Abstract
O-linked β-N-acetylglucosaminylation (O-GlcNAcylation) is involved in the regulation of many cellular cascades and neurological diseases such as Alzheimer’s disease (AD), Parkinson’s disease (PD), and stroke. In the brain, the expression of O-GlcNAcylation is notably heightened, as is that of O-linked N-acetylglucosaminyltransferase (OGT) and β-N-acetylglucosaminidase (OGA), the presence of which is prominent in many regions of neurological importance. Most importantly, O-GlcNAcylation is believed to contribute to the normal functioning of neurons; conversely, its dysregulation participates in the pathogenesis of neurological disorders. In neurodegenerative diseases, O-GlcNAcylation of the brain’s key proteins, such as tau and amyloid-β, interacts with their phosphorylation, thereby triggering the formation of neurofibrillary tangles and amyloid plaques. An increase of O-GlcNAcylation by pharmacological intervention prevents neuronal loss. Additionally, O-GlcNAcylation is stress sensitive, and its elevation is cytoprotective. Increased O-GlcNAcylation ameliorated brain damage in victims of both trauma-hemorrhage and stroke. In this review, we summarize the current understanding of O-GlcNAcylation’s physiological and pathological roles in the nervous system and provide a foundation for development of a therapeutic strategy for neurological disorders.
Keywords: O-GlcNAcylation, Neurological disorders, Alzheimer’s disease, Parkinson’s disease, Amyotrophic lateral sclerosis, Stroke
Introduction
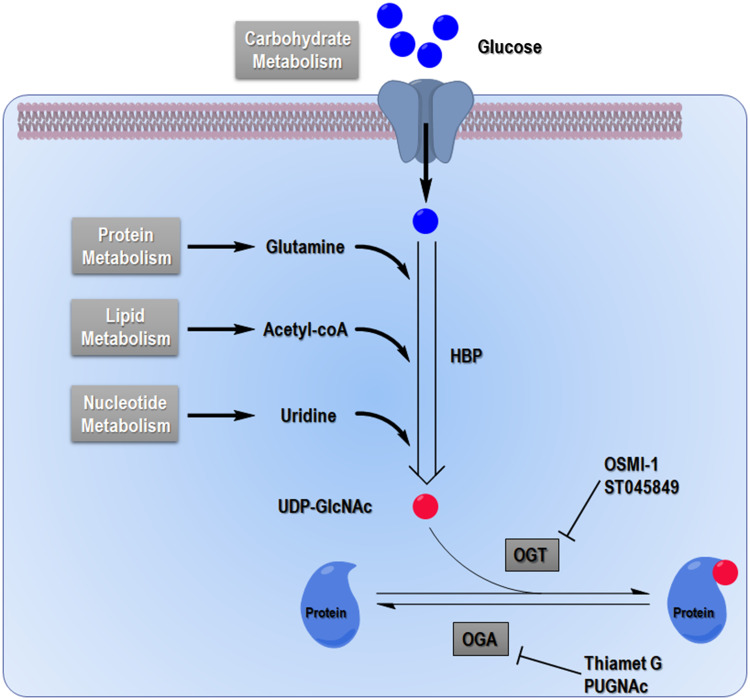
Glycosylation is the covalent attachment of glycans to proteins or lipids with an O- or N- (or occasionally a C- or S-) linkage, usually associated with complex polysaccharides with branches. The spectrum of glycosylation’s activities expanded when O-linked β-N-acetylglucosaminylation (O-GlcNAcylation) was first found in murine lymphocytes during the early 1980s [1, 2]. This event was recognized as a single monosaccharide’s attachment to serine and threonine of nucleocytoplasmic proteins without subsequent elongation [3]. After decades of study, more than one thousand proteins bearing O-GlcNAcylation were identified in species such as viruses, bacteria, filamentous fungi, insects, plants, and animals. Protein O-GlcNAcylation acts much like phosphorylation, which is maintained in a delicate and dynamic homeostatic state in vivo and mediates cellular signaling transduction by modulating protein function [4]. This scenario plays an essential role in the process of cell signaling, protein homeostatic maintenance, and gene expression [5–8]. The interaction between protein O-GlcNAcylation and phosphorylation was highly emphasized in an early report that proposed this bipartisan activity as “Yin-Yang theory” [9, 10]. Crosstalk between protein O-GlcNAcylation and other protein translational modifications, such as protein acetylation, ubiquitination, and sumoylation, was also found in recent years [11–13]. Moreover, protein O-GlcNAcylation is commonly referred to as a nutrient sensor for cells owing to its close connection with the hexosamine biosynthesis pathway (HBP) (Fig. 1). The end product of HBP is UDP-GlcNAc, which is used as a donor substrate for O-linked N-acetylglucosaminyltransferase (OGT) and regulates OGT’s enzymatic activity, in turn providing substrate selectivity, as well as regulating O-GlcNAclyation levels in cells [14, 15]. The cellular UDP-GlcNAc level is restricted not only by glucose flux but also, mainly, by amino acid flux. Lipid metabolism and nucleotide metabolism are also involved [16] (Fig. 1). As the essential role of O-GlcNAcylation in the regulatory networks of cellular biological process came to light, its importance grew as a regulator of cell signaling and as a bridge between extracellular stimuli and cellular stress responses.
Fig. 1.
Hexosamine biosynthetic pathway (HBP). Glucose flux enters mainly the glycolysis pathway and glycogen synthesis pathway, as well as using the pentose phosphate pathway. Approximately 5% of glucose flux is redirected into the HBP. Glucose is converted into UDP-GlcNAc by a series of enzymes including l-glutamine:fructose-6-phosphate amidotransferase (GFAT), glucosamine-6-phosphate acetyltransferase (Emeg32), phosphate-acetylglucosamine mutase (mutase) and UDP-GlcNAc pyrophosphorylase (pyrophosphorylase), which is a sugar donor to the O-GlcNAcylation reaction. OGT catalyzes transfer of the O-GlcNAc moiety to serine or threonine residues of proteins, whereas OGA catalyzes removal of the O-GlcNAc group. Using the end product of HBP, O-GlcNAcylation is linked with carbohydrate, protein, lipid, and nucleotide metabolism. OSMI-1 and ST045849 are OGT inhibitors; Thiamet G and PUGNAc are OGA inhibitors
Protein O-GlcNAcylation and O-GlcNAc cycling enzymes are widely distributed in brain tissues; in particular, OGT is highly expressed at neuronal synapses. As a unique metabolic pathway that responds to conditions of cellular stress, i.e., oxidative, osmotic and chemical stress, O-GlcNAcylation is notably responsive [17]. Accordingly, changes in its level may exert a protective effect during acute stress conditions [18, 19]. Aberrant O-GlcNAcylation abolishes this protection and promotes the progression of multiple diseases, the choice of which depends on the cellular context. In this review, we will summarize the physiological and pathological roles of O-GlcNAcylation in neurological disorders, as well as the implications of exploiting this phenomenon for therapeutic development.
Protein O-GlcNAcylation: general overview
Functional consequences of O-GlcNAcylation
Differing from classical N- or O-linked glycosylation, which transpires mainly during protein biosynthesis in the endoplasmic reticulum and Golgi apparatus, O-GlcNAcylation occurs in the milieu of a great diversity of proteins localized in nuclei, cytoplasm, and mitochondria. Some of those proven to bear O-GlcNAcylation include Nup62, RNA polymerase II, c-Myc, Sp1, AMPK, HIF-1α, and AKT.
As a monosaccharide attachment, O-GlcNAcylation presents a technical challenge with respect to identification and site mapping of protein owing to the long-time lack of appropriate research tools. The classical methods of biochemistry were initially used to detect protein O-GlcNAcylation, involving H3-labeled UDP-Galactose which is transferred to the O-GlcNAc moiety of proteins by β1-4-galactosyltransferase (GalT). Autoradiography was then applied to identify the modified proteins [1]. Subsequently, a series of antibodies against O-GlcNAcylation were developed, although only a few of those reported were site specific [20–24]. Among those, CTD110.6 and RL2 were widely used for immunoblotting, immunofluorescence, and immunohistochemistry [20, 25, 26]. Recently, a fluorescein-conjugated antibody became commercially available for flow cytometry detection [27]. Now, after adapting biological mass spectrometry (MS) for this purpose, several methods based on affinity chromatography or chemical/chemoenzymatic derivatization were applied to enrich O-GlcNAcylated proteins and peptides before sequence analysis by MS. Combined with several MS detection approaches, such as electron-transfer dissociation (ETD)-MS/MS, varying number of proteins with O-GlcNAcylation and O-GlcNAcylation sites were identified in mammalian cells [28–31]. An earlier proteomic research identified 30 O-GlcNAcylated proteins from an extract of rat brain and provided site mapping information using Michael addition with dithiothreitol (BEMAD) technique followed by affinity chromatography and liquid chromatography (LC)–MS/MS [32]. Khidekel and colleagues developed a strategy of quantitative MS-based proteomics, namely quantitative isotopic and chemoenzymatic tagging, to monitor the dynamics of O-GlcNAcylation in rat brains after external stimulation [33]. Using a modified chemical/enzymatic photochemical cleavage method for enrichment, a total of 458 O-GlcNAc sites in 195 proteins were identified in the samples from mouse brains [34]. The astonishing result that followed was obtained by combining lectin weak-affinity chromatography and reversed-phase liquid chromatography (RPLC)–ETD-MS/MS, with which a total number of 1750 O-GlcNAc sites was identified in the synaptosomes from mouse brains [35]. These results prove that about 10–20% of proteins are O-GlcNAcylated, whereas 40–60% of proteins are phosphorylated. Moreover, quantitation methods based on MS have been developed and favor elucidating the function of O-GlcNAcylation in cellular processes [36, 37].
The biological effects of O-GlcNAcylation on proteins have been studied extensively in diverse settings, and emerging evidence suggests that O-GlcNAcylation impacts numerous protein functions. Akin to N-glycans, O-GlcNAcylation is proposed to stabilize proteins by enhancing their resistance to thermal unfolding or aggregation. O-GlcNAcylations are mostly found in intrinsically disordered regions of proteins, which are apt to be misfolded and clumped in aggregates. In that context, the protective role of O-GlcNAcylation against misfolding and aggregation, including such neural proteins as tau and β-amyloid, is detailed below. As an example of this point, less protein aggregation in CHO cell lines was found after transfection with an OGT overexpression construct [38]. In contrast, the thermal stability of Sp1 was decreased by β-N-acetylglucosaminidase (OGA) overexpression in cells [39]. Additionally, the transcriptional activator β-catenin also became stabilized by O-GlcNAcylation in cancer cells, dependent on nutrient conditions [40].
Regarding proteins with catalytic activity, O-GlcNAcylation influences mainly their enzymatic activity and substrate specificity. Protein O-GlcNAcylation of endothelial nitric oxide synthase (eNOS), which is responsible for nitric oxide (NO) production in cultured bovine aortic endothelial cells and human coronary artery endothelial cells, inhibits its phosphorylation by Akt and suppresses its ability to generate NO [41, 42]. Similarly, the rate-limiting enzyme of glycolysis, phosphofructokinase 1 (PFK1) is O-GlcNAcylated at Ser529 in response to hypoxia to redirect glucose flux into the pentose phosphate pathway, contributing to the biosynthesis of biomacromolecules in cancer cells [43]. Moreover, O-GlcNAcylation on co-activator-associated arginine methyltransferase 1 regulates its substrate specificity in addition to its stability, dimerization, and cellular localization [44].
Of its many qualities, the ability of O-GlcNAcylation to influence the binding of proteins to their ligands is particularly noteworthy. Signal transducer and activator of transcription (Stat) 5a belongs to a protein family composed of seven isoforms involved in mediating cellular responses to cytokines, hormones, and growth factors [45]. Stat5a was found to bear O-GlcNAcylation at Thr92 in HC11 mammary epithelial cells. However, substitution of Thr92 with an alanine ablates the glycosylation of Stat5a as well as its translational activity due to a loss of interaction with CREB-binding proteins [46]. Yin Yang 1 (YY1) is a zinc finger transcription factor that participates in the regulation of development [47]. Upon elevated O-GlcNAcylation induced by glucose, the formation of protein complexes by YY1 and hypophosphorylated retinoblastoma protein Rb was disrupted, and then YY1 was free to bind to DNA promoters [48].
Interplay between O-GlcNAcylation and other post-translational modifications
Interactions between O-GlcNAcylation and phosphorylation have been examined extensively for decades. The dynamic balance of O-GlcNAcylation and phosphorylation modulates the activity of various proteins in cells. Caenorhabditis elegans-bearing ogt-1 or oga-1 showed dramatically altered patterns of phosphorylation [49]. Elevation of O-GlcNAcylation in mammalian cells also affected the phosphorylation status of proteins. Proteomic studies identified about 400 proteins with both phosphorylation and O-GlcNAcylation including chaperone, cytoskeletal and regulatory proteins, metabolic enzymes, kinases, transcription factors, and RNA-processing proteins [50]. O-GlcNAcylation sites commonly occur at the same sites or near to those of phosphorylation. Because of the proposal that the O-GlcNAc moiety provides spatial resistance to the addition of phosphate, the theory was called “Yin-Yang” and related investigations on many proteins, including tau protein and CAMKII, have been performed with results that support this hypothesis. However, recent proteomic studies provided opposing evidence to overthrow this theory [35]. In summary, the apparent ability of O-GlcNAcylation to suppress phosphorylation may derive from a regulatory effect on kinase and phosphatase, rather than direct hindrance to the addition of phosphate.
Other post-translational modifications are also involved in the interplay with O-GlcNAcylation such as ubiquitination. For example, inhibition with glucosamine or O-(2-acetamido-2-deoxy-d-glucopyranosylidene) amino-N-phenylcarbamate (PUGNAc) promotes the global increase of ubiquitination [23]. In contrast, O-GlcNAcylation on β-catenin decreases its ubiquitination, which may be caused by direct competition. Moreover, O-GlcNAcylation of p53 at Ser149 could block phosphorylation at Thr155, leading to resistance against degradation induced by ubiquitination [51]. Regarding the complexity of crosstalk among these and other post-translational modifications, further research is needed to explore their relationship with O-GlcNAcylation.
O-GlcNAc cycling enzymes
Unlike the foregoing modifications, O-GlcNAcylation on myriad proteins is regulated by a unique pair of enzymes, OGT and OGA, which are responsible for the addition and removal of O-GlcNAc, respectively [52]. Both enzymes are highly conserved from C. elegans to humans, which proved to be of immense importance in understanding vertebrate physiology. OGT and OGA are distributed extensively in many cell types of humans and other higher eukaryotes. Compared with other organs, the pancreas and brain express greater amounts of OGT and OGA [53, 54], and both enzymes are sensitive to glucose levels in plasma.
O-linked N-acetylglucosaminyltransferase (OGT)
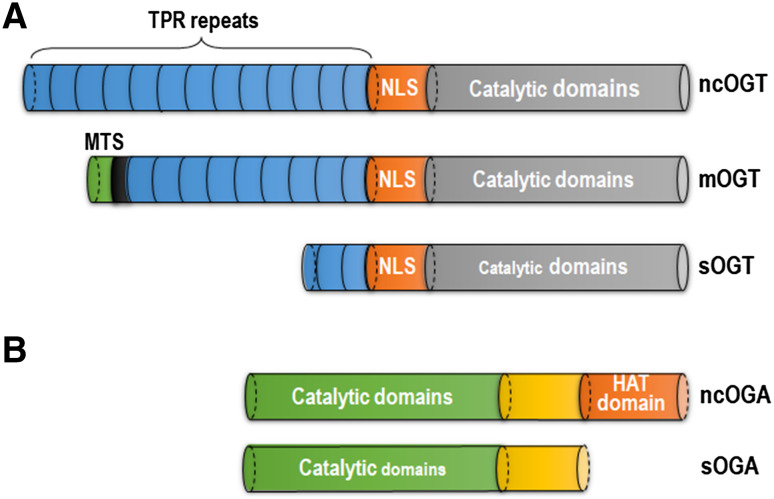
OGT is encoded by a single gene in Xq13 of the human genome, which is spliced and translated into three variable isoforms: nucleocytoplasmic OGT (ncOGT), mitochondrial OGT (mOGT), and short OGT (sOGT) [55] (Fig. 2a). At the N terminus of OGT is a tetratricopeptide repeat (TPR) domain differing in the three isoforms and proposed to interact with other partners, as well as to form self-trimerization. The catalytic domain of OGT localizes at the C-terminus and is linked with the TPR domain by a putative nuclear localization sequence (NLS) [53, 56]. Variable numbers of TPR repeats provide plasticity for OGT isoforms to modulate their substrate specificity [57, 58]. Interactions with partners or substrates such as OGA [59] and p38 MAPK [60] are associated with the regulation of OGT’s activity and localization [61, 62], affecting its recruitment toward target proteins such as RNA polymerase II, transcription complexes, and neurofilament H.
