Abstract
Carbohydrates are essential nutrients that are used as a primary source of energy. Carbohydrate utilization should be properly controlled, as abnormal regulation of carbohydrate metabolism is associated with diseases, such as diabetes, cardiovascular diseases, and stroke. These metabolic syndromes have become a serious problem in developed countries, and there is an increased need for research examining the influence of carbohydrates on animal physiology. Diets enriched in glucose, a major carbohydrate, are also associated with accelerated aging in several model organisms, including yeast and Caenorhabditis elegans (C. elegans). Genetic factors that mediate the effects of high glucose diets on aging have been identified during the last decade, mostly through the use of C. elegans. In this review, we describe studies that determine the effects of carbohydrate-enriched diets on aging by focusing on the mechanisms through which evolutionarily conserved pathways mediate the lifespan-altering effects of glucose in C. elegans. These include the insulin/insulin-like growth factor-1, sterol-regulatory element-binding protein, and AMP-activated protein kinase signaling pathways. We also discuss the effects of various carbohydrates and carbohydrate-derived metabolites on aging in model organisms and cultured mammalian cells. Finally, we discuss how dietary carbohydrates influence health and aging in humans.
Keywords: Sugar, FOXO, MDT-15, Dihydroxyacetone phosphate, Reactive oxygen species, Longevity
Introduction
Carbohydrates are crucial molecules that are used for various cellular processes, including energy production. Diverse forms of carbohydrates are converted to glucose, which is an essential cellular nutrient and a primary source of energy. However, excessive glucose is associated with metabolic complications. In humans, high blood glucose levels contribute to the development of many chronic diseases, such as diabetes mellitus. Glucose is also one of the most extensively studied dietary nutrients that influence lifespan in several model organisms. In simple eukaryotic organisms, such as Caenorhabditis elegans (C. elegans) and yeast, glucose-enriched diets decrease lifespan. During the last decade, various genetic components that mediate the lifespan-altering effects conferred by high glucose diets have been identified, primarily through the use of C. elegans as a model.
The roundworm C. elegans has been a popular model for aging research for more than 30 years. The main advantage of C. elegans for aging research is its short lifespan of approximately 3 weeks in standard culture conditions. C. elegans is also a powerful system for molecular genetics research, with numerous available mutants, a feeding RNAi system, and transgenesis or genome editing using CRISPR/Cas9 techniques (reviewed in [1, 2]). Importantly, various signaling pathways, including insulin/insulin-like growth factor-1 (IGF-1) and target of rapamycin (TOR) signaling, are evolutionarily conserved and influence aging in C. elegans and complex organisms, such as mammals (reviewed in [3, 4]). Therefore, research on C. elegans aging has provided important insights into the molecular mechanisms by which mammalian aging is regulated. In standard laboratory conditions, C. elegans is cultured on solid agar media with E. coli as a food source [5]. The effects of specific dietary nutrients, including carbohydrates, on C. elegans aging can be tested by supplementing media or E. coli culture with specific nutrients. Using the C. elegans system, researchers have found that dietary carbohydrates, including glucose, and carbohydrate-derived metabolites influence aging and lifespan through various genes and signaling pathways.
Here, we will review studies on the effects of carbohydrates on lifespan, focusing on how dietary glucose affects lifespan in C. elegans. We will review various genes and signaling pathways that mediate the effects of dietary glucose on lifespan in C. elegans. We will also describe studies on the effects of non-glucose carbohydrates on aging and related findings in other organisms and cultured mammalian cells. We will further discuss the implications of studies using model organisms in human health and aging.
Glucose-enriched diets shorten lifespan via inhibition of forkhead box O (FOXO) and heat shock factor-1 (HSF-1)
The insulin/IGF-1 signaling (IIS) pathway is a major regulator of glucose and carbohydrate metabolism (reviewed in [6]). The insulin and IGF-1 receptors transduce signals to downstream transcription factors, through the phosphoinositide 3-kinase (PI3K) cascade (reviewed in [3, 7]). The IIS receptor in C. elegans is DAF-2, whose name originated from the phenotypes of daf-2 mutants, which display constitutive dauer (an alternative hibernation-like juvenile larva) formation [8]. At least three longevity-promoting transcription factors, DAF-16/FOXO, HSF-1, and SKN-1/nuclear factor-erythroid-related factor (Nrf), function to regulate the expression of various target genes in the IIS pathway (reviewed in [3, 7]). These target genes are implicated in a broad range of physiological processes, including lifespan, development, metabolism, stress responses, and immunity [9–13].
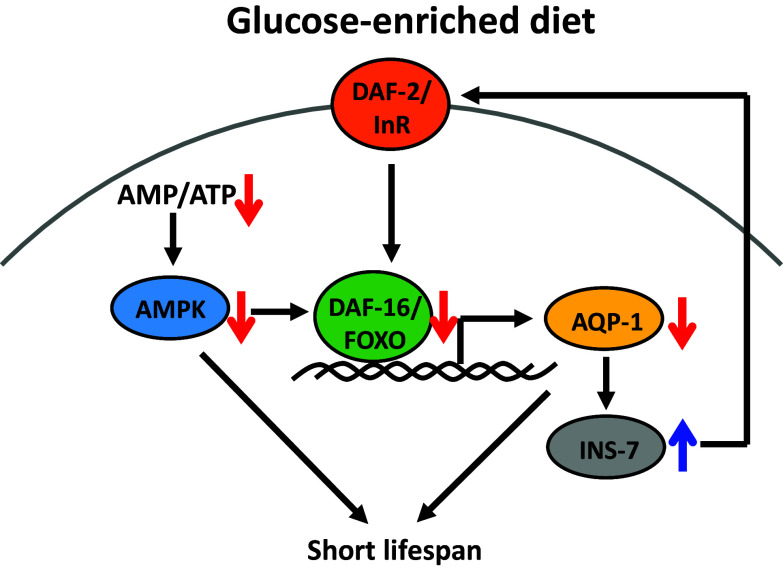
One mechanism by which glucose-enriched diets shorten the lifespan of C. elegans is through down-regulation of DAF-16/FOXO and HSF-1 transcription factors in IIS (Fig. 1) [14]. Glucose-rich diets (111 mM in growth media) reduce the lifespan of wild-type worms, but do not further decrease the short lifespan of daf-16/FOXO or hsf-1 mutant worms [14]. Thus, glucose diets, DAF-16/FOXO, and HSF-1 function in the same pathway to regulate lifespan. In addition, glucose feeding prevents dauer formation, which requires DAF-16/FOXO activity (reviewed in [15]), and reduces the expression of DAF-16/FOXO target genes, including a superoxide dismutase gene (sod-3) [14]. These data suggest that glucose-rich diets reduce the lifespan of C. elegans through IIS, which also regulates glucose metabolism in mammals (reviewed in [6]). However, it remains unknown how dietary glucose down-regulates DAF-16/FOXO, and therefore it will be important to address the underlying mechanisms in future studies.
Fig. 1.
Glucose-enriched diets shorten lifespan through insulin/IGF-1 signaling and AMPK pathways in C. elegans. Glucose feeding decreases the activity of DAF-16/FOXO, a downstream transcription factor of DAF-2/insulin/IGF-1 signaling (IIS) receptor (InR). Down-regulation of DAF-16/FOXO and its target AQP-1/aquaporin 1 on a glucose-rich diet results in short lifespan and increases the expression of INS-7/insulin-like peptide that amplifies the IIS. In addition, decreased AMP/ATP ratio on a glucose-rich diet reduces the activity of AMPK, and this leads to short lifespan. AMPK is also known to increase the activity of DAF-16/FOXO [46]
Interestingly, a glycerol channel AQP-1/aquaporin, a target of DAF-16/FOXO and HSF-1, mediates the lifespan-shortening effects of glucose-rich diets [14]. Glucose-rich diet feeding or aqp-1 mutation increases the expression of INS-7/insulin-like peptide [14], which acts as an agonist of DAF-2 [12, 16]. This event subsequently decreases the activity of DAF-16/FOXO and in turn reduces the expression of aqp-1 [14]. Thus, the physiological consequence of glucose feeding appears to be an insulin-mediated endocrine positive feedback loop that amplifies IIS [14]. Importantly, two mammalian glycerol channels, AQP7 and AQP9, are implicated in glucose metabolism [17, 18]; Aqp7-knockout mice display obesity and insulin resistance [17], and Aqp9 deficiency causes reduced blood glucose levels in diabetic mice [18]. Therefore, C. elegans AQP-1 may play evolutionarily conserved roles in glucose metabolism and lifespan regulation.
