Abstract
BACE2 (β-site APP-cleaving enzyme 2) is a protease expressed in the brain, but also in the pancreas, where it seems to play a physiological role. Amyloidogenic diseases, including Alzheimer’s disease and type 2 diabetes (T2D), share the accumulation of abnormally folded and insoluble proteins that interfere with cell function. In T2D, islet amyloid polypeptide (IAPP) deposits have been shown to be a pathogenic key feature of the disease. The aim of the present study was to investigate the effect of BACE2 modulation on β-cell alterations in a mouse model of T2D induced by IAPP overexpression. Heterozygous mice carrying the human transcript of IAPP (hIAPP-Tg) were used as a model to study the deleterious effects of IAPP upon β-cell function. These animals showed glucose intolerance and impaired insulin secretion. When crossed with BACE2-deficient mice, the animals presented a significant improvement in glucose tolerance accompanied with an enhanced insulin secretion, as compared to hIAPP-Tg mice. BACE2 deficiency also partially reverted gene expression changes observed in islets from hIAPP-Tg mice, including a set of genes related to inflammation. Moreover, homozygous hIAPP mice presented a severe hyperglycemia and a high lethality rate from 8 weeks onwards due to a massive destruction of β-cell mass. This process was significantly reduced when crossed with the BACE2-KO model, improving the survival rate of the animals. Altogether, the absence of BACE2 ameliorates glucose tolerance defects induced by IAPP overexpression in the β-cell and promotes β-cell survival. Thus, targeting BACE2 may represent a promising therapeutic strategy to improve β-cell function in T2D.
Keywords: BACE activity, Glucose tolerance, Islet inflammation, Proliferation, Type 2 diabetes, Survival
Introduction
Beta-site amyloid precursor protein cleaving enzymes (BACEs) are eukaryotic transmembrane aspartic proteases related to the pepsin family. Two genes have been identified for the two homologue enzymes: Bace1, which is located on chromosome 11, and its close homologue Bace2, belonging to the same family but located on chromosome 21 [1]. As they do not present homology in the promoter region and [2, 3], levels are very low in the brain and higher in the pancreas and other peripheral tissues [4–7]. BACE1 was identified as the enzyme responsible for initiating the amyloid cascade in Alzheimer’s disease (AD) by cleaving the amyloid precursor protein (APP) in the brain [8], an important feature in the development and progression of this disease. On the contrary, although BACE2 can cleave APP at the β-site in vitro, it seems to be more efficient at sites within the amyloid-β (Aβ) region, which argues against its implication in amyloid production [8, 9]. Moreover, it has been described that BACE2-deficient mice present higher β-cell mass and proliferation and improved glucose homeostasis [10].
Human islet amyloid polypeptide (hIAPP) is a 37-aminoacid peptide and a neuroendocrine hormone co-secreted with insulin in response to glucose and other secretagogues [11] that plays a role in maintaining glucose homeostasis. Moreover, it has anorexigenic effects as a neuroendocrine hormone [12] and also exerts an autocrine function in β-cell proliferation and signalling [13]. However, in addition to its physiological role, another well-described feature is its toxicity upon the β-cell due to misfolding and accumulation [14]. Indeed, hIAPP is the main component of pancreatic amyloid deposits [15] which are considered a hallmark of type 2 diabetes (T2D) [16, 17]. hIAPP can aggregate into oligomers and interfere with the proper functioning of the cell by blocking the secretory machinery [18]. Oligomer formation and amyloid deposits are common features of other amyloidogenic diseases such as AD that share a similar process of aggregation of amyloid fibrils with different proteins. In the case of T2D, there is a strong correlation between the process of amyloidogenesis and the progressive loss of pancreatic β-cell mass [19] and insulin secretion deficiency. Thus, understanding the molecular mechanisms by which the β-cell responds to amyloidosis will be essential to identify the pro-survival genes specifically expressed and regulated in β-cells that may represent a target for new therapeutic approaches.
Previously, our group demonstrated that the inhibition of secretase enzymatic activity in pancreatic cells affects the intracellular trafficking of the insulin receptor as well as insulin gene expression and content, suggesting that β-secretase plays a significant role in maintaining β-cell function [20]. More recently, using a rat pancreatic β-cell line INS1E stably transfected with human IAPP (hIAPP-INS1E cells) that elicits defects related to the insulin secretory machinery [21], we showed that BACE2 expression and function is increased in the cell model described and that its inhibition counteracts hIAPP-induced insulin secretory defects by increasing insulin secretion [7].
Taking these data together, the present study set out to investigate whether BACE2 modulation could revert the process of β-cell dysfunction induced by anomalous expression of IAPP within the pancreatic β-cell in vivo, and consequently restore glucose homeostasis.
Materials and methods
Transgenic mice
Heterozygous male transgenic mice with β-cell specific expression of hIAPP (hIAPP-Tg) on FVB background (FVB/N-Tg(Ins2-IAPP)RFHSoel/J) [22] were purchased from The Jackson Laboratory (BarHarbour, ME, USA) and backcrossed to a C57Bl6J background. Homozygous male with a global knock-down of BACE2 (BACE2-KO) on a mixed background (B6;129P2-Bace2tm1Bdes/J) were purchased from The Jackson Laboratory. Double transgenic hIAPP-TgxBACE2-KO animals were obtained by crossing hIAPP-Tg mice on a C57Bl6J background with BACE2-KO mice. Non-transgenic (wild type, WT) littermates were used as controls. Mice were maintained under a 12-h light–dark cycle and in compliance with current Spanish and European legislation. Studies were approved by the Universitat de Barcelona Ethics Committee (ID: 588/12).
Glucose tolerance tests
In vivo islet function was assessed by i.p. glucose tolerance test. Mice were fasted for 12 h and injected with D50 glucose (2 g/kg body weight) to perform glucose tolerance test. Tail blood glucose was measured at 0, 15, 30, 60, and 120 min using a clinical glucometer and Accutrend Check test strips (Roche Diagnostics, Switzerland). Blood samples were taken at 0 and 15 min to measure insulin release. Blood samples were obtained at 0, 15, 30, and 60 min from the tail vein, and glucose levels were measured as described above. Insulin levels were measured in plasma obtained by blood centrifugation and kept at −80 °C, using an insulin ELISA kit (Mercodia, Uppsala, Sweden), according to the manufacturer’s protocol.
Immunofluorescence and quantitative image analysis
Pancreata of WT, BACE2-KO, hIAPP-Tg, and hIAPP-TgxBACE2-KO mice (three animals per group) were fixed overnight in 10% formalin and paraffin-embedded. Six non-consecutive 3-µm-thick pancreatic sections 150 µm apart (six sections/animal) were permeabilized in paraformaldehyde, and unspecific binding was blocked by incubation in 3% donkey serum (Jackson ImmunoResearch, Cambridgeshire, UK). After overnight incubation at 4 °C, primary antibodies were revealed with selected secondary antibodies. Pancreatic tissues were counterstained with Hoechst (1 µg/mL; Sigma-Aldrich) and mounted with Vectashield (Vector Laboratories, Burlingame, CA, USA). Fluorescent slides were viewed using a Leica fluorescence microscope (Leica Microsystems, Wetzlar, Germany). Images were acquired using the Leica LAS Image Analysis software (Leica) and quantified using the ImageJ software (National Institutes of Health, USA; http://rsb.info.nih.gov/ij/). For morphometric analysis, at least 100 islets from all genotypes were manually traced and analysed using the Image J software. β-Cell mass was quantified blindly as β-cell volume density multiplied by pancreas weight.
