Abstract
There is an ongoing need for effective and targeted cancer treatments that can overcome the detrimental side effects presented by current treatment options. One class of novel anticancer molecules with therapeutic potential currently under investigation are cationic antimicrobial peptides (CAPs). CAPs are small innate immunity peptides found ubiquitously throughout nature that are typically membrane-active against a wide range of pathogenic microbes. A number of CAPs can also target mammalian cells and often display selective activity towards tumor cells, making them attractive candidates as novel anticancer agents warranting further investigation. This current and comprehensive review describes key examples of naturally occurring membrane-targeting CAPs and their modified derivatives that have demonstrated anticancer activity, across multiple species of origin and structural subfamilies. In addition, we address recent advances made in the field and the ongoing challenges faced in translating experimental findings into clinically relevant treatments.
Keywords: Cationic antimicrobial peptide, Cancer, Membrane, Phospholipid
Structural and functional properties of membrane-targeting cationic antimicrobial peptides
It is widely understood that, although chemotherapy and radiotherapy remain the most common non-surgical cancer treatment options, they present major drawbacks. These include adverse side effects such as cardiotoxicity and neurotoxicity due to low tumor cell-specificity [1] and multidrug resistance, resulting in reduced chemotherapeutic drug efficacy [2]. Whilst the development of targeted therapy options such as immunotherapy show significant promise with demonstrated improved outcomes, they remain expensive and are often not applicable to all cancers. Due to advances in available treatment options and increased awareness leading to earlier detection, the 5-year survival rate across all cancers in Australia reached 66% by 2010, a 19% increase since the 1980s [3]. Although significant, this figure translates to one third of all diagnoses leading to death within 5 years and reflects an urgent and ongoing need for more effective, targeted cancer treatments.
A novel family of anticancer molecules that has attracted significant and increasing interest over the past decade is the cationic antimicrobial peptides (CAPs) [4, 5]. CAPs are small (typically <50 amino acids), positively charged peptides present in all forms of life, that comprise a major component of the innate immune system [6]. Either constitutively expressed or produced in response to microbial attack, CAPs act as a ‘first line of defense’ against invading microbes. CAPs are active against a wide range of pathogenic organisms including Gram-positive and Gram-negative bacteria, fungi and viruses, often targeting the plasma membrane to form pores, modify ion channels or induce membrane rupture [7, 8]. The cationic charge of CAPs suitably targets them to negatively-charged membranes and cell walls, such as those present in bacteria or fungi.
In addition to antimicrobial targeting, many CAPs also display membranolytic activity towards mammalian cells. The relative increase in anionicity of tumor cell membranes compared with healthy cells has been suggested to promote the selectivity often observed for tumor cells, making CAPs attractive candidates for development of novel cancer therapies [4, 5, 9]. Furthermore, since the ability of CAPs to target tumor cells is largely charge-based, their efficacy is not likely to be influenced by multi-drug resistance, whilst the ability of CAPs to target cells that are not actively dividing means they could potentially be used to kill “dormant” tumor cells, unlike traditional chemotherapeutics that usually only work on actively dividing cells. In addition to plasma membrane targeting (typically resulting in necrotic cell death), a number of CAPs have also demonstrated the ability to elicit anticancer activity via mechanisms that are not membrane-dependent, in particular via inducing apoptosis [10, 11]. Although the wider antimicrobial peptide field now encompasses peptides ranging from naturally-occurring through to synthetic de novo peptides, this review will focus primarily on the different classes of membranolytic CAPs found endogenously across species, as well as related derivatives. In particular, this review will examine CAPs that are known to exhibit anticancer activity via membrane targeting (and via alternative mechanisms, where applicable) and address the value and challenges in pursuing the development of cationic peptides for therapeutic use.
CAPs are structurally diverse
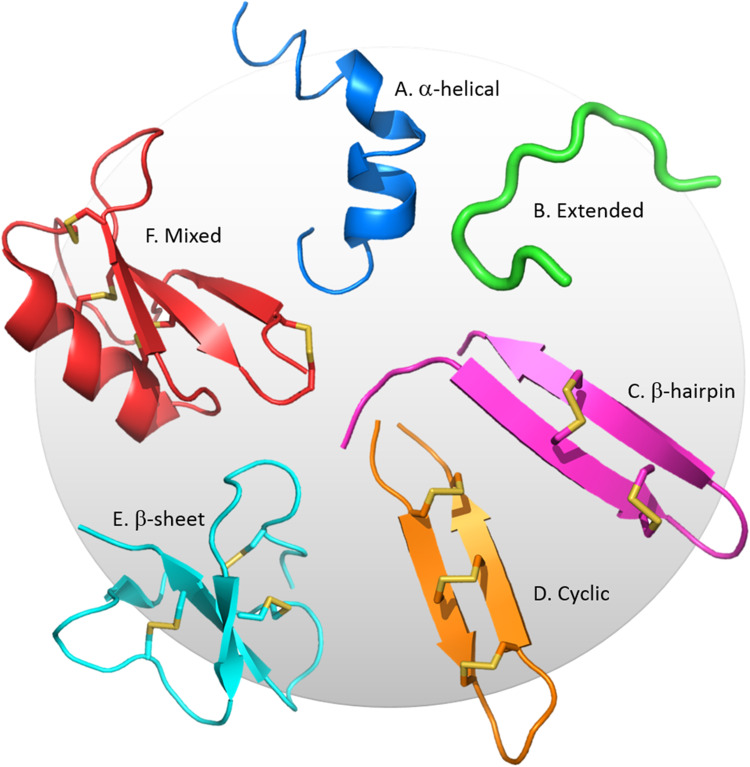
CAPs are a diverse peptide class, with approximately 2400 identified or predicted to date [18]. CAPs mediate host defense against pathogen infection through a wide range of processes, predominantly by the direct killing of microorganisms via plasma membrane disruption and/or targeting of intracellular pathways upon CAP internalization [19] but also by other mechanisms including immune modulation (e.g. induction of inflammation and enhanced bacterial clearance). CAPs exist in a range of tertiary structures, including α-helical, β-sheet (including β-hairpin and a combination of α-helix and β-sheet), extended (neither α-helix or β-sheet), and can be cyclical [7]. Defensins, which are the most widely distributed class of CAP and are present in all forms of life, are typically disulfide-rich and contain β-sheets with some also featuring α-helices (Fig. 1). Approximately 100 CAPs of known tertiary structure from various species are listed in the Antimicrobial Peptide Database (ADP3) as having activity against cancer cells [18]. Of these, the two major structural groups are the α-helical CAPs such as cathelicidins, magainins, cecropins and the disulfide-rich, β-sheet and mixed α-helical/β-sheet peptides such as defensins. Other smaller groups include β-hairpin, cyclic and extended (unstructured) peptides. This review will focus on the α-helical, β-hairpin, β-sheet and mixed peptide classes.
Fig. 1.
Structural classes of CAPs. Representative structures of the six main classes are shown. a The African clawed frog peptide, magainin, is α-helical (PDB code: 2LSA). b The bovine neutrophil peptide, indolicidin, does not contain β-strands or α-helices [12] (PDB code: 1QXQ). c The porcine leukocyte peptide, protegrin-1 adopts a β-hairpin structure stabilized by two disulfide bonds [13] (PDB code: 1PG1). d The synthetic human θ-defensin, HTD-2, is similar to a β-hairpin but pseudo-cyclized by a third, connecting disulfide bond [14] (PDB code: 2LZI). e The human β-defensin, HBD-2, contains a disulfide-bonded, triple-stranded β-sheet [15] (PDB code: 1FD4). f The ornamental tobacco defensin, NaD1, is comprised of a triple-stranded β-sheet with one α-helix. This pseudocyclic peptide is stabilized by four disulfide bonds [16] (PDB code: 1MR4). Images generated using Pymol [17]
Mechanisms of CAP-mediated membrane disruption
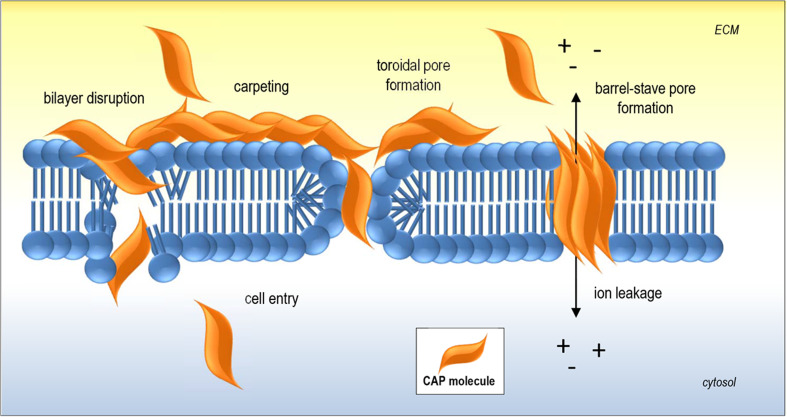
Two common features of membranolytic CAPs are a net positive charge and amphipathicity. These properties allow CAPs to interact with negatively charged cell wall/membrane components (such as glycoproteins and/or plasma membrane phospholipids of bacterial or fungal cells) and bury into the bilayer to ultimately induce membrane destabilization and permeabilization or peptide internalization [20]. A number of models have been proposed to describe the various methods of CAP-mediated membrane interactions, which vary depending on both the physical properties of the CAP as well as target cell membrane composition, reviewed extensively by Brogden [8] (Fig. 2). It should be noted that these models have been devised predominantly through studies involving α-helical CAPs with artificial bilayers or bacterial membranes. Briefly, the carpet model involves parallel accumulation of peptides via electrostatic attractions to the anionic cell surface in a carpet-like fashion. When a critical concentration threshold is reached, membrane curvature stress induces detergent-like disruption of the membrane and the formation of micelles, leading to membrane destabilization. The toroidal pore model involves the formation of ‘wormhole-like’ pores in the membrane, in which phospholipid head-groups of membrane lipids remain associated with the hydrophilic portion of the peptides continuously from the outer to inner leaflets of the membrane. The barrel-stave model involves vertical insertion of oligomeric α-helical peptides to form a bundle into the bilayer, aligned hydrophobically with lipid acyl chains to form ion-permeable pores in the membrane, also capable of resulting in membrane rupture [8]. It is worth noting that these models are not mutually exclusive, that is, certain CAPs may adopt features of more than one model.
Fig. 2.
Key examples of CAP-membrane interactions. Initial electrostatic interactions between CAP and negatively charged membrane components are followed by accumulation of CAP molecules on the plasma membrane leading to transient pore formation. Alternatively, insertion of CAPs into the membrane resulting in membrane disruption and/or internalization may occur as depicted in classic models of CAP-mediated membrane permeabilization including ‘carpeting’, ‘toroidal pore’ (or wormhole) and ‘barrel-stave’ models
β-Sheet-rich CAPs adopt more diverse tertiary structures, including two-stranded anti-parallel β-hairpin, such as protegrins, or triple-stranded antiparallel β-sheet that may or may not also contain an α-helix, such as defensins. Less molecular detail has been elucidated with regard to how β-sheet CAPs interact with the plasma membrane of target cells, although there is considerable emerging evidence for the role of specific membrane lipids such as sphingolipids and phosphatidylinositols, as well as the ability to form dimers and/or higher order oligomers, to facilitate membrane disruption by these peptides. In the next section, the tumour cell-specific targeting mechanisms of CAPs will be discussed.
