1a, b) [43]. Clones labeled by the MADM system become adjacent red and green clusters after clonal expansion and migration [44]. With Mesp1–Cre, cells from a single clone (twin spots) populated discreet anatomic locations including the left ventricle, right ventricle, outflow tract, atria-left ventricle, and interventricular septum, but twin spots never spanned the right and left ventricles. Thus, a common progenitor of the heart is not present in Mesp1+ cells, and subpopulations of Mesp1+ cells have already been pre-fated to different compartments of the future heart. Subsequently, the Mesp1 progeny expresses Smarcd3, prior to other markers of cardiac progenitors (Nkx2-5, Tbx5, Isl1). Expression of Tbx5 and the Mef2AHF enhancer further subdivides the early population into the first and second heart fields (FHF and SHF), respectively [43].
Fig. 1.
Tracking Mesp1-expressing cells at clonal level. a The principle of mosaic analysis with double markers (MADM). Before the expression of Cre recombinase, full-length GFP and tomato fluorescent proteins are not produced. Interchromosomal recombination is induced by Cre, restoring the expression of GFP and tomato. Recombination in G1 or postmitotic G0 cells leads to yellow color (expression of both GFP and tomato). Recombination in G2 cells and the subsequent mitosis leads to two scenarios. In X segregation, the two daughter cells are labeled either red or green. In Z segregation, the two daughter cells are labeled colorless or yellow. b Representative findings of Mesp1–Cre clonal labeling using the MADM system. c The strategy of clonal lineage tracking of Mesp1-expressing cells using the Rosa–Confetti reporter. d Representative findings in Mesp1-rtTA/TetO-Cre/Rosa–Confetti clonal labeling. A low dose of Dox is titrated and used to obtain unicolor embryos at the time of analysis
Clonal analyses of the Mesp1-expressing cells were also performed with a Mesp1-rtTA/TetO-Cre/Rosa–Confetti strategy, in which a low dose of dox is given between E6.25 and E7.25, leading to a single fluorescent color per heart (Fig. 1c, d) [45]. Out of 27 unicolor hearts analyzed at E12.5, no unicolor clones spanned both FHF and SHF, supporting absence of a common Mesp1+ progenitor for the two heart fields. Further, dox administration at earlier time points (E6.25 and E6.75) led to preferential labeling of FHF, while at a later time point (E7.25) preferentially labeled SHF. Thus, the FHF and SHF may be specified at two Mesp1 waves. In a given unicolor patch in FHF, the Mesp1-derived clones give rise to either CMs or ECs, suggesting unipotency, whereas clones in SHF are either unipotent (differentiating into CMs or ECs) or bipotent (differentiating into CMs and ECs, or CMs and SMCs) [45].
These delicate works strongly argue that mesoderm is rapidly specified at or before E6.0–7.5 into discrete fates with anticipated anatomical localization; also, if a common progenitor for the two heart fields does exist, it has to be at the pre-gastrulation stage, and not Mesp1+. However, it is not answered if the cells are prepatterned or gradually restricted to sublineages by signaling cues during gastrulation. Technically, it is not known whether the Cre recombination causes a delay [46], which would result in missed labeling of the earliest wave of Mesp1-expressing cells. Despite possible limitations, clonal labeling of Mesp1+ cells brings specification of the two heart fields to much earlier stages than previously recognized, which is important for our understanding of congenital heart diseases, and provides conceptual guide to cell-based heart regeneration.
Broad involvement in the development of mesoderm derivatives
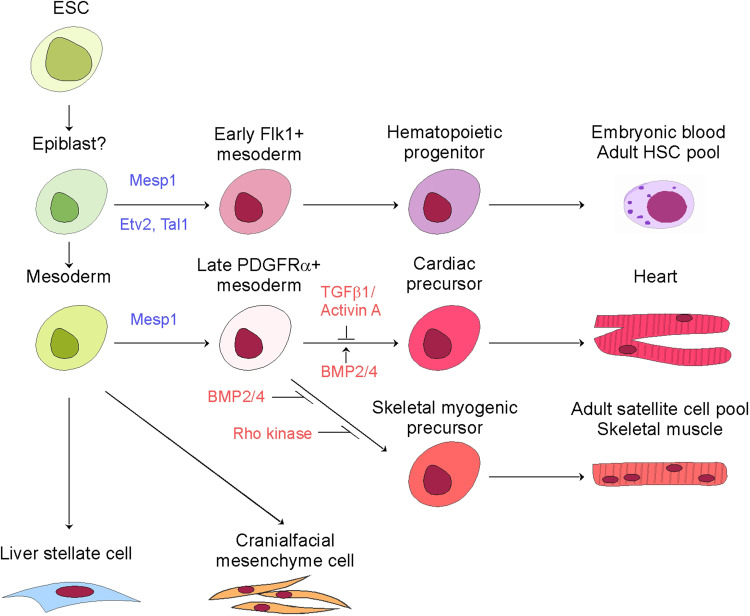
Saga’s pioneering works depicted that Mesp1 not only is expressed in the nascent mesoderm within the primitive streak, but also in the extraembryonic mesoderm. It was postulated at the time that the Mesp1-expressing cells contribute to processes beyond cardiogenesis and somitogenesis [1, 3–5]. This notion has gained support from data in ESC differentiation and detailed lineage tracking. The broad Mesp1-lineage now includes hematopoietic cells, skeletal muscle cells, craniofacial mesenchyme, and liver mesothelium, in addition to cardiomyocytes, smooth muscle cells, and endothelial cells in the cardiovascular system (Fig. 2).
Fig. 2.
Lineage differentiation map of Mesp1-expressing cells
Hematopoietic cells
In contrast to the findings that prolonged ectopic Mesp1 expression in ESCs promoted CMs, SMCs and ECs, but suppressed hematopoiesis [6, 8], Mesp1 lineage tracking repeatedly labeled the extraembryonic mesoderm, including the blood islands [1, 5]. Chan et al. revisited the role of Mesp1 in driving additional mesoderm derivatives in ESCs [47]. Ectopic Mesp1 expression in an early window (day 2–3 since differentiation) initiated hematopoietic differentiation, while in a late time window (day 3–4 since differentiation) triggered cardiovascular lineages. In E9.5 Mesp1Cre/+; R26fl − stop−lacZ/+ embryos, Mesp1+ cells contribute to hematopoiesis in both yolk sacs and the embryo proper. In 6w Mesp1Cre/+; R26fl − stop−lacZ/+ mice, 30% bone marrow hematopoietic stem cells are Mesp1+ derived, and transfusion of which repopulates the hematopoietic compartment of irradiated hosts. It is worth noting that Mesp1 reactivation has not happened in adult hematopoiesis, therefore, the early embryonic Mesp1+ cells contribute to both embryonic and adult hematopoiesis. In a separate study, all hematopoietic cells are labeled by Mesp1–Cre with variable efficiencies among individual animals (10–99%) [48]. The labeling is across all hematopoietic lineages, including hematopoietic stem cells (HSCs), multipotent progenitors, and mature lineages derived from these progenitors.
Tal1 and Etv2 may mediate the function of Mesp1 in hematopoiesis. A Mesp1-binding site is located in a +40 kb downstream enhancer of Tal1. Mesp1 depends on E12 to form a heterodimer and bind to this enhancer, thus activating Tal1 gene expression [47]. Ets variant 2 (Etv2) is an important factor in the differentiation of endothelial cells and hematopoietic lineages [49]. There is a CRE motif in the proximal promoter of Etv2, which mediates the cooperation between Creb1 and Mesp1 in activating Etv2 transcription [50]. Creb1 and Mesp1 directly interact with each other; the responsible domains are bHLH on Mesp1 and the C-terminus bZIP domain on Creb1, respectively. Further, Etv2+ cells at E8.5 and E9.5 are mainly derived from Mesp1+ cells in the embryo as well as the yolk sac. Conditional knockout of Etv2 in the Mesp1-lineage results in vascular and hematopoietic defects and lethality at E9.5, similar to global deletion [50–52].
The sequential activation of Mesp1 in driving the hematopoietic and cardiac lineages is coherent with the notion that mesoderm arises in distinct waves [53–55]. The first wave comprises Flk-1+ cells that give rise to hematopoietic lineages, whereas the second wave comprises PDGFRα+ cells that populate the heart. The differentiation status is likely the source of differential responsiveness to Mesp1. Consistently, single-cell transcriptomes of day 4 embryoid bodies, following a one-day induction of Mesp1, indicate that Mesp1 induces a heterogeneous mesoderm population containing both hematopoietic-primed and cardiac-primed subsets [56]. How the two fates are selected is unclear. A recent report suggests the expression of Tal1 or not determines if the yolk sac cells take a hematopoietic or cardiac fate [57], which has raised an interesting question, how Mesp1 activates Tal1 in some cells but not in others?
Skeletal muscle
In contrast to the early and short windows for activation of the hematopoietic and cardiac programs, extended Mesp1 expression (day 3–8 since differentiation) induced skeletal myogenic progenitors in the absence of serum-derived factors [47]. Induction of Mesp1 in an early time window (day 3–5) in serum-free conditions generates bipotent cardiac/skeletal myogenic progenitors [58]. A Mesp1/PDGFRα double-positive subpopulation resembles the cardiopharyngeal mesoderm, a common progenitor of the cranial muscles and the second heart field [59, 60]. Subsequent cardiogenesis is favored by inhibition of TGFβ1/Activin A signaling and stimulation of BMP2/4 signaling, while skeletal myogenesis is favored by Rho kinase inhibition [58]. Co-labeling of head muscles and SHF was found in 11% of embryos in clonal Mesp1-lineage tracking, supporting the existence of a common cardiopharyngeal mesoderm progenitor, but this was not seen in the MADM clonal analyses [43, 45].
