Abstract
While the biological effects of high-dose-ionizing radiation on human health are well characterized, the consequences of low-dose radiation exposure remain poorly defined, even though they are of major importance for radiological protection. Lymphocytes are very radiosensitive, and radiation-induced health effects may result from immune cell loss and/or immune system impairment. To decipher the mechanisms of effects of low doses, we analyzed the modulation of the T-cell receptor gene repertoire in mice exposed to a single low (0.1 Gy) or high (1 Gy) dose of radiation. High-throughput T-cell receptor gene profiling was used to visualize T-lymphocyte dynamics over time in control and irradiated mice. Radiation exposure induces “aging-like” effects on the T-cell receptor gene repertoire, detectable as early as 1 month post-exposure and for at least 6 months. Surprisingly, these effects are more pronounced in animals exposed to 0.1 Gy than to 1 Gy, where partial correction occurs over time. Importantly, we found that low-dose radiation effects are partially due to the hematopoietic stem cell impairment. Collectively, our findings show that acute low-dose radiation exposure specifically results in long-term alterations of the T-lymphocyte repertoire.
Electronic supplementary material
The online version of this article (doi:10.1007/s00018-017-2581-2) contains supplementary material, which is available to authorized users.
Keywords: Low-dose radiation, Immune system, T cell receptor, Lymphocytes, Hematopoietic stem cells
Introduction
Lymphocytes are extremely sensitive to the toxic effects of radiation exposure [1]. T lymphocytes play a central role in the immune defenses by their ability to recognize antigens (Ags) derived from micro-organisms or transformed cells via their clonogenic antigenic αβ T-cell receptor (TR) [2, 3]. A decrease in TR repertoire diversity and/or T-lymphocyte activation may contribute to the increase in recurrent infections and in non-infectious chronic diseases such as cardiovascular diseases and neoplasia observed in the elderly [3–6]. Cardiovascular diseases and cancer are also well-recognized detrimental consequences of high-dose-ionizing radiation (IR) exposure [7, 8]. Acute myeloid leukaemia (AML) is the predominant radiation-associated leukaemia form, accounting for ~80% of excess leukaemia cases in Japanese A-bombs survivors [9]. In mice, around 20% of CBA/Ca mice exposed to a single 3 Gy IR dose develop AML [10] with characteristics similar to human AML [11, 12]. In contrast, uncertainties remain on the health risks of low-dose and low dose rate radiation [13] which are particularly relevant for nuclear workers. Furthermore, in light of the increase in medical radiation exposure [14], low-dose radiation health risk is also becoming increasingly important for the general population. The biological effects of low-dose exposures are, therefore, of major importance in the field of radiological protection.
The influence of high-dose IR on the different peripheral blood αβ+ T-lymphocyte sub-populations has been observed in A bomb survivors [15–18], workers chronically exposed to γ-radiation [19, 20], and after accidental protracted irradiation [21], but the effects of low radiation (<100 mGy) are not so well established in human and mice [19, 22–24]. A transient increase in splenic effector and regulatory T cells was observed 24 h after exposure to 10 mGy, but not to other low doses such as 50 and 100 mGy, and all populations were decreased 6 days later [25]. Continuous exposure of lymphoma-prone SJL mice at very low dose rate only resulted in minor sporadic changes, indicating either marginal effects or a recovery/adaptation of the immune system in this setting [26]. Thus, radiation exposure across a wide range of acute or protracted doses durably affects the T-lymphocyte compartment in human and mice. Whether these changes at the cellular level result in changes in the diversity of the expressed αβTR repertoire has not yet been addressed.
TR chain genes are assembled in T-lymphocyte progenitors from discrete V, D, and J genes located in distinct genetic loci. The modification of V, D, and J gene ends by random nucleotide deletion and/or addition during V(D)J recombination [27] results in the creation of a unique rearranged TR gene in each locus. Because of this randomness, only one out of three rearrangements is productive, i.e., maintains an open reading frame between conserved amino-acids in the V and J genes. In addition, the processing of V, D, and J gene ends sometimes results in the creation of a stop codon in V(D)J joints where the open reading frame is maintained, precluding expression of a protein from these rearrangements. Thus, V(D)J recombination generates both functional (i.e., that can code for a functional protein that can be expressed onto the cell surface) and non-functional (or non-productive, i.e., that cannot code for a functional protein) TR genes in developing lymphocytes. Only cells able to express a functional TR gene receive the survival signals required to complete their maturation in the thymus [28].
TRB genes, coding for the TRβ chain, are sequentially rearranged on the two homolog chromosomes in pro-T cells. If the rearrangement on the first chromosome is productive, expression of the TRβ chain represses TRB gene recombination on the second chromosome and induces pro-T-cell survival, proliferation, and differentiation to the pre-T-cell stage [29]. Pro-T cells with non-productive TRB genes can be rescued from cell death by rearranging their second TRB allele. If it is productive, differentiation resumes. Pre-T cells then rearrange their TRA genes, eventually express an αβTR and differentiate into mature αβ+ CD4+ or αβ+ CD8+ thymocytes, which are exported into the periphery to become naïve CD4+ helper/regulatory and CD8+ cytotoxic T lymphocytes, respectively [2, 29]. Part of these T lymphocytes have one of their TRB loci rearranged productively and the other in germ-line configuration (TRB+/GL cells), while the others have rearranged their TRB genes on both chromosomes: the first one non-productively and the second one productively (TRB−/+ cells).
In the periphery, naïve T lymphocytes survive for a few weeks [30] before eventually being activated by TR-mediated Ag stimulation, through recognition by the complementary determining region 3 (CDR3) of the TRα and TRβ chains, or after non-standard interactions with super-antigens able to bind to certain TR Vβ domains independently of the CDR3. T-lymphocyte activation results in the intensive clonal proliferation of the antigen-specific lymphocyte(s), followed by a contraction of this population due to apoptosis, which spares only of a few antigen-experienced memory cells. The net outcome is that a few memory T cells are generated for each antigen-specific T lymphocyte that did encounter its cognate antigen. The peripheral T-cell repertoire, therefore, results from (1) de novo generation of T lymphocytes in the thymus from hematopoietic stem cells (HSC) derived progenitors, (2) homeostatic proliferation and/or death of naïve T cells in the periphery, and (3) T-lymphocyte clonal amplification resulting from TR-mediated activation [3, 31]. Changes in the representation of the different T-cell sub-populations after IR exposure may result from IR effects on T-cell differentiation, homeostasis, or TR-induced activation in response to new Ags, or super-Ags following irradiation, or from a combination of these factors. To better understand the long-term effects of low- and high-dose IR exposure on T lymphocytes, we analyzed the peripheral TR repertoire in mice exposed to a single low (0.1 Gy) or high (1 Gy) total body irradiation (TBI).
Materials and methods
Mice
Sixteen-week-old CBA/Ca male mice were either sham irradiated or exposed to 0.1 or 1 Gy at a dose rate of 0.5 Gy/min at room temperature with an A.G.O. HS X-ray system (Aldermaston, Reading, UK). The X-ray generator was set to an output of 13 mA, running at 250 V, constant potential, with a Cu/Al filter producing a beam of 1.2 mm HVL Cu to provide a dose rate of 0.5 Gy/min. All animal procedures conformed to the United Kingdom Animals (Scientific Procedures) Act 1986, Amendment Regulations 2012. Experimental protocols were approved by the Home Office and institutional animal welfare and ethical review body.
Long-term transplantation assay
Ten-week-old CBA/HmCherry male mice were sham irradiated or exposed to 0.01 Gy at a dose rate of 5 mGy/min or to 0.1, 1, 3, or 7 Gy as above. Seven days later, their femurs, tibias, iliac crests, and spine were removed and crushed, and the hematopoietic stem and progenitor cells (HSPC) were purified by immunomagnetic isolation with the Lineage− cells EasySep kit (Stem Cell Technologies, France) using manufacturer’s protocol. One million Lin− HSPC cells resuspended in 150 μL IMDM (Sigma, United Kingdom) were transplanted via tail-vein injection into un-irradiated 10-week-old immunodeficient NOD scid gamma (NSG) mice housed in containment isolators and habituated for 2 weeks prior to experimental use as described [32]. Mice were provided with sterile water and food ad libitum, and subjected to a 12-h light/12-h dark cycle.