What makes tumor cells susceptible to CAPs?
As mentioned above, a key feature of tumor cells that increases their susceptibility to CAP-mediated cytotoxicity is a higher overall net negative charge relative to their non-tumor counterparts, resulting from a number of key factors.
Increased exposure of anionic phospholipids in the plasma membrane
Changed conditions within the tumor microenvironment including elevated reactive oxygen species (ROS) and hypoxia can lead to dysregulation of phospholipid transporters that are responsible for maintaining plasma membrane phospholipid asymmetry under normal conditions [21]. In particular, the maintenance of the higher concentration of the anionic phospholipids phosphatidylserine (PS) and phosphatidylethanolamine (PE) in the inner membrane can be lost, leading to their elevated presence in the outer membrane [22, 23]. This phenomenon is seen both in the tumor vascular endothelium as well as in epithelial and other cells of the tumor microenvironment and has been associated with increased cell survival and cancer progression, such as through activation of the blood coagulation cascade that can trigger pro-survival signalling [24]. The notion that CAPs may target cancer cells with increased anionic phospholipid exposure has been investigated, predominantly through experiments involving both liposomes of different phospholipid compositions, as well as tumor cell lines with known PS exposure. The requirement for PS (or other anionic phospholipids) to induce lysis or cytotoxicity has been demonstrated in this context for several CAPs [25–29].
Increased expression of anionic cell surface glycoproteins
In addition to anionic membrane phospholipids, various anionic glycoproteins are commonly overexpressed in cancers, contributing to the overall negative charge on the outer tumor cell surface. One example are mucins, glycoproteins present on the apical surface of epithelial cells that contain repeated regions of O-glycosylation, giving them a net negative charge [30]. Over expression of transmembrane mucins has been reported in carcinomas of the breast, prostate, lung and pancreas [30]. Similarly, heparan sulfate proteoglycans (HSPGs) are negatively-charged molecules present at the cell surface that interact with numerous ligands including growth factors, cytokines and enzymes and are overexpressed in breast, lung, brain, pancreatic, skin, and colorectal cancers [31]. The negative charge of both mucins and HSPGs has been suggested to electrostatically enhance CAP interactions at the surface of tumor cells to promote binding [5, 9].
Other factors
Alterations in the physical properties of tumor cell membranes have also been suggested to increase their susceptibility to CAP-mediated interactions. For example, an increase in membrane fluidity (e.g. due to lowered cholesterol concentrations), has been commonly reported in malignant cell types [32–35] and may facilitate penetration of CAPs into the lipid bilayer. In addition, increased levels of microvilli, thereby leading to an increase in the overall surface area of the tumor cell plasma membrane, could draw a higher concentration of CAP molecules to the cell surface to facilitate destabilization [36–38].
CAPs with anticancer activity
As mentioned, many CAPs display anticancer activity, as assessed by a number of approaches ranging from in vitro cell viability assays through to in vivo tumor regression trials using xenograft models of cancer. In addition to CAPs isolated directly from natural sources, many de novo peptides have been designed and synthesized through modification of specific residues or regions of the native peptide to enhance function, increase selectivity and reduce off-target effects. The following are selected examples of membranolytic CAPs from various species and their optimized derivatives that have demonstrated the ability to kill cancer cells. Tables 1, 2 and 3 contain extended lists of anticancer CAPs with Tables 1 and 2 describing in vitro anticancer activity of α-helical CAPs and β-sheet/β-hairpin CAPs, respectively. Table 3 describes in vivo anticancer activity of both α-helical and β-sheet/β-hairpin CAPs.
Table 1.
In vitro anticancer activity of α-helical cationic antimicrobial peptides
| Peptide class | Species/species derived from | Peptide name | Tumor cells tested | Proposed mechanism | Reference[s] |
|---|---|---|---|---|---|
| Mammals | |||||
| Cathelicidin | Human (Homo sapiens) | LL-37 | U937 | Membrane permeabilization | [43] |
| Human (H. sapiens) | LL-37 | Jurkat | Caspase-independent apoptosis | [45] | |
| Rhesus macaque (Macaca mulatta) | RL-37 | U937 | Membrane permeabilization | [43] | |
| Human (H. sapiens) | FK-16 (LL-37 derivative) | HCT-116, LoVo | Apoptosis, autophagy, growth inhibition | [103] | |
| Cow (Bos taurus) | BMAP-27/BMAP-28 | HL60, U937, K562, JURKAT, CEM, CEM-VLB, activated human lymphocytes | Plasma/mitochondrial membrane permeabilization | [46, 47] | |
| Reptiles, amphibians and fish | |||||
| Cathelicidin | Snake (Bungarus fasciatus) | BF-30 | B16F10 | Membrane permeabilization | [104] |
| Snake (B. fasciatus) | Cbf-K16 (BF-30 derivative) | H460 | Membrane permeabilization | [105] | |
| Magainin | African clawed frog (Xenopus laevis) | Magainin II (MG2), MSI-136, MSI-238 (MG2 derivatives) | A549, mouse Ehlrich ascites | Growth inhibition | [48] |
| African clawed frog (X. laevis) | Magainin II (MG2) | RT4, 647V, 486P | Cell lysis, growth inhibition | [106] | |
| African clawed frog (X. laevis) | MAG A, MAG G (MG2) | NC-H82, NCI-H526, NCI-H678, NCI-H735, NCI-H841, NCI-H889 | Growth inhibition | [107] | |
| African clawed frog (X. laevis) | Pexiganan MSI-78 (magainin derivative) | U937 | Growth inhibition | [49] | |
| African claw frog (X. laevis), European fire-bellied toad (Bombina bombina) | MG2B (MG2-Bombesin conjugate) | MCF7, A357 | Membrane permeabilization, caspase-dependent apoptosis, mitochondrial damage | [52] | |
| African claw frog (X. laevis), fruit fly (Drosophila melanogaster) | MG2A (MG2-penetratin conjugate) | HeLa, A549 | Membrane permeabilization, caspase-dependent apoptosis | [108] | |
| Buforin | Asian toad (Bufo bufo gargarizans) | Buforin IIb | Ganglioside-mediated cell-penetration/apoptosis | [109] | |
| Epinicidin | Grouper fish (Epinephelus coioides) | Epinicidin-1 | HT1080, U937 | Membrane permeabilization, growth inhibition | [54] |
| Grouper fish (E. coioides) | Epinicidin-1 | U937 | Caspase-dependent apoptosis | [10] | |
| Hepcidin | Mozambique tilapia fish (Oreochromis mossambicus) | TH2-3 | HT1080 | Membrane permeabilization, growth inhibition | [110] |
| Pleurocidin | Atlantic flounder (Pseudopleuronectes americanus) | NRC03,NRC07 | MDA-MB-231 | proteoglycan-mediated membrane lysis, necrosis | [93, 111] |
| Temporin | Chinese brown frog (Rana chensinensis) | Temporin-1CEa | MCF7, MDA-MB-231 | Membrane permeabilization | [112] |
| Chinese brown frog (R. chensinensis) | Temporin-1CEa | Bcap-37 | Membrane permeabilization, intracellular pathway modification | [113] | |
| Insects | |||||
| Cecropin | Cecropia moth (Hualophoria cecropia) | Cecropin A | HL-60 | Caspase-independent apoptosis | [114] |
| Chinese oak silkmoth (Antheraea pernyi) | Cecropin B, Cecropin B-1 and B2 (Cecropin B derivatives) | HL-60, K-562, Jurkat, CCRF-CEM | Growth inhibition, membrane permeabilization | [36] | |
| Chinese oak silkmoth (A. pernyi), Cecropia moth (H. cecropia) | Cecropin A, Cecropin B | 486P, RT4, 647V, J82 | Growth inhibition, membrane permeabilization | [55] | |
| Housefly (Musca domestica) | Cecropin | BEL-7402 | Caspase-dependent apoptosis | [115] | |
| Melittin | Honey bee (Apis mellifica) | Melittin | HL60, l1210 | Membrane permeabilization | [58, 59] |
| Honey bee (A. mellifica), pitviper (Glydius ussuriensis) | DLM (Melittin-disintegrin conjugate) | BT-549, MDA-MB-231, MCF-7, SKOV-3, SMMC-7721 | Growth inhibition, membrane disruption | [62] | |
Table 2.
In vitro anticancer activity of β-sheet and β-hairpin cationic antimicrobial peptides
| Peptide class | Species/species derived from | Peptide name | Tumor cells tested | Proposed mechanism | Reference[s] |
|---|---|---|---|---|---|
| Mammals | |||||
| Neutrophil peptides | Human (H. sapiens) | HNP-1, HNP-2, HNP-3 | Raji, U937, K562, IM-9, WIL-2 | Membrane permeabilization | [72] |
| Human (H. sapiens) | HNP-1 | Oral squamous cell carcinoma | Growth inhibition | [116] | |
| Human (H. sapiens) | HNP-1 | NMB-7, LAN-5, LAN-1 | Membrane permeabilization | [117] | |
| Rabbit (Lepus curpaeums) | NP-1, NP-2, NP-3a, NP-3b | Raji, U937, K562, IM-9, WIL-2 | Membrane permeabilization | [72] | |
| Lactoferricin | Cow (B. taurus) | Lactoferricin B (LfcinB) | A range of leukemia, breast, colon and ovarian cancers | Apoptosis, mitochondrial membrane damage | [63] |
| Cow (B. taurus) | Lactoferricin B (LfcinB) | Jurkat | Membrane permeabilization, apoptosis | [11] | |
| Cow (B. taurus) | Lactoferricin B (LfcinB) | Kelly, SK-N-DZ, IMR-32, 1640, SHEP-1 | Plasma and mitochondrial membrane permeabilization | [66] | |
| Cow (B. taurus) | Lactoferricin B (LfcinB) | MDA-MB-435 (used synergistically with tamoxifen) | Apoptosis | [118] | |
| Cow (B. taurus) | Lactoferricin B (LfcinB) | Jurkat, CCRF-CEM | Ceramide-enhanced apoptosis | [119] | |
| Cow (B. taurus) | Lactoferricin B (LfcinB) | AGS | Apoptosis, late-stage inhibition of autophagy | [64] | |
| Human (H. sapiens) | LF11-322, 6-MO-LF11-322, R-DIM-P-LF11-322 (Human lactoferricin derivatives) | SBcl-2 | Phosphatidylserine-dependent membrane permeabilization, apoptosis | [26, 29] | |
| Protegrin | Pig (Sus scrofa) | Protegrin-1 | U937 | Membrane permeabilization, growth inhibition | [49] |
| Human β-defensin | Human (H. sapiens) | HBD-3 | U937, Jurkat, HL-60, HeLa, PC3 | PIP2-mediated membrane permeabilization, growth inhibition | [88] |
| Other | |||||
| Gomesin | Brazilian tarantula (Acanthoscurria gomesiana) | Gomesin | B16F10, SKBr3, LS180, HeLa, SKMel 19, A2058 | Membrane permeabilization | [69] |
| Brazilian tarantula (A. gomesiana) | Gomesin | SH-5YSY | Membrane permeabilization/necrotic cell death | [68] | |
| Tachyplesin | Horseshoe crab (Tachypleus tridentatus) | Tachyplesin-RGD | TSU, B16 | Growth inhibition, apoptosis | [70] |
| Horseshoe crab (T. tridentatus) | Tachyplesin | TSU | Activation of complement pathway, proteoglycan-mediated membrane permeabilization | [71] | |
| Plant defensins | Ornamental tobacco (Nicotiana alata) | NaD1 | U937, HeLa, PC3, Jurkat | PIP2-mediated membrane permeabilization, growth inhibition | [16] |
| Tomato (Solanum lycopersicum) | TPP3 | U937, HeLa, Jurkat, PC3 | PIP2-mediated membrane permeabilization | [83] | |
| Yardlong bean (Vigna sesquinpedalis) | Sesquin | M1 (leukemia), MCF-7 | Growth inhibition | [79] | |
| String bean (Phaseolus vulgaris) | Spotted bean defensin | L1210, MBL2 | Growth inhibition | [81] | |
| Lima bean (Phaseolus lutanus) | Lunatusin | MCF-7 | Growth inhibition | [80] | |
Table 3.
