Abstract
Melatonin protects the electron transport chain (ETC) in multiple ways. It reduces levels of ·NO by downregulating inducible and inhibiting neuronal nitric oxide synthases (iNOS, nNOS), thereby preventing excessive levels of peroxynitrite. Both ·NO and peroxynitrite-derived free radicals, such as ·NO2, hydroxyl (·OH) and carbonate radicals (CO3·−) cause blockades or bottlenecks in the ETC, by ·NO binding to irons, protein nitrosation, nitration and oxidation, changes that lead to electron overflow or even backflow and, thus, increased formation of superoxide anions (O2·−). Melatonin improves the intramitochondrial antioxidative defense by enhancing reduced glutathione levels and inducing glutathione peroxidase and Mn-superoxide dismutase (Mn-SOD) in the matrix and Cu,Zn-SOD in the intermembrane space. An additional action concerns the inhibition of cardiolipin peroxidation. This oxidative change in the membrane does not only initiate apoptosis or mitophagy, as usually considered, but also seems to occur at low rate, e.g., in aging, and impairs the structural integrity of Complexes III and IV. Moreover, elevated levels of melatonin inhibit the opening of the mitochondrial permeability transition pore and shorten its duration. Additionally, high-affinity binding sites in mitochondria have been described. The assumption of direct binding to the amphipathic ramp of Complex I would require further substantiation. The mitochondrial presence of the melatonin receptor MT1 offers the possibility that melatonin acts via an inhibitory G protein, soluble adenylyl cyclase, decreased cAMP and lowered protein kinase A activity, a signaling pathway shown to reduce Complex I activity in the case of a mitochondrial cannabinoid receptor.
Keywords: Electron leakage, Melatonin, NADPH oxidase, Reactive nitrogen species, Reactive oxygen species
Introduction
Mitochondria represent a major source of free radicals, which can be considerably augmented under conditions of pathophysiological challenges that may lead, in the extreme, to mitochondrial dysfunction, mitophagy or apoptosis. With regard to involvement of malfunctioning mitochondria in numerous pathologies, they have been called “powerhouses of disease” [1]. The general topic of this multi-author review that relates melatonin to these organelles is based on many reports on beneficial effects that can be interpreted as improvements of mitochondrial function and integrity. These findings may gain particular importance with regard to the observation that melatonin can accumulate in mitochondria [2–5] and may, thus, be considered as a protective compound of enhanced importance to these organelles. As melatonin levels decrease in numerous diseases and, progressively, in the course of aging [6, 7], its mitochondrial role deserves special attention in the context of health maintenance.
Mitochondrial formation of reactive oxygen species (ROS) is mainly based on two processes, electron dissipation and activation of NADPH oxidase (NOX). The former occurs by electron transfer to molecular oxygen, which results in the formation of superoxide anions (O2·−), free radicals of comparably lower reactivity, which are, however, a source of highly reactive, more dangerous compounds. Electron leakage is especially associated with the Complexes I and III [8–11]. At Complex III, this process has been attributed to electron bifurcation from ubiquinol [12] and seems to take place at the Qo site, from where electrons are released as the consequence of an interruption of electron transfer between two b L hemes in the dimeric cytochrome bc1 complex [13]. At Complex III, dissipating electrons are released to both sides of the inner mitochondrial membrane [9]. Superoxide anions formed thereby on the intermembrane side can be converted by Cu,Zn-superoxide dismutase (SOD1) located in the intermembrane space, whereas those formed on the side of the matrix are metabolized by Mn-SOD (SOD2). Notably, both the intermembrane and matrix SOD forms are influenced by melatonin [14, 15]. In Complex I, the iron–sulfur cluster N2 is known as the site of electron leakage [16–20]. N2 is located in the amphipathic ramp that extends into the matrix. Therefore, superoxide anions formed in this place are released into the matrix. As a consequence, the extrusion towards the matrix makes the amphipathic ramp particularly vulnerable to secondary radicals of higher reactivity. On the other hand, this area may be accessible to regulatory molecules influencing electron flux, perhaps including melatonin.
The other ROS sources, members of the NOX family, contribute differently to mitochondrial oxidative stress and dysfunction. The family contains five NOX and two DUOX (dual oxidase) subforms, which differ with regard to cell type and intracellular localization [21, 22]. They typically generate O2·−, which, being a charged molecule, does not cross membranes at substantial rates, whereas its SOD product, H2O2, being a symmetric apolar compound, is well capable to do this. Damage to the mitochondrial electron transport chain (ETC) by extramitochondrially formed H2O2 presumably represents an exception that only occurs under conditions of severe oxidative stress. The subform NOX4 differs from the others by the fact that it is also found in mitochondria, in addition to localization in other compartments such as the endoplasmic reticulum. The mitochondrial localization, discovered in 2009 [23], has meanwhile been confirmed [24–26]. Another peculiarity repeatedly mentioned concerns the assumed direct formation of H2O2, instead of O2·− [25], a possibly precocious interpretation, because this was found in compartments in which O2·− may have been rapidly dismutated [21]. The earlier assumption that NOX4 may be associated with Complex IV [23] has not been entirely ruled out, but stronger evidence has been more recently obtained for an interaction with Complex I [24–26]. The interaction was reported to inactivate Complex I [24], an effect that should have considerable consequences to electron flow as well as to the balance of electron supply between Complexes I and II. However, the importance of NOX4 for ROS mitochondrial formation under basal conditions has been questioned [26]. Nevertheless, as will be discussed later in the context of melatonin’s actions, mitochondrial NOX upregulation seems to be highly relevant in oxidotoxin-induced mitochondrial dysfunction [27]. In this case, strong enhancements of NOX have been observed in the mitochondrial fraction, but the subform, which may be with some likelihood NOX4, remains to be determined.
To what extent mitochondrial ROS formation may impair the electron flux through the ETC and, thereby, cause further ROS generation by enhanced electron leakage depends mainly on the balance between protective and damaging factors. On the protective side, the antioxidant capacity is decisive. Without discussing in this place already the role of melatonin, a major protective factor is the availability of reduced glutathione (GSH). Moreover, substantial protection is provided intramitochondrially by the peroxide-eliminating capacity of peroxiredoxin-3 (PRX3), which requires activation by the thioredoxin-2 (TRX2)/thioredoxin reductase-2 (TRXR2) cascade [28, 29]. Moreover, TRX2 is a protein that connects ROS levels with apoptosis, as it binds to apoptosis signal-regulating kinase 1 (ASK1). This complex dissociates when specific cysteine residues in TRX2 are oxidized by ROS, an effect that allows the auto-activated ASK1 to induce mitochondrial-dependent apoptotic pathways [29, 30]. On the damaging and electron flux-interrupting side, enhanced levels of reactive nitrogen species (RNS) can become a decisive parameter. Moreover, CO2, anyway present at high concentrations in the matrix because of its formation by the citric acid cycle, can contribute to mitochondrial dysfunction, an aspect that is frequently disregarded. A primary critical factor that initiates ETC blockades is ·NO, a free radical of moderate reactivity, which, nonetheless, can directly interact with ETC components and additionally generate other interacting compounds. Under conditions of neuronal overexcitation or of inflammation, ·NO is excessively formed by neuronal and inducible NO synthase isoforms (nNOS, iNOS), [15, 31, 32]. ·NO can directly act as an iron ligand at iron/sulfur clusters or hemes. It is known to form of S-nitrosothiols, in both protein residues and small organic thiols. Soluble S-nitrosothiols generated in this way, such as S-nitrosoglutathione, are capable of transnitrosating protein thiols in ETC Complexes [11, 33, 34], a type of reaction that may be of even higher importance than direct protein nitrosation by ·NO [34]. Complex I is particularly vulnerable to S-nitrosation, which leads to enhanced electron leakage and, thus, superoxide formation at this site [34]. It may be also noted that ·NO can give rise to other nitrosating compounds such as its redox congeners NO+ and HNO (protonated NO−) or N2O3 [35, 36]. A much more reactive compound of a considerably higher destructive potential is peroxynitrite (ONOO−), which is easily formed by combination of ·NO and O2·−, a process that can take place at substantial rates, because O2·− has similar affinities to superoxide dismutases and to ·NO. As O2·− is abundantly available in mitochondria because of electron leakage, the limiting factor of adduct formation is ·NO. Despite its high reactivity, direct effects of peroxynitrite have remained to a certain degree unclear, since it easily generates free radicals of elevated reactivity. Therefore, direct and indirect, radical-mediated actions are frequently difficult to distinguish under biological conditions. The main modes of free radical formation from peroxynitrite occur after either protonation or interaction with CO2. Upon protonation to ONOOH, the molecule decomposes to ·NO2 and the hydroxyl radical, ·OH. Formation of a peroxynitrite-CO2 adduct (ONOOCO2 −) leads, correspondingly, to ·NO2 and the carbonate radical (CO3·−). CO3·− is less reactive than ·OH, but, owing to its resonance stabilization, it has a considerably longer life time than ·OH and a much more far-reaching radius of action. Its reactivity is still sufficient to oxidize many compounds that are otherwise attacked by ·OH. Under in vitro conditions, addition of hydrogen carbonate as a source of CO2 frequently enhances peroxynitrite-dependent reactions with other compounds. The mixture of ·NO2 and CO3·− generated from ONOOCO2 − represents a highly effective, but nonclassic nitration reagent [37]. Aromate nitration is normally believed to represent a nonradical mechanism, but this radical-based variant should not be overlooked in biological systems, especially under pathophysiological conditions of tyrosine nitration.
Both nitration and oxidation reactions, as caused by RNS and their decay products, or by ·OH formed from H2O2, are sources of ETC dysfunction. This may concern protein subunits of the ETC complexes, but can also result from peroxidation of cardiolipin, which is required for the structural integrity of especially Complexes III and IV [38–41]. However, cardiolipin peroxidation largely represents a nonradical mechanism catalyzed by cytochrome c, which gains peroxidase activity upon interaction with cardiolipin. Cardiolipin peroxidation is a crucial step in mitochondrial dysfunction, breakdown of the mitochondrial membrane potential, cytochrome c release and, thus, initiation of apoptosis. This lipid is earlier and more strongly peroxidized than other mitochondrial lipids [42–44]. As outlined elsewhere [32], antioxidants that interrupt cardiolipin peroxidation have been concluded to not act by scavenging free radicals, but rather by inhibiting the peroxidase activity of the cytochrome c/cardiolipin complex. However, this view may require further support. Nonenzymatic low-rate cardiolipin peroxidation that does not immediately cause apoptosis or mitophagy will be discussed below.
The described processes of oxidation, nitrosation and nitration lead to the occurrence of bottlenecks in the ETC and, thus, to enhanced electron dissipation, which primarily results in O2·− formation, but secondarily to more reactive and more damaging intermediates. There are, however, additional possibilities of malfunctioning electron flow. For instance, imbalance of electron feeding between Complexes I and II can also cause electron overflow, especially at Complex I, and an excess of succinate can increase electron leakage by an order of magnitude [11, 31, 45]. Bottlenecks either at Complex III by internal electron transfer disruption or at Complex IV, as occurring under conditions of oxygen deficiency, will lead to electron dissipation at complex III [11, 31]. Moreover, cytochrome c is subject to acetylation [46], another modification assumed to enhance electron dissipation, which is reportedly reversed by a mitochondrially localized protein deacetylase, the sirtuin subform SIRT5 [47]. Additionally, the association of NOX4 with ETC components inhibits electron flux at Complex I, but the roles of on-site oxidant formation and of protein–protein interactions would require further clarification. This is even more the case for the previously reported interaction with Complex IV, which might cause another bottleneck. Anyway, reduced oxygen availability at Complex IV can lead to an electron tailback and, thus, cause enhanced superoxide formation, e.g., at Complex III. The relationship between oxygen supply and mitochondrial function may become critical under some conditions, such as in ischemia/reperfusion or already in atherosclerosis. This may even lead to vicious cycles, if compromised mitochondria produce an excess of oxidants, while ·NO formation is upregulated, either because of inflammatory responses or in attempts of improving blood supply, thereby leading to peroxynitrite and radicals deriving thereof.