Long-term transplantation success was assessed by analyzing donor chimerism 6-m post-transplantation to study the long-term fate of exogenous hematopoietic stem cells (HSC) and their progeny. Most transplanted NSG mice showed myeloid and lymphoid cell chimerism (data not shown). The blood from those showing the highest percentage of T lymphocytes (11–15% for mice transplanted with control HSC, 4–13% and 3.5–10% for mice transplanted with HSC exposed to 0.01 Gy and 0.1 mGy, respectively), only derived from donor HSCs, was used for TRB sequencing. Only a very low number of unique rearranged TRB gene sequences could be obtained from one of the NSG mice reconstituted with 100-mGy-exposed HSC, in which T lymphocytes represented only 3.5% of the leukocytes. These mice were identified as an outlier according to Hubert and Vandervieren test [33] and were rejected from further analysis.
DNA preparation and TRB gene sequencing
Blood DNA was prepared using the DNeasy® blood and tissue kit (Qiagen, Hilden, Germany) from samples collected post-mortem 1, 3, or 6 months (m) post-exposure, and sent to Adaptive Biotechnologies (Seattle, USA), where rearranged TRB genes were amplified and sequenced using the ImmunoSeq Assay (Adaptive Biotechnologies). TRB sequences were automatically annotated according to (1) their status (in-frame, out-of-frame, and having stop codon), (2) identification of their V, D, and J gene segments, and (3) their CDR3 sequence at the VDJ junction. IMGT® [34] nomenclature was used to refer to V, D, and J genes and their functionality. TRB sequence status and CDR3 location were checked. Sequences with unresolved assignments to V or J genes were identified and filtered out. To strengthen statistical inference, only sequences with functional V and J genes were considered.
Data preprocessing
The skewness of sequence distributions was evaluated using medcouple statistics for each endpoint. Hubert and Vandervieren criterion was applied to detect outlying in shape sequence distributions [33]. Statistical analysis was performed following pooled analysis design with regard to the dose of irradiation and mouse age. Results were verified by single mouse analysis.
Statistical analysis
Some of the gene segments occur with very low frequency and might even not be observed in every experimental condition. In this latter situation, k-NN algorithm was used to identify genes with the most similar dose- and age-response profile in the process of class merging.
Pielou’s J index was chosen as a measure of sequence diversity [35] to compare several parameters of the TRB repertoire in the different groups of mice:
| 1a |
where S denotes number of classes and H—Basharin’s unbiased estimator of entropy calculated as
| 1b |
where p i denotes the frequency of the ith class and N—total number of sequences in a group.
This approach allows the comparison of large groups of data differing in size. J index value varies between 0 (completely heterogeneous distribution, one class of events dominates all the other classes) and 1 (completely homogeneous distribution, all classes of events are evenly represented). The hypothesis on equality of Pielou’s J indices in two experimental conditions (H 0: J 1 = J 2 against H 1: J 1 ≠ J 2) was verified by modified Hutcheson test [36], where originally analyzed entropy H was substituted by Pielou’s J index:
| 2 |
where J 1 and J 2 are the sample based estimates of Pielou’s indices calculated for two groups under comparison, Var(J k), and k = 1, 2 denotes the variance of the estimator of J k calculated as
| 3 |
where test statistics t follows Student t distribution with noninteger degrees of freedom df:
| 4 |
Bonferroni’s algorithm was used to correct p values for multiple testing [37]. Results with corrected p value smaller than 0.05 were considered statistically significant.
An effect size statistics was calculated to support quantitatively the comparative analysis of J indices. Generalized Cohen’s d statistics [38] in the domain of Pielou’s indices was calculated as
| 5 |
with the pooled standard deviation SD estimated by
| 6 |
The estimates of the variance of Pielou’s index for each group (k = 1, 2) can be found as follows:
| 7 |
where S k denotes the number of classes in group k, p k,i is the frequencies of the ith class in the kth group, H k is the entropy value in the kth group, and N k is the total number of sequences in the kth group.
The above signal analyzing pipeline was applied to comparisons of sequence distribution, functionality status, and V gene distributions. All graphs were generated with the R software.
Results
The distribution of rearranged TRB sequences obtained from the different mice was found to be right skewed in each animal. One mouse exposed to 1 Gy was detected as an outlier [33] and, therefore, removed from further analysis. Erroneous definition of the CDR3 in sequences using the TRBV26 gene was manually corrected. We were left with a database of 16,173,605 sequences in total with 494,401 unique sequences (Table 1). The number of TRB gene sequences obtained for the different experimental groups ranged from 9.5 × 104 to 1.9 × 106 (Table 1). More sequences were obtained from each mouse at 3 and 6 m than in the 1 m group, indicating that more peripheral T-lymphocyte genomes were amplified and sequenced from these blood samples. We analyzed three parameters in these data sets: the number of times each rearranged TRB sequence is found (referred to as Sequence Diversity), reflecting the clonality of the TRB repertoire, the repertoire of TRBV genes used in rearranged TRB genes (V Gene Diversity), and the evolution of the proportion of productive and non-productive TRB rearrangements (Status Diversity). In each mice and group of mice, these parameters were described by calculating their Pielou’s J diversity index, which can then be compared between experimental conditions. Importantly, the value of this index is robust and does not vary against the number of events considered, and, therefore, allows the comparison of TRB repertoires obtained from different numbers of T lymphocytes.
Table 1.
Number of TRB sequences obtained from control and irradiated CBA/Ca mice at the different radiation doses and timepoints
| All mice | Total counts | Unique counts | |||
|---|---|---|---|---|---|
| 16,173,605 | 494,401 | ||||
| Time (months) | Dose (Gy) | Productive | Non-productive | Productive | Non-productive |
| 11,573,626 | 4,599,979 | 344,932 | 149,466 | ||
| 1 | 0 | 514,882 | 227,754 | 9075 | 4230 |
| 1 | 0.1 | 328,254 | 138,377 | 9579 | 2922 |
| 1 | 1 | 220,499 | 95,621 | 5019 | 2136 |
| 3 | 0 | 1,497,500 | 595,408 | 44,112 | 19,285 |
| 3 | 0.1 | 1,931,907 | 750,039 | 58,306 | 25,188 |
| 3 | 1 | 1,809,168 | 733,392 | 54,402 | 23,928 |
| 6 | 0 | 1,845,466 | 724,802 | 63,292 | 27,060 |
| 6 | 0.1 | 1,764,866 | 672,879 | 51,834 | 22,041 |
| 6 | 1 | 1,661,084 | 661,707 | 52,316 | 22,556 |
The numbers of sequences shown in the table were obtained from four (at 1 m) or three (at 3 and 6 m) mice per experimental condition
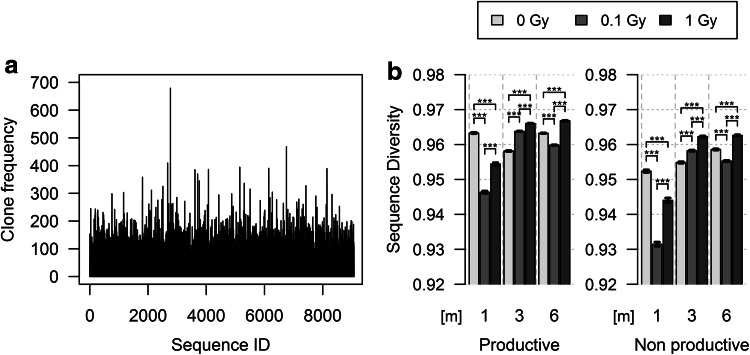
The comparison of Pielou’s index for Sequence Diversity obtained for the different experimental conditions shows the evolution of the repertoire homogeneity over time and after exposure (Fig. 1a; supplemental Fig. 1). Productive and non-productive TRB gene rearrangements were analyzed separately to discriminate aging and radiation effects at the cellular level, which affects similarly the expressed and non-expressed repertoires, from the effects resulting specifically from TR expression/reactivity. Sequence Diversity for productively rearranged TRB genes in control mice was high (0.963, Fig. 1b). The TR repertoire is very homogenously distributed, and stays quite stable over time: J value slightly decreases at 3 m, to raise back at 6 m. The differences between J indices are in the range of small effect size (0.001–0.263), but are, however, statistically significant. They most probably reflect the normal T-cell repertoire dynamics in mice. These small oscillations are amplified following radiation exposure. One month post-exposure, Sequence Diversity value for mice exposed to 0.1 Gy and to 1 Gy is lower than in controls, with the J index value lower for low-dose-exposed animals than for mice exposed to 1 Gy. Thus, the TRB sequence distribution is more heterogeneous in exposed mice than in controls, and this heterogeneity is more important in low-dose-irradiated mice. This decrease is transient. Sequence Diversity raises back later to values in the range of those found in un-irradiated controls, suggesting that the TRB repertoire is as homogeneous in control and in irradiated mice. Thus, the small homeostatic oscillations in Sequence Diversity observed in un-irradiated animals are more pronounced in irradiated animals, but radiation-induced heterogeneity in TRB sequence distribution is attenuated over time. For the non-productive TRB gene repertoire, Sequence Diversity for each of the experimental conditions was slightly lower in all groups. Overall, the modulation of J index value with time and exposure was largely similar to that observed for productive rearrangements, suggesting that the effects of age and radiation on TR gene distribution are independent of TR gene expression. Interestingly, the initial effects of radiation exposure were stronger in mice exposed to low-dose radiation.