Fig. 2.
Structures of OGT and OGA isoforms. a Ogt allele is transcribed and spliced into three isoforms: nucleocytoplasmic OGT (ncOGT), mitochondrial OGT (mOGT) and short OGT (sOGT). These isoforms share similar structures, composed of tetratricopeptide (TPR) repeats, putative nuclear localization sequence (NLS) and catalytic domains and varying in the number of TPR repeats. b Two variants of OGA are nucleocytoplasmic OGA (ncOGA) and short-form OGA (sOGA). By comparing with sOGA, ncOGA contains a domain that is putatively responsible for histone acetyltransferase (HAT) activity
OGT is highly expressed in the brain, consequently leading to an augmented content of O-GlcNAcylated proteins. OGT mediating O-GlcNAcylation engages in an intense crosstalk with phosphorylation of neuronal proteins and plays a significant role in myriad cellular processes including transcriptional regulation, signaling, proteasomal degradation, and organelle trafficking. Aberrant O-GlcNAcylation-induced chaos in these pathways is linked to neuronal dysfunction. An early genetic research project established a putative link between an ogt allele and a rare neurological disorder, which was designated as a form of Parkinson-dystonia [63]. Another genome-wide associated study performed in peripheral blood mononuclear cells from patients with multiple sclerosis revealed an alteration in their expression of OGT compared with healthy volunteers, indicating that O-GlcNAc cycling may participate in the pathogenesis of multiple sclerosis [64]. As an X-linked gene, ogt allele expression in male and female victims may differ and yield a bias toward the appearance of a disease such as systemic lupus erythematosus [65]. OGT is an active player in the modulation of neuronal functions under physiological and pathological conditions.
β-N-Acetylglucosaminidase (OGA)
OGA is encoded by a single gene of meningioma-expressed antigen 5 (MGEA5) on chromosome 10 [66], which is spliced into two variants: nuclear and cytoplasmic isoform (ncOGA) and a short-form version (sOGA) (Fig. 2b). ncOGA is the long form and consists of a catalytic domain at the N terminus and a putative histone acetyltransferase (HAT) domain at the C terminus, as well as a link region between the two parts. In contrast, sOGA shares identical domains but lacks the HAT region. In the structure of OGA, caspase 3 recognizes the site between the catalytic domain and link region during apoptosis, whereas the enzymatic activity of OGA does not change after cleavage [67]. Like OGT, OGA is widely expressed in all tissues, highly conserved throughout evolution and abundant in the pancreas and brain [54, 68, 69]. The region of the mgea5 locus is reported to be associated with late-onset AD [70, 71].
As is well known from research on neurological disorders, OGA inhibitors are widely used to increase the O-GlcNAcylation in cultured cells and animal models. A series of studies uncovered a number of OGA inhibitors, such as PUGNAc, 1,2-dideoxy-2′-propyl-alpha-d-glucopyranoso-[2,1-d]-Delta 2′-thiazoline (NButGT) and Thiamet G. PUGNAc was first described as an inhibitor of another β-N-acetylhexosaminidase and later recognized as an OGA inhibitor [72, 73]. Although PUGNAc inhibits OGA, at low doses it also inhibits human lysosomal hexosaminidase B [73, 74]. After a period of exploration, the highly effective and selective inhibitor named NButGT was screened based on previous knowledge. NButGT showed a relatively potent 800-fold selectivity for OGA over the lysosomal β-hexosaminidases. Experiments in vivo and in vitro proved that NButGT treatment more effectively increased O-GlcNAcylation than a change in ganglioside levels [75–77]. Afterward, NButGT was modified to generate the compound Thiamet G, which is highly potent and selective for human OGA. Further, Thiamet G is extraordinarily stable when distributed in solution, capable of penetrating the blood–brain barrier and suitable for administration to cell cultures and intact animals [24, 78–83]. All these chemical agents offer the much needed opportunity to manipulate O-GlcNAcylation levels in cells and animal models of neurological diseases.
O-GlcNAcylation, OGT, and OGA in brain
O-GlcNAc is widely distributed in almost all types of cells and tissues in metazoan animals. The content of transcript-encoding OGA is enlarged in brain tissue, whereas that encoding OGT are highly expressed in the brain and pancreas [53, 54, 84]. With highly active OGT compared with peripheral organs, most proteins in the brain are modified with O-GlcNAc [85, 86]. In fact, several studies focused on the dynamics and topographic distribution of O-GlcNAcylation and O-GlcNAc cycling enzymes have linked their fluctuant levels to development of the rat brain [87, 88]. Monitoring of the O-GlcNAcylation levels in rat brains from embryos to 2-year-old indicated a decrease of O-GlcNAcylation level after birth and a subsequent stable level. The expression of OGT and OGA splicing isoforms was then compared during development of the rat brain, as well as the enzymatic activity of OGT and OGA. After birth, the rats gained greater activity of OGT even as the activity of OGA simultaneously changed in an opposite trend. Immunohistochemical staining of rat brains showed an extensive distribution of O-GlcNAcylation as well as OGT and OGA in every type of neuron across all the regions of these specimens [87]. Related research indicated an upregulation of O-GlcNAcylation in the cerebellar cortex and hippocampus, in which OGT was highly expressed [83, 85, 89, 90]. The ubiquitous distribution of O-GlcNAcylation in the cytoplasm and nuclei suggests a tight correlation between O-GlcNAcylation and neuronal functions.
Early studies showed that the neuron-specific mutation of OGT led to neuronal apoptosis [91]. Recent evidence now indicates that O-GlcNAcylation may be involved in neuronal apoptosis by modulating the activity of p53 or cyclin-dependent kinase 5 (CDK5) [89, 92]. In addition, upregulated O-GlcNAcylation of NF-κB was described as an arch-criminal for promoting apoptosis of retinal ganglion cells in a diabetic retinopathy model [93]. Other experimental data suggested that OGT was involved in the process of mitochondrial transport within neurons [94] and was deemed as a molecular mechanism in the regulation of excitatory synaptic function and synapse maturity [95, 96]. In the C. elegans model of tauopathy, amyloidosis and polyglutamine expansion, an alteration of O-GlcNAcylation by direct mutagenesis significantly modulated the severity of each phenotype [94]. Finally, since the O-GlcNAcylation level and the expression of O-GlcNAc cycling enzymes fluctuated during development of the CNS and became dysregulated as part of neurological disorders, O-GlcNAcylation was deemed to be responsible for the senile decline of brain functional recovery and could be a potential target for therapeutic development of neurological disorders [97, 98].
O-GlcNAcylation in neurological disorders
Alzheimer’s disease (AD)
AD, as the most prevalent of all neurodegenerative diseases, afflicts millions of patients with memory loss and an impaired recognition ability [86, 99]. At the histopathological level, extracellular senile plaques containing β-amyloid peptide (Aβ), intracellular neurofibrillary tangles of tau proteins, and neuronal loss are the major signatures of AD.
Increasing amounts of evidence has convinced researchers to follow the hypothesis that glucose uptake and utilization are impaired in AD, analogous to that in other metabolic diseases. During the pathogenesis and development of AD, glucose metabolism and supply of energy to the brain are both downregulated accordingly [100–104]. Many studies of AD models (transgenic mice and rats) have indicated that the amyloid-associated pathophysiologic process is closely linked to abnormal glucose uptake in the brain. In fact, Aβ toxicity is deemed the villain leading to glucose hypometabolism [105]. Similarly, in the human body, functionality study has shown that neurodegeneration may relate to glucose hypometabolism [106]. Like type 2 diabetes, AD may develop from an insulin deficiency and dysregulated signal transduction that impair cerebral glucose metabolism. Clinical data also support the possibility that insulin resistance results in an increased risk of dementia [107–112], indicating a similar potential in AD. In brains from patients with AD, insulin receptors are profuse [113], although insulin levels in cerebral spinal fluid (CSF) and/or the CSF/plasma insulin ratio are downregulated [114]. Additionally, the insulin signaling pathway in AD brains typically loses the ability to respond to insulin/insulin-like growth factor stimulation [115], and the expression level and activity of several key proteins such as Akt and GSK-3β lessen [112, 116]. Furthermore, the presence of GLUT1 and GLUT3 predominately expressed in neurons declines in brains of AD patients [108], possibly resulting from the decrease of GLUT3 localization to the plasma membrane caused by insulin deficiency. Collectively, emerging evidence emphasizes the importance of glucose hypometabolism and insulin signaling deficiency as essential factors in the etiology of AD, although the precise mechanism is yet to be understood.
To fill the information gap between impairment of glucose metabolism in the brain and the development of AD, O-GlcNAcylation is assigned the role of a promising molecular link that acts as a nutrient sensor with a close association with glucose metabolism [86, 117]. A decrease of O-GlcNAcylation level in AD brains is proposed to result from impaired glucose metabolism induced by Aβ toxicity and type 2 diabetes. The subsequent loss of O-GlcNAcylation protection leads to the progression of AD. Now, because of its well-known protective effect during the cellular stress response [18], more and more research devotees are inclined to support the likelihood that the elevation of O-GlcNAcylation is a promising therapeutic target for managing AD.
The two protein aggregates considered as historical hallmarks of AD are amyloid plaques and neurofibrillary tangles. Amyloid plaques are formed as the byproducts of extracellular amyloid precursor protein (APP) digestion by β- and γ-secretases, namely Aβ peptide, and intracellular neurofibrillary tangles are composed of hyperphosphorylated tau proteins (Fig. 3). The latter are much better indicators for the cognitive deficit of AD.
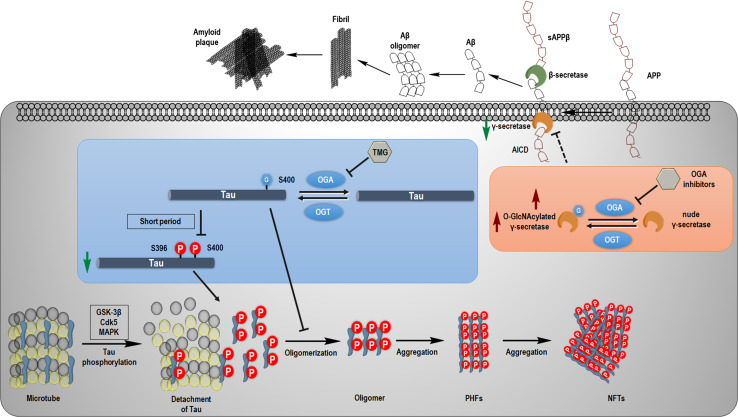
Fig. 3.
Molecular cascade of tau and Aβ pathology. Normal tau proteins bind to microtubules to stabilize the complex of α-tubulins and β-tubulins. When kinases such as GSK-3β, CDK5, and MAPK are activated under the conditions of diseases, tau is phosphorylated and detaches from the microtubule to form oligomers, and subsequently aggregates as paired helical filaments (PHFs) and further as neurofibrillary tangles (NFTs). Inhibition of OGA by Thiamet G (TMG) evokes the O-GlcNAcylation at Ser400 of tau, which may suppress the phosphorylation at Ser396 and S400 of tau within a short period of administration and directly prevent the aggregation of tau. On the other hand, transmembrane amyloid precursor protein (APP) is cleaved by β- and γ-secretase to produce soluble amyloid precursor protein β (sAPPβ) and Aβ, and release the APP intracellular domain (AICD). Aβ could self-organize to aggregate and form amyloid plaques. Inhibition of OGA may reduce the activity of γ-secretase to diminish the secretion of Aβ
O-GlcNAcylation and tau
Tau protein is a microtubule-associated protein (MAP) encoded by a single MAPT gene in humans, and that gene has six variants differing in the length of their N-terminal projection region and the number of microtubule-binding repeats in the C terminus. Tau proteins are highly soluble and intrinsically disordered, interacting with various partners as a hub in the cellular protein–protein interaction network [118]. Phosphorylation occurs on many different residues of tau proteins, and its dysregulation is the most prevalent driving factor associated with neurotoxicity. In this situation, the ratio of phosphorylated tau is increased about six- to eightfold above that at physiological conditions [119]. Hyperphosphorylation of tau leads to its detachment from the microtubule, subsequently increasing the tangle of microtubules as well as free tau proteins in the cytoplasm [120–122]. Accumulations of free tau proteins begin to form phosphorylated helical filaments, as reported from research performed in vitro and in vivo [123–125] (Fig. 3). Data from studies in transgenic mice in which specific kinases were inhibited have proven that hyperphosphorylation of tau is of critical significance in its toxicity [126, 127]. Intriguingly, soluble small oligomers of tau are found to be the toxic species, whereas the aggregations of tau as neurofibrillary tangles are non-toxic in the brain [128, 129]. Therefore, the massive aggregation of tau proteins is proposed as a pathologic cell stress response.
O-GlcNAcylation on tau proteins, when first discovered in samples of bovine tissues, indicated a stoichiometry of about 4 moles of O-GlcNAc per mole of tau proteins [130]. Subsequent studies then surveyed the precise residues of O-GlcNAcylation on tau proteins. Four sites on human tau proteins have been confirmed as Thr123, Ser208, Ser400, and Ser409/Ser412/Ser413 [81, 131]. O-GlcNAcylation at Ser400 is also found on tau proteins from mice and rats [81, 132, 133]. At an early stage of exploration on this topic, O-GlcNAcylation was thought to be a component in the regulation of tau activity and a mutual interaction with phosphorylation. However, several later efforts identified the reciprocity between phosphorylation and O-GlcNAcylation on tau proteins. Gong et al. described the O-GlcNAcylation of tau in humans and showed the antagonistic effect of O-GlcNAcylation against phosphorylation of tau at different residues in cultured cells and rat brain slices. Surprisingly, no O-GlcNAcylation was found on aggregated tau proteins in samples from AD patients [134]. Experimental data from fasting mice and a triple transgenic mouse model of AD indicated an increase of phosphorylation on tau [135, 136], and blocking of protein phosphatase led to a decrease of O-GlcNAcylation [137]. Tau phosphorylation was also increased in transgenic mice subjected to neuron-specific OGT deletion [91]. Additionally, Aβ-induced S-nitrosylated OGT led to the hyper-O-GlcNAcylation of tau protein in neurons [138].
Overall, these discoveries have stimulated efforts to explore the ability of elevations in O-GlcNAcylation to provide protection against tau toxicity in AD. Many kinds of tauopathy mouse models have been generated by genetic manipulation, including JNPL3 mice [139], Tau.P301L mice [140] and Tg4510 mice [128]. In JNPL3 mice, the administration of Thiamet G for several months suppressed the aggregation of tau, reduced the amount of neurofibrillary tangles in the brain and protected against neuronal loss [81]. A striking revelation was that an increase of O-GlcNAcylation at Ser400 directly inhibited tau aggregation in vitro rather than affecting the conformation of tau by suppression of its phosphorylation, an outcome that was recently confirmed [141]. Treatment of Tg451 mice with Thiamet G for 4 months resulted in a similar aftermath. Notably, a monoclonal antibody against Ser400 O-GlcNAcylation of tau was generated and applied for use in this study [82]. Thiamet G was also used for the Tau.P301L model. A 2.5-month-long treatment with Thiamet G decreased the distribution of neurons bearing neurofibrillary tangles, offset weight loss, improved motor defects, and lengthened survival rates as well [142]. The controversial item in this model was that tau proteins were not modified by O-GlcNAc, when applying a methodology that differs from that of previous studies.