In contrast to DAF-16/FOXO and HSF-1, SKN-1/Nrf2 does not appear to directly affect the shortened lifespan caused by a glucose-enriched diet. Instead, SKN-1/Nrf2 prevents fat accumulation on a high glucose diet (111 mM) [19]. The relationship between dietary glucose and SKN-1/Nrf2 was based on the finding that high glucose feeding does not increase fat levels in worms with gain-of-function mutations in the skn-1 gene. SKN-1 activation promotes fatty acid oxidation, a catabolic process that decreases fat levels and generates energy from stored fats [19]. Because of this increased fatty acid oxidation, worms with the gain-of-function skn-1 mutations maintain normal fat levels during high glucose feeding [19]. A correlation between SKN-1/Nrf2-regulated fat metabolism and glucose-rich diet-induced shortened lifespan has not been demonstrated. However, SKN-1/Nrf2-regulated fat metabolism may contribute to longevity in other dietary conditions. Indeed, SKN-1/Nrf2 is crucial for lifespan extension in C. elegans conferred by dietary restriction (DR) [20], an evolutionarily well-conserved anti-aging regimen (reviewed in [21]). SKN-1/Nrf2 is activated in specific sensory neurons during DR [20]. SKN-1/Nrf2 transmits signals from sensory neurons to non-neuronal tissues and increases organismal lifespan [20]. Thus, SKN-1 mediates the longevity effect of DR and lipid metabolism in response to DR.
Sterol-regulatory element-binding protein (SREBP)/MDT-15 moderates the life-shortening effects of glucose by preventing accumulation of saturated fatty acids and toxic intermediate metabolites
High sugar diets promote fatty acid synthesis and storage of excessive energy through SREBPs, which are major fat metabolism-regulatory transcription factors. SREBPs belong to a basic-helix-loop-helix leucine zipper class family and play key roles in cholesterol and fatty acid synthesis (reviewed in [22]). In mammals, SREBPs are located on the endoplasmic reticulum (ER) membrane during normal metabolic conditions. Under conditions of low cholesterol or high carbohydrate, SREBPs are released from the ER membrane by proteolytic cleavage. This cleavage triggers the translocation of SREBPs into the nucleus with subsequent induction of SREBP target genes, including acetyl-CoA carboxylase (ACC), fatty acid synthase (FAS), and steroyl-CoA desaturases (SCDs) [23]. ACC and FAS are key enzymes that regulate rate-limiting steps for de novo fat synthesis. SCDs govern the conversion of saturated fatty acids (SFAs) to unsaturated fatty acids (UFAs) by inserting double bonds. Thus, SREBPs mediate the increased fat levels and conversion of SFAs to UFAs under high carbohydrate conditions in mammalian cells.
The role of SREBPs in fat and carbohydrate metabolism is generally conserved in C. elegans [24–27]. Increased concentrations (111 mM) of dietary glucose or fructose, another major sugar that shortens the lifespan of C. elegans, lead to the up-regulation of SBP-1, a C. elegans homolog of SREBP [27]. Glucose-rich diets increase SBP-1/SREBP protein levels both in the nucleus and the cytosol without elevating sbp-1 mRNA levels, indicating post-transcriptional up-regulation of SBP-1/SREBP [27]. This up-regulation of SBP-1/SREBP induces expression of lipogenic genes, including fatty acid desaturases, homologs of SCDs. Conversely, down-regulation of SBP-1/SREBP causes decreased expression of lipogenic genes, which in turn leads to developmental defects, short lifespan, and sterility [24, 27–29]. These studies indicate that SBP-1/SREBP is an evolutionarily conserved transcription factor that governs lipid/glucose metabolism and animal physiology. As it remains unknown how dietary glucose up-regulates SBP-1/SREBP, it will be important to identify the underlying mechanisms in future research.
Mediator is a large protein complex that interacts with transcription factors for proper transcription (reviewed in [30]). Mediator complex subunit 15 (MED15) acts as a co-regulator for the transcriptional activity of SREBP, and the roles of MED15 are also conserved in C. elegans [29]. MDT-15, the MED15 homolog in C. elegans, physically interacts with SBP-1/SREBP and regulates the expression of SBP-1/SREBP target genes [29]. MDT-15/MED15 also functions in fatty acid oxidation as a co-regulator for other transcription factors, including nuclear receptor 49 (NHR-49) and SKN-1/Nrf2 [19, 31, 32]. Thus, MDT-15/MED15 is a crucial transcriptional co-regulator that mediates fat metabolism in response to changes in metabolic status.
SBP-1/SREBP and MDT-15/MED15 play critical roles in lifespan regulation in C. elegans on high glucose diets (Fig. 2) [27]. C. elegans fed with a high glucose diet has extremely short lifespan and displays accelerated aging phenotypes, including age-dependent declines in motility, when SBP-1/SREBP or MDT-15/MED15 is genetically inhibited [27]. Conversely, up-regulation of SBP-1/SREBP or MDT-15/MED15 mitigates the life-shortening effect of dietary glucose [27]. These results indicate that SBP-1/SREBP and MDT-15/MED15 protect worms from the life-shortening effects of glucose-enriched diets [27]. Expression of SBP-1/SREBP or MDT-15/MED15 is mainly observed in the intestine of C. elegans [24, 27, 31], which is a major organ that regulates metabolism, similar to the mammalian liver [33]. Consistent with the expression pattern, the intestine is the most crucial organ for the protective roles of SBP-1/SREBP and MDT-15/MED15 in aging under high glucose-fed conditions [27]. SREBP-1-induced lipogenesis in the mouse liver can lower blood glucose levels and reduce glucose toxicity [34, 35]. Thus, the protective roles of SBP-1/SREBP and MDT-15/MED15 in the intestine are similar to those of mammalian SREBP in the liver.
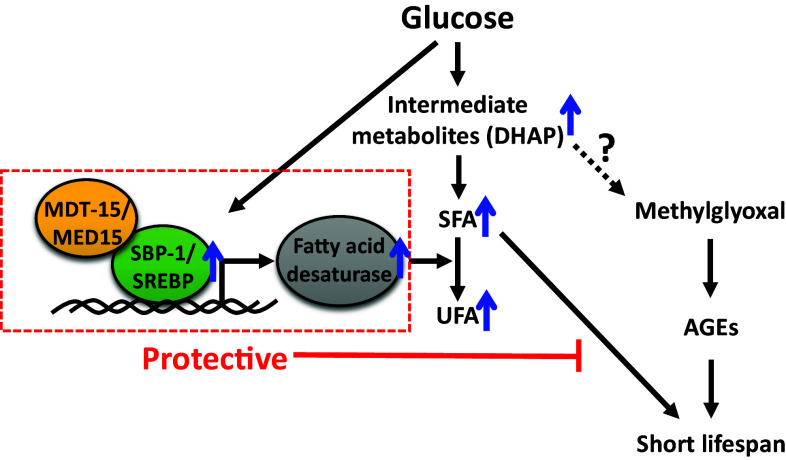
Fig. 2.
The life-shortening effect of dietary glucose is moderated by SBP-1/SREBP and MDT-15/MED15 complex in C. elegans. SBP-1/SREBP and MDT-15/MED15 protect animals from lifespan-shortening effects of glucose-rich diets by inducing fatty acid desaturases that promote saturated fatty acid (SFA) to unsaturated fatty acid (UFA) conversion in C. elegans. This event also prevents the accumulation of glucose-derived toxic intermediate metabolites, including dihydroxyacetone phosphate (DHAP), which potentially converts to methylglyoxal and advanced glycation end products (AGEs) that lead to short lifespan [40]
The major metabolic function of SBP-1/SREBP and MDT-15/MED15 under glucose-rich conditions is the conversion of SFAs to UFAs by increasing the expression of SCDs/fatty acid desaturases [27]. Genetic inhibition of SBP-1/SREBP or MDT-15/MED15 decreases the levels of SCDs/fatty acid desaturases, leading to the accumulation of SFAs and reduction of UFAs [27]. These data suggest that the accumulation of SFAs or the reduction of UFAs shortens lifespan in glucose-fed animals upon depletion of SBP-1/SREBP or MDT-15/MED15. Additional experiments show that SFA treatments greatly decrease the lifespan of glucose-fed worms, similar to the genetic inhibition of SBP-1/SREBP or MDT-15/MED-15. In contrast, UFA treatments do not suppress the very short lifespan of glucose-fed worms depleted in SBP-1/SREBP or MDT-15/MED15 [27]. Interestingly, the roles of SBP-1/SREBP on a high glucose diet in C. elegans share similarities to glucolipotoxicity in human diseases. High levels of glucose and fatty acids induce glucolipotoxicity that causes a functional decline and cell death in pancreatic β-cells (reviewed in [36]). Similarly, the accumulation of SFA by the inhibition of SBP-1/SREBP or SFA treatment under glucose-rich conditions causes a very short lifespan in C. elegans [27]. Together, these findings suggest that SBP-1/SREBP and MDT-15/MED15 protect animals on glucose-enriched diets from rapid aging by preventing the accumulation of SFAs.
