Abstract
Chemotherapy is one of the most effective and broadly used approaches for cancer management and many modern regimes can eliminate the bulk of the cancer cells. However, recurrence and metastasis still remain a major obstacle leading to the failure of systemic cancer treatments. Therefore, to improve the long-term eradication of cancer, the cellular and molecular pathways that provide targets which play crucial roles in drug resistance should be identified and characterised. Multidrug resistance (MDR) and the existence of tumor-initiating cells, also referred to as cancer stem cells (CSCs), are two major contributors to the failure of chemotherapy. MDR describes cancer cells that become resistant to structurally and functionally unrelated anti-cancer agents. CSCs are a small population of cells within cancer cells with the capacity of self-renewal, tumor metastasis, and cell differentiation. CSCs are also believed to be associated with chemoresistance. Thus, MDR and CSCs are the greatest challenges for cancer chemotherapy. A significant effort has been made to identify agents that specifically target MDR cells and CSCs. Consequently, some agents derived from nature have been developed with a view that they may overcome MDR and/or target CSCs. In this review, natural products-targeting MDR cancer cells and CSCs are summarized and clustered by their targets in different signaling pathways.
Keywords: Cancer therapy, Drug treatment, Drug efflux pumps, Cancer-initiating cells, Multidrug resistance, Cancer stem cells, Natural products
Introduction
Cancer is a complex array of diseases with a morbidity of around 7.5 million deaths per year worldwide, and these numbers are rising, owing partly to aging populations and environment pollution [1]. Chemotherapy using anti-cancer agents with structural and functional diversity has been developed to treat cancers. These drugs have been applied alone or in combination to prolong life or to alleviate the symptoms of cancer for decades. However, chemotherapy has failed to completely eradicate cancers for several reasons. Multidrug resistance (MDR) is one of the major obstacles for chemotherapy. MDR is a phenomenon, in which cancer cells become resistant to mechanistically and structurally unrelated anti-cancer drugs [2]. Various factors can contribute to MDR, including inappropriate drug delivery and genetic alterations, that prolong cell surviving [3]. The overexpression of drug efflux pumps, such as the ATP-binding cassette (ABC) transporter family of proteins, is one of the major factors that confer drug resistance [2]. It is the largest transporter superfamily which exports specific molecules through cell membranes. For example, the activation of one of the ABC transporter P-glycoproteins (P-gp), the product of the MDR1 (also referred to as ABCB1) gene, can cause active drug release from cells [4]. The overexpression of ABC transporters can reduce the cellular concentration of the drugs, such as the well-known anti-cancer compounds vinblastine, vincristine, and doxorubicin (DOX) [5–7]. Therefore, ABC transporters play an important role in the development of drug resistance in cancers.
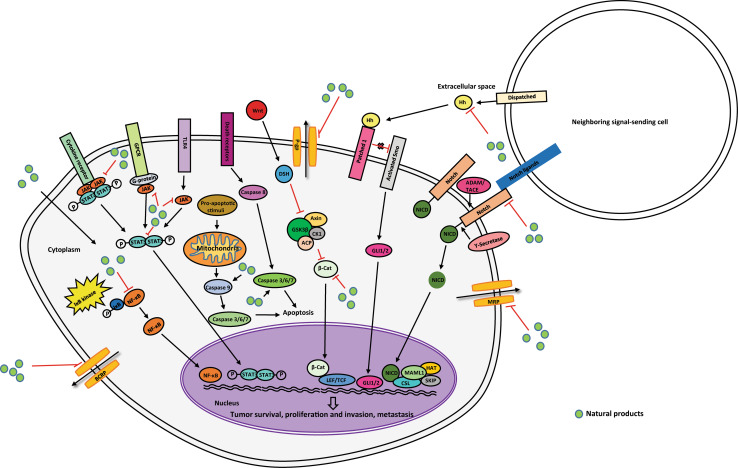
Cancer stem cells (CSC) are another obstacle for chemotherapy. A large body of evidence indicates that there exists a small subset of tumor cells within a cancer population which are heterogeneous in proliferation rates and clonogenic potency (reviewed in [8]). This subset of tumor cells was termed cancer stem cells. It is believed that this subset of cells has the capacity of self-renewal, differentiation, cancer relapse, and tumor metastasis [9]. Subsequently, more and more CSCs were identified in various tumors, including colorectal cancer [10], breast cancer [11], prostate cancer [12], colon cancer [13], and brain cancer [14]. How do CSCs drive the failure of cancer therapy? CSCs share the capacity of self-renewal and differentiation with normal stem cells and can be characterised by the expression of specific stem-cell markers. Some cell-surface markers used to identify and enrich the CSCs are also markers for normal stem cells. For example, CD133 was characterised as a marker for the identification of brain CSCs [15] and colorectal carcinoma [16], but it is also a marker of normal neural stem cells [17] and probably pancreatic stem cells [18]. Therefore, CSCs might share properties with normal stem cells, including the expression of some specific ABC drug transporters, prosurvival and anti-apoptotic molecules, and other factors. This review discusses how CSC and chemoresistance may be correlated. The following sections give a brief outline of some of the mechanisms utilized by CSC and MDR cells with a view to highlighting cellular pathways that can be targeted by natural products and their derivatives in the fight to halt cancer progression (Fig. 1).
Fig. 1.
Depicts the major pathways that are targeted by natural products in multidrug-resistant cancer cells and cancer stem cells. The figure depicts natural products (green circles) interacting with their target cell-surface receptor (labelled rectangles) or protein (labelled circles) within signaling pathways
The ABC transporter family
The ABC transporter family acts by pumping drugs to either the external leaflet of the membrane or the extracellular domain and requires ATP hydrolysis to do so [4]. To date, 49 human ABC genes have been identified and were clustered in seven subfamilies (ABCA–ABCG) according to the homology of amino-acid sequences, the constitution of transmembrane (TM) domains, and ATP-binding sites [19]. There are three major transporters correlated with MDR, including P-glycoprotein (MDR1/ABCB1), MDR-associated protein (MRP/ABCC1), and breast cancer resistance protein (BCRP/ABCG2) [3], and these are discussed in the following sections.
P-glycoprotein in cancer (ABCB1)
P-glycoprotein (P-gp), a 170 kDa TM phosphoglycoprotein, is one of the most important ABC transporters and its overexpression is directly associated with MDR in humans. Similar to several other ABC transporter members, P-gp consists of two TM domains, each of which comprises six helices and two ATP-binding sites. Two paralogs, expressed by the MDR1/ABCB1 and MDR2/3/ABCB4 genes, have been identified so far. The P-gp expressed from MDR1/ABCB1 was initially demonstrated to have a protective role from toxins in susceptible tissues, such as the brain, inner ear, testis, and mammary tissue [20–22]. When substrates or drugs enter the binding site, ATP will be hydrolyzed by ATPase to supply the energy to release the substrates/drugs to the outer leaflet or the extracellular space. There are three patterns that modulate the ATPase activity of P-gp by drug substrates and modulators; many drugs stimulate the activity of ATPase at low concentrations while inhibiting activity at higher concentrations, other drugs can only inhibit the activity of ATPase, whereas some of the drugs stimulate the ATPase activity. P-gp can transport a large range of compounds from chemotherapeutics to peptides; however, priority is given to amphipathic and relatively hydrophobic drugs, including the large complex drugs, such as paclitaxel, vinblastine and small molecules, such as daunorubicin and DOX. Most of the commonly recognized constitutions of the substrates of P-gp contained planar aromatic rings and positively charged tertiary N atoms. Therefore, a therapeutic approach exists which targets P-gp, so that efflux-driven drug resistance of cancer cells could be reversed. In a cellular environment, ABCB1 expression can be regulated by both transcriptional regulation and the translation or stability of the mRNA. For example, P-gp has been shown to bypass translational silencing [23] in specialized Ribonucleoprotein particles RNP called stress granules (reviewed in [24]. ABCB1 can also be regulated via its upstream signaling pathways, such as protein kinase C and mitogen-activated protein kinase cascades, fluxion of Ca2+, and NF-κB [25], and some drugs were developed to regulate these pathways. For instance, curcumin and capsaicin were used to block NF-κB activation. With a greater understanding of the mechanisms of ABCB1 activation, new therapeutics can be developed to target these pathways.
MDR-associated proteins (ABCC1)
The existence of P-gp by itself cannot explain all the occurrences of MDR, since the overexpression of P-gp was not found in all types of MDR cells. Further studies discovered other efflux pumps, such as multidrug-resistance-associated proteins (MRPs) and breast cancer resistant protein (BCRP/ABCG2). The MRP family is comprised of 13 members, 9 of which were associated with drug resistance. MRP1 and MRP9 played the most important roles in drug resistance among the MRPs [26]. MRP1 shares 15 % identity, at the protein level, with P-gp, and consists of one membrane-spanning domain (MSD) with five TM helices, and two other MSDs with six TM helices. Subsequently, MRP1 has an overlapping resistance with P-gp. MRP1 is a 190 kDa protein encoded by the ABCC1 gene, which is expressed in normal tissues, such as lung [27], testis [22], kidney [28], placenta [29], macrophages [30], and skeletal and cardiac muscles [31]. Primarily, the expression of MRP1 can make a contribution to some drug protection in these tissues of humans. For example, the defective expression of MRP1 can enhance the damage caused by etoposide in the mucosa of the oropharyngeal cavity and the seminiferous tubules of the testis [32]. In parallel, the diversity of substrates of MRP1 resulted in decreasing accumulation of drugs, including certain anti-cancer drugs, such as antharacyclines, vinca alkaloids, cisplatin, epipodophyllotoxins, camptothecins, saquinavir, methotrexate, and mitoxantrone [26]. MRP9 is another member within the family which is related to drug resistance and is comprised of two MSD and 12 helices. The high expression of an unusual truncated MRP9 mRNA was discovered in breast cancer, and MRP9 could be a significant target for breast cancer treatment, because of the relatively low-expression level in normal breast tissue [26].
Breast cancer resistance protein (ABCG2)
Interestingly, the overexpression of P-gp or MRP cannot be detected in the drug-resistant cell line, MCF7/AdrVp. However, a related 2.4 kb mRNA which encodes a 663 amino-acid protein was identified in the cell line and was termed as the breast cancer resistance protein (BCRP/ABCG2) [33]. The G subfamily of ABC transporters is comprised of six half-transporters with a nucleotide-binding domain (NBD) at the N-terminus and a TM domain at the C-terminus [34]. The expression level of ABCG2 mRNA in some tissues may shed some light on its potential function. There were no ABCG2 transcripts detected in heart, lung, skeletal muscle, kidney, pancreas, spleen, thymus, and peripheral blood leukocytes, while approximately 100 times more expression was detected in placental tissue than that in the brain, prostate, small intestine, testis, ovary, colon, or liver [35]. It appears to play a protective role in human stem cells, because ABCG2 has been reported as a potential phenotypic marker for stem cells in normal lung [36] and breast tissue [37]. Some anti-cancer therapeutics were identified as the substrates of ABCG2, including mitoxantrone, topotecan, isrinotecan, flavopiridol, and methotrexate [38]. In addition, bioflavonoid kaempferol is a substrate of ABCG2 and inhibits ABCG2-mediated quercetin efflux [39]. To overcome the resistance caused by ABCG2, some inhibitors were developed. The first reported inhibitor of ABCG2 was fumitremorgin C (FTC), 5 µM of which reversed cellular resistance to mitoxantrone, DOX, and topotecan in drug-selected colon carcinoma cells [40]. However, FTC can cause severe side effects, such as neurotoxicity, which precluded its clinical application. Ko143, an analog of FTC, caused an increase in intracellular drug accumulation and reversed mouse BCRP1- and human BCRP-mediated MDR without neurotoxic effects [41]. In recent years, more and more inhibitors have been discovered to reverse the effects of BCRP and these will be discussed below.
The Hedgehog receptor, patched, functions in multidrug resistance
The Hedgehog (Hh) gene was discovered in 1980 in fruit fly [42], and since then, three Hh genes have been identified in mammals, including Desert hedgehog (Dhh), Indian hedgehog (Ihh), and Sonic hedgehog (Shh) [43]. Hedgehog-activated signal transduction occurs via its receptor, patched (Ptch), which is a 12-transmembrane transporter-like protein [44] that has been implicated in multidrug transport in some cancer cell lines [45]. The Hh/patched pathway plays a crucial role in the early embryonic development as well as the tumor development, progression, and metastasis. In particular, the dysregulation of the hedgehog pathway has been observed in a number of cancers, especially in cells showing drug resistance [46]. The analysis of Ptch revealed sequence and topology conservation with the resistance-nodulation-division (RND) family of prokaryotic permeases. Furthermore, the GXXXD motif in the RND bacterial drug efflux pumps was highly conserved in the fourth putative transmembrane segment of human and drosophila Ptch. The RND family transports a broad range of agents, and therefore, Ptch has also been proposed to be an efflux transporter. Not surprisingly, yeast-expressing Ptch showed resistance to certain chemotherapeutics, such as DOX, methotrexate, temozolomide, and 5-FU [47]. Therefore, the development of new agents to target Ptch has been considered as a strategy for the treatment of cancer.
The ABC family in CSCs
The current research trends suggest that several characteristics of CSCs are shared with common features identified in normal stem cells, including the overexpression of ABC transporter family members, protecting the cells from drugs and the toxins. Glioblastoma CSCs were characterised by the expression level of the MDR gene BCRP1 [48]. Normal stem cells were believed to possess drug resistance by the expression of ATP-binding cassette transporters, DNA-repair systems, and anti-apoptosis systems to provide protection to these cells. For example, a high level of ABCG2 expression was detected in hematopoietic stem cells (HSC) as compared with most committed progenitor cells and mature blood cells [49]. RBM3 is a proto-oncogene that encodes for an RNA-binding protein that promoted a stem-like phenotype and spheroid formation in the colorectal cancer cell line HCT116. The overexpression of RBM3 also enhanced drug resistance to DOX and paclitaxel by upregulating the gene expression of MRP2 and P-gp [50]. Another ABC transporter member ABCB5, identified in oral squamous carcinoma cells (OSCC), was associated with tumor formation, metastasis, and a putative CSC compartment through gene expression studies [51]. In a study of leukemia, leukemia stem cells exhibited MDR by the expression of ABC transporters, such as P-gp, BCRP, and MRP8. This resistance was reversed by the agent salinomycin [52]. The occurrence of ABC expression in CSC and the evidence which suggests that they can be blocked with drugs make this family of genes an interesting target for future lead therapeutics.
Anti-apoptotic pathways in CSCs
Apoptosis can be triggered by the extrinsic and intrinsic pathways. The extrinsic pathway requires extracellular ligands, such as TNF-α, FasL, and TNF-related apoptosis-inducing ligand (TRAIL) binding to the cell-surface receptor, which is termed the death receptor. The intrinsic pathway is activated by the stimuli, such as cytotoxic signals, resulting in cell death. Bcl2 family members function as important regulators of programmed cell death pathways by blocking the extrinsic and intrinsic pathways and inhibiting downstream caspase activity. Bcl2 was a predicted target of miR-1915 in human colorectal carcinoma cells. In accordance with this, it was observed that the increased expression of miR-1915 reduced Bcl2 protein levels and increased cell sensitivity to some anti-cancer drugs [53]. The cellular FLICE-like inhibitory protein (cFLIP) is another regulator of apoptosis and works by blocking the activated death receptor signals, consequently inhibiting the activation of caspase 8. Importantly, cFLIP is not only expressed in many cancer cells but also in CSCs [54], such as glioblastoma stem cells which overexpress cFLIP. A study on glioblastoma CSCs demonstrated that the CD133+ cells not only overexpress BCRP1, but also the anti-apoptosis genes Bcl2, Bcl-X L, XIAP, and FLIP, which have been correlated to their chemoresistance [55]. NF-κB has been the most extensively examined transcription factors. It controls the expression of more than 500 different products that are relevant to inflammation, cellular transformation, tumor cell survival, proliferation, apoptosis suppression, invasion, and metastasis [56]. NF-κB signaling is multifunctional in many cells depending on its signaling context. In the immune system, NF-κB-mediated activation has been potent to inhibit tumor growth, in part through the production of growth inhibitory cytokines [57]. NF-κB is constitutively active in a wide range of cancers, including acute lymphocyte leukemia [58], chronic myelogenous leukemia [59], prostate [60], and breast cancers [61]. The previous study suggested that the activation of NF-κB could potentiate the expression level of anti-apoptosis genes, including Bcl2, Bcl-X L, XIAP, cFLIP, suvivin, cIAP-1, and cIAP-2 [62]. A study on the breast CSCs indicated that blocking NF-κB signaling by parthenolide (PTL), pyrrolidinedithiocarbamate (PDTC), and its analog diethyldithiocarbamate (DETC) resulted in the inhibition of the proliferation of sphere cells [63]. The examples described above are only a snapshot of the roles that anti-apoptotic systems play in cancer CSC survival but provide us with some of the scope of survival mechanisms available to CSC.
Prosurvival signaling in CSCs
In addition to the expression of efflux pumps and anti-apoptotic genes, drug resistance in cancer is also mediated by signaling cascades that control self-renewal, differentiation, and survival, such as the Notch, Wnt, and Hedgehog pathways. Notch induces the high expression of the anti-apoptotic gene BIRC5 (survivin) as well as the upregulation of the cyclin D1 protein [64]. It has been shown that Notch might be involved in the resistance to antitumor agents, such as trastuzumab, a HER2 inhibitor, that was used for the treatment of ErbB-2-positive breast cancer. The resistance to trastuzumab could be prevented and reversed by the inhibition of the Notch pathway [65]. The blockage of Notch by γ-secretase inhibitors (GSIs) in glioblastoma-derived neurospheres reduced tumor growth and expression of CSC markers, including CD133, NESTIN, BMI1, and OLIG2. Therefore, a high level of Notch expression maintained the CSC properties in CSCs and promoted proliferation, decreased apoptosis, and caused chemoresistance.