Remarkably, Mesp1+ cells contribute to the satellite cell pool in skeletal muscles with a head muscle bias: Mesp1Cre;RosaYFP clearly labels the masseter and associated satellite cells, while Pax3Cre;RosaYFP labels trunk muscles and associated satellite cells but not craniofacial muscles [61]. The head satellite cells express higher Nkx2.5 than trunk satellite cells; and BMP4 induces higher expression of cardiac genes including Isl1 and Tbx20 in head satellite cells. To this end, Isl1+ and Nkx2.5+ cells contribute to the myocardium and the distal myogenic core of the first branchial arch, and Isl1 holds a nodal point where distinct mesoderm progenitors are diverged. In a separate study, more than 70% of facial and masseter muscle (both are of craniofacial mesoderm origin) satellite cells are derived from Mesp1+ cells. To a much lesser extent, Mesp1+ progenitor cells also generate satellite cells in hindlimb and trunk muscles [47]. Together, Mesp1 induction may be a viable approach in generating craniofacial myogenic precursors, which may be employed to understand muscle diseases specific to these muscles [62].
Craniofacial mesenchyme
The craniofacial structures are either cranial neural crest-derived or cranial mesoderm-derived. Mesp1+ mesoderm cells contribute to the muscles of the face and neck, endothelial cells of the blood vessels, and bones of the neurocranium and the posterior skull base [63]. Twist transcript is expressed in the mesoderm during gastrulation. It is among the first transcripts responsive to Mesp1 induction in ESCs, and mediates epithelial-to-mesenchymal transition [8]. Ablation of Twist by Mesp1–Cre results in loss of mesenchymal cells, and the cells transit toward an epithelial architecture in craniofacial structures. It also leads to deficient extracellular matrix (ECM) formation, with a secondary effect of impaired neural tube closure. Meantime, loss of Twist results in defective bone formation from both mesoderm and neural crest origins [64]. Mesp1-mediated EMT, thus, plays crucial roles in craniofacial morphogenesis through both cell-autonomous and cell non-autonomous mechanisms.
Mesothelium
Mesothelium is the single epithelial layer that covers the surface of visceral organs and body cavities. It secretes lubricating fluids to facilitate movement between organs. It is a major source of myofibroblasts which contribute to organ fibrosis after injury. Mesp1+ mesoderm derives liver mesothelial cells (MCs) but not peritoneal mesothelium [65]. Liver MCs further differentiate into hepatic stellate cells (HSCs) and portal fibroblasts (PFs), which become myofibroblasts after injury [66, 67]. Though there were reports that HSCs are residential stem cells, differentiating into hepatocytes, cholangiocytes and oval cells [68], this is not supported by Mesp1 lineage tracing. In sum, Mesp1+ derived MCs can go through a mesothelial-mesenchymal transition to become myofibroblasts, but cannot go through a mesenchymal-epithelial transition to become hepatocytes. Myofibroblasts participate in fibrogenesis by syntheses of proinflammatory cytokines and extracellular matrices. Selective blocking of MC differentiation may be explored for the suppression of fibrosis and cirrhosis. To this end, it would be interesting to determine if Mesp1+ cells give rise to cardiac myofibroblasts and contribute to fibrosis in post-MI remodeling [69].
Mesp1 sitting in the interface of cell fate specification and progenitor cell migration
Ablation of Mesp1 leads to cardia bifida, a defect in progenitor cell migration [5], which brings up the issue whether Mesp1 plays a more important role in driving the directional migration of progenitor cells than specifying cell fates. Indeed, induction of Mesp1 in ESCs triggers EMT, a prerequisite step in migration [8]. Early Mesp1 GFP+ cells in Mesp1-rtTA/TetO-H2B-GFP embryos show elevated expression of genes related to cell polarity and migration [45]. In hESCs, Mesp1+ cells have elevated expression of ECM protein genes, including several collagens, integrins, laminins, fibronectin, as well as ECM-receptors, including integrin α5 [70, 71]. Transcriptome surveys are highly supportive that Mesp1 activates the gene network responsible for cell migration.
The function of Mesp1 in cell fate specification and cell migration may be mechanistically independent. In Ciona savignyi, Cs-Mesp is essential for both heart specification and progenitor migration [25]. In Ciona intestinalis, Mesp is first expressed in the B7.5 blastomeres, under the control of Tbx6c. When a Mesp–VP16 fusion gene is expressed in B7.5 cells, cell migration is inhibited, but beating cardiac tissues are formed at the site where the tail is normally histolyzed [72]. This portrays inhibition of Mesp as a required step for cell migration. RhoDF is the only Rho GTPase expressed in the trunk ventral cells (cardiac precursor cells), which contribute to the protrusive activity of migrating cells. RhoDF is regulated by the Mesp-Ets1/2-FoxF cascade together with fibroblast growth factor signaling [73]. The simplicity of Ciona cell lineages would continue to offer great opportunities in systematic identification of the signals underlying the early migration of heart progenitor cells. In vertebrates, Mesp1 is only transiently expressed; perhaps the downregulation of Mesp1 is essential for cell migration or cell fate specification.
Regulation over progenitor cell migration appears to be unique for Mesp1, but not Mesp2 [74]. When Mesp1 and Mesp2 are expressed in ESCs at comparable levels, both induce indistinguishable extents of cardiomyocyte and endothelial cell formation. Mesp1, but not Mesp2, induces rapid cell migration in a cell-autonomous fashion. In addition, polarity and directionality of cell migration are promoted only by Mesp1. Mesp1 and Mesp2 induce similar levels of EMT-related genes, including Snail, Slug, Twist1 and Twist2, but Mesp1 induces more Prickle1 and RasGRP3 expression. Prickle1 is a member of the planar cell polarity (PCP) pathway, lack of which causes defective primitive streak formation. Prickle1 and Mesp2 co-expression leads to directional migration, while knockout of Prickle1 abolishes the directional migration of Mesp1-expressing cells. A Mesp1/RasGRP3/ERK axis regulates the speed of cell migration, responding to extracellular cues such as FGFs, VEGFs, and PDGFs [74]. It is intriguing that Mesp1 is endowed with a unique promigratory function, while Mesp1 and Mesp2 are indistinguishable in promoting cardiac progenitor specification and differentiation.
Cell migration involves the interaction between the migrating cells and the environmental changes along the migration path. This interaction is exemplified by two recent studies. Mesp1–Cre-mediated deletion of Msx1 and Msx2 led to defective primordial germ cell (PGC) migration, although PGCs are not known to derive from Mesp1-expressing cells, suggesting that Mesp1-expressing cells guide PGC migration [75]. In another study, ablation of integrin α5β1 in Mesp1-expressing cells resulted in defects related to the abnormal development of the neural crest, including defective remodeling of the aortic arch arteries [76]. Thus, mesodermal integrin α5β1 works through non-cell-autonomous mechanisms to regulate the structures derived from neural crest. Future studies designed to delineate the mechanisms of Mesp1 in promoting cell migration in vivo would have important implications for understanding congenital heart defects and other organ malformations resulted from defective cell migration.
Upstream regulators of Mesp1 transcription
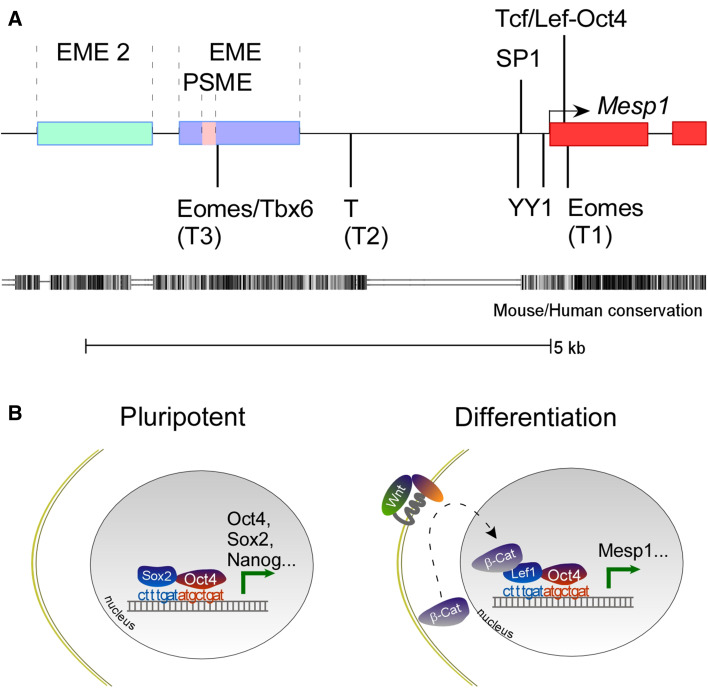
The Mesp1 and Mesp2 genes run back to back off a regulatory intergenic region. Mesp2 knocked into the Mesp1 locus completely rescues the defect in the mesp1-null embryo (stated but unpublished data by Saga), supporting a notion that the cis-regulatory elements play a key role in distinguishing the functions of Mesp1 and Mesp2 [77]. Mesp1 is sequentially activated in the early mesoderm and presomitic mesoderm (PSM). A common enhancer (EME, standing for early mesoderm enhancer) regulates the transcription of both Mesp1 and Mesp2 at early gastrulation stage: deletion of a 6-kb upstream fragment (containing the common enhancer) of the Mesp1 gene resulted in identical phenotypes as in Mesp1/Mesp2 double KO [77]. Using BAC-based reporters, the enhancer responsible for early mesoderm Mesp1 expression was expanded to include an upstream region next to the original EME, and the new region does not affect mesoderm expression of Mesp2 [78]. Within the mesoderm enhancer, a 200-bp conserved region is responsible for PSM expression of mesp1. The region contains a T-box element and an E-box element, and both are essential for PSM expression of Mesp1. The PSM enhancer of Mesp2 is independent from that of Mesp1, located immediately upstream of the Mesp2 coding sequence.
Several transcription factors bind to Mesp1 promoter/enhancer and regulate Mesp1 expression. Yin Yang 1 (YY1) and Specific Protein 1 (SP1) bind the immediate proximal promoter region and synergistically transactivate Mesp1 expression [79]. There are several T-box elements in Mesp1 promoter/enhancer regions which mediate the binding and transactivation by Eomes, brachyury and Tbx6 [80–82]. Our group recently identified an Oct4/Tcf composite binding site immediate downstream of the transcription start site (TSS) [83]. A schematic map of the cis-regulatory regions and elements is summed in Fig. 3. These TFs have ubiquitous or broad expression patterns in mouse embryos, thus, cannot individually account for the unique expression pattern of Mesp1.
