Abstract
Immunoglobulin gamma (IgG) antibodies are key effector proteins of the immune system. They recognize antigens with high specificity and are indispensable for immunological memory following pathogen exposure or vaccination. The constant, crystallizable fragment (Fc) of IgG molecules mediates antibody effector functions such as complement-dependent cytotoxicity, antibody-mediated cellular cytotoxicity, and antibody-dependent cell-mediated phagocytosis. These functions are regulated by a single N-linked, biantennary glycan of the heavy chain, which resides just below the hinge region, and the presence of specific sugar moieties on the glycan has profound implications on IgG effector functions. Emerging knowledge of how Fc glycans contribute to IgG structure and functions has opened new avenues for the therapeutic exploitation of defined antibody glycoforms in the treatment of cancer and autoimmune diseases. Here, we review recent advances in understanding proinflammatory IgG effector functions and their regulation by Fc glycans.
Keywords: Immunology, Antibody, Immunoglobulin, Glycan, Glycobiology, Immunotherapy
Immunoglobulins
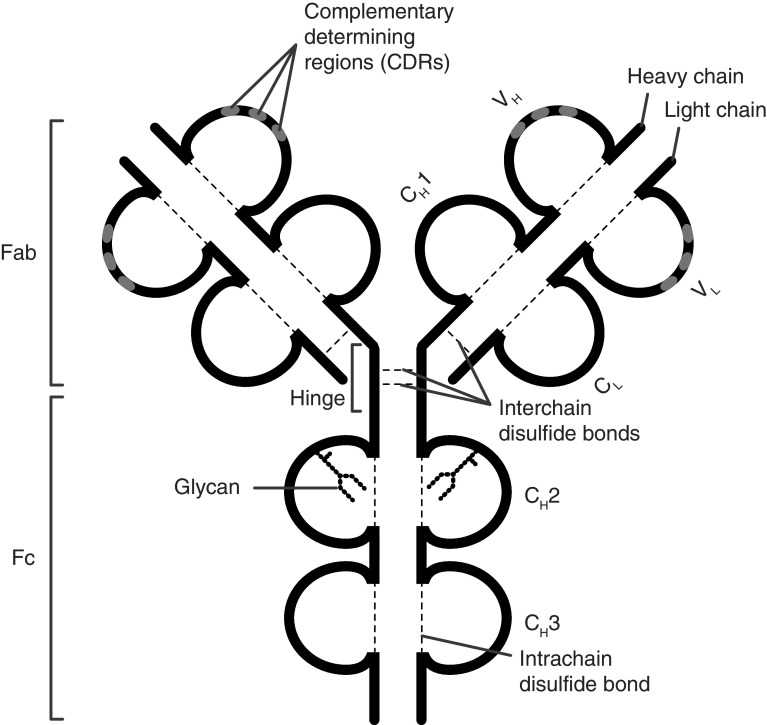
Immunoglobulins (Igs) are glycoproteins secreted by B cells and plasma cells and constitute one of the main effector mechanisms of the adaptive immune system. Igs evolved to specifically recognize target structures (antigens) and mediate appropriate actions by communicating with cellular and humoral components of the immune system. Antigen recognition is mediated by the “fragment antigen binding” (Fab) domains which contain the complementarity-determining regions (CDRs) located in the N-terminal region of heavy chains (HCs) and light chains (LCs) (Fig. 1, IgG). These areas are characterized by a high degree of variability in amino acid composition in between antibodies, which in turn leads to a broad spectrum of potential binding partners. The characteristic architecture of Igs, composed of disulfide bond-stabilized β-sheets as well as inter-chain disulfide bonds, guarantees the structural integrity required for the functionality of antibodies. The C-terminal regions of the two HCs constitute the “fragment crystallizable” (Fc), which contains the binding sites for immune effector molecules such as the complement system or Fc receptors. In some antibody types, including IgG, the Fab and Fc regions are separated by a less-structured stretch of amino acids called the “hinge region” which provides flexibility to the antibody and contains the disulfide bond(s) linking the two HCs [1, 2].
Fig. 1.
Structure of the immunoglobulin G (IgG) molecule. IgG is composed of two heavy and two light chains linked by disulfide bonds. The antigen-binding fragment (Fab) consists of two moieties with identical structure which define the antigen-specificity through their complementarity-determining regions (CDR). The crystallizable fragment (Fc) mediates antibody effector functions through binding to Fc receptors and interaction with the C1q component of the complement system. Each IgG molecule contains a single, highly conserved IgG-Fc N-glycan in each of the two CH2 domains (Fc glycan) and may carry additional glycans in the antigen-binding sites (Fab glycans). CH constant heavy, CL constant light, Fab antigen-binding fragment, Fc crystallizable fragment
Being amongst the most abundant serum proteins [3], immunoglobulins fulfill essential functions in protecting our body against invading pathogens. Depending on the type and stage of an infection as well as the anatomical site, different functional properties are required. This functional diversification is achieved by a process called antibody class switching. Thereby, tightly regulated DNA recombination events lead to the excision and replacement of the antibody’s constant region while keeping the CDRs and, therefore, the specificity of the antibody largely unchanged. The different constant regions are called isotypes and are differentiated in five classes (IgA, IgD, IgE, IgG and IgM) and six subclasses (IgG1–4 and IgA1–2). The decision which type of antibody is produced is dependent on the signals a B cell encounters during its maturation towards an antibody-secreting cell (ACS). A naïve mature B cell, previously selected in the bone marrow and spleen for functional integrity of the cell-surface-bound antibody (B-cell receptor, BCR) and low self-reactivity, expresses cell-surface IgD and/or IgM. Alternative splicing of the primary VH transcript results in the secreted antibody, which now lacks the cytoplasmic tail and the transmembrane region of the BCR. This allows B cells to simultaneously produce cell-surface-bound- (BCR) and secreted (antibodies) immunoglobulins with identical binding specificities. Upon cognate antigen encounter, B cells can enter several developmental programs eventually resulting in further diversification of the antibody repertoire by somatic hypermutation (SHM), a process which leads to mutation of the CDR sequences, and differentiation into long-lived memory B cells, terminally differentiated long-lived ASC (plasma cells) or short-lived ASC (plasmablasts). The sites of antigen-induced B-cell differentiation are secondary lymphoid organs such as the spleen, lymph nodes or gut-associated lymphoid tissues. Differentiation can take place independent of T cells, resulting in little SHM and limited class switch or in a T-cell dependent manner, which typically involves the generation of germinal centers where extensive SHM takes place and switching to all classes of antibodies can occur. The nature of the antigen, anatomic location, signaling via pattern recognition receptors and the cytokine milieu are crucial for the choice of the differentiation program and the decision which antibody isotypes develop [4]. Antibodies fulfill important functions within the immune system such as neutralization of toxins or microbes and assisting the killing of transformed cells or bacteria by opsonization, antibody-dependent cell-mediated cytotoxicity (ADCC), antibody-dependent cell-mediated phagocytosis (ADCP) or complement-dependent cytotoxicity (CDC) (Fig. 2). This review puts a focus on the generation and functions of the most abundant human antibody class in circulation, immunoglobulin G (IgG) and highlights the aspects of how these effector functions are regulated.