Fig. 1.
Distribution of rearranged TRB sequences in control and irradiated mice. a Distribution of productively rearranged TRB genes in un-irradiated control mice at 1 m. The x axis indicates the index of each sequence, and the y axis indicates the number of times that it was found (clonality). The profiles for all experimental groups are shown in Supplemental Fig. 1. b Representation of Pielou’s J index for Sequence Diversity of the productive (left) and non-productive (right) rearranged TRB genes in the different experimental groups at the different times of analysis. Differences between the different experimental groups at a given timepoint or between the different timepoints of a given experimental group significant (p < 0.002), albeit in the small effect size range (0.001–0.263)
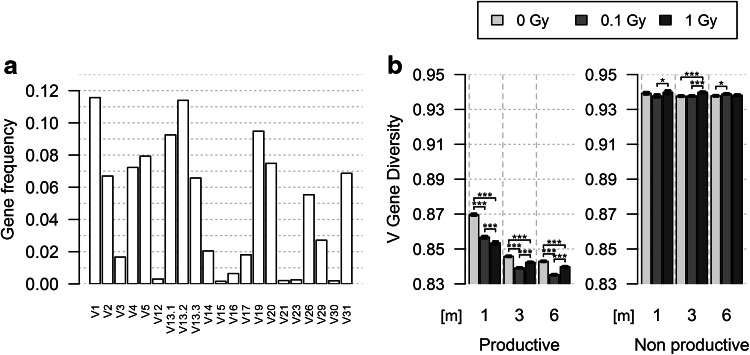
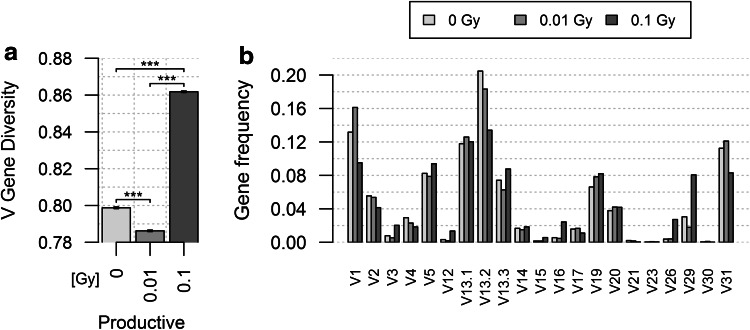
We next analyzed the evolution of V Gene Diversity in TRB gene rearrangements. The murine TRB locus encompasses 22 functional TRBV genes [34]. Hence, the hundreds of thousands of TRB sequences in each group are now distributed over the same number of classes (Fig. 2a; supplemental Fig. 2). Pielou’s index was calculated for these classes, and we can now, therefore, formally exclude the possibility that differences observed between groups result from eventual biases generated by differences in the number of events in each group or rare classes of non-functional V genes. Pielou’s index for V Gene Diversity in productive rearrangements is lower than that found for Sequence Diversity, as expected given the much lower number of classes. In control mice, the V gene repertoire evolution is biphasic: the J index value decreases sharply from 1 to 3 m, and then more slowly until 6 m (Fig. 2b). The V gene repertoire becomes more heterogeneous with age, and most of this heterogeneity is already established in the first 3 months of our experiments. The same biphasic pattern is observed in irradiated mice. However, J index value is already lower at 1-m post-exposure than in control animals, indicating that radiation exposure already increased the V gene repertoire heterogeneity in this short period of time, and these early changes are dose-dependent. However, for the 3- and 6-m timepoints, the V Gene Diversity value is clearly lower (p < 10−7) in low-dose-irradiated animals than in control and 1-Gy irradiated mice. V genes are much more homogeneously distributed in non-productive TRB rearrangements, and in sharp contrast to what is observed in productive TRB genes, this distribution barely changes over time and after irradiation. Together, these results indicate that similar to natural aging, lymphocytes using different V genes in their TR are differentially affected by radiation exposure. Whereas the initial effects of radiation are dose-dependent, the long lasting effects are stronger following low-dose irradiation.
Fig. 2.
