Abstract
CFTR protein is an ion channel regulated by cAMP-dependent phosphorylation and expressed in many types of epithelial cells. CFTR-mediated chloride and bicarbonate secretion play an important role in the respiratory and gastrointestinal systems. Pharmacological modulators of CFTR represent promising drugs for a variety of diseases. In particular, correctors and potentiators may restore the activity of CFTR in cystic fibrosis patients. Potentiators are also potentially useful to improve mucociliary clearance in patients with chronic obstructive pulmonary disease. On the other hand, CFTR inhibitors may be useful to block fluid and electrolyte loss in secretory diarrhea and slow down the progression of polycystic kidney disease.
Keywords: CFTR, Cystic fibrosis, Chloride channel, Channel blocker
Introduction
CFTR is a plasma membrane protein belonging to the superfamily of ABC transporters [1, 2]. In these proteins, binding/hydrolysis of ATP by specialized structures (nucleotide-binding domains, NBDs) is coupled to conformational changes of transmembrane helices that result in unidirectional active transport of various types of substrates. However, CFTR behaves differently, since conformation changes induced by NBDs control the opening of a pathway that allows bidirectional flow of ions. Indeed, CFTR works as an ion channel permeable to Cl−, bicarbonate, thiocyanate, and other anions [1, 2].
CFTR structure includes: 12 transmembrane helices that form the conductive pathway and probably the gate; two NBDs, NBD1, and NBD2 that face the cytosolic side and dimerize by binding to two molecules of intracellular ATP; a cytosolic region, the R domain, not present in other ABC proteins, that is the site of cAMP-dependent phosphorylation [1, 2]. Intracellular cAMP elevation is the trigger for CFTR activation.
Being localized in the apical membrane of many types of epithelial cells, CFTR represents a major route for anion secretion in the airways, intestine, exocrine pancreas, and liver [3]. Loss of CFTR function is the cause of the multi-organ defects that characterize cystic fibrosis (CF), a genetic disease due to CFTR gene mutations [3]. CF manifestations include: progressive loss of respiratory function, pancreatic insufficiency, biliary cirrhosis, male infertility, and excessive salt loss by sweating [3]. CF pathology is also reproduced in pigs and ferrets with genetic loss of CFTR [4–6]. Interestingly, these animal models have also revealed a CFTR role in non-epithelial cells as in endocrine pancreas and smooth muscle [7, 8]. CFTR also plays an important role in the polycystic kidney disease [9].
CFTR pharmacology has tremendously evolved in the last 15 years with the identification of a large number of small molecules that act as inhibitors, potentiators, and correctors. These molecules are important as tools of research and as possible drugs. In this respect, highly selective inhibitors are required to investigate the role of CFTR in a variety of physiological processes and may represent potential drugs to treat human diseases characterized by upregulation of CFTR function. On the other hand, defective CFTR function is the basis of CF and of non-genetic chronic respiratory diseases. In this case, rescue of CFTR-dependent anion transport may be obtained by potentiators and/or correctors, depending on the type of defect to target. Potentiators increase the time spent by CFTR in the open state, thus resulting particularly effective in correcting the intrinsic channel gating defect caused by class 3 CF mutations. Correctors are instead important to improve the folding and stability of CFTR.
CFTR inhibitors
Low-affinity inhibitors
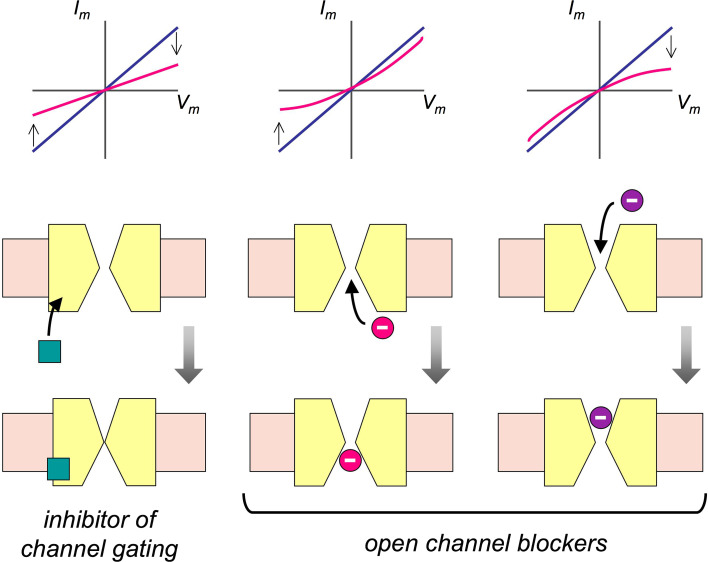
Before the era of discovery of CFTR modulators by high-throughput screening, CFTR was known to be inhibited by a set of organic small molecules, such as DPC, NPPB, niflumic acid, and glibenclamide. All these molecules are characterized by low potency (with IC50 values in the high micromolar range) and a mechanism of CFTR inhibition based on direct block of the channel pore from the intracellular side [10–14]. Since most low-affinity CFTR inhibitors have a negative charge, their mechanism of block is affected by the transmembrane electrical potential [10–14]. In particular, the extent of CFTR inhibition is enhanced and decreased by shifting the membrane potential in the negative and positive directions, respectively (Fig. 1). Additional features associated with a low-affinity mechanism of pore block are: apparent reduction of the mean open time in single-channel recordings (due to appearance of rapid closure events) and dose dependence affected by the transmembrane Cl− concentration gradient [10, 11, 13, 14].
Fig. 1.
Effect of inhibitors on CFTR-dependent currents. Compounds acting as inhibitors of CFTR gating (left) equally reduce CFTR-dependent current at all membrane potentials. The current–voltage (I–V) relationship remains linear even in the presence of the inhibitor (e.g. CFTRinh-172). In contrast, compounds acting as open channel blockers (center and right) interact with the CFTR pore. They can reach the pore from the extracellular side or the intracellular side. If they are electrically charged, their block is affected by membrane potential. For low-affinity CFTR blockers (e.g. NPPB, DPC, and niflumic acid), which are negatively charged and act from the inside, negative membrane potentials make the block stronger, thus changing I–V relationship from linear to outwardly rectifying. For compounds like GlyH-101, which acts from the outside, the effect of membrane potential is opposite and the I–V relationship becomes inwardly rectifying
High-affinity inhibitors
The development of a high-throughput method to screen large chemical libraries was the basis for the identification of novel and potent CFTR inhibitors. The method was based on a halide-sensitive yellow fluorescent protein (HS-YFP) that allows fast and automated determination of CFTR activity [15, 16]. This assay was initially applied to the screening of 50,000 compounds using FRT cells expressing wild-type CFTR [17]. An entirely novel compound, the thiazolidinone CFTRinh-172, was found. Main characteristics of CFTRinh-172 were: IC50 around 300 nM and voltage-independent block despite the presence of a negative charge [17]. The latter characteristic suggested that CFTRinh-172 does not act as open channel blocker, like low-affinity inhibitors, but probably affects the mechanism of CFTR gating. CFTRinh-172 is inactive on Ca2+-activated Cl− channels, volume-sensitive Cl− channels, ATP-sensitive K+ channels, MDR-1 multidrug transporter, and SLC26A4 anion exchanger [17–19]. Because of this quite specific type of activity for CFTR relative to other Cl− channels and transporters, CFTRinh-172 has widely been used as a tool of research in hundreds of studies.
The mechanism of action of CFTRinh-172 was investigated in patch-clamp experiments. In an initial study, the compound appeared to mainly prolong the CFTR closed state [20]. In a second study, CFTRinh-172 was shown to also reduce the duration of the open time [21]. In particular, CFTRinh-172 was demonstrated to be highly effective on CFTR mutants characterized by very long open times [21]. Mutagenesis of CFTR amino-acid residues in the sixth transmembrane helix led to the identification of arginine 347 (R347) as key position [22]. Replacement of R347 with alanine or aspartic acid strongly decreased CFTRinh-172 potency, with IC50 shifting to high micromolar values [22]. Importantly, R347 does not contribute directly to the formation of CFTR pore, but may be important in the mechanism of pore gating.
CFTRinh-172 has a maximum solubility in saline solution of about 20 µM. Solubility may be a limiting factor under some particular experimental conditions and for tissues and animal models in which potency of CFTRinh-172 seems to be lower. To improve solubility, 58 chemical analogs of CFTRinh-172 were later synthesized and tested [23]. This search identified two compounds, tetrazolo-172 and oxo-172, with more than tenfold improved solubility and satisfactory potency (IC50 ~1 µM).
To find additional CFTR inhibitors, a second high-throughput screening was later done on a novel chemical library containing 100,000 compounds [24]. This second campaign led to the identification of the glycine hydrazide GlyH-101. Interestingly, this compound showed voltage-dependent block but in a way opposite to that of low-affinity CFTR inhibitors. Block by GlyH-101 was actually relieved by negative membrane potentials and IC50, therefore, changed from 1.4 to 5.6 µM at +60 and −60 mV, respectively [24]. In the presence of GlyH-101, the CFTR current–voltage relationship changed from linear to inwardly rectifying (Fig. 1). The characteristics of GlyH-101 mechanism of action indicated that the compound enters the CFTR pore from the extracellular side. This conclusion was further supported by results from another study [25]. A 3D model of CFTR was generated using the Sav1866 bacterial transporter as a homologous protein. This model allowed the investigation of putative GlyH-101 sites of action using the ligand-docking techniques. A region in the narrowest part of CFTR pore was identified. Interestingly, mutagenesis of a critical residue (change of phenylalanine 342 to alanine) increased the apparent affinity of the blocker by ~200-fold [25]. Probably, replacement of phenylalanine with alanine removes a predicted steric clash that impedes an optimal interaction between GlyH-101 and its binding site.
