Abstract
Chronic kidney disease is an incurable to date pathology, with renal replacement therapy through dialysis or transplantation being the only available option for end-stage patients. A deeper understanding of the molecular mechanisms governing the progression of kidney diseases will permit the identification of unknown mediators and potential novel markers or targets of therapy which promise more efficient diagnostic and therapeutic applications. Over the last years, periostin was established by several studies as a novel key player in the progression of renal disease. Periostin is de novo expressed focally by the injured kidney cells during the development of renal disease. In diverse cohorts of renal disease patients, the expression levels of periostin in the kidney and urine were highly correlated with the stage of the pathology and the decline of renal function. Subsequent studies in animal models demonstrated that periostin is centrally involved in mediating renal inflammation and fibrosis, contributing to the deterioration of renal structure and function. Genetic or pharmaco-genetic inhibition of periostin in animal models of renal disease was efficient in arresting the progression of the pathology. This review will summarize the recent advances on periostin in the field of kidney diseases and will discuss its utility of as a novel target of therapy for chronic kidney disease.
Keywords: Chronic kidney disease, Periostin, Target of therapy, Inflammation, Fibrosis
Introduction
Chronic kidney disease (CKD) is a major societal and economic burden for national health systems, with a continuously rising incidence that contributes to the increase of the number of deaths worldwide [1]. With currently no efficient prognostic or therapeutic options being available, the only possibility for the treatment of end-stage renal disease remains the renal replacement therapy through dialysis or transplantation. CKD may originate from various causes, such as diabetes, hypertension, immune or toxic stimuli attacking different kidney compartments; however, it is characterized by common pathologic pathways which involve chronic inflammation and excessive production of extracellular matrix (ECM) within the kidney, leading to gradual impairment of renal structure and function [2].
During the last years, a major focus in kidney research has been the deeper understanding of the pathophysiological mechanisms governing CKD progression, which will allow the development of early prognostic markers and targeted therapeutic treatments. This collective effort has led to the discovery of several novel mediators and promising targets of renal disease [3–7]. Drugs that were developed against some of these molecules, including well-known profibrotic and proinflammatory factors such as TGF-β1, CTGF, CCL2/CCR2, ET-1, TNF-α, have been efficient in preclinical studies and have entered early phase clinical trials on subgroups of CKD patients [8].
Periostin was identified by us and other investigators as a novel biomarker and/or mediator of CKD. Periostin is a 90 kDa matricellular protein with high expression in bone and dental tissues [9]. As is the case for other such proteins, the expression of periostin is high in development while restricted in adult tissues, but it is considerably upregulated in injury and wound-healing conditions [10]. The protein structure of periostin comprises distinct regions, including a tandem repeat of four fasciclin-I domains derived from the homologous insect domain which has been associated with neuronal adhesion. Periostin was shown to interact via its different domains with several proteins of the ECM, such as collagen-1, Notch-1, tenascin-C, BMP-1 and various cell-surface integrins [11–15]. Through these interactions periostin is capable to mediate signals both in the extracellular and the intracellular environment, thus modulating ECM assembly and cellular responses associated to adhesion, migration and differentiation.
Periostin has been shown to mediate inflammation and fibrosis the during disease of several organs, including the heart, lung, kidney, skin, liver, skeletal muscle and retina, while inhibiting periostin in animal models of these diseases was efficient in arresting the progression of the pathology [16–26]. The scope of this review is to summarize the latest research on the role(s) of periostin in kidney diseases and discuss the potential of periostin as a future target of therapy of CKD.
Involvement of periostin in renal disease
Insights from patient biopsies
Periostin was first implicated in the progression of renal disease by a study that identified high expression of the protein in cyst-lining cells and cystic fluid from autosomal dominant polycystic kidney disease patients [27]. Subsequently, given the known involvement of matricellular proteins in fibrotic diseases, Sen et al. performed a transcriptomic analysis in isolated glomeruli specimens from patients with proteinuric diseases, including focal segmental glomerulosclerosis, IgA nephropathy, lupus nephritis and mesangial nephropathy, to discover differentially expressed matricellular transcripts [28]. This study identified periostin as the most highly expressed protein among the targets and demonstrated a broad association of its expression with the progression of human nephropathies. In immunohistochemical analyses in biopsies of these patients, periostin was localized in areas of mesangial expansion, the tubulointerstitium and sites of fibrosis, while the degree of periostin expression was negatively correlated with renal function. Interestingly, the profibrotic factor TGF-β1 was shown as a potent inducer of periostin in cultured mesangial cells, while treatment of the cells with exogenous periostin was associated with increased proliferation and reduced apoptosis.
