Abstract
To sustain the bio-energetic demands of growth, proliferation, and effector functions, the metabolism of immune cells changes dramatically in response to immunologic stimuli. In this review, I focus on B cell metabolism, especially regarding the production of intestinal IgA antibody. Accumulating evidence has implicated not only host-derived factors (e.g., cytokines) but also gut environmental factors, including the possible involvement of commensal bacteria and diet, in the control of B cell metabolism during intestinal IgA antibody production. These findings yield new insights into the regulation of immunosurveillance and homeostasis in the gut.
Keywords: B cell, Immunometabolism, IgA antibody, Lipid, Commensal bacteria, Glycolysis, TCA cycle, Vitamin B1, Mucosal vaccine, Signaling
Introduction
The metabolic processes in immune cells and their effects on overall immune regulation, including immune diseases, have become the focus of intense investigation in the emerging field of immunometabolism [1, 2]. The core function of metabolic pathways is the synthesis or degradation of sugars, fatty acids, nucleic acids, or proteins coupled with the consumption or generation of ATP by oxidative phosphorylation or glycolysis. Signals associated with infection, inflammation, and other biologic states are mediated through the pattern recognition receptors, cytokine receptors, and antigen receptors (e.g., T and B cell receptors) of immune cells, reprogramming these cells and leading to changes in their function, growth, proliferation, and metabolism [3–5]. By providing energy and precursor molecules, these metabolic changes support biosynthetic activities associated with immune cell activation, such as the production of effector molecules, including cytokines, chemokines, and antibodies. However, recent studies demonstrate that, in addition to their actions in energy generation and general biosynthesis, metabolic processes play critical roles in controlling the specific functions and differentiation of immune cells [3–5]. Therefore, the metabolic pathways of immune cells might be manipulated to alter their immune functions (e.g., T cell differentiation) and thus provide immunotherapy by modulating activation and differentiation of immune cells [6, 7].
The regulation of immunometabolism in T cells is well studied [8, 9]. In general, naïve and memory T cells use the TCA (tricarboxylic acid) cycle and fatty acid oxidation (FAO) for energy production. Upon activation, the metabolic machinery of T cells typically is shifted toward glycolysis [10]. This shift is favorable for obtaining the intermediates necessary for building the components required for cell proliferation (e.g., membranes, proteins, and nucleotides). In response to the cytokine environment, activated T cells then differentiate into various subsets, including Th1, Th2, Th17, and regulatory T (Treg) cells and cytotoxic T lymphocytes (CTL). Several lines of evidence have shown that energy metabolism varies markedly among T cell subsets [8]. For instance, like naïve and memory T cells, Treg cells show high levels of FAO rates while the other effector T cells such as Th1, Th2, Th17 cells and CTL preferentially use aerobic glycolysis pathway [11]. In addition, a recent study showed that follicular helper T cells, another important effector cells for antibody production, exhibited less glycolysis [12]. Thus, distinct metabolic requirement is defined by the consequences of cell differentiation.
Macrophages are another example of immune cells with subset-specific metabolism which is also associated with the cell differentiation (reviewed in Ref. [4]). Currently, macrophages are divided into at least two subpopulations: M1 macrophages, which have a pro-inflammatory phenotype, and M2 macrophages, which are anti-inflammatory [13]. The metabolic properties of each of these subsets differ greatly. Polarization into M1 macrophages is accompanied by a rapid shift toward aerobic glycolysis, whereas M2 macrophages preferentially use FAO-mediated oxidative phosphorylation [14].
As in T cells, the rates of glycolysis increase in activated B cells in response to various stimuli in vitro [15–17]; however, little is known about the metabolic changes that occur during the differentiation of B cells into antibody-producing plasma cells (PCs), especially in vivo. To address this issue, we exploited the unique environment of the intestine, where immunologic stimuli associated with environmental factors such as commensal microorganisms and diet induce the spontaneous differentiation of B cells into immunoglobulin A (IgA)-producing PCs (IgA PCs) [18]. These features allowed us to reveal the metabolic changes during B cell differentiation into antibody-producing PCs in the intestine.
Preferential differentiation of B cells into IgA PCs in the intestine
IgA is the primary antibody type in the intestine, where it protects the host against pathogenic infections [19] and binds to commensal microorganisms to maintain its homeostatic communities [20]. The gut-associated lymphoid tissues (GALTs) are the primary sites for the initiation and induction of intestinal IgA antibody production [21].
