Abstract
Notwithstanding the enormous reproductive potential encapsulated within a mature mammalian oocyte, these cells present only a limited window for fertilization before defaulting to an apoptotic cascade known as post-ovulatory oocyte aging. The only cell with the capacity to rescue this potential is the fertilizing spermatozoon. Indeed, the union of these cells sets in train a remarkable series of events that endows the oocyte with the capacity to divide and differentiate into the trillions of cells that comprise a new individual. Traditional paradigms hold that, beyond the initial stimulation of fluctuating calcium (Ca2+) required for oocyte activation, the fertilizing spermatozoon plays limited additional roles in the early embryo. While this model has now been drawn into question in view of the recent discovery that spermatozoa deliver developmentally important classes of small noncoding RNAs and other epigenetic modulators to oocytes during fertilization, it is nevertheless apparent that the primary responsibility for oocyte activation rests with a modest store of maternally derived proteins and mRNA accumulated during oogenesis. It is, therefore, not surprising that widespread post-translational modifications, in particular phosphorylation, hold a central role in endowing these proteins with sufficient functional diversity to initiate embryonic development. Indeed, proteins targeted for such modifications have been linked to oocyte activation, recruitment of maternal mRNAs, DNA repair and resumption of the cell cycle. This review, therefore, seeks to explore the intimate relationship between Ca2+ release and the suite of molecular modifications that sweep through the oocyte to ensure the successful union of the parental germlines and ensure embryogenic fidelity.
Keywords: Zygote, Oocyte activation, Phosphorylation, Protein kinase, DNA repair, DNA protection
Introduction
A defining feature of the mature, ovulated, metaphase II (MII) oocyte is the narrow window of opportunity that it presents to undergo successful fertilization and initiate embryogenesis [1]. Indeed, without the union of an oocyte and a functionally mature spermatozoon, the oocyte will rapidly undergo apoptosis and degradation via a process known as post-ovulatory oocyte aging [2]. Immediately following fertilization, however, the fertilizing sperm cell initiates a series of irreversible biochemical and physiological modifications to the oocyte’s cortex and cytoplasm, thus rescuing the cell from its otherwise predestined apoptotic fate and immortalizing its genetic contribution within the conceptus [3, 4]. Accordingly, the molecular basis of the sequential interactions between the fertilizing spermatozoon, the oocyte, and the subsequent events that they set in train has been the subject of considerable attention spanning many decades. Such intense research effort has provided compelling evidence that gamete fusion is followed by a rapid release of intracellular calcium from internal stores [5]. This initial elevation generally occurs within 1–3 min post-fusion [6] and, in turn, stimulates oscillating Ca2+ transients that can persist for a further 3–4 h [7, 8]. It is during this period of fluctuating Ca2+ transients that oocyte activation is initiated (reviewed in [9]).
Coinciding with pronuclear formation and syngamy, these Ca2+ oscillations briefly pause, only to transiently resume during the first phase of mitosis [7]. Recent work has provided evidence that the pause in calcium ion fluxes may also occur synchronously with a critical round of DNA repair and a concomitant upregulation of protective machinery [10, 11]. Such mechanisms have been postulated to ensure the zygote is in optimal condition to undergo embryogenesis [12] and appear to be causally linked to the post-translational modification, and hence activation, of an impressive suite of reparative and protective enzymes [10]. Notwithstanding these exciting data, the identity of a majority of the targets, the complex signaling pathways that underpin their activation, and their diverse roles in preparing the oocyte for embryogenesis remain to be fully investigated and, in some instances, are the subject of considerable controversy [13, 14]. In this review, we seek to integrate the leading theories and emerging data to provide a comprehensive appraisal of the role of fertilization and oocyte activation in extending the viability of the oocyte with a focus on highlighting the prominent role of post translation modifications (PTMs), in particular phosphorylation, in these events.
Post-ovulatory oocyte aging
Immediately following ovulation into the female reproductive tract, the mature oocyte remains viable for a relatively short period of time (~16 h) before defaulting to a terminal pathway of post-ovulatory aging [4, 15]. Oocytes progressing through this degradative process bear the well-characterized hallmarks of apoptosis and recent literature has implicated mitochondrial dysfunction and a consequential generation of reactive oxygen species (ROS) as a key mediator of this process [2, 4, 15, 16]. Produced as an intermediary product of normal cellular metabolism, ROS are well known to play fundamental roles in physiological signaling [17–20]. However, imbalances created by elevated levels of ROS that overwhelm the inherent antioxidant defenses within a cell can lead to the oxidation and alkylation of cellular components, such as DNA, lipids, and proteins with dire consequences for cell viability [21, 22]. Post-ovulatory oocyte aging appears to be orchestrated, at least in part, by these oxidative processes that progress through a cascade of events encompassing elevated ROS and the peroxidation of lipids comprising the cellular membranes (not excluding the mitochondrial membrane) [2, 23]. The ensuing production of electrophilic lipid aldehydes leads to widespread adduction of vulnerable proteins and DNA causing extensive cellular damage [21]. Even prior to the final induction of apoptosis, these profound insults prevent, or severely reduce the capacity of the oocyte to participate in fertilization and subsequently support embryonic development [1, 2, 4, 23, 24]. Significant biochemical abnormalities result in the reduced activity of critical proteins [e.g., maturation-promoting factor (MPF) and mitogen-activating protein kinase (MAPK)], impaired Ca2+ homeostasis, increased autophagy-related activity, mitochondrial dysfunction and disruptions to cell-cycle and stress response pathways [2, 15, 16, 24–28].
In this context, recent work in the mouse has shown that the onset of post-ovulatory oocyte aging is precipitated by an imbalance in cellular antioxidants and ROS levels [4]. Furthermore, in vitro studies have shown that mouse oocytes can be induced to display an aging phenotype following exposure to electrophilic aldehydes such as acrolein and 4-hydroxynonenal (4HNE) resulting in a dramatic decline in the fertilizability of these cells [2, 28]. Such consequences are not surprising, considering the integral contribution electrophilic aldehydes play in a number of oxidative stress-associated diseases, including: diabetes, cancer, atherosclerosis, acute lung injury, chronic alcohol exposure, and in neurodegenerative diseases, such as Alzheimer’s and Parkinson’s disease (reviewed in [29–31]). In addition, this model may also account for reduced pregnancy rates and higher incidences of cytogenetic abnormalities in humans associated with the use of in vitro aged oocytes following ‘rescue ICSI’ strategies [32, 33]. Although not specifically reviewed here, it is also important to note that oocytes from individuals of increasing maternal age also display many of the characteristic hallmarks of oxidative stress and the corresponding loss in fertility of those which undergo post ovulatory aging (for review, see [34–38]).
In the search for strategies to prevent oxidative threat to female fertility, sirtuins [silent information regulator 2 (Sir2) proteins] and antioxidant supplementation have emerged as candidates to modulate oxidative assault. Sirtuins appear to play a role in sensing and modulating cellular redox status as well as directly deacetylating key proteins involved in the cellular stress response, thus having been shown to provide protective effects in cells and tissues exposed to oxidative stressors in vitro and in vivo [39, 40]. Supplementation of antioxidants, such as melatonin or caffeine, also appears to provide a valuable, yet temporary, solution to oxidative insult in oocytes. However, while such interventions can delay oocyte aging, they do not appear to be able to prevent this phenomenon entirely [2, 4, 23, 27, 41]. While it is clear that ROS can precipitate a number of negative consequences within oocytes, other factors, such as in vitro handling techniques and oocyte age (as a result of maternal age), have also been correlated with degradation of oocyte quality. This not only encompasses the consequences listed previously, but also manifests in a reduction in abundance of maternal effect proteins, loss of RNA-binding proteins, critical alterations of pericentromeric proteins, aneuploidy, and epigenetic changes inherited by the derivative generation(s) [42–44]. Undeniably, it is only the act of fertilization that can effectively truncate the post-ovulatory aging phenotype and the inevitable induction of apoptosis [4, 15]. Thus, understanding the precise biochemical and physiological modifications that are initiated upon gamete fusion may hold the key to our attempts to prolong the viability of oocytes and the developmental competence of early embryos.
The role of ions as post-fertilization signaling molecule
At the moment of fertilization, the spermatozoon is responsible for activating embryonic development by virtue of its ability to promote a transient elevation and subsequent oscillating waves of intracellular Ca2+ levels within the oocyte [5, 6]. Such events release the oocyte from its MII stage arrest and drive it toward embryogenesis via stimulation of meiotic resumption, cortical granule exocytosis, decondensation of the sperm nucleus, recruitment of maternal mRNAs, and pronuclear development [45].
In mammals, the initial burst of Ca2+ is of a longer duration and amplitude than that of the subsequent transients [8, 46]. More importantly, while Ca2+ has distinctive short-term effects on the initiation and completion of oocyte activation events, it has also been implicated in the downstream events encompassed by peri-implantation development and gene expression [47–49]. Support for the central role of Ca2+ in stimulating embryonic development rests with a series of elegant studies incorporating intracellular Ca2+ chelating agents [such as 1,2-bis(o-aminophenoxy) ethane-N,N,N’,N’-tetraacetic acid (BAPTA-BA)], which prevents cellular depolarization, metaphase II exit, cortical granule exocytosis, and pronuclear formation [50, 51]. Additional supporting evidence has been secured from experiments involving the judicious use of chemicals such as strontium chloride (SrCl2) and ethanol (EtOH) to artificially stimulate an increase in intracellular Ca2+ concentration, and thus drive the chemical or parthenogenetic activation of an oocyte in the absence of a fertilizing spermatozoon [15, 52–55]. The utility of such an approach is recognized by the routine use of artificial activators, together with a spermatozoon, as a supplement in ART settings when the male gamete is unable to activate the oocyte [15, 52–55]. Despite recognition of the importance of Ca2+ in these events, the specific sperm factor(s) and signal transduction pathways responsible for triggering its initial release after sperm–oocyte fusion remain unclear and the subject of considerable controversy [13, 14, 56, 57].
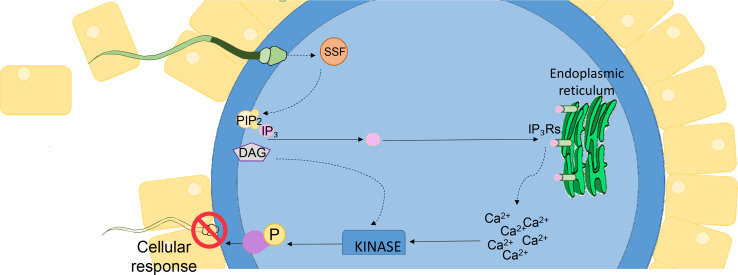
What is clear, is that at the moment of fertilization, the hydrolysis of the phosphatidylinositol 4,5-bisphosphate (PIP2) phospholipid is initiated resulting in the release of cleaved inositol trisphosphate (IP3) and diacylglycerol (DAG), the former of which binds to IP3 receptors (IP3Rs) located in the endoplasmic reticulum (ER), thereby stimulating the release of stored Ca2+ [58, 59]. Indeed, the injection of native IP3 or IP3 analogs is sufficient to induce Ca2+ release in mammalian oocytes [8, 60]. Conversely, inhibitory antibodies and pharmacological reagents that prevent IP3 binding to IP3Rs are able to elicit a potent suppression of fertilization induced Ca2+ oscillations and subsequently arrest fertilization and downstream embryonic development [58, 61]. The factor(s) that link these signaling phenomena to upstream sperm fusion may be expected to take the form of either oolemmal receptor(s) and/or soluble factor(s) delivered by the fertilizing spermatozoon [62, 63]. Indeed, numerous hypotheses have been put forward to account for the origin of the signal that stimulates the early events of fertilization [13, 14, 56].
Presently, the most widely accepted model centers on sperm specific factor(s) (SSF) that rouse the oocyte by promoting the initial surge in [Ca2+]i. Two purported sperm borne factors that putatively fulfill this role are phospholipase C zeta (PLCζ) and post-acrosomal WW-domain binding protein (PAWP) (Fig. 1) [8, 64, 65]. Indeed, experiments in which either purified PLCζ or PAWP has been injected into an MII stage oocyte have successfully stimulated the production of Ca2+ transients that are akin to those that ensue after sperm fusion [60, 64]. Since their initial identification however, numerous research groups have independently reported evidence conferring support for the role of PLCζ in oocyte activation and release of stored Ca2+ [8, 66–68]. In this regard, a considerable evidence base has now been established supporting a strong correlation between abnormalities in the structure, expression, and localization pattern of human PLCζ with that of oocyte activation deficiency (OAD) and total fertilisation failure (TFF) [69]. In contrast, since its original identification in 2007, independent research groups have yet to corroborate the ability of PAWP to successfully activate oocytes and/or induce Ca2+ oscillations (reviewed in [13]). Indeed, with the generation of a PAWP knockout mouse, it now appears that depletion of PAWP does not elicit the anticipated quantitative change in Ca2+ oscillations or in the subsequent rates of embryo development [57].
Fig. 1.
