Abstract
In addition to being multi-potent, mesenchymal stem cells (MSCs) possess immunomodulatory functions that have been investigated as potential treatments in various immune disorders. MSCs can robustly interact with cells of the innate and adaptive immune systems, either through direct cell–cell contact or through their secretome. In this review, we discuss current findings regarding the interplay between MSCs and different immune cell subsets. We also draw attention to the mechanisms involved.
Keywords: Mesenchymal stem cell, Inflammation, Immune regulation, Plasticity
Introduction
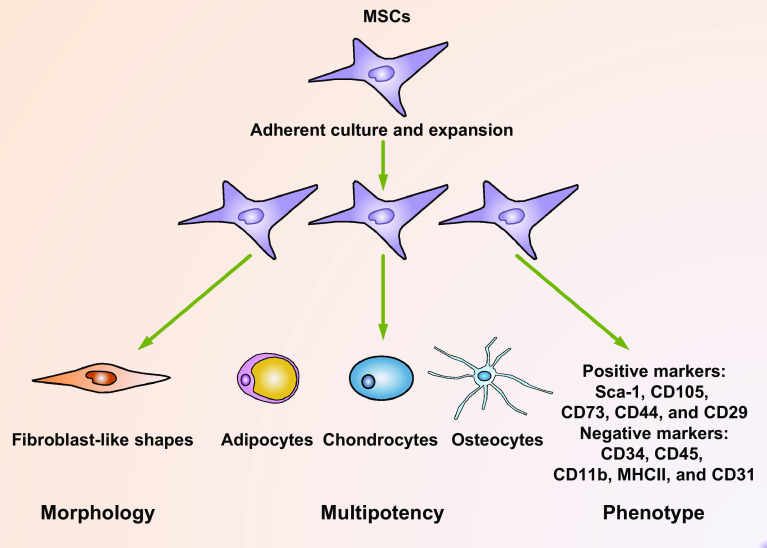
Mesenchymal stem cells (MSCs) are multi-potent cells that can be isolated from various adult tissues, such as the bone marrow, umbilical cord, adipose, peripheral blood, liver, and tooth root [1, 2]. In vitro, these cells are adherent to plastic dishes and can be passaged consecutively for 30–40 generations while retaining their multipotency [3, 4]. They can be induced to differentiate into cells of mesodermal lineages, such as adipocytes, chondrocytes, and osteoblasts [3]. Interestingly, they also have the potential to trans-differentiate into ectodermal or endodermal cell lineages [5]. Phenotypically, MSCs are positive for cell surface antigens, including stem cell antigen-1 (Sca-1), CD105, CD73, and CD90, and they do not express markers of hematopoietic cell lineage, such as CD34, CD45, CD11b, major histocompatibility complex class II (MHCII), and endothelial marker CD31 [6–9] (Fig. 1).
Fig. 1.
Multiple criteria for the definition of MSCs. MSCs can be isolated from various origins, such as the bone marrow, adipose tissues, and peripheral blood. In culture, they are adherent to plastic dishes, thus can be purified and expanded by consecutive passaging. Usually, MSCs exhibit heterogeneous population of fibroblast-like shapes. MSCs are multi-potential which can differentiate into cells of mesenchymal tissues, including adipocytes, chondrocytes, and osteocytes. These cells can also trans-differentiate into cells of non-mesenchymal tissues. Usually, mouse and human MSCs are positive for such markers as Sca-1, CD105, CD73, CD44, and CD29, while they are negative for such markers as CD34, CD45, CD11b, MHCII, and CD31. These criteria combined to form a strict definition of MSCs
Stem cell-based investigations have increased hope for the treatment of many diseases. Nevertheless, the clinical applications of embryonic stem cells (ESCs) and induced pluripotent stem cells (iPSCs) are hindered by their teratoma-generating ability in vivo [10, 11] and, most importantly, ethical concerns [12]. MSCs bring new enthusiasm for regenerative medicine and immune disorder-related diseases, because they are convenient to isolate, have strong self-renewal abilities, and have multi-potent differentiation abilities. Moreover, MSCs are free of the complications that can emerge with the use of ESCs and iPSCs. Much has been learnt about the functions of MSCs during tissue repair and in the control of immune disorders. They can directly replace damaged tissues through differentiation, even though this is less effective than that of ESCs or iPSCs. Several studies have reported the successful promotion of tissue regeneration, including the liver [13], kidney [14], heart [15], and pancreas [16] through the administration of MSCs. Most importantly, MSCs modulate tissue regeneration and various immune disorders through their immunoregulatory properties. These cells are capable of interacting with various types of immune cells, including T cells, B cells, natural killer (NK) cells, macrophages, dendritic cells (DCs), neutrophils, and mast cells. These interactions occur through direct cell–cell contact or their specific secretome, which consists of various growth factors and immunomodulatory factors. This balances the immune response and regulates inflammation profiles, thus promoting the successful treatment of various immune cell-associated diseases, as reviewed in detail elsewhere [17–19]. In this study, we primarily discuss the current findings on the immunomodulatory properties of MSCs and the associated mechanisms.
T cells
T cells are extensively distributed throughout tissues. In the thymus, hematopoietic stem cell-derived progenitors develop into T cells through a series of distinct developmental stages [20]. Activation of naïve T cells requires two signals, namely, T cell receptor signaling and co-stimulatory signaling [21, 22]. Upon activation, CD4+ T cells can differentiate into T-helper 1 (Th1), Th2, Th9, Th17, or regulatory T cell (Treg) subsets, depending on the strength of the stimulation and the cytokine milieu [23–26]. Various infections also activate and promote the differentiation of CD8+ T cells into cytotoxic T lymphocytes that secrete granzymes, perforins, and various cytokines to kill infected cells [27]. T-cell-mediated immunity is the key component of the adaptive immune system, protecting against infections and malignancies but also mediating a number of autoimmune diseases [28].
The interplay between MSCs and T cells has been intensively studied. It was found that MSCs potently inhibited T cell proliferation in several models. A study investigating baboon MSCs highlighted their proliferation-suppressive feature, which could also be applied to an in vivo graft-versus-host disease (GVHD) model [29]. Moreover, human bone-marrow-derived MSCs efficiently inhibited the proliferation of T lymphocytes in vitro. The proliferation-inhibiting effect of MSCs on T cells is thought to be mediated by the release of transforming growth factor beta (TGF-β) and hepatocyte growth factor (HGF), which leads to the decrease of cyclin D2 and the increase of p27kip1 expression in T cells, resulting in arrest of proliferation in the G1 phase [30, 31]. MSCs are also capable of inducing apoptosis of activated T cells, a process associated with the conversion of tryptophan into kynurenine [32], and with the Fas/Fas ligand-dependent pathway [33].
In addition to affecting T-cell proliferation and apoptosis, MSCs can also alter the activation and differentiation process of T cells. Several lines of evidence have exhibited that MSCs suppressed interferon (IFN)-γ and IL-17 secretion but promoted IL-10 production of T cells by antagonizing the differentiation of Th1 and Th17 cells, thereby inducing the generation of Tregs [34, 35]. MSCs also suppressed effector T-cell priming indirectly through the regulation of DCs and NK cells [36]. These findings were applicable to several in vivo models, as MSC transplantation efficiently improved several inflammatory diseases, such as experimental autoimmune encephalomyelitis (EAE) [34], arthritis [37], experimental autoimmune uveitis [38], transplant arteriosclerosis [39], acute hepatitis [40], systemic lupus erythematosus (SLE) [41], and GVHD [42].
Interestingly, MSCs are not capable of suppressing T cells unless they are pre-stimulated by certain inflammatory cytokines, such as IFN-γ and at least one other cytokine, specifically tumor necrosis factor (TNF)-α, interleukin (IL-)1α, or IL-1β [43, 44]. In response to stimulation by these inflammatory cytokines, MSCs upregulated their inducible nitric oxide synthase (iNOS) and cyclooxygenase (COX)-2 expression levels, which resulted in robust production of the immunosuppressive molecules nitric oxide (NO) and prostaglandin E2 (PGE2) to modulate immune responses [43, 45]. In addition, these MSCs produced a variety of chemokines and adhesion molecules, such as CXC chemokine receptor 3 (CXCR3) ligands, C-C chemokine receptor type 5 (CCR5) ligands, intercellular adhesion molecule 1 (ICAM-1), and vascular cell adhesion molecule 1 (VCAM-1). These chemokines are critical for lymphocyte recruitment to injured sites in close proximity, thus ensuring their optimum suppressive function [17, 43, 44, 46]. The induced expression of soluble immunoregulatory molecules and adhesion molecules was both indispensable for effective T-cell inhibition, since blocking either of them would greatly reverse the suppressive effects of MSCs [43, 44].
However, the immunosuppressive ability of MSCs is not always achieved, as several contradictory findings showed that MSCs were unable to suppress or even enhance T cell responses under several conditions. Indeed, the immunomodulatory capacity of MSCs is dependent upon the types and strengths of the inflammatory signals they receive. This plasticity of MSCs in immunomodulation was demonstrated in a study investigating how different concentrations of IFN-γ and TNF affected the functions of MSCs in immune regulation [47]. In this study, low proinflammatory cytokine levels led to inadequate production of NO from murine MSCs, whereas high proinflammatory cytokine levels resulted in adequate production of NO and guaranteed their inhibitory effects on T cells. The plasticity of murine MSCs was also applicable in human MSCs [47] (Fig. 2). This was confirmed in vivo in several murine models, including models for delayed-type hypersensitivity response, tumor growth, and heart transplantation [47, 48].
Fig. 2.
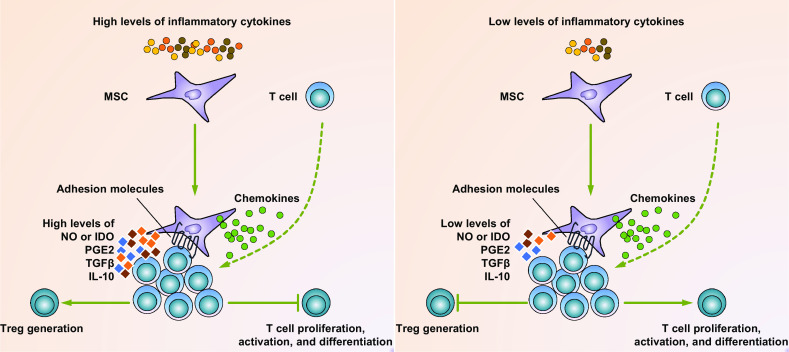
MSC plasticity in immuno-modulation. In response to high levels of proinflammatory cytokines that exist in the acute phase of inflammatory diseases, MSCs are licensed to secrete large amounts of immuno-suppressive factors, such as NO (mice) or IDO (humans), PGE2, TGFβ, and IL-10. In addition, these MSCs also produce various chemokines and express adhesion molecules that are responsible for T-cell recruitment and keeping T cells in close proximity with them. As a result, T cells are suppressed in proliferation, activation, and differentiation. Moreover, CD4+CD25+Foxp3+ Tregs can also be generated by these suppressive MSCs (left panel). In response to low levels of proinflammatory cytokines that exist in various chronic diseases, MSCs still produce considerable amounts of chemokines and adhesion molecules that recruit T cells in close proximity with them. However, they produce only low levels of the immuno-suppressive factors. Thus, the recruited T cells are unchecked and become activated (right panel)
Notably, the key molecules mediating the immunosuppressive function of MSCs are species dependent, with iNOS being a key molecule in mice, whereas indoleamine 2,3-dioxygenase (IDO) is a key molecule in humans [49, 50]. iNOS is a synthase that catalyzes the production of NO in vivo, which is highly immunosuppressive at high concentrations [51]. In murine models of GVHD and experimental arthritis, iNos −/− or iNOS inhibitor treated MSCs failed to suppress T cells and thus did not exert therapeutic effects [43, 52]. IDO strongly inhibited immune responses by depleting tryptophan and promoting the accumulation of tryptophan metabolites [53, 54]. Similar to iNOS, IDO in human MSCs exerted immunosuppressive functions in a few models [50, 54, 55]. Human MSCs also secreted a considerable amount of soluble human leukocyte antigen class I molecule G5 (HLA-G5) to mediate their immunosuppressive functions [35]. Nevertheless, murine and human MSCs also share some common molecules in mediating T-cell immunosuppression. One of the most important molecules may be PGE2, whose role was highlighted in a number of studies. Mouse bone-marrow MSCs secrete large amounts of PGE2, which is correlated with higher efficacy to EAE inhibition, collagen-induced arthritis mitigation, and mixed lymphocyte reaction suppression [39, 52, 56]. In addition, the emerging roles of MSC-derived extracellular vesicles (EVs) in T-cell suppression are attracting increasing interest [57–61]. Similar to the functions of MSCs, EVs may also inhibit effector T cell differentiation, activation, and proliferation [62–66], induce T cell apoptosis [66, 67], and promote Treg generation [65–68]. Other soluble factors, such as TGF-β, HGF, tumor necrosis factor-induced protein 6 (TSG6), and IL-10, have also been implicated in suppression of T cells [30, 69–71]. However, the role of TGF-β has not been completely defined, since TGF-β may act directly on MSCs by inhibiting iNOS expression, thus antagonizing their immunosuppressive effects [69].