TRBV gene use in the TR repertoire of control and irradiated mice. The V12-02 gene was not found in TRB sequences obtained from mice analyzed at 1 m. In productive TRB sequences, the nearest category was found to be sequences using the V12-01 gene. Thus, these two classes were merged and represented as V12 for both productive and non-productive groups resulting in 21 classes of V genes to analyze to compare the same number of classes of events as explained in the “Materials and methods” section. a Profile of V gene use in productive TRB genes in control mice at 1 m. The profiles for all experimental groups are shown in Supplemental Fig. 2. b Representation of Pielou’s J index for V Gene Diversity of the productive (left) and non-productive (right) rearranged TRB genes in the different experimental groups at the different times of analysis. All differences are significant (p < 10−7) in productive subset, albeit in the small effect size range (0.011–0.128). In non-productive subset, only half of the indices differ significantly between groups, whereas the other comparisons do not show significant differences. The analysis of individual mice shows the same trend
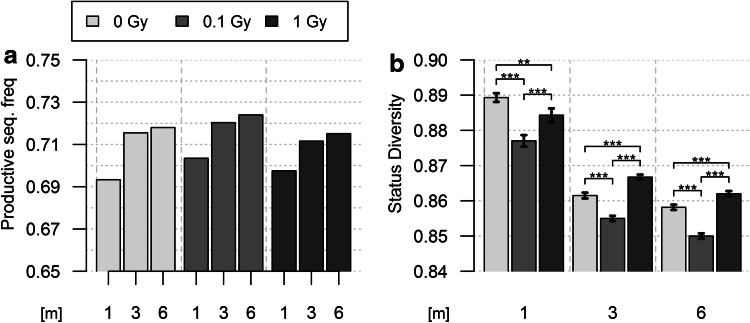
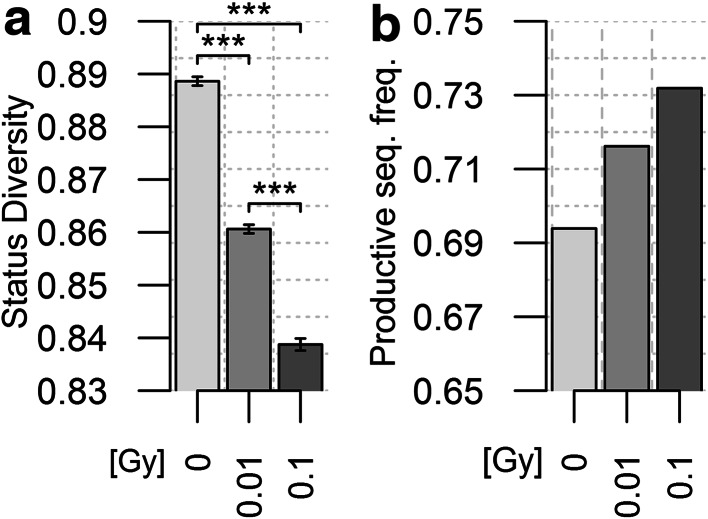
To further investigate the importance of TR expression in the effects of radiation exposure, we compared the status of TRB gene (productive vs non-productive) in the different groups. The proportion of productive rearrangements increases in un-irradiated mice from 1 to 6 m (Fig. 3b). Consequently, the J index value for Status Diversity decreases, in a biphasic pattern as observed for the V gene repertoire. All peripheral T lymphocytes have a productively rearranged TRB gene (TR+/GL and TR−/+ cells), and about 43% (Table 1) also carry a non-productive TRB gene (TR−/+ cells). The increase in productive TRB genes indicates that TR+/GL cells are preferentially expanded over time or that TR−/+ cells are disappearing faster. Low-dose radiation exposure amplifies this phenomenon. The productive TRB rearrangement frequency is higher and Status Diversity value is lower for each timepoint in 0.1 Gy irradiated mice compared to controls (Fig. 3). The effects of 1 Gy exposure are, however, clearly different: the frequency of productive TR genes first increases over the value found in control mice at 1 m, but at 3 and 6 m it is slightly below that of non-irradiated animals (Fig. 3). Consequently, after an initial decrease below the value of control mice at 1 m, but less important than in 0.1-Gy-exposed animals, Status Diversity value raises back to value higher than those for control mice at 3 and 6 m. Thus, high-dose exposure first results in mild aging-like effects which are compensated over time whereas, in line with our previous findings, low-dose exposure accentuates aging effects at all timepoints.
Fig. 3.
Evolution of rearranged TRB gene status in control and irradiated mice. a Representation of the frequency of productive TRB gene rearrangements in the blood of mice from the different experimental groups. b Representation of the corresponding Pielou’s J Status Diversity index for the different groups of mice. Differences between the different experimental groups at a given timepoint or between the different timepoints of a given experimental group significant (p < 1.9 × 10−6), albeit in the small effect size range (0.005–0.053). The analysis of individual mice shows the same trend
To find out whether radiation exposure affects the contribution of HSC to the peripheral blood TR repertoire, we transferred HSPC isolated from the bone-marrow of un-irradiated and irradiated CBA/HmCherry mice into non-irradiated NSG recipient mice. These immunodeficient mice do not have mature peripheral B and T lymphocytes. This lymphopenia results from a defect in NHEJ which blocks lymphocyte development. Thus, in this system, the T cells found in the blood of reconstituted NSG mice are generated only from the implanted HSPC and we can compare the TR repertoire generated by control and irradiated HSPC in a non-irradiated environment without any interference from the pre-existing mature peripheral T cells.
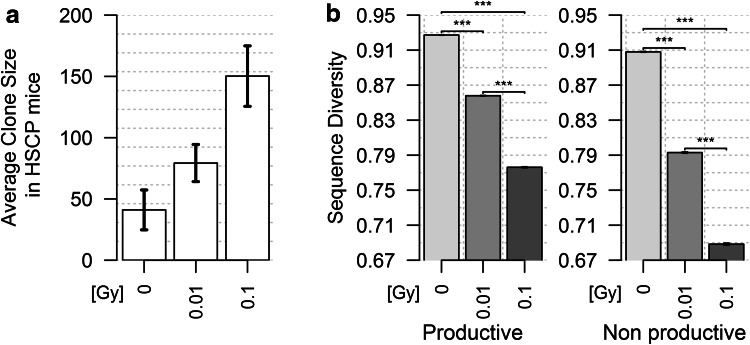
The TR repertoire was analyzed in reconstituted NSG mice 6 months after transplantation with HSPC isolated from un-irradiated control animals or CBA/HmCherry mice 7 day post-exposure to 0.01 or 0.1 Gy. The total number of TRB sequences obtained from the peripheral blood DNA extracted from the three groups of mice is quite similar (less than twofold variation, Table 2), whereas the difference in unique sequence numbers is much more important (almost six times). The number of unique sequences is lower in mice reconstituted with irradiated HSPCs, and this number decreases when the dose received by these cells increases. Consequently, the average T-cell clone size (number of times each TRB sequence is found) is higher in mice which received HSPCs isolated from irradiated CBA/Ca mice (Fig. 4a), indicating that T lymphocytes proliferated more vigorously in these recipient mice.
Table 2.
Number of TRB sequences obtained from NSG mice reconstituted with HSPC purified from control and low-dose-irradiated mice
| All mice | Total counts | Unique counts | |||
|---|---|---|---|---|---|
| 4,941,611 | 80,510 | ||||
| Time (months) | Dose (Gy) | Productive | Non-productive | Productive | Non-productive |
| 3,521,362 | 1,420,249 | 55,083 | 25,427 | ||
| 6 | 0 | 1,139,709 | 502,783 | 30,083 | 14,724 |
| 6 | 0.1 | 1,499,614 | 594,338 | 18,652 | 8368 |
| 6 | 1 | 882,039 | 323,128 | 5470 | 2335 |
The numbers of sequences shown in the table were obtained from three (for mice reconstituted with control and 0.01-Gy-exposed HSC) or three (for mice reconstituted with 0.1-Gy-exposed HSC) mice per experimental condition. “Dose (Gy)” refers to the dose of radiation received by the mice from which the HSPC used for NSG mice reconstitution were purified
Fig. 4.
Analysis of TRB clone size and TRB gene Status Diversity in reconstituted NSG mice. a This graph represents the average (±SEM) TRB clone size in the different groups of mice. The total number of sequences obtained in each mouse was divided by the number of unique sequences in that mouse. These results were obtained from three (control and 0.01-Gy-exposed HSPC) or two (0.1-Gy-exposed HSPC) mice. b Pielou’s J Sequence Diversity index for productive (left) and non-productive (right) TRB genes in the same experimental groups. The differences between groups are significant (p < 10−7). b Frequency of productive and non-productive TRB genes in the peripheral blood of NSG mice reconstituted with control and irradiated HSCP
Sequence Diversity, lower in mice reconstituted with un-irradiated HSPC than in un-manipulated CBA/Ca mice (compare Figs. 4b and 1b), was found to decrease sharply when HSPCs were isolated from irradiated mice, in a dose-dependent manner (Fig. 4b). This pattern shows that TRB gene representation is more uneven in HSPC-reconstituted NSG mice than in wild-type animals and that, in reconstituted NSG mice, the TRB repertoire generated from HSPC in more heterogeneous when the HSPC have been isolated from irradiated CBA/Ca animals. Some T-cell clones are much more represented than other. Together, the results presented in Fig. 4a, b suggest that the T lymphocytes proliferate with different rates or to different extent in NSG mice reconstituted with irradiated HSPC, and these differences are more marked when the radiation dose is higher. This conclusion is further supported by the observation that the V gene repertoire evolves differently in reconstituted NSG mice: V Gene Diversity of productive TRB rearrangements decreases slightly in mice reconstituted with 0.01-Gy-exposed HSPC, and raises sharply in mice reconstituted with 0.1-Gy-exposed HSPC (Fig. 5a). The difference in rate and/or extent of proliferation of the different T-lymphocyte clones in mice reconstituted with irradiated HSPCs induces in oligoclonal T-cell expansion which disrupts the V gene distribution in the TR repertoire. Hence, the relative frequency of T cells using V3, V12, V13.3, V16, V26, and V29 is strongly increased in mice reconstituted with HSPCs isolated from 100-mGy-exposed mice (Fig. 5b).