Fertilization from fusion to activation. The signaling phenomena necessary for oocyte activation and embryonic development encompasses the hydrolysis of the phosphatidylinositol 4,5-bisphosphate (PIP2) phospholipid anchored in the plasma membrane of the oocyte triggering the subsequent release of cleaved inositol trisphosphate (IP3) and diacylglycerol (DAG). The released IP3 is then able to bind IP3 receptors (IP3Rs) embedded within the endoplasmic reticulum (ER) and stimulate the release of stored Ca2+ to initiate the cellular responses required for oocyte activation. Among the putative sperm-specific factor(s) (SSF) that link sperm fusion to PIP2 hydrolysis, phospholipase C zeta (PLCζ) and/or post-acrosomal WW-domain binding protein (PAWP) have emerged as key contenders (Adapted from [13])
Ultimately, the definitive identification of the key sperm factor(s) and their mode of action may be crucial for the development of therapeutic intervention strategies to extend the viability of the oocyte, and may also hold value as a prognostic biomarker for the diagnosis of male factor infertility [56, 68, 70, 71]. In this context, strong correlations have been drawn between PLCζ and PAWP protein levels and the success of assisted reproductive cycles, with many instances of infertile human spermatozoa having been found to be deficient in their ability to stimulate the Ca2+ oscillations necessary for successful fertilization [72–75]. This work would also benefit from further analysis of the putative synergistic roles that have recently been assigned to dynamic fluxes in alternative ions such as zinc (Zn2+) [76–78]. While still in their relative infancy, the study of the newly coined ‘zinc-sparks’ has revealed a striking redistribution of Zn2+ loaded vesicles immediately at fertilization and demonstrated an inverse relationship between declining Zn2+ levels and the all-important increase in Ca2+ that is required for successful fertilization, oocyte activation and egg–embryo transition [76–78].
Indeed, during the final hours of meiotic maturation, the mouse oocyte accumulates an impressive twenty billion Zn2+ ions (representing an approximate 50 % increase in total Zn2+ content) [79, 80], via the two maternally derived and cortically distributed zinc transporters, ZIP6 and ZIP10 [77, 80–82]. Despite this, it is now widely accepted that release from MII arrest requires a dramatic decrease in intracellular Zn2+ content. Thus, the act of fertilization must trigger the coordinated release of billions of Zn2+ ions, and appears to do so via a novel exocytotic event referred to as a ‘zinc-spark.’ This process is necessary to re-establish cell cycle progression, oocyte activation and induce the egg-to-embryo transition [76–78, 81]. Accordingly, zinc-sparks appear to be evolutionarily conserved in all mammalian species studied to date, including humans, rodents, and nonhuman primates [77, 79]. The importance of Zn2+ homeostasis for oocyte biology is further emphasized by recent studies in which the sequestration of zinc using the heavy metal chelator N,N,N’,N’-tetrakis-(2-pyridylmethyl)-ethylenediamine (TPEN) [83], or the targeted disruption of Zn2+ transporters (ZIP6 and ZIP10), both led to immature telophase I-like cell cycle arrest; a response that could be reversed by Zn2+ supplementation [77]. Similarly, oocyte zinc-spark profiles have been positively correlated with mouse embryonic development and embryo quality. Thus, those oocytes that released higher concentrations of Zn2+ immediately following fertilization displayed the greatest embryonic development potential [84]. In view of such information, zinc-spark profiles hold considerable promise as a novel extracellular physicochemical biomarker of embryonic developmental potential [84].
Despite the clear biological and clinical importance of Ca2+, and now Zn2+, a rapid induction of transient fluxes in the intracellular concentration of either ion, would not in themselves be sufficient to support conception. Rather, it is likely that these ion(s) act in either an indirect and/or direct manner to promote widespread post-translational modifications (PTMs) across a suite of key enzymes (e.g., protein kinases (PK), phosphatases, and acetyltransferases) that themselves are responsible for promoting the changes in cellular physiology necessary for oocyte activation and embryonic development [85].
Phosphorylation and fertilization
Protein post-translational modifications (PTMs) increase the functional diversity of the cellular proteome via the covalent addition of functional groups, proteolytic cleavage of regulatory subunits or degradation of entire proteins. These chemical modifications influence almost all aspects of normal cell biology and pathogenesis [86], and their requirement during fertilization is driven at least in part, by the unique dependence of the early embryo on a modest store of maternally derived proteins and mRNA to support all of the early events during embryogenesis [87, 88]. Indeed, until recently, the leading paradigms have held that beyond the initial stimulation of oocyte activation, the fertilizing spermatozoon plays limited additional roles in the cleavage stage embryo [88]. Instead, this role is believed to rest predominantly with maternal factors that accumulate during oogenesis and are responsible for directing zygotic genome activation, the cleavage stages of embryogenesis, as well as the establishment of the initial cell lineages [87, 88]. In keeping with this notion, an autonomous transcription program is not established until the 2 (mouse) or 4 cell stages of embryonic development (bovine, ovine, and human) [89–91].
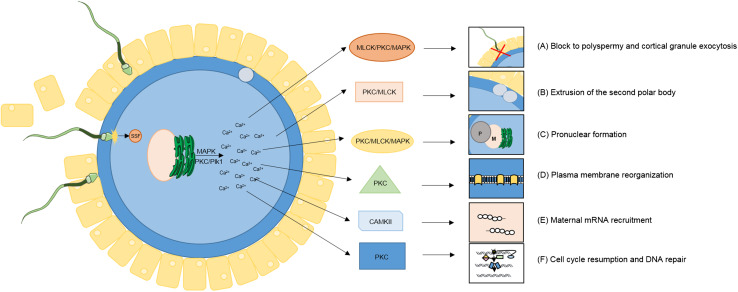
Among a complement of some 300 forms of PTMs, those involving the selective activation and inactivation of substrates via phosphorylation appear to hold a central position in coordinating the regulation of early embryo development (reviewed in [85]). Protein phosphorylation is a dynamic PTM that is mediated by kinases and phosphatases, which selectively phosphorylate and dephosphorylate substrates, respectively. Principally targeting serine, threonine or tyrosine residues, phosphorylation is one of the most important and well-studied PTMs with estimates suggesting that as many as one-third of the proteins in the human proteome are substrates for phosphorylation [92]. Such substrates extend to the fertilized oocyte, where they have been implicated in a diverse suite of physiological responses that encompass: a rapid block to polyspermy, cortical granule exocytosis, polar body extrusion, pronuclear development, plasma membrane reorganization, recruitment of maternal mRNAs, DNA repair, and resumption of the cell cycle (Fig. 2, Table 1).
Fig. 2.
Protein kinases are intimately tied to each essential event of oocyte activation. Protein kinases and phosphorylation events have been directly implicated in transducing the calcium (Ca2+) signal into many of the necessary activation events required for successful fertilization. Such events encompass a swift block to polyspermy, cortical granule exocytosis, polar body extrusion, pronuclear development, plasma membrane reorganization, recruitment of maternal mRNAs, DNA repair and resumption of the cell cycle (See Table 1) (Adapted from [85])
Table 1.
Phosphorylation directs key physiological events required for successful fertilization and early embryonic development. Widespread post-translational modifications, in particular phosphorylation has been implicated in directing a diverse suite of physiological responses required for oocyte activation and early embryonic development. A remarkable degree of redundancy exists between kinases at fertilization suggesting the highly integrated nature of these processes for the successful activation of development. A number of key kinases, their regulators, and proposed functions are listed in this table
| Kinase | Regulated by | Proposed function(s) | References |
|---|---|---|---|
| Conventional protein kinase C (cPKC) |
Intracellular Ca2+ DAG Phosphatidylserine |
Stimulation/maintenance of Ca2+ oscillations Extrusion of the second PB Cell cycle resumption/MII exit PN formation Spindle dynamics/organization Cytoskeletal reorganization DNA repair Block to polyspermy Cortical granule translocation/exocytosis Differential activation of kinases |
[85, 95–98, 172–174] |
| Novel protein kinase C (nPKC) |
DAG Phosphatidylserine |
Meiotic spindle dynamics Formation/extrusion of the second PN Cortical granule translocation |
[95, 175, 176] |
| Atypical protein kinase C (aPKC) |
Phosphatidylserine Negatively charged phospholipids |
Regulation of nuclear activity Cortical granule exocytosis |
[177] |
| Calmodulin-dependent protein kinase II (CAMKII) | Intracellular Ca2+ bound to calmodulin (CAM) |
Protein degradation by ubiquitination Decrease in MPF/resumption of meiosis Cortical granule translocation/exocytosis Maternal mRNA recruitment MII exit Cyclin B1 and securin destruction Decreases in the activity of MAPK Second PB extrusion Formation of maternal PN Phosphorylation of IP3R1s Regulation of apoptosis Sister chromatid segregation/decondensation |
[85, 99, 174, 178–182] |
| Polo-like kinase1 (PLK1) | Intracellular Ca2+ |
Redistribution of the ER Phosphorylation of IP3R1s Regulation of Ca2+ Enhance receptor function on ER Removing an APC/C inhibitor |
[85, 112, 183, 184] |
| Myosin light chain kinases (MLCK) |
Intracellular Ca2+ phosphorylation of myosin II |
Block to polyspermy PN formation Cytoskeletal remodeling PB formation/extrusion Cleavage/cytokinesis Redistribution of the ER and IP3R1s Calcium oscillations Regulator of myosin II in non-muscle cells Spindle rotation Morula-to-blastocyst transition |
[85, 102–107, 185] |
| Mitogen-activating protein kinase (MAPK) and Extracellular signal-regulated kinases (ERK) |
Intracellular Ca2+ CSF activity |
Activation and regulation of Ca2+ release Formation of PN Disintegration of the pronuclear envelope Cortical re-organization prior to fertilization Translocation of the meiotic spindle Redistribution of the ER and IP3R1s Formation of ER and IP3R1 cortical clusters phosphorylation of IP3R1 |
[85, 93, 102, 106, 111, 186, 187] |
| M-phase kinases or Maturation promoting factor (MPF) |
Intracellular Ca2+ ATP Cyclin |
Stimulation/maintenance of Ca2+ oscillations promoting resumption of meiosis and cell-cycle transitions Nuclear membrane breakdown |
[85, 186] |
| Rho-kinase (ROCK) | Intracellular Ca2+ |
Rho-furrowing phase of cytokinesis Spindle rotation Polar body formation Phosphorylation of IP3R1 Cytoskeletal rearrangement Ooplasmic segregation Spindle rotation |
[86, 112, 186, 188–190, 107] |
| Zipper-interacting protein kinase (ZIP kinase) | Intracellular Ca2+ | Regulator of myosin II required for cytokinesis | [101] |
| Protein kinase A (PKA) | Intracellular Ca2+ | Phosphorylate IP3R1 | [175, 191] |
| Protein kinase G (PKG) | Intracellular Ca2+ | Phosphorylate IP3R1 | [192] |
| Tyrosine kinases (FYN and LYN) | Intracellular Ca2+ | Phosphorylate IP3R1 | [193, 194] |
| Protein kinase B (PKB) | Intracellular Ca2+ | Phosphorylate IP3R1 | [195] |
The vital role of protein kinases in oocyte activation has been eloquently described in Drosophila melanogaster, where 311 proteins were shown to exhibit a change in phosphorylation status between mature and activated oocytes [93], suggesting that phosphorylation might simultaneously and rapidly modulate the activity of many proteins. Chief among these protein targets were those integral to Ca2+ binding, proteolysis, and protein translation, as well as those required for general oocyte activation and post-fertilization developmental stages. A number of kinases and their regulatory subunits were also identified amongst the candidates, including extracellular signal-regulated kinases (ERK) [also known as MAPK (mitogen-activated protein kinases)] and A-kinase anchor protein 200 (a regulatory subunit required for PKA localization) [93].
Like that of D. melanogaster, the mouse oocyte experiences a swift and dramatic alteration to its global phosphorylation status following both in vivo and in vitro activation [94]. Of particular interest, protein kinase C (PKC) activity has been linked to fertilization-induced Ca2+ oscillations (Table 1) using fluorescent C-kinase activity reporter (CKAR) probes in tandem with the selective PKC inhibitor, Gö6976. Interestingly, in vitro manipulation of PKC has revealed an additional role for this enzyme in the stimulation and maintenance of the Ca2+ oscillations that drive its activation, suggesting that it forms part of an important regulatory feedback loop [95]. As an extension of this model, Gonzalez-Garcia et al. [96] also showed that PKC-induced phosphorylation outlasts each Ca2+ transient, thus raising the possibility that it has a prolonged influence over such downstream events as pronuclear formation, spindle dynamics, cytoskeletal reorganization, cell cycle resumption, and DNA repair [97, 98]. In addition to PKC, oscillating Ca2+ has also been implicated in stimulation of calmodulin-dependent protein kinase II (CAMII), a response that triggers the phosphorylation and systematic degradation of non-essential proteins through ubiquitination [85, 99], resumption of meiosis and cortical granule exocytosis [100] (Table 1). Accordingly, CAMII activity was also found to spike almost immediately prior to, and remain elevated following, the extrusion of the second polar body (1.5 h after insemination of oocytes with spermatozoa) [100]. In a similar manner, additional kinases, such as myosin light chain kinases (MLCK), zipper-interacting protein kinase (ZIP), and Rho-kinase (ROCK), have each been implicated as playing integral roles in cytoskeletal arrangement during the fertilization cascade (Table 1). Such activity appears to be mediated, at least in part, via a conserved mechanism involving the phosphorylation of serine 19 (Ser19) on myosin regulatory light chains [101]. This phosphorylation event stimulates actin-mediated ATPase activity and the assembly of myosin II into filaments, thereby promoting cytoskeletal remodeling, cortical granule exocytosis, cytokinesis, polar body extrusion, and cleavage [85, 101]. In support of such functions, it has been demonstrated that selective pharmacological inhibition of MLCK, ZIP, and ROCK (by blebbistatin, ML-7 or Y-27632, respectively) is able to ablate the formation of the second polar body and the correct spindle rotation required for normal cytokinesis [102–107]. The role of myosin phosphorylation during fertilization is further underscored by studies in which mouse oocytes were microinjected with nonphosphorylatable myosin regulatory light chain peptides [108]. Such a strategy has been shown to effectively block sperm incorporation cone disassembly and obstruct cell cycle progression with pronounced consequences for successful fertilization [108].
