Abstract
ATPases Associated with various cellular Activities (AAA+ ATPases) are molecular motors that use the energy of ATP binding and hydrolysis to remodel their target macromolecules. The majority of these ATPases form ring-shaped hexamers in which the active sites are located at the interfaces between neighboring subunits. Structural changes initiate in an active site and propagate to distant motor parts that interface and reshape the target macromolecules, thereby performing mechanical work. During the functioning cycle, the AAA+ motor transits through multiple distinct states. Ring architecture and placement of the catalytic sites at the intersubunit interfaces allow for a unique level of coordination among subunits of the motor. This in turn results in conformational differences among subunits and overall asymmetry of the motor ring as it functions. To date, a large amount of structural information has been gathered for different AAA+ motors, but even for the most characterized of them only a few structural states are known and the full mechanistic cycle cannot be yet reconstructed. Therefore, the first part of this work will provide a broad overview of what arrangements of AAA+ subunits have been structurally observed focusing on diversity of ATPase oligomeric ensembles and heterogeneity within the ensembles. The second part of this review will concentrate on methods that assess structural and functional heterogeneity among subunits of AAA+ motors, thus bringing us closer to understanding the mechanism of these fascinating molecular motors.
Keywords: AAA+ ATPase, AAA+ motor, Mechanochemical enzyme, Ring ATPase
Introduction
The extended family of ATPases Associated with various cellular Activities (AAA+ ATPases) is a broad class of versatile molecular motors that utilize the energy of ATP binding and hydrolysis to reshape their target molecules. These ATPases are ubiquitous among all kingdoms of life and perform a vast variety of functions, hence the broad family name. Due to their ability to direct or induce conformational changes in other molecules, AAA+ proteins are often referred to as AAA+ motors or mechanochemical enzymes, a name given to enzymes that convert chemical energy into mechanical work [1–7].
As mentioned above, AAA+ ATPases remodel other macromolecules such as nucleic acids and proteins. They are crucial in DNA transcription, replication, recombination, and repair. For example, bacterial DnaA and papillomavirus E1 helicases are responsible for unwinding double-stranded DNA for replication initiation. AAA+ motors also assist in protein folding, complex formation, disassembling protein aggregates and degradation of misfolded proteins. Classic examples for protein remodeling by AAA+ motors are unfoldases: ClpX unfolds proteins to pass them into protease chamber and ClpB helps to disaggregate proteins and works in collaboration with chaperones. Fusion and fission of membranes and transport of macromolecules across lipid layers also depend upon AAA+ ATPases. For example, one function of p97 protein is involved in membrane fusion while Vps4 ATPase participates in membrane deformation and fission events. AAA+ ATPases are some of the most abundant P-loop ATPases and are involved in numerous essential cellular processes [2, 8]. Mutations in many AAA+ ATPases, involved in crucial cell functions, give rise to multiple human genetic diseases (reviewed in [2, 9]). Recently two of the most studied AAA+ ATPases in this regard are p97 and dynein. Mutations in p97 (VCP, CDC48 homolog), a key player in protein quality control system, are linked with amyotrophic lateral sclerosis (ALS), inclusion body myopathy associated with Paget’s disease of the bone and frontotemporal dementia (IBMPFD), and certain cancers [10, 11]. Dynein is the major molecular transporter of cargoes along the microtubules and thus is essential for mitosis, autophagy, and structure of several cellular organelles. Disruption of the dynein function was implicated in numerous neurodegenerative and neurodevelopmental diseases [12–14]. Some AAA+ proteins are required for virulence and survival of pathogenic bacteria and therefore may potentially serve as targets for development of new antimicrobial strategies. For example, an inhibitor of major quorum-sensing regulator LuxO was recently shown to reduce virulence of a notorious human pathogen Vibrio cholerae [15].
This work first focuses on general structural features of the AAA+ ATPases that define this broad family of molecular motors and then describes how this general fold is used in different ATPases to produce observed diversity of functions. The second part gives a brief overview of experimental approaches uniquely suitable to assess heterogeneity of AAA+ ring subunits and thus to interrogate mechanism of the motor function. Overall, this review is intended as an introduction to general properties of the AAA+ motors, as a guide for further reading about detailed structural work, and for planning experimental studies.
Canonical structure of AAA+ motors
Structural core of AAA+ ATPases
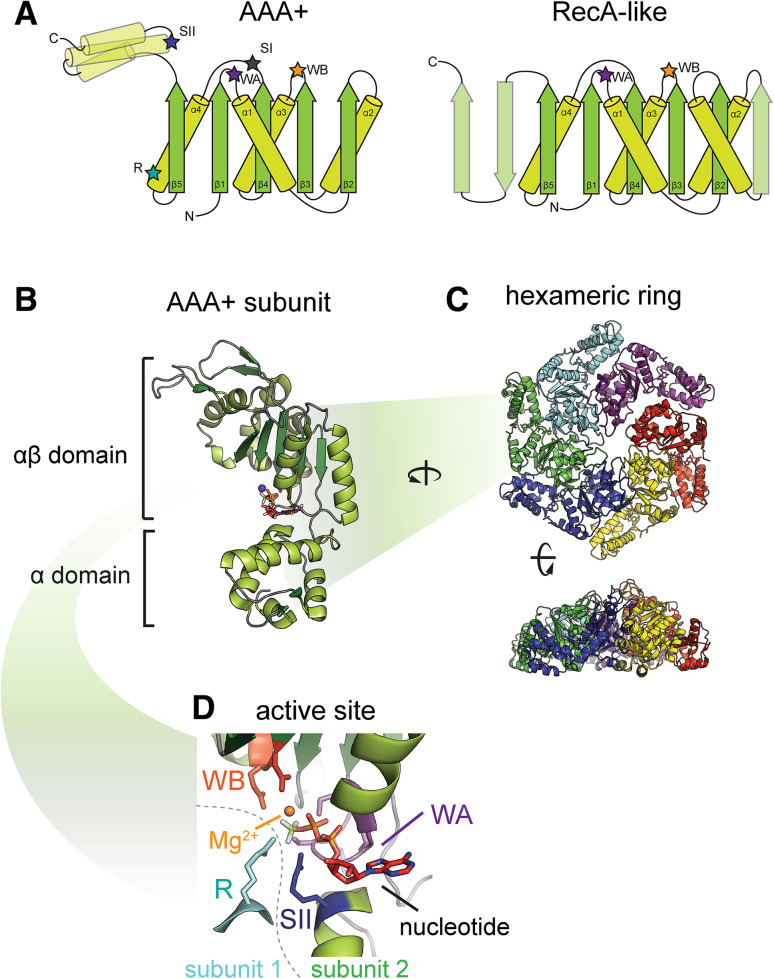
AAA+ ATPase motors belong to the Additional Strand Catalytic glutamate E (ASCE) family of P-loop NTPases, one of the most abundant types of enzymes in any living cell [2, 8]. In many aspects of their structures and functions, AAA+ ATPases resemble RecA-like ATPase motors and are often discussed as a single family of RecA-related molecular motors. Evolution, topology, similarities and differences of AAA+ and RecA families are beautifully analyzed in several detailed reviews to which I would refer the interested reader [6, 8, 16–18]. Briefly, ASCE ATPases all contain a conserved core αβα fold that is created by five parallel β-strands which are arranged in order 5-1-4-2-3 (numbered from N to C terminus) and connected by loops and short α-helices (Fig. 1a). Different families of these ASCE ATPases vary in additional core subdomains and inserted elements. In the case of the RecA family, the core fold features additional β-strands, while AAA+ ATPases possess a conserved α-helical bundle at the C terminus [16, 17, 19].
Fig. 1.
Conserved AAA+ ATPase core. a Topology of core AAA+ and RecA-like folds. b Structure of an AAA+ subunit in cartoon rendering in example of NtrC1 from Aquifex aeolicus (4LZZ, [87]). Active site is located in the cleft between two subdomains. c AAA+ hexameric ring shown in top-down and side views. All six subunits are colored differently. d Active site of an AAA+ ATPase (NtrC1, 4LZZ) with conserved elements from two neighboring subunits: (1) Walker A (WA, purple), Walker B (WB, dark orange), Sensor II (SII, dark blue) and (2) arginine finger (R, cyan). There is an ATP analog molecule (in stick rendering) bound to the active site as an example of ligand interaction. Please note that in this example, the WA contains GKE rather than canonical GK[S/T] residues. All high-resolution structures are illustrated using PyMOL program (The PyMOL Molecular Graphics System, Version 1.8 Schrödinger, LLC)
The core of the AAA+ subunit usually consists of about 220–240 residues arranged in a bigger N-terminal αβ and a smaller C-terminal all α-helical subdomains (Fig. 1b). This core features several conserved motifs responsible for interaction between the enzyme and nucleotide and for performing catalysis (Fig. 1a, b, d). First described by Walker and colleagues, there are conserved motifs characterizing many mononucleotide-binding enzymes, including the RecA and AAA+ ATPases—now termed Walker A (WA) and Walker B (WB) motifs [20]. The WA motif is a glycine-rich stretch followed by a critical lysine residue GXXGXGK[S/T]. This motif is responsible for interaction with phosphate groups of the nucleotide and is therefore often dubbed as the phosphate loop (P-loop) [7]. The P-loop usually follows a hydrophobic β-strand (β1) and precedes an α-helix (α1) within the subunit structure. The WB motif is less conserved, but usually consists of acidic aspartate and glutamate residues following a short hydrophobic (h) stretch—hhhhDE. WB is crucial for ATP hydrolysis as its aspartate (D) participates in Mg2+ coordination and glutamate (E) is thought to serve as a catalytic base for hydrolysis [20, 21].
In addition to WA and WB motifs, there are several other motifs of the AAA+ core such as arginine finger (R-finger or R), Sensor I (SI) and Sensor II (SII) (Fig. 1d, [6, 7, 16, 22, 23]). These motifs are not absolutely conserved throughout the family. The R-finger senses the presence of γ-phosphate and is required for coordinating the leaving phosphate during hydrolysis of ATP. The R-finger is contributed to the active site from the neighboring ATPase subunit, not the subunit containing the WA and WB elements (cyan residue in Fig. 1d). SI is a polar amino acid that participates in hydrolysis by coordinating water required for catalysis. SII is a positively charged residue, usually arginine, that is positioned to interact with the phosphates of nucleotide thus performing “sensing” or binding functions. SII usually originates from the tip of the α7 helix in the C-terminal α-helical bundle. Structural analyses also identified a glutamate switch that constitutes a pair of residues: a glutamate from WB and a conserved asparagine in the β2 strand [22, 24]. Interaction between the glutamate switch residues might be regulated by substrate binding resulting in restricting the catalytic glutamate in an ‘inactive’ configuration or releasing it. Structural and functional features of the nucleotide binding site of AAA+ motors were recently reviewed by Wendler and co-authors [22].
AAA+ motors function as ring-shaped hexamers
The majority of the well-studied ATPases were observed to form hexamers and initial structural investigation revealed that these AAA+ hexamers are highly symmetric ring-like ensembles (Figs. 1c, 2a). Indeed, the first of the structurally characterized AAA+ motors were crystallized as hexameric rings saturated with ATP or ADP nucleotides with rare exceptions of partly occupied rings (see, for example, first review on AAA+ structural information by Ogura and Wilkinson in 2001 [2] and references within). From then, the number of solved crystal structures of AAA+ motors grew from a few at the beginning of the millennium to hundreds today, according to the PDB and PFAM databases (http://www.rcsb.org; http://pfam.xfam.org). These numerous observations of sixfold symmetric rings clearly illustrate that the hexameric state is one of the most stable and relevant arrangements for the majority of AAA+ proteins. In the ring-shaped hexamers active sites are located at the intersubunit interfaces (Fig. 1d). This way the R-finger of one subunit is brought into vicinity to the WA and WB motifs of the neighboring protomer, completing the active site architecture, as mentioned above.
Fig. 2.
Diversity of AAA+ motors through additional structural elements and interaction with substrate. a An example of comparison of structural elements for HslU and NtrC1 ATPases showing that conserved functional pore loops originate from the same location of the AAA+ core (1G4A, 4LZZ, [87, 168]). In both of these ATPases high-resolution structural data revealed that the pore loops undergo significant movement upon nucleotide binding in the active sites of the ATPase. In combination with biochemical data, it is shown that the loops interact with the cognate substrates and are hypothesized to exhibit force to re-shape these substrates upon ATP hydrolysis. b Various AAA+ motors bind their cognate macromolecular substrates differently. For example, E1 helicase threads DNA substrate through the ring pore (schematically presented on the left side), while AAA+ modules of dynein are hypothesized to exert force on the linker domain, residing on one side of the AAA+ ring (schematics only shows six AAA+ domains and abstract linker, middle). Recently characterized transposase IstB ATPase forms a double pentameric “clamshell” that sandwiches dsDNA substrate (right)
Diversity of AAA+ motors
While the core subunit structure of all AAA+ ATPases is well conserved, there are at least three major features that lead to tremendous diversity of AAA+ functions. These are additional inserts into the conserved core, interaction with the macromolecular substrate, and mode of subunit arrangement in an AAA+ oligomer. This way, multiple insertions of functional loops and structural elements define diverse clades of the AAA+ motors. Figure 2a gives a couple of examples of how AAA+ core can be modified by the addition of loops, β-hairpins and α helices. Detailed description of the differences among various AAA+ families can be found in thorough reviews on the subject by Iyer et al., Erzberger and Berger, Ammelburg et al., and others [6, 8, 16]. Such classification of AAA+ motors based on structural topology is extremely useful if one wants to trace similarities and differences among known structural details about different protein classes because it correlates conserved features of one protein subclass to the features of another. For instance, comparison of topologies of the NtrC and HslU/ClpX families, as defined in [16], clearly indicates that proteins from both families contain an additional insertion of a stem loop between α3 helix and β4 strand—so-called “pre-sensor I insert” (PS-I). Therefore, functional loop “L2” in the NtrC bacterial transcription activators is analogous to the “aromatic paddle” (pore-1, GYVG loop) in the proteasomal ATPases from the HslU/ClpX family [25, 26] (Fig. 2a). Although this similarity does not imply an analogous functional mechanism it does help to identify the key portions of the ATPase that must interact with its common core. This knowledge can be applied to guide investigation of specific mechanisms.
