Abstract
Type 2 phosphatidic acid phosphatases (PAP2s) can be either soluble or integral membrane enzymes. In bacteria, integral membrane PAP2s play major roles in the metabolisms of glycerophospholipids, undecaprenyl-phosphate (C55-P) lipid carrier and lipopolysaccharides. By in vivo functional experiments and biochemical characterization we show that the membrane PAP2 coded by the Bacillus subtilis yodM gene is the principal phosphatidylglycerol phosphate (PGP) phosphatase of B. subtilis. We also confirm that this enzyme, renamed bsPgpB, has a weaker activity on C55-PP. Moreover, we solved the crystal structure of bsPgpB at 2.25 Å resolution, with tungstate (a phosphate analog) in the active site. The structure reveals two lipid chains in the active site vicinity, allowing for PGP substrate modeling and molecular dynamic simulation. Site-directed mutagenesis confirmed the residues important for substrate specificity, providing a basis for predicting the lipids preferentially dephosphorylated by membrane PAP2s.
Electronic supplementary material
The online version of this article (doi:10.1007/s00018-017-2464-6) contains supplementary material, which is available to authorized users.
Keywords: Membrane protein structure, Bacterial lipids metabolism, Undecaprenyl phosphate, Peptidoglycan-related lipid
Introduction
The type 2 phosphatidic acid phosphatases (PAP2s) constitute a diverse family of enzymes found in the three kingdoms of life. Some are soluble proteins (class A nonspecific acid phosphatases, NSAPs), others are integral membrane proteins (lipid phosphatase/phosphotransferase family) [1]. PAP2s dephosphorylate a variety of compounds such as lipids [2] and carbohydrates [3, 4]. In addition, some soluble PAP2s bind a vanadate prosthetic group to their catalytic histidine to gain a haloperoxidase activity [5]. These activities play a role in a wide range of processes such as blood glucose homeostasis [3], energy storage [6], protein glycosylation, large carbohydrate and lipid biosynthesis [2], bacterial virulence [4], environmental adaptation [7] or vesicular trafficking, secretion, and endocytosis [8].
In bacteria, transmembrane PAP2s catalyze steps in the metabolism of the three major types of lipids: the membrane glycerophospholipids, the undecaprenyl-phosphate (C55-P) lipid carrier and the lipopolysaccharides (LPS).
The membrane glycerophospholipids are involved in several processes such as permeability of hydrophobic molecules, active solute transport and protein–protein interactions. Bacteria can modify their phospholipids composition to adapt to environmental changes or escape the host innate immune system [9]. Modifications in phospholipids composition also disturb cellular processes such as stress response, antimicrobial resistance and virulence [10, 11]. In Gram-negative bacteria, such as Escherichia coli, the major phospholipids are phosphatidylethanolamine, phosphatidylglycerol (PG) and cardiolipin (CL) [12], while in Gram-positive species such as Staphylococcus aureus and Bacillus subtilis, the major phospholipids include PG, lysyl-phosphatidylglycerol (L-PG) and CL [11, 13]. Of these lipids, PG is especially important because of its abundance and because it is an intermediate in CL and L-PG synthesis. It is also involved in the metabolism of membrane-derived oligosaccharides [14], the major murein lipoproteins and wall teichoic acids [15]. The biosynthesis of these glycerophospholipids is well described in E. coli [12], and one PAP2 enzyme, PgpB, is involved in the dephosphorylation of phosphatidylglycerol phosphate (PGP) to PG [16]. PgpA and PgpC are two additional redundant enzymes of unrelated fold sharing the same function, and the co-inactivation of the three PGP phosphatases is required to get a lethal phenotype [17]. The equivalent enzymes in other bacterial species have not been clearly identified yet.
C55-P plays a key role in the biogenesis of bacterial cell wall polysaccharides such as peptidoglycan, lipopolysaccharides (LPS) O-antigen and wall teichoic acids [2]. In eukaryotic cells, dolichyl-phosphate, the C55-P equivalent, is involved in protein glycosylation [18]. Undecaprenyl-pyrophosphate (C55-PP), the C55-P precursor, is either synthesized de novo by the undecaprenyl-pyrophosphate synthase UppS [19] or released after each round of polymerization of a cell wall polysaccharide component. C55-PP is the target of bacitracin, a mixture of small cyclic antibiotic peptides produced by certain strains of Bacillus licheniformis [20].
In E. coli, resistance to bacitracin was used to identify BacA, the principal C55-PP phosphatase and the founding member of the BacA/UppP protein family [21]. Three PAP2 proteins with C55-PP phosphatase activity (PgpB, YbjG and LpxT) were later identified [22]. The co-inactivation of multiple C55-PP phosphatase-encoding genes is required to elicit a lethal phenotype, i.e., deletion of bacA together with pgpB and ybjG is lethal and lpxT cannot complement this mutant. This redundancy seems to be shared in the bacterial world as suggested by the BLAST search for BacA and membrane PAP2 enzymes in bacteria, raising the question of the substrate specificities of the different enzymes. PgpB is indeed involved in the synthesis of both PG and C55-P, and LpxT transfers the terminal phosphate group from C55-PP to the lipid A moiety of LPS [7]. LpxE and LpxF from the carcinogenic bacteria Helicobacter pylori are two other examples of PAP2 enzymes involved in LPS metabolism. They dephosphorylate the lipid A at two positions, inducing an increase of the resistance to cationic antimicrobial peptides and a decrease of the recognition of the H. pylori LPS by the host innate immune system [4].
In B. subtilis, two genes, bcrC and yubB, code for two C55-PP phosphatases that belong to the PAP2 and BacA/UppP families, respectively [23]. Recently, it has been reported that the presence of at least one of these two genes is essential for cell survival [24]. The authors also identified the yodM gene encoding a second PAP2 type phosphatase, whose overexpression overcame the lethal effect of the bcrC and yubB deletion.
The low-resolution crystal structure of a transmembrane PAP2, the E. coli PgpB, has been reported in its apo form [25] and in complex with phosphatidylethanolamine [26]. This structure contains the three PAP2 signature motifs [K(X6)RP–PSGH–SR(X5)H(X3)D] and a core helix bundle. Remarkably, the same features are observed in the structures of soluble PAP2s such as the vanadium-dependent haloperoxidases [5, 27] and the bacterial NSAPs [28]. Sequence identity between all PAP2 sub-families is below 20%, resulting in significant structural differences in the loops connecting the core bundle helices as well as in large insertions at various positions. These variations may likely play important roles in substrate specificity and function of the different enzymes. However, the available structural information remains insufficient to cover the wide heterogeneity of substrates and functions of the enzymes of the PAP2 family, especially for the transmembrane members. This makes it difficult to predict which type of lipid is substrate of the various eukaryotic or bacterial transmembrane PAP2s. Additional structural information is also of great interest for the development of new antibiotics targeting these enzymes.
In this work we studied the membrane PAP2 protein of B. subtilis coded by the yodM gene. This includes in vivo functional complementation of the PGP and C55-PP dephosphorylation steps, as well as phosphatase assays on various substrates with membrane extracts or purified protein. All results point to a principal PGP phosphatase activity for this enzyme. The crystal structure of this membrane protein at 2.25 Å resolution suggests, in combination with a molecular dynamic simulation (MDS) and site-directed mutagenesis, the position of PGP in the active site, providing an explanation for the substrate specificity of the enzyme.
Results
Role of the B. subtilis yodM gene product in C55-P and/or PG metabolisms
The B. subtilis strain with the chromosomal copy of yodM deleted (BS502, Online Resource 1), displays the same growth phenotype in rich or minimal media as the parental strain. No increase in bacitracin susceptibility was observed (data not shown), in contrast to a ΔbcrC strain (BS173) that presented an eightfold decrease of its IC50 (50% of growth inhibition concentration) for this antibiotic [29]. Moreover, the overexpression of yodM from an ectopic copy of yodM under the control of an IPTG-inducible promoter (BS525 strain) did not induce any growth defect or increased bacitracin resistance, indicating that the yodM gene product does not compete with bacitracin for the C55-PP binding and does not significantly dephosphorylate C55-PP in vivo.
This is coherent with the recent data form Zhao et al. who found that the basal chromosomal expression of the yodM gene is not sufficient to overcome the lethal effect of bcrC yubB deletion [24]. We further addressed this issue by cloning the bcrC gene on a pMAD vector that possesses a thermosensitive replicon to generate the ΔbcrC ΔyubB double mutant at 30 °C in the presence of the pMAD:bcrC plasmid. This resulting strain (BS533 strain) displayed a thermosensitive phenotype, i.e., was unable to grow above 40 °C. This growth defect was not restored upon yodM overexpression with IPTG.
