Abstract
The detrimental effects of drug abuse are apparently not limited to individuals but may also impact the vulnerability of their progenies to develop addictive behaviours. Epigenetic signatures, early life experience and environmental factors, converge to influence gene expression patterns in addiction phenotypes and consequently may serve as mediators of behavioural trait transmission between generations. The majority of studies investigating the role of epigenetics in addiction do not consider the influence of social interactions. This shortcoming in current experimental approaches necessitates developing social models that reflect the addictive behaviour in a free-living social environment. Furthermore, this review also reports on the advancement of interventions for drug addiction and takes into account the emerging roles of histone deacetylase (HDAC) inhibitors in the etiology of drug addiction and that HDAC may be a potential therapeutic target at nucleosomal level to improve treatment outcomes.
Keywords: Cocaine and alcohol abuse, DNA methylation, Chromatin remodeling, Epigenomic programming and inheritance, Environmental stimuli, Social stress
Introduction
Drug addiction is a chronic relapsing disorder, where the individual is unable to control his or her drug-seeking and drug-taking behaviours despite severe adverse consequences [1]. Clinically, drug addiction presents with behavioural, cognitive, and physiological symptoms that reflect the involvement of complex control systems of the brain [2]. Due to the increase in risky behaviours, this disorder is often associated with trauma and death [3]. Epidemiological surveys have shown that not only is addiction highly prevalent on its own, but that the disorder also frequently occurs as a comorbidity with other mental disorders [4]. The hundreds of research projects conducted all over the world underline the importance of studying addictive behaviour. In fact, many of the current trends of addiction research now have a well-defined focus that attempts to understand the neural mechanisms that underpin the conventional change from recreational drug use to a chronic addicted state with persistent addictive behaviours even after long abstinence and relapse propensity in drug addicts [5]. Many studies on chromatin remodeling and transcriptional regulation have shown that exposure to drugs of abuse induces changes in the expression of specific genes, such as brain-derived neurotrophic factor (BDNF), activator of G-protein signaling 3 (AGS3), and transcription factors (e.g., ∆FosB) in key reward areas of the brain, such as the ventral tegmental area (VTA), nucleus accumbens (NAc), and prefrontal cortex (PFC) [6–10]. Despite the substantial progress that has been made, our understanding of the molecular underpinnings of drug addiction remains incomplete. For instance, the impact of epigenetic alterations in combination with social environmental factors within the context of addictive behaviour appears underexplored. The purpose of this article is, therefore, to provide an overview of the current status of research in the field of drugs of abuse with specific reference to these factors, with a focus on how they might play a role in the etiology of drug abuse and its inheritance. We also report on current pharmacotherapies and highlight possible novel treatments that could be considered for drug-related disorders (Table 1).
Table 1.
Overview of studies on the epigenetic regulation by drugs of abuse
| Drug of abuse | Brain region | Epigenetic mechanism | Residues | Implication | References |
|---|---|---|---|---|---|
| Ethanol | AMY | Methylation | H3K27/H3K4 | Susceptibility to alcohol dependence | [119] |
| AMY | Acetylation | H3K9 | Susceptibility to alcohol dependence | [119] | |
| VTA | Acetylation | H3K9 | Ethanol withdrawal | [120] | |
| NAc | Acetylation | H4K12 | Persistence of ethanol-related behaviours | [121] | |
| Cocaine | NAc | Acetylation | Gen H3 | Cocaine addiction-related behaviours | [105, 122, 123] |
| VTA | Acetylation | Gen H3 | Motivation for drug reinforcement | [124] | |
| NAc | Acetylation | H3K14/H4K12 | Cocaine addiction-related behaviours | [105, 125] | |
| NAc | Methylation | H3K9 | Cocaine addiction-related behaviours | [75, 105] | |
| NAc | Acetylation | Gen H4 | Motivation for drug reinforcement | [74, 122] |
AMY amygdala, VTA ventral tegmental area, NAc nucleus accumbens, DG/Hipp dentate gyrus/Hippocampus
Epigenetic mechanisms
Both genes and the environment are important determinants of developmental processes. Subtle differences in the interaction between genes and the environment may be responsible for altering developmental trajectories that confer vulnerability or resilience to mental conditions, such as addiction. At the molecular level, epigenetics provides invaluable insight into the interaction between an individual’s genome and the environment [11]. Epigenetics, also known as chromatin remodeling, is defined as heritable chemical modifications to DNA capable of influencing transcriptional activity independent of DNA-coding sequence [12]. Principally, epigenetic mechanisms include DNA methylation and various chemical modifications of the histone proteins (H2A, H2B, H3, and H4) (see Fig. 1a). Most complex and coordinated series of epigenetic modifications, such as acetylation, methylation, phosphorylation, ubiquitylation, and ADP ribosylation, occur at the N-terminal tail of histones [13–15]. It is worth noting that recent findings have revealed additional mechanisms involving RNA interference and prion proteins which also contribute to epigenetic regulation [16]. In general, DNA methylation and histone acetylation remain the most recognized, widely studied, and established epigenetic modifications [17]. These two mechanisms work in tandem to cause chromatin remodeling and thus regulate gene expression.
Fig. 1.
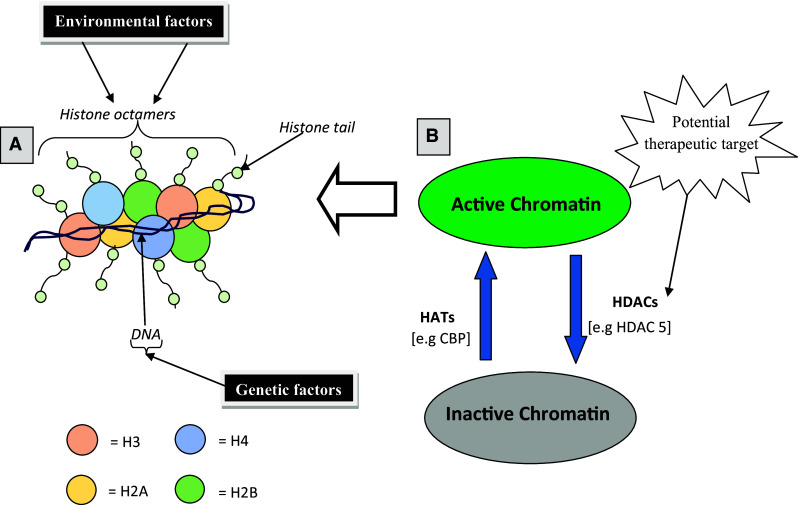
Schematic view of post-translational modifications of histones. a In eukaryotic cells, the DNA (consists of 147 base pairs) wraps around histone octamers (two copies each of H2A, H2B, H3, and H4) to form the nucleosome which is the functional unit of the chromatin. Projecting from the core of the histone octamers is the amino acid (-N-) terminal tails on which significant transcriptional modification occurs. b Transcriptional active or inactive states of the chromatin are balanced by the opposing actions of HATs and HDACs, respectively. Enhanced action of HATs promotes histone acetylation which allows assessibility of the DNA to the transcription factors by relaxing the chromatin resulting in an enhanced gene activity. Conversely, increased action of HDACs represses transcription (gene silencing) by deacetylating the histones. HDAC5 is one the II HDACs that shuttles between the nucleus and the cytoplasm and actively mediates gene silencing mechanism by binding hormone co-repressors. Consequently, nuclear export of HDAC5 which results in histone hyperacetylation as well as increased mRNA expression of its target genes (e.g., substance P and neurokinin 1) have been critically implicated in sensitized behavioural responses to addictive drugs [57]. In addition, toxicants such as drugs or endocrine disruptors can induce epimutations of the histone octamers [73] resulting in chromosomal abnormalities that are fundamental to addiction phenotypes and disease
DNA methylation
DNA methylation is generally linked to gene silencing due to its repressive effect on gene transcription. It either disrupts association of DNA binding factors with their target sequence or recruits transcriptional co-repressors by binding to methyl-CpG-binding protein-2 (MeCP2) [18] to induce an inactivated, condensed (silenced) chromatin state. Studies have shown that MeCP2 mediates behavioural responses to addictive properties of alcohol and cocaine mainly by altering BDNF expression in specific regions of the brain [19–21]. DNA methylation modification occurs only within specific gene promoters to produce stable epigenetic changes as opposed to histone tail modifications which are readily reversible [22, 23]. DNA methyltransferases (Dnmt) catalyze and maintain the sequence of gene expression events induced by DNA methylation. Dnmt1, Dnmt3a, and Dnmt3b are highly expressed in postmitotic nerve cells [24]. Altered expression of these Dnmt has been implicated in drug dependence and other related psychiatric disorders. For instance, Dnmt1 is highly expressed in GABAergic neurons whose dysfunction characterizes development of schizophrenia [25, 26]. Both Dnmt3a and Dnmt3b are capable of inducing methylation on naked DNA [27]. Expression of Dnmt3a in the NAc was increased by chronic social defeat stress and cocaine infusion suggesting its importance in regulating emotional behaviour and cocaine addiction [17, 28]. In response to cocaine treatment, it appears that Dnmt3a expression is biphasically regulated. Evidence from quantitative PCR analysis of NAc tissue from mice acutely and chronically pretreated with cocaine suggested that early withdrawal (4 h after injection of cocaine) upregulated Dnmt3a expression, whereas after 24 h, it was downregulated [28]. Mice lacking both Dnmt3a and 3b in mature forebrain neurons displayed impaired long-term plasticity and performed poorly in learning and memory tasks which were attributed in part to dysregulated gene expression. Other studies have shown that murine Dnmt3b knockouts exhibited neural tube defects that led to early lethality [29].