Fig. 3.
a Transcription factors and cis-regulatory regions/elements in the Mesp1 gene. b A Tcf/Lef-Oct4 composite site mediates cell fate decisions. It recruits Oct4 and Sox2 to the cis-regulatory regions of pluripotent genes and maintains undifferentiated state. It mediates the converge of Oct4 and canonical Wnt signaling, thus spatially and temporally restricts Mesp1 expression
Work on the regulation over Mesp in Ciona intestinalis constitutes an interesting theory, “gut-muscle origin of the heart” [84]. Tbx6 genes are broadly expressed in B7.5 and muscle precursor cells in Ciona intestinalis. Lhx3 is a beta-catenin target gene expressed in the presumptive endoderm and the B7.5 cells. The only sites where Lhx3 and Tbx6 overlap are the pair of B7.5 cells. Further, Lhx3 and Tbx6b share a composite binding site and both are sufficient and necessary for Mesp expression in B.5 cells [84]. This represents a simple logic in cell differentiation where two activators spatio-temporally overlap to drive a specified fate. Echoing the “gut-muscle origin of the heart” theory, the vertebrate heart may be specified in the mesendoderm. One of the responsible mesendoderm factors may be Eomes, expressed as early as E5.75 in epiblast. Eomes+ cells give rise to definitive endoderm (DE) and the cardiac mesoderm. Eomes−/− embryos are defective in the development of both DE and cardiac lineages [81]. High levels of Activin enhance Eomes-dependent endoderm specification, while inhibiting the cardiac mesoderm [80]. The “mesendoderm origin of heart” theory is consistent with the gene expression signature in early Mesp1+ cells and transactivating targets of Mesp1. Eomes, T, Snail, Gata4, Gata6, Hand1, Meis2, et al., signatures of mesendoderm and EMT, are enriched in Mesp1 GFP+ cells in Mesp1-rtTA/TetO-H2B-GFP embryos following dox administration at E6.25 or E7.25 [45]. High-throughput sequencing of endogenous MESP1-bound DNA revealed that MESP1 activates critical mesendoderm modulators, including Eomes, Gata4, Wnt5a, Wnt5b, Mixl1, T, Gsc and Wnt3 [85].
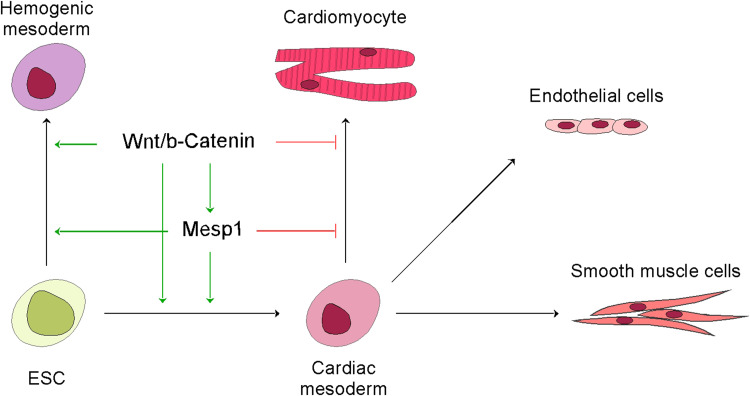
A hypothesis: biphasic requirement of a Wnt/beta-catenin/Mesp1 cascade in cardiomyocyte differentiation
Dynamic regulation over the canonical Wnt pathway is critical for cardiomyocyte differentiation: activation of Wnt is required for the formation of the cardiac mesoderm, and inhibition of it facilitates further differentiation into cardiomyocytes [86–91]. A cell differentiation protocol mimicking this dynamic pattern is widely used in producing cardiomyocytes in human ESCs [92, 93]. The expression of Mesp1 follows a similar biphasic pattern. Only a short window, not prolonged ectopic Mesp1, drives cardiac differentiation in ESCs [6, 47, 94]. In some studies, cardiac gene induction depends on the transient induction of mesp1 and subsequent inhibition of Wnt [8]. We recently tracked the Mesp1+ cells in ESCs, and found while BMP4 drives the expression of Mesp1 and the expansion of the Mesp1+ cells, the cells cannot further differentiate into cardiomyocytes. Subsequent addition of Wnt inhibitors effectively downregulates Mesp1 expression and stops the expansion of Mesp1+ cells, which leads the cells to the cardiomyocyte fate [94]. Strikingly, Mesp–VP16 fusion gene leads to defective cardiac progenitor migration in Ciona intestinalis [72]. These findings are highly suggestive that Mesp1 relays the Wnt signals during cardiomyocyte differentiation.
We recently identified a Tcf/Lef-Oct4 composite binding site in the regulatory region of Mesp1, which provides an explanation to the timely activation and shutdown of Mesp1 transcription [83]. This composite site is located immediately downstream of the TSS. It mediates ternary complex formation between Lef1, Oct4 and the cis-elements. Point mutations on either the Oct4 or the Tcf/lef1 site abolish embryonic Mesp1 expression. According to this model, the diminishing Oct4 signal and the burst of Wnt signal meet in the late epiblast/early mesoderm, another example of spatio-temporal overlap of activators, drives Mesp1 expression. The disappearance of Oct4 and Wnt quickly ensures, shutting down Mesp1 expression. Intriguingly, the Tcf/Lef-Oct4 composite elements also mediate the maintenance of pluripotency by recruiting Sox2 and Oct4 to pluripotent marker genes, suggesting that the interaction between Oct4 and high mobility group (HMG, Tcf/Lef and Sox are closely related within the HMG superfamily) proteins plays important roles in pluripotency vs. differentiation decisions (Fig. 3b) [95–102]. Other mechanisms contributing to the inhibition of Mesp1 include a negative feedback loop by Mesp1 itself [78], induction of Dkk1 by Mesp1 [7], and active degradation of Mesp1 at protein level (RJ Schwartz, personal communication). In sum, sequential activation and inactivation of the Wnt/beta-catenin/Mesp1 cascade are necessary steps in specifying subpopulations in the mesoderm and driving cardiomyocyte differentiation, respectively (Fig. 4). Further work is required to prove this hypothesis, such as genetically extending Mesp1 expression in the presence or absence of canonical Wnt, and examining the differentiation outcome of the Mesp1-lineage.
Fig. 4.
Dynamic regulation upon a Wnt/beta-catenin/Mesp1 cascade in cardiomyocyte differentiation. The cascade of Wnt/beta-catenin/Mesp1 is required in gastrulation, generating the cardiac and hemogenic mesoderm. While the further differentiation of the cardiac mesoderm into ECs and SMCs may be independent from the cascade, active shutdown of the cascade is required for CM formation
Downstream targets of Mesp1
Does Mesp1 directly target the cardiac core transcription network?
In Mesp1-overexpressing ESCs, many genes (Hand2, Gata4, Gata6, Tbx20, Myocardin, Nkx2-5 and Mef2c) of the core cardiac transcriptional machinery acutely responded to Mesp1 induction. Some of these genes recruited the ectopic Mesp1 to their promoters [6]. These and other evidences position Mesp1 to the top of the hierarchy of the cardiovascular transcriptional network. However, this notion has been challenged by recent findings.
Den Hartogh et al. used MESP1mCherry/w-NKX2-5eGFP/w, a double knock-in hESC line in which fluorescent reporters were inserted into the endogenous loci of Mesp1 and Nkx2-5, respectively, to monitor the sequential events in cardiogenesis [71]. Mesp1-mCherry peaks around day 3, while Nkx2-5-eGFP starts to appear around day 7. Such a wide temporal gap makes direct transactivation unlikely. This gap also exists in mouse ESC differentiation models, though narrower [85, 94]. In zebrafish, overexpression of mespaa promotes cardiac fates in the animal cap, but not the margin, which is the site where cardiac progenitors normally arise. There is a relatively long period present between the requirement for mespaa (4–5 hpf) and the induction of gata5 (>10 hpf) [22].
In human and mouse ESCs, Mesp1+ cells bear gene expression signatures of the mesendoderm and EMT. Enrichment of core cardiac transcriptional network is low [70, 71, 85, 94]. In our recent survey of endogenous Mesp1-targeted genomic loci, we identified core mesendoderm genes as Mesp1 targets, but not late TFs like Nkx2-5 or Mef2c [85]. Members of the core cardiac transcriptional network appear in a specific order. Tbx5 is considered the earliest marker of FHF which appears as early as the PM stage [43]. Single-cell sequencing suggests that Tbx5+ cells gradually change to Tbx5+/Nkx2-5+ [103]. Thus, Mesp1 may directly transactivate early cardiac TFs, but not the core cardiac transcriptional network. Instead, it may be required to “poise” mesoderm to a cardiac fate.
How Mesp1 poises the mesoderm cells toward a cardiac fate is unclear. It may act as a pioneer factor, drastically modifying the epigenome in favor of its downstream lineages. In mESCs, we observed a global correlation between Mesp1 binding and the H3K27Ac signature [85]. A subset of Mesp1-lineage cells subsequently activate Smarcd3, a SWI/SNF chromatin remodeling factor that is essential for cardiac development [43]. Chromodomain-helicase-DNA-binding protein 7 (CHD7) is an ATP-dependent chromatin remodeler, spontaneous mutations of which causes the CHARGE syndrome with cardiovascular malformations being the chief anomalies. Ablation of CHD7 in the Mesp1-expressing mesoderm caused cardiovascular structural defects, akin to those seen in CHARGE patients [104]. CHD7 interacts with BMP signaling pathway nuclear mediators, SMAD1/5/8, in activating the enhancers of a critical cardiac transcription factor, Nkx2.5 [105]. The histone acetyltransferase monocytic leukaemia zinc finger protein, MOZ (MYST3/KAT6A), is essential for the expression of Tbx1 and Tbx5, likely through histone acetylation in the respective loci. Ablation of MOZ in Mesp1-expressing cells caused decreased expression of Tbx1 and Tbx5, and high penetrance cardiac anomalies including ventricular septal defects (VSDs) [106, 107]. MOZ could be another epigenetic effector of Mesp1. However, it is not known if Mesp1 directly regulates the aforementioned chromatin remodelers.
