Abstract
Neuropathic pain resulting from damage or dysfunction of the nervous system is a highly debilitating chronic pain state and is often resistant to currently available treatments. It has become clear that neuroinflammation, mainly mediated by proinflammatory cytokines and chemokines, plays an important role in the establishment and maintenance of neuropathic pain. Chemokines were originally identified as regulators of peripheral immune cell trafficking and were also expressed in neurons and glial cells in the central nervous system. In recent years, accumulating studies have revealed the expression, distribution and function of chemokines in the spinal cord under chronic pain conditions. In this review, we provide evidence showing that several chemokines are upregulated after peripheral nerve injury and contribute to the pathogenesis of neuropathic pain via different forms of neuron–glia interaction in the spinal cord. First, chemokine CX3CL1 is expressed in primary afferents and spinal neurons and induces microglial activation via its microglial receptor CX3CR1 (neuron-to-microglia signaling). Second, CCL2 and CXCL1 are expressed in spinal astrocytes and act on CCR2 and CXCR2 in spinal neurons to increase excitatory synaptic transmission (astrocyte-to-neuron signaling). Third, we recently identified that CXCL13 is highly upregulated in spinal neurons after spinal nerve ligation and induces spinal astrocyte activation via receptor CXCR5 (neuron-to-astrocyte signaling). Strategies that target chemokine-mediated neuron-glia interactions may lead to novel therapies for the treatment of neuropathic pain.
Keywords: Neuroinflammation, Astrocytes, Microglia, Spinal cord, Chronic pain
Introduction
Neuropathic pain, resulting from a direct consequence of various lesions or diseases affecting the somatosensory nervous system, is common clinically but there is a lack of effective treatments [1]. Neuropathic pain is manifested as spontaneous pain, hyperalgesia (enhanced pain evoked by a noxious stimulus), and allodynia (pain evoked by innocuous stimulus) [1]. It has been thought that neural plasticity, which includes both peripheral sensitization in the dorsal root ganglion (DRG), and central sensitization in the spinal cord and supraspinal areas, is responsible for the development and maintenance of neuropathic pain [2–4]. Based on this concept, neuronal mechanisms underlying neuropathic pain have been widely investigated. Indeed, after nerve injury, a variety of receptors, transmitters, and inflammatory mediators are upregulated in DRG neurons, which increase the neuronal excitability and contribute to peripheral sensitization [2, 5–7]. In the spinal cord, nerve injury increases NMDA receptor- and AMPA receptor-mediated excitatory synaptic transmission, and decreases GABA receptor- and glycine receptor-mediated inhibitory synaptic transmission in dorsal horn neurons which contribute to central sensitization [8–10].
In recent years, it has been accepted that besides neurons, non-neuronal cells such as immune cells (macrophages and lymphocytes) and glial cells [Schwann cells and satellite cells in the peripheral nervous system (PNS), and astrocytes and microglia in the central nervous system (CNS)] play an important role in the induction and maintenance of neuropathic pain [11–15]. Nerve injury induces sequential activation of microglia and astrocytes in the spinal cord. Microglia marker (CD11b) mRNA is increased at 4 h post nerve injury, continued to increase at day 14, and returned to basal levels at day 28 [16]. In contrast, astrocyte marker (GFAP) mRNA is increased from day 4 post nerve injury, maintained at high level at day 28 [16]. Inhibition of microglial activation by minocycline prevents or delays the development of neuropathic pain [12, 17, 18], whereas inhibition of astrocytes by fluorocitrate or l-α-aminoadipate alleviates established neuropathic pain [11, 17]. Given that both astrocytes and microglia have physiological functions and inhibition of glial activation by toxins may cause side effects [19], a deep understanding of the communication between glial cells and neurons under chronic pain conditions is essential for the development of novel therapies.
Neuroinflammation has been well demonstrated to play an important role in the pathogenesis of neurodegenerative diseases and chronic pain [4]. Glial cells contribute to neuroinflammation by releasing proinflammatory cytokines [such as the tumor necrosis factor (TNF-α) and interleukin-1β (IL-1β)], growth factors, and chemokines (such as CCL2 and CXCL1) [4, 11, 20–22]. On the other hand, inflammatory mediators are also released from primary afferents and spinal neurons to induce glial activation [4, 23–25]. Among the inflammatory mediators, chemokines are implicated in peripheral sensitization and central sensitization after nerve injury or tissue damage [4, 21, 22, 24–26]. In this review, we summarize the evidence for the involvement of chemokines and chemokine receptors in neuropathic pain, focusing on how chemokines regulate nerve injury-induced neuropathic pain via different forms neuron–glia interaction in the spinal cord.
Chemokines and receptors
Chemokines are small secreted proteins (8–14 kDa) that were initially characterized as chemotactic peptides to control the trafficking of leukocytes [6, 27, 28]. Chemokines have conserved cysteine residues in their amino acid sequences. Based on the presence and position of the first cysteine residues, chemokines are classified into four subfamilies: CC, CXC, XC, and CX3C [2]. The CC group has the first two of total four cysteines in adjacent position, the CXC group has the two of the four cysteines separated by an intervening animo acid, the XC group has only two cysteine residues, and the CX3C group has three amino acids between two cysteine residues. Approximately 50 chemokines have been identified in mammalian species. The CC group and CXC group are the largest groups containing 28 (CCL1-28) and 16 (CXCL1-16) members, respectively. The XC group includes two members (XCL1 and XCL2), and the CX3C group has only one member CX3CL1.
The functions of chemokines are exerted through their respective receptors, which are seven-transmembrane-domain G-protein-coupled receptors (GPCRs) with a serine/threonine-rich intracellular C-terminal domain and an acidic N-terminal extracellular domain. Chemokine receptors are divided into different families, CC chemokine receptors (CCR1-10), CXC chemokine receptors (CXCR1-7), XC chemokine receptors (XCR1), and CX3C chemokine receptors (CX3CR1), that correspond to the four distinct subfamilies of chemokines they bind. Except for some monogamous chemokine–chemokine receptor pairs, such as CXCL13–CXCR5, CXCL16–CXCR6, and CX3CL1–CX3CR1, most chemokines activate multiple receptors. A single receptor can also be activated by diverse chemokines [2, 29]. The large number of chemokines and the promiscuous interaction of chemokines and receptors make their functions complex.
It is well known that chemokines play an important role in the immune system. Studies show that chemokines are also involved in several other processes, including cardiogenesis, vascular development, cell proliferation, angiogenesis, and metastasis [30–33]. In particular, chemokines and their receptors are involved in the pathogenesis of neurodegenerative diseases such as multiple sclerosis (MS), Alzheimer’s disease (AD), as well as in neurological disorders, such as stroke and trauma [34–36]. In recent years, the expression, distribution, and function of chemokines and chemokine receptors in neuropathic pain have been investigated in different animal models, such as spinal nerve ligation (SNL) [37], spared nerve injury (SNI) [38], chronic constriction of sciatic nerve (CCI) [39], partial sciatic nerve ligation (pSNL) [40], and chronic compression of the DRG (CCD) [41]. Chemokine pairs CX3CL1/CX3CR1 has been demonstrated to be involved in neuropathic pain via neuron–microglia interaction in the spinal cord (see reviews [2, 25, 27, 29, 42]). Recent studies showed that CXCL1/CXCR2 and CXCL13/CXCR5 contribute to neuropathic pain via different forms of neuron-glia interaction.
Chemokines contribute to the pathogenesis of neuropathic pain via different forms of neuron–glia interaction in the spinal cord
CX3CL1–CX3CR1 regulates neuropathic pain via neuron–microglia interaction
CX3CL1 (also called fractalkine), the only member of the CX3C group, is constitutively expressed in the normal PNS and CNS, where it is found in neurons [43, 44]. This profile was also confirmed by the CX3CL1-mCherry transgenic mice [45]. However, CX3CL1 can be induced in spinal astrocytes by peripheral nerve injury [46]. CX3CL1 is a unique chemokine in that it binds to only one known receptor (CX3CR1), and that chemokine receptor binds only CX3CL1 [47, 48].
The expression and distribution of CX3CL1 and CX3CR1 after nerve injury
CX3CL1 is also unique as it has two forms (a ~ 100 kDa large form and a ~ 80 kDa small form) corresponding to membrane-bound and soluble form, respectively. Neuronal injury or peripheral nerve injury does not change the total expression of CX3CL1 mRNA in brain and spinal neurons, but the membrane-associated CX3CL1 protein is decreased [25, 43, 44, 49]. However, a recent report showed that CX3CL1 protein is upregulated in spinal neurons via NFκB-dependent H4 acetylation in paclitaxel-induced neuropathic pain [50]. Peripheral nerve injury also induces a marked reduction of membrane-bound form and an increase of soluble form in the DRG [51]. Dorsal rhizotomy greatly reduces CX3CL1 immunoreactivity in nerve fibers in the spinal dorsal horn, suggesting that DRG neurons are also the source of the CX3CL1 in the spinal dorsal horn [43]. The shedding of mature CX3CL1 is executed by various proteases, including cathepsin S (CatS), metalloproteases, ADAM10 (a closely related disintegrin-like metalloproteinase 10), and ADAM-17 (TACE, tumor necrosis factor-alpha converting enzyme) [25, 52–54]. CatS is expressed in vascular smooth cells and can cleave CX3CL1 into a soluble smaller size form (~55 kDa) [55]. In vitro study shows that CatS incubation reduces the level of sensory neuron-associated CX3CL1 on the neuron membrane [56]. In the spinal cord, CatS is secreted by activated spinal microglia and upregulated after nerve injury to cleave the soluble CX3CL1 from spinal local neurons and primary afferent fibers [49, 56]. The metalloproteases also play an important role in shedding of mature CX3CL1. Application of the general matrix metalloprotease inhibitor batimastat dose-dependently inhibits the cleavage of CX3CL1 from the membranes of glutamate-treated primary cortical neurons [44]. Whether ADAM-17 or ADAM-10 contributes to soluble CX3CL1 shedding in the spinal cord has not been evaluated.
CX3CR1 is found on the microglia of CNS, satellite glial cells of DRG, and macrophages of peripheral nerves, but not in astrocytes, neurons, or oligodendrocytes [25, 57, 58]. In naïve or CFA (Complete Freund’s adjuvant)-injected animals, the immunoreactivity of CX3CR1 in spinal microglia is weak [46]. However, following peripheral nerve injury or bone cancer, the extensive upregulation of CX3CR1 occurs in microglia of the dorsal horn [43, 46, 51, 58].
CX3CL1–CX3CR1 mediates neuron–microglia interaction in the spinal cord
Currently, the mechanisms underlying CX3CL1-induced pain facilitation are well demonstrated. In peripheral nerve, the CX3CR1-expressing macrophages are critical for the initial development of chemotherapy-induced neuropathic pain [59]. In the DRG, CX3CR1-expressing satellites contribute to the genesis of inflammatory pain [60]. In the spinal cord, the CatS from activated microglia results in the production of soluble CX3CL1, which acts on CX3CR1 of microglia [49, 61]. Therefore, CatS-CX3CL1–CX3CR1 forms a positive feedback loop in peripheral nerve injury-induced neuropathic pain [49, 56, 61].
P38, a member of mitogen-activated protein kinases (MAPKs), is an important downstream kinase of CX3CL1/CX3CR1 signaling [51, 56]. Intrathecal delivery of a CX3CR1 neutralizing antibody decreases the activation of p38 in spinal microglia following SNL [51]. Conversely, intrathecal injection of CX3CL1 induces p38 activation in microglia [51, 56]. Activation of p38 induces synthesis of proinflammatory cytokines such as TNF-α, IL-1β, and IL-6 in microglia [62], which can regulate synaptic transmission in the superficial spinal dorsal horn [2]. Accumulating evidence has shown that activated microglia contribute to spinal long-term potentiation (LTP) and pathological pain [63, 64]. CX3CL1/CX3CR1 signaling is also involved in tetanic stimulation of the sciatic nerve-induced pain hypersensitivity and LTP in the spinal dorsal horn [65, 66].
