Abstract
Transposable elements or transposons are DNA pieces that can move around within the genome and are, therefore, potential threat to genome stability and faithful transmission of the genetic information in the germline. Accordingly, self-defense mechanisms have evolved in the metazoan germline to silence transposons, and the primary mechanism requires the germline-specific non-coding small RNAs, named Piwi-interacting RNA (piRNAs), which are in complex with Argonaute family of PIWI proteins (the piRNA–RISC complexes), to silence transposons. piRNA-mediated transposon silencing occurs at both transcriptional and post-transcriptional levels. With the advantages of genetic manipulation and advances of sequencing technology, much progress has been made on the molecular mechanisms of piRNA-mediated transposon silencing in Drosophila melanogaster, which will be the focus of this review. Because piRNA-mediated transposon silencing is evolutionarily conserved in metazoan, model organisms, such as Drosophila, will continue to be served as pioneer systems towards the complete understanding of transposon silencing in the metazoan germline.
Keywords: Transposon silencing, Drosophila, piRNA, Piwi
Introduction
Transposable elements (TEs), also known as “jumping genes”, are discrete genetic elements which can move within the genome. Although they were first described by Barbara McClintock through studying mosaic coloration in maize in the 1940s [1], it took more than 30 years for the presence of mobile DNA in the eukaryotic species idea to be widely accepted. Now, we know transposons, and their remnants occupy as much as 42 % of the human genome [2], and they can be divided into two major classes, Class I and II, according to whether or not their transposition uses an RNA intermediate. Class II elements are DNA transposons with inverted terminal repeat and direct repeat, and use a “cut and paste” process to excise themselves from the genome and insert into a new genomic site without copy number increase [3]. Class I elements are retrotransposons which employ a “copy and paste” manner to retain the original copy and integrate a new copy at a new genomic location via the RNA transcripts as transposition intermediates which most of them synthesized by PolII [3–5]. Retrotransposons can be separated into two groups on the basis of molecular structure: LTRs (long terminal repeats) and non-LTR retrotransposons. Most of them encode several proteins which are required for transposition machinery assembly, such as virus capsid-like Gag protein and Pol protein, that are responsible for reverse-transcriptase, RNase H and integrase activity, respectively.
The transposition of a typical LTR retrotransposon is that the retrotransposon is transcribed by PolII to give rise to the mRNA with poly(A) tail in the nucleus. The mRNA is exported to the cytoplasm to encode several proteins, including the chaperon protein and the protein with reverse-transcriptase or endonuclease activity. These proteins together with mRNA form a ribonucleoprotein (RNP) particle and re-enter the nucleus, where the transposable element will integrate into a new genomic locus (see reviews [6, 7]).
Repetitive DNA and retrotransposons can generate genomic instability in many ways. The most straightforward way is insertion mutagenesis which can alter genome function and impact nearby gene expression. DNA double-strand breaks that occur during transposition contribute to a fraction of genomic instability, and are highly mutagenic and prone to recombination [8–10]. The “copy and paste” mechanism results in extremely high copy number of retrotransposons in the genome which have profound impact on the genome, such as producing insertion-mediated deletions and ectopic recombination [11, 12]. Therefore, the hyper-active transposition is likely deleterious. Not surprisingly, host evolves parallel defenses to combat rampant retrotransposons spread. Among these, the most important one in the metazoan is Piwi-interacting RNAs (piRNAs)-mediated transposon silencing, a mechanism that has been initially and most-extensively studied in Drosophila melanogaster. Therefore, this review will mainly focus on the current understanding of the piRNA pathway in the Drosophila ovary, more comprehensive reviews on the piRNA pathways, including that in mammals can be found elsewhere (see reviews [13–16]).
Argonaute proteins
To execute the gene silencing function, small RNA must be loaded onto the Argonaute proteins to form the RNA-induced silencing complex (RISC). The Argonaute proteins are belonged to a highly conserved gene family from bacteria to mammals [17]. Based on the phylogenetic studies, the eukaryotic Argonaute family can be divided into three distinct clades: the AGO clade, the PIWI clade, and a worm-specific WAGO clade [17]. In a C. elegans screen, rde-1, the worm Argonaute gene was first identified as being essential for RNAi response [18]. Thereafter, numerous studies demonstrate Argonaute proteins lie at the center of small RNA processes. Structural studies reveal that Argonaute proteins have highly specialized domains for their functions, including the Piwi-Argonaute-Zwille (PAZ) domain, the MID domain, and the PIWI domain [19]. The overall construction of Argonaute proteins is extraordinarily conserved: the N-PAZ and the MID-PIWI domains form two lobes of the crescent-shaped structure, respectively [19–21]. The PAZ domain binds the 3′ end of single-stranded RNA in a hydrophobic cleft that is lined with a highly conserved module [22]. The PAZ domains of PIWI clade members could accommodate the methylated 3′ ends into a hydrophobic cavity [23]. The MID domain anchors the 5′ end of small RNA guide in a basic pocket [24]. The PIWI domain adopts an RNaseH-like fold, and it, indeed, could function as an endonuclease to cleave target RNA with enough base complementarity [19, 25].
The fly genome encodes two AGO family members (ago1 and ago2) and three PIWI family members (piwi, aub, and ago3). Aub and Ago3 are exclusively expressed in the germline nurse cells and localized at the perinuclear electron-dense nuage structure [26]. Piwi localizes to the nucleus in both germline nurse cells and surrounding soma-derived follicle cells [26]. Loss of any these three PIWI members will lead to the dramatic derepression of transposons and compromised piRNA biogenesis [26–28].
PIWI proteins have a conserved post-translational modification: the symmetric dimethylarginine (sDMA) modification in their N-terminal regions by the methyltransferase dPRMT5 [29, 30]. The loss of dPRMT5 leads to a reduction of piRNAs and accumulation of transposons, suggesting a functional importance of sDMA modification in PIWI proteins [29, 30].
piRNA identification
The first identified piRNAs were known as rasiRNAs (repeat-associated small interfering RNAs) in Drosophila through studying stellate gene in male testes and the early embryos [28, 31, 32]. The tandem-repeated stellate gene is located on the X chromosome, which encodes a casein-kinase 2-like protein without known function [33]. It is silenced by the Y chromosome-located Suppressor of Stellate [Su(Ste)] locus. The deletion of Su(Ste) locus leads to abundant Ste protein accumulated in the primary spermatocytes, which forms needle-like crystals and causes the reduction of male fertility [33]. It is now clear that the paralogous Su(Ste) locus produces antisense piRNAs to silence the protein-coding ste gene [34]. Profiling of small RNAs in Drosophila tissues has identified 23–27 (now with accuracy, the range should be 23–30) nucleotide RNAs that are specifically expressed in gonad tissues and their sequences match transposable elements and repeats [31, 32]. Interestingly, unlike miRNAs and siRNAs, piRNAs are produced in a Dcr-1 or Dcr-2 independent pathway [35] and lack either a 2′ or a 3′ terminal hydroxyl group [28]. Instead, they undergo Hen-1-mediated methylation of their terminal 2′ oxygen [36]. In addition, these small RNAs are evolutionarily conserved, specifically associated with and functionally rely on PIWI clade Argonaute proteins and are distinguishable from miRNAs and siRNAs, which bind to Argonaute subfamilies, Ago1 and Ago2, respectively [26, 28, 35]. Thus, this new class of small RNAs was renamed as piRNAs [26, 37].
piRNA clusters
piRNAs are derived from the discrete pieces of DNA sequence in the genome, which are known as piRNA clusters. There are about 142 piRNA clusters in Drosophila [26], whose products contribute to more than 80 % of uniquely mapped piRNAs, and 92 % of all sequenced piRNAs, including multiply-mapped ones [26]. piRNA clusters comprise only 3.5 % of the whole genome, the majority of which are found in pericentromeric or subtelomeric heterochromatin, and the rest in dispersed euchromatic regions, including 3′UTR of coding genes, such as traffic jam (TJ) [26, 38, 39].
There are two classes of piRNA clusters based on strand bias. The uni-strand clusters are uni-directionally transcribed from one genomic strand, and they produce most somatic piRNAs that function to silence active transposons in the somatic follicle cells surrounding the germline. The 179 kb flamenco locus is the major cluster responsible for transposon silencing in the soma, 87 % of which is consisted of nested transposable elements with biased orientation. On the other hand, the dual-strand clusters are transcribed from both strands of genome which give rise to piRNAs predominantly in the germline [26]. The 42AB cluster is the largest one, spanning 240 kb in the pericentromeric heterochromatin of chromosome 2R, which produces 20.8 % of all uniquely mapped piRNA sequences [26].
It is still not clear which transcription factor regulates the transcription of piRNA clusters, but recent studies suggest an essential role for local chromatin context in the generation of piRNAs. Maternally deposited piRNAs are found to be involved in the specification of a piRNA cluster [40]. A phenomenon that the hybrid progeny are sterile only if the transposon is paternally inherited is known as hybrid dysgenesis [41, 42]. The lack of maternal deposited piRNAs leads to silencing escape of corresponding transposons in the germline of progeny [41]. These dysgenic hybrids partially restore fertility with age, as the new transposons are inserted into the piRNA cluster, and piRNAs against this new transposon are produced de novo [42]. Using the paramutation assay, Vanssay et al. found that a transgene cluster could induce strong trans-silencing effect (TSE), which can convert another homologous transgene cluster normally incapable of TSE into strong silencers in a maternally deposited piRNA-dependent manner. In addition, this capability could transmit through generations [40]. It implies that the inherited piRNA complexes are required for the reestablishment of piRNA clusters. Another insight comes from the studies on Drosophila histone 3 lysine9 methyltransferase dSETDB1 (egg), in which Rangan et al. found that the deposition of H3K9me3 is required for piRNA cluster transcription. In the absence of egg, transcription of cluster precursor will be collapsed in both germline and somatic gonad cells [43]. As Piwi-piRNA complex is required to establish repressive H3K9me3 mark and heterochromatin formation on the target sites and their genomic surroundings [44, 45], the maternally transmitted transgenerationally inherited piRNAs may act as epigenetic memory carrier to the installment of H3K9me3 mark on genomic piRNA clusters, which provides a permissive chromatin environment for piRNA precursor synthesis [40, 43, 46] (Fig. 1). Recent studies demonstrate that the sequence fragments within 5′ end of flamenco and 3′ UTR of TJ could serve as somatic piRNA trigger sequences [47, 48]. This raises the possibility that uni-strand piRNA could be produced independent of chromatin status.