Another AAA+ property resulting in the diversity of functions is the different ways of interacting with cognate substrate (Fig. 2b). As mentioned above, the AAA+ motors reshape nucleic acids, proteins and protein complexes. Moreover, the substrate can bind differently to the ATPase oligomer. Many AAA+ motors were shown to bind their main substrate in close proximity to the ring pore and to thread substrate through the pore. For example, the papillomavirus replication helicase E1 binds to its DNA substrate via a β-hairpin (the pre-sensor I β-hairpin) loops that line the pore of the E1 hexameric ring (Fig. 3b). A single strand of DNA is being threaded through the pore opening [27]. Similarly, protein unfoldase ClpX has three conserved pore loops that engage substrate protein chain and pull it into the protease chamber [28]. Nevertheless, there are known examples when the substrate does not bind to the ring pore of AAA+ motor and rather interacts laterally. Dynein and IstB encoded in the IS21 transposon can serve as examples of this atypical mode of substrate binding (Fig. 2b, [29–31]). For example, AAA+ motor of dynein is thought to exert force on the linker domain that in turn transmits conformational changes through the stalk to microtubule binding domain (MTBD) resulting in detaching of MTBD from the microtubule. Coordinated binding and detachment of MTBD domains of the dimeric dyneins results in “walking” of this motor along the microtubule path. In this case, the linker domain serves as an intramolecular substrate for the dynein’s AAA+ ring.
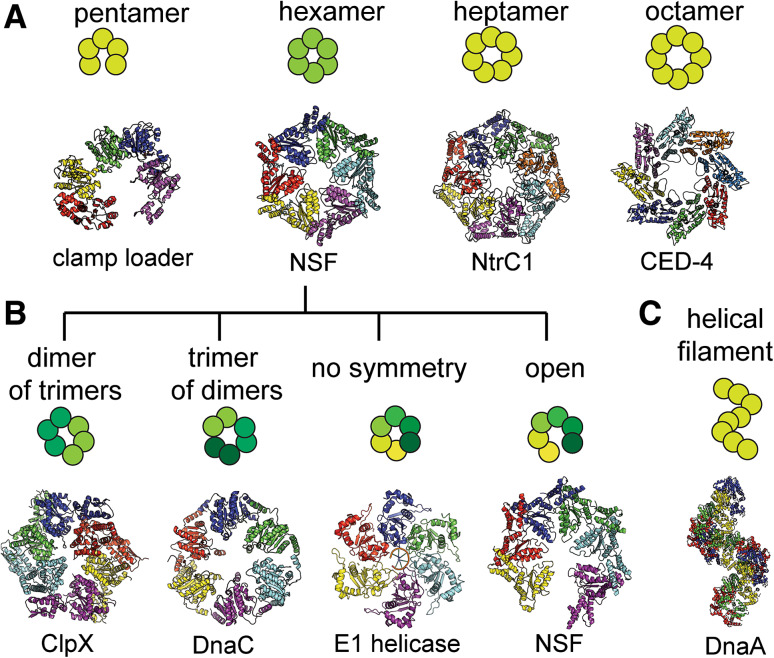
Fig. 3.
Polymorphism of AAA+ motors. a Different observed stoichiometry: from pentamers to octamers. Presented examples: clamp loader (1XXH, [50]); D2 of NSF (1NSF, [65]); NtrC1(3M0E, [32]); CED-4 (3LQQ, [58]). b Low-symmetry hexameric forms: ClpX (3HWS, [78]); DnaC (2VYE, [169]); E1 helicase (2GXA, [27]); D1 of NSF (3J95, [88]). c An example of an AAA+ motor [DnaA (2HCB, [63])] that forms a helical filament
In addition to inserted elements and mode of substrate binding, the AAA+ subunits can form rather different oligomeric ensembles. Even if one were to consider subunit packing within only ring hexamers, it is notable that orientation of the AAA+ core within the ring varies a lot. Relative packing of the small α helical domain with respect to the αβ core also differs for different AAA+ motors [5, 16]. Moreover, data accumulated to date show that AAA+ subunits are capable of forming various oligomeric ensembles that differ from a ring with sixfold symmetry and some AAA+ motors are thought to function as non-hexameric oligomers.
Non-hexameric ensembles of ATPase subunits
In addition to the observed hexameric state, AAA+ ATPases have been observed as helical filaments, pentamers, heptamers, octamers, and double rings of variable stoichiometry (Fig. 3a, c). Moreover, under equivalent conditions many AAA+ motors were shown to exist as a heterologous population with different conformations or stoichiometry. For instance, coexistence of hexamer and heptamer populations was observed for NtrC1 [25, 32], NtrC4 [33], FlrC [34], HslU [35]. Such stoichiometry flexibility was also observed for other multimeric ATPases from the RecA-type family, i.e., T7 helicase (gene 4 product; [36, 37]) and human mtDNA helicase (TWINKLE, [38]). Both oligomeric states were observed under various conditions for mtMCM (Methanobacterium thermoautothrophicum, [39, 40]), ClpB [41–44], p97 [45, 46], and HslU [35]. Both hexameric and heptameric ensembles were crystallized for p97 (D1 domain; [45, 46]), Lon protease [47, 48] and NtrC1 activator [25, 32]. These mixed populations highlight the flexibility of crystal packing of AAA+ modules as well as their exquisite sensitivity to the presence of nucleotides and macromolecular substrates. Indeed, a shift in the mixed population composition can be caused by the presence of nucleotides or other substrates, such as nucleic acid in case of helicases. For instance, RuvB predominantly forms hexamers in the presence of DNA and forms heptamers in the absence of this substrate [49].
One other well-known deviation from the hexameric geometry is the pentameric clamp loader (RFC) which has been shown to function as a highly asymmetric open pentameric ring [50, 51]. Clamp loaders consist of five polypeptides and thus each subunit of the pentamer is highly specialized. Multiple crystal structures revealed that the subunits adopt highly specific conformations and each position in the pentamer is unique (for example, reviewed in [52]). RNA/DNA packaging motors of viruses were shown by EM to form closed pentamers within the viral capsid while being observed as hexamers in separation [53–57]. Most recently IstB transposase ATPase was shown to form pentameric “clamshell” consisting of two AAA+ pentamers sandwiching double-stranded DNA(dsDNA) in between [29].
There are also few examples of ATPases forming octamers. Program cell death-related CED4 AAA+ ATPase was crystallized as a closed octameric ring (Fig. 3a, [58]). Rep68 helicase was shown to form different arrangements including double octameric rings [59]. The double rings are not unique for Rep68, having also been detected for other motors with single or double AAA+ modules (see below).
DnaA is an example of AAA+ ATPase that forms long helical filaments (Fig. 3c). It is one of the proteins that are thought to function as a helical oligomer. Such assembly strongly resembles polymerization of RecA homologous recombination protein and its homologs as well as other proteins from the RecA family, despite quite different effects these ATPases inflict to the substrate DNA. Apart from DnaA family, it is a generally accepted opinion that AAA+ ATPases function as closed hexameric rings. Nevertheless, quite many AAA+ proteins were found to form filaments, at least in vitro as shown by crystallography and EM. PspF, ClpX, ClpB, RuvB, and DnaA were crystallized with helical arrangements of AAA+ subunits [42, 60–64]. EM studies demonstrated filament formation for mtMCM and Rep68. Surprisingly, there are different types of helices formed by AAA+ subunits. For example, RuvB of Thermotoga maritima and PspF from Escherichia coli form left-handed helical filaments with six subunits per turn (i.e., 65, [60, 64]), while A. aeolicus DnaA forms right-handed helices with eight subunits per helical turn (81, [63]).
While some of these versatile assemblies of AAA+ subunits may potentially represent non-physiological states, they offer insight on what stable interfaces and subunit conformations are possible for a given ATPase protomer. One may argue that these structural features might eventually be found in some of the multiple, functionally relevant states of AAA+ motors. Therefore, analyses of different arrangements of ATPase subunits may help characterize potential structural states of a subunit and active site. By itself, existence of the non-hexameric arrangements evidently illustrates flexibility of AAA+ ring structures.
AAA+ domains forming double-tiered rings
There are many examples of proteins containing two tandem AAA+ modules in polypeptide chains. These ATPase motors have been shown to function as double-tiered rings. For instance, there are two ATPases involved in membrane fusion events, NSF and p97, which both contain two ATPase modules (denoted D1 and D2) that form two-tiered rings. Crystal structures of these two proteins were among the first solved and have since served as prototypical AAA+ fold [46, 65, 66]. Other examples of double-tiered ring ATPases are bacterial ClpB, heat shock unfoldase Hsp104 and heterocomplex Pex1/Pex6 [42, 67–71]. For double-tiered rings it is often observed that one ATPase domain regulates oligomerization while the other is catalytically active. For example, the D1 domain of p97 ATPases (CDC48, VAT homologs) hexamerizes upon nucleotide binding while D2 is responsible for substrate interactions and ATP hydrolysis. Double-stacked rings were also observed for some single AAA+ module ATPases (i.e., Rvb, Vps4 [72, 73]). Unlike the ATPase motors containing two AAA+ modules as part of one polypeptide chain, the double-layered hexamers of the single ATPase domains are mostly believed to be artifacts of in vitro work and non-physiological conditions.
There are also examples of ATPases that have three covalently linked ATPase subunits (D1–D3) such ESX ATPases required for Type VII/ESX protein secretion in bacteria [74–76]. In these cases, the organization of the respective oligomers formed by D1 through D3 is unknown. It is hypothesized that the three linked ATPase subunits may form hexamers with three rings stacked on top of each other or a pseudohexamer out of two ESX ATPase molecules, resulting in a single-layer hexameric ring. Interestingly, similar to the case of double-layered rings and heterohexamers, D1–3 domains possess different functions [74–77].
Heterogeneity of subunits within AAA+ motors
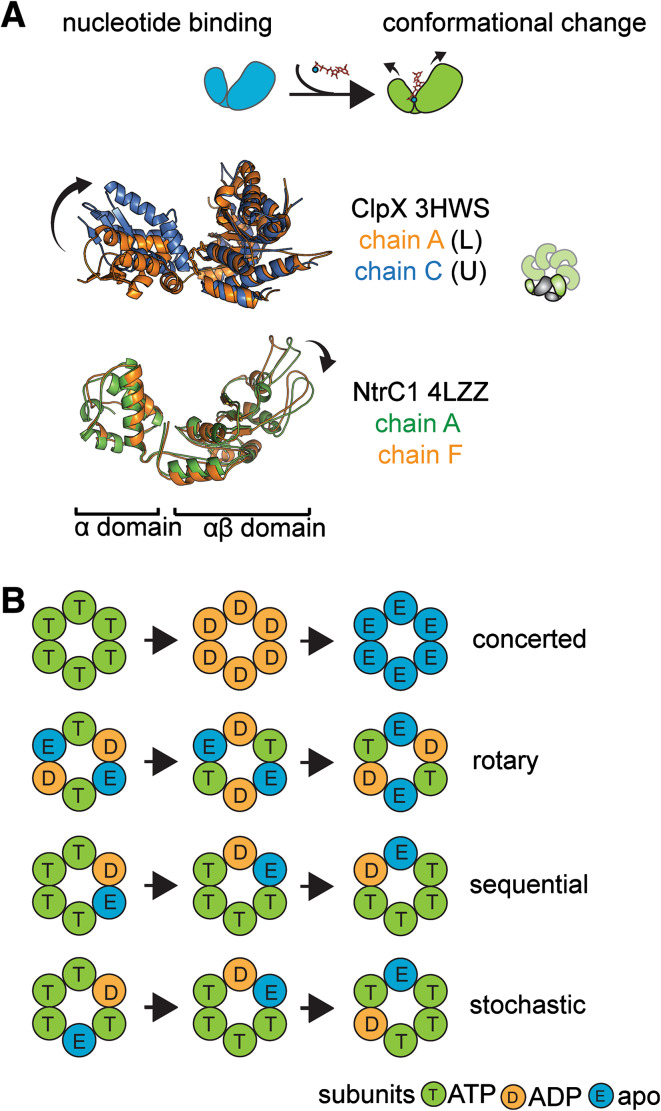
A unique feature of all AAA+ motors is the positioning of the active sites at the interfaces of an oligomeric ensemble. In such an ensemble, nucleotide processing in an active site initiates structural changes that propagate to distant motor parts, such as substrate binding loops and other active sites. The functioning cycle of an AAA+ motor should then consist of multiple distinct conformational states. Numerous studies showed that AAA+ subunit is exquisitely sensitive to the presence and identity of a nucleotide in the active site. With the active site positioned in the cleft between small α and large αβ domains (Fig. 1b) nucleotide-induced differences of ATPase subunits in their apo, ADP- or ATP-bound states are often characterized by rotation of the two subdomains considered to be “rigid bodies” due to less pronounced structural rearrangements within the subdomains (Fig. 4a). For example, ClpX ATPase subunit was shown to adopt two drastically different states with about 80° rotation of the small subdomain with respect to the large subdomain (Fig. 4a, [78]). The two states are termed L and U, as the active site of the U subunit is occluded for nucleotide binding and thus “unloadable”. Interestingly, in the ClpX hexamer, it was observed that the interface between the small α-helical domain of one subunit and the large AAA+ domain of the neighboring subunit is almost undisturbed by nucleotide-induced changes and therefore these two domains from the neighboring ATPase subunits are considered as a “rigid body” (grey in the ring cartoon insert in Fig. 4a, [78]). In some other AAA+ motors, the nucleotide binding induces a relatively small subdomain rearrangement that nevertheless results in a significant movement of the functional pore loops with respect to the motor complex (Fig. 4a). The relocation of the conserved loops is considered to be a key step in AAA+ motor function, conveying change in the nucleotide status into action on the macromolecular substrate.
