Abstract
Defensins are a well-characterised group of small, disulphide-rich, cationic peptides that are produced by essentially all eukaryotes and are highly diverse in their sequences and structures. Most display broad range antimicrobial activity at low micromolar concentrations, whereas others have other diverse roles, including cell signalling (e.g. immune cell recruitment, self/non-self-recognition), ion channel perturbation, toxic functions, and enzyme inhibition. The defensins consist of two superfamilies, each derived from an independent evolutionary origin, which have subsequently undergone extensive divergent evolution in their sequence, structure and function. Referred to as the cis- and trans-defensin superfamilies, they are classified based on their secondary structure orientation, cysteine motifs and disulphide bond connectivities, tertiary structure similarities and precursor gene sequence. The utility of displaying loops on a stable, compact, disulphide-rich core has been exploited by evolution on multiple occasions. The defensin superfamilies represent a case where the ensuing convergent evolution of sequence, structure and function has been particularly extreme. Here, we discuss the extent, causes and significance of these convergent features, drawing examples from across the eukaryotes.
Electronic supplementary material
The online version of this article (doi:10.1007/s00018-016-2344-5) contains supplementary material, which is available to authorized users.
Keywords: Disulphide-rich protein, Protein superfamily, Evolutionary constraint, Divergent evolution, Evolvability, Antimicrobial peptide
Introduction
Defensins are one of the best-described groups of antimicrobial peptides, and are expressed by a wide array of plants, animals and fungi for host defence. These proteins are small (less than 10 kDa), cysteine-rich (forming three to six disulphide bonds) and are typically cationic (net charge inter-quartile range of +1 to +5). The defensins are best known for their antimicrobial activity at low micromolar concentrations against Gram-positive and Gram-negative bacteria, fungi, viruses, and parasitic protozoa [1–3]. Additionally, the defensin fold has proved highly evolvable, with defensin-like protein (DLP) families having divergently evolved to perform alternative functions to antimicrobial activity. Diverse cell signalling roles via interaction with cell-surface receptors have been described, such as involvement in immune cell recruitment in vertebrates [4] and self/non-self-recognition during fertilisation in plants [5–7]. The venoms from scorpions, spiders, platypus, snakes and lizards all contain protein families with defensin-like structures that disrupt ion channels [8–10]. Plants and sessile animals have also adapted them for enzyme inhibition functions to deter grazers and predators [11, 12].
The defensins from across the animal, plant and fungal kingdoms have recently been classified into two superfamilies, the cis- and trans-defensins, each of which has an independent evolutionary origin [13]. The separation was established by analysis of 2714 defensin and defensin-like sequences and structures, covering 27 distinct disulphide connectivities (discussed in more detail in later sections) [13], in addition to the recently described sea anemone DLP, which constitutes a fifth trans-defensin fold [12]. Because their sequences are so divergent, sequence similarity and cysteine motifs are insufficient to resolve the more ancient evolutionary relationships; however, structural information has proved more useful in resolving these questions. Networks of structural similarity and topology separate the defensins into two groups, within each of which homology is statistically supported, but between which similarities are below the threshold of chance. This split results from incompatible differences in secondary structure and disulphide order and orientation between the two superfamilies [13].
The larger superfamily is named the cis-defensins, derived from the two parallel disulphides that bond the final β-strand to an α-helix. This superfamily, which is dominated by plant defensins, contains 11 of the structurally characterised defensin motifs and 11 motifs with currently unsolved structures. Conversely, members of the trans-defensin superfamily (accounting for the five remaining disulphide motifs) have two analogous disulphides that point in opposite directions from the final β-strand and thus bond to different secondary structure elements (Fig. 1a, b) [13].
Fig. 1.
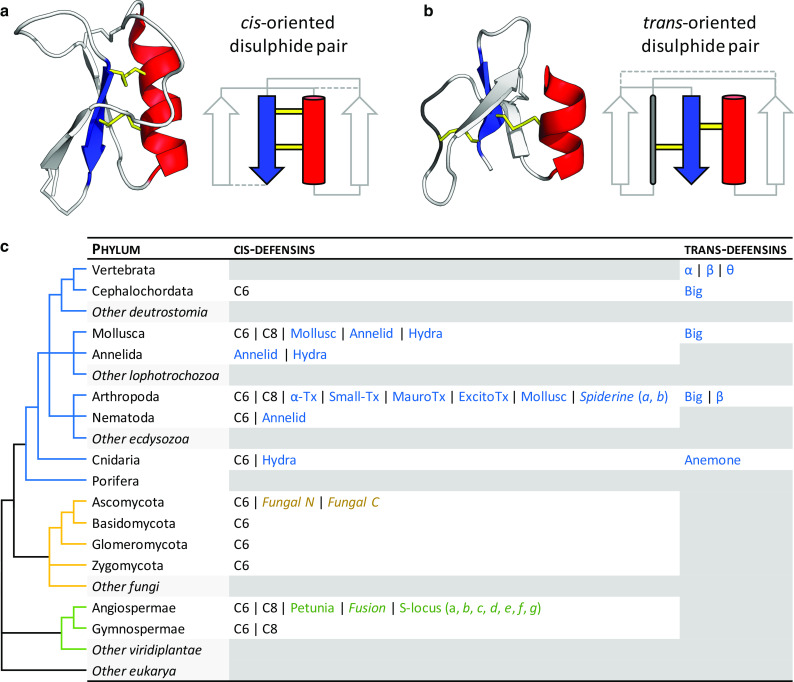
Architecture and taxonomic distribution of cis- and trans-defensins. a The plant defensin NaD1 (PDB:1MR4) is a typical cis-defensin in which both of the most conserved disulphides (yellow) from the final β-strand (blue) point in the same direction and bond to the same α-helix (red). b The human β-defensin HBD-1 (PDB:1IJV) is a typical trans-defensin in which the disulphides from the final β-strand point in opposite directions, therefore, bonding to different secondary structure elements. Non-conserved disulphides are represented as dashed lines in the secondary structure diagrams. Adapted from [13]. c A simplified phylogeny of eukaryotic phyla, annotated with the occurrence of different structural classes and cysteine motifs (in italics) from each defensin superfamily. Classes specific to a kingdom are coloured as in the phylogeny. Classes are described in more detail in Figs. 4 and 5. Phyla with no known defensins from each of the superfamilies are filled in grey
This evolutionary analysis has addressed the historical difficulties in classifying defensins by coupling primary sequence information, with secondary structure orientation, disulphide bond connectivities and tertiary structure similarities. This extends the classifications in the existing defensin-specific databases (e.g. the Defensins Knowledgebase [14] and iDPF-PseRAAAC [15]). It is also relevant for resolving relationships within the ever-expanding small cysteine-rich protein and peptide databases such as the manually curated APD (and its subsequent updates) [16–18] and machine-learning databases and prediction servers, such as CAMP [19], iAMP-2L [20], LAMP [21], PhytAMP [22], YADAMP [23], and ATDB [24].
The extant defensin structural classes, therefore, represent the divergent evolution of two ancestral folds to a variety of elaborated structures that specialise the defensins to their diverse functions. Within these structural classes, the inter-cysteine regions have undergone further extensive divergent evolution, to the extent that defensins of the same fold often display only chance sequence identity. Given their independent evolutionary origins and subsequent divergent evolution, the cis- and trans-defensin superfamilies display remarkable convergent evolution of a diverse array of traits. This review explores the known distribution of defensins in the two superfamilies, and how members have undergone convergences at the levels of gene and precursor protein organisation, protein sequence and structure, and how this has translated to functional and mechanistic convergences. Furthermore, the evolutionary pressures, constraints and solutions that have caused this convergence and divergence are discussed.
Phylogenetic distribution
With a few notable exceptions, cis- and trans-defensins are produced by different phyla [13] (Fig. 1c). Most trans-defensins occur in vertebrates (fish, reptiles, birds and mammals), with big defensins produced in some molluscs, arthropods and basal chordates (lancelets), and anemone DLPs produced in cnidarians. The greatest exception to this distribution is the presence of transcripts encoding cis β-defensins in two spiny lobster species of arthropods [25]. Conversely, cis-defensins occur in a wider array of animals: hydra, annelids, nematodes, arthropods, molluscs, and lancelets. They are also common in fungi, and spermatophyte plants [26, 27].
Genes from both defensin superfamilies are present in lancelets, and some arthropods and molluscs [27–30]. Within the multicellular eukaryotes, defensins have yet to be described in the non-spermatophyte plants (e.g. bryophytes, monilophytes), non-chordate deuterostomes (e.g. echinoderms), and the non-arthropod/nematode ecdysozoans (e.g. tardigrades). This broad and patchy distribution of defensins may be the result of repeated gene loss in multiple lineages [31] or extensive horizontal gene transfer between phyla, as has been documented for other host defence genes [32, 33]. Although “defensin-like” sequences of prokaryotic origin have been reported, these have only four cysteines and lack any other sequence similarity [34]. Therefore, in the absence of structural information, it is not yet possible to assert their relatedness [13].
Most of the disulphide connectivities are unique to a phylum; however, two cis-defensin disulphide connectivities are broadly distributed across multiple eukaryotic kingdoms. They are termed the C6 and C8 defensins in reference to their number of cysteines (Fig. 1c). The C8 defensins are found in plants, molluscs and insects and are mostly antimicrobial (Table 1). C6 defensins are distributed even more broadly and contain members with antimicrobial activity in invertebrates, plants and fungi, as well as members with signalling roles in plants and toxic roles in chelicerates.
Table 1.
Distribution and functions of examples from the shared C6 and C8 cis-defensin scaffolds
| Taxon | Function | Example | Species | Accession | References | |
|---|---|---|---|---|---|---|
| C6 | Plant | Unknown | Nodule defensin | Astragalus sinicus | 77994681 | [ds] |
| Fertilisation | LURE1 | Torenia fournieri | 225320707 | [7] | ||
| Fungus | Antibacterial | Micasin | Arthroderma otae | 2LR5 | [35] | |
| Cnidarian | Antifungal | Galiomicin | Helicoverpa zea | 528880428 | [ds] | |
| Cephalochordate | Unknown | BfD1 | Branchiostoma floridae | 260803302 | [29] | |
| Nematode | Unknown | CreD1 | Caenorhabditis remanei | 308463700 | [ds] | |
| Mollusc | Antibacterial | MGD-1 | Mytilus galloprovincialis | 1FJN | [36] | |
| Insect | Antibacterial | Nasonin-1 | Nasonia vitripennis | 2KOZ | [37] | |
| Ixodid | Antibacterial | Varisin A1 | Dermacentor variabilis | 37999545 | [38] | |
| Arachnid | Antibacterial, antifungal | oh-Defensin | Ornithoctonus hainana | none | [39] | |
| Chelicerate | Antibacterial | LqD1 | Leiurus quinquestriatus | 1169262 | [40] | |
| Ion channel toxin | Bmtx2 | Mesobuthus martensii | 2BMT | [41] | ||
| C8 | Plant | Antifungal | NaD1 | Nicotiana alata | 1MR4 | [42] |
| Serine protease inhibitor | ATT | Arabidopsis thaliana | 1JXC | [11] | ||
| Sweet taste | Brazzein | Pentadiplandra brazzeana | 1BRZ | [43] | ||
| Mollusc | Induced by bacteria | Hs-defn | Hyriopsis schlegelii | 339646140 | [44] | |
| Insect | Antifungal | Drosomycin | Drosophila melanogaster | 1MYN | [45] |
ds direct submission to NCBI database
Gene and precursor protein convergence
Gene copy number
Defensins from both superfamilies can be present in multiple copies in the genome of an organism, having evolved by tandem gene duplication with subsequent sequence diversification [46–49]. For instance, over 300 defensin and defensin-like sequences have been identified in Arabidopsis and Medicago [48, 50, 51]. Orthologues frequently derive new functions (i.e. neofunctionalise) due to positive selection, a common feature of host immune proteins co-evolving against pathogens or parasites as the host competes in an arms race [52, 53].
The β-defensin gene clusters on chromosome 8p23.1 form one of the most copy number variable regions in the human genome [54] and gene copy number variation correlates with a range of disease susceptibilities. The DEFB4 gene, encoding human β-defensin-2 (HBD-2) has been particularly well studied in this regard where increased DEFB4 gene copy number was associated with psoriasis [55, 56]. For Crohn’s disease, the findings have been mixed with separate studies reporting correlations with low [57] or high [58] DEFB4 gene copy number. In addition, β-defensin gene copy number may also contribute to susceptibility to other conditions and diseases such as HIV infection [59], cervical cancer [60] and ankylosing spondylitis [54].
Protein biosynthesis, processing and trafficking
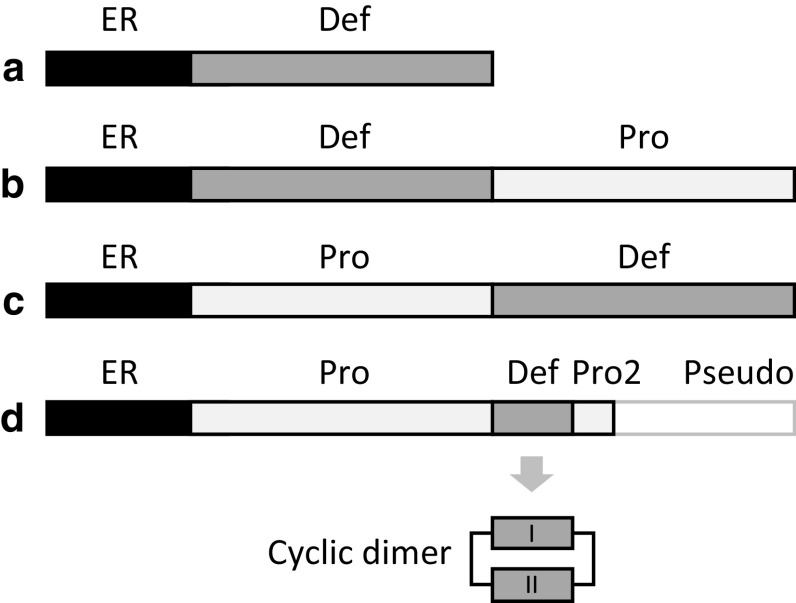
All known cis- and trans-defensins are processed from precursor proteins during maturation and trafficking. Given the presence of cysteine residues that participate in disulphide bonds, defensins are produced with N-terminal endoplasmic reticulum (ER) signal sequences (Fig. 2a). The mature defensin is secreted in the absence of any other signalling information.