Fig. 1.
piRNA precursor transcription and delivery. In follicle cells, the piRNA cluster regions are deposited with H3K9me3. After transcription by PolII, the single long-strand piRNA precursor is exported to the cytoplasm for cascade enzymatic cuts. In the germline, The Rhi–Del–Cuff complex binds to H3K9me3 via Rhi’s chromo domain which defines the germline piRNA clusters. piRNA clusters are dual stranded transcribed via readthrough of PolII from convergent neighboring genes or non-canonical PolII initiation. After the 3′ end processing of the upstream transcript, the newly formed 5′ end binds to Cuff which prevents degradation and allows PolII continue transcription. UAP56 binding to nascent transcripts suppresses splicing and escorts the precursor to the nuclear pore, in which, together with Vasa, it will be organized a piRNA-processing compartment in the perinuclear nuage
In the germline cells, Rhino (Rhi), a heterochromatin Protein 1 homolog, is associated with the dual-strand 42AB, 38C, and 80F clusters [49], together with Cutoff (Cuff), a protein related to yeast transcription termination factor Rai1, to promote transcription [50] (Fig. 1). Indeed, Deadlock (Del) acts as a flexible linker between Rhi and Cuff, as its N terminus interacts with Rhi’s chromo-shadow domain and C terminus interacts with Cuff [51]. The Rhi, Del, and Cuff (RDC) complex is anchored to H3K9me3 marked chromatin by Rhi’s chromo domain, which differentiates genome-wide dual-strand piRNA clusters from the H3K9me3-carrying heterochromatic regions in Drosophila ovaries [51, 52]. Interestingly, the binding of Rhi is dependent on Piwi protein at some loci. As Piwi is able to guide H3K9 methylation, a feed forward loop is, therefore, established for the recruitment of Rhi and the formation of a Piwi sensitive piRNA locus [51]. Interestingly, with the presence of the DEAD box protein UAP56, the Rhi/Del/Cuff complex is able to suppress splicing during the transcription of the piRNA clusters, which makes piRNA precursors distinct from mRNAs. This drives piRNA biogenesis from loci-expressing complementary transcripts [52].
piRNA precursor transcription and delivery
In somatic follicle cells, the long, single-stranded precursor transcripts are produced by PolII from H3K9me3-deposited piRNA clusters [26, 43], such as the flamenco locus and the 3′UTR of TJ. These loci exhibit canonical PolII transcription signature, including a defined Pol transcription start site (TSS), enrichment of active histone marker H3K4me2 at their putative promoter, and the expression of 5′ methyl-guanosine-capped and terminated RNAs [51, 53] (Fig. 1).
Unlike the somatic primary piRNA pathway, the germline piRNA precursors are produced from dual-strand piRNA clusters, such as 42AB. The genome-wide ChIP-seq data argued against the existence of defined promoters or TSSs at nearly all germline piRNA clusters which also lack of enrichment of H3K4me2 and 5′ methyl-guanosine caps [51, 52]. The current model favors that dual-strand precursor transcripts are generated by PolII readthrough from convergent neighboring gene pairs or non-canonical transcription initiation [51]. Rhi binding to H3K9me3 provides a licensing signal, since its binding strongly correlates with cluster expression and piRNA production [49, 51, 52]. The RDC complex binds to H3K9me3 in piRNA cluster region, which brings the putative termination cofactor Cuff in close proximity to the nascent piRNA precursor transcript. Therefore, Cuff stabilizes the 5′ phosphorylated nascent transcripts after the 3′ end processing of the upstream protein-coding transcript, which allows PolII continue transcription of an otherwise non-transcribed region [51]. In addition, Rhi, Cuff, and the DEAD box protein UAP56 suppress splicing of piRNA precursor transcript [52]. It might be a consequence of protection by the RDC complex from the binding of the cap-binding complex to the nascent 5′ end, which will recruit spliceosome and facilitate poly(A) site cleavage [51]. Next, UAP56 escorts the primary transcripts through the nuclear pore to nuage, a piRNA-processing compartment that spans the nuclear envelope [54] (Fig. 1).
piRNA precursor processing in somatic follicle cells
After being exported to the cytoplasm, the precursor transcripts will be processed into mature piRNAs by a cascade of enzymatic cuts (Fig. 2). As Piwi-bound piRNAs have a strong preference for 5′ terminal uridine [26, 27], it is believed that the 5′ end of the piRNA with a 5′U is generated first, loaded into Piwi, and followed by 3′ exonucleolytic trimming. Zucchini (Zuc) is the principal candidate for monophosphorylated piRNA 5′ formation, and it is a member of the phospholipase-D superfamily which is required for TE silencing and piRNA production [55]. The crystal structure reveals that Zuc has a narrow catalytic groove at the dimer interface which could accommodate a single-stranded RNA [56], a feature can also be found in the mouse Zuc [57]. In vitro assay shows that Zuc has the single-strand-specific nuclease activity, and the RNA cleavage products bear a 5′-monophosphate group, a hallmark of mature piRNAs [56]. Depletion of Zuc in OSC cells, which is derived from ovarian somatic cells results in the accumulation of ribonucleoprotein (RNP) complexes, composed of unprocessed piRNA intermediates [58]. Importantly, this phenotype can be rescued by the ectopic expression of wild-type Zuc but not the endonuclease-deficient Zuc [56]. Recent studies on phased piRNAs (described later) suggest that 3′ ends and 5′ ends of adjacent piRNA are produced in a single cleavage mediated by Zuc [59, 60].
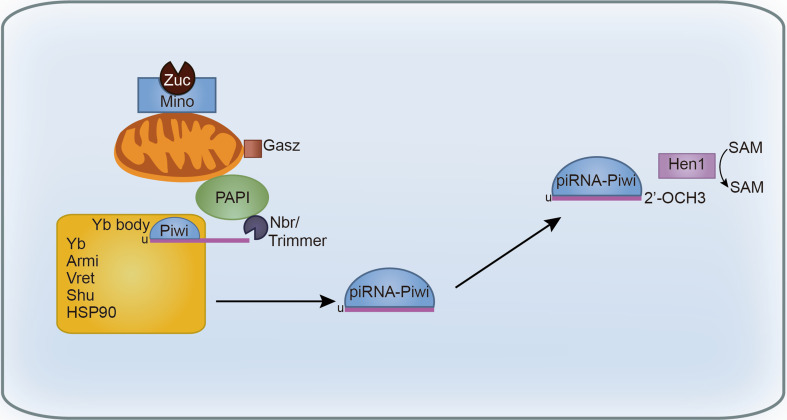
Fig. 2.
piRNA processing. First, the mitochondria outer membrane protein Zuc generates the 5′ end of piRNAs. Then piRNA loaded into Piwi with 1U bias in the Yb body with the help of the TUDOR protein Yb and Vret, helicase Armi, chaperone Shu, Hsp90, Mino, and Gasz. Next, piRNA 3′ end is trimmed to the mature length by Nbr or Trimmer. The trimming activity requires the cooperation of mitochondria outer membrane protein PAPI and Trimmer. After that, piRNA 3′ end is modified with 2′-O-methylation by Hen1. The mature Piwi-piRISC is imported into the nucleus
The maturing piRNA precursor then enters the perinuclear Yb body, which is a soma-specific discrete spherical structure, named after Yb protein. Yb is a TUDOR-domain protein-containing an RNA helicase motif and is expressed only in somatic cells [61]. The Yb bodies are adjacent to Zuc which localizes to the mitochondrial membrane and the RNA processing P-body [62]. The current model supports that Piwi is loaded with mature piRNAs before being imported into nucleus [63], although the precise loading process is still unclear. Yb directly binds piRNA intermediates through its N-terminal RecA-like domain, thereby forming the Yb body [64]. The further processing steps take place in Yb body with the help of Yb and other loading components, including the RNA helicase Armitage (Armi) [62, 63], the co-chaperone Shutdown (Shu) [65], the TUDOR-domain protein Vreteno (Vret) [66], the chaperone Hsp90 [67], Minotaur (Mino)/GPAT2 [68, 69], and Gasz [70]. In zuc mutants, due to the failure of piRNA 5′ end formation, the loading machinery components accumulate in enlarged Yb bodies with Piwi [56, 63].
