Abstract
Human adipose-derived mesenchymal stem cells (hASCs) are an ideal cell source for regenerative medicine due to their capabilities of multipotency and the readily accessibility of adipose tissue. They have been found residing in a relatively low oxygen tension microenvironment in the body, but the physiological condition has been overlooked in most studies. In light of the escalating need for culturing hASCs under their physiological condition, this review summarizes the most recent advances in the hypoxia effect on hASCs. We first highlight the advantages of using hASCs in regenerative medicine and discuss the influence of hypoxia on the phenotype and functionality of hASCs in terms of viability, stemness, proliferation, differentiation, soluble factor secretion, and biosafety. We provide a glimpse of the possible cellular mechanism that involved under hypoxia and discuss the potential clinical applications. We then highlight the existing challenges and discuss the future perspective on the use of hypoxic-treated hASCs.
Keywords: Hypoxia, hASCs, Phenotype, Functionality, Clinical applications, Challenges
Introduction
Stem cells are undifferentiated or unspecialized cells found in all the multicellular organisms, which possess the unique ability of self-renewal and multilineage differentiation [1, 2]. Among various types of human stem cells, human mesenchymal stem cells (hMSCs) have emerged as a promising cell source for cell-based therapy [3–5]. They can be isolated from bone marrow, adipose tissue, and other parts of the body (e.g., umbilical cord blood and periosteum). Human adipose-derived mesenchymal stem cells (hASCs) hold more advantages than human bone-marrow-derived mesenchymal stem cells (hBMSCs) as adipose tissue is abundant, readily accessible through liposuction or lipectomy, and is easily harvested with minimal donor site morbidity, making it a useful source of hMSCs for clinical applications [6, 7]. Lipectomy is best suited for slim individuals, which offers surgical removal of subcutaneous fatty tissues from any part of the body. Therefore, subcutaneous adipose tissues can be obtained from the slim individuals through lipectomy [8, 9]. It is not advisable to isolate hASCs from the obese patients as obese-derived hASCs might not be normal. For instance, it has been reported that obese-derived hASCs are insulin resistant and display enhanced secretion of proinflammatory cytokines [10, 11], which might negatively affect the outcome of patients receiving therapy using hASCs. Additional steps are required to reverse the profile of obese-derived hASCs prior to being used for clinical applications [10].
Interestingly, hASCs reside in the microenvironment with low oxygen tension or hypoxia in the human body [8]. Human adipose tissue physiologically experiences an oxygen tension (i.e., 1–5% O2) which is lower than the atmospheric oxygen tension (i.e., 20–21% O2) [12]. Following the O2 inhalation, the O2 level progressively reduces once it enters the lungs and circulates throughout the body. It has been found that the level of O2 is 14, 12, and 5% in alveolar air, arterial blood, and venous blood, respectively, and drops to 1–5% when it arrives in organs and tissues. In fact, hASCs have been found residing in a hypoxic niche with the oxygen level between 1–5% [13, 14]. However, they are cultured at atmospheric oxygen tension in most experimental studies, which do not reflect their normal physiological condition [8]. Therefore, low oxygen tension has to be taken into account for the cultivation of hASCs in scientific research to obtain a more accurate and reliable experimental result.
Several studies have demonstrated the effect of hypoxia on functionality of hASCs, including proliferation, differentiation, survival rate, cytokines or growth factors secretions, and cell biosafety [8, 12, 15, 16]. Theoretically, culturing hASCs under their physiological condition (1–5% O2) may benefit their proliferation, differentiation, cytokine, or growth factor secretions while maintaining their stemness. However, there has been controversy in several research findings, which makes that their effect remains uncertain to date. For instance, hypoxia has been reported to reduce osteogenic and adipogenic differentiations of hASCs [12], contradicting the data of a few studies [17, 18]. Apart from oxygen level, it has been reported that the discrepancies may be due to other factors, such as different sexes and ages of human and the tissue origin of hASCs. Therefore, developing the most favorable microenvironment of hASCs for future clinical applications remains challenging to date.
There exist a number of reviews on hypoxia effect on stem cells. However, most of them focused more on bone-marrow-derived mesenchymal stem cells, without emphasizing the use of the most abundant and readily available hASCs [19–23]. To date, in view of the growing need for culturing readily accessible hASCs under their physiological oxygen microenvironment (1–5% O2), we review the most recent advances in the effect of hypoxia on hASCs. We first highlight the advantages of using hASCs in regenerative medicine and discuss the impact of hypoxia on the phenotype and functionality of hASCs in terms of viability, stemness, proliferation, differentiation, soluble factor secretion, and biosafety. We also provide a glimpse of the possible cellular mechanism that involved under hypoxic condition and discuss the potential clinical applications. We then highlight the existing challenges and discuss the future perspective on the use of hypoxic-treated hASCs.
Advantages of hASCs
Among various types of hMSCs, hASCs offer more advantages for future regenerative medicine [24, 25]. In addition, hASCs are normally isolated through simple isolation method and are cultured through easy cell culture procedures. The hASCs isolation procedure was pioneered by Rodbell in the 1960s [26]. The adipose tissue was initially minced and washed to remove hematopoietic cells, digested with collagenase, and centrifuged to collect the pelleted stromal vascular fraction (SVF) [27]. SVF consists of a heterogenous cell population, such as blood cells, fibroblasts, endothelial cells, and pericytes. The cell isolation is followed by commonly used stem cell culture technique to culture the hASCs and produce a high quantity of high-quality cells [8]. The simple enzyme-based isolation procedure and easy culture techniques with high yields of viable cells make hASCs a more attractive source for clinical applications among various MSCs. It has been reported that about 1 × 107 to 6 × 108 hASCs have been isolated from 300 mL of lipoaspirate, with >90% of viable cells as compared to <0.1% of hBMSCs isolated from bone marrow [28]. The yield of hASCs is approximately 5000 fibroblast colony-forming units (CFU-F)/g of adipose tissue, whereas the yield of hBMSCs is approximately 100–1000 CFU-F/mL of bone marrow [29].
In general, there are two types of adipose tissues: white adipose tissues that store triglyceride and brown adipose tissues that metabolize fat and generate heat [30]. White adipose tissues are much more abundant than brown adipose tissues. White adipose tissues are found throughout the human body, whereas brown adipose tissues are only found in the interscapular areas, mediastinum, and neck [31]. ASCs derived from both tissues possess some similar and different features [30]. Human white ASCs and brown ASCs express similar common MSC markers, such as CD44, CD73, CD90, and CD105 [32]. It is known that human white ASCs have the capability to differentiate into mesodermal lineage-like cells, including chondrocytes, osteocytes, and adipocytes. Human white ASCs could also differentiate into non-mesodermal lineage-like cells, including pancreatic cells, hepatocytes, cardiomyocytes, neurons and vascular endothelial cells, etc [33, 34] with the expression of related biomarkers. For example, it has been known that the Sox17 and IPF-1 are expressed in differentiated pancreatic cells but not in undifferentiated cells [35]. However, the specific functions of differentiated cells are still under investigation. On the other hand, human brown ASCs express brown adipose specific genes, such as PRDM 16, UCP-1, IRS2, and NRF1, indicating their greater potential to differentiate into brown adipocytes [32], which can potentially be used to counteract obesity.
Besides having multipotency, hASCs are of great interest due to its immunocompatibility [36]. It has been reported that hASCs suppress immunoreactions, and transplanted hASCs may not elicit a strong immune response (e.g., cytotoxic T-cell response) [36]. hASCs are able to secrete soluble factors which help to regulate immune response and initiate tissue repair [37, 38], hence having a profound impact on hASC application in regenerative medicine.
Effects of hypoxia on human adipose-derived mesenchymal stem cells
Due to the abundance of white adipose tissues and the great potential of differentiating into mesodermal and non-mesodermal lineage-like cells, human white ASCs with low oxygen tension have currently attracted a significant research interest [12, 39]. Numerous studies have investigated hASCs treated with various oxygen concentrations (0.1–5%) in various durations of hypoxic treatment (e.g., 1, 3, 7, 14, 21, and 28 days) [18, 40–44]. They have demonstrated the effects of hypoxia in terms of oxygen concentration on the cell phenotype, proliferation, differentiation, viability, soluble factors secretion, and biosafety. It was found that the duration of hypoxic treatment does not have any significant effect on the phenotype and functionality of hASCs.
Cell phenotype
Morphological examination and evaluation of surface marker expression are normally performed to assess the phenotype of hASCs. In general, hASCs have fibroblast-like or spindle shapes [45, 46]. It has been demonstrated that hypoxia does not cause morphological changes to hASCs. On the other hand, hASCs are positive for mesenchymal-associated markers, including CD73, CD105, and CD90, while they are negative for hematopoietic-associated markers, including CD14, CD45, CD19, CD34, and HLA DRDPDQ [47]. Oxygen tension at 1 and 2% was found to maintain the expression of those markers [12, 15, 41, 48]. In addition, hypoxia has a greater ability to preserve the stemness of hASCs, as indicated by the increased expression levels of stemness genes (e.g., NANOG, SOX-2, OCT-4, and REX-1) [12, 18]. Collectively, the existing evidences demonstrate that hypoxia (1–2% O2) has no significant effect on the phenotype of hASCs.