Accumulation of certain metabolites can block upstream metabolic enzymes, because metabolism is tightly regulated through a negative feedback mechanism. For example, acetyl-CoA carboxylase (ACC) and fatty acid synthase (FAS), which regulate rate-limiting steps in the de novo SFA synthesis pathway, are inhibited by their product palmitoyl-CoA through a feedback mechanism [37, 38]. Similar to mammals, C. elegans POD-2/ACC and FASN-1/FAS are crucial for de novo SFA synthesis. If the accumulation of SFAs inhibits upstream metabolic processes in a negative feedback manner, these two enzymes can be down-regulated by SFAs. This implies that the accumulation of SFAs caused by the genetic inhibition of SBP-1/SREBP or MDT-15/MED15 in glucose-rich conditions can accelerate aging by decreasing the activity of POD-2/ACC or FASN-1/FAS. Indeed, depletion of POD-2/ACC or FASN-1/FAS causes a very short lifespan on a glucose-enriched diet, similar to the genetic inhibition of SBP-1/SREBP and MDT-15/MED15 [27]. Because POD-2/ACC and FASN-1/FAS link glucose metabolism and fatty acid synthesis, depletion of POD-2/ACC or FASN-1/FAS may result in the accumulation of glucose-derived intermediate metabolites. Thus, increased SFA levels by the inhibition of SBP-1/SREBP or MDT-15/MED15 appear to cause the accumulation of glucose-derived intermediates, which also contribute to an accelerated aging.
What are the lifespan-shortening intermediate metabolites that are generated from glucose? Among the potential metabolites generated from dietary glucose via glycolysis, dihydroxyacetone phosphate (DHAP) feeding drastically shortens the lifespan of C. elegans [27]. In addition, genetic inhibition of the glycolytic enzymes that regulate the upstream steps of DHAP, such as glucose-6-phosphate isomerases and aldolases, ameliorates the lifespan-shortening effects of glucose [27]. These data suggest that DHAP is a glucose-derived intermediate metabolite that accelerates aging. It will be interesting to determine if the inhibition of SBP-1/SREBP or MDT-15/MED15 increases the levels of DHAP on a glucose-rich diet.
The short lifespan caused by DHAP treatments can be explained by the accumulation of advanced glycation end products (AGEs). DHAP is non-enzymatically degraded to methylglyoxal (MG), a precursor of AGEs, which are associated with cellular oxidative stress as well as diabetes and other age-related chronic diseases (reviewed in [39]). Thus, the lifespan-shortening effects of dietary glucose can result from the accumulation of AGEs by increasing the levels of DHAP and MG. Indeed, glucose-enriched diets (40 mM) increase the levels of MG-derived AGEs and reactive oxygen species (ROS) in C. elegans [40]. This study indicates that the glucose concentration in C. elegans body extracts reaches 10–15 mM on glucose-enriched diets (40 mM) and is similar to the glucose concentration in diabetic patients [40]. Glucose feeding also seems to cause the accumulation of AGEs by down-regulation of the activity of glyoxalase-1, one of the two enzymes that detoxify MG (glyoxalase-1 and glyoxalase-2); however, the mechanisms by which glucose-rich diets down-regulate glyoxalase-1 activity are not clear [40]. During this detoxification, MG and reduced glutathione (GSH) spontaneously form a hemithioacetal. Glyoxalase-1 then converts hemithioacetal to S-lactoylglutathione, which is subsequently metabolized to d-lactate by glyoxalase-2 (reviewed in [39]). Overexpression of glyoxalase-1 reduces MG formation upon glucose treatment and diminishes the lifespan-shortening effects of glucose-rich diets in C. elegans [40]. Therefore, glucose-derived AGE accumulation is one of the lifespan-decreasing mechanisms of high glucose diets, and reducing the AGE levels can be beneficial for ameliorating the life-shortening effects of glucose-enriched diets.
Glucose restriction increases lifespan by up-regulating AMP-activated protein kinase (AMPK) and mitochondrial ROS
AMPK acts as a cellular energy sensor (reviewed in [41]). Low energy status such as DR decreases the level of ATP and increases the ratio of AMP/ATP. This leads to AMP binding to AMPK and the activation of AMPK via a conformational change. Conversely, when cellular energy levels are high, such as under glucose-rich diet conditions, the AMP to ATP ratio is low and AMPK becomes inactive. Activated AMPK transduces cellular signals through phosphorylation of kinase substrates to adapt to a low-energy status. AMPK promotes many catabolic processes, including glucose uptake, mitochondrial respiration, glycolysis, and fatty acid oxidation. AMPK also reduces overall anabolic processes, including lipogenesis and protein synthesis.
AMPK regulates metabolism and longevity in C. elegans. Mutations in aak-2, which encodes a catalytic α subunit of AMPK, shorten lifespan, whereas overexpression or constitutive activation of AMPK extends lifespan [42–46]. AMPK also acts downstream of IIS for lifespan extension [45] and mediates DR-induced longevity by phosphorylating and activating DAF-16/FOXO [46]. In addition, AMPK mediates lifespan changes conferred by interventions that potentially affect metabolic status. For example, glucose surplus and glucose restriction result in opposite effects on AMPK activity and lifespan. Glucose restriction activates AMPK and extends lifespan (Fig. 1) [47]. Conversely, a high glucose diet (5 or 50 mM) decreases AMPK activity and shortens lifespan [47]. However, another study showed that glucose-rich diets still shorten the lifespan of aak-2/AMPK mutants [14], and therefore the life-decreasing effects of high glucose diets do not seem to be dependent on down-regulation of AMPK. Overall, these studies indicate that AMPK is one of the crucial factors that mediate lifespan changes depending on physiological nutritional status.
Under glucose-restricted conditions, animals may need to increase their energy utilization efficiency due to decreased available nutrient levels. Consistent with this scenario, glucose restriction enhances mitochondrial respiration and AMPK activity in C. elegans [47]. This enhanced mitochondrial respiration increases the generation of ROS, perhaps as a by-product, which paradoxically leads to enhanced oxidative stress resistance and longevity [47]. Mildly increased mitochondrial ROS levels equip organisms against severe oxidative stresses and contribute to longevity; this phenomenon is called “mitohormesis” (reviewed in [48]). Our group showed that mitochondrial ROS increase the activity of AMPK, preventing a further increase in ROS levels through negative feedback [44]. These findings suggest that glucose restriction lengthens lifespan by increasing AMPK activity and mitochondrial ROS levels, which should be tightly regulated to exert a beneficial effect.
Other factors that mediate the effects of dietary glucose on animal physiology
Other genetic factors that mediate the effects of dietary glucose on C. elegans physiology have been identified. These include pro-apoptotic genes, glucose transporters, adiponectin receptors, and mitochondrial unfolded protein response (UPRmt) genes [49–53]. Apoptosis contributes to lifespan reduction by glucose-rich diets (6 μM) [49]. In C. elegans, most apoptotic processes occur during development, but glucose-rich diets induce apoptosis during adulthood [49]. Moreover, genetic inhibition of pro-apoptotic genes ameliorates the short lifespan of glucose-fed worms [49]. It seems likely that glucose-rich foods cause apoptotic cell death, which results in decreased lifespan at an organismal level.
Glucose is metabolized to produce other organic molecules and energy. Recent studies identified a C. elegans-facilitated glucose transporter-1 (FGT-1) as a functional glucose transporter [50, 51]. Genetic inhibition of fgt-1 decreases glucose uptake and glucose oxidation [51]. Moreover, fgt-1 knockdown extends lifespan [51], similar to glucose restriction, which also increases lifespan [47]. However, FGT-1 does not seem to affect the lifespan of C. elegans on glucose-enriched diets (20 mM) [51], suggesting that the lifespan-decreasing effects of glucose-rich diets bypass this glucose transporter possibly because of redundancy. Overall, FGT-1 is a functionally important glucose transporter that regulates metabolism and physiology in C. elegans.