Histone acetylation
Histone acetylation is considered a good marker of actively transcribed genes [30]. Most genomic studies involving histone acetylation have focussed on the amino acid (-N-) terminal lysine residues in histones H3 and H4 [15]. Increased or low levels of histone acetylation within specific promoter regions correlates with enhanced or repressed gene activity, respectively [31]. These dynamic processes are actively controlled by two key enzymes histone acetyltransferases (HATs) and histone deacetylases (HDACs) (see Fig. 1b). The roles that these enzymes play in drug addiction are described below.
Social environmental factors that increase susceptibility to drug addiction
Human and animal studies suggest that there may be a direct relationship between environmental stress prior to drug exposure and the development of addiction-like behaviours. A meta-analysis showed that a degraded home environment significantly increases the risk for drug-related disorders to develop [31], while an enriched environment, such as positive family relationships, involvement, and attachment, appear to discourage drug use and prevent drug addiction [32]. The relationship between an adverse environment and drug dependence is underpinned by the interaction of multiple endocrine, paracrine, and intracellular systems [33], since these systems have been shown to be sensitive to social experience. It is, therefore, not surprising that drug addiction is highly prevalent in vulnerable populations undergoing social stress [34]. Many factors confound the study of drug addiction-related social stress and physiology in human populations, including long life spans, inaccuracy of self-reporting, ethical considerations, and high levels of genetic variation [35]. Animal models circumvent many of these challenges, and provide more tractable systems to study the interplay of social factors and drug addiction. For example, exposure to stress in utero has been shown to modify the behavioural reactivity of rats to drugs with addictive potential [36]. Similarly, an increase in drug seeking has been observed during adulthood in offspring of maternally stressed dams. Experiments utilising isolation stress and social deprivation yielded comparable results [37]. Poor maternal behaviour towards the pups and/or maternal separation during the early postnatal period increased ethanol consumption in adult rats [38]. Maternal licking and grooming correlated negatively with vulnerability to cocaine and ethanol use in rats [39]. Repeated social defeat during adolescence increased cocaine self-administration and cocaine-induced conditioned place preference (CPP) in rodents [40–43]. Furthermore, these stress events were suggested to share common epigenetic process with drugs of abuse to influence addiction-like behaviours. Indeed, it has been demonstrated that both early life stress and methamphetamine alter the expression of the epigenetic regulator methyl CpG binding protein 2 (MeCP2) in the nucleus accumbens to influence the motivational effects of methamphetamine and natural reinforcement [44–46]. These basic studies offer valuable insights into the effect of adverse environmental conditions on addictive behaviour. Similar studies have demonstrated the involvement of epigenetic changes in these effects [43, 47], such as the epigenetic mechanisms responsible for dysregulation of the hippocampal glucocorticoid receptors [48] and upregulation of histone acetylation by social defeat [43]. Epigenetic alterations induced by early life experiences have been shown to accumulate over time and have consequently been considered serious risk factors for mental disease development [49, 50]. This line of work on epigenetic regulation related to environmental stimuli has raised many questions, including and among others, (1) how the pharmacological effects of drugs with addictive potential may vary depending on the associated environmental conditions to which subjects may be exposed, or (2) to what extent social stress influences drug consumption, or (3) which genes are altered and in what way, by various environmental circumstances.
Mild stress and environmental enrichment have also been shown to protect and sometimes reverse addictive phenotypes. The chronic mild stress of neonatal handling prevented reinstatement of morphine CPP in adulthood [51] and environmental enrichment blocked reinstatement of ethanol-induced CPP [52]. It also reduced cocaine seeking and reinstatement induced by cues and stress [53, 54]. Similar to environmental enrichment, overexpression of ∆FosB decreased cocaine self-administration, enhanced extinction of cocaine seeking, and decreased cocaine-induced reinstatement of intravenous cocaine self-administration [55].
Interestingly, there is evidence that suggests that neurons do not only respond to various environmental signals via dynamic changes in epigenetic modifications [31], but also vary in sensitivity to drugs of abuse subject to altered states in the environment (see Fig. 2) [32]. Therefore, while there is no doubt of an interaction between the environment and addictive behaviour, the underlying mechanisms that facilitate this interaction continue to be poorly understood.
Fig. 2.
Model of possible factors that influence drug intake. Altered states in the environment influenced by various factors, such as physical contact, gender, family history, social or early life experience all converge to impact on the individual’s sensitivity and vulnerability to addictive compounds. In contrast, escalated or chronic drug intake especially at high doses possibly induces changes that accumulate over time to promote further drug use or addictive behaviours and sometimes may be passed down the germline to the next generation. Invariably, behavioural (drug intake) and psychological (vulnerability) balance largely depends on the level of exposure to drugs and associated factorial states within the environment
Inheritance of epigenetic imprints and trans-generational effects
The vulnerability of progeny to drug-induced maladaptive behaviours or neural plasticity is jointly influenced by both genetic and non-genetic factors. Many authors share the opinion that drugs of abuse are likely to induce epigenetic changes in parent sex cells (ova and sperm) which are passed down to future generations [18] and thereby predispose the offspring to subsequent drug effects and/or addiction. The fact that some epigenetic imprints, particularly maternal DNA methylation, can escape the epigenomic reprogramming that occurs during gametogenesis and fertilization [56] provides a mechanism that may enable the transgenerational transfer of parental traits. Some epigenetic imprints may, therefore, accumulate over a lifetime and be conserved between generations. This implies that the inheritance of acquired traits resulting from environmental exposure that alters a phenotype in one generation can be transmitted epigenetically to unexposed offspring [57]. This notion has profound implications for understanding how diseases may be prevalent in families as well as how the genome functions as an etiological factor in hereditary diseases.
The impact of social environment on the epigenome and its transgenerational transfer
Drug intake related to social context involves long-lasting epigenetic underpinnings that may be transmissible from one generation to another. The genes affected by social factors are mostly related to regulatory networks that control the hypothalamic-pituitary-gonadal axis and a variety of social behaviours [58]. Paternal transmission of epigenetic variation may manifest only in later life when the social environment changes [59]. In male mice, chronic social stress (achieved through instability of social hierarchy) experienced during adolescence through to adulthood induced social deficits and increased anxiety-like behaviours in up to two succeeding generations [60].
These observations in animal models find their counterpart in human studies. Recently, it was shown that differences in socio-economic status early in life are imprinted on the epigenome and maintained into adulthood. Several hundred promoters showed different levels of DNA methylation in blood profiles of adults who experienced social adversity early in life when compared to those that did not [61]. Similarly, global DNA hypomethylation was observed in blood samples of socio-economically deprived subjects [62]. These clinical observations highlighted the ‘epigenome’ as an interface between the social environment and the genome. However, genome-wide assays (GWA) of epigenetic changes in different regions and cell types of the brain are necessary to fully understand how specific epigenetic modifications may both influence and be caused by social behaviour. These previous studies indicate that the social component may have tremendous impact on the physiological and behavioural responses of an individual to environmental factors.
The impact of substances of abuse on the genome and its transgenerational transfer
Adding to the transgenerational effects of social conditions, chronic exposure to several drugs of abuse, including alcohol and cocaine, has been reported to induce epigenetic changes which points to a dysregulation of gene expression in both the brain and periphery [5, 57].