Identification of Mesp1-triggered microRNAs
microRNAs (miRNAs) are small non-coding RNAs 18 to 24 nucleotides in length that regulate gene expression. There are a few miRNAs (“myomiRs”) specifically expressed in the heart and skeletal muscles, namely, miR-1, miR-133, miR-206, miR-208 and miR-499. Genetic loss and gain of function studies have revealed critical roles of the miR-1-133 cluster in the mouse heart [108–111]. Deletion of miR1-2 resulted in lethality in the embryos due to a VSD and in the postnatal due to conduction system defects [110]. miR-208 and miR-499 are encoded in the intron of cardiac structure genes [112, 113]. miR-208a deletion resulted in a generally normal phenotype, but it is required for stress-induced cardiac pathophysiological response. The expression of myomiRs is relatively late, so their functions lean toward physiological properties, rather than cell fate determination. The miRNAs associated with early cardiac progenitor cells (CPC), which are often depicted by Mesp1 and Flk1/PDGFRα expression [4, 114], are largely unknown.
We have isolated the Mesp1-lineage of CPCs and captured the enriched miRNAs [115]. As we anticipated, the myomiRs are not enriched in Mesp1+ cells. We have generated a CPC-enriched miRNA list including 140 miRNAs, with an arbitrary cutoff of fold change > 2 and reads >1000. Out of this list, 92 (65.7%) miRNA genes bear both MESP1 and H3K4me3 signatures in their regulatory regions, strongly suggesting that Mesp1 directly targets miRNAs in relaying its lineage-specifying function. Next, we set up a calcium transient-based screening assay for miRNAs that drive cardiomyocyte differentiation in ESCs. Starting from day 5, ESCs transduced with miR-322/503 or miR-17–92 showed multiple (>4) calcium transients after pulse. Other miRNAs which consistently displayed calcium transients (at any time point) include the miR-130b/301b cluster, the miR-23a/24–2/27a cluster, miR-340, -378, -335, -31, -708, -542, -152, and -382. Table 1 summarizes selected CPC-enriched miRNAs, their folds in enrichment, the presence of MESP1-transactivating sites, and activities in the screening assay. These miRNAs represent early regulators of cardiac fate, likely at the stages of cardiac mesoderm formation and cardiac program initiation. Agreeing with this notion, miRNAs that specify mesoderm (let-7, miR-18, miR-302) and/or essential for cardiac development (the miR-17–92 super family) are among the highest enriched [116–118]. Mesp1-lineage-enriched miRNAs represent a valuable resource for dissecting early cell fate and cardiomyocyte differentiation. However, it would be critical to distinguish which miRNAs act at “prerequisite steps”, and which are directly involved in cardiac fate establishment, in the future.
Table 1.
Mesp1-lineage-enriched miRNAs
| MicroRNAs | Fold change | Mesp1-target | Calcium transient |
|---|---|---|---|
| miR-322/503 | 25.0 | Y | ++ |
| miR-17–92 | 4.6–7.7 | Y | ++ |
| miR-130/301 | 7.4–9.6 | Y | + |
| miR-23a–27a–24–2 | 7.2–11.0 | Y | + |
| let-7 family | 3.2–16.0 | Y | N/A |
| miR-340 | 15.89 | Y | + |
| miR-378 | 14.62 | Y | + |
| miR-21 | 14.29 | Y | +/− |
| miR-335 | 13.36 | Y | + |
| miR-31 | 13.23 | N | + |
| miR-708 | 11.98 | Y | + |
| miR-28 | 11.36 | Y | +/− |
| miR-148b | 11.17 | Y | N/A |
| miR-542 | 10.14 | Y | + |
| miR-152 | 9.71 | Y | + |
| miR-126 | 8.38 | Y | − |
| miR-99b | 6.28 | Y | +/− |
| miR-369 | 6.13 | N | +/− |
| miR-382 | 5.44 | N | + |
N/A excluded due to low expression
miR-322 and miR-503 are the most highly enriched miRNAs in Mesp1+ CPCs [115]. They are encoded as a cluster on Xq26.2, a locus closely associated with the imprinting paradigm non-coding RNA, X19 [119]. The “seed” sequences of miR-322 and miR-503 differ by only one nucleotide, suggesting overlapping functions in regulating target mRNAs. miR-322/503 promotes cardiomyocyte differentiation at the cost of neuroectoderm. One of their targets is Celf1, an RNA decay and alternative splicing factor well known for its role in myotonic dystrophy [120, 121]. During embryogenesis, Celf1 and miR-322/503 have mutually exclusive expression patterns. Celf1 is restricted in neuroectoderm-derived tissues, whereas miR-322/503 is expressed in cardiac and skeletal muscle progenitor cells, and persists in striated muscle lineages. The mechanisms of miR-322/503 in striated muscle differentiation exemplify a notion that Mesp1 employs miRNAs to inhibit other cell fate decisions at the same developmental stage. As myomiR-based cardiomyocyte reprogramming has been of low efficiency, it would be interesting to study if the addition of early CPC miRNAs would greatly improve cardiomyocyte reprogramming.
Mesp1 mutations in congenital heart diseases
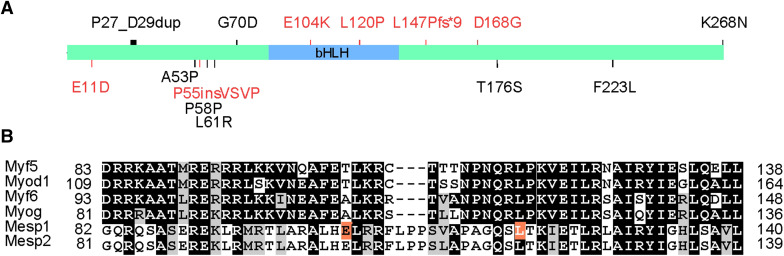
Congenital heart defects (CHD) are the most common type of major birth defects. The etiology of CHD likely includes genetic components and environmental insults. Previously, mutations in TFs of the core cardiac transcription network were linked to CHD predisposition, but Mesp1 mutations had not been recorded until recently (Fig. 5). Lahm et al. analyzed 215 patients with CHDs including mainly septal defects, left heart lesions and right heart lesions [122]. Five highly variable base pair positions (c.157_G>C A53P; c.174_A>C P58P; c.182_T>G L61R; c.669_C>G F223L; c.687_T>G P229P) and a frequent 12-bp insertion after position 55 (c.165_166insGTGCCGAGCCCC P55insVPSP) coding for VPSP are identified. The insertion variant is correlated with further amino acid changes (c.157_G>C A53P, c.182_T>G L61R, c.669_C>G F223L). In functional tests, a c.33_G>C E11D mutation enhanced the transactivating function of MESP1, whereas the insertion variant without an accompanying change of c.182_T>G L61R reduced it. The enhanced activity of the c.33_G>C E11D variant is intriguing, as it may as well be detrimental to cardiac differentiation, as discussed earlier. From 647 patients of congenital conotruncal and related heart diseases (mainly Tetralogy of Fallot and ventricular septal defects), Werner et al. identified seven nonsynonymous variants: p.P27_D29dup (duplication of a.a. 27–a.a. 29), p.G70D, p.E104K, p.L120P, p.L147Pfs*9, p.D168G, and p.K268N [123]. Among the seven nonsynonymous variants, three alter the function of Mesp1: a c.310G>A mutation resulted in p.E104K, a c.359T>C resulted in p.L120P, a c.436_437delAG caused a frameshift and premature termination, p.L147Pfs*9. The mutant proteins have impaired transactivation activity in a reporter assay, and p.E104K, p.L120P, p.L147Pfs*9, and p.D168G have impaired interaction with E47 in a mammalian two-hybrid assay [13, 123]. Additional studies will be required to define the range of Mesp1-related CHDs, but the works described here provided the first evidence that Mesp1 may contribute to CHDs. Importantly, biochemical and genetic analyses of these variants may lead to better understanding of MESP1 protein structure, stability, and its interacting partners, and how they contribute to the specification of the diverse Mesp1-lineage.
Fig. 5.
Mesp1 mutations identified in congenital heart diseases. a Schematic diagram of MESP1 protein and the mutations identified by Werner et al. (top) and Lahm et al. (below). The mutations with altered functions are indicated in orange. b Alignment of the bHLH domains in several bHLHc TFs. Two residues subjected to mutation in Werner’s findings are indicated in orange
Future perspectives
Like its closest cousins, the bHLH factor Mesp1 plays important roles in early cell fate decisions. Recent evidences have placed the role of Mesp1 to even earlier, at the stage of late epiblast, and even broader, governing cell fate decisions of hematopoietic, skeletal muscle, craniofacial, and mesothelial cells, in addition to cardiovascular cells. Another important advancement is the interface of cell fate decision and migration. Mesp1 not only regulates key cell differentiation processes through cell-autonomous mechanisms, but it also guides progenitor cell migration through cell-cell and cell–extracellular matrix interactions. Further, Mesp1 is dynamically regulated. Extended expression or increased activity may also contribute to developmental anomalies. This notion may have practical significance in cell fate reprogramming. It will be interesting to know if the shutdown of Mesp1 at late stage of reprogramming helps the efficiency of iCM generation.
An important future direction is investigating if Mesp1 works as a pioneer factor. Though Mesp1 directly regulates a number of core cardiac transcription factors, in many species, there is a temporal gap between Mesp1 expression and the subsequent activation of the putative downstream genes, indicating additional mechanisms. Mesp1 may function as a pioneer factor, attacking “non-permissive” genomic regions, or recruit professional chromatin remodelers to change the epigenetic landscape of its target genes.
An understudied but critical area is the biochemical properties of MESP1 protein, which may benefit from the recent identification of Mesp1 mutations in CHDs. Knowledge of the structure, stability and interacting partners of MESP1 protein may bring in substantial advancement in our understanding of its function in specifying the diverse scope of lineages.