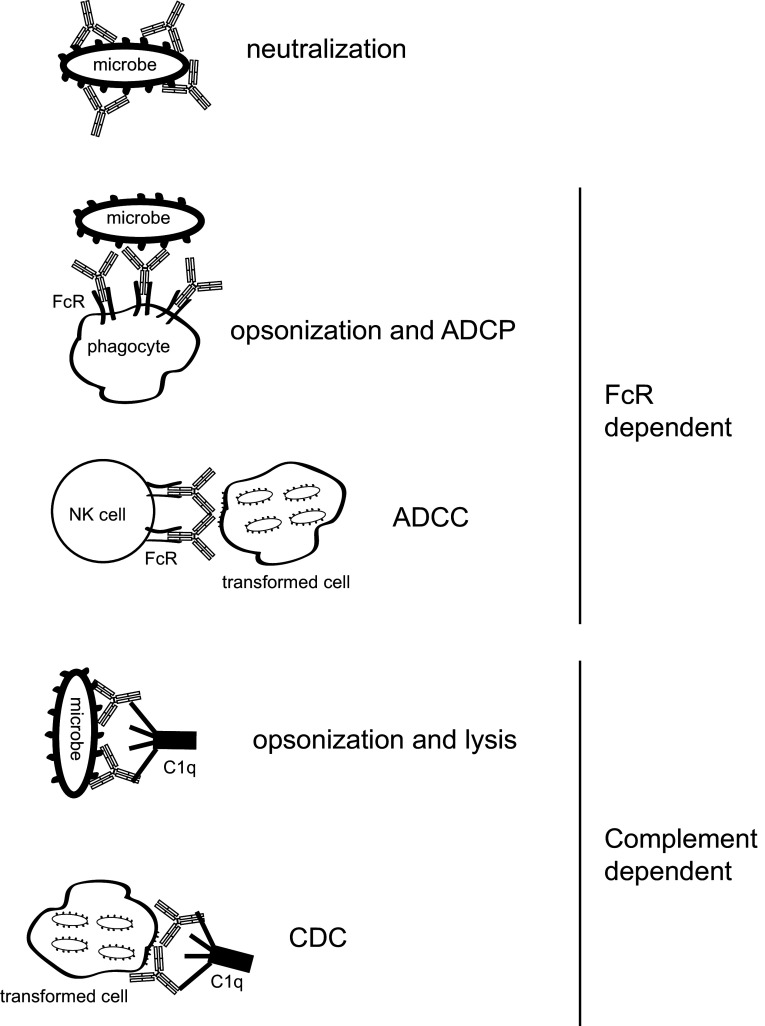
Fig. 2.
Effector functions of IgG antibodies. In addition to neutralization initiated by binding of the Fab domain to target molecules, the antibodies’ Fc fragment mediates IgG effector functions such as killing of transformed cells or bacteria by opsonization, antibody-dependent cell-mediated cytotoxicity (ADCC), antibody-dependent cell-mediated phagocytosis (ADCP) or complement-dependent cytotoxicity (CDC)
Immunoglobulin G
IgG is the prototypic antibody composed of two HCs and two LCs linked by disulfide bonds. Each IgG molecule contains a single, highly conserved IgG-Fc N-glycan in each of the two CH2 domains and may carry additional glycans in the antigen-binding sites [5, 6]. One reason for its predominance in serum is the exceptionally long half-life of 21 days due to recycling via the neonatal Fc receptor (FcRn) as loss of FcRn dramatically reduces the half-life of IgG [7–9]. FcRn binds to internalized IgG at low pH (6.5) and recycles to the cell surface where higher pH leads to the release of IgG back into circulation [8].
The four human IgG subclasses are named according to their frequency in serum IgG-1, -2, -3, and -4 and share more than 90 % amino acid (AA) sequence homology with some important differences: the cysteine- and proline-rich hinge region, which contains inter-HC disulfide bonds and determines Fab-arm flexibility, is the hotspot of diversity. IgG3 has the longest hinge constituted of up to 62 AA and 11 disulfide bonds (exact numbers vary in allelic variants called allotypes). The extended length allows the Fab domain of IgG3 to have a great amount of conformational flexibility relative to its Fc [2, 10]. In contrast, IgG2 has a rigid hinge composed of only 12 AA containing four disulfide bonds making it the IgG isotype with the most restricted Fab-arm flexibility [2, 10]. IgG1 (15 AA hinge, 2 disulfide bonds) and IgG4 (12 AA, 2 disulfide bonds) have intermediate properties [2, 10]. Further important AA differences are located in the binding region for complement proteins and Fc receptors, the CH2 domain of the HC. Additional AA sequence variations stem from allelic differences in the human population. These, if immunogenic, are called allotypes, some of which can influence functional properties, in particular for IgG3 [2, 11].
Fc-dependent effector functions of IgG
In addition to binding antigen via their Fab fragments, IgG antibodies regulate immune responses through their Fc domain (Fig. 2). First, IgG can initiate the activation of the complement pathway, resulting in the generation of the proinflammatory anaphylatoxins C3a and C5a and the membrane attack complex which may lead to lysis of the target cell by complement-dependent cytotoxicity (CDC). Second, IgG autoantibodies can cross-link cellular Fc receptors specific for IgG (FcγRs) that are present on most innate immune effector cells, including neutrophils, mast cells and macrophages. FcγRs mediate important IgG effector functions such as induction of antibody-dependent cellular cytotoxicity and opsonization and phagocytosis of antigens.
Receptors binding to the Fc domain of IgG are called Fc-gamma receptors (FcγRs) (Fig. 3). Humans express five or six classical FcγRs which are grouped into activating [FcγRI, FcγRIIa (FcγRIIc expressed by some individuals [12]) and FcγRIIIa], inhibitory (FcγRIIb) and glycosylphosphatidylinositol (GPI)-anchored (FcγRIIIb) Fc receptors (Fig. 3). With the exception of T cells, all major immune cells express FcγRs, which allows antibodies to explore the functions of many cells and exhibit a wide range of effector mechanisms. IgG isotypes bind to FcγRs with their CH2 domain [13–16]. Differences in the CH2 AA composition [17], Fc glycan structure [18, 19] and hinge region [15, 17] influence IgG binding to FcγRs. Activating FcγRs contain immunoreceptor tyrosine-based activation motifs (ITAM) in their intracellular domain or their adaptor proteins, whereas FcγRIIb contains an immunoreceptor tyrosine-based inhibitory motif (ITIM), which allows it to counteract activating signals. Based on the receptor`s affinity for IgG, FcγRI is referred to as “high affinity FcγR”, whereas all other FcγRs are considered “low affinity FcγRs”. The high affinity results in FcγRI being constantly associated with IgG, whereas firm binding to low-affinity FcγRs requires the formation of an immune complex [20]. Although due to the high serum-IgG concentration, even low-affinity FcγRs are almost saturated with IgG in blood [21], the faster off-rates allow cells expressing these receptors to rapidly sample IgGs in solution [22–25].
Fig. 3.
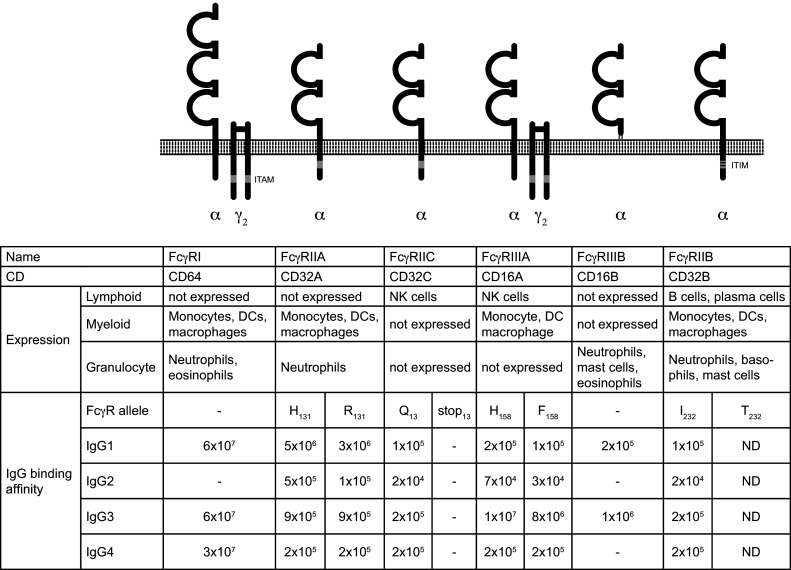
Human Fc receptors for IgG (FcγR). FcγRs differ in their cellular distribution, their function and their affinity for the IgG-Fc fragment
FcγRI (CD64) is expressed by myeloid cells (monocytes, DCs, macrophages) and granulocytes (neutrophils, mast cells) and binds with very high affinity to IgG1, 3 and 4 but does not bind IgG2. Signaling by FcγRI leads to the activation and differentiation of monocytes towards monocyte-derived DCs and may contribute to antigen presentation to T cells [26]. FcγRI is deregulated in several antibody-mediated autoimmune diseases implying a potential role in disease pathology [27, 28]. FcγRI-bound monomeric IgG is constantly internalized and recycled to the cell surface, whereas cross-linking with the cognate antigen leads to internalization and degradation [29]. Despite its high affinity for monomeric IgG, mice transgenic for human FcγRI suggest that the receptor retains its ability to bind IgG-ICs in vivo and contributes to IC-mediated cell activation [30].