Wnt/β-catenin proteins play an important role in embryonic development and the maintenance of stem-cell properties. Not surprisingly, the β-catenin signaling cascade was also described as an essential pathway for sustaining the CSC phenotype in cutaneous cancer. In contrast, the depletion of the β-catenin gene led to the loss of CSCs and tumor regression [66]. Furthermore, it was reported that Wnt signaling played an important role in spheroidal CSC cultures that contained heterogeneity [67]. In haematopoietic stem cells, proliferation and self-renewal can be promoted by the overexpression of the β-catenin or stimulation with Wnt protein in vitro [68]. In addition, the expression of Wnt in CSCs derived from the intestine and mammary gland was also essential for their maintenance, which subsequently gave rise to the drug resistance [69]. Thus, the Wnt signaling pathway could be an important target to overcoming drug resistance.
The Hedgehog (Hh) signaling pathway is also critical for the embryonic development, regulating cell proliferation, metastasis, and differentiation in a tightly controlled mechanism. Emerging evidence based on the study of human cancers, such as glioblastoma, breast cancer, pancreatic adenocarcinoma, multiple myeloma, and chronic myeloid leukemia indicated that Hh signaling pathway was involved in the regulation of CSCs undergoing self-renewal and differentiation. Hh-Gli signaling affected stem-like gene expression and self-renewal in glioma CSCs, sustaining glioma proliferation, and survival [70]. The inhibition of the Hh signaling pathway by cyclopamine resulted in the ablation of stem-like cancer cells in glioblastoma [71]. A recent study demonstrated that Hh-Gli1 drove the UDP glucuronosyltransferase (GUT1A)-dependent glucuronidation of ribavirin and Ara-C, leading to the drug resistance [72]. On the other hand, the stimulation of the Hh pathway produced chemoresistance partly due to the upregulation of drug efflux by ABC transporters, including P-gp and BCRP [73]. In addition, the expression of the transcription factor Oct4 in CSCs played an important role in the maintenance of the survival of CSCs, and in drug resistance in prostate and liver cancer through the Oct4-AKT-ABCG2 signaling pathway [74, 75].
Aldehyde dehydrogenases in CSCs
Aldehyde dehydrogenases (ALDHs) are a family of intracellular enzymes, which can be used as molecular markers to identify normal stem cells and CSCs, such as the ovarian CSCs [76]. This superfamily consists of 19 genes in humans, including 11 families and four subfamilies. The most common studied members in normal stem cells and CSCs include ALDH1, ALDH2, and ALDH3A1. ALDH1 is commonly used to identify and enrich CSCs from many cancers, including liver cancer [77], head and neck cancer [78], breast cancer [79], colon cancer [80], and bladder cancer [81].
In CSCs, ALDHs are crucial for the maintenance of stemness. Treating lung adenoma stem cells with ALDH1A1 siRNA resulted in a lower capacity of clonogenicity, which suggested that ALDH1A1 was involved in the maintenance of stem-like properties by inhibiting the Notch/CDK2/CCNE pathway [82]. Blockage of ALDH in breast CSCs inhibited cell growth. Furthermore, the ALDH inhibitor, diethylaminobenzaldehyde (DEAB) obstructs tumor metastasis to the lung. In conclusion, a high level of ALDH expression promotes stemness features in breast cancer [83]. In addition, ALDHs play a detoxifying role, functioned in self-protection and conferred drug resistance to alkylating agents by metabolic inactivation [84]. Malignant pleural mesothelioma (MPM) cells with resistance to cisplatin and DEAB exhibited the overexpression of ALDH1A2, ALDH1A3 isozymes, and CD44, which suggest that ALDHhighCD44+ cells were implicated in conferring drug resistance [85]. ALDH inhibition and CD44 knockdown resulted in decreased stem-like gene expression and the enhancement of sensitivity to chemotherapeutics in lung cancer [86]. In accordance, stem-like ALDHhighCD44+ human breast cancer cells exhibited chemotherapy and radiation resistance, which was reversed by the inhibition of ALDH [87]. In these cases, ALDHs can be used as therapeutic targets, and subsequently, some ALDH inhibitors were developed to kill CSCs. For example, one of the inhibitors, copper (Cu)-dependent disulfiram (DS), was toxic to glioblastoma multiforme (GBM) stem-like cells by inhibiting the ALDH and NF-κB pathways, and enhanced the antitumor activity of gemcitabine (dFdC) in a synergistic manner [88]. Another CSC killer, salinomycin, also used for the reversal of MDR in cancer, was identified and found to be specifically toxic on the ALDHhigh population of stem-like cells in gastric cancer [89].
DNA repair and quiescence in CSCs
Genotoxic damage in mammalian cells, caused by endogenous and exogenous chemical, physical, and biological mutagens, results in DNA degradation and cell apoptosis. Under the normal conditions, the cellular integrity of genomic material is repaired to maintain the normal functions of the cells. This is especially true in the stem cells, as they have the capacity of self-renewal and give rise to a daughter cell that must retain an identical genome, and the potential to differentiate into tissue-specific cells. Thus, to ensure the original stemness properties, stem cells are endowed with multiple DNA-repair mechanisms, such as nucleotide excision repair, base excision repair, mismatch repair, direct repair, and the double-strand break recombinational repair [90].
The malignant counterpart of stem cells, CSCs, exhibits similar protective mechanisms. Accumulating evidence has demonstrated that CSCs show radioresistance and chemoresistance which were mediated by the DNA-repair mechanisms. Chronic myelogenous leukemia is mainly caused by the acquisition of BCR/ABL in HSCs. BCR/ABL expression increased reactive oxygen species (ROS) expression, which subsequently promoted oxidative stress and DNA damage. When DNA damage occurs, the inhibition of apoptosis can also be mediated by BCR/ABL, which subsequently induces the acquisition of radioresistance and chemoresistance [91]. In the breast CSCs, high levels of gene expression are observed for genes involved in DNA damage response and repair, such as Nek1, Brac1, Chek1, Hus1, Ung, Xrcc5, Sfpq, and Uhrf1, suggesting that breast CSCs are also resistant to chemotherapy and radiotherapy [92]. Compared with normal glioblastoma multiform cells, glioblastoma multiform CSCs were more radioresistant to cell death by the down-regulation of DNA damage checkpoint proteins, including ATM, Chk1, and Chk2 [93]. In recent research, prostate CSCs (PCSCs) exhibited chemoresistance in response to etoposide and docetaxel, the most commonly used chemotherapeutic drugs. This was achieved through the elevated expression of γH2AX (a marker for DNA double-strand breaks and genomic instability) and G2/M arrest. This study demonstrated that the upregulation of DNA damage responses made a contribution to chemoresistance in PCSCs [94].
Another mechanism to prevent apoptosis is for a cell to remain quiescent. Leukemia CSCs, transplanted into immunodeficient mice, conferred chemoresistance by quiescence as a defensive mechanism [95]. Esophageal CSCs (ECSCs) also remain in quiescence and, as a result, are more resistant to DNA damage agents. ECSCs demonstrated a low-expression level of EGFR, phosphorylated STAT3, and c-Myc, but elevated expression of p27. These factors appear to maintain quiescence and attenuate DNA damage responses and contributed to cell survival [96].
The studies described above highlighted a few of the mechanisms, such as the expression of DNA damage response and repair genes, as well as quiescence, that are utilized by cells to achieve chemoresistance which helped cells avoid apoptosis.
In conclusion, CSCs acts as one of the major obstacles to chemotherapy mainly due to their highly tumorigenic and chemoresistance properties. Understanding the mechanisms to achieve chemoresistance in CSCs has been exploited for the development of novel anti-cancer drugs. Therefore, the development of the new ABC inhibitors, anti-apoptotic antagonists, prosurvival pathway inhibitors, ALDH inhibitors, and the agents that target DNA-repair pathways holds promise for effective treatment of CSCs.
Natural products as MDR-reversing agents
MDR in cancer is a major obstacle for cancer therapy. To reverse MDR in cancer cells, researchers have developed antisense therapies, such as MDR1-antisense RNA [97], TAT-conjugated mesoporous silica nanoparticle drug delivery systems [98], and adjuvant therapy (i.e., fluoxetine synergies). Chemotherapeutics have also been developed to directly inhibit the activity of P-gp, MRPs, and BCRPs. For example, vinblastine and azidopine bound to the transport sites of P-gp, leading to the intracellular accumulation of the substrates [99–101], while amoxapene and Ioxapine non-competitively bound at the allosteric modulatory sites of P-gp, resulting in a 3.5-fold reduction of the DOX GI50 in K562Dox cells [102]. To date, a number of natural products have been identified to reverse drug resistance by modulating ABC transporters, including P-gp, MRP1, and BCRP.
