Abstract
All olfactory receptors identified in teleost fish are expressed in a single sensory surface, whereas mammalian olfactory receptor gene families segregate into different olfactory organs, chief among them the main olfactory epithelium expressing ORs and TAARs, and the vomeronasal organ expressing V1Rs and V2Rs. A transitional stage is embodied by amphibians, with their vomeronasal organ expressing more ‘modern’, later diverging V2Rs, whereas more ‘ancient’, earlier diverging V2Rs are expressed in the main olfactory epithelium. During metamorphosis, the main olfactory epithelium of Xenopus tadpoles transforms into an air-filled cavity (principal cavity, air nose), whereas a newly formed cavity (middle cavity) takes over the function of a water nose. We report here that larval expression of ancient V2Rs is gradually lost from the main olfactory epithelium as it transforms into the air nose. Concomitantly, ancient v2r gene expression begins to appear in the basal layers of the newly forming water nose. We observe the same transition for responses to amino acid odorants, consistent with the hypothesis that amino acid responses may be mediated by V2R receptors.
Keywords: Metamorphosis, Olfactory receptors, Evolution, V2R family, Calcium imaging, Amino acid odorants
Introduction
Amphibians occupy an intermediate stage in the water-to-land transition that occurred during vertebrate evolution. Thus, they have to cope with the drastically different demands placed by these two environments on their olfactory systems (see [1]). The olfactory organ of an aquatic species needs to sense water-borne, hydrophilic odorants, whereas a terrestrial species has to detect a mostly non-overlapping set of hydrophobic, airborne odorants. Amphibians solve this problem by adapting the larval olfactory organ during metamorphosis to meet the requirements of the adult lifestyle [2]. Anurans reconstruct the main olfactory epithelium (MOE) of their aquatic tadpoles into a so-called air nose (principal cavity) during metamorphosis [2]. Secondarily aquatic pipid frogs such as Xenopus laevis have an additional olfactory epithelium, the so-called water nose (middle cavity), which develops newly during metamorphosis [3–5], and is responsible for detecting water-borne odors (in terrestrial anurans, the middle cavity is non-sensory [2]).
During this metamorphotic reorganization, massive apoptotic cell death occurs, former larval olfactory receptor neurons (ORNs) are replaced and newly generated neurons form the middle cavity [4]. Thus, one expects massive changes in the molecular and functional response characteristics of olfactory sensory neurons during metamorphosis. However, so far, these changes have not been investigated, either at the molecular or the functional level.
We have recently identified an early diverging subclade of vomeronasal type 2 receptors (V2Rs) that was surprisingly expressed in the MOE of tadpoles [6], together with transient receptor potential cation channel (TRPC2), an element of the signal transduction cascade for V2Rs [7]. Remarkably, ORNs activated by amino acids, a major odor group for fish and frogs [8–11], show a similar spatial distribution to the V2R and TRPC2 expression [6]. It may be expected that amino acid responses and possibly V2R expression do not remain unaltered during metamorphosis, as there appears to be no use for receptors to detect water-borne odorants such as amino acids in the air nose of an adult frog.
We have therefore analyzed the amino acid responses of both water and air nose during metamorphosis and in post-metamorphotic frogs and report a gradual loss of response in what used to be larval MOE and a concomitant increase in amino acid responses in the newly formed water nose. Exactly the same transition is seen for the expression pattern of larval MOE-specific v2r genes, with a gradual loss of expression in the larval MOE and a concomitant increase of expression in the middle cavity. Moreover, spatial segregation within the middle cavity is very similar for amino acid responses and V2R expression. These results strengthen the hypothesis of V2R receptors carrying the olfactory response to amino acids in the amphibian sense of smell.
Materials and methods
Animal handling and preparation of acute slices
Xenopus laevis (of either sex, larval stages 57–58; 61–62 and post-metamorphotic froglets stage 66+ ; see [12]) were cooled in iced water to produce complete immobility and killed by transection of the brain at its transition to the spinal cord, as approved by the Göttingen University Committee for Ethics in Animal Experimentation. A block of tissue containing the olfactory organ, the olfactory nerves and the forebrain was cut out, and in post-metamorphotic animals parts of the skull were removed.
For acute slices, the tissue block was glued onto the stage of a vibroslicer (VT 1200s, Leica, Bensheim, Germany), covered with amphibian Ringer’s solution (see below) and sliced horizontally into 130–150 µm-thick slices. Slices included sensory epithelium of either only MC or PC or both.
In situ hybridization
For in situ hybridization, tissue blocks containing MOE and vomeronasal organ were cut horizontally, fixed in 4% (wt/vol) formaldehyde solution for 2 h at room temperature, equilibrated in 30% saccharose and embedded in Jung tissue-freezing medium (Leica, Bensheim, Germany). Cryostat sections of 10–12 μm (Leica CM1900) were dried at 55 °C and postfixed in 4% (wt/vol) paraformaldehyde for 10–15 min at room temperature. Hybridizations were performed overnight at 60 °C in 50% (vol/vol) formamide using standard protocols. Anti-DIG primary antibody coupled to alkaline phosphatase and NBT–BCIP (4-nitro blue tetrazolium chloride and 5-bromo-4-chloro-3-indolyl-phosphate, both from Roche Molecular Biochemicals) were used for signal detection. For each stage and gene, the olfactory tissue of ten animals was used for in situ hybridization, amounting to over 300 sections.