CX3CL1 and CX3CR1 contribute to neuropathic pain
A growing body of evidence supports the involvement of CX3CL1 in neuropathic pain. Intrathecal administration of CX3CL1 induces dose-dependent mechanical allodynia and thermal hyperalgesia in naive rats and mice [56, 67]. In addition, intrathecal administration of CX3CL1 neutralizing antibody reverses neuropathic pain and CatS-induced mechanical hyperalgesia [56]. Intrathecal delivery of CX3CR1-neutralizing antibody attenuates the development of neuropathic pain and also blocks CX3CL1-induced mechanical allodynia and thermal hyperalgesia in naïve animals [60, 67]. In Cx3cr1 knockout (KO) mice, microglial activation is reduced in the spinal cord, mechanical allodynia is not developed, and thermal hypersensitivity is delayed following pSNL compared to their C57BL/6 background wild type (WT) littermates [58]. However, it was also reported that Cx3cr1 KO mice display an increase in allodynia for 3 weeks after SNI compared to strain-matched Balb/c controls. In addition, intra-neural injection into the sciatic nerve of CX3CL1 delayed the development of alldynia for 3 days following SNI [68]. The reason for this discrepancy is not clear, but it could be due to the differences in the mice (C57Bl/6 vs. Balb/c) and the models (pSNL vs. SNI). In addition, as most of chemokines and receptors are widely expressed in different cell types in the PNS and CNS, cre-lox systems are needed in future studies to clarify the specific role of a chemokine/receptor in different cell type and different anatomical area.
CCL2–CCR2 and CXCL1–CXCR2 regulate neuropathic pain via astrocyte–neuron interaction in the spinal cord
CCL2–CCR2
CCL2 is also known as monocyte chemoattractant protein 1 (MCP-1). CCL2 can activate CCR2 and CCR11. However, CCR2 is its preferred receptor [69–71]. Besides CCL2, CCR2 can also be activated by CCL7, CCL8, and CCL13. Binding analysis indicates that CCR2 binds CCL2 with ten times higher affinity than CCL7 and CCL8 in mouse tissues [72]. A recent study showed that CCL7 (monocyte chemotactic protein 3, MCP-3), which is produced by spinal astrocytes, acts on CCR2 in microglia to induce spinal microglial activation [73], suggesting that CCR2 can also be recognized by CCL7 in the spinal cord.
The expression and distribution of CCL2 and CCR2 after nerve injury
CCL2 is constitutively expressed in DRG neurons [74]. After peripheral nerve injury, CCL2 expression in ipsilateral DRG is dramatically upregulated in small and large neurons [75–78]. In addition, CCL2 in DRG neurons can also be transported to central terminal in the spinal cord. CCL2 is found in SP (Substance P)- and CGRP (Calcitonin rene-related peptide)-positive primary afferents in the superficial dorsal horn [21, 23, 70, 74, 79–81]. In vivo immunohistochemistry shows that CCL2 is also expressed in astrocytes of the spinal cord and is upregulated after nerve injury [21]. The increase of CCL2 expression in astrocytes is shown at days 3 and 10, and peaked at day 3 in the spinal cord after SNL [21]. In cultured astrocytes, TNF-α induces a c-Jun N-terminal kinase (JNK)-dependent expression and release of CCL2 [21]. Intrathecal injection of TNF-α also induces JNK-dependent CCL2 upregulation in the spinal cord [21] (Fig. 1). CCL2 is also expressed in astrocytes in the medulla oblongata after the inferior alveolar and mental nerve transection (IAMNT) or experimental tooth movement (ETM) [26, 82], as well as in brain after demyelinating injury [83], focal cerebral ischemia [84], and entorhinodentate lesions [85].
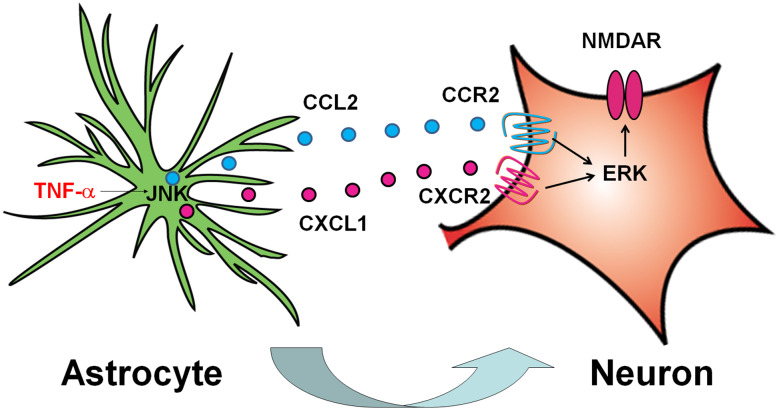
Fig. 1.
Schematic shows CCL2/CCR2 and CXCL1/CXCR2 mediate astrocyte-to-neuron crosstalk in the spinal cord. SNL induces rapid TNF-α expression and release, which activates JNK and further induces CCL2 and CXCL1 expression in astrocytes. These chemokines are released from astrocytes to act on spinal neurons via CCR2 and CXCR2, respectively. The activated CCR2 and CXCR2 activates ERK, which may phosphorylate NMDA receptor and enhance synaptic transmission
CCR2 is expressed and upregulated in the DRG after sciatic nerve demyelination [70, 76, 77]. Chronic constriction of DRG (CCD) induces CCR2 mRNA expression in neurons and non-neuronal cells in DRG [77]. In addition, CCR2 is partially colocalized with CCL2, suggesting a possible autocrine/paracrine role for CCL2/CCR2 signaling within the DRG [70]. In the spinal cord, CCR2 has low expression under normal conditions [21] and is upregulated in spinal microglia after peripheral nerve injury [23, 73, 79, 86]. However, our in situ hybridization data show that CCR2 is expressed and increased in spinal neurons after peripheral nerve injury [21]. Jung et al. also reported a weak but clear GFP signal in spinal dorsal horn neurons in CCR2-GFP reporter mice [76]. CCR2 is also upregulated in neurons of the ipsilateral medulla oblongata after IAMNT [26].
CCL2–CCR2 mediates astrocyte–neuron interaction in the spinal cord
Previous studies showed that intrathecal injection of high-dose CCL2 induces microglial activation and mechanical allodynia in WT mice, but not in Ccr2 KO mice [21, 87, 88]. In addition, microglial activation is not developed after peripheral nerve injury in Ccr2 KO mice [79, 86]. Nerve injury-induced p38 activation in microglia is also attenuated in Ccr2 KO mice [79, 86]. These data suggest that CCL2 is involved in microglial activation via CCR2 in the spinal cord under neuropathic pain condition.
However, accumulating evidence also supports that CCL2 and CCR2 mediate astrocyte–neuron interaction in the spinal cord. Electrophysiological studies show that CCL2 can immediately (within 2 min) enhance NMDA- and AMPA-induced current and increase the frequency of sEPSCs in spinal cord lamina II neurons [21]. Perfusion of spinal cord slices with CCL2 induces rapid phosphorylation of extracellular-signal-regulated kinase (ERK), a nociceptive-specific marker [89, 90] in superficial dorsal horn neurons within 5 min [21]. In addition, co-stimulation of CCL2 with GABA to cultured spinal cord neurons results in rapid dose-dependent reduction of GABA-induced inward currents [91]. These data suggest that CCL2, released from primary afferents and astrocytes, can act on CCR2 in neurons and regulate synaptic transmission in the spinal cord (Fig. 1).
CCL2 and CCR2 contribute to neuropathic pain
Behavioral evidence shows that intrathecal administration of CCL2 induces rapid heat hyperalgesia, starting at 15 min and recovering at 24 h [21]. Mice over-expressing CCL2 in astrocytes display enhanced nociceptive responses [92]. Intrathecal injection of CCL2 neutralizing antibody reduces SNL- or CCI-induced mechanical allodynia [21, 88]. The siRNA targeting Ccr2 reverses the nociceptive behaviors induced by CCL2 intrathecal injection or CFA intraplantar injection [93]. In addition, the development of mechanical allodynia is totally abrogated after peripheral nerve injury in Ccr2 KO mice [79, 86]. The magnitude of the nocifensive behavior induced by IAMNT or ETM is reduced by intracisternal injection of CCR2 antagonists in a dose-dependent manner [26, 82].
CXCL1–CXCR2
CXCL1 is one member of the CXC family and also known as keratinocyte-derived chemokines (KC), growth-related oncogene (GRO), or cytokine-induced neutrophil chemoattractant-1 (CINC-1). CXCL1 in rodents has similar biological functions as interleukin-8 (IL-8) in human [94]. In the peripheral tissue, CXCL1 is expressed and secreted by activated macrophages, endothelial cells, and fibroblast cells. CXCL1 is involved in neutrophil chemotaxis and degranulation at the early stage of inflammation and is involved in inflammatory pain and acute post-incisional pain [95, 96]. The role of CXCL1 is dependent on its primary receptor CXCR2 [95, 96]. In brain tissue, CXCL1 is expressed by neurons, microglia, and oligodendrocytes [97–99]. CXCR2 has been detected on neurons, microglia, and oligodendrocyte progenitors in the brain [97–99].
The expression and distribution of CXCL1 and CXCR2 after nerve injury
In the spinal cord, CXCL1 is weakly expressed in naïve animal and increased after peripheral nerve injury [22, 100]. ELISA shows that CXCL1 expression is increased from day 3, peaked at day 10, and maintained at day 21 after SNL. The upregulation of TNF-α is earlier than the increase of CXCL1 after SNL, and TNF-α inhibitor blocks SNL-induced CXCL1 upregulation [22], suggesting that TNF-α is an important trigger for CXCL1 expression in the spinal cord. In addition, immunostaining shows that CXCL1 is primarily expressed by astrocytes in naive mice and increased after SNL (Fig. 2a–c), spinal cord injury, or inoculation of cancer cells into the bone [22, 101, 102]. CXCL1 is induced in human astrocytes in the active multiple sclerosis lesions [103]. In cultured astrocytes, TNF-α increases the expression of connexin 43 (Cx43), a hemichannel expressed on astrocytes, and controls the release of astrocytic mediators. TNF-α-induced release of CXCL1 is blocked by carbenoxolone (a non-selective hemichannel blocker), Gap26/Gap27 (selective Cx43 blockers), or Cx43 siRNA [104], suggesting that CXCL1 is expressed in astrocytes and the release is controlled by gap junction.
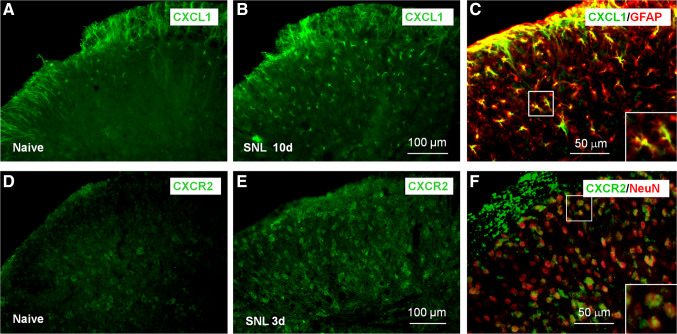
Fig. 2.
SNL induces the upregulation of CXCL1 and CXCR2 in the spinal cord. a, b Immunostaining shows the expression of CXCL1 in the spinal cord of naïve mice (a) and SNL 10d mice (b). c Double staining shows that CXCL1 is colocalized with astrocytic marker GFAP in the spinal dorsal horn. d, e Immunostaining shows the expression of CXCR2 in the spinal cord of naïve mice (d) and SNL 3d mice (e). f Double staining shows that CXCR2 is colocalized with neuronal marker NeuN in the spinal dorsal horn. The images are reproduced from a paper by Zhang et al. [22] (Pain) with permission
CXCR2 mRNA and protein are also upregulated in the spinal cord in peripheral nerve injury-induced neuropathic pain, incisional postoperative pain, CFA-induced inflammatory pain, and bone cancer pain (BCP) [22, 102, 105, 106]. Immunostaining shows the CXCR2 is located in the spinal neurons (Fig. 2d–f), but not in spinal astrocytes or microglia in mice 10 days after SNL or 14 days after cancer cells inoculation into the bone [22, 102].
CXCL1–CXCR2 mediates astrocyte–neuron interaction in the spinal cord
CXCL1 and CXCR2 are, respectively, expressed in astrocytes and neurons in the spinal cord, indicating that they may be involved in the interaction of astrocytes and neurons (Fig. 1). Indeed, intrathecal injection of CXCL1 not only induces CXCR2-dependent thermal hyperalgesia, but also induces ERK activation and c-Fos expression in spinal neurons [22]. Electrophysiological recording shows that CXCL1 increases NMDA-induced currents via CXCR2 in neurons of dorsal horn lamina II [22, 105]. Furthermore, nerve injury-induced sEPSC in spinal lamina IIo nociceptive synapses in the late phase is suppressed by carbenoxolone and Gap27 and recapitulated by CXCL1 [104]. Compared to the rapid (1 day) onset of neuropathic pain after SNL, CXCL1 is increased slowly (3 days) [22], indicating that CXCL1 may not initiate the neuropathic pain, but involves in the maintenance of neuropathic pain.