Acknowledgements
I thank Robert J. Schwartz, Shuxing Zhang and M. David Stewart for helpful discussions; Research in my laboratory is supported by a startup fund from University of Houston, multiple American Heart Association grants (11SDG5260033, 16GRNT27760164) and a grant from US Department of Defense (LC140601).
References
- 1.Saga Y, Hata N, Kobayashi S, Magnuson T, Seldin MF, Taketo MM (1996) MesP1: a novel basic helix–loop–helix protein expressed in the nascent mesodermal cells during mouse gastrulation. Development 122 (9):2769–2778 [DOI] [PubMed]
- 2.Skinner MK, Rawls A, Wilson-Rawls J, Roalson EH. Basic helix-loop-helix transcription factor gene family phylogenetics and nomenclature. Differentiation. 2010;80(1):1–8. doi: 10.1016/j.diff.2010.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kitajima S, Takagi A, Inoue T, Saga Y (2000) MesP1 and MesP2 are essential for the development of cardiac mesoderm. Development 127 (15):3215–3226 [DOI] [PubMed]
- 4.Saga Y, Kitajima S, Miyagawa-Tomita S. Mesp1 expression is the earliest sign of cardiovascular development. Trends Cardiovasc Med. 2000;10(8):345–352. doi: 10.1016/S1050-1738(01)00069-X. [DOI] [PubMed] [Google Scholar]
- 5.Saga Y, Miyagawa-Tomita S, Takagi A, Kitajima S, Miyazaki J, Inoue T (1999) MesP1 is expressed in the heart precursor cells and required for the formation of a single heart tube. Development 126 (15):3437–3447 [DOI] [PubMed]
- 6.Bondue A, Lapouge G, Paulissen C, Semeraro C, Iacovino M, Kyba M, Blanpain C. Mesp1 acts as a master regulator of multipotent cardiovascular progenitor specification. Cell Stem Cell. 2008;3(1):69–84. doi: 10.1016/j.stem.2008.06.009. [DOI] [PubMed] [Google Scholar]
- 7.David R, Brenner C, Stieber J, Schwarz F, Brunner S, Vollmer M, Mentele E, Muller-Hocker J, Kitajima S, Lickert H, Rupp R, Franz WM. MesP1 drives vertebrate cardiovascular differentiation through Dkk-1-mediated blockade of Wnt-signalling. Nat Cell Biol. 2008;10(3):338–345. doi: 10.1038/ncb1696. [DOI] [PubMed] [Google Scholar]
- 8.Lindsley RC, Gill JG, Murphy TL, Langer EM, Cai M, Mashayekhi M, Wang W, Niwa N, Nerbonne JM, Kyba M, Murphy KM. Mesp1 coordinately regulates cardiovascular fate restriction and epithelial-mesenchymal transition in differentiating ESCs. Cell Stem Cell. 2008;3(1):55–68. doi: 10.1016/j.stem.2008.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Islas JF, Liu Y, Weng KC, Robertson MJ, Zhang S, Prejusa A, Harger J, Tikhomirova D, Chopra M, Iyer D, Mercola M, Oshima RG, Willerson JT, Potaman VN, Schwartz RJ. Transcription factors ETS2 and MESP1 transdifferentiate human dermal fibroblasts into cardiac progenitors. Proc Natl Acad Sci USA. 2012;109(32):13016–13021. doi: 10.1073/pnas.1120299109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wada R, Muraoka N, Inagawa K, Yamakawa H, Miyamoto K, Sadahiro T, Umei T, Kaneda R, Suzuki T, Kamiya K, Tohyama S, Yuasa S, Kokaji K, Aeba R, Yozu R, Yamagishi H, Kitamura T, Fukuda K, Ieda M. Induction of human cardiomyocyte-like cells from fibroblasts by defined factors. Proc Natl Acad Sci USA. 2013;110(31):12667–12672. doi: 10.1073/pnas.1304053110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fu JD, Stone NR, Liu L, Spencer CI, Qian L, Hayashi Y, Delgado-Olguin P, Ding S, Bruneau BG, Srivastava D. Direct Reprogramming of Human Fibroblasts toward a Cardiomyocyte-like State. Stem Cell Rep. 2013;1(3):235–247. doi: 10.1016/j.stemcr.2013.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bondue A, Blanpain C. Mesp1: a key regulator of cardiovascular lineage commitment. Circ Res. 2010;107(12):1414–1427. doi: 10.1161/CIRCRESAHA.110.227058. [DOI] [PubMed] [Google Scholar]
- 13.Saga Y. Segmental border is defined by the key transcription factor Mesp2, by means of the suppression of Notch activity. Dev Dyn. 2007;236(6):1450–1455. doi: 10.1002/dvdy.21143. [DOI] [PubMed] [Google Scholar]
- 14.Morimoto M, Takahashi Y, Endo M, Saga Y. The Mesp2 transcription factor establishes segmental borders by suppressing Notch activity. Nature. 2005;435(7040):354–359. doi: 10.1038/nature03591. [DOI] [PubMed] [Google Scholar]
- 15.Takahashi Y, Koizumi K, Takagi A, Kitajima S, Inoue T, Koseki H, Saga Y. Mesp2 initiates somite segmentation through the Notch signalling pathway. Nat Genet. 2000;25(4):390–396. doi: 10.1038/78062. [DOI] [PubMed] [Google Scholar]
- 16.Saga Y, Hata N, Koseki H, Taketo MM. Mesp2: a novel mouse gene expressed in the presegmented mesoderm and essential for segmentation initiation. Genes Dev. 1997;11(14):1827–1839. doi: 10.1101/gad.11.14.1827. [DOI] [PubMed] [Google Scholar]
- 17.Saga Y. Genetic rescue of segmentation defect in MesP2-deficient mice by MesP1 gene replacement. Mech Dev. 1998;75(1–2):53–66. doi: 10.1016/S0925-4773(98)00077-X. [DOI] [PubMed] [Google Scholar]
- 18.Buchberger A, Bonneick S, Klein C, Arnold HH. Dynamic expression of chicken cMeso2 in segmental plate and somites. Dev Dyn. 2002;223(1):108–118. doi: 10.1002/dvdy.1240. [DOI] [PubMed] [Google Scholar]
- 19.Buchberger A, Seidl K, Klein C, Eberhardt H, Arnold HH. cMeso-1, a novel bHLH transcription factor, is involved in somite formation in chicken embryos. Dev Biol. 1998;199(2):201–215. doi: 10.1006/dbio.1998.8919. [DOI] [PubMed] [Google Scholar]
- 20.Kriegmair MC, Frenz S, Dusl M, Franz WM, David R, Rupp RA. Cardiac differentiation in Xenopus is initiated by mespa. Cardiovasc Res. 2013;97(3):454–463. doi: 10.1093/cvr/cvs354. [DOI] [PubMed] [Google Scholar]
- 21.Hitachi K, Kondow A, Danno H, Nishimura Y, Okabayashi K, Asashima M. Molecular analyses of Xenopus laevis Mesp-related genes. Integr Zool. 2009;4(4):387–394. doi: 10.1111/j.1749-4877.2009.00110.x. [DOI] [PubMed] [Google Scholar]
- 22.Deshwar AR, Onderisin JC, Aleksandrova A, Yuan X, Burrows JT, Scott IC. Mespaa can potently induce cardiac fates in zebrafish. Dev Biol. 2016;418(1):17–27. doi: 10.1016/j.ydbio.2016.08.022. [DOI] [PubMed] [Google Scholar]
- 23.Cutty SJ, Fior R, Henriques PM, Saude L, Wardle FC. Identification and expression analysis of two novel members of the Mesp family in zebrafish. Int J Dev Biol. 2012;56(4):285–294. doi: 10.1387/ijdb.113447sc. [DOI] [PubMed] [Google Scholar]
- 24.Sawada A, Fritz A, Jiang YJ, Yamamoto A, Yamasu K, Kuroiwa A, Saga Y, Takeda H. Zebrafish Mesp family genes, mesp-a and mesp-b are segmentally expressed in the presomitic mesoderm, and Mesp-b confers the anterior identity to the developing somites. Development. 2000;127(8):1691–1702. doi: 10.1242/dev.127.8.1691. [DOI] [PubMed] [Google Scholar]
- 25.Satou Y, Imai KS, Satoh N. The ascidian Mesp gene specifies heart precursor cells. Development. 2004;131(11):2533–2541. doi: 10.1242/dev.01145. [DOI] [PubMed] [Google Scholar]
- 26.Hirano T, Nishida H. Developmental fates of larval tissues after metamorphosis in ascidian Halocynthia roretzi. I. Origin of mesodermal tissues of the juvenile. Dev Biol. 1997;192(2):199–210. doi: 10.1006/dbio.1997.8772. [DOI] [PubMed] [Google Scholar]
- 27.Davidson B, Levine M. Evolutionary origins of the vertebrate heart: specification of the cardiac lineage in Ciona intestinalis . Proc Natl Acad Sci USA. 2003;100(20):11469–11473. doi: 10.1073/pnas.1634991100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Beh J, Shi W, Levine M, Davidson B, Christiaen L. FoxF is essential for FGF-induced migration of heart progenitor cells in the ascidian Ciona intestinalis . Development. 2007;134(18):3297–3305. doi: 10.1242/dev.010140. [DOI] [PubMed] [Google Scholar]
- 29.Davidson B, Shi W, Beh J, Christiaen L, Levine M. FGF signaling delineates the cardiac progenitor field in the simple chordate, Ciona intestinalis . Genes Dev. 2006;20(19):2728–2738. doi: 10.1101/gad.1467706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ieda M, Fu JD, Delgado-Olguin P, Vedantham V, Hayashi Y, Bruneau BG, Srivastava D. Direct reprogramming of fibroblasts into functional cardiomyocytes by defined factors. Cell. 2010;142(3):375–386. doi: 10.1016/j.cell.2010.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Qian L, Huang Y, Spencer CI, Foley A, Vedantham V, Liu L, Conway SJ, Fu JD, Srivastava D. In vivo reprogramming of murine cardiac fibroblasts into induced cardiomyocytes. Nature. 2012;485(7400):593–598. doi: 10.1038/nature11044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Song K, Nam YJ, Luo X, Qi X, Tan W, Huang GN, Acharya A, Smith CL, Tallquist MD, Neilson EG, Hill JA, Bassel-Duby R, Olson EN. Heart repair by reprogramming non-myocytes with cardiac transcription factors. Nature. 2012;485(7400):599–604. doi: 10.1038/nature11139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cao N, Huang Y, Zheng J, Spencer CI, Zhang Y, Fu JD, Nie B, Xie M, Zhang M, Wang H, Ma T, Xu T, Shi G, Srivastava D, Ding S. Conversion of human fibroblasts into functional cardiomyocytes by small molecules. Science. 2016;352(6290):1216–1220. doi: 10.1126/science.aaf1502. [DOI] [PubMed] [Google Scholar]