Fig. 4.
Nucleotide binding and hydrolysis in AAA+ motors. a Examples of nucleotide-induced conformational changes in AAA+ subunits: a large-scale rotation of subdomains in ClpX hexamer (top) and a smaller scale repositioning of the functional loops within the large subdomain in NtrC1 (bottom). The illustrations were prepared from the hexameric NtrC1 and ClpX structures (4LZZ, 3HWS) by automatic alignment in PyMOL program. In each case, the two indicated chains from respective hexamers were chosen to show the extent of nucleotide-induced asymmetry within the AAA+ motors. The inset on the right depicts a ClpX “rigid body” (in grey) that consists of the small α-helical domain of one subunit and the large domain of the clockwise-next neighbor (when viewed from the top of the hexamer as in Fig. 1c). b Proposed mechanisms of ATP hydrolysis coordination within the AAA+ motors: concerted, rotary, sequential and stochastic. Subunits of an AAA+ hexamer are labeled and colored according to the code: green T subunit—transition state of the ATP, orange D subunit—ADP-bound, post-hydrolysis state, blue E—product release, apo state (this notation is based on [2, 100–102])
Despite the large amount of structural information that is currently available, the full mechanistic cycle cannot be yet described even for most characterized AAA+ motors. From the high-resolution structural data, it is clear that substrate binding, non-saturating nucleotide and metal binding, or interaction with regulatory subunits may trigger asymmetric conformational changes within the oligomers. Therefore, the ATPase ring becomes heterogeneous even if all AAA+ subunits within the oligomer were chemically and structurally identical.
Hexameric ATPase ensembles with lower than sixfold symmetry
Recently, asymmetry has been suggested to be important in the functioning of AAA+ ATPases because deviations from the sixfold symmetry were observed in many ATPases (Fig. 3b). The asymmetry results in transient specialization of the subunits upon binding with nucleotides and substrates. Two of the most commonly met departures from sixfold symmetry of the ideal hexameric ring are packing with 2- or 3-fold axes. Such hexamers can be considered as dimers of trimers and trimers of dimers, respectively. These symmetries, C2 and C3, were observed by crystallography and EM more often than other deviations from the sixfold hexamers, for example for ClpX, HslU, FtsH, Vps4, and Lon [78–83].
The symmetry of some hexameric ATPases was found to be completely broken by formation of open or “notched” rings. E1 helicase was crystallized twice as an asymmetric hexamer in the presence and absence of DNA and ADP [27, 84]. “Opening” of the ring ensembles was also observed in MCM, NtrC1, double-tiered Ltag and NSF proteins, and similarly in RecA-like T7 helicase and DnaB, as shown both by EM and crystallographic studies [37, 85–88]. For nucleic acid translocases and helicases, opening of the ring or losing one or more subunits was hypothesized to be an important step for loading the ring ensemble onto the DNA/RNA strand [85, 89–91]. In those cases, it is clear that without the available free end of the nucleic acid, loading of the closed ring ATPase would not be topologically possible.
Sequence-defined subunit specialization in AAA+ motors
In the homooligomeric ATPases, reduction of the ensemble symmetry arises from partial nucleotide binding, hydrolysis and release and/or substrate interactions. Such specialization is fixed in heterooligomeric ATPases in which a functional AAA+ motor consists of non-identical subunits. There are several modes of formation of heterohexamers: a complex of two or six non-identical subunits or a pseudohexamer created by a single polypeptide chain. Most of these examples are found in eukaryotic ATPases. In such heterooligomeric ATPases non-equality of the subunits is encoded in the primary sequence of the ATPase subunits. In the heterohexameric AAA+ ATPases or covalently linked oligomers, asymmetry of the complex is intrinsic to the protein sequence and not necessarily defined by the presence of substrates. Moreover, sequence-defined asymmetry allows for additional layers of specific regulation of the activity of a given AAA+ motor. For example, peroxisomal Pex1/Pex6 ATPase hexamer is formed by interchanging Pex1 and Pex6 subunits [71, 92]. Similarly to Pex1/Pex6 heterohexamer, Rvb1 and Rvb2 ATPases from the chromatin remodeling complex form a single heterohexameric ring [73].
The minichromosome maintenance protein (MCM) that is essential for DNA replication in archaea and eukaryotes can serve as an example of AAA+ motors created by six different ATPase subunits. While six identical MCM subunits form a functional helicase in archaea, six different subunits, MCM2 through MCM7, come together in a functional heterohexamer MCM2–7 in eukaryotes [93]. Another example of a heterooligomeric ATPase motor is the complex formed by six different AAA+ subunits, Rpt1 through Rpt6, within the regulatory 19S particle of eukaryotic proteasome. This complex is analogous to the prokaryotic and archaeal proteasomal ATPases that consist of identical protomers, such as ClpX and PAN [28, 94].
Similar to heterohexameric ATPases formed by six non-identical subunits, there are pseudohexamers in which different ATPase subunits belong to a single polypeptide chain. Dyneins are the most characterized of such pseudohexameric ATPase motors, but there is also an example of ribosome biogenesis factor midasin/Rea1 [95, 96]. Dyneins and midasin contain six different AAA+ modules that are shown to arrange in a ring. Therefore, the dynein ring is an intrinsically asymmetric hexamer of covalently linked ATPase subunits [30, 31]. Sequence alignments and extensive biochemical studies showed that some of the ATPase subunits in dynein are unable to hydrolyze ATP confirming sequence-defined heterogeneity of these motors (i.e., [95, 97–99]).
Coordination of ATP hydrolysis within AAA+ ensembles
Based on the combination of biochemical and structural data several possible mechanisms were proposed for different AAA+ motors to describe succession of ATP hydrolysis events within the protein oligomer [2, 100–102]. These are concerted, rotary, sequential and stochastic mechanisms with variations (Fig. 4b). The concerted (or synchronized) mechanism was proposed in 2004 for some AAA+ motors, such as Ltag of SV40, for which only symmetric oligomeric structures were observed at the time. In these symmetric hexameric structures all sites are fully saturated with nucleotides and all subunits are structurally identical. This fact led to a hypothesis that all sites should be occupied before some signal can trigger a concerted hydrolysis in all subunits simultaneously [34, 103]. The rotary mechanism was proposed for the AAA+ ATPases because of the notable similarity of the ring-shaped motors to the F1-ATPase, for which the rotational mechanism was first hypothesized [104]. F1-ATPase is a heterohexamer consisting of three alternating β- and α-subunits with three catalytic sites located at the interfaces of the α and β pairs. ATP is thought to bind to the β subunits with hydrolysis occurring in one β subunit causing ADP in the second β subunit to escape and ATP to bind to its site in the apo form of the third β subunit. Thus the arrangement of the apo, ATP, and (ADP + Pi) sites rotates around the heterohexamer. Unlike F1-ATPase, AAA+ motors often have six active subunits. A rotational mechanism for such a hexamer would imply that due to allosteric effects every other subunit of the hexamer is inactive and the remaining three are in different states: hydrolysis, product release, and ATP binding. The sequential or asymmetric rotary mechanism was suggested for AAA + ATPases which were found to form asymmetrical oligomers in their crystal lattices. For instance, HslU was found to contain four nucleotides in some crystal structures and the hypothesis followed that all subunits are active but the hydrolysis steps are synchronized only for two subunits on opposite sides of the ring. Later, asymmetric structures were observed for other AAA+ motors showing that there are at least three classes of binding sites similar to those suggested earlier but distributed differently around the hexameric rings. The sequential and rotary mechanisms can both be considered as organized and directional movement of catalytically active site around the hexameric ensemble. Finally, recent biochemical studies of many AAA+ ATPases showed that ATP hydrolysis may be a stochastic (random) process with the “firing” happening randomly amongst ring active sites [100, 105].
Experimental approaches for characterization of dynamic and heterogeneous states of AAA+ motors
Traditionally, ring ATPases were analyzed with classic biochemical and biophysical methods. Most of these methods provide bulk, averaged information about AAA+ subunits as the output cannot distinguish behavior of individual subunits. For example, ATPase activity assays result in measuring apparent enzymatic activity per subunits or per ring. Using this approach, it is impossible to distinguish the order of subunits’ firing or difference in subunits’ activities. Other methods have a potential for characterizing individual protomers within the ring, but the modeling tools for such analyses are not developed or theoretically proven to be possible. Isothermal titration calorimetry (ITC) and fluorescence stopped-flow (SF) methods may serve as examples here, because not all complex dependencies can be fitted with existing models. Overall, models of cooperativity and allostery within the ring ensembles are not as well developed as for simpler oligomeric states of enzymes.
EM and X-ray crystallography are the only methods that can provide full atomic details of AAA+ motors and therefore shed light on structure of the intermediates of the ATPase cycle. In combination with solution state studies such as gel filtration, native gel electrophoresis, non-specific crosslinking and analytical ultracentrifugation, structural studies have brought us to the current state of understanding of AAA+ motor mechanisms. Nevertheless, there are obvious methodological biases in classic EM and crystallographic methods towards symmetric or fully saturated AAA+ ensembles. Before a few years ago, EM reconstruction methods often relied on picking certain particle configurations for analyses and applying high-symmetry restraints for modeling. The crystallization process itself is biased in selecting a uniform population of the macromolecules that form crystals, therefore “purifying” a particular subset from potentially heterogeneous population. Crystal nucleation may also require a very uniform population of macromolecules to begin with, such as fully saturated and thus uniform AAA+ rings. Despite these crystallization biases, the extensive body of work shows that AAA+ ring heterogeneity can be successfully captured using subsaturating nucleotide concentrations, nucleotide analogs [106], complexes with macromolecular substrate, as well as specific functional inhibitors. Moreover, recent developments allow for some time-resolved crystallographic experiments when crystals are amiable to soaking with small substrates [107].
Recent advances in structural methods and development of novel approaches might allow us to gain better understanding of AAA+ motors expanding beyond their oligomeric state and bulk biochemical behavior. These approaches are applicable not only for AAA+ but other types of ATPase motors. In the following section of the review, I collected examples of analyses of multimeric ATPases with classic or novel methods that allow one to distinguish behavior of individual subunits in the ring or characterize heterogeneity of the complex.
Electron microscopy
Earlier structure reconstruction methods from EM data in many cases relied on modeling with symmetry restraints, such as sixfold symmetry for the ring ATPases, similar to modeling data from solution X-ray scattering experiments [108]. Moreover, observed conformational heterogeneity in the particles was averaged by analyses. Recent developments in EM and single-particle analyses methods allow for more flexible approaches.
Cryo-EM analyses of AAA+ rings of protein-remodeling factor Hsp104 were done both with and without applying sixfold symmetry. Model maps built without symmetry restraints showed strong asymmetry within the two tandem AAA+ rings [70]. Based on structural analyses, different nucleotide occupancies within the asymmetric ring were hypothesized to originate from a sequential ATP hydrolysis around the ring. Similarly, more recent work on ClpB ATPase used modeling of negative stain EM datasets with and without sixfold symmetrization [109]. Asymmetric maps of AAA+ rings, obtained without C6 restraints, clearly show heterogeneity within the hexamer subunits and positions of adjacent middle domains (MD). Such asymmetry was then again attributed to partial ring occupancy with nucleotides based on following structure analyses, crystal structure docking, and characterization of nucleotide binding by ITC. Similar symmetry relaxation was done for mitochondrial replicative helicase of RecA-type, TWINKLE [110].