High glucose feeding changes lipid composition [27]. Lipids are crucial components of the plasma membrane and important for maintaining membrane fluidity. Thus, organisms are equipped with mechanisms that maintain proper membrane fluidity under various physiological conditions, such as high sugar conditions. A recent paper shows that an adiponectin receptor homolog PAQR-2/progestin and adipoQ receptor 2 and its binding partner IGLR-2/Ig (immunoglobulin) and LRR (leucine-rich repeat) domain 2 regulate membrane fluidity under glucose-enriched (20 mM) conditions in C. elegans [52]. Wild-type worms can homeostatically maintain membrane fluidity at a low temperature or on a high glucose diet. However, when PAQR-2 or IGLR-2 is depleted at a low temperature or on a glucose diet, the plasma membrane becomes rigid [52], resulting in developmental arrest at the larval stages [27, 52, 54]. Because adiponectin signaling is important for glucose and fat metabolism-associated pathology (reviewed in [55]), this study implies conserved roles for adiponectin receptors in metabolism and physiology in C. elegans and mammals.
As discussed above, glucose-enriched diet feeding throughout life or during adulthood shortens lifespan in C. elegans [27, 40, 47]. Unexpectedly, however, glucose feeding (111 mM or 222 mM) during larval developmental stages extends lifespan [53]. This lifespan extension by glucose feeding during larval stages requires UPRmt, a protective process that induces nuclear-encoded mitochondrial chaperones for reducing mitochondrial stress caused by the accumulation of unfolded proteins [53]. Growing evidence indicates that UPRmt contributes to longevity in diverse organisms (reviewed in [56]). Thus, glucose treatment during early juvenile stages may extend adult lifespan by increasing mitochondrial protein homeostasis via UPRmt.
Glucose-rich diets also protect animals from an age-dependent increase in proteotoxicity. Transgenic C. elegans that expresses toxic proteins, such as expanded polyglutamine (polyQ), mTDP-43 (human mutant transactive response DNA-binding protein (TDP) 43: an amyotrophic lateral sclerosis (ALS) disease model), or mFUS (ALS-associated fused in sarcoma mutant), displays the accumulation of proteins in an insoluble fraction, age-dependent neurodegeneration, and defects in touch sensation and motility [57]. Glucose-enriched diets (111 mM) delay the age-associated phenotypes in these transgenic animals by reducing the accumulation of toxic insoluble proteins [57]. In addition, the neuroprotective effects of glucose-rich diet feeding require DAF-16/FOXO, HSF-1, UPRER, and the ubiquitin–proteasome system [57]. These results suggest that dietary glucose can reduce proteotoxicity through multiple protein homeostasis mechanisms [57]. Moreover, a glucose-rich diet enhances resistance against oxidative stress, heat stress, and osmotic stress [57]. These data imply that the negative effects of dietary glucose on lifespan can be separated from its protective effects against various internal or external stresses.
Other carbohydrates that affect lifespan
In addition to glucose, other dietary carbohydrates affect the lifespan of C. elegans. Trehalose is glucose disaccharide found in many invertebrate organisms and an important component of physiological responses to various stresses [58–62]. Trehalose feeding increases lifespan and enhances thermotolerance in C. elegans [62]. Trehalose also contributes to the long lifespan of daf-2/insulin/IGF-1 receptor mutants, which contain more trehalose than wild-type animals [62]. Glucose is metabolized to produce energy through glycolysis, the tricarboxylic acid (TCA) cycle, and the mitochondrial electron transport chain (ETC). Elevation of pyruvate levels by direct feeding or by genetic inhibition of pyruvate kinase, pyk-1, prolongs lifespan [63, 64]. Several glucose-derived metabolites in the TCA cycle increase lifespan; these include malate, fumarate, succinate, and α-ketoglutarate (α-KG) [65, 66]. Among them, α-KG treatment extends the lifespan of C. elegans by inhibiting ATP synthase subunit β and TOR signaling [66]. This suggests that an intermediate metabolite in energy metabolism can act as a signaling molecule, which interacts with a specific protein that regulates longevity.
N-acetylglucosamine (GlcNAc), a glucose-derived metabolite used as a precursor for N- and O-glycans in the hexosamine pathway, a branch of glycolysis that generates amino sugars, extends lifespan [67]. Moreover, gain-of-function mutations in gfat-1/glutamine-fructose-6-phosphate aminotransferase, an enzyme that governs a rate-limiting step in the hexosamine pathway, increase lifespan seemingly without causing defects in development, feeding capacity, or reproduction [67]. Mechanisms by which metabolites in the hexosamine pathway extend lifespan are linked to protein quality control, one of the most crucial lifespan-influencing factors. The gfat-1 gain-of-function mutants were first identified from a genetic screen for tunicamycin-induced ER stress-resistant mutants [67]. Therefore, the activated hexosamine pathway likely leads to better ER-regulated protein homeostasis. Indeed, the gfat-1 gain-of-function mutation or GlcNAc supplementation increases diverse protein quality control processes, including ER-associated protein degradation (ERAD), autophagy, and proteasome-mediated degradation [67]. Thus, GlcNAc seems to extend lifespan by promoting protein quality control. It will be interesting to determine if the gain-of-function mutations in gfat-1 protect worms from short lifespan on a glucose-enriched diet by reducing glucose levels through increased synthesis of beneficial GlcNAc.
Differential effects of various carbohydrates on lifespan
Among the carbohydrates that we discussed, glucose, fructose, and DHAP are pro-aging factors [14, 27, 40, 47], whereas several other intermediate metabolites, including pyruvate, malate, fumarate, succinate, and α-KG, are anti-aging factors [63–66]. What causes the different effects of these metabolites on lifespan? Glucose is metabolized in the cytosol through glycolysis. The product of glycolysis, pyruvate, enters the mitochondria for further metabolism through the TCA cycle. Thus, treatment with metabolites in the TCA cycle can activate mitochondrial respiration without accumulating potentially toxic glucose-derived glycolytic metabolites, such as glucose, fructose, or DHAP.
Organisms catabolize stored fats under energy-limited conditions (e.g., glucose restriction) to generate acetyl-CoA via fatty acid oxidation. The acetyl-CoA molecules are used for energy production through the TCA cycle and ETC in the mitochondria. This potentially results in increased ROS generation, as mitochondrial ETC is a major source of cellular ROS. Slightly elevated ROS may extend lifespan by mitohormetic mechanisms. Indeed, malate and fumarate treatments elevate oxygen consumption rates [65], which correlate with increased ROS levels. Moreover, mutations in slcf-1, which encodes a solute carrier family protein, promote longevity and increase internal pyruvate and ROS levels [63]. In addition, the longevity of slcf-1 mutants is completely suppressed by antioxidant treatment [63], suggesting that mildly up-regulated mitochondrial ROS mediate the lifespan-extending effects of pyruvate. Thus, several TCA cycle metabolites appear to prolong lifespan by activating mitochondrial respiration and mildly increasing ROS levels. In the case of glucose feeding, metabolites in both glycolysis and the TCA cycle may be accumulated. Therefore, in glucose-fed worms, toxic effects of glycolytic metabolites may be greater than the beneficial effects of TCA cycle metabolites on longevity.