P-gp inhibitors
A number of flavonoids have shown to be MDR reversal agents by inhibiting P-gp. Baicalein (Table 1, 1), a flavonoid from Scutellariae radix, was shown to exert strong anti-cancer activity against ovarian cancer with LD50 values ranging from 25 to 40 µM [103]. It induced G0/G1 phase arrest in hepatocellular carcinoma cells and obstructed H22 xenograft tumor growth. It has been shown that baicalein can inhibit tumor growth and apoptosis by affecting the phosphatidylinositol 3-kinase-AKT, Bcl-2, Bax, NF-κB, and p53 pathways [104]. Baicalein can also enhance the cytotoxicity of other anti-cancer chemotherapeutics, such as cisplatin, by increasing gap junction intercellular communication [105]. Hypoxia-induced 5-fluorouracil resistance in gastric cancer AGS cells can be reversed by baicalein via the suppression of glycolysis through the regulation of the PTEN/Akt/HIF-1α signaling pathway [106]. Baicalein increased nimodipine bioavailability by inhibiting cytochrome P450 3 A4 (CYP3A4)-mediated metabolism of nimodipine in the small intestine and/or in the liver. Baicalein also elevated intracellular rhodamine-123 (the substrate of P-gp, RH123) concentration in P-gp overexpressed MCF7/ADR cells in a dose-dependent manner [107]. Icaritin (Table 1, 2), a flavonoid isolated from Herba epimedii, exhibited a broad range of pharmacological and biological activities, including anti-cancer activity in hepatoma cells SMMC-7721 with an IC50 of 9.6 µM [108]. Icaritin can reduce renal cell carcinoma (RCC) by reducing the activation of the protein, signal transducer, and transcription-3 (STAT3), which is critical for tumor survival, proliferation, and angiogenesis [109]. Further studies indicated that icaritin can also reverse MDR in HepG2/adriamycin (HepG2/ADR) human hepatoma cells by down-regulating the expression of MDR1. Icaritin reversed the resistance to ADR by more than 7-folds at the concentration of 30 µM [110]. Icariin (Table 1, 3), a flavonoid glycoside, isolated from the same plant, is commonly prescribed for the treatment of cardiovascular diseases, osteoporosis, and cancer in China [111, 112]. The MDR reversal activity study revealed that icariin enhanced the sensitivity of MCF7/ADR cells to ADR by about twofold at the concentration of 25 µM [113]. Recent study suggested that icariin inhibited P-gp-mediated efflux pump by competitively binding at the P-gp drug-binding site [114]. Other flavonoids, including quercetin (Table 1, 4), biochanin A (Table 1, 5), phloretin (Table 1, 6), silymarin (Table 1, 7), and morin (Table 1, 8), also showed activity in reversing the drug resistance by inhibiting P-gp-mediated drug efflux [115, 116].
Table 1.
Natural products that have been implicated in the reversal of multidrug resistance in cancers
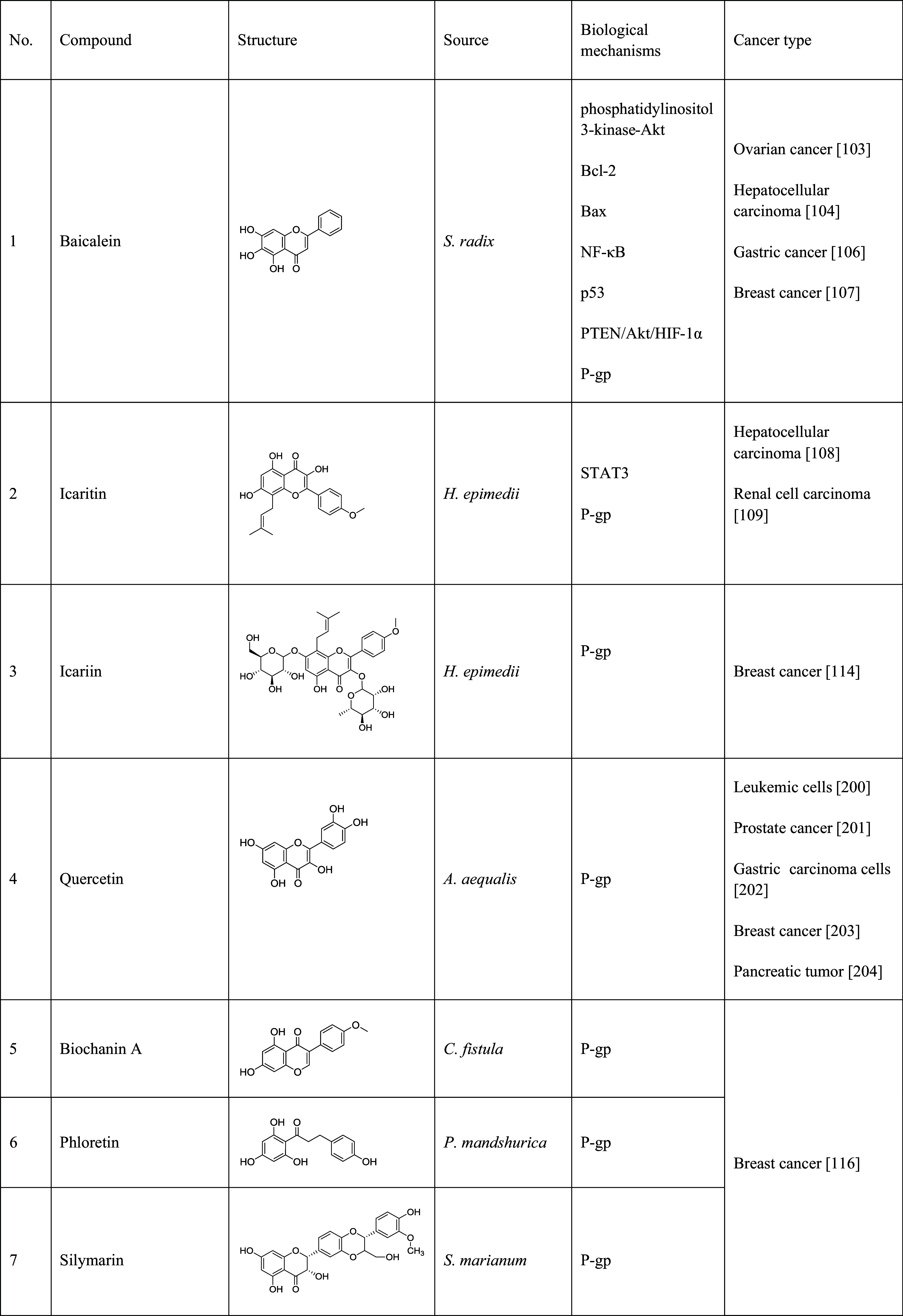
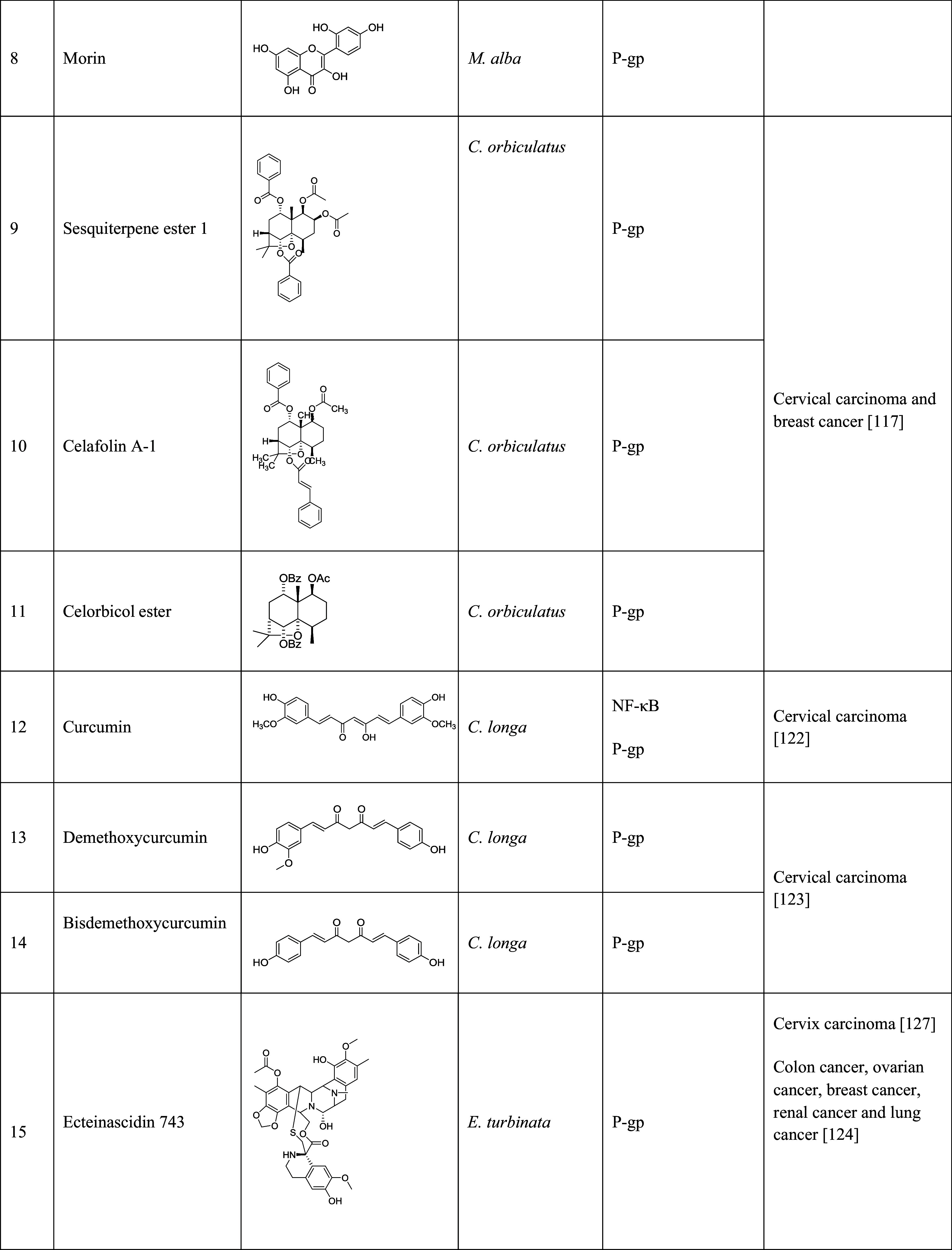
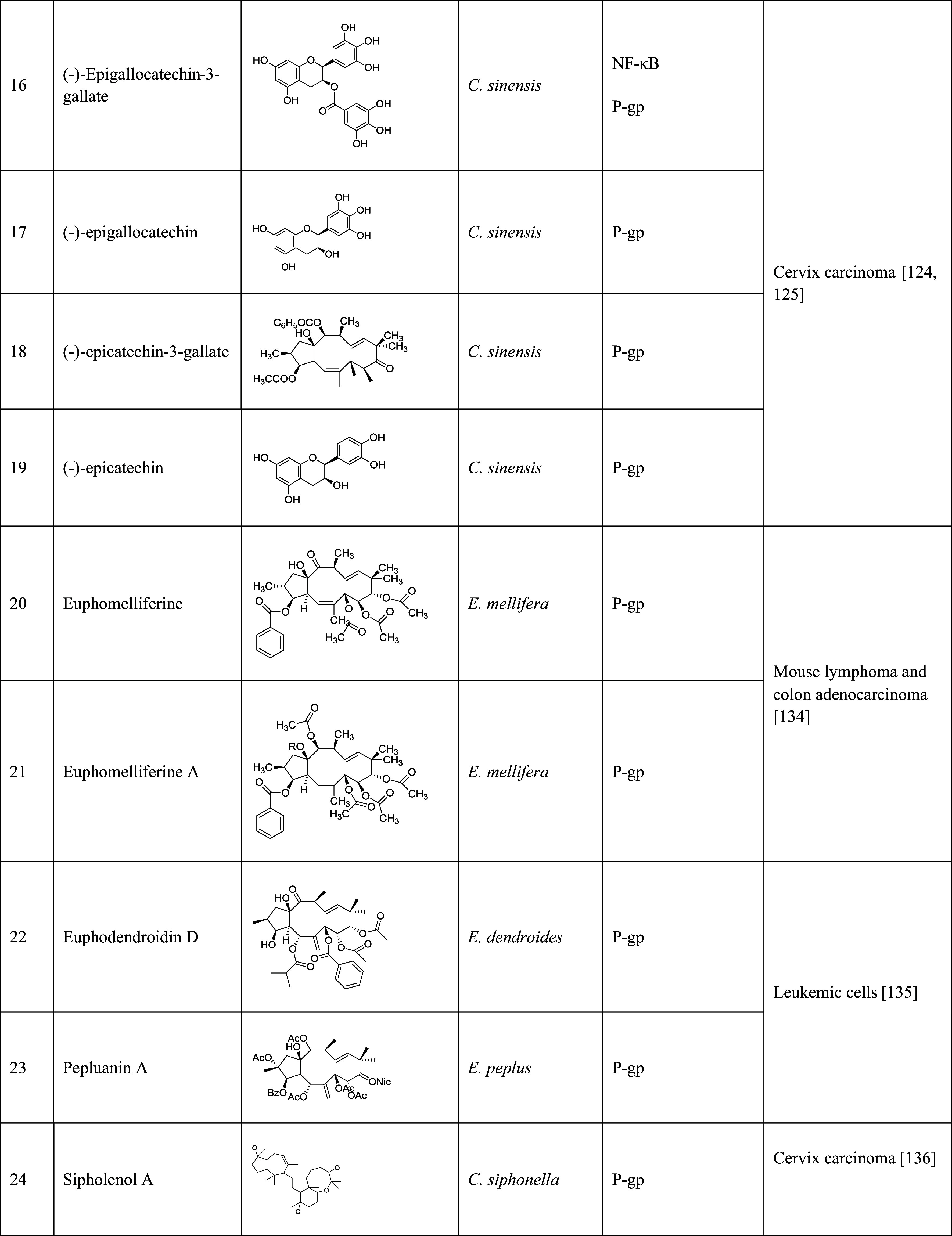
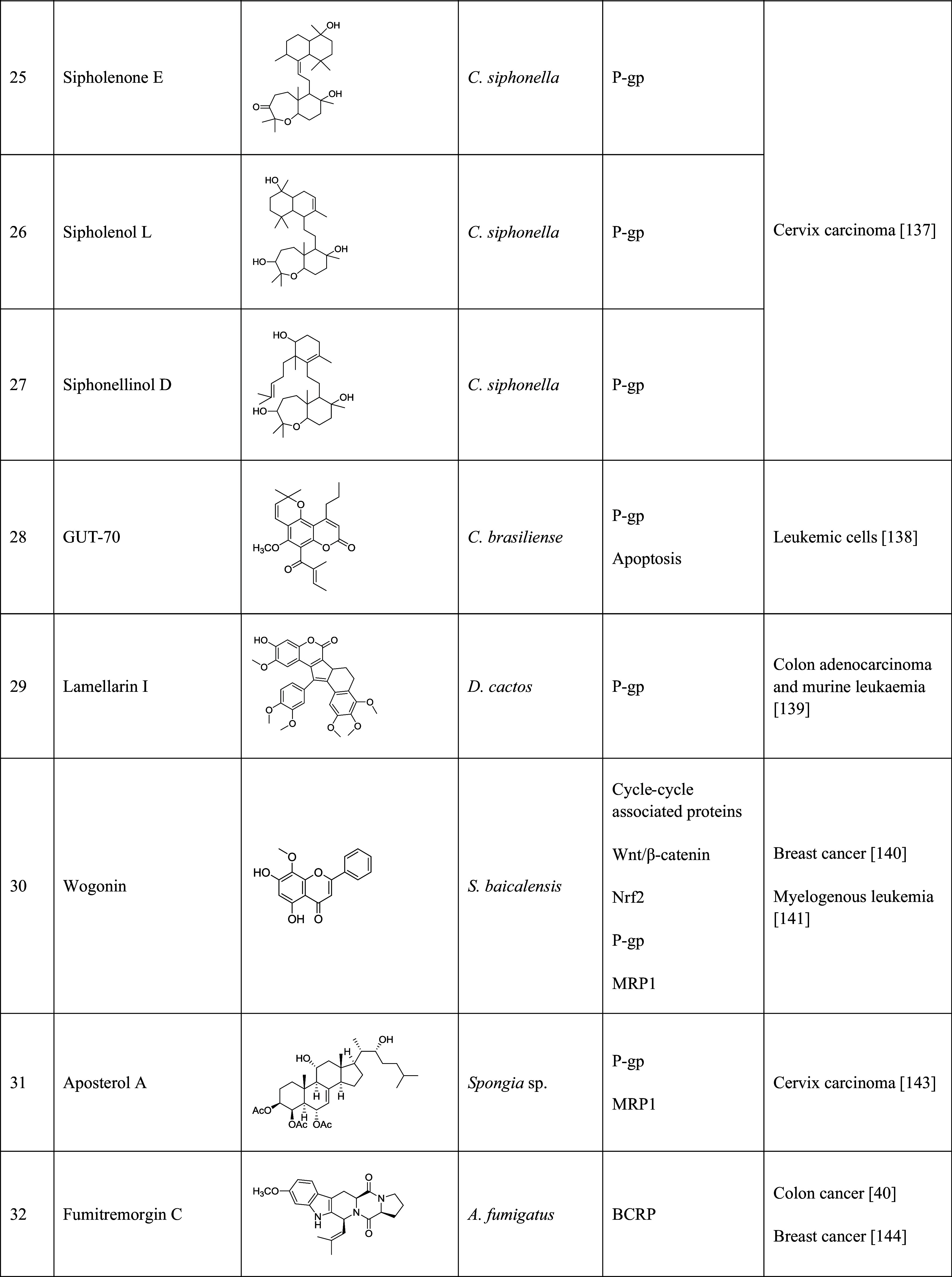
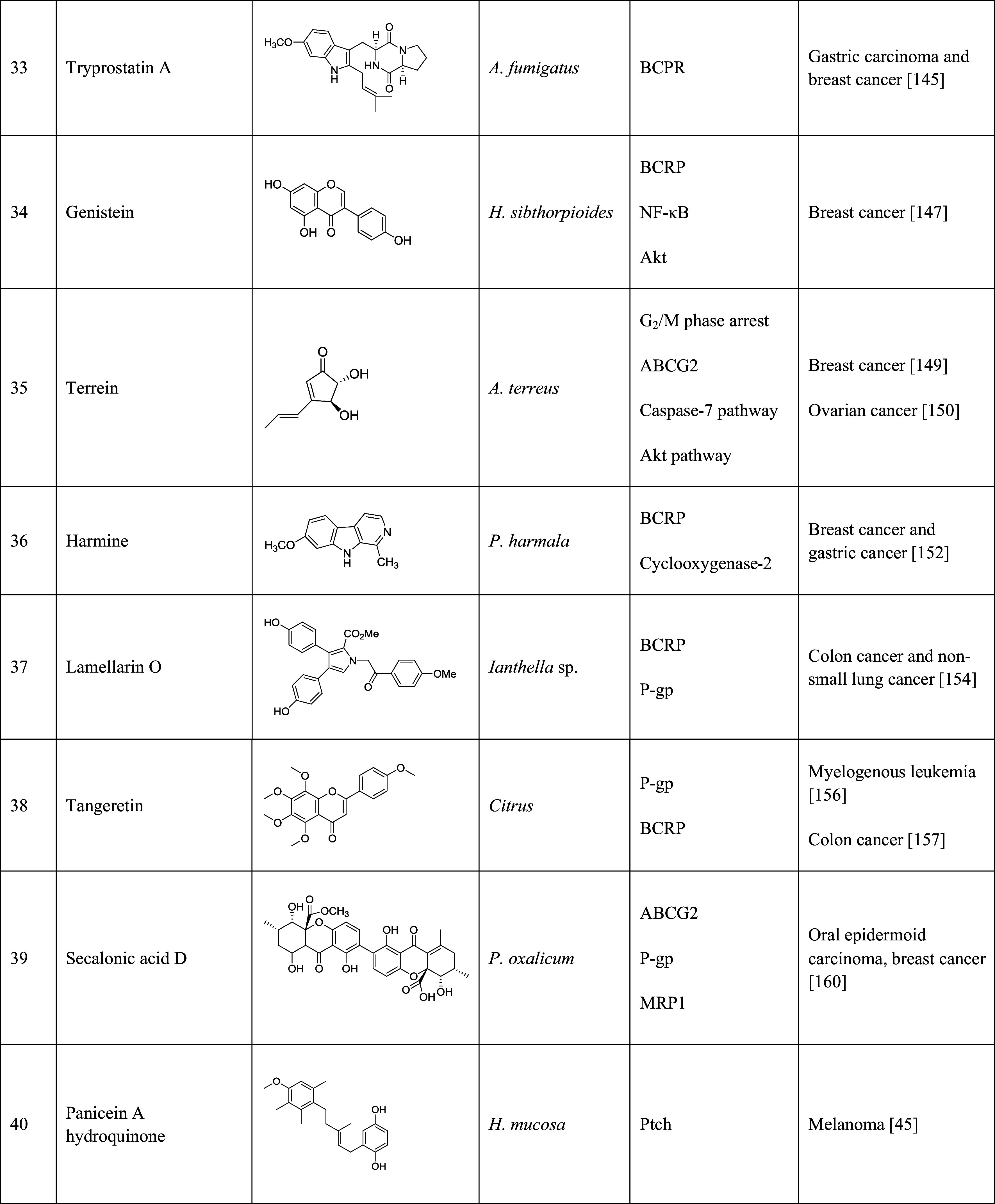
The table lists the compound, its structure, the natural source of the compound, the biological mechanism or signaling pathway targeted by the compound, and the types of cancers that have been targeted by the compound
Sesquiterpenes from the plant family Celastraceae have also been used for the treatment of MDR cells in cancers. Sesquiterpene ester 1 (Table 1, 9), celafolin A-1 (Table 1, 10), and celorbicol ester (Table 1, 11) all showed drug-resistance reversal activities with an IC50 value of 61.91, 14.00, and 14.36 µM against MCF7/ADR, respectively [117]. Munoz-Martinez et al. investigated the MDR reversal potency of 28 dihydro-β-agarofuran sesquiterpenes in human MDR1-transfected NIH-3T3 cells in vitro. The results suggested that the agarofuran sesquiterpenes reversed the MDR phenotype by interacting with the TM domain of P-gp and modulating P-gp ATPase activity [118].
Curcumin (diferuloylmethane, Table 1, 12) is a polyphenol natural product isolated from the rhizomes of Curcuma longa. The compound is known to regulate signaling pathways related to cell growth, differentiation, and apoptosis [119, 120], and has widely been used as antioxidant, anti-inflammatory, anti-cancer, and antimetastatic agent. It was reported that curcumin inhibited NF-κB signaling pathway activated by TNF, TPA, and hydrogen peroxide in human myelomonoblastic leukemia cell line ML-1a [121]. In a different study, treatment of human cervical carcinoma cells, KB-V1, with low concentrations (1, 5, and 10 µM) of curcumin reduced the expression of MDR1 gene. Curcumin also increased the accumulation of RH123 by inhibiting P-gp [122]. Demethoxycurcumin (Table 1, 13) and bisdemethoxycurcumin (Table 1, 14) from C. longa also modulated the MDR1 gene expression in human cervical carcinoma cells. Bisdemethoxycurcumin reduced P-gp expression by 88 % at 5 µM for three days [123].
Ecteinascidin 743 (Et-743, Table 1, 15), a marine natural product from Caribbean sea squirt Ecteinascidia turbinate, was used for the treatment of several cancers, including ovarian, breast, non-small lung, and renal cancers [124–126]. It has been shown that Et-734 can down-regulate P-gp expression at a concentration of 0.1 nM. In addition, Et-743 increased the cellular accumulation of DOX/VCR in P-gp-overexpressed cervix cells [127].
Polyphenolic catechins, such as (−)-epigallo catechin-3-gallate (EGCG, Table 1, 16), (−)-epigallocatechin (EGC, Table 1, 17), (−)-epicatechin-3-gallate (ECG, Table 1, 18), and (−)-epicatechin (EC, Table 1, 19), from tea, can be used for the treatment of cancers, including skin, lung, oral cavity, esophagus, breast, stomach, small intestine, colon, liver, pancreas, and mammary glands [128]. EGCG, one of the major water-soluble compounds in green tea (Camellia sinensis), has been shown to have the chemopreventive, anti-carcinogenic, anti-atherogenic, and antioxidant effects [129]. EGCG was used as the chemopreventive agent by inducing apoptosis and increasing cell growth arrest via regulating the expression of cell-cycle regulation proteins. The previous studies have shown that EGCG inhibited carcinogenesis by regulating a wide range of signaling pathways. It can activate NF-κB, and then induce the expression of more than 200 genes that inhibited apoptosis, and promoted cell proliferation, invasion, metastasis, and chemoresistance [130]. It was also reported that EGCG inhibited NF-κB signaling pathway by blocking TPA- and UV-induced phosphorylation of IκB [131, 132]. In a study, catechins, including EGC, ECG, and EGCG, enhanced the cellular accumulation of P-gp substrates, RH123, and daunorubicin, in P-gp overexpressed KB-C2 cells. Therefore, the catechins played an important role in reversing drug resistance by inhibiting the activity of P-gp.
Three macrocyclic jatrophane diterpenes and one tetracyclic triterpene were isolated from Euphorbia mellifera. Their ability to reverse drug resistance was evaluated through the RH123 accumulation test on human MDR1gene-transfected mouse lymphoma cells (L5178Y MDR) and multidrug-resistant human colon adenocarcinoma cells (COLO 320). Macrocyclic jatrophane diterpenes, euphomelliferine (Table 1, 20), and euphomelliferine A (Table 1, 21) exhibited high MDR reversal activity with the fluorescence activity ratio (FAR) values of 72.9 and 82.2 at 60 µM, respectively [133]. Two other compounds, euphodendroidin D (Table 1, 22) and pepluanin A (Table 1, 23), isolated from genus Euphorbia were also studies. At the concentration of 5 µM, euphodendroidin D inhibited daunomycin-efflux activity twofold (183 ± 17 %) more than cyclosporine A, a conventional modulator, by specifically modulating P-gp activity. Pepluanin A also showed higher inhibition (207 ± 17 %) [134].
Sipholenol A (Table 1, 24), one of the sipholane triterpenes isolated from the Red Sea sponge Callyspongia siphonella, reversed MDR in P-gp overexpressed cervix carcinoma cells. At the concentration of 2.5, 5, and 10 µM, sipolenol A potentiated the toxicity of P-gp substrates, colchicine, vinblastine, and paclitaxel in a dose-dependent manner [135]. Three other triterpenoids isolated from the same sponge, sipholenone E (Table 1, 25), sipholenol L (Table 1, 26), and siphonellinol D (Table 1, 27) also enhanced the inhibitory effect of colchicine, vinblastine, and paclitaxel, and reversed the MDR in P-gp-overexpressing MDR cancer cell line KB-C2 at 1, 3 and 10 µM [136].
A tricyclic coumarin, GUT-70 (Table 1, 28), derived from the stem bark of Brazilian plant Calophyllum brasiliense, exhibited the inhibitory effect on six human leukemic cell lines, including P-gp overexpressed cells in a concentration- and time-dependent manner. GUT-70 induced caspase-mediated and p53-independent apoptosis to overcome MDR [137]. Anti-cancer effects of several lamellarins isolated from genus Didemnum were also studied on P-gp-mediated MDR cancer cell lines. Lamellarin I (Table 1, 29) reversed MDR by inhibiting P-gp efflux. This compound completely reversed resistance of DOX, vinblastine, and daunorubicin at 2 µM in multidrug-resistant P388/Shabel cells [138].
