Abstract
Chromosome 17 abnormalities are often observed in medulloblastomas (MBs), particularly those classified in the consensus Groups 3 and 4. Herein we review MB signature genes associated with chromosome 17 and the relationship of these signature genes to the ubiquitin-proteasome system. While clinical investigators have not focused on the ubiquitin-proteasome system in relation to MB, a substantial amount of data on the topic has been hidden in the form of supplemental datasets of gene expression. A supplemental dataset associated with the Thompson classification of MBs shows that a subgroup of MB with 17p deletions is characterized by reduced expression of genes for several core particle subunits of the beta ring of the proteasome (β1, β4, β5, β7). One of these genes (PSMB6, the gene for the β1 subunit) is located on chromosome 17, near the telomeric end of 17p. By comparison, in the WNT group of MBs only one core proteasome subunit, β6, associated with loss of a gene (PSMB1) on chromosome 6, was down-regulated in this dataset. The MB subgroups with the worst prognosis have a significant association with chromosome 17 abnormalities and irregularities of APC/C cyclosome genes. We conclude that the expression of proteasome subunit genes and genes for ubiquitin ligases can contribute to prognostic classification of MBs. The therapeutic value of targeting proteasome subunits and ubiquitin ligases in the various subgroups of MB remains to be determined separately for each classification of MB.
Keywords: Medulloblastoma, Chromosome 17, Ubiquitin ligase, APC/C cyclosome, Proteasome subunits
Introduction
Medulloblastoma (MB) is a malignant embryonic brain tumor that typically constitutes two-thirds of all pediatric brain cancer cases [1, 2]. MB is a cerebellar-specific tumor of the posterior fossa that originates from cerebellar granule cell precursors [3]. Cerebellar granule cell precursors originate from the developing rhombic lip (or caudomedial germinal zone) and the external germinal layer (EGL) within the hindbrain of embryos [4]. These cells undergo proliferation for an extensive period, well into postnatal stages of development, to form the major cell type of the cerebellum, which is the most populous neuron within the brain. Granule cell precursors and the molecular mechanisms involved in controlling their proliferation have been shown to be involved in the pathogenesis of MB. Granule cells are generated in response to molecular regulators of cell proliferation and differentiation during development [3].
Several subgroups of MB have been described, based on histopathology, genomic features, clinical characteristics, and cell type of origin. While there are various classifications of MB, in the consensus classification of 2012 [5, 6] four subgroups of MB are recognized: the WNT (Wingless-related Integration) group, the SHH (Sonic Hedgehog) group, and consensus Group 3 and Group 4. In the WNT group there is upregulation of WNT signaling while in the SHH group there is upregulation of SHH signaling, PTCH1 (Patched 1) and GLI1 (glioma-associated oncogene 1) [7, 8]. Groups 3 and 4 have been defined partly in terms of Myc (avian myelocytomatosis viral oncogene homolog) and MycN (neuroblastoma MYC oncogene) expression [9]. The relative percentages of the four groups are approximately 11 % (WNT), 28 % (SHH), 28 % Group 3 and 34 % Group 4 [10]. The distribution of the four groups differs with age [11]. Group 3 MBs are less likely to appear in the adult, whereas the WNT MBs are less likely to appear in the infant. The cellular origins of MB subtypes have been reviewed by Northcott et al. [5], Gilbertson and Ellison [12], Gibson et al. [13], and Grill and Dufour [14]. WNT MBs arise from precursors in the lower rhombic lip while SHH MBs originate from granule cell precursors in the EGL of the cerebellum [13, 15]. The cellular origins of Group 3 (often associated with Myc expression) and Group 4 MBs have not been definitively determined, but are identified by marker genes [5, 13].
Various genetic aberrations have been reported in the various MB subgroups [5–7]. The loss of the short arm of chromosome 17 (17p) [16, 17] and the deletion of alleles in the telomeric region of 17p [18, 19] are sometimes associated with duplication of 17q (isochromosome 17q) [7, 20, 21]. The abnormalities of chromosome 17 have been associated with consensus Groups 3 and 4 [6], the groups with the worst outcomes. While research reports often refer to ‘loss of 17p’, the breakpoint may be some distance from the centromere [16, 22, 23]. Loss of heterozygosity in 17p occurs in approximately 50 % of the cases [24–26]. Allelic loss of 17p thus may be the most frequent genetic aberration in MB [19]. Thompson et al. [7] reported chromosome 17p deletions primarily in their Group C tumors (included in consensus classification Group 4), in which 10 of 11 tested had deletions of 17p (in 4 cases associated with isochromosome 17q). Their Group C MBs showed a gene expression pattern consistent with deletion of 17p and gain of 17q, whereas their SHH MBs (Group D) under expressed genes on 17q compared to the other tumor groups. McCabe et al. [27] reported that isolated 17p loss was a genetic marker of poor outcome. In the consensus classification of MBs 17p deletion and isochromosome 17q are seen in Groups 3 and 4 (isochromosome 17q more often in consensus Group 4). Nicholson et al. [20] reported loss of 17p (40 %) in 11 of 27 MBs and gain of 17q (52 %) in 14 of 27.
Herein we review genes on chromosome 17 reported as MB signature genes and susceptibility genes. Furthermore, we examine the literature for evidence of a role of the ubiquitin-proteasome system in MBs associated with chromosome 17 abnormalities. For comparison purposes we note that WNT MBs are reported to selectively delete chromosome 6 (monosomy of chromosome 6) [7, 28]. Increased proteasome activity has been reported in various cancers [29–31]. It has been suggested that dysfunction of the ubiquitin-proteasome system may be significant in the development of MBs [32, 33]. While clinical investigators have not focused on this issue, substantial data is available in the form of supplemental tables [7, 26, 34]. One striking example was found in the Nature report of Pomeroy and Tamayo [34]. In a supplemental table they list PSMB5 (Proteasome Subunit Beta 5), the gene that codes for the chymotrypsin-like component of the proteasome, as one of the top predictors that distinguish classic from desmoplastic MBs. Considering that the degradation of most of the marker proteins for the various types of MB is regulated by the proteasome, this finding deserves some comment. This gene was also listed, without comment or interpretation, as a signature gene distinguishing various subtypes of MB in the Thompson classification [7] (appendix, Table 2). The supplemental table in the Thompson dataset, furthermore, included 16 additional genes for proteasome subunits (on various chromosomes) as among the MB signature genes. Thus we raise the question whether Chromosome 17 genes associated with structural and functional aspects of the ubiquitin-proteasome system are related to the development of MBs. Of particular interest is the loss or mutation of genes in the cytogenetic region 17p13.3–17p13.1 of the short arm of this chromosome. We also discuss tumor-related genes on 17q, the long arm of chromosome 17 that have been, or could be, implicated in MB, due to the frequency of isochromosome 17q. Isochromosome 17q tends to occur in consensus classification Group 4 MBs [10, 35], but it also occurs in consensus Group 3 tumors [36]. We identified, using GeneCards, Thompson MB signature genes located in Chromosome 17 and have listed them in Tables 1 and 2. Next we examine the literature for variations in ubiquitin ligases and deubiquitinases as they relate to the distinct groups of MBs. Among the MB signature genes listed in the Thompson dataset we found at least 60 genes coding for ubiquitin ligases and at least 10 genes coding for deubiquitinases. We review these data to examine the potential prognostic value of proteins of the ubiquitin-proteasome system as markers of subgroups, as well as the potential for targeting specific genes and their proteins in MBs, particularly those with poor prognosis.
Table 1.
Medulloblastoma signature genes on 17p in the Thompson dataset
| Gene symbol | Gene name | Location | Function |
|---|---|---|---|
| GEMINI4 | Gem-associated protein 4 | 17p13.3 | Splicing mRNA |
| ABR | Active BCR-related | 17p13.3 | GTPase activation |
| YWHAE | 17p13.3 | Signal transduction | |
| CRK | Proto-oncogene C-Crk | 17p13.3 | Binds tyrosine-phosphorylated proteins |
| SERPINF1 | Serpin peptidase inhibitor | 17p13.3 | Induce neural differentiation |
| HIC1 | Hypermethylated in cancer 1 | 17p13.3 | Tumor suppressor |
| MNT | MAX network transcriptional repressor | 17p13.3 | Myc antagonist |
| GARNL4 | RAP1 GTPase activating protein 2 | 17p13.3 | GTPase activation |
| TIP-1 | Tax interaction protein 1 | 17p13.2 | Inhibitor of WNT signaling |
| ARRB2 | Arrestin, Beta 2 | 17p13.2 | GPCR regulation |
| PSMB6 | Proteasome subunit Beta 6 | 17p13.2 | Endopeptidase activity |
| PLD2 | Phospholipase D2 | 17p13.2 | Hydrolysis of phosphatidylcholine |
| RNF167 | Ring finger protein 167 | 17p13.2 | Ubiquitin E3 ligase |
| PFN1 | Profilin 1 | 17p13.2 | Actin-binding protein |
| SPAG7 | Sperm-associated antigen 7 | 17p13.2 | Nucleic acid binding |
| NUP88 | Nucleoporin 88 kDa | 17p13.2 | Nucleoporin protein |
| C1QBP | Complement component 1, Q-binding protein | 17p13.2 | Complement component and splicing mRNA |
| FJ10156 | Family with sequence similarity 64, member A | 17p13.2 | Regulate chromosome segregation |
| GABARAP | GABA (A) receptor-associated protein | 17p13.1 | Regulate GABA (A) receptor |
| Dullard | CND Nuclear envelope phosphatase (aka CTDNEP1) | 17p13.1 | Serine/threonine phosphatase |
| POLR2A | DNA-directed RNA polymerase II, subunit A | 17p13.1 | DNA transcription into RNA |
| TNFSF12 | Tumor necrosis factor, superfamily, subunit 12 | 17p13.1 | Cytokine |
| EIF4A1 | Eukaryotic translation initiation factor 4A1 | 17p13.1 | mRNA binding to ribosome |
| ATP1B2 | Sodium–potassium ATPase subunit B2 | 17p13.1 | Sodium pump subunit |
| TP53 | Tumor suppressor P53 | 17p13.1 | Tumor suppressor |
| CHD3 | Zinc finger helicase | 17p13.1 | Histone deacetylase component |
| PFAS | FGAM synthase | 17p13.1 | Synthesis of inosine monophosphate |
| PMP22 | Peripheral myelin protein 22 | 17p12 | Myelin component |
| NCOR1 | Nuclear receptor corepressor 1 | 17p11.2 | Protein phosphatase |
| KIAA0565 | Coiled-coil domain containing 144A | 17p11.2 | Interacts with ubiquitin C |
| COPS3 | COP9 signalosome subunit 3 | 17p11.2 | Regulation of SCF-type ubiquitin ligases |
| MFAP4 | Microfibrillar-associated protein 4 | 17p11.2 | Intercellular interactions |
| ULK2 | Unc-51 like autophagy activating kinase 2 | 17p11.2 | Serine-threonine kinase |
| MAP2K3 | Mitogen-activated protein kinase kinase 3 | 17p11.2 | Protein kinase |
Table 2.
Chromosome 17q signature genes
| AATK | HAP1 | NSF |
| ACOX1 | HCNGP | NXPH3 |
| ACTG1 | HCRT | P4HB |
| ADAM11 | HOXB6 | PIP5K2B |
| AOC2 | HT008 | PLXDC1 |
| AP2B1 | KCNJ2 | PRPSAP1 |
| ARHN | KIAA1447 | PSMD11a |
| AUTS2 | KRT17 | PSMD12a |
| CA4 | LHX1 | RARA |
| CACNA1G | LLGL2 | RGS9 |
| CCT6B | LLGL2 | RHOT1 |
| CDC27 | LOC146712 | RPL19 |
| CGI-48 | LOC51136 | RPL27 |
| CLTC | M17S2 | SLC9A3R1 |
| COX11 | MAP2K6 | SMARCE1 |
| CRHR1 | MAPT | SP2 |
| CSH1 | MFSD11 | SPAG9 |
| CYB561 | MGC15396 | SPHK1 |
| DHX40 | MGC33887 | STARD3 |
| DLX4 | MMD | SUMO2 |
| DNAJC7 | MPO | TBC1D16 |
| ERBB2 | NARF | THRAP1 |
| EVER1 | NBR1 | TLK2 |
| FALZ | NEUROD2 | TRAD |
| FLJ20315 | NF1 | TRIM37 |
| FLJ21347 | NGRF | TUBD1 |
| FLJ22729 | NLK | UNC119 |
| GFAP | NME2 | WIRE |
| GK001 | NPEPPS | XYLT2 |
| H3F3B | NPTX1 | ZNF403 |
aProteasome subunits
Finally, we discuss reported differences in expression of genes coding for proteasome subunits in the classification of MB subgroups, a discussion that takes us beyond chromosome 17. While the concept that the subgroups of MBs may vary in their expression of genes coding for proteasome subunits has not, to the best of our knowledge, been reported as such, we will show that data hidden in supplemental tables of signature genes support this view. In Table 3 we list the proteasome subunits and indicate those that were significantly up or down-regulated in the various MB subgroups of the Thompson dataset.
Table 3.