Fig. 2.
Organisation of defensin precursor proteins. All defensins are produced with N-terminal endoplasmic reticulum (ER) signal sequences (to direct them to the ER for disulphide bond formation) in addition to the mature defensin domain (Def). Examples of defensins that adopt this structure include a scorpion C6 and plant C8 class I defensins. Other defensins are produced with additional prodomains (Pro) that can be positioned b C-terminally (e.g. mussel, and plant C8 class II defensins) or c N-terminally (e.g. insect C6 and vertebrate α- and β-defensins) of the mature domain. d θ-defensin precursors are truncated α-defensin prologues with a premature stop codon after the first 12 residues, from which a 9-mer fragment is excised, dimerised, and ligated to create the backbone-cyclised θ-defensin. The sequence after the stop codon is still highly similar to the α-defensin (Pseudo). Domain lengths not to scale
Defensins that are targeted to intracellular locations (e.g. vacuole or phagolysosome) have additional prodomains (also referred to as propeptides, prosequences, prosegments or propieces) [61–66]. This targeting prodomain can either precede or follow the mature defensin domain (Fig. 2b, c). For instance, plant class II defensins (mainly represented in the Solanaceae family) have long anionic prodomains on the C-terminal side of their C8 cis-defensin domains, in contrast to the more common plant class I defensins, which lack a prodomain and are secreted [42, 62]. Analogous negative prodomains are located at the N-terminus of mammalian α-defensins from the trans-defensin superfamily [67]. The fungal N-terminal and C-terminal defensin classes only occur as a two-domain fusion, and are proteolytically processed into two mature defensins [68] (Fig. S1). The plant ‘fusion’ class is similarly only found fused to a C8 defensin in a two-domain gene and it is currently unknown whether or not it is proteolytically processed.
Reconstruction of ancestral α-defensin sequences indicates their acidic prodomains have co-evolved to compensate for the basic amino acids in the mature defensins [67]. Therefore, in addition to its role as a targeting signal, the prodomain either has a chaperone function to assist folding or protects against autocytotoxicity by shielding the extreme positive charge of the mature defensin from deleterious interactions with lipids or other cellular proteins [63, 69–73]. Similar targeting sequences are located between the N-terminal ER signal and mature defensin domains of other non-secreted defensins [28, 64, 74].
The complete activation of preprodefensins often involves a two-step process: cleavage of the ER signal peptide producing an inactive prodefensin, followed by removal of the prodomain [62, 63, 65]. The mature defensins can be stored as fully processed active proteins such as in HNP-1–4, which reside primarily in the intracellular compartment of the phagolysosome [64, 75, 76]. This is akin to the mature class II plant defensins that are stored in the plant vacuole [42, 62, 73]. In contrast, other defensins (e.g. human Paneth cell α-defensins HD-5 and HD-6) are stored as inactive prodefensins in secretory granules that are destined for extracellular activities in the intestinal lumen [77–79]. These defensins are activated proteolytically by a Paneth cell-derived trypsin after they are secreted [75]. In mice, the Paneth cell α-defensins (known as cryptdins) [80, 81] are activated by removal of the prodomain by matrix metalloproteinase-7 (matrilysin, MMP-7) [82] before secretion [69]. The importance of proteolytic removal of the prodomain for defensin activation is highlighted by the observation that mice deficient in the MMP-7 protease do not produce mature cryptdins and are more susceptible to oral challenges with Salmonella typhimurium bacteria [82].
The precursor proteins of θ-defensins are especially unusual. The prodomains are homologous to full-length α-defensins and undergo unique processing, in which two nine amino acid segments from two prodefensins are cyclised head-to-tail by transpeptidation to form a single 18-amino acid mature cyclic protein [83] (Fig. 2d). The cyclic product can consist of a homodimer produced by ligation of two identical precursors, or heterodimers from ligations of different precursors [84, 85]. Heterodimers are strongly favoured, although the mechanisms controlling their ligation are not yet known [86, 87]. Human θ-defensin pseudogenes are not expressed due to a premature stop codon in their precursor, which may contribute to the human susceptibility to HIV as compared to the resistance in old world monkeys [83].
Individual defensins from the cis- and trans- superfamilies are expressed under specific circumstances or at specific sites. For instance, they often have distinct, organ-specific expression patterns, particularly in tissues that are vulnerable to microbial attack, such as nutrient-rich reproductive tissues, root nodules and seeds in plants or epithelial tissues and neutrophils in animals. They can also be expressed constitutively or induced by infection and inflammatory factors [42, 48, 51, 88]. Mice cryptdins, for instance, constitute ~70 % of the bactericidal activity that is secreted by the Paneth cells, with the concentration of cryptdins at the point of secretion in the intestinal mucosa reaching levels that are at least 1000 times greater than the antibacterial minimal inhibitory concentration (MIC) [89]. In humans, α-defensin HD-5 is stored at approximately 90–450 μg/cm2 of the surface of the intestinal mucosa, sufficient to generate microbicidal concentrations in the lumen [75].
Structural convergence
Primary structure
The cis- and trans-defensin superfamilies have convergent features across their primary, secondary and tertiary structures. Both superfamilies are extremely sequence diverse. The inter-cysteine loops of homologues from the same phylogenetic order often share less than 20 % amino acid sequence identity and have multiple insertions and deletions. Despite this, there are several convergent sequence features between the 1820 cis-defensins and 894 trans-defensins [90]. Foremost, their sequence composition is highly biased towards cysteines, positively charged amino acids (arginine and lysine) and glycine, at the expense of the aliphatic hydrophobic residues (valine, leucine, isoleucine and methionine) which form the hydrophobic cores of globular proteins [91] (Fig. 3a). This amino acid bias parallels the overall hydrophilic and net positive charge distributions of proteins from both superfamilies (Fig. 3b–g).
Fig. 3.
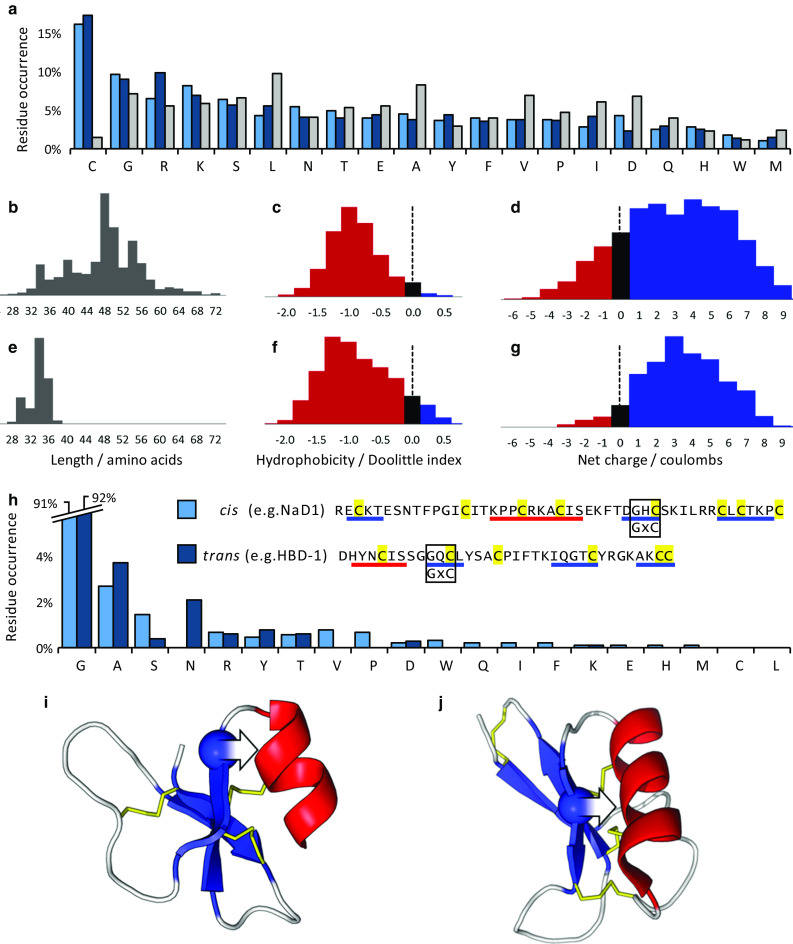
Amino acid sequence properties of cis- and trans-defensins. a Average amino acid residue occurrence for the cis-defensins (light blue), trans-defensins (dark blue) and whole Uniprot database (grey). Distributions of length, hydrophobicity and charge for b–d 1820 cis-defensins and e–g 894 trans-defensins. The common GxC motif occurs in both cis-defensins (e.g. NaD1) and trans-defensins (e.g. HBD-1). h Residue bias in the first position of the GxC motif in the cis-defensins (excluding S-locus and spiderines, which have an additional disulphide at this location) and the trans-defensins (excluding α- and θ-defensins, which lack an α-helix and so are unconstrained at this location). In both i cis-defensins (PDB:1MR4) and j trans-defensins (PDB:1IJV), the glycine (sphere) is oriented such that a non-hydrogen R-group on any other amino acid in this position (arrow) would clash with the α-helix. β-Strands in blue, α-helices in red, disulphide bonds in yellow
The only non-cysteine residue that is broadly conserved within each superfamily is a glycine in a GxC motif. This motif occurs in 91 % of cis-defensins (excluding the S-locus proteins which have an additional disulphide at the homologous location) and 92 % of the α-helix-containing trans-defensins, with alanine being the most common alternative (Fig. 3h). This motif is a consequence of the disulphides, which constrain the β-strand such that the glycine’s hydrogen side chain points back towards the α-helix (Fig. 3i, j). The R-groups of other residues cannot be accommodated in such a confined space and thus the potential steric clash causes them to be selected against. In this way, the constraints intrinsic to building similar cationic, disulphide-rich proteins cause convergence of both an overall residue bias, and the independent appearance of a defined GxC arrangement.
Secondary and tertiary structure and disulphide connectivity
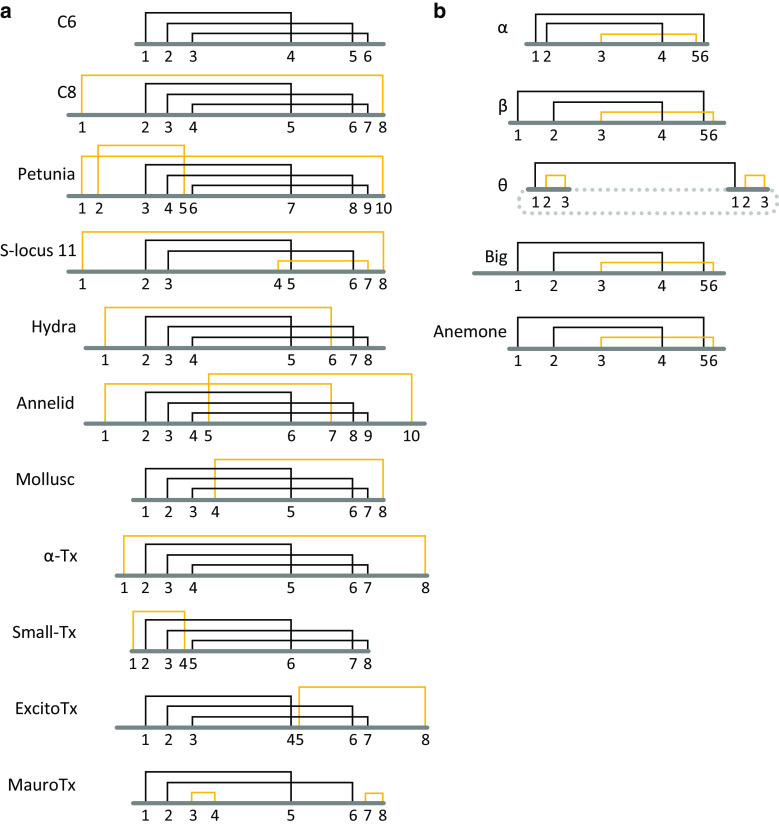
Both defensin superfamilies convergently use a double- or triple-stranded β-sheet (typically with an α-helix), cross-linked by a disulphide network into a compact core (Fig. 1a, b). Residues characterised as functionally important typically have highly solvent-exposed cationic side chains that bind to anionic ligands on the target. They may be located in the core (as in charybdotoxin) or on the displayed loops (as in NaD1) [26, 92–94]. Each superfamily has a conserved disulphide connectivity, which has been elaborated by divergent evolution to produce 22 cis-defensin and five trans-defensin classes with distinct, additional disulphides (Figs. 4, S2).
Fig. 4.
Defensin disulphide connectivities. Disulphide connectivities for the a cis-defensins and b trans-defensins. The most highly conserved disulphides are indicated in black and disulphides that are unique to each class are indicated in yellow. The dashed line indicates cyclisation of the θ-defensin
The disulphide bonding imparts another common feature to the defensins: their high stability to temperature, pH and proteolysis [42, 95–97]. The presence of disulphides limits the conformation entropy of the unfolded state and sterically occludes proteases. This may also account for the evolvability of the defensins, as disulphides make the structure robust to mutations in the loop regions, allowing extreme sequence diversification of the superfamilies [98].
The diversity of disulphide connectivities in the cis-defensins (Fig. 4) is generally derived from elaboration of the common C6 motif found in all eukaryotic kingdoms (Fig. 5a). Although such ancient evolutionary relationships are unresolved, it is tempting to speculate that the C6 class represents the ancestral fold of the cis-defensin superfamily. The C8 defensins, for example, have an additional disulphide compared to the C6 class, which constrains their longer N- and C-termini. The C10 petunia cis-defensins further elaborate on their C8 counterparts with a fifth disulphide which does not change the orientation of secondary structure elements, but substitutes for the network of non-covalent interactions that are present in the C8 defensins [42, 99]. Conversely, fewer trans-defensin structural classes have been identified, but each is far more distinct from other classes of the superfamily (Fig. 5b).
Fig. 5.