Cell proliferation
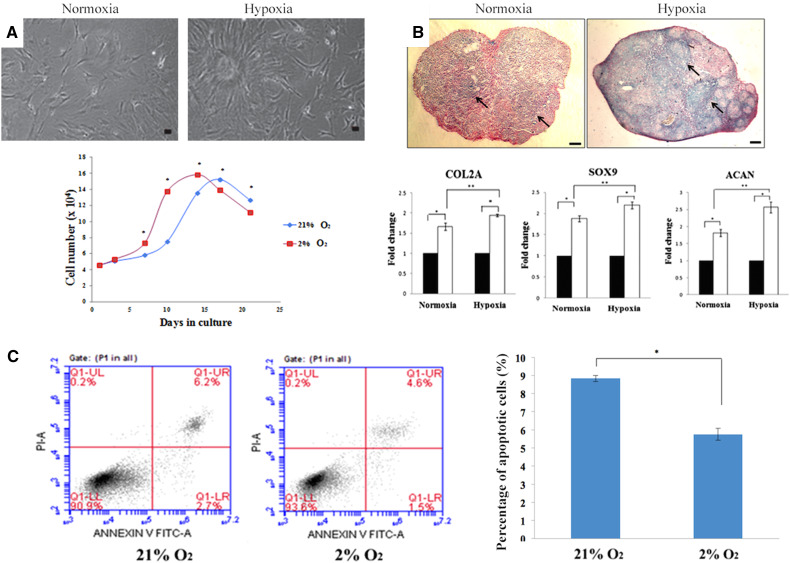
The proliferation rate of hASCs can be evaluated through their growth kinetic assays (e.g., growth curve analysis and doubling time), bromodeoxyuridine (BrdU) incorporation assay, total DNA content analysis, and Resazurin red assay. A study has demonstrated an increased rate of hASC proliferation 2% O2 through morphology observation and growth curve plotting [8] (Fig. 1a). Most studies have shown that 1–2% O2 enhances the proliferation rate of hASCs [8, 15, 18, 39, 41]. Only a few studies reported that proliferation rate of hASCs is maintained following exposure to 1 and 5% O2, respectively [48, 49].
Fig. 1.
Hypoxia enhances cell growth, chondrogenic differentiation, and cell viability. a It has been reported that hypoxia enhances cell proliferation by more number of cells observed under hypoxia [8]. b Hypoxia also enhances chondrogenic differentiation as shown by more glycosaminoglycans formed and a high expression level of chondrogenic markers [12]. This image was adapted from [12] with permission from Elsevier, copyright 2014. c Hypoxia enhances the viability of cells as demonstrated by a lower percentage of apoptotic cells as compared to that of normoxia [8]. a and c were adapted from [8] published under the Creative Commons license)
Cell differentiation
The differentiation of hASCs into chondrocytes, osteocytes, and osteocytes can be evaluated through histochemical staining, immunofluorescence staining, and gene expression analysis [12]. Several studies that investigated the impact of hypoxia on differentiation of hASCs yielded inconsistent and contrasting results (Table 1). Oxygen tension at 1 and 1.5% was found to maintain adipogenic, osteogenic, and chondrogenic differentiations of hASCs, whereas oxygen tension at 2 and 5% was found to significantly affect (either reduce or enhance) the differentiation.
Table 1.
Effect of hypoxia on differentiation of hASCs
| O2 level | Osteogenic differentiation | Adipogenic differentiation | Chondrogenic differentiation | References |
|---|---|---|---|---|
| 1% | Maintained | Maintained | Maintained | [18] |
| 1.5% | Maintained | Maintained | Maintained | [39] |
| 2% | Reduced | Reduced | Enhanced | [12] |
| 2% | – | Enhanced | – | [17] |
| 5% | Reduced | Reduced | Enhanced | [14, 42, 44, 49–51] |
| 5% | – | Maintained | – | [17] |
In general, the presence of proteoglycan and cartilage stained by Alcian Blue and Toluidine Blue, respectively, was observed after hypoxic-treated hASCs which were induced to differentiate into chondrogenic-like cells [12, 14, 18, 42, 50]. Through immunostaining, it was found that these cells which are cultured in 5% O2 highly express chondrogenic markers, such as collagen type II, chondroitin sulfate, and aggrecan [14, 42]. Furthermore, gene expression analysis revealed that these cells which are cultured under 2 and 5% O2 also highly express chondrogenic transcription factors (e.g., L-SOX-5, SOX-6, and SOX-9), collagen (e.g., types II, IX, X, and XI), and proteoglycan (e.g., aggrecan and versican) [12, 14, 50]. Studies have reported the presence of more glycosaminoglycans in hASCs culture under 2% O2 through alcian blue staining with a higher expression level of chondrogenic genes, including COL-2, SOX-9, and ACAN [12] (Fig. 1b). Additional assays demonstrated that the total sulfated glycosaminoglycan and cartilage contents in the chondrogenic induction medium of hypoxic-treated hASCs (5% O2) are higher than those in hASCs cultured under atmospheric condition (normoxic-treated hASCs) [44, 51]. The chondrogenic potential enhancement is proposed to be associated with the upregulation of hypoxia inducible factor 1-alpha (HIF-1α) or HIF-2α [12, 44, 52]. Given that cartilage tissue is avascular and adapted to a lower oxygen level in the body as compared to other tissues, it is likely that chondrogenic differentiation takes place in a hypoxic condition [8]. Overall, most existing evidences show that hypoxia (2–5% O2) enhances the chondrogenic potential of hASCs.
To evaluate the osteogenic potential of hypoxic-treated hASCs, Alizarin Red or Von Kossa staining is normally performed to detect the presence of calcium deposits upon osteogenic induction [12, 41, 42, 53]. Through gene expression analysis, it was found that osteogenic-like cells derived from hypoxic-treated hASCs (2 and 5% O2) display low expression levels of osteogenic markers (e.g., RUNX2, osteocalcin, and alkaline phosphatase) as compared to those derived from normoxic-treated hASCs [12, 42, 53]. In addition, alkaline phosphatase activity after osteogenic induction of hypoxic-treated hASCs (5% O2) was lower than that of normoxic-treated hASCs [42]. Taken together, most studies have reported a reduced osteogenic potential of hASCs under hypoxia (2–5% O2). In addition to chondrogenic and osteogenic differentiation, adipogenic differentiation of hypoxic-treated ASCs is normally confirmed by Oil Red O staining [12, 18, 41, 49]. This assay is performed to detect the presence of lipid droplets after adipogenic induction. It has been reported that hypoxic-treated cells (2 and 5% O2) expressed low levels of adipogenic genes (e.g., PPAR-γ, LPL, and FABP4) as compared to those of normoxic-treated hASCs [12, 49]. In addition, hypoxic-treated cells (5% O2) also present a low triglyceride content and a low glycerol-3-phosphate dehydrogenase (GADH) activity [49]. In short, most findings suggested that hypoxia (2–5% O2) reduces adipogenic potential of hASCs.
Interestingly, when hypoxic-treated ASCs were re-cultured in a normoxic condition, the inhibitory effect of hypoxia (2% O2) on osteogenesis and adipogenesis can be reversed [41]. It has been suggested that the reduced osteogenic and adipogenic differentiations of hASCs might be contributed by the upregulation of HIF-1α in response to hypoxia [12]. Given the fact that human bone tissues and adipose tissues are vascular, osteogenesis and adipogenesis of hASCs are believed to take place in a condition with a higher oxygen tension.
Cell viability
Viability assays, such as [3-(4,5-dimethy-2-thiazolyl)-2,5-diphenyl-2H-tetrazolium bromide] (MTT) assay and annexin V-propidium iodide (annexin V-PI) assay, have been used to assess the viability of hypoxic-treated hASCs [8]. Most findings showed that hypoxia (0.1–2% O2) enhances the viability of hASCs [15, 39–41]. It has been reported that hypoxic-treated hASCs (1.5 and 2% O2) display a lower percentage of apoptotic cells as compared to normoxic-treated hASCs through annexin V-PI assay [15, 39, 41] (Fig. 1c). Hypoxia was found to downregulate cleaved caspase-3 and upregulate prolyl hydroxylase 3, leading to the decreased activation of caspase-3 (a mediator of apoptosis) which in turn reduces cell apoptosis [54]. Furthermore, the protective effect of hypoxia on the viability of hASCs was also found to be contributed by VEGF-A. VEGF-A activates the phosphoinositide 3-kinase pathway which in turn phosphorylates protein kinase B (Akt) and subsequently increases the survival of hASCs under hypoxic condition [40].