Proteasome subunits as signature genes as per the Thompson dataset
| Gene | Location | Subunit | Reference | Group A | Group B (WNT) | Group C | Group D (SHH) | Group E |
|---|---|---|---|---|---|---|---|---|
| PSMA1 | 11p15.2 | α6 | ||||||
| PSMA2 | 7p14.1 | α2 | T | Up | Up | Down* | ||
| PSMA3 | 14q23.1 | α7 | ||||||
| PSMA4 | 15q25.1 | α3 | T | Up | Down* | |||
| PSMA5 | 1p13.3 | α5 | ||||||
| PSMA6 | 14q13.2 | α1 | T | Up* | ||||
| PSMA7 | 20q13.33 | α4 | ||||||
| PSMB1 | 6q27 | β6 | T | Up* | Down | |||
| PSMB2 | 1p34.3 | β4 | T | Up | Down* | Up | ||
| PSMB3 | 17q12 | β3 | ||||||
| PSMB4 | 1q21.3 | β7 | T | Up | Down* | Up | ||
| PSMB5 | 14q11.2 | β5–chy | T | Up | Down* | Up | ||
| PSMB6 | 17p13 | β1–cas | T | Up | Down* | |||
| PSMB7 | 9q33.3 | β2–tryp | ||||||
| PSMB8 | 6p21.3 | β5i | ||||||
| PSMB9 | 6p21.3 | Β6i | ||||||
| PSMC1 | 14q32.11 | Rpt2 | ||||||
| PSMC2 | 7q22.1 | Rpt1 | T | Up | Up* | Down | ||
| PSMC3 | 11p11.2 | Rpt5 | ||||||
| PSMC4 | 19q13.2 | Rpt3 | T | Down* | ||||
| PSMC5 | 17q23.3 | Rpt6 | ||||||
| PSMC6 | 14q22.1 | Rpt4 | ||||||
| PSMD1 | 2q37.1 | Rpn2 | T | Up | Down | Down | ||
| PSMD2 | 3q27.1 | Rpn1 | T | Down* | ||||
| PSMD3 | 17q.21.1 | Rpn3 | ||||||
| PSMD4 | 1q21.3 | Rpn10 | T | Up* | Down | |||
| PSMD5 | 9q33.2 | |||||||
| PSMD6 | 3p14.1 | Rpn7 | T | Up* | Down | Down | ||
| PSMD7 | 16q23–24 | |||||||
| PSMD8 | 19q13.2 | Rpn12 | ||||||
| PSMD9 | 12q24.31–32 | Rpn4 | T | Down* | Up | |||
| PSMD10 | xq22.3 | |||||||
| PSMD11 | 17q11.2 | Rpn6 | T | Up | Down | |||
| PSMD12 | 17q24.2 | Rpn5 | T | Up | Up* | Down | ||
| PSMD13 | 11p15.5 | Rpn9 | ||||||
| PSMD14 | 2q14.2 | Rpn11 | T | Up | Down | |||
| PSME1 | 14q11.2 | T | Down | |||||
| PSME2 | 14q12 | T | Up | Down* | ||||
| PSME3 | 17q21.31 | PA28γ |
T located gene is in the Thompson dataset; Up/down gene expression was reported as significantly increased or decreased relative to that of the other groups
* High level of significance at p < 0.0001
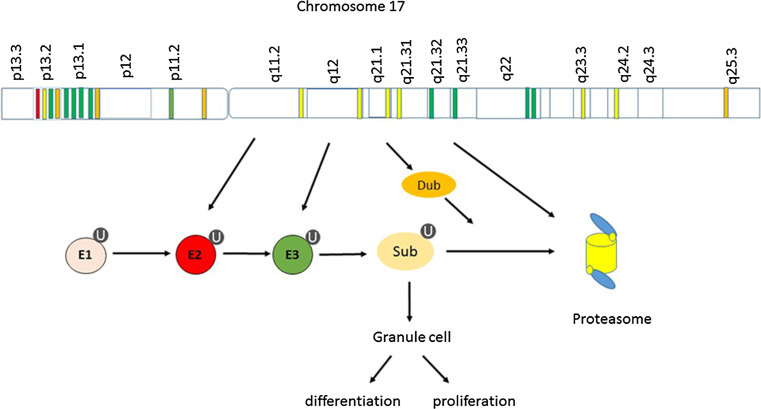
Chromosome 17 contains MB signature genes associated with the ubiquitin-proteasome system
An estimate of MB signature genes on chromosome 17 can be obtained by noting the chromosomal location of MB signature genes listed in the supplementary table (appendix Table 2) of Thompson et al. 2006 [7]. Based on information from GeneCards, the number of MB signature genes on chromosome 17 was 123 genes; of these 34 were located on 17p and 89 on 17q. We list the 17p signature genes in Table 1 and the 17q signature genes in Table 2. Thompson et al. [7] noted that their Group C MBs showed a gene pattern consistent with deletion of 17p and gain of 17q (isochromosome 17q). Thus expression of 17p signature genes for their Group C MBs were mostly down-regulated (when statistically significant); 17p signature genes for their group D (SHH group MBs) however were mostly up-regulated compared to other groups, while 17q MB signature genes for the SHH group were mostly down-regulated compared to other groups. Thus gain or loss of 17p or 17q was shown to be an important factor in differentiating two major groups of MBs. In comparison, loss of chromosome 6 (monosomy of chromosome 6) was shown to be a significant factor in distinguishing their Group B MBs (the WNT group) [7, 28].
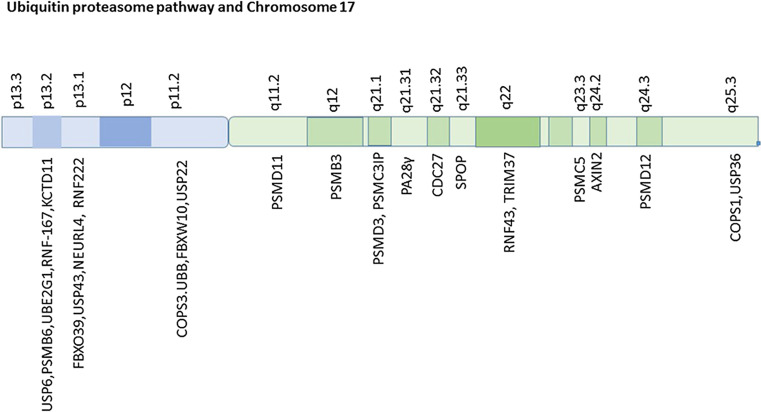
Several protein subunits (at least 9) of the proteasome have been listed, but not discussed, by Anagnostopoulos et al. (Supporting Information, Supplementary Tables 1–3) [26] as either over or under-expressed in MBs. Furthermore, the supplemental dataset of Thompson et al. [7], showed significant differential expression of numerous genes associated with the ubiquitin-proteasome system between the subsets of MB, but again were not discussed. Table 3 lists the genes for the proteasome subunits with their chromosomal locations, indicates those included by Thompson as MB signature genes, and shows those that were reported as significantly up or down-regulated in the five different MB groups of the Thompson classification. Leaving aside for later (see below) the question of whether the expression of proteasome subunit genes can be used to classify MBs, we note that in Thompson Group C tumors (17p deletion group) the expression of genes for several core beta ring components of the proteasome (genes PSMB2, PSMB4, PSMB5, and PSMB6) were reported as down-regulated (at a very high level of statistical significance), but up-regulated in Groups A and E (Thompson Appendix, Supplemental Table 2). The location of the PSMB6 gene, which codes for a core proteasome catalytic enzyme, is cytogenetic region 17p13.2 (Fig. 1). It has been listed by Anagnostopoulos et al. [26] as a protein that is commonly expressed in all childhood MBs (see their Supplemental Table 1). Also in cytogenetic region 17p13.2 are other genes associated with the ubiquitin-proteasome system: UBE2G1 (ubiquitin-conjugating enzyme 2G1), USP6 (ubiquitin specific peptidase 6) (a deubiquitinase), and the gene for at least two ubiquitin ligases, RNF167 (ring finger protein 167) and KCTD11 (potassium channel tetramerization domain containing 11). RNF167 is listed in the Thompson dataset as a MB signature gene. The gene for the ubiquitin ligase KCTD11 codes for a ubiquitin ligase that plays a role in the SHH pathway (see below). The 17p13.2 region of chromosome 17 is considered by various investigators as a region containing a MB tumor suppressor gene [19].
Fig. 1.
Ubiquitin-proteasome system and chromosome 17
The Thompson dataset also shows that among the MB signature genes in chromosome 17 are the genes (PSMD11 and PSMD12) for two proteasome subunits, located on 17q (17q11.2 and 17q24.2, respectively) (see Table 3; Fig. 1). Both genes code for components of the 19S regulatory particle of the proteasome, the proteins RPN6 and RPN5 respectively, both of which are required for assembly of the proteasome [37, 38]. These two genes PSMD11 and PSMD12 were significantly up-regulated in MB group C, and down-regulated in Group D (SHH group) (Table 3). Genes on 17q for additional proteasome subunits, not included in the Thompson supplemental table, comprise PSMC5 (which codes for Rpt6 (regulatory protease 6), a unit of the ATPase ring), PSMD3 (which codes for Rpn3 (proteasome regulatory particle lid subunit 3), a non-ATPase subunit of the proteasome lid) and PSMB3 (which codes for the core particle subunit β3) [39]. A gene, PSME3, which codes for the proteasome activator PA28γ is also located on 17q (17q21.31). Finally, the gene PSMC3IP, which interacts with proteasome subunit PSMC3, is located at 17q21.2 (Fig. 1). Therefore, it is likely that deletions and/or duplications of parts of chromosome 17 differentially influence the activity of the proteasome in the MB groups associated with chromosome 17 aberrations. Since the proteasome regulates the degradation of many proteins as well as the activity of many transcription factors, it is probable that variations in the expression of proteasome subunits contribute to the expression of many MB marker and MB driver genes.
For comparison purposes we note that in Group B (the WNT group) the expression of the gene (PSMB1) for only the proteasome subunit, β6, was significantly down-regulated (Table 2), presumably due to monosomy of chromosome 6. The PSMB9 gene coding for subunit β6i, the β6 subunit found in the immunoproteasome, is also located on chromosome 6 [40]. The importance of chromosome 17 and chromosome 6 in predicting survival outcome has been shown by Pfister et al. [41] and recognized by other leaders in the field [42]. We discuss marker genes on 17p and 17q separately and list the Thompson signature genes on 17p and 17q in Tables 1 and 2.
Chromosome 17p and MB signature genes
One 17p gene, described as a MB driver gene, is the serine/threonine phosphatase, CTDNEP1 (C-terminal domain nuclear envelope phosphatase 1) (aka Dullard) [5]. It is located at cytogenetic region 17p13.1.
Recently microRNAs of chromosome 17 have been studied in relationship to MBs. Lopez-Ochoa et al. [43] reported that at least 19 genes encoding for miRNAs were localized to various bands of chromosome 17 in both the 17p and 17q arms. At least four of them were considered relevant to development and growth of MBs. Two of them, MIR3183 and MIR1253, are located at cytogenetic region 17p13.3.
Several tumor suppressor genes are located on the short arm of chromosome 17. The well-known suppressor gene, p53 (tumor protein p53) (aka TP53), is located on the short arm of 17 [44] at 17p13.1. Mutation of p53, however, occurs only in a small number of MBs [45]. Adesina et al. [45] and others [17, 18, 43] suggested that it is unlikely that p53 is the putative MB tumor suppressor gene at 17p13. Northcott et al. [5], however, list p53 as a driver gene for the WNT subgroup of MBs. Cogen et al. [18] suggested that there are loci on chromosome 17p, other than p53, which are involved in MB. The gene for the ubiquitin ligase and tumor suppressor RNF43 (ring finger protein 43) is also located in cytogenetic region 17p13.1.
The tumor suppressor gene, HIC-1 (hypermethylated in cancer 1), located at 17p13.3, has been reported to synergize with p53 [46]. Briggs et al. [47] provided evidence that the HIC-1 protein inhibits transformation of granule cell precursor differentiation in the cerebellum by inhibition of Atoh1 (Atonal BHLH Transcription Factor) (aka Math-1) expression. Math-1 is a transcription factor essential for differentiation and proliferation of cerebellar granule cells. Disruption of the Math-1 gene results in loss of granule cell development [48] due to a disruption of the balance between proliferation and differentiation. The turnover of Math-1 is regulated by HUWE1 (HECT, UBA and WWE domain containing 1), a ubiquitin ligase, whose gene is located on the X chromosome. Math-1 gene expression has been used as a predictor for outcome in MB [49]. It is listed in the Thompson dataset as up-upregulated in the SHH group of MBs compared to the other groups, at a very high level of significance [7]. Since Math-1 is an early marker of developing granule cells in the EGL [50] the Thompson data are consistent with the view that SHH MBs arise from the EGL. CAMKK1 (calcium/calmodulin-dependent protein kinase kinase 1) is another 17p gene (at 17p13.2) that reportedly is required for normal granule cell development [51]. The enzyme coded for by this gene phosphorylates CAMK4, a calcium/calmodulin-dependent protein kinase.
The DPH1 (diphthamide biosynthesis protein 2 homolog-like 1) gene, located at 17p13.3, reported to code for a tumor suppressor [52], was found to inhibit proliferation of ovarian cancer cells [53]. Bruening et al. suggested that a reduction in expression of this gene could result in dysregulation of the cell cycle and tumorigenesis. The DPH1 gene has also been associated with breast cancer [54]. Chen and Behringer [54] also showed that this tumor suppressor can also modify p53-induced tumorigenesis. DPH1 has also been considered as a candidate tumor suppressor gene for MB in the 17p13.3-13.2 region [52].