We also measured the levels of C55-PP and PGP phosphatase activities in membrane protein extracts of the wild-type strain, ΔyodM mutant strain (BS502) and wild-type strain overexpressing yodM (BS525, Online Resource 1). The C55-PP phosphatase-specific activity was very similar in membrane extracts of the wild-type and ΔyodM strains and it was increased by 20-fold in the extracts from the yodM-overexpressing strain (Table 1). In contrast, a 3.5-fold decrease of the PGP phosphatase activity was measured in the membrane extracts of the ΔyodM mutant strain as compared to the wild-type strain and this activity increased by 328-fold upon yodM overexpression (Table 1). These data show that the yodM gene product does not significantly participate to the synthesis of the C55-P lipid carrier through C55-PP dephosphorylation, but contributes to about 70% of the PGP phosphatase activity present in the membrane of B. subtilis (Table 1).
Table 1.
Phosphatase activities measured in B. subtilis membrane extracts
| Strain | C55-PP | PGP | ||
|---|---|---|---|---|
| Specific activity (nmol/min/mg) | Relative activitya | Specific activity (nmol/min/mg) | Relative activitya | |
| B. subtilis 168 | 1.06 ± 0.18 | 1 | 0.84 ± 0.12 | 1 |
| B. subtilis overexpressing yodM (BS525)b | 22.0 ± 2.2 | 20 | 275.6 ± 67.6 | 328 |
| B. subtilis ΔyodM (BS502) | 1.22 ± 0.12 | 1.1 | 0.24 ± 0.04 | 0.29 |
aRelative activities as compared to the wild-type strain membrane protein extracts
b yodM expression was induced by the addition of 1 mM IPTG during exponential growth, 1 h before membrane extraction. Each value is the mean of three independent measurements
Because of the clear contribution of YodM to the dephosphorylation of PGP and because it displays the same three PAP2 conserved motifs as ecPgpB, this protein was renamed bsPgpB (B. subtilis PgpB).
bsPgpB complements both C55-P and PG metabolisms in E. coli
The pTrcHis30:yodM plasmid, allowing the expression of an N-terminally His6-tagged version of bsPgpB under the control of an IPTG-inducible promoter, was used for functional complementation of thermosensitive E. coli mutant strains (BWTsbacA and BWPGPTs strains), which are deficient in C55-PP and PGP dephosphorylation, respectively, above 40 °C. This plasmid perfectly sustained the growth of the BWTsbacA strain at 42 °C in the presence of 0.1 mM IPTG (Table 2), but it did not complement the strain in the absence or in the presence of 1 mM IPTG. For the BWPGPTs strain, the complementation already occurred in the absence of IPTG, indicating that a basal level of yodM expression allows a supply of PG that is appropriate for optimal growth. A lack of growth of all strains containing the plasmid pTrcHis30:yodM, including wild-type E. coli BW25113, was observed in the presence of 1 mM IPTG at 42 °C, likely indicating a strong toxic effect of the bsPgpB overproduction (Table 2).
Table 2.
Complementation of E. coli conditional strains by yodM
| Strain | CFU numeration | |||||
|---|---|---|---|---|---|---|
| −IPTG | +IPTG 1 mM | +IPTG 0.1 mM | ||||
| 30 °C | 42 °C | 30 °C | 42 °C | 30 °C | 42 °C | |
| BWTsbacA | 142 | 0 (0%) | 129 | 0 (0%) | 190 | 152 (80%) |
| BWPGPTs | 401 | 404 (100%) | 566 | 0 (0%) | 502 | 426 (85%) |
| BW25113 | 48 | 46 (96%) | 55 | 0 (0%) | 36 | 47 (130%) |
The two E. coli thermosensitive strains were transformed with the pTrcHis30:yodM plasmid and aliquots were plated onto two ampicillin-containing 2YT agar plates incubated at either 30 °C or 42 °C. The CFU were counted after 24 h incubation. Values in parentheses are ratios between the numbers of clones obtained at 42 °C and 30 °C.
These results show that, in addition to the significant PGP dephosphorylation activity observed in B. subtilis, bsPgpB is sufficiently active on C55-PP to restore the viability of the E. coli cells devoid of the endogenous C55-PP phosphatases.
In vitro substrate specificity of bsPgpB
The recombinant His6-bsPgpB membrane protein was overproduced in C43(DE3) E. coli cells from the pET28d:yodM plasmid and purified to homogeneity. The phosphatase activity of the detergent-solubilized bsPgpB towards different substrates was examined by measuring the amount of released inorganic phosphate with the Malachite Green assay [C15-PP, C5-PP, dioctanoylglycerol pyrophosphate (DGPP) and dioctanoylglycerol phosphate (phosphatidic acid)] or using enzymatically synthesized radiolabeled substrates ([14C]PGP and [14C]C55-PP) with quantification of the released radiolabeled lipids by TLC analysis. bsPgpB dephosphorylates the three isoprenoid compounds (C55-PP, C5-PP and C15-PP) and PGP with increasing specific activities (12.5 ± 3.6, 19.8 ± 0.5, 30.6 ± 3.5 and 54.7 ± 4.6 μmoles/min/mg, respectively), PGP and C55-PP, therefore, being the best and worse tested substrates, respectively. bsPgpB is also able to dephosphorylate DGPP (19.5 ± 4.3 μmoles/min/mg) with an efficiency similar to the one measured with C5-PP. Hydrolysis of phosphatidic acid was not detected when up to 2 µg of pure protein (1000-fold more than for the other substrates) was used per assay.
Interestingly, no further dephosphorylation of C55-P was observed, i.e., no radiolabeled [14C]C55-OH was detectable by TLC analysis under standard assay conditions. Undecaprenol (C55-OH) accounts for a third and half of the undecaprenyl phosphate derivatives in S. aureus [30] and B. subtilis, respectively (data not shown); however, its origin and function are not yet established.
bsPgpB is, therefore, able to dephosphorylate both physiologically important lipids C55-PP and PGP in vitro, with a fourfold preference for the latter, but does not contribute to the production of undecaprenol.
Overall structure of bsPgpB
The structural characterization of bsPgpB was carried on to better understand the determinants of its substrate specificity and mechanism of action. The 2.25 Å resolution structure of bsPgpB was solved using the single wavelength anomalous dispersion (SAD) method at the tungsten absorption edge with a crystal obtained by crystallization in lipid cubic phase in the presence of tungstate, an analog of phosphate. The crystal belonged to the I222 space group and the final Rcryst and Rfree values are 20.1% and 23.1%, respectively (Table 3). The asymmetric unit contains one molecule of bsPgpB. The final model includes residues Met1 to Arg201. The last two residues are missing. The recombinant protein also included an N-terminal His6 tag and a TEV protease cleavage site that are not observed in the electron density map.
Table 3.
Crystallographic data and model refinement statistics
| bsPgpB-W04 | |
|---|---|
| PDB code | 5JKI |
| Data collection | |
| Wavelength (Å) | 1.214580 |
| Space group | I222 |
| a, b, c (Å) | 70.07, 76.96, 99.63 |
| α, β, γ (°) | 90, 90, 90 |
| Resolution range (Å)a | 38.48–2.25 (2.32–2.25) |
| Rmerge (%)a | 13.7 (102) |
| < I >/< σI > a | 11.3 (2.3) |
| Completeness (%)a | 100 (100) |
| Redundancy a | 12.8 (12.9) |
| Refinement | |
| Resolution range (Å) | 38.48–2.25 |
| No. of unique reflections | 13,107 |
| R work (%) | 20.1 |
| R free (%) | 23.1 |
| No. of non-hydrogen atoms | 3401 |
| Number of water molecules | 39 |
| RMS deviations from ideal stereochemistry | |
| Bond lengths (Å) | 0.014 |
| Bond angles (o) | 0.86 |
| Mean B factor (protein) (Å2) | 47.6 |
| Mean B factor (ligand) (Å2) | 46.2 |
| Mean B factor (solvent) (Å2) | 56.3 |
| Ramachandran plot | |
| Favored region (%) | 99 |
| Allowed regions (%) | 1 |
| Outlier regions (%) | 0 |
aValues in parentheses refer to the high-resolution shell
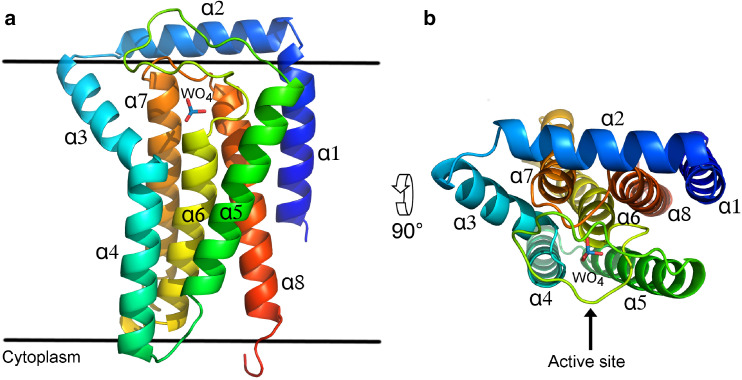
The overall structure of bsPgpB contains eight α-helices (Fig. 1), six of them being tightly packed transmembrane segments (α1, α4–α8). α2 is amphiphilic with its lower hydrophobic face inserted in the membrane and the upper polar face oriented towards the outside of the cell. The α3 helix makes a tilt of approximately 45° compared to the membrane plane and forms a transmembrane segment with α4. The orientation of the protein in the membrane has not been determined experimentally but according to the PredictProtein server [31], the three active site motifs of bsPgpB are located outside of the cell. This is also consistent with the fact that all PAP2 enzymes from E. coli have been experimentally demonstrated to have their active site in the periplasm [32, 33]. The active site of bsPgpB is located at the N-terminal end of helices α4 and α6, surrounded by portions of helices α5, α7 and α8, and covered by the α5α6 loop.
