Abstract
Intercellular communications play a vital role during tissue patterning, tissue repair, and immune reactions, in homeostasis as well as in disease. Exosomes are cell-derived secreted vesicles, extensively studied for their role in intercellular communication. Exosomes have the intrinsic ability to package multiple classes of proteins and nucleic acids within their lumens and on their membranes. Here, we explore the hypothesis that exosomal targeting may represent a cellular strategy that has evolved to deliver specific combinations of signals to specific target cells and influence normal or pathological processes. This review aims to evaluate the available evidence for this hypothesis and to identify open questions whose answers will illuminate our understanding and applications of exosome biology.
Keywords: Nanoscale, miRNA, Morphogen, Heterogeneity, Extracellular vesicles, Microdomains, Cancer, Signaling
Introduction
Most cells secrete different types of vesicles into the extracellular milieu. Different protocols have been used to isolate the different pools of extracellular vesicles, broadly classified as apoptotic bodies, microvesicles and exosomes [1]. Exosomes are a major group of these extracellular vesicles and are most extensively studied amongst all the different types. Exosomes have been purified from the secretions of several cell types including epithelial cells, neurons, immune cells, mesenchymal stem cells (MSCs) and fibroblasts, suggesting that every cell type that can make multivesicular bodies is capable of generating exosomes. Their ubiquitous presence also suggests that exosomes potentially represent a major mode of communication between different cell types. The recent surge in the exosome literature indicates that this pool of extracellular vesicles is highly heterogeneous. Their size, contents, mechanism of biogenesis as well as their functional output depend on the cell type of origin and physiological state [2–8]. Due to this heterogeneity, classifying and distinguishing exosomes amongst the other extracellular vesicles is a major task. In recent years, there has been significant emphasis on exosome-mediated intercellular interactions during tissue patterning, tissue maintenance and disease [9, 10]. Until 2007, exosomes were mainly appreciated for their contribution in activating juxtacrine signaling [11]. In 2007, yet another variable and heterogeneous component, i.e., cellular RNA, was found to be packaged in exosomes [12], greatly increasing the informational content and potential regulatory impact of these vesicles. Since then, exosomal fusion and the ability of exosomal RNA to regulate gene expression in target cells have been extensively investigated [13].
While a variety of specific exosome-mediated signals have been identified, it is likely that exosomes carry multiple juxtacrine and cytosolic signaling proteins, as well as other regulators. Indeed, exosomes may act as platforms to bring together different molecules with the required affinity to activate synergistic signals and steer the cellular program of target cells. Exosome-mediated signaling might, therefore, be more complex than previously appreciated. Here, we review the literature that reflects this idea with reference to exosome-mediated paracrine signaling in development and disease.
Exosome-mediated intercellular communications
The mechanisms of exosome biogenesis and their signaling roles have been extensively covered by several excellent reviews [10, 11, 14] and will not be covered here in detail. From a historical perspective, exosomes were initially thought to be involved in disposal of non-essential proteins [15]. However, subsequent studies have illuminated their role as mediators of several types of intercellular communication. For example, exosomes play an important role in antigen presentation via MHCI/II and activation of T-cells [16], in stimulating dendritic cells [17], as well as in the intercellular transfer and spread of pathogens [18]. Conversely, pathogen-derived exosomes are involved in immune-suppression and manipulation of host immune responses [19]. In cancer, exosome-mediated crosstalk between tumor and stromal compartments has been implicated in disease progression, metastasis and drug resistance [8, 20–22]. Clinically, the presence of exosomes in body fluids such as urine, plasma, saliva and breast milk has also received significant recent attention, since disease-specific alterations in exosomal cargo reflecting the patho-physiological state of the individual suggest an enormous potential for exosomes as diagnostic markers [6, 8, 23, 24]. Finally, though still in its early days, engineering of exosomal compartments to express specific signals has given rise to the concept of designer exosomes that may be therapeutically deployed [25, 26].
Exosomal proteins mediate intercellular interactions
Apart from their classical role in the range of different immunological processes, recently, exosomes have also been implicated in tissue patterning by ferrying biologically active morphogens in developing tissues to sculpt them in species-specific patterns. Exosome-mediated apical secretion of membrane-anchored morphogens, i.e., Hedgehog (Hh)-related proteins (Wrt-2, Wrt-8), was first reported in C. elegans [27]. Since then, several studies have identified exosomes as vehicles for biologically active morphogens in tissue patterning [28–31]. Apart from the membrane-anchored morphogens, several active secreted morphogens such as TGF-β, FGF and VEGF have also been identified on exosomes, probably due to their ability to interact with extracellular matrix proteins (ECM) [32–34]. Indeed, the available evidence also suggests that the sites of the most active cellular crosstalk are also sites for active exosomal communication. For example, exosomes play a major role in neuron–glia crosstalk. Exosomes secreted by neurons are involved in regulating synaptic plasticity and strength [35]; astrocyte-derived exosomes provide neuro-protective functions [36]; exosomal release at the synapse is modulated by glutamatergic activity and might play a regulatory role in normal and pathological conditions [37]. Further, microglia-derived exosomes are involved in intercellular pathogen transfer and immune surveillance in the brain, and exosomes secreted from oligodendrocytes play an important role in myelination [38]. Wg, a major player in patterning the developing nervous system, is secreted on exosomes by Drosophila cells in culture and at neuromuscular junctions by presynaptic neurons [39, 40]. The Notch ligand, Delta-like 4 (Dll4) is also secreted on exosomes to activate paracrine Notch signaling [41]. Using an in vitro tube formation assay, it has been demonstrated that Dll4-containing exosomes secreted by endothelial cells may participate in promoting blood vessel branching [41]. Apart from surface proteins, exosomes are also known to ferry several proteins inside their lumens, including transcription factors, RNA-binding proteins, metabolites, and cytoskeletal proteins (http://www.exocarta.org/). It has been demonstrated that pro-tumorigenic transcription factors as well as metabolites can be transferred to recipient cells to promote metastasis and cancer progression [42, 43]. Brain MSCs-derived exosomes also ferry cellular miRNA (miR-133b) to regulate neurite growth [44]. Thus, exosome-based intercellular communications involve contact-dependent (juxtacrine) signaling via surface molecules as well as their fusion and delivery of internal contents.
