Abstract
We found for the first time that IL-4 and IL-13, signature type 2 cytokines, are able to induce periostin expression. We and others have subsequently shown that periostin is highly expressed in chronic inflammatory diseases―asthma, atopic dermatitis, eosinophilc chronic sinusitis/chronic rhinosinusitis with nasal polyp, and allergic conjunctivitis—and that periostin plays important roles in the pathogenesis of these diseases. The epithelial/mesenchymal interaction via periostin is important for the onset of allergic inflammation, in which periostin derived from fibroblasts acts on epithelial cells or fibroblasts, activating their NF-κB. Moreover, the immune cell/non-immune cell interaction via periostin may be also involved. Now the significance of periostin has been expanded into other inflammatory or fibrotic diseases such as scleroderma and pulmonary fibrosis. The cross-talk of periostin with TGF-β or pro-inflammatory cytokines is important for the underlying mechanism of these diseases. Because of its pathogenic importance and broad expression, diagnostics or therapeutic drugs can be potentially developed to target periostin as a means of treating these diseases.
Keywords: Periostin, Matricellular protein, Allergy, Biomarker, Asthma, Atopic dermatitis, Allergic conjunctivitis, Scleroderma, Pulmonary fibrosis, Epithelial/mesenchymal interaction, Cross-talk, IL-4, IL-13, TGF-β
Introduction
Periostin is an extracellular matrix (ECM) protein belonging to the fasciclin family [1]. Periostin also acts as a matricellular protein by binding to cell-surface receptors belonging to the integrin family, followed by transducing the signals in cells. The roles of periostin as both an ECM and a matricellular protein contribute together to developing or maintaining various tissues or organs. For example, periostin is involved in the process of wound healing in skin as both an ECM and a matricellular protein; a genetic deficiency of periostin in mice delays the closure of wounds [2–4]. The dual functions of periostin as an ECM and a matricellular protein are important also for the onset of inflammation, particularly for allergic inflammation. Periostin is deposited in inflamed sites showing fibrosis, whereas it activates immune and non-immune cells as a matricellular protein, further augmenting inflammation. The epithelial/mesenchymal interaction and/or the immune cell/non-immune cell interaction is important for periostin to exert its effects in the setting of inflammation. Based on these findings, great attention has been paid to periostin as a biomarker or a target to develop therapeutic agents against inflammation. In this article, we focus on the roles of periostin in inflammation and allergy, as well as on the utility of periostin as a biomarker and a target to develop therapeutic agents for inflammation and allergy.
Expression of periostin in inflammation and allergy
Induction of periostin by IL-4 or IL-13
It is known that various stimuli―IL-4/IL-13, TGF-β, angiotensin II, connective tissue growth factor 2, bone morphogenetic protein 2, mechanical stretch, and cancer-derived factors―induce periostin expression [1]. We found for the first time that IL-4 and IL-13, signature type 2 cytokines, can induce periostin expression [5]. IL-4 binds to two types of IL-4 receptors (IL-4Rs), composed of the IL-4R α chain and either the common γ chain (type 1 IL-4R) or the IL-13R α1 chain (type 2 IL-4R), whereas IL-13 binds to type 2 IL-4R, also called IL-13R [6]. Engagement of type 1 IL-4R or type 2 IL-4R/IL-13R with the ligands activates the JAK-STAT pathway; the former activates JAK1 and JAK3 followed by activation of STAT6, whereas the latter activates JAK1 and TYK2 followed by activation of STAT6 and STAT3. We have already found that inhibition or downregulation of STAT6 decreased periostin expression, suggesting that STAT6 activation is critical, as are other IL-13-induced molecules [7, 8] (Mitamura, unpublished data). However, it remains undetermined whether STAT6 induces periostin expression directly by a cis-regulatory mechanism or indirectly by a trans-regulatory mechanism via transcription factors.
Expression of periostin in inflamed sites
It is now widely accepted that type 2 immunity is dominant in allergic inflammation [9]. Based on this knowledge, several agents targeting IL-4 and/or IL-13 for allergic diseases are now under development [10, 11]. IL-4 and IL-13, as well as IL-5, are produced by TH2 cells, follicular helper T cells, type 2 innate lymphoid cells, eosinophils, mast cells, and basophils [7, 12–14]. Since periostin is downstream of IL-4 and IL-13, we reasoned that periostin would be highly expressed in the inflamed sites of allergic diseases. It turned out that periostin is highly expressed in the subepithelial regions of many chronic inflammatory diseases―asthma [15], atopic dermatitis (AD) [16], eosinophilc chronic sinusitis/chronic rhinosinusitis with nasal polyp (CRSwNP) [17, 18], and allergic conjunctivitis [19] (Fig. 1). Additionally, Blanchard et al. have reported that periostin was highly expressed in the esophagus of patients with eosinophilic esophagitis [20]. Thus, high periostin expression downstream of the IL-4/IL-13 signals is an event common in allergic diseases.
Fig. 1.
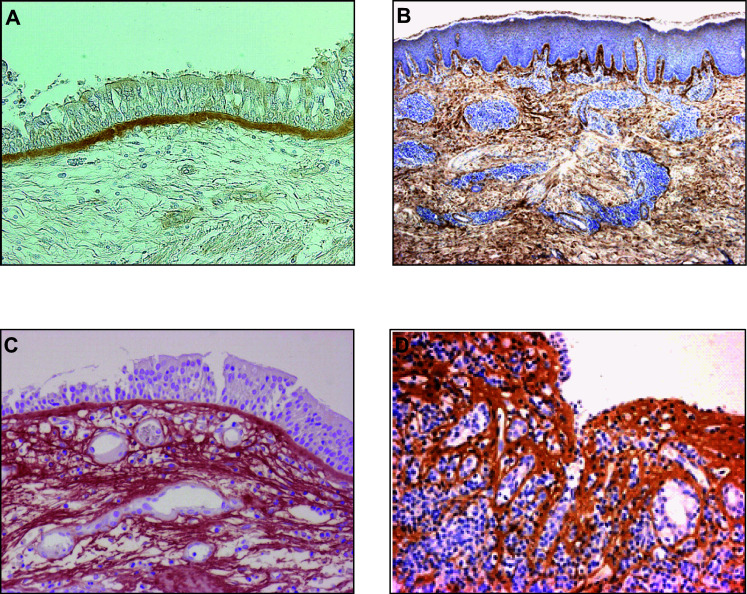
High expression of periostin in inflamed sites of allergic diseases. Expression of periostin in bronchial tissue from an asthma patient (a) [15], in skin tissue from an AD patient (b) [16], in a nasal polyp from an eosinophilc chronic sinusitis/CRSwNP patient (c) [17], and in conjunctival tissue from an AKC patient (d) [19]. It is of note that periostin is highly expressed in the subepithelial regions of each tissue
Periostin-producing cells
To our knowledge thus far, three kinds of tissue-resident cells―fibroblasts, epithelial cells, and endothelial cells―are known to produce periostin protein by stimulating IL-4 or IL-13. We first identified periostin as a downstream molecule of IL-13 by applying airway epithelial cells to DNA microarray [5]. However, we did not detect production of periostin protein in either airway epithelial cells or keratinocytes cultured in liquid medium [15, 16]. In contrast, pulmonary or dermal fibroblasts secrete robust amounts of periostin by stimulation of IL-4 or IL-13. Sidhu et al. have shown that when they culture airway epithelial cells in the presence of IL-13 using an air/liquid interface method in which one side of the airway epithelial cells faces air, whereas the other side faces liquid medium, periostin was secreted in the basal direction, but not into the apical side [21]. This result suggests that secretion of periostin from airway epithelial cells by stimulation of IL-13 would have a polarity. Moreover, Shoda et al. have reported that microvascular endothelial cells derived from lung and skin can secrete periostin protein by stimulation of IL-4 or IL-13 [22]. It is of interest that IL-4- or IL-13-induced periostin production in endothelial cells was resistant to corticosteroids, whereas that of fibroblasts was sensitive to it.