Soluble factor secretion
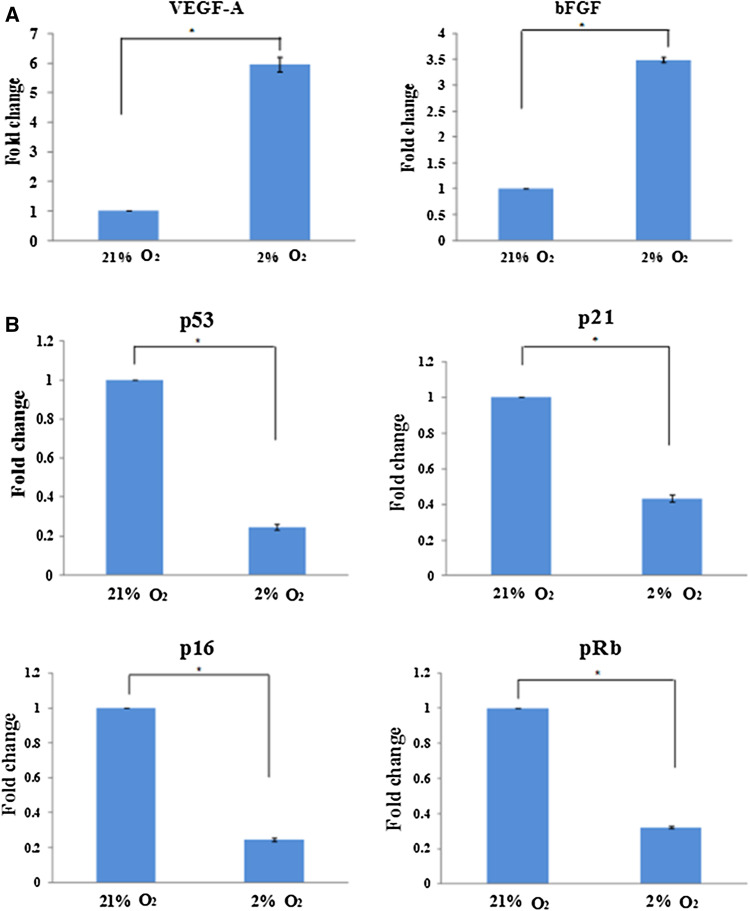
Molecular-based assays (e.g., quantitative real-time PCR (qPCR) and western blotting) and secretome-based assays (e.g., proteome profiler and ELISA) have been used to evaluate the ability of hASCs to secrete soluble factors [8]. Hypoxia (0.1–2% O2) was found to enhance the ability of hASCs to secrete soluble factors, including VEGF-A, bFGF, etc [40, 55–57]. It has been reported that the expression of VEGF and FGF increased under 2% O2 [8] (Fig. 2a). These factors may contribute to major functions of hASCs in potential clinical therapies, such as angiogenesis, immunosuppressive capacity, tissue repair and tissue regeneration, etc [48, 56]. It has been demonstrated that hypoxia (0.1–2% O2) upregulates HIF-1α in hASCs, which in turn increases the activation of its downstream targets, e.g., soluble factors, such as VEGF-A and FGF [8, 13, 15, 40], which may potentially contribute to an increased cell proliferation. The possible mechanism involved will be explicitly discussed in “Cellular mechanism under hypoxia”.
Fig. 2.
Hypoxia increases the expression of growth factors and reduces tumor suppressor genes. a It has been reported that low oxygen tension enhances the growth factors VEGF and FGF, which could potentially contribute to the growth and viability of hASCs. b hASCs have also been found to display low levels of p53, p16, p21, and pRb under hypoxia, suggesting its low risk of tumorigenesis [8]. a and b were adapted from [8] published under the Creative Commons license)
Biosafety (genetic stability and tumorigenicity)
As mentioned, most studies have reported that hypoxia enhances cell proliferation and viability of hASCs, which may be correlated with the increased secretion of growth factors [8, 15]. However, the increased proliferation and survival rate of hASCs may lead to the risk of tumor formation. Therefore, biosafety evaluation is important in hASCs under low oxygen tension.
Analysis of aneuploidy in chromosomes 8, 11, and 17 is essential for evaluating genetic instability in hMSCs, as these chromosomes contain vital oncogenes and tumor suppressors (e.g., p53 in chromosome 17) [58]. To evaluate the effect of hypoxia on the aneuploidy, hASCs were subjected to fluorescence in situ hybridization (FISH) using centromeric probes for chromosomes 8, 11, and 17. Incidence of aneuploidy was lower in hypoxic-treated hASCs than that in normoxic-treated hASCs, indicating that hypoxic-treated hASCs have a low risk of genetic instability or chromosomal aberration [58].
To evaluate the effect of hypoxia on the tumorigenicity, hASCs were subjected to tumorigenic potential assessment, including DNA damage, expression levels of tumor suppressors and human telomerase reverse transcriptase (hTERT), and in vivo tumorigenic assessment [8]. Degree of DNA damage in hypoxic-treated hASCs (3% O2) was assessed by immunofluorescence staining against p53 binding protein (53BP1) (marker indicating double stranded DNA break) and comet assay. Less 53BP1-positive cells were observed in hypoxia as compared to normoxia, indicating that hypoxia causes suboptimal double-stranded DNA breaks in hASCs [58]. On the other hand, comet assay revealed that tail length, tail DNA percentage, and tail moment, respectively, are lower in hypoxic-treated hASCs (2% O2) as compared to that in normoxic-treated hASCs. These findings demonstrated that hypoxic-treated hASCs have a low degree of DNA damage. In addition, hypoxic-treated hASCs (2% O2) displayed low levels of tumor suppressor genes (e.g., p53, p16, p21, and pRb) (Fig. 2b) and absence of hTERT, suggesting that hypoxia has a low risk of tumor formation [8]. It has also been reported that hypoxic-treated hASCs (1.5% O2) did not undergo in vivo malignant transformation [39]. Collectively, hypoxic-treated hASCs (1.5–3% O2) have a low tumorigenic potential, which may be safe to be used in clinical applications.
Cellular mechanism under hypoxia
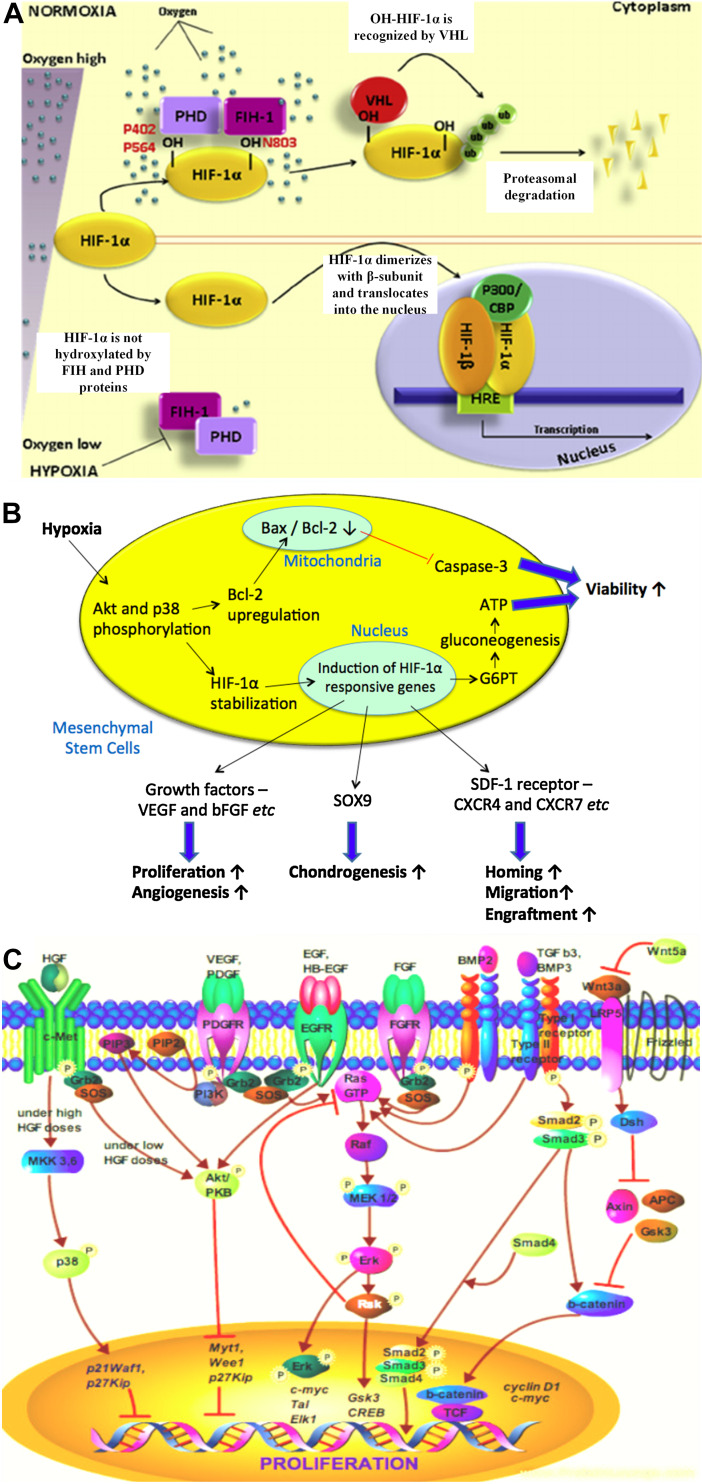
Oxygen-sensing mechanisms exist in the human body to maintain tissue homeostasis [59]. When oxygen drops to a certain level, hypoxia inducible factor-1 (HIF-1), a heterodimeric transcription factor, would play a vital role in regulating several cellular processes [20, 60] (Fig. 3a). In general, HIF-1 consists of an oxygen-dependent alpha-subunit and an oxygen-independent beta-subunit. The latter is known as an elementally expressed aryl hydrocarbon receptor nuclear translocator (ARNT). HIF-1α regulates the HIF-1 transcriptional activity. Under normoxia, HIF-1α is ubiquitinated by the von Hippel–Lindau (vHL) tumor-suppressor gene product and degraded by proteasome in the cytosol after the translation process, inhibiting HIF-1α-mediated gene transcription. However, under hypoxia, the binding between HIF-1α and vHL is inhibited, leading to the stabilization of HIF-1α and its translocation into the nucleus. HIF-1α would dimerize with HIF-1b, forming HIF-1 complex. The complex would then bind to a DNA sequence termed hypoxia-responsive elements (HREs) in the promoters of target genes, triggering the activation of hypoxia-regulated genes that involve in various cellular processes of MSCs, such as cell viability, secretion of soluble factors, cell proliferation, cell differentiation, cell migration, and so forth [61].