Exosomal RNA-mediated intercellular communication
Apart from signaling proteins secreted within exosomes and on their membranes, the finding that exosomes package coding and non-coding RNA molecules that are also transferred and functional in the recipient cells has enormous implications, and has fueled a new appreciation of the regulatory influence of nucleic acid transport beyond cellular boundaries [12, 45]. There are several example of exosomal miRNA-dependent gene regulation in target cells especially in cancers [12, 46, 47]. In breast cancer, exosomal miRs secreted by tumor cells target the endothelial tight-junction protein zonula occludens-1 or VE-cadherin, compromising the integrity of the endothelial layer to facilitate infiltration of cancer cells into other organs [48, 49]. Another study on breast cancer suggests that resistance to chemotherapy and radiotherapy involves transfer of stromal-cell-derived exosomal RNA and juxtacrine Notch3 signaling in tumor cells [50]. The role of exosomal RNA and proteins in malignant transformation of normal cells by manipulating their target gene expression has also been reported [20, 51]. Cancer cell metastasis is another complex cellular process where transformed cells leave the primary tumor site and home into other tissues. In this process, exosome-mediated miRNA transfer from the tissue microenvironment has been implicated in the adaptation and proliferation of cancer cells [22]. For example, transfer of exosomal mir-19a by astrocytes to tumor cells has been demonstrated to down-regulate the tumor suppressor, PTEN, in cancer cells during brain metastasis to facilitate homing. In another example of tumor–microenvironment interaction, it has been demonstrated that cancer cells secrete extracellular vesicles containing miRNAs to manipulate gene expression in dermal fibroblasts, converting them into specialized cancer-associated fibroblasts (CAF) and thereby facilitating tumor growth and progression [52]. In the case of acute myeloid leukemia, tumor-cell-derived exosomal miRNA signaling and regulation of residual hematopoietic stem cells and progenitor cell function by targeting proto-oncogene, c-MYB, levels strengthens the view that exosome-mediated miRNA transfer has pathological functions [53].
Useful applications of exosome signaling include expansion of hematopoietic precursor cells (HPCs) in vitro by co-culture with embryonic stem cells (ESCs), where ESC-derived vesicles enriched for Wnt-3 protein and mRNA for several pluripotent transcription factors promote efficient expansion of HPCs [45]. Thus, the available evidence implicates exosomal RNA and proteins in tissue patterning, homeostasis as well as in disease progression. While RNA packaging and transfer in the recipient cells have been demonstrated, regulation of gene expression by exosomal RNA in recipient cells is still surrounded by controversies, as the approaches used so far are not able to rule out cell-autonomous regulatory events [54]. However, this aspect is an active area of research, given its implications in development and disease [54]. Extending the findings that exosomes can ferry active proteins and RNA, it is worth exploring whether exosomes/extracellular vesicles are used by cells as platforms for the assembly of particular proteins and RNA molecules in a targeted manner that assigns specific fates to the signal-receiving cells. Conceivably, exosomes might be used by cells to send molecular combinations to activate and/or inhibit specific signaling events. Some questions to explore in this hypothesis would be: how might the assembly of molecules on exosomes be regulated? Can surface proteins of exosomes target them to specific cell types? Can some molecules on or in exosomes help in customizing the exosomal contents? Does exosomal heterogeneity play a role in differential targeting?
While several studies have correlated exosomal RNA delivery and gene regulation in target cells [6, 12, 45], it is far from clear as to how different types of cellular RNAs are sorted into exosomes. A recent study has estimated that there might be less than one miRNA per exosome [55]. While this estimate may not represent the realistic numbers due to technical limitations, it suggests that all exosomes might not carry miRNAs, reflecting the inherently heterogeneous nature of these vesicles. Quantitative estimation of single exosomal contents is a necessary detail that remains to be achieved. When performed on a pool of exosomes, current methods fail to represent the heterogeneity in the exosomal contents [55]. If executed on distinct exosomal populations or at single-exosome level, such analysis could provide better resolution of the inherent heterogeneity in the exosomal pool. The ability to resolve single exosomal contents and designing single-exosome assays will go a long way in unraveling exosomal functions, with major implications for basic and therapeutic applications.
Is heterogeneity in exosomal contents required for their targeted and synergistic signaling?
Exosomes are known to exhibit a variable size that ranges from 30 to 100 nm. There have been suggestions that cells can secrete different types of exosomes with varying protein and RNA contents [7, 56]. Recently, there has been an attempt to study single exosomes to identify heterogeneity using Laser Tweezers Raman Spectroscopy (LTRS) [57]. This study was able to group exosomes produced by a given cell type into four major groups based on their membrane cholesterol and phospholipid content. Though such approaches are essential to resolve the heterogeneity of exosomes [57], other methods will also be needed to quantify the contents of single exosomes.
As outlined above, the available evidence supports the view that exosomes not only activate juxtacrine signaling, but also deliver cytosolic and regulatory molecules such as mRNAs and miRNAs into recipient cells. We speculate that molecules on exosomal surfaces and within exosomes work together to confer the final signaling/regulatory outcome to recipient cells (Fig. 1a). In our study, we discovered that exosomes generated from chick notochord cells not only contain Sonic hedgehog (Shh) but also contain miRNAs [7]. Based on multiple pathway prediction algorithms, the potential target mRNAs in recipient cells are not known targets of Shh signaling. Instead, these exosomal miRNAs from chick notochord cells target other transcripts governing signaling pathways such as Wnt, FGF, retinoic acid [7]. The emerging picture suggests that while Shh creates its own signaling domain by juxtacrine signaling, it also deploys miRNAs that inhibit the effects of other signaling pathways from neighboring tissues. This type of mechanism might be required to insulate the Shh-responsive domain from influences by other signaling molecules in the region. However, experimental validations of this scenario are still lacking. Genetic perturbations that alter miRNA biogenesis in exosome-producing cells or alter incorporation of specific miRNAs into exosomes, combined with analysis of subsequent paracrine effects on target cells are required to resolve this hypothesis.