The roles of periostin in the pathogenesis of allergic diseases
Analyses of periostin-deficient model mice have provided us direct evidence concerning the roles of periostin in the pathogenesis of asthma and AD. Moreover, comparisons of periostin expression in the inflamed sites of asthma and AD with their clinical severities have given us important and useful, although indirect, information about the roles of periostin in the pathogenesis of asthma and AD.
Asthma
The results of applying periostin-deficient mice to the mouse model of asthma are controversial. Initial studies for this purpose showed that ovalbumin- or Aspergillus-challenge in periostin-deficient mice enhanced airway hyperresponsiveness (AHR), type 2 inflammation, and mucus production, suggesting that periostin plays a protective role against airway allergic inflammation in the model mice [23, 24]. Gordon et al. speculated that this might be because TGF-β-induced differentiation of regulatory T cells was impaired in periostin-deficient mice. In contrast, it has been more recently shown that periostin deficiency decreased AHR, type 2 inflammation, and mucus production in house dust mite (HDM)-challenged mice [25]. Moreover, administration of neutralizing anti-periostin antibodies inhibited asthma-like phenotypes. These results suggest that periostin helps to accelerate airway allergic inflammation. Adoptive transfer of HDM-treated bone-marrow-derived dendritic cells (DCs) from wild-type mice into periostin-deficient mice restored HDM-induced asthma-like phenotypes, suggesting the importance of periostin in DCs in this context. Thus far, the reason for this discrepancy has been elusive. On the other hand, Kanemitsu et al. analyzed the correlation between periostin expression and the change of pulmonary function in asthma patients followed for long terms [26]. They found that deposition of periostin in bronchial subepithelium in the biopsy samples that they took more than 20 years ago was well inversely correlated with decline of ∆FEV1, which strongly, although indirectly, supports the role of periostin in accelerating airway allergic inflammation.
AD
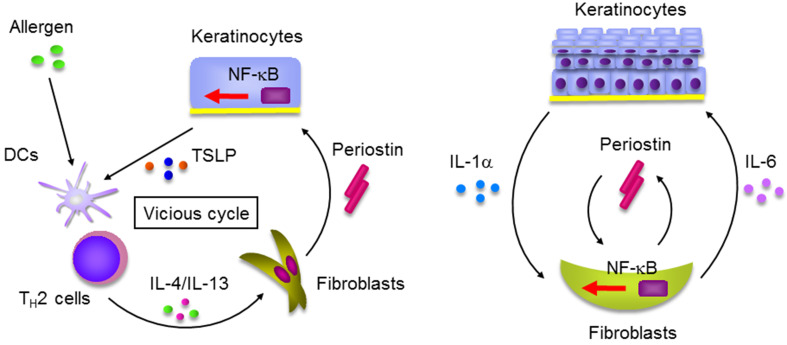
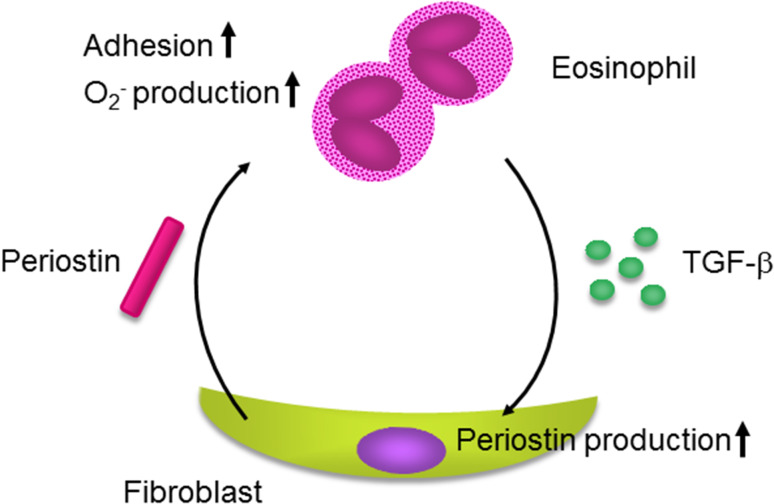
We have previously shown that periostin plays a critical role in skin allergic inflammation using HDM-painted mice [16, 27]; the features reminiscent of AD patients―skin swelling, epidermal hyperplasia, type 2 inflammation, and dermal fibrosis―were diminished in HDM-painted, periostin-deficient mice. Consistent with this, we and Kou et al. have shown that periostin was expressed in inflamed sites or serum of AD patients according to its clinical severity [16, 28], which strongly suggests that periostin is an accelerating mediator for AD. Using the 3-dimensional organotypic co-culture system in which keratinocytes are seeded on the fibroblast-embedded collagen gel, we found that the epithelial/mesenchymal interaction is important in the underlying mechanism; periostin derived from fibroblasts acts on keratinocytes via αV integrin, inducing production of pro-inflammatory cytokines including thymic stromal lymphopoietin (TSLP) from keratinocytes (Fig. 2a). Periostin activates NF-κB, a transcriptional factor critical for inflammation, in keratinocytes. We furthermore found the importance of another epithelial/mesenchymal interaction in which periostin derived from fibroblasts targets fibroblasts themselves [29] (Fig. 2b). In this interaction, IL-1α derived from keratinocytes cooperates with periostin derived from fibroblasts, acting on fibroblasts themselves activating NF-κB followed by induction of pro-inflammatory cytokines such as IL-6. Moreover, it has been reported that periostin augments adhesion, superoxide anion (O2 −) generation, and TGF-β production in eosinophils [20, 30] (Fig. 3). Activated eosinophils may reciprocally induce periostin protein in fibroblasts by TGF-β. Thus, in addition to the epithelial/mesenchymal interaction, the immune cell/non-immune cell interaction via periostin would be involved in the onset of allergic diseases, including AD.
Fig. 2.
Epithelial/mesenchymal interaction via periostin in the pathogenesis of skin allergic diseases (modified from [16, 29]). a IL-4/IL-13 produced by TH2 cells activated by exposure to allergens induces periostin production in fibroblasts. Periostin acts on keratinocytes activating NF-κB followed by production of pro-inflammatory cytokines including TSLP, which acts on dendritic cells (DCs), accelerating type 2 inflammation. Thus, IL-4/IL-13, periostin, and TSLP generate a vicious cycle in the pathogenesis of skin allergic diseases. b IL-1α and periostin produced by keratinocytes and fibroblasts, respectively, cooperate to act on fibroblasts activating NF-κB. Activated fibroblasts produce IL-6 accelerating proliferation of keratinocytes
Fig. 3.
Immune cell/non-immune cell interaction via periostin in the pathogenesis of allergic diseases. Periostin augments adhesion, superoxide anion (O2 −) generation, and TGF-β production in eosinophils. Reciprocally, activated eosinophils may induce periostin protein in fibroblasts
Usefulness of periostin as a biomarker for allergic diseases
Periostin is now receiving much attention as a biomarker for inflammatory diseases. The advantages of using periostin as a biomarker are listed as follows [31] (Fig. 4). (1) Periostin is characterized as a molecule easily moving or secreted from inflamed sites into various body fluids such as blood [26, 32, 33], urine [34–36], sputum [37, 38], and tears [19], although the precise mechanism of the moving or secretion still remains elusive. (2) The serum concentration (~tens ng/mL) is appropriate for reflecting the increase in the inflamed sites, and for detecting accurate concentrations. Since the serum concentration is not high, with other ECM proteins such as fibronection or vitronectin (~hundreds μg/mL), the increase in the inflamed area can be more easily reflected to it, whereas since the serum concentration is not low compared with cytokines (~tens pg/mL), it can be detected accurately. It must be noted that the disadvantage of using periostin as a biomarker is that basal levels of serum periostin in childhood are high [39]; this is probably because serum periostin in children is mostly derived from bone, and its levels are sustained high until bone growth stops. Therefore, the window width of the increase in teens by various diseases is relatively narrow (Fig. 4).