B cells
B cells are another hallmark effector cells of the adaptive immune system. These cells are differentiated from hematopoietic stem cells through a series of coordinated stages [72]. Following the recognition of specific antigens by B cell receptors, naïve B cells will proliferate and differentiate into activated antibody-producing cells and memory cells to mediate and sustain protection against foreign pathogens [73–75]. Distinct from the conventional B cells which are termed B2 cells, there is a population of B1 cells enriched in the pleural and peritoneal cavities in mice. These cells respond effectively to innate immune signals and play a role in the elimination of pathogens and in providing long-term protection for the host [76]. Regulatory B cells (Bregs) are another subset of B cells; they produce IL-10 and exert immunomodulatory functions in several models [77].
Both human and murine MSCs are capable of suppressing the proliferation, differentiation, and activation of B cells. Several lines of evidence demonstrated that B cells co-cultured with MSCs exhibited cell cycle arrest, impaired plasma cell generation, compromised immunoglobulin-secreting ability, and reduced chemotactic properties [78–81]. Soluble factors are of critical importance to exert this suppressive function [78, 81–83]. CCL2 is one such factor mediating these actions, as metalloproteinase-processed CCL2 derived from MSCs inhibited signal transducer and activator of transcription 3 (STAT3) activation in plasma cells, leading to PAX5 expression and thus suppression of immunoglobulin synthesis [80, 82]. In a recent study, IL-1 receptor antagonist (IL-1Ra) derived from MSCs was shown to control B-cell differentiation and arthritis progression [83]. In addition, EVs derived from MSCs were also important in suppressing B-cell proliferation, differentiation, and antibody production, which were observed in a dose-dependent manner [60, 84]. In addition, cell–cell contact was also crucial, and was associated with the PD-1/PD-L1 pathway [85]. In addition to these findings, the modulation of several other signaling pathways, such as Akt, extracellular response kinase 1/2, p38, and B lymphocyte-induced maturation protein 1 (Blimp1) signaling, was highlighted in other studies [79–81].
There is evidence that MSCs also regulate B-cell responses through the induction of Bregs, which are CD19+CD24highCD38high in humans and CD19+CD1dhighCD5+ in mice. These cells secrete a considerable amount of anti-inflammatory cytokine IL-10, resulting in suppressed immune responses [86]. Indeed, the induction of Bregs by MSCs was shown to be efficient in treating several diseases in mouse models, such as GVHD, SLE, and EAE [86–89].
As in T cells, inflammatory stimulation of MSCs enhances their inhibitory effects on B cells. Potent IFN-γ signaling is crucial to stimulate the suppressive function of MSCs [85]. Moreover, sufficient inflammatory signals, such as signals from the bacterium Mycoplasma arginini, efficiently enhanced the ability of MSCs to suppress the antibody secretion of B cells [90]. In contrast, insufficient inflammatory signal-stimulated MSCs, such as those derived from lupus-like mice or SLE patients, are compromised in suppressing B-cell proliferation and differentiation, or can even increase the number of antibody-secreting B cells [91, 92]. Thus, it is understandable that several conflicting results have been observed, as some researchers report that the proliferation, activation, differentiation, and antibody production of B cells could be enhanced through the addition of MSCs [93, 94]. Although it is suggested that these disparities might result from variances in B cell purity, stimuli, source of MSCs, and the MSC-to-B cell ratio [95], the plasticity of MSCs as a result of the different intensities of the inflammatory signals they receive should also be carefully considered.
DCs
DCs play crucial roles in the acquisition, processing, transporting, and presentation of various antigens and comprise the most potent antigen-presenting cells (APCs) in the body [96]. These cells are specialized in antigen presentation and, therefore, are of critical importance in directing the responses of the adaptive immune system [4].
Increasing evidence demonstrates that MSCs have potent immunosuppressive effects on DCs. In an in vitro study, it was found that both MSCs and their culture supernatants inhibited the activation of DCs, down-regulated their endocytosis and IL-12 secreting ability, prevented their maturation, and decreased their ability to activate alloreactive T cells [97]. Similar findings were obtained in another in vitro study, which demonstrated that MSCs strongly inhibited the differentiation of monocytes to DCs, and skewed mature DCs to an immature state by suppressing their expression of MHCII, CD1-α, CD80, and CD86, and by inhibiting their IL-12 production [98]. In addition to inhibiting the differentiation of DCs from monocytes, MSCs also profoundly inhibit the differentiation and function of CD34-positive hematopoietic progenitor cell-derived DCs [99, 100]. Similar findings have also been demonstrated by several other studies [101–103]. MSCs can also skew mature DCs into a regulatory phenotype dependent on Jagged1, Jagged2, or IL-10-SOCS3 signaling [104–106]. In addition, the migration of DCs can be impaired by MSCs, which downregulated molecules associated with DC migration, such as CCR7 and CD49dβ1, and decreased their antigen presentation and inflammatory cytokine secretion ability, making them less efficient in activating T cells [36, 103, 107, 108]. In accordance with these in vitro findings, it was found that administration of MSCs effectively improved fulminant hepatic failure induced by Propionibacterium acnes and LPS by inducing the generation of regulatory liver DCs and Tregs [109]. In addition, infusion of ex-vivo MSC-stimulated DCs alleviated colitis in mice by increasing Treg amounts and decreasing lymphocyte proliferation [110]. The suppressive effects of MSCs on DCs also resulted in mitigation of several other immune disorders, including acute GVHD [108], allograft rejection [111], and type 1 diabetes [65].
In exploring possible mechanisms, it was found that IL-6 was involved in the suppressive effects of MSCs on DC differentiation, even though its strength was controversial [100, 112–114]. M-CSF was another candidate in this process, but was tested in combination with IL-6 [99]. Further mechanistic studies indicated that MSC-derived PGE2 and its receptor EP4 played a major role in the inhibitory effects of MSCs on DCs [109]. Notably, PGE2 levels were upregulated in MSC-monocyte co-cultures, and the addition of PGE2 inhibitor NS-398 restored DC function and differentiation, whereas direct addition of PGE2 blocked monocyte differentiation toward DCs [114]. In addition, EVs derived from MSCs were also shown to promote the induction of immature IL-10-secreting DCs, which were indicated in suppression of inflammatory T-cell responses to islet antigens [65]. Importantly, direct cell–cell contact of MSCs and DCs was also suggested in the suppression of DC generation, a process mediated by activation of Notch signaling in DCs [100]. Another finding showed that MSCs blocked cell cycle progression of DCs which may account for the impaired differentiation and function of DCs co-cultured with MSCs [115]. Interestingly, in certain circumstances, the survival of MSCs is dependent on DCs, which has been emphasized in a recent study showing that lymphotoxin-β expression in DCs assisted adipose-derived MSC survival in mouse models of scleroderma skin fibrosis [116].
Macrophages
It is well known that macrophages are critical cells within the innate immune system [117]. Contrary to the long-held view that all macrophages are derived from monocytes in the bone marrow, recent studies have suggested the distinct origins of tissue resident macrophages and circulating macrophages; the former are derived from the yolk-sac and self-maintain independently of the bone marrow contribution during adulthood, whereas the latter are differentiated and replenished from bone-marrow monocytes [118, 119]. Macrophages have prominent plasticity and can be polarized into classically activated M1 or alternatively activated M2 macrophages, depending on the specific micro-environment they are in. In general, M1 macrophages are proinflammatory and possess remarkable antimicrobial abilities via the secretion of various inflammatory cytokines and chemokines, whereas M2 macrophages are immunomodulatory by releasing IL-10 and trophic factors to promote tissue repair and resolve inflammation [120].
Various in vitro studies have demonstrated that co-culture of macrophages with MSCs led to the generation of M2 macrophages, which secreted high levels of IL-10, and low levels of various inflammatory cytokines, such as IL-12, TNF-α, IL-1β, and IL-23, had increased phagocytic ability while displaying decreased co-stimulatory molecule CD86 and MHCII expressions [121–124]. Moreover, proinflammatory stimulation-licensed MSCs promoted further M2 macrophage polarization [123, 125]. The biological relevance of these in vitro findings has been investigated in vivo in several recent studies. In an elegant study investigating sepsis, it was demonstrated that administration of bone-marrow MSCs effectively improved organ function and reduced mortality. This beneficial effect was eliminated by macrophage depletion or IL-10 signaling abrogation [125]. In a model of cutaneous wound healing, the transplantation of human gingiva MSCs formed a spatial interaction with macrophages in the wound site, thus suppressing their TNF-α and IL-6 secretion while promoting IL-10 production to mitigate local inflammation [121]. Similar effects of MSCs were observed in several other immune disorders, such as peritonitis [126], ischemia–reperfusion injury [127], acute liver injury [128], atherosclerosis [128], endotoxemia [129], type 2 diabetes [130], asthma [131], and arthritis [83]. MSCs are also capable of enhancing recruitment of macrophages to injured sites, thus promoting tissue regeneration or improving immune disorders [132, 133].
In investigating the mechanisms, it was found that this effect resulted from a combination of soluble factor-dependent signaling, including the release of PGE2 functioning through the EP2 and EP4 receptors on macrophages, and cell-contact-mediated signaling [125]. Inflammatory signals, such as IFN-γ, TNF-α, and LPS, stimulated the expressions of IDO and COX2 in MSCs, which further enhanced the suppressive functions of MSCs [95, 134, 135]. IL-1Ra was another factor mediating the immunomodulatory effect [83, 128]. IL-1Ra-deficient MSCs were less effective than wild-type MSCs in inducing M2 macrophage polarization and were unable to mitigate arthritic progression in a collagen-induced arthritis model [83]. In addition, MSC-derived exosomes were shown to induce generation of IL-10- and TGF-β-secreting M2-like macrophages from primary human and mouse monocytes [68]. TGF-β signaling was indicated in the mediation of M2 polarization of macrophages in a mouse model of asthma [136]. In a model of zymosan-induced peritonitis, inflammation-activated MSCs secreted TNF-stimulated gene 6 (TSG-6), which interacted through CD44 on macrophages to decrease zymoson/TLR2-mediated nuclear translocation of NF-κB, creating a negative feedback loop to attenuate macrophage activation [126].
NK cells
Natural killer (NK) cells are the key effector cells of the innate immune system; they are developed from a common lymphoid progenitor that is capable of giving rise to all lymphocyte subsets in or outside of the bone marrow [137, 138]. The activities of NK cells are finely regulated by the interaction of various activating and inhibitory receptors expressed on their surfaces with cognate ligands [139]. NK cells are critically involved in the control of various types of microbial infections and tumors by inducing direct cytotoxicity of target cells and/or proinflammatory cytokine production [140, 141].
A number of studies have demonstrated that MSCs are potent inhibitors of NK cells, as they are capable of suppressing the proliferation, cytokine production, and cytotoxicity of NK cells under specific circumstances [142–147]. For this, the ratio of MSCs and NK cells is important, since such suppressive effects could only be exerted at high MSC-to-NK ratios [144]. The significance of these findings was investigated in vivo, in which MSC administration hindered the trafficking and activation of NK cells in the liver, thus ameliorating Poly(I:C)-induced liver injury [146]. To clarify the mechanisms, soluble factors, such as IDO, PGE2, HLA-5, and EVs, have been shown to play critical roles [35, 60, 145]. Notably, blocking the synthesis or activities of either IDO or PGE2 significantly reversed the suppressive effect, with the two factors acting synergistically in this process [144, 145]. CD73 can dephosphorylate AMP into adenosine, and is crucial in the induction of an anti-inflammatory environment mediated by adenosine [148]. It was found that up-regulation of CD73 on NK cells by MSCs led to such inhibition [149, 150]. In addition, direct cell–cell contact is also necessary for the inhibition of NK cells, which is involved in expression of TLR4 on MSCs [147, 151]. Nevertheless, disparities concerning the role of MSCs in modulating NK cells have been noted, as several studies reported opposite effects. It was shown that MSCs, when irradiated as a feeder layer, stimulated the proliferation of NK cell progenitors significantly [152]. Another study observed that MSCs efficiently enhanced the IFN-γ levels secreted by NK cells when stimulated by IL-12/IL-18 [153]. Moreover, NK cells and MSCs interacted in a positive feedback manner, in which NK cell-derived IFN-γ stimulated the CCL2 synthesis of MSCs, which in turn primed NK cells for the further release of IFN-γ [154]. NK cells also stimulated MSC recruitment, a process dependent on chemokines CCL5 and CXCL7 secreted by NK cells [155]. In addition to these findings, MSCs are lysis-sensitive targets for activated NK cells. It has been shown in several studies that MSCs could be efficiently lysed by activated NK cells, which was involved with the various activating receptors on NK cells [144, 156].
Taken together, these findings suggest that the interplay between MSCs and NK cells strongly depends on the stimulation of both cells, their microenvironment, and their ratios. Even so, more in vivo investigations should be conducted to determine the significance of these observations.
Neutrophils
Neutrophils are polymorphonuclear leukocytes and are recognized as one of the key players during acute inflammation [157]. They are abundantly found in the bloodstream and can be recruited to sites of injury within minutes. Neutrophils eliminate pathogens through multiple mechanisms, such as phagocytosis, secretion of bactericidal molecules, and neutrophil extracellular traps [4, 157, 158].