The gene for profilin1 (PFN1), which codes for a protein required for cerebellar granule cell migration, is located at cytogenetic location 17p13.2. Profilin 1 is an actin-binding protein. The data of Yao et al. [55] supported a role for Profilin 1 as a tumor suppressor in pancreatic cancer. PFN1 is listed in the Thompson supplemental dataset (Supplemental Data, Table 2) as a MB signature gene. In this dataset, its expression is shown as down-regulated (at a high level of significance) in their Group C MBs compared to the other groups. In their Group D MBs (SHH group) expression of this gene was significantly up-regulated. In the Anagnostopoulos dataset (supplemental Table 2), this gene is listed as commonly overexpressed in MBs compared to normal brain tissue controls [26]. Their dataset, as noted above, did not distinguish the subtypes of MB.
GABARAP (GABA receptor A-associated protein) interacts with GABA (A) receptors and is involved in intracellular transport of these receptors. It also contributes to the mechanisms of apoptosis [56] and autophagy [57]. GABARAP protein belongs to a family of proteins that are similar to ubiquitin [58]. This gene, located at 17p13.1, has been reported as a tumor suppressor in breast cancer [59]. In the Thompson dataset, GABARAP expression is significantly reduced in their Group C MBs compared to the other groups.
Allelic loss of 17p would result in loss of the gene, DVL2 (dishevelled segment polarity protein 2), which codes for the protein, Dsh (Disheveled). Thus loss of 17p could be expected to interfere with WNT signaling. The DVL2 gene is located at the cytogenetic band 17p13.1. Since Dsh contributes to the WNT signaling pathway it dysregulation might be expected to contribute to WNT type MBs. It is, however, not listed in either the Thompson or Anagnostopoulos datasets of MB signature genes. In addition to serving as part of the WNT signaling pathway, Dsh is also reported to be required for normal cell migration during neural development [60].
The gene for EIF5A (eukaryotic translation factor 5A) is located at 17p13.1. This translation initiation factor regulates a number of cancer related genes referred to as the EIF5A regulon [61] in studies of cervical cancer. In this model EIF5A controls cell proliferation via the proteins it regulates. These include HSP27 (heat shock protein 27), NM23 (metastasis inhibition factor Nm23), DJ-1 (Oncogene DJ1, aka Parkinson Protein 7), TrpRS (tryptophan TRNA ligase) and PRDX2 (Peroxiredoxin 2). EIF5A is listed as among those proteins commonly expressed in all childhood MBs [26] (see Anagnostopoulos Supplemental Table 1).
The GPS2 (G protein pathway suppressor 2) gene, located at 17p13.1, is a protein described as a nuclear transcription repressor and is involved in MAPK signaling. It is listed by Pugh et al. [62] as a gene with a mutation associated with MB, along with other more well-known mutations such as CTNNB1 (β-catenin), PTCH1 and p53.
Another 17p gene associated with MB is NTN-1, which codes for the axon guidance molecule netrin-1 [63]. The gene for netrin-1 is located in the 17p13.1 cytogenetic band. Akino et al. [63] reported that netrin-1 stimulates invasion of MB tumor cells. Netrin-1 levels were five to ten times higher in MB cells than in normal cerebellar tissue, and were found to be nine times higher in urine of MB patients than in urine of normal controls. The membrane receptor of netrin, DCC (deleted in colorectal cancer), was found to be down-regulated by netrin-1 stimulation, an action dependent on the ubiquitin-proteasome system [64]. DCC is a tumor suppressor located on chromosome 18. While this gene is not in the Thompson list of MB signature genes, one of the genes of the netrin family of receptors, UNC5C (Unc-5 Netrin Receptor C), is listed as a MB signature gene.
TIP-1 (Tax-Interacting Protein 1) is located at 17p13.2. It is involved in several signaling pathways including inhibition of WNT/β-catenin [65]. Under some circumstances it can act as a tumor suppressor [61]. Tip-1 has a PDZ domain that allows it to interact with many proteins that have a PDZ domain binding site. It also interacts with the ubiquitin conjugating enzyme, UBE2D2 [66]; both are listed in the Thompson dataset of MB signature genes.
The tumor suppressor gene, MNT (MAX network transcriptional repressor) is located at 17p13.3. As a Myc antagonist, it inhibits Myc-dependent transcription. In the Thompson dataset it is significantly up-regulated in their Group E MBs [5]. We would, therefore, expect this gene to be up-regulated in the consensus MB group 3, into which the Thompson Group E has been placed. Myc has been described as one of the driver genes for consensus Group 3 MBs, but its expression may also be increased in the WNT group of MBs [67]. Myc stability is regulated by the ubiquitin-proteasome system. It is a substrate for the ubiquitin SCF ligase FBW7 (F-box and WD repeat domain containing 7) [68, 69], the SCF ligase SKP2 (S-phase kinase-associated protein 2) [70] and, reportedly, the ligase TRPC4AP (transient receptor potential cation channel subfamily C member 4 associated protein) [70]; its ubiquitination and degradation are thus influenced by more than one ubiquitin ligase. Both FBW7 and SKP2 are significant MB signature genes in the Thompson dataset. They are located on chromosomes 4 and 5, respectively.
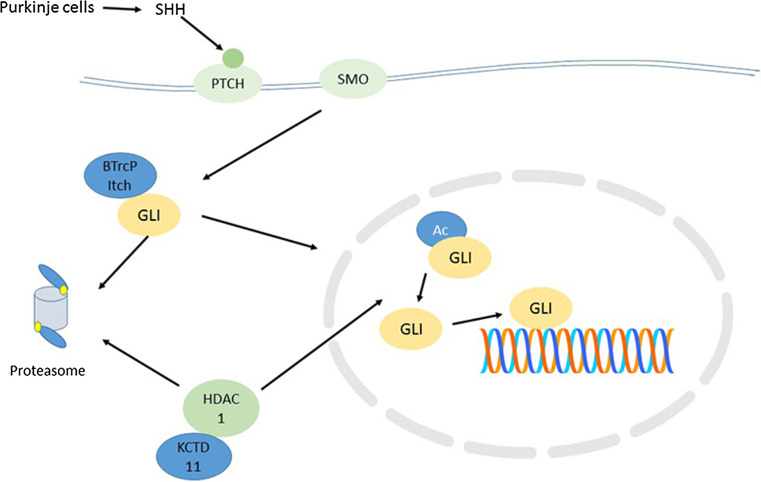
The KCTD11 gene (aka KCASH1) on chromosome 17p is among those that are frequently deleted in MB. The gene for the KCTD11 protein has been reported as located on chromosome 17 at 17p13.2 [71]. It has been described as a tumor suppressor gene in studies of prostate cancer [72]. This gene is lost in 17p deletions in MBs. It is also down-regulated in a variety of other tumors [73]. In the cerebellum it regulates the transcription factor GLI, which stimulates cerebellar granule cell proliferation in the cerebellum (Fig. 2). Di Marcotullio et al. [19] identified KCTD11 as a suppressor of SHH signaling and suggested that its loss or inactivation (through 17p deletion) may lead to activation of the SHH pathway in the SHH group of MBs. The region 17p13.2–17p13.3 is described by Di Marcotullio [19] as a ‘common region’ probably involved in a tumor suppressor pathway. As shown in Fig. 1, the gene for KCTD11 is located in this region.
Fig. 2.
Regulation of GLI by ubiquitin ligases and deacetylation. *E3 ligases include BTrCP, Itch, and SPOP. KCTD11, also an E3 ligase is located on chromosome 17p. SPOP is located on chromosome 17q. HDAC-1 is a deacetylase for GLI, which may also play a role in Notch, WNT, and MYC signaling
Ubiquitin-proteasome system genes on 17p
KCTD11 has been identified as the gene for part of an E3 ubiquitin ligase [74] which functions to ubiquitinate histone deacetylase (HDAC1) in preparation for degradation [75, 76]. Loss of KCTD11 has been associated with SHH dependent proliferation of granule cell precursors and development through activation of the GLI transcription factors [19, 76]. The GLI transcription factor, thus, is regulated by acetylation/deacetylation and by ubiquitination/deubiquitination [19, 76]. This is illustrated in Fig. 2. It should be noted that HDAC1 may also regulate Notch signaling [77, 78], WNT signaling [79, 80] and MYC signaling [81] in various contexts.
Three additional ubiquitin ligases, BTrCP (Beta-transducin repeat containing) [82], Itch (Itchy E3 ubiquitin-protein ligase) [32], and SPOP (speckle-type BTB/POZ protein) [83, 84] have been reported to regulate the degradation of GLI1 by the proteasome (Fig. 2). The genes for BTrCP and Itch are located on chromosomes 10 and 20, while the gene for SPOP is located on 17q21.33.
RNF-167 (DKFZP566H073) (ring finger protein 167) is a ubiquitin ligase gene listed in the Thompson dataset of MB signature genes. It is also located at 17p13.2 and is reported as regulating the tumor suppressor TSSC5 [85]. Thus the 17p13.2 cytogenetic region may regulate the degradation of a number of proteins including tumor suppressor proteins.
Three additional genes associated with the ubiquitin-proteasome system (UPS) are located at 17p13.2 (Fig. 1), the gene for an ubiquitin conjugating enzyme, UBE2G1, a gene for the deubiquitinase USP6 (ubiquitin-specific peptidase 6) (aka Tre-oncogene, or Tre-2) [85, 86] and a gene for the proteasome core particle subunit, PSMB6 (also known as β1) [87]. PSMB6 is among those proteins reported as regularly expressed in childhood MBs [26]. In Thompsons Group C tumors, which have a significant number of 17p deletions, PSMB6 gene expression is reported as significantly reduced [7] (Thompson supplementary data, Table 2). Thus a 17p deletion could account for reduced PSMB6 expression, since this gene is on 17p. (Note that we discuss below the fact that 6 genes for proteasome subunits are located on 17q).
Finally, also located on 17p are the genes for the deubiquitinase USP43 (ubiquitin-specific peptidase 43) (at 17p13.1), the gene for ubiquitin B, UBB (at 17p11.1), and the gene COPS3 (signalosome subunit 3) (at 17p 11.2), which codes for a component of the COPS9 signalosome, a complex that regulates the activity of Cullin-Ring ubiquitin ligases. Another component of the signalosome is located on 17q (see below).
MB and the long arm of chromosome 17 (17q)
As noted above, a frequent finding in MB is isochromosome 17q [86]. This aberration may be a key event in consensus Group 4 MBs, and in some consensus Group 3 tumors. Isochromosome 17q is also found in other cancers including breast cancer [88, 89], colorectal cancer [90], chronic myeloid leukemia [91], prostate cancer [92], and gastric cancer [93]. Since chromosome copy number leads to substantial differences in expression of proteins [8], isochromosome 17q has an impact on the expression of a variety proteins coded for by 17q, including tumor suppressors and oncoproteins. We will discuss some of the MB signature genes located on 17q and relate them to the subtypes of MB. Table 2 lists the MB signature genes on 17q identified in the Thompson dataset. Not included in this list are any of the 25 MB driver genes reported by Northcott et al. [5]; this list of driver genes did not include any genes on 17q.
Among the genes located on chromosome 17q, is BRCA1 (breast cancer 1, early onset) [94]. It has been reported that this gene acts as a tumor suppressor for MB [95]. It is not identified as a MB signature gene in the Thompson dataset while this dataset does list NBR1 (nearest neighbor of BRCA1, aka M17S2) as a MB signature gene (Table 2). Another 17q gene associated with breast cancer, ERBB2 (Erb-B2 receptor tyrosine kinase 2) (aka HER2), codes for a protein which functions as a receptor tyrosine kinase. While this gene is better known for its role in breast cancer, it is overexpressed in many MBs [96, 97]. It is identified in the Thompson dataset as up-regulated in their subgroup E MBs.
The MB marker, HDAC5 (histone deacetylase 5) is located at 17q21.31. This gene is regarded as a risk marker for MB [98]. It is frequently overexpressed in MBs [10]. Morales and Hatten [99] discuss evidence that Lhx1/5 proteins are involved in early stages of Purkinje cell differentiation. LHX1 (LIM homeobox protein 1) maps to cytogenetic band 17q12. It was identified by the Thompson dataset as an MB signature gene.
WNT3 and WNT15 are two genes associated with the WNT signaling pathway. WNT3 is located at 17q21.32, while WNT15 is at 17q21.32, in the same cytogenetic region [100]. WNT3 has been implicated in various cancers including breast cancer, lung cancer, rectal cancer, and gastric cancer. It has been implicated in neural differentiation in NT2 cells. Neither of these genes was identified as a MB signature gene in the Thompson dataset.
The Musashi-2 gene (which codes for MSI2) is located at 17q22. The Musashi protein is reported to regulate the balance between stem cell renewal and differentiation [101]. MSI2 contributes to maintaining the hemopoietic stem cell population [102] but its role in differentiation and proliferation of any of the cell populations giving rise to MB has not been determined.
NGFR (nerve growth factor receptor) (aka p75NTR) is located to 17q21.33. It has been implicated in MB tumor invasion and progression [103, 104]. According to the data (Supplementary Data Table 2) of Thompson et al. [7], the gene for this protein is significantly down-regulated in their Group A and Group D (SHH) MBs.
NME2 (NME nucleoside diphosphate kinase 2) is an myc gene transcription factor. This gene is also located at cytogenetic region 17q21.33. In the Thompson dataset it is a significant MB signature gene.