Effects of carbohydrates on lifespan in yeast
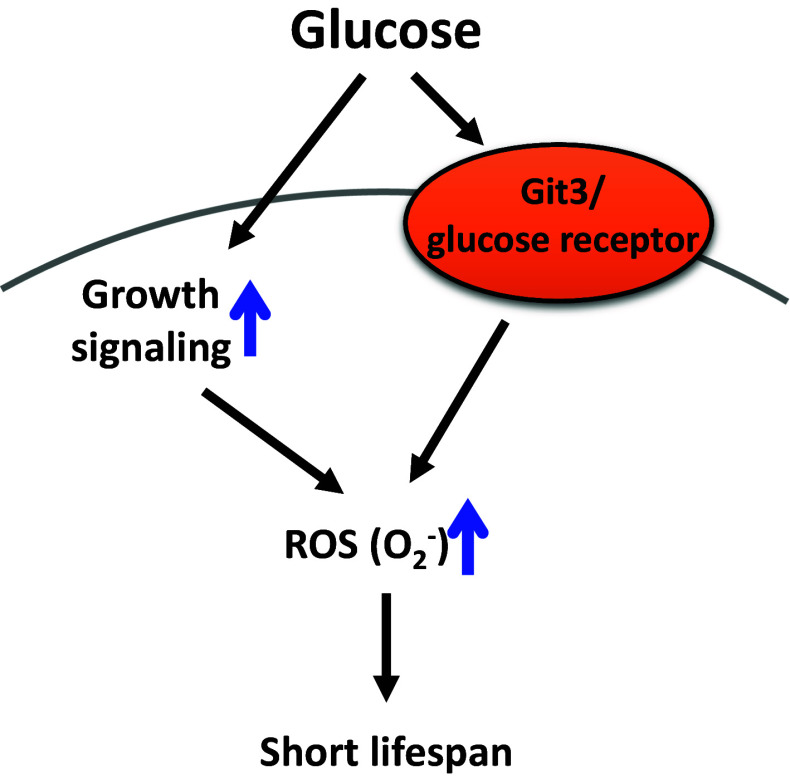
High sugar conditions shorten the chronological lifespan (CLS) of budding and fission yeasts (Fig. 3) [68, 69]. Glucose increases the levels of toxic superoxide ions (O2 −) through activation of growth signaling, which results in decreased CLS in budding yeast [69]. The CLS of fission yeast is decreased by high glucose through the activation of Git3/a glucose receptor GPCR (G protein-coupled receptor) and ROS formation, which may decrease lifespan [68]. In addition to glucose, the aging rate of budding yeast is accelerated by another monosaccharide, fructose [70], which also shortens the lifespan of C. elegans [27]. Interestingly, high fructose-treated yeast contains more carbonylated proteins and ROS, and displays higher mortality rates than those of high glucose-treated yeast [70]. Because fructose is more reactive than glucose for protein glycation [71], fructose may exert more toxic effects than glucose on yeast physiology. Overall, these studies suggest that high carbohydrate conditions accelerate the aging of yeast, and the increased levels of toxic ROS appear to underlie the carbohydrate-induced accelerated aging.
Fig. 3.
High sugar conditions reduce chronological lifespan in budding and fission yeasts. In budding yeast, glucose increases growth signaling and superoxide ion (O2 −) levels, which lead to short lifespan. In fission yeast, glucose increases the activity of glucose receptor and reactive oxygen species (ROS) levels, which result in decreased lifespan
Effects of glucose on senescence in human cells
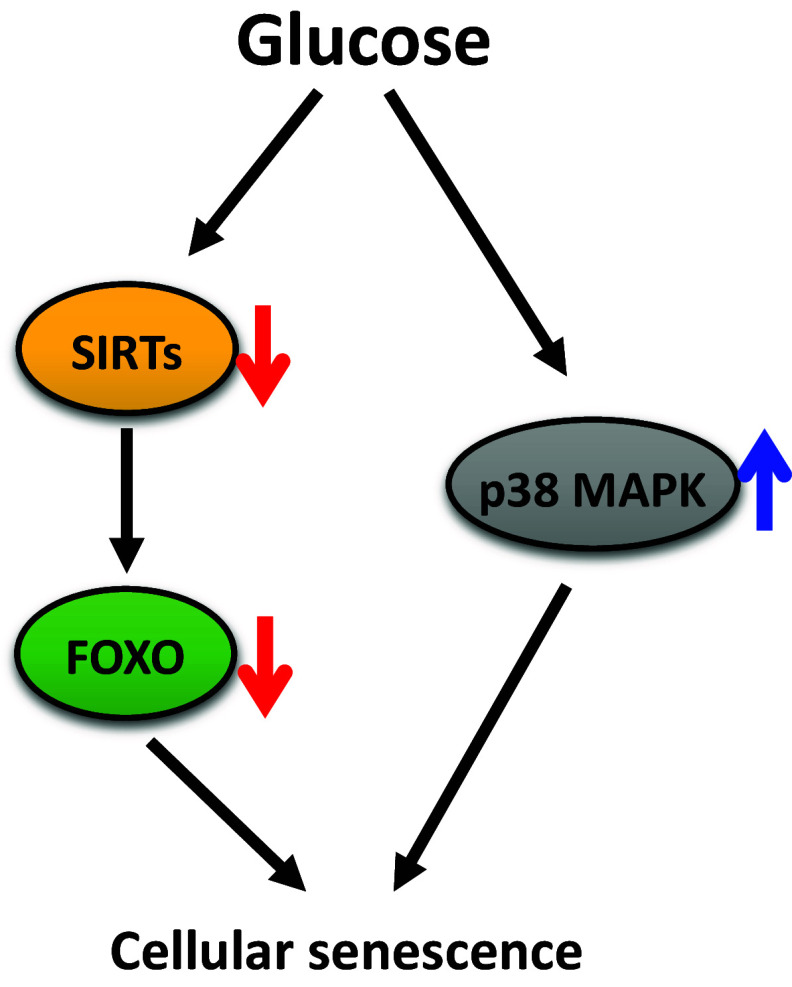
High glucose treatments also have negative effects on aging in human endothelial cells and fibroblasts (Fig. 4) [72, 73]. In these human cells, treatment with high glucose accelerates various aging-related phenotypes, such as increased levels of senescence-associated (SA) β-gal staining, reduced proliferation, irregular morphology, and increased ROS levels [72, 73]. Sirtuins (Sir2-like proteins: SIRTs) appear to mediate glucose-induced accelerated cellular aging. SIRTs are NAD+-dependent protein deacetylases implicated in the aging of many organisms (reviewed in [74]). Glucose treatments down-regulate SIRTs and FOXO1 [72, 73]. Conversely, treatment with a SIRT1 activator or SIRT3 overexpression restores FOXO1 activity and delays glucose-induced accelerated aging [72, 73]. As observed in human cells, glucose feeding down-regulates DAF-16/FOXO in C. elegans [14]. Thus, it will be interesting to test whether glucose-enriched diets decrease the activity of DAF-16/FOXO through SIRTs in C. elegans.
Fig. 4.
High glucose treatments accelerate senescence in cultured human cells. High glucose treatments down-regulate sirtuins (SIRTs), and this leads to decreased activity of FOXO and accelerates cellular senescence. High glucose also increases p38 MAP kinase (p38 MAPK) activity, which leads to cellular senescence
High glucose also accelerates the senescence of human endothelial progenitor cells (EPCs) by activating p38 mitogen-activated protein kinase (MAPK) (Fig. 4) [75]. Under glucose-rich conditions, cellular aging phenotypes, such as increased levels of SA β-gal staining and reduced cellular proliferation, are observed in EPCs, and p38 MAPK is activated [75]. These aging phenotypes are restored by treatment with a p38 MAPK inhibitor, suggesting that p38 MAPK mediates the pro-aging effects of high glucose [75]. Together, these studies show that the pro-aging effects of high glucose are also observed in human cells.
Human implications
Although we do not have direct evidence regarding whether high or low levels of carbohydrate intake influence aging in humans, several clinical studies show that low carbohydrate diets can have beneficial effects on human health. For example, consumption of a low carbohydrate diet for 3 or 6 months causes significant weight loss, improvement in insulin sensitivity and low levels of several risk factors for heart diseases in obese people [76, 77]. Low carbohydrate diets also improve serum factors associated with aging in elderly people [78]. These findings suggest that a low carbohydrate intake can be beneficial for reducing risk factors associated with aging and aging-related diseases in humans.
Conclusions and perspectives
To study how dietary carbohydrates influence aging, researchers have used model organisms, including C. elegans and yeast. Several studies identified genes and pathways, including insulin/IGF-1 signaling, SREBP and MED15, and AMPK, which mediate or counteract the aging-accelerating effects of high carbohydrate diets, particularly high dietary glucose in C. elegans. All of these genetic factors are evolutionarily well conserved aging- and/or metabolism-regulatory genes. Thus, the findings may provide insights into the conserved roles of genetic factors in lifespan regulation on a carbohydrate-rich diet in mammals, including humans. Because carbohydrate metabolism is the sum of complicated processes, and almost all metabolites are interconnected, changes in specific intermediate metabolites may affect the levels of other metabolites. Interestingly, diverse carbohydrates or carbohydrate-derived metabolites have different effects on lifespan. For example, glucose, fructose, and DHAP shorten the lifespan of C. elegans. In contrast, trehalose, certain TCA cycle metabolites, and GlcNAc extend lifespan. These suggest that each metabolite has a specific function in physiology rather than simply increasing energy levels as a carbon source.