In 2008, it was first reported that MSCs had beneficial effects on neutrophils. Human bone-marrow MSCs from healthy donors, even at very low MSC to neutrophil ratios, significantly suppressed the apoptosis of resting or IL-8-activated neutrophils, a process largely dependent on IL-6 secretion [159]. Similarly, MSCs pre-treated with TLR3 stimulator Poly (I:C) exerted potent anti-apoptotic effects on neutrophils, primarily mediated by the combined action of IL-6, IFN-β, and GM-CSF [160]. In addition to their anti-apoptotic functions, MSCs also secreted IL-8 and macrophage migration inhibitory factor (MIF) to recruit neutrophils in vitro [161]. These findings were corroborated by several in vivo assays [162–164]. It was reported that neutrophils were effectively recruited by subcutaneously injected LPS-stimulated MSCs [162]. In addition, TNF-α-stimulated or gastric cancer-derived MSCs strikingly recruited neutrophils into the tumor, fostering tumor metastasis, and angiogenesis [163]. Through these mechanisms, it is speculated that MSCs may help preserve the storage pool of neutrophils in the bone marrow, and can also facilitate neutrophil migration to inflammatory sites, contributing to the resolution of infection and inflammation [165]. Nevertheless, conflicting findings also exist. In a murine vasculitis model, MSCs inhibited neutrophil activation, prevented neutrophil extracellular trap formation and excessive spillage of tissue-damaging proteases, thus dampening unrestrained inflammation and attenuating tissue damage. In this model, the therapeutic effect of MSCs was mediated by the constitutive release of superoxide dismutase-3 [166]. In another model of neutrophil recruitment induced by cytokine-stimulated endothelial cells, MSCs from various origins suppressed neutrophil recruitment effectively [167]. Moreover, MSC-derived EVs were also shown to inhibit the influx of neutrophils to the lung in an endotoxin-induced lung injury model [168]. It would be of interest to explore why these discrepancies exist, whether this is model-specific or is due to a different MSC dose or other aspects.
Mast cells
Mast cells are generally considered as the major effector cells in allergic reactions [169]. Several lines of evidence also implicated their role in inflammatory diseases, where they are activated by non-allergic triggers to contribute to hose defense or autoimmunity [170, 171].
When mast cells were co-cultured with bone-marrow-derived MSCs, their degranulation, inflammatory cytokine secretion, and chemotaxis abilities were suppressed, an effect dependent on the upregulation of COX2 in MSCs. This finding was confirmed in vivo as MSC administration significantly hindered mast cell degranulation in mouse skin and the peritoneal cavity [172]. In a murine model of atopic dermatitis, administration of MSCs suppressed both the infiltration and degranulation of mast cells, which was mediated by the production of PGE2 and TGF-β1 from the MSCs [173]. Similar findings were noted in several other studies [174–176]. MSC-produced PGE2 also suppressed mast cell infiltration and de novo synthesis of inflammatory cytokines in a murine contact hypersensitivity model [177]. Interestingly, MSCs could also in turn be activated by IgE-stimulated mast cells, thus releasing thymic stromal lymphopoietin and hematopoietic growth factors, regulating the lineage commitment and proliferation of CD34+ precursor cells [178]. In addition, mast cells also affected MSCs by promoting their proliferation and accumulation while inhibiting their differentiation via the activation of platelet-derived growth factor, which may play a role in improving the process of cardiac regeneration [179].
Conclusions and future perspectives
We have discussed our current understanding of the interactions between MSCs and the immune system and the underlying mechanisms (Fig. 3; Table 1). MSCs possess potent immunomodulatory properties, which is dependent on the types and intensities of inflammatory stimulus present in the microenvironment. However, human and murine MSCs may utilize distinct effector molecules to exert their functions (see Table 2 for a detailed comparison of human and murine MSCs). Through the immunomodulatory properties, MSCs are capable of interacting with cells of both the innate and adaptive immune systems and can affect the progression of various inflammatory diseases.
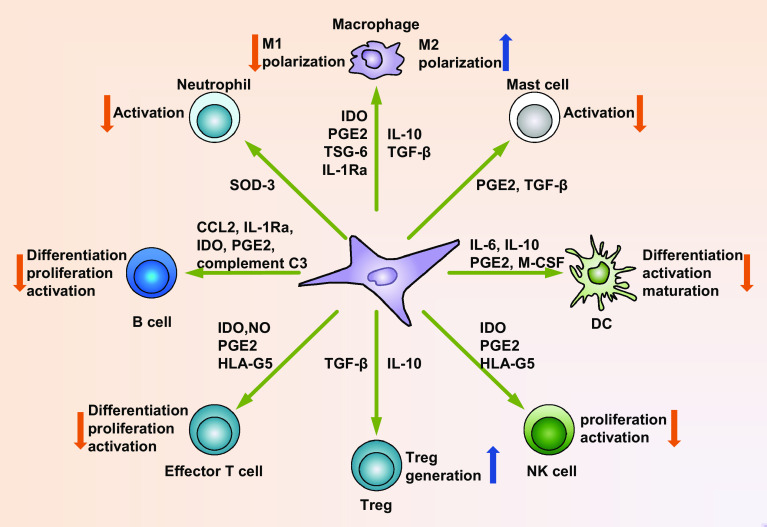
Fig. 3.
Mechanisms of immunomodulatory functions of MSCs. MSCs possess broad immunomodulatory properties. After activation, MSCs can secrete a variety of soluble factors, such as NO (mice) or IDO (humans), PGE2, TGF-β, HLA-G5, TSG-6, CCL2, IL-1Ra, and IL-10. Production of these factors can suppress the differentiation, proliferation, activation of various immune cell subsets, including T cells, B cells, DCs, macrophages, NK cells, neutrophils, and mast cells. In addition, Tregs may be generated in response to TGF-β and IL-10 production from MSCs. As a result, the immune response will be inhibited and local inflammation is suppressed by MSCs (also refer to Table 1 for more detailed information regarding the mechanisms of immunomodulatory functions of MSCs)
Table 1.
The functions of MSCs in regulating different immune cells and the related mechanisms
| Immune cell type | MSC function | Mechanism | References |
|---|---|---|---|
| T cell | Suppressing T cell differentiation, proliferation, activation, and survival | TGF-β, HGF, IDO, NO, PGE2, HLA-G5, TSG6, IL-10, EVs | [30, 31, 34, 35, 43, 62–66, 69–71, 180] |
| Cell–cell contact: Fas/FasL signaling | [33] | ||
| Promoting T cell recruitment | CXCR3 ligands, CCR5 ligands, ICAM-1, VCAM-1 | [17, 43, 44, 46] | |
| B cell | Suppressing B cell differentiation, proliferation, activation, and chemotaxis; Breg induction | CCL2, IL-1Ra, IDO, PGE2, complement C3, EVs | [60, 78, 81–84, 87, 90, 180] |
| Cell–cell contact: PD-1/PD-L1 | [85] | ||
| Promoting B cell differentiation, proliferation, and activation | VEGF | [93, 94] | |
| DC | Suppressing DC differentiation, activation, endocytosis, migration, and maturation | IL-6, IL-10, M-CSF, PGE2, EVs | [36, 65, 99, 101–103, 107–109, 113, 114] |
| Cell–cell contact: notch pathway activation | [100] | ||
| Macrophage | Suppressing M1 while inducing M2 polarization | PGE2, IDO, IL-1Ra, IL-10, TSG-6, TGF-β, exosomes | [68, 83, 123, 125, 126, 131, 135, 136] |
| NK cell | Suppressing NK cell proliferation, migration, and activation | IDO, PGE2, HLA5, EVs | [35, 60, 142–147] |
| Cell–cell contact: CD73, TLR4 | [147, 149–151] | ||
| Promoting NK cell progenitor proliferation and NK activation | CCL2 | [152–154] | |
| Neutrophil | Suppressing neutrophil activation, recruitment, neutrophil extracellular trap formation, and protease secretion | Superoxide dismutase-3, EVs | [166–168] |
| Promoting survival and recruitment | IL-6, IL-8, MIF, IFN-β, and GM-CSF | [159–162] | |
| Mast cell | Suppressing mast cell degranulation, inflammatory cytokine secretion, and chemotaxis | PGE2 and TGF-β1 | [172–177] |
Table 2.
Comparison of human and murine MSCs
| Items | Human MSCs | Murine MSCs |
|---|---|---|
| Differences in specific markers | Stro-1, CD146, alkaline phosphatase, CD49a, CD271, and HLA-DR [3, 8] | Nestin, CD105, vascular cell adhesion protein, CD90, MHCII [5, 19, 46] |
| Common markers |
Positive: Sca-1, CD105, CD73, CD29, and CD44 Negative: CD45, CD34, CD11b, CD31, and MHCII [3, 5, 8, 19, 46] |
|
| Differences in effector molecules for immune regulation | IDO, HLA-G5 [35, 47, 50] | NO [47, 50] |
| Common effector molecules for immune regulation | PGE2, IL-6, IL-10, TGFβ, TSG-6, CCL2, IL-1Ra [30, 39, 69–71, 80, 82, 83] | |
| Key cytokines for induction of immunosuppressive capacity | IFN-γ and TNF-α [43, 47] | IFN-γ [43] |
The immunomodulatory capabilities of MSCs have provided considerable possibilities to improve tissue regeneration and to treat immune disorders. In fact, the clinical virtues of MSC therapy have been tested in a variety of clinical trials for diseases, such as GVHD, SLE, rheumatoid arthritis, Type 1 diabetes, and Crohn’s disease, as reviewed by Paul S. et al. and Hafsa et al. [18, 19], with effective outcomes in several cases [41, 181–184]. Moreover, MSC-based products, such as Prochymal and Cupistem, have also been commercially used for treatment of various diseases [185, 186]. Nevertheless, there are still challenges in the application of MSC therapy, as the clinical outcome varies between trials and reports exist that show the therapeutic effects of MSCs cannot be obtained in some cases [187, 188]. However, given the potent plasticity nature of MSCs, these discrepancies may result from the timing, dose, infusion route, and pretreatment of MSCs in different trials. Thus, establishing standardized methods is necessary to avoid such discrepancies and guarantee the efficacy of MSC therapy.
Choosing the most appropriate type of MSC is also important for positive clinical effects. Even though human MSCs are primarily isolated from bone marrow [54, 63, 182, 188, 189], there are increasing publications highlighting the function of MSCs from other tissues, such as the umbilical cord [130, 173], gingiva [121, 189], or adipose tissues [37, 86]. How these MSCs differ in terms of repair capacities and immunomodulatory properties are largely unknown, and whether MSCs from certain source(s) are more efficient in treating specific diseases remains unexplored. Thus, we suggest that future efforts should be made to fully define the range of sources from which human MSCs can be isolated and suggest further works to identify the most appropriate types of MSCs for specific disease treatment.
Naturally, there are concerns from the scientific community about the efficacy and safety of MSC-based therapies. However, MSC-based therapy still merits further investigation due to the advantages discussed above. Undoubtedly, we are now bridging the translational gap between the basic research of MSCs and their clinical applications for disease treatment. With the increasing explorations in MSCs, we may expect that all these concerns will be addressed over time once a better understanding of the immunomodulatory properties of MSCs is achieved and when MSCs can be exploited appropriately to optimize their therapeutic effects.
Acknowledgements
This study was done with the support of Grants from the China National Basic Research Program (JFYS 2016YFA0100203), the National Natural Science Foundation of China (31272518, 31572399), and the Program of the Shaanxi Province (2015NY157).
Abbreviations
- MSCs
Mesenchymal stem cells;
- Sca-1
Stem cell antigen-1
- ESCs
Embryonic stem cells
- iPSCs
Induced pluripotent stem cells
- Th
T-helper
- TGF-β
Transforming growth factor beta
- HGF
Hepatocyte growth factor
- IFN
Interferon
- Treg
Regulatory T cell
- NK
Natural killer
- DC
Dendritic cell
- EAE
Experimental autoimmune encephalomyelitis
- SLE
Systemic lupus erythematosus
- GVHD
Graft-versus-host disease
- TNF
Tumor necrosis factor
- IL
Interleukin
- iNOS
Nitric oxide synthase
- COX
Cyclooxygenase
- NO
Nitric oxide
- PGE2
Prostaglandin E2
- CXCR3
CXC chemokine receptor3
- CCR5
C-C chemokine receptor type 5
- ICAM-1
Intercellular adhesion molecule 1
- VCAM-1
Vascular cell adhesion molecule 1
- IDO
Indoleamine 2,3-dioxygenase
- HLA-G5
Human leukocyte antigen class I molecule G5
- EV
Extracellular vesicle
- TSG6
Tumor necrosis factor-induced protein 6
- Breg
Regulatory B cell
- STAT3
Signal transducer and activator of transcription 3
- Blimp1
B lymphocyte-induced maturation protein 1
- IL-1Ra
IL-1 receptor antagonist
- APC
Antigen-presenting cell
- MIF
Macrophage migration inhibitory factor.