Genes of the ubiquitin-proteasome system on chromosome 17q
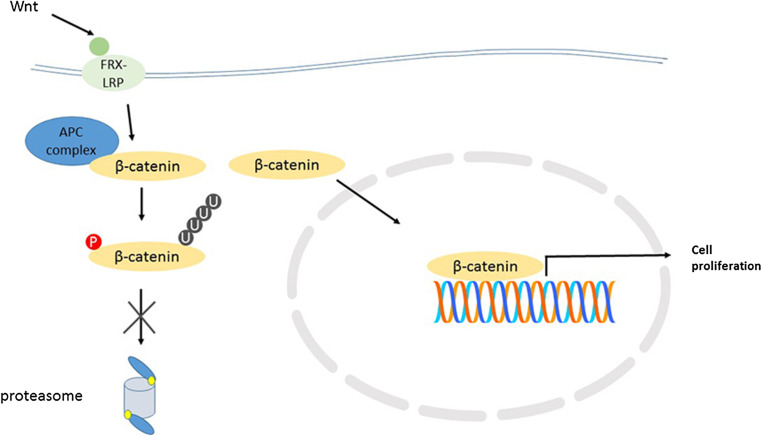
There are several genes associated with the ubiquitin-proteasome system on chromosome 17q. The gene for axin2 is located at 17q.24.1. It forms a component of the APC (adenomatous polyposis coli) ubiquitin ligase which recognizes β-catenin as substrate [105] and therefore contributes to regulation of the stability of β-catenin. It is part of a signaling mechanism that regulates the concentration of β-catenin that accumulates in the cytoplasm and translocates to the nucleus (Fig. 3). As part of this mechanism, Axin2 interacts with Dsh (whose gene DVL2 is on 17p, see above). This is illustrated in a review by MacDonald et al. [106].
Fig. 3.
Proteasomal dysregulation in WNT MBs. FRX-LRP: components of WNT receptor; APC: adenomatous polyposis coli ubiquitin ligase; * monosomy of chromosome 6 results in deficiency of proteasome subunit β6
Among the Thompson MB signature genes coding for ubiquitin ligases (see Table 4), four are located in chromosome 17, CDC27 (cell division cycle 27), RNF43 (ring finger 43), RNF167 (ring finger 167) and TRIM37 (tripartite motif containing 37). The ubiquitin ligases, RNF43 and TRIM37, are located in 17q22 (Fig. 1). RNF43 is a tumor suppressor gene (at 17q22) associated with WNT signaling and colorectal cancer [107]. It is a paralog of RNF167, located at 17p. CDC27 is located at q21.32. It is part of the APC/C ubiquitin ligase (see below). TRIM37 regulates proteins that control centriole duplication [108] during the cell cycle. It is listed as up-regulated in Thompson Group C MBs (Table 4). An additional MB signature gene on 17q, COPS1 (signalosome subunit 1) (aka CSN1), codes for a component of the COPS9 signalosome which regulates the activity of Cullin-Ring ubiquitin ligases by deneddylation [109]. Thus aberrations of chromosome 17q are likely to interfere with the activity of multiple ubiquitin ligases.
Table 4.
Ubiquitin ligases as marker genes in medulloblastoma
| Gene symbol | Gene name | Thompson subgroupsa | Robinson—copy number variationb | ||||
|---|---|---|---|---|---|---|---|
| A | B | C | D | E | |||
| Anapc5 | Anaphase promoting complex subunit 5 | D | D | ||||
| Anapc13 | Anaphase promoting complex subunit 13 | D | |||||
| ARIH1 | Protein ariadne-1 homolog | U | |||||
| ASB13 | Ankyrin repeat and SOCS box containing 13 | U | Loss | ||||
| Brap | BRCA1-associated protein | D | |||||
| Ccnb1ip1 | Cyclin B1 interacting protein 1 | D | U | ||||
| Cdc20 | Cell division cycle 20 | D | U | ||||
| Cdc27 | Cell division cycle 27 (aka Anapc3) | U | Gain | ||||
| Cul3 | Cullin 3 | U | D | Gain | |||
| Cul4b | Cullin 4b | D | Loss | ||||
| Cops3 | Signalosome subunit 3 | U | Gain or Loss | ||||
| Enc1 | Ectodermal-neural cortex 1 | U | D | Gain | |||
| Fbxo2 | F-box protein 2 | U | |||||
| Fbxo9 | F-box protein 9 | U | D | U | |||
| Fbxo26 | F-box protein 26 | U | U | ||||
| Fbxo28 | F-box protein 28 | U | D | Gain | |||
| Fbxw1B | F-box and WD repeat domain containing 11 | U | |||||
| Fbxw2 | F-box and WD repeat domain containing 2 | U | Loss | ||||
| Fbxw7 | F-box and WD repeat domain containing 7 | U | Loss | ||||
| Fbxl7 | F-box and leucine-rich repeat protein 7 | D | U | Gain | |||
| Fbxl14 | F-box and leucine-rich repeat protein 14 | D | U | Gain | |||
| Fem1B | FEM-1-like death receptor binding protein | D | Gain | ||||
| Herc3 | HECT And RLD domain containing | U | |||||
| Kbtbd2 | Kelch repeat and BTB domain containing 2 | D | Gain | ||||
| Kbtbd4 | Kelch repeat and BTB domain containing 4 | U | |||||
| Kbtbd9 | Kelch repeat and BTB domain containing 9 | D | |||||
| Mid1 | Midline 1 (aka Trim18) | U | Loss | ||||
| Mycbp2 | MYC binding protein 2 | U | Gain or loss | ||||
| Neurl | Neuralized E3 ubiquitin-protein ligase 1 | D | U | ||||
| Parc | P53-associated Parkin-like cytoplasmic protein | D | |||||
| Peli1 | Pellino E3 ubiquitin-protein ligase 1 | U | Gain | ||||
| Phip | Pleckstrin homology interacting protein | D | Gain or loss | ||||
| Pias1 | Protein inhibitor of activated STAT 1 | U | D | Gain | |||
| PPil2 | Peptidylprolyl isomerase-like 2 | D | Gain | ||||
| Rabgef1 | RAB guanine nucleotide exchange factor 1 | U | D | Gain | |||
| Ring1 | Really interesting new gene 1 protein | D | Gain | ||||
| RNF5 | Ring finger protein 5 | D | |||||
| RNF43 | Ring finger protein 43 | U | Gain | ||||
| RNF44 | Ring finger protein 44 | D | |||||
| RNF130 | Ring finger protein 130 | D | U | Gain or loss | |||
| RNF146 | Ring finger protein 146 | U | |||||
| RNF166 | Ring finger protein 167 | D | |||||
| Rnf173 | Ring finger protein 173 | D | U | ||||
| Skp1A | S-phase kinase-associated protein 1 | U | D | Gain | |||
| Skp2 | S-phase kinase-associated protein 2 | D | |||||
| Socs2 | Suppressor of cytokine signaling 2 | U | Gain | ||||
| Socs5 | Suppressor of cytokine signaling 5 | U | |||||
| Socs6 | Suppressor of cytokine signaling 6 | U | Gain | ||||
| Traf3 | TNF receptor-associated factor 3 | D | |||||
| Trim2 | Tripartite motif containing 2 | D | |||||
| Trim26 | Tripartite motif containing 26 | D | Loss | ||||
| Trim28 | Tripartite motif containing 28 | D | U | Gain or loss | |||
| Trim37 | Tripartite motif containing 37 | U | Gain | ||||
| Trim45 | Tripartite motif containing 45 | D | |||||
| TTC3 | Tetratricopeptide repeat domain 3 | U | |||||
| Tulp3 | Tubby-like protein 3 | U | Gain | ||||
aSignificantly up (U) or down (D) in Thompson (Ref 7) subgroups
bAssociated with copy number gain or loss in Robinson data (Ref 21)
We have already noted that the gene PSMB6, which codes for the β1 core catalytic subunit of the proteasome, is located at 17p13. In addition, the proteasome subunit genes located on chromosome 17 include genes for the subunits β3, Rpt6, Rpn3, Rpn5, Rpn6 (all on 17q), as well as the gene for the proteasome activator PA28γ (on 17q as well) (see Fig. 1; Table 3). Rpt6, a subunit of the proteasome (coded for by PSMC5) is reported to have a role during proteasome assembly, as well as a role in activating the mature proteasome together with Rpt3 [110]. The proteasome subunits, Rpn3, Rpn5 and Rpn6 (coded for by PSMD3, PSMD12, and PSMD11, respectively) form part of the regulatory particle of the proteasome. These genes are included in Fig. 1.
The PSME3 (proteasome activator subunit 3) gene, which codes for PA28γ is a proteasome activator. This gene is located at 17q21.31. An increase in this protein might be expected with an extra copy of chromosome 17.
Ubiquitin ligases and deubiquitinases in MB
MB and ubiquitin ligases
Among the Thompson MB signature genes we identified 61 ubiquitin ligases, including the MYC-binding protein, MYCBP2. In Table 4 we indicate those that significantly distinguished one MB group from the others at a level of statistical significance of p < 0.001 in the Thompson dataset. We have noted above that several of the ligases in this table are located on chromosome 17, including TRIM37, RNF43, RNF167, and CDC27, a component of the APC/C anaphase-promoting ligase complex (discussed below). We also note that genes for seven of the ubiquitin ligases that serve as signature genes for the WNT group were located on chromosome 6 (FBXO9 (F-box protein 9), PARC (P53-associated Parkin-like cytoplasmic protein), PHIP (Pleckstrin homology domain interacting protein), RING1 (ring finger 1 protein), RNF5 (ring finger 5 protein), RNF146 (ring finger 146 protein), and TRIM26 (tripartite motif containing 26). Their expressions were all significantly reduced in the WNT group, as would be expected with loss of one copy of chromosome 6. Of the 61 different ubiquitin ligases listed in Table 4 as MB signature genes, 30 were also listed in the supplemental Table 7 of Robinson et al., as genes in the coding region of copy number variations in MB [21].
Thus the datasets of Thomson et al. [7] and Robinson et al. [21] are strong evidence that ubiquitin ligases are genetic markers for the various types of MBs (Table 4). Included among these are MYCBP2, the MYC binding protein; RING1, an E3 ligase that regulates gene expression by interaction with histones [111]; FBXW7 (F-box and WD repeat containing 7), whose substrates include cyclin E, Myc, Jun and Notch; FBXl7; SKP2 (S-phase kinase-associated protein 2), a ubiquitin ligase involved in cell cycle control [112] and overexpressed in a variety of cancers [113]; Cul3 (Cullin 3), another E3 ubiquitin ligase involved in cell cycle control [112], and Ube3A (ubiquitin-protein ligase 3A) (aka E6-AP) a maternally expressed gene [114, 115]. Also included in the list of MB signature genes are ANAPC5, ANAPC13, CDC27, and CDC20, all coding for components of the APC/C (anaphase promoting complex/cyclosome) ubiquitin ligase complex known as the cyclosome. The components of the cyclosome and its substrates have been illustrated in several reviews [116, 117]. The APC/C complex should not be confused with the APC complex (adenomatous polyposis coli) which also functions as an ubiquitin ligase (see above).
MB and the APC/C complex
We suggest based on the information in Table 4 that the ubiquitin ligase APC/C plays a significant role in MB, in Thompson Groups C and E MBs (hence in consensus Groups 3 and 4 MBs). ANAPC5 a component of the cyclosome, was significantly reduced in these groups of MBs; CDC20, another cyclosome component was also down-regulated in Group C MBs while CDC27 (aka APC3) expression was up-regulated. Furthermore, the expression of the gene for a ubiquitin E2 conjugase associated with the cyclosome, UBE3C (ubiquitin conjugating enzyme E2) (aka UBCH10) [118] was also significantly down-regulated in this group of MBs. The APC/C complex regulates entry of the cell into anaphase and the exit from mitosis [117] via the spindle assembly checkpoint (mitotic checkpoint) [119]. Primorac and Musacchio [120] have illustrated the molecular interaction that occurs between the APC/C and substrates during various stages of the cell cycle. The major substrates of APC/C during the cell cycle are Securin, Cyclin B, Aurora kinase A, Skp2 and Casein kinase 1δ (CK1δ) [121]. Interference with APC/C subunits can lead to dysfunction of the cyclosome, abnormal proliferation, and cancer [122]. It should be noted that the deubiquitinase, USP39 (ubiquitin-specific peptidase 39), which opposes the action of APC/C during spindle assembly checkpoint [123], was also significantly decreased in Group C MBs compared to the other groups (Table 5).
Table 5.
Deubiquitinase expression in MD groups of Thomson et al. [7]
| Gene | Location | Group A | Group B (WNT) | Group C | Group D (SHH) | Group E |
|---|---|---|---|---|---|---|
| USP1 | 1p31.3 | Down | ||||
| USP15 | 12q14.1 | Up | ||||
| USP24 | 1p32.3 | Down | ||||
| USP33 | 1p31.1 | Up | ||||
| USP34 | 2p.15 | Down | ||||
| USP39 | 2p11.2 | Down | ||||
| USP46 | 4q12 | Up | ||||
| USP48 | 1p36.12 | Down | ||||
| USP9X | Xp11.4 | Down | ||||
| UCHL1 | 4p13 | Down | ||||
| CYLD | 16q12.1 | Up | Down | |||
| PSMD14 | 2q24.2 | Up | Down |
Genes whose expressions were significantly different from the other groups at p < 0.001
We note that a component of the APC/C cyclosome, ANAPC13, is also a significant marker for Thompson Group E MBs. Another protein that interacts with the APC/C cyclosome, BUB3 (BUB3, mitotic checkpoint protein), was also significantly reduced in Thompson Group E MBs. Thus MBs with the worst prognosis appear to have a highly significant association with variations in cyclosome function, as well as with chromosome 17 abnormalities.