To summarize briefly, increasing tau O-GlcNAcylation by the inhibition of OGA protects tau proteins against aggregation independent of phosphorylation, although the results from previous similar studies are still controversial. A putative proposal to explain the difference is that acute administration of an OGA inhibitor suppressed the activity of kinases and phosphatases, which would be responsible for phosphorylation and dephosphorylation of tau proteins. After a long term of perturbation, cells may adapt to these changes. On the other hand, sustained action of kinases could overcome the barrier of O-GlcNAc blockade to restore the phosphorylation level. Overall, these studies provide solid evidence that chronic treatment with OGA inhibitor ameliorates neuronal loss and motor defects by elevating O-GlcNAcylation at Ser400 and possibly other residues, shedding light on the development of a therapeutic strategy for AD [86].
O-GlcNAcylation and APP
Amyloid plaques are typical lesions in the brain of a patient with AD where Aβ peptides have aggregated [143]. Aβ peptides are produced by the cleavage of APP by β- and γ-secretase, whereas soluble APP is generated by cleavage by α- and γ-secretase. It is well accepted that dysregulation of Aβ processing and generation of Aβ peptides are the earliest phenomena to initiate the pathogenesis of AD, apparently with the aid of other factors [144, 145].
As noted previously, Aβ toxicity may lead to impaired glucose metabolism in the brain, but type 2 diabetes could be the accelerant in AD. APP was reported to be O-GlcNAcylated decades ago, although this information was not brought to the forefront at that time [146, 147]. Much later, application of the OGA inhibitor, PUGNAc, increased O-GlcNAcylation and visibly reduced the production of Aβ [148, 149]. Recent study using another OGA inhibitor, NButGT [150], in the 5xFAD Aβ mouse model also supported the protective effect of long-term treatment with OGA inhibitor that inhibited Aβ toxicity. As a result, the levels of Aβ40 and Aβ42 decreased as did plaque formation and neuroinflammation followed by improved cognition. In vitro, experiments with CHO cells expressing the Swedish mutation in APP also showed a decrease of that protein’s C-terminal fragment. Presumably, these effects result mainly from the suppression of γ-secretase by O-GlcNAcylation [150]. However, the precise sites of O-GlcNAcylation on γ-secretase have not yet been mapped, and how O-GlcNAcylation functions in the regulation of γ-secretase remains to be explored (Fig. 3). In bigenetic TAPP mice that combine the pathologic effects of amyloid and tau, the outcome of long-term OGA inhibition induced by Thiamet G was also described. The prevention of cognitive defects resulted from OGA inhibition, along with decreases of Aβ levels and plaque numbers in the brain, as well as the stabilization of tau [151]. Interestingly, Ryu et al. reported that O-GlcNAcylation could be elevated by Aβ-induced S-nitrosylation of OGT, whereas hyper-O-GlcNAcylation within cells was protective against Aβ neurotoxicity [138]. Although these studies provide convincing evidence of the protection that OGA inhibition provides against Aβ toxicity, exactly how the long-term treatment of OGA inhibitors affects the processing of APP remains open to further validation.
Additionally, impairments were noted in several other cellular signaling pathways of AD, in which key proteins were modified with O-GlcNAc. Protein kinase A catalytic subunits (PKAcs) were reported to be O-GlcNAcylated at both α and β subunits, which positively regulate their enzymatic activity. A decrease of O-GlcNAcylation during AD downregulates the activity of PKAcs, leading to the suppression of PKA-CREB signaling, which causes deficiencies in learning and memory [152].
Parkinson’s disease (PD)
The common neurodegenerative disorder, PD, occurs in approximately 1% of persons over 65 years old with no bias as to race or geographical location. The characteristic clinical syndrome includes muscle rigidity, bradykinesia, and resting tremor, accompanied by the secondary syndromes of cognitive dysfunction and postural instability [153, 154]. The pathophysiology of PD is attributed to the loss of dopaminergic neurons leading to a disability of motor function-related nerves in the central nervous system, along with the accumulation of Lewy bodies in surviving neurons [155, 156].
α-Synuclein is thought to be of critical importance in the etiology of PD, although the precise underlying basis of this disorder remains ambiguous. Genetic research has implicated an association between α-synuclein and familial PD [157, 158]. Further, several mutations in α-synuclein, such as A30P, A53T, and E46K, were reported as causes of inherited PD [157, 159–162]. During the pathogenesis of PD, α-synuclein undergoes a conformational change and forms dimer and oligomers, subsequently aggregated as fibrils that are toxic to cells and model organisms [163–165]. A series of post-translational modifications such as phosphorylation [166–171], ubiquitination [172, 173], and glycosylation [34, 132, 174] are proposed to be involved in this process. Shimura et al. described a protein complex composed of parkin and UbcH7, as well as an O-glcosylated α-synuclein, which implies the existence of a linkage between the O-glcosylated form of α-synuclein and parkin [174]. However, an opposing result was also cited indicating that parkin interacts with the nonglycosylated form of α-synuclein in the embryonic hippocampal cell line, H19-7. Initially, when specific antibodies against O-GlcNAc were applied to identify the immunoprecipitated proteins, glycosylation on α-synuclein was not confirmed [175]. However, several years later, the O-GlcNAcylation site on α-synuclein was established as Thr72 in humans and located in a region that is resistant to protein degradation [132]. On its homologous protein in murine neurons, O-GlcNAcylation sites were mapped to Thr53, Thr64, and Thr72, but to Ser87 in human erythrocytes [10, 34]. Marotta et al. first used a chemically synthesized peptide containing O-GlcNAcylation to validate the proposal that O-GlcNAcylation at Thr72 could inhibit α-synuclein aggregations. They found the full length of α-synuclein with or without O-GlcNAcylation and experimental data confirmed the inhibitory effect of O-GlcNAcylation at Thr72 on fibril formation, as well as the reduction of α-synuclein’s toxicity for living cells [176]. Recently, Matthew’s group identified a less inhibitory effect of O-GlcNAcylation at Ser87 of α-synuclein against protein aggregation using a strategy of synthetic protein chemistry [177] (Fig. 4). Although a vivid picture of PD’s etiology is yet to come, blocking of α-synuclein aggregation by O-GlcNAcylation could be a potential target for therapeutic control of PD. Inhibitors of OGA might serve as the agents, since they have a proven capacity to raise the O-GlcNAcylation level in the brain [82, 151].
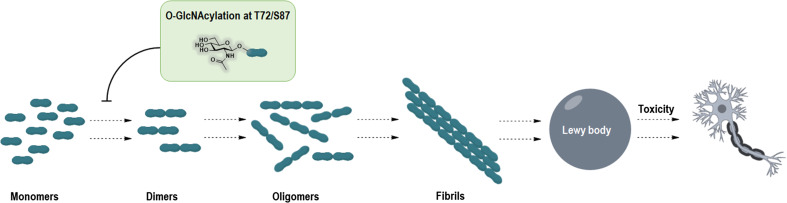
Fig. 4.
Schematic diagram of α-synuclein aggregation. Genetic mutations of α-synuclein are associated with the pathologic effects of PD. Misfolded α-synuclein is susceptible to forming dimers and oligomers, as well as aggregating fibrils, leading to the formation of Lewy bodies, which are toxic for neurons. Strikingly, a single O-GlcNAcylation at Thr72 or Ser 87 of α-synuclein inhibits the aggregation and reduces the toxicity of aggregated α-synuclein for adjacent cells
Several other clues hint that the link between O-GlcNAc and PD is factual. A recent report asserted that tyrosine 3-monooxygenase is modified by O-GlcNAc, which is responsible for dopamine secretion. Stimulation of N-propanoylmannosamine (an unnatural precursor of neuraminic acid) downregulated the O-GlcNAcylation of tyrosine 3-monooxygenase and promoted its activation, leading to an increase of dopamine secretion [178]. Since this outcome might be controversial with respect to previous reports, further validation is warranted.
Stress and stroke
O-GlcNAcylation is believed to be an important mitigating factor of cells’ stress response. An early report showed the prolonged survival times of rats undergoing experimentally induced hemorrhages after suppression of stress-induced hyperglycemia [179]. At first, this protection was proposed to result from either the physical effect of maintaining homeostasis [180, 181] or the provision of an adequate energy supply [182]. Glucose flux through HBP was highlighted afterward because the production of UDP-GlcNAc was the end product. Other experimental data suggested that an elevated O-GlcNAcylation level induced the increased survival of mammalian cells ex vivo after exposure to stress [18]. However, O-GlcNAc modification has been closely associated with vascular ischemic/reperfusion injury [183]. In an animal model of trauma hemorrhage, intraperitoneal injection of glucosamine induced an increase of O-GlcNAcylation level, improved blood flow in critical organs, and decreased the production of inflammatory cytokines [184]. Subsequently, PUGNAc, an inhibitor of OGA, was used as an alternative agent to upregulate the O-GlcNAcylation level in a rat model of trauma hemorrhage and similar results were obtained [185]. In other studies, an increase of O-GlcNAc levels again improved cardiac function and perfusion of critical organ systems as well as reducing the amount of inflammatory cytokines in plasma [186–189]. Treatment with glucosamine (GlcN) and PUGNAc during resuscitation dramatically decreased the trauma-hemorrhage-induced increase of TNF-α, IL-6, NF-κB, IκB-α phosphorylation and NF-κB DNA binding activity in cardiac tissues [190]. Outcomes from all these studies support the concept that protection is offered by O-GlcNAcylation under conditions of physiologic stress.
Elsewhere, a dramatic increase of O-GlcNAc modification in the cortex was recorded after stroke [191, 192]. Supposedly, an elevation of O-GlcNAc modification was associated with neuronal apoptosis in the early stage of stroke and the extent of recovery after ischemic injury. Moreover, Hwang et al. provided evidence that treatment with GlcN could inhibit the production of inflammatory cytokines and activation of microglia in a rat model of stroke [193]. As an alternative way to increase O-GlcNAcylation, our group pursued the application of a highly selective, blood–brain barrier-permeable inhibitor of OGA, Thiamet G, in mice manipulated to model ischemic stroke [194]. We then confirmed the neuroprotective effect of Thiamet G, a novel inflammation antagonist, in mice imbued with transient middle cerebral artery occlusion. Experimental data showed that Thiamet G significantly reduced infarct size and diminished the neurological deficits and lessened motor coordination impairment in subjects of experimental stroke. The results achieved in vivo and confirmed in vitro indicate that the neuroprotective effect of Thiamet G is associated predominantly with a shift of microglia polarization in an NF-κB-dependent manner (Fig. 5).
Fig. 5.
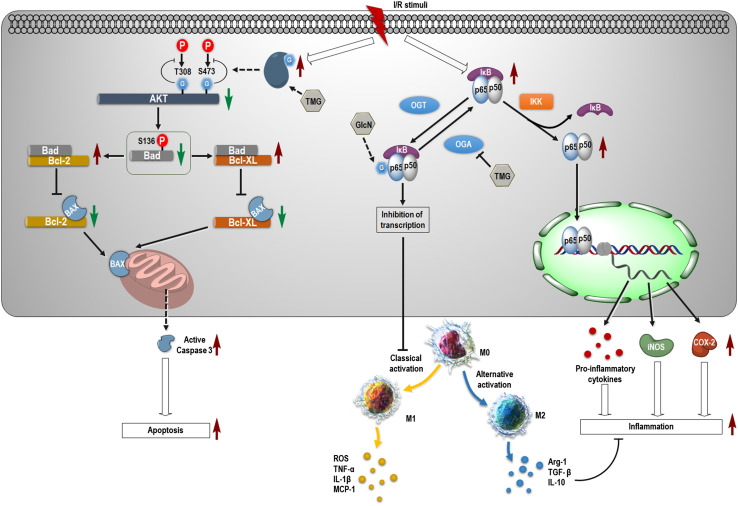
Proposed molecular events after ischemia and reperfusion. After stimulating ischemia and performing reperfusion, the O-GlcNAcylation level rises dramatically in a short period of time. O-GlcNAcylation at Thr308 and Ser473 of Akt suppresses its phosphorylation and activation. Decreased Akt activity results in the loss of phosphorylation of Bad at Ser136, leading to its increased binding with the Bcl-2 family of proteins. This action downregulates the formation of protein complexes composed of Bcl-2 proteins and BAX. Subsequently, more BAXs attack the mitochondrial membrane to activate caspase-3, which causes apoptosis of neurons. Inhibition of OGA by Thiamet G (TMG) accelerates this process. On the contrary, the NF-κB pathway is activated, and the complex of p65 and p50 translocates into nuclei to initiate transcription of target genes including pro-inflammatory cytokines, iNOS and COX-2. Increase of O-GlcNAcylation on p65 by pharmacological intervention with glucosamine (GlcN) and TMG inhibits transcription initiation and suppresses the classical activation of macrophage/microglia, resulting in their shift of phenotype into M2. BAX, BCL2-associated X, apoptosis regulator; I/R, ischemia/reperfusion; OGT, O-linked N-acetylglucosaminyltransferase; OGA, β-N-acetylglucosaminidase; IKK, inhibitor of nuclear factor κB kinase; IκB, inhibitor of nuclear factor κB; “G” indicates the moiety of GlcNAc; TMG, Thiamet G
After ischemic stroke, apoptosis is trigged by ischemia-induced stress, leading to neuronal cell death and preliminary tissue damage. At the initial stage of ischemic stroke, apoptosis appears around the penumbra. Afterwards, the ischemic area expands as ischemic time lengthens, and numerous cells in the central area of ischemia die of necrosis [195, 196]. In a mouse model of ischemic stroke, O-GlcNAcylation was found to promote apoptosis by attenuating phosphorylation/activation of AKT and the BCL2-associated agonist of cell death (Bad). In the process, both Thr308 and Ser473 of AKT were modified with O-GlcNAc as identified using co-immunoprecipitation and mutagenesis techniques. However, O-GlcNAcylation-induced apoptosis was attenuated by the overexpression of AKT in cultured cells. A negative correlation between AKT phosphorylation and O-GlcNAcylation in brain tissues of ischemic mice became evident by immunoblotting. These results indicate that cerebral ischemia induces a rapid increase of O-GlcNAcylation that promotes apoptosis through down-regulation of AKT activity and provide a novel mechanism with which O-GlcNAcylation regulates ischemia-induced neuronal apoptosis through AKT signaling [191] (Fig. 5). Similarly, a preliminary study performed in a model of intracerebral hemorrhage (ICH) in vitro indicated that O-GlcNAcylation of CDK5 protected against neuronal apoptosis by suppressing the p53 pathway [92]. An observation showed that O-GlcNAcylation of neuronal nitric oxide synthase (nNOS) slowly increased at 3 h after ischemic stroke in a rat model of middle cerebral artery occlusion. This result indicated a possible association of O-GlcNAcylation with excitotoxicity induced by brain injury. Subsequent experiments in vitro hinted that O-GlcNAcylation of nNOS leads to neuronal death by modulating the interaction between OGT and nNOS [197]. This work provides new evidence on the role of O-GlcNAcylation in the pathology of ischemic stroke and contributes to the development of a useful therapeutic strategy.