Mouse islet isolation and culture
Pancreatic islets were isolated from the pancreata of 12-week-old males by collagenase digestion (Roche, Basel, Switzerland) and a density gradient using Histopaque (Sigma-Aldrich), as previously described [23]. Handpicked islets were cultured overnight in 11.1-mM d-glucose RPMI 1640 medium (Sigma-Aldrich) supplemented with 10% fetal bovine serum (vol/vol), 2-mM l-glutamine, 100 units/mL of penicillin, and 100 μg/mL of streptomycin, to allow them to recover from the isolation procedure.
Glucose-stimulated insulin and IAPP secretion assays
After 24 h recovery, ten islets per animal were preincubated in triplicate with Krebs–Ringer bicarbonate buffer (KRBB) containing 140-mM NaCl, 4.5-mM KCl, 2.5-mM CaCl2, 1-mM MgCl2, 20-mM HEPES, and pH 7.4. They were then supplemented with 0.1% BSA and 2.8-mM d-glucose for 30 min, followed by stimulation with 2.8 or 16.7-mM glucose for 1 h at 37 °C. Supernatant was recovered, and islets were lysed in 200 μL of acid–ethanol solution to measure insulin content. Insulin and IAPP levels in supernatant and lysates were determined by insulin ELISA kit (Mercodia) and IAPP ELISA kit (Merck Millipore, Madrid, Spain), respectively, according to the manufacturer’s protocol, and measured using a spectrophotometer (Synergy, Bio-Tek, Winooski, VT, USA).
RNA isolation and real-time PCR
RNA was isolated using Trizol® reagent (Invitrogen, Paisley, UK), according to the manufacturer’s instructions. Then, cDNA was synthesized from 1 µg RNA using SuperScript III reverse transcriptase (Invitrogen). Genes studied were amplified using SYBR Green PCR Core Reagents (Eurogentec, Liege, Belgium). PCR was run with 1 ng of cDNA using the ABI Prism 7900HT Sequence Detection System (Applied Biosystems, Foster City, CA, USA), according to the manufacturer’s instructions. Expression levels were normalised to tbp-1 (Applied Biosystems) and expressed in arbitrary units.
ACCCTTCACCAATGACTCCTATG and ATGATGACTGCAGCAAATCGC for Tbp-1; TGGCCCAAGCAAAGATTCC and TCAATCCCACCCAGGACAAG for Bace2; TGTGCTCTCTGTTGCATTGA and GGATCCCACGTTGGTAGATG for hIapp; TTAAAAACCTGGATCGGAACCAA and GCATTAGCTTCAGATTTACGGGT for Ccl2; CAGGAGAGAAAGGTTGCCCA and GTGGTCGATCCCACAACTCC for Tgfbi and AAGATGAGCGGGTGGCAGCG and GCACGTGGACCAGGTTCTGCT for Chop.
Global gene expression profiling
mRNA from mouse pancreatic islets was amplified through two cycles of cDNA synthesis. Labelled cRNA from biological triplicates was hybridised to Affymetrix GeneChip® Mouse HT_MG-430_PM arrays (Affymetrix, Santa Clara, CA, USA). Expression data were normalised with a robust multi-array average (RMA).
The LIMMA software package available from Bioconductor (http://www.bioconductor.org) was used for statistical analysis to identify differences in gene expression as previously described [23]. A fold change of ±1.5 with a p < 0.05 was considered significant. Data have been deposited in Gene Expression Omnibus (http://www.ncbi.nlm.nih.gov/geo), accession number GSE94672.
Functional category analysis
The DAVID Functional Annotation Tool (http://david.abcc.ncifcrf.gov, v6.8 Beta) was used to identify enriched functional categories in differentially expressed genes.
Antibodies
Antibodies directed toward the following proteins were used: insulin (Dako, Glostrup, Denmark), glucagon (Calbiochem, MERCK, Whitehouse Station, NJ, USA), CD68 (Bio-Rad Laboratories, Barcelona, Spain), and Ki67 (Thermo Fisher Scientific, Rockford, IL, USA).
Mouse survival curves
Kaplan–Meier curves were used to represent homozygous hIAPP-Tg survival. Glycaemia of homozygous hIAPP-Tg male mice was followed up weekly from week 6 to the endpoint.
Statistical analysis
Data are presented as means ± SEM for at least three independent experiments in triplicate, as indicated in each figure. Statistical significance between two groups was determined using Student’s two-tailed t test, and differences among more than two groups were analysed by ANOVA. A p value <0.05 was accepted as significant (*).