FcγRIIa is expressed by granulocytes, monocytes, macrophages, DCs and platelets [31]. FcγRIIc is encoded by a gene that resulted from the crossover of fcgr2a and fcgr2b [32]. It is only found on NK cells, and allelic polymorphisms result in FcγRIIc being expressed by only approximately 45 % of individuals [12, 33] where it can contribute to cytotoxicity [33].
The FcγRIII (CD16) exists in two alternative forms encoded by two different genes, a transmembrane FcγRIIIa expressed on natural killer (NK) cells and macrophages, and a GPI-linked FcγRIIIb present on neutrophils [34, 35]. The activating low-affinity FcγRIIIa (CD16) mediates antibody-dependent cellular cytotoxicity (ADCC) and is highly expressed on the cytotoxic CD56dim CD16+ NK-cell subset as well as on other hematopoietic cells (Fig 3). NK cells are thought to be the key mediators of ADCC, a mechanism harnessed in monoclonal antibody treatments of various cancers overexpressing unique antigens, such as neuroblastoma, breast cancer, B-cell lymphoma, and others.
FcγRIIb is the only FcγR expressed on B cells and plasma cells. Its co-engagement with the BCR delivers an inhibitory signal and can, therefore, be envisioned as a negative feedback from circulating antibodies, which may limit B-cell differentiation and antibody production [36]. Its importance for B-cell homeostasis is highlighted by the autoimmune susceptibility of mice lacking FcγRIIb [37] and reduced expression on B cells derived from patients with various antibody-mediated autoimmune diseases [27, 38, 39]. FcγRIIb expression on murine plasma cells regulates their persistence and apoptosis [40] and limits IgG autoantibody production [41].
On myeloid cells and granulocytes, such as monocytes, dendritic cells, neutrophils and basophils, FcγRIIb can be co-expressed with activating FcγRs [32]. Immune complex-mediated activation of monocytes was shown to be negatively regulated by FcγRIIb [42, 43], and consequently, absence of FcγRIIb on murine DC results in increased T-cell priming [36]. These studies suggest that loss of balanced FcγR signaling could result in uncontrolled responses that can lead to the damage of healthy tissues and the initiation of autoimmune disease.
Complement-mediated IgG effector functions
Complement activation is one of the most effective defense mechanisms of the immune system. It is initiated by the binding of so-called initiators of the complement system such as C1q, ficolins or mannose-binding lectin to target surfaces like infected cells or microbes. Their binding leads to a cascade of events ultimately culminating in the formation of the membrane attack complex and lyses of the target. IgG can assist the initiation of the complement system in three ways. (1) C1q binds with high affinity to antigen-bound, but not monomeric IgG [44, 45], (2) IgG carrying IgG-Fc N-glycan terminating in mannose may additionally be able to bind mannose-binding lectin [46, 47] and (3) C3b, a downstream component of the complement cascade, can directly bind to IgG [48]. IgG isotypes have different C1q binding affinities with IgG3 binding most potently followed by IgG1, very weak binding for IgG2 and none for IgG4 [49]. The main interaction points of C1q are located in IgG’s CH2 and are constituted by residues which are mostly conserved between isotypes [13, 50]. The structure of the hinge region, the IgG-Fc N-glycan and the relative orientation of the Fab domain influences the affinity of C1q and may explain binding differences between IgG isotypes [44, 51].
Fc glycan-modulated IgG effector functions
Human immunoglobulins are Fc-glycosylated and can, depending on the isotype and the sequence of the antigen-binding regions (complementarity-determining regions, CDR), carry additional glycans in the Fab domains. IgG is unique with respect to a single, highly conserved asparagine 297 (N297)-glycosylation site which points towards a hole in the Fc region formed by the CH2 and CH3 domains.
During protein translation, a pre-formed lipid-linked glycan is transferred and covalently attached to N297 in the lumen of the endoplasmic reticulum (ER). This initial glycan is composed of two N-acetylglucosamines (GlcNAc) followed by nine mannose (Man) and three glucose (Glc) residues [52, 53]. Its structure is highly conserved in eukaryotes and serves as an important mechanism for protein folding and quality control of proteins carrying N-glycans [52]. If folded properly, the IgG polypeptide is transferred from the ER to the Golgi, where glycosyl-hydrolases and -transferases can modify the glycan structure leading to such diverse and highly complex glycans as seen in the IgG Fc. In addition to the oligosaccharide core, more than 95 % of the biantennary complex-type structure of the final IgG glycan carries an N-acetylglucosamine on both arms [54, 55] and 85 % are fucosylated [56] (Fig. 4). In contrast, the presence of galactose is less homogenous with 40 % of glycans carrying one galactose (G1 glycan) and the frequency of non-galactosylated (G0) or bi-galactosylated glycans (G2) ranging between 20 and 40 % depending on age and gender [55, 57, 58]. The most distal sugar on the glycan is sialic acid (neuraminic acid, Neu5Ac). Around 5–10 % of glycans carry sialic acid on one arm, and approximately 1 % of serum IgG-Fc glycans are bi-sialylated [5, 58, 59]. In addition to the Fc domain, roughly 15–20 % of human serum IgGs are glycosylated in their Fab domain [60, 61]. The functional significance of Fab glycosylation is incompletely understood, but it has been suggested that it might impact binding affinities of antigen–antibody interactions [62, 63].
Fig. 4.
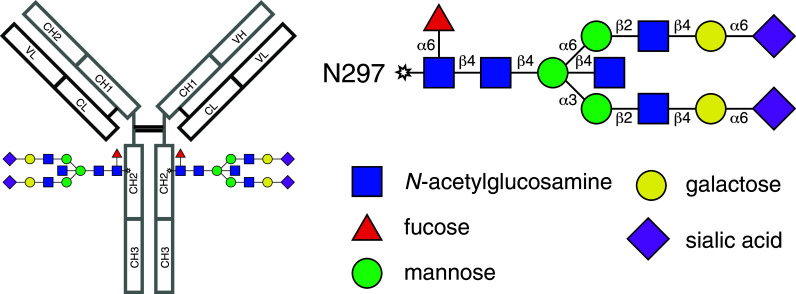
Structure of a fully processed IgG-Fc N-glycan. The Asparagine 297-linked complex-type glycan is located within the CH2 domains of the Fc fragment and consists of a complex, biantennary structure. In vivo, such a fully processed glycan will be found only in trace amounts as the majority of antibodies will carry either no, one or two galactose residues and a fraction of those carrying galactose will additionally possess sialic acid
Removal of the entire Fc N-glycan impairs antibody effector functions [13, 64], and the presence or absence of distinct IgG-Fc monosaccharides was shown to regulate IgG effector functions.
Fc fucose
The majority of circulating IgG antibodies are fucosylated [56] which, compared to afucosylated isotypes, reduces IgG´s binding affinitiy for the activating FcγRIII (CD16) and thereby its potential to induce antibody-dependent cellular cytotoxicity [19]. Fucosylation also appears to impair antibody-dependent cell-mediated phagocytosis [65, 66]. Consequently, clinical trials using afucosylated monoclonal antibodies were initiated and showed improved efficacy in target cell depletion [67–69].