The following primers were used to generate probes: V2R-C 5′-CGCACAATAGCCAGTGA-3′ 5′-CTGAACTGCAAAGCCAA-3′, V2R-A1a 5′-GCCTTCTCCTGCTTTCC-3′ 5′-TGTCAGGGAGGCGTCT-3′, V2R-A1b 5′-CTTCTCATCTCCCTCATG-3′ 5′-AAATGTGTCAGGGAGCT-3′, OMP2 5′-CTTTCTTAGATGGCGCTGACC-3′ 5′-ACACACTTTTTTGTCTTGGG-3′, V2R-A3 E-1 5′-TGAGCTTCCTCCTCCTTGTC-3′ 5′-GGTAATGTCCGAGCTAAAAATGC-3′ [6] and TRPC2 5′-AAGGGATTAAGATGGACATCAA-3′ 5′-GCAATGCCCTTGTAGGTGTT-3′ [7].
Quantification of the spatial expression pattern
The position of cells was evaluated in the basal-to-apical dimension according to [6]. The relative height of the cell was defined as the distance of the cell soma center from the basal border of the epithelium divided by the total thickness of the epithelial layer at the position of the cell (h rel = h cell/h layer). Cell positions were measured using ImageJ (http://rsbweb.nih.gov/ij/). Distributions are visualized as histograms with 10 bins (x value given corresponds to the bin center), or unbinned as empirical cumulative distribution function (ECDF). Median, skewness and half-width of the spatial distributions were calculated from unbinned values using Open Office (version 3.2; http://www.openoffice.org/). Half-width of a height distribution was defined as the difference between the height values for the upper quartile and the lower quartile. The peak value was taken from the graphical representation of the histograms. To estimate whether two spatial distributions were significantly different, we performed Kolmogorov–Smirnov tests on the unbinned distributions (see [6]). This test is particularly suitable for continuous distributions and makes no assumptions about the nature of the distributions investigated, which is essential because the observed distributions are not Gaussian. Due to the sensitive nature of the test for large distributions (n > 100), we selected p < 0.01 as cutoff criterion for significant difference (see [6]). To count the total cells per section, slides were mounted with VectaShield containing DAPI (Vector). Fluorescence was analyzed using a Keyence BZ-9000 fluorescence microscope.
Calcium imaging, solutions, staining protocol and stimulus application
Amphibian Ringer’s consisted of (in mM): 98 NaCl, 2 KCl, 1 CaCl2, 2 MgCl2, 5 glucose, 5 Na-pyruvate, 10 HEPES (pH 7.8; osmolarity 230 mOsmol/l). Tissue slices (see above) were stained with the Ca2+-sensitive dye Fluo-4/AM (Molecular Probes, Leiden, The Netherlands) as described in our previous work [13]. The slices were then placed in a recording chamber, which was constantly perfused with amphibian Ringer’s solution applied by gravity feed from a storage syringe through a funnel applicator placed directly above the olfactory epithelia. Amino acid odors and forskolin were applied into the funnel without stopping the flow. Amphibian Ringer’s solution was constantly removed from the recording chamber through a syringe needle. All experiments were conducted at room temperature. The reproducibility of the responses was verified by regularly repeating the applications at least twice. The minimum interstimulus interval was at least 2 min in all of the experiments. Amino acid odors were applied as a mixture of 19 l-amino acids (for a detailed list of the amino acids, see [10]), all purchased from Sigma (Deisenhofen, Germany). Forskolin was also purchased from Sigma. The amino acids and forskolin were dissolved in amphibian Ringer’s solution and DMSO (10 mM stock), respectively, aliquoted and frozen. Aliquots were thawed only once and the working solution (mixture of amino acids, 100 µM; forskolin, 50 µM) was freshly prepared before performing the experiments.
Calcium imaging and data evaluation
Changes of intracellular calcium concentrations of individual ORNs of the epithelia of the PC ) and MC of the olfactory organ were monitored using a laser-scanning confocal microscope (LSM 510/Axiovert 100 M, Zeiss, Jena, Germany). Fluorescence images (excitation at 488 nm; emission >505 nm) of the epithelia of the principal and middle cavity were acquired at 1 Hz, with about ten images taken as control before the onset of stimulus delivery. The thickness of the optical slices excluded fluorescence detection from more than one cell layer. The data were analyzed using custom-written programs in MATLAB (Mathworks, Natick, USA). To facilitate selection of regions of interest, a “pixel correlation map” was obtained (see [14]). The fluorescence changes for individual ORNs are given as ΔF/F values. For more detailed information, see our previous work [13].