- 34.Nam YJ, Song K, Luo X, Daniel E, Lambeth K, West K, Hill JA, DiMaio JM, Baker LA, Bassel-Duby R, Olson EN. Reprogramming of human fibroblasts toward a cardiac fate. Proc Natl Acad Sci USA. 2013;110(14):5588–5593. doi: 10.1073/pnas.1301019110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Evans SM, Yelon D, Conlon FL, Kirby ML. Myocardial lineage development. Circ Res. 2010;107(12):1428–1444. doi: 10.1161/CIRCRESAHA.110.227405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bruneau BG. Signaling and transcriptional networks in heart development and regeneration. Cold Spring Harb Perspect Biol. 2013;5(3):a008292. doi: 10.1101/cshperspect.a008292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Parameswaran M, Tam PP. Regionalisation of cell fate and morphogenetic movement of the mesoderm during mouse gastrulation. Dev Genet. 1995;17(1):16–28. doi: 10.1002/dvg.1020170104. [DOI] [PubMed] [Google Scholar]
- 38.Tam PP, Parameswaran M, Kinder SJ, Weinberger RP. The allocation of epiblast cells to the embryonic heart and other mesodermal lineages: the role of ingression and tissue movement during gastrulation. Development. 1997;124(9):1631–1642. doi: 10.1242/dev.124.9.1631. [DOI] [PubMed] [Google Scholar]
- 39.Meilhac SM, Esner M, Kelly RG, Nicolas JF, Buckingham ME. The clonal origin of myocardial cells in different regions of the embryonic mouse heart. Dev Cell. 2004;6(5):685–698. doi: 10.1016/S1534-5807(04)00133-9. [DOI] [PubMed] [Google Scholar]
- 40.Kinder SJ, Loebel DA, Tam PP. Allocation and early differentiation of cardiovascular progenitors in the mouse embryo. Trends Cardiovasc Med. 2001;11(5):177–184. doi: 10.1016/S1050-1738(01)00091-3. [DOI] [PubMed] [Google Scholar]
- 41.Meilhac SM, Kelly RG, Rocancourt D, Eloy-Trinquet S, Nicolas JF, Buckingham ME (2003) A retrospective clonal analysis of the myocardium reveals two phases of clonal growth in the developing mouse heart. Development 130(16):3877–3889 [DOI] [PubMed]
- 42.Buckingham M, Meilhac S, Zaffran S. Building the mammalian heart from two sources of myocardial cells. Nat Rev Genet. 2005;6(11):826–835. doi: 10.1038/nrg1710. [DOI] [PubMed] [Google Scholar]
- 43.Devine WP, Wythe JD, George M, Koshiba-Takeuchi K, Bruneau BG (2014) Early patterning and specification of cardiac progenitors in gastrulating mesoderm. Elife:3 [DOI] [PMC free article] [PubMed]
- 44.Zong H, Espinosa JS, Su HH, Muzumdar MD, Luo L. Mosaic analysis with double markers in mice. Cell. 2005;121(3):479–492. doi: 10.1016/j.cell.2005.02.012. [DOI] [PubMed] [Google Scholar]
- 45.Lescroart F, Chabab S, Lin X, Rulands S, Paulissen C, Rodolosse A, Auer H, Achouri Y, Dubois C, Bondue A, Simons BD, Blanpain C. Early lineage restriction in temporally distinct populations of Mesp1 progenitors during mammalian heart development. Nat Cell Biol. 2014;16(9):829–840. doi: 10.1038/ncb3024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Nagy A. Cre recombinase: the universal reagent for genome tailoring. Genesis. 2000;26(2):99–109. doi: 10.1002/(SICI)1526-968X(200002)26:2<99::AID-GENE1>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- 47.Chan SS, Shi X, Toyama A, Arpke RW, Dandapat A, Iacovino M, Kang J, Le G, Hagen HR, Garry DJ, Kyba M. Mesp1 patterns mesoderm into cardiac, hematopoietic, or skeletal myogenic progenitors in a context-dependent manner. Cell Stem Cell. 2013;12(5):587–601. doi: 10.1016/j.stem.2013.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Cai M, Langer EM, Gill JG, Satpathy AT, Albring JC, Kc W, Murphy TL, Murphy KM. Dual actions of Meis1 inhibit erythroid progenitor development and sustain general hematopoietic cell proliferation. Blood. 2012;120(2):335–346. doi: 10.1182/blood-2012-01-403139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lammerts van Bueren K, Black BL. Regulation of endothelial and hematopoietic development by the ETS transcription factor Etv2. Curr Opin Hematol. 2012;19(3):199–205. doi: 10.1097/MOH.0b013e3283523e07. [DOI] [PubMed] [Google Scholar]
- 50.Shi X, Zirbes KM, Rasmussen TL, Ferdous A, Garry MG, Koyano-Nakagawa N, Garry DJ. The transcription factor Mesp1 interacts with cAMP-responsive element binding protein 1 (Creb1) and coactivates Ets variant 2 (Etv2) gene expression. J Biol Chem. 2015;290(15):9614–9625. doi: 10.1074/jbc.M114.614628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ferdous A, Caprioli A, Iacovino M, Martin CM, Morris J, Richardson JA, Latif S, Hammer RE, Harvey RP, Olson EN, Kyba M, Garry DJ. Nkx2-5 transactivates the Ets-related protein 71 gene and specifies an endothelial/endocardial fate in the developing embryo. Proc Natl Acad Sci USA. 2009;106(3):814–819. doi: 10.1073/pnas.0807583106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lee D, Park C, Lee H, Lugus JJ, Kim SH, Arentson E, Chung YS, Gomez G, Kyba M, Lin S, Janknecht R, Lim DS, Choi K. ER71 acts downstream of BMP, Notch, and Wnt signaling in blood and vessel progenitor specification. Cell Stem Cell. 2008;2(5):497–507. doi: 10.1016/j.stem.2008.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kattman SJ, Huber TL, Keller GM. Multipotent flk-1 + cardiovascular progenitor cells give rise to the cardiomyocyte, endothelial, and vascular smooth muscle lineages. Dev Cell. 2006;11(5):723–732. doi: 10.1016/j.devcel.2006.10.002. [DOI] [PubMed] [Google Scholar]
- 54.Kouskoff V, Lacaud G, Schwantz S, Fehling HJ, Keller G. Sequential development of hematopoietic and cardiac mesoderm during embryonic stem cell differentiation. Proc Natl Acad Sci USA. 2005;102(37):13170–13175. doi: 10.1073/pnas.0501672102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Irion S, Clarke RL, Luche H, Kim I, Morrison SJ, Fehling HJ, Keller GM. Temporal specification of blood progenitors from mouse embryonic stem cells and induced pluripotent stem cells. Development. 2010;137(17):2829–2839. doi: 10.1242/dev.042119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Chan SS, Chan HH, Kyba M. Heterogeneity of Mesp1+ mesoderm revealed by single-cell RNA-sEq. Biochem Biophys Res Commun. 2016;474(3):469–475. doi: 10.1016/j.bbrc.2016.04.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Van Handel B, Montel-Hagen A, Sasidharan R, Nakano H, Ferrari R, Boogerd CJ, Schredelseker J, Wang Y, Hunter S, Org T, Zhou J, Li X, Pellegrini M, Chen JN, Orkin SH, Kurdistani SK, Evans SM, Nakano A, Mikkola HK. Scl represses cardiomyogenesis in prospective hemogenic endothelium and endocardium. Cell. 2012;150(3):590–605. doi: 10.1016/j.cell.2012.06.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Chan SS, Hagen HR, Swanson SA, Stewart R, Boll KA, Aho J, Thomson JA, Kyba M. Development of bipotent cardiac/skeletal myogenic progenitors from MESP1+ mesoderm. Stem Cell Rep. 2016;6(1):26–34. doi: 10.1016/j.stemcr.2015.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lescroart F, Kelly RG, Le Garrec JF, Nicolas JF, Meilhac SM, Buckingham M (2010) Clonal analysis reveals common lineage relationships between head muscles and second heart field derivatives in the mouse embryo. Development 137(19):3269–3279 [DOI] [PubMed]
- 60.Tirosh-Finkel L, Elhanany H, Rinon A, Tzahor E. Mesoderm progenitor cells of common origin contribute to the head musculature and the cardiac outflow tract. Development. 2006;133(10):1943–1953. doi: 10.1242/dev.02365. [DOI] [PubMed] [Google Scholar]
- 61.Harel I, Nathan E, Tirosh-Finkel L, Zigdon H, Guimaraes-Camboa N, Evans SM, Tzahor E. Distinct origins and genetic programs of head muscle satellite cells. Dev Cell. 2009;16(6):822–832. doi: 10.1016/j.devcel.2009.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Emery AE. The muscular dystrophies. Lancet. 2002;359(9307):687–695. doi: 10.1016/S0140-6736(02)07815-7. [DOI] [PubMed] [Google Scholar]
- 63.Yoshida T, Vivatbutsiri P, Morriss-Kay G, Saga Y, Iseki S. Cell lineage in mammalian craniofacial mesenchyme. Mech Dev. 2008;125(9–10):797–808. doi: 10.1016/j.mod.2008.06.007. [DOI] [PubMed] [Google Scholar]