Fc galactose
In adoptive transfer models of autoimmune diseases, non-pathogenic doses of autoantibodies become pathogenic when present as agalactosyl glycoforms [18, 70, 71]. Decreased levels of galactosylation are associated with several chronic inflammatory diseases such as rheumatoid arthritis, systemic lupus erythematosus, multiple sclerosis, and in patients with tuberculosis [55, 72–74]. In contrast, increased IgG-Fc galactosylation is detectable during pregnancy, and in rheumatoid arthritis patients who experience pregnancy-induced remission [75, 76] suggesting that Fc galactosylation might exert anti-inflammatory functions. In line with this hypothesis, a recent study showed that high galactosylation of IgG immune complexes in mice promotes the association of FcγRIIB and dectin-1, which blocks the proinflammatory effector functions of C5aR and CXCR2 [77]. The observation that antiviral activity and spontaneous control of HIV infection are associated with increased prevalence of total and antigen-specific agalactosylated antibodies additionally argues for a functional significance of antibody galactosylation in humans and is in line with the assumption that lack or loss of IgG Fc-linked galactose occurs during or promotes inflammation [6, 78]. Along these lines, Ho et al. recently reported that in patients with chronic hepatitis B low IgG-Fc galactosylation levels are associated with high-grade liver inflammation and fibrosis, suggesting that IgG-Fc galactosylation might be a potential noninvasive indicator of severe liver necroinflammation and fibrosis [79]. One important aspect to consider when investigating the impact of galactose is that it provides the basis for the addition of sialic acid, the most distal sugar moiety on the IgG-Fc glycan.
Fc sialic acid
Similar to Fc galactosylation, decreased levels of IgG sialylation are observed in chronic autoimmune diseases such as rheumatoid arthritis, juvenile idiopathic arthritis and Wegener’s granulomatosis [55, 80–82]. It has also been demonstrated that IgG sialylation increases during pregnancy and that this increase may be associated with the remission of rheumatoid arthritis during pregnancy [83]. While the aforementioned data clearly support the hypothesis that these terminal sugar residues are involved in modulating antibody activity, they suggest that it is not the lack of galactose residues itself but, rather, the concomitant absence of terminal sialic acid residues that may be responsible for the enhanced inflammatory activity exerted by aglycosylated glycoforms [18, 70]. The relevance of sialic acid residues for modulating immune responses is highlighted by the finding that intravenous immunoglobulins (IVIG) completely lose their immunosuppressive capacity upon removal of Fc-sialic acid residues by neuraminidase treatment in experimental autoimmune disease models [18]. Conversely, IVIG preparations as well as isolated Fc fragments enriched for terminal sialic acid residues appear to have a more than tenfold higher anti-inflammatory activity [70, 84]. Proposed mechanisms that mediate anti-inflammatory activities of Fc sialylation include the induction of an anti-inflammatory cytokine milieu following binding of sialylated IgGs to the murine C-type lectin receptor SIGNR1, which in turn induces interleukin (IL)-33 and IL-4 production and eventually leads to the upregulation of the inhibitory FcγRIIb on macrophages, thereby limiting antibody-mediated immunopathologies [85]. However, conflicting results exist concerning the ability of SIGNR1 and its human homolog DC-SIGN to recognize the sialylated IgG-Fc glycan [86, 87] and if these receptors are required for the anti-inflammatory properties of sialylated Fc [88]. In humans, Fc sialylation reduces proinflammatory IgG effector functions such as complement-dependent cytotoxicity (CDC) by inhibiting the binding of the antibody’s CH2 domain to C1q [89]. Thus, the mechanisms that mediate anti-inflammatory properties of Fc sialylation are not fully understood and might involve Fc receptor-dependent and -independent mechanisms. Therapeutic implications of the aforementioned findings lie in the possibility to modify Fc glycosylation to increase the anti-inflammatory efficacy of both IVIG and monoclonal antibody-mediated immunotherapies. It remains to be evaluated whether Fc sialylation can be harnessed to improve anti-inflammatory efficacy and the clinical response to IgG-mediated treatment strategies.
Therapeutic relevance and concluding remarks
The aforementioned studies clearly demonstrated that minor structural changes in IgG-Fc glycosylation profoundly affect antibody effector functions and opened up new opportunities for designing therapeutic antibodies with increased efficacies. Defucosylated antibodies which enhance ADCC are currently evaluated and increasingly utilized in cancer therapy. Obinutuzumab, a glycoengineered anti-CD20 antibody with reduced fucosylation and increased bisecting GlcNAc, has recently been approved as first-line treatment for patients with chronic lymphocytic leukemia (CLL) and follicular lymphoma (FL) who did not benefit from treatment with a fucosylated anti-CD20 antibody, i.e., rituximab [90]. Removal of the N-glycan impairs FcγR binding and complement activation, and this strategy has been increasingly recognized as a targeted treatment for autoimmune conditions. In vivo administration of the bacterial IgG glycan-hydrolysing enzyme EndoS, which cleaves the linkage between the two GlcNAc residues in the core of the N-linked glycan, was shown to ameliorate the development of various experimental models of autoimmune diseases [91–94], and this appears to be safe and well-tolerated in preclinical models [95] and represents a potential strategy to limit antibody-mediated autoimmune disease conditions [96].
The anti-inflammatory activity of sialylated IgGs was first demonstrated for intravenous immunoglubulins (IVIG): in contrast to fully sialylated IVIG preparations, desialylated IVIG failed to suppress autoimmune disease development in an antibody-mediated experimental arthritis model [18]. Subsequent studies confirmed the protective and crucial role of IVIG sialylation in various experimental autoimmune disease conditions [84, 87, 97, 98]. Fc-sialylated glycovariants were shown to mediate upregulation of the inhibitory FcgRIIB [18], to block B-cell proliferation independent of Fc receptors [99] in mice and to limit proinflammatory IgG effector functions through impairment of CDC in humans [89]. A scalable process to produce fully sialylated IVIG with consistent enhanced anti-inflammatory activity has recently been described, and the safety and efficacy of fully sialylated IgG will soon be evaluated in clinical trials [84]. Such trials, if well designed, are instrumental to evaluate the biological significance of IgG-Fc N-glycan modifications in human diseases and might generate strategies for tailoring IgG-based recombinant antibodies for the treatment of cancer and autoimmune diseases.
Acknowledgments
I.Q. was supported by a DOC scholarship provided by the Austrian Academy of Sciences (ÖAW). J.D.L. receives funding by the Swiss National Multiple Sclerosis Society, the Sassella Foundation, The Hartmann Müller Foundation, and the Novartis Foundation for medical-biological research.
Compliance with ethical standards
Conflict of interest
The authors declare no conflict of interest.