Alcohol has been shown to interfere with the epigenetic regulation of gene expression. A recent transcriptome study, comparing human post-mortem brain samples of alcoholics and age-matched controls, showed profound epigenetic effects of alcohol abuse. A notable difference between the two groups was that endogenous retroviral sequences that maintain DNA methylation throughout gametogenesis and fertilization, which are normally silenced by DNA methylation, were less methylated, coinciding with dramatically increased transcription of their host genes [63]. In utero studies in mice have shown that exposure to alcohol induces teratogenic effects related to epigenetic changes in the foetus. In female mice, free access to 10% (v/v) ethanol for 4 days per week for 10 weeks affected the adult offspring phenotype by altering the epigenotype of the early embryo. These alterations in the epigenome were associated with postnatal growth restriction and craniofacial dysmorphology reminiscent of foetal alcohol syndrome [64]. Alcohol exposure in utero also reduced DNA methylation at the differentially methylated domain of the paternally imprinted growth-related gene H19 in the sperm of exposed mice (F1). Most notably, a similar decrease at the same CpG sites was observed in the brains of the offspring (F2) [65]. These epigenetic changes caused by alcohol exposure resulted in a variety of developmental disturbances ranging from reduced litter size and birth weight to behavioural alterations, such as lower fearfulness and higher aggression. These findings from animal studies demonstrating how parental alcohol consumption may induce epigenetic aberrations that negatively affect the normal structure and function of their offspring were corroborated by clinical observations. For example, moderate and heavy drinkers show subtle reductions in DNA methylation at the H19 imprinted gene in their sperm compared to non-drinkers [66], and alcohol-induced parental epigenome changes such as these have been suggested to have detrimental effects on the cognitive performance of their children [67].
Changes in histone acetylation in the PFC of ethanol-exposed adolescent rats are associated with ethanol-induced place conditioning [68]. Intermittent alcohol exposure upregulated HAT activity in adolescent rat PFC and increased histone acetylation and dimethylation in the promoter region of cFos, Cdk5, and FosB [69]. Alcohol exposure during adulthood has been shown to be associated with downregulation of genes implicated in neural plasticity, such as cut-like 2 (cutl2), insulin-like growth factor 1 (Igf1), epidermal growth factor–containing fibulin-like extracellular matrix protein 1 (Efemp1), SRY-box-containing gene 7 (Sox 7) and many others, as well upregulation of SWI/SNF (an ATP dependent chromatin remodeling complex that mobilizes histone octamers) related, matrix associated, actin dependent regulator of chromatin, subfamily a, member 2 (Smarca2), the cytosolic enzyme DiGeorge syndrome critical region gene 2 (Dgcr2), and Pard6a which are implicated in the migration of immature granule neurons and neuroblast cell polarity [57]. In addition, cocaine and alcohol exposure have been associated with significant decreases in the mRNA levels of enzymes responsible for DNA methylation in the testes and sperm of adult male rodents, presenting a high risk induction factor for heritable epigenetic changes [70, 71]. Altered methylation related to alcohol was associated with dysregulation of genes known to play a role in metabolism, such as (Cyp4f13) and decreased methylation of genes associated with development (Nlgn3, Elavl2, Sox21, and Sim1), imprinting (Igf2r), and chromatin (Hist1h3d) which contribute to abnormal foetal development [70].
Cocaine has a profound effect on chromatin remodeling in brain areas, such as the NAc and PFC—brain regions key in processing reward and implicated in addiction [72]. Chronic cocaine treatment and self-administration induced epigenetic dysregulation of expression of several genes associated with neural plasticity, such as the immediate–early gene cFos, BDNF, cyclin-dependent kinase 5 (cdk5), and myocyte enhancing factor 2 (MEF2) [5, 73]. Chronic cocaine-induced hyperlocomotor activity and expression of CPP in rats was associated with a decrease in gene expression in the NAc caused by an increase in DNA methylation and a decrease in global levels of histone H3 acetylation [74]. Moreover, using conditional mutagenesis and viral mediated gene transfer, Maze et al. [27] showed that histone methyltransferase G9a downregulation increases dendritic spine plasticity of NAc neurons and enhances rewarding responses to cocaine by decreasing repressive H3K9me2 at specific target genes thus increasing the expression of those genes [75]. Vassoler et al. [28], using histone H3 acetylation in BDNF promoters as an epigenetic marker, showed that voluntary paternal ingestion of cocaine resulted in epigenetic reprogramming of the germline that changed medial PFC gene expression to such an extent that the male offspring became resistant to cocaine reinforcement [76]. This observation suggests that epigenetic reprogramming of the genome in offspring of addicted parents may serve as a protective mechanism in rendering the next generation less susceptible to future addiction susceptibility. However, administration of cocaine during gestation has been shown to alter global DNA methylation in several promoter regions for genes implicated in crucial cellular functions [77]. For instance, maternal cocaine consumption during gestation caused increased CpG methylation at two SP1 binding sites in the promoter region of protein kinase C (PKC) that precipitated downregulation of PKC expression in the heart of adult offspring. This change in PKC expression rendered these animals more sensitive to ischemia and reperfusion injury [78]. The genomic effects of parental drug intake and how these effects impact on the well-being of the offspring, therefore, remain controversial.
Animal models of drug addiction
Animal models of drug addiction have enabled the implementation of protocols that are used to characterise addictive behaviour, as well as facilitating the study of trans-generational effects over short periods. Over time, these models have undergone a number of refinements that allowed a deeper understanding of the circuitry involved in drug craving, relapse and loss of control in several behavioural paradigms, in particular, tests of behavioural sensitization, CPP, and drug self-administration. Despite the advances made, these classical paradigms do not allow a continuous assessment of addictive behaviour, nor do they consider the social contexts of addicts in the neuropathology of addiction [79]. It has been repeatedly shown that social factors that trigger craving and drug-seeking behaviour in animals and humans induce lasting behavioural and neurogenic changes. Moreover, recent behavioural sensitization studies of housing conditions demonstrated that social isolation alters the neuronal functioning of the dopaminergic and serotonergic systems, evoking changes in sympathetic neurotransmission [80–82]. Such environmentally induced changes may potentiate the post-sensitization conditioned locomotor response to cocaine that is said to be mediated by alterations in dopamine D2 receptor density, and can be further modified by ethanol treatment. Furthermore, animals subjected to overcrowded conditions appear to consume more ethanol than isolated animals that in turn drink more than animals housed under the standard conditions (four animals per cage). In line with these studies, McCormick et al. [37] have shown that daily social isolation for 1 h followed by pair housing with an unfamiliar partner induced anxiety and endocrine changes [83] that was associated with suppressed hippocampal cell proliferation and impaired adult object recognition in a spatial memory test [84]. One neural mechanism that may underlie the role of social context in increased risk for drug abuse is the imbalance between mineralocorticoid and glucocorticoid receptor levels in the limbic system and in the hypothalamic-pituitary-gonadal axis [57, 85–87]. Thus, development of laboratory animal models for drug addiction should allocate more consideration to the social component in order to generate data that ethically and practically represent a valid construct of the human condition and closely resemble the componential behaviours of an addicted subject in a free-living state.
Pharmacological treatment of addictive disorders
Current medications and novel therapeutic approaches
Attempts to manage drug addiction have included behavioural and psychosocial interventions [88, 89] as well as various medications [90–93] (see Table 2 for tested medications for cocaine and alcohol addiction). Biologics, such as monoclonal antibodies, vaccines, and engineered enzymes, are currently being proposed as alternatives to addiction pharmacotherapy [94–96]. For example, cocaine esterases (CocE-L169K/G173Q or CocE-T172R/G173Q) have been shown to robustly antagonize cocaine rewarding effects in rats [97–99]. Most biologics prevent central rewarding effects of drugs but fail to address the question of drug craving and relapse [100]. This may be attributed to their inability to rectify impaired components of the addiction neurocircuitry and/or neuroplasticity. A more recent therapeutic approach employed the use of small interfering RNA (siRNA) coupled with gold nanorods to silence DARRP-32 (dopamine and cyclic-AMP regulated protein phosphatase inhibitor) gene, in dopaminergic cells [101], generally known as one of the key regulators of histone phosporylation. Studies have demonstrated that genetic disruption of DARPP-32 has dramatic effects on behavioural responses to cocaine [15], whereas inhibition breaks the addiction cycle possibly by down-regulating extracellular signal-regulated kinase (ERK) and protein phosphatase-1 (PP-1)—factors that play key roles in the addiction signaling pathway [101]. siRNA complexes may, therefore, serve as pharmaceutical vehicles that could enable effective delivery of specific compounds, such as biologics or approved drugs, directly into brain sites relevant to addictive behaviour.
Table 2.