Much progress has been made toward understanding the mechanisms of piRNA 3′ end generation. A cell-free system was established using lysate from BmN4, a silkworm-ovary cell line, to recapitulate the key steps of piRNA biogenesis [71], which provides an opportunity to biochemically identify the “trimmer”. With this system, Kawaoka et al. observed that Siwi (silkworm Piwi) first incorporated single long piRNA precursor with 5′-U bias, and the precursor could be trimmed to mature length by an exonuclease in a Mg2+-dependent manner [71]. Interestingly, it seems that the Tudor-domain protein PAPI also participates in this step [72]. Tudor-domain proteins could recognize and bind sDMA to mediate protein interactions [73]. PAPI localizes at the cytoplasm, nuage, and outer membrane of mitochondria. It interacts with Ago3 and is required for the localization of Ago3 to the nuage [72, 74]. The depletion of PAPI in BmN4 cells results in the 3′-terminal extension of mature piRNAs [72]. The 2′-O-methylation at 3′ end by the methyltransferase Hen1 is tightly coupled with trimming. The importance of the 3′ terminal modification is yet to be determined, as loss of Hen1 or blockage of 2′-O-methylation has no detectable phenotype, and the length of the piRNA products remains unchanged as well [36, 71, 75]. Nibbler (Nbr) is another potential candidate for 3′ end trimmer. Nbr is a 3′-to-5′ exoribonuclease, and it is found to be responsible for 3′ trimming of certain miRNAs [76, 77]. In Nbr mutant ovaries, piRNA profile has a longer mean length [78]. It seems that Nbr and Hen1 play antagonistic roles in piRNA 3′ end biogenesis [79], but direct biochemical evidence is needed. Using the BmN4 in vitro system and nucleases screen, respectively, two groups have identified the conserved PARN family exonucleases that trim 3′ end of piRNAs to mature size [80, 81]. In silkworm, PNLDC1/Trimmer is a 3′-5′ exonuclease which enriched in the mitochondrial fraction and associated with BmPapi. Trimmer cooperates with BmPapi to trim pre-piRNA both in vivo and in vitro. The depletion of BmPapi or Trimmer causes 3′ extension of piRNA, and overexpression of the catalytically inactive Trimmer could inhibit the trimming reaction. Importantly, mature piRNAs are more efficient than pre-piRNAs in target cleavage assay [80]. In C. elegans, PARN-1 localizes to nuage and possesses 3′–5′ exonuclease activity in vitro. In parn-1 mutant strain, animals produce longer piRNAs compared with the mature 21U-RNAs, which is the mature piRNAs in C. elegans, and are partially defective in transgene silencing [81]. However, there is lack of apparent PARN-like exonucleases in flies and zebrafish.
The Ping-Pong cycle and post-transcriptional gene silencing (PTGS)
After germline precursors being exported to the nuage, the further processing engages Aub and Ago3 centered an adaptive, slicer-dependent Ping-Pong cycle (Fig. 3). Based on the deep sequencing data of the three Argonaute protein libraries, piRNAs are found to have the maximal probability to find a partner, whose 5′ end can be mapped 10 nt away on the complementary strand. In fact, nearly a half small RNAs in the Ago3 library had complementary partners in the Aub library [26]. These ideas are finally incorporated into the Ping-Pong model [26, 27], which is described as follows: Aub binds antisense piRNAs and recognizes active transposon transcripts, cleaving them to generate a new sense piRNA. This is subsequently loaded into the Ago3 protein and targets piRNA precursor. Ago3 cleavage gives rise to an additional piRNA which is loaded back into the Aub protein, amplifying the initial piRNA population. This results in a feedforward amplification loop and represents the adaptive RNA-based immune system that could selectively amplify the piRNA which targeting the most active transposon transcripts. Qin, a Tudor-domain protein, co-localizes with Aub and Ago3 in the nuage to ensure the association of Aub and Ago3. Therefore, Qin regulates the heterotypic ping-pong cycle by preventing Aub’s cleavage products from becoming substrates for Piwi or Aub [82, 83]. Krimper, a stable nuage component, that recruits Ago3 to nuage, can interact with methylated Aub and Ago3 to ensure proper loading of Ago3 and establish the antisense bias in the Ping-Pong amplification loop [84, 85]. Taking advantage of the in vitro BmN4 cell culture system, the detailed mechanism of how endonucleolytic cleavage during transposon silencing is linked to the generation of a new piRNA is unveiled. Vasa, an RNA helicase, together with the two Ping-Pong Piwi partners and Qin, forms a transient piRNA amplifier complex in the nuage [86, 87]. Vasa has two different conformations. When Vasa binds RNA in its ATP-bound form, Vasa’s RNA helicase domain takes up a closed conformation which provides a binding platform for Amplifier components at the domain surface. After Siwi, the Aub homolog in silkworm, slicing the transposon transcript, and ATP hydrolysis in Vasa trigger the transfer of sliced 5′ processed precursors to Ago3 and eventual disassembly of the Amplifier. After the further 3′ end maturation, the secondary piRNA loaded with Ago3 enters the next Ping-Pong cycle to generate more Aub-bound piRNA [86, 87].
Fig. 3.
Ping-Pong cycle and post-transcriptional silencing. Aub binds to antisense piRNA and cleaves active transposon transcripts. The ATP-bound Vasa takes a closed conformation which provides a binding platform for Qin and Ago3 to form a transient Amplifier complex. After ATP hydrolysis, Vasa opens its helicase domains, resulting in Amplifier disassembly and 5′ processed transcript handover to Ago3 to further generate a new sense piRNA. Krimper dimer interacts with Aub and Ago3 to ensure the proper loading of new generated RNA substrate to Ago3. Ago3-piRISC, in turn, targets piRNA precursor and cleaves them to generate additional piRNA. It forms a reciprocal ping-pong cycle
Phased piRNA biogenesis
Recently, two groups independently reveal that piRNAs could be produced in a strictly phased manner (phased piRNA), immediate downstream of the initial piRNA formation site [59, 60] (Fig. 4). Aub and Ago3 are central components of reciprocal Ping-Pong cycle, and, therefore, in the aub and ago3 double mutants, there is only maternal and primary piRNAs. In these mutants, Han et al. found that the 5′ ends of piRNAs mapping to the same genomic strand typically lay 25–28 nt apart [59]. In rhi mutant ovaries, Mohn et al. found that piRNAs have abrupt nucleotide start sites. Most of them are the cleavage production of Ago3-bound piRNAs (trigger-piRNA) which mediate Ping-Pong cycle, the so-called responder-piRNA [60, 83]. Surprisingly, the piRNAs (trail-piRNA/phased piRNA) mapping to the downstream of responder-piRNA display pronounced ~27 nt phasing [60]. This is also observed in mouse germline [59, 60]. These phased piRNAs are prone to bound Piwi and others bound Aub but not Ago3. Their 5′ ends are immediately downstream of responder-piRNA 3′ ends, and both accuracy and levels decrease with increasing distance from the trigger site [60]. Both groups find that the production of phased piRNAs depends on Zuc. The Piwi/Aub-bound piRNAs display 1U bias. By analyzing the genomic nucleotide following a piRNA (+1U percentage), the 3′ end and following 5′ end of phased piRNAs are simultaneously produced [59, 60]. Mohn et al. further used a piRNA biogenesis reporter-containing defined intervals of Us to confirm that 3′ and 5′ ends of adjacent-phased piRNAs are formed by a single endonucleolytic cleavage upstream of a U residue. They also noticed that additional downstream trigger sites could bypass Zuc to produce phased piRNAs, while there is additional exonucleolytic trimming to mature length. Consistent with that, Wang et al. observed that there was a slicing-independent pathway which generated Piwi-bound piRNAs for some transposons [83]. Phased piRNAs were also observed in the ovarian somatic cells where Ping-Pong cycle is absent. Because Zuc has no nucleotide preference in vitro [56, 57], some factors may replace the role of Ago3-trigger-piRISC to specify the initial sites of phased piRNAs. Wang et al. also found that Ago3 or Aub cleavage initiates the majority production of germline Piwi-bound piRNAs [83]. The spreading mechanism of phased piRNAs greatly increases the sequence diversity of piRNA pool, and provides an adaptive mechanism to defend against versatile transposons.
Fig. 4.
Phased piRNA biogenesis. In the germline cells, piRNA precursors are exported into the cytoplasm. Ago3-piRISC (trigger-piRNA) recognizes the complementary piRNA precursors, cleaving them to generate Aub-bound responder-piRNAs. Zuc-mediated cleavage occurs immediately upstream of a U residue, generating the 3′ end of responder-piRNA and 5′ end of the next trail-piRNA simultaneously. After several rounds, Zuc-dependent cleavage, trail-piRNAs are funneled into Piwi and moderately into Aub. Or, additional Ago3 target sites also provide opportunity to generate phased piRNAs independent of Zuc. Instead, these phased piRNAs need exonucleolytic trimming to mature length. In the somatic follicle cells, some unknown factors specify the initial site of Zuc cleavage, and then loaded into Piwi to generate Piwi-bound piRNAs
Piwi-mediated transcriptional gene silencing
The mechanisms of transposon silencing are complicated. For several reasons, it had long been believed that transcriptional gene silencing of transposon is mediated by Piwi. First, among three PIWI clade Argonaute proteins in Drosophila, only Piwi is nuclear located, moreover, this nuclear localization is essential for Piwi’s transposon silencing function. A mutant of Piwi, which lacking the N-terminal nuclear localization signal, results in its cytoplasmic localization and incapability of TE silencing without notable change of piRNA-bound ability compared with wild-type Piwi [39, 44, 88]. In addition, the slicer activity is dispensable for TE silencing, as the catalytic dead Piwi can still be efficiently loaded with piRNAs similar to the wild type, and even rescues the piwi null mutant phenotypes [39, 44].
Although the biologically relevant genomic occupancy pattern of Piwi remains unknown, the studies on salivary polytene chromosome demonstrate that Piwi binds to chromatin in an RNA-dependent manner, and the chromatin localization pattern suggests that Piwi overlaps and probably interacts with the heterochromatin marker HP1a [89].
Furthermore, taking advantage of OSC cell line, several essential components involved in Piwi-centered TE silencing pathway have been identified, including Asterix(Arx)/DmGtsf1, Maelstrom(Mael), and Panoramix(Panx)/Silencio (Fig. 5).