For example, it is well documented that p97 ATPase shows a significant conformational heterogeneity upon EM analyses [111] some of which concerns not only the added flexible N-terminal domain, but rather the conformation of the two ATPase rings (D1 and D2 rings). In a recent cryo-EM study of p97 ATPase [111], it was noted that there are two types of side view class averages for the particles and these two types are present in all three tested nucleotide states of the protein. To model the differences, researchers split the overall image set into two subsets that were then processed separately and led to reconstructions that show two co-existing populations of p97 particles as well as new type of structural re-arrangement in the juxtaposition of the D1–D2 rings [111]. Similar to prior studies [112], the analyses were still done with imposing C6 symmetry. Despite the improvement in the reconstruction method, the authors note that for more detailed characterization of the observed structural heterogeneity in p97 particles, new methodological developments are required [111]. In the most recent EM studies, authors applied this method to reconstruct three different co-existing conformational states in the presence of ATPγS nucleotide and modeled these states without imposed symmetry [113]. Interestingly, despite abundant crystallographic data for modelling and enhanced resolution, some state that EM cannot fully capture the heterogeneity of the rings and their conformational flexibility [113–115]. Most recent advances in cryo-EM data collection and modeling resulted in establishing atomic resolution structures of several AAA+ motors without imposing any symmetry constraints, and the resulting structures show a great level of asymmetry (i.e., p97, 26S proteasome, and NSF [88, 113, 116, 117]; also see EM-derived NSF structure in Fig. 3b).
X-ray and neutron solution scattering
Small-angle X-ray and neutron scattering (SAXS and SANS, respectively) are techniques that allow one to follow the conformation of a macromolecule in solution, close to the native physiological environment. SAXS showed a great potential in monitoring ATPase oligomers in solution upon various conditions. Moreover, for several ATPases, SAXS was used to establish that nucleotide binding dramatically affected the overall shape of the ring [32, 87, 118, 119]. For example, bacterial transcription activator NtrC1 and p97 protein were analyzed in the presence of different nucleotides and showed significant conformational changes that were later detected by EM and crystallography methods. Moreover, time-resolved SAXS is a developing unique method allowing one to follow conformational dynamics of biomolecules in solutions [120, 121]. It was used to follow conformational changes in NtrC1, GroEL, and KaiC ATPases over time [87, 122, 123].
Unfortunately, due to the random orientations of the particles of interest, such as ring ATPases, in solution there is limited amount of structural information that can be extracted from SAXS/SANS data [124]. Analysis of the complex mixtures of differently occupied ring ATPases and/or asymmetric ensembles is complicated, because it is not easy to model mixed populations of ATPases in different states and hard to model asymmetric ensembles without establishing a unique structure solution. Currently, new modeling methods have been developed that allow for modeling more complex structures and mixtures (i.e., reviewed in [125]). Moreover, recently described metrics for gauging the quality of the modeling have been introduced [126] and online SAXS data and models databases are now available (http://www.bioisis.net and http://www.sasbdb.org [127]) which in future might allow for easy comparison and modeling improvements.
Despite the fact that SAXS data modeling still represents significant challenge it is one of the methods that allows the following of conformational and oligomerization changes over time. Other methods such as fluorescence and heat effect measurements usually cannot assess structural features (with exception of distance information from FRET and quenching methods) upon measurement of temporal dependencies. Dynamic light scattering and gel filtration methods on the other hand might provide general sizing parameters such as Stokes radii but it is not feasible to add time dimension to these methods. Overall, using a combination of several structural methods (EM, crystallography, NMR, SAXS) with biochemical studies shows the best results in assessing AAA+ function (see for example, [87, 92, 128]).
Mixing different variants of AAA+ subunits
It is tricky to study nucleotide–protein and subunits interactions within the ring-like ensembles of the multimeric ATPases. Similar to studies of other multimeric enzymes, AAA+ function was often probed by mixing differently modified ATPase subunits. Such mixing experiments are sometimes referred to as “mutant titration experiments” or “mutant doping” (Fig. 5a). This approach is often applied to estimate how many catalytically active subunits among the ring are required for proper functioning of the motor, to establish functional oligomerization, to map intersubunit coordination or to evaluate how many subunits are involved in substrate binding. Extensive analyses for PspF transcription activator were done by mixing differently modified inactive subunits to identify resulting ATPase activity or interaction with the substrate [101, 129]. For example, mixing equimolar amounts of WB mutants with subunits substituted in putative R-fingers (all these mutant versions are catalytically inactive by themselves) in several cases resulted in partial restoration of ATPase activity [129].
Fig. 5.
Experimental approaches to analyze subunit heterogeneity of the AAA+ motors. a Controlling identities of AAA+ ring subunits using site-directed mutagenesis and covalent linking. Biochemical and structural information can be used to create ATPase variants that have altered functionality (orange). For example, substitutions in the WB motif were shown to abrogate ATP hydrolysis. By doping wild-type protein (green) with altered copies, one can assess AAA+ properties (I). By encoding single-chain oligomers, researchers successfully created ATPase complexes with certain distribution of altered AAA+ subunits (II, IV–VI). With guidance of structural data, site-specific crosslinking can be used to restrain target conformational changes (III–IV) and to affix the oligomers (IV–V). b Using fluorophore labeling to monitor conformational changes within an AAA+ subunit by measuring FRET signal or fluorescence quenching. c Fluorophore labeling of AAA+ protein can be used to measure nucleotide binding. In combination with methods from a this approach was successfully used to study heterogeneity of nucleotide binding to the AAA+ ring
In another work, Eckert and colleagues use wild-type and disease-relevant WB mutant of spastin AAA+ motors and show that even a low number of mutant subunits can inhibit spastin’s hexamer function [130]. Mathematical modeling of the data supported the simplest explanation that the interaction of two, possibly neighboring, subunits dominates cooperative effects. Similar dominant-negative effect of non-hydrolyzing subunits were observed in ClpB protein in the presence of its co-chaperone DnaK system [131]. Interestingly in the absence of DnaK, an equal ratio of wild type and inactive subunits resulted in maximal enzymatic activity. Analogous approaches were applied to analyze the function of many ATPases, such as ClpX, ClpB, Rubisco activase Rca, viral DNA packaging ATPase gp16 of Phi29 [56, 131–134].
The downside of this approach is the complicated mathematical analysis and uncertainty of the subunit arrangements. For analyses of mixing experiments, the subunit arrangement is often assumed to follow random mixing based on subunit ratio, but in practice it is often severely biased. For example, strong bias can be introduced by the presence of additional to AAA+ functional domains with dimerization interfaces, such as the N-domain in ClpX and receiver and DNA binding domains in NtrC homologs [28, 135, 136]. Moreover, mutant subunits might be trapped in a particular nucleotide state resulting in preferential oligomerization. As the result, measured biochemical properties would not reflect the behavior of assumed by random mixing ensembles and would be hard to interpret.
Covalently linked ATPase oligomers
Martin and colleagues used an elegant way to probe ClpX ATPase function, but avoid biases and uncertainties of mixing experiments [100]. The researchers encoded six ClpX subunits within a single gene producing covalently linked ATPase oligomers (Fig. 5a). The AAA+ ATPase subunits were spaced with linkers between subunits long enough to allow hexamer assembly. Such linkage allowed for fixing the position of wild-type and mutant protomers within the ring with respect to each other. This approach allowed the investigation of how many catalytically active subunits within the ClpX hexamer are required for full activity and resulted in a model of stochastic “firing” within the ring (Fig. 4b, [100]). Unfortunately, only few proteins thus far have been shown to be amenable to this technique and until now the main hurdle is the solubility of the covalently linked oligomer. In most cases, dimers and trimers of an ATPase can be obtained in suitable quantities and with native activity. Since these early studies, a covalent linkage approach has been used to crystallize dimer of covalent trimers of ClpX resulting in establishing a distorted hexamer with a twofold symmetry axis [78] mentioned above (Fig. 3b). For example, working with covalent trimers of ClpX, Sauer’s group was able to crystallize several new states of the enzyme [78, 137] that resemble those seen by EM and exhibit a significant asymmetry of the pseudohexamers with individual ClpX subunits being in open (L) or closed, unloadable (U) states (Fig. 4a). Similar fusion approach was applied for transcription activator PspF as well as RecA-like ATPases, FtsK and RecA itself [138–140].
Site-specific crosslinking
When expression of a single-chain ATPase oligomer is not possible but detailed structural information is available, site-directed crosslinking can serve as a good alternative. Specific crosslinking covalently fixes an oligomeric assembly or restrict conformational changes within a macromolecule or a complex yielding some insights into an AAA+ motor function (Fig. 5a).
With relative ease, cysteine residues can be introduced into an ATPase subunit via standard site-directed mutagenesis. Cysteine pairs, located in close proximity at the intersubunit interfaces within the ATPase ring, can be oxidized into disulfides or be linked by bifunctional crosslinkers such as 1,6-bismaleimidohexane (BMH). Such crosslinking results in covalently linked ATPase oligomers [105, 128, 137, 141, 142]. Cysteine pairs might also be positioned to lock a certain subunit conformation [105]. Due to this property of restraining certain conformations or complexes, the approach is sometimes dubbed as “cysteine staples”. Using shorter-than-hexamer linear oligomers of ATPase subunits in combination with cysteine staples opens up a broad range of available experiments.
Cysteine staples have been used to fix the assembly of ClpB subunits [142]. The Cys-crosslinked ClpB oligomer did not show lower activity in substrate unfolding and therefore showed that disassembly of the ring ATPase is not required for efficient function. Similarly, Glynn and colleagues also used available high-resolution information about ClpX ATPase to create covalent cysteine-crosslinked pseudohexamers from monomeric, dimeric, or trimeric constructs of ClpX ATPase domain [141]. By introducing Cys mutations at the subunit interfaces researchers tested how these substitutions and their crosslinking affected the ATPase and unfolding activities and showed importance of intersubunit interactions for force generation [141].
Most recently, several peptide tags were developed for specific covalent crosslinking of proteins using sortase and SpyCatcher that do not rely on cysteine residues [143, 144]. Added to AAA+ ATPase, sortase signals and specific TEV protease sites were successfully used to control oligomerization states of ClpX ATPase [105].
The restrictions of the method of the cysteine staples are evident. For example, the ATPase should be active without any natively present cysteine residues and with newly introduced ones. Therefore, only ATPases that are well structurally characterized benefit from the targeted crosslinking. Without existing structural information, placing cysteine residues within the ATPase subunits may require a lot of screening or may not be interpretable. Even though with cysteine crosslinking some arrangements of the active and non-active subunits might be not achievable, this method provides a new powerful tool for investigating the mechanism of ring ATPases.
Bulk solution fluorescence methods—Foerster resonance energy transfer and fluorescence quenching
Several fluorescence-based methods were used to analyze the AAA+ mechanisms by assaying structural changes at different length scales. Fluorescence (or Foerster) resonance energy transfer (FRET) allows one to monitor conformational changes of scale of 20–80 Å while quenching of fluorescence dyes can be used to monitor structural changes at a few Å scale. In the FRET approach, the most well-known examples include numerous single molecule studies for AAA+ translocases of nucleic acids and protein unfoldases. Since these FRET-based and other single-molecule measurements are extremely versatile I would refer the reader to recent reviews that go into details of the approaches (i.e., [145–150]). Single molecule studies of well structurally and biochemically characterized ATPase motors yield unique mechanistic insights including, but not limited to measured force generation and number of ATP hydrolyzed per functional step.
Apart from single molecule studies there are several methods that can assess AAA+ mechanisms via bulk fluorescence measurements in solution or even in living cells. For example, a recent study of ClpX ATPase employed modifications of transition metal ion FRET (tmFRET) to assess function of this AAA+ motor [137, 151]. tmFRET utilizes ability of some metal ions (Ni2+, Co2+, and Cu2+) to serve as energy acceptors for a short-range FRET (10–20 Å) from visible light fluorophores, resulting in fluorescence quenching [151].
The first method cCoMET (where CoMET stands for coordinated metal energy transfer) employs rhodamine dye and Ni2+ that quenches fluorescence when brought into the dye proximity. cCoMET can be used to gauge short range conformational changes in a protein (Fig. 5b). The second method nCoMET utilizes quenching of cobalt cation (Co2+) fluorescence by proximal Oregon Green fluorophore to analyze nucleotide binding of an AAA+ motor (Fig. 5c). In this approach, one protein residue is labeled with Oregon Green dye while Co2+ substitutes for usual Mg2+ in the nucleotide complex with a divalent metal. The residue to label is chosen to ensure that binding of Co2+-ATP results in metal ion being closer than the calculated Foerster radius of about 13 Å (i.e., Stinson et al., 2013 attach fluorophore to the cysteine-substituted 363th residue of ClpX unfoldase). Importantly, both cCoMET and nCoMET measurements of subunit-specific nucleotide binding were possible with using of covalently linked subunits providing fixed spatial distribution of wild-type and nucleotide-binding incompetent subunits as well as positioning of fluorophores in specific locations within the AAA+ ring. Stinson and colleagues also used fluorescence quenching of two TAMRA dyes attached to one residue in the small domain of ClpX subunit and to the second residue in the large domain of the next subunit to address stoichiometry of active and inactive ATPase subunits within the motor ring [137].