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
References
- 1.Hass R, Kasper C, Bohm S, Jacobs R. Different populations and sources of human mesenchymal stem cells (MSC): a comparison of adult and neonatal tissue-derived MSC. Cell Commun Signal. 2011;9:12. doi: 10.1186/1478-811X-9-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Elahi KC, Klein G, Avci-Adali M, Sievert KD, MacNeil S, Aicher WK. Human mesenchymal stromal cells from different sources diverge in their expression of cell surface proteins and display distinct differentiation patterns. Stem Cells Int. 2016;2016:5646384. doi: 10.1155/2016/5646384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pittenger MF, Mackay AM, Beck SC, Jaiswal RK, Douglas R, Mosca JD, Moorman MA, Simonetti DW, Craig S, Marshak DR. Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284(5411):143–147. doi: 10.1126/science.284.5411.143. [DOI] [PubMed] [Google Scholar]
- 4.Le Blanc K, Mougiakakos D. Multipotent mesenchymal stromal cells and the innate immune system. Nat Rev Immunol. 2012;12(5):383–396. doi: 10.1038/nri3209. [DOI] [PubMed] [Google Scholar]
- 5.Jiang Y, Jahagirdar BN, Reinhardt RL, Schwartz RE, Keene CD, Ortiz-Gonzalez XR, Reyes M, Lenvik T, Lund T, Blackstad M, Du J, Aldrich S, Lisberg A, Low WC, Largaespada DA, Verfaillie CM. Pluripotency of mesenchymal stem cells derived from adult marrow. Nature. 2002;418(6893):41–49. doi: 10.1038/nature00870. [DOI] [PubMed] [Google Scholar]
- 6.Keating A. Mesenchymal stromal cells: new directions. Cell stem cell. 2012;10(6):709–716. doi: 10.1016/j.stem.2012.05.015. [DOI] [PubMed] [Google Scholar]
- 7.Horwitz EM, Le Blanc K, Dominici M, Mueller I, Slaper-Cortenbach I, Marini FC, Deans RJ, Krause DS, Keating A, International Society for Cellular T Clarification of the nomenclature for MSC: the International Society for Cellular Therapy position statement. Cytotherapy. 2005;7(5):393–395. doi: 10.1080/14653240500319234. [DOI] [PubMed] [Google Scholar]
- 8.Lv FJ, Tuan RS, Cheung KM, Leung VY. Concise review: the surface markers and identity of human mesenchymal stem cells. Stem Cells. 2014;32(6):1408–1419. doi: 10.1002/stem.1681. [DOI] [PubMed] [Google Scholar]
- 9.Houlihan DD, Mabuchi Y, Morikawa S, Niibe K, Araki D, Suzuki S, Okano H, Matsuzaki Y. Isolation of mouse mesenchymal stem cells on the basis of expression of Sca-1 and PDGFR-alpha. Nat Protoc. 2012;7(12):2103–2111. doi: 10.1038/nprot.2012.125. [DOI] [PubMed] [Google Scholar]
- 10.Gutierrez-Aranda I, Ramos-Mejia V, Bueno C, Munoz-Lopez M, Real PJ, Macia A, Sanchez L, Ligero G, Garcia-Parez JL, Menendez P. Human induced pluripotent stem cells develop teratoma more efficiently and faster than human embryonic stem cells regardless the site of injection. Stem Cells. 2010;28(9):1568–1570. doi: 10.1002/stem.471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ben-David U, Benvenisty N. The tumorigenicity of human embryonic and induced pluripotent stem cells. Nature Rev Cancer. 2011;11(4):268–277. doi: 10.1038/nrc3034. [DOI] [PubMed] [Google Scholar]
- 12.Lo B, Parham L. Ethical issues in stem cell research. Endocr Rev. 2009;30(3):204–213. doi: 10.1210/er.2008-0031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cho KA, Ju SY, Cho SJ, Jung YJ, Woo SY, Seoh JY, Han HS, Ryu KH. Mesenchymal stem cells showed the highest potential for the regeneration of injured liver tissue compared with other subpopulations of the bone marrow. Cell Biol Int. 2009;33(7):772–777. doi: 10.1016/j.cellbi.2009.04.023. [DOI] [PubMed] [Google Scholar]
- 14.Qian H, Yang H, Xui WR, Yan YM, Chen QL, Zhu W, Cao HL, Yin Q, Zhou HX, Mao F, Chen YC. Bone marrow mesenchymal stem cells ameliorate rat acute renal failure by differentiation into renal tubular epithelial-like cells. Int J Mol Med. 2008;22(3):325–332. [PubMed] [Google Scholar]
- 15.Rose RA, Jiang H, Wang X, Helke S, Tsoporis JN, Gong N, Keating SC, Parker TG, Backx PH, Keating A. Bone marrow-derived mesenchymal stromal cells express cardiac-specific markers, retain the stromal phenotype, and do not become functional cardiomyocytes in vitro. Stem Cells. 2008;26(11):2884–2892. doi: 10.1634/stemcells.2008-0329. [DOI] [PubMed] [Google Scholar]
- 16.Han F, Wang CY, Yang L, Zhan SD, Zhang M, Tian K. Contribution of murine bone marrow mesenchymal stem cells to pancreas regeneration after partial pancreatectomy in mice. Cell Biol Int. 2012;36(9):823–831. doi: 10.1042/CBI20110680. [DOI] [PubMed] [Google Scholar]
- 17.Wang Y, Chen X, Cao W, Shi Y. Plasticity of mesenchymal stem cells in immunomodulation: pathological and therapeutic implications. Nat Immunol. 2014;15(11):1009–1016. doi: 10.1038/ni.3002. [DOI] [PubMed] [Google Scholar]
- 18.Munir H, McGettrick HM. Mesenchymal stem cell therapy for autoimmune disease: risks and rewards. Stem Cells Dev. 2015;24(18):2091–2100. doi: 10.1089/scd.2015.0008. [DOI] [PubMed] [Google Scholar]
- 19.Frenette PS, Pinho S, Lucas D, Scheiermann C. Mesenchymal stem cell: keystone of the hematopoietic stem cell niche and a stepping-stone for regenerative medicine. Annu Rev Immunol. 2013;31:285–316. doi: 10.1146/annurev-immunol-032712-095919. [DOI] [PubMed] [Google Scholar]
- 20.Koch U, Radtke F. Mechanisms of T cell development and transformation. Annu Rev Cell Dev Biol. 2011;27:539–562. doi: 10.1146/annurev-cellbio-092910-154008. [DOI] [PubMed] [Google Scholar]
- 21.Mueller DL, Jenkins MK, Schwartz RH. Clonal expansion versus functional clonal inactivation: a costimulatory signalling pathway determines the outcome of T cell antigen receptor occupancy. Annu Rev Immunol. 1989;7:445–480. doi: 10.1146/annurev.iy.07.040189.002305. [DOI] [PubMed] [Google Scholar]
- 22.June CH, Ledbetter JA, Gillespie MM, Lindsten T, Thompson CB. T-cell proliferation involving the Cd28 pathway is associated with cyclosporine-resistant interleukin-2 gene-expression. Mol Cell Biol. 1987;7(12):4472–4481. doi: 10.1128/MCB.7.12.4472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.O’Garra A. Cytokines induce the development of functionally heterogeneous T helper cell subsets. Immunity. 1998;8(3):275–283. doi: 10.1016/S1074-7613(00)80533-6. [DOI] [PubMed] [Google Scholar]
- 24.Soroosh P, Doherty TA. Th9 and allergic disease. Immunology. 2009;127(4):450–458. doi: 10.1111/j.1365-2567.2009.03114.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Langrish CL, Chen Y, Blumenschein WM, Mattson J, Basham B, Sedgwick JD, McClanahan T, Kastelein RA, Cua DJ. IL-23 drives a pathogenic T cell population that induces autoimmune inflammation. J Exp Med. 2005;201(2):233–240. doi: 10.1084/jem.20041257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hori S, Nomura T, Sakaguchi S. Control of regulatory T cell development by the transcription factor Foxp3. Science. 2003;299(5609):1057–1061. doi: 10.1126/science.1079490. [DOI] [PubMed] [Google Scholar]
- 27.Kaech SM, Cui WG. Transcriptional control of effector and memory CD8(+) T cell differentiation. Nat Rev Immunol. 2012;12(11):749–761. doi: 10.1038/nri3307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dimeloe S, Burgener AV, Grahlert J, Hess C. T-cell metabolism governing activation, proliferation and differentiation; a modular view. Immunology. 2016 doi: 10.1111/imm.12655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bartholomew A, Sturgeon C, Siatskas M, Ferrer K, McIntosh K, Patil S, Hardy W, Devine S, Ucker D, Deans R, Moseley A, Hoffman R. Mesenchymal stem cells suppress lymphocyte proliferation in vitro and prolong skin graft survival in vivo. Exp Hematol. 2002;30(1):42–48. doi: 10.1016/S0301-472X(01)00769-X. [DOI] [PubMed] [Google Scholar]
- 30.Di Nicola M, Carlo-Stella C, Magni M, Milanesi M, Longoni PD, Matteucci P, Grisanti S, Gianni AM. Human bone marrow stromal cells suppress T-lymphocyte proliferation induced by cellular or nonspecific mitogenic stimuli. Blood. 2002;99(10):3838–3843. doi: 10.1182/blood.V99.10.3838. [DOI] [PubMed] [Google Scholar]
- 31.Glennie S, Soeiro I, Dyson PJ, Lam EW, Dazzi F. Bone marrow mesenchymal stem cells induce division arrest anergy of activated T cells. Blood. 2005;105(7):2821–2827. doi: 10.1182/blood-2004-09-3696. [DOI] [PubMed] [Google Scholar]
- 32.Plumas J, Chaperot L, Richard MJ, Molens JP, Bensa JC, Favrot MC. Mesenchymal stem cells induce apoptosis of activated T cells. Leukemia. 2005;19(9):1597–1604. doi: 10.1038/sj.leu.2403871. [DOI] [PubMed] [Google Scholar]
- 33.Akiyama K, Chen C, Wang D, Xu X, Qu C, Yamaza T, Cai T, Chen W, Sun L, Shi S. Mesenchymal-stem-cell-induced immunoregulation involves FAS-ligand-/FAS-mediated T cell apoptosis. Cell Stem Cell. 2012;10(5):544–555. doi: 10.1016/j.stem.2012.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Luz-Crawford P, Kurte M, Bravo-Alegria J, Contreras R, Nova-Lamperti E, Tejedor G, Noel D, Jorgensen C, Figueroa F, Djouad F, Carrion F. Mesenchymal stem cells generate a CD4+CD25+Foxp3+ regulatory T cell population during the differentiation process of Th1 and Th17 cells. Stem Cell Res Ther. 2013;4(3):65. doi: 10.1186/scrt216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Selmani Z, Naji A, Zidi I, Favier B, Gaiffe E, Obert L, Borg C, Saas P, Tiberghien P, Rouas-Freiss N, Carosella ED, Deschaseaux F. Human leukocyte antigen-G5 secretion by human mesenchymal stem cells is required to suppress T lymphocyte and natural killer function and to induce CD4(+)CD25(high)FOXP3(+) regulatory T cells. Stem Cells. 2008;26(1):212–222. doi: 10.1634/stemcells.2007-0554. [DOI] [PubMed] [Google Scholar]
- 36.Consentius C, Akyuz L, Schmidt-Lucke JA, Tschope C, Pinzur L, Ofir R, Reinke P, Volk HD, Juelke K. Mesenchymal stromal cells prevent allostimulation in vivo and control checkpoints of Th1 priming: migration of human DC to lymph nodes and NK cell activation. Stem Cells. 2015;33(10):3087–3099. doi: 10.1002/stem.2104. [DOI] [PubMed] [Google Scholar]
- 37.Lopez-Santalla M, Mancheno-Corvo P, Menta R, Lopez-Belmonte J, DelaRosa O, Bueren JA, Dalemans W, Lombardo E, Garin MI. Human adipose-derived mesenchymal stem cells modulate experimental autoimmune arthritis by modifying early adaptive T cell responses. Stem Cells. 2015;33(12):3493–3503. doi: 10.1002/stem.2113. [DOI] [PubMed] [Google Scholar]
- 38.Tasso R, Ilengo C, Quarto R, Cancedda R, Caspi RR, Pennesi G. Mesenchymal stem cells induce functionally active T-regulatory lymphocytes in a paracrine fashion and ameliorate experimental autoimmune uveitis. Invest Ophthalmol Vis Sci. 2012;53(2):786–793. doi: 10.1167/iovs.11-8211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hsu WT, Lin CH, Chiang BL, Jui HY, Wu KK, Lee CM. Prostaglandin E2 potentiates mesenchymal stem cell-induced IL-10+ IFN-gamma+ CD4+ regulatory T cells to control transplant arteriosclerosis. J Immunol. 2013;190(5):2372–2380. doi: 10.4049/jimmunol.1202996. [DOI] [PubMed] [Google Scholar]
- 40.Ryu KH, Kim SY, Kim YR, Woo SY, Sung SH, Kim HS, Jung SC, Jo I, Park JW. Tonsil-derived mesenchymal stem cells alleviate concanavalin A-induced acute liver injury. Exp Cell Res. 2014;326(1):143–154. doi: 10.1016/j.yexcr.2014.06.007. [DOI] [PubMed] [Google Scholar]