Fig. 5.
Piwi-piRISC transcriptional represses transposons. Piwi-piRISC, together with its binding partner Arx, recognizes the nascent transcripts of TE by base pair complementarity, thereby recruits the scaffold protein Panx which will recruit the general silencing machinery to induce H3K9me3 of the transcript source regions, H4K9me2 demethylation in the promoter region, and loss of PolII occupancy. Mael acts in parallel or downstream of H3K9me3 to make these regions silencing status probably though heterochromatin formation with HP1a binding
Sienski and colleagues have provided strong evidence to support that transcriptional gene silencing (TGS) is the major route through which Piwi represses TEs and this is accompanied by local heterochromatin formation. Using OSC cell line, the authors closely compared the PolII occupancy, H3K9me3 status, nascent transcribed RNA, and steady-state RNAs at genome-wide level in Piwi KD cells and control cells [44]. Knocking down Piwi in OSCs caused increased PolII occupancy in transposon loci, nascent TE transcripts, and steady-state RNAs. In contrast, the heterochromatin marker H3K9me3 decreased in these regions. Importantly, nearly all dispersed H3K9me3 islands in euchromatin correlate with neighboring TE insertions, and the H3K9me3 island formation is dependent on piRNA pathway. Furthermore, the underlying TE insertions are often in sense orientation to introns of expressed genes, and, therefore, make them the targets for Piwi-guided H3K9 trimethylation. These data strongly implicate an RNA-recognition mode for Piwi-dependent silencing.
None of Mael, Arx, and Panx is required for piRNA biogenesis [44, 90–94]. Genetic studies show that Mael acts downstream of Piwi in transcriptional silencing of transposons [44]. Mael protein is localized to cytoplasm, nuage, and nucleus, but it can shuttle between the cytoplasm and nucleus, especially enriched in the nucleus of OSCs [44, 95]. It is worthy to note that H3K9me3 itself cannot be the final silencing mark, as Sienski et al. observed strong TE derepression upon loss of Mael despite no or only very modest changes in H3K9me3 [44]. It suggests that Mael works downstream or in parallel with H3K9me3 to silence transposons. Co-IP and pull-down in OSCs show that Arx physically interacts with Piwi via its C-terminal tail but not Mael [90, 92]. Both the Zinc finger and C-tail are required for Arx TE silencing function, even disruption of four conserved cysteines in Arx’s CHHC-type Zinc finger motifs compromises the TE silencing activity [90, 92]. Arx does not affect Argonaute proteins and Mael expression levels, but its nuclear localization is dependent on Piwi [92]. ChIP-seq data show that arx homozygous mutant ovaries and RNAi OSCs have less-efficient H3K9me3 deposition, in the meanwhile, PolII accumulation increases in both strongly derepressed TE loci and flanking region of euchromatic TE insertion [90, 92]. The studies on Panx by two independent groups have provided important insights on how recruitment of Piwi-piRISC to nascent TE transcripts leads to heterochromatin formation [93, 94]. Panx also interacts with Piwi. Knockdown of Panx caused a sharp rise in TE transcripts and losses of H3K9me3 marks over transposons, which is nearly identical to the effect of Piwi. Using the λN-BoxB reporter assay in OSCs and ovaries, it is found that Panx λN-fused protein could considerately reduce the reporter activity, but Piwi and Arx could not [93, 94]. Further knockdown of repressive epigenetic proteins, such as HP1a, dLSD1, CoRest, Eggless, and Wde, could weaken the repression effect of λN-Panx, rather than Piwi and Arx [93]. It suggests that Panx acts as a central bridging factor which functions the downstream of Piwi and its binding partner Arx to silence transposons at transcriptional level. When Panx bounds to nascent transcripts, it will recruit the general silencing machinery to deposit the repressive chromatin marks to the source of the locus [93].
The current model of Piwi-mediated TGS proposes that Piwi-piRISC and its binding partner Arx recognizes nascent TE transcripts by base pair complementarity, thereby recruits the adaptor protein Panx, then the general silencing machinery triggers histone modification of the source locus. Mael probably acts in parallel or downstream of H3K9me3. At last, the target chromatic loci become silencing status which probably induces heterochromatin formation. It is still poorly understood how exactly Piwi recruits the silencing machinery to induce the heterochromatin formation. TGS is likely the major mechanism underlying Piwi-mediated TE silencing. However, studies also suggest that Piwi might also participate in germline piRNA biogenesis and exert an effect on post-transcriptional gene silencing [96].
Other transposon silencing mechanisms
Besides piRNAs, the endogenous short interfering RNAs (esiRNAs) are responsible for transposon silencing in both gonadal and somatic tissues as well [97, 98]. The mature esiRNAs are typically ~21 nt in length, and the processing of long dsRNA precursors into esiRNA duplexes requires the type III RNase Dicer-2 [97, 98]. The further processing of esiRNA precursor requires the dsRNA-binding protein Loquacious (Loqs) but not the canonical Dicer-2 partner R2D2 [99, 100]. The esiRNAs are finally predominantly loaded onto Ago2 and target both transposon and protein-coding genes [97, 98].
Small RNA usually plays multiple roles in repressing target genes expression. Numerous studies addressed the mechanism of miRNA-mediated translation repression, including translation initiation block, ribosome elongation block, and ribosome drop-off [101]. The majority of retrotransposons harbor their own protein-coding units, and encode several proteins to facilitate transposition. If the transposon transcript escapes from the TGS and PTGS surveillance pathway, furthermore, we can do to control the transposition? It is found that piRNAs are associated with ribosomes and regulate translation during mouse spermatogenesis [102, 103]. Aub-piRISC could interact with the hundreds of maternal mRNAs and promote them undergo Aub-dependent degradation in the early embryo [104, 105]. It could also trap mRNAs to germplasm of oocyte using partial base-pairing [106]. These functions seem to analogous to miRNA-mediated translation repression. Recently, our group discovered that an mRNA surveillance complex Pelo-Hbs1 is required for transposon silencing [107] (Fig. 6). The complex was initially identified in yeast, in which the mRNA with stalled ribosomes during translational elongation can be recognized by Dom34, the ortholog of Pelo in yeast, and Hbs1 to trigger mRNA endonucleolytic cleavage, a process known as No-go decay (NGD) [108]. Further biochemical studies find that Dom34-Hbs1 complex binds to the empty ribosomal A site to promote subsequent peptide release and dissociation of ribosome subunits [109]. In addition to NGD, Dom34-Hbs1 also plays important roles in a variety of cellular processes, such as non-stop decay, non-functional 18S rRNA decay, and ribosome arrested in 3′UTR [110–112]. Therefore, Dom34-Hbs1 is a general RNA surveillance pathway in eukaryote to detect and rescue stalled ribosomes. We observe that transposon mRNAs are moderately increased in pelo mutant ovaries and pelo RNAi testes, in the meanwhile, transposon proteins are accumulated. Pelo is neither required for piRNA biogenesis nor subcellular localization of piRNA proteins. Interestingly, this function of Pelo requires its interaction with Hbs1, and the overexpression of ribosome protein RpS30a, which can complement the NGD defect in Dom34Δ yeast [113], also rescues the transposon-silencing defect in pelo mutant. These observations indicate that Pelo-Hbs1 may function at the translational level through an NGD-like mechanism to silence transposons (Fig. 6) [107].
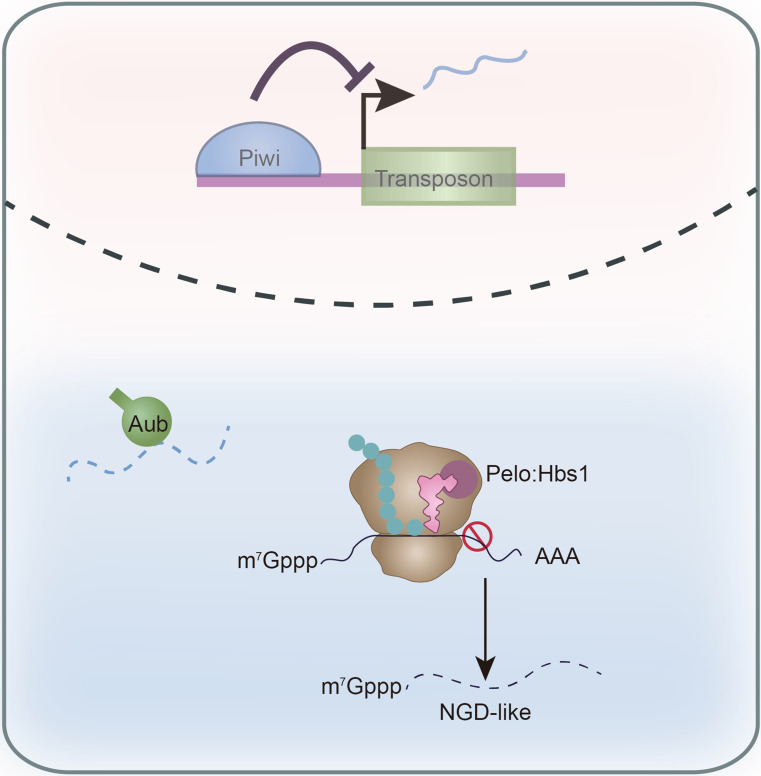
Fig. 6.