Results
hIAPP-Tg mice are glucose intolerant and present decreased insulin secretion
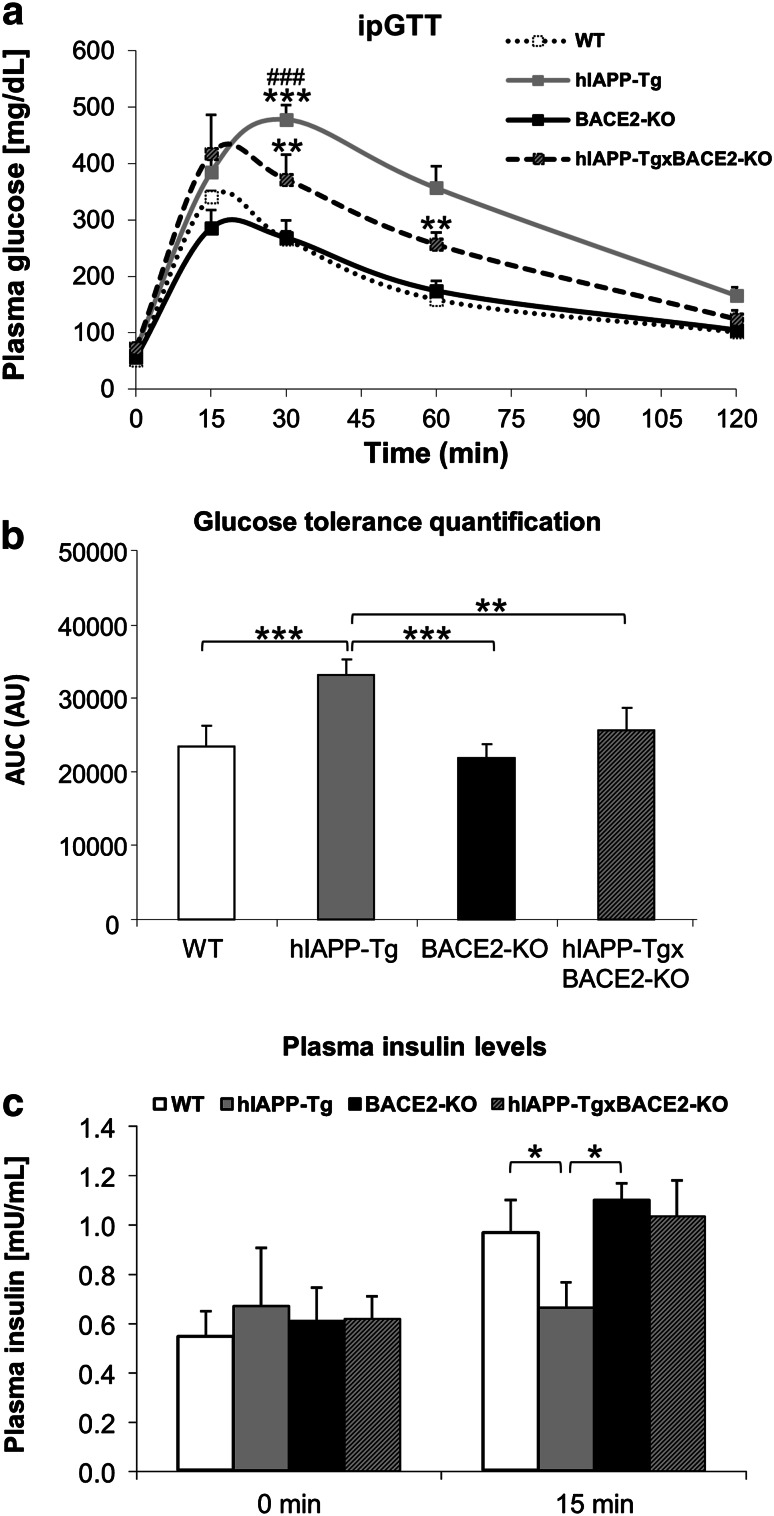
To characterize the metabolic phenotype of hIAPP-Tg mice, seven animals from each group (10-week-old hIAPP-Tg and wild-type control mice) were challenged with intraperitoneal glucose tolerance test to uncover the effects of hIAPP overexpression on whole body glucose homeostasis. Insulin secretion upon glucose stimulation was also examined at basal level and 15 min after glucose challenge. When compared, hIAPP-Tg animals showed a decrease in glucose tolerance with respect to control (WT) littermates (Fig. 1a). This effect was quantified with the calculation of the area under the curve (AUC) (Fig. 1b), which was statistically significant higher for the hIAPP-Tg vs. WT animals. This decrease in glucose tolerance was accompanied by a reduction in the plasma insulin levels of the hIAPP-Tg animals 15 min after glucose injection (Fig. 1c).
Fig. 1.
Metabolic phenotype of transgenic animals. a–c Intraperitoneal glucose tolerance test (ipGTT) was performed at 10 weeks of age. Plasma glucose levels (mg/dL) at indicated times (a) and area under the curve (b) are shown. c Absolute plasma insulin levels at 0 and 15 min after glucose injection. Results are presented as mean ± SEM. n = 7 animals per group. a **p < 0.01 and ***p < 0.001 relative to WT; ### p < 0.001 relative to hIAPP-TgxBACE2-KO
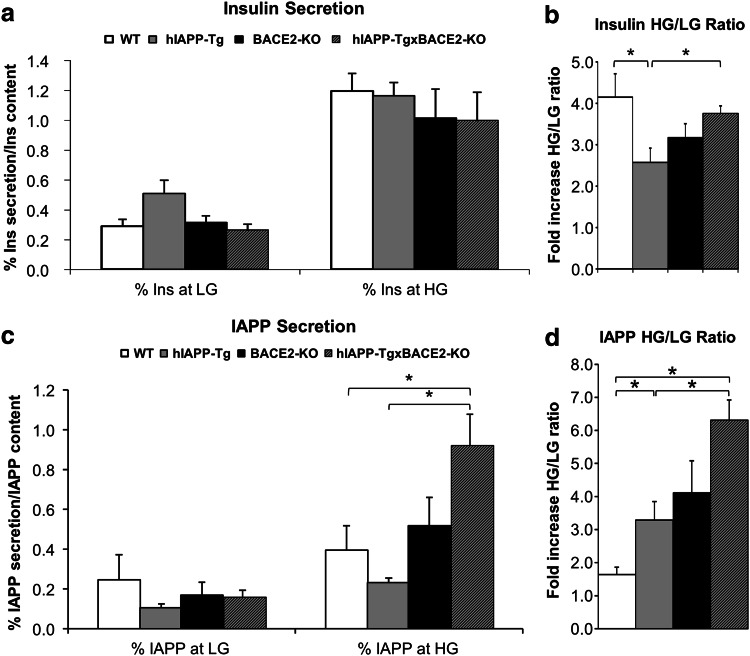
Two weeks after the glucose tolerance test, the animals were sacrificed and the pancreas extracted, as described in “Materials and methods”. Pancreatic islets from three different animals per group were isolated and exposed to glucose-stimulated insulin secretion (GSIS) (see Materials and methods for details). hIAPP-Tg islets showed a decrease in the insulin secretory response compared to WT islets (Fig. 2a, b), estimated as the ratio between insulin secretion at high glucose concentration and levels at low glucose concentration. Then, we examined the functionality of the islets by analysing IAPP secretion, as IAPP is co-secreted with insulin in response to glucose and other secretagogues. The same pancreatic islets from hIAPP-Tg and WT animals were used for IAPP secretion. hIAPP-Tg islets presented a significant increase in IAPP release (Fig. 2c, d) compared to WT animals.
Fig. 2.
Pancreatic islet insulin and IAPP secretion. Mouse islets were isolated from the pancreata of 12-week-old animals, cultured overnight and subjected to glucose-stimulated insulin and IAPP secretion. a % of insulin secretion respect to insulin content. b Fold increase of insulin release at high glucose (HG, 16.7 mM glucose) vs. low glucose (LG, 2.8 mM glucose). c % of IAPP secretion respect to IAPP content. b Fold increase of IAPP release at high glucose (HG, 16.7 mM glucose) vs. low glucose (LG, 2.8 mM glucose). Results are presented as mean ± SEM. n = 3 in triplicate batches of ten islets. *p < 0.05
hIAPP-Tg mice present increased β-cell mass and β-cell proliferation
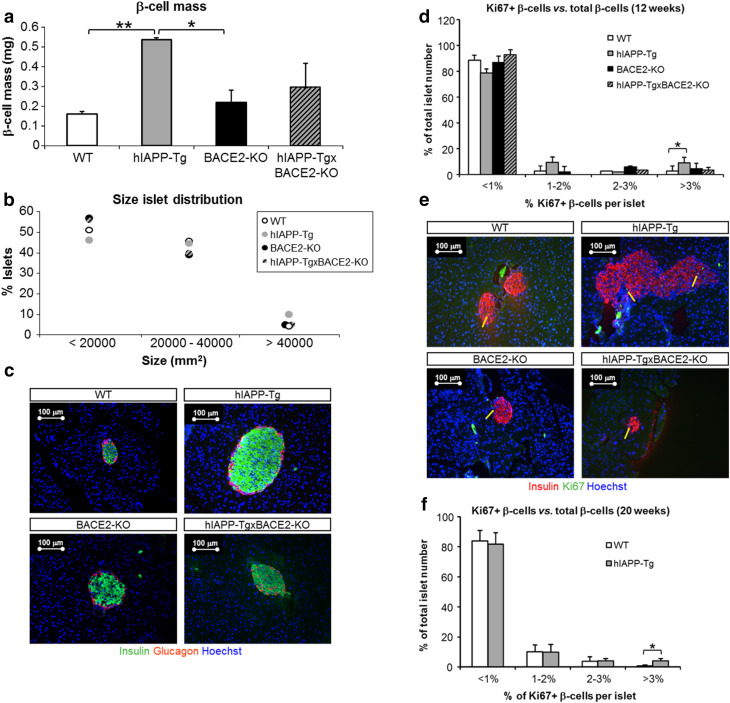
Morphometric analysis of slices of whole pancreas revealed a threefold increase in the β-cell mass of hIAPP-Tg animals compared to WT littermates (Fig. 3a). The increase in the insulin-positive area observed in hIAPP-Tg animals prompted us to question whether proliferation could have a role in this increase. For this, we performed an immunostaining for the cell proliferation marker Ki67, which revealed that hIAPP-Tg animals contained a higher percentage of islets presenting an increase in β-cell proliferation (Fig. 3d, e). Interestingly, we verified that, at 20 weeks of age, hIAPP-Tg mice still presented an increase in β-cell mass (not shown) and a higher percentage of islets showing high rates of β-cell proliferation (Fig. 3f).