Interestingly, in a parallel study focused on the change of protein post-translational modifications including O-GlcNAcylation, ubiquitination and SUMO conjunction in young and aged mice subjected to surgery to induce middle cerebral artery occlusion, results suggested that impaired O-GlcNAcylation in brains of aged mice may be responsible for slow recovery compared with that of young mice [192]. This indicated that protein O-GlcNAcylation is associated with functional recovery after ischemic stroke and is proposed as an age-related post-translational modification. Further investigation may provide more information about the perturbation of O-GlcNAc cycling during the onset and progression of stroke.
Other neurological disorders
In addition to the diseases mentioned above, the impact of protein O-GlcNAcylation has been extended. Wang and co-workers found that a decrease of O-GlcNAc cycling reduced neuronal loss in a model of Huntington’s disease (HD) in transgenic mice expressing a fusion human Huntington protein with an expansion of polyglutamines and vice versa [94]. Recently, Jonathan et al. confirmed that mutant Huntingtin gene-induced disruption of nucleocytoplasmic transport was responsible for HD-like pathology in multiple models of HD, and an increase of O-GlcNAcylation by Thiamet G rescued nucleocytoplasmic trafficking defects and alleviated neurotoxicity in HD [198]. These data suggest that hyper-O-GlcNAcylation is neuroprotective, which is consistent with foregoing research. Further investigations are still requisite to elucidate the role of O-GlcNAcylation in the etiology and pathogenesis of HD. In a genome-wide association analysis of a Drosophila melanogaster model of trauma brain injury, results also indicated that O-GlcNAc cycling enzymes and HBP are associated with glucose flux after brain injury [199].
Amyotrophic lateral sclerosis (ALS), another typical neurodegenerative disease with adult onset, is characterized by a loss of neurons, particularly motor neurons in the central nervous system. ALS of humans and mice is closely linked to dysregulation of protein kinase function and an increase of phosphorylated products [200, 201]. Under the condition of ALS, an essential protein, neurofilament, is reputedly hyperphosphorylated, a status that is also modified by O-GlcNAc [202, 203]. As is usual on other proteins, such as tau, O-GlcNAcylation and phosphorylation of neurofilaments are mutually reciprocal [204]. In a rat model of ALS, a decrease of O-GlcNAcylation in the spinal cord proved to be associated with the pathogenesis of ALS and acted against phosphorylation on neurofilament-M, whereas the inhibition of OGA attenuated the tangle formation intrinsic to this disease [22]. Shan and co-workers used NButGT to identify a similar result in mice carrying a mutation in superoxide dismutase 1 (SOD1) [77]. These experiments suggest that upregulation of O-GlcNAcylation by intervention with OGA inhibitors is a promising therapeutic method for ALS.
Multiple sclerosis, a chronic autoimmune and inflammatory disease of the central nervous system, is characterized by demyelinating lesions and progressive axon loss [205]. Recently, the remarkable impact of O-GlcNAc modification on immunity and inflammation was identified [206]. Several studies have provided preliminary clues that O-GlcNAc modification is a participant in the pathogenesis of autoimmune diseases [65, 207, 208]. However, no further revelations have yielded the precise role of O-GlcNAc modification in CNS inflammation. Yet we identified microRNA-15b as a key regulator of T helper 17 cell-associated effects in a mouse model of multiple sclerosis; those effects modulated the transcriptional regulation of retinoic acid-related orphan receptor γT through O-linked N-acetylglucosamine glycosylation of NF-κB by targeting OGT [209]. These data indicate that O-GlcNAcylation supposedly contributes to the etiology and pathogenesis of multiple sclerosis.
Conclusions
In this review, we highlight the physiological and pathological functions of protein O-GlcNAcylation in the nervous system. Compared with the long-established effects of phosphorylation, O-GlcNAcylation is an emerging actor in the theater of neurological diseases. The “playbook” of these performances and related studies is just beginning to open. As an indispensable link between metabolism and neuronal activity, protein O-GlcNAcylation is a putatively essential instrument in the development of neurological diseases. A growing body of evidence from research studies performed in vivo and in vitro show that O-GlcNAcylation fluctuates and changes, and O-GlcNAc cycling enzymes depend as well on the cellular context and stress conditions in neurological disorders that underlie the capability for learning and memory. Although researchers have mapped the critical O-GlcNAcylation site on tau protein to Ser400 and deciphered its biological effect during the pathogenesis of AD, most experimental data provide a description of changes in O-GlcNAcylation status rather than in the molecular mechanisms beneath the surface. Our challenge is to figure out solutions to these problems in view of the multiple factors involved in the development of neurological disease.
In the near future, emerging research studies in this area will provide more precise information about the regulation of O-GlcNAcylation and specificity of O-GlcNAcylation. We await understanding of O-GlcNAcylation in the nervous system and neurological disorders, so as to build a complete network of metabolic and neuronal activity that restores health in the aftermath of neurological damage. Moreover, pharmacological intervention with inhibitors of O-GlcNAc cycling enzymes in the experimental setting may shed light on the “sweet spot” of developing therapeutic methods for alleviating neurological diseases.
Acknowledgements
This work was supported by the National Natural Science Foundation of China (81571600, 81322018 and 81273287 to J.W.H., and 81401361 to X.F.M.) and the Youth Top-notch Talent Support Program (to J.W.H.).
Abbreviations
- Aβ
β-Amyloid peptide
- AD
Alzheimer’s disease
- ALS
Amyotrophic lateral sclerosis
- AICD
Amyloid precursor protein intracellular domain
- APP
Amyloid precursor protein
- Bad
BCL2-associated agonist of cell death
- BAX
BCL2 associated X
- BEMAD
Michael addition with dithiothreitol
- CDK5
Cyclin-dependent kinase 5
- CSF
Cerebral spinal fluid
- eNOS
Endothelial nitric oxide synthase
- ETD
Electron-transfer dissociation
- GalT
β1-4-Galactosyltransferase
- GFAT
l-Glutamine:fructose-6-phosphate amidotransferase
- GlcN
Glucosamine
- HAT
Histone acetyltransferase
- HBP
Hexosamine biosynthetic pathway
- HD
Huntington’s disease
- ICH
Intracerebral hemorrhage
- LC
Liquid chromatography
- IKK
Inhibitor of nuclear factor κB kinase
- IκB
Inhibitor of nuclear factor κB
- I/R
Ischemia/reperfusion
- MAP
Microtubule-associated protein
- MGEA5
Meningioma expressed antigen 5
- mOGT
Mitochondrial OGT
- MS
Mass spectrometry
- NButGT
1,2-Dideoxy-2′-propyl-alpha-d-glucopyranoso-[2,1-d]-Delta 2′-thiazoline
- ncOGA
Nuclear and cytoplasmic OGA
- ncOGT
Nucleocytoplasmic OGT
- NFTs
Neurofibrillary tangles
- NLS
Nuclear localization signal
- NO
Nitric oxide
- OGA
β-N-Acetylglucosaminidase
- nNOS
Neuronal nitric oxide synthase
- O-GlcNAcylation
O-linked β-N-acetylglucosaminylation
- OGT
O-linked N-acetylglucosaminyltransferase
- PD
Parkinson’s disease
- PKAcs
Protein kinase A catalytic subunit
- PFK1
Phosphofructokinase 1
- PHFs
Paired helical filaments
- PUGNAc
O-(2-Acetamido-2-deoxy-d-glucopyranosylidene) amino-N-phenylcarbamate
- RPLC
Reversed-phase liquid chromatography
- sAPPβ
Soluble amyloid precursor protein β
- SOD1
Superoxide dismutase 1
- sOGA
Short-form OGA
- sOGT
Short OGT
- Stat
Signal transducer and activator of transcription
- TPR
Tetratricopeptide
- YY1
Yin Yang 1
Footnotes
Xiaofeng Ma and He Li contributed equally to this work.
References
- 1.Torres CR, Hart GW. Topography and polypeptide distribution of terminal N-acetylglucosamine residues on the surfaces of intact lymphocytes. Evidence for O-linked GlcNAc. J Biol Chem. 1984;259(5):3308–3317. [PubMed] [Google Scholar]
- 2.Holt GD, Hart GW. The subcellular distribution of terminal N-acetylglucosamine moieties. Localization of a novel protein-saccharide linkage, O-linked GlcNAc. J Biol Chem. 1986;261(17):8049–8057. [PubMed] [Google Scholar]
- 3.Haltiwanger RS, Holt GD, Hart GW. Enzymatic addition of O-GlcNAc to nuclear and cytoplasmic proteins. Identification of a uridine diphospho-N-acetylglucosamine:peptide beta-N-acetylglucosaminyltransferase. J Biol Chem. 1990;265(5):2563–2568. [PubMed] [Google Scholar]
- 4.Hart GW, Housley MP, Slawson C. Cycling of O-linked beta-N-acetylglucosamine on nucleocytoplasmic proteins. Nature. 2007;446(7139):1017–1022. doi: 10.1038/nature05815. [DOI] [PubMed] [Google Scholar]
- 5.Comer FI, Hart GW. Reciprocity between O-GlcNAc and O-phosphate on the carboxyl terminal domain of RNA polymerase II. Biochemistry. 2001;40(26):7845–7852. doi: 10.1021/bi0027480. [DOI] [PubMed] [Google Scholar]
- 6.Groves JA, Lee A, Yildirir G, Zachara NE. Dynamic O-GlcNAcylation and its roles in the cellular stress response and homeostasis. Cell Stress Chaperones. 2013;18(5):535–558. doi: 10.1007/s12192-013-0426-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Harwood KR, Hanover JA. Nutrient-driven O-GlcNAc cycling - think globally but act locally. J Cell Sci. 2014;127(Pt 9):1857–1867. doi: 10.1242/jcs.113233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vaidyanathan K, Durning S, Wells L. Functional O-GlcNAc modifications: implications in molecular regulation and pathophysiology. Crit Rev Biochem Mol Biol. 2014;49(2):140–163. doi: 10.3109/10409238.2014.884535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hart GW, Greis KD, Dong LY, Blomberg MA, Chou TY, Jiang MS, Roquemore EP, Snow DM, Kreppel LK, Cole RN, et al. O-linked N-acetylglucosamine: the “yin-yang” of Ser/Thr phosphorylation? Nuclear and cytoplasmic glycosylation. Adv Exp Med Biol. 1995;376:115–123. doi: 10.1007/978-1-4615-1885-3_10. [DOI] [PubMed] [Google Scholar]
- 10.Hu P, Shimoji S, Hart GW. Site-specific interplay between O-GlcNAcylation and phosphorylation in cellular regulation. FEBS Lett. 2010;584(12):2526–2538. doi: 10.1016/j.febslet.2010.04.044. [DOI] [PubMed] [Google Scholar]
- 11.Guinez C, Mir AM, Dehennaut V, Cacan R, Harduin-Lepers A, Michalski JC, Lefebvre T. Protein ubiquitination is modulated by O-GlcNAc glycosylation. FASEB J. 2008;22(8):2901–2911. doi: 10.1096/fj.07-102509. [DOI] [PubMed] [Google Scholar]
- 12.Sakabe K, Wang Z, Hart GW. Beta-N-acetylglucosamine (O-GlcNAc) is part of the histone code. Proc Natl Acad Sci USA. 2010;107(46):19915–19920. doi: 10.1073/pnas.1009023107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lu L, Fan D, Hu CW, Worth M, Ma ZX, Jiang J. Distributive O-GlcNAcylation on the highly repetitive C-terminal domain of RNA polymerase II. Biochemistry. 2016;55(7):1149–1158. doi: 10.1021/acs.biochem.5b01280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Marshall S, Bacote V, Traxinger RR. Discovery of a metabolic pathway mediating glucose-induced desensitization of the glucose transport system. Role of hexosamine biosynthesis in the induction of insulin resistance. J Biol Chem. 1991;266(8):4706–4712. [PubMed] [Google Scholar]
- 15.Slawson C, Copeland RJ, Hart GW. O-GlcNAc signaling: a metabolic link between diabetes and cancer? Trends Biochem Sci. 2010;35(10):547–555. doi: 10.1016/j.tibs.2010.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Banerjee PS, Hart GW, Cho JW. Chemical approaches to study O-GlcNAcylation. Chem Soc Rev. 2013;42(10):4345–4357. doi: 10.1039/C2CS35412H. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Martinez MR, Dias TB, Natov PS, Zachara NE. Stress-induced O-GlcNAcylation: an adaptive process of injured cells. Biochem Soc Trans. 2017;45(1):237–249. doi: 10.1042/BST20160153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zachara NE, O’Donnell N, Cheung WD, Mercer JJ, Marth JD, Hart GW. Dynamic O-GlcNAc modification of nucleocytoplasmic proteins in response to stress. A survival response of mammalian cells. J Biol Chem. 2004;279(29):30133–30142. doi: 10.1074/jbc.M403773200. [DOI] [PubMed] [Google Scholar]
- 19.Nagel AK, Ball LE. Intracellular protein O-GlcNAc modification integrates nutrient status with transcriptional and metabolic regulation. Adv Cancer Res. 2015;126:137–166. doi: 10.1016/bs.acr.2014.12.003. [DOI] [PubMed] [Google Scholar]
- 20.Comer FI, Vosseller K, Wells L, Accavitti MA, Hart GW. Characterization of a mouse monoclonal antibody specific for O-linked N-acetylglucosamine. Anal Biochem. 2001;293(2):169–177. doi: 10.1006/abio.2001.5132. [DOI] [PubMed] [Google Scholar]
- 21.Kamemura K, Hayes BK, Comer FI, Hart GW. Dynamic interplay between O-glycosylation and O-phosphorylation of nucleocytoplasmic proteins: alternative glycosylation/phosphorylation of THR-58, a known mutational hot spot of c-Myc in lymphomas, is regulated by mitogens. J Biol Chem. 2002;277(21):19229–19235. doi: 10.1074/jbc.M201729200. [DOI] [PubMed] [Google Scholar]
- 22.Ludemann N, Clement A, Hans V, Leschik J, Behl C, Brandt R. O-glycosylation of the tail domain of neurofilament protein M in human neurons and in spinal cord tissue of a rat model of amyotrophic lateral sclerosis (ALS) J Biol Chem. 2005;280(36):31648–31658. doi: 10.1074/jbc.M504395200. [DOI] [PubMed] [Google Scholar]