Model for Pelo-Hbs1-mediated TE silencing at translational level. The transposon transcripts have escaped the transcriptional and post-transcriptional gene silencing and have loaded to ribosomes for translation, and could be degraded by the Pelo-Hbs1 complex via an NGD-like mechanism. During the ribosome elongation, some unknown factors, possibly piRNAs, could cause ribosome stall, followed by Pelo-Hbs1 recruitment and mRNA decay
DNA modification is important for transposon silencing in mammals [114, 115]; however, 5-methylcytosine (5mC) levels in D. melanogaster are extremely low. Recently, using an extremely sensitive mass spectrometry approach and dot blot, Adenine N6 methylation (6mA) was detected at an extremely low level in D. melanogaster genomic DNA [116]. DNA immunoprecipitation results show that 6mA is enriched in transposon region and transposon transcripts are increased in DMAD mutant ovaries which are a newly identified 6mA demethylase [116]. It seems that 6mA promotes transposon expression, but the underlying mechanism, especially the relationship with other histone markers, awaits further investigation.
Concluding remarks
The piRNA pathway is an evolutionary conserved surveillance mechanism to suppress transposon activity in the metazoan germline. Although piRNA was identified nearly a decade ago, there are many questions remained on the various steps of piRNA biogenesis and functional regulation. For example, what are the additional factors that define the piRNA cluster and maintain the precursor transcription? How are the piRNA precursors transported to the cytoplasm? During the process of piRNA biogenesis, piRNAs are matured with the participation of several factors localized at different organelles, such as nuage, mitochondria, and Yb body, but the molecular processes are largely unclear. In piRNA-directed TGS, the detailed recruitment mechanism of general silencing machinery is still unclear. The mechanism that bridges the piRISC to general silencing machinery is still not fully understood. The establishment, molecular, and regulatory relationships between H3K9 methylation and H3K4 demethylation during transposon silencing are still yet to be defined. Beyond the transposon silencing, piRNAs are also found to participate in many developmental processes, such as translational regulation [105], mRNA trapping [106], and virus-induced production [117]. It is worthy further work to get a better understanding of the whole function of piRNAs.
The introduction of somatic follicle cell-derived cell line and new generation RNAi tools have allowed scientists to systematically screen for genes required for transposon silencing [91, 118–122]. The development of new biochemical methods, for example, the establishment of a cell free in vitro system, will boost further biochemical characterization of the piRNA biogenesis processes [71]. With the advances of deep sequencing technology and the application of the CRISPR-Cas9 system in genome editing, model organisms, such as D. melanogaster, will continue serving as pioneer systems towards complete understanding of molecular and biochemical frameworks of the piRNA pathway.
Acknowledgments
We thank Kyra Yang for proof reading of the manuscript, and Mengli Shi for graphic illustrations. This work is supported by the National Basic Science 973 Grant (2014CB850002) from the Chinese Ministry of Science and Technology. F.Y. is supported by a grant from the National Natural Science Foundation of China (No.31601059).
References
- 1.Mc CB. The origin and behavior of mutable loci in maize. Proc Natl Acad Sci USA. 1950;36:344–355. doi: 10.1073/pnas.36.6.344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lander ES, Linton LM, Birren B, Nusbaum C, Zody MC, Baldwin J, Devon K, Dewar K, Doyle M, FitzHugh W, et al. Initial sequencing and analysis of the human genome. Nature. 2001;409:860–921. doi: 10.1038/35057062. [DOI] [PubMed] [Google Scholar]
- 3.Craig NL. Mobile DNA II. Washington, D.C.: ASM Press; 2002. [Google Scholar]
- 4.Minnick MF, Stillwell LC, Heineman JM, Stiegler GL. A highly repetitive DNA sequence possibly unique to canids. Gene. 1992;110:235–238. doi: 10.1016/0378-1119(92)90654-8. [DOI] [PubMed] [Google Scholar]
- 5.Bentolila S, Bach JM, Kessler JL, Bordelais I, Cruaud C, Weissenbach J, Panthier JJ. Analysis of major repetitive DNA sequences in the dog (Canis familiaris) genome. Mamm Genome. 1999;10:699–705. doi: 10.1007/s003359901074. [DOI] [PubMed] [Google Scholar]
- 6.Beauregard A, Curcio MJ, Belfort M. The take and give between retrotransposable elements and their hosts. Annu Rev Genet. 2008;42:587–617. doi: 10.1146/annurev.genet.42.110807.091549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cordaux R, Batzer MA. The impact of retrotransposons on human genome evolution. Nat Rev Genet. 2009;10:691–703. doi: 10.1038/nrg2640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Morrish TA, Gilbert N, Myers JS, Vincent BJ, Stamato TD, Taccioli GE, Batzer MA, Moran JV. DNA repair mediated by endonuclease-independent LINE-1 retrotransposition. Nat Genet. 2002;31:159–165. doi: 10.1038/ng898. [DOI] [PubMed] [Google Scholar]
- 9.Gasior SL, Wakeman TP, Xu B, Deininger PL. The human LINE-1 retrotransposon creates DNA double-strand breaks. J Mol Biol. 2006;357:1383–1393. doi: 10.1016/j.jmb.2006.01.089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen Y, Pane A, Schupbach T. Cutoff and aubergine mutations result in retrotransposon upregulation and checkpoint activation in Drosophila . Curr Biol. 2007;17:637–642. doi: 10.1016/j.cub.2007.02.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sen SK, Han K, Wang J, Lee J, Wang H, Callinan PA, Dyer M, Cordaux R, Liang P, Batzer MA. Human genomic deletions mediated by recombination between Alu elements. Am J Hum Genet. 2006;79:41–53. doi: 10.1086/504600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Han K, Lee J, Meyer TJ, Remedios P, Goodwin L, Batzer MA. L1 recombination-associated deletions generate human genomic variation. Proc Natl Acad Sci USA. 2008;105:19366–19371. doi: 10.1073/pnas.0807866105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Czech B, Hannon GJ. One loop to rule them all: the ping-pong cycle and piRNA-guided silencing. Trends Biochem Sci. 2016;41:324–337. doi: 10.1016/j.tibs.2015.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pillai RS, Chuma S. piRNAs and their involvement in male germline development in mice. Dev Growth Differ. 2012;54:78–92. doi: 10.1111/j.1440-169X.2011.01320.x. [DOI] [PubMed] [Google Scholar]
- 15.Ishizu H, Siomi H, Siomi MC. Biology of PIWI-interacting RNAs: new insights into biogenesis and function inside and outside of germlines. Genes Dev. 2012;26:2361–2373. doi: 10.1101/gad.203786.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Crichton JH, Dunican DS, Maclennan M, Meehan RR, Adams IR. Defending the genome from the enemy within: mechanisms of retrotransposon suppression in the mouse germline. Cell Mol Life Sci. 2014;71:1581–1605. doi: 10.1007/s00018-013-1468-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tolia NH, Joshua-Tor L. Slicer and the argonautes. Nat Chem Biol. 2007;3:36–43. doi: 10.1038/nchembio848. [DOI] [PubMed] [Google Scholar]
- 18.Tabara H, Sarkissian M, Kelly WG, Fleenor J, Grishok A, Timmons L, Fire A, Mello CC. The rde-1 gene, RNA interference, and transposon silencing in C. elegans . Cell. 1999;99:123–132. doi: 10.1016/S0092-8674(00)81644-X. [DOI] [PubMed] [Google Scholar]
- 19.Song JJ, Smith SK, Hannon GJ, Joshua-Tor L. Crystal structure of Argonaute and its implications for RISC slicer activity. Science. 2004;305:1434–1437. doi: 10.1126/science.1102514. [DOI] [PubMed] [Google Scholar]
- 20.Nakanishi K, Weinberg DE, Bartel DP, Patel DJ. Structure of yeast Argonaute with guide RNA. Nature. 2012;486:368–374. doi: 10.1038/nature11211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schirle NT, MacRae IJ. The crystal structure of human Argonaute2. Science. 2012;336:1037–1040. doi: 10.1126/science.1221551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lingel A, Simon B, Izaurralde E, Sattler M. Structure and nucleic-acid binding of the Drosophila Argonaute 2 PAZ domain. Nature. 2003;426:465–469. doi: 10.1038/nature02123. [DOI] [PubMed] [Google Scholar]
- 23.Simon B, Kirkpatrick JP, Eckhardt S, Reuter M, Rocha EA, Andrade-Navarro MA, Sehr P, Pillai RS, Carlomagno T. Recognition of 2′-O-methylated 3′-end of piRNA by the PAZ domain of a Piwi protein. Structure. 2011;19:172–180. doi: 10.1016/j.str.2010.11.015. [DOI] [PubMed] [Google Scholar]