Support of mitochondrial function by reducing RNS formation
With regard to the crucial role of RNS in the interruption of electron flux, the reduction of ·NO formation is of utmost importance for the maintenance of a well-operating ETC. Since ·NO is a highly diffusible compound that easily crosses membranes, its rates of formation are not only relevant to mitochondria in the cell hosting them, but also in the surrounding tissue. Moreover, ·NO is also generated by a mitochondrially targeted iNOS subform (mt-iNOS) [48–52]. Melatonin has been shown to inhibit nNOS in the context of preventing neuronal overexcitation and hypoxic insults [53–59] and to downregulate iNOS in numerous cell types, including astrocytes and microglia [3, 49, 50, 58, 60–65]. In the CNS and, correspondingly, in microglial cell lines, these effects counteract low-grade inflammation and, thus, reduce proinflammatory cytokine release [32, 66–70]. Highly impressive effects of iNOS downregulation by melatonin have been obtained in models of endotoxemia and sepsis, actions that were unambiguously related to maintenance of mitochondrial function and cell survival [48, 49, 51, 52, 60–63, 71]. Notably, these effects were prominently observed in mt-iNOS [48, 49, 51, 52].
Although low or moderate ·NO levels have also been reported to be cell protective [35, 72–74], the prevention of excessive ·NO synthesis can be assumed to have various beneficial consequences. As outlined in the Introduction, this avoids enhanced production of peroxynitrite and its secondary and tertiary products, such as peroxynitrite-derived free radicals (·OH, CO3·−, ·NO2) and the strongly nitrosating agent N2O3 formed by combination of ·NO and ·NO2. Moreover, keeping ·NO below a critical level prevents vicious cycles that involve progressively increasing inflammatory responses, damage by leukocyte-derived ROS and RNS, attraction of further immune cells by DNA-damaged cells and aggravating mitochondrial malfunction [32, 70]. Corresponding vicious cycles existing in the CNS are likewise interrupted. These involve neuronal Ca2+ overload, overexcitation, activation of astro- and microglia, lead to losses in peripheral mitochondria, disturbances of the fission/fusion balance, reduced neuronal connectivity and cell loss [32, 33, 68, 70]. An overview of the various mechanisms of antinitrosative, antinitrative and associated antioxidative protection by melatonin with relevance to the ETC as well as the consequences of reducing electron dissipation is given in Fig. 1. It should also be noted that endothelial nitric oxide synthase (eNOS) can also substantially contribute to ·NO levels, especially under hypoxic conditions such as ischemia or atherosclerosis. However, this has been omitted from the figure and further discussion, since eNOS is poorly influenced by melatonin.
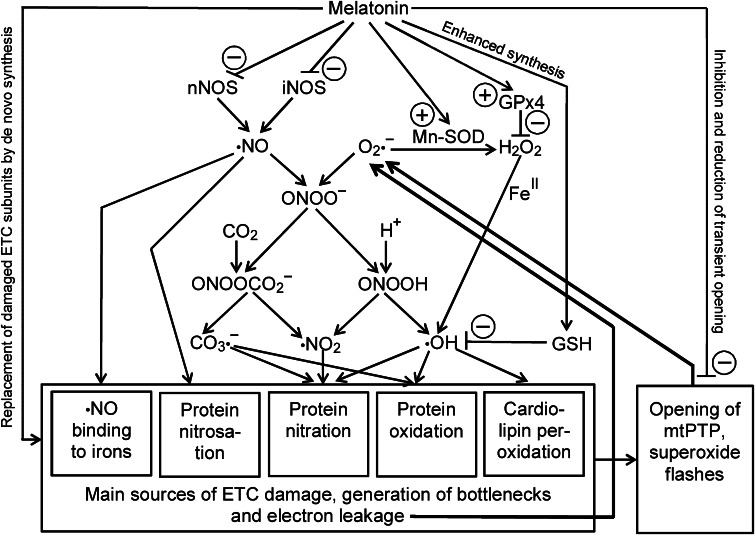
Fig. 1.
Summary of the main processes leading to damage of ETC components with consequences to bottlenecks of electron flux and formation of superoxide anions (O2·−) by electron dissipation. The scheme also emphasizes the role of ·NO formation for ETC malfunction. To avoid further complexity, extramitochondrial sources of H2O2, the role of Cu,Zn-SOD in the intermembrane space, endothelial nitric oxide synthase, scavenging of ROS and RNS by melatonin and all actions of melatonin metabolites have been omitted. ETC electron transport chain, GPx4 glutathione peroxidase subform 4, GSH reduced glutathione, iNOS inducible nitric oxide synthase, Mn-SOD manganese superoxide dismutase, mtPTP mitochondrial permeability transition pore, nNOS, neuronal nitric oxide synthase. For further details, especially concerning chemical compounds, see current text. Plus signs indicate upregulation, minus signs inhibition (nNOS, mtPTP), downregulation (iNOS) or direct detoxification by scavenging (GSH)
Support by direct and indirect antioxidant actions
Since the discovery of this property [75], melatonin is well known as a potent ·OH scavenger. The major problem with this direct antioxidant action is, however, to distinguish between high pharmacological and physiological levels. Nanomolar levels as in the circulation are certainly not sufficient for conveying a substantial protection. However, concentrations in melatonin-synthesizing cells may well be high enough for antioxidative purposes. Although melatonin can accumulate in mitochondria [2–5], the concentrations may still be too low for a relevant contribution in other cell types. Nevertheless, another theoretical possibility may follow from the fact that melatonin generates radical-scavenging metabolites, such as cyclic 3-hydroxymelatonin [76, 77] and the substituted kynuramines, N 1-acetyl-N 2-formyl-5-methoxykynuramine (AFMK) and N 1-acetyl-5-methoxykynuramine (AMK) [78–80]. Their sequential action can constitute a radical-scavenging cascade, which allows the elimination of up to 10 free radicals per melatonin molecule [81]. However, this oxidative pathway is composed of several compounds that differ in their reactivity to free radicals and some of the steps are also catalyzed by enzymatic or pseudoenzymatic mechanisms. Therefore, such a mixture of different oxidative processes may also lead to intramitochondrial accumulation of melatonin metabolites. This possibility can be excluded as long as all steps of the scavenging cascade are caused by reactions with ·OH. However, melatonin can be also mitochondrially catabolized to AFMK via the pseudoperoxidase activity of cytochrome c [82]. Since AFMK is much more inert than melatonin [83], an accumulation of this metabolite may not be impossible, provided that rates of ·OH generation are not high enough to eliminate AFMK. In this context, another case of AFMK accumulation may be of interest: In the cerebrospinal fluid of patients with brain inflammation (viral meningitis), AFMK accumulated to levels by orders of magnitude higher than melatonin [84]. Therefore, AFMK may represent a source of melatonin-derived metabolites which may be used for purposes of antioxidative protection under conditions of suddenly enhanced oxidative stress. The further radical-mediated catabolism would give rise to compounds more potent than AFMK, in particular, the efficient radical scavenger AMK [79, 83]. However, this consideration is to date not more than a possibility and would require confirmation by metabolite quantification in mitochondria. Nevertheless, this line should be worth further experimental analyses, especially because of several findings concerning AMK. This compound, apart from being a potent inhibitor of nNOS [57, 85] and downregulator of iNOS, including mt-iNOS [50], efficiently scavenges ·NO as well as its redox congeners NO+ and HNO, contrary to melatonin without re-donating ·NO [37, 86, 87], and has been shown to protect mitochondria at relatively low concentrations [88]. Moreover, AMK efficiently interacts with peroxynitrite-derived free radicals, in particular, CO3·− and ·NO2, as demonstrated by CO2/HCO3 −-dependent formation of 3-nitro-AMK [37] and oxidation by CO3·− [83]. AMK was also shown to be a potent scavenger of hydroxyl radicals [80, 83], peroxyl radicals [79] and singlet oxygen [89]. In all these properties, AMK is clearly superior to AFMK, but to melatonin only in the case of singlet oxygen. The property of efficiently scavenging CO3·− [37, 79, 83] is shared with melatonin [90–92]. With regard to the high mitochondrial CO2 levels, this property may deserve more future attention.
As in many other cases, indirect antioxidant actions of melatonin are of particular importance to the protection of mitochondria, too. Without referring to frequently discussed endpoints of protection, such as reduction of lipid peroxidation, of DNA damage in the mitochondrial chromosome, stabilization of membrane fluidity, and prevention of apoptosis induction, the focus should rather be laid on the melatonin-induced improvements of the enzymatic part of the antioxidant system, including the consequences to the availability of GSH, a major low molecular weight antioxidant in mitochondria. In fact, melatonin has been shown to increase, in various organs, the mitochondrial content of GSH and the GSH/GSSG ratio, effects that were attributable to increases in glutathione synthesis and glutathione reductase activity, changes that were accompanied by augmentations of glutathione peroxidase activity [48, 49, 63, 93–95]. Notably, these protective actions were observed under conditions of sepsis as well as aging, in both normally aging and senescence-accelerated animals. Another typical improvement documented in the publications cited concerns enhancements of ATP formation and respiratory efficiency. Another protective effect of melatonin consists in the upregulation of Mn-SOD, a result obtained under diverse conditions and in various cell types [96–102]. It would be of greatest interest to know whether increased levels of H2O2, which occur in oxidative stress and at upregulated Mn-SOD, would be accompanied and eliminated by corresponding rises in the mitochondrially targeted peroxiredoxin-3 activity (cf. Introduction), but to date this subform has not been studied under the influence of melatonin. It should be noted that such a study may not lead to conclusive results by only determining expression levels, but presumably requires activity measurements, including those of thioredoxin-2 and thioredoxin reductase-2.
A particular aspect of antioxidant actions in mitochondria concerns cardiolipin. As outlined above, this lipid is crucial for the functionality of ETC complexes and, thus, electron flux. Additionally, cardiolipin peroxidation represents an important step in cytochrome c release and, therefore, apoptosis induction. Moreover, cardiolipin from damaged membranes is externalized by phospholipid scramblase-3 to the outer membrane and, thereby, serves as mitophagy signal [103]. The fact that this peroxidation reaction is catalyzed by the cardiolipin peroxidase side activity of cytochrome c has led to the conclusion that this type of lipid peroxidation is entirely enzymatic and independent of free radicals. Nevertheless, it seems difficult to understand why sufficiently reactive free radicals should be unable to peroxidize this lipid, too. In fact, a study on Aβ peptide-induced mitochondrial dysfunction concluded that free radicals can deplete cardiolipin and that melatonin reduced both free-radical formation and cardiolipin peroxidation [104]. Presumably, a free-radical mechanism cannot be totally excluded, but a decisive difference presumably exists in the velocity and the rates of enzymatic compared to radical-mediated processes of cardiolipin peroxidation. Moreover, low-rate cardiolipin peroxidation, as it occurs, e.g., in aging, should be distinguished from a high-rate process that causes cytochrome c release and apoptosis. Melatonin has been repeatedly reported to prevent or strongly reduce cardiolipin peroxidation [4, 104–109]. It may depend on the system studied whether radical detoxification plays a role in this inhibitory action of melatonin. However, there is no convincing evidence for a direct interaction of melatonin with the cardiolipin peroxidase. As discussed elsewhere [15, 32], an explanation may be based on the upregulation of mitochondrial glutathione peroxidase subform, GPx4, by melatonin. Although the subform has not been explicitly identified in the various studies on mitochondrial GPx upregulation by melatonin [48, 49, 63, 93–95], this should have been GPx4 with high likelihood. Overexpression of GPx4 has been shown to strongly inhibit both cardiolipin peroxidation and cytochrome c release [110], findings that would be in favor of a free radical mechanism, at least in the low-rate variant of this peroxidation process.