The extracellular side of action of GlyH-101 suggested the possibility to develop analogs with minimal membrane permeability [26]. For this purpose, a malonic hydrazide (MalH) derivative of GlyH-101 was linked to polyethylene glycols (PEGs) of various molecular weights. These molecules were, therefore, membrane impermeable but kept the ability to block CFTR in a voltage-dependent manner. Such results indicated that the inhibitory part of the molecule was able to enter the CFTR pore despite the link with a large polymer [26]. Interestingly, divalent molecules (MalH-PEG-MalH), having two inhibitory units, showed a significantly improved potency. The concept of non-absorbable CFTR inhibitors was further tested by conjugating MalH to lectins [27]. This modification led to inhibitors with (nanomolar) potency and able to bind to cell-surface glycocalyx and resist to washout for several hours.
The assay based on the HS-YFP was used to screen a new chemical library containing nearly 110,000 synthetic and natural compounds [28]. The screening identified a new class of CFTR inhibitors, pyrimido-pyrrolo-quinaxolinediones (PPQs). The most interesting compound was PPQ-102, an uncharged molecule which fully inhibited CFTR with an IC50 of 90 nM [28]. As for CFTRinh-172, inhibition by PPQ-102 was voltage-independent and probably due to a modification of CFTR gating. A subsequent study on the structure–activity relationship of PPQs led to an optimized derivative, BPO-27, having very high potency (IC50 ~8 nM) and improved metabolic stability and aqueous solubility [29].
Use of CFTR inhibitors
Inhibitors are important as research tools to understand the CFTR structure–function relationship and physiological role. Regarding the latter point, inhibitors are useful in different types of experiments to demonstrate the involvement of CFTR in a particular physiological process. However, caution needs to be taken when applying inhibitors in long-term experiments. Low-affinity inhibitors may have multiple effects on other types of proteins, such as DPC on cyclooxygenase [30]. GlyH-101 was found to also block Ca2+-activated Cl− channels [18] and to perturb mitochondrial function [31]. Even for more selective inhibitors, such as CFTRinh-172, we cannot rule out that they have activity on other targets, including non-channel proteins. Finally, the stability of the inhibitor during a long duration experiment needs to be evaluated. In this respect, CFTRinh-172 is known to strongly bind to serum proteins and cell-culture plasticware. Therefore, particular conditions need to be taken into account for long-term treatments [32].
Besides the utility as tools of research, CFTR inhibitors have a great potential for the treatment of human diseases. Because of its key role in intestinal electrolyte and fluid secretion, CFTR is an important target for inhibitors to treat secretory diarrhea [33]. Bacterial enterotoxins, such as cholera toxin from Vibrio cholerae and heat-stable enterotoxin from Escherichia coli, cause a large elevation in intracellular cAMP and cGMP levels and hence massive CFTR activation. The resulting loss of water and salts may be life-threatening, particularly in children. Therefore, pharmacological inhibition of CFTR could be beneficial. In this respect, several CFTR inhibitors were effective in blocking intestinal fluid secretion in animal models. CFTRinh-172 was found to strongly block fluid accumulation induced by cholera toxin in a rat intestine closed-loop model [17]. The particular pharmacokinetics of CFTRinh-172, with a low level of distribution in key organs, such as lungs and high concentration in intestine due to enterohepatic recirculation, indicated that oral administration of the compound could result in effective anti-diarrheal activity without causing CF-like symptoms.
As stated previously, the discovery of GlyH-101, which acts from the extracellular side, offered the possibility to generate non-absorbable anti-diarrheal drugs. Such drugs could be administered orally and remain in the intestinal lumen with minimal side effects. Non-absorbable GlyH-101 derivatives, including MalH-PEGs and MalH-lectins, showed anti-diarrheal activity [26, 27]. In particular, conjugation to lectins, allowing binding to the glycocalyx of intestinal epithelial cells, resulted in a higher ability of the inhibitor to resist washout caused by fluid secretion [27].
CFTR inhibitors may also be useful to treat polycystic kidney disease (PKD). This is because renal cyst enlargement in PKD involves CFTR-dependent fluid secretion [9, 34, 35]. Thiazolidinones and glycine hydrazide compounds were found to inhibit cyst growth in an in vitro model based on canine MDCK cells [35, 36]. In particular, tetrazolo-172 and Ph-GlyH-101 strongly blocked cyst growth without altering cell proliferation, thus implying a direct effect on fluid secretion [36]. Such compounds and PPQ compounds were also effective in an embryonic kidney cyst model [28, 29, 36]. In particular, PPQ-102 and BPO-27 were quite potent, with IC50 values in the 100–500 nM range, and even reduced the size of pre-formed cysts.
CFTR potentiators
Druggability of class 3 CFTR mutants
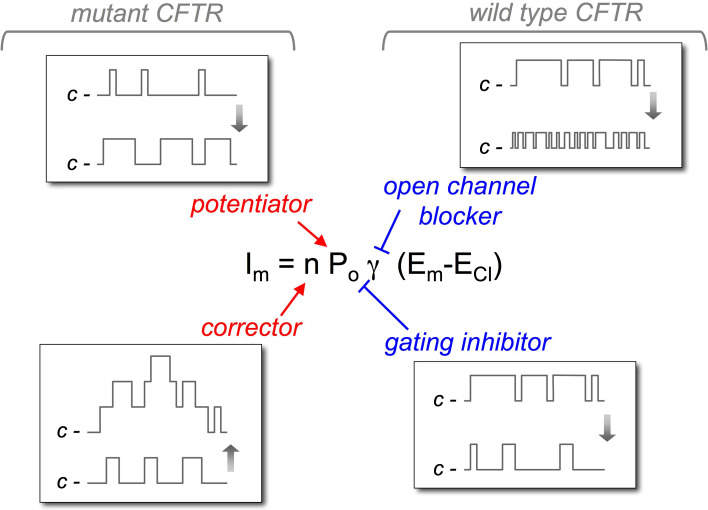
In 2011, ivacaftor, previously known as VX-770 [37], was the first small molecule shown to be effective on CF patients with G551D, a severe mutation that impairs the opening of CFTR channel. The positive results obtained by ivacaftor in clinical trials [38, 39] led to its approval for the treatment of patients with at least one copy of G551D allele. Ivacaftor belongs to the class of potentiators, i.e., small molecules that stimulate CFTR channel activity (Fig. 2). Such molecules are particularly suited for mutations that belong to class 3 like G551D and G1349D [40]. CFTR proteins with such mutations show a channel gating defect, consisting of a very low open channel probability (P o), i.e., the fraction of time spent by the protein in the open/conducting state. The most frequent mutation in CF, F508del, has also a gating defect, but is classified as a class 2 mutation, since it causes a severe impairment in CFTR protein stability and folding [40, 41].
Fig. 2.
Mechanism of action of CFTR modulators. The figure shows the site of action of potentiators, correctors, and inhibitors of CFTR. Potentiators act on open channel probability (P o) favoring the open state vs. the closed state of the channel, as shown by the idealized single-channel traces. Instead, correctors increase n, i.e., the number of CFTR channels in the plasma membrane (the idealized trace shows simultaneous openings of multiple channels). Open channel inhibitors block the pore causing the appearance of fast closure events. Depending on the sampling time and filtering, the effect may appear as a reduction in single-channel conductance (g). Inhibitors of CFTR gating reduce P o. The effect consists of a lengthening of closure events and/or shortening of openings
The first indications that small chemical compounds can restore channel activity in CFTR mutants with gating defect came shortly after the discovery of CFTR gene. Indeed, xanthines like 3-isobutyl-1-methylxanthine (IBMX), flavones (apigenin), and isoflavones (genistein) were found to increase the activity of wild-type CFTR and that of G551D-CFTR [42–46]. The effect of genistein was confirmed in vivo by measuring nasal potential difference on G551D-CF patients [46]. Xanthines were found to increase the activity of CFTR incorporated into planar lipid bilayers, suggesting that these drugs act by a direct interaction with the CFTR protein itself and by a cAMP-independent mechanism [43, 47]. Studies of xanthines and isoflavones effects on CFTR protein bearing mutations in NBDs revealed a functional role of these domains in the activation of CFTR [48, 49].
The benzimidazolone NS004 and NS1619 were also found to increase the activity of CFTR mutants [50]. Interestingly, the study concluded that these compounds act only on phosphorylated CFTR. Therefore, benzimidazolones and other molecules acting similarly, such as genistein, were called “potentiators” and not activators. Another important observation was that the mechanism shared by both benzimidazolones and genistein consists of an increase in P o. Other compounds, including phloxine B and benzo(c)quinoliziniums, were also found to act as potentiators of mutant and wild-type CFTR [51, 52]. The benzo(c)quinolizinium MPB-07 was found to act on CFTR without modifying intracellular cAMP and ATP levels or phosphatase activity, thus suggesting a direct effect of potentiators on CFTR protein.