Subsequent studies in patient biopsies and urine samples confirmed the elevated expression of periostin in diverse renal diseases and highlighted the potential utility of periostin as a tissue or urine biomarker of the disease progression. In lupus nephritis patients, periostin was highly expressed in periglomerular areas, fibrous vessels and areas with interstitial fibrosis, while its expression levels were correlated with the chronicity of the disease and the decline of renal function [29]. Another study revealed high expression of periostin in sclerotic glomeruli and tubular epithelium of type 2 diabetes patients. Urinary periostin levels of these patients were significantly upregulated compared with healthy controls, whereas significant variations of the urine periostin values were also observed between normo-, micro- or macroalbuminuric diabetes patients [30]. In a large cohort of IgA nephropathy patients, high urinary periostin concentration was associated with an advanced stage of the disease and could predict worsening renal outcomes [31]. Accordingly, in a group of transplant recipients, urine periostin levels could accurately distinguish chronic allograft nephropathy patients from transplant subjects with normal renal function [32]. Interestingly, another study showed that urine periostin/creatinine ratio is significantly higher in patients with both proteinuric and non-proteinuric renal diseases, uncoupling urine periostin excretion from the dysfunction of the glomerular filtration barrier [33].
Insights from animal models of CKD
Models of vascular injury
To identify factors crucially implicated in the progression of renal disease, we administered losartan, an angiotensin-II receptor blocker, to l-NAME-treated hypertensive rats at the non-return point of the disease. This method resulted in either reversal or progression of renal disease after losartan treatment, which was followed by comparative analysis of the kidney transcriptome of the two experimental groups, aiming to reveal novel putative targets of therapy. Periostin was identified in this analysis as the target with the highest expression in the progression compared to the reversal group [34]. Moreover, periostin was predominantly localized in the injured fibrotic regions of the diseased kidneys, while its expression levels showed a close correlation with classical parameters of renal function such as plasma creatinine, proteinuria and renal blood flow. This was the first study to denote periostin as a potential diagnostic target for the evaluation of the progression or response to therapy of kidney diseases.
Our results where more recently confirmed in the two-kidney, one-clip (2K1C) rat model of hypertensive nephrosclerosis, where proteomic analysis of the renal cortex identified periostin as one of the most highly upregulated proteins, with a principal expression around fibrotic vessels [35]. Independent in vitro studies showed that angiotensin-II, a major factor involved in hypertensive nephropathy, is a potent inducer of periostin in vascular smooth muscle cells and cardiac fibroblasts through complex pathways mediated by PI3 kinase and Ras/ERK/TGFβ1 signaling, respectively [36, 37].
Models of tubular aggression
A first study investigating the expression and function of periostin in the models of 5/6 nephrectomy and unilateral ureteral obstruction (UUO), identified high expression of periostin in distal tubular epithelium and in tubulointerstitial areas [33]. Periostin overexpression in cultured mouse distal tubular cells downregulated the expression of E-cadherin and induced expression of mesenchymal markers like fibroblast specific protein-1 (FSP-1) and matrix metalloproteinase-9 (MMP-9), associating periostin function with the induction of a mesenchymal phenotype in tubular cells.
Subsequent studies by our group revealed a critical role for periostin in the progression of renal disease. Mice with genetic deletion of periostin displayed reduced interstitial fibrosis and inflammation associated with preservation of the renal epithelial phenotype in the UUO model, compared with their wild-type littermates [38]. Expression of periostin by renal epithelial cells was attributed to TGF-β1, while treatment of the cells with periostin could upregulate the expression of collagen I and stimulate mitogen-activated protein kinase (MAPK) pathways.
In parallel, other investigators identified a role for periostin in the progression of polycystic kidney diseases (PKD). Periostin was found to be highly expressed by cyst-lining epithelial cells and accumulate in the tubulointerstitial matrix adjacent to cysts of PKD patients. Deletion of the periostin gene in a genetic mouse model of PKD resulted in the reduced number of cysts, decreased proliferation and autophagy in the cystic kidneys, which were accompanied by reduction of interstitial fibrosis and preservation of renal function [39].