Fig. 5.
Analysis of TRB gene V Sequence Diversity in the peripheral blood T cells of NSG mice reconstituted with control and irradiated HSPC. a Pielou’s index for V gene diversity in NSG mice reconstituted with un-irradiated HSPCs, and HSPCs isolated from mice exposed to 10 and 100 mGy. b Representation of functional V gene frequency in the repertoire of productive TRB genes in the different groups of mice
The consequences of the sustained T-cell proliferation in mice reconstituted with irradiated HSPCs are also visible on TRB gene Status Diversity. The J index value is similar in NSG mice reconstituted with un-irradiated HSPC and in CBA/Ca mice (compare Figs. 3a and 6). However, Status Diversity was found to decrease in a dose-dependent manner when the HSPC used for reconstitution have been isolated from irradiated mice (Fig. 6a); this higher heterogeneity results from an increased frequency of productively rearranged TRB genes (Fig. 6b), indicating that TR+/GL T lymphocytes are preferentially expanded over TR−/+ cells. Altogether, these results show that the generation of the T lymphocytes repertoires from low-dose-exposed HSPC in a lymphopenic non-irradiated environment is durably affected by radiation exposure. Therefore, the effects of radiation on HSPC most probably contribute to the long-term effects of radiation on the TR repertoire of peripheral blood lymphocyte observed in irradiated mice.
Fig. 6.
Analysis of TRB gene Status Diversity in reconstituted NSG mice. a Pielou’s J Status Diversity index for NSG mice reconstituted with un-irradiated HSPCs, and HSPCs isolated from mice exposed to 10 and 100 mGy. b Frequency of productive and non-productive TRB genes in the peripheral blood of NSG mice reconstituted with control and irradiated HSCPs
Discussion
In this study, we compared IR effects with those of aging in settings precluding variations due to age at exposure, gender, genetic variability, or environmental factors. In un-irradiated control mice, the TR gene distribution remains homogeneous over time. The mechanisms controlling T-lymphocyte homeostasis maintain a near constant T-lymphocyte clone size in each age group. In sharp contrast, we observed age-dependent variations in the repertoire of V genes used in productive TR and in productive TR genes frequency. The composition of the expressed TR repertoire changes with age. The V gene repertoire becomes gradually more heterogeneous, indicating preferential over representation of lymphocytes using certain V genes. These changes probably result, at least in part, from the reactivity of their antigenic T-cell receptors towards certain Ags/super-Ags, which induces a gradual increase in memory T cells over time [39]. Beside this shift in the proportion of naïve and memory T cells, the evolution of the relative proportion of CD4+ and CD8+ lymphocytes with age is another contributing factor [40], as helper and cytotoxic lymphocytes use different V gene repertoires [41]. In any case, the absence of modulation of the V gene repertoire in non-productive TR rearrangements in aging control mice shows that this selection requires cell surface TR expression.
Our finding of a relative increase in productive TRB genes in older mice is more surprising. To the best of our knowledge, this phenomenon has never been described so far. Because of the allelic exclusion of TRB gene rearrangement and the requirement of expression of a TRB gene onto pro-T cells during thymocyte development [42], peripheral T lymphocytes fall into two categories: TR+/GL and TR−/+ cells. These two cell types differ only by the time they took to differentiate in the thymus. TRB+/GL thymocytes were able to express a TRB gene from their first allele and progressed to the pre-T-cell stage without delay, whereas in TRB−/+ thymocytes, TRB gene rearrangement on the first allele was not productive and these cells had to go through a second round of V(D)J recombination. Thus, they probably spent more time in thymus, “waiting” for their second rearranged TRB gene to be eventually expressed, at a time when it is essential for them to receive survival signals. Indeed, expression of a TRB gene within the pre-TCR rescues pro-T cell from cell death and induces their maturation to the pre-T-cell stage. This transition is characterized by an intense proliferation and the high energetic demand required for this process is met by an increase in glucose uptake and metabolism resulting from the activation of the PI(3)K/Akt pathway [43, 44]. In addition, T-lymphocyte differentiation in the thymus is spatially regulated; developing thymocytes migrate through different thymic micro-environments to receive different signals [45]. The time required to rearrange and express the second TRB allele may delay the delivery of these important developmental cues, either because the pre-TCR is not expressed or because the thymocytes are not in the right location, and result in a sub-optimal thymocyte development program which would generate T lymphocytes less fit or more prone to a premature exhaustion and disappearance. A prolonged stay in the pro-T-cell compartment in the absence of pre-TCR signaling can, for example, impair the initiation of the complex chromatin remodeling program governing T-cell development [46], and T lymphocytes generated from these progenitors may not be able to respond optimally to homeostatic/proliferative/survival signals in the thymus or the periphery. As a consequence, the proportion of TR+/GL cells increases gradually in the aged T-cell repertoire, as observed here.
While the consequences of low-dose radiation exposure can be assimilated to accelerated aging for all the parameters analyzed at all timepoints, several aspects of the response to 1 Gy suggest that compensatory mechanisms are able to halt or partially reverse these aging-like effects at this dose. The modulation of V gene repertoire homogeneity in 1-Gy irradiated animals is, for example, less important at 3 and 6 m than in animals exposed to 0.1 Gy, suggesting that high-dose radiation imposes a slower “rate” of aging than low dose to the T-lymphocyte repertoire. However, the initial effects of both low- and high-dose exposure are similar: Sequence Diversity, V Gene Diversity, and Status Diversity are more heterogeneous in 0.1- and 1-Gy irradiated animals than in control mice at 1 m, but only changes in the V Gene Diversity are radiation-dose dependent at that time, suggesting that these three aspects of the TR repertoire are not linked. This conclusion is also supported by the observation that later in time, these parameters evolve differently: the effects of low- and high-dose exposure clearly diverge and proceed with different kinetics. Compensatory mechanisms are not observed in 0.1-Gy-exposed animals, where radiation always seems to amplify the effects of age.
Low- and high-dose exposures induce quantitatively and/or qualitatively different effects in irradiated mice. 1 Gy certainly induces more cell death than 0.1 Gy, possibly triggering a stronger compensatory proliferation to restore homeostasis. Alternatively, 1 Gy can result in more severe effects, leading to a more efficient active clearance of damaged/affected cells. Hence, only relatively less affected cells would remain, resulting in a milder apparent effect. Alternatively, the immune system may be able to recover better, to the point of attenuating or limiting the effects of aging, because the initial effects were potent enough to activate a checkpoint inducing a strong recovery program after exposure. This situation would be reminiscent of—and could be linked with—the development of an inflammatory response, where “the beginning programs the end” [47]: some of the pro-inflammatory cytokines produced at the onset of the inflammatory response are needed to actively program the resolution of inflammation and the restoration of tissue homeostasis and function. Similarly, the outcome of the response to radiation could depend on the level of initial radiation-induced damage and to the cellular response triggered. A stronger activation of the DNA damage response (DDR) checkpoint and the ATM/p53 axis after high rather than low-dose exposure could be more efficient in coordinating damage management and restoration of tissue homeostasis.