CXCL1 and CXCR2 contribute to neuropathic pain
Inhibition of CXCL1 alleviates neuropathic pain induced by peripheral nerve injury. Intrathecal injection of CXCL1 neutralizing antibody partially reduces pSNL- and SNL-induced mechanical allodynia [22, 100]. Intraspinal injection of CXCL1 shRNA lentivirus vectors leads to persistent knockdown of CXCL1 expression, produces a marked and persistent anti-hyperalgesic and anti-allodynic effect in SNL mice [22], indicating the role of CXCL1 in the maintenance of neuropathic pain.
Intrathecal injection of CXCR2 antagonist SB225002 blocks CXCL1-induced heat hyperalgesia [22]. Intrathecal injection of SB225002 also dose-dependently reduces mechanical allodynia and heat hyperalgesia induced by SNL or hind paw incision [22, 107]. Intraperitoneal injection of SB225002 displays prominent and long-lasting antinociceptive effect in pSNL mice [108]. These data indicate that CXCL1/CXCR2 signaling is important in the pathogenesis of neuropathic pain.
Interestingly, CXCL1 level is increased in the cerebrospinal fluid (CSF) samples from opioid-tolerant cancer patients [109]. Rats with morphine tolerance also show increased CXCL1 mRNA in the spinal cord. In addition, the development of tolerance of morphine in rats is accelerated by coadministration of CXCL1 and attenuated by coadministration of CXCL1-neutralizing antibody or CXCR2 antagonist [109], suggesting that CXCL1/CXCR2 signaling may be a novel target for the treatment of opioid tolerance.
CXCL13–CXCR5 contributes to neuropathic pain via neuron–astrocyte interaction
CXCL13, also known as B lymphocyte chemoattractant (BLC), was originally identified to be produced by stromal cells in B cell follicles [110, 111]. CXCR5, the only receptor for CXCL13 [110] and also called CD185 (cluster of differentiation 185) or Burkitt lymphoma receptor 1 (BLR1), is expressed on all B cells and a subset of T cells in blood, lymphatic tissue, and CSF [112, 113]. CXCL13 strongly attracts B lymphocytes and some T cells and macrophages via CXCR5 in peripheral lymphoid organs such as spleen, lymph nodes, and Peyer’s patches [114]. CXCL13 was thought not to be expressed in the healthy CNS but upregulated due to the infiltration of immune cells under pathological conditions [115–117]. For example, CXCL13 was found in infiltrating dendritic cells in the inflamed brain meninges and spinal cord in mice with experimental autoimmune encephalomyelitis (EAE) [115, 116]. Several studies showed that CXCL13 level correlates directly with the number of B cells in brain tissue and CSF [113], while successful treatment of multiple sclerosis patients is associated with parallel declines of CXCL13 levels and CSF B cell counts [118–120]. However, it was also reported that CXCL13-deficient animals show normal CNS B cell recruitment but mild, self-limited form of EAE [115, 121], indicating that CXCL13/CXCR5 in the CNS may regulate this neuroinflammatory disease via B cell-independent mechanisms.
The expression and distribution of CXCL13 and CXCR5 after nerve injury
A recent study showed that spinal cord has low level of CXCL13 and CXCR5 in naïve mice and non-diseased humans [24]. In addition, CXCL13 is the most upregulated gene in 37 detectable chemokines in the spinal cord 10 days after SNL. The 47-fold increase of CXCL13 is more than that of CCL7, CCL2, and CXCL1, which are known to be involved in neuropathic pain [21, 22, 73]. CXCL13 mRNA expression is increased as early as 1 day after SNL and persists for more than 21 days [24]. The protein level of this chemokine is also elevated in the ipsilateral dorsal horn of spinal cord (Fig. 3a, b), as well as in the CSF 10 days after SNL. Furthermore, CXCL13 is restricted to neurons (Fig. 3c), with no expression in astrocytes or microglia in the spinal cord [24]. Interestingly, the increase in CXCL13 gene transcription after SNL is regulated by a non-coding microRNA, miR-186-5p, which is downregulated after SNL via NMDA receptor in spinal neurons (Fig. 4) [122]. Besides the spinal cord, CXCL13 mRNA is increased in the DRG 14 days after local inflammation of DRG or 3 days after SNL or modified SNL model in rats [123]. CXCL13 mRNA and protein expression is also increased in the trigeminal ganglion (TG) after partial infraorbital nerve ligation (pIONL) [122].
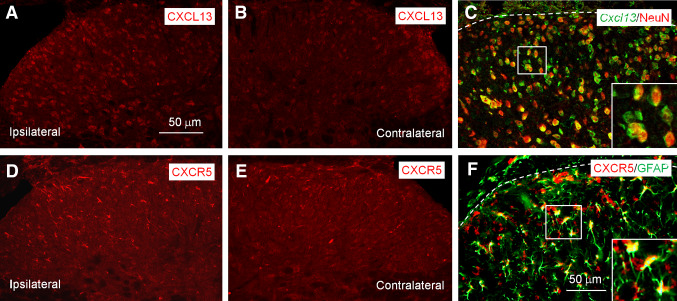
Fig. 3.
SNL induces CXCL13 and CXCR5 expression in spinal neurons and astrocytes, respectively. a, b SNL increases CXCL13 expression in the ipsilateral spinal dorsal horn (a), but not in the contralateral spinal dorsal horn (b). c Double staining shows that CXCL13 is colocalized with NeuN in the spinal cord dorsal horn. d, e SNL increases CXCR5 expression in the ipsilateral spinal dorsal horn (d), but not in the contralateral spinal dorsal horn (e). f Double staining shows CXCR5 is colocalized with GFAP in the spinal cord. The images are re-produced from a paper by Jiang et al. [24] (The Journal of Clinical Investigation) with permission
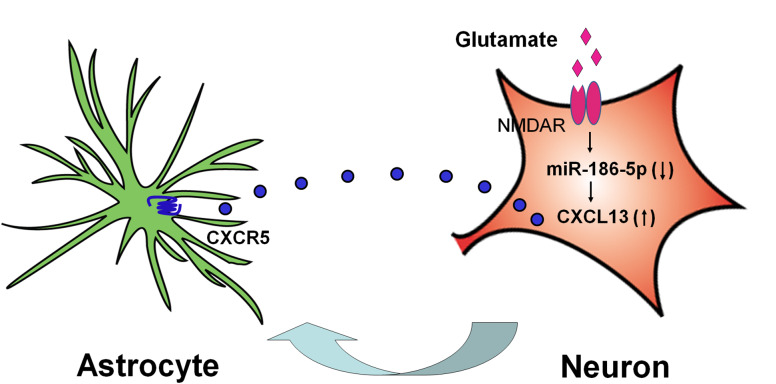
Fig. 4.
Schematic shows CXCL13/CXCR5 mediates neuron-to-astrocyte crosstalk in the spinal cord. SNL increases glutamate release from presynaptic terminals to activate NMDAR on postsynaptic neurons, leading to downregulation of miR-186-5p, and this decrease results in CXCL13 upregulation in spinal neurons. Upon release, CXCL13 acts on CXCR5 in astrocytes to induce astrocytic activation
The sole receptor of CXCL13, CXCR5 has low expression in naïve spinal cord but is increased in the ipsilateral dorsal horn after SNL (Fig. 3d, e), which is started from 1 day and maintained for more than 21 days. Interestingly, CXCR5 is mainly localized in astrocytes (Fig. 3f), with a few in neurons in the superficial dorsal horn [24]. Conversely, CXCR5 is expressed in neurons of TG and increased after pIONL [122]. These data suggest that CXCL13/CXCR5 may mediate pain processing via different mechanisms in the CNS and PNS.
CXCL13–CXCR5 mediates neuron–astrocyte interaction in the spinal cord
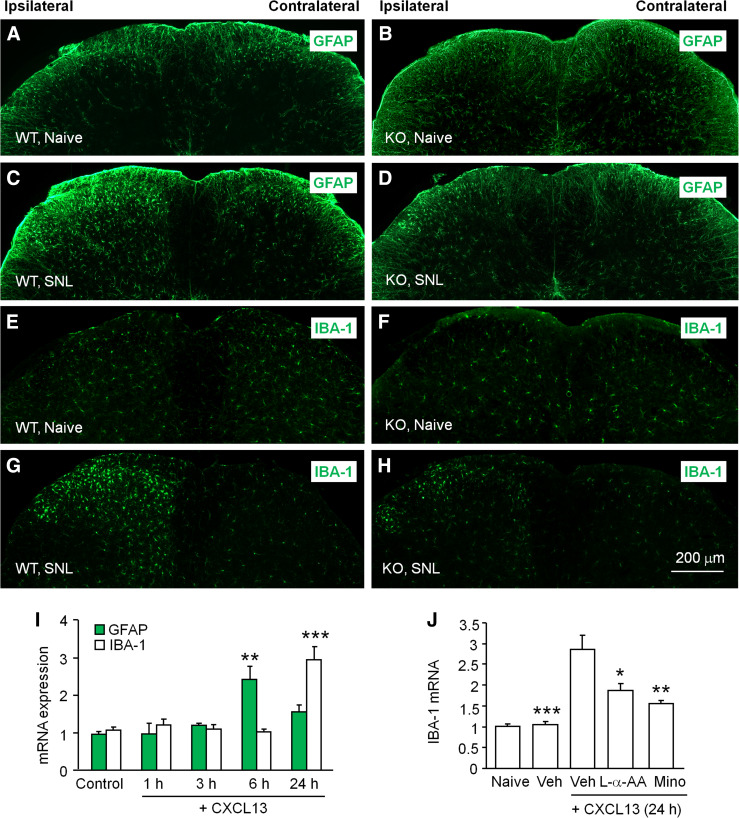
Due to the respective expression of CXCL13 and CXCR5 in spinal neurons and astrocytes, they may contribute to neuropathic pain via neuron–astrocyte interaction (Fig. 4). Indeed, astrocyte activation in the spinal cord was remarkably reduced in Cxcr5-deficient mice (Fig. 5a–d). Intrathecal injection of CXCL13 increases GFAP expression in the spinal cord 6 h after injection. Intrathecal CXCL13-induced pain hypersensitivity is inhibited by astrocyte functional inhibitor, l-α-aminoadipate [24]. Furthermore, CXCL13 induces CXCR5-dependent ERK activation in spinal astrocytes. SNL-induced ERK activation is reduced in the spinal cord in Cxcr5-deficient mice [24]. Importantly, CXCL13 induces ERK activation in cultured astrocytes. In addition, intrathecal injection of CXCL13-activated astrocytes from WT mice, but not from Cxcr5-deficient mice, induces mechanical allodynia in naive mice [24]. These data suggest that CXCL13 may activate astrocytes via CXCR5.
Fig. 5.
SNL-induced activation of glial cells in the spinal cord is inhibited in CXCR5 KO mice. a–d GFAP staining in the spinal cord of naive WT and Cxcr5 KO mice or mice after SNL (7 days). e–h IBA-1 staining in the spinal cord in naive WT and Cxcr5 KO mice or mice after SNL (7 days). Scale bar 200 µm. i Intrathecal injection of CXCL13 increased GFAP mRNA at 6 h and IBA-1 mRNA at 24 h. **P < 0.01, ***P < 0.001, vs. control. One-way ANOVA followed by Bonferroni’s test. j Intrathecal injection of astroglial function inhibitor l-α-aminoadipate (L-α-AA) or microglial inhibitor minocycline (Mino) decreased CXCL13-induced IBA-1 mRNA upregulation in the spinal cord 24 h after CXCL13 injection. *P < 0.05, **P < 0.01, ***P < 0.001, compared to Vehicle (Veh) + CXCL13. One-way ANOVA followed by Bonferroni’s test. The images are re-produced from a paper by Jiang et al. [24] (The Journal of Clinical Investigation) with permission
Interestingly, although CXCR5 is not expressed in spinal microglia, SNL-induced microglial activation is reduced in Cxcr5-deficient mice (Fig. 5e–h). Moreover, spinal injection of CXCL13 not only induces rapid astroglial activation at 6 h, but also induces delayed microglial activation at 24 h (Fig. 5i). Inhibition of either astrocyte function or microglia function inhibits microglia marker IBA-1 expression (Fig. 5j). These results suggest that activated astrocytes may further activate microglia in the spinal cord [24].