Recently, in 2015, it has been shown that CK1δ (Casein kinase 1 delta) regulates cerebellar granule cell proliferation during neurogenesis [121] and that APC/C down-regulates CK1δ in cerebellar granule cell precursors during cell-cycle exit. These investigators concluded that APC/C regulates CK1δ levels to control the balance between proliferation and cell cycle exit in the developing cerebellum. In this context Penas et al. [121] described APC/C as a tumor suppressor. Furthermore they suggested the possibility that CK1δ could serve as a therapeutic target in MB.
MB and deubiquitinases
Table 5 lists genes coding for deubiquitinases (DUBs) which we identified in the Thompson dataset of MB signature genes. USP1 (ubiquitin-specific peptidase 1) is reported to deubiquitinate inhibitors of DNA binding, which would otherwise be degraded by the APC/C complex [124]. Statistically it was significant in distinguishing Group E MBs from the others in the Thompson dataset. USP15 is reported to regulate TGF-beta signaling [125] and to stabilize the silencing transcription factor REST (RE1 silencing transcription factor) [126], a significant regulator in MB [127]. USP24 was reported a DUB for the UV damage-specific DNA-binding protein, DDB2 (damage-specific DNA-binding protein 2) [128] and more recently as a DUB for p53 during the DNA damage response [129]. USP33 (aka VDU1) contributes to activation of Dio2 (type 2 deiodinase), the enzyme that converts the thyroid hormone T4 to the active hormone T3 [130] in local tissue. USP34 contributes to the WNT signaling pathway by deubiquitination of axin and thus controlling its stability [131]. USP39 is a DUB that counteracts the activity of the APC/C complex particularly during the mitotic spindle checkpoint [123]. The substrates of USP46 and USP48 apparently have not been definitely defined. On the other hand, a recent review has listed a number of substrates for USP9x and has discussed the contexts in which USP9x can be oncogenic or tumor suppressor [132]. As a marker it was significant in distinguishing Thompson subtype E MBs (Table 5). UCHL1 (ubiquitin C-terminal hydrolase) is a DUB highly expressed in neurons. It has been described as an oncogene [133]. CYLD (Cylindramatosis) is a DUB that is required for normal cell cycle entry into mitosis [134]. PSMD14 is a proteasome regulatory particle subunit that functions as a DUB for proteins prior to degradation [135].
A classification of MB based on proteasome subunits
The proteasome is a proteolytic structure that regulates the stability of most cellular proteins, and hence the most basic cellular processes. The structure of the proteasome and the function of its subunits have been reviewed in detail by several investigators [136, 137, 138]. The proteasome is a barrel-shaped structure slightly smaller than the ribosome. It consists of a proteolytic core particle of 28 subunits (the 20S core) and a regulatory particle (the 19S particle) at each end of at least 19 subunits. The 20S core consists of two sets of seven beta subunits with two sets of seven alpha subunits arranged at each end. The alpha ring has a gating function [139] while the beta ring contains the proteolytic subunits β1, β2, and β3 [137]. In immune cells these three subunits are substituted by β1i, β2i, and β3i. The regulatory particle on each end of the core particle is composed of a base formed by six ATPase subunits (Rpt1–Rpt6) and a lid composed of an additional 13 subunits. The regulatory particle functions to recognize ubiquitinated proteins, unfold them, translocate them to the core particle, and to remove the ubiquitin for recycling [137].
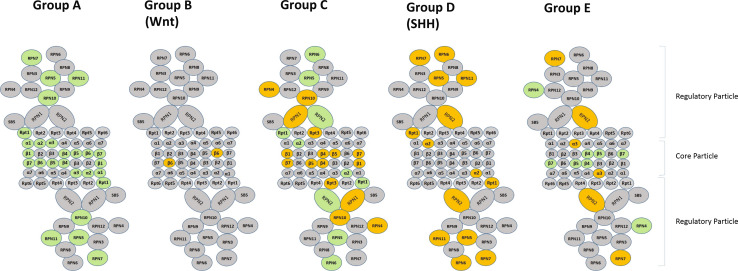
A number of genes on chromosomes other than chromosome 17, code for proteasome subunits and serve as markers for MBs as well (Table 3). These data suggest the possibility of classifying MBs based on expression of genes for proteasome subunits. While Anagnostopoulos et al. [26] have listed several proteins (in their supplemental data) for proteasome subunits as those that are overexpressed or under expressed in MB compared to control tissue, there have been no systematic studies of proteasome subunits in the various MB subgroups. Although Thompson et al. [7] did not discuss the issue their supplemental dataset of MB signature genes provided some remarkable data on the topic. Seventeen of the genes that we identified as 26S proteasome subunits were listed by Thompson et al. [7] as signature genes for MB subgroups (Thompson supplemental data Table 2). In Table 3 of this manuscript we have listed the proteasome subunits and indicated which, by the Thompson dataset, are MB signature genes. Also indicated in this table is whether these 17 genes are significantly up-regulated or down-regulated by subgroup compared to other subgroups. We illustrate the differential expression of proteasome subunits in Fig. 4.
Fig. 4.
Differentiation expression of proteasome subunits in MB subtypes in Thompson supplemental dataset. Green significantly increased. Orange significantly decreased compared to other groups. Grey not in dataset or not significant
Thompson’s Group A tumors have up-regulated proteasome subunits within the alpha ring, the beta ring, and in the 19 s regulatory particle including the ATPase ring. Thompson’s Group B tumors (WNT group) show no change in proteasome subunit genes except for PSMB1 (the gene for the core particle subunit β6), which is on chromosome 6, a chromosome which show loss of heterozygosity in many of the tumors in this group. Neben et al. list this gene among 54 genes whose expression levels in MBs correlate with unfavorable outcome [140].
Thompson’s Group C (the isochromosome 17 group) tumors have down-regulated genes (PSMB6, PSMB2, PSMB5, PSMB4) which code for several beta-ring core particle proteins (β1, β4, β5, β7). PSMC4 and PSMD2 were also significantly down-regulated in this group as were the genes for the proteasome activators, PSME1 and PSME2. However the genes for several subunits within the regulatory particle (Rpn 2, 5 and 6) were up-regulated in this group. The gene for Rpn4, which reportedly functions as a transcription factor for proteasome assembly [141], was also decreased in Group C. Group D tumors (SHH group), however, were characterized by highly significant down-regulation of genes for Rpn 2, 5, 6, 7 and 11. Many of SHH MBs have loss of chromosome 9q [42]. Since the genes for the β2 subunit and for the PSMD5 subunit are on 9q we can predict SHH MBs will have a statistical tendency towards down-regulation of these two subunits as well. The Thompson dataset of MB signature genes, however, does not include data on these two genes. Group E tumors were associated with down-regulation of PSMA4 (the gene for subunit α3), and upregulation of several core beta-ring subunits. Thus the Thompson supplemental dataset shows that a classification of MB tumors based on expression of genes for proteasome subunits was possible in 2006 based on 50 % of the genes for subunits of the 26S proteasome. The classification was further supported by genetic data associating loss of chromosome 6 and loss of chromosome 9q with decreased expression of genes for proteasome subunits in the WNT and SHH groups of MB.
A classification based on proteasome subunits could enhance and clarify the current consensus classification (WNT Group, SHH Group, Groups 3 and 4). The expression of proteasome subunits in the different types of MBs is physiologically important since the proteasome regulates the turnover of many proteins, including those that are clinically relevant.
Summary and conclusions
Hereditable epigenetic modifications have been identified as playing a role in cancer differentiation and proliferation [142]. In this context we have discussed the role of Chromosome 17 and the ubiquitin-proteasome system as they relate to MB and differentiation of granules cells (Fig. 5). Chromosome 17 contains more than 100 genes that are marker genes or ‘signature genes’ for MB subtypes. These include genes that code for several proteins that are subunits of the proteasome, including the core catalytic enzyme coded for by PSMB6. Chromosome 17 also codes for CDC20, a component of the APC/C ubiquitin E3 ligase complex also known as the cyclosome. The APC/C cyclosome plays a key role in regulating the events of the cell cycle. It regulates the exit from mitosis and entry into anaphase. Based on evidence that several components of the APC/C are MB signature genes, we suggest that dysfunction of the APC/C cyclosome plays a key role in at least one of the MB subtypes (Thompson subgroup C), a subgroup included in the consensus MB Group 4. This conclusion is supported by the recent work of Penas et al. [121]. Regulating ubiquitin ligases, including the APC/C cyclosome, in specific MB subtypes may prove therapeutically useful.
Fig. 5.
Chromosome 17 and the ubiquitin-proteasome system in MB. E1-ubiquitin activating enzyme; E2-ubiquitin conjugating enzyme; E3-ubiquitin ligase; Dub-deubiquitinase; Sub-substrate; U-Ubiquitin. Color coding for genes: red ubiquitin conjugase (Ube2G1); green ubiquitin ligase (Rnf167, Kctd11, Fbxo29, Neurl4, Rnf222, Fbxw10, Cdc27, Spop, Rnf43, Trim27, respectively); gold, deubiquitinase (Usp6, Usp39, Usp36, respectively); yellow proteasome subunits (PSMB6, PSMD11, PSMB3, PSMD3, PA28γ, PSMC5, PSMD12, respectively)
Furthermore, the supplementary dataset of Thompson et al. [7] provides evidence that all MB subgroups can be classified based on the expression of proteasome subunits. The MB subgroups can also be distinguished based on differential expression of ubiquitin ligases and deubiquitinases. Proteasome components may prove to be effective therapeutic targets based on specific MB subtypes. The drug periplocin, for example, is reported to inhibit substantially PSMB6 in lung cancer cells [143]. Ubiquitin ligases, such as the F-BOX proteins SKP2 and FBXW7 [144], that have been suspected to be of significance in other tumors also appear to play a role in MBs (Table 4). Recently evidence has accumulating showing that deubiquitinases also play a role in signal pathways that are relevant to cancer [145, 146] and that DUB inhibitors may be useful in cancer therapy [147]. Classifying MBs based on the functional expression of various components of the ubiquitin-proteasome system will help in understanding the cellular mechanisms in the subgroups of MBs and in developing specific therapeutic approaches for the various types of MBs. It would be useful to have a microarray chip specifically designed to classify MBs and other tumors based on gene expression of components of the ubiquitin-proteasome system.
Acknowledgments
The authors received no funding for this project.
Compliance with ethical standards
Conflict of interest
The authors declare no conflict of interest.