Peyer’s patches (PPs) are the predominant GALT. The follicle-associated epithelium overlying PPs contains antigen-sampling M cells for the uptake of antigens and their translocation to underlying regions, where antigen-presenting cells, such as dendritic cells (DCs), reside [22, 23]. In the unique cytokine environment (e.g., IL-4, IL-6, and TGF-β) of the intestine, the immunologic interactions among DCs, T cells, and B cells activate B cells for their commitment into IgA+ B cells [21]. Thus, the B cells in the PPs are naïve B cells predominantly, with a minor component of activated IgA+ B cells (Fig. 1).
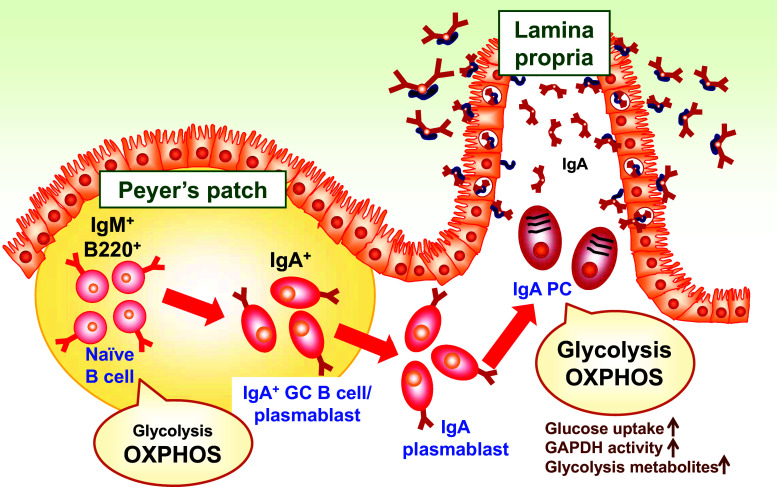
Fig. 1.
Shift of energy metabolism during the differentiation of B cells into IgA-producing plasma cells. Naïve B cells, the predominant B cell population in Peyer’s patches, obtain biosynthetic energy from oxidative phosphorylation (OXPHOS) through the TCA cycle. After the activation of B cells and their differentiation into germinal center (GC) B cells and IgA+ plasmablasts, they traffic into the intestinal lamina propria, where they ultimately differentiate into IgA-producing plasma cells (PCs). IgA PCs utilize both glycolysis and OXPHOS for energy generation. This metabolic shift is associated with changes in glucose uptake, GAPDH activity, and glycolytic metabolites
After their differentiation in the PPs, IgA+ B cells begin to express receptors for sphingosine 1-phosphate, chemokines (e.g., CCR9), and adhesion molecules (e.g., α4β7 integrin), allowing the cells to emigrate from the PPs and subsequently traffic into the intestinal lamina propria (iLP) [24, 25]. Upon their arrival at the iLP, IgA+ B cells further differentiate into IgA PCs under the influence of IL-5, IL-6, IL-10, IL-15, a proliferation-inducing ligand (APRIL), and B cell-activating factor (BAFF) (Fig. 1) [26].
B cells from peritoneum and isolated lymphoid follicle (ILF) are the other sources of intestinal IgA PCs [27, 28]. Unlike B cells in the PPs which produce the IgA antibody against T cell-dependent antigens with the help by DCs and T cells, B cells from peritoneum and ILF uniquely recognize T cell-independent antigens such as polysaccharide and phosphorylcholine [27, 28]. Upon the activation, these B cells exhibit class switching to express IgA and subsequently traffic to the intestine, where they further differentiate into IgA PCs.
Changes of energy metabolism during cell differentiation from naïve B cells into IgA PCs
We performed metabolic analysis using capillary electrophoresis–mass spectrometry, revealing that the energy–metabolite profile of naïve B cells in the PPs differed from that of IgA PCs in the iLP [29]. Specifically, both naïve B cells and IgA PCs exhibited similar levels of TCA cycle metabolites (e.g., citrate and succinate). In contrast, metabolic intermediates of glycolysis (e.g., glucose mono-phosphate and fructose bis-phosphate) were detected preferentially in IgA PCs compared with naïve B cells (Fig. 1). In addition, glucose uptake and the activity of glyceraldehyde 3-phosphate dehydrogenase, a key glycolytic enzyme, were consistently higher in IgA PCs than in naïve B cells [29]. Therefore, unlike naïve B cells in the PPs, IgA PCs in the iLP appear to use glycolysis preferentially as an energy metabolic pathway (Fig. 1).