CXCL13 and CXCR5 contribute to neuropathic pain
Behavioral studies show that intraspinal injection of CXCL13 shRNA persistently attenuated SNL-induced mechanical allodynia. In addition, overexpression of spinal miR-186-5p decreases CXCL13 expression and attenuates SNL-induced pain hypersensitivity [24]. In contrast, inhibition of miR-186-5p is sufficient to increase CXCL13 expression and induce pain hypersensitivity. Knockdown of CXCR5 in the spinal cord by shRNA lentivirus also reverses SNL-induced pain hypersensitivity in WT mice for more than 4 weeks. Furthermore, the second injection of CXCR5 shRNA is still effective in reversing late-phase neuropathic pain [24]. Consistently, Cxcr5 KO mice show marked deficits in SNL-induced heat hyperalgesia and mechanical allodynia [24]. In addition, inhibition of CXCL13/CXCR5 in the TG before or after pIONL attenuates pIONL-induced mechanical allodynia [122]. These data suggest that CXCL13/CXCR5 is necessary for the maintenance of neuropathic pain. Moreover, intrathecal injection of CXCL13 induces CXCR5-dependent heat hyperalgesia and mechanical allodynia [24]. Intra-TG injection of CXCL13 also induces orofacial pain [122], indicating that activation of CXCR5 is also sufficient to induce pain hypersensitivity. It is noteworthy that different from the cellular distribution of CXCL13 and CXCR5 in the spinal cord, CXCL13 and CXCR5 are both expressed in neurons of the trigeminal ganglion. Intra-TG injection of CXCL13 induces CXCR5-dependent ERK and pp38 activation and proinflammatory cytokine production [122, 124], suggesting a different mechanism of CXCL13/CXCR5 in the TG in mediating neuropathic pain.
Other chemokines that involve in neuropathic pain
Besides the chemokines discussed above, some other chemokiens are also involved in neuropathic pain.
CXCL10 (IFN-γ-inducible protein 10) is one of highly upregulated chemokines in the spinal cord after SNL [24]. It mainly recognizes CXCR3, which is also recognized by CXCL9 and CXCL11 [35]. Our recent study shows that CXCL10 is constitutively expressed in spinal neurons in naive mice, but increased in neurons and astrocytes after SNL [125]. SNL or cancer cells inoculation also increases CXCR3 expression in spinal neurons [125, 126]. Cxcr3-deficient mice show reduced mechanical allodynia and heat hyperalgesia after SNL [125]. In addition, intrathecal injection of CXCR3 antagonist attenuates both neuropathic pain and bone cancer pain [125, 126]. Although it has been demonstrated that CXCL10 can induce pain via CXCR3 [125], the role of CXCL9 and CXCL11 has not been identified yet.
CXCL12 (stromal cell-derived factor 1, SDF-1), exerts function through its primary CXCR4 receptor. In the spinal cord, CXCL12 and CXCR4 are mainly colocalized with spinal neurons and also in spinal astrocytes and microglia [127–131]. Intrathecal injection of a CXCL12 neutralizing antibody or CXCR4 antagonist AMD3100 can delay or reverse the initiation and persistence of bone cancer pain and nerve injury-induced neuropathic pain [127, 129, 130, 132, 133].
CCL21, also named secondary lymphoid-tissue chemokine (SLC), is specifically located in large dense-core vesicles in damaged DRG neurons and transported into axons and pre-synaptic terminals [134]. CCR7, as the receptor of CCL21, can be induced in activated microglia in EAE animals [135]. The Ccl21-deficient mice do not develop neuropathic pain and fail to upregulate the P2X4 expression in activated microglia after peripheral nerve injury [136].
CCL3, also named macrophage inflammatory protein-1 alpha (MIP-1α), is found to participate in the development of neuropathic pain through its dominant receptors CCR1 and CCR5 [137]. In spinal dorsal horn, the distribution of CCL3 is still unclear. In the spinal dorsal horn, CCL3 and its receptors CCR1, but not CCR5 are increased after pSNL [138]. Intrathecal injection of CCL3 neutralizing antibody can prevent the mechanical allodynia and thermal hyperalgesia induced by pSNL [138, 139]. Ccr5 KO mice do not develop mechanical allodynia and thermal hyperalgesia, and the spinal microglial reaction to the peripheral nerve injury is also impaired [139]. Considering there is no expression of CCR5 in the spinal cord, the analgesia effects in Ccr5 KO mice may be due to the inhibition of inflammation that occurred in injured peripheral nerve.
Conclusion
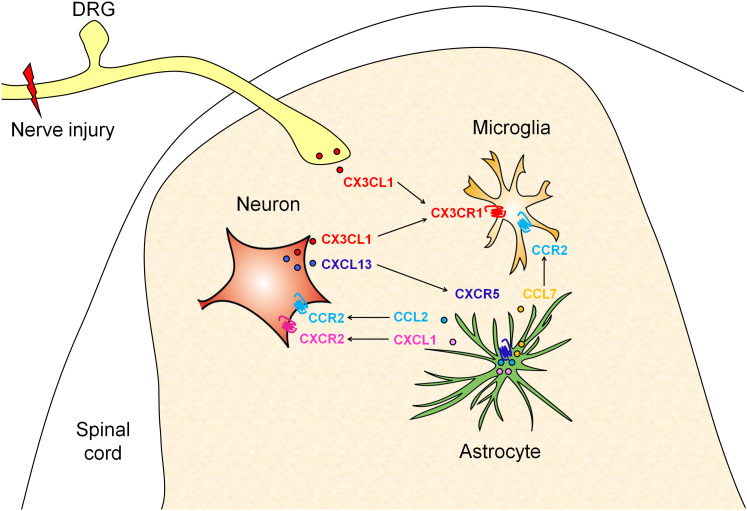
It is clear that neuropathic pain states are associated with a profound neuroinflammation, in which chemokines play an important role in the development and maintenance of neuropathic pain. By the different distribution in neurons and glial cells of the ligand and receptor, chemokines can either induce glial activation or facilitate excitatory synaptic transmission of spinal neurons, which exaggerates central sensitization (Fig. 6). Moreover, evidence supports that nerve injury induced a rapid microglial activation and a delayed and persistent astrocytic activation. Consistently, chemokines, such as CX3CL1, which induces microglia activation, are upregulated in the first few days after nerve injury, whereas CCL2, CXCL1, and CXCL10, which induce astrocyte activation, are upregulated after 3 days, and maintained for more than 21 days (Table 1). These findings suggest that targeting chemokine receptors, such as CX3CR1 after nerve injury may prevent the development of neuropathic pain. In contrast, blocking the chemokine receptors, such as CCR2, CXCR2, CXCR3, and CXCR5, may alleviate the established late phase neuropathic pain.
Fig. 6.
A schematic shows the interactions between several chemokines and their receptors in the spinal cord in the neuropathic pain condition. After nerve injury, CX3CL1 is shed from primary afferents and spinal neurons and acts on its receptor CX3CR1 to induce microglia activation. CXCL13 releases from spinal neurons and acts on CXCR5 to induce astrocyte activation. The activated astrocytes release CCL2 and CXCL1, which act on their major receptor CCR2 and CXCR2 on spinal neurons to enhance excitatory synaptic transmission. CCL7 may be released from astrocytes and acts on CCR2 on microglia to activate microglia. Thus, microglia, astrocytes, neurons constitute a positive feedback loop via chemokine and receptor interactions to contribute to the pathogenesis of neuropathic pain
Table 1.
The expression, distribution, and function of chemokine/receptor in the spinal cord under neuropathic pain condition
| Pain model | Chemokine/receptor | Expression | Distribution | Phenotype of receptor KO mice | Pharmacological intervention to pain behavior (i.t.) | Neuronal-glial interaction | Ref. |
|---|---|---|---|---|---|---|---|
|
CCI SNI SNL pSNL |
CX3CL1 | CX3CL1 Soluble form POD (1–7) (↑) | Primary afferents Neuron Astrocyte |
Normal basal pain threshold pSNL-Mechanical allodynia (↓) pSNL-Heat hyperalgesia (↓) SNI-Mechanical allodynia (↑) |
CX3CL1 neutralizing antibody (↓) CX3CR1neutralizing antibody (↓) |
Neuron–microglia interaction |
43, 46 51, 56 58, 61 68 |
| CX3CR1 | POD (1–10) (↑) | Microglia | |||||
|
CCI SNL IAMNT ETM |
CCL2 | POD (3–21) (↑) | Primary afferents Astrocyte |
Normal basal pain threshold pSNL-Mechanical allodynia (↓) |
CCL2 neutralizing antibody (↓) CCR2 siRNA (↓) CCR2 antagonist (INCB344, RS504393) (↓) |
Neuron–astrocyte interaction |
21, 78 79, 82 85, 86 88 |
| CCR2 | POD (3–21) (↑) |
Microglia Neuron |
|||||
|
SNL BCP |
CXCL1 | POD (3–21) (↑) | Astrocyte | Unknown |
CXCL1 neutralizing antibody (↓) CXCL1 shRNA (↓) CXCR2 antagonist (SB225002) (↓) |
Astrocyte–neuron interaction |
22, 100 102, 104 |
| CXCR2 | POD (3–21) (↑) | Neuron | |||||
| SNL | CXCL13 | POD (1–21) (↑) | Neuron |
Normal basal pain threshold SNL-Mechanical allodynia (↓) SNL-Heat hyperalgesia (↓) |
CXCL13 shRNA (↓) CXCR5 shRNA (↓) |
Neuron–astrocyte interaction | 24 |
| CXCR5 | POD (3–21) (↑) | Astrocyte | |||||
|
SNL BCP |
CXCL10 | POD (3–21) (↑) |
Neuron Astrocyte |
Normal basal pain threshold SNL-Mechanical allodynia (↓) SNL-Heat hyperalgesia (↓) |
CXCR3 shRNA (↓) CXCR3 antagonist (NBI-74330) (↓) |
Astrocyte–neuron interaction |
24, 125 126 |
| CXCR3 | POD (3–21) (↑) | Neuron | |||||
|
pSNL SNL SNI BCP |
CXCL12 | POD (3–21) (↑) |
Neuron Astrocyte Microglia |
Unknown |
CXCL12 neutralizing antibody (↓) CXCR4 antagonist (AMD3100, AMD3465) (↓) |
Unknown |
127, 129 130, 132 133 |
| CXCR4 | POD (5–21) (↑) |
Neuron Astrocyte Microglia |
|||||
| SNL | CCL21 | POD (1–2) (↑) | Primary afferents |
Normal basal pain threshold SNL-Mechanical allodynia (↓) |
CCL21 neutralizing antibody (↓) | Unknown | 136 |
| CCR7 | POD (?) | Microglia | |||||
|
pSNL SNL |
CCL3 | POD (3–14) (↑) | Unknown |
Normal basal pain threshold SNL-Mechanical allodynia (↓) SNL-Heat hyperalgesia (↓) |
CCL3 neutralizing antibody (↓) | Unknown | 138, 139 |
| CCR1,CCR5 | POD (3–7) (↑) | Unknown |
CCI chronic constriction injury, IAMNT inferior alveolar and mental nerve transection, pSNL partial sciatic nerve ligation, SNL spinal nerve ligation, SNI spared nerve injury, BCP bone cancer pain, ETM experimental tooth movement, POD post-operative days, i.t. intrathecal injection
As we summarized in Table 1, siRNA or neutralizing antibody was used in most of the studies to block the function of chemokine signaling, and only a few chemokine receptor antagonists were tested. In recent years, pharmaceutical companies have developed a number of chemokine receptor antagonists (including antagonists for CCR1, CCR2, CCR3, CCR4, CCR5, CCR8, CCR9, CXCR1/CXCR2, CXCR2, CXCR3, and CXCR4) for the treatment of autoimmune-related or inflammation-related diseases, such as asthma, atopic diseases, AIDS, rheumatoid arthritis, and diabetes [140–142]. However, only two chemokine receptor antagonists have been licensed for clinical use: CCR5 inhibitor, Mavaviroc and CXCR4 inhibitor, Plerixafor. They show efficacy in the inhibition of HIV-1 entry (Miraviroc) and stem cell mobilization (Plerixafor) [143, 144]. Most of the chemokine receptor antagonists have not been tested for analgesic efficacy clinically. A CCR2 antagonist, AZD2423 (AstraZeneca), was tested for the treatment of posttraumatic neuralgia. Different from the anti-hyperalgesia effect on animals with neuropathic pain, it did not show efficacy on NRS (numerical rating scale) average pain scores of patients [145]. The reasons for the failures may attribute to species selectivity, the redundancy of the drug target, pharmacokinetic properties, and drug metabolism [143]. In addition, most of the preclinical studies checked the analgesic effect of drugs on stimulus-evoked pain, but NRS was evaluated in human. Thus, it is important to evaluate the efficacy of chemokine receptor antagonists on animals’ spontaneous pain in the future. Given the important role of spinal chemokines and receptors in neuropathic pain and the redundancy in the chemokine network, development of receptor antagonists that have CNS permeability and can target several chemokine receptors may be beneficial for the treatment of neuropathic pain.