Extending beyond the induction of fertilization, anomalous kinase activity and thus the fidelity of downstream phosphorylation events have been implicated in the etiology of infertility as well as gross phenotypic and developmental abnormalities in offspring [108–110]. Of particular concern, disruption of global phosphorylation by pharmacological or antibody inhibition has been shown to lead to abnormal cortical granule exocytosis, redistribution of the ER and IP3R1s, disruption of the second polar body formation and extrusion, as well as aberrant cytoskeletal reorganization and cleavage during embryogenesis [100]. For instance, mutation of MAPK signaling pathways abrogates normal IP3R1 phosphorylation required for the optimal release of Ca2+ ions at fertilization; those oocytes deprived of the MAPK signaling pathway during maturation fail to mount normal Ca2+ oscillations and show compromised IP3R1 function leading to compromised or arrested development [111]. Not surprisingly, phosphorylation of IP3R1 by Polo-like kinase1 (PLK1) also appears to underlie the spatial and temporal regulation of intracellular Ca2+ signals required for oocyte maturation. In fact, PLK1 has been shown to co-localize with MAPK and its activity is reduced in the absence of MAPK/ERK activity [112] with devastating consequences for fertilization. Similarly, inhibition of CAM kinase II by pharmacological means [myristoylated-AIP (autocamtide-2-related inhibitory peptide)] disrupted the inactivation of MPF (maturing promoting factor), preventing cell cycle resumption and cortical granule exocytosis in both fertilized and ethanol-activated oocytes [100].
Collectively, these data highlight the importance of phosphorylation cascades in several pivotal aspects of oocyte activation. First, this form of PTM directly affects the activity of a diverse suite of protein targets with dominant roles in transducing the initial Ca2+ signal into embryonic activation. Second, even subtle disruptions to these integral pathways can cause dramatic and irreversible consequences for future offspring. Moreover, when these disruptions occur on a broad scale, they can elicit gross biochemical, phenotypic and genomic abnormalities culminating in arrested embryonic development. Such a situation arises, at least in part, because a majority of the enzymes involved in intrinsic cellular repair pathways, such as base excision repair (BER) and homologous recombination (HR), require activation via phosphorylation (or other forms of PTMs) prior to being able to engage in the detection and mitigation of DNA damage (reviewed in [113–115]).
DNA repair within the oocyte
At the moment of fertilization, the majority of oocyte activation events either occur in parallel with, or in quick succession after, the initiation of Ca2+ oscillations. However, at the time of pronuclear formation, the conceptus experiences a transient arrest of Ca2+ oscillations [7]. Recent evidence has raised the possibility that this phenomenon is staged to allow a critical round of DNA repair to occur prior to DNA replication during the first mitotic division [12]. In addition, this temporary suspension of Ca2+ oscillations enables the reconfiguration of the sperm chromatin prior to pronuclear formation and syngamy [116]. However, the capacity of preimplantation stage embryos to repair damaged DNA remains to be fully characterized, and it seems that phosphorylation of existing repair proteins and maternal (and more even recently paternal) mRNAs capable of damage detection and cell cycle control are likely responsible for directing DNA repair [117–121]. Fittingly, the effects of paternal miRNAs seem to be mediated, at least in part, by post-transcriptional regulation over maternal and early zygotic mRNAs (e.g., miRNA mediated mRNA stability control; refer to section ‘Epigenetics and DNA repair’) [117, 122]. The quality of the paternal and maternal genetic contributions at fertilization greatly influences the developmental competency of the derivative organism [123]. It follows that the absence of efficient DNA repair at this critical developmental phase can either result in complete embryonic arrest or poor embryo development associated with increased risks of immunodeficiency, neurological disorders, and cancer within offspring and a concomitant reduction in life expectancy [124–127].
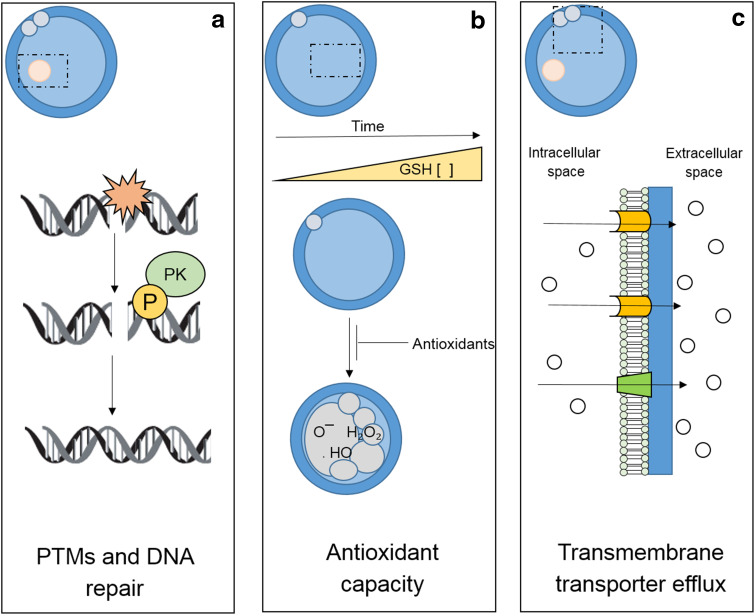
While many somatic cells experience a consequential increase in the synthesis of DNA repair enzymes and protective proteins in response to DNA damage [128, 129], the ovulatory stage oocyte is, in contrast, transcriptionally silent and incapable of mounting such a response [130]. Instead, these cells depend on stores of pre-synthesized proteins and/or mRNA transcripts to drive repair pathways during fertilization and early embryo development [131], which additionally may work in combination with paternal epigenetic modulators [118] (see section ‘Epigenetics and DNA repair’). During these transcriptionally inert stages of development, PTM of pre-synthesized and maternally stored repair enzymes are likely to facilitate the activation of the fundamental machinery necessary for mitigating DNA and/or cellular damage. In this context, an impressive array of PTMs (including acetylation, phosphorylation, methylation, ubiquitination, and sumoylation, as well as histone modification) have been implicated in cellular repair capacity and have been found to dramatically influence the kinetics of DNA repair [23, 127, 132]. Recent research within our own laboratory has provided evidence for the induction of a dramatic series of post-fertilization reparative (and protective) events involved in ensuring oocyte viability prior to embryogenesis [10, 133, 134]. Furthermore, we have also provided evidence for DNA repair and protection pathways with stage-dependent activity in the mammalian oocyte [10, 133, 134]. As a culmination of this work, it has been suggested that oocytes experience accelerated repair of oxidative DNA damage following fertilization driven by post-translational modifications of proteins that participate in the BER pathway [10] (Fig. 3a). This pathway, which includes the key elements of oxoguanine glycosylase (OGG1), apurinic/apyrimidinic endonuclease (APE1), and X-ray repair cross complementing protein 1 (XRCC1), is capable of repairing the common oxidative DNA base adduct, 8-hydroxyguanosine.
Fig. 3.
Fertilization induces a dynamic suite of protective and reparative events to ensure successful egg–embryo transition. These include: a post-translational modifications (PTMs) of pre-synthesized maternally derived repair enzymes that provide the fundamental machinery necessary for mitigating DNA and/or cellular damage in the early embryo; b significant alterations in glutathione (GSH) and glutathione peroxidase activity, thus truncating the effects of post-ovulatory oocyte aging and defending the embryo against the accumulation of oxidative DNA damage; and c a complex assortment of transmembrane transporter molecules implicated in facilitating the removal of genotoxic agents from the cytosol (Adapted from [2, 10, 126, 127])
While the BER pathway is considered the primary orchestrator of oxidative damage repair in the zygotic genome, a host of other enzymes have the capacity to contribute to these and other repair processes [134]. A recent proteomic comparison of murine MII oocytes and zygotes identified a total of 53, and in primate oocytes, approximately 37 proteins involved in DNA damage and DNA repair related processes [121, 130]. In the MII oocyte, enzymes required for nucleotide excision repair (NER), single strand break repair (SSB), double strand break repair (DSB), and BER are all highly expressed [121, 130], thus highlighting their potential for activation and use immediately following fertilization. In the same manner, a proteomic analysis of high- and low-quality oocytes in a porcine model indicated a positive correlation between the abundance of DNA repair proteins and the quality of the oocyte [135]. Microarray and bioinformatic approaches have also confirmed the expression of a similar profile of proteins in human oocytes and early embryos [136].
In addition to direct DNA repair, the oocyte is endowed with a host of alternative protective systems that may serve as the first line of defense against various forms of cellular damage including oxidative insult [10, 126, 127, 137]. In some instances, these protective systems are differentially expressed and/or activated in peri- and post-fertilized oocytes, generally rendering these cells less susceptible after fertilization has commenced. Examples include transmembrane transporter proteins involved in drug exclusion and antioxidant defenses that act to resolve oxidative DNA base adducts and prevent protein alkylation and mitochondrial disruption [10, 131, 133, 134]. In this context, accumulating evidence indicates that immediately prior to fertilization, concentrations of the antioxidant glutathione (GSH) are significantly increased in the oocyte, and remain elevated during the zygote stage, before precipitously falling by the 2-cell embryo stage [138]. Consistent with these data, it has also been shown that glutathione peroxidase activity is increased in zygotes compared to MII stage oocytes, thus providing additional protection to the zygote against hydrogen peroxide-induced DNA strand breakage [10; Fig. 3b] in conjunction with its essential role in paternal pronucleus formation and successful preimplantation development [139].
Epigenetics and DNA repair
Perhaps not surprisingly, embryonic quality and the effective maintenance of genomic integrity are influenced by the epigenetic signatures of both parental gametes [140]. Indeed, it appears that the correct establishment of epigenetic modifications and subsequent chromatin scaffolding are crucial steps in regulating the fidelity of the first mitotic cleavages with their absence leading to pronounced consequences for the development of a preimplantation embryo [127]. In this context, it has been shown that the inhibition of H3K4 demethylation can inhibit cleavage to the 4-cell stage embryo [127], while the inactivation of histone lysine methyltransferase (KMT5A) induces early embryonic lethality prior to the 8-cell stage [141]. Similarly, the deletion of Jumonji C domain-containing demethylase (JMJD2C) arrests embryo development prior to the formation of a blastocyst [142]. Recent evidence also suggests that the propensity of epigenetic modifications to modulate contact between nucleosomes and chromatin may serve to influence the compaction and/or relaxation state of the DNA fiber [143, 127]. In this capacity, epigenetic modifications may not only ‘open’ and ‘close’ regions of the DNA for transcription, but they may also act as important molecular gatekeepers during DNA decondensation and repair by providing the requisite enzymes with appropriate access to the DNA template [143–145]. In this respect, an enrichment of proteins responsible for epigenetic modification and chromatin remodeling (e.g., SMARCA5, CHD3, and CHD4) has been found in the mouse MII oocyte and early embryo, a particularly important consideration given the transcriptionally inert nature of immature oocytes prior to fertilization [123]. In further support of this notion, a dramatic loss in DNA demethylation [as evidenced by a reduction in 5-methyl-cytosine (5mC) content] has been recorded shortly after the protamine‐histone exchange that occurs within the paternal genome following fertilization [146, 147]. This demethylation phenomenon appears to be closely associated with the appearance of DNA DSBs (γH2A.X) and DNA repair markers [Poly(ADP-Ribose) Polymerase 1 (PARP-1)] [148]. Furthermore, the distinctive co-localization of γH2A.X foci formation with the sites of demethylation within the paternal pronucleus during the pre-replicative stages of development, has led some to postulate that DNA demethylation may be regulated by indirect DNA repair‐induced mechanisms such as the BER or NER pathways [149]. Such a model is consistent with the fact that the paternal gamete must decondense before syngamy [116], and that the maternal gamete is responsible for ensuring the genetic integrity of both nuclear contributions [87, 88].