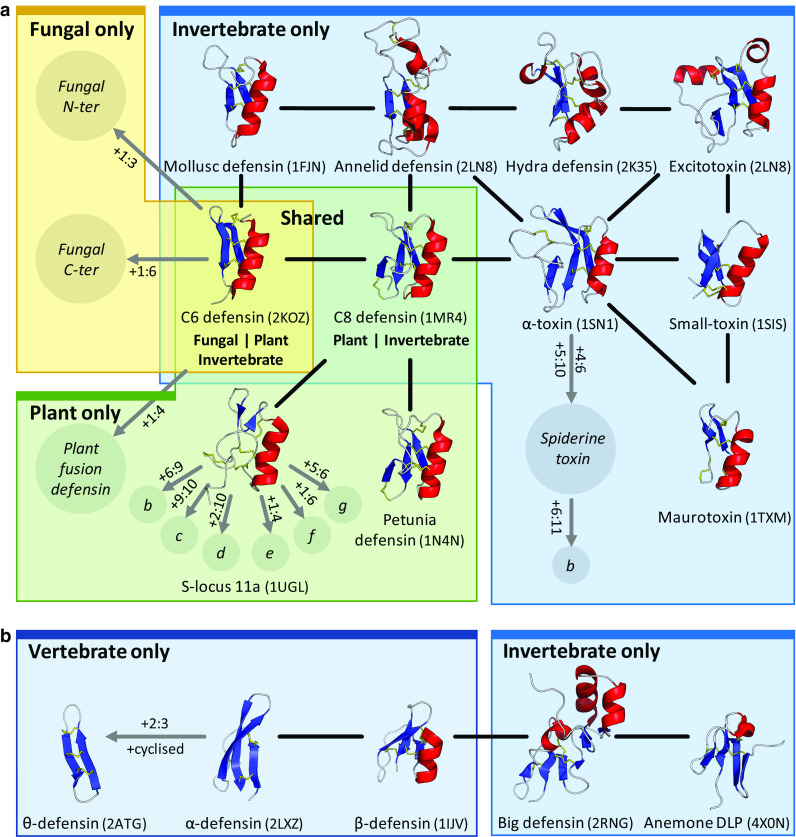
Relatedness within the cis- and trans-defensins. Evidence for common origin in the a cis-defensins and b trans-defensins. Structures are shown for cysteine patterns with solved structures, classes with unresolved structures are represented by italicised names in circles. Putative disulphides unique to a class are denoted as x:y where x and y are the additional cysteines involved in the disulphide. Uncharacterised variants with additional disulphides are denoted by single letters (e.g. S-locus 11b, etc.). Black lines indicate homology evidence from structural similarity, grey lines indicate evidence from gene structure and organisation. The PDB codes for the proteins are given in parentheses. Structures are organised by kingdom, with a fungal representative as an example of the shared C6 defensins and a plant representative for the shared C8 defensins (colours as used in Fig. 1)
Within each superfamily, insertion of secondary structure elements has generated different elaborations on the same core structure. Some such exemplifiers include the annelid and hydra ‘macin’ defensins and the big defensins (Fig. 5). These structures can be twice the size of the smaller members and have multiple insertions within their loops relative to smaller antimicrobial defensins, although they retain a similar charge density and hydrophobicity [13]. Even within each scaffold, families have divergent inter-cysteine loop lengths and composition, which adapts them to alternative functions. For example, scorpion toxins that use the C6 defensin fold have a shorter and more hydrophilic first loop for binding to their target ion channels [92]. Conversely, several cysteine pattern classes can be involved in the same function; for example, signalling by the seven S-locus 11 disulphide variant subclasses a–g (Figs. 5a, S2).
Quaternary dimerisation and oligomerisation
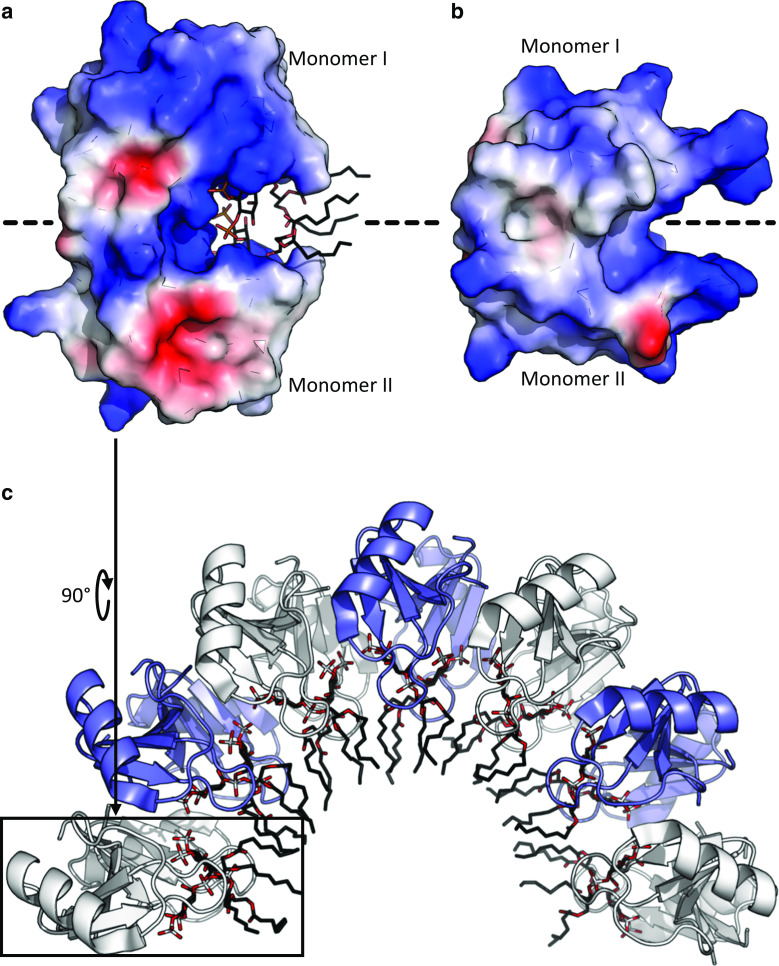
Several cis- and trans-defensins form homodimers or higher order oligomers [100–104]. The increased local charge density on the multimers is proposed to contribute to their high potency, broad-spectrum antimicrobial activity (elaborated further in the next section) [102, 105–107]. For such defensins in homogeneous solutions, there is an equilibrium between dimers and higher oligomers but dimers are the most prominent form adopted by the plant cis-defensins NaD1 (Fig. 6a) and TPP3 [100, 101], and the human trans-defensins including β-defensin HBD-2 (Fig. 6b) [102], and α-defensins HNP-3, HNP-4, HD-5 and HD-6 [103, 104]. The solved structures of specific human α-, β- and plant defensins are dimers with a six-stranded antiparallel β-sheet across the dimer interfaces. These dimeric structures have been proposed to provide a platform for lipid bilayer attachment and permeabilisation for innate defence against pathogens [2, 100]. It remains to be ascertained whether oligomerisation is a common feature for other cis- and trans-defensins.
Fig. 6.
Defensin dimerisation and lipid-mediated oligomerisation. a The plant C8 defensin NaD1 (PDB:4CQK) forms a homodimer that binds negatively charged phospholipid head groups via a cationic grip [94]. b The human β-defensin HBD-2 (PDB:1FD4) forms a structurally similar dimer [102]. Protein surface charge is indicated by blue (positive) and red (negative). Lipids are shown as sticks with phosphate in white and oxygen in red. c NaD1 dimers assemble into an arching oligomeric structure after interaction with the anionic head groups of PIP2 within an extended cationic groove on the surface of the NaD1 oligomer (PDB:4CQK). Alternating dimers in white and blue
NaD1 and TPP3 homodimers display a grip-shaped, cationic binding pocket, termed the “cationic grip” [94, 101]. The inner face of the cationic grip for NaD1 binds the anionic head group of the phospholipid phosphatidylinositol 4,5-bisphosphate (PIP2) via a network of ionic and hydrogen bond interactions (Fig. 6a). The grip region is comprised mainly of loop residues 36–40 (KILRR) in NaD1 and residues 37–41 (KLQRK) in TPP3. These loops are critical for lipid binding as well as for the antifungal and anticancer activities of these defensins [94, 100, 101]. The homologous loop of the Medicago truncatula defensin (MtDef4) consists of RGFRRR, which has been proposed to mediate antifungal activity by binding to phosphatidic acid (PA) as well as promoting entry into the fungal cell [108]. Whether dimerisation and oligomerisation is important for this PA interaction and antifungal activity has yet to be determined.
Human HBD-2 contains an analogous cationic loop that connects the first two β-strands. This loop, comprised of residues 22–25 (RRYK), forms a strikingly similar cationic grip structure in the HBD-2 dimer to that of the plant defensins NaD1 and TPP3 [102] (Fig. 6b). Despite a distinctly different dimer arrangement compared with HBD-2, HBD-6 also dimerises and forms a positively charged binding groove in the presence of glycosaminoglycan [109]. Therefore, the cationic binding pocket may be a common convergent feature in the structure–function relationship of defensin dimers.
For some defensins, high concentrations or the presence of ligand can promote the formation of higher order oligomers, illustrating an emerging role for defensin oligomers in innate host defence. HBD-2 oligomerises at high concentrations [102], NaD1 oligomerises in the presence of PIP2 [94], and α-defensins HNP-1, HNP-2 and HD-6 oligomerise upon contact with artificial lipid membranes [110–112].
A high-resolution structure of an NaD1:PIP2 complex was determined by X-ray crystallography and revealed an intriguing oligomeric arrangement. The oligomer comprises seven “cationic-grip” dimers of NaD1 in complex with the anionic head groups of 14 PIP2 molecules. The seven NaD1 dimers assemble into an arch-shaped configuration with an extended cationic grove in which the anionic lipid head groups are bound via a cooperative network of hydrogen bonds [94] (Fig. 6c). Interestingly, NaD1:PIP2 complexes can assemble into long string-like fibrils in vitro, as revealed by transmission electron microscopy [94]. Whether such large oligomeric complexes can form in vivo and their functional importance remains to be determined.
A distinct oligomerisation event has been described for the α-defensin HD-6, which lacks direct antimicrobial activity, but self-assembles into ordered fibrils and nanonets to entrap bacteria [112]. The formation of multimeric transmembrane pores has also long been proposed for α-defensins, such as HNP-2 [111] and the C6 defensin, phormicin [113].
Functional and mechanistic convergence
Antimicrobial activity by targeting membrane lipids
Host defence by antimicrobial activity was the first described activity and is the most commonly reported function for members of both defensin superfamilies. It is also the likely ancestral role of each superfamily, with other functions having divergently evolved in various eukaryote taxa. Antimicrobial action is often achieved via complex, multi-step interaction mechanisms, which remain poorly characterised for the majority of defensins. These diverse mechanisms include interaction with cell wall carbohydrates, membrane transport machineries, cytoplasmic cell components, nucleic acids, or induction of reactive oxygen species, and typically cannot be generalised across even closely related defensins [114–121]. However, the most common antimicrobial mechanism of both superfamilies involves lipid binding that either directly disrupts membranes, inhibits lipid-dependent cell wall synthesis or aggregates pathogens. Specific defensin-lipid interactions distinguish between host and pathogen by taking advantage of differences in cell wall and membrane composition. Vertebrate defensins additionally regulate the interaction between innate and adaptive immunity via signalling mechanisms, and are described in more detail in the next section [122, 123].
Lipid targeting is a property that extends throughout the antifungal C8 plant and insect cis-defensins. For example, glucosylceramide lipids located in the cell walls and plasma membranes of filamentous fungi are targeted by the plant defensins RsAFP2 [117], Psd1 [124] and MsDef1 [125] and the insect C6 defensin heliomicin [117]. Other plant defensins interact with structurally related membrane lipids such as mannosyl-diinositolphospho-ceramide (bound by DmAMP1) [126] and phosphatidic acid (bound by MtDef4) [108]. As mentioned, the cis-defensins NaD1 and TPP3 bind the phospholipid PIP2 as does the trans-defensin HBD-3 [94, 101, 127, 128]. Indeed, the above-mentioned lipid binding loops of NaD1 and TPP3 are strikingly analogous to that of HBD-3 (residues 36-39, RGRK), suggesting a convergent ‘phospholipid recognition code’ between the defensin superfamilies [129].
Both superfamilies also contain members that bind the membrane-anchored lipid II peptidoglycan precursor, to block cell wall biosynthesis. Fungal C6 cis-defensins, including plectasin [130], oryzeasin [131] and eurocin [132] use this mechanism, and lipid II binding has also evolved in the mollusc cis-defensins MGD-1 and Cg-Def [36, 133]. Convergent use of lipid II binding is reported for the trans-defensins, human α-defensin 1 (HNP-1) [134] and human β-defensin 3 (HBD-3) [135].
Finally, binding to the cell surfaces of potential microbial pathogens by several cis- and trans-defensins may block pathogen adsorption and entry into host cells [136, 137], or cause aggregation of the microbes [112, 138]. Proposed aggregation mechanisms include the simultaneous binding of two microbial cells by a defensin with two hydrophobic interfaces, or by the formation of extended fibril networks [112, 138].
Achieving high affinity as well as specificity for a particular lipid in a pathogen’s membrane requires high binding energy, whether for membrane disruption [128] or lipid extraction (of the order of 100 kJ mol−1 [139]). Hydrophobic interactions are typically neither energetic, nor specific enough to achieve this [140, 141] and consequently the proteins rely on multiple electrostatic contacts with the charged head groups (on the order of 10 kJ mol−1 energy each [142]. This contrasts with the non-specific plant lipid transfer proteins, which use an extensive binding tunnel to form hydrophobic interactions with fatty acyl lipid tails [143].
Signalling by receptor interaction
Both cis- and trans-defensin superfamilies have convergently evolved members with signalling activities. The two most common signalling functions are immune cell recruitment and self-recognition, mediated by interactions with cell-surface receptors. In the trans-defensin superfamily, multiple human α- and β-defensins selectively chemoattract leukocytes and stimulate cytokine release [144–147]. These immunomodulatory effects are mediated by interaction with a number of chemokine receptors. For human β-defensins, these receptors include CCR6 (immature DCs, neutrophil, T cells), CCR2 (monocytes), and Toll-like receptors (TLR) 1, 2 and 4 (monocytes, myeloid DCs and immature DCs) [147–151]. In contrast, human α-defensins HNP-1, 2 and 3 potently inhibit the phospholipid/Ca2+ protein kinase C-mediated signalling pathway [152]. They, therefore, link innate and adaptive immunity, and effectively enhance pathogen killing and clearance.