It is likely that the differences in the long-term effects of low- and high-dose radiation have both cell autonomous and extrinsic causes. Indeed, mice were exposed to whole body irradiation, and we should not exclude the influence of T-lymphocyte environment. For example, the responses elicited after ex vivo exposure of whole blood to low and high X-ray doses were somewhat different: low doses induced inflammatory signaling, whereas high-doses showed a more typical p53-dependent DDR [48]. Monocytes probably play a role in the induction of this inflammatory response [49]. However, the dichotomy between low- and high-dose exposures may not be so clear. Indeed, opposing activities have been reported about the involvement of p53 and ATM in the expression of inflammatory cytokines [50–52], and it is, therefore, difficult at that time to predict the consequences of the activation of the DDR checkpoint by radiation in vivo, which undoubtedly influences T-lymphocyte fate, independent of the outcome. Some inflammatory cytokines have been shown to control the survival/proliferation of irradiated T lymphocytes [53] or the premature aging of the thymus [54]. The kinetics of DNA damage repair in response to low and high IR dose can be quite different in quiescent cells [55]. As the thymic stroma is largely composed of non-dividing cells, radiation-induced damage repair may be delayed or impaired in stromal cells exposed to 0.1 Gy, resulting in premature senescence/aging or loss of function. Moreover, thymocytes are exquisitely sensitive to radiation-induced apoptosis at the time when they rearrange their TRB genes. The extent of apoptosis and the delay before recovery begins have both been shown to increase with the dose received [56]. Therefore, the effects of radiation exposure probably also result from impaired de novo T-lymphocyte generation in the thymus, at least at the early times. In addition, the reconstitution of lymphopenic un-irradiated NSG mice with HSPC isolated from irradiated mice clearly demonstrated that the TR repertoire generated in these conditions is affected when compared to the repertoire generated from non-irradiated HSPCs. The peripheral T lymphocyte resulting from the differentiation of exposed HSPCs proliferated more. A high-level homeostatic proliferation of mature T lymphocytes in a lymphopenic environment is well known (reviewed in [57]). This proliferation is suppressed by the presence of sufficient numbers of mature T lymphocytes [58]. In NSG mice, the lymphoid compartment, originally totally empty, is progressively replenished over time by the differentiation of the transplanted HSPCs. The difference in proliferation observed between mice reconstituted with control and irradiated HSPCs may reflect the fact that the latter stayed lymphopenic for longer after transplantation, either because of a lower number of transplanted functional HSPcs or because of a lower efficiency of implantation or differentiation of these cells. As a result, the few T cells generated proliferate more, and this expansion leads to the generation of an oligoclonal TR repertoire. Thus, low-dose radiation alters HSPCs survival and/or functionality and impairs their ability to generate functional T lymphocytes. It is of course difficult to reconcile the effects observed in this system with those observed in TBI mice, as in the latest irradiated HSPC develop in an irradiated environment, but these results, nevertheless, demonstrate that acute low-dose radiation exposure of HSPC induces long lasting, measurable changes in the TR repertoire expressed in peripheral blood lymphocytes generated from these progenitors. Finally, the modulation of the TR repertoire observed in irradiated mice could also be a consequence of the role of T lymphocytes in the immune system. The immune system helps to preserve the integrity of the organisms against infectious and non-infectious trauma [59, 60]. Radiation-induced cell and tissue damage may uncover neo-antigens in irradiated mice. Natural aging of the immune system is attributed at least in part to a loss of function in response to persistent exposure to oxidative conditions generated by chronic inflammation [40]. The activation of T lymphocytes directed against viral or neo-antigens in the context of a rich inflammatory milieu can participate in the acceleration of age-related changes in the TR repertoire. Our observation that age-related changes are more pronounced in mice exposed to low dose may be due to the fact that comparatively more T lymphocytes in these mice are living with the consequences of radiation-induced oxidative stress than in high-dose-exposed animals, where T lymphocytes are more prone to dying after exposure.
In summary, the differences observed in the long-term effects of acute low- and high-dose radiation exposure on the TR repertoire can have multiple non-exclusive origins. As it is likely that all these events do not proceed with the same kinetics and dynamics, changes in the TR repertoire may result from different combinations of these complex events over time. However, even if the exact nature of the radiation-induced events responsible for the modulation of the TR repertoire cannot be precisely defined at that time, it is clear these events mimic aging, with a discontinuous dose–response dynamics: low-dose exposure accelerates aging, whereas the effects of high dose are partially compensated over time. Aging of the immune system probably contributes to the increase in infectious and non-infectious diseases observed in the elderly [4]. Thus, it seems reasonable to speculate that exposure to radiation could increase the susceptibility to the same spectrum of diseases, especially after exposure to low dose, where effects persist longer. However, peripheral T-lymphocyte homeostasis and functions were recently found to be un-affected 1 year after acute exposure of 5-m-old mice to a single high (1–4 Gy) IR dose [61]. Naïve T-cell counts were depressed until 30 day post-irradiation, but raised back to control levels in the next months, showing again the resilience of the immune system. This pattern is reminiscent to the TR repertoire dynamics that we observed after 1 Gy exposure. Our results then suggest that the transient alterations of the TR repertoire observed after 1 Gy exposure are not sufficient to impair T-lymphocyte functions on the long term. It remains to be determined whether low-dose radiation exposure affects the functionality of the immune system in the same time frame.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
This work has been supported by the European Commissions [DoReMi, European Atomic Energy Community’s Seventh Framework Program (FP7/2007–2011) under Grant Agreement No. 249689] and National Science Centre Grants HARMONIA 4 No. 2013/08/M/ST6/00924 and OPUS No. 2015/19/B/ST6/01736 (JP). JM was supported by GeCONil project (POIG.02.03.01-24-099).
Contributor Information
Serge M. Candéias, Email: serge.candeias@cea.fr
Christophe Badie, Email: christophe.badie@phe.gov.uk.
References
- 1.Trowell OA. The sensitivity of lymphocytes to ionising radiation. J Pathol Bacteriol. 1952;64(4):687–704. doi: 10.1002/path.1700640403. [DOI] [PubMed] [Google Scholar]
- 2.Goldrath AW, Bevan MJ. Selecting and maintaining a diverse T-cell repertoire. Nature. 1999;402(6759):255–262. doi: 10.1038/46218. [DOI] [PubMed] [Google Scholar]
- 3.Nikolich-Zugich J, Slifka MK, Messaoudi I. The many important facets of T-cell repertoire diversity. Nat Rev Immunol. 2004;4(2):123–132. doi: 10.1038/nri1292. [DOI] [PubMed] [Google Scholar]