Previous studies help understand the complexity of carbohydrate metabolism and its roles in animal physiology. However, it remains unclear how various genetic and dietary factors interact with each other, or how these interactions influence lifespan in higher organisms. Geometric framework studies on insects and rodents, and observational research in humans have shown that low protein with high carbohydrate diets have beneficial effects on lifespan or health (reviewed in [79]). These studies suggest that various ratios of nutritional components, not a single nutritional component, differentially affect health and aging.
Diverse organisms have different nutritional sources and metabolic processes. For instance, carbohydrate sources of C. elegans under standard conditions are limited to specific bacteria, but humans consume various foods as carbohydrate sources. In C. elegans glucose-enriched diets may also affect the proportion of macronutrients and total calorie intake, which can affect lifespan. In addition, normal glucose intake in C. elegans remains unknown, due to technical issues involved in culturing C. elegans with defined media. These issues should be considered when interpreting C. elegans studies on how carbohydrates or nutritional components influence lifespan and aging. More comprehensive research using mammalian systems will solve these remaining issues and provide therapeutic implications for human diseases and aging.
Acknowledgements
We thank Dr. Murat Artan and other Lee lab members for helpful comments. This research was supported by the National Research Foundation of Korea (NRF) Grant funded by the Korean government (Ministry of Science, ICT, and Future Planning; NRF-2012R1A4A1028200) and a Grant of the Korean Health Technology R&D Project, Ministry of Health and Welfare (HI14C2337 to S.-J.V.L.).
References
- 1.Dickinson DJ, Goldstein B. CRISPR-based methods for Caenorhabditis elegans genome engineering. Genetics. 2016;202(3):885–901. doi: 10.1534/genetics.115.182162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Corsi AK, Wightman B, Chalfie M. A transparent window into biology: a primer on Caenorhabditis elegans . Genetics. 2015;200(2):387–407. doi: 10.1534/genetics.115.176099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kenyon CJ. The genetics of ageing. Nature. 2010;464(7288):504–512. doi: 10.1038/nature08980. [DOI] [PubMed] [Google Scholar]
- 4.Lee Y, An SWA, Artan M, Seo M, Hwang AB, Jeong D-E, Son HG, Hwang W, Lee D, Seo K, Altintas O, Park S, Lee S-JV (2015) Genes and pathways that influence longevity in Caenorhabditis elegans. In: Mori N, Mook-Jung I (eds) Aging mechanisms: longevity, metabolism, and brain aging. Springer, Tokyo, pp 123–169. doi:10.1007/978-4-431-55763-0_8
- 5.Stiernagle T. Maintenance of C. elegans . WormBook. 2006 doi: 10.1895/wormbook.1.101.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Saltiel AR, Kahn CR. Insulin signalling and the regulation of glucose and lipid metabolism. Nature. 2001;414(6865):799–806. doi: 10.1038/414799a. [DOI] [PubMed] [Google Scholar]
- 7.Altintas O, Park S, Lee SJ. The role of insulin/IGF-1 signaling in the longevity of model invertebrates, C. elegans and D. melanogaster . BMB Rep. 2016;49(2):81–92. doi: 10.5483/BMBRep.2016.49.2.261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Riddle DL, Swanson MM, Albert PS. Interacting genes in nematode dauer larva formation. Nature. 1981;290(5808):668–671. doi: 10.1038/290668a0. [DOI] [PubMed] [Google Scholar]
- 9.Morley JF, Morimoto RI. Regulation of longevity in Caenorhabditis elegans by heat shock factor and molecular chaperones. Mol Biol Cell. 2004;15(2):657–664. doi: 10.1091/mbc.E03-07-0532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hsu AL, Murphy CT, Kenyon C. Regulation of aging and age-related disease by DAF-16 and heat-shock factor. Science. 2003;300(5622):1142–1145. doi: 10.1126/science.1083701. [DOI] [PubMed] [Google Scholar]
- 11.Lee SS, Kennedy S, Tolonen AC, Ruvkun G. DAF-16 target genes that control C. elegans life-span and metabolism. Science. 2003;300(5619):644–647. doi: 10.1126/science.1083614. [DOI] [PubMed] [Google Scholar]
- 12.Murphy CT, McCarroll SA, Bargmann CI, Fraser A, Kamath RS, Ahringer J, Li H, Kenyon C. Genes that act downstream of DAF-16 to influence the lifespan of Caenorhabditis elegans . Nature. 2003;424(6946):277–283. doi: 10.1038/nature01789. [DOI] [PubMed] [Google Scholar]
- 13.Tullet JM, Hertweck M, An JH, Baker J, Hwang JY, Liu S, Oliveira RP, Baumeister R, Blackwell TK. Direct inhibition of the longevity-promoting factor SKN-1 by insulin-like signaling in C. elegans . Cell. 2008;132(6):1025–1038. doi: 10.1016/j.cell.2008.01.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lee SJ, Murphy CT, Kenyon C. Glucose shortens the life span of C. elegans by downregulating DAF-16/FOXO activity and aquaporin gene expression. Cell Metab. 2009;10(5):379–391. doi: 10.1016/j.cmet.2009.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hu PJ. Dauer. WormBook. 2007 doi: 10.1895/wormbook.1.144.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Murphy CT, Lee SJ, Kenyon C. Tissue entrainment by feedback regulation of insulin gene expression in the endoderm of Caenorhabditis elegans . Proc Natl Acad Sci USA. 2007;104(48):19046–19050. doi: 10.1073/pnas.0709613104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hibuse T, Maeda N, Funahashi T, Yamamoto K, Nagasawa A, Mizunoya W, Kishida K, Inoue K, Kuriyama H, Nakamura T, Fushiki T, Kihara S, Shimomura I. Aquaporin 7 deficiency is associated with development of obesity through activation of adipose glycerol kinase. Proc Natl Acad Sci USA. 2005;102(31):10993–10998. doi: 10.1073/pnas.0503291102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rojek AM, Skowronski MT, Fuchtbauer EM, Fuchtbauer AC, Fenton RA, Agre P, Frokiaer J, Nielsen S. Defective glycerol metabolism in aquaporin 9 (AQP9) knockout mice. Proc Natl Acad Sci USA. 2007;104(9):3609–3614. doi: 10.1073/pnas.0610894104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pang S, Lynn DA, Lo JY, Paek J, Curran SP. SKN-1 and Nrf2 couples proline catabolism with lipid metabolism during nutrient deprivation. Nat Commun. 2014;5:5048. doi: 10.1038/ncomms6048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bishop NA, Guarente L. Two neurons mediate diet-restriction-induced longevity in C. elegans . Nature. 2007;447(7144):545–549. doi: 10.1038/nature05904. [DOI] [PubMed] [Google Scholar]
- 21.Fontana L, Partridge L. Promoting health and longevity through diet: from model organisms to humans. Cell. 2015;161(1):106–118. doi: 10.1016/j.cell.2015.02.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Walker AK, Näär AM. SREBPs: regulators of cholesterol/lipids as therapeutic targets in metabolic disorders, cancers and viral diseases. Clin Lipidol. 2012;7(1):27–36. doi: 10.2217/clp.11.67. [DOI] [Google Scholar]