An example of signalling by an antimicrobial cis-defensin is the plant C8 defensin Psd1, which mediates its antifungal action against Neurospora crassa, not from direct membrane disruption but rather from protein internalisation and signalling via cyclin F, which interferes with nuclear division and disrupts the cell cycle [153]. This mechanism was supported by localisation of Psd1 to the nucleus and its interaction with cyclin F [153].
The largest group of signalling cis-defensins (the S-locus 11 class) functions in self/non-self-recognition during fertilisation in angiosperm plants and lack antimicrobial activity. S-locus protein 11 (SP11) variants, also referred to as S-locus Cysteine-Rich (SCR) proteins, are important signalling mediators of plant self/non-self-recognition in the sporophytic incompatibility system that prevents inbreeding [154]. SP11 variants or haplotypes are expressed by polymorphic genes that reside at the multi-allelic S-locus and serve as the pollen S-haplotype specificity determinants. They are paired with cognate stigmatic S-haplotype specificity determinants known as S-locus receptor kinases (SRKs), which are single-pass serine/threonine receptor kinases present in the plasma membrane of stigmatic papilla cells [6, 155]. In a self-pollination, binding of the pollen SP11 protein to the “self” SRK on the stigma leads to SRK autophosphorylation and results in pollen rejection. This is mediated by the initiation of a transduction pathway that results in the inhibition of pollen hydration and penetration of the pollen tube through the epidermal cell walls of the stigma. During cross-pollination, there is no interaction between SP11 and SRK and fertilisation proceeds unimpeded [6]. Binding to cognate SRKs is largely determined by the exposed loop regions between the third and fourth as well as the fifth and sixth cysteine residues in SP11, as identified by site-directed alanine mutagenesis and loop swapping experiments [6]. This suggests that new allelic specificity has evolved readily in the SP11 folds. Indeed, over 100 haplotypes can exist in a given species, and consequently as many SP11 and SRK proteins [154, 156]. This is reflected in the extraordinarily high variation in protein sequence and seven different disulphide connectivities (Figs. 4a, S2) [13].
An additional plant fertilisation role is played by another group of defensins called LUREs (C6 defensin fold). LUREs are secreted by the two synergid cells on the side of the egg cell and act as diffusible, species-specific signals that chemoattract and guide the pollen tube to the ovule for fertilisation [7, 157].
The diverse signalling interactions by defensins from both superfamilies are a product of the small and stable defensin folds, which allow for the display of highly divergent loop sequences and the selection of molecules that form specific receptor interactions.
Toxic function by ion channel perturbation
Within the two defensin superfamilies, several subfamilies of animal DLPs have been converted to neurotoxic functions. These DLPs retain a defensin-like scaffold, but inter-cysteine loop sequences have been selected that enable specific interactions with ion channels, and they have typically radiated into large, diverse multigene families [158].
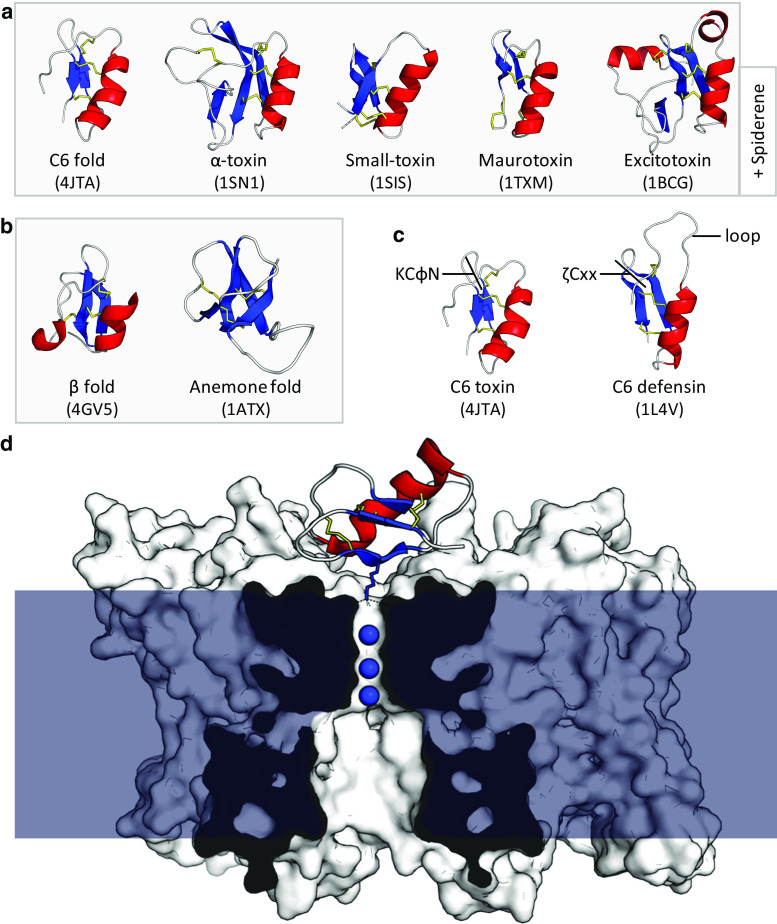
The cis-defensins include several classes from scorpions and spiders that are uniquely used for toxic functions, such as the α-toxins (note that α-toxins are not related to α-defensins) [159, 160] (Fig. 7a). Spiderines are based on an α-toxin scaffold with additional cysteines (up to 12 total) and, in some cases, an additional and unique N-terminal domain [8].
Fig. 7.
Blocking of ion channels by defensin-like peptides. a The common cis-defensin C6 fold is adapted in some scorpion toxins, along with four toxin-specific structural classes with distinct additional disulphides. A structurally uncharacterised cis-defensin is also present in lynx spider venom (spiderine). b The trans-defensin fold has been recruited to toxic function such as crotamine in snakes, OvDLP from platypus and helofensin from bearded lizards. The anemone fold is also used in sea anemone neurotoxins. c Comparison of the C6 defensin fold with different functions. Toxins contain a conserved KCφN motif, whereas antimicrobial defensins contain the broader ζCxx motif at the same location, in addition to a large, flexible loop (φ = hydrophobe, ζ = hydrophile). d Charybdotoxin binds to the tetrameric K v channel (white surface) and inserts a lysine residue into the first of the channel’s four K+ binding sites, blocking the transport of K+ ions (blue spheres) through the cell membrane (blue) (PDB:4JTA) [93]
In addition to the classes that uniquely perform toxic functions, distinct subfamilies specialised to toxic function are present in both C6 cis-defensins and β-trans-defensins (Fig. 7a, b). In line with their highly divergent function, their sequences are clearly specialised [92]. Toxins of the C6 class, such as charybdotoxin, contain the conserved KCφN motif for ion channel binding, not present in antimicrobial defensins (Fig. 7c). They also lack the segregated, amphiphilic, cationic surface charge distribution typical of antimicrobial defensins, and have altered loop lengths to allow for specific interactions with ion channels, rather than lipids. Similarly, the β-defensin fold has been adapted to a toxic function in the well-characterised snake crotamines, as well as the putative toxins helofensin from bearded lizard venom and ovDLP-A from platypus venom [161, 162].
The toxic members from both superfamilies act by binding cation channels (K+, Na+, Ca2+) with the exception of chlorotoxin, which binds Cl− channels [163]. The best characterised of these is the scorpion charybdotoxin, which binds to voltage-gated K+ channels and inserts a lysine to block the channel’s selectivity filter (Fig. 7d) [92, 93]. The snake toxin crotamine (trans-defensin fold) is also proposed to bind and block voltage-gated K+ channels via analogous residues R31-Y32 [164]. Conversely, a different region of the cis-defensin scaffold has been repurposed for toxic function in the scorpion excitatory toxins, such as Bj-xtrIT. These toxins bind Na+ channels using the opposite surface to charybdotoxin [165], and likely interact with the channel’s regulatory regions, rather than the pore itself [163].
It is, therefore, likely that ancestral antimicrobial defensins from both superfamilies were convergently neofunctionalised to toxicity by extensive adaptation of the sequence and length of their inter-cysteine loops for specific interaction with new ion channel targets. This convergent recruitment is indicative of the versatility of defensin folds when subjected to suitable selection pressures. Their short length and secretion is suitable for the large-scale expression required for toxin production [166]. The stability afforded by the disulphide-rich structures is beneficial for storage in venom sacs and persistence in prey, as well as allowing the sequence diversification of the loop regions [167]. Indeed, similar evolution from innate immunity function to toxicity has also occurred in other defence gene families where their mechanisms for pathogen defence are repurposed for offence [166].
Enzyme inhibition
Some plant defensins exhibit proteinase [11, 168, 169] and α-amylase [170–172] inhibitory activities, probably evolved to fend against insect predation. The tight, stable disulphide-linked topology of the defensins appears to make them well suited to enzyme inhibition. For instance, the A. thaliana trypsin inhibitor ATT uses the cis- plant C8 defensin fold to competitively inhibit PA clan proteases, such as trypsin [11]. The putative reactive site P1–P1′ residues are contained in the first solvent-exposed loop [11] (Fig. 8a). Trans-defensins including α- and θ-defensins are also protease inhibitors, but by non-competitive mechanisms, whereby they bind to the active site at a location other than the substrate binding site [173, 174].
Fig. 8.
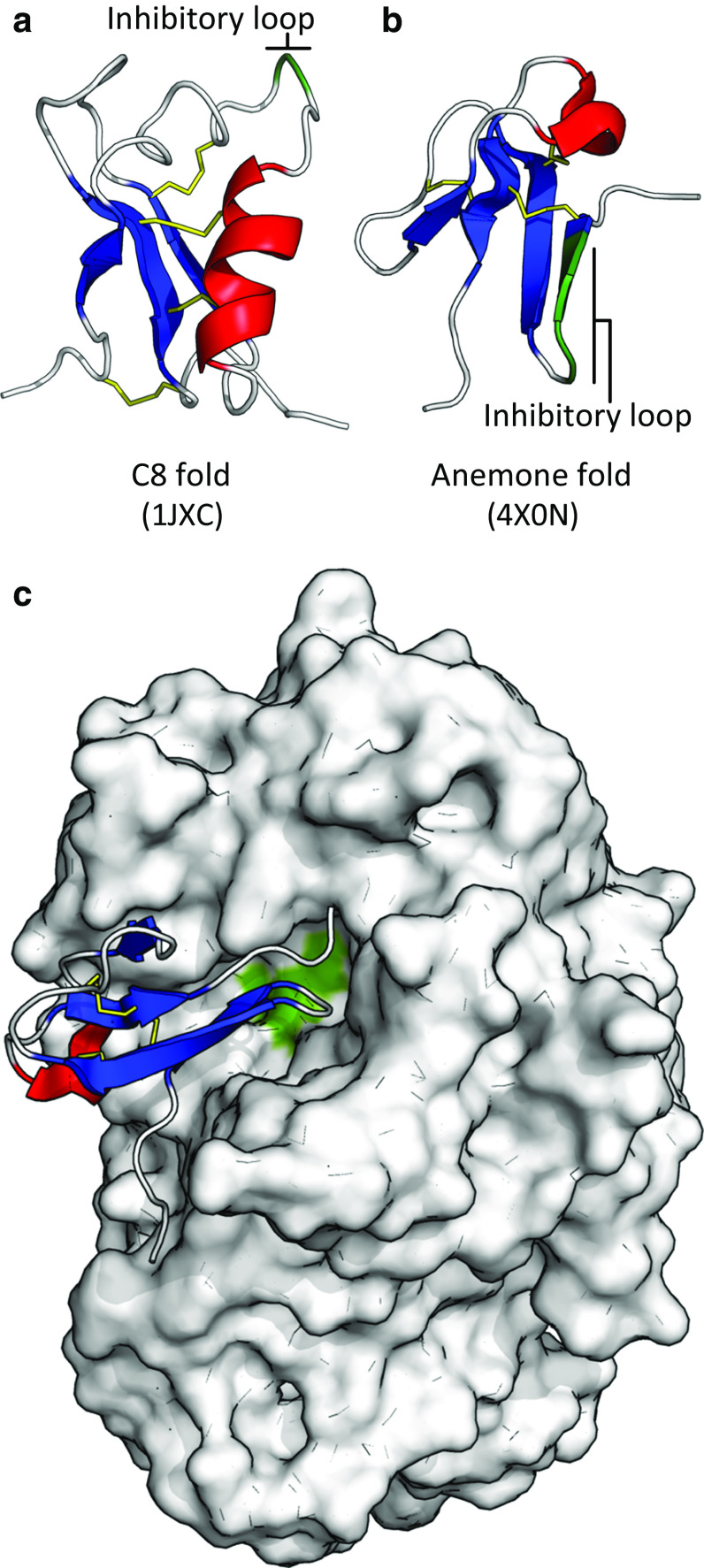
Enzyme inhibition by defensin-like peptides. a The C8 cis-defensins fold has been adapted to enzyme inhibitory function in the Arabidopsis thaliana trypsin inhibitor (ATT) (PDB:1JXC) [11], and b the trans-defensins contain an α-amylase inhibitor, helianthamide, from sea anemones (PDB:4X0N) [12]. Inhibitory loop highlighted in green (putative for ATT) [11]. c The enzyme α-amylase (white surface) uses an aspartate-glutamate dyad in its active site for hydrolysis (green), which is competitively inhibited by the bound helianthamide (PDB:4X0N)
More recently, a big-defensin-like protein from the sea anemone Stichodactyla helianthus, helianthamide, was identified with highly potent (K i = 10 pM) and selective inhibitory activity against human pancreatic α-amylase [12]. Helianthamide adopts a four-stranded trans-defensin fold highly similar to the big defensins and binds into and across the α-amylase active site and is thought to act as an antifeedant. Three aromatic residues (Y7, Y9, and H10) constitute all of the important polar contacts of helianthamide with the enzyme’s catalytic machinery, along with I11 and V12, which create a nonpolar interface to complement the hydrophobic ridges bordering the active site of the enzyme [12]. It is interesting that both plants and sessile animals independently converted defensins to effective antifeedant activities to deter their respective grazers or predators.