Acknowledgements
This study was supported by the grants from the National Natural Science Foundation of China (NSFC 31371121, 81400915, 81571070, and 31671091), the National Science Foundation for Young Scientists of Jiangsu Province (BK20140427), the Qing Lan Project, and the Priority Academic Program Development of Jiangsu Higher Education Institutions.
Abbreviations
- AD
Alzheimer’s disease
- AMPA
Alpha-amino-3-hydroxyl-5-methyl-4-isoxazole-propionate
- BCP
Bone cancer pain
- CCI
Chronic constriction injury
- CFA
Complete Freund’s adjuvant
- CNS
Central nervous system
- CSF
Cerebrospinal fluid
- DRG
Dorsal root ganglion
- EAE
Experimental autoimmune encephalomyelitis
- ERK
Extracellular signal-regulated kinase
- EPSC
Excitatory postsynaptic currents
- GABA
Gamma-aminobutyric acid
- IL
Interleukin
- IAMNT
Inferior alveolar nerve and mental nerve transection
- JNK
c-Jun N-terminal kinase
- MAPK
Mitogen-activated protein kinase
- MCP-1
Monocytes chemoattractant protein-1
- MS
Multiple sclerosis
- LTP
Long-term potentiation
- NMDA
N-methyl-d-aspartic acid
- pIONL
Partial infraorbital nerve ligation
- PNS
Peripheral nervous system
- pSNL
Partial sciatic nerve ligation
- SNL
Spinal nerve ligation
- SNI
Spared nerve injury
- TNF-α
Tumor necrosis factor-alpha
- TG
Trigeminal ganglion
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
References
- 1.Baron R (2009) Neuropathic pain: a clinical perspective. Handbook of experimental pharmacology, vol 194. pp 3–30. doi:10.1007/978-3-540-79090-7_1 [DOI] [PubMed]
- 2.Gao YJ, Ji RR. Chemokines, neuronal-glial interactions, and central processing of neuropathic pain. Pharmacol Ther. 2010;126(1):56–68. doi: 10.1016/j.pharmthera.2010.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ji RR, Woolf CJ. Neuronal plasticity and signal transduction in nociceptive neurons: implications for the initiation and maintenance of pathological pain. Neurobiol Dis. 2001;8(1):1–10. doi: 10.1006/nbdi.2000.0360. [DOI] [PubMed] [Google Scholar]
- 4.Ji RR, Xu ZZ, Gao YJ. Emerging targets in neuroinflammation-driven chronic pain. Nat Rev Drug Discov. 2014;13(7):533–548. doi: 10.1038/nrd4334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ji RR, Strichartz G. Cell signaling and the genesis of neuropathic pain. Sci STKE. 2004;2004(252):reE14. doi: 10.1126/stke.2522004re14. [DOI] [PubMed] [Google Scholar]
- 6.Abbadie C. Chemokines, chemokine receptors and pain. Trends Immunol. 2005;26(10):529–534. doi: 10.1016/j.it.2005.08.001. [DOI] [PubMed] [Google Scholar]
- 7.Sommer C, Kress M. Recent findings on how proinflammatory cytokines cause pain: peripheral mechanisms in inflammatory and neuropathic hyperalgesia. Neurosci Lett. 2004;361(1–3):184–187. doi: 10.1016/j.neulet.2003.12.007. [DOI] [PubMed] [Google Scholar]
- 8.Ji RR, Kohno T, Moore KA, Woolf CJ. Central sensitization and LTP: do pain and memory share similar mechanisms? Trends Neurosci. 2003;26(12):696–705. doi: 10.1016/j.tins.2003.09.017. [DOI] [PubMed] [Google Scholar]
- 9.Coull JA, Beggs S, Boudreau D, Boivin D, Tsuda M, Inoue K, Gravel C, Salter MW, De Koninck Y. BDNF from microglia causes the shift in neuronal anion gradient underlying neuropathic pain. Nature. 2005;438(7070):1017–1021. doi: 10.1038/nature04223. [DOI] [PubMed] [Google Scholar]
- 10.Woolf CJ, Mannion RJ. Neuropathic pain: aetiology, symptoms, mechanisms, and management. Lancet. 1999;353(9168):1959–1964. doi: 10.1016/S0140-6736(99)01307-0. [DOI] [PubMed] [Google Scholar]
- 11.Gao YJ, Ji RR. Targeting astrocyte signaling for chronic pain. Neurother. 2010;7(4):482–493. doi: 10.1016/j.nurt.2010.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Aldskogius H, Kozlova EN. Microglia and neuropathic pain. CNS Neurol Disord Drug Targets. 2013;12(6):768–772. doi: 10.2174/18715273113126660168. [DOI] [PubMed] [Google Scholar]
- 13.Cao H, Zhang YQ. Spinal glial activation contributes to pathological pain states. Neurosci Biobehav Rev. 2008;32(5):972–983. doi: 10.1016/j.neubiorev.2008.03.009. [DOI] [PubMed] [Google Scholar]
- 14.Scholz J, Woolf CJ. The neuropathic pain triad: neurons, immune cells and glia. Nat Neurosci. 2007;10(11):1361–1368. doi: 10.1038/nn1992. [DOI] [PubMed] [Google Scholar]
- 15.Ji RR, Chamessian A, Zhang YQ. Pain regulation by non-neuronal cells and inflammation. Science (New York, NY) 2016;354(6312):572–577. doi: 10.1126/science.aaf8924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tanga FY, Raghavendra V, DeLeo JA. Quantitative real-time RT-PCR assessment of spinal microglial and astrocytic activation markers in a rat model of neuropathic pain. Neurochem Int. 2004;45(2–3):397–407. doi: 10.1016/j.neuint.2003.06.002. [DOI] [PubMed] [Google Scholar]
- 17.Zhang X, Xu Y, Wang J, Zhou Q, Pu S, Jiang W, Du D. The effect of intrathecal administration of glial activation inhibitors on dorsal horn BDNF overexpression and hind paw mechanical allodynia in spinal nerve ligated rats. J Neural Transm (Vienna) 2012;119(3):329–336. doi: 10.1007/s00702-011-0713-7. [DOI] [PubMed] [Google Scholar]
- 18.Ledeboer A, Sloane EM, Milligan ED, Frank MG, Mahony JH, Maier SF, Watkins LR. Minocycline attenuates mechanical allodynia and proinflammatory cytokine expression in rat models of pain facilitation. Pain. 2005;115(1–2):71–83. doi: 10.1016/j.pain.2005.02.009. [DOI] [PubMed] [Google Scholar]
- 19.Hayakawa K, Nakano T, Irie K, Higuchi S, Fujioka M, Orito K, Iwasaki K, Jin G, Lo EH, Mishima K, Fujiwara M. Inhibition of reactive astrocytes with fluorocitrate retards neurovascular remodeling and recovery after focal cerebral ischemia in mice. J Cereb Blood Flow Metab. 2010;30(4):871–882. doi: 10.1038/jcbfm.2009.257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ji RR, Berta T, Nedergaard M. Glia and pain: is chronic pain a gliopathy? Pain. 2013;154(Suppl 1):S10–S28. doi: 10.1016/j.pain.2013.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gao YJ, Zhang L, Samad OA, Suter MR, Yasuhiko K, Xu ZZ, Park JY, Lind AL, Ma Q, Ji RR. JNK-induced MCP-1 production in spinal cord astrocytes contributes to central sensitization and neuropathic pain. J Neurosci. 2009;29(13):4096–4108. doi: 10.1523/JNEUROSCI.3623-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhang ZJ, Cao DL, Zhang X, Ji RR, Gao YJ. Chemokine contribution to neuropathic pain: respective induction of CXCL1 and CXCR2 in spinal cord astrocytes and neurons. Pain. 2013;154(10):2185–2197. doi: 10.1016/j.pain.2013.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Biber K, Boddeke E. Neuronal CC chemokines: the distinct roles of CCL21 and CCL2 in neuropathic pain. Front Cell Neurosci. 2014;8:210. doi: 10.3389/fncel.2014.00210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jiang BC, Cao DL, Zhang X, Zhang ZJ, He LN, Li CH, Zhang WW, Wu XB, Berta T, Ji RR, Gao YJ. CXCL13 drives spinal astrocyte activation and neuropathic pain via CXCR5. J Clin Invest. 2016;126(2):745–761. doi: 10.1172/JCI81950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Clark AK, Malcangio M. Fractalkine/CX3CR1 signaling during neuropathic pain. Front Cell Neurosci. 2014;8:121. doi: 10.3389/fncel.2014.00121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhang ZJ, Dong YL, Lu Y, Cao S, Zhao ZQ, Gao YJ. Chemokine CCL2 and its receptor CCR2 in the medullary dorsal horn are involved in trigeminal neuropathic pain. J Neuroinflammation. 2012;9:136. doi: 10.1186/1742-2094-9-136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Abbadie C, Bhangoo S, De Koninck Y, Malcangio M, Melik-Parsadaniantz S, White FA. Chemokines and pain mechanisms. Brain Res Rev. 2009;60(1):125–134. doi: 10.1016/j.brainresrev.2008.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Charo IF, Ransohoff RM. The many roles of chemokines and chemokine receptors in inflammation. N Engl J Med. 2006;354(6):610–621. doi: 10.1056/NEJMra052723. [DOI] [PubMed] [Google Scholar]
- 29.Old EA, Malcangio M. Chemokine mediated neuron-glia communication and aberrant signalling in neuropathic pain states. Curr Opin Pharmacol. 2012;12(1):67–73. doi: 10.1016/j.coph.2011.10.015. [DOI] [PubMed] [Google Scholar]
- 30.Rossi D, Zlotnik A. The biology of chemokines and their receptors. Annu Rev Immunol. 2000;18:217–242. doi: 10.1146/annurev.immunol.18.1.217. [DOI] [PubMed] [Google Scholar]
- 31.Bonecchi R, Galliera E, Borroni EM, Corsi MM, Locati M, Mantovani A. Chemokines and chemokine receptors: an overview. Front Biosci. 2009;14:540–551. doi: 10.2741/3261. [DOI] [PubMed] [Google Scholar]
- 32.Giovannelli A, Limatola C, Ragozzino D, Mileo AM, Ruggieri A, Ciotti MT, Mercanti D, Santoni A, Eusebi F. CXC chemokines interleukin-8 (IL-8) and growth-related gene product alpha (GROalpha) modulate Purkinje neuron activity in mouse cerebellum. J Neuroimmunol. 1998;92(1–2):122–132. doi: 10.1016/S0165-5728(98)00192-1. [DOI] [PubMed] [Google Scholar]
- 33.Limatola C, Giovannelli A, Maggi L, Ragozzino D, Castellani L, Ciotti MT, Vacca F, Mercanti D, Santoni A, Eusebi F. SDF-1alpha-mediated modulation of synaptic transmission in rat cerebellum. Eur J Neurosci. 2000;12(7):2497–2504. doi: 10.1046/j.1460-9568.2000.00139.x. [DOI] [PubMed] [Google Scholar]
- 34.Mennicken F, Maki R, de Souza EB, Quirion R. Chemokines and chemokine receptors in the CNS: a possible role in neuroinflammation and patterning. Trends Pharmacol Sci. 1999;20(2):73–78. doi: 10.1016/S0165-6147(99)01308-5. [DOI] [PubMed] [Google Scholar]
- 35.Savarin-Vuaillat C, Ransohoff RM. Chemokines and chemokine receptors in neurological disease: raise, retain, or reduce? Neurother. 2007;4(4):590–601. doi: 10.1016/j.nurt.2007.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ubogu EE, Cossoy MB, Ransohoff RM. The expression and function of chemokines involved in CNS inflammation. Trends Pharmacol Sci. 2006;27(1):48–55. doi: 10.1016/j.tips.2005.11.002. [DOI] [PubMed] [Google Scholar]
- 37.Kim SH, Chung JM. An experimental model for peripheral neuropathy produced by segmental spinal nerve ligation in the rat. Pain. 1992;50(3):355–363. doi: 10.1016/0304-3959(92)90041-9. [DOI] [PubMed] [Google Scholar]
- 38.Decosterd I, Woolf CJ. Spared nerve injury: an animal model of persistent peripheral neuropathic pain. Pain. 2000;87(2):149–158. doi: 10.1016/S0304-3959(00)00276-1. [DOI] [PubMed] [Google Scholar]