- 23.Horton JD, Shimomura I, Brown MS, Hammer RE, Goldstein JL, Shimano H. Activation of cholesterol synthesis in preference to fatty acid synthesis in liver and adipose tissue of transgenic mice overproducing sterol regulatory element-binding protein-2. J Clin Invest. 1998;101(11):2331–2339. doi: 10.1172/JCI2961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.McKay RM, McKay JP, Avery L, Graff JM. C elegans: a model for exploring the genetics of fat storage. Dev Cell. 2003;4(1):131–142. doi: 10.1016/S1534-5807(02)00411-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Walker AK, Jacobs RL, Watts JL, Rottiers V, Jiang K, Finnegan DM, Shioda T, Hansen M, Yang F, Niebergall LJ, Vance DE, Tzoneva M, Hart AC, Naar AM. A conserved SREBP-1/phosphatidylcholine feedback circuit regulates lipogenesis in metazoans. Cell. 2011;147(4):840–852. doi: 10.1016/j.cell.2011.09.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Walker AK, Yang F, Jiang K, Ji JY, Watts JL, Purushotham A, Boss O, Hirsch ML, Ribich S, Smith JJ, Israelian K, Westphal CH, Rodgers JT, Shioda T, Elson SL, Mulligan P, Najafi-Shoushtari H, Black JC, Thakur JK, Kadyk LC, Whetstine JR, Mostoslavsky R, Puigserver P, Li X, Dyson NJ, Hart AC, Naar AM. Conserved role of SIRT1 orthologs in fasting-dependent inhibition of the lipid/cholesterol regulator SREBP. Genes Dev. 2010;24(13):1403–1417. doi: 10.1101/gad.1901210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lee D, Jeong DE, Son HG, Yamaoka Y, Kim H, Seo K, Khan AA, Roh TY, Moon DW, Lee Y, Lee SJ. SREBP and MDT-15 protect C. elegans from glucose-induced accelerated aging by preventing accumulation of saturated fat. Genes Dev. 2015;29(23):2490–2503. doi: 10.1101/gad.266304.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nomura T, Horikawa M, Shimamura S, Hashimoto T, Sakamoto K. Fat accumulation in Caenorhabditis elegans is mediated by SREBP homolog SBP-1. Genes Nutr. 2010;5(1):17–27. doi: 10.1007/s12263-009-0157-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yang F, Vought BW, Satterlee JS, Walker AK, Jim Sun ZY, Watts JL, DeBeaumont R, Saito RM, Hyberts SG, Yang S, Macol C, Iyer L, Tjian R, van den Heuvel S, Hart AC, Wagner G, Naar AM. An ARC/mediator subunit required for SREBP control of cholesterol and lipid homeostasis. Nature. 2006;442(7103):700–704. doi: 10.1038/nature04942. [DOI] [PubMed] [Google Scholar]
- 30.Allen BL, Taatjes DJ. The mediator complex: a central integrator of transcription. Nat Rev Mol Cell Biol. 2015;16(3):155–166. doi: 10.1038/nrm3951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Taubert S, Van Gilst MR, Hansen M, Yamamoto KR. A Mediator subunit, MDT-15, integrates regulation of fatty acid metabolism by NHR-49-dependent and -independent pathways in C. elegans . Genes Dev. 2006;20(9):1137–1149. doi: 10.1101/gad.1395406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Goh GY, Martelli KL, Parhar KS, Kwong AW, Wong MA, Mah A, Hou NS, Taubert S. The conserved Mediator subunit MDT-15 is required for oxidative stress responses in Caenorhabditis elegans . Aging Cell. 2014;13(1):70–79. doi: 10.1111/acel.12154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.McGhee JD. The C. elegans intestine. WormBook. 2007 doi: 10.1895/wormbook.1.133.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Becard D, Hainault I, Azzout-Marniche D, Bertry-Coussot L, Ferre P, Foufelle F. Adenovirus-mediated overexpression of sterol regulatory element binding protein-1c mimics insulin effects on hepatic gene expression and glucose homeostasis in diabetic mice. Diabetes. 2001;50(11):2425–2430. doi: 10.2337/diabetes.50.11.2425. [DOI] [PubMed] [Google Scholar]
- 35.Takahashi A, Shimano H, Nakagawa Y, Yamamoto T, Motomura K, Matsuzaka T, Sone H, Suzuki H, Toyoshima H, Yamada N. Transgenic mice overexpressing SREBP-1a under the control of the PEPCK promoter exhibit insulin resistance, but not diabetes. Biochim Biophys Acta. 2005;1740(3):427–433. doi: 10.1016/j.bbadis.2004.11.006. [DOI] [PubMed] [Google Scholar]
- 36.Poitout V, Robertson RP. Glucolipotoxicity: fuel excess and beta-cell dysfunction. Endocr Rev. 2008;29(3):351–366. doi: 10.1210/er.2007-0023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Goodridge AG. Regulation of the activity of acetyl coenzyme A carboxylase by palmitoyl coenzyme A and citrate. J Biol Chem. 1972;247(21):6946–6952. [PubMed] [Google Scholar]
- 38.Ogiwara H, Tanabe T, Nikawa J, Numa S. Inhibition of rat-liver acetyl-coenzyme-A carboxylase by palmitoyl-coenzyme A. Formation of equimolar enzyme-inhibitor complex. Eur J Biochem. 1978;89(1):33–41. doi: 10.1111/j.1432-1033.1978.tb20893.x. [DOI] [PubMed] [Google Scholar]
- 39.Rabbani N, Thornalley PJ. Dicarbonyl stress in cell and tissue dysfunction contributing to ageing and disease. Biochem Biophys Res Commun. 2015;458(2):221–226. doi: 10.1016/j.bbrc.2015.01.140. [DOI] [PubMed] [Google Scholar]
- 40.Schlotterer A, Kukudov G, Bozorgmehr F, Hutter H, Du X, Oikonomou D, Ibrahim Y, Pfisterer F, Rabbani N, Thornalley P, Sayed A, Fleming T, Humpert P, Schwenger V, Zeier M, Hamann A, Stern D, Brownlee M, Bierhaus A, Nawroth P, Morcos M. C. elegans as model for the study of high glucose- mediated life span reduction. Diabetes. 2009;58(11):2450–2456. doi: 10.2337/db09-0567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hardie DG, Ross FA, Hawley SA. AMPK: a nutrient and energy sensor that maintains energy homeostasis. Nat Rev Mol Cell Biol. 2012;13(4):251–262. doi: 10.1038/nrm3311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mair W, Morantte I, Rodrigues AP, Manning G, Montminy M, Shaw RJ, Dillin A. Lifespan extension induced by AMPK and calcineurin is mediated by CRTC-1 and CREB. Nature. 2011;470(7334):404–408. doi: 10.1038/nature09706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sagi D, Kim SK. An engineering approach to extending lifespan in C. elegans . PLoS Genet. 2012;8(6):e1002780. doi: 10.1371/journal.pgen.1002780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hwang AB, Ryu EA, Artan M, Chang HW, Kabir MH, Nam HJ, Lee D, Yang JS, Kim S, Mair WB, Lee C, Lee SS, Lee SJ. Feedback regulation via AMPK and HIF-1 mediates ROS-dependent longevity in Caenorhabditis elegans . Proc Natl Acad Sci USA. 2014;111(42):E4458–E4467. doi: 10.1073/pnas.1411199111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Apfeld J, O’Connor G, McDonagh T, DiStefano PS, Curtis R. The AMP-activated protein kinase AAK-2 links energy levels and insulin-like signals to lifespan in C. elegans . Genes Dev. 2004;18(24):3004–3009. doi: 10.1101/gad.1255404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Greer EL, Dowlatshahi D, Banko MR, Villen J, Hoang K, Blanchard D, Gygi SP, Brunet A. An AMPK-FOXO pathway mediates longevity induced by a novel method of dietary restriction in C. elegans . Curr Biol. 2007;17(19):1646–1656. doi: 10.1016/j.cub.2007.08.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Schulz TJ, Zarse K, Voigt A, Urban N, Birringer M, Ristow M. Glucose restriction extends Caenorhabditis elegans life span by inducing mitochondrial respiration and increasing oxidative stress. Cell Metab. 2007;6(4):280–293. doi: 10.1016/j.cmet.2007.08.011. [DOI] [PubMed] [Google Scholar]