Upregulation of ETC complex activities and its components
Many studies focused on mitochondrial protection by melatonin have also investigated the activities of the respiratory complexes, either in the context of preventing ETC damage, under conditions of sepsis [48, 49, 51, 63, 111], endotoxemia [52, 112], ischemia/reperfusion [105, 113] and application of mitochondrial toxins [50, 114–116], or with regard to renormalization of aging-related losses [94, 95, 107, 117–121]. Upregulations were detected in various organs/tissues, such as brain [107, 113–115, 120], nigrostriatum [50, 116], heart [49, 51, 94, 105, 111], skeletal muscle [63], diaphragm [48, 95], liver [3, 52, 112, 114, 115, 117–119], and lung [121]. Collectively, one can state that the activities of all ETC complexes can be increased or, under oxidative challenge, normalized by melatonin. However, differences exist between organs, conditions and studies. Very frequently, Complex I and Complex IV activities are strongly upregulated, but this is not generally so. In other cases, substantial increases have also been observed in Complexes II and III. General rules concerning design of the study and tissue cannot be deduced from the data. Therefore, these details shall not be discussed here. Instead, the general statement seems appropriate that, in principle, all ETC complexes can be upregulated or protected by melatonin, though, to a variable degree.
A specific problem concerning the meaning of these findings needs to be addressed. Most of the respective experiments have been conducted using submitochondrial particles. This is entirely acceptable, but is important to remain aware of the fact that the measurements on these particles reflect their activity capacity, but not the in vivo activities in a functional ETC. Therefore, aspects on electron overflow or backflow, electron leakage rates including the occurrence of superoxide flashes [122, 123] can only be roughly or not at all deduced. Even experiments in isolated mitochondria may not reflect physiological conditions, because these organelles may be differently energized in vitro and in vivo. If, just for experimental convenience, mitochondria are mainly energized via Complex II, the circumvention of the bottleneck of electron flux at Complex I may lead to results differing from those obtained when the ETC is mainly fed through Complex I and, in the extreme, may cause electron backflow and superoxide formation [11, 31].
In principle, all protective mechanisms summarized in the two preceding sections may contribute to the observed increases or normalizations of ETC complex activities and the associated improvements of respiratory efficiency, ATP formation and reduced superoxide formation. However, an additional effect that contributes to ETC well functioning concerns the upregulation of subunits of ETC complexes by melatonin. This was first shown for the subunits 1–3 of Complex IV [88]. Similar data were obtained in another study for the subunits 1 and 3, as well as upregulation of two Complex I subunits, ND1 and ND4 [124]. Moreover, melatonin normalized the expression levels of the Complex I subunits ND1, ND2, ND4 and ND4L in ob/ob mice, which, in the untreated state, exhibit strongly reduced levels of these proteins and low activities of all ETC complexes because of enhanced oxidative and nitrosative stress originating from the visceral fat [125]. Interestingly, all four subunits mentioned are encoded by mitochondrial DNA. Additional experiments from the same study showed that various isolated subunits exposed to peroxynitrite were damaged, but protection by melatonin required very high concentrations (0.5–3 mM), according to the strong challenge. Therefore, these results only underline the damaging effects of oxidative and nitrosative stress and melatonin’s potential of eliminating peroxynitrite-derived free radicals, but do not reflect physiological or even pathophysiological conditions.
In summary, protection from damage by ROS and RNS and effects on expression levels of mitochondrial complex subunits by melatonin seem to jointly contribute to the preservation of ETC integrity and to support electron flux.
Reports on mitochondrial binding sites of melatonin
Although melatonin may enter mitochondria by virtue of its amphiphilicity, this property which is believed to allow the molecule to cross membranes should not suffice to explain the observed mitochondrial accumulation [2–5]. The lipophilicity of melatonin is not high enough to explain an accumulation by uptake into mitochondrial membranes. Otherwise, one would also observe a melatonin enrichment in other membranes, which may not be entirely ruled out, but is obviously not substantial. The alternative is sequestration of melatonin by proteins, a suggestion that has also been forwarded for explaining the poor release of melatonin from extrapineal sites of synthesis [126]. However, with regard to the relatively high quantities that can accumulate in mitochondria, these sequestering proteins should not be expected to represent receptors, but rather factors with low-affinity binding sites. A further low-affinity binding site will be discussed in the subsequent section. The uptake of melatonin by mitochondria has been recently reported to be mediated by the oligopeptide transporters PEPT1/2 [127]. Whether their activities are sufficient for explaining the accumulation or whether additional intramitochondrial sequestration is required remains to be studied. Moreover, their presence and abundance in cells different from the cancer cell lines studied in that investigation would be of particular interest.
High-affinity binding sites, including those with receptor properties, seem to exist, too. Pigeon brain mitochondria were shown to bind [125I]iodomelatonin with high affinity, which was displaced by melatonin and structurally related indoles, and to be loaded with 39% of the total cellular radioligand [128]. In a study on effects of MPP+ in isolated rat liver mitochondria, authors concluded that melatonin physically interacts with Complex I [129]. These findings would be in accordance with other results on the existence of a high-affinity binding site in the amphipathic ramp of Complex I, close to the iron–sulfur cluster N2. The dissociation constant was determined to be 150 pM, at a total number of specific binding sites of 30 fmol/mg protein. In rat brain preparations, about 42% of iodomelatonin binding were attributed to mitochondria. These findings including the criteria for localization were cited a couple of times [11, 130, 131], but the original data have not been published by the investigators. More recently, the MT1 receptor was reported to be present in mouse brain mitochondria [132]. It would be of greatest interest to know whether the mitochondrial MT1 is identical with the aforementioned binding site, how it is targeted to mitochondria and by which protein modification, which may also modulate the binding properties. However, there are substantial problems in relating MT1 to a binding site in the amphipathic ramp. As an integral membrane protein containing seven transmembrane domains, MT1 may be too distant from this Complex I intrusion into the matrix. Moreover, as will be discussed below, the melatonin binding site of MT1 may be on the other face of the inner membrane. Either MT1 may be different from the assumed binding site in the amphipathic ramp or the criteria for localizing the binding site, which are based on displacement by known ligands that bind close to N2, have been misleading.
The most important unsettled questions related to a mitochondrial role of MT1 concern the orientation of the receptor and its signal transduction. Although substantial quantities of MT1 were clearly present in the mitochondrial fraction, contrary to MT2 [132], it has not been directly clarified whether agonist binding occurs from the matrix side or from the intermembrane space and, respectively, where, on the other face of the membrane, the C terminus is located and G protein interaction takes place. This would have profound consequences for the subsequent steps of G protein-dependent signaling. It has also remained unclear whether a mitochondrial MT1 receptor acts via the classic signal transduction pathways or, via other G protein variants, on nonclassic effector proteins. Although these problems have not yet been directly investigated, a potentially important hint may come from a recent study on the mitochondrial type 1 cannabinoid receptor (mtCB1) [133]. In this investigation, the mtCB1 receptor was shown to interact with an intramitochondrial Gαi protein that inhibits the soluble adenylyl cyclase (sAC) in the matrix. The mitochondrial sAC is known to be activated by CO2/HCO3 −, a process by which enhanced activity of the citric acid cycle stimulates electron throughput and, thus, adapts respiration in the ETC [134] (Fig. 2). The further signaling steps in this compartment comprise activation of protein kinase A (PKA) by cAMP binding, and phosphorylation of subunits of, at least, Complex I and Complex IV [133, 135]. In the case of mtCB1 activation, the reduced phosphorylation of the Complex I subunit NDUFS2 was concluded to cause a decrease in electron flux and respiration [133]. This subunit is, notably, located at the amphipathic ramp. In other studies, phosphorylation of NDUSF4 was reported to be critical to Complex I activity [135]. If these findings can be translated to MT1, this could mean that melatonin may enter the receptor from the intermembrane space and lead, via a mitochondrial variant of its classic signaling pathway, to Gαi-mediated sAC inhibition, reduction of cAMP and PKA activity in the matrix, with the consequence of decreased phosphorylation of Complex I subunit(s) and, finally, electron entry at Complex I into the ETC. This reduction of electron throughput would avoid electron overflow at possible bottlenecks at Complexes III and, perhaps, IV and, therefore, decrease superoxide formation by the ETC.
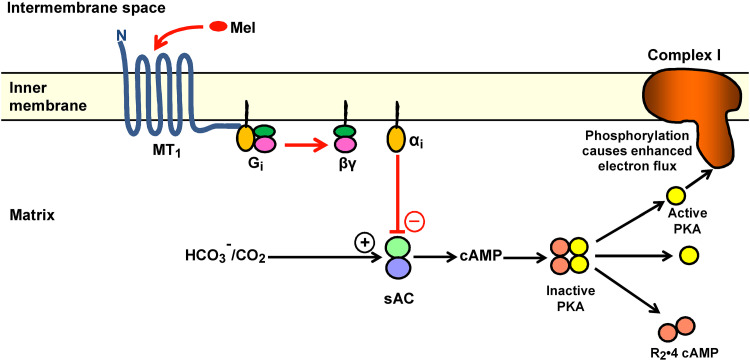
Fig. 2.
A model proposing an MT1-mediated action of melatonin that leads to reduced electron flux by decreasing phosphorylation at the amphipathic ramp of Complex I. The model has been designed by adapting a recently demonstrated corresponding action of the cannabinoid receptor (mtCB1) [134]. If the analogy to mtCB1 is correct, the C terminus of MT1 should be on the matrix side of the inner membrane, to allow interaction with the Gi protein and inhibition of sAC by the αi subunit. As a consequence, melatonin should bind to MT1 from the side of the intermembrane space. The presence of melatonin in the intermembrane compartment would also be well compatible with its actions on the mtPTP. Black arrows enhancement of Complex I activity via HCO3 −/CO2-mediated activation of sAC; red arrows inhibitory pathway of melatonin. α i inhibitory subunit of Gi protein, βγ dissociated dimer of G protein, cAMP cyclic adenosine 3′,5′-monophosphate, G i inhibitory G protein, Mel melatonin, MT 1 melatonin receptor 1, N amino terminus, PKA protein kinase A, R 2 dimer of regulatory PKA subunits, sAC soluble adenylyl cyclase
Although these considerations require experimental confirmation, they would conform to observations on mitochondrial actions of melatonin. In particular, a decreased electron flux at Complex I would presumably avoid ETC bottlenecks and reduce electron leakage [31]. Although further details on intramitochondrial sAC/PKA signaling remain to be clarified, it should be noted that this pathway has been related, in recent studies, to other aspects relevant to melatonin’s mitochondrial actions, such as ROS generation, calcium accumulation, permeability transition, cell death, and effects of sepsis [134, 136–138].