Interest is also beginning to focus on the epigenetic regulation imposed by the fertilizing spermatozoon [150, 151]. In this regard, it is now recognized that, in addition to delivering the signal(s) that initiate the fertilization cascade, the male gamete may also contribute developmentally important epigenetic modulators (e.g., DNA methylation, sperm-specific histones, and other chromatin-associated proteins), as well as a number of small noncoding RNAs (sncRNAs), to the oocyte during fertilization that may all participate in successful embryogenesis [122, 151–153]. A strong case for this form of regulation has been mounted on the basis of experiments involving the use of conditional germline specific, Dicer and Drosha knockout mouse models. These studies have revealed that an embryos developmental potential, transcriptomic homeostasis and early zygotic gene activation are each dependent on paternally derived sncRNA cargo [117, 122, 151]. It has also been suggested that a specific sub-class of sncRNAs, known as the small interfering RNAs (siRNAs), may participate in key developmental processes encompassing pronuclear formation, DNA repair, orchestrating oocyte activation, the transition from maternal to embryonic gene control, and the establishment of imprints in early embryos [154]. Such findings offer many exciting avenues for future research, not the least of which will be to determine how epigenetic mechanisms of regulation are seamlessly integrated with maternally mediated PTM of signaling pathways to support early embryonic development.
Additional protective mechanisms of the oocyte and early embryo
In recent years, a variety of additional mechanisms have been identified that may prevent the propagation of damage in the fertilized oocyte. In marine invertebrates as in some mammalian species, post-fertilization activation has been implicated in promoting the synthesis of transmembrane transporter molecules, such as the ABCB protein, P-glycoprotein (PGP), and an ABCC protein similar to the multidrug resistant (MDR)-associated protein (MRP)-like transporter [154]. Though poorly understood, these proteins appear to be trafficked to the plasma membrane where they become functionally active and thereafter increase the bi-directional transport capacity of the cell [133, 134, 154]. Such proteins have been implicated in the transport of genotoxic agents from the intracellular environment and away from the vulnerable genomic material [133, 134, 155], as well as shuttling hormones and amino acids from the surrounding environment to the zygote to facilitate growth and development [156]. In somatic and cancer cell models, a similar complement of transmembrane transporter proteins has been implicated in multidrug resistance [157]. In these cells, the transport activity of the proteins appears to be intimately tied to their activation by post-translational phosphorylation events driven by Ca2+ and the serine/threonine kinases of PKC and/or PKA [133, 134, 158–160]. These results are particularly interesting given the notable increase in PKC activity that flows from the elevated levels of intracellular Ca2+ as well as a secondary stimulus of DAG, which are present at the time of fertilization. Taken together, it is tempting to speculate that PKC may hold a key role in the increased activation of transmembrane transporters to help protect mammalian oocytes and early embryos (Fig. 3c).
If this hypothesis was correct, it may afford unique opportunities to increase the protection of maturing oocytes with important implications for assisted reproductive strategies. In this regard, the ‘drugability’ of kinases [161] and their protein targets could provide avenues for the improvement of oocyte and early embryonic quality in vitro. The merits of this approach have been emphasized by recent proof-of-concept studies. For instance, artificial upregulation of transmembrane transporter molecules in bovine and porcine oocytes has been shown to have a dramatic positive impact on the post-cryopreservation viability of late stage embryos [162, 163]. In addition, recent literature indicates that numerous pharmacological (bovine embryos: rifampicin and forskolin) and hormonal (porcine GV stage oocytes: progesterone) signals can significantly elevate membrane transporter protein expression and/or activity, respectively [162, 163]. This paradigm provides an exciting prospect in clinical IVF settings whereby culture medium could be supplemented with compounds to increase the efflux activity of transmembrane transport proteins and thus reduce the intracellular availability of potentially damaging agents. This may also prove to be a valuable mechanism for the protection of the ovary and immature oocytes from both fresh and frozen sources in clinical fertility management.
In the event that the innate prevention and repair systems are inadequate or overwhelmed by a particular insult, mechanisms that are responsible for avoiding the propagation of damage take center stage. This is particularly important when considering the unique role that the oocyte plays in the continuation of a species when, in extreme cases, significant DNA damage could lead to chromosomal loss, translocation or duplication. Cumulatively, such damage could also significantly increase the mutational load borne by the embryo and thus heighten the risk of carcinogenesis in the offspring [164]. Correspondingly, a variety of mechanisms have been identified as being responsible for the removal of severely damaged oocytes or early embryos. These are usually characterized by significant cellular senescence, DNA fragmentation and degradation, and eventually either cell atresia or apoptosis (reviewed in [165–167]). Atresia dominates the mechanisms involved in removal of immature oocytes from the ovarian reservoir and involves ligand–receptor complex systems, including tumor necrosis factor alpha (TNFα), TNF-related apoptosis-inducing ligand (TRAIL or APO-2), Fas ligand, APO-3 ligand, PFG-5 ligand, and associated receptors; on the other hand, mature oocyte and early embryo loss are primarily mediated by Bcl-2 family members, Apaf-1 and caspase activation leading to apoptosis [154, 167–171].
The endowment of such elaborate systems for the detection, prevention, repair, or as a last resort, removal of a compromised cell clearly has evolutionarily benefits for a species. However, the increasing instance of delayed childbirth in our own species means that these mechanisms are working against us, particularly considering the expression of several key repair proteins has been found to be drastically reduced between ‘young’ and ‘aged’ oocytes [172] leading to a further potential increase in the demand for assisted reproductive procedures. While the advent of increasingly sophisticated assisted reproductive technologies to bypass such biological obstacles has been remarkably beneficial to many couples wishing to conceive, the reliance on this technology carries the very real risk of exacerbating genetic and cellular damage through gamete handling and culturing procedures in cells that would otherwise fail to participate in fertilization events [36].
Concluding remarks
The process of fertilization elicits a complex suite of biochemical and physiological modifications that prepare the oocyte for sustained embryonic development. These events are intimately tied to a prominent rise in intracellular Ca2+ at the moment of fertilization and the concomitant stimulation of an extensive range of oocyte activation events. Chief among these appears to be the activation of kinases that mediate the post-translational modification of a suite of key developmental proteins. It is now known that such events are likely to work in unison with paternally derived small non-coding RNA species, which regulate the stability/translation of the limited pool of maternally stored mRNA transcripts. This review contextualizes the role of fertilization and oocyte activation in extending the viability of the oocyte, initiating a series of biochemical alterations required for embryonic development and highlighting the prominent role that protein PTMs, and in particular phosphorylation, hold in their execution.
Acknowledgments
The authors gratefully acknowledge the editorial advice provided by Prof. Eileen McLaughlin, Dr. Tessa Lord and Mrs. Aleona Swegen.
Compliance with ethical standards
Conflict of interest
The authors have nothing to declare.
Funding
This work was supported by the University of Newcastle’s Priority Research Center for Reproductive Science. Jacinta Martin is a recipient of an Australian Postgraduate Award (APA).
Footnotes
R. J. Aitken and B. Nixon contributed equally to this work.
References
- 1.Wilcox AJ, Weinberg CR, Baird DD. Post-ovulatory ageing of the human oocyte and embryo failure. Hum Reprod. 1998;13:394–397. doi: 10.1093/humrep/13.2.394. [DOI] [PubMed] [Google Scholar]
- 2.Lord T, Aitken RJ. Fertilization stimulates 8-hydroxy-2′-deoxyguanosine repair and antioxidant activity to prevent mutagenesis in the embryo. Dev Biol. 2015;406:1–13. doi: 10.1016/j.ydbio.2015.07.024. [DOI] [PubMed] [Google Scholar]
- 3.Plachot M, de Grouchy J, Junca AM, Mandelbaum J, Salat-Baroux J, Cohen J. Chromosome analysis of human oocytes and embryos: does delayed fertilization increase chromosome imbalance? Hum Reprod. 1988;3:125–127. doi: 10.1093/humrep/3.suppl_1.125. [DOI] [PubMed] [Google Scholar]
- 4.Lord T, Nixon B, Jones KT, Aitken RJ. Melatonin prevents postovulatory oocyte aging in the mouse and extends the window for optimal fertilization in vitro. Biol Reprod. 2013;88:67. doi: 10.1095/biolreprod.112.106450. [DOI] [PubMed] [Google Scholar]
- 5.Swann K, Lai FA. Egg activation at fertilization by a soluble sperm protein. Physiol Rev. 2016;96:127–149. doi: 10.1152/physrev.00012.2015. [DOI] [PubMed] [Google Scholar]
- 6.Lawrence Y, Whitaker M, Swann K. Sperm-egg fusion is the prelude to the initial Ca2+ increase at fertilization in the mouse. Development. 1997;124:233–241. doi: 10.1242/dev.124.1.233. [DOI] [PubMed] [Google Scholar]
- 7.Marangos P, FitzHarris G, Carroll J. Ca2+ oscillations at fertilization in mammals are regulated by the formation of pronuclei. Development. 2003;130:1461–1472. doi: 10.1242/dev.00340. [DOI] [PubMed] [Google Scholar]
- 8.Saunders CM, Larman MG, Parrington J, Cox LJ, Royse J, Blayney LM, Swann K, Lai FA. PLC zeta: a sperm-specific trigger of Ca(2+) oscillations in eggs and embryo development. Development. 2002;129:3533–3544. doi: 10.1242/dev.129.15.3533. [DOI] [PubMed] [Google Scholar]
- 9.Miyazaki S, Ito M. Calcium signals for egg activation in mammals. J Pharmacol Sci. 2006;100:545–552. doi: 10.1254/jphs.CPJ06003X. [DOI] [PubMed] [Google Scholar]
- 10.Lord T, Martin JH, Aitken RJ. Accumulation of electrophilic aldehydes during postovulatory aging of mouse oocytes causes reduced fertility, oxidative stress, and apoptosis. Biol Reprod. 2015;92:33. doi: 10.1095/biolreprod.114.122820. [DOI] [PubMed] [Google Scholar]
- 11.Marchetti F, Bishop J, Gingerich J, Wyrobek AJ. Meiotic interstrand DNA damage escapes paternal repair and causes chromosomal aberrations in the zygote by maternal misrepair. Sci Rep. 2015;5:7689. doi: 10.1038/srep07689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Derijck A, van der Heijden G, Giele M, Philippens M, de Boer P. DNA double-strand break repair in parental chromatin of mouse zygotes, the first cell cycle as an origin of de novo mutation. Hum Mol Genet. 2008;17:1922–1937. doi: 10.1093/hmg/ddn090. [DOI] [PubMed] [Google Scholar]
- 13.Nomikos M, Swann K, Lai FA. Is PAWP the “real” sperm factor? Asian J Androl. 2015;17:444–446. doi: 10.4103/1008-682X.142145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vadnais ML, Gerton GL. From PAWP to “Pop”: opening up new pathways to fatherhood. Asian J Androl. 2015;17:443–444. doi: 10.4103/1008-682X.142140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Takahashi T, Takahashi E, Igarashi H, Tezuka N, Kurachi H. Impact of oxidative stress in aged mouse oocytes on calcium oscillations at fertilization. Mol Reprod Dev. 2003;66:143–152. doi: 10.1002/mrd.10341. [DOI] [PubMed] [Google Scholar]