Fig. 3.
HIF-1a regulates cellular process in hypoxia. a Under normoxia, hypoxia inducible factor (HIF)-1α is ubiquitinated by the von Hippel–Lindau (vHL) tumor-suppressor gene product and degraded by proteasome in the cytosol, inhibiting HIF-1α-mediated gene transcription. Under hypoxia, the binding between HIF-1α and vHL is inhibited, leading to the stabilization of HIF-1α and its translocation into the nucleus, where it dimerizes with HIF-1b to form HIF-1 complex. The complex then binds to the hypoxia-responsive elements (HREs) and triggers various cellular processes in MSCs [60]. This image was adapted from [60] with permission from Elsevier, copyright 2015. b MSCs response to hypoxia, triggering various cellular activities, including enhanced viability, proliferation, and differentiation. c Growth factors, especially VEGF and FGF, play a significant role in regulating cell growth of MSCs under hypoxia [67]. This image was adapted from [67] published under the Creative Commons license)
Figure 3b shows the MSCs response to hypoxia. MSCs should have the ability to survive in a microenvironment that is deprived of oxygen if they are used for repair of ischemic tissues. Therefore, when availability of oxygen is limited, cells are able to switch metabolic pathway from aerobic to anaerobic to generate energy independently of oxygen using the abundant glucose which is generated through gluconeogenesis [62]. This anaerobic pathway is called glycolytic pathway, which is likely employed by MSCs to maintain their viability under hypoxic conditions [63]. When MSCs encounter hypoxic challenges, HIF-1α activates genes that code for glucose-6-phosphatase transporter, lactate dehydrogenase-A, glycolytic enzymes, and glucose transporters to facilitate the glycolytic pathway. In addition, HIF-1α upregulates antiapoptotic factors, e.g., Bcl-2 and bcl-xL, and downregulates the activation of caspase-3 (protein involved in cell apoptosis) to protect MSCs from apoptosis, which enhances their viability under hypoxic conditions [64]. These beneficial effects seem to be modulated by the glucose metabolism of MSCs through the signaling pathways, such as ERK, p38, and Akt pathways [19, 65]. Prolonged viability of MSCs under hypoxic conditions is imperative for angiogenesis to successfully repair ischemic tissues [66].
On the other hand, HIF-1α also activates genes that code for growth factors, e.g., VEGF, platelet-derived growth factor (PDGF), epidermal growth factor (EGF), and bFGF [65]. It has been reported that such growth factors mediate the proliferation of MSCs [8, 67]. Once the growth factors are activated, their binding to the respective receptors would lead to the receptor phosphorylation, which results in the recruitment of growth factor receptor-bound protein 2, Grb2. The Grb2 would bind to the phosphotyrosine residue of the activated receptors via Src Homology 2 (SH2) domain. Grb2 would also bind to the guanine nucleotide exchange factor, Son of Sevenless (SOS) via two Src Homology 3 (SH3) domains, resulting in activation of SOS. The activated SOS in turn stimulates activation of downstream pathways, including phosphoinositide-3 kinase (PI3K)-Akt/protein kinase B (PKB) pathway and mitogen activated protein kinase (MAPK)-extracellular-signal-regulated kinase 1 (ERK) pathway [67]. Briefly, SOS would induce the guanosine diphosphate (GDP) removal from Ras protein. Ras would bind to guanosine triphosphate (GTP) and become activated. The activated Ras would in turn induce the protein kinase activity of RAF kinase. RAF kinase would phosphorylate and activate Map/Erk kinase (MEK)-1 and MEK2 dual-specificity protein kinases. MEK 1 or 2 would in turn phosphorylate and activate MAPK-ERK1 and -ERK2. The activated ERKs would either translocate into the nucleus and activate the transcription of cellular proliferation genes, such as c-myc, transcription activator-like (Tal) and EPH-like kinase 1 (Elk1), or activate downstream receptors, such as Rsk, which would in turn activate the cellular proliferation genes, such as glycogen synthase kinase 3 (Gsk3) and cyclic adenosine monophosphate (cAMP) response element-binding protein (CREB), leading to cell proliferation [67]. Figure 3c shows the potential hASCs growth regulation through growth factor signaling pathway. In short, these growth factors play a significant role in regulating the growth of MSCs at low oxygen tension.
Differentiation of MSCs has been shown to be regulated in a HIF-dependent manner. Under hypoxic conditions, HIF-1α and HIF-2α (which also can dimerize with HIF-1b) were shown to bind to SOX9 promoter and upregulate SOX9, a transcription factor for chondrogenesis, leading to enhanced chondrogenic differentiation of MSCs through p38MAPK and Akt pathways [68–70]. On the contrary, FABP4 (the core regulator of adipogenesis) and RUNX2 (a transcription factor for osteogenesis) were found to be repressed by HIF-1α under low oxygen tension [71, 72]. When hypoxic-treated MSCs were re-cultured in a normoxic condition or HIF-1α was silenced by gene-specific small interfering RNA (siRNA), MSCs restored their adipogenic and osteogenic differentiation potential [72, 73]. Taken together, HIF-1α plays a vital role in enhancing chondrogenic differentiation of MSCs while decreasing adipogenic and osteogenic differentiation of MSCs under hypoxia.
Furthermore, HIF-1 also regulates the expression of SDF-1 (a chemokine) and its receptors (e.g., CXCR4, CXCR7, and CX3CR1), which are essential for migration, homing, and engraftment of MSCs to the injured or ischemic tissues [74, 75]. These receptors expressed on the surface of MSCs interact with SDF-1 secreted by injured tissues, leading to migration and engraftment of transplanted MSCs to the target site [19, 20]. It has been demonstrated that the expression of SDF-1 and its receptors are hypoxia-inducible, as ischemic tissues and hypoxic-treated MSCs were found to highly express SDF-1 and its receptors respectively [19, 74]. This suggests that hypoxic treatment may improve the migration and engraftment potential of MSCs, thus increase the success rate of transplanted MSCs in the cell-based therapies [20].
Potential clinical applications of hypoxic-treated hASCs
As mentioned, hypoxic-treated hASCs were found to increase secretion of angiogenin factors (e.g., VEGF-A and angiogenin), which would contribute to angiogenesis. It has been demonstrated that conditioned medium of hypoxic-treated hASCs enhances the proliferation and viability of endothelial cells [40, 43]. It also supports endothelial tube formation in vitro [40]. It has been found that the formation of endothelial (CD31+) vessels in the murine models of wound healing was enhanced upon treatment with conditioned medium of hypoxic-treated hASCs [55]. Furthermore, implantation of hypoxic-treated hASCs improved perfusion in the nude mice with ischemic hindlimbs [43], which was consistent with other findings using hypoxic-treated MSCs (Fig. 4a, b) [76]. In addition to paracrine effects of hypoxic-treated hASCs, they were also found to be differentiated into endothelial-like cells in the presence of VEGF and leptin [54]. These cells highly expressed markers of endothelial cells, including CD31, vascular endothelial cadherin, Flk-1 (VEGF receptor), Tie2 (angiopoietin receptor), Von Willebrand factor, and endothelial cell nitric oxide synthase [54]. These findings showed that hypoxic-treated hASCs may enhance angiogenesis in a paracrine fashion or by differentiating into endothelial cells. Collectively, hypoxic-treated hASCs could be a potential therapeutic option for enhancement of angiogenesis or wound healing.
Fig. 4.
Potential application of hypoxic-treated hASCs in angiogenesis and cartilage repair. Hypoxic-treated hASCs enhanced formation of endothelial vessels and blood perfusion, which were in accordance with other study using hypoxic-treated MSCs derived from bone marrow, as indicated by a increased number of CD31+ marker (brown) and b improved blood flow recovery in the nude mice with ischemic hindlimbs [76]. a and b were adapted from [76] with permission from Elsevier, copyright 2010). c Implantation of hypoxic-treated hASCs-laden (pre-cultured in chondrogenic induction medium) hydroxylpropyl methycellulose (Si-HPMC) hydrogel demonstrated formation of new cartilaginous tissues in the nude mice with articular cartilage defect, as indicated by the formation of proteoglycan (purple) and collagen type II (brown) [14]. This image was adapted from [14] published under the Creative Commons license
Due to the restricted proliferation rate of postnatal cardiomyocytes, cardiomyocytes lost upon myocardial infarction cannot be sufficiently replenished, leading to post-myocardial infarction complications, such as heart failure [77, 78]. To alleviate such complications, a novel therapy that could promote the proliferation of cardiomyocytes is essential. It has been suggested that stem cells might improve the cardioprotective outcome in the damaged cardiac microenvironment through the secretion of soluble factors [79, 80]. One of the soluble factors readily secreted by hASCs is interleukin-6 (IL-6) [28, 81]. Hypoxic-treated hASCs were found to increase secretion of IL-6 which enhances the proliferation rate of cardiomyocytes [57]. IL-6 has also been demonstrated to inhibit cardiomyocyte apoptosis and reduce infarct size [82]. These demonstrate the potential application of hypoxic-treated hASCs in the treatment of post-myocardial infarction complications.