Consistently, various in vitro studies demonstrated that stimulation of B cells with various stimuli in vitro induced the increase of glycolysis activity with the changes in the proliferation, cell size, survival, gene expression and antibody production [16, 30–32]. It was reported that glucose-initiated energy generation is essential for antibody production [17]. Indeed, impairment of glucose uptake by B cell-specific deletion of GLUT1 results in decreased antibody production [17]. Therefore, it is likely that the metabolic changes are not simply a consequence of cell activation but is also essential for the cell differentiation-associated immunological and biological processes including antibody production. Additionally, as mentioned above, these activation signals, especially in IgA+ B cells in the intestine, associate with the increased expression of CCR9 and α4β7 integrin, which determine their trafficking into the intestine [24, 25]. So, it is possible that the metabolic changes are related with B cell migration, which is a subject of future study.
The shift to glycolysis-mediated energy metabolism likely is useful for generating metabolic intermediates for cell activation and growth. For instance, the glucose mono-phosphate and 3-phosphoglycerate generated through glycolysis are used in the pentose phosphate and serine biosynthetic pathways for nucleotide and amino acid synthesis, respectively [33]. In addition, glucose is metabolized into pyruvate and then acetyl-CoA, to join the TCA cycle by condensing with oxaloacetate to form citrate. In addition to its use in the TCA cycle, citrate can be exported into the cytoplasm, where it is converted to acetyl-CoA and used in fatty acid synthesis [33], which is required for B cell differentiation [34]. Hence, the shift to glycolysis in IgA PCs is considered to support the generation and production of IgA antibody without requiring the consumption of preexisting amino acids and fatty acids.
Molecular mechanisms underlying the energy metabolic changes in B cells
Several key molecules involved in the regulation of immunometabolism in T cells have been identified [35]. Upon the activation-induced shift to aerobic glycolysis, T cells express glucose transporter Glut1 on the cell surface to facilitate glucose uptake [10]. Similarly, B cells stimulated with LPS or through the B cell receptor begin to express Glut1, which is an essential step for antibody production [17]. As mentioned above, B cell-specific deletion of Glut1 reduced B cell numbers and impaired antibody production [17].
In both T and B cells, Glut1 expression is dependent on the activation of the kinase Akt by phosphatidylinositol 3-kinase (PI3K) [10, 15]. In addition, PI3K–Akt signaling activates the mechanistic target of rapamycin (mTOR) and supports effector T cell differentiation, growth, and function by enhancing glycolytic metabolism [36]. Whereas Th1, Th2, and Th17 cells engage glycolysis through mTOR signaling, Treg cells depend on FAO [11]. In agreement with these findings, rapamycin-induced suppression of mTOR promoted the generation of Treg cells [37, 38]. Of the two predominant mTOR complexes, termed mTORC1 and mTORC2, mTORC1 is required for Th1 and Th17 cells, whereas Th2 cells use mTORC2 [39]. Indeed, impairment of mTORC1- and mTORC2-mediated signaling led to defects in the development of Th1/Th17 and Th2 cells, respectively [39]. Like T cells, the complete deletion of mTOR in B cells resulted in the suppression of germinal center responses, including class switching [40]. In addition, B cell-specific deletion of the tuberous sclerosis complex, a negative regulator mTORC1, led to the activation of mTORC1, thus increasing PC differentiation and antibody secretion [41].
Requirement of vitamin B1 for the maintenance of intestinal IgA
Diet is well known to affect energy metabolism and also immune responses including intestinal IgA production [42, 43]. Among various dietary components, vitamins are essential factors for immune responses [44]. For example, it is well known that vitamin A is a key factor in the control of lymphocyte trafficking into the intestine by inducing the expression of α4β7 integrin and chemokine receptor CCR9 on activated B and T cells, which is mediated by conversion of vitamin A to retinotic via retinaldehyde dehydrogenase expressed in intestinal DC [24, 25]. Another example is vitamin B9 (also known as folate), which is required for the maintenance of Treg cells [45].