Fig. 3.
Pancreas morphometry and β-cell proliferation of transgenic animals. β-Cell mass quantified in mg as β-cell volume density, multiplied by pancreas weight (a) and profile of islet size distribution (b). c Representative images of insulin (green) and glucagon (red) immunostaining from whole pancreas slices with differentiating β-cell area between the hIAPP-Tg and the rest of the experimental groups. Nuclei stained with Hoechst (blue). Bar scale 100 µm. Six sections per animal and three animals per group. *p < 0.05 and **p < 0.01. d β-Cell proliferation was analysed in transgenic and WT islets from 12-week-old mice. The percentage of proliferating β-cells was calculated as the percentage (%) of Ki67 positive β-cells with respect to total β-cells. Islet distribution according to the percentage of proliferating β-cells is shown. e Representative images of insulin (red) and Ki67 (green) immunostaining from representative islets with differentiated proliferation between the hIAPP-Tg and the other groups analysed in c. Nuclei stained with Hoechst (blue). Yellow arrows point to Ki67-positive nuclei. Bar scale 100 µm. Six sections per animal and three animals per group. f β-Cell proliferation was analysed in hIAPP-Tg and WT islets from 20-week-old mice. The percentage of proliferating β-cells was calculated as the percentage (%) of Ki67-positive β-cells with respect to total β-cells. Islet distribution according to the percentage of proliferating β-cells is shown. Data are presented as mean ± SEM. *p < 0.05, **p < 0.01
BACE2 deficiency reverts glucose intolerance in hIAPP-Tg mice
We next wondered whether the absence of BACE2 could ameliorate the pathological effects induced by the overexpression of hIAPP in the β-cell, as we previously described in hIAPP-overexpressing β-cell lines [7]. To achieve this objective, we crossed hIAPP-Tg animals with BACE2-deficient mice (BACE2-KO) to obtain double transgenic animals that overexpressed hIAPP and were deficient for the secretase BACE2 at the same time. The resulting animals (hIAPP-TgxBACE2-KO) and their respective controls were monitored for glucose tolerance at the age of 10 weeks (seven animals per group). The BACE2-KO animals showed no difference in glucose tolerance with respect to the WT animals (Fig. 1a, b). Interestingly, intraperitoneal glucose tolerance test showed that the hIAPP-TgxBACE2-KO mice were less glucose intolerant than their hIAPP-Tg counterparts, since blood glucose levels at 30 and 60 min after glucose injection were lower in the double transgenic animals compared to the hIAPP-Tg mice (Fig. 1a). Consistently, the AUC of the glucose tolerance test of the hIAPP-TgxBACE2-KO animals presented a significant decrease in relation to the hIAPP-Tg animals (Fig. 1b). When studying insulin secretion during the glucose tolerance test, we observed that the hIAPP-TgxBACE2-KO animals showed similar insulin response (quantified as the insulin secretion at basal levels and 15 min after the glucose injection) compared to WT mice (Fig. 1c).
The increase in insulin secretion was also evident in the ex vivo experiments. Pancreatic islets were isolated from three mice of 12 weeks of age per group and left to recover overnight. Glucose-stimulated insulin secretion assay revealed that hIAPP-TgxBACE2-KO islets showed a significant improvement on insulin secretion with respect to hIAPP-Tg islets when comparing the high glucose/low glucose ratio of the percentage of insulin secreted at high stimulating glucose concentration (16.7 mM) with respect to low glucose concentration (2.8 mM) (Fig. 2b). Following the same rationale as in the case of hIAPP-Tg animals, we next checked whether IAPP secretion was affected in hIAPP-TgxBACE2-KO mice. We found that hIAPP-TgxBACE2-KO (the double transgenic animals) presented a significant increase in IAPP secretion ratio (Fig. 2c, d) as compared to both WT and hIAPP-Tg animals.
To check if the improvement in glucose metabolism could be due to a change in β-cell mass, we performed morphometric analyses of the pancreases of the four groups of animals (WT, BACE2-KO, hIAPP-Tg, and hIAPP-TgxBACE2-KO). We observed that BACE2-KO animals showed no differences in β-cell mass in relation to WT animals. However, in the case of the double transgenic animals, we observed a high dispersion in the insulin-positive areas between animals, without a clear tendency to either increase or decrease, as compared to hIAPP-Tg mice (Fig. 3a, c). To understand this discrepancy, we split the islets according to their size. We found that hIAPP-Tg animals presented a higher number of large islets than the rest of the groups and a lower percentage of small islets. The distribution of islets per size showed that the profile of hIAPP-TgxBACE2-KO islets was more similar to that of the WT or BACE2-KO mice than to that of the hIAPP-Tg mice (Fig. 3b). Interestingly, contrary to the hIAPP-Tg model, hIAPP-TgxBACE2-KO mice presented a similar distribution of islets according to the percentage of β-cell proliferation as did WT animals (Fig. 3d, e).
Global gene expression analysis in pancreatic islets
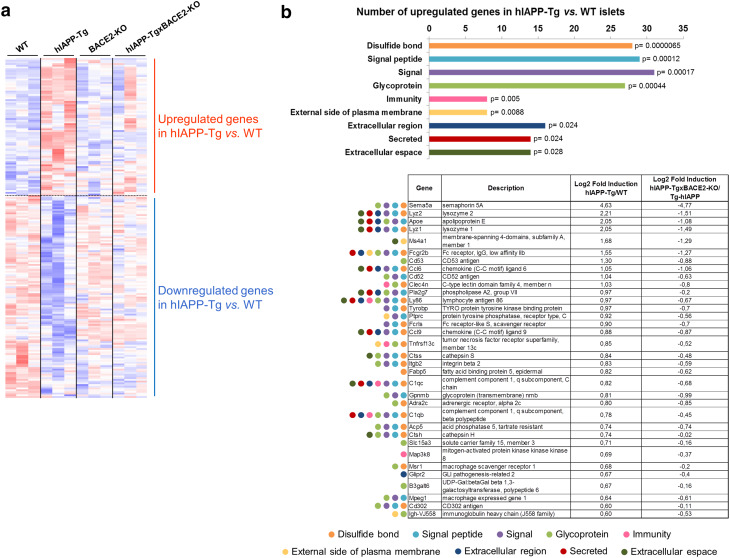
To uncover the global effects of hIAPP in pancreatic islets, we compared the transcriptional profiles of the pancreatic islets from 12-week-old hIAPP-Tg mice with those of WT mice, using Affymetrix GeneChip® Mouse HT_MG-430_PM arrays.