Adaption to abiotic stresses
In addition to their induction by biotic stresses such as pathogen infection, the plant cis-defensins have been co-opted for response to abiotic stressors, including drought [175], salinity [176, 177], cold [178, 179] and metals [180]. For instance, the AhPDF1.1 defensin from Arabidopsis halleri (the only Arabidopsis species adapted to metal-contaminated soils and displaying high zinc and cadmium tolerance and hyper-accumulation capacities) has been functionally linked to conferring zinc tolerance in studies with yeast (Saccharomyces cerevisiae) and transgenic plants (A. thaliana) [180].
Anticancer activity
Several defensins from both superfamilies have specific cytotoxic anti-proliferative activities on cancer cell lines as well as solid and haematological tumours, and have minimal effects on healthy cells [181–183]. Examples include human α-defensins HNP-1 to HNP-3 [184, 185], their rabbit orthologues NP-1 to NP-3 [184], human β-defensin HBD-1 and HBD-3 [127, 186], frog defensin brevinin-2 [187] and the plant cis-defensins NaD1 [94] and TPP3 [101]. They are cytotoxic to several cancer cell lines such as Raji and WIL-2 (lymphoma), L1210 and Jurkat (leukaemia), human HeLa (cervical carcinoma) and MCF-7 (breast carcinoma) and do so via direct membrane disruption and cell lysis [127, 184] or DNA damage [188]. Greater activity towards cancerous over healthy cells is likely to be due, in part, to an increase in affinity for the dysregulated tumour cell plasma membranes. The changes to the membrane include an increase in negatively charged phospholipids [189, 190] and glycoproteins [191, 192] in the outer leaflet, as well as increased surface area and fluidity [193, 194].
In contrast, murine β-defensins and human HBD-2 exert indirect anticancer activity via chemotactic and immunoadjuvant activities that promote adaptive immune responses [195–197]. Furthermore, HBD-3 and HNP-1, 2 and 3 additionally exert inhibitory effects on metastasis and angiogenesis [198–200]. These activities may be physiologically relevant in mammalian defensins, but are certainly a promiscuous side-activity in plant defensins, and reflect the propensity of antimicrobial defensins to interact with cell membrane targets.
Causes and significance of convergence
Convergent evolution occurs when similar selection pressures coincide with biophysical constraints that favour only a small number of accessible, adaptive solutions within a fitness landscape. In such cases, selection funnels evolutionary lineages towards similar solutions in that fitness landscape [201, 202]. Convergent evolution has been widely described for a range of biological phenomena: from physiology and behaviour to gene organisation and recruitment; however, examples at the protein level are rare [201–203].
Convergent evolution of specific sequences or structural folds is less common than functions, since equivalent functions can typically be achieved by different structural folds, and equivalent structures can be formed by many different sequences [204]. For example, the ability to cleave peptide bonds by a variety of chemical mechanisms has convergently evolved in the different classes of proteases. Indeed, even the same mechanism of covalent proteolysis using the same catalytic triad geometry has evolved independently at least 24 times in distinct superfamilies of serine and cysteine proteases [205]. Sequence convergence has also occurred in the transmembrane (TM) helix of the mitochondrial import receptor subunit TOM20. The plant and fungal analogues have evolved the same sequence motif, but in reverse order, as the TM helix passes though the membrane in opposite directions in the different analogues [206]. Similarly, sequence convergence of linear motif peptides in pathogens is driven by selection to mimic their host’s sequences and so bind host targets and disrupt cellular processes [207]. Finally, the convergent evolution of particularly favourable protein folds is thought to be extremely rare, and possible examples (such as the β-barrel fold) are highly contentious [206, 208, 209].
The extent of convergence between the cis- and trans-defensins is, therefore, particularly remarkable and derives from several intrinsic constraints of fold and function. First, the structures of small cysteine-rich proteins (CRPs) are freed from the requirements of a hydrophobic core, but have additional packing constraints when secondary structural elements are forced into close proximity. Second, the activities of the defensins dictate additional biophysical requirements that further constrain functional structures. Yet there are simultaneous evolutionary pressures that favour very high sequence divergence within these constraints, largely driven by specialisation to different targets and subsequent co-evolution with those targets [210].
The constraints of small CRPs impose a limited number of viable secondary structure orientations and disulphide topologies [211–213]. Consequently, similar CRP structures can be converged upon by evolution whereas larger globular proteins remain more diverse. This constraint is strong enough that additional unrelated proteins such as the MARCO receptor have also converged on similar folds [13].
The use of a compact, disulphide-stabilised core to display a set of cationic loops has, therefore, proven to be remarkably evolvable for a number of functions. Both the cis- and trans-defensin superfamilies independently evolved innate immune functions, using positively charged loops for membrane disruption. Analogous loops can interact with negatively charged lipid head groups in a ‘cationic grip’ formed by protein dimers. Each superfamily also contains members that have evolved to bind cell surface receptors to perform a variety of signalling roles, some immune related, some as divergent as fertilisation. Similarly, on multiple occasions, both folds have been recruited to a toxic function using exposed positive residues for ion channel blocking. The two defensin superfamilies, therefore, represent one of the most extensive occurrences of convergent evolution and demonstrate how evolution can favour extremely similar solutions to a selection pressure even when run independently.
Concluding remarks
In summary, this review provides insights into the unique features of the two defensin superfamilies, which arose by extensive convergent evolution from independent origins. It highlights how the defensins represent a thorough case study in the evolvability of small CRP scaffolds, which is unlike that for more commonly studied globular proteins [214]. This recent work establishes the foundation for understanding evolutionary relatedness in defensins, and highlights how the elaboration of stable scaffolds has enabled both superfamilies to span an uncommonly wide array of biological roles [13].
With new sequences and structures being described at an ever-increasing rate, it is inevitable that additional defensin structural classes will be discovered. The integration of evolutionary, structural and functional data will inform design principles to enable the engineering of improved or novel variants for therapeutic and agricultural applications.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgments
The authors would like to thank the financial support of the Australian Research council (Grant DP150104386), Hexima Ltd, and La Trobe University.
Footnotes
Thomas M. A. Shafee and Fung T. Lay contributed equally to the work.
Contributor Information
Marilyn A. Anderson, Email: m.anderson@latrobe.edu.au
Mark D. Hulett, Email: m.hulett@latrobe.edu.au
References
- 1.Brogden KA. Antimicrobial peptides: pore formers or metabolic inhibitors in bacteria? Nat Rev Microbiol. 2005;3(3):238–250. doi: 10.1038/nrmicro1098. [DOI] [PubMed] [Google Scholar]
- 2.Ganz T. Defensins: antimicrobial peptides of innate immunity. Nat Rev Immunol. 2003;3(9):710–720. doi: 10.1038/nri1180. [DOI] [PubMed] [Google Scholar]
- 3.Boulanger N, et al. Characterization of a defensin from the sand fly Phlebotomus duboscqi induced by challenge with bacteria or the protozoan parasite Leishmania major . Infect Immun. 2004;72(12):7140–7146. doi: 10.1128/IAI.72.12.7140-7146.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pushpanathan M, Gunasekaran P, Rajendhran J. Antimicrobial peptides: versatile biological properties. Int J Pept. 2013;2013:675391. doi: 10.1155/2013/675391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Assadi-Porter FM, et al. Key amino acid residues involved in multi-point binding interactions between brazzein, a sweet protein, and the T1R2-T1R3 human sweet receptor. J Mol Biol. 2010;398(4):584–599. doi: 10.1016/j.jmb.2010.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chookajorn T, et al. Specificity determinants and diversification of the Brassica self-incompatibility pollen ligand. Proc Natl Acad Sci USA. 2004;101(4):911–917. doi: 10.1073/pnas.2637116100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Okuda S, et al. Defensin-like polypeptide LUREs are pollen tube attractants secreted from synergid cells. Nature. 2009;458(7236):357–362. doi: 10.1038/nature07882. [DOI] [PubMed] [Google Scholar]
- 8.Sachkova MY, et al. Genes and evolution of two-domain toxins from lynx spider venom. FEBS Lett. 2014;588(5):740–745. doi: 10.1016/j.febslet.2014.01.018. [DOI] [PubMed] [Google Scholar]
- 9.Sunagar K, et al. Evolution stings: the origin and diversification of scorpion toxin peptide scaffolds. Toxins. 2013;5(12):2456–2487. doi: 10.3390/toxins5122456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Whittington C, Belov K. Platypus venom: a review. Aust Mammal. 2007;29(1):57–62. doi: 10.1071/AM07006. [DOI] [Google Scholar]
- 11.Zhao Q, Chae YK, Markley JL. NMR solution structure of ATT(p), an Arabidopsis thaliana trypsin inhibitor. Biochemistry. 2002;41(41):12284–12296. doi: 10.1021/bi025702a. [DOI] [PubMed] [Google Scholar]
- 12.Tysoe C, et al. Potent human alpha-amylase inhibition by the beta-defensin-like protein helianthamide. ACS Cent Sci. 2016;2(3):154–161. doi: 10.1021/acscentsci.5b00399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shafee TMA, et al. The defensins consist of two independent, convergent protein superfamilies. Mol Biol Evol. 2016;33(9):2345–2356. doi: 10.1093/molbev/msw106. [DOI] [PubMed] [Google Scholar]
- 14.Seebah S, et al. Defensins knowledgebase: a manually curated database and information source focused on the defensins family of antimicrobial peptides. Nucleic Acids Res. 2007;35:D265–D268. doi: 10.1093/nar/gkl866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zuo YC, et al. iDPF-PseRAAAC: a web-server for identifying the defensin peptide family and subfamily using pseudo reduced amino acid alphabet composition. PLoS One. 2015;10(12):e0145541. doi: 10.1371/journal.pone.0145541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang G, Li X, Wang Z. APD3: the antimicrobial peptide database as a tool for research and education. Nucleic Acids Res. 2016;44:D1087–D1093. doi: 10.1093/nar/gkv1278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang GS, Li X, Wang Z. APD2: the updated antimicrobial peptide database and its application in peptide design. Nucleic Acids Res. 2009;37:D933–D937. doi: 10.1093/nar/gkn823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang Z, Wang GS. APD: the antimicrobial peptide database. Nucleic Acids Res. 2004;32:D590–D592. doi: 10.1093/nar/gkh025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Thomas S, et al. CAMP: a useful resource for research on antimicrobial peptides. Nucleic Acids Res. 2010;38:D774–D780. doi: 10.1093/nar/gkp1021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Xiao X, et al. iAMP-2L: a two-level multi-label classifier for identifying antimicrobial peptides and their functional types. Anal Biochem. 2013;436(2):168–177. doi: 10.1016/j.ab.2013.01.019. [DOI] [PubMed] [Google Scholar]
- 21.Zhao X, et al. LAMP: a database linking antimicrobial peptides. PLoS One. 2013;8(6):e66557. doi: 10.1371/journal.pone.0066557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hammami R, et al. PhytAMP: a database dedicated to antimicrobial plant peptides. Nucleic Acids Res. 2009;37:D963–D968. doi: 10.1093/nar/gkn655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Piotto SP, et al. YADAMP: yet another database of antimicrobial peptides. Int J Antimicrob Ag. 2012;39(4):346–351. doi: 10.1016/j.ijantimicag.2011.12.003. [DOI] [PubMed] [Google Scholar]
- 24.He QY, et al. ATDB: a uni-database platform for animal toxins. Nucleic Acids Res. 2008;36:D293–D297. doi: 10.1093/nar/gkm832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tassanakajon A, Somboonwiwat K, Amparyup P. Sequence diversity and evolution of antimicrobial peptides in invertebrates. Dev Comp Immunol. 2015;48(2):324–341. doi: 10.1016/j.dci.2014.05.020. [DOI] [PubMed] [Google Scholar]
- 26.van der Weerden NL, Anderson MA. Plant defensins: common fold, multiple functions. Fungal Biol Rev. 2013;26(4):121–131. doi: 10.1016/j.fbr.2012.08.004. [DOI] [Google Scholar]
- 27.Wu J, Gao B, Zhu S. The fungal defensin family enlarged. Pharmaceuticals. 2014;7(8):866–880. doi: 10.3390/ph7080866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gerdol M, et al. Big defensins and mytimacins, new AMP families of the Mediterranean mussel Mytilus galloprovincialis . Dev Comp Immunol. 2012;36(2):390–399. doi: 10.1016/j.dci.2011.08.003. [DOI] [PubMed] [Google Scholar]
- 29.Putnam NH, et al. The amphioxus genome and the evolution of the chordate karyotype. Nature. 2008;453(7198):1064–1072. doi: 10.1038/nature06967. [DOI] [PubMed] [Google Scholar]