Summary of pharmacotherapeutics for drug addiction
| Drugs of abuse | Medications | Class | Action | Consequences/clinical implications | FDA approved? (±) | Indications/remarks | References |
|---|---|---|---|---|---|---|---|
| Alcohol | Naltrexone | Opioid receptor antagonist | Blocks mu-opioid receptors | Not effective in all patients | + | Less effective against stress-induced relapse | [126–130] |
| Disulfiram | ALDH inhibitor | Inhibits ALDH and prevents acetaldehyde metabolism | Low compliance due to aversive reactions and other side effects | + | Effective against alcohol and cocaine dependence | [100, 127, 129–132] | |
| Acamprosate | NMDA glutamate receptor antagonist | Normalizes hyperglutamatergic states | Adverse reactions e.g., diarrhea, suicidal ideation | + | Reduces withdrawal symptoms and prevents relapse | [127, 130, 131, 133, 134] | |
| Topiramate, Ondansetron and Baclofen | − | Have promising therapeutic potential | [130, 133, 135, 136] | ||||
| Cocaine | NAC | Thiol antioxidant | Regulates X(c) system activity and GSH biosynthesis | Reduces respiratory burst and may cause liver damage | − | Low bio-distribution | [137–139] |
| Modafinil | GABA/glutaminergic agent | Upregulates brain glutamate system | Causes euphoria | − | Approved for other indications | [127] | |
| Topiramate | Antiepileptic drug | Mainly antagonizes AMPA receptors and kainate glutamate receptors | Causes anorexia, paresthesia, reversible cognitive impairment and taste aversion | − | Approved for other indications | [127, 135, 136] | |
| Baclofen, Propranolol, α-adrenergic agonists and Rimonabant | − | Have promising therapeutic potential | [127, 140–142] |
Drug addiction, also called substance use disorder, is generally considered a brain disease. It is measured on a continuum scale that ranges from mild to severe. According to SAMHSA, diagnosis using DSM-5 criteria is based on evidence of social and control impairments, risky use, and pharmacological implications [143]. However, treatment approaches should involve biological, behavioural, and social context components [144] to produce effective results
X(c) system cystine–glutamate antiporter system, NAC N-acetylcysteine, GSH glutathione, DA dopamine, ALDH aldehyde dehydrogenase, AMPA alpha-amino-3-hydroxy-5-methylisoxazole-4-propionic acid, NMDA N-methyl-d-aspartate, FDA food and drug administration, ± yes/no, DSM-5 diagnostic and statistical manual of mental disorders 5, SAMHSA substance abuse and mental health services administration
HDAC inhibitors (HDACi): a target therapeutic for drug addiction
Recent advances in the field of chromatin remodeling and epigenetic regulation improved our understanding of how genes interact and are regulated by the environment. Although pathological alterations in the brain transcriptome that underlie psychiatric and neurodegenerative disorders are incompletely understood, GWA studies have established genetic associations between specific genes or chromosomal regions with various brain diseases which have in common the hallmark of cognitive impairment [102]. Genetic-based studies have suggested critical roles for the epigenetic modifiers—HATs and HDACs in maintaining brain homeostatsis in various disease conditions. Targeting histone acetylation may provide benefit for the treatment of a broad range of human diseases, such as depression, schizophrenia, anxiety disorders, and drug addiction [103]. Of the various HATs, CREB-binding protein (CBP) has been implicated in drug addiction, but the evidence is contradictory [104]. CBP deletion in the NAc attenuated cocaine sensitivity and CPP [105], whereas striatal deletion increased sensitivity to cocaine and amphetamine [106]. In contrast, evidence to support a role for HDACS in drug addiction is convincing. Previous studies have shown that HDAC activity was increased in the PFC and NAc of rodents following cocaine self-administration. Now, much attention is given to HDACs, especially HDAC5 due to its antidepressant activity [103, 107] and unique response to chronic cocaine administration which critically implicates its involvement in behavioural transitions from drug experimentation to compulsive drug use [15]. Moreover, HDAC5 has been identified to centrally integrate chromatin changes and gene alterations induced by drugs and stress stimuli [57]. In addition, the regulation of saliency circuit, which mediates behavioural responses to various environmental stimuli, critically implicates HDAC5. For example, increased response to rewarding effects of chronic cocaine exposure as well as chronic neuropathic pain and social defeat stress were observed in HDAC5 knockout mice [5, 57, 108]. Although the effects of HDAC5 on cocaine rewarding effects still remains unclear, it has previously been shown that its overexpresion blunts cocaine-induced place conditioning and locomotor-activating effects [57]. However, the behavioural effects of HDAC5 on cocaine-induced reward and locomotion requires further investigation, since it is uncertain whether its action is due to interaction with HDAC3 on the same catalytic deacetylase domain and blocking this site prevents its inhibitory action on cocaine reward [109].
Currently, there is considerable and growing interest in the use of HDAC inhibitors to activate the expression of mRNAs that are downregulated in various neurological disorders and psychiatric conditions [31] which include drug addiction [110]. HDAC inhibitors have been considered for many years as potent anticancer agents [111, 112]. HDAC inhibitors competitively inhibit HDACs from deacetylating lysines on the histone tails resulting in hyperacetylated and transcriptionally active chromatin states—giving rise to increased gene expression in the cell. Trichostatin A (TSA), valproic acid (VPA), sodium butyrate (NaBut), and suberoylanilide hydroxamic acid (SAHA) are among pharmaceutical compounds known to have HDAC inhibitor activity [113]. The usefulness of these compounds is exemplified by administration of SAHA restoring memory function in mice lacking appropriate CBP activity [102]. Similarly, it has also been reported that the HDAC inhibitor TSA improved long-term memory and synaptic plasticity in a mouse model of Rubinstein-Taybi Syndrome that is characterized by mental retardation due to mutations of CBP and p300 [114]. Another HDAC inhibitor NaBut has been used to strengthen memory associated with learning events [115]. Malvaez et al. have demonstrated that transgenic mice treated with NaBut extingished cocaine-induced CPP more quickly and in a more persistent manner than their vehicle-treated controls [116]. An exciting application of these findings that incorporate pharmacologic enhancement of extinction learning by modulating memory components of substance use disorders via HDAC inhibitor is of potential therapeutic interest. Besides, it was previously shown that HDAC inhibitors reversed long-term chromatin changes and persistent behavioural alterations at adulthood in maternally stressed rats [12]. This action coupled with other evidence of its neuroprotective and neuroregenerative properties in animal models [102] further support the speculations that HDAC inhibitors might be useful in the treatment of neuropsychiatric diseases.
Some controversial reports about the therapeutic action of HDAC inhibitors have also been recorded in the literature. It is, therefore, important to stress that HDAC inhibitors may sometimes exhibit opposing actions on drug-seeking behaviours subject to the manner in which they are administered. For instance, HDAC inhibitors increased drug intake in animals trained to self-administer cocaine but reduced intake when given before drug acquisition [117, 118]. The bioavailability and half-life of HDAC inhibitors may also account for some of their disparate actions in vivo [111]. Hence, relatively high or low concentrations may be required for desired action when HDAC inhibitors with short (e.g., valproic acid) or longer plasma half-lives (e.g., SAHA and TSA) are used.
Valproic acid which mimics the action of the HDAC inhibitor, TSA, has been used for the treatment of schizophrenia and bipolar disorder in humans for decades and other HDAC inhibitors are currently in different phases of human clinical trials for CNS disorders [103].
Moreover, it is beyond any doubt that altered histone acetylation is one of the main contributors to transition to an addicted state. However, research in the field of drug addiction needs to focus more on specific HDAC inhibitors that target extinction memory of drugs as this may later translate to effective medications for preventing drug relapse.
Conclusion/future directions
It has been shown that the majority of drug–induced behavioural alterations result not only from genetic but also epigenetic interactions giving rise to a breaking point situation that is beyond the individual’s adjustment capacity to curtail further use [5]. Some authors have suggested that these changed behaviours are inheritable by succeeding generations through mechanisms that also implicate epigenetic modifications. Other proposed strategies include epigenetic mechanisms which may be at play in the germline and subsequently interfere with normal embryonic–epigenomic programming. In the addicted parents, this suggests an intriguing and potentially alarming possibility that exposure to drugs of abuse may produce transmissible epigenetic changes that result in profound alterations to the physiology and behaviour of the offspring, raising the interesting question as to whether non-exposed children of addicts are “programmed” to become addicts themselves. The few studies that have looked beyond the first generation suggest that many phenotypes persist. Regardless of the number of future generations, the impact of drug use on the first generation offspring alone is sufficient to justify further research defining the extent of epigenetic heritability of phenotypes associated with parental drug abuse and the specific mechanisms underlying these effects. However, preventing, curtailing, or even rolling back the scourge of cocaine or alcohol-induced maladaptive disorders still remains a notable challenge today. It is, therefore, imperative that the search for better treatment outcomes continues. We, therefore, propose that combining psychosocial intervention with gene therapy involving pharmacological manipulations of HDACs, especially HDAC5, may further enhance current therapies and perhaps result in a more successful management of drug addiction.
Acknowledgements
We wish to express our appreciation to the College of Health Sciences (CHS) of University of KwaZulu-Natal for granting a PhD Scholarship to D.C Ajonijebu and a postdoctoral fellowship to O. Abboussi. The authors also wish to acknowledge the National Research Foundation (NRF) of South Africa who supports the research of MV Mabandla and WMU Daniels.
Compliance with ethical standards
Conflict of interest
All authors have no conflict of interest to disclose.