References
- 1.Woof JM, Russell MW. Structure and function relationships in IgA. Mucosal Immunol. 2011;4(6):590–597. doi: 10.1038/mi.2011.39. [DOI] [PubMed] [Google Scholar]
- 2.Vidarsson G, Dekkers G, Rispens T. IgG subclasses and allotypes: from structure to effector functions. Front Immunol. 2014;5:520. doi: 10.3389/fimmu.2014.00520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Anderson NL, Anderson NG. The human plasma proteome: history, character, and diagnostic prospects. Mol Cell Proteom. 2002;1(11):845–867. doi: 10.1074/mcp.R200007-MCP200. [DOI] [PubMed] [Google Scholar]
- 4.Pone EJ, Zhang J, Mai T, White CA, Li G, Sakakura JK, Patel PJ, Al-Qahtani A, Zan H, Xu Z, Casali P. BCR-signalling synergizes with TLR-signalling for induction of AID and immunoglobulin class-switching through the non-canonical NF-kappaB pathway. Nat Commun. 2012;3:767. doi: 10.1038/ncomms1769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Arnold JN, Wormald MR, Sim RB, Rudd PM, Dwek RA. The impact of glycosylation on the biological function and structure of human immunoglobulins. Annu Rev Immunol. 2007;25:21–50. doi: 10.1146/annurev.immunol.25.022106.141702. [DOI] [PubMed] [Google Scholar]
- 6.Quast I, Lunemann JD. Fc glycan-modulated immunoglobulin G effector functions. J Clin Immunol. 2014;34(Suppl 1):S51–S55. doi: 10.1007/s10875-014-0018-3. [DOI] [PubMed] [Google Scholar]
- 7.Chaudhury C, Mehnaz S, Robinson JM, Hayton WL, Pearl DK, Roopenian DC, Anderson CL. The major histocompatibility complex-related Fc receptor for IgG (FcRn) binds albumin and prolongs its lifespan. J Exp Med. 2003;197(3):315–322. doi: 10.1084/jem.20021829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ober RJ, Martinez C, Vaccaro C, Zhou J, Ward ES. Visualizing the site and dynamics of IgG salvage by the MHC class I-related receptor. FcRn. J Immunol. 2004;172(4):2021–2029. doi: 10.4049/jimmunol.172.4.2021. [DOI] [PubMed] [Google Scholar]
- 9.Dickinson BL, Badizadegan K, Wu Z, Ahouse JC, Zhu X, Simister NE, Blumberg RS, Lencer WI. Bidirectional FcRn-dependent IgG transport in a polarized human intestinal epithelial cell line. J Clin Invest. 1999;104(7):903–911. doi: 10.1172/JCI6968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Roux KH, Strelets L, Michaelsen TE. Flexibility of human IgG subclasses. J Immunol. 1997;159(7):3372–3382. [PubMed] [Google Scholar]
- 11.Lefranc MP, Lefranc G. Human Gm, Km, and Am allotypes and their molecular characterization: a remarkable demonstration of polymorphism. Methods Mol Biol. 2012;882:635–680. doi: 10.1007/978-1-61779-842-9_34. [DOI] [PubMed] [Google Scholar]
- 12.Ernst LK, Metes D, Herberman RB, Morel PA. Allelic polymorphisms in the FcgammaRIIC gene can influence its function on normal human natural killer cells. J Mol Med (Berl) 2002;80(4):248–257. doi: 10.1007/s00109-001-0294-2. [DOI] [PubMed] [Google Scholar]
- 13.Duncan AR, Woof JM, Partridge LJ, Burton DR, Winter G. Localization of the binding site for the human high-affinity Fc receptor on IgG. Nature. 1988;332(6164):563–564. doi: 10.1038/332563a0. [DOI] [PubMed] [Google Scholar]
- 14.Chappel MS, Isenman DE, Everett M, Xu YY, Dorrington KJ, Klein MH. Identification of the Fc gamma receptor class I binding site in human IgG through the use of recombinant IgG1/IgG2 hybrid and point-mutated antibodies. Proc Natl Acad Sci USA. 1991;88(20):9036–9040. doi: 10.1073/pnas.88.20.9036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sondermann P, Huber R, Oosthuizen V, Jacob U. The 3.2-A crystal structure of the human IgG1 Fc fragment-Fc gammaRIII complex. Nature. 2000;406(6793):267–273. doi: 10.1038/35018508. [DOI] [PubMed] [Google Scholar]
- 16.Ierino FL, Hulett MD, McKenzie IF, Hogarth PM. Mapping epitopes of human Fc gamma RII (CDw32) with monoclonal antibodies and recombinant receptors. J Immunol. 1993;150(5):1794–1803. [PubMed] [Google Scholar]
- 17.Canfield SM, Morrison SL. The binding affinity of human IgG for its high affinity Fc receptor is determined by multiple amino acids in the CH2 domain and is modulated by the hinge region. J Exp Med. 1991;173(6):1483–1491. doi: 10.1084/jem.173.6.1483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kaneko Y, Nimmerjahn F, Ravetch JV. Anti-inflammatory activity of immunoglobulin G resulting from Fc sialylation. Science. 2006;313(5787):670–673. doi: 10.1126/science.1129594. [DOI] [PubMed] [Google Scholar]
- 19.Shields RL, Lai J, Keck R, O’Connell LY, Hong K, Meng YG, Weikert SH, Presta LG. Lack of fucose on human IgG1 N-linked oligosaccharide improves binding to human Fcgamma RIII and antibody-dependent cellular toxicity. J Biol Chem. 2002;277(30):26733–26740. doi: 10.1074/jbc.M202069200. [DOI] [PubMed] [Google Scholar]
- 20.Bruhns P, Iannascoli B, England P, Mancardi DA, Fernandez N, Jorieux S, Daeron M. Specificity and affinity of human Fcgamma receptors and their polymorphic variants for human IgG subclasses. Blood. 2009;113(16):3716–3725. doi: 10.1182/blood-2008-09-179754. [DOI] [PubMed] [Google Scholar]
- 21.van der Poel CE, Spaapen RM, van de Winkel JG, Leusen JH. Functional characteristics of the high affinity IgG receptor, FcgammaRI. J Immunol. 2011;186(5):2699–2704. doi: 10.4049/jimmunol.1003526. [DOI] [PubMed] [Google Scholar]
- 22.Miller KL, Duchemin AM, Anderson CL. A novel role for the Fc receptor gamma subunit: enhancement of Fc gamma R ligand affinity. J Exp Med. 1996;183(5):2227–2233. doi: 10.1084/jem.183.5.2227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sondermann P, Jacob U, Kutscher C, Frey J. Characterization and crystallization of soluble human Fc gamma receptor II (CD32) isoforms produced in insect cells. Biochemistry. 1999;38(26):8469–8477. doi: 10.1021/bi982889q. [DOI] [PubMed] [Google Scholar]
- 24.Vance BA, Huizinga TW, Wardwell K, Guyre PM. Binding of monomeric human IgG defines an expression polymorphism of Fc gamma RIII on large granular lymphocyte/natural killer cells. J Immunol. 1993;151(11):6429–6439. [PubMed] [Google Scholar]
- 25.Li P, Jiang N, Nagarajan S, Wohlhueter R, Selvaraj P, Zhu C. Affinity and kinetic analysis of Fcgamma receptor IIIa (CD16a) binding to IgG ligands. J Biol Chem. 2007;282(9):6210–6221. doi: 10.1074/jbc.M609064200. [DOI] [PubMed] [Google Scholar]
- 26.Tanaka M, Krutzik SR, Sieling PA, Lee DJ, Rea TH, Modlin RL. Activation of Fc gamma RI on monocytes triggers differentiation into immature dendritic cells that induce autoreactive T cell responses. J Immunol. 2009;183(4):2349–2355. doi: 10.4049/jimmunol.0801683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Quast I, Cueni F, Nimmerjahn F, Tackenberg B, Lunemann JD. Deregulated Fcgamma receptor expression in patients with CIDP. Neurol Neuroimmunol Neuroinflamm. 2015;2(5):e148. doi: 10.1212/NXI.0000000000000148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kikuchi-Taura A, Yura A, Tsuji S, Ohshima S, Kitatoube A, Shimizu T, Nii T, Katayama M, Teshigawara S, Yoshimura M, Kudo-Tanaka E, Harada Y, Matsushita M, Hashimoto J, Saeki Y. Monocyte CD64 expression as a novel biomarker for the disease activity of systemic lupus erythematosus. Lupus. 2015 doi: 10.1177/0961203315579093. [DOI] [PubMed] [Google Scholar]