We recently found that vitamin B1 (thiamine) is also important for the intestinal IgA responses, which was linked to energy metabolism. Vitamin B1 plays a pivotal role in energy generation by acting as a cofactor of pyruvate dehydrogenase and α-ketoglutarate dehydrogenase, which are essential enzymes in the TCA cycle [46]. Because they are unable to synthesize vitamin B1, mammals must obtain this cofactor from their diet [47]. We confirmed that the TCA cycle is selectively impaired in mice that received a diet deficient in vitamin B1, which is coincident with decreased numbers of naïve B cells and consequent atrophy of PPs (Fig. 2) [29]. In contrast, IgA PCs in the iLP of these mice were unchanged in both frequency and absolute cell number (Fig. 2) [29]. In agreement with these findings, the expression level of THTR1, a transporter of vitamin B1, was higher in the naïve B cells than in the IgA PCs of mice, and in vitro treatment with a vitamin B1 antagonist decreased the number and viability of purified naïve B cells, whereas the cell number of purified IgA PCs remained unchanged [29]. As mentioned above, IgA PCs use glycolysis for the energy generation (Fig. 1), which could support energy supply in the absence of vitamin B1-dependent energy generation (Fig. 2) [29]. Consistently, glycolytic metabolites (e.g., glucose mono-phosphate and fructose bis-phosphate) and glycolysis-associated enzymes (e.g., glyceraldehyde 3-phosphate dehydrogenase) were preferentially noted in IgA PCs.
Fig. 2.
Relationship between energy metabolism and vitamin B1 dependency during the differentiation of B cells into IgA-producing plasma cells. Naïve B cells in the Peyer’s patches (PP) generate ATP from the amino acid (AA)- or fatty acid (FA)-originated TCA cycle, whereas IgA plasma cells utilize the glycolysis-initiated TCA cycle. Because vitamin B1 deficiency impairs enzymatic activity in the TCA cycle, naïve B cells cannot generate ATP in the absence of vitamin B1 and thus fail to survive and proliferate. In contrast, despite impairment of the TCA cycle, IgA plasma cells remain able to synthesize energy through glycolysis, allowing them to survive in the absence of vitamin B1
As mentioned earlier, intestinal IgA responses against orally administered antigen are initiated in the PPs [21]. Indeed, vitamin B1 dependency during intestinal B cell differentiation influenced antigen-specific IgA responses against oral vaccine; that is, mice fed the vitamin B1-deficient diet during oral immunization showed reduced numbers of naïve B cells and consequently decreased levels of antigen-specific fecal IgA production compared with those of mice that received a complete diet [29]. Taken together, these studies show that vitamin B1 directly affects the survival and proliferation of naïve B cells in the PPs, which are required for efficient IgA antibody responses against oral vaccine.
Similarities and differences in B cell energy metabolism between the systemic and intestinal immune compartments
Intestinal B cells share some metabolic characteristics with their counterparts in systemic immune compartments, such as the spleen. For example, the levels of TCA cycle metabolites were identical between intestinal and splenic B cells [21]. Consistent with this finding, the spleen decreased in size due to the decreased numbers of naïve B cells that resulted when mice received a vitamin B1-deficient diet [21]. These vitamin B1-deficient mice also had decreased serum IgG responses against intraperitoneally immunized antigen [21]. Therefore, the energy metabolism and vitamin B1 dependency of naïve B cells in the spleen appear to be similar to those of naïve B cells in the PPs. However, unlike naïve B cells, splenic PCs had low levels of glycolytic metabolites [21], suggesting that the glycolysis-mediated pathway is a specific phenomenon of intestinal IgA PCs and may reflect features of the unique immunologic environment of the intestine, such as continuous exposure to commensal bacteria and diets.
In addition to the intestine, respiratory tissues provide IgA antibody. For instance, tonsils in human and nasopharynx-associated lymphoid tissue (NALT) in rodents show similar immunological phenotypes to Peyer’s patches as inductive lymphoid tissues for IgA production [48]. Furthermore, infection and inflammation induce the development of inducible bronchus-associated lymphoid tissue (iBALT), which also initiates IgA-mediated immune responses [49]. Since some immunologic phenotypes are different between respiratory and intestinal tissues (e.g., lack of germinal center formation in NALT and less numbers of commensal bacteria) [48], it is interesting to examine whether metabolic phenotypes and associated dependency on vitamin B1 were similar between respiratory and intestinal IgA PCs.