One of the first studies done with potentiators on primary human airway epithelial cells involved the comparison of three molecules: genistein, CPX, and MPB-07 [53]. This study identified genistein as the most effective potentiator, with a particular efficacy on the G551D mutant. In contrast, CPX and MBP-07 displayed low activity on mutant CFTR. The study also demonstrated that the concentrations of genistein needed to increase efficiently the activity of G551D-CFTR were significantly higher (~200 µM) than those used for wild-type CFTR (~30 µM), thus suggesting that the Gly551 residue is close to the binding site for potentiators. Despite the modest activity shown in vitro, CPX was tested as a CFTR potentiator in a multicenter clinical trial that included 37 subjects homozygous for F508del mutation [54]. The study followed a protocol of increasing doses (1, 3, 10, 30, 100, 300, and 1000 mg) to evaluate safety, pharmacokinetics, and efficacy of CPX. Efficacy was determined using nasal PD and sweat chloride measurements at 1–4 h post-dose. The treatment resulted safe, but there was no apparent effect on either nasal PD or sweat chloride measurements [54, 55]. Two reasons may explain the failure of CPX in vivo. First, as discussed above, CPX is not a very effective potentiator. Second, the folding/stability defect is caused by F508del predominates over the gating defect. Therefore, a significant rescue of F508del-CFTR function cannot be obtained with a potentiator alone.
Development of CFTR potentiators by high-throughput screening
The low potency and/or efficacy of many potentiators like genistein, CPX, MPB-07, and benzimidazolones indicated the need to find molecules with new chemical scaffold and better properties. This goal could be achieved by screening large chemical libraries with a functional assay. For this purpose, a yellow fluorescent protein with high sensitivity to halides was developed [56]. Halide-sensitive yellow fluorescent proteins (HS-YFPs) were used in automated cell-based assays to screen hundreds of thousands of molecules in different rounds. In this way, several chemical families were identified, including 7,8-benzoflavones, isoxazole and isoxazoline heterocyles, trifluoromethylphenylbenzamines, tetrahydrobenzothiophenes, benzofurans, pyramidinetriones, 1,4-dihydropyridines, anthraquinones, phenylglycines, and sulfonamides [57–63]. Patch-clamp experiments demonstrated that the new potentiators identified by high-throughput screening increase P o and improve CFTR gating [61].
Other types of potentiators were found by Vertex pharmaceutical company using a different functional assay based on membrane potential sensitive fluorescent probes. An initial report described VRT-532 as an effective potentiator, although with modest potency [64]. In a subsequent study, the Vertex team described the discovery of a very potent and effective molecule [37]. By screening hundreds of thousands of molecules, one of the hits was improved by rounds of chemical modification and functional evaluation [37, 65]. The final result was VX-770, a potentiator highly effective on F508del and G551D mutants with nanomolar affinity. In patch-clamp experiments, VX-770 increased P o of F508del- and G551D-CFTR. Importantly, VX-770 was also effective on cultured human bronchial epithelial cells with G551D mutation on one allele. Stimulation of cells with VX-770 resulted in a tenfold increase in transepithelial Cl− secretion reaching a value of nearly 50 % of that observed in non-CF cells [37]. Furthermore, VX-770 elicited positive effects on airway surface fluid height and on cilia beating frequency, two parameters that are relevant as surrogate markers of CFTR function in the airways.
The very positive results obtained in vitro rapidly led to clinical trials with VX-770/ivacaftor on G551D patients. In a first phase 2 trial, VX-770 treatment was associated with a significant improvement in lung function after 2–4 weeks treatment [38]. In a subsequent phase 3 clinical trial, involving 84 subjects and lasting 48 weeks, VX-770 elicited significant positive effects on pulmonary function, body weight, and other clinically relevant parameters [39]. As stated at the beginning of this paragraph, the results from clinical trials allowed approval of VX-770/ivacaftor by FDA and EMA for the treatment of G551D patients. Interestingly, VX-770 is also effective on many other CFTR mutants. In a study published in 2014, efficacy of VX-770 was tested on 54 different missense mutations [66]. Ivacaftor potentiated the activity of a variety of mutant CFTR proteins, including those with mild defects in CFTR processing or mild defects in CFTR single-channel conductance. Now, ivacaftor is approved for the treatment of patients with other eight class 3 mutations in addition to G551D. The in vitro and in vivo data indicate that VX-770/ivacaftor is a broad acting molecule, effective on many types of CFTR mutations. The exception is represented by F508del and other (class 2 mutations) with severe folding/stability defects. In these cases, VX-770, as well other potentiators, is only effective if mutant CFTR trafficking to the cell surface is helped by another type of treatment.
Putative mechanism of action
So far, the precise of mechanism of action of potentiators is unclear, but there have been various studies pointing out to a direct effect on CFTR protein. In one of these studies [67], the large number of compounds identified as potentiators and the crystal structure of the murine NBD1 previously determined [68], permitted to test the hypothesis of a common binding site. The apparent dissociation constant (K d) for 18 potentiators was estimated from experiments done on cells expressing with wild-type or mutant (G551D and G1349D) CFTR [67]. A decrease in potency (higher K d) was observed when compounds were tested on mutant CFTR, thus suggesting that the binding site could reside in the regions surrounding G551 and G1349. A model of the NBD1–NBD2 complex was generated in silico by overlaying monomers of a bacterial ATP transporter NBD dimer in the head-to-tail conformation, and binding sites were predicted by molecular docking [67]. Comparison of theoretical-binding free energy in the model with the free energy deduced from the apparent K d yielded a very good correlation coefficient for a site located at the interface between NBD1 and NBD2 [67]. The involvement of this site was successively confirmed by the mutation analysis, indicating a role of residues R553 and V1293 [69]. Most of CFTR potentiators exhibit at least two effects on CFTR channel: an increase in activity at low concentrations and a decrease of activity when the concentrations are further raised. The consequence of this is a bell-shaped dose–response curve. The functional analysis of mutations in the putative-binding site showed that there is an inverse correlation between the activation dissociation constant and the inhibition dissociation constant, indicating that these sites are not independent of each other [69].
More recently, Linsdell and colleagues [70], using patch-clamp analysis, found that extracellular pseudo-halide anions are able to increase CFTR conductance in intact cells, as well as increase anion secretion in airway epithelial cells. This effect appeared to reflect the interaction of these substances with an extracellular site on the CFTR protein by a previously undescribed molecular mechanism. Therefore, the authors suggested that future drugs could utilize this mechanism to increase CFTR activity in CF, possibly in conjunction with known intracellularly acting potentiators.
In biochemical studies, the potentiator VRT-532 affected the ATPase activity of the purified and reconstituted mutant CFTR protein. Authors found that ATP turnover was decreased by VRT-532 treatment, an effect that probably accounts for the increase in channel open time induced by this compound [71]. In another study, a potentiator was directly tested on an NBD1/NBD2 complex [72]. The potentiator reduced the rate of ATP hydrolysis by the NBD1/NBD2 complex. In addition, small-angle X-ray scattering revealed that the potentiator induced a conformational change in the NBD heterodimer. These results led authors to propose that the potentiator-induced conformational changes could modify the NBDs-intracellular loop interactions in a way that would facilitate the open state of the channel [72].
In subsequent studies, Christine Bear’s group found that VX-770 is effective on purified CFTR reconstituted in artificial membranes [73]. This is a strong line of evidence supporting a direct mechanism of action of potentiator(s) on CFTR. Intriguingly, VX-770 also enhanced the channel activity in the nominal absence of Mg-ATP. This finding suggested that VX-770 can induce CFTR channel opening through a non-conventional ATP-independent mechanisms [73]. Using the patch-clamp technique, Jih and Hwang [74] also found that VX-770 enhances spontaneous, ATP-independent activity of WT-CFTR. This finding may explain how VX-770 can be effective on the G551D mutant, which is actually insensitive to ATP. The effect of VX-770 was also analysed on R352C-CFTR, a mutant that allows direct observation of hydrolysis-triggered gating events. The conclusions from this study were that VX-770 promotes decoupling between gating cycle and ATP hydrolysis cycle [74]. Interestingly, these authors also proposed the alternative hypothesis that VX-770 acts on the transmembrane domains of CFTR.
Potentiators may also be useful to treat other, non-genetic, pulmonary diseases [75]. It has been shown that a deficit in CFTR function may exist in chronic obstructive pulmonary diseases characterized by mucus accumulation [76]. Therefore, potentiators could promote CFTR activity, thus improving fluid secretion and mucociliary clearance.
Summarizing, CFTR potentiators are an important class of molecules that are highly effective in restoring mutant CFTR activity. Their mechanism of action is probably based on a direct binding to the CFTR protein, but their precise binding site remains to be clarified.
CFTR correctors
The term “corrector” was coined to define small molecules that are able to increase the amount of CFTR protein on the plasma membrane. This is an effect that is particularly important for those mutations, in particular F508del, that cause a severe defect in CFTR protein folding and stability [41]. Such mutants are trapped in the endoplasmic reticulum and rapidly degraded. A small fraction of the mutant protein can actually reach the plasma membrane, but the lifetime on cell surface is significantly reduced [41]. Therefore, correctors may act in different ways: by directly improving the folding and stability of mutant CFTR, by specific modulation (inhibition or activation) of a protein involved in CFTR protein processing, or by broad modulation of the proteostasis network to create an environment more favorable to mutant CFTR (thus reducing its degradation and improving trafficking).
Several CFTR correctors have been identified in the last years, particularly by high-throughput screening of chemical libraries. The search is frequently done by incubating the cells with test compounds for 24 h and then looking for enhanced function that results from an increase of mutant CFTR in the plasma membrane [77]. Other types of high-throughput assays directly measure the presence of CFTR protein on cell surface instead of function [78].