This global gene expression analysis showed 83 upregulated and 153 downregulated genes in hIAPP-Tg islets with respect to WT islets (Fig. 4a). We did not detect significantly enriched categories among the downregulated genes, revealing that these genes were representative of a pleiotropic response to hIAPP overexpression in islets. In contrast, upregulated genes were enriched in categories related to signalling, immunity, and secretion, among others. Many of the genes belonging to these categories were related to inflammation (such as Ccl6, Ccl9, and Ly86) and macrophage markers (Lyz1, Lyz2, and Mpeg1) (Fig. 4b). These results pointed to inflammation and macrophage activation as one of the main consequences of hIAPP overexpression in pancreatic islets. Interestingly, the global gene expression analysis revealed that almost 50% (39 out of 83) of the upregulated genes in the hIAPP-Tg mice were not altered in the hIAPP-TgxBACE2-KO, including a number of genes related to signalling and inflammation (Fig. 4b).
Fig. 4.
Global gene expression profile of pancreatic islets. a Heatmap of differentially expressed genes in pancreatic islets of the four experimental groups (highest expression in red and lowest expression in blue). b Functional category analysis of modulated pathways in hIAPP-Tg pancreatic islets in relation to WT islets. The table shows representative genes of each category with the fold induction of hIAPP-Tg vs. WT, and the reversion of hIAPP-TgxBACE2-KO vs. hIAPP-Tg. Results from global gene expression profiling data and DAVID Functional Annotation Tool. n = 3 animals per group
Next, we studied and validated the expression of selected genes related to inflammation and macrophage activation in the hIAPP-TgxBACE2-KO mouse model. Remarkably, while ER stress or extracellular matrix-related genes such as Chop and Tgfbi were not significantly altered (Fig. 5a, b), expression of Ccl2, which encodes a chemokine involved in inflammation, was found to be modulated in the different experimental groups (Fig. 5c). The significant increase observed in the hIAPP-Tg islets, indicative of an inflammated condition, was abolished in the hIAPP-TgxBACE2-KO islets, pointing to a potential reversion of the inflammation. Moreover, while the expression of cytokine Il-1b was not modulated in the hIAPP-Tg model, other inflammation markers such as Txnip and the macrophage-related genes Lyz1 and Mpeg1 followed the increase pattern in the hIAPP-Tg animals, along with the subsequent reversion in the hIAPP-TgxBACE2-KO model (Fig. 5e–g). Consistent with these findings in gene expression, the number of CD68+ cells, which corresponds to the macrophage lineage, was significantly reduced in hIAPP-TgxBACE2-KO islets (Fig. 5h).
Fig. 5.
Lower inflammation profile in hIAPP-TgxBACE2-KO islets. Fold induction of mRNA expression (related to WT animals) of selected genes in pancreatic islets from the four experimental groups. Chop (a) and Tgfbi (b) gene expression were not significantly modulated in any group. Gene expression of Ccl2 inflammation marker was significantly enhanced in hIAPP-Tg islets, but the increase was reversed to control values in hIAPP-TgxBACE2-KO animals (c). Cytokine Il-1β (d) was not significantly modulated in any model, while the inflammation-related gene Txnip (e) and the macrophage activation markers Lyz1 (f) and Mpeg1 (g) were increased in hIAPP-Tg islets and reverted in hIAPP-TgxBACE2-KO islets. Tbp-1 expression was used as a housekeeping gene. h. CD68 protein expression was quantified by immunostaining in whole pancreas slices. Results are represented as CD68-positive spots inside the pancreatic islet corrected by the total number of islets. Bars are the mean of triplicates from at least three independent experiments ±SEM. *p < 0.05
BACE2-deficiency prolongs survival in homozygous hIAPP-Tg mice
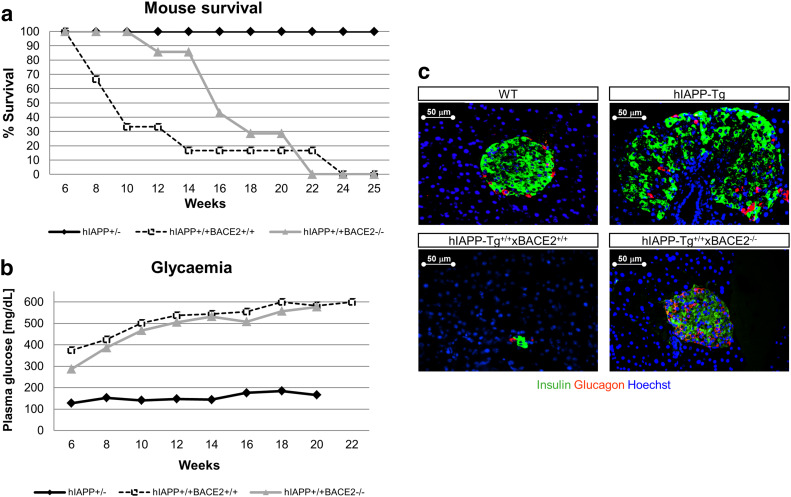
Homozygous hIAPP-Tg+/+ animals present a low survival rate after birth. These animals become highly hyperglycaemic and diabetic at early ages (3–4 weeks). We wondered whether BACE2 deficiency could prevent this deleterious effect, so we backcrossed hemizygous hIAPP-Tg+/−xBACE2-KO mice to obtain homozygous hIAPP-Tg+/+ animals that were also deficient (or not) in BACE2. A follow-up of blood glucose levels showed that, regardless of the presence of BACE2, hIAPP-Tg+/+ animals became hyperglycaemic starting at week 8 of age (Fig. 6b). However, interestingly, hIAPP-Tg+/+ animals that were deficient for BACE2 presented an increased life span (Fig. 6a). The probability of survival at 12 weeks of age increased significantly in the hIAPP-Tg+/+xBACE2−/− mice compared with the hIAPP-Tg+/+xBACE2+/+ mice (86 vs. 33%). This improvement in survival correlated with an increase in insulin-positive area in the homozygous hIAPP-Tg+/+xBACE2−/− mice when compared with the single homozygous hIAPP-Tg+/+ animals, which showed massive β-cell destruction (Fig. 6c).
Fig. 6.