Possible involvement of gut environment in the control of B cell metabolism
Intestinal tissue creates the unique environment for the preferential IgA production. For instance, it was demonstrated that interaction between IgA PCs and the other cells (e.g., DCs, stromal cells, and epithelial cells) is required for the efficient production of intestinal IgA, which is at least partly mediated by APRIL and BAFF [26]. Additionally, other cytokines such as IL-5, IL-6, IL-10, IL-15 and thymic stromal lymphopoietin (TSLP) are also involved in the intestinal IgA production [26]. As mentioned above, stimulation of B cells with these cytokines leads to the changes of energy metabolism, particularly preferential usage of glycolysis [16, 30–32]. Thus, it is likely that unique immunologic gut environment promotes efficient production of intestinal IgA together with preferential usage of glycolysis-initiated energy metabolism.
In addition to the internal factors, commensal bacteria likely affect the induction and function of intestinal IgA [18]. Indeed, germ-free mice have decreased intestinal IgA responses and structurally immature PPs [50]. We previously identified a subset of intestinal IgA PCs that is induced by commensal bacteria, CD11b+ IgA PCs, which show unique characteristics such as vigorous proliferation and the production of high amounts of IgA [51]. Our study showed that they require the lymphoid structure of PP and IL-10, abundant cytokine in the iLP [51], implicating that the phenotype CD11b+ IgA plasma cells may be obtained during terminal differentiation. Given that metabolic changes alter cell proliferation and IgA antibody production, commensal bacteria likely influence the metabolism of intestinal IgA PCs, especially CD11b+ IgA PCs.
Like vitamins, dietary fatty acids influence host immune function and metabolism [43, 52–54]. Indeed, overnutrition due to a high-fat diet leads to the development of inflammation in adipose tissue, which is frequently associated with obesity and atherosclerosis [55]. Recent findings from our laboratory and others suggest that in addition to the quantity of dietary oil, the FA composition of dietary oils is another important factor in various immunologic and inflammatory conditions [43, 52–54]. For example, we reported that intestinal IgA production was increased in mice maintained on a palmitic acid-enriched oil (e.g., palm oil) and palmitic acid directly promoted IgA production from PCs and stimulated cell proliferation through the conversion of palmitic acid into sphingolipids by serine palmitoyltransferase [56]. These activation pathways may be coincident with the changes of energy metabolism that occur in intestinal IgA PCs.
Conclusion
As in other immune cells, the functions of B cells are dependent on their metabolism. The switching of B cell metabolic pathways is controlled not only by host-originated factors associated with cell activation and differentiation but also possibly by gut external environmental factors (e.g., commensal microorganisms and diet); these control mechanisms are especially important for the intestinal IgA antibody production pathway. Given that intestinal IgA antibody is a primary effector molecule in preventing intestinal infection and maintaining an appropriate composition of commensal microorganisms, understanding B cell metabolism in the intestine not only provides fundamental information about B cell biology but also new insights into the development of immunotherapy and mucosal vaccines.
Acknowledgements
The author’s work featured in this review article was supported by grants from MEXT/JSPS KAKENHI Grant Numbers 26293111, 16H01373, and 15H05790; from The Ministry of Health, Labour and Welfare (MHLW) and the Research on Development of New Drugs, the Japan Agency for Medical Research and Development (AMED); the Science and Technology Research Promotion Program for Agriculture, Forestry, Fisheries, and Food Industry; Astellas Foundation for Research on Metabolic Disorders, Terumo Foundation for Life Sciences and Arts and Suzuken Memorial Foundation.