- 41.Sun L, Akiyama K, Zhang H, Yamaza T, Hou Y, Zhao S, Xu T, Le A, Shi S. Mesenchymal stem cell transplantation reverses multiorgan dysfunction in systemic lupus erythematosus mice and humans. Stem Cells. 2009;27(6):1421–1432. doi: 10.1002/stem.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wu KH, Chan CK, Tsai C, Chang YH, Sieber M, Chiu TH, Ho M, Peng CT, Wu HP, Huang JL. Effective treatment of severe steroid-resistant acute graft-versus-host disease with umbilical cord-derived mesenchymal stem cells. Transplantation. 2011;91(12):1412–1416. doi: 10.1097/TP.0b013e31821aba18. [DOI] [PubMed] [Google Scholar]
- 43.Ren GW, Zhang LY, Zhao X, Xu GW, Zhang YY, Roberts AI, Zhao RC, Shi YF. Mesenchymal stem cell-mediated immunosuppression occurs via concerted action of chemokines and nitric oxide. Cell Stem Cell. 2008;2(2):141–150. doi: 10.1016/j.stem.2007.11.014. [DOI] [PubMed] [Google Scholar]
- 44.Ren GW, Zhao X, Zhang LY, Zhang JM, L’Huillier A, Ling WF, Roberts AI, Le AD, Shi ST, Shao CS, Shi YF. Inflammatory cytokine-induced intercellular adhesion molecule-1 and vascular cell adhesion molecule-1 in mesenchymal stem cells are critical for immunosuppression. J Immunol. 2010;184(5):2321–2328. doi: 10.4049/jimmunol.0902023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Crop MJ, Baan CC, Korevaar SS, Ijzermans JN, Pescatori M, Stubbs AP, van Ijcken WF, Dahlke MH, Eggenhofer E, Weimar W, Hoogduijn MJ. Inflammatory conditions affect gene expression and function of human adipose tissue-derived mesenchymal stem cells. Clin Exp Immunol. 2010;162(3):474–486. doi: 10.1111/j.1365-2249.2010.04256.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ma S, Xie N, Li W, Yuan B, Shi Y, Wang Y. Immunobiology of mesenchymal stem cells. Cell Death Differ. 2014;21(2):216–225. doi: 10.1038/cdd.2013.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Li W, Ren G, Huang Y, Su J, Han Y, Li J, Chen X, Cao K, Chen Q, Shou P, Zhang L, Yuan ZR, Roberts AI, Shi S, Le AD, Shi Y. Mesenchymal stem cells: a double-edged sword in regulating immune responses. Cell Death Differ. 2012;19(9):1505–1513. doi: 10.1038/cdd.2012.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Renner P, Eggenhofer E, Rosenauer A, Popp FC, Steinmann JF, Slowik P, Geissler EK, Piso P, Schlitt HJ, Dahlke MH. Mesenchymal stem cells require a sufficient, ongoing immune response to exert their immunosuppressive function. Transplant Proc. 2009;41(6):2607–2611. doi: 10.1016/j.transproceed.2009.06.119. [DOI] [PubMed] [Google Scholar]
- 49.Su J, Chen X, Huang Y, Li W, Li J, Cao K, Cao G, Zhang L, Li F, Roberts AI, Kang H, Yu P, Ren G, Ji W, Wang Y, Shi Y. Phylogenetic distinction of iNOS and IDO function in mesenchymal stem cell-mediated immunosuppression in mammalian species. Cell Death Differ. 2014;21(3):388–396. doi: 10.1038/cdd.2013.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ren G, Su J, Zhang L, Zhao X, Ling W, L’Huillie A, Zhang J, Lu Y, Roberts AI, Ji W, Zhang H, Rabson AB, Shi Y. Species variation in the mechanisms of mesenchymal stem cell-mediated immunosuppression. Stem Cells. 2009;27(8):1954–1962. doi: 10.1002/stem.118. [DOI] [PubMed] [Google Scholar]
- 51.Bogdan C. Nitric oxide and the immune response. Nat Immunol. 2001;2(10):907–916. doi: 10.1038/ni1001-907. [DOI] [PubMed] [Google Scholar]
- 52.Bouffi C, Bony C, Courties G, Jorgensen C, Noel D. IL-6-dependent PGE2 secretion by mesenchymal stem cells inhibits local inflammation in experimental arthritis. PLoS One. 2010;5(12):e14247. doi: 10.1371/journal.pone.0014247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Munn DH. Blocking IDO activity to enhance anti-tumor immunity. Front Biosci (Elite Ed) 2012;4:734–745. doi: 10.2741/e414. [DOI] [PubMed] [Google Scholar]
- 54.Meisel R, Zibert A, Laryea M, Gobel U, Daubener W, Dilloo D. Human bone marrow stromal cells inhibit allogeneic T-cell responses by indoleamine 2,3-dioxygenase-mediated tryptophan degradation. Blood. 2004;103(12):4619–4621. doi: 10.1182/blood-2003-11-3909. [DOI] [PubMed] [Google Scholar]
- 55.Ling W, Zhang J, Yuan Z, Ren G, Zhang L, Chen X, Rabson AB, Roberts AI, Wang Y, Shi Y. Mesenchymal stem cells use IDO to regulate immunity in tumor microenvironment. Cancer Res. 2014;74(5):1576–1587. doi: 10.1158/0008-5472.CAN-13-1656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Matysiak M, Orlowski W, Fortak-Michalska M, Jurewicz A, Selmaj K. Immunoregulatory function of bone marrow mesenchymal stem cells in EAE depends on their differentiation state and secretion of PGE2. J Neuroimmunol. 2011;233(1–2):106–111. doi: 10.1016/j.jneuroim.2010.12.004. [DOI] [PubMed] [Google Scholar]
- 57.Bruno S, Deregibus MC, Camussi G. The secretome of mesenchymal stromal cells: role of extracellular vesicles in immunomodulation. Immunol Lett. 2015;168(2):154–158. doi: 10.1016/j.imlet.2015.06.007. [DOI] [PubMed] [Google Scholar]
- 58.Burrello J, Monticone S, Gai C, Gomez Y, Kholia S, Camussi G. Stem cell-derived extracellular vesicles and immune-modulation. Front Cell Dev Biol. 2016;4:83. doi: 10.3389/fcell.2016.00083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Stephen J, Bravo EL, Colligan D, Fraser AR, Petrik J, Campbell JDM. Mesenchymal stromal cells as multifunctional cellular therapeutics—a potential role for extracellular vesicles. Transfus Apher Sci. 2016;55(1):62–69. doi: 10.1016/j.transci.2016.07.011. [DOI] [PubMed] [Google Scholar]
- 60.Di Trapani M, Bassi G, Midolo M, Gatti A, Kamga PT, Cassaro A, Carusone R, Adamo A, Krampera M (2016) Differential and transferable modulatory effects of mesenchymal stromal cell-derived extracellular vesicles on T, B and NK cell functions. Sci Rep UK 6. doi:10.1038/srep24120 [DOI] [PMC free article] [PubMed]
- 61.Zhang B, Yin Y, Lai RC, Lim SK. Immunotherapeutic potential of extracellular vesicles. Front Immunol. 2014;5:518. doi: 10.3389/fimmu.2014.00518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Blazquez R, Sanchez-Margallo FM, de la Rosa O, Dalemans W, Alvarez V, Tarazona R, Casado JG. Immunomodulatory potential of human adipose mesenchymal stem cells derived exosomes on in vitro stimulated T cells. Front Immunol. 2014;5:556. doi: 10.3389/fimmu.2014.00556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Amarnath S, Foley JE, Farthing DE, Gress RE, Laurence A, Eckhaus MA, Metais JY, Rose JJ, Hakim FT, Felizardo TC, Cheng AV, Robey PG, Stroncek DE, Sabatino M, Battiwalla M, Ito S, Fowler DH, Barrett AJ. Bone marrow-derived mesenchymal stromal cells harness purinergenic signaling to tolerize human Th1 cells in vivo. Stem Cells. 2015;33(4):1200–1212. doi: 10.1002/stem.1934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wang L, Gu Z, Zhao X, Yang N, Wang F, Deng A, Zhao S, Luo L, Wei H, Guan L, Gao Z, Li Y, Wang L, Liu D, Gao C. Extracellular vesicles released from human umbilical cord-derived mesenchymal stromal cells prevent life-threatening acute graft-versus-host disease in a mouse model of allogeneic hematopoietic stem cell transplantation. Stem Cells Dev. 2016;25(24):1874–1883. doi: 10.1089/scd.2016.0107. [DOI] [PubMed] [Google Scholar]
- 65.Favaro E, Carpanetto A, Caorsi C, Giovarelli M, Angelini C, Cavallo-Perin P, Tetta C, Camussi G, Zanone MM. Human mesenchymal stem cells and derived extracellular vesicles induce regulatory dendritic cells in type 1 diabetic patients. Diabetologia. 2016;59(2):325–333. doi: 10.1007/s00125-015-3808-0. [DOI] [PubMed] [Google Scholar]
- 66.Mokarizadeh A, Delirezh N, Morshedi A, Mosayebi G, Farshid AA, Mardani K. Microvesicles derived from mesenchymal stem cells: potent organelles for induction of tolerogenic signaling. Immunol Lett. 2012;147(1–2):47–54. doi: 10.1016/j.imlet.2012.06.001. [DOI] [PubMed] [Google Scholar]
- 67.Del Fattore A, Luciano R, Pascucci L, Goffredo BM, Giorda E, Scapaticci M, Fierabracci A, Muraca M. Immunoregulatory effects of mesenchymal stem cell-derived extracellular vesicles on T lymphocytes. Cell Transplant. 2015;24(12):2615–2627. doi: 10.3727/096368915X687543. [DOI] [PubMed] [Google Scholar]
- 68.Zhang B, Yin Y, Lai RC, Tan SS, Choo AB, Lim SK. Mesenchymal stem cells secrete immunologically active exosomes. Stem Cells Dev. 2014;23(11):1233–1244. doi: 10.1089/scd.2013.0479. [DOI] [PubMed] [Google Scholar]
- 69.Xu C, Yu P, Han X, Du L, Gan J, Wang Y, Shi Y. TGF-beta promotes immune responses in the presence of mesenchymal stem cells. J Immunol. 2014;192(1):103–109. doi: 10.4049/jimmunol.1302164. [DOI] [PubMed] [Google Scholar]
- 70.Sala E, Genua M, Petti L, Anselmo A, Arena V, Cibella J, Zanotti L, D’Alessio S, Scaldaferri F, Luca G, Arato I, Calafiore R, Sgambato A, Rutella S, Locati M, Danese S, Vetrano S. Mesenchymal stem cells reduce colitis in mice via release of TSG6, independently of their localization to the intestine. Gastroenterology. 2015;149(1):163 e120–176 e120. doi: 10.1053/j.gastro.2015.03.013. [DOI] [PubMed] [Google Scholar]
- 71.Choi JJ, Yoo SA, Park SJ, Kang YJ, Kim WU, Oh IH, Cho CS. Mesenchymal stem cells overexpressing interleukin-10 attenuate collagen-induced arthritis in mice. Clin Exp Immunol. 2008;153(2):269–276. doi: 10.1111/j.1365-2249.2008.03683.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Pieper K, Grimbacher B, Eibel H. B-cell biology and development. J Allergy Clin Immunol. 2013;131(4):959–971. doi: 10.1016/j.jaci.2013.01.046. [DOI] [PubMed] [Google Scholar]
- 73.O’Connor BP, Vogel LA, Zhang WJ, Loo W, Shnider D, Lind EF, Ratliff M, Noelle RJ, Erickson LD. Imprinting the fate of antigen-reactive B cells through the affinity of the B cell receptor. J Immunol. 2006;177(11):7723–7732. doi: 10.4049/jimmunol.177.11.7723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.De Silva NS, Klein U. Dynamics of B cells in germinal centres. Nat Rev Immunol. 2015;15(3):137–148. doi: 10.1038/nri3804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Depoil D, Weber M, Treanor B, Fleire SJ, Carrasco YR, Harwood NE, Batista FD. Early events of B cell activation by antigen. Sci Signal. 2009;2(63):pt1. doi: 10.1126/scisignal.263pt1. [DOI] [PubMed] [Google Scholar]
- 76.Hayakawa K, Hardy RR, Herzenberg LA, Herzenberg LA. Progenitors for Ly-1 B cells are distinct from progenitors for other B cells. J Exp Med. 1985;161(6):1554–1568. doi: 10.1084/jem.161.6.1554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Yang M, Sun L, Wang S, Ko KH, Xu H, Zheng BJ, Cao X, Lu L. Novel function of B cell-activating factor in the induction of IL-10-producing regulatory B cells. J Immunol. 2010;184(7):3321–3325. doi: 10.4049/jimmunol.0902551. [DOI] [PubMed] [Google Scholar]
- 78.Corcione A, Benvenuto F, Ferretti E, Giunti D, Cappiello V, Cazzanti F, Risso M, Gualandi F, Mancardi GL, Pistoia V, Uccelli A. Human mesenchymal stem cells modulate B-cell functions. Blood. 2006;107(1):367–372. doi: 10.1182/blood-2005-07-2657. [DOI] [PubMed] [Google Scholar]