Articular cartilage has a restricted potential of self-repair after injury [83]. To treat cartilage defect, a strategy, which can regenerate cartilage tissue is required. Cartilage repair strategies involving the integration of biomaterials and in vitro chondrogenic differentiated MSCs have been explored [84, 85]. Most existing evidences demonstrate that hypoxic-treated hASCs display enhanced in vitro chondrogenic differentiation [12, 14, 42, 44, 50, 51], suggesting their great advantage in cartilage repair using such strategies. In a preclinical trial, hypoxic-treated hASCs cultured in a silanized hydroxypropyl methylcellulose (Si-HPMC) hydrogel and chondrogenic induction medium was implanted into nude mice with articular cartilage defect to determine their in vivo efficacy in cartilage repair. The implantation has displayed newly formed cartilaginous tissues, which were positively stained by collagen type II and Alcian Blue (Fig. 4c) [14]. These findings indicate that hypoxic-treated hASCs could be potentially used to repair cartilage defect. In the future, hypoxic-treated hASCs should be prompted to clinical trial for applications, such as wound healing, cardiomyocyte regeneration and cartilage repair.
To evaluate the potential therapeutic applications of hMSCs, the clinical trials database has demonstrated 685 registered clinical trials of hMSCs (including 167 clinical trials of hASCs) in different clinical phases from year 2010 to 2014 (http://www.clinicaltrials.gov). Since 2010, there has been an increasing number of registered clinical trial of hMSCs. A significant number of the trials are in Phase I which involves the assessment of safety, Phase II which aims to compare the efficacy of hMSCs against a placebo, or a mixture of Phase I/II studies. A minority of the trials includes Phase III, a mixture of Phases II/III or Phase IV. Phase III compares the efficacy of hMSCs against other commonly used treatments, while Phase IV outlines supplementary information, such as the risks and optimal dosage of the treatment [86]. They are mainly aimed to treat immune system diseases (e.g., multiple sclerosis and GvHD), neurological diseases (e.g., amyotrophic lateral sclerosis and spinal cord injury), musculoskeletal diseases (e.g., rheumatic diseases and osteoarthritis), and cardiovascular diseases (e.g., stroke and myocardial ischemia). Most trials have demonstrated that no sign of significant adverse effect was observed in the patients receiving therapies using hMSCs [87]. Most outcome showed the efficacy of hMSCs in the clinical applications, including acute myocardial infarction, stroke, liver cirrhosis, amyotrophic sclerosis, and GvHD [88]. To date, hypoxic-treated hBMSCs have been used in clinical trials for ischemic heart disease and pulmonary emphysema. However, hypoxic-treated hASCs have not been subjected to any clinical trial yet. Therefore, future work should focus on developing more defined strategies of using hypoxic-treated hASCs in clinical trials. Different hypoxic preconditioning strategies could be used for different clinical indications. For instance, prior transplantation of hASCs, undifferentiated hASCs, can be expanded in a hypoxic condition (ideally 0.1–2% O2) to maintain their high viability, high proliferation rate, and enhanced ability of secreting soluble factors, which can promote angiogenesis in patient with ischemic injury. Furthermore, hypoxic-treated hASCs could migrate and engraft better to the targeted ischemic tissues, which increase the success rate of transplanted hASCs in such clinical application. On the other hand, hASCs can be induced into chondrogenic cells in vitro in a hypoxic condition (ideally 2–5% O2) as hypoxia enhances their chondrogenic differentiation potential. These cells can be transplanted into patient with cartilage defect for cartilage repair and regeneration. Concentration of O2 can be easily adjusted using an oxygen-controlled cell culture incubator, which is supplied with nitrogen gas to maintain low O2 concentration [8, 12, 15].
Conclusion and future perspective
hASCs are readily accessible, expandable, and greatly usable with the capabilities of multipotency and secretion of various soluble factors, presenting an ideal cell source for regenerative medicine. Hypoxia represents the physiological conditions found in the microenvironment of hASCs, but most studies are still performed under atmospheric conditions. To date, several studies have evaluated the effects of hypoxia (0.1–5% O2) on hASCs in terms of phenotype, viability, and functional properties, including proliferation, differentiation, and secretion of soluble factors, as well as the risk of tumorigenesis and genetic instability. Most studies have performed evaluation of effect on hASCs cultured in 2% O2, revealing that 2% O2 enhances their viability, proliferation, and ability of secreting soluble factors with a low risk of tumorigenesis and genetic instability. However, tri-lineage differentiation potential of hASCs was found to be varied under different concentrations of oxygen. Oxygen concentration at 1% and 1.5% was found to maintain adipogenic, osteogenic and chondrogenic differentiation of hASCs, whereas oxygen concentration at 2 and 5% was found to enhance their chondrogenic potential while reduce their adipogenic and osteogenic potential. These findings suggest that different hypoxic conditions may be suitable for different applications. For instance, hASCs cultured in 2% O2 are more suitable to be induced into chondrogenic cells in vitro prior to transplantation into human body for cartilage regeneration. On the other hand, oxygen concentrations range from 0.1 to 2% can be used to expand undifferentiated hASCs for clinical use as these concentrations enhance their viability, proliferation, and ability of secreting soluble factors. Apart from the oxygen level selected, the outcome discrepancies in some previous studies could be due to the different sexes and ages of human and the tissue origin of hASCs. The effects of hypoxia on hASCs isolated from: (1) adipose tissues taken from different body parts (subcutaneous fat versus visceral fat); (2) human with different sexes (male versus female); and (3) age (young versus adult) should also be investigated. Apart from that, while assessing the hypoxic effect on hASCs, the cells can be seeded onto appropriate scaffolds, which support their growth to produce a more desirable outcome. Future study also should focus on testing of new bioactive and biocompatible polymers to produce the appropriate scaffolds, which provide a better surface for cell adherence and create a desired three-dimensional tissue constructs under their physiological oxygen microenvironment (1–5% O2), which could potentially be applied to human clinical applications in the future. Therefore, extensive studies are still required to optimize the culture conditions of hypoxic-treated hASCs for future clinical applications.
Studies have demonstrated that the beneficial effects of hypoxia on MSCs, including ASCs, are mainly attributed to HIF-1. Hypoxia upregulates HIF-1, which in turn enhances the viability, proliferation, secretion of soluble factors, chondrogenic potential, migration, and engraftment potential of MSCs. To further optimize the therapeutic benefits of hypoxic-treated MSCs, further investigation is still required to elucidate the comprehensive mechanism of hypoxia on MSCs. Preclinical trials have shown that hypoxic-treated hASCs are effective in wound healing, cardiomyocyte regeneration, and cartilage repair, indicating their great potential therapeutic application in ischemic diseases and cartilage defect. However, hypoxic-treated hASCs have not been subjected to any clinical trial yet to date. Future work should focus on developing more defined strategies of using hypoxic-treated hASCs in clinical applications.
Acknowledgements
The work was supported by the UM High Impact Research Grant UM-MOHE (UM.C/HIR/MOHE/ENG/44) from the Ministry of Higher Education Malaysia and the University of Malaya Research Grant (UMRG: RP040B-15HTM).
Footnotes
J. R. Choi and K. W. Yong contributed equally to this work.
Contributor Information
Jane Ru Choi, Phone: +603-79672718, Email: janeruchoi@gmail.com.
Wan Kamarul Zaman Wan Safwani, Phone: +603-79677628, Email: wansafwani@um.edu.my.