Fig. 4.
Advantages and disadvantages of periostin as a biomarker. Several advantages and one disadvantage of periostin as a biomarker are depicted
Many commercial and non-commercial periostin detection kits are now available; however, the correlations and absolute values differ among the kits. We have recently compared our kit (the Shino-Test assay) and the Elecsys® periostin assay provided by Genentech Inc. [40], showing that these two assays have a positive correlation (r = 0.9236, P < 0.0001) significantly, and that the absolute values are a little higher in the Shino-test assay than in the Elecsys® periostin assay. Standardization of each periostin detection kit is needed to develop these kits for diagnostics.
Asthma
We and others have made a lot of effort to characterize serum periostin as a biomarker for asthma during the past several years, because no good serum biomarker is available for asthma, and several anti-asthma drugs targeting IL-13 have been developed. Some surrogate biomarker reflecting IL-13 production in the body is needed to predict the efficacy of such drugs, and serum periostin is considered to be a candidate. We have already presented several review articles about this topic [10, 31].
A lot of evidence has supported the idea that serum periostin reflects type 2 inflammation in asthma. Serum periostin is reproducibly correlated with eosinophilia, fractional inhaled NO (FeNO), and IgE in some studies, all of which are type 2 biomarkers [32, 33, 38, 40–44]. Moreover, serum periostin is correlated with aspirin intolerance, nasal disorders, and late onset, in which eosinophilic inflammation is often detected [32, 33, 41], supporting the characterization of periostin as a type 2 biomarker as well. Furthermore, cluster analysis has shown that high periostin is a characteristic of the eosinophil-dominant group of asthma [43].
Although periostin belongs to type 2 biomarkers along with eosinophils, FeNO, and IgE, its differentiating characteristic is that it is involved in generating a thickened basement membrane, in other words tissue remodeling, which serum periostin reflects in asthma. The finding that of serum periostin was correlated with thickening of bronchial walls also supports this notion [45]. The characterization of serum periostin as a biomarker for remodeling of asthma would lead to its correlation with hyporesponsiveness to inhaled corticosteroids (ICSs); several clinical studies have reported that serum periostin is high in ICS-resistant asthma patients, particularly in eosinophil-dominant groups [32, 40, 41, 43] and that periostin-high asthma patients showed a high frequency of exacerbation after tapering ICSs [46]. Introducing ICSs to asthma patients promptly decreased FeNO levels, whereas it sustained high serum periostin levels [47]. This suggests that ICSs improve superficial inflammation, but not the inflammation of deep layers. Treatment with ICSs decreases FeNO secreted from epithelial cells, whereas it does not change periostin deposited in subepithelial regions. It is important to clarify the different characteristics of type 2 biomarkers and to combine them appropriately in treating asthma.
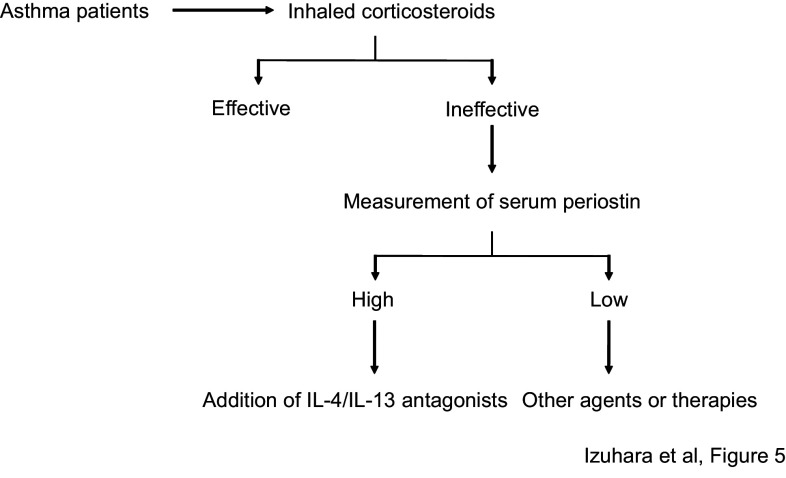
We can take advantage of the characteristics of serum periostin as a biomarker for type 2 inflammation and remodeling in asthma to predict the efficacy of anti-asthma drugs targeting type 2 inflammation-correlating molecules. Particularly, since serum periostin is a surrogate biomarker for IL-13, it is reasonable to think that serum periostin can be applied to predicting the efficacy of anti-IL-13 drugs (Fig. 5). These molecularly targeted drugs are given only to ICS-resistant patients showing high remodeling, thus it is reasonable to use serum periostin in the selection of patients for whom these drugs should be prescribed. It was first demonstrated that serum periostin is useful to predict the efficacy of lebrikizumab, an anti-IL-13 Ab developed by Roche/Genentech in its phase II trial [47, 48]. Although the development of lebrikizumab ended after its phase III trial, the utility of serum periostin to predict its efficacy was still confirmed in the trial [49]. A clinical trial of phase IIb for tralokinumab, another anti-IL-13 Ab developed by AstraZeneca/Medimmune, was performed using serum periostin and dipeptidyl peptidase 4 (DPP4), another type 2 biomarker. The finding was that both these biomarkers show a good positive correlation with responsiveness to this drug [50]. We have analyzed the usefulness of serum periostin as estimated by our kit (Shino-Test kit) to predict the efficacy of dupilumab, an anti-IL-4Rα Ab developed by Sanofi/Regeneron, finding a positive correlation with improvement of ∆FEV1 (Wentzel, unpublished data). Moreover, it has been shown that serum periostin is well associated with efficacy of the anti-IgE Ab omalizumab, which is now used for asthma patients [51, 52]. These results suggest a potential for using serum periostin to estimate the efficacy of not only anti-IL-13 drugs, but also agents targeting other type 2 inflammation-correlating molecules.
Fig. 5.
Algorithm for the treatment of asthma (modified from [10]). A first-line of anti-asthma drugs is inhaled corticosteroids. If they are ineffective, measurement of serum periostin is recommended. If the level is high, anti-IL-4/IL-13 antagonists should be added
AD
After we found in a pilot study that serum periostin was up-regulated in AD patients [16], Kou et al. performed a large-scale study, enrolling 257 AD patients, to ascertain the correlation between serum periostin and clinical parameters of AD [28]. Overall, serum periostin was significantly higher in the AD patients [median 144 ng/mL interquartile range (96–224 ng/mL)] than in either those with psoriasis vulgaris [69 ng/mL (60–93 ng/mL)] or in healthy donors [56 ng/mL (49–63 ng/mL)]. Serum periostin was high depending on disease severity and clinical types of AD, particularly in the erythroderma type. Serum periostin was associated with other type 2 biomarkers―thymus and activation-regulated chemokine (TARC), LDH, and eosinophils―but not with IgE, and serum periostin declined with improvement of disease activity by additional treatments. Thus, measuring serum periostin would be useful for stratification of AD patients. However, the difference between periostin and other type 2 biomarkers such as TARC and eosinophils remains unknown. We also do not yet know whether serum periostin is useful for predicting the efficacy of molecularly targeted drugs for AD such as dupilumab.
Allergic conjunctivitis
We have reported the usefulness of measuring tear periostin in ocular allergic diseases―atopic keratoconjunctivitis (AKC), vernal keratoconjunctivitis (VKC), and seasonal allergic conjunctivitis (SAC) [19]. Tear periostin was significantly higher in patients with AKC (median 444.0 ng/mL), VKC (67.0 ng/mL), and SAC (12.2 ng/mL) in descending order than in healthy donors (0.2 ng/mL). Tear periostin levels were positively correlated with complications of AKC such as papilla formation or corneal damage. Tear periostin could clearly distinguish AKC patients from AD patients without conjunctivitis with the sensitivity of 96.8% and the specificity of 93.8%. Moreover, topical treatment with immune suppressants decreased tear periostin in some AKC patients along with improvement of clinical features. These results suggest that measuring tear periostin is useful in diagnosing allergic conjunctivitis, evaluating disease activity, and establishing the efficacy of immune suppressants in AKC.