Models of glomerular injury
In a recent study, we investigated the mechanism of induction and the role of periostin in a severe model of nephrotoxic serum (NTS)-induced glomerulonephritis. As with the aforementioned models, deletion of the periostin gene resulted in a marked reduction of proinflammatory and profibrotic mediators in the diseased kidney accompanied by preservation of renal structure and function [40]. In addition, we used a pharmacogenetic approach via administration of antisense oligonucleotides to block the expression of periostin after the onset of the disease in the NTS model. This strategy revealed that inhibition of periostin at a later stage of the disease after establishment of proteinuria is efficient in arresting the progression of the pathology and preserving renal function, demonstrating the utility of periostin as a future potential therapeutic target of CKD. Moreover, using a combination of bioinformatics analysis, in vitro reporter assays and in vivo chromatin immunoprecipitation analyses, we showed that NFκΒ and other proinflammatory transcription factors are the major inducers of periostin in the NTS model [40]. This is in accordance with other studies showing that periostin is highly induced by the interleukins IL-4 and IL-13 in inflammatory conditions such as bronchial asthma [18, 41]. Moreover, periostin was shown to mediate immune responses and promote production of proinflammatory chemokines in allergic inflammation and inflammatory lung diseases [19, 42]. The function of periostin in the NTS model was associated to activation of the integrin αvβ3 signaling cascade in glomerular podocytes and parietal epithelial cells, inducing migration, survival and proliferation pathways and amplifying the inflammatory and fibrotic responses [40]. Periostin has been previously shown to mediate adhesion and migration of different cell types, such as cancer cells, vascular smooth muscle cells and macrophages, though interactions with cell-surface integrins [15, 43, 44].
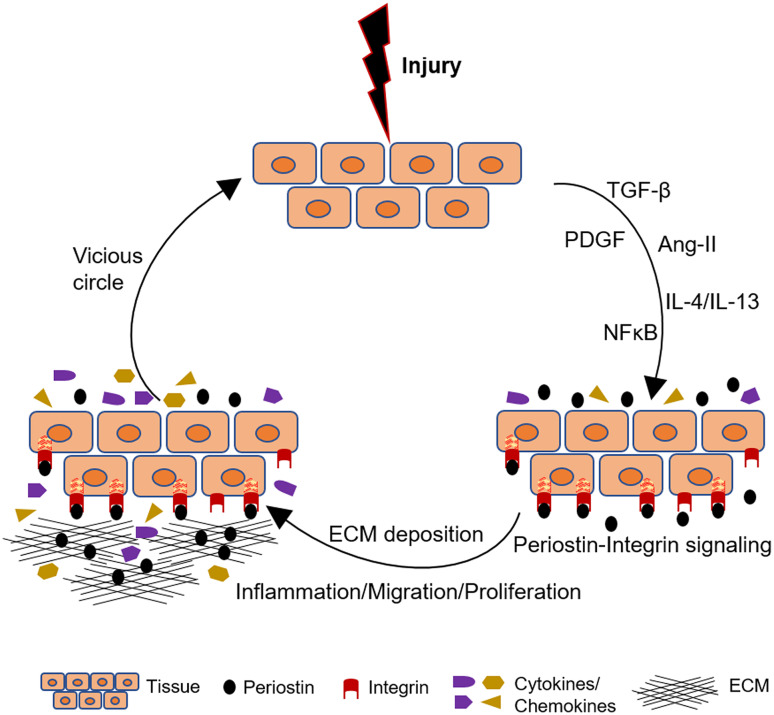
Another study examined the role of periostin in a genetic mouse model of lupus nephritis [45]. Lupus mice displayed upregulated periostin expression in glomerular mesangial cells accompanied by increased proliferation, ECM production and expression of platelet-derived growth factor-B (PDGF-B). The expression of periostin was induced by PDGF-B in cultured mouse mesangial cells and was shown to mediate increased proliferation and secretion of fibronectin downstream PDGF-B and the activation of PI3 kinase/Akt pathways. The described mechanisms of periostin induction and function in renal disease are summarized in Fig. 1 and Table 1.