- 29.Harrison PT, Davis W, Norman JC, Hockaday AR, Allen JM. Binding of monomeric immunoglobulin G triggers Fc gamma RI-mediated endocytosis. J Biol Chem. 1994;269(39):24396–24402. [PubMed] [Google Scholar]
- 30.Mancardi DA, Albanesi M, Jonsson F, Iannascoli B, Van Rooijen N, Kang X, England P, Daeron M, Bruhns P. The high-affinity human IgG receptor FcgammaRI (CD64) promotes IgG-mediated inflammation, anaphylaxis, and antitumor immunotherapy. Blood. 2013;121(9):1563–1573. doi: 10.1182/blood-2012-07-442541. [DOI] [PubMed] [Google Scholar]
- 31.King M, McDermott P, Schreiber AD. Characterization of the Fc gamma receptor on human platelets. Cell Immunol. 1990;128(2):462–479. doi: 10.1016/0008-8749(90)90041-O. [DOI] [PubMed] [Google Scholar]
- 32.Veri MC, Gorlatov S, Li H, Burke S, Johnson S, Stavenhagen J, Stein KE, Bonvini E, Koenig S. Monoclonal antibodies capable of discriminating the human inhibitory Fcgamma-receptor IIB (CD32B) from the activating Fcgamma-receptor IIA (CD32A): biochemical, biological and functional characterization. Immunology. 2007;121(3):392–404. doi: 10.1111/j.1365-2567.2007.02588.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Metes D, Ernst LK, Chambers WH, Sulica A, Herberman RB, Morel PA. Expression of functional CD32 molecules on human NK cells is determined by an allelic polymorphism of the FcgammaRIIC gene. Blood. 1998;91(7):2369–2380. [PubMed] [Google Scholar]
- 34.Ravetch JV, Perussia B. Alternative membrane forms of Fc gamma RIII(CD16) on human natural killer cells and neutrophils. Cell type-specific expression of two genes that differ in single nucleotide substitutions. J Exp Med. 1989;170(2):481–497. doi: 10.1084/jem.170.2.481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Edberg JC, Barinsky M, Redecha PB, Salmon JE, Kimberly RP. Fc gamma RIII expressed on cultured monocytes is a N-glycosylated transmembrane protein distinct from Fc gamma RIII expressed on natural killer cells. J Immunol. 1990;144(12):4729–4734. [PubMed] [Google Scholar]
- 36.Li F, Smith P, Ravetch JV. Inhibitory Fcgamma receptor is required for the maintenance of tolerance through distinct mechanisms. J Immunol. 2014;192(7):3021–3028. doi: 10.4049/jimmunol.1302934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bolland S, Ravetch JV. Spontaneous autoimmune disease in Fc(gamma)RIIB-deficient mice results from strain-specific epistasis. Immunity. 2000;13(2):277–285. doi: 10.1016/S1074-7613(00)00027-3. [DOI] [PubMed] [Google Scholar]
- 38.Tackenberg B, Jelcic I, Baerenwaldt A, Oertel WH, Sommer N, Nimmerjahn F, Lunemann JD. Impaired inhibitory Fcgamma receptor IIB expression on B cells in chronic inflammatory demyelinating polyneuropathy. Proc Natl Acad Sci USA. 2009;106(12):4788–4792. doi: 10.1073/pnas.0807319106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mackay M, Stanevsky A, Wang T, Aranow C, Li M, Koenig S, Ravetch JV, Diamond B. Selective dysregulation of the FcgammaIIB receptor on memory B cells in SLE. J Exp Med. 2006;203(9):2157–2164. doi: 10.1084/jem.20051503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Xiang Z, Cutler AJ, Brownlie RJ, Fairfax K, Lawlor KE, Severinson E, Walker EU, Manz RA, Tarlinton DM, Smith KG. FcgammaRIIb controls bone marrow plasma cell persistence and apoptosis. Nat Immunol. 2007;8(4):419–429. doi: 10.1038/ni1440. [DOI] [PubMed] [Google Scholar]
- 41.Fukuyama H, Nimmerjahn F, Ravetch JV. The inhibitory Fcgamma receptor modulates autoimmunity by limiting the accumulation of immunoglobulin G+ anti-DNA plasma cells. Nat Immunol. 2005;6(1):99–106. doi: 10.1038/ni1151. [DOI] [PubMed] [Google Scholar]
- 42.Boruchov AM, Heller G, Veri MC, Bonvini E, Ravetch JV, Young JW. Activating and inhibitory IgG Fc receptors on human DCs mediate opposing functions. J Clin Invest. 2005;115(10):2914–2923. doi: 10.1172/JCI24772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Clynes R, Maizes JS, Guinamard R, Ono M, Takai T, Ravetch JV. Modulation of immune complex-induced inflammation in vivo by the coordinate expression of activation and inhibitory Fc receptors. J Exp Med. 1999;189(1):179–185. doi: 10.1084/jem.189.1.179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gaboriaud C, Juanhuix J, Gruez A, Lacroix M, Darnault C, Pignol D, Verger D, Fontecilla-Camps JC, Arlaud GJ. The crystal structure of the globular head of complement protein C1q provides a basis for its versatile recognition properties. J Biol Chem. 2003;278(47):46974–46982. doi: 10.1074/jbc.M307764200. [DOI] [PubMed] [Google Scholar]
- 45.Duncan AR, Winter G. The binding site for C1q on IgG. Nature. 1988;332(6166):738–740. doi: 10.1038/332738a0. [DOI] [PubMed] [Google Scholar]
- 46.Malhotra R, Wormald MR, Rudd PM, Fischer PB, Dwek RA, Sim RB. Glycosylation changes of IgG associated with rheumatoid arthritis can activate complement via the mannose-binding protein. Nat Med. 1995;1(3):237–243. doi: 10.1038/nm0395-237. [DOI] [PubMed] [Google Scholar]
- 47.Nimmerjahn F, Anthony RM, Ravetch JV. Agalactosylated IgG antibodies depend on cellular Fc receptors for in vivo activity. Proc Natl Acad Sci USA. 2007;104(20):8433–8437. doi: 10.1073/pnas.0702936104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Shohet JM, Pemberton P, Carroll MC. Identification of a major binding site for complement C3 on the IgG1 heavy chain. J Biol Chem. 1993;268(8):5866–5871. [PubMed] [Google Scholar]
- 49.Tao MH, Smith RI, Morrison SL. Structural features of human immunoglobulin G that determine isotype-specific differences in complement activation. J Exp Med. 1993;178(2):661–667. doi: 10.1084/jem.178.2.661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Idusogie EE, Presta LG, Gazzano-Santoro H, Totpal K, Wong PY, Ultsch M, Meng YG, Mulkerrin MG. Mapping of the C1q binding site on rituxan, a chimeric antibody with a human IgG1 Fc. J Immunol. 2000;164(8):4178–4184. doi: 10.4049/jimmunol.164.8.4178. [DOI] [PubMed] [Google Scholar]
- 51.Brekke OH, Michaelsen TE, Aase A, Sandin RH, Sandlie I. Human IgG isotype-specific amino acid residues affecting complement-mediated cell lysis and phagocytosis. Eur J Immunol. 1994;24(10):2542–2547. doi: 10.1002/eji.1830241042. [DOI] [PubMed] [Google Scholar]
- 52.Schwarz F, Aebi M. Mechanisms and principles of N-linked protein glycosylation. Curr Opin Struct Biol. 2011;21(5):576–582. doi: 10.1016/j.sbi.2011.08.005. [DOI] [PubMed] [Google Scholar]
- 53.Moremen KW, Tiemeyer M, Nairn AV. Vertebrate protein glycosylation: diversity, synthesis and function. Nat Rev Mol Cell Biol. 2012;13(7):448–462. doi: 10.1038/nrm3383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Parekh R, Roitt I, Isenberg D, Dwek R, Rademacher T. Age-related galactosylation of the N-linked oligosaccharides of human serum IgG. J Exp Med. 1988;167(5):1731–1736. doi: 10.1084/jem.167.5.1731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Parekh RB, Dwek RA, Sutton BJ, Fernandes DL, Leung A, Stanworth D, Rademacher TW, Mizuochi T, Taniguchi T, Matsuta K, et al. Association of rheumatoid arthritis and primary osteoarthritis with changes in the glycosylation pattern of total serum IgG. Nature. 1985;316(6027):452–457. doi: 10.1038/316452a0. [DOI] [PubMed] [Google Scholar]