CRSwNP
Comprehensive analyses have shown that periostin is one of the highest expression genes in chronic sinusitis [18, 53, 54]. Consistent with these results, we and others found that expression of periostin was high in the subepithelial regions of CRSwNP patients [17, 18, 55]. However, the usefulness of serum periostin as a biomarker for CRSwNP still remains elusive. We recently found high periostin levels in CRSwNP patients using our kit (the Shino-Test assay, Ninomiya et al., unpublished data), although Wang et al. have reported that they could not detect upregulation of serum periostin in CRSwNP patients [55], which would probably be due to differences in the assay kits.
Involvement of periostin in other inflammatory diseases
Since fibrosis is a cardinal histological feature of inflammation, it is no wonder that periostin is involved in the pathogenesis of various inflammatory diseases other than allergic diseases. Here we mention two inflammatory or fibrotic diseases―scleroderma and pulmonary fibrosis―in which periostin is known to play important roles.
Scleroderma
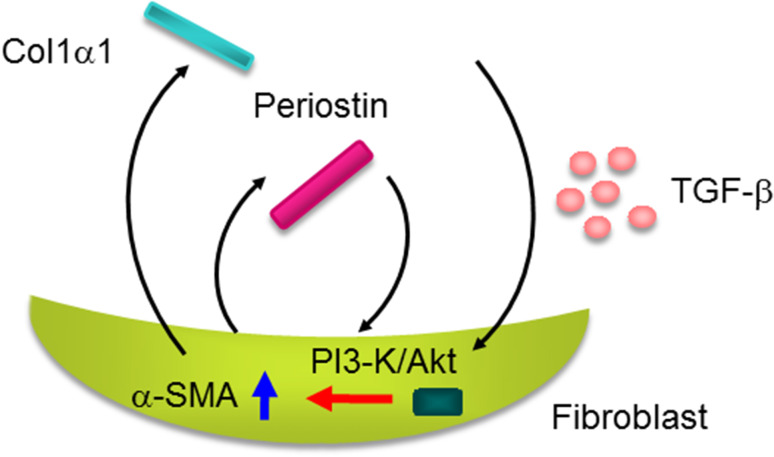
Yang et al. found that periostin expression was significantly up-regulated in the dermis of scleroderma patients [56]. They applied periostin-deficient mice to the model of scleroderma by administering bleomycin, finding that bleomycin-administered periostin-deficient mice impaired skin fibrosis with a decrease of α-SMA‒positive myofibroblasts. They have also demonstrated, using in vitro experiments, that periostin cooperated with TGF-β, inducing expression of α-SMA or collagen 1α1, and that activation of the phosphatidylinositol-3 kinase/Akt pathway downstream of αV integrin is important for the periostin signal (Fig. 6). Yamaguchi et al. confirmed high expression of periostin in the skin of scleroderma patients and found that periostin was co-localized with α-SMA‒positive myofibroblasts [57]. Taken together, these results suggest that periostin and TGF-β may cooperatively accelerate skin fibrosis; myofibroblasts induced by TGF-β produce more periostin than quiescent fibroblasts, whereas periostin up-regulates differentiation of quiescent fibroblasts into myofibroblasts cooperating with TGF-β.
Fig. 6.
Cross-talk of periostin and TGF-β in the pathogenesis of scleroderma. Periostin cooperates with TGF-β activating the phosphatidylinositol-3 kinase (PI3-K)/Akt pathway in fibroblasts followed by induction of collagen 1α1 and α-SMA, which would accelerate scleroderma
Yamaguchi et al, moreover, analyzed serum periostin in scleroderma patients, finding that serum periostin was significantly high in these patients, particularly in systemic scleroderma compared to limited scleroderma [57]. It is of note that systemic scleroderma patients with disease duration of less than 5 years showed higher periostin than those whose disease had lasted longer than 5 years. It is known that systemic scleroderma is not a progressive disease, but relatively high disease activity such as severe organ damage is recognized within the first 3 years of disease onset [58]. These results suggest that serum periostin can become a biomarker not only to diagnose scleroderma, but also to estimate the activity of the disease.
Pulmonary fibrosis
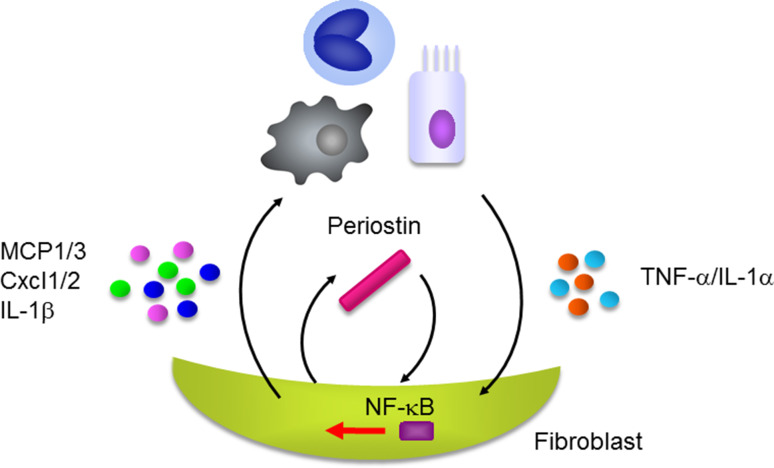
We first found, in a small-scale study, that periostin was highly expressed in the lungs of IPF patients and that serum periostin was up-regulated in these patients [59]. We then examined the underlying mechanism of how periostin contributes to pulmonary fibrosis by investigating bleomycin-administered mice [60]. High expression of periostin was observed also in the mice, particularly in the fibrotic foci adjacent to α-SMA-positive myofibroblasts, such as scleroderma; periostin deficiency protected against bleomycin-induced pulmonary fibrosis. Naik et al. confirmed high expression of periostin in lungs and serum in IPF patients and that fibroblasts derived from IPF patients produced higher levels of periostin [61]. They also showed that either genetic deficiency or administration of neutralizing antibodies against periostin protected against bleomycin-administered pulmonary fibrosis. We found that TNFα-induced chemokines/pro-inflammatory cytokines—Ccl2/MCP1, Ccl4/MIP1β, Ccl7/MCP3, Cxcl1/KC, Cxcl2/MIP1α, and IL-1β—and recruitment of neutrophils and macrophages in the bronchoalveolar liquid fluids were impaired in periostin-deficient fibroblasts and in mice. These results suggest that cooperative production of periostin with TNFα causes production of various pro-inflammatory cytokines/chemokines, followed by recruitment of neutrophils and macrophages, and leading to the formation of pulmonary fibrosis (Fig. 7). Ashley et al., moreover, have demonstrated that periostin accelerates the actions of fibrocytes to promote myofibroblast differentiation leading to pulmonary fibrosis [62].
Fig. 7.