- 79.Tabera S, Perez-Simon JA, Diez-Campelo M, Sanchez-Abarca LI, Blanco B, Lopez A, Benito A, Ocio E, Sanchez-Guijo FM, Canizo C, San Miguel JF. The effect of mesenchymal stem cells on the viability, proliferation and differentiation of B-lymphocytes. Haematologica. 2008;93(9):1301–1309. doi: 10.3324/haematol.12857. [DOI] [PubMed] [Google Scholar]
- 80.Che N, Li X, Zhou S, Liu R, Shi D, Lu L, Sun L. Umbilical cord mesenchymal stem cells suppress B-cell proliferation and differentiation. Cell Immunol. 2012;274(1–2):46–53. doi: 10.1016/j.cellimm.2012.02.004. [DOI] [PubMed] [Google Scholar]
- 81.Asari S, Itakura S, Ferreri K, Liu CP, Kuroda Y, Kandeel F, Mullen Y. Mesenchymal stem cells suppress B-cell terminal differentiation. Exp Hematol. 2009;37(5):604–615. doi: 10.1016/j.exphem.2009.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Rafei M, Hsieh J, Fortier S, Li M, Yuan S, Birman E, Forner K, Boivin MN, Doody K, Tremblay M, Annabi B, Galipeau J. Mesenchymal stromal cell-derived CCL2 suppresses plasma cell immunoglobulin production via STAT3 inactivation and PAX5 induction. Blood. 2008;112(13):4991–4998. doi: 10.1182/blood-2008-07-166892. [DOI] [PubMed] [Google Scholar]
- 83.Luz-Crawford P, Djouad F, Toupet K, Bony C, Franquesa M, Hoogduijn MJ, Jorgensen C, Noel D. Mesenchymal stem cell-derived interleukin 1 receptor antagonist promotes macrophage polarization and inhibits B cell differentiation. Stem Cells. 2016;34(2):483–492. doi: 10.1002/stem.2254. [DOI] [PubMed] [Google Scholar]
- 84.Conforti A, Scarsella M, Starc N, Giorda E, Biagini S, Proia A, Carsetti R, Locatelli F, Bernardo ME. Microvescicles derived from mesenchymal stromal cells are not as effective as their cellular counterpart in the ability to modulate immune responses in vitro. Stem Cells Dev. 2014;23(21):2591–2599. doi: 10.1089/scd.2014.0091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Schena F, Gambini C, Gregorio A, Mosconi M, Reverberi D, Gattorno M, Casazza S, Uccelli A, Moretta L, Martini A, Traggiai E. Interferon-gamma-dependent inhibition of B cell activation by bone marrow-derived mesenchymal stem cells in a murine model of systemic lupus erythematosus. Arthritis Rheum. 2010;62(9):2776–2786. doi: 10.1002/art.27560. [DOI] [PubMed] [Google Scholar]
- 86.Franquesa M, Mensah FK, Huizinga R, Strini T, Boon L, Lombardo E, DelaRosa O, Laman JD, Grinyo JM, Weimar W, Betjes MG, Baan CC, Hoogduijn MJ. Human adipose tissue-derived mesenchymal stem cells abrogate plasmablast formation and induce regulatory B cells independently of T helper cells. Stem Cells. 2015;33(3):880–891. doi: 10.1002/stem.1881. [DOI] [PubMed] [Google Scholar]
- 87.Peng Y, Chen X, Liu Q, Zhang X, Huang K, Liu L, Li H, Zhou M, Huang F, Fan Z, Sun J, Liu Q, Ke M, Li X, Zhang Q, Xiang AP. Mesenchymal stromal cells infusions improve refractory chronic graft versus host disease through an increase of CD5+ regulatory B cells producing interleukin 10. Leukemia. 2015;29(3):636–646. doi: 10.1038/leu.2014.225. [DOI] [PubMed] [Google Scholar]
- 88.Guo Y, Chan KH, Lai WH, Siu CW, Kwan SC, Tse HF, Wing-Lok Ho P, Wing-Man Ho J. Human mesenchymal stem cells upregulate CD1dCD5(+) regulatory B cells in experimental autoimmune encephalomyelitis. Neuroimmunomodulation. 2013;20(5):294–303. doi: 10.1159/000351450. [DOI] [PubMed] [Google Scholar]
- 89.Park MJ, Kwok SK, Lee SH, Kim EK, Park SH, Cho ML. Adipose tissue-derived mesenchymal stem cells induce expansion of interleukin-10-producing regulatory B cells and ameliorate autoimmunity in a murine model of systemic lupus erythematosus. Cell Transplant. 2015;24(11):2367–2377. doi: 10.3727/096368914X685645. [DOI] [PubMed] [Google Scholar]
- 90.Lee DS, Yi TG, Lee HJ, Kim SN, Park S, Jeon MS, Song SU. Mesenchymal stem cells infected with Mycoplasma arginini secrete complement C3 to regulate immunoglobulin production in B lymphocytes. Cell Death Dis. 2014;5:e1192. doi: 10.1038/cddis.2014.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Che N, Li X, Zhang L, Liu R, Chen HF, Gao X, Shi ST, Chen WJ, Sun LY. Impaired B cell inhibition by lupus bone marrow mesenchymal stem cells is caused by reduced CCL2 expression. J Immunol. 2014;193(10):5306–5314. doi: 10.4049/jimmunol.1400036. [DOI] [PubMed] [Google Scholar]
- 92.Rasmusson I, Le Blanc K, Sundberg B, Ringden O. Mesenchymal stem cells stimulate antibody secretion in human B cells. Scand J Immunol. 2007;65(4):336–343. doi: 10.1111/j.1365-3083.2007.01905.x. [DOI] [PubMed] [Google Scholar]
- 93.Traggiai E, Volpi S, Schena F, Gattorno M, Ferlito F, Moretta L, Martini A. Bone marrow-derived mesenchymal stem cells induce both polyclonal expansion and differentiation of B cells isolated from healthy donors and systemic lupus erythematosus patients. Stem Cells. 2008;26(2):562–569. doi: 10.1634/stemcells.2007-0528. [DOI] [PubMed] [Google Scholar]
- 94.Healy ME, Bergin R, Mahon BP, English K Mesenchymal stromal cells protect against caspase 3-mediated apoptosis of CD19(+) peripheral B cells through contact-dependent upregulation of VEGF. Stem Cells Dev. 2015;24(20):2391–2402. doi: 10.1089/scd.2015.0089. [DOI] [PubMed] [Google Scholar]
- 95.Franquesa M, Hoogduijn MJ, Bestard O, Grinyo JM (2012) Immunomodulatory effect of mesenchymal stem cells on B cells. Front Immunol 3. doi:10.3389/fimmu.2012.00212 [DOI] [PMC free article] [PubMed]
- 96.Mildner A, Jung S. Development and function of dendritic cell subsets. Immunity. 2014;40(5):642–656. doi: 10.1016/j.immuni.2014.04.016. [DOI] [PubMed] [Google Scholar]
- 97.Zhang W, Ge W, Li C, You S, Liao L, Han Q, Deng W, Zhao RC. Effects of mesenchymal stem cells on differentiation, maturation, and function of human monocyte-derived dendritic cells. Stem Cells Dev. 2004;13(3):263–271. doi: 10.1089/154732804323099190. [DOI] [PubMed] [Google Scholar]
- 98.Jiang XX, Zhang Y, Liu B, Zhang SX, Wu Y, Yu XD, Mao N. Human mesenchymal stem cells inhibit differentiation and function of monocyte-derived dendritic cells. Blood. 2005;105(10):4120–4126. doi: 10.1182/blood-2004-02-0586. [DOI] [PubMed] [Google Scholar]
- 99.Nauta AJ, Kruisselbrink AB, Lurvink E, Willemze R, Fibbe WE. Mesenchymal stem cells inhibit generation and function of both CD34+-derived and monocyte-derived dendritic cells. J Immunol. 2006;177(4):2080–2087. doi: 10.4049/jimmunol.177.4.2080. [DOI] [PubMed] [Google Scholar]
- 100.Li YP, Paczesny S, Lauret E, Poirault S, Bordigoni P, Mekhloufi F, Hequet O, Bertrand Y, Ou-Yang JP, Stoltz JF, Miossec P, Eljaafari A. Human mesenchymal stem cells license adult CD34(+) hemopoietic progenitor cells to differentiate into regulatory dendritic cells through activation of the notch pathway. J Immunol. 2008;180(3):1598–1608. doi: 10.4049/jimmunol.180.3.1598. [DOI] [PubMed] [Google Scholar]
- 101.Chen HW, Chen HY, Wang LT, Wang FH, Fang LW, Lai HY, Chen HH, Lu J, Hung MS, Cheng Y, Chen MY, Liu SJ, Chong P, Lee OKS, Hsu SC. Mesenchymal stem cells tune the development of monocyte-derived dendritic cells toward a myeloid-derived suppressive phenotype through growth-regulated oncogene chemokines. J Immunol. 2013;190(10):5065–5077. doi: 10.4049/jimmunol.1202775. [DOI] [PubMed] [Google Scholar]
- 102.Abomaray FM, Al Jumah MA, Kalionis B, AlAskar AS, Al Harthy S, Jawdat D, Al Khaldi A, Alkushi A, Knawy BA, Abumaree MH. Human chorionic villous mesenchymal stem cells modify the functions of human dendritic cells, and induce an anti-inflammatory phenotype in CD1+ dendritic cells. Stem Cell Rev. 2015;11(3):423–441. doi: 10.1007/s12015-014-9562-8. [DOI] [PubMed] [Google Scholar]
- 103.English K, Barry FP, Mahon BP. Murine mesenchymal stem cells suppress dendritic cell migration, maturation and antigen presentation. Immunol Lett. 2008;115(1):50–58. doi: 10.1016/j.imlet.2007.10.002. [DOI] [PubMed] [Google Scholar]
- 104.Zhang B, Liu R, Shi D, Liu XX, Chen Y, Dou XW, Zhu XS, Lu CH, Liang W, Liao LM, Zenke M, Zhao RCH. Mesenchymal stem cells induce mature dendritic cells into a novel Jagged-2-dependent regulatory dendritic cell population. Blood. 2009;113(1):46–57. doi: 10.1182/blood-2008-04-154138. [DOI] [PubMed] [Google Scholar]
- 105.Liu X, Qu X, Chen Y, Liao L, Cheng K, Shao C, Zenke M, Keating A, Zhao RC. Mesenchymal stem/stromal cells induce the generation of novel IL-10-dependent regulatory dendritic cells by SOCS3 activation. J Immunol. 2012;189(3):1182–1192. doi: 10.4049/jimmunol.1102996. [DOI] [PubMed] [Google Scholar]
- 106.Cahill EF, Tobin LM, Carty F, Mahon BP, English K (2015) Jagged-1 is required for the expansion of CD4(+)CD25(+)FoxP3(+) regulatory T cells and tolerogenic dendritic cells by murine mesenchymal stromal cells. Stem Cell Res Ther 6. doi:10.1186/s13287-015-0021-5 [DOI] [PMC free article] [PubMed]
- 107.Chiesa S, Morbelli S, Morando S, Massollo M, Marini C, Bertoni A, Frassoni F, Bartolome ST, Sambuceti G, Traggiai E, Uccelli A. Mesenchymal stem cells impair in vivo T-cell priming by dendritic cells. P Natl Acad Sci USA. 2011;108(42):17384–17389. doi: 10.1073/pnas.1103650108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Li H, Guo Z, Jiang X, Zhu H, Li X, Mao N. Mesenchymal stem cells alter migratory property of T and dendritic cells to delay the development of murine lethal acute graft-versus-host disease. Stem Cells. 2008;26(10):2531–2541. doi: 10.1634/stemcells.2008-0146. [DOI] [PubMed] [Google Scholar]
- 109.Zhang Y, Cai W, Huang Q, Gu Y, Shi Y, Huang J, Zhao F, Liu Q, Wei X, Jin M, Wu C, Xie Q, Zhang Y, Wan B, Zhang Y. Mesenchymal stem cells alleviate bacteria-induced liver injury in mice by inducing regulatory dendritic cells. Hepatology. 2014;59(2):671–682. doi: 10.1002/hep.26670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Liu XX, Ren SD, Ge CZ, Cheng K, Zenke M, Keating A, Zhao RCH. Sca-1(+)Lin()CD117() mesenchymal stem/stromal sells induce the generation of novel IRF8-controlled regulatory dendritic cells through Notch-RBP-J signaling. J Immunol. 2015;194(9):4298–4308. doi: 10.4049/jimmunol.1402641. [DOI] [PubMed] [Google Scholar]
- 111.Huang YF, Chen P, Zhang CB, Ko GJ, Ruiz M, Fiorina P, Hussain MA, Wasowska BA, Rabb H, Womer KL. Kidney-derived mesenchymal stromal cells modulate dendritic cell function to suppress alloimmune responses and delay allograft rejection. Transplantation. 2010;90(12):1307–1311. doi: 10.1097/TP.0b013e3181fdd9eb. [DOI] [PubMed] [Google Scholar]
- 112.Djouad F, Charbonnier LM, Bouffi C, Louis-Plence P, Bony C, Apparailly F, Cantos C, Jorgensen C, Noel D. Mesenchymal stem cells inhibit the differentiation of dendritic cells through an interleukin-6-dependent mechanism. Stem Cells. 2007;25(8):2025–2032. doi: 10.1634/stemcells.2006-0548. [DOI] [PubMed] [Google Scholar]
- 113.Deng Y, Yi S, Wang G, Cheng J, Zhang Y, Chen W, Tai Y, Chen S, Chen G, Liu W, Zhang Q, Yang Y. Umbilical cord-derived mesenchymal stem cells instruct dendritic cells to acquire tolerogenic phenotypes through the IL-6-mediated upregulation of SOCS1. Stem Cells Dev. 2014;23(17):2080–2092. doi: 10.1089/scd.2013.0559. [DOI] [PubMed] [Google Scholar]