One of the first reports of CFTR correctors resulted from the screening of 150,000 compounds using the HS-YFP assay [79]. Compounds belonging to four chemical classes were found to rescue F508del-CFTR in cell lines, but only one of them, bisaminomethylbithiazoles, was effective in primary bronchial epithelial cells from CF patients with F508del/F508del genotype. The best compound was corr-4a. This compound increased cAMP-dependent Cl− secretion to nearly 8 % of normal value [79]. The structure–activity relationship of corr-4a analogs was investigated in subsequent studies leading to compounds with improved efficacy and potency [80–82].
The Vertex pharmaceutical company has also used their method of screening to look for correctors. In a first report, two compounds, VRT-325 and VRT-422, with modest corrector activity, were found [64]. In a subsequent paper, the same team described the discovery of a very effective corrector [83]. As for VX-770, the chemical structure of the initial hit identified by high-throughput screening was modified to improve its pharmacological properties. The final result was VX-809, a compound with relatively high efficacy in cell lines and primary bronchial epithelial cells from F508del/F508del patients [83]. In the latter type of cells, VX-809 increased cAMP-dependent Cl− secretion to 14 % of normal function. When the gating defect of F508del was overcome with the VX-770 potentiator, the cAMP-dependent Cl− secretion in cells treated with VX-809 reached 25 % of normal function [83].
Another team of investigators has also screened multiple chemical libraries, in this case with a trafficking assay that utilizes CFTR tagged with an extracellular epitope [78]. The epitope allows the detection of cell-surface F508del-CFTR exposure induced by correctors. With the CFTR trafficking assay, several active compounds have been discovered: sildenafil analogs [84], glafenine [85], and latonduine [86].
CFTR correctors have also been identified by hypothesis-driven projects. In one approach, the histone deacetylase inhibitor SAHA was used because of its broad capacity to change cell transcriptome [87]. The rationale was to modulate the intracellular proteome to create an environment more favorable for mutant CFTR folding and processing. Treatment of cells with SAHA induced a nearly threefold increase in F508del-CFTR function at the cell surface [87]. In other studies, investigators chose to target kinases to modify the proteostasis network. Various inhibitors of receptor tyrosine kinases, RAS/Raf/MEK/ERK or p38, appeared to rescue F508del-CFTR [88]. In another study, roscovitine, an inhibitor of cyclin dependent kinases (CDKs), was also able to correct mutant CFTR [89]. However, the analysis of roscovitine mechanism of action excluded CDK involvement and rather pointed out to a modulation of protein degradation machinery [89].
Modulation of autophagy is another possible strategy to correct F508del-CFTR. Actually, autophagy was found to be defective in CF cells. It was postulated that CFTR defect causes upregulation of reactive oxygen species, and activation of transglutaminase with consequent entrapment of beclin 1 causing impairment in autophagy process and inflammation [90]. This cascade can be blocked and CFTR rescued by compounds like cysteamine [91].
Recently, new correctors for F508del-CFTR were found by considering the detrimental interaction that occurs between CFTR-NBD1 and keratin-8. Pharmacological inhibitors of this interaction were found by in silico screenings. Such compounds rescued F508del-CFTR [92].
Another possible strategy to CFTR correctors is by investigating the interactome of CFTR with proteomic and bioinformatic methods [93, 94]. Identification of key networks and signaling pathways linked to CFTR processing could reveal ways to modulate such processes with pharmacological agents.
An intriguing aspect of mutant CFTR rescue is the completely separate activity of potentiators and correctors. Although potentiators overcome the gating defect associated with F508del mutation, they have no activity at all on its trafficking problem. Actually, chronic treatment with potentiators may even decrease the efficacy of correctors [95, 96]. An exception to this rule is trimethylangelicin. This compound was reported to work both as a potentiator and a corrector [97]. Other compounds with possible dual activity are aminoarylthiazoles, AATs [98]. However, it is possible that the dual activity of AATs arises from two separate mechanisms of action [99].
The mechanism of action of most correctors, particularly of those identified by high-throughput screening, remains to be elucidated. It is possible that such compounds act on CFTR protein as pharmacological chaperones. However, mechanisms based on the modulation of another protein cannot be excluded. For example, latonduine, identified by screening a marine extract collection, was found to act through members of the poly(ADP-ribose) polymerase (PARP) family [86, 100].
It is important to note that efficacy of correctors is always far from reaching 100 % of F508del-CFTR rescue. There are multiple reasons to explain this partial activity. For compounds acting as pharmacological chaperones, it should be considered that F508del causes at least two types of main defects to CFTR protein: intrinsic instability of NBD1 and impairment of NBD1 interaction with the intracellular loops (ICLs) that connect NBDs to transmembrane domains. It has been shown that both types of defect need to be corrected to achieve a very high rescue of mutant CFTR, but that known correctors target only one defect [101]. For example, VX-809 improves the NBD1–ICL4 interaction, but does not correct NBD1 instability [101, 102]. Corr-4a acts differently, probably through interaction with the second half of CFTR, possibly involving NBD2 [101, 103]. Importantly, only combination of one or two correctors with the chemical chaperone glycerol elicited a nearly total F508del-CFTR rescue [101]. Therefore, corrector combination is probably needed to obtain a therapeutically relevant effect in vivo. For compounds acting as proteostasis regulators, partial activity is inherent to the multiple pathways that control CFTR folding, trafficking, and degradation [41]. There are no compounds that can modulate all these processes together in an optimal way.
Partial efficacy of single corrector therapy is the probable reason of the modest effects obtained by correctors in clinical trials. Treatment of F508del/F508del patients with VX-809 (also known as Lumacaftor) did not result in a significant clinical benefit [104]. Better findings were found by combining Lumacaftor with the potentiator Ivacaftor [105]. This drug combination, named Orkambi, was approved for patients carrying two copies of F508del. However, efficacy of Orkambi is lower than that of Ivacaftor on patients with G551D and other class 3 mutations.
Summarizing, rescue of F508del-CFTR is a particularly difficult to task and probably not feasible with a single molecule. New correctors are probably going to be discovered in the near future. In the mean time, the large number of compounds discovered so far in a variety of studies offers the possibility to test all of them on a comparative basis using the same cell models and assays. Identification of the best compounds could help in the design of corrector combinations having particularly strong additive/synergic effects on F508del-CFTR rescue.
References
- 1.Riordan JR. CFTR function and prospects for therapy. Annu Rev Biochem. 2008;77:701–726. doi: 10.1146/annurev.biochem.75.103004.142532. [DOI] [PubMed] [Google Scholar]
- 2.Mornon JP, Hoffmann B, Jonic S, Lehn P. Callebaut I (2015) Full-open and closed CFTR channels, with lateral tunnels from the cytoplasm and an alternative position of the F508 region, as revealed by molecular dynamics. Cell Mol Life Sci. 2015;72:1377–1403. doi: 10.1007/s00018-014-1749-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Elborn JS. Cystic fibrosis. Lancet. 2016 doi: 10.1016/S0140-6736(16)00576-6. [DOI] [PubMed] [Google Scholar]