References
- 1.Harandi OF, Ambros VR. Control of stem cell self-renewal and differentiation by the heterochronic genes and the cellular asymmetry machinery in Caenorhabditis elegans . Proc Natl Acad Sci. 2015;112(3):E287–E296. doi: 10.1073/pnas.1422852112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Weissman IL. Stem cells are units of natural selection for tissue formation, for germline development, and in cancer development. Proc Natl Acad Sci. 2015;112(29):8922–8928. doi: 10.1073/pnas.1505464112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Frenette PS, Pinho S, Lucas D, Scheiermann C. Mesenchymal stem cell: keystone of the hematopoietic stem cell niche and a stepping-stone for regenerative medicine. Annu Rev Immunol. 2013;31:285–316. doi: 10.1146/annurev-immunol-032712-095919. [DOI] [PubMed] [Google Scholar]
- 4.Ankrum JA, Ong JF, Karp JM. Mesenchymal stem cells: immune evasive, not immune privileged. Nat Biotechnol. 2014;32(3):252–260. doi: 10.1038/nbt.2816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yong KW, Wan Safwani WK, Xu F, Wan Abas WA, Choi JR, Pingguan-Murphy B. Cryopreservation of human mesenchymal stem cells for clinical applications: current methods and challenges. Biopreserv Biobank. 2015;13(4):231–239. doi: 10.1089/bio.2014.0104. [DOI] [PubMed] [Google Scholar]
- 6.Duscher D, Luan A, Rennert RC, Atashroo D, Maan ZN, Brett EA, Whittam AJ, Ho N, Lin M, Hu MS. Suction assisted liposuction does not impair the regenerative potential of adipose derived stem cells. J Transl Med. 2016;14(1):1. doi: 10.1186/s12967-016-0881-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Duscher D, Atashroo D, Maan ZN, Luan A, Brett EA, Barrera J, Khong SM, Zielins ER, Whittam AJ, Hu MS. Ultrasound-assisted liposuction does not compromise the regenerative potential of adipose-derived stem cells. Stem Cells Transl Med. 2016;5(2):248–257. doi: 10.5966/sctm.2015-0064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Choi JR, Pingguan-Murphy B, Abas WABW, Yong KW, Poon CT, Azmi MAN, Omar SZ, Chua KH, Xu F, Safwani WKZW. In situ normoxia enhances survival and proliferation rate of human adipose tissue-derived stromal cells without increasing the risk of tumourigenesis. PLoS One. 2015;10(1):e0115034. doi: 10.1371/journal.pone.0115034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yong KW, Safwani WKZW, Xu F, Zhang X, Choi JR, Abas WABW, Omar SZ, Azmi MAN, Chua KH, Pingguan-Murphy B (2016) Assessment of tumourigenic potential in long-term cryopreserved human adipose-derived stem cells. J Tissue Eng Regen Med. doi:10.1002/term.2120 [DOI] [PubMed]
- 10.Pérez LM, Bernal A, San Martín N, Lorenzo M, Fernández-Veledo S, Gálvez BG. Metabolic rescue of obese adipose-derived stem cells by Lin28/Let7 pathway. Diabetes. 2013;62(7):2368–2379. doi: 10.2337/db12-1220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Eljaafari A, Robert M, Chehimi M, Chanon S, Durand C, Vial G, Bendridi N, Madec A-M, Disse E, Laville M. Adipose tissue-derived stem cells from obese subjects contribute to inflammation and reduced insulin response in adipocytes through differential regulation of the Th1/Th17 balance and monocyte activation. Diabetes. 2015;64(7):2477–2488. doi: 10.2337/db15-0162. [DOI] [PubMed] [Google Scholar]
- 12.Choi JR, Pingguan-Murphy B, Wan Abas WA, Noor Azmi MA, Omar SZ, Chua KH, Wan Safwani WK. Impact of low oxygen tension on stemness, proliferation and differentiation potential of human adipose-derived stem cells. Biochem Biophys Res Commun. 2014;448(2):218–224. doi: 10.1016/j.bbrc.2014.04.096. [DOI] [PubMed] [Google Scholar]
- 13.Skiles ML, Sahai S, Rucker L, Blanchette JO. Use of culture geometry to control hypoxia-induced vascular endothelial growth factor secretion from adipose-derived stem cells: optimizing a cell-based approach to drive vascular growth. Tissue Eng Part A. 2013;19(21–22):2330–2338. doi: 10.1089/ten.tea.2012.0750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Portron S, Merceron C, Gauthier O, Lesoeur J, Sourice S, Masson M, Fellah BH, Geffroy O, Lallemand E, Weiss P, Guicheux J, Vinatier C. Effects of in vitro low oxygen tension preconditioning of adipose stromal cells on their in vivo chondrogenic potential: application in cartilage tissue repair. PLoS One. 2013;8:4. doi: 10.1371/journal.pone.0062368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Choi JR, Pingguan-Murphy B, Abas WABW, Azmi MAN, Omar SZ, Chua KH, Safwani WKZW. Hypoxia promotes growth and viability of human adipose-derived stem cells with increased growth factors secretion. J Asian Sci Res. 2014;4(7):328–338. [Google Scholar]
- 16.Safwani WKZW, Wong CW, Yong KW, Choi JR, Adenan NAM, Omar SZ, Abas WABW, Pingguan-Murphy B (2016) The effects of hypoxia and serum-free conditions on the stemness properties of human adipose-derived stem cells. Cytotechnology:1–14 [DOI] [PMC free article] [PubMed]
- 17.Kim JH, Kim SH, Song SY, Kim WS, Song SU, Yi T, Jeon MS, Chung HM, Xia Y, Sung JH. Hypoxia induces adipocyte differentiation of adipose-derived stem cells by triggering reactive oxygen species generation. Cell Biol Int. 2014;38(1):32–40. doi: 10.1002/cbin.10170. [DOI] [PubMed] [Google Scholar]
- 18.Fotia C, Massa A, Boriani F, Baldini N, Granchi D. Hypoxia enhances proliferation and stemness of human adipose-derived mesenchymal stem cells. CytoTechnology. 2015;67(6):1073–1084. doi: 10.1007/s10616-014-9731-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Das R, Jahr H, van Osch GJ, Farrell E. The role of hypoxia in bone marrow-derived mesenchymal stem cells: considerations for regenerative medicine approaches. Tissue Eng Part B Rev. 2009;16(2):159–168. doi: 10.1089/ten.teb.2009.0296. [DOI] [PubMed] [Google Scholar]
- 20.Haque N, Rahman MT, Abu Kasim NH, Alabsi AM (2013) Hypoxic culture conditions as a solution for mesenchymal stem cell based regenerative therapy. Sci World J. doi:10.1155/2013/632972 [DOI] [PMC free article] [PubMed]
- 21.Buravkova L, Andreeva E, Gogvadze V, Zhivotovsky B. Mesenchymal stem cells and hypoxia: where are we? Mitochondrion. 2014;19:105–112. doi: 10.1016/j.mito.2014.07.005. [DOI] [PubMed] [Google Scholar]
- 22.Ejtehadifar M, Shamsasenjan K, Movassaghpour A, Akbarzadehlaleh P, Dehdilani N, Abbasi P, Molaeipour Z, Saleh M. The effect of hypoxia on mesenchymal stem cell biology. Adv Pharm Bull. 2015;5(2):141. doi: 10.15171/apb.2015.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Buravkova L, Andreeva E, Grigoriev A. The impact of oxygen in physiological regulation of human multipotent mesenchymal cell functions. Hum Physiol. 2012;38(4):444–452. doi: 10.1134/S0362119712040032. [DOI] [PubMed] [Google Scholar]
- 24.Krähenbühl S, Grognuz A, Michetti M, Raffoul W, Applegate L. Enhancement of human adipose-derived stem cell expansion and stability for clinical use. Int J Stem Cell Res Ther. 2015;2:007. [Google Scholar]
- 25.Walmsley GG, Atashroo DA, Maan ZN, Hu MS, Zielins ER, Tsai JM, Duscher D, Paik K, Tevlin R, Marecic O. High-throughput screening of surface marker expression on undifferentiated and differentiated human adipose-derived stromal cells. Tissue Eng Part A. 2015;21(15–16):2281–2291. doi: 10.1089/ten.tea.2015.0039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rodbell M. Metabolism of isolated fat cells II. The similar effects of phospholipase C (Clostridium perfringens α toxin) and of insulin on glucose and amino acid metabolism. J Biol Chem. 1966;241(1):130–139. [PubMed] [Google Scholar]
- 27.Aronowitz JA, Lockhart RA, Hakakian CS, Hicok KC. Clinical safety of stromal vascular fraction separation at the point of care. Ann Plast Surg. 2015;75(6):666–671. doi: 10.1097/SAP.0000000000000594. [DOI] [PubMed] [Google Scholar]
- 28.Mizuno H, Tobita M, Uysal AC. Concise review: adipose-derived stem cells as a novel tool for future regenerative medicine. Stem Cells. 2012;30(5):804–810. doi: 10.1002/stem.1076. [DOI] [PubMed] [Google Scholar]
- 29.Strem BM, Hicok KC, Zhu M, Wulur I, Alfonso Z, Schreiber RE, Fraser JK, Hedrick MH. Multipotential differentiation of adipose tissue-derived stem cells. Keio J Med. 2005;54(3):132–141. doi: 10.2302/kjm.54.132. [DOI] [PubMed] [Google Scholar]