FRET measurements were also done with two different fluorophore-labeled nucleotides that can bind to neighboring or other pair of subunits and result in detectable energy transfer from one fluorophore to another. For example, mant-ATP and TNP-ATP were used to gauge the interaction of subunits in archaeal and eukaryotic proteasomal ATPases [152]. Authors used WB mutants of the ATPases with estimated ~36 to 75 Å distances between various sets of nucleotide binding pockets within the hexamer, allowing for FRET between nucleotides.
Labeling separate subunits with fluorophores, such as green fluorescent protein derivatives is also possible. In vivo FRET life-time microscopy (FLIM) was applied to assay homooligomerization of CDC48 ATPase that was labeled with either cerulean or yellow fluorescent proteins (CrFP and YFP, respectively; [153]). As a result, the authors could observe in vivo oligomerization of the ATPase. Though only interaction of two subunits can be detected this way, it is one of the few in vivo assessments of an AAA+ motor function.
Mass spectrometric studies of subunits and nucleotide stoichiometry and conformational changes
Advanced mass spectrometry (MS) methods address stoichiometry of the multimeric ATPases and their interaction with nucleotides directly. Such MS analyses are done with soft evaporation techniques that preserve protein complexes and protein-nucleotide interactions.
For example, subunit–subunit quaternary structures of bacterial enhancer binding protein (EBP) NtrC4 was analyzed by MS to establish that the presence of accessory domains in the full length protein shifts the oligomeric state of the NtrC4 ATPase from seven to six [33]. Another EBP PspF was analyzed using similar MS approach to observe which nucleotides are bound and to what oligomer of PspF upon the ATPase’s interaction with the target sigma54 protein [154].
Allostery of nucleotide binding to the chaperone ATPase GroEL was studied via MS [155], because structural MS allows quantifying populations of the ATPase oligomers differently occupied with nucleotides. Such a study would be impossible in bulk solution biochemical assays. This method is also shown to be useful for analyses of equilibrium mixtures that are often found in preparations of multimeric ATPases (see notion about the EM studies above).
Differential hydrogen/deuterium exchange (HDX) mass spectrometric analyses is a method that accurately measures peptide mass increases due to amide hydrogen exchanging to deuterium [156, 157]. Because the HD exchange rates strongly depend on conformation of the biomolecules and their accessibility to solvent, this technique was successfully applied to analyze conformational changes and complexation of ATPase motors [158–160]. For instance, HDX was recently applied to elucidate conformational changes within DnaB ATPase in complex with DnaC ATPase. By analyses of which surfaces of the ATPase motors are more exposed to the solvent, authors concluded that DnaC keeps DnaB in an open conformation [159] consistent with prior reports.
High-speed atomic force microscopy (HS-AFM)
High-speed atomic force microscopy (HS-AFM) is a scanning probe microscopy technique that was developed to observe undisturbed dynamics of biological macromolecules [161–163]. In tapping mode, HS-AFM obtains protein dynamic records with spatiotemporal resolution unachievable by other methods delivering sub-second to sub-100 ms temporal resolution at nm to sub-nm spatial resolution. The scanning probe of a nanoscale cantilever vibrates with resonance frequency and feeds back the changes in height of a biological molecule at the surface. One of the disadvantages of the AFM method is the necessity of affixing the sample protein onto mica surface via covalent linkage. Due to the AAA+ ring geometry it usually flattens the complexes on the surface, which allows top- or bottom-view observations but might affect the progression of structural changes. There are a few studies that utilized this novel technique to address structural changes within ATPases of AAA+ and RecA-type families. CDC48/p97 protein was analyzed by HS-AFM method [164], and the results show that in presence of ATP, but not ADP, one of the two AAA+ rings repeatedly rotates about 23°. When HS-AFM applied to F1-ATPase, the experiments showed that catalytic β subunits cycle between open and closed states such that only one of the three β subunits is open at a time, suggestive of sequential reaction cycle mechanism [165]. To date this may be the closest to what is available to direct observation of full cycle of conformation changes associated with ATP catalysis.
Additional considerations of experimental setup
Since the AAA+ motors are exquisitely flexible and sensitive ensembles, several factors should be taken into consideration in choosing the experimental approach to study them.
AAA+ motors usually contain functional domains other than the ATPase domain itself. While studying a particular AAA+ protein in isolation is a common and very effective practice, it is important to keep in mind that presence of accessory domains, certain truncations of the ATPase fold, and presence of affinity tags were often shown to affect the assembly of the multimeric ATPases. For example, mass spectrometry analysis of truncated mutants and the full-length form of NtrC4 transcription activator showed that heptamer and other stoichiometries are formed only for the truncated mutants, while the full-length protein organizes into a hexamer [33]. However, there are instances when smaller fragments of an ATPase form hexamers and longer fragments in fact assemble into heptamers. For example, fragment 4D of the T7 helicase forms hexamers and the full-length T7 helicase-primase oligomerizes into heptamers [36, 37]. Another example is Rvb1 protein which formed double hexameric rings when purified with His-tag. Without the His-tag, Rvb1 formed single-layered rings [72].
Since ring ATPases are exquisitely sensitive to the nucleotide state, one may observe different quaternary structures upon presence of different nucleotides (i.e., [166]. and other examples throughout the review). It was shown that due to the ring allostery ATPase oligomers often display sub-stoichiometric nucleotide binding even in the presence of saturating concentrations of nucleotides and “forcing full occupancy” might thus result in non-physiological states of the ATPase motors. Moreover, in some cases use of non-hydrolyzable analogs, while being a general practice in work with the ring ATPases, may result in altered oligomeric state or conformation (i.e., Vps4 example described in [167]). The other standard technique of using catalytic mutants of the ATPases may also result in changed state that might be non-physiological.
In many cases the presence of non-hexameric ensembles cannot be easily attributed to an artifact because the full-length protein is being analyzed under close to physiological conditions and in the presence of nucleotides or native substrates. The co-existence of hexamers and heptamers of T7 helicases and transition of RuvB from one form to another suggest that multiple oligomeric forms can be functional and are yet to be appreciated. These instances also demonstrate that the presence of nucleotides and substrate play a significant role in promoting a particular organization of the subunits within an AAA+ motor. In most of the cases, the significance of the AAA+ polymorphism is unknown.
Lastly, limitations of the experimental methods used for analyses of an AAA+ motor should be considered, such as need for imposing symmetry for data processing (EM, SAXS, SANS) and building adequate models (ITC, SF, SPR, mixing subunits, ATPase activity and such).
Concluding remarks
Owing to two decades of structural and biochemical studies, there is great progress made in our understanding of how the AAA+ motors work. Nevertheless, there are still many unanswered questions. While this review focuses on diversity of AAA+ ensembles and heterogeneity of the subunits within these ensembles, it does not cover other important aspects of the AAA+ functioning. It is well established that structural coupling in the AAA+ ensembles allow for various levels of coordination of catalysis events among the ring active sites. Moreover, dynamic and specific interactions of AAA+ motors with their substrates affect the ATPase activity, ensemble stoichiometry, and structure. To date, the molecular details of substrate binding are elucidated for relatively low number of AAA+ ATPases. On top of regulation by nucleotide and substrate binding, AAA+ function is often modulated by interaction with multiple adaptor proteins. Further studies into these unique molecular machines will help us better understand how AAA+ motors work, develop methods to regulate their function as well as design novel molecular motors on demand.
Acknowledgments
I would like to thank Drs. Marina Besprozvannaya, Lia Cardarelli, Baoyu Chen, Prashanti Iyer, Ryan Tsoi, and my anonymous reviewers for their helpful comments and suggestions on the manuscript. I apologize for not including a significant number of relevant and detailed studies from my colleagues in the field on account of limited space.
References
- 1.Neuwald AF, Aravind L, Spouge JL, Koonin EV. AAA+: a class of chaperone-like ATPases associated with the assembly, operation, and disassembly of protein complexes. Genome Res. 1999;9:27–43. [PubMed] [Google Scholar]
- 2.Ogura T, Wilkinson AJ. AAA+ superfamily ATPases: common structure–diverse function. Genes Cells. 2001;6:575–597. doi: 10.1046/j.1365-2443.2001.00447.x. [DOI] [PubMed] [Google Scholar]
- 3.Maurizi MR, Li CC. AAA proteins: in search of a common molecular basis. International meeting on cellular functions of AAA proteins. EMBO Rep. 2001;2:980–985. doi: 10.1093/embo-reports/kve229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lupas AN, Martin J. AAA proteins. Curr Opin Struct Biol. 2002;12:746–753. doi: 10.1016/S0959-440X(02)00388-3. [DOI] [PubMed] [Google Scholar]
- 5.Wang J. Nucleotide-dependent domain motions within rings of the RecA/AAA(+) superfamily. J Struct Biol. 2004;148:259–267. doi: 10.1016/j.jsb.2004.07.003. [DOI] [PubMed] [Google Scholar]
- 6.Ammelburg M, Frickey T, Lupas AN. Classification of AAA+ proteins. J Struct Biol. 2006;156:2–11. doi: 10.1016/j.jsb.2006.05.002. [DOI] [PubMed] [Google Scholar]
- 7.Hanson PI, Whiteheart SW. AAA+ proteins: have engine, will work. Nat Rev Mol Cell Biol. 2005;6:519–529. doi: 10.1038/nrm1684. [DOI] [PubMed] [Google Scholar]
- 8.Iyer LM, Leipe DD, Koonin EV, Aravind L. Evolutionary history and higher order classification of AAA+ ATPases. J Struct Biol. 2004;146:11–31. doi: 10.1016/j.jsb.2003.10.010. [DOI] [PubMed] [Google Scholar]
- 9.White SR, Lauring B. AAA+ ATPases: achieving diversity of function with conserved machinery. Traffic. 2007;8:1657–1667. doi: 10.1111/j.1600-0854.2007.00642.x. [DOI] [PubMed] [Google Scholar]
- 10.Barthelme D, Jauregui R, Sauer RT. An ALS disease mutation in Cdc48/p97 impairs 20S proteasome binding and proteolytic communication. Protein Sci. 2015;24:1521–1527. doi: 10.1002/pro.2740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Xia D, Tang WK, Ye Y. Structure and function of the AAA+ ATPASE p97/Cdc48p. Gene. 2016;583:64–77. doi: 10.1016/j.gene.2016.02.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hafezparast M, Klocke R, Ruhrberg C, Marquardt A, et al. Mutations in dynein link motor neuron degeneration to defects in retrograde transport. Science. 2003;300:808–812. doi: 10.1126/science.1083129. [DOI] [PubMed] [Google Scholar]
- 13.Eschbach J, Dupuis L. Cytoplasmic dynein in neurodegeneration. Pharmacol Ther. 2011;130:348–363. doi: 10.1016/j.pharmthera.2011.03.004. [DOI] [PubMed] [Google Scholar]
- 14.Chen XJ, Xu H, Cooper HM, Liu Y. Cytoplasmic dynein: a key player in neurodegenerative and neurodevelopmental diseases. Sci China Life Sci. 2014;57:372–377. doi: 10.1007/s11427-014-4639-9. [DOI] [PubMed] [Google Scholar]