- 16.Benkhalifa M, Ferreira YJ, Chahine H, Louanjli N, Miron P, Merviel P, Copin H. Mitochondria: participation to infertility as source of energy and cause of senescence. Int J Biochem Cell Biol. 2014;55:60–64. doi: 10.1016/j.biocel.2014.08.011. [DOI] [PubMed] [Google Scholar]
- 17.Aitken RJ, Gordon E, Harkiss D, Twigg JP, Milne P, Jennings Z, Irvine DS. Relative impact of oxidative stress on the functional competence and genomic integrity of human spermatozoa. Biol Reprod. 1998;59:1037–1046. doi: 10.1095/biolreprod59.5.1037. [DOI] [PubMed] [Google Scholar]
- 18.Aitken RJ, Harkiss D, Knox W, Paterson M, Irvine DS. A novel signal transduction cascade in capacitating human spermatozoa characterised by a redox-regulated, cAMP-mediated induction of tyrosine phosphorylation. J Cell Sci. 1998;111:645–656. doi: 10.1242/jcs.111.5.645. [DOI] [PubMed] [Google Scholar]
- 19.Griveau JF, Renard P, Le Lannou D. Superoxide anion production by human spermatozoa as a part of the ionophore-induced acrosome reaction process. Int J Androl. 1995;18:67–74. doi: 10.1111/j.1365-2605.1995.tb00388.x. [DOI] [PubMed] [Google Scholar]
- 20.Houston B, Curry B, Aitken RJ. Human spermatozoa possess an IL4I1 l-amino acid oxidase with a potential role in sperm function. Reproduction. 2015;149:587–596. doi: 10.1530/REP-14-0621. [DOI] [PubMed] [Google Scholar]
- 21.Aitken RJ, Whiting S, De Iuliis GN, McClymont S, Mitchell LA, Baker MA. Electrophilic aldehydes generated by sperm metabolism activate mitochondrial reactive oxygen species generation and apoptosis by targeting succinate dehydrogenase. J Biol Chem. 2012;287:33048–33060. doi: 10.1074/jbc.M112.366690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Moazamian R, Polhemus A, Connaughton H, Fraser B, Whiting S, Gharagozloo P, Aitken RJ. Oxidative stress and human spermatozoa: diagnostic and functional significance of aldehydes generated as a result of lipid peroxidation. Mol Hum Reprod. 2015;21:502–515. doi: 10.1093/molehr/gav014. [DOI] [PubMed] [Google Scholar]
- 23.Tarin JJ, Ten J, Vendrell FJ, Cano A. Dithiothreitol prevents age-associated decrease in oocyte/conceptus viability in vitro. Hum Reprod. 1998;13:381–386. doi: 10.1093/humrep/13.2.381. [DOI] [PubMed] [Google Scholar]
- 24.McGinnis LK, Pelech S, Kinsey WH. Post-ovulatory aging of oocytes disrupts kinase signaling pathways and lysosome biogenesis. Mol Reprod Dev. 2014;81:928–945. doi: 10.1002/mrd.22413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kikuchi K, Naito K, Noguchi J, Kaneko H, Tojo H. Maturation/M-phase promoting factor regulates aging of porcine oocytes matured in vitro. Cloning Stem Cells. 2002;4:211–222. doi: 10.1089/15362300260339494. [DOI] [PubMed] [Google Scholar]
- 26.Takahashi T, Igarashi H, Kawagoe J, Amita M, Hara S, Kurachi H. Poor embryo development in mouse oocytes aged in vitro is associated with impaired calcium homeostasis1. Biol Reprod. 2008;80:493–502. doi: 10.1095/biolreprod.108.072017. [DOI] [PubMed] [Google Scholar]
- 27.Zhang N, Wakai T, Fissore RA. Caffeine alleviates the deterioration of Ca(2+) release mechanisms and fragmentation of in vitro-aged mouse eggs. Mol Reprod Dev. 2011;78:684–701. doi: 10.1002/mrd.21366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cecconi S, Rossi G, Deldar H, Cellini V, Patacchiola F, Carta G, Macchiarelli G, Canipari R. Post-ovulatory ageing of mouse oocytes affects the distribution of specific spindle-associated proteins and Akt expression levels. Reprod Fertil Dev. 2014;26:562–569. doi: 10.1071/RD13010. [DOI] [PubMed] [Google Scholar]
- 29.Jacobs AT, Marnett LJ. Systems analysis of protein modification and cellular responses induced by electrophile stress. Acc Chem Res. 2010;43:673–683. doi: 10.1021/ar900286y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yin H, Xu L, Porter NA. Free radical lipid peroxidation: mechanisms and analysis. Chem Rev. 2011;111:5944–5972. doi: 10.1021/cr200084z. [DOI] [PubMed] [Google Scholar]
- 31.Voulgaridou GP, Anestopoulos I, Franco R, Panayiotidis MI, Pappa A. DNA damage induced by endogenous aldehydes: current state of knowledge. Mutat Res. 2011;711:13–27. doi: 10.1016/j.mrfmmm.2011.03.006. [DOI] [PubMed] [Google Scholar]
- 32.Chen C, Kattera S. Rescue ICSI of oocytes that failed to extrude the second polar body 6 h post-insemination in conventional IVF. Hum Reprod. 2003;18(10):2118–2121. doi: 10.1093/humrep/deg325. [DOI] [PubMed] [Google Scholar]
- 33.Pehlivan T, Rubio C, Ruiz A, Navarro J, Remohí J, Pellicer A, Simón C. Embryonic chromosomal abnormalities obtained after rescue intracytoplasmic sperm injection of 1-day-old unfertilized oocytes. J Assist Reprod Genet. 2004;21(2):55–57. doi: 10.1023/B:JARG.0000025939.26834.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Li Q, Geng X, Zheng W, Tang J, Xu B, Shi Q. Current understanding of ovarian aging. Sci China Life Sci. 2012;55:659–669. doi: 10.1007/s11427-012-4352-5. [DOI] [PubMed] [Google Scholar]
- 35.Perry JR, Murray A, Day FR, Ong KK. Molecular insights into the aetiology of female reproductive ageing. Nat Rev Endocrinol. 2015;11:725–734. doi: 10.1038/nrendo.2015.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tatone C, Di Emidio G, Vitti M, Di Carlo M, Santini S, Jr, D’Alessandro AM, Falone S, Amicarelli F. Sirtuin functions in female fertility: possible role in oxidative stress and aging. Oxid Med Cell Longev. 2015;2015:659687. doi: 10.1155/2015/659687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tatone C, Amicarelli F, Carbone MC, Monteleone P, Caserta D, Marci R, Artini PG, Piomboni P, Focarelli R. Cellular and molecular aspects of ovarian follicle ageing. Hum Reprod Update. 2008;14:131–142. doi: 10.1093/humupd/dmm048. [DOI] [PubMed] [Google Scholar]
- 38.Eichenlaub-Ritter U, Wieczorek M, Luke S, Seidel T. Age related changes in mitochondrial function and new approaches to study redox regulation in mammalian oocytes in response to age or maturation conditions. Mitochondrion. 2011;11:783–796. doi: 10.1016/j.mito.2010.08.011. [DOI] [PubMed] [Google Scholar]
- 39.Fan H, Yang HC, You L, Wang YY, He WJ, Hao CM. The histone deacetylase, SIRT1, contributes to the resistance of young mice to ischemia/reperfusion-induced acute kidney injury. Kidney Int. 2013;83:404–413. doi: 10.1038/ki.2012.394. [DOI] [PubMed] [Google Scholar]
- 40.Kao CL, Chen LK, Chang YL, Yung MC, Hsu CC, Chen YC, Lo WL, Chen SJ, Ku HH, Hwang SJ. Resveratrol protects human endothelium from H(2)O(2)-induced oxidative stress and senescence via SirT1 activation. J Atheroscler Thromb. 2010;17:970–979. doi: 10.5551/jat.4333. [DOI] [PubMed] [Google Scholar]
- 41.Rausell F, Pertusa JF, Gomez-Piquer V, Hermenegildo C, Garcia-Perez MA, Cano A, Tarin JJ. Beneficial effects of dithiothreitol on relative levels of glutathione S-transferase activity and thiols in oocytes, and cell number, DNA fragmentation and allocation at the blastocyst stage in the mouse. Mol Reprod Dev. 2007;74:860–869. doi: 10.1002/mrd.20569. [DOI] [PubMed] [Google Scholar]
- 42.Combelles CM, Gupta S, Agarwal A. Could oxidative stress influence the in-vitro maturation of oocytes? Reprod Biomed Online. 2009;18:864–880. doi: 10.1016/S1472-6483(10)60038-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ge ZJ, Schatten H, Zhang CL, Sun QY. Oocyte ageing and epigenetics. Reproduction. 2015;149:R103–R114. doi: 10.1530/REP-14-0242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Trapphoff T, Heiligentag M, Dankert D, Demond H, Deutsch D, Frohlich T, Arnold GJ, Grummer R, Horsthemke B, Eichenlaub-Ritter U. Postovulatory aging affects dynamics of mRNA, expression and localization of maternal effect proteins, spindle integrity and pericentromeric proteins in mouse oocytes. Hum Reprod. 2016;31:133–149. doi: 10.1093/humrep/dev279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ducibella T, Huneau D, Angelichio E, Xu Z, Schultz RM, Kopf GS, Fissore R, Madoux S, Ozil J-P. Egg-to-embryo transition is driven by differential responses to Ca2+ oscillation number. Dev Biol. 2002;250:280–291. doi: 10.1006/dbio.2002.0788. [DOI] [PubMed] [Google Scholar]
- 46.Deng MQ, Sheng SS. A specific inhibitor of p34 (cdc2)/cyclin B suppresses fertilization-induced calcium oscillations in mouse eggs. Biol Reprod. 2000;62:873–878. doi: 10.1095/biolreprod62.4.873. [DOI] [PubMed] [Google Scholar]
- 47.Ducibella T, Schultz RM, Ozil JP. Role of calcium signals in early development. Semin Cell Dev Biol. 2006;17:324–332. doi: 10.1016/j.semcdb.2006.02.010. [DOI] [PubMed] [Google Scholar]
- 48.Ozil JP, Banrezes B, Toth S, Pan H, Schultz RM. Ca2+ oscillatory pattern in fertilized mouse eggs affects gene expression and development to term. Dev Biol. 2006;300:534–544. doi: 10.1016/j.ydbio.2006.08.041. [DOI] [PubMed] [Google Scholar]
- 49.Ozil JP, Markoulaki S, Toth S, Matson S, Banrezes B, Knott JG, Schultz RM, Huneau D, Ducibella T. Egg activation events are regulated by the duration of a sustained [Ca2+]cyt signal in the mouse. Dev Biol. 2005;282:39–54. doi: 10.1016/j.ydbio.2005.02.035. [DOI] [PubMed] [Google Scholar]
- 50.Kline D, Kline JT. Repetitive calcium transients and the role of calcium in exocytosis and cell cycle activation in the mouse egg. Dev Biol. 1992;149:80–89. doi: 10.1016/0012-1606(92)90265-I. [DOI] [PubMed] [Google Scholar]
- 51.Miao YL, Stein P, Jefferson WN, Padilla-Banks E, Williams CJ. Calcium influx-mediated signaling is required for complete mouse egg activation. Proc Natl Acad Sci U S A. 2012;109:4169–4174. doi: 10.1073/pnas.1112333109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Didion BA, Martin MJ, Markert CL. Parthenogenetic activation of mouse and pig oocytes matured in vitro. Theriogenology. 1990;33:1165–1175. doi: 10.1016/0093-691X(90)90035-R. [DOI] [Google Scholar]
- 53.Ibanez E, Albertini DF, Overstrom EW. Effect of genetic background and activating stimulus on the timing of meiotic cell cycle progression in parthenogenetically activated mouse oocytes. Reproduction. 2005;129:27–38. doi: 10.1530/rep.1.00452. [DOI] [PubMed] [Google Scholar]
- 54.Zhang N, Yoon SY, Parys JB, Fissore RA. Effect of M-phase kinase phosphorylations on type 1 inositol 1,4,5-trisphosphate receptor-mediated Ca2+ responses in mouse eggs. Cell Calcium. 2015;58:476–488. doi: 10.1016/j.ceca.2015.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Yeste M, Jones C, Amdani SN, Patel S, Coward K. Oocyte activation deficiency: a role for an oocyte contribution? Hum Reprod Update. 2016;22:23–47. doi: 10.1093/humupd/dmv040. [DOI] [PubMed] [Google Scholar]
- 56.Aarabi M, Balakier H, Bashar S, Moskovtsev SI, Sutovsky P, Librach CL, Oko R. Sperm-derived WW domain-binding protein, PAWP, elicits calcium oscillations and oocyte activation in humans and mice. FASEB J. 2014;28:4434–4440. doi: 10.1096/fj.14-256495. [DOI] [PubMed] [Google Scholar]
- 57.Satouh Y, Nozawa K, Ikawa M. Sperm postacrosomal WW domain-binding protein is not required for mouse egg activation. Biol Reprod. 2015;93:94. doi: 10.1095/biolreprod.115.131441. [DOI] [PubMed] [Google Scholar]
- 58.Nomikos M, Swann K, Lai FA. Starting a new life: sperm PLC-zeta mobilizes the Ca2+ signal that induces egg activation and embryo development. Bioessays. 2012;34:126–134. doi: 10.1002/bies.201100127. [DOI] [PubMed] [Google Scholar]
- 59.Swann K, Yu Y. The dynamics of calcium oscillations that activate mammalian eggs. Int J Dev Biol. 2008;52:585–594. doi: 10.1387/ijdb.072530ks. [DOI] [PubMed] [Google Scholar]
- 60.Yoon SY, Eum JH, Lee JE, Lee HC, Kim YS, Han JE, Won HJ, Park SH, Shim SH, Lee WS, et al. Recombinant human phospholipase C zeta 1 induces intracellular calcium oscillations and oocyte activation in mouse and human oocytes. Hum Reprod. 2012;27:1768–1780. doi: 10.1093/humrep/des092. [DOI] [PubMed] [Google Scholar]