- 64.Bildsoe H, Loebel DA, Jones VJ, Hor AC, Braithwaite AW, Chen YT, Behringer RR, Tam PP. The mesenchymal architecture of the cranial mesoderm of mouse embryos is disrupted by the loss of Twist1 function. Dev Biol. 2013;374(2):295–307. doi: 10.1016/j.ydbio.2012.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Lua I, Li Y, Pappoe LS, Asahina K. Myofibroblastic conversion and regeneration of mesothelial cells in peritoneal and liver fibrosis. Am J Pathol. 2015;185(12):3258–3273. doi: 10.1016/j.ajpath.2015.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Li Y, Wang J, Asahina K. Mesothelial cells give rise to hepatic stellate cells and myofibroblasts via mesothelial-mesenchymal transition in liver injury. Proc Natl Acad Sci USA. 2013;110(6):2324–2329. doi: 10.1073/pnas.1214136110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Lua I, James D, Wang J, Wang KS, Asahina K. Mesodermal mesenchymal cells give rise to myofibroblasts, but not epithelial cells, in mouse liver injury. Hepatology. 2014;60(1):311–322. doi: 10.1002/hep.27035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kordes C, Sawitza I, Muller-Marbach A, Ale-Agha N, Keitel V, Klonowski-Stumpe H, Haussinger D. CD133 + hepatic stellate cells are progenitor cells. Biochem Biophys Res Commun. 2007;352(2):410–417. doi: 10.1016/j.bbrc.2006.11.029. [DOI] [PubMed] [Google Scholar]
- 69.Kanisicak O, Khalil H, Ivey MJ, Karch J, Maliken BD, Correll RN, Brody MJ, SC JL, Aronow BJ, Tallquist MD, Molkentin JD. Genetic lineage tracing defines myofibroblast origin and function in the injured heart. Nat Commun. 2016;7:12260. doi: 10.1038/ncomms12260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.den Hartogh SC, Wolstencroft K, Mummery CL, Passier R. A comprehensive gene expression analysis at sequential stages of in vitro cardiac differentiation from isolated MESP1-expressing-mesoderm progenitors. Scientific reports. 2016;6:19386. doi: 10.1038/srep19386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Den Hartogh SC, Schreurs C, Monshouwer-Kloots JJ, Davis RP, Elliott DA, Mummery CL, Passier R. Dual reporter MESP1 mCherry/w-NKX2-5 eGFP/w hESCs enable studying early human cardiac differentiation. Stem Cells. 2015;33(1):56–67. doi: 10.1002/stem.1842. [DOI] [PubMed] [Google Scholar]
- 72.Davidson B, Shi W, Levine M. Uncoupling heart cell specification and migration in the simple chordate Ciona intestinalis . Development. 2005;132(21):4811–4818. doi: 10.1242/dev.02051. [DOI] [PubMed] [Google Scholar]
- 73.Christiaen L, Davidson B, Kawashima T, Powell W, Nolla H, Vranizan K, Levine M. The transcription/migration interface in heart precursors of Ciona intestinalis . Science. 2008;320(5881):1349–1352. doi: 10.1126/science.1158170. [DOI] [PubMed] [Google Scholar]
- 74.Chiapparo G, Lin X, Lescroart F, Chabab S, Paulissen C, Pitisci L, Bondue A, Blanpain C. Mesp1 controls the speed, polarity, and directionality of cardiovascular progenitor migration. J Cell Biol. 2016;213(4):463–477. doi: 10.1083/jcb.201505082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Sun J, Ting MC, Ishii M, Maxson R. Msx1 and Msx2 function together in the regulation of primordial germ cell migration in the mouse. Dev Biol. 2016;417(1):11–24. doi: 10.1016/j.ydbio.2016.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Liang D, Wang X, Mittal A, Dhiman S, Hou SY, Degenhardt K, Astrof S. Mesodermal expression of integrin alpha5beta1 regulates neural crest development and cardiovascular morphogenesis. Dev Biol. 2014;395(2):232–244. doi: 10.1016/j.ydbio.2014.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Haraguchi S, Kitajima S, Takagi A, Takeda H, Inoue T, Saga Y. Transcriptional regulation of Mesp1 and Mesp2 genes: differential usage of enhancers during development. Mech Dev. 2001;108(1–2):59–69. doi: 10.1016/S0925-4773(01)00478-6. [DOI] [PubMed] [Google Scholar]
- 78.Oginuma M, Hirata T, Saga Y. Identification of presomitic mesoderm (PSM)-specific Mesp1 enhancer and generation of a PSM-specific Mesp1/Mesp2-null mouse using BAC-based rescue technology. Mech Dev. 2008;125(5–6):432–440. doi: 10.1016/j.mod.2008.01.010. [DOI] [PubMed] [Google Scholar]
- 79.Beketaev I, Zhang Y, Weng KC, Rhee S, Yu W, Liu Y, Mager J, Wang J. cis-regulatory control of Mesp1 expression by YY1 and SP1 during mouse embryogenesis. Dev Dyn. 2016;245(3):379–387. doi: 10.1002/dvdy.24349. [DOI] [PubMed] [Google Scholar]
- 80.van den Ameele J, Tiberi L, Bondue A, Paulissen C, Herpoel A, Iacovino M, Kyba M, Blanpain C, Vanderhaeghen P. Eomesodermin induces Mesp1 expression and cardiac differentiation from embryonic stem cells in the absence of Activin. EMBO Rep. 2012;13(4):355–362. doi: 10.1038/embor.2012.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Costello I, Pimeisl IM, Drager S, Bikoff EK, Robertson EJ, Arnold SJ. The T-box transcription factor Eomesodermin acts upstream of Mesp1 to specify cardiac mesoderm during mouse gastrulation. Nat Cell Biol. 2011;13(9):1084–1091. doi: 10.1038/ncb2304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.David R, Jarsch VB, Schwarz F, Nathan P, Gegg M, Lickert H, Franz WM. Induction of MesP1 by Brachyury(T) generates the common multipotent cardiovascular stem cell. Cardiovasc Res. 2011;92(1):115–122. doi: 10.1093/cvr/cvr158. [DOI] [PubMed] [Google Scholar]
- 83.Li Y, Yu W, Cooney AJ, Schwartz RJ, Liu Y. Brief report: Oct4 and canonical Wnt signaling regulate the cardiac lineage factor Mesp1 through a Tcf/Lef-Oct4 composite element. Stem Cells. 2013;31(6):1213–1217. doi: 10.1002/stem.1362. [DOI] [PubMed] [Google Scholar]
- 84.Christiaen L, Stolfi A, Davidson B, Levine M. Spatio-temporal intersection of Lhx3 and Tbx6 defines the cardiac field through synergistic activation of Mesp. Dev Biol. 2009;328(2):552–560. doi: 10.1016/j.ydbio.2009.01.033. [DOI] [PubMed] [Google Scholar]
- 85.Soibam B, Benham A, Kim J, Weng KC, Yang L, Xu X, Robertson M, Azares A, Cooney AJ, Schwartz RJ, Liu Y. Genome-wide identification of MESP1 targets demonstrates primary regulation over mesendoderm gene activity. Stem Cells. 2015;33(11):3254–3265. doi: 10.1002/stem.2111. [DOI] [PubMed] [Google Scholar]
- 86.Ai D, Fu X, Wang J, Lu MF, Chen L, Baldini A, Klein WH, Martin JF. Canonical Wnt signaling functions in second heart field to promote right ventricular growth. Proc Natl Acad Sci USA. 2007;104(22):9319–9324. doi: 10.1073/pnas.0701212104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Cohen ED, Wang Z, Lepore JJ, Lu MM, Taketo MM, Epstein DJ, Morrisey EE. Wnt/beta-catenin signaling promotes expansion of Isl-1-positive cardiac progenitor cells through regulation of FGF signaling. J Clin Invest. 2007;117(7):1794–1804. doi: 10.1172/JCI31731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Nakamura T, Sano M, Songyang Z, Schneider MD. A Wnt- and beta -catenin-dependent pathway for mammalian cardiac myogenesis. Proc Natl Acad Sci USA. 2003;100(10):5834–5839. doi: 10.1073/pnas.0935626100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Lindsley RC, Gill JG, Kyba M, Murphy TL, Murphy KM. Canonical Wnt signaling is required for development of embryonic stem cell-derived mesoderm. Development. 2006;133(19):3787–3796. doi: 10.1242/dev.02551. [DOI] [PubMed] [Google Scholar]
- 90.Schneider VA, Mercola M. Wnt antagonism initiates cardiogenesis in Xenopus laevis . Genes Dev. 2001;15(3):304–315. doi: 10.1101/gad.855601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Foley AC, Mercola M. Heart induction by Wnt antagonists depends on the homeodomain transcription factor Hex. Genes Dev. 2005;19(3):387–396. doi: 10.1101/gad.1279405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Lian X, Hsiao C, Wilson G, Zhu K, Hazeltine LB, Azarin SM, Raval KK, Zhang J, Kamp TJ, Palecek SP. Robust cardiomyocyte differentiation from human pluripotent stem cells via temporal modulation of canonical Wnt signaling. Proc Natl Acad Sci USA. 2012;109(27):E1848–E1857. doi: 10.1073/pnas.1200250109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Lian X, Zhang J, Azarin SM, Zhu K, Hazeltine LB, Bao X, Hsiao C, Kamp TJ, Palecek SP. Directed cardiomyocyte differentiation from human pluripotent stem cells by modulating Wnt/beta-catenin signaling under fully defined conditions. Nat Protoc. 2013;8(1):162–175. doi: 10.1038/nprot.2012.150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Liu Y, Chen L, Diaz AD, Benham A, Xu X, Wijaya CS, Fa’ak F, Luo W, Soibam B, Azares A, Yu W, Lyu Q, Stewart MD, Gunaratne P, Cooney A, McConnell BK, Schwartz RJ. Mesp1 marked cardiac progenitor cells repair infarcted mouse hearts. Sci Rep. 2016;6:31457. doi: 10.1038/srep31457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Loh YH, Wu Q, Chew JL, Vega VB, Zhang W, Chen X, Bourque G, George J, Leong B, Liu J, Wong KY, Sung KW, Lee CW, Zhao XD, Chiu KP, Lipovich L, Kuznetsov VA, Robson P, Stanton LW, Wei CL, Ruan Y, Lim B, Ng HH. The Oct4 and Nanog transcription network regulates pluripotency in mouse embryonic stem cells. Nat Genet. 2006;38(4):431–440. doi: 10.1038/ng1760. [DOI] [PubMed] [Google Scholar]