Fig. 1.
Overall structure of bsPgpB. a bsPgpB is shown in cartoon representation with rainbow coloring from the blue N-terminus to the red C-terminus. All α helices are numbered and the tungstate ion in the active site is displayed as sticks. Horizontal black lines represent the membrane boundaries. b Same as a with a 90° rotation around the horizontal axis
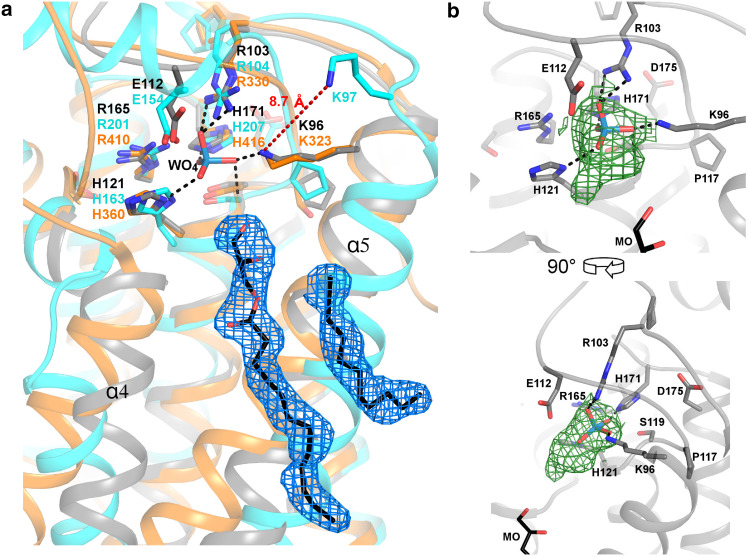
One monoolein molecule coming from the lipid cubic phase was identified below the active site. It runs parallel to helix α4 and covers helix α5. However, only 12 of the 18 carbons could be observed in the density. Four other aliphatic chains between 8 and 11 carbon atoms long were also refined in the model, one of them being parallel to the lipid molecule close to the active site (Fig. 2a). These aliphatic chains derive from other monoolein molecules for which the glycerol moiety could not be identified.
Fig. 2.
bsPgpB active site features. a bsPgpB (gray) is superposed on ecPgpB (pdb code 4PX7) in cyan and Z. galactivorans VHPO (pdb code 4USZ) in orange. Residues conserved in the three PAP2 sequence motifs are displayed as sticks in their respective colors. The monoolein and unidentified aliphatic chain present in the bsPgpB structure are shown in black with the corresponding feature enhanced map (see “Experimental procedures” section) displayed as blue mesh at 1 σ. Black dashed lines represent hydrogen bonds involving tungstate. The red dashed line highlights the 8.7 Å distance between the bsPgpB Lys96 Nζ atom and its counterpart in ecPgpB (Lys97). b bsPgpB is shown as cartoon representation in two different orientations. Carbons in active site residues and monoolein are shown as gray and black sticks, respectively. The composite omit map (green mesh) is displayed at 1 σ around the tungstate ion in the active site of the bsPgpB structure. Tungstate mimics the phosphate removed from the substrates by the bsPgpB enzyme
Differences with available membrane and soluble PAP2 protein structures
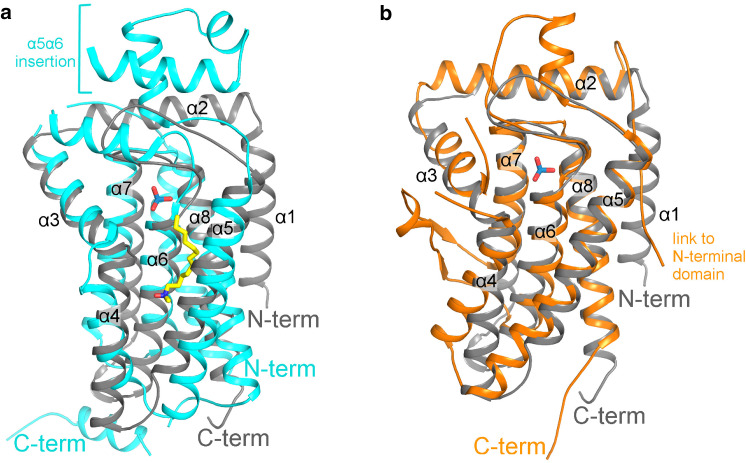
The results of the superposition of bsPgpB to other PAP2 structures available in the Protein Data Bank are summarized in the Online Resource 2. The membrane ecPgpB protein has the highest percentage of sequence identity with bsPgpB (20%), but a relatively poor rms deviation (3.9 Å). Four significant differences separate ecPgpB and bsPgpB (Fig. 3a): at the N-terminus, ecPgpB is missing an amphiphilic helix equivalent to α2, and α1 does not pack at the same position on the core helix bundle; ecPgpB contains an extra C-terminal helix parallel to the membrane plane on the cytoplasmic side; ecPgpB has a 60 amino acid insertion in the α5α6 loop that forms a small periplasmic domain absent in bsPgpB; the ecPgpB helix equivalent to α5 is not tightly packed on the core helix bundle like in bsPgpB.
Fig. 3.
Superposition of bsPgpB with two PAP2 type structures. bsPgpB (gray) is superposed on: a the membrane protein ecPgpB (pdb code 4PX7) in cyan, the LDAO molecule present in the ecPgpB structure being displayed as yellow sticks, b the soluble protein Z. galactanivorans VHPO (pdb code 4USZ) in orange. The VHPO N-terminal domain (residues 22–207) is omitted for clarity
Compared to membrane ecPgpB and bsPgpB, the soluble vanadium haloperoxidases (VHPO) contain an additional N-terminal domain of about 200 amino acids packed on the helices equivalent to α4 and α5 of the core helix bundle and partially covering the active site. The rms deviation between the vanadium iodoperoxidase from Zobellia galactanivorans and bsPgpB is about 2 Å with a sequence identity of 14% (Online Resource 2). Despite the low sequence identity and except for the additional N-terminal domain, this structure has the closest similarity to that of bsPgpB and only diverges with the absence of an equivalent for the N-terminal helix, short insertions in some loops and a tilted α4 (Fig. 3b).
Active site description
The three bsPgpB conserved motifs specific to the PAP2 catalytic site (96K(X6)RP104, 118PSGH121 and 164SR(X5)H(X3)D175) are located at both ends of the α5α6 loop and at the N-terminal end of helix α8, respectively. These motifs superpose very well with their equivalent in the soluble NSAP and VHPO structures (Online Resource 2) [5, 27, 28]. For the membrane ecPgpB, the three motifs also superposed well, except for Lys97 in the first motif that is involved in the hydrolysis of some substrates and has its Nζ located 8.7 Å away from its counterpart Lys96 in bsPgpB (Fig. 2a). This difference is correlated with the shift of the helix α5 bearing this amino acid observed in the ecPgpB structures [25, 26]. bsPgpB and ecPgpB share the presence of a glutamate, Glu112 and Glu154, respectively, six residues before the second motif (Fig. 2a). This residue is conserved in PgpB homologs in 14 out of 30 bacterial phyla/classes (Online Resource 3). This amino acid, recently identified as critical in ecPgpB for the hydrolysis of some substrates but not PGP [26], is located only 3.5 Å away from the tungstate ion in the bsPgpB structure.
The bsPgpB structure was indeed obtained in the presence of tungstate, a phosphate analog that was used for experimental phasing. This compound is found in the active site in close proximity with the Lys96, Arg103, His121 and His171 side chains like the WO4 and PO4 ions seen in two different structures of the soluble Salmonella typhimurium NSAP [28]. However, the electron density seems to extend beyond a WO4 ion, indicating a potential modification, which could not be identified and modeled (Fig. 2b).