- 24.Ma JB, Yuan YR, Meister G, Pei Y, Tuschl T, Patel DJ. Structural basis for 5′-end-specific recognition of guide RNA by the A. fulgidus Piwi protein. Nature. 2005;434:666–670. doi: 10.1038/nature03514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liu J, Carmell MA, Rivas FV, Marsden CG, Thomson JM, Song JJ, Hammond SM, Joshua-Tor L, Hannon GJ. Argonaute2 is the catalytic engine of mammalian RNAi. Science. 2004;305:1437–1441. doi: 10.1126/science.1102513. [DOI] [PubMed] [Google Scholar]
- 26.Brennecke J, Aravin AA, Stark A, Dus M, Kellis M, Sachidanandam R, Hannon GJ. Discrete small RNA-generating loci as master regulators of transposon activity in Drosophila . Cell. 2007;128:1089–1103. doi: 10.1016/j.cell.2007.01.043. [DOI] [PubMed] [Google Scholar]
- 27.Gunawardane LS, Saito K, Nishida KM, Miyoshi K, Kawamura Y, Nagami T, Siomi H, Siomi MC. A slicer-mediated mechanism for repeat-associated siRNA 5′ end formation in Drosophila . Science. 2007;315:1587–1590. doi: 10.1126/science.1140494. [DOI] [PubMed] [Google Scholar]
- 28.Saito K, Nishida KM, Mori T, Kawamura Y, Miyoshi K, Nagami T, Siomi H, Siomi MC. Specific association of Piwi with rasiRNAs derived from retrotransposon and heterochromatic regions in the Drosophila genome. Genes Dev. 2006;20:2214–2222. doi: 10.1101/gad.1454806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kirino Y, Kim N, de Planell-Saguer M, Khandros E, Chiorean S, Klein PS, Rigoutsos I, Jongens TA, Mourelatos Z. Arginine methylation of Piwi proteins catalysed by dPRMT5 is required for Ago3 and Aub stability. Nat Cell Biol. 2009;11:652–658. doi: 10.1038/ncb1872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nishida KM, Okada TN, Kawamura T, Mituyama T, Kawamura Y, Inagaki S, Huang H, Chen D, Kodama T, Siomi H, et al. Functional involvement of Tudor and dPRMT5 in the piRNA processing pathway in Drosophila germlines. EMBO J. 2009;28:3820–3831. doi: 10.1038/emboj.2009.365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Aravin AA, Lagos-Quintana M, Yalcin A, Zavolan M, Marks D, Snyder B, Gaasterland T, Meyer J, Tuschl T. The small RNA profile during Drosophila melanogaster development. Dev Cell. 2003;5:337–350. doi: 10.1016/S1534-5807(03)00228-4. [DOI] [PubMed] [Google Scholar]
- 32.Aravin AA, Klenov MS, Vagin VV, Bantignies F, Cavalli G, Gvozdev VA. Dissection of a natural RNA silencing process in the Drosophila melanogaster germ line. Mol Cell Biol. 2004;24:6742–6750. doi: 10.1128/MCB.24.15.6742-6750.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Livak KJ. Detailed structure of the Drosophila melanogaster stellate genes and their transcripts. Genetics. 1990;124:303–316. doi: 10.1093/genetics/124.2.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Aravin AA, Naumova NM, Tulin AV, Vagin VV, Rozovsky YM, Gvozdev VA. Double-stranded RNA-mediated silencing of genomic tandem repeats and transposable elements in the D. melanogaster germline. Curr Biol. 2001;11:1017–1027. doi: 10.1016/S0960-9822(01)00299-8. [DOI] [PubMed] [Google Scholar]
- 35.Vagin VV, Sigova A, Li C, Seitz H, Gvozdev V, Zamore PD. A distinct small RNA pathway silences selfish genetic elements in the germline. Science. 2006;313:320–324. doi: 10.1126/science.1129333. [DOI] [PubMed] [Google Scholar]
- 36.Horwich MD, Li C, Matranga C, Vagin V, Farley G, Wang P, Zamore PD. The Drosophila RNA methyltransferase, DmHen1, modifies germline piRNAs and single-stranded siRNAs in RISC. Curr Biol. 2007;17:1265–1272. doi: 10.1016/j.cub.2007.06.030. [DOI] [PubMed] [Google Scholar]
- 37.Yin H, Lin H. An epigenetic activation role of Piwi and a Piwi-associated piRNA in Drosophila melanogaster . Nature. 2007;450:304–308. doi: 10.1038/nature06263. [DOI] [PubMed] [Google Scholar]
- 38.Robine N, Lau NC, Balla S, Jin Z, Okamura K, Kuramochi-Miyagawa S, Blower MD, Lai EC. A broadly conserved pathway generates 3′UTR-directed primary piRNAs. Curr Biol. 2009;19:2066–2076. doi: 10.1016/j.cub.2009.11.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Saito K, Inagaki S, Mituyama T, Kawamura Y, Ono Y, Sakota E, Kotani H, Asai K, Siomi H, Siomi MC. A regulatory circuit for piwi by the large Maf gene traffic jam in Drosophila . Nature. 2009;461:1296–1299. doi: 10.1038/nature08501. [DOI] [PubMed] [Google Scholar]
- 40.de Vanssay A, Bouge AL, Boivin A, Hermant C, Teysset L, Delmarre V, Antoniewski C, Ronsseray S. Paramutation in Drosophila linked to emergence of a piRNA-producing locus. Nature. 2012;490:112–115. doi: 10.1038/nature11416. [DOI] [PubMed] [Google Scholar]
- 41.Brennecke J, Malone CD, Aravin AA, Sachidanandam R, Stark A, Hannon GJ. An epigenetic role for maternally inherited piRNAs in transposon silencing. Science. 2008;322:1387–1392. doi: 10.1126/science.1165171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Khurana JS, Wang J, Xu J, Koppetsch BS, Thomson TC, Nowosielska A, Li C, Zamore PD, Weng Z, Theurkauf WE. Adaptation to P element transposon invasion in Drosophila melanogaster . Cell. 2011;147:1551–1563. doi: 10.1016/j.cell.2011.11.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rangan P, Malone CD, Navarro C, Newbold SP, Hayes PS, Sachidanandam R, Hannon GJ, Lehmann R. piRNA production requires heterochromatin formation in Drosophila . Curr Biol. 2011;21:1373–1379. doi: 10.1016/j.cub.2011.06.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sienski G, Donertas D, Brennecke J. Transcriptional silencing of transposons by Piwi and maelstrom and its impact on chromatin state and gene expression. Cell. 2012;151:964–980. doi: 10.1016/j.cell.2012.10.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Le Thomas A, Rogers AK, Webster A, Marinov GK, Liao SE, Perkins EM, Hur JK, Aravin AA, Toth KF. Piwi induces piRNA-guided transcriptional silencing and establishment of a repressive chromatin state. Genes Dev. 2013;27:390–399. doi: 10.1101/gad.209841.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Le Thomas A, Stuwe E, Li S, Du J, Marinov G, Rozhkov N, Chen YC, Luo Y, Sachidanandam R, Toth KF, et al. Transgenerationally inherited piRNAs trigger piRNA biogenesis by changing the chromatin of piRNA clusters and inducing precursor processing. Genes Dev. 2014;28:1667–1680. doi: 10.1101/gad.245514.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Homolka D, Pandey RR, Goriaux C, Brasset E, Vaury C, Sachidanandam R, Fauvarque MO, Pillai RS. PIWI slicing and RNA elements in precursors instruct directional primary piRNA biogenesis. Cell Rep. 2015;12:418–428. doi: 10.1016/j.celrep.2015.06.030. [DOI] [PubMed] [Google Scholar]
- 48.Ishizu H, Iwasaki YW, Hirakata S, Ozaki H, Iwasaki W, Siomi H, Siomi MC. Somatic primary piRNA biogenesis driven by cis-acting RNA Elements and trans-acting Yb. Cell Rep. 2015;12:429–440. doi: 10.1016/j.celrep.2015.06.035. [DOI] [PubMed] [Google Scholar]
- 49.Klattenhoff C, Xi H, Li C, Lee S, Xu J, Khurana JS, Zhang F, Schultz N, Koppetsch BS, Nowosielska A, et al. The Drosophila HP1 homolog Rhino is required for transposon silencing and piRNA production by dual-strand clusters. Cell. 2009;138:1137–1149. doi: 10.1016/j.cell.2009.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Pane A, Jiang P, Zhao DY, Singh M, Schupbach T. The Cutoff protein regulates piRNA cluster expression and piRNA production in the Drosophila germline. EMBO J. 2011;30:4601–4615. doi: 10.1038/emboj.2011.334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mohn F, Sienski G, Handler D, Brennecke J. The rhino-deadlock-cutoff complex licenses noncanonical transcription of dual-strand piRNA clusters in Drosophila . Cell. 2014;157:1364–1379. doi: 10.1016/j.cell.2014.04.031. [DOI] [PubMed] [Google Scholar]
- 52.Zhang Z, Wang J, Schultz N, Zhang F, Parhad SS, Tu S, Vreven T, Zamore PD, Weng Z, Theurkauf WE. The HP1 homolog rhino anchors a nuclear complex that suppresses piRNA precursor splicing. Cell. 2014;157:1353–1363. doi: 10.1016/j.cell.2014.04.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Goriaux C, Desset S, Renaud Y, Vaury C, Brasset E. Transcriptional properties and splicing of the flamenco piRNA cluster. EMBO Rep. 2014;15:411–418. doi: 10.1002/embr.201337898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zhang F, Wang J, Xu J, Zhang Z, Koppetsch BS, Schultz N, Vreven T, Meignin C, Davis I, Zamore PD, et al. UAP56 couples piRNA clusters to the perinuclear transposon silencing machinery. Cell. 2012;151:871–884. doi: 10.1016/j.cell.2012.09.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Pane A, Wehr K, Schupbach T. Zucchini and squash encode two putative nucleases required for rasiRNA production in the Drosophila germline. Dev Cell. 2007;12:851–862. doi: 10.1016/j.devcel.2007.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Nishimasu H, Ishizu H, Saito K, Fukuhara S, Kamatani MK, Bonnefond L, Matsumoto N, Nishizawa T, Nakanaga K, Aoki J, et al. Structure and function of Zucchini endoribonuclease in piRNA biogenesis. Nature. 2012;491:284–287. doi: 10.1038/nature11509. [DOI] [PubMed] [Google Scholar]