In vivo anticancer activity of α-helical and β-sheet cationic antimicrobial peptides
| Peptide class | Species/species derived from | Peptide name | In vivo model(s) used | Effects/proposed mechanisms | Reference[s] |
|---|---|---|---|---|---|
| α-Helical | |||||
| Cathelicidin | Snake (Bungarus fasciatus) | BF-30 | B16F10 mouse allograft model | Suppression of tumor growth | [104] |
| Magainin | African claw frog (X. laevis) | Magainin II (MG2), MSI-136, MSI-238 (MG2 derivatives) | P388/L1210, S180 and SOT mouse allograft models | Increased lifespan, tumor shrinkage | [48] |
| African claw frog (X. laevis), European fire-bellied toad. (Bombina bombina) | MG2B (MG2-Bombesin conjugate) | MCF-7 mouse xenograft model | Suppression of tumor growth | [52] | |
| African claw frog (X. laevis), Drosophila | MG2A (MG2-penetratin conjugate) | HeLa, A549 mouse xenograft model | Suppression of tumor growth (necrosis) | [108] | |
| Buforin | Asian toad (B. gargarizans) | Buforin IIb | NCI-H460 mouse xenofraft model | Suppression of tumor growth (apoptosis) | [109] |
| Cecropin | Housefly (M. domestica) | Cecropin | BEL-7402 mouse xenograft | Suppression of tumor growth | [56] |
| Pleurocidin | Atlantic flounder (Pseudopleuronectes americanus) | NRC03,NRC07 | MDA-MB-231 mouse xenograft model | Suppression of tumor growth (necrosis) | [93, 111] |
| β-Sheet and β-hairpin | |||||
| Neutrophil peptide | Human (H. sapiens) | HNP-1, HNP-2, HNP-3 | MOT mouse allograft model | Suppression of tumor growth (of pre-treated cells) | [72] |
| Human (H. sapiens) | HNP-1 | A549 mouse xenograft model (in vivo expression of HNP-1 within A549 cells) | Tumor shrinkage (apoptosis) | [75] | |
| Lactoferricin | Cow (B. taurus) | LfcinB | SH-SY-5Y rat xenograft model | Suppression of tumor growth | [66] |
| Cow (B. taurus) | LfcinB | L5178Y-ML25 xenograft mouse model of liver, spleen and lung metastasis, B16BL6 mouse model of angiogenesis | Inhibition of tumor metastasis, angiogenesis; tumor regression | [65] | |
| Cow (B. taurus) | LfcinB(20–25)4 (tetrameric LfcinB derivative) | Oral squamous cell carcinoma xenograft hamster model | Suppression of tumor growth (necrosis) | [67] | |
| Gomesin | Brazilian spider (A. gomesiana) | Gomesin | B16F10 mouse allograft model, topically treated | Increased lifespan | [69] |
| Tachyplesin | Horseshoe crab (T. tridentatus) | Tachyplesin-RGD | TSU and B16 mouse xenografts | Suppression of tumor growth | [70] |
α-Helical peptides
Several α-helical CAPs from mammals, amphibians and insects have exhibited membranolytic activity towards tumor cells, with some of these also demonstrating the ability to target intracellular pathways, thereby inducing apoptosis or other forms of cell death (Tables 1, 3).
Mammalian α-helical peptides
Cathelicidins are a major class of mammalian CAP, found in the lysosomes of macrophages and polymorphonuclear leukocytes (PMNs). LL-37 is the only known human cathelicidin, and has diverse roles in innate and adaptive immunity, including microbial membrane permeabilization and growth inhibition [39, 40], chemotaxis [41] and wound healing [42]. The propensity of LL-37 to form oligomers in vitro has also been demonstrated [43] (Box 1) and this has been suggested to contribute to the formation of toroidal pores to induce membrane destabilization [44] (Fig. 3a). In terms of anticancer activity, LL-37 has been reported to induce membrane permeabilization of U937 cells at low micromolar concentrations whilst displaying only low-level haemolytic activity, indicating selectivity towards the neoplastic cell line [43]. LL-37 was also reported to induce calpain-mediated, caspase-independent apoptosis in Jurkat cells [45]. The bovine homologs of LL-37, BMAP-27 and BMAP-28, display membrane permeabilizing activity towards a range of myeloid and lymphoid leukemia cells lines at low micromolar concentrations, with minimal effect on normal human lymphocytes at these concentrations [46]. Interestingly, treating U937 cells with neuraminidase to cleave sialylated glycoproteins, significantly reduced the activity of both peptides in this study, suggesting mucins may play a role in their anticancer activity. BMAP-28 was subsequently shown to induce mitochondrial permeability transition pore opening and cytochrome c release, lending further support that this peptide can activate apoptosis [47] (Fig. 3b).
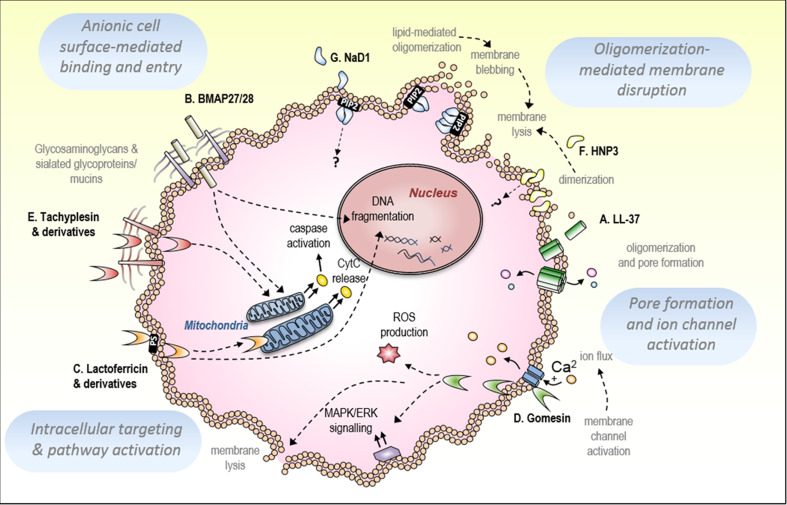
Fig. 3.
Mechanisms of CAP-mediated tumor cell killing. a The human cathelicidin, LL-37, induces membrane permeabilization of tumor cells possibly via forming oligomeric membrane pores of up to seven peptides. b Bovine cathelicidins BMAP 27/28 activate apoptotic pathways in tumor cells, demonstrated via DNA fragmentation and cytochrome c release, possibly dependent on an interaction with cell-surface mucins. c The bovine β-haripin peptide, lactoferricin B (LfcinB) induces mitochondrial membrane permeabilization, cytochrome c release and DNA fragmentation, with phosphatidylserine-mediated plasma membrane permeabilization demonstrated for the synthetic derivative of human lactoferricin, R-DIM-P-LF11-322. d The β-hairpin tarantula peptide, gomesin, induces necrotic cell death via l-type calcium channel activation, ROS production and activation of the MAPK/ERK signalling pathway. e The apoptosis-inducing activity of the horseshoe crab peptide, tachyplesin, has been suggested to involve an interaction with glycosaminoglycans at the tumor cell membrane. f Human neutrophil peptide 3 (HNP-3) dimerizes and may form higher oligomers to destabilize target membranes. g The plant defensin, NaD1, oligomerizes at the inner membrane of tumor cells with PIP2, inducing membrane blebbing and lysis
Amphibian and fish α-helical CAPs
Several CAPs isolated from fish and amphibian species exhibit anticancer activity via membrane targeting (Tables 1, 3). The α-helical CAP isolated from the African clawed frog, magainin 2 (MG2) and a number of synthetic derivatives of this peptide, exhibit anticancer activity on a range of mammalian cancer cell lines. Human lung A549 cells and murine Ehlrich ascites tumor cells displayed reduced cell viability in vitro following treatment with MG2 [48], whilst a synthetic analog of magainin known as Pexiganan MSI-78 displayed growth inhibition as well as membrane disruption of U937 cells accompanied by the release of TNF-α [49]. Significantly increased cytotoxic activity was observed with MSI-136, a synthetic MG2 homolog with enhanced amphipathic α-helical structure, when compared with wildtype MG2. Similarly, an all d-amino acid version of MSI-136, MSI-238, designed for improved protection from proteolytic cleavage [50, 51], displayed even greater efficacy [48].
The ability of MG2-based peptides to more efficiently target the membranes of tumor cells was later investigated by designing a MG2 conjugate with bombesin, a 14-amino acid peptide from frog skin, which binds a range of tumor cells with high affinity [52]. The resultant MG2-bombesin conjugate (MG2B) displayed potent cytotoxicity, with IC50 values reduced by up to 16-fold on MCF-7 (breast) and A357 (melanoma) tumor cell lines, compared with MG2 alone [52]. It was further shown in the same study that MG2B induced caspase-dependent cell death in a proportion of MCF7 cells. This indicated that, in addition to inducing necrosis via membrane permeabilization, MG2B can also induce apoptosis, possibly via internalizing and causing mitochondrial damage. In addition, a xenograft mouse model of cancer using MCF-7 cells revealed that MG2B could significantly reduce tumor growth in vivo, compared with MG2 alone, further highlighting the value in modifying native CAPs such as magainin to achieve optimized hybrid peptides with superior function [52].
Anticancer CAPs isolated from a small number of fish species have also been reported. Of note is epinecidin-1, a CAP synthesized from cDNA isolated from the grouper fish (Epinephelus coioides), predicted to be α-helical in structure [53]. Epinecidin-1 displayed growth inhibitory activity towards a range of human and mouse tumor and non-tumor cell lines, with HT1080 (fibrosarcoma) and U937 cells displaying the greatest sensitivity [54]. The apoptosis-inducing effects of epinecidin-1 were also reported, revealing that epinecidin-1 induced DNA fragmentation and caspase activation in U937 cells [10].
Insect α-helical CAPs
Cecropins are a group of α-helical insect CAPs first identified in the Cecropia moth (Hyalpohoria cecropia). Several cecropins and their derivatives have demonstrated anticancer activity, including growth inhibition, membrane permeabilization and induction of apoptosis [36, 55, 56]. Cecropin B, isolated from the Chinese oak silk moth (Antheraea pernyi) as well as two synthetically designed derivatives with increased cationicity, Cecropin B-1 and B-2, were tested on a range of human leukemic and normal fibroblast cell lines. Membrane lysis of leukemic cells was observed for all three peptides with greater potency observed for the derivatives with higher positive charge [36]. A more recent in vivo study investigating the effects of the Musca domestica (house fly) cecropin on a BEL-7402 human hepatocellular carcinoma xenograft mouse model, demonstrated that this cecropin could successfully suppress tumor growth [56].