- 39.Bennett GJ, Xie YK. A peripheral mononeuropathy in rat that produces disorders of pain sensation like those seen in man. Pain. 1988;33(1):87–107. doi: 10.1016/0304-3959(88)90209-6. [DOI] [PubMed] [Google Scholar]
- 40.Seltzer Z, Dubner R, Shir Y. A novel behavioral model of neuropathic pain disorders produced in rats by partial sciatic nerve injury. Pain. 1990;43(2):205–218. doi: 10.1016/0304-3959(90)91074-S. [DOI] [PubMed] [Google Scholar]
- 41.Hu SJ, Xing JL. An experimental model for chronic compression of dorsal root ganglion produced by intervertebral foramen stenosis in the rat. Pain. 1998;77(1):15–23. doi: 10.1016/S0304-3959(98)00067-0. [DOI] [PubMed] [Google Scholar]
- 42.Milligan ED, Sloane EM, Watkins LR. Glia in pathological pain: a role for fractalkine. J Neuroimmunol. 2008;198(1–2):113–120. doi: 10.1016/j.jneuroim.2008.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Verge GM, Milligan ED, Maier SF, Watkins LR, Naeve GS, Foster AC. Fractalkine (CX3CL1) and fractalkine receptor (CX3CR1) distribution in spinal cord and dorsal root ganglia under basal and neuropathic pain conditions. Eur J Neurosci. 2004;20(5):1150–1160. doi: 10.1111/j.1460-9568.2004.03593.x. [DOI] [PubMed] [Google Scholar]
- 44.Chapman GA, Moores K, Harrison D, Campbell CA, Stewart BR, Strijbos PJ. Fractalkine cleavage from neuronal membranes represents an acute event in the inflammatory response to excitotoxic brain damage. J Neurosci. 2000;20(15):RC87. doi: 10.1523/JNEUROSCI.20-15-j0004.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kim KW, Vallon-Eberhard A, Zigmond E, Farache J, Shezen E, Shakhar G, Ludwig A, Lira SA, Jung S. In vivo structure/function and expression analysis of the CX3C chemokine fractalkine. Blood. 2011;118(22):e156–e167. doi: 10.1182/blood-2011-04-348946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lindia JA, McGowan E, Jochnowitz N, Abbadie C. Induction of CX3CL1 expression in astrocytes and CX3CR1 in microglia in the spinal cord of a rat model of neuropathic pain. J Pain. 2005;6(7):434–438. doi: 10.1016/j.jpain.2005.02.001. [DOI] [PubMed] [Google Scholar]
- 47.Schall T. Fractalkine–a strange attractor in the chemokine landscape. Immunol Today. 1997;18(4):147. doi: 10.1016/S0167-5699(97)84655-5. [DOI] [PubMed] [Google Scholar]
- 48.Hesselgesser J, Horuk R. Chemokine and chemokine receptor expression in the central nervous system. J Neurovirol. 1999;5(1):13–26. doi: 10.3109/13550289909029741. [DOI] [PubMed] [Google Scholar]
- 49.Clark AK, Malcangio M. Microglial signalling mechanisms: Cathepsin S and Fractalkine. Exp Neurol. 2012;234(2):283–292. doi: 10.1016/j.expneurol.2011.09.012. [DOI] [PubMed] [Google Scholar]
- 50.Li D, Huang ZZ, Ling YZ, Wei JY, Cui Y, Zhang XZ, Zhu HQ, Xin WJ. Up-regulation of CX3CL1 via nuclear factor-kappaB-dependent histone acetylation is involved in paclitaxel-induced peripheral neuropathy. Anesthesiology. 2015;122(5):1142–1151. doi: 10.1097/ALN.0000000000000560. [DOI] [PubMed] [Google Scholar]
- 51.Zhuang ZY, Kawasaki Y, Tan PH, Wen YR, Huang J, Ji RR. Role of the CX3CR1/p38 MAPK pathway in spinal microglia for the development of neuropathic pain following nerve injury-induced cleavage of fractalkine. Brain Behav Immun. 2007;21(5):642–651. doi: 10.1016/j.bbi.2006.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hundhausen C, Misztela D, Berkhout TA, Broadway N, Saftig P, Reiss K, Hartmann D, Fahrenholz F, Postina R, Matthews V, Kallen KJ, Rose-John S, Ludwig A. The disintegrin-like metalloproteinase ADAM10 is involved in constitutive cleavage of CX3CL1 (fractalkine) and regulates CX3CL1-mediated cell-cell adhesion. Blood. 2003;102(4):1186–1195. doi: 10.1182/blood-2002-12-3775. [DOI] [PubMed] [Google Scholar]
- 53.Hundhausen C, Schulte A, Schulz B, Andrzejewski MG, Schwarz N, von Hundelshausen P, Winter U, Paliga K, Reiss K, Saftig P, Weber C, Ludwig A. Regulated shedding of transmembrane chemokines by the disintegrin and metalloproteinase 10 facilitates detachment of adherent leukocytes. J Immunol. 2007;178(12):8064–8072. doi: 10.4049/jimmunol.178.12.8064. [DOI] [PubMed] [Google Scholar]
- 54.Garton KJ, Gough PJ, Blobel CP, Murphy G, Greaves DR, Dempsey PJ, Raines EW. Tumor necrosis factor-alpha-converting enzyme (ADAM17) mediates the cleavage and shedding of fractalkine (CX3CL1) J Biol Chem. 2001;276(41):37993–38001. doi: 10.1074/jbc.M106434200. [DOI] [PubMed] [Google Scholar]
- 55.Fonovic UP, Jevnikar Z, Kos J. Cathepsin S generates soluble CX3CL1 (fractalkine) in vascular smooth muscle cells. Biol Chem. 2013;394(10):1349–1352. doi: 10.1515/hsz-2013-0189. [DOI] [PubMed] [Google Scholar]
- 56.Clark AK, Yip PK, Grist J, Gentry C, Staniland AA, Marchand F, Dehvari M, Wotherspoon G, Winter J, Ullah J, Bevan S, Malcangio M. Inhibition of spinal microglial cathepsin S for the reversal of neuropathic pain. Proc Natl Acad Sci USA. 2007;104(25):10655–10660. doi: 10.1073/pnas.0610811104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Souza GR, Talbot J, Lotufo CM, Cunha FQ, Cunha TM, Ferreira SH. Fractalkine mediates inflammatory pain through activation of satellite glial cells. Proc Natl Acad Sci USA. 2013;110(27):11193–11198. doi: 10.1073/pnas.1307445110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Staniland AA, Clark AK, Wodarski R, Sasso O, Maione F, D’Acquisto F, Malcangio M. Reduced inflammatory and neuropathic pain and decreased spinal microglial response in fractalkine receptor (CX3CR1) knockout mice. J Neurochem. 2010;114(4):1143–1157. doi: 10.1111/j.1471-4159.2010.06837.x. [DOI] [PubMed] [Google Scholar]
- 59.Old EA, Nadkarni S, Grist J, Gentry C, Bevan S, Kim KW, Mogg AJ, Perretti M, Malcangio M. Monocytes expressing CX3CR1 orchestrate the development of vincristine-induced pain. J Clin Invest. 2014;124(5):2023–2036. doi: 10.1172/JCI71389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Milligan E, Zapata V, Schoeniger D, Chacur M, Green P, Poole S, Martin D, Maier SF, Watkins LR. An initial investigation of spinal mechanisms underlying pain enhancement induced by fractalkine, a neuronally released chemokine. Eur J Neurosci. 2005;22(11):2775–2782. doi: 10.1111/j.1460-9568.2005.04470.x. [DOI] [PubMed] [Google Scholar]
- 61.Clark AK, Yip PK, Malcangio M. The liberation of fractalkine in the dorsal horn requires microglial cathepsin S. J Neurosci. 2009;29(21):6945–6954. doi: 10.1523/JNEUROSCI.0828-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Jin SX, Zhuang ZY, Woolf CJ, Ji RR. p38 mitogen-activated protein kinase is activated after a spinal nerve ligation in spinal cord microglia and dorsal root ganglion neurons and contributes to the generation of neuropathic pain. J Neurosci. 2003;23(10):4017–4022. doi: 10.1523/JNEUROSCI.23-10-04017.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Gong QJ, Li YY, Xin WJ, Zang Y, Ren WJ, Wei XH, Zhang T, Liu XG. ATP induces long-term potentiation of C-fiber-evoked field potentials in spinal dorsal horn: the roles of P2 × 4 receptors and p38 MAPK in microglia. Glia. 2009;57(6):583–591. doi: 10.1002/glia.20786. [DOI] [PubMed] [Google Scholar]
- 64.Chu YX, Zhang Y, Zhang YQ, Zhao ZQ. Involvement of microglial P2 × 7 receptors and downstream signaling pathways in long-term potentiation of spinal nociceptive responses. Brain Behav Immun. 2010;24(7):1176–1189. doi: 10.1016/j.bbi.2010.06.001. [DOI] [PubMed] [Google Scholar]
- 65.Bian C, Zhao ZQ, Zhang YQ, Lu N. Involvement of CX3CL1/CX3CR1 signaling in spinal long term potentiation. PloS One. 2015;10(3):e0118842. doi: 10.1371/journal.pone.0118842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Bian C, Wang ZC, Yang JL, Lu N, Zhao ZQ, Zhang YQ. Up-regulation of interleukin-23 induces persistent allodynia via CX3CL1 and interleukin-18 signaling in the rat spinal cord after tetanic sciatic stimulation. Brain Behav Immun. 2014;37:220–230. doi: 10.1016/j.bbi.2013.12.011. [DOI] [PubMed] [Google Scholar]
- 67.Milligan ED, Zapata V, Chacur M, Schoeniger D, Biedenkapp J, O’Connor KA, Verge GM, Chapman G, Green P, Foster AC, Naeve GS, Maier SF, Watkins LR. Evidence that exogenous and endogenous fractalkine can induce spinal nociceptive facilitation in rats. Eur J Neurosci. 2004;20(9):2294–2302. doi: 10.1111/j.1460-9568.2004.03709.x. [DOI] [PubMed] [Google Scholar]
- 68.Holmes FE, Arnott N, Vanderplank P, Kerr NC, Longbrake EE, Popovich PG, Imai T, Combadiere C, Murphy PM, Wynick D. Intra-neural administration of fractalkine attenuates neuropathic pain-related behaviour. J Neurochem. 2008;106(2):640–649. doi: 10.1111/j.1471-4159.2008.05419.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.White FA, Jung H, Miller RJ. Chemokines and the pathophysiology of neuropathic pain. Proc Natl Acad Sci USA. 2007;104(51):20151–20158. doi: 10.1073/pnas.0709250104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Jung H, Bhangoo S, Banisadr G, Freitag C, Ren D, White FA, Miller RJ. Visualization of chemokine receptor activation in transgenic mice reveals peripheral activation of CCR2 receptors in states of neuropathic pain. J Neurosci. 2009;29(25):8051–8062. doi: 10.1523/JNEUROSCI.0485-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Gosselin RD, Dansereau MA, Pohl M, Kitabgi P, Beaudet N, Sarret P, Melik Parsadaniantz S. Chemokine network in the nervous system: a new target for pain relief. Curr Med Chem. 2008;15(27):2866–2875. doi: 10.2174/092986708786242822. [DOI] [PubMed] [Google Scholar]
- 72.Kurihara T, Bravo R. Cloning and functional expression of mCCR2, a murine receptor for the C–C chemokines JE and FIC. J Biol Chem. 1996;271(20):11603–11607. doi: 10.1074/jbc.271.20.11603. [DOI] [PubMed] [Google Scholar]
- 73.Imai S, Ikegami D, Yamashita A, Shimizu T, Narita M, Niikura K, Furuya M, Kobayashi Y, Miyashita K, Okutsu D, Kato A, Nakamura A, Araki A, Omi K, Nakamura M, James Okano H, Okano H, Ando T, Takeshima H, Ushijima T, Kuzumaki N, Suzuki T. Epigenetic transcriptional activation of monocyte chemotactic protein 3 contributes to long-lasting neuropathic pain. Brain. 2013;136(Pt 3):828–843. doi: 10.1093/brain/aws330. [DOI] [PubMed] [Google Scholar]