Results
Metamorphotic transition of amino acid odor sensitivity from the larval principal cavity to the emerging middle cavity
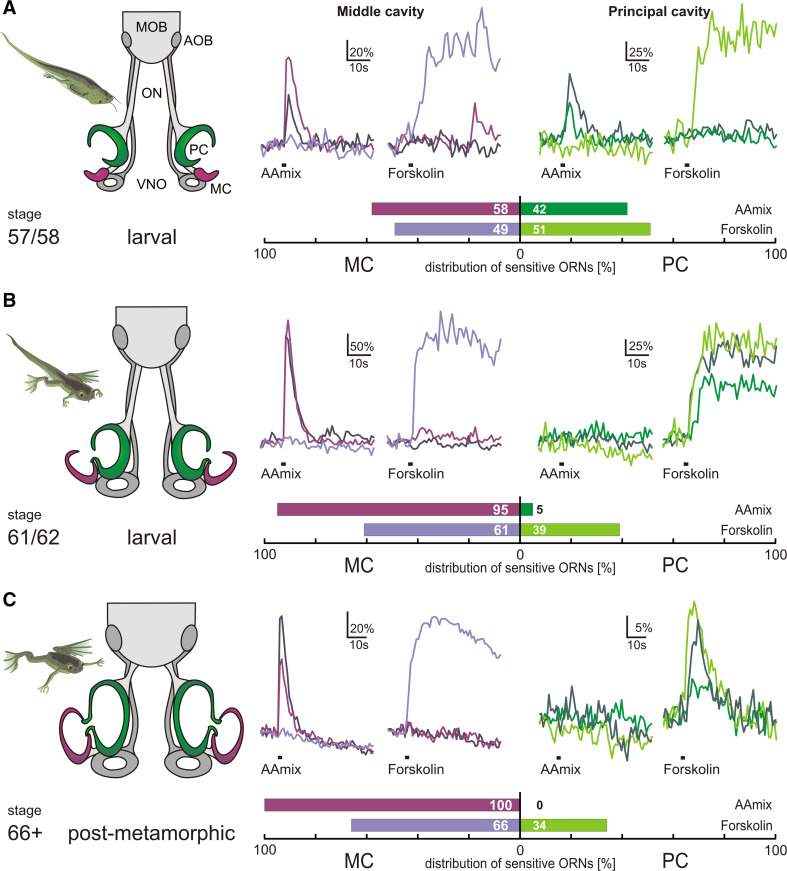
Amino acids constitute an important odor class in aquatic organisms, signaling the presence of food [8, 11]. To examine the fate of olfactory responses during metamorphosis of Xenopus laevis, we monitored responses to amino acids at three ontogenetic stages, 57/58, 61/62 and 66+ (beginning metamorphosis with functional ORNs already present in the MC, mid-metamorphosis, and post-metamorphosis, respectively; see Fig. 1 and [15, 16]). At the same stages, we concomitantly measured forskolin responses to visualize neurons that use a cAMP-mediated signal transduction cascade and are presumably ciliated [10]. Amino acid odor- and forskolin-induced responses of ORNs were measured as somatic Ca2+ transients in acute slice preparations of the epithelia of the MC and PC. Representative traces from individual ORNs are shown for all stages (Fig. 1).
Fig. 1.
Metamorphotic shift of the sensitivity to amino acid odors from the principal cavity to the newly formed middle cavity. The metamorphotic stages examined for Xenopus laevis are visualized as schematic drawings to the left. Calcium transients induced by amino acid mix (100 µM) and forskolin (50 µM) in acute slices are represented as ∆F/F, with responses of three representative ORNs overlaid for each stimulus (amino acid mix, forskolin) and olfactory cavity (middle and principal cavity). Shades of magenta are used for traces of middle cavity neurons, whereas shades of green are used for neurons located in the principal cavity. Within one panel, traces with the same color originate from the same neuron. The relative abundance of neurons analyzed is given as horizontal bar graphs below the representative traces; dark shades, amino acid responses; light shades, forskolin responses. a Late prometamorphotic stage, note the forming MC (magenta), the PC (green) and the vomeronasal organ (VNO; gray). At this stage, ORNs of the MC already show responsiveness to amino acid odors and forskolin, while the PC still shows responses characteristic of the PC of premetamorphotic larvae (see [10]). Amino acid odor and forskolin-sensitive ORNs are almost equally distributed between the epithelia of the two cavities. b Mid-metamorphosis stage, MC (magenta), PC (green) and VNO (gray) have grown in size. In this stage, the responsiveness to amino acid odors has almost completely shifted to the epithelium of the MC (see bar diagram). Forskolin-sensitive ORNs still coexist in both the epithelia of the MC and PC. c Post-metamorphotic froglet: all three epithelia have further grown in size and the olfactory nerve (ON) has become shorter. The responsiveness to amino acid odors has completely shifted to the epithelium of the MC. Forskolin-sensitive ORNs continue to coexist in both the epithelia of the MC and PC (see bar diagram). AOB accessory olfactory bulb, MOB main olfactory bulb, ON olfactory nerve, ORNs olfactory receptor neurons, PC principal cavity, MC middle cavity, VNO vomeronasal organ, AAmix amino acid mixture
In all stages, the two stimuli activated mutually exclusive subsets of ORNs (Fig. 1; Table 1), with the sole exception of two neurons found at stage 66+, which reacted to both stimuli (Table 1). The MC contained amino acid-responsive as well as forskolin-responsive cells at all stages examined, similar to the larval MOE [10]. In contrast, the developing PC retains forskolin responses, but gradually loses all amino acid responses (Fig. 1).
Table 1.
Specificity of olfactory receptor neuron (ORN) responses during metamorphosis
| Developmental stage | # ORNs only responsive to amino acids Total (MC, PC) |
# ORNs only responsive to forskolin Total (MC, PC) |
# ORNs responsive to both Total (MC, PC) |
# Slices evaluated Total (MC, PC) |
|---|---|---|---|---|
| 57/58 | 24 (14, 10) | 43 (21, 22) | 0 (0, 0) | 19 (10, 9) |
| 61/62 | 22 (21, 1) | 61 (37, 24) | 0 (0, 0) | 13 (7, 6) |
| 66+ | 26 (26, 0) | 35 (23, 12) | 2 (2, 0) | 16 (10, 6) |
Forskolin-responsive ORNs were found in roughly similar frequency in the middle and principal cavity during all metamorphosis stages analyzed (Fig. 1). Likewise, in early metamorphosis (stage 57/58), the responses to amino acids were nearly equally distributed across the middle and principal cavity (Fig. 1). However, during mid-metamorphosis the balance shifted massively, with only 5% of amino acid-responsive neurons located in the principal cavity (Fig. 1). Finally, post-metamorphosis (stage 66+) not a single amino acid-responsive neuron was found in the principal cavity, whereas many such cells were present in the newly formed middle cavity (Fig. 1).