The European honey bee CAP, melittin, which constitutes up to 50% dry weight of bee venom, has been widely described as possessing anticancer properties including growth inhibition, apoptosis and membrane permeabilization [57–59]. However, the comparable haemolytic effects of melittin [59] have posed challenges in further developing this peptide as an anticancer therapeutic. One approach to overcome this poor selectivity for tumor cells has been the design of hybrid peptides comprised of the N-terminal regions of melittin with other lytic peptides such as cecropin [60, 61]. Indeed, fusion of the Gloydius ussuriensis pitviper peptide, disintegrin, via a cleavable linker with melittin to form DLM (disintegrin–linker–melittin) resulted in selective growth inhibition and membrane disruption of human breast and ovarian cancer cell lines whilst displaying minimal haemolytic activity [62].
β-Sheet-rich CAPs
In addition to the numerous α-helical CAPs investigated for anticancer properties, a number of β-sheet-rich CAPs that are active against tumor cells have also been characterized, including those with β-hairpin, larger β-sheet, and mixed β-sheet/α-helix tertiary structures (Table 2, 3).
β-Hairpin CAPs
A handful of β-hairpin CAPs from mammalian and invertebrate species have demonstrated anticancer activity. Lactoferricin is an antimicrobial peptide resulting from trypsin cleavage of the 80 kDa milk glycoprotein lactoferrin, present in humans and cows. Bovine lactoferricin (LfcinB) has been widely shown to induce apoptosis in tumor cells. Mader and colleagues reported caspase-dependent DNA fragmentation, mitochondrial association and cytochrome c release following LfcinB treatment [11, 63] (Fig. 3c). The selectivity of LfcinB for tumor cell lines over primary cells was determined with a range of leukemia, breast, colon and ovarian tumor cell lines displaying between 20 and 90% increases in growth inhibition compared with untransformed cell types [63]. Another study reported that induction of apoptosis by LfcinB in gastric carcinoma cells was shown to involve late-stage inhibition of autophagy, as determined by caspase cleavage of the autophagy-associated protein, beclin-1 [64]. The in vivo anticancer effects of LfcinB have also been demonstrated in rodents. Significant inhibition of both metastasis and angiogenesis was observed in a lymphoma xenograft model of liver, spleen and lung metastasis and in a B16-BL6 model of angiogenesis in mice [65], whilst a neuroblastoma xenograft model in rats demonstrated significant LfcinB-mediated tumor growth inhibition compared with controls [66]. More recently, modification of LcfinB to a tetrameric peptide comprised of the active ‘core sequence’ known as LfcinB(20–25)4 has also been investigated for its anticancer effects in vivo. Intratumoral administration of the peptide in an oral squamous cell carcinoma golden Syrian hamster model of disease demonstrated significant antitumor effects compared with control peptides, proposed to be via an enhanced cytotoxic effect of the peptide [67]. Investigations into the anticancer properties of human lactoferricin have also been reported. Riedl and colleagues showed that R-DIM-P-LF11-322, a human lactoferricin-derived peptide was able to induce apoptosis via the specific targeting of exposed PS in the outer membrane of melanoma and glioblastoma cells as well as in PS-containing liposomes [26, 29].
Other β-hairpin CAPs of note include protegrin, gomesin and tachyplesin. Protegrin-1, isolated from pig leukocytes, displayed both growth inhibitory and membrane permeabilizing activity towards U937 cells [49]. Gomesin, isolated from the Brazilian tarantula spider (Acanthoscurria gomesiana) induced necrotic cell death in SH-5YSY neuroblastoma cells, involving l-type calcium channel-mediated intracellular calcium flux, ROS production and MAPK/ERK, PKC and PI3K signalling [68] (Fig. 3d). In vivo, topical treatment of gomesin on mice with B16F10 melanomas resulted in significantly increased survival times compared with controls [69]. Lastly, the horseshoe crab (Tachypleus tridentatus) peptide, tachyplesin, has also demonstrated both in vitro and in vivo anticancer activity. A synthetic conjugate of tachyplesin, RGD-tachyplesin, that contains an integrin binding motif proposed to facilitate internalization, displayed selective membrane permeabilization and caspase activation [70]. Interestingly, tachyplesin (unconjugated to RGD) interacted with C1q, a component of the complement pathway in vitro, which was proposed to facilitate its anticancer activity. The authors suggested that glycosaminoglycans such as hyaluronan may play a role in the interaction between tachyplesin and the tumor cell membrane [71] (Fig. 3e).
Neutrophil peptides (α-defensins)
Early studies describe the membranolytic effects of neutrophil peptides, also known as α-defensins, from humans and rabbits [72]. Human neutrophil peptides HNP-1, HNP-2 and HNP-3 as well as a range of rabbit neutrophil peptides displayed cytolytic activity towards Raji, U937, K562, IM-9 and WIL-2 tumor cell lines, with varying degrees of lysis reported [72]. It is currently unknown how α-defensins, which are structurally comprised of a triple-stranded antiparallel β-sheet, interact with the plasma membrane of target cells, although crystal structures of HNP-3, HNP-4, HD-5 and HD-6 have been solved, revealing that these defensins form dimers (or tetramers, in the case of HD-6) which may facilitate membrane lysis [73, 74] (Fig. 3f, Box 1). More recently, the intracellular effects of HNP-1 were investigated by expressing HNP-1 in A549 lung adenocarcinoma cells, which resulted in apoptosis induction over 48 h. The in vivo activity of HNP-1 was also examined in a xenograft mouse model of lung cancer, in which intracellular expression of HNP-1 in A549 cells resulted in significant tumor shrinkage compared with controls, proposed to be via apoptosis induction. [75].
Plant defensins, β-defensins and related CAPs
Plant defensins, previously known as γ-thionins before being recognized as a distinct class of peptides [76], adopt a tertiary structure that is highly conserved across species and consists of a triple-stranded antiparallel β-sheet with an α-helix, stabilized by four (or five) disulfide bridges, folded into a “cysteine-stabilized alpha–beta” (CSαβ) configuration [77, 78]. Whilst a number of plant defensins and defensin-like peptides derived from legume species have been shown to exhibit growth inhibitory activity on various tumor cell lines in vitro [79–82], no further investigations into the specific mechanism of action has been reported in these examples. However, insights into the molecular interactions between defensins and the plasma membrane of target cells have recently been described for two related defensins from the Solanaceae plant family, demonstrating selective membrane permeabilizing anticancer activity towards a range of mammalian tumor cell lines [16, 83]. The first, the ornamental tobacco defensin, NaD1, displayed cytotoxic activity toward U937, Jurkat, Hela and PC3 cells at low micromolar concentrations (~10 μM) via rapid membrane lysis, accompanied by large plasma membrane blebs, suggesting the induction of necrotic cell death [16]. The activity of NaD1, not only against mammalian tumor cells but also filamentous fungi and yeast cells, was shown to involve the presence of the negatively-charged plasma membrane phosphatidylinositol 4,5-bisphosphate (PIP2) [16, 84]. Importantly, through solving the crystal structure of NaD1 in complex with PIP2, valuable molecular information was gained regarding the peptide–lipid interaction and how this may contribute to membrane destabilization. The formation of an arch-shaped oligomer was revealed, comprised of seven NaD1 dimers in a configuration termed the ‘cationic grip’, interacting with 14 PIP2 molecules within the inner cationic groove of the arch (Box 1). A number of biophysical and cell-based analyses presented within this study supported the hypothesis that in a cellular context, this oligomeric interaction could facilitate NaD1-mediated membrane lysis upon contact with PIP2 molecules in the tumor cell membrane [16] (Fig. 3g). NaD1 was subsequently investigated for its potential to induce apoptosis in mammalian tumor cells across a broad range of time frames and concentrations, revealing NaD1 does not induce apoptosis and is solely a necrosis-inducing peptide that acts via a PIP2-dependent membranolytic pathway [85]. More recently, the anticancer activity of the related tomato defensin, TPP3, was also investigated [83]. TPP3 displayed comparable cytolytic activity towards tumor cells as seen with NaD1. The activity of TPP3 was also shown to be dependent on plasma membrane PIP2, whilst the solved crystal structure of TPP3 revealed the formation of a dimer highly homologous to the ‘cationic grip’ dimer previously observed for NaD1, capable of binding PIP2 and highlighting a conserved mechanism of action for these two solanaceous defensins (Box 1) [83]. Significantly, these were the first reports of lipid-mediated oligomerization to induce target cell death by a CAP and the first examples of PIP2 being identified as the target lipid of a CAP in its membranolytic mechanism of tumor cell-killing. Such findings highlight the value in structural exploration of CAP-mediated membrane interactions (e.g. via X-ray crystallography), through which specific interactions with membrane targets can be revealed (Box 1).
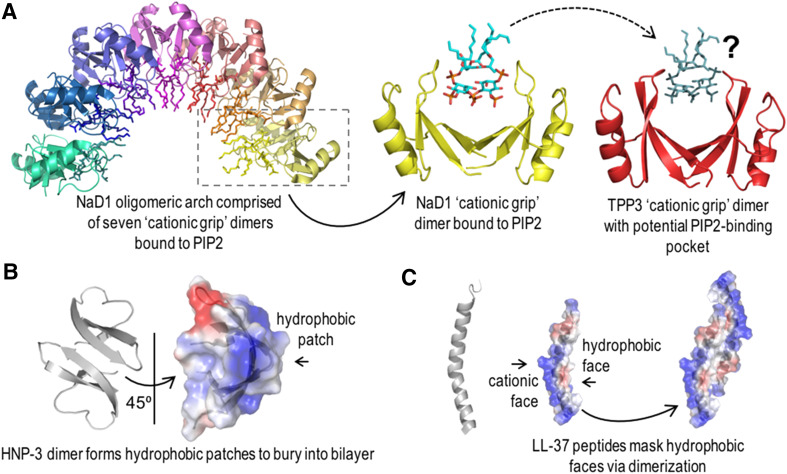
Power in numbers: molecular structures of peptides provide insights into dimer-driven membrane interactions.
The ability of CAPs to dimerize has been implicated in the process of target membrane disruption for a number of peptides. The related solanaceous plant defensins, NaD1 and TPP3, both form a ‘cationic grip’ dimer, which has been implicated in the process of tumor cell lysis by these defensins [16, 83]. For NaD1, X-ray crystallography revealed that seven NaD1 dimers each bound to two PIP2 molecules interact to form a large oligomer, suggesting that in vivo, NaD1 dimers may interact with membrane PIP2 to form lipid–peptide oligomeric complexes that disrupt both fungal and mammalian tumor cell membranes [16]. Whilst the crystal structure of the TPP3 dimer was solved in the absence of PIP2, site-directed mutagenesis studies revealed that, as with NaD1, both PIP2 binding and oligomerization were important for tumor cell lysis by this peptide. Interestingly, tetramers of this peptide were observed in the crystal unit, further suggesting that higher-order oligomers may form in vivo [83] (Fig. 4a).
Fig. 4.