- 15.Boyaci H, Shah T, Hurley A, Kokona B, et al. Structure, regulation, and inhibition of the quorum-sensing signal integrator LuxO. PLoS Biol. 2016;14:e1002464. doi: 10.1371/journal.pbio.1002464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Erzberger JP, Berger JM. Evolutionary relationships and structural mechanisms of AAA plus proteins. Annu Rev Biophys Biomol Struct. 2006;35:93–114. doi: 10.1146/annurev.biophys.35.040405.101933. [DOI] [PubMed] [Google Scholar]
- 17.Thomsen ND, Berger JM. Structural frameworks for considering microbial protein- and nucleic acid-dependent motor ATPases. Mol Microbiol. 2008;69:1071–1090. doi: 10.1111/j.1365-2958.2008.06364.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Frickey T, Lupas AN. Phylogenetic analysis of AAA proteins. J Struct Biol. 2004;146:2–10. doi: 10.1016/j.jsb.2003.11.020. [DOI] [PubMed] [Google Scholar]
- 19.Iyer LM, Makarova KS, Koonin EV, Aravind L. Comparative genomics of the FtsK-HerA superfamily of pumping ATPases: implications for the origins of chromosome segregation, cell division and viral capsid packaging. Nucleic Acids Res. 2004;32:5260–5279. doi: 10.1093/nar/gkh828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Walker JE, Saraste M, Runswick MJ, Gay NJ. Distantly related sequences in the alpha- and beta-subunits of ATP synthase, myosin, kinases and other ATP-requiring enzymes and a common nucleotide binding fold. EMBO J. 1982;1:945–951. doi: 10.1002/j.1460-2075.1982.tb01276.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Snider J, Houry WA. AAA+ proteins: diversity in function, similarity in structure. Biochem Soc Trans. 2008;36:72–77. doi: 10.1042/BST0360072. [DOI] [PubMed] [Google Scholar]
- 22.Wendler P, Ciniawsky S, Kock M, Kube S. Structure and function of the AAA+ nucleotide binding pocket. Biochim Biophys Acta. 2012;1823:2–14. doi: 10.1016/j.bbamcr.2011.06.014. [DOI] [PubMed] [Google Scholar]
- 23.Ogura T, Whiteheart SW, Wilkinson AJ. Conserved arginine residues implicated in ATP hydrolysis, nucleotide-sensing, and inter-subunit interactions in AAA and AAA+ ATPases. J Struct Biol. 2004;146:106–112. doi: 10.1016/j.jsb.2003.11.008. [DOI] [PubMed] [Google Scholar]
- 24.Zhang X, Wigley DB. The ‘glutamate switch’ provides a link between ATPase activity and ligand binding in AAA+ proteins. Nat Struct Mol Biol. 2008;15:1223–1227. doi: 10.1038/nsmb.1501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lee SY, De La Torre A, Yan D, Kustu S, et al. Regulation of the transcriptional activator NtrC1: structural studies of the regulatory and AAA+ ATPase domains. Genes Dev. 2003;17:2552–2563. doi: 10.1101/gad.1125603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Park E, Rho YM, Koh OJ, Ahn SW, et al. Role of the GYVG pore motif of HslU ATPase in protein unfolding and translocation for degradation by HslV peptidase. J Biol Chem. 2005;280:22892–22898. doi: 10.1074/jbc.M500035200. [DOI] [PubMed] [Google Scholar]
- 27.Enemark EJ, Joshua-Tor L. Mechanism of DNA translocation in a replicative hexameric helicase. Nature. 2006;442:270–275. doi: 10.1038/nature04943. [DOI] [PubMed] [Google Scholar]
- 28.Sauer RT, Baker TA. AAA+ proteases: ATP-fueled machines of protein destruction. Annu Rev Biochem. 2011;80:587–612. doi: 10.1146/annurev-biochem-060408-172623. [DOI] [PubMed] [Google Scholar]
- 29.Arias-Palomo E, Berger JM. An atypical AAA+ ATPase assembly controls efficient transposition through DNA remodeling and transposase recruitment. Cell. 2015;162:860–871. doi: 10.1016/j.cell.2015.07.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kon T, Sutoh K, Kurisu G. X-ray structure of a functional full-length dynein motor domain. Nat Struct Mol Biol. 2011;18:638–642. doi: 10.1038/nsmb.2074. [DOI] [PubMed] [Google Scholar]
- 31.Carter AP, Cho C, Jin L, Vale RD. Crystal structure of the dynein motor domain. Science. 2011;331:1159–1165. doi: 10.1126/science.1202393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chen B, Sysoeva TA, Chowdhury S, Guo L, et al. Engagement of arginine finger to ATP triggers large conformational changes in NtrC1 AAA+ ATPase for remodeling bacterial RNA polymerase. Structure. 2010;18:1420–1430. doi: 10.1016/j.str.2010.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Batchelor JD, Sterling HJ, Hong E, Williams ER, et al. Receiver domains control the active-state stoichiometry of Aquifex aeolicus sigma54 activator NtrC4, as revealed by electrospray ionization mass spectrometry. J Mol Biol. 2009;393:634–643. doi: 10.1016/j.jmb.2009.08.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dey S, Biswas M, Sen U, Dasgupta J. Unique ATPase site architecture triggers cis-mediated synchronized ATP binding in heptameric AAA+-ATPase domain of flagellar regulatory protein FlrC. J Biol Chem. 2015;290:8734–8747. doi: 10.1074/jbc.M114.611434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rohrwild M, Pfeifer G, Santarius U, Muller SA, et al. The ATP-dependent HslVU protease from Escherichia coli is a four-ring structure resembling the proteasome. Nat Struct Biol. 1997;4:133–139. doi: 10.1038/nsb0297-133. [DOI] [PubMed] [Google Scholar]
- 36.Toth EA, Li Y, Sawaya MR, Cheng Y, et al. The crystal structure of the bifunctional primase-helicase of bacteriophage T7. Mol Cell. 2003;12:1113–1123. doi: 10.1016/S1097-2765(03)00442-8. [DOI] [PubMed] [Google Scholar]
- 37.Singleton MR, Sawaya MR, Ellenberger T, Wigley DB. Crystal structure of T7 gene 4 ring helicase indicates a mechanism for sequential hydrolysis of nucleotides. Cell. 2000;101:589–600. doi: 10.1016/S0092-8674(00)80871-5. [DOI] [PubMed] [Google Scholar]
- 38.Ziebarth TD, Gonzalez-Soltero R, Makowska-Grzyska MM, Nunez-Ramirez R, et al. Dynamic effects of cofactors and DNA on the oligomeric state of human mitochondrial DNA helicase. J Biol Chem. 2010;285:14639–14647. doi: 10.1074/jbc.M109.099663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yu X, VanLoock MS, Poplawski A, Kelman Z, et al. The Methanobacterium thermoautotrophicum MCM protein can form heptameric rings. EMBO Rep. 2002;3:792–797. doi: 10.1093/embo-reports/kvf160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gomez-Llorente Y, Fletcher RJ, Chen XS, Carazo JM, et al. Polymorphism and double hexamer structure in the archaeal minichromosome maintenance (MCM) helicase from Methanobacterium thermoautotrophicum . J Biol Chem. 2005;280:40909–40915. doi: 10.1074/jbc.M509760200. [DOI] [PubMed] [Google Scholar]
- 41.Kim KI, Cheong GW, Park SC, Ha JS, et al. Heptameric ring structure of the heat-shock protein ClpB, a protein-activated ATPase in Escherichia coli . J Mol Biol. 2000;303:655–666. doi: 10.1006/jmbi.2000.4165. [DOI] [PubMed] [Google Scholar]
- 42.Lee S, Sowa ME, Watanabe YH, Sigler PB, et al. The structure of ClpB: a molecular chaperone that rescues proteins from an aggregated state. Cell. 2003;115:229–240. doi: 10.1016/S0092-8674(03)00807-9. [DOI] [PubMed] [Google Scholar]
- 43.Watanabe YH, Takano M, Yoshida M. ATP binding to nucleotide binding domain (NBD)1 of the ClpB chaperone induces motion of the long coiled-coil, stabilizes the hexamer, and activates NBD2. J Biol Chem. 2005;280:24562–24567. doi: 10.1074/jbc.M414623200. [DOI] [PubMed] [Google Scholar]
- 44.Akoev V, Gogol EP, Barnett ME, Zolkiewski M. Nucleotide-induced switch in oligomerization of the AAA+ ATPase ClpB. Protein Sci. 2004;13:567–574. doi: 10.1110/ps.03422604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Davies JM, Brunger AT, Weis WI. Improved structures of full-length p97, an AAA ATPase: implications for mechanisms of nucleotide-dependent conformational change. Structure. 2008;16:715–726. doi: 10.1016/j.str.2008.02.010. [DOI] [PubMed] [Google Scholar]
- 46.Zhang X, Shaw A, Bates PA, Newman RH, et al. Structure of the AAA ATPase p97. Mol Cell. 2000;6:1473–1484. doi: 10.1016/S1097-2765(00)00143-X. [DOI] [PubMed] [Google Scholar]
- 47.Cha SS, An YJ, Lee CR, Lee HS, et al. Crystal structure of Lon protease: molecular architecture of gated entry to a sequestered degradation chamber. EMBO J. 2010;29:3520–3530. doi: 10.1038/emboj.2010.226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Stahlberg H, Kutejova E, Suda K, Wolpensinger B, et al. Mitochondrial Lon of Saccharomyces cerevisiae is a ring-shaped protease with seven flexible subunits. Proc Natl Acad Sci USA. 1999;96:6787–6790. doi: 10.1073/pnas.96.12.6787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Miyata T, Yamada K, Iwasaki H, Shinagawa H, et al. Two different oligomeric states of the RuvB branch migration motor protein as revealed by electron microscopy. J Struct Biol. 2000;131:83–89. doi: 10.1006/jsbi.2000.4290. [DOI] [PubMed] [Google Scholar]
- 50.Kazmirski SL, Podobnik M, Weitze TF, O’Donnell M, et al. Structural analysis of the inactive state of the Escherichia coli DNA polymerase clamp-loader complex. Proc Natl Acad Sci USA. 2004;101:16750–16755. doi: 10.1073/pnas.0407904101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kelch BA, Makino DL, O’Donnell M, Kuriyan J. How a DNA polymerase clamp loader opens a sliding clamp. Science. 2011;334:1675–1680. doi: 10.1126/science.1211884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hedglin M, Kumar R, Benkovic SJ. Replication clamps and clamp loaders. Cold Spring Harb Perspect Biol. 2013;5:a010165. doi: 10.1101/cshperspect.a010165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Alam TI, Rao VB. The ATPase domain of the large terminase protein, gp17, from bacteriophage T4 binds DNA: implications to the DNA packaging mechanism. J Mol Biol. 2008;376:1272–1281. doi: 10.1016/j.jmb.2007.12.041. [DOI] [PubMed] [Google Scholar]
- 54.Mao H, Saha M, Reyes-Aldrete E, Sherman MB, et al. Structural and molecular basis for coordination in a viral DNA packaging motor. Cell Rep. 2016;14:2017–2029. doi: 10.1016/j.celrep.2016.01.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Moffitt JR, Chemla YR, Aathavan K, Grimes S, et al. Intersubunit coordination in a homomeric ring ATPase. Nature. 2009;457:446–450. doi: 10.1038/nature07637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Schwartz C, De Donatis GM, Fang H, Guo P. The ATPase of the phi29 DNA packaging motor is a member of the hexameric AAA+ superfamily. Virology. 2013;443:20–27. doi: 10.1016/j.virol.2013.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Schwartz C, De Donatis GM, Zhang H, Fang H, et al. Revolution rather than rotation of AAA+ hexameric phi29 nanomotor for viral dsDNA packaging without coiling. Virology. 2013;443:28–39. doi: 10.1016/j.virol.2013.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Qi S, Pang Y, Hu Q, Liu Q, et al. Crystal structure of the Caenorhabditis elegans apoptosome reveals an octameric assembly of CED-4. Cell. 2010;141:446–457. doi: 10.1016/j.cell.2010.03.017. [DOI] [PubMed] [Google Scholar]
- 59.Mansilla-Soto J, Yoon-Robarts M, Rice WJ, Arya S, et al. DNA structure modulates the oligomerization properties of the AAV initiator protein Rep68. PLoS Pathog. 2009;5:e1000513. doi: 10.1371/journal.ppat.1000513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Putnam CD, Clancy SB, Tsuruta H, Gonzalez S, et al. Structure and mechanism of the RuvB Holliday junction branch migration motor. J Mol Biol. 2001;311:297–310. doi: 10.1006/jmbi.2001.4852. [DOI] [PubMed] [Google Scholar]
- 61.Sawaya MR, Guo S, Tabor S, Richardson CC, et al. Crystal structure of the helicase domain from the replicative helicase-primase of bacteriophage T7. Cell. 1999;99:167–177. doi: 10.1016/S0092-8674(00)81648-7. [DOI] [PubMed] [Google Scholar]
- 62.Kim DY, Kim KK. Crystal structure of ClpX molecular chaperone from Helicobacter pylori . J Biol Chem. 2003;278:50664–50670. doi: 10.1074/jbc.M305882200. [DOI] [PubMed] [Google Scholar]
- 63.Erzberger JP, Mott ML, Berger JM. Structural basis for ATP-dependent DnaA assembly and replication-origin remodeling. Nat Struct Mol Biol. 2006;13:676–683. doi: 10.1038/nsmb1115. [DOI] [PubMed] [Google Scholar]
- 64.Rappas M, Schumacher J, Beuron F, Niwa H, et al. Structural insights into the activity of enhancer-binding proteins. Science. 2005;307:1972–1975. doi: 10.1126/science.1105932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Yu RC, Hanson PI, Jahn R, Brunger AT. Structure of the ATP-dependent oligomerization domain of N-ethylmaleimide sensitive factor complexed with ATP. Nat Struct Biol. 1998;5:803–811. doi: 10.1038/1843. [DOI] [PubMed] [Google Scholar]