During metamorphosis, expression of the broadly expressed olfactory receptor V2R-C ceases in the principal cavity and concomitantly appears in the newly forming middle cavity
We have previously suggested that the amphibian olfactory response to amino acids is carried by V2R receptors, based on spatial co-segregation within the tadpole olfactory organ [6]. Here, we show that olfactory amino acid responses migrate to the newly forming middle cavity during metamorphosis. If V2R receptors do mediate these responses, we expect their expression pattern to switch in parallel with the amino acid responses during metamorphosis.
Amphibian v2r genes are subdivided into three subfamilies (V2R-A1, A2, and A3), plus V2R-C as the most ancestral gene of the family. This gene is broadly expressed in possibly all microvillous neurons of the tadpole MOE, whereas other v2r genes are expressed in sparse subsets of neurons [6, 7]. Thus, V2R-C can serve as a general indicator for V2R expression. We performed in situ hybridization on tissue sections of the olfactory organ to follow the expression of V2R-C over the course of metamorphosis up to the young post-metamorphotic frog stage.
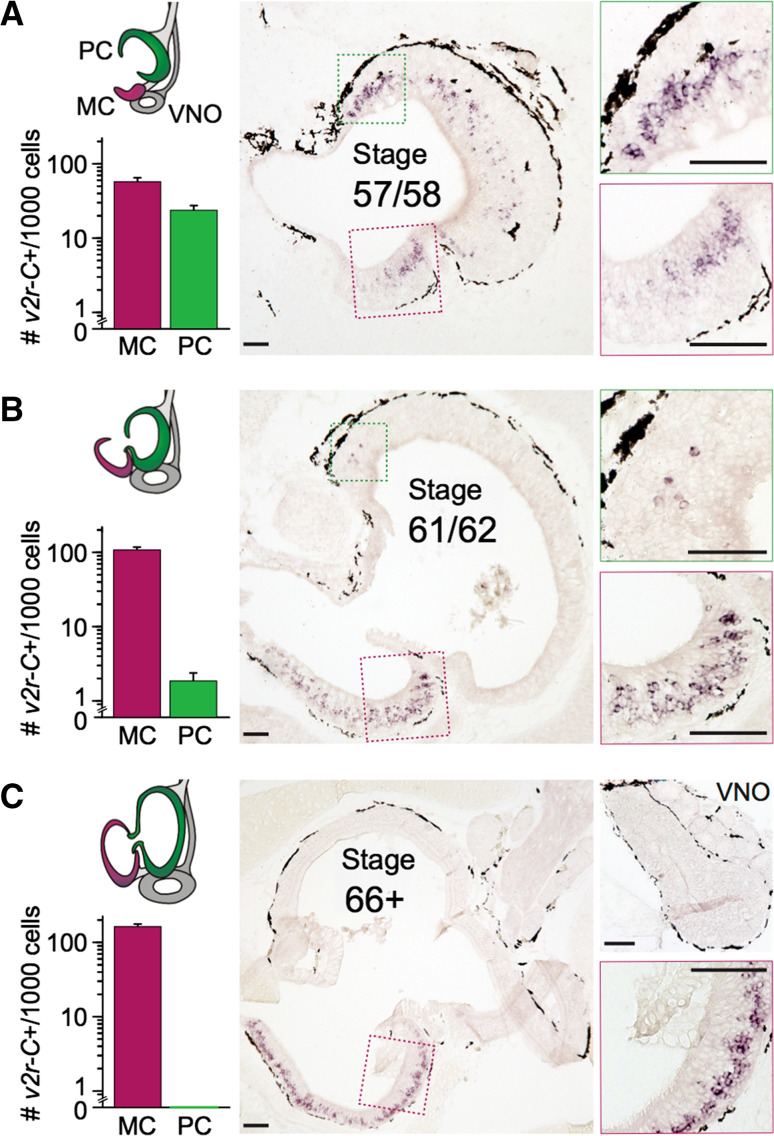
We report that during metamorphosis, the expression of V2R-C becomes gradually less frequent in the MOE (Fig. 2). At stage 57/58, V2R-C-expressing neurons are found in high frequency in both the MOE and the newly forming middle cavity (water nose). At stage 61/62, i.e., during mid-metamorphosis of the former MOE into the adult principal cavity (air nose), very little expression remains in the MOE. Post-metamorphosis, at stage 66+, not a single V2R-C-expressing cell could be found in the principal cavity among several hundred labeled neurons examined (Fig. 2). For TRPC2, a component of the signal transduction pathway for V2Rs, a very similar gradual shift in expression from the MOE to the middle cavity was observed (data not shown). Incidentally, V2R-C is also completely absent in the VNO (Fig. 2).
Fig. 2.
Gradual transition from the MOE to the MC for the broadly expressed v2r-C gene. Expression of V2R-C was monitored by in situ hybridization of cryostat sections throughout metamorphosis. A schematic representation of the ontogenetic stages is shown to the left (see also Fig. 1), with quantitative results for the respective stage shown as bar graphs just below. Labeled cells were counted in MC/PC in 6/6 sections (stage 57/58) or 4/4 sections (stage 61/62 and stage 66+), and frequency is given as mean ± SEM of labeled cells per 1000 total cells (counted as DAPI signals in three sections). Total cell numbers roughly double in the observed time window, with about fourfold more cells in PC compared to MC. The color code is identical to that used in Fig. 1 for schemes and bar graphs, respectively. Middle column, representative tissue sections are shown, with magnified subregions shown to the right. In the prometamorphotic stage (top row), similar numbers of labeled cells are present in the principal and middle cavity. In mid-metamorphosis (middle row), very few V2R-C-expressing cells remain in the principal cavity. Post-metamorphosis (bottom row), not a single V2R-C-expressing cell is detected in the principal cavity, with over 15% of total cells concomitantly expressing V2R-C in the middle cavity. Bottom right subpanel, the post-metamorphotic VNO also does not contain V2R-C-expressing cells. Scale bar 100 µm. PC principal cavity, MC middle cavity, VNO vomeronasal organ
Taken together, V2R-C-expressing cells show a very similar transition from larval MOE to middle cavity, as observed for the amino acid responses (Fig. 1), supporting the hypothesis that V2R-expressing neurons mediate amino acid responses.