Dimer formation of different CAPs, which may contribute to CAP-mediated tumor cell membrane lysis. a NaD1 and TPP3 form cationic grip dimers that oligomerize with PIP2 in the membrane of tumor cells, leading to cell lysis. b HNP-3 forms dimers in solution and may form tetramers or higher oligomers at target membranes. The hydrophobic dimer faces have been suggested to point into the bilayer to form membrane pores. c The proposed dimerization of LL-37 masks the hydrophobic face of the peptide to enable electrostatic membrane interaction and subsequent formation of membrane pores. Images were generated using Pymol
In addition to elucidating specific molecular interactions at the peptide–membrane interface, structural investigations can also reveal how dimerization of peptides leads to the formation of polar or uncharged surface regions that can facilitate membrane penetration. For example, the human α-defensin HNP-3 can form dimers in solution [73], with analysis of the crystal structure of HNP-3 providing insights into how this peptide may interact with anionic lipid bilayers [73, 74]. It has been suggested that two or more HNP-3 dimers may disrupt the bilayer via interactions between cationic residues and anionic phospholipid head groups, as well as via hydrophobic patches on the dimer, to enable the formation of membrane pores [73] (Fig. 4b). Likewise, the human cathelicidin, LL-37, has been proposed to dimerize at the membrane of target cells to facilitate membrane disruption. It has been suggested that LL-37 dimers form by masking their hydrophobic faces towards each other and away from the membrane to allow electrostatic membrane interactions and the subsequent formation of oligomeric membrane pores [44] (Fig. 4c). Whilst models of dimer-mediated membrane interactions for both HNP-3 and LL-37 have only been studied in bacterial membranes thus far, an increase in anionicity on the tumor cell surface could facilitate this process in the context of mammalian tumor cells, for both of these peptides. Thus, the propensity of CAPs to form dimers or higher oligomers can contribute to their membrane-lytic abilities.
Since these initial findings, the ability of another closely related defensin from Nicotiana suaveolens, NsD7, to form oligomers with the membrane phospholipid, phosphatidic acid (PA), has been demonstrated, suggesting that these peptides may have evolved to target a range of membrane lipids to facilitate membrane disruption [86]. It is worth noting, however, that recent investigations into the antifungal mechanism of NaD1 by Bleackley and colleagues indicate a multi-faceted process in which cell wall binding, membrane lysis and cell entry by NaD1 are required to elicit fungal cell death [87]. In this setting, PIP2 is implicated as just one of several cellular ligands involved in the antifungal mechanism of NaD1.
Stemming from these investigations into the lipid-binding activity of plant defensins has been the discovery that a human defensin also mediates tumor cell membrane permeabilization via PIP2-binding [88]. The triple-stranded β-sheet peptide, human β-defensin 3 (HBD-3), which is structurally very similar to NaD1 and TPP3, displays selective anticancer activity against a range of mammalian tumor cell types through membrane lysis which is dependent on the presence of plasma membrane PIP2 [88]. It remains to be determined whether HBD-3 also undergoes oligomerization in its PIP2-binding mechanism of membrane lysis. Despite adopting a similar fold and a similar mechanism of PIP2-mediated membrane targeting, human β-defensins including HBD-3 have been recently described as evolutionarily unrelated to plant defensins [89, 90]. Thus, the ability of defensins across species and kingdoms to elicit cytotoxic activity via a common lipid ligand, suggests that PIP2-binding may remarkably be a convergent evolutionary trait of these peptides with potential for further development therapeutically.
Prospects for CAPs as anticancer therapeutics
Identification and characterization of the numerous CAPs that display anticancer properties, as well as the de novo synthesis of optimized derivatives, provides a rich source of knowledge from which a potentially valuable class of novel anticancer drugs may evolve. Although the majority of reports on CAP-mediated anticancer activity are still based on in vitro findings, much of the in vivo data available illustrates that many CAPs, including both those of α-helical and β-sheet structure, can effectively suppress tumor growth in mouse models. However, major challenges are faced in determining the therapeutic efficacy of CAPs, such as maintaining peptide stability in serum and overcoming toxicity within therapeutic windows. To date, there have been no CAPs that have reached human clinical trial status as anticancer drugs, further highlighting the progress yet to be made in this field.
Overcoming poor stability in serum
Poor stability of CAPs in human serum due to the presence of serum proteases can cause peptide degradation and therefore reduce bioavailability [91]. In addition, anionic serum proteins may interfere with charge-based peptide activity, leading to reduced efficacy. One approach to overcome this has been vector-mediated gene delivery of anticancer peptides in vivo [75]. Based on previous evidence that HNP-1 displayed immunomodulatory effects towards renal cell carcinoma and cervical cancer, Xu and colleagues intratumorally delivered HNP-1 in the form of plasmid DNA in an A549 xenograft model in mice. They demonstrated that vector-mediated expression of HNP-1 could effectively inhibit tumor growth via the induction of apoptosis, increase lymphocyte infiltration and inhibit angiogenesis [75]. For some amphipathic α-helical CAPs, the development of all-d amino acid derivatives has demonstrated improved stability in serum [48, 92, 93]. In the case of both the magainin derivative, MSI-238 and the pleurocidin-like [d]-NRC03, it was proposed that the observed increase in in vivo anticancer efficacy was owing to this stability [48, 93]. Other CAPs such as the antimicrobial, CSαβ-configured defensin from the Pesudoplectania nigrella fungus, plectasin, display natural stability in serum [94]. Whilst plectasin has not been investigated for its anticancer properties, it exhibits promising in vivo activity as an antibacterial agent against Staphlococcus aureus in combination with other commercial antibiotics [95]. This supports the notion that other peptides possessing the CSαβ architecture (such as NaD1 or TPP3) may also be suitably stable in serum for use in vivo as anticancer agents.
Overcoming toxicity, improving specificity
Some CAPs are effective against cancer cells but also display toxicity towards healthy cells or only demonstrate a low level of specificity for tumor cells. In vivo, this may translate to significant negative off-target effects and, for this reason, may not prove viable for therapeutic use. Therefore, where applicable, improving tumor cell specificity, or the possibility of specific tumor targeting, must also be considered. For example, in addition to the use of vector-mediated gene delivery of CAPs as a means of overcoming serum instability, this method has also been employed for its potential to reduce toxicity towards healthy cells and/or improving target cell specificity in vivo [96–98]. Intratumoral expression of HNP-1 used in combination with doxorubicin showed improved efficacy in tumor shrinkage and reduced lung metastasis in a 4T1 mouse model of breast cancer compared with single agent treatment [96]. More recently, an inducible adenovirus–melittin transgene vector designed to specifically target hepatocellular carcinoma cells in a xenograft mouse model, was able to significantly shrink tumor volume in mice and prolong lifespans, compared with controls [98]. An alternative approach to improve specificity with α-helical CAPs has been to develop hybrid peptides combining active regions of multiple peptides to improve specificity, such as the MG2-bombesin peptide and the cecropin–melittin hybrids, described above [52, 60, 61]. In addition, based on the discovery that all-d amino acid α-helical peptides increase stability in serum, the de novo design of diastereomeric peptides, that is, membranolytic peptides bearing a combination of both d- and l-amino acids, has also been investigated. In such studies, improved tumor cell specificity in vivo was observed due to optimized helical charge distribution [99, 100]. For example, the diastereomeric peptide l3,10,13k7,8K4R2L9 designed by Shai et al., when administered intravenously to tumor-bearing mice, displayed the ability to shrink tumor growth of both lung carcinoma and melanoma tumors, with minimal negative side effects in mice [99]. More recently, Khono et al. targeted EGFR-overexpressing tumor cells by designing a diastereomeric hybrid peptide consisting of EGFP binding peptide conjugated to an arginine-rich lytic peptide, called ‘EGFR-lytic’. This peptide was reported to successfully arrest tumor growth in athymic nude mouse xenograft models of human pancreatic and breast cancer, also with minimal side effects [101]. Rationally-designed novel peptides such as these continue to be developed for their potential both as anticancer and other therapeutic agents and are reviewed extensively elsewhere [102].
In summary, in addition to those CAPs that display potent in vivo activity in their native forms, the design of modified or novel peptides that can enhance activity or reduce negative side effects is proving a worthy pursuit for the future prospect of CAPs reaching the clinic as anticancer agents. Importantly, at the heart of successfully developing CAPs as cancer therapeutics is the need to fully uncover the molecular basis of their anticancer activity, both at the plasma membrane level as well as through determining their intracellular targets. Therefore, continued efforts are required to fully define the molecular mechanisms of CAP-mediated cell killing that will provide such insights in the future.