- 30.Teng L, Gao B, Zhang SC. The first chordate big defensin: identification, expression and bioactivity. Fish Shellfish Immun. 2012;32(4):572–577. doi: 10.1016/j.fsi.2012.01.007. [DOI] [PubMed] [Google Scholar]
- 31.Hughes AL, Friedman R. Shedding genomic ballast: extensive parallel loss of ancestral gene families in animals. J Mol Evol. 2004;59(6):827–833. doi: 10.1007/s00239-004-0115-7. [DOI] [PubMed] [Google Scholar]
- 32.Crisp A, et al. Expression of multiple horizontally acquired genes is a hallmark of both vertebrate and invertebrate genomes. Genome Biol. 2015;16(1):50. doi: 10.1186/s13059-015-0607-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Melnikov A, et al. Comprehensive mutational scanning of a kinase in vivo reveals substrate-dependent fitness landscapes. Nucleic Acids Res. 2014;42(14):e112. doi: 10.1093/nar/gku511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhu S. Evidence for myxobacterial origin of eukaryotic defensins. Immunogenetics. 2007;59(12):949–954. doi: 10.1007/s00251-007-0259-x. [DOI] [PubMed] [Google Scholar]
- 35.Zhu S, et al. Dermatophytic defensin with antiinfective potential. Proc Natl Acad Sci USA. 2012;109(22):8495–8500. doi: 10.1073/pnas.1201263109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yang YS, et al. Solution structure and activity of the synthetic four-disulfide bond Mediterranean mussel defensin (MGD-1) Biochemistry. 2000;39(47):14436–14447. doi: 10.1021/bi0011835. [DOI] [PubMed] [Google Scholar]
- 37.Tian C, et al. Antimicrobial peptide-like genes in Nasonia vitripennis: a genomic perspective. BMC Genom. 2010;11:187. doi: 10.1186/1471-2164-11-187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ceraul SM, et al. An arthropod defensin expressed by the hemocytes of the American dog tick, Dermacentor variabilis (Acari: Ixodidae) Insect Biochem Mol Biol. 2003;33(11):1099–1103. doi: 10.1016/S0965-1748(03)00122-X. [DOI] [PubMed] [Google Scholar]
- 39.Zhao H, et al. A defensin-like antimicrobial peptide from the venoms of spider, Ornithoctonus hainana . J Pept Sci. 2011;17(7):540–544. doi: 10.1002/psc.1370. [DOI] [PubMed] [Google Scholar]
- 40.Cociancich S, et al. Purification and characterization of a scorpion defensin, a 4 kDa antibacterial peptide presenting structural similarities with insect defensins and scorpion toxins. Biochem Biophys Res Commun. 1993;194(1):17–22. doi: 10.1006/bbrc.1993.1778. [DOI] [PubMed] [Google Scholar]
- 41.Blanc E, et al. Solution structure of two new toxins from the venom of the Chinese scorpion Buthus martensi Karsch blockers of potassium channels. Biochemistry. 1998;37(36):12412–12418. doi: 10.1021/bi9809371. [DOI] [PubMed] [Google Scholar]
- 42.Lay FT, Brugliera F, Anderson MA. Isolation and properties of floral defensins from ornamental tobacco and petunia. Plant Physiol. 2003;131(3):1283–1293. doi: 10.1104/pp.102.016626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Caldwell JE, et al. Solution structure of the thermostable sweet-tasting protein brazzein. Nat Struct Biol. 1998;5(6):427–431. doi: 10.1038/nsb0698-427. [DOI] [PubMed] [Google Scholar]
- 44.Peng K, et al. Molecular characterization and immune analysis of a defensin from freshwater pearl mussel, Hyriopsis schlegelii . Aquaculture. 2012;334:45–50. doi: 10.1016/j.aquaculture.2011.12.039. [DOI] [Google Scholar]
- 45.Landon C, et al. Solution structure of drosomycin, the first inducible antifungal protein from insects. Protein Sci. 1997;6(9):1878–1884. doi: 10.1002/pro.5560060908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Patil A, Hughes AL, Zhang GL. Rapid evolution and diversification of mammalian alpha-defensins as revealed by comparative analysis of rodent and primate genes. Physiol Genomics. 2004;20(1):1–11. doi: 10.1152/physiolgenomics.00150.2004. [DOI] [PubMed] [Google Scholar]
- 47.Semple CA, et al. The changing of the guard: molecular diversity and rapid evolution of beta-defensins. Mol Divers. 2006;10(4):575–584. doi: 10.1007/s11030-006-9031-7. [DOI] [PubMed] [Google Scholar]
- 48.Silverstein KAT, et al. Genome organization of more than 300 defensin-like genes in Arabidopsis . Plant Physiol. 2005;138(2):600–610. doi: 10.1104/pp.105.060079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Linzmeier RM, Ganz T. Human defensin gene copy number polymorphisms: comprehensive analysis of independent variation in alpha- and beta-defensin regions at 8p22-p23. Genomics. 2005;86(4):423–430. doi: 10.1016/j.ygeno.2005.06.003. [DOI] [PubMed] [Google Scholar]
- 50.Mergaert P, et al. A novel family in Medicago truncatula consisting of more than 300 nodule-specific genes coding for small, secreted polypeptides with conserved cysteine motifs. Plant Physiol. 2003;132(1):161–173. doi: 10.1104/pp.102.018192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Tesfaye M, et al. Spatio-temporal expression patterns of Arabidopsis thaliana and Medicago truncatula defensin-like genes. PLoS One. 2013;8(3):e58992. doi: 10.1371/journal.pone.0058992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zhang JZ. Evolution by gene duplication: an update. Trends Ecol Evol. 2003;18(6):292–298. doi: 10.1016/S0169-5347(03)00033-8. [DOI] [Google Scholar]
- 53.Nei M, Gu X, Sitnikova T. Evolution by the birth-and-death process in multigene families of the vertebrate immune system. Proc Natl Acad Sci USA. 1997;94(15):7799–7806. doi: 10.1073/pnas.94.15.7799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ding N, et al. Association of beta-defensin gene copy number variations with ankylosing spondylitis in Chinese population: a case-control study. Mod Rheumatol. 2016;26(1):146–150. doi: 10.3109/14397595.2015.1056930. [DOI] [PubMed] [Google Scholar]
- 55.Stuart PE, et al. Association of beta-defensin copy number and psoriasis in three cohorts of European origin. J Invest Dermatol. 2012;132(10):2407–2413. doi: 10.1038/jid.2012.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hollox EJ, et al. Psoriasis is associated with increased beta-defensin genomic copy number. Nat Genet. 2008;40(1):23–25. doi: 10.1038/ng.2007.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Fellermann K, et al. A chromosome 8 gene-cluster polymorphism with low human beta-defensin 2 gene copy number predisposes to Crohn disease of the colon. Am J Hum Genet. 2006;79(3):439–448. doi: 10.1086/505915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Bentley RW, et al. Association of higher DEFB4 genomic copy number with Crohn’s disease. Am J Gastroenterol. 2010;105(2):354–359. doi: 10.1038/ajg.2009.582. [DOI] [PubMed] [Google Scholar]
- 59.Hardwick RJ, et al. Beta-defensin genomic copy number is associated with HIV load and immune reconstitution in sub-saharan africans. J Infect Dis. 2012;206(7):1012–1019. doi: 10.1093/infdis/jis448. [DOI] [PubMed] [Google Scholar]
- 60.Abe S, et al. Copy number variation of the antimicrobial-gene, defensin beta 4, is associated with susceptibility to cervical cancer. J Hum Genet. 2013;58(5):250–253. doi: 10.1038/jhg.2013.7. [DOI] [PubMed] [Google Scholar]
- 61.Wilson CL, et al. Differential processing of alpha- and beta-defensin precursors by matrix metalloproteinase-7 (MMP-7) J Biol Chem. 2009;284(13):8301–8311. doi: 10.1074/jbc.M809744200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Lay FT, Anderson MA. Defensins—components of the innate immune system in plants. Curr Protein Pept Sci. 2005;6(1):85–101. doi: 10.2174/1389203053027575. [DOI] [PubMed] [Google Scholar]
- 63.Satchell DP, et al. Interactions of mouse Paneth cell alpha-defensins and alpha-defensin precursors with membranes. Prosegment inhibition of peptide association with biomimetic membranes. J Biol Chem. 2003;278(16):13838–13846. doi: 10.1074/jbc.M212115200. [DOI] [PubMed] [Google Scholar]
- 64.Valore EV, Ganz T. Posttranslational processing of defensins in immature human myeloid cells. Blood. 1992;79(6):1538–1544. [PubMed] [Google Scholar]
- 65.Wu Z, et al. From pro defensins to defensins: synthesis and characterization of human neutrophil pro alpha-defensin-1 and its mature domain. J Pept Res. 2003;62(2):53–62. doi: 10.1034/j.1399-3011.2003.00068.x. [DOI] [PubMed] [Google Scholar]
- 66.Selsted ME, Ouellette AJ. Mammalian defensins in the antimicrobial immune response. Nat Immunol. 2005;6(6):551–557. doi: 10.1038/ni1206. [DOI] [PubMed] [Google Scholar]
- 67.Hughes AL, Yeager M. Coordinated amino acid changes in the evolution of mammalian defensins. J Mol Evol. 1997;44(6):675–682. doi: 10.1007/PL00006191. [DOI] [PubMed] [Google Scholar]
- 68.Zhu SY. Discovery of six families of fungal defensin-like peptides provides insights into origin and evolution of the CS alpha beta defensins. Mol Immunol. 2008;45(3):828–838. doi: 10.1016/j.molimm.2007.06.354. [DOI] [PubMed] [Google Scholar]
- 69.Ayabe T, et al. Activation of Paneth cell alpha-defensins in mouse small intestine. J Biol Chem. 2002;277(7):5219–5228. doi: 10.1074/jbc.M109410200. [DOI] [PubMed] [Google Scholar]
- 70.Liu L, Ganz T. The pro region of human neutrophil defensin contains a motif that is essential for normal subcellular sorting. Blood. 1995;85(4):1095–1103. [PubMed] [Google Scholar]
- 71.Michaelson D, et al. Cationic defensins arise from charge-neutralized propeptides—a mechanism for avoiding leukocyte autocytotoxicity. J Leukocyte Biol. 1992;51(6):634–639. doi: 10.1002/jlb.51.6.634. [DOI] [PubMed] [Google Scholar]
- 72.Valore EV, et al. Intramolecular inhibition of human defensin HNP-1 by its propiece. J Clin Invest. 1996;97(7):1624–1629. doi: 10.1172/JCI118588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Lay FT, et al. The C-terminal propeptide of a plant defensin confers cytoprotective and subcellular targeting functions. BMC Plant Biol. 2014;14:41. doi: 10.1186/1471-2229-14-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Dimarcq JL, et al. Cysteine-rich antimicrobial peptides in invertebrates. Biopolymers. 1998;47(6):465–477. doi: 10.1002/(SICI)1097-0282(1998)47:6<465::AID-BIP5>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- 75.Ghosh D, et al. Paneth cell trypsin is the processing enzyme for human defensin-5. Nat Immunol. 2002;3(6):583–590. doi: 10.1038/ni797. [DOI] [PubMed] [Google Scholar]
- 76.Harwig SSL, Park ASK, Lehrer RI. Characterization of defensin precursors in mature human neutrophils. Blood. 1992;79(6):1532–1537. [PubMed] [Google Scholar]
- 77.Cunliffe RN, et al. Human defensin 5 is stored in precursor form in normal Paneth cells and is expressed by some villous epithelial cells and by metaplastic Paneth cells in the colon in inflammatory bowel disease. Gut. 2001;48(2):176–185. doi: 10.1136/gut.48.2.176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Porter EM, et al. Localization of human intestinal defensin 5 in Paneth cell granules. Infect Immun. 1997;65(6):2389–2395. doi: 10.1128/iai.65.6.2389-2395.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Porter EM, et al. Broad-spectrum antimicrobial activity of human intestinal defensin 5. Infect Immun. 1997;65(6):2396–2401. doi: 10.1128/iai.65.6.2396-2401.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Ouellette AJ, Selsted ME. Paneth cell defensins: endogenous peptide components of intestinal host defense. FASEB J. 1996;10(11):1280–1289. doi: 10.1096/fasebj.10.11.8836041. [DOI] [PubMed] [Google Scholar]
- 81.Selsted ME, et al. Enteric defensins—antibiotic peptide components of intestinal host defense. J Cell Biol. 1992;118(4):929–936. doi: 10.1083/jcb.118.4.929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Wilson CL, et al. Regulation of intestinal alpha-defensin activation by the metalloproteinase matrilysin in innate host defense. Science. 1999;286(5437):113–117. doi: 10.1126/science.286.5437.113. [DOI] [PubMed] [Google Scholar]
- 83.Li DY, et al. Evolution of primate alpha and theta defensins revealed by analysis of genomes. Mol Biol Rep. 2014;41(6):3859–3866. doi: 10.1007/s11033-014-3253-z. [DOI] [PubMed] [Google Scholar]
- 84.Leonova L, et al. Circular minidefensins and posttranslational generation of molecular diversity. J Leukocyte Biol. 2001;70(3):461–464. [PubMed] [Google Scholar]
- 85.Tang YQ, et al. A cyclic antimicrobial peptide produced in primate leukocytes by the ligation of two truncated alpha-defensins. Science. 1999;286(5439):498–502. doi: 10.1126/science.286.5439.498. [DOI] [PubMed] [Google Scholar]
- 86.Garcia AE, et al. Isolation, synthesis, and antimicrobial activities of naturally occurring theta-defensin isoforms from baboon leukocytes. Infect Immun. 2008;76(12):5883–5891. doi: 10.1128/IAI.01100-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Tran D, et al. Homodimeric theta-defensins from Rhesus macaque leukocytes—isolation, synthesis, antimicrobial activities, and bacterial binding properties of the cyclic peptides. J Biol Chem. 2002;277(5):3079–3084. doi: 10.1074/jbc.M109117200. [DOI] [PubMed] [Google Scholar]
- 88.Gallo RL, Hooper LV. Epithelial antimicrobial defence of the skin and intestine. Nat Rev Immunol. 2012;12(7):503–516. doi: 10.1038/nri3228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Ayabe T, et al. Secretion of microbicidal alpha-defensins by intestinal Paneth cells in response to bacteria. Nat Immunol. 2000;1(2):113–118. doi: 10.1038/77783. [DOI] [PubMed] [Google Scholar]
- 90.Shafee TMA, et al. Structural homology guided alignment of cysteine rich proteins. Springer Plus. 2016;5(1):27. doi: 10.1186/s40064-015-1609-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Rose GD, et al. Hydrophobicity of amino acid residues in globular proteins. Science. 1985;229(4716):834–838. doi: 10.1126/science.4023714. [DOI] [PubMed] [Google Scholar]
- 92.Zhu SY, et al. Experimental conversion of a defensin into a neurotoxin: implications for origin of toxic function. Mol Biol Evol. 2014;31(3):546–559. doi: 10.1093/molbev/msu038. [DOI] [PubMed] [Google Scholar]