- 56.Mizuochi T, Taniguchi T, Shimizu A, Kobata A. Structural and numerical variations of the carbohydrate moiety of immunoglobulin G. J Immunol. 1982;129(5):2016–2020. [PubMed] [Google Scholar]
- 57.Shikata K, Yasuda T, Takeuchi F, Konishi T, Nakata M, Mizuochi T. Structural changes in the oligosaccharide moiety of human IgG with aging. Glycoconj J. 1998;15(7):683–689. doi: 10.1023/A:1006936431276. [DOI] [PubMed] [Google Scholar]
- 58.Zauner G, Selman MH, Bondt A, Rombouts Y, Blank D, Deelder AM, Wuhrer M. Glycoproteomic analysis of antibodies. Mol Cell Proteom. 2013;12(4):856–865. doi: 10.1074/mcp.R112.026005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wuhrer M, Stam JC, van de Geijn FE, Koeleman CA, Verrips CT, Dolhain RJ, Hokke CH, Deelder AM. Glycosylation profiling of immunoglobulin G (IgG) subclasses from human serum. Proteomics. 2007;7(22):4070–4081. doi: 10.1002/pmic.200700289. [DOI] [PubMed] [Google Scholar]
- 60.Wormald MR, Rudd PM, Harvey DJ, Chang SC, Scragg IG, Dwek RA. Variations in oligosaccharide-protein interactions in immunoglobulin G determine the site-specific glycosylation profiles and modulate the dynamic motion of the Fc oligosaccharides. Biochemistry. 1997;36(6):1370–1380. doi: 10.1021/bi9621472. [DOI] [PubMed] [Google Scholar]
- 61.Holland M, Yagi H, Takahashi N, Kato K, Savage CO, Goodall DM, Jefferis R. Differential glycosylation of polyclonal IgG, IgG-Fc and IgG-Fab isolated from the sera of patients with ANCA-associated systemic vasculitis. Biochim Biophys Acta. 2006;1760(4):669–677. doi: 10.1016/j.bbagen.2005.11.021. [DOI] [PubMed] [Google Scholar]
- 62.Jefferis R. Glycosylation of recombinant antibody therapeutics. Biotechnol Prog. 2005;21(1):11–16. doi: 10.1021/bp040016j. [DOI] [PubMed] [Google Scholar]
- 63.van de Bovenkamp FS, Hafkenscheid L, Rispens T, Rombouts Y. The emerging importance of IgG Fab glycosylation in immunity. J Immunol. 2016;196(4):1435–1441. doi: 10.4049/jimmunol.1502136. [DOI] [PubMed] [Google Scholar]
- 64.Nose M, Wigzell H. Biological significance of carbohydrate chains on monoclonal antibodies. Proc Natl Acad Sci USA. 1983;80(21):6632–6636. doi: 10.1073/pnas.80.21.6632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Herter S, Birk MC, Klein C, Gerdes C, Umana P, Bacac M. Glycoengineering of therapeutic antibodies enhances monocyte/macrophage-mediated phagocytosis and cytotoxicity. J Immunol. 2014;192(5):2252–2260. doi: 10.4049/jimmunol.1301249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Golay J, Da Roit F, Bologna L, Ferrara C, Leusen JH, Rambaldi A, Klein C, Introna M. Glycoengineered CD20 antibody obinutuzumab activates neutrophils and mediates phagocytosis through CD16B more efficiently than rituximab. Blood. 2013;122(20):3482–3491. doi: 10.1182/blood-2013-05-504043. [DOI] [PubMed] [Google Scholar]
- 67.Niwa R, Hatanaka S, Shoji-Hosaka E, Sakurada M, Kobayashi Y, Uehara A, Yokoi H, Nakamura K, Shitara K. Enhancement of the antibody-dependent cellular cytotoxicity of low-fucose IgG1 Is independent of FcgammaRIIIa functional polymorphism. Clin Cancer Res. 2004;10(18 Pt 1):6248–6255. doi: 10.1158/1078-0432.CCR-04-0850. [DOI] [PubMed] [Google Scholar]
- 68.Mori K, Iida S, Yamane-Ohnuki N, Kanda Y, Kuni-Kamochi R, Nakano R, Imai-Nishiya H, Okazaki A, Shinkawa T, Natsume A, Niwa R, Shitara K, Satoh M. Non-fucosylated therapeutic antibodies: the next generation of therapeutic antibodies. Cytotechnology. 2007;55(2–3):109–114. doi: 10.1007/s10616-007-9103-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Gasdaska JR, Sherwood S, Regan JT, Dickey LF. An afucosylated anti-CD20 monoclonal antibody with greater antibody-dependent cellular cytotoxicity and B-cell depletion and lower complement-dependent cytotoxicity than rituximab. Mol Immunol. 2012;50(3):134–141. doi: 10.1016/j.molimm.2012.01.001. [DOI] [PubMed] [Google Scholar]
- 70.Anthony RM, Nimmerjahn F, Ashline DJ, Reinhold VN, Paulson JC, Ravetch JV. Recapitulation of IVIG anti-inflammatory activity with a recombinant IgG Fc. Science. 2008;320(5874):373–376. doi: 10.1126/science.1154315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Rademacher TW, Williams P, Dwek RA. Agalactosyl glycoforms of IgG autoantibodies are pathogenic. Proc Natl Acad Sci USA. 1994;91(13):6123–6127. doi: 10.1073/pnas.91.13.6123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Bond A, Alavi A, Axford JS, Bourke BE, Bruckner FE, Kerr MA, Maxwell JD, Tweed KJ, Weldon MJ, Youinou P, Hay FC. A detailed lectin analysis of IgG glycosylation, demonstrating disease specific changes in terminal galactose and N-acetylglucosamine. J Autoimmun. 1997;10(1):77–85. doi: 10.1006/jaut.1996.0104. [DOI] [PubMed] [Google Scholar]
- 73.Vuckovic F, Kristic J, Gudelj I, Teruel M, Keser T, Pezer M, Pucic-Bakovic M, Stambuk J, Trbojevic-Akmacic I, Barrios C, Pavic T, Menni C, Wang Y, Zhou Y, Cui L, Song H, Zeng Q, Guo X, Pons-Estel BA, McKeigue P, Leslie Patrick A, Gornik O, Spector TD, Harjacek M, Alarcon-Riquelme M, Molokhia M, Wang W, Lauc G (2015) Association of systemic lupus erythematosus with decreased immunosuppressive potential of the IgG glycome. Arthritis Rheumatol (Hoboken, NJ) 67(11):2978–2989. doi:10.1002/art.39273 [DOI] [PMC free article] [PubMed]
- 74.Wuhrer M, Selman MHJ, McDonnell LA, Kümpfel T, Derfuss T, Khademi M, Olsson T, Hohlfeld R, Meinl E, Krumbholz M. Pro-inflammatory pattern of IgG1 Fc glycosylation in multiple sclerosis cerebrospinal fluid. J Neuroinflamm. 2015;12(1):1–14. doi: 10.1186/s12974-015-0450-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Rook GA, Steele J, Brealey R, Whyte A, Isenberg D, Sumar N, Nelson JL, Bodman KB, Young A, Roitt IM, et al. Changes in IgG glycoform levels are associated with remission of arthritis during pregnancy. J Autoimmun. 1991;4(5):779–794. doi: 10.1016/0896-8411(91)90173-A. [DOI] [PubMed] [Google Scholar]
- 76.Bondt A, Selman MH, Deelder AM, Hazes JM, Willemsen SP, Wuhrer M, Dolhain RJ. Association between galactosylation of immunoglobulin G and improvement of rheumatoid arthritis during pregnancy is independent of sialylation. J Proteome Res. 2013;12(10):4522–4531. doi: 10.1021/pr400589m. [DOI] [PubMed] [Google Scholar]
- 77.Karsten CM, Pandey MK, Figge J, Kilchenstein R, Taylor PR, Rosas M, McDonald JU, Orr SJ, Berger M, Petzold D, Blanchard V, Winkler A, Hess C, Reid DM, Majoul IV, Strait RT, Harris NL, Kohl G, Wex E, Ludwig R, Zillikens D, Nimmerjahn F, Finkelman FD, Brown GD, Ehlers M, Kohl J. Anti-inflammatory activity of IgG1 mediated by Fc galactosylation and association of FcgammaRIIB and dectin-1. Nat Med. 2012;18(9):1401–1406. doi: 10.1038/nm.2862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Ackerman ME, Crispin M, Yu X, Baruah K, Boesch AW, Harvey DJ, Dugast AS, Heizen EL, Ercan A, Choi I, Streeck H, Nigrovic PA, Bailey-Kellogg C, Scanlan C, Alter G. Natural variation in Fc glycosylation of HIV-specific antibodies impacts antiviral activity. J Clin Invest. 2013;123(5):2183–2192. doi: 10.1172/JCI65708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Ho CH, Chien RN, Cheng PN, Liu JH, Liu CK, Su CS, Wu IC, Li IC, Tsai HW, Wu SL, Liu WC, Chen SH, Chang TT. Aberrant serum immunoglobulin G glycosylation in chronic hepatitis B is associated with histological liver damage and reversible by antiviral therapy. J Infect Dis. 2015;211(1):115–124. doi: 10.1093/infdis/jiu388. [DOI] [PubMed] [Google Scholar]