- 23.Fujiki R, Hashiba W, Sekine H, Yokoyama A, Chikanishi T, Ito S, Imai Y, Kim J, He HH, Igarashi K, Kanno J, Ohtake F, Kitagawa H, Roeder RG, Brown M, Kato S. GlcNAcylation of histone H2B facilitates its monoubiquitination. Nature. 2011;480(7378):557–560. doi: 10.1038/nature10656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cameron A, Giacomozzi B, Joyce J, Gray A, Graham D, Ousson S, Neny M, Beher D, Carlson G, O’Moore J, Shearman M, Hering H. Generation and characterization of a rabbit monoclonal antibody site-specific for tau O-GlcNAcylated at serine 400. FEBS Lett. 2013;587(22):3722–3728. doi: 10.1016/j.febslet.2013.09.042. [DOI] [PubMed] [Google Scholar]
- 25.Holt GD, Snow CM, Senior A, Haltiwanger RS, Gerace L, Hart GW. Nuclear pore complex glycoproteins contain cytoplasmically disposed O-linked N-acetylglucosamine. J Cell Biol. 1987;104(5):1157–1164. doi: 10.1083/jcb.104.5.1157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Snow CM, Senior A, Gerace L. Monoclonal antibodies identify a group of nuclear pore complex glycoproteins. J Cell Biol. 1987;104(5):1143–1156. doi: 10.1083/jcb.104.5.1143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Swamy M, Pathak S, Grzes KM, Damerow S, Sinclair LV, van Aalten DM, Cantrell DA. Glucose and glutamine fuel protein O-GlcNAcylation to control T cell self-renewal and malignancy. Nat Immunol. 2016;17(6):712–720. doi: 10.1038/ni.3439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Khidekel N, Ficarro SB, Peters EC, Hsieh-Wilson LC. Exploring the O-GlcNAc proteome: direct identification of O-GlcNAc-modified proteins from the brain. Proc Natl Acad Sci USA. 2004;101(36):13132–13137. doi: 10.1073/pnas.0403471101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhao P, Viner R, Teo CF, Boons GJ, Horn D, Wells L. Combining high-energy C-trap dissociation and electron transfer dissociation for protein O-GlcNAc modification site assignment. J Proteome Res. 2011;10(9):4088–4104. doi: 10.1021/pr2002726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Myers SA, Panning B, Burlingame AL. Polycomb repressive complex 2 is necessary for the normal site-specific O-GlcNAc distribution in mouse embryonic stem cells. Proc Natl Acad Sci USA. 2011;108(23):9490–9495. doi: 10.1073/pnas.1019289108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zaro BW, Yang YY, Hang HC, Pratt MR. Chemical reporters for fluorescent detection and identification of O-GlcNAc-modified proteins reveal glycosylation of the ubiquitin ligase NEDD4-1. Proc Natl Acad Sci USA. 2011;108(20):8146–8151. doi: 10.1073/pnas.1102458108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wells L, Vosseller K, Cole RN, Cronshaw JM, Matunis MJ, Hart GW. Mapping sites of O-GlcNAc modification using affinity tags for serine and threonine post-translational modifications. Mol Cell Proteom. 2002;1(10):791–804. doi: 10.1074/mcp.M200048-MCP200. [DOI] [PubMed] [Google Scholar]
- 33.Khidekel N, Ficarro SB, Clark PM, Bryan MC, Swaney DL, Rexach JE, Sun YE, Coon JJ, Peters EC, Hsieh-Wilson LC. Probing the dynamics of O-GlcNAc glycosylation in the brain using quantitative proteomics. Nat Chem Biol. 2007;3(6):339–348. doi: 10.1038/nchembio881. [DOI] [PubMed] [Google Scholar]
- 34.Alfaro JF, Gong CX, Monroe ME, Aldrich JT, Clauss TR, Purvine SO, Wang Z, Camp DG, 2nd, Shabanowitz J, Stanley P, Hart GW, Hunt DF, Yang F, Smith RD. Tandem mass spectrometry identifies many mouse brain O-GlcNAcylated proteins including EGF domain-specific O-GlcNAc transferase targets. Proc Natl Acad Sci USA. 2012;109(19):7280–7285. doi: 10.1073/pnas.1200425109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Trinidad JC, Barkan DT, Gulledge BF, Thalhammer A, Sali A, Schoepfer R, Burlingame AL. Global identification and characterization of both O-GlcNAcylation and phosphorylation at the murine synapse. Mol Cell Proteom. 2012;11(8):215–229. doi: 10.1074/mcp.O112.018366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Maury JJ, Ng D, Bi X, Bardor M, Choo AB. Multiple reaction monitoring mass spectrometry for the discovery and quantification of O-GlcNAc-modified proteins. Anal Chem. 2014;86(1):395–402. doi: 10.1021/ac401821d. [DOI] [PubMed] [Google Scholar]
- 37.Wang X, Yuan ZF, Fan J, Karch KR, Ball LE, Denu JM, Garcia BA. A novel quantitative mass spectrometry platform for determining protein O-GlcNAcylation dynamics. Mol Cell Proteom. 2016;15(7):2462–2475. doi: 10.1074/mcp.O115.049627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kazemi Z, Chang H, Haserodt S, McKen C, Zachara NE. O-linked beta-N-acetylglucosamine (O-GlcNAc) regulates stress-induced heat shock protein expression in a GSK-3beta-dependent manner. J Biol Chem. 2010;285(50):39096–39107. doi: 10.1074/jbc.M110.131102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lim KH, Chang HI. O-linked N-acetylglucosamine suppresses thermal aggregation of Sp1. FEBS Lett. 2006;580(19):4645–4652. doi: 10.1016/j.febslet.2006.07.040. [DOI] [PubMed] [Google Scholar]
- 40.Olivier-Van Stichelen S, Dehennaut V, Buzy A, Zachayus JL, Guinez C, Mir AM, El Yazidi-Belkoura I, Copin MC, Boureme D, Loyaux D, Ferrara P, Lefebvre T. O-GlcNAcylation stabilizes beta-catenin through direct competition with phosphorylation at threonine 41. FASEB J. 2014;28(8):3325–3338. doi: 10.1096/fj.13-243535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Du XL, Edelstein D, Dimmeler S, Ju Q, Sui C, Brownlee M. Hyperglycemia inhibits endothelial nitric oxide synthase activity by posttranslational modification at the Akt site. J Clin Investig. 2001;108(9):1341–1348. doi: 10.1172/JCI11235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Federici M, Menghini R, Mauriello A, Hribal ML, Ferrelli F, Lauro D, Sbraccia P, Spagnoli LG, Sesti G, Lauro R. Insulin-dependent activation of endothelial nitric oxide synthase is impaired by O-linked glycosylation modification of signaling proteins in human coronary endothelial cells. Circulation. 2002;106(4):466–472. doi: 10.1161/01.CIR.0000023043.02648.51. [DOI] [PubMed] [Google Scholar]
- 43.Yi W, Clark PM, Mason DE, Keenan MC, Hill C, Goddard WA, 3rd, Peters EC, Driggers EM, Hsieh-Wilson LC. Phosphofructokinase 1 glycosylation regulates cell growth and metabolism. Science (New York, NY) 2012;337(6097):975–980. doi: 10.1126/science.1222278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Charoensuksai P, Kuhn P, Wang L, Sherer N, Xu W. O-GlcNAcylation of co-activator-associated arginine methyltransferase 1 regulates its protein substrate specificity. Biochem J. 2015;466(3):587–599. doi: 10.1042/BJ20141072. [DOI] [PubMed] [Google Scholar]
- 45.Schindler C, Levy DE, Decker T. JAK-STAT signaling: from interferons to cytokines. J Biol Chem. 2007;282(28):20059–20063. doi: 10.1074/jbc.R700016200. [DOI] [PubMed] [Google Scholar]
- 46.Gewinner C, Hart G, Zachara N, Cole R, Beisenherz-Huss C, Groner B. The coactivator of transcription CREB-binding protein interacts preferentially with the glycosylated form of Stat5. J Biol Chem. 2004;279(5):3563–3572. doi: 10.1074/jbc.M306449200. [DOI] [PubMed] [Google Scholar]
- 47.Gordon S, Akopyan G, Garban H, Bonavida B. Transcription factor YY1: structure, function, and therapeutic implications in cancer biology. Oncogene. 2006;25(8):1125–1142. doi: 10.1038/sj.onc.1209080. [DOI] [PubMed] [Google Scholar]
- 48.Hiromura M, Choi CH, Sabourin NA, Jones H, Bachvarov D, Usheva A. YY1 is regulated by O-linked N-acetylglucosaminylation (O-glcNAcylation) J Biol Chem. 2003;278(16):14046–14052. doi: 10.1074/jbc.M300789200. [DOI] [PubMed] [Google Scholar]
- 49.Forsythe ME, Love DC, Lazarus BD, Kim EJ, Prinz WA, Ashwell G, Krause MW, Hanover JA. Caenorhabditis elegans ortholog of a diabetes susceptibility locus: oga-1 (O-GlcNAcase) knockout impacts O-GlcNAc cycling, metabolism, and dauer. Proc Natl Acad Sci USA. 2006;103(32):11952–11957. doi: 10.1073/pnas.0601931103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wang Z, Gucek M, Hart GW. Cross-talk between GlcNAcylation and phosphorylation: site-specific phosphorylation dynamics in response to globally elevated O-GlcNAc. Proc Natl Acad Sci USA. 2008;105(37):13793–13798. doi: 10.1073/pnas.0806216105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Slawson C, Hart GW. O-GlcNAc signalling: implications for cancer cell biology. Nat Rev Cancer. 2011;11(9):678–684. doi: 10.1038/nrc3114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Love DC. Hanover JA (2005) The hexosamine signaling pathway: deciphering the “O-GlcNAc code”. Science’s STKE. 2005;312:re13. doi: 10.1126/stke.3122005re13. [DOI] [PubMed] [Google Scholar]
- 53.Kreppel LK, Blomberg MA, Hart GW. Dynamic glycosylation of nuclear and cytosolic proteins. Cloning and characterization of a unique O-GlcNAc transferase with multiple tetratricopeptide repeats. J Biol Chem. 1997;272(14):9308–9315. doi: 10.1074/jbc.272.14.9308. [DOI] [PubMed] [Google Scholar]
- 54.Gao Y, Wells L, Comer FI, Parker GJ, Hart GW. Dynamic O-glycosylation of nuclear and cytosolic proteins: cloning and characterization of a neutral, cytosolic beta-N-acetylglucosaminidase from human brain. J Biol Chem. 2001;276(13):9838–9845. doi: 10.1074/jbc.M010420200. [DOI] [PubMed] [Google Scholar]
- 55.Nolte D, Muller U. Human O-GlcNAc transferase (OGT): genomic structure, analysis of splice variants, fine mapping in Xq13.1. Mamm Genome. 2002;13(1):62–64. doi: 10.1007/s00335-001-2108-9. [DOI] [PubMed] [Google Scholar]
- 56.Lubas WA, Hanover JA. Functional expression of O-linked GlcNAc transferase. Domain structure and substrate specificity. J Biol Chem. 2000;275(15):10983–10988. doi: 10.1074/jbc.275.15.10983. [DOI] [PubMed] [Google Scholar]
- 57.Blatch GL, Lassle M. The tetratricopeptide repeat: a structural motif mediating protein-protein interactions. BioEssays. 1999;21(11):932–939. doi: 10.1002/(SICI)1521-1878(199911)21:11<932::AID-BIES5>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- 58.Jinek M, Rehwinkel J, Lazarus BD, Izaurralde E, Hanover JA, Conti E. The superhelical TPR-repeat domain of O-linked GlcNAc transferase exhibits structural similarities to importin alpha. Nat Struct Mol Biol. 2004;11(10):1001–1007. doi: 10.1038/nsmb833. [DOI] [PubMed] [Google Scholar]
- 59.Whisenhunt TR, Yang X, Bowe DB, Paterson AJ, Van Tine BA, Kudlow JE. Disrupting the enzyme complex regulating O-GlcNAcylation blocks signaling and development. Glycobiology. 2006;16(6):551–563. doi: 10.1093/glycob/cwj096. [DOI] [PubMed] [Google Scholar]
- 60.Cheung WD, Hart GW. AMP-activated protein kinase and p38 MAPK activate O-GlcNAcylation of neuronal proteins during glucose deprivation. J Biol Chem. 2008;283(19):13009–13020. doi: 10.1074/jbc.M801222200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Yang X, Ongusaha PP, Miles PD, Havstad JC, Zhang F, So WV, Kudlow JE, Michell RH, Olefsky JM, Field SJ, Evans RM. Phosphoinositide signalling links O-GlcNAc transferase to insulin resistance. Nature. 2008;451(7181):964–969. doi: 10.1038/nature06668. [DOI] [PubMed] [Google Scholar]
- 62.Lazarus MB, Nam Y, Jiang J, Sliz P, Walker S. Structure of human O-GlcNAc transferase and its complex with a peptide substrate. Nature. 2011;469(7331):564–567. doi: 10.1038/nature09638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Nemeth AH, Nolte D, Dunne E, Niemann S, Kostrzewa M, Peters U, Fraser E, Bochukova E, Butler R, Brown J, Cox RD, Levy ER, Ropers HH, Monaco AP, Muller U. Refined linkage disequilibrium and physical mapping of the gene locus for X-linked dystonia-parkinsonism (DYT3) Genomics. 1999;60(3):320–329. doi: 10.1006/geno.1999.5929. [DOI] [PubMed] [Google Scholar]
- 64.Kemppinen AK, Kaprio J, Palotie A, Saarela J. Systematic review of genome-wide expression studies in multiple sclerosis. BMJ Open. 2011;1(1):e000053. doi: 10.1136/bmjopen-2011-000053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Hewagama A, Gorelik G, Patel D, Liyanarachchi P, McCune WJ, Somers E, Gonzalez-Rivera T, Strickland F, Richardson B. Overexpression of X-linked genes in T cells from women with lupus. J Autoimmun. 2013;41:60–71. doi: 10.1016/j.jaut.2012.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Comtesse N, Maldener E, Meese E. Identification of a nuclear variant of MGEA5, a cytoplasmic hyaluronidase and a beta-N-acetylglucosaminidase. Biochem Biophys Res Commun. 2001;283(3):634–640. doi: 10.1006/bbrc.2001.4815. [DOI] [PubMed] [Google Scholar]
- 67.Butkinaree C, Cheung WD, Park S, Park K, Barber M, Hart GW. Characterization of beta-N-acetylglucosaminidase cleavage by caspase-3 during apoptosis. J Biol Chem. 2008;283(35):23557–23566. doi: 10.1074/jbc.M804116200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Hart GW, West CM, et al. Nucleocytoplasmic Glycosylation. In: Varki A, Cummings RD, Esko JD, et al., editors. Essentials of glycobiology. New York: Cold Spring Harbor Laboratory Press; 2009. [PubMed] [Google Scholar]
- 69.Hart GW, Akimoto Y, et al. The O-GlcNAc modification. In: Varki A, Cummings RD, Esko JD, et al., editors. Essentials of glycobiology. New York: Cold Spring Harbor Laboratory Press; 2009. [Google Scholar]
- 70.Bertram L, Blacker D, Mullin K, Keeney D, Jones J, Basu S, Yhu S, McInnis MG, Go RC, Vekrellis K, Selkoe DJ, Saunders AJ, Tanzi RE. Evidence for genetic linkage of Alzheimer’s disease to chromosome 10q. Science (New York, NY) 2000;290(5500):2302–2303. doi: 10.1126/science.290.5500.2302. [DOI] [PubMed] [Google Scholar]