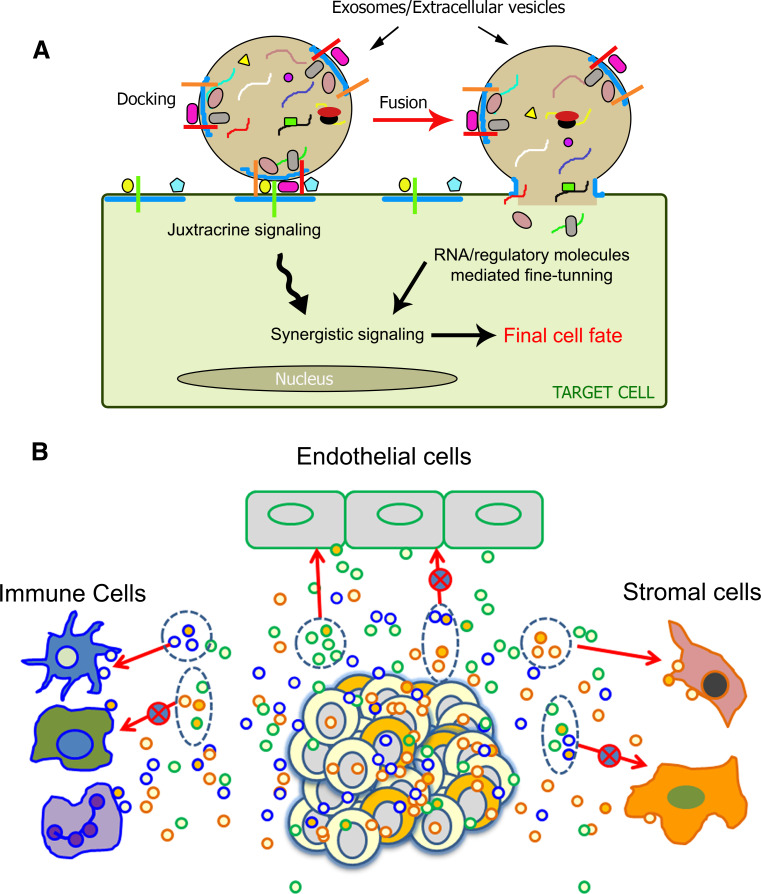
Fig. 1.
Schematic representations of targeted and synergistic exosomal signaling. a Model representing synergistic signaling via molecules on and in exosomes for final fate determination of target cell: docking of exosomes via extracellular and surface proteins (represented by different shapes on the membrane of exosomes and target cells) could result in activation of one or more juxtacrine signals, leading to their fusion with the target cell. Upon fusion, molecules within the exosomes, i.e., cytoplasmic proteins and metabolites (represented by different colored shapes in the lumen of exosomes), RNA (represented by different colored strands inside the exosomes) are delivered in the target cell for synergistic signal activation and regulating the target cells at multiple levels for final cell fate determination. Microdomains on the exosomes and cell membrane (represented by blue lines) might be involved in the assemblage of molecules in and on the exosomes for efficient, synergistic signaling. b Hypothetical model representing exosomal heterogeneity and targeted delivery of molecules. Tumor cells, depicted by a cluster of cells in white and yellow color, might secrete different types of exosomes or extracellular vesicles to target specific combination of molecules to specific target cells. Heterogeneity in the composition of exosomes might be involved in their targeted delivery. Exosomal ECM and cell surface molecules could play a major role in docking and fusion of exosomes with target cells containing the appropriate binding partners. Binding partners depicted by color coded outlines and red arrow; red arrows with cross represent incompatible interactions
Targeting exosomes via their surface proteins
As might be expected, the list of proteins packaged into exosomes is large (http://www.exocarta.org/). In vivo, exosomes can be made by several cell types, and recent efforts to direct these vesicles to specific cells of interest have significant implications for targeted therapy [23]. However, the evidence that exosomes can be targeted to specific cell types is sparse. Valadi et al. have shown that exosomes derived from mast cells could fuse to other mast cells but not with CD4 cells [12]. Neuronal stem cell-derived exosomes ferry both interferon-γ and its receptor, and require the presence of interferon-γ receptor in recipient cells to activate Stat-1 signaling [58]. In our studies, we find that vertebrate Hh (i.e., Shh) is secreted on two types of exosomes, and the ability of Shh to induce genes in target cells depends on the composition of the vesicles on which it is secreted [7]. Organotropic metastasis has been reported in several cancers [59], suggesting specific homing mechanisms. However, a precise mechanism for such a process is yet to be resolved. In a recent study, it has been demonstrated that organotropic metastasis involves interaction of cancer exosomes with specific cell types based on their surface-specific integrin molecules [60]. Once again, this study implicates membrane proteins in exosomal targeting. Taken together, these findings may suggest that molecular components displayed by exovesicles and target cells may influence effective docking of extracellular vesicles to activate juxtacrine signaling in the recipient cells that subsequently leads to their fusion with the target cells (Fig. 1a).
A key question that arises is whether cells engineer distinct classes of exosomes to communicate with different cell types. Recent studies suggest that cancer cell-derived exosomes evade immune cells [61], promote neo-vascularization, metastasis [48, 60] and are involved in crosstalk with surrounding stroma [62]. In such a scenario, preferential docking could ensure that tumor-derived exosomes targeted for endothelial cells to promote metastasis do not interact with immune cells or other stromal cells and vice versa (Fig. 1b). However, the major limitation in proving cell-specific exosomal effects/interactions is that it is difficult to identify the source of any specific class of exosomes in a complex mixture such as serum or other extracellular fluids. Based on the limited information on exosome targeting, several research groups have attempted to direct the targeting of engineered exosomes to specific cells using specific surface proteins. Mouse bone marrow dendritic cells were engineered to produce exosomes that can be targeted to neurons and deliver packaged, labeled siRNAs, as a read-out for exosomal targeting [25]. Systemic administration of exosomes with an EGF-mimetic peptide on their surface could target the delivery of miRNAs to EGF receptor-expressing cancer xenografts in mice [26]. However, apart from fusion with the plasma membrane, extracellular vesicles are also known to be endocytosed by the receiving cells and trafficked to internal organelles such as endoplasmic reticulum and lysosomes [63]. A recent study suggests that CAFs secret exosomes containing intact metabolites which could be utilized by cancer cells for their growth advantage [42]. Thus, exosomes that do not seem to engage in the expected manner with cells may play other roles. Resolving such signaling mechanisms will require the ability to reliably detect the heterogeneity in exosomal pools, single-exosome studies and methods to reliably detect individual signaling events from individual classes of exosomes. Second, exosomes could work at different levels of sensitivity with different fate and functions depending on the target cell. Hence, while these studies indicate that exosomes might be targeted via their surface molecules to fuse with specific cell types, current approaches do not allow us to reliably detect and distinguish these possibilities. More sophisticated assays are needed to distinguish juxtacrine signaling, endocytosis and fusion of exosomes with recipient cells. Here, single exosome-based studies using multiple markers and super-resolution microscopy might be able to resolve these abilities of exosomes. While immuno-electron microscopy can resolve structures in the 10–20 nm range, this technique compromises membrane quality due to harsh fixation effects, and destroys the functional properties of exosomes. Apart from LTRS, super-resolution fluorescence microcopy techniques such as photoactivated localization microscopy (PALM; [64]) and stochastic optical reconstruction microscopy (STORM; [65]) capable of efficiently resolving structures <100 nm may revolutionize our ability to resolve different subsets of exosomes that are 40–100 nm in size. These super-resolution microscopy techniques overcome the diffraction barrier using photo-switchable fluorescent dyes. By photo-activating and bleaching the probes, molecules that are located within the diffraction limit can also be resolved. The single molecules, thus, imaged by repeated cycles of fluorophore excitation and beaching are used to derive a final super-resolution image. Combined with live cell imaging [66], these methods hold great promise in defining exosome trafficking and function. Overall, while targeted, synergistic signaling via exosomes is an exciting possibility; the complex nature of exosomes requires further exploration to delineate the mechanisms that control their distribution and deployment.