Homozygous hIAPP-Tg animals. a Kaplan–Meier curves for mouse survival. Hemizygous hIAPP-Tg+/− mice were taken as controls. hIAPP-Tg+/+xBACE2−/− presented a higher survival rate than hIAPP-Tg+/+BACE2+/+. b Follow-up of fed plasma glucose levels in the three experimental groups. All curves were started with at least 20 animals per group. c Representative images of insulin (green) and glucagon (red) immunostaining from whole pancreas slices with differentiating β-cell area between the hIAPP-Tg+/+ and hIAPP-Tg+/+xBACE2−/−. Nuclei stained with Hoechst (blue). Bar scale 50 µm
Discussion
In this work, based on our previous results where we demonstrated that silencing BACE2 increases insulin secretion in an in vitro model of rat pancreatic β-cells, we wondered whether reducing BACE2 levels cells could ameliorate β-cell dysfunction in vivo. Thus, we aimed to investigate whether BACE2 plays a role in the alteration of glucose homeostasis in a model of T2D induced by the anomalous expression of human IAPP within the pancreatic β-cell. To achieve this objective, we crossed hIAPP-Tg animals with BACE2-deficient animals and studied whole body glucose metabolism.
We found that hIAPP overexpression was associated with glucose intolerance, a decrease in insulin secretion and an increase of β-cell mass in vivo, the latter possibly in an attempt to compensate for the observed decrease in insulin secretion. This increase in β-cell mass may be explained by an enhanced number of pancreatic islets that present a higher percentage of proliferating β-cells. This increment in β-cell proliferation correlated with an increase of IAPP secretion in hIAPP-Tg islets. Indeed, IAPP has been described to induce β-cell proliferation via an autocrine loop [13], which is in accordance with other studies showing that IAPP induces proliferation in neonatal β-cells [24] and that IAPP-deficient mice exhibit a greater reduction in β-cell mass when exposed to cytotoxic agents [25]. Of note, while insulin secretory response was reduced in hIAPP-Tg animals, IAPP secretory response was increased. Nevertheless, this increase in IAPP response is due to both mouse and human IAPP, which is indeed overexpressed in the hIAPP-Tg model. On the other hand, it has been recently found that IAPP could be a potential substrate of BACE2 [26]. In fact, we observed a trend of increased IAPP release in the BACE2-KO animals compared to WT mice. We could speculate that this increase could be related to IAPP being a BACE2 substrate. However, in these current experiments, we are not able to confirm the potential direct effect of BACE2 upon IAPP.
It has been previously described that a reduction in BACE2 enhances β-cell proliferation in vivo and increases GSIS ex vivo [10]. In our study, we move a step forward, demonstrating that BACE2-deficiency improves additionally glucose tolerance in a model of T2D induced by the overexpression of hIAPP. Furthermore, the double transgenic animals presented an increase in insulin secretion compared with the pathological model hIAPP-Tg mice, pointing to BACE2 inhibition as a promising strategy to overcome insulin secretion defects observed in T2D. Remarkably, the novelty here relies on the fact that BACE2 deficiency is able to counteract the detrimental effects that hIAPP exerts on pancreatic β-cells, highlighting the potential of BACE2 inhibition as a therapeutic strategy for T2D treatment.
In last years, several studies have demonstrated that inflammation is an important cause of dysfunction in pancreatic islets in T2D [27]. It is already known that IAPP accumulation can induce inflammation in the pancreatic islets [28–30]. Indeed, in our work, the global gene expression profile of the hIAPP-Tg islets showed an increase in inflammation and macrophage-related genes. Interestingly, this phenotype could be reverted when hIAPP-Tg animals were crossed with BACE2 deficient animals. In this case, the increase observed in Ccl2, Txnip, Lyz1, and Mpeg1 gene expression and CD68 protein in the hIAPP-Tg animals was normalised to control (WT) values in the hIAPP-TgxBACE2-KO mice, demonstrating amelioration in the inflammation profile of the pancreatic islets. These results go in the same line that previous data demonstrating that a blockade of the receptor of the proinflammatory cytokine IL-1β is able to ameliorate β-cell dysfunction by reducing the release of other proinflammatory cytokines [31]. Moreover, it has been suggested that the amyloid deposits in the islets may promote the recruitment of macrophages and the release of cytokines that are toxic for the cells [32]. Certainly, the development of therapeutic strategies to block or counteract the inflammation associated with T2D is an emerging field, where the scientists are paying close attention [33, 34], and this potential is already been used in human clinical trials [35].
Beta-cell death and function plays a critical role in the development of the two types of diabetes [36, 37], and the maintenance of β-cell mass is critical for the development of new therapeutic strategies [38]. However, none of the current treatments can prevent the loss of β-cell mass [39]. Homozygous hIAPP+/+-Tg mice present a massive destruction of β-cell mass and become highly diabetic at early ages, leading to a low rate of survival. However, in our work, we show that when crossed with BACE2-deficient mice, even presenting high plasma glucose levels, hIAPP+/+xBACE2−/− mice partially recovered β-cell mass and showed a significant increase in life span, compared to homozygous hIAPP+/+xBACE2+/+. Thus, here, we demonstrate that BACE2 deficiency not only improves glucose tolerance in a model of mild hyperglycemia such as the hemizygous overexpression of hIAPP, but also increases the life span in a more severe model of diabetes characterized by β-cell destruction. How BACE2 deficiency promotes β-cell survival in this model is still unknown. We can anticipate that this process is related to the several substrates of BACE2 and thus mediated through different mechanisms. First, BACE2 has been reported to cleave and inactivate the transmembrane TMEM27, the overexpression of which in β-cells leads to increased β-cell mass in vivo [40] and to an augment of glucose-stimulated insulin secretion [41]. Nevertheless, it is important to mention that BACE2 has many more substrates that may mediate the increased β-cell survival observed in BACE2-KO mice even in the presence of hIAPP, since 55 potential BACE2 targets have been identified [42, 43]. Among these targets, we find receptors of growth factors that have been shown to promote β-cell proliferation, such as the receptor for the insulin growth factor 2 [44] or the hepatocyte growth factor receptor [45]. An increased activity of the signalling pathways in pancreatic β-cells induced by the mentioned BACE2 targets and others still to be identified may underlay the beneficial effects of BACE2 inhibition on β-cell survival, leading to the high survival rates observed for the hIAPP+/+xBACE2−/− mice.
Altogether, our results show that BACE2 modulation can ameliorate glucose homeostasis defects induced by the overexpression of hIAPP. However, more studies are needed to decipher all of the factors playing a role in insulin secretory defects and β-cell mass maintenance. A better understanding of the pathogenic mechanisms involved in T2D is essential to find therapeutic targets that will be useful for preventing and treating the disease. In this regard, the increasing number of BACE2 inhibitors being developed and patented [46–48] also points (in the same direction as our results do) to BACE2 as a new emerging target in the treatment of T2D.
Acknowledgements
The authors thank Ms. Kimberly Katte for valuable assistance and comments in the preparation of the manuscript. This work was supported by Sardà Farriol Research Programme and by Grants FIS PI11/00679 and PI14/00447, from the Instituto de Salud Carlos III–Subdirección General de Evaluación y Fomento de la Investigación, Fondo Europeo de Desarrollo Regional (FEDER funds), Unión Europea, Una manera de hacer Europa. It also received support from Centro de Investigación Biomédica en Red de Diabetes y Enfermedades Metabólicas Asociadas (CIBERDEM) and from the Project 2014_SGR_520 of the Department of Universities, Research and Information Society of the Government of Catalonia. JM is a recipient of an IDIBAPS Postdoctoral Fellowship-BIOTRACK, supported by the European Community’s Seventh Framework Programme (ECFP7/2007–2013) under Grant Agreement Number 229673.