Fig. 1.
Schematic illustration of how periostin mediates progression of renal disease. After an initial aggression, different growth factors, cytokines and signaling pathways upregulate periostin expression. Periostin interacts with its integrin receptors on the cell surface, inducing inflammation and matrix assembly and promoting cell adhesion, migration and proliferation. These pathways amplify the inflammatory and fibrotic responses inducing a vicious circle of renal damage
Table 1.
Physiopathological functions of periostin in animal models of CKD
| Animal model | Periostin expression sites | Function | References |
|---|---|---|---|
| l-NAME-induced hypertensive nephropathy | Renal vessels, vascular smooth muscle cells, injured tubules | Periostin levels are correlated with the decline of renal function. Treatment of animals with periostin antisense protects from glomerulosclerosis and perivascular/interstitial fibrosis | [34, 38] |
| 2-Kidney, 1-clip (2K1C) rat model of hypertensive nephrosclerosis | Fibrotic vessels | Relative abundance of periostin is sufficient to differentiate 2K1C animals from controls. | [35] |
| 5/6 Nephrectomy (5/6 Nx) | Distal tubular cells, tubular interstitium | Periostin induces a mesenchymal phenotype in tubular cells by decreasing E-cadherin and upregulating MMP-9 and FSP1 | [33] |
| Unilateral ureteral obstruction (UUO) | Tubular cells, tubular interstitium | Periostin treatment of cultured tubular cells stimulates expression of collagen I and MAPK pathways. Periostin null mice are protected from renal inflammation and interstitial fibrosis | [33, 38] |
| Polycystic kidney disease (pcy/pcy mice) | Cyst-lining tubular epithelial cells | Periostin activates the mTOR pathway to mediate cyst proliferation, autophagy and interstitial fibrosis | [27, 39] |
| Glomerulonephritis induced by nephrotoxic serum (NTS-induced GN) | Glomerular podocytes, parietal epithelial cells, vascular smooth muscle cells | Periostin is induced by proinflammatory transcription factors to activate integrin αvβ3 signaling pathway. Null mice display reduced inflammation and fibrosis and preservation of renal function | [40] |
| Lupus nephritis (MRL/lpr mice) | Glomerular mesangial cells | Periostin is induced by PDGF-B to promote mesangial cell proliferation and matrix production | [45] |
Periostin as a target of therapy in CKD
During the last decades, a main focus of renal research has been the identification of novel biomarkers and/or targets of therapy for kidney diseases, which promise a more effective diagnosis and treatment of CKD patients. Although a lot of progress has been made towards the discovery of unknown mediators of renal disease, many among them were proven inefficient in clinical practice, while the considerable time and cost required for the generation and testing of potential drugs further impedes the clinical application of the new candidate targets.
Periostin fulfils several criteria for representing a promising biomarker of CKD. Although its expression is minimal in healthy tissue, periostin is highly upregulated in the kidney in diverse renal pathologies, both in animal models and human renal disease, while its expression levels were found to correlate with the decline of renal function [28–30, 34] (Table 1). Given the nature of periostin as a secreted molecule, it can be easily detectable in plasma or urine samples. Accordingly, periostin levels were found elevated in the urine of several types of CKD patients, including diabetes, IgA nephropathy, chronic allograft nephropathy, lupus nephritis, focal segmental glomerulonephritis and polycystic kidney disease [30–33]. Further studies are necessary to investigate whether measurement of periostin levels in biological fluids can be applied in the clinic as an early and sensitive marker of kidney damage.
The localization of periostin in the ECM renders the protein an easily accessible target of potential drugs. Moreover, the expression of periostin is focal in the damaged tissue, associated to the sites of injury, e.g. periostin is expressed by renal vessels in hypertensive nephropathy [34, 35], by renal tubules in UUO, 5/6 nephrectomy and polycystic kidney disease [33, 38, 39] and by glomerular cells in several glomerulopathies [28, 40]. Inhibition of periostin using knock-out mice or antisense oligonucleotides preserved renal structure and function in several animal models of CKD, demonstrating that periostin has the potential of being a target of therapy for kidney diseases [34, 38–40] (Table 1). However, targeting periostin in human renal disease requires the generation and validation of targeted drugs, for example specific neutralizing antibodies, blocking peptides or antisense with increased stability suitable for use in humans, which may necessitate further elaboration on the specific interplay between periostin and its interaction partners as well as collaboration of different fields of research.