- 4.Rogers CS, Stoltz DA, Meyerholz DK, Ostedgaard LS, Rokhlina T, Taft PJ, Rogan MP, Pezzulo AA, Karp PH, Itani OA, Kabel AC, Wohlford-Lenane CL, Davis GJ, Hanfland RA, Smith TL, Samuel M, Wax D, Murphy CN, Rieke A, Whitworth K, Uc A, Starner TD, Brogden KA, Shilyansky J, McCray PB, Jr, Zabner J, Prather RS, Welsh MJ. Disruption of the CFTR gene produces a model of cystic fibrosis in newborn pigs. Science. 2008;321:1837–1841. doi: 10.1126/science.1163600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Stoltz DA, Meyerholz DK, Pezzulo AA, Ramachandran S, Rogan MP, Davis GJ, Hanfland RA, Wohlford-Lenane C, Dohrn CL, Bartlett JA, Nelson GA, 4th, Chang EH, Taft PJ, Ludwig PS, Estin M, Hornick EE, Launspach JL, Samuel M, Rokhlina T, Karp PH, Ostedgaard LS, Uc A, Starner TD, Horswill AR, Brogden KA, Prather RS, Richter SS, Shilyansky J, McCray PB, Jr, Zabner J, Welsh MJ. Cystic fibrosis pigs develop lung disease and exhibit defective bacterial eradication at birth. Sci Transl Med. 2010;2:29ra31. doi: 10.1126/scitranslmed.3000928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sun X, Sui H, Fisher JT, Yan Z, Liu X, Cho HJ, Joo NS, Zhang Y, Zhou W, Yi Y, Kinyon JM, Lei-Butters DC, Griffin MA, Naumann P, Luo M, Ascher J, Wang K, Frana T, Wine JJ, Meyerholz DK, Engelhardt JF. Disease phenotype of a ferret CFTR-knockout model of cystic fibrosis. J Clin Invest. 2010;120:3149–3160. doi: 10.1172/JCI43052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Olivier AK, Yi Y, Sun X, Sui H, Liang B, Hu S, Xie W, Fisher JT, Keiser NW, Lei D, Zhou W, Yan Z, Li G, Evans TI, Meyerholz DK, Wang K, Stewart ZA, Norris AW, Engelhardt JF. Abnormal endocrine pancreas function at birth in cystic fibrosis ferrets. J Clin Invest. 2012;122:3755–3768. doi: 10.1172/JCI60610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cook DP, Rector MV, Bouzek DC, Michalski AS, Gansemer ND, Reznikov LR, Li X, Stroik MR, Ostedgaard LS, Abou Alaiwa MH, Thompson MA, Prakash YS, Krishnan R, Meyerholz DK, Seow CY, Stoltz DA. Cystic fibrosis transmembrane conductance regulator in sarcoplasmic reticulum of airway smooth muscle. Implications for airway contractility. Am J Respir Crit Care Med. 2016;193:417–426. doi: 10.1164/rccm.201508-1562OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brill SR, Ross KE, Davidow CJ, Ye M, Grantham JJ, Caplan MJ. Immunolocalization of ion transport proteins in human autosomal dominant polycystic kidney epithelial cells. Proc Natl Acad Sci USA. 1996;93:10206–10211. doi: 10.1073/pnas.93.19.10206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Scott-Ward TS, Li H, Schmidt A, Cai Z, Sheppard DN. Direct block of the cystic fibrosis transmembrane conductance regulator Cl− channel by niflumic acid. Mol Membr Biol. 2004;21:27–38. doi: 10.1080/09687680310001597758. [DOI] [PubMed] [Google Scholar]
- 11.Sheppard DN, Robinson KA. Mechanism of glibenclamide inhibition of cystic fibrosis transmembrane conductance regulator Cl− channels expressed in a murine cell line. J Physiol. 1997;503:333–346. doi: 10.1111/j.1469-7793.1997.333bh.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Walsh KB, Long KJ, Shen X. Structural and ionic determinants of 5-nitro-2-(3-phenylprophyl-amino)-benzoic acid block of the CFTR chloride channel. Br J Pharmacol. 1999;127:369–376. doi: 10.1038/sj.bjp.0702562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhang ZR, Zeltwanger S, McCarty NA. Direct comparison of NPPB and DPC as probes of CFTR expressed in Xenopus oocytes. J Membr Biol. 2000;175:35–52. doi: 10.1007/s002320001053. [DOI] [PubMed] [Google Scholar]
- 14.Zhou Z, Hu S, Hwang TC. Probing an open CFTR pore with organic anion blockers. J Gen Physiol. 2002;120:647–662. doi: 10.1085/jgp.20028685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jayaraman S, Haggie P, Wachter RM, Remington SJ, Verkman AS. Mechanism and cellular applications of a green fluorescent protein-based halide sensor. J Biol Chem. 2000;275:6047–6050. doi: 10.1074/jbc.275.9.6047. [DOI] [PubMed] [Google Scholar]
- 16.Galietta LV, Jayaraman S, Verkman AS. Cell-based assay for high-throughput quantitative screening of CFTR chloride transport agonists. Am J Physiol. 2001;281:C1734–C1742. doi: 10.1152/ajpcell.2001.281.5.C1734. [DOI] [PubMed] [Google Scholar]
- 17.Ma T, Thiagarajah JR, Yang H, Sonawane ND, Folli C, Galietta LJ, Verkman AS. Thiazolidinone CFTR inhibitor identified by high-throughput screening blocks cholera toxin-induced intestinal fluid secretion. J Clin Invest. 2002;110:1651–1658. doi: 10.1172/JCI0216112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Caputo A, Caci E, Ferrera L, Pedemonte N, Barsanti C, Sondo E, Pfeffer U, Ravazzolo R, Zegarra-Moran O, Galietta LJ. TMEM16A, a membrane protein associated with calcium-dependent chloride channel activity. Science. 2008;322:590–594. doi: 10.1126/science.1163518. [DOI] [PubMed] [Google Scholar]
- 19.Pedemonte N, Caci E, Sondo E, Caputo A, Rhoden K, Pfeffer U, Di Candia M, Bandettini R, Ravazzolo R, Zegarra-Moran O, Galietta LJ. Thiocyanate transport in resting and IL-4-stimulated human bronchial epithelial cells: role of pendrin and anion channels. J Immunol. 2007;178:5144–5153. doi: 10.4049/jimmunol.178.8.5144. [DOI] [PubMed] [Google Scholar]
- 20.Taddei A, Folli C, Zegarra-Moran O, Fanen P, Verkman AS, Galietta LJ. Altered channel gating mechanism for CFTR inhibition by a high-affinity thiazolidinone blocker. FEBS Lett. 2004;558:52–56. doi: 10.1016/S0014-5793(04)00011-0. [DOI] [PubMed] [Google Scholar]
- 21.Kopeikin Z, Sohma Y, Li M, Hwang TC. On the mechanism of CFTR inhibition by a thiazolidinone derivative. J Gen Physiol. 2010;136:659–671. doi: 10.1085/jgp.201010518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Caci E, Caputo A, Hinzpeter A, Arous N, Fanen P, Sonawane N, Verkman AS, Ravazzolo R, Zegarra-Moran O, Galietta LJ. Evidence for direct CFTR inhibition by CFTRinh-172 based on Arg347 mutagenesis. Biochem J. 2008;413:135–142. doi: 10.1042/BJ20080029. [DOI] [PubMed] [Google Scholar]
- 23.Sonawane ND, Verkman AS. Thiazolidinone CFTR inhibitors with improved water solubility identified by structure-activity analysis. Bioorg Med Chem. 2008;16:8187–8195. doi: 10.1016/j.bmc.2008.07.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Muanprasat C, Sonawane ND, Salinas D, Taddei A, Galietta LJ, Verkman AS. Discovery of glycine hydrazide pore-occluding CFTR inhibitors: mechanism, structure-activity analysis, and in vivo efficacy. J Gen Physiol. 2004;124:125–137. doi: 10.1085/jgp.200409059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Norimatsu Y, Ivetac A, Alexander C, O’Donnell N, Frye L, Sansom MS, Dawson DC. Locating a plausible binding site for an open-channel blocker, GlyH-101, in the pore of the cystic fibrosis transmembrane conductance regulator. Mol Pharmacol. 2012;82:1042–1055. doi: 10.1124/mol.112.080267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sonawane ND, Zhao D, Zegarra-Moran O, Galietta LJ, Verkman AS. Nanomolar CFTR inhibition by pore-occluding divalent polyethylene glycol-malonic acid hydrazides. Chem Biol. 2008;15:718–728. doi: 10.1016/j.chembiol.2008.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sonawane ND, Zhao D, Zegarra-Moran O, Galietta LJ, Verkman AS. Lectin conjugates as potent, nonabsorbable CFTR inhibitors for reducing intestinal fluid secretion in cholera. Gastroenterology. 2007;132:1234–1244. doi: 10.1053/j.gastro.2007.02.018. [DOI] [PubMed] [Google Scholar]
- 28.Tradtrantip L, Sonawane ND, Namkung W, Verkman AS. Nanomolar potency pyrimido-pyrrolo-quinoxalinedione CFTR inhibitor reduces cyst size in a polycystic kidney disease model. J Med Chem. 2009;52:6447–6455. doi: 10.1021/jm9009873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Snyder DS, Tradtrantip L, Yao C, Kurth MJ, Verkman AS. Potent, metabolically stable benzopyrimido-pyrrolo-oxazine-dione (BPO) CFTR inhibitors for polycystic kidney disease. J Med Chem. 2011;54:5468–5477. doi: 10.1021/jm200505e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Stutts MJ, Henke DC, Boucher RC. Diphenylamine-2-carboxylate (DPC) inhibits both Cl− conductance and cyclooxygenase of canine tracheal epithelium. Pflugers Arch. 1990;415:611–616. doi: 10.1007/BF02583514. [DOI] [PubMed] [Google Scholar]
- 31.Kelly M, Trudel S, Brouillard F, Bouillaud F, Colas J, Nguyen-Khoa T, Ollero M, Edelman A, Fritsch J. Cystic fibrosis transmembrane regulator inhibitors CFTRinh-172 and GlyH-101 target mitochondrial functions, independently of chloride channel inhibition. J Pharmacol Exp Ther. 2010;333:60–69. doi: 10.1124/jpet.109.162032. [DOI] [PubMed] [Google Scholar]