- 57.Ipsaro JJ, Haase AD, Knott SR, Joshua-Tor L, Hannon GJ. The structural biochemistry of Zucchini implicates it as a nuclease in piRNA biogenesis. Nature. 2012;491:279–283. doi: 10.1038/nature11502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Voigt F, Reuter M, Kasaruho A, Schulz EC, Pillai RS, Barabas O. Crystal structure of the primary piRNA biogenesis factor Zucchini reveals similarity to the bacterial PLD endonuclease Nuc. RNA. 2012;18:2128–2134. doi: 10.1261/rna.034967.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Han BW, Wang W, Li C, Weng Z, Zamore PD. Noncoding RNA. piRNA-guided transposon cleavage initiates Zucchini-dependent, phased piRNA production. Science. 2015;348:817–821. doi: 10.1126/science.aaa1264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Mohn F, Handler D, Brennecke J. Noncoding RNA. piRNA-guided slicing specifies transcripts for Zucchini-dependent, phased piRNA biogenesis. Science. 2015;348:812–817. doi: 10.1126/science.aaa1039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Szakmary A, Reedy M, Qi H, Lin H. The Yb protein defines a novel organelle and regulates male germline stem cell self-renewal in Drosophila melanogaster . J Cell Biol. 2009;185:613–627. doi: 10.1083/jcb.200903034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Olivieri D, Sykora MM, Sachidanandam R, Mechtler K, Brennecke J. An in vivo RNAi assay identifies major genetic and cellular requirements for primary piRNA biogenesis in Drosophila . EMBO J. 2010;29:3301–3317. doi: 10.1038/emboj.2010.212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Saito K, Ishizu H, Komai M, Kotani H, Kawamura Y, Nishida KM, Siomi H, Siomi MC. Roles for the Yb body components Armitage and Yb in primary piRNA biogenesis in Drosophila . Genes Dev. 2010;24:2493–2498. doi: 10.1101/gad.1989510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Murota Y, Ishizu H, Nakagawa S, Iwasaki YW, Shibata S, Kamatani MK, Saito K, Okano H, Siomi H, Siomi MC. Yb integrates piRNA intermediates and processing factors into perinuclear bodies to enhance piRISC assembly. Cell Rep. 2014;8:103–113. doi: 10.1016/j.celrep.2014.05.043. [DOI] [PubMed] [Google Scholar]
- 65.Olivieri D, Senti KA, Subramanian S, Sachidanandam R, Brennecke J. The cochaperone shutdown defines a group of biogenesis factors essential for all piRNA populations in Drosophila . Mol Cell. 2012;47:954–969. doi: 10.1016/j.molcel.2012.07.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Zamparini AL, Davis MY, Malone CD, Vieira E, Zavadil J, Sachidanandam R, Hannon GJ, Lehmann R. Vreteno, a gonad-specific protein, is essential for germline development and primary piRNA biogenesis in Drosophila . Development. 2011;138:4039–4050. doi: 10.1242/dev.069187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Izumi N, Kawaoka S, Yasuhara S, Suzuki Y, Sugano S, Katsuma S, Tomari Y. Hsp90 facilitates accurate loading of precursor piRNAs into PIWI proteins. RNA. 2013;19:896–901. doi: 10.1261/rna.037200.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Shiromoto Y, Kuramochi-Miyagawa S, Daiba A, Chuma S, Katanaya A, Katsumata A, Nishimura K, Ohtaka M, Nakanishi M, Nakamura T, et al. GPAT2, a mitochondrial outer membrane protein, in piRNA biogenesis in germline stem cells. RNA. 2013;19:803–810. doi: 10.1261/rna.038521.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Vagin VV, Yu Y, Jankowska A, Luo Y, Wasik KA, Malone CD, Harrison E, Rosebrock A, Wakimoto BT, Fagegaltier D, et al. Minotaur is critical for primary piRNA biogenesis. RNA. 2013;19:1064–1077. doi: 10.1261/rna.039669.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Ma L, Buchold GM, Greenbaum MP, Roy A, Burns KH, Zhu H, Han DY, Harris RA, Coarfa C, Gunaratne PH, et al. GASZ is essential for male meiosis and suppression of retrotransposon expression in the male germline. PLoS Genet. 2009;5:e1000635. doi: 10.1371/journal.pgen.1000635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kawaoka S, Izumi N, Katsuma S, Tomari Y. 3′ end formation of PIWI-interacting RNAs in vitro. Mol Cell. 2011;43:1015–1022. doi: 10.1016/j.molcel.2011.07.029. [DOI] [PubMed] [Google Scholar]
- 72.Honda S, Kirino Y, Maragkakis M, Alexiou P, Ohtaki A, Murali R, Mourelatos Z, Kirino Y. Mitochondrial protein BmPAPI modulates the length of mature piRNAs. RNA. 2013;19:1405–1418. doi: 10.1261/rna.040428.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Maurer-Stroh S, Dickens NJ, Hughes-Davies L, Kouzarides T, Eisenhaber F, Ponting CP. The Tudor domain ‘Royal Family’: Tudor, plant Agenet, Chromo, PWWP and MBT domains. Trends Biochem Sci. 2003;28:69–74. doi: 10.1016/S0968-0004(03)00004-5. [DOI] [PubMed] [Google Scholar]
- 74.Liu L, Qi H, Wang J, Lin H. PAPI, a novel TUDOR-domain protein, complexes with AGO3, ME31B and TRAL in the nuage to silence transposition. Development. 2011;138:1863–1873. doi: 10.1242/dev.059287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Saito K, Sakaguchi Y, Suzuki T, Suzuki T, Siomi H, Siomi MC. Pimet, the Drosophila homolog of HEN1, mediates 2′-O-methylation of Piwi-interacting RNAs at their 3′ ends. Genes Dev. 2007;21:1603–1608. doi: 10.1101/gad.1563607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Han BW, Hung JH, Weng Z, Zamore PD, Ameres SL. The 3′-to-5′ exoribonuclease Nibbler shapes the 3′ ends of microRNAs bound to Drosophila Argonaute1. Curr Biol. 2011;21:1878–1887. doi: 10.1016/j.cub.2011.09.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Liu N, Abe M, Sabin LR, Hendriks GJ, Naqvi AS, Yu Z, Cherry S, Bonini NM. The exoribonuclease Nibbler controls 3′ end processing of microRNAs in Drosophila . Curr Biol. 2011;21:1888–1893. doi: 10.1016/j.cub.2011.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Feltzin VL, Khaladkar M, Abe M, Parisi M, Hendriks GJ, Kim J, Bonini NM. The exonuclease Nibbler regulates age-associated traits and modulates piRNA length in Drosophila . Aging Cell. 2015;14:443–452. doi: 10.1111/acel.12323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Wang H, Ma Z, Niu K, Xiao Y, Wu X, Pan C, Zhao Y, Wang K, Zhang Y, Liu N. Antagonistic roles of Nibbler and Hen1 in modulating piRNA 3′ ends in Drosophila . Development. 2016;143:530–539. doi: 10.1242/dev.128116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Izumi N, Shoji K, Sakaguchi Y, Honda S, Kirino Y, Suzuki T, Katsuma S, Tomari Y. Identification and functional analysis of the Pre-piRNA 3′ trimmer in silkworms. Cell. 2016;164:962–973. doi: 10.1016/j.cell.2016.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Tang W, Tu S, Lee HC, Weng Z, Mello CC. The RNase PARN-1 Trims piRNA 3′ ends to promote transcriptome surveillance in C. elegans . Cell. 2016;164:974–984. doi: 10.1016/j.cell.2016.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Zhang Z, Xu J, Koppetsch BS, Wang J, Tipping C, Ma S, Weng Z, Theurkauf WE, Zamore PD. Heterotypic piRNA Ping-Pong requires qin, a protein with both E3 ligase and Tudor domains. Mol Cell. 2011;44:572–584. doi: 10.1016/j.molcel.2011.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Wang W, Han BW, Tipping C, Ge DT, Zhang Z, Weng Z, Zamore PD. Slicing and BInding by Ago3 or Aub trigger Piwi-bound piRNA production by distinct mechanisms. Mol Cell. 2015;59:819–830. doi: 10.1016/j.molcel.2015.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Sato K, Iwasaki YW, Shibuya A, Carninci P, Tsuchizawa Y, Ishizu H, Siomi MC, Siomi H. Krimper enforces an antisense bias on piRNA pools by binding AGO3 in the Drosophila germline. Mol Cell. 2015;59:553–563. doi: 10.1016/j.molcel.2015.06.024. [DOI] [PubMed] [Google Scholar]
- 85.Webster A, Li S, Hur JK, Wachsmuth M, Bois JS, Perkins EM, Patel DJ, Aravin AA. Aub and Ago3 are recruited to Nuage through two mechanisms to form a ping-pong complex assembled by krimper. Mol Cell. 2015;59:564–575. doi: 10.1016/j.molcel.2015.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Xiol J, Spinelli P, Laussmann MA, Homolka D, Yang Z, Cora E, Coute Y, Conn S, Kadlec J, Sachidanandam R, et al. RNA clamping by Vasa assembles a piRNA amplifier complex on transposon transcripts. Cell. 2014;157:1698–1711. doi: 10.1016/j.cell.2014.05.018. [DOI] [PubMed] [Google Scholar]
- 87.Nishida KM, Iwasaki YW, Murota Y, Nagao A, Mannen T, Kato Y, Siomi H, Siomi MC. Respective functions of two distinct Siwi complexes assembled during PIWI-interacting RNA biogenesis in Bombyx germ cells. Cell Rep. 2015;10:193–203. doi: 10.1016/j.celrep.2014.12.013. [DOI] [PubMed] [Google Scholar]