P-gp and MRP1 inhibitors
Wogonin (Table 1, 30), a flavonoid isolated from Scutellaria baicalensis, reversed DOX resistance in MCF7/DOX cells by inhibiting nuclear factor erythroid 2-related factor 2 (Nrf2), which played a vital role in cell survival and MDR. Wogonin increased the sensitivity of MCF7/DOX cells to DOX by 1.24-, 1.93-, and 3.24-fold at 20, 40, and 60 µM, respectively [139]. A recent study implicated that wogonin can reverse MDR in human myelogenous leukemia K562/A02 cells. Wogonin promoted the sensitivity of K562/A02 cells to ADR by 1.22-, 2.31-, and 3.85-fold at the concentrations of 10, 20, and 40 µM, respectively. It also repressed the Nrf2 signaling pathway, resulting in the down-regulation of MRP1 expression [140]. In addition, wogonin showed P-gp inhibitory activity and suppressed excretion of calcein-AM, a substrate of P-gp, in Jurkat cells, and A549 cells at a concentration of 10 µM [141]. Aposterol A (Table 1, 31), a polyhydroxylated sterol acetate, was isolated from a marine sponge Spongia sp. [142]. Aposterol A increased the vincristine accumulation in both P-gp-mediated MDR cells (KB-C2) and MRP1-mediated MDR cells (KB-CV60) at 3 µM and showed reversal effect on P-gp and MRP1 [142].
BCRP inhibitors
Fumitremorgin C (FTC, Table 1, 32), a prenylated indole alkaloid isolated from Aspergillus fumigatus, was the first reported BCRP inhibitor. FTC significantly potentiated the sensitivity of S1-M1-3.2, a BCRP-overexpressed colon carcinoma cell line, to mitoxantrone (93-fold), DOX (26-fold), and topotecan (24-fold) at the concentration of 5 µM [40]. At the same concentration, FTC sensitized BCRP-overexpressed MCF7 cells to DOX (6.6-fold), mitoxantrone (29.4-fold), and topotecan (6.5-fold) [143]. However, FTC can cause severe side effects, such as neurotoxicity, which limited its clinical application. In recent years, FTC analogs have been developed and showed inhibitory activity against BCRP. One of the natural analogs, tryprostatin A (Table 1, 33), reversed mitoxantrone-resistant gastric carcinoma cell line EPG85-257RNOV, breast cancer cell line MCF7/AdrVp, and BCRP cDNA-transfected breast cancer cell line MCF7/BCRP at the concentrations of 10–50 µM [144]. An isoflavone, genistein (Table 1, 34) isolated from Hydrocotyle sibthorpioides [145], competitively inhibited BCRP-mediated drug efflux. Genistein potentiated cytotoxicity of SN-38 (7.23-fold) and mitoxantrone (6.28-fold) at the concentration of 3 µM [146]. The marine sponge-derived fungal metabolite, terrain (Table 1, 35), was isolated from Aspergillus terreus [147]. Terrein significantly decreased ABCG2-expressed MCF7 cells at the concentrations of 1 or 10 nM. It induced apoptosis by potentiating the caspase-7 signaling pathway and repressing the Akt signaling pathway. Terrein showed 100-fold more toxicity against MCF7 cells than paclitaxel. The IC50 values of terrain against breast cancer MCF7 cells, pancreatic cancer PANC-1 cells, and liver cancer HepG2 cells were 1.1 nM, 9.8 µM, and 66.8 nM, respectively [148]. A later study suggested that terrein can also be used in the treatment of ovarian cancer, since the compound induced G2/M phase cell cycle arrest in the ovarian CSCs [149]. The β-carboline alkaloid, harmine (Table 1, 36), was isolated from Peganum harmala [150], and was identified as a BCRP inhibitor in a BCRP-overexpressed breast cancer cell line MDA-MB-231. The anti-cancer activity of harmine was evaluated in gastric cancer and it induced apoptosis and inhibited cell proliferation, migration, and invasion [151]. Harmine showed some side effects, such as neurotoxicity and cytotoxicity; however, it could be used as a lead compound for the development of BCRP inhibitors [152].
P-gp and BCRP inhibitors
Lamellarin O (Table 1, 37), isolated from an Australian marine sponge Ianthella sp., showed growth inhibition against P-gp overexpressed colon cancer cell line SW620 Ad300 with an IC50 of 22.3 µM. Treatment with lamellarin O increased sensitivity towards DOX, a P-gp substrate by 4.8-fold. Calcein-AM accumulation- and cell flow cytometry-based assays indicated that lamellarin O acted as a potent and selective BCRP inhibitor in non-small lung cancer with an IC50 of 4.7 µM [153]. Tangeretin (Table 1, 38) is a natural Citrus flavonoid known with antiproliferative activity [154]. Tangeretin was previously identified as MDR reversal agent by inhibiting P-gp in K562/ADM human myelogenous leukemia with an EC25 of 12.84 µM [155]. Tangeretin induced apoptosis by caspase-3 activation, and reversed multidrug resistance in colon cancer by inhibiting P-gp [156]. A recent study suggested that tangeretin showed potent inhibitory effect on BCRP with an EC50 of 1.19 µM against human BCRP transfected MDCK-II cells. Tangeretin also significantly enhanced dasatinib intracellular accumulation (341 % in mean value) by inhibiting P-gp at 50 µM [157].
P-gp, MRP1, and BCRP inhibitors
Secalonic acid D (SAD, Table 1, 39) was isolated from Penicillium oxalicum [158]. It showed potent inhibitory effect against P-gp-, MRP1-, and BCRP-overexpressed MDR cells and their parental cells, with an IC50 value of 0.27, 1.20, 0.13, and 1.04 µM against ABCB1-overexpressed oral epidermoid carcinoma cell line KBv200, ABCB1-overexpressed breast cancer cell line MCF7/Adr, ABCC1-overexpressed epidermoid carcinoma cell line CA120, and ABCG2-overexpressed colon carcinoma cell line S1-M1-80, respectively. Particularly, SAD suppressed the expression of ABCG2 and shortened the half-life of ABCG2 protein via the activation of calpain 1. Therefore, SAD was implicated in the treatment of cancer as a MDR inhibitor [159].
Ptch inhibitors
Four natural products, panicein A hydroquinone, panicein B2, panicein B3, and panicein C, were isolated from Haliclona (Soestella) mucosa. These four compounds inhibited the growth of yeast-expressing Ptch in the presence of DOX with IC50 values of 1, 2, 0.8, and 4.9 µM, respectively. Treatment with Panicein A hydroquinone significantly increased cell death 5–8-fold after treatment by DOX in melanoma MEWO cells and caused a 2–3-fold increase in A375 cells. Further studies indicated that Panicein A hydroquinone inhibited 40 % of the DOX efflux activity of Ptch [45].
Natural products-targeting CSCs
The past few decades have seen many achievements in cancer prevention and therapy. Our better understanding of the CSCs and the mechanisms of chemoresistance have helped researchers to explore new strategies for cancer treatment. Many natural products have shown biological activities against CSCs by interacting with apoptotic genes, survival genes, and cell cycles.
Berberine from Berberis aristata or Coptis chinensis
Berberine (Table 2, 1) from the roots and bark of Berberis aristata or Coptis chinensis [160] has been shown to have antitumor activity. In an early study, berberine was shown to inhibit cell proliferation of human tumor U937 and murine melanoma B16 cell lines, inducing apoptosis in the U937 cells with an IC100 of 100 µg/mL and causing cytoplasmic membrane damage on the B16 cells with an IC100 of 1 µg/mL [161]. Recent studies on CSCs suggested that berberine was an effective inhibitor of CSCs; it suppressed cancer invasion and metastasis in A549 lung cancer with an IC50 of 56.15 µM, up-regulated epithelial phenotype marker E-cadherin, down-regulated the expression of mesenchymal phenotype marker Vimentin, and suppressed TGF-β1-induced epithelial-to-mesenchymal (EMT) cell transition [162]. Berberine decreased the proportion of pancreatic CSCs from 9.7 to 5.7 % at the concentration of 15 µM. Stem-cell-associated genes (SOX2, POU5F1, and NANOG) were down-regulated by the treatment of berberine [163]. In contrast, berberine increased the side-population fraction, referred to as CSCs, by 7.6 % in H460 lung cancer cells [164].
Table 2.
Natural products that have shown inhibition of cancer stem cells
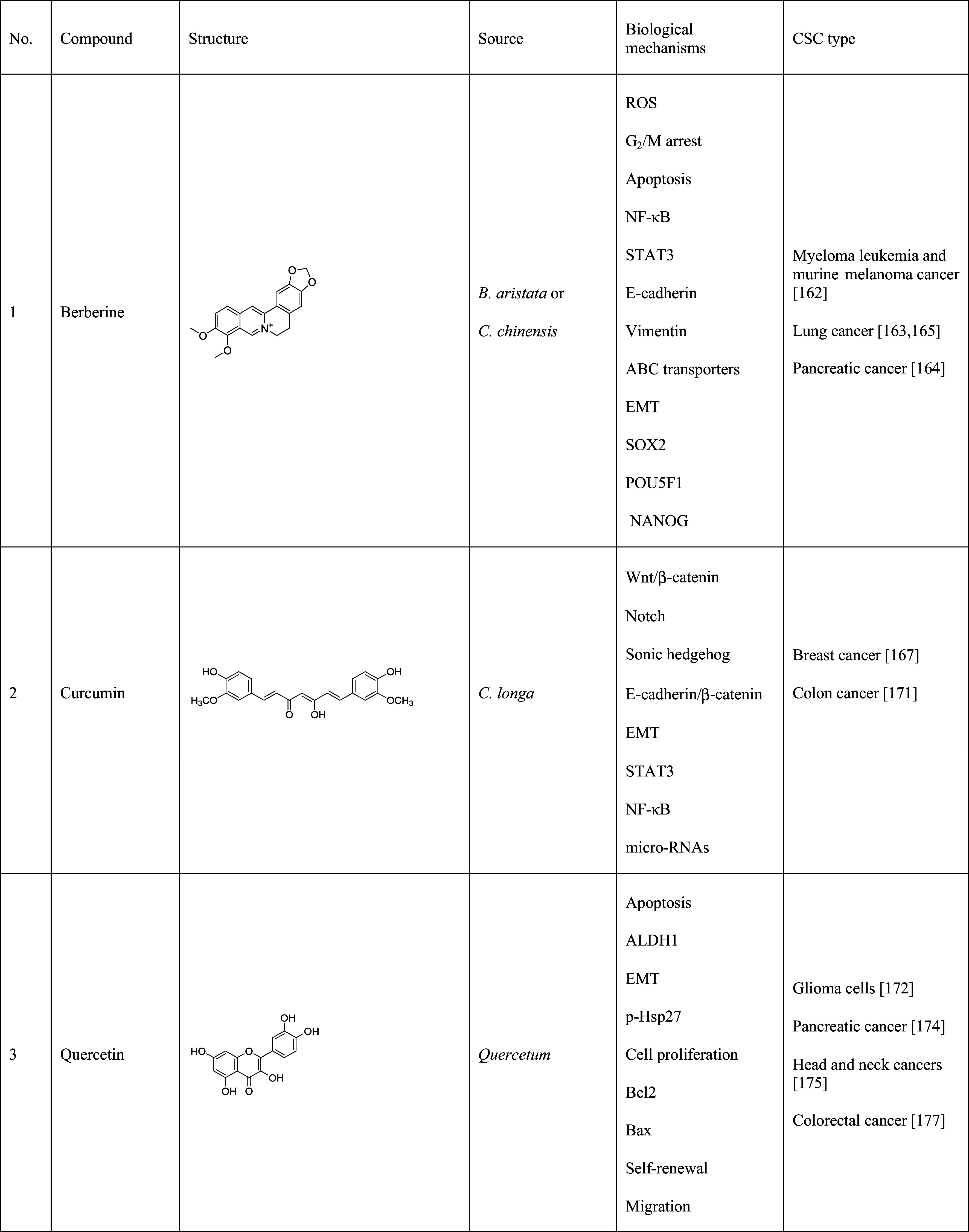
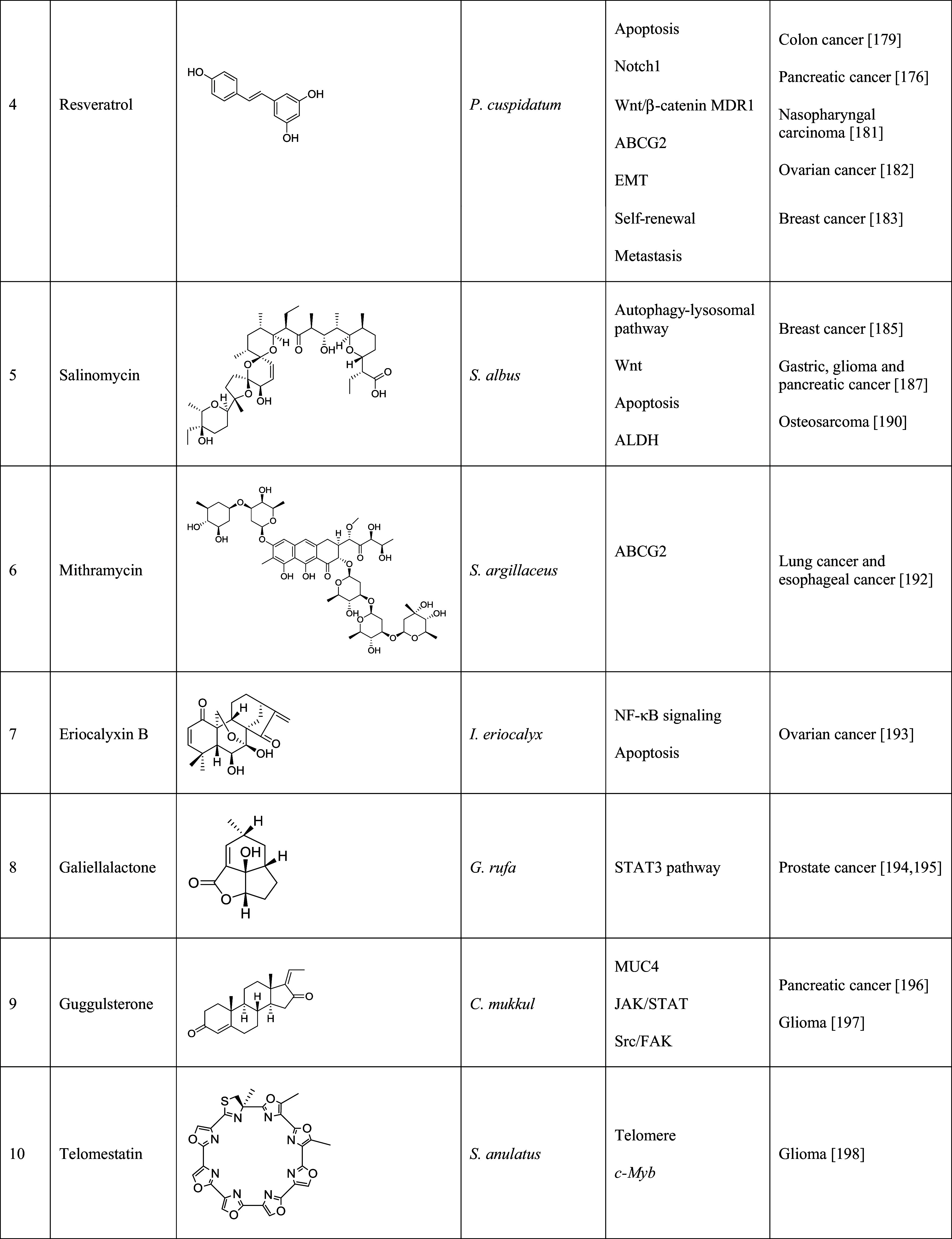
The table lists the compound, its structure, the natural source of the compound, the biological mechanism or signaling pathway targeted by the compound, and the types of cancer stem cells that have been targeted by the compound
Curcumin from Curcuma longa
More and more evidence suggested that curcumin (Table 2, 2) can target CSCs by regulating self-renewal pathways, such as Wnt/β-catenin, Notch and sonic hedgehog, and specific microRNAs [165]. Curcumin inhibited the mammosphere formation in breast cancer cells by 50 % at the concentration of 5 µM and reduced the proportion of ALDH1A1-expressing cells by 5.8 % at the concentration of 10 µM [166]. Another study suggested that curcumin dramatically decreased the percentage of CD44+/CD24− expressing cells after 6-h treatment at the concentration of 50 µM [167]. Studies of curcumin on breast CSCs suggested that curcumin caused the apoptotic effects on mammospheres of MCF7 and T47D cells at the concentration of 15 µM. Curcumin suppressed β-catenin nuclear translocation, subsequently increased E-cadherin/β-catenin complex formation, down-regulated EMT-promoting target gene expression, and, thus, inhibited the migration of breast CSCs [168]. The combinational application of curcumin and EGCG reduced CSC population in breast cancer by targeting STAT3 and NF-κB signaling pathways [169]. To improve the bioavailability of curcumin, analogs, and combinational therapy were developed. For example, difluoro-curcumin (CDF), in combination with 5-FU and oxaliplatin (Ox), inhibited cell growth, yet promoted apoptosis and disintegration of colon CSCs by down-regulating ABCG2 and attenuating EGFR, IGF-IR, and NF-κB signaling pathways [170].
Quercetin from Quercetum (oak forest)
Quercetin (Table 2, 3) was a ubiquitous flavonoid that was originally identified from Quercetum (forest oak). It was also isolated from a wide range of fruits and vegetables. It showed inhibitory effects on various cancers. For example, quercetin down-regulated the expression of survivin and anti-apoptotic proteins in human glioma cells, resulting in caspase-dependent apoptosis [171]. The mechanism of action study suggested that quercetin inhibited cell proliferation by regulating Bcl2 and Bax expression [172]. Quercetin also affected CSC properties. Using an in vitro and in vivo pancreatic CSC model, quercetin reduced the capacity of self-renewal, ALDH1 activity, and apoptosis resistance. Quercetin, together with sulforaphane, had synergistic effects on the prevention of EMT [173]. Quercetin was an effective inhibitor of CSCs in head and neck cancers with the concentrations ranging from 25 to 100 µM. Further studies suggested that quercetin suppressed ALDH1 activity, and reduced self-renewal and migration ability of CSCs [174]. Other studies on tongue cancer-derived stem cells showed that quercetin promoted cell apoptosis by inhibiting p-Hsp27 expression, which led to the reversal of drug resistance triggered by the activation of p38 MAPK signaling pathway [175]. Recent study on CD133+ cancer stem cells derived from human colorectal HT29 cancer cells suggested that quercetin increased cytotoxicity and apoptosis induction of DOX with an IC50 concentration of 75 µM. Quercetin, in combination with DOX, induced G2/M cell cycle arrest in HT29 cells [176].