Abbreviations
- AD
Alzheimer disease
- BACE1
β-Site APP-cleaving enzyme 1
- BACE2
β-Site APP-cleaving enzyme 2
- GSIS
Glucose-stimulated insulin secretion
- GTT
Glucose tolerance test
- IAPP
Islet amyloid polypeptide
- PCR
Polymerase chain reaction
- mRNA
Messenger RNA
- T2D
Type 2 diabetes mellitus
Contributor Information
Joan-Marc Servitja, Email: servitja@clinic.cat.
Anna Novials, Email: anovials@clinic.cat.
References
- 1.Saunders A, Kim T-W, Tanzi R. BACE maps to chromosome 11 and a BACE homolog, BACE2, reside in the obligate Down Syndrome region of chromosome 21. Science. 1999;286:1255a. doi: 10.1126/science.286.5443.1255a. [DOI] [Google Scholar]
- 2.Sun X, Wang Y, Qing H, et al. Distinct transcriptional regulation and function of the human BACE2 and BACE1 genes. FASEB J. 2005;19:739–749. doi: 10.1096/fj.04-3426com. [DOI] [PubMed] [Google Scholar]
- 3.Lahiri D, Maloney B, Ge Y. Functional domains of the BACE1 and BACE2 promoters and mechanisms of transcriptional suppression of the BACE2 promoter in normal neuronal cells. J Mol Neurosci. 2006;29:65–80. doi: 10.1385/JMN:29:1:65. [DOI] [PubMed] [Google Scholar]
- 4.Sinha S, Anderson JP, Barbour R, et al. Purification and cloning of amyloid precursor protein beta-secretase from human brain. Nature. 1999;402:537–540. doi: 10.1038/990114. [DOI] [PubMed] [Google Scholar]
- 5.Yan R, Bienkowski MJ, Shuck ME, et al. Membrane-anchored aspartyl protease with Alzheimer’s disease beta-secretase activity. Nature. 1999;402:533–537. doi: 10.1038/990107. [DOI] [PubMed] [Google Scholar]
- 6.Bennett BD, Babu-Khan S, Loeloff R, et al. Expression analysis of BACE2 in brain and peripheral tissues. J Biol Chem. 2000;275:20647–20651. doi: 10.1074/jbc.M002688200. [DOI] [PubMed] [Google Scholar]
- 7.Alcarraz-Vizán G, Casini P, Cadavez L, et al. Inhibition of BACE2 counteracts hIAPP-induced insulin secretory defects in pancreatic b-cells. FASEB J. 2015;29:95–104. doi: 10.1096/fj.14-255489. [DOI] [PubMed] [Google Scholar]
- 8.Vassar R, Bennett BD, Babu-Khan S, et al. Beta-secretase cleavage of Alzheimer’s amyloid precursor protein by the transmembrane aspartic protease BACE. Science. 1999;286:735–741. doi: 10.1126/science.286.5440.735. [DOI] [PubMed] [Google Scholar]
- 9.Farzan M, Schnitzler CE, Vasilieva N, et al. BACE2, a beta -secretase homolog, cleaves at the beta site and within the amyloid-beta region of the amyloid-beta precursor protein. Proc Natl Acad Sci USA. 2000;97:9712–9717. doi: 10.1073/pnas.160115697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Esterhazy D, Stutzer I, Wang H, et al. Bace2 is a b cell-enriched protease that regulates pancreatic b cell function and mass. Cell Metab. 2011;14:365–377. doi: 10.1016/j.cmet.2011.06.018. [DOI] [PubMed] [Google Scholar]
- 11.Kahn SE, Alessio DAD, Schwartz MW, et al. Evidence of cosecretion of islet amyloid polypeptide and insulin by b-cells. Diabetes. 1990;39:634–638. doi: 10.2337/diab.39.5.634. [DOI] [PubMed] [Google Scholar]
- 12.Zhang X-X, Pan Y-H, Huang Y-M. Neuroendocrine hormone amylin in diabetes. World J Diabetes. 2016;7:189–197. doi: 10.4239/wjd.v7.i9.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Visa M, Alcarraz-Vizan G, Montane J, et al. Islet amyloid polypeptide exerts a novel autocrine action in b-cell signaling and proliferation. FASEB J. 2015;29:2970–2979. doi: 10.1096/fj.15-270553. [DOI] [PubMed] [Google Scholar]
- 14.Mukherjee A, Morales-Scheihing D, Butler PC, Soto C. Type 2 diabetes as a protein misfolding disease. Trends Mol Med. 2015;21:439–449. doi: 10.1016/j.molmed.2015.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cooper GJS, Willis AC, Sim RB, Reid KBM. Purification and characterization of a peptide from amyloid-rich pancreases of type 2 diabetic patients. Med Sci Purif. 1987;84:8628–8632. doi: 10.1073/pnas.84.23.8628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Clark A, Charge SB, Badman MK, et al. Islet amyloid polypeptide: actions and role in the pathogenesis of diabetes. Biochem Soc Trans. 1996;24:594–599. doi: 10.1042/bst0240594. [DOI] [PubMed] [Google Scholar]
- 17.Westermark P, Andersson A, Westermark GT. Islet amyloid polypeptide, islet amyloid, and diabetes mellitus. Physiol Rev. 2011;91:795–826. doi: 10.1152/physrev.00042.2009. [DOI] [PubMed] [Google Scholar]
- 18.Casas S, Gomis R, Gribble F, Altirriba J. Impairment of the ubiquitin-proteasome pathway is a downstream endoplasmic reticulum stress response induced by extracellular human islet amyloid polypeptide and contributes to pancreatic β-cell apoptosis. Diabetes. 2007;56:2284–2294. doi: 10.2337/db07-0178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Prentki M, Nolan CJ. Islet b cell failure in type 2 diabetes. J Clin Invest. 2006;116:1802–1812. doi: 10.1172/JCI29103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Casas S, Casini P, Piquer S, et al. BACE2 plays a role in the insulin receptor trafficking in pancreatic β-cells. Am J Physiol Endocrinol Metab. 2010;299:E1087–E1095. doi: 10.1152/ajpendo.00420.2010. [DOI] [PubMed] [Google Scholar]
- 21.Soty M, Visa M, Soriano S, et al. Involvement of ATP-sensitive potassium (K ATP) channels in the loss of beta-cell function induced by human islet amyloid polypeptide. J Biol Chem. 2011;286:40857–40866. doi: 10.1074/jbc.M111.232801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Janson J, Soeller W, Roche P, et al. Spontaneous diabetes mellitus in transgenic mice expressing human islet amyloid polypeptide. Proc Natl Acad Sci USA. 1996;93:7283–7288. doi: 10.1073/pnas.93.14.7283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Moreno-Asso A, Castaño C, Grilli A, et al. Glucose regulation of a cell cycle gene module is selectively lost in mouse pancreatic islets during ageing. Diabetologia. 2013;56:1761–1772. doi: 10.1007/s00125-013-2930-0. [DOI] [PubMed] [Google Scholar]