Assemblage of molecules in and on exosomes for synergistic signaling
A key question raised by the observed heterogeneity of exosome populations and the possibility of their targeted and synergistic signaling concerns whether molecules on exosome surfaces can be organized in a guided or template-dependent manner. Two possibilities will be considered: first, the organization and sorting of molecules on exosomal membrane, and second, the ability of some exosomal proteins to be actively involved in regulating the content of these vesicles.
Organization of molecules on exosomal membrane
The plasma membrane of cells contains cholesterol- and sphingolipid-rich microdomains [67–69]. These membrane domains are known to be involved in organizing molecules at the nanometer scale [69, 70]. Using detergent resistance assays and fluorescence resonance energy transfer (FRET) techniques, it has been demonstrated that tetraspanins, cell adhesion molecules and signaling molecules are involved in generating microdomains and oligomers at plasma membrane [71]. Ability of tetraspanins to associate with nucleic acids, histones, ribosomal proteins, signaling protein and cytoskeletal proteins has also been investigated using whole-cell extracts [72]. Tetraspanins are a major component of exosomes, and hence, it has been speculated that they might be involved in generating cell-specific microdomains on exosomes as they do on the plasma membrane [73]. Further, lipid modification(s) are also implicated in oligomerization and exosomal incorporation of proteins [74, 75]. Since exosomes are endocytically derived, they too sample the plasma membrane and might contain equivalent lipid microdomains. The occurrence of detergent-resistant regions (a hallmark of plasma membrane microdomains) on exosomes has been reported [76]. Whether these microdomains are reassembled on the internal limiting membrane of the precursors of exosomes—multivesicular bodies (MVB)—or whether they are plasma membrane-derived is not known. Nevertheless, lipid microdomains that are found on exosomes could serve as platforms to bring together different proteins in an organized manner, as they do on the cell surface. Organization and packaging of signaling molecules and their partners on exosomes as well as on the plasma membrane of signal-receiving cells might be necessary not only to facilitate proper docking, but also to ensure that signaling molecules are present at physiologically effective concentrations.
In support of this hypothesis, it has been reported that cell surface nanoscale organization of Hh proteins is critical for their precise signaling function [70]. This nanoscale organization of Hh molecules is essential for their long-range signaling ability but not for their autocrine signaling function [70]. Importantly, Hh molecules that are organized at nanometer scale are also secreted on exosomes for long-range signaling [29, 31]. A mutant of Hh, HhK132D that is deficient in nanoscale organization also exhibits defective long-range signaling ability [70], and cannot be efficiently released on classical exosomes [29]. It is conceivable that K132-dependent nanoscale organization of Hh proteins ensures that the correct binding partners are sorted together for efficient signaling. However, resolving the proposed nanoscale organization of molecules and domains on exosomes will require application of new tools. Live super-resolution microscopy equipped with anisotropy measurements and mathematical modeling might be able to resolve the possible domains on limiting membrane of exosomes. Such membrane microdomains could potentially act as platforms for sorting and organizing proteins on exosome-limiting membrane, leading to synergistic signaling and cellular fate determination in the signal-receiving cells.
Can the composition of exosomes be customized?
Since the landmark report of miRNA-packaging in exosomes [12], there have been suggestions that the process might be regulated. The original study found enrichment of specific mRNA and miRNA in exosomes derived from mast cells: 270 transcripts identified in mast cell-derived exosomes were not detectable in the cells, and the most abundant transcripts in the donor cells and their exosomes did not overlap [12]. In cancer cells, miRNA incorporation into exosomes appears to far exceed the amount of miRNA in exosomes derived from normal cells, and could not be explained simply by increased cellular mRNA/miRNA levels [6, 51]. Depletion of endosomal sorting complex required for transport (ESCRT) proteins does not seem to affect miRNA secretion; rather, it was found to be sensitive to changes in cellular sphingomyelin levels [77]. Recently, it has also been demonstrated that a particular class of RNA-binding proteins such as heterogeneous nuclear ribonucleoprotein (hnRNPs), can control the subcellular localization and loading of miRNA into exosomes by binding to exosome-recognition motifs identified at the 3′-end of miRNAs [78, 79]. Further, post-transcriptional modification of the 3′-end of miRNAs (uridinylation vs. adenylation) is correlated for sorting miRNAs into exosomes [80]. In breast cancer cells, enrichment of Dicer (and hence perhaps miRNAs) in exosomes is regulated by tumor-cell-specific trans-membrane protein CD43 [51]. Surface glycoproteins such as CD44, involved in cell adhesion and cell migration, as well as CD59 are also thought to be involved in incorporation of specific miRNAs in exosomes [81]. In Drosophila S2 cells as well, ectopic expression of Wg results in the incorporation of several unique proteins including Argonaute, a key player in miRNA biogenesis and several other RNA-binding proteins into exosomes [82]. It is, therefore, conceivable that specific proteins such as Wg or CD43 tailor the content of exosomes. In a recent study, it has been confirmed that exosomes from pancreatic cancer cells (tumor-derived cell lines as well as human patient samples) are highly enriched for glypican-1 (GPC-1), unlike those from healthy patients or non-tumorigenic cells [8]. These GPC-1-containing cancer exosomes also selectively incorporated oncogenic mutant K-Ras mRNA, i.e., K-RasG12D [8]. Conversely, oncogenic K-Ras protein plays a role in regulating the content of exosomal-miRNA in colorectal cancer [83]. Thus, there is growing evidence to suggest that RNA packaging in exosomes does not result merely from a passive invagination of cytoplasmic contents. However, the identity and source of signals that might control RNA-binding proteins or packaging of different classes of RNA into exosomes are still obscure. Further, the mechanisms that may control deployment of RNA for regulation/translation upon delivery to target cells are yet to be identified.