- 114.Spaggiari GM, Abdelrazik H, Becchetti F, Moretta L. MSCs inhibit monocyte-derived DC maturation and function by selectively interfering with the generation of immature DCs: central role of MSC-derived prostaglandin E2. Blood. 2009;113(26):6576–6583. doi: 10.1182/blood-2009-02-203943. [DOI] [PubMed] [Google Scholar]
- 115.Ramasamy R, Fazekasova H, Lam EWF, Soeiro I, Lombardi G, Dazzi F. Mesenchymal stem cells inhibit dendritic cell differentiation and function by preventing entry into the cell cycle. Transplantation. 2007;83(1):71–76. doi: 10.1097/01.tp.0000244572.24780.54. [DOI] [PubMed] [Google Scholar]
- 116.Chia JJ, Zhu T, Chyou S, Dasoveanu DC, Carballo C, Tian S, Magro CM, Rodeo S, Spiera RF, Ruddle NH, McGraw TE, Browning JL, Lafyatis R, Gordon JK, Lu TT. Dendritic cells maintain dermal adipose-derived stromal cells in skin fibrosis. J Clin Invest. 2016 doi: 10.1172/JCI85740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Wynn TA, Vannella KM. Macrophages in tissue repair, regeneration, and fibrosis. Immunity. 2016;44(3):450–462. doi: 10.1016/j.immuni.2016.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Perdiguero EG, Geissmann F. The development and maintenance of resident macrophages. Nat Immunol. 2016;17(1):2–8. doi: 10.1038/ni.3341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Schulz C, Gomez Perdiguero E, Chorro L, Szabo-Rogers H, Cagnard N, Kierdorf K, Prinz M, Wu B, Jacobsen SE, Pollard JW, Frampton J, Liu KJ, Geissmann F. A lineage of myeloid cells independent of Myb and hematopoietic stem cells. Science. 2012;336(6077):86–90. doi: 10.1126/science.1219179. [DOI] [PubMed] [Google Scholar]
- 120.Glass CK, Natoli G. Molecular control of activation and priming in macrophages. Nat Immunol. 2016;17(1):26–33. doi: 10.1038/ni.3306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Zhang QZ, Su WR, Shi SH, Wilder-Smith P, Xiang AP, Wong A, Nguyen AL, Kwon CW, Le AD. Human gingiva-derived mesenchymal stem cells elicit polarization of M2 macrophages and enhance cutaneous wound healing. Stem Cells. 2010;28(10):1856–1868. doi: 10.1002/stem.503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Cho DI, Kim MR, Jeong HY, Jeong HC, Jeong MH, Yoon SH, Kim YS, Ahn Y. Mesenchymal stem cells reciprocally regulate the M1/M2 balance in mouse bone marrow-derived macrophages. Exp Mol Med. 2014 doi: 10.1038/emm.2013.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Francois M, Romieu-Mourez R, Li M, Galipeau J. Human MSC suppression correlates with cytokine induction of indoleamine 2,3-dioxygenase and bystander M2 macrophage differentiation. Mol Ther. 2012;20(1):187–195. doi: 10.1038/mt.2011.189. [DOI] [PubMed] [Google Scholar]
- 124.Selleri S, Bifsha P, Civini S, Pacelli C, Dieng MM, Lemieux W, Jin P, Bazin R, Patey N, Marincola FM, Moldovan F, Zaouter C, Trudeau LE, Benabdhalla B, Louis I, Beausejour C, Stroncek D, Le Deist F, Haddad E. Human mesenchymal stromal cell-secreted lactate induces M2-macrophage differentiation by metabolic reprogramming. Oncotarget. 2016;7(21):30193–30210. doi: 10.18632/oncotarget.8623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Nemeth K, Leelahavanichkul A, Yuen PST, Mayer B, Parmelee A, Doi K, Robey PG, Leelahavanichkul K, Koller BH, Brown JM, Hu XZ, Jelinek I, Star RA, Mezey E. Bone marrow stromal cells attenuate sepsis via prostaglandin E-2-dependent reprogramming of host macrophages to increase their interleukin-10 production. Nat Med. 2009;15(1):42–49. doi: 10.1038/nm.1905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Choi H, Lee RH, Bazhanov N, Oh JY, Prockop DJ. Anti-inflammatory protein TSG-6 secreted by activated MSCs attenuates zymosan-induced mouse peritonitis by decreasing TLR2/NF-kappa B signaling in resident macrophages. Blood. 2011;118(2):330–338. doi: 10.1182/blood-2010-12-327353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Wise AF, Williams TM, Kiewiet MBG, Payne NL, Siatskas C, Samuel CS, Ricardo SD. Human mesenchymal stem cells alter macrophage phenotype and promote regeneration via homing to the kidney following ischemia-reperfusion injury. Am J Physiol Renal. 2014;306(10):F1222–F1235. doi: 10.1152/ajprenal.00675.2013. [DOI] [PubMed] [Google Scholar]
- 128.Lee KC, Lin HC, Huang YH, Hung SC. Allo-transplantation of mesenchymal stem cells attenuates hepatic injury through IL1Ra dependent macrophage switch in a mouse model of liver disease. J Hepatol. 2015;63(6):1405–1412. doi: 10.1016/j.jhep.2015.07.035. [DOI] [PubMed] [Google Scholar]
- 129.Zullo JA, Nadel EP, Rabadi MM, Baskind MJ, Rajdev MA, Demaree CM, Vasko R, Chugh SS, Lamba R, Goligorsky MS, Ratliff BB. The secretome of hydrogel-coembedded endothelial progenitor cells and mesenchymal stem cells instructs macrophage polarization in endotoxemia. Stem Cells Transl Med. 2015;4(7):852–861. doi: 10.5966/sctm.2014-0111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Xie ZY, Hao HJ, Tong C, Cheng Y, Liu JJ, Pang YP, Si YL, Guo YL, Zang L, Mu YM, Han WD. Human umbilical cord-derived mesenchymal stem cells elicit macrophages into an anti-inflammatory phenotype to alleviate insulin resistance in type 2 diabetic rats. Stem Cells. 2016;34(3):627–639. doi: 10.1002/stem.2238. [DOI] [PubMed] [Google Scholar]
- 131.Braza F, Dirou S, Forest V, Sauzeau V, Hassoun D, Chesne J, Cheminant-Muller MA, Sagan C, Magnan A, Lemarchand P. Mesenchymal stem cells induce suppressive mcrophages through phagocytosis in a mouse model of asthma. Stem Cells. 2016;34(7):1836–1845. doi: 10.1002/stem.2344. [DOI] [PubMed] [Google Scholar]
- 132.Chaturvedi P, Gilkes DM, Takano N, Semenza GL. Hypoxia-inducible factor-dependent signaling between triple-negative breast cancer cells and mesenchymal stem cells promotes macrophage recruitment. P Natl Acad Sci USA. 2014;111(20):E2120–E2129. doi: 10.1073/pnas.1406655111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Liu WX, Zhang S, Gu SZ, Sang LX, Dai C. Mesenchymal stem cells recruit macrophages to alleviate experimental colitis through TGF beta 1. Cell Physiol Biochem. 2015;35(3):858–865. doi: 10.1159/000369743. [DOI] [PubMed] [Google Scholar]
- 134.Braza F, Dirou S, Forest V, Sauzeau V, Hassoun D, Chesne J, Cheminant-Muller MA, Sagan C, Magnan A, Lemarchand P. Mesenchymal stem cells induce suppressive macrophages through phagocytosis in a mouse model of asthma. Stem Cells. 2016;34(7):1836–1845. doi: 10.1002/stem.2344. [DOI] [PubMed] [Google Scholar]
- 135.Ylostalo JH, Bartosh TJ, Coble K, Prockop DJ. Human mesenchymal stem/stromal cells cultured as spheroids are self-activated to produce prostaglandin E2 that directs stimulated macrophages into an anti-inflammatory phenotype. Stem Cells. 2012;30(10):2283–2296. doi: 10.1002/stem.1191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Song XL, Xie SS, Lu K, Wang CH. Mesenchymal stem cells alleviate experimental asthma by inducing polarization of alveolar macrophages. Inflammation. 2015;38(2):485–492. doi: 10.1007/s10753-014-9954-6. [DOI] [PubMed] [Google Scholar]
- 137.Fathman JW, Bhattacharya D, Inlay MA, Seita J, Karsunky H, Weissman IL. Identification of the earliest natural killer cell-committed progenitor in murine bone marrow. Blood. 2011;118(20):5439–5447. doi: 10.1182/blood-2011-04-348912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Res P, MartinezCaceres E, Jaleco AC, Staal F, Noteboom E, Weijer K, Spits H. CD34(+)CD38(dim) cells in the human thymus can differentiate into T, natural killer, and dendritic cells but are distinct from pluripotent stem cells. Blood. 1996;87(12):5196–5206. [PubMed] [Google Scholar]
- 139.Pegram HJ, Andrews DM, Smyth MJ, Darcy PK, Kershaw MH. Activating and inhibitory receptors of natural killer cells. Immunol Cell Biol. 2011;89(2):216–224. doi: 10.1038/icb.2010.78. [DOI] [PubMed] [Google Scholar]
- 140.Childs RW, Carlsten M. Therapeutic approaches to enhance natural killer cell cytotoxicity against cancer: the force awakens. Nat Rev Drug Discov. 2015;14(7):487–498. doi: 10.1038/nrd4506. [DOI] [PubMed] [Google Scholar]
- 141.Fauriat C, Long EO, Ljunggren HG, Bryceson YT. Regulation of human NK-cell cytokine and chemokine production by target cell recognition. Blood. 2010;115(11):2167–2176. doi: 10.1182/blood-2009-08-238469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Aggarwal S, Pittenger MF. Human mesenchymal stem cells modulate allogeneic immune cell responses. Blood. 2005;105(4):1815–1822. doi: 10.1182/blood-2004-04-1559. [DOI] [PubMed] [Google Scholar]
- 143.Spaggiari GM, Capobianco A, Becchetti S, Mingari MC, Moretta L. Mesenchymal stem cell-natural killer cell interactions: evidence that activated NK cells are capable of killing MSCs, whereas MSCs can inhibit IL-2-induced NK-cell proliferation. Blood. 2006;107(4):1484–1490. doi: 10.1182/blood-2005-07-2775. [DOI] [PubMed] [Google Scholar]
- 144.Sotiropoulou PA, Perez SA, Gritzapis AD, Baxevanis CN, Papamichail M. Interactions between human mesenchymal stem cells and natural killer cells. Stem Cells. 2006;24(1):74–85. doi: 10.1634/stemcells.2004-0359. [DOI] [PubMed] [Google Scholar]
- 145.Spaggiari GM, Capobianco A, Abdelrazik H, Becchetti F, Mingari MC, Moretta L. Mesenchymal stem cells inhibit natural killer-cell proliferation, cytotoxicity, and cytokine production: role of indoleamine 2,3-dioxygenase and prostaglandin E2. Blood. 2008;111(3):1327–1333. doi: 10.1182/blood-2007-02-074997. [DOI] [PubMed] [Google Scholar]
- 146.Qu MM, Cui J, Zhu J, Ma YH, Yuan X, Shi JM, Guo DY, Li CY. Bone marrow-derived mesenchymal stem cells suppress NK cell recruitment and activation in PolyI:C-induced liver injury. Biochem Biophys Res Commun. 2015;466(2):173–179. doi: 10.1016/j.bbrc.2015.08.125. [DOI] [PubMed] [Google Scholar]
- 147.Michelo CM, Fasse E, Van Cranenbroek B, Linda K, van der Meer A, Abdelrazik H, Joosten I. Added effects of dexamethasone and mesenchymal stem cells on early Natural Killer cell activation. Transpl Immunol. 2016;37:1–9. doi: 10.1016/j.trim.2016.04.008. [DOI] [PubMed] [Google Scholar]
- 148.Chen X, Shao H, Zhi Y, Xiao Q, Su C, Dong L, Liu X, Li X, Zhang X. CD73 pathway contributes to the immunosuppressive ability of mesenchymal stem cells in intraocular autoimmune responses. Stem Cells Dev. 2016;25(4):337–346. doi: 10.1089/scd.2015.0227. [DOI] [PubMed] [Google Scholar]
- 149.Chatterjee D, Tufa DM, Baehre H, Hass R, Schmidt RE, Jacobs R. Natural killer cells acquire CD73 expression upon exposure to mesenchymal stem cells. Blood. 2014;123(4):594–595. doi: 10.1182/blood-2013-09-524827. [DOI] [PubMed] [Google Scholar]
- 150.El Omar R, Xiong Y, Dostert G, Louis H, Gentils M, Menu P, Stoltz JF, Velot E, Decot V. Immunomodulation of endothelial differentiated mesenchymal stromal cells: impact on T and NK cells. Immunol Cell Biol. 2016;94(4):342–356. doi: 10.1038/icb.2015.94. [DOI] [PubMed] [Google Scholar]
- 151.Lu Y, Liu J, Liu Y, Qin YR, Luo Q, Wang QL, Duan HF. TLR4 plays a crucial role in MSC-induced inhibition of NK cell function. Biochem Biophys Res Commun. 2015;464(2):541–547. doi: 10.1016/j.bbrc.2015.07.002. [DOI] [PubMed] [Google Scholar]