Cross-talk of periostin and TNFα/IL-1α in the pathogenesis of pulmonary fibrosis. Periostin cooperates with TNFα or IL-1α derived from epithelial cells or inflammatory cells activating NF-κB in fibroblasts followed by induction of various chemokines/pro-inflammatory cytokines such as Ccl2/MCP1, Ccl4/MCP1, Ccl7/MCP3, Cxcl1/KC, Cxcl2/MIP-1α, and IL-1β. These chemokines/pro-inflammatory cytokines recruit neutrophils and macrophages, accelerating pulmonary fibrosis
Following the preceding analyses of serum periostin in IPF patients [59, 61], we performed a multi-center analysis to examine the usefulness of measuring serum periostin in IPF patients [63]. Serum periostin mostly exists in the oligomeric form, with only small amounts in the monomeric form. The oligomeric form of periostin is assembled by intramolecular disulfide bonds. We found that monomeric periostin was relatively up-regulated compared to oligomeric periostin in IPF patients, which might be explained by the aberrant redox status in IPF. Monomeric periostin showed an ability to diagnose IPF better than total (oligomeric + monomeric) periostin. This capability was comparable to the conventional biomarkers for IPF such as KL-6 and SP-D. Both monomeric and total periostin were well correlated with decline of %VC and %D L,CO suggesting that serum periostin is useful for predicting IPF progression. Moreover, Tajiri et al. have shown that measuring periostin was useful for predicting not only short-term progression of IPF, i.e., the decline of lung function, but also long-term progression in terms of the survival time [64]. On the other hand, Nance et al. have reported that spliced-out exon 21 highly occured in IPF patients [65]. Thus, we may apply serum periostin to treatment for IPF patients based on their serum periostin levels.
Potential of periostin as a therapeutic target
Given that deficiency or inhibition of periostin protects against various inflammatory or fibrotic diseases such as AD, pulmonary fibrosis, and skin fibrosis, drugs can potentially be developed to target periostin as a means of treating these diseases. Particularly, since there are only a few drugs available for IPF or scleroderma, it is strongly hoped that some agent targeting periostin against these diseases will be developed. There are two strategies to block the functions of periostin in vivo: targeting periostin itself and targeting its receptor, integrin. Since Moore’s group has succeeded in improving asthma- or pulmonary fibrosis-like phenotypes by administering neutralizing anti-periostin antibodies (OC-20) [24, 61], development of biologics targeting periostin would be a promising option. We have shown that administration of neutralizing antibodies against αV integrin improved allergic skin inflammation [16]. Moreover, it has been previously shown that neutralizing antibodies against αV integrin improved pulmonary fibrosis in bleomycin-administered mice as well [66]. These results suggest that targeting αV integrin would be another option for developing therapeutic agents for inflammatory or fibrosis diseases. Neutralizing antibodies against αVβ3 which is thought to be the main periostin receptor (MEDI-522), have already been developed as therapeutic agents against malignancies. The safety of MEDI-522 has been confirmed [67], although there is a problem in redundancy in β subunits used by αV for the periostin receptor [68].
Conclusion
In this article, we describe the roles of periostin in inflammation and allergy and the utility and potential of periostin in developing diagnostics and therapeutic agents against inflammatory or fibrotic diseases. This field has developed dramatically in these past several years. It is hoped that in the near future, these studies will give us a new concept of the pathogenesis of inflammation and allergy, and yield useful diagnostics or therapeutic agents against these diseases.
Acknowledgements
We thank Dr. Dovie R. Wylie for the critical review of this manuscript. We also thank the colleagues and collaborators as follows for contributing to the present work: Go Takayama, Masaru Uchida, Miho Masuoka, Hiroshi Shiraishi, Minako Ontsuka, Kazuto Taniguchi, Kazuhiko Arima, Shoichi Suzuki, Shoichiro Ohta, Go Kato, Koichiro Takahashi, Shin-ichiro Hayashi (Saga Medical School), Noriko Yuyama (Genox Research, Inc.), Akihiro Ishida, Nobuo Ohta (Yamagata University), Takahiro Ninomiya, Shigeharu Fujieda (Fukui University), Hiroshi Fujishima (Tsurumi University), Naoko Okada, Kenji Matsumoto (National Research Institute for Child Health and Development Laboratory), Yuzaburo Inoue, Naoki Shimojo (Chiba University), Yoshihiro Kanemitsu, Tadao Nagasaki, Tomoko Tajiri, Hisako Matsumoto (Kyoto University), Masako Matsuzaka, Koichi Fukunaga (Keio University), Koichiro Asano (Tokai University), Yorihisa Kotobuki, Ichiro Katayama (Osaka University), Kenzen Kou, Yukie Yamaguchi, Michiko Aihara (Yokohama City University), Timothy Hinks, Peter Howarth (Southampton University Hospital), Mi-Ae Kim, Hae-Sim Park (Ajou University), Anna James, Sven-Erik Dahlen (Karolinska Institutet), Ayami Kamei, Masayuki Takai, Yoshinori Azuma (Shino-Test Co.), Simon J. Conway (Indiana University), Masaki Okamoto, Tomoaki Hoshino, Kiminori Fujimoto (Kurume University), Naoko Kushima, Hiroshi Ishii, Junichi Kadota (Oita University), Hiroshi Yamamoto, Masayuki Hanaoka (Shinshu University), Noriho Sakamoto, Shigeru Kohno (Nagasaki University), Keiichi Akasaka, Hironori Sagara (Dokkyo Medical University Koshigaya Hospital), and Takeshi Johkoh (Kinki Central Hospital of Mutual Aid Association of Public Teachers).
References
- 1.Conway SJ, Izuhara K, Kudo Y, Litvin J, Markwald R, Ouyang G, Arron JR, Holweg CT, Kudo A. The role of periostin in tissue remodeling across health and disease. Cell Mol Life Sci. 2014;71:1279–1288. doi: 10.1007/s00018-013-1494-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ontsuka K, Kotobuki Y, Shiraishi H, Serada S, Ohta S, Tanemura A, Yang L, Fujimoto M, Arima K, Suzuki S, Murota H, Toda S, Kudo A, Conway SJ, Narisawa Y, Katayama I, Izuhara K, Naka T. Periostin, a matricellular protein, accelerates cutaneous wound repair by activating dermal fibroblasts. Exp Dermatol. 2012;21:331–336. doi: 10.1111/j.1600-0625.2012.01454.x. [DOI] [PubMed] [Google Scholar]