References
- 1.Chu E, DeVita VT, Jr, DeVita VT., Jr . Principles of cancer chemotherapy. Physicians’ cancer chemotherapy drug manual 2016. Burlington: Jones & Bartlett Publishers; 2015. pp. 1–4. [Google Scholar]
- 2.Wu Q, Yang Z, Nie Y, Shi Y, Fan D. Multi-drug resistance in cancer chemotherapeutics: mechanisms and lab approaches. Cancer Lett. 2014;347(2):159–166. doi: 10.1016/j.canlet.2014.03.013. [DOI] [PubMed] [Google Scholar]
- 3.WHO (2015) Cancer fact sheet 2015. http://www.who.int/mediacentre/factsheets/fs297/en/. Accessed 1 Dec 2016
- 4.Gaspar D, Castanho MA. Anticancer peptides: prospective innovation in cancer therapy. Host defense peptides and their potential as therapeutic agents. Berlin: Springer; 2016. pp. 95–109. [Google Scholar]
- 5.Riedl S, Zweytick D, Lohner K. Membrane-active host defense peptides—challenges and perspectives for the development of novel anticancer drugs. Chem Phys Lipid. 2011;164(8):766–781. doi: 10.1016/j.chemphyslip.2011.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Phoenix DA, Dennison SR, Harris F (2013) Cationic antimicrobial peptides. Antimicrobial Peptides, Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim, Germany, pp 39–81
- 7.Hancock RE, Diamond G. The role of cationic antimicrobial peptides in innate host defences. Trends Microbiol. 2000;8(9):402–410. doi: 10.1016/S0966-842X(00)01823-0. [DOI] [PubMed] [Google Scholar]
- 8.Brogden KA. Antimicrobial peptides: pore formers or metabolic inhibitors in bacteria? Nat Rev Microbiol. 2005;3(3):238–250. doi: 10.1038/nrmicro1098. [DOI] [PubMed] [Google Scholar]
- 9.Mader JS, Hoskin DW. Cationic antimicrobial peptides as novel cytotoxic agents for cancer treatment. Expert Opin Investig Drugs. 2006;15(8):933–946. doi: 10.1517/13543784.15.8.933. [DOI] [PubMed] [Google Scholar]
- 10.Chen J-Y, Lin W-J, Wu J-L, Her GM, Hui C-F. Epinecidin-1 peptide induces apoptosis which enhances antitumor effects in human leukemia U937 cells. Peptides. 2009;30(12):2365–2373. doi: 10.1016/j.peptides.2009.08.019. [DOI] [PubMed] [Google Scholar]
- 11.Mader JS, Richardson A, Salsman J, Top D, de Antueno R, Duncan R, et al. Bovine lactoferricin causes apoptosis in Jurkat T-leukemia cells by sequential permeabilization of the cell membrane and targeting of mitochondria. Exp Cell Res. 2007;313(12):2634–2650. doi: 10.1016/j.yexcr.2007.05.015. [DOI] [PubMed] [Google Scholar]
- 12.Rozek A, Pocwers JP-S, Friedrich CL, Hancock RE. Structure-based design of an indolicidin peptide analogue with increased protease stability. Biochemistry. 2003;42(48):14130–14138. doi: 10.1021/bi035643g. [DOI] [PubMed] [Google Scholar]
- 13.Fahrner RL, Dieckmann T, Harwig SS, Lehrer RI, Eisenberg D, Feigon J. Solution structure of protegrin-1, a broad-spectrum antimicrobial peptide from porcine leukocytes. Chem Biol. 1996;3(7):543–550. doi: 10.1016/S1074-5521(96)90145-3. [DOI] [PubMed] [Google Scholar]
- 14.Conibear AC, Rosengren KJ, Harvey PJ, Craik DJ. Structural characterization of the cyclic cystine ladder motif of θ-defensins. Biochemistry. 2012;51(48):9718–9726. doi: 10.1021/bi301363a. [DOI] [PubMed] [Google Scholar]
- 15.Hoover DM, Rajashankar KR, Blumenthal R, Puri A, Oppenheim JJ, Chertov O, et al. The structure of human β-defensin-2 shows evidence of higher order oligomerization. J Biol Chem. 2000;275(42):32911–32918. doi: 10.1074/jbc.M006098200. [DOI] [PubMed] [Google Scholar]
- 16.Poon IK, Baxter AA, Lay FT, Mills GD, Adda CG, Payne JA, et al. Phosphoinositide-mediated oligomerization of a defensin induces cell lysis. eLife. 2014;3:e01808. doi: 10.7554/eLife.01808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schrödinger (2016) PyMOL Molecular Graphics System, Version 1.8
- 18.Wang G, Li X, Wang Z. APD3: the antimicrobial peptide database as a tool for research and education. Nucleic Acids Res. 2016;44(D1):D1087–D1093. doi: 10.1093/nar/gkv1278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hancock RE, Sahl H-G. Antimicrobial and host-defense peptides as new anti-infective therapeutic strategies. Nat Biotechnol. 2006;24(12):1551–1557. doi: 10.1038/nbt1267. [DOI] [PubMed] [Google Scholar]
- 20.Yin LM, Edwards MA, Li J, Yip CM, Deber CM. Roles of hydrophobicity and charge distribution of cationic antimicrobial peptides in peptide-membrane interactions. J Biol Chem. 2012;287(10):7738–7745. doi: 10.1074/jbc.M111.303602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ran S, Downes A, Thorpe PE. Increased exposure of anionic phospholipids on the surface of tumor blood vessels. Can Res. 2002;62(21):6132–6140. [PubMed] [Google Scholar]
- 22.Balasubramanian K, Schroit AJ. Aminophospholipid asymmetry: a matter of life and death. Annu Rev Physiol. 2003;65(1):701–734. doi: 10.1146/annurev.physiol.65.092101.142459. [DOI] [PubMed] [Google Scholar]
- 23.Ran S, Thorpe PE. Phosphatidylserine is a marker of tumor vasculature and a potential target for cancer imaging and therapy. Int J Radiat Oncol Biol Phys. 2002;54(5):1479–1484. doi: 10.1016/S0360-3016(02)03928-7. [DOI] [PubMed] [Google Scholar]
- 24.Lima LG, Monteiro RQ. Activation of blood coagulation in cancer: implications for tumour progression. Biosci Rep. 2013;33(5):701–710. doi: 10.1042/BSR20130057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schröder-Borm H, Bakalova R, Andrä J. The NK-lysin derived peptide NK-2 preferentially kills cancer cells with increased surface levels of negatively charged phosphatidylserine. FEBS Lett. 2005;579(27):6128–6134. doi: 10.1016/j.febslet.2005.09.084. [DOI] [PubMed] [Google Scholar]
- 26.Riedl S, Rinner B, Schaider H, Lohner K, Zweytick D. Killing of melanoma cells and their metastases by human lactoferricin derivatives requires interaction with the cancer marker phosphatidylserine. Biometals. 2014;27(5):981–997. doi: 10.1007/s10534-014-9749-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Matsuzaki K, Harada M, Handa T, Funakoshi S, Fujii N, Yajima H, et al. Magainin 1-induced leakage of entrapped calcein out of negatively-charged lipid vesicles. Biochim Biophys Acta Biomembr. 1989;981(1):130–134. doi: 10.1016/0005-2736(89)90090-4. [DOI] [PubMed] [Google Scholar]
- 28.Iwasaki T, Ishibashi J, Tanaka H, Sato M, Asaoka A, Taylor D, et al. Selective cancer cell cytotoxicity of enantiomeric 9-mer peptides derived from beetle defensins depends on negatively charged phosphatidylserine on the cell surface. Peptides. 2009;30(4):660–668. doi: 10.1016/j.peptides.2008.12.019. [DOI] [PubMed] [Google Scholar]
- 29.Riedl S, Leber R, Rinner B, Schaider H, Lohner K, Zweytick D. Human lactoferricin derived di-peptides deploying loop structures induce apoptosis specifically in cancer cells through targeting membranous phosphatidylserine. Biochim Biophys Acta Biomembr. 2015;1848(11):2918–2931. doi: 10.1016/j.bbamem.2015.07.018. [DOI] [PubMed] [Google Scholar]
- 30.Kufe DW. Mucins in cancer: function, prognosis and therapy. Nat Rev Cancer. 2009;9(12):874–885. doi: 10.1038/nrc2761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Raman K, Kuberan B. Chemical tumor biology of heparan sulfate proteoglycans. Curr Chem Biol. 2010;4(1):20. doi: 10.2174/187231310790226206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sok M, Šentjurc M, Schara M. Membrane fluidity characteristics of human lung cancer. Cancer Lett. 1999;139(2):215–220. doi: 10.1016/S0304-3835(99)00044-0. [DOI] [PubMed] [Google Scholar]
- 33.Deliconstantinos G. Physiological aspects of membrane lipid fluidity in malignancy. Anticancer Res. 1986;7(5B):1011–1021. [PubMed] [Google Scholar]
- 34.Zeisig R, Koklič T, Wiesner B, Fichtner I, Sentjurč M. Increase in fluidity in the membrane of MT3 breast cancer cells correlates with enhanced cell adhesion in vitro and increased lung metastasis in NOD/SCID mice. Arch Biochem Biophys. 2007;459(1):98–106. doi: 10.1016/j.abb.2006.09.030. [DOI] [PubMed] [Google Scholar]
- 35.Taraboletti G, Perin L, Bottazzi B, Mantovani A, Giavazzi R, Salmona M. Membrane fluidity affects tumor-cell motility, invasion and lung-colonizing potential. Int J Cancer. 1989;44(4):707–713. doi: 10.1002/ijc.2910440426. [DOI] [PubMed] [Google Scholar]
- 36.Chen HM, Wang W, Smith D, Chan SC. Effects of the anti-bacterial peptide cecropin B and its analogs, cecropins B-1 and B-2, on liposomes, bacteria, and cancer cells. Biochim Biophys Acta Gen Subj. 1997;1336(2):171–179. doi: 10.1016/S0304-4165(97)00024-X. [DOI] [PubMed] [Google Scholar]
- 37.Chaudhary J, Munshi M. Scanning electron microscopic analysis of breast aspirates. Cytopathology. 1995;6(3):162–167. doi: 10.1111/j.1365-2303.1995.tb00469.x. [DOI] [PubMed] [Google Scholar]
- 38.Ren J, Ji Hamada, Okada F, Takeichi N, Morikawa K, Hosokawa M, et al. Correlation between the presence of microvilli and the growth or metastatic potential of tumor cells. Jpn J Cancer Res. 1990;81(9):920–926. doi: 10.1111/j.1349-7006.1990.tb02668.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Turner J, Cho Y, Dinh N-N, Waring AJ, Lehrer RI. Activities of LL-37, a cathelin-associated antimicrobial peptide of human neutrophils. Antimicrob Agents Chemother. 1998;42(9):2206–2214. doi: 10.1128/aac.42.9.2206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gordon YJ, Huang LC, Romanowski EG, Yates KA, Proske RJ, McDermott AM. Human cathelicidin (LL-37), a multifunctional peptide, is expressed by ocular surface epithelia and has potent antibacterial and antiviral activity. Curr Eye Res. 2005;30(5):385–394. doi: 10.1080/02713680590934111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Niyonsaba F, Iwabuchi K, Someya A, Hirata M, Matsuda H, Ogawa H, et al. A cathelicidin family of human antibacterial peptide LL-37 induces mast cell chemotaxis. Immunology. 2002;106(1):20–26. doi: 10.1046/j.1365-2567.2002.01398.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Carretero M, Escámez MJ, García M, Duarte B, Holguín A, Retamosa L, et al. In vitro and in vivo wound healing-promoting activities of human cathelicidin LL-37. J Investig Dermatol. 2008;128(1):223–236. doi: 10.1038/sj.jid.5701043. [DOI] [PubMed] [Google Scholar]