References
- 1.Everitt BJ, Robbins TW. Neural systems of reinforcement for drug addiction: from actions to habits to compulsion. Nat Neurosci. 2005;8(11):1481–1489. doi: 10.1038/nn1579. [DOI] [PubMed] [Google Scholar]
- 2.Lieb R (2015) Epidemiological perspectives on comorbidity between substance use disorders and other mental disorders. In: Dom G, Moggi F (eds) Co-occurring Addictive and Psychiatric Disorders. Springer, Heidelberg, pp 3–12
- 3.Nelson PK, et al. Global epidemiology of hepatitis B and hepatitis C in people who inject drugs: results of systematic reviews. Lancet. 2011;378(9791):571–583. doi: 10.1016/S0140-6736(11)61097-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Compton WM, et al. Developments in the epidemiology of drug use and drug use disorders. Am J Psychiatry. 2005;162(8):1494–1502. doi: 10.1176/appi.ajp.162.8.1494. [DOI] [PubMed] [Google Scholar]
- 5.Renthal W, Nestler EJ. Epigenetic mechanisms in drug addiction. Trends Mol Med. 2008;14(8):341–350. doi: 10.1016/j.molmed.2008.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Graham DL, et al. Dynamic BDNF activity in nucleus accumbens with cocaine use increases self-administration and relapse. Nat Neurosci. 2007;10(8):1029–1037. doi: 10.1038/nn1929. [DOI] [PubMed] [Google Scholar]
- 7.Lu L, et al. A single infusion of brain-derived neurotrophic factor into the ventral tegmental area induces long-lasting potentiation of cocaine seeking after withdrawal. J Neurosci. 2004;24(7):1604–1611. doi: 10.1523/JNEUROSCI.5124-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ting-A-Kee R, et al. Infusion of brain-derived neurotrophic factor into the ventral tegmental area switches the substrates mediating ethanol motivation. Eur J Neurosci. 2013;37(6):996–1003. doi: 10.1111/ejn.12105. [DOI] [PubMed] [Google Scholar]
- 9.Bowers MS, et al. Activator of G protein signaling 3: a gatekeeper of cocaine sensitization and drug seeking. Neuron. 2004;42(2):269–281. doi: 10.1016/S0896-6273(04)00159-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kelz MB, et al. Expression of the transcription factor ∆FosB in the brain controls sensitivity to cocaine. Nature. 1999;401(6750):272–276. doi: 10.1038/45790. [DOI] [PubMed] [Google Scholar]
- 11.Jaenisch R, Bird A. Epigenetic regulation of gene expression: how the genome integrates intrinsic and environmental signals. Nat Genet. 2003;33:245–254. doi: 10.1038/ng1089. [DOI] [PubMed] [Google Scholar]
- 12.Weaver IC, et al. Epigenetic programming by maternal behavior. Nat Neurosci. 2004;7(8):847–854. doi: 10.1038/nn1276. [DOI] [PubMed] [Google Scholar]
- 13.Abifadel M, et al. Mutations in PCSK9 cause autosomal dominant hypercholesterolemia. Nat Genet. 2003;34(2):154–156. doi: 10.1038/ng1161. [DOI] [PubMed] [Google Scholar]
- 14.Jenuwein T, Allis CD. Translating the histone code. Science. 2001;293(5532):1074–1080. doi: 10.1126/science.1063127. [DOI] [PubMed] [Google Scholar]
- 15.Renthal W, Nestler EJ. Chromatin regulation in drug addiction and depression. Dialog Clin Neurosci. 2009;11(3):257. doi: 10.31887/DCNS.2009.11.3/wrenthal. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Houri-Zeevi L, Rechavi O. A matter of time: small RNAs regulate the duration of epigenetic inheritance. Trends in Genetics . 2017;33(1):46–57. doi: 10.1016/j.tig.2016.11.001. [DOI] [PubMed] [Google Scholar]
- 17.Starkman BG, Sakharkar AJ, Pandey SC. Epigenetics—beyond the genome in alcoholism. Alcohol Res-Curr Rev. 2011;34(3):293. [PMC free article] [PubMed] [Google Scholar]
- 18.Nestler EJ. Epigenetic mechanisms of drug addiction. Neuropharmacology. 2014;76:259–268. doi: 10.1016/j.neuropharm.2013.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Im H-I, et al. MeCP2 controls BDNF expression and cocaine intake through homeostatic interactions with microRNA-212. Nat Neurosci. 2010;13(9):1120–1127. doi: 10.1038/nn.2615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.He D-Y, Neasta J, Ron D. Epigenetic regulation of BDNF expression via the scaffolding protein RACK1. J Biol Chem. 2010;285(25):19043–19050. doi: 10.1074/jbc.M110.100693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Moonat S, et al. The role of amygdaloid brain-derived neurotrophic factor, activity-regulated cytoskeleton-associated protein and dendritic spines in anxiety and alcoholism. Addict Biol. 2011;16(2):238–250. doi: 10.1111/j.1369-1600.2010.00275.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Antequera F. Structure, function and evolution of CpG island promoters. Cell Mol Life Sci CMLS. 2003;60(8):1647–1658. doi: 10.1007/s00018-003-3088-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Maunakea AK, et al. Conserved role of intragenic DNA methylation in regulating alternative promoters. Nature. 2010;466(7303):253–257. doi: 10.1038/nature09165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Feng J, Fan G. The role of DNA methylation in the central nervous system and neuropsychiatric disorders. Int Rev Neurobiol. 2009;89:67–84. doi: 10.1016/S0074-7742(09)89004-1. [DOI] [PubMed] [Google Scholar]
- 25.Dong E, et al. Reviewing the role of DNA (cytosine-5) methyltransferase overexpression in the cortical GABAergic dysfunction associated with psychosis vulnerability. Epigenetics. 2007;2(1):29–36. doi: 10.4161/epi.2.1.4063. [DOI] [PubMed] [Google Scholar]
- 26.Costa E, Grayson DR, Guidotti A. Epigenetic downregulation of GABAergic function in schizophrenia: potential for pharmacological intervention? Mol Interv. 2003;3(4):220. doi: 10.1124/mi.3.4.220. [DOI] [PubMed] [Google Scholar]
- 27.Moore LD, Le T, Fan G. DNA methylation and its basic function. Neuropsychopharmacology. 2013;38(1):23–38. doi: 10.1038/npp.2012.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.LaPlant Q, et al. Dnmt3a regulates emotional behavior and spine plasticity in the nucleus accumbens. Nat Neurosci. 2010;13(9):1137–1143. doi: 10.1038/nn.2619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lagali P, Corcoran C, Picketts D. Hippocampus development and function: role of epigenetic factors and implications for cognitive disease. Clin Genet. 2010;78(4):321–333. doi: 10.1111/j.1399-0004.2010.01503.x. [DOI] [PubMed] [Google Scholar]
- 30.Jiang Y, et al. Epigenetics in the nervous system. J Neurosci. 2008;28(46):11753–11759. doi: 10.1523/JNEUROSCI.3797-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Grayson DR, Kundakovic M, Sharma RP. Is there a future for histone deacetylase inhibitors in the pharmacotherapy of psychiatric disorders? Mol Pharmacol. 2010;77(2):126–135. doi: 10.1124/mol.109.061333. [DOI] [PubMed] [Google Scholar]
- 32.Robinson TE, Berridge KC. Incentive-sensitization and addiction. Addiction. 2001;96(1):103–114. doi: 10.1046/j.1360-0443.2001.9611038.x. [DOI] [PubMed] [Google Scholar]
- 33.Fattore L, Melis M. Sex differences in impulsive and compulsive behaviors: a focus on drug addiction. Addict Biol . 2016;21(5):1043–1051. doi: 10.1111/adb.12381. [DOI] [PubMed] [Google Scholar]
- 34.Chavkin C, Koob GF. Dynorphin, Dysphoria, and Dependence: the Stress of Addiction. Neuropsychopharmacology. 2016;41(1):373–374. doi: 10.1038/npp.2015.258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ahmed S. The science of making drug-addicted animals. Neuroscience. 2012;211:107–125. doi: 10.1016/j.neuroscience.2011.08.014. [DOI] [PubMed] [Google Scholar]
- 36.Sinha R. Chronic stress, drug use, and vulnerability to addiction. Ann N Y Acad Sci. 2008;1141(1):105–130. doi: 10.1196/annals.1441.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Deminière JM, et al. Increased locomotor response to novelty and propensity to intravenous amphetamine self-administration in adult offspring of stressed mothers. Brain Res. 1992;586(1):135–139. doi: 10.1016/0006-8993(92)91383-P. [DOI] [PubMed] [Google Scholar]
- 38.Isengulova A, Kalmykova Z, Miroshnichenko I. The significance of maternal care for the formation of ethanol preference in rats periodically separated from mothers during the first half of the nest period. Bull Exp Biol Med. 2009;147(4):390–393. doi: 10.1007/s10517-009-0533-z. [DOI] [PubMed] [Google Scholar]