- 3.Nishiyama T, Kii I, Kashima TG, Kikuchi Y, Ohazama A, Shimazaki M, Fukayama M, Kudo A. Delayed re-epithelialization in periostin-deficient mice during cutaneous wound healing. PLoS One. 2011;6:e18410. doi: 10.1371/journal.pone.0018410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Elliott CG, Wang J, Guo X, Xu SW, Eastwood M, Guan J, Leask A, Conway SJ, Hamilton DW. Periostin modulates myofibroblast differentiation during full-thickness cutaneous wound repair. J Cell Sci. 2012;125:121–132. doi: 10.1242/jcs.087841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yuyama N, Davies DE, Akaiwa M, Matsui K, Hamasaki Y, Suminami Y, Yoshida NL, Maeda M, Pandit A, Lordan JL, Kamogawa Y, Arima K, Nagumo F, Sugimachi M, Berger A, Richards I, Roberds SL, Yamashita T, Kishi F, Kato H, Arai K, Ohshima K, Tadano J, Hamasaki N, Miyatake S, Sugita Y, Holgate ST, Izuhara K. Analysis of novel disease-related genes in bronchial asthma. Cytokine. 2002;19:287–296. doi: 10.1006/cyto.2002.1972. [DOI] [PubMed] [Google Scholar]
- 6.Izuhara K, Arima K, Yasunaga S. IL-4 and IL-13: their pathological roles in allergic diseases and their potential in developing new therapies. Curr Drug Targets Inflamm Allergy. 2002;1:263–269. doi: 10.2174/1568010023344661. [DOI] [PubMed] [Google Scholar]
- 7.Izuhara K, Arima K, Kanaji S, Ohta S, Kanaji T. IL-13: a promising therapeutic target for bronchial asthma. Curr Med Chem. 2006;13:2291–2298. doi: 10.2174/092986706777935140. [DOI] [PubMed] [Google Scholar]
- 8.Goenka S, Kaplan MH. Transcriptional regulation by STAT6. Immunol Res. 2011;50:87–96. doi: 10.1007/s12026-011-8205-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pulendran B, Artis D. New paradigms in type 2 immunity. Science. 2012;337:431–435. doi: 10.1126/science.1221064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Izuhara K, Ohta S, Ono J. Using periostin as a biomarker in the treatment of asthma. Allergy Asthma Immunol Res. 2016;8:491–498. doi: 10.4168/aair.2016.8.6.491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Furue M, Chiba T, Tsuji G, Ulzii D, Kido-Nakahara M, Nakahara T, Kadono T. Atopic dermatitis: immune deviation, barrier dysfunction, IgE autoreactivity and new therapies. Allergol Int. 2017;66:398–403. doi: 10.1016/j.alit.2016.12.002. [DOI] [PubMed] [Google Scholar]
- 12.Kubo M. T follicular helper and TH2 cells in allergic responses. Allergol Int. 2017;66:377–381. doi: 10.1016/j.alit.2017.04.006. [DOI] [PubMed] [Google Scholar]
- 13.Kabata H, Moro K, Koyasu S, Asano K. Group 2 innate lymphoid cells and asthma. Allergol Int. 2015;64:227–234. doi: 10.1016/j.alit.2015.03.004. [DOI] [PubMed] [Google Scholar]
- 14.Miyake K, Karasuyama H. Emerging roles of basophils in allergic inflammation. Allergol Int. 2017;66:382–391. doi: 10.1016/j.alit.2017.04.007. [DOI] [PubMed] [Google Scholar]
- 15.Takayama G, Arima K, Kanaji T, Toda S, Tanaka H, Shoji S, McKenzie AN, Nagai H, Hotokebuchi T, Izuhara K. Periostin: a novel component of subepithelial fibrosis of bronchial asthma downstream of IL-4 and IL-13 signals. J Allergy Clin Immunol. 2006;118:98–104. doi: 10.1016/j.jaci.2006.02.046. [DOI] [PubMed] [Google Scholar]
- 16.Masuoka M, Shiraishi H, Ohta S, Suzuki S, Arima K, Aoki S, Toda S, Inagaki N, Kurihara Y, Hayashida S, Takeuchi S, Koike K, Ono J, Noshiro H, Furue M, Conway SJ, Narisawa Y, Izuhara K. Periostin promotes chronic allergic inflammation in response to Th2 cytokines. J Clin Invest. 2012;122:2590–2600. doi: 10.1172/JCI58978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ishida A, Ohta N, Suzuki Y, Kakehata S, Okubo K, Ikeda H, Shiraishi H, Izuhara K. Expression of pendrin and periostin in allergic rhinitis and chronic rhinosinusitis. Allergol Int. 2012;61:589–595. doi: 10.2332/allergolint.11-OA-0370. [DOI] [PubMed] [Google Scholar]
- 18.Stankovic KM, Goldsztein H, Reh DD, Platt MP, Metson R. Gene expression profiling of nasal polyps associated with chronic sinusitis and aspirin-sensitive asthma. Laryngoscope. 2008;118:881–889. doi: 10.1097/MLG.0b013e31816b4b6f. [DOI] [PubMed] [Google Scholar]
- 19.Fujishima H, Okada N, Matsumoto K, Fukagawa K, Igarashi A, Matsuda A, Ono J, Ohta S, Mukai H, Yoshikawa M, Izuhara K. The usefulness of measuring tear periostin for the diagnosis and management of ocular allergic diseases. J Allergy Clin Immunol. 2016;138(459–467):e2. doi: 10.1016/j.jaci.2015.11.039. [DOI] [PubMed] [Google Scholar]
- 20.Blanchard C, Mingler MK, McBride M, Putnam PE, Collins MH, Chang G, Stringer K, Abonia JP, Molkentin JD, Rothenberg ME. Periostin facilitates eosinophil tissue infiltration in allergic lung and esophageal responses. Mucosal Immunol. 2008;1:289–296. doi: 10.1038/mi.2008.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sidhu SS, Yuan S, Innes AL, Kerr S, Woodruff PG, Hou L, Muller SJ, Fahy JV. Roles of epithelial cell-derived periostin in TGF-β activation, collagen production, and collagen gel elasticity in asthma. Proc Natl Acad Sci USA. 2010;107:14170–14175. doi: 10.1073/pnas.1009426107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shoda T, Futamura K, Kobayashi F, Saito H, Matsumoto K, Matsuda A. Cell type-dependent effects of corticosteroid on periostin production by primary human tissue cells. Allergy. 2013;68:1467–1470. doi: 10.1111/all.12240. [DOI] [PubMed] [Google Scholar]
- 23.Sehra S, Yao W, Nguyen ET, Ahyi AN, Tuana FM, Ahlfeld SK, Snider P, Tepper RS, Petrache I, Conway SJ, Kaplan MH. Periostin regulates goblet cell metaplasia in a model of allergic airway inflammation. J Immunol. 2011;186:4959–4966. doi: 10.4049/jimmunol.1002359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gordon ED, Sidhu SS, Wang ZE, Woodruff PG, Yuan S, Solon MC, Conway SJ, Huang X, Locksley RM, Fahy JV. A protective role for periostin and TGF-β in IgE-mediated allergy and airway hyperresponsiveness. Clin Exp Allergy. 2012;42:144–155. doi: 10.1111/j.1365-2222.2011.03840.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bentley JK, Chen Q, Hong JY, Popova AP, Lei J, Moore BB, Hershenson MB. Periostin is required for maximal airways inflammation and hyperresponsiveness in mice. J Allergy Clin Immunol. 2014;134:1433–1442. doi: 10.1016/j.jaci.2014.05.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kanemitsu Y, Ito I, Niimi A, Izuhara K, Ohta S, Ono J, Iwata T, Matsumoto H, Mishima M. Osteopontin and periostin are associated with a 20-year decline of pulmonary function in patients with asthma. Am J Respir Crit Care Med. 2014;190:472–474. doi: 10.1164/rccm.201403-0562LE. [DOI] [PubMed] [Google Scholar]
- 27.Shiraishi H, Masuoka M, Ohta S, Suzuki S, Arima K, Taniguchi K, Aoki S, Toda S, Yoshimoto T, Inagaki N, Conway SJ, Narisawa Y, Izuhara K. Periostin contributes to the pathogenesis of atopic dermatitis by inducing TSLP production from keratinocytes. Allergol Int. 2012;61:563–572. doi: 10.2332/allergolint.10-OA-0297. [DOI] [PubMed] [Google Scholar]