Resveratrol from Polygonum cuspidatum
Resveratrol (Table 2, 4) is a polyphenol molecule present in various fruits and foods, such as grape skins. The compound can also be found in Polygonum cuspidatum and is used in oriental folk medicine [177]. Grape seed extract, in combination with resveratrol, potentiated the cell apoptosis and suppressed the proliferation of human colon CSCs via the activation of p53-dependent pathway [178]. Resveratrol also suppressed the self-renewal ability of pancreatic CSCs which derived from human tumor and Notch1, MDR1 and ABCG2 overexpressed KrasG12D mice [179]. In addition, resveratrol hampered stem-cell properties, including drug resistance, self-renewal ability, tumor initiation ability and metastasis potency, and EMT through the activation of p53 in nasopharyngeal carcinoma CSCs [180]. A recent study suggested that resveratrol, at 60 µM, also inhibited EMT, induced by CDDP treatment in ovarian cancer cell lines. It caused cell death in an apoptotic-independent manner [181]. Resveratrol also dramatically inhibited the proliferation of breast CSCs and induced the autophagy through the suppression of Wnt/β-catenin signaling pathway [182].
Salinomycin from Streptomyces albus
Salinomycin (Table 2, 5) was recently isolated from Streptomyces albus [183]. It selectively inhibited the cell growth of CD44+/CD24− breast CSCs by 20-fold relative to vehicle control. In addition, salinomycin reduced the tumor-seeding, tumor formation, and metastatic ability [184]. Salinomycin was found to suppress the autophagy-lysosomal pathway, which was crucial for the tumorigenicity of breast CSCs [185]. It was also proven that salinomycin was not only effective on breast CSCs, but also on gastric, pancreatic, and glioma CSCs in different ways [186]. In the gastric and pancreatic CSCs, salinomycin inhibited the proliferation of the CSC population by suppressing the Wnt signaling pathway, which was essential for the self-renewal capacity of gastric and pancreatic CSCs [187, 188]. Salinomycin killed glioma CSCs with an IC50 value of 0.06 µM [186]. In a recent study on osteosarcoma stem cells, salinomycin selectively killed tumor stem cells with an IC50 value of <5 µM. This inhibition effects also involved Wnt/β-catenin signaling pathway [189].
Other CSC inhibitors
Mithramycin (Table 2, 6), a major product from Streptocyces argillaceus, exhibited remarkable cytotoxicity against various cancers [190]. It inhibited stem-cell signaling in lung and esophageal cancer cells and suppressed ABCG2 expression [191]. An ent-daurene diterpenoid from Isodon eriocalyx var. Laxiflora, eriocalyxin B (EriB, Table 2, 7), was shown to target p50 by inducing apoptosis via NF-κB signaling inhibition. The compound was also an effective inhibitor of ovarian CSCs with a GI50 of 0.5–1 µM [192]. A secondary metabolite from fungus Galeilla rufa, galiellalactone (Table 2, 8), was a promising therapeutic agent for both prostate cancer cells and prostate CSCs by targeting the STAT3 pathway [193, 194]. Guggulsterone (GS, Table 2, 9), isolated from the plant Commiphora mukkul, showed anti-proliferation activity against various cancers. In a study on pancreatic cancer cells, GS inhibited cell growth and metastasis by down-regulating MUC4 expression involved in cancer cell fate, invasion, and drug resistance [195]. Meanwhile, GS, in the cooperation with SANT-1, a novel inhibitor on glioma cells, can target stem and non-stem glioma cells [196]. Telomestatin (Table 2, 10), a macrocyclic compound from Streptomyces anulatus 3533-SV4, exhibited strong inhibition against glioma CSCs via telomere disruption and c-Myb inhibition [197].
Prospects
Natural products derived from microbes, plants, and marine organisms have played a dominate role in cancer drug discovery. The membrane transporters, a major contributor to MDR in cancer, have been demonstrated as one of the most important targets in cancer therapy. A growing number of natural products have been discovered to reverse MDR in cancers; however, problems with these compounds still remain. In the reversal of MDR in cancer, the effects on the normal cells, especially the cells expressing ABC transporter family, should be considered. High cytotoxicity from these compounds also limits their potential application in clinical practice. For example, cyclosporine A (CsA), a well-known P-gp inhibitor, reduced the efflux of DOX in tumor cells. However, it cannot be administrated for long period, because of the side effects it caused, including immunosuppression and severe nephrotoxicity. Therefore, searching for the drugs with specific activity against MDR is still a major barrier to the success of chemotherapy. Hunting for new therapeutics from nature, which hosts a vast resource of natural products that target MDR cells, is a potential strategy for cancer treatments. In the future, to overcome the MDR in cancer, the targets can be concentrated on ABC transporters, as they are widely expressed in cancers, but this does not exclude other targets that may be expressed in specific cancer cell types.
The existence of CSCs is another prominent challenge for cancer therapy. More and more evidence suggests that CSCs play a critical role in the aetiology of metastasis, the main cause of the mortality of cancer patients. A better understanding of the origins of CSCs and their molecular mechanisms will make important contribution to the treatment of CSCs. Though many products from nature were discovered to eliminate CSCs, they still displayed high toxicity against normal cells. For example, salinomycin was effective on many cancers, but it displayed neurotoxic effects [198]. On the other hand, some of the drugs have physical and chemical property limitations, resulting in the failure of bioavailability. Curcumin had prominent anti-cancer properties, but was limited in clinical applications due to its insolubility in water and instability.
In this review, the cellular mechanisms of MDR and CSCs and the developed natural products against MDR cancer cells and CSCs were discussed with a view that we can obtain a better understanding of the mechanisms which will ultimately help in the development of new targets in the battle against cancer. To overcome these hurdles, a significant effort has to be devoted to the discovery of new agents that eliminate MDR cancer cells and CSCs.
References
- 1.Gilbertson RJ. Mapping cancer origins. Cell. 2011;145(1):25–29. doi: 10.1016/j.cell.2011.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.O’Connor R, Clynes M, Dowling P, O’Donovan N, O’Driscoll L. Drug resistance in cancer—searching for mechanisms, markers and therapeutic agents. Expert Opin Drug Metab Toxicol. 2007;3(6):805–817. doi: 10.1517/17425255.3.6.805. [DOI] [PubMed] [Google Scholar]
- 3.Gottesman MM, Fojo T, Bates SE. Multidrug resistance in cancer: role of ATP-dependent transporters. Nat Rev Cancer. 2002;2(1):48–58. doi: 10.1038/nrc706. [DOI] [PubMed] [Google Scholar]
- 4.Ramachandra M, Ambudkar SV, Chen D, Hrycyna CA, Dey S, Gottesman MM, Pastan I. Human P-glycoprotein exhibits reduced affinity for substrates during a catalytic transition state. Biochemistry. 1998;37(14):5010–5019. doi: 10.1021/bi973045u. [DOI] [PubMed] [Google Scholar]
- 5.Tsuruo T, Iida H, Tsukagoshi S, Sakurai Y. Overcoming of vincristine resistance in P388 leukemia in vivo and in vitro through enhanced cytotoxicity of vincristine and vinblastine by verapamil. Cancer Res. 1981;41(5):1967–1972. [PubMed] [Google Scholar]
- 6.Thimmaiah KN, Horton JK, Qian XD, Beck WT, Houghton JA, Houghton PJ. Structural determinants of phenoxazine type compounds required to modulate the accumulation of vinblastine and vincristine in multidrug-resistant cell lines. Cancer Commun. 1990;2(7):249–259. doi: 10.3727/095535490820874308. [DOI] [PubMed] [Google Scholar]
- 7.Belinsky MG, Chen ZS, Shchaveleva I, Zeng H, Kruh GD. Characterization of the drug resistance and transport properties of multidrug resistance protein 6 (MRP6, ABCC6) Cancer Res. 2002;62(21):6172–6177. [PubMed] [Google Scholar]
- 8.McCormack E, Bruserud O, Gjertsen BT. Animal models of acute myelogenous leukaemia—development, application and future perspectives. Leukemia. 2005;19(5):687–706. doi: 10.1038/sj.leu.2403670. [DOI] [PubMed] [Google Scholar]
- 9.Yu Z, Pestell TG, Lisanti MP, Pestell RG. Cancer stem cells. Int J Biochem Cell Biol. 2012;44(12):2144–2151. doi: 10.1016/j.biocel.2012.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vaiopoulos AG, Kostakis ID, Koutsilieris M, Papavassiliou AG. Colorectal cancer stem cells. Stem Cells. 2012;30(3):363–371. doi: 10.1002/stem.1031. [DOI] [PubMed] [Google Scholar]
- 11.Velasco-Velazquez MA, Homsi N, De La Fuente M, Pestell RG. Breast cancer stem cells. Int J Biochem Cell Biol. 2012;44(4):573–577. doi: 10.1016/j.biocel.2011.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tu SM, Lin SH. Prostate cancer stem cells. Clin Genitourin Cancer. 2012;10(2):69–76. doi: 10.1016/j.clgc.2012.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ricci-Vitiani L, Fabrizi E, Palio E, De Maria R. Colon cancer stem cells. J Mol Med. 2009;87(11):1097–1104. doi: 10.1007/s00109-009-0518-4. [DOI] [PubMed] [Google Scholar]
- 14.Piccirillo SG, Binda E, Fiocco R, Vescovi AL, Shah K. Brain cancer stem cells. J Mol Med. 2009;87(11):1087–1095. doi: 10.1007/s00109-009-0535-3. [DOI] [PubMed] [Google Scholar]
- 15.Singh SK, Hawkins C, Clarke ID, Squire JA, Bayani J, Hide T, Henkelman RM, Cusimano MD, Dirks PB. Identification of human brain tumour initiating cells. Nature. 2004;432(7015):396–401. doi: 10.1038/nature03128. [DOI] [PubMed] [Google Scholar]
- 16.O’Brien CA, Pollett A, Gallinger S, Dick JE. A human colon cancer cell capable of initiating tumour growth in immunodeficient mice. Nature. 2007;445(7123):106–110. doi: 10.1038/nature05372. [DOI] [PubMed] [Google Scholar]
- 17.Uchida N, Buck DW, He D, Reitsma MJ, Masek M, Phan TV, Tsukamoto AS, Gage FH, Weissman IL. Direct isolation of human central nervous system stem cells. Proc Natl Acad Sci USA. 2000;97(26):14720–14725. doi: 10.1073/pnas.97.26.14720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Oshima Y, Suzuki A, Kawashimo K, Ishikawa M, Ohkohchi N, Taniguchi H. Isolation of mouse pancreatic ductal progenitor cells expressing CD133 and c-Met by flow cytometric cell sorting. Gastroenterology. 2007;132(2):720–732. doi: 10.1053/j.gastro.2006.11.027. [DOI] [PubMed] [Google Scholar]
- 19.Dean M, Hamon Y, Chimini G. The human ATP-binding cassette (ABC) transporter superfamily. J Lipid Res. 2001;42(7):1007–1017. [PubMed] [Google Scholar]
- 20.Beaulieu E, Demeule M, Ghitescu L, Beliveau R. P-glycoprotein is strongly expressed in the luminal membranes of the endothelium of blood vessels in the brain. Biochem J. 1997;326(Pt 2):539–544. doi: 10.1042/bj3260539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ruetz S, Gros P. Phosphatidylcholine translocase: a physiological role for the mdr2 gene. Cell. 1994;77(7):1071–1081. doi: 10.1016/0092-8674(94)90446-4. [DOI] [PubMed] [Google Scholar]
- 22.Melaine N, Lienard MO, Dorval I, Le Goascogne C, Lejeune H, Jegou B. Multidrug resistance genes and p-glycoprotein in the testis of the rat, mouse, Guinea pig, and human. Biol Reprod. 2002;67(6):1699–1707. doi: 10.1095/biolreprod.102.003558. [DOI] [PubMed] [Google Scholar]
- 23.Yague E, Raguz S. Escape from stress granule sequestration: another way to drug resistance? Biochem Soc Trans. 2010;38(6):1537–1542. doi: 10.1042/BST0381537. [DOI] [PubMed] [Google Scholar]
- 24.Smith R, Rathod RJ, Rajkumar S, Kennedy D. Nervous translation, do you get the message? A review of mRNPs, mRNA-protein interactions and translational control within cells of the nervous system. Cell Mol Life Sci. 2014;71(20):3917–3937. doi: 10.1007/s00018-014-1660-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shtil AA, Azare J. Redundancy of biological regulation as the basis of emergence of multidrug resistance. Int Rev Cytol. 2005;246:1–29. doi: 10.1016/S0074-7696(05)46001-5. [DOI] [PubMed] [Google Scholar]
- 26.Sodani K, Patel A, Kathawala RJ, Chen ZS. Multidrug resistance associated proteins in multidrug resistance. Chin J Cancer. 2012;31(2):58–72. doi: 10.5732/cjc.011.10329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Scheffer GL, Pijnenborg AC, Smit EF, Muller M, Postma DS, Timens W, van der Valk P, de Vries EG, Scheper RJ. Multidrug resistance related molecules in human and murine lung. J Clin Pathol. 2002;55(5):332–339. doi: 10.1136/jcp.55.5.332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Peng KC, Cluzeaud F, Bens M, Duong Van Huyen JP, Wioland MA, Lacave R, Vandewalle A. Tissue and cell distribution of the multidrug resistance-associated protein (MRP) in mouse intestine and kidney. J Histochem Cytochem. 1999;47(6):757–768. doi: 10.1177/002215549904700605. [DOI] [PubMed] [Google Scholar]
- 29.St-Pierre MV, Serrano MA, Macias RI, Dubs U, Hoechli M, Lauper U, Meier PJ, Marin JJ. Expression of members of the multidrug resistance protein family in human term placenta. Am J Physiol Regul Integr Comp Physiol. 2000;279(4):R1495–R1503. doi: 10.1152/ajpregu.2000.279.4.R1495. [DOI] [PubMed] [Google Scholar]
- 30.Jorajuria S, Dereuddre-Bosquet N, Becher F, Martin S, Porcheray F, Garrigues A, Mabondzo A, Benech H, Grassi J, Orlowski S, Dormont D, Clayette P. ATP binding cassette multidrug transporters limit the anti-HIV activity of zidovudine and indinavir in infected human macrophages. Antivir Ther. 2004;9(4):519–528. [PubMed] [Google Scholar]
- 31.Gibson NM, Quinn CJ, Pfannenstiel KB, Hydock DS, Hayward R. Effects of age on multidrug resistance protein expression and doxorubicin accumulation in cardiac and skeletal muscle. Xenobiot Fate Foreign Compd Biol Syst. 2014;44(5):472–479. doi: 10.3109/00498254.2013.846489. [DOI] [PubMed] [Google Scholar]
- 32.Wijnholds J, Scheffer GL, van der Valk M, van der Valk P, Beijnen JH, Scheper RJ, Borst P. Multidrug resistance protein 1 protects the oropharyngeal mucosal layer and the testicular tubules against drug-induced damage. J Exp Med. 1998;188(5):797–808. doi: 10.1084/jem.188.5.797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Doyle LA, Yang W, Abruzzo LV, Krogmann T, Gao Y, Rishi AK, Ross DD. A multidrug resistance transporter from human MCF-7 breast cancer cells. Proc Natl Acad Sci USA. 1998;95(26):15665–15670. doi: 10.1073/pnas.95.26.15665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dean M, Rzhetsky A, Allikmets R. The human ATP-binding cassette (ABC) transporter superfamily. Genome Res. 2001;11(7):1156–1166. doi: 10.1101/gr.GR-1649R. [DOI] [PubMed] [Google Scholar]
- 35.Doyle L, Ross DD. Multidrug resistance mediated by the breast cancer resistance protein BCRP (ABCG2) Oncogene. 2003;22(47):7340–7358. doi: 10.1038/sj.onc.1206938. [DOI] [PubMed] [Google Scholar]
- 36.Summer R, Kotton DN, Sun X, Ma B, Fitzsimmons K, Fine A. Side population cells and Bcrp1 expression in lung. Am J Physiol Lung Cell Mol Physiol. 2003;285(1):L97–L104. doi: 10.1152/ajplung.00009.2003. [DOI] [PubMed] [Google Scholar]
- 37.Alvi AJ, Clayton H, Joshi C, Enver T, Ashworth A, Vivanco M, Dale TC, Smalley MJ. Functional and molecular characterisation of mammary side population cells. Breast Cancer Res. 2003;5(1):R1–R8. doi: 10.1186/bcr547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Robey RW, Polgar O, Deeken J, To KW, Bates SE. ABCG2: determining its relevance in clinical drug resistance. Cancer Metastas Rev. 2007;26(1):39–57. doi: 10.1007/s10555-007-9042-6. [DOI] [PubMed] [Google Scholar]