- 39.Francis D, Kuhar M. Frequency of maternal licking and grooming correlates negatively with vulnerability to cocaine and alcohol use in rats. Pharmacology Biochemistry Behavior. 2008;90(3):497–500. doi: 10.1016/j.pbb.2008.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yap JJ, Miczek KA. Social defeat stress, sensitization, and intravenous cocaine self-administration in mice. Psychopharmacology (Berl) 2007;192(2):261–273. doi: 10.1007/s00213-007-0712-4. [DOI] [PubMed] [Google Scholar]
- 41.Burke AR, Miczek KA. Escalation of cocaine self-administration in adulthood after social defeat of adolescent rats: role of social experience and adaptive coping behavior. Psychopharmacology (Berl) 2015;232(16):3067–3079. doi: 10.1007/s00213-015-3947-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.odríguez-Arias M. Effects of repeated social defeat on adolescent mice on cocaine-induced CPP and self-administration in adulthood: integrity of the blood–brain barrier. Addict Biol . 2017;22:129–141. doi: 10.1111/adb.12301. [DOI] [PubMed] [Google Scholar]
- 43.Montagud-Romero S, et al. Up-regulation of histone acetylation induced by social defeat mediates the conditioned rewarding effects of cocaine. Prog Neuropsychopharmacol Biol Psychiatry. 2016;70:39–48. doi: 10.1016/j.pnpbp.2016.04.016. [DOI] [PubMed] [Google Scholar]
- 44.Lewis CR, et al. Interactions between Early Life Stress, Nucleus Accumbens MeCP2 Expression, and Methamphetamine Self-Administration in Male Rats. Neuropsychopharmacology. 2016;41(12):2851–2861. doi: 10.1038/npp.2016.96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lewis CR, et al. The Effects of Maternal Separation on Adult Methamphetamine Self-Administration, Extinction, Reinstatement, and MeCP2 Immunoreactivity in the Nucleus Accumbens. Front Psychiatry. 2013;4:55. doi: 10.3389/fpsyt.2013.00055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tesone-Coelho C, et al. Vulnerability to opiate intake in maternally deprived rats: implication of MeCP2 and of histone acetylation. Addict Biol. 2015;20(1):120–131. doi: 10.1111/adb.12084. [DOI] [PubMed] [Google Scholar]
- 47.Champagne FA, Curley JP. Epigenetic mechanisms mediating the long-term effects of maternal care on development. Neuroscience Biobehavioral Reviews. 2009;33(4):593–600. doi: 10.1016/j.neubiorev.2007.10.009. [DOI] [PubMed] [Google Scholar]
- 48.McGowan PO, et al. Epigenetic regulation of the glucocorticoid receptor in human brain associates with childhood abuse. Nat Neurosci. 2009;12(3):342–348. doi: 10.1038/nn.2270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Feinberg AP, Fallin MD. Epigenetics at the crossroads of genes and the environment. Jama. 2015;314(11):1129–1130. doi: 10.1001/jama.2015.10414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lewis CR, Olive MF. Early life stress interactions with the epigenome: potential mechanisms driving vulnerability towards psychiatric illness. Behav Pharmacol. 2014;25(5 0 6):341. doi: 10.1097/FBP.0000000000000057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Schwarz JM, Hutchinson MR, Bilbo SD. Early-life experience decreases drug-induced reinstatement of morphine CPP in adulthood via microglial-specific epigenetic programming of anti-inflammatory IL-10 expression. The Journal of Neuroscience. 2011;31(49):17835–17847. doi: 10.1523/JNEUROSCI.3297-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Li X, et al. Environmental enrichment blocks reinstatement of ethanol-induced conditioned place preference in mice. Neurosci Lett. 2015;599:92–96. doi: 10.1016/j.neulet.2015.05.035. [DOI] [PubMed] [Google Scholar]
- 53.Chauvet C, et al. Environmental enrichment reduces cocaine seeking and reinstatement induced by cues and stress but not by cocaine. Neuropsychopharmacology. 2009;34(13):2767–2778. doi: 10.1038/npp.2009.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Thiel KJ, et al. Anti-craving effects of environmental enrichment. Int J Neuropsychopharmacolog. 2009;12(9):1151–1156. doi: 10.1017/S1461145709990472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zhang Y, et al. Overexpression of DeltaFosB in nucleus accumbens mimics the protective addiction phenotype, but not the protective depression phenotype of environmental enrichment. Front Behav Neurosci. 2014;8:297. doi: 10.3389/fnbeh.2014.00297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Richards EJ. Inherited epigenetic variation—revisiting soft inheritance. Nat Rev Genet. 2006;7(5):395–401. doi: 10.1038/nrg1834. [DOI] [PubMed] [Google Scholar]
- 57.Renthal W, et al. Histone deacetylase 5 epigenetically controls behavioral adaptations to chronic emotional stimuli. Neuron. 2007;56(3):517–529. doi: 10.1016/j.neuron.2007.09.032. [DOI] [PubMed] [Google Scholar]
- 58.Maruska KP, Fernald RD. Social regulation of gene expression in the hypothalamic-pituitary-gonadal axis. Physiology. 2011;26(6):412–423. doi: 10.1152/physiol.00032.2011. [DOI] [PubMed] [Google Scholar]
- 59.Champagne FA. Epigenetic influence of social experiences across the lifespan. Dev Psychobiol. 2010;52(4):299–311. doi: 10.1002/dev.20436. [DOI] [PubMed] [Google Scholar]
- 60.Champagne FA, Meaney MJ. Transgenerational effects of social environment on variations in maternal care and behavioral response to novelty. Behav Neurosci. 2007;121(6):1353. doi: 10.1037/0735-7044.121.6.1353. [DOI] [PubMed] [Google Scholar]
- 61.Borghol N, et al. Associations with early-life socio-economic position in adult DNA methylation. Int J Epidemiol. 2012;41(1):62–74. doi: 10.1093/ije/dyr147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.McGuinness D, et al. Socio-economic status is associated with epigenetic differences in the pSoBid cohort. Int J Epidemiol. 2012;41:151–160. doi: 10.1093/ije/dyr215. [DOI] [PubMed] [Google Scholar]
- 63.Ponomarev I, et al. Gene coexpression networks in human brain identify epigenetic modifications in alcohol dependence. J Neurosci. 2012;32(5):1884–1897. doi: 10.1523/JNEUROSCI.3136-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kaminen-Ahola N, et al. Maternal ethanol consumption alters the epigenotype and the phenotype of offspring in a mouse model. PLoS Genet. 2010;6(1):e1000811. doi: 10.1371/journal.pgen.1000811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ouko LA, et al. Effect of alcohol consumption on CpG methylation in the differentially methylated regions of H19 and IG-DMR in male gametes—implications for fetal alcohol spectrum disorders. Alcohol Clin Exp Res. 2009;33(9):1615–1627. doi: 10.1111/j.1530-0277.2009.00993.x. [DOI] [PubMed] [Google Scholar]
- 66.Bohacek J, Mansuy IM. Epigenetic inheritance of disease and disease risk. Neuropsychopharmacology. 2013;38(1):220–236. doi: 10.1038/npp.2012.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Haycock PC. Fetal alcohol spectrum disorders: the epigenetic perspective. Biol Reprod. 2009;81(4):607–617. doi: 10.1095/biolreprod.108.074690. [DOI] [PubMed] [Google Scholar]
- 68.Pascual M, et al. Changes in histone acetylation in the prefrontal cortex of ethanol-exposed adolescent rats correlate with ethanol-induced place conditioning. Alcohol Clin Exp Res. 2012;36:84. doi: 10.1016/j.neuropharm.2012.01.011. [DOI] [PubMed] [Google Scholar]
- 69.Pascual M, et al. Changes in histone acetylation in the prefrontal cortex of ethanol-exposed adolescent rats are associated with ethanol-induced place conditioning. Neuropharmacology. 2012;62(7):2309–2319. doi: 10.1016/j.neuropharm.2012.01.011. [DOI] [PubMed] [Google Scholar]