References
- 1.Coluccia D, Figuereido C, Isik S, Smith C, Rutka JT. Medulloblastoma: tumor biology and relevance to treatment and prognosis paradigm. Curr Neurol Neurosci Rep. 2016;16(5):43. doi: 10.1007/s11910-016-0644-7. [DOI] [PubMed] [Google Scholar]
- 2.Vaquero E, Gomez CM, Quintero EA, Gonzalez-Rosa JJ, Marquez J. Differential prefrontal-like deficit in children after cerebellar astrocytoma and medulloblastoma tumor. Behav Brain Funct. 2008;4:18. doi: 10.1186/1744-9081-4-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Behesti H, Marino S. Cerebellar granule cells: insights into proliferation, differentiation, and role in medulloblastoma pathogenesis. Int J Biochem Cell Biol. 2009;41(3):435–445. doi: 10.1016/j.biocel.2008.06.017. [DOI] [PubMed] [Google Scholar]
- 4.Marzban H, Del Bigio MR, Alizadeh J, Ghavami S, Zachariah RM, Rastegar M. Cellular commitment in the developing cerebellum. Front Cell Neurosci. 2014;8:450. doi: 10.3389/fncel.2014.00450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Northcott PA, Jones DT, Kool M, Robinson GW, Gilbertson RJ, Cho YJ, et al. Medulloblastomics: the end of the beginning. Nat Rev Cancer. 2012;12(12):818–834. doi: 10.1038/nrc3410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Taylor MD, Northcott PA, Korshunov A, Remke M, Cho YJ, Clifford SC, et al. Molecular subgroups of medulloblastoma: the current consensus. Acta Neuropathol. 2012;123(4):465–472. doi: 10.1007/s00401-011-0922-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Thompson MC, Fuller C, Hogg TL, Dalton J, Finkelstein D, Lau CC, et al. Genomics identifies medulloblastoma subgroups that are enriched for specific genetic alterations. J Clin Oncol Off J Am Soc Clin Oncol. 2006;24(12):1924–1931. doi: 10.1200/JCO.2005.04.4974. [DOI] [PubMed] [Google Scholar]
- 8.Kool M, Koster J, Bunt J, Hasselt NE, Lakeman A, van Sluis P, et al. Integrated genomics identifies five medulloblastoma subtypes with distinct genetic profiles, pathway signatures and clinicopathological features. PLoS One. 2008;3(8):e3088. doi: 10.1371/journal.pone.0003088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vo BT, Wolf E, Kawauchi D, Gebhardt A, Rehg JE, Finkelstein D, et al. The interaction of Myc with Miz1 defines medulloblastoma subgroup identity. Cancer Cell. 2016;29(1):5–16. doi: 10.1016/j.ccell.2015.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.DeSouza RM, Jones BR, Lowis SP, Kurian KM. Pediatric medulloblastoma—update on molecular classification driving targeted therapies. Front Oncol. 2014;4:176. doi: 10.3389/fonc.2014.00176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gajjar AJ, Robinson GW. Medulloblastoma-translating discoveries from the bench to the bedside. Nature reviews Clinical oncology. 2014;11(12):714–722. doi: 10.1038/nrclinonc.2014.181. [DOI] [PubMed] [Google Scholar]
- 12.Gilbertson RJ, Ellison DW. The origins of medulloblastoma subtypes. Ann Rev Pathol. 2008;3:341–365. doi: 10.1146/annurev.pathmechdis.3.121806.151518. [DOI] [PubMed] [Google Scholar]
- 13.Gibson P, Tong Y, Robinson G, Thompson MC, Currle DS, Eden C, et al. Subtypes of medulloblastoma have distinct developmental origins. Nature. 2010;468(7327):1095–1099. doi: 10.1038/nature09587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Grill J, Dufour C. Neuro-oncology: stability of medulloblastoma subgroups at tumour recurrence. Nat Rev Neurol. 2014;10(1):5–6. doi: 10.1038/nrneurol.2013.256. [DOI] [PubMed] [Google Scholar]
- 15.McCarthy N. Medulloblastoma: origins. Nat Rev Cancer. 2011;11(2):79. doi: 10.1038/nrc3010. [DOI] [PubMed] [Google Scholar]
- 16.Huse JT, Holland EC. Targeting brain cancer: advances in the molecular pathology of malignant glioma and medulloblastoma. Nat Rev Cancer. 2010;10(5):319–331. doi: 10.1038/nrc2818. [DOI] [PubMed] [Google Scholar]
- 17.Burnett ME, White EC, Sih S, von Haken MS, Cogen PH. Chromosome arm 17p deletion analysis reveals molecular genetic heterogeneity in supratentorial and infratentorial primitive neuroectodermal tumors of the central nervous system. Cancer Genet Cytogenet. 1997;97(1):25–31. doi: 10.1016/S0165-4608(96)00319-6. [DOI] [PubMed] [Google Scholar]
- 18.Cogen PH, Daneshvar L, Metzger AK, Duyk G, Edwards MS, Sheffield VC. Involvement of multiple chromosome 17p loci in medulloblastoma tumorigenesis. Am J Hum Genet. 1992;50(3):584–589. [PMC free article] [PubMed] [Google Scholar]
- 19.Di Marcotullio L, Ferretti E, De Smaele E, Argenti B, Mincione C, Zazzeroni F, et al. REN(KCTD11) is a suppressor of Hedgehog signaling and is deleted in human medulloblastoma. Proc Natl Acad Sci USA. 2004;101(29):10833–10838. doi: 10.1073/pnas.0400690101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nicholson J, Wickramasinghe C, Ross F, Crolla J, Ellison D. Imbalances of chromosome 17 in medulloblastomas determined by comparative genomic hybridisation and fluorescence in situ hybridisation. Mol Pathol MP. 2000;53(6):313–319. doi: 10.1136/mp.53.6.313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Robinson G, Parker M, Kranenburg TA, Lu C, Chen X, Ding L, et al. Novel mutations target distinct subgroups of medulloblastoma. Nature. 2012;488(7409):43–48. doi: 10.1038/nature11213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Scheurlen WG, Seranski P, Mincheva A, Kuhl J, Sorensen N, Krauss J, et al. High-resolution deletion mapping of chromosome arm 17p in childhood primitive neuroectodermal tumors reveals a common chromosomal disruption within the Smith-Magenis region, an unstable region in chromosome band 17p11.2. Genes Chromosom Cancer. 1997;18(1):50–58. doi: 10.1002/(SICI)1098-2264(199701)18:1<50::AID-GCC6>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- 23.Fioretos T, Strombeck B, Sandberg T, Johansson B, Billstrom R, Borg A, et al. Isochromosome 17q in blast crisis of chronic myeloid leukemia and in other hematologic malignancies is the result of clustered breakpoints in 17p11 and is not associated with coding TP53 mutations. Blood. 1999;94(1):225–232. [PubMed] [Google Scholar]
- 24.Emadian SM, McDonald JD, Gerken SC, Fults D. Correlation of chromosome 17p loss with clinical outcome in medulloblastoma. Clin Cancer Res Off J Am Assoc Cancer Res. 1996;2(9):1559–1564. [PubMed] [Google Scholar]
- 25.Steichen-Gersdorf E, Baumgartner M, Kreczy A, Maier H, Fink FM. Deletion mapping on chromosome 17p in medulloblastoma. Br J Cancer. 1997;76(10):1284–1287. doi: 10.1038/bjc.1997.549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Anagnostopoulos AK, Papathanassiou C, Karamolegou K, Anastasiadou E, Dimas KS, Kontos H, et al. Proteomic studies of pediatric medulloblastoma tumors with 17p deletion. J Proteome Res. 2015;14(2):1076–1088. doi: 10.1021/pr501219f. [DOI] [PubMed] [Google Scholar]
- 27.McCabe MG, Backlund LM, Leong HS, Ichimura K, Collins VP. Chromosome 17 alterations identify good-risk and poor-risk tumors independently of clinical factors in medulloblastoma. Neuro-Oncol. 2011;13(4):376–383. doi: 10.1093/neuonc/noq192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Clifford SC, Lusher ME, Lindsey JC, Langdon JA, Gilbertson RJ, Straughton D, et al. Wnt/Wingless pathway activation and chromosome 6 loss characterize a distinct molecular sub-group of medulloblastomas associated with a favorable prognosis. Cell Cycle. 2006;5(22):2666–2670. doi: 10.4161/cc.5.22.3446. [DOI] [PubMed] [Google Scholar]
- 29.Chen L, Madura K. Increased proteasome activity, ubiquitin-conjugating enzymes, and eEF1A translation factor detected in breast cancer tissue. Cancer Res. 2005;65(13):5599–5606. doi: 10.1158/0008-5472.CAN-05-0201. [DOI] [PubMed] [Google Scholar]
- 30.Boland K, Flanagan L, McCawley N, Pabari R, Kay EW, McNamara DA, et al. Targeting the 19S proteasomal subunit, Rpt4, for the treatment of colon cancer. Eur J Pharmacol. 2016;780:53–64. doi: 10.1016/j.ejphar.2016.03.031. [DOI] [PubMed] [Google Scholar]
- 31.Bazzaro M, Lee MK, Zoso A, Stirling WL, Santillan A, Shih Ie M, et al. Ubiquitin-proteasome system stress sensitizes ovarian cancer to proteasome inhibitor-induced apoptosis. Cancer Res. 2006;66(7):3754–3763. doi: 10.1158/0008-5472.CAN-05-2321. [DOI] [PubMed] [Google Scholar]
- 32.Di Marcotullio L, Greco A, Mazza D, Canettieri G, Pietrosanti L, Infante P, et al. Numb activates the E3 ligase Itch to control Gli1 function through a novel degradation signal. Oncogene. 2011;30(1):65–76. doi: 10.1038/onc.2010.394. [DOI] [PubMed] [Google Scholar]
- 33.Vriend J, Ghavami S, Marzban H. The role of the ubiquitin proteasome system in cerebellar development and medulloblastoma. Mol Brain. 2015;8(1):64. doi: 10.1186/s13041-015-0155-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pomeroy SL, Tamayo P, Gaasenbeek M, Sturla LM, Angelo M, McLaughlin ME, et al. Prediction of central nervous system embryonal tumour outcome based on gene expression. Nature. 2002;415(6870):436–442. doi: 10.1038/415436a. [DOI] [PubMed] [Google Scholar]
- 35.Jones DT, Jager N, Kool M, Zichner T, Hutter B, Sultan M, et al. Dissecting the genomic complexity underlying medulloblastoma. Nature. 2012;488(7409):100–105. doi: 10.1038/nature11284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rusert JM, Wu X, Eberhart CG, Taylor MD, Wechsler-Reya RJ. SnapShot: medulloblastoma. Cancer Cell. 2014;26(6):940-e1. doi: 10.1016/j.ccell.2014.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Vilchez D, Boyer L, Morantte I, Lutz M, Merkwirth C, Joyce D, et al. Increased proteasome activity in human embryonic stem cells is regulated by PSMD11. Nature. 2012;489(7415):304–308. doi: 10.1038/nature11468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yen HC, Espiritu C, Chang EC. Rpn5 is a conserved proteasome subunit and required for proper proteasome localization and assembly. J Biol Chem. 2003;278(33):30669–30676. doi: 10.1074/jbc.M302093200. [DOI] [PubMed] [Google Scholar]
- 39.Gomes AV. Genetics of proteasome diseases. Scientifica (Cairo) 2013;2013:637629. doi: 10.1155/2013/637629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Groettrup M, Kirk CJ, Basler M. Proteasomes in immune cells: more than peptide producers? Nat Rev Immunol. 2010;10(1):73–78. doi: 10.1038/nri2687. [DOI] [PubMed] [Google Scholar]
- 41.Pfister S, Remke M, Benner A, Mendrzyk F, Toedt G, Felsberg J, et al. Outcome prediction in pediatric medulloblastoma based on DNA copy-number aberrations of chromosomes 6q and 17q and the MYC and MYCN loci. J Clin Oncol Off J Am Soc Clin Oncol. 2009;27(10):1627–1636. doi: 10.1200/JCO.2008.17.9432. [DOI] [PubMed] [Google Scholar]
- 42.Northcott PA, Korshunov A, Witt H, Hielscher T, Eberhart CG, Mack S, et al. Medulloblastoma comprises four distinct molecular variants. J Clin Oncol Off J Am Soc Clin Oncol. 2011;29(11):1408–1414. doi: 10.1200/JCO.2009.27.4324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lopez-Ochoa S, Ramirez-Garcia M, Castro-Sierra E, Arenas-Huertero F. Analysis of chromosome 17 miRNAs and their importance in medulloblastomas. BioMed Res Int. 2015;2015:717509. doi: 10.1155/2015/717509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Miller C, Mohandas T, Wolf D, Prokocimer M, Rotter V, Koeffler HP. Human p53 gene localized to short arm of chromosome 17. Nature. 1986;319(6056):783–784. doi: 10.1038/319783a0. [DOI] [PubMed] [Google Scholar]
- 45.Adesina AM, Nalbantoglu J, Cavenee WK. p53 gene mutation and mdm2 gene amplification are uncommon in medulloblastoma. Cancer Res. 1994;54(21):5649–5651. [PubMed] [Google Scholar]
- 46.Chopin V, Leprince D (2006) Chromosome arm 17p13.3: could HIC1 be the one? Med Sci 22(1):54–61 [DOI] [PubMed]
- 47.Briggs KJ, Corcoran-Schwartz IM, Zhang W, Harcke T, Devereux WL, Baylin SB, et al. Cooperation between the Hic1 and Ptch1 tumor suppressors in medulloblastoma. Genes Dev. 2008;22(6):770–785. doi: 10.1101/gad.1640908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wang VY, Zoghbi HY. Genetic regulation of cerebellar development. Nat Rev Neurosci. 2001;2(7):484–491. doi: 10.1038/35081558. [DOI] [PubMed] [Google Scholar]
- 49.Zakrzewska M, Gresner SM, Zakrzewski K, Zalewska-Szewczyk B, Liberski PP (2013) Novel gene expression model for outcome prediction in paediatric medulloblastoma. J Mol Neurosci 51(2):371–379 [DOI] [PubMed]