Concluding remarks and perspectives
Understanding the molecular basis of kidney diseases will advance our knowledge on the mechanisms participating in the development of CKD and will provide us with novel tools to counteract the progression of end-stage renal disease. Periostin has been identified as a novel mediator of renal disease centrally involved in the processes of renal inflammation and fibrosis, with potency to constitute a future biomarker or therapeutic target of CKD. Periostin is de novo expressed in the kidney after renal damage while its inhibition efficiently protects from the progression of renal disease in animal models, which renders periostin an appealing biomarker or target of therapy. Targeting periostin in clinical practice will require the development of validated drugs for use in humans and the follow-up of large cohorts of patients, which is subject of further investigation.
Acknowledgements
This work was financially supported by funds from Institut National de la Santé Et de la Recherche Médicale (INSERM) and the Agence Nationale de la Recherche (ANR).
References
- 1.Naghavi M, Wang H, Lozano R, Davis A, Liang X, Zhou M, et al. Global, regional, and national age-sex specific all-cause and cause-specific mortality for 240 causes of death, 1990–2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet. 2015;385(9963):117–171. doi: 10.1016/S0140-6736(14)61682-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.López-Novoa JM, Martínez-Salgado C, Rodríguez-Peña AB, López-Hernández FJ. Common pathophysiological mechanisms of chronic kidney disease: therapeutic perspectives. Pharmacol Ther. 2010;128(1):61–81. doi: 10.1016/j.pharmthera.2010.05.006. [DOI] [PubMed] [Google Scholar]
- 3.Devarajan P. The use of targeted biomarkers for chronic kidney disease. Adv Chronic Kidney Dis. 2010;17(6):469–479. doi: 10.1053/j.ackd.2010.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wong MG, Pollock CA. Biomarkers in kidney fibrosis: are they useful? Kidney Int Suppl. 2014;4(1):79–83. doi: 10.1038/kisup.2014.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Prakoura N, Chatziantoniou C. Periostin and discoidin domain receptor 1: new biomarkers or targets for therapy of renal disease. Front Med. 2017;4:52. doi: 10.3389/fmed.2017.00052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kavvadas P, Dussaule JC, Chatziantoniou C. Searching novel diagnostic markers and targets for therapy of CKD. Kidney Int Suppl. 2014;4(1):53–57. doi: 10.1038/kisup.2014.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Boor P, Floege J. Renal allograft fibrosis: biology and therapeutic targets. Am J Transplant. 2015;15(4):863–886. doi: 10.1111/ajt.13180. [DOI] [PubMed] [Google Scholar]
- 8.Lee SY, Kim SI, Choi ME. Therapeutic targets for treating fibrotic kidney diseases. Transl Res. 2015;165(4):512–530. doi: 10.1016/j.trsl.2014.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Horiuchi K, Amizuka N, Takeshita S, Takamatsu H, Katsuura M, Ozawa H, Toyama Y, Bonewald LF, Kudo A. Identification and characterization of a novel protein, periostin, with restricted expression to periosteum and periodontal ligament and increased expression by transforming growth factor beta. J Bone Miner Res. 1999;14(7):1239–1249. doi: 10.1359/jbmr.1999.14.7.1239. [DOI] [PubMed] [Google Scholar]
- 10.Prakoura N, Chatziantoniou C. Matricellular proteins and organ fibrosis. Curr Pathobiol Rep. 2017 [Google Scholar]