- 4.Gardner ID. The effect of aging on susceptibility to infection. Rev Infect Dis. 1980;2(5):801–810. doi: 10.1093/clinids/2.5.801. [DOI] [PubMed] [Google Scholar]
- 5.Yu HT, Park S, Shin EC, Lee WW. T cell senescence and cardiovascular diseases. Clin Exp Med. 2015 doi: 10.1007/s10238-015-0376-z. [DOI] [PubMed] [Google Scholar]
- 6.Haynes L, Swain SL. Aged-related shifts in T cell homeostasis lead to intrinsic T cell defects. Semin Immunol. 2012;24(5):350–355. doi: 10.1016/j.smim.2012.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kamiya K, Ozasa K, Akiba S, Niwa O, Kodama K, Takamura N, Zaharieva EK, Kimura Y, Wakeford R. Long-term effects of radiation exposure on health. Lancet. 2015;386(9992):469–478. doi: 10.1016/S0140-6736(15)61167-9. [DOI] [PubMed] [Google Scholar]
- 8.Preston DL, Pierce DA, Shimizu Y, Cullings HM, Fujita S, Funamoto S, Kodama K. Effect of recent changes in atomic bomb survivor dosimetry on cancer mortality risk estimates. Radiat Res. 2004;162(4):377–389. doi: 10.1667/RR3232. [DOI] [PubMed] [Google Scholar]
- 9.Hsu WL, Preston DL, Soda M, Sugiyama H, Funamoto S, Kodama K, Kimura A, Kamada N, Dohy H, Tomonaga M, Iwanaga M, Miyazaki Y, Cullings HM, Suyama A, Ozasa K, Shore RE, Mabuchi K. The incidence of leukemia, lymphoma and multiple myeloma among atomic bomb survivors: 1950-2001. Radiat Res. 2013;179(3):361–382. doi: 10.1667/RR2892.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Major IR. Induction of myeloid leukaemia by whole-body single exposure of CBA male mice to X-rays. Br J Cancer. 1979;40(6):903–913. doi: 10.1038/bjc.1979.284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Suraweera N, Meijne E, Moody J, Carvajal-Carmona LG, Yoshida K, Pollard P, Fitzgibbon J, Riches A, van Laar T, Huiskamp R, Rowan A, Tomlinson IP, Silver A. Mutations of the PU.1 Ets domain are specifically associated with murine radiation-induced, but not human therapy-related, acute myeloid leukaemia. Oncogene. 2005;24(22):3678–3683. doi: 10.1038/sj.onc.1208422. [DOI] [PubMed] [Google Scholar]
- 12.Verbiest T, Bouffler S, Nutt SL, Badie C. PU.1 downregulation in murine radiation-induced acute myeloid leukaemia (AML): from molecular mechanism to human AML. Carcinogenesis. 2015;36(4):413–419. doi: 10.1093/carcin/bgv016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Goodhead DT. Fifth Warren K. Sinclair Keynote Address: issues in quantifying the effects of low-level radiation. Health Phys. 2009;97(5):394–406. doi: 10.1097/HP.0b013e3181ae8acf. [DOI] [PubMed] [Google Scholar]
- 14.Mettler FA, Jr, Bhargavan M, Faulkner K, Gilley DB, Gray JE, Ibbott GS, Lipoti JA, Mahesh M, McCrohan JL, Stabin MG, Thomadsen BR, Yoshizumi TT. Radiologic and nuclear medicine studies in the United States and worldwide: frequency, radiation dose, and comparison with other radiation sources—1950–2007. Radiology. 2009;253(2):520–531. doi: 10.1148/radiol.2532082010. [DOI] [PubMed] [Google Scholar]
- 15.Kusunoki Y, Hayashi T, Morishita Y, Yamaoka M, Maki M, Bean MA, Kyoizumi S, Hakoda M, Kodama K. T-cell responses to mitogens in atomic bomb survivors: a decreased capacity to produce interleukin 2 characterizes the T cells of heavily irradiated individuals. Radiat Res. 2001;155(1 Pt 1):81–88. doi: 10.1667/0033-7587(2001)155[0081:TCRTMI]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 16.Kusunoki Y, Yamaoka M, Kasagi F, Hayashi T, MacPhee DG, Kyoizumi S. Long-lasting changes in the T-cell receptor V beta repertoires of CD4 memory T-cell populations in the peripheral blood of radiation-exposed people. Br J Haematol. 2003;122(6):975–984. doi: 10.1046/j.1365-2141.2003.04520.x. [DOI] [PubMed] [Google Scholar]
- 17.Kusunoki Y, Yamaoka M, Kubo Y, Hayashi T, Kasagi F, Douple EB, Nakachi K. T-cell immunosenescence and inflammatory response in atomic bomb survivors. Radiat Res. 2010;174(6):870–876. doi: 10.1667/RR1847.1. [DOI] [PubMed] [Google Scholar]
- 18.Yamaoka M, Kusunoki Y, Kasagi F, Hayashi T, Nakachi K, Kyoizumi S. Decreases in percentages of naive CD4 and CD8 T cells and increases in percentages of memory CD8 T-cell subsets in the peripheral blood lymphocyte populations of A-bomb survivors. Radiat Res. 2004;161(3):290–298. doi: 10.1667/RR3143. [DOI] [PubMed] [Google Scholar]
- 19.Gyuleva IM, Penkova KI, Rupova IT, Panova DY, Djounova JN. Assessment of some immune parameters in occupationally exposed nuclear power plant workers: flow cytometry measurements of T lymphocyte subpopulations and immunoglobulin determination. Dose Response. 2015;13(4):1–11. doi: 10.2203/dose-response.14-041.Gyuleva. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rybkina VL, Azizova TV, Scherthan H, Meineke V, Doerr H, Adamova GV, Teplyakova OV, Osovets SV, Bannikova MV, Zurochka AV. Expression of blood serum proteins and lymphocyte differentiation clusters after chronic occupational exposure to ionizing radiation. Radiat Environ Biophys. 2014;53(4):659–670. doi: 10.1007/s00411-014-0556-3. [DOI] [PubMed] [Google Scholar]
- 21.Scherthan H, Abend M, Muller K, Beinke C, Braselmann H, Zitzelsberger H, Kohn FM, Pillekamp H, Schiener R, Das O, Peter RU, Herzog G, Tzschach A, Dorr HD, Fliedner TM, Meineke V. Radiation-induced late effects in two affected individuals of the Lilo radiation accident. Radiat Res. 2007;167(5):615–623. doi: 10.1667/RR0774.1. [DOI] [PubMed] [Google Scholar]
- 22.Chambers KA, Harrington NP, Ross WM, Filion LG. Relative alterations in blood mononuclear cell populations reflect radiation injury in mice. Cytometry. 1998;31(1):45–52. doi: 10.1002/(SICI)1097-0320(19980101)31:1<45::AID-CYTO6>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- 23.Mullenders L, Atkinson M, Paretzke H, Sabatier L, Bouffler S. Assessing cancer risks of low-dose radiation. Nat Rev Cancer. 2009;9(8):596–604. doi: 10.1038/nrc2677. [DOI] [PubMed] [Google Scholar]
- 24.Rees GS, Daniel CP, Morris SD, Whitehouse CA, Binks K, MacGregor DH, Tawn EJ. Occupational exposure to ionizing radiation has no effect on T- and B-cell total counts or percentages of helper, cytotoxic and activated T-cell subsets in the peripheral circulation of male radiation workers. Int J Radiat Biol. 2004;80(7):493–498. doi: 10.1080/09553000410001725099. [DOI] [PubMed] [Google Scholar]
- 25.Bogdandi EN, Balogh A, Felgyinszki N, Szatmari T, Persa E, Hildebrandt G, Safrany G, Lumniczky K. Effects of low-dose radiation on the immune system of mice after total-body irradiation. Radiat Res. 2010;174(4):480–489. doi: 10.1667/RR2160.1. [DOI] [PubMed] [Google Scholar]
- 26.Lacoste-Collin L, Jozan S, Cances-Lauwers V, Pipy B, Gasset G, Caratero C, Courtade-Saidi M. Effect of continuous irradiation with a very low dose of gamma rays on life span and the immune system in SJL mice prone to B-cell lymphoma. Radiat Res. 2007;168(6):725–732. doi: 10.1667/RR1007.1. [DOI] [PubMed] [Google Scholar]
- 27.Davis MM, Bjorkman PJ. T-cell antigen receptor genes and T-cell recognition. Nature. 1988;334(6181):395–402. doi: 10.1038/334395a0. [DOI] [PubMed] [Google Scholar]
- 28.Hoffman ES, Passoni L, Crompton T, Leu TM, Schatz DG, Koff A, Owen MJ, Hayday AC. Productive T-cell receptor beta-chain gene rearrangement: coincident regulation of cell cycle and clonality during development in vivo. Genes Dev. 1996;10(8):948–962. doi: 10.1101/gad.10.8.948. [DOI] [PubMed] [Google Scholar]
- 29.Outters P, Jaeger S, Zaarour N, Ferrier P. Long-range control of V(D)J recombination and allelic exclusion: modeling views. Adv Immunol. 2015;128:363–413. doi: 10.1016/bs.ai.2015.08.002. [DOI] [PubMed] [Google Scholar]
- 30.Witherden D, van Oers N, Waltzinger C, Weiss A, Benoist C, Mathis D. Tetracycline-controllable selection of CD4(+) T cells: half-life and survival signals in the absence of major histocompatibility complex class II molecules. J Exp Med. 2000;191(2):355–364. doi: 10.1084/jem.191.2.355. [DOI] [PubMed] [Google Scholar]