Analysis of the bsPgpB surface around the active site reveals a clear groove below the catalytic site. The amino acids involved are located on the transmembrane helices α4 and α5 (Fig. 4). Ser55, Ser56 and Leu59 from α4, and Leu79, Leu82 and Phe85 from α5 define the left side of this groove. The bottom is composed of Leu83, Gly86 and Arg89, and the right side of Leu90 and Lys93; all these residues belonging to the α5 helix. In the bsPgpB structure, two distinct electron densities were observed in this shallow channel and modeled as a monoolein molecule and an unidentified aliphatic chain (Fig. 2a).
Fig. 4.
Modeling of bsPgpB complexes and mutation results. a Surface electrostatic representation of bsPgpB obtained by charge smoothing (PyMOL), with positively and negatively charged regions in blue and red, respectively. The tungstate molecule is displayed as light blue and red sticks. The PGP molecule modeled in the bsPgpB active site is shown in yellow and the monoolein and unidentified aliphatic chain present in the bsPgpB structure are shown in black. b Cartoon representation of bsPgpB (gray). Residues defining the substrate binding site are displayed as sticks. The PGP and C55-PP molecules modeled in the active site are shown with their carbon in yellow and green, respectively. Residues modified by site-directed mutagenesis in this study are underlined in red. c Characterization of bsPgpB mutations in the substrate binding site through phosphatase activity measurements using four different substrates PGP, C55-PP, C15-PP and C5-PP. The activity is represented as a percentage compared to the wild-type enzyme
Modeling of PGP
Because of the striking similarity between PGP, the preferred substrate of bsPgpB, and the two aliphatic chains observed closed to the bsPgpB active site, we manually modeled a PGP molecule in the active site. In this model, the PGP terminal phosphate is placed at the same position as the tungstate ion, and the acylated glycerol is superposed to the monoolein glycerol moiety observed in the structure and the two aliphatic chains. The geometry was then optimized and followed by an energy minimization step (Fig. 4a). The accommodation of this PGP molecule in the active site of bsPgpB only induced a slight displacement of the Arg89 side chain closer to the core helix bundle. This arginine mainly interacts with the PGP phosphate bound to the acylated glycerol and potentially participates in the substrate recognition. However, it is not conserved in the other PgpB homologs (Online Resource 3). A similar procedure was used to model C55-PP in the active site of bsPgpB (Fig. 4b). In this model, the only modification compared to the crystallographic structure is a slight displacement (1.5 Å) of the Arg89 side chain towards Asn92.
A 100-ns molecular dynamic simulation (MDS) was performed to verify the stability of the bsPgpB:PGP complex (Online Movie 4). The simulation shows a good stability of the overall protein fold and the PGP molecule. As expected, the terminal phosphate is tightly bound to the positively charged residues of the three conserved motifs. The interaction between the second phosphate of PGP and Arg89 is also stable throughout the simulation and acts as a second anchor for the substrate. The two aliphatic chains remain inserted in the hydrophobic groove identified by two lipid molecules in bsPgpB structure. Sliding of the two aliphatic chains in the groove and some flexibility of their extremities are, however, observed.
Site-directed mutagenesis of the residues potentially involved in the enzyme specificity
Based on the structure and the MDS, seven mutations of residues defining the hydrophobic groove and the polar pocket adjacent to the catalytic site were created (Fig. 4b). These proteins were purified and enzymatically characterized (Fig. 4c). The mutation of Arg89 into alanine slightly reduced the hydrolysis of PGP. For the Lys93Ala mutant, the PGP hydrolysis was not significantly affected. However, the activity against C55-PP and C15-PP was more than doubled for these two mutants. Asn124, which is located inside the protein next to Arg89, is not directly accessible by the substrate but its mutation into alanine likely allows a displacement of Arg89 toward the core of the protein, inducing an effect similar to the R89A mutation (slight decrease in PGP hydrolysis and almost doubling of C55-PP hydrolysis). The mutation of Ser55, the third residue part of the polar pocket, did not reveal a significant role of this residue in enzyme specificity. The hydrolysis of C5-PP was not affected by these four mutations.
Our attempts to modify the substrate specificity by partially blocking the hydrophobic groove with mutations G86F, L82W and K93W were not successful. The first two mutants behave like the wild-type enzyme and K93W like K93A. However, the L82W mutant displayed a reduced activity against C15-PP and C5-PP, which cannot be explained by an interference with these short substrates but potentially indicates a lower stability of this enzyme.
Distribution of PgpB enzymes in the bacterial kingdom
To identify bsPgpB and ecPgpB homologs in the bacterial kingdom, protein–protein blasts (http://blast.ncbi.nlm.nih.gov/) were performed with these two sequences for 19 bacterial phyla. For the Firmicutes and the Proteobacteria, the search was performed at the class level. At least one homolog of bsPgpB was found in each phylum and class tested except in the Deferribacteres (Online Resource 3). The percentage of sequence identity ranged from 27 to 35% except for the Chlorobi phylum where the sequence identity was only 22%.
For ecPgpB, homologs could only be identified in seven phyla and an insertion in the α5α6 loop was only found in the Acidobacteria, Bacteroidetes and Proteobacteria phylums, with the beta-, gamma-, delta- and epsilon-proteobacteria classes represented (Online Resource 5). The fact that bsPgpB homologs are found in 18 out of the 19 major bacterial phyla tested, while the same blast search using the ecPgpB sequence found homologs with a similar α5α6 loop in only 3 phyla, shows that the structure of bsPgpB is representative of a larger set of bacterial membrane PAP2 enzymes than ecPgpB.
The list of proteins selected is summarized in the Online Resource 6.
Discussion
bsPgpB is the principal B. subtilis PGP phosphatase
Our in vitro assays with the purified protein and B. subtilis membrane extracts show the preference of bsPgpB for the dephosphorylation of PGP over C55-PP. This result is confirmed by the ability of yodM gene to complement the thermosensitive E. coli strain BWPGPTs (ΔpgpA ΔpgpB ΔpgpC) in the absence of induction, while the complementation of the thermosensitive strain BWTsbacA (ΔbacA ΔpgpB ΔybjG) only occurs in induction conditions. In B. subtilis, activity measurements performed with membrane protein extracts show that yodM is responsible for 70% of the PGP dephosphorylation but only contributes significantly to C55-PP hydrolysis when overexpressed (Table 1). This overexpression is not sufficient for in vivo complementation of the B. subtilis ΔyubB ΔbcrC conditional mutant strain. However, Zhao et al. recently observed such a complementation by further increasing the yodM overexpression with a strong ribosome binding site and a different start codon.
bsPgpB is, therefore, a PAP2 phosphatase exhibiting a preference for PGP over C55-PP and is the principal PGP phosphatase in this species (if no soluble protein contributes to this activity, as it is the case in E. coli).
Analysis of the bsPgpB overall structure
The bsPgpB structure is the highest resolution structure for a transmembrane PAP2 protein elucidated so far. The core bundle of helices and the active site architecture of this fold are conserved in all available structures of soluble and membrane PAP2 proteins. The similarity is particularly striking with the soluble VHPO from Z. galactanivorans (Fig. 3b). To our knowledge, this represents the best example of soluble and α-helical membrane proteins with extremely similar folds despite a poor sequence identity. Combined with the broader distribution of bsPgpB homologs in bacterial phyla compared to ecPgpB (Online Resources 3 and 5), this likely indicates that the bsPgpB structure better represents a wider set of the membrane PAP2 enzymes. Based on the sequence identity, this structure is indeed the best model so far for eukaryotic membrane PAP2 enzymes such as human dolichyl pyrophosphate phosphatase (27% sequence identity), human phospholipid phosphatases (PLPP1, PLPP2 and PLPP3 with 27%, 24% and 23% sequence identity, respectively) and human glucose 6 phosphatase (22% sequence identity).
Determinant of the bsPgpB substrate specificity
The bsPgpB structure, with electron densities corresponding to two aliphatic chains observed in the hydrophobic groove located below the active site, suggests the putative positioning of its substrates (Figs. 2, 3). This groove has an ideal width and its surface seems to be made of two shallow channels, a long straight one and a kinked shorter one that can optimally accommodate the two acyl chains of PGP. Although wide enough to accept the isopentenyl units of C55-PP, this groove does not seem to be as tailor-made for this single branched chain substrate (groove too wide and shallow channels too narrow), which could be partially responsible for the higher activity of bsPgpB for PGP.