Melatonin and mitochondrial permeability transition
In the understanding of many researchers, the breakdown of the mitochondrial membrane potential (ΔΨmt) is indicative of a profound loss of mitochondrial functioning that may either induce apoptotic cell death or mitophagy. Although these consequences are a frequent reality under experimental conditions, the sudden decrease of ΔΨmt does not necessarily initiate such drastic processes, but rather seems to be a frequent event under unchallenged conditions. This became apparent by the discovery of the so-called superoxide flashes [122, 123], events in which the ΔΨmt breakdown is associated with a massive release of O2·−. It actually seems that duration and frequency of the opening of the mitochondrial permeability transition pore (mtPTP) are decisive and that openings of short duration do not lead to apoptosis. As discussed elsewhere [15, 32], a transient ΔΨmt breakdown may be, instead, favorable because it allows the release of Ca2+ from the mitochondrial matrix, thereby avoiding mitochondrial Ca2+ overload that would initiate cell death. Moreover, the high amounts of O2·− entering the intermembrane space are presumably readily detoxified by the superoxide-activated Cu,Zn-superoxide dismutase present in this compartment.
However, prolonged or overcritically frequent mtPTP openings do induce apoptosis. In the heart, the frequency of superoxide flashes has been shown to be increased by ischemia/reperfusion [123] and corresponding changes should be expected to occur under other conditions of oxidative stress, too. Interestingly, melatonin interferes with the processes related to permeability transition in several ways. Melatonin was reported to activate Cu,Zn-SOD in the intermembrane space by a mechanism involving a mitochondrial cytochrome P450 subform [14]. This response is certainly relevant to the detoxification of O2·− entering by electron leakage via Complex III. It may contribute to O2·− elimination upon permeability transition, although Cu,Zn-SOD activation by O2·− should prevail under these conditions. Moreover, melatonin was shown to directly inhibit mtPTP opening, at an IC50 of 0.8 µM [139]. The relatively high concentrations of melatonin required for this effect can be attained using high pharmacological doses. Whether or not physiological accumulation of melatonin in mitochondria, as discussed above, may also suffice is still uncertain because the site of melatonin binding to the mtPTP or to an adjacent regulatory protein is still unknown. In this context, it would be important to identify the compartment in which melatonin is enriched. If melatonin accumulates in the matrix, an eventual mtPTP-related binding site accessible from the intermembrane space may not be saturated by physiological levels, whereas this may be possible with localization at the matrix side. Another effect of melatonin seems to be of potentially high relevance, namely, that by reducing the duration of permeability transition, an effect observed in astrocytes [140]. In this study, melatonin was shown to not prevent mtPTP opening, but to reduce its duration, thereby keeping the permeability transition below the threshold for inducing apoptosis. Again, the dose dependence may be critically judged, since melatonin was used at a concentration of 100 µM. This high level was necessary to overcome an ionomycin-mediated Ca2+ overload. Therefore, future studies should identify the dose-dependent efficacy of melatonin under less challenging conditions. Nevertheless, the principle of action that discriminates between transient and long-lasting or even permanent ΔΨmt depolarizations may be of fundamental importance and should not only be seen under the aspect of Ca2+ distribution, but also under that of O2·− release.
Conclusion
Various different actions of melatonin have been shown to support the electron flux through the ETC, by preventing or, at least, reducing the occurrence of bottlenecks that cause electron leakage. This concerns especially blockades by nitrosation, nitration and oxidation, in which RNS such as ·NO and peroxynitrite are strongly involved. Formation of O2·− by electron transfer can happen in several different ways. Electron leakage at Complexes I or III can be caused by local malfunction due to protein modification or ·NO binding to irons. Moreover, electron backflow towards Complex I may happen when the flux is hindered by a bottleneck at Complex III, which may also occur when electrons are entering the ETC excessively via Complex II. To what extent this latter possibility takes place under physiological conditions may require further experimental analysis, but is observed in isolated mitochondria energized in an unbalanced way via Complex II relative to Complex I [31]. High amounts of O2·− are released in superoxide flashes that occur regularly under physiological conditions, but seem to be more frequent in oxidative stress. The opening of the mtPTP remains relatively harmless if it remains temporally restricted. As outlined above, melatonin can protect the ETC and support electron flux by various mechanisms, from reducing RNS formation, de novo synthesis of subunits of ETC complexes and antioxidant actions to the prevention of a long-lasting ΔΨmt breakdown. These latter observations may be of particular importance, but would require further clarification concerning the efficacy of physiologically possible melatonin levels in mitochondria. A further demand for experimental analysis seems appropriate in the case of cardiolipin peroxidation. Although its crucial role seems to be well founded in the processes of apoptosis induction [42–44] or initiation of mitophagy [103], in which the reaction is catalyzed by the cardiolipin peroxidase activity of cytochrome c, the likely possibility of low-rate cardiolipin peroxidation by usual lipid peroxidation mechanisms involving ROS and ROS-induced alkyl or alkoxyl radicals should not be left out of sight. This might contribute to peroxidation rates below threshold for apoptosis or mitophagy, but carry the potential of disturbing the electron flux because of cardiolipin’s importance for the structural and, thus, functional integrity of Complexes III and IV [38–41]. Melatonin was shown to reduce cardiolipin peroxidation, whereas a direct inhibitory effect on cardiolipin peroxidase has not yet been demonstrated. Therefore, the additional possibility of cardiolipin protection by other known antioxidant actions of melatonin should still be taken into consideration, especially in the context of aging and low-grade inflammation. A further gap to be closed concerns the detoxification of intramitochondrial H2O2, which might also be influenced by melatonin. This would require measurements, at the activity level, of peroxiredoxin-3 as well as its regulators thioredoxin-2 and thioredoxin reductase-2.
Another area that deserves further clarification concerns the role of melatonin metabolites. Apart from the fact that several of them display antioxidant properties [77, 80, 81], their relationship to ·NO formation and metabolism deserves particular attention. This concerns especially AMK, which is not only a potent scavenger of peroxyl and carbonate radicals [79, 83] as well as singlet oxygen [89], but also of ·NO [37, 86, 87], its redox congeners [87] and nitrating radical mixtures [37]. Moreover, its properties as an extremely potent inhibitor of nNOS [57, 58] and downregulator of iNOS including its mitochondrial subform [50] seem to be, under experimental conditions, a major cause of mitochondrial protection that has been already described [50, 88]. In this context, the major question is that of the physiologically occurring quantities of AMK. These potentially important data are still missing. Relatively unsuccessful determinations in the circulation and some tissues may not yet be fully conclusive, since possibilities of AFMK formation and accumulation in mitochondria have not been taken into consideration. Mitochondrial AFMK production [82] and AFMK accumulation under inflammatory conditions [84] might give rise to substantial AMK levels, but this remains to be demonstrated.
Finally, the regulatory role of melatonin on electron flux, especially by modulation of Complex I activity, requires future attention and elaboration. It would be important to distinguish between melatonin binding at the amphipathic ramp of Complex I, as previously assumed [11, 131, 132], and the role of mitochondrial MT1 [133]. As discussed above, Complex I activity may be regulated by a mitochondrial variant of the classic signaling pathway consisting of Gαi binding, inhibition of sAC, decreased cAMP and reduced PKA activity. This pathway including the control of Complex I has been shown to exist [134, 135], but to date not as originating from MT1 but rather mtCB1 [134] activation.
References
- 1.Lane MN. Mitochondrial disease: powerhouse of disease. Nature. 2006;440:600–602. doi: 10.1038/440600a. [DOI] [PubMed] [Google Scholar]
- 2.Messner M, Hardeland R, Rodenbeck A, Huether G. Tissue retention and subcellular distribution of continuously infused melatonin in rats under near physiological conditions. J Pineal Res. 1998;25:251–259. doi: 10.1111/j.1600-079X.1998.tb00395.x. [DOI] [PubMed] [Google Scholar]
- 3.López A, García JA, Escames G, Venegas C, Ortíz F, López LC, Acuña-Castroviejo D. Melatonin protects the mitochondria from oxidative damage reducing oxygen consumption, membrane potential, and superoxide anion production. J Pineal Res. 2009;46:188–198. doi: 10.1111/j.1600-079X.2008.00647.x. [DOI] [PubMed] [Google Scholar]
- 4.Paradies G, Petrosillo G, Paradies V, Reiter RJ, Ruggiero FM. Melatonin, cardiolipin and mitochondrial bioenergetics in health and disease. J Pineal Res. 2010;48:297–310. doi: 10.1111/j.1600-079X.2010.00759.x. [DOI] [PubMed] [Google Scholar]