- 96.Chew JL, Loh YH, Zhang W, Chen X, Tam WL, Yeap LS, Li P, Ang YS, Lim B, Robson P, Ng HH. Reciprocal transcriptional regulation of Pou5f1 and Sox2 via the Oct4/Sox2 complex in embryonic stem cells. Mol Cell Biol. 2005;25(14):6031–6046. doi: 10.1128/MCB.25.14.6031-6046.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Rodda DJ, Chew JL, Lim LH, Loh YH, Wang B, Ng HH, Robson P. Transcriptional regulation of nanog by OCT4 and SOX2. J Biol Chem. 2005;280(26):24731–24737. doi: 10.1074/jbc.M502573200. [DOI] [PubMed] [Google Scholar]
- 98.Ambrosetti DC, Basilico C, Dailey L. Synergistic activation of the fibroblast growth factor 4 enhancer by Sox2 and Oct-3 depends on protein–protein interactions facilitated by a specific spatial arrangement of factor binding sites. Mol Cell Biol. 1997;17(11):6321–6329. doi: 10.1128/MCB.17.11.6321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Dailey L, Basilico C. Coevolution of HMG domains and homeodomains and the generation of transcriptional regulation by Sox/POU complexes. J Cell Physiol. 2001;186(3):315–328. doi: 10.1002/1097-4652(2001)9999:9999<000::AID-JCP1046>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- 100.Nishimoto M, Fukushima A, Okuda A, Muramatsu M. The gene for the embryonic stem cell coactivator UTF1 carries a regulatory element which selectively interacts with a complex composed of Oct-3/4 and Sox-2. Mol Cell Biol. 1999;19(8):5453–5465. doi: 10.1128/MCB.19.8.5453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Tomioka M, Nishimoto M, Miyagi S, Katayanagi T, Fukui N, Niwa H, Muramatsu M, Okuda A. Identification of Sox-2 regulatory region which is under the control of Oct-3/4-Sox-2 complex. Nucleic Acids Res. 2002;30(14):3202–3213. doi: 10.1093/nar/gkf435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Tokuzawa Y, Kaiho E, Maruyama M, Takahashi K, Mitsui K, Maeda M, Niwa H, Yamanaka S. Fbx15 is a novel target of Oct3/4 but is dispensable for embryonic stem cell self-renewal and mouse development. Mol Cell Biol. 2003;23(8):2699–2708. doi: 10.1128/MCB.23.8.2699-2708.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Kokkinopoulos I, Ishida H, Saba R, Ruchaya P, Cabrera C, Struebig M, Barnes M, Terry A, Kaneko M, Shintani Y, Coppen S, Shiratori H, Ameen T, Mein C, Hamada H, Suzuki K, Yashiro K. Single-cell expression profiling reveals a dynamic state of cardiac precursor cells in the early mouse embryo. PLoS One. 2015;10(10):e0140831. doi: 10.1371/journal.pone.0140831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Payne S, Burney MJ, McCue K, Popal N, Davidson SM, Anderson RH, Scambler PJ. A critical role for the chromatin remodeller CHD7 in anterior mesoderm during cardiovascular development. Dev Biol. 2015;405(1):82–95. doi: 10.1016/j.ydbio.2015.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Liu Y, Harmelink C, Peng Y, Chen Y, Wang Q, Jiao K. CHD7 interacts with BMP R-SMADs to epigenetically regulate cardiogenesis in mice. Hum Mol Genet. 2014;23(8):2145–2156. doi: 10.1093/hmg/ddt610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Vanyai HK, Thomas T, Voss AK. Mesodermal expression of Moz is necessary for cardiac septum development. Dev Biol. 2015;403(1):22–29. doi: 10.1016/j.ydbio.2015.04.011. [DOI] [PubMed] [Google Scholar]
- 107.Voss AK, Vanyai HK, Collin C, Dixon MP, McLennan TJ, Sheikh BN, Scambler P, Thomas T. MOZ regulates the Tbx1 locus, and Moz mutation partially phenocopies DiGeorge syndrome. Dev Cell. 2012;23(3):652–663. doi: 10.1016/j.devcel.2012.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Kalsotra A, Singh RK, Gurha P, Ward AJ, Creighton CJ, Cooper TA. The Mef2 transcription network is disrupted in myotonic dystrophy heart tissue, dramatically altering miRNA and mRNA expression. Cell Rep. 2014;6(2):336–345. doi: 10.1016/j.celrep.2013.12.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Liu N, Bezprozvannaya S, Williams AH, Qi X, Richardson JA, Bassel-Duby R, Olson EN. microRNA-133a regulates cardiomyocyte proliferation and suppresses smooth muscle gene expression in the heart. Genes Dev. 2008;22(23):3242–3254. doi: 10.1101/gad.1738708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Zhao Y, Ransom JF, Li A, Vedantham V, von Drehle M, Muth AN, Tsuchihashi T, McManus MT, Schwartz RJ, Srivastava D. Dysregulation of cardiogenesis, cardiac conduction, and cell cycle in mice lacking miRNA-1-2. Cell. 2007;129(2):303–317. doi: 10.1016/j.cell.2007.03.030. [DOI] [PubMed] [Google Scholar]
- 111.Mishima Y, Stahlhut C, Giraldez AJ. miR-1-2 gets to the heart of the matter. Cell. 2007;129(2):247–249. doi: 10.1016/j.cell.2007.04.008. [DOI] [PubMed] [Google Scholar]
- 112.van Rooij E, Quiat D, Johnson BA, Sutherland LB, Qi X, Richardson JA, Kelm RJ, Jr, Olson EN. A family of microRNAs encoded by myosin genes governs myosin expression and muscle performance. Dev Cell. 2009;17(5):662–673. doi: 10.1016/j.devcel.2009.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.van Rooij E, Sutherland LB, Qi X, Richardson JA, Hill J, Olson EN. Control of stress-dependent cardiac growth and gene expression by a microRNA. Science. 2007;316(5824):575–579. doi: 10.1126/science.1139089. [DOI] [PubMed] [Google Scholar]
- 114.Kattman SJ, Witty AD, Gagliardi M, Dubois NC, Niapour M, Hotta A, Ellis J, Keller G. Stage-specific optimization of activin/nodal and BMP signaling promotes cardiac differentiation of mouse and human pluripotent stem cell lines. Cell Stem Cell. 2011;8(2):228–240. doi: 10.1016/j.stem.2010.12.008. [DOI] [PubMed] [Google Scholar]
- 115.Shen X, Soibam B, Benham A, Xu X, Chopra M, Peng X, Yu W, Bao W, Liang R, Azares A, Liu P, Gunaratne PH, Mercola M, Cooney AJ, Schwartz RJ, Liu Y. miR-322/-503 cluster is expressed in the earliest cardiac progenitor cells and drives cardiomyocyte specification. Proc Natl Acad Sci USA. 2016;113(34):9551–9556. doi: 10.1073/pnas.1608256113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Chen J, Huang ZP, Seok HY, Ding J, Kataoka M, Zhang Z, Hu X, Wang G, Lin Z, Wang S, Pu WT, Liao R, Wang DZ. mir-17-92 cluster is required for and sufficient to induce cardiomyocyte proliferation in postnatal and adult hearts. Circ Res. 2013;112(12):1557–1566. doi: 10.1161/CIRCRESAHA.112.300658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Mendell JT. miRiad roles for the miR-17-92 cluster in development and disease. Cell. 2008;133(2):217–222. doi: 10.1016/j.cell.2008.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Ventura A, Young AG, Winslow MM, Lintault L, Meissner A, Erkeland SJ, Newman J, Bronson RT, Crowley D, Stone JR, Jaenisch R, Sharp PA, Jacks T. Targeted deletion reveals essential and overlapping functions of the miR-17 through 92 family of miRNA clusters. Cell. 2008;132(5):875–886. doi: 10.1016/j.cell.2008.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Necsulea A, Soumillon M, Warnefors M, Liechti A, Daish T, Zeller U, Baker JC, Grutzner F, Kaessmann H. The evolution of lncRNA repertoires and expression patterns in tetrapods. Nature. 2014;505(7485):635–640. doi: 10.1038/nature12943. [DOI] [PubMed] [Google Scholar]
- 120.Udd B, Krahe R. The myotonic dystrophies: molecular, clinical, and therapeutic challenges. Lancet Neurol. 2012;11(10):891–905. doi: 10.1016/S1474-4422(12)70204-1. [DOI] [PubMed] [Google Scholar]
- 121.Wang GS, Kearney DL, De Biasi M, Taffet G, Cooper TA. Elevation of RNA-binding protein CUGBP1 is an early event in an inducible heart-specific mouse model of myotonic dystrophy. J Clin Invest. 2007;117(10):2802–2811. doi: 10.1172/JCI32308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Lahm H, Deutsch MA, Dressen M, Doppler S, Werner A, Horer J, Cleuziou J, Schreiber C, Bohm J, Laugwitz KL, Lange R, Krane M. Mutational analysis of the human MESP1 gene in patients with congenital heart disease reveals a highly variable sequence in exon 1. Eur J Med Genet. 2013;56(11):591–598. doi: 10.1016/j.ejmg.2013.09.001. [DOI] [PubMed] [Google Scholar]
- 123.Werner P, Latney B, Deardorff MA, Goldmuntz E. MESP1 mutations in patients with congenital heart defects. Hum Mutat. 2016;37(3):308–314. doi: 10.1002/humu.22947. [DOI] [PMC free article] [PubMed] [Google Scholar]