Post-metamorphosis ancient v2r genes and a component of their signal transduction cascade are completely absent in the principal cavity
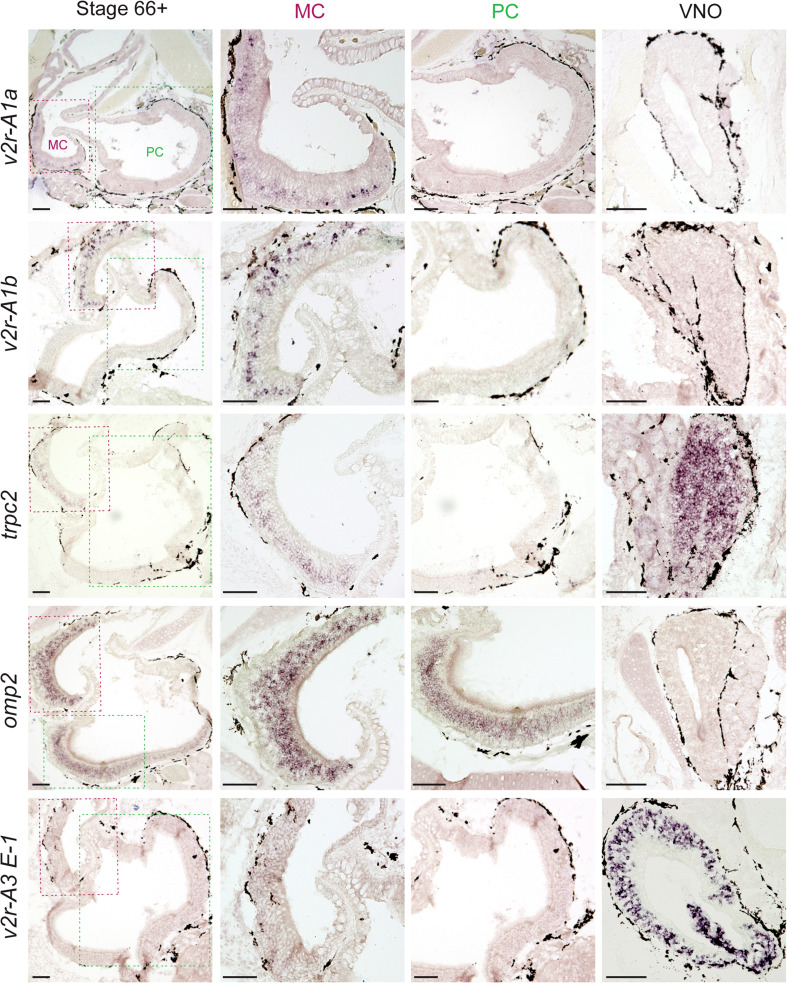
Next, we evaluated the spatial expression pattern of two ancient v2r genes, V2R-A1a, and V2R-A1b, after metamorphosis was completed. In tadpoles, ORNs expressing these genes appear to constitute a subpopulation of V2R-C-expressing cells [6]. Both genes were found to be exclusively expressed in the middle cavity and absent in the principal cavity and VNO (Figs. 3, 4). Thus, expression of these genes co-migrates both with the V2R-C expression and the amino acid responses. In contrast, a late diverging v2r gene known to be expressed exclusively in the VNO of tadpoles (V2R-A3 E1), is still exclusively found in VNO after metamorphosis (Fig. 3). The probe for V2R-A3 E1 cross-reacts with 95 other late diverging v2r genes [6]; in other words, it reflects the expression pattern of up to 95 v2r genes. This suggests that the larval split in the expression pattern between early and late diverging v2r genes is faithfully reproduced during metamorphosis, even though the site of expression changes for the early diverging genes.
Fig. 3.