- 5.Venegas C, García JA, Escames G, Ortiz F, López A, Doerrier C, García-Corzo L, López LC, Reiter RJ, Acuña-Castroviejo D. Extrapineal melatonin: analysis of its subcellular distribution and daily fluctuations. J Pineal Res. 2012;52:217–227. doi: 10.1111/j.1600-079X.2011.00931.x. [DOI] [PubMed] [Google Scholar]
- 6.Hardeland R. Melatonin in aging and disease—multiple consequences of reduced secretion, options and limits of treatment. Aging Dis. 2012;3:194–225. [PMC free article] [PubMed] [Google Scholar]
- 7.Hardeland R. Neurobiology, pathophysiology, and treatment of melatonin deficiency and dysfunction. ScientificWorldJournal. 2012;2012:640389. doi: 10.1100/2012/640389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Choksi KB, Boylston WH, Rabek JP, Widger WR, Papaconstantinou J. Oxidatively damaged proteins of heart mitochondrial electron transport complexes. Biochim Biophys Acta. 2004;1688:95–101. doi: 10.1016/j.bbadis.2003.11.007. [DOI] [PubMed] [Google Scholar]
- 9.Miwa S, Brand MD. The topology of superoxide production by complex III and glycerol 3-phosphate dehydrogenase in Drosophila mitochondria. Biochim Biophys Acta. 2005;1709:214–219. doi: 10.1016/j.bbabio.2005.08.003. [DOI] [PubMed] [Google Scholar]
- 10.Panee J, Liu W, Nakamura K, Berry MJ. The responses of HT22 cells to the blockade of mitochondrial complexes and potential protective effect of selenium supplementation. Int J Biol Sci. 2007;3:335–341. doi: 10.7150/ijbs.3.335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hardeland R, Poeggeler B, Pappolla MA. Mitochondrial actions of melatonin—an endeavor to identify their adaptive and cytoprotective mechanisms. Proc Saxon Acad Sci. 2009;65(Pt 3):14–31. [Google Scholar]
- 12.Staniek K, Gille L, Kozlov AV, Nohl H. Mitochondrial superoxide radical formation is controlled by electron bifurcation to the high and low potential pathways. Free Radic Res. 2002;36:381–387. doi: 10.1080/10715760290021225. [DOI] [PubMed] [Google Scholar]
- 13.Gong X, Yu L, Xia D, Yu CA. Evidence for electron equilibrium between the two hemes bL in the dimeric cytochrome bc1 complex. J Biol Chem. 2005;280:9251–9257. doi: 10.1074/jbc.M409994200. [DOI] [PubMed] [Google Scholar]
- 14.Iñarrea P, Casanova A, Alava MA, Iturralde M, Cadenas E. Melatonin and steroid hormones activate Cu, Zn-superoxide dismutase by means of mitochondrial cytochrome P450. Free Radic Biol Med. 2011;50:1575–1581. doi: 10.1016/j.freeradbiomed.2011.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hardeland R. Melatonin and the theories of aging: a critical appraisal of melatonin’s role in antiaging mechanisms. J Pineal Res. 2013;55:325–356. doi: 10.1111/jpi.12090. [DOI] [PubMed] [Google Scholar]
- 16.Genova ML, Ventura B, Giuliano G, Bovina C, Formiggini G, Parenti Castelli G, Lenaz G. The site of production of superoxide radical in mitochondrial Complex I is not a bound ubisemiquinone but presumably iron-sulfur cluster N2. FEBS Lett. 2001;505:364–368. doi: 10.1016/S0014-5793(01)02850-2. [DOI] [PubMed] [Google Scholar]
- 17.Lenaz G, Bovina C, D’Aurelio M, Fato R, Formiggini G, Genova ML, Giuliano G, Merlo Pich M, Paolucci U, Parenti Castelli G, Ventura B. Role of mitochondria in oxidative stress and aging. Ann NY Acad Sci. 2002;959:199–213. doi: 10.1111/j.1749-6632.2002.tb02094.x. [DOI] [PubMed] [Google Scholar]
- 18.Genova ML, Merlo Pich M, Bernacchia A, Bianchi C, Biondi A, Bovina C, Falasca AI, Formiggini G, Parenti Castelli G, Lenaz G. The mitochondrial production of reactive oxygen species in relation to aging and pathology. Ann NY Acad Sci. 2004;1011:86–100. doi: 10.1196/annals.1293.010. [DOI] [PubMed] [Google Scholar]
- 19.Ohnishi ST, Ohnishi T, Muranaka S, Fujita H, Kimura H, Uemura K, Yoshida K, Utsumi K. A possible site of superoxide generation in the complex I segment of rat heart mitochondria. J Bioenerg Biomembr. 2005;37:1–15. doi: 10.1007/s10863-005-4117-y. [DOI] [PubMed] [Google Scholar]
- 20.Lenaz G, Fato R, Genova ML, Bergamini C, Bianchi C, Biondi A. Mitochondrial complex I: structural and functional aspects. Biochim Biophys Acta. 2006;1757:1406–1420. doi: 10.1016/j.bbabio.2006.05.007. [DOI] [PubMed] [Google Scholar]
- 21.Bedard K, Krause K-H. The NOX family of ROS-generating NADPH oxidases: physiology and pathophysiology. Physiol Rev. 2007;87:245–313. doi: 10.1152/physrev.00044.2005. [DOI] [PubMed] [Google Scholar]
- 22.Ma MW, Wang J, Zhang Q, Wang R, Dhandapani KM, Vadlamudi RK, Brann DW. NADPH oxidase in brain injury and neurodegenerative disorders. Mol Neurodegener. 2017;12:7. doi: 10.1186/s13024-017-0150-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Block K, Gorin Y, Abboud HE. Subcellular localization of Nox4 and regulation in diabetes. Proc Natl Acad Sci USA. 2009;106:14385–14390. doi: 10.1073/pnas.0906805106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kozieł R, Pircher H, Kratochwil M, Lener B, Hermann M, Dencher NA, Jansen-Dürr P. Mitochondrial respiratory chain complex I is inactivated by NADPH oxidase Nox4. Biochem J. 2013;452:231–239. doi: 10.1042/BJ20121778. [DOI] [PubMed] [Google Scholar]
- 25.Wolin MS. Evidence for novel aspects of Nox4 oxidase regulation of mitochondrial function and peroxide generation in an endothelial cell model of senescence. Biochem J. 2013;452:e1–e2. doi: 10.1042/BJ20130484. [DOI] [PubMed] [Google Scholar]
- 26.Hirschhäuser C, Bornbaum J, Reis A, Böhme S, Kaludercic N, Menabò R, Di Lisa F, Boengler K, Shah AM, Schulz R, Schmidt HH. NOX4 in mitochondria: yeast two-hybrid-based interaction with complex I without relevance for basal reactive oxygen species? Antioxid Redox Signal. 2015;23:1106–1112. doi: 10.1089/ars.2014.6238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Asensio-López MC, Soler F, Sánchez-Más J, Pascual-Figal D, Fernández-Belda F, Lax A. Early oxidative damage induced by doxorubicin: source of production, protection by GKT137831 and effect on Ca2+ transporters in HL-1 cardiomyocytes. Arch Biochem Biophys. 2016;594:26–36. doi: 10.1016/j.abb.2016.02.021. [DOI] [PubMed] [Google Scholar]
- 28.Forred BJ, Daugaard DR, Titus BK, Wood RR, Floen MJ, Booze ML, Vitiello PF. Detoxification of mitochondrial oxidants and apoptotic signaling are facilitated by thioredoxin-2 and peroxiredoxin-3 during hyperoxic injury. PLoS One. 2017;12:e0168777. doi: 10.1371/journal.pone.0168777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Huang Q, Zhou HJ, Zhang H, Huang Y, Hinojosa-Kirschenbaum F, Fan P, Yao L, Belardinelli L, Tellides G, Giordano FJ, Budas GR, Min W. Thioredoxin-2 inhibits mitochondrial reactive oxygen species generation and apoptosis stress kinase-1 activity to maintain cardiac function. Circulation. 2015;131:1082–1897. doi: 10.1161/CIRCULATIONAHA.114.012725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pober JS, Min W, Bradley JR. Mechanisms of endothelial dysfunction, injury, and death. Annu Rev Pathol. 2009;4:71–95. doi: 10.1146/annurev.pathol.4.110807.092155. [DOI] [PubMed] [Google Scholar]
- 31.Hardeland R. Melatonin, mitochondrial electron flux and leakage: recent findings and resolution of contradictory results. Adv Stud Biol. 2009;1:207–230. [Google Scholar]
- 32.Hardeland R, Cardinali DP, Brown GM, Pandi-Perumal SR. Melatonin and brain inflammaging. Prog Neurobiol. 2015;127–128:46–63. doi: 10.1016/j.pneurobio.2015.02.001. [DOI] [PubMed] [Google Scholar]
- 33.Brown GC, Bal-Price A. Inflammatory neurodegeneration mediated by nitric oxide, glutamate, and mitochondria. Mol Neurobiol. 2003;27:325–355. doi: 10.1385/MN:27:3:325. [DOI] [PubMed] [Google Scholar]
- 34.Dahm CC, Moore K, Murphy MP. Persistent S-nitrosation of complex I and other mitochondrial membrane proteins by S-nitrosothiols but not nitric oxide or peroxynitrite: implications for the interaction of nitric oxide with mitochondria. J Biol Chem. 2006;281:10056–10065. doi: 10.1074/jbc.M512203200. [DOI] [PubMed] [Google Scholar]
- 35.Hardeland R. Neuroprotection by radical avoidance: search for suitable agents. Molecules. 2009;14:5054–5102. doi: 10.3390/molecules14125054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hardeland R, Coto-Montes A. New vistas on oxidative damage and aging. Open Biol J. 2010;3:39–52. doi: 10.2174/1874196701003010039. [DOI] [Google Scholar]
- 37.Guenther AL, Schmidt SI, Laatsch H, Fotso S, Ness H, Ressmeyer A-R, Poeggeler B, Hardeland R. Reactions of the melatonin metabolite AMK (N 1-acetyl-5-methoxykynuramine) with reactive nitrogen species: formation of novel compounds, 3-acetamidomethyl-6-methoxycinnolinone and 3-nitro-AMK. J Pineal Res. 2005;39:251–260. doi: 10.1111/j.1600-079X.2005.00242.x. [DOI] [PubMed] [Google Scholar]
- 38.Klingen AR, Palsdottir H, Hunte C, Ullmann GM. Redox-linked protonation state changes in cytochrome bc1 identified by Poisson-Boltzmann electrostatics calculations. Biochim Biophys Acta. 2007;1767:204–221. doi: 10.1016/j.bbabio.2007.01.016. [DOI] [PubMed] [Google Scholar]
- 39.Lesnefsky EJ, Hoppel CL. Cardiolipin as an oxidative target in cardiac mitochondria in the aged rat. Biochim Biophys Acta. 2008;1777:1020–1027. doi: 10.1016/j.bbabio.2008.05.444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lesnefsky EJ, Minkler P, Hoppel CL. Enhanced modification of cardiolipin during ischemia in the aged heart. J Mol Cell Cardiol. 2009;46:1008–1015. doi: 10.1016/j.yjmcc.2009.03.007. [DOI] [PubMed] [Google Scholar]
- 41.Wenz T, Hielscher R, Hellwig P, Schägger H, Richers S, Hunte C. Role of phospholipids in respiratory cytochrome bc1 complex catalysis and supercomplex formation. Biochim Biophys Acta. 2009;1787:609–616. doi: 10.1016/j.bbabio.2009.02.012. [DOI] [PubMed] [Google Scholar]