- 50.Grimmer MR, Weiss WA. BMPs oppose Math1 in cerebellar development and in medulloblastoma. Genes Dev. 2008;22(6):693–699. doi: 10.1101/gad.1657808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kokubo M, Nishio M, Ribar TJ, Anderson KA, West AE, Means AR. BDNF-mediated cerebellar granule cell development is impaired in mice null for CaMKK2 or CaMKIV. J Neurosci Off J Soc Neurosci. 2009;29(28):8901–8913. doi: 10.1523/JNEUROSCI.0040-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Chen CM, Behringer RR. OVCA1: tumor suppressor gene. Curr Opin Genet Dev. 2005;15(1):49–54. doi: 10.1016/j.gde.2004.12.006. [DOI] [PubMed] [Google Scholar]
- 53.Bruening W, Prowse AH, Schultz DC, Holgado-Madruga M, Wong A, Godwin AK. Expression of OVCA1, a candidate tumor suppressor, is reduced in tumors and inhibits growth of ovarian cancer cells. Cancer Res. 1999;59(19):4973–4983. [PubMed] [Google Scholar]
- 54.Chen CM, Behringer RR. Ovca1 regulates cell proliferation, embryonic development, and tumorigenesis. Genes Dev. 2004;18(3):320–332. doi: 10.1101/gad.1162204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Yao W, Ji S, Qin Y, Yang J, Xu J, Zhang B, et al. Profilin-1 suppresses tumorigenicity in pancreatic cancer through regulation of the SIRT3-HIF1alpha axis. Mol Cancer. 2014;13:187. doi: 10.1186/1476-4598-13-187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lee JH, Rho SB, Chun T. GABAA receptor-associated protein (GABARAP) induces apoptosis by interacting with DEAD (Asp-Glu-Ala-Asp/His) box polypeptide 47 (DDX 47) Biotechnol Lett. 2005;27(9):623–628. doi: 10.1007/s10529-005-3628-2. [DOI] [PubMed] [Google Scholar]
- 57.Slobodkin MR, Elazar Z. The Atg8 family: multifunctional ubiquitin-like key regulators of autophagy. Essays Biochem. 2013;55:51–64. doi: 10.1042/bse0550051. [DOI] [PubMed] [Google Scholar]
- 58.Shpilka T, Weidberg H, Pietrokovski S, Elazar Z. Atg8: an autophagy-related ubiquitin-like protein family. Genome Biol. 2011;12(7):226. doi: 10.1186/gb-2011-12-7-226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Klebig C, Seitz S, Arnold W, Deutschmann N, Pacyna-Gengelbach M, Scherneck S, et al. Characterization of {gamma}-aminobutyric acid type A receptor-associated protein, a novel tumor suppressor, showing reduced expression in breast cancer. Cancer Res. 2005;65(2):394–400. [PubMed] [Google Scholar]
- 60.Wallingford JB, Habas R. The developmental biology of Dishevelled: an enigmatic protein governing cell fate and cell polarity. Development. 2005;132(20):4421–4436. doi: 10.1242/dev.02068. [DOI] [PubMed] [Google Scholar]
- 61.Memin E, Hoque M, Jain MR, Heller DS, Li H, Cracchiolo B, et al. Blocking eIF5A modification in cervical cancer cells alters the expression of cancer-related genes and suppresses cell proliferation. Cancer Res. 2014;74(2):552–562. doi: 10.1158/0008-5472.CAN-13-0474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Pugh TJ, Weeraratne SD, Archer TC, Pomeranz Krummel DA, Auclair D, Bochicchio J, et al. Medulloblastoma exome sequencing uncovers subtype-specific somatic mutations. Nature. 2012;488(7409):106–110. doi: 10.1038/nature11329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Akino T, Han X, Nakayama H, McNeish B, Zurakowski D, Mammoto A, et al. Netrin-1 promotes medulloblastoma cell invasiveness and angiogenesis, and demonstrates elevated expression in tumor tissue and urine of patients with pediatric medulloblastoma. Cancer Res. 2014;74(14):3716–3726. doi: 10.1158/0008-5472.CAN-13-3116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kim TH, Lee HK, Seo IA, Bae HR, Suh DJ, Wu J, et al. Netrin induces down-regulation of its receptor, Deleted in Colorectal Cancer, through the ubiquitin-proteasome pathway in the embryonic cortical neuron. J Neurochem. 2005;95(1):1–8. doi: 10.1111/j.1471-4159.2005.03314.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Mohanty S, Ovee M, Banerjee M. pdz domain recognition: insight from human tax-interacting protein 1 (TIP-1) interaction with target proteins. Biology. 2015;4(1):88–103. doi: 10.3390/biology4010088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Havugimana PC, Hart GT, Nepusz T, Yang H, Turinsky AL, Li Z, et al. A census of human soluble protein complexes. Cell. 2012;150(5):1068–1081. doi: 10.1016/j.cell.2012.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Roussel MF, Robinson GW (2013) Role of MYC in Medulloblastoma. Cold Spring Harbor perspectives in medicine 3(11) [DOI] [PMC free article] [PubMed]
- 68.Popov N, Schulein C, Jaenicke LA, Eilers M. Ubiquitylation of the amino terminus of Myc by SCF(beta-TrCP) antagonizes SCF(Fbw7)-mediated turnover. Nat Cell Biol. 2010;12(10):973–981. doi: 10.1038/ncb2104. [DOI] [PubMed] [Google Scholar]
- 69.Yada M, Hatakeyama S, Kamura T, Nishiyama M, Tsunematsu R, Imaki H, et al. Phosphorylation-dependent degradation of c-Myc is mediated by the F-box protein Fbw7. EMBO J. 2004;23(10):2116–2125. doi: 10.1038/sj.emboj.7600217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kim SY, Herbst A, Tworkowski KA, Salghetti SE, Tansey WP. Skp2 regulates Myc protein stability and activity. Mol Cell. 2003;11(5):1177–1188. doi: 10.1016/S1097-2765(03)00173-4. [DOI] [PubMed] [Google Scholar]
- 71.De Smaele E, Di Marcotullio L, Ferretti E, Screpanti I, Alesse E, Gulino A. Chromosome 17p deletion in human medulloblastoma: a missing checkpoint in the Hedgehog pathway. Cell Cycle. 2004;3(10):1263–1266. doi: 10.4161/cc.3.10.1200. [DOI] [PubMed] [Google Scholar]
- 72.Zazzeroni F, Nicosia D, Tessitore A, Gallo R, Verzella D, Fischietti M, et al. KCTD11 tumor suppressor gene expression is reduced in prostate adenocarcinoma. BioMed research international. 2014;2014:380398. doi: 10.1155/2014/380398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Mancarelli MM, Zazzeroni F, Ciccocioppo L, Capece D, Po A, Murgo S, et al. The tumor suppressor gene KCTD11REN is regulated by Sp1 and methylation and its expression is reduced in tumors. Molecular cancer. 2010;9:172. doi: 10.1186/1476-4598-9-172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Correale S, Pirone L, Di Marcotullio L, De Smaele E, Greco A, Mazza D, et al. Molecular organization of the cullin E3 ligase adaptor KCTD11. Biochimie. 2011;93(4):715–724. doi: 10.1016/j.biochi.2010.12.014. [DOI] [PubMed] [Google Scholar]
- 75.Canettieri G, Di Marcotullio L, Greco A, Coni S, Antonucci L, Infante P, et al. Histone deacetylase and Cullin3-REN(KCTD11) ubiquitin ligase interplay regulates Hedgehog signalling through Gli acetylation. Nat Cell Biol. 2010;12(2):132–142. doi: 10.1038/ncb2013. [DOI] [PubMed] [Google Scholar]
- 76.Gulino A, Di Marcotullio L, Canettieri G, De Smaele E, Screpanti I. Hedgehog/Gli control by ubiquitination/acetylation interplay. Vitam Horm. 2012;88:211–227. doi: 10.1016/B978-0-12-394622-5.00009-2. [DOI] [PubMed] [Google Scholar]
- 77.Lightman EG, Harrison MR, Cunliffe VT. Opposing actions of histone deacetylase 1 and Notch signalling restrict expression of erm and fgf20a to hindbrain rhombomere centres during zebrafish neurogenesis. Int J Dev Biol. 2011;55(6):597–602. doi: 10.1387/ijdb.113315el. [DOI] [PubMed] [Google Scholar]
- 78.Harrison MR, Georgiou AS, Spaink HP, Cunliffe VT. The epigenetic regulator Histone Deacetylase 1 promotes transcription of a core neurogenic programme in zebrafish embryos. BMC Genom. 2011;12:24. doi: 10.1186/1471-2164-12-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Nambiar RM, Ignatius MS, Henion PD. Zebrafish colgate/hdac1 functions in the non-canonical Wnt pathway during axial extension and in Wnt-independent branchiomotor neuron migration. Mech Dev. 2007;124(9–10):682–698. doi: 10.1016/j.mod.2007.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Chen S, Yao X, Li Y, Saifudeen Z, Bachvarov D, El-Dahr SS. Histone deacetylase 1 and 2 regulate Wnt and p53 pathways in the ureteric bud epithelium. Development. 2015;142(6):1180–1192. doi: 10.1242/dev.113506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Hwang IY, Roe JS, Seol JH, Kim HR, Cho EJ, Youn HD. pVHL-mediated transcriptional repression of c-Myc by recruitment of histone deacetylases. Mol Cells. 2012;33(2):195–201. doi: 10.1007/s10059-012-2268-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Huntzicker EG, Estay IS, Zhen H, Lokteva LA, Jackson PK, Oro AE. Dual degradation signals control Gli protein stability and tumor formation. Genes Dev. 2006;20(3):276–281. doi: 10.1101/gad.1380906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Schwend T, Jin Z, Jiang K, Mitchell BJ, Jia J, Yang J. Stabilization of speckle-type POZ protein (Spop) by Daz interacting protein 1 (Dzip1) is essential for Gli turnover and the proper output of Hedgehog signaling. J Biol Chem. 2013;288(45):32809–32820. doi: 10.1074/jbc.M113.512962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Infante P, Alfonsi R, Botta B, Mori M, Di Marcotullio L. Targeting GLI factors to inhibit the Hedgehog pathway. Trends Pharmacol Sci. 2015;36(8):547–558. doi: 10.1016/j.tips.2015.05.006. [DOI] [PubMed] [Google Scholar]
- 85.Yamada HY, Gorbsky GJ. Tumor suppressor candidate TSSC5 is regulated by UbcH6 and a novel ubiquitin ligase RING105. Oncogene. 2006;25(9):1330–1339. doi: 10.1038/sj.onc.1209167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Giordana MT, Migheli A, Pavanelli E. Isochromosome 17q is a constant finding in medulloblastoma. An interphase cytogenetic study on tissue sections. Neuropathol Appl Neurobiol. 1998;24(3):233–238. doi: 10.1046/j.1365-2990.1998.00102.x. [DOI] [PubMed] [Google Scholar]
- 87.Kisselev AF, Garcia-Calvo M, Overkleeft HS, Peterson E, Pennington MW, Ploegh HL, et al. The caspase-like sites of proteasomes, their substrate specificity, new inhibitors and substrates, and allosteric interactions with the trypsin-like sites. J Biol Chem. 2003;278(38):35869–35877. doi: 10.1074/jbc.M303725200. [DOI] [PubMed] [Google Scholar]
- 88.Orsetti B, Nugoli M, Cervera N, Lasorsa L, Chuchana P, Ursule L, et al. Genomic and expression profiling of chromosome 17 in breast cancer reveals complex patterns of alterations and novel candidate genes. Cancer Res. 2004;64(18):6453–6460. doi: 10.1158/0008-5472.CAN-04-0756. [DOI] [PubMed] [Google Scholar]
- 89.Phelan CM, Borg A, Cuny M, Crichton DN, Baldersson T, Andersen TI, et al. Consortium study on 1280 breast carcinomas: allelic loss on chromosome 17 targets subregions associated with family history and clinical parameters. Cancer Res. 1998;58(5):1004–1012. [PubMed] [Google Scholar]
- 90.Cornelis RS, Devilee P, van Vliet M, Kuipers-Dijkshoorn N, Kersenmaeker A, Bardoel A, et al. Allele loss patterns on chromosome 17q in 109 breast carcinomas indicate at least two distinct target regions. Oncogene. 1993;8(3):781–785. [PubMed] [Google Scholar]
- 91.Sousa JC, Germano RT, Castro CC, Magalhaes SM, Pinheiro RF. Case report of isochromosome 17q in acute myeloid leukemia with myelodysplasia-related changes after treatment with a hypomethylating agent. Genet Mol Res GMR. 2012;11(3):2045–2050. doi: 10.4238/2012.August.6.8. [DOI] [PubMed] [Google Scholar]
- 92.Gudmundsson J, Sulem P, Steinthorsdottir V, Bergthorsson JT, Thorleifsson G, Manolescu A, et al. Two variants on chromosome 17 confer prostate cancer risk, and the one in TCF2 protects against type 2 diabetes. Nat Genet. 2007;39(8):977–983. doi: 10.1038/ng2062. [DOI] [PubMed] [Google Scholar]
- 93.Ciesielski M, Kruszewski WJ, Smialek U, Walczak J, Szajewski M, Szefel J, et al. The HER2 gene and HER2 protein status and chromosome 17 polysomy in gastric cancer cells in own material. Appl Immunohistochem Mol Morphol AIMM/Off Publ Soc Appl Immunohistochem. 2015;23(2):113–117. doi: 10.1097/PAI.0000000000000070. [DOI] [PubMed] [Google Scholar]
- 94.Miki Y, Swensen J, Shattuck-Eidens D, Futreal PA, Harshman K, Tavtigian S, et al. A strong candidate for the breast and ovarian cancer susceptibility gene BRCA1. Science. 1994;266(5182):66–71. doi: 10.1126/science.7545954. [DOI] [PubMed] [Google Scholar]
- 95.Bayrakli F, Akgun B, Soylemez B, Kaplan M, Gurelik M. Variation in the BRCA2 gene in a child with medulloblastoma and a family history of breast cancer. J Neurosurg Pediatr. 2011;8(5):476–478. doi: 10.3171/2011.8.PEDS11210. [DOI] [PubMed] [Google Scholar]