Between this hydrophobic groove and the active site core occupied by tungstate in the bsPgpB structure lies the second area that may be important for substrate specificity. This region contains three surface residues with polar side chains (Ser55, Arg89 and Lys93). MDS points to Arg89 as the most important residue in terms of accommodating the PGP phosphate bound to the bis-acylated glycerol (Online Movie 4) but mutational analysis shows that Arg89 and Lys93 are both important for substrate specificity. Indeed, the mutation of each residue reduces the polarity of the bsPgpB surface, better matching the characteristics of C55-PP (Fig. 4b), and induces a twofold increase of the activity against this substrate. Together, these two residues likely account for most of the approximately fourfold difference between the PGP and C55-PP hydrolysis rates. On the other hand, Ser55 does not seem to participate in substrate specificity.
In vivo and in membrane protein extracts, the difference between C55-PP and PGP dephosphorylation by bsPgpB seems to be significantly larger than the difference in specific activities measured in vitro. The factors responsible for this effect have not been identified but could, for example, be protein–protein interactions or cell localization.
How similar are E. coli and B. subtilis PgpBs?
In the two ecPgpB structures available, the helix equivalent to α5 of bsPgpB is separated from the rest of the helix bundle by either a detergent molecule in 4PX7 (Fig. 3a) or a phosphatidylethanolamine molecule in 5JWY [25, 26]. Fan et al. and Tong et al. suggest that this different position compared to other PAP2 enzymes is part of an induced fit mechanism that would bring this helix back to its functional position (packed onto the helix bundle) upon binding of most substrates for a canonical PAP2 hydrolysis mechanism. For PGP hydrolysis, active site mutations suggest a possibly different mechanism with a different proton transfer route, but the authors failed to obtain a meaningful docking of PGP based on these structures, suggesting that some protein reorganization is further required.
In our structure of bsPgpB obtained in lipid cubic phase, there is no indication of such mechanisms. The bsPgpB active site configuration is indeed similar to that of all the other soluble PAP2 structures, with Lys96, from the first conserved motif (and located on the α5 helix), binding the tungstate ion present in the active site (Fig. 2a). MDS did also not provide any hint for a similar structural reorganization in bsPgpB. Moreover, thanks to the monoolein fragments and tungstate molecule observed in our structure, we were able to model a functional PGP–bsPgpB complex showing sufficient stability in MDS without the need of any structural reorganization.
Among the various PgpB mutations studied so far, only two can be compared in bsPgpB and ecPgpB. S55A in bsPgpB and Q50A in ecPgpB have similar localization on the proteins surface, and none of these two mutations affects the dephosphorylation of the substrates tested. K93A (or K93W) located in the active site surrounding bsPgpB could be compared to K93A in ecPgpB for the hydrolysis of PGP; K93A does not significantly affect the PGP dephosphorylation by bsPgpB while a 50% increase of activity is observed in ecPgpB [26]. This difference could be the sign of different hydrolysis mechanisms in the two enzymes.
Recent results on ecPgpB and our results on bsPgpB highlight significant differences between the two enzymes. A potential induced fit mechanism has indeed been observed in ecPgpB that could have potential role in the dephosphorylation of PGP and in the regulation of the enzyme by a phosphatidylethanolamine inhibition. In bsPgpB, the results obtained so far do not indicate a similar mechanism. On the contrary, they support a specificity of this enzyme that is dependent on the extension and nature of the polar pocket surrounding the catalytic site and potentially the amino acids defining the size and shape of the hydrophobic grove accommodating the different aliphatic chain(s). In bsPgpB, we indeed identified Arg89 and Lys93 as important residues to favor PGP over C55-PP as substrate. The bsPgpB structure also reveals the hydrophobic grove well suited for the two aliphatic chains of PGP. The 60-amino acid insertion in the α5α6 loop that follows the first motif and forms a small periplasmic domain in ecPgpB (Fig. 3a) could potentially play an important role in the different behaviors of these two closely related enzymes.
Experimental procedures
Chemicals
Detergents n-dodecyl β-d-maltoside (DDM) and cymal-5 were purchased from Anatrace and isopropyl-β-d-thiogalactopyranoside (IPTG) from Eurogentec. Farnesyl-pyrophosphate (C15-PP), isopentenyl-pyrophosphate (C5-PP), glycerol-3′-phosphate, phosphatidic acid (dioctanoylglycerol phosphate), tungstate and antibiotics were from Sigma. [14C]C5-PP and [14C]glycerol-3′-phosphate were purchased from Perkin Elmer Life Sciences. Dioctanoylglycerol pyrophosphate and CDP-diacylglycerol were from Avanti Polar Lipids. The phosphatidylglycerol phosphate (PGP) was synthesized using purified E. coli PgsA enzyme, essentially as described by Lu et al. [17] Briefly, the PGP synthase-encoding gene, pgsA, from E. coli was cloned under the control of T7 IPTG-inducible promoter in vector pTrcHis60 allowing the expression of C-terminal His6-tagged protein [34] (Online Resource 1). The purification of the PgsA membrane protein was performed following the same procedure as used for bsPgpB, yielding mg quantities of pure PgsA enzyme. The [14C]PGP was synthesized by mixing 200 µM CDP-diacylglycerol and 200 µM [14C]glycerol-3′-phosphate (120,000 Bq) in 100 mM Tris–HCl, pH 8.0, 100 mM MgCl2, 1% Triton X-100 and 3 µg of DDM-purified PgsA, in a final volume of 0.5 ml. PGP synthesis was followed by TLC analysis on silica plates using the appropriate mobile phase (chloroform, pyridine, 88% formic acid, water, 50:50:16:5, v/v) allowing the separation of [14C]glycerol-3′-phosphate from [14C]PGP (R f values of 0.14 and 0.68, respectively). A total conversion of glycerol-3′-phosphate into PGP was achieved after 2 h incubation at 37 °C. The PGP was then extracted from the reaction mixture via a Bligh–Dyer extraction procedure [35] before being tested for hydrolysis. Radiolabeled [14C]C55-PP synthesis and purification were performed as described previously [21].
Bacterial strains and growth conditions
The E. coli strains were grown in 2YT or terrific broth (TB) media and B. subtilis strains in Luria–Bertani broth medium. Ampicillin, kanamycin and chloramphenicol were used for E. coli strains at the final concentrations of 100, 60 and 25 µg/ml, respectively. Tetracycline, spectinomycin, kanamycin and erythromycin were used for B. subtilis strains at the final concentrations of 10, 100, 20 and 0.3 µg/ml, respectively.
The yodM, bcrC and yubB B. subtilis single deletion mutants were constructed following the modified long flanking homology-PCR method [36] (Online Resource 1). For its overexpression in B. subtilis, the yodM gene was amplified by PCR using the appropriate pair of primers and cloned in the pDR111 vector at the HindIII and NheI restriction sites. The resulting plasmid was then used to transform B. subtilis 168 wild-type strain, allowing the insertion of the yodM gene at the amyE locus under the control of a strong IPTG-inducible promoter, yielding strains BS525 (Online Resource 1). bcrC was also cloned in the pMAD plasmid containing a thermosensitive replicon in the BamHI site using appropriate primers, leading to BS528 (Online Resource 1). For double and triple mutant strains, the chromosomic DNA of single mutants was sequentially used to transform the BS528 strain (Online Resource 1) cultured at 30 °C and selected with the appropriate antibiotic.
The E. coli-thermosensitive strain BWTsbacA carrying a triple chromosomal gene deletion (ΔbacA ΔybjG ΔpgpB::kanR) and harboring a bacA-expressing plasmid pMAKbacA (CamR) whose replication is impaired at 42 °C has been previously described [22]. The E. coli-thermosensitive strain BWPGPTs displaying a triple chromosomal gene deletion (ΔpgpA ΔpgpB::camR ΔpgpC) and carrying a pgpB-expressing plasmid pMAKkanpgpB (KanR) was constructed for this study as follows. First, the pgpB gene was inactivated by a CamR cassette in the BW25113 strain chromosome by the classical procedure of Datsenko and Wanner [37], using the pKD3 plasmid as template and oligonucleotides inact1-pgpB and inact2-pgpB [22] for the CamR cassette PCR amplification step. This yielded the BW25113 ΔpgpB::camR strain. For the construction of the plasmid pMAKkan:pgpB, the pgpB gene was PCR-amplified from the E. coli chromosome using appropriate oligonucleotides and the PCR product was cleaved by BamHI and PstI, and cloned between the same restriction sites of the plasmid pMAKkan. The latter plasmid was derived from the pMAK705 plasmid [38] (CamR) which bears a thermosensitive replicon, in which the CamR cassette was replaced by the KanR cassette from the pUC4K plasmid vector (Pharmacia). Briefly, the KanR cassette was PCR-amplified from pUC4K and inserted between EcoRI restriction sites of pMAK705. The previously described DMEG5 strain (BW25113 ΔpgpA::camR) [22] was then used, from which the antibiotic resistance cassette was excised by the expression of the Flp recombinase from the pCP20 plasmid, as described previously [37]. Thereafter, the pgpC::kanR mutation from the JW5408 strain of the Keio collection [39] was transferred into the ΔpgpA strain by P1vir transduction. The KanR resistance cassette of the resulting strain BW25113 ΔpgpA ΔpgpC::kanR was subsequently removed via Flp recombinase expression. The ΔpgpA ΔpgpC mutant was transformed by the pMAKkan:pgpB plasmid (KanR) and finally, the pgpB::camR mutation was transduced in this strain, yielding the BWPGPTs strain. The disruption of the three chromosomal genes was verified by PCR using oligonucleotides flanking each encoding regions. As expected [17], the resulting BWPGPTs strain displayed a thermosensitive phenotype, being able to grow normally at 30 °C as compared to the wild-type strain but unable to form colonies above 40 °C.