Post-metamorphosis ancient v2r genes and a component of their signal transduction cascade are completely absent from the PC. PC and MC as well as VNO was examined for the expression of V2R-A1a, V2R-A1b, V2R-A3 E1, TRPC2 and OMP2 at stage 66+, using in situ hybridization. Left column low magnification view shows both PC and MC; second column middle cavity at higher magnification; third column principal cavity at higher magnification; right column VNO. Scale bar 100 µm. PC principal cavity, MC middle cavity, VNO vomeronasal organ
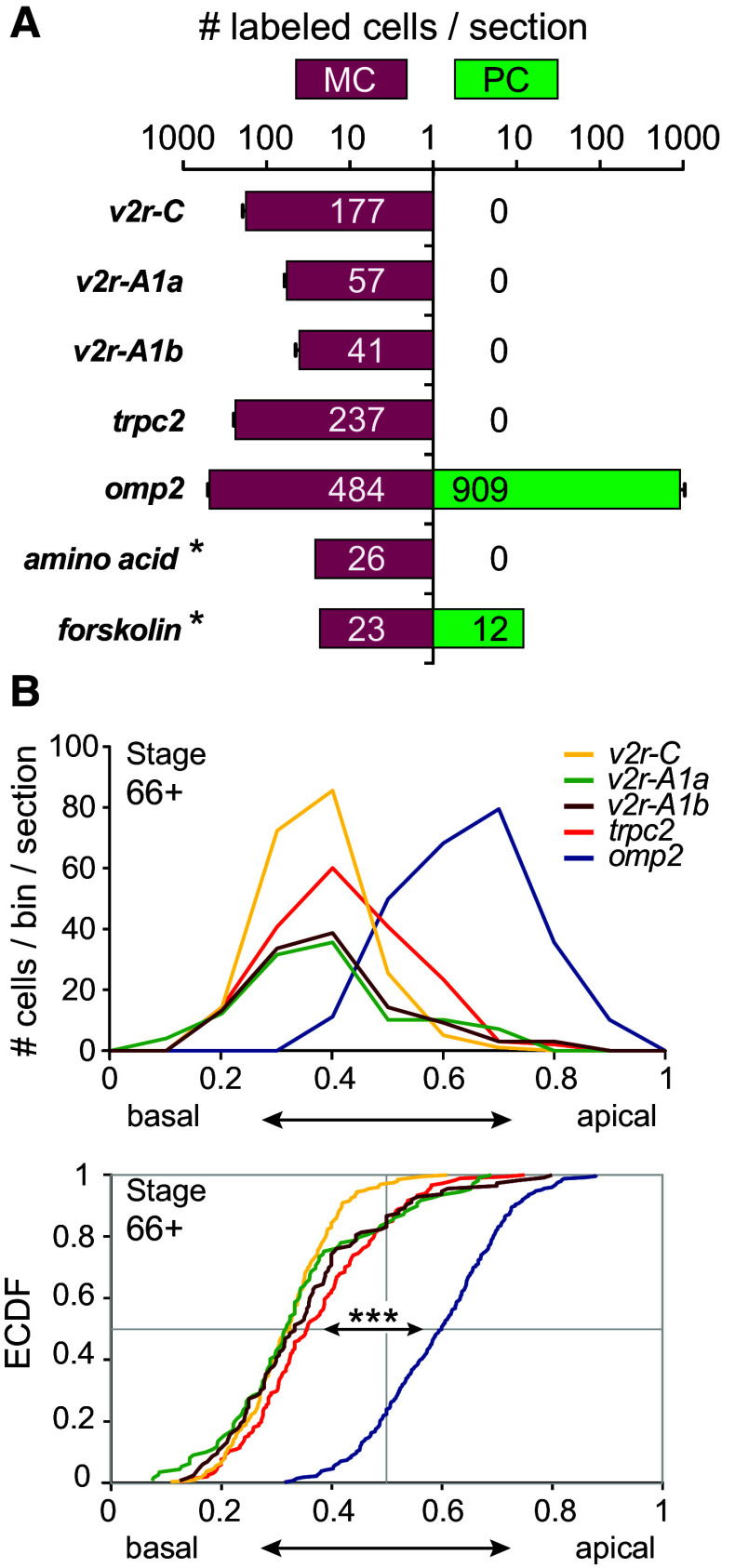
Fig. 4.
Ancient V2Rs form a basal expression domain in the post-metamorphotic MC. a Quantification of cells expressing V2R-A1a, V2R-A1b, V2R-C, TRPC2, and OMP2 as well as cells responding to forskolin and amino acids at stage 66+. Numbers of labeled cells per section are given as mean ± SD for v2r genes and TRPC2 (MC: n = 4 and n = 3, respectively; PC: n = 10). Numbers for OMP are from three sections (mean ± SD); asterisk cumulative values (from ten sections for MC and six for PC). At this stage, total cells per section in the MC are about one-fourth of those in the PC (990 ± 120 vs. 4040 ± 190; mean ± SD, n = 3). Note the broad distribution of OMP2-expressing cells and of forskolin-responsive cells, in contrast to the ancient v2r genes, TRPC2 and amino acid responses, which are restricted to the MC. b Height distribution of cells expressing V2R-A1a, V2R-A1b, V2R-C, TRPC2 and OMP2 within the MC at stage 66+. Top panel shows data as histogram, and bottom panel as empirical cumulative distribution function. Note the apical OMP peak and distribution clearly segregating from the more basal distributions for v2r genes and TRPC2, whereas all v2r gene distributions are not significantly different from each other; ***p < 10 −10 as evaluated by Kolmogorov–Smirnov test (see [6])
Finally, we examined the expression pattern of two marker genes for ciliated and microvillous receptor neurons (OMP and TRPC2, respectively; [6, 7]). Strong OMP expression was seen in both middle and principal cavity post-metamorphosis (Figs. 3, 4). This result is consistent with the presence of forskolin-responsive neurons in both middle and principal cavity (Fig. 1). As expected, the VNO did not exhibit OMP expression (Fig. 3). On the other hand, TRPC2, a signal transduction component of microvillous neurons [7, 17], was found to be expressed in the MC and the VNO, but absent in the PC (Figs. 3, 4). Thus, the MC mimics the mixed ORN population of the larval MOE, with both ciliated and microvillous receptor neurons, and responses to forskolin as well as amino acids, even though this organ is built de novo during metamorphosis [4].
Ancient V2Rs form a basal expression domain in the middle cavity very similar to that observed for expression in tadpole main olfactory epithelium
In the tadpole main olfactory epithelium, V2R-, TRPC2-, and OMP-expressing receptor neurons largely segregate according to height, with V2R- and TRPC2-expressing neurons found in a basal layer, whereas OMP-positive neurons occupy more apical positions [6, 7]. Presumably, these populations resemble microvillous and ciliated ORNs, respectively.
We were interested to see whether this spatial expression pattern would re-form in the de novo generated MC after metamorphosis. The relative height within the organ was measured for post-metamorphotic stage 66+. V2R-C-expressing cells show a basal peak at 0.4 relative height and the same peak was observed for V2R-A1a and V2R-A1b expression as well as for TRPC2. In contrast, OMP expression showed an apical peak at 0.7 relative height (Fig. 4). These values are very similar to those observed in the tadpole MOE (0.3-0.4 for V2R-C, V2R-A1a, and V2R-A1b compared to 0.8 for OMP2 [6]). Thus, the segregation in a basal V2R-expressing domain and an apical domain for OMP-expressing neurons is conserved in the de novo formed post-metamorphotic MC.