- 66.Lenzen CU, Steinmann D, Whiteheart SW, Weis WI. Crystal structure of the hexamerization domain of N-ethylmaleimide-sensitive fusion protein. Cell. 1998;94:525–536. doi: 10.1016/S0092-8674(00)81593-7. [DOI] [PubMed] [Google Scholar]
- 67.Lee S, Choi JM, Tsai FT. Visualizing the ATPase cycle in a protein disaggregating machine: structural basis for substrate binding by ClpB. Mol Cell. 2007;25:261–271. doi: 10.1016/j.molcel.2007.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Lee S, Sielaff B, Lee J, Tsai FT. CryoEM structure of Hsp104 and its mechanistic implication for protein disaggregation. Proc Natl Acad Sci USA. 2010;107:8135–8140. doi: 10.1073/pnas.1003572107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Wendler P, Shorter J, Plisson C, Cashikar AG, et al. Atypical AAA+ subunit packing creates an expanded cavity for disaggregation by the protein-remodeling factor Hsp104. Cell. 2007;131:1366–1377. doi: 10.1016/j.cell.2007.10.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Wendler P, Shorter J, Snead D, Plisson C, et al. Motor mechanism for protein threading through Hsp104. Mol Cell. 2009;34:81–92. doi: 10.1016/j.molcel.2009.02.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Gardner BM, Chowdhury S, Lander GC, Martin A. The Pex1/Pex6 complex is a heterohexameric AAA+ motor with alternating and highly coordinated subunits. J Mol Biol. 2015;427:1375–1388. doi: 10.1016/j.jmb.2015.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Cheung KL, Huen J, Kakihara Y, Houry WA, et al. Alternative oligomeric states of the yeast Rvb1/Rvb2 complex induced by histidine tags. J Mol Biol. 2010;404:478–492. doi: 10.1016/j.jmb.2010.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Nguyen VQ, Ranjan A, Stengel F, Wei D, et al. Molecular architecture of the ATP-dependent chromatin-remodeling complex SWR1. Cell. 2013;154:1220–1231. doi: 10.1016/j.cell.2013.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Champion PA, Stanley SA, Champion MM, Brown EJ, et al. C-terminal signal sequence promotes virulence factor secretion in Mycobacterium tuberculosis . Science. 2006;313:1632–1636. doi: 10.1126/science.1131167. [DOI] [PubMed] [Google Scholar]
- 75.Ramsdell TL, Huppert LA, Sysoeva TA, Fortune SM, et al. Linked domain architectures allow for specialization of function in the FtsK/SpoIIIE ATPases of ESX secretion systems. J Mol Biol. 2015;427:1119–1132. doi: 10.1016/j.jmb.2014.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Rosenberg OS, Dovala D, Li X, Connolly L, et al. Substrates control multimerization and activation of the multi-domain ATPase motor of type VII secretion. Cell. 2015;161:501–512. doi: 10.1016/j.cell.2015.03.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Ates LS, Ummels R, Commandeur S, van de Weerd R, et al. Essential role of the ESX-5 secretion system in outer membrane permeability of pathogenic mycobacteria. PLoS Genet. 2015;11:e1005190. doi: 10.1371/journal.pgen.1005190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Glynn SE, Martin A, Nager AR, Baker TA, et al. Structures of asymmetric ClpX hexamers reveal nucleotide-dependent motions in a AAA+ protein-unfolding machine. Cell. 2009;139:744–756. doi: 10.1016/j.cell.2009.09.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Fouts ET, Yu X, Egelman EH, Botchan MR. Biochemical and electron microscopic image analysis of the hexameric E1 helicase. J Biol Chem. 1999;274:4447–4458. doi: 10.1074/jbc.274.7.4447. [DOI] [PubMed] [Google Scholar]
- 80.Bochtler M, Hartmann C, Song HK, Bourenkov GP, et al. The structures of HsIU and the ATP-dependent protease HsIU-HsIV. Nature. 2000;403:800–805. doi: 10.1038/35001629. [DOI] [PubMed] [Google Scholar]
- 81.Bieniossek C, Niederhauser B, Baumann UM. The crystal structure of apo-FtsH reveals domain movements necessary for substrate unfolding and translocation. Proc Natl Acad Sci USA. 2009;106:21579–21584. doi: 10.1073/pnas.0910708106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Caillat C, Macheboeuf P, Wu Y, McCarthy AA, et al. Asymmetric ring structure of Vps4 required for ESCRT-III disassembly. Nat Commun. 2015;6:8781. doi: 10.1038/ncomms9781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Lin CC, Su SC, Su MY, Liang PH, et al. Structural insights into the allosteric operation of the lon AAA+ protease. Structure. 2016;24:667–675. doi: 10.1016/j.str.2016.03.001. [DOI] [PubMed] [Google Scholar]
- 84.Sanders CM, Kovalevskiy OV, Sizov D, Lebedev AA, et al. Papillomavirus E1 helicase assembly maintains an asymmetric state in the absence of DNA and nucleotide cofactors. Nucleic Acids Res. 2007;35:6451–6457. doi: 10.1093/nar/gkm705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Itsathitphaisarn O, Wing RA, Eliason WK, Wang J, et al. The hexameric helicase DnaB adopts a nonplanar conformation during translocation. Cell. 2012;151:267–277. doi: 10.1016/j.cell.2012.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Cuesta I, Nunez-Ramirez R, Scheres SH, Gai D, et al. Conformational rearrangements of SV40 large T antigen during early replication events. J Mol Biol. 2010;397:1276–1286. doi: 10.1016/j.jmb.2010.02.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Sysoeva TA, Chowdhury S, Guo L, Nixon BT. Nucleotide-induced asymmetry within ATPase activator ring drives sigma54-RNAP interaction and ATP hydrolysis. Genes Dev. 2013;27:2500–2511. doi: 10.1101/gad.229385.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Zhao M, Wu S, Zhou Q, Vivona S, et al. Mechanistic insights into the recycling machine of the SNARE complex. Nature. 2015;518:61–67. doi: 10.1038/nature14148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Crampton DJ, Ohi M, Qimron U, Walz T, et al. Oligomeric states of bacteriophage T7 gene 4 primase/helicase. J Mol Biol. 2006;360:667–677. doi: 10.1016/j.jmb.2006.05.037. [DOI] [PubMed] [Google Scholar]
- 90.O’Shea VL, Berger JM. Loading strategies of ring-shaped nucleic acid translocases and helicases. Curr Opin Struct Biol. 2014;25:16–24. doi: 10.1016/j.sbi.2013.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Arias-Palomo E, O’Shea VL, Hood IV, Berger JM. The bacterial DnaC helicase loader is a DnaB ring breaker. Cell. 2013;153:438–448. doi: 10.1016/j.cell.2013.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Blok NB, Tan D, Wang RY, Penczek PA, et al. Unique double-ring structure of the peroxisomal Pex1/Pex6 ATPase complex revealed by cryo-electron microscopy. Proc Natl Acad Sci USA. 2015;112:E4017–E4025. doi: 10.1073/pnas.1500257112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Tye BK. MCM proteins in DNA replication. Annu Rev Biochem. 1999;68:649–686. doi: 10.1146/annurev.biochem.68.1.649. [DOI] [PubMed] [Google Scholar]
- 94.Bar-Nun S, Glickman MH. Proteasomal AAA-ATPases: structure and function. Biochim Biophys Acta. 2012;1823:67–82. doi: 10.1016/j.bbamcr.2011.07.009. [DOI] [PubMed] [Google Scholar]
- 95.Cho C, Vale RD. The mechanism of dynein motility: insight from crystal structures of the motor domain. Biochim Biophys Acta. 2012;1823:182–191. doi: 10.1016/j.bbamcr.2011.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Garbarino JE, Gibbons IR. Expression and genomic analysis of midasin, a novel and highly conserved AAA protein distantly related to dynein. BMC Genom. 2002;3:18. doi: 10.1186/1471-2164-3-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Silvanovich A, Li MG, Serr M, Mische S, et al. The third P-loop domain in cytoplasmic dynein heavy chain is essential for dynein motor function and ATP-sensitive microtubule binding. Mol Biol Cell. 2003;14:1355–1365. doi: 10.1091/mbc.E02-10-0675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Kon T, Nishiura M, Ohkura R, Toyoshima YY, et al. Distinct functions of nucleotide-binding/hydrolysis sites in the four AAA modules of cytoplasmic dynein. Biochemistry. 2004;43:11266–11274. doi: 10.1021/bi048985a. [DOI] [PubMed] [Google Scholar]
- 99.Cho C, Reck-Peterson SL, Vale RD. Regulatory ATPase sites of cytoplasmic dynein affect processivity and force generation. J Biol Chem. 2008;283:25839–25845. doi: 10.1074/jbc.M802951200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Martin A, Baker TA, Sauer RT. Rebuilt AAA+ motors reveal operating principles for ATP-fuelled machines. Nature. 2005;437:1115–1120. doi: 10.1038/nature04031. [DOI] [PubMed] [Google Scholar]
- 101.Joly N, Buck M. Engineered interfaces of an AAA+ ATPase reveal a new nucleotide-dependent coordination mechanism. J Biol Chem. 2010;285:15178–15186. doi: 10.1074/jbc.M110.103150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Lyubimov AY, Strycharska M, Berger JM. The nuts and bolts of ring-translocase structure and mechanism. Curr Opin Struct Biol. 2011;21:240–248. doi: 10.1016/j.sbi.2011.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Gai D, Zhao R, Li D, Finkielstein CV, et al. Mechanisms of conformational change for a replicative hexameric helicase of SV40 large tumor antigen. Cell. 2004;119:47–60. doi: 10.1016/j.cell.2004.09.017. [DOI] [PubMed] [Google Scholar]
- 104.Boyer PD. A perspective of the binding change mechanism for ATP synthesis. FASEB J. 1989;3:2164–2178. doi: 10.1096/fasebj.3.10.2526771. [DOI] [PubMed] [Google Scholar]
- 105.Stinson BM, Baytshtok V, Schmitz KR, Baker TA, et al. Subunit asymmetry and roles of conformational switching in the hexameric AAA+ ring of ClpX. Nat Struct Mol Biol. 2015;22:411–416. doi: 10.1038/nsmb.3012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Danko SJ, Suzuki H. The use of metal fluoride compounds as phosphate analogs for understanding the structural mechanism in P-type ATPases. Methods Mol Biol. 2016;1377:195–209. doi: 10.1007/978-1-4939-3179-8_19. [DOI] [PubMed] [Google Scholar]
- 107.Ogawa H, Cornelius F, Hirata A, Toyoshima C. Sequential substitution of K(+) bound to Na(+), K(+)-ATPase visualized by X-ray crystallography. Nat Commun. 2015;6:8004. doi: 10.1038/ncomms9004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Wendler P, Saibil HR. Cryo electron microscopy structures of Hsp100 proteins: crowbars in or out? Biochem Cell Biol. 2010;88:89–96. doi: 10.1139/O09-164. [DOI] [PubMed] [Google Scholar]
- 109.Carroni M, Kummer E, Oguchi Y, Wendler P, et al. Head-to-tail interactions of the coiled-coil domains regulate ClpB activity and cooperation with Hsp70 in protein disaggregation. Elife. 2014;3:e02481. doi: 10.7554/eLife.02481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Fernandez-Millan P, Lazaro M, Cansiz-Arda S, Gerhold JM, et al. The hexameric structure of the human mitochondrial replicative helicase Twinkle. Nucleic Acids Res. 2015;43:4284–4295. doi: 10.1093/nar/gkv189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Yeung HO, Forster A, Bebeacua C, Niwa H, et al. Inter-ring rotations of AAA ATPase p97 revealed by electron cryomicroscopy. Open Biol. 2014;4:130142. doi: 10.1098/rsob.130142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Beuron F, Flynn TC, Ma J, Kondo H, et al. Motions and negative cooperativity between p97 domains revealed by cryo-electron microscopy and quantised elastic deformational model. J Mol Biol. 2003;327:619–629. doi: 10.1016/S0022-2836(03)00178-5. [DOI] [PubMed] [Google Scholar]
- 113.Banerjee S, Bartesaghi A, Merk A, Rao P, et al. 2.3 Å resolution cryo-EM structure of human p97 and mechanism of allosteric inhibition. Science. 2016;351:871–875. doi: 10.1126/science.aad7974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Hanzelmann P, Schindelin H. Structural basis of ATP hydrolysis and intersubunit signaling in the AAA+ ATPase p97. Structure. 2016;24:127–139. doi: 10.1016/j.str.2015.10.026. [DOI] [PubMed] [Google Scholar]
- 115.Schuller JM, Beck F, Lossl P, Heck AJ, et al. Nucleotide-dependent conformational changes of the AAA+ ATPase p97 revisited. FEBS Lett. 2016;590:595–604. doi: 10.1002/1873-3468.12091. [DOI] [PubMed] [Google Scholar]
- 116.Huang X, Luan B, Wu J, Shi Y. An atomic structure of the human 26S proteasome. Nat Struct Mol Biol. 2016;23:778–785. doi: 10.1038/nsmb.3273. [DOI] [PubMed] [Google Scholar]
- 117.Schweitzer A, Aufderheide A, Rudack T, Beck F, et al. Structure of the human 26S proteasome at a resolution of 3.9 A. Proc Natl Acad Sci USA. 2016;113:7816–7821. doi: 10.1073/pnas.1608050113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Davies JM, Tsuruta H, May AP, Weis WI. Conformational changes of p97 during nucleotide hydrolysis determined by small-angle X-ray scattering. Structure. 2005;13:183–195. doi: 10.1016/j.str.2004.11.014. [DOI] [PubMed] [Google Scholar]