Construction of yodM-expressing plasmids
For the production of a N-terminally His6-tagged version of bsPgpB under the control of strong IPTG-inducible promoters (T7 or trc) in E. coli strains, the plasmids pET28b:yodM and pTrcHis30:yodM were constructed. The recombinant protein produced from pET28b:yodM plasmid contained a TEV cleavage site following the His6-tag, although the purified recombinant protein was used with the His-tag in place. For the pET28b:yodM construction, the yodM sequence was PCR-amplified from the B. subtilis 168 chromosome, introducing NdeI and XhoI restriction sites at the 5′ and 3′ ends of yodM gene, respectively. After cleavage by the appropriate endonucleases, the PCR product was inserted into the corresponding sites of the expression vector pET28b-MHL (Invitrogen). For pTrcHis30:yodM construction, yodM gene was similarly amplified using restriction site BamHI (5′ end) and HindIII (3′ end), and inserted into pTrcHis30 vector [34].
Functional complementation
The E. coli mutants BWTsbacA and BWPGPTs were transformed by the pTrcHis30:yodM plasmid and aliquots from the transformation mixture were plated onto two ampicillin-2YT agar plates that were then incubated at either 30 °C or 42 °C. After 24-h incubation, the CFU were numerated on each plate and the functional complementation of the thermosensitive mutants was based on the comparison of the CFUs obtained at the two temperatures. Each experiment was reproduced three times with similar results but the numerical values of only one of each experiment is presented in Table 2.
B. subtilis membrane extracts preparation
B. subtilis strains were grown at 37 °C in LB medium (200 ml cultures). For the overexpression of yodM with the BS525 strain, IPTG was added at 1 mM final concentration when the OD600 of the culture had reached 1, and growth was continued for 1 h. Cells were harvested and washed with 30 ml of cold 20 mM Tris–HCl buffer, pH 7.4, containing 0.4 M NaCl, 10 mM β-mercaptoethanol and 10% glycerol. The cells were finally resuspended in 6 ml of the same buffer and disrupted by sonication in the cold. The membranes were then pelleted by centrifugation at 4 °C for 20 min at 200,000×g, washed with the same buffer and subjected to solubilization during 1 h in the cold in the same buffer supplemented with 2% (w/v) DDM. The solubilized proteins were then recovered in the supernatant after centrifugation at 4 °C for 20 min at 200,000×g. The protein concentration was estimated using the absorbance at 280 nm with an absorbance coefficient of 1.
Expression and purification of bsPgpB
E. coli C43(DE3) cells carrying pET28b:yodM were grown in TB medium supplemented with kanamycin (50 µg/ml) until the OD600 reached 0.9. The culture was induced with 1 mM IPTG for 12 h at 20 °C. Cells were harvested by centrifugation at 4 °C for 20 min at 4000×g, and the pellet was resuspended in the lysis buffer (20 mM KH2PO4, pH 6, 400 mM NaCl, 10% glycerol, 2 mM DTT, 2 mM MgSO4, 1.5 U/ml benzonase) before disruption using an Emulsiflex C3 homogenizer. The resulting suspension was centrifuged at 4 °C for 1 h at 100,000×g. The pellet containing the membranes was resuspended in 30 ml of the solubilizing buffer (20 mM KH2PO4, pH 6, 400 mM NaCl, 10% glycerol, 2 mM DTT and 2% DDM). The mixture was incubated for 2 h at 4 °C under gentle agitation before being centrifuged at 4 °C for 1 h at 100,000×g. The recovered supernatant was filtered through a 0.22-µm membrane (Millex-GP, Millipore) before purification. Solubilized membrane proteins were loaded on a Histrap column (GE Healthcare) equilibrated with buffer A (20 mM KH2PO4, pH 6, 400 mM NaCl, 10% glycerol, 2 mM DTT and 0.05% DDM). The column was washed with increasing imidazole concentrations (from 10 to 40 mM), and the elution of proteins was performed with 200 mM of imidazole. After SDS-PAGE analysis, pure fractions were pooled and dialyzed for 2 h against buffer A. The DDM detergent was replaced by cymal-5 using an additional Ni–NTA purification step. Purified proteins were loaded onto a Histrap column (GE Healthcare) equilibrated with buffer A. The column was washed with 50 ml of buffer B (20 mM KH2PO4, pH 6, 400 mM NaCl, 10% glycerol, 2 mM DTT and 7.2 mM cymal-5). Proteins were eluted with 200 mM imidazole in buffer B. Buffer exchange and desalting were performed by dialysis against buffer C (30 mM Na citrate, pH 6, 30 mM NaCl, 20% sucrose, 2 mM DTT and 7.2 mM cymal-5). An additional purification step using a SPHP cation-exchange column was performed to enhance the purity and increase the protein concentration. The column was washed with 20 ml of buffer C and the protein was eluted with a buffer containing 30 mM Na citrate, pH 6, 30 mM NaCl, 500 mM Na2SO4, 5% sucrose, 2 mM DTT and 7.2 mM cymal-5. Desalting of the samples and buffer exchange were carried out using PD-10 desalting column (GE Healthcare) using buffer D (30 mM Na citrate, pH 6, 30 mM NaCl, 200 mM Na2SO4, 5% sucrose, 2 mM DTT and 7.2 mM cymal-5). Each purification step was analyzed by SDS-PAGE. Proteins were concentrated to 12 mg/ml using concentrators (Sartorius 30-kDa cutoff). Protein concentration was determined by measuring the absorbance at 280 nm using an extinction coefficient of 25,440 M−1 cm−1. The absence of aggregates was verified by Dynamic Light Scattering using a Wyatt Dynapro Nanostar system. Cleavage of the N-terminal His6-tag could not be performed because of the extremely low efficiency of the TEV protease on our construct of bsPgpB.
C55-PP and PGP phosphatase assays
The C55-PP and PGP phosphatase assays were carried out in a 10-µl reaction mixture containing 20 mM Tris–HCl, pH 7.4, 10 mM β-mercaptoethanol, 150 mM NaCl, 0.2% DDM, 50 µM [14C]C55-PP or 50 µM [14C]PGP (900 Bq) and enzyme. Appropriate dilutions of purified bsPgpB wild-type and mutant proteins, or of membrane extracts, were used to achieve less than 30% substrate hydrolysis. The reaction was incubated for 10 min at 37 °C and subsequently stopped by freezing in liquid nitrogen. The substrates and products were then separated and quantified by TLC analysis, as previously described for C55-PP [21] and PGP [17] hydrolysis. The activity of bsPgpB towards other non-radiolabeled substrates [C5-PP, C15-PP, diacyl(C8)glycerol-PP] was determined by measuring the amount of released inorganic phosphate during catalysis. The reaction mixture was as described above with 50 µM substrate in a final volume of 100 µl. After 10 min of incubation at 37 °C, the reaction was stopped by the addition of 0.9 ml of Malachite green solution (Biomol green™, Enzo Life Sciences), and the released phosphate was quantified by measurement of the absorbance at 620 nm.
In meso reconstitution and crystallization
bsPgpB was reconstituted into the bilayer of the cubic phase following a standard protocol [40]. The protein solution, at 12 mg/ml in buffer D, was homogenized with monoolein in a coupled syringe device [41] at room temperature (RT, 19–24 °C), using two volumes of protein solution for every three of monoolein. Crystallization trials were set up by transferring 50 nl of the protein-loaded mesophase onto a siliconized 96-well glass crystallization plate which was subsequently covered with 800 nl of precipitant solution using an in meso robot [42]. Wells were sealed with a glass cover slide. The glass sandwich plates were stored at 20 °C in an imager (Rock Imager 1500, Formulatrix, Inc, Waltham, MA) for crystal growth. Crystallization progress was monitored automatically in the imager and manually using bright field and polarized light microscopy (Eclipse E400 Pol, Nikon, Melville, NY). The protein was incubated with 100 mM sodium tungstate for 30 min at 4 °C before making the cubic phase to obtain crystals suitable for SAD phasing. The composition of precipitant solution that yielded crystals was 40% (v/v) PEG-400, 0.1 M HEPES pH 7 and 0.1 M lithium citrate tribasic tetrahydrate. X-shaped plates grew to a maximum size of 240 × 60 × 10 µm3 in 14 days. After 20 days, wells were opened with a tungsten-carbide glass cutter (Silverline) and the crystals were harvested using 250 µm micromounts (MiTeGen, Ithaca, NY, USA) at 20 °C. Crystals were snap-cooled directly in liquid nitrogen [43].