- 61.He CL, Damiani P, Ducibella T, Takahashi M, Tanzawa K, Parys JB, Fissore RA. Isoforms of the inositol 1,4,5-trisphosphate receptor are expressed in bovine oocytes and ovaries: the type-1 isoform is down-regulated by fertilization and by injection of adenophostin A. Biol Reprod. 1999;61:935–943. doi: 10.1095/biolreprod61.4.935. [DOI] [PubMed] [Google Scholar]
- 62.Homa ST, Swann K. Fertilization and early embryology: a cytosolic sperm factor triggers calcium oscillations and membrane hyperpolarizations in human oocytes. Hum Reprod. 1994;9:2356–2361. doi: 10.1093/oxfordjournals.humrep.a138452. [DOI] [PubMed] [Google Scholar]
- 63.Stricker SA. Comparative biology of calcium signaling during fertilization and egg activation in animals. Dev Biol. 1999;211:157–176. doi: 10.1006/dbio.1999.9340. [DOI] [PubMed] [Google Scholar]
- 64.Wu AT, Sutovsky P, Manandhar G, Xu W, Katayama M, Day BN, Park KW, Yi YJ, Xi YW, Prather RS, et al. PAWP, a sperm-specific WW domain-binding protein, promotes meiotic resumption and pronuclear development during fertilization. J Biol Chem. 2007;282:12164–12175. doi: 10.1074/jbc.M609132200. [DOI] [PubMed] [Google Scholar]
- 65.Anifandis G, Messini CI, Dafopoulos K, Daponte A, Messinis IE. Sperm contributions to oocyte activation: more that meets the eye. J Assist Reprod Genet. 2016;33:313–316. doi: 10.1007/s10815-016-0653-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Cox LJ, Larman MG, Saunders CM, Hashimoto K, Swann K, Lai FA. Sperm phospholipase Czeta from humans and cynomolgus monkeys triggers Ca2+ oscillations, activation and development of mouse oocytes. Reproduction. 2002;124:611–623. doi: 10.1530/rep.0.1240611. [DOI] [PubMed] [Google Scholar]
- 67.Kouchi Z, Fukami K, Shikano T, Oda S, Nakamura Y, Takenawa T, Miyazaki S. Recombinant phospholipase C zeta has high Ca2+ sensitivity and induces Ca2+ oscillations in mouse eggs. J Biol Chem. 2004;279:10408–10412. doi: 10.1074/jbc.M313801200. [DOI] [PubMed] [Google Scholar]
- 68.Nomikos M, Yu Y, Elgmati K, Theodoridou M, Campbell K, Vassilakopoulou V, Zikos C, Livaniou E, Amso N, Nounesis G, et al. Phospholipase C zeta rescues failed oocyte activation in a prototype of male factor infertility. Fertil Steril. 2013;99:76–85. doi: 10.1016/j.fertnstert.2012.08.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Amdani SN, Yeste M, Jones C, Coward K. Phospholipase C zeta (PLCzeta) and male infertility: clinical update and topical developments. Adv Biol Regul. 2016;61:58–67. doi: 10.1016/j.jbior.2015.11.009. [DOI] [PubMed] [Google Scholar]
- 70.Lee HC, Arny M, Grow D, Dumesic D, Fissore RA, Jellerette-Nolan T. Protein phospholipase C zeta1 expression in patients with failed ICSI but with normal sperm parameters. J Assist Reprod Genet. 2014;31:749–756. doi: 10.1007/s10815-014-0229-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Yelumalai S, Yeste M, Jones C, Amdani SN, Kashir J, Mounce G, Da Silva SJ, Barratt CL, McVeigh E, Coward K. Total levels, localization patterns, and proportions of sperm exhibiting phospholipase C zeta are significantly correlated with fertilization rates after intracytoplasmic sperm injection. Fertil Steril. 2015;104(561–8):e4. doi: 10.1016/j.fertnstert.2015.05.018. [DOI] [PubMed] [Google Scholar]
- 72.Yoon S-Y, Jellerette T, Salicioni AM, Lee HC, Yoo M-S, Coward K, Parrington J, Grow D, Cibelli JB, Visconti PE, et al. Human sperm devoid of PLC, zeta 1 fail to induce Ca2+ release and are unable to initiate the first step of embryo development. J Clin Invest. 2008;118:3671–3681. doi: 10.1172/JCI36942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Heytens E, Parrington J, Coward K, Young C, Lambrecht S, Yoon S-Y, Fissore RA, Hamer R, Deane CM, Ruas M, et al. Reduced amounts and abnormal forms of phospholipase C zeta (PLCζ) in spermatozoa from infertile men. Hum Reprod. 2009;24:2417–2428. doi: 10.1093/humrep/dep207. [DOI] [PubMed] [Google Scholar]
- 74.Javadian-Elyaderani S, Ghaedi K, Tavalaee M, Rabiee F, Deemeh MR, Nasr-Esfahani MH. Diagnosis of genetic defects through parallel assessment of PLCzeta and CAPZA3 in infertile men with history of failed oocyte activation. Iran J Basic Med Sci. 2016;19:281–289. [PMC free article] [PubMed] [Google Scholar]
- 75.Tavalaee M, Nasr-Esfahani MH. Expression profile of PLCzeta, PAWP, and TR-KIT in association with fertilization potential, embryo development, and pregnancy outcomes in globozoospermic candidates for intra-cytoplasmic sperm injection and artificial oocyte activation. Andrology. 2016;4:850–856. doi: 10.1111/andr.12179. [DOI] [PubMed] [Google Scholar]
- 76.Que EL, Bleher R, Duncan FE, Kong BY, Gleber SC, Vogt S, Chen S, Garwin SA, Bayer AR, Dravid VP, et al. Quantitative mapping of zinc fluxes in the mammalian egg reveals the origin of fertilization-induced zinc sparks. Nat Chem. 2015;7:130–139. doi: 10.1038/nchem.2133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Kong BY, Duncan FE, Que EL, Kim AM, O’Halloran TV, Woodruff TK. Maternally-derived zinc transporters ZIP6 and ZIP10 drive the mammalian oocyte-to-egg transition. Mol Hum Reprod. 2014;20:1077–1089. doi: 10.1093/molehr/gau066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Duncan FE, Que EL, Zhang N, Feinberg EC, O’Halloran TV, Woodruff TK. The zinc spark is an inorganic signature of human egg activation. Sci Rep. 2016;6:24737. doi: 10.1038/srep24737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Kim AM, Bernhardt ML, Kong BY, Ahn RW, Vogt S, Woodruff TK, O’Halloran TV. Zinc sparks are triggered by fertilization and facilitate cell cycle resumption in mammalian eggs. ACS Chem Biol. 2011;6:716–723. doi: 10.1021/cb200084y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Kim AM, Vogt S, O’Halloran TV, Woodruff TK. Zinc availability regulates exit from meiosis in maturing mammalian oocytes. Nat Chem Biol. 2010;6:674–681. doi: 10.1038/nchembio.419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Bernhardt ML, Kong BY, Kim AM, O’Halloran TV, Woodruff TK. A zinc-dependent mechanism regulates meiotic progression in mammalian oocytes. Biol Reprod. 2012;86:114. doi: 10.1095/biolreprod.111.097253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Bernhardt ML, Kim AM, O’Halloran TV, Woodruff TK. Zinc requirement during meiosis I-meiosis II transition in mouse oocytes is independent of the MOS-MAPK pathway. Biol Reprod. 2011;84:526–536. doi: 10.1095/biolreprod.110.086488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Kong BY, Duncan FE, Que EL, Xu Y, Vogt S, O’Halloran TV, Woodruff TK. The inorganic anatomy of the mammalian preimplantation embryo and the requirement of zinc during the first mitotic divisions. Dev Dyn. 2015;244:935–947. doi: 10.1002/dvdy.24285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Zhang N, Duncan FE, Que EL, O’Halloran TV, Woodruff TK. The fertilization-induced zinc spark is a novel biomarker of mouse embryo quality and early development. Sci Rep. 2016;6:22772. doi: 10.1038/srep22772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Ducibella T, Fissore R. The roles of Ca2+, downstream protein kinases, and oscillatory signaling in regulating fertilization and the activation of development. Dev Biol. 2008;315:257–279. doi: 10.1016/j.ydbio.2007.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Karve TM, Cheema AK. Small changes huge impact: the role of protein posttranslational modifications in cellular homeostasis and disease. J Amino Acids. 2011;2011:207691. doi: 10.4061/2011/207691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Kim DA, Suh EK. Defying DNA double-strand break-induced death during prophase I meiosis by temporal TAp63alpha phosphorylation regulation in developing mouse oocytes. Mol Cell Biol. 2014;34:1460–1473. doi: 10.1128/MCB.01223-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Li L, Zheng P, Dean J. Maternal control of early mouse development. Development. 2010;137:859–870. doi: 10.1242/dev.039487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Flach G, Johnson MH, Braude PR, Taylor RA, Bolton VN. The transition from maternal to embryonic control in the 2-cell mouse embryo. EMBO J. 1982;1:681–686. doi: 10.1002/j.1460-2075.1982.tb01230.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Schultz RM. Regulation of zygotic gene activation in the mouse. Bioessays. 1993;15:531–538. doi: 10.1002/bies.950150806. [DOI] [PubMed] [Google Scholar]
- 91.Telford NA, Watson AJ, Schultz GA. Transition from maternal to embryonic control in early mammalian development: a comparison of several species. Mol Reprod Dev. 1990;26:90–100. doi: 10.1002/mrd.1080260113. [DOI] [PubMed] [Google Scholar]
- 92.Cohen P. The regulation of protein function by multisite phosphorylation–a 25 year update. Trends Biochem Sci. 2000;25:596–601. doi: 10.1016/S0968-0004(00)01712-6. [DOI] [PubMed] [Google Scholar]
- 93.Kranuchunas AR, Horner VL, Wolfner MF. Protein phosphorylation changes reveal new candidates in the regulation of egg activation and early embryogenesis in D. melanogaster . Dev Biol. 2012;370:125–134. doi: 10.1016/j.ydbio.2012.07.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Endo Y, Kopf GS, Schultz RM. Stage-specific changes in protein phosphorylation accompanying meiotic maturation of mouse oocytes and fertilization of mouse eggs. J Exp Zool. 1986;239:401–409. doi: 10.1002/jez.1402390311. [DOI] [PubMed] [Google Scholar]
- 95.Halet G, Tunwell R, Parkinson SJ, Carroll J. Conventional PKCs regulate the temporal pattern of Ca2+ oscillations at fertilization in mouse eggs. J Cell Biol. 2004;164:1033–1044. doi: 10.1083/jcb.200311023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Gonzalez-Garcia JR, Machaty Z, Lai FA, Swann K. The dynamics of PKC-induced phosphorylation TRiggered by Ca(2+) oscillations in mouse eggs. J Cell Physiol. 2013;228:110–119. doi: 10.1002/jcp.24110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Baluch DP, Koeneman BA, Hatch KR, McGaughey RW, Capco DG. PKC isotypes in post-activated and fertilized mouse eggs: association with the meiotic spindle. Dev Biol. 2004;274:45–55. doi: 10.1016/j.ydbio.2004.05.030. [DOI] [PubMed] [Google Scholar]
- 98.Luria A, Tennenbaum T, Sun QY, Rubinstein S, Breitbart H. Differential localization of conventional protein kinase C isoforms during mouse oocyte development. Biol Reprod. 2000;62:1564–1570. doi: 10.1095/biolreprod62.6.1564. [DOI] [PubMed] [Google Scholar]
- 99.Markoulaki S, Matson S, Ducibella T. Fertilization stimulates long-lasting oscillations of CaMKII activity in mouse eggs. Dev Biol. 2004;272:15–25. doi: 10.1016/j.ydbio.2004.04.008. [DOI] [PubMed] [Google Scholar]
- 100.Tatone C, Delle Monache S, Iorio R, Caserta D, Di Cola M, Colonna R. Possible role for Ca(2+) calmodulin-dependent protein kinase II as an effector of the fertilization Ca(2+) signal in mouse oocyte activation. Mol Hum Reprod. 2002;8:750–757. doi: 10.1093/molehr/8.8.750. [DOI] [PubMed] [Google Scholar]
- 101.Komatsu S, Ikebe M. ZIP kinase is responsible for the phosphorylation of myosin II and necessary for cell motility in mammalian fibroblasts. J Cell Biol. 2004;165:243–254. doi: 10.1083/jcb.200309056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Deng M, Williams CJ, Schultz RM. Role of MAP kinase and myosin light chain kinase in chromosome-induced development of mouse egg polarity. Dev Biol. 2005;278:358–366. doi: 10.1016/j.ydbio.2004.11.013. [DOI] [PubMed] [Google Scholar]
- 103.Matson S, Markoulaki S, Ducibella T. Antagonists of myosin light chain kinase and of myosin II inhibit specific events of egg activation in fertilized mouse eggs. Biol Reprod. 2006;74:169–176. doi: 10.1095/biolreprod.105.046409. [DOI] [PubMed] [Google Scholar]
- 104.Zhong ZS, Huo LJ, Liang CG, Chen DY, Sun QY. Small GTPase RhoA is required for ooplasmic segregation and spindle rotation, but not for spindle organization and chromosome separation during mouse oocyte maturation, fertilization, and early cleavage. Mol Reprod Dev. 2005;71:256–261. doi: 10.1002/mrd.20253. [DOI] [PubMed] [Google Scholar]