Perspective
Exosome-mediated juxtacrine signaling and delivery of regulatory molecules via their fusion with the target cells could be a central mechanism in intercellular communication (Fig. 1a). In this review, we propose that the observed heterogeneity in exosomes might represent a mechanism that tailors exosome targeting to specific cell types for specific outcome. Lipid microdomains on exosomes might be involved in regulating this process. Exosomes could, thus, act as platforms to assemble different molecules with specific binding patterns on their surface as well as within their lumens. During developmental patterning as well as in disease, a specific cell fate is achieved by activating and inhibiting specific signaling events. Exosome-mediated intercellular signaling might play a major role in this process by bringing together combinations of surface proteins that target these long-range signal transporters, and deliver signaling molecules and miRNAs to inhibit alternative fates. The specific assemblage of molecules in and on exosomes might together enable steering of target cell programs at multiple levels, resulting in regulated cell fates during embryonic tissue patterning or disease-associated remodeling and homeostasis. The presence of secreted molecules such as TGF-β, interferons and other growth factors on exosomes might suggest that the freely secreted pool of these proteins plays a distinct role, while the fraction of these signaling molecules that is delivered in a precise exosomal milieu may facilitate other roles in a specific context. The ability to resolve single exosomes, reliably mark them and consistently score the distribution of the exosomal cargo would go a long way in resolving the physiological significance of exosomal heterogeneity and the details of their targeted delivery. Though exosomes vary significantly in their contents, some exosomal proteins are relatively constant and serve as bonafide markers of these extracellular vesicles. Whether these exosomal marker proteins and/or lipids play a structural role in presenting signaling molecules in a more perceivable form is not known. While the targeting capabilities of exosome-associated morphogens, secreted signaling molecules, ECM proteins or trans-membrane proteins are apparent, whether they also regulate exosomal contents is not clear, and remains an exciting possibility. If secreted signaling molecules are also involved in “customizing” the composition of exosomes, a new level of signaling complexities will emerge.
Acknowledgements
We thank Dr. Arjun Guha for his critical inputs on the manuscript. This work was supported by an Early Career Fellowship from the Wellcome Trust/DBT India Alliance to NV, support to NV from DST-ECR award grant (Grant No. ECR/2016/000251) and core support from St. John’s Research Institute. JD is supported by an Indo-Danish Collaborative grant and core funds to InStem (Govt. of India Dept. of Biotechnology) and CSIR-CCMB. Authors have no competing interests.
Appendix
Box 1.
Brief description of exosome biogenesis. Exosomes are endocytically derived vesicles initially thought to be secreted only by immune cells. However, subsequent studies suggest that these vesicles are secreted by all cell types tested, including fibroblasts, epithelial cells, endothelial cells, neurons, glia, stem cells, and tumor cells. The ESCRT machinery is involved in biogenesis of exosomes, and comprises four major groups of proteins ESCRT 0, ESCRT I, ESCRT II, and ESCRT III. ESCRT 0 group includes Hrs (hepatocyte growth factor-regulated tyrosine kinase substrate) and STAM (signal transducing adaptor molecule). These proteins are predominantly found on endosomal membranes via FYVE domain-mediated interaction with the PI3P lipids on the membrane [84]. Hrs and STAM form hetero-tetramers in membranes and interact with ubiquitinated proteins in cells [85]. ESCRT I group is a hetero-tetramer of Tsg101/Vps23, Vps28, Vps37 and Mvb12: this complex helps in bridging ESCRT 0 and ESCRT II complexes during MVB biogenesis and is involved in vesicle budding along with ESCRT II members. ESCRT II is also a hetero-tetramer consisting of two molecules of Vps25 and one molecule of Vps22 and Vps36 each. ESCRT III is a complex polymer and is highly dynamic in its composition. Members of ESCRT III group include core subunits consisting of Vps20, SNF7, Vps24 and Vps2. Three parallel subunits of ESCRT III include Did2, Vps60 and Ist1. ESCRT III complex is required for scission of the vesicle buds [86]. Finally, AAA + ATPaseVps4 terminates the process by disassembling ESCRT III complex and recycling the subunits for next cycle of vesicle budding and scission. Inward budding MVB limiting membrane and subsequent scission results in the formation of intra-luminal vesicles, approximately 30–100 nm in diameter; these vesicles are released in the extracelluar milieu by the fusion of the MVBs with plasma membrane and are termed as exosomes. Exosomes are also known to be generated by ESCRT-independent mechanisms [10, 87].
Box 2.
A survey of reports suggesting that exosomes might be targeted to specific cell types for synergistic signaling
| Category | Description | References |
|---|---|---|
| Exosomes can be targeted to specific cell types via their surface protein for exosomal RNA-mediated gene regulation in recipient cells | Mast cells derived exosomes containing functional cellular RNA fuse with other mast cells but not with CD4 cells | [12] |
| Exosomes can be engineered to ferry specific surface proteins to facilitate cell-specific docking and fusion | [25], [26] | |
| Exosomes secreted by primary tumor cells use surface Integrins to interact with cells of specific organs and promote organtropic metastasis | [60] | |
| Specifc stromal cells deliver exosomes to specific breast cancer cells (i.e. IRDS-R cells) for developing resistance to chemotherapy and radiotherapy | [50] | |
| Grafted neural stem cells may interacts with host cells via exosomes containing IFN-gamma/Ifrngr1 complexes | [58] | |
| Some exosomal proteins might be involved in customizing their composition. | Some transmembrane and signaling proteins when incorporated in exosomes can modify their contents | [51, 81, 82] |