- 152.Boissel L, Tuncer HH, Betancur M, Wolfberg A, Klingemann H. Umbilical cord mesenchymal stem cells increase expansion of cord blood natural killer cells. Biol Blood Marrow Transplant. 2008;14(9):1031–1038. doi: 10.1016/j.bbmt.2008.06.016. [DOI] [PubMed] [Google Scholar]
- 153.Thomas H, Jager M, Mauel K, Brandau S, Lask S, Flohe SB. Interaction with mesenchymal stem cells provokes natural killer cells for enhanced IL-12/IL-18-induced interferon-gamma secretion. Mediat Inflamm. 2014 doi: 10.1155/2014/143463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Cui RT, Rekasi H, Hepner-Schefczyk M, Fessmann K, Petri RM, Bruderek K, Brandau S, Jager M, Flohe SB (2016) Human mesenchymal stromal/stem cells acquire immunostimulatory capacity upon cross-talk with natural killer cells and might improve the NK cell function of immunocompromised patients. Stem Cell Res Ther 7. doi:10.1186/s13287-016-0353-9 [DOI] [PMC free article] [PubMed]
- 155.Almeida CR, Caires HR, Vasconcelos DP, Barbosa MA. NAP-2 secreted by human NK cells can stimulate mesenchymal stem/stromal cell recruitment. Stem Cell Rep. 2016;6(4):466–473. doi: 10.1016/j.stemcr.2016.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Gotherstrom C, Lundqvist A, Duprez IR, Childs R, Berg L, le Blanc K. Fetal and adult multipotent mesenchymal stromal cells are killed by different pathways. Cytotherapy. 2011;13(3):269–278. doi: 10.3109/14653249.2010.523077. [DOI] [PubMed] [Google Scholar]
- 157.Kolaczkowska E, Kubes P. Neutrophil recruitment and function in health and inflammation. Nat Rev Immunol. 2013;13(3):159–175. doi: 10.1038/nri3399. [DOI] [PubMed] [Google Scholar]
- 158.Brinkmann V, Reichard U, Goosmann C, Fauler B, Uhlemann Y, Weiss DS, Weinrauch Y, Zychlinsky A. Neutrophil extracellular traps kill bacteria. Science. 2004;303(5663):1532–1535. doi: 10.1126/science.1092385. [DOI] [PubMed] [Google Scholar]
- 159.Raffaghello L, Bianchi G, Bertolotto M, Montecucco F, Busca A, Dallegri F, Ottonello L, Pistoia V. Human mesenchymal stem cells inhibit neutrophil apoptosis: a model for neutrophil preservation in the bone marrow niche. Stem cells. 2008;26(1):151–162. doi: 10.1634/stemcells.2007-0416. [DOI] [PubMed] [Google Scholar]
- 160.Cassatella MA, Mosna F, Micheletti A, Lisi V, Tamassia N, Cont C, Calzetti F, Pelletier M, Pizzolo G, Krampera M. Toll-like receptor-3-activated human mesenchymal stromal cells significantly prolong the survival and function of neutrophils. Stem cells. 2011;29(6):1001–1011. doi: 10.1002/stem.651. [DOI] [PubMed] [Google Scholar]
- 161.Brandau S, Jakob M, Hemeda H, Bruderek K, Janeschik S, Bootz F, Lang S. Tissue-resident mesenchymal stem cells attract peripheral blood neutrophils and enhance their inflammatory activity in response to microbial challenge. J Leukoc Biol. 2010;88(5):1005–1015. doi: 10.1189/jlb.0410207. [DOI] [PubMed] [Google Scholar]
- 162.Romieu-Mourez R, Francois M, Boivin MN, Bouchentouf M, Spaner DE, Galipeau J. Cytokine modulation of TLR expression and activation in mesenchymal stromal cells leads to a proinflammatory phenotype. J Immunol. 2009;182(12):7963–7973. doi: 10.4049/jimmunol.0803864. [DOI] [PubMed] [Google Scholar]
- 163.Yu PF, Huang Y, Han YY, Lin LY, Sun WH, Rabson AB, Wang Y, Shi YF. TNFalpha-activated mesenchymal stromal cells promote breast cancer metastasis by recruiting CXCR2+ neutrophils. Oncogene. 2016 doi: 10.1038/onc.2016.217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Isakova IA, Baker KC, Dufour J, Phinney DG. Mesenchymal stem cells yield transient improvements in motor function in an infant rhesus macaque with severe early-onset krabbe disease. Stem Cells Transl Med. 2016 doi: 10.5966/sctm.2015-0317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Brandau S, Jakob M, Bruderek K, Bootz F, Giebel B, Radtke S, Mauel K, Jager M, Flohe SB, Lang S. Mesenchymal stem cells augment the anti-bacterial activity of neutrophil granulocytes. PLoS One. 2014;9(9):e106903. doi: 10.1371/journal.pone.0106903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Jiang D, Muschhammer J, Qi Y, Kugler A, de Vries JC, Saffarzadeh M, Sindrilaru A, Beken SV, Wlaschek M, Kluth MA, Ganss C, Frank NY, Frank MH, Preissner KT, Scharffetter-Kochanek K. Suppression of neutrophil-mediated tissue damage—a novel skill of mesenchymal stem cells. Stem Cells. 2016;34(9):2393–2406. doi: 10.1002/stem.2417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Munir H, Luu NT, Clarke LS, Nash GB, McGettrick HM. Comparative ability of mesenchymal stromal cells from different tissues to limit neutrophil recruitment to inflamed endothelium. PLoS One. 2016;11(5):e0155161. doi: 10.1371/journal.pone.0155161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Zhu YG, Feng XM, Abbott J, Fang XH, Hao Q, Monsel A, Qu JM, Matthay MA, Lee JW. Human mesenchymal stem cell microvesicles for treatment of Escherichia coli endotoxin-induced acute lung injury in mice. Stem Cells. 2014;32(1):116–125. doi: 10.1002/stem.1504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Amin K. The role of mast cells in allergic inflammation. Respir Med. 2012;106(1):9–14. doi: 10.1016/j.rmed.2011.09.007. [DOI] [PubMed] [Google Scholar]
- 170.Theoharides TC, Alysandratos KD, Angelidou A, Delivanis DA, Sismanopoulos N, Zhang B, Asadi S, Vasiadi M, Weng Z, Miniati A, Kalogeromitros D. Mast cells and inflammation. Biochim Biophys Acta. 2012;1822(1):21–33. doi: 10.1016/j.bbadis.2010.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Theoharides TC, Valent P, Akin C. Mast cells, mastocytosis, and related disorders. N Engl J Med. 2015;373(2):163–172. doi: 10.1056/NEJMra1409760. [DOI] [PubMed] [Google Scholar]
- 172.Brown JM, Nemeth K, Kushnir-Sukhov NM, Metcalfe DD, Mezey E. Bone marrow stromal cells inhibit mast cell function via a COX2-dependent mechanism. Clin Exp Allergy. 2011;41(4):526–534. doi: 10.1111/j.1365-2222.2010.03685.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Kim HS, Yun JW, Shin TH, Lee SH, Lee BC, Yu KR, Seo Y, Lee S, Kang TW, Choi SW, Seo KW, Kang KS. Human umbilical cord blood mesenchymal stem cell-derived PGE2 and TGF-beta1 alleviate atopic dermatitis by reducing mast cell degranulation. Stem Cells. 2015;33(4):1254–1266. doi: 10.1002/stem.1913. [DOI] [PubMed] [Google Scholar]
- 174.Liu J, Kuwabara A, Kamio Y, Hu S, Park J, Hashimoto T, Lee JW. Human mesenchymal stem cell-derived microvesicles prevent the rupture of intracranial aneurysm in part by suppression of mast cell activation via a PGE2-dependent mechanism. Stem Cells. 2016 doi: 10.1002/stem.2448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Su W, Wan Q, Huang J, Han L, Chen X, Chen G, Olsen N, Zheng SG, Liang D. Culture medium from TNF-alpha-stimulated mesenchymal stem cells attenuates allergic conjunctivitis through multiple antiallergic mechanisms. J Allergy Clin Immunol. 2015;136(2):423 e428–432 e428. doi: 10.1016/j.jaci.2014.12.1926. [DOI] [PubMed] [Google Scholar]
- 176.Kim A, Yu HY, Heo J, Song M, Shin JH, Lim J, Yoon SJ, Kim Y, Lee S, Kim SW, Oh W, Choi SJ, Shin DM, Choo MS. Mesenchymal stem cells protect against the tissue fibrosis of ketamine-induced cystitis in rat bladder. Sci Rep. 2016;6:30881. doi: 10.1038/srep30881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Su WR, Zhang QZ, Shi SH, Nguyen AL, Le AD. Human gingiva-derived mesenchymal stromal cells attenuate contact hypersensitivity via prostaglandin E2-dependent mechanisms. Stem Cells. 2011;29(11):1849–1860. doi: 10.1002/stem.738. [DOI] [PubMed] [Google Scholar]
- 178.Allakhverdi Z, Comeau MR, Armant M, Agrawal R, Woodfolk JA, Sehmi R, Howie KJ, Gauvreau GM, Delespesse G. Mast cell-activated bone marrow mesenchymal stromal cells regulate proliferation and lineage commitment of CD34(+) progenitor cells. Front Immunol. 2013;4:461. doi: 10.3389/fimmu.2013.00461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Nazari M, Ni NC, Ludke A, Li SH, Guo J, Weisel RD, Li RK. Mast cells promote proliferation and migration and inhibit differentiation of mesenchymal stem cells through PDGF. J Mol Cell Cardiol. 2016;94:32–42. doi: 10.1016/j.yjmcc.2016.03.007. [DOI] [PubMed] [Google Scholar]
- 180.Le Blanc K, Rasmusson I, Sundberg B, Gotherstrom C, Hassan M, Uzunel M, Ringden O. Treatment of severe acute graft-versus-host disease with third party haploidentical mesenchymal stem cells. Lancet. 2004;363(9419):1439–1441. doi: 10.1016/S0140-6736(04)16104-7. [DOI] [PubMed] [Google Scholar]
- 181.Chen SL, Fang WW, Ye F, Liu YH, Qian J, Shan SJ, Zhang JJ, Chunhua RZ, Liao LM, Lin S, Sun JP. Effect on left ventricular function of intracoronary transplantation of autologous bone marrow mesenchymal stem cell in patients with acute myocardial infarction. Am J Cardiol. 2004;94(1):92–95. doi: 10.1016/j.amjcard.2004.03.034. [DOI] [PubMed] [Google Scholar]
- 182.He XW, He XS, Lian L, Wu XJ, Lan P. Systemic infusion of bone marrow-derived mesenchymal stem cells for treatment of experimental colitis in mice. Dig Dis Sci. 2012;57(12):3136–3144. doi: 10.1007/s10620-012-2290-5. [DOI] [PubMed] [Google Scholar]
- 183.Carlsson PO, Schwarcz E, Korsgren O, Le Blanc K. Preserved beta-cell function in type 1 diabetes by mesenchymal stromal cells. Diabetes. 2015;64(2):587–592. doi: 10.2337/db14-0656. [DOI] [PubMed] [Google Scholar]
- 184.Kurtzberg J, Prockop S, Teira P, Bittencourt H, Lewis V, Chan KW, Horn B, Yu L, Talano JA, Nemecek E, Mills CR, Chaudhury S. Allogeneic human mesenchymal stem cell therapy (remestemcel-L, Prochymal) as a rescue agent for severe refractory acute graft-versus-host disease in pediatric patients. Biol Blood Marrow Transplant. 2014;20(2):229–235. doi: 10.1016/j.bbmt.2013.11.001. [DOI] [PubMed] [Google Scholar]
- 185.Wohn DY. Korea okays stem cell therapies despite limited peer-reviewed data. Nat Med. 2012;18(3):329–329. doi: 10.1038/nm0312-329a. [DOI] [PubMed] [Google Scholar]
- 186.Allison M. Genzyme backs Osiris, despite Prochymal flop. Nat Biotechnol. 2009;27(11):966–967. doi: 10.1038/nbt1109-966. [DOI] [PubMed] [Google Scholar]
- 187.Yamout B, Hourani R, Salti H, Barada W, El-Hajj T, Al-Kutoubi A, Herlopian A, Baz EK, Mahfouz R, Khalil-Hamdan R, Kreidieh NM, El-Sabban M, Bazarbachi A. Bone marrow mesenchymal stem cell transplantation in patients with multiple sclerosis: a pilot study. J Neuroimmunol. 2010;227(1–2):185–189. doi: 10.1016/j.jneuroim.2010.07.013. [DOI] [PubMed] [Google Scholar]
- 188.Lazarus HM, Haynesworth SE, Gerson SL, Rosenthal NS, Caplan AI. Ex vivo expansion and subsequent infusion of human bone marrow-derived stromal progenitor cells (mesenchymal progenitor cells): implications for therapeutic use. Bone Marrow Transplant. 1995;16(4):557–564. [PubMed] [Google Scholar]
- 189.Chen M, Su W, Lin X, Guo Z, Wang J, Zhang Q, Brand D, Ryffel B, Huang J, Liu Z, He X, Le AD, Zheng SG. Adoptive transfer of human gingiva-derived mesenchymal stem cells ameliorates collagen-induced arthritis via suppression of Th1 and Th17 cells and enhancement of regulatory T cell differentiation. Arthritis Rheum. 2013;65(5):1181–1193. doi: 10.1002/art.37894. [DOI] [PMC free article] [PubMed] [Google Scholar]