- 42.Basova LV, Kurnikov IV, Wang L, Ritov VB, Belikova NA, Vlasova II, Pacheco AA, Winnica DE, Peterson J, Bayir H, Waldeck DH, Kagan VE. Cardiolipin switch in mitochondria: shutting off the reduction of cytochrome c and turning on the peroxidase activity. Biochemistry. 2007;46:3423–3434. doi: 10.1021/bi061854k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kagan VE, Bayir A, Bayir H, Stoyanovsky D, Borisenko GG, Tyurina YY, Wipf P, Atkinson J, Greenberger JS, Chapkin RS, Belikova NA. Mitochondria-targeted disruptors and inhibitors of cytochrome c/cardiolipin peroxidase complexes: a new strategy in anti-apoptotic drug discovery. Mol Nutr Food Res. 2009;53:104–114. doi: 10.1002/mnfr.200700402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kagan VE, Bayir HA, Belikova NA, Kapralov O, Tyurina YY, Tyurin VA, Jiang J, Stoyanovski DA, Wipf P, Kochanek PM, Greenberger JS, Pitt B, Shvedova AA, Borisenko G. Cytochrome c/cardiolipin relations in mitochondria: a kiss of death. Free Radic Biol Med. 2009;46:1439–1453. doi: 10.1016/j.freeradbiomed.2009.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zoccarato F, Cavallini L, Alexandre A. Succinate is the controller of O2 −/H2O2 release at mitochondrial complex I: negative modulation by malate, positive by cyanide. J Bioenerg Biomembr. 2009;41:387–393. doi: 10.1007/s10863-009-9238-2. [DOI] [PubMed] [Google Scholar]
- 46.Kim SC, Sprung R, Chen Y, Xu Y, Ball H, Pei J, Cheng T, Kho Y, Xiao H, Xiao L, Grishin NV, White M, Yang XJ, Zhao Y. Substrate and functional diversity of lysine acetylation revealed by a proteomics survey. Mol Cell. 2006;23:607–618. doi: 10.1016/j.molcel.2006.06.026. [DOI] [PubMed] [Google Scholar]
- 47.Schlicker C, Gertz M, Papatheodorou P, Kachholz B, Becker CF, Steegborn C. Substrates and regulation mechanisms for the human mitochondrial sirtuins Sirt3 and Sirt5. J Mol Biol. 2008;382:790–801. doi: 10.1016/j.jmb.2008.07.048. [DOI] [PubMed] [Google Scholar]
- 48.López LC, Escames G, Tapias V, Utrilla P, León J, Acuña-Castroviejo D. Identification of an inducible nitric oxide synthase in diaphragm mitochondria from septic mice: its relation with mitochondrial dysfunction and prevention by melatonin. Int J Biochem Cell Biol. 2006;38:267–278. doi: 10.1016/j.biocel.2005.09.008. [DOI] [PubMed] [Google Scholar]
- 49.Escames G, López LC, Ortíz F, López A, García JA, Ros E, Acuña-Castroviejo D. Attenuation of cardiac mitochondrial dysfunction by melatonin in septic mice. FEBS J. 2007;274:2135–2147. doi: 10.1111/j.1742-4658.2007.05755.x. [DOI] [PubMed] [Google Scholar]
- 50.Tapias V, Escames G, López LC, López A, Camacho E, Carrión MD, Entrena A, Gallo MA, Espinosa A, Acuña-Castroviejo D. Melatonin and its brain metabolite N 1-acetyl-5-methoxykynuramine prevent mitochondrial nitric oxide synthase induction in parkinsonian mice. J Neurosci Res. 2009;87:3002–3010. doi: 10.1002/jnr.22123. [DOI] [PubMed] [Google Scholar]
- 51.Ortiz F, García JA, Acuña-Castroviejo D, Doerrier C, López A, Venegas C, Volt H, Luna-Sánchez M, López LC, Escames G. The beneficial effects of melatonin against heart mitochondrial impairment during sepsis: inhibition of iNOS and preservation of nNOS. J Pineal Res. 2014;56:71–81. doi: 10.1111/jpi.12099. [DOI] [PubMed] [Google Scholar]
- 52.García JA, Ortiz F, Miana J, Doerrier C, Fernández-Ortiz M, Rusanova I, Escames G, García JJ, Acuña-Castroviejo D. Contribution of inducible and neuronal nitric oxide synthases to mitochondrial damage and melatonin rescue in LPS-treated mice. J Physiol Biochem. 2017 doi: 10.1007/s13105-017-0548-2. [DOI] [PubMed] [Google Scholar]
- 53.León J, Vives F, Crespo E, Camacho E, Espinosa A, Gallo MA, Escames G, Acuña-Castroviejo D. Modification of nitric oxide synthase activity and neuronal response in rat striatum by melatonin and kynurenine derivatives. J Neuroendocrinol. 1998;10:297–302. doi: 10.1046/j.1365-2826.1998.00203.x. [DOI] [PubMed] [Google Scholar]
- 54.León J, Macías M, Escames G, Camacho E, Khaldy H, Martín M, Espinosa A, Gallo MA, Acuña-Castroviejo D. Structure-related inhibition of calmodulin-dependent neuronal nitric-oxide synthase activity by melatonin and synthetic kynurenines. Mol Pharmacol. 2000;58:967–975. doi: 10.1124/mol.58.5.967. [DOI] [PubMed] [Google Scholar]
- 55.Chang HM, Ling EA, Chen CF, Lue H, Wen CY, Shieh JY. Melatonin attenuates the neuronal NADPH-d/NOS expression in the nodose ganglion of acute hypoxic rats. J Pineal Res. 2002;32:65–73. doi: 10.1034/j.1600-079x.2002.1816.x. [DOI] [PubMed] [Google Scholar]
- 56.Acuña-Castroviejo D, Escames G, López LC, Hitos AB, León J. Melatonin and nitric oxide: two required antagonists for mitochondrial homeostasis. Endocrine. 2005;27:159–168. doi: 10.1385/ENDO:27:2:159. [DOI] [PubMed] [Google Scholar]
- 57.Entrena A, Camacho ME, Carrión MD, López-Cara LC, Velasco G, León J, Escames G, Acuña-Castroviejo D, Tapias V, Gallo MA, Vivo A, Espinosa A. Kynurenamines as neural nitric oxide synthase inhibitors. J Med Chem. 2005;48:8174–8181. doi: 10.1021/jm050740o. [DOI] [PubMed] [Google Scholar]
- 58.Jiménez-Ortega V, Cano P, Cardinali DP, Esquifino AI. 24-Hour variation in gene expression of redox pathway enzymes in rat hypothalamus: effect of melatonin treatment. Redox Rep. 2009;14:132–138. doi: 10.1179/135100009X392548. [DOI] [PubMed] [Google Scholar]
- 59.Tjong YW, Li MF, Hung MW, Fung ML. Melatonin ameliorates hippocampal nitric oxide production and large conductance calcium-activated potassium channel activity in chronic intermittent hypoxia. J Pineal Res. 2008;44:234–243. doi: 10.1111/j.1600-079X.2007.00515.x. [DOI] [PubMed] [Google Scholar]
- 60.Escames G, Acuña-Castroviejo D, López LC, Tan D-X, Maldonado MD, Sánchez-Hidalgo M, León J, Reiter RJ. Pharmacological utility of melatonin in the treatment of septic shock: experimental and clinical evidence. J Pharm Pharmacol. 2006;58:1153–1165. doi: 10.1211/jpp.58.9.0001. [DOI] [PubMed] [Google Scholar]
- 61.López LC, Escames G, Ortíz F, Ros E, Acuña-Castroviejo D. Melatonin restores the mitochondrial production of ATP in septic mice. Neuroendocrinol Lett. 2006;27:623–630. [PubMed] [Google Scholar]
- 62.Escames G, López LC, Ortíz F, Ros E, Acuña-Castroviejo D. Age-dependent lipopolysaccharide-induced iNOS expression and multiorgan failure in rats: effects of melatonin treatment. Exp Gerontol. 2006;41:1165–1173. doi: 10.1016/j.exger.2006.09.002. [DOI] [PubMed] [Google Scholar]
- 63.Escames G, López LC, Tapias V, Utrilla P, Reiter RJ, Hitos AB, León J, Rodríguez MI, Acuña-Castroviejo D. Melatonin counteracts inducible mitochondrial nitric oxide synthase-dependent mitochondrial dysfunction in skeletal muscle of septic mice. J Pineal Res. 2006;40:71–78. doi: 10.1111/j.1600-079X.2005.00281.x. [DOI] [PubMed] [Google Scholar]
- 64.Deng WG, Tang ST, Tseng HP, Wu KK. Melatonin suppresses macrophage cyclooxygenase-2 and inducible nitric oxide synthase expression by inhibiting p52 acetylation and binding. Blood. 2006;108:518–524. doi: 10.1182/blood-2005-09-3691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Korkmaz A, Rosales-Corral S, Reiter RJ. Gene regulation by melatonin linked to epigenetic phenomena. Gene. 2012;503:1–11. doi: 10.1016/j.gene.2012.04.040. [DOI] [PubMed] [Google Scholar]
- 66.Tocharus J, Chongthammakun S, Govitrapong P. Melatonin inhibits amphetamine-induced nitric oxide synthase mRNA overexpression in microglial cell lines. Neurosci Lett. 2008;439:134–137. doi: 10.1016/j.neulet.2008.05.036. [DOI] [PubMed] [Google Scholar]
- 67.Tocharus J, Khonthun C, Chongthammakun S, Govitrapong P. Melatonin attenuates methamphetamine-induced overexpression of pro-inflammatory cytokines in microglial cell lines. J Pineal Res. 2010;48:347–352. doi: 10.1111/j.1600-079X.2010.00761.x. [DOI] [PubMed] [Google Scholar]
- 68.Hardeland R, Cardinali DP, Srinivasan V, Spence DW, Brown GM, Pandi-Perumal SR. Melatonin—a pleiotropic, orchestrating regulator molecule. Prog Neurobiol. 2011;93:350–384. doi: 10.1016/j.pneurobio.2010.12.004. [DOI] [PubMed] [Google Scholar]
- 69.Campbell A, Sharman E, Bondy SC. Age-related differences in the response of the brain to dietary melatonin. Age (Dordr) 2014;36:49–55. doi: 10.1007/s11357-013-9542-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Hardeland R. Deacceleration of brain aging by melatonin. In: Bondy SC, Campbell A, editors. Inflammation, aging, and oxidative stress. New York: Humana Press; 2016. pp. 345–376. [Google Scholar]
- 71.Srinivasan V, Pandi-Perumal SR, Spence DW, Kato H, Cardinali DP. Melatonin in septic shock: some recent concepts. J Crit Care. 2010;25:656.e1–656.e6. doi: 10.1016/j.jcrc.2010.03.006. [DOI] [PubMed] [Google Scholar]
- 72.Farinelli SE, Park DS, Greene LA. Nitric oxide delays the death of trophic factor-deprived PC12 cells and sympathetic neurons by a cGMP-mediated mechanism. J Neurosci. 1996;16:2325–2334. doi: 10.1523/JNEUROSCI.16-07-02325.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Estévez AG, Spear N, Thompson JA, Cornwell TL, Radi R, Barbeito L, Beckman JS. Nitric oxide-dependent production of cGMP supports the survival of rat embryonic motor neurons cultured with brain-derived neurotrophic factor. J Neurosci. 1998;18:3708–3714. doi: 10.1523/JNEUROSCI.18-10-03708.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Robb SJ, Connor JR. Nitric oxide protects astrocytes from oxidative stress. Ann NY Acad Sci. 2002;962:93–102. doi: 10.1111/j.1749-6632.2002.tb04059.x. [DOI] [PubMed] [Google Scholar]
- 75.Tan D-X, Chen L-D, Poeggeler B, Manchester LC, Reiter RJ. Melatonin: a potent, endogenous hydroxyl radical scavenger. Endocr J. 1993;1:57–60. [Google Scholar]
- 76.Tan D-X, Manchester LC, Reiter RJ, Plummer BF, Hardies LJ, Weintraub ST, Vijayalaxmi Shepherd AM. A novel melatonin metabolite, cyclic 3-hydroxymelatonin: a biomarker of in vivo hydroxyl radical generation. Biochem Biophys Res Commun. 1998;253:614–620. doi: 10.1006/bbrc.1998.9826. [DOI] [PubMed] [Google Scholar]