| Glypican-1-containing cancer exosomes selectively incorporated oncogenic mutant K-Ras mRNA | [8] | |
| Oncogenic K-Ras protein regulates incorporation of exosomal miRNAs in colorectal cancer cells | [83] | |
| Certain RNA binding proteins, hnRNPs, can selectively incorporate miRNAs into exosomes | [78], [79] |
References
- 1.Crescitelli R et al (2013) Distinct RNA profiles in subpopulations of extracellular vesicles: apoptotic bodies, microvesicles and exosomes. J Extracell Vesicles 2. doi:10.3402/jev.v2i0.20677 [DOI] [PMC free article] [PubMed]
- 2.Kowal J, et al. Proteomic comparison defines novel markers to characterize heterogeneous populations of extracellular vesicle subtypes. Proc Natl Acad Sci USA. 2016;113(8):E968–E977. doi: 10.1073/pnas.1521230113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Colombo M, Raposo G, Thery C. Biogenesis, secretion, and intercellular interactions of exosomes and other extracellular vesicles. Annu Rev Cell Dev Biol. 2014;30:255–289. doi: 10.1146/annurev-cellbio-101512-122326. [DOI] [PubMed] [Google Scholar]
- 4.Kowal J, Tkach M, Thery C. Biogenesis and secretion of exosomes. Curr Opin Cell Biol. 2014;29:116–125. doi: 10.1016/j.ceb.2014.05.004. [DOI] [PubMed] [Google Scholar]
- 5.Colombo M, et al. Analysis of ESCRT functions in exosome biogenesis, composition and secretion highlights the heterogeneity of extracellular vesicles. J Cell Sci. 2013;126(Pt 24):5553–5565. doi: 10.1242/jcs.128868. [DOI] [PubMed] [Google Scholar]
- 6.Skog J, et al. Glioblastoma microvesicles transport RNA and proteins that promote tumour growth and provide diagnostic biomarkers. Nat Cell Biol. 2008;10(12):1470–1476. doi: 10.1038/ncb1800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vyas N, et al. Vertebrate Hedgehog is secreted on two types of extracellular vesicles with different signaling properties. Sci Rep. 2014;4:7357. doi: 10.1038/srep07357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Melo SA, et al. Glypican-1 identifies cancer exosomes and detects early pancreatic cancer. Nature. 2015;523(7559):177–182. doi: 10.1038/nature14581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Thery C (2011) Exosomes: secreted vesicles and intercellular communications. F1000 Biol Rep 3:15 [DOI] [PMC free article] [PubMed]
- 10.Raposo G, Stoorvogel W. Extracellular vesicles: exosomes, microvesicles, and friends. J Cell Biol. 2013;200(4):373–383. doi: 10.1083/jcb.201211138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Stoorvogel W, et al. The biogenesis and functions of exosomes. Traffic. 2002;3(5):321–330. doi: 10.1034/j.1600-0854.2002.30502.x. [DOI] [PubMed] [Google Scholar]
- 12.Valadi H, et al. Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nat Cell Biol. 2007;9(6):654–659. doi: 10.1038/ncb1596. [DOI] [PubMed] [Google Scholar]
- 13.Chen X, et al. Secreted microRNAs: a new form of intercellular communication. Trends Cell Biol. 2012;22(3):125–132. doi: 10.1016/j.tcb.2011.12.001. [DOI] [PubMed] [Google Scholar]
- 14.Lo Cicero A, Stahl PD, Raposo G. Extracellular vesicles shuffling intercellular messages: for good or for bad. Curr Opin Cell Biol. 2015;35:69–77. doi: 10.1016/j.ceb.2015.04.013. [DOI] [PubMed] [Google Scholar]
- 15.Johnstone RM, Bianchini A, Teng K. Reticulocyte maturation and exosome release: transferrin receptor containing exosomes shows multiple plasma membrane functions. Blood. 1989;74(5):1844–1851. [PubMed] [Google Scholar]
- 16.Raposo G, et al. B lymphocytes secrete antigen-presenting vesicles. J Exp Med. 1996;183(3):1161–1172. doi: 10.1084/jem.183.3.1161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Denzer K, et al. Follicular dendritic cells carry MHC class II-expressing microvesicles at their surface. J Immunol. 2000;165(3):1259–1265. doi: 10.4049/jimmunol.165.3.1259. [DOI] [PubMed] [Google Scholar]
- 18.Fevrier B, et al. Cells release prions in association with exosomes. Proc Natl Acad Sci USA. 2004;101(26):9683–9688. doi: 10.1073/pnas.0308413101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schorey JS, et al. Exosomes and other extracellular vesicles in host–pathogen interactions. EMBO Rep. 2015;16(1):24–43. doi: 10.15252/embr.201439363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kahlert C, Kalluri R. Exosomes in tumor microenvironment influence cancer progression and metastasis. J Mol Med (Berl) 2013;91(4):431–437. doi: 10.1007/s00109-013-1020-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hoshino D, et al. Exosome secretion is enhanced by invadopodia and drives invasive behavior. Cell Rep. 2013;5(5):1159–1168. doi: 10.1016/j.celrep.2013.10.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhang L, et al. Microenvironment-induced PTEN loss by exosomal microRNA primes brain metastasis outgrowth. Nature. 2015;527(7576):100–104. doi: 10.1038/nature15376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tan A, Rajadas J, Seifalian AM. Exosomes as nano-theranostic delivery platforms for gene therapy. Adv Drug Deliv Rev. 2013;65(3):357–367. doi: 10.1016/j.addr.2012.06.014. [DOI] [PubMed] [Google Scholar]
- 24.Simpson RJ, et al. Exosomes: proteomic insights and diagnostic potential. Expert Rev Proteomics. 2009;6(3):267–283. doi: 10.1586/epr.09.17. [DOI] [PubMed] [Google Scholar]
- 25.Alvarez-Erviti L, et al. Delivery of siRNA to the mouse brain by systemic injection of targeted exosomes. Nat Biotechnol. 2011;29(4):341–1179. doi: 10.1038/nbt.1807. [DOI] [PubMed] [Google Scholar]
- 26.Ohno S, et al. Systemically injected exosomes targeted to EGFR deliver antitumor microRNA to breast cancer cells. Mol Therapy. 2013;21(1):185–191. doi: 10.1038/mt.2012.180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Liegeois S, et al. The V0-ATPase mediates apical secretion of exosomes containing Hedgehog-related proteins in Caenorhabditis elegans . J Cell Biol. 2006;173(6):949–961. doi: 10.1083/jcb.200511072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gross JC, et al. Active Wnt proteins are secreted on exosomes. Nat Cell Biol. 2012;14(10):1036–1045. doi: 10.1038/ncb2574. [DOI] [PubMed] [Google Scholar]