- 93.Banerjee A et al (2013) Structure of a pore-blocking toxin in complex with a eukaryotic voltage-dependent K+ channel. eLife 2:e00594 [DOI] [PMC free article] [PubMed]
- 94.Poon IKH et al (2014) Phosphoinositide-mediated oligomerization of a defensin induces cell lysis. eLlife 3:e01808 [DOI] [PMC free article] [PubMed]
- 95.Conibear AC, et al. The cyclic cystine ladder in θ-defensins is important for structure and stability, but not antibacterial activity. J Biol Chem. 2013;288(15):10830–10840. doi: 10.1074/jbc.M113.451047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Chan YS, Ng TB. Northeast red beans produce a thermostable and pH-stable defensin-like peptide with potent antifungal activity. Cell Biochem Biophys. 2013;66(3):637–648. doi: 10.1007/s12013-012-9508-1. [DOI] [PubMed] [Google Scholar]
- 97.Zou J, et al. Discovery of multiple beta-defensin like homologues in teleost fish. Mol Immunol. 2007;44(4):638–647. doi: 10.1016/j.molimm.2006.01.012. [DOI] [PubMed] [Google Scholar]
- 98.Bloom JD, et al. Protein stability promotes evolvability. Proc Natl Acad Sci USA. 2006;103(15):5869–5874. doi: 10.1073/pnas.0510098103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Janssen BJC, et al. Structure of Petunia hybrida defensin 1, a novel plant defensin with five disulfide bonds. Biochemistry. 2003;42(27):8214–8222. doi: 10.1021/bi034379o. [DOI] [PubMed] [Google Scholar]
- 100.Lay FT, et al. Dimerization of plant defensin NaD1 enhances its antifungal activity. J Biol Chem. 2012;287(24):19961–19972. doi: 10.1074/jbc.M111.331009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Baxter AA, et al. The tomato defensin TPP3 binds phosphatidylinositol (4,5)-bisphosphate via a conserved dimeric cationic grip conformation to mediate cell lysis. Mol Cell Biol. 2015;35(11):1964–1978. doi: 10.1128/MCB.00282-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Hoover DM, et al. The structure of human beta-defensin-2 shows evidence of higher order oligomerization. J Biol Chem. 2000;275(42):32911–32918. doi: 10.1074/jbc.M006098200. [DOI] [PubMed] [Google Scholar]
- 103.Hill CP, et al. Crystal structure of defensin HNP-3, an amphiphilic dimer: mechanisms of membrane permeabilization. Science. 1991;251(5000):1481–1485. doi: 10.1126/science.2006422. [DOI] [PubMed] [Google Scholar]
- 104.Szyk A, et al. Crystal structures of human alpha-defensins HNP4, HD5, and HD6. Protein Sci. 2006;15(12):2749–2760. doi: 10.1110/ps.062336606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Pazgier M, et al. Human beta-defensins. Cell Mol Life Sci. 2006;63(11):1294–1313. doi: 10.1007/s00018-005-5540-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Suresh A, Verma C. Modelling study of dimerization in mammalian defensins. BMC Bioinformatics. 2006;7(Suppl 5):S17. doi: 10.1186/1471-2105-7-S5-S17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Bauer F, et al. Structure determination of human and murine beta-defensins reveals structural conservation in the absence of significant sequence similarity. Protein Sci. 2001;10(12):2470–2479. doi: 10.1110/ps.ps.24401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Sagaram US, et al. Structural and functional studies of a phosphatidic acid-binding antifungal plant defensin MtDef4: identification of an RGFRRR motif governing fungal cell entry. PLoS One. 2013;8(12):e82485. doi: 10.1371/journal.pone.0082485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.De Paula VS, Pomin VH, Valente AP. Unique properties of human beta-defensin 6 (hBD6) and glycosaminoglycan complex: sandwich-like dimerization and competition with the chemokine receptor 2 (CCR2) binding site. J Biol Chem. 2014;289(33):22969–22979. doi: 10.1074/jbc.M114.572529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Pardi A, et al. NMR studies of defensin antimicrobial peptides. 2. Three-dimensional structures of rabbit NP-2 and human HNP-1. Biochemistry. 1992;31(46):11357–11364. doi: 10.1021/bi00161a013. [DOI] [PubMed] [Google Scholar]
- 111.Wimley WC, Selsted ME, White SH. Interactions between human defensins and lipid bilayers: evidence for formation of multimeric pores. Protein Sci. 1994;3(9):1362–1373. doi: 10.1002/pro.5560030902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Chu H, et al. Human alpha-defensin 6 promotes mucosal innate immunity through self-assembled peptide nanonets. Science. 2012;337(6093):477–481. doi: 10.1126/science.1218831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Cociancich S, et al. Insect defensin, an inducible antibacterial peptide, forms voltage-dependent channels in Micrococcus luteus . J Biol Chem. 1993;268(26):19239–19245. [PubMed] [Google Scholar]
- 114.Aerts AM, et al. The antifungal plant defensin HsAFP1 from Heuchera sanguinea induces apoptosis in Candida albicans . Front Microbiol. 2011;2:47. doi: 10.3389/fmicb.2011.00047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Lu W, de Leeuw E. Pro-inflammatory and pro-apoptotic properties of human defensin 5. Biochem Biophys Res Commun. 2013;436(3):557–562. doi: 10.1016/j.bbrc.2013.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Guilhelmelli F, et al. Antibiotic development challenges: the various mechanisms of action of antimicrobial peptides and of bacterial resistance. Front Microbiol. 2013;4:353. doi: 10.3389/fmicb.2013.00353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Thevissen K, et al. Defensins from insects and plants interact with fungal glucosylceramides. J Biol Chem. 2004;279(6):3900–3905. doi: 10.1074/jbc.M311165200. [DOI] [PubMed] [Google Scholar]
- 118.Thomma BP, Cammue BP, Thevissen K. Plant defensins. Planta. 2002;216(2):193–202. doi: 10.1007/s00425-002-0902-6. [DOI] [PubMed] [Google Scholar]
- 119.Hayes BME, et al. Identification and mechanism of action of the plant defensin NaD1 as a new member of the antifungal drug arsenal against Candida albicans . Antimicrob Agents Chemother. 2013;57(8):3667–3675. doi: 10.1128/AAC.00365-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.van der Weerden NL, Hancock REW, Anderson MA. Permeabilization of fungal hyphae by the plant defensin NaD1 occurs through a cell wall-dependent process. J Biol Chem. 2010;285(48):37513–37520. doi: 10.1074/jbc.M110.134882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.van der Weerden NL, Lay FT, Anderson MA. The plant defensin, NaD1, enters the cytoplasm of Fusarium oxysporum hyphae. J Biol Chem. 2008;283(21):14445–14452. doi: 10.1074/jbc.M709867200. [DOI] [PubMed] [Google Scholar]
- 122.Ganz T. The role of antimicrobial peptides in innate immunity. Integr Comp Biol. 2003;43(2):300–304. doi: 10.1093/icb/43.2.300. [DOI] [PubMed] [Google Scholar]
- 123.Ulm H, et al. Antimicrobial host defensins—specific antibiotic activities and innate defense modulation. Front Immunol. 2012;3:249. doi: 10.3389/fimmu.2012.00249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Gonçalves S, et al. Evaluation of the membrane lipid selectivity of the pea defensin Psd1. BBA Biomembranes. 2012;1818(5):1420–1426. doi: 10.1016/j.bbamem.2012.02.012. [DOI] [PubMed] [Google Scholar]
- 125.Ramamoorthy V, et al. Glucosylceramide synthase is essential for alfalfa defensin-mediated growth inhibition but not for pathogenicity of Fusarium graminearum . Mol Microbiol. 2007;66(3):771–786. doi: 10.1111/j.1365-2958.2007.05955.x. [DOI] [PubMed] [Google Scholar]
- 126.Thevissen K, et al. A gene encoding a sphingolipid biosynthesis enzyme determines the sensitivity of Saccharomyces cerevisiae to an antifungal plant defensin from dahlia (Dahlia merckii) Proc Natl Acad Sci USA. 2000;97(17):9531–9536. doi: 10.1073/pnas.160077797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Phan TK, et al. Human β-defensin 3 contains an oncolytic motif that binds PI(4,5)P2 to mediate tumour cell permeabilisation. Oncotarget. 2015 doi: 10.18632/oncotarget.6520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Payne JA, et al. The plant defensin NaD1 introduces membrane disorder through a specific interaction with the lipid, phosphatidylinositol 4,5 bisphosphate. Biochim Biophys Acta. 2016;1858(6):1099–1109. doi: 10.1016/j.bbamem.2016.02.016. [DOI] [PubMed] [Google Scholar]
- 129.Baxter AA, Hulett MD, Poon I. The phospholipid code: a key component of dying cell recognition, tumor progression and host-microbe interactions. Cell Death Differ. 2015;22(12):1893–1905. doi: 10.1038/cdd.2015.122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Schneider T, et al. Plectasin, a fungal defensin, targets the bacterial cell wall precursor lipid II. Science. 2010;328(5982):1168–1172. doi: 10.1126/science.1185723. [DOI] [PubMed] [Google Scholar]
- 131.Schneider T, et al. Plectasin, a fungal defensin antibiotic peptide, targets the bacterial cell wall precursor Lipid II. Int J Med Microbiol. 2009;299:20. [Google Scholar]
- 132.Oeemig JS, et al. Eurocin, a new fungal defensin structure, lipid binding, and its mode of action. J Biol Chem. 2012;287(50):42361–42372. doi: 10.1074/jbc.M112.382028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Gueguen Y, et al. Characterization of a defensin from the oyster Crassostrea gigas—recombinant production, folding, solution structure, antimicrobial activities, and gene expression. J Biol Chem. 2006;281(1):313–323. doi: 10.1074/jbc.M510850200. [DOI] [PubMed] [Google Scholar]
- 134.de Leeuw E, et al. Functional interaction of human neutrophil peptide-1 with the cell wall precursor lipid II. FEBS Lett. 2010;584(8):1543–1548. doi: 10.1016/j.febslet.2010.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Sass V, et al. Human β-defensin 3 inhibits cell wall biosynthesis in staphylococci. Infect Immun. 2010;78(6):2793–2800. doi: 10.1128/IAI.00688-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Smith JG, Nemerow GR. Mechanism of adenovirus neutralization by human alpha-defensins. Cell Host Microbe. 2008;3(1):11–19. doi: 10.1016/j.chom.2007.12.001. [DOI] [PubMed] [Google Scholar]
- 137.Munoz A, et al. Specific domains of plant defensins differentially disrupt colony initiation, cell fusion and calcium homeostasis in Neurospora crassa . Mol Microbiol. 2014;92(6):1357–1374. doi: 10.1111/mmi.12634. [DOI] [PubMed] [Google Scholar]
- 138.Michalek M, et al. Hydramacin-1 in action: scrutinizing the barnacle model. Antimicrob Agents Chemother. 2013;57(7):2955–2966. doi: 10.1128/AAC.02498-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Marrink SJ, et al. Adhesion forces of lipids in a phospholipid membrane studied by molecular dynamics simulations. Biophys J. 1998;74(2):931–943. doi: 10.1016/S0006-3495(98)74016-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Fersht AR. Basis of biological specificity. Trends Biochem Sci. 1984;9(4):145–147. doi: 10.1016/0968-0004(84)90122-1. [DOI] [Google Scholar]
- 141.Lee AG. Lipid-protein interactions in biological membranes: a structural perspective. Biochim Biophys Acta. 2003;1612(1):1–40. doi: 10.1016/S0005-2736(03)00056-7. [DOI] [PubMed] [Google Scholar]
- 142.Gubernator K et al (1995) Structure-based ligand design. In: Computer aided drug design in industrial research. Springer Berlin Heidelberg, pp 61–77
- 143.Cheng HC, et al. Lipid binding in rice nonspecific lipid transfer protein-1 complexes from Oryza sativa . Protein Sci. 2004;13(9):2304–2315. doi: 10.1110/ps.04799704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Grigat J, et al. Chemoattraction of macrophages, T lymphocytes, and mast cells is evolutionarily conserved within the human alpha-defensin family. J Immunol. 2007;179(6):3958–3965. doi: 10.4049/jimmunol.179.6.3958. [DOI] [PubMed] [Google Scholar]
- 145.Semple F, Dorin JR. beta-Defensins: multifunctional modulators of infection, inflammation and more? J Innate Immun. 2012;4(4):337–348. doi: 10.1159/000336619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Silva PM, Goncalves S, Santos NC. Defensins: antifungal lessons from eukaryotes. Front Microbiol. 2014;5:97. doi: 10.3389/fmicb.2014.00097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Soruri A, et al. beta-Defensins chemoattract macrophages and mast cells but not lymphocytes and dendritic cells: CCR6 is not involved. Eur J Immunol. 2007;37(9):2474–2486. doi: 10.1002/eji.200737292. [DOI] [PubMed] [Google Scholar]
- 148.Funderburg, N., et al. Human beta-defensin-3 activates professional antigen-presenting cells via Toll-like receptors 1 and 2. Proc Natl Acad Sci USA. 2007;104(47):18631–18635. doi: 10.1073/pnas.0702130104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Morgera F, et al. Effects on antigen-presenting cells of short-term interaction with the human host defence peptide beta-defensin 2. Biochem J. 2011;436(3):537–546. doi: 10.1042/BJ20101977. [DOI] [PubMed] [Google Scholar]
- 150.Niyonsaba F, Ogawa H, Nagaoka I. Human beta-defensin-2 functions as a chemotactic agent for tumour necrosis factor-alpha-treated human neutrophils. Immunology. 2004;111(3):273–281. doi: 10.1111/j.0019-2805.2004.01816.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Petrov V, et al. Human beta defensin-3 induces chemokines from monocytes and macrophages: diminished activity in cells from HIV-infected persons. Immunology. 2013;140(4):413–420. doi: 10.1111/imm.12148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Charp PA, et al. Inhibition of protein kinase C by defensins, antibiotic peptides from human neutrophils. Biochem Pharmacol. 1988;37(5):951–956. doi: 10.1016/0006-2952(88)90187-6. [DOI] [PubMed] [Google Scholar]
- 153.Lobo DS, et al. Antifungal Pisum sativum defensin 1 interacts with Neurospora crassa cyclin F related to the cell cycle. Biochemistry. 2007;46(4):987–996. doi: 10.1021/bi061441j. [DOI] [PubMed] [Google Scholar]