- 48.Yun J, Finkel T. Mitohormesis. Cell Metab. 2014;19(5):757–766. doi: 10.1016/j.cmet.2014.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Choi SS. High glucose diets shorten lifespan of Caenorhabditis elegans via ectopic apoptosis induction. Nutr Res Pract. 2011;5(3):214–218. doi: 10.4162/nrp.2011.5.3.214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kitaoka S, Morielli AD, Zhao FQ. FGT-1 is a mammalian GLUT2-like facilitative glucose transporter in Caenorhabditis elegans whose malfunction induces fat accumulation in intestinal cells. PLoS One. 2013;8(6):e68475. doi: 10.1371/journal.pone.0068475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Feng Y, Williams BG, Koumanov F, Wolstenholme AJ, Holman GD. FGT-1 is the major glucose transporter in C. elegans and is central to aging pathways. Biochem J. 2013;456(2):219–229. doi: 10.1042/BJ20131101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Svensk E, Devkota R, Stahlman M, Ranji P, Rauthan M, Magnusson F, Hammarsten S, Johansson M, Boren J, Pilon M. Caenorhabditis elegans PAQR-2 and IGLR-2 protect against glucose toxicity by modulating membrane lipid composition. PLoS Genet. 2016;12(4):e1005982. doi: 10.1371/journal.pgen.1005982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Tauffenberger A, Vaccaro A, Parker JA. Fragile lifespan expansion by dietary mitohormesis in C. elegans . Aging (Albany, NY) 2016;8(1):50–61. doi: 10.18632/aging.100863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Svensk E, Stahlman M, Andersson CH, Johansson M, Boren J, Pilon M. PAQR-2 regulates fatty acid desaturation during cold adaptation in C. elegans . PLoS Genet. 2013;9(9):e1003801. doi: 10.1371/journal.pgen.1003801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Iwabu M, Okada-Iwabu M, Yamauchi T, Kadowaki T. Adiponectin/adiponectin receptor in disease and aging. NPJ Aging Mech Dis. 2015;1:15013. doi: 10.1038/npjamd.2015.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Jensen MB, Jasper H. Mitochondrial proteostasis in the control of aging and longevity. Cell Metab. 2014;20(2):214–225. doi: 10.1016/j.cmet.2014.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Tauffenberger A, Vaccaro A, Aulas A, Vande Velde C, Parker JA. Glucose delays age-dependent proteotoxicity. Aging Cell. 2012;11(5):856–866. doi: 10.1111/j.1474-9726.2012.00855.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.De Virgilio C, Hottiger T, Dominguez J, Boller T, Wiemken A. The role of trehalose synthesis for the acquisition of thermotolerance in yeast. I. Genetic evidence that trehalose is a thermoprotectant. Eur J Biochem. 1994;219(1–2):179–186. doi: 10.1111/j.1432-1033.1994.tb19928.x. [DOI] [PubMed] [Google Scholar]
- 59.Watanabe M, Kikawada T, Minagawa N, Yukuhiro F, Okuda T. Mechanism allowing an insect to survive complete dehydration and extreme temperatures. J Exp Biol. 2002;205(Pt 18):2799–2802. doi: 10.1242/jeb.205.18.2799. [DOI] [PubMed] [Google Scholar]
- 60.Sakurai M, Furuki T, Akao K, Tanaka D, Nakahara Y, Kikawada T, Watanabe M, Okuda T. Vitrification is essential for anhydrobiosis in an African chironomid, Polypedilum vanderplanki . Proc Natl Acad Sci USA. 2008;105(13):5093–5098. doi: 10.1073/pnas.0706197105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Elbein AD, Pan YT, Pastuszak I, Carroll D. New insights on trehalose: a multifunctional molecule. Glycobiology. 2003;13(4):17R–27R. doi: 10.1093/glycob/cwg047. [DOI] [PubMed] [Google Scholar]
- 62.Honda Y, Tanaka M, Honda S. Trehalose extends longevity in the nematode Caenorhabditis elegans . Aging Cell. 2010;9(4):558–569. doi: 10.1111/j.1474-9726.2010.00582.x. [DOI] [PubMed] [Google Scholar]
- 63.Mouchiroud L, Molin L, Kasturi P, Triba MN, Dumas ME, Wilson MC, Halestrap AP, Roussel D, Masse I, Dalliere N, Segalat L, Billaud M, Solari F. Pyruvate imbalance mediates metabolic reprogramming and mimics lifespan extension by dietary restriction in Caenorhabditis elegans . Aging Cell. 2011;10(1):39–54. doi: 10.1111/j.1474-9726.2010.00640.x. [DOI] [PubMed] [Google Scholar]
- 64.Cho SC, Park MC, Keam B, Choi JM, Cho Y, Hyun S, Park SC, Lee J. DDS, 4,4′-diaminodiphenylsulfone, extends organismic lifespan. Proc Natl Acad Sci USA. 2010;107(45):19326–19331. doi: 10.1073/pnas.1005078107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Edwards CB, Copes N, Brito AG, Canfield J, Bradshaw PC. Malate and fumarate extend lifespan in Caenorhabditis elegans . PLoS One. 2013;8(3):e58345. doi: 10.1371/journal.pone.0058345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Chin RM, Fu X, Pai MY, Vergnes L, Hwang H, Deng G, Diep S, Lomenick B, Meli VS, Monsalve GC, Hu E, Whelan SA, Wang JX, Jung G, Solis GM, Fazlollahi F, Kaweeteerawat C, Quach A, Nili M, Krall AS, Godwin HA, Chang HR, Faull KF, Guo F, Jiang M, Trauger SA, Saghatelian A, Braas D, Christofk HR, Clarke CF, Teitell MA, Petrascheck M, Reue K, Jung ME, Frand AR, Huang J. The metabolite alpha-ketoglutarate extends lifespan by inhibiting ATP synthase and TOR. Nature. 2014;510(7505):397–401. doi: 10.1038/nature13264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Denzel MS, Storm NJ, Gutschmidt A, Baddi R, Hinze Y, Jarosch E, Sommer T, Hoppe T, Antebi A. Hexosamine pathway metabolites enhance protein quality control and prolong life. Cell. 2014;156(6):1167–1178. doi: 10.1016/j.cell.2014.01.061. [DOI] [PubMed] [Google Scholar]
- 68.Roux AE, Leroux A, Alaamery MA, Hoffman CS, Chartrand P, Ferbeyre G, Rokeach LA. Pro-aging effects of glucose signaling through a G protein-coupled glucose receptor in fission yeast. PLoS Genet. 2009;5(3):e1000408. doi: 10.1371/journal.pgen.1000408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Weinberger M, Mesquita A, Caroll T, Marks L, Yang H, Zhang Z, Ludovico P, Burhans WC. Growth signaling promotes chronological aging in budding yeast by inducing superoxide anions that inhibit quiescence. Aging (Albany NY) 2010;2(10):709–726. doi: 10.18632/aging.100215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Semchyshyn HM, Lozinska LM, Miedzobrodzki J, Lushchak VI. Fructose and glucose differentially affect aging and carbonyl/oxidative stress parameters in Saccharomyces cerevisiae cells. Carbohydr Res. 2011;346(7):933–938. doi: 10.1016/j.carres.2011.03.005. [DOI] [PubMed] [Google Scholar]
- 71.Suarez G, Rajaram R, Oronsky AL, Gawinowicz MA. Nonenzymatic glycation of bovine serum albumin by fructose (fructation). Comparison with the Maillard reaction initiated by glucose. J Biol Chem. 1989;264(7):3674–3679. [PubMed] [Google Scholar]
- 72.Mortuza R, Chen S, Feng B, Sen S, Chakrabarti S. High glucose induced alteration of SIRTs in endothelial cells causes rapid aging in a p300 and FOXO regulated pathway. PLoS One. 2013;8(1):e54514. doi: 10.1371/journal.pone.0054514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Zhang B, Cui S, Bai X, Zhuo L, Sun X, Hong Q, Fu B, Wang J, Chen X, Cai G. SIRT3 overexpression antagonizes high glucose accelerated cellular senescence in human diploid fibroblasts via the SIRT3-FOXO1 signaling pathway. Age (Dordr) 2013;35(6):2237–2253. doi: 10.1007/s11357-013-9520-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Imai S, Guarente L. NAD+ and sirtuins in aging and disease. Trends Cell Biol. 2014;24(8):464–471. doi: 10.1016/j.tcb.2014.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Kuki S, Imanishi T, Kobayashi K, Matsuo Y, Obana M, Akasaka T. Hyperglycemia accelerated endothelial progenitor cell senescence via the activation of p38 mitogen-activated protein kinase. Circ J. 2006;70(8):1076–1081. doi: 10.1253/circj.70.1076. [DOI] [PubMed] [Google Scholar]
- 76.Samaha FF, Iqbal N, Seshadri P, Chicano KL, Daily DA, McGrory J, Williams T, Williams M, Gracely EJ, Stern L. A low-carbohydrate as compared with a low-fat diet in severe obesity. N Engl J Med. 2003;348(21):2074–2081. doi: 10.1056/NEJMoa022637. [DOI] [PubMed] [Google Scholar]
- 77.Foster GD, Wyatt HR, Hill JO, McGuckin BG, Brill C, Mohammed BS, Szapary PO, Rader DJ, Edman JS, Klein S. A randomized trial of a low-carbohydrate diet for obesity. N Engl J Med. 2003;348(21):2082–2090. doi: 10.1056/NEJMoa022207. [DOI] [PubMed] [Google Scholar]
- 78.Rosedale R, Westman EC, Konhilas JP. Clinical experience of a diet designed to reduce aging. J Appl Res. 2009;9(4):159–165. [PMC free article] [PubMed] [Google Scholar]
- 79.Le Couteur DG, Solon-Biet S, Cogger VC, Mitchell SJ, Senior A, de Cabo R, Raubenheimer D, Simpson SJ. The impact of low-protein high-carbohydrate diets on aging and lifespan. Cell Mol Life Sci. 2016;73(6):1237–1252. doi: 10.1007/s00018-015-2120-y. [DOI] [PMC free article] [PubMed] [Google Scholar]