- 39.An G, Gallegos J, Morris ME. The bioflavonoid kaempferol is an Abcg2 substrate and inhibits Abcg2-mediated quercetin efflux. Drug Metab Dispos Biol Fate Chem. 2011;39(3):426–432. doi: 10.1124/dmd.110.035212. [DOI] [PubMed] [Google Scholar]
- 40.Rabindran SK, He H, Singh M, Brown E, Collins KI, Annable T, Greenberger LM. Reversal of a novel multidrug resistance mechanism in human colon carcinoma cells by fumitremorgin C. Cancer Res. 1998;58(24):5850–5858. [PubMed] [Google Scholar]
- 41.Allen JD, van Loevezijn A, Lakhai JM, van der Valk M, van Tellingen O, Reid G, Schellens JH, Koomen GJ, Schinkel AH. Potent and specific inhibition of the breast cancer resistance protein multidrug transporter in vitro and in mouse intestine by a novel analogue of fumitremorgin C. Mol Cancer Ther. 2002;1(6):417–425. [PubMed] [Google Scholar]
- 42.Nusslein-Volhard C, Wieschaus E. Mutations affecting segment number and polarity in Drosophila. Nature. 1980;287(5785):795–801. doi: 10.1038/287795a0. [DOI] [PubMed] [Google Scholar]
- 43.Echelard Y, Epstein DJ, St-Jacques B, Shen L, Mohler J, McMahon JA, McMahon AP. Sonic hedgehog, a member of a family of putative signaling molecules, is implicated in the regulation of CNS polarity. Cell. 1993;75(7):1417–1430. doi: 10.1016/0092-8674(93)90627-3. [DOI] [PubMed] [Google Scholar]
- 44.Kawamura S, Hervold K, Ramirez-Weber FA, Kornberg TB. Two patched protein subtypes and a conserved domain of group I proteins that regulates turnover. J Biol Chem. 2008;283(45):30964–30969. doi: 10.1074/jbc.M806242200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Fiorini L, Tribalat MA, Sauvard L, Cazareth J, Lalli E, Broutin I, Thomas OP, Mus-Veteau I. Natural paniceins from mediterranean sponge inhibit the multidrug resistance activity of Patched and increase chemotherapy efficiency on melanoma cells. Oncotarget. 2015;6(26):22282–22297. doi: 10.18632/oncotarget.4162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Scales SJ, de Sauvage FJ. Mechanisms of Hedgehog pathway activation in cancer and implications for therapy. Trends Pharmacol Sci. 2009;30(6):303–312. doi: 10.1016/j.tips.2009.03.007. [DOI] [PubMed] [Google Scholar]
- 47.Bidet M, Tomico A, Martin P, Guizouarn H, Mollat P, Mus-Veteau I. The hedgehog receptor patched functions in multidrug transport and chemotherapy resistance. Mol Cancer Res. 2012;10(11):1496–1508. doi: 10.1158/1541-7786.MCR-11-0578. [DOI] [PubMed] [Google Scholar]
- 48.Yu JS, Liu GT, Morris-Irvin D, Black KL. Glioblastoma cancer stem cells exhibit chemoresistance with overexpression of multidrug resistance gene BCRP-1. Neurosurgery. 2005;57(2):428. doi: 10.1093/neurosurgery/57.2.428. [DOI] [Google Scholar]
- 49.Chaudhary PM, Roninson IB. Expression and activity of P-glycoprotein, a multidrug efflux pump, in human hematopoietic stem cells. Cell. 1991;66(1):85–94. doi: 10.1016/0092-8674(91)90141-K. [DOI] [PubMed] [Google Scholar]
- 50.Venugopal A, Kwatra D, Stecklein S, Ramalingam S, Subramaniam D, Anant S (2012) RNA binding protein RBM3 promotes a cancer stem cell phenotype with multidrug resistance. FASEB J:26
- 51.Grimm M, Krimmel M, Polligkeit J, Alexander D, Munz A, Kluba S, Keutel C, Hoffmann J, Reinert S, Hoefert S. ABCB5 expression and cancer stem cell hypothesis in oral squamous cell carcinoma. Eur J Cancer. 2012;48(17):3186–3197. doi: 10.1016/j.ejca.2012.05.027. [DOI] [PubMed] [Google Scholar]
- 52.Fuchs D, Daniel V, Sadeghi M, Opelz G, Naujokat C. Salinomycin overcomes ABC transporter-mediated multidrug and apoptosis resistance in human leukemia stem cell-like KG-1a cells. Biochem Biophys Res Commun. 2010;394(4):1098–1104. doi: 10.1016/j.bbrc.2010.03.138. [DOI] [PubMed] [Google Scholar]
- 53.Xu K, Liang X, Cui D, Wu Y, Shi W, Liu J. miR-1915 inhibits Bcl-2 to modulate multidrug resistance by increasing drug-sensitivity in human colorectal carcinoma cells. Mol Carcinog. 2013;52(1):70–78. doi: 10.1002/mc.21832. [DOI] [PubMed] [Google Scholar]
- 54.Signore M, Ricci-Vitiani L, De Maria R. Targeting apoptosis pathways in cancer stem cells. Cancer Lett. 2013;332(2):374–382. doi: 10.1016/j.canlet.2011.01.013. [DOI] [PubMed] [Google Scholar]
- 55.Liu G, Yuan X, Zeng Z, Tunici P, Ng H, Abdulkadir IR, Lu L, Irvin D, Black KL, Yu JS. Analysis of gene expression and chemoresistance of CD133+ cancer stem cells in glioblastoma. Mol Cancer. 2006;5:67. doi: 10.1186/1476-4598-5-67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Aggarwal BB. Nuclear factor-κB: the enemy within. Cancer Cell. 2004;6(3):203–208. doi: 10.1016/j.ccr.2004.09.003. [DOI] [PubMed] [Google Scholar]
- 57.Aggarwal BB, Vijayalekshmi RV, Sung B. Targeting inflammatory pathways for prevention and therapy of cancer: short-term friend, long-term foe. Clin Cancer Res. 2009;15(2):425–430. doi: 10.1158/1078-0432.CCR-08-0149. [DOI] [PubMed] [Google Scholar]
- 58.Griffin JD. Leukemia stem cells and constitutive activation of NF-kappaB. Blood. 2001;98(8):2291. doi: 10.1182/blood.V98.8.2291a. [DOI] [PubMed] [Google Scholar]
- 59.Baron F, Turhan AG, Giron-Michel J, Azzarone B, Bentires-Alj M, Bours V, Bourhis JH, Chouaib S, Caignard A. Leukemic target susceptibility to natural killer cytotoxicity: relationship with BCR-ABL expression. Blood. 2002;99(6):2107–2113. doi: 10.1182/blood.V99.6.2107. [DOI] [PubMed] [Google Scholar]
- 60.Palayoor ST, Youmell MY, Calderwood SK, Coleman CN, Price BD. Constitutive activation of IkappaB kinase alpha and NF-kappaB in prostate cancer cells is inhibited by ibuprofen. Oncogene. 1999;18(51):7389–7394. doi: 10.1038/sj.onc.1203160. [DOI] [PubMed] [Google Scholar]
- 61.Nakshatri H, Bhat-Nakshatri P, Martin DA, Goulet RJ, Jr, Sledge GW., Jr Constitutive activation of NF-kappaB during progression of breast cancer to hormone-independent growth. Mol Cell Biol. 1997;17(7):3629–3639. doi: 10.1128/MCB.17.7.3629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Aggarwal BB, Sung B. NF-kappaB in cancer: a matter of life and death. Cancer discovery. 2011;1(6):469–471. doi: 10.1158/2159-8290.CD-11-0260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Zhou J, Zhang H, Gu P, Bai J, Margolick JB, Zhang Y. NF-kappaB pathway inhibitors preferentially inhibit breast cancer stem-like cells. Breast Cancer Res Treat. 2008;111(3):419–427. doi: 10.1007/s10549-007-9798-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Stahl M, Ge C, Shi S, Pestell RG, Stanley P. Notch1-induced transformation of RKE-1 cells requires up-regulation of cyclin D1. Cancer Res. 2006;66(15):7562–7570. doi: 10.1158/0008-5472.CAN-06-0974. [DOI] [PubMed] [Google Scholar]
- 65.Osipo C, Patel P, Rizzo P, Clementz AG, Hao L, Golde TE, Miele L. ErbB-2 inhibition activates Notch-1 and sensitizes breast cancer cells to a gamma-secretase inhibitor. Oncogene. 2008;27(37):5019–5032. doi: 10.1038/onc.2008.149. [DOI] [PubMed] [Google Scholar]
- 66.Malanchi I, Peinado H, Kassen D, Hussenet T, Metzger D, Chambon P, Huber M, Hohl D, Cano A, Birchmeier W, Huelsken J. Cutaneous cancer stem cell maintenance is dependent on beta-catenin signalling. Nature. 2008;452(7187):650–653. doi: 10.1038/nature06835. [DOI] [PubMed] [Google Scholar]
- 67.Vermeulen L, De Sousa EMF, van der Heijden M, Cameron K, de Jong JH, Borovski T, Tuynman JB, Todaro M, Merz C, Rodermond H, Sprick MR, Kemper K, Richel DJ, Stassi G, Medema JP. Wnt activity defines colon cancer stem cells and is regulated by the microenvironment. Nat Cell Biol. 2010;12(5):468–476. doi: 10.1038/ncb2048. [DOI] [PubMed] [Google Scholar]
- 68.Reya T, Duncan AW, Ailles L, Domen J, Scherer DC, Willert K, Hintz L, Nusse R, Weissman IL. A role for Wnt signalling in self-renewal of haematopoietic stem cells. Nature. 2003;423(6938):409–414. doi: 10.1038/nature01593. [DOI] [PubMed] [Google Scholar]
- 69.Wend P, Holland JD, Ziebold U, Birchmeier W. Wnt signaling in stem and cancer stem cells. Semin Cell Dev Biol. 2010;21(8):855–863. doi: 10.1016/j.semcdb.2010.09.004. [DOI] [PubMed] [Google Scholar]
- 70.Clement V, Sanchez P, de Tribolet N, Radovanovic I, Ruiz i Altaba A. HEDGEHOG-GLI1 signaling regulates human glioma growth, cancer stem cell self-renewal, and tumorigenicity. Curr Biol. 2007;17(2):165–172. doi: 10.1016/j.cub.2006.11.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Bar EE, Chaudhry A, Lin A, Fan X, Schreck K, Matsui W, Piccirillo S, Vescovi AL, DiMeco F, Olivi A, Eberhart CG. Cyclopamine-mediated hedgehog pathway inhibition depletes stem-like cancer cells in glioblastoma. Stem Cells. 2007;25(10):2524–2533. doi: 10.1634/stemcells.2007-0166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Zahreddine HA, Culjkovic-Kraljacic B, Assouline S, Gendron P, Romeo AA, Morris SJ, Cormack G, Jaquith JB, Cerchietti L, Cocolakis E, Amri A, Bergeron J, Leber B, Becker MW, Pei S, Jordan CT, Wilson HM, Jr, Katherine LBB. The sonic hedgehog factor GLI1 imparts drug resistance through inducible glucuronidation. Nature. 2014;511(7507):90. doi: 10.1038/nature13283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Sims-Mourtada J, Izzo JG, Ajani J, Chao KSC. Sonic Hedgehog promotes multiple drug resistance by regulation of drug transport. Oncogene. 2007;26(38):5674–5679. doi: 10.1038/sj.onc.1210356. [DOI] [PubMed] [Google Scholar]
- 74.Linn DE, Yang X, Sun F, Xie Y, Chen H, Jiang R, Chen H, Chumsri S, Burger AM, Qiu Y. A role for OCT4 in tumor initiation of drug-resistant prostate cancer cells. Genes Cancer. 2010;1(9):908–916. doi: 10.1177/1947601910388271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Wang XQ, Ongkeko WM, Chen L, Yang ZF, Lu P, Chen KK, Lopez JP, Poon RTP, Fan ST. Octamer 4 (Oct4) mediates chemotherapeutic drug resistance in liver cancer cells through a potential Oct4–AKT–ATP-binding cassette G2 pathway. Hepatology. 2010;52(2):528–539. doi: 10.1002/hep.23692. [DOI] [PubMed] [Google Scholar]
- 76.Landen CN, Jr, Goodman B, Katre AA, Steg AD, Nick AM, Stone RL, Miller LD, Mejia PV, Jennings NB, Gershenson DM, Bast RC, Jr, Coleman RL, Lopez-Berestein G, Sood AK. Targeting aldehyde dehydrogenase cancer stem cells in ovarian cancer. Mol Cancer Ther. 2010;9(12):3186–3199. doi: 10.1158/1535-7163.MCT-10-0563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Lingala S, Cui Y-Y, Chen X, Ruebner BH, Qian X-F, Zern MA, Wu J. Immunohistochemical staining of cancer stem cell markers in hepatocellular carcinoma. Exp Mol Pathol. 2010;89(1):27–35. doi: 10.1016/j.yexmp.2010.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Clay MR, Tabor M, Owen JH, Carey TE, Bradford CR, Wolf GT, Wicha MS, Prince ME. Single-marker identification of head and neck squamous cell carcinoma cancer stem cells with aldehyde dehydrogenase. Head Neck. 2010;32(9):1195–1201. doi: 10.1002/hed.21315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Tanei T, Morimoto K, Shimazu K, Kim SJ, Tanji Y, Taguchi T, Tamaki Y, Noguchi S. Association of breast cancer stem cells identified by aldehyde dehydrogenase 1 expression with resistance to sequential paclitaxel and epirubicin-based chemotherapy for breast cancers. Clin Cancer Res. 2009;15(12):4234–4241. doi: 10.1158/1078-0432.CCR-08-1479. [DOI] [PubMed] [Google Scholar]
- 80.Lugli A, Iezzi G, Hostettler I, Muraro MG, Mele V, Tornillo L, Carafa V, Spagnoli G, Terracciano L, Zlobec I. Prognostic impact of the expression of putative cancer stem cell markers CD133, CD166, CD44s, EpCAM, and ALDH1 in colorectal cancer. Br J Cancer. 2010;103(3):382–390. doi: 10.1038/sj.bjc.6605762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Su Y, Qiu Q, Zhang X, Jiang Z, Leng Q, Liu Z, Stass SA, Jiang F. Aldehyde dehydrogenase 1 A1-positive cell population is enriched in tumor-initiating cells and associated with progression of bladder cancer. Cancer Epidemiol Biomark Prev. 2010;19(2):327–337. doi: 10.1158/1055-9965.EPI-09-0865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Li ZJ, Xiang Y, Xiang LX, Xiao YN, Li FJ, Hao P. ALDH maintains the stemness of lung adenoma stem cells by suppressing the Notch/CDK2/CCNE pathway. PLoS One. 2014;9(3):e92669. doi: 10.1371/journal.pone.0092669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Kim R-J, Park J-R, Roh K-J, Choi AR, Kim S-R, Kim P-H, Yu JH, Lee JW, Ahn S-H, Gong G, Hwang J-W, Kang K-S, Kong G, Sheen YY, Nam J-S. High aldehyde dehydrogenase activity enhances stem cell features in breast cancer cells by activating hypoxia-inducible factor-2α. Cancer Lett. 2013;333(1):18–31. doi: 10.1016/j.canlet.2012.11.026. [DOI] [PubMed] [Google Scholar]
- 84.Vasiliou V, Nebert DW. Analysis and update of the human aldehyde dehydrogenase (ALDH) gene family. Hum Genom. 2005;2(2):138–143. doi: 10.1186/1479-7364-2-2-138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Cortes-Dericks L, Froment L, Boesch R, Schmid RA, Karoubi G. Cisplatin-resistant cells in malignant pleural mesothelioma cell lines show ALDHhighCD44+ phenotype and sphere-forming capacity. BMC Cancer. 2014;14(1):304. doi: 10.1186/1471-2407-14-304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Liu J, Xiao ZJ, Wong SKM, Tin VPC, Ho KY, Wang JW, Sham MH, Wong MP. Lung cancer tumorigenicity and drug resistance are enhanced through ALDH(hi)CD44(hi) tumor initiating cells. Oncotarget. 2013;4(10):1686–1699. doi: 10.18632/oncotarget.1265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Croker AK, Allan AL. Inhibition of aldehyde dehydrogenase (ALDH) activity reduces chemotherapy and radiation resistance of stem-like ALDHhiCD44+ human breast cancer cells. Breast Cancer Res Treat. 2012;133(1):75–87. doi: 10.1007/s10549-011-1692-y. [DOI] [PubMed] [Google Scholar]
- 88.Liu P, Brown S, Goktug T, Channathodiyil P, Kannappan V, Hugnot JP, Guichet PO, Bian X, Armesilla AL, Darling JL, Wang W. Cytotoxic effect of disulfiram/copper on human glioblastoma cell lines and ALDH-positive cancer-stem-like cells. Br J Cancer. 2012;107(9):1488–1497. doi: 10.1038/bjc.2012.442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Zhi QM, Chen XH, Ji J, Zhang JN, Li JF, Cai Q, Liu BY, Gu QL, Zhu ZG, Yu YY. Salinomycin can effectively kill ALDHhigh stem-like cells on gastric cancer. Biomed Pharmacother. 2011;65(7):509–515. doi: 10.1016/j.biopha.2011.06.006. [DOI] [PubMed] [Google Scholar]
- 90.Maugeri-Saccà M, Bartucci M, De Maria R. DNA damage repair pathways in cancer stem cells. Mol Cancer Ther. 2012;11(8):1627–1636. doi: 10.1158/1535-7163.MCT-11-1040. [DOI] [PubMed] [Google Scholar]
- 91.Burke BA, Carroll M. BCR-ABL: a multi-faceted promoter of DNA mutation in chronic myelogeneous leukemia. Leukemia. 2010;24(6):1105–1112. doi: 10.1038/leu.2010.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Zhang M, Behbod F, Atkinson RL, Landis MD, Kittrell F, Edwards D, Medina D, Tsimelzon A, Hilsenbeck S, Green JE, Michalowska AM, Rosen JM. Identification of tumor-initiating cells in a p53-null mouse model of breast cancer. Cancer Res. 2008;68(12):4674–4682. doi: 10.1158/0008-5472.CAN-07-6353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Bao S, Dewhirst MW, Hjelmeland AB, Bigner DD, Wu Q, Hao Y, Rich JN, McLendon RE, Shi Q. Glioma stem cells promote radioresistance by preferential activation of the DNA damage response. Nature. 2006;444(7120):756–760. doi: 10.1038/nature05236. [DOI] [PubMed] [Google Scholar]
- 94.Yan J, Tang DM. Prostate cancer stem-like cells proliferate slowly and resist etoposide-induced cytotoxicity via enhancing DNA damage response. Exp Cell Res. 2014;328(1):132–142. doi: 10.1016/j.yexcr.2014.08.016. [DOI] [PubMed] [Google Scholar]