- 154.Fobis-Loisy I, Ivanov R, Gaude T (2012) The S-locus cysteine-rich protein (SCR): a small peptide with a high impact on the evolution of flowering plants. In: Signaling and communication in plants. Springer-Verlag Berlin Heidelberg, pp 77–92
- 155.Shimosato H, et al. Characterization of the SP11/SCR high-affinity binding site involved in self/nonself recognition in brassica self-incompatibility. Plant Cell. 2007;19(1):107–117. doi: 10.1105/tpc.105.038869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Watanabe M, et al. Highly divergent sequences of the pollen self-incompatibility (S) gene in class-I S haplotypes of Brassica campestris (syn. rapa) L. FEBS Lett. 2000;473(2):139–144. doi: 10.1016/S0014-5793(00)01514-3. [DOI] [PubMed] [Google Scholar]
- 157.Higashiyama T, Takeuchi H. The mechanism and key molecules involved in pollen tube guidance. Annu Rev Plant Biol. 2015;66:393–413. doi: 10.1146/annurev-arplant-043014-115635. [DOI] [PubMed] [Google Scholar]
- 158.Zhu SY, Bosmans F, Tytgat J. Adaptive evolution of scorpion sodium channel toxins. J Mol Evol. 2004;58(2):145–153. doi: 10.1007/s00239-003-2534-2. [DOI] [PubMed] [Google Scholar]
- 159.Ma YB, et al. Extreme diversity of scorpion venom peptides and proteins revealed by transcriptomic analysis: implication for proteome evolution of scorpion venom arsenal. J Proteomics. 2012;75(5):1563–1576. doi: 10.1016/j.jprot.2011.11.029. [DOI] [PubMed] [Google Scholar]
- 160.Vassilevski AA, et al. Spider toxins comprising disulfide-rich and linear amphipathic domains: a new class of molecules identified in the lynx spider Oxyopes takobius . FEBS J. 2013;280(23):6247–6261. doi: 10.1111/febs.12547. [DOI] [PubMed] [Google Scholar]
- 161.Fry BG, et al. Novel venom proteins produced by differential domain-expression strategies in beaded lizards and gila monsters (genus Heloderma) Mol Biol Evol. 2010;27(2):395–407. doi: 10.1093/molbev/msp251. [DOI] [PubMed] [Google Scholar]
- 162.Whittington CM, et al. Expression patterns of platypus defensin and related venom genes across a range of tissue types reveal the possibility of broader functions for OvDLPs than previously suspected. Toxicon. 2008;52(4):559–565. doi: 10.1016/j.toxicon.2008.07.002. [DOI] [PubMed] [Google Scholar]
- 163.Mouhat S, et al. Diversity of folds in animal toxins acting on ion channels. Biochem J. 2004;378:717–726. doi: 10.1042/bj20031860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Peigneur S, et al. Crotamine pharmacology revisited: novel insights based on the inhibition of Kv channels. Toxicon. 2012;60(2):102–103. doi: 10.1016/j.toxicon.2012.04.014. [DOI] [PubMed] [Google Scholar]
- 165.Cohen L, et al. Dissection of the functional surface of an anti-insect excitatory toxin illuminates a putative “hot spot” common to all scorpion beta-toxins affecting Na+ channels. J Biol Chem. 2004;279(9):8206–8211. doi: 10.1074/jbc.M307531200. [DOI] [PubMed] [Google Scholar]
- 166.Wong ESW, Belov K. Venom evolution through gene duplications. Gene. 2012;496(1):1–7. doi: 10.1016/j.gene.2012.01.009. [DOI] [PubMed] [Google Scholar]
- 167.Fry BG, et al. The toxicogenomic multiverse: convergent recruitment of proteins into animal venoms. Annu Rev Genom Hum Gen. 2009;10:483–511. doi: 10.1146/annurev.genom.9.081307.164356. [DOI] [PubMed] [Google Scholar]
- 168.Melo FR, et al. Inhibition of trypsin by cowpea thionin: characterization, molecular modeling, and docking. Proteins. 2002;48(2):311–319. doi: 10.1002/prot.10142. [DOI] [PubMed] [Google Scholar]
- 169.Wijaya R, et al. Defense proteins from seed of Cassia fistula include a lipid transfer protein homologue and a protease inhibitory plant defensin. Plant Sci. 2000;159(2):243–255. doi: 10.1016/S0168-9452(00)00348-4. [DOI] [PubMed] [Google Scholar]
- 170.Bloch C, Richardson M. A new family of small (5 kDa) protein inhibitors of insect alpha-amylases from seeds of sorghum (Sorghum-bicolor (L) Moench) have sequence homologies with wheat gamma-purothionins. FEBS Lett. 1991;279(1):101–104. doi: 10.1016/0014-5793(91)80261-Z. [DOI] [PubMed] [Google Scholar]
- 171.Zhang NY, Jones BL, Tao HP. Purification and characterization of a new class of insect alpha-amylase inhibitors from barley. Cereal Chem. 1997;74(2):119–122. doi: 10.1094/CCHEM.1997.74.2.119. [DOI] [Google Scholar]
- 172.Pelegrini PB, et al. Novel insights on the mechanism of action of alpha-amylase inhibitors from the plant defensin family. Proteins. 2008;73(3):719–729. doi: 10.1002/prot.22086. [DOI] [PubMed] [Google Scholar]
- 173.Kim C, et al. Human α-defensins neutralize anthrax lethal toxin and protect against its fatal consequences. Proc Natl Acad Sci USA. 2005;102(13):4830–4835. doi: 10.1073/pnas.0500508102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Wang W, et al. Retrocyclins kill bacilli and germinating spores of Bacillus anthracis and inactivate anthrax lethal toxin. J Biol Chem. 2006;281(43):32755–32764. doi: 10.1074/jbc.M603614200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Maitra N, Cushman JC. Characterization of a drought-induced soybean cDNA encoding a plant defensin (Accession no. U12150) (PGR98-213) Plant Physiol. 1998;118:1536. doi: 10.1104/pp.106.2.805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Yamada S, Komori T, Imaseki H. cDNA cloning of gamma-thionin from Nicotiana excelsior (accession no. AB005266) (PGR97-131) Plant Physiol. 1997;115:314. [Google Scholar]
- 177.Komori T, Yamada S, Imaseki H. A cDNA clone for gamma-thionin from Nicotiana paniculata (accession no. AB005250) (PGR97-132) Plant Physiol. 1997;115:314. [Google Scholar]
- 178.Gaudet DA, et al. Cold induced expression of plant defensin and lipid transfer protein transcripts in winter wheat. Physiol Plant. 2003;117(2):195–205. doi: 10.1034/j.1399-3054.2003.00041.x. [DOI] [Google Scholar]
- 179.Koike M, et al. A novel plant defensin-like gene of winter wheat is specifically induced during cold acclimation. Biochem Biophys Res Commun. 2002;298(1):46–53. doi: 10.1016/S0006-291X(02)02391-4. [DOI] [PubMed] [Google Scholar]
- 180.Mirouze M, et al. A putative novel role for plant defensins: a defensin from the zinc hyper-accumulating plant, Arabidopsis halleri, confers zinc tolerance. Plant J. 2006;47(3):329–342. doi: 10.1111/j.1365-313X.2006.02788.x. [DOI] [PubMed] [Google Scholar]
- 181.Gaspar D, Veiga AS, Castanho MA. From antimicrobial to anticancer peptides. A review. Front Microbiol. 2013;4:294. doi: 10.3389/fmicb.2013.00294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Mulder KC, et al. Current scenario of peptide-based drugs: the key roles of cationic antitumor and antiviral peptides. Front Microbiol. 2013;4:321. doi: 10.3389/fmicb.2013.00321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Hoskin DW, Ramamoorthy A. Studies on anticancer activities of antimicrobial peptides. Biochim Biophys Acta. 2008;1778(2):357–375. doi: 10.1016/j.bbamem.2007.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Lichtenstein A, et al. In vitro tumor cell cytolysis mediated by peptide defensins of human and rabbit granulocytes. Blood. 1986;68(6):1407–1410. [PubMed] [Google Scholar]
- 185.Aarbiou J, et al. Mechanisms of cell death induced by the neutrophil antimicrobial peptides alpha-defensins and LL-37. Inflamm Res. 2006;55(3):119–127. doi: 10.1007/s00011-005-0062-9. [DOI] [PubMed] [Google Scholar]
- 186.Bullard RS, et al. Functional analysis of the host defense peptide human beta defensin-1: new insight into its potential role in cancer. Mol Immunol. 2008;45(3):839–848. doi: 10.1016/j.molimm.2006.11.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Ghavami S, et al. Brevinin-2R(1) semi-selectively kills cancer cells by a distinct mechanism, which involves the lysosomal-mitochondrial death pathway. J Cell Mol Med. 2008;12(3):1005–1022. doi: 10.1111/j.1582-4934.2008.00129.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Gera JF, Lichtenstein A. Human neutrophil peptide defensins induce single strand DNA breaks in target cells. Cell Immunol. 1991;138(1):108–120. doi: 10.1016/0008-8749(91)90136-Y. [DOI] [PubMed] [Google Scholar]
- 189.Ran S, Thorpe PE. Phosphatidylserine is a marker of tumor vasculature and a potential target for cancer imaging and therapy. Int J Radiat Oncol Biol Phys. 2002;54(5):1479–1484. doi: 10.1016/S0360-3016(02)03928-7. [DOI] [PubMed] [Google Scholar]
- 190.Utsugi T, et al. Elevated expression of phosphatidylserine in the outer membrane leaflet of human tumor cells and recognition by activated human blood monocytes. Cancer Res. 1991;51(11):3062–3066. [PubMed] [Google Scholar]
- 191.Blackhall FH, et al. Heparan sulfate proteoglycans and cancer. Br J Cancer. 2001;85(8):1094–1098. doi: 10.1054/bjoc.2001.2054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Hollingsworth MA, Swanson BJ. Mucins in cancer: protection and control of the cell surface. Nat Rev Cancer. 2004;4(1):45–60. doi: 10.1038/nrc1251. [DOI] [PubMed] [Google Scholar]
- 193.Yamazaki D, Kurisu S, Takenawa T. Regulation of cancer cell motility through actin reorganization. Cancer Sci. 2005;96(7):379–386. doi: 10.1111/j.1349-7006.2005.00062.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Barnett RE, Furcht LT, Scott RE. Differences in membrane fluidity and structure in contact-inhibited and transformed cells. Proc Natl Acad Sci USA. 1974;71(5):1992–1994. doi: 10.1073/pnas.71.5.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Biragyn A, et al. Toll-like receptor 4-dependent activation of dendritic cells by beta-defensin 2. Science. 2002;298(5595):1025–1029. doi: 10.1126/science.1075565. [DOI] [PubMed] [Google Scholar]
- 196.Biragyn A, et al. Mediators of innate immunity that target immature, but not mature, dendritic cells induce antitumor immunity when genetically fused with nonimmunogenic tumor antigens. J Immunol. 2001;167(11):6644–6653. doi: 10.4049/jimmunol.167.11.6644. [DOI] [PubMed] [Google Scholar]
- 197.Lapteva N, et al. Attraction and activation of dendritic cells at the site of tumor elicits potent antitumor immunity. Mol Ther. 2009;17(9):1626–1636. doi: 10.1038/mt.2009.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Wang K, et al. Effect of human beta-defensin-3 on head and neck cancer cell migration using micro-fabricated cell islands. Head Neck Oncol. 2012;4:41. doi: 10.1186/1758-3284-4-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Economopoulou M, et al. Inhibition of pathologic retinal neovascularization by alpha-defensins. Blood. 2005;106(12):3831–3838. doi: 10.1182/blood-2005-03-0889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Chavakis T, et al. Regulation of neovascularization by human neutrophil peptides (alpha-defensins): a link between inflammation and angiogenesis. FASEB J. 2004;18(11):1306–1308. doi: 10.1096/fj.03-1009fje. [DOI] [PubMed] [Google Scholar]
- 201.Doolittle RF. Convergent evolution: the need to be explicit. Trends Biochem Sci. 1994;19(1):15–18. doi: 10.1016/0968-0004(94)90167-8. [DOI] [PubMed] [Google Scholar]
- 202.Stern DL. The genetic causes of convergent evolution. Nat Rev Genet. 2013;14(11):751–764. doi: 10.1038/nrg3483. [DOI] [PubMed] [Google Scholar]
- 203.Gough J. Convergent evolution of domain architectures (is rare) Bioinformatics. 2005;21(8):1464–1471. doi: 10.1093/bioinformatics/bti204. [DOI] [PubMed] [Google Scholar]
- 204.Koonin EV, Wolf YI, Karev GP. The structure of the protein universe and genome evolution. Nature. 2002;420(6912):218–223. doi: 10.1038/nature01256. [DOI] [PubMed] [Google Scholar]
- 205.Buller AR, Townsend CA. Intrinsic evolutionary constraints on protease structure, enzyme acylation, and the identity of the catalytic triad. Proc Natl Acad Sci USA. 2013;110(8):E653–E661. doi: 10.1073/pnas.1221050110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Perry AJ, et al. Convergent evolution of receptors for protein import into mitochondria. Curr Biol. 2006;16(3):221–229. doi: 10.1016/j.cub.2005.12.034. [DOI] [PubMed] [Google Scholar]
- 207.Chemes LB, de Prat-Gay G, Sanchez IE. Convergent evolution and mimicry of protein linear motifs in host-pathogen interactions. Curr Opin Struct Biol. 2015;32:91–101. doi: 10.1016/j.sbi.2015.03.004. [DOI] [PubMed] [Google Scholar]
- 208.Dokholyan NV, Shakhnovich EI. Understanding hierarchical protein evolution from first principles. J Mol Biol. 2001;312(1):289–307. doi: 10.1006/jmbi.2001.4949. [DOI] [PubMed] [Google Scholar]
- 209.Kopec KO, Lupas AN. Beta-propeller blades as ancestral peptides in protein evolution. PLoS One. 2013;8(10):e77074. doi: 10.1371/journal.pone.0077074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Whittington CM, et al. Defensins and the convergent evolution of platypus and reptile venom genes. Genome Res. 2008;18(6):986–994. doi: 10.1101/gr.7149808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Heitz A, Le-Nguyen D, Chiche L. Min-21 and Min-23, the smallest peptides that fold like a cystine-stabilized beta-sheet motif: design, solution structure, and thermal stability. Biochemistry. 1999;38(32):10615–10625. doi: 10.1021/bi990821k. [DOI] [PubMed] [Google Scholar]
- 212.Nicoll AJ, et al. De novo design of a stable N-terminal helical foldamer. Org Biomol Chem. 2005;3(24):4310–4315. doi: 10.1039/b513891d. [DOI] [PubMed] [Google Scholar]
- 213.Tamaoki H, et al. Folding motifs induced and stabilized by distinct cystine frameworks. Protein Eng. 1998;11(8):649–659. doi: 10.1093/protein/11.8.649. [DOI] [PubMed] [Google Scholar]
- 214.Undheim EAB, Mobli M, King GF. Toxin structures as evolutionary tools: using conserved 3D folds to study the evolution of rapidly evolving peptides. BioEssays. 2016;38(6):539–548. doi: 10.1002/bies.201500165. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.