- 105.Zhang JY, Dong HS, Oqani RK, Lin T, Kang JW, Jin DI. Distinct roles of ROCK1 and ROCK2 during development of porcine preimplantation embryos. Reproduction. 2014;148:99–107. doi: 10.1530/REP-13-0556. [DOI] [PubMed] [Google Scholar]
- 106.McGinnis LA, Lee HJ, Robinson DN, Evans JP. MAPK3/1 (ERK1/2) and myosin light chain kinase in mammalian eggs affect myosin-II function and regulate the metaphase II state in a calcium- and zinc-dependent manner. Biol Reprod. 2015;92(146):1–14. doi: 10.1095/biolreprod.114.127027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Liang QX, Zhang QH, Qi ST, Wang ZW, Hu MW, Ma XS, Zhu MS, Schatten H, Wang ZB, Sun QY. Deletion of Mylk1 in oocytes causes delayed morula-to-blastocyst transition and reduced fertility without affecting folliculogenesis and oocyte maturation in mice. Biol Reprod. 2015;92:97. doi: 10.1095/biolreprod.114.122127. [DOI] [PubMed] [Google Scholar]
- 108.Simerly C, Nowak G, de Lanerolle P, Schatten G. Differential expression and functions of cortical myosin IIA and IIB isotypes during meiotic maturation, fertilization, and mitosis in mouse oocytes and embryos. Mol Biol Cell. 1998;9:2509–2525. doi: 10.1091/mbc.9.9.2509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Bement WM, Benink HA, von Dassow G. A microtubule-dependent zone of active RhoA during cleavage plane specification. J Cell Biol. 2005;170:91–101. doi: 10.1083/jcb.200501131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Lucero A, Stack C, Bresnick AR, Shuster CB. A global, myosin light chain kinase-dependent increase in myosin II contractility accompanies the metaphase-anaphase transition in sea urchin eggs. Mol Biol Cell. 2006;17:4093–4104. doi: 10.1091/mbc.E06-02-0119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Lee B, Vermassen E, Yoon S-Y, Vanderheyden V, Ito J, Alfandari D, De Smedt H, Parys JB, Fissore RA. Phosphorylation of IP(3)R1 and the regulation of [Ca(2+)](i) responses at fertilization: a role for the MAP kinase pathway. Dev (Cambridge, England) 2006;133:4355–4365. doi: 10.1242/dev.02624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Ito J, Yoon S-Y, Lee B, Vanderheyden V, Vermassen E, Wojcikiewicz R, Alfandari D, De Smedt H, Parys JB, Fissore RA. Inositol 1,4,5-trisphosphate receptor 1, a widespread Ca2+ channel, is a novel substrate of polo-like kinase 1 in eggs. Dev Biol. 2008;320:402–413. doi: 10.1016/j.ydbio.2008.05.548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Almeida KH, Sobol RW. A unified view of base excision repair: lesion-dependent protein complexes regulated by post-translational modification. DNA Repair. 2007;6:695–711. doi: 10.1016/j.dnarep.2007.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Hakem R. DNA-damage repair; the good, the bad, and the ugly. EMBO J. 2008;27:589–605. doi: 10.1038/emboj.2008.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Maiani E, Diederich M, Gonfloni S. DNA damage response: the emerging role of c-Abl as a regulatory switch? Biochem Pharmacol. 2011;82:1269–1276. doi: 10.1016/j.bcp.2011.07.001. [DOI] [PubMed] [Google Scholar]
- 116.Morgan WF, Corcoran J, Hartmann A, Kaplan MI, Limoli CL, Ponnaiya B. DNA double-strand breaks, chromosomal rearrangements, and genomic instability. Mutat Res. 1998;404:125–128. doi: 10.1016/S0027-5107(98)00104-3. [DOI] [PubMed] [Google Scholar]
- 117.Yuan S, Tang C, Zhang Y, Wu J, Bao J, Zheng H, Xu C, Yan W. mir-34b/c and mir-449a/b/c are required for spermatogenesis, but not for the first cleavage division in mice. Biol Open. 2015;4:212–223. doi: 10.1242/bio.201410959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Ostermeier GC, Miller D, Huntriss JD, Diamond MP, Krawetz SA. Reproductive biology: delivering spermatozoan RNA to the oocyte. Nature. 2004;429:154. doi: 10.1038/429154a. [DOI] [PubMed] [Google Scholar]
- 119.Kumar M, Kumar K, Jain S, Hassan T, Dada R. Novel insights into the genetic and epigenetic paternal contribution to the human embryo. Clinics. 2013;68:5–14. doi: 10.6061/clinics/2013(Sup01)02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Rodgers AB, Morgan CP, Leu NA, Bale TL. Transgenerational epigenetic programming via sperm microRNA recapitulates effects of paternal stress. Proc Nat Acad Sci. 2015;112:13699–13704. doi: 10.1073/pnas.1508347112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Zheng P, Schramm RD, Latham KE. Developmental regulation and in vitro culture effects on expression of DNA repair and cell cycle checkpoint control genes in rhesus monkey oocytes and embryos. Biol Reprod. 2005;72:1359–1369. doi: 10.1095/biolreprod.104.039073. [DOI] [PubMed] [Google Scholar]
- 122.Liu W-M, Pang RTK, Chiu PCN, Wong BPC, Lao K, Lee K-F, Yeung WSB. Sperm-borne microRNA-34c is required for the first cleavage division in mouse. Proc Natl Acad Sci U S A. 2012;109:490–494. doi: 10.1073/pnas.1110368109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Gawecka JE, Marh J, Ortega M, Yamauchi Y, Ward MA, Ward WS. Mouse zygotes respond to severe sperm DNA damage by delaying paternal DNA replication and embryonic development. PLoS One. 2013;8:e56385. doi: 10.1371/journal.pone.0056385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Di Giacomo M, Barchi M, Baudat F, Edelmann W, Keeney S, Jasin M. Distinct DNA-damage-dependent and -independent responses drive the loss of oocytes in recombination-defective mouse mutants. Proc Nat Acad Sci U S A. 2005;102:737–742. doi: 10.1073/pnas.0406212102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Subba Rao K. Mechanisms of disease: DNA repair defects and neurological disease. Nat Clin Pract Neuro. 2007;3:162–172. doi: 10.1038/ncpneuro0448. [DOI] [PubMed] [Google Scholar]
- 126.O’Driscoll M, Jeggo PA. The role of double-strand break repair - insights from human genetics. Nat Rev Genet. 2006;7:45–54. doi: 10.1038/nrg1746. [DOI] [PubMed] [Google Scholar]
- 127.Beaujean N. Epigenetics, embryo quality and developmental potential. Reprod Fertil Dev. 2014;27:53–62. doi: 10.1071/RD14309. [DOI] [PubMed] [Google Scholar]
- 128.Kastan MB, Onyekwere O, Sidransky D, Vogelstein B, Craig RW. Participation of p53 protein in the cellular response to DNA damage. Cancer Res. 1991;51:6304–6311. [PubMed] [Google Scholar]
- 129.Meng S, Lin L, Lama S, Qiao M, Tuor UI. Cerebral expression of DNA repair protein, Ku70, and its association with cell proliferation following cerebral hypoxia-ischemia in neonatal rats. Int J Dev Neurosci. 2009;27:129–134. doi: 10.1016/j.ijdevneu.2008.12.002. [DOI] [PubMed] [Google Scholar]
- 130.Wang S, Kou Z, Jing Z, Zhang Y, Guo X, Dong M, Wilmut I, Gao S. Proteome of mouse oocytes at different developmental stages. Proc Natl Acad Sci U S A. 2010;107:17639–17644. doi: 10.1073/pnas.1013185107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Kocabas AM, Crosby J, Ross PJ, Otu HH, Beyhan Z, Can H, Tam W-L, Rosa GJM, Halgren RG, Lim B, Fernandez E, Cibelli JB. The transcriptome of human oocytes. Proc Nat Acad Sci. 2006;103:14027–14032. doi: 10.1073/pnas.0603227103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Oberle C, Blattner C. Regulation of the DNA damage response to DSBs by post-translational modifications. Curr Genomics. 2010;11:184–198. doi: 10.2174/138920210791110979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Martin JH, Nixon B, Lord T, Bromfield EG, Aitken RJ. Identification of a key role for permeability glycoprotein in enhancing the cellular defense mechanisms of fertilized oocytes. Dev Biol. 2016;417:63–76. doi: 10.1016/j.ydbio.2016.06.035. [DOI] [PubMed] [Google Scholar]
- 134.Martin JH, Bromfield EG, Aitken RJ, Lord T, Nixon B. Data on the concentrations of etoposide, PSC833, BAPTA-AM, and cycloheximide that do not compromise the vitality of mature mouse oocytes, parthenogencially activated and fertilized embryos. Data Brief. 2016;8:1215–1220. doi: 10.1016/j.dib.2016.07.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Powell MD, Manandhar G, Spate L, Sutovsky M, Zimmerman S, Sachdev SC, Hannink M, Prather RS, Sutovsky P. Discovery of putative oocyte quality markers by comparative ExacTag proteomics. Proteomics Clin Appl. 2010;4:337–351. doi: 10.1002/prca.200900024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Jaroudi S, Kakourou G, Cawood S, Doshi A, Ranieri DM, Serhal P, Harper JC, SenGupta SB. Expression profiling of DNA repair genes in human oocytes and blastocysts using microarrays. Hum Reprod. 2009;24:2649–2655. doi: 10.1093/humrep/dep224. [DOI] [PubMed] [Google Scholar]
- 137.Harrouk W, Codrington A, Vinson R, Robaire B, Hales BF. Paternal exposure to cyclophosphamide induces DNA damage and alters the expression of DNA repair genes in the rat preimplantation embryo. Mutat Res. 2000;461:229–241. doi: 10.1016/S0921-8777(00)00053-7. [DOI] [PubMed] [Google Scholar]
- 138.Zuelke KA, Jeffay SC, Zucker RM, Perreault SD. Glutathione (GSH) concentrations vary with the cell cycle in maturing hamster oocytes, zygotes, and pre-implantation stage embryos. Mol Reprod Dev. 2003;64:106–112. doi: 10.1002/mrd.10214. [DOI] [PubMed] [Google Scholar]
- 139.Nakamura BN, Fielder TJ, Hoang YD, Lim J, McConnachie LA, Kavanagh TJ, Luderer U. Lack of maternal glutamate cysteine ligase modifier subunit (Gclm) decreases oocyte glutathione concentrations and disrupts preimplantation development in mice. Endocrinology. 2011;152:2806–2815. doi: 10.1210/en.2011-0207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Anifandis G, Messini CI, Dafopoulos K, Messinis IE. Genes and conditions controlling mammalian pre- and post-implantation embryo development. Curr Genomics. 2015;16:32–46. doi: 10.2174/1389202916666141224205025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Shao GB, Ding HM, Gong AH. Role of histone methylation in zygotic genome activation in the preimplantation mouse embryo. In Vitro Cell Dev Biol Anim. 2008;44:115–120. doi: 10.1007/s11626-008-9082-4. [DOI] [PubMed] [Google Scholar]
- 142.Wang J, Zhang M, Zhang Y, Kou Z, Han Z, Chen DY, Sun QY, Gao S. The histone demethylase JMJD2C is stage-specifically expressed in preimplantation mouse embryos and is required for embryonic development. Biol Reprod. 2010;82:105–111. doi: 10.1095/biolreprod.109.078055. [DOI] [PubMed] [Google Scholar]
- 143.Oda H, Okamoto I, Murphy N, Chu J, Price SM, Shen MM, Torres-Padilla ME, Heard E, Reinberg D. Monomethylation of histone H4-lysine 20 is involved in chromosome structure and stability and is essential for mouse development. Mol Cell Biol. 2009;29:2278–2295. doi: 10.1128/MCB.01768-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Schneider R, Grosschedl R. Dynamics and interplay of nuclear architecture, genome organization, and gene expression. Genes Dev. 2007;21:3027–3043. doi: 10.1101/gad.1604607. [DOI] [PubMed] [Google Scholar]
- 145.Santos F, Hendrich B, Reik W, Dean W. Dynamic reprogramming of DNA methylation in the early mouse embryo. Dev Biol. 2002;241:172–182. doi: 10.1006/dbio.2001.0501. [DOI] [PubMed] [Google Scholar]
- 146.Santos F, Dean W. Epigenetic reprogramming during early development in mammals. Reproduction. 2004;127:643–651. doi: 10.1530/rep.1.00221. [DOI] [PubMed] [Google Scholar]
- 147.Peterson CL, Laniel MA. Histones and histone modifications. Curr Biol. 2004;14:R546–R551. doi: 10.1016/j.cub.2004.07.007. [DOI] [PubMed] [Google Scholar]
- 148.Kouzarides T. Chromatin modifications and their function. Cell. 2007;128:693–705. doi: 10.1016/j.cell.2007.02.005. [DOI] [PubMed] [Google Scholar]
- 149.Wossidlo M, Arand J, Sebastiano V, Lepikhov K, Boiani M, Reinhardt R, Scholer H, Walter J. Dynamic link of DNA demethylation, DNA strand breaks and repair in mouse zygotes. EMBO J. 2010;29:1877–1888. doi: 10.1038/emboj.2010.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Nixon B, Stanger SJ, Mihalas BP, Reilly JN, Anderson AL, Tyagi S, Holt JE, McLaughlin EA. The microRNA signature of mouse spermatozoa is substantially modified during epididymal maturation. Biol Reprod. 2015;93:91. doi: 10.1095/biolreprod.115.132209. [DOI] [PubMed] [Google Scholar]