- 11.Norris RA, Damon B, Mironov V, Kasyanov V, Ramamurthi A, Moreno-Rodriguez R, Trusk T, Potts JD, Goodwin RL, Davis J, Hoffman S, Wen X, Sugi Y, Kern CB, Mjaatvedt CH, Turner DK, Oka T, Conway SJ, Molkentin JD, Forgacs G, Markwald RR. Periostin regulates collagen fibrillogenesis and the biomechanical properties of connective tissues. J Cell Biochem. 2007;101(3):695–711. doi: 10.1002/jcb.21224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tanabe H, Takayama I, Nishiyama T, Shimazaki M, Kii I, Li M, Amizuka N, Katsube K, Kudo A. Periostin associates with Notch1 precursor to maintain Notch1 expression under a stress condition in mouse cells. PLoS One. 2010;5:e12234. doi: 10.1371/journal.pone.0012234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Maruhashi T, Kii I, Saito M, Kudo A. Interaction between periostin and BMP-1 promotes proteolytic activation of lysyl oxidase. J Biol Chem. 2010;285(17):13294–13303. doi: 10.1074/jbc.M109.088864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kii I, Nishiyama T, Li M, Matsumoto K, Saito M, Amizuka N, Kudo A. Incorporation of tenascin-C into the extracellular matrix by periostin underlies an extracellular meshwork architecture. J Biol Chem. 2010;285(3):2028–2039. doi: 10.1074/jbc.M109.051961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gillan L, Matei D, Fishman DA, Gerbin CS, Karlan BY, Chang DD. Periostin secreted by epithelial ovarian carcinoma is a ligand for alpha(V)beta(3) and alpha(V)beta(5) integrins and promotes cell motility. Cancer Res. 2002;62(18):5358–5364. [PubMed] [Google Scholar]
- 16.Oka T, Xu J, Kaiser RA, Melendez J, Hambleton M, Sargent MA, Lorts A, Brunskill EW, Dorn GW, 2nd, Conway SJ, Aronow BJ, Robbins J, Molkentin JD. Genetic manipulation of periostin expression reveals a role in cardiac hypertrophy and ventricular remodeling. Circ Res. 2007;101(3):313–321. doi: 10.1161/CIRCRESAHA.107.149047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shimazaki M, Nakamura K, Kii I, Kashima T, Amizuka N, Li M, Saito M, Fukuda K, Nishiyama T, Kitajima S, Saga Y, Fukayama M, Sata M, Kudo A. Periostin is essential for cardiac healing after acute myocardial infarction. J Exp Med. 2008;205(2):295–303. doi: 10.1084/jem.20071297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sidhu SS, Yuan S, Innes AL, Kerr S, Woodruff PG, Hou L, Muller SJ, Fahy JV. Roles of epithelial cell-derived periostin in TGF-beta activation, collagen production, and collagen gel elasticity in asthma. Proc Natl Acad Sci USA. 2010;107(32):14170–14175. doi: 10.1073/pnas.1009426107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Uchida M, Shiraishi H, Ohta S, Arima K, Taniguchi K, Suzuki S, Okamoto M, Ahlfeld SK, Ohshima K, Kato S, Toda S, Sagara H, Aizawa H, Hoshino T, Conway SJ, Hayashi S, Izuhara K. Periostin, a matricellular protein, plays a role in the induction of chemokines in pulmonary fibrosis. Am J Respir Cell Mol Biol. 2012;46(5):677–686. doi: 10.1165/rcmb.2011-0115OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Naik PK, Bozyk PD, Bentley JK, Popova AP, Birch CM, Wilke CA, Fry CD, White ES, Sisson TH, Tayob N, Carnemolla B, Orecchia P, Flaherty KR, Hershenson MB, Murray S, Martinez FJ, Moore BB, COMET Investigators Periostin promotes fibrosis and predicts progression in patients with idiopathic pulmonary fibrosis. Am J Physiol Lung Cell Mol Physiol. 2012;303(12):L1046–L1056. doi: 10.1152/ajplung.00139.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhou HM, Wang J, Elliott C, Wen W, Hamilton DW, Conway SJ. Spatiotemporal expression of periostin during skin development and incisional wound healing: lessons for human fibrotic scar formation. J Cell Commun Signal. 