- 32.Perez A, Issler AC, Cotton CU, Kelley TJ, Verkman AS, Davis PB. CFTR inhibition mimics the cystic fibrosis inflammatory profile. Am J Physiol. 2007;292:L383–L395. doi: 10.1152/ajplung.00403.2005. [DOI] [PubMed] [Google Scholar]
- 33.Thiagarajah JR, Verkman AS. Chloride channel-targeted therapy for secretory diarrheas. Curr Opin Pharmacol. 2013;13:888–894. doi: 10.1016/j.coph.2013.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Davidow CJ, Maser RL, Rome LA, Calvet JP, Grantham JJ. The cystic fibrosis transmembrane conductance regulator mediates transepithelial fluid secretion by human autosomal dominant polycystic kidney disease epithelium in vitro. Kidney Int. 1996;50:208–218. doi: 10.1038/ki.1996.304. [DOI] [PubMed] [Google Scholar]
- 35.Li H, Findlay IA, Sheppard DN. The relationship between cell proliferation, Cl− secretion, and renal cyst growth: a study using CFTR inhibitors. Kidney Int. 2004;66:1926–1938. doi: 10.1111/j.1523-1755.2004.00967.x. [DOI] [PubMed] [Google Scholar]
- 36.Yang B, Sonawane ND, Zhao D, Somlo S, Verkman AS. Small-molecule CFTR inhibitors slow cyst growth in polycystic kidney disease. J Am Soc Nephrol. 2008;19:1300–1310. doi: 10.1681/ASN.2007070828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Van Goor F, Hadida S, Grootenhuis PD, Burton B, Cao D, Neuberger T, Turnbull A, Singh A, Joubran J, Hazlewood A, Zhou J, McCartney J, Arumugam V, Decker C, Yang J, Young C, Olson ER, Wine JJ, Frizzell RA, Ashlock M, Negulescu P. Rescue of CF airway epithelial cell function in vitro by a CFTR potentiator, VX-770. Proc Natl Acad Sci USA. 2009;106:18825–18830. doi: 10.1073/pnas.0904709106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Accurso FJ, Rowe SM, Clancy JP, Boyle MP, Dunitz JM, Durie PR, Sagel SD, Hornick DB, Konstan MW, Donaldson SH, Moss RB, Pilewski JM, Rubenstein RC, Uluer AZ, Aitken ML, Freedman SD, Rose LM, Mayer-Hamblett N, Dong Q, Zha J, Stone AJ, Olson ER, Ordoñez CL, Campbell PW, Ashlock MA, Ramsey BW. Effect of VX-770 in persons with cystic fibrosis and the G551D-CFTR mutation. N Engl J Med. 2010;363:1991–2003. doi: 10.1056/NEJMoa0909825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ramsey BW, Davies J, McElvaney NG, Tullis E, Bell SC, Dřevínek P, Griese M, McKone EF, Wainwright CE, Konstan MW, Moss R, Ratjen F, Sermet-Gaudelus I, Rowe SM, Dong Q, Rodriguez S, Yen K, Ordoñez C, Elborn JS, VX08-770-102 Study Group A CFTR potentiator in patients with cystic fibrosis and the G551D mutation. N Engl J Med. 2011;365:1663–1672. doi: 10.1056/NEJMoa1105185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Veit G, Avramescu RG, Chiang AN, Houck SA, Cai Z, Peters KW, Hong JS, Pollard HB, Guggino WB, Balch WE, Skach WR, Cutting GR, Frizzell RA, Sheppard DN, Cyr DM, Sorscher EJ, Brodsky JL, Lukacs GL. From CFTR biology toward combinatorial pharmacotherapy: expanded classification of cystic fibrosis mutations. Mol Biol Cell. 2016;27:424–433. doi: 10.1091/mbc.E14-04-0935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lukacs GL, Verkman AS. CFTR: folding, misfolding and correcting the ΔF508 conformational defect. Trends Mol Med. 2012;18:81–91. doi: 10.1016/j.molmed.2011.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Drumm ML, Wilkinson DJ, Smit LS, Worrell RT, Strong TV, Frizzell RA, Dawson DC, Collins FS. Chloride conductance expressed by delta F508 and other mutant CFTRs in Xenopus oocytes. Science. 1991;254:1797–1799. doi: 10.1126/science.1722350. [DOI] [PubMed] [Google Scholar]
- 43.Arispe N, Ma J, Jacobson KA, Pollard HB. Direct activation of cystic fibrosis transmembrane conductance regulator channels by 8-cyclopentyl-1,3-dipropylxanthine (CPX) and 1,3-diallyl-8-cyclohexylxanthine (DAX) J Biol Chem. 1998;273:5727–5734. doi: 10.1074/jbc.273.10.5727. [DOI] [PubMed] [Google Scholar]
- 44.Al-Nakkash L, Hwang TC. Activation of wild-type and deltaF508-CFTR by phosphodiesterase inhibitors through cAMP-dependent and -independent mechanisms. Pflugers Arch. 1999;437:553–561. doi: 10.1007/s004240050817. [DOI] [PubMed] [Google Scholar]
- 45.French PJ, Bijman J, Bot AG, Boomaars WE, Scholte BJ, de Jonge HR. Genistein activates CFTR Cl− channels via a tyrosine kinase- and protein phosphatase-independent mechanism. Am J Physiol. 1997;273:C747–C753. doi: 10.1152/ajpcell.1997.273.2.C747. [DOI] [PubMed] [Google Scholar]
- 46.Illek B, Zhang L, Lewis NC, Moss RB, Dong JY, Fischer H. Defective function of the cystic fibrosis-causing missense mutation G551D is recovered by genistein. Am J Physiol. 1999;277:C833–C839. doi: 10.1152/ajpcell.1999.277.4.C833. [DOI] [PubMed] [Google Scholar]
- 47.He Z, Raman S, Guo Y, Reenstra WW. Cystic fibrosis transmembrane conductance regulator activation by cAMP-independent mechanisms. Am J Physiol. 1998;275:C958–C966. doi: 10.1152/ajpcell.1998.275.4.C958. [DOI] [PubMed] [Google Scholar]
- 48.Smit LS, Wilkinson DJ, Mansoura MK, Collins FS, Dawson DC. Functional roles of the nucleotide-binding folds in the activation of the cystic fibrosis transmembrane conductance regulator. Proc Natl Acad Sci USA. 1993;90:9963–9967. doi: 10.1073/pnas.90.21.9963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Weinreich F, Wood PG, Riordan JR, Nagel G. Direct action of genistein on CFTR. Pflugers Arch. 1997;434:484–491. doi: 10.1007/s004240050424. [DOI] [PubMed] [Google Scholar]
- 50.Al-Nakkash L, Hu S, Li M, Hwang TC. A common mechanism for cystic fibrosis transmembrane conductance regulator protein activation by genistein and benzimidazolone analogs. J Pharmacol Exp Ther. 2001;296:464–472. [PubMed] [Google Scholar]
- 51.Cai Z, Sheppard DN. Phloxine B interacts with the cystic fibrosis transmembrane conductance regulator at multiple sites to modulate channel activity. J Biol Chem. 2002;277:19546–19553. doi: 10.1074/jbc.M108023200. [DOI] [PubMed] [Google Scholar]
- 52.Becq F, Mettey Y, Gray MA, Galietta LJ, Dormer RL, Merten M, Métayé T, Chappe V, Marvingt-Mounir C, Zegarra-Moran O, Tarran R, Bulteau L, Dérand R, Pereira MM, McPherson MA, Rogier C, Joffre M, Argent BE, Sarrouilhe D, Kammouni W, Figarella C, Verrier B, Gola M, Vierfond JM. Development of substituted Benzo[c]quinolizinium compounds as novel activators of the cystic fibrosis chloride channel. J Biol Chem. 1999;274:27415–27425. doi: 10.1074/jbc.274.39.27415. [DOI] [PubMed] [Google Scholar]
- 53.Zegarra-Moran O, Romio L, Folli C, Caci E, Becq F, Vierfond JM, Mettey Y, Cabrini G, Fanen P, Galietta LJ. Correction of G551D-CFTR transport defect in epithelial monolayers by genistein but not by CPX or MPB-07. Br J Pharmacol. 2002;137:504–512. doi: 10.1038/sj.bjp.0704882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.McCarty NA, Standaert TA, Teresi M, Tuthill C, Launspach J, Kelley TJ, Milgram LJ, Hilliard KA, Regelmann WE, Weatherly MR, Aitken ML, Konstan MW, Ahrens RC. A phase I randomized, multicenter trial of CPX in adult subjects with mild cystic fibrosis. Pediatr Pulmonol. 2002;33:90–98. doi: 10.1002/ppul.10041. [DOI] [PubMed] [Google Scholar]
- 55.Ahrens RC, Standaert TA, Launspach J, Han SH, Teresi ME, Aitken ML, Kelley TJ, Hilliard KA, Milgram LJ, Konstan MW, Weatherly MR, McCarty NA. Use of nasal potential difference and sweat chloride as outcome measures in multicenter clinical trials in subjects with cystic fibrosis. Pediatr Pulmonol. 2002;33:142–150. doi: 10.1002/ppul.10043. [DOI] [PubMed] [Google Scholar]
- 56.Galietta LJ, Haggie PM, Verkman AS. Green fluorescent protein-based halide indicators with improved chloride and iodide affinities. FEBS Lett. 2001;499:220–224. doi: 10.1016/S0014-5793(01)02561-3. [DOI] [PubMed] [Google Scholar]
- 57.Galietta LJ, Springsteel MF, Eda M, Niedzinski EJ, By K, Haddadin MJ, Kurth MJ, Nantz MH, Verkman AS. Novel CFTR chloride channel activators identified by screening of combinatorial libraries based on flavone and benzoquinolizinium lead compounds. J Biol Chem. 2001;276:19723–19728. doi: 10.1074/jbc.M101892200. [DOI] [PubMed] [Google Scholar]
- 58.Sammelson RE, Ma T, Galietta LJ, Verkman AS, Kurth MJ. 3-(2-Benzyloxyphenyl)isoxazoles and isoxazolines: synthesis and evaluation as CFTR activators. Bioorg Med Chem Lett. 2003;13:2509–2512. doi: 10.1016/S0960-894X(03)00482-7. [DOI] [PubMed] [Google Scholar]