- 29.Parchure A, et al. Oligomerization and endocytosis of Hedgehog is necessary for its efficient exovesicular secretion. Mol Biol Cell. 2015;26(25):4700–4717. doi: 10.1091/mbc.E15-09-0671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gradilla A-C, et al. Exosomes as Hedgehog carriers in cytoneme-mediated transport and secretion. Nat Commun. 2014;5:5649. doi: 10.1038/ncomms6649. [DOI] [PubMed] [Google Scholar]
- 31.Matusek T, et al. The ESCRT machinery regulates the secretion and long-range activity of Hedgehog. Nature. 2014;516(7529):99–103. doi: 10.1038/nature13847. [DOI] [PubMed] [Google Scholar]
- 32.Webber J, et al. Cancer exosomes trigger fibroblast to myofibroblast differentiation. Cancer Res. 2010;70(23):9621–9630. doi: 10.1158/0008-5472.CAN-10-1722. [DOI] [PubMed] [Google Scholar]
- 33.Schiera G, et al. Neurons produce FGF2 and VEGF and secrete them at least in part by shedding extracellular vesicles. J Cell Mol Med. 2007;11(6):1384–1394. doi: 10.1111/j.1582-4934.2007.00100.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Proia P, et al. Astrocytes shed extracellular vesicles that contain fibroblast growth factor-2 and vascular endothelial growth factor. Int J Mol Med. 2008;21(1):63–67. [PubMed] [Google Scholar]
- 35.Faure J, et al. Exosomes are released by cultured cortical neurones. Mol Cell Neurosci. 2006;31(4):642–648. doi: 10.1016/j.mcn.2005.12.003. [DOI] [PubMed] [Google Scholar]
- 36.Wang S, et al. Synapsin I is an oligomannose-carrying glycoprotein, acts as an oligomannose-binding lectin, and promotes neurite outgrowth and neuronal survival when released via glia-derived exosomes. J Neurosci. 2011;31(20):7275–7290. doi: 10.1523/JNEUROSCI.6476-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lachenal G, et al. Release of exosomes from differentiated neurons and its regulation by synaptic glutamatergic activity. Mol Cell Neurosci. 2011;46(2):409–418. doi: 10.1016/j.mcn.2010.11.004. [DOI] [PubMed] [Google Scholar]
- 38.Fruhbeis C, Frohlich D, Kramer-Albers EM. Emerging roles of exosomes in neuron-glia communication. Front Physiol. 2012;3:119. doi: 10.3389/fphys.2012.00119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Korkut C, et al. Trans-synaptic transmission of vesicular Wnt signals through Evi/Wntless. Cell. 2009;139(2):393–404. doi: 10.1016/j.cell.2009.07.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Koles K, et al. Mechanism of evenness interrupted (Evi)-exosome release at synaptic boutons. J Biol Chem. 2012;287(20):16820–16834. doi: 10.1074/jbc.M112.342667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sheldon H, et al. New mechanism for notch signaling to endothelium at a distance by delta-like 4 incorporation into exosomes. Blood. 2010;116(13):2385–2394. doi: 10.1182/blood-2009-08-239228. [DOI] [PubMed] [Google Scholar]
- 42.Zhao H, et al. Tumor microenvironment derived exosomes pleiotropically modulate cancer cell metabolism. Elife. 2016;5:e10250. doi: 10.7554/eLife.10250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Aga M, et al. Exosomal HIF1[alpha] supports invasive potential of nasopharyngeal carcinoma-associated LMP1-positive exosomes. Oncogene. 2014;33(37):4613–4622. doi: 10.1038/onc.2014.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Xin H, et al. Exosome-mediated transfer of miR-133b from multipotent mesenchymal stromal cells to neural cells contributes to neurite outgrowth. Stem Cells. 2012;30(7):1556–1564. doi: 10.1002/stem.1129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ratajczak J, et al. Embryonic stem cell-derived microvesicles reprogram hematopoietic progenitors: evidence for horizontal transfer of mRNA and protein delivery. Leukemia. 2006;20(5):847–856. doi: 10.1038/sj.leu.2404132. [DOI] [PubMed] [Google Scholar]
- 46.Umezu T, et al. Leukemia cell to endothelial cell communication via exosomal miRNAs. Oncogene. 2013;32(22):2747–2755. doi: 10.1038/onc.2012.295. [DOI] [PubMed] [Google Scholar]
- 47.van Balkom BW et al (2013) Endothelial cells require miR-214 to secrete exosomes that suppress senescence and induce angiogenesis in human and mouse endothelial cells. Blood 121(19):3997–4006 (S1–15) [DOI] [PubMed]
- 48.Zhou W, et al. Cancer-secreted miR-105 destroys vascular endothelial barriers to promote metastasis. Cancer Cell. 2014;25(4):501–515. doi: 10.1016/j.ccr.2014.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Di Modica M, et al. Breast cancer-secreted miR-939 downregulates VE-cadherin and destroys the barrier function of endothelial monolayers. Cancer Lett. 2016;384:94–100. doi: 10.1016/j.canlet.2016.09.013. [DOI] [PubMed] [Google Scholar]
- 50.Boelens MC, et al. Exosome transfer from stromal to breast cancer cells regulates therapy resistance pathways. Cell. 2014;159(3):499–513. doi: 10.1016/j.cell.2014.09.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Melo SA, et al. Cancer exosomes perform cell-independent microRNA biogenesis and promote tumorigenesis. Cancer Cell. 2014;26(5):707–721. doi: 10.1016/j.ccell.2014.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Dror S, et al. Melanoma miRNA trafficking controls tumour primary niche formation. Nature Cell Biology. 2016;18(9):1006–1017. doi: 10.1038/ncb3399. [DOI] [PubMed] [Google Scholar]
- 53.Hornick NI et al (2016) AML suppresses hematopoiesis by releasing exosomes that contain microRNAs targeting c-MYB. Sci Signal 9(444):ra88 [DOI] [PubMed]
- 54.Tkach M, Théry C Communication by extracellular vesicles: where we are and where we need to go. Cell 164(6):1226–1232 [DOI] [PubMed]
- 55.Chevillet JR, et al. Quantitative and stoichiometric analysis of the microRNA content of exosomes. Proc Natl Acad Sci USA. 2014;111(41):14888–14893. doi: 10.1073/pnas.1408301111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Willms E, et al. Cells release subpopulations of exosomes with distinct molecular and biological properties. Sci Rep. 2016;6:22519. doi: 10.1038/srep22519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Smith ZJ et al (2015) Single exosome study reveals subpopulations distributed among cell lines with variability related to membrane content. J Extracell Vesicles 4. doi:10.3402/jev.v4.28533 [DOI] [PMC free article] [PubMed]
- 58.Cossetti C, et al. Extracellular vesicles from neural stem cells transfer IFN-gamma via Ifngr1 to activate Stat1 signaling in target cells. Mol Cell. 2014;56(2):193–204. doi: 10.1016/j.molcel.2014.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Nguyen DX, Bos PD, Massague J. Metastasis: from dissemination to organ-specific colonization. Nat Rev Cancer. 2009;9(4):274–284. doi: 10.1038/nrc2622. [DOI] [PubMed] [Google Scholar]