2010;4(2):99–107. doi: 10.1007/s12079-010-0090-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yang L, Serada S, Fujimoto M, Terao M, Kotobuki Y, Kitaba S, Matsui S, Kudo A, Naka T, Murota H, Katayama I. Periostin facilitates skin sclerosis via PI3K/Akt dependent mechanism in a mouse model of scleroderma. PLoS One. 2012;7(7):e41994. doi: 10.1371/journal.pone.0041994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lorts A, Schwanekamp JA, Baudino TA, McNally EM, Molkentin JD. Deletion of periostin reduces muscular dystrophy and fibrosis in mice by modulating the transforming growth factor-β pathway. Proc Natl Acad Sci USA. 2012;109(27):10978–10983. doi: 10.1073/pnas.1204708109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ozdemir C, Akpulat U, Sharafi P, Yıldız Y, Onbaşılar I, Kocaefe C. Periostin is temporally expressed as an extracellular matrix component in skeletal muscle regeneration and differentiation. Gene. 2014;553(2):130–139. doi: 10.1016/j.gene.2014.10.014. [DOI] [PubMed] [Google Scholar]
- 25.Sugiyama A, Kanno K, Nishimichi N, Ohta S, Ono J, Conway SJ, Izuhara K, Yokosaki Y, Tazuma S. Periostin promotes hepatic fibrosis in mice by modulating hepatic stellate cell activation via αv integrin interaction. J Gastroenterol. 2016;51(12):1161–1174. doi: 10.1007/s00535-016-1206-0. [DOI] [PubMed] [Google Scholar]
- 26.Nakama T, Yoshida S, Ishikawa K, Kobayashi Y, Zhou Y, Nakao S, Sassa Y, Oshima Y, Takao K, Shimahara A, Yoshikawa K, Hamasaki T, Ohgi T, Hayashi H, Matsuda A, Kudo A, Nozaki M, Ogura Y, Kuroda M, Ishibashi T. Inhibition of choroidal fibrovascular membrane formation by new class of RNA interference therapeutic agent targeting periostin. Gene Ther. 2015;22(2):127–137. doi: 10.1038/gt.2014.112. [DOI] [PubMed] [Google Scholar]
- 27.Wallace DP, Quante MT, Reif GA, Nivens E, Ahmed F, Hempson SJ, Blanco G, Yamaguchi T. Periostin induces proliferation of human autosomal dominant polycystic kidney cells through alphaV-integrin receptor. Am J Physiol Renal Physiol. 2008;295(5):F1463–F1471. doi: 10.1152/ajprenal.90266.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sen K, Lindenmeyer MT, Gaspert A, Eichinger F, Neusser MA, Kretzler M, Segerer S, Cohen CD. Periostin is induced in glomerular injury and expressed de novo in interstitial renal fibrosis. Am J Pathol. 2011;179(4):1756–1767. doi: 10.1016/j.ajpath.2011.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wantanasiri P, Satirapoj B, Charoenpitakchai M, Aramwit P. Periostin: a novel tissue biomarker correlates with chronicity index and renal function in lupus nephritis patients. Lupus. 2015;24(8):835–845. doi: 10.1177/0961203314566634. [DOI] [PubMed] [Google Scholar]
- 30.Satirapoj B, Tassanasorn S, Charoenpitakchai M, Supasyndh O. Periostin as a tissue and urinary biomarker of renal injury in type 2 diabetes mellitus. PLoS One. 2015;10(4):e0124055. doi: 10.1371/journal.pone.0124055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hwang JH, Lee JP, Kim CT, Yang SH, Kim JH, An JN, Moon KC, Lee H, Oh YK, Joo KW, Kim DK, Kim YS, Lim CS. Urinary periostin excretion predicts renal outcome in IgA nephropathy. Am J Nephrol. 2016;44(6):481–492. doi: 10.1159/000452228. [DOI] [PubMed] [Google Scholar]
- 32.Satirapoj B, Witoon R, Ruangkanchanasetr P, Wantanasiri P, Charoenpitakchai M, Choovichian P. Urine periostin as a biomarker of renal injury in chronic allograft nephropathy. Transplant Proc. 2014;46(1):135–140. doi: 10.1016/j.transproceed.2013.07.069. [DOI] [PubMed] [Google Scholar]
- 33.Satirapoj B, Wang Y, Chamberlin MP, Dai T, LaPage J, Phillips L, Nast CC, Adler SG. Periostin: novel tissue and urinary biomarker of progressive renal injury induces a coordinated mesenchymal phenotype in tubular cells. Nephrol Dial Transplant. 2012;27(7):2702–2711. doi: 10.1093/ndt/gfr670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Guerrot D, Dussaule JC, Mael-Ainin M, Xu-Dubois YC, Rondeau E, Chatziantoniou C, Placier S. Identification of periostin as a critical marker of progression/reversal of hypertensive nephropathy. PLoS One. 2012;7(3):e31974. doi: 10.1371/journal.pone.0031974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Vethe H, Finne K, Skogstrand T, Vaudel M, Vikse BE, Hultström M, Placier S, Scherer A, Tenstad O, Marti HP. Distinct protein signature of hypertension-induced damage in the renal proteome of the two-kidney, one-clip rat model. J Hypertens. 2015;33(1):126–135. doi: 10.1097/HJH.0000000000000370. [DOI] [PubMed] [Google Scholar]