- 43.Xhindoli D, Pacor S, Guida F, Antcheva N, Tossi A. Native oligomerization determines the mode of action and biological activities of human cathelicidin LL-37. Biochem J. 2014;457(2):263–275. doi: 10.1042/BJ20131048. [DOI] [PubMed] [Google Scholar]
- 44.Xhindoli D, Pacor S, Benincasa M, Scocchi M, Gennaro R, Tossi A. The human cathelicidin LL-37—a pore-forming antibacterial peptide and host-cell modulator. Biochim Biophys Acta Biomembr. 2016;1858(3):546–566. doi: 10.1016/j.bbamem.2015.11.003. [DOI] [PubMed] [Google Scholar]
- 45.Mader JS, Mookherjee N, Hancock RE, Bleackley RC. The human host defense peptide LL-37 induces apoptosis in a calpain-and apoptosis-inducing factor-dependent manner involving bax activity. Mol Cancer Res. 2009;7(5):689–702. doi: 10.1158/1541-7786.MCR-08-0274. [DOI] [PubMed] [Google Scholar]
- 46.Risso A, Zanetti M, Gennaro R. Cytotoxicity and apoptosis mediated by two peptides of innate immunity. Cell Immunol. 1998;189(2):107–115. doi: 10.1006/cimm.1998.1358. [DOI] [PubMed] [Google Scholar]
- 47.Risso A, Braidot E, Sordano MC, Vianello A, Macrì F, Skerlavaj B, et al. BMAP-28, an antibiotic peptide of innate immunity, induces cell death through opening of the mitochondrial permeability transition pore. Mol Cell Biol. 2002;22(6):1926–1935. doi: 10.1128/MCB.22.6.1926-1935.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Baker MA, Maloy WL, Zasloff M, Jacob LS. Anticancer efficacy of Magainin2 and analogue peptides. Can Res. 1993;53(13):3052–3057. [PubMed] [Google Scholar]
- 49.Koszałka P, Kamysz E, Wejda M, Kamysz W, Bigda J. Antitumor activity of antimicrobial peptides against U937 histiocytic cell line. Acta Biochim Pol. 2011;58(1):111–117. [PubMed] [Google Scholar]
- 50.Bessalle R, Kapitkovsky A, Gorea A, Shalit I, Fridkin M. All-d-magainin: chirality, antimicrobial activity and proteolytic resistance. FEBS Lett. 1990;274(1):151–155. doi: 10.1016/0014-5793(90)81351-n. [DOI] [PubMed] [Google Scholar]
- 51.Bland JM, De Lucca AJ, Jacks TJ, Vigo CB. All-d-cecropin B: synthesis, conformation, lipopolysaccharide binding, and antibacterial activity. Mol Cell Biochem. 2001;218(1):105–111. doi: 10.1023/A:1007293816634. [DOI] [PubMed] [Google Scholar]
- 52.Liu S, Yang H, Wan L, H-w Cai, S-f Li, Y-p Li, et al. Enhancement of cytotoxicity of antimicrobial peptide magainin II in tumor cells by bombesin-targeted delivery. Acta Pharmacol Sin. 2011;32(1):79–88. doi: 10.1038/aps.2010.162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Yin Z-X, He W, Chen W-J, Yan J-H, Yang J-N, Chan S-M, et al. Cloning, expression and antimicrobial activity of an antimicrobial peptide, epinecidin-1, from the orange-spotted grouper, Epinephelus coioides . Aquaculture. 2006;253(1):204–211. doi: 10.1016/j.aquaculture.2005.10.002. [DOI] [Google Scholar]
- 54.Lin W-J, Chien Y-L, Pan C-Y, Lin T-L, Chen J-Y, Chiu S-J, et al. Epinecidin-1, an antimicrobial peptide from fish (Epinephelus coioides) which has an antitumor effect like lytic peptides in human fibrosarcoma cells. Peptides. 2009;30(2):283–290. doi: 10.1016/j.peptides.2008.10.007. [DOI] [PubMed] [Google Scholar]
- 55.Suttmann H, Retz M, Paulsen F, Harder J, Zwergel U, Kamradt J, et al. Antimicrobial peptides of the Cecropin-family show potent antitumor activity against bladder cancer cells. BMC Urol. 2008;8(1):5. doi: 10.1186/1471-2490-8-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Jin X-B, Wang Y-J, Liang L-L, Pu Q-H, Shen J, Lu X-M, et al. Cecropin suppresses human hepatocellular carcinoma BEL-7402 cell growth and survival in vivo without side-toxicity. Asian Pac J Cancer Prev. 2014;15(13):5433. doi: 10.7314/APJCP.2014.15.13.5433. [DOI] [PubMed] [Google Scholar]
- 57.Gajski G, Garaj-Vrhovac V. Melittin: a lytic peptide with anticancer properties. Environ Toxicol Pharmacol. 2013;36(2):697–705. doi: 10.1016/j.etap.2013.06.009. [DOI] [PubMed] [Google Scholar]
- 58.Killion JJ, Dunn JD. Differential cytolysis of murine spleen, bone-marrow and leukemia cells by melittin reveals differences in membrane topography. Biochem Biophys Res Commun. 1986;139(1):222–227. doi: 10.1016/S0006-291X(86)80102-4. [DOI] [PubMed] [Google Scholar]
- 59.Midoux P, Mayer R, Monsigny M. Membrane permeabilization by α-helical peptides: a flow cytometry study. Biochim Biophys Acta Biomembr. 1995;1239(2):249–256. doi: 10.1016/0005-2736(95)00163-W. [DOI] [PubMed] [Google Scholar]
- 60.Andreu D, Ubach J, Boman A, Wåhlin B, Wade D, Merrifield R, et al. Shortened cecropin A-melittin hybrids significant size reduction retains potent antibiotic activity. FEBS Lett. 1992;296(2):190–194. doi: 10.1016/0014-5793(92)80377-S. [DOI] [PubMed] [Google Scholar]
- 61.Schlamadinger DE, Wang Y, McCammon JA, Kim JE. Spectroscopic and computational study of melittin, cecropin A, and the hybrid peptide CM15. J Phys Chem B. 2012;116(35):10600–10608. doi: 10.1021/jp304021t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Sun D, Sun M, Zhu W, Wang Z, Li Y, Ma J. The anti-cancer potency and mechanism of a novel tumor-activated fused toxin. DLM Toxins. 2015;7(2):423–438. doi: 10.3390/toxins7020423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Mader JS, Salsman J, Conrad DM, Hoskin DW. Bovine lactoferricin selectively induces apoptosis in human leukemia and carcinoma cell lines. Mol Cancer Ther. 2005;4(4):612–624. doi: 10.1158/1535-7163.MCT-04-0077. [DOI] [PubMed] [Google Scholar]
- 64.Pan W-R, Chen P-W, Chen Y-L, Hsu H-C, Lin C-C, Chen W-J. Bovine lactoferricin B induces apoptosis of human gastric cancer cell line AGS by inhibition of autophagy at a late stage. J Dairy Sci. 2013;96(12):7511–7520. doi: 10.3168/jds.2013-7285. [DOI] [PubMed] [Google Scholar]
- 65.Yoo YC, Watanabe S, Watanabe R, Hata K, Shimazaki KI, Azuma I. Bovine lactoferrin and lactoferricin, a peptide derived from bovine lactoferrin, inhibit tumor metastasis in mice. Jpn J Cancer Res. 1997;88(2):184–190. doi: 10.1111/j.1349-7006.1997.tb00364.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Eliassen LT, Berge G, Leknessund A, Wikman M, Lindin I, Løkke C, et al. The antimicrobial peptide, lactoferricin B, is cytotoxic to neuroblastoma cells in vitro and inhibits xenograft growth in vivo. Int J Cancer. 2006;119(3):493–500. doi: 10.1002/ijc.21886. [DOI] [PubMed] [Google Scholar]
- 67.Solarte VA, Conget P, Vernot J-P, Rosas JE, Rivera ZJ, García JE, et al. A tetrameric peptide derived from bovine lactoferricin as a potential therapeutic tool for oral squamous cell carcinoma: a preclinical model. PLoS One. 2017;12(3):e0174707. doi: 10.1371/journal.pone.0174707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Soletti RC, Del Barrio L, Daffre S, Miranda A, Borges HL, Moura-Neto V, et al. Peptide gomesin triggers cell death through l-type channel calcium influx, MAPK/ERK, PKC and PI3K signaling and generation of reactive oxygen species. Chem Biol Interact. 2010;186(2):135–143. doi: 10.1016/j.cbi.2010.04.012. [DOI] [PubMed] [Google Scholar]
- 69.Rodrigues EG, Dobroff AS, Cavarsan CF, Paschoalin T, Nimrichter L, Mortara RA, et al. Effective topical treatment of subcutaneous murine B16F10-Nex2 melanoma by the antimicrobial peptide gomesin. Neoplasia. 2008;10(1):61–68. doi: 10.1593/neo.07885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Chen Y, Xu X, Hong S, Chen J, Liu N, Underhill CB, et al. RGD-Tachyplesin inhibits tumor growth. Can Res. 2001;61(6):2434–2438. [PubMed] [Google Scholar]
- 71.Chen J, Xu X-M, Underhill CB, Yang S, Wang L, Chen Y, et al. Tachyplesin activates the classic complement pathway to kill tumor cells. Can Res. 2005;65(11):4614–4622. doi: 10.1158/0008-5472.CAN-04-2253. [DOI] [PubMed] [Google Scholar]
- 72.Lichtenstein A, Ganz T, Selsted M, Lehrer R. In vitro tumor cell cytolysis mediated by peptide defensins of human and. Blood. 1986;68(6):1407–1410. [PubMed] [Google Scholar]
- 73.Hill CP, Yee J, Selsted ME, Eisenberg D. Crystal structure of defensin HNP-3, an amphiphilic dimer: mechanisms of membrane permeabilization. Science. 1991;251(5000):1481–1485. doi: 10.1126/science.2006422. [DOI] [PubMed] [Google Scholar]
- 74.Szyk A, Wu Z, Tucker K, Yang D, Lu W, Lubkowski J. Crystal structures of human α-defensins HNP4, HD5, and HD6. Protein Sci. 2006;15(12):2749–2760. doi: 10.1110/ps.062336606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Xu N, Y-s Wang, W-b Pan, Xiao B, Y-j Wen, X-c Chen, et al. Human α-defensin-1 inhibits growth of human lung adenocarcinoma xenograft in nude mice. Mol Cancer Ther. 2008;7(6):1588–1597. doi: 10.1158/1535-7163.MCT-08-0010. [DOI] [PubMed] [Google Scholar]
- 76.Terras FR, Eggermont K, Kovaleva V, Raikhel NV, Osborn RW, Kester A, et al. Small cysteine-rich antifungal proteins from radish: their role in host defense. Plant Cell. 1995;7(5):573–588. doi: 10.1105/tpc.7.5.573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Cornet B, Bonmatin J-M, Hetru C, Hoffmann JA, Ptak M, Vovelle F. Refined three-dimensional solution structure of insect defensin A. Structure. 1995;3(5):435–448. doi: 10.1016/S0969-2126(01)00177-0. [DOI] [PubMed] [Google Scholar]
- 78.Thomma BP, Cammue BP, Thevissen K. Plant defensins. Planta. 2002;216(2):193–202. doi: 10.1007/s00425-002-0902-6. [DOI] [PubMed] [Google Scholar]
- 79.Wong JH, Ng TB. Sesquin, a potent defensin-like antimicrobial peptide from ground beans with inhibitory activities toward tumor cells and HIV-1 reverse transcriptase. Peptides. 2005;26(7):1120–1126. doi: 10.1016/j.peptides.2005.01.003. [DOI] [PubMed] [Google Scholar]
- 80.Wong JH, Ng TB. Lunatusin, a trypsin-stable antimicrobial peptide from lima beans (Phaseolus lunatus L.) Peptides. 2005;26(11):2086–2092. doi: 10.1016/j.peptides.2005.03.004. [DOI] [PubMed] [Google Scholar]
- 81.Wang H, Ng T. Isolation and characterization of an antifungal peptide with antiproliferative activity from seeds of Phaseolus vulgaris cv. ‘Spotted Bean’. Appl Microbiol Biotechnol. 2007;74(1):125–130. doi: 10.1007/s00253-006-0650-9. [DOI] [PubMed] [Google Scholar]
- 82.Lin P, Wong JH, Ng TB. A defensin with highly potent antipathogenic activities from the seeds of purple pole bean. Biosci Rep. 2010;30(2):101–109. doi: 10.1042/BSR20090004. [DOI] [PubMed] [Google Scholar]