- 59.Ma T, Vetrivel L, Yang H, Pedemonte N, Zegarra-Moran O, Galietta LJ, Verkman AS. High-affinity activators of cystic fibrosis transmembrane conductance regulator (CFTR) chloride conductance identified by high-throughput screening. J Biol Chem. 2002;277:37235–37241. doi: 10.1074/jbc.M205932200. [DOI] [PubMed] [Google Scholar]
- 60.Yang H, Shelat AA, Guy RK, Gopinath VS, Ma T, Du K, Lukacs GL, Taddei A, Folli C, Pedemonte N, Galietta LJ, Verkman AS. Nanomolar affinity small molecule correctors of defective Delta F508-CFTR chloride channel gating. J Biol Chem. 2003;278:35079–35085. doi: 10.1074/jbc.M303098200. [DOI] [PubMed] [Google Scholar]
- 61.Pedemonte N, Sonawane ND, Taddei A, Hu J, Zegarra-Moran O, Suen YF, Robins LI, Dicus CW, Willenbring D, Nantz MH, Kurth MJ, Galietta LJ, Verkman AS. Phenylglycine and sulfonamide correctors of defective ∆F508 and G551D cystic fibrosis transmembrane conductance regulator chloride-channel gating. Mol Pharmacol. 2005;67:1797–1807. doi: 10.1124/mol.105.010959. [DOI] [PubMed] [Google Scholar]
- 62.Pedemonte N, Diena T, Caci E, Nieddu E, Mazzei M, Ravazzolo R, Zegarra-Moran O, Galietta LJ. Antihypertensive 1,4-dihydropyridines as correctors of the cystic fibrosis transmembrane conductance regulator channel gating defect caused by cystic fibrosis mutations. Mol Pharmacol. 2005;68:1736–1746. doi: 10.1124/mol.105.015149. [DOI] [PubMed] [Google Scholar]
- 63.Pedemonte N, Boido D, Moran O, Giampieri M, Mazzei M, Ravazzolo R, Galietta LJ. Structure-activity relationship of 1,4-dihydropyridines as potentiators of the cystic fibrosis transmembrane conductance regulator chloride channel. Mol Pharmacol. 2007;72:197–207. doi: 10.1124/mol.107.034702. [DOI] [PubMed] [Google Scholar]
- 64.Van Goor F, Straley KS, Cao D, González J, Hadida S, Hazlewood A, Joubran J, Knapp T, Makings LR, Miller M, Neuberger T, Olson E, Panchenko V, Rader J, Singh A, Stack JH, Tung R, Grootenhuis PD, Negulescu P. Rescue of ∆F508-CFTR trafficking and gating in human cystic fibrosis airway primary cultures by small molecules. Am J Physiol. 2006;290:L1117–L1130. doi: 10.1152/ajplung.00169.2005. [DOI] [PubMed] [Google Scholar]
- 65.Hadida S, Van Goor F, Zhou J, Arumugam V, McCartney J, Hazlewood A, Decker C, Negulescu P, Grootenhuis PD. Discovery of N-(2,4-di-tert-butyl-5-hydroxyphenyl)-4-oxo-1,4-dihydroquinoline-3-carboxamide (VX-770, ivacaftor), a potent and orally bioavailable CFTR potentiator. J Med Chem. 2014;57:9776–9795. doi: 10.1021/jm5012808. [DOI] [PubMed] [Google Scholar]
- 66.Van Goor F, Yu H, Burton B, Hoffman B. Effect of ivacaftor on CFTR forms with missense mutations associated with defects in protein processing or function. J Cyst Fibros. 2014;13:29–36. doi: 10.1016/j.jcf.2013.06.008. [DOI] [PubMed] [Google Scholar]
- 67.Moran O, Galietta LJ, Zegarra-Moran O. Binding site of activators of the cystic fibrosis transmembrane conductance regulator in the nucleotide binding domains. Cell Mol Life Sci. 2005;62:446–460. doi: 10.1007/s00018-004-4422-3. [DOI] [PubMed] [Google Scholar]
- 68.Lewis HA, Buchanan SG, Burley SK, Conners K, Dickey M, Dorwart M, Fowler R, Gao X, Guggino WB, Hendrickson WA, Hunt JF, Kearins MC, Lorimer D, Maloney PC, Post KW, Rajashankar KR, Rutter ME, Sauder JM, Shriver S, Thibodeau PH, Thomas PJ, Zhang M, Zhao X, Emtage S. Structure of nucleotide-binding domain 1 of the cystic fibrosis transmembrane conductance regulator. EMBO J. 2004;23:282–293. doi: 10.1038/sj.emboj.7600040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Zegarra-Moran O, Monteverde M, Galietta LJ, Moran O. Functional analysis of mutations in the putative binding site for cystic fibrosis transmembrane conductance regulator potentiators. Interaction between activation and inhibition. J Biol Chem. 2007;282:9098–9104. doi: 10.1074/jbc.M611411200. [DOI] [PubMed] [Google Scholar]
- 70.Li MS, Cowley EA, Linsdell P. Pseudohalide anions reveal a novel extracellular site for potentiators to increase CFTR function. Br J Pharmacol. 2012;167:1062–1075. doi: 10.1111/j.1476-5381.2012.02041.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Wellhauser L, Kim Chiaw P, Pasyk S, Li C, Ramjeesingh M, Bear CE. A small-molecule modulator interacts directly with deltaPhe508-CFTR to modify its ATPase activity and conformational stability. Mol Pharmacol. 2009;75:1430–1438. doi: 10.1124/mol.109.055608. [DOI] [PubMed] [Google Scholar]
- 72.Galfrè E, Galeno L, Moran O. A potentiator induces conformational changes on the recombinant CFTR nucleotide binding domains in solution. Cell Mol Life Sci. 2012;69:3701–3713. doi: 10.1007/s00018-012-1049-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Eckford PD, Li C, Ramjeesingh M, Bear CE. Cystic fibrosis transmembrane conductance regulator (CFTR) potentiator VX-770 (ivacaftor) opens the defective channel gate of mutant CFTR in a phosphorylation-dependent but ATP-independent manner. J Biol Chem. 2012;287:36639–36649. doi: 10.1074/jbc.M112.393637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Jih KY, Hwang TC. Vx-770 potentiates CFTR function by promoting decoupling between the gating cycle and ATP hydrolysis cycle. Proc Natl Acad Sci USA. 2013;110:4404–4409. doi: 10.1073/pnas.1215982110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Sloane PA, Shastry S, Wilhelm A, Courville C, Tang LP, Backer K, Levin E, Raju SV, Li Y, Mazur M, Byan-Parker S, Grizzle W, Sorscher EJ, Dransfield MT, Rowe SM. A pharmacologic approach to acquired cystic fibrosis transmembrane conductance regulator dysfunction in smoking related lung disease. PLoS One. 2012;7:e39809. doi: 10.1371/journal.pone.0039809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Dransfield MT, Wilhelm AM, Flanagan B, Courville C, Tidwell SL, Raju SV, Gaggar A, Steele C, Tang LP, Liu B, Rowe SM. Acquired cystic fibrosis transmembrane conductance regulator dysfunction in the lower airways in COPD. Chest. 2013;144:498–506. doi: 10.1378/chest.13-0274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Pedemonte N, Zegarra-Moran O, Galietta LJ. High-throughput screening of libraries of compounds to identify CFTR modulators. Methods Mol Biol. 2011;741:13–21. doi: 10.1007/978-1-61779-117-8_2. [DOI] [PubMed] [Google Scholar]
- 78.Carlile GW, Robert R, Zhang D, Teske KA, Luo Y, Hanrahan JW, Thomas DY. Correctors of protein trafficking defects identified by a novel high-throughput screening assay. ChemBioChem. 2007;8:1012–1020. doi: 10.1002/cbic.200700027. [DOI] [PubMed] [Google Scholar]
- 79.Pedemonte N, Lukacs GL, Du K, Caci E, Zegarra-Moran O, Galietta LJ, Verkman AS. Small-molecule correctors of defective DeltaF508-CFTR cellular processing identified by high-throughput screening. J Clin Invest. 2005;115:2564–2571. doi: 10.1172/JCI24898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Yoo CL, Yu GJ, Yang B, Robins LI, Verkman AS, Kurth MJ. 4′-Methyl-4,5′-bithiazole-based correctors of defective delta F508-CFTR cellular processing. Bioorg Med Chem Lett. 2008;18:2610–2614. doi: 10.1016/j.bmcl.2008.03.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Yu GJ, Yoo CL, Yang B, Lodewyk MW, Meng L, El-Idreesy TT, Fettinger JC, Tantillo DJ, Verkman AS, Kurth MJ. Potent s-cis-locked bithiazole correctors of ∆F508 cystic fibrosis transmembrane conductance regulator cellular processing for cystic fibrosis therapy. J Med Chem. 2008;51:6044–6054. doi: 10.1021/jm800533c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Coffman KC, Nguyen HH, Phuan PW, Hudson BM, Yu GJ, Bagdasarian AL, Montgomery D, Lodewyk MW, Yang B, Yoo CL, Verkman AS, Tantillo DJ, Kurth MJ. Constrained bithiazoles: small molecule correctors of defective ΔF508-CFTR protein trafficking. J Med Chem. 2014;57:6729–6738. doi: 10.1021/jm5007885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Van Goor F, Hadida S, Grootenhuis PD, Burton B, Stack JH, Straley KS, Decker CJ, Miller M, McCartney J, Olson ER, Wine JJ, Frizzell RA, Ashlock M, Negulescu PA. Correction of the F508del-CFTR protein processing defect in vitro by the investigational drug VX-809. Proc Natl Acad Sci USA. 2011;108:18843–18848. doi: 10.1073/pnas.1105787108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Robert R, Carlile GW, Pavel C, Liu N, Anjos SM, Liao J, Luo Y, Zhang D, Thomas DY, Hanrahan JW. Structural analog of sildenafil identified as a novel corrector of the F508del-CFTR trafficking defect. Mol Pharmacol. 2008;73:478–489. doi: 10.1124/mol.107.040725. [DOI] [PubMed] [Google Scholar]