- 24.Karlsson E, Sandler S. Islet amyloid polypeptide promotes beta-cell proliferation in neonatal rat pancreatic islets. Cell. 2001;44:1015–1018. doi: 10.1007/s001250100601. [DOI] [PubMed] [Google Scholar]
- 25.Mulder H, Gebre-medhin S, Betsholtz C, et al. Islet amyloid polypeptide (amylin)-deficient mice develop a more severe form of alloxan-induced diabetes. Am J Physiol ENdocrinol Metab. 2000;278(4):E684–E691. doi: 10.1152/ajprenal.2000.278.4.F684. [DOI] [PubMed] [Google Scholar]
- 26.Rulifson IC, Cao P, Miao L, et al. Identification of human islet amyloid polypeptide as a BACE2 substrate. PLoS One. 2016 doi: 10.1371/journal.pone.0147254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Boni-Schnetzler M, Thorne J, Parnaud G, et al. Increased interleukin (IL)-1b messenger ribonucleic acid expression in b-cells of individuals with type 2 diabetes and regulation of IL-1b in human islets by glucose and autostimulation. J Clin Endocrinol Metab. 2008;93:4065–4074. doi: 10.1210/jc.2008-0396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Westwell-Roper CY, Chehroudi CA, Denroche HC, et al. IL-1 mediates amyloid-associated islet dysfunction and inflammation in human islet amyloid polypeptide transgenic mice. Diabetologia. 2014;58:575–585. doi: 10.1007/s00125-014-3447-x. [DOI] [PubMed] [Google Scholar]
- 29.Meier DT, Morcos M, Samarasekera T, et al. Islet amyloid formation is an important determinant for inducing islet inflammation in high-fat-fed human IAPP transgenic mice. Diabetologia. 2014;57:1884–1888. doi: 10.1007/s00125-014-3304-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Westwell-Roper C, Denroche HC, Ehses JA, Verchere CB. Differential activation of innate immune pathways by distinct islet amyloid polypeptide (IAPP) aggregates. J Biol Chem. 2016;291:jbc.M115.712455. doi: 10.1074/jbc.M115.712455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Westwell-Roper C, Dai DL, Soukhatcheva G, et al. IL-1 blockade attenuates islet amyloid polypeptide-induced proinflammatory cytokine release and pancreatic islet graft dysfunction. J Immunol. 2011;187:2755–2765. doi: 10.4049/jimmunol.1002854. [DOI] [PubMed] [Google Scholar]
- 32.Masters SL, Dunne A, Subramanian SL, et al. Activation of the Nlrp3 inflammasome by islet amyloid polypeptide provides a mechanism for enhanced IL-1b in type 2 diabetes. Nat Immunol. 2011;11:897–904. doi: 10.1038/ni.1935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ehses Ja, Perren A, Eppler E, et al. Increased number of islet associated macrophages in type 2 diabetes. Diabetes. 2007 doi: 10.2337/db06-1650. [DOI] [PubMed] [Google Scholar]
- 34.Westwell-Roper CY, Ehses JA, Verchere CB. Resident macrophages mediate islet amyloid polypeptide-induced islet IL-1 b production and b-cell dysfunction. Diabetes. 2014;63:1698–1711. doi: 10.2337/db13-0863. [DOI] [PubMed] [Google Scholar]
- 35.Larsen C, Faulenbach M, Vaag A, et al. Interleukin-1-receptor antagonist in type 2 diabetes mellitus. N Engl J Med. 2007;357:302–303. doi: 10.1056/NEJMc071324. [DOI] [PubMed] [Google Scholar]
- 36.Butler AE, Janson J, Bonner-Weir S, et al. Beta-cell deficit and increased beta-cell apoptosis in humans with type 2 diabetes. Diabetes. 2003;52:102–110. doi: 10.2337/diabetes.52.1.102. [DOI] [PubMed] [Google Scholar]
- 37.Mathis D, Vence L, Benoist C. B-cell death during progression to diabetes. Nature. 2001;414:792–798. doi: 10.1038/414792a. [DOI] [PubMed] [Google Scholar]
- 38.Tonne JM, Sakuma T, Munoz-Gomez M, et al. Beta cell regeneration after single-round immunological destruction in a mouse model. Diabetologia. 2014;58:313–323. doi: 10.1007/s00125-014-3416-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ardestani A, Maedler K. MST1: a promising therapeutic target to restore functional beta cell mass in diabetes. Diabetologia. 2016;59:1843–1849. doi: 10.1007/s00125-016-3892-9. [DOI] [PubMed] [Google Scholar]
- 40.Akpinar P, Kuwajima S, Krützfeldt J, Stoffel M. Tmem27: a cleaved and shed plasma membrane protein that stimulates pancreatic beta cell proliferation. Cell Metab. 2005;2:385–397. doi: 10.1016/j.cmet.2005.11.001. [DOI] [PubMed] [Google Scholar]
- 41.Fukui K, Yang Q, Cao Y, et al. The HNF-1 target Collectrin controls insulin exocytosis by SNARE complex formation. Cell Metab. 2005;2:373–384. doi: 10.1016/j.cmet.2005.11.003. [DOI] [PubMed] [Google Scholar]
- 42.Stützer I, Selevsek N, Esterházy D, et al. Systematic proteomic analysis identifies β-site amyloid precursor protein cleaving enzyme 2 and 1 (BACE2 and BACE1) substrates in pancreatic β-cells. J Biol Chem. 2013;288:10536–10547. doi: 10.1074/jbc.M112.444703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hemming ML, Elias JE, Gygi SP, Selkoe DJ. Identification of b-secretase (BACE1) substrates using quantitative proteomics. PLoS One. 2009 doi: 10.1371/journal.pone.0008477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hill D, Petrik J, Arany E. Growth factors and the regulation of fetal growth. Diabetes Care. 1998;21:B60–B69. doi: 10.2337/diacare.21.1.S60. [DOI] [PubMed] [Google Scholar]
- 45.Garcia-Ocaña A, Takane KK, Syed MA, et al. Hepatocyte growth factor overexpression in the islet of transgenic mice increases beta cell proliferation, enhances islet mass, and induces mild hypoglycemia. J Biol Chem. 2000;275:1226–1232. doi: 10.1074/jbc.275.2.1226. [DOI] [PubMed] [Google Scholar]
- 46.Gilbert EJ. 1,3-Oxazines as BACE1 and/or BACE2 inhibitors: a patent evaluation (WO2012156284) Expert Opin Ther Patents. 2013;23:1069–1073. doi: 10.1517/13543776.2013.818134. [DOI] [PubMed] [Google Scholar]
- 47.Southan C. BACE2 as a new diabetes target: a patent review (2010–2012) Expert Opin Ther Patents. 2013;23:649–663. doi: 10.1517/13543776.2013.780032. [DOI] [PubMed] [Google Scholar]
- 48.Southan C, Hancock JM. A tale of two drug targets: the evolutionary history of BACE1 and BACE2. Front Genet. 2013;4:1–20. doi: 10.3389/fgene.2013.00293. [DOI] [PMC free article] [PubMed] [Google Scholar]