- 30.Hu HH, Yin L, Aggabao PC, Perkins TG, Chia JM, Gilsanz V. Comparison of brown and white adipose tissues in infants and children with chemical-shift-encoded water-fat MRI. J Magn Reson Imaging. 2013;38(4):885–896. doi: 10.1002/jmri.24053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tsuji W, Rubin JP, Marra KG. Adipose-derived stem cells: implications in tissue regeneration. World. J Stem Cells. 2014;6(3):312–321. doi: 10.4252/wjsc.v6.i3.312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Silva FJ, Holt DJ, Vargas V, Yockman J, Boudina S, Atkinson D, Grainger DW, Revelo MP, Sherman W, Bull DA. Metabolically active human brown adipose tissue derived stem cells. Stem Cells. 2014;32(2):572–581. doi: 10.1002/stem.1595. [DOI] [PubMed] [Google Scholar]
- 33.Silva AC, Percegona LS, Franca AL, Dos Santos TM, Perini CC, Gonzalez P, Rebelatto CL, Camara NO, Aita CA. Expression of pancreatic endocrine markers by mesenchymal stem cells from human adipose tissue. Transplant Proc. 2012;44(8):2495–2496. doi: 10.1016/j.transproceed.2012.07.036. [DOI] [PubMed] [Google Scholar]
- 34.Dzobo K, Turnley T, Wishart A, Rowe A, Kallmeyer K, van Vollenstee FA, Thomford NE, Dandara C, Chopera D, Pepper MS. Fibroblast-derived extracellular matrix induces chondrogenic differentiation in human adipose-derived mesenchymal stromal/stem cells in vitro. Int J Mol Sci. 2016;17(8):1259. doi: 10.3390/ijms17081259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lee J, Han D-J, Kim S-C. In vitro differentiation of human adipose tissue-derived stem cells into cells with pancreatic phenotype by regenerating pancreas extract. Biochem Biophys Res Commun. 2008;375(4):547–551. doi: 10.1016/j.bbrc.2008.08.064. [DOI] [PubMed] [Google Scholar]
- 36.Gimble JM, Katz AJ, Bunnell BA. Adipose-derived stem cells for regenerative medicine. Circ Res. 2007;100(9):1249–1260. doi: 10.1161/01.RES.0000265074.83288.09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Liu L, Gao J, Yuan Y, Chang Q, Liao Y, Lu F. Hypoxia preconditioned human adipose derived mesenchymal stem cells enhance angiogenic potential via secretion of increased VEGF and bFGF. Cell Biol Int. 2013;37(6):551–560. doi: 10.1002/cbin.10097. [DOI] [PubMed] [Google Scholar]
- 38.Yong KW, Li Y, Liu F, Gao B, Lu TJ, Abas WABW, Safwani WKZW, Pingguan-Murphy B, Ma Y, Xu F (2016) Paracrine effects of adipose-derived stem cells on matrix stiffness-induced cardiac myofibroblast differentiation via angiotensin II type 1 receptor and Smad7. Sci Rep. doi:10.1038/srep33067 [DOI] [PMC free article] [PubMed]
- 39.Feng Y, Zhu M, Dangelmajer S, Lee YM, Wijesekera O, Castellanos CX, Denduluri A, Chaichana KL, Li Q, Zhang H, Levchenko A, Guerrero-Cazares H, Quinones-Hinojosa A. Hypoxia-cultured human adipose-derived mesenchymal stem cells are non-oncogenic and have enhanced viability, motility, and tropism to brain cancer. Cell Death Dis. 2014;11(5):521. doi: 10.1038/cddis.2014.521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Stubbs SL, Hsiao ST, Peshavariya HM, Lim SY, Dusting GJ, Dilley RJ. Hypoxic preconditioning enhances survival of human adipose-derived stem cells and conditions endothelial cells in vitro. Stem Cells Dev. 2012;21(11):1887–1896. doi: 10.1089/scd.2011.0289. [DOI] [PubMed] [Google Scholar]
- 41.Valorani MG, Montelatici E, Germani A, Biddle A, D’Alessandro D, Strollo R, Patrizi MP, Lazzari L, Nye E, Otto WR, Pozzilli P, Alison MR. Pre-culturing human adipose tissue mesenchymal stem cells under hypoxia increases their adipogenic and osteogenic differentiation potentials. Cell Prolif. 2012;45(3):225–238. doi: 10.1111/j.1365-2184.2012.00817.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Merceron C, Vinatier C, Portron S, Masson M, Amiaud J, Guigand L, Cherel Y, Weiss P, Guicheux J. Differential effects of hypoxia on osteochondrogenic potential of human adipose-derived stem cells. Am J Physiol Cell Physiol. 2010;298(2):25. doi: 10.1152/ajpcell.00398.2009. [DOI] [PubMed] [Google Scholar]
- 43.Rehman J, Traktuev D, Li J, Merfeld-Clauss S, Temm-Grove CJ, Bovenkerk JE, Pell CL, Johnstone BH, Considine RV, March KL. Secretion of angiogenic and antiapoptotic factors by human adipose stromal cells. Circulation. 2004;109(10):1292–1298. doi: 10.1161/01.CIR.0000121425.42966.F1. [DOI] [PubMed] [Google Scholar]
- 44.Khan WS, Adesida AB, Hardingham TE (2007) Hypoxic conditions increase hypoxia-inducible transcription factor 2alpha and enhance chondrogenesis in stem cells from the infrapatellar fat pad of osteoarthritis patients. Arthritis Res Ther 9(3) [DOI] [PMC free article] [PubMed]
- 45.Wan Safwani WK, Makpol S, Sathapan S, Chua KH. The changes of stemness biomarkers expression in human adipose-derived stem cells during long-term manipulation. Biotechnol Appl Biochem. 2011;58(4):261–270. doi: 10.1002/bab.38. [DOI] [PubMed] [Google Scholar]
- 46.Yong KW, Pingguan-Murphy B, Xu F, Abas WA, Choi JR, Omar SZ, Azmi MA, Chua KH, Wan Safwani WK. Phenotypic and functional characterization of long-term cryopreserved human adipose-derived stem cells. Sci Rep. 2015;5:9596. doi: 10.1038/srep09596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Dominici M, Le Blanc K, Mueller I, Slaper-Cortenbach I, Marini F, Krause D, Deans R, Keating A, Prockop D, Horwitz E. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. CytoTherapy. 2006;8(4):315–317. doi: 10.1080/14653240600855905. [DOI] [PubMed] [Google Scholar]
- 48.Roemeling-van Rhijn M, Mensah FK, Korevaar SS, Leijs MJ, van Osch GJ, Ijzermans JN, Betjes MG, Baan CC, Weimar W, Hoogduijn MJ. Effects of hypoxia on the immunomodulatory properties of adipose tissue-derived mesenchymal stem cells. Front Immunol. 2013;4:203. doi: 10.3389/fimmu.2013.00203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Schiller ZA, Schiele NR, Sims JK, Lee K, Kuo CK. Adipogenesis of adipose-derived stem cells may be regulated via the cytoskeleton at physiological oxygen levels in vitro. Stem Cell Res Ther. 2013;4:4. doi: 10.1186/scrt230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Munir S, Foldager CB, Lind M, Zachar V, Soballe K, Koch TG. Hypoxia enhances chondrogenic differentiation of human adipose tissue-derived stromal cells in scaffold-free and scaffold systems. Cell Tissue Res. 2014;355(1):89–102. doi: 10.1007/s00441-013-1732-5. [DOI] [PubMed] [Google Scholar]
- 51.Wang DW, Fermor B, Gimble JM, Awad HA, Guilak F. Influence of oxygen on the proliferation and metabolism of adipose derived adult stem cells. J Cell Physiol. 2005;204(1):184–191. doi: 10.1002/jcp.20324. [DOI] [PubMed] [Google Scholar]
- 52.Wan Safwani WKZ, Choi JR, Yong KW, Ting I, Adenan NAM, Pingguan-Murphy B (2017) Hypoxia enhances the viability, growth and chondrogenic potential of cryopreserved human adipose-derived stem cells. Cryobiology. doi:10.1016/j.cryobiol.2017.01.006 [DOI] [PubMed]
- 53.Wan Safwani WK, Wong CW, Yong KW, Choi JR, Mat Adenan NA, Omar SZ, Wan Abas WA, Pingguan-Murphy B. The effects of hypoxia and serum-free conditions on the stemness properties of human adipose-derived stem cells. CytoTechnology. 2016;4:4. doi: 10.1007/s10616-015-9939-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bekhite MM, Finkensieper A, Rebhan J, Huse S, Schultze-Mosgau S, Figulla HR, Sauer H, Wartenberg M. Hypoxia, leptin, and vascular endothelial growth factor stimulate vascular endothelial cell differentiation of human adipose tissue-derived stem cells. Stem Cells Dev. 2014;23(4):333–351. doi: 10.1089/scd.2013.0268. [DOI] [PubMed] [Google Scholar]
- 55.Hsiao ST, Lokmic Z, Peshavariya H, Abberton KM, Dusting GJ, Lim SY, Dilley RJ. Hypoxic conditioning enhances the angiogenic paracrine activity of human adipose-derived stem cells. Stem Cells Dev. 2013;22(10):1614–1623. doi: 10.1089/scd.2012.0602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Linero I, Chaparro O. Paracrine effect of mesenchymal stem cells derived from human adipose tissue in bone regeneration. PLoS One. 2014;9:9. doi: 10.1371/journal.pone.0107001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Przybyt E, Krenning G, Brinker MG, Harmsen MC. Adipose stromal cells primed with hypoxia and inflammation enhance cardiomyocyte proliferation rate in vitro through STAT3 and Erk1/2. J Transl Med. 2013;11(39):1479–5876. doi: 10.1186/1479-5876-11-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Estrada JC, Albo C, Benguria A, Dopazo A, Lopez-Romero P, Carrera-Quintanar L, Roche E, Clemente EP, Enriquez JA, Bernad A, Samper E. Culture of human mesenchymal stem cells at low oxygen tension improves growth and genetic stability by activating glycolysis. Cell Death Differ. 2012;19(5):743–755. doi: 10.1038/cdd.2011.172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Semenza GL. Oxygen sensing, hypoxia-inducible factors, and disease pathophysiology. Annu Rev Pathol Mech Dis. 2014;9:47–71. doi: 10.1146/annurev-pathol-012513-104720. [DOI] [PubMed] [Google Scholar]