- 77.Tan D-X, Hardeland R, Manchester LC, Galano A, Reiter RJ. Cyclic-3-hydroxymelatonin (C3HOM), a potent antioxidant, scavenges free radicals and suppresses oxidative reactions. Curr Med Chem. 2014;21:1557–1565. doi: 10.2174/0929867321666131129113146. [DOI] [PubMed] [Google Scholar]
- 78.Tan D-X, Manchester LC, Burkhardt S, Sainz RM, Mayo JC, Kohen R, Shohami E, Huo Y-S, Hardeland R, Reiter RJ. N 1-Acetyl-N 2-formyl-5-methoxykynuramine, a biogenic amine and melatonin metabolite, functions as a potent antioxidant. FASEB J. 2001;15:2294–2296. doi: 10.1096/fj.01-0309fje. [DOI] [PubMed] [Google Scholar]
- 79.Ressmeyer A-R, Mayo JC, Zelosko V, Sáinz RM, Tan D-X, Poeggeler B, Antolín I, Zsizsik BK, Reiter RJ, Hardeland R. Antioxidant properties of the melatonin metabolite N 1-acetyl-5-methoxykynuramine (AMK): scavenging of free radicals and prevention of protein destruction. Redox Rep. 2003;8:205–213. doi: 10.1179/135100003225002709. [DOI] [PubMed] [Google Scholar]
- 80.Hardeland R, Tan D-X, Reiter RJ. Kynuramines, metabolites of melatonin and other indoles: the resurrection of an almost forgotten class of biogenic amines. J Pineal Res. 2009;47:109–126. doi: 10.1111/j.1600-079X.2009.00701.x. [DOI] [PubMed] [Google Scholar]
- 81.Rosen J, Than NN, Koch D, Poeggeler B, Laatsch H, Hardeland R. Interactions of melatonin and its metabolites with the ABTS cation radical: extension of the radical scavenger cascade and formation of a novel class of oxidation products, C2-substituted 3-indolinones. J Pineal Res. 2006;41:374–381. doi: 10.1111/j.1600-079X.2006.00379.x. [DOI] [PubMed] [Google Scholar]
- 82.Semak I, Naumova M, Korik E, Terekhovich V, Wortsman J, Slominski A. A novel metabolic pathway of melatonin: oxidation by cytochrome C. Biochemistry. 2005;44:9300–9307. doi: 10.1021/bi050202d. [DOI] [PubMed] [Google Scholar]
- 83.Hardeland R, Ressmeyer A-R, Zelosko V, Burkhardt S, Poeggeler B. Metabolites of melatonin: Formation and properties of the methoxylated kynuramines AFMK and AMK. In: Haldar C, Singh SS, editors. Recent advances in endocrinology and reproduction: evolutionary, biotechnological and clinical applications. Varanasi: Banaras Hindu University; 2004. pp. 21–38. [Google Scholar]
- 84.Silva SO, Ximenes VF, Livramento JA, Catalani LH, Campa A. High concentrations of the melatonin metabolite, N 1-acetyl-N 2-formyl-5-methoxykynuramine, in cerebrospinal fluid of patients with meningitis: a possible immunomodulatory mechanism. J Pineal Res. 2005;39:302–306. doi: 10.1111/j.1600-079X.2005.00247.x. [DOI] [PubMed] [Google Scholar]
- 85.León J, Escames G, Rodríguez MI, López LC, Tapias V, Entrena A, Camacho E, Carrión MD, Gallo MA, Espinosa A, Tan D-X, Reiter RJ, Acuña-Castroviejo D. Inhibition of neuronal nitric oxide synthase activity by N 1-acetyl-5-methoxykynuramine, a brain metabolite of melatonin. J Neurochem. 2006;98:2023–2033. doi: 10.1111/j.1471-4159.2006.04029.x. [DOI] [PubMed] [Google Scholar]
- 86.Hardeland R, Backhaus C, Fadavi A, Hess M. N 1-acetyl-5-methoxykynuramine contrasts with other tryptophan metabolites by a peculiar type of NO scavenging: cyclization to a cinnolinone prevents formation of unstable nitrosamines. J Pineal Res. 2007;43:104–105. doi: 10.1111/j.1600-079X.2007.00431.x. [DOI] [PubMed] [Google Scholar]
- 87.Hardeland R, Backhaus C, Fadavi A. Reactions of the NO redox forms NO+, ·NO and HNO (protonated NO−) with the melatonin metabolite N 1-acetyl-5-methoxykynuramine (AMK) J Pineal Res. 2007;43:382–388. doi: 10.1111/j.1600-079X.2007.00489.x. [DOI] [PubMed] [Google Scholar]
- 88.Acuña-Castroviejo D, Escames G, León J, Carazo A, Khaldy H. Mitochondrial regulation by melatonin and its metabolites. Adv Exp Med Biol. 2003;527:549–557. doi: 10.1007/978-1-4615-0135-0_63. [DOI] [PubMed] [Google Scholar]
- 89.Schaefer M, Hardeland R. The melatonin metabolite N 1-acetyl-5-methoxykynuramine is a potent singlet oxygen scavenger. J Pineal Res. 2009;46:49–52. doi: 10.1111/j.1600-079X.2008.00614.x. [DOI] [PubMed] [Google Scholar]
- 90.Hardeland R, Niebergall R, Schoenke M, Poeggeler B. Carbonate radicals as initiators of melatonin oxidation: chemiluminescence and formation of oxidation products. In: Hardeland R, editor. Actions and redox properties of melatonin and other aromatic amino acid metabolites. Göttingen: Cuvillier; 2001. pp. 49–55. [Google Scholar]
- 91.Zelosko V, Libau K, Hardeland R. Product analyses reveal rapid and preferential conversion of melatonin to AFMK under the influence of carbonate radicals. In: Hardeland R, editor. Actions and redox properties of melatonin and other aromatic amino acid metabolites. Göttingen: Cuvillier; 2001. pp. 56–57. [Google Scholar]
- 92.Hardeland R, Poeggeler B, Niebergall R, Zelosko V. Oxidation of melatonin by carbonate radicals and chemiluminescence emitted during pyrrole ring cleavage. J Pineal Res. 2003;34:17–25. doi: 10.1034/j.1600-079X.2003.02941.x. [DOI] [PubMed] [Google Scholar]
- 93.Rodriguez MI, Escames G, López LC, García JA, Ortiz F, López A, Acuña-Castroviejo D. Melatonin administration prevents cardiac and diaphragmatic mitochondrial oxidative damage in senescence-accelerated mice. J Endocrinol. 2007;194:637–643. doi: 10.1677/JOE-07-0260. [DOI] [PubMed] [Google Scholar]
- 94.Rodríguez MI, Carretero M, Escames G, López LC, Maldonado MD, Tan DX, Reiter RJ, Acuña-Castroviejo D. Chronic melatonin treatment prevents age-dependent cardiac mitochondrial dysfunction in senescence-accelerated mice. Free Radic Res. 2007;41:15–24. doi: 10.1080/10715760600936359. [DOI] [PubMed] [Google Scholar]
- 95.Rodríguez MI, Escames G, López LC, López A, García JA, Ortiz F, Sánchez V, Romeu M, Acuña-Castroviejo D. Improved mitochondrial function and increased life span after chronic melatonin treatment in senescent prone mice. Exp Gerontol. 2008;43:749–756. doi: 10.1016/j.exger.2008.04.003. [DOI] [PubMed] [Google Scholar]
- 96.Jung KH, Hong SW, Zheng HM, Lee HS, Lee H, Lee DH, Lee SY, Hong SS. Melatonin ameliorates cerulein-induced pancreatitis by the modulation of nuclear erythroid 2-related factor 2 and nuclear factor-kappaB in rats. J Pineal Res. 2010;48:239–250. doi: 10.1111/j.1600-079X.2010.00748.x. [DOI] [PubMed] [Google Scholar]
- 97.García-Macia M, Vega-Naredo I, De Gonzalo-Calvo D, Rodríguez-González SM, Camello PJ, Camello-Almaraz C, Martín-Cano FE, Rodríguez-Colunga MJ, Pozo MJ, Coto-Montes AM. Melatonin induces neural SOD2 expression independent of the NF-kappaB pathway and improves the mitochondrial population and function in old mice. J Pineal Res. 2011;50:54–63. doi: 10.1111/j.1600-079X.2010.00809.x. [DOI] [PubMed] [Google Scholar]
- 98.Chen Y, Qing W, Sun M, Lv L, Guo D, Jiang Y. Melatonin protects hepatocytes against bile acid-induced mitochondrial oxidative stress via the AMPK-SIRT3-SOD2 pathway. Free Radic Res. 2015;49:1275–1284. doi: 10.3109/10715762.2015.1067806. [DOI] [PubMed] [Google Scholar]
- 99.Pi H, Xu S, Reiter RJ, Guo P, Zhang L, Li Y, Li M, Cao Z, Tian L, Xie J, Zhang R, He M, Lu Y, Liu C, Duan W, Yu Z, Zhou Z. SIRT3-SOD2-mROS-dependent autophagy in cadmium-induced hepatotoxicity and salvage by melatonin. Autophagy. 2015;11:1037–1051. doi: 10.1080/15548627.2015.1052208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Remião MH, Lucas CG, Domingues WB, Silveira T, Barther NN, Komninou ER, Basso AC, Jornada DS, Beck RC, Pohlmann AR, Junior AS, Seixas FK, Campos VF, Guterres SS, Collares T. Melatonin delivery by nanocapsules during in vitro bovine oocyte maturation decreased the reactive oxygen species of oocytes and embryos. Reprod Toxicol. 2016;63:70–81. doi: 10.1016/j.reprotox.2016.05.016. [DOI] [PubMed] [Google Scholar]
- 101.Song C, Peng W, Yin S, Zhao J, Fu B, Zhang J, Mao T, Wu H, Zhang Y. Melatonin improves age-induced fertility decline and attenuates ovarian mitochondrial oxidative stress in mice. Sci Rep. 2016;6:35165. doi: 10.1038/srep35165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Yu L, Gong B, Duan W, Fan C, Zhang J, Li Z, Xue X, Xu Y, Meng D, Li B, Zhang M, Zhang Bin, Jin Z, Yu S, Yang Y, Wang H. Melatonin ameliorates myocardial ischemia/reperfusion injury in type 1 diabetic rats by preserving mitochondrial function: role of AMPK-PGC-1α-SIRT3 signaling. Sci Rep. 2017;7:41337. doi: 10.1038/srep41337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Chu CT, Ji J, Dagda RK, Jiang JF, Tyurina YY, Kapralov AA, Tyurin VA, Yanamala N, Shrivastava IH, Mohammadyani D, Qiang Wang KZ, Zhu J, Klein-Seetharaman J, Balasubramanian K, Amoscato AA, Borisenko G, Huang Z, Gusdon AM, Cheikhi A, Steer EK, Wang R, Baty C, Watkins S, Bahar I, Bayir H, Kagan VE. Cardiolipin externalization to the outer mitochondrial membrane acts as an elimination signal for mitophagy in neuronal cells. Nat Cell Biol. 2013;15:1197–1205. doi: 10.1038/ncb2837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Hsiao CW, Peng TI, Peng AC, Reiter RJ, Tanaka M, Lai YK, Jou MJ. Long-term Aβ exposure augments mCa2+-independent mROS-mediated depletion of cardiolipin for the shift of a lethal transient mitochondrial permeability transition to its permanent mode in NARP cybrids: a protective targeting of melatonin. J Pineal Res. 2013;54:107–125. doi: 10.1111/jpi.12004. [DOI] [PubMed] [Google Scholar]
- 105.Petrosillo G, Di Venosa N, Pistolese M, Casanova G, Tiravanti E, Colantuono G, Federici A, Paradies G, Ruggiero FM. Protective effect of melatonin against mitochondrial dysfunction associated with cardiac ischemia-reperfusion: role of cardiolipin. FASEB J. 2006;20:269–276. doi: 10.1096/fj.05-4692com. [DOI] [PubMed] [Google Scholar]
- 106.Luchetti F, Canonico B, Mannello F, Masoni C, D’Emilio A, Battistelli M, Papa S, Falcieri E. Melatonin reduces early changes in intramitochondrial cardiolipin during apoptosis in U937 cell line. Toxicol In Vitro. 2007;21:293–301. doi: 10.1016/j.tiv.2006.08.003. [DOI] [PubMed] [Google Scholar]