- 119.Chen B, Doucleff M, Wemmer DE, De Carlo S, et al. ATP ground- and transition states of bacterial enhancer binding AAA+ ATPases support complex formation with their target protein, sigma54. Structure. 2007;15:429–440. doi: 10.1016/j.str.2007.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Roessle M, Manakova E, Laure I, Nawroth T, et al. Time-resolved small angle scattering: kinetic and structural data from proteins in solution. J Appl Crystallogr. 2000;33:548–551. doi: 10.1107/S0021889800001369. [DOI] [Google Scholar]
- 121.Kirby NM, Cowieson NP. Time-resolved studies of dynamic biomolecules using small angle X-ray scattering. Curr Opin Struct Biol. 2014;28:41–46. doi: 10.1016/j.sbi.2014.07.007. [DOI] [PubMed] [Google Scholar]
- 122.Murayama Y, Mukaiyama A, Imai K, Onoue Y, et al. Tracking and visualizing the circadian ticking of the cyanobacterial clock protein KaiC in solution. EMBO J. 2011;30:68–78. doi: 10.1038/emboj.2010.298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Inobe T, Arai M, Nakao M, Ito K, et al. Equilibrium and kinetics of the allosteric transition of GroEL studied by solution X-ray scattering and fluorescence spectroscopy. J Mol Biol. 2003;327:183–191. doi: 10.1016/S0022-2836(03)00087-1. [DOI] [PubMed] [Google Scholar]
- 124.Svergun DI, Stuhrmann HB. New developments in direct shape determination from small-angle scattering. 1. Theory and model-calculations. Acta Crystallogr Sect A. 1991;47:736–744. doi: 10.1107/S0108767391006414. [DOI] [Google Scholar]
- 125.Schneidman-Duhovny D, Kim SJ, Sali A. Integrative structural modeling with small angle X-ray scattering profiles. BMC Struct Biol. 2012;12:17. doi: 10.1186/1472-6807-12-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Rambo RP, Tainer JA. Accurate assessment of mass, models and resolution by small-angle scattering. Nature. 2013;496:477–481. doi: 10.1038/nature12070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Valentini E, Kikhney AG, Previtali G, Jeffries CM, et al. SASBDB, a repository for biological small-angle scattering data. Nucleic Acids Res. 2015;43:D357–D363. doi: 10.1093/nar/gku1047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Huang R, Ripstein ZA, Augustyniak R, Lazniewski M, et al. Unfolding the mechanism of the AAA+ unfoldase VAT by a combined cryo-EM, solution NMR study. Proc Natl Acad Sci USA. 2016;113:E4190–E4199. doi: 10.1073/pnas.1603980113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Joly N, Zhang N, Buck M. ATPase site architecture is required for self-assembly and remodeling activity of a hexameric AAA+ transcriptional activator. Mol Cell. 2012;47:484–490. doi: 10.1016/j.molcel.2012.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Eckert T, Link S, Le DT, Sobczak JP, et al. Subunit interactions and cooperativity in the microtubule-severing AAA ATPase spastin. J Biol Chem. 2012;287:26278–26290. doi: 10.1074/jbc.M111.291898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Hoskins JR, Doyle SM, Wickner S. Coupling ATP utilization to protein remodeling by ClpB, a hexameric AAA+ protein. Proc Natl Acad Sci USA. 2009;106:22233–22238. doi: 10.1073/pnas.0911937106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Werbeck ND, Schlee S, Reinstein J. Coupling and dynamics of subunits in the hexameric AAA+ chaperone ClpB. J Mol Biol. 2008;378:178–190. doi: 10.1016/j.jmb.2008.02.026. [DOI] [PubMed] [Google Scholar]
- 133.Stotz M, Mueller-Cajar O, Ciniawsky S, Wendler P, et al. Structure of green-type Rubisco activase from tobacco. Nat Struct Mol Biol. 2011;18:1366–1370. doi: 10.1038/nsmb.2171. [DOI] [PubMed] [Google Scholar]
- 134.Lundqvist J, Braumann I, Kurowska M, Muller AH, et al. Catalytic turnover triggers exchange of subunits of the magnesium chelatase AAA+ motor unit. J Biol Chem. 2013;288:24012–24019. doi: 10.1074/jbc.M113.480012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Batchelor JD, Doucleff M, Lee CJ, Matsubara K, et al. Structure and regulatory mechanism of Aquifex aeolicus NtrC4: variability and evolution in bacterial transcriptional regulation. J Mol Biol. 2008;384:1058–1075. doi: 10.1016/j.jmb.2008.10.024. [DOI] [PubMed] [Google Scholar]
- 136.De Carlo S, Chen B, Hoover TR, Kondrashkina E, et al. The structural basis for regulated assembly and function of the transcriptional activator NtrC. Genes Dev. 2006;20:1485–1495. doi: 10.1101/gad.1418306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Stinson BM, Nager AR, Glynn SE, Schmitz KR, et al. Nucleotide binding and conformational switching in the hexameric ring of a AAA+ machine. Cell. 2013;153:628–639. doi: 10.1016/j.cell.2013.03.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Lowe J, Ellonen A, Allen MD, Atkinson C, et al. Molecular mechanism of sequence-directed DNA loading and translocation by FtsK. Mol Cell. 2008;31:498–509. doi: 10.1016/j.molcel.2008.05.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Crozat E, Meglio A, Allemand JF, Chivers CE, et al. Separating speed and ability to displace roadblocks during DNA translocation by FtsK. EMBO J. 2010;29:1423–1433. doi: 10.1038/emboj.2010.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Chen Z, Yang H, Pavletich NP. Mechanism of homologous recombination from the RecA-ssDNA/dsDNA structures. Nature. 2008;453:489–494. doi: 10.1038/nature06971. [DOI] [PubMed] [Google Scholar]
- 141.Glynn SE, Nager AR, Baker TA, Sauer RT. Dynamic and static components power unfolding in topologically closed rings of a AAA+ proteolytic machine. Nat Struct Mol Biol. 2012;19:616–622. doi: 10.1038/nsmb.2288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Biter AB, Lee S, Sung N, Tsai FT. Structural basis for intersubunit signaling in a protein disaggregating machine. Proc Natl Acad Sci USA. 2012;109:12515–12520. doi: 10.1073/pnas.1207040109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Zakeri B, Fierer JO, Celik E, Chittock EC, et al. Peptide tag forming a rapid covalent bond to a protein, through engineering a bacterial adhesin. Proc Natl Acad Sci USA. 2012;109:E690–E697. doi: 10.1073/pnas.1115485109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Popp MW, Antos JM, Grotenbreg GM, Spooner E, et al. Sortagging: a versatile method for protein labeling. Nat Chem Biol. 2007;3:707–708. doi: 10.1038/nchembio.2007.31. [DOI] [PubMed] [Google Scholar]
- 145.Liu S, Chistol G, Bustamante C. Mechanical operation and intersubunit coordination of ring-shaped molecular motors: insights from single-molecule studies. Biophys J. 2014;106:1844–1858. doi: 10.1016/j.bpj.2014.03.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Besprozvannaya M, Burton BM. Do the same traffic rules apply? Directional chromosome segregation by SpoIIIE and FtsK. Mol Microbiol. 2014;93:599–608. doi: 10.1111/mmi.12708. [DOI] [PubMed] [Google Scholar]
- 147.Olivares AO, Baker TA, Sauer RT. Mechanistic insights into bacterial AAA+ proteases and protein-remodelling machines. Nat Rev Microbiol. 2016;14:33–44. doi: 10.1038/nrmicro.2015.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Belyy V, Yildiz A. Processive cytoskeletal motors studied with single-molecule fluorescence techniques. FEBS Lett. 2014;588:3520–3525. doi: 10.1016/j.febslet.2014.05.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Chemla YR, Smith DE. Single-molecule studies of viral DNA packaging. Adv Exp Med Biol. 2012;726:549–584. doi: 10.1007/978-1-4614-0980-9_24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Fili N. Single-molecule and single-particle imaging of molecular motors in vitro and in vivo. EXS. 2014;105:131–159. doi: 10.1007/978-3-0348-0856-9_7. [DOI] [PubMed] [Google Scholar]
- 151.Taraska JW, Puljung MC, Olivier NB, Flynn GE, et al. Mapping the structure and conformational movements of proteins with transition metal ion FRET. Nat Methods. 2009;6:532–537. doi: 10.1038/nmeth.1341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Kim YC, Snoberger A, Schupp J, Smith DM. ATP binding to neighbouring subunits and intersubunit allosteric coupling underlie proteasomal ATPase function. Nat Commun. 2015;6:8520. doi: 10.1038/ncomms9520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Aker J, Hesselink R, Engel R, Karlova R, et al. In vivo hexamerization and characterization of the Arabidopsis AAA ATPase CDC48A complex using Forster resonance energy transfer-fluorescence lifetime imaging microscopy and fluorescence correlation spectroscopy. Plant Physiol. 2007;145:339–350. doi: 10.1104/pp.107.103986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Zhang N, Gordiyenko Y, Joly N, Lawton E, et al. Subunit dynamics and nucleotide-dependent asymmetry of an AAA(+) transcription complex. J Mol Biol. 2014;426:71–83. doi: 10.1016/j.jmb.2013.08.018. [DOI] [PubMed] [Google Scholar]
- 155.Dyachenko A, Gruber R, Shimon L, Horovitz A, et al. Allosteric mechanisms can be distinguished using structural mass spectrometry. Proc Natl Acad Sci USA. 2013;110:7235–7239. doi: 10.1073/pnas.1302395110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Rand KD, Zehl M, Jorgensen TJ. Measuring the hydrogen/deuterium exchange of proteins at high spatial resolution by mass spectrometry: overcoming gas-phase hydrogen/deuterium scrambling. Acc Chem Res. 2014;47:3018–3027. doi: 10.1021/ar500194w. [DOI] [PubMed] [Google Scholar]
- 157.Konermann L, Pan J, Liu YH. Hydrogen exchange mass spectrometry for studying protein structure and dynamics. Chem Soc Rev. 2011;40:1224–1234. doi: 10.1039/C0CS00113A. [DOI] [PubMed] [Google Scholar]
- 158.Zhang Q, Chen J, Kuwajima K, Zhang HM, et al. Nucleotide-induced conformational changes of tetradecameric GroEL mapped by H/D exchange monitored by FT-ICR mass spectrometry. Sci Rep. 2013;3:1247. doi: 10.1038/srep01247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Chodavarapu S, Jones AD, Feig M, Kaguni JM. DnaC traps DnaB as an open ring and remodels the domain that binds primase. Nucleic Acids Res. 2016;44:210–220. doi: 10.1093/nar/gkv961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Snijder J, Burnley RJ, Wiegard A, Melquiond AS, et al. Insight into cyanobacterial circadian timing from structural details of the KaiB-KaiC interaction. Proc Natl Acad Sci USA. 2014;111:1379–1384. doi: 10.1073/pnas.1314326111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Ando T. Molecular machines directly observed by high-speed atomic force microscopy. FEBS Lett. 2013;587:997–1007. doi: 10.1016/j.febslet.2012.12.024. [DOI] [PubMed] [Google Scholar]
- 162.Ando T. High-speed atomic force microscopy. Microscopy (Oxf) 2013;62:81–93. doi: 10.1093/jmicro/dfs093. [DOI] [PubMed] [Google Scholar]
- 163.Eghiaian F, Rico F, Colom A, Casuso I, et al. High-speed atomic force microscopy: imaging and force spectroscopy. FEBS Lett. 2014;588:3631–3638. doi: 10.1016/j.febslet.2014.06.028. [DOI] [PubMed] [Google Scholar]
- 164.Noi K, Yamamoto D, Nishikori S, Arita-Morioka K, et al. High-speed atomic force microscopic observation of ATP-dependent rotation of the AAA+ chaperone p97. Structure. 2013;21:1992–2002. doi: 10.1016/j.str.2013.08.017. [DOI] [PubMed] [Google Scholar]
- 165.Uchihashi T, Iino R, Ando T, Noji H. High-speed atomic force microscopy reveals rotary catalysis of rotorless F(1)-ATPase. Science. 2011;333:755–758. doi: 10.1126/science.1205510. [DOI] [PubMed] [Google Scholar]
- 166.Bujalowski W, Klonowska MM, Jezewska MJ. Oligomeric structure of Escherichia coli primary replicative helicase DnaB protein. J Biol Chem. 1994;269:31350–31358. [PubMed] [Google Scholar]
- 167.Hurley JH, Yang B. Making sense of Vps4. J Mol Biol. 2014;426:503–506. doi: 10.1016/j.jmb.2013.10.038. [DOI] [PubMed] [Google Scholar]
- 168.Wang J, Song JJ, Franklin MC, Kamtekar S, et al. Crystal structures of the HslVU peptidase-ATPase complex reveal an ATP-dependent proteolysis mechanism. Structure. 2001;9:177–184. doi: 10.1016/S0969-2126(01)00570-6. [DOI] [PubMed] [Google Scholar]
- 169.Lo YH, Tsai KL, Sun YJ, Chen WT, et al. The crystal structure of a replicative hexameric helicase DnaC and its complex with single-stranded DNA. Nucleic Acids Res. 2009;37:804–814. doi: 10.1093/nar/gkn999. [DOI] [PMC free article] [PubMed] [Google Scholar]