- 60.Borsi E, Terragna C, Brioli A, Tacchetti P, Martello M, Cavo M. Therapeutic targeting of hypoxia and hypoxia-inducible factor 1 alpha in multiple myeloma. Transl Res. 2015;165(6):641–650. doi: 10.1016/j.trsl.2014.12.001. [DOI] [PubMed] [Google Scholar]
- 61.Palomäki S, Pietilä M, Laitinen S, Pesälä J, Sormunen R, Lehenkari P, Koivunen P. HIF-1α is upregulated in human mesenchymal stem cells. Stem Cells. 2013;31(9):1902–1909. doi: 10.1002/stem.1435. [DOI] [PubMed] [Google Scholar]
- 62.Lord-Dufour S, Copland IB, Levros LC, Post M, Das A, Khosla C, Galipeau J, Rassart E, Annabi B. Evidence for transcriptional regulation of the glucose-6-phosphate transporter by HIF-1α: targeting G6PT with Mumbaistatin analogs in hypoxic mesenchymal stromal cells. Stem Cells. 2009;27(3):489–497. doi: 10.1634/stemcells.2008-0855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Grayson WL, Zhao F, Izadpanah R, Bunnell B, Ma T. Effects of hypoxia on human mesenchymal stem cell expansion and plasticity in 3D constructs. J Cell Physiol. 2006;207(2):331–339. doi: 10.1002/jcp.20571. [DOI] [PubMed] [Google Scholar]
- 64.Hu X, Yu SP, Fraser JL, Lu Z, Ogle ME, Wang J-A, Wei L. Transplantation of hypoxia-preconditioned mesenchymal stem cells improves infarcted heart function via enhanced survival of implanted cells and angiogenesis. J Thorac Cardiovasc Surg. 2008;135(4):799–808. doi: 10.1016/j.jtcvs.2007.07.071. [DOI] [PubMed] [Google Scholar]
- 65.Hung SC, Pochampally RR, Chen SC, Hsu SC, Prockop DJ. Angiogenic effects of human multipotent stromal cell conditioned medium activate the PI3K-Akt pathway in hypoxic endothelial cells to inhibit apoptosis, increase survival, and stimulate angiogenesis. Stem Cells. 2007;25(9):2363–2370. doi: 10.1634/stemcells.2006-0686. [DOI] [PubMed] [Google Scholar]
- 66.Shintani S, Murohara T, Ikeda H, Ueno T, Honma T, Katoh A, Sasaki K-i, Shimada T, Oike Y, Imaizumi T. Mobilization of endothelial progenitor cells in patients with acute myocardial infarction. Circulation. 2001;103(23):2776–2779. doi: 10.1161/hc2301.092122. [DOI] [PubMed] [Google Scholar]
- 67.Rodrigues M, Griffith LG, Wells A. Growth factor regulation of proliferation and survival of multipotential stromal cells. Stem Cell Res Ther. 2010;1(4):1. doi: 10.1186/scrt32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Robins JC, Akeno N, Mukherjee A, Dalal RR, Aronow BJ, Koopman P, Clemens TL. Hypoxia induces chondrocyte-specific gene expression in mesenchymal cells in association with transcriptional activation of Sox9. Bone. 2005;37(3):313–322. doi: 10.1016/j.bone.2005.04.040. [DOI] [PubMed] [Google Scholar]
- 69.Duval E, Baugé C, Andriamanalijaona R, Bénateau H, Leclercq S, Dutoit S, Poulain L, Galéra P, Boumédiene K. Molecular mechanism of hypoxia-induced chondrogenesis and its application in in vivo cartilage tissue engineering. Biomaterials. 2012;33(26):6042–6051. doi: 10.1016/j.biomaterials.2012.04.061. [DOI] [PubMed] [Google Scholar]
- 70.Shang J, Liu H, Li J, Zhou Y. Roles of hypoxia during the chondrogenic differentiation of mesenchymal stem cells. Curr Stem Cell Res Ther. 2014;9(2):141–147. doi: 10.2174/1574888X09666131230142459. [DOI] [PubMed] [Google Scholar]
- 71.Fehrer C, Brunauer R, Laschober G, Unterluggauer H, Reitinger S, Kloss F, Gully C, Gassner R, Lepperdinger G. Reduced oxygen tension attenuates differentiation capacity of human mesenchymal stem cells and prolongs their lifespan. Aging Cell. 2007;6(6):745–757. doi: 10.1111/j.1474-9726.2007.00336.x. [DOI] [PubMed] [Google Scholar]
- 72.Xu N, Liu H, Qu F, Fan J, Mao K, Yin Y, Liu J, Geng Z, Wang Y. Hypoxia inhibits the differentiation of mesenchymal stem cells into osteoblasts by activation of Notch signaling. Exp Mol Pathol. 2013;94(1):33–39. doi: 10.1016/j.yexmp.2012.08.003. [DOI] [PubMed] [Google Scholar]
- 73.Lin Q, Lee Y-J, Yun Z. Differentiation arrest by hypoxia. J Biol Chem. 2006;281(41):30678–30683. doi: 10.1074/jbc.C600120200. [DOI] [PubMed] [Google Scholar]
- 74.Liu H, Liu S, Li Y, Wang X, Xue W, Ge G, Luo X. The role of SDF-1-CXCR4/CXCR7 axis in the therapeutic effects of hypoxia-preconditioned mesenchymal stem cells for renal ischemia/reperfusion injury. PLoS One. 2012;7(4):e34608. doi: 10.1371/journal.pone.0034608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Shi M, Li J, Liao L, Chen B, Li B, Chen L, Jia H, Zhao RC. Regulation of CXCR4 expression in human mesenchymal stem cells by cytokine treatment: role in homing efficiency in NOD/SCID mice. Haematologica. 2007;92(7):897–904. doi: 10.3324/haematol.10669. [DOI] [PubMed] [Google Scholar]
- 76.Leroux L, Descamps B, Tojais NF, Séguy B, Oses P, Moreau C, Daret D, Ivanovic Z, Boiron J-M, Lamazière J-MD. Hypoxia preconditioned mesenchymal stem cells improve vascular and skeletal muscle fiber regeneration after ischemia through a Wnt4-dependent pathway. Mol Ther. 2010;18(8):1545–1552. doi: 10.1038/mt.2010.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Bergmann O, Bhardwaj RD, Bernard S, Zdunek S, Barnabe-Heider F, Walsh S, Zupicich J, Alkass K, Buchholz BA, Druid H, Jovinge S, Frisen J. Evidence for cardiomyocyte renewal in humans. Science. 2009;324(5923):98–102. doi: 10.1126/science.1164680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Kajstura J, Rota M, Cappetta D, Ogorek B, Arranto C, Bai Y, Ferreira-Martins J, Signore S, Sanada F, Matsuda A, Kostyla J, Caballero MV, Fiorini C, D’Alessandro DA, Michler RE, del Monte F, Hosoda T, Perrella MA, Leri A, Buchholz BA, Loscalzo J, Anversa P. Cardiomyogenesis in the aging and failing human heart. Circulation. 2012;126(15):1869–1881. doi: 10.1161/CIRCULATIONAHA.112.118380. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 79.Hwang H, Kloner RA. Improving regenerating potential of the heart after myocardial infarction: factor-based approach. Life Sci. 2010;86(13–14):461–472. doi: 10.1016/j.lfs.2010.01.004. [DOI] [PubMed] [Google Scholar]
- 80.Willems E, Lanier M, Forte E, Lo F, Cashman J, Mercola M. A chemical biology approach to myocardial regeneration. J Cardiovasc Transl Res. 2011;4(3):340–350. doi: 10.1007/s12265-011-9270-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.J Salgado A, L Reis R, Sousa N, M Gimble J. Adipose tissue derived stem cells secretome: soluble factors and their roles in regenerative medicine. Curr Stem Cell Res Ther. 2010;5(2):103–110. doi: 10.2174/157488810791268564. [DOI] [PubMed] [Google Scholar]
- 82.Matsushita K, Iwanaga S, Oda T, Kimura K, Shimada M, Sano M, Umezawa A, Hata J, Ogawa S. Interleukin-6/soluble interleukin-6 receptor complex reduces infarct size via inhibiting myocardial apoptosis. Lab Invest. 2005;85(10):1210–1223. doi: 10.1038/labinvest.3700322. [DOI] [PubMed] [Google Scholar]
- 83.Buckwalter JA, Mankin HJ. Articular cartilage repair and transplantation. Arthritis Rheum. 1998;41(8):1331–1342. doi: 10.1002/1529-0131(199808)41:8<1331::AID-ART2>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- 84.Marquass B, Schulz R, Hepp P, Zscharnack M, Aigner T, Schmidt S, Stein F, Richter R, Osterhoff G, Aust G, Josten C, Bader A. Matrix-associated implantation of predifferentiated mesenchymal stem cells versus articular chondrocytes: in vivo results of cartilage repair after 1year. Am J Sports Med. 2011;39(7):1401–1412. doi: 10.1177/0363546511398646. [DOI] [PubMed] [Google Scholar]
- 85.Zscharnack M, Hepp P, Richter R, Aigner T, Schulz R, Somerson J, Josten C, Bader A, Marquass B. Repair of chronic osteochondral defects using predifferentiated mesenchymal stem cells in an ovine model. Am J Sports Med. 2010;38(9):1857–1869. doi: 10.1177/0363546510365296. [DOI] [PubMed] [Google Scholar]
- 86.Yong KW, Choi JR, Safwani WKZW (2016) Biobanking of human mesenchymal stem cells: future strategy to facilitate clinical applications. In: Biobanking and cryopreservation of stem cells. Springer, pp 99–110 [DOI] [PubMed]
- 87.Otto WR, Wright NA. Mesenchymal stem cells: from experiment to clinic. Fibrogenesis Tissue Repair. 2011;4(20):1755–1536. doi: 10.1186/1755-1536-4-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Wang S, Qu X, Zhao RC. Clinical applications of mesenchymal stem cells. J Hematol Oncol. 2012;5(19):1756–8722. doi: 10.1186/1756-8722-5-19. [DOI] [PMC free article] [PubMed] [Google Scholar]