- 60.Hoshino A, et al. Tumour exosome integrins determine organotropic metastasis. Nature. 2015;527(7578):329–335. doi: 10.1038/nature15756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Liu C, et al. Murine mammary carcinoma exosomes promote tumor growth by suppression of NK cell function. J Immunol. 2006;176(3):1375–1385. doi: 10.4049/jimmunol.176.3.1375. [DOI] [PubMed] [Google Scholar]
- 62.Suetsugu A, et al. Imaging exosome transfer from breast cancer cells to stroma at metastatic sites in orthotopic nude-mouse models. Adv Drug Deliv Rev. 2013;65(3):383–390. doi: 10.1016/j.addr.2012.08.007. [DOI] [PubMed] [Google Scholar]
- 63.Heusermann W, et al. Exosomes surf on filopodia to enter cells at endocytic hot spots, traffic within endosomes, and are targeted to the ER. J Cell Biol. 2016;213(2):173–184. doi: 10.1083/jcb.201506084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Betzig E, et al. Imaging intracellular fluorescent proteins at nanometer resolution. Science. 2006;313(5793):1642–1645. doi: 10.1126/science.1127344. [DOI] [PubMed] [Google Scholar]
- 65.Rust MJ, Bates M, Zhuang X. Sub-diffraction-limit imaging by stochastic optical reconstruction microscopy (STORM) Nat Methods. 2006;3(10):793–795. doi: 10.1038/nmeth929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Hess ST, et al. Dynamic clustered distribution of hemagglutinin resolved at 40 nm in living cell membranes discriminates between raft theories. Proc Natl Acad Sci USA. 2007;104(44):17370–17375. doi: 10.1073/pnas.0708066104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Simons K, Sampaio JL. Membrane organization and lipid rafts. Cold Spring Harb Perspect Biol. 2011;3(10):a004697. doi: 10.1101/cshperspect.a004697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Coskun U, Simons K. Membrane rafting: from apical sorting to phase segregation. Febs Lett. 2010;584(9):1685–1693. doi: 10.1016/j.febslet.2009.12.043. [DOI] [PubMed] [Google Scholar]
- 69.Mayor S, Rao M. Rafts: scale-dependent, active lipid organization at the cell surface. Traffic. 2004;5(4):231–240. doi: 10.1111/j.1600-0854.2004.00172.x. [DOI] [PubMed] [Google Scholar]
- 70.Vyas N, et al. Nanoscale organization of hedgehog is essential for long-range signaling. Cell. 2008;133(7):1214–1227. doi: 10.1016/j.cell.2008.05.026. [DOI] [PubMed] [Google Scholar]
- 71.Barreiro O, et al. Endothelial adhesion receptors are recruited to adherent leukocytes by inclusion in preformed tetraspanin nanoplatforms. J Cell Biol. 2008;183(3):527–542. doi: 10.1083/jcb.200805076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Perez-Hernandez D, et al. The intracellular interactome of tetraspanin-enriched microdomains reveals their function as sorting machineries toward exosomes. J Biol Chem. 2013;288(17):11649–11661. doi: 10.1074/jbc.M112.445304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Yanez-Mo M, et al. Tetraspanin-enriched microdomains: a functional unit in cell plasma membranes. Trends Cell Biol. 2009;19(9):434–446. doi: 10.1016/j.tcb.2009.06.004. [DOI] [PubMed] [Google Scholar]
- 74.Shen B, et al. Protein targeting to exosomes/microvesicles by plasma membrane anchors. J Biol Chem. 2011;286(16):14383–14395. doi: 10.1074/jbc.M110.208660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Fang Y, et al. Higher-order oligomerization targets plasma membrane proteins and HIV gag to exosomes. Plos Biol. 2007;5(6):e158. doi: 10.1371/journal.pbio.0050158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.de Gassart A, et al. Lipid raft-associated protein sorting in exosomes. Blood. 2003;102(13):4336–4344. doi: 10.1182/blood-2003-03-0871. [DOI] [PubMed] [Google Scholar]
- 77.Kosaka N, et al. Secretory mechanisms and intercellular transfer of microRNAs in living cells. J Biol Chem. 2010;285(23):17442–17452. doi: 10.1074/jbc.M110.107821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Villarroya-Beltri C, et al. Sumoylated hnRNPA2B1 controls the sorting of miRNAs into exosomes through binding to specific motifs. Nat Commun. 2013;4:2980. doi: 10.1038/ncomms3980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Santangelo L, et al. The RNA-binding protein SYNCRIP is a component of the hepatocyte exosomal machinery controlling microRNA sorting. Cell Rep. 2016;17(3):799–808. doi: 10.1016/j.celrep.2016.09.031. [DOI] [PubMed] [Google Scholar]
- 80.Koppers-Lalic D, et al. Nontemplated nucleotide additions distinguish the small RNA composition in cells from exosomes. Cell Rep. 2014;8(6):1649–1658. doi: 10.1016/j.celrep.2014.08.027. [DOI] [PubMed] [Google Scholar]
- 81.Palma J, et al. MicroRNAs are exported from malignant cells in customized particles. Nucleic Acids Res. 2012;40(18):9125–9138. doi: 10.1093/nar/gks656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Beckett K, et al. Drosophila S2 cells secrete wingless on exosome-like vesicles but the wingless gradient forms independently of exosomes. Traffic. 2013;14(1):82–96. doi: 10.1111/tra.12016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Cha DJ, et al. KRAS-dependent sorting of miRNA to exosomes. Elife. 2015;4:e07197. doi: 10.7554/eLife.07197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Raiborg C, et al. FYVE and coiled-coil domains determine the specific localisation of Hrs to early endosomes. J Cell Sci. 2001;114(Pt 12):2255–2263. doi: 10.1242/jcs.114.12.2255. [DOI] [PubMed] [Google Scholar]
- 85.Mayers JR, et al. ESCRT-0 assembles as a heterotetrameric complex on membranes and binds multiple ubiquitinylated cargoes simultaneously. J Biol Chem. 2011;286(11):9636–9645. doi: 10.1074/jbc.M110.185363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Hurley JH, Hanson PI. Membrane budding and scission by the ESCRT machinery: it’s all in the neck. Nat Rev Mol Cell Biol. 2010;11(8):556–566. doi: 10.1038/nrm2937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Trajkovic K, et al. Ceramide triggers budding of exosome vesicles into multivesicular endosomes. Science. 2008;319(5867):1244–1247. doi: 10.1126/science.1153124. [DOI] [PubMed] [Google Scholar]