- 71.Van Tine BA, Patterson A, Kudlow JE. Assignment of N-acetyl-d-glucosaminidase (Mgea5) to rat chromosome 1q5 by tyramide fluorescence in situ hybridization (T-FISH): synteny between rat, mouse and human with Insulin Degradation Enzyme (IDE) Cytogenet Genome Res. 2003;103(1–2):202b. doi: 10.1159/000076313. [DOI] [PubMed] [Google Scholar]
- 72.Horsch M, Hoesch L, Vasella A, Rast DM. N-acetylglucosaminono-1,5-lactone oxime and the corresponding (phenylcarbamoyl)oxime. Novel and potent inhibitors of beta-N-acetylglucosaminidase. Eur J Biochem. 1991;197(3):815–818. doi: 10.1111/j.1432-1033.1991.tb15976.x. [DOI] [PubMed] [Google Scholar]
- 73.Dong DL, Hart GW. Purification and characterization of an O-GlcNAc selective N-acetyl-beta-d-glucosaminidase from rat spleen cytosol. J Biol Chem. 1994;269(30):19321–19330. [PubMed] [Google Scholar]
- 74.Macauley MS, Whitworth GE, Debowski AW, Chin D, Vocadlo DJ. O-GlcNAcase uses substrate-assisted catalysis: kinetic analysis and development of highly selective mechanism-inspired inhibitors. J Biol Chem. 2005;280(27):25313–25322. doi: 10.1074/jbc.M413819200. [DOI] [PubMed] [Google Scholar]
- 75.Ji S, Park SY, Roth J, Kim HS, Cho JW. O-GlcNAc modification of PPARgamma reduces its transcriptional activity. Biochem Biophys Res Commun. 2012;417(4):1158–1163. doi: 10.1016/j.bbrc.2011.12.086. [DOI] [PubMed] [Google Scholar]
- 76.Mehdy A, Morelle W, Rosnoblet C, Legrand D, Lefebvre T, Duvet S, Foulquier F. PUGNAc treatment leads to an unusual accumulation of free oligosaccharides in CHO cells. J Biochem. 2012;151(4):439–446. doi: 10.1093/jb/mvs012. [DOI] [PubMed] [Google Scholar]
- 77.Shan X, Vocadlo DJ, Krieger C. Reduced protein O-glycosylation in the nervous system of the mutant SOD1 transgenic mouse model of amyotrophic lateral sclerosis. Neurosci Lett. 2012;516(2):296–301. doi: 10.1016/j.neulet.2012.04.018. [DOI] [PubMed] [Google Scholar]
- 78.Yuzwa SA, Macauley MS, Heinonen JE, Shan X, Dennis RJ, He Y, Whitworth GE, Stubbs KA, McEachern EJ, Davies GJ, Vocadlo DJ. A potent mechanism-inspired O-GlcNAcase inhibitor that blocks phosphorylation of tau in vivo. Nat Chem Biol. 2008;4(8):483–490. doi: 10.1038/nchembio.96. [DOI] [PubMed] [Google Scholar]
- 79.Andres-Bergos J, Tardio L, Larranaga-Vera A, Gomez R, Herrero-Beaumont G, Largo R. The increase in O-linked N-acetylglucosamine protein modification stimulates chondrogenic differentiation both in vitro and in vivo. J Biol Chem. 2012;287(40):33615–33628. doi: 10.1074/jbc.M112.354241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Yu Y, Zhang L, Li X, Run X, Liang Z, Li Y, Liu Y, Lee MH, Grundke-Iqbal I, Iqbal K, Vocadlo DJ, Liu F, Gong CX. Differential effects of an O-GlcNAcase inhibitor on tau phosphorylation. PLoS One. 2012;7(4):e35277. doi: 10.1371/journal.pone.0035277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Yuzwa SA, Shan X, Macauley MS, Clark T, Skorobogatko Y, Vosseller K, Vocadlo DJ. Increasing O-GlcNAc slows neurodegeneration and stabilizes tau against aggregation. Nat Chem Biol. 2012;8(4):393–399. doi: 10.1038/nchembio.797. [DOI] [PubMed] [Google Scholar]
- 82.Graham DL, Gray AJ, Joyce JA, Yu D, O’Moore J, Carlson GA, Shearman MS, Dellovade TL, Hering H. Increased O-GlcNAcylation reduces pathological tau without affecting its normal phosphorylation in a mouse model of tauopathy. Neuropharmacology. 2014;79:307–313. doi: 10.1016/j.neuropharm.2013.11.025. [DOI] [PubMed] [Google Scholar]
- 83.Taylor EW, Wang K, Nelson AR, Bredemann TM, Fraser KB, Clinton SM, Puckett R, Marchase RB, Chatham JC, McMahon LL. O-GlcNAcylation of AMPA receptor GluA2 is associated with a novel form of long-term depression at hippocampal synapses. J Neurosci. 2014;34(1):10–21. doi: 10.1523/JNEUROSCI.4761-12.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Lubas WA, Frank DW, Krause M, Hanover JA. O-Linked GlcNAc transferase is a conserved nucleocytoplasmic protein containing tetratricopeptide repeats. J Biol Chem. 1997;272(14):9316–9324. doi: 10.1074/jbc.272.14.9316. [DOI] [PubMed] [Google Scholar]
- 85.Okuyama R, Marshall S. UDP-N-acetylglucosaminyl transferase (OGT) in brain tissue: temperature sensitivity and subcellular distribution of cytosolic and nuclear enzyme. J Neurochem. 2003;86(5):1271–1280. doi: 10.1046/j.1471-4159.2003.01939.x. [DOI] [PubMed] [Google Scholar]
- 86.Gong CX, Liu F, Iqbal K. O-GlcNAcylation: a regulator of tau pathology and neurodegeneration. Alzheimer’s Dementia. 2016 doi: 10.1016/j.jalz.2016.02.011. [DOI] [PubMed] [Google Scholar]
- 87.Liu Y, Li X, Yu Y, Shi J, Liang Z, Run X, Li Y, Dai CL, Grundke-Iqbal I, Iqbal K, Liu F, Gong CX. Developmental regulation of protein O-GlcNAcylation, O-GlcNAc transferase, and O-GlcNAcase in mammalian brain. PLoS One. 2012;7(8):e43724. doi: 10.1371/journal.pone.0043724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Rex-Mathes M, Werner S, Strutas D, Griffith LS, Viebahn C, Thelen K, Schmitz B. O-GlcNAc expression in developing and ageing mouse brain. Biochimie. 2001;83(7):583–590. doi: 10.1016/S0300-9084(01)01305-0. [DOI] [PubMed] [Google Scholar]
- 89.Liu K, Paterson AJ, Zhang F, McAndrew J, Fukuchi K, Wyss JM, Peng L, Hu Y, Kudlow JE. Accumulation of protein O-GlcNAc modification inhibits proteasomes in the brain and coincides with neuronal apoptosis in brain areas with high O-GlcNAc metabolism. J Neurochem. 2004;89(4):1044–1055. doi: 10.1111/j.1471-4159.2004.02389.x. [DOI] [PubMed] [Google Scholar]
- 90.Yang YR, Song S, Hwang H, Jung JH, Kim SJ, Yoon S, Hur JH, Park JI, Lee C, Nam D, Seo YK, Kim JH, Rhim H, Suh PG. Memory and synaptic plasticity are impaired by dysregulated hippocampal O-GlcNAcylation. Sci Rep. 2017;7:44921. doi: 10.1038/srep44921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.O’Donnell N, Zachara NE, Hart GW, Marth JD. Ogt-dependent X-chromosome-linked protein glycosylation is a requisite modification in somatic cell function and embryo viability. Mol Cell Biol. 2004;24(4):1680–1690. doi: 10.1128/MCB.24.4.1680-1690.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Ning X, Tao T, Shen J, Ji Y, Xie L, Wang H, Liu N, Xu X, Sun C, Zhang D, Shen A, Ke K. The O-GlcNAc modification of CDK5 involved in neuronal apoptosis following in vitro intracerebral hemorrhage. Cell Mol Neurobiol. 2017;37(3):527–536. doi: 10.1007/s10571-016-0391-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Kim SJ, Yoo WS, Choi M, Chung I, Yoo JM, Choi WS. Increased O-GlcNAcylation of NF-kappaB enhances retinal ganglion cell death in streptozotocin-induced diabetic retinopathy. Curr Eye Res. 2016;41(2):249–257. doi: 10.3109/02713683.2015.1006372. [DOI] [PubMed] [Google Scholar]
- 94.Wang P, Lazarus B, Forsythe M, Love D, Krause M, Hanover J. O-GlcNAc cycling mutants modulate proteotoxicity in Caenorhabditis elegans models of human neurodegenerative diseases. Proc Natl Acad Sci USA. 2012;109(43):17669–17674. doi: 10.1073/pnas.1205748109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Lagerlof O, Slocomb JE, Hong I, Aponte Y, Blackshaw S, Hart GW, Huganir RL. The nutrient sensor OGT in PVN neurons regulates feeding. Science (New York, NY) 2016;351(6279):1293–1296. doi: 10.1126/science.aad5494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Lagerlof O, Hart GW, Huganir RL. O-GlcNAc transferase regulates excitatory synapse maturity. Proc Natl Acad Sci USA. 2017;114(7):1684–1689. doi: 10.1073/pnas.1621367114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Lagerlof O, Hart GW. O-GlcNAcylation of neuronal proteins: roles in neuronal functions and in neurodegeneration. Adv Neurobiol. 2014;9:343–366. doi: 10.1007/978-1-4939-1154-7_16. [DOI] [PubMed] [Google Scholar]
- 98.Wang AC, Jensen EH, Rexach JE, Vinters HV, Hsieh-Wilson LC. Loss of O-GlcNAc glycosylation in forebrain excitatory neurons induces neurodegeneration. Proc Natl Acad Sci USA. 2016;113(52):15120–15125. doi: 10.1073/pnas.1606899113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.As Association. 2016 Alzheimer’s disease facts and figures. Alzheimer’s Dementia. 2016;12(4):459–509. doi: 10.1016/j.jalz.2016.03.001. [DOI] [PubMed] [Google Scholar]
- 100.Fukuyama H, Ogawa M, Yamauchi H, Yamaguchi S, Kimura J, Yonekura Y, Konishi J. Altered cerebral energy metabolism in Alzheimer’s disease: a PET study. J Nucl Med. 1994;35(1):1–6. [PubMed] [Google Scholar]
- 101.Drzezga A, Lautenschlager N, Siebner H, Riemenschneider M, Willoch F, Minoshima S, Schwaiger M, Kurz A. Cerebral metabolic changes accompanying conversion of mild cognitive impairment into Alzheimer’s disease: a PET follow-up study. Eur J Nucl Med Mol Imaging. 2003;30(8):1104–1113. doi: 10.1007/s00259-003-1194-1. [DOI] [PubMed] [Google Scholar]
- 102.Hoyer S. Glucose metabolism and insulin receptor signal transduction in Alzheimer disease. Eur J Pharmacol. 2004;490(1–3):115–125. doi: 10.1016/j.ejphar.2004.02.049. [DOI] [PubMed] [Google Scholar]
- 103.Cai H, Cong WN, Ji S, Rothman S, Maudsley S, Martin B. Metabolic dysfunction in Alzheimer’s disease and related neurodegenerative disorders. Curr Alzheimer Res. 2012;9(1):5–17. doi: 10.2174/156720512799015064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Protas HD, Chen K, Langbaum JB, Fleisher AS, Alexander GE, Lee W, Bandy D, de Leon MJ, Mosconi L, Buckley S, Truran-Sacrey D, Schuff N, Weiner MW, Caselli RJ, Reiman EM. Posterior cingulate glucose metabolism, hippocampal glucose metabolism, and hippocampal volume in cognitively normal, late-middle-aged persons at 3 levels of genetic risk for Alzheimer disease. JAMA Neurol. 2013;70(3):320–325. doi: 10.1001/2013.jamaneurol.286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Edison P, Archer HA, Hinz R, Hammers A, Pavese N, Tai YF, Hotton G, Cutler D, Fox N, Kennedy A, Rossor M, Brooks DJ. Amyloid, hypometabolism, and cognition in Alzheimer disease: an [11C]PIB and [18F]FDG PET study. Neurology. 2007;68(7):501–508. doi: 10.1212/01.wnl.0000244749.20056.d4. [DOI] [PubMed] [Google Scholar]
- 106.Burns CM, Chen K, Kaszniak AW, Lee W, Alexander GE, Bandy D, Fleisher AS, Caselli RJ, Reiman EM. Higher serum glucose levels are associated with cerebral hypometabolism in Alzheimer regions. Neurology. 2013;80(17):1557–1564. doi: 10.1212/WNL.0b013e31828f17de. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Yaffe K, Blackwell T, Kanaya AM, Davidowitz N, Barrett-Connor E, Krueger K. Diabetes, impaired fasting glucose, and development of cognitive impairment in older women. Neurology. 2004;63(4):658–663. doi: 10.1212/01.WNL.0000134666.64593.BA. [DOI] [PubMed] [Google Scholar]
- 108.Liu Y, Liu F, Iqbal K, Grundke-Iqbal I, Gong CX. Decreased glucose transporters correlate to abnormal hyperphosphorylation of tau in Alzheimer disease. FEBS Lett. 2008;582(2):359–364. doi: 10.1016/j.febslet.2007.12.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.de la Monte SM, Wands JR. Alzheimer’s disease is type 3 diabetes-evidence reviewed. J Diabetes Sci Technol. 2008;2(6):1101–1113. doi: 10.1177/193229680800200619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Liu Y, Liu F, Grundke-Iqbal I, Iqbal K, Gong CX. Brain glucose transporters, O-GlcNAcylation and phosphorylation of tau in diabetes and Alzheimer’s disease. J Neurochem. 2009;111(1):242–249. doi: 10.1111/j.1471-4159.2009.06320.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Raffaitin C, Feart C, Le Goff M, Amieva H, Helmer C, Akbaraly TN, Tzourio C, Gin H, Barberger-Gateau P. Metabolic syndrome and cognitive decline in French elders: the Three-City Study. Neurology. 2011;76(6):518–525. doi: 10.1212/WNL.0b013e31820b7656. [DOI] [PubMed] [Google Scholar]
- 112.Liu Y, Liu F, Grundke-Iqbal I, Iqbal K, Gong CX. Deficient brain insulin signalling pathway in Alzheimer’s disease and diabetes. J Pathol. 2011;225(1):54–62. doi: 10.1002/path.2912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Frolich L, Blum-Degen D, Bernstein HG, Engelsberger S, Humrich J, Laufer S, Muschner D, Thalheimer A, Turk A, Hoyer S, Zochling R, Boissl KW, Jellinger K, Riederer P. Brain insulin and insulin receptors in aging and sporadic Alzheimer’s disease. J Neural Transm. 1998;105(4–5):423–438. doi: 10.1007/s007020050068. [DOI] [PubMed] [Google Scholar]
- 114.Moloney AM, Griffin RJ, Timmons S, O’Connor R, Ravid R, O’Neill C. Defects in IGF-1 receptor, insulin receptor and IRS-1/2 in Alzheimer’s disease indicate possible resistance to IGF-1 and insulin signalling. Neurobiol Aging. 2010;31(2):224–243. doi: 10.1016/j.neurobiolaging.2008.04.002. [DOI] [PubMed] [Google Scholar]
- 115.Talbot K, Wang HY, Kazi H, Han LY, Bakshi KP, Stucky A, Fuino RL, Kawaguchi KR, Samoyedny AJ, Wilson RS, Arvanitakis Z, Schneider JA, Wolf BA, Bennett DA, Trojanowski JQ, Arnold SE. Demonstrated brain insulin resistance in Alzheimer’s disease patients is associated with IGF-1 resistance, IRS-1 dysregulation, and cognitive decline. J Clin Investig. 2012;122(4):1316–1338. doi: 10.1172/JCI59903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Steen E, Terry BM, Rivera EJ, Cannon JL, Neely TR, Tavares R, Xu XJ, Wands JR, de la Monte SM. Impaired insulin and insulin-like growth factor expression and signaling mechanisms in Alzheimer’s disease—is this type 3 diabetes? J Alzheimer’s Dis. 2005;7(1):63–80. doi: 10.3233/JAD-2005-7107. [DOI] [PubMed] [Google Scholar]
- 117.Liu F, Shi J, Tanimukai H, Gu J, Gu J, Grundke-Iqbal I, Iqbal K, Gong CX. Reduced O-GlcNAcylation links lower brain glucose metabolism and tau pathology in Alzheimer’s disease. Brain. 2009;132(Pt 7):1820–1832. doi: 10.1093/brain/awp099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Guo T, Noble W, Hanger DP. Roles of tau protein in health and disease. Acta Neuropathol. 2017 doi: 10.1007/s00401-017-1707-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Ksiezak-Reding H, Liu WK, Yen SH. Phosphate analysis and dephosphorylation of modified tau associated with paired helical filaments. Brain Res. 1992;597(2):209–219. doi: 10.1016/0006-8993(92)91476-U. [DOI] [PubMed] [Google Scholar]