- 31.Attaf M, Legut M, Cole DK, Sewell AK. The T cell antigen receptor: the Swiss army knife of the immune system. Clin Exp Immunol. 2015;181(1):1–18. doi: 10.1111/cei.12622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Verbiest T, Finnon R, Brown N, Finnon P, Bouffler S, Badie C. NOD scid gamma mice are permissive to allogeneic HSC transplantation without prior conditioning. Int J Mol Sci. 2016;17(11):E1850. doi: 10.3390/ijms17111850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Vandervieren E, Hubert M. An adjusted boxplot for skewed distribution. In: Antoch J, editor. Proceedings in computational statistics. Heidelberg: Springer; 2004. pp. 1933–1940. [Google Scholar]
- 34.Lefranc MP, Giudicelli V, Ginestoux C, Jabado-Michaloud J, Folch G, Bellahcene F, Wu Y, Gemrot E, Brochet X, Lane J, Regnier L, Ehrenmann F, Lefranc G, Duroux P. IMGT, the international ImMunoGeneTics information system. Nucleic Acids Res. 2009;37(Database issue):D1006–D1012. doi: 10.1093/nar/gkn838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pielou EC. Measurement of diversity in different types of biological collections. J Theor Biol. 1966;13:131. doi: 10.1016/0022-5193(66)90013-0. [DOI] [Google Scholar]
- 36.Hutcheson K. A test for comparing diversities based on Shannon formula. J Theor Biol. 1970;29(1):151. doi: 10.1016/0022-5193(70)90124-4. [DOI] [PubMed] [Google Scholar]
- 37.Bonferroni CE (1936) Teoria statistica delle classi e calcolo delle probabilità. Pubblicazioni del R Istituto Superiore di Scienze Economiche e Commerciali di Firenze
- 38.Cohen J. The t test for means. In: Press A, editor. Statistical power analysis for the behavioral sciences, revised edition. New York: Academic Press; 1977. pp. 19–74. [Google Scholar]
- 39.Nikolich-Zugich J. Aging of the T cell compartment in mice and humans: from no naive expectations to foggy memories. J Immunol. 2014;193(6):2622–2629. doi: 10.4049/jimmunol.1401174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bauer ME, Fuente Mde L. The role of oxidative and inflammatory stress and persistent viral infections in immunosenescence. Mech Ageing Dev. 2016;158:27–37. doi: 10.1016/j.mad.2016.01.001. [DOI] [PubMed] [Google Scholar]
- 41.McLean-Tooke A, Barge D, Spickett GP, Gennery AR. T cell receptor Vbeta repertoire of T lymphocytes and T regulatory cells by flow cytometric analysis in healthy children. Clin Exp Immunol. 2008;151(1):190–198. doi: 10.1111/j.1365-2249.2007.03536.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Dudley EC, Petrie HT, Shah LM, Owen MJ, Hayday AC. T cell receptor beta chain gene rearrangement and selection during thymocyte development in adult mice. Immunity. 1994;1(2):83–93. doi: 10.1016/1074-7613(94)90102-3. [DOI] [PubMed] [Google Scholar]
- 43.Chen H, Yang T, Zhu L, Zhao Y. Cellular metabolism on T-cell development and function. Int Rev Immunol. 2015;34(1):19–33. doi: 10.3109/08830185.2014.902452. [DOI] [PubMed] [Google Scholar]
- 44.Ciofani M, Zuniga-Pflucker JC. Notch promotes survival of pre-T cells at the beta-selection checkpoint by regulating cellular metabolism. Nat Immunol. 2005;6(9):881–888. doi: 10.1038/ni1234. [DOI] [PubMed] [Google Scholar]
- 45.Bleul CC, Boehm T. Chemokines define distinct microenvironments in the developing thymus. Eur J Immunol. 2000;30(12):3371–3379. doi: 10.1002/1521-4141(2000012)30:12<3371::AID-IMMU3371>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- 46.Rothenberg EV. The chromatin landscape and transcription factors in T cell programming. Trends Immunol. 2014;35(5):195–204. doi: 10.1016/j.it.2014.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Serhan CN, Savill J. Resolution of inflammation: the beginning programs the end. Nat Immunol. 2005;6(12):1191–1197. doi: 10.1038/ni1276. [DOI] [PubMed] [Google Scholar]
- 48.El-Saghire H, Thierens H, Monsieurs P, Michaux A, Vandevoorde C, Baatout S. Gene set enrichment analysis highlights different gene expression profiles in whole blood samples X-irradiated with low and high doses. Int J Radiat Biol. 2013;89(8):628–638. doi: 10.3109/09553002.2013.782448. [DOI] [PubMed] [Google Scholar]
- 49.El-Saghire H, Michaux A, Thierens H, Baatout S. Low doses of ionizing radiation induce immune-stimulatory responses in isolated human primary monocytes. Int J Mol Med. 2013;32(6):1407–1414. doi: 10.3892/ijmm.2013.1514. [DOI] [PubMed] [Google Scholar]
- 50.Candeias SM, Testard I. The many interactions between the innate immune system and the response to radiation. Cancer Lett. 2015;368(2):173–178. doi: 10.1016/j.canlet.2015.02.007. [DOI] [PubMed] [Google Scholar]
- 51.Lowe JM, Menendez D, Bushel PR, Shatz M, Kirk EL, Troester MA, Garantziotis S, Fessler MB, Resnick MA. p53 and NF-kappaB coregulate proinflammatory gene responses in human macrophages. Cancer Res. 2014;74(8):2182–2192. doi: 10.1158/0008-5472.CAN-13-1070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sabatel H, Di Valentin E, Gloire G, Dequiedt F, Piette J, Habraken Y. Phosphorylation of p65(RelA) on Ser(547) by ATM represses NF-kappaB-dependent transcription of specific genes after genotoxic stress. PLoS One. 2012;7(6):e38246. doi: 10.1371/journal.pone.0038246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Seki H, Iwai K, Kanegane H, Konno A, Ohta K, Ohta K, Yachie A, Taniguchi N, Miyawaki T. Differential protective action of cytokines on radiation-induced apoptosis of peripheral lymphocyte subpopulations. Cell Immunol. 1995;163(1):30–36. doi: 10.1006/cimm.1995.1095. [DOI] [PubMed] [Google Scholar]
- 54.Sempowski GD, Hale LP, Sundy JS, Massey JM, Koup RA, Douek DC, Patel DD, Haynes BF. Leukemia inhibitory factor, oncostatin M, IL-6, and stem cell factor mRNA expression in human thymus increases with age and is associated with thymic atrophy. J Immunol. 2000;164(4):2180–2187. doi: 10.4049/jimmunol.164.4.2180. [DOI] [PubMed] [Google Scholar]
- 55.Rothkamm K, Lobrich M. Evidence for a lack of DNA double-strand break repair in human cells exposed to very low X-ray doses. Proc Natl Acad Sci USA. 2003;100(9):5057–5062. doi: 10.1073/pnas.0830918100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Gentil Dit Maurin A, Lemercier C, Collin-Faure V, Marche PN, Jouvin-Marche E, Candeias SM. Developmental regulation of p53-dependent radiation-induced thymocyte apoptosis in mice. Clin Exp Immunol. 2015;179(1):30–38. doi: 10.1111/cei.12329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sprent J, Surh CD. Normal T cell homeostasis: the conversion of naive cells into memory-phenotype cells. Nat Immunol. 2011;12(6):478–484. doi: 10.1038/ni.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Min B, McHugh R, Sempowski GD, Mackall C, Foucras G, Paul WE. Neonates support lymphopenia-induced proliferation. Immunity. 2003;18(1):131–140. doi: 10.1016/S1074-7613(02)00508-3. [DOI] [PubMed] [Google Scholar]
- 59.Chen GY, Nunez G. Sterile inflammation: sensing and reacting to damage. Nat Rev Immunol. 2010;10(12):826–837. doi: 10.1038/nri2873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Schaue D, Kachikwu EL, McBride WH. Cytokines in radiobiological responses: a review. Radiat Res. 2012;178(6):505–523. doi: 10.1667/RR3031.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Pugh JL, Foster SA, Sukhina AS, Petravic J, Uhrlaub JL, Padilla-Torres J, Hayashi T, Nakachi K, Smithey MJ, Nikolich-Zugich J. Acute systemic DNA damage in youth does not impair immune defense with aging. Aging Cell. 2016;15(4):686–693. doi: 10.1111/acel.12478. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.