- 96.de Bont JM, Packer RJ, Michiels EM, den Boer ML, Pieters R. Biological background of pediatric medulloblastoma and ependymoma: a review from a translational research perspective. Neuro-Oncol. 2008;10(6):1040–1060. doi: 10.1215/15228517-2008-059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Gilbertson RJ, Perry RH, Kelly PJ, Pearson AD, Lunec J. Prognostic significance of HER2 and HER4 coexpression in childhood medulloblastoma. Cancer Res. 1997;57(15):3272–3280. [PubMed] [Google Scholar]
- 98.Milde T, Oehme I, Korshunov A, Kopp-Schneider A, Remke M, Northcott P, et al. HDAC5 and HDAC9 in medulloblastoma: novel markers for risk stratification and role in tumor cell growth. Clin Cancer Res Off J Am Assoc Cancer Res. 2010;16(12):3240–3252. doi: 10.1158/1078-0432.CCR-10-0395. [DOI] [PubMed] [Google Scholar]
- 99.Morales D, Hatten ME. Molecular markers of neuronal progenitors in the embryonic cerebellar anlage. J Neurosci Off J Soc Neurosci. 2006;26(47):12226–12236. doi: 10.1523/JNEUROSCI.3493-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Bergstein I, Eisenberg LM, Bhalerao J, Jenkins NA, Copeland NG, Osborne MP, et al. Isolation of two novel WNT genes, WNT14 and WNT15, one of which (WNT15) is closely linked to WNT3 on human chromosome 17q21. Genomics. 1997;46(3):450–458. doi: 10.1006/geno.1997.5041. [DOI] [PubMed] [Google Scholar]
- 101.Siddall NA, McLaughlin EA, Marriner NL, Hime GR. The RNA-binding protein Musashi is required intrinsically to maintain stem cell identity. Proc Natl Acad Sci USA. 2006;103(22):8402–8407. doi: 10.1073/pnas.0600906103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.de Andres-Aguayo L, Varas F, Kallin EM, Infante JF, Wurst W, Floss T, et al. Musashi 2 is a regulator of the HSC compartment identified by a retroviral insertion screen and knockout mice. Blood. 2011;118(3):554–564. doi: 10.1182/blood-2010-12-322081. [DOI] [PubMed] [Google Scholar]
- 103.Marchetti D, Mrak RE, Paulsen DD, Sinnappah-Kang ND. Neurotrophin receptors and heparanase: a functional axis in human medulloblastoma invasion. Journal of experimental & clinical cancer research: CR. 2007;26(1):5–23. [PubMed] [Google Scholar]
- 104.Sinnappah-Kang ND, Mrak RE, Paulsen DB, Marchetti D. Heparanase expression and TrkC/p75NTR ratios in human medulloblastoma. Clin Exp Metastasis. 2006;23(1):55–63. doi: 10.1007/s10585-006-9017-y. [DOI] [PubMed] [Google Scholar]
- 105.De Sousa EMF, Medema JP. Axing Wnt signals. Cell Res. 2012;22(1):9–11. doi: 10.1038/cr.2011.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.MacDonald BT, Tamai K, He X. Wnt/beta-catenin signaling: components, mechanisms, and diseases. Dev Cell. 2009;17(1):9–26. doi: 10.1016/j.devcel.2009.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Wang G, Fu Y, Yang X, Luo X, Wang J, Gong J, et al. Brg-1 targeting of novel miR550a-5p/RNF43/Wnt signaling axis regulates colorectal cancer metastasis. Oncogene. 2016;35(5):651–661. doi: 10.1038/onc.2015.124. [DOI] [PubMed] [Google Scholar]
- 108.Balestra FR, Strnad P, Fluckiger I, Gonczy P. Discovering regulators of centriole biogenesis through siRNA-based functional genomics in human cells. Dev Cell. 2013;25(6):555–571. doi: 10.1016/j.devcel.2013.05.016. [DOI] [PubMed] [Google Scholar]
- 109.Cavadini S, Fischer ES, Bunker RD, Potenza A, Lingaraju GM, Goldie KN, et al. Cullin-RING ubiquitin E3 ligase regulation by the COP9 signalosome. Nature. 2016;531(7596):598–603. doi: 10.1038/nature17416. [DOI] [PubMed] [Google Scholar]
- 110.Sokolova V, Li F, Polovin G, Park S. Proteasome activation is mediated via a functional switch of the Rpt6 C-terminal tail following chaperone-dependent assembly. Sci Rep. 2015;5:14909. doi: 10.1038/srep14909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.van der Stoop P, Boutsma EA, Hulsman D, Noback S, Heimerikx M, Kerkhoven RM, et al. Ubiquitin E3 ligase Ring1b/Rnf2 of polycomb repressive complex 1 contributes to stable maintenance of mouse embryonic stem cells. PLoS One. 2008;3(5):e2235. doi: 10.1371/journal.pone.0002235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Nakayama KI, Nakayama K. Ubiquitin ligases: cell-cycle control and cancer. Nat Rev Cancer. 2006;6(5):369–381. doi: 10.1038/nrc1881. [DOI] [PubMed] [Google Scholar]
- 113.Gstaiger M, Jordan R, Lim M, Catzavelos C, Mestan J, Slingerland J, et al. Skp2 is oncogenic and overexpressed in human cancers. Proc Natl Acad Sci USA. 2001;98(9):5043–5048. doi: 10.1073/pnas.081474898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Vu TH, Hoffman AR. Imprinting of the Angelman syndrome gene, UBE3A, is restricted to brain. Nat Genet. 1997;17(1):12–13. doi: 10.1038/ng0997-12. [DOI] [PubMed] [Google Scholar]
- 115.Rougeulle C, Glatt H, Lalande M. The Angelman syndrome candidate gene, UBE3A/E6-AP, is imprinted in brain. Nat Genet. 1997;17(1):14–15. doi: 10.1038/ng0997-14. [DOI] [PubMed] [Google Scholar]
- 116.Mo M, Shahar S, Fleming SB, Mercer AA. How viruses affect the cell cycle through manipulation of the APC/C. Trends Microbiol. 2012;20(9):440–448. doi: 10.1016/j.tim.2012.05.007. [DOI] [PubMed] [Google Scholar]
- 117.Sivakumar S, Gorbsky GJ. Spatiotemporal regulation of the anaphase-promoting complex in mitosis. Nat Rev Mol Cell Biol. 2015;16(2):82–94. doi: 10.1038/nrm3934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Thornton BR, Toczyski DP. Precise destruction: an emerging picture of the APC. Genes Dev. 2006;20(22):3069–3078. doi: 10.1101/gad.1478306. [DOI] [PubMed] [Google Scholar]
- 119.Peters JM. The anaphase promoting complex/cyclosome: a machine designed to destroy. Nat Rev Mol Cell Biol. 2006;7(9):644–656. doi: 10.1038/nrm1988. [DOI] [PubMed] [Google Scholar]
- 120.Primorac I, Musacchio A. Panta rhei: the APC/C at steady state. J Cell Biol. 2013;201(2):177–189. doi: 10.1083/jcb.201301130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Penas C, Govek EE, Fang Y, Ramachandran V, Daniel M, Wang W, et al. Casein kinase 1delta is an APC/C(Cdh1) substrate that regulates cerebellar granule cell neurogenesis. Cell reports. 2015;11(2):249–260. doi: 10.1016/j.celrep.2015.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Penas C, Ramachandran V, Ayad NG. The APC/C Ubiquitin ligase: from cell biology to tumorigenesis. Front Oncol. 2011;1:60. doi: 10.3389/fonc.2011.00060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.van Leuken RJ, Luna-Vargas MP, Sixma TK, Wolthuis RM, Medema RH. Usp39 is essential for mitotic spindle checkpoint integrity and controls mRNA-levels of aurora B. Cell Cycle. 2008;7(17):2710–2719. doi: 10.4161/cc.7.17.6553. [DOI] [PubMed] [Google Scholar]
- 124.Williams SA, Maecker HL, French DM, Liu J, Gregg A, Silverstein LB, et al. USP1 deubiquitinates ID proteins to preserve a mesenchymal stem cell program in osteosarcoma. Cell. 2011;146(6):918–930. doi: 10.1016/j.cell.2011.07.040. [DOI] [PubMed] [Google Scholar]
- 125.Eichhorn PJ, Rodon L, Gonzalez-Junca A, Dirac A, Gili M, Martinez-Saez E, et al. USP15 stabilizes TGF-beta receptor I and promotes oncogenesis through the activation of TGF-beta signaling in glioblastoma. Nat Med. 2012;18(3):429–435. doi: 10.1038/nm.2619. [DOI] [PubMed] [Google Scholar]
- 126.Xirodimas DP. DUBs “found in translation”: uSP15 controls stability of newly synthesized REST. Cell Cycle. 2013;12(16):2536–2537. doi: 10.4161/cc.25844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Lawinger P, Venugopal R, Guo ZS, Immaneni A, Sengupta D, Lu W, et al. The neuronal repressor REST/NRSF is an essential regulator in medulloblastoma cells. Nat Med. 2000;6(7):826–831. doi: 10.1038/77565. [DOI] [PubMed] [Google Scholar]
- 128.Zhang L, Lubin A, Chen H, Sun Z, Gong F. The deubiquitinating protein USP24 interacts with DDB2 and regulates DDB2 stability. Cell Cycle. 2012;11(23):4378–4384. doi: 10.4161/cc.22688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Zhang L, Nemzow L, Chen H, Lubin A, Rong X, Sun Z, et al. The deubiquitinating enzyme USP24 is a regulator of the UV damage response. Cell Rep. 2015;10(2):140–147. doi: 10.1016/j.celrep.2014.12.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Curcio-Morelli C, Zavacki AM, Christofollete M, Gereben B, de Freitas BC, Harney JW, et al. Deubiquitination of type 2 iodothyronine deiodinase by von Hippel-Lindau protein-interacting deubiquitinating enzymes regulates thyroid hormone activation. J Clin Investig. 2003;112(2):189–196. doi: 10.1172/JCI18348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Lui TT, Lacroix C, Ahmed SM, Goldenberg SJ, Leach CA, Daulat AM, et al. The ubiquitin-specific protease USP34 regulates axin stability and Wnt/beta-catenin signaling. Mol Cell Biol. 2011;31(10):2053–2065. doi: 10.1128/MCB.01094-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Murtaza M, Jolly LA, Gecz J, Wood SA. La FAM fatale: USP9X in development and disease. Cell Mol Life Sci CMLS. 2015;72(11):2075–2089. doi: 10.1007/s00018-015-1851-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Hussain S, Foreman O, Perkins SL, Witzig TE, Miles RR, van Deursen J, et al. The de-ubiquitinase UCH-L1 is an oncogene that drives the development of lymphoma in vivo by deregulating PHLPP1 and Akt signaling. Leukemia. 2010;24(9):1641–1655. doi: 10.1038/leu.2010.138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Stegmeier F, Sowa ME, Nalepa G, Gygi SP, Harper JW, Elledge SJ. The tumor suppressor CYLD regulates entry into mitosis. Proc Natl Acad Sci USA. 2007;104(21):8869–8874. doi: 10.1073/pnas.0703268104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Yao T, Cohen RE. A cryptic protease couples deubiquitination and degradation by the proteasome. Nature. 2002;419(6905):403–407. doi: 10.1038/nature01071. [DOI] [PubMed] [Google Scholar]
- 136.Kish-Trier E, Hill CP. Structural biology of the proteasome. Annu Rev Biophys. 2013;42:29–49. doi: 10.1146/annurev-biophys-083012-130417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Bhattacharyya S, Yu H, Mim C, Matouschek A. Regulated protein turnover: snapshots of the proteasome in action. Nat Rev Mol Cell Biol. 2014;15(2):122–133. doi: 10.1038/nrm3741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Dahlmann B. Mammalian proteasome subtypes: their diversity in structure and function. Arch Biochem Biophys. 2016;591:132–140. doi: 10.1016/j.abb.2015.12.012. [DOI] [PubMed] [Google Scholar]
- 139.Choi WH, de Poot SA, Lee JH, Kim JH, Han DH, Kim YK, et al. Open-gate mutants of the mammalian proteasome show enhanced ubiquitin-conjugate degradation. Nat Commun. 2016;7:10963. doi: 10.1038/ncomms10963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Neben K, Korshunov A, Benner A, Wrobel G, Hahn M, Kokocinski F, et al. Microarray-based screening for molecular markers in medulloblastoma revealed STK15 as independent predictor for survival. Cancer Res. 2004;64(9):3103–3111. doi: 10.1158/0008-5472.CAN-03-3968. [DOI] [PubMed] [Google Scholar]
- 141.Shirozu R, Yashiroda H, Murata S. Identification of minimum Rpn4-responsive elements in genes related to proteasome functions. FEBS Lett. 2015;589(8):933–940. doi: 10.1016/j.febslet.2015.02.025. [DOI] [PubMed] [Google Scholar]
- 142.Parsons DW, Li M, Zhang X, Jones S, Leary RJ, Lin JC, et al. The genetic landscape of the childhood cancer medulloblastoma. Science. 2011;331(6016):435–439. doi: 10.1126/science.1198056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Lu Z, Song Q, Yang J, Zhao X, Zhang X, Yang P, et al. Comparative proteomic analysis of anti-cancer mechanism by periplocin treatment in lung cancer cells. Cell Physiol Biochem Int J Exp Cell Physiol Biochem Pharmacol. 2014;33(3):859–868. doi: 10.1159/000358658. [DOI] [PubMed] [Google Scholar]
- 144.Uddin S, Bhat AA, Krishnankutty R, Mir F, Kulinski M, Mohammad RM. Involvement of F-BOX proteins in progression and development of human malignancies. Semin Cancer Biol. 2016;36:18–32. doi: 10.1016/j.semcancer.2015.09.008. [DOI] [PubMed] [Google Scholar]
- 145.Sacco JJ, Coulson JM, Clague MJ, Urbe S. Emerging roles of deubiquitinases in cancer-associated pathways. IUBMB Life. 2010;62(2):140–157. doi: 10.1002/iub.300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Wei R, Liu X, Yu W, Yang T, Cai W, Liu J, et al. Deubiquitinases in cancer. Oncotarget. 2015;6(15):12872–12889. doi: 10.18632/oncotarget.3671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.D’Arcy P, Wang X, Linder S. Deubiquitinase inhibition as a cancer therapeutic strategy. Pharmacol Ther. 2015;147:32–54. doi: 10.1016/j.pharmthera.2014.11.002. [DOI] [PubMed] [Google Scholar]