- 151.Reilly JN, McLaughlin EA, Stanger SJ, Anderson AL, Hutcheon K, Church K, Mihalas BP, Tyagi S, Holt JE, Eamens AL, et al. Characterisation of mouse epididymosomes reveals a complex profile of microRNAs and a potential mechanism for modification of the sperm epigenome. Sci Rep. 2016;6:31794. doi: 10.1038/srep31794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Wu Q, Song R, Ortogero N, Zheng H, Evanoff R, Small CL, Griswold MD, Namekawa SH, Royo H, Turner JM, et al. The RNase III enzyme DROSHA is essential for MicroRNA production and spermatogenesis. J Biol Chem. 2012;287:25173–25190. doi: 10.1074/jbc.M112.362053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Yamauchi Y, Shaman JA, Ward WS. Non-genetic contributions of the sperm nucleus to embryonic development. Asian J Androl. 2011;13:31–35. doi: 10.1038/aja.2010.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Krawetz SA. Paternal contribution: new insights and future challenges. Nat Rev Genet. 2005;6:633–642. doi: 10.1038/nrg1654. [DOI] [PubMed] [Google Scholar]
- 155.Roepke TA, Hamdoun AM, Cherr GN. Increase in multidrug transport activity is associated with oocyte maturation in sea stars. Dev Growth Differ. 2006;48:559–573. doi: 10.1111/j.1440-169X.2006.00893.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Elbling L, Berger W, Rehberger A, Waldhor T, Micksche M. P-glycoprotein regulates chemosensitivity in early developmental stages of the mouse. FASEB J. 1993;7:1499–1506. doi: 10.1096/fasebj.7.15.7903262. [DOI] [PubMed] [Google Scholar]
- 157.Hamdoun AM, Cherr GN, Roepke TA, Epel D. Activation of multidrug efflux transporter activity at fertilization in sea urchin embryos (Strongylocentrotus purpuratus) Dev Biol. 2004;276:452–462. doi: 10.1016/j.ydbio.2004.09.013. [DOI] [PubMed] [Google Scholar]
- 158.Mechetner E, Kyshtoobayeva A, Zonis S, Kim H, Stroup R, Garcia R, Parker RJ, Fruehauf JP. Levels of multidrug resistance (MDR1) P-glycoprotein expression by human breast cancer correlate with in vitro resistance to taxol and doxorubicin. Clin Cancer Res. 1998;4:389–398. [PubMed] [Google Scholar]
- 159.Idriss HT, Hannun YA, Boulpaep E, Basavappa S. Regulation of volume-activated chloride channels by P-glycoprotein: phosphorylation has the final say! J Physiol. 2000;524:629–636. doi: 10.1111/j.1469-7793.2000.00629.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Lelong-Rebel IH, Cardarelli CO. Differential phosphorylation patterns of P-glycoprotein reconstituted into a proteoliposome system: insight into additional unconventional phosphorylation sites. Anticancer Res. 2005;25:3925–3935. [PubMed] [Google Scholar]
- 161.Vanoye CG, Castro AF, Pourcher T, Reuss L, Altenberg GA. Phosphorylation of P-glycoprotein by PKA and PKC modulates swelling-activated Cl- currents. Am J Physiol. 1999;276:C370–C378. doi: 10.1152/ajpcell.1999.276.2.C370. [DOI] [PubMed] [Google Scholar]
- 162.Kfir-Erenfeld S, Sionov RV, Spokoini R, Cohen O, Yefenof E. Protein kinase networks regulating glucocorticoid-induced apoptosis of hematopoietic cancer cells: fundamental aspects and practical considerations. Leuk Lymphoma. 2010;51:1968–2005. doi: 10.3109/10428194.2010.506570. [DOI] [PubMed] [Google Scholar]
- 163.Mori M, Kasa S, Isozaki Y, Kamori T, Yamaguchi S, Ueda S, Kuwano T, Eguchi M, Isayama K, Nishimura S, et al. Improvement of the cellular quality of cryopreserved bovine blastocysts accompanied by enhancement of the ATP-binding cassette sub-family B member 1 expression. Reprod Toxicol. 2013;35:17–24. doi: 10.1016/j.reprotox.2012.11.002. [DOI] [PubMed] [Google Scholar]
- 164.Yokota K, Hirano T, Urata N, Yamauchi N, Hattori MA. Upregulation of P-glycoprotein activity in porcine oocytes and granulosa cells during in vitro maturation. J Reprod Dev. 2011;57:322–326. doi: 10.1262/jrd.10-137M. [DOI] [PubMed] [Google Scholar]
- 165.Degtyareva NP, Chen L, Mieczkowski P, Petes TD, Doetsch PW. Chronic oxidative DNA damage due to DNA repair defects causes chromosomal instability in Saccharomyces cerevisiae . Mol Cell Biol. 2008;28:5432–5445. doi: 10.1128/MCB.00307-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Aitken RJ, Findlay JK, Hutt KJ, Kerr JB. Apoptosis in the germ line. Reproduction. 2011;141:139–150. doi: 10.1530/REP-10-0232. [DOI] [PubMed] [Google Scholar]
- 167.Hutt KJ. The role of BH3-only proteins in apoptosis within the ovary. Reproduction. 2015;149:R81–R89. doi: 10.1530/REP-14-0422. [DOI] [PubMed] [Google Scholar]
- 168.Reynaud K, Driancourt MA. Oocyte attrition. Mol Cell Endocrinol. 2000;163:101–108. doi: 10.1016/S0303-7207(99)00246-4. [DOI] [PubMed] [Google Scholar]
- 169.Grive KJ, Freiman RN. The developmental origins of the mammalian ovarian reserve. Development. 2015;142:2554–2563. doi: 10.1242/dev.125211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Pangas SA. Regulation of the ovarian reserve by members of the transforming growth factor beta family. Mol Reprod Dev. 2012;79:666–679. doi: 10.1002/mrd.22076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Wood MA, Rajkovic A. Genomic markers of ovarian reserve. Semin Reprod Med. 2013;31:399–415. doi: 10.1055/s-0033-1356476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.deSouza N, Reiken S, Ondrias K, Yang Y-M, Matkovich S, Marks AR. Protein kinase A and two phosphatases are components of the inositol 1,4,5-trisphosphate receptor macromolecular signaling complex. J Biol Chem. 2002;277:39397–39400. doi: 10.1074/jbc.M207059200. [DOI] [PubMed] [Google Scholar]
- 173.Kalive M, Faust JJ, Koeneman BA, Capco DG. Involvement of the PKC family in regulation of early development. Mol Reprod Dev. 2010;77:95–104. doi: 10.1002/mrd.21112. [DOI] [PubMed] [Google Scholar]
- 174.Vermassen E, Fissore RA, Nadif Kasri N, Vanderheyden V, Callewaert G, Missiaen L, Parys JB, De Smedt H. Regulation of the phosphorylation of the inositol 1,4,5-trisphosphate receptor by protein kinase C. Biochem Biophys Res Commun. 2004;319:888–893. doi: 10.1016/j.bbrc.2004.05.071. [DOI] [PubMed] [Google Scholar]
- 175.Gangeswaran R, Jones KT. Unique protein kinase C profile in mouse oocytes: lack of calcium-dependent conventional isoforms suggested by rtPCR and Western blotting. FEBS Lett. 1997;412:309–312. doi: 10.1016/S0014-5793(97)00782-5. [DOI] [PubMed] [Google Scholar]
- 176.Eliyahu E, Shalgi R. A role for protein kinase C during rat egg activation. Biol Reprod. 2002;67:189–195. doi: 10.1095/biolreprod67.1.189. [DOI] [PubMed] [Google Scholar]
- 177.Fan H-Y, Tong C, Li M-Y, Lian L, Chen D-Y, Schatten H, Sun Q-Y. Translocation of the classic protein kinase C isoforms in porcine oocytes: implications of protein kinase C involvement in the regulation of nuclear activity and cortical granule exocytosis. Exp Cell Res. 2002;277:183–191. doi: 10.1006/excr.2002.5547. [DOI] [PubMed] [Google Scholar]
- 178.Johnson J, Bierle BM, Gallicano GI, Capco DG. Calcium/calmodulin-dependent protein kinase II and calmodulin: regulators of the meiotic spindle in mouse eggs. Dev Biol. 1998;204:464–477. doi: 10.1006/dbio.1998.9038. [DOI] [PubMed] [Google Scholar]
- 179.Jones KT. Mammalian egg activation: from Ca2+ spiking to cell cycle progression. Reproduction. 2005;130:813–823. doi: 10.1530/rep.1.00710. [DOI] [PubMed] [Google Scholar]
- 180.Madgwick S, Levasseur M, Jones KT. Calmodulin-dependent protein kinase II, and not protein kinase C, is sufficient for triggering cell-cycle resumption in mammalian eggs. J Cell Sci. 2005;118:3849–3859. doi: 10.1242/jcs.02506. [DOI] [PubMed] [Google Scholar]
- 181.McCoy F, Darbandi R, Lee HC, Bharatham K, Moldoveanu T, Grace CR, Dodd K, Lin W, Chen SI, Tangallapally RP, et al. Metabolic activation of CaMKII by coenzyme A. Mol Cell. 2013;52:325–339. doi: 10.1016/j.molcel.2013.08.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Tong C, Fan HY, Lian L, Li SW, Chen DY, Schatten H, Sun QY. Polo-like kinase-1 is a pivotal regulator of microtubule assembly during mouse oocyte meiotic maturation, fertilization, and early embryonic mitosis. Biol Reprod. 2002;67:546–554. doi: 10.1095/biolreprod67.2.546. [DOI] [PubMed] [Google Scholar]
- 183.Jia J-L, Han Y-H, Kim H-C, Ahn M, Kwon J-W, Luo Y, Gunasekaran P, Lee S-J, Lee KS, Kyu Bang J, et al. Structural basis for recognition of Emi2 by Polo-like kinase 1 and development of peptidomimetics blocking oocyte maturation and fertilization. Sci Rep. 2015;5:14626. doi: 10.1038/srep14626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Chang H-Y, Minahan K, Merriman JA, Jones KT. Calmodulin-dependent protein kinase gamma 3 (CamKIIγ3) mediates the cell cycle resumption of metaphase II eggs in mouse. Development. 2009;136:4077–4081. doi: 10.1242/dev.042143. [DOI] [PubMed] [Google Scholar]
- 185.Kline D, Mehlmann L, Fox C, Terasaki M. The cortical endoplasmic reticulum (ER) of the mouse egg: localization of ER clusters in relation to the generation of repetitive calcium waves. Dev Biol. 1999;215:431–442. doi: 10.1006/dbio.1999.9445. [DOI] [PubMed] [Google Scholar]
- 186.Sun QY, Wu GM, Lai L, Bonk A, Cabot R, Park KW, Day BN, Prather RS, Schatten H. Regulation of mitogen-activated protein kinase phosphorylation, microtubule organization, chromatin behavior, and cell cycle progression by protein phosphatases during pig oocyte maturation and fertilization in vitro. Biol Reprod. 2002;66:580–588. doi: 10.1095/biolreprod66.3.580. [DOI] [PubMed] [Google Scholar]
- 187.Malathi K, Kohyama S, Ho M, Soghoian D, Li X, Silane M, Berenstein A, Jayaraman T. Inositol 1,4,5-trisphosphate receptor (type 1) phosphorylation and modulation by Cdc2. J Cell Biochem. 2003;90:1186–1196. doi: 10.1002/jcb.10720. [DOI] [PubMed] [Google Scholar]
- 188.Singleton PA, Bourguignon LY. CD44v10 interaction with Rho-kinase (ROK) activates inositol 1,4,5-triphosphate (IP3) receptor-mediated Ca2+ signaling during hyaluronan (HA)-induced endothelial cell migration. Cell Motil Cytoskeleton. 2002;53:293–316. doi: 10.1002/cm.10078. [DOI] [PubMed] [Google Scholar]
- 189.Lu DP, Tian L, O’Neill C, King NJ. Regulation of cellular adhesion molecule expression in murine oocytes, peri-implantation and post-implantation embryos. Cell Res. 2002;12:373–383. doi: 10.1038/sj.cr.7290139. [DOI] [PubMed] [Google Scholar]
- 190.Yoshida M, Horiuchi Y, Sensui N, Morisawa M. Signaling pathway from [Ca2+]i transients to ooplasmic segregation involves small GTPase rho in the ascidian egg. Dev Growth Differ. 2003;45:275–281. doi: 10.1046/j.1524-4725.2003.695.x. [DOI] [PubMed] [Google Scholar]
- 191.Ferris CD, Huganir RL, Bredt DS, Cameron AM, Snyder SH. Inositol trisphosphate receptor: phosphorylation by protein kinase C and calcium calmodulin-dependent protein kinases in reconstituted lipid vesicles. Proc Natl Acad Sci U S A. 1991;88:2232–2235. doi: 10.1073/pnas.88.6.2232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Koga T, Yoshida Y, Cai JQ, Islam MO, Imai S. Purification and characterization of 240-kDa cGMP-dependent protein kinase substrate of vascular smooth muscle. Close resemblance to inositol 1,4,5-trisphosphate receptor. J Biol Chem. 1994;269:11640–11647. [PubMed] [Google Scholar]
- 193.Jayaraman T, Ondrias K, Ondriasova E, Marks AR. Regulation of the inositol 1,4,5-trisphosphate receptor by tyrosine phosphorylation. Science. 1996;272:1492–1494. doi: 10.1126/science.272.5267.1492. [DOI] [PubMed] [Google Scholar]
- 194.Yokoyama K, Su IH, Tezuka T, Yasuda T, Mikoshiba K, Tarakhovsky A, Yamamoto T. BANK regulates BCR-induced calcium mobilization by promoting tyrosine phosphorylation of IP(3) receptor. EMBO J. 2002;21:83–92. doi: 10.1093/emboj/21.1.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Khan MT, Wagner L, 2nd, Yule DI, Bhanumathy C, Joseph SK. Akt kinase phosphorylation of inositol 1,4,5-trisphosphate receptors. J Biol Chem. 2006;281:3731–3737. doi: 10.1074/jbc.M509262200. [DOI] [PubMed] [Google Scholar]