- 95.Saito Y, Najima Y, Takagi S, Uchida N, Wake A, Taniguchi S, Sone A, Ishikawa F, Tanaka S, Tomizawa-Murasawa M, Aoki Y, Suzuki N, Shultz LD. Induction of cell cycle entry eliminates human leukemia stem cells in a mouse model of AML. Nat Biotechnol. 2010;28(3):275–280. doi: 10.1038/nbt.1607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Chen Y, Li D, Wang D, Liu X, Yin N, Song Y, Lu SH, Ju Z, Zhan Q. Quiescence and attenuated DNA damage response promote survival of esophageal cancer stem cells. J Cell Biochem. 2012;113(12):3643–3652. doi: 10.1002/jcb.24228. [DOI] [PubMed] [Google Scholar]
- 97.Chan JY, Chu AC, Fung KP. Inhibition of P-glycoprotein expression and reversal of drug resistance of human hepatoma HepG2 cells by multidrug resistance gene (mdr1) antisense RNA. Life Sci. 2000;67(17):2117–2124. doi: 10.1016/S0024-3205(00)00798-0. [DOI] [PubMed] [Google Scholar]
- 98.Pan L, Liu J, He Q, Wang L, Shi J. Overcoming multidrug resistance of cancer cells by direct intranuclear drug delivery using TAT-conjugated mesoporous silica nanoparticles. Biomaterials. 2013;34(11):2719–2730. doi: 10.1016/j.biomaterials.2012.12.040. [DOI] [PubMed] [Google Scholar]
- 99.Ford JM. Modulators of multidrug resistance. Preclinical studies. Hematol Oncol Clin North Am. 1995;9(2):337–361. [PubMed] [Google Scholar]
- 100.Cornwell MM, Safa AR, Felsted RL, Gottesman MM, Pastan I. Membrane vesicles from multidrug-resistant human cancer cells contain a specific 150- to 170-kDa protein detected by photoaffinity labeling. Proc Natl Acad Sci USA. 1986;83(11):3847–3850. doi: 10.1073/pnas.83.11.3847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Gottesman MM, Pastan I. Biochemistry of multidrug resistance mediated by the multidrug transporter. Annu Rev Biochem. 1993;62:385–427. doi: 10.1146/annurev.bi.62.070193.002125. [DOI] [PubMed] [Google Scholar]
- 102.Palmeira A, Rodrigues F, Sousa E, Pinto M, Vasconcelos MH, Fernandes MX. New uses for old drugs: pharmacophore-based screening for the discovery of P-glycoprotein inhibitors. Chem Biol Drug Des. 2011;78(1):57–72. doi: 10.1111/j.1747-0285.2011.01089.x. [DOI] [PubMed] [Google Scholar]
- 103.Chen J, Li Z, Chen AY, Ye X, Luo H, Rankin GO, Chen YC. Inhibitory effect of baicalin and baicalein on ovarian cancer cells. Int J Mol Sci. 2013;14(3):6012–6025. doi: 10.3390/ijms14036012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Zheng YH, Yin LH, Grahn TH, Ye AF, Zhao YR, Zhang QY. Anticancer effects of baicalein on hepatocellular carcinoma cells. Phytother Res. 2014;28(9):1342–1348. doi: 10.1002/ptr.5135. [DOI] [PubMed] [Google Scholar]
- 105.Wang Y, Wang Q, Zhang S, Zhang Y, Tao L. Baicalein increases the cytotoxicity of cisplatin by enhancing gap junction intercellular communication. Mol Med Rep. 2014;10(1):515–521. doi: 10.3892/mmr.2014.2157. [DOI] [PubMed] [Google Scholar]
- 106.Chen F, Zhuang M, Zhong C, Peng J, Wang X, Li J, Chen Z, Huang Y. Baicalein reverses hypoxia-induced 5-FU resistance in gastric cancer AGS cells through suppression of glycolysis and the PTEN/Akt/HIF-1alpha signaling pathway. Oncol Rep. 2015;33(1):457–463. doi: 10.3892/or.2014.3550. [DOI] [PubMed] [Google Scholar]
- 107.Cho YA, Choi JS, Burm JP. Effects of the antioxidant baicalein on the pharmacokinetics of nimodipine in rats: a possible role of P-glycoprotein and CYP3A4 inhibition by baicalein. Pharmacol Rep. 2011;63(4):1066–1073. doi: 10.1016/S1734-1140(11)70624-7. [DOI] [PubMed] [Google Scholar]
- 108.Sun L, Peng Q, Qu L, Gong L, Si J. Anticancer agent icaritin induces apoptosis through caspase-dependent pathways in human hepatocellular carcinoma cells. Mol Med Rep. 2015;11(4):3094–3100. doi: 10.3892/mmr.2014.3007. [DOI] [PubMed] [Google Scholar]
- 109.Li S, Priceman SJ, Xin H, Zhang W, Deng J, Liu Y, Huang J, Zhu W, Chen M, Hu W, Deng X, Zhang J, Yu H, He G. Icaritin inhibits JAK/STAT3 signaling and growth of renal cell carcinoma. PLoS One. 2013;8(12):e81657. doi: 10.1371/journal.pone.0081657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Sun L, Chen W, Qu L, Wu J, Si J. Icaritin reverses multidrug resistance of HepG2/ADR human hepatoma cells via downregulation of MDR1 and Pglycoprotein expression. Mol Med Rep. 2013;8(6):1883–1887. doi: 10.3892/mmr.2013.1742. [DOI] [PubMed] [Google Scholar]
- 111.Lee KS, Lee HJ, Ahn KS, Kim SH, Nam D, Kim DK, Choi DY, Ahn KS, Lu J, Kim SH. Cyclooxygenase-2/prostaglandin E2 pathway mediates icariside II induced apoptosis in human PC-3 prostate cancer cells. Cancer Lett. 2009;280(1):93–100. doi: 10.1016/j.canlet.2009.02.024. [DOI] [PubMed] [Google Scholar]
- 112.Sze SC, Tong Y, Ng TB, Cheng CL, Cheung HP. Herba Epimedii: anti-oxidative properties and its medical implications. Molecules. 2010;15(11):7861–7870. doi: 10.3390/molecules15117861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Liu DF, Li YP, Ou TM, Huang SL, Gu LQ, Huang M, Huang ZS. Synthesis and antimultidrug resistance evaluation of icariin and its derivatives. Bioorg Med Chem Lett. 2009;19(15):4237–4240. doi: 10.1016/j.bmcl.2009.05.103. [DOI] [PubMed] [Google Scholar]
- 114.Zhang Y, Wang QS, Cui YL, Meng FC, Lin KM. Changes in the intestinal absorption mechanism of icariin in the nanocavities of cyclodextrins. Int J Nanomed. 2012;7:4239–4249. doi: 10.2147/IJN.S33014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Conseil G, Baubichon-Cortay H, Dayan G, Jault JM, Barron D, Di Pietro A. Flavonoids: a class of modulators with bifunctional interactions at vicinal ATP- and steroid-binding sites on mouse P-glycoprotein. Proc Natl Acad Sci USA. 1998;95(17):9831–9836. doi: 10.1073/pnas.95.17.9831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Zhang S, Morris ME. Effects of the flavonoids biochanin A, morin, phloretin, and silymarin on P-glycoprotein-mediated transport. J Pharmacol Exp Therap. 2003;304(3):1258–1267. doi: 10.1124/jpet.102.044412. [DOI] [PubMed] [Google Scholar]
- 117.Kim SE, Kim YH, Lee JJ, Kim YC. A new sesquiterpene ester from Celastrus orbiculatus reversing multidrug resistance in cancer cells. J Nat Prod. 1998;61(1):108–111. doi: 10.1021/np9702392. [DOI] [PubMed] [Google Scholar]
- 118.Munoz-Martinez F, Lu P, Cortes-Selva F, Perez-Victoria JM, Jimenez IA, Ravelo AG, Sharom FJ, Gamarro F, Castanys S. Celastraceae sesquiterpenes as a new class of modulators that bind specifically to human P-glycoprotein and reverse cellular multidrug resistance. Cancer Res. 2004;64(19):7130–7138. doi: 10.1158/0008-5472.CAN-04-1005. [DOI] [PubMed] [Google Scholar]
- 119.Aggarwal BB, Kumar A, Bharti AC. Anticancer potential of curcumin: preclinical and clinical studies. Anticancer Res. 2003;23(1A):363–398. [PubMed] [Google Scholar]
- 120.Han SS, Chung ST, Robertson DA, Ranjan D, Bondada S. Curcumin causes the growth arrest and apoptosis of B cell lymphoma by downregulation of egr-1, c-myc, bcl-XL, NF-kappa B, and p53. Clin Immunol. 1999;93(2):152–161. doi: 10.1006/clim.1999.4769. [DOI] [PubMed] [Google Scholar]
- 121.Singh S, Aggarwal BB. Activation of transcription factor NF-kappa B is suppressed by curcumin (diferuloylmethane) [corrected] J Biol Chem. 1995;270(42):24995–25000. doi: 10.1074/jbc.270.42.24995. [DOI] [PubMed] [Google Scholar]
- 122.Anuchapreeda S, Leechanachai P, Smith MM, Ambudkar SV, Limtrakul PN. Modulation of P-glycoprotein expression and function by curcumin in multidrug-resistant human KB cells. Biochem Pharmacol. 2002;64(4):573–582. doi: 10.1016/S0006-2952(02)01224-8. [DOI] [PubMed] [Google Scholar]
- 123.Limtrakul P, Anuchapreeda S, Buddhasukh D. Modulation of human multidrug-resistance MDR-1 gene by natural curcuminoids. BMC Cancer. 2004;4:13. doi: 10.1186/1471-2407-4-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Izbicka E, Lawrence R, Raymond E, Eckhardt G, Faircloth G, Jimeno J, Clark G, Von Hoff DD. In vitro antitumor activity of the novel marine agent, ecteinascidin-743 (ET-743, NSC-648766) against human tumors explanted from patients. Ann Oncol. 1998;9(9):981–987. doi: 10.1023/A:1008224322396. [DOI] [PubMed] [Google Scholar]
- 125.Ghielmini M, Colli E, Erba E, Bergamaschi D, Pampallona S, Jimeno J, Faircloth G, Sessa C. In vitro schedule-dependency of myelotoxicity and cytotoxicity of Ecteinascidin 743 (ET-743) Ann Oncol. 1998;9(9):989–993. doi: 10.1023/A:1008430827281. [DOI] [PubMed] [Google Scholar]
- 126.Valoti G, Nicoletti MI, Pellegrino A, Jimeno J, Hendriks H, D’Incalci M, Faircloth G, Giavazzi R. Ecteinascidin-743, a new marine natural product with potent antitumor activity on human ovarian carcinoma xenografts. Clin Cancer Res. 1998;4(8):1977–1983. [PubMed] [Google Scholar]
- 127.Kanzaki A, Takebayashi Y, Ren XQ, Miyashita H, Mori S, Akiyama S, Pommier Y. Overcoming multidrug drug resistance in P-glycoprotein/MDR1-overexpressing cell lines by ecteinascidin 743. Mol Cancer Ther. 2002;1(14):1327–1334. [PubMed] [Google Scholar]
- 128.Yang CS, Wang X. Green tea and cancer prevention. Nutr Cancer. 2010;62(7):931–937. doi: 10.1080/01635581.2010.509536. [DOI] [PubMed] [Google Scholar]
- 129.Lambert JD, Yang CS. Cancer chemopreventive activity and bioavailability of tea and tea polyphenols. Mutat Res. 2003;523–524:201–208. doi: 10.1016/S0027-5107(02)00336-6. [DOI] [PubMed] [Google Scholar]
- 130.Lin Y, Bai L, Chen W, Xu S. The NF-kappaB activation pathways, emerging molecular targets for cancer prevention and therapy. Expert Opin Therap Targets. 2010;14(1):45–55. doi: 10.1517/14728220903431069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Afaq F, Adhami VM, Ahmad N, Mukhtar H. Inhibition of ultraviolet B-mediated activation of nuclear factor kappaB in normal human epidermal keratinocytes by green tea Constituent (−)-epigallocatechin-3-gallate. Oncogene. 2003;22(7):1035–1044. doi: 10.1038/sj.onc.1206206. [DOI] [PubMed] [Google Scholar]
- 132.Nomura M, Ma W, Chen N, Bode AM, Dong Z. Inhibition of 12-O-tetradecanoylphorbol-13-acetate-induced NF-kappaB activation by tea polyphenols, (−)-epigallocatechin gallate and theaflavins. Carcinogenesis. 2000;21(10):1885–1890. doi: 10.1093/carcin/21.10.1885. [DOI] [PubMed] [Google Scholar]
- 133.Valente I, Reis M, Duarte N, Serly J, Molnar J, Ferreira MJ. Jatrophane diterpenes from Euphorbia mellifera and their activity as P-glycoprotein modulators on multidrug-resistant mouse lymphoma and human colon adenocarcinoma cells. J Nat Prod. 2012;75(11):1915–1921. doi: 10.1021/np3004003. [DOI] [PubMed] [Google Scholar]
- 134.Corea G, Di Pietro A, Dumontet C, Fattorusso E, Lanzotti V. Jatrophane diterpenes from Euphorbia spp. as modulators of multidrug resistance in cancer therapy. Phytochem Rev. 2009;8(2):431–447. doi: 10.1007/s11101-009-9126-8. [DOI] [Google Scholar]
- 135.Shi Z, Jain S, Kim IW, Peng XX, Abraham I, Youssef DT, Fu LW, El Sayed K, Ambudkar SV, Chen ZS. Sipholenol A, a marine-derived sipholane triterpene, potently reverses P-glycoprotein (ABCB1)-mediated multidrug resistance in cancer cells. Cancer Sci. 2007;98(9):1373–1380. doi: 10.1111/j.1349-7006.2007.00554.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Abraham I, Jain S, Wu CP, Khanfar MA, Kuang Y, Dai CL, Shi Z, Chen X, Fu L, Ambudkar SV, El Sayed K, Chen ZS. Marine sponge-derived sipholane triterpenoids reverse P-glycoprotein (ABCB1)-mediated multidrug resistance in cancer cells. Biochem Pharmacol. 2010;80(10):1497–1506. doi: 10.1016/j.bcp.2010.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Kimura S, Ito C, Jyoko N, Segawa H, Kuroda J, Okada M, Adachi S, Nakahata T, Yuasa T, Filho VC, Furukawa H, Maekawa T. Inhibition of leukemic cell growth by a novel anti-cancer drug (GUT-70) from calophyllum brasiliense that acts by induction of apoptosis. Int J Cancer. 2005;113(1):158–165. doi: 10.1002/ijc.20505. [DOI] [PubMed] [Google Scholar]
- 138.Quesada AR, Garcia Gravalos MD, Fernandez Puentes JL. Polyaromatic alkaloids from marine invertebrates as cytotoxic compounds and inhibitors of multidrug resistance caused by P-glycoprotein. Br J Cancer. 1996;74(5):677–682. doi: 10.1038/bjc.1996.421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Zhong Y, Zhang F, Sun Z, Zhou W, Li ZY, You QD, Guo QL, Hu R. Drug resistance associates with activation of Nrf2 in MCF-7/DOX cells, and wogonin reverses it by down-regulating Nrf2-mediated cellular defense response. Mol Carcinog. 2013;52(10):824–834. doi: 10.1002/mc.21921. [DOI] [PubMed] [Google Scholar]
- 140.Xu X, Zhang Y, Li W, Miao H, Zhang H, Zhou Y, Li Z, You Q, Zhao L, Guo Q. Wogonin reverses multi-drug resistance of human myelogenous leukemia K562/A02 cells via downregulation of MRP1 expression by inhibiting Nrf2/ARE signaling pathway. Biochem Pharmacol. 2014;92(2):220–234. doi: 10.1016/j.bcp.2014.09.008. [DOI] [PubMed] [Google Scholar]
- 141.Enomoto R, Koshiba C, Suzuki C, Lee E. Wogonin potentiates the antitumor action of etoposide and ameliorates its adverse effects. Cancer Chemother Pharmacol. 2011;67(5):1063–1072. doi: 10.1007/s00280-010-1396-8. [DOI] [PubMed] [Google Scholar]
- 142.Aoki S, Chen ZS, Higasiyama K, Setiawan A, Akiyama S, Kobayashi M. Reversing effect of agosterol A, a spongean sterol acetate, on multidrug resistance in human carcinoma cells. Jpn J Cancer Res Gann. 2001;92(8):886–895. doi: 10.1111/j.1349-7006.2001.tb01177.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Rabindran SK, Ross DD, Doyle LA, Yang W, Greenberger LM. Fumitremorgin C reverses multidrug resistance in cells transfected with the breast cancer resistance protein. Cancer Res. 2000;60(1):47–50. [PubMed] [Google Scholar]
- 144.Woehlecke H, Osada H, Herrmann A, Lage H. Reversal of breast cancer resistance protein-mediated drug resistance by tryprostatin A. Int J Cancer. 2003;107(5):721–728. doi: 10.1002/ijc.11444. [DOI] [PubMed] [Google Scholar]
- 145.Huang Q, Huang R, Zhang S, Lin J, Wei L, He M, Zhuo L, Lin X. Protective effect of genistein isolated from Hydrocotyle sibthorpioides on hepatic injury and fibrosis induced by chronic alcohol in rats. Toxicol Lett. 2013;217(2):102–110. doi: 10.1016/j.toxlet.2012.12.014. [DOI] [PubMed] [Google Scholar]
- 146.Imai Y, Tsukahara S, Asada S, Sugimoto Y. Phytoestrogens/flavonoids reverse breast cancer resistance protein/ABCG2-mediated multidrug resistance. Cancer Res. 2004;64(12):4346–4352. doi: 10.1158/0008-5472.CAN-04-0078. [DOI] [PubMed] [Google Scholar]
- 147.Wang Y, Zheng J, Liu P, Wang W, Zhu W. Three new compounds from Aspergillus terreus PT06-2 grown in a high salt medium. Mar Drugs. 2011;9(8):1368–1378. doi: 10.3390/md9081368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Liao WY, Shen CN, Lin LH, Yang YL, Han HY, Chen JW, Kuo SC, Wu SH, Liaw CC. Asperjinone, a nor-neolignan, and terrein, a suppressor of ABCG2-expressing breast cancer cells, from thermophilic Aspergillus terreus . J Nat Prod. 2012;75(4):630–635. doi: 10.1021/np200866z. [DOI] [PubMed] [Google Scholar]
- 149.Chen YF, Wang SY, Shen H, Yao XF, Zhang FL, Lai D. The marine-derived fungal metabolite, terrein, inhibits cell proliferation and induces cell cycle arrest in human ovarian cancer cells. Int J Mol Med. 2014;34(6):1591–1598. doi: 10.3892/ijmm.2014.1964. [DOI] [PubMed] [Google Scholar]
- 150.Zeng Y, Zhang Y, Weng Q, Hu M, Zhong G. Cytotoxic and insecticidal activities of derivatives of harmine, a natural insecticidal component isolated from Peganum harmala . Molecules. 2010;15(11):7775–7791. doi: 10.3390/molecules15117775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Zhang H, Sun K, Ding J, Xu H, Zhu L, Zhang K, Li X, Sun W. Harmine induces apoptosis and inhibits tumor cell proliferation, migration and invasion through down-regulation of cyclooxygenase-2 expression in gastric cancer. Phytomedicine Int J Phytother Phytopharmacol. 2014;21(3):348–355. doi: 10.1016/j.phymed.2013.09.007. [DOI] [PubMed] [Google Scholar]