Data collection, structure solution and refinement
The data set used for the bsPgpB structure was collected on the ID23-D beamline of the Advance Photon Source synchrotron (Argonne, IL, USA). The diffraction data relating to the crystal grown in the presence of tungstate were acquired at the LIII tungsten absorption edge (1.214580 Å). The data were indexed, integrated and scaled using XDS [44]. The crystal belongs to the orthorhombic space group I222 and was used to obtain initial phases using the SAD method implemented in Phenix AutoSol [45, 46]. This stage yielded a structure containing 195 amino acids. The subsequent refinement and model building cycles were performed with phenix.refine and Coot [47], respectively. A summary of the data collection and refinement statistics is given in Table 3. Protein superposition was performed using the Secondary Structure Matching algorithm implemented in Coot [47]. The figures were prepared using PyMOL (The PyMOL Molecular Graphics System, Version 1.7.4.3 Enhanced for Mac OS X, Schrödinger, LLC). A Feature Enhanced Map [48] was used for Fig. 2.
Modeling of the bsPgpB–phosphatidylglycerol phosphate complex
The X-ray structure of bsPgpB was used to model the complex between bsPgpB and PGP. PGP was modeled with its two acyl chains fitting the electronic densities of the two parallel presumed monoolein chains found close to the active site and the terminal phosphate interacting with active site residues (Arg103, Ser119, His121, Arg165 and His171). The model was energy minimized with the backbone atoms fixed to generate Fig. 4. A similar procedure was used to obtain the bsPgpB:C55–PP complex (Fig. 4b). A molecular dynamic simulation was conducted on the bsPgpB:PGP model with the program Yasara [49]. The initial structure was embedded into a phosphatidylethanolamine (1-hexadecanoyl, 2-(Z9-octadecaenoyl)-sn-glycero-3-phosphoethanolamine) membrane and subjected to a 250-ps molecular dynamic equilibration simulation followed by a 100-ns run. Simulation parameters included use of the AMBER03 force field [50], a cutoff distance of 7.86 Å, particle mesh Ewald (PME) long-range electrostatics [51], periodic boundary conditions and simulation cell filled with water outside the membrane.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
This work was supported by the Belgian program of Interuniversity Attraction Poles initiated by the Federal Office for Scientific Technical and Cultural Affairs (IAP no. P7/44), the FRS-FNRS (MIS F.4518.12, IISN 4.4503.11), the Tournesol/Hubert Curien partnership between Belgium and France (R.CFRA.1567), the Science Foundation Ireland (Grant Number 12/IA/1255), the Agence Nationale de la Recherche (Bactoprenyl project, ANR-11-BSV3-002), the Centre National de la Recherche Scientifique and the University of Paris-Sud (UMR 9198) and the Aix-Marseille University. The assistance and support of beamline scientists at the Advanced Photon Source (23-ID) are acknowledged.
Compliance with ethical standards
Conflict of interest
The authors declare no competing financial interests.
Footnotes
Meriem El Ghachi, Nicole Howe, Rodolphe Auger and Alexandre Lambion contributed equally.
Data deposition: coordinates of the bsPgpB crystal structure have been deposited in the Protein Data Bank (PDB id code 5JKI).
Contributor Information
Thierry Touzé, Phone: +33169156428, Email: thierry.touze@i2bc.paris-saclay.fr.
Martin Caffrey, Phone: +353-1-8964253, Email: martin.caffrey@tcd.ie.
Frédéric Kerff, Phone: +3243663620, Email: fkerff@ulg.ac.be.
References
- 1.Sigal YJ, McDermott MI, Morris AJ. Integral membrane lipid phosphatases/phosphotransferases: common structure and diverse functions. Biochem J. 2005;387:281–293. doi: 10.1042/BJ20041771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Manat G, Roure S, Auger R, et al. Deciphering the metabolism of undecaprenyl-phosphate: the bacterial cell-wall unit carrier at the membrane frontier. Microb Drug Resist. 2014;20:199–214. doi: 10.1089/mdr.2014.0035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lei KJ, Shelly LL, Pan CJ, et al. Mutations in the glucose-6-phosphatase gene that cause glycogen storage disease type 1a. Science. 1993;262:580–583. doi: 10.1126/science.8211187. [DOI] [PubMed] [Google Scholar]
- 4.Cullen TW, Giles DK, Wolf LN, et al. Helicobacter pylori versus the host: remodeling of the bacterial outer membrane is required for survival in the gastric mucosa. PLoS Pathog. 2011;7:e1002454. doi: 10.1371/journal.ppat.1002454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fournier J-B, Rebuffet E, Delage L, et al. The Vanadium Iodoperoxidase from the marine flavobacteriaceae species Zobellia galactanivorans reveals novel molecular and evolutionary features of halide specificity in the vanadium haloperoxidase enzyme family. Appl Environ Microbiol. 2014;80:7561–7573. doi: 10.1128/AEM.02430-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Comba S, Menendez-Bravo S, Arabolaza A, Gramajo H. Identification and physiological characterization of phosphatidic acid phosphatase enzymes involved in triacylglycerol biosynthesis in Streptomyces coelicolor . Microb Cell Fact. 2013;12:9. doi: 10.1186/1475-2859-12-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Touzé T, Tran AX, Hankins JV, et al. Periplasmic phosphorylation of lipid A is linked to the synthesis of undecaprenyl phosphate. Mol Microbiol. 2008;67:264–277. doi: 10.1111/j.1365-2958.2007.06044.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chae M, Carman GM. Characterization of the yeast actin patch protein app1p phosphatidate phosphatase. J Biol Chem. 2013;288:6427–6437. doi: 10.1074/jbc.M112.449629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Peschel A. How do bacteria resist human antimicrobial peptides? Trends Microbiol. 2002;10:179–186. doi: 10.1016/S0966-842X(02)02333-8. [DOI] [PubMed] [Google Scholar]
- 10.Nishi H, Komatsuzawa H, Fujiwara T, et al. Reduced content of lysyl-phosphatidylglycerol in the cytoplasmic membrane affects susceptibility to moenomycin, as well as vancomycin, gentamicin, and antimicrobial peptides, in Staphylococcus aureus . Antimicrob Agents Chemother. 2004;48:4800–4807. doi: 10.1128/AAC.48.12.4800-4807.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kuhn S, Slavetinsky CJ, Peschel A. Synthesis and function of phospholipids in Staphylococcus aureus . Int J Med Microbiol. 2015;305:196–202. doi: 10.1016/j.ijmm.2014.12.016. [DOI] [PubMed] [Google Scholar]
- 12.Raetz CR. Molecular genetics of membrane phospholipid synthesis. Annu Rev Genet. 1986;20:253–295. doi: 10.1146/annurev.ge.20.120186.001345. [DOI] [PubMed] [Google Scholar]
- 13.Ratledge C, Wilkinson SG. Microbial lipids. London: Academic Presss; 1988. [Google Scholar]
- 14.Goldberg DE, Rumley MK, Kennedy EP. Biosynthesis of membrane-derived oligosaccharides: a periplasmic phosphoglyceroltransferase. Proc Natl Acad Sci USA. 1981;78:5513–5517. doi: 10.1073/pnas.78.9.5513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Koch HU, Haas R, Fischer W. The role of lipoteichoic acid biosynthesis in membrane lipid metabolism of growing Staphylococcus aureus . Eur J Biochem. 1984;138:357–363. doi: 10.1111/j.1432-1033.1984.tb07923.x. [DOI] [PubMed] [Google Scholar]
- 16.Icho T, Raetz CRH. Multiple genes for membrane-bound phosphatases in Escherichia coli and their action on phospholipid precursors. J Bacteriol. 1983;153:722–730. doi: 10.1128/jb.153.2.722-730.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lu Y-H, Guan Z, Zhao J, Raetz CRH. Three phosphatidylglycerol-phosphate phosphatases in the inner membrane of Escherichia coli . J Biol Chem. 2011;286:5506–5518. doi: 10.1074/jbc.M110.199265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cantagrel V, Lefeber DJ. From glycosylation disorders to dolichol biosynthesis defects: a new class of metabolic diseases. J Inherit Metab Dis. 2011;34:859–867. doi: 10.1007/s10545-011-9301-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Apfel CM, Takács B, Fountoulakis M, et al. Use of genomics to identify bacterial undecaprenyl pyrophosphate synthetase: cloning, expression, and characterization of the essential uppS gene. J Bacteriol. 1999;181:483–492. doi: 10.1128/jb.181.2.483-492.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Siewert G, SJ Bacitracin: an inhibitor of the dephosphorylation of lipid pyrophosphate, an intermediate in the biosynthesis of the peptidoglycan of bacterial cell wall. Proc Natl Acad Sci USA. 1967;57:767–773. doi: 10.1073/pnas.57.3.767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.El Ghachi M, Bouhss A, Blanot D, Mengin-Lecreulx D. The bacA gene of Escherichia coli encodes an undecaprenyl pyrophosphate phosphatase activity. J Biol Chem. 2004;279:30106–30113. doi: 10.1074/jbc.M401701200. [DOI] [PubMed] [Google Scholar]