- 83.Baxter AA, Richter V, Lay FT, Poon IK, Adda CG, Veneer PK, et al. The tomato defensin TPP3 binds phosphatidylinositol (4,5)-bisphosphate via a conserved dimeric cationic grip conformation to mediate cell lysis. Mol Cell Biol. 2015;35(11):1964–1978. doi: 10.1128/MCB.00282-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Payne JA, Bleackley MR, Lee T-H, Shafee TM, Poon IK, Hulett MD, et al. The plant defensin NaD1 introduces membrane disorder through a specific interaction with the lipid, phosphatidylinositol 4,5 bisphosphate. Biochim Biophys Acta Biomembr. 2016;1858(6):1099–1109. doi: 10.1016/j.bbamem.2016.02.016. [DOI] [PubMed] [Google Scholar]
- 85.Baxter AA, Poon IK, Hulett MD. The plant defensin NaD1 induces tumor cell death via a non-apoptotic, membranolytic process. Cell Death Discov. 2017;3:16102. doi: 10.1038/cddiscovery.2016.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Kvansakul M, Lay FT, Adda CG, Veneer PK, Baxter AA, Phan TK, et al. Binding of phosphatidic acid by NsD7 mediates the formation of helical defensin–lipid oligomeric assemblies and membrane permeabilization. Proc Natl Acad Sci. 2016;113(40):11202–11207. doi: 10.1073/pnas.1607855113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Bleackley MR, Payne JA, Hayes BM, Durek T, Craik DJ, Shafee TM, et al. Nicotiana alata defensin chimeras reveal differences in the mechanism of fungal and tumour cell killing and an enhanced antifungal variant. Antimicrob Agents Chemother. 2016;60(10):6302–6312. doi: 10.1128/AAC.01479-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Phan TK, Lay FT, Poon IK, Hinds MG, Kvansakul M, Hulett MD. Human β-defensin 3 contains an oncolytic motif that binds PI(4,5)P2 to mediate tumour cell permeabilisation. Oncotarget. 2016;7(2):2054. doi: 10.18632/oncotarget.6520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Shafee TM, Lay FT, Hulett MD, Anderson MA. The defensins consist of two independent, convergent protein superfamilies. Mol Biol Evol. 2016;33(9):2345–2356. doi: 10.1093/molbev/msw106. [DOI] [PubMed] [Google Scholar]
- 90.Shafee TM, Lay FT, Phan TK, Anderson MA, Hulett MD. Convergent evolution of defensin sequence, structure and function. Cell Mol Life Sci. 2016;74(4):663–682. doi: 10.1007/s00018-016-2344-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Nguyen LT, Chau JK, Perry NA, De Boer L, Zaat S, Vogel HJ. Serum stabilities of short tryptophan-and arginine-rich antimicrobial peptide analogs. PLoS One. 2010;5(9):e12684. doi: 10.1371/journal.pone.0012684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Molhoek EM, Van Dijk A, Veldhuizen EJ, Haagsman HP, Bikker FJ. Improved proteolytic stability of chicken cathelicidin-2 derived peptides by d-amino acid substitutions and cyclization. Peptides. 2011;32(5):875–880. doi: 10.1016/j.peptides.2011.02.017. [DOI] [PubMed] [Google Scholar]
- 93.Hilchie AL, Haney EF, Pinto DM, Hancock RE, Hoskin DW. Enhanced killing of breast cancer cells by a d-amino acid analog of the winter flounder-derived pleurocidin NRC-03. Exp Mol Pathol. 2015;99(3):426–434. doi: 10.1016/j.yexmp.2015.08.021. [DOI] [PubMed] [Google Scholar]
- 94.Mygind PH, Fischer RL, Schnorr KM, Hansen MT, Sönksen CP, Ludvigsen S, et al. Plectasin is a peptide antibiotic with therapeutic potential from a saprophytic fungus. Nature. 2005;437(7061):975–980. doi: 10.1038/nature04051. [DOI] [PubMed] [Google Scholar]
- 95.Zhang Y, Teng D, Mao R, Wang X, Xi D, Hu X, et al. High expression of a plectasin-derived peptide NZ2114 in Pichia pastoris and its pharmacodynamics, postantibiotic and synergy against Staphylococcus aureus . Appl Microbiol Biotechnol. 2014;98(2):681–694. doi: 10.1007/s00253-013-4881-2. [DOI] [PubMed] [Google Scholar]
- 96.Li D, Qin Q, Wang X-Y, Shi H-S, Luo M, Guo F-C, et al. Intratumoral expression of mature human neutrophil peptide-1 potentiates the therapeutic effect of doxorubicin in a mouse 4T1 breast cancer model. Oncol Rep. 2014;31(3):1287–1295. doi: 10.3892/or.2013.2947. [DOI] [PubMed] [Google Scholar]
- 97.Winder D, Günzburg WH, Erfle V, Salmons B. Expression of antimicrobial peptides has an antitumour effect in human cells. Biochem Biophys Res Commun. 1998;242(3):608–612. doi: 10.1006/bbrc.1997.8014. [DOI] [PubMed] [Google Scholar]
- 98.Qian C-Y, Wang K-L, Fang F-F, Gu W, Huang F, Wang F-Z, et al. Triple-controlled oncolytic adenovirus expressing melittin to exert inhibitory efficacy on hepatocellular carcinoma. Int J Clin Exp Pathol. 2015;8(9):10403. [PMC free article] [PubMed] [Google Scholar]
- 99.Papo N, Shahar M, Eisenbach L, Shai Y. A novel lytic peptide composed of dl-amino acids selectively kills cancer cells in culture and in mice. J Biol Chem. 2003;278(23):21018–21023. doi: 10.1074/jbc.M211204200. [DOI] [PubMed] [Google Scholar]
- 100.Huang Y, He L, Li G, Zhai N, Jiang H, Chen Y. Role of helicity of α-helical antimicrobial peptides to improve specificity. Protein Cell. 2014;5(8):631–642. doi: 10.1007/s13238-014-0061-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Kohno M, Horibe T, Haramoto M, Yano Y, Ohara K, Nakajima O, et al. A novel hybrid peptide targeting EGFR-expressing cancers. Eur J Cancer. 2011;47(5):773–783. doi: 10.1016/j.ejca.2010.10.021. [DOI] [PubMed] [Google Scholar]
- 102.Li H, Anuwongcharoen N, Malik AA, Prachayasittikul V, Wikberg JE, Nantasenamat C. Roles of d-amino acids on the bioactivity of host defense peptides. Int J Mol Sci. 2016;17(7):1023. doi: 10.3390/ijms17071023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Ren SX, Shen J, Cheng AS, Lu L, Chan RL, Li ZJ, et al. FK-16 derived from the anticancer peptide LL-37 induces caspase-independent apoptosis and autophagic cell death in colon cancer cells. PLoS One. 2013;8(5):e63641. doi: 10.1371/journal.pone.0063641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Wang H, Ke M, Tian Y, Wang J, Li B, Wang Y, et al. BF-30 selectively inhibits melanoma cell proliferation via cytoplasmic membrane permeabilization and DNA-binding in vitro and in B16F10-bearing mice. Eur J Pharmacol. 2013;707(1):1–10. doi: 10.1016/j.ejphar.2013.03.028. [DOI] [PubMed] [Google Scholar]
- 105.Tian Y, Wang H, Li B, Ke M, Wang J, Dou J, et al. The cathelicidin-BF Lys16 mutant Cbf-K16 selectively inhibits non-small cell lung cancer proliferation in vitro. Oncol Rep. 2013;30(5):2502–2510. doi: 10.3892/or.2013.2693. [DOI] [PubMed] [Google Scholar]
- 106.Lehmann J, Retz M, Sidhu SS, Suttmann H, Sell M, Paulsen F, et al. Antitumor activity of the antimicrobial peptide magainin II against bladder cancer cell lines. Eur Urol. 2006;50(1):141–147. doi: 10.1016/j.eururo.2005.12.043. [DOI] [PubMed] [Google Scholar]
- 107.Ohsaki Y, Gazdar AF, Chen H-C, Johnson BE. Antitumor activity of magainin analogues against human lung cancer cell lines. Can Res. 1992;52(13):3534–3538. [PubMed] [Google Scholar]
- 108.Liu S, Yang H, Wan L, Cheng J, Lu X. Penetratin-mediated delivery enhances the antitumor activity of the cationic antimicrobial peptide magainin II. Cancer Biother Radiopharm. 2013;28(4):289–297. doi: 10.1089/cbr.2012.1328. [DOI] [PubMed] [Google Scholar]
- 109.Lee HS, Park CB, Kim JM, Jang SA, Park IY, Kim MS, et al. Mechanism of anticancer activity of buforin IIb, a histone H2A-derived peptide. Cancer Lett. 2008;271(1):47–55. doi: 10.1016/j.canlet.2008.05.041. [DOI] [PubMed] [Google Scholar]
- 110.Chen J-Y, Lin W-J, Lin T-L. A fish antimicrobial peptide, tilapia hepcidin TH2-3, shows potent antitumor activity against human fibrosarcoma cells. Peptides. 2009;30(9):1636–1642. doi: 10.1016/j.peptides.2009.06.009. [DOI] [PubMed] [Google Scholar]
- 111.Hilchie AL, Doucette CD, Pinto DM, Patrzykat A, Douglas S, Hoskin DW. Pleurocidin-family cationic antimicrobial peptides are cytolytic for breast carcinoma cells and prevent growth of tumor xenografts. Breast Cancer Res. 2011;13(5):1. doi: 10.1186/bcr3043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Wang C, Tian L-L, Li S, Li H-B, Zhou Y, Wang H, et al. Rapid cytotoxicity of antimicrobial peptide tempoprin-1CEa in breast cancer cells through membrane destruction and intracellular calcium mechanism. PLoS One. 2013;8(4):e60462. doi: 10.1371/journal.pone.0060462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Wang C, Zhou Y, Li S, Li H, Tian L, Wang H, et al. Anticancer mechanisms of temporin-1CEa, an amphipathic α-helical antimicrobial peptide, in Bcap-37 human breast cancer cells. Life Sci. 2013;92(20):1004–1014. doi: 10.1016/j.lfs.2013.03.016. [DOI] [PubMed] [Google Scholar]
- 114.Cerón JM, Contreras-Moreno J, Puertollano E, De Cienfuegos GA, Puertollano MA, De Pablo MA. The antimicrobial peptide cecropin A induces caspase-independent cell death in human promyelocytic leukemia cells. Peptides. 2010;31(8):1494–1503. doi: 10.1016/j.peptides.2010.05.008. [DOI] [PubMed] [Google Scholar]
- 115.Jin X, Mei H, Li X, Ma Y, A-H Zeng, Wang Y, et al. Apoptosis-inducing activity of the antimicrobial peptide cecropin of Musca domestica in human hepatocellular carcinoma cell line BEL-7402 and the possible mechanism. Acta Biochim Biophys Sin. 2010;42(2):259–265. doi: 10.1093/abbs/gmq021. [DOI] [PubMed] [Google Scholar]
- 116.McKeown ST, Lundy FT, Nelson J, Lockhart D, Irwin CR, Cowan CG, et al. The cytotoxic effects of human neutrophil peptide-1 (HNP1) and lactoferrin on oral squamous cell carcinoma (OSCC) in vitro. Oral Oncol. 2006;42(7):685–690. doi: 10.1016/j.oraloncology.2005.11.005. [DOI] [PubMed] [Google Scholar]
- 117.Barker E, Reisfeld RA. A mechanism for neutrophil-mediated lysis of human neuroblastoma cells. Can Res. 1993;53(2):362–367. [PubMed] [Google Scholar]
- 118.Furlong SJ, Mader JS, Hoskin DW. Lactoferricin-induced apoptosis in estrogen-nonresponsive MDA-MB-435 breast cancer cells is enhanced by C6 ceramide or tamoxifen. Oncol Rep. 2006;15(5):1385–1390. [PubMed] [Google Scholar]
- 119.Furlong SJ, Ridgway ND, Hoskin DW. Modulation of ceramide metabolism in T-leukemia cell lines potentiates apoptosis induced by the cationic antimicrobial peptide bovine lactoferricin. Int J Oncol. 2008;32(3):537–544. [PubMed] [Google Scholar]