References
- 1.Pearce EL, Pearce EJ. Metabolic pathways in immune cell activation and quiescence. Immunity. 2013;38:633–643. doi: 10.1016/j.immuni.2013.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mathis D, Shoelson SE. Immunometabolism: an emerging frontier. Nat Rev Immunol. 2011;11:81. doi: 10.1038/nri2922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Buck MD, O’Sullivan D, Pearce EL. T cell metabolism drives immunity. J Exp Med. 2015;212:1345–1360. doi: 10.1084/jem.20151159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.O’Neill LA, Pearce EJ. Immunometabolism governs dendritic cell and macrophage function. J Exp Med. 2015;213:15–23. doi: 10.1084/jem.20151570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Aronov M, Tirosh B. Metabolic control of plasma cell differentiation- what we know and what we don’t know. J Clin Immunol. 2016;36(Suppl 1):12–17. doi: 10.1007/s10875-016-0246-9. [DOI] [PubMed] [Google Scholar]
- 6.Chang CH, Pearce EL. Emerging concepts of T cell metabolism as a target of immunotherapy. Nat Immunol. 2016;17:364–368. doi: 10.1038/ni.3415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mockler MB, Conroy MJ, Lysaght J. Targeting T cell immunometabolism for cancer immunotherapy; understanding the impact of the tumor microenvironment. Front Oncol. 2014;4:107. doi: 10.3389/fonc.2014.00107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.MacIver NJ, Michalek RD, Rathmell JC. Metabolic regulation of T lymphocytes. Annu Rev Immunol. 2013;31:259–283. doi: 10.1146/annurev-immunol-032712-095956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Delgoffe GM, Powell JD. Sugar, fat, and protein: new insights into what T cells crave. Curr Opin Immunol. 2015;33:49–54. doi: 10.1016/j.coi.2015.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Frauwirth KA, et al. The CD28 signaling pathway regulates glucose metabolism. Immunity. 2002;16:769–777. doi: 10.1016/S1074-7613(02)00323-0. [DOI] [PubMed] [Google Scholar]
- 11.Michalek RD, et al. Cutting edge: distinct glycolytic and lipid oxidative metabolic programs are essential for effector and regulatory CD4+ T cell subsets. J Immunol. 2011;186:3299–3303. doi: 10.4049/jimmunol.1003613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ray JP, et al. The Interleukin-2-mTORc1 kinase axis defines the signaling, differentiation, and metabolism of T helper 1 and follicular B helper T cells. Immunity. 2015;43:690–702. doi: 10.1016/j.immuni.2015.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Porta C, Riboldi E, Ippolito A, Sica A. Molecular and epigenetic basis of macrophage polarized activation. Semin Immunol. 2015;27:237–248. doi: 10.1016/j.smim.2015.10.003. [DOI] [PubMed] [Google Scholar]
- 14.Jha AK, et al. Network integration of parallel metabolic and transcriptional data reveals metabolic modules that regulate macrophage polarization. Immunity. 2015;42:419–430. doi: 10.1016/j.immuni.2015.02.005. [DOI] [PubMed] [Google Scholar]
- 15.Doughty CA, et al. Antigen receptor-mediated changes in glucose metabolism in B lymphocytes: role of phosphatidylinositol 3-kinase signaling in the glycolytic control of growth. Blood. 2006;107:4458–4465. doi: 10.1182/blood-2005-12-4788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dufort FJ, et al. Cutting edge: IL-4-mediated protection of primary B lymphocytes from apoptosis via Stat6-dependent regulation of glycolytic metabolism. J Immunol. 2007;179:4953–4957. doi: 10.4049/jimmunol.179.8.4953. [DOI] [PubMed] [Google Scholar]
- 17.Caro-Maldonado A, et al. Metabolic reprogramming is required for antibody production that is suppressed in anergic but exaggerated in chronically BAFF-exposed B cells. J Immunol. 2014;192:3626–3636. doi: 10.4049/jimmunol.1302062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gutzeit C, Magri G, Cerutti A. Intestinal IgA production and its role in host-microbe interaction. Immunol Rev. 2014;260:76–85. doi: 10.1111/imr.12189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Brandtzaeg P. Function of mucosa-associated lymphoid tissue in antibody formation. Immunol Investig. 2010;39:303–355. doi: 10.3109/08820131003680369. [DOI] [PubMed] [Google Scholar]