- 36.Li G, Oparil S, Sanders JM, Zhang L, Dai M, Chen LB, Conway SJ, McNamara CA, Sarembock IJ. Phosphatidylinositol-3-kinase signaling mediates vascular smooth muscle cell expression of periostin in vivo and in vitro. Atherosclerosis. 2006;188(2):292–300. doi: 10.1016/j.atherosclerosis.2005.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Li L, Fan D, Wang C, Wang JY, Cui XB, Wu D, Zhou Y, Wu LL. Angiotensin II increases periostin expression via Ras/p38 MAPK/CREB and ERK1/2/TGF-β1 pathways in cardiac fibroblasts. Cardiovasc Res. 2011;91(1):80–89. doi: 10.1093/cvr/cvr067. [DOI] [PubMed] [Google Scholar]
- 38.Mael-Ainin M, Abed A, Conway SJ, Dussaule JC, Chatziantoniou C. Inhibition of periostin expression protects against the development of renal inflammation and fibrosis. J Am Soc Nephrol. 2014;25(8):1724–1736. doi: 10.1681/ASN.2013060664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wallace DP, White C, Savinkova L, Nivens E, Reif GA, Pinto CS, Raman A, Parnell SC, Conway SJ, Fields TA. Periostin promotes renal cyst growth and interstitial fibrosis in polycystic kidney disease. Kidney Int. 2014;85(4):845–854. doi: 10.1038/ki.2013.488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Prakoura N, Kavvadas P, Kormann R, Dussaule JC, Chadjichristos C, Chatziantoniou C. NFκB-induced periostin activates integrin-β3 signaling to promote renal injury in GN. J Am Soc Nephrol. 2017;28(5):1475–1490. doi: 10.1681/ASN.2016070709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Takayama G, Arima K, Kanaji T, Toda S, Tanaka H, Shoji S, McKenzie AN, Nagai H, Hotokebuchi T, Izuhara K. Periostin: a novel component of subepithelial fibrosis of bronchial asthma downstream of IL-4 and IL-13 signals. J Allergy Clin Immunol. 2006;118(1):98–104. doi: 10.1016/j.jaci.2006.02.046. [DOI] [PubMed] [Google Scholar]
- 42.Masuoka M, Shiraishi H, Ohta S, Suzuki S, Arima K, Aoki S, Toda S, Inagaki N, Kurihara Y, Hayashida S, Takeuchi S, Koike K, Ono J, Noshiro H, Furue M, Conway SJ, Narisawa Y, Izuhara K. Periostin promotes chronic allergic inflammation in response to Th2 cytokines. J Clin Investig. 2012;122(7):2590–2600. doi: 10.1172/JCI58978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Li G, Jin R, Norris RA, Zhang L, Yu S, Wu F, Markwald RR, Nanda A, Conway SJ, Smyth SS, Granger DN. Periostin mediates vascular smooth muscle cell migration through the integrins alphavbeta3 and alphavbeta5 and focal adhesion kinase (FAK) pathway. Atherosclerosis. 2010;208(2):358–365. doi: 10.1016/j.atherosclerosis.2009.07.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zhou W, Ke SQ, Huang Z, Flavahan W, Fang X, Paul J, Wu L, Sloan AE, McLendon RE, Li X, Rich JN, Bao S. Periostin secreted by glioblastoma stem cells recruits M2 tumour-associated macrophages and promotes malignant growth. Nat Cell Biol. 2015;17(2):170–182. doi: 10.1038/ncb3090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zhao X, Hao J, Duan H, Rong Z, Li F. Phosphoinositide 3-kinase/protein kinase B/periostin mediated platelet-derived growth factor-induced cell proliferation and extracellular matrix production in lupus nephritis. Exp Biol Med (Maywood) 2017;242(2):160–168. doi: 10.1177/1535370216668050. [DOI] [PMC free article] [PubMed] [Google Scholar]