- 85.Robert R, Carlile GW, Liao J, Balghi H, Lesimple P, Liu N, Kus B, Rotin D, Wilke M, de Jonge HR, Scholte BJ, Thomas DY, Hanrahan JW. Correction of the ∆phe508 cystic fibrosis transmembrane conductance regulator trafficking defect by the bioavailable compound glafenine. Mol Pharmacol. 2010;77:922–930. doi: 10.1124/mol.109.062679. [DOI] [PubMed] [Google Scholar]
- 86.Carlile GW, Keyzers RA, Teske KA, Robert R, Williams DE, Linington RG, Gray CA, Centko RM, Yan L, Anjos SM, Sampson HM, Zhang D, Liao J, Hanrahan JW, Andersen RJ, Thomas DY. Correction of F508del-CFTR trafficking by the sponge alkaloid latonduine is modulated by interaction with PARP. Chem Biol. 2012;19:1288–1299. doi: 10.1016/j.chembiol.2012.08.014. [DOI] [PubMed] [Google Scholar]
- 87.Hutt DM, Herman D, Rodrigues AP, Noel S, Pilewski JM, Matteson J, Hoch B, Kellner W, Kelly JW, Schmidt A, Thomas PJ, Matsumura Y, Skach WR, Gentzsch M, Riordan JR, Sorscher EJ, Okiyoneda T, Yates JR, 3rd, Lukacs GL, Frizzell RA, Manning G, Gottesfeld JM, Balch WE. Reduced histone deacetylase 7 activity restores function to misfolded CFTR in cystic fibrosis. Nat Chem Biol. 2010;6:25–33. doi: 10.1038/nchembio.275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Trzcinska-Daneluti AM, Nguyen L, Jiang C, Fladd C, Uehling D, Prakesch M, Al-awar R, Rotin D. Use of kinase inhibitors to correct ΔF508-CFTR function. Mol Cell Proteomics. 2012;11:745–757. doi: 10.1074/mcp.M111.016626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Norez C, Vandebrouck C, Bertrand J, Noel S, Durieu E, Oumata N, Galons H, Antigny F, Chatelier A, Bois P, Meijer L, Becq F. Roscovitine is a proteostasis regulator that corrects the trafficking defect of F508del-CFTR by a CDK-independent mechanism. Br J Pharmacol. 2014;171:4831–4849. doi: 10.1111/bph.12859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Luciani A, Villella VR, Esposito S, Brunetti-Pierri N, Medina D, Settembre C, Gavina M, Pulze L, Giardino I, Pettoello-Mantovani M, D’Apolito M, Guido S, Masliah E, Spencer B, Quaratino S, Raia V, Ballabio A, Maiuri L. Defective CFTR induces aggresome formation and lung inflammation in cystic fibrosis through ROS-mediated autophagy inhibition. Nat Cell Biol. 2010;12:863–875. doi: 10.1038/ncb2090. [DOI] [PubMed] [Google Scholar]
- 91.De Stefano D, Villella VR, Esposito S, Tosco A, Sepe A, De Gregorio F, Salvadori L, Grassia R, Leone CA, De Rosa G, Maiuri MC, Pettoello-Mantovani M, Guido S, Bossi A, Zolin A, Venerando A, Pinna LA, Mehta A, Bona G, Kroemer G, Maiuri L, Raia V. Restoration of CFTR function in patients with cystic fibrosis carrying the F508del-CFTR mutation. Autophagy. 2014;10:2053–2074. doi: 10.4161/15548627.2014.973737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Odolczyk N, Fritsch J, Norez C, Servel N, da Cunha MF, Bitam S, Kupniewska A, Wiszniewski L, Colas J, Tarnowski K, Tondelier D, Roldan A, Saussereau EL, Melin-Heschel P, Wieczorek G, Lukacs GL, Dadlez M, Faure G, Herrmann H, Ollero M, Becq F, Zielenkiewicz P, Edelman A. Discovery of novel potent ΔF508-CFTR correctors that target the nucleotide binding domain. EMBO Mol Med. 2013;5:1484–1501. doi: 10.1002/emmm.201302699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Hegde RN, Parashuraman S, Iorio F, Ciciriello F, Capuani F, Carissimo A, Carrella D, Belcastro V, Subramanian A, Bounti L, Persico M, Carlile G, Galietta L, Thomas DY, Di Bernardo D, Luini A. Unravelling druggable signalling networks that control F508del-CFTR proteostasis. Elife. 2015;4:e10365. doi: 10.7554/eLife.10365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Pankow S, Bamberger C, Calzolari D, Martínez-Bartolomé S, Lavallée-Adam M, Balch WE, Yates JR., 3rd ∆F508 CFTR interactome remodelling promotes rescue of cystic fibrosis. Nature. 2015;528:510–516. doi: 10.1038/nature15729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Veit G, Avramescu RG, Perdomo D, Phuan PW, Bagdany M, Apaja PM, Borot F, Szollosi D, Wu YS, Finkbeiner WE, Hegedus T, Verkman AS, Lukacs GL. Some gating potentiators, including VX-770, diminish ΔF508-CFTR functional expression. Sci Transl Med. 2014;6:246ra97. doi: 10.1126/scitranslmed.3008889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Cholon DM, Quinney NL, Fulcher ML, Esther CR, Jr, Das J, Dokholyan NV, Randell SH, Boucher RC, Gentzsch M. Potentiator ivacaftor abrogates pharmacological correction of ΔF508 CFTR in cystic fibrosis. Sci Transl Med. 2014;6:246ra96. doi: 10.1126/scitranslmed.3008680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Favia M, Mancini MT, Bezzerri V, Guerra L, Laselva O, Abbattiscianni AC, Debellis L, Reshkin SJ, Gambari R, Cabrini G, Casavola V. Trimethylangelicin promotes the functional rescue of mutant F508del CFTR protein in cystic fibrosis airway cells. Am J Physiol. 2014;307:L48–L61. doi: 10.1152/ajplung.00305.2013. [DOI] [PubMed] [Google Scholar]
- 98.Pedemonte N, Tomati V, Sondo E, Caci E, Millo E, Armirotti A, Damonte G, Zegarra-Moran O, Galietta LJ. Dual activity of aminoarylthiazoles on the trafficking and gating defects of the cystic fibrosis transmembrane conductance regulator chloride channel caused by cystic fibrosis mutations. J Biol Chem. 2011;286:15215–15226. doi: 10.1074/jbc.M110.184267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Pesce E, Bellotti M, Liessi N, Guariento S, Damonte G, Cichero E, Galatini A, Salis A, Gianotti A, Pedemonte N, Zegarra-Moran O, Fossa P, Galietta LJ, Millo E. Synthesis and structure-activity relationship of aminoarylthiazole derivatives as correctors of the chloride transport defect in cystic fibrosis. Eur J Med Chem. 2015;99:14–35. doi: 10.1016/j.ejmech.2015.05.030. [DOI] [PubMed] [Google Scholar]
- 100.Carlile GW, Robert R, Matthes E, Yang Q, Solari R, Hatley R, Edge CM, Hanrahan JW, Andersen R, Thomas DY, Birault V. Latonduine analogs restore F508del-cystic fibrosis transmembrane conductance regulator trafficking through the modulation of poly-ADP ribose polymerase 3 and poly-ADP ribose polymerase 16 activity. Mol Pharmacol. 2016;90:65–79. doi: 10.1124/mol.115.102418. [DOI] [PubMed] [Google Scholar]
- 101.Okiyoneda T, Veit G, Dekkers JF, Bagdany M, Soya N, Xu H, Roldan A, Verkman AS, Kurth M, Simon A, Hegedus T, Beekman JM, Lukacs GL. Mechanism-based corrector combination restores ΔF508-CFTR folding and function. Nat Chem Biol. 2013;9:444–454. doi: 10.1038/nchembio.1253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Farinha CM, King-Underwood J, Sousa M, Correia AR, Henriques BJ, Roxo-Rosa M, Da Paula AC, Williams J, Hirst S, Gomes CM, Amaral MD. Revertants, low temperature, and correctors reveal the mechanism of F508del-CFTR rescue by VX-809 and suggest multiple agents for full correction. Chem Biol. 2013;20:943–955. doi: 10.1016/j.chembiol.2013.06.004. [DOI] [PubMed] [Google Scholar]
- 103.Grove DE, Rosser MF, Ren HY, Naren AP, Cyr DM. Mechanisms for rescue of correctable folding defects in CFTR∆F508. Mol Biol Cell. 2009;20:4059–4069. doi: 10.1091/mbc.E08-09-0929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Clancy JP, Rowe SM, Accurso FJ, Aitken ML, Amin RS, Ashlock MA, Ballmann M, Boyle MP, Bronsveld I, Campbell PW, De Boeck K, Donaldson SH, Dorkin HL, Dunitz JM, Durie PR, Jain M, Leonard A, McCoy KS, Moss RB, Pilewski JM, Rosenbluth DB, Rubenstein RC, Schechter MS, Botfield M, Ordoñez CL, Spencer-Green GT, Vernillet L, Wisseh S, Yen K, Konstan MW. Results of a phase IIa study of VX-809, an investigational CFTR corrector compound, in subjects with cystic fibrosis homozygous for the F508del-CFTR mutation. Thorax. 2012;67:12–18. doi: 10.1136/thoraxjnl-2011-200393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Wainwright CE, Elborn JS, Ramsey BW, Marigowda G, Huang X, Cipolli M, Colombo C, Davies JC, De Boeck K, Flume PA, Konstan MW, McColley SA, McCoy K, McKone EF, Munck A, Ratjen F, Rowe SM, Waltz D, Boyle MP, TRAFFIC Study Group; TRANSPORT Study Group Lumacaftor-Ivacaftor in Patients with Cystic Fibrosis Homozygous for Phe508del CFTR. N Engl J Med. 2015;373:220–231. doi: 10.1056/NEJMoa1409547. [DOI] [PMC free article] [PubMed] [Google Scholar]