- 20.Macpherson AJ, Koller Y, McCoy KD. The bilateral responsiveness between intestinal microbes and IgA. Trends Immunol. 2015;36:460–470. doi: 10.1016/j.it.2015.06.006. [DOI] [PubMed] [Google Scholar]
- 21.Kunisawa J, Kurashima Y, Kiyono H. Gut-associated lymphoid tissues for the development of oral vaccines. Adv Drug Deliv Rev. 2012;64:523–530. doi: 10.1016/j.addr.2011.07.003. [DOI] [PubMed] [Google Scholar]
- 22.Ohno H. Intestinal M cells. J Biochem. 2016;159:151–160. doi: 10.1093/jb/mvv121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bekiaris V, Persson EK, Agace WW. Intestinal dendritic cells in the regulation of mucosal immunity. Immunol Rev. 2014;260:86–101. doi: 10.1111/imr.12194. [DOI] [PubMed] [Google Scholar]
- 24.Gohda M, et al. Sphingosine 1-phosphate regulates the egress of IgA plasmablasts from Peyer’s patches for intestinal IgA responses. J Immunol. 2008;180:5335–5343. doi: 10.4049/jimmunol.180.8.5335. [DOI] [PubMed] [Google Scholar]
- 25.Mora JR, et al. Generation of gut-homing IgA-secreting B cells by intestinal dendritic cells. Science. 2006;314:1157–1160. doi: 10.1126/science.1132742. [DOI] [PubMed] [Google Scholar]
- 26.Fagarasan S, Kawamoto S, Kanagawa O, Suzuki K. Adaptive immune regulation in the gut: T cell-dependent and T cell-independent IgA synthesis. Annu Rev Immunol. 2010;28:243–273. doi: 10.1146/annurev-immunol-030409-101314. [DOI] [PubMed] [Google Scholar]
- 27.Kunisawa J, et al. Sphingosine 1-phosphate regulates peritoneal B-cell trafficking for subsequent intestinal IgA production. Blood. 2007;109:3749–3756. doi: 10.1182/blood-2006-08-041582. [DOI] [PubMed] [Google Scholar]
- 28.Tsuji M, et al. Requirement for lymphoid tissue-inducer cells in isolated follicle formation and T cell-independent immunoglobulin A generation in the gut. Immunity. 2008;29:261–271. doi: 10.1016/j.immuni.2008.05.014. [DOI] [PubMed] [Google Scholar]
- 29.Kunisawa J, et al. Mode of bioenergetic metabolism during B cell differentiation in the intestine determines the distinct requirement for vitamin B1. Cell Rep. 2015;13:122–131. doi: 10.1016/j.celrep.2015.08.063. [DOI] [PubMed] [Google Scholar]
- 30.Patke A, Mecklenbrauker I, Erdjument-Bromage H, Tempst P, Tarakhovsky A. BAFF controls B cell metabolic fitness through a PKC beta- and Akt-dependent mechanism. J Exp Med. 2006;203:2551–2562. doi: 10.1084/jem.20060990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Otipoby KL, et al. BAFF activates Akt and Erk through BAFF-R in an IKK1-dependent manner in primary mouse B cells. Proc Natl Acad Sci USA. 2008;105:12435–12438. doi: 10.1073/pnas.0805460105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Woodland RT, et al. Multiple signaling pathways promote B lymphocyte stimulator dependent B-cell growth and survival. Blood. 2008;111:750–760. doi: 10.1182/blood-2007-03-077222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Vander Heiden MG, et al. Metabolic pathway alterations that support cell proliferation. Cold Spring Harb Symp Quant Biol. 2011;76:325–334. doi: 10.1101/sqb.2012.76.010900. [DOI] [PubMed] [Google Scholar]
- 34.Dufort FJ, et al. Glucose-dependent de novo lipogenesis in B lymphocytes: a requirement for atp-citrate lyase in lipopolysaccharide-induced differentiation. J Biol Chem. 2014;289:7011–7024. doi: 10.1074/jbc.M114.551051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pollizzi KN, Powell JD. Integrating canonical and metabolic signalling programmes in the regulation of T cell responses. Nat Rev Immunol. 2014;14:435–446. doi: 10.1038/nri3701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Liu C, Chapman NM, Karmaus PW, Zeng H, Chi H (2015) mTOR and metabolic regulation of conventional and regulatory T cells. J Leukoc Biol 97:837–847 [DOI] [PMC free article] [PubMed]