- 152.Ma Y, Wink M. The beta-carboline alkaloid harmine inhibits BCRP and can reverse resistance to the anticancer drugs mitoxantrone and camptothecin in breast cancer cells. Phytother Res. 2010;24(1):146–149. doi: 10.1002/ptr.2860. [DOI] [PubMed] [Google Scholar]
- 153.Huang XC, Xiao X, Zhang YK, Talele TT, Salim AA, Chen ZS, Capon RJ. Lamellarin O, a pyrrole alkaloid from an Australian marine sponge, Ianthella sp., reverses BCRP mediated drug resistance in cancer cells. Mar Drugs. 2014;12(7):3818–3837. doi: 10.3390/md12073818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Seo J, Lee HS, Ryoo S, Seo JH, Min BS, Lee JH. Tangeretin, a citrus flavonoid, inhibits PGDF-BB-induced proliferation and migration of aortic smooth muscle cells by blocking AKT activation. Eur J Pharmacol. 2011;673(1–3):56–64. doi: 10.1016/j.ejphar.2011.10.011. [DOI] [PubMed] [Google Scholar]
- 155.Ikegawa T, Ushigome F, Koyabu N, Morimoto S, Shoyama Y, Naito M, Tsuruo T, Ohtani H, Sawada Y. Inhibition of P-glycoprotein by orange juice components, polymethoxyflavones in adriamycin-resistant human myelogenous leukemia (K562/ADM) cells. Cancer Lett. 2000;160(1):21–28. doi: 10.1016/S0304-3835(00)00549-8. [DOI] [PubMed] [Google Scholar]
- 156.Wesolowska O, Wisniewski J, Sroda-Pomianek K, Bielawska-Pohl A, Paprocka M, Dus D, Duarte N, Ferreira MJ, Michalak K. Multidrug resistance reversal and apoptosis induction in human colon cancer cells by some flavonoids present in citrus plants. J Nat Prod. 2012;75(11):1896–1902. doi: 10.1021/np3003468. [DOI] [PubMed] [Google Scholar]
- 157.Fleisher B, Unum J, Shao J, An G. Ingredients in fruit juices interact with dasatinib through inhibition of BCRP: a new mechanism of beverage-drug interaction. J Pharm Sci. 2015;104(1):266–275. doi: 10.1002/jps.24289. [DOI] [PubMed] [Google Scholar]
- 158.Steyn PS. The isolation, structure and absolute configuration of secalonic acid D, the toxic metabolite of Penicillium oxalicum . Tetrahedron. 1970;26(1):51–57. doi: 10.1016/0040-4020(70)85006-2. [DOI] [PubMed] [Google Scholar]
- 159.Hu YP, Tao LY, Wang F, Zhang JY, Liang YJ, Fu LW. Secalonic acid D reduced the percentage of side populations by down-regulating the expression of ABCG2. Biochem Pharmacol. 2013;85(11):1619–1625. doi: 10.1016/j.bcp.2013.04.003. [DOI] [PubMed] [Google Scholar]
- 160.Tsai P-L, Tsai T-H. Hepatobiliary excretion of berberine. Drug Metab Dispos. 2004;32(4):405–412. doi: 10.1124/dmd.32.4.405. [DOI] [PubMed] [Google Scholar]
- 161.Letašiová S, Jantová S, Čipák Lu, Múčková M. Berberine—antiproliferative activity in vitro and induction of apoptosis/necrosis of the U937 and B16 cells. Cancer Lett. 2006;239(2):254–262. doi: 10.1016/j.canlet.2005.08.024. [DOI] [PubMed] [Google Scholar]
- 162.Qi HW, Xin LY, Xu X, Ji XX, Fan LH. Epithelial-to-mesenchymal transition markers to predict response of Berberine in suppressing lung cancer invasion and metastasis. J Transl Med. 2014;12(1):22. doi: 10.1186/1479-5876-12-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Park SH, Sung JH, Chung N. Berberine diminishes side population and down-regulates stem cell-associated genes in the pancreatic cancer cell lines PANC-1 and MIA PaCa-2. Mol Cell Biochem. 2014;394(1–2):209–215. doi: 10.1007/s11010-014-2096-1. [DOI] [PubMed] [Google Scholar]
- 164.Sung JH, Kim JB, Park SH, Park SY, Lee JK, Lee H-S, Chung N. Berberine decreases cell growth but increases the side population fraction of H460 lung cancer cells. J Korean Soc Appl Biol Chem. 2012;55(4):491–495. doi: 10.1007/s13765-012-2119-0. [DOI] [Google Scholar]
- 165.Li YY, Zhang T. Targeting cancer stem cells by curcumin and clinical applications. Cancer Lett. 2014;346(2):197–205. doi: 10.1016/j.canlet.2014.01.012. [DOI] [PubMed] [Google Scholar]
- 166.Kakarala M, Brenner DE, Korkaya H, Cheng C, Tazi K, Ginestier C, Liu S, Dontu G, Wicha MS. Targeting breast stem cells with the cancer preventive compounds curcumin and piperine. Breast Cancer Res Treat. 2010;122(3):777–785. doi: 10.1007/s10549-009-0612-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Charpentier MS, Whipple RA, Vitolo MI, Boggs AE, Slovic J, Thompson KN, Bhandary L, Martin SS. Curcumin targets breast cancer stem-like cells with microtentacles that persist in mammospheres and promote reattachment. Cancer Res. 2014;74(4):1250–1260. doi: 10.1158/0008-5472.CAN-13-1778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Mukherjee S, Mazumdar M, Chakraborty S, Manna A, Saha S, Khan P, Bhattacharjee P, Guha D, Adhikary A, Mukhjerjee S, Das T. Curcumin inhibits breast cancer stem cell migration by amplifying the E-cadherin/β-catenin negative feedback loop. Stem Cell Res Therapy. 2014;5(5):116. doi: 10.1186/scrt506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Chung SS, Vadgama JV. Curcumin and epigallocatechin gallate inhibit the cancer stem cell phenotype via down-regulation of STAT3-NFκB signaling. Anticancer Res. 2015;35(1):39. [PMC free article] [PubMed] [Google Scholar]
- 170.Kanwar SS, Yu Y, Nautiyal J, Patel BB, Padhye S, Sarkar FH, Majumdar APN. Difluorinated-curcumin (CDF): a novel curcumin analog is a potent inhibitor of colon cancer stem-like cells. Pharm Res. 2011;28(4):827–838. doi: 10.1007/s11095-010-0336-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Kim EJ, Choi CH, Park JY, Kang SK, Kim YK. Underlying mechanism of quercetin-induced cell death in human glioma cells. Neurochem Res. 2008;33(6):971–979. doi: 10.1007/s11064-007-9416-8. [DOI] [PubMed] [Google Scholar]
- 172.Duo J, Ying GG, Wang GW, Zhang L. Quercetin inhibits human breast cancer cell proliferation and induces apoptosis via Bcl-2 and Bax regulation. Mol Med Rep. 2012;5(6):1453–1456. doi: 10.3892/mmr.2012.845. [DOI] [PubMed] [Google Scholar]
- 173.Zhou W, Kallifatidis G, Baumann B, Rausch V, Mattern J, Gladkich J, Giese N, Moldenhauer G, Wirth T, Buchler MW, Salnikov AV, Herr I. Dietary polyphenol quercetin targets pancreatic cancer stem cells. Int J Oncol. 2010;37(3):551–561. doi: 10.3892/ijo_00000704. [DOI] [PubMed] [Google Scholar]
- 174.Chang WW, Hu FW, Yu CC, Wang HH, Feng HP, Lan C, Tsai LL, Chang YC. Quercetin in elimination of tumor initiating stem-like and mesenchymal transformation property in head and neck cancer. Head Neck. 2013;35(3):413–419. doi: 10.1002/hed.22982. [DOI] [PubMed] [Google Scholar]
- 175.Chen SF, Nieh S, Jao SW, Liu CL, Wu CH, Chang YC, Yang CY, Lin YS. Quercetin suppresses drug-resistant spheres via the p38 MAPK-Hsp27 apoptotic pathway in oral cancer cells. PLoS One. 2012;7(11):e49275. doi: 10.1371/journal.pone.0049275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Atashpour S, Fouladdel S, Movahhed TK, Barzegar E, Ghahremani MH, Ostad SN, Azizi E. Quercetin induces cell cycle arrest and apoptosis in CD133(+) cancer stem cells of human colorectal HT29 cancer cell line and enhances anticancer effects of doxorubicin. Iran J Basic Med Sci. 2015;18(7):635–643. [PMC free article] [PubMed] [Google Scholar]
- 177.Szkudelski T. Resveratrol inhibits insulin secretion from rat pancreatic islets. Eur J Pharmacol. 2006;552(1–3):176–181. doi: 10.1016/j.ejphar.2006.09.046. [DOI] [PubMed] [Google Scholar]
- 178.Vanamala J, Charepalli V, Radhakrishnan S, Reddivari L (2012) Resveratrol and grape seed extract combination elevates apoptosis in the colon cancer stem cells, even in the presence of IGF-1, via P53 dependent pathway. FASEB J:26
- 179.Shankar S, Nall D, Tang SN, Meeker D, Passarini J, Sharma J, Srivastava RK. Resveratrol inhibits pancreatic cancer stem cell characteristics in human and KrasG12D transgenic mice by inhibiting pluripotency maintaining factors and epithelial-mesenchymal transition. PLoS One. 2011;6(1):e16530. doi: 10.1371/journal.pone.0016530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Shen YA, Lin CH, Chi WH, Wang CY, Hsieh YT, Wei YH, Chen YJ. Resveratrol impedes the stemness, epithelial-mesenchymal transition, and metabolic reprogramming of cancer stem cells in nasopharyngeal carcinoma through p53 activation. Evid Complement Altern Med. 2013;2013:590393. doi: 10.1155/2013/590393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Baribeau S, Chaudhry P, Parent S, Asselin E. Resveratrol inhibits cisplatin-induced epithelial-to-mesenchymal transition in ovarian cancer cell lines. PLoS One. 2014;9(1):e86987. doi: 10.1371/journal.pone.0086987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Fu Y, Chang H, Peng X, Bai Q, Yi L, Zhou Y, Zhu J, Mi M. Resveratrol inhibits breast cancer stem-like cells and induces autophagy via suppressing Wnt/beta-catenin signaling pathway. PLoS One. 2014;9(7):e102535. doi: 10.1371/journal.pone.0102535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Kocieński PJ, Brown RCD, Pommier A, Procter M, Schmidt B. Synthesis of salinomycin. J Chem Soc Perkin Trans. 1998;1(1):9–39. doi: 10.1039/a705385a. [DOI] [Google Scholar]
- 184.Gupta PB, Onder TT, Jiang G, Tao K, Kuperwasser C, Weinberg RA, Lander ES. Identification of selective inhibitors of cancer stem cells by high-throughput screening. Cell. 2009;138(4):645–659. doi: 10.1016/j.cell.2009.06.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Yue W, Hamai A, Tonelli G, Bauvy C, Nicolas V, Tharinger H, Codogno P, Mehrpour M. Inhibition of the autophagic flux by salinomycin in breast cancer stem-like/progenitor cells interferes with their maintenance. Autophagy. 2013;9(5):714–729. doi: 10.4161/auto.23997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Chen T, Yi L, Li F, Hu R, Hu S, Yin Y, Lan C, Li Z, Fu C, Cao L, Chen Z, Xian J, Feng H. Salinomycin inhibits the tumor growth of glioma stem cells by selectively suppressing glioma-initiating cells. Mol Med Rep. 2015;11(4):2407–2412. doi: 10.3892/mmr.2014.3027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Aykut B, Schenk M, Giese N, Kleber S, Martin-Villalba A, Welsch T. Salinomycin is effective against pancreatic cancer stem cells and targets metastasis-promoting fascin. Pancreatology. 2013;13(2):e15. doi: 10.1016/j.pan.2012.12.105. [DOI] [Google Scholar]
- 188.Mao J, Fan S, Ma W, Fan P, Wang B, Zhang J, Wang H, Tang B, Zhang Q, Yu X, Wang L, Song B, Li L. Roles of Wnt/beta-catenin signaling in the gastric cancer stem cells proliferation and salinomycin treatment. Cell Death Dis. 2014;5:e1039. doi: 10.1038/cddis.2013.515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Tang QL, Zhao ZQ, Li JC, Liang Y, Yin JQ, Zou CY, Xie XB, Zeng YX, Shen JN, Kang T, Wang J. Salinomycin inhibits osteosarcoma by targeting its tumor stem cells. Cancer Lett. 2011;311(1):113–121. doi: 10.1016/j.canlet.2011.07.016. [DOI] [PubMed] [Google Scholar]
- 190.Wohlert SE, Künzel E, Machinek R, Méndez C, Salas JA, Rohr J. The structure of mithramycin reinvestigated. J Nat Prod. 1999;62(1):119–121. doi: 10.1021/np980355k. [DOI] [PubMed] [Google Scholar]
- 191.Zhang M, Mathur A, Zhang Y, Xi S, Atay S, Hong JA, Datrice N, Upham T, Kemp CD, Ripley RT, Wiegand G, Avital I, Fetsch P, Mani H, Zlott D, Robey R, Bates SE, Li X, Rao M, Schrump DS. Mithramycin represses basal and cigarette smoke-induced expression of ABCG2 and inhibits stem cell signaling in lung and esophageal cancer cells. Cancer Res. 2012;72(16):4178–4192. doi: 10.1158/0008-5472.CAN-11-3983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Leizer AL, Alvero AB, Fu HH, Holmberg JC, Cheng YC, Silasi DA, Rutherford T, Mor G. Regulation of inflammation by the NF-kappa B pathway in ovarian cancer stem cells. Am J Reprod Immunol. 2011;65(4):438–447. doi: 10.1111/j.1600-0897.2010.00914.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Don-Doncow N, Escobar Z, Johansson M, Kjellstrom S, Garcia V, Munoz E, Sterner O, Bjartell A, Hellsten R. Galiellalactone Is a direct inhibitor of the transcription factor STAT3 in prostate cancer cells. J Biol Chem. 2014;289(23):15969–15978. doi: 10.1074/jbc.M114.564252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Hellsten R, Johansson M, Dahlman A, Sterner O, Bjartell A, Pediatrics/Urology/Gynecology/Endocrinology, Sektionen för BUKE, Medicin, Pathology, Department of Laboratory Medicine M, Institutionen för kliniska vetenskaper M, Faculty of M, Department of Clinical Sciences M, Division of Urological C, Urologi, Enheten för urologisk c, Institutionen för laboratoriemedicin M, Lunds u, Lund U, Urology, Patologi M (2011) Galiellalactone inhibits stem cell-like ALDH-positive prostate cancer cells. PloS One 6 (7):e22118. doi:10.1371/journal.pone.0022118 [DOI] [PMC free article] [PubMed]
- 195.Macha MA, Rachagani S, Gupta S, Pai P, Ponnusamy MP, Batra SK, Jain M. Guggulsterone decreases proliferation and metastatic behavior of pancreatic cancer cells by modulating JAK/STAT and Src/FAK signaling. Cancer Lett. 2013;341(2):166–177. doi: 10.1016/j.canlet.2013.07.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Dixit D, Ghildiyal R, Anto NP, Ghosh S, Sharma V, Sen E. Guggulsterone sensitizes glioblastoma cells to Sonic hedgehog inhibitor SANT-1 induced apoptosis in a Ras/NF kappa B dependent manner. Cancer Lett. 2013;336(2):347–358. doi: 10.1016/j.canlet.2013.03.025. [DOI] [PubMed] [Google Scholar]
- 197.Miyazaki T, Pan Y, Joshi K, Purohit D, Hu B, Demir H, Mazumder S, Okabe S, Yamori T, Viapiano M, Shin-ya K, Seimiya H, Nakano I. Telomestatin impairs glioma stem cell survival and growth through the disruption of telomeric G-quadruplex and inhibition of the proto-oncogene, c-Myb. Clin Cancer Res. 2012;18(5):1268–1280. doi: 10.1158/1078-0432.CCR-11-1795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Boehmerle W, Muenzfeld H, Springer A, Huehnchen P, Endres M. Specific targeting of neurotoxic side effects and pharmacological profile of the novel cancer stem cell drug salinomycin in mice. J Mol Med. 2014;92(8):889–900. doi: 10.1007/s00109-014-1155-0. [DOI] [PubMed] [Google Scholar]
- 199.Russo GL, Spagnuolo C, Russo M, Volpe S, Tedesco I, Bilotto S. Synergistic response induced by quercetin and ABT-737 in leukemic cell lines and in B-cells isolated from chronic lymphocytic leukemia. Eur J Cancer. 2012;48:S200. doi: 10.1016/S0959-8049(12)71467-3. [DOI] [Google Scholar]
- 200.Ward AB, Mir H, Kapur N, Singh S. Quercetin inhibits prostate cancer by modulating molecules involved in apoptosis and cell proliferation. Cancer Res. 2015 [Google Scholar]
- 201.Borska S, Chmielewska M, Wysocka T, Drag-Zalesinska M, Zabel M, Dziegiel P. In vitro effect of quercetin on human gastric carcinoma: targeting cancer cells death and MDR. Food Chem Toxicol. 2012;50(9):3375–3383. doi: 10.1016/j.fct.2012.06.035. [DOI] [PubMed] [Google Scholar]
- 202.Srinivasan A, Thangavel C, Liu Y, Shoyele S, Den RB, Selvakumar P, Lakshmikuttyamma A. Quercetin regulates β-catenin signaling and reduces the migration of triple negative breast cancer: qUERCETIN INHIBITS CELL MIGRATION. Mol Carcinog. 2015 doi: 10.1002/mc.22318. [DOI] [PubMed] [Google Scholar]
- 203.Borska S, Drag-Zalesinska M, Wysocka T, Sopel M, Dumanska M, Zabel M, Dziegiel P. Antiproliferative and pro-apoptotic effects of quercetin on human pancreatic carcinoma cell lines EPP85-181P and EPP85-181RDB. Folia Histochem Cytobiol. 2010;48(2):222–229. doi: 10.2478/v10042-08-0109-1. [DOI] [PubMed] [Google Scholar]