Discussion
During metamorphosis, an extensive remodeling of the amphibian bauplan takes place, including a reconstruction of the olfactory system reflecting the transition from an aquatic to a terrestrial habitat. The larval MOE of anurans transforms into the PC, the so-called air nose, dedicated to the detection of airborne smells. In secondarily aquatic anurans such as Xenopus, a newly formed MC, the so-called water nose, takes over the detection of water-borne odors.
Here, we report that olfactory neuronal responses to a behaviorally important class of aquatic odors—amino acids, which serve as food odor—migrate during metamorphosis from the larval MOE to the MC. After metamorphosis, the PC retains not a single amino acid-responsive neuron. In parallel, the expression of early-derived V2Rs disappears from the larval MOE, as it transforms into the adult PC and concomitantly appears in the newly generated MC. This is in line with our previous findings that neuronal populations of the olfactory organ change substantially during metamorphosis: larval ORNs undergo apoptotic cell death, do not persist in the long run and are replaced by newly generated neurons [4]. The basal enrichment of V2R-expressing cells within the MC is indistinguishable from that of TRPC2-expressing neurons, very similar to the situation observed in the tadpole MOE [7]. Thus, the correlation between V2R and TRPC2 expression is retained in a newly formed organ [4], the MC. In the same vein, the distribution of amino acid-responsive cells between the MC and the PC closely parallels the distribution of V2R-C-expressing cells, consistent with the hypothesis that V2Rs expressed in microvillous neurons mediate the amino acid responses in amphibians, similar to what has been shown for teleost fish [18].
Like the larval MOE, the MC contains both amino acid-responsive and forskolin-responsive neurons. In larval MOE, amino acid responses have been shown to be mediated primarily by microvillous neurons, but to some extent also by forskolin-responsive (and forskolin-insensitive) ciliated neurons [7, 10, 19]. A small minority of both forskolin- and amino acid-responsive neurons is also observed in the MC (this study), but not in the post-metamorphotic PC. Thus this subpopulation is dying out in the PC, but to some extent replaced during ontogenesis of the MC. Considering that forskolin responsiveness is a hallmark of the adenylate cyclase signaling pathway characteristic for OR-expressing ciliated neurons and that OMP expression closely parallels forskolin responsiveness ([6, 10], this study), it may be assumed that the forskolin-responsive neurons of the MC represent ciliated neurons, whose ligands are to be found among water-borne odors, and may also include amino acids. Such a dichotomic neuronal representation of amino acids appears to be an evolutionary ancient feature of neuronal representation of amino acids, since a contribution of both ciliated and microvillous ORNs has been suggested for several teleost fish species [20].
Previous work by the Breer group [21] has suggested that the adult MC contains OR class I-expressing neurons (subgroup alpha in Niimura and Nei nomenclature, [22, 23]). For one of these OR class I receptors, amino acid responses have been shown [24], so this class of receptors would be a plausible candidate for mediating amino acid responses in ciliated neurons.
Taken together, our results confirm and extend the molecular and functional segregation of olfaction in the middle vs. the principal cavity. Furthermore, they show that the unusual division of a large olfactory receptor gene family between two main olfactory organs is faithfully kept during metamorphosis: only those v2r genes expressed in tadpoles’ MOE later become expressed in the MC (water nose). Thus, the dichotomy of V2R expression in two different organs is not an immature feature of the Xenopus olfactory system, but stably maintained in adulthood. As both V2R subclades are expressed in microvillous ORNs, it will be informative to identify the regulatory elements which direct microvillous neurons of the MOE toward expression of early diverging V2Rs (subfamilies C and A1) and to compare them with those resulting in late diverging V2Rs (subfamilies A2 and A3) being expressed in the vomeronasal microvillous neurons.
Another feature of V2R expression, which is stable during metamorphosis, is their basal expression, suggesting the same segregation of basal microvillous and apical ciliated ORNs in larval MOE and post-metamorphotic MC. Interestingly, a similar segregation between ciliated and microvillous neurons is found in the single olfactory surface of teleost fish, although the relative positions are inversed, with microvillous neurons occupying apical positions [18].
Finally, it is worth noting that in adult secondarily aquatic amphibians, detection of non-volatile odors is performed by two olfactory organs, the middle cavity/water nose and the vomeronasal organ. We describe here post-metamorphosis, the persistent expression of ancient V2Rs in the water nose and of ‘modern’ V2Rs in the VNO, suggesting a corresponding segregation of functional properties between these two organs. It is tempting to speculate that the odors detected by the water nose are no longer relevant for strictly terrestrial species such as reptiles and mammals, whereas the VNO with few exceptions has kept its relevance throughout vertebrate evolution as detector of non-volatile pheromones.
Acknowledgements
This work was supported by DFG Grants 4113/3-1 (I.M.), KO1046/10-1 (S. I. K.), Schwerpunktprogramm 1392 (I. M. and S. I. K.), Cluster of Excellence and DFG Research Center Nanoscale Microscopy and Molecular Physiology of the Brain (I. M.), and German Ministry of Research and Education (BMBF), Grant Number: 1364480 (I. M.).
Compliance with ethical standards
Conflict of interest
The authors declare no competing financial interests.