- 120.Lindwall G, Cole RD. Phosphorylation affects the ability of tau protein to promote microtubule assembly. J Biol Chem. 1984;259(8):5301–5305. [PubMed] [Google Scholar]
- 121.Sengupta A, Kabat J, Novak M, Wu Q, Grundke-Iqbal I, Iqbal K. Phosphorylation of tau at both Thr 231 and Ser 262 is required for maximal inhibition of its binding to microtubules. Arch Biochem Biophys. 1998;357(2):299–309. doi: 10.1006/abbi.1998.0813. [DOI] [PubMed] [Google Scholar]
- 122.Cho JH, Johnson GV. Primed phosphorylation of tau at Thr231 by glycogen synthase kinase 3beta (GSK3beta) plays a critical role in regulating tau’s ability to bind and stabilize microtubules. J Neurochem. 2004;88(2):349–358. doi: 10.1111/j.1471-4159.2004.02155.x. [DOI] [PubMed] [Google Scholar]
- 123.Grundke-Iqbal I, Iqbal K, Quinlan M, Tung YC, Zaidi MS, Wisniewski HM. Microtubule-associated protein tau. A component of Alzheimer paired helical filaments. J Biol Chem. 1986;261(13):6084–6089. [PubMed] [Google Scholar]
- 124.Nukina N, Kosik KS, Selkoe DJ. Recognition of Alzheimer paired helical filaments by monoclonal neurofilament antibodies is due to crossreaction with tau protein. Proc Natl Acad Sci USA. 1987;84(10):3415–3419. doi: 10.1073/pnas.84.10.3415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Alonso A, Zaidi T, Novak M, Grundke-Iqbal I, Iqbal K. Hyperphosphorylation induces self-assembly of tau into tangles of paired helical filaments/straight filaments. Proc Natl Acad Sci USA. 2001;98(12):6923–6928. doi: 10.1073/pnas.121119298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Le Corre S, Klafki HW, Plesnila N, Hubinger G, Obermeier A, Sahagun H, Monse B, Seneci P, Lewis J, Eriksen J, Zehr C, Yue M, McGowan E, Dickson DW, Hutton M, Roder HM. An inhibitor of tau hyperphosphorylation prevents severe motor impairments in tau transgenic mice. Proc Natl Acad Sci USA. 2006;103(25):9673–9678. doi: 10.1073/pnas.0602913103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Uno Y, Iwashita H, Tsukamoto T, Uchiyama N, Kawamoto T, Kori M, Nakanishi A. Efficacy of a novel, orally active GSK-3 inhibitor 6-Methyl-N-[3-[[3-(1-methylethoxy)propyl]carbamoyl]-1H-pyrazol-4-yl]pyridine-3-carboxamide in tau transgenic mice. Brain Res. 2009;1296:148–163. doi: 10.1016/j.brainres.2009.08.034. [DOI] [PubMed] [Google Scholar]
- 128.Santacruz K, Lewis J, Spires T, Paulson J, Kotilinek L, Ingelsson M, Guimaraes A, DeTure M, Ramsden M, McGowan E, Forster C, Yue M, Orne J, Janus C, Mariash A, Kuskowski M, Hyman B, Hutton M, Ashe KH. Tau suppression in a neurodegenerative mouse model improves memory function. Science (New York, NY) 2005;309(5733):476–481. doi: 10.1126/science.1113694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Frost B, Jacks RL, Diamond MI. Propagation of tau misfolding from the outside to the inside of a cell. J Biol Chem. 2009;284(19):12845–12852. doi: 10.1074/jbc.M808759200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Arnold CS, Johnson GV, Cole RN, Dong DL, Lee M, Hart GW. The microtubule-associated protein tau is extensively modified with O-linked N-acetylglucosamine. J Biol Chem. 1996;271(46):28741–28744. doi: 10.1074/jbc.271.46.28741. [DOI] [PubMed] [Google Scholar]
- 131.Yuzwa SA, Yadav AK, Skorobogatko Y, Clark T, Vosseller K, Vocadlo DJ. Mapping O-GlcNAc modification sites on tau and generation of a site-specific O-GlcNAc tau antibody. Amino Acids. 2011;40(3):857–868. doi: 10.1007/s00726-010-0705-1. [DOI] [PubMed] [Google Scholar]
- 132.Wang Z, Udeshi ND, O’Malley M, Shabanowitz J, Hunt DF, Hart GW. Enrichment and site mapping of O-linked N-acetylglucosamine by a combination of chemical/enzymatic tagging, photochemical cleavage, and electron transfer dissociation mass spectrometry. Mol Cell Proteom. 2010;9(1):153–160. doi: 10.1074/mcp.M900268-MCP200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Morris M, Knudsen GM, Maeda S, Trinidad JC, Ioanoviciu A, Burlingame AL, Mucke L. Tau post-translational modifications in wild-type and human amyloid precursor protein transgenic mice. Nat Neurosci. 2015;18(8):1183–1189. doi: 10.1038/nn.4067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Liu F, Iqbal K, Grundke-Iqbal I, Hart GW, Gong CX. O-GlcNAcylation regulates phosphorylation of tau: a mechanism involved in Alzheimer’s disease. Proc Natl Acad Sci USA. 2004;101(29):10804–10809. doi: 10.1073/pnas.0400348101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Li X, Lu F, Wang JZ, Gong CX. Concurrent alterations of O-GlcNAcylation and phosphorylation of tau in mouse brains during fasting. Eur J Neurosci. 2006;23(8):2078–2086. doi: 10.1111/j.1460-9568.2006.04735.x. [DOI] [PubMed] [Google Scholar]
- 136.Gatta E, Lefebvre T, Gaetani S, dos Santos M, Marrocco J, Mir AM, Cassano T, Maccari S, Nicoletti F, Mairesse J. Evidence for an imbalance between tau O-GlcNAcylation and phosphorylation in the hippocampus of a mouse model of Alzheimer’s disease. Pharmacol Res. 2016;105:186–197. doi: 10.1016/j.phrs.2016.01.006. [DOI] [PubMed] [Google Scholar]
- 137.Lefebvre T, Ferreira S, Dupont-Wallois L, Bussiere T, Dupire MJ, Delacourte A, Michalski JC, Caillet-Boudin ML. Evidence of a balance between phosphorylation and O-GlcNAc glycosylation of Tau proteins—a role in nuclear localization. Biochem Biophys Acta. 2003;1619(2):167–176. doi: 10.1016/S0304-4165(02)00477-4. [DOI] [PubMed] [Google Scholar]
- 138.Ryu IH, Lee KY, Do SI. Abeta-affected pathogenic induction of S-nitrosylation of OGT and identification of Cys-NO linkage triplet. Biochem Biophys Acta. 2016;1864(5):609–621. doi: 10.1016/j.bbapap.2016.02.003. [DOI] [PubMed] [Google Scholar]
- 139.Lewis J, McGowan E, Rockwood J, Melrose H, Nacharaju P, Van Slegtenhorst M, Gwinn-Hardy K, Paul Murphy M, Baker M, Yu X, Duff K, Hardy J, Corral A, Lin WL, Yen SH, Dickson DW, Davies P, Hutton M. Neurofibrillary tangles, amyotrophy and progressive motor disturbance in mice expressing mutant (P301L) tau protein. Nat Genet. 2000;25(4):402–405. doi: 10.1038/78078. [DOI] [PubMed] [Google Scholar]
- 140.Terwel D, Lasrado R, Snauwaert J, Vandeweert E, Van Haesendonck C, Borghgraef P, Van Leuven F. Changed conformation of mutant Tau-P301L underlies the moribund tauopathy, absent in progressive, nonlethal axonopathy of Tau-4R/2N transgenic mice. J Biol Chem. 2005;280(5):3963–3973. doi: 10.1074/jbc.M409876200. [DOI] [PubMed] [Google Scholar]
- 141.Yuzwa SA, Cheung AH, Okon M, McIntosh LP, Vocadlo DJ. O-GlcNAc modification of tau directly inhibits its aggregation without perturbing the conformational properties of tau monomers. J Mol Biol. 2014;426(8):1736–1752. doi: 10.1016/j.jmb.2014.01.004. [DOI] [PubMed] [Google Scholar]
- 142.Borghgraef P, Menuet C, Theunis C, Louis JV, Devijver H, Maurin H, Smet-Nocca C, Lippens G, Hilaire G, Gijsen H, Moechars D, Van Leuven F. Increasing brain protein O-GlcNAc-ylation mitigates breathing defects and mortality of Tau. P301L mice. PLoS One. 2013;8(12):e84442. doi: 10.1371/journal.pone.0084442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Masters CL, Simms G, Weinman NA, Multhaup G, McDonald BL, Beyreuther K. Amyloid plaque core protein in Alzheimer disease and Down syndrome. Proc Natl Acad Sci USA. 1985;82(12):4245–4249. doi: 10.1073/pnas.82.12.4245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Hardy JA, Higgins GA. Alzheimer’s disease: the amyloid cascade hypothesis. Science (New York, NY) 1992;256(5054):184–185. doi: 10.1126/science.1566067. [DOI] [PubMed] [Google Scholar]
- 145.Hardy J. The discovery of Alzheimer-causing mutations in the APP gene and the formulation of the “amyloid cascade hypothesis”. FEBS J. 2017;284(7):1040–1044. doi: 10.1111/febs.14004. [DOI] [PubMed] [Google Scholar]
- 146.Griffith LS, Mathes M, Schmitz B. Beta-amyloid precursor protein is modified with O-linked N-acetylglucosamine. J Neurosci Res. 1995;41(2):270–278. doi: 10.1002/jnr.490410214. [DOI] [PubMed] [Google Scholar]
- 147.Zheng BW, Yang L, Dai XL, Jiang ZF, Huang HC. Roles of O-GlcNAcylation on amyloid-beta precursor protein processing, tau phosphorylation, and hippocampal synapses dysfunction in Alzheimer’s disease. Neurol Res. 2016;38(2):177–186. doi: 10.1080/01616412.2015.1133485. [DOI] [PubMed] [Google Scholar]
- 148.Jacobsen KT, Iverfeldt K. O-GlcNAcylation increases non-amyloidogenic processing of the amyloid-beta precursor protein (APP) Biochem Biophys Res Commun. 2011;404(3):882–886. doi: 10.1016/j.bbrc.2010.12.080. [DOI] [PubMed] [Google Scholar]
- 149.Chun YS, Park Y, Oh HG, Kim TW, Yang HO, Park MK, Chung S. O-GlcNAcylation promotes non-amyloidogenic processing of amyloid-beta protein precursor via inhibition of endocytosis from the plasma membrane. J Alzheimer’s Dis. 2015;44(1):261–275. doi: 10.3233/JAD-140096. [DOI] [PubMed] [Google Scholar]
- 150.Kim C, Nam DW, Park SY, Song H, Hong HS, Boo JH, Jung ES, Kim Y, Baek JY, Kim KS, Cho JW, Mook-Jung I. O-linked beta-N-acetylglucosaminidase inhibitor attenuates beta-amyloid plaque and rescues memory impairment. Neurobiol Aging. 2013;34(1):275–285. doi: 10.1016/j.neurobiolaging.2012.03.001. [DOI] [PubMed] [Google Scholar]
- 151.Yuzwa SA, Shan X, Jones BA, Zhao G, Woodward ML, Li X, Zhu Y, McEachern EJ, Silverman MA, Watson NV, Gong CX, Vocadlo DJ. Pharmacological inhibition of O-GlcNAcase (OGA) prevents cognitive decline and amyloid plaque formation in bigenic tau/APP mutant mice. Mol Neurodegeneration. 2014;9:42. doi: 10.1186/1750-1326-9-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Xie S, Jin N, Gu J, Shi J, Sun J, Chu D, Zhang L, Dai CL, Gu JH, Gong CX, Iqbal K, Liu F. O-GlcNAcylation of protein kinase A catalytic subunits enhances its activity: a mechanism linked to learning and memory deficits in Alzheimer’s disease. Aging Cell. 2016;15(3):455–464. doi: 10.1111/acel.12449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Samaranch L, Lorenzo-Betancor O, Arbelo JM, Ferrer I, Lorenzo E, Irigoyen J, Pastor MA, Marrero C, Isla C, Herrera-Henriquez J, Pastor P. PINK1-linked parkinsonism is associated with Lewy body pathology. Brain. 2010;133(Pt 4):1128–1142. doi: 10.1093/brain/awq051. [DOI] [PubMed] [Google Scholar]
- 154.Postuma RB, Berg D, Stern M, Poewe W, Olanow CW, Oertel W, Obeso J, Marek K, Litvan I, Lang AE, Halliday G, Goetz CG, Gasser T, Dubois B, Chan P, Bloem BR, Adler CH, Deuschl G. MDS clinical diagnostic criteria for Parkinson’s disease. Mov Disord. 2015;30(12):1591–1601. doi: 10.1002/mds.26424. [DOI] [PubMed] [Google Scholar]
- 155.Lubbe S, Morris HR. Recent advances in Parkinson’s disease genetics. J Neurol. 2014;261(2):259–266. doi: 10.1007/s00415-013-7003-2. [DOI] [PubMed] [Google Scholar]
- 156.Henchcliffe C, Beal MF. Mitochondrial biology and oxidative stress in Parkinson disease pathogenesis. Nat Clin Pract Neurol. 2008;4(11):600–609. doi: 10.1038/ncpneuro0924. [DOI] [PubMed] [Google Scholar]
- 157.Chartier-Harlin MC, Kachergus J, Roumier C, Mouroux V, Douay X, Lincoln S, Levecque C, Larvor L, Andrieux J, Hulihan M, Waucquier N, Defebvre L, Amouyel P, Farrer M, Destee A. Alpha-synuclein locus duplication as a cause of familial Parkinson’s disease. Lancet (London, England) 2004;364(9440):1167–1169. doi: 10.1016/S0140-6736(04)17103-1. [DOI] [PubMed] [Google Scholar]
- 158.Klein C, Westenberger A. Genetics of Parkinson’s disease. Cold Spring Harbor Perspect Med. 2012;2(1):a008888. doi: 10.1101/cshperspect.a008888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Polymeropoulos MH, Lavedan C, Leroy E, Ide SE, Dehejia A, Dutra A, Pike B, Root H, Rubenstein J, Boyer R, Stenroos ES, Chandrasekharappa S, Athanassiadou A, Papapetropoulos T, Johnson WG, Lazzarini AM, Duvoisin RC, Di Iorio G, Golbe LI, Nussbaum RL. Mutation in the alpha-synuclein gene identified in families with Parkinson’s disease. Science (New York, NY) 1997;276(5321):2045–2047. doi: 10.1126/science.276.5321.2045. [DOI] [PubMed] [Google Scholar]
- 160.Kruger R, Kuhn W, Muller T, Woitalla D, Graeber M, Kosel S, Przuntek H, Epplen JT, Schols L, Riess O. Ala30Pro mutation in the gene encoding alpha-synuclein in Parkinson’s disease. Nat Genet. 1998;18(2):106–108. doi: 10.1038/ng0298-106. [DOI] [PubMed] [Google Scholar]
- 161.Singleton AB, Farrer M, Johnson J, Singleton A, Hague S, Kachergus J, Hulihan M, Peuralinna T, Dutra A, Nussbaum R, Lincoln S, Crawley A, Hanson M, Maraganore D, Adler C, Cookson MR, Muenter M, Baptista M, Miller D, Blancato J, Hardy J, Gwinn-Hardy K. Alpha-synuclein locus triplication causes Parkinson’s disease. Science (New York, NY) 2003;302(5646):841. doi: 10.1126/science.1090278. [DOI] [PubMed] [Google Scholar]
- 162.Zarranz JJ, Alegre J, Gomez-Esteban JC, Lezcano E, Ros R, Ampuero I, Vidal L, Hoenicka J, Rodriguez O, Atares B, Llorens V, Gomez Tortosa E, del Ser T, Munoz DG, de Yebenes JG. The new mutation, E46K, of alpha-synuclein causes Parkinson and Lewy body dementia. Ann Neurol. 2004;55(2):164–173. doi: 10.1002/ana.10795. [DOI] [PubMed] [Google Scholar]