- 70.Liu Y, et al. Alcohol exposure alters DNA methylation profiles in mouse embryos at early neurulation. Epigenetics. 2009;4(7):500–511. doi: 10.4161/epi.4.7.9925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Anier K, et al. DNA methylation regulates cocaine-induced behavioral sensitization in mice. Neuropsychopharmacology. 2010;35(12):2450–2461. doi: 10.1038/npp.2010.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Sadri-Vakili G, et al. Cocaine-induced chromatin remodeling increases brain-derived neurotrophic factor transcription in the rat medial prefrontal cortex, which alters the reinforcing efficacy of cocaine. J Neurosci. 2010;30(35):11735–11744. doi: 10.1523/JNEUROSCI.2328-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Skinner MK, Manikkam M, Guerrero-Bosagna C. Epigenetic transgenerational actions of environmental factors in disease etiology. Trends Endocrinol Metabol. 2010;21(4):214–222. doi: 10.1016/j.tem.2009.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Wang L, et al. Chronic cocaine-induced H3 acetylation and transcriptional activation of CaMKIIα in the nucleus accumbens is critical for motivation for drug reinforcement. Neuropsychopharmacology. 2010;35(4):913–928. doi: 10.1038/npp.2009.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Maze I, et al. Essential role of the histone methyltransferase G9a in cocaine-induced plasticity. Science. 2010;327(5962):213–216. doi: 10.1126/science.1179438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Vassoler FM, et al. Epigenetic inheritance of a cocaine-resistance phenotype. Nat Neurosci. 2013;16(1):42–47. doi: 10.1038/nn.3280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Novikova SI, et al. Maternal cocaine administration in mice alters DNA methylation and gene expression in hippocampal neurons of neonatal and prepubertal offspring. PLoS One. 2008;3(4):e1919–e1919. doi: 10.1371/journal.pone.0001919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Meyer K, Zhang H, Zhang L. Direct effect of cocaine on epigenetic regulation of PKCɛ gene repression in the fetal rat heart. J Mol Cell Cardiol. 2009;47(4):504–511. doi: 10.1016/j.yjmcc.2009.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Caprioli D, et al. Modeling the role of environment in addiction. Prog Neuropsychopharmacol Biol Psychiatry. 2007;31(8):1639–1653. doi: 10.1016/j.pnpbp.2007.08.029. [DOI] [PubMed] [Google Scholar]
- 80.Araujo NP, et al. The importance of housing conditions on behavioral sensitization and tolerance to ethanol. Pharmacol Biochem Behav. 2005;82(1):40–45. doi: 10.1016/j.pbb.2005.07.009. [DOI] [PubMed] [Google Scholar]
- 81.Matsuda T, et al. Functional alteration of brain dopaminergic system in isolated aggressive mice. Nihon shinkei seishin yakurigaku zasshi = Jpn J Psychopharmacol. 2001;21(3):71–76. [PubMed] [Google Scholar]
- 82.D’Arbe M, Einstein R, Lavidis N. Stressful animal housing conditions and their potential effect on sympathetic neurotransmission in mice. Am J Physiol-Regul Integr Comp Physiol. 2002;282(5):R1422–R1428. doi: 10.1152/ajpregu.00805.2000. [DOI] [PubMed] [Google Scholar]
- 83.McCormick CM, et al. Long-lasting, sex-and age-specific effects of social stressors on corticosterone responses to restraint and on locomotor responses to psychostimulants in rats. Horm Behav. 2005;48(1):64–74. doi: 10.1016/j.yhbeh.2005.01.008. [DOI] [PubMed] [Google Scholar]
- 84.McCormick CM, et al. Hippocampal cell proliferation and spatial memory performance after social instability stress in adolescence in female rats. Behav Brain Res. 2010;208(1):23–29. doi: 10.1016/j.bbr.2009.11.003. [DOI] [PubMed] [Google Scholar]
- 85.Shahbazi M, et al. Age-and sex-dependent amphetamine self-administration in rats. Psychopharmacology (Berl) 2008;196(1):71–81. doi: 10.1007/s00213-007-0933-6. [DOI] [PubMed] [Google Scholar]
- 86.Lin E-JD, et al. Environmental enrichment exerts sex-specific effects on emotionality in C57BL/6 J mice. Behav Brain Res. 2011;216(1):349–357. doi: 10.1016/j.bbr.2010.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Sterlemann V, et al. Long-term behavioral and neuroendocrine alterations following chronic social stress in mice: implications for stress-related disorders. Horm Behav. 2008;53(2):386–394. doi: 10.1016/j.yhbeh.2007.11.001. [DOI] [PubMed] [Google Scholar]
- 88.McHugh RK, Hearon BA, Otto MW. Cognitive behavioral therapy for substance use disorders. Psychiatr Clin North Am. 2010;33(3):511–525. doi: 10.1016/j.psc.2010.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Krupnick J (2009) Computer-assisted delivery of cognitive-behavioral therapy for addiction. Year Book of Psychiatry and Applied Mental Health, 72–73
- 90.Anton RF, et al. Combined pharmacotherapies and behavioral interventions for alcohol dependence: the COMBINE study: a randomized controlled trial. Jama. 2006;295(17):2003–2017. doi: 10.1001/jama.295.17.2003. [DOI] [PubMed] [Google Scholar]
- 91.Carroll KM (2003) Integrating psychotherapy and pharmacotherapy in substance abuse treatment. In: Rotgers F, Morgenstern, J, Walters ST (eds) Treating Substance Abuse: Theory and Technique. Guilford Press, New York, pp 314–342
- 92.Volkow ND, Skolnick P. New medications for substance use disorders: challenges and opportunities. Neuropsychopharmacology. 2012;37(1):290–292. doi: 10.1038/npp.2011.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Shorter D, Kosten TR. Novel pharmacotherapeutic treatments for cocaine addiction. BMC Med. 2011;9(1):119. doi: 10.1186/1741-7015-9-119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Zheng F, Zhan C-G. Recent progress in protein drug design and discovery with a focus on novel approaches to the development of anticocaine medications. Future Med Chem. 2009;1(3):515–528. doi: 10.4155/fmc.09.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Zheng F, Zhan C-G. Enzyme-therapy approaches for the treatment of drug overdose and addiction. Future Med Chem. 2011;3(1):9–13. doi: 10.4155/fmc.10.275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Kinsey BM, Jackson DC, Orson FM. Anti-drug vaccines to treat substance abuse. Immunol Cell Biol. 2009;87(4):309–314. doi: 10.1038/icb.2009.17. [DOI] [PubMed] [Google Scholar]
- 97.Zhan C-G. Novel pharmacological approaches to treatment of drug overdose and addiction. Expert Rev Clin Pharmacol. 2009;2(1):1. doi: 10.1586/17512433.2.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Collins GT, et al. Cocaine esterase prevents cocaine-induced toxicity and the ongoing intravenous self-administration of cocaine in rats. J Pharmacol Exp Ther. 2009;331(2):445–455. doi: 10.1124/jpet.108.150029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Brim RL, et al. A thermally stable form of bacterial cocaine esterase: a potential therapeutic agent for treatment of cocaine abuse. Mol Pharmacol. 2010;77(4):593–600. doi: 10.1124/mol.109.060806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Montoya ID (2015) Biologics (vaccines, antibodies, enzymes) to treat drug addictions. In: Nady elGuebaly GC, Galanter M (eds) Textbook of Addiction Treatment: International Perspectives. Springer, New York, pp 683–692
- 101.Bonoiu AC, et al. Nanotechnology approach for drug addiction therapy: gene silencing using delivery of gold nanorod-siRNA nanoplex in dopaminergic neurons. Proc Natl Acad Sci. 2009;106(14):5546–5550. doi: 10.1073/pnas.0901715106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Fischer A, et al. Targeting the correct HDAC (s) to treat cognitive disorders. Trends Pharmacol Sci. 2010;31(12):605–617. doi: 10.1016/j.tips.2010.09.003. [DOI] [PubMed] [Google Scholar]
- 103.Kazantsev AG, Thompson LM. Therapeutic application of histone deacetylase inhibitors for central nervous system disorders. Nat Rev Drug Discovery. 2008;7(10):854–868. doi: 10.1038/nrd2681. [DOI] [PubMed] [Google Scholar]
- 104.Cadet JL. Epigenetics of stress, addiction, and resilience: therapeutic implications. Mol Neurobiol. 2016;53(1):545–560. doi: 10.1007/s12035-014-9040-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Malvaez M, et al. CBP in the nucleus accumbens regulates cocaine-induced histone acetylation and is critical for cocaine-associated behaviors. J Neurosci. 2011;31(47):16941–16948. doi: 10.1523/JNEUROSCI.2747-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Madsen HB, et al. CREB1 and CREB-binding protein in striatal medium spiny neurons regulate behavioural responses to psychostimulants. Psychopharmacology (Berl) 2012;219(3):699–713. doi: 10.1007/s00213-011-2406-1. [DOI] [PubMed] [Google Scholar]
- 107.Tsankova NM, et al. Sustained hippocampal chromatin regulation in a mouse model of depression and antidepressant action. Nat Neurosci. 2006;9(4):519–525. doi: 10.1038/nn1659. [DOI] [PubMed] [Google Scholar]