References
- 1.Korsching SI (2016) Aquatic olfaction. In: Zufall F, Munger SD (eds) Chemosensory transduction: the detection of odors, tastes, and other chemostimuli, Academic Press, Elsevier, Amsterdam, Netherland, pp 82–100
- 2.Reiss JO, Eisthen HL (2008) Comparative anatomy and physiology of chemical senses in amphibians. In: Thewissen JGM, Nummela S (eds) Sensory evolution on the threshold. Adaptations in secondarily aquatic vertebrates, University of California Press, Berkeley, CA, USA, pp 43–63
- 3.Hansen A, Reiss JO, Gentry CL, Burd GD. Ultrastructure of the olfactory organ in the clawed frog, Xenopus laevis, during larval development and metamorphosis. J Comp Neurol. 1998;398:273–288. doi: 10.1002/(SICI)1096-9861(19980824)398:2<273::AID-CNE8>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- 4.Dittrich K, Kuttler J, Hassenklöver T, Manzini I. Metamorphic remodeling of the olfactory organ of the African clawed frog, Xenopus laevis . J Comp Neurol. 2016;524:986–998. doi: 10.1002/cne.23887. [DOI] [PubMed] [Google Scholar]
- 5.Higgs DM, Burd GD. Neuronal turnover in the Xenopus laevis olfactory epithelium during metamorphosis. J Comp Neurol. 2001;433:124–130. doi: 10.1002/cne.1130. [DOI] [PubMed] [Google Scholar]
- 6.Syed AS, Sansone A, Nadler W, et al. Ancestral amphibian v2rs are expressed in the main olfactory epithelium. Proc Natl Acad Sci USA. 2013;110:7714–7719. doi: 10.1073/pnas.1302088110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sansone A, Syed AS, Tantalaki E, et al. Trpc2 is expressed in two olfactory subsystems, the main and the vomeronasal system of larval Xenopus laevis . J Exp Biol. 2014;217:2235–2238. doi: 10.1242/jeb.103465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hara T. Feeding behaviour in some teleosts is triggered by single amino acids through olfaction. J Fish Biol. 2006;68:810–825. doi: 10.1111/j.0022-1112.2006.00967.x. [DOI] [Google Scholar]
- 9.Manzini I, Brase C, Chen T-W, Schild D. Response profiles to amino acid odorants of olfactory glomeruli in larval Xenopus laevis . J Physiol. 2007;581:567–579. doi: 10.1113/jphysiol.2007.130518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gliem S, Syed AS, Sansone A, et al. Bimodal processing of olfactory information in an amphibian nose: odor responses segregate into a medial and a lateral stream. Cell Mol Life Sci. 2013;70:1965–1984. doi: 10.1007/s00018-012-1226-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zielinski BS, Hara T (2007) Olfaction. In: Hara T, Zielinski BS (eds) Fish physiology: sensory systems neuroscience, vol. 25. Academic Press, Elsevier, Amsterdam, Netherland, pp 1–44
- 12.Nieuwkoop PD, Faber J. Normal table of Xenopus laevis (Daudin) New York: Garland Publishing Inc; 1994. [Google Scholar]
- 13.Hassenklöver T, Kurtanska S, Bartoszek I, et al. Nucleotide-induced Ca2+ signaling in sustentacular supporting cells of the olfactory epithelium. Glia. 2008;56:1614–1624. doi: 10.1002/glia.20714. [DOI] [PubMed] [Google Scholar]
- 14.Junek S, Chen T-W, Alevra M, Schild D. Activity correlation imaging: visualizing function and structure of neuronal populations. Biophys J. 2009;96:3801–3809. doi: 10.1016/j.bpj.2008.12.3962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Etkin W. Growth and resorption phenomena in anuran metamorphosis. Physiol Zool. 1932;5:275–300. doi: 10.1086/physzool.5.2.30152791. [DOI] [Google Scholar]
- 16.Mezler M, Konzelmann S, Freitag J, et al. Expression of olfactory receptors during development in Xenopus laevis . J Exp Biol. 1999;202:365–376. doi: 10.1242/jeb.202.4.365. [DOI] [PubMed] [Google Scholar]
- 17.Liberles SD. Mammalian pheromones. Annu Rev Physiol. 2014;76:151–175. doi: 10.1146/annurev-physiol-021113-170334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sato Y, Miyasaka N, Yoshihara Y. Mutually exclusive glomerular innervation by two distinct types of olfactory sensory neurons revealed in transgenic zebrafish. J Neurosci. 2005;25:4889–4897. doi: 10.1523/JNEUROSCI.0679-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sansone A, Hassenklöver T, Syed AS, et al. Phospholipase C and diacylglycerol mediate olfactory responses to amino acids in the main olfactory epithelium of an amphibian. PLoS One. 2014;9:e87721. doi: 10.1371/journal.pone.0087721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hansen A, Rolen SH, Anderson K, et al. Correlation between olfactory receptor cell type and function in the channel catfish. J Neurosci. 2003;23:9328–9339. doi: 10.1523/JNEUROSCI.23-28-09328.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Freitag J, Krieger J, Strotmann J, Breer H. Two classes of olfactory receptors in Xenopus laevis . Neuron. 1995;15:1383–1392. doi: 10.1016/0896-6273(95)90016-0. [DOI] [PubMed] [Google Scholar]
- 22.Niimura Y, Nei M. Evolutionary dynamics of olfactory receptor genes in fishes and tetrapods. Proc Natl Acad Sci USA. 2005;102:6039–6044. doi: 10.1073/pnas.0501922102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Niimura Y. Olfactory receptor multigene family in vertebrates: from the viewpoint of evolutionary genomics. Curr Genomics. 2012;13:103–114. doi: 10.2174/138920212799860706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mezler M, Fleischer J, Breer H. Characteristic features and ligand specificity of the two olfactory receptor classes from Xenopus laevis . J Exp Biol. 2001;204:2987–2997. doi: 10.1242/jeb.204.17.2987. [DOI] [PubMed] [Google Scholar]