- 163.Luk KC, Song C, O’Brien P, Stieber A, Branch JR, Brunden KR, Trojanowski JQ, Lee VM. Exogenous alpha-synuclein fibrils seed the formation of Lewy body-like intracellular inclusions in cultured cells. Proc Natl Acad Sci USA. 2009;106(47):20051–20056. doi: 10.1073/pnas.0908005106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Luk KC, Kehm VM, Zhang B, O’Brien P, Trojanowski JQ, Lee VM. Intracerebral inoculation of pathological alpha-synuclein initiates a rapidly progressive neurodegenerative alpha-synucleinopathy in mice. J Exp Med. 2012;209(5):975–986. doi: 10.1084/jem.20112457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Cremades N, Cohen SI, Deas E, Abramov AY, Chen AY, Orte A, Sandal M, Clarke RW, Dunne P, Aprile FA, Bertoncini CW, Wood NW, Knowles TP, Dobson CM, Klenerman D. Direct observation of the interconversion of normal and toxic forms of alpha-synuclein. Cell. 2012;149(5):1048–1059. doi: 10.1016/j.cell.2012.03.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Fujiwara H, Hasegawa M, Dohmae N, Kawashima A, Masliah E, Goldberg MS, Shen J, Takio K, Iwatsubo T. alpha-Synuclein is phosphorylated in synucleinopathy lesions. Nat Cell Biol. 2002;4(2):160–164. doi: 10.1038/ncb748. [DOI] [PubMed] [Google Scholar]
- 167.Paleologou KE, Schmid AW, Rospigliosi CC, Kim HY, Lamberto GR, Fredenburg RA, Lansbury PT, Jr, Fernandez CO, Eliezer D, Zweckstetter M, Lashuel HA. Phosphorylation at Ser-129 but not the phosphomimics S129E/D inhibits the fibrillation of alpha-synuclein. J Biol Chem. 2008;283(24):16895–16905. doi: 10.1074/jbc.M800747200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Chen L, Periquet M, Wang X, Negro A, McLean PJ, Hyman BT, Feany MB. Tyrosine and serine phosphorylation of alpha-synuclein have opposing effects on neurotoxicity and soluble oligomer formation. J Clin Investig. 2009;119(11):3257–3265. doi: 10.1172/JCI39088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Paleologou KE, Oueslati A, Shakked G, Rospigliosi CC, Kim HY, Lamberto GR, Fernandez CO, Schmid A, Chegini F, Gai WP, Chiappe D, Moniatte M, Schneider BL, Aebischer P, Eliezer D, Zweckstetter M, Masliah E, Lashuel HA. Phosphorylation at S87 is enhanced in synucleinopathies, inhibits alpha-synuclein oligomerization, and influences synuclein-membrane interactions. J Neurosci. 2010;30(9):3184–3198. doi: 10.1523/JNEUROSCI.5922-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Hejjaoui M, Butterfield S, Fauvet B, Vercruysse F, Cui J, Dikiy I, Prudent M, Olschewski D, Zhang Y, Eliezer D, Lashuel HA. Elucidating the role of C-terminal post-translational modifications using protein semisynthesis strategies: alpha-synuclein phosphorylation at tyrosine 125. J Am Chem Soc. 2012;134(11):5196–5210. doi: 10.1021/ja210866j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Brahmachari S, Ge P, Lee SH, Kim D, Karuppagounder SS, Kumar M, Mao X, Shin JH, Lee Y, Pletnikova O, Troncoso JC, Dawson VL, Dawson TM, Ko HS. Activation of tyrosine kinase c-Abl contributes to alpha-synuclein-induced neurodegeneration. J Clin Investig. 2016;126(8):2970–2988. doi: 10.1172/JCI85456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Gomez-Tortosa E, Newell K, Irizarry MC, Sanders JL, Hyman BT. alpha-Synuclein immunoreactivity in dementia with Lewy bodies: morphological staging and comparison with ubiquitin immunostaining. Acta Neuropathol. 2000;99(4):352–357. doi: 10.1007/s004010051135. [DOI] [PubMed] [Google Scholar]
- 173.Alexopoulou Z, Lang J, Perrett RM, Elschami M, Hurry ME, Kim HT, Mazaraki D, Szabo A, Kessler BM, Goldberg AL, Ansorge O, Fulga TA, Tofaris GK. Deubiquitinase Usp8 regulates alpha-synuclein clearance and modifies its toxicity in Lewy body disease. Proc Natl Acad Sci USA. 2016;113(32):E4688–E4697. doi: 10.1073/pnas.1523597113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Shimura H, Schlossmacher MG, Hattori N, Frosch MP, Trockenbacher A, Schneider R, Mizuno Y, Kosik KS, Selkoe DJ. Ubiquitination of a new form of alpha-synuclein by parkin from human brain: implications for Parkinson’s disease. Science (New York, NY) 2001;293(5528):263–269. doi: 10.1126/science.1060627. [DOI] [PubMed] [Google Scholar]
- 175.Kim SJ, Sung JY, Um JW, Hattori N, Mizuno Y, Tanaka K, Paik SR, Kim J, Chung KC. Parkin cleaves intracellular alpha-synuclein inclusions via the activation of calpain. J Biol Chem. 2003;278(43):41890–41899. doi: 10.1074/jbc.M306017200. [DOI] [PubMed] [Google Scholar]
- 176.Marotta NP, Lin Y, Lewis YE, Ambroso MR, Zaro BW, Roth MT, Arnold DB, Langen R, Pratt MR. O-GlcNAc modification blocks the aggregation and toxicity of the protein α-synuclein associated with Parkinson’s disease. Nat Chem. 2015;7(11):913–920. doi: 10.1038/nchem.2361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Lewis YE, Galesic A, Levine PM, De Leon CA, Lamiri N, Brennan CK, Pratt MR. O-GlcNAcylation of alpha-synuclein at serine 87 reduces aggregation without affecting membrane binding. ACS Chem Biol. 2017 doi: 10.1021/acschembio.7b00113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Bork K, Kannicht C, Nohring S, Reutter W, Weidemann W, Hart GW, Horstkorte R. N-propanoylmannosamine interferes with O-GlcNAc modification of the tyrosine 3-monooxygenase and stimulates dopamine secretion. J Neurosci Res. 2008;86(3):647–652. doi: 10.1002/jnr.21526. [DOI] [PubMed] [Google Scholar]
- 179.Nettelbladt CG, Alibergovic A, Ljungqvist O. Pre-stress carbohydrate solution prevents fatal outcome after hemorrhage in 24-hour food-deprived rats. Nutrition (Burbank, Los Angeles County, Calif) 1996;12(10):696–699. doi: 10.1016/S0899-9007(96)00165-7. [DOI] [PubMed] [Google Scholar]
- 180.Jarhult J. Osmotic fluid transfer from tissue to blood during hemorrhagic hypotension. Acta Physiol Scand. 1973;89(2):213–226. doi: 10.1111/j.1748-1716.1973.tb05514.x. [DOI] [PubMed] [Google Scholar]
- 181.Ware J, Ljungqvist O, Norberg KA, Nylander G. Osmolar changes in haemorrhage: the effects of an altered nutritional status. Acta Chir Scand. 1982;148(8):641–646. [PubMed] [Google Scholar]
- 182.Mizock BA. Alterations in fuel metabolism in critical illness: hyperglycaemia. Best Pract Res Clin Endocrinol Metab. 2001;15(4):533–551. doi: 10.1053/beem.2001.0168. [DOI] [PubMed] [Google Scholar]
- 183.Dassanayaka S, Jones SP. O-GlcNAc and the cardiovascular system. Pharmacol Ther. 2014;142(1):62–71. doi: 10.1016/j.pharmthera.2013.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Yang SL, Zou LY, Bounelis P, Chaudry I, Chatham JC, Marchase RB. Glucosamine administration during resuscitation improves organ function after trauma hemorrhage. Shock. 2006;25(6):600–607. doi: 10.1097/01.shk.0000209563.07693.db. [DOI] [PubMed] [Google Scholar]
- 185.Not LG, Marchase RB, Fulop N, Brocks CA, Chatham JC. Glucosamine administration improves survival rate after severe hemorrhagic shock combined with trauma in rats. Shock. 2007;28(3):345–352. doi: 10.1097/shk.0b013e3180487ebb. [DOI] [PubMed] [Google Scholar]
- 186.Fulop N, Zhang Z, Marchase R, Chatham J. Glucosamine cardioprotection in perfused rat hearts associated with increased O-linked N-acetylglucosamine protein modification and altered p38 activation. Am J Physiol Heart Circ Physiol. 2007;292(5):H2227–H2236. doi: 10.1152/ajpheart.01091.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Zou L, Yang S, Hu S, Chaudry I, Marchase R, Chatham J. The protective effects of PUGNAc on cardiac function after trauma-hemorrhage are mediated via increased protein O-GlcNAc levels. Shock. 2007;27(4):402–408. doi: 10.1097/01.shk.0000245031.31859.29. [DOI] [PubMed] [Google Scholar]
- 188.Laczy B, Marsh S, Brocks C, Wittmann I, Chatham J. Inhibition of O-GlcNAcase in perfused rat hearts by NAG-thiazolines at the time of reperfusion is cardioprotective in an O-GlcNAc-dependent manner. Am J Physiol Heart Circ Physiol. 2010;299(5):H1715–H1727. doi: 10.1152/ajpheart.00337.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Lunde I, Aronsen J, Kvaloy H, Qvigstad E, Sjaastad I, Tonnessen T, Christensen G, Gronning-Wang L, Carlson C. Cardiac O-GlcNAc signaling is increased in hypertrophy and heart failure. Physiol Genom. 2011;44(2):162–172. doi: 10.1152/physiolgenomics.00016.2011. [DOI] [PubMed] [Google Scholar]
- 190.Zou LY, Yang SL, Champattanachai V, Hu SH, Chaudry IH, Marchase RB, Chatham JC. Glucosamine improves cardiac function following trauma-hemorrhage by increased protein O-GlcNAcylation and attenuation of NF-kappa B signaling. Am J Physiol Heart Circ Physiol. 2009;296(2):H515–H523. doi: 10.1152/ajpheart.01025.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Shi J, Gu JH, Dai CL, Gu J, Jin X, Sun J, Iqbal K, Liu F, Gong CX. O-GlcNAcylation regulates ischemia-induced neuronal apoptosis through AKT signaling. Sci Rep. 2015;5:14500. doi: 10.1038/srep14500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Liu S, Sheng H, Yu Z, Paschen W, Yang W. O-linked β-N-acetylglucosamine modification of proteins is activated in post-ischemic brains of young but not aged mice: implications for impaired functional recovery from ischemic stress. J Cereb Blood Flow Metab. 2016;36(2):393–398. doi: 10.1177/0271678X15608393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Hwang S, Shin J, Hwang J, Kim S, Shin J, Oh E, Oh S, Kim J, Lee J, Han I. Glucosamine exerts a neuroprotective effect via suppression of inflammation in rat brain ischemia/reperfusion injury. Glia. 2010;58(15):1881–1892. doi: 10.1002/glia.21058. [DOI] [PubMed] [Google Scholar]
- 194.He Y, Ma X, Li D, Hao J. Thiamet G mediates neuroprotection in experimental stroke by modulating microglia/macrophage polarization and inhibiting NF-kappaB p65 signaling. J Cereb Blood Flow Metab. 2016 doi: 10.1177/0271678X16679671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Charriaut-Marlangue C, Margaill I, Represa A, Popovici T, Plotkine M, Ben-Ari Y. Apoptosis and necrosis after reversible focal ischemia: an in situ DNA fragmentation analysis. J Cereb Blood Flow Metab. 1996;16(2):186–194. doi: 10.1097/00004647-199603000-00002. [DOI] [PubMed] [Google Scholar]
- 196.Yamashima T. Hsp70.1 and related lysosomal factors for necrotic neuronal death. J Neurochem. 2012;120(4):477–494. doi: 10.1111/j.1471-4159.2011.07596.x. [DOI] [PubMed] [Google Scholar]
- 197.Chen R, Gong P, Tao T, Gao Y, Shen J, Yan Y, Duan C, Wang J, Liu X. O-GlcNAc glycosylation of nNOS promotes neuronal apoptosis following glutamate excitotoxicity. Cell Mol Neurobiol. 2017 doi: 10.1007/s10571-017-0477-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Grima JC, Daigle JG, Arbez N, Cunningham KC, Zhang K, Ochaba J, Geater C, Morozko E, Stocksdale J, Glatzer JC, Pham JT, Ahmed I, Peng Q, Wadhwa H, Pletnikova O, Troncoso JC, Duan W, Snyder SH, Ranum LP, Thompson LM, Lloyd TE, Ross CA, Rothstein JD. Mutant Huntingtin disrupts the nuclear pore complex. Neuron. 2017;94(1):93–107. doi: 10.1016/j.neuron.2017.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Katzenberger RJ, Chtarbanova S, Rimkus SA, Fischer JA, Kaur G, Seppala JM, Swanson LC, Zajac JE, Ganetzky B, Wassarman DA. Death following traumatic brain injury in Drosophila is associated with intestinal barrier dysfunction. eLife. 2015 doi: 10.7554/eLife.04790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Hu JH, Chernoff K, Pelech S, Krieger C. Protein kinase and protein phosphatase expression in the central nervous system of G93A mSOD over-expressing mice. J Neurochem. 2003;85(2):422–431. doi: 10.1046/j.1471-4159.2003.01669.x. [DOI] [PubMed] [Google Scholar]
- 201.Hu JH, Zhang H, Wagey R, Krieger C, Pelech SL. Protein kinase and protein phosphatase expression in amyotrophic lateral sclerosis spinal cord. J Neurochem. 2003;85(2):432–442. doi: 10.1046/j.1471-4159.2003.01670.x. [DOI] [PubMed] [Google Scholar]
- 202.Honchar MP, Bunge MB, Agrawal HC. In vivo phosphorylation of neurofilament proteins in the central nervous system of immature rat and rabbit. Neurochem Res. 1982;7(4):365–372. doi: 10.1007/BF00965490. [DOI] [PubMed] [Google Scholar]
- 203.Dong DL, Xu ZS, Chevrier MR, Cotter RJ, Cleveland DW, Hart GW. Glycosylation of mammalian neurofilaments. Localization of multiple O-linked N-acetylglucosamine moieties on neurofilament polypeptides L and M. J Biol Chem. 1993;268(22):16679–16687. [PubMed] [Google Scholar]
- 204.Deng Y, Li B, Liu F, Iqbal K, Grundke-Iqbal I, Brandt R, Gong CX. Regulation between O-GlcNAcylation and phosphorylation of neurofilament-M and their dysregulation in Alzheimer disease. FASEB J. 2008;22(1):138–145. doi: 10.1096/fj.07-8309com. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Hauser SL, Chan JR, Oksenberg JR. Multiple sclerosis: prospects and promise. Ann Neurol. 2013;74(3):317–327. doi: 10.1002/ana.24009. [DOI] [PubMed] [Google Scholar]
- 206.Baudoin L, Issad T. O-GlcNAcylation and inflammation: a vast territory to explore. Front Endocrinol. 2014;5:235. doi: 10.3389/fendo.2014.00235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Zhang D, Cai Y, Chen M, Gao L, Shen Y, Huang Z. OGT-mediated O-GlcNAcylation promotes NF-kappaB activation and inflammation in acute pancreatitis. Inflamm Res. 2015;64(12):943–952. doi: 10.1007/s00011-015-0877-y. [DOI] [PubMed] [Google Scholar]
- 208.Kim HB, Lee SW, Mun CH, Yoon JY, Pai J, Shin I, Park YB, Lee SK, Cho JW. O-linked N-acetylglucosamine glycosylation of p65 aggravated the inflammation in both fibroblast-like synoviocytes stimulated by tumor necrosis factor-alpha and mice with collagen induced arthritis. Arthritis Res Ther. 2015;17:248. doi: 10.1186/s13075-015-0762-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Liu R, Ma X, Chen L, Yang Y, Zeng Y, Gao J, Jiang W, Zhang F, Li D, Han B, Han R, Qiu R, Huang W, Wang Y, Hao J. MicroRNA-15b suppresses Th17 differentiation and is associated with pathogenesis of multiple sclerosis by targeting O-GlcNAc transferase. J Immunol. 2017;198(7):2626–2639. doi: 10.4049/jimmunol.1601727. [DOI] [PubMed] [Google Scholar]