- 108.Levenson JM, et al. Regulation of histone acetylation during memory formation in the hippocampus. J Biol Chem. 2004;279(39):40545–40559. doi: 10.1074/jbc.M402229200. [DOI] [PubMed] [Google Scholar]
- 109.Renthal W, Nestler EJ (2009) Histone acetylation in drug addiction. In Seminars in cell & developmental biology. Academic Press 20(4):387–394 [DOI] [PMC free article] [PubMed]
- 110.Lewis CR, Olive MF. Epigenetic modifications as novel targets for drug addiction. Front CNS Drug Discov. 2013;17:26–42. doi: 10.2174/9781608057672113020004. [DOI] [Google Scholar]
- 111.Johnstone RW. Histone-deacetylase inhibitors: novel drugs for the treatment of cancer. Nat Rev Drug Discov. 2002;1(4):287–299. doi: 10.1038/nrd772. [DOI] [PubMed] [Google Scholar]
- 112.Minucci S, Pelicci PG. Histone deacetylase inhibitors and the promise of epigenetic (and more) treatments for cancer. Nat Rev Cancer. 2006;6(1):38–51. doi: 10.1038/nrc1779. [DOI] [PubMed] [Google Scholar]
- 113.McQuown SC, Wood MA. Epigenetic regulation in substance use disorders. Curr Psychiatry Rep. 2010;12(2):145–153. doi: 10.1007/s11920-010-0099-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Vecsey CG, et al. Histone deacetylase inhibitors enhance memory and synaptic plasticity via CREB: CBP-dependent transcriptional activation. J Neurosci. 2007;27(23):6128–6140. doi: 10.1523/JNEUROSCI.0296-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Stefanko DP, et al. Modulation of long-term memory for object recognition via HDAC inhibition. Proc Natl Acad Sci. 2009;106(23):9447–9452. doi: 10.1073/pnas.0903964106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Malvaez M, et al. Modulation of chromatin modification facilitates extinction of cocaine-induced conditioned place preference. Biol Psychiatry. 2010;67(1):36–43. doi: 10.1016/j.biopsych.2009.07.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Sun J, et al. The effects of sodium butyrate, an inhibitor of histone deacetylase, on the cocaine-and sucrose-maintained self-administration in rats. Neurosci Lett. 2008;441(1):72–76. doi: 10.1016/j.neulet.2008.05.010. [DOI] [PubMed] [Google Scholar]
- 118.Romieu P, et al. Histone deacetylase inhibitors decrease cocaine but not sucrose self-administration in rats. J Neurosci. 2008;28(38):9342–9348. doi: 10.1523/JNEUROSCI.0379-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.D’Addario C, et al. Ethanol induces epigenetic modulation of prodynorphin and pronociceptin gene expression in the rat amygdala complex. J Mol Neurosci. 2013;49(2):312–319. doi: 10.1007/s12031-012-9829-y. [DOI] [PubMed] [Google Scholar]
- 120.Arora DS, et al. Hyposensitivity to gamma-aminobutyric acid in the ventral tegmental area during alcohol withdrawal: reversal by histone deacetylase inhibitors. Neuropsychopharmacology. 2013;38(9):1674–1684. doi: 10.1038/npp.2013.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Botia B, et al. Expression of ethanol-induced behavioral sensitization is associated with alteration of chromatin remodeling in mice. PloS one. 2012;7(10):e47527. doi: 10.1371/journal.pone.0047527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Kumar A, et al. Chromatin remodeling is a key mechanism underlying cocaine-induced plasticity in striatum. Neuron. 2005;48(2):303–314. doi: 10.1016/j.neuron.2005.09.023. [DOI] [PubMed] [Google Scholar]
- 123.Wang L, et al. Chronic cocaine-induced H3 acetylation and transcriptional activation of CaMKII[alpha] in the nucleus accumbens is critical for motivation for drug reinforcement. Neuropsychopharmacology. 2009;35(4):913–928. doi: 10.1038/npp.2009.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Schmidt HD, et al. Increased brain-derived neurotrophic factor (BDNF) expression in the ventral tegmental area during cocaine abstinence is associated with increased histone acetylation at BDNF exon I-containing promoters. J Neurochem. 2012;120(2):202–209. doi: 10.1111/j.1471-4159.2011.07571.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Kennedy PJ, et al. Class I HDAC inhibition blocks cocaine-induced plasticity by targeted changes in histone methylation. Nat Neurosci. 2013;16(4):434–440. doi: 10.1038/nn.3354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Montoya ID, Vocci F. Novel medications to treat addictive disorders. Curr Psychiatry Rep. 2008;10(5):392–398. doi: 10.1007/s11920-008-0063-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Jorenby DE, et al. A controlled trial of sustained-release bupropion, a nicotine patch, or both for smoking cessation. N Engl J Med. 1999;340(9):685–691. doi: 10.1056/NEJM199903043400903. [DOI] [PubMed] [Google Scholar]
- 128.Johnson BA, et al. Dose-ranging kinetics and behavioral pharmacology of naltrexone and acamprosate, both alone and combined, in alcohol-dependent subjects. J Clin Psychopharmacol. 2003;23(3):281–293. doi: 10.1097/01.jcp.0000084029.22282.bb. [DOI] [PubMed] [Google Scholar]
- 129.Substance Abuse and Mental Health Services Administration (SAMHSA) (2009) Incorporating alcohol pharmacotherapies into medical practice. Treatment improvement protocol (TIP) series 49. HHS publication no. (SMA) 12–4380 [PubMed]
- 130.Johnson BA. Update on neuropharmacological treatments for alcoholism: Scientific basis and clinical findings. Biochem Pharmacol. 2008;75(1):34–56. doi: 10.1016/j.bcp.2007.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Fishman MJ, et al. Treatment of opioid dependence in adolescents and young adults with extended release naltrexone: preliminary case-series and feasibility. Addiction. 2010;105(9):1669–1676. doi: 10.1111/j.1360-0443.2010.03015.x. [DOI] [PubMed] [Google Scholar]
- 132.Marsch LA, et al. Predictors of outcome in LAAM, buprenorphine, and methadone treatment for opioid dependence. Exp Clin Psychopharmacol. 2005;13(4):293. doi: 10.1037/1064-1297.13.4.293. [DOI] [PubMed] [Google Scholar]
- 133.Leung CM. Handbook of clinical alcoholism treatment. Hong Kong J Psychiatry. 2003;13(3):31–32. [Google Scholar]
- 134.Heilig M, Egli M. Pharmacological treatment of alcohol dependence: target symptoms and target mechanisms. Pharmacol Ther. 2006;111(3):855–876. doi: 10.1016/j.pharmthera.2006.02.001. [DOI] [PubMed] [Google Scholar]
- 135.Le Foll B, Goldberg SR. Cannabinoid CB1 receptor antagonists as promising new medications for drug dependence. J Pharmacol Exp Ther. 2005;312(3):875–883. doi: 10.1124/jpet.104.077974. [DOI] [PubMed] [Google Scholar]
- 136.Karila L, et al. Pharmacological approaches to methamphetamine dependence: a focused review. Br J Clin Pharmacol. 2010;69(6):578–592. doi: 10.1111/j.1365-2125.2010.03639.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Berk M, et al. The promise of N-acetylcysteine in neuropsychiatry. Trends Pharmacol Sci. 2013;34(3):167–177. doi: 10.1016/j.tips.2013.01.001. [DOI] [PubMed] [Google Scholar]
- 138.Murray J, Lacoste J, Belin D (2012) N-Acetylcysteine as a treatment for addiction. In: Belin D (ed) Addictions: from pathophysiology to treatment. InTech, Rijeka, pp 335–380
- 139.O’Brien CP. Anticraving medications for relapse prevention: a possible new class of psychoactive medications. Am J Psychiatry. 2005;162(8):1423–1431. doi: 10.1176/appi.ajp.162.8.1423. [DOI] [PubMed] [Google Scholar]
- 140.Leavitt S (2002) Evidence for the efficacy of naltrexone in the treatment of alcohol dependence (alcoholism). In: Addiction Treatment Forum: Naltrexone Clinical Update, pp 1–8
- 141.Sinha R, et al. Sex steroid hormones, stress response, and drug craving in cocaine-dependent women: implications for relapse susceptibility. Exp Clin Psychopharmacol. 2007;15(5):445. doi: 10.1037/1064-1297.15.5.445. [DOI] [PubMed] [Google Scholar]
- 142.Walsh SL, et al. Effects of naltrexone on response to intravenous cocaine, hydromorphone and their combination in humans. J Pharmacol Exp Ther. 1996;279(2):524–538. [PubMed] [Google Scholar]
- 143.Health, U.D.o. and H. Services, Substance Abuse and Mental Health Services Administration (2014) National registry of evidence-based programs and practices (NREPP), 2013
- 144.Leshner AI. Addiction is a brain disease, and it matters. Science. 1997;278(5335):45–47. doi: 10.1126/science.278.5335.45. [DOI] [PubMed] [Google Scholar]