- 74.Dansereau MA, Gosselin RD, Pohl M, Pommier B, Mechighel P, Mauborgne A, Rostene W, Kitabgi P, Beaudet N, Sarret P, Melik-Parsadaniantz S. Spinal CCL2 pronociceptive action is no longer effective in CCR2 receptor antagonist-treated rats. J Neurochem. 2008;106(2):757–769. doi: 10.1111/j.1471-4159.2008.05429.x. [DOI] [PubMed] [Google Scholar]
- 75.Tanaka T, Minami M, Nakagawa T, Satoh M. Enhanced production of monocyte chemoattractant protein-1 in the dorsal root ganglia in a rat model of neuropathic pain: possible involvement in the development of neuropathic pain. Neurosci Res. 2004;48(4):463–469. doi: 10.1016/j.neures.2004.01.004. [DOI] [PubMed] [Google Scholar]
- 76.Jung H, Toth PT, White FA, Miller RJ. Monocyte chemoattractant protein-1 functions as a neuromodulator in dorsal root ganglia neurons. J Neurochem. 2008;104(1):254–263. doi: 10.1111/j.1471-4159.2007.04969.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.White FA, Sun J, Waters SM, Ma C, Ren D, Ripsch M, Steflik J, Cortright DN, Lamotte RH, Miller RJ. Excitatory monocyte chemoattractant protein-1 signaling is up-regulated in sensory neurons after chronic compression of the dorsal root ganglion. Proc Natl Acad Sci USA. 2005;102(39):14092–14097. doi: 10.1073/pnas.0503496102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Zhang J, De Koninck Y. Spatial and temporal relationship between monocyte chemoattractant protein-1 expression and spinal glial activation following peripheral nerve injury. J Neurochem. 2006;97(3):772–783. doi: 10.1111/j.1471-4159.2006.03746.x. [DOI] [PubMed] [Google Scholar]
- 79.Zhang J, Shi XQ, Echeverry S, Mogil JS, De Koninck Y, Rivest S. Expression of CCR2 in both resident and bone marrow-derived microglia plays a critical role in neuropathic pain. J Neurosci. 2007;27(45):12396–12406. doi: 10.1523/JNEUROSCI.3016-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Miller RJ, Jung H, Bhangoo SK, White FA (2009) Cytokine and chemokine regulation of sensory neuron function. Handbook of experimental pharmacology, vol 194. pp 417–449. doi:10.1007/978-3-540-79090-7_12 [DOI] [PMC free article] [PubMed]
- 81.Van Steenwinckel J, Reaux-Le Goazigo A, Pommier B, Mauborgne A, Dansereau MA, Kitabgi P, Sarret P, Pohl M, Melik Parsadaniantz S. CCL2 released from neuronal synaptic vesicles in the spinal cord is a major mediator of local inflammation and pain after peripheral nerve injury. J Neurosci. 2011;31(15):5865–5875. doi: 10.1523/JNEUROSCI.5986-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Luo W, Fu R, Tan Y, Fang B, Yang Z. Chemokine CCL2 up-regulated in the medullary dorsal horn astrocytes contributes to nocifensive behaviors induced by experimental tooth movement. Eur J Oral Sci. 2014;122(1):27–35. doi: 10.1111/eos.12099. [DOI] [PubMed] [Google Scholar]
- 83.Tanuma N, Sakuma H, Sasaki A, Matsumoto Y. Chemokine expression by astrocytes plays a role in microglia/macrophage activation and subsequent neurodegeneration in secondary progressive multiple sclerosis. Acta Neuropathol. 2006;112(2):195–204. doi: 10.1007/s00401-006-0083-7. [DOI] [PubMed] [Google Scholar]
- 84.Yan YP, Sailor KA, Lang BT, Park SW, Vemuganti R, Dempsey RJ. Monocyte chemoattractant protein-1 plays a critical role in neuroblast migration after focal cerebral ischemia. J Cereb Blood Flow Metab. 2007;27(6):1213–1224. doi: 10.1038/sj.jcbfm.9600432. [DOI] [PubMed] [Google Scholar]
- 85.Babcock AA, Kuziel WA, Rivest S, Owens T. Chemokine expression by glial cells directs leukocytes to sites of axonal injury in the CNS. J Neurosci. 2003;23(21):7922–7930. doi: 10.1523/JNEUROSCI.23-21-07922.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Abbadie C, Lindia JA, Cumiskey AM, Peterson LB, Mudgett JS, Bayne EK, DeMartino JA, MacIntyre DE, Forrest MJ. Impaired neuropathic pain responses in mice lacking the chemokine receptor CCR2. Proc Natl Acad Sci USA. 2003;100(13):7947–7952. doi: 10.1073/pnas.1331358100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Moreno M, Bannerman P, Ma J, Guo F, Miers L, Soulika AM, Pleasure D. Conditional ablation of astroglial CCL2 suppresses CNS accumulation of M1 macrophages and preserves axons in mice with MOG peptide EAE. J Neurosci. 2014;34(24):8175–8185. doi: 10.1523/JNEUROSCI.1137-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Thacker MA, Clark AK, Bishop T, Grist J, Yip PK, Moon LD, Thompson SW, Marchand F, McMahon SB. CCL2 is a key mediator of microglia activation in neuropathic pain states. Eur J Pain. 2009;13(3):263–272. doi: 10.1016/j.ejpain.2008.04.017. [DOI] [PubMed] [Google Scholar]
- 89.Ji RR, Baba H, Brenner GJ, Woolf CJ. Nociceptive-specific activation of ERK in spinal neurons contributes to pain hypersensitivity. Nat Neurosci. 1999;2(12):1114–1119. doi: 10.1038/16040. [DOI] [PubMed] [Google Scholar]
- 90.Gao YJ, Ji RR. c-Fos and pERK, which is a better marker for neuronal activation and central sensitization after noxious stimulation and tissue injury? Open Pain J. 2009;2:11–17. doi: 10.2174/1876386300902010011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Gosselin RD, Varela C, Banisadr G, Mechighel P, Rostene W, Kitabgi P, Melik-Parsadaniantz S. Constitutive expression of CCR2 chemokine receptor and inhibition by MCP-1/CCL2 of GABA-induced currents in spinal cord neurones. J Neurochem. 2005;95(4):1023–1034. doi: 10.1111/j.1471-4159.2005.03431.x. [DOI] [PubMed] [Google Scholar]
- 92.Menetski J, Mistry S, Lu M, Mudgett JS, Ransohoff RM, Demartino JA, Macintyre DE, Abbadie C. Mice overexpressing chemokine ligand 2 (CCL2) in astrocytes display enhanced nociceptive responses. Neuroscience. 2007;149(3):706–714. doi: 10.1016/j.neuroscience.2007.08.014. [DOI] [PubMed] [Google Scholar]
- 93.Begin-Lavallee V, Midavaine E, Dansereau MA, Tetreault P, Longpre JM, Jacobi AM, Rose SD, Behlke MA, Beaudet N, Sarret P. Functional inhibition of chemokine receptor CCR2 by dicer-substrate-siRNA prevents pain development. Mol Pain. 2016 doi: 10.1177/1744806916653969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Verri WA, Jr, Cunha TM, Parada CA, Poole S, Cunha FQ, Ferreira SH. Hypernociceptive role of cytokines and chemokines: targets for analgesic drug development? Pharmacol Ther. 2006;112(1):116–138. doi: 10.1016/j.pharmthera.2006.04.001. [DOI] [PubMed] [Google Scholar]
- 95.Cunha TM, Verri WA, Jr, Schivo IR, Napimoga MH, Parada CA, Poole S, Teixeira MM, Ferreira SH, Cunha FQ. Crucial role of neutrophils in the development of mechanical inflammatory hypernociception. J Leukoc Biol. 2008;83(4):824–832. doi: 10.1189/jlb.0907654. [DOI] [PubMed] [Google Scholar]
- 96.Carreira EU, Carregaro V, Teixeira MM, Moriconi A, Aramini A, Verri WA, Jr, Ferreira SH, Cunha FQ, Cunha TM. Neutrophils recruited by CXCR1/2 signalling mediate post-incisional pain. Eur J Pain. 2013;17(5):654–663. doi: 10.1002/j.1532-2149.2012.00240.x. [DOI] [PubMed] [Google Scholar]
- 97.Valles A, Grijpink-Ongering L, de Bree FM, Tuinstra T, Ronken E. Differential regulation of the CXCR2 chemokine network in rat brain trauma: implications for neuroimmune interactions and neuronal survival. Neurobiol Dis. 2006;22(2):312–322. doi: 10.1016/j.nbd.2005.11.015. [DOI] [PubMed] [Google Scholar]
- 98.Popivanova BK, Koike K, Tonchev AB, Ishida Y, Kondo T, Ogawa S, Mukaida N, Inoue M, Yamashima T. Accumulation of microglial cells expressing ELR motif-positive CXC chemokines and their receptor CXCR2 in monkey hippocampus after ischemia-reperfusion. Brain Res. 2003;970(1–2):195–204. doi: 10.1016/S0006-8993(03)02343-6. [DOI] [PubMed] [Google Scholar]
- 99.Nguyen D, Stangel M. Expression of the chemokine receptors CXCR1 and CXCR2 in rat oligodendroglial cells. Brain Res Dev Brain Res. 2001;128(1):77–81. doi: 10.1016/S0165-3806(01)00128-6. [DOI] [PubMed] [Google Scholar]
- 100.Manjavachi MN, Costa R, Quintao NL, Calixto JB. The role of keratinocyte-derived chemokine (KC) on hyperalgesia caused by peripheral nerve injury in mice. Neuropharmacology. 2014;79:17–27. doi: 10.1016/j.neuropharm.2013.10.026. [DOI] [PubMed] [Google Scholar]
- 101.Pineau I, Sun L, Bastien D, Lacroix S. Astrocytes initiate inflammation in the injured mouse spinal cord by promoting the entry of neutrophils and inflammatory monocytes in an IL-1 receptor/MyD88-dependent fashion. Brain Behav Immun. 2010;24(4):540–553. doi: 10.1016/j.bbi.2009.11.007. [DOI] [PubMed] [Google Scholar]
- 102.Xu J, Zhu MD, Zhang X, Tian H, Zhang JH, Wu XB, Gao YJ. NFkappaB-mediated CXCL1 production in spinal cord astrocytes contributes to the maintenance of bone cancer pain in mice. J Neuroinflammation. 2014;11:38. doi: 10.1186/1742-2094-11-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Omari KM, John G, Lango R, Raine CS. Role for CXCR2 and CXCL1 on glia in multiple sclerosis. Glia. 2006;53(1):24–31. doi: 10.1002/glia.20246. [DOI] [PubMed] [Google Scholar]
- 104.Chen G, Park CK, Xie RG, Berta T, Nedergaard M, Ji RR. Connexin-43 induces chemokine release from spinal cord astrocytes to maintain late-phase neuropathic pain in mice. Brain. 2014;137(Pt 8):2193–2209. doi: 10.1093/brain/awu140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Cao DL, Zhang ZJ, Xie RG, Jiang BC, Ji RR, Gao YJ. Chemokine CXCL1 enhances inflammatory pain and increases NMDA receptor activity and COX-2 expression in spinal cord neurons via activation of CXCR2. Exp Neurol. 2014;261:328–336. doi: 10.1016/j.expneurol.2014.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Yang LH, Xu GM, Wang Y. Up-regulation of CXCL1 and CXCR2 contributes to remifentanil-induced hypernociception via modulating spinal NMDA receptor expression and phosphorylation in rats. Neurosci Lett. 2016;626:135–141. doi: 10.1016/j.neulet.2015.12.044. [DOI] [PubMed] [Google Scholar]
- 107.Sun Y, Sahbaie P, Liang DY, Li WW, Li XQ, Shi XY, Clark JD. Epigenetic regulation of spinal CXCR2 signaling in incisional hypersensitivity in mice. Anesthesiology. 2013;119(5):1198–1208. doi: 10.1097/ALN.0b013e31829ce340. [DOI] [PubMed] [Google Scholar]
- 108.Manjavachi MN, Quintao NL, Campos MM, Deschamps IK, Yunes RA, Nunes RJ, Leal PC, Calixto JB. The effects of the selective and non-peptide CXCR2 receptor antagonist SB225002 on acute and long-lasting models of nociception in mice. Eur J Pain. 2010;14(1):23–31. doi: 10.1016/j.ejpain.2009.01.007. [DOI] [PubMed] [Google Scholar]