- 28.Kou K, Okawa T, Yamaguchi Y, Ono J, Inoue Y, Kohno M, Matsukura S, Kambara T, Ohta S, Izuhara K, Aihara M. Periostin levels correlate disease severity and chronicity in patients with atopic dermatitis. Br J Dermatol. 2014;171:283–291. doi: 10.1111/bjd.12943. [DOI] [PubMed] [Google Scholar]
- 29.Taniguchi K, Arima K, Masuoka M, Ohta S, Shiraishi H, Ontsuka K, Suzuki S, Inamitsu M, Yamamoto K, Simmons O, Toda S, Conway SJ, Hamasaki Y, Izuhara K. Periostin controls keratinocyte proliferation and differentiation by interacting with the paracrine IL-1α/IL-6 loop. J Invest Dermatol. 2014;134:1295–1304. doi: 10.1038/jid.2013.500. [DOI] [PubMed] [Google Scholar]
- 30.Noguchi T, Nakagome K, Kobayashi T, Uchida Y, Soma T, Nakamoto H, Nagata M. Periostin upregulates the effector functions of eosinophils. J Allergy Clin Immunol. 2016;138(1449–1452):e5. doi: 10.1016/j.jaci.2016.05.020. [DOI] [PubMed] [Google Scholar]
- 31.Izuhara K, Matsumoto H, Ohta S, Ono J, Arima K, Ogawa M. Recent developments regarding periostin in bronchial asthma. Allergol Int. 2015;64(Suppl):S3–S10. doi: 10.1016/j.alit.2015.04.012. [DOI] [PubMed] [Google Scholar]
- 32.Matsusaka M, Kabata H, Fukunaga K, Suzuki Y, Masaki K, Mochimaru T, Sakamaki F, Oyamada Y, Inoue T, Oguma T, Sayama K, Koh H, Nakamura M, Umeda A, Ono J, Ohta S, Izuhara K, Asano K, Betsuyaku T. Phenotype of asthma related with high serum periostin levels. Allergol Int. 2015;64:175–180. doi: 10.1016/j.alit.2014.07.003. [DOI] [PubMed] [Google Scholar]
- 33.Kim MA, Izuhara K, Ohta S, Ono J, Yoon MK, Ban GY, Yoo HS, Shin YS, Ye YM, Nahm DH, Park HS. Association of serum periostin with aspirin-exacerbated respiratory disease. Ann Allergy Asthma Immunol. 2014;113:314–320. doi: 10.1016/j.anai.2014.06.014. [DOI] [PubMed] [Google Scholar]
- 34.Satirapoj B, Wang Y, Chamberlin MP, Dai T, LaPage J, Phillips L, Nast CC, Adler SG. Periostin: novel tissue and urinary biomarker of progressive renal injury induces a coordinated mesenchymal phenotype in tubular cells. Nephrol Dial Transplant. 2012;27:2702–2711. doi: 10.1093/ndt/gfr670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Satirapoj B, Witoon R, Ruangkanchanasetr P, Wantanasiri P, Charoenpitakchai M, Choovichian P. Urine periostin as a biomarker of renal injury in chronic allograft nephropathy. Transplant Proc. 2014;46:135–140. doi: 10.1016/j.transproceed.2013.07.069. [DOI] [PubMed] [Google Scholar]
- 36.Satirapoj B, Tassanasorn S, Charoenpitakchai M, Supasyndh O. Periostin as a tissue and urinary biomarker of renal injury in type 2 diabetes mellitus. PLoS One. 2015;10:e0124055. doi: 10.1371/journal.pone.0124055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bobolea I, Barranco P, Del Pozo V, Romero D, Sanz V, Lopez-Carrasco V, Canabal J, Villasante C, Quirce S. Sputum periostin in patients with different severe asthma phenotypes. Allergy. 2015;70:540–546. doi: 10.1111/all.12580. [DOI] [PubMed] [Google Scholar]
- 38.Simpson JL, Yang IA, Upham JW, Reynolds PN, Hodge S, James AL, Jenkins C, Peters MJ, Jia G, Holweg CT, Gibson PG. Periostin levels and eosinophilic inflammation in poorly-controlled asthma. BMC Pulm Med. 2016;16:67. doi: 10.1186/s12890-016-0230-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Inoue T, Akashi K, Watanabe M, Ikeda Y, Ashizuka S, Motoki T, Suzuki R, Sagara N, Yanagida N, Sato S, Ebisawa M, Ohta S, Ono J, Izuhara K, Katsunuma T. Periostin as a biomarker for the diagnosis of pediatric asthma. Pediatr Allergy Immunol. 2016;27:521–526. doi: 10.1111/pai.12575. [DOI] [PubMed] [Google Scholar]
- 40.James A, Janson C, Malinovschi A, Holweg C, Alving K, Ono J, Ohta S, Ek A, Middelveld R, Dahlen B, Forsberg B, Izuhara K, Dahlen SE. Serum periostin relates to type-2 inflammation and lung function in asthma; data from the large population-based cohort Swedish GA(2)LEN. Allergy. 2017 doi: 10.1111/all.13181. [DOI] [PubMed] [Google Scholar]
- 41.Kanemitsu Y, Matsumoto H, Izuhara K, Tohda Y, Kita H, Horiguchi T, Kuwabara K, Tomii K, Otsuka K, Fujimura M, Ohkura N, Tomita K, Yokoyama A, Ohnishi H, Nakano Y, Oguma T, Hozawa S, Nagasaki T, Ito I, Inoue H, Tajiri T, Iwata T, Izuhara Y, Ono J, Ohta S, Tamari M, Hirota T, Yokoyama T, Niimi A, Mishima M. Increased periostin associates with greater airflow limitation in patients receiving inhaled corticosteroids. J Allergy Clin Immunol. 2013;132:305–312. doi: 10.1016/j.jaci.2013.04.050. [DOI] [PubMed] [Google Scholar]
- 42.Nagasaki T, Matsumoto H, Kanemitsu Y, Izuhara K, Tohda Y, Horiguchi T, Kita H, Tomii K, Fujimura M, Yokoyama A, Nakano Y, Hozawa S, Ito I, Oguma T, Izuhara Y, Tajiri T, Iwata T, Ono J, Ohta S, Yokoyama T, Niimi A, Mishima M. Using exhaled nitric oxide and serum periostin as a composite marker to identify severe/steroid-insensitive asthma. Am J Respir Crit Care Med. 2014;190:1449–1452. doi: 10.1164/rccm.201407-1290LE. [DOI] [PubMed] [Google Scholar]
- 43.Hinks TS, Brown T, Lau LC, Rupani H, Barber C, Elliott S, Ward JA, Ono J, Ohta S, Izuhara K, Djukanovic R, Kurukulaaratchy RJ, Chauhan A, Howarth PH. Multidimensional endotyping in patients with severe asthma reveals inflammatory heterogeneity in matrix metalloproteinases and chitinase 3-like protein 1. J Allergy Clin Immunol. 2016;138:61–75. doi: 10.1016/j.jaci.2015.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Johansson MW, Evans MD, Crisafi GM, Holweg CT, Matthews JG, Jarjour NN. Serum periostin is associated with type 2 immunity in severe asthma. J Allergy Clin Immunol. 2016;137:1904–1907. doi: 10.1016/j.jaci.2015.12.1346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hoshino M, Ohtawa J, Akitsu K. Effect of treatment with inhaled corticosteroid on serum periostin levels in asthma. Respirology. 2016;21:297–303. doi: 10.1111/resp.12687. [DOI] [PubMed] [Google Scholar]
- 46.Kato G, Takahashi K, Izuhara K, Komiya K, Kimura S, Hayashi S. Markers that can reflect asthmatic activity before and after reduction of inhaled corticosteroids: a pilot study. Biomark Insights. 2013;8:97–105. doi: 10.4137/BMI.S12537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Corren J, Lemanske RF, Hanania NA, Korenblat PE, Parsey MV, Arron JR, Harris JM, Scheerens H, Wu LC, Su Z, Mosesova S, Eisner MD, Bohen SP, Matthews JG. Lebrikizumab treatment in adults with asthma. N Engl J Med. 2011;365:1088–1098. doi: 10.1056/NEJMoa1106469. [DOI] [PubMed] [Google Scholar]