- 80.Parekh RB, Roitt IM, Isenberg DA, Dwek RA, Ansell BM, Rademacher TW (1988) Galactosylation of IgG associated oligosaccharides: reduction in patients with adult and juvenile onset rheumatoid arthritis and relation to disease activity. Lancet (London, England) 1 (8592):966–969 [DOI] [PubMed]
- 81.Scherer HU, van der Woude D, Ioan-Facsinay A, el Bannoudi H, Trouw LA, Wang J, Haupl T, Burmester GR, Deelder AM, Huizinga TW, Wuhrer M, Toes RE. Glycan profiling of anti-citrullinated protein antibodies isolated from human serum and synovial fluid. Arthritis Rheum. 2010;62(6):1620–1629. doi: 10.1002/art.27414. [DOI] [PubMed] [Google Scholar]
- 82.Espy C, Morelle W, Kavian N, Grange P, Goulvestre C, Viallon V, Chereau C, Pagnoux C, Michalski JC, Guillevin L, Weill B, Batteux F, Guilpain P. Sialylation levels of anti-proteinase 3 antibodies are associated with the activity of granulomatosis with polyangiitis (Wegener’s) Arthritis Rheum. 2011;63(7):2105–2115. doi: 10.1002/art.30362. [DOI] [PubMed] [Google Scholar]
- 83.van de Geijn FE, Wuhrer M, Selman MH, Willemsen SP, de Man YA, Deelder AM, Hazes JM, Dolhain RJ. Immunoglobulin G galactosylation and sialylation are associated with pregnancy-induced improvement of rheumatoid arthritis and the postpartum flare: results from a large prospective cohort study. Arthritis Res Ther. 2009;11(6):R193. doi: 10.1186/ar2892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Washburn N, Schwab I, Ortiz D, Bhatnagar N, Lansing JC, Medeiros A, Tyler S, Mekala D, Cochran E, Sarvaiya H, Garofalo K, Meccariello R, Meador JW, 3rd, Rutitzky L, Schultes BC, Ling L, Avery W, Nimmerjahn F, Manning AM, Kaundinya GV, Bosques CJ. Controlled tetra-Fc sialylation of IVIg results in a drug candidate with consistent enhanced anti-inflammatory activity. Proc Natl Acad Sci USA. 2015;112(11):E1297–E1306. doi: 10.1073/pnas.1422481112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Anthony RM, Kobayashi T, Wermeling F, Ravetch JV. Intravenous gammaglobulin suppresses inflammation through a novel T(H)2 pathway. Nature. 2011;475(7354):110–113. doi: 10.1038/nature10134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Yu X, Vasiljevic S, Mitchell DA, Crispin M, Scanlan CN. Dissecting the molecular mechanism of IVIg therapy: the interaction between serum IgG and DC-SIGN is independent of antibody glycoform or Fc domain. J Mol Biol. 2013;425(8):1253–1258. doi: 10.1016/j.jmb.2013.02.006. [DOI] [PubMed] [Google Scholar]
- 87.Anthony RM, Wermeling F, Karlsson MC, Ravetch JV. Identification of a receptor required for the anti-inflammatory activity of IVIG. Proc Natl Acad Sci USA. 2008;105(50):19571–19578. doi: 10.1073/pnas.0810163105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Bayry J, Bansal K, Kazatchkine MD, Kaveri SV. DC-SIGN and α2,6-sialylated IgG Fc interaction is dispensable for the anti-inflammatory activity of IVIg on human dendritic cells. Proc Natl Acad Sci. 2009;106(9):E24. doi: 10.1073/pnas.0900016106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Quast I, Keller CW, Maurer MA, Giddens JP, Tackenberg B, Wang LX, Munz C, Nimmerjahn F, Dalakas MC, Lunemann JD. Sialylation of IgG Fc domain impairs complement-dependent cytotoxicity. J Clin Invest. 2015;125(11):4160–4170. doi: 10.1172/JCI82695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Sehn LH, Goy A, Offner FC, Martinelli G, Caballero MD, Gadeberg O, Baetz T, Zelenetz AD, Gaidano G, Fayad LE, Buckstein R, Friedberg JW, Crump M, Jaksic B, Zinzani PL, Padmanabhan Iyer S, Sahin D, Chai A, Fingerle-Rowson G, Press OW. Randomized phase II trial comparing obinutuzumab (GA101) with rituximab in patients with relapsed CD20+ indolent B-cell non-Hodgkin lymphoma: final analysis of the GAUSS study. J Clin Oncol. 2015;33(30):3467–3474. doi: 10.1200/JCO.2014.59.2139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Albert H, Collin M, Dudziak D, Ravetch JV, Nimmerjahn F. In vivo enzymatic modulation of IgG glycosylation inhibits autoimmune disease in an IgG subclass-dependent manner. Proc Natl Acad Sci USA. 2008;105(39):15005–15009. doi: 10.1073/pnas.0808248105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Benkhoucha M, Molnarfi N, Santiago-Raber ML, Weber MS, Merkler D, Collin M, Lalive PH. IgG glycan hydrolysis by EndoS inhibits experimental autoimmune encephalomyelitis. J Neuroinflamm. 2012;9:209. doi: 10.1186/1742-2094-9-209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Hirose M, Vafia K, Kalies K, Groth S, Westermann J, Zillikens D, Ludwig RJ, Collin M, Schmidt E. Enzymatic autoantibody glycan hydrolysis alleviates autoimmunity against type VII collagen. J Autoimmun. 2012;39(4):304–314. doi: 10.1016/j.jaut.2012.04.002. [DOI] [PubMed] [Google Scholar]
- 94.Nandakumar KS, Collin M, Olsen A, Nimmerjahn F, Blom AM, Ravetch JV, Holmdahl R. Endoglycosidase treatment abrogates IgG arthritogenicity: importance of IgG glycosylation in arthritis. Eur J Immunol. 2007;37(10):2973–2982. doi: 10.1002/eji.200737581. [DOI] [PubMed] [Google Scholar]
- 95.Collin M, Shannon O, Bjorck L. IgG glycan hydrolysis by a bacterial enzyme as a therapy against autoimmune conditions. Proc Natl Acad Sci USA. 2008;105(11):4265–4270. doi: 10.1073/pnas.0711271105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Lood C, Allhorn M, Lood R, Gullstrand B, Olin AI, Ronnblom L, Truedsson L, Collin M, Bengtsson AA. IgG glycan hydrolysis by endoglycosidase S diminishes the proinflammatory properties of immune complexes from patients with systemic lupus erythematosus: a possible new treatment? Arthritis Rheum. 2012;64(8):2698–2706. doi: 10.1002/art.34454. [DOI] [PubMed] [Google Scholar]
- 97.Schwab I, Mihai S, Seeling M, Kasperkiewicz M, Ludwig RJ, Nimmerjahn F. Broad requirement for terminal sialic acid residues and FcgammaRIIB for the preventive and therapeutic activity of intravenous immunoglobulins in vivo. Eur J Immunol. 2014;44(5):1444–1453. doi: 10.1002/eji.201344230. [DOI] [PubMed] [Google Scholar]
- 98.Schwab I, Lux A, Nimmerjahn F. Pathways responsible for human autoantibody and therapeutic intravenous IgG activity in humanized mice. Cell Rep. 2015;13(3):610–620. doi: 10.1016/j.celrep.2015.09.013. [DOI] [PubMed] [Google Scholar]
- 99.Hess C, Winkler A, Lorenz AK, Holecska V, Blanchard V, Eiglmeier S, Schoen AL, Bitterling J, Stoehr AD, Petzold D, Schommartz T, Mertes MM, Schoen CT, Tiburzy B, Herrmann A, Kohl J, Manz RA, Madaio MP, Berger M, Wardemann H, Ehlers M. T cell-independent B cell activation induces immunosuppressive sialylated IgG antibodies. J Clin Invest. 2013;123(9):3788–3796. doi: 10.1172/JCI65938. [DOI] [PMC free article] [PubMed] [Google Scholar]