- 37.Kopf H, de la Rosa GM, Howard OM, Chen X. Rapamycin inhibits differentiation of Th17 cells and promotes generation of FoxP3+ T regulatory cells. Int Immunopharmacol. 2007;7:1819–1824. doi: 10.1016/j.intimp.2007.08.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Delgoffe GM, et al. The mTOR kinase differentially regulates effector and regulatory T cell lineage commitment. Immunity. 2009;30:832–844. doi: 10.1016/j.immuni.2009.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Delgoffe GM, et al. The kinase mTOR regulates the differentiation of helper T cells through the selective activation of signaling by mTORC1 and mTORC2. Nat Immunol. 2011;12:295–303. doi: 10.1038/ni.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhang S, et al. B cell-specific deficiencies in mTOR limit humoral immune responses. J Immunol. 2013;191:1692–1703. doi: 10.4049/jimmunol.1201767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Benhamron S, Pattanayak SP, Berger M, Tirosh B. mTOR activation promotes plasma cell differentiation and bypasses XBP-1 for immunoglobulin secretion. Mol Cell Biol. 2015;35:153–166. doi: 10.1128/MCB.01187-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lamichhane A, Kiyono H, Kunisawa J. Nutritional components regulate the gut immune system and its association with intestinal immune disease development. J Gastroenterol Hepatol. 2013;28(Suppl 4):18–24. doi: 10.1111/jgh.12259. [DOI] [PubMed] [Google Scholar]
- 43.Kunisawa J, Kiyono H. Sphingolipids and epoxidized lipid metabolites in the control of gut immunosurveillance and allergy. Front Nutr. 2016;3:3. doi: 10.3389/fnut.2016.00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kunisawa J, Kiyono H. Vitamins mediate immunological homeostasis and diseases at the surface of the body. Endocr Metab Immune Disord Drug Targets. 2015;15:25–30. doi: 10.2174/1871530314666141021114651. [DOI] [PubMed] [Google Scholar]
- 45.Kunisawa J, Kiyono H. Vitamin-mediated regulation of intestinal immunity. Front Immunol. 2013;4:189. doi: 10.3389/fimmu.2013.00189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Frank RA, Leeper FJ, Luisi BF. Structure, mechanism and catalytic duality of thiamine-dependent enzymes. Cell Mol Life Sci. 2007;64:892–905. doi: 10.1007/s00018-007-6423-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Webb ME, Marquet A, Mendel RR, Rebeille F, Smith AG. Elucidating biosynthetic pathways for vitamins and cofactors. Nat Prod Rep. 2007;24:988–1008. doi: 10.1039/b703105j. [DOI] [PubMed] [Google Scholar]
- 48.Kunisawa J, Nochi T, Kiyono H. Immunological commonalities and distinctions between airway and digestive immunity. Trends Immunol. 2008;29:505–513. doi: 10.1016/j.it.2008.07.008. [DOI] [PubMed] [Google Scholar]
- 49.Randall TD. Bronchus-associated lymphoid tissue (BALT) structure and function. Adv Immunol. 2010;107:187–241. doi: 10.1016/B978-0-12-381300-8.00007-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Weinstein PD, Cebra JJ. The preference for switching to IgA expression by Peyer’s patch germinal center B cells is likely due to the intrinsic influence of their microenvironment. J Immunol. 1991;147:4126–4135. [PubMed] [Google Scholar]
- 51.Kunisawa J, et al. Microbe-dependent CD11b+ IgA+ plasma cells mediate robust early-phase intestinal IgA responses in mice. Nat Commun. 2013;4:1772. doi: 10.1038/ncomms2718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Galli C, Calder PC. Effects of fat and fatty acid intake on inflammatory and immune responses: a critical review. Ann Nutr Metab. 2009;55:123–139. doi: 10.1159/000228999. [DOI] [PubMed] [Google Scholar]
- 53.Margioris AN. Fatty acids and postprandial inflammation. Curr Opin Clin Nutr Metab Care. 2009;12:129–137. doi: 10.1097/MCO.0b013e3283232a11. [DOI] [PubMed] [Google Scholar]
- 54.Arita M. Mediator lipidomics in acute inflammation and resolution. J Biochem. 2012;152:313–319. doi: 10.1093/jb/mvs092. [DOI] [PubMed] [Google Scholar]
- 55.Jin C, Flavell RA. Innate sensors of pathogen and stress: linking inflammation to obesity. J Allergy Clin Immunol. 2013;132:287–294. doi: 10.1016/j.jaci.2013.06.022. [DOI] [PubMed] [Google Scholar]
- 56.Kunisawa J, et al. Regulation of intestinal IgA responses by dietary palmitic acid and its metabolism. J Immunol. 2014;193:1666–1671. doi: 10.4049/jimmunol.1302944. [DOI] [PubMed] [Google Scholar]