- 88.Klenov MS, Sokolova OA, Yakushev EY, Stolyarenko AD, Mikhaleva EA, Lavrov SA, Gvozdev VA. Separation of stem cell maintenance and transposon silencing functions of Piwi protein. Proc Natl Acad Sci USA. 2011;108:18760–18765. doi: 10.1073/pnas.1106676108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Brower-Toland B, Findley SD, Jiang L, Liu L, Yin H, Dus M, Zhou P, Elgin SC, Lin H. Drosophila PIWI associates with chromatin and interacts directly with HP1a. Genes Dev. 2007;21:2300–2311. doi: 10.1101/gad.1564307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Ohtani H, Iwasaki YW, Shibuya A, Siomi H, Siomi MC, Saito K. DmGTSF1 is necessary for Piwi-piRISC-mediated transcriptional transposon silencing in the Drosophila ovary. Genes Dev. 2013;27:1656–1661. doi: 10.1101/gad.221515.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Muerdter F, Guzzardo PM, Gillis J, Luo Y, Yu Y, Chen C, Fekete R, Hannon GJ. A genome-wide RNAi screen draws a genetic framework for transposon control and primary piRNA biogenesis in Drosophila . Mol Cell. 2013;50:736–748. doi: 10.1016/j.molcel.2013.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Donertas D, Sienski G, Brennecke J. Drosophila Gtsf1 is an essential component of the Piwi-mediated transcriptional silencing complex. Genes Dev. 2013;27:1693–1705. doi: 10.1101/gad.221150.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Yu Y, Gu J, Jin Y, Luo Y, Preall JB, Ma J, Czech B, Hannon GJ. Panoramix enforces piRNA-dependent cotranscriptional silencing. Science. 2015;350:339–342. doi: 10.1126/science.aab0700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Sienski G, Batki J, Senti KA, Donertas D, Tirian L, Meixner K, Brennecke J. Silencio/CG9754 connects the Piwi-piRNA complex to the cellular heterochromatin machinery. Genes Dev. 2015;29:2258–2271. doi: 10.1101/gad.271908.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Findley SD, Tamanaha M, Clegg NJ, Ruohola-Baker H. Maelstrom, a Drosophila spindle-class gene, encodes a protein that colocalizes with Vasa and RDE1/AGO1 homolog, Aubergine, in nuage. Development. 2003;130:859–871. doi: 10.1242/dev.00310. [DOI] [PubMed] [Google Scholar]
- 96.Rozhkov NV, Hammell M, Hannon GJ. Multiple roles for Piwi in silencing Drosophila transposons. Genes Dev. 2013;27:400–412. doi: 10.1101/gad.209767.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Czech B, Malone CD, Zhou R, Stark A, Schlingeheyde C, Dus M, Perrimon N, Kellis M, Wohlschlegel JA, Sachidanandam R, et al. An endogenous small interfering RNA pathway in Drosophila . Nature. 2008;453:798–802. doi: 10.1038/nature07007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Kawamura Y, Saito K, Kin T, Ono Y, Asai K, Sunohara T, Okada TN, Siomi MC, Siomi H. Drosophila endogenous small RNAs bind to Argonaute 2 in somatic cells. Nature. 2008;453:793–797. doi: 10.1038/nature06938. [DOI] [PubMed] [Google Scholar]
- 99.Zhou R, Czech B, Brennecke J, Sachidanandam R, Wohlschlegel JA, Perrimon N, Hannon GJ. Processing of Drosophila endo-siRNAs depends on a specific Loquacious isoform. RNA. 2009;15:1886–1895. doi: 10.1261/rna.1611309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Miyoshi K, Miyoshi T, Hartig JV, Siomi H, Siomi MC. Molecular mechanisms that funnel RNA precursors into endogenous small-interfering RNA and microRNA biogenesis pathways in Drosophila . RNA. 2010;16:506–515. doi: 10.1261/rna.1952110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Fabian MR, Sonenberg N, Filipowicz W. Regulation of mRNA translation and stability by microRNAs. Annu Rev Biochem. 2010;79:351–379. doi: 10.1146/annurev-biochem-060308-103103. [DOI] [PubMed] [Google Scholar]
- 102.Grivna ST, Pyhtila B, Lin H. MIWI associates with translational machinery and PIWI-interacting RNAs (piRNAs) in regulating spermatogenesis. Proc Natl Acad Sci USA. 2006;103:13415–13420. doi: 10.1073/pnas.0605506103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Unhavaithaya Y, Hao Y, Beyret E, Yin H, Kuramochi-Miyagawa S, Nakano T, Lin H. MILI, a PIWI-interacting RNA-binding protein, is required for germ line stem cell self-renewal and appears to positively regulate translation. J Biol Chem. 2009;284:6507–6519. doi: 10.1074/jbc.M809104200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Rouget C, Papin C, Boureux A, Meunier AC, Franco B, Robine N, Lai EC, Pelisson A, Simonelig M. Maternal mRNA deadenylation and decay by the piRNA pathway in the early Drosophila embryo. Nature. 2010;467:1128–1132. doi: 10.1038/nature09465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Barckmann B, Pierson S, Dufourt J, Papin C, Armenise C, Port F, Grentzinger T, Chambeyron S, Baronian G, Desvignes JP, et al. Aubergine iCLIP reveals piRNA-dependent decay of mRNAs involved in germ cell development in the early embryo. Cell Rep. 2015;12:1205–1216. doi: 10.1016/j.celrep.2015.07.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Vourekas A, Alexiou P, Vrettos N, Maragkakis M, Mourelatos Z. Sequence-dependent but not sequence-specific piRNA adhesion traps mRNAs to the germ plasm. Nature. 2016;531:390–394. doi: 10.1038/nature17150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Yang F, Zhao R, Fang X, Huang H, Xuan Y, Ma Y, Chen H, Cai T, Qi Y, Xi R. The RNA surveillance complex Pelo-Hbs1 is required for transposon silencing in the Drosophila germline. EMBO Rep. 2015;16:965–974. doi: 10.15252/embr.201540084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Doma MK, Parker R. Endonucleolytic cleavage of eukaryotic mRNAs with stalls in translation elongation. Nature. 2006;440:561–564. doi: 10.1038/nature04530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Shoemaker CJ, Eyler DE, Green R. Dom34:Hbs1 promotes subunit dissociation and peptidyl-tRNA drop-off to initiate no-go decay. Science. 2010;330:369–372. doi: 10.1126/science.1192430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Cole SE, LaRiviere FJ, Merrikh CN, Moore MJ. A convergence of rRNA and mRNA quality control pathways revealed by mechanistic analysis of nonfunctional rRNA decay. Mol Cell. 2009;34:440–450. doi: 10.1016/j.molcel.2009.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Tsuboi T, Kuroha K, Kudo K, Makino S, Inoue E, Kashima I, Inada T. Dom34:hbs1 plays a general role in quality-control systems by dissociation of a stalled ribosome at the 3′ end of aberrant mRNA. Mol Cell. 2012;46:518–529. doi: 10.1016/j.molcel.2012.03.013. [DOI] [PubMed] [Google Scholar]
- 112.Guydosh NR, Green R. Dom34 rescues ribosomes in 3′ untranslated regions. Cell. 2014;156:950–962. doi: 10.1016/j.cell.2014.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Passos DO, Doma MK, Shoemaker CJ, Muhlrad D, Green R, Weissman J, Hollien J, Parker R. Analysis of Dom34 and its function in no-go decay. Mol Biol Cell. 2009;20:3025–3032. doi: 10.1091/mbc.E09-01-0028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Aravin AA, Sachidanandam R, Bourc’his D, Schaefer C, Pezic D, Toth KF, Bestor T, Hannon GJ. A piRNA pathway primed by individual transposons is linked to de novo DNA methylation in mice. Mol Cell. 2008;31:785–799. doi: 10.1016/j.molcel.2008.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Kuramochi-Miyagawa S, Watanabe T, Gotoh K, Totoki Y, Toyoda A, Ikawa M, Asada N, Kojima K, Yamaguchi Y, Ijiri TW, et al. DNA methylation of retrotransposon genes is regulated by Piwi family members MILI and MIWI2 in murine fetal testes. Genes Dev. 2008;22:908–917. doi: 10.1101/gad.1640708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Zhang G, Huang H, Liu D, Cheng Y, Liu X, Zhang W, Yin R, Zhang D, Zhang P, Liu J, et al. N6-methyladenine DNA modification in Drosophila . Cell. 2015;161:893–906. doi: 10.1016/j.cell.2015.04.018. [DOI] [PubMed] [Google Scholar]
- 117.Miesen P, Ivens A, Buck AH, van Rij RP. Small RNA profiling in dengue virus 2-infected aedes mosquito cells reveals viral piRNAs and novel host miRNAs. PLoS Negl Trop Dis. 2016;10:e0004452. doi: 10.1371/journal.pntd.0004452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Niki Y, Yamaguchi T, Mahowald AP. Establishment of stable cell lines of Drosophila germ-line stem cells. Proc Natl Acad Sci USA. 2006;103:16325–16330. doi: 10.1073/pnas.0607435103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Lau NC, Robine N, Martin R, Chung WJ, Niki Y, Berezikov E, Lai EC. Abundant primary piRNAs, endo-siRNAs, and microRNAs in a Drosophila ovary cell line. Genome Res. 2009;19:1776–1785. doi: 10.1101/gr.094896.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Ni JQ, Zhou R, Czech B, Liu LP, Holderbaum L, Yang-Zhou D, Shim HS, Tao R, Handler D, Karpowicz P, et al. A genome-scale shRNA resource for transgenic RNAi in Drosophila . Nat Methods. 2011;8:405–407. doi: 10.1038/nmeth.1592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Czech B, Preall JB, McGinn J, Hannon GJ. A transcriptome-wide RNAi screen in the Drosophila ovary reveals factors of the germline piRNA pathway. Mol Cell. 2013;50:749–761. doi: 10.1016/j.molcel.2013.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Handler D, Meixner K, Pizka M, Lauss K, Schmied C, Gruber FS, Brennecke J. The genetic makeup of the Drosophila piRNA pathway. Mol Cell. 2013;50:762–777. doi: 10.1016/j.molcel.2013.04.031. [DOI] [PMC free article] [PubMed] [Google Scholar]