- 107.Petrosillo G, Fattoretti P, Matera M, Ruggiero FM, Bertoni-Freddari C, Paradies G. Melatonin prevents age-related mitochondrial dysfunction in rat brain via cardiolipin protection. Rejuvenation Res. 2008;11:935–943. doi: 10.1089/rej.2008.0772. [DOI] [PubMed] [Google Scholar]
- 108.Petrosillo G, De Benedictis V, Ruggiero FM, Paradies G. Decline in cytochrome c oxidase activity in rat-brain mitochondria with aging. Role of peroxidized cardiolipin and beneficial effect of melatonin. J Bioenerg Biomembr. 2013;45:431–440. doi: 10.1007/s10863-013-9505-0. [DOI] [PubMed] [Google Scholar]
- 109.Paradies G, Paradies V, Ruggiero FM, Petrosillo G. Protective role of melatonin in mitochondrial dysfunction and related disorders. Arch Toxicol. 2015;89:923–939. doi: 10.1007/s00204-015-1475-z. [DOI] [PubMed] [Google Scholar]
- 110.Liang H, Ran Q, Jang YC, Holstein D, Lechleiter J, McDonald-Marsh T, Musatov A, Song W, Van Remmen H, Richardson A. Glutathione peroxidase 4 differentially regulates the release of apoptogenic proteins from mitochondria. Free Radic Biol Med. 2009;47:312–320. doi: 10.1016/j.freeradbiomed.2009.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Doerrier C, García JA, Volt H, Díaz-Casado ME, Luna-Sánchez M, Fernández-Gil B, Escames G, López LC, Acuña-Castroviejo D. Permeabilized myocardial fibers as model to detect mitochondrial dysfunction during sepsis and melatonin effects without disruption of mitochondrial network. Mitochondrion. 2016;27:56–63. doi: 10.1016/j.mito.2015.12.010. [DOI] [PubMed] [Google Scholar]
- 112.Escames G, León J, Macías M, Khaldy H, Acuña-Castroviejo D. Melatonin counteracts lipopolysaccharide-induced expression and activity of mitochondrial nitric oxide synthase in rats. FASEB J. 2003;17:932–934. doi: 10.1096/fj.02-0692fje. [DOI] [PubMed] [Google Scholar]
- 113.Yang Y, Jiang S, Dong Y, Fan C, Zhao L, Yang X, Li J, Di S, Yue L, Liang G, Reiter RJ, Qu Y. Melatonin prevents cell death and mitochondrial dysfunction via a SIRT1-dependent mechanism during ischemic-stroke in mice. J Pineal Res. 2015;58:61–70. doi: 10.1111/jpi.12193. [DOI] [PubMed] [Google Scholar]
- 114.Martín M, Macías M, Escames G, Reiter RJ, Agapito MT, Ortiz GG, Acuña-Castroviejo D. Melatonin-induced increased activity of the respiratory chain complexes I and IV can prevent mitochondrial damage induced by ruthenium red in vivo. J Pineal Res. 2000;28:242–248. doi: 10.1034/j.1600-079X.2000.280407.x. [DOI] [PubMed] [Google Scholar]
- 115.Martín M, Macías M, León J, Escames G, Khaldy H, Acuña-Castroviejo D. Melatonin increases the activity of the oxidative phosphorylation enzymes and the production of ATP in rat brain and liver mitochondria. Int J Biochem Cell Biol. 2002;34:348–357. doi: 10.1016/S1357-2725(01)00138-8. [DOI] [PubMed] [Google Scholar]
- 116.Khaldy H, Escames G, León J, Bikjdaouene L, Acuña-Castroviejo D. Synergistic effects of melatonin and deprenyl against MPTP-induced mitochondrial damage and DA depletion. Neurobiol Aging. 2003;24:491–500. doi: 10.1016/S0197-4580(02)00133-1. [DOI] [PubMed] [Google Scholar]
- 117.Okatani Y, Wakatsuki A, Reiter RJ. Melatonin protects hepatic mitochondrial respiratory chain activity in senescence-accelerated mice. J Pineal Res. 2002;32:143–148. doi: 10.1034/j.1600-079x.2002.1o106.x. [DOI] [PubMed] [Google Scholar]
- 118.Okatani Y, Wakatsuki A, Reiter RJ, Miyahara Y. Hepatic mitochondrial dysfunction in senescence-accelerated mice: correction by long-term, orally administered physiological levels of melatonin. J Pineal Res. 2002;33:127–133. doi: 10.1034/j.1600-079X.2002.02109.x. [DOI] [PubMed] [Google Scholar]
- 119.Okatani Y, Wakatsuki A, Reiter RJ, Miyahara Y. Acutely administered melatonin restores hepatic mitochondrial physiology in old mice. Int J Biochem Cell Biol. 2003;35:367–375. doi: 10.1016/S1357-2725(02)00260-1. [DOI] [PubMed] [Google Scholar]
- 120.Carretero M, Escames G, López LC, Venegas C, Dayoub JC, García L, Acuña-Castroviejo D. Long-term melatonin administration protects brain mitochondria from aging. J Pineal Res. 2009;47:192–200. doi: 10.1111/j.1600-079X.2009.00700.x. [DOI] [PubMed] [Google Scholar]
- 121.Acuña-Castroviejo D, Carretero M, Doerrier C, López LC, García-Corzo L, Tresguerres JAF, Escames G. Melatonin protects lung mitochondria from aging. Age (Dordr) 2012;34:681–692. doi: 10.1007/s11357-011-9267-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Wang W, Fang H, Groom L, Cheng A, Zhang W, Liu J, Wang X, Li K, Han P, Zheng M, Yin J, Wang W, Mattson MP, Kao JP, Lakatta EG, Sheu SS, Ouyang K, Chen J, Dirksen RT, Cheng H. Superoxide flashes in single mitochondria. Cell. 2008;134:279–290. doi: 10.1016/j.cell.2008.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Sheu SS, Wang W, Cheng H, Dirksen RT. Superoxide flashes: illuminating new insights into cardiac ischemia/reperfusion injury. Future Cardiol. 2008;4:551–554. doi: 10.2217/14796678.4.6.551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Anisimov VN, Popovich IG, Zabezhinski MA, Anisimov SV, Vesnushkin GM, Vinogradova IA. Melatonin as antioxidant, geroprotector and anticarcinogen. Biochim Biophys Acta. 2006;57:573–589. doi: 10.1016/j.bbabio.2006.03.012. [DOI] [PubMed] [Google Scholar]
- 125.Solís-Muñoz P, Solís-Herruzo JA, Fernández-Moreira D, Gómez-Izquierdo E, García-Consuegra I, Muñoz-Yagüe T, García Ruiz I. Melatonin improves mitochondrial respiratory chain activity and liver morphology in ob/ob mice. J Pineal Res. 2011;51:113–123. doi: 10.1111/j.1600-079X.2011.00868.x. [DOI] [PubMed] [Google Scholar]
- 126.Hardeland R, Poeggeler B. Melatonin beyond its classical functions. Open Physiol J. 2008;1:1–23. doi: 10.2174/1874360900901010001. [DOI] [Google Scholar]
- 127.Huo X, Wang C, Yu Z, Peng Y, Wang S, Feng S, Zhang S, Tian X, Sun C, Liu K, Deng S, Ma X. Human transporters, PEPT1/2, facilitate melatonin transportation into mitochondria of cancer cells: an implication of the therapeutic potential. J Pineal Res. 2017 doi: 10.1111/jpi.12390. [DOI] [PubMed] [Google Scholar]
- 128.Yuan H, Pang SF. [125I]Iodomelatonin-binding sites in the pigeon brain: binding characteristics, regional distribution and diurnal variation. J Endocrinol. 1991;128:475–482. doi: 10.1677/joe.0.1280475. [DOI] [PubMed] [Google Scholar]
- 129.Absi E, Ayala A, Machado A, Parrado J. Protective effect of melatonin against the 1-methyl-4-phenylpyridinium-induced inhibition of complex I of the mitochondrial respiratory chain. J Pineal Res. 2000;29:40–47. doi: 10.1034/j.1600-079X.2000.290106.x. [DOI] [PubMed] [Google Scholar]
- 130.Hardeland R, Poeggeler B. Actions of melatonin, its structural and functional analogs in the central nervous system and the significance of metabolism. Cent Nerv Syst Agents Med Chem. 2007;7:289–303. doi: 10.2174/187152407783220823. [DOI] [Google Scholar]
- 131.Hardeland R, Poeggeler B, Pappolla MA (2008) New vistas on mitochondrial electron flux rates and aging. Cell: comment: http://www.cell.com/content/article/comments?uid=PIIS0092867408000627. Accessed 26 Mar 2008
- 132.Wang X, Sirianni A, Pei Z, Cormier K, Smith K, Jiang J, Zhou S, Wang H, Zhao R, Yano H, Kim JE, Li W, Kristal BS, Ferrante RJ, Friedlander RM. The melatonin MT1 receptor axis modulates mutant Huntingtin-mediated toxicity. J Neurosci. 2011;31:14496–14507. doi: 10.1523/JNEUROSCI.3059-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Hebert-Chatelain E, Desprez T, Serrat R, Bellocchio L, Soria-Gomez E, Busquets-Garcia A, Pagano Zottola AC, Delamarre A, Cannich A, Vincent P, Varilh M, Robin LM, Terral G, García-Fernández MD, Colavita M, Mazier W, Drago F, Puente N, Reguero L, Elezgarai I, Dupuy JW, Cota D, Lopez-Rodriguez ML, Barreda-Gómez G, Massa F, Grandes P, Bénard G, Marsicano G. A cannabinoid link between mitochondria and memory. Nature. 2016;539:555–559. doi: 10.1038/nature20127. [DOI] [PubMed] [Google Scholar]
- 134.Wang Z, Liu D, Varin A, Nicolas V, Courilleau D, Mateo P, Caubere C, Rouet P, Gomez AM, Vandecasteele G, Fischmeister R, Brenner C. A cardiac mitochondrial cAMP signaling pathway regulates calcium accumulation, permeability transition and cell death. Cell Death Dis. 2016;7:e2198. doi: 10.1038/cddis.2016.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Valsecchi F, Konrad C, Manfredi G. Role of soluble adenylyl cyclase in mitochondria. Biochim Biophys Acta. 2014;1842:2555–2560. doi: 10.1016/j.bbadis.2014.05.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Ladilov Y, Appukuttan A. Role of soluble adenylyl cyclase in cell death and growth. Biochim Biophys Acta. 2014;1842:2646–2655. doi: 10.1016/j.bbadis.2014.06.034. [DOI] [PubMed] [Google Scholar]
- 137.Lark DS, Reese LR, Ryan TE, Torres MJ, Smith CD, Lin CT, Neufer PD. Protein kinase A governs oxidative phosphorylation kinetics and oxidant emitting potential at Complex I. Front Physiol. 2015;6:332. doi: 10.3389/fphys.2015.00332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Neviere R, Delguste F, Durand A, Inamo J, Boulanger E, Preau S. Abnormal mitochondrial cAMP/PKA signaling is involved in sepsis-induced mitochondrial and myocardial dysfunction. Int J Mol Sci. 2016;17:2075. doi: 10.3390/ijms17122075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Andrabi SA, Sayeed I, Siemen D, Wolf G, Horn TF. Direct inhibition of the mitochondrial permeability transition pore: a possible mechanism responsible for anti-apoptotic effects of melatonin. FASEB J. 2004;18:869–871. doi: 10.1096/fj.03-1031fje. [DOI] [PubMed] [Google Scholar]
- 140.Jou MJ. Melatonin preserves the transient mitochondrial permeability transition for protection during mitochondrial Ca2+ stress in astrocyte. J Pineal Res. 2011;50:427–435. doi: 10.1111/j.1600-079X.2011.00861.x. [DOI] [PubMed] [Google Scholar]