- 22.El Ghachi M, Derbise A, Bouhss A, Mengin-Lecreulx D. Identification of multiple genes encoding membrane proteins with undecaprenyl pyrophosphate phosphatase (UppP) activity in Escherichia coli . J Biol Chem. 2005;280:18689–18695. doi: 10.1074/jbc.M412277200. [DOI] [PubMed] [Google Scholar]
- 23.Bernard R, El Ghachi M, Mengin-Lecreulx D, et al. BcrC from Bacillus subtilis acts as an undecaprenyl pyrophosphate phosphatase in bacitracin resistance. J Biol Chem. 2005;280:28852–28857. doi: 10.1074/jbc.M413750200. [DOI] [PubMed] [Google Scholar]
- 24.Zhao H, Sun Y, Peters JM, et al. Depletion of undecaprenyl pyrophosphate phosphatases (UPP-Pases) disrupts cell envelope biogenesis in Bacillus subtilis . J Bacteriol. 2016 doi: 10.1128/JB.00507-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fan J, Jiang D, Zhao Y, et al. Crystal structure of lipid phosphatase Escherichia coli phosphatidylglycerophosphate phosphatase B. Proc Natl Acad Sci USA. 2014;111:7636–7640. doi: 10.1073/pnas.1403097111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tong S, Lin Y, Lu S, et al. Structural insight into substrate selection and catalysis of lipid phosphate phosphatase PgpB in the cell membrane. J Biol Chem. 2016 doi: 10.1074/jbc.M116.737874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Isupov MN, Dalby AR, Brindley AA, et al. Crystal structure of dodecameric vanadium-dependent bromoperoxidase from the red algae Corallina officinalis . J Mol Biol. 2000;299:1035–1049. doi: 10.1006/jmbi.2000.3806. [DOI] [PubMed] [Google Scholar]
- 28.Makde RD, Mahajan SK, Kumar V. Structure and mutational analysis of the PhoN protein of Salmonella typhimurium provide insight into mechanistic details. BioChemistry. 2007;46:2079–2090. doi: 10.1021/bi062180g. [DOI] [PubMed] [Google Scholar]
- 29.Bernard R, Joseph P, Guiseppi A, et al. YtsCD and YwoA, two independent systems that confer bacitracin resistance to Bacillus subtilis . FEMS Microbiol Lett. 2003;228:93–97. doi: 10.1016/S0378-1097(03)00738-9. [DOI] [PubMed] [Google Scholar]
- 30.Barreteau H, Magnet S, Ghachi MEl, et al. Quantitative high-performance liquid chromatography analysis of the pool levels of undecaprenyl phosphate and its derivatives in bacterial membranes. J Chromatogr B Anal Technol Biomed Life Sci. 2009;877:213–220. doi: 10.1016/j.jchromb.2008.12.010. [DOI] [PubMed] [Google Scholar]
- 31.Rost B, Yachdav G, Liu J. The PredictProtein server. Nucleic Acids Res. 2004;32:W321–W326. doi: 10.1093/nar/gkh377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Touzé T, Blanot D, Mengin-Lecreulx D. Substrate specificity and membrane topology of Escherichia coli PgpB, an undecaprenyl pyrophosphate phosphatase. J Biol Chem. 2008;283:16573–16583. doi: 10.1074/jbc.M800394200. [DOI] [PubMed] [Google Scholar]
- 33.Tatar LD, Marolda CL, Polischuk AN, et al. An Escherichia coli undecaprenyl-pyrophosphate phosphatase implicated in undecaprenyl phosphate recycling. Microbiology. 2007;153:2518–2529. doi: 10.1099/mic.0.2007/006312-0. [DOI] [PubMed] [Google Scholar]
- 34.Pompeo F, Van Heijenoort J, Mengin-Lecreulx D. Probing the role of cysteine residues in glucosamine-1-phosphate acetyltransferase activity of the bifunctional glmU protein from Escherichia coli: Site-directed mutagenesis and characterization of the mutant enzymes. J Bacteriol. 1998;180:4799–4803. doi: 10.1128/jb.180.18.4799-4803.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bligh EG, Dyer WJ. A rapid method of total lipid extraction and purification. Can J Biochem Physiol. 1959;37:911–917. doi: 10.1139/o59-099. [DOI] [PubMed] [Google Scholar]
- 36.Mascher T, Margulis NG, Wang T, et al. Cell wall stress responses in Bacillus subtilis: the regulatory network of the bacitracin stimulon. Mol Microbiol. 2003;50:1591–1604. doi: 10.1046/j.1365-2958.2003.03786.x. [DOI] [PubMed] [Google Scholar]
- 37.Datsenko KA, Wanner BL. One-step inactivation of chromosomal genes in Escherichia coli K-12 using PCR products. Proc Natl Acad Sci USA. 2000;97:6640–6645. doi: 10.1073/pnas.120163297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hamilton CM, Aldea M, Washburn BK, et al. New method for generating deletions and gene replacements in Escherichia coli . J Bacteriol. 1989;171:4617–4622. doi: 10.1128/jb.171.9.4617-4622.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Baba T, Ara T, Hasegawa M, et al. Construction of Escherichia coli K-12 in-frame, single-gene knockout mutants: the Keio collection. Mol Syst Biol. 2006;2(2006):0008. doi: 10.1038/msb4100050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Caffrey M, Cherezov V. Crystallizing membrane proteins using lipidic mesophases. Nat Protoc. 2009;4:706–731. doi: 10.1038/nprot.2009.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cheng A, Hummel B, Qiu H, Caffrey M. A simple mechanical mixer for small viscous lipid-containing samples. Chem Phys Lipids. 1998;95:11–21. doi: 10.1016/S0009-3084(98)00060-7. [DOI] [PubMed] [Google Scholar]
- 42.Li D, Boland C, Walsh K, Caffrey M (2012) Use of a robot for high-throughput crystallization of membrane proteins in lipidic mesophases. J Vis Exp e4000. doi:10.3791/4000 [DOI] [PMC free article] [PubMed]
- 43.Li D, Boland C, Aragao D, et al (2012) Harvesting and cryo-cooling crystals of membrane proteins grown in lipidic mesophases for structure determination by macromolecular crystallography. J Vis Exp e4001. doi:10.3791/4001 [DOI] [PMC free article] [PubMed]
- 44.Kabsch W. XDS. Acta Crystallogr Sect D Biol Crystallogr. 2010;66:125–132. doi: 10.1107/S0907444909047337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Terwilliger TC, Adams PD, Read RJ, et al. Decision-making in structure solution using Bayesian estimates of map quality: the PHENIX AutoSol wizard. Acta Crystallogr Sect D Biol Crystallogr. 2009;65:582–601. doi: 10.1107/S0907444909012098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Afonine PV, Grosse-Kunstleve RW, Echols N, et al. Towards automated crystallographic structure refinement with phenix.refine. Acta Crystallogr Sect D Biol Crystallogr. 2012;68:352–367. doi: 10.1107/S0907444912001308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Emsley P, Lohkamp B, Scott WG, Cowtan K. Features and development of Coot. Acta Crystallogr Sect D Biol Crystallogr. 2010;66:486–501. doi: 10.1107/S0907444910007493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Afonine PV, Moriarty NW, Mustyakimov M, et al. ) FEM: feature-enhanced map. Acta Crystallogr Sect D Biol Crystallogr. 2015;71:646–666. doi: 10.1107/S1399004714028132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Krieger E, Darden T, Nabuurs SB, et al. Making optimal use of empirical energy functions: force-field parameterization in crystal space. Proteins Struct Funct Genet. 2004;57:678–683. doi: 10.1002/prot.20251. [DOI] [PubMed] [Google Scholar]
- 50.Best RB, Hummer G. Optimized molecular dynamics force fields applied to the helix-coil transition of polypeptides. J Phys Chem B. 2009;113:9004–9015. doi: 10.1021/jp901540t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Essmann U, Perera L, Berkowitz ML, et al. A smooth particle mesh Ewald method. J Chem Phys. 1995;103:8577–8593. doi: 10.1063/1.470117. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.