- 48.Hanania NA, Noonan M, Corren J, Korenblat P, Zheng Y, Fischer SK, Cheu M, Putnam WS, Murray E, Scheerens H, Holweg CT, Maciuca R, Gray S, Doyle R, McClintock D, Olsson J, Matthews JG, Yen K. Lebrikizumab in moderate-to-severe asthma: pooled data from two randomised placebo-controlled studies. Thorax. 2015;70:748–756. doi: 10.1136/thoraxjnl-2014-206719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hanania NA, Korenblat P, Chapman KR, Bateman ED, Kopecky P, Paggiaro P, Yokoyama A, Olsson J, Gray S, Holweg CT, Eisner M, Asare C, Fischer SK, Peng K, Putnam WS, Matthews JG. Efficacy and safety of lebrikizumab in patients with uncontrolled asthma (LAVOLTA I and LAVOLTA II): replicate, phase 3, randomised, double-blind, placebo-controlled trials. Lancet Respir Med. 2016;4:781–796. doi: 10.1016/S2213-2600(16)30265-X. [DOI] [PubMed] [Google Scholar]
- 50.Brightling CE, Chanez P, Leigh R, O’Byrne PM, Korn S, She D, May RD, Streicher K, Ranade K, Piper E. Efficacy and safety of tralokinumab in patients with severe uncontrolled asthma: a randomised, double-blind, placebo-controlled, phase 2b trial. Lancet Respir Med. 2015;3:692–701. doi: 10.1016/S2213-2600(15)00197-6. [DOI] [PubMed] [Google Scholar]
- 51.Hanania NA, Wenzel S, Rosen K, Hsieh HJ, Mosesova S, Choy DF, Lal P, Arron JR, Harris JM, Busse W. Exploring the effects of omalizumab in allergic asthma: an analysis of biomarkers in the EXTRA study. Am J Respir Crit Care Med. 2013;187:804–811. doi: 10.1164/rccm.201208-1414OC. [DOI] [PubMed] [Google Scholar]
- 52.Tajiri T, Matsumoto H, Gon Y, Ito R, Hashimoto S, Izuhara K, Suzukawa M, Ohta K, Ono J, Ohta S, Ito I, Oguma T, Inoue H, Iwata T, Kanemitsu Y, Nagasaki T, Niimi A, Mishima M. Utility of serum periostin and free IgE levels in evaluating responsiveness to omalizumab in patients with severe asthma. Allergy. 2016;71:1472–1479. doi: 10.1111/all.12922. [DOI] [PubMed] [Google Scholar]
- 53.Daines SM, Wang Y, Orlandi RR. Periostin and osteopontin are overexpressed in chronically inflamed sinuses. Int Forum Allergy Rhinol. 2011;1:101–105. doi: 10.1002/alr.20031. [DOI] [PubMed] [Google Scholar]
- 54.Zhang W, Hubin G, Endam LM, Al-Mot S, Filali-Mouhim A, Desrosiers M. Expression of the extracellular matrix gene periostin is increased in chronic rhinosinusitis and decreases following successful endoscopic sinus surgery. Int Forum Allergy Rhinol. 2012;2:471–476. doi: 10.1002/alr.21056. [DOI] [PubMed] [Google Scholar]
- 55.Wang M, Wang X, Zhang N, Wang H, Li Y, Fan E, Zhang L, Zhang L, Bachert C. Association of periostin expression with eosinophilic inflammation in nasal polyps. J Allergy Clin Immunol. 2015;136:1700–1703. doi: 10.1016/j.jaci.2015.09.005. [DOI] [PubMed] [Google Scholar]
- 56.Yang L, Serada S, Fujimoto M, Terao M, Kotobuki Y, Kitaba S, Matsui S, Kudo A, Naka T, Murota H, Katayama I. Periostin facilitates skin sclerosis via PI3K/Akt dependent mechanism in a mouse model of scleroderma. PLoS One. 2012;7:e41994. doi: 10.1371/journal.pone.0041994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Yamaguchi Y, Ono J, Masuoka M, Ohta S, Izuhara K, Ikezawa Z, Aihara M, Takahashi K. Serum periostin levels are correlated with progressive skin sclerosis in patients with systemic sclerosis. Br J Dermatol. 2013;168:717–725. doi: 10.1111/bjd.12117. [DOI] [PubMed] [Google Scholar]
- 58.Steen VD, Medsger TA., Jr Severe organ involvement in systemic sclerosis with diffuse scleroderma. Arthritis Rheum. 2000;43:2437–2444. doi: 10.1002/1529-0131(200011)43:11<2437::AID-ANR10>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- 59.Okamoto M, Hoshino T, Kitasato Y, Sakazaki Y, Kawayama T, Fujimoto K, Ohshima K, Shiraishi H, Uchida M, Ono J, Ohta S, Kato S, Izuhara K, Aizawa H. Periostin, a matrix protein, is a novel biomarker for idiopathic interstitial pneumonias. Eur Respir J. 2011;37:1119–1127. doi: 10.1183/09031936.00059810. [DOI] [PubMed] [Google Scholar]
- 60.Uchida M, Shiraishi H, Ohta S, Arima K, Taniguchi K, Suzuki S, Okamoto M, Ahlfeld SK, Ohshima K, Kato S, Toda S, Sagara H, Aizawa H, Hoshino T, Conway SJ, Hayashi S, Izuhara K. Periostin, a matricellular protein, plays a role in the induction of chemokines in pulmonary fibrosis. Am J Respir Cell Mol Biol. 2012;46:677–686. doi: 10.1165/rcmb.2011-0115OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Naik PK, Bozyk PD, Bentley JK, Popova AP, Birch CM, Wilke CA, Fry CD, White ES, Sisson TH, Tayob N, Carnemolla B, Orecchia P, Flaherty KR, Hershenson MB, Murray S, Martinez FJ, Moore BB. Periostin promotes fibrosis and predicts progression in patients with idiopathic pulmonary fibrosis. Am J Physiol Lung Cell Mol Physiol. 2012;303:L1046–L1056. doi: 10.1152/ajplung.00139.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ashley SL, Wilke CA, Kim KK, Moore BB. Periostin regulates fibrocyte function to promote myofibroblast differentiation and lung fibrosis. Mucosal Immunol. 2017;10:341–351. doi: 10.1038/mi.2016.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ohta S, Okamoto M, Fujimoto K, Sakamoto N, Takahashi K, Yamamoto H, Kushima H, Ishii H, Akasaka K, Ono J, Kamei A, Azuma Y, Matsumoto H, Yamaguchi Y, Aihara M, Johkoh T, Kawaguchi A, Ichiki M, Sagara H, Kadota JI, Hanaoka M, Hayashi SI, Kohno S, Hoshino T, Izuhara K. The usefulness of monomeric periostin as a biomarker for idiopathic pulmonary fibrosis. PLoS One. 2017;12:e0174547. doi: 10.1371/journal.pone.0174547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Tajiri M, Okamoto M, Fujimoto K, Johkoh T, Ono J, Tominaga M, Azuma K, Kawayama T, Ohta S, Izuhara K, Hoshino T. Serum level of periostin can predict long-term outcome of idiopathic pulmonary fibrosis. Respir Investig. 2015;53:73–81. doi: 10.1016/j.resinv.2014.12.003. [DOI] [PubMed] [Google Scholar]
- 65.Nance T, Smith KS, Anaya V, Richardson R, Ho L, Pala M, Mostafavi S, Battle A, Feghali-Bostwick C, Rosen G, Montgomery SB. Transcriptome analysis reveals differential splicing events in IPF lung tissue. PLoS One. 2014;9:e92111. doi: 10.1371/journal.pone.0092111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Takahashi F, Takahashi K, Okazaki T, Maeda K, Ienaga H, Maeda M, Kon S, Uede T, Fukuchi Y. Role of osteopontin in the pathogenesis of bleomycin-induced pulmonary fibrosis. Am J Respir Cell Mol Biol. 2001;24:264–271. doi: 10.1165/ajrcmb.24.3.4293. [DOI] [PubMed] [Google Scholar]
- 67.McNeel DG, Eickhoff J, Lee FT, King DM, Alberti D, Thomas JP, Friedl A, Kolesar J, Marnocha R, Volkman J, Zhang J, Hammershaimb L, Zwiebel JA, Wilding G. Phase I trial of a monoclonal antibody specific for αvβ3 integrin (MEDI-522) in patients with advanced malignancies, including an assessment of effect on tumor perfusion. Clin Cancer Res. 2005;11:7851–7860. doi: 10.1158/1078-0432.CCR-05-0262. [DOI] [PubMed] [Google Scholar]
- 68.Izuhara K, Arima K, Ohta S, Suzuki S, Inamitsu M, Yamamoto K. Periostin in allergic inflammation. Allergol Int. 2014;63:143–151. doi: 10.2332/allergolint.13-RAI-0663. [DOI] [PubMed] [Google Scholar]