- 109.Lin CP, Kang KH, Lin TH, Wu MY, Liou HC, Chuang WJ, Sun WZ, Fu WM. Role of spinal CXCL1 (GROalpha) in opioid tolerance: a human-to-rodent translational study. Anesthesiology. 2015;122(3):666–676. doi: 10.1097/ALN.0000000000000523. [DOI] [PubMed] [Google Scholar]
- 110.Ansel KM, Ngo VN, Hyman PL, Luther SA, Forster R, Sedgwick JD, Browning JL, Lipp M, Cyster JG. A chemokine-driven positive feedback loop organizes lymphoid follicles. Nature. 2000;406(6793):309–314. doi: 10.1038/35018581. [DOI] [PubMed] [Google Scholar]
- 111.Katayama T, Tanaka H, Yoshida T, Uehara T, Minami M. Neuronal injury induces cytokine-induced neutrophil chemoattractant-1 (CINC-1) production in astrocytes. J Pharmacol Sci. 2009;109(1):88–93. doi: 10.1254/jphs.08298FP. [DOI] [PubMed] [Google Scholar]
- 112.Kim CH, Rott LS, Clark-Lewis I, Campbell DJ, Wu L, Butcher EC. Subspecialization of CXCR5+ T cells: B helper activity is focused in a germinal center-localized subset of CXCR5+ T cells. J Exp Med. 2001;193(12):1373–1381. doi: 10.1084/jem.193.12.1373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Krumbholz M, Theil D, Cepok S, Hemmer B, Kivisakk P, Ransohoff RM, Hofbauer M, Farina C, Derfuss T, Hartle C, Newcombe J, Hohlfeld R, Meinl E. Chemokines in multiple sclerosis: CXCL12 and CXCL13 up-regulation is differentially linked to CNS immune cell recruitment. Brain. 2006;129(Pt 1):200–211. doi: 10.1093/brain/awh680. [DOI] [PubMed] [Google Scholar]
- 114.Gunn MD, Ngo VN, Ansel KM, Ekland EH, Cyster JG, Williams LT. A B-cell-homing chemokine made in lymphoid follicles activates Burkitt’s lymphoma receptor-1. Nature. 1998;391(6669):799–803. doi: 10.1038/35876. [DOI] [PubMed] [Google Scholar]
- 115.Bagaeva LV, Rao P, Powers JM, Segal BM. CXC chemokine ligand 13 plays a role in experimental autoimmune encephalomyelitis. J Immunol. 2006;176(12):7676–7685. doi: 10.4049/jimmunol.176.12.7676. [DOI] [PubMed] [Google Scholar]
- 116.Magliozzi R, Columba-Cabezas S, Serafini B, Aloisi F. Intracerebral expression of CXCL13 and BAFF is accompanied by formation of lymphoid follicle-like structures in the meninges of mice with relapsing experimental autoimmune encephalomyelitis. J Neuroimmunol. 2004;148(1–2):11–23. doi: 10.1016/j.jneuroim.2003.10.056. [DOI] [PubMed] [Google Scholar]
- 117.Smith JR, Braziel RM, Paoletti S, Lipp M, Uguccioni M, Rosenbaum JT. Expression of B-cell-attracting chemokine 1 (CXCL13) by malignant lymphocytes and vascular endothelium in primary central nervous system lymphoma. Blood. 2003;101(3):815–821. doi: 10.1182/blood-2002-05-1576. [DOI] [PubMed] [Google Scholar]
- 118.Wallace VC, Cottrell DF, Brophy PJ, Fleetwood-Walker SM. Focal lysolecithin-induced demyelination of peripheral afferents results in neuropathic pain behavior that is attenuated by cannabinoids. J Neurosci. 2003;23(8):3221–3233. doi: 10.1523/JNEUROSCI.23-08-03221.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Ljostad U, Mygland A. CSF B–lymphocyte chemoattractant (CXCL13) in the early diagnosis of acute Lyme neuroborreliosis. J Neurol. 2008;255(5):732–737. doi: 10.1007/s00415-008-0785-y. [DOI] [PubMed] [Google Scholar]
- 120.Polomano RC, Mannes AJ, Clark US, Bennett GJ. A painful peripheral neuropathy in the rat produced by the chemotherapeutic drug, paclitaxel. Pain. 2001;94(3):293–304. doi: 10.1016/S0304-3959(01)00363-3. [DOI] [PubMed] [Google Scholar]
- 121.Rainey-Barger EK, Rumble JM, Lalor SJ, Esen N, Segal BM, Irani DN. The lymphoid chemokine, CXCL13, is dispensable for the initial recruitment of B cells to the acutely inflamed central nervous system. Brain Behav Immun. 2011;25(5):922–931. doi: 10.1016/j.bbi.2010.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Zhang Q, Cao DL, Zhang ZJ, Jiang BC, Gao YJ. Chemokine CXCL13 mediates orofacial neuropathic pain via CXCR5/ERK pathway in the trigeminal ganglion of mice. J Neuroinflammation. 2016;13(1):183. doi: 10.1186/s12974-016-0652-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Strong JA, Xie W, Coyle DE, Zhang JM. Microarray analysis of rat sensory ganglia after local inflammation implicates novel cytokines in pain. PloS One. 2012;7(7):e40779. doi: 10.1371/journal.pone.0040779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Zhang Q, Zhu MD, Cao DL, Bai XQ, Gao YJ, Wu XB. Chemokine CXCL13 activates p38 MAPK in the trigeminal ganglion after infraorbital nerve injury. Inflammation. 2017 doi: 10.1007/s10753-017-0520-x. [DOI] [PubMed] [Google Scholar]
- 125.Jiang BC, He LN, Wu XB, Shi H, Zhang WW, Zhang ZJ, Cao DL, Li CH, Gu J, Gao YJ. Promoted Interaction of C/EBPalpha with Demethylated Cxcr3 gene promoter contributes to neuropathic pain in mice. J Neurosci. 2017;37(3):685–700. doi: 10.1523/JNEUROSCI.2262-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Guan XH, Fu QC, Shi D, Bu HL, Song ZP, Xiong BR, Shu B, Xiang HB, Xu B, Manyande A, Cao F, Tian YK. Activation of spinal chemokine receptor CXCR3 mediates bone cancer pain through an Akt-ERK crosstalk pathway in rats. Exp Neurol. 2015;263:39–49. doi: 10.1016/j.expneurol.2014.09.019. [DOI] [PubMed] [Google Scholar]
- 127.Luo X, Tai WL, Sun L, Pan Z, Xia Z, Chung SK, Cheung CW. Crosstalk between astrocytic CXCL12 and microglial CXCR4 contributes to the development of neuropathic pain. Mol Pain. 2016 doi: 10.1177/1744806916636385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Hu XM, Liu YN, Zhang HL, Cao SB, Zhang T, Chen LP, Shen W. CXCL12/CXCR4 chemokine signaling in spinal glia induces pain hypersensitivity through MAPKs-mediated neuroinflammation in bone cancer rats. J Neurochem. 2015;132(4):452–463. doi: 10.1111/jnc.12985. [DOI] [PubMed] [Google Scholar]
- 129.Shen W, Hu XM, Liu YN, Han Y, Chen LP, Wang CC, Song C. CXCL12 in astrocytes contributes to bone cancer pain through CXCR4-mediated neuronal sensitization and glial activation in rat spinal cord. J Neuroinflammation. 2014;11:75. doi: 10.1186/1742-2094-11-75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Bai L, Wang X, Li Z, Kong C, Zhao Y, Qian JL, Kan Q, Zhang W, Xu JT. Upregulation of chemokine CXCL12 in the dorsal root ganglia and spinal cord contributes to the development and maintenance of neuropathic pain following spared nerve injury in rats. Neurosci Bull. 2016;32(1):27–40. doi: 10.1007/s12264-015-0007-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Knerlich-Lukoschus F, von der Ropp-Brenner B, Lucius R, Mehdorn HM, Held-Feindt J. Spatiotemporal CCR1, CCL3(MIP-1alpha), CXCR4, CXCL12(SDF-1alpha) expression patterns in a rat spinal cord injury model of posttraumatic neuropathic pain. J Neurosurg Spine. 2011;14(5):583–597. doi: 10.3171/2010.12.SPINE10480. [DOI] [PubMed] [Google Scholar]
- 132.Luo X, Tai WL, Sun L, Qiu Q, Xia Z, Chung SK, Cheung CW. Central administration of C-X-C chemokine receptor type 4 antagonist alleviates the development and maintenance of peripheral neuropathic pain in mice. PloS One. 2014;9(8):e104860. doi: 10.1371/journal.pone.0104860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Xie F, Wang Y, Li X, Chao YC, Yue Y. Early repeated administration of CXCR4 antagonist AMD3100 dose-dependently improves neuropathic pain in rats after L5 spinal nerve ligation. Neurochem Res. 2016;41(9):2289–2299. doi: 10.1007/s11064-016-1943-8. [DOI] [PubMed] [Google Scholar]
- 134.de Jong EK, Vinet J, Stanulovic VS, Meijer M, Wesseling E, Sjollema K, Boddeke HW, Biber K. Expression, transport, and axonal sorting of neuronal CCL21 in large dense-core vesicles. Faseb J. 2008;22(12):4136–4145. doi: 10.1096/fj.07-101907. [DOI] [PubMed] [Google Scholar]
- 135.Dijkstra IM, de Haas AH, Brouwer N, Boddeke HW, Biber K. Challenge with innate and protein antigens induces CCR7 expression by microglia in vitro and in vivo. Glia. 2006;54(8):861–872. doi: 10.1002/glia.20426. [DOI] [PubMed] [Google Scholar]
- 136.Biber K, Tsuda M, Tozaki-Saitoh H, Tsukamoto K, Toyomitsu E, Masuda T, Boddeke H, Inoue K. Neuronal CCL21 up-regulates microglia P2 × 4 expression and initiates neuropathic pain development. EMBO J. 2011;30(9):1864–1873. doi: 10.1038/emboj.2011.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Kiguchi N, Kobayashi Y, Kishioka S. Chemokines and cytokines in neuroinflammation leading to neuropathic pain. Curr Opin Pharmacol. 2012;12(1):55–61. doi: 10.1016/j.coph.2011.10.007. [DOI] [PubMed] [Google Scholar]
- 138.Kiguchi N, Kobayashi Y, Maeda T, Saika F, Kishioka S. CC-chemokine MIP-1alpha in the spinal cord contributes to nerve injury-induced neuropathic pain. Neurosci Lett. 2010;484(1):17–21. doi: 10.1016/j.neulet.2010.07.085. [DOI] [PubMed] [Google Scholar]
- 139.Padi SS, Shi XQ, Zhao YQ, Ruff MR, Baichoo N, Pert CB, Zhang J. Attenuation of rodent neuropathic pain by an orally active peptide, RAP-103, which potently blocks CCR2- and CCR5-mediated monocyte chemotaxis and inflammation. Pain. 2012;153(1):95–106. doi: 10.1016/j.pain.2011.09.022. [DOI] [PubMed] [Google Scholar]
- 140.Horuk R. Chemokine receptor antagonists: overcoming developmental hurdles. Nat Rev Drug Discov. 2009;8(1):23–33. doi: 10.1038/nrd2734. [DOI] [PubMed] [Google Scholar]
- 141.Pease JE, Horuk R. Chemokine receptor antagonists: Part 1. Expert Opin Ther Pat. 2009;19(1):39–58. doi: 10.1517/13543770802641346. [DOI] [PubMed] [Google Scholar]
- 142.Pease JE, Horuk R. Chemokine receptor antagonists: part 2. Expert Opin Ther Pat. 2009;19(2):199–221. doi: 10.1517/13543770802641353. [DOI] [PubMed] [Google Scholar]
- 143.Pease JE, Horuk R. Recent progress in the development of antagonists to the chemokine receptors CCR3 and CCR4. Expert Opin Drug Discov. 2014;9(5):467–483. doi: 10.1517/17460441.2014.897324. [DOI] [PubMed] [Google Scholar]
- 144.Pease J, Horuk R. Chemokine receptor antagonists. J Med Chem. 2012;55(22):9363–9392. doi: 10.1021/jm300682j. [DOI] [PubMed] [Google Scholar]
- 145.Kalliomaki J, Attal N, Jonzon B, Bach FW, Huizar K, Ratcliffe S, Eriksson B, Janecki M, Danilov A, Bouhassira D. A randomized, double-blind, placebo-controlled trial of a chemokine receptor 2 (CCR2) antagonist in posttraumatic neuralgia. Pain. 2013;154(5):761–767. doi: 10.1016/j.pain.2